Abstract
Background
Granulocyte‐colony stimulating factor (G‐CSF) seems to play an important role in the process of embryo implantation and continuation of pregnancy. It has been used during in vitro fertilisation (IVF) treatment for subfertile women with chronically thin endometrium and those with previous multiple IVF failures. It is currently unknown whether G‐CSF is effective in improving results following assisted reproductive technology (ART).
Objectives
To evaluate the effectiveness and safety of G‐CSF in women undergoing ART.
Search methods
We searched the Cochrane Gynaecology and Fertility Group Specialised Register, CENTRAL, MEDLINE, Embase, ClinicalTrials.gov, and the World Health Organization International Clinical Trials Registry Platform in February 2019. We searched reference lists of relevant articles and handsearched relevant conference proceedings.
Selection criteria
Randomised controlled trials (RCTs) comparing G‐CSF administration versus no treatment or placebo in subfertile women undergoing IVF treatment.
Data collection and analysis
Two review authors independently screened studies, extracted data, and assessed risk of bias. The primary outcomes were live‐birth rate and miscarriage rate following G‐CSF administration. We have reported ongoing pregnancy rate in cases where studies did not report live birth but reported ongoing pregnancy. Secondary outcomes were clinical pregnancy rate, multiple pregnancy rate, adverse events, ectopic pregnancy rate, small for gestational age at birth, abnormally adherent placenta, and congenital anomaly rate. We analysed data using risk ratio (RR), Peto odds ratio and a fixed‐effect model. We assessed the quality of the evidence using the GRADE criteria.
Main results
We included 13 trials involving 522 women who received G‐CSF and 528 women who received placebo or no additional treatment during IVF. The main limitations in the quality of the evidence were inadequate reporting of study methods and high risk of performance bias due to lack of blinding. We assessed only two of the 13 included trials as at a low risk of bias. None of the trials reported the primary effectiveness outcome of live‐birth rate.
We are uncertain whether G‐CSF administration improves ongoing pregnancy rate compared to control in subfertile women undergoing ART (RR 1.62, 95% confidence interval (CI) 0.86 to 3.08; 1 RCT; participants = 150; very low‐quality evidence). For a typical clinic with 16% ongoing pregnancy rate, G‐CSF administration would be expected to result in ongoing pregnancy rates between 14% and 50%. We are uncertain whether G‐CSF administration reduces miscarriage rate (Peto odds ratio 0.40, 95% CI 0.09 to 1.77; 2 RCTs; participants = 291; I² = 0%; very low‐quality evidence) compared to the control group in subfertile women undergoing ART.
We are uncertain whether G‐CSF administration improves overall clinical pregnancy rate compared to control in subfertile women undergoing ART (RR 1.65, 95% CI 1.32 to 2.06; 12 RCTs; participants = 1050; I² = 19%; very low‐quality evidence). For a typical clinic with 18% clinical pregnancy rate, G‐CSF administration would be expected to result in clinical pregnancy rates between 23% and 37%. In the unselected IVF population, we are uncertain whether G‐CSF administration improves clinical pregnancy rate compared to the control group (RR 1.12, 95% CI 0.75 to 1.68; 2 RCTs; participants = 291; I² = 32%; low‐quality evidence).
Sensitivity analysis restricted to studies at low‐risk of bias suggests no evidence of difference in clinical pregnancy after G‐CSF administration versus control group in an unselected IVF population ( RR 1.46, 95% CI 0.81 to 2.65; 1 RCT; participants = 150).
G‐CSF administration may improve clinical pregnancy rate in women with two or more previous IVF failures compared to the control group (RR 2.10, 95% CI 1.53 to 2.89; 6 RCTs; participants = 553; I² = 0%; low‐quality evidence). However, sensitivity analysis restricted to studies at low‐risk of selection bias suggests that there is no evidence of difference in clinical pregnancy rate after G‐CSF administration versus control group in women with two or more IVF failures (RR 2.00, 95% CI 0.75 to 5.33; 1 RCT; participants =100). In subfertile women with thin endometrium undergoing ART, we are uncertain whether G‐CSF administration improves clinical pregnancy rate compared to the control group (RR 1.58, 95% CI 0.95 to 2.63; 4 RCTs; participants = 206; I² = 30%; low‐quality evidence). No studies in this subgroup remained in a sensitivity analysis restricted to studies at low‐risk of selection bias.
No study reported on multiple pregnancy rate. Only four trials reported adverse events as an outcome, and none of them reported any major adverse events following either G‐CSF administration or placebo/no treatment.
Authors' conclusions
In subfertile women undergoing ART, we are uncertain whether the administration of G‐CSF improves ongoing pregnancy or overall clinical pregnancy rates or reduces miscarriage rate compared to no treatment or placebo, whether in all women or those with thin endometrium, based on very low‐quality evidence. Low‐quality evidence suggests that G‐CSF administration may improve clinical pregnancy rate in women with two or more IVF failures, but the included studies had unclear allocation concealment or were at high risk of performance bias.
Plain language summary
Use of granulocyte‐colony stimulating factor during in vitro fertilisation treatment
Review question
To assess the safety and usefulness of giving granulocyte‐colony stimulating factor (G‐CSF) in women undergoing in vitro fertilisation (IVF).
Background
It has been suggested that in women who have persistent thin endometrium (inner lining of the womb) or who have experienced multiple failed IVF, giving G‐CSF during treatment may improve IVF outcomes. G‐CSF is a type of growth factor that stimulates bone marrow to produce certain types of white blood cells. In the endometrium, G‐CSF promotes regenerative activity of the cells and helps in increasing the blood supply. It is proposed that G‐CSF may increase IVF success by helping to improve embryo implantation (adherence to the lining of the womb) and facilitating continuation of pregnancy. It can be given either by injecting it inside the uterus (womb) with the help of a syringe around the time of embryo transfer or subcutaneously (under the skin) after embryo transfer.
Study characteristics
We found 13 trials (1070 women) comparing G‐CSF with placebo or no treatment. Nine trials evaluated the role of G‐CSF in women undergoing IVF, with a majority of trials including those women with two or more failed attempts. The remaining four trials investigated the role of G‐CSF in women with thin endometrium undergoing IVF. The evidence is current to February 2019.
Key results
We are uncertain whether giving G‐CSF in women undergoing IVF improves chances of ongoing pregnancy or overall clinical pregnancy rates or reduces miscarriage rate compared to placebo or no treatment. For a typical clinic with 16% ongoing pregnancy rate, G‐CSF administration would be expected to result in ongoing pregnancy rates between 14% and 50%. No study reported on multiple pregnancy rate. Only four trials reported adverse events as an outcome, and none of them reported any major adverse events following either G‐CSF administration or placebo/no treatment.
Quality of evidence
We are uncertain whether giving G‐CSF improves ongoing pregnancy or reduces miscarriage rates in women undergoing IVF based on very low‐quality evidence. The quality of the evidence was reduced because of risk of bias.
Summary of findings
Summary of findings 1. G‐CSF compared to no treatment or placebo in subfertile women undergoing assisted reproduction.
| G‐CSF compared to no treatment or placebo in subfertile women undergoing assisted reproduction | ||||||
| Patient or population: subfertile women undergoing assisted reproduction Setting: private clinic or academic setting Intervention: G‐CSF Comparison: no treatment or placebo | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Risk with no treatment or placebo in subfertile women undergoing assisted reproduction | Risk with G‐CSF | |||||
| Ongoing pregnancy rate per woman randomised |
162 per 1000 | 263 per 1000 (139 to 499) | RR 1.62 (0.86 to 3.08) | 150 (1 RCT) | ⊕⊝⊝⊝ VERY LOW 1 2 | |
| Miscarriage rate per woman randomised | 35 per 1000 | 14 per 1000 (3 to 61) | Peto OR 0.40 (0.09 to 1.77) | 291 (2 RCTs) | ⊕⊝⊝⊝ VERY LOW 2 3 4 | |
| Clinical pregnancy rate per woman randomised |
178 per 1000 | 294 per 1000 (235 to 367) | RR 1.65 (1.32 to 2.06) | 1050 (12 RCTs) | ⊕⊝⊝⊝ VERY LOW 2 5 6 | |
| Clinical pregnancy rate ‐ unselected or unstated IVF number |
232 per 1000 | 260 per 1000 (174 to 390) | RR 1.12 (0.75 to 1.68) | 291 (2 RCTs) | ⊕⊕⊝⊝ LOW 2 3 | |
| Clinical pregnancy rate ‐ 2 or more IVF failures | 148 per 1000 | 312 per 1000 (227 to 429) | RR 2.10 (1.53 to 2.89) | 553 (6 RCTs) | ⊕⊕⊝⊝ LOW 2 7 | |
| Clinical pregnancy rate ‐ women with thin endometrium | 184 per 1000 | 291 per 1000 (175 to 485) | RR 1.58 (0.95 to 2.63) | 206 (4 RCTs) |
⊕⊕⊝⊝ LOW 2 8 | |
| Multiple pregnancy rate | None of the included trials reported multiple pregnancy rate as an outcome. | |||||
| Adverse events per woman randomised | 0 per 1000 | 0 per 1000 (0 to 0) |
Not estimable | 410 (4 RCTs) |
‐ | None of the 4 trials reported any major adverse effect. |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; G‐CSF: granulocyte‐colony stimulating factor; IVF: in vitro fertilisation; OR: odds ratio; RCT: randomised controlled trial; RR: risk ratio | ||||||
| GRADE Working Group grades of evidence High quality: We are very confident that the true effect lies close to that of the estimate of the effect. Moderate quality: We are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low quality: Our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low quality: We have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
1Very serious indirectness, downgraded by two levels: only one study reported ongoing pregnancy rate and the study included only unselected population, limiting applicability of results to the other subpopulations. 2Serious imprecision, downgraded by one level due to wide confidence interval and low number of events. 3Serious risk of bias, downgraded by one level: one study had low risk of bias for all domains. One study was at high risk of reporting bias. 4Serious indirectness, downgraded by one level: only two studies reported miscarriage rates, and both the studies included an unselected IVF population. The reported miscarriages in the studies only captured first trimester losses. 5Serious risk of bias, downgraded by one level: only two studies were at low risk of bias for all domains. Most of the remaining studies were at high risk of performance bias and unclear risk of bias for allocation concealment. There is a likelihood of overestimation of the treatment effect. 6 Serious inconsistency, downgraded by two levels: there was substantial variability between effect estimates between the two subgroups, P = 0.06. 7Serious risk of bias, downgraded by one level: only one study was at low risk of bias for all domains. Most of the remaining studies were at high risk of performance bias and unclear risk of bias for allocation concealment. There is a likelihood of overestimation of the treatment effect. 8Serious risk of bias, downgraded by one level: three studies were at unclear risk of bias for allocation concealment. Two studies were at high risk of other potential sources of bias. There is a likelihood of overestimation of the treatment effect.
Background
Description of the condition
Assisted reproductive technology (ART) is widely used to treat subfertility, and it is estimated that more than 8 million babies have been born using this treatment worldwide (Adamson 2018). Despite many technological breakthroughs over the years, live‐birth rates following ART remain modest, at between 20% and 29% (Dyer 2016; Kushnir 2017). Embryo implantation (embryo adherence to uterine lining) is a complex process involving interaction between the genetically competent embryo and endometrium and is influenced by local and systemic immune factors. Endometrial receptivity (window of implantation) and the final process of embryo implantation remains a rate‐limiting step for the success of ART (Gnainsky 2014). During an ART treatment cycle, endometrial thickness is commonly measured using an ultrasound to indirectly assess endometrial receptivity. A systematic review suggests that an endometrial thickness of 7 mm or less during ART is suboptimal and associated with lower clinical pregnancy rates (Kasius 2014). A chronically thin endometrium that does not respond to various treatment modalities is a difficult clinical situation. Similarly, in women with two or more in vitro fertilisation (IVF) failures, where there is a failure of implantation despite the transfer of good‐quality embryos, endometrial receptivity remains the main focus for evaluation and intervention (Coughlan 2014; Macklon 2017; Valdes 2017). Treating chronically thin endometrium and repeated IVF failures despite transfer of good‐quality embryos remains a challenge for clinicians. Numerous strategies and interventions have been suggested to improve endometrial receptivity and thereby embryo implantation.
Description of the intervention
Granulocyte‐colony stimulating factor (G‐CSF) is a polypeptide that belongs to the colony stimulating factor glycoprotein group (Demetri 1991). Other members of the colony stimulating factor group include granulocyte macrophage‐colony stimulating factor (GM‐CSF) and macrophage‐colony stimulating factor (M‐CSF) (McNiece 1989). Granulocyte‐colony stimulating factor is produced by cells of the immune system such as monocytes, macrophages, endothelial and bone marrow cells, and stimulates the proliferation, differentiation, survival, and function of neutrophils (Demetri 1991). Granulocyte‐colony stimulating factor binds to a specific receptor expressed on the surfaces of the target cells (such as neutrophils, vascular endothelium, and trophoblastic cells) and triggers growth signals within it (Demetri 1991). Recombinant human G‐CSF became available in the late 1980s and is mainly used to treat haematological disorders (Bonilla 1989; Tabbara 1997). In reproductive medicine, G‐CSF is being administered locally or systemically mainly in women with thin unresponsive endometrium undergoing IVF, repeated IVF failures following transfer of good‐quality embryos, and unexplained recurrent pregnancy losses (Scarpellini 2009; Gleicher 2011; Würfel 2015). In chronically thin endometrium, the most common route of administration of G‐CSF is intrauterine instillation or perfusion, whilst the subcutaneous route is preferred in recurrent pregnancy loss (Scarpellini 2009; Gleicher 2011; Gleicher 2013).
How the intervention might work
At the endometrial level, G‐CSF seems to play an important role in the process of embryo implantation and continuation of pregnancy. Granulocyte‐colony stimulating factor is involved in controlling gene expression, thereby influencing local embryo adhesions, cell migration, apoptotic (programmed cell death) activity, angiogenesis (formation of newer blood vessels), and endometrial remodelling, which are important for successful implantation (Rahmati 2014). Promotion of endometrial regeneration, anti‐apoptotic activity, and increased vascularisation (formation of blood vessels) are some of the proposed mechanisms for the beneficial effect of G‐CSF on thin endometrium (Tanaka 2000; Schneider 2005). It also helps in continuation of pregnancy by temporarily modulating response of T‐helper cells (Th‐1 and Th‐2), which play an important role in immunity, helping mediate maternal immune tolerance against the semi‐allogenic foetus, which shares some maternal genes, but not all. The decidua (uterine lining during pregnancy) plays an important role in embryo implantation, and a balanced immunoregulation of different immune cells is crucial. Granulocyte‐colony stimulating factor predominantly promotes Th‐2 response in the foetal‐maternal interface, thereby blocking any maternal Th‐1 cell attacks against the embryo and helping continuation of pregnancy (Rutella 2005). It also promotes generation of interleukin 10‐producing T regulatory cells, which assist in transplantation tolerance, an important immunoregulatory event around the peri‐implantation period (Morris 2004).
Granulocyte‐colony stimulating factor is a potential biomarker for oocyte (female egg) competence (ability of an oocyte to develop and sustain as an early embryo). Granulocyte‐colony stimulating factor receptors have been located on granulosa and luteal cells, which are closely associated with female oocyte development. Low levels of G‐CSF in follicular fluid (fluid surrounding the female oocyte) are linked to lower oocyte competence (Lédée 2008). It is hypothesised that the follicular fluid G‐CSF is linked to mRNA content of oocyte and that it may influence the production of adhesion molecules involved in the attachment of the future potential embryo to the endometrial cells (Lédée 2008). Granulocyte‐colony stimulating factor may also influence cumulus granulosa cells to produce growth factors that are essential for the development and implantation of the embryo.
The use of GM‐CSF as a supplement in embryo culture medium has also been explored in repeated IVF failures to mimic in vivo conditions (Tevkin 2014), but our current review focused only on G‐CSF.
Why it is important to do this review
The first published study on the use of G‐CSF in reproductive medicine was a case series in which the authors reported successful IVF outcomes after using intrauterine instillation of G‐CSF in women with thin endometrium that did not respond to standard treatment (Gleicher 2011). The same authors subsequently published a larger, uncontrolled study involving 21 women with chronic unresponsive endometrium and reported an increase in endometrial thickness after intrauterine instillation of G‐CSF (Gleicher 2013). Some randomised trials were published evaluating the effectiveness of G‐CSF in women undergoing IVF with chronically thin endometrium and recurrent implantation failures (RIF) as well as in women with recurrent pregnancy loss (Scarpellini 2009; Kunicki 2014; Aleyasin 2016). The results of these trials varied, with some showing a benefit of G‐CSF, and others showing no improvement in outcomes (Barad 2014; Kunicki 2014; Aleyasin 2016; Kunicki 2017). A recently published systematic review evaluated the effectiveness of G‐CSF in women with thin endometrium and RIF and included a total of four trials (two in each subgroup) (Kamath 2017). This review suggested a possible benefit of G‐CSF in women with thin endometrium and RIF undergoing IVF. Another systematic review that included six trials after searching two databases also suggested a possible benefit of G‐CSF in women with thin endometrium and RIF (Li 2017). Both of these reviews conducted limited searches and suggested the need for further validation of their findings before G‐CSF can be used in routine clinical practice for women undergoing IVF.
Newer trials have been published since the above reviews were conducted (Aleyasin 2016; Sarvi 2017). Furthermore, some trials have evaluated the role of G‐CSF in fresh‐embryo transfer cycles, whilst other trials have included only frozen‐embryo transfer cycles (Barad 2014; Eftekhar 2014; Kunicki 2017; Sarvi 2017). The effect of the intervention (G‐CSF) as assessed by clinical pregnancy rate and obstetric outcomes might differ in fresh‐ and frozen‐embryo transfer cycles due to differences in hormonal milieu at the endometrial level (Evans 2014). A more comprehensive search of the literature and appraisal of the current evidence was needed, hence we planned a systematic review for evaluating the role of G‐CSF in women undergoing assisted reproduction.
Objectives
To evaluate the effectiveness and safety of granulocyte‐colony stimulating factor (G‐CSF) in women undergoing assisted reproductive technology (ART).
Methods
Criteria for considering studies for this review
Types of studies
Published and unpublished randomised controlled trials (RCTs) were eligible for inclusion. We excluded non‐randomised studies as they are associated with a high risk of bias. We included data from only the first phase of cross‐over trials (pre‐cross‐over data) in the meta‐analyses, as the cross‐over trial is not a valid study design in the context of subfertility.
Types of participants
Subfertile women undergoing IVF and fresh‐ or frozen‐embryo transfer cycles.
Types of interventions
We included RCTs comparing administration of G‐CSF versus either no treatment or placebo.
Types of outcome measures
Primary outcomes
Effectiveness: live‐birth rate or ongoing pregnancy rate per woman randomised. We defined 'live birth' as delivery of a live foetus after 20 completed weeks of gestation (duration of pregnancy). We counted the delivery of single, twin, or multiple pregnancies as one live birth. We used ongoing pregnancy, defined as a viable pregnancy of 12 or more weeks of gestation, instead of live birth in cases where studies did not report live birth but reported ongoing pregnancy.
Adverse events: miscarriage rate per woman randomised defined as the spontaneous loss of a clinical pregnancy that occurs before 20 completed weeks of gestation.
Secondary outcomes
Effectiveness: clinical pregnancy rate per woman randomised (clinical pregnancy defined as evidence of gestational sac on ultrasound).
Adverse events: multiple pregnancy rate per woman randomised.
Adverse events: incidence of side effects or adverse reaction due to administration of G‐CSF, analysed as a composite measure of any adverse events (including anaphylactic reaction (serious allergic reaction), fever, headache, infection following intrauterine instillation or perfusion, etc.).
Adverse events: ectopic pregnancy rate.
Adverse events: small for gestational age at birth (defined as birthweight less than the 10th percentile for gestational age and infant sex).
Adverse events: abnormally adherent placenta (e.g. placenta accreta, increta, or percreta).
Adverse events: congenital anomaly (or birth defect) rates
Search methods for identification of studies
We searched for all published and unpublished RCTs evaluating the effectiveness of G‐CSF in infertile women undergoing IVF, with no language restriction and in consultation with the Cochrane Gynaecology and Fertilty Group (CGF) Information Specialist.
Electronic searches
We searched the following electronic databases and websites, from their inception to 7 February 2019.
Cochrane Gynaecology and Fertility Group Specialised Register of controlled trials; ProCite platform, searched 7 February 2019 (Appendix 1).
The Cochrane Central Register of Controlled Trials (CENTRAL) via the Cochrane Register of Studies Online (CRSO); web platform, searched 7 February 2019 (Appendix 2).
MEDLINE; Ovid platform, searched from 1946 to 7 February 2019 (Appendix 3).
Embase; Ovid platform, searched from 1980 to 7 February 2019 (Appendix 4).
CINAHL (Cumulative Index to Nursing and Allied Health Literature); EBSCO platform, searched from 1961 to 7 February 2019 (Appendix 5).
The MEDLINE search was combined with the Cochrane Highly Sensitive Search Strategy for identifying randomised trials, described in Section 6.4.11 of the Cochrane Handbook for Systematic Reviews of Interventions (Lefebvre 2011). The Embase search was combined with trial filters developed by the Scottish Intercollegiate Guidelines Network (SIGN). There were no language restrictions in these searches.
In the Cochrane Library we searched DARE (Database of Abstracts of Reviews of Effects) to identify reviews with potentially relevant RCTs.
We searched for ongoing and registered trials in the following trial registers on 7 February 2019:
World Health Organization International Clinical Trials Registry Platform (WHO ICTRP) (www.who.int/trialsearch/default.aspx);
US National Institutes of Health Ongoing Trials Register ClinicalTrials.gov (clinicaltrials.gov);
Current Controlled Trials (www.controlled-trials.com).
We also searched:
Citation indexes (scientific.thomson.com/products/sci/);
Conference abstracts in the ISI Web of Knowledge (isiwebofknowledge.com/);
OpenSigle database for grey literature (opensigle.inist.fr/);
PubMed and Google for any recently published trials not yet indexed in the major databases.
Searching other resources
Two review authors (MSK and SKS) handsearched reference lists of articles retrieved by the search and contacted experts in the field to obtain additional data. We also handsearched relevant journals and conference abstracts that were not covered in the CGF register, in liaison with the Information Specialist.
Data collection and analysis
We formatted a data extraction sheet to retrieve data from the included studies. Two review authors (MSK and SKS) independently extracted the data onto the data extraction sheet. Any discrepancies was resolved by involving the third review author (RK).
Selection of studies
Two review authors (MSK and SKS) initially screened the titles and abstracts retrieved by the search for potentially relevant studies. We retrieved the full texts of all potentially eligible studies, and two review authors (MSK and SKS) independently examined the full‐text articles for compliance with the inclusion criteria and selected studies for inclusion in the review. We corresponded with study investigators as required to clarify study eligibility. Any disagreements as to study eligibility were resolved by discussion or by involving a third review author (RK). We documented the selection process with a PRISMA flow chart.
Data extraction and management
Two review authors (one a methodologist (MSK) and one a topic‐area specialist (SKS)) independently extracted data from the included studies using a data extraction form designed and pilot‐tested by the review authors. Any disagreements were resolved by discussion or by involving a third review author (RK). The extracted data included study characteristics and outcome data (see the data extraction table in Appendix 6 for details). Where studies had multiple publications, we collated multiple reports of the same study so that each study, rather than each report, was the unit of interest in the review; such studies had a single study ID with multiple references. We corresponded with study investigators for further data on methods or results, or both, as required. We included studies irrespective of whether outcomes were reported in a 'usable' way. For multi‐arm studies, we excluded data from arms that did not meet the eligibility criteria.
Assessment of risk of bias in included studies
Two review authors (MSK and SKS) independently assessed the included studies for risk of bias using the Cochrane 'Risk of bias' tool, according to the criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We assessed the following 'Risk of bias' domains.
Selection bias (random sequence generation and allocation concealment)
Performance bias (blinding of participants and personnel)
Detection bias (blinding of outcome assessors)
Attrition bias (incomplete outcome data)
Reporting bias (selective reporting)
Other bias (including unplanned interim analysis)
We considered lack of blinding of personnel (clinician or embryologist, or both) as high risk for performance bias. However, we considered lack of blinding as low risk for detection bias due to the objective nature of outcomes.
Any disagreements were resolved by discussion or by involving a third review author (RK). We described all judgements fully and presented the conclusions in the 'Risk of bias' table, which we incorporated into the interpretation of review findings by means of sensitivity analyses (see below).
Selective reporting is a type of reporting bias that affects the internal validity of an individual study. It refers to the selective reporting of some outcomes (e.g. positive outcomes) and the failure to report others (e.g. adverse events). We took care to search for within‐trial selective reporting, such as trials failing to report obvious outcomes, or reporting them in insufficient detail to permit inclusion. We sought published protocols and compared the outcomes in the protocol with those in the final published study. Where identified studies failed to report the primary outcome of live birth, but did report interim outcomes such as pregnancy, we undertook informal assessment to determine whether the interim values (e.g. pregnancy rates) were similar to those reported in studies that also reported live birth.
Measures of treatment effect
For dichotomous data (e.g. pregnancy or live‐birth rates), we used the number of events in the control and intervention groups of each study to calculate Mantel‐Haenszel risk ratios (RR). We used Peto odds ratio for outcomes with low event rates. We reversed the direction of effect of individual studies to ensure consistency across trials where required. We presented 95% confidence intervals (CIs) for all outcomes. Where data to calculate risk ratios were not available, we utilised the most detailed numerical data available that facilitated similar analyses of included studies (e.g. test statistics, P values). We compared the magnitude and direction of effect reported in studies with how they were presented in the review, taking account of legitimate differences.
Unit of analysis issues
The primary analysis was per woman randomised; per‐pregnancy data were to be included for some outcomes as secondary analysis (for the outcome miscarriage). If studies reported only per‐cycle data, we attempted to contact the study authors for per‐woman data. We counted multiple live births (e.g. twins or triplets) as one live‐birth event. We included only first‐phase data from cross‐over trials.
Dealing with missing data
We analysed data on an intention‐to‐treat basis to the greatest degree possible and attempted to obtain missing data from the original study authors. Where this information was unobtainable, we undertook imputation of individual values for the primary outcomes only. We assumed live births not to have occurred in participants without a reported outcome. For other outcomes, we analysed only the available data.
Assessment of heterogeneity
We considered whether the clinical and methodological characteristics of the included studies were sufficiently similar for meta‐analysis to provide a clinically meaningful summary. We used the I² statistic to measure statistical heterogeneity amongst the trials in each analysis.
We used the rough guide to interpretation as outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011), considering an I² measurement greater than 55% as indicative of substantial heterogeneity.
Assessment of reporting biases
In view of the difficulty of detecting and correcting for publication bias and other reporting biases, we aimed to minimise the potential impact of these biases by ensuring a comprehensive search for eligible studies and by being alert for duplication of data. If there were 10 or more studies in an analysis, we used a funnel plot to explore the possibility of small‐study effects (a tendency for estimates of the intervention effect to be more beneficial in smaller studies).
Data synthesis
One review author (MSK) entered data into and performed statistical analysis using Review Manager 5 (RevMan 2014). We combined the data using a fixed‐effect model for the following comparison.
G‐CSF versus no treatment or placebo in subfertile women undergoing ART.
We stratified the comparison further into three groups, as follows.
Unselected IVF population
Two or more IVF failures
Women with thin endometrium
Any increase in the risk of a particular outcome, which may be beneficial (e.g. live birth) or detrimental (e.g. adverse effects of G‐CSF), was displayed graphically in the meta‐analyses to the right of the centre‐line, and a decrease in the risk of an outcome to the left of the centre‐line. The aim was to define analyses that are comprehensive and mutually exclusive, so that all results can be slotted into one stratum only, and trials within the same stratum can be sensibly pooled. Stratification is not a requirement, but allows consideration of effects within each stratum as well as, or instead of, an overall estimate for the comparison.
Subgroup analysis and investigation of heterogeneity
We planned that if substantial heterogeneity was present and data were available for the primary outcomes, we would conduct subgroup analyses to determine the separate evidence within the following subgroups.
Fresh versus frozen ART‐ IVF cycles.
Route of administration: local versus systemic administration of G‐CSF
Sensitivity analysis
We carried out sensitivity analyses for the primary outcomes to determine whether the conclusions were robust to arbitrary decisions made regarding the eligibility and analysis. These analyses included consideration of whether the review conclusions would have differed if:
eligibility was restricted to studies without high risk of bias (without high or unclear risk of bias in any domain);
a random‐effects model had been adopted;
the summary effect measure had been odds ratio rather than risk ratio;
alternative imputation strategies had been implemented;
restricting the analysis by excluding any unpublished studies.
Summary of findings and assessment of the certainty of the evidence
We used the GRADE approach to summarise and interpret findings and GRADEpro GDT to import data from Review Manager 5 to create 'Summary of findings' tables (GRADEpro GDT 2015). These tables provide outcome‐specific information concerning within‐study risk of bias (methodological quality), directness of evidence, heterogeneity, precision of effect estimates, and risk of publication bias, and the sum of available data on all outcomes rated as important to patient care and decision making. The GRADE approach specifies four levels of quality: high, moderate, low, and very low.
We used the methods and recommendations described in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011), employing GRADEpro GDT software (GRADEpro GDT 2015). We justified all decisions to down‐ or upgrade the quality of the evidence using footnotes and made comments to aid reader's understanding of the review where necessary. Two review authors independently made decisions about evidence quality, with any disagreements resolved by discussion.
The comparison was 'G‐CSF versus no treatment or placebo in subfertile women undergoing ART'.
We included the following outcomes in the 'Summary of findings' tables.
Live‐birth/ongoing pregnancy rate per woman randomised
Miscarriage rate per woman randomised
Clinical pregnancy per woman randomised
Multiple pregnancy per woman randomised
Adverse events per woman randomised
Results
Description of studies
Results of the search
We ran our electronic search on 7 February 2019. The targeted search resulted in 585 records, from which 168 duplicate records were removed. Two review authors (MSK and SKS) simultaneously and independently screened the records and examined the titles and abstracts to identify potentially eligible studies among the remaining 417 records. The two review authors identified 51 potentially eligible studies. We excluded 23 studies (Würfel 2000; Scarpellini 2009; Cambiaghi 2012b; Gleicher 2013; Eftekhar 2014; Sbracia 2014; Xu 2014; Jung 2015; Singh 2015b; Lukaszuk 2016; Mishra 2016; Kunicki 2017; Li 2017; Sen 2017; Singh 2017; Wasim 2017; Zabardoust 2017; Arefi 2018; Eapen 2018; Obidniak 2018; Wu 2018; Zhang 2018; Mehrafza 2019) and included 13 trials in the review (Scarpellini 2011; Cambiaghi 2012a; Scarpellini 2013; Barad 2014; Singh 2015; Aleyasin 2016; Davari‐Tanha 2016; Obidniak 2016; Sarvi 2017; Jain 2018; Jalilvand 2018; Singh 2018; NCT01202643). Three studies are awaiting classification (Kim 2011; Eftekhar 2016a; Eftekhar 2016b). Records from five included trials (Scarpellini 2011; Barad 2014; Aleyasin 2016; Davari‐Tanha 2016; Jain 2018) and two trials (Eftekhar 2016a; Eftekhar 2016b) awaiting classification were also published as conference abstracts. We identified five ongoing trials (NCT01715974; NCT02149277; NCT03023774; NCT03163862; NCT03549728). The search results are summarised in the PRISMA flow chart (Figure 1).
1.
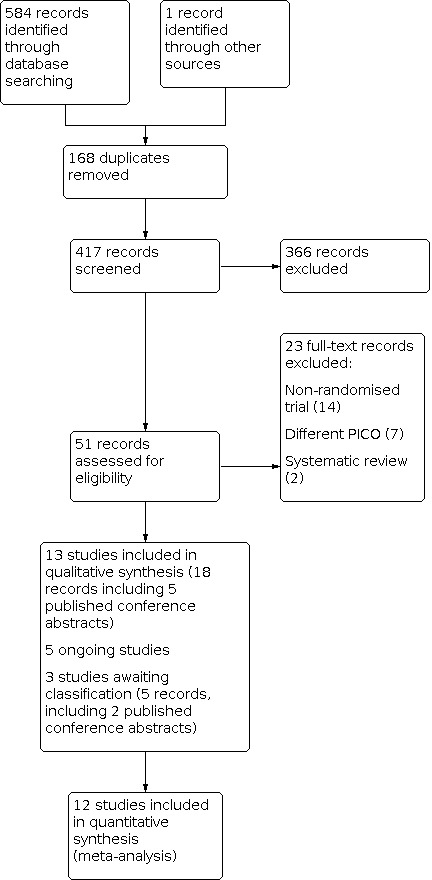
Study flow diagram.
Included studies
See Characteristics of included studies.
Design
We included a total of 13 RCTs in the review. Six of these trials were published as full articles (Barad 2014; Aleyasin 2016; Davari‐Tanha 2016; Sarvi 2017; Jain 2018; Jalilvand 2018), six as conference abstracts (Scarpellini 2011; Cambiaghi 2012a; Scarpellini 2013; Singh 2015; Obidniak 2016; Singh 2018), and one was an unpublished trial (NCT01202643). Two trials were multicentre (Aleyasin 2016; Davari‐Tanha 2016), whilst the remaining 11 trials were conducted at single centres. Two trials were conducted in the USA (Barad 2014; NCT01202643), four in Iran (Aleyasin 2016; Davari‐Tanha 2016; Sarvi 2017; Jalilvand 2018), three in India (Singh 2015; Jain 2018; Singh 2018), two in Italy (Scarpellini 2011; Scarpellini 2013), one in Russia (Obidniak 2016), and one in Brazil (Cambiaghi 2012a).
Two studies were funded by Tehran University of Medical Sciences (Aleyasin 2016; Davari‐Tanha 2016). The two studies from the USA were supported by the Foundation for Reproductive Medicine, a not‐for‐profit medical research foundation, and by intramural funds from the Center for Human Reproduction (Barad 2014; NCT01202643). One study was funded by BTTB Centre (Singh 2015). One study did not receive any funding support (Jain 2018). No funding information was available for seven studies (Scarpellini 2011; Cambiaghi 2012a; Scarpellini 2013; Obidniak 2016; Sarvi 2017; Jalilvand 2018; Singh 2018).
Participants
Nine trials included women undergoing fresh transfer IVF cycles (Scarpellini 2011; Cambiaghi 2012a; Scarpellini 2013; Singh 2015; Aleyasin 2016; Sarvi 2017; Jain 2018; Singh 2018; NCT01202643), whilst two trials included women undergoing frozen‐embryo transfer cycles (Obidniak 2016; Jalilvand 2018). The remaining two trials included women undergoing both fresh and frozen cycles (Barad 2014; Davari‐Tanha 2016). Most trials included women aged between 18 and 40 years. The characteristics of the participants amongst the IVF population are shown in Table 2.
1. Cycle characteristics of included studies.
| Study ID | Population |
Fresh or frozen |
Intervention | Control | Outcomes |
| Aleyasin 2016 | 2 or more IVF failures; 3 IVF attempts with 3 high‐grade embryos in each attempt |
Fresh | G‐CSF, 300 µg 1 hour before ET; subcutaneously; single dose |
No additional treatment | Clinical pregnancy rate; ectopic pregnancy rate |
| Barad 2014 | Unselected IVF population | Fresh and frozen cycles |
G‐CSF, 300 µg, 1 mL on the day of hCG trigger; intrauterine infusion; single dose |
Normal saline, 1 mL, on the day of hCG trigger; intrauterine infusion |
Clinical pregnancy rate; miscarriage rate; adverse effects |
| Cambiaghi 2012a | 2 or more IVF failures | Fresh | G‐CSF, 300 µg; subcutaneously; from day of ET to 6 weeks later until evidence of clinical pregnancy, alternate days |
No additional treatment | Implantation rate; clinical pregnancy rate |
| Davari‐Tanha 2016 | 2 or more IVF failures; 3 consecutive IVF with transference of at least 4 good‐quality embryos in a minimum of 3 fresh or frozen cycles |
Fresh and frozen |
G‐CSF, 300 µg, 1 mL, day of oocyte retrieval and day of progesterone initiation for frozen cycle; intrauterine infusion; single dose |
2 control arms: first arm: normal saline intrauterine infusion on the day of oocyte retrieval; second arm: only passage of catheter through cervix without injecting saline |
Clinical pregnancy rate; miscarriage rate |
| Jain 2018 | Unselected IVF population | Fresh | G‐CSF, 300 µg, 0.5 mL on the day of hCG trigger; intrauterine infusion; single dose |
Normal saline, 0.5 mL, on the day of hCG trigger; intrauterine infusion |
Ongoing pregnancy rate; clinical pregnancy rate; miscarriage rate |
| Jalilvand 2018 | 2 or more IVF failures | Frozen | G‐CSF, 100 µg, from the day of progesterone initiation until ET; intrauterine infusion; multiple dose |
No additional treatment | Clinical pregnancy rate |
| NCT01202643 | Women with thin endometrium | Fresh (pre‐cross‐over data only) |
G‐CSF, 300 mcg/mL, on the day of hCG trigger; intrauterine infusion; single dose |
Normal saline, 1 cm3, on the day of hCG trigger; intrauterine infusion |
Clinical pregnancy rate; adverse effect |
| Obidniak 2016 | 2 or more IVF failures; at least 2 cycles of IVF in which good‐quality embryos were transferred in each cycle |
Frozen | 2 arms: first arm: G‐CSF, 30 mIU, 1 mL, 5 days prior to transfer; intrauterine infusion; single dose second arm: 30 mIU, 1 mL, day of embryo transfer; single dose |
No additional treatment | Clinical pregnancy rate; adverse effect |
| Sarvi 2017 | Women with thin endometrium | Fresh | G‐CSF, 300 µg, 1 mL, on the day of hCG trigger; intrauterine infusion; a second dose of G‐CSF was injected 2 to 3 days after oocyte retrieval day if endometrial thickness < 7 mm |
Normal saline (1 cm3) was injected into the uterus. |
Clinical pregnancy rate |
| Scarpellini 2011 | 2 or more IVF failures; at least 3 failed ET with at least 7 good embryos transferred |
Fresh | G‐CSF, 1.5 mg/kg/day (60 to 100 mg), daily from day of ET until day of results and continued for 40 days if positive; subcutaneously; multiple dose |
Similar saline placebo injection in control group subcutaneously |
Clinical pregnancy rate; adverse effect |
| Scarpellini 2013 | 2 or more IVF failures; at least 3 failed ET with at least 8 good embryos transferred |
Fresh | G‐CSF, 60 μg, daily from day of ET until day of results; subcutaneously; multiple dose |
Similar saline placebo injection in control group subcutaneously |
Clinical pregnancy rate; adverse effect |
| Singh 2015 | Women with thin endometrium | Fresh | G‐CSF, 300 μg, before embryo transfer, repeated 48 hours later if ET < 7 mm; intrauterine infusion; 1 to 2 doses |
Saline infusion as placebo intrauterine infusion |
Clinical pregnancy rate |
| Singh 2018 | Women with thin endometrium | Fresh | G‐CSF, 300 μg/mL on the day of trigger, repeated 48 hours later if ET < 7 mm; intrauterine infusion or subcutaneously; 1 or 2 doses |
Saline infusion intrauterine or subcutaneous |
Clinical pregnancy rate |
ET: embryo transfer G‐CSF: granulocyte‐colony stimulating factor hCG: human chorionic gonadotropin IVF: in vitro fertilisation
Seven trials included women with two or more IVF failures (Scarpellini 2011; Cambiaghi 2012a; Scarpellini 2013; Aleyasin 2016; Davari‐Tanha 2016; Obidniak 2016; Jalilvand 2018). Two trials included an unselected IVF population (Barad 2014; Jain 2018). In four trials, women with thin endometrium (< 7 mm) were evaluated (Singh 2015; Sarvi 2017; Singh 2018; NCT01202643). Most trials excluded women with renal disease, sickle cell disease, chronic neutropenia, or a history of malignancy, since G‐CSF is contraindicated in these conditions.
Interventions
Nine trials compared G‐CSF in the intervention arm with placebo, normal saline in the control arm (Scarpellini 2011; Scarpellini 2013; Barad 2014; Singh 2015; Davari‐Tanha 2016; Sarvi 2017; Jain 2018; Singh 2018; NCT01202643), whilst four trials had no placebo in the control arm (Cambiaghi 2012a; Aleyasin 2016; Obidniak 2016; Jalilvand 2018). The route, dose, and duration of G‐CSF administration varied in the included trials (Table 2). In seven trials, G‐CSF was administered by intrauterine infusion (300 μg/mL) either around the time of day of human chorionic gonadotropin (hCG) trigger or oocyte retrieval in fresh transfer IVF cycles or on the day of progesterone initiation in the frozen‐embryo transfer cycles (Barad 2014; Singh 2015; Davari‐Tanha 2016; Sarvi 2017; Jain 2018; Jalilvand 2018; NCT01202643). In four trials, G‐CSF was administered subcutaneously, mostly on the day of embryo transfer either as a single dose (Aleyasin 2016), or continued daily or alternate days, either until the day of a pregnancy test, Scarpellini 2013, or up to the day when clinical pregnancy was documented by ultrasound scan (Scarpellini 2011; Cambiaghi 2012a). In two trials, G‐CSF was administered by both routes, intrauterine infusion and subcutaneously (Obidniak 2016; Singh 2018), in two separate arms. In one trial with three arms (one intervention and two control arms), intrauterine infusion of G‐CSF was performed in the intervention arm, whilst intrauterine infusion of saline was used in one control arm, and only a catheter passed through the cervix without injecting any fluid in the second control arm (Davari‐Tanha 2016).
Outcomes
None of the included trials reported the primary outcome of live birth, with one trial reporting ongoing pregnancy rate (Jain 2018). Two trials reported the primary outcome of adverse events, miscarriage rate (Barad 2014; Jain 2018). All 13 included trials reported clinical pregnancy as an outcome.
Excluded studies
We excluded a total of 23 studies after examining full‐text reports and obtaining clarifications from study authors. Of the excluded studies, 14 were non‐randomised trials (Würfel 2000; Cambiaghi 2012b; Gleicher 2013; Eftekhar 2014; Xu 2014; Jung 2015; Singh 2015b; Lukaszuk 2016; Mishra 2016; Kunicki 2017; Sen 2017; Singh 2017; Arefi 2018; Mehrafza 2019); seven evaluated a different population or interventions (Scarpellini 2009; Sbracia 2014; Wasim 2017; Zabardoust 2017; Eapen 2018; Obidniak 2018; Wu 2018); and two were systematic reviews (Li 2017; Zhang 2018). See Characteristics of excluded studies.
Risk of bias in included studies
We assessed the included studies for methodological quality using the Cochrane ’Risk of bias’ tool (Higgins 2011). See the ’Risk of bias’ graph (Figure 2) and ’Risk of bias’ summary (Figure 3).
2.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
3.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Generation of random sequence
Ten studies reported adequate methods for random sequence generation and were at low risk of selection bias (Scarpellini 2011; Cambiaghi 2012a; Scarpellini 2013; Barad 2014; Singh 2015; Aleyasin 2016; Davari‐Tanha 2016; Sarvi 2017; Jain 2018; NCT01202643). The remaining three trials did not report the method of randomisation and were assessed as at unclear risk of bias (Obidniak 2016; Jalilvand 2018; Singh 2018).
Allocation concealment
Only five trials clearly reported the method of allocation concealment and were at low risk of selection bias (Barad 2014; Aleyasin 2016; Davari‐Tanha 2016; Sarvi 2017; Jain 2018). The remaining eight trials did not state the method of allocation concealment and were judged as at unclear risk of bias (Scarpellini 2011; Cambiaghi 2012a; Scarpellini 2013; Singh 2015; Obidniak 2016; Jalilvand 2018; Singh 2018; NCT01202643).
Blinding
Performance bias
We judged six trials as at high risk of performance bias due to lack of blinding of the participants or clinicians (Scarpellini 2011; Cambiaghi 2012a; Scarpellini 2013; Aleyasin 2016; Obidniak 2016; Jalilvand 2018). In four of these trials, no additional intervention (sham procedure or placebo treatment) was given in the control arm, hence blinding was not possible (Cambiaghi 2012a; Aleyasin 2016; Obidniak 2016; Jalilvand 2018). In the remaining two trials placebo was used in the control arm, but only participants were blinded (Scarpellini 2011; Scarpellini 2013).
In four trials, either a placebo was given or a sham procedure was performed in the control arm with both participants and clinicians blinded, hence we assessed these trials as at low risk of bias for this domain (Barad 2014; Davari‐Tanha 2016; Jain 2018; NCT01202643). No clear information was available for the remaining three trials regarding blinding of participants or personnel, even when a placebo was given or a sham procedure was performed in the control group, therefore we judged these studies as at unclear risk of bias (Singh 2015; Sarvi 2017; Singh 2018).
Detection bias
Only two trials reported that the outcome assessor was blinded (Barad 2014; NCT01202643). The remaining 11 trials did not specify if outcome assessors were blinded, however given that absence of blinding was unlikely to have influenced the findings for our primary and secondary outcomes, we categorised these studies as at low risk for detection bias.
Incomplete outcome data
We deemed two studies to be at unclear risk of attrition bias, Cambiaghi 2012a; Jalilvand 2018, and the remaining 11 trials as at low risk of bias (Scarpellini 2011; Scarpellini 2013; Barad 2014; Singh 2015; Aleyasin 2016; Davari‐Tanha 2016; Obidniak 2016; Sarvi 2017; Jain 2018; Singh 2018; NCT01202643).
Selective reporting
We judged one trial as at high risk of reporting bias because live birth, which was a prespecified outcome (as per clinical trial registry site), was not reported in the final published manuscript (Barad 2014). We assessed six trials as at unclear risk of reporting bias, Scarpellini 2011; Cambiaghi 2012a; Singh 2015; Obidniak 2016; Jalilvand 2018; Singh 2018, and six trials as at low risk of bias (Scarpellini 2013; Aleyasin 2016; Davari‐Tanha 2016; Sarvi 2017; Jain 2018; NCT01202643).
Other potential sources of bias
Three included trials were prematurely terminated before the calculated sample size could be reached and were assessed as at high risk of bias (Scarpellini 2013; Sarvi 2017; NCT01202643). We judged six trials (of which five were conference abstracts) as at unclear risk of bias (Scarpellini 2011; Cambiaghi 2012a; Singh 2015; Obidniak 2016; Jalilvand 2018; Singh 2018). The remaining four trials were at low risk of bias (Barad 2014; Aleyasin 2016; Davari‐Tanha 2016; Jain 2018).
Effects of interventions
See: Table 1
1. G‐CSF versus no treatment or placebo in women undergoing ART
A total of 13 trials were included in this comparison (Scarpellini 2011; Cambiaghi 2012a; Scarpellini 2013; Barad 2014; Singh 2015; Aleyasin 2016; Davari‐Tanha 2016; Obidniak 2016; Sarvi 2017; Jain 2018; Jalilvand 2018; Singh 2018; NCT01202643). For one trial, we could not obtain information on the exact number of women randomised in each group, hence data from this study could not be pooled (Cambiaghi 2012a).
Primary outcomes
1.1 Live‐birth/ongoing pregnancy rate
None of the trials reported live‐birth rate as an outcome. Only one trial reported ongoing pregnancy rates (Jain 2018). We used these data as a surrogate outcome for the primary outcome of effectiveness, live‐birth rate. The quality of the evidence was very low, and we are uncertain whether G‐CSF administration improves ongoing pregnancy rate compared to the control group in subfertile women undergoing ART (risk ratio (RR) 1.62, 95% confidence interval (CI) 0.86 to 3.08; 1 RCT; participants = 150; Analysis 1.1; Figure 4). For a typical clinic with 16% ongoing pregnancy rate, G‐CSF administration would be expected to result in ongoing pregnancy rates between 14% and 50%.
1.1. Analysis.

Comparison 1: G‐CSF versus placebo or no treatment in women undergoing assisted reproduction, Outcome 1: Ongoing pregnancy rate
4.

Forest plot of comparison: 1 G‐CSF versus placebo/no treatment in women undergoing assisted reproduction, outcome: 1.1 Ongoing pregnancy rate.
Sensitivity analysis
Sensitivity analyses on the choice of the summary effect measure (odds ratio versus RR) or the analysis model (fixed‐effect versus random‐effects model) did not demonstrate differences in the direction of the treatment effect or the statistical significance tests.
Subgroup analysis
We did not perform planned subgroup analyses for number of IVF attempts or heterogeneity as only one trial (Jain 2018) reported ongoing pregnancy rate.
1.2 Miscarriage rate
Two trials reported miscarriage rate (Barad 2014; Jain 2018). The quality of the evidence was very low, and we are uncertain whether G‐CSF administration reduces miscarriage rate per woman randomised (Peto odds ratio 0.40, 95% CI 0.09 to 1.77; 2 RCTs; participants = 291; I² = 0%; Analysis 1.2) compared to control group in subfertile women undergoing ART. We obtained a similar pooled result between the two groups when miscarriage rate per pregnancy was calculated (Peto odds ratio 0.33, 95% CI 0.07 to 1.58; 2 RCTs; number of clinical pregnancies = 72; I² = 0%). For a typical clinic with 3% miscarriage rate per woman, G‐CSF administration would be expected to result in miscarriage rates between 0% and 6%.
1.2. Analysis.

Comparison 1: G‐CSF versus placebo or no treatment in women undergoing assisted reproduction, Outcome 2: Miscarriage rate
Sensitivity analysis
We performed sensitivity analysis by removing one trial with high risk of bias for any 'Risk of bias' domain (Barad 2014); the results did not change (Peto odds ratio 0.50, 95% CI 0.05 to 4.83; 1 RCT; participants = 150).
Subgroup analysis
We planned a subgroup analysis according to the number of IVF attempts. However, both the trials reporting miscarriage rates included only an unselected IVF population (Barad 2014; Jain 2018).
We did not perform our planned subgroup analysis for heterogeneity due to no substantial heterogeneity in this analysis.
Secondary outcomes
1.3 Clinical pregnancy rate
Twelve trials reported clinical pregnancy rate (Scarpellini 2011; Scarpellini 2013; Barad 2014; Singh 2015; Aleyasin 2016; Davari‐Tanha 2016; Obidniak 2016; Sarvi 2017; Jain 2018; Jalilvand 2018; Singh 2018; NCT01202643). The quality of the evidence was very low, and we are uncertain whether G‐CSF administration improves clinical pregnancy rate compared to the control group in subfertile women undergoing ART (RR 1.65, 95% CI 1.32 to 2.06; 12 RCTs; participants = 1050; I² = 19%; Analysis 1.3; Figure 5). For a typical clinic with 18% clinical pregnancy rate, G‐CSF administration would be expected to result in clinical pregnancy rates between 23% and 37%.
1.3. Analysis.

Comparison 1: G‐CSF versus placebo or no treatment in women undergoing assisted reproduction, Outcome 3: Clinical pregnancy rate
5.

Forest plot of comparison: 1 G‐CSF versus placebo/no treatment in women undergoing assisted reproduction, outcome: 1.3 Clinical pregnancy rate.
Sensitivity analysis
We performed sensitivity analysis by removing 10 trials with unclear or high risk of bias for any 'Risk of bias' domain (Scarpellini 2011; Scarpellini 2013; Barad 2014; Singh 2015; Aleyasin 2016; Obidniak 2016; Sarvi 2017; Jalilvand 2018; Singh 2018; NCT01202643); the pooled results showed no evidence of a difference in clinical pregnancy rate after G‐CSF administration versus the control group (RR 1.60, 95% CI 0.96 to 2.65; 2 RCTs; participants = 250; I² = 0%). Sensitivity analyses on the choice of summary effect measure (odds ratio versus RR) or the analysis model (fixed‐effect versus random‐effects model) did not demonstrate differences in the direction of the treatment effect or the statistical significance tests. A funnel plot for this outcome showed no evidence of publication bias (Figure 6).
6.

Funnel plot of comparison: 1 G‐CSF versus placebo/no treatment in women undergoing assisted reproduction, outcome: 1.3 Clinical pregnancy rate.
Subgroup analysis
We conducted a subgroup analysis according to the number of IVF attempts and women with thin endometrium, which showed no evidence of a difference between the subgroups: test for subgroup differences: Chi² = 5.70, df = 2 (P = 0.06), I² = 64.9%
1.3.1 Clinical pregnancy rate in an unselected IVF population
Two trials that included an unselected IVF population reported clinical pregnancy rate. We are uncertain whether G‐CSF administration improves clinical pregnancy rate in an unselected IVF population compared to the control group (RR 1.12, 95% CI 0.75 to 1.68; 2 RCTs; participants = 291; I² = 32 %; low‐quality evidence; Analysis 1.3; Figure 5).
Sensitivity analysis restricted to studies at low‐risk of bias (Jain 2018) suggests no evidence of difference in clinical pregnancy after G‐CSF administration versus control group in an unselected IVF population (RR 1.46, 95% CI 0.81 to 2.65; 1 RCT; participants = 150).
1.3.2 Clinical pregnancy rate after two or more IVF failures
Six trials that included women with two or more IVF failures reported clinical pregnancy rate. Granulocyte‐colony stimulating factor administration may improve clinical pregnancy rate in women with two or more IVF failures compared to the control group (RR 2.10, 95% CI 1.53 to 2.89; 6 RCTs; participants = 553; I² = 0%; low‐quality evidence; Analysis 1.3; Figure 5).
Sensitivity analysis restricted to studies at low‐risk of selection bias (Davari‐Tanha 2016) suggests that there is no evidence of difference in clinical pregnancy rate after G‐CSF administration versus control group in women with two or more IVF failures (RR 2.00, 95% CI 0.75 to 5.33; 1 RCT; participants =100).
1.3.3 Clinical pregnancy in women with thin endometrium
Four trials reported clinical pregnancy rate in women with thin endometrium (Singh 2015; Sarvi 2017; Singh 2018; NCT01202643). We are uncertain whether G‐CSF administration improves clinical pregnancy rate compared to the control group in subfertile women with thin endometrium undergoing ART (RR 1.58, 95% CI 0.95 to 2.63; 4 RCTs; participants = 206; I² = 30%; low‐quality evidence; Analysis 1.3; Figure 5). No studies in this subgroup remained in a sensitivity analysis restricted to studies at low‐risk of selection bias.
1.4 Multiple pregnancy rate
None of the included trials reported multiple pregnancy rate.
1.5 Adverse events
Four trials reported adverse events (Scarpellini 2011; Scarpellini 2013; Barad 2014; Obidniak 2016). All four trials did not report any major side effects such as anaphylactic or serious drug reaction, fever, or headache in either treatment arm. The authors of two trials conducted in the same centre reported minor side effects (skin rashes) (Scarpellini 2011; Scarpellini 2013). In one of these trials (Scarpellini 2011), five out of 45 participants in the G‐CSF arm and two out of 44 participants in the control arm reported skin rashes. In the other trial (Scarpellini 2013), two out of 25 participants in the G‐CSF arm and no participants in the control arm developed skin rashes. Since no major adverse events were noted in all four trials, the effect estimate could not be calculated (Analysis 1.4).
1.4. Analysis.

Comparison 1: G‐CSF versus placebo or no treatment in women undergoing assisted reproduction, Outcome 4: Adverse events
1.6 Ectopic pregnancy rate
Only one trial reported ectopic pregnancy rate (Aleyasin 2016). We are uncertain whether G‐CSF administration reduces ectopic pregnancy rate compared to the control group (Peto odds ratio 1.97, 95% CI 0.20 to 19.35; 1 RCT; participants = 112; Analysis 1.5).
1.5. Analysis.

Comparison 1: G‐CSF versus placebo or no treatment in women undergoing assisted reproduction, Outcome 5: Ectopic pregnancy rate
1.7 Small for gestational age
None of the included trials reported this outcome.
1.8 Abnormally adherent placenta
None of the included trials reported this outcome.
1.9 Congenital anomaly
None of the included trials reported this outcome.
Discussion
Summary of main results
G‐CSF versus no treatment or placebo in women undergoing ART
None of the trials reported the primary outcome for effectiveness of live‐birth rate. We are uncertain whether G‐CSF administration improves ongoing pregnancy rate compared to no intervention or placebo in subfertile women undergoing ART (very low‐quality evidence) (Table 1). We are uncertain whether G‐CSF administration reduces miscarriage rate or improves clinical pregnancy rate compared to no intervention or placebo in subfertile women undergoing ART (very low‐quality evidence). We are uncertain whether G‐CSF administration improves clinical pregnancy rate compared to no treatment or placebo in the subpopulations of unselected IVF group and women with thin endometrium (low‐quality evidence). Low‐quality evidence suggests that G‐CSF administration may improve clinical pregnancy rate compared to no treatment or placebo in women with two or more IVF failures. Only four trials reported adverse events as an outcome, and none of them reported any major adverse events following G‐CSF administration or placebo.
Overall completeness and applicability of evidence
The current review suggests that there is uncertainty regarding whether G‐CSF administration in subfertile women undergoing ART improves ongoing pregnancy or clinical pregnancy or reduces miscarriage rate compared to no treatment or placebo. However, the applicability of the evidence may have some limitations and may vary according to different subpopulations. In women with two or more IVF failures, G‐CSF administration may improve clinical pregnancy rate versus placebo.
We observed variability in administration of G‐CSF in terms of dose (100 μg versus 300 μg), frequency (one versus multiple doses), duration (once around the time of embryo transfer versus from day of embryo transfer until day of the pregnancy test versus from day of embryo transfer until ultrasound confirmation of clinical pregnancy), and route (intrauterine infusion versus subcutaneous administration). The optimal dose, frequency, duration or route of G‐CSF administration is therefore not clear. In some trials, normal saline was used as placebo in the control arm, whilst in others no placebo was used. Some trials included only fresh‐embryo transfer, others included only frozen‐embryo transfer, and still other trials included both fresh and frozen transfers. We had planned a subgroup analysis (fresh versus frozen; intrauterine versus subcutaneous) as an investigative tool in case statistical heterogeneity was detected after pooling the results; however, this was precluded as we did not detect substantial statistical heterogeneity across the pooled studies for the comparisons. Stratification of the analysis into different subpopulations (unselected IVF group, women with two or more IVF failures, and women with thin endometrium) resulted in a difference between the pooled estimates for clinical pregnancy rate.
There could be some degree of clinical heterogeneity due to different eligibility criteria used in the studies. In the studies with an unselected IVF population, both women undergoing first IVF and those with one or more IVF failures were included. In the studies that included women with two or more IVF failures, different criteria were applied to define IVF failure (number of embryos transferred and stage of embryo transfer). Importantly, few studies reported adverse events following G‐CSF. Due to limited data availability, no definitive conclusions can be made about the safety of G‐CSF administration.
Quality of the evidence
For the comparison of G‐CSF versus no treatment or placebo in subfertile women undergoing ART, we reported ongoing pregnancy, clinical pregnancy, miscarriage rate, and adverse events. The quality of the evidence was very low for the main comparison overall. In the majority of trials the description of allocation concealment was unclear (unclear risk of selection bias) or the treatment providers were not blinded (high risk of performance bias), hence we downgraded the quality of the evidence by one level for risk of bias. We downgraded the quality of the evidence for most outcomes by one level for imprecision due to wide confidence intervals. For ongoing pregnancy rate, we further downgraded the evidence by two levels for indirectness, as pooled results were obtained from only one study that included an unselected IVF population. For miscarriage rate, we downgraded the quality of the evidence by one level for indirectness because the reported miscarriages in the studies only captured first trimester losses. We further downgraded the quality of the evidence for clinical pregnancy rate by one level for inconsistency due to substantial variability between effect estimates between the three subpopulations. Within the subpopulations of an unselected IVF group, women with two or more IVF failures, and women with thin endometrium, the quality of evidence was low for clinical pregnancy rate. We did not find statistical heterogeneity across studies within each subpopulation.
Potential biases in the review process
We aimed to identify all studies that met the eligibility criteria for this current review. We performed a comprehensive search, but the possibility remains that there may have been studies that our search did not find. We attempted to contact authors of ongoing trials for clarification and unpublished data as well as authors of published conference abstracts. Of six published conference abstracts, we got satisfactory responses for two (Scarpellini 2011; Scarpellini 2013), partial responses for another two (Singh 2015; Singh 2018), and no response from the authors of the remaining two abstracts (Cambiaghi 2012a; Obidniak 2016). Amongst the six published trials, we requested clarification regarding data from three author groups, Barad 2014; Davari‐Tanha 2016; Sarvi 2017, and received satisfactory responses from three of them. We obtained all the necessary data and clarification from the author of an unpublished trial (NCT01202643). We obtained translated information and data for one trial that was published in the Persian language (Jalilvand 2018). Two authors (MSK and RK) of the current Cochrane Review were also authors of an earlier systematic review that evaluated the role of G‐CSF in women with recurrent implantation failure (RIF) and thin endometrium.
Agreements and disagreements with other studies or reviews
An earlier systematic review evaluated the effectiveness of G‐CSF in women with RIF and thin endometrium undergoing ART (Kamath 2017). The review included randomised (N = 3) and non‐randomised prospective trial (N =1) data. The authors pooled results from two studies evaluating the role of G‐CSF in RIF and reported a significantly higher clinical pregnancy rate (RR 2.51, 95% CI 1.36 to 4.63; I2 = 0%) following G‐CSF administration compared to placebo (low‐quality evidence). These findings are similar to the pooled results obtained for the subpopulation of women with two or more IVF failures in the current Cochrane Review. The same review combined results from two studies evaluating G‐CSF in women with thin endometrium and found a significantly higher clinical pregnancy rate following G‐CSF administration (RR 2.43, 95% CI 1.09 to 5.40; I2 = 0%; very low‐quality evidence). In the current Cochrane Review, we are uncertain whether G‐CSF administration improves clinical pregnancy rate when compared to placebo in women with thin endometrium (four RCTs). The differences in the results could be explained by the inclusion of non‐randomised trial data in the earlier review. We did not include non‐randomised data in the current Cochrane Review, as they tend to overestimate the effect size.
Another systematic review also aimed to evaluate the role of G‐CSF in infertile women undergoing IVF (Li 2017). That review included data from three RCTs and three non‐randomised trials. The authors pooled results from all six studies and reported a significantly higher (RR 1.56, 95% CI 1.12 to 2.18) clinical pregnancy rate following G‐CSF administration versus no treatment or placebo. The authors concluded that G‐CSF may have important role to play in human reproduction, especially in women with thin endometrium and RIF. The reason for the difference in findings compared with current Cochrane Review could be that the authors pooled studies with different study designs (randomised and non‐randomised trials) as well as different populations (women with thin endometrium, RIF, and an unselected population).
A third systematic review explored the role of G‐CSF in infertile women undergoing ART and included only RCTs (N = 10) (Zhang 2018). The authors of that review reported significantly improved clinical pregnancy rate following G‐CSF administration (RR 1.89, 95% CI 1.53 to 2.33) compared to placebo. The majority of studies (8/10) that contributed to the pooled results included women with RIF. The pooled results in the RIF subpopulation also showed a significantly higher clinical pregnancy rate (RR 2.07, 95% CI 1.64 to 2.61) following G‐CSF administration compared to placebo. The authors concluded that G‐CSF administration has a beneficial role in women undergoing ART, especially in the RIF population.
Authors' conclusions
Implications for practice.
In subfertile women undergoing assisted reproductive technology (ART), we are uncertain whether the administration of granulocyte‐colony stimulating factor (G‐CSF) improves ongoing pregnancy or clinical pregnancy rates or reduces miscarriage rate compared to no treatment or placebo, whether in all women or those with thin endometrium (very low‐quality evidence). Low‐quality evidence suggests that G‐CSF administration may improve clinical pregnancy rate in women with two or more IVF failures, but the included studies had an unclear risk of bias for allocation concealment or were at high risk of performance bias.
Implications for research.
There is a need for high‐quality randomised trials to assess the effectiveness and safety of G‐CSF administration in subfertile women undergoing ART. Further investigation is also required into its role in women with thin endometrium undergoing ART. The optimum dose, duration, and route of G‐CSF administration needs to be established. Trial designs should include an identical placebo in the control arm to reduce performance bias. Future trials should report live‐birth or ongoing pregnancy rate as an outcome. The major and minor adverse effects of G‐CSF administration should also be captured in any future studies.
What's new
| Date | Event | Description |
|---|---|---|
| 4 November 2020 | Amended | We have moved 2 formerly included studies to Awaiting classification pending provision of further details on the studies. Minot edits to Abstract. No change in conclusions. |
History
Protocol first published: Issue 12, 2018 Review first published: Issue 1, 2020
| Date | Event | Description |
|---|---|---|
| 19 December 2018 | Amended | Names of referees added to Acknowledgements section. |
Acknowledgements
We thank Helen Nagels (Managing Editor), Marian Showell (Information Specialist), and Professor Cindy Farquahar, Cochrane Gynaecology and Fertilty Group Co‐ordinating Editor, for their valuable support in developing this Cochrane Review. We also acknowledge the contributions of referees Ms Katie Stocking, Dr Tze Wong, and Dr Ioannis Sfontouris.
We thank Dr Sharin Asadi who helped in translating and extracting data from one manuscript. Finally, we would like to express our gratitude to the following investigators, who provided essential information for the preparation of this review: Dr Marzieh Ghasemi, Dr Randhir Singh, Dr Fatemah Davari, Dr David H Barad, Dr Mahdi Sheikh, and Dr Marco Sbracia.
Appendices
Appendix 1. Cochrane Gynaecology and Fertility Group Specialised Register search strategy
Searched 7 February 2019
Procite platform
Keywords CONTAINS "IVF" or "ICSI" or "ET" or "intracytoplasmic sperm injection techniques" or "intracytoplasmic sperm injection" or "in‐vitro fertilisation " or "in vitro fertilization" or "Embryo Transfer" or "blastocyst transfer" or "ovarian stimulation" or "ovarian stimulation controlled ovarian stimulation" or "ovulation induction" or "ovarian hyperstimulation" or "poor prognostic patients" or "controlled ovarian hyperstimulation" or "controlled ovarian stimulation" or "COH" or "recurrent miscarriage" or "recurrent implantation failure" or "recurrent pregnancy loss" or "recurrent spontaneous miscarriage" or Title CONTAINS "IVF" or "ICSI" or "ET" or "intracytoplasmic sperm injection techniques" or "intracytoplasmic sperm injection" or "in‐vitro fertilisation " or "in vitro fertilization" or "Embryo Transfer" or "ovarian stimulation" or "ovarian stimulation controlled ovarian stimulation" or "recurrent miscarriage" or "recurrent implantation failure" or "recurrent pregnancy loss" or "recurrent spontaneous miscarriage"
AND
Keywords CONTAINS "granulocyte colony‐stimulating factor" or "G‐CSF" or Title CONTAINS "granulocyte colony‐stimulating factor" or "G‐CSF"
(27 records)
Appendix 2. CENTRAL CRSO search strategy
Searched 7 February 2019
Web platform
#1 MESH DESCRIPTOR Granulocyte Colony‐Stimulating Factor EXPLODE ALL TREES 1119 #2 (Granulocyte‐colony stimulating factor*):TI,AB,KY 2349 #3 filgrastim*:TI,AB,KY 906 #4 (G‐CSF or GCSF):TI,AB,KY 2121 #5 (colony‐stimulating factor 3):TI,AB,KY 2 #6 (CSF 3):TI,AB,KY 22 #7 n?upogen:TI,AB,KY 70 #8 (r methug csf):TI,AB,KY 58 #9 topneuter:TI,AB,KY 2 #10 zarxio:TI,AB,KY 3 #11 #1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7 OR #8 OR #9 OR #10 3589 #12 MESH DESCRIPTOR Embryo Transfer EXPLODE ALL TREES 1029 #13 MESH DESCRIPTOR Fertilization in Vitro EXPLODE ALL TREES 1959 #14 MESH DESCRIPTOR Sperm Injections, Intracytoplasmic EXPLODE ALL TREES 510 #15 embryo*: TI,AB,KY 5579 #16 (vitro fertili?ation):TI,AB,KY 2564 #17 ivf:TI,AB,KY 3819 #18 icsi:TI,AB,KY 1704 #19 (intracytoplasmic sperm injection*):TI,AB,KY 1498 #20 blastocyst*:TI,AB,KY 945 #21 infertil* or subfertil*:TI,AB,KY 6613 #22 assisted reproducti*:TI,AB,KY 908 #23 (endometri* adj3 thin*):TI,AB,KY 25 #24 (endometri* adj3 thick*):TI,AB,KY 1025 #25 RIF:TI,AB,KY 273 #26 (implant* adj3 failure*):TI,AB,KY 992 #27 (recurrent adj3 abortion*):TI,AB,KY 210 #28 (recurrent adj3 miscarriage*):TI,AB,KY 167 #29 #12 OR #13 OR #14 OR #15 OR #16 OR #17 OR #18 OR #19 OR #20 OR #21 OR #22 OR #23 OR #24 OR #25 OR #26 OR #27 OR #28 13790 #30 #11 AND #29 55
Appendix 3. MEDLINE search strategy
Searched from 1946 to 7 February 2019
Ovid platform
1 exp granulocyte colony‐stimulating factor/ or filgrastim/ (15000) 2 filgrastim*.tw. (1790) 3 Granulocyte‐colony stimulating factor*.tw. (13815) 4 (G‐CSF or GCSF).tw. (14449) 5 colony‐stimulating factor 3.tw. (71) 6 CSF 3.tw. (182) 7 n?upogen.tw. (198) 8 r methug csf.tw. (87) 9 topneuter.tw. (1) 10 zarxio.tw. (14) 11 or/1‐10 (23818) 12 vitro fertili?ation.tw. (21565) 13 (ivf or et).tw. (258518) 14 icsi.tw. (7674) 15 (embryo* adj2 transfer*).tw. (15018) 16 (blastocyst* adj2 transfer*).tw. (1486) 17 exp Reproductive Techniques, Assisted/ (65327) 18 intracytoplasmic sperm injection*.tw. (6650) 19 assisted reproducti* techn*.tw. (9138) 20 (endometri* adj3 thin*).tw. (384) 21 (endometri* adj3 thick*).tw. (2998) 22 RIF.tw. (3997) 23 (implant* adj3 failure*).tw. (8100) 24 (recurrent adj3 abortion*).tw. (2322) 25 or/12‐24 (330592) 26 11 and 25 (257) 27 randomized controlled trial.pt. (475791) 28 controlled clinical trial.pt. (92898) 29 randomized.ab. (434012) 30 randomised.ab. (86557) 31 placebo.tw. (200574) 32 clinical trials as topic.sh. (185956) 33 randomly.ab. (304976) 34 trial.ti. (193886) 35 (crossover or cross‐over or cross over).tw. (79135) 36 or/27‐35 (1256577) 37 exp animals/ not humans.sh. (4544569) 38 36 not 37 (1156155) 39 26 and 38 (38)
Appendix 4. Embase search strategy
Searched from 1980 to 7 February 2019
Ovid platform
1 exp granulocyte colony stimulating factor/ (40941) 2 exp filgrastim/ (1898) 3 Granulocyte‐colony stimulating factor*.tw. (17226) 4 (G‐CSF or GCSF).tw. (24112) 5 colony‐stimulating factor 3.tw. (104) 6 CSF 3.tw. (268) 7 filgrastim*.tw. (4514) 8 n?upogen.tw. (2566) 9 r methug csf.tw. (105) 10 topneuter.tw. (2) 11 zarxio.tw. (111) 12 or/1‐11 (54787) 13 exp infertility therapy/ (95300) 14 vitro fertili?ation.tw. (29185) 15 (ivf or et).tw. (644805) 16 icsi.tw. (16126) 17 intracytoplasmic sperm injection*.tw. (9503) 18 assisted reproducti* techn*.tw. (14422) 19 (embryo* adj2 transfer*).tw. (25044) 20 (blastocyst* adj2 transfer*).tw. (3141) 21 (endometri* adj3 thin*).tw. (666) 22 (endometri* adj3 thick*).tw. (5246) 23 RIF.tw. (5316) 24 (implant* adj3 failure*).tw. (11212) 25 (recurrent adj3 abortion*).tw. (3208) 26 (recurrent adj3 miscarriage*).tw. (3712) 27 or/13‐26 (745520) 28 12 and 27 (2021) 29 Clinical Trial/ (944181) 30 Randomized Controlled Trial/ (530653) 31 exp randomization/ (81171) 32 Single Blind Procedure/ (33811) 33 Double Blind Procedure/ (154793) 34 Crossover Procedure/ (58004) 35 Placebo/ (316159) 36 Randomi?ed controlled trial$.tw. (195858) 37 Rct.tw. (31138) 38 random allocation.tw. (1856) 39 randomly.tw. (396386) 40 randomly allocated.tw. (31541) 41 allocated randomly.tw. (2395) 42 (allocated adj2 random).tw. (798) 43 Single blind$.tw. (22015) 44 Double blind$.tw. (187683) 45 ((treble or triple) adj blind$).tw. (892) 46 placebo$.tw. (278582) 47 prospective study/ (498402) 48 or/29‐47 (2197098) 49 case study/ (58845) 50 case report.tw. (361827) 51 abstract report/ or letter/ (1045652) 52 or/49‐51 (1457062) 53 48 not 52 (2146638) 54 (exp animal/ or animal.hw. or nonhuman/) not (exp human/ or human cell/ or (human or humans).ti.) (5621596) 55 53 not 54 (1997760) 56 28 and 55 (421)
Appendix 5. CINAHL search strategy
Searched from 1961 to 7 February 2019
EBSCO platform
S28 S11 AND S27 43 S27 S12 OR S13 OR S14 OR S15 OR S16 OR S17 OR S18 OR S19 OR S20 OR S21 OR S22 OR S23 OR S24 OR S25 OR S26 15,364 S26 TX (recurrent N3 miscarriage*) 399 S25 TX (recurrent N3 abortion*) 194 S24 TX (implant* N3 failure*) 2,336 S23 TX RIF 554 S22 TX (endometri* N3 thick*) 578 S21 TX (endometri* N3 thin*) 72 S20 TX assisted reproducti* techn* 3,215 S19 TX blastocyst* N3 transfer* 325 S18 TX embryo* N3 transfer* 2,775 S17 TX ovar* N3 hyperstimulat* 776 S16 TX ovari* N3 stimulat* 893 S15 TX IVF or TX ICSI 4,521 S14 (MM "Fertilization in Vitro") 3,154 S13 TX COH 217 S12 TX vitro fertilisation 6,402 S11 S1 OR S2 OR S3 OR S4 OR S5 OR S6 OR S7 OR S8 OR S9 OR S10 5,438 S10 TX zarxio 16 S9 TX r methug csf 95 S8 TX r methug csf 95 S7 TX filgrastim* 374 S6 TX n?upogen 38 S5 TX CSF 3 2,955 S4 TX colony‐stimulating factor 3 1,222 S3 TX (G‐CSF or GCSF) 1,049 S2 TX Granulocyte‐colony stimulating factor* 2,213 S1 (MM "Granulocyte Colony‐Stimulating Factor") 931
Appendix 6. Data extraction form
| Study information | |||||||
| 1. Ref ID | |||||||
| 2. First author | |||||||
| 3. Year | |||||||
| 4. Published | |||||||
| 5. Language | |||||||
| Criteria for eligibility: | YES | NO | |||||
| Patients: | Couples undergoing IVF/ ICSI ( fresh or frozen) Fresh Frozen |
q q q |
q q q |
||||
| Intervention | Instillation of GCSF or administration of GCSF a) Thin endometrium b) Two or more IVF failures |
q | q | ||||
| Comparison | No treatment / placebo / other treatment like Sildenafil / aspirin | q | q | ||||
| Outcome |
Primary: Live birth rate (per randomised couple) Adverse events (e.g anaphylactic reaction) Secondary: Clinical pregnancy rate (per randomised couple) (positive pregnancy test, gestational sac on ultrasound) Multiple pregnancy rate (per randomised couple) Miscarriage rate (per randomised couple and clinical pregnancy) Ectopic pregnancy ( per clinical pregnancy) Congenital anamoly rate ( per clinical pregnancy |
q q q q q q |
q q q q q q |
||||
| Study characteristics | |||||||
| Design | |||||||
| 1. Study design | q RCT q Parallel (intervention vs. control) q Cross‐over (participants used as intervention and control group) q ………………………………. Quotes: ……………………………………………………………………………………………………………………………………………………………………………………………………………………………… |
||||||
| 4. Setting | q Single‐centre | q Multi‐centre | |||||
| Country: .……………………………………………………………………………………………………………… | |||||||
| Participants: in‐ and exclusion | |||||||
| 5. Study criteria for patient inclusion | ____________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________ | ||||||
| 6. Study criteria for patient exclusion | _____________________________________________________________________________________________________________________________________________________________________________________________ | ||||||
| 7. Description control/ comparison treatment | ……………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………… | ||||||
| Baseline Characteristics | |||||||
| Intervention | |||||||
| Embryo transfer after IVF, ICSI | |||||||
| 1. Time of randomisation during cycle | q Prior to commencement of treatment cycle | ||||||
| 2. Nature of intervention | q GCSF q no treatment/ placebo/ other treatments like sildenafil/ aspirin |
||||||
| 3. Nature of cycle | q fresh q frozen |
||||||
Data and analyses
Comparison 1. G‐CSF versus placebo or no treatment in women undergoing assisted reproduction.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1.1 Ongoing pregnancy rate | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1.1 Unselected IVF population | 1 | 150 | Risk Ratio (M‐H, Random, 95% CI) | 1.62 [0.86, 3.08] |
| 1.2 Miscarriage rate | 2 | 291 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.40 [0.09, 1.77] |
| 1.2.1 Unselected IVF population | 2 | 291 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.40 [0.09, 1.77] |
| 1.3 Clinical pregnancy rate | 12 | 1050 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.65 [1.32, 2.06] |
| 1.3.1 Unselected IVF population | 2 | 291 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.12 [0.75, 1.68] |
| 1.3.2 2 or more IVF failures | 6 | 553 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.10 [1.53, 2.89] |
| 1.3.3 Women with thin endometrium | 4 | 206 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.58 [0.95, 2.63] |
| 1.4 Adverse events | 4 | 410 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Not estimable |
| 1.4.1 Unselected IVF population | 1 | 141 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Not estimable |
| 1.4.2 2 or more IVF failures | 3 | 269 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Not estimable |
| 1.5 Ectopic pregnancy rate | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Subtotals only | |
| 1.5.1 Unselected IVF population | 0 | 0 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Not estimable |
| 1.5.2 2 or more IVF failures | 1 | 112 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.97 [0.20, 19.35] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Aleyasin 2016.
| Study characteristics | ||
| Methods | Randomized controlled trial (RCT) Multicentre Iran |
|
| Participants | Women with repeated in vitro fertilization (IVF) failure defined as failure of implantation in at least 3 consecutive IVF attempts in which 3 high‐grade embryos were transferred in each cycle, undergoing subsequent IVF. Women were considered eligible if they met the following inclusion criteria.
Exclusion criteria:
|
|
| Interventions | Intervention group (N = 56): subcutaneous administration of G‐CSF 300 µg 1 hour before embryo transfer (ET) Control group (N = 56): participants in the control group did not receive any additional treatment before the embryo transfer |
|
| Outcomes | Implantation rate, biochemical pregnancy rate, clinical pregnancy rate, ectopic pregnancy rate | |
| Notes | Trial was registered (IRCT201503119568N11). We contacted the authors and received satisfactory replies to our queries. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | A computerised random number generator was used for sequence generation. Simple randomisation with a 1:1 allocation ratio was used. |
| Allocation concealment (selection bias) | Low risk | Consecutive opaque envelopes for the allocation concealment were used. The envelopes were opaque when held to the light, opened sequentially, and only after the participant’s name and other details were written on the appropriate envelope and implementation of assignments was carried out. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No placebo was given to the control group; does not mention blinding of participant/clinician/embryologist. High risk for performance bias |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Does not specify if outcome assessors were blinded. However, absence of blinding is unlikely to have influenced the findings for our primary and secondary outcomes, hence categorised as low risk for detection bias. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No attrition, all randomised women had primary outcomes reported. |
| Selective reporting (reporting bias) | Low risk | All prespecified outcomes reported as per clinical trial website information. |
| Other bias | Low risk | Baseline characteristics similar, funding mentioned (Tehran University of Medical Sciences). Original sample size was 100 (as per clinical trial registry information). Final published study included number was 112, which is unlikely to have introduced any bias. |
Barad 2014.
| Study characteristics | ||
| Methods | RCT Single centre USA |
|
| Participants | Women aged 18 to 38 years with normal endometrium undergoing fresh IVF. Exclusion criteria: women with renal disease, sickle cell disease, or a history of malignancy were considered ineligible for medical reasons. |
|
| Interventions | Intervention arm (N = 73): G‐CSF used was Nupogen (300 μg/1.0 mL, filgrastim; Amgen) Control arm (N = 68): placebo used (normal saline) 1 mL of G‐CSF or placebo (normal saline) was administered on the morning of hCG administration before noon by slow transcervical intrauterine infusion, similar in technique to an intrauterine insemination. Endometrial thickness was assessed by routine vaginal ultrasound before infusion and again 5 days later at the time of embryo transfer. Only data from pre‐cross‐over phase of the study was included. |
|
| Outcomes | Clinical pregnancy rate, implantation rate, miscarriage rate | |
| Notes | Registered trial (NCT01202656). We contacted authors for clarification, who responded with data clarification and provided other details. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | A computer‐generated randomisation table was used with separate randomisation blocks for women undergoing IVF and frozen‐embryo transfer in the first study cycle. |
| Allocation concealment (selection bias) | Low risk | Individual randomisation cards were sealed in numbered, opaque envelopes that were only accessible to the single staff member who administered the randomisation table and prepared the study materials. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Treatment assignment was blinded to participants, physicians, and nursing staff. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | The outcome assessors were also blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No dropouts during the first phase of the treatment, therefore considered to be at low risk of attrition bias |
| Selective reporting (reporting bias) | High risk | Live birth as a prespecified outcome (as per trial registry file) was not reported in the published manuscript. |
| Other bias | Low risk | None; funding mentioned (Foundation for Reproductive Medicine, a not‐for‐profit medical research foundation, and by intramural funds from the Center for Human Reproduction). No difference in baseline characteristics |
Cambiaghi 2012a.
| Study characteristics | ||
| Methods | RCT Single centre Brazil |
|
| Participants | Women undergoing IVF who met the following criteria.
|
|
| Interventions | Intervention group: subcutaneous administration of G‐CSF, 0.25 mL of filgrastim (300 μg/mL) every other day from day of ET until transvaginal ultrasound (TVS) confirmation of gestational sac with foetal heartbeat on ultrasound 6 weeks after ET Control group: women in the control group did not receive any additional treatment apart from the usual luteal phase support A total of 20 women were included in the trial, however the number of women randomised to each group was not mentioned. As we were unable to contact authors through email for data clarification, we could not include the data from this study in quantitative analysis. |
|
| Outcomes | Implantation rate, clinical pregnancy rate | |
| Notes | Conference abstract. Not registered. Contacted authors by email, no response received. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | A computer‐based randomisation was used to allocate the women to the 2 groups. |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Control arm, no additional intervention. Does not mention blinding of participant/clinician/embryologist. High risk for performance bias |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Does not specify if outcome assessors were blinded. However, absence of blinding is unlikely to have influenced the findings for our primary and secondary outcomes, hence categorised as low risk for detection bias. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Insufficient information to permit a judgement |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit a judgement |
| Other bias | Unclear risk | Insufficient information to permit a judgement |
Davari‐Tanha 2016.
| Study characteristics | ||
| Methods | RCT Multicentre (2 centres) Iran |
|
| Participants | Women with RIF undergoing subsequent fresh and frozen IVF treatment. Inclusion criteria:
Exclusion criteria:
|
|
| Interventions | 3 groups: Intervention group (N = 40): at the time of oocyte retrieval 1 mL of G‐CSF (300 μg/mL, filgrastim) was administered by a transcervical Cook catheter for embryo transfer slowly into uterine cavity. In frozen embryo transfer (FET) cycle intervention was scheduled at the day of starting progesterone. Control group 1 (N = 40): at the time of oocyte retrieval 1 mL of normal saline was administered by a transcervical Cook catheter for embryo transfer slowly into uterine cavity Control group 2 (N = 20): for placebo group a catheter was passed through the cervix without any injection |
|
| Outcomes | Implantation rate, biochemical pregnancy rate, clinical pregnancy rate | |
| Notes | Registered trial (IRCT201108212576N5). Authors responded to all email queries. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was performed by a computer‐generated randomisation block table. |
| Allocation concealment (selection bias) | Low risk | Randomisation cards were offered to the participants by a nurse who was blinded to the study groups. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Participants and clinician were blinded regarding the study groups. Author response: embryologist blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Does not specify if outcome assessors were blinded. However, absence of blinding is unlikely to have influenced the findings for our primary and secondary outcomes, hence categorised as low risk for detection bias. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All randomised participants were included in the final analysis. |
| Selective reporting (reporting bias) | Low risk | All prespecified outcomes reported (as per trial registry information). |
| Other bias | Low risk | Baseline characteristics similar. Funding and support from Tehran University of Medical Sciences. No other bias detected. |
Jain 2018.
| Study characteristics | ||
| Methods | RCT Single centre India |
|
| Participants | Women undergoing fresh IVF. Inclusion criteria:
Exclusion criteria:
|
|
| Interventions | Women in the intervention group (N = 76) received intrauterine perfusion of 300 μg (0.5 mL) of G‐CSF on the day of ovulation trigger. Women in placebo group (N = 74) received intrauterine perfusion of 0.5 mL normal saline on the day of ovulation trigger. |
|
| Outcomes | Clinical pregnancy rate, ongoing pregnancy rate, implantation rate, endometrial thickness, endometrial cavity volume and vascularity on the day of embryo transfer | |
| Notes | Retrospectively registered trial (CTRI/2017/10/010310) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Patients were randomised into two groups; intervention and control groups, according to the computer generated randomisation table" |
| Allocation concealment (selection bias) | Low risk | Quote: "Individual randomisation cards were sealed in numbered opaque envelopes which were accessible to a single person who administered the randomisation table and prepared the study materials." |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Treatment assignment was blinded to participants, doctors administering treatment and performing ultrasounds, and nursing staff. No information as to whether embryologists were blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Does not specify if outcome assessors were blinded. However, absence of blinding is unlikely to have influenced the findings for our primary and secondary outcomes, hence categorised as low risk for detection bias. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Similar dropouts in both groups (intervention 6/76 vs control 11/74) mentioned with reasons. Intention‐to‐treat analysis done. |
| Selective reporting (reporting bias) | Low risk | All outcomes prespecified at the time of trial registration were reported. |
| Other bias | Low risk | Retrospectively registered trial. No funding support taken for conduct of the study. |
Jalilvand 2018.
| Study characteristics | ||
| Methods | RCT Single centre Iran |
|
| Participants | Women undergoing frozen‐embryo transfer. Inclusion criteria: women aged 18 to 45 years with history of 2 or more implantation failures (RIF) Exclusion criteria: women with renal disease, sickle cell disease, or a history of malignancy were considered ineligible for medical reasons |
|
| Interventions | Intervention group (N = 34): G‐CSF, 100 μg intrauterine on the day of progesterone until ET Control group (N = 38): no additional treatment |
|
| Outcomes | Positive pregnancy rates, clinical pregnancy rates | |
| Notes | Trial not registered. The manuscript was written in the Persian language (except abstract which was in English). We thank editorial team and Dr Sharin Asadi who helped in translating the manuscript and with data extraction. We attempted to contact the authors through emails but did not receive a reply. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information to permit a judgement |
| Allocation concealment (selection bias) | Unclear risk | No information to permit a judgement |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Blinding of participants and personnel not mentioned. Assessed as high risk as the control arm had no placebo treatment or additional treatment. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | No information to permit a judgement. However, absence of blinding is unlikely to have influenced the findings for our primary and secondary outcomes, hence categorised as low risk for detection bias. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No information to permit a judgement |
| Selective reporting (reporting bias) | Unclear risk | No information to permit a judgement |
| Other bias | Unclear risk | No information to permit a judgement |
NCT01202643.
| Study characteristics | ||
| Methods | RCT Single centre USA |
|
| Participants | Female IVF patients aged between 21 and 45 years who were willing to be randomised to treatment and, in either IVF treatment, FET or donor IVF cycles, 5 days before ET, and have inadequate endometrial thickness (< 7 mm). Exclusion criteria:
|
|
| Interventions | Intervention group (N = 6): G‐CSF 300 mcg/mL administered by transcervical infusion 1 time on day of hCG trigger for ovulation Control group (N = 6): normal saline administered by transcervical infusion 1 time on day of hCG trigger for ovulation 1 cm3 Only data from phase 1 were included (pre‐cross‐over), which were fresh transfers. |
|
| Outcomes | Clinical pregnancy rates, implantation rates, endometrial thickness | |
| Notes | Trial was terminated prematurely. Data were available on trial registry site. Authors responded and provided clarification regarding study details. Study was not published. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: “Computer generated randomisation table with 50:50 distributions” |
| Allocation concealment (selection bias) | Unclear risk | No information on method of allocation concealment |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Participant, care provider, investigator, outcome assessor were blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Participant, care provider, investigator, outcome assessor were blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All randomised participants completed phase 1 of the trial. We only included data from phase 1 in the meta‐analysis (pre‐cross‐over). |
| Selective reporting (reporting bias) | Low risk | Data for all prespecified outcomes were reported. |
| Other bias | High risk | The trial was terminated prematurely before the calculated sample size could be achieved due to insufficient recruitment. The trial was not peer reviewed/published. |
Obidniak 2016.
| Study characteristics | ||
| Methods | RCT Single centre Russia |
|
| Participants | Women undergoing frozen embryo transfer. Inclusion criteria:
Exclusion criteria:
|
|
| Interventions | 3 arms: Group 1 (N = 40): intrauterine perfusion with G‐CSF (filgrastim 30 mIU, 1 mL) using insemination catheter 5 days prior to embryo transfer (intervention arm) Group 2 (N = 30): G‐CSF (filgrastim 30 mIU, 1 mL) was administered subcutaneously once at the day of embryo transfer (intervention arm) Group 3 (N = 60): no therapy (control arm) |
|
| Outcomes | Implantation rates, clinical pregnancy rates | |
| Notes | Conference abstract. No clinical registration number available. Authors could not be contacted. Doses of G‐CSF stated in the abstract to be 30 million IU; FDA information suggests dose of 300 mcg/mL which is equivalent to 30 milli‐international units (mIU); intervention text amended accordingly. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Three groups was formed on the basis of randomisation" Method of randomisation not mentioned. |
| Allocation concealment (selection bias) | Unclear risk | Method of allocation concealment not mentioned. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Blinding of participants and personnel not mentioned. Assessed as high risk as the control arm had no placebo treatment or sham procedure. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Does not specify if outcome assessors were blinded. However, absence of blinding is unlikely to have influenced the findings for our primary and secondary outcomes, hence categorised as low risk for detection bias. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Data for all participants were considered in the reporting of results. |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit a judgement |
| Other bias | Unclear risk | Insufficient information to permit a judgement |
Sarvi 2017.
| Study characteristics | ||
| Methods | RCT Single centre Iran |
|
| Participants | Women under fresh IVF cycle. Long agonist protocol was used for all participants. Inclusion criteria:
Exclusion criteria:
|
|
| Interventions | In the investigation group (N = 17), a dosage of G‐CSF (300 μg in 1 cm3) was infused into the uterus by embryo transfer catheter within 5 minutes on the day of hCG trigger. If endometrial thickness was < 6 mm, a second dosage of G‐CSF was injected 2 to 3 days after oocyte retrieval day. In the control group (N = 17), 1 cm3 of normal saline was injected into the uterus with the same type of catheter. |
|
| Outcomes | Endometrial thickness, implantation rate, clinical pregnancy rate | |
| Notes | Registered trial (IRCT201406046063N2). The authors responded to our queries through email. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: “Patients were randomly allocated into two groups using a balanced block randomisation technique” |
| Allocation concealment (selection bias) | Low risk | Participant allocation was performed with online application described as “Sealed Envelope”. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Placebo was used in the control group. No specific information on whether participants/clinician/embryologist were blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "GCSF injection was done by one of the researchers at all stages of the project, but ultrasound was done by another researcher who was an infertility sub specialist that had worked for more than eight years in the IVF ward. She was unaware of the study groups and was a blinded observer" Overall presence or absence of blinding is unlikely to have influenced the findings for our primary and secondary outcomes, hence categorised as low risk for detection bias. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Out of 17 randomised in each group, only 13 in intervention group and 15 in control group were included in the final analyses. Participants who did not complete the study were excluded from the analysis. However, this is unlikely to have introduced any attrition bias due to the small numbers. |
| Selective reporting (reporting bias) | Low risk | All outcomes prespecified at the time of trial registration were reported. Information obtained from trial registry website. |
| Other bias | High risk | Original planned sample size was 46 at the time of trial registration (as per information obtained from trial registry website), but trial eventually included 34 participants. No clear explanation provided regarding reduced sample size. |
Scarpellini 2011.
| Study characteristics | ||
| Methods | RCT Single centre Italy |
|
| Participants | Women undergoing fresh IVF with recurrent implantation failure (at least 3 failed embryo transfers with 7 good embryos transferred).
|
|
| Interventions | Intervention group (N = 45): G‐CSF given subcutaneously from day of transfer till day of result and continued for another 40 days if positive (1.5 mg/kg/daily (60 to 100 mg)) Control group (N = 44): saline placebo injection in control group subcutaneously for same duration |
|
| Outcomes | Clinical pregnancy rates | |
| Notes | Conference abstract. No trial registration number available. Author responded to email queries regarding methodology and data clarification. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: “were randomly divided” Email response from authors: “computer generated sequence used." |
| Allocation concealment (selection bias) | Unclear risk | Email response: "envelopes for used to allocate groups." Unclear if envelopes were sequentially numbered and opaque |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Placebo injection was used. Only participants were blinded (as per response from authors). Clinician blinding not mentioned. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | No information on outcome assessor blinding status. However, absence of blinding is unlikely to have influenced the findings for our primary and secondary outcomes, hence categorised as low risk for detection bias. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No information was available on dropouts, however all randomised women were included in final analysis. |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit a judgement |
| Other bias | Unclear risk | Conference abstract; insufficient information to permit a judgement |
Scarpellini 2013.
| Study characteristics | ||
| Methods | RCT Single centre Italy |
|
| Participants | Women undergoing fresh IVF with RIF (at least 3 failed ET with at least 8 good embryos transferred).
Exclusion criteria:
|
|
| Interventions | Intervention group (N = 25): G‐CSF given subcutaneously from day of transfer till day of results (60 μg/daily) Control group (N = 25): saline placebo injection in control group subcutaneously for the same duration |
|
| Outcomes | Pregnancy rates | |
| Notes | Conference abstract. Registered trial (NCT01715974). Author responded to email queries regarding methodology and data clarification. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: “were randomly divided” Email response from authors: “computer generated sequence used." |
| Allocation concealment (selection bias) | Unclear risk | Email response from authors: "envelopes for used to allocate groups." Unclear if envelopes were sequentially numbered and opaque |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Placebo injection was used. Only participants were blinded (as per response from authors). Clinician blinding not mentioned. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | No information on outcome assessor blinding status. However, absence of blinding is unlikely to have influenced the findings for our primary and secondary outcomes, hence categorised as low risk for detection bias. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No information was available on dropouts, however all randomised women were included in final analysis. |
| Selective reporting (reporting bias) | Low risk | Prespecified outcomes reported as per clinical trial registry information. |
| Other bias | High risk | Calculated sample size was 100 (as per clinical trial registry information). However, no clear information was available on premature termination of the study before actual sample size could be achieved (n = 50). |
Singh 2015.
| Study characteristics | ||
| Methods | RCT Single centre India |
|
| Participants | Women undergoing fresh IVF cycle with thin endometrium. Previous traditional methods of treating thin endometrium have been unsuccessful (oestradiol and sildenafil). |
|
| Interventions | Intervention group (N = 24): intrauterine infusion of G‐CSF (300 μg/mL) on day of trigger, repeated after 48 hours if endometrial thickness was < 7 mm Control group (N = 24): saline intrauterine infusion on day of trigger |
|
| Outcomes | Implantation and pregnancy rates, endometrial thickness | |
| Notes | Study was published as conference abstract only. Partial response to queries on data was received from authors. No clinical trial registration number available. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: “Patient were randomly divided into two groups using computer generated list” |
| Allocation concealment (selection bias) | Unclear risk | No information on allocation concealment |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Placebo (intrauterine saline infusion) was used in the control group. No specific information as to whether participants/clinician/embryologist were blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Does not specify if outcome assessors were blinded. However, absence of blinding is unlikely to have influenced the findings for our primary and secondary outcomes, hence categorised as low risk for detection bias. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No information was available on dropouts, however all randomised women were included in final analysis. |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit a judgement |
| Other bias | Unclear risk | Conference abstract; insufficient information to permit a judgement. Funding mentioned (BTTB Centre). |
Singh 2018.
| Study characteristics | ||
| Methods | RCT Single centre India |
|
| Participants | Women undergoing fresh IVF cycle with thin endometrium | |
| Interventions | Intervention group (N = 56): intrauterine infusion of G‐CSF (300 μg/mL) or subcutaneous G‐CSF administration on day of trigger, repeated after 48 hours if endometrial thickness was < 7 mm Control group (N = 56): saline intrauterine infusion on day of trigger or subcutaneous administration |
|
| Outcomes | Implantation and pregnancy rates, endometrial thickness | |
| Notes | Study was published as conference abstract only. Partial response to queries on data was received from authors. No clinical trial registration number available. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of randomisation not mentioned. |
| Allocation concealment (selection bias) | Unclear risk | No information on allocation concealment |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Placebo (intrauterine saline infusion or subcutaneous administration) was used in control group. No specific information as to whether participants/clinician/embryologist were blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Does not specify if outcome assessors were blinded. However, absence of blinding is unlikely to have influenced the findings for our primary and secondary outcomes, hence categorised as low risk for detection bias. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No information was available on dropouts, however all randomised women were included in final analysis. |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit a judgement |
| Other bias | Unclear risk | Conference abstract; insufficient information to permit a judgement |
AFC: antral follicle count AMH: anti‐Müllerian hormone BMI: body mass index ET: embryo transfer FET: frozen embryo transfer FSH: follicle‐stimulating hormone G‐CSF: granulocyte‐colony stimulating factor hCG: human chorionic gonadotrophin IUI: intrauterine insemination IVF: in vitro fertilization µg/L: microgram/litre mIU: milli‐international unit RIF: recurrent implantation failure TVS: transvaginal ultrasound
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Arefi 2018 | Quasi‐randomised trial. Randomised participants based on hospital number (per email response from author) |
| Cambiaghi 2012b | Non‐randomised design (self control) |
| Eapen 2018 | Different population, intervention, comparison and outcomes (PICO); women with recurrent pregnancy loss |
| Eftekhar 2014 | Non‐randomised design |
| Gleicher 2013 | Non‐randomised design (cohort study) |
| Jung 2015 | Conference abstract. Non‐randomised design Authors mention prospective study only. Could not contact authors for further clarification (no email address) |
| Kunicki 2017 | Non‐randomised design |
| Li 2017 | Systematic review |
| Lukaszuk 2016 | Non‐randomised design |
| Mehrafza 2019 | Non‐randomised design (retrospective cohort study) |
| Mishra 2016 | Non‐randomised design |
| Obidniak 2018 | Different PICO (evaluation of colony stimulating factor in uterine fluid). |
| Sbracia 2014 | Different PICO (granulocyte‐macrophage colony stimulating factor (GM‐CSF) medium in culture media) |
| Scarpellini 2009 | Different population (women with recurrent pregnancy loss) and not in vitro fertilization (IVF) intervention |
| Sen 2017 | Cohort study |
| Singh 2015b | Cohort study |
| Singh 2017 | Case control study |
| Wasim 2017 | Different PICO (GM‐CSF medium) |
| Wu 2018 | Different PICO |
| Würfel 2000 | Quasi‐randomised trial. Trial nurse randomised participants based on order of reporting: first in intervention, second in control, third in intervention, and so on (email response from author). |
| Xu 2014 | Non‐randomised trial. Compared intervention with historical control |
| Zabardoust 2017 | Different population (women with recurrent pregnancy loss) and different intervention (ovulation induction) |
| Zhang 2018 | Systematic review |
Characteristics of studies awaiting classification [ordered by study ID]
Eftekhar 2016a.
| Methods | RCT Single centre Iran |
| Participants | Women undergoing fresh IVF. Inclusion criteria:
Exclusion criteria: Women with sickle cell disease, chronic neutropenia, malignancy history, renal failure, congenital fructose intolerance, respiratory infection, endometriosis, and severe male factor |
| Interventions | Interventional group (N = 45): received uterine infusion of 300 μg (0.5 mL) recombinant human G‐CSF by use of intrauterine insemination (IUI) catheter after ovarian puncture Control group (N = 45): standard treatment was continued in the 45 women (control group) and did not receive G‐CSF treatment |
| Outcomes | Implantation rates and clinical pregnancy rates |
| Notes | Registered trial (IRCT201510086420N15). We tried contacting the authors for clarification on study methods but did not receive a reply. |
Eftekhar 2016b.
| Methods | RCT Single centre Iran |
| Participants | Women undergoing fresh IVF Inclusion criteria:
Exclusion criteria:
|
| Interventions | Intervention group (N = 55): 300 μg transcervical intrauterine of G‐CSF was administered at the oocyte retrieval day Control group (N =58): no additional intervention |
| Outcomes | Implantation rate, clinical pregnancy rate, ongoing pregnancy rate, miscarriage rate |
| Notes | Registered trial (IRCT201508236420N13). We tried contacting the authors for clarification on study methods but did not receive a reply. |
Kim 2011.
| Methods | Prospective study (unclear if randomised trial) |
| Participants | Women undergoing in vitro fertilization (IVF) with recurrent implantation failure (RIF), aged between 29 and 40 years |
| Interventions | Intervention arm: granulocyte‐macrophage colony stimulating factor (GM‐CSF) (100 μg) was administered on day of embryo transfer (ET) and 4 days later Control arm: no additional intervention mentioned |
| Outcomes | Implantation rate and clinical pregnancy rates |
| Notes | Conference abstract. We tried contacting the author through workplace email address, however we were unable to obtain the email address since the author was no longer working in the same institute where the study was conducted. We were unable to obtain clarification or confirmation on randomisation/design. |
Characteristics of ongoing studies [ordered by study ID]
NCT01715974.
| Study name | Administration of granulocyte‐colony stimulating factor (G‐CSF) in women with recurrent implantation failure in in vitro fertilization (IVF) cycles |
| Methods | Allocation: randomised Intervention model: parallel assignment Masking: single (participant) |
| Participants | Total 100 participants with recurrent implantation failure. Inclusion criteria:
Exclusion criteria:
|
| Interventions | Intervention: 60 μg/day of G‐CSF from the day of embryo transfer through the day of beta human chorionic gonadotrophin (hCG) test Control: saline infusion every day from the day of embryo transfer through the day of beta hCG test |
| Outcomes | Primary outcome:
Secondary outcome:
|
| Starting date | December 2012 |
| Contact information | Location: Italy Contact: Marco Sbracia, MD Centre for Endocrinology and Reproductive Medicine, Italy |
| Notes | Recruitment status: completed Completion date: March 2016 Sponsor: Centre for Endocrinology and Reproductive Medicine, Italy We contacted the author about the status of the trial. The study is still ongoing as per author reply. |
NCT02149277.
| Study name | Effects of exogenous recombinant G‐CSF in patients with repeated implantation failure ‐ a randomized single‐blind trial |
| Methods | Allocation: randomised Intervention model: parallel assignment Masking: double (participant, investigator) |
| Participants | Total 290 participants. Inclusion criteria:
Exclusion criteria:
|
| Interventions | Active comparator: filgrastim
Placebo comparator: sodium chloride
|
| Outcomes | Dosage of G‐CSF [ Time Frame: up to 3 years ] Measure dosage of G‐CSF in the bloodstream on the day of oocyte retrieval and measure the implantation rate as well as pregnancy rate following the G‐CSF injection Pregnancy and implantation [ Time Frame: up to 3 years ] Measure the pregnancy and embryo implantation rate post‐G‐CSF injection |
| Starting date | March 2013 |
| Contact information | Location: Canada Contact: Nelly Delouya, Nurse 514‐798‐2000 ext 759n delouya@cliniqueovo.com |
| Notes | Recruitment status: recruiting Estimated study completion date: December 2020 Sponsor: OVO R & D |
NCT03023774.
| Study name | Effects of granulocyte‐colony stimulating factor (G‐CSF) Neupogen on cases with thin endometrium or previous implantation failure in intracytoplasmic sperm injection (ICSI) Cycles |
| Methods | Allocation: not stated Intervention model: single‐group assignment Masking: none (open‐label) |
| Participants | Total sample size: 20 Inclusion criteria:
Exclusion criteria:
|
| Interventions | Drug: Neupogen Neupogen 30 internation unit (IU) once intrauterine at the time of ovum pickup. Other name: granulocyte colony‐stimulating factor Control: will be treated with enoxaparin sodium (Clexane) 40 mg and oestrogen |
| Outcomes | Primary outcome:
Secondary outcome:
|
| Starting date | June 2018 |
| Contact information | Location: Cairo, Egypt Contact: Dr Wessam Magdi Abuelghar, principal investigator, Ain Shams Maternity Hospital. No email address available on website. |
| Notes | Recruitment status: completed Estimated study completion date: December 2016 Sponsor: Ain Shams Maternity Hospital |
NCT03163862.
| Study name | Granulocyte‐colony stimulating factor (G‐CSF) to increase the rate of implantation success in IVF patients in preferable preceptive endometrium score |
| Methods | Allocation: randomised Intervention model: parallel assignment Masking: single (care provider) |
| Participants | Total sample size: 100 Inclusion criteria:
Exclusion criteria:
|
| Interventions | Experimental: G‐CSF group Women treated with G‐CSF if the biopsy adhesive score 1 to 3 only, by endometrial scratching and adhesive factor scoring on day 21 to 24 cycle prior to IVF/or day 3 of IVF cycle not planned before. The dose of G‐CSF is 300 µg by transcervical intrauterine route administered at the oocyte retrieval day, Ans subcutaneous 300 µg G‐CSF on the day of embryo transfer Sham comparator: comparative group Women not treated with G‐CSF after scratching if the biopsy adhesive score 4 only. Placebo comparator: placebo Women treated with infusion of placebo (saline solution) from the day of embryo transfer through the day of beta hCG test. |
| Outcomes | Primary outcome:
Secondary outcome:
|
| Starting date | August 2017 |
| Contact information | Location: Rihan, Lebanon Contact: Areej Khatib, Pathology & clinical labs head department, Istishari Arab Hospital |
| Notes | Recruitment status: unknown Estimated study completion: January 2019 Sponsor: Istishari Arab Hospital |
NCT03549728.
| Study name | Effect of granulocyte colony‐stimulating factor on clinical pregnancy rate in patients with endometriosis undergoing in vitro fertilization after recurrent implantation failure |
| Methods | Allocation: randomised Intervention model: parallel assignment Masking: triple (participant, care provider, investigator) |
| Participants | A total of 88 women with endometriosis will be enrolled in the study divided into 2 groups, as follows.
Inclusion criteria:
Exclusion criteria:
|
| Interventions | Experimental: Group A
Placebo comparator: Group B
|
| Outcomes | Primary outcome :
Secondary outcome :
|
| Starting date | June 2018 |
| Contact information | Location: Cairo, Egypt Contact: Amira Magdi Guergues Selim, Resident of obstetrics and gynaecology, Ain Shams Maternity Hospital. No email address available on website. |
| Notes | Recruitment status: not yet recruiting Estimated study completion date: December 2018 Study sponsor: Ain Shams Maternity Hospital |
PRL: prolactin TSH: thyroid stimulating hormone
Differences between protocol and review
In the protocol, the planned sensitivity analysis was restricted to studies without high risk of bias (not at high risk of bias in any domain and at low risk of bias for randomisation procedures). We changed the eligibility to studies at low risk of bias (without high or unclear risk of bias in any domain) as per recent Cochrane editorial guidelines.
We removed the second comparison (subfertile women with thin endometrium) and incorporated those data under subgroup analysis in the main review comparison.
We used Peto odds ratio for outcomes with low event rates (miscarriage and ectopic pregnancy rates).
Contributions of authors
MSK and SKS screened the studies, extracted and analysed data, and wrote the review; RK contributed to data extraction, gave methodological advice, and commented on the review.
Sources of support
Internal sources
Cochrane South Asia, Prof. BV Moses Centre for Evidence‐Informed Health Care and Health Policy, Christian Medical College, Vellore, India, Other
External sources
None, Other
Declarations of interest
Mohan S Kamath and Richard Kirubakaran have no conflicts of interest to declare. Sesh Kamal Sunkara has participated as speaker in symposia by Merck, MSD, and Ferring.
Edited (no change to conclusions)
References
References to studies included in this review
Aleyasin 2016 {published data only}
- Abedi Asl Z. The efficacy of systemic administration of granulocyte colony stimulating factor (GCSF) on the in vitro fertilization (IVF) success in women with repeated implantation failure. Fertility and Sterility 2015;104(3 Suppl):e 61. [Google Scholar]
- Aleyasin A, Abediasl Z, Nazari A, Sheikh M. Granulocyte colony-stimulating factor in repeated IVF failure, a randomized trial. Reproduction 2016;151(6):637-42. [DOI] [PubMed] [Google Scholar]
Barad 2014 {published data only}
- Barad DH, Kushnir VA, Shohat-Tal A, Lazzaroni E, Lee HJ, Gleicher N. Prospective randomized study of endometrial perfusion with granulocyte colony-stimulating factor (G-CSF) in unselected IVF cycles: impact on endometrial thickness and clinical pregnancy rates. Fertility and Sterility 2013;100(3 Suppl):S144. [DOI] [PubMed] [Google Scholar]
- Barad DH, Yu Y, Kushnir VA, Shohat-Tal A, Lazzaroni E, Lee HJ, et al. A randomized clinical trial of endometrial perfusion with granulocyte colony-stimulating factor in in vitro fertilization cycles: impact on endometrial thickness and clinical pregnancy rates. Fertility and Sterility 2014;101(3):710-15. [DOI] [PubMed] [Google Scholar]
Cambiaghi 2012a {published data only}
- Cambiaghi AS, Leao RBF. Granulocyte-colony stimulation factor (G-CSF) may improve pregnancy rate in patients with repeated implantation failures. Fertility and Sterility 2012;98(3 Suppl):S186-7. [Google Scholar]
Davari‐Tanha 2016 {published data only}
- Davari-Tanha F, Shahrokh Tehraninejad E, Ghazi M, Shahraki Z. The role of G-CSF in recurrent implantation failure: a randomized double blind placebo control trial. International Journal of Reproductive Biomedicine 2016;14(12):737-42. [PMC free article] [PubMed] [Google Scholar]
- Davari-Tanha FD, Kaveh M, Kaveh Z, Tehraninejad E. The role of G-CSF in recurrent implantation failure - a randomized double blind placebo control trial. Placenta 2016;45:116. [PMC free article] [PubMed] [Google Scholar]
Jain 2018 {published data only}
- Jain S, Mahey R, Malhotra N, Kalaivani M, Sangeeta P, Bhatt A, et al. Effect of intrauterine perfusion of granulocyte colony-stimulating factor on endometrial parameters and in vitro fertilization outcome in women undergoing in vitro fertilization/Intracytoplasmic sperm injection cycles: a randomized controlled trial. Journal of Human Reproductive Sciences 2018;11(3):254-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kriplani A, Jain S, Mahey R, Malhotra N, Signh N, Kalaivani M. Effect of intrauterine perfusion of granulocyte colony stimulating factor on endometrial parameters, implantation and pregnancy rate in women undergoing IVF cycles: randomised controlled trial. Human Reproduction 2018;33(Suppl 1):i356. [DOI] [PMC free article] [PubMed] [Google Scholar]
Jalilvand 2018 {published data only}
- Jalilvand F, Gasemzadeh A, Hamdi K, Navali N, Pia H, Farzadi L. Efficacy of uteral injection of G-CSF in repeated implantation failure: a clinical trial study. Medical Journal of Tabriz University of Medical Sciences and Health Services 2018;40(1):16-21. [Google Scholar]
NCT01202643 {unpublished data only}
- NCT01202643. Effect of colony stimulating factor on poor endometrial development during IVF [G-CSF and endometrial growth, embryo implantation and pregnancy following FET or donor ET]. clinicaltrials.gov/ct2/show/NCT01202643 (first received 16 September 2010).
Obidniak 2016 {published data only}
- Obidniak D, Gzgzyan A, Dzhemlikhanova L, Feoktistov A. Effect of colony stimulating growth factor on outcome of frozen thawed embryo transfer in patients with repeated implantation failure. Fertility and Sterility 2016;106(3 Suppl):e134-5. [Google Scholar]
Sarvi 2017 {published data only}
- Sarvi F, Arabahmadi M, Alleyassin A, Aghahosseini M, Ghasemi M. Effect of increased endometrial thickness and implantation rate by granulocyte colony-stimulating factor on unresponsive thin endometrium in fresh in vitro fertilization cycles: a randomized clinical trial. Obstetrics and Gynecology International 2017;2017:3596079. [DOI] [PMC free article] [PubMed] [Google Scholar]
Scarpellini 2011 {published data only}
- Scarpellini F, Sbracia M. G-CSF treatment improves IVF outcome in women with recurrent implantation failure in IVF. Journal of Reproductive Immunology 2012;94(1):103. [Google Scholar]
- Scarpellini F, Sbracia M. The use of G-CSF for implantation failure in IVF: a clinical trial. Fertility and Sterility 2011;96(3 Suppl):S93. [Google Scholar]
Scarpellini 2013 {published data only}
- Scarpellini F, Sbracia M. G-CSF treatment in the implantation failure with a fixed dose of 60 mcg/day: preliminary data of a controlled trial. Human Reproduction 2013;28(Suppl 1):i145-46. [Google Scholar]
Singh 2015 {published data only}
- Singh R, Singh M, Jindal A, Jindal PC. A prospective randomized controlled study (RCT) of intra-uterine administration of granulocyte colony-stimulating factor (G-CSF) before embryo-transfer on resistant thin endometrium in IVF cycles. Human Reproduction 2015;30(Suppl 1):i280. [Google Scholar]
Singh 2018 {published data only}
- Singh R, Singh M. RCT of intra-uterine administration or subcutaneous injection of GCSF (granulocyte colony-stimulating factor) before embryo-transfer on resistant thin endometrium in IVF. Human Reproduction 2018;33(Suppl 1):i275-76. [Google Scholar]
References to studies excluded from this review
Arefi 2018 {published data only}
- Arefi S, Fazeli E, Esfahani M, Borhani N, Yamini N, Hosseini A, et al. Granulocyte-colony stimulating factor may improve pregnancy outcome in patients with history of unexplained recurrent implantation failure: an RCT. International Journal of Reproductive Biomedicine 2018;16(5):299-304. [PMC free article] [PubMed] [Google Scholar]
Cambiaghi 2012b {published data only}
- Cambiaghi AS, Leao RBF. Granulocyte-colony stimulation factor (G-CSF): an option to improve in vitro fertilization (IVF) outcomes in poor responders. Fertility and Sterility 2012;98(3 Suppl 1):S174. [Google Scholar]
Eapen 2018 {published data only}
- Eapen A, Joing M, Lissauer D, Coomarasamy A. Recombinant human granulocyte-colony stimulating factor (rhG-CSF) in women with unexplained recurrent pregnancy losses (RESPONSE Study): randomised, double blind, multicentre, placebo controlled trial. Human Reproduction 2018;33(Suppl 1):i83-4. [Google Scholar]
Eftekhar 2014 {published data only}
- Eftekhar M, Sayadi M, Arabjahvani F. Transvaginal endometrial perfusion of granulocyte colony-stimulating factor for infertile women with thin endometrium in frozen embryo transfer program. Iranian Journal of Reproductive Medicine 2014;12(6 Suppl 1):3. [PMC free article] [PubMed] [Google Scholar]
Gleicher 2013 {published data only}
- Gleicher N, Kim A, Michaeli T, Lee HJ, Shohat-Tal A, Lazzaroni E, et al. A pilot cohort study of granulocyte colony-stimulating factor in the treatment of unresponsive thin endometrium resistant to standard therapies. Human Reproduction 2013;28(1):172-7. [DOI] [PubMed] [Google Scholar]
Jung 2015 {published data only}
- Jung YH, Kim SS, Kim YY, Yoo YJ, Kim MH, Kim SH, et al. Combined treatments of intrauterine perfusion with G-CSF and injection of hCG enhance the endometrial growth, implantation and pregnancy rates in thin endometrium patients. Human Reproduction 2015;30(Suppl 1):i285-6. [Google Scholar]
Kunicki 2017 {published data only}
- Kunicki M, Łukaszuk K, Liss J, Skowrońska P, Szczyptańska J. Granulocyte colony stimulating factor treatment of resistant thin endometrium in women with frozen-thawed blastocyst transfer. Systems Biology in Reproductive Medicine 2017;63(1):49-57. [DOI] [PubMed] [Google Scholar]
Li 2017 {published data only}
- Li J, Mo S, Chen Y. The effect of G-CSF on infertile women undergoing IVF treatment: a meta-analysis. Systems Biology in Reproductive Medicine 2017;63:239-47. [DOI] [PubMed] [Google Scholar]
Lukaszuk 2016 {published data only}
- Lukaszuk K, Kunicki M, Liss J, Skowronska P, Szczyptanska J. G-CSF effects on treatment-resistant thin endometrium in women with frozen transfer/h2. Journal of Assisted Reproduction and Genetics 2016;33(12):1706. [Google Scholar]
Mehrafza 2019 {published data only}
- Mehrafza M, Kabodmehri R, Nikpouri Z, Pourseify G, Raoufi A, Eftekhari A, et al. Comparing the impact of autologous platelet-rich plasma and granulocyte colony stimulating factor on pregnancy outcome in patients with repeated implantation failure. Journal of Reproduction & Infertility 2019;20(1):34-41. [PMC free article] [PubMed] [Google Scholar]
Mishra 2016 {published data only}
- Mishra VV, Choudhary S, Sharma U, Aggarwal R, Agarwal R, Gandhi K, et al. Effects of granulocyte colony-stimulating factor (GCSF) on persistent thin endometrium in frozen embryo transfer (FET) cycles. Journal of Obstetrics and Gynecology of India 2016;66(Suppl 1):407-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
Obidniak 2018 {published data only}
- Obidniak D, Gzgzyan A, Niauri D, Kalugina A. Pilot study investigating concentration of colony stimulating growth factor (CSF) in uterine flushing as prognostic criterion of IVF cycle outcome in patients with recurrent implantation failure. Human Reproduction 2018;33(Suppl 1):i10. [Google Scholar]
Sbracia 2014 {published data only}
- Sbracia M, Morgia F, Torti M, Amodei M, Corizza R, Scarpellini F. Use of GM-CSF supplemented IVF medium in patients with recurrent implantation failure: a controlled trial. Human Reproduction 2014;29(Suppl 1):i176. [Google Scholar]
Scarpellini 2009 {published data only}
- Scarpellini F, Sbracia M. Use of granulocyte colony-stimulating factor for the treatment of unexplained recurrent miscarriage: a randomised controlled trial. Human Reproduction 2009;24(11):2703-8. [DOI] [PubMed] [Google Scholar]
Sen 2017 {published data only}
- Sen U, Khastgir G. Clinical pregnancy outcome after double dose intrauterine instillation of granulocyte colony-stimulating factor(G-CSF) in the unresponsive thin endometrium in frozen embryo transfer. Human Reproduction 2017;32(Suppl 1):i344. [Google Scholar]
Singh 2015b {published data only}
- Swati S, Sankalp S, Ashraf CM. Granulocyte colony stimulating factor(G-CSF): does it really improve the endometrial thickness in women with persistent thin endometrial thickness undergoing frozen endometrial transfer? Human Reproduction 2015;30:i265. [Google Scholar]
Singh 2017 {published data only}
- Singh S, Ashraf M, Laddad D. Effect of intra-uterine G-CSF on endometrial thickness in patients with persistently thin endometrium: A retrospective study of 244 thawed embryo transfer cycles. Human Reproduction 2017;32(Suppl 1):i345-6. [Google Scholar]
Wasim 2017 {published data only}
- Wasim S, Chattopadhyay R, Ghosh S, Goswami SK, Sharma S, Bathwal S, et al. Analysis of implantation and clinical pregnancy in repeated implantation failure undergoing frozen transfer using transfer media with granulocyte macrophage colony stimulating factor or hyaluronan. Fertility and Sterility 2017;108(3):e176. [Google Scholar]
Wu 2018 {published data only}
- Wu W. Study on the protective effect of G-CSF on RIF and RA patients with low HCG level in early pregnancy. Fertility and Sterility 2018;110(4):e257-8. [Google Scholar]
Würfel 2000 {published data only}
- Würfel W. Approaches to a better implantation. Journal of Assisted Reproduction and Genetics 2000;17(8):473. [Google Scholar]
Xu 2014 {published data only}
- Xu B, Zhang Q, Hao J, Xu D, Li Y. Two protocols to treat thin endometrium with granulocyte colony-stimulating factor during frozen embryo transfer cycles. Reproductive Biomedicine Online 2014;30(4):349-58. [DOI] [PubMed] [Google Scholar]
Zabardoust 2017 {published data only}
- Zafardoust S, Akhondi MM, Sadeghi MR, Mohammadzadeh A, Karimi A, Jouhari S, et al. Efficacy of intrauterine injection of granulocyte colony stimulating factor (G-CSF) on treatment of unexplained recurrent miscarriage: A Pilot RCT Study. Journal of Reproduction and Infertility 2017;18(4):379-85. [PMC free article] [PubMed] [Google Scholar]
Zhang 2018 {published data only}
- Zhang L, Xu WH, Fu XH, Huang QX, Guo XY, Zhang L, et al. Therapeutic role of granulocyte colony-stimulating factor (G-CSF) for infertile women under in vitro fertilization and embryo transfer (IVF-ET) treatment: a meta-analysis. Archives of Gynecology and Obstetrics 2018;298(5):861-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to studies awaiting assessment
Eftekhar 2016a {published data only}
- Eftekhar M, Miraj S, Farid Mojtahedi M, Neghab N. Efficacy of intrauterine infusion of granulocyte colony stimulating factor on patients with history of implantation failure: a randomized control trial. International Journal of Reproductive BioMedicine 2016;14(11):687-90. [PMC free article] [PubMed] [Google Scholar]
- Miraj S, Eftekhar M, Farid Mojtahedi M. Efficacy of transvaginal perfusion of granulocyte colony stimulating factor on recurrent implantation failure: randomized control trial. Iranian Journal of Reproductive Medicine 2015;13(1 Suppl):16. [Google Scholar]
Eftekhar 2016b {published data only}
- Eftekhar M, Hosseinisadat R, Baradaran R, Naghshineh E. Effect of granulocyte colony stimulating factor (G-CSF) on IVF outcomes in infertile women: an RCT. International Journal of Reproductive Biomedicine 2016;14(5):341-46. [PMC free article] [PubMed] [Google Scholar]
- Hosseinisadat R. Effect of granulocyte colony stimulating factor (G-CSF) on IVF outcomes in normal infertile women. International Journal of Reproductive Biomedicine 2017;15:45. [PMC free article] [PubMed] [Google Scholar]
Kim 2011 {published data only}
- Kim CH, You RM, Nah HY, Kang HJ, Kim S, Chae HD, et al. Effect of granulocyte colony-stimulating factor on pregnancy outcome following IVF/ICSI in patients with repeated implantation failure. Human Reproduction 2011;26(Suppl 1):i244. [Google Scholar]
References to ongoing studies
NCT01715974 {unpublished data only}
- NCT01715974. Use of G-CSF treatment in recurrent implantation failure [Administration of G-CSF in women with recurrent implantation failure in IVF cycles]. clinicaltrials.gov/show/NCT01715974 (first received 29 October 2012).
NCT02149277 {unpublished data only}
- NCT02149277. Gcsf injection in women with repeated implantation failure [Effects of exogenous recombinant GCSF in patients with repeated implantation failure - a randomized single-blind trial]. clinicaltrials.gov/show/NCT02149277 (first received 29 May 2014).
NCT03023774 {unpublished data only}
- NCT03023774. Use of Gcsf in patients with recurrent Ivf/Icsi failure [Effects of granulocyte colony-stimulating factor (GCSF)-Neupogen on cases with thin endometrium or previous implantation failure in ICSI Cycles]. clinicaltrials.gov/show/NCT03023774 (first received 18 January 2017).
NCT03163862 {unpublished data only}
- NCT03163862. G-CSF administration in IVF in a preferable preceptive endometrium score [Granulocyte Stimulating Factor (G-CSF) to Increase the rate of implantation success in IVF patients in preferable preceptive endometrium score]. clinicaltrials.gov/show/NCT03163862 (first received 23 May 2017).
NCT03549728 {unpublished data only}
- NCT03549728. Effect of granulocyte colony-stimulating factor on clinical pregnancy rate in patients with endometriosis [Effect of granulocyte colony-stimulating factor on clinical pregnancy rate in patients with endometriosis undergoing In-vitro fertilization after recurrent implantation failure]. clinicaltrials.gov/show/NCT03549728 (first received 8 June 2018).
Additional references
Adamson 2018
- Adamson GD, Mouzon J, Chambers GM, Zegers-Hochschild F, Mansour R, Ishihara O, Banker M, Dyer S. International Committee for Monitoring Assisted Reproductive Technology: world report on assisted reproductive technology, 2011. Fertility and Sterility 2018;110(6):1067-80. [DOI] [PubMed] [Google Scholar]
Bonilla 1989
- Bonilla MA, Gillio AP, Ruggeiro M, Kernan NA, Brochstein JA, Abboud M, et al. Effects of recombinant human granulocyte colony-stimulating factor on neutropenia in patients with congenital agranulocytosis. New England Journal of Medicine 1989;320(24):1574-80. [DOI] [PubMed] [Google Scholar]
Coughlan 2014
- Coughlan C, Ledger W, Wang Q, Liu F, Demirol A, Gurgan T, et al. Recurrent implantation failure: definition and management. Reproductive Biomedicine Online 2014;28(1):14-38. [DOI] [PubMed] [Google Scholar]
Demetri 1991
- Demetri GD, Griffin JD. Granulocyte colony-stimulating factor and its receptor. Blood 1991;78(11):2791-808. [PubMed] [Google Scholar]
Dyer 2016
- Dyer S, Chambers GM, Mouzon J, Nygren KG, Zegers-Hochschild F, Mansour R, et al. International Committee for Monitoring Assisted Reproductive Technologies world report: Assisted Reproductive Technology 2008, 2009 and 2010. Human Reproduction 2016;31(7):1588-609. [DOI] [PubMed] [Google Scholar]
Evans 2014
- Evans J, Hannan NJ, Edgell TA, Vollenhoven BJ, Lutjen PJ, Osianlis T, et al. Fresh versus frozen embryo transfer: backing clinical decisions with scientific and clinical evidence. Human Reproduction Update 2014;20(6):808-21. [DOI] [PubMed] [Google Scholar]
Gleicher 2011
- Gleicher N, Vidali A, Barad DH. Successful treatment of unresponsive thin endometrium. Fertility and Sterility 2011;95(6):2123.e13-17. [DOI] [PubMed] [Google Scholar]
Gnainsky 2014
- Gnainsky Y, Dekel N, Granot I. Implantation: mutual activity of sex steroid hormones and the immune system guarantee the maternal-embryo interaction. Seminars in Reproductive Medicine 2014;32(5):337-45. [DOI] [PubMed] [Google Scholar]
GRADEpro GDT 2015 [Computer program]
- McMaster University (developed by Evidence Prime) GRADEpro GDT. Version accessed 11 August 2019. Hamilton (ON): McMaster University (developed by Evidence Prime), 2015. Available at gradepro.org.
Higgins 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration 2011. Available from handbook.cochrane.org.
Kamath 2017
- Kamath MS, Chittawar PB, Kirubakaran R, Mascarenhas M. Use of granulocyte-colony stimulating factor in assisted reproductive technology: a systematic review and meta-analysis. European Journal of Obstetrics, Gynecology, and Reproductive Biology 2017;214:16-24. [DOI] [PubMed] [Google Scholar]
Kasius 2014
- Kasius A, Smit JG, Torrance HL, Eijkemans MJC, Mol BW, Opmeer BC, et al. Endometrial thickness and pregnancy rates after IVF: a systematic review and meta-analysis. Human Reproduction Update 2014;20(4):530-41. [DOI] [PubMed] [Google Scholar]
Kunicki 2014
- Kunicki M, Łukaszuk K, Woclawek-Potocka I, Liss J, Kulwikowska P, Szczyptańska J. Evaluation of granulocyte colony-stimulating factor effects on treatment-resistant thin endometrium in women undergoing in vitro fertilization. BioMed Research International 2014;2014:913235. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kushnir 2017
- Kushnir VA, Barad DH, Albertini DF, Darmon SK, Gleicher N. Systematic review of worldwide trends in assisted reproductive technology 2004-2013. Reproductive Biology and Endocrinology 2017;15(1):6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lédée 2008
- Lédée N, Lombroso R, Lombardelli L, Selva J, Dubanchet S, Chaouat G, et al. Cytokines and chemokines in follicular fluids and potential of the corresponding embryo: the role of granulocyte colony-stimulating factor. Human Reproduction 2008;23(9):2001-9. [DOI] [PubMed] [Google Scholar]
Lefebvre 2011
- Lefebvre C, Manheimer E, Glanville J. Chapter 6: Searching for studies. In: Higgins JPT, Green S, editors(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration. Available from www.cochrane-handbook.org, 2011. [Google Scholar]
Macklon 2017
- Macklon N. Recurrent implantation failure is a pathology with a specific transcriptomic signature. Fertility and Sterility 2017;108(1):9-14. [DOI] [PubMed] [Google Scholar]
McNiece 1989
- McNiece I, Andrews R, Stewart M, Clark S, Boone T, Quesenberry P. Action of interleukin-3, G-CSF, and GM-CSF on highly enriched human hematopoietic progenitor cells: synergistic interaction of GM-CSF plus G-CSF. Blood 1989;74:110-4. [PubMed] [Google Scholar]
Morris 2004
- Morris ES, MacDonald KP, Rowe V, Johnson DH, Banovic T, Clouston AD, et al. Donor treatment with pegylated G-CSF augments the generation of IL-10-producing regulatory T cells and promotes transplantation tolerance. Blood 2004;103(9):3573-81. [DOI] [PubMed] [Google Scholar]
Rahmati 2014
- Rahmati M, Petitbarat M, Dubanchet S, Bensussan A, Chaouat G, Ledee N. Granulocyte-colony stimulating factor related pathways tested on an endometrial ex-vivo model. PLoS ONE 2014;9:e102286. [DOI] [PMC free article] [PubMed] [Google Scholar]
RevMan 2014 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Rutella 2005
- Rutella S, Zavala F, Danese S, Kared H, Leone G. Granulocyte colony-stimulating factor: a novel mediator of T cell tolerance. Journal of Immunology 2005;175(11):7085-91. [DOI] [PubMed] [Google Scholar]
Schneider 2005
- Schneider A, Krüger C, Steigleder T, Weber D, Pitzer C, Laage R, et al. The hematopoietic factor G-CSF is a neuronal ligand that counteracts programmed cell death and drives neurogenesis. Journal of Clinical Investigation 2005;115(8):2083-98. [DOI] [PMC free article] [PubMed] [Google Scholar]
Tabbara 1997
- Tabbara IA, Ghazal CD, Ghazal HH. The role of granulocyte colony-stimulating factor in hematopoietic stem cell transplantation. Cancer Investigation 1997;15(4):353-7. [DOI] [PubMed] [Google Scholar]
Tanaka 2000
- Tanaka T, Miyama M, Masuda M, Mizuno K, Sakamoto T, Umesaki N. Production and physiological function of granulocyte colony-stimulating factor in non-pregnant human endometrial stromal cells. Gynecological Endocrinology 2000;14(6):399-404. [DOI] [PubMed] [Google Scholar]
Tevkin 2014
- Tevkin S, Lokshin V, Shishimorova M, Polumiskov V. The frequency of clinical pregnancy and implantation rate after cultivation of embryos in a medium with granulocyte macrophage colony-stimulating factor (GM-CSF) in patients with preceding failed attempts of ART. Gynecology Endocrinology 2014;30 Suppl 1:9-12. [DOI] [PubMed] [Google Scholar]
Valdes 2017
- Valdes CT, Schutt A, Simon C. Implantation failure of endometrial origin: it is not pathology, but our failure to synchronize the developing embryo with a receptive endometrium. Fertility and Sterility 2017;108(1):15-8. [DOI] [PubMed] [Google Scholar]
Würfel 2015
- Würfel W. Treatment with granulocyte colony-stimulating factor in patients with repetitive implantation failures and/or recurrent spontaneous abortions. Journal of Reproductive Immunology 2015;108:123-35. [DOI] [PubMed] [Google Scholar]


