Abstract
Background
Endometriosis is a common gynaecological condition affecting approximately 10% of women of reproductive age (Ozkan 2008). Common symptoms are dysmenorrhoea, pelvic pain, infertility or a pelvic mass. Diagnosis by laparoscopy or laparotomy enables identification of the location, extent and severity of the disease. Surgery may include removal (excision) or destruction (ablation) of endometriotic tissue, division of adhesions and removal of endometriotic cysts. Laparoscopic excision or ablation of endometriosis has been shown to be effective in the management of pain in mild to moderate endometriosis. Adjunctive medical treatment pre or post‐operatively may prolong the symptom‐free interval.
Objectives
To determine the effectiveness of medical therapies for hormonal suppression before or after surgery for endometriosis for improving painful symptoms, reducing disease recurrence and increasing pregnancy rates.
Search methods
We searched the Cochrane Menstrual Disorders and Subfertility Group Trials Register (searched Sept 2010), the Cochrane Central Register of Controlled Trials (CENTRAL) in The Cochrane Library (Sept 2010), MEDLINE (January 1966 to September 2010), EMBASE (January 1985 to September 2010) and reference lists of articles.
Selection criteria
Trials were included if they were randomised controlled trials comparing medical therapies for hormonal suppression before or after or before and after, surgery for endometriosis.
Data collection and analysis
Data extraction and assessment of risk of bias were performed independently by two authors. Where possible, data were combined using relative risk (RR), standardised mean difference or mean difference and 95% confidence intervals (CI).
Main results
Sixteen trials were included. Two trials of pre‐surgical medical therapy showed no evidence of benefit compared to surgery alone. There was no evidence of benefit for post‐surgical hormonal suppression of endometriosis compared to surgery alone for the outcomes of pain, disease recurrence or pregnancy rates (RR 0.84, 95% CI 0.59 to 1.18). There were no trials identified in the search that compared hormonal suppression of endometriosis before and after surgery with surgery alone. One trial found no evidence that pre‐surgical hormonal suppression was different from post‐surgical hormonal suppression for the outcome of pain. Another single trial comparing post‐surgical medical therapy with both pre and post‐surgery found no difference in the outcomes of American Fertility Society (AFS) scores and pregnancy rate.
Authors' conclusions
There is no evidence of benefit associated with post surgical medical therapy and insufficient evidence to determine whether there is a benefit from pre‐surgical medical therapy with regard to the outcomes evaluated.
Plain language summary
There is no evidence that hormonal suppression either before or after surgery for endometriosis is associated with a benefit
Endometriosis is caused by the lining of the uterus (endometrium) spreading outside the uterus. It can cause pelvic pain, painful periods and infertility. Common treatments are hormonal suppression with medical therapy to reduce the size of endometrial implants or laparoscopic surgery (where small incisions are made in the abdomen) to remove visible areas of endometriosis. There is no evidence that hormonal suppression either before or after surgery is associated with a benefit compared with surgery alone.
(Synopsis prepared by Cochrane Menstrual Disorders and Subfertility Group)
Summary of findings
Background
Description of the condition
Endometriosis is a common gynaecological condition which affects women during their reproductive years. Endometriosis occurs when endometrial tissue, which usually grows in the uterus (normal), is found in other parts of the body, for example the ovaries, fallopian tubes and pelvis. A range of symptoms are evident and women most commonly present with dysmenorrhoea (painful periods), pelvic pain, infertility or a pelvic mass. Endometriosis also responds to hormonal changes associated with the menstrual cycle and cyclical growth of endometriotic implants or cysts is thought to be associated with pelvic pain and the development of adhesions (scar tissue). Estimates of the prevalence of endometriosis amongst women vary but a recent article (Ozkan 2008) reported a prevalence of 0.5% to 5% in fertile women and 25% to 40% in a subfertile population, with the peak incidence between 30 and 45 years of age (Guzick 1989). Endometriosis may be asymptomatic (no symptoms) or associated with chronic pelvic pain and subfertility (Cook 1991).
The 'gold standard' for the diagnosis of endometriosis is visualisation of endometriosis lesions or cysts during a surgical procedure, either laparoscopy or laparotomy. The extent of the disease can be graded according to a scale developed by the American Fertility Society (AFS 1985), although there is no direct correlation between the severity of the disease and the severity of the symptoms experienced (Vercellini 1996). Concern over the reproducibility of the scoring system resulted in the publication of a new scoring system in 1997 by the American Society of Reproductive Medicine (ASRM 1997).
Description of the intervention
Current treatments that are available for endometriosis include both surgery and medical therapy. Surgical therapy can be performed concurrently with diagnostic surgery and may involve the destruction of endometriotic tissue (ablation), division of adhesions (scar tissue) or removal of endometriotic cysts. In advanced endometriosis (AFS stage III or IV), laparoscopic surgery to remove (excise) visible endometrial implants, divide adhesions (scar tissue) or surgically interrupt neural pathways is the treatment of choice (Muzii 1996; Proctor 1999; Rana 1996). This is because large (> 3 cm) lesions respond poorly to medical therapy and hormonal suppression does not influence the extent of the adhesions which are often associated with large lesions (Shaw 1992).
Medical therapies for systemic hormonal suppression of endometriosis include danazol (a synthetic testosterone hormone derivative), gonadotrophin releasing hormone analogues (GnRHas), progestogens, gestrinone and the oral contraceptive pill. These therapies may be effective for relief of pain associated with endometriosis but they also decrease fertility. Both danazol and GnRHas are associated with side effects related to hyperandrogenism (increased hair growth and a deepening of the voice) and hypoestrogenism (such as hot flushes and vaginitis), respectively, and should only be used for periods of up to six months. Recurrence of endometriosis symptoms and disease is common after the cessation of medical therapy (Barbieri 1990).
How the intervention might work
Over recent years there has been interest in combining medical and surgical therapy in an attempt to reduce recurrence of endometriosis. The pre‐operative use of GnRHas may decrease the extent of endometriosis and the size of endometriomas (ovarian endometriosis) making complete removal of endometriosis easier during laparoscopic surgery and increasing subsequent pregnancy rates (Hemmings 1998; Donnez 1987). However possible disadvantages of pre‐operative medical therapy, especially with danazol or GnRHas, are the adverse effects associated with these medications (for example hot flushes or vaginal dryness), which may influence women's willingness to use the therapy and result only in a delay of surgery. Post‐operative medical therapy appears be an effective treatment of microscopic endometriosis which may not have been evident to the surgeon. It induces suppression of lesions that can not be surgically removed and reduces the risk of recurrence of endometriosis as a result of surgery (Kettel 1989; Thomas 1992).
Why it is important to do this review
Although the combination of surgery and medical therapy would appear to be beneficial, it is necessary to evaluate the benefits and consider the harms prior to this strategy being recommended. This review aims to evaluate the use of medical therapy before or after, or before and after, surgery for endometriosis.
Objectives
To determine the effectiveness of medical therapies for hormonal suppression before or after or before and after surgery for endometriosis for improving painful symptoms, reducing disease recurrence and increasing pregnancy rates.
Methods
Criteria for considering studies for this review
Types of studies
All randomised controlled trials of the use of medical hormonal suppression therapies used:
pre‐surgery for endometriosis compared with surgery alone or placebo prior to surgery for the treatment of endometriosis;
post‐surgery for endometriosis compared with surgery alone or surgery and placebo;
pre and post‐surgery for endometriosis compared with surgery alone or surgery and placebo;
pre‐surgery for endometriosis compared with medical therapies used post‐surgery for endometriosis.
Types of participants
The study population included women of reproductive age who were undergoing surgery for endometriosis. The diagnosis of endometriosis could have been made provisionally by clinical examination and confirmed during the surgery, or could have been confirmed endometriosis where women were undergoing second or subsequent surgery. They would have further medical treatment either before or after surgery. Studies in the hospital care setting were considered.
Types of interventions
All systemic medical treatments for the hormonal suppression of endometriosis including GnRHas, danazol, progestogens, gestrinone or the oral contraceptive pill (or combinations of these) administered before surgery, after surgery or before and after surgery for endometriosis compared to medical treatment after surgery, before surgery, no medical treatment, or placebo were studied. The use of medical therapy was considered at any dosage and for a period of at least three months duration before or after surgery. Only agents used with the aim of hormonal suppression were included. Medical treatment with analgesics, anti‐inflammatory drugs or antibiotics were excluded. Alternative, dietary or complementary therapeutic strategies were also excluded. Modulation of the immune system via pentoxifylline treatment is considered in a separate systematic review (Lv 2009). All surgical procedures for the treatment of endometriosis that conserve the pelvic organs (such as ovarian cystectomy, drainage of endometriosis, excision or ablation of endometriosis) were included.
Types of outcome measures
The effectiveness of the use and timing of medical therapy as an adjunct to surgery for endometriosis was compared to surgery alone (no medical treatment or placebo) and was assessed by the following outcome measures where the data were available.
Primary outcomes
Painful symptoms of endometriosis as measured by a visual analogue scale (VAS) of pain, other validated scales or dichotomous outcomes
Recurrence of disease as evidenced by rAFS (revised American Fertility Society) or rASRM scores at second look laparoscopy
Pregnancy rate per woman (measured by either urinary human chorionic gonadotrophin (HCG) levels or foetal heart detected by ultrasound)
Secondary outcomes
Ease of surgery, duration of surgery, post‐operative complications
Levels of satisfaction of women participants
Adverse effects (proportion of women with one or more reported adverse effects associated with medical treatment)
Search methods for identification of studies
Reports that described or might have described randomised controlled trials of hormonal suppression in the treatment of endometriosis before or after surgery were obtained using the following search strategy.
Electronic searches
(1) We searched the Menstrual Disorders and Subfertility Group Specialised Register of controlled trials (10 September 2003) for any trials of hormonal suppression in the treatment of endometriosis before or after surgery. The Cochrane Menstrual Disorders and Subfertility Group Specialised Register is based on regular searches of CENTRAL, MEDLINE, EMBASE, CINAHL and PsycINFO, the handsearching of 20 relevant journals and conference proceedings, and searches of several key grey literature sources. A full description is given in the Group's module on The Cochrane Library.
(2) The following electronic databases were searched using Ovid software: MEDLINE (1966 to September 2010) (Appendix 1); EMBASE (1980 to September 2010) (Appendix 3); CINAHL (1982 to September 2010); Biological Abstracts (1980 to September 2010); PsycINFO (1872 to September 2010).
The search strategy was developed for MEDLINE Ovid and adapted for use on the other Ovid databases listed above.
(3) We searched the Cochrane Central Register of Controlled Trials (CENTRAL) on The Cochrane Library (20 September 2010) in all fields using the search terms listed in Appendix 2.
(4) We searched controlled trials.com for ongoing and recently completed trials.
Searching other resources
(5) We searched the reference lists and bibliographies of all relevant articles to identify additional trials for inclusion in this review.
(6) We sent letters to experts within the field, pharmaceutical companies producing the products being reviewed and authors of unpublished abstracts to identify unpublished trials of medical therapy before or after surgery for endometriosis.
Data collection and analysis
Selection of studies
The selection of trials for inclusion in the latest update of the review was performed by the two review authors (YC and SF) after employing the search strategy previously described. The titles and abstracts were screened and studies that were clearly ineligible were discarded but we aimed to be overly inclusive rather than risk losing relevant studies. Copies of the full articles were obtained. Both review authors then independently assessed whether the studies met the inclusion criteria. Disagreements were resolved by discussion. Further information was sought from the authors where papers contained insufficient information to make a decision about eligibility.
Data extraction and management
Included trials were analysed for the following methodological details.
(1) Duration, timing and location of the study. (2) Inclusion and exclusion criteria for the trial. (3) Number of patients randomised, excluded or lost to follow up.
(4) Whether a power calculation was done.
(5) Source of funding for the trial.
This information is presented in the Characteristics of included studies table, which describes the included studies and provides a context for discussing the reliability of results.
Characteristics of the study participants
(1) Method of diagnosis of endometriosis (2) Severity of endometriosis (rAFS scores) (3) Severity of painful symptoms associated with endometriosis (pain scales) (4) Age and parity of study participants (5) General demographic characteristics of study participants
Interventions used
(1) Type of medical treatment used, dosage, duration of treatment, mode of administration (2) Type of control or placebo used (3) Timing of medical treatment (before or after surgery, or before and after surgery)
Outcomes
(1) Methods used to measure recurrence of disease (rAFS or rASRM scores at second look laparoscopy) (2) Methods used to measure pain relief achieved by treatment (e.g. VAS pain scores, other validated pain scores, dichotomous outcomes) (3) Pregnancy rate per woman during follow up (4) Methods used to measure adverse effects (including post‐operative complications) and types of adverse effects reported (5) Methods used to measure ease of surgery, duration of surgery (6) Length of follow up and timing of outcome measurement relative to timing of treatment (7) Methods used to measure levels of satisfaction of the women participants
Assessment of risk of bias in included studies
For the studies included in this review, assessment of risk of bias was conducted by two review authors using the Cochrane risk of bias assessment tool (Higgins 2009). We assessed six domains for each included study: sequence generation, allocation concealment, blinding of outcome assessor, completeness of outcome data, risk of selective outcome reporting and risk of other potential sources of bias .
For this systematic review we assessed risk of bias according to the following criteria.
Adequate sequence generation: use of a random number table, use of a computerised system, central randomisation by statistical coordinating centre, randomisation by an independent service using minimisation technique, permuted block allocation or Zelan technique were considered adequate. If the paper merely stated 'randomised' or 'randomly allocated' with no further information this was assessed as being unclear.
Allocation concealment: centralised allocation including access by telephone call or fax, or pharmacy controlled randomisation, using sequentially numbered, sealed opaque envelopes were considered adequate. Where there was no mention of allocation concealment methods, this domain was assessed as unclear.
Blinding: for these treatments, even when blinding of patients and clinicians to treatment allocation was part of the trial protocol, the adverse effects of medical therapies make it difficult for blinding to be maintained. Therefore we have focused on whether the outcome assessment was blinded. Unless the trial was specifically described as double blind, or there was a statement about blinding in the methods section of the paper, it was assumed that blinding of patients, clinical staff and outcome assessors did not occur.
Outcome data: outcome data were considered complete if all patients randomised were included in the analysis of the outcome(s).
Selective outcome reporting: a trial was assessed as being at low risk of bias due to selective outcome reporting if the outcomes of interest described in the methods section were systematically reported in the results section. Where reported outcomes did not include those outcomes specified or expected in trials of treatments for endometriosis, this domain was assessed as unclear.
Other bias: imbalance in potentially important prognostic factors between the treatment groups at baseline, or the use of a co‐intervention in only one group (for example analgesics) were examples of potential sources of bias that were noted.
Additional information on trial methodology or original trial data was sought from the principal authors of trials which appeared to meet the eligibility criteria but were unclear in aspects of methodology or outcomes, or where the data were in a form unsuitable for meta‐analysis.
Measures of treatment effect
This review used both positive and negative outcome measures. This was taken into account when the meta‐analysis was interpreted. For example with regard to the outcome of pain, a positive treatment effect was associated with a reduction in pain scores indicated by a WMD less than zero. However with regard to pregnancy rates, which was a desirable outcome in the treatment of subfertility, a positive treatment effect was associated with an increase in pregnancy rate indicated by a relative risk (RR) greater than one. The data were entered so that in positive outcomes (for example pregnancy) points to the left of the 'line of no effect' favoured the control, and in the negative outcomes (for example pain) points to the left of the 'line of no effect' favoured treatment.
When neither continuous nor dichotomous data suitable for the calculation of mean difference, standardised mean difference or relative risk could be extracted from a trial, any available data were reported descriptively in additional data Table 11. It is expected that the data for outcomes of pain are skewed rather than normally distributed. Any skew ness was described in the results section.
1. Descriptive data for trials not included in the meta‐analysis.
| Study ID | Comparison | Outcome | n | Conclusion |
| Donnez 1994 | pre‐surgical GNRHa (goserelin) versus no medical therapy | mean endometrioma size | 40/40 | favouring goserelin mean difference ‐1.81cm (95% CI ‐2.05 to 1.57) |
| Shaw 2001 | pre‐surgical GNRHa (goserelin) versus no medical treatment | change in endometrioma size | 21/27 | favouring goserelin adj mean difference ‐1.25 cm (95% CI ‐2.42 to ‐0.08) |
| Shaw 2001 | complete excision of cyst | 21/27 | no difference 13/21 (72%) and 16/27 (73%) had cysts completely excised at surgery |
|
| Shaw 2001 | recurrence of residual cysts at 6 months | 21/27 | favours goserelin 2/21 (10%) and 4/27 (15%) had recurrence of residual cysts |
|
| Shaw 2001 | mean rAFS scores | 21/27 | no difference 41.7, 42.5 (no SD given) |
|
| Telimaa 1987 | MPA versus placebo | pain | 17/8 | pain scores after 12 months assessed with 4 point scales; MPA 1.8; Placebo 4.4 "significant difference" |
| Telimaa 1987 | danazol versus placebo | pain | 18/8 | pain scores after 12 months assessed with 4 point scales; danazol 2.5; placebo 4.4, "significant difference" |
| Telimaa 1987 | MPA versus placebo | patient satisfaction | 17/8 | patient satisfaction achieved in MPA 84% vs placebo 24% |
| Telimaa 1987 | danazol versus placebo | patient satisfaction | 18/8 | patient satisfaction achieved in danazol 84% vs placebo 24% |
| Tsai 2004 | post‐surgical leuprolide/danazol versus no treatment | cumulative pregnancy rate at 12 months after clomiphene stimulation in both groups | 15/30 | no difference 56.7% and 54.5% |
| Yang 2006 | post‐surgical gestrinone versus no medical treatment | disease recurrence at 6‐30 months | 19/13 | favours medical treatment 1/19 and 4/13 (p<0.05) |
| Audebert 1998 | pre‐surgical versus post‐surgical GnRHa (nafarelin) | AFS scores | 25/28 | total AFS score after 6 months was 0 and 6 in pre and post groups respectively (p= 0.007); no SD or SE given and not calculable. |
| Audebert 1998 | AFS scores | 25/28 | adhesion AFS score after 6 months was 0 and 2 in pre and post groups respectively (p= 0.007), no SD or SE given and not calculable. | |
| Audebert 1998 | AFS scores | 25/28 | implant AFS score after 6 months was 0 and 4 in pre and post groups respectively (p= 0.05), no SD or SE given and not calculable. | |
| Audebert 1998 | ease of surgery | 25/28 | surgery was easy in 56% of patients with GnRHa treatment pre‐surgery (Grp II) compared to 35.7% in the post‐surgery group (Grp I) |
Unit of analysis issues
The primary analysis was per woman randomised to treatment. Reported data that was based on a different unit of analysis (for example per endometrioma cyst) were not included in the meta‐analysis but were to be summarised in an additional table.
Dealing with missing data
Data were analysed on an intention‐to‐treat basis, where possible, and attempts were made to contact authors to obtain missing data. Where studies reported data by type of medical therapy, these treatment groups were combined and compared to placebo or no treatment using mean difference and the standard deviation for continuous outcomes. Where the mean and standard deviation for the combined groups was not reported this was estimated using the formulae described in Table 7.7a in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2009).
Assessment of heterogeneity
A fixed‐effect analysis was used unless the number of trials in a meta‐analysis was greater than three. It was planned that heterogeneity among the results of different studies would examined by inspecting the scatter in the data points and the overlap in their confidence intervals and, more formally, by checking the results of the Chi2 test and the I2 statistic.
Data synthesis
Statistical analysis was performed in accordance with the guidelines for statistical analysis developed by the Cochrane Menstrual Disorders and Subfertility Group.
Where possible, the outcomes were pooled statistically. For dichotomous data (for example proportion of patients with pain recurrence at 12 months), results for each study was expressed as a relative risk (RR) with 95% confidence intervals (CI) and combined for meta‐analysis with RevMan software using the Peto‐modified Mantel‐Haenszel method.
For continuous outcomes (for example multidimensional pain scores) means and standard deviations for each group were combined in the meta‐analysis and shown as a mean difference (MD) or standardised mean difference (SMD) and 95% confidence interval (CI).
Subgroup analysis and investigation of heterogeneity
A priori, it was planned to look at the possible contribution of differences in trial design, medical treatment used, timing of treatment, dosage, mode of administration and duration of treatment to any heterogeneity identified. Sensitivity analysis based on these criteria was planned to investigate the robustness of the data.
Sensitivity analysis
Sensitivity analysis was conducted where there were sufficient trials included, in order to determine whether the conclusions were robust that is whether conclusions would have differed if the inclusion of trials was restricted to those with low risk of bias.
Results
Description of studies
Results of the search
The original search identified 11 trials that met the inclusion criteria (Audebert 1998; Batioglu 1997; Bianchi 1999; Busacca 2001; Donnez 1994; Hornstein 1997; Loverro 2001; Muzii 2000; Parazzini 1994; Telimaa 1987; Vercellini 1999). The updated search (in September 2010) identified a further six trials which met the inclusion criteria (Loverro 2008; Sesti 2007; Shaw 2001; Shawki 2002; Tsai 2004; Yang 2006). One trial (Shawki 2002) was published only as an abstract, with insufficient information available to include it in this review. Attempts to contact the author to obtain additional information have been unsuccessful. This study is listed under studies awaiting classification. Five new trials have been included in the updated review.
Of the 16 trials now included in this review, eight were conducted in Italy (Bianchi 1999; Busacca 2001; Loverro 2001; Loverro 2008; Muzii 2000; Parazzini 1994; Sesti 2007; Vercellini 1999 ) and one study was conducted in each of Belgium (Donnez 1994), China (Yang 2006), Finland (Telimaa 1987), France (Audebert 1998), Taiwan (Tsai 2004), Turkey (Batioglu 1997), UK/Republic of Ireland (Shaw 2001) and USA (Hornstein 1997). A total of 1410 women with endometriosis were randomly allocated to medical treatments which included gonadotrophin releasing hormone analogues (goserelin, leuprorelin, nafarelin, triptorelin), danazol, progestogen (gestrinone), and the combined oral contraceptive pill.
Included studies
Pre‐surgical medical therapy
Two trials compared pre‐surgical medical therapy for endometriosis to surgery alone (no medical therapy) (Donnez 1994; Shaw 2001). Donnez 1994 included 80 women with infertility who were < 35 years of age with laparoscopically confirmed ovarian endometriotic cysts which were drained and flushed out laparoscopically. The patients were then randomised to receive a subcutaneous goserelin implant four‐weekly for 12 weeks or no treatment. Twelve weeks after the first‐look laparoscopy, another laparoscopy was performed during which a biopsy was done, endometriosis and the cyst wall vaporised. AFS scoring was done by the same two observers.
Shaw 2001 randomised 48 women aged 18 to 50 years who had been referred for management of symptoms or infertility due to endometrioma. After the cysts were aspirated women received either goserelin four‐weekly for three months or no medical treatment. Following an ultrasound measurement of the residual cysts women underwent definitive excision and were then followed for a further six months. Outcomes included size of endometrioma pre‐surgery, proportion who had complete excision of cysts, AFS scores and recurrence of cysts at six months.
Post‐surgical medical therapy versus placebo or no treatment
Twelve studies assessed post‐surgical medical therapy for endometriosis. Five of these compared post‐operative medical therapy to placebo (Hornstein 1997; Loverro 2008; Parazzini 1994; Sesti 2007; Telimaa 1987) and in the remaining seven trials the control group received surgery alone with no medical therapy (Bianchi 1999; Busacca 2001; Loverro 2001; Muzii 2000; Tsai 2004; Vercellini 1999; Yang 2006).
Three different medical therapies were compared with placebo, in five trials (Hornstein 1997; Loverro 2008; Parazzini 1994; Sesti 2007; Telimaa 1987) and seven trials in this group compared post‐operative medical therapy with either GNRHa, danazol, progestogen or oral contraceptive pills to no post‐operative medical treatment (Bianchi 1999; Busacca 2001; Loverro 2001; Muzii 2000; Tsai 2004; Vercellini 1999; Yang 2006).
Hornstein 1997 and Parazzini 1994 both compared intranasal nafarelin (400 uG/day) with placebo over a period of six months and three months, respectively. Loverro 2008 randomised 60 women with a mean age of 28.6 years to three months of a post‐operative triptorelin depot or placebo and followed them for five years to evaluate persistence of pain, recurrence of endometrioma and pregnancy. In a large trial in Rome, Sesti 2007 randomly allocated 234 women with endometriosis to post‐operative medical treatment with GNRHa (either triptorelin or leuprorelin), continuous estroprogestin (OCP), dietary therapy (vitamins, minerals, lactic ferments and fish oil) or placebo and evaluated pain (dysmenorrhoea, non‐menstrual pelvic pain and dyspareunia) and quality of life. Data from the two hormonal suppression arms were combined and compared to placebo in the meta‐analysis. The other placebo controlled trial of post‐surgical medical therapy (Telimaa 1987) had two treatment arms, medroxyprogesterone acetate (MPA) 100 mg/day taken orally for six months (n = 17) and danazol 600 mg/day (200 mg tds) for six months (n = 18), and a placebo arm (n = 16). Data have been reported separately for each group compared to placebo in Table 11. In the meta‐analysis of the subgroup of 22 patients in this trial desiring pregnancy, data from the medical therapy groups have been combined.
In Bianchi 1999, post‐surgical danazol, 600 mg/day for three months, was compared with surgery alone in 53 women. Busacca 2001, Loverro 2001 and Vercellini 1999 compared post‐surgical GnRHa (leuprolide, triptorelin and goserelin respectively), administered subcutaneously every four weeks for a period of 12 weeks, with surgery alone in groups of 89, 62 and 210 women with endometriosis respectively. Tsai 2004 randomly allocated 15 women to post‐operative treatment with either GNRHa (leuprolide, n = 8) or danazol (n = 7) and the remaining 30 to no post‐operative medical treatment prior to controlled ovarian hyperstimulation with clomiphene followed by intrauterine insemination or in vitro fertilisation. In China, Yang 2006 compared post‐operative treatment with traditional Chinese medicine, gestrinone or no treatment in 52 women and reported the pregnancy rate and recurrence of endometriosis with a nine and 30 months follow up. Muzii 2000 compared surgery plus six months of therapy with low‐dose cyclical oral contraceptives to surgery alone.
Pre‐surgical medical therapy compared with post‐surgical medical therapy
One study compared pre‐surgical medical therapy with post‐surgical medical therapy. Audebert 1998 compared medical therapy with intranasal nafarelin administered for six months before surgery with intranasal nafarelin administered for six months after surgery. Outcomes of pain, AFS scores and ease of surgery were assessed.
Post‐surgical medical therapy compared with pre and post‐surgical medical therapy
One study compared medical therapy pre and post‐surgery with medical therapy post‐surgery. Batioglu 1997 compared medical therapy with triptorelin commenced post‐surgery to medical therapy with triptorelin started in the luteal phase of the menstrual cycle pre‐surgery and continued after surgery. The duration and dose of medical therapy was the same in both groups; the difference between the groups was the time that medical therapy started relative to surgery.
Risk of bias in included studies
Only one of the 16 trials included in this review could be considered as at low risk of bias (Parazzini 1994) (see Figure 1). Details of the risk of bias assessments are summarised under the headings below.
1.
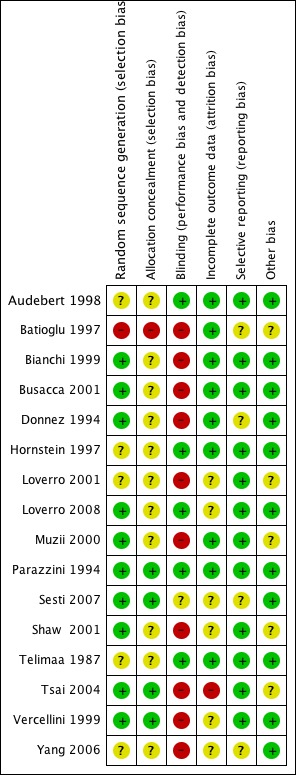
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Out of the 16 included studies, nine used computer generated randomisation (Bianchi 1999; Busacca 2001; Loverro 2008; Muzii 2000; Parazzini 1994; Sesti 2007; Shaw 2001; Tsai 2004; Vercellini 1999), one used randomisation tables (Donnez 1994) and one used odd and even numbers (Batioglu 1997). These studies were assessed as at low risk of bias for this domain. The remainder of studies did not state their method of randomisation (Audebert 1998; Hornstein 1997; Loverro 2001; Telimaa 1987; Yang 2006) and were therefore assessed as unclear for this domain.
Two studies reported adequate allocation concealment using telephone allocation (Parazzini 1994; Vercellini 1999). Sesti 2007 allocated patients by using serially numbered opaque, sealed envelopes whilst Tsai 2004 allocated patients according to list "unknown to physicians". These four studies were therefore assessed as being at low risk of bias. The remainder of the included studies did not describe their allocation methods and were therefore assessed as unclear.
Blinding
Four studies were double blinded (Audebert 1998; Hornstein 1997; Parazzini 1994; Telimaa 1987). In Loverro 2008 patients were blinded to treatment allocation and placebo injections were used. The trial by Sesti 2007 stated that "neither the patients nor the surgeons were aware of the regimen prescribed during the evaluation of improvement of endometriosis‐related pelvic pain and health related quality of life during the study period". However in this study patients received either placebo, GNRHa, oral contraceptive pills or dietary therapy so it is considered likely that maintaining blinding to treatment would have been difficult.
One study was open label (Vercellini 1999). Blinding was not described in the remaining studies. In all the studies included in this review the adverse effects of the medication may have alerted the investigators and the participants to the type of medical intervention.
Incomplete outcome data
Outcome data were incomplete in one study (Tsai 2004) with four women (27%) withdrawing from one group, which may have introduced a bias. A further six studies were assessed as being at unclear risk of bias for this domain (Loverro 2001; Loverro 2008; Sesti 2007; Shaw 2001; Vercellini 1999; Yang 2006). The remaining studies were assessed as being at low risk of bias for this domain, five had no post‐randomisation losses (Batioglu 1997; Bianchi 1999; Busacca 2001; Donnez 1994; Parazzini 1994) and three trials had few post‐randomisation losses (Audebert 1998: 3%; Muzii 2000: 4%; Telimaa 1987: 2%). One trial (Hornstein 1997) had a 15% post‐randomisation loss, approximately equal in the medical therapy and placebo groups.
Pregnancy is a desired outcome for some of the women included in these trials. Seven studies looked at pregnancy rates as an outcome measure. In Batioglu 1997, Parazzini 1994 and Telimaa 1987 pregnancy rates 12 months after treatment commenced were reported as an outcome for all participants in the trials; losses to follow up were small (0%, 9% and 2% respectively). Busacca 2001 and Loverro 2001 reported pregnancy rates after 18 months follow up in a subgroup of participants (30% and 40 % of participants respectively). Busacca 2001 reported no losses to follow up in the group desiring pregnancy and Loverro 2001 did not state whether there were any losses to follow up. In Vercellini 1999 pregnancy outcomes were reported in a subgroup of 152 women desiring fertility (56% of participants) after two years of follow up; losses to follow up in these groups were very small.
Other potential sources of bias
Five reports declared pharmaceutical support for their studies (Audebert 1998; Hornstein 1997; Parazzini 1994; Shaw 2001; Vercellini 1999) while Donnez 1994 had independent funding from Fonds de la Recherche Scientifique and Yang 2006 received funding from the Natural Science Foundation. The remainder did not describe any form of funding or support.
One trial (Tsai 2004), which was described as a randomised controlled trial, reported that patients were "randomly selected to receive" post‐operative medical treatment prior to ovarian stimulation over a period of 13 years (1988 to 2001). During this time period there have been significant advancements in endoscopic technology. It is unclear whether this resulted in any bias in the results of the study.
Two studies (Batioglu 1997; Shaw 2001) reported differences between the groups at baseline with regard to disease severity, which may have introduced a bias into the results from these studies. Loverro 2001 and Muzii 2000 did not report the characteristics of each group at baseline.
Effects of interventions
See: Table 1; Table 2; Table 3; Table 4; Table 5; Table 6
Summary of findings for the main comparison. Post surgical medical therapies compared to No treatment or placebo for infertility associated with endometriosis.
| Post surgical medical therapies compared to No treatment or placebo for infertility associated with endometriosis | ||||||
| Patient or population: patients with infertility associated with endometriosis Intervention: Post surgical medical therapies Comparison: No treatment or placebo | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| No treatment or placebo | Post surgical medical therapies | |||||
| Pregnancy | 246 per 1000 | 207 per 1000 (145 to 290) | RR 0.84 (0.59 to 1.18) | 420 (8 studies) | ⊕⊕⊝⊝ low1,2 | |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio; | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 Allocation concealment was not adequately explained in six of eight trials, there was no evidence of blinding in five trials and four trials did not explain incomplete outcome data 2 Only some of the trial participants were seeking treatment for fertility‐ numbers unclear
Summary of findings 2. Pre and post operative medical therapies versus placebo for endometriosis.
| Pre and post operative medical therapies versus placebo for endometriosis | ||||||
| Patient or population: patients with infertility associated with endometriosis Intervention: Post surgical medical therapies Comparison: Pre and post‐surgical medical therapy with GnRHa | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Pre and post‐surgical medical therapy with GnRHa | Post surgical medical therapies | |||||
| Pregnancy Rate | 500 per 1000 | 0 per 1000 (0 to 0) | RR 0 (0 to 0) | 25 (1 study) | ⊕⊕⊝⊝ low1 | |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio; | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 Description of randomisation , allocation concealment and blinding not adequate.
Summary of findings 3. Pre‐surgical medical therapy compared to no medical therapy for endometriosis surgery.
| Pre‐surgical medical therapy compared to no medical therapy for endometriosis surgery | ||||||
| Patient or population: patients with endometriosis surgery Intervention: Pre‐surgical medical therapy Comparison: no medical therapy | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| no medical therapy | Pre‐surgical medical therapy | |||||
| Recurrence ‐ AFS Score ‐ Total AFS | The mean Recurrence ‐ AFS Score ‐ Total AFS in the intervention groups was 9.6 lower (11.42 to 7.78 lower) | 80 (1 study) | ⊕⊕⊝⊝ low1,2 | |||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 No blinding and trial lacked details on allocation concealment 2 Evidence based on a single trial
Summary of findings 4. Post‐surgical medical therapy compared to placebo or no treatment for endometriosis.
| Post‐surgical medical therapy compared to placebo or no treatment for endometriosis | ||||||
| Patient or population: patients with endometriosis Intervention: Post‐surgical medical therapy Comparison: placebo or no treatment | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| placebo or no treatment | Post‐surgical medical therapy | |||||
| Pain (VAS) ‐ Dysmenorrhoea at 12 months | The mean Pain (VAS) ‐ Dysmenorrhoea at 12 months in the intervention groups was 0.58 standard deviations lower (0.87 to 0.28 lower) | 187 (1 study) | ⊕⊕⊝⊝ low1,2 | SMD ‐0.58 (‐0.87 to ‐0.28) | ||
| Pain (dichotomous) ‐ Pain recurrence </= 12 months | 273 per 1000 | 207 per 1000 (142 to 300) | RR 0.76 (0.52 to 1.1) | 332 (3 studies) | ⊕⊕⊝⊝ low3 | |
| Pain (dichotomous) ‐ pain persistence/recurrence 5 years | 480 per 1000 | 446 per 1000 (254 to 797) | RR 0.93 (0.53 to 1.66) | 54 (1 study) | ⊕⊝⊝⊝ very low2,4 | |
| Recurrence ‐ AFS Score ‐ Total AFS score (12 months) | The mean Recurrence ‐ AFS Score ‐ Total AFS score (12 months) in the intervention groups was 2.29 lower (4.69 lower to 0.11 higher) | 43 (1 study) | ⊕⊕⊝⊝ low2,5 | |||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio; | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 There are inadequate details on blinding and attrition 2 Evidence is based on a single study 3 Two of the trials did not provide adequate details for allocation concealment or attrition and there was no blinding 4 The trial lacked details on all methodological aspects and there was no blinding 5 The trial lacked details on allocation concealment and randomisation
Summary of findings 5. Pre‐surgical compared to Post‐surgical medical therapy for endometriosis surgery.
| Pre‐surgical compared to Post‐surgical medical therapy for endometriosis surgery | ||||||
| Patient or population: patients with endometriosis surgery Intervention: Pre‐surgical Comparison: Post‐surgical medical therapy | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Post‐surgical medical therapy | Pre‐surgical | |||||
| Pain (Dichotomous) ‐ Dysmenorrhoea | See comment | See comment | Not estimable | 53 (1 study) | ⊕⊕⊝⊝ low1,2 | There were no events reported in either the intervention or the control group |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio; | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 The trial did not provide adequate details on allocation concealment or randomisation 2 Evidence based on a single trial
Summary of findings 6. Post‐surgical medical therapy compared to Pre and post‐surgical medical therapy with GnRHa for endometriosis surgery.
| Post‐surgical medical therapy compared to Pre and post‐surgical medical therapy with GnRHa for endometriosis surgery | ||||||
| Patient or population: patients with endometriosis surgery Intervention: Post‐surgical medical therapy Comparison: Pre and post‐surgical medical therapy with GnRHa | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Pre and post‐surgical medical therapy with GnRHa | Post‐surgical medical therapy | |||||
| Recurrence ‐ AFS Score ‐ Total AFS score | The mean Recurrence ‐ AFS Score ‐ Total AFS score in the intervention groups was 3.49 higher (5.1 lower to 12.08 higher) | 25 (1 study) | ⊕⊝⊝⊝ very low1,2,3 | |||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 No adequate explanation of randomisation and allocation concealment 2 CI crossed line of no effect and substantive harm and benefit. 3 Evidence based on a single trial
Pre‐surgical medical therapy
1.1. Analysis.
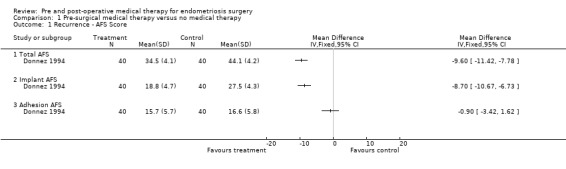
Comparison 1 Pre‐surgical medical therapy versus no medical therapy, Outcome 1 Recurrence ‐ AFS Score.
Disease recurrence
Both trials used AFS scores as the outcome measure in comparing medical treatment pre‐surgery with surgery alone. Donnez 1994 showed a statistically significant reduction in endometrioma cyst size (Table 11), total AFS scores and implant AFS scores favouring the goserelin treated group but there was no statistically significant difference between the groups with regard to adhesion AFS scores (Analysis 1.1).
Shaw 2001 found a decrease in endometrioma size and a decreased recurrence rate in the goserelin group with no difference in mean total AFS scores post‐treatment (but no estimate of precision was stated so these data were not included in the meta‐analysis: the raw data only are presented in Table 11).
These two trials showed that pre‐operative GnRH agonist treatment decreased the size of endometrial cysts, although the clinical significance of this is unknown; however these trials were assessed as being at significant risk of bias and showed conflicting AFS results.
Based on these two trials there is insufficient evidence to conclude that pre‐surgical medical therapy was better than surgery alone.
Post‐surgical medical therapy versus placebo or no treatment
Pain
(Analysis 2.1; Analysis 2.2; Table 11)
2.1. Analysis.
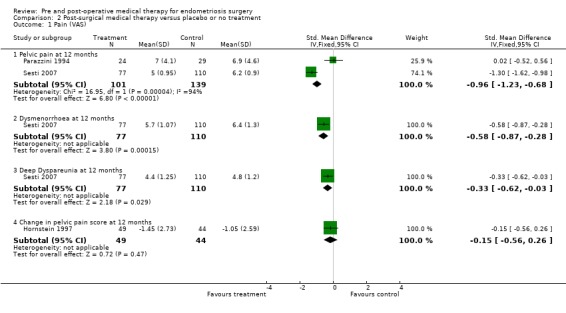
Comparison 2 Post‐surgical medical therapy versus placebo or no treatment, Outcome 1 Pain (VAS).
2.2. Analysis.
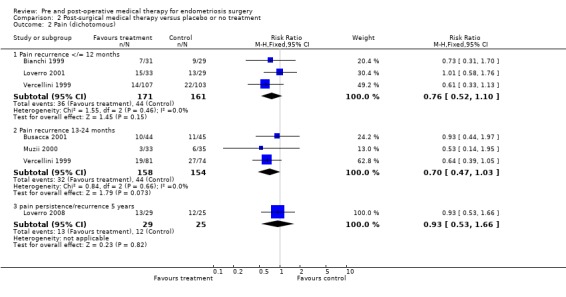
Comparison 2 Post‐surgical medical therapy versus placebo or no treatment, Outcome 2 Pain (dichotomous).
The outcome of pain was reported in 10 trials (Bianchi 1999; Busacca 2001; Hornstein 1997; Loverro 2001; Loverro 2008; Parazzini 1994; Muzii 2000; Sesti 2007; Telimaa 1987; Vercellini 1999).
Meta‐analysis was possible for the continuous outcome, mean VAS score of pelvic pain at 12 months, for two studies (Parazzini 1994; Sesti 2007) and the pooled estimate showed a statistically significant reduction in pelvic pain at 12 months (SMD ‐0.96, 95% CI ‐1.23 to ‐0.68) favouring medical therapy compared to placebo. However these studies show inconsistent effects and considerable statistical heterogeneity (I2 = 94%, P<0.0001). A third study (Telimaa 1987) reported pain after 12 months using a four‐point scale and presented mean scores without estimates of precision. The paper stated that there was a "significant difference between both danazol and placebo and MPA and placebo favouring medical therapy". It was not possible to include these data in the meta‐analysis but the estimates are recorded in Table 11.
Sesti 2007 also reported the outcomes of dysmenorrhoea and deep dyspareunia. When the groups receiving medical therapy were combined there was a statistically significant difference favouring medical therapy over placebo for both dysmenorrhoea and dyspareunia (Analysis 2.1.2; Analysis 2.1.3). In the trial by Hornstein 1997 the change in pain score from baseline to 12 months after surgery was presented and showed no statistically significant difference between medical therapy and placebo (Analysis 2.1.4).
Pain recurrence was measured during the first year after surgical treatment and reported in Bianchi 1999; Loverro 2001 and Vercellini 1999. The pooled estimate from these three trials showed no statistically significant difference between medical therapy and surgery alone (RR 0.76, 95% CI 0.52 to 1.10) (Analysis 2.2.1).
Pain recurrence during the second year after surgery was reported in three trials (Busacca 2001; Muzii 2000; Vercellini 2003) and the pooled estimate also showed no statistically significant difference between medical therapy after surgery and surgery alone (RR 0.70, 95% CI 0.47 to 1.03) (Analysis 2.2.2).
Five years after treatment, a single study (Loverro 2008) found no statistically significant difference in pain persistence or recurrence between medical therapy and placebo (Analysis 2.2.3).
Disease recurrence
The only study in this group to report AFS scores for disease recurrence was Telimaa 1987. After 12 months, a second‐look laparoscopy showed a statistically significant reduction in AFS scores from baseline in all three groups: MPA, danazol and placebo. When each active treatment group was compared to placebo, there was a statistically significant mean difference favouring MPA, but the difference between danazol and placebo was not statistically significant (Table 11) and the combined mean AFS score for both medical therapies was not statistically significantly different from placebo (Analysis 2.3).
2.3. Analysis.
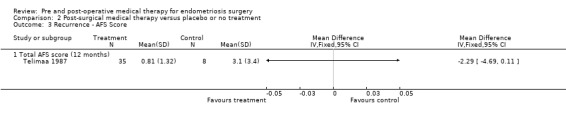
Comparison 2 Post‐surgical medical therapy versus placebo or no treatment, Outcome 3 Recurrence ‐ AFS Score.
Disease or symptom recurrence, evaluated by gynaecological examination or ultrasonography, was measured at two time points: one year and two years after surgery . There was no statistically significant difference in disease recurrence at one year (Bianchi 1999; Busacca 2001) between surgery plus medical therapy and surgery alone (RR 0.76, 95% CI 0.30 to 1.90) (Analysis 2.4.1) and no difference at two years in the one study (Tsai 2004) that reported this outcome (Analysis 2.4.2).
2.4. Analysis.
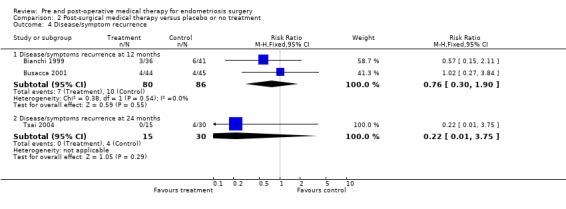
Comparison 2 Post‐surgical medical therapy versus placebo or no treatment, Outcome 4 Disease/symptom recurrence.
Pregnancy
Pregnancy was a desired outcome in some of the patients in eight studies (Bianchi 1999; Busacca 2001; Loverro 2001; Loverro 2008; Parazzini 1994; Telimaa 1987; Vercellini 1999; Yang 2006). There was no difference between surgery plus medical therapy and either surgery plus placebo or no treatment with regard to pregnancy rate following treatment (RR 0.84, 95% CI 0.59 to 1.18) (Analysis 2.5).
2.5. Analysis.
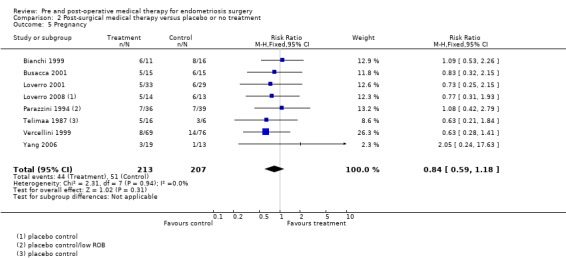
Comparison 2 Post‐surgical medical therapy versus placebo or no treatment, Outcome 5 Pregnancy.
Patient satisfaction
Only one study reported the outcome of patients satisfaction. Telimaa 1987 reported an increase in patient satisfaction in both active treatment groups, which was statistically significantly greater compared to the placebo group but there was no difference between the danazol and MPA groups for either of these outcomes (Table 11).
Pre‐surgical medical therapy compared with post‐surgical medical therapy
3.1. Analysis.
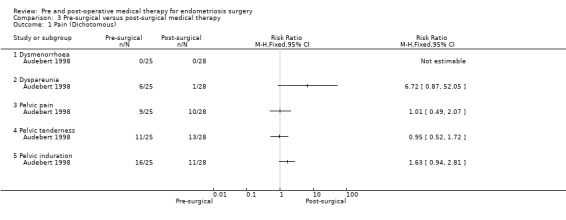
Comparison 3 Pre‐surgical versus post‐surgical medical therapy, Outcome 1 Pain (Dichotomous).
In the single study in this comparison (Audebert 1998) there was no statistically significant difference between the groups for the outcome of pain (Analysis 3.1).
The trial reported that the pre‐operative nafarelin group had statistically significantly lower global AFS scores, adhesion scores and 'endometriosis scores' compared to the post‐operative nafarelin group, but data were presented in a form that was not suitable for inclusion in a forest plot so these data are recorded in Table 11.
Surgery was described as 'easy' in a higher proportion of the pre‐operative nafarelin group compared to the post‐operative nafarelin group, but no indication of any statistical significance was given.
Post‐surgical medical therapy compared with pre and post‐surgical medical therapy
In this small study (Batioglu 1997) (n = 25) no statistically significant differences were found between the groups with respect to the outcomes of recurrence (total AFS scores, implant AFS scores, adhesion AFS scores) (Analysis 4.1) and pregnancy rate (Analysis 4.2).
4.1. Analysis.
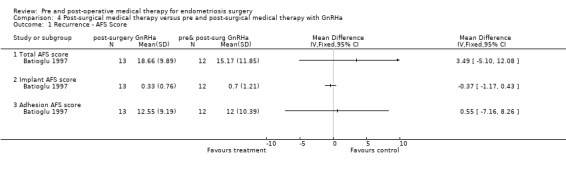
Comparison 4 Post‐surgical medical therapy versus pre and post‐surgical medical therapy with GnRHa, Outcome 1 Recurrence ‐ AFS Score.
4.2. Analysis.
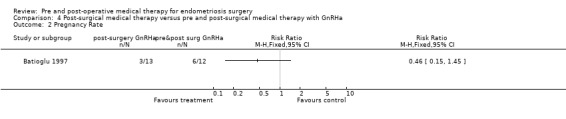
Comparison 4 Post‐surgical medical therapy versus pre and post‐surgical medical therapy with GnRHa, Outcome 2 Pregnancy Rate.
Adverse effects
The adverse effects data were summarised in a 'Table of adverse drug effects' in the additional tables section (Table 12). Adverse effects were described in some trials but data were not presented in a way that allowed any quantitative analysis.
2. Table of adverse drug effects.
| Trial ID | Adverse Drug Effects | Withdrawals‐ADE |
| Audebert 1998 | Side effects were reported with equal frequency in both groups and were consistent with those published by other investigators | 2 withdrawals after randomisation from hot flushes and headaches |
| Batioglu 1997 | Not described | None |
| Bianchi 1999 | Hyperandrogenism 16.7%, weight gain ≥3kg 8.3% | None |
| Busacca 2001 | Most experienced menopausal symptoms, all became amenorrhoeic | 1 withdrawal from unacceptable side effects |
| Donnez 1994 | Not described | None |
| Hornstein 1997 | Not described | Not due to ADE |
| Loverro 2001 | Not described | None |
| Loverro 2008 | Not described | None |
| Muzii 2000 | Not described | Not due to ADE |
| Parazzini 1994 | Amenorrhoea in all actively treated, none in placebo group | None |
| Sesti 2007 | Menopausal symptoms, spotting, bloating, weight gain and headache reported but "well tolerated" | 4 withdrew from hormonal suppression group due to AE |
| Shaw 2001 | Hot flushes (62%), headaches (29%), dysmenorrhoea (14%) in goserelin group and 33% had dysmenorrhoea in no treatment group | 4 withdrew from goserelin gp due to serious AE (1 treated to treatment) |
| Telimaa 1987‐Danazol and Telimaa 1987‐MPA |
Weight increase MPA 1.9±1.3kg, danazol 3.4±2.3kg, placebo 0.4±2.6kg; breakthrough bleeding at 6/12: MPA 65%, danazol 56%, placebo 6%; acne at 6/12: danazol 56%, placebo 6% | Not due to ADE |
| Tsai 2004 | Not described | Reasons for withdrawals not given |
| Vercellini 1999 | Not described | None |
| Yang 2006 | Not described | None |
Sensitivity analysis
Planned sensitivity analysis was not undertaken because only one of the 16 trials was assessed as being at low risk of bias.
Discussion
Summary of main results
(1) There is insufficient evidence to support the view that medical therapy for hormonal suppression of endometriosis prior to surgery is more effective than surgery alone. Two studies compared pre‐surgical medical therapy with surgery alone. AFS scores were significantly improved in the medical treatment group in one study and not in the other. Meta‐analysis of the data from these studies was not possible. Medical therapy may or may not be associated with better outcomes for the patients.
(2) Post‐surgical hormonal suppression of endometriosis compared to surgery alone (either no medical therapy or placebo) showed some reduction in pain after 12 months but results were inconsistent and pain recurrence in both groups indicated that there was no evidence of a benefit for pain beyond 12 months. There was no evidence of benefit for the outcomes of disease recurrence (AFS scores) or pregnancy (RR 0.84, 95% CI 0.59 to 1.18).
(3) There were no trials identified from the search that compared hormonal suppression of endometriosis before and after surgery with surgery alone.
(4) There was no significant difference between pre‐surgery hormonal suppression and post‐surgery hormonal suppression for the outcome of pain in the one trial identified. This trial reported a statistically significant reduction in recurrence at six months as measured by AFS scores but ease of surgery was reported only as a descriptive summary so any difference between the groups can not be quantified from the information given in the report of this trial.
(5) There was insufficient evidence to support the view that medical therapy for hormonal suppression of endometriosis pre and post‐surgery was more effective than medical therapy post‐surgery only.
In summary, the use of medical treatment after surgery was not associated with a long‐term statistically significant difference in pain from endometriosis. When used prior to surgery, medical therapy was shown to decrease cyst size but the effect on AFS scores was conflicting. There is no evidence that medical therapy pre or post‐surgery improves pregnancy rates. No conclusions can be drawn with respect to the outcomes of facilitating surgery, duration of surgery, post‐operative complications or levels of satisfaction of women participants from the trials included in this review. Adverse effects were described but not quantified, so no direct comparisons between the included trials were possible.
Quality of the evidence
A strength of this review is that all of the included studies involved women with laparoscopically diagnosed endometriosis and laparoscopic assessment of the extent of the endometriosis. Weaknesses of this review are that the included studies were small and many were at risk of bias. There was a spread of different times at which a range of different outcomes were measured, which does not allow for studies to be combined in a meta‐analysis. Only one study used a quality of life measure as an outcome (Sesti 2007) although this may well be the outcome of most interest to women with endometriosis.
There were some methodological issues in the trials included in this review. Of the 16 reports of randomised controlled trials, 11 described the randomisation process used but only four reported adequate allocation concealment. Four of the 16 studies were double blinded and in two studies the outcome assessors were blinded to the intervention status. The side effects associated with medical therapies for endometriosis are such that maintaining blinding of participants and investigators is likely to have been difficult in these trials.
Authors' conclusions
Implications for practice.
There is no evidence from the studies identified to conclude that hormonal suppression in association with surgery for endometriosis is associated with a significant benefit with regard to any of the outcomes identified. From two studies of pre‐surgical medical therapy there is no evidence that pre‐surgical medical therapy is better than surgery alone. There was no evidence that post‐surgical medical therapy was associated with a benefit compared to surgery alone.
Implications for research.
Research done to assess the effects of medical treatment pre or post‐surgery for endometriosis is associated with many difficulties. Not many women will consent to undergo second‐look laparoscopy to assess the results of previous treatment modalities. Hence, recruiting large numbers of participants for randomised trials is difficult. Maintaining blinding is also difficult due to the adverse effects associated with hormonal suppression, which would be obvious to both the patient and investigator. Blinded outcome assessment is possible and desirable. Women with subfertility due to endometriosis may also not accept treatment that may improve pain and other symptoms but reduces or delays their chance of conceiving.
Despite these difficulties, it would be valuable to have well designed, adequately powered and well conducted trials to determine if there is a significant benefit in adjunctive medical therapy before or after surgery for endometriosis. Consistency in the methods of assessing outcomes, with respect to pain and the extent of endometriosis from AFS scores, would also facilitate meta‐analysis of data across trials. Data to quantify the number and degree of adverse events experienced as a result of medical therapy would enable better assessment of the comparative benefits and harms of medical treatment.
What's new
| Date | Event | Description |
|---|---|---|
| 2 May 2011 | Amended | Summary of findings tables added |
History
Protocol first published: Issue 2, 2002 Review first published: Issue 3, 2004
| Date | Event | Description |
|---|---|---|
| 20 September 2010 | New search has been performed | Substantive update September 2010 ‐ 5 new trials included. Risk of bias assessment on all included studies. Minor changes to the objectives ‐ hypotheses deleted |
| 7 November 2008 | Amended | Converted to new review format. |
| 26 May 2004 | New citation required and conclusions have changed | Substantive amendment |
Acknowledgements
We would like to thank the Menstrual Disorders and Subfertility Group (MDSG) in Auckland, New Zealand, for their help, advice and support during the preparation of the original review. For the current update of the review we would like to thank Marian Showell (Trials Search Coordinator) who updated the searches.
Appendices
Appendix 1. MEDLINE search strategy
1 Endometriosis/ (14076) 2 endometrio$.ti,ab,sh. (19246) 3 adenomyosis.tw. (1325) 4 or/1‐3 (19555) 5 exp Contraceptives, Oral/ (38769) 6 (oral contraceptive$ or contraceptive$ pill$).tw. (20171) 7 OCP.tw. (1048) 8 exp Gonadotropin‐Releasing Hormone/ (26314) 9 (GnRH or lhrh or gn‐rh or lfrh or lh‐rh or lhfshrh).tw. (24157) 10 Gonadotropin‐Releasing Hormone.ti,ab,sh. (26055) 11 (gonadorelin or luliberin or luteinizing hormone‐releasing hormone or cystorelin).tw. (5201) 12 (dirigestran or factrel or gonadoliberin).tw. (135) 13 danazol/ or danazol.tw. (2626) 14 progestins/ or gestrinone/ or progesterone/ (55370) 15 (progestogen$ or gestrinone).tw. (4456) 16 or/5‐15 (129407) 17 specialties, surgical/ or gynecology/ or surgery/ (42306) 18 surg$.tw. (1064203) 19 Laparoscopy/ (45637) 20 Laparoscop$.ti,ab,sh. (68764) 21 celioscop$.tw. (534) 22 peritoneoscop$.tw. (617) 23 Surgical Procedures, Minimally Invasive/ (12402) 24 exp Surgical Procedures, Operative/ (1965124) 25 or/17‐24 (2517350) 26 4 and 16 and 25 (1367) 27 randomized controlled trial.pt. (299824) 28 controlled clinical trial.pt. (82501) 29 randomized.ab. (213949) 30 placebo.tw. (129005) 31 clinical trials as topic.sh. (151072) 32 randomly.ab. (158040) 33 trial.ti. (91956) 34 (crossover or cross‐over or cross over).tw. (49316) 35 or/27‐34 (728695) 36 (animals not (humans and animals)).sh. (3453357) 37 35 not 36 (674119) 38 26 and 37 (219) 39 2010$.ed. (775885) 40 38 and 39 (8)
Appendix 2. CENTRAL search strategy
1 Endometriosis/ (392) 2 endometrio$.ti,ab,sh. (746) 3 adenomyosis.tw. (23) 4 or/1‐3 (761) 5 exp Contraceptives, Oral/ (2713) 6 (oral contraceptive$ or contraceptive$ pill$).tw. (1478) 7 OCP.tw. (43) 8 exp Gonadotropin‐Releasing Hormone/ (1664) 9 (GnRH or lhrh or gn‐rh or lfrh or lh‐rh or lhfshrh).tw. (1803) 10 Gonadotropin‐Releasing Hormone.ti,ab,sh. (1247) 11 (gonadorelin or luliberin or luteinizing hormone‐releasing hormone or cystorelin).tw. (239) 12 (dirigestran or factrel or gonadoliberin).tw. (5) 13 danazol/ or danazol.tw. (294) 14 progestins/ or gestrinone/ or progesterone/ (1257) 15 (progestogen$ or gestrinone).tw. (617) 16 or/5‐15 (6967) 17 specialties, surgical/ or gynecology/ or surgery/ (257) 18 surg$.tw. (56109) 19 Laparoscopy/ (2020) 20 Laparoscop$.ti,ab,sh. (4319) 21 celioscop$.tw. (9) 22 peritoneoscop$.tw. (13) 23 Surgical Procedures, Minimally Invasive/ (392) 24 exp Surgical Procedures, Operative/ (65773) 25 or/17‐24 (94838) 26 4 and 16 and 25 (170) 27 limit 26 to yr="2010 ‐Current" (3)
Appendix 3. EMBASE
1 Endometriosis/ (17794) 2 endometrio$.ti,ab,sh. (23228) 3 adenomyosis.tw. (1571) 4 or/1‐3 (24065) 5 exp Contraceptives, Oral/ (44979) 6 (oral contraceptive$ or contraceptive$ pill$).tw. (18807) 7 OCP.tw. (1147) 8 exp Gonadotropin‐Releasing Hormone/ (24685) 9 (GnRH or lhrh or gn‐rh or lfrh or lh‐rh or lhfshrh).tw. (25582) 10 Gonadotropin‐Releasing Hormone.ti,ab,sh. (9813) 11 (gonadorelin or luliberin or luteinizing hormone‐releasing hormone or cystorelin).tw. (4930) 12 (dirigestran or factrel or gonadoliberin).tw. (270) 13 danazol/ or danazol.tw. (6487) 14 progestins/ or gestrinone/ or progesterone/ (75274) 15 (progestogen$ or gestrinone).tw. (4557) 16 or/5‐15 (153928) 17 laparoscopy/ (37548) 18 laparoscop$.mp. (88603) 19 celioscop$.mp. (964) 20 peritoneoscop$.mp. (655) 21 surgical procedures, Minimally invasive/ (16214) 22 exp surgical procedures, operative/ (2443651) 23 gynaecologic surgery/ or endoscopic surgery/ (30299) 24 or/17‐23 (2448911) 25 4 and 16 and 24 (1983) 26 Clinical Trial/ (806389) 27 Randomized Controlled Trial/ (280762) 28 exp randomization/ (52428) 29 Single Blind Procedure/ (13288) 30 Double Blind Procedure/ (99074) 31 Crossover Procedure/ (29228) 32 Placebo/ (167897) 33 Randomi?ed controlled trial$.tw. (56110) 34 Rct.tw. (5927) 35 random allocation.tw. (988) 36 randomly allocated.tw. (14617) 37 allocated randomly.tw. (1666) 38 (allocated adj2 random).tw. (676) 39 Single blind$.tw. (10363) 40 Double blind$.tw. (113147) 41 ((treble or triple) adj blind$).tw. (224) 42 placebo$.tw. (150524) 43 prospective study/ (155097) 44 or/26‐43 (1083621) 45 case study/ (10221) 46 case report.tw. (190983) 47 abstract report/ or letter/ (753390) 48 or/45‐47 (951065) 49 44 not 48 (1052070) 50 25 and 49 (494) 51 2010$.em. (849128) 52 50 and 51 (44)
Data and analyses
Comparison 1. Pre‐surgical medical therapy versus no medical therapy.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Recurrence ‐ AFS Score | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 1.1 Total AFS | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 Implant AFS | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.3 Adhesion AFS | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
Comparison 2. Post‐surgical medical therapy versus placebo or no treatment.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Pain (VAS) | 3 | Std. Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 1.1 Pelvic pain at 12 months | 2 | 240 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.96 [‐1.23, ‐0.68] |
| 1.2 Dysmenorrhoea at 12 months | 1 | 187 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.58 [‐0.87, ‐0.28] |
| 1.3 Deep Dyspareunia at 12 months | 1 | 187 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.33 [‐0.62, ‐0.03] |
| 1.4 Change in pelvic pain score at 12 months | 1 | 93 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.15 [‐0.56, 0.26] |
| 2 Pain (dichotomous) | 6 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.1 Pain recurrence </= 12 months | 3 | 332 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.76 [0.52, 1.10] |
| 2.2 Pain recurrence 13‐24 months | 3 | 312 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.70 [0.47, 1.03] |
| 2.3 pain persistence/recurrence 5 years | 1 | 54 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.93 [0.53, 1.66] |
| 3 Recurrence ‐ AFS Score | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 3.1 Total AFS score (12 months) | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4 Disease/symptom recurrence | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 4.1 Disease/symptoms recurrence at 12 months | 2 | 166 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.76 [0.30, 1.90] |
| 4.2 Disease/symptoms recurrence at 24 months | 1 | 45 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.22 [0.01, 3.75] |
| 5 Pregnancy | 8 | 420 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.84 [0.59, 1.18] |
Comparison 3. Pre‐surgical versus post‐surgical medical therapy.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Pain (Dichotomous) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 1.1 Dysmenorrhoea | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 Dyspareunia | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.3 Pelvic pain | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.4 Pelvic tenderness | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.5 Pelvic induration | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
Comparison 4. Post‐surgical medical therapy versus pre and post‐surgical medical therapy with GnRHa.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Recurrence ‐ AFS Score | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 1.1 Total AFS score | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 Implant AFS score | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.3 Adhesion AFS score | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Pregnancy Rate | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Audebert 1998.
| Methods | Location: France
No. of centres: multi centre Recruitment period: December 1990 to March 1993 |
|
| Participants | Inclusion criteria: < 40 years, stage III‐IV endometriosis, pelvic pain, dysmenorrhoea or dyspareunia Exclusion criteria: > 40 years, hormonal treatment for endometriosis within 3/12 (including OCP, progestins), significant medical illness e.g. liver, heart, renal disease, abnormal PAP smear, pregnancy, surgery for endometriosis within 6/12 No. randomised: 55 No. analysed: 53 | |
| Interventions | Gr A (n=28) Pre‐surgery medical treatment with nafarelin nasal 400 uG daily x 6/12 Gr B (n=25) Post‐surgery medical treatment with nafarelin nasal 400 uG daily x 6/12 | |
| Outcomes | Pain: dysmenorrhoea, dyspareunia, pelvic pain, pelvic tenderness, pelvic induration AFS scores: global, adhesions, endometriosis Ease of surgery | |
| Notes | Power calculation: NS Funding: Syntex Pharmaceuticals International for supply of Nafarelin, grant for trial | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "randomised" no details of method of sequence generation provided |
| Allocation concealment (selection bias) | Unclear risk | not mentioned |
| Blinding (performance bias and detection bias) All outcomes | Low risk | double blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | all randomised patients included in analysis |
| Selective reporting (reporting bias) | Low risk | important outcomes ‐ AFS scores, recurrence, surgical difficulty reported |
| Other bias | Low risk | groups appear comparable at baseline |
Batioglu 1997.
| Methods | Location: Ankara Turkey
No. of Centres: 1 Recruitment period: NS |
|
| Participants | Inclusion criteria: ovarian endometriomas >3cm unilateral/bilateral No. randomised: 25 No. analysed: 25 |
|
| Interventions | Post‐surgery medical treatment with triptorelin 3.75 mg IM x 4 weekly for 6 months (n = 13) versus Pre‐surgery and post‐surgery treatment with triptorelin 3.75 mg IM x 4 weekly for 6 months (n = 12) | |
| Outcomes | AFS scores at 6 months Pregnancy rate at 1 year follow up | |
| Notes | Power calculation: NS Funding: NS | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | "randomly allocated into one of the treatment groups, odd numbers in the first group and even numbers in the second |
| Allocation concealment (selection bias) | High risk | no allocation concealment |
| Blinding (performance bias and detection bias) All outcomes | High risk | not mentioned, no placebo |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | pregnancy rate reported for all randomised patients |
| Selective reporting (reporting bias) | Unclear risk | mean AFS scores reported per endometrioma not per patient, pregnancy rate |
| Other bias | Unclear risk | there were differences between the groups at baseline with regard to mean adhesion scores |
Bianchi 1999.
| Methods | No. of centres: 1
Location: University of Milan, Italy Recruitment period: July 1994 to October 1996 |
|
| Participants | Inclusion criteria: < 40 yrs Exclusion criteria: medical or surgical treatment for endometriosis, concurrent disease that might affect fertility or cause pelvic pain, women without pain symptoms, women not seeking pregnancy, liver or endocrine disease No. randomised: 77 No. analysed: 77 | |
| Interventions | Post‐surgical medical therapy 1. Danazol oral 600 mg daily x 3/12 (n = 36) 2. No treatment (n = 41) | |
| Outcomes | Pain recurrence AFS scores Pregnancy rates Adverse events of medication | |
| Notes | Power calculation: NS Funding: NS | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Randomization was done according to a computer generated list" |
| Allocation concealment (selection bias) | Unclear risk | not mentioned |
| Blinding (performance bias and detection bias) All outcomes | High risk | not mentioned, no placebo |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | all randomised patients included in analysis |
| Selective reporting (reporting bias) | Low risk | important outcomes ‐ pregnancy rate and recurrence of endometriosis pain, adverse effects reported |
| Other bias | Low risk | groups appear comparable at baseline |
Busacca 2001.
| Methods | Location: University of Milan, Italy No. of centres: 1 Recruitment period: July 1997 to December 1999 |
|
| Participants | Inclusion criteria: < 40 yrs, laparoscopic diagnosis of endometriosis stage III‐IV Exclusion criteria: previous medical or surgical therapy for endometriosis, other diseases that might affect fertility or cause pelvic pain; liver, endocrine or neoplastic disease No. randomised: 89 No. analysed: 89 | |
| Interventions | Post‐surgical medical therapy Gr A (n=44): leuprolide acetate SC 3.5 mg 4 weekly x 3 doses Gr B (n=45): no treatment | |
| Outcomes | Pain: pelvic pain recurrence at 18/12 AFS scores: objective disease recurrence Cumulative pregnancy rates at 18/12 | |
| Notes | Power calculation: yes Funding: NS | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "randomization was performed according to a computer generated list unknown to the physicians" |
| Allocation concealment (selection bias) | Unclear risk | not described |
| Blinding (performance bias and detection bias) All outcomes | High risk | not mentioned, no placebo |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | all randomised patients included in the analysis |
| Selective reporting (reporting bias) | Low risk | important outcomes of pregnancy and recurrence of endometriosis and pain reported |
| Other bias | Low risk | groups appear comparable at baseline |
Donnez 1994.
| Methods | No. of centres: 1
Location: Catholic University of Louvain, Belgium Recruitment period: January 1990 to December 1990 |
|
| Participants | Inclusion criteria: age < 35 yrs, infertility, laparoscopic confirmed ovarian endometriotic cysts (AFS moderate n = 41, AFS severe n = 39) Exclusion criteria: none No. randomised: 80 No. analysed: 80 | |
| Interventions | Medical therapy pre‐surgery 1. Goserelin S/C 4 weekly x 4 (n = 40) 2. No therapy (n = 40) | |
| Outcomes | AFS scores: total, implants, adhesions, moderate, severe scores Ovarian cyst diameter Degree of active endometriosis as determined histologically from ovarian cyst wall biopsy | |
| Notes | Power calculation: NS Funding: Fonds de la Recherche Scientifique | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "randomised according to official randomisation tables" |
| Allocation concealment (selection bias) | Unclear risk | not mentioned |
| Blinding (performance bias and detection bias) All outcomes | High risk | not mentioned, no placebo used |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | all randomised patients included in outcome assessment |
| Selective reporting (reporting bias) | Unclear risk | AFS scores at second look laparoscopy ‐ no pregnancy data reported |
| Other bias | Low risk | groups appear comparable at baseline |
Hornstein 1997.
| Methods | No. of centres: 13
Location: 13 clinics (North America) Recruitment period: not stated |
|
| Participants | Inclusion criteria: age 18‐47, normal menstrual cycles of 24‐36 days, clinical pelvic pain, dysmenorrhoea, dyspareunia Exclusion criteria: received medical therapy for endometriosis within 3 months, abnormal bone density, significant medical illness, laboratory abnormalities, pregnancy and lactation No. randomised: 109 No. analysed: 93 7 in nafarelin group and 8 in placebo group withdrew after 90 days of therapy 1 in placebo group excluded because missed 5 days of medication | |
| Interventions | Post‐surgery medical therapy 1. Nafarelin nasal 400 uG daily x 6/12 (n = 49) 2. Placebo (n = 44) | |
| Outcomes | Pain Physician scores for tenderness and induration on physical examination at end of treatment and 6/12 after treatment | |
| Notes | Power calculation: NS Funding: Syntex laboratory, California | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "multicenter, prospective, randomized, double‐blind study" method of sequence generation not described |
| Allocation concealment (selection bias) | Unclear risk | not mentioned |
| Blinding (performance bias and detection bias) All outcomes | Low risk | "double blind" but authors acknowledge difficulty of maintaining blinding with this treatment |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 7 and 8 patients were excluded from analyses of treatment and placebo groups because they withdrew before completing 90 days of therapy. Remaining 93 patients included in analysis even though 39 and 43 patients from treatment and placebo groups terminated the study early |
| Selective reporting (reporting bias) | Low risk | primary outcome was time to requiring alternative treatment; pain & recurrence also reported |
| Other bias | Low risk | groups appear comparable at baseline |
Loverro 2001.
| Methods | No. of centres: 1
Location: Bari, Italy Recruitment period: January 1996 to January 1997 |
|
| Participants | Inclusion criteria: AFS score III‐IV Exclusion criteria: NS No. randomised: 62 No. analysed: 62? | |
| Interventions | Post‐surgery medical therapy 1. Triptorelin SC 3.75 mg every 4 weeks x 3 months (n = 33) 2. No treatment (n = 29) | |
| Outcomes | Pain Pregnancy rates | |
| Notes | Power calculation: NS Funding: NS Pregnancy outcomes and pain recurrence were only expressed as percentages in each group so numbers calculated were rounded up to the nearest whole number | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "prospective and randomized" ‐ no details of method of sequence generation |
| Allocation concealment (selection bias) | Unclear risk | not mentioned |
| Blinding (performance bias and detection bias) All outcomes | High risk | not mentioned, no placebo used |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | not stated how many women are included in the outcomes ‐ only percentages reported. |
| Selective reporting (reporting bias) | Low risk | important outcomes ‐ time to relapse (pelvic pain) and pregnancy reported |
| Other bias | Unclear risk | no information on comparability of groups at baseline given |
Loverro 2008.
| Methods | Location: Italy
No. of centres: one Recruitment period: January 1998 to January 1999 |
|
| Participants | Inclusion criteria: women of reproductive age with stage III ‐ IV endometriosis, associated with chronic pelvic pain,adnexial mass or infertility, who had undergone complete laparoscopic excision, had rAFS score > 15 and no previous hormonal treatment Exclusion criteria: No. randomised: 60 No. analysed: 54 | |
| Interventions | Post‐operative triptorelin versus placebo Gr A (n=29): triptorelin 3.75 mg depot monthly on day 20 of cycle for 3 months Gr B (n=25): placebo monthly on day 20 of cycle for 3 months | |
| Outcomes | Pain persistence, pregnancy | |
| Notes | Power calculation: NS Funding: NS Email sent to contact author Feb 2010 requesting further information ‐ no reply received |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "using a computer generated randomization table" |
| Allocation concealment (selection bias) | Unclear risk | not mentioned |
| Blinding (performance bias and detection bias) All outcomes | Low risk | patients were blinded to treatment allocation. placebo injections used |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 1 and 5 patients lost to follow up from triptorelin and no treatment groups respectively. Possibility of bias |
| Selective reporting (reporting bias) | Low risk | pain, relapse and pregnancy reported (for those who desired pregnancy) |
| Other bias | Low risk | groups appear similar at baseline |
Muzii 2000.
| Methods | Location: University departments, Rome, Italy
No. of centres: 2 Recruitment period: January 1994 to June 1997 |
|
| Participants | Inclusion criteria: 20‐35 yrs, moderate to severe dysmenorrhoea and/or chronic pelvic pain, not desiring fertility Exclusion criteria: treatment for endometriosis in previous 6 months No. randomised: 70 No. analysed: 68 | |
| Interventions | Post‐surgical medical therapy Gr A (n=35): cyclic monophasic oral contraceptive pill (ethinyl estradiol 0.03 mg, gestodene 0.075 mg) for 21 days with 7 pill free days x 6/12 Gr B (n=35): no treatment | |
| Outcomes | Recurrence of pain and time to recurrence Recurrence of cysts | |
| Notes | Power calculation: yes Funding: NS. Drugs supplied by Population Council | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "randomly allocated to one of two management arms on the basis of a computer generated sequence" |
| Allocation concealment (selection bias) | Unclear risk | not described |
| Blinding (performance bias and detection bias) All outcomes | High risk | not mentioned, no placebo |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | two post‐randomisation withdrawals. Unlikely to have introduced a bias |
| Selective reporting (reporting bias) | Low risk | important outcomes reported ‐ recurrence of endometriosis, pain, AFS scores. Patients not desiring pregnancy |
| Other bias | Unclear risk | no information of the baseline characteristics of the groups reported |
Parazzini 1994.
| Methods | Location: University centres in Italy
No. of centres: 6 Recruitment period: January 1990 to July 1991 |
|
| Participants | Inclusion criteria: age < 38 yrs, normal medical examination, unexplained infertility for at least 1 year, with/without chronic pelvic pain, endometriosis stage III‐IV, partners with normal sperm analysis and post‐coital tests Exclusion criteria: previous laparoscopic/clinical diagnosis of endometriosis, other diseases that might cause infertility or pelvic pain, previous treatment for endometriosis or infertility No. randomised: 75 No. analysed: 75 (pregnancy rates), 68 (pain scores) | |
| Interventions | Post‐surgical medical therapy Gr A (n=36): nafarelin nasal 400 μg daily x 3/12 Gr B (n=39): placebo | |
| Outcomes | Pain (multidimensional and 10‐point linear scale) score at 12 months Pregnancy rates Adverse drug outcome (amenorrhoea) | |
| Notes | Power calculation: yes (post‐hoc?) Funding: Recordati Milan provided nafarelin | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "computer generated randomization list" |
| Allocation concealment (selection bias) | Low risk | assigned by telephone call 7 days from surgery |
| Blinding (performance bias and detection bias) All outcomes | Low risk | double blind but authors acknowledge that adverse effects of treatment make maintaining blinding difficult |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | no losses to follow up, all randomised patients included in analyses |
| Selective reporting (reporting bias) | Low risk | pregnancy rate and pelvic pain reported |
| Other bias | Low risk | groups appear comparable at baseline |
Sesti 2007.
| Methods | Location: Rome, Italy
No. of centres: one Recruitment period: January 1999 to May 2005 |
|
| Participants | Inclusion criteria: women of reproductive age <40, with endometriosis related symptoms (dysmenorrhoea, pelvic pain, deep dyspareunia), laparoscopic diagnosis of St III ‐IV endometriosis, desiring pregnancy, nulliparous Exclusion criteria: concurrent disease, such as cancer or pelvic inflammatory disease, previous surgery for endometriosis, contraindications to estrogens/progestins No. randomised: 234 No. analysed: 222 | |
| Interventions | Gr A (n=115 ): placebo for 6 months
Gr B (n=119 ): post‐operative medical or dietary therapy patients in group B received either
|
|
| Outcomes | Pelvic pain | |
| Notes | Power calculation: yes Funding: NS | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "randomized according to a computer generated randomization sequence" |
| Allocation concealment (selection bias) | Low risk | allocated by serially numbered opaque sealed envelopes |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | "neither the surgeons not the patients were aware of the regimen prescribed during the study period". Hoever placebo not described and it seems unlikely that blinding of patients could be maintained when treatments are either SC, oral medication or diet plus supplements |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 5 and 3 lost to follow up from placebo and GNRHa groups and reasons given. 2 lost to follow up from each of OCP and diet groups but reasons not given. 222 evaluated |
| Selective reporting (reporting bias) | Unclear risk | pain and health related quality of life reported. No pregnancy outcome in a group of women desiring pregnancy |
| Other bias | Low risk | groups appear comparable at baseline |
Shaw 2001.
| Methods | Location: UK and Republic of Ireland
No. of centres: 7 Recruitment period: not stated |
|
| Participants | Inclusion criteria: 18‐50 year old women referred for symptom management or infertility Exclusion criteria: cervical intraepithelial neoplasia (CIN) No. randomised: 48 No. analysed: 40 | |
| Interventions | Pre‐operative goserelin versus no treatment Gr A (n=21 ): goserelin 3.6 mg SC monthly for 3 months pre‐operatively Gr B (n=27 ): no medical therapy | |
| Outcomes | Change in endometrioma size, recurrence, pregnancy | |
| Notes | Power calculation: yes Funding: Astra Zeneca, Macclesfield, UK | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | participants were stratified by endometrioma size and "randomly allocated using computer generated randomization lists" |
| Allocation concealment (selection bias) | Unclear risk | not mentioned |
| Blinding (performance bias and detection bias) All outcomes | High risk | not mentioned, no placebo |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 33%(7) and 41% (11) of patients in Gr A & B withdrew from trial before surgery. Reasons given but are different in each group. 4 withdrawals from goserelin group pre surgery due to serious AE ‐ migraine/headache/hot flushes, groin pain, muscle cramps, pain iliac fossa/sciatica. However all patients are included in outcome evaluation provided there is >1 post baseline measurement of endometrioma, but these numbers are not stated |
| Selective reporting (reporting bias) | Low risk | primary outcome is change in size of endometrioma, range of other outcomes including ease of surgery and pregnancy reported |
| Other bias | Unclear risk | some differences between the groups at baseline in mean endometrioma size. Difficulty in recruiting patients made trial underpowered, with different numbers in each group |
Telimaa 1987.
| Methods | Location: University of Oulu, Finland
No. of centres: 1 Recruitment period: not stated |
|
| Participants | Inclusion criteria: advanced endometriosis Exclusion criteria: NS No. randomised: 60 No. analysed: 51 (pain), 59 (pregnancy) | |
| Interventions | Post‐surgical medical therapy Gr A (n = 20): danazol oral 600 mg daily x 180 days Gr B (n = 20): MPA 100 mg daily x 180 days Gr C (n=20): placebo | |
| Outcomes | Pain scores AFS scores Pregnancy rates Patient satisfaction Adverse drug reactions: weight gain, breakthrough bleeding, acne | |
| Notes | Power calculation: NS Funding: Research and Science Foundation Farmos Ltd, Turku; Cultural Foundation of Keski‐Pohjanmaa, Finland; Farmos Group, Turku, Finland supplied drugs | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "randomized" no information on method of sequence generation |
| Allocation concealment (selection bias) | Unclear risk | not mentioned |
| Blinding (performance bias and detection bias) All outcomes | Low risk | double blind |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Numbers and reasons for post randomisation exclusions similar in each group. 3,2,3 excluded in groups A,B & C due to pregnancy. 1 adverse event in placebo group |
| Selective reporting (reporting bias) | Low risk | important outcomes of pregnancy recurrence and pain reported |
| Other bias | Low risk | groups appear comparable at baseline and authors state that" no other medication was used during the trial" |
Tsai 2004.
| Methods | Location: Taiwan
No. of centres: one Recruitment period: June 1988 to December 2001 |
|
| Participants | Inclusion criteria: women of reproductive age with infertility and stage III or IV endometriosis planning to undergo controlled ovarian hyperstimulation and intrauterine insemination or in vitro fertilisation and embryo transfer. All had surgery for endometriosis ‐ either laparotomy or laparoscopy for cystectomy, adhesiolysis, ablation of endometriosis Exclusion criteria: NS No. randomised: 45 No. analysed: 41 | |
| Interventions | Post‐operative medical therapy (either danazol or GNRH analogue) Gr A (n=15 ): either 3 months 400 mg danazol orally, twice daily for 3 months or 3.75 mg leuprolide acetate depot SC every 28 days for 3 months Gr B (n= 30): no post‐operative medical treatment | |
| Outcomes | Pregnancy rate, recurrence | |
| Notes | Power calculation: NS Funding: NS | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "simple randomisation with a computer generated list unknown to physicians" |
| Allocation concealment (selection bias) | Low risk | list "unknown to physicians" |
| Blinding (performance bias and detection bias) All outcomes | High risk | not mentioned, no placebo |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 4 lost to follow up from Gr A (27%) |
| Selective reporting (reporting bias) | Low risk | pregnancy and recurrence reported |
| Other bias | Unclear risk | 13 years of recruitment ‐ ? associated changes in surgical techniques over this time |
Vercellini 1999.
| Methods | Location: Italy No. of centres: 19 Recruitment period: February 1992 to June 1994 |
|
| Participants | Inclusion criteria: pre‐menopausal, endometriosis score >/= 4 points, chronic pelvic pain Exclusion criteria: NS No. randomised: 269 No. analysed: 210 | |
| Interventions | Post‐surgical medical therapy Gr A (n= 133): goserelin SC 3.6 mg every 4 weeks x 6 months Gr B (n=134): no treatment | |
| Outcomes | Pain recurrence Pregnancy rates | |
| Notes | Power calculation: Yes Funding: Zeneca Pharmaceuticals provided drugs and financial support | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "randomised in a proportion of 1:1 ... in accordance with a computer‐generated randomisation sequence" |
| Allocation concealment (selection bias) | Low risk | centralised randomisation, allocation obtained by phone call |
| Blinding (performance bias and detection bias) All outcomes | High risk | not mentioned, no placebo |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 269 patients randomised, 2 excluded because case record forms not completed, 26 & 31patients (22%) withdrew from treatment and control groups respectively for reasons other than symptom recurrence or were excluded due to major protocol violations. Reasons for exclusion similar in each group‐ may have introduced bias |
| Selective reporting (reporting bias) | Low risk | important outcomes of recurrence, dysmenorrhoea and pregnancy reported |
| Other bias | Low risk | groups appear comparable at baseline |
Yang 2006.
| Methods | Location: Harbin, China
No. of centres: one Recruitment period:March 2002 to March 2004 |
|
| Participants | Inclusion criteria: women with stage 3 endometriosis who had undergone conservative or semi‐conservative surgery, with normal renal function and blood count, aged 23 to 42 Exclusion criteria: history of hypertension, heart disease, diabetes mellitis, had undergone hormone therapy in 6 months prior to surgery No. randomised: 52 No. analysed: unclear | |
| Interventions | Post‐operative
Gr A (n=20): yiweining (YWN) 200 ml orally twice daily, for 3 months, starting on 7th post‐operative day
Gr B (n=19): gestrinone 2.5 mg twice weekly, SC? for 3‐6 months, starting on 7th post‐operative day Gr C (n=13): control ‐ no treatment |
|
| Outcomes | Pregnancy, recurrence of endometriosis, adverse effects | |
| Notes | Power calculation: not stated
Funding: Fund of National Science of Heilongjiang Province Email sent to corresponding author requesting additional information April 2010 ‐ mailbox not found |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "randomly divided" ‐ no details of method of sequence generation given |
| Allocation concealment (selection bias) | Unclear risk | not mentioned |
| Blinding (performance bias and detection bias) All outcomes | High risk | not mentioned, no placebo used |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | not clear how many of the randomised patients are included in the outcomes (% only given) |
| Selective reporting (reporting bias) | Unclear risk | pregnancy and recurrence reported ‐ no details as criteria for measuring these |
| Other bias | Low risk | groups are similar in age and type of surgery at baseline |
NS = not stated IM = intramuscular
SC = subcutaneous
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Morgante 1999 | This trial was excluded because all the patient received triptorelin for 6 months post‐surgery before randomisation to danazol or no treatment. |
| Schindler 1998 | This is a prospective multi centre phase three study published in German. Preliminary translation suggests however that treatment was not randomly assigned. |
| Vercellini 2003 | This is a pilot study using the levonorgestrel‐releasing intrauterine system for treatment of endometriosis post‐surgery. This is a locally effective hormonal suppressive therapy and has low systemic effects. |
| Ylanen 2003 | This is a dose finding study with no comparison of treatment modality with placebo or no treatment. |
Characteristics of studies awaiting assessment [ordered by study ID]
Shawki 2002.
| Methods | Location: Egypt Recruitment: February 1999 to March 2000 |
| Participants | 68 women with stage I or II endometriosis, subfertility, failed clomiphene citrate stimulation, known tubal patency and partner with fertile semen analysis |
| Interventions | Post‐operative goserelin 3.6 mg injection every 28 days for 6 months versus no medical therapy, followed by 3 months of no treatment. If no pregnancy occurs then 2 cycles of clomiphene citrate stimulation follow |
| Outcomes | Pregnancy rate (spontaneous or following stimulation) |
| Notes | Published abstract only. Outcomes expressed as percentage only, no details of randomisation, allocation concealment, withdrawals, duration of follow up. Emailed author requesting more information (February and March 2010). No reply received |
Differences between protocol and review
Clarifications to the original protocol
We consider that levonorgestrel‐releasing intrauterine devices do not meet the inclusion requirement for systemic hormonal suppression and we therefore excluded the trial by Vercellini 2003.
Quality assessment of included studies has been updated as 'Assessment of risk of bias of included studies' in line with the latest version of the Cochrane Handbook (Higgins 2009). Additional headings available in RevMan 5 have been utilised to make the structure of the Methods, Results and Discussion sections of the review clearer.
It was planned to undertake sensitivity analysis in this update to investigate whether the conclusions would differ if analysis was restricted to trials with low risk of bias.
Pentoxyfylline is a medical therapy for endometriosis which is evaluated in a separate systematic review (Lv 2009).
Minor changes were made to the format of the objectives of this review ‐ the hypotheses were deleted from the updated review.
Contributions of authors
Christine Yap: took the lead in writing the protocol, developed objectives, selection criteria, methods, background, results and discussion. Sue Furness: contributed to the background, search strategy, data extraction, risk of bias, analysis, results and discussion of the review and this update. Cindy Farquhar: initiated and conceptualised the protocol, assisted in resolution of issues raised during the preparation of the original review, contributed to the updated review.
Ying Cheong: contributed to the data extraction, risk of bias, analysis, results and discussion of the updated review.
Sources of support
Internal sources
Singhealth Research, Singapore General Hospital, Singapore.
External sources
No sources of support supplied
Declarations of interest
None known
Edited (no change to conclusions)
References
References to studies included in this review
Audebert 1998 {published data only}
- Audebert A, Descamps P, Marret H, Ory‐Lavollee L, Bailleul F, Hamamah S. Pre or post‐operative medical treatment with nafarelin in stage III‐IV endometriosis: a French multicenter study. Obstetrics and Gynecology 1998;79:145‐8. [DOI] [PubMed] [Google Scholar]
Batioglu 1997 {published data only}
- Batioglu S, Haberai A, Celikkanat H. Comparison of GnRH agonist administration before and after laparoscopic drainage of endometriomas. Journal of Gynecologic Surgery 1997;13(1):17‐21. [Google Scholar]
Bianchi 1999 {published data only}
- Bianchi S, Busacca M, Agnoli B, Candiani M, Calia C, Vignali M. Effects of 3 month therapy with danazol after laparoscopic surgery for stage III/IV endometriosis: a randomized study. Human Reproduction 1999;14(5):1335‐7. [DOI] [PubMed] [Google Scholar]
Busacca 2001 {published data only}
- Busacca M, Somigliana E, Bianchi S, Marinis SD, Calia C, Candiani M, et al. Post‐operative GnRH analogue treatment after conservative surgery for symptomatic endometriosis stage III‐IV: a randomized controlled trial. Human Reproduction 2001;16(11):2399‐402. [DOI] [PubMed] [Google Scholar]
Donnez 1994 {published data only}
- Donnez J, Anaf V, Nisolle M, Clerckx‐Braun F, Gillerot S, Casanas‐Roux F. Ovarian endometrial cysts : the role of gonadotropin‐releasing hormone agonist and/or drainage. Fertility and Sterility 1994;62(1):63‐6. [DOI] [PubMed] [Google Scholar]
Hornstein 1997 {published data only}
- Hornstein MD, Hemmings R, Yuzpe AA, Heinrichs WL. Useo o nafarelin versus placebo after reductive laparoscopic surgery for endometriosis. Fertility and Sterility 1997;68(5):860‐4. [DOI] [PubMed] [Google Scholar]
Loverro 2001 {published data only}
- Loverro G, Santillo V, Pansini MV, Lorusso F, Depalo R, Selvaggi L. Are GnRH agonists helpful in the therapy of endometriosis after surgical treatment?. Human Reproduction 2001;16 Suppl(1):96. [Google Scholar]
Loverro 2008 {published data only}
- Loverro G, Carriero C, Rossi AC, Putignano G, Nicolardi V, Selvaggi L. A randomized study comparing triptorelin or expectant management following conservative laparoscopic surgery for symptomatic stage III‐IV endometriosis. European Journal of Obstetrics, Gynecology, and Reproductive Biology 2008; Vol. 136, issue 2:194‐8. [DOI] [PubMed]
Muzii 2000 {published data only}
- Muzii L, Marana R, Caruana P, Catalano GF, Margutti F, Panici PB. Postoperative administration of monophasic combined oral contraceptives after laparoscopic treatment of ovarian endometriomas: a prospective, randomized trial. American Journal of Obstetrics and Gynecology 1999;183(3):588‐92. [DOI] [PubMed] [Google Scholar]
Parazzini 1994 {published data only}
- Parazzini F, Fedele L, Busacca M, Falsetti L, Pellegrini S, Venturini PL, et al. Postsurgical medical treatment of advanced endometriosis: Results of a randomized clinical trial. American Journal of Obstetrics and Gynecology 1994;171:1205‐7. [DOI] [PubMed] [Google Scholar]
Sesti 2007 {published data only}
- Sesti F, Pietropolli A, Capozzolo T, Broccoli P, Pierangeli S, Bollea MR, et al. Hormonal suppression treatment or dietary therapy versus placebo in the control of painful symptoms after conservative surgery for endometriosis stage III‐IV. A randomized comparative trial. Fertility and Sterility 2007; Vol. 88, issue 6:1541‐7. [DOI] [PubMed]
Shaw 2001 {published data only}
- Shaw R, Garry R, McMillan L, Sutton C, Wood S, Harrison R, Das R. A prospective randomized open study comparing goserelin (Zoladex) plus surgery and surgery alone in the management of ovarian endometriomas. Gynaecological Endoscopy 2001;10:151‐7. [Google Scholar]
Telimaa 1987 {published data only}
- Telimaa S, Ronnberg L, Kauppila A. Placebo‐controlled comparison of danazol and high‐dose medroxyprogesterone acetate in the treatment of endometriosis after conservative surgery. Gynecological Endocrinology 1987;1(4):363‐71. [DOI] [PubMed] [Google Scholar]
Tsai 2004 {published data only}
- Tsai Y‐L, Hwang J‐L, Loo T‐C, Cheng W‐C, Chuang J, Seow K‐M. Short‐term postoperative GnRH analogue or danazol treatment after conservative surgery for stage III or IV endometriosis before ovarian stimulation: a prospective, randomized study. Journal of Reproductive Medicine 2004; Vol. 49, issue 12:955‐9. [PubMed]
Vercellini 1999 {published data only}
- Vercellini P, Crosignani PG, Fadini R, Radici E, Belloni C, Sismondi P. A gonadotrophin‐releasing hormone agonist compared with expectant management after conservative surgery for symptomatic endometriosis. British Journal of Obstetrics and Gynaecology 1999;106:672‐7. [DOI] [PubMed] [Google Scholar]
Yang 2006 {published data only (unpublished sought but not used)}
- Yang D, Ma W, Qu F, MA B. Comparative study on the efficiency of yiweining and gestrinone for post‐operational treatment of stage III endometriosis. Chinese Journal of Integrative Medicine 2006;12(3):218‐20. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Morgante 1999 {published data only}
- Morgante G, Ditto A, Marca AL, Leo VD. Low‐dose danazol after combined surgucal and medical therapy reduces the incidence of pelvic pain in women with moderate and severe endometriosis. Human Reproduction 1999;14(9):2371‐4. [DOI] [PubMed] [Google Scholar]
Schindler 1998 {published data only}
- Schindler AE, Buhler K, Lubben G, Kienle E. Managment of endometriosis through a combined medical‐surgical approach [Was leistet die kombinierte chirurgisch‐hormonell Therapie zum Management der Endometriose]. Zentralblatt fur Gynakologie 1998;120:183‐90. [PubMed] [Google Scholar]
Vercellini 2003 {published data only}
- Vercellini P, Frontino G, Giorgi OD, Aimi G, Zaina B, Crosignani PG. Comparison of a levonorgestrel‐releasing intrauterine device versus expectant management after conservative surgery for symptomatic endometriosis: a pilot study. Fertility and Sterility 2003;80(2):305‐9. [DOI] [PubMed] [Google Scholar]
Ylanen 2003 {published data only}
- Ylanen K, Laatikainen T, Lahteenmaki P, Moo‐Young AJ. Subdermal progestin implant (Nestorone) in the treatment of endometriosis: clinical response to various doses. Acta Obstetricia et Gynecologica Scandinavica 2003;82:167‐72. [PubMed] [Google Scholar]
References to studies awaiting assessment
Shawki 2002 {published data only (unpublished sought but not used)}
- Shawki O, Hamza H, Sattar M. Mild endometriosis, to treat or not treat: randomized controlled trial comparing diagnostic laparoscopy with no further treatment versus post operative Zoladex in cases with infertility associated with Stage I, II endometriosis. Fertility and Sterility 2002;77 Suppl 1:13. [Google Scholar]
Additional references
AFS 1985
- American Fertility Society. Revised American Fertility Society classification of endometriosis. Fertility & Sterility 1985;43:351‐2. [DOI] [PubMed] [Google Scholar]
ASRM 1997
- American Society for Reproductive Medicine. Revised American Society for Reproductive Medicine classification of endometriosis. Fertility and Sterility 1997;67:817‐21. [DOI] [PubMed] [Google Scholar]
Barbieri 1990
- Barbieri RL. Endometriosis 1990. Current treatment approaches. Drugs 1990;39(4):502‐10. [DOI] [PubMed] [Google Scholar]
Cook 1991
- Cook FS, Rock JA. The role of laparoscopy in the treatment of endometriosis. Fertility and Stertility 1991;55:663‐80. [DOI] [PubMed] [Google Scholar]
Donnez 1987
- Donnez J, Lemaire‐Rubbers M, Karaman Y, Nisolle‐Pochet M, Casanas‐Roux F. Combined (hormonal and microsurgical) therapy in infertilie women with endometriosis. Fertility and Sterility 1987;48(2):239‐42. [DOI] [PubMed] [Google Scholar]
Guzick 1989
- Guzick DS. Clinical epidemiology of endometriosis and infertility. Obstetrics and Gynecology Clinics of North America 1989;16:43‐59. [PubMed] [Google Scholar]
Hemmings 1998
- Hemmings R. Combined treatment of endometriosis, GnRH agonists and laparoscopic surgery. Journal of Reproductive Medicine 1998;43 Suppl(2):316‐20. [PubMed] [Google Scholar]
Higgins 2009
- Higgins JPT, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions Version 5.0.2 [updated September 2009]. The Cochrane Collaboration, 2009. [Google Scholar]
Kettel 1989
- Kettel LM, Murphy AA. Combination medical and surgical therapy for infertile patients with endometriosis. Obstetrics and Gynecology Clinics of North America 1989;16(1):167‐77. [PubMed] [Google Scholar]
Lv 2009
- Lv D, Song H, Li Y, Clarke J, Shi G. Pentoxifylline versus medical therapies for subfertile women with endometriosis. Cochrane Database of Systematic Reviews 2009, Issue 2. [DOI: 10.1002/14651858.] [DOI] [PubMed] [Google Scholar]
Muzii 1996
- Muzii L, Marana R, Caruana P, Mancuso S. The impact of preoperative gonadotropin‐releasing hormone agonist treatment on laparoscopic excision of ovarian endometriotic cysts. Fertility and Sterility 1996;65(6):1235‐7. [DOI] [PubMed] [Google Scholar]
Ozkan 2008
- Ozkan S, Murk W, Arici A. Endometriosis and infertility: epidemiology and evidence‐based treatments. Annals of New York Academy of Science 2008;1127:92‐100. [DOI] [PubMed] [Google Scholar]
Proctor 1999
- Proctor ML, Farquhar CM, Sinclair OJ, Johnson NP. Surgical interruption of pelvic nerve pathways for primary and secondary dysmenorrhoea. Cochrane Database of Systematic Reviews 2004, Issue 1. [DOI: 10.1002/14651858] [DOI] [PubMed] [Google Scholar]
Rana 1996
- Rana N, Thomas S, Rotman C, Dmowski WP. Decrease in the size of ovarian and endometriomas during ovarian suppression in stage IV endometriosis. Role of preoperative medical treatment. Journal of Reproductive Medicine 1996;41(6):384‐92. [PubMed] [Google Scholar]
Shaw 1992
- Shaw RW. Treatment of endometriosis. Lancet 1992;340(8830):1267‐71. [DOI] [PubMed] [Google Scholar]
Thomas 1992
- Thomas EJ. Combining medical and surgical treatment for endometriosis: the best of both worlds?. British Journal of Obstetrics and Gynaecology 1992;99 Suppl 7:5‐8. [DOI] [PubMed] [Google Scholar]
Vercellini 1996
- Vercellini P, Trespidi L, Giorgi O, Cortesi I, Parazzini F, Crosignani PG. Endometriosis and pelvic pain: relation to disease stage and localization. Fertility and Sterility 1996;65(2):299‐304. [PubMed] [Google Scholar]


