Notes
Editorial note
This Cochrane Review has been superseded by Interventions for myopia control in children: a living systematic review and network meta‐analysis (https://doi.org/10.1002/14651858.CD014758).
Abstract
Background
Nearsightedness (myopia) causes blurry vision when one is looking at distant objects. Interventions to slow the progression of myopia in children include multifocal spectacles, contact lenses, and pharmaceutical agents.
Objectives
To assess the effects of interventions, including spectacles, contact lenses, and pharmaceutical agents in slowing myopia progression in children.
Search methods
We searched CENTRAL; Ovid MEDLINE; Embase.com; PubMed; the LILACS Database; and two trial registrations up to February 2018. A top up search was done in February 2019.
Selection criteria
We included randomized controlled trials (RCTs). We excluded studies when most participants were older than 18 years at baseline. We also excluded studies when participants had less than ‐0.25 diopters (D) spherical equivalent myopia.
Data collection and analysis
We followed standard Cochrane methods.
Main results
We included 41 studies (6772 participants). Twenty‐one studies contributed data to at least one meta‐analysis. Interventions included spectacles, contact lenses, pharmaceutical agents, and combination treatments. Most studies were conducted in Asia or in the United States. Except one, all studies included children 18 years or younger. Many studies were at high risk of performance and attrition bias.
Spectacle lenses: undercorrection of myopia increased myopia progression slightly in two studies; children whose vision was undercorrected progressed on average ‐0.15 D (95% confidence interval [CI] ‐0.29 to 0.00; n = 142; low‐certainty evidence) more than those wearing fully corrected single vision lenses (SVLs). In one study, axial length increased 0.05 mm (95% CI ‐0.01 to 0.11) more in the undercorrected group than in the fully corrected group (n = 94; low‐certainty evidence). Multifocal lenses (bifocal spectacles or progressive addition lenses) yielded small effect in slowing myopia progression; children wearing multifocal lenses progressed on average 0.14 D (95% CI 0.08 to 0.21; n = 1463; moderate‐certainty evidence) less than children wearing SVLs. In four studies, axial elongation was less for multifocal lens wearers than for SVL wearers (‐0.06 mm, 95% CI ‐0.09 to ‐0.04; n = 896; moderate‐certainty evidence). Three studies evaluating different peripheral plus spectacle lenses versus SVLs reported inconsistent results for refractive error and axial length outcomes (n = 597; low‐certainty evidence).
Contact lenses: there may be little or no difference between vision of children wearing bifocal soft contact lenses (SCLs) and children wearing single vision SCLs (mean difference (MD) 0.20D, 95% CI ‐0.06 to 0.47; n = 300; low‐certainty evidence). Axial elongation was less for bifocal SCL wearers than for single vision SCL wearers (MD ‐0.11 mm, 95% CI ‐0.14 to ‐0.08; n = 300; low‐certainty evidence). Two studies investigating rigid gas permeable contact lenses (RGPCLs) showed inconsistent results in myopia progression; these two studies also found no evidence of difference in axial elongation (MD 0.02mm, 95% CI ‐0.05 to 0.10; n = 415; very low‐certainty evidence). Orthokeratology contact lenses were more effective than SVLs in slowing axial elongation (MD ‐0.28 mm, 95% CI ‐0.38 to ‐0.19; n = 106; moderate‐certainty evidence). Two studies comparing spherical aberration SCLs with single vision SCLs reported no difference in myopia progression nor in axial length (n = 209; low‐certainty evidence).
Pharmaceutical agents: at one year, children receiving atropine eye drops (3 studies; n = 629), pirenzepine gel (2 studies; n = 326), or cyclopentolate eye drops (1 study; n = 64) showed significantly less myopic progression compared with children receiving placebo: MD 1.00 D (95% CI 0.93 to 1.07), 0.31 D (95% CI 0.17 to 0.44), and 0.34 (95% CI 0.08 to 0.60), respectively (moderate‐certainty evidence). Axial elongation was less for children treated with atropine (MD ‐0.35 mm, 95% CI ‐0.38 to ‐0.31; n = 502) and pirenzepine (MD ‐0.13 mm, 95% CI ‐0.14 to ‐0.12; n = 326) than for those treated with placebo (moderate‐certainty evidence) in two studies. Another study showed favorable results for three different doses of atropine eye drops compared with tropicamide eye drops (MD 0.78 D, 95% CI 0.49 to 1.07 for 0.1% atropine; MD 0.81 D, 95% CI 0.57 to 1.05 for 0.25% atropine; and MD 1.01 D, 95% CI 0.74 to 1.28 for 0.5% atropine; n = 196; low‐certainty evidence) but did not report axial length. Systemic 7‐methylxanthine had little to no effect on myopic progression (MD 0.07 D, 95% CI ‐0.09 to 0.24) nor on axial elongation (MD ‐0.03 mm, 95% CI ‐0.10 to 0.03) compared with placebo in one study (n = 77; moderate‐certainty evidence). One study did not find slowed myopia progression when comparing timolol eye drops with no drops (MD ‐0.05 D, 95% CI ‐0.21 to 0.11; n = 95; low‐certainty evidence).
Combinations of interventions: two studies found that children treated with atropine plus multifocal spectacles progressed 0.78 D (95% CI 0.54 to 1.02) less than children treated with placebo plus SVLs (n = 191; moderate‐certainty evidence). One study reported ‐0.37 mm (95% CI ‐0.47 to ‐0.27) axial elongation for atropine and multifocal spectacles when compared with placebo plus SVLs (n = 127; moderate‐certainty evidence). Compared with children treated with cyclopentolate plus SVLs, those treated with atropine plus multifocal spectacles progressed 0.36 D less (95% CI 0.11 to 0.61; n = 64; moderate‐certainty evidence). Bifocal spectacles showed small or negligible effect compared with SVLs plus timolol drops in one study (MD 0.19 D, 95% CI 0.06 to 0.32; n = 97; moderate‐certainty evidence). One study comparing tropicamide plus bifocal spectacles versus SVLs reported no statistically significant differences between groups without quantitative results.
No serious adverse events were reported across all interventions. Participants receiving antimuscarinic topical medications were more likely to experience accommodation difficulties (Risk Ratio [RR] 9.05, 95% CI 4.09 to 20.01) and papillae and follicles (RR 3.22, 95% CI 2.11 to 4.90) than participants receiving placebo (n=387; moderate‐certainty evidence).
Authors' conclusions
Antimuscarinic topical medication is effective in slowing myopia progression in children. Multifocal lenses, either spectacles or contact lenses, may also confer a small benefit. Orthokeratology contact lenses, although not intended to modify refractive error, were more effective than SVLs in slowing axial elongation. We found only low or very low‐certainty evidence to support RGPCLs and sperical aberration SCLs.
Plain language summary
Interventions to slow progression of nearsightedness in children
What was the aim of this review? To find out if there are treatments that can slow the progress of nearsightedness (myopia) in children. Myopia is a vision condition in which people can see close objects clearly, but objects farther away appear blurred.
Key message Eye drop medication, such as atropine, probably slows myopia progression in children. Children taking these eye drops may have blurred near vision, sensitivity to light, and some itching and discomfort. Multifocal lenses, either spectacles or contact lenses, may also confer a small benefit.
What did we study in this review? During childhood and adolescence, the eyeballs can grow too long and can develop myopia. Treatments can slow growth of the eye, thereby slowing down the progression of myopia.
Cochrane researchers assessed how certain the evidence was for each review finding, factoring in problems such as the ways studies were done, inclusion of very small studies, and inconsistent findings across studies. They also looked for factors that can make the evidence more certain, including very large effects. They graded each finding as very low, low, moderate, or high certainty.
What were the main results of this review? Cochrane researchers found 41 studies of treatments to slow myopia progression. These studies included a total of 6772 children. The review found that the following treatments may slow the progression of myopia, compared with wearing ordinary spectacles.
• Eye drops, in particular antimuscarinic drugs such as atropine, pirenzepine gel, and cyclopentolate (moderate‐certainty evidence).
• Multifocal spectacles (either bifocal or progressive addition lenses) (moderate‐certainty evidence).
• Bifocal soft contact lenses (low‐certainty evidence).
• Orthokeratology contact lenses (moderate‐certainty evidence).
• Combinations of eye drops and multifocal spectacles (moderate‐certainty evidence).
The review found that the following treatments may have a small effect, or no effect, on myopia progression.
• Spherical aberration soft contact lenses (low‐certainty evidence).
• Systematic adenosine antagonists (moderate‐certainty evidence).
Children who wear undercorrected spectacles may have an increased chance of myopia progression compared with children who wear fully corrected spectacles (low‐certainty evidence). Only very low‐certainty evidence on rigid gas permeable contact lenses was available.
Antimuscarinic eye drops may result in blurred near vision, sensitivity to light, some discomfort and itching, and medication residue on the eyelids or eyelashes. Some children may develop small nodules or bumps under the eyelid. Spectacles and contact lenses, if used properly, are safe and effective.
How up‐to‐date is the review? Cochrane researchers reviewed studies published up to February 2018.
Summary of findings
Summary of findings 1. Interventions to slow progression of myopia in children.
| Interventions to slow progression of myopia in children | ||||
|
Population: children with myopia (nearsightedness) Settings: ophthalmology or optometry clinics | ||||
| Outcome: change in refractive error, measured in diopters (D), from baseline to 1‐year follow‐up | ||||
|
Comparison (intervention vs comparator) |
Mean difference (95% CI) Positive values represent slower progression of myopia in the treatment group than in the comparison group | No. of participants (studies) | Certainty of the evidence (GRADE) | Comments |
| Undercorrected vs fully corrected spectacles | ‐0.15 D (‐0.29 to 0.00) | 142 (2) | ⊕⊕⊝⊝ lowa,b | A third study did not report this outcome at 1 year |
| Multifocal vs single vision lens spectacles | 0.14 D (0.08 to 0.21) | 1463 (9) | ⊕⊕⊕⊝ moderateb | Five studies not included in the meta‐analyses also showed mostly favorable effects of multifocal lenses for slowing myopia progression |
| Peripheral plus spectacles vs single vision lens spectacles | See comment | 597 (3) | ⊕⊕⊝⊝ lowb,c | No meta‐analysis was conducted because of clinical and methodological heterogeneity among the 3 studies; furthermore, the results from these studies were inconsistent |
| Bifocal vs single vision soft contact lenses | 0.20 D (‐0.06 to 0.47) | 300 (4) | ⊕⊕⊝⊝ lowb,c | ‐ |
| Rigid gas permeable contact lenses vs spectacles or soft contact lenses | See comment | 420 (2) | ⊕⊝⊝⊝ very lowa,b,c | No meta‐analysis was conducted due to differences among 2 studies that reported inconsistent results |
| Orthokeratology contact lenses vs single vision lenses | See comment | ‐ | ‐ | Because orthokeratology contact lenses temporarily reduce myopia, their myopia control treatment effect can be measured only by axial elongation. We did not analyze the changes in refractive error for this comparison |
| Spherical aberration soft contact lenses vs single vision soft contact lenses | See comment | 209 (2) | ⊕⊕⊝⊝ lowb,d | No meta‐analysis was conducted because 1 of the studies did not provide effect estimates; however, 2 studies comparing spherical aberration SCLs with single vision SCLs reported no difference in myopia progression |
| Antimuscarinic agents vs placebo | Atropine: 1.00 D (0.93 to 1.07) Pirenzepine: 0.31 D (0.17 to 0.44) Cyclopentolate: 0.34 D (0.08 to 0.60) | 629 (3) 326 (2) 64 (1) | ⊕⊕⊕⊝ moderateb | We stratified the analysis by types of antimuscarinic agents due to statistical inconsistency |
| Atropine vs tropicamide | Atropine 0.1%: 0.78 D (0.49 to 1.07) Atropine 0.25%: 0.81 D (0.57 to 1.05) Atropine 0.5%: 1.01 D (0.74 to 1.28) |
196 (1) | ⊕⊕⊕⊝ lowb | ‐ |
| Systemic 7‐methylxanthine vs placebo | 0.07 D (‐0.09 to 0.24) | 77 (1) | ⊕⊕⊕⊝ moderatea | ‐ |
| Timolol drops vs no drops | ‐0.05 D (‐0.21 to 0.11) | 95 (1) | ⊕⊕⊝⊝ lowa,b | ‐ |
| Atropine plus multifocal spectacles vs placebo plus SVLs | 0.78 D (0.54 to 1.02) | 191 (2) | ⊕⊕⊕⊝ moderateb | ‐ |
| Atropine plus bifocal spectacles vs cyclopentolate plus SVLs | 0.36 D (0.11 to 0.61) | 64 (1) | ⊕⊕⊕⊝ moderateb | ‐ |
| Bifocal spectacles vs SVLs with timolol drops | 0.19 D (0.06 to 0.32) | 97 (1) | ⊕⊕⊕⊝ moderateb | ‐ |
| Tropicamide plus bifocal spectacles vs SVLs | See comment | 50 (1) | ‐ | No estimate of effect was reported |
| Outcome: change in axial length, measured in millimeters (mm), from baseline to 1‐year follow‐up | ||||
|
Comparison (intervention vs comparator) |
Mean difference (95% CI) Negative values represent less axial elongation in the treatment group than in the comparison group | No. of participants (studies) | Certainty of the evidence (GRADE) | Comments |
| Undercorrected vs fully corrected spectacles | 0.05 mm (‐0.01 to 0.11) | 94 (1) | ⊕⊕⊝⊝ lowa,b | Two studies did not report this outcome at 1 year |
| Multifocal vs single vision lens spectacles | ‐0.06 mm (‐0.09 to ‐0.04) | 896 (4) | ⊕⊕⊕⊝ moderateb | Four studies (not included in the meta‐analysis) showed mostly favorable effects of multifocal lenses and 6 studies did not report this outcome |
| Peripheral plus spectacles vs single vision lens spectacles | See comment | 597 (3) | ⊕⊕⊝⊝ lowb,c | ‐ |
| Bifocal vs single vision soft contact lenses | ‐0.11 mm (‐0.14 to ‐0.08) | 300 (4) | ⊕⊕⊝⊝ lowb,c | ‐ |
| Rigid gas permeable contact lenses vs spectacles or soft contact lenses | 0.02 mm (‐0.05 to 0.10) | 415 (2) | ⊕⊕⊝⊝ lowa,b | ‐ |
| Orthokeratology contact lenses vs single vision lenses | ‐0.28 mm (‐0.38 to ‐0.19) | 106 (2) | ⊕⊕⊕⊝ moderateb | One other study reported this outcome; however, the study did not report sufficient data for analysis |
| Spherical aberration soft contact lenses vs single vision soft contact lenses | See comment | 209 (2) | ⊕⊝⊝⊝ very lowa,b,d | No meta‐analysis was conducted due to clinical, methodological, and statistical differences between the 2 studies; however, 2 studies comparing spherical aberration SCLs with single vision SCLs reported no difference in axial length |
| Antimuscarinic agents vs placebo | Atropine: ‐0.35 mm (‐0.38 to ‐0.31) Pirenzepine: ‐0.13 mm (‐0.14 to ‐0.12) | 502 (2) 326 (2) | ⊕⊕⊕⊝ moderatec | We did not combine results for all antimuscarinic agents due to statistical inconsistency; outcome was not reported by 2 studies |
| Atropine vs tropicamide | See comment | 196 (1) | ‐ | Outcome was not reported |
| Systemic 7‐methylxanthine vs placebo | ‐0.03 mm (‐0.10 to 0.03) | 77 (1) | ⊕⊕⊕⊝ moderatea | ‐ |
| Timolol drops vs no drops | See comment | 95 (1) | ‐ | Outcome was not reported |
| Atropine plus multifocal spectacles vs placebo plus SVLs | ‐0.37 mm (‐0.47 to ‐0.27) | 127 (1) | ⊕⊕⊕⊝ moderateb | One study did not report this outcome |
| Atropine plus bifocal spectacles vs cyclopentolate plus SVLs | See comment | 64 (1) | ‐ | Outcome was not reported |
| Bifocal spectacles vs SVLs with timolol drops | See comment | 97 (1) | ‐ | Outcome was not reported |
| Tropicamide plus bifocal spectacles vs SVLs | See comment | 50 (1) | ‐ | Outcome was not reported |
| Adverse effects | ||||
| No serious adverse events were reported across all interventions. Two studies showed that participants receiving antimuscarinic topical medications (n=259) were more likely to experience accommodation difficulties (Risk Ratio 9.05, 95% CI 4.09 to 20.01), papillae and follicles (RR 3.22, 95% CI 2.11 to 4.90) than participants receiving placebo (n=128), but no difference in medication residue on the eyelids or eyelashes (RR 0.91, 95% CI 0.73 to 1.12). Certainty of a body of evidence was moderate, downgraded for imprecision of results (‐1). | ||||
| GRADE Working Group grades of evidence. High certainty: further research is very unlikely to change our confidence in the estimate of effect. Moderate certainty: further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low certainty: further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low certainty: we are very uncertain about the estimate. | ||||
CI: confidence interval; D: diopters.
aDowngraded for imprecision (i.e. wide confidence interval). bDowngraded for risk of bias among included trials. cDowngraded for inconsistency.
dDowngraded for indirectness due to averaging values over time assuming linear change (e.g. reporting the change per year using data collected at baseline and at 2 years of follow‐up).
Background
Description of the condition
Myopia, also known as nearsightedness, occurs because the cornea or the lens is too powerful or the eyeball is longer than normal; this causes distant objects to be focused in front of the retina instead of on it, as occurs in nonmyopic individuals. In myopia, near objects are seen clearly but distant objects appear blurred.
Epidemiology
Myopia is an important cause of reduced vision in populations throughout the world and is one of the five immediate priorities for the "Vision 2020" initiative of the World Health Organization (WHO) (Pararajasegaram 1998). Approximately 33% of persons in the United States are myopic, reflecting an increase from approximately 25% in the early 1970s (Vitale 2009). It is estimated that half of the world’s population will be myopic by 2050 (Holden 2016). Racial and ethnic differences in the magnitude and prevalence of myopia have been observed (Garner 1999; Lin 1999; Maul 2000; Voo 1998; Zhan 2000), with both greater in Asia than in other parts of the world (Lin 1999; Zhan 2000).
Juvenile‐onset myopia in the United States typically develops at approximately six to eight years of age and progresses at a rate of approximately 0.50 D (diopters) per year through 15 to 16 years (COMET Study 2003; Fulk 2002; Goss 1987; Perrigin 1990). The progression of myopia is typically faster at younger ages (Braun 1996; Goss 1987; Goss 1990; Pärssinen 1989; Saw 2000), but myopia onset, progression, and stabilization vary widely among individuals (Braun 1996; Pärssinen 1989; Saw 2000). Similar proportions of boys and girls are affected by myopia, and the degree of myopia is similar between the two genders (Zadnik 2003).
Etiology and risk factors
Several factors have been suggested to have a role in the development of myopia. Many models estimate greater genetic effects than environmental effects for myopia (Chen 1985; Hammond 2001). Children with two myopic parents have greater axial lengths; this indicates higher risk of myopia than for children with one or no myopic parents (Zadnik 1994). Environmental influences are related to prolonged reading or near work, which has inconsistently been associated with increased myopia prevalence (Saw 2001; Young 1969). Fewer hours spent outdoors has also been associated with myopia (Dirani 2009; Guggenheim 2012; Guo 2013; Jones 2007; Rose 2008). Children randomly assigned to additional outdoor time exhibit a lower incidence of myopia onset but do not exhibit slowed progression of myopia after onset (He 2015; Wu 2010).
Presentation and diagnosis
The primary symptom of myopia is blurred distance vision. Children often present to an eye care practitioner after they have failed a vision screening at school or after a parent or teacher has noticed the child squinting or having difficulty seeing distant objects.
An eye care practitioner using autorefraction or retinoscopy may confirm the diagnosis of myopia objectively, or the practitioner can confirm the diagnosis by performing a subjective refraction, which requires responses from the child. To diagnose myopia in a child, cycloplegic drops should be placed in the child's eyes, hindering his or her ability to focus the eyes, so that an accurate prescription can be determined.
Description of the intervention
Spectacles are often the initial treatment for children with myopia because they provide clear vision with few potential side effects. Spectacles for myopia correction use concave lenses that focus light more posteriorly, resulting in a clear image focused on the retina.
Contact lenses are typically a secondary treatment option for children because they require greater dexterity and responsibility when compared to spectacles. They also bear greater risks than spectacles, which range from innocuous redness of the eyes to severe pain and vision loss due to corneal ulcers (Fonn 1988; MacRae 1991; Schein 1989). However, young children are at lower risk for problems associated with contact lens wear than are college‐age adults (Chalmers 2011; Wagner 2011). There are different types of contact lenses. Soft contact lenses are made of gel‐like, water‐containing, flexible plastics that allow oxygen to pass through the cornea. Spherical aberration soft contact lenses aims to correct an optical problem that occurs when incoming light rays end up focusing at different points after passing through a spherical surface (in this case the ocular system). Rigid gas permeable contact lenses (RGPCLs) are rigid, more durable and less likely to tear compared to soft contact lenses, and resistant to deposit buildup; however, they may be less comfortable to wear initially. Orthokeratology is a lens fitting procedures that uses specially designed RGPCLs to change the curvature of the cornea to temporarily improve the eye's ability to focus on objects. Most orthokeratology lenses are worn at night and then removed during the day. When orthokeratology is discontinued, the cornea will return to its original curvature and the eye to its original amount of nearsightedness.
Lastly, both spectacles and contact lenses can contain more than one power zone; they are called bifocal, multifocal, or progressive addition lenses.
There are currently no pharmaceutical agents approved by the US Food and Drug Administration for use as myopia treatments, although antimuscarinic agents, such as atropine, pirenzepine, tropicamide, and scopolamine, as well as 7‐methylxanthine (7‐mx), a non‐antimuscarinic agent, have been used off‐label and targeted in recent clinical trials.
Laser refractive surgery, such as laser in situ keratomileusis (LASIK) or photorefractive keratectomy (PRK), causes permanent flattening of the central corneal curvature resulting from removal of stromal tissue with a laser once myopia has developed (Duffey 2003; Shortt 2006), but it is not routinely performed in children.
Other forms of myopia correction, such as placement of a lens inside the eye and clear rings into the cornea, also are not used routinely in children because of the risk of potential myopia progression (Barsam 2010).
How the intervention might work
In terms of slowing myopia progression, use of multifocal spectacles and undercorrection of myopic refractive error are thought to reduce accommodative error, which may act as a stimulus for increased eye growth. Myopic patients exhibit greater accommodative lag than nonmyopic patients (COMET Study 2003; Mutti 2006). Accommodative lag results in light focused behind the retina during near work, which may act as a signal to increase eye growth and may result in myopia. If the accommodative error can be reduced with bifocals or myopic undercorrection, then the stimulus for eye growth will be reduced, and this may slow myopia progression.
Antimuscarinic agents were thought to reduce myopic progression by eliminating accommodation, but this has been shown to be a local retinal effect that slows myopia progression (Troilo 1987). Antimuscarinic receptor binding may lead to a biochemical change that slows eye growth, but the exact mechanism is unknown.
Multifocal contact lenses provide myopic defocus of light in the periphery while allowing clear vision by focusing light on the central retina (Charman 2006; Kang 2011; Moore 2017; Ticak 2013). The myopic defocus (light focused in front of the retina) may act as a signal to slow eye growth and reduce myopia progression (Smith 2009). Orthokeratology works by flattening the center cornea to temporarily improve the eye's ability to focus on objects.
Why it is important to do this review
Myopia has been reported to have reached epidemic proportions in parts of the world (Park 2004). Strategies to control progression of myopia gain importance in the context of the "Vision 2020" initiative by the WHO, which seeks to eliminate preventable causes of blindness, including risks associated with high myopia, by the year 2020 (Pararajasegaram 1998). Interventions that have been explored for this purpose include bifocal spectacles, cycloplegic eye drops, intraocular pressure–lowering drugs, muscarinic receptor antagonists, and contact lenses. In this review, we systematically assessed the effectiveness of strategies to control progression of myopia in children.
Objectives
To assess the effects of interventions, including spectacles, contact lenses, and pharmaceutical agents, such as muscarinic receptor antagonists, cycloplegic eye drops, and intraocular pressure–lowering medications, in slowing myopia progression in children.
Methods
Criteria for considering studies for this review
Types of studies
This review included randomized controlled trials (RCTs).
Types of participants
We included trials in which participants were treated with spectacles, contact lenses, or pharmaceutical agents for controlling progression of myopia. We excluded trials in which most participants were older than 18 years at the start of the trial. We also excluded trials that included participants with less than ‐0.25 D spherical equivalent myopia at baseline. (The spherical equivalent is an optical measurement based on a mathematical calculation: the sum of the spherical power plus half the cylindrical power of the refractive error.)
Types of interventions
We included trials in which any of the following interventions for slowing the progression of myopia were compared with a control treatment of single vision spectacle lenses, single vision soft contact lenses (SVSCLs), or placebo treatment, or with each other.
Undercorrection of myopia, bifocal lenses (spectacles), progressive addition lenses (PALs), and other modifications to spectacle lenses.
Bifocal soft contact lenses (BSCLs), RGPCLs, and corneal reshaping (orthokeratology) contact lenses.
Pharmaceutical agents (e.g. atropine, pirenzepine).
Types of outcome measures
Primary outcomes
Progression of myopia assessed as the mean change in refractive error (spherical equivalent) from baseline to each year of follow‐up and measured by any method
Secondary outcomes
Mean change in axial length, measured by any method
Mean change in corneal radius of curvature, measured by any method
We analyzed the secondary outcomes for each year of follow‐up when sufficient data were available.
Adverse effects
We summarized reported adverse effects related to the interventions as described in the included studies, including but not limited to blurry vision, red eyes, infection, and conjunctival reactions.
Economic data
We documented reported cost analyses and other data on economic outcomes when reported by the included trials.
Quality of life measures
We documented any quality of life information when reported by the included trials.
Follow‐up
We reported outcomes for follow‐up at one year, at two years, and as available throughout the study periods. We imposed no restrictions based on length of follow‐up.
Search methods for identification of studies
Electronic searches
The Cochrane Eyes and Vision Information Specialist searched the following electronic databases for RCTs and controlled clinical trials, with no language or publication year restrictions up to Febrary 2018 (and all relevant studies up to Febrary 2018 were included in the current version). A top up search was done on February 26, 2019. We listed potentially relevant studies from the top up search in the tables for "Characteristics of studies awaiting classification" and "Characteristics of ongoing studies".
Cochrane Central Register of Controlled Trials (CENTRAL; Issue 2, 2019) (which contains the Cochrane Eyes and Vision Trials Register), in the Cochrane Library (searched February 26, 2019) (Appendix 1).
MEDLINE Ovid (1946 to February 26, 2019) (Appendix 2).
Embase (1947 to February 26, 2019) (Appendix 3).
PubMed (1948 to February 26, 2019) (Appendix 4).
Latin American and Caribbean Health Science Information Database (LILACS) (1982 to February 26, 2019) (Appendix 5).
International Standard Randomized Controlled Trials Number (ISRCTN) registry (www.isrctn.com/editAdvancedSearch; searched February 26, 2019) (Appendix 6).
US National Institutes of Health Ongoing Trials Register ClinicalTrials.gov (www.clinicalTrials.gov; searched February 26, 2019) (Appendix 7).
World Health Organization (WHO) International Clinical Trials Registry Platform (ICTRP) (www.who.int/ictrp; searched February 26, 2019) (Appendix 8).
Searching other resources
We searched the reference lists of identified trial reports to find additional trials. We used the Science Citation Index (last assessed April 12, 2013) to find studies that had cited the identified trials. We contacted the primary investigators of identified trials for details of other potentially relevant trials not identified by the electronic searches, and of recently completed or ongoing trials. We did not conduct manual searches of abstracts of conference proceedings and optometry literature specifically for this review, as these sources are searched by the Cochrane Eyes and Vision Group and are listed in CENTRAL.
Data collection and analysis
Selection of studies
Two review authors, including at least one clinician and one methodologist, independently assessed the titles and abstracts of records identified by electronic and manual searches as per the Criteria for considering studies for this review. We classified records as (1) definitely relevant, (2) possibly relevant, or (3) definitely not relevant. We obtained and assessed the full‐text reports of records classified as (1) or (2) by at least one review author. After assessing the full‐text reports, we classified studies as (A) include, (B) awaiting assessment, or (C) exclude. A third review author resolved disagreements. Review authors were unmasked to report authors, authors' institutions, and trial results during this assessment. We included and further assessed studies identified as (A) for study design and risk of bias. We contacted the authors of studies classified as (B) for clarification and reassessed these studies as per the inclusion criteria, as further information became available. We excluded studies identified as (C) and documented the reasons for exclusion in this review.
We initially included Cheng 2010, but after data extraction and risk of bias assessment, we assessed this study to be quasi‐randomized and thus deemed it ineligible for the review. However, as we initially included the study, we did not exclude it post hoc but instead conducted sensitivity analyses for inadequate randomization when applicable.
Data extraction and management
Two review authors independently extracted the data for primary and secondary outcomes on two paper data collection forms developed by the Cochrane Eyes and Vision Group. We resolved discrepancies by discussion. We contacted primary investigators for data reported unclearly or incompletely. One review author entered the data into Review Manager 5 (RevMan 5) (Review Manager 2014), and a second review author verified the data entered.
Assessment of risk of bias in included studies
Two review authors independently assessed potential sources of bias in trials according to the methods described in Chapter 8 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2017). We resolved disagreements between authors through discussion.
We considered the following parameters.
Selection bias (random sequence generation, quality of allocation concealment).
Performance bias (masking of participants).
Detection bias (masking of outcome assessors and data analyzers).
Attrition bias (completeness of follow‐up, intention‐to‐treat [ITT] analysis).
Reporting bias (selective outcome reporting, incomplete reporting of results).
Other potential sources of bias (e.g. funding source).
For attrition bias, we considered whether or not reasons for losses to follow‐up were comparable between treatment arms, and whether or not all participants were analyzed as randomized. If studies reported that an ITT analysis was performed, we assessed whether (1) all randomized participants were included in the analysis, even when no outcome data were collected, and (2) participants were analyzed in the intervention groups to which they were randomized, regardless of the intervention they actually received. We interpreted a true ITT analysis to have been undertaken only when both of these criteria were fulfilled.
We classified the risk of bias for each parameter as "low risk of bias," "unclear risk of bias," or "high risk of bias." For example, we considered studies using allocation concealment by centralized randomization and use of sequential opaque envelopes (which provided reasonable confidence that participating eye care providers and patients were not aware of the randomization sequence) to be at low risk of bias. We contacted the authors of trials when we needed additional information to assess risk of bias. If trial authors did not respond within an eight‐week period, we classified the trial based on available information.
Measures of treatment effect
We reported mean differences (MDs) for continuous outcome measures and risk ratios (RRs) for dichotomous outcomes.
Unit of analysis issues
When only one eye per participant was randomized, the unit of analysis was the individual eye (and participant). When both eyes from the same participant were randomized (either to the same intervention or to different interventions), we used estimates that had accounted for the correlation between the two eyes. For cross‐over design and cluster‐randomized design, we analyzed only estimates that had accounted for the design.
Dealing with missing data
We contacted the authors of trial reports for any missing data. When we did not receive a response within eight weeks, we analyzed the studies based on available information. We will include any new information in future updates of the review.
Assessment of heterogeneity
We assessed methodological and clinical heterogeneity by examining the characteristics and design of included studies. We assessed statistical heterogeneity by using the Chi² test and the I² statistic. We considered a P value less than 0.1 as significant for the test of heterogeneity. We assessed the inconsistency of effect estimates across studies using the I² statistic. An I² value greater than 50% was an indication of substantial statistical heterogeneity.
Assessment of reporting biases
We assessed reporting biases based on communications with trial authors regarding any outcomes assessed but not reported.
Data synthesis
We used a fixed‐effect model for meta‐analyses including fewer than three studies, and a random‐effects model for meta‐analyses including three or more studies. Change‐from‐baseline data were combined in meta‐analyses with mean outcome data at annual measurement time points based on the generic inverse variance (unstandardized) MD method, as outlined in Chapter 9 of the Cochrane Handbook for Systematic Reviews of Interventions (Deeks 2017). When we assessed substantial clinical, methodological, or statistical heterogeneity, we did not combine individual trials in meta‐analysis but instead reported study results separately.
Subgroup analysis and investigation of heterogeneity
We undertook subgroup analyses for types of intervention modalities (i.e. bifocals, PALs, and specific pharmaceutical agents). In the future, if sufficient evidence becomes available, we will also conduct subgroup analyses according to age, degree of myopia at baseline, and type of contact lens (soft vs rigid gas permeable).
Sensitivity analysis
We conducted a sensitivity analysis for meta‐analyses in which more than three studies were included and when change‐from‐baseline outcomes were combined in analysis with mean outcomes at annual measurement time points. We combined studies using autorefraction in analysis with subjective refraction or when analyses included the Cheng 2010 study.
"Summary of findings"
We prepared a "Summary of findings" table including all comparisons for each of the following outcomes: change in refractive error, change in axial length, change in corneal curvature, and adverse effects. Additionally, we presented adverse effects by intervention in the Additional tables section, as the data were insufficient for quantitative analysis. We used the GRADE approach to assess the overall certainty of evidence for each outcome based on five criteria: risk of bias, imprecision, inconsistency, indirectness, and publication bias (Guyatt 2011).
Results
Description of studies
Results of the search
Details of results of the 2011 version of this review were published previously (Walline 2011). Briefly, we included 89 records (from 23 studies), excluded 82 records (from 61 studies), identified four records awaiting classification (from three studies: Anstice 2011; ATOM 2 Study 2012; COMET2 Study 2011), and identified one ongoing study (STAMP Study 2012).
In February 2018, we conducted an update of the electronic literature search, handsearched the reference lists of included studies, and used the Science Citation Index to identify additional studies. We identified 4064 additional records, 10 of which we identified by manual searching. After omitting duplicates and screening 4052 titles and abstracts, we excluded 3678 records and obtained full‐text reports of 374 records for further review. Upon full‐text review, we excluded 127 reports. Of them, six excluded reports belonged to a study previously assessed as awaiting classification because it did not include a single vision control group (ATOM 2 Study 2012). We also identified 66 reports for studies listed as ongoing. We listed seven reports as awaiting classification. We included the remaining 174 reports: 27 reported 15 newly included studies (Cambridge Anti‐Myopia Study 2013; Charm 2013; Cheng 2016; DISC Study 2011; Fujikado 2014; Han 2018; Hasebe 2014; Koomson 2016; Lu 2015; ROMIO Study 2012; Swarbrick 2015; Trier 2008; Wang 2005; Wang 2017; Yi 2015), six reported results for the previously assessed ongoing study (STAMP Study 2012), two reported results for studies previously assessed as awaiting classification (Anstice 2011; COMET2 Study 2011), 50 reported new results for studies already included in the review, and 89 reported results included in the previously published review (Walline 2011).
In an additional top‐up search conducted on February 26, 2019, we screened 724 titles and abstracts, of which we excluded 668 records. We excluded nine reports upon full‐text review. We identified 10 reports of 10 studies listed as ongoing and 18 records as awaiting assessment.
Overall, we included 41 studies (174 reports), excluded 91 studies (136 reports), identified 74 ongoing studies (76 reports), and 25 records awaiting classification (Figure 1).
1.
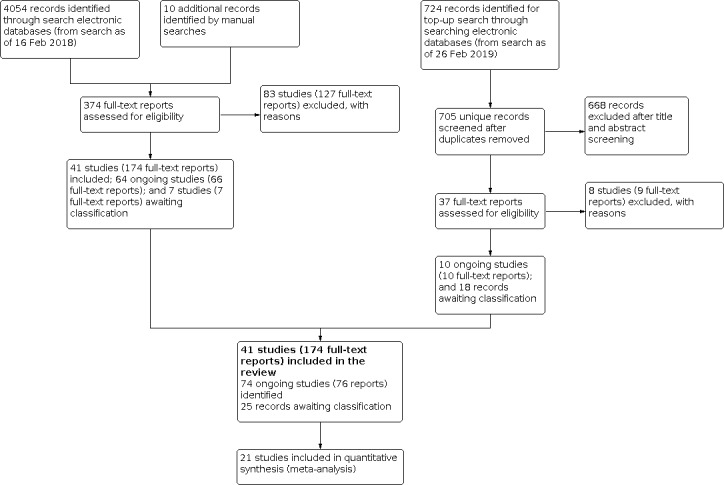
Study flow diagram.
Included studies
We included 41 studies (6772 total participants) in this review. The studies evaluated varying interventions, including spectacles, contact lenses, and pharmaceutical agents (Table 2). With the exception of interventions, study characteristics and outcomes were comparable among the included studies. Except one study (Cambridge Anti‐Myopia Study 2013; n=147), all other studies included children 18 years or younger. No participant had myopia less than 0.25 D. Progression of myopia, measured as the change in refractive error, was assessed as the primary outcome in 37 studies, and as a secondary outcome in three studies (Charm 2013; Swarbrick 2015; Trier 2008). ROMIO Study 2012 was the only study that did not report refractive error as an outcome. Thirty‐eight studies measured refraction under cycloplegia, of which 33 used autorefraction. No study reported quality of life or economic outcomes. Outcomes by intervention are summarized in Table 3Table 4 and Table 5.
1. Interventions of included studies.
| Study | Spectacles | Contact lenses | Pharmaceutical agents | Combination of interventions | |||||||||
| Undercorrected SVLs | Multifocal lenses | Fully corrected SVLs | Soft bifocal lenses | RGP | Ortho‐k | SA‐SCL | SVSCL | Test group | Reference group | ||||
| Bifocal lenses | PALs | Peripheral plus lenses | |||||||||||
| Adler 2006; 2 study arms | X | X | |||||||||||
| Chung 2002; 2 study arms | X | X | |||||||||||
| Koomson 2016; 2 study arms | X | X | |||||||||||
| Cheng 2010; 3 study arms | +1.50 and +1.50 prism | X | |||||||||||
| Fulk 1996; 2 study arms | +1.25 | X | |||||||||||
| Fulk 2002; 2 study arms | +1.50 | X | |||||||||||
| Houston Study 1987; 3 study arms | +1.00 and +2.00 | X | |||||||||||
| Jensen 1991; 3 study arms | +2.00 | X | Timolol + SVLs | ||||||||||
| Pärssinen 1989; 3 study arms | +1.75 | Continous use and distance only | |||||||||||
| COMET Study 2003; 2 study arms | +2.00 | X | |||||||||||
| COMET2 Study 2011; 2 study arms | +2.00 | X | |||||||||||
| Edwards 2002; 2 study arms | +1.50 | X | |||||||||||
| Hasebe 2008; 2 study armsa | +1.50 | X | |||||||||||
| MIT Study 2001; 3 study arms | Plus placebo drops | Plus placebo drops | Atropine + PALs | ||||||||||
| STAMP Study 2012; 2 study arms | +2.00 | X | |||||||||||
| Wang 2005; 2 study arms | Add NR | X | |||||||||||
| Yang 2009; 2 study arms | +1.50 | X | |||||||||||
| Lu 2015; 2 study arms | +2.50 | X | |||||||||||
| Hasebe 2014; 3 study arms | +1.00 and +1.50 | X | |||||||||||
| Sankaridurg 2010; 4 study arms | +1.00, +1.90, and +2.00 | X | |||||||||||
| Anstice 2011; 2 study armsa | +2.00 | X | |||||||||||
| CONTROL Study 2016; 2 study arms | Add NR | X | |||||||||||
| DISC Study 2011; 2 study arms | +2.50 | X | |||||||||||
| Fujikado 2014; 2 study armsa | +0.50 | X | |||||||||||
| CLAMP Study 2004; 2 study arms | X | X | |||||||||||
| Katz 2003; 2 study arms | X | X | |||||||||||
| Charm 2013; 2 study arms | X | X | |||||||||||
| ROMIO Study 2012; 2 study arms | X | X | |||||||||||
| Swarbrick 2015; 2 study armsa | X | X | |||||||||||
| Cambridge Anti‐Myopia Study 2013; 4 study arms | With and without vision training | With and without vision training | |||||||||||
| Cheng 2016; 2 study arms | X | X | |||||||||||
| ATOM Study 2006; 2 study arms | 1% atropine | Placebo drops | |||||||||||
| Yi 2015; 2 study arms | 1% atropine | Placebo drops | |||||||||||
| Yen 1989; 3 study arms | 1% atropine + bifocals | Saline + SVLs | Cyclopentolate + SVLs | ||||||||||
| Shih 1999; 4 study arms | 0.1%, 0.25%, and 0.5% atropine | 0.5% tropicamide | |||||||||||
| PIR‐205 Study 2004; 2 study arms | 2% pirenzepine gel | Placebo gel | |||||||||||
| Tan 2005; 3 study arms | 2% pirenzepine gel once and twice daily | Placebo gel | |||||||||||
| Trier 2008; 2 study arms | Systemic 7‐methylxanthine | Placebo tablet | |||||||||||
| Schwartz 1981; 2 study arms | X | Tropicamide + bifocals | |||||||||||
NR: not reported. Ortho‐k: orthokeratology lenses. PALs: progressive addition lenses. RGP: rigid gas permeable contact lenses. SA‐SCL: spherical aberration soft contact lenses. SVLs: single vision lenses. SVSCL: single vision soft contact lenses.
aCross‐over trial.
2. Outcomes reported by studies of spectacle interventionsa.
| Outcomes | Interventions studied | ||
| Undercorrected lenses: 3studies | Multifocal lenses: 14studies | Peripheral plus spectacles: 3studies | |
| Primary outcome: change in refractive error | Analysis 1.1 | Analysis 2.1; Analysis 2.2; Analysis 2.3 | Analysis 3.1; Analysis 3.2 |
| Secondary outcome: change in axial length | Analysis 1.2 | Analysis 2.4; Analysis 2.5; Analysis 2.6 | Analysis 3.3; Analysis 3.4 |
| Secondary outcome: change in corneal radius of curvature | Not reported by 2 studies and reported only as nonsignificant by Chung 2002 | Analysis 2.7 | Not reported |
| Adverse effects | Two participants who were undercorrected complained of blurred vision (Adler 2006) | Three participants using PALs in 1 study had conjunctivitis, distance blur, or dizziness (COMET2 Study 2011) | Participants reported blurred side vision, visual distortion, dizziness, headaches, and falls (Sankaridurg 2010) |
aCompared with fully corrected single vision lenses.
3. Outcomes reported by studies of contact lens interventionsa.
| Outcomes | Interventions studied | |||
| Soft bifocal contact lenses: 4studies | Rigid gas permeable contact lenses: 2 studies | Orthokeratology: 3 studies | Spherical aberration soft contact lenses: 2 studies | |
| Primary outcome: change in refractive error | Analysis 4.1 | Analysis 5.1 | No data for analysis | Data reported by both studies, but not meta‐analyzable |
| Secondary outcome: change in axial length | Analysis 4.2 | Analysis 5.2 | Analysis 6.1 | Data reported by both studies, but not meta‐analyzable |
| Secondary outcome: change in corneal radius of curvature | Analysis 4.3 | Analysis 5.3 | No data for analysis | Not reported |
| Adverse effects | Six children in 1 study withdrew from the study, 3 from each group (CONTROL Study 2016) | Not reported | Adverse effects reported from all 3 studies | One study reported 1 child with allergic conjunctivitis and 1 with contact dermatitis |
aCompared with fully corrected single vision lenses or contact lenses.
4. Outcomes reported by studies of pharmaceutical interventionsa.
| Outcomes | Interventions studied | ||||
| Antimuscarinic agents: 6studies | Atropine vs tropicamide: 1study | Systemic adenosine antagonists: 1study | Timolol: 1 study | Tropicamide (plus bifocals): 1 study | |
| Primary outcome: change in refractive error | Analysis 7.1; Analysis 7.2 | Analysis 8.1; Analysis 8.2 | Analysis 9.1 | Analysis 10.1 | Control twins showed more progression in myopia than their co‐twins who received tropicamide and bifocals, but this difference was not statistically significant (Schwartz 1981) |
| Secondary outcome: change in axial length | Analysis 7.3; Analysis 7.4 | Not reported | Analysis 9.2 | Not reported | Not reported |
| Secondary outcome: change in corneal radius of curvature | Not reported | Not reported | Analysis 9.3 | Not reported | Not reported |
aCompared with placebo or no drops.
The most common methods of handling unit of analysis issues were to use the average of both eyes (15 studies); to use data from the right eye only (15 studies); and to use data from the eye with more severe myopia (one study) (Table 6). Nine studies were funded primarily by industry, were conducted by employees of the manufacturer of the intervention, or both (Anstice 2011; Cheng 2010; Cheng 2016; CONTROL Study 2016; Fujikado 2014; Hasebe 2014; PIR‐205 Study 2004; Tan 2005; Trier 2008). An additional 14 studies were funded partially by industry or received materials from the manufacturer (Adler 2006; ATOM Study 2006; Charm 2013; CLAMP Study 2004; COMET Study 2003; COMET2 Study 2011; Edwards 2002; Hasebe 2008; ROMIO Study 2012; Sankaridurg 2010; Schwartz 1981; STAMP Study 2012; Swarbrick 2015; Yang 2009).
5. Unit of analysis for included studies.
| Unit of analysis | Studies reporting each type of unit of analysis |
| Average of both eyes | 15 studies: Adler 2006; Chung 2002; COMET Study 2003a; COMET2 Study 2011; CONTROL Study 2016; Fujikado 2014; Fulk 1996; Fulk 2002; Hasebe 2008a; PIR‐205 Study 2004; Sankaridurg 2010; Schwartz 1981; Shih 1999; Tan 2005; Trier 2008 |
| Right eye only | 15 studies: Cambridge Anti‐Myopia Study 2013; Charm 2013; Cheng 2010; Cheng 2016; CLAMP Study 2004; DISC Study 2011; Edwards 2002; Houston Study 1987; Katz 2003; Koomson 2016; MIT Study 2001; ROMIO Study 2012; STAMP Study 2012; Yen 1989; Yi 2015 |
| Right and left eyes reported as separate analyses | 2 studies: Jensen 1991; Pärssinen 1989 |
| One study eye randomized and treated per child | 1 study: ATOM Study 2006 |
| Child randomized and both eyes analyzed as independent units | 2 studies: Hasebe 2014; Lu 2015 |
| Paired‐eye design | 2 studies: Anstice 2011; Swarbrick 2015 |
| Eye with more severe myopia | 1 study: Wang 2017 |
| Not reported | 3 studies: Han 2018; Wang 2005; Yang 2009 |
aAverage values of both eyes were used if the correlation coefficient was > 0.85 between eyes and the mean difference (MD) was not statistically significant; otherwise the eye with more myopic change was used for each child (COMET Study 2003). Mean of both eyes or of right eye only (Hasebe 2008).
Spectacles
Undercorrected versus fully corrected spectacles
Three studies compared the use of undercorrected spectacles versus fully corrected spectacles. In two studies, one in Israel and one in Ghana, children up to 15 years old were randomized to receive spectacles blurred by +0.50 D or spectacles with full correction (Adler 2006; Koomson 2016). In the third study, 106 Malay and Chinese children were evenly randomized to receive spectacles undercorrected by approximately +0.75 D or fully corrected spectacles (Chung 2002). Study follow‐up periods were 18 months in Adler 2006 and two years in Chung 2002 and Koomson 2016.
Multifocal versus single vision lenses
Fourteen studies included in the review compared multifocal spectacles versus single vision lenses (SVLs) (spectacles) for slowing progression of myopia in children: six used bifocal lenses (Cheng 2010; Fulk 1996; Fulk 2002; Houston Study 1987; Jensen 1991; Pärssinen 1989), and eight used progressive addition lenses (PALs) (COMET Study 2003; COMET2 Study 2011; Edwards 2002; Hasebe 2008; MIT Study 2001; STAMP Study 2012; Wang 2005; Yang 2009). All studies enrolled children from 6 to 15 years of age, used a plus addition lens from +1.00 D to +2.00 D, and had at least 18 months of follow‐up (maximum three years). All bifocal studies were conducted outside of Asia (Canada, Denmark, Finland, or USA), although the Canadian study included only children of Chinese ancestry (Cheng 2010); five PAL studies were conducted in Asia (China, Hong Kong, Japan, or Taiwan), and three in the USA (COMET Study 2003; COMET2 Study 2011; STAMP Study 2012).
Of the six bifocal studies, two were two‐arm trials that directly compared bifocal spectacles to SVLs for slowing the progression of myopia in children. One study, conducted in Tahlequah, Oklahoma, USA, randomized 32 children to receive bifocals with +1.25 D addition or SVLs (Fulk 1996). The children were 6 to 13 years old and were followed for 18 months. Following this pilot study, study authors initiated a larger study with slight modifications to the study design (Fulk 2002). For their second study, study authors added another study center in Tulsa, Oklahoma, USA; enrolled 82 children aged 6 to 12 years; changed the bifocal addition to +1.50 D; and extended the follow‐up period to 30 months.
The remaining four bifocal studies were three‐arm trials with at least one bifocal group and one SVL group. In the Houston Myopia Control Study (Houston Study 1987), 207 children ages 6 to 15 years were randomized to one of three treatment groups and were followed for three years. Treatment groups included two intervention groups that received bifocals with either +1.00 D or +2.00 D addition and a standard treatment group that received SVLs. A three‐arm trial including interventions of bifocals, timolol maleate, and SVLs was completed in Odense, Denmark (Jensen 1991). For two years, 159 schoolchildren with a mean age of 10.9 years were followed after they were randomized to one of three treatment groups. The bifocal group received bifocal lenses with +2.00 D addition for constant wear. The timolol group received one drop of 0.25% timolol maleate (an intraocular pressure [IOP]‐reducing beta‐blocker) in each eye twice daily in addition to SVLs for constant wear. The control group received only SVLs for constant wear. Another study compared the effects of bifocal lenses (+1.50 D) with or without three‐prism diopters of base‐in prism in the near segment with single vision distance lenses for slowing the progression of myopia over two years in 150 Chinese Canadian children (aged 8 to 13 years) (Cheng 2010). A study from central Finland enrolled myopic schoolchildren referred by local doctors and nurses after routine vision check‐ups (Pärssinen 1989). In all, 240 children with a mean age of 10.9 years were randomized to one of three treatment groups and were followed for three years. The first intervention group, the distant use group, received full myopic correction and were advised to use glasses for distance vision only and to read at the greatest distance possible. The second intervention group, the bifocal group, received bifocal lenses with +1.75 D addition for continuous use. The third group was the control group and received minus lenses with full correction for continuous use.
All eight PAL studies directly compared use of PALs (multifocal lenses with gradual and progressive changes in power) to SVLs. The three USA‐based studies used +2.00 addition PALs, and four of the five Asia‐based studies used +1.50 addition PALs (the fifth Asian study did not specify the addition power). The Correction of Myopia Evaluation Trial (COMET) was a three‐year, multicenter trial conducted in four major US cities (COMET Study 2003). In all, 469 children aged 6 to 11 years were randomized to receive either PALs or SVLs. The COMET 2 study was conducted to evaluate effectiveness in slowing myopia progression among children (n = 118) aged 8 to 11 years with low baseline myopia, high accommodative lag, and near esophoria (COMET2 Study 2011). Follow‐up was provided for three years. In the third USA‐based study, 85 children aged 6 to 11 years between ‐0.75D and ‐4.50 D of myopia, high accommodative lag, and near esophoria wore either PALs or SVLs for one year; all children wore SVLs in the second year of the study (STAMP Study 2012). A Japanese cross‐over trial followed up children aged 6 to 12 years for 18 months after randomization to PALs or SVLs (Hasebe 2008). After 18 months, each child was switched to receive the alternate type of lens and was followed up for another 18 months. The Myopia Intervention Trial (MIT) included 227 Taiwanese children and investigated SVLs, PALs, and PALs in combination with atropine drops for controlling the progression of myopia (MIT Study 2001). The children, who were between 6 and 13 years of age, were randomized to one of three treatment groups and were followed up for 18 months: (1) SVLs and placebo eye drops; (2) PALs and placebo eye drops; and (3) PALs and 0.5% atropine instilled once a day at bedtime. Studies of 298 children from 7 to 10.5 years of age and of 178 children from 7 to 13 years of age were completed in Hong Kong and China (Edwards 2002; Yang 2009), respectively. The children in both studies were randomized to receive PALs or SVLs and were followed up for two years. Finally, another Chinese study, reported only in the form of a conference abstract, enrolled 104 children aged 6 to 15 years; the addition power used in the PAL lenses was not reported (Wang 2005).
Peripheral plus spectacles versus single vision lenses
Four studies compared various types of peripheral plus spectacles versus SVLs (Han 2018; Hasebe 2014; Lu 2015; Sankaridurg 2010). Peripheral plus spectacles are designed to reduce peripheral hyperopic defocus (peripheral vision farsightedness). As such they consist of lenses that correct for central vision as SVLs do, as well as for peripheral vision using positively aspherized and increasing peripheral power. The addition of the peripheral plus spectacles in these three trials ranged from +1.00 D to +2.50 D. All trials were conducted in China (Hasebe 2014 was a multicenter trial with additional sites in Japan and South Korea) and enrolled children aged 6 to 14 years. Hasebe 2014 randomized 197 children to one of three treatment groups: peripheral plus spectacles with +1.00 D addition, peripheral plus spectacles with +1.50 D addition, and SVLs. Lu 2015 randomized 80 children to either peripheral plus spectacles with up to +2.50 D addition or SVLs. Sankaridurg 2010 randomized 210 children to lens designs that had (1) a symmetrical, clear central aperture (20 mm) with increasing peripheral power to +1.00 D; (2) a symmetrical, clear central aperture (14 mm) with increasing peripheral power to +2.00 D; (3) an asymmetrical, clear central aperture with increasing peripheral power to +1.90 D; or (4) SVLs. The study was planned for two years of follow‐up but was terminated at year one because the older age of participants resulted in slower than expected myopia progression among all study participants. Study follow‐up periods were one year in Lu 2015 and two years in Hasebe 2014. Finally, Han 2018 included 240 children who were randomized to (1) peripheral defocus‐reducing spectacles, (2) single vision lenses, or (3) orthokeratology lenses.
Contact lenses
Bifocal soft contact lenses versus single vision soft contact lenses
Four studies compared bifocal soft contact lenses (BSCLs) to single vision soft contact lenses (SVSCLs) for controlling myopia progression; one each was conducted in China (DISC Study 2011), Japan (Fujikado 2014), New Zealand (Anstice 2011), and the USA (CONTROL Study 2016). The age of children included in all trials ranged from 6 to 18 years. The addition powers for BSCLs ranged from +0.50 to +2.50 D across trials.
The New Zealand study was a paired‐eye, cross‐over study in which one eye of each child aged 11 to 14 years was randomized to receive +2.00 D addition BSCLs or SVSCLs. Fellow eyes received the other type of lens. After 10 months, the types of lenses worn in each eye were switched and children were followed for another 10 months. The Japanese study also was a cross‐over trial in which children aged 6 to 16 years were randomized to wear +0.50 D addition BSCLs or SVSCLs in both eyes for one year, then were switched to the other type of lens for the second year. The remaining two studies were parallel‐group trials. In the first, children aged 8 to 13 years wore either +2.50 D addition BSCLs or SVSCLs for two years. In the second parallel‐group study, 78 children from California, USA, ages 8 to 18 years with eso (convergent) fixation disparity, were randomized to wear BSCLs or SVSCLs every day for one year; the BSCL power prescribed was that needed to eliminate the child's eso fixation disparity while looking at near.
Rigid gas permeable contact lenses versus single vision lenses
Two studies included in the review compared rigid gas permeable contact lenses (RGPCLs) to either SVSCLs or spectacles (SVLs). The Contact Lens and Myopia Progression (CLAMP) study was a three‐year trial that compared RGPCLs to SVSCLs for controlling myopia in school‐aged children (CLAMP Study 2004). All participants had to complete a run‐in period successfully before enrollment to exclude those who could not adapt to wearing rigid contact lenses. After the run‐in period, 116 children aged 8 to 12 were randomized to RGPCL or SVSCL treatment groups. A study of 564 children aged 6 to 12 years in Singapore compared RGPCLs to SVL spectacles for controlling myopia over a two‐year period (Katz 2003). After a three‐month adaptation period, 383 participants remained in the study.
Orthokeratology contact lenses versus single vision lenses
Four studies investigated overnight orthokeratology contact lenses for controlling myopia progression. Studies enrolled children from 6 to 16 years of age with East Asian ethnicity; two studies were conducted in Hong Kong (Charm 2013; ROMIO Study 2012), one in China (Han 2018), and one in Australia (Swarbrick 2015). Charm 2013 evaluated high myopia (‐5.00 D or worse), whereas ROMIO Study 2012 and Swarbrick 2015 included children with low to moderate myopia (no worse than ‐4.50 D). Axial length was the primary outcome in three studies (Charm 2013; ROMIO Study 2012; Swarbrick 2015).
The two studies from Hong Kong compared orthokeratology contact lenses worn overnight versus single vision spectacles. In Charm 2013, 26 of 52 children randomized were assigned to wear partial reduction orthokeratology contact lenses (target 4.00 D) and single vision spectacles during the daytime if needed. In ROMIO Study 2012, 51 of 102 children randomized were assigned to wear orthokeratology contact lenses (target not reported). The Australian study was a paired‐eye, cross‐over study in which one eye of each child was randomized to wear orthokeratology contact lenses during the night and the other eye to wear an RGPCL during the day. After six months, the type of contact lens worn in each eye was switched and children were followed for another six months. In Han 2018, 90 of 240 children were randomized to wear orthokeratology lenses with a "four‐district seven‐arc reverse geometric design."
Spherical aberration soft contact lenses versus single vision soft contact lenses
Two studies compared SVSCLs with or without an additional design to alter spherical aberration. The Cambridge Anti‐Myopia Study 2013 was a 2 × 2 factorial trial testing a spherical aberration design and vision training against SVSCLs in 147 British participants aged 14 to 22 years. Although this trial included adults, most participants were 18 years of age or younger, thus we decided to include it in this review. We did not include in the analysis the two groups with vision training as an intervention because the review is limited to devices and pharmaceuticals. In Cheng 2016, 127 children aged 8 to 11 years were randomized to receive soft daily disposable contact lenses either with positive spherical aberration or without positive spherical aberration. The trial was conducted in the USA and enrolled mostly Asian children (91%). Both studies were planned for two years, but Cheng 2016 was stopped early and reported only one‐year data.
Pharmaceutical agents
Antimuscarinic agents versus placebo
Use of three different topical antimuscarinic agents was compared with use of placebo for control of myopia progression in six studies: three studies evaluated atropine eye drops (ATOM Study 2006; MIT Study 2001; Yi 2015); two evaluated pirenzepine gel (PIR‐205 Study 2004; Tan 2005); and one evaluated both atropine and cyclopentolate eye drops (Yen 1989). All studies were conducted in Asia, except for PIR‐205 Study 2004, which was conducted in the USA. Studies included children from 6 to 14 years of age at enrollment who had low to moderate myopia (up to ‐6.00 D). The atropine studies used one of two doses, 0.5% or 1.0%; the pirenzepine studies used a 2% gel formulation; and the cyclopentolate study used a dosage of 1%.
Two studies compared daily 1% atropine with placebo. The Atropine in the Treatment of Myopia (ATOM) Study enrolled 400 Singaporean children aged 6 to 12 years (ATOM Study 2006). Once each child was randomized to a treatment group, one eye of each child was randomized to receive treatment and the other eye served as a natural control. Follow‐up for this study was two years. In Yi 2015, 140 children with low myopia (‐0.50 D to ‐2.00 D) instilled either 1% atropine or placebo drops in both eyes every night for one year.
One study compared daily 0.5% atropine with placebo (Wang 2017); 126 children aged 5 to 10 years with myopia ranging from ‐0.5 D to ‐2.00 D were randomized to the two interventions. In both groups, the intervention was administered once daily at night for one year.
Two three‐arm studies investigated using atropine in conjunction with wearing multifocal lenses. The Myopia Intervention Trial, described above in the "Spectacles" section, evaluated SVLs, PALs, and PALs in combination with 0.5% atropine drops for controlling progression of myopia (MIT Study 2001). Yen 1989 randomized 247 Taiwanese children aged 6 to 14 years to one of three treatment groups: (1) 1% atropine eye drops every other night and bifocal spectacles prescribed after two weeks of treatment; (2) 1% cyclopentolate eye drops every night and SVLs prescribed if necessary; and (3) normal saline eye drops every night and SVLs as prescribed if necessary. Follow‐up was at one year for Yen 1989 and at 18 months for the MIT Study 2001.
Two studies compared pirenzepine gel (an antimuscarinic) to placebo gel for control of myopia progression. The first was a multicenter US study that enrolled 174 children 8 to 12 years old and followed them up for one year (PIR‐205 Study 2004). Children were randomized at a 2:1 ratio to 2% pirenzepine ophthalmic gel or placebo gel twice a day. An additional year of follow‐up was provided for children who completed the first year. The final study was a three‐arm, multicenter trial from Singapore, Hong Kong, and Thailand (Tan 2005). For one year, 353 children aged 6 to 13 years were randomly treated with (1) 2% pirenzepine gel applied twice daily (gel/gel); (2) placebo once daily and 2% pirenzepine gel once daily (placebo/gel); or (3) placebo gel twice daily (placebo/placebo).
Antimuscarinic agents versus tropicamide
One study, completed in Taiwan, investigated the effectiveness of low concentrations of atropine for controlling progression of myopia in children aged 6 to 13 years (Shih 1999). Two hundred children were randomized to one of three atropine groups or to a control group: (1) daily drop of 0.5% atropine and advised to wear bifocal spectacles; (2) daily drop of 0.25% atropine and advised to wear slightly undercorrected SVLs; (3) daily drop of 0.1% atropine and advised to wear fully corrective SVLs; or (4) daily drop of 0.5% tropicamide.
Systemic adenosine antagonists versus placebo
One study investigated the effectiveness of systemic 7‐mx, an adenosine receptor antagonist, for slowing axial elongation and thus controlling the progression of myopia (Trier 2008). In the first year of this Danish study, 83 children aged 8 to 13 years were randomized to take a 7‐mx or placebo tablet once daily. After one year, all children had the option to receive 7‐mx and to continue follow‐up for two more years.
Combinations of interventions
Tropicamide plus bifocal spectacles versus single vision lenses
In a study of 26 twin pairs, the combined use of bifocal lenses and tropicamide ophthalmic solution for controlling myopia progression was compared to the use of SVLs over a 3½‐year period (Schwartz 1981). This Washington DC area study included monozygotic twin pairs between the ages of 7 and 14 with similar myopia. From each twin pair, one twin was randomized to receive combined treatment of bifocal spectacles with a +1.25 D addition and two drops of 1% tropicamide ophthalmic solution (a short‐acting cycloplegic) instilled into each eye nightly; the other twin received a standard myopic spectacle correction.
Other combinations of interventions
The following combinations of interventions were compared by studies described in the previous sections.
Atropine plus multifocal spectacles versus placebo plus SVLs (MIT Study 2001Yen 1989).
Atropine plus bifocal spectacles versus cyclopentolate plus SVLs (Yen 1989).
Bifocal spectacles versus SVLs with timolol eye drops (Jensen 1991).
Ongoing studies
We identified 74 ongoing studies, which are described under Characteristics of ongoing studies. These studies compared multifocal contact lenses, orthokeratology lenses, progressive addition lenses, spectacle lenses, full correction, and undercorrection. We will incorporate their findings in future updates of this review.
Excluded studies
We excluded 91 studies from this review after full‐text assessment. The complete list of studies and reasons for exclusion are provided in the Characteristics of excluded studies table. Our reasons for exclusion were based on four categories: (1) the study was not an RCT (58 studies); (2) study interventions were not intended to control myopia progression (13 studies); (3) study interventions were not within the scope of this review (13 studies); and (4) the study population was not eligible for this review (seven studies).
We excluded from this review two RCTs comparing SVSCLs with spectacles in myopic children and adolescents because SVSCLs and spectacles are not meant to control the progression of myopia (ACHIEVE Study 2008; Horner 1999). The purpose of the ACHIEVE study was to compare the effects of contact lens wear versus spectacle wear on children’s self‐perception.
Risk of bias in included studies
Allocation
Thirty‐three (81%) of the 41 included studies described the randomization procedure used to allocate participants to treatment groups; we judged them as having adequate sequence generation and thus low risk of allocation bias (Figure 2). Methods employed for adequate sequence generation included using block randomization schemes, computer‐generated randomization lists, or independently prepared randomization lists or tables, and flipping coins or drawing lots. Seven studies stated that children were randomized but did not report other details on how randomization was implemented, and we judged unclear risks of bias for sequence generation (Charm 2013; Cheng 2016; Lu 2015; Wang 2005; Yang 2009; Yi 2015; Han 2018). This review included RCTs only; however, we included one study that was reported to be an RCT but was confirmed to be a quasi‐randomized study based on information provided by the study author (Cheng 2010). The first 50 numbers pulled out were assigned to the control group, the second 50 to the bifocal group, and the remaining 50 to the bifocal plus prism group. This method of sequence generation was inadequate because participants did not have equal chances of being assigned to all treatment groups once the first 50 numbers were drawn. In addition, because treatment assignments were consecutive, allocation concealment was inadequate. We judged this study as having inadequate sequence generation and allocation concealment because treatment assignment was determined by selecting from a container pieces of paper with patient numbers written on them.
2.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
We judged 13 studies to have adequate allocation concealment. Methods considered to be at low risk of bias for this domain included using sequentially numbered, sealed, and opaque envelopes, and calling a centralized coordinating center. Twenty‐five studies did not provide sufficient information on whether and how allocation was concealed. These studies were judged to be unclear risk of bias for allocation concealment. Two studies stated that the person assigning participants to treatment groups was aware of the allocation sequence (ROMIO Study 2012; Cambridge Anti‐Myopia Study 2013), and they were judged as high risk of bias for this domain.
Masking (performance bias and detection bias)
We assessed the use of masking (blinding) for three types of roles: study participants, outcome assessors, and data analysts (Figure 2). Furthermore, we considered separately the masking of outcome assessors for primary (change in refractive error) and secondary (changes in axial length and corneal radius of curvature) outcomes.
Due to the interventions under investigation, masking of participants was not feasible for many of the studies included in this review. Interventions from 34 (83%) of the 41 included studies had significant physical (e.g. contact lenses vs spectacles), functional (e.g. multifocal lenses vs SVLs), or performance (e.g. undercorrected vs fully corrected spectacles) differences between control interventions. Despite these differences, six studies reported masking participants, but we judged them as having unclear risk of bias because it was not clear whether masking was effective (Cambridge Anti‐Myopia Study 2013; Cheng 2016; CONTROL Study 2016; DISC Study 2011; Hasebe 2014; Sankaridurg 2010). Of six studies evaluating pharmaceutical agents exclusively, four studies masked participants adequately by distributing identically packaged and coded bottles or tablets (ATOM Study 2006; PIR‐205 Study 2004; Tan 2005; Trier 2008), and two did not implement masking of participants (Shih 1999; Yi 2015).
Adequate methods of masking outcome assessors involved having participants examined by an investigator who was unaware of treatment assignments. This method was implemented for spectacle or contact lens studies by having participants remove contact lenses and spectacles before they were examined or distributing SVLs for all participants to wear during office visits. Use of coded, identical packaging was considered adequate masking for pharmaceutical studies. Overall, masking of primary outcome assessors was done for 28 (72%) of the 39 included studies. Of the 28 studies that masked primary outcome assessors, 25 were masked similarly for secondary outcome assessors and three did not measure secondary outcomes related to this review. ROMIO Study 2012 did not assess change in refractive error as an outcome but masked assessments for our review's secondary outcomes. We assessed Charm 2013 as having unclear risk of bias for not reporting masking of primary outcome assessment and low risk for masking of secondary outcomes.
We judged five included studies as being at high risk of bias for not masking primary outcome assessors adequately. In a three‐armed study comparing bifocal lenses or timolol with SVLs, there was only one study investigator, who therefore could not be masked to treatment assignments (Jensen 1991). Refractive errors for this study were measured by cycloplegic autorefraction. In another three‐armed trial comparing bifocals or distance‐use spectacles versus continuous‐use single vision spectacles, it was reported that the examining ophthalmologist did not look at group assignments before the examination, but often for different reasons group assignments were revealed (Pärssinen 1989). However, three‐year follow‐up examinations were conducted by two different ophthalmologists, one of whom did not know the group assignments. Refractive errors for this study were measured by subjective cycloplegic refraction. The Houston Myopia Control Study included a team of masked observers (evaluation team) and a team of unmasked observers (patient care team) to measure outcomes in a trial of bifocal lenses versus SVLs (Houston Study 1987). Results presented in the final analysis of the primary outcome were derived from the nonmasked group; therefore we judged the study as having inadequate masking of primary outcome assessors. Refractive errors for this study's results were measured by subjective noncycloplegic refraction. Two included studies did not attempt to mask primary outcome assessors; one measured refractive errors by cycloplegic autorefraction (Cheng 2010), and the other measured refractive errors by subjective cycloplegic refraction (Katz 2003). With the exception of the Houston study, secondary outcome assessors were the same as primary outcome assessors. Data for secondary outcomes in the Houston study were collected by the masked evaluation team; therefore we considered these studies to have low risk of bias.
Five studies did not report masking of primary or secondary outcome assessors; we judged them to have unclear risk of bias (Han 2018; Lu 2015; Swarbrick 2015; Wang 2005; Yen 1989).
The final assessments for masking were applied to study data analysts. How data were handled and whether or not data analysts were masked to treatment groups were not reported in 26 (63%) of the 41 included studies. Two studies explicitly stated that masked investigators analyzed the data independently (Edwards 2002; Hasebe 2008). Additionally, study authors contacted for clarification replied that data analysts were masked for Cheng 2010, MIT Study 2001, and Shih 1999. Although three studies stated that data were analyzed independently after the conclusion of the trial, we considered masking of data analysts to be unclear because treatment assignments may have been accessible in the data (Adler 2006; Chung 2002; Yang 2009). One study could not be masked because only one investigator was involved (Jensen 1991). Study authors for five studies informed us that data analysts were not masked (via email communications) (CLAMP Study 2004; COMET Study 2003; Katz 2003; PIR‐205 Study 2004; Tan 2005). We assessed studies in which data analysts were not masked or in which masking of data analysts was not reported to have unclear risk of bias for this parameter.
Incomplete outcome data
Attrition rates reported by the included studies varied from 0% to 61%. Seven studies followed the intention‐to‐treat (ITT) analysis as defined by this review: (1) participants were analyzed in the intervention groups to which they were randomized, regardless of the intervention they actually received; and (2) all randomized participants were included in the analysis, even participants for whom no outcome data were collected. Three studies provided follow‐up data for all participants at the final follow‐up visit (CLAMP Study 2004; Lu 2015; Han 2018), and four used statistical methods to account for all randomized patients by imputing values for missing data (COMET Study 2003; COMET2 Study 2011; Fulk 2002; Koomson 2016).
A total of 14 studies analyzed participants in the intervention groups to which they were randomized but did not include all randomized participants in the analysis due to attrition. In none of these studies were participants excluded from the analysis due to noncompliance, switching of intervention groups, or failure to adhere to treatment protocols. In 11 of these studies, outcome data were missing for both intervention groups but dropouts were balanced across groups and participants who dropped out were similar to those who remained (Anstice 2011; Chung 2002; CONTROL Study 2016; Edwards 2002; Fujikado 2014; Fulk 1996; Hasebe 2008; Pärssinen 1989; STAMP Study 2012; Trier 2008; Yi 2015). The attrition rate for each of these studies was between 6.5% and 15%. For these considerations, we judged these 11 studies as having low risk of bias due to minimal quantities of incomplete outcome data. The other three studies had unclear risk of attrition bias due to unbalanced dropout rates between treatment groups, or because there were statistically significant differences between participants who dropped out compared with those who remained in the study (ATOM Study 2006; Katz 2003; Yang 2009). One additional study included all randomized participants in the analyses but did not report the methods used to address missing data.
One study published only as an abstract reported the number of patients included in the analyses, but it is not clear whether this number represents the total number initially enrolled in the study and randomized to treatment (Wang 2005).
We judged the remaining 18 studies to have high risk of bias due to incomplete outcome data. The percentage of missing data ranged from 4% to 61%. In all of these studies, a proportion of participants were excluded after randomization for not adhering to the treatment protocol, for having adverse events, or for withdrawing consent. In a study evaluating undercorrected spectacles with full correction spectacles, participants were excluded for not wearing spectacles continuously (Adler 2006). Two studies comparing bifocal lenses with SVLs excluded children from the analysis: one study excluded children who were randomized to receive SVLs but dropped out because their parents wanted them to receive bifocals (Cheng 2010), and another study dismissed noncompliant patients and patients who were fitted with contact lenses without informing study personnel, along with patients who were fitted with contact lenses without informing study personnel (Houston Study 1987). Two studies that investigated peripheral plus spectacles excluded participants for committing protocol violations, withdrawing because of an adverse event, or withdrawing consent (Hasebe 2014; Sankaridurg 2010). One study of BSCLs versus SVSCLs excluded 42% of participants because they did not want to wear the contact lenses (DISC Study 2011). All three orthokeratology studies excluded participants due to issues with wearing the orthokeratology lenses (Charm 2013; ROMIO Study 2012; Swarbrick 2015). Both studies of spherical aberration soft contact lenses excluded 17%—Cheng 2016—or 33%—Cambridge Anti‐Myopia Study 2013—of participants from analyses. The other seven studies evaluated a pharmaceutical agent in at least one treatment arm (seven of the eight pharmaceutical studies included in this review). Five of these studies excluded participants for not using the eye drops or gel, or for not using them consistently (MIT Study 2001; PIR‐205 Study 2004; Shih 1999; Tan 2005; Yen 1989). Another study evaluating timolol plus SVLs versus bifocals or SVLs excluded patients for switching to contact lenses, or because they could not adapt to bifocal lenses (Jensen 1991). The last study, a co‐twin study in which one twin received bifocal spectacles and 1% tropicamide ophthalmic solution and the other twin received SVLs, excluded one twin pair from the study for noncompliance (Schwartz 1981).
In addition to excluding participants for nonadherence, two studies reported an imbalance in dropout rates (PIR‐205 Study 2004; Tan 2005). The PIR‐205 Study 2004 reported that there were significantly more dropouts in the pirenzepine arm compared with the placebo arm, although three additional analytical methods used to impute missing values for those who discontinued the study revealed similar or more beneficial treatment effects for pirenzepine compared with the analysis censoring dropouts. Tan 2005 also reported more dropouts in the pirenzepine‐treated groups than in the placebo‐only group. Although differences were not statistically significant, all participants who dropped out because of adverse events were included in the pirenzepine group.
Two studies excluded participants for lack of efficacy of treatment (MIT Study 2001; Tan 2005). The MIT study excluded two children for having myopic progression greater than 2.00 D per year: one child was from the SVL group and the other child was from the PAL group (none were from the atropine plus PAL group). One child was dropped from the placebo group of the Tan 2005 study for inadequate efficacy.
Finally, the study with the highest percentage of missing data enrolled 247 children, but data were missing for 151 (61%) children (Yen 1989). Reasons for missing data were not reported. Study authors stated that "patients who used the eye drops continuously for one year received another complete ophthalmologic examination," and "96 such patients were collected for evaluation, 32 in each group." It is not clear whether the 96 patients analyzed included all children who were examined at one year or a subset of those examined.
Selective reporting
We assessed 31 (76%) studies as having low risk of bias for selective reporting: 16 studies reported results for study outcomes defined a priori (i.e. in a design and methods publication, baseline report, or clinical trial registry), and 15 studies reported results for outcomes described in the methods section of each paper (Figure 2).
We assessed three studies as having unclear risk of reporting bias. One study had unclear risk of bias due to inadequate reporting for one of the two outcomes measured (Fulk 1996). For this study, the refractive error outcome was reported by treatment assignment; however, the axial length outcome was presented only as it correlated with myopia progression and results by treatment groups were not given. One study was planned for two years but was terminated early and reported results for only one year of follow‐up; 18.9% of children completed two years of follow‐up before the study was terminated early (Cheng 2016). Another study was published as a conference abstract only (Wang 2005).
We considered seven studies to have high risk of reporting bias. One study reported results for only one intervention group (Charm 2013). Another study stated in its methods section that results would be discussed only if they were exceptional (Jensen 1991). In one study, not all outcomes described in the methods section were reported (Yen 1989), and in another study, all outcomes identified in the study methods were reported but the numbers of participants included in the analyses were not consistent between outcomes (Katz 2003). Two studies showed differences between outcomes specified in the clinical trial registration and in the journal publication: one study switched a secondary outcome in the clinical trial registration record to the primary outcome of the journal publication (Fujikado 2014), and the other study did not report results for secondary outcomes listed in the clinical trial registration record (Swarbrick 2015). The final study did not report results for evaluation team (masked observers) measurements nor for other secondary outcomes outlined in the design paper (Houston Study 1987). The methods paper stated that an evaluation team report would be based on (1) cycloplegic retinoscopy, (2) noncycloplegic autorefraction, and (3) cycloplegic autorefraction performed by masked examiners. However, findings from masked examinations were not reported in the outcomes paper; instead results from the patient team were reported (unmasked observers). Also, secondary outcomes such as change in axial length were not reported.
Other potential sources of bias
We assessed 18 (44%) studies as free of other potential sources of bias, 13 (33%) studies as having unclear risk of bias, and 10 (26%) studies as having high risk of bias (Figure 2).
We assessed all four cross‐over trials as having high risk of bias because data were not analyzed according to the cross‐over design, carry‐over effects were not investigated, and some participants who completed the first period dropped out during the second period (Anstice 2011; Fujikado 2014; Hasebe 2008; Swarbrick 2015). Thus, we used only data from the first period to estimate treatment effects. Additionally, three of these studies reported financial conflicts of interest.
We assessed two additional studies as having high risk of bias due to unit of analysis issues (i.e. not accounting for the nonindependence of eyes) (Hasebe 2014; Lu 2015), and two studies as having unclear risk of bias for not reporting the unit of analysis (Wang 2005; Yang 2009).
Two studies planned for two years' duration were terminated early. We assessed one study as having high risk of bias because it was the funder's decision to stop early based on its own interests (Cheng 2016), and the other study as having unclear risk of bias because the decision to stop was made by investigators because they observed lower than expected progression in myopia among all study participants (Sankaridurg 2010).
One study reported imbalances at baseline between treatment groups in gender, corneal curvature, and refractive error (Katz 2003). This study reported unequal losses to follow‐up between treatment groups and by gender. In addition to these considerations, and because 32% of participants dropped out of the study between randomization and the end of the adaptation period, we judged this study to have high risk of bias. Two other studies incorporated prerandomization administration of an intervention into their study design (ATOM Study 2006; CLAMP Study 2004). Run‐in periods may enhance or diminish the effects of a subsequent randomized intervention; thus we assessed these studies as having unclear risk of bias.
The remaining two of 10 studies that we judged to be at high risk of other sources of bias were Shih 1999 and Yen 1989. In Shih 1999, participants in different treatment groups were advised to wear different types of spectacle lenses depending on the concentration of atropine received. The rationale for recommending different types of spectacles for different atropine doses (bifocals in the 0.5% group; undercorrected lenses in the 0.25% group; and fully corrective lenses in the 0.1% group) was not explained. In Yen 1989, it was unknown why equal numbers of participants dropped out of each group, or how equal numbers of participants per group were selected for analysis ("96 such patients were collected for evaluation, 32 in each group").
Eight other studies were fully or partially funded by companies with financial interests in at least one of the interventions being studied. We considered these studies to have unclear risk of bias (Cheng 2010; COMET Study 2003; CONTROL Study 2016; PIR‐205 Study 2004; ROMIO Study 2012; STAMP Study 2012; Tan 2005; Trier 2008).
Effects of interventions
See: Table 1
We compared several interventions to SVLs (spectacles) or SCLs to determine which treatments are effective in slowing the progression of myopia in children. We meta‐analyzed results for prespecified outcomes when appropriate; otherwise we reported study‐specific results. For the primary outcome of this review, progression of myopia assessed as the mean change in refractive error (spherical equivalent) from baseline for each year of follow‐up, negative mean differences (MDs) represented faster progression of myopia in the treatment group compared with progression in the control group. Thus, point estimates to the left of null on the forest plots favor the control group for this outcome. For axial length, negative MDs represent less axial elongation for treatment group participants compared with control group participants (point estimates to the left of null on the forest plots favor the treatment group for this outcome). The unit of analysis reported by each study is shown in Table 6.
Spectacles
1. Undercorrected versus fully corrected spectacles
Three studies with a total of 292 participants compared spectacles that undercorrected myopia by approximately ‐0.50 to ‐0.75 D with SVLs that fully corrected myopia (Adler 2006; Chung 2002; Koomson 2016).
Change in refractive error (Analysis 1.1)
At one year, two studies reported that 72 children who were undercorrected progressed, on average, by ‐0.15 D (95% CI ‐0.29 to 0.00) more than the 70 SVL wearers (Figure 3). At two years, progression of myopia from baseline for the undercorrection group was 0.02 D (95% CI ‐0.05 to 0.09) compared with the full correction group in two studies with 244 children. We graded the certainty of evidence for refractive error as low, downgrading for imprecision (‐1) and risk of bias (‐1).
3.

Forest plot of comparison: 1 Undercorrection vs full correction spectacles, outcome: 1.2 Change in refractive error from baseline (1 year).
Change in axial length (Analysis 1.2)
Changes in axial length were measured by Chung 2002 and Koomson 2016. The undercorrected group showed greater axial elongation than the fully corrected group in one study at one year (MD 0.05 mm, 95% CI ‐0.01 to 0.11), and the studies showed no difference at two years (MD ‐0.01 mm, 95% CI ‐0.06 to 0.03). We graded the certainty of evidence for axial length as low, downgrading for imprecision (‐1) and risk of bias (‐1).
Change in corneal radius of curvature
Changes in corneal radius of curvature were not measured by Adler 2006 nor Koomson 2016, and were reported to be statistically nonsignificant during the two‐year study by Chung 2002.
Adverse effects
Two participants who were undercorrected complained of blurred vision in the study by Adler 2006. No other adverse effects were reported by any study.
2. Multifocal spectacles versus single vision lens spectacles
Fourteen studies compared multifocal spectacles versus single vision lens spectacles to slow the progression of myopia in children. Six studies evaluated bifocal lenses (Cheng 2010; Fulk 1996; Fulk 2002; Houston Study 1987; Jensen 1991; Pärssinen 1989), and eight studies used progressive addition lenses (PALs) (COMET Study 2003; COMET2 Study 2011; Edwards 2002; Hasebe 2008; MIT Study 2001; STAMP Study 2012; Wang 2005; Yang 2009). Ten studies were included in the quantitative analysis, and four studies did not provide adequate data for meta‐analysis: two studies did not report data for each year of follow‐up (Hasebe 2008; Wang 2005), and two studies reported outcomes as rates of change per year based on varying follow‐up times (Fulk 1996; Houston Study 1987). Of the 10 studies that we analyzed quantitatively, eight reported mean changes from baseline and two reported only final values (Edwards 2002; MIT Study 2001). Because the studies were randomized with no significant imbalance in potential confounders between groups at baseline, we combined MDs based on changes from baseline with MDs based on final measurements, given the assumption that these measures address the same underlying between‐group effects. With the exception of Pärssinen 1989, which measured refractive error by subjective cycloplegic refraction, studies included in the analysis used cycloplegic autorefraction for refraction measurements. We included Cheng 2010 in the review following full‐text assessment, but we subsequently classified it as not adequately randomized; we examined the impact of excluding this study from meta‐analysis by performing a sensitivity analysis when appropriate.
Change in refractive error (Analysis 2.1; Analysis 2.2; Analysis 2.3)
At one‐year follow‐up, the average progression was 0.14 D slower (95% CI 0.08 to 0.21; I² = 40%) for 729 multifocal (+1.50 D to +2.00 D near addition) spectacle wearers than for 734 SVL wearers in nine studies (Figure 4). The effect, from subgroup analysis based on type of lens, was similar among PAL wearers (MD 0.15 D, 95% CI 0.09 to 0.21; five studies) and bifocal lens wearers (MD 0.16 D, 95% CI 0.01 to 0.32; four studies). One study with quantitative data did not report data at one year (Yang 2009). Excluding from the analysis the two studies with MDs based on final values did not influence the results substantively (MD 0.14 D, 95% CI 0.07 to 0.22). Excluding Pärssinen 1989, which used subjective refraction, from the analysis did not influence the result substantively (MD 0.16 D, 95% CI 0.10 to 0.23). Excluding Cheng 2010, which was not randomized adequately, from the analysis did not influence the overall result substantively (MD 0.13 D, 95% CI 0.08 to 0.18); however, when Cheng 2010 was excluded from the analysis, the I² was reduced from 40% to 0%.
4.

Forest plot of comparison: 2 Multifocal lenses vs single vision lenses, outcome: 2.1 Change in refractive error from baseline (1 year).
Eight of the ten studies with meta‐analyzable data followed up participants for at least two years; of these, four evaluated bifocal lenses and four evaluated PALs. At two years, average progression was 0.19 D slower (95% CI 0.08 to 0.30; I² = 55%) for 696 multifocal (+1.50 to +2.00 near addition) spectacle wearers than for 705 SVL wearers. Excluding from the analysis Pärssinen 1989, which used subjective refraction and was the only study that favored SVLs, did not influence the result substantively (MD 0.22 D, 95% CI 0.15 to 0.29); however, when Pärssinen 1989 was excluded from the analysis, the I² was reduced from 55% to 0%.
Three of the ten studies with quantitative data followed up participants for three years (COMET Study 2003; COMET2 Study 2011; Pärssinen 1989). We did not combine these studies in an overall meta‐analysis due to statistical heterogeneity (I² = 84.4%). For the PAL subgroup, average progression was 0.21 D slower (95% CI 0.08 to 0.34; I² = 0%) for 287 multifocal (+2.00 D near addition) spectacle wearers than for 292 SVL wearers. Pärssinen 1989 reported a nonsignificant MD in the opposite direction for bifocal wearers compared with SVL wearers (MD ‐0.19, 95% CI ‐0.47 to 0.09).
Four studies not included in the meta‐analyses showed mostly favorable effects of multifocal lenses for slowing myopia progression. In a cross‐over study of +1.50 D PALs versus SVLs, children wearing PALs during the first 18‐month treatment period showed significantly less progression than children wearing SVLs (MD 0.31 D, 95% CI 0.11 to 0.51); however, no difference between groups was evident for the second 18‐month period (MD 0.02 D, 95% CI ‐0.17 to 0.21) (Hasebe 2008). In an 18‐month study reported only by a conference abstract, children wearing PALs showed significantly less progression than children wearing SVLs (MD 0.39 D, 95% CI 0.21 to 0.57; 104 children) (Wang 2005). In another 18‐month study of 14 children assigned to wear +1.25 D bifocal lenses and 14 children assigned to wear SVLs, bifocal wearers progressed by ‐0.39 D per year and SVL wearers progressed by ‐0.57 D per year (P = 0.26) (Fulk 1996). Trial authors noted that during the first year of the study, the rate of progression was equal between groups, but during the last six months of the study, the SVL group progressed more rapidly than the bifocal group. In a three‐arm trial of +1.00 D bifocals, +2.00 D bifocals, and SVLs, no significant differences were observed between groups after three years of follow‐up (Houston Study 1987). The reported average change in refraction error per year during the three‐year study for +1.00 D bifocals was ‐0.36 D per year (n = 41), for +2.00 D bifocals was ‐0.32 D per year (n = 44), and for SVLs was ‐0.34 D per year (n = 39).
We graded the certainty of evidence for refractive error as moderate, downgrading for risk of bias (‐1).
Change in axial length (Analysis 2.4; Analysis 2.5; Analysis 2.6)
Eight studies reported axial length outcomes, four of which reported results for one‐year follow‐up (Cheng 2010; COMET Study 2003; Edwards 2002; STAMP Study 2012). At one year, the summary MD was ‐0.06 mm (95% CI ‐0.09 to ‐0.04) for 445 PAL wearers compared with 451 SVL wearers. This was similar to the summary results after two years of follow‐up (MD ‐0.05 mm, 95% CI ‐0.10 to ‐0.01) for two of these studies (COMET Study 2003; Edwards 2002). After three years of follow‐up, participants in the COMET study wearing PALs continued to have less axial elongation compared with participants wearing SVLs (MD ‐0.11 mm, 95% CI ‐0.17 to ‐0.05).
The four studies that did not report one‐year data showed treatment effects in the same direction as the studies included in the meta‐analysis, although results were not significant in two studies. In a three‐arm trial of PALs with or without atropine compared with SVLs, a pairwise comparison showed that participants who wore PALs without atropine had on average 0.10 mm (95% CI 0.00 to 0.20) less axial elongation compared with participants who wore SVLs at 18‐month follow‐up (MIT Study 2001). In an 18‐month study reported only by a conference abstract, children wearing PALs showed significantly less axial elongation than children wearing SVLs (MD ‐0.21 D, 95% CI ‐0.34 to ‐0.08; 104 children) (Wang 2005). In the cross‐over trial Hasebe 2008, axial length was not measured at baseline; however, there was no significant difference in axial length between groups after the first 18‐month study period (MD ‐0.08 mm, 95% CI ‐0.41 to 0.25), and no significant change in axial length was reported between groups after the second 18‐month study period (MD ‐0.01 mm, 95% CI ‐0.09 to 0.07). In the third study, changes in axial length were not significantly different between bifocal wearers and SVL wearers after 30 months of follow‐up (MD ‐0.09 mm, 95% CI ‐0.24 to 0.06) (Fulk 2002).
We graded the certainty of evidence for axial length as moderate, downgrading for risk of bias (‐1).
Change in corneal radius of curvature (Analysis 2.7)
Changes in corneal radius of curvature outcomes were reported in four studies. Two studies stated only that differences were not significantly different between treatment and control groups (Edwards 2002; Hasebe 2008). In the COMET Study 2003, neither horizontal measurements nor vertical measurements differed between groups after three years of follow‐up (MD 0.00 D, 95% CI ‐0.15 to 0.15; MD 0.00 D, 95% CI ‐0.14 to 0.14, respectively). In an 18‐month study reported only by a conference abstract, children wearing PALs showed significantly greater change in horizontal corneal curvature when compared with children wearing SVLs (MD 0.03 D, 95% CI 0.01 to 0.05; 104 children) (Wang 2005).
Adverse effects
Only one study that compared multifocal lenses with SVLs reported adverse effects (COMET2 Study 2011). Three adverse effects were reported in the PAL group (one each of conjunctivitis, distance blur, and dizziness) and 14 in the SVL group (nine cases of dizziness, three of reduced visual acuity, and one each of floaters and eye pain).
3. Peripheral plus spectacles versus single vision lens spectacles
Three studies compared peripheral plus spectacles versus SVLs (Hasebe 2014; Lu 2015; Sankaridurg 2010). An additional study compared peripheral defocus‐reducing spectacles versus SVLs. Three studies reported outcomes at only one‐year follow‐up—Han 2018Lu 2015Sankaridurg 2010—and one at only two‐year follow‐up—Hasebe 2014. All but one study reported using cycloplegic autorefraction; Han 2018 did not specify the method of measurement of refractive error. Due to differences in lens design and in follow‐up across studies, we did not combine individual trial data in the meta‐analysis.
Change in refractive error (Analysis 3.1; Analysis 3.2)
At one year, there were no significant differences in myopia progression among three peripheral plus lens types when compared with each other or with SVLs, as reported by Sankaridurg 2010. At one year, Lu 2015 reported a nearly 1.00‐D difference between the peripheral plus group (mean change from baseline ‐0.35 D; SD 0.32; 80 eyes of 40 children) and the SVL group (mean change from baseline ‐1.32 D; SD 0.24; 80 eyes of 40 children) (MD 0.97, 95% CI 0.88 to 1.06). At two years, Hasebe 2014 reported similar mean changes from baseline for the peripheral plus +1.00 D group compared with the SVL group (MD 0.06, 95% CI ‐0.09 to 0.21) but less myopia progression for the peripheral plus +1.50 D group compared with the SVL group (MD 0.19, 95% CI 0.05 to 0.33). At one year, Han 2018 reported estimates of change from baseline within groups, at ‐0.43 (SD 0.14) with peripheral defocus‐reducing spectacles and ‐1.15 (SD 0.46) with SVLs. We graded the certainty of evidence for refractive error as low, downgrading for inconsistency (‐1) and risk of bias (‐1).
Change in axial length (Analysis 3.3; Analysis 3.4)
At one year, there were no significant differences in axial elongation among three peripheral plus lens types when compared with each other or with SVLs, as reported by Sankaridurg 2010, or between peripheral plus lenses compared with SVLs, as reported by Lu 2015 (MD 0.03, 95% CI ‐0.15 to 0.21). At two years, Hasebe 2014 reported similar mean changes from baseline for both the peripheral plus +1.00 D compared with SVL group (MD ‐0.05, 95% CI ‐0.16 to 0.06) and the peripheral plus +1.50 D compared with SVL group (MD ‐0.08, 95% CI ‐0.19 to 0.03). We graded the certainty of evidence for axial length as low, downgrading for inconsistency (‐1) and risk of bias (‐1).
Change in corneal radius of curvature
Corneal radius of curvature was not assessed by Hasebe 2014Lu 2015Sankaridurg 2010, or Han 2018.
Adverse effects
Sankaridurg 2010 conducted telephone questionnaires at one week post distribution of lenses. At this time, 2/50 participants in the type I group, 2/60 participants in the type II group, 5/50 participants in the type III group, and 1/50 participants in the SVL group reported blurred side vision. Three participants reported visual distortion—one in the type I group and two in the SVL group. Two participants in the type II group experienced dizziness; for one participant, dizziness resolved after one month; for the other participant, dizziness was accompanied by headaches causing the participant to withdraw from the study. Two falls were reported during the study period; both occurred in the type II lens group during the first weeks of the study. Hasebe 2014 reported that "no serious adverse events were reported during the 2‐year follow‐up," and that "children generally recognized the usability of wearing the study glasses as good (score 4) or very good (5). There was no difference in the median score in any of the questions among the study groups." Lu 2015 did not report adverse effects as an outcome.
Contact lenses
4. Bifocal soft contact lenses versus single vision soft contact lenses
Four studies compared bifocal soft contact lenses (BSCLs) versus single vision soft contact lenses (SVSCLs) (Anstice 2011; CONTROL Study 2016; DISC Study 2011; Fujikado 2014). Anstice 2011 was a paired‐eye, cross‐over study with two 10‐month periods, and Fujikado 2014 was a cross‐over study with two 12‐month periods. Because data were not reported appropriately for within‐person or cross‐over designs, we included data for only the first phase of each trial, considered as one‐year follow‐up, and acknowledged that between‐group estimates did not account for intraperson correlations for the paired‐eye study. CONTROL Study 2016 followed children with myopia and eso fixation disparity at near vision for one year, and DISC Study 2011 followed children for two years. All trials used cycloplegic autorefraction.
Change in refractive error (Analysis 4.1)
At one year, myopia in the BSCL group (n = 149) progressed slightly slower than in the SVSCL group (n = 151) (MD 0.20 D, 95% CI ‐0.06 to 0.47; I² = 86%). Only one trial assessed change in refractive error at two years; DISC Study 2011 reported a similar effect between the BSCL group (n = 65) and the SVSCL group (n = 63) (MD 0.20 D, 95% CI 0.02 to 0.38). We graded the certainty of evidence for refractive error as low, downgrading for inconsistency (‐1) and risk of bias (‐1).
Change in axial length (Analysis 4.2)
At one year, axial elongation in the BSCL group was significantly less than in the SVSCL group (MD ‐0.11 mm, 95% CI ‐0.14 to ‐0.08; I² = 67%). At two years, DISC Study 2011 reported a similar effect between the BSCL group (n = 65) and the SVSCL group (n = 63) (MD ‐0.12 mm, 95% CI ‐0.20 to ‐0.04). We graded the certainty of evidence for axial length as low, downgrading for inconsistency (‐1) and risk of bias (‐1).
Change in corneal radius of curvature (Analysis 4.3)
At one year, in CONTROL Study 2016, the corneal radius of curvature in the BSCL group showed similar change compared with the SVSCL group (MD ‐0.05 D, 95% CI ‐0.15 to 0.05). Corneal radius of curvature was not assessed by the other three trials.
Adverse effects
Six (15%) of 40 children in the Anstice 2011 study—three from each group—did not complete follow‐up; four children withdrew due to difficulties handling the contact lenses, one due to negative publicity on contact lens solutions, and one due to dislike of cycloplegia. The other three studies did not report any adverse effect.
5. Rigid gas permeable contact lenses versus spectacles or soft contact lenses
Two studies investigated the use of rigid gas permeable contact lenses (RGPCLs) in slowing the progression of myopia in children. RGPCLs were compared with soft contact lenses (SCLs) in one study (CLAMP Study 2004), and they were compared with SVLs in the other group (Katz 2003). The CLAMP Study 2004 followed up participants for three years, and the Katz 2003 study followed up participants for two years. We assessed the CLAMP Study 2004 as having generally low risk of bias, and the Katz 2003 study as having generally high risk of bias.
Change in refractive error (Analysis 5.1)
The CLAMP Study 2004 evaluated the use of RGPCLs to slow the progression of myopia in children compared with SCLs. At one (MD 0.40 D, 95% CI 0.19 to 0.61), two (MD 0.54 D, 95% CI 0.27 to 0.81), and three (MD 0.63 D, 95% CI 0.30 to 0.96) years of follow‐up, participants wearing RGPCLs had significantly less progression of myopia compared with participants wearing SCLs (Figure 5). After one year and two years of follow‐up, no difference in myopia progression was observed between RGPCL wearers and SVL wearers in Katz 2003 (MD ‐0.02, 95% CI ‐0.14 to 0.10; MD ‐0.05 D, 95% CI ‐0.25 to 0.15, respectively). Data were not pooled for these studies due to statistical heterogeneity (I² = 91% at one year and 92% at two years). We graded the certainty of evidence for refractive error as very low, downgrading for imprecision (‐1), inconsistency (‐1), and risk of bias (‐1).
5.

Forest plot of comparison: 5 Rigid gas permeable contact lenses vs control, outcome: 5.1 Change in refractive error from baseline [D].
Change in axial length (Analysis 5.2)
At one year, meta‐analysis of the two studies showed that axial elongation was 0.02 mm (95% CI ‐0.05 to 0.10) greater for the 176 RGPCL wearers than for 239 control participants. After two years, it was 0.03 mm greater (95% CI ‐0.05 to 0.12) for the 154 RGPCL wearers than for 240 control participants who were followed up by the two studies. After three years, it was 0.05 mm greater (95% CI ‐0.12 to 0.22) for the 59 RGPCL wearers than for 57 SCL participants in the CLAMP Study 2004. We graded the certainty of evidence for axial length as low, downgrading for impression (‐1) and risk of bias (‐1).
Change in corneal radius of curvature (Analysis 5.3)
Data from the CLAMP Study 2004 suggest that use of RGPCLs may prevent increases in the corneal radius of curvature compared with SCLs. At one, two, and three years of follow‐up, the MD between participants wearing RGPCLs and participants wearing SCLs was ‐0.24 D (95% CI ‐0.43 to ‐0.05), ‐0.38 D (95% CI ‐0.56 to ‐0.20), and ‐0.26 D (95% CI ‐0.48 to ‐0.04), respectively. After one year of follow‐up, Katz 2003 also suggested that RGPCLs may be beneficial compared with SCLs (MD ‐0.08 D, 95% CI ‐0.14 to ‐0.01); however, these results were not statistically significant after two years of follow‐up (MD ‐0.06 D, 95% CI ‐0.14 to 0.02). Data were not pooled for these studies due to statistical heterogeneity (I² = 60% for year one results and 90% for year two results).
Adverse effects
None were reported.
6a. Orthokeratology contact lenses versus single vision lenses
Four studies investigated orthokeratology contact lenses to slow the progression of myopia. Charm 2013 and ROMIO Study 2012 followed up participants wearing either orthokeratology contact lenses or SVLs for two years. Swarbrick 2015 compared orthokeratology contact lenses with RGPCLs in a paired‐eye, cross‐over study with two six‐month periods. Analysis for this study did not account for the within‐person, cross‐over design of the study; therefore no data were included in any meta‐analysis. While three studies had overall high risk of bias, one study had unclear risk of bias (Han 2018).
Change in refractive error
Because orthokeratology contact lenses temporarily reduce myopia, their myopia control treatment effect can be measured only by axial elongation. We did not analyze changes in refractive error for this comparison.
Change in axial length
Three of the four studies reported axial length as an outcome; however, as with outcomes of refractive error, Swarbrick 2015 did not report data eligible for analysis. A fourth study did not report data on axial length (Han 2018).
Summary estimates suggest that the change in axial length is significantly different in favor of the orthokeratology group at two‐year follow‐up (MD ‐0.28, 95% CI ‐0.38 to ‐0.19; Figure 6; Analysis 6.1).
6.

Forest plot of comparison: 6 Orthokeratology contact lenses versus single vision lenses, outcome: 6.1 Change in axial length from baseline (2 years).
6.1. Analysis.

Comparison 6: Orthokeratology contact lenses vs single vision lenses, Outcome 1: Change in axial length from baseline (2 years)
We graded the certainty of evidence for axial length as moderate, downgrading for risk of bias (‐1).
Change in corneal radius of curvature
Neither Charm 2013 nor ROMIO Study 2012 reported outcomes related to changes in corneal radius of curvature; Swarbrick 2015 reported inconsistent findings at the end of each six‐month cross‐over period.
Adverse effects
All three studies reported adverse effects; however, these effects were different across studies. Of 26 participants assigned to wear orthokeratology contact lenses in Charm 2013 five withdrew from the study because they could not achieve a proper lens fitting despite repeated modifications, one withdrew due to lens discomfort, and another withdrew due to not wearing the lenses as instructed. Of the remaining 19 participants, six reported issues with lens binding at the beginning of the study and six showed pigmented corneal arc formation at one‐month follow‐up. Additionally, all participants wore SVLs to correct for residual refractive error during the daytime.
In ROMIO Study 2012, mild rhinitis (3/51 participants), increased conjunctival hyperemia (1/51 participants), and chalazion (1/51 participants) were observed in the orthokeratology group and one case of recurrent corneal inflammation was observed in the SVL group during two years of follow‐up.
Swarbrick 2015 reported that one of 26 eyes in the orthokeratology group had lens adherence.
6b. Spherical aberration soft contact lenses versus single vision soft contact lenses
Two studies investigated spherical aberration soft contact lenses to slow the progression of myopia. The Cambridge Anti‐Myopia Study 2013 followed up participants for two years. Quantitative results for this study were reported for the combined cohort of participants (not by treatment group) or were graphically represented in figures only. Thus, we were not able to calculate between‐group effects for this study but instead describe the results as available. Cheng 2016 planned to follow participants for two years but ended the trial early and reported results for one year only. We identified issues impacting risk of bias in both studies.
Change in refractive error
Neither study reported clinically meaningful differences between treatment groups. Cheng 2016 reported that the least squares mean (LSM) difference in the mean change in refractive error was 0.137 D (95% CI ‐0.007 to 0.281) among 52 children in the spherical aberration group compared with 57 children in the SVSCL group at one‐year follow‐up. The Cambridge Anti‐Myopia Study 2013 reported, "The mean progression was found to be 0.33 Dioptres (D) over the 2 years of the study," and "There was no significant treatment effect of either Vision Training or Contact Lens Spherical Aberration control on myopia progression." We graded the certainty of evidence for refractive error as low, downgrading for risk of bias (‐1) and indirectness (‐1).
Change in axial length
At one year, Cheng 2016 reported that axial elongation was 0.143 mm (95% CI ‐0.188 to ‐0.098) less for children in the spherical aberration group compared with control participants. The Cambridge Anti‐Myopia Study 2013 reported, "Axial length increased steadily over the 2 years of the study by 0.15 mm (SD 0.14) in both right and left eyes," and "There were no significant differences between axial length increases in the different groups." We graded the certainty of evidence for axial length as very low, downgrading for imprecision (‐1), risk of bias (‐1), and indirectness (‐1).
Change in corneal radius of curvature
Corneal radius of curvature was not assessed by Cambridge Anti‐Myopia Study 2013 nor by Cheng 2016.
Adverse effects
Adverse effects were not reported by Cambridge Anti‐Myopia Study 2013. Cheng 2016 reported that one child in the spherical aberration group had allergic conjunctivitis and one child in the control group had contact dermatitis.
Pharmaceutical agents
7. Antimuscarinic agents versus placebo
Six studies assessed topical antimuscarinic agents for slowing the progression of myopia in children. Three studies evaluated an atropine ophthalmic solution, one at 0.5% (MIT Study 2001), and two at 1% (ATOM Study 2006; Yi 2015), versus placebo; two studies evaluated 2% pirenzepine gel versus placebo (PIR‐205 Study 2004; Tan 2005); and one study evaluated 1% cyclopentolate ophthalmic solution versus placebo (Yen 1989). Study participants included in these analyses from the MIT Study 2001 also were provided with PALs. With the exception of Yen 1989, which measured refractive error by subjective cycloplegic refraction, these studies used cycloplegic autorefraction for refraction measurements. We did not include data from one study because it did not report data eligible for meta‐analysis. Specifically, estimates of changes from baseline reported in the paper were of the same magnitude as those reported at baseline, suggesting that they were not, in fact, changes from baseline (Wang 2017).
Change in refractive error (Analysis 7.1; Analysis 7.2)
Due to statistical heterogeneity (I² = 98%), we did not combine results for all antimuscarinic agents but instead pooled the subgroups separately. At one‐year follow‐up, average progression was 1.00 D slower (95% CI 0.93 to 1.07) for participants treated with atropine, 0.31 D slower (95% CI 0.17 to 0.44) for participants treated with pirenzepine, and 0.34 D slower (95% CI 0.08 to 0.60) for participants treated with cyclopentolate (Figure 7). The difference in progression between groups continued among participants in the two studies with two years of follow‐up (MD 0.92 D, 95% CI 0.75 to 1.09 for atropine; ATOM Study 2006 MD 0.41 D, 95% CI 0.13 to 0.69 for pirenzepine; PIR‐205 Study 2004). We graded the certainty of evidence for refractive error as moderate, downgrading for risk of bias (‐1).
7.

Forest plot of comparison: 6 Antimuscarinic agents vs placebo, outcome: 6.1 Change in refractive error from baseline (1 year).
Change in axial length (Analysis 7.3; Analysis 7.4)
Four studies reported axial length outcomes; however, we did not combine results for all antimuscarinic agents due to statistical heterogeneity (I² = 99%). At one‐year follow‐up, the two atropine studies—ATOM Study 2006 and Yi 2015—reported significantly less axial elongation for participants assigned to atropine than for participants assigned to placebo (MD ‐0.35 mm, 95% CI ‐0.38 to ‐0.31). This effect persisted at the end of two years (MD ‐0.40 mm, 95% CI ‐0.48 to ‐0.32) in the ATOM Study 2006. In the pirenzepine gel studies, Tan 2005 reported that after one year, the mean increase in axial length was greatest in the placebo/placebo‐treated group (0.33 mm) than in the placebo/gel (0.30 mm) and gel/gel (0.20 mm) groups. Although standard deviations (SDs) for mean changes in axial length were shown only on a graph, the paper reported that there was a statistically significant treatment effect at one year (repeated‐measures analysis of variance; P = 0.008). No significant changes in axial length were observed at one year in the PIR‐205 Study 2004 (MD ‐0.04 mm, 95% CI ‐0.15 to 0.07). We graded the certainty of evidence for axial length as moderate, downgrading for inconsistency (‐1).
Change in corneal radius of curvature
Corneal radius of curvature outcomes were not assessed by studies comparing topical antimuscarinic agents versus placebo.
Adverse effects (Analysis 7.5)
Both of the studies evaluating pirenzepine documented ocular and systemic adverse events that occurred during the trials (Table 7). Both studies used a significance level of P < 0.15 for reporting adverse events. The three systemic adverse events most frequently reported were headache, common cold, and flu syndrome in the PIR‐205 Study 2004, and increased cough, respiratory infection, and rhinitis in Tan 2005. In the PIR‐205 Study 2004, events of common cold, rhinitis, and sinusitis differed statistically between groups (P < 0.15) and occurred more frequently in the placebo group than in the pirenzepine group. Tan 2005 reported more complaints of abdominal pain in the gel/gel group than in the placebo/placebo group (P = 0.065) and more incidents of rash in the placebo/gel group than in the placebo/placebo group (P = 0.104). The three ocular adverse events most frequently reported by both studies (n=387) were symptoms of decreased accommodation (RR 9.05, 95% CI 4.09 to 20.01), papillae/follicles (RR 3.22, 95% CI 2.11 to 4.90), and medication residue on the eyelids or eyelashes (RR 0.91, 95% CI 0.73 to 1.12). Six ocular adverse events differed significantly (P < 0.15) between groups in the PIR‐205 Study 2004; symptoms of decreased accommodation, papillae/follicles, decreases in visual acuity, eye discomfort, and mydriasis occurred more frequently in the pirenzepine‐treated group, and medication residue on the eyelids or eyelashes occurred more frequently in the placebo group. Four ocular adverse events differed significantly (P < 0.15) between groups in Tan 2005: symptoms of decreased accommodation, papillae/follicles, and decreases in visual acuity occurred more frequently in the gel/gel and placebo/gel groups, and itchy eyes occurred more frequently in the placebo/gel group than in the placebo group. We graded the certainty of evidence for adverse effects as moderate, downgrading for imprecision of results (‐1).
6. Adverse effects reported by studies of pharmaceutical interventions.
| Study | Interventions studied | Details |
| PIR‐205 Study 2004 | Pirenzepine gel vs placebo gel | Reported 6 ocular adverse events with P ≤ 0.15
|
| Tan 2005 | Pirenzepine gel and placebo gel
|
Reported 4 ocular adverse events with P ≤ 0.15 (compared to PLC/PLC)
|
| ATOM Study 2006 | Atropine 1% vs placebo eye drops | No serious adverse events reported, but reasons for withdrawal among atropine users included allergic or hypersensitivity reactions or discomfort (4.5%), glare (1.5%), blurred near vision (1%), and logistical difficulties (3.5%) |
| Yen 1989 | Atropine 1% + bifocals vs cyclopentolate + SVLs vs placebo + SVLs | All atropine users reported photophobia; most reported that they stopped gym classes and did not like going outdoors. No other systemic or ocular complications were observed |
| Shih 1999 | Atropine 0.5%, 0.25%, 0.1%, and tropicamide 0.5% | Three events reported in the atropine 0.5% group: 2 patients complained of photophobia, 1 with allergic blepharitis |
PIR: pirenzepine gel. PLC: placebo gel. SVLs: single vision lenses.
Five studies included in this review evaluated atropine in at least one study arm (ATOM Study 2006; MIT Study 2001; Shih 1999; Yen 1989; Yi 2015); however, only three studies compared atropine with placebo directly (ATOM Study 2006; MIT Study 2001; Yi 2015), and four studies reported adverse effect data. In the ATOM Study 2006, no serious adverse events were reported, although the four most common reasons for study withdrawal in the atropine group were allergic or hypersensitivity reactions or discomfort (4.5%), logistical difficulties (3.5%), glare (1.5%), and blurred near vision (1%). No instances of decreased visual acuity; intraocular pressure changes over 5.5 mmHg; or lenticular, optic disc, or macular changes were reported. Shih 1999 reported three adverse events, all of which occurred in the highest‐dose atropine group (0.5%); two participants complained of photophobia and one participant had allergic blepharitis. Yen 1989 reported that all patients in the atropine (plus bifocal lenses) group had photophobia, which was not reported in the cyclopentolate (plus SVLs) or placebo (plus SVLs) groups. Yi 2015 reported that no adverse effects were observed for children in either atropine or placebo groups.
8. Antimuscarinic agents versus tropicamide
One study compared three doses of atropine versus tropicamide (Shih 1999). In the four‐arm trial, participants were assigned to receive 0.5% atropine drops plus bifocals, 0.25% atropine drops plus slightly undercorrected lenses, 0.1% atropine drops plus fully corrected SVLs, or 0.5% tropicamide drops (control group).
Change in refractive error (Analysis 8.1; Analysis 8.2)
At one‐year follow‐up, myopia progression measured with cycloplegic autorefraction was significantly slowed for each atropine group compared with the tropicamide group, with the highest atropine dose showing the least progression (MD 0.78 D, 95% CI 0.49 to 1.07 for 0.1% atropine; MD 0.81 D, 95% CI 0.57 to 1.05 for 0.25% atropine; and MD 1.01 D, 95% CI 0.74 to 1.28 for 0.5% atropine). This effect was also observed at two‐year follow‐up for each atropine group compared with the tropicamide group (MD 1.95, 95% CI 1.60 to 2.30 for 0.1% atropine; MD 1.98, 95% CI 1.68 to 2.28 for 0.25% atropine; and MD 2.42, 95% CI 2.16 to 2.68 for 0.5% atropine). We graded the certainty of evidence as moderate, downgrading for risk of bias (‐1).
Shih 1999 did not report on axial length, corneal radius of curvature, or adverse effects.
9. Systemic adenosine antagonists versus placebo
One study compared systemic 7‐methylxanthine (7‐mx), an adenosine receptor antagonist, versus placebo for one year (Trier 2008). Participants in both groups (35 in the 7‐mx group and 42 in the placebo group) wore SVLs. Refractive error was measured by cycloplegic autorefraction.
Change in refractive error (Analysis 9.1)
At one‐year follow‐up, the mean difference in myopia progression when 7‐mx was compared with placebo was 0.07 D (95% CI ‐0.09 to 0.24). We graded the certainty of evidence as moderate, downgrading for imprecision (‐1).
Change in axial length (Analysis 9.2)
The 7‐mx group showed less or the same amount of change in axial length compared with the placebo group at one‐year follow‐up (MD ‐0.03 mm, 95% CI ‐0.10 to 0.03). We graded the certainty of evidence as moderate, downgrading for imprecision (‐1).
Change in corneal radius of curvature (Analysis 9.3)
The mean change in corneal radius of curvature between 7‐mx and placebo groups was not significantly different at one‐year follow‐up (MD 0.02 D, 95% CI ‐0.03 to 0.07).
Adverse effects
Trial authors reported that "no subjective side effects were reported."
10. Timolol drops versus no drops
One study compared 0.25% timolol drops versus no drops for slowing the progression of myopia in children (Jensen 1991). Participants in both groups wore SVLs. Refractive error was measured by cycloplegic autorefraction.
Change in refractive error (Analysis 10.1)
There were no statistically significant differences in myopia progression for 46 participants who used timolol compared with 49 participants who did not, at one year (MD ‐0.05 D, 95% CI ‐0.21 to 0.11) and at two years (MD ‐0.04 D, 95% CI ‐0.30 to 0.22). We graded the certainty of evidence as low, downgrading for imprecision (‐1) and risk of bias (‐1).
Jensen 1991 did not report on axial length, corneal radius of curvature, or adverse effects.
Comparisons of combinations of interventions
11. Atropine plus multifocal spectacles versus placebo plus SVLs
Two studies compared atropine drops plus multifocal lenses versus placebo drops plus SVLs to slow the progression of myopia in children. The MIT Study 2001 used 0.5% atropine plus PALs, and Yen 1989 used 1% atropine plus bifocal lenses.
Change in refractive error (Analysis 11.1)
At one year, both studies showed less progression among atropine plus multifocal lens users compared with placebo plus SVL users (MD 0.78 D, 95% CI 0.54 to 1.02). We graded the certainty of evidence as moderate, downgrading for risk of bias (‐1).
Change in axial length (Analysis 11.2)
At the end of the 18‐month MIT Study 2001, participants in the atropine plus multifocal lens group had significantly less axial elongation compared with participants in the placebo plus SVL group (MD ‐0.37 mm, 95% CI ‐0.47 to ‐0.27). We graded the certainty of evidence as moderate, downgrading for risk of bias (‐1).
Change in corneal radius of curvature
Neither the MIT Study 2001 nor Yen 1989 reported outcomes for corneal radius of curvature.
Adverse effects
Yen 1989 reported that all participants in the atropine plus bifocal lenses group had photophobia, which was not reported in the placebo plus SVLs group. The MIT Study 2001 did not report on adverse effects.
12. Atropine plus bifocal spectacles versus cyclopentolate plus SVLs
One study compared 1% atropine drops plus bifocal lenses versus 1% cyclopentolate drops plus SVLs (Yen 1989).
Change in refractive error (Analysis 12.1)
At one year, participants in the atropine plus bifocal lens group had significantly less myopia progression compared with participants in the cyclopentolate plus SVLs group (MD 0.36 D, 95% CI 0.11 to 0.61). We graded the certainty of evidence as moderate, downgrading for risk of bias (‐1).
Axial length and corneal radius of curvature were not measured by Yen 1989. It was reported that all participants in the atropine plus bifocal lenses group had photophobia, which was not observed in the cyclopentolate plus SVLs group.
13. Bifocal spectacles versus SVLs with timolol drops
Change in refractive error (Analysis 13.1)
In a three‐arm trial of +2.00 D bifocal lenses, 0.25% timolol drops plus SVLs, and SVLs (Jensen 1991), a pairwise comparison of bifocal and SVL plus timolol groups suggested that use of bifocals slowed the progression of myopia more effectively than SVLs plus timolol drops at one year (MD 0.19 D, 95% CI 0.06 to 0.32) and at two years (MD 0.23 D, 95% CI 0.00 to 0.46). Neither intervention when compared with the SVL‐only group was statistically significant for this study (see Analysis 2.1; Analysis 2.2; Analysis 9.1). We graded the certainty of evidence as moderate, downgrading for risk of bias (‐1).
2.1. Analysis.

Comparison 2: Multifocal lenses vs single vision lenses, Outcome 1: Change in refractive error from baseline (1 year)
2.2. Analysis.

Comparison 2: Multifocal lenses vs single vision lenses, Outcome 2: Change in refractive error from baseline (2 years)
9.1. Analysis.

Comparison 9: Systemic 7‐methylxanthine vs placebo, Outcome 1: Change in refractive error from baseline (1 year)
Jensen 1991 did not report on axial length, corneal radius of curvature, or adverse effects.
Tropicamide plus bifocal spectacles versus SVLs
In a co‐twin study, one twin from each twin pair was randomized to receive either 1% tropicamide once per day and +1.25 D bifocals or SVLs; follow‐up was provided for 3.5 years (Schwartz 1981). No numerical results were presented in the paper. Study authors stated that control twins showed more progression in myopia than their co‐twins who received tropicamide and bifocals, but that this difference was not statistically significant.
Discussion
Summary of main results
Our review included 41 studies that investigated 15 comparisons of interventions to slow progression of myopia in children.
Our findings suggest that there is limited evidence favoring full correction of myopia over undercorrection. Trials have also shown a statistically significant but clinically unimportant benefit of multifocal spectacle lenses compared with single vision lenses (SVLs) for both myopia progression and axial elongation.
We found consistent evidence favoring antimuscarinic drugs compared with placebo for reducing progression of myopia and elongation of axial length in children with myopia. Atropine resulted in an effect of larger magnitude than was seen with pirenzepine or cyclopentolate. No trial directly compared the three different antimuscarinic drugs. These drugs are associated with frequent side effects, such as accommodation difficulties, papillae and follicles, sensitivity to light, and eye discomfort, which may lead to approximately 15% of children quitting therapy (ATOM Study 2006). One study directly compared atropine versus tropicamide and found a significant beneficial effect with atropine; however, the concentration of atropine (0.1% and 0.25%) was much lower than that used in other trials.
Studies investigating peripheral plus spectacle lenses, bifocal soft contact lenses, rigid gas permeable contact lenses, overnight orthokeratology contact lenses, spherical aberration soft contact lenses, systemic adenosine antagonists (7‐methylxanthine), and topical timolol eye drops provided limited or inconclusive evidence as to the effectiveness of these interventions for slowing the progression of myopia compared with single vision lenses alone.
In summary, we found consistent evidence of meaningful benefit when antimuscarinic drugs were used for slowing progression of myopia in children. Neither the optimal dose of antimuscarinic drug nor the additional value of using an antimuscarinic drug along with spectacles or contact lenses has been adequately answered by available evidence. Evidence regarding beneficial effects of the other interventions included in this review is neither consistent nor confirmatory.
Overall completeness and applicability of evidence
Several interventions have been investigated by more than one reporting source (journal publication, conference abstract, trial registry, etc.), which provided sufficient evidence to determine the applicability of treatment for slowing myopia progression. However, reporting of results was inconsistent among studies, so grouping of findings was difficult. Antimuscarinic pharmaceutical agents hold the greatest promise for slowing myopia progression in children, but not all studies provided complete data for inclusion in the meta‐analysis.
The included trials have been conducted across diverse geographic locations. The effects that we observed for antimuscarinic drugs were consistent across studies conducted in Caucasian populations as well as in Asian populations.
Evidence regarding the beneficial effects of various myopic control agents may be related to the ethnicity of participants and/or the comparator intervention in the included trials. For example, Asian children are more likely to be myopic and their myopia progresses faster than that in Caucasian children (Lin 1999; Zhan 2000), so any myopia control agent may be more or less effective for Asian children than for Caucasian children because the cause of their myopia may be different.
The primary outcome for myopia progression studies typically has been change in refractive error over time; however, as new methods of assessing and treating myopia have become available, the primary outcome has been switched to axial growth of the eye. For example, all three studies evaluating orthokeratology lenses—Charm 2013ROMIO Study 2012Swarbrick 2015—and one study assessing systemic 7‐methylxanthine—Trier 2008—defined axial length as their primary outcome. In some studies, both methods have been measured and reported, which may enhance confusion if the two methods yield differing information. For example, the rigid gas permeable contact lenses (RGPCLs) trial by Walline and colleagues found that RGPCLs significantly slowed myopia progression but did not slow axial eye growth (CLAMP Study 2004). The change in the primary outcome of myopia control studies also may lead to reporting bias, as was observed in one study that planned to report refractive error as the primary outcome but switched the primary outcome to axial length in the journal publication based on significant findings for axial length but not for refractive error (Fujikado 2014).
Quality of the evidence
Overall, the certainty of evidence ranged from very low to high across all comparisons and outcomes. In terms of the primary outcome for this review—change in refractive error—we assessed two comparisons (multifocal vs single vision lens spectacles and atropine plus multifocal spectacles vs placebo plus SVLs) to provide high‐certainty evidence in favor of the treatment group, finding no reason to downgrade. We downgraded for imprecision, inconsistency, or risk of bias for most analyses. Imprecision and inconsistency may reflect comparisons with only one or two small trials and underlying differences among studies, respectively.
This review was limited to randomized controlled trials (RCTs), minimizing the chance of treatment selection bias based on participants' desires for a specific correction. However, not all biases were completely eliminated, and many children dropped out of studies due to dissatisfaction with the intervention received. For many interventions, participants could not be masked with regard to treatment when they were assigned randomly to spectacles versus contact lenses or to one of two types of contact lenses. Although it is unlikely that participants could influence the outcome of myopic eye growth, they may have been more likely to halt participation in a study if they received a treatment that did not interest them, which could potentially increase the risk of bias. Thus, high risk of performance bias, attrition bias, or both was the most common reason for downgrading the certainty of evidence when risk of bias was an issue.
Potential biases in the review process
We reduced the risk of bias during the review process by utilizing a thorough literature search and by not limiting reviewed studies on the basis of language or dates. Two review authors, including at least one clinician and one methodologist, independently assessed the search results for eligibility and extracted data. There is little reason to believe that investigations would have been missed by the search methods unless the study results were never reported.
Overall, despite improvement seen in trials following the CONSORT statement for RCTs (Schulz 2010), many studies still lack the required rigor of reporting necessary to allow the reader to assess the risk of bias in individual trials. The vast majority of studies either did not mask the person analyzing the study data or did not report whether this occurred. It is important for statisticians to make decisions based solely on available data that should not include treatment allocation to reduce or eliminate the potential for reporting bias.
Of the 25 records awaiting for classification, eight studies with published results are likely to be included in the future update of the review. Five of these eight studies are unlikely to contribute any quantitative data for synthesis because outcomes and timepoints for outcomes are out of the scope of the review (Cheung 2018; Lam 2018; Tan 2019; Tilia 2018; Wu 2018). The remaining three studies are likely to contribute quantitative data (Pärssinen 2017; Ren 2017; Wei 2017); however, because the findings in these three studies are consistent with what we report herein, we anticipate they will not change the effect sizes in any meaningful way.
Agreements and disagreements with other studies or reviews
Saw 2002a, Saw 2002b, and Gwiazda 2009 did not include a systematic and comprehensive literature search nor any meta‐analyses. The conclusions of these three reviews are consistent with our observations in this systematic review. The 2017 report by the American Academy of Ophthalmology also concluded that there is "high‐level evidence" to support the use of atropine to prevent myopia progression, although reports have described rebound of myopia after treatment is discontinued (Pineles 2017). Other treatments, such as undercorrection of myopia, multifocal spectacles, and RGPCLs, do not slow growth of the eye in a clinically meaningful manner (i.e. slowing growth of the eye by 50% or more).
Authors' conclusions
Implications for practice.
Based on available evidence, antimuscarinic topical medications are effective in slowing myopia progression, but they lead to ocular adverse effects, such as reduced accommodation, papillae or follicles, and medication residue on the eyelids or eyelashes. Further investigations of myopia control must be conducted to find a treatment that is clinically meaningful and beneficial with fewer adverse effects.
Implications for research.
Until recently, few RCTs have been conducted to investigate myopia control. Reporting of results from RCTs has been extremely variable. Investigators must compare results to those of previous investigations and must report findings according to the CONSORT statement to maximize the potential for combining results from a variety of studies. Future investigators should consider findings from this systematic review in determining the comparisons that should be examined and the patient populations that should be studied. We have not found conclusive evidence of the effects of most of the interventions included in this review despite our consistent findings on the effects of antimuscarinic drugs. For example, there is limited evidence on an optimal dose of antimuscarinic drugs for use in children. The evidence that we examined was limited in several ways including the potential for bias. Future trials should be designed to reduce the potential for bias and should be reported in light of the potential for application of novel analytical methods such as multiple‐treatment meta‐analyses. The added value of using antimuscarinic drugs along with spectacles or contact lenses and the effects of other combinations of interventions in slowing the progression of myopia in children need to be clarified. If future investigators find a clinically and statistically significant treatment effect, they should determine whether the effect continues to be sustained after treatment is discontinued and should attempt to determine the true mechanism of the treatment effect.
What's new
| Date | Event | Description |
|---|---|---|
| 3 September 2021 | Amended | Editorial note added |
History
Protocol first published: Issue 1, 2008 Review first published: Issue 12, 2011
| Date | Event | Description |
|---|---|---|
| 12 December 2019 | New search has been performed | Issue 1, 2020: searches updated |
| 12 December 2019 | New citation required and conclusions have changed | Issue 1, 2020: 16 new studies added (Anstice 2011; Cambridge Anti‐Myopia Study 2013; Charm 2013; Cheng 2016; COMET2 Study 2011; DISC Study 2011; Fujikado 2014; Hasebe 2014; Koomson 2016; Lu 2015; ROMIO Study 2012; STAMP Study 2012; Swarbrick 2015; Trier 2008; Wang 2005; Yi 2015); data added for 1 included study from previous version (CONTROL Study 2016); 6 ongoing trials identified (BLINK Study 2017a; Li 2013; JPRN‐UMIN000005054; PACT Study 2017; ACTRN12611000499987; 11000582954) |
| 30 January 2013 | New search has been performed | Search updated; new studies added |
| 25 June 2008 | Amended | Scope of the review changed to assess all interventions for slowing myopia progression in children |
| 25 June 2008 | Amended | Review converted to new review format |
Notes
Acknowledgements
Iris Gordon, Information Specialist for Cochrane Eyes and Vision, designed and conducted the original electronic searches for the original version of the review; Lori Rosman, Information Specialist for Cochrane Eyes and Vision, updated and conducted the electronic searches for this version of the review. We acknowledge Milan Mathew's contribution to the original published protocol as well as earlier contributions made by Karla Zadnik and Mark A. Bullimore. Drs. Barbara Hawkins and Jenny Evans provided statistical guidance and general comments throughout the process of this review update. We would also like to acknowledge Satoshi Hasebe (Okayama University Medical School), Akiko Miki (Johns Hopkins School of Medicine), and Sueko Matsumura Ng (Johns Hopkins School of Public Health) for their assistance with screening Japanese language articles. We thank Cesar Augusto, Ugarte Gil, and Yuanxi Jia for assistance with data collection. We are grateful to the following peer reviewers for their time and comments: Lindsay Sicks (Illinois College of Optometry), Courtney Kraus (Wilmer Eye Institute), and Bradly D'Souza.
We acknowledge gratefully the following study authors for providing additional study information and/or data: Aller TA, Cheng D, Chua WH, Eiden S, Gwiazda J, Hasebe S, Katz J, Millodot M, Shih YF, and Yen MY.
The 2020 review update was managed by CEV@US and was signed off for publication by Tianjing Li and Richard Wormald.
Appendices
Appendix 1. CENTRAL search strategy
#1 MeSH descriptor: [Myopia] explode all trees #2 myop* #3 (short near/3 sight*) or ("near" near/3 sight*) #4 nearsighted* #5 {or #1‐#4} #6 MeSH descriptor: [Eyeglasses] explode all trees #7 spectacles or glasses or eyeglass* #8 (progressive or single or vision or addition or bifocal or spectacle or corrective) near/2 lens* #9 MeSH descriptor: [Contact Lenses] explode all trees #10 (contact or "gas permeable") near/2 lens* #11 {or #6‐#10} #12 MeSH descriptor: [Mydriatics] explode all trees #13 mydriat* or cycloplegic* #14 "7‐methylxanthine" or "552‐62‐5" #15 MeSH descriptor: [Cholinergic Antagonists] explode all trees #16 Cholinergic* next/2 (antagonist* or block* or inhibitor*) #17 cholinolytic* #18 acetylcholine* next/2 (antagonist* or block* or inhibitor*) #19 anticholinergic* or "anti cholinergic" or "anti cholinergics" #20 muscarinic* next/2 (antagonist* or block* or inhibitor*) #21 antimuscarinic* or "anti muscarinic" or "anti muscarinics" #22 MeSH descriptor: [Parasympatholytics] explode all trees #23 parasympathetic* next/2 (antagonist* or block* or inhibitor*) #24 parasympathicolytic* or parasympaticolytic* or Parasympatholytic* #25 pharmaceutical* or pharmacologic* #26 MeSH descriptor: [Atropine] explode all trees #27 Atropine or atrinal or "atro‐polygyl" or atrop or atropen or atropin or atropina or "atropini sulfas" or atropinol or atropisol or atropt or atroptol or atrospan or "atrosulf‐1" or "bar bropin" or "bellpino‐artin" or "cendo tropine" or "dextro levo hyosciamine" or "ichtho bellol" or "isopto" or isoptoAtropine or "ocu‐tropine" or "sal‐tropine" or skiatropine or "tropine dextro levo tropate" or ximex or "51‐55‐8" or "55‐48‐1" #28 berefrine or POPD or "105567‐83‐7" #29 MeSH descriptor: [Cyclopentolate] explode all trees #30 Cyclopentolate or "ak‐pentolate" or akpentolate or "bell pentolate" or ciclolux or cyclogyl or cyclomydri or cyclopentol or cyclopentolat or cylate or cyplegin or diopentolate or midriodavi or mydrilate or "ocu‐pentolate" or ocucyclo or "oftan‐syklo" or pentolair or "refractyl ofeno" or skiacol or zyklolat or "512‐15‐2" or "5870‐29‐1" #31 MeSH descriptor: [Epinephrine] explode all trees #32 Epinephrine or Adrenaline or adrenalin or Epitrate or Lyophrin or Epifrin or adnephrin or adnephrine or adrenaclick or "adrenal hydrochloride" or adrenalina or adrenamine or adrenapax or adrenazin or adrenine or adrin or adrine or advaradin or balmadren or biorenine or bosmin or chelafrin or dylephrin or epiglaufrin or epimephrine or epinefrina or epinephran or epinephrin or epirenamine or epirenan or exadrin or glaucon or glaucosan or glaufrin or "glin epin" or glycirenan or haemostatin or hemisine or hemostasin or hemostatin or hypernephrin or "isopto epinal" or levoadrenalin or levoadrenaline or levoepinephrine or levorenin or levorenine or methylaminoethanolcatechol or methylarterenol or mucidrina or myosthenine or "n methylnoradrenalin" or nephridine or nieraline or paranephrin or posumin or renaglandin or renaglandulin or renaleptine or renalina or renaline or renoform or renostypticin or renostyptin or scurenaline or simplene or soladren or sphygmogenin or styptirenal or supracapsulin or supranephrane or supranephrin or supranol or suprarenaline or suprarenin or suprarenine or suprel or surenine or surrenine or "sus‐phrine sulfite‐free" or susphrine or "sympathin I" or takamina or tonogen or vasoconstrictine or vasodrine or vasotonin or weradren or "51‐43‐4" or "55‐31‐2" or "6912‐68‐1" #33 MeSH descriptor: [Ethylmorphine] explode all trees #34 Ethylmorphine or Ethomorphine or Trachyl or codethyline or diolan or dionine or "ethyl morphine" or ethylmorfine or ethylmorphin or "morphine ethyl ether" or "125‐30‐4" or "76‐58‐4" #35 Eucatropine or euphthalmine or "100‐91‐4" or "536‐93‐6" #36 Homatropine or homatro or homatrocil or homatropaire or homatropin or homatropina or isoptoHomatropine or "I Homatrine" or "mandelyl tropeine" or mandelyltropeine or "mydryn eye" or "omatropina lux" or "tropine mandelate" or "51‐56‐9" or "87‐00‐3" #37 MeSH descriptor: [Hyoscyamine] explode all trees #38 Hyoscyamine or anaspaz or cystospaz or cytospaz or daturine or donnamar or duboisine or egacen or hyoscamine or hyosciamine or hyoscyanin or hyosyne or "ib‐stat" or levbid or levsin or "levsinex sr" or neosol or nulev or spasdel or "symax sl" or "symax sr" or "tropine l tropate" or "101‐31‐5" or "306‐03‐6" #39 Ibopamine or "N‐methyldopamine diisobutyrate" or "SB 7505" or "SB7505" or Escandine or Inopamil or "diisobutyric n methyldopamine ester" or scandine or "skf 100168" or "skf 100168 a" or "66195‐31‐1" or "75011‐65‐3" #40 Methylatropine or "8‐methylatropinium nitrate" or "31610‐87‐4" #41 MeSH descriptor: [Naphazoline] explode all trees #42 Naphazoline or "Afazol Grin" or "AK Con" or AKCon or Albalon or albasol or "All Clear" or allersol or antan or benil or cefasan or "Clear Eyes" or coldan or "Colirio Alfa" or "comfort eye drops" or dazolin or "degest 2" or derinox or Idril or imidin or minha or Miraclar or mirafrin or Nafazair or nafazoline or naftazolina or "naphacel ofteno" or naphasal or naphazolin or Naphcon or "naphozoline hydrochloride" or naphtears or naphthazoline or naphthizine or naphthyzin or nastizol or "nazil ofteno" or niazol or "ocu‐zoline" or opcon or Optazine or Privin or privina or Privine or privine or Proculin or rhinantin or rhinazin or rhinoperd or rimidol or sanorin or sanotin or Siozwo or strictylon or "Tele Stulln" or TeleStulln or Vasoclear or Vasocon or "Vasoconstrictor Pensa" or VasoNit or vistalbalon or vistobalon or "5144‐52‐5" or "550‐99‐2" or "835‐31‐4" #43 Oxedrine or Synephrine or Sympaethamin or Synephrin or aetaphen or pentedrine or vasoton or "94‐07‐5" #44 MeSH descriptor: [Synephrine] explode all trees #45 MeSH descriptor: [Oxyphenonium] explode all trees #46 Oxyphenonium or Methacin or Oxyphenon or Atrenyl or Spastrex or antrenyl or "ba 5473" or ba5473 or "c 5473" or c5473 or helkamon or metacin or metacinum or oxyphenium or "oxyphenomium bromide" or spasmofen or spasmophen or "14214‐84‐7" or "50‐10‐2" #47 MeSH descriptor: [Phenylephrine] explode all trees #48 Phenylephrine or adrianol or "af‐taf" or "ak‐dilate" or "albalon relief" or alconefrin or almefrin or altafrin or biomidrin or biomydrin or derizene or "despec‐sf" or "disneumon pernasal" or drosin or "efrin‐10" or efrisel or fenylephrine or idrianol or isonefrine or isophrin or isophrine or "isopto frin" or isoptofrin or lexatol or "m synephrine" or mesaton or "meta sympathol" or "meta synephrine" or Metaoxedrin or metaoxedrine or Metasympatol or metasynephrine or Mezaton or "murucoll 2" or mydfrin or "n 105 to" or "nefrin‐ofteno" or "Neo Synephrine" or neofrin or neooxedrine or neophryn or neosynephrin or Neosynephrine or "neosynephrin‐pos" or neosynesin or neosynesine or "ocu‐phrin" or "oftan‐metaoksedrin" or optistin or phenoptic or phenylefrine or phenylephedrine or prefrin or "pupiletto forte" or rectasol or "rhinall 10" or "slv 325" or slv325 or sucraphen or vazculep or visadron or vistafrin or vistosan or "532‐38‐7" or "59‐42‐7" or "61‐76‐7" #49 Pholedrine or "4 hydroxy n methylamphetamine" or "4 hydroxymethamphetamine" or adyston or "para hydroxymethamphetamine" or "p‐hydroxymethamphetamine" or paredrinol or "Pholedrin liquidum" or "Pholedrin‐longo‐Isis" or pulsotyl or venosan or veritol or "370‐14‐9" #50 MeSH descriptor: [p‐Hydroxyamphetamine] explode all trees #51 p‐Hydroxyamphetamine or "1 para hydroxyphenyl 2 propylamine" or "alpha methyl para tyramine" or "alpha methyl tyramine" or "dl 1 p hydroxyphenyl 2 propylamine" or "dl 1 para hydroxyphenyl 2 propylamine" or "dl p hydroxy alpha methylphenethylamine" or "dl para hydroxy alpha methylphenethylamine" or "h 66 37" or "para hydroxy alpha methylphenethylamine" or Hydroxyamfetamine or Hydroxyamphetamin or Hydroxyamphetamine or Hydroxyphenylisopropylamine or Methyltyramine or Norpholedrin or norpholedrine or oxamphetamine or Oxyamphetamine or paradrine or parahydroxyamphetamine or Paredrine or paredrinea or paredrinex or pedrolone or pulsoton or "103‐86‐6" or "1518‐86‐1" or "306‐21‐8" #52 MeSH descriptor: [Racepinephrine] explode all trees #53 Racepinephrine or asthmanefrin or Micronefrin or micronefrine or Micronephrine or mikronephrin or racadrenalin or "Racepinefrine Hydrochloride" or racinephrine or Vaponefrin or vaponefrine or vaponephrin or "329‐65‐7" #54 MeSH descriptor: [Scopolamine Hydrobromide] explode all trees #55 Scopolamine or "Boro Scopol" or BoroScopol or Hyoscine or Kwells or "levo hyoscinehydrobromide" or Scoburen or Scopace or scopos or "Travacalm HO" or Vorigeno or "114‐49‐8" or atrochin or atroquin or atroscine or hyosceine or hysco or "kimite‐patch" or "l epoxytropine tropate" or "n methylhyoscine" or oscine or scopalamine or "scopine tropate" or scopoderm or scopolamin or transcop or "transderm scop" or "transderm v" or "tropic acid ester with scopine" or "138‐12‐5" or "51‐34‐3" or "55‐16‐3" #56 MeSH descriptor: [Tropicamide] explode all trees #57 Tropicamide or "alcon‐mydril" or bistropamide or "cendo mydriatyl" or "Colircusi Tropicamida" or midriaticum or mydiacyl or mydral or mydramide or mydriacyl or Mydriafair or Mydriaticum or "mydrin m" or "mydrin p" or Mydrum or "n ethyl 2 phenyl n pyrid 4 ylmethylhydracrylamide" or "n ethyl n 4 picolyltropamide" or "n ethyl n gamma picolyltropamide" or "n ethyl n pyrid 4 ylmethyltropamide" or "Ocu‐Tropic" or OcuTropic or opticyl or sandol or sintropic or "tropamid forte" or "tropic acid n ethyl n gamma picolyl amide" or Tropicacyl or tropicamid or "tropico eye" or tropicol or tropikamid or tropimil or visumidriatic or "1508‐75‐4" #58 MeSH descriptor: [Tyramine] explode all trees #59 Tyramine or "4 hydroxyphenethylamine" or lyramine or mydrial or "para hydroxyphenethylamine" or paratyramine or systogene or tiramine or tocosine or tyramin or tyrosamine or uteramine or "51‐67‐2" or "60‐19‐5" #60 Vibrocil or "8059‐14‐1" #61 MeSH descriptor: [Yohimbine] explode all trees #62 Yohimbine or actibine or aphrodine or aphrodyne or Corynanthine or "corynine hydrochloride" or "dayto‐himbin" or "methyl yohimbine 16alpha carboxylate" or "methylyohimbane 16alpha carboxylate" or Pluriviron or quebrachin or "quebrachine hydrochloride" or Rauhimbine or Rauwolscine or urobine or yobin or yobinol or yocan or yocaral or Yocon or yocon or yohimbe or "yohimbic acid methyl ester" or yohimbin or Yohimex or yohimex or yohimibin or yovital or "146‐48‐5" or "65‐19‐0" #63 MeSH descriptor: [Timolol] explode all trees #64 timolol* or "apo timol" or "apo timop" or apotimol or apotimolol or apotimop or betimol or Blocadren or "chibro timoptol" or istalol or "l 714465" or l714465 or "MK 950" or MK950 or moducren or nyolol or ofal or ofan or optimol or timolo or Timoptic or Timoptol or Timacar or titol or "26839‐75‐8" #65 MeSH descriptor: [Pirenzepine] explode all trees #66 pirenzepin* or abrinac or azuzepin or bisvanil or "cl 2" or cl2 or gastricur or gastrocepin or gastrozepin or gastrozepina or gastrozepine or leblon or "ls 519" or "ls 519c12" or ls519 or ls519c12 or maghen or tabe or zinc00538196 or Pyrenzepine or "L S 519" or Ulgescum or "Piren basan" or Gastrotsepin or Ulcoprotect or "28797‐61‐7" or "29868‐97‐1" #67 {or #12‐#66} #68 #11 or #67 #69 #5 and #68
Appendix 2. MEDLINE Ovid search strategy
1. Randomized Controlled Trial.pt. 2. Controlled Clinical Trial.pt. 3. (randomized or randomised).ab,ti. 4. placebo.ab,ti. 5. drug therapy.fs. 6. randomly.ab,ti. 7. trial.ab,ti. 8. groups.ab,ti. 9. 1 or 2 or 3 or 4 or 5 or 6 or 7 or 8 10. exp animals/ not humans.sh. 11. 9 not 10 12. exp myopia/ 13. myop*.tw. 14. ((short or near) adj3 sight*).tw. 15. nearsighted*.tw. 16. or/12‐15 17. exp Eyeglasses/ 18. (spectacles or glasses or eyeglass*).tw. 19. ((progressive or single or vision or addition or bifocal or spectacle or corrective) adj2 lens*).tw. 20. exp contact lenses/ 21. ((contact or "gas permeable") adj2 lens*).tw. 22. or/17‐21 23. exp Mydriatics/ 24. (mydriat* or cycloplegic*).tw. 25. ("7‐methylxanthine" or "552‐62‐5").tw,rn. 26. exp cholinergic antagonists/ 27. (cholinergic adj2 (antagonist* or block* or inhibitor*)).tw. 28. cholinolytic*.tw. 29. (acetylcholine* adj2 (antagonist* or block* or inhibitor*)).tw. 30. (anticholinergic* or "anti cholinergic" or "anti cholinergics").tw. 31. (muscarinic* adj2 (antagonist* or block* or inhibitor*)).tw. 32. (antimuscarinic* or "anti muscarinic" or "anti muscarinics").tw. 33. exp Parasympatholytics/ 34. (parasympathetic* adj2 (antagonist* or block* or inhibitor*)).tw. 35. (parasympathicolytic* or parasympaticolytic* or Parasympatholytic*).tw. 36. (pharmaceutical* or pharmacologic*).tw. 37. exp Atropine/ 38. (Atropine or atrinal or "atro‐polygyl" or atrop or atropen or atropin or atropina or "atropini sulfas" or atropinol or atropisol or atropt or atroptol or atrospan or "atrosulf‐1" or "bar bropin" or "bellpino‐artin" or "cendo tropine" or "dextro levo hyosciamine" or "ichtho bellol" or "isopto" or isoptoAtropine or "ocu‐tropine" or "sal‐tropine" or skiatropine or "tropine dextro levo tropate" or ximex or "51‐55‐8" or "55‐48‐1").tw,rn. 39. (berefrine or POPD or "105567‐83‐7").tw,rn. 40. exp Cyclopentolate/ 41. (Cyclopentolate or "ak‐pentolate" or akpentolate or "bell pentolate" or ciclolux or cyclogyl or cyclomydri or cyclopentol or cyclopentolat or cylate or cyplegin or diopentolate or midriodavi or mydrilate or "ocu‐pentolate" or ocucyclo or "oftan‐syklo" or pentolair or "refractyl ofeno" or skiacol or zyklolat or "512‐15‐2" or "5870‐29‐1").tw,rn. 42. exp Epinephrine/ 43. (Epinephrine or Adrenaline or adrenalin or Epitrate or Lyophrin or Epifrin or adnephrin or adnephrine or adrenaclick or "adrenal hydrochloride" or adrenalina or adrenamine or adrenapax or adrenazin or adrenine or adrin or adrine or advaradin or balmadren or biorenine or bosmin or chelafrin or dylephrin or epiglaufrin or epimephrine or epinefrina or epinephran or epinephrin or epirenamine or epirenan or exadrin or glaucon or glaucosan or glaufrin or "glin epin" or glycirenan or haemostatin or hemisine or hemostasin or hemostatin or hypernephrin or "isopto epinal" or levoadrenalin or levoadrenaline or levoepinephrine or levorenin or levorenine or methylaminoethanolcatechol or methylarterenol or mucidrina or myosthenine or "n methylnoradrenalin" or nephridine or nieraline or paranephrin or posumin or renaglandin or renaglandulin or renaleptine or renalina or renaline or renoform or renostypticin or renostyptin or scurenaline or simplene or soladren or sphygmogenin or styptirenal or supracapsulin or supranephrane or supranephrin or supranol or suprarenaline or suprarenin or suprarenine or suprel or surenine or surrenine or "sus‐phrine sulfite‐free" or susphrine or "sympathin I" or takamina or tonogen or vasoconstrictine or vasodrine or vasotonin or weradren or "51‐43‐4" or "55‐31‐2" or "6912‐68‐1").tw,rn. 44. exp Ethylmorphine/ 45. (Ethylmorphine or Ethomorphine or Trachyl or codethyline or diolan or dionine or "ethyl morphine" or ethylmorfine or ethylmorphin or "morphine ethyl ether" or "125‐30‐4" or "76‐58‐4").tw,rn. 46. (Eucatropine or euphthalmine or "100‐91‐4" or "536‐93‐6").tw,rn. 47. (Homatropine or homatro or homatrocil or homatropaire or homatropin or homatropina or isoptoHomatropine or "I Homatrine" or "mandelyl tropeine" or mandelyltropeine or "mydryn eye" or "omatropina lux" or "tropine mandelate" or "51‐56‐9" or "87‐00‐3").tw,rn. 48. exp Hyoscyamine/ 49. (Hyoscyamine or anaspaz or cystospaz or cytospaz or daturine or donnamar or duboisine or egacen or hyoscamine or hyosciamine or hyoscyanin or hyosyne or "ib‐stat" or levbid or levsin or "levsinex sr" or neosol or nulev or spasdel or "symax sl" or "symax sr" or "tropine l tropate" or "101‐31‐5" or "306‐03‐6").tw,rn. 50. (Ibopamine or "N‐methyldopamine diisobutyrate" or "SB 7505" or "SB7505" or Escandine or Inopamil or "diisobutyric n methyldopamine ester" or scandine or "skf 100168" or "skf 100168 a" or "66195‐31‐1" or "75011‐65‐3").tw,rn. 51. (Methylatropine or "8‐methylatropinium nitrate" or "31610‐87‐4").tw,rn. 52. exp Naphazoline/ 53. (Naphazoline or "Afazol Grin" or "AK Con" or AKCon or Albalon or albasol or "All Clear" or allersol or antan or benil or cefasan or "Clear Eyes" or coldan or "Colirio Alfa" or "comfort eye drops" or dazolin or "degest 2" or derinox or Idril or imidin or minha or Miraclar or mirafrin or Nafazair or nafazoline or naftazolina or "naphacel ofteno" or naphasal or naphazolin or Naphcon or "naphozoline hydrochloride" or naphtears or naphthazoline or naphthizine or naphthyzin or nastizol or "nazil ofteno" or niazol or "ocu‐zoline" or opcon or Optazine or Privin or privina or Privine or privine or Proculin or rhinantin or rhinazin or rhinoperd or rimidol or sanorin or sanotin or Siozwo or strictylon or "Tele Stulln" or TeleStulln or Vasoclear or Vasocon or "Vasoconstrictor Pensa" or VasoNit or vistalbalon or vistobalon or "5144‐52‐5" or "550‐99‐2" or "835‐31‐4").tw,rn. 54. (Oxedrine or Synephrine or Sympaethamin or Synephrin or aetaphen or pentedrine or vasoton or "94‐07‐5").tw,rn. 55. exp Synephrine/ 56. exp Oxyphenonium/ 57. (Oxyphenonium or Methacin or Oxyphenon or Atrenyl or Spastrex or antrenyl or "ba 5473" or ba5473 or "c 5473" or c5473 or helkamon or metacin or metacinum or oxyphenium or "oxyphenomium bromide" or spasmofen or spasmophen or "14214‐84‐7" or "50‐10‐2").tw,rn. 58. exp Phenylephrine/ 59. (Phenylephrine or adrianol or "af‐taf" or "ak‐dilate" or "albalon relief" or alconefrin or almefrin or altafrin or biomidrin or biomydrin or derizene or "despec‐sf" or "disneumon pernasal" or drosin or "efrin‐10" or efrisel or fenylephrine or idrianol or isonefrine or isophrin or isophrine or "isopto frin" or isoptofrin or lexatol or "m synephrine" or mesaton or "meta sympathol" or "meta synephrine" or Metaoxedrin or metaoxedrine or Metasympatol or metasynephrine or Mezaton or "murucoll 2" or mydfrin or "n 105 to" or "nefrin‐ofteno" or "Neo Synephrine" or neofrin or neooxedrine or neophryn or neosynephrin or Neosynephrine or "neosynephrin‐pos" or neosynesin or neosynesine or "ocu‐phrin" or "oftan‐metaoksedrin" or optistin or phenoptic or phenylefrine or phenylephedrine or prefrin or "pupiletto forte" or rectasol or "rhinall 10" or "slv 325" or slv325 or sucraphen or vazculep or visadron or vistafrin or vistosan or "532‐38‐7" or "59‐42‐7" or "61‐76‐7").tw,rn. 60. (Pholedrine or "4 hydroxy n methylamphetamine" or "4 hydroxymethamphetamine" or adyston or "para hydroxymethamphetamine" or "p‐hydroxymethamphetamine" or paredrinol or "Pholedrin liquidum" or "Pholedrin‐longo‐Isis" or pulsotyl or venosan or veritol or "370‐14‐9").tw,rn. 61. exp p‐Hydroxyamphetamine/ 62. (p‐Hydroxyamphetamine or "1 para hydroxyphenyl 2 propylamine" or "alpha methyl para tyramine" or "alpha methyl tyramine" or "dl 1 p hydroxyphenyl 2 propylamine" or "dl 1 para hydroxyphenyl 2 propylamine" or "dl p hydroxy alpha methylphenethylamine" or "dl para hydroxy alpha methylphenethylamine" or "h 66 37" or "para hydroxy alpha methylphenethylamine" or Hydroxyamfetamine or Hydroxyamphetamin or Hydroxyamphetamine or Hydroxyphenylisopropylamine or Methyltyramine or Norpholedrin or norpholedrine or oxamphetamine or Oxyamphetamine or paradrine or parahydroxyamphetamine or Paredrine or paredrinea or paredrinex or pedrolone or pulsoton or "103‐86‐6" or "1518‐86‐1" or "306‐21‐8").tw,rn. 63. exp Racepinephrine/ 64. (Racepinephrine or asthmanefrin or Micronefrin or micronefrine or Micronephrine or mikronephrin or racadrenalin or "Racepinefrine Hydrochloride" or racinephrine or Vaponefrin or vaponefrine or vaponephrin or "329‐65‐7").tw,rn. 65. exp Scopolamine Hydrobromide/ 66. (Scopolamine or "Boro Scopol" or BoroScopol or Hyoscine or Kwells or "levo hyoscinehydrobromide" or Scoburen or Scopace or scopos or "Travacalm HO" or Vorigeno or "114‐49‐8" or atrochin or atroquin or atroscine or hyosceine or hysco or "kimite‐patch" or "l epoxytropine tropate" or "n methylhyoscine" or oscine or scopalamine or "scopine tropate" or scopoderm or scopolamin or transcop or "transderm scop" or "transderm v" or "tropic acid ester with scopine" or "138‐12‐5" or "51‐34‐3" or "55‐16‐3").tw,rn. 67. exp Tropicamide/ 68. (Tropicamide or "alcon‐mydril" or bistropamide or "cendo mydriatyl" or "Colircusi Tropicamida" or midriaticum or mydiacyl or mydral or mydramide or mydriacyl or Mydriafair or Mydriaticum or "mydrin m" or "mydrin p" or Mydrum or "n ethyl 2 phenyl n pyrid 4 ylmethylhydracrylamide" or "n ethyl n 4 picolyltropamide" or "n ethyl n gamma picolyltropamide" or "n ethyl n pyrid 4 ylmethyltropamide" or "Ocu‐Tropic" or OcuTropic or opticyl or sandol or sintropic or "tropamid forte" or "tropic acid n ethyl n gamma picolyl amide" or Tropicacyl or tropicamid or "tropico eye" or tropicol or tropikamid or tropimil or visumidriatic or "1508‐75‐4").tw,rn. 69. exp Tyramine/ 70. (Tyramine or "4 hydroxyphenethylamine" or lyramine or mydrial or "para hydroxyphenethylamine" or paratyramine or systogene or tiramine or tocosine or tyramin or tyrosamine or uteramine or "51‐67‐2" or "60‐19‐5").tw,rn. 71. (Vibrocil or "8059‐14‐1").tw,rn. 72. exp Yohimbine/ 73. (Yohimbine or actibine or aphrodine or aphrodyne or Corynanthine or "corynine hydrochloride" or "dayto‐himbin" or "methyl yohimbine 16alpha carboxylate" or "methylyohimbane 16alpha carboxylate" or Pluriviron or quebrachin or "quebrachine hydrochloride" or Rauhimbine or Rauwolscine or urobine or yobin or yobinol or yocan or yocaral or Yocon or yocon or yohimbe or "yohimbic acid methyl ester" or yohimbin or Yohimex or yohimex or yohimibin or yovital or "146‐48‐5" or "65‐19‐0").tw,rn. 74. exp Timolol/ 75. (timolol* or "apo timol" or "apo timop" or apotimol or apotimolol or apotimop or betimol or Blocadren or "chibro timoptol" or istalol or "l 714465" or l714465 or "MK 950" or MK950 or moducren or nyolol or ofal or ofan or optimol or timolo or Timoptic or Timoptol or Timacar or titol or "26839‐75‐8").tw,rn. 76. exp Pirenzepine/ 77. (pirenzepin* or abrinac or azuzepin or bisvanil or "cl 2" or cl2 or gastricur or gastrocepin or gastrozepin or gastrozepina or gastrozepine or leblon or "ls 519" or "ls 519c12" or ls519 or ls519c12 or maghen or tabe or zinc00538196 or Pyrenzepine or "L S 519" or Ulgescum or "Piren basan" or Gastrotsepin or Ulcoprotect or "28797‐61‐7" or "29868‐97‐1").tw,rn. 78. or/23‐77 79. 22 or 78 80. 11 and 16 and 79
The search filter for trials at the beginning of the MEDLINE strategy is from the published paper by Glanville (Glanville 2006).
Appendix 3. Embase.com search strategy
#1 'randomized controlled trial'/exp #2 'randomization'/exp #3 'double blind procedure'/exp #4 'single blind procedure'/exp #5 random*:ab,ti #6 #1 OR #2 OR #3 OR #4 OR #5 #7 'animal'/exp OR 'animal experiment'/exp #8 'human'/exp #9 #7 AND #8 #10 #7 NOT #9 #11 #6 NOT #10 #12 'clinical trial'/exp #13 (clin* NEAR/3 trial*):ab,ti #14 ((singl* OR doubl* OR trebl* OR tripl*) NEAR/3 (blind* OR mask*)):ab,ti #15 'placebo'/exp #16 placebo*:ab,ti #17 random*:ab,ti #18 'experimental design'/exp #19 'crossover procedure'/exp #20 'control group'/exp #21 'latin square design'/exp #22 #12 OR #13 OR #14 OR #15 OR #16 OR #17 OR #18 OR #19 OR #20 OR #21 #23 #22 NOT #10 #24 #23 NOT #11 #25 'comparative study'/exp #26 'evaluation'/exp #27 'prospective study'/exp #28 control*:ab,ti OR prospectiv*:ab,ti OR volunteer*:ab,ti #29 #25 OR #26 OR #27 OR #28 #30 #29 NOT #10 #31 #30 NOT (#11 OR #23) #32 #11 OR #24 OR #31 #33 'myopia'/exp #34 myop*:ab,ti #35 ((short OR near) NEAR/3 sight*):ab,ti #36 nearsighted*:ab,ti #37 #33 OR #34 OR #35 OR #36 #38 'spectacles'/exp #39 spectacles:ab,ti OR glasses:ab,ti OR eyeglass*:ab,ti #40 ((progressive OR single OR vision OR addition OR bifocal OR spectacle OR corrective) NEAR/2 lens*):ab,ti #41 'contact lens'/exp #42 ((contact OR 'gas permeable') NEAR/2 lens*):ab,ti #43 #38 OR #39 OR #40 OR #41 OR #42 #44 'mydriatic agent'/exp #45 mydriat*:ab,ti OR cycloplegic*:ab,ti #46 'cholinergic receptor blocking agent'/exp #47 (cholinergic* NEXT/2 (antagonist* OR block* OR inhibitor*)):ab,ti #48 cholinolytic*:ab,ti #49 (acetylcholine* NEXT/2 (antagonist* OR block* OR inhibitor*)):ab,ti #50 anticholinergic*:ab,ti OR 'anti cholinergic':ab,ti OR 'anti cholinergics':ab,ti #51 (muscarinic* NEXT/2 (antagonist* OR block* OR inhibitor*)):ab,ti #52 antimuscarinic*:ab,ti OR 'anti muscarinic':ab,ti OR 'anti muscarinics':ab,ti #53 (parasympathetic* NEXT/2 (antagonist* OR block* OR inhibitor*)):ab,ti #54 parasympathicolytic*:ab,ti OR parasympaticolytic*:ab,ti OR parasympatholytic*:ab,ti #55 pharmaceutical*:ab,ti OR pharmacologic*:ab,ti #56 atropine:tn,ab,ti OR atrinal:tn,ab,ti OR 'atro‐polygyl':tn,ab,ti OR atrop:tn,ab,ti OR atropen:tn,ab,ti OR atropin:tn,ab,ti OR atropina:tn,ab,ti OR 'atropini sulfas':tn,ab,ti OR atropinol:tn,ab,ti OR atropisol:tn,ab,ti OR atropt:tn,ab,ti OR atroptol:tn,ab,ti OR atrospan:tn,ab,ti OR 'atrosulf‐1':tn,ab,ti OR 'bar bropin':tn,ab,ti OR 'bellpino‐artin':tn,ab,ti OR 'cendo tropine':tn,ab,ti OR 'dextro levo hyosciamine':tn,ab,ti OR 'ichtho bellol':tn,ab,ti OR 'isopto':tn,ab,ti OR isoptoatropine:tn,ab,ti OR 'ocu‐tropine':tn,ab,ti OR 'sal‐tropine':tn,ab,ti OR skiatropine:tn,ab,ti OR 'tropine dextro levo tropate':tn,ab,ti OR ximex:tn,ab,ti OR '51‐55‐8':tn,ab,ti OR '55‐48‐1':tn,ab,ti #57 berefrine:tn,ab,ti OR popd:tn,ab,ti OR '105567‐83‐7':tn,ab,ti #58 cyclopentolate:tn,ab,ti OR 'ak‐pentolate':tn,ab,ti OR akpentolate:tn,ab,ti OR 'bell pentolate':tn,ab,ti OR ciclolux:tn,ab,ti OR cyclogyl:tn,ab,ti OR cyclomydri:tn,ab,ti OR cyclopentol:tn,ab,ti OR cyclopentolat:tn,ab,ti OR cylate:tn,ab,ti OR cyplegin:tn,ab,ti OR diopentolate:tn,ab,ti OR midriodavi:tn,ab,ti OR mydrilate:tn,ab,ti OR 'ocu‐pentolate':tn,ab,ti OR ocucyclo:tn,ab,ti OR 'oftan‐syklo':tn,ab,ti OR pentolair:tn,ab,ti OR 'refractyl ofeno':tn,ab,ti OR skiacol:tn,ab,ti OR zyklolat:tn,ab,ti OR '512‐15‐2':tn,ab,ti OR '5870‐29‐1':tn,ab,ti #59 'adrenalin'/exp #60 epinephrine:tn,ab,ti OR adrenaline:tn,ab,ti OR adrenalin:tn,ab,ti OR epitrate:tn,ab,ti OR lyophrin:tn,ab,ti OR epifrin:tn,ab,ti OR adnephrin:tn,ab,ti OR adnephrine:tn,ab,ti OR adrenaclick:tn,ab,ti OR 'adrenal hydrochloride':tn,ab,ti OR adrenalina:tn,ab,ti OR adrenamine:tn,ab,ti OR adrenapax:tn,ab,ti OR adrenazin:tn,ab,ti OR adrenine:tn,ab,ti OR adrin:tn,ab,ti OR adrine:tn,ab,ti OR advaradin:tn,ab,ti OR balmadren:tn,ab,ti OR biorenine:tn,ab,ti OR bosmin:tn,ab,ti OR chelafrin:tn,ab,ti OR dylephrin:tn,ab,ti OR epiglaufrin:tn,ab,ti OR epimephrine:tn,ab,ti OR epinefrina:tn,ab,ti OR epinephran:tn,ab,ti OR epinephrin:tn,ab,ti OR epirenamine:tn,ab,ti OR epirenan:tn,ab,ti OR exadrin:tn,ab,ti OR glaucon:tn,ab,ti OR glaucosan:tn,ab,ti OR glaufrin:tn,ab,ti OR 'glin epin':tn,ab,ti OR glycirenan:tn,ab,ti OR haemostatin:tn,ab,ti OR hemisine:tn,ab,ti OR hemostasin:tn,ab,ti OR hemostatin:tn,ab,ti OR hypernephrin:tn,ab,ti OR 'isopto epinal':tn,ab,ti OR levoadrenalin:tn,ab,ti OR levoadrenaline:tn,ab,ti OR levoepinephrine:tn,ab,ti OR levorenin:tn,ab,ti OR levorenine:tn,ab,ti OR methylaminoethanolcatechol:tn,ab,ti OR methylarterenol:tn,ab,ti OR mucidrina:tn,ab,ti OR myosthenine:tn,ab,ti OR 'n methylnoradrenalin':tn,ab,ti OR nephridine:tn,ab,ti OR nieraline:tn,ab,ti OR paranephrin:tn,ab,ti OR posumin:tn,ab,ti OR renaglandin:tn,ab,ti OR renaglandulin:tn,ab,ti OR renaleptine:tn,ab,ti OR renalina:tn,ab,ti OR renaline:tn,ab,ti OR renoform:tn,ab,ti OR renostypticin:tn,ab,ti OR renostyptin:tn,ab,ti OR scurenaline:tn,ab,ti OR simplene:tn,ab,ti OR soladren:tn,ab,ti OR sphygmogenin:tn,ab,ti OR styptirenal:tn,ab,ti OR supracapsulin:tn,ab,ti OR supranephrane:tn,ab,ti OR supranephrin:tn,ab,ti OR supranol:tn,ab,ti OR suprarenaline:tn,ab,ti OR suprarenin:tn,ab,ti OR suprarenine:tn,ab,ti OR suprel:tn,ab,ti OR surenine:tn,ab,ti OR surrenine:tn,ab,ti OR 'sus‐phrine sulfite‐free':tn,ab,ti OR susphrine:tn,ab,ti OR 'sympathin i':tn,ab,ti OR takamina:tn,ab,ti OR tonogen:tn,ab,ti OR vasoconstrictine:tn,ab,ti OR vasodrine:tn,ab,ti OR vasotonin:tn,ab,ti OR weradren:tn,ab,ti OR '51‐43‐4':tn,ab,ti OR '55‐31‐2':tn,ab,ti OR '6912‐68‐1':tn,ab,ti #61 ethylmorphine:tn,ab,ti OR ethomorphine:tn,ab,ti OR trachyl:tn,ab,ti OR codethyline:tn,ab,ti OR diolan:tn,ab,ti OR dionine:tn,ab,ti OR 'ethyl morphine':tn,ab,ti OR ethylmorfine:tn,ab,ti OR ethylmorphin:tn,ab,ti OR 'morphine ethyl ether':tn,ab,ti OR '125‐30‐4':tn,ab,ti OR '76‐58‐4':tn,ab,ti #62 eucatropine:tn,ab,ti OR euphthalmine:tn,ab,ti OR '100‐91‐4':tn,ab,ti OR '536‐93‐6':tn,ab,ti #63 homatropine:tn,ab,ti OR homatro:tn,ab,ti OR homatrocil:tn,ab,ti OR homatropaire:tn,ab,ti OR homatropin:tn,ab,ti OR homatropina:tn,ab,ti OR isoptohomatropine:tn,ab,ti OR 'i homatrine':tn,ab,ti OR 'mandelyl tropeine':tn,ab,ti OR mandelyltropeine:tn,ab,ti OR 'mydryn eye':tn,ab,ti OR 'omatropina lux':tn,ab,ti OR 'tropine mandelate':tn,ab,ti OR '51‐56‐9':tn,ab,ti OR '87‐00‐3':tn,ab,ti #64 'hyoscyamine'/exp #65 hyoscyamine:tn,ab,ti OR anaspaz:tn,ab,ti OR cystospaz:tn,ab,ti OR cytospaz:tn,ab,ti OR daturine:tn,ab,ti OR donnamar:tn,ab,ti OR duboisine:tn,ab,ti OR egacen:tn,ab,ti OR hyoscamine:tn,ab,ti OR hyosciamine:tn,ab,ti OR hyoscyanin:tn,ab,ti OR hyosyne:tn,ab,ti OR 'ib‐stat':tn,ab,ti OR levbid:tn,ab,ti OR levsin:tn,ab,ti OR 'levsinex sr':tn,ab,ti OR neosol:tn,ab,ti OR nulev:tn,ab,ti OR spasdel:tn,ab,ti OR 'symax sl':tn,ab,ti OR 'symax sr':tn,ab,ti OR 'tropine l tropate':tn,ab,ti OR '101‐31‐5':tn,ab,ti OR '306‐03‐6':tn,ab,ti #66 'ibopamine'/exp #67 ibopamine:tn,ab,ti OR 'n‐methyldopamine diisobutyrate':tn,ab,ti OR 'sb 7505':tn,ab,ti OR 'sb7505':tn,ab,ti OR escandine:tn,ab,ti OR inopamil:tn,ab,ti OR 'diisobutyric n methyldopamine ester':tn,ab,ti OR scandine:tn,ab,ti OR 'skf 100168':tn,ab,ti OR 'skf 100168 a':tn,ab,ti OR '66195‐31‐1':tn,ab,ti OR '75011‐65‐3':tn,ab,ti #68 methylatropine:tn,ab,ti OR '8‐methylatropinium nitrate':tn,ab,ti OR '31610‐87‐4':tn,ab,ti #69 naphazoline:tn,ab,ti OR 'afazol grin':tn,ab,ti OR 'ak con':tn,ab,ti OR akcon:tn,ab,ti OR albalon:tn,ab,ti OR albasol:tn,ab,ti OR 'all clear':tn,ab,ti OR allersol:tn,ab,ti OR antan:tn,ab,ti OR benil:tn,ab,ti OR cefasan:tn,ab,ti OR 'clear eyes':tn,ab,ti OR coldan:tn,ab,ti OR 'colirio alfa':tn,ab,ti OR 'comfort eye drops':tn,ab,ti OR dazolin:tn,ab,ti OR 'degest 2':tn,ab,ti OR derinox:tn,ab,ti OR idril:tn,ab,ti OR imidin:tn,ab,ti OR minha:tn,ab,ti OR miraclar:tn,ab,ti OR mirafrin:tn,ab,ti OR nafazair:tn,ab,ti OR nafazoline:tn,ab,ti OR naftazolina:tn,ab,ti OR 'naphacel ofteno':tn,ab,ti OR naphasal:tn,ab,ti OR naphazolin:tn,ab,ti OR naphcon:tn,ab,ti OR 'naphozoline hydrochloride':tn,ab,ti OR naphtears:tn,ab,ti OR naphthazoline:tn,ab,ti OR naphthizine:tn,ab,ti OR naphthyzin:tn,ab,ti OR nastizol:tn,ab,ti OR 'nazil ofteno':tn,ab,ti OR niazol:tn,ab,ti OR 'ocu‐zoline':tn,ab,ti OR opcon:tn,ab,ti OR optazine:tn,ab,ti OR privin:tn,ab,ti OR privina:tn,ab,ti OR privine:tn,ab,ti OR proculin:tn,ab,ti OR rhinantin:tn,ab,ti OR rhinazin:tn,ab,ti OR rhinoperd:tn,ab,ti OR rimidol:tn,ab,ti OR sanorin:tn,ab,ti OR sanotin:tn,ab,ti OR siozwo:tn,ab,ti OR strictylon:tn,ab,ti OR 'tele stulln':tn,ab,ti OR telestulln:tn,ab,ti OR vasoclear:tn,ab,ti OR vasocon:tn,ab,ti OR 'vasoconstrictor pensa':tn,ab,ti OR vasonit:tn,ab,ti OR vistalbalon:tn,ab,ti OR vistobalon:tn,ab,ti OR '5144‐52‐5':tn,ab,ti OR '550‐99‐2':tn,ab,ti OR '835‐31‐4':tn,ab,ti #70 oxedrine:tn,ab,ti OR synephrine:tn,ab,ti OR sympaethamin:tn,ab,ti OR synephrin:tn,ab,ti OR aetaphen:tn,ab,ti OR pentedrine:tn,ab,ti OR vasoton:tn,ab,ti OR '94‐07‐5':tn,ab,ti #71 'oxyphenonium bromide'/exp #72 oxyphenonium:tn,ab,ti OR methacin:tn,ab,ti OR oxyphenon:tn,ab,ti OR atrenyl:tn,ab,ti OR spastrex:tn,ab,ti OR antrenyl:tn,ab,ti OR 'ba 5473':tn,ab,ti OR ba5473:tn,ab,ti OR 'c 5473':tn,ab,ti OR c5473:tn,ab,ti OR helkamon:tn,ab,ti OR metacin:tn,ab,ti OR metacinum:tn,ab,ti OR oxyphenium:tn,ab,ti OR 'oxyphenomium bromide':tn,ab,ti OR spasmofen:tn,ab,ti OR spasmophen:tn,ab,ti OR '14214‐84‐7':tn,ab,ti OR '50‐10‐2':tn,ab,ti #73 phenylephrine:tn,ab,ti OR adrianol:tn,ab,ti OR 'af‐taf':tn,ab,ti OR 'ak‐dilate':tn,ab,ti OR 'albalon relief':tn,ab,ti OR alconefrin:tn,ab,ti OR almefrin:tn,ab,ti OR altafrin:tn,ab,ti OR biomidrin:tn,ab,ti OR biomydrin:tn,ab,ti OR derizene:tn,ab,ti OR 'despec‐sf':tn,ab,ti OR 'disneumon pernasal':tn,ab,ti OR drosin:tn,ab,ti OR 'efrin‐10':tn,ab,ti OR efrisel:tn,ab,ti OR fenylephrine:tn,ab,ti OR idrianol:tn,ab,ti OR isonefrine:tn,ab,ti OR isophrin:tn,ab,ti OR isophrine:tn,ab,ti OR 'isopto frin':tn,ab,ti OR isoptofrin:tn,ab,ti OR lexatol:tn,ab,ti OR 'm synephrine':tn,ab,ti OR mesaton:tn,ab,ti OR 'meta sympathol':tn,ab,ti OR 'meta synephrine':tn,ab,ti OR metaoxedrin:tn,ab,ti OR metaoxedrine:tn,ab,ti OR metasympatol:tn,ab,ti OR metasynephrine:tn,ab,ti OR mezaton:tn,ab,ti OR 'murucoll 2':tn,ab,ti OR mydfrin:tn,ab,ti OR 'n 105 to':tn,ab,ti OR 'nefrin‐ofteno':tn,ab,ti OR 'neo synephrine':tn,ab,ti OR neofrin:tn,ab,ti OR neooxedrine:tn,ab,ti OR neophryn:tn,ab,ti OR neosynephrin:tn,ab,ti OR neosynephrine:tn,ab,ti OR 'neosynephrin‐pos':tn,ab,ti OR neosynesin:tn,ab,ti OR neosynesine:tn,ab,ti OR 'ocu‐phrin':tn,ab,ti OR 'oftan‐metaoksedrin':tn,ab,ti OR optistin:tn,ab,ti OR phenoptic:tn,ab,ti OR phenylefrine:tn,ab,ti OR phenylephedrine:tn,ab,ti OR prefrin:tn,ab,ti OR 'pupiletto forte':tn,ab,ti OR rectasol:tn,ab,ti OR 'rhinall 10':tn,ab,ti OR 'slv 325':tn,ab,ti OR slv325:tn,ab,ti OR sucraphen:tn,ab,ti OR vazculep:tn,ab,ti OR visadron:tn,ab,ti OR vistafrin:tn,ab,ti OR vistosan:tn,ab,ti OR '532‐38‐7':tn,ab,ti OR '59‐42‐7':tn,ab,ti OR '61‐76‐7':tn,ab,ti #74 pholedrine:tn,ab,ti OR '4 hydroxy n methylamphetamine':tn,ab,ti OR '4 hydroxymethamphetamine':tn,ab,ti OR adyston:tn,ab,ti OR 'para hydroxymethamphetamine':tn,ab,ti OR 'p‐hydroxymethamphetamine':tn,ab,ti OR paredrinol:tn,ab,ti OR 'pholedrin liquidum':tn,ab,ti OR 'pholedrin‐longo‐isis':tn,ab,ti OR pulsotyl:tn,ab,ti OR venosan:tn,ab,ti OR veritol:tn,ab,ti OR '370‐14‐9':tn,ab,ti #75 'hydroxyamphetamine'/exp #76 'p hydroxyamphetamine':tn,ab,ti OR '1 para hydroxyphenyl 2 propylamine':tn,ab,ti OR 'alpha methyl para tyramine':tn,ab,ti OR 'alpha methyl tyramine':tn,ab,ti OR 'dl 1 p hydroxyphenyl 2 propylamine':tn,ab,ti OR 'dl 1 para hydroxyphenyl 2 propylamine':tn,ab,ti OR 'dl p hydroxy alpha methylphenethylamine':tn,ab,ti OR 'dl para hydroxy alpha methylphenethylamine':tn,ab,ti OR 'h 66 37':tn,ab,ti OR 'para hydroxy alpha methylphenethylamine':tn,ab,ti OR hydroxyamfetamine:tn,ab,ti OR hydroxyamphetamin:tn,ab,ti OR hydroxyamphetamine:tn,ab,ti OR hydroxyphenylisopropylamine:tn,ab,ti OR methyltyramine:tn,ab,ti OR norpholedrin:tn,ab,ti OR norpholedrine:tn,ab,ti OR oxamphetamine:tn,ab,ti OR oxyamphetamine:tn,ab,ti OR paradrine:tn,ab,ti OR parahydroxyamphetamine:tn,ab,ti OR paredrine:tn,ab,ti OR paredrinea:tn,ab,ti OR paredrinex:tn,ab,ti OR pedrolone:tn,ab,ti OR pulsoton:tn,ab,ti OR '103‐86‐6':tn,ab,ti OR '1518‐86‐1':tn,ab,ti OR '306‐21‐8':tn,ab,ti #77 'racepinefrine'/exp #78 racepinephrine:tn,ab,ti OR asthmanefrin:tn,ab,ti OR micronefrin:tn,ab,ti OR micronefrine:tn,ab,ti OR micronephrine:tn,ab,ti OR mikronephrin:tn,ab,ti OR racadrenalin:tn,ab,ti OR 'racepinefrine hydrochloride':tn,ab,ti OR racinephrine:tn,ab,ti OR vaponefrin:tn,ab,ti OR vaponefrine:tn,ab,ti OR vaponephrin:tn,ab,ti OR '329‐65‐7':tn,ab,ti #79 'scopolamine bromide'/exp #80 scopolamine:tn,ab,ti OR 'boro scopol':tn,ab,ti OR boroscopol:tn,ab,ti OR hyoscine:tn,ab,ti OR kwells:tn,ab,ti OR 'levo hyoscinehydrobromide':tn,ab,ti OR scoburen:tn,ab,ti OR scopace:tn,ab,ti OR scopos:tn,ab,ti OR 'travacalm ho':tn,ab,ti OR vorigeno:tn,ab,ti OR '114‐49‐8':tn,ab,ti OR atrochin:tn,ab,ti OR atroquin:tn,ab,ti OR atroscine:tn,ab,ti OR hyosceine:tn,ab,ti OR hysco:tn,ab,ti OR 'kimite‐patch':tn,ab,ti OR 'l epoxytropine tropate':tn,ab,ti OR 'n methylhyoscine':tn,ab,ti OR oscine:tn,ab,ti OR scopalamine:tn,ab,ti OR 'scopine tropate':tn,ab,ti OR scopoderm:tn,ab,ti OR scopolamin:tn,ab,ti OR transcop:tn,ab,ti OR 'transderm scop':tn,ab,ti OR 'transderm v':tn,ab,ti OR 'tropic acid ester with scopine':tn,ab,ti OR '138‐12‐5':tn,ab,ti OR '51‐34‐3':tn,ab,ti OR '55‐16‐3':tn,ab,ti #81 tropicamide:tn,ab,ti OR 'alcon‐mydril':tn,ab,ti OR bistropamide:tn,ab,ti OR 'cendo mydriatyl':tn,ab,ti OR 'colircusi tropicamida':tn,ab,ti OR midriaticum:tn,ab,ti OR mydiacyl:tn,ab,ti OR mydral:tn,ab,ti OR mydramide:tn,ab,ti OR mydriacyl:tn,ab,ti OR mydriafair:tn,ab,ti OR mydriaticum:tn,ab,ti OR 'mydrin m':tn,ab,ti OR 'mydrin p':tn,ab,ti OR mydrum:tn,ab,ti OR 'n ethyl 2 phenyl n pyrid 4 ylmethylhydracrylamide':tn,ab,ti OR 'n ethyl n 4 picolyltropamide':tn,ab,ti OR 'n ethyl n gamma picolyltropamide':tn,ab,ti OR 'n ethyl n pyrid 4 ylmethyltropamide':tn,ab,ti OR 'ocu‐tropic':tn,ab,ti OR ocutropic:tn,ab,ti OR opticyl:tn,ab,ti OR sandol:tn,ab,ti OR sintropic:tn,ab,ti OR 'tropamid forte':tn,ab,ti OR 'tropic acid n ethyl n gamma picolyl amide':tn,ab,ti OR tropicacyl:tn,ab,ti OR tropicamid:tn,ab,ti OR 'tropico eye':tn,ab,ti OR tropicol:tn,ab,ti OR tropikamid:tn,ab,ti OR tropimil:tn,ab,ti OR visumidriatic:tn,ab,ti OR '1508‐75‐4':tn,ab,ti #82 tyramine:tn,ab,ti OR '4 hydroxyphenethylamine':tn,ab,ti OR lyramine:tn,ab,ti OR mydrial:tn,ab,ti OR 'para hydroxyphenethylamine':tn,ab,ti OR paratyramine:tn,ab,ti OR systogene:tn,ab,ti OR tiramine:tn,ab,ti OR tocosine:tn,ab,ti OR tyramin:tn,ab,ti OR tyrosamine:tn,ab,ti OR uteramine:tn,ab,ti OR '51‐67‐2':tn,ab,ti OR '60‐19‐5':tn,ab,ti #83 vibrocil:tn,ab,ti OR '8059‐14‐1':tn,ab,ti #84 'yohimbine'/exp #85 yohimbine:tn,ab,ti OR actibine:tn,ab,ti OR aphrodine:tn,ab,ti OR aphrodyne:tn,ab,ti OR corynanthine:tn,ab,ti OR 'corynine hydrochloride':tn,ab,ti OR 'dayto‐himbin':tn,ab,ti OR 'methyl yohimbine 16alpha carboxylate':tn,ab,ti OR 'methylyohimbane 16alpha carboxylate':tn,ab,ti OR pluriviron:tn,ab,ti OR quebrachin:tn,ab,ti OR 'quebrachine hydrochloride':tn,ab,ti OR rauhimbine:tn,ab,ti OR rauwolscine:tn,ab,ti OR urobine:tn,ab,ti OR yobin:tn,ab,ti OR yobinol:tn,ab,ti OR yocan:tn,ab,ti OR yocaral:tn,ab,ti OR yocon:tn,ab,ti OR yohimbe:tn,ab,ti OR 'yohimbic acid methyl ester':tn,ab,ti OR yohimbin:tn,ab,ti OR yohimex:tn,ab,ti OR yohimibin:tn,ab,ti OR yovital:tn,ab,ti OR '146‐48‐5':tn,ab,ti OR '65‐19‐0':tn,ab,ti #86 'timolol'/exp #87 timolol*:ab,ti,tn OR 'apo timol':ab,ti,tn OR 'apo timop':ab,ti,tn OR apotimol:ab,ti,tn OR apotimolol:ab,ti,tn OR apotimop:ab,ti,tn OR betimol:ab,ti,tn OR blocadren:ab,ti,tn OR 'chibro timoptol':ab,ti,tn OR istalol:ab,ti,tn OR 'l 714465':ab,ti,tn OR l714465:ab,ti,tn OR 'mk 950':ab,ti,tn OR mk950:ab,ti,tn OR moducren:ab,ti,tn OR nyolol:ab,ti,tn OR ofal:ab,ti,tn OR ofan:ab,ti,tn OR optimol:ab,ti,tn OR timolo:ab,ti,tn OR timoptic:ab,ti,tn OR timoptol:ab,ti,tn OR timacar:ab,ti,tn OR titol:ab,ti,tn OR '26839‐75‐8':ab,ti,tn #88 pirenzepin*:ab,ti,tn OR abrinac:ab,ti,tn OR azuzepin:ab,ti,tn OR bisvanil:ab,ti,tn OR 'cl 2':ab,ti,tn OR cl2:ab,ti,tn OR gastricur:ab,ti,tn OR gastrocepin:ab,ti,tn OR gastrozepin:ab,ti,tn OR gastrozepina:ab,ti,tn OR gastrozepine:ab,ti,tn OR leblon:ab,ti,tn OR 'ls 519':ab,ti,tn OR 'ls 519c12':ab,ti,tn OR ls519:ab,ti,tn OR ls519c12:ab,ti,tn OR maghen:ab,ti,tn OR tabe:ab,ti,tn OR zinc00538196:ab,ti,tn OR pyrenzepine:ab,ti,tn OR 'l s 519':ab,ti,tn OR ulgescum:ab,ti,tn OR 'piren basan':ab,ti,tn OR gastrotsepin:ab,ti,tn OR ulcoprotect:ab,ti,tn OR '28797‐61‐7':ab,ti,tn OR '29868‐97‐1':ab,ti,tn #89 '7 methylxanthine'/exp #90 '7 methylxanthine':ti,ab,tn #91 #44 OR #45 OR #46 OR #47 OR #48 OR #49 OR #50 OR #51 OR #52 OR #53 OR #54 OR #55 OR #56 OR #57 OR #58 OR #59 OR #60 OR #61 OR #62 OR #63 OR #64 OR #65 OR #66 OR #67 OR #68 OR #69 OR #70 OR #71 OR #72 OR #73 OR #74 OR #75 OR #76 OR #77 OR #78 OR #79 OR #80 OR #81 OR #82 OR #83 OR #84 OR #85 OR #86 OR #87 OR #88 OR #89 OR #90 #92 #43 OR #91 #93 #37 AND #92 #94 #32 AND #93
Appendix 4. PubMed search strategy
1. ((randomized controlled trial[pt]) OR (controlled clinical trial[pt]) OR (randomised[tiab] OR randomized[tiab]) OR (placebo[tiab]) OR (drug therapy[sh]) OR (randomly[tiab]) OR (trial[tiab]) OR (groups[tiab])) NOT (animals[mh] NOT humans[mh]) 2. myop*[tw] NOT Medline[sb] 3. (short sight*[tw] OR near sight*[tw]) NOT Medline[sb] 4. nearsighted*[tw] NOT Medline[sb] 5. #2 OR #3 OR #4 6. (Spectacles[tw] OR glasses[tw] OR eyeglass*[tw]) NOT Medline[sb] 7. ((progressive[tw] OR single[tw] OR vision[tw] OR addition[tw] OR bifocal[tw] OR spectacle[tw] OR corrective[tw] OR contact[tw] OR "gas permeable"[tw]) AND lens*[tw]) NOT Medline[sb] 8. (mydriatic*[tw] OR cycloplegic*[tw]) NOT Medline[sb] 9. ("7‐methylxanthine"[tw] OR "552‐62‐5"[tw]) NOT Medline[sb] 10. ((Cholinergic*[tw] OR acetylcholine*[tw] OR muscarinic[tw] OR parasympathetic*[tw]) AND (antagonist*[tw] OR block*[tw] OR inhibitor*[tw])) NOT Medline[sb] 11. cholinolytic*[tw] NOT Medline[sb] 12. (anticholinergic*[tw] OR "anti cholinergic"[tw] OR "anti cholinergics"[tw]) NOT Medline[sb] 13. (antimuscarinic*[tw] OR "anti muscarinic"[tw] OR "anti muscarinics"[tw]) NOT Medline[sb] 14. (parasympathicolytic*[tw] OR parasympaticolytic*[tw] OR Parasympatholytic*[tw]) NOT Medline[sb] 15. (pharmaceutical*[tw] OR pharmacologic*[tw]) NOT Medline[sb] 16. (Atropine [tw] OR atrinal [tw] OR "atro‐polygyl" [tw] OR atrop [tw] OR atropen [tw] OR atropin [tw] OR atropina [tw] OR "atropini sulfas" [tw] OR atropinol [tw] OR atropisol [tw] OR atropt [tw] OR atroptol [tw] OR atrospan [tw] OR "atrosulf‐1" [tw] OR "bar bropin" [tw] OR "bellpino‐artin" [tw] OR "cendo tropine" [tw] OR "dextro levo hyosciamine" [tw] OR "ichtho bellol" [tw] OR "isopto" [tw] OR isoptoAtropine [tw] OR "ocu‐tropine" [tw] OR "sal‐tropine" [tw] OR skiatropine [tw] OR "tropine dextro levo tropate" [tw] OR ximex [tw] OR "51‐55‐8" [tw] OR "55‐48‐1"[tw]) NOT Medline[sb] 17. (berefrine [tw] OR POPD [tw] OR "105567‐83‐7"[tw]) NOT Medline[sb] 18. (Cyclopentolate [tw] OR "ak‐pentolate" [tw] OR akpentolate [tw] OR "bell pentolate" [tw] OR ciclolux [tw] OR cyclogyl [tw] OR cyclomydri [tw] OR cyclopentol [tw] OR cyclopentolat [tw] OR cylate [tw] OR cyplegin [tw] OR diopentolate [tw] OR midriodavi [tw] OR mydrilate [tw] OR "ocu‐pentolate" [tw] OR ocucyclo [tw] OR "oftan‐syklo" [tw] OR pentolair [tw] OR "refractyl ofeno" [tw] OR skiacol [tw] OR zyklolat [tw] OR "512‐15‐2" [tw] OR "5870‐29‐1"[tw]) NOT Medline[sb] 19. (Epinephrine [tw] OR Adrenaline [tw] OR adrenalin [tw] OR Epitrate [tw] OR Lyophrin [tw] OR Epifrin [tw] OR adnephrin [tw] OR adnephrine [tw] OR adrenaclick [tw] OR "adrenal hydrochloride" [tw] OR adrenalina [tw] OR adrenamine [tw] OR adrenapax [tw] OR adrenazin [tw] OR adrenine [tw] OR adrin [tw] OR adrine [tw] OR advaradin [tw] OR balmadren [tw] OR biorenine [tw] OR bosmin [tw] OR chelafrin [tw] OR dylephrin [tw] OR epiglaufrin [tw] OR epimephrine [tw] OR epinefrina [tw] OR epinephran [tw] OR epinephrin [tw] OR epirenamine [tw] OR epirenan [tw] OR exadrin [tw] OR glaucon [tw] OR glaucosan [tw] OR glaufrin [tw] OR "glin epin" [tw] OR glycirenan [tw] OR haemostatin [tw] OR hemisine [tw] OR hemostasin [tw] OR hemostatin [tw] OR hypernephrin [tw] OR "isopto epinal" [tw] OR levoadrenalin [tw] OR levoadrenaline [tw] OR levoepinephrine [tw] OR levorenin [tw] OR levorenine [tw] OR methylaminoethanolcatechol [tw] OR methylarterenol [tw] OR mucidrina [tw] OR myosthenine [tw] OR "n methylnoradrenalin" [tw] OR nephridine [tw] OR nieraline [tw] OR paranephrin [tw] OR posumin [tw] OR renaglandin [tw] OR renaglandulin [tw] OR renaleptine [tw] OR renalina [tw] OR renaline [tw] OR renoform [tw] OR renostypticin [tw] OR renostyptin [tw] OR scurenaline [tw] OR simplene [tw] OR soladren [tw] OR sphygmogenin [tw] OR styptirenal [tw] OR supracapsulin [tw] OR supranephrane [tw] OR supranephrin [tw] OR supranol [tw] OR suprarenaline [tw] OR suprarenin [tw] OR suprarenine [tw] OR suprel [tw] OR surenine [tw] OR surrenine [tw] OR "sus‐phrine sulfite‐free" [tw] OR susphrine [tw] OR "sympathin I" [tw] OR takamina [tw] OR tonogen [tw] OR vasoconstrictine [tw] OR vasodrine [tw] OR vasotonin [tw] OR weradren [tw] OR "51‐43‐4" [tw] OR "55‐31‐2" [tw] OR "6912‐68‐1"[tw]) NOT Medline[sb] 20. (Ethylmorphine[tw] OR Ethomorphine[tw] OR Trachyl[tw] OR codethyline[tw] OR diolan[tw] OR dionine[tw] OR "ethyl morphine"[tw] OR ethylmorfine[tw] OR ethylmorphin[tw] OR "morphine ethyl ether"[tw] OR "125‐30‐4"[tw] OR "76‐58‐4"[tw]) NOT Medline[sb] 21. (Eucatropine[tw] OR euphthalmine[tw] OR "100‐91‐4"[tw] OR "536‐93‐6"[tw]) NOT Medline[sb] 22. (Homatropine[tw] OR homatro[tw] OR homatrocil[tw] OR homatropaire[tw] OR homatropin[tw] OR homatropina[tw] OR isoptoHomatropine[tw] OR "I Homatrine"[tw] OR "mandelyl tropeine"[tw] OR mandelyltropeine[tw] OR "mydryn eye"[tw] OR "omatropina lux"[tw] OR "tropine mandelate"[tw] OR "51‐56‐9"[tw] OR "87‐00‐3"[tw]) NOT Medline[sb] 23. (Hyoscyamine[tw] OR anaspaz[tw] OR cystospaz[tw] OR cytospaz[tw] OR daturine[tw] OR donnamar[tw] OR duboisine[tw] OR egacen[tw] OR hyoscamine[tw] OR hyosciamine[tw] OR hyoscyanin[tw] OR hyosyne[tw] OR "ib‐stat"[tw] OR levbid[tw] OR levsin[tw] OR "levsinex sr"[tw] OR neosol[tw] OR nulev[tw] OR spasdel[tw] OR "symax sl"[tw] OR "symax sr"[tw] OR "tropine l tropate"[tw] OR "101‐31‐5"[tw] OR "306‐03‐6"[tw]) NOT Medline[sb] 24. (Ibopamine[tw] OR "N‐methyldopamine diisobutyrate"[tw] OR "SB 7505"[tw] OR "SB7505"[tw] OR Escandine[tw] OR Inopamil[tw] OR "diisobutyric n methyldopamine ester"[tw] OR scandine[tw] OR "skf 100168"[tw] OR "skf 100168 a"[tw] OR "66195‐31‐1"[tw] OR "75011‐65‐3"[tw]) NOT Medline[sb] 25. (Methylatropine[tw] OR "8‐methylatropinium nitrate"[tw] OR "31610‐87‐4"[tw]) NOT Medline[sb] 26. (Naphazoline[tw] OR "Afazol Grin"[tw] OR "AK Con"[tw] OR AKCon[tw] OR Albalon[tw] OR albasol[tw] OR "All Clear"[tw] OR allersol[tw] OR antan[tw] OR benil[tw] OR cefasan[tw] OR "Clear Eyes"[tw] OR coldan[tw] OR "Colirio Alfa"[tw] OR "comfort eye drops"[tw] OR dazolin[tw] OR "degest 2"[tw] OR derinox[tw] OR Idril[tw] OR imidin[tw] OR minha[tw] OR Miraclar[tw] OR mirafrin[tw] OR Nafazair[tw] OR nafazoline[tw] OR naftazolina[tw] OR "naphacel ofteno"[tw] OR naphasal[tw] OR naphazolin[tw] OR Naphcon[tw] OR "naphozoline hydrochloride"[tw] OR naphtears[tw] OR naphthazoline[tw] OR naphthizine[tw] OR naphthyzin[tw] OR nastizol[tw] OR "nazil ofteno"[tw] OR niazol[tw] OR "ocu‐zoline"[tw] OR opcon[tw] OR Optazine[tw] OR Privin[tw] OR privina[tw] OR Privine[tw] OR privine[tw] OR Proculin[tw] OR rhinantin[tw] OR rhinazin[tw] OR rhinoperd[tw] OR rimidol[tw] OR sanorin[tw] OR sanotin[tw] OR Siozwo[tw] OR strictylon[tw] OR "Tele Stulln"[tw] OR TeleStulln[tw] OR Vasoclear[tw] OR Vasocon[tw] OR "Vasoconstrictor Pensa"[tw] OR VasoNit[tw] OR vistalbalon[tw] OR vistobalon[tw] OR "5144‐52‐5"[tw] OR "550‐99‐2"[tw] OR "835‐31‐4"[tw]) NOT Medline[sb] 27. (Oxedrine[tw] OR Synephrine[tw] OR Sympaethamin[tw] OR Synephrin[tw] OR aetaphen[tw] OR pentedrine[tw] OR vasoton[tw] OR "94‐07‐5"[tw]) NOT Medline[sb] 28. (Oxyphenonium[tw] OR Methacin[tw] OR Oxyphenon[tw] OR Atrenyl[tw] OR Spastrex[tw] OR antrenyl[tw] OR "ba 5473"[tw] OR ba5473[tw] OR "c 5473"[tw] OR c5473[tw] OR helkamon[tw] OR metacin[tw] OR metacinum[tw] OR oxyphenium[tw] OR "oxyphenomium bromide"[tw] OR spasmofen[tw] OR spasmophen[tw] OR "14214‐84‐7"[tw] OR "50‐10‐2"[tw]) NOT Medline[sb] 29. (Phenylephrine[tw] OR adrianol[tw] OR "af‐taf"[tw] OR "ak‐dilate"[tw] OR "albalon relief"[tw] OR alconefrin[tw] OR almefrin[tw] OR altafrin[tw] OR biomidrin[tw] OR biomydrin[tw] OR derizene[tw] OR "despec‐sf"[tw] OR "disneumon pernasal"[tw] OR drosin[tw] OR "efrin‐10"[tw] OR efrisel[tw] OR fenylephrine[tw] OR idrianol[tw] OR isonefrine[tw] OR isophrin[tw] OR isophrine[tw] OR "isopto frin"[tw] OR isoptofrin[tw] OR lexatol[tw] OR "m synephrine"[tw] OR mesaton[tw] OR "meta sympathol"[tw] OR "meta synephrine"[tw] OR Metaoxedrin[tw] OR metaoxedrine[tw] OR Metasympatol[tw] OR metasynephrine[tw] OR Mezaton[tw] OR "murucoll 2"[tw] OR mydfrin[tw] OR "n 105 to"[tw] OR "nefrin‐ofteno"[tw] OR "Neo Synephrine"[tw] OR neofrin[tw] OR neooxedrine[tw] OR neophryn[tw] OR neosynephrin[tw] OR Neosynephrine[tw] OR "neosynephrin‐pos"[tw] OR neosynesin[tw] OR neosynesine[tw] OR "ocu‐phrin"[tw] OR "oftan‐metaoksedrin"[tw] OR optistin[tw] OR phenoptic[tw] OR phenylefrine[tw] OR phenylephedrine[tw] OR prefrin[tw] OR "pupiletto forte"[tw] OR rectasol[tw] OR "rhinall 10"[tw] OR "slv 325"[tw] OR slv325[tw] OR sucraphen[tw] OR vazculep[tw] OR visadron[tw] OR vistafrin[tw] OR vistosan[tw] OR "532‐38‐7"[tw] OR "59‐42‐7"[tw] OR "61‐76‐7"[tw]) NOT Medline[sb] 30. (Pholedrine[tw] OR "4 hydroxy n methylamphetamine"[tw] OR "4 hydroxymethamphetamine"[tw] OR adyston[tw] OR "para hydroxymethamphetamine"[tw] OR "p‐hydroxymethamphetamine"[tw] OR paredrinol[tw] OR "Pholedrin liquidum"[tw] OR "Pholedrin‐longo‐Isis"[tw] OR pulsotyl[tw] OR venosan[tw] OR veritol[tw] OR "370‐14‐9"[tw]) NOT Medline[sb] 31. (p‐Hydroxyamphetamine [tw] OR "1 para hydroxyphenyl 2 propylamine" [tw] OR "alpha methyl para tyramine" [tw] OR "alpha methyl tyramine" [tw] OR "dl 1 p hydroxyphenyl 2 propylamine" [tw] OR "dl 1 para hydroxyphenyl 2 propylamine" [tw] OR "dl p hydroxy alpha methylphenethylamine" [tw] OR "dl para hydroxy alpha methylphenethylamine" [tw] OR "h 66 37" [tw] OR "para hydroxy alpha methylphenethylamine" [tw] OR Hydroxyamfetamine [tw] OR Hydroxyamphetamin [tw] OR Hydroxyamphetamine [tw] OR Hydroxyphenylisopropylamine [tw] OR Methyltyramine [tw] OR Norpholedrin [tw] OR norpholedrine [tw] OR oxamphetamine [tw] OR Oxyamphetamine [tw] OR paradrine [tw] OR parahydroxyamphetamine [tw] OR Paredrine [tw] OR paredrinea [tw] OR paredrinex [tw] OR pedrolone [tw] OR pulsoton [tw] OR "103‐86‐6" [tw] OR "1518‐86‐1" [tw] OR "306‐21‐8"[tw]) NOT Medline[sb] 32. (Racepinephrine[tw] OR asthmanefrin [tw] OR Micronefrin[tw] OR micronefrine[tw] OR Micronephrine[tw] OR mikronephrin[tw] OR racadrenalin[tw] OR "Racepinefrine Hydrochloride"[tw] OR racinephrine[tw] OR Vaponefrin[tw] OR vaponefrine[tw] OR vaponephrin[tw] OR "329‐65‐7"[tw]) NOT Medline[sb] 33. (Scopolamine[tw] OR "Boro Scopol"[tw] OR BoroScopol[tw] OR Hyoscine[tw] OR Kwells[tw] OR "levo hyoscinehydrobromide"[tw] OR Scoburen[tw] OR Scopace[tw] OR scopos[tw] OR "Travacalm HO"[tw] OR Vorigeno[tw] OR "114‐49‐8"[tw] OR atrochin[tw] OR atroquin[tw] OR atroscine[tw] OR hyosceine[tw] OR hysco[tw] OR "kimite‐patch"[tw] OR "l epoxytropine tropate"[tw] OR "n methylhyoscine"[tw] OR oscine[tw] OR scopalamine[tw] OR "scopine tropate"[tw] OR scopoderm[tw] OR scopolamin[tw] OR transcop[tw] OR "transderm scop"[tw] OR "transderm v"[tw] OR "tropic acid ester with scopine"[tw] OR "138‐12‐5"[tw] OR "51‐34‐3"[tw] OR "55‐16‐3"[tw]) NOT Medline[sb] 34. (Tropicamide[tw] OR "alcon‐mydril"[tw] OR bistropamide[tw] OR "cendo mydriatyl"[tw] OR "Colircusi Tropicamida"[tw] OR midriaticum[tw] OR mydiacyl[tw] OR mydral[tw] OR mydramide[tw] OR mydriacyl[tw] OR Mydriafair[tw] OR Mydriaticum[tw] OR "mydrin m"[tw] OR "mydrin p"[tw] OR Mydrum[tw] OR "n ethyl 2 phenyl n pyrid 4 ylmethylhydracrylamide"[tw] OR "n ethyl n 4 picolyltropamide"[tw] OR "n ethyl n gamma picolyltropamide"[tw] OR "n ethyl n pyrid 4 ylmethyltropamide"[tw] OR "Ocu‐Tropic"[tw] OR OcuTropic[tw] OR opticyl[tw] OR sandol[tw] OR sintropic[tw] OR "tropamid forte"[tw] OR "tropic acid n ethyl n gamma picolyl amide" [tw] OR Tropicacyl[tw] OR tropicamid[tw] OR "tropico eye"[tw] OR tropicol[tw] OR tropikamid[tw] OR tropimil[tw] OR visumidriatic[tw] OR "1508‐75‐4"[tw]) NOT Medline[sb] 35. (Tyramine[tw] OR "4 hydroxyphenethylamine"[tw] OR lyramine[tw] OR mydrial[tw] OR "para hydroxyphenethylamine"[tw] OR paratyramine[tw] OR systogene[tw] OR tiramine[tw] OR tocosine[tw] OR tyramin[tw] OR tyrosamine[tw] OR uteramine[tw] OR "51‐67‐2"[tw] OR "60‐19‐5"[tw]) NOT Medline[sb] 36. (Vibrocil[tw] OR "8059‐14‐1"[tw]) NOT Medline[sb] 37. (Yohimbine[tw] OR actibine[tw] OR aphrodine[tw] OR aphrodyne[tw] OR Corynanthine[tw] OR "corynine hydrochloride"[tw] OR "dayto‐himbin"[tw] OR "methyl yohimbine 16alpha carboxylate"[tw] OR "methylyohimbane 16alpha carboxylate"[tw] OR Pluriviron[tw] OR quebrachin[tw] OR "quebrachine hydrochloride"[tw] OR Rauhimbine[tw] OR Rauwolscine[tw] OR urobine[tw] OR yobin[tw] OR yobinol[tw] OR yocan[tw] OR yocaral[tw] OR Yocon[tw] OR yocon[tw] OR yohimbe[tw] OR "yohimbic acid methyl ester"[tw] OR yohimbin[tw] OR Yohimex[tw] OR yohimex[tw] OR yohimibin[tw] OR yovital[tw] OR "146‐48‐5"[tw] OR "65‐19‐0"[tw]) NOT Medline[sb] 38. (Timolol*[tw] OR "apo timol"[tw] OR "apo timop"[tw] OR apotimol[tw] OR apotimolol[tw] OR apotimop[tw] OR betimol[tw] OR Blocadren[tw] OR "chibro timoptol"[tw] OR istalol[tw] OR "l 714465"[tw] OR l714465[tw] OR "MK 950"[tw] OR MK950[tw] OR moducren[tw] OR nyolol[tw] OR ofal[tw] OR ofan[tw] OR optimol[tw] OR timolo[tw] OR Timoptic[tw] OR Timoptol[tw] OR Timacar[tw] OR titol[tw] OR "26839‐75‐8"[tw]) NOT Medline[sb] 39. (Pirenzepin*[tw] OR abrinac[tw] OR azuzepin[tw] OR bisvanil[tw] OR "cl 2"[tw] OR cl2[tw] OR gastricur[tw] OR gastrocepin[tw] OR gastrozepin[tw] OR gastrozepina[tw] OR gastrozepine[tw] OR leblon[tw] OR "ls 519"[tw] OR "ls 519c12"[tw] OR ls519[tw] OR ls519c12[tw] OR maghen[tw] OR tabe[tw] OR zinc00538196[tw] OR Pyrenzepine[tw] OR "L S 519"[tw] OR Ulgescum[tw] OR "Piren basan"[tw] OR Gastrotsepin[tw] OR Ulcoprotect[tw] OR "28797‐61‐7"[tw] OR "29868‐97‐1"[tw]) NOT Medline[sb] 40. #6 OR #7 OR #8 OR #9 OR #10 OR #11 OR #12 OR #13 OR #14 OR #15 OR #16 OR #17 OR #18 OR #19 OR #20 OR #21 OR #22 OR #23 OR #24 OR #25 OR #26 OR #27 OR #28 OR #29 OR #30 OR #31 OR #32 OR #33 OR #34 OR #35 OR #36 OR #37 OR #38 OR #39 41. #5 AND #40 42. #1 AND #41
Appendix 5. LILACS search terms
(Myopi$ OR Miopi$ OR MH:C11.744.636$ OR (near sight$) OR (short sight$) OR nearsighted$) AND (MH:E07.632.500.300$ OR MH:VS2.006.001.009.002$ OR spectacles OR glasses OR eyeglass$ OR Anteojos OR Óculos OR MH:E07.632.500.276$ OR MH:VS2.006.001.009.001$ OR ((progressive OR single OR vision OR addition OR bifocal OR spectacle OR corrective OR contact OR "gas permeable") AND lens$) OR MH:D27.505.519.625.120.200$ OR MH:D27.505.696.577.120.200$ OR ((Cholinergic$ OR acetylcholine$ OR muscarinic$ OR parasympathetic$) AND (antagonist$ OR block$ OR inhibitor$)) OR cholinolytic$ OR anticholinergic$ OR "anti cholinergic" OR "anti cholinergics" OR antimuscarinic$ OR "anti muscarinic" OR "anti muscarinics" OR Parasympathicolytic$ OR parasympaticolytic$ OR Parasympatholytic$ OR pharmaceutical$ OR pharmacologic$ OR MH:D27.505.696.663.050.650$ OR Parasympatholytic$ OR Parasimpatolítico$ OR Parassimpatolítico$ OR mydriatic$ OR Midriáticos OR cycloplegic$ OR "7‐methylxanthine" OR Atropin$ OR MH:D02.145.074.722.229.199$ OR MH:D03.132.760.180.572.199$ OR MH:D03.132.889.180.648.199$ OR MH:D03.605.869.229.199$ OR MH:D04.075.080.875.099.722.229.199 OR berefrine OR Cyclopentolate OR Ciclopentolato OR MH:D02.241.223.601.200$ OR Epinephrine OR Epinefrina OR MH:D02.033.100.291.310$ OR MH:D02.092.063.291.310$ OR MH:D02.092.211.215.311.461$ OR MH:D02.092.311.461$ OR Ethylmorphine OR Etilmorfina OR MH:D03.132.577.249.562.430$ OR MH:D03.549.686.607.460$ OR MH:D03.605.497.607.460$ OR MH:D04.615.723.795.576.430$ OR Eucatropine OR Homatropine OR Hyoscyamine OR Hiosciamina OR MH:D02.145.074.722.229.199.500$ OR MH:D03.132.760.180.572.199.500$ OR MH:D03.132.889.180.648.199.500$ OR MH:D03.605.869.229.199.500$ OR MH:D04.075.080.875.099.722.229.199.500$ OR Ibopamine OR Methylatropine OR Naphazoline OR Nafazolina OR MH:D03.383.129.308.585$ OR Oxedrine OR Synephrine OR Sinefrina OR MH:D02.033.100.291.870$ OR MH:D02.092.063.291.870$ OR MH:D02.092.211.215.811.875$ OR Oxyphenonium OR Oxifenonio OR MH:D02.092.877.648$ OR MH:D02.675.276.648$ OR Phenylephrine OR Fenilefrina OR MH:D02.033.100.291.617$ OR MH:D02.092.063.291.617$ OR Pholedrine OR "p‐Hydroxyamphetamine" OR "p‐Hidroxianfetamina" OR MH:D02.092.471.683.152.500$ OR Racepinephrine OR Racepinefrina OR MH:D02.033.100.291.310.500$ OR MH:D02.092.063.291.310.500$ OR MH:D02.092.211.215.311.461.700$ OR MH:D02.092.311.461.825$ OR Scopolamine OR Escopolamina OR MH:D02.145.074.722.822.775$ OR MH:D03.132.760.180.848$ OR MH:D03.132.889.601.775$ OR MH:D03.605.869.822.775$ OR MH:D04.075.080.875.099.722.822.775$ OR Tropicamide OR Tropicamida OR MH:D03.383.725.942$ OR Tyramine OR Tiramina OR MH:D02.092.211.215.811$ OR Vibrocil OR Yohimbine OR Yohimbina OR Ioimbina OR MH:D03.132.436.681.933$ OR MH:D03.438.473.402.681.933$ OR Timolol$ OR MH:D02.033.100.624.915$ OR MH:D02.033.755.624.915$ OR MH:D02.092.063.624.915$ OR MH:D02.886.675.867.768$ OR MH:D03.383.129.708.867.768$ OR MH:D03.383.533.640.775$ OR Pirenzepin$ OR MH:D03.438.079.080.070.750$)
Appendix 6. ISRCTN search strategy
myopia
Appendix 7. ClinicalTrials.gov search strategy
myopia OR myopic
Appendix 8. WHO ICTRP search strategy
myopia OR myopic
Data and analyses
Comparison 1. Undercorrection vs full correction spectacles.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1.1 Change in refractive error from baseline | 3 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 1.1.1 At 1 year | 2 | 142 | Mean Difference (IV, Fixed, 95% CI) | ‐0.15 [‐0.29, ‐0.00] |
| 1.1.2 At 2 years | 2 | 244 | Mean Difference (IV, Fixed, 95% CI) | 0.02 [‐0.05, 0.09] |
| 1.2 Change in axial length from baseline | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 1.2.1 At 1 year | 1 | 94 | Mean Difference (IV, Fixed, 95% CI) | 0.05 [‐0.01, 0.11] |
| 1.2.2 At 2 years | 2 | 244 | Mean Difference (IV, Fixed, 95% CI) | ‐0.01 [‐0.06, 0.03] |
1.1. Analysis.

Comparison 1: Undercorrection vs full correction spectacles, Outcome 1: Change in refractive error from baseline
1.2. Analysis.

Comparison 1: Undercorrection vs full correction spectacles, Outcome 2: Change in axial length from baseline
Comparison 2. Multifocal lenses vs single vision lenses.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 2.1 Change in refractive error from baseline (1 year) | 9 | 1463 | Mean Difference (IV, Random, 95% CI) | 0.14 [0.08, 0.21] |
| 2.1.1 Bifocal lenses | 4 | 421 | Mean Difference (IV, Random, 95% CI) | 0.16 [0.01, 0.32] |
| 2.1.2 Progressive addition lenses | 5 | 1042 | Mean Difference (IV, Random, 95% CI) | 0.15 [0.09, 0.21] |
| 2.2 Change in refractive error from baseline (2 years) | 8 | 1401 | Mean Difference (IV, Random, 95% CI) | 0.19 [0.08, 0.30] |
| 2.2.1 Bifocal lenses | 4 | 416 | Mean Difference (IV, Random, 95% CI) | 0.20 [‐0.09, 0.49] |
| 2.2.2 Progressive addition lenses | 4 | 985 | Mean Difference (IV, Random, 95% CI) | 0.20 [0.12, 0.28] |
| 2.3 Change in refractive error from baseline (3 years) | 3 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 2.3.1 Bifocal lenses | 1 | 158 | Mean Difference (IV, Fixed, 95% CI) | ‐0.19 [‐0.47, 0.09] |
| 2.3.2 Progressive addition lenses | 2 | 579 | Mean Difference (IV, Fixed, 95% CI) | 0.21 [0.08, 0.34] |
| 2.4 Change in axial length from baseline (1 year) | 4 | 896 | Mean Difference (IV, Random, 95% CI) | ‐0.06 [‐0.09, ‐0.04] |
| 2.5 Change in axial length from baseline (2 years) | 2 | 723 | Mean Difference (IV, Fixed, 95% CI) | ‐0.05 [‐0.10, ‐0.01] |
| 2.6 Change in axial length from baseline (3 years) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2.7 Change in corneal radius of curvature from baseline, horizontal (3 years) | 2 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2.7.1 At 3 years, horizontal | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2.7.2 At 3 years, vertical | 2 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected |
2.3. Analysis.

Comparison 2: Multifocal lenses vs single vision lenses, Outcome 3: Change in refractive error from baseline (3 years)
2.4. Analysis.

Comparison 2: Multifocal lenses vs single vision lenses, Outcome 4: Change in axial length from baseline (1 year)
2.5. Analysis.

Comparison 2: Multifocal lenses vs single vision lenses, Outcome 5: Change in axial length from baseline (2 years)
2.6. Analysis.

Comparison 2: Multifocal lenses vs single vision lenses, Outcome 6: Change in axial length from baseline (3 years)
2.7. Analysis.

Comparison 2: Multifocal lenses vs single vision lenses, Outcome 7: Change in corneal radius of curvature from baseline, horizontal (3 years)
Comparison 3. Peripheral plus spectacles vs single vision lenses.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 3.1 Change in refractive error from baseline (1 year) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 3.1.1 Type I | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 3.1.2 Type II | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 3.1.3 Type III | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 3.2 Change in refractive error from baseline (2 years) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 3.2.1 +1.0 Diopters | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 3.2.2 +1.5 Diopters | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 3.3 Change in axial length from baseline (1 year) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 3.3.1 Type I | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 3.3.2 Type II | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 3.3.3 Type III | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 3.4 Change in axial length from baseline (2 years) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 3.4.1 +1.0 Diopters | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 3.4.2 +1.5 Diopters | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected |
3.1. Analysis.

Comparison 3: Peripheral plus spectacles vs single vision lenses, Outcome 1: Change in refractive error from baseline (1 year)
3.2. Analysis.

Comparison 3: Peripheral plus spectacles vs single vision lenses, Outcome 2: Change in refractive error from baseline (2 years)
3.3. Analysis.

Comparison 3: Peripheral plus spectacles vs single vision lenses, Outcome 3: Change in axial length from baseline (1 year)
3.4. Analysis.

Comparison 3: Peripheral plus spectacles vs single vision lenses, Outcome 4: Change in axial length from baseline (2 years)
Comparison 4. Bifocal soft contact lenses vs single vision soft contact lenses.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 4.1 Change in refractive error from baseline (1 year) | 4 | 300 | Mean Difference (IV, Random, 95% CI) | 0.20 [‐0.06, 0.47] |
| 4.2 Change in axial length from baseline (1 year) | 4 | 300 | Mean Difference (IV, Fixed, 95% CI) | ‐0.11 [‐0.14, ‐0.08] |
| 4.3 Change in corneal radius of curvature from baseline (1 year) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected |
4.1. Analysis.

Comparison 4: Bifocal soft contact lenses vs single vision soft contact lenses, Outcome 1: Change in refractive error from baseline (1 year)
4.2. Analysis.

Comparison 4: Bifocal soft contact lenses vs single vision soft contact lenses, Outcome 2: Change in axial length from baseline (1 year)
4.3. Analysis.

Comparison 4: Bifocal soft contact lenses vs single vision soft contact lenses, Outcome 3: Change in corneal radius of curvature from baseline (1 year)
Comparison 5. Rigid gas permeable contact lenses vs control.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 5.1 Change in refractive error from baseline | 2 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 5.1.1 At 1 year | 2 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 5.1.2 At 2 years | 2 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 5.1.3 At 3 years | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 5.2 Change in axial length from baseline | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 5.2.1 At 1 year | 2 | 415 | Mean Difference (IV, Fixed, 95% CI) | 0.02 [‐0.05, 0.10] |
| 5.2.2 At 2 years | 2 | 394 | Mean Difference (IV, Fixed, 95% CI) | 0.03 [‐0.05, 0.12] |
| 5.2.3 At 3 years | 1 | 116 | Mean Difference (IV, Fixed, 95% CI) | 0.05 [‐0.12, 0.22] |
| 5.3 Change in corneal radius of curvature from baseline | 2 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 5.3.1 At 1 year | 2 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 5.3.2 At 2 years | 2 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 5.3.3 At 3 years | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected |
5.1. Analysis.

Comparison 5: Rigid gas permeable contact lenses vs control, Outcome 1: Change in refractive error from baseline
5.2. Analysis.

Comparison 5: Rigid gas permeable contact lenses vs control, Outcome 2: Change in axial length from baseline
5.3. Analysis.

Comparison 5: Rigid gas permeable contact lenses vs control, Outcome 3: Change in corneal radius of curvature from baseline
Comparison 6. Orthokeratology contact lenses vs single vision lenses.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 6.1 Change in axial length from baseline (2 years) | 2 | 106 | Mean Difference (IV, Fixed, 95% CI) | ‐0.28 [‐0.38, ‐0.19] |
Comparison 7. Antimuscarinic agents vs placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 7.1 Change in refractive error from baseline (1 year) | 6 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 7.1.1 Atropine eye drops | 3 | 629 | Mean Difference (IV, Fixed, 95% CI) | 1.00 [0.93, 1.07] |
| 7.1.2 Pirenzepine 2% gel | 2 | 326 | Mean Difference (IV, Fixed, 95% CI) | 0.31 [0.17, 0.44] |
| 7.1.3 Cyclopentolate eye drops | 1 | 64 | Mean Difference (IV, Fixed, 95% CI) | 0.34 [0.08, 0.60] |
| 7.2 Change in refractive error from baseline (2 years) | 2 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 7.2.1 Atropine eye drops | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 7.2.2 Pirenzepine 2% gel | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 7.3 Change in axial length from baseline (1 year) | 4 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 7.3.1 Atropine eye drops | 2 | 502 | Mean Difference (IV, Fixed, 95% CI) | ‐0.35 [‐0.38, ‐0.31] |
| 7.3.2 Pirenzepine 2% gel | 2 | 326 | Mean Difference (IV, Fixed, 95% CI) | ‐0.13 [‐0.14, ‐0.12] |
| 7.4 Change in axial length from baseline (2 years) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 7.5 Incidence of adverse events | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 7.5.1 Accomodation abnormality symptoms | 2 | 387 | Risk Ratio (M‐H, Fixed, 95% CI) | 9.05 [4.09, 20.01] |
| 7.5.2 Papillae/follicles | 2 | 387 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.22 [2.11, 4.90] |
| 7.5.3 Medication residue on the eyelids/eyelashes | 2 | 387 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.91 [0.73, 1.12] |
7.1. Analysis.

Comparison 7: Antimuscarinic agents vs placebo, Outcome 1: Change in refractive error from baseline (1 year)
7.2. Analysis.

Comparison 7: Antimuscarinic agents vs placebo, Outcome 2: Change in refractive error from baseline (2 years)
7.3. Analysis.

Comparison 7: Antimuscarinic agents vs placebo, Outcome 3: Change in axial length from baseline (1 year)
7.4. Analysis.

Comparison 7: Antimuscarinic agents vs placebo, Outcome 4: Change in axial length from baseline (2 years)
7.5. Analysis.

Comparison 7: Antimuscarinic agents vs placebo, Outcome 5: Incidence of adverse events
Comparison 8. Atropine vs tropicamide.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 8.1 Change in refractive error from baseline (1 year) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 8.1.1 Atropine 0.5% | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 8.1.2 Atropine 0.25% | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 8.1.3 Atropine 0.1% | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 8.2 Change in refractive error from baseline (2 years) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 8.2.1 Atropine 0.5% | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 8.2.2 Atropine 0.25% | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 8.2.3 Atropine 0.1% | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected |
8.1. Analysis.

Comparison 8: Atropine vs tropicamide, Outcome 1: Change in refractive error from baseline (1 year)
8.2. Analysis.

Comparison 8: Atropine vs tropicamide, Outcome 2: Change in refractive error from baseline (2 years)
Comparison 9. Systemic 7‐methylxanthine vs placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 9.1 Change in refractive error from baseline (1 year) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 9.2 Change in axial length from baseline (1 year) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 9.3 Change in corneal radius of curvature from baseline (1 year) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected |
9.2. Analysis.

Comparison 9: Systemic 7‐methylxanthine vs placebo, Outcome 2: Change in axial length from baseline (1 year)
9.3. Analysis.

Comparison 9: Systemic 7‐methylxanthine vs placebo, Outcome 3: Change in corneal radius of curvature from baseline (1 year)
Comparison 10. Timolol eye drops vs no eye drops.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 10.1 Change in refractive error from baseline | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 10.1.1 At 1 year | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 10.1.2 At 2 years | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected |
10.1. Analysis.

Comparison 10: Timolol eye drops vs no eye drops, Outcome 1: Change in refractive error from baseline
Comparison 11. Atropine + multifocal lenses vs placebo + single vision lenses.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 11.1 Change in refractive error from baseline (1 year) | 2 | 191 | Mean Difference (IV, Fixed, 95% CI) | 0.78 [0.54, 1.02] |
| 11.2 Change in axial length from baseline (1 year) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected |
11.1. Analysis.

Comparison 11: Atropine + multifocal lenses vs placebo + single vision lenses, Outcome 1: Change in refractive error from baseline (1 year)
11.2. Analysis.

Comparison 11: Atropine + multifocal lenses vs placebo + single vision lenses, Outcome 2: Change in axial length from baseline (1 year)
Comparison 12. Atropine + multifocal lenses vs cyclopentolate + single vision lenses.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 12.1 Change in refractive error from baseline (1 year) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected |
12.1. Analysis.

Comparison 12: Atropine + multifocal lenses vs cyclopentolate + single vision lenses, Outcome 1: Change in refractive error from baseline (1 year)
Comparison 13. Bifocal spectacles vs single vision lenses + timolol.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 13.1 Change in refractive error from baseline | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 13.1.1 At 1 year | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 13.1.2 At 2 years | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected |
13.1. Analysis.

Comparison 13: Bifocal spectacles vs single vision lenses + timolol, Outcome 1: Change in refractive error from baseline
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Adler 2006.
| Study characteristics | ||
| Methods | Study design: parallel‐group RCT Study center: urban private optometric practice in Jerusalem, Israel Number randomized: 62 children Study follow‐up: 18 months Exclusions and losses to follow‐up: 5 (8%) children who were randomized were excluded from the analyses; 9 (14.5%) were lost to follow‐up |
|
| Participants | Age: mean = 10.08 years (range 6 to 15 years) Gender: 34 boys, 14 girls Culture: most children were orthodox Jews who attended school year‐round and performed a study method of swaying back and forth while learning and reading Inclusion criteria: pediatric patients aged 6 to 15 years from study centers with early‐onset myopia Exclusion criteria: (1) strabismus; (2) amblyopia; (3) VA < 6/9; (4) spherical equivalent > ‐6.00 D or < ‐0.50 D in either eye; (5) astigmatism > 1.50 D in either eye; (6) anisometropia > 1.50 D; (7) a difference between objective and subjective refraction findings ≥ 0.75 D; (8) any ocular pathological manifestations; and (9) premature birth |
|
| Interventions | Undercorrected group (n = 25): blurred by +0.50 D; glasses were to be worn continuously Fully corrected group (n = 23): glasses were to be worn continuously Note: changes in prescription were made if the subjective refraction had changed by ≥ 0.50 D for 1 or both eyes |
|
| Outcomes |
Progression of early‐onset myopia:
Measurements taken at baseline, 6 months, 12 months, and 18 months Unit of analysis: average values of both eyes used for all results |
|
| Notes | Study dates: enrollment occurred over an 8‐month period
Trial registration: not reported Materials: free spectacle lenses were supplied by Einit Optical Clinic Additional data: study author provided unpublished data via email correspondence |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | A coin was tossed to determine group assignments |
| Allocation concealment (selection bias) | Unclear risk | The assignment for each participant was determined after enrollment by tossing a coin, but it was unclear how the allocation was concealed |
| Masking of participants (performance bias) | High risk | Masking of participants was not applicable due to performance differences between the interventions studied |
| Masking of outcome assessors (detection bias) Progression of myopia | Low risk | The optometrist conducting the examination was masked to the treatment group and to previous results for each participant |
| Masking of outcome assessors (detection bias) Secondary outcomes | Unclear risk | N/A (study did not measure secondary outcomes of this review) |
| Masking of data analyzers | Unclear risk | Analysis of the results was carried out by the other member of the team only after all data had been collected |
| Incomplete outcome data (attrition bias) Incomplete outcome(s) data | High risk | Of the 62 children recruited, 48 are included in the analysis; 5 were excluded (3 did not wear their glasses continually, 2 were twins born prematurely) and 9 were lost to follow‐up |
| Selective reporting (reporting bias) | Low risk | Results were reported for outcomes described in the methods section of the paper |
| Other bias | Low risk | None was identified |
Anstice 2011.
| Study characteristics | ||
| Methods | Study design: paired‐eye, cross‐over RCT Study center: 1 Number randomized: 40 children Study follow‐up: 20 months (10 months for each period) Exclusions and losses to follow‐up: no exclusions; 5 (12.5%) and 6 (15.0%) were lost to follow‐up at 10‐month visit and 20‐month visit, respectively |
|
| Participants | Age: mean = 13.4 years (range 11 to 14 years) Gender: 11 boys, 29 girls Culture: New Zealand, including East Asian ethnicity and others (European, Indian, and Maori/Pacifica) Inclusion criteria: (1) 11 to 14 years old at recruitment; (2) spherical equivalent between ‐1.25 and ‐4.50 D in the least myopic eye as determined by noncycloplegic subjective refraction; (3) myopia progression ≥ 0.50 D in the previous 12 months; (4) best‐corrected spectacle visual acuity of Snellen 6/6 or better in each eye; (5) willingness to wear contact lenses for ≥ 8 hours per day during the study Exclusion criteria: history of (1) astigmatism ≥ 1.25 D; (2) anisometropia ≥ 1.00 D; (3) strabismus at distance or near as assessed by cover test; (4) ocular or systemic pathology likely to affect refractive development or successful contact lens wear; (5) birth weight ≤ 1250 g |
|
| Interventions | Group 1 (n = 21): 10 months wearing 2.00 D dual‐focus (DF) contact lens in the dominant eye and SVSCL in the contralateral eye, followed by 10 months wearing the swapped lens assignment Group 2 (n = 19): 10 months wearing DF contact lens in the nondominant eye and SVSCL in the contralateral eye, followed by 10 months wearing the swapped lens assignment |
|
| Outcomes |
Primary outcome:
Secondary outcome:
Measurements taken at baseline and every 5 months for 20 months Unit of analysis: data analyzed by dominant eye |
|
| Notes | Study dates: 2005 to not reported
Trial registration: ACTRN12605000633684 Funding source: Maurice and Phyllis Paykel Trust; New Zealand Optometric and Vision Research Foundation; Cornea and Contact Lens Society of New Zealand Notes: study is also known as the Dual‐focus Inhibition of Myopia Evaluation in New Zealand (DIMENZ) study |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Children were randomized into group 1 or group 2 using a permuted block design with a block size of 4" |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment was not reported |
| Masking of participants (performance bias) | High risk | "The participants and the optometrist responsible for clinical care were not masked to lens assignment" |
| Masking of outcome assessors (detection bias) Progression of myopia | Low risk | The "investigating optometrists responsible for making outcome measures were masked" |
| Masking of outcome assessors (detection bias) Secondary outcomes | Low risk | The "investigating optometrists responsible for making outcome measures were masked" |
| Masking of data analyzers | Unclear risk | This was not reported |
| Incomplete outcome data (attrition bias) Incomplete outcome(s) data | Low risk | Six participants (15%)—3 from each group—were lost to followup and were excluded from the analysis: 4 children due to difficulties handling the contact lenses, 1 due to negative publicity on contact lens solutions, and 1 due to dislike of cycloplegia |
| Selective reporting (reporting bias) | Low risk | All outcomes specified prospectively on a clinical trials registry were reported |
| Other bias | High risk | One of the study authors disclosed an inventor patent for the contact lens design. Data were not appropriately analyzed for paired‐eye nor cross‐over design |
ATOM Study 2006.
| Study characteristics | ||
| Methods | Study design: parallel‐group RCT, with 2‐week run‐in period Study center: 1 Number randomized: 400 children Study follow‐up: 2 years Exclusions and losses to follow‐up: no exclusions; 54 (13.5%) were lost to follow‐up |
|
| Participants | Age: mean = 9.2 years (range 6 to 12 years) Gender: 220 boys, 180 girls Culture: Chinese (94%) and Indian children (4%) in Singapore Inclusion criteria: (1) age 6 to 12 years; (2) myopia with spherical equivalent refractive error between ‐1.00 D and ‐6.00 D in each eye as measured by cycloplegic autorefraction; (3) distance vision correctable to logMAR 0.2 or better in both eyes; (4) normal ocular health; (5) good general health with no history of cardiac or significant respiratory disease; (6) normal binocular function and stereopsis; (7) willingness and ability to tolerate monocular cycloplegia and mydriasis Exclusion criteria: (1) astigmatism > ‐1.50 D by cycloplegic autorefraction; (2) IOP 21 mmHg or greater; (3) allergies to atropine, cyclopentolate, proparacaine, or benzalkonium chloride; (4) previous or current use of contact lenses, bifocals, PALs, or other forms of myopia treatment; (5) amblyopia or manifest strabismus, including intermittent tropia |
|
| Interventions | Atropine (n = 200): 1 eye was randomized to 1 drop of 1% atropine sulfate nightly; the other eye received nothing Placebo control (n = 200): 1 eye was randomized to 1 drop of vehicle nightly; the other eye received nothing Note: all children received single vision photochromatic lenses for correction of refractive errors |
|
| Outcomes |
Primary efficacy outcome:
Secondary efficacy outcome:
Primary safety outcome:
Secondary safety outcomes:
Measurements taken at baseline and annually for 2 years Note: baseline measurements recorded 2 weeks after treatment began to allow for stabilization of the cycloplegic effect of atropine Unit of analysis: only 1 eye per patient randomized to receive treatment (fellow eyes were controls) |
|
| Notes | Study dates: enrollment between April 1999 and September 2000 Trial registration: not reported Materials: vehicle drops were prepared by Alcon Laboratories; spectacles were SOLA Transitions SVLs Funding source: National Medical Research Council, Singapore Additional data: study author provided unpublished data via email correspondence |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Children were allocated to groups based on a computer‐generated randomization list |
| Allocation concealment (selection bias) | Unclear risk | Methods section stated "allocated with concealment", but it was unclear how allocation concealment was conducted |
| Masking of participants (performance bias) | Low risk | Study was placebo‐controlled, and identical appearing bottles with coded labels were distributed |
| Masking of outcome assessors (detection bias) Progression of myopia | Low risk | Use of identical appearing bottles with coded labels and dilation of both pupils before examination |
| Masking of outcome assessors (detection bias) Secondary outcomes | Low risk | Primary and secondary outcomes were assessed by the same examiners |
| Masking of data analyzers | Unclear risk | This was not reported |
| Incomplete outcome data (attrition bias) Incomplete outcome(s) data | Unclear risk | "All statistical analyses were based on the intention‐to‐treat principle" Study authors noted (via personal communication) that there was a typographical error in the publication (54 were lost to follow‐up—34 from the atropine group and 20 from the placebo group); the paper reports that those who did not complete the study were characteristically similar to those who completed the study for each group |
| Selective reporting (reporting bias) | Low risk | Results were reported for outcomes described in the methods section of the paper |
| Other bias | Unclear risk | Pre‐randomization administration of the intervention may have enhanced or diminished the effect of the intervention during the subsequent randomized evaluation period |
Cambridge Anti‐Myopia Study 2013.
| Study characteristics | ||
| Methods | Study design: 2 × 2 factorial RCT Study center: 1 (Anglia Ruskin University) Number randomized: 147 children Study follow‐up: 24 months Exclusions and losses to follow‐up: 47 (33%) were excluded or lost to follow‐up |
|
| Participants | Age: mean = 17 years (range 14 to 22 years) Gender: 69 boys, 78 girls Culture: British Inclusion criteria: (1) age 14 to 22 years; (2) myopia with cycloplegic spherical equivalent ‐0.75 to ‐10.00 D; (3) astigmatism ≤ 0.75 D; 0.0 logMAR or less visual acuity with spectacles in each eye; no strabismus or uncompensated phoria; no ocular pathology; no systemic pathology that may affect myopia progression; zero or positive spherical aberration at distance; able to wear soft contact lenses throughout trial; no previous myopia control in study participation |
|
| Interventions | SCLs + SA + VT group (n = 25): SCLs with design to alter spherical aberration (fourth order), with vision training (VT) consisting of lens flipper exercises VT‐only group (n = 31): unaltered SCLs, with VT SCL + SA‐only group (n = 41): SCLs with design to alter spherical aberration SCL group (n = 45): unaltered SCLs, without VT Note: SCLs were replaced when refractive error changed by 0.25 D or more |
|
| Outcomes |
Primary outcome:
Secondary outcome:
Measurements taken at 6‐month intervals for 2 years Unit of analysis: child‐based (right eye) |
|
| Notes | Study dates: no dates provided; manuscript submitted October 2012 Trial registration: NCT00317551 Funding source: Australian government CRC Scheme via the Vision Cooperative Research Centre Disclosures: "the authors report no conflicts of interest and have no proprietary interest in any of the materials mentioned in this article" |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "When a participant was the first (and third etc.) member of a block, they were assigned by coin‐toss to the control or treatment groups, and the second (and fourth etc.) member of a block were assigned to the alternate group" |
| Allocation concealment (selection bias) | High risk | "One experimenter, who was unmasked, allocated participants to groups. Participants were referred to this experimenter by others who were enrolling participants in the trial" |
| Masking of participants (performance bias) | Unclear risk | "All participants wore contact lenses, either treatment or control, for the duration of the study. In order to mask the participants with regard to the treatment that they were getting, the participants were told that, in addition to wearing contact lenses, there were a range of possible things they might be required to do (including vision training), but this was not described in a way where they would see themselves as being in another treatment group or not for the purposes of the clinical trial" |
| Masking of outcome assessors (detection bias) Progression of myopia | Low risk | "The masked experimenters had no information about the way individual participants were allocated to treatment groups, and remained masked for the duration of the study"; "Completed data records for each visit were stored securely and were not available to the masked examiners. Clinical care records contained no information regarding the treatment assignments or data from the baseline or follow‐up visits" |
| Masking of outcome assessors (detection bias) Secondary outcomes | Low risk | Same as for primary outcomes |
| Masking of data analyzers | Unclear risk | This was not reported |
| Incomplete outcome data (attrition bias) Incomplete outcome(s) data | High risk | "Over the course of the trial 33% of participants dropped out"; "Only participants with a full dataset are presented"; "There was no significant difference in the number of subjects who dropped out of the study from each treatment group (Chi square P = 0.81) or in the gender of participants who dropped out (Chi square P = 0.49)" |
| Selective reporting (reporting bias) | Low risk | Results were reported for outcomes described in the methods section of the paper and in the clinical trial registry record |
| Other bias | Low risk | None was identified |
Charm 2013.
| Study characteristics | ||
| Methods | Study design: parallel‐group RCT Study center: 1 (Hong Kong Polytechnic University) Number randomized: 52 children Study follow‐up: 2 years Exclusions and losses to follow‐up: 14 (27%) children who were randomized, 7 in each group, were excluded or lost to follow‐up |
|
| Participants | Age: median = 10 years (range 8 to 11 years) Gender: not reported Culture: children "recruited via advertisements posted on local newspapers and leaflets in the Optometry Clinic of the School of Optometry" Inclusion criteria: (1) age 8 to 11 years; (2) myopia with spherical equivalent refractive error ≥ ‐5.00 D by cycloplegic manifest refraction; (3) monocular Snellen VA 20/25 or better; (4) willingness to wear orthokeratology and to be available for monthly follow‐up Exclusion criteria: (1) astigmatism > 1.25 D; (2) binocular vision problems; (3) any ocular or systemic condition that may affect vision or vision development; (4) contraindications for contact lens wear; (5) previous experience with refractive surgery, PALs, or orthokeratology |
|
| Interventions | Orthokeratology (n = 26): partial reduction orthokeratology contact lenses of target 4.00 D (DreamLite, Procornea Ltd, The Netherlands); "residual refractive errors were corrected by a pair of single vision spectacles to be worn during daytime" SVLs (n = 26): single vision spectacles Note: "spectacle prescription would be updated at any subsequent visit for either group of subjects if difference in residual refractive errors (sphere or astigmatism) obtained at that visit exceeded 0.50 D" |
|
| Outcomes |
Primary outcome:
Secondary outcomes:
Measurements taken every 6 months for 2 years Unit of analysis: child‐based (right eye) |
|
| Notes | Study dates: not reported Trial registration: NCT00977236 Funding source: "this study was supported by a Collaborative Research Agreement between The Hong Kong Polytechnic University (PolyU) and Procornea Nederland B.V. and a Niche Area Funding (J‐BB7P) from PolyU. We thank Menicon Company Limited for supplying Menicon O2 Care for the study" Conflict of interest: "the authors have no proprietary interest in any of the products used in the study" |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of randomization was not reported |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment before randomization was not reported |
| Masking of participants (performance bias) | High risk | "Single‐masked" study; participants were not masked |
| Masking of outcome assessors (detection bias) Progression of myopia | Unclear risk | This was not reported |
| Masking of outcome assessors (detection bias) Secondary outcomes | Low risk | "Measurements of AL were performed with the Zeiss IOLMaster (Carl Zeiss Meditec, Inc., USA) by masked examiners"; "This study was a single‐masked design to eliminate any examiner bias on myopic progression. The masked examiner only measured and recorded the AL which was the primary outcome of the study" |
| Masking of data analyzers | Unclear risk | This was not reported |
| Incomplete outcome data (attrition bias) Incomplete outcome(s) data | High risk | "Seven subjects in the control group decided to quit the study after baseline examination. One PR ortho‐k subject quitted after two days of lens wear due to lens discomfort, five were terminated due to poor lens fitting despite repeated lens modifications, and another was terminated after one week of lens wear due to noncompliance with aftercare schedule" |
| Selective reporting (reporting bias) | High risk | Results were reported for the intervention group only |
| Other bias | Low risk | None was identified |
Cheng 2010.
| Study characteristics | ||
| Methods | Study design: parallel‐group RCT Study center: 1 (optometric practice in Mississauga, Ontario, Canada) Number randomized: 150 children Study follow‐up: 2 years Exclusions and losses to follow‐up: 15 (10%) children who were randomized were excluded from the analyses; 4 (3%) were lost to follow‐up |
|
| Participants | Age: mean = 10 years (range 8 to13 years) Gender: 62 boys and 73 girls received treatment Culture: Chinese Canadian children were recruited by reviewing clinical records and mailing invitation letters addressed to their parents, or by responding to poster in the practice or during regular eye examinations Inclusion criteria: (1) Chinese Canadian children who were seen at the practice in the last 9 to 18 months; (2) age 8 to 13 years; (3) myopia between ‐1.00 D and ‐5.50 D; (4) myopia progression ≥ 0.50 D in the preceding year; (5) distance monocular visual acuity of 6/6 or better; (6) near monocular visual acuity of 6/6 or better; (7) stereoacuity ≤ 40 s of arc at 40 cm; (8) single vision distance lens wear; (9) consent of child and parent for study participation Exclusion criteria: (1) astigmatism > 1.50 D; (2) anisometropia > 1.50 D; (3) strabismus; (4) inability to respond to subjective testing; (5) history of systemic or ocular disease; (6) history of bifocal lens wear and/or contact lens use |
|
| Interventions | SVLs (n = 50): single vision distance lenses Bifocal lenses (n = 50): bifocal lenses with +1.50 D near addition Prismatic bifocal lenses (n = 50): prismatic bifocal lenses with +1.50 D addition and a 3‐prism diopter base‐in prism in the near segment Note: distance prescription changes were made if subjective refraction changed by ≥ 0.50 D in either eye |
|
| Outcomes |
Primary outcome:
Secondary outcome:
Measurements taken at baseline and every 6 months for 2 years Unit of analysis: child‐based (right eye) |
|
| Notes | Study dates: April 2003 to April 2008
Trial registration: NCT00787579 Funding source: Essilor International of France Auxiliary data: parents and/or guardians completed questionnaires related to vision habits of the enrolled child and the child's birth parents' refractive errors. The number of years the children were myopic before entering the study was estimated from clinical records. Auxiliary data were used as covariates for regression statistics and to test the hypothesis that bifocal treatment is more effective with a shorter duration of myopia Additional data: study author provided unpublished data via email correspondence |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | "Randomization was implemented by putting the subjects' file numbers on slips of paper and drawing them from a container at random...The first 50 subjects drawn were assigned to the control group; the second 50 were assigned to the bifocal group, and so forth" |
| Allocation concealment (selection bias) | High risk | "The first 50 subjects drawn were assigned to the control group; the second 50 were assigned to the bifocal group, and so forth" |
| Masking of participants (performance bias) | High risk | "The subjects and the investigator were aware of the treatment assignments." Masking was not applicable due to visual and functional differences between the interventions studied |
| Masking of outcome assessors (detection bias) Progression of myopia | High risk | "The subjects and the investigator were aware of the treatment assignments. Masking was difficult to achieve in a practice‐based intervention, particularly when the lens treatments were visually very different." The primary study investigator dispensed lenses and performed examinations |
| Masking of outcome assessors (detection bias) Secondary outcomes | High risk | "...the primary and secondary outcome variables were measured by objective methods to minimize possible bias of the unmasked investigator" |
| Masking of data analyzers | Low risk | "The data analyst discerned the study investigated the effect of three types of lenses on ocular refraction, but he was masked to the possible effect of bifocal or prismatic bifocal lens on myopia control" (via email communication with study author) |
| Incomplete outcome data (attrition bias) Incomplete outcome(s) data | High risk | "The analysis of the data followed the intention‐to‐treat approach, and we used the last progression information (i.e. carry forward) method for subjects lost to follow‐up". Although study authors stated that they used intention‐to‐treat analysis, 15 of the 150 children randomized were not included in the analysis: 9 children randomized to single vision lenses dropped out because their parents wanted them to receive bifocals; and 2 children in the bifocals group and 4 in the prismatic bifocals group were excluded due to adverse reactions following cycloplegia |
| Selective reporting (reporting bias) | Low risk | Results were reported for outcomes described in the methods section of the paper |
| Other bias | Unclear risk | This study was funded by a company that produces the types of lenses being investigated |
Cheng 2016.
| Study characteristics | ||
| Methods | Study design: parallel‐group RCT Study center: Korb and Associates in Boston, Massachusetts Number randomized: 127 children Study follow‐up: 12 months (planned for 24 months) Exclusions and losses to follow‐up: 6 (4.7%) children who were randomized were excluded from the analyses; 15 (11.8%) were lost to follow‐up |
|
| Participants | Age: mean = 9.7 years (range 8 to 11 years) Gender: 59 boys, 68 girls Culture: 90.6% were Asian and 8.7% were white Inclusion criteria: (1) ages 8 to 11 years; (2) myopia ‐0.75 to ‐4.00 D sphere by cycloplegic refraction; (3) 1.00 D or less astigmatism; (4) 1.00 D or less difference between eyes in spherical equivalent; (5) 20/25 + 2 or better visual acuity in each eye with spherocylindrical refraction; (6) 20/25 or better visual acuity with best sphere Exclusion criteria: (1) ocular or systemic pathology; (2) history of eye surgery; (3) history of myopia control |
|
| Interventions | SCL + SA group (n = 64): soft daily disposable contact lenses with positive spherical aberration (0.175 μm) SCL group (n = 63): soft daily disposable contact lenses without the positive spherical aberration Note: control and test lenses had identical material and appearance; spherical aberration was chosen to negate the negative spherical aberration that occurred in myopes during accommodation |
|
| Outcomes |
Primary outcome:
Secondary outcome:
Measurements taken every 6 months for 2 years Unit of analysis: child‐based (right eye) |
|
| Notes | Study dates: April 2008 to October 2011 Trial registration: NCT01829191; NCT01829230 Funding source: Johnson and Johnson Vision Care, Inc. Disclosures of interest: "Xu Cheng, Jing Xu, Khaled Chehab, and Noel Brennan are all paid employees of Johnson and Johnson Vision Care, Inc. Joan Exford of Korb & Associates is a contract principal investigator paid by Johnson and Johnson Vision Care, Inc."; "We thank Dr. Jichang He of New England College of Optometry and Dr. Victor Finnemore of Korb & Associates for collecting data for the study and Dr. Myles Jaffe of Innova Medical Communications, LLC, who is a contract medical writer paid by Johnson and Johnson Vision Care, Inc. for preparing this manuscript" Notes: "the study was terminated because sufficient data had been collected from concurrent internal studies of similar designs" |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of randomization was not reported |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment before randomization was not reported |
| Masking of participants (performance bias) | Unclear risk | "Both the subjects and investigators involved in gathering data in the withdrawal phase continued to be masked as to the initial treatment assignment in the earlier double‐masked component of this investigation"; "The test lenses were identical to the control lenses in every aspect except that they were designed with aspheric front surfaces incorporating +SA" |
| Masking of outcome assessors (detection bias) Progression of myopia | Low risk | "Both the subjects and investigators involved in gathering data in the withdrawal phase continued to be masked as to the initial treatment assignment in the earlier double‐masked component of this investigation"; "Three investigators were involved in data collection. Two conducted lens fittings and provided ongoing care, and a separate masked investigator was responsible for refraction and AL measurements" |
| Masking of outcome assessors (detection bias) Secondary outcomes | Low risk | Same as for the primary outcome |
| Masking of data analyzers | Unclear risk | This was not reported |
| Incomplete outcome data (attrition bias) Incomplete outcome(s) data | High risk | Children who were randomized but who dropped out were excluded from analyses; "During the entire course of the treatment phase, a total of 21 (17%) subjects were discontinued from the study, among which 14 (22%) and 7 (11%) were from the test cohort and control cohort, respectively" |
| Selective reporting (reporting bias) | Unclear risk | Results were reported only for 12‐month data; 24 (18.9%) children completed 24 months of follow‐up before the study was terminated early |
| Other bias | High risk | This study was funded by a company that produces the types of lenses being investigated; the authors of the study are employees of the company; and "the study was terminated because sufficient data had been collected from concurrent internal studies of similar designs" |
Chung 2002.
| Study characteristics | ||
| Methods | Study design: parallel‐group RCT Study center: patient care unit at the Department of Optometry, Faculty of Allied Health Science, National University of Malaysia Number randomized: 106 children Study follow‐up: 2 years Exclusions and losses to follow‐up: no exclusions; 12 (11%) were lost to follow‐up |
|
| Participants | Age: mean = 11.56 years (range 9 to 14 years) Gender: 39 boys, 55 girls Culture: Malay and Chinese ethnic origin Inclusion criteria: (1) age 9 to 14 years; (2) myopia with spherical equivalent refractive error ≥ ‐0.50 D in both eyes, with no principal meridian being plano or having any amount of plus power; (3) corrected VA of 6/6 or better in each eye; (4) normal ocular health; (5) willingness to give written consent Exclusion criteria: (1) more than 2 diopters of astigmatism in each eye; (2) binocular vision problems, including anisometropia over 2.00 D, problems requiring refractive therapy, strabismus, and amblyopia; (3) previous contact lens wear; (4) family was planning to leave the area before the end of the study period |
|
| Interventions | Undercorrected group (n = 47): monocular VA blurred to 6/12 (approximately +0.75 D) in each eye with spectacles Fully corrected group (n = 47): monocular VA maintained at 6/6 or better in each eye with spectacles Note: in the fully corrected group, changes in prescription were made if subjective refraction had changed by ≥ 0.50 D for 1 or both eyes. For the undercorrected group, changes in prescription were made to maintain a vision of 6/12 in each eye |
|
| Outcomes |
Progression of early‐onset myopia:
Measurements taken at baseline and every 6 months for 2 years Unit of analysis: average values of both eyes used for all results |
|
| Notes | Study dates: not reported Trial registration: not reported Funding source: IRPA grant Compliance in wearing glasses was monitored via questionnaires. Compliance was defined as wearing glasses for at least 8 hours a day (40 patients in the undercorrected group vs 41 in the fully corrected group). Partial compliance was defined as wearing glasses 6 to 8 hours a day (7 patients in the undercorrected group vs 6 in the fully corrected group) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Block randomization based on age, sex, race, and refractive error. Treatment and control pairings were made to complete cells based on 3 age categories, 4 refractive error categories, 2 racial groups, and 2 gender groups (3 x 4 x 2 x 2 = 48 cells). Patients were designated as subject 1 or subject 2 for each cell based on a predetermined randomization procedure |
| Allocation concealment (selection bias) | Low risk | Once the patients were paired, a coin toss determined which patient was assigned to the treatment or control group. Heads meant subject 1 was allocated to undercorrection and subject 2 received full correction. Tails meant subject 1 was allocated to full correction and subject 2 received undercorrection. A coin toss was performed for each pair |
| Masking of participants (performance bias) | High risk | Masking of participants was not applicable due to performance differences between the interventions studied |
| Masking of outcome assessors (detection bias) Progression of myopia | Low risk | During all evaluations, the examining optometrist was not aware of the group assignment |
| Masking of outcome assessors (detection bias) Secondary outcomes | Low risk | Primary and secondary outcomes were assessed by the same examiners |
| Masking of data analyzers | Unclear risk | Results were analyzed only after the last reading of the last patient was collected |
| Incomplete outcome data (attrition bias) Incomplete outcome(s) data | Low risk | Of the 106 children recruited, 94 completed the study and were included in the analyses; 12 (11.3%) dropped out and were excluded from the analyses |
| Selective reporting (reporting bias) | Low risk | Results were reported for outcomes described in the methods section of the paper |
| Other bias | Low risk | None was identified |
CLAMP Study 2004.
| Study characteristics | ||
| Methods | Study design: parallel‐group RCT, with run‐in period Study center: 1 (The Ohio State University College of Optometry) Number randomized: 116 children Study follow‐up: 3 years Exclusions and losses to follow‐up: none |
|
| Participants | Age: mean = 10.7 years (range 8 to 12 years) Gender: 47 boys, 69 girls Culture: Columbus, Ohio, USA; 84.5% white (not of Hispanic origin), 8.6% Asian or Pacific Islander, 4.3% Black (not of Hispanic origin) Inclusion criteria: (1) 8 to 11 years old at time of randomization; (2) myopia with spherical equivalent refractive error between ‐0.75 D and ‐4.00 D in each eye, as measured by cycloplegic refraction; (3) corrected VA of 20/20 or better in each eye Exclusion criteria: (1) astigmatism > 1.50 DC in each eye by cycloplegic refraction or > 1.00 DC on manifest refraction; (2) previous or attempted history of contact lens wear; (3) anisometropia > 1.00 D between eyes; (4) eye disease and binocular vision problems; (5) systemic disease that may affect vision or vision development Note: all participants had to successfully complete a run‐in period before enrollment into the study to exclude those who could not adapt to rigid contact lenses; 32 children did not complete the run‐in period and were excluded. Success for the run‐in period was defined as wearing the lenses at least 40 hours/week and stating that the lenses were "always comfortable" or "usually comfortable" |
|
| Interventions | (n = 59): RGPCLs worn during waking hours for 3 years (n = 57): SCLs worn during waking hours for 3 years Note: prescription changes were made by an unmasked examiner based on participant complaints and improvement in visual acuity |
|
| Outcomes |
Primary outcome:
Secondary outcomes:
Measurements taken at baseline and every 6 months for 3 years Unit of analysis: data analyzed for right eye only |
|
| Notes | Study dates: enrollment July 8, 1998 to February 26, 2000 Trial registration: NCT00009529 Funding source: National Eye Institute, National Institutes of Health; Menicon Co, Ltd.; CIBA Vision Corporation; SOLA Optical; and Essilor |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomized participants were stratified by gender and in treatment blocks of 3. A list of randomized treatment assignments was prepared by an independent person before the study began |
| Allocation concealment (selection bias) | Unclear risk | Individual treatment assignments from the list were placed in sequentially numbered envelopes that were sealed. Envelopes were drawn from the pool in sequential order according to the participant's gender. It was unclear whether the envelopes were opaque. |
| Masking of participants (performance bias) | High risk | Masking of participants was not applicable due to material differences between the interventions studied |
| Masking of outcome assessors (detection bias) Progression of myopia | Low risk | Masked examiners conducted the primary outcome procedure and all secondary outcomes except visual acuity. When the masked examiner was in the room, participants wore only spectacle correction or no correction and were told not to mention any contact lens wear to the masked examiner. No assessment of masking was reported |
| Masking of outcome assessors (detection bias) Secondary outcomes | Low risk | Outcome measures were performed by examiners masked to the mode of correction worn by the participant with the exception of visual acuity measurements |
| Masking of data analyzers | Unclear risk | This was not reported in the paper, but the persons conducting the analyses were not masked to treatment group allocation (JW). Outcome measures were not presented by treatment group until the conclusion of the trial. Data were managed via a dual‐entry format |
| Incomplete outcome data (attrition bias) Incomplete outcome(s) data | Low risk | All data were analyzed according to the original result of the random assignment, and no data were missing. "We analyzed all data using intention‐to‐treat methods" |
| Selective reporting (reporting bias) | Low risk | All outcomes outlined in the study protocol were reported |
| Other bias | Unclear risk | Pre‐randomization administration of the intervention may have enhanced or diminished the effect of the intervention during the subsequent, randomized evaluation period This study was partially funded by companies that produce the interventions being investigated |
COMET2 Study 2011.
| Study characteristics | ||
| Methods | Study design: parallel‐group RCT Study centers: 8 (including 7 optometry colleges and schools and 1 community‐based ophthalmology practice) Number randomized: 118 children Study follow‐up: 3 years Exclusions and losses to follow‐up: no exclusions; 8 (7%) were lost to follow‐up |
|
| Participants | Age: mean = 10.1 years (range 8 to 12 years) Gender: 54 boys, 64 girls Culture: USA Inclusion criteria: (1) age 8 to < 12 years; (2) refractive error determined by cycloplegic autorefraction, which meets all of the following: spherical equivalent ‐0.50 to ‐3.00 D in both eyes; astigmatism ≤ 1.5 D in both eyes; anisometropia ≤ 1.00 D difference between eyes in spherical equivalent; (3) visual acuity at least 20/20 with best subjective refraction in both eyes; (4) accommodative response at near vision (33 cm) is less than 2.0 D by noncycloplegic autorefraction; (5) near esophoria (≥ 2.0 PD) present by alternate prism and cover test (APCT) at near vision using best refractive correction determined from noncycloplegic subjective refraction Exclusion criteria: (1) history of strabismus; (2) current or prior use of PALs, bifocals, or contact lenses in either eye (prior or current use of SVLs was permitted) |
|
| Interventions | PAL group (n = 59): Varilux Ellipse progressive addition lenses with a +2.00 D near addition; worn during all waking hours for 3 years SVL group (n = 59): standard single vision lenses (spectacles); worn during all waking hours for 3 years Notes: the distance correction was changed if the endpoint of the noncycloplegic subjective refraction differed from the current prescription by 0.50 D or more in spherical equivalent. Prescription changes could be made for smaller differences at investigator discretion if the new prescription improved the patient’s visual acuity by at least 1 line over that in their current correction |
|
| Outcomes |
Primary outcome:
Secondary outcomes:
Measurements taken at baseline and every 6 months for 3 years Unit of analysis: child‐based (median for each eye averaged to obtain the spherical equivalent used for analysis) |
|
| Notes | Study dates: enrollment from April 2005 to March 2007 Trial registration: NCT00320593 Funding source: National Institutes of Health, Department of Health and Human Services, USA Materials: Essilor of America and Eyewear Designs provided spectacles at a reduced cost Study name: progressive addition lenses vs single vision lenses for slowing progression of myopia in children with high accommodative lag and near esophoria |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "In a permuted block design stratified by site and by history of previous SVL wear, each subject was randomly assigned with equal probability to receive spectacles that were either PALs with a +2.00 D near addition or SVLs" |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment was not reported |
| Masking of participants (performance bias) | High risk | Masking of participants was not applicable due to functional differences between the interventions studied |
| Masking of outcome assessors (detection bias) Progression of myopia | Low risk | "All testing procedures were performed by a study‐certified optometrist or ophthalmologist who was masked to the subject’s lens assignment. To maintain masking of the investigators, the subject saw an unmasked coordinator before the examination who collected the subject’s spectacles and told the optometrist or ophthalmologist performing the eye examination what distance refractive correction to use in trial frames for the examination" |
| Masking of outcome assessors (detection bias) Secondary outcomes | Low risk | Primary and secondary outcomes were assessed by the same examiners |
| Masking of data analyzers | Unclear risk | Masking of persons analyzing results was not reported |
| Incomplete outcome data (attrition bias) Incomplete outcome(s) data | Low risk | "All analyses followed the intent‐to‐treat principle. The Monte Carlo Markov Chain (MCMC) 18 method of multiple imputation was used to impute data for subjects who did not complete the 3‐year visit. To evaluate the effect of imputation on the primary results, we also performed the primary analysis (1) using the last‐observation carried‐forward method and (2) using only data from subjects who completed the 3‐year visit"; 1 (2%) and 7 (12%) children withdrew in SVLs and PALs groups, respectively; 3 were lost to follow‐up, 1 moved to another state, and 4 withdrew by clinical site |
| Selective reporting (reporting bias) | Low risk | All outcomes specified prospectively on a clinical trials registry were reported |
| Other bias | Low risk | None was identified |
COMET Study 2003.
| Study characteristics | ||
| Methods | Study design: parallel‐group RCT Study center: multicenter, including (1) a study chair, (2) a coordinating center, (3) 4 clinical centers, and (4) the National Eye Institute, USA Number randomized: 469 children Study follow‐up: 3 years Exclusions and losses to follow‐up: no exclusions; 7 (1.5%) were lost to follow‐up |
|
| Participants | Age: mean = 9.3 years (range 6 to 11 years) Gender: 223 boys, 246 girls Culture: 4 major cities in the USA (Birmingham, Alabama: n = 133; Boston, Massachusetts: n =110; Philadelphia, Pennsylvania: n = 108; and Houston, Texas: n = 118) Inclusion criteria: (1) 6 to 11 years old; (2) myopia with spherical equivalent refractive error between ‐1.25 D and ‐4.50 D in both eyes, as measured by cycloplegic autorefraction; (3) astigmatism ≤ 1.50 D; (4) no anisometropia (difference in spherical equivalent < 1.00 D between eyes); (5) best‐corrected VA of 20/32 or better; (6) no strabismus by cover test for far (4.0 m) and/or near (0.33 m) fixation; (7) willingness to not wear contact lenses for study duration Exclusion criteria: (1) strabismus detected by cover test; (2) any ocular, systemic, or neurodevelopmental conditions that could influence refractive development; (3) chronic medication use that might affect myopia progression or visual acuity; (4) birth weight < 1250 g; (5) previous use of bifocals, PALs, or contact lenses; (6) problems with adherence to the protocol or the follow‐up period |
|
| Interventions | PAL group (n = 235): multifocal lenses (no‐line bifocals) with gradual and progressive change toward less negative or more positive power from the distance portion to the near portion of the lens (power +2.00 D); worn during waking hours for 3 years SVL (n = 234): single vision lenses with same focal power throughout the lens area; worn during waking hours for 3 years Note: prescription changes were made if the subjective refraction had changed by at least 0.50 D for 1 or both eyes. Smaller prescription changes were made if clinically indicated. Both groups were offered single vision sports glasses to use while participating in sports activities |
|
| Outcomes |
Primary outcome:
Magnitude of change in spherical equivalent refractive error relative to baseline measured by cycloplegic autorefraction with 2 drops of 1% tropicamide Secondary outcomes:
Measurements taken at baseline and every 6 months for 3 years Unit of analysis: child‐based Average values of both eyes used if the correlation coefficient was > 0.85 between eyes and the mean difference was not statistically significant; otherwise the eye with greater myopic change used for each child |
|
| Notes | Study dates: enrollment was from September 1997 to September 1998; follow‐up was designed for 3 years but continued for 7 years, including 5 years wearing original lens assignments and 2 years wearing either glasses or contact lenses Trial registration: NCT00000113 Funding source: NEI grants, Essilor of America, Marchon Eyewear, Marco Technologies, and Welch Allyn Sample of 150 children were followed up at 1 month to evaluate possible lens‐induced phoria changes; no problems were detected in either group Compliance in wearing glasses was monitored via separate questionnaires for children and parents (93% compliance in PAL group, 96% compliance in SVL group). Attitude toward wearing glasses and self‐esteem were also measured Additional data: study author provided unpublished data via email correspondence |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomization was derived by permuted block design with preset block size and was stratified by clinical center by the coordinating center |
| Allocation concealment (selection bias) | Low risk | Randomization assignments were allocated by the coordinating center after the eligibility of each participant was verified |
| Masking of participants (performance bias) | High risk | Masking of participants was not applicable due to functional differences between the interventions studied |
| Masking of outcome assessors (detection bias) Progression of myopia | Low risk | Optometrists responsible for assessing study outcomes were unaware of lens assignments |
| Masking of outcome assessors (detection bias) Secondary outcomes | Low risk | Primary and secondary outcomes were assessed by the same examiners |
| Masking of data analyzers | Unclear risk | "The data analysts were not masked to treatment assignment when analyzing the data" (via email communication with study author) |
| Incomplete outcome data (attrition bias) Incomplete outcome(s) data | Low risk | "Follow‐up data were analyzed by applying an intention‐to‐treat principle according to the child’s original lens assignment and the last known value of the outcome measures. For the seven children lost to follow‐up and thus without data at the third annual visit, progression information from the latest follow‐up visit was used" |
| Selective reporting (reporting bias) | Low risk | All outcomes published a priori in the design paper (Hyman 2001) were reported in results papers |
| Other bias | Unclear risk | This study was partially funded by companies that produce the interventions being investigated |
CONTROL Study 2016.
| Study characteristics | ||
| Methods | Study design: parallel‐group RCT Study center: 1 Number randomized: 86 children Study follow‐up: 1 year Exclusions and losses to follow‐up: 8 children did not complete the study |
|
| Participants | Age: mean = 13 years (range 8 to 18 years) Gender: 26 boys, 60 girls Culture: California, USA Inclusion criteria: (1) myopia between ‐0.50 D and ‐6.00 D, with documented progression of ‐0.50 D or more since last examination; (2) eso fixation disparity at 33 cm with distance correction; (3) astigmatism 1.00 D or less; (4) anisometropia 2.00 D or less; (5) best‐corrected visual acuity 20/20 or better in each eye; (6) ability to wear SCLs and attend follow‐up visits Exclusion criteria: (1) presence of ocular disease affecting eye growth or preventing wear of contacts; (2) prior ocular surgery; (3) history of wearing RGPs in previous 2 years or extended wear SCLs in previous 6 months: (4) pregnancy or nursing; (5) use of certain medications |
|
| Interventions | BSCL group (n = 39): Vistakon Acuvue Bifocal lenses (distance center, alternating 5‐ring), worn on a daily basis SVSCL group (n = 40): Vistakon Acuvue 2, worn on a daily basis |
|
| Outcomes |
Primary outcomes:
Secondary outcomes:
Measurements taken at baseline, 6 months, and 12 months Unit of analysis: average values for both eyes |
|
| Notes | Study dates: start date was October 2003; study was completed in 2006 Trial registration: NCT00214487 Funding source: Vistakon Additional information: study author provided unpublished information via email correspondence |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Because of the relatively small group (sample) sizes involved, a modified covariate adaptive randomization approach was used to allocate treatments. Specifically, subjects were randomly assigned a treatment by the masked off‐site clinical trial coordinator who used an adaptive biased coin toss design to increase the probability that successive subjects were assigned to the group with the smaller sample size with respect to age, refractive error, amount of eso‐associated near phoria, sex, and Asian versus non‐Asian ethnicity" |
| Allocation concealment (selection bias) | Low risk | Contact lens prescriptions for eligible participants were transmitted to an off‐site research assistant for allocation (via email communication with study author) |
| Masking of participants (performance bias) | Unclear risk | "Masking was aided by the choice of lenses; both were 58% water, two‐week disposable lenses, identical in appearance and supplied in masked packaging" "The study examiner, office staff, subjects, and parents were not aware of the lens assignments before the end of the study" |
| Masking of outcome assessors (detection bias) Progression of myopia | Low risk | "Masking was aided by the choice of lenses; both were 58% water, two‐week disposable lenses, identical in appearance and supplied in masked packaging" "The study examiner, office staff, subjects, and parents were not aware of the lens assignments before the end of the study" |
| Masking of outcome assessors (detection bias) Secondary outcomes | Low risk | Primary and secondary outcomes were assessed by the same examiners |
| Masking of data analyzers | Unclear risk | This was not reported |
| Incomplete outcome data (attrition bias) Incomplete outcome(s) data | Low risk | 79/86 (92%) randomized participants were included in final analyses; attrition was balanced between groups |
| Selective reporting (reporting bias) | Low risk | All outcomes specified on a clinical trials registry and in a conference abstract were reported |
| Other bias | Unclear risk | This study was funded by a company that produces the types of lenses being investigated |
DISC Study 2011.
| Study characteristics | ||
| Methods | Study design: parallel‐group RCT Study center: 1 (Hong Kong Polytechnic University) Number randomized: 221 children Study follow‐up: 2 years Exclusions and losses to follow‐up: 38 (34.2 %) in BSCL group and 36 (32.7%) in SVSCL group were excluded; 8 (7.2 %) in BSCL group and 11 (10.0%) in SVSCL group were lost to follow‐up |
|
| Participants | Age: mean = 11 years (range 8 to 13 years) Gender: 85 boys, 136 girls Culture: Hong Kong, China Inclusion criteria: (1) age 8 to 13 years; (2) spherical equivalent ‐1.00 to ‐5.00 D; (3) astigmatism 1.00 D or less; (4) anisometropia 1.25 D or less; (5) spectacle‐corrected monocular visual acuity 0.0 logMAR or better; (6) contact lens–corrected monocular visual acuity 0.1 logMAR or better; (7) willingness to wear contact lenses regularly and parents' understanding and acceptance of random allocation of intervention Exclusion criteria: (1) ocular or systemic abnormalities affecting visual function or refractive development; (2) prior use of PALs or bifocal contact lenses; (3) contraindication for contact lens wear |
|
| Interventions | BSCL group (n = 111): dual‐focus incorporated soft contact (DISC) lenses, which were custom‐made BSCLs with distance correction in the center and alternating rings of defocusing (+2.50 D addition) and distance correction zones SVSCL group (n = 110): single vision soft contact lenses Note: children were instructed to wear lenses for 5 to 10 hours per day and to wear spectacles with full prescription when not wearing contact lenses |
|
| Outcomes |
Primary outcomes:
Secondary outcome:
Measurements taken every 6 months over 2 years Unit of analysis: individual (right eye used for analysis) |
|
| Notes | Study dates: September 2007 and October 2009 Trial registration: NCT00919334 Funding source: "the study was supported by grants of RGC GRF (B‐Q04G) and Niche Areas Fund (J‐BB7P) from The Hong Kong Polytechnic University" Conflict of interest: reported "none" |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Allocation was determined by a random software sequence in ex‐cell" |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment before randomization was not reported |
| Masking of participants (performance bias) | Unclear risk | "The children and their parents were not told which lens design was prescribed" It is unclear whether children would remain masked during the treatment period given the different designs of the lenses |
| Masking of outcome assessors (detection bias) Progression of myopia | Low risk | "One investigator was masked from grouping and was responsible for refracting and relevant ocular data measurement. The other investigator was unmasked and responsible for group allocation, lens fitting and aftercare, measuring lens performance, record keeping and compliance checking" |
| Masking of outcome assessors (detection bias) Secondary outcomes | Low risk | "One investigator was masked from grouping and was responsible for refracting and relevant ocular data measurement. The other investigator was unmasked and responsible for group allocation, lens fitting and aftercare, measuring lens performance, record keeping and compliance checking" |
| Masking of data analyzers | Unclear risk | This was not reported |
| Incomplete outcome data (attrition bias) Incomplete outcome(s) data | High risk | 46 (41.4%) in the BSCL group and 47 (42.7%) in the SVSCL group were not included in the final analyses. The most common reason for exclusion of children from the analyses—22 in the BSCL group and 22 in the SVSCL group—was that they did not want to wear the contact lenses |
| Selective reporting (reporting bias) | Low risk | All outcomes specified prospectively on a clinical trials registry were reported |
| Other bias | Low risk | None was identified |
Edwards 2002.
| Study characteristics | ||
| Methods | Study design: parallel‐group RCT Study center: 1 (Centre for Myopia Research) Number randomized: 298 children Study follow‐up: 2 years Exclusions and losses to follow‐up: no exclusions; 44 (15%) were lost to follow‐up |
|
| Participants | Age: mean = 9.09 years (range 7 to 10.5 years) Gender: 122 boys, 132 girls Culture: Hong Kong children, recruited through newspaper advertisements Inclusion criteria: (1) 7 to 10.5 years old; (2) spherical equivalent refractive error between ‐1.25 D and ‐4.50 D, as measured under cycloplegia; (3) best‐corrected VA of 0.00 logMAR or better; (4) no previous use of contact lenses and willingness to not wear contact lenses; (5) willingness to wear glasses constantly; (6) parents' acceptance of randomization Exclusion criteria: (1) astigmatism > 1.50 D; (2) anisometropia > 1.50 D in spherical or cylindrical error; (3) any ocular or systemic condition that might affect refractive development; (4) previous use of bifocals or PALs; (5) problems with adherence to the protocol or the follow‐up period |
|
| Interventions | PAL group (n = 138): SOLA MC progressive addition lenses (add +1.50 D); worn constantly for 2 years SVL (n = 160): SOLA single vision lenses; worn constantly for 2 years Note: prescription changes were made if there was a reduction in aided vision of ≥ 0.10 logMAR units |
|
| Outcomes |
Primary outcomes:
Secondary outcomes:
Measurements taken at baseline and every 6 months for 2 years Unit of analysis: only data from right eyes reported |
|
| Notes | Study dates: not reported Trial registration: not reported Materials: lenses provided by Sola (Hong Kong) Ltd Funding source: Centre for Myopia Research (Area of Strategic Development), The Hong Kong Polytechnic University |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Predetermined random sequence |
| Allocation concealment (selection bias) | Unclear risk | The investigator, who was involved in assigning children, was not aware of group allocation until a child was enrolled in the study. It was unclear how the allocation was concealed |
| Masking of participants (performance bias) | High risk | Masking of participants was not applicable due to functional differences between the interventions studied |
| Masking of outcome assessors (detection bias) Progression of myopia | Low risk | The investigator measuring refractive error was masked to treatment assignment |
| Masking of outcome assessors (detection bias) Secondary outcomes | Low risk | The investigator measuring axial length was masked to treatment assignment |
| Masking of data analyzers | Low risk | Masked and unmasked investigators independently analyzed the data |
| Incomplete outcome data (attrition bias) Incomplete outcome(s) data | Low risk | There were no exclusions after randomization. There were 44 patients lost to follow‐up: n = 17 in the PAL group, n = 27 in the SVL group. It was reported that whether or not a patient was retained in the study was not statistically associated with treatment allocation |
| Selective reporting (reporting bias) | Low risk | Results for study outcomes were reported at 2‐year follow‐up |
| Other bias | Low risk | None was identified |
Fujikado 2014.
| Study characteristics | ||
| Methods | Study design: cross‐over RCT Study center: 1 (Osaka University School of Medicine) Number randomized: 24 children Study follow‐up: 12 months for each phase Exclusions and losses to follow‐up: "in the second year, two children dropped out from the study because their families moved to another city" |
|
| Participants | Age: mean = 14 years (range 6 to 16 years) Gender: 7 boys, 17 girls Culture: Japan Inclusion criteria: (1) 6 to 16 years of age; (2) myopic refractive error between ‐0.75 D and ‐3.50 D; (3) anisometropia ≤ 1.0 D; (4) astigmatism ≤ 1.0 D; (5) best‐corrected visual acuity 20/20 or better; (6) willingness to wear lenses Exclusion criteria: (1) amblyopia, strabismus, or other ocular disease other than refractive error; (2) history of orthokeratology, bifocal spectacles, or progression spectacles in past 12 months |
|
| Interventions | BSCL group (n = 11 in phase 1): progressive addition soft contact lenses (+0.50 D) with 8.6 mm base curve, 14.5 mm diameter, 3.25 mm central zone, and horizontal thick zones to prevent rotation (Mipafilcon A; Menicon, Nagoya, Japan) SVSCL group (n = 13 in phase 1): single vision soft contact lenses |
|
| Outcomes |
Primary outcomes:
Secondary outcomes:
Measurements taken months 1, 3, 6, 9, and 12 in each phase Unit of analysis: individual (average of both eyes except for 1 child whose right eye was enrolled only) |
|
| Notes | Study dates: January 2011 to March 2013 Trial registration: JPRN‐UMIN000007989 Funding sources: Menicon Corp., Itami Central Ophthalmology Clinic (Japan) Conflict of interest: "AS and MN are employees of Menicon. The authors report no other conflicts of interest in this work" |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Randomization was done by a random‐number table" |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment was not reported |
| Masking of participants (performance bias) | High risk | Masking of participants was not applicable due to performance differences between the interventions studied |
| Masking of outcome assessors (detection bias) Progression of myopia | Low risk | "The outcome measurements were made by masked examiners"; "the measurements were taken by orthoptists masked to the type of CLs worn" |
| Masking of outcome assessors (detection bias) Secondary outcomes | Low risk | Same as for primary outcomes |
| Masking of data analyzers | Unclear risk | This was not reported |
| Incomplete outcome data (attrition bias) Incomplete outcome(s) data | Low risk | No children were lost to follow‐up in the first year; "the data for the children who attended at least the 12‐monthly visit were included in the analysis of the progression of myopia" |
| Selective reporting (reporting bias) | High risk | Axial length was listed as a secondary outcome in the clinical trial registry record, but it was stated as a primary outcome in the journal publication. Trials authors' main conclusion that "this pilot study suggests that low‐addition soft CLs with decentered optical design can reduce the degree of axial elongation in myopic children after an initial transient phase of CL wear" does not correspond to the study's objective |
| Other bias | High risk | Study was funded by the manufacturers of the lenses under investigation. Inappropriate analysis of cross‐over data was performed |
Fulk 1996.
| Study characteristics | ||
| Methods | Study design: parallel‐group RCT Study center: 1 (Indian Health Service Hospital, Optometry Department, Tahlequah, Oklahoma, USA) Number randomized: 32 children Study follow‐up: 18 months Exclusions and losses to follow‐up: no exclusions; 4 (12.5%) were lost to follow‐up |
|
| Participants | Age: range 6 to 13 years Gender: included boys and girls (numbers not reported) Culture: children with myopia and near point esophoria identified from medical records and referred by local optometrists Inclusion criteria: (1) at least 0.50 D of myopia in both principal meridians of both eyes; (2) ages 6 to 13.99 years for boys and 6 to 12.99 years for girls; (3) near point esophoria; (4) corrected acuity of at least 20/25 in each eye, distance and near, with SVLs; (5) ability to respond to subjective tests Exclusion criteria: (1) strabismus; (2) astigmatism greater than 2.00 D in either eye; (3) anisometropia greater than 2 D; (4) convergence insufficiency accompanied by symptoms; (5) diabetes or other systemic disease with potential effects on refractive error; (6) ocular disease other than mild inflammation of the adnexa |
|
| Interventions | Bifocals (n = 16): bifocal lenses with +1.25 D addition SVLs (n = 16): single vision lenses Note: prescription changes were made if the spherical equivalent in either eye had changed by 0.50 D |
|
| Outcomes |
Primary outcomes:
Measurements taken at baseline and every 6 months for 18 months Unit of analysis: average values of both eyes |
|
| Notes | Study dates: not reported Trial registration: not reported Funding source: Northeastern State University Faculty Research Committee (Tahlequah, Oklahoma, USA) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Block randomization process was used with reference to Zelen |
| Allocation concealment (selection bias) | Unclear risk | "The optician kept envelopes containing the assignment and fitted the appropriate glasses at the end of the base‐line examination" It was unclear if sequentially numbered, sealed, and opaque envelopes were used. |
| Masking of participants (performance bias) | High risk | Masking of participants was not applicable due to visual and functional differences between the interventions studied |
| Masking of outcome assessors (detection bias) Progression of myopia | Low risk | "A research assistant who did not know what type of glasses the child wore, measured..." However, the success of masking the examiner was not addressed in the paper |
| Masking of outcome assessors (detection bias) Secondary outcomes | Low risk | Primary and secondary outcomes were assessed by the same examiners |
| Masking of data analyzers | Unclear risk | This was not reported |
| Incomplete outcome data (attrition bias) Incomplete outcome(s) data | Low risk | 32 participants were enrolled; 4 dropped out—2 from each treatment group; does not address reason for dropouts. Analysis is for the 28 remaining participants, so not “intention to treat” in terms of including all enrolled participants for analysis at the end; however, equal dropouts in each arm and patients were analyzed by the group to which they were randomized. It was not stated whether all 28 participants completed all 3 follow‐up visits, although it was stated that they completed the study |
| Selective reporting (reporting bias) | Unclear risk | Refractive error outcome was reported. Axial length outcome (in mm) was plotted against myopia progression in D, but mean values by treatment groups were not given |
| Other bias | Low risk | None was identified |
Fulk 2002.
| Study characteristics | ||
| Methods | Study design: parallel‐group RCT and study of variables that may influence myopia progression in children Study centers: 2 (Tahlequah and Tulsa, Oklahoma, USA) Number randomized: 82 children Study follow‐up: 30 months Exclusions and losses to follow‐up: no exclusions; 7 (8.5%) were lost to follow‐up |
|
| Participants | Age: mean = 10.7 years (range 6 to 12 years) Gender: 43 boys, 39 girls Culture: children with myopia and near point esophoria recruited locally and through clinics operated by the Cherokee Nation: 58% Caucasian, 29% American Indian, 5% Hispanic, 4% African American, 3% other, 1% Asian/Pacific Islander Inclusion criteria: (1) at least 0.50 D of myopia in both principal meridians of both eyes; (2) ages 6 to 12.99 years for boys and 6 to 11.99 years for girls; (3) near point esophoria; (4) corrected VA of at least 20/25 in each eye at distance and binocularly with SVLs; (5) corrected stereoacuity of at least 40 second arc with SVLs at 40 cm; (6) assent of child and consent to participate Exclusion criteria: (1) strabismus; (2) astigmatism or anisometropia greater than 2.00 D; (3) diabetes or other systemic disease with potential effects on refractive error; (4) ocular disease other than mild inflammation of the adnexa; (5) known history of allergic reaction to proparacaine or tropicamide; (6) history of use of RGPs; (7) current use of bifocals or use within the last year; (8) high myopia of ‐6.00 D or more for children younger than 9 years or ‐8.00 D or more for children 9 years or older; (9) inability to respond to subjective testing or hold fixation sufficiently to allow for study measurements |
|
| Interventions | Bifocals (n = 42): bifocal lenses with +1.50 D add SVLs (n = 40): single vision lenses Note: prescription changes were made if (1) the spherical equivalent in either eye had changed by 0.50 D, or (2) any combination of sphere or cylinder change could improve the distance acuity by 3 or more letters in either eye |
|
| Outcomes |
Primary outcome:
Secondary outcomes:
Measurements taken at baseline and every 6 months for 30 months Unit of analysis: average values of both eyes |
|
| Notes | Study dates: enrollment between August 20 and October 15, 1996; original follow‐up was for 30 months; some children remained for 54 months Trial registration: NCT00000128 Funding source: National Eye Institute, National Institutes of Health Notes: study was also known as the Myopia Progression Study |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomized permuted block design was used with separate number sequences for each of the 2 sites stratified by gender to assign participants in approximately equal allocation to the 2 treatments. |
| Allocation concealment (selection bias) | Unclear risk | "Sealed envelopes containing the treatment assignments were maintained at each site and opened by the optician after a subject was enrolled" It was unclear whether opaque envelopes were used. |
| Masking of participants (performance bias) | High risk | Masking of participants was not applicable due to visual and functional differences between the interventions studied |
| Masking of outcome assessors (detection bias) Progression of myopia | Low risk | "A research assistant who did not know what type of glasses the child wore, measured..." However, the success of masking the examiner was not addressed in the paper |
| Masking of outcome assessors (detection bias) Secondary outcomes | Low risk | Primary and secondary outcomes were assessed by the same examiners |
| Masking of data analyzers | Unclear risk | This was not reported |
| Incomplete outcome data (attrition bias) Incomplete outcome(s) data | Low risk | "This is an intention‐to‐treat analysis, with all subjects being classified according to their original treatment assignment ‐ disregarding the fact that many discontinued that mode of correction during the last year" Seven participants did not complete the study: 6 of 42 were randomized to bifocals (2 died, 1 drowned, and 1 died in an auto accident; 4 were "unwilling"), and 1 of 40 were randomized to SVL (participant moved). "In a secondary analysis, estimates of myopia progression were imputed for children who did not complete the study; each subject who left the study prematurely was assumed to have myopia progression equal to that of mean progression observed in the SVL group for the time period for which their data were missing." This weakened the treatment effect |
| Selective reporting (reporting bias) | Low risk | Primary and secondary outcomes were reported a priori |
| Other bias | Low risk | None was identified |
Han 2018.
| Study characteristics | ||
| Methods | Study design: parallel‐group RCT Study center: 1 (Affiliated Yixing People Hospital of Jiangsu University) Number randomized: 240 children Study follow‐up: 1 year Exclusions and losses to follow‐up: none |
|
| Participants | Age: mean = 9.8 years (range 9 to 14 years) Gender: 117 boys, 123 girls Culture: China Inclusion criteria: children with myopia treated in the study authors’ hospital Exclusion criteria: not reported |
|
| Interventions | OFG (n = 90): ordinary frame glasses M‐OK lenses (n = 90): Mouldway orthokeratology lenses; described as “four‐district seven‐arc reverse geometric design. The main component is Boston XO (Bausch + Lomb, USA [Hexafocon A, main component fluorosiliconepropenylphenol ester]) and the standard piece was the Mouldway IV‐DF type” ML (n = 60): Medcall lenses (ML) “fitted with a new paracentral defocus‐reducing lens” Note: none |
|
| Outcomes |
Primary outcome:
Secondary outcome:
Measurements taken at 1 year Unit of analysis: individual (1 eye per person enrolled) |
|
| Notes | Study dates: between May 2013 and May 2015 Trial registration: not reported. Funding source: “the authors have no funding or conflicts of interest to disclose” |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of randomization was not reported |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment before randomization was not reported |
| Masking of participants (performance bias) | Unclear risk | Masking of participants was not possible because of the nature of the interventions. Masking of study personnel was not reported |
| Masking of outcome assessors (detection bias) Progression of myopia | Unclear risk | Masking of outcome assessors was not reported |
| Masking of outcome assessors (detection bias) Secondary outcomes | Unclear risk | Masking of outcome assessors was not reported |
| Masking of data analyzers | Unclear risk | Masking of data analyzers was not reported |
| Incomplete outcome data (attrition bias) Incomplete outcome(s) data | Low risk | No details on attrition were provided; all participants seem to have been analyzed |
| Selective reporting (reporting bias) | Low risk | Results were reported for outcomes described in the methods section of the paper |
| Other bias | Low risk | None was identified |
Hasebe 2008.
| Study characteristics | ||
| Methods | Study design: cross‐over RCT Study center: 1 (Okayama University Medical School) Number randomized: 92 children Study follow‐up: 3 years Exclusions and losses to follow‐up: no exclusions; 6 (6.5%) were lost to follow‐up |
|
| Participants | Age: mean = 9.85 years (range 6 to 12 years) Gender: 47 boys, 45 girls Culture: Okayama, Japan Inclusion criteria: (1) age 6 to 12 years; (2) spherical equivalent refractive error between ‐1.25 D and ‐6.00 D in both eyes, as measured by noncycloplegic autorefraction; (3) best‐corrected VA of 20/20 or better in each eye; (4) no other eye disease; (5) experience wearing spectacles; (6) willingness to wear glasses constantly and attend follow‐up visits; (7) acceptance of randomization Exclusion criteria: (1) astigmatism > 1.50 D in both eyes; (2) anisometropia > 1.50 D; (3) manifest strabismus; (4) birth weight < 1250 g; (5) heterotropia or severe ophthalmic disease that may affect refractive development; (6) previous use of PALs or contact lenses |
|
| Interventions | PALs (n = 46): 18 months wearing PALs (add +1.50 D), followed by 18 months wearing SVLs SVLs (n = 46): 18 months wearing SVLs, followed by 18 months wearing PALs (addition +1.50 D) Note: prescription changes were made if corrected distance visual acuity was less than 20/30 in at least 1 eye |
|
| Outcomes |
Primary outcome:
Secondary outcomes:
Measurements taken at baseline and every 6 months for 3 years Unit of analysis: child‐based (mean of both eyes or right eye only) |
|
| Notes | Study dates: enrolled July 2002 to June 2003 Trial registration: ISRCTN28611140 Funding source: Japanese Ministry of Education, Culture, Sports, Science and Technology, and Megane Tanaka Chain, Ltd |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomly allocated to Group 1 or Group 2 by drawing lots. “Participants drew lots (number of 1‐80 was described in each card) at initial inspection and participant number was randomly decided” (Hasebe 2002) |
| Allocation concealment (selection bias) | Low risk | Physicians conducting examinations did not know the allocation. Participants drew lots from numbered cards; then the principal investigator and 3 opticians determined allocation based on the number drawn (Hasebe 2002) |
| Masking of participants (performance bias) | High risk | Masking of participants was not applicable due to visual and functional differences between the interventions studied |
| Masking of outcome assessors (detection bias) Progression of myopia | Low risk | The examiners collecting data or prescribing spectacles were masked to lens assignment |
| Masking of outcome assessors (detection bias) Secondary outcomes | Low risk | Primary and secondary outcomes were assessed by the same examiners |
| Masking of data analyzers | Low risk | Methods paper stated that the statistician was masked to lens assignments (Hasebe 2002) |
| Incomplete outcome data (attrition bias) Incomplete outcome(s) data | Low risk | "Only six children, two in group 1 and four in group 2, failed to return for the final visit. The reasons for being lost to follow‐up or excluded from the analysis included a problem in using cycloplegic eye drops (two children), moving to another prefecture (two children), desire to wear contact lenses (one child), or the occurrence of exotropia (one child)" |
| Selective reporting (reporting bias) | Low risk | All outcomes published a priori in the design paper were reported in the results papers |
| Other bias | High risk | Design‐specific risk of bias: cross‐over trial. Four children dropped out during the second study period The study was partially funded by a company that produces the types of lenses being investigated |
Hasebe 2014.
| Study characteristics | ||
| Methods | Study design: parallel‐group RCT Study centers: 3 (Okayama University Medical School, Japan; Eye Hospital of Wenzhou Medical College, China; Eulji University, South Korea) Number randomized: 197 children (120 from China and 77 from Japan) Study follow‐up: 2 years Exclusions and losses to follow‐up: the trial in South Korea was terminated after 12 months due to protocol violation and the data were not included; 28/197 (14%) did not complete 2 years of follow‐up |
|
| Participants | Age: mean = 10 years (range 6 to 12 years) Gender: 95 boys, 74 girls Culture: Chinese and Japanese children Inclusion criteria: (1) age 6 to 12 years; (2) spherical equivalent refractive error between ‐0.50 D and ‐4.50 D; (3) astigmatism ≤ 1.50 D; (4) anisometropia ≤ 1.50 in spherical or cylindrical error; (5) best‐corrected visual acuity of 6/9 (20/30) or better in each eye; (6) normal ocular and general health; (7) willingness to wear spectacle lenses continuously; (8) willingness and ability to tolerate cycloplegia; (9) informed parental consent Exclusion criteria: (1) amblyopia or manifested squint; (2) history of rigid contact lens or bifocal contact lens wear; (3) use of bifocal or progressive lenses or other myopia treatment in previous 12 months; (4) abnormal binocular function; (5) vestibular disorders or motor imbalance; (6) any systemic condition affecting refractive development or vision, or any condition precluding adherence to the study protocol (e.g. not available for follow‐up for 2 years) |
|
| Interventions | PA‐PALs +1.0 D (n = 67): positively aspherized progressive addition lenses with +1.00 D add PA‐PALs +1.5 D (n = 63): positively aspherized progressive addition lenses with +1.50 D add SVLs (n = 67) Note: all lenses are worn during normal waking hours |
|
| Outcomes |
Primary outcomes:
Secondary outcome: peripheral refractive error, measured using an open field autorefractor Measurements taken at baseline and at 6, 12, 18, and 24 months Unit of analysis: eye (both eyes of each child analyzed) |
|
| Notes | Study dates: between July 2008 and June 2009 Trial registration: ACTRN12608000566336 Funding source: "supported by Carl Zeiss Vision" Conflict of interest: "S. Hasebe, Carl Zeiss Vision Australia Holdings Ltd. (F); J. Jun, Carl Zeiss Vision Australia Holdings Ltd. (F); S.R. Varnas, Carl Zeiss Vision Australia Holdings Ltd. (E), P" |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "One of the authors (unmasked investigator) made a lens allocation table by using the Microsoft Excel (Microsoft, Redmond, WA, USA) function 'INT(RAND()*2.99),' which was used to generate random integers between 0 and 2. These numbers denote each lens design. This page was refreshed to change the seed of the RAND function until the allocation ratio was approximately 1:1:1" |
| Allocation concealment (selection bias) | Low risk | "The results were copied and pasted as values into a new spreadsheet (master allocation table). The masked investigator at each study center sent prescriptions with the subject ID to the unmasked investigator who, in turn, assigned the lens design from the master allocation table according to the subject ID and placed the order with the Zeiss surfacing laboratory" |
| Masking of participants (performance bias) | Unclear risk | "Enrolled children and their parents were not told of their group allocation; we emphasized the importance of full‐time proper wear of the assigned spectacles, as if they were wearing PA‐PALs" "However, the rate of unmasking and its potential impact on the study results are not known" |
| Masking of outcome assessors (detection bias) Progression of myopia | Low risk | "All lenses had semivisible engravings indicating the lens design, but no masked investigator or optician having direct contact with the participants was allowed to check the engravings or was told their meaning. However, the rate of unmasking and its potential impact on the study results are not known" |
| Masking of outcome assessors (detection bias) Secondary outcomes | Low risk | Same outcome assessors as primary outcome |
| Masking of data analyzers | Unclear risk | This was not reported |
| Incomplete outcome data (attrition bias) Incomplete outcome(s) data | High risk | All children at 1 site were excluded. Only those who completed 2‐year follow‐up at the remaining 2 sites were included in the analysis; 9 (13.4%) children in PA‐PALs +1.0 D group, 12 (19.0%) in the PA‐PALs +1.5 D group, and 7 (10.4%) in the control group did not complete the study; 23 (19%) children from China and 5 (6%) from Japan discontinued due to complaint (10 children), moving to another city (4), refusal to undergo cycloplegia (2), loss to follow‐up (2), inability to adapt the test lenses (1), or unknown (5) |
| Selective reporting (reporting bias) | Low risk | All outcomes specified prospectively on a clinical trials registry were reported |
| Other bias | High risk | Children were randomized and both eyes were analyzed without appropriate methods to account for within‐person correlation. Study was funded by the manufacturers of the lenses under investigation |
Houston Study 1987.
| Study characteristics | ||
| Methods | Study design: parallel‐group RCT Study center: 1 (University of Houston, Texas, USA) Number randomized: 207 children Study follow‐up: 3 years Exclusions and losses to follow‐up: 83 (40%) children were excluded from or dropped out of the study |
|
| Participants | Age: range 6 to 15 years Gender: 58 boys and 66 girls completed the study Culture: children were recruited from patients, from family members of faculty and staff, and from the racially diverse Houston community Inclusion criteria: (1) myopia of ‐0.25 D in one or both eyes; (2) ages 6 to 15 years; (3) best corrected VA of 20/20 or 20/15; (4) normal ocular health; (5) ability to provide informed consent Exclusion criteria: (1) strabismus or amblyopia; (2) contact lens wearers; (3) astigmatism of 2.00 D or more; (4) particularly high or low gradient AC/A ratios |
|
| Interventions | Bifocals 1: bifocal lenses with +1.00 D addition Bifocals 2: bifocal lenses with +2.00 D addition SVLs Note: prescription changes were made if (1) there was a change in spherical power of 0.50 D or more in one or both eyes, or (2) there was an improvement of one line of visual acuity. One participant was allowed to wear contact lenses when playing basketball |
|
| Outcomes |
Patient care team outcomes (unmasked):
Evaluation team outcomes (masked):
Measurements taken at baseline and every 6 months for 3 years Unit of analysis: data from right eyes |
|
| Notes | Study dates: "subjects were admitted to the study over a period of 20 months, in five 'accrual groups.' The first group of subjects entered the study in February, 1981 and completed the study in February, 1984, whereas the last group of subjects entered the study in October, 1982," and completed the study in October, 1985 Trial registration: not reported Materials: bifocals were executive 1‐piece lenses in CR‐39 plastic (American Optical Corporation); SVLs were polycarbonate lenses (Gentex Corporation) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Placed in treatment group "on basis of a table of random numbers" via block randomization technique |
| Allocation concealment (selection bias) | Unclear risk | Allocation was done via a random numbers table, but it was unclear whether and how the allocation was concealed before randomization |
| Masking of participants (performance bias) | High risk | Masking of participants was not applicable due to visual and functional differences between the interventions studied |
| Masking of outcome assessors (detection bias) Progression of myopia | High risk | This study involved a team of masked observers (evaluation team) and a team of unmasked observers (patient care team). The results presented in the final analysis are from the unmasked group |
| Masking of outcome assessors (detection bias) Secondary outcomes | Low risk | Evaluation team members collecting the data were masked |
| Masking of data analyzers | Unclear risk | This was not reported |
| Incomplete outcome data (attrition bias) Incomplete outcome(s) data | High risk | Patients were dismissed for "noncompliance" and some were fitted with contact lenses without letting study personnel know, so they were also dropped. Participants who did not return and those who moved were dropped. Out of 207 enrolled, 83 (40%) dropped out. It is not clear from which treatment groups the dropouts came. Incomplete data were reported as only 60% remained in the study |
| Selective reporting (reporting bias) | High risk | Results were not reported for evaluation team measurements or for other secondary outcomes outlined in the design paper. The methods paper stated that an evaluation team report would be based on (1) cycloplegic retinoscopy, (2) noncycloplegic autorefraction, and (3) cycloplegic autorefraction performed by masked examiners. However, these were never reported in the outcome paper. Secondary outcomes, including axial length, were never reported in the outcome paper |
| Other bias | Low risk | None was identified |
Jensen 1991.
| Study characteristics | ||
| Methods | Study design: parallel‐group RCT Study center: 1 (Odense University Hospital, Denmark) Number randomized: 159 children Study follow‐up: 2 years Exclusions and losses to follow‐up: 4 (2.5%) children who were randomized were excluded from the analyses; 16 (10%) were lost to follow‐up |
|
| Participants | Age: mean = 10.9 years Gender: 87 boys, 72 girls Culture: medical records of children from schools in Odense, Denmark, were screened for myopia (n = 8769). Possible cases of myopia underwent a primary examination (n = 1216). Myopic children with at least ‐1.0 D in either eye, and in 2nd to 5th grades, were examined at the eye clinic (n = 361). Children meeting inclusion/exclusion criteria at the eye exam were mailed invitations to participate in the trial (n = 227) Inclusion criteria: (1) in 2nd to 5th grades at screening; (2) myopia with spherical equivalent refractive error between ‐1.25 D and ‐6.00 D in both eyes; (3) normal corrected vision; (4) Danish parents; (5) affirmative response to mailed invitation for study Exclusion criteria: (1) unilateral myopia; (2) eye disease or general illness, especially heart/lung disease; (3) experience in pilot study |
|
| Interventions | Bifocals (n = 57): constant wear of bifocals with +2.0 D addition to upper edge of reading segment Timolol (n = 51): 1 drop of 0.25% timolol maleate in each eye twice daily and constant wear of SVLs for corrected visual acuity ≥ 0.8 Control (n = 51): constant wear of SVLs for corrected visual acuity ≥ 0.8 Note: participants were permitted to wear their own SVLs if corrected visual acuity was ≥ 0.8 |
|
| Outcomes |
Primary outcomes:
Secondary outcomes:
Measurements taken at baseline and every 6 months for 2 years Unit of analysis: right eyes and left eyes analyzed separately |
|
| Notes | Study dates: screening January to April 1983; eye clinic exams October 1984 to April 1985 Trial registration: not reported Notes: children who chose not to participate in the study (n = 44) did not statistically differ from those examined with regard to age and degree of myopia |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Block randomization based on age, sex, and refractive error. Intervention and control groups were made by completing cells based on 3 age categories, 3 refractive error categories, and 2 gender groups (3 x 3 x 2 = 18 cells). Participants were assigned to each cell after baseline examinations |
| Allocation concealment (selection bias) | Unclear risk | For each cell, the children in groups of 3 were allocated to study groups by drawing numbers 1 to 6 for the first assignment. The second and third assignments were dependent on the first assignment. It was unclear how allocation was concealed before randomization. |
| Masking of participants (performance bias) | High risk | Masking of participants was not applicable due to visual and functional differences between the interventions studied |
| Masking of outcome assessors (detection bias) Progression of myopia | High risk | Masking was not reported, but there was only 1 study investigator |
| Masking of outcome assessors (detection bias) Secondary outcomes | High risk | Primary and secondary outcomes were assessed by the same examiner |
| Masking of data analyzers | Unclear risk | Data were analyzed by the study investigator |
| Incomplete outcome data (attrition bias) Incomplete outcome(s) data | High risk | 159 children were enrolled: 51 SVLs, 57 bifocals, 51 timolol. At the first year visit, 13 (8%) children were excluded or lost to follow‐up: 1 patient given contact lenses was excluded and 1 patient dropped out from the control group; 6 patients dropped out from the bifocal group (3 because they could not adapt to the bifocals); and 2 patients were excluded and 3 dropped out from the timolol group. At the second year visit, an additional 7 (4.5%) children were excluded or lost to follow‐up: 1 patient given contact lenses was excluded from the control group; 3 patients dropped out from the bifocal group because they could not adapt to the bifocals; and 3 dropped out from the timolol group |
| Selective reporting (reporting bias) | High risk | The methods section of the article described that results would be discussed only if exceptional |
| Other bias | Low risk | None was identified |
Katz 2003.
| Study characteristics | ||
| Methods | Study design: parallel‐group RCT, with 3‐month adaptation period Study center: 1 (Myopia Clinic of the Singapore Eye Research Institute) Number randomized: 564 children (428 children attended initial visit; 383 children completed the adaptation period) Study follow‐up: 2 years Exclusions and losses to follow‐up: 136 (24%) children who were randomized did not attend the initial visit, and 45 (8%) more did not complete the adaptation period; 86 (22%) of the 383 children who completed the adaptation period were lost to follow‐up |
|
| Participants | Age: mean = 8.3 years (range 6 to 12 years) Gender: 204 boys, 179 girls Culture: Singaporean children with Chinese ethnicity Inclusion criteria: (1) age 6 to 12 years; (2) myopia with spherical equivalent refractive error between ‐1.0 D and ‐4.0 D; (3) Chinese ethnicity; (4) provided informed consent Exclusion criteria: (1) astigmatism > 2.0 D; (2) previous contact lens wear; (3) other ocular pathologies Note: all participants were provided a 3‐month period to adapt to assigned intervention |
|
| Interventions | Contact lenses (n = 158): RGPCLs worn daily for at least 8 hours per day Spectacles (n = 225): SVLs worn daily for at least 8 hours per day Note: prescription changes were made if corrected visual acuity fell below 20/40 |
|
| Outcomes |
Primary outcome:
Measured by subjective cycloplegic refraction from post adaption through 2 years of follow‐up Secondary outcomes:
Measurements taken at baseline and every 3 months over a 24‐month period Unit of analysis: only data from right eyes reported |
|
| Notes | Materials: Asian Design Lens, Baush and Lomb, Rochester, New York, USA Trial registration: not reported Adherence to treatment was measured for children and parents (agreement was almost 100%) and was defined as use of contact lenses or spectacle use for at least 8 hours per day, 7 days per week Notes: study is also known as the Contact Lens‐Myopia Treatment Study (CL‐MTS) Additional data: study author provided unpublished data via email correspondence |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomization schedule of block size 6, generated from random number tables in Baltimore, USA |
| Allocation concealment (selection bias) | Unclear risk | Assignments were placed in sealed envelopes with sequential patient numbers. It was unclear whether the opaque envelopes were used. |
| Masking of participants (performance bias) | High risk | Masking of participants was not applicable due to visual and functional differences between the interventions studied |
| Masking of outcome assessors (detection bias) Progression of myopia | High risk | Clinical observers were not masked to treatment group |
| Masking of outcome assessors (detection bias) Secondary outcomes | High risk | Primary and secondary outcomes were assessed by the same examiners |
| Masking of data analyzers | Unclear risk | The data analyst was not masked (via email communication with study author) |
| Incomplete outcome data (attrition bias) Incomplete outcome(s) data | Unclear risk | Of the 564 children randomized, 297 (53%) completed the study. There were 86 (22%) children who completed the adaptation period who were lost to follow‐up or censored from the study for not attending the final study visit. Statistical comparisons between those lost to follow‐up and those who completed the study showed "a higher proportion of girls in the contact lens (59%) than spectacle group (42%) completed the study (P = 0.004)." It was also reported that "axial length and astigmatism were similar between the treatment groups that completed the study, but the contact lens group that completed the study had 0.3 diopters more myopia at baseline than did those in the spectacle group that completed the study (P = 0.003)." |
| Selective reporting (reporting bias) | High risk | Although all outcomes identified in the study methods were reported, it is unclear why some participants were not included in the analyses. 105 RGPCL and 192 SVL wearers should be examined over 2 years, but only 97 RGPCL and 188 SVL wearers were included in the analyses |
| Other bias | High risk | Unequal loss to follow‐up; imbalance in gender, corneal curvature, and refractive error at baseline visit (controlled for in analyses); many participants lost from study before they were examined for outcomes |
Koomson 2016.
| Study characteristics | ||
| Methods | Study design: parallel‐group RCT Study center: 1 (Kumasi, Ghana) Number randomized: 150 children Study follow‐up: 24 months Exclusions and losses to follow‐up: 1 child in the fully corrected group dropped out before the 24‐month visit |
|
| Participants | Age: mean = 12.39 years (range 10 to 15 years) Gender: 60 boys, 90 girls Culture: recruited from "eight purposively chosen high socioeconomic schools in the Kumasi metropolis" in Ghana Inclusion criteria: (1) healthy children, ages 10 to 15 years; (2) spherical equivalent ‐1.25 to ‐4.50 D as measured by cycloplegic refraction; (3) visual acuity of 0.20 logMAR or worse with habitual spectacles and logMAR 0.00 or better with full correction; (4) willingness to wear study spectacles only and to wear them during waking hours Exclusion criteria: (1) strabismus; (2) amblyopia; (3) astigmatism over 1.25 D; (4) anisometropia over 1.00 D; (5) parental myopia; (6) allergy to cycloplegic agents; (7) use of multifocal optical lenses or pharmacological agents; (8) history of contact lens wear |
|
| Interventions | Undercorrected group (n = 75): SVLs blurred by +0.50 D Fully corrected group (n = 75): SVLs Note: changes in prescription were made if refraction had changed by at least 0.50 D for 1 or both eyes |
|
| Outcomes |
Primary outcome:
Secondary outcomes:
Measurements taken at 6‐month intervals for 2 years Unit of analysis: child‐based (right eye) |
|
| Notes | Study dates: enrollment September 2010 to March 2011
Trial registration: not reported Funding source: not reported Disclosures of interest: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Subjects for the study were matched in cells derived by using randomized block design based on three criteria (age, sex, and school) as suggested by Chung et al. The study design comprised three age groups (10‐11, 12‐13, and 14‐15 years), created from the ages of the subjects recorded at baseline; two gender categories; and four schools. In total, 24 different cells of at most 10 members (due to the matched design, cells had even number of members starting from 4 to 10) were formed. In each block, numbers generated from random tables and placed in sealed envelopes with sequential patient identification numbers were used to pair the children. A coin was then tossed to separate each pair into either a control group (FC) or the treatment group (UC). After tossing the coin, if a head came up, the first subject in the pair was assigned to UC and the second to FC. On the contrary, if a tail came up, the first subject was allocated to FC and the second to UC" |
| Allocation concealment (selection bias) | Unclear risk | Used sealed envelopes and randomized subsequent to enrollment. It was unclear whether the opaque envelopes were used. |
| Masking of participants (performance bias) | High risk | Masking of participants was not applicable due to performance differences between the interventions studied |
| Masking of outcome assessors (detection bias) Progression of myopia | Low risk | "During all examinations, the optometrist who took all measurements was not aware of the treatment group the child belonged and no child was supposed to discuss any problem on the spectacles with the optometrist" |
| Masking of outcome assessors (detection bias) Secondary outcomes | Low risk | "During all examinations, the optometrist who took all measurements was not aware of the treatment group the child belonged and no child was supposed to discuss any problem on the spectacles with the optometrist" |
| Masking of data analyzers | Unclear risk | Masking of data analyzers was not reported |
| Incomplete outcome data (attrition bias) Incomplete outcome(s) data | Low risk | "Follow‐up data were analyzed using an intent‐to‐treat principle according to the child’s original lens assignment and the last measured value of the outcome measures" |
| Selective reporting (reporting bias) | Low risk | Results were reported for outcomes described in the methods section of the paper |
| Other bias | Low risk | None was identified |
Lu 2015.
| Study characteristics | ||
| Methods | Study design: parallel‐group RCT Study center: 1 (Guangzhou Red Cross Hospital, School of Medicine, Jinan University, China) Number randomized: 80 children Study follow‐up: 1 year Exclusions and losses to follow‐up: not reported |
|
| Participants | Age: mean = 11.21 years (range 9 to 14 years) Gender: 43 boys, 37 girls Culture: Chinese Inclusion criteria: (1) age 9 to 14 years; (2) progressive (0.50 D or more change) myopia from ‐1.00 to ‐5.00 D; (3) astigmatism with 1.50 D or less with‐rule, 0.75 D or less against‐rule; (4) best‐corrected visual acuity 1.0 or better in both eyes by Snellen chart; (5) ocular pressure less than 21 mmHg; (6) compliance with examination and treatment Exclusion criteria: (1) other ocular condition (glaucoma, cataract, iritis, congenital small cornea, keratoconus, fundus lesions, congenital amblyopia, dominant strabismus); (2) family history of hereditary eye disease (e.g. high myopia, Leber disease); (3) recent or current use of drugs that may affect myopia development; (4) previous RGP wear; (5) other systemic disease (diabetes, Marfan syndrome, albinism, severe sinusitis, etc.) |
|
| Interventions | Mid‐periphery additional lenses (n = 40): addition up to +2.50 D and adjustment training SVLs (n = 40): frame glasses |
|
| Outcomes |
Primary outcomes:
Secondary outcomes:
Measurements taken every 3 months for 1 year Unit of analysis: eye (both eyes of each child analyzed) |
|
| Notes | Study dates: January 2014 to July 2015 Trial registration: not reported Funding source: Guangdong Medical Science and Technology Research Foundation (No. A2014557); Department of Ophthalmology, Guangzhou Red Cross Hospital Affiliated to School of Medicine, Jinan University, China |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "The patients were randomly divided into two groups: treatment group and control group, 40 cases in each group" |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment before randomization was not reported |
| Masking of participants (performance bias) | High risk | Masking of participants was not applicable due to functional differences between the interventions studied |
| Masking of outcome assessors (detection bias) Progression of myopia | Unclear risk | Masking was not reported |
| Masking of outcome assessors (detection bias) Secondary outcomes | Unclear risk | Masking was not reported |
| Masking of data analyzers | Unclear risk | Masking was not reported |
| Incomplete outcome data (attrition bias) Incomplete outcome(s) data | Low risk | No attrition was reported |
| Selective reporting (reporting bias) | Low risk | Results were reported for outcomes described in the methods section of the paper |
| Other bias | High risk | Children were randomized and both eyes were analyzed without appropriate methods to account for within‐person correlation |
MIT Study 2001.
| Study characteristics | ||
| Methods | Study design: parallel‐group RCT Study center: 1 (National Taiwan University Hospital vision care center) Number randomized: 227 children Study follow‐up: 18 months Exclusions and losses to follow‐up: 39 (17%) children were excluded or lost to follow‐up |
|
| Participants | Age: range 6 to 13 years Gender: 105 boys, 122 girls Culture: school children in Taiwan with an average myopia of ‐3.27 D Inclusion criteria: (1) age 6 to 13 years; (2) provided informed consent; (3) willing to wear glasses; (4) available for follow‐up period Exclusion criteria: (1) tropia or amblyopia; (2) increase of more than 2 D in any eye during the treatment period |
|
| Interventions | SVLs (n = 76): regular SVLs worn all the time and placebo drops PALs (n = 75): multifocal lenses with the near addition part for reading and placebo drops PALs plus atropine (n = 76): 0.5% atropine instilled once a day at bedtime, in addition to PALs Note: an intervention group given atropine and SVLs was omitted from the study design because difficulty while reading due to the intervention would have induced poor compliance. Prescription changes were made for any child whose refractive error increased by more than 0.75 D |
|
| Outcomes |
Primary outcome:
Secondary outcomes:
Measurements taken at baseline and every 3 months over an 18‐month period Unit of analysis: data from right eyes analyzed |
|
| Notes | Study dates: 1997 to 2000 Trial registration: not reported Materials: HOYALUX3 plastic lenses were used for PALs; polycarbonate plastic lenses were used for SVLs Additional data: study author provided unpublished data via email correspondence |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Stratification was based on age (younger than 9.5 years or not), sex (boy or girl), and myopic severity (more than ‐4.0 D for both eyes or not) at the baseline visit. This resulted in the total of 8 strata. Each participant was categorized into 1 of the strata and then was randomized to receive 1 of the 3 treatments. A block size of 9 was used to balance the number of patients in the 3 treatment categories for each stratum (via email communication with study author) |
| Allocation concealment (selection bias) | Low risk | Group assignments were unknown to study personnel when participants were being enrolled by using coded bottles with sealed envelopes (via email communication with study author) |
| Masking of participants (performance bias) | High risk | Masking of participants was not applicable due to functional differences between the interventions studied |
| Masking of outcome assessors (detection bias) Progression of myopia | Low risk | Ophthalmologists were masked, and it would be improbable to guess patients' treatment by examining the pupils because the ocular examination was performed only after the cycloplegic agent was given to each patient at each visit (via email communication with study author) |
| Masking of outcome assessors (detection bias) Secondary outcomes | Low risk | Ophthalmologists (outcome assessors) were masked (via email communication with study author) |
| Masking of data analyzers | Low risk | Data analysts were masked (via email communication with study author) |
| Incomplete outcome data (attrition bias) Incomplete outcome(s) data | High risk | Attritions were reported by group: atropine, n = 10 (13%); PALs, n = 14 (19%); SVLs, n = 15 (20%). Reasons for attrition were poor follow‐up, switching to contact lenses, poor compliance, myopic progression greater than 2.00 D per year (1 patient from the PAL group and 1 from the SVL group), and loss to follow‐up with no specified reason |
| Selective reporting (reporting bias) | Low risk | Results were reported for outcomes described in the methods section of the paper |
| Other bias | Low risk | None was identified |
Pärssinen 1989.
| Study characteristics | ||
| Methods | Study design: parallel‐group RCT Study center: 1 (outpatient clinic of the Central Hospital of Central Finland) Number randomized: 240 children Study follow‐up: 3 years Exclusions and losses to follow‐up: 1 (0.4%) child who was randomized was excluded from the analyses; 2 (0.8%) were lost to follow‐up |
|
| Participants | Age: mean = 10.9 years (range 8.8 to 12.8 years) Gender: 119 boys, 121 girls Culture: schoolchildren with suspected myopia were referred by school nurses and doctors after routine vision check‐ups Inclusion criteria: (1) in 3rd to 5th grade; (2) myopia with spherical equivalent refractive error between ‐0.25 D and ‐3.0 D in both eyes and ≥ ‐0.50 D in the worst eye; (3) corrected VA of 6/6 or better in both eyes Exclusion criteria: (1) astigmatism > 2.0 D; (2) anisometropia > 2.0 D; (3) manifest strabismus; (4) horizontal phorias more than ‐10 or +9 Δ or vertical more than 1 Δ; (5) previous use of spectacles for myopia; (6) eye disease or serious general disease; (7) plans to move out of the area in the near future or the child not wanting to have spectacles |
|
| Interventions | Distant use (n = 80): minus lenses with full correction to be used for distant vision only; advised to read at greatest distance possible Bifocals (n = 80): clear plastic bifocal lenses with +1.75 D addition for continuous use Continuous use (n = 79): minus lenses with full correction for continuous use; advised to remove spectacles only if there was danger of breaking them Note: prescription changes were made if corrected visual acuity fell below 20/40 |
|
| Outcomes |
Primary outcome:
Secondary outcomes:
Measurements taken at baseline and annually for 3 years Unit of analysis: right eyes and left eyes analyzed separately |
|
| Notes | Study dates: enrollment March 1983 to April 1985 Trial registration: not reported Funding source: Academy of Finland Compliance was measured by questionnaires and patients were classified as compliant, partly compliant, or noncompliant |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random, sex‐stratified codes were used |
| Allocation concealment (selection bias) | Unclear risk | Treatment assignments were sealed in envelopes, but it was unclear whether opaque envelopes were used. |
| Masking of participants (performance bias) | High risk | Masking of participants was not applicable due to visual and functional differences between the interventions studied |
| Masking of outcome assessors (detection bias) Progression of myopia | High risk | The ophthalmologist did not look at the group assignment before the examination, but often, for different reasons, the group was revealed. The 3‐year examinations were conducted by 2 different ophthalmologists, 1 of whom did not know the group assignments |
| Masking of outcome assessors (detection bias) Secondary outcomes | High risk | Primary and secondary outcomes were assessed by the same examiners |
| Masking of data analyzers | Unclear risk | This was not reported |
| Incomplete outcome data (attrition bias) Incomplete outcome(s) data | Low risk | "After allocation one of the boys in the continuous use group was excluded from comparison with the other treatment groups when we found that his sister has been included previously in a different group. Two children moved from the area, and their refraction values could not be obtained" The remaining participants were analyzed by their original treatment assignments |
| Selective reporting (reporting bias) | Low risk | Results were reported for outcomes described in a study design paper |
| Other bias | Low risk | None was identified |
PIR‐205 Study 2004.
| Study characteristics | ||
| Methods | Study design: parallel‐group RCT Study centers: 13 (US academic clinics and private practices) Number randomized: 174 children Study follow‐up: 1 year (planned), plus 1 year extension Exclusions and losses to follow‐up: 27 (15.5%) children who were randomized were excluded from the analyses; 2 (1%) were lost to follow‐up |
|
| Participants | Age: mean = 9.9 ± 1.3 years (range 8 to 12 years) Gender: 71 boys, 103 girls Culture: children from US cities of study centers: 73% white, 7% black, 4% Asian, 12% Hispanic, 4% other Inclusion criteria: (1) age 8 to 12 years; (2) myopia of ‐0.75 D to ‐4.00 D; (3) best‐corrected VA of 20/25 or better; (4) normal pupils; (5) good general health Exclusion criteria: (1) anisometropia or astigmatism greater than 1.00 D; (2) any manifest tropia; (3) current use of either contact lenses or bifocals; (4) history of ocular surgery, trauma, or chronic ocular disease, including allergic conjunctivitis; (5) disease requiring long‐term or regular intermittent medication; (6) behavioral or neurological disorder that would interfere with the study; (7) participation in any study that involved an investigational drug within 1 month of enrollment; (8) intolerance or hypersensitivity to topical anesthetics, mydriatics, or components of the formulations; (9) contraindications to antimuscarinic agents; (10) pregnancy or planned pregnancy |
|
| Interventions | Pirenzepine (n = 117): 2% pirenzepine ophthalmic gel applied twice a day Control (n = 57): vehicle‐placebo gel applied twice a day |
|
| Outcomes |
Primary outcome:
Secondary outcome:
Measurements taken at baseline and every 3 months for 1 year Unit of analysis: average of both eyes |
|
| Notes | Study dates: March 1, 2000 to February 28, 2002 Trial registration: not reported Funding source: Valley Forge Pharmaceuticals, Inc. Notes: study is also known as the Collaborative Assessment of Myopia Progression with Pirenzepine (CAMPP) study |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Eligible patients were randomized via a sponsor‐prepared, computer‐generated list stratified by site. Randomization was done at a 2:1 ratio of pirenzepine to placebo |
| Allocation concealment (selection bias) | Low risk | "Eligible patients were randomized via a sponsor‐prepared, computer‐generated list stratified by site...Study sites used coded lists to determine which tube of medication to administer to the next enrolled subject. The pirenzepine gels and placebo gels were packaged in identical tubes and the gels themselves appeared identical. Thus study personnel had no way of knowing which tubes had which medications, same for patients" (SC) |
| Masking of participants (performance bias) | Low risk | Study was placebo‐controlled, and identical bottles with coded labels were distributed |
| Masking of outcome assessors (detection bias) Progression of myopia | Low risk | "Since the pirenzepine gels and placebo gels were packaged in identical tubes and the gels themselves appeared identical, the study personnel had no way of knowing which tubes had which medications" (SC) |
| Masking of outcome assessors (detection bias) Secondary outcomes | Low risk | Primary and secondary outcomes were assessed by the same examiners |
| Masking of data analyzers | Unclear risk | Data analyzers were not masked (personal communication with biostatistician) |
| Incomplete outcome data (attrition bias) Incomplete outcome(s) data | High risk | Screened 277 patients and enrolled 174. Attrition was reported: 145 of 174 patients completed the trial. There were significantly more dropouts in the pirenzepine arm: 26/117 (26%), compared with placebo arm: 3/57 (5%). Reasons for dropout included occurrence of adverse events, nonadherence, and loss to follow‐up "Additional methods were used to impute missing values due to patients who discontinued the study. In all 3 methods used (last observation carried forward, visit‐to‐visit extrapolation using median of respective treatment, and visit‐to‐visit extrapolation using median of placebo group), the treatment effect was similar to or greater than in the primary analysis method" |
| Selective reporting (reporting bias) | Low risk | Primary and secondary outcomes were chosen a priori and all were reported |
| Other bias | Unclear risk | Some study authors were employed by the pharmaceutical company funding the study |
ROMIO Study 2012.
| Study characteristics | ||
| Methods | Study design: parallel‐group RCT Study center: 1 (Hong Kong Polytechnic University) Number randomized: 102 children Study follow‐up: 2 years Exclusions and losses to follow‐up: 24 (24%) children who were randomized (14 in the orthokeratology group and 10 in the control group) were excluded from the analyses, of whom, 9 (8.8%) were lost to follow‐up |
|
| Participants | Age: mean = 9 years (range 6 to 10 years) Gender: 52 boys, 50 girls Culture: Hong Kong Inclusion criteria: (1) ages 6 to 10 years; (2) myopia between 0.50 D and 4.00 D in at least 1 eye and between 0.50 D and 4.50 D in both eyes; (3) astigmatism < 1.50 D, with‐the‐rule astigmatism (axes 180 ± 30) ≤ 1.25 D, astigmatism of other axes ≤ 0.50 D in both eyes; (4) anisometropia ≤ 1.50 D); (5) best‐corrected logMAR visual acuity 0.10 or better in both eyes; (6) symmetrical corneal topography with corneal toricity < 2.00 D in either eye; (7) agree to randomization Exclusion criteria: (1) strabismus at distance or near; (2) history of contact lens wear or myopia control treatment; (3) contraindication for contact lens wear and orthokeratology; (4) history of ocular surgery, trauma, or chronic ocular disease; (5) concurrent use of medications that may affect tear quality; (6) systemic or ocular conditions that may affect tear quality or contact lens wear or that may affect refractive development; (7) poor compliance with tests; (8) lack of willingness to comply with allocated treatment and follow‐up schedule |
|
| Interventions | Orthokeratology (n = 51): orthokeratology (ortho‐k) lenses SVLs (n = 51): single vision spectacles Participants wore assigned treatment on a daily basis |
|
| Outcomes |
Primary outcome:
Secondary outcome:
Measurements taken at baseline and at 6, 12, 18, and 24 months Unit of analysis: child‐based (right eye) |
|
| Notes | Study dates: enrollment March 2008 to November 2009 Trial registration: NCT00962208 Funding source: "supported by a collaborative agreement between The Hong Kong Polytechnic University and Menicon Co. Ltd., Japan; contact lenses and solutions and spectacles were sponsored by Menicon Co. Ltd., NKL Contactlenzen B.V., Alcon Hong Kong, Bausch & Lomb Hong Kong, Skyview Optical Co. Ltd., Hong Kong, and Hong Kong Optical Lens Co., Ltd.; and Niche Myopia Funding Grant J‐BB7P for facilities at the Centre for Myopia Research" |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Randomization was performed in blocks of two using a commercial spreadsheet random number generator (Excel; Microsoft, Redmond, WA)" |
| Allocation concealment (selection bias) | High risk | "The random allocation sequence was revealed to the unmasked examiner who would proceed to prescribe the assigned treatment to the subjects accordingly" |
| Masking of participants (performance bias) | High risk | Participants were not masked |
| Masking of outcome assessors (detection bias) Progression of myopia | Unclear risk | This was not assessed |
| Masking of outcome assessors (detection bias) Secondary outcomes | Low risk | "The primary outcome measure (i.e., the axial length) was masked in the study" Masking of persons measuring adverse events was not reported |
| Masking of data analyzers | Unclear risk | This was not reported |
| Incomplete outcome data (attrition bias) Incomplete outcome(s) data | High risk | 24 (24%) children who were randomized (14 in the orthokeratology group and 10 in the control group) were excluded from the analyses: 5 children in the orthokeratology group and 1 in the control group due to ocular health problem; 9 children in the orthokeratology group due to poor correction or undercorrection; and 9 children in the control group due to loss to follow‐up |
| Selective reporting (reporting bias) | Low risk | Results were reported for outcomes described in the methods section of the paper |
| Other bias | Unclear risk | Study was funded by a company producing the lenses under investigation in the study |
Sankaridurg 2010.
| Study characteristics | ||
| Methods | Study design: parallel‐group RCT Study center: 1 (Zhongshan Ophthalmic Center, Sun Yet Sen University, China) Number randomized: 210 children Study follow‐up: 12 months (study was originally planned to be 2 years in duration) Exclusions and losses to follow‐up at 12‐month visit: 2 children who were randomized were excluded from the analyses; 7 (3.3%) were lost to follow‐up |
|
| Participants | Age: mean = 11 years (range 6 to 16 years) Gender: 110 boys, 100 girls Culture: Chinese children in Guangzhou, China Inclusion criteria: (1) age 6 to 16 years; (2) bilaterally myopic (spherical component range from ‐0.75 D to ‐3.50 D inclusive) with astigmatism not exceeding ‐1.50 D and maximum of 1.00 D of anisometropia; (3) vision correctable to 6/9.5 or better in each eye; (4) ocular findings considered to be normal; (5) willingness to wear study spectacles and adhere to the protocol schedule |
|
| Interventions | Novel spectacle lens type I (n = 50): a rotationally symmetrical design; featured a clear central aperture of 20 mm diameter, with maximum spherical equivalent of +1.0 D relative peripheral power achieved 25 mm from its axis Novel spectacle lens type II (n = 60): a rotationally symmetrical design; featured a clear central aperture of 14 mm diameter, with maximum spherical equivalent of +2.00 D relative peripheral power achieved 25 mm from its axis Novel spectacle lens type III (n = 50): an asymmetrical design; a clear central aperture extended approximately 10 mm either side of center along the horizontal meridian and a similar distance inferiorly, with positive additional peripheral power of 1.9 D 25 mm from the axis in that meridian SVLs (n = 50): conventional, single vision design Note: lenses were fitted to spectacle frames that ranged in eye‐size from 45 mm to 55 mm with depths from 27 mm to 33 mm |
|
| Outcomes |
Primary outcome:
Secondary outcome:
Measurements taken at baseline, 6 months, and 12 months Unit of analysis: average of both eyes |
|
| Notes | Study dates: recruitment October 2007 to January 2009 Trial registration: not reported Funding source: Australian Federal Government; Institute for Eye Research, Sydney, Australia; Vision CRC, Australia Lenses were provided by industry |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Subjects were randomized to a single treatment using the method of randomly permuted blocks of a constant size of 20" The website randomization.com was used |
| Allocation concealment (selection bias) | Low risk | Central allocation by web‐based randomization was performed: "the four designs (3 novel and one standard) were coded A, B, C, and D and the randomization scheme generated using the website randomization.com (http://www.randomization.com)" |
| Masking of participants (performance bias) | Unclear risk | Although masking was reported for participants, study authors noted that the novel lenses were substantially different from SVLs. For this reason, 10 additional participants were enrolled for the group allocated to lens type II |
| Masking of outcome assessors (detection bias) Progression of myopia | Low risk | "Access to the study randomization table was restricted to the study optical dispenser who allocated the randomization and coordinated with the laboratory for the delivery of the spectacles. The dispenser was masked to the lens design. Also, the participants and investigators were masked to the spectacle lenses used in the study" |
| Masking of outcome assessors (detection bias) Secondary outcomes | Low risk | "Access to the study randomization table was restricted to the study optical dispenser who allocated the randomization and coordinated with the laboratory for the delivery of the spectacles. The dispenser was masked to the lens design. Also, the participants and investigators were masked to the spectacle lenses used in the study" |
| Masking of data analyzers | Unclear risk | This was not reported |
| Incomplete outcome data (attrition bias) Incomplete outcome(s) data | High risk | Two children who were randomized were excluded from the analyses, and 7 (3.3 %) were lost to follow‐up at 12 months (2 in type I, 1 each in type II and control, and 3 in type III). One child in type II withdrew due to an adverse event, and 1 in type III lenses withdrew consent. They were not included in the analysis, and there was no re‐inclusion |
| Selective reporting (reporting bias) | Low risk | Results were reported for outcomes described in the methods section of the paper |
| Other bias | Unclear risk | Study originally was planned for 2 years but was stopped early at 1 year Lenses being investigated were provided by industry |
Schwartz 1981.
| Study characteristics | ||
| Methods | Study design: parallel‐group RCT in twins Study center: 1 Number randomized: 52 children (26 twin pairs) Study follow‐up: 3 years (planned), extended 6 months Exclusions and losses to follow‐up: 2 (4%) children (1 twin pair) who were randomized were excluded from the study; none were lost to follow‐up |
|
| Participants | Age: mean = 11.2 years (range 7 to 14 years) Gender: 26 boys (13 twin pairs) and 24 girls (12 twin pairs) completed the study Culture: pairs of monozygotic (MZ) twins identified from the Twin Registry of Eye Examinations from the Washington, DC area; all were Caucasian Inclusion criteria: (1) MZ twins with bilateral myopia; (2) ages 7 to 13 years; (3) shared domicile in local area; (4) good general health; (5) vision correctable to 20/20 or better; (6) third‐degree fusion; (7) no other significant abnormality Exclusion criteria: (1) astigmatism or anisometropia greater than 1.00 D; (2) difference in refraction between co‐twins of 1.50 D or more in the more advanced eye |
|
| Interventions | Treatment group (n = 26): combined treatment of bifocal spectacles with 1.25 D addition and 2 drops of 1% tropicamide ophthalmic solution instilled to each eye nightly Control group (n = 26): standard spectacle correction (SVLs) Note: full cycloplegic correction in the treatment group was sometimes reduced up to 0.50 D when it did not impair vision below 20/20 |
|
| Outcomes |
Primary outcome:
Secondary outcome:
Measurements taken at baseline and every 6 months for 3 years Unit of analysis: average values of both eyes |
|
| Notes | Study dates: not reported Trial registration: not reported Materials: 1% tropicamide (Mydriacyl) ophthalmic solution supplied by Alcon Laboratories Inc. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "The assignment of special treatment or control status to members of each twinship was based on a strict randomization protocol" |
| Allocation concealment (selection bias) | Unclear risk | "Treatment or control status was randomly assigned only after the twin pair and the parents expressed willingness to accept the rigorous requirements and the desire to participate" does not explain how allocation was concealed. |
| Masking of participants (performance bias) | High risk | Masking of participants was not applicable due to visual and functional differences between the interventions studied |
| Masking of outcome assessors (detection bias) Progression of myopia | Low risk | "All refractions were performed by the author, who was unaware of the treatment or control status of examinees" However, there was only 1 study investigator, and it was not reported who reviewed participants' activities when they came in for follow‐up and how the examiner remained masked at follow‐up visits |
| Masking of outcome assessors (detection bias) Secondary outcomes | Unclear risk | N/A (study did not measure secondary outcomes of this review) |
| Masking of data analyzers | Unclear risk | This was not reported |
| Incomplete outcome data (attrition bias) Incomplete outcome(s) data | High risk | Of 26 twin pairs enrolled, 25 pairs completed the study and 1 pair was excluded due to noncompliance after 1 year |
| Selective reporting (reporting bias) | Low risk | Outcomes were chosen a priori and presented in the methods and design paper |
| Other bias | Low risk | None was identified |
Shih 1999.
| Study characteristics | ||
| Methods | Study design: parallel‐group RCT Study center: 1 (National Taiwan University Hospital) Number randomized: 200 children Study follow‐up: 2 years Exclusions and losses to follow‐up: 14 (7%) children who were randomized were excluded from the study; none were lost to follow‐up |
|
| Participants | Age: mean = 9.2 years (range 6 to 13 years) Gender: included boys and girls Culture: children recruited from the vision care center at National Taiwan University Hospital Inclusion criteria: (1) age 6 to 13 years; (2) myopia with refractive error between ‐0.50 D and ‐6.75 D Exclusion criteria: (1) amblyopia or tropia; (2) astigmatism ‐2.00 D or greater; (3) anisometropia ‐2.00 D or greater |
|
| Interventions | Atropine 0.5% (n = 50): 1 drop of 0.5% atropine nightly; advised to wear bifocal spectacles Atropine 0.25% (n = 50): 1 drop of 0.25% atropine nightly; advised to wear slightly undercorrected spectacles Atropine 0.1% (n = 50): 1 drop of 0.1% atropine nightly; advised to wear fully corrective spectacles Control (n = 50): 1 drop of 0.5% tropicamide nightly Note: all children were advised to wear sunglasses with UV protection in bright light |
|
| Outcomes |
Primary outcome:
Measurements taken at baseline and every 3 months for 2 years Unit of analysis: average values of both eyes |
|
| Notes | Study dates: 1994 Trial registration: not reported Funding source: Department of Health grant (Taiwan) Additional data: study author provided unpublished data via email correspondence |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Block randomization: each participant was categorized into 1 of the strata and then was randomized to receive 1 of the 4 treatments. A block size of 12 was used to balance the number of patients in the 4 treatment categories for each stratum (via email communication with study author) |
| Allocation concealment (selection bias) | Low risk | Group assignments were unknown to study personnel when participants were being enrolled via coded bottles with sealed envelopes (via email communication with study author) |
| Masking of participants (performance bias) | High risk | Masking of participants was not applicable due to visual and functional differences between the interventions studied |
| Masking of outcome assessors (detection bias) Progression of myopia | Low risk | All study personnel, physicians, examiners, and data analysts were masked to treatment assignment (via email communication with study author) |
| Masking of outcome assessors (detection bias) Secondary outcomes | Unclear risk | N/A (study did not measure secondary outcomes of this review) |
| Masking of data analyzers | Low risk | All study personnel, physicians, examiners, and data analysts were masked to treatment assignment (via email communication with study author) |
| Incomplete outcome data (attrition bias) Incomplete outcome(s) data | High risk | 14 (7%) children were excluded from the study: 9 from the 0.5% atropine group (4 had no patience for eye drops, 2 for photophobia, 2 from fear of drops, 1 for allergic blepharitis); 3 from the 0.25% atropine group (no patience for eye drops); 1 from the 0.1% atropine group (no patience for eye drops); and 1 from the 0.5% tropicamide group (no patience for eye drops) |
| Selective reporting (reporting bias) | Low risk | Results were reported for outcomes described in the methods section of the paper |
| Other bias | High risk | Children were advised to wear different types of spectacle lenses depending on the concentration of atropine received |
STAMP Study 2012.
| Study characteristics | ||
| Methods | Study design: parallel‐group RCT Study center: 1 (The Ohio State University College of Optometry) Number randomized: 85 children Study follow‐up: 2 years Exclusions and losses to follow‐up: 2 (2.3%) children did not complete the study |
|
| Participants | Age: mean = 9.8 years (range 6 to 11 years) Gender: 41 boys, 44 girls Culture: Ohio, USA: 20% Black, 68% White, 7% Asian, 5% other Inclusion criteria: (1) 6 to 11 years of age; (2) at least ‐0.75 D myopia in each meridian measured with cycloplegic autorefraction but not more than ‐4.50 D in each meridian in each eye; (3) ≥ 1.30 D accommodative lag (4 D stimulus) without correction; (4) esophoria at near if more than ‐2.25 D spherical equivalent; (5) astigmatism ≤ 2.00 DC in each eye; (6) anisometropia ≤ 2.00 D; (7) best‐corrected VA of at least 20/32 logMAR equivalent; (8) birth weight ≥ 1250 g by parental report Exclusion criteria: (1) strabismus; (2) history of contact lens wear or previous bifocal wear; (3) diabetes mellitus |
|
| Interventions | PALs (n = 42): PALs with + 2.00 D addition (Varilux Ellipse; Essilor of America, Dallas, TX) SVLs (n = 43) Note: children were randomly assigned to wear either PALs or SVLs for the first year of the study; all children wore SVLs for the second year of the study |
|
| Outcomes |
Primary outcome:
Secondary outcomes:
Measurements taken at baseline and at 6‐month intervals for 2 years Unit of analysis: the individual (right eye only) |
|
| Notes | Study dates: study recruitment from December 2006 to May 2008 Trial registration: NCT00335049 Funding source: National Eye Institute, National Institutes of Health, USA; Essilor of America, Inc.; American Optometric Foundation Ezell Fellowship Study name: study of theories about myopia progression (STAMP) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "The randomization sequence used random, even block sizes and was generated by the Optometry Coordinating Center at The Ohio State University" |
| Allocation concealment (selection bias) | Low risk | "Confirmation of eligibility and randomization of children to either SVLs or PALs was administered through a Web portal. A child's group assignment could not be accessed until all required baseline visit data were entered" |
| Masking of participants (performance bias) | High risk | Masking of participants was not applicable due to functional differences between the interventions studied |
| Masking of outcome assessors (detection bias) Progression of myopia | Low risk | "All outcome data were collected by an examiner masked to the treatment assignment”; “All outcome data were collected by an examiner masked to the treatment assignment. At each visit, subjects were reminded not to talk about their spectacles or vision when the examiner was in the room. The child’s spectacles were removed and hidden from view before the examiner entered the room" |
| Masking of outcome assessors (detection bias) Secondary outcomes | Low risk | Same outcome assessors as for the primary outcome |
| Masking of data analyzers | Unclear risk | Masking of persons analyzing results was not reported |
| Incomplete outcome data (attrition bias) Incomplete outcome(s) data | Low risk | 2 (2.3%) children did not complete the study and were excluded from the analysis |
| Selective reporting (reporting bias) | Low risk | Design and baseline data were published; results for all prespecified outcomes were reported |
| Other bias | Unclear risk | Study was funded by a company producing the PAL lenses under investigation in the study; trial authors were financially compensated by the company |
Swarbrick 2015.
| Study characteristics | ||
| Methods | Study design: paired‐eye, cross‐over RCT Study center: 1 (School of Optometry and Vision Science, University of New South Wales, Australia) Number randomized: 32 children Study follow‐up: 12 months (two 6‐month periods) Exclusions and losses to follow‐up: 6 (19%) during first period and 8 (25%) during 12‐month study |
|
| Participants | Age: mean = 13.4 years (range 8 to 16 years) Gender: 14 boys, 12 girls Culture: East Asian ethnicity Inclusion criteria: (1) 8 to 16 years of age; (2) myopic refractive error between ‐1.00 D and ‐4.00 D in both eyes with < 0.75 D difference between eyes; (3) evidence of myopic progression in 12 months before enrollment; (4) with‐the‐rule astigmatism < 1.50 D and no against‐the‐rule astigmatism; (5) anisometropia ≤ 0.75 D; (6) best‐corrected visual acuity of 6/9 or better; (7) East Asian ethnicity; (8) good general and ocular health Exclusion criteria: (1) contraindications for rigid contact lens wear; (2) history of previous rigid contact lens wear; (3) abnormal corneal topography; (4) abnormal binocular function; (5) ocular pathology or active ocular surface disease precluding contact lens wear |
|
| Interventions | Orthokeratology (n = 26): orthokeratology lens in 1 eye (overnight wear) RGP (n = 26): rigid gas permeable (RGP) contact lens in the other eye (daily or extended wear) Note: children were randomly assigned to wear the orthokeratology lens in 1 eye and the RGP lens in the other eye for 6 months; at 6 months, the lenses were switched for each eye. The clinical trial registry record also mentioned a matched control group of children who wore spectacles for 12 months; this group was not mentioned in the journal article |
|
| Outcomes |
Primary outcome:
Secondary outcomes:
Measurements taken at baseline and at 3, 6, 9, and 12 months Unit of analysis: the eye |
|
| Notes | Study dates: not reported Trial registration: ACTRN12608000007336 Funding sources: Australian Research Council (ARC) Linkage Project Grant Scheme, BE Enterprises Pty Ltd., Capricornia Contact Lens Pty Ltd. (Australia); Boston Products Group of Bausch & Lomb (USA) Disclosures of interest: "the authors have no proprietary or commercial interest in any materials discussed in this article" |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "At study commencement, subjects were dispensed an OK lens for overnight wear only (with no lens wear during the day) in 1 eye chosen at random by coin toss (the “night” lens) and a conventional GP lens for the contralateral eye for daytime wear (the “day” lens)" |
| Allocation concealment (selection bias) | Unclear risk | Allocation of both eyes was determined at time of coin toss, but does not explain how the allocation was concealed. |
| Masking of participants (performance bias) | High risk | Masking of participants was not applicable due to functional differences between the interventions studied |
| Masking of outcome assessors (detection bias) Progression of myopia | Unclear risk | Masking of outcome assessors was not reported |
| Masking of outcome assessors (detection bias) Secondary outcomes | Unclear risk | Masking of outcome assessors was not reported |
| Masking of data analyzers | Unclear risk | This was not reported |
| Incomplete outcome data (attrition bias) Incomplete outcome(s) data | High risk | 8 (25%) of 32 children were not included in the analyses: 4 due to inconvenience of study visits; 2 due to poor adaptation to GP lens wear; 1 due to persistent GP contact lens adherence; and 1 due to lenses not being consistently worn in the correct eye |
| Selective reporting (reporting bias) | High risk | Outcomes listed in the clinical trial registry record were not reported in the journal publication: corneal epithelial cell exfoliation during gentle eye wash with sterile saline, amount of bacterial binding, peripheral refractive status |
| Other bias | High risk | Data were not appropriately analyzed for paired‐eye nor cross‐over design |
Tan 2005.
| Study characteristics | ||
| Methods | Study design: parallel‐group RCT Study centers: 7 (academic centers and clinical practices in Singapore, Hong Kong, and Thailand) Number randomized: 353 children Study follow‐up: 1 year Exclusions and losses to follow‐up: 55 (16%) children who were randomized were dropped from the analyses |
|
| Participants | Age: mean = 8.7 years (range 6 to 13 years) Gender: 177 boys, 176 girls Culture: 99.4% Asian Inclusion criteria: (1) age 6 to 12 years; (2) myopia of ‐0.75 D and ‐4.00 D; (3) good general health; (4) round pupils; (5) refractive to light; (6) best‐corrected VA of 20/25 or better in each eye Exclusion criteria: (1) astigmatism greater than 1.00 D; (2) anisometropia greater than 1.00 D; (3) strabismus; (4) current use of either contact lenses or bifocals; (5) history of ocular surgery, trauma, or chronic ocular disease, including allergic conjunctivitis; (6) previous use of atropine for myopia; (7) disease requiring long‐term or regular intermittent medication; (8) behavioral or neurological disorder that would interfere with the study; (9) participation in any study that involved an investigational drug within 1 month of enrollment; (10) intolerance or hypersensitivity to topical anesthetics, mydriatics, or components of the formulations; (11) contraindications to antimuscarinic agents; (12) pregnancy or planned pregnancy |
|
| Interventions | Gel/gel (n = 142): 2% pirenzepine ophthalmic gel applied twice a day Placebo/gel (n = 140): 2% pirenzepine ophthalmic gel applied once a day and placebo gel applied once a day Placebo/placebo (n = 71): vehicle‐placebo gel applied twice a day |
|
| Outcomes |
Primary outcome:
Secondary outcome:
Measurements taken at baseline and every 3 months for 1 year Unit of analysis: average of both eyes |
|
| Notes | Study dates: November 2000 to July 2002 Trial registration: not reported Funding source: Valley Forge Pharmaceuticals, Inc., and Novartis Ophthalmics AG |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Eligible patients were randomized via a sponsor‐prepared, computer‐generated list stratified by site. Randomization was done at a 2:2:1 ratio of pirenzepine twice daily, pirenzepine once daily, and placebo |
| Allocation concealment (selection bias) | Low risk | Study sites used coded lists to determine which tubes of medication to administer to the next enrolled participant. The pirenzepine gels and placebo gels were packaged in identical tubes for morning and night applications, and the gels themselves appeared identical. All morning tubes had yellow labels, and all evening tubes had blue labels. Thus study personnel had no way of knowing which tubes had which medications; same for patients |
| Masking of participants (performance bias) | Low risk | Study was placebo‐controlled, and identical appearing bottles with coded labels were distributed |
| Masking of outcome assessors (detection bias) Progression of myopia | Low risk | Because the pirenzepine gels and placebo gels were packaged in identical tubes and the gels themselves appeared identical, study personnel had no way of knowing which tubes had which medications. The study reported that "no treatment code was unmasked for any subject during the study" |
| Masking of outcome assessors (detection bias) Secondary outcomes | Low risk | Primary and secondary outcomes were assessed by the same examiners |
| Masking of data analyzers | Unclear risk | Data analyzers were not masked (personal communication with biostatistician) |
| Incomplete outcome data (attrition bias) Incomplete outcome(s) data | High risk | 55 (16%) children were dropped from the study after randomization: 25 (18%) were gel/gel, 21 (15%) were placebo/gel, and 9 (13%) were placebo/placebo (this difference was not statistically different). Of these 55 patients, 31 discontinued the study because of adverse events (20 gel/gel, 11 placebo/gel, 0 placebo); 5 were not adherent to the study medication regimen; and 1 was dropped for inadequate efficacy (progression of myopia) "We considered statistical methods to impute missing values due to patients who discontinued treatment. However, as the proportion of patients not completing the study was similar across treatment groups, any correction would apply similarly to all groups, and thus we did not conduct these analyses" |
| Selective reporting (reporting bias) | Low risk | Primary and secondary outcomes were chosen a priori |
| Other bias | Unclear risk | Some study authors were employed by the pharmaceutical company funding the study |
Trier 2008.
| Study characteristics | ||
| Methods | Study design: parallel‐group RCT Study center: 1 Number randomized: 83 children Study follow‐up: 3 years (intervention 12 months) Exclusions and losses to follow‐up: 6 (7.2%), 9 (10.8%), and 7 (8.4%) were lost to follow‐up during the first year, the second year, and the third year, respectively |
|
| Participants | Age: mean 11.3 years (range 8 to 13 years) Gender: not reported Culture: Denmark Inclusion criteria: (1) age 8 to 13 years; (2) minimum myopia of ‐0.75 D in 1 eye; (3) average axial length growth rate 0.075 to 0.39 mm per 6‐month period Exclusion criteria: (1) severe general ailment (e.g. diabetes, epilepsy, psychiatric disease); (2) other eye disease (e.g. cataract, keratoconus, chronic iritis, glaucoma) |
|
| Interventions | Systemic 7‐methylxanthine (n = 35): one 400 mg 7‐methylxanthine (7‐mx) tablet every morning Placebo (n = 42): 1 placebo tablet every morning Notes: children received either 7‐mx or placebo for the first 12 months; all participants received 7‐mx after 12 months (400 mg 7‐mx tablet once or twice per day); "all children used single vision lenses" |
|
| Outcomes |
Primary outcome:
Secondary outcome:
Measurements taken at ‐6, 0, 12, 24, and 36 months Unit of analysis: the individual (average of both eyes) |
|
| Notes | Study dates: October 2003 Trial registration: NCT00263471 Funding source: "supported by grants from 'Jørgen Bagenkop Nielsens Myopi‐Fond' and 'Generalkonsul Einar Høyvalds Fond', and by 'Øjenlæge Klaus Trier ApS'" Declarations of interest: 2 authors affiliated with Trier Research Laboratories |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "The allocation sequence was generated in the pharmacy and sealed and numbered containers consecutively handed out to the participants" |
| Allocation concealment (selection bias) | Low risk | "The allocation sequence was generated in the pharmacy and sealed and numbered containers consecutively handed out to the participants" |
| Masking of participants (performance bias) | Low risk | "Outcomes were assessed unaware of group assignment at Trier Research Laboratories. No participants or investigators became unmasked during the first 12 months of the trial" |
| Masking of outcome assessors (detection bias) Progression of myopia | Low risk | "Outcomes were assessed unaware of group assignment at Trier Research Laboratories. No participants or investigators became unmasked during the first 12 months of the trial" |
| Masking of outcome assessors (detection bias) Secondary outcomes | Low risk | "Outcomes were assessed unaware of group assignment at Trier Research Laboratories. No participants or investigators became unmasked during the first 12 months of the trial" |
| Masking of data analyzers | Unclear risk | This was not reported |
| Incomplete outcome data (attrition bias) Incomplete outcome(s) data | Low risk | 6 (7.2%), 9 (10.8%), and 7 (8.4%) children missed the first year, second year, and third year follow‐up visits, respectively; reasons for missed visits were not reported |
| Selective reporting (reporting bias) | Low risk | Results of all outcomes prespecified in the clinical trial registry record were reported |
| Other bias | Unclear risk | Study was conducted by author's research company |
Wang 2005.
| Study characteristics | ||
| Methods | Study design: parallel‐group RCT Study center: 1 (Shanghai, China) Number randomized: 104 children Study follow‐up: 18 months Exclusions and losses to follow‐up: not reported |
|
| Participants | Age: mean = 11.6 years (range 6 to 15 years) Gender: 51 boys, 53 girls Culture: recruited from outpatient department of Eye & Ear, Nose, Throat Hospital in Shanghai, China Inclusion criteria: (1) age 6 to 15 years; (2) myopia Exclusion criteria: not reported |
|
| Interventions | PAL group (n = 50): add not reported SVL (n = 54) |
|
| Outcomes |
Primary outcomes:
Secondary outcomes:
Measurements taken at baseline and every 6 months for 18 months Unit of analysis: not reported |
|
| Notes | Study period: enrollment from April 1999 to April 2000 Trial registration: not reported Funding source: not reported We were not able to make contact with study authors for additional information; we report the data available in the conference abstract only |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "A randomized control trial (RCT) was conducted. Children were distributed into PALs group or control randomly" |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment before randomization was not reported |
| Masking of participants (performance bias) | High risk | Masking of participants was not applicable due to functional differences between the interventions studied |
| Masking of outcome assessors (detection bias) Progression of myopia | Unclear risk | Masking was not reported |
| Masking of outcome assessors (detection bias) Secondary outcomes | Unclear risk | Masking was not reported |
| Masking of data analyzers | Unclear risk | Masking was not reported |
| Incomplete outcome data (attrition bias) Incomplete outcome(s) data | Unclear risk | Trial was reported only as a conference abstract with limited information to assess attrition bias |
| Selective reporting (reporting bias) | Unclear risk | Trial was reported only as a conference abstract with limited information to assess outcomes |
| Other bias | Unclear risk | Reasons for trial authors not publishing full‐length report of the study are not clear |
Wang 2017.
| Study characteristics | ||
| Methods | Study design: parallel‐group RCT Study center: 1 (The People’s Hospital of Yan’an and Affiliated Hospital of Yan’an Medical University) Number randomized: 126 children Study follow‐up: 1 year Exclusions and losses to follow‐up: 7 (11.1%) in intervention group and 5 (7.9%) in control group discontinued intervention; 2 (3.2%) in intervention group and 3 (4.8%) in control group were lost to follow‐up |
|
| Participants | Age mean (SD): 9.1 (1.4) years in intervention group; 8.7 (1.5) years in control group Gender: 36 (57.1%) boys and 27 (42.9%) girls in intervention group; 31 (49.2%) boys and 32 (50.8%) girls in control group Culture: China Inclusion criteria: (1) diagnosis of low myopia (spherical equivalent between ‐0.50 and ‐2.00 D by cycloplegic autorefraction); (2) age 5 to 10 years; (3) normal intraocular pressure (IOP; < 21 mmHg); (4) not on any other treatment within 1 month before study enrollment; (5) provided informed consent Exclusion criteria: (1) abnormal binocular function or stereopsis; (2) other eye disease; (3) history of hemostatic or other systemic disorder; (4) contact lens or any other intervention for myopia; (5) allergy to atropine |
|
| Interventions | Atropine (n = 63): 0.5% eye drops once daily at night Placebo (n = 63): vehicle eye drops once daily at night Note: none |
|
| Outcomes |
Primary outcome:
Secondary outcome:
Safety outcome:
Measurements taken at 4, 8, and 12 months Unit of analysis: individual (eye with more severe myopia used) |
|
| Notes | Study dates: January 2014 to December 2016 Trial registration: not reported Funding source: none |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | “The stratified randomization schedule was operated by a computerized number generated using SAS package (Version 9.1; SAS Institute Inc., Cary, NC)” |
| Allocation concealment (selection bias) | Low risk | “The information of all assignments and its allocation were concealed in sequentially numbered, opaque, sealed envelopes” |
| Masking of participants (performance bias) | Low risk | “The participants and investigators were not informed whether a participant was assigned to the intervention or control group”; “The placebo eyedrops had similar labels and appearances as the ATE” |
| Masking of outcome assessors (detection bias) Progression of myopia | Low risk | “We also blinded the outcome assessors and data analysts” |
| Masking of outcome assessors (detection bias) Secondary outcomes | Low risk | “We also blinded the outcome assessors and data analysts” |
| Masking of data analyzers | Low risk | “We also blinded the outcome assessors and data analysts” |
| Incomplete outcome data (attrition bias) Incomplete outcome(s) data | Unclear risk | “Of those included participants, 17 were excluded because of the discontinued intervention (n = 12) and loss to contact (n = 5). Thus, 109 participants completed all treatment. Fortunately, we used ITT approach to analyze all outcome data” Unclear how ITT analysis was performed |
| Selective reporting (reporting bias) | Low risk | Results were reported for outcomes described in the methods section of the paper |
| Other bias | Low risk | None was identified |
Yang 2009.
| Study characteristics | ||
| Methods | Study design: parallel‐group RCT Study center: 1 (Guangzhou City, China) Number randomized: 178 children Study follow‐up: 2 years Exclusions and losses to follow‐up: no exclusions; 29 (16%) were lost to follow‐up |
|
| Participants | Age: range 7 to 13 years Gender: 94 boys, 84 girls Culture: urban children from Guangzhou City, China Inclusion criteria: (1) age 7 to 13 years; (2) myopia with spherical equivalent refractive error between ‐0.50 D and ‐3.00 D in both eyes, as measured under cycloplegia; (3) astigmatism ≤ 1.50 D; (4) no anisometropia (difference in spherical equivalent ≤ 1.00 D between eyes); (5) best‐corrected VA 6/6 or better; (6) no strabismus; (7) normal IOP; (8) willingness to wear glasses constantly for study duration; (9) understanding of random assignment and willingness to not use other medications Exclusion criteria: (1) any ocular or systemic condition known to influence refractive development; (2) use of medication that might affect refractive development; (3) moderately or highly myopic (< ‐3.00 D) parents; (4) birth weight ≤ 1250 g; (5) previous use of bifocals, PALs, or contact lenses |
|
| Interventions | PAL group (n = 89): multifocal lenses with +1.50 D near addition worn constantly SVL group (n = 89): single vision lenses worn constantly Note: prescription changes were made if subjective refraction had changed by at least 0.50 D for 1 or both eyes or if clinically indicated |
|
| Outcomes |
Primary outcome:
Change in spherical equivalent refractive error relative to baseline measured by cycloplegic autorefraction with 0.5% tropicamide + 0.5% phenylephrine hydrochloride Secondary outcomes:
Measurements taken at baseline and every 6 months for 2 years Unit of analysis: not reported |
|
| Notes | Study dates: enrollment was from July 2004 to March 2005 Trial registration: not reported Funding source: National Natural Science Grant, China Materials: lenses provided by Sola (China) Ltd. Compliance in wearing glasses was monitored with separate questionnaires for children and parents (87% overall compliance) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Enrolled subjects were assigned randomly to either the SV group or PAL group" |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment before randomization was not reported |
| Masking of participants (performance bias) | High risk | Masking of participants was not applicable due to functional differences between the interventions studied |
| Masking of outcome assessors (detection bias) Progression of myopia | Low risk | Masked investigators were unaware of allocation groups during evaluation, although an unmasked investigator was available if clinical consultations were needed |
| Masking of outcome assessors (detection bias) Secondary outcomes | Low risk | Primary and secondary outcomes were assessed by the same examiners |
| Masking of data analyzers | Unclear risk | "All statistical analysis was carried out independently" |
| Incomplete outcome data (attrition bias) Incomplete outcome(s) data | Unclear risk | 29 (16%) patients dropped out of the study: 15 in the PAL group and 14 in the SVL group. Statistical analyses comparing the retained participants to those lost to follow‐up showed that dropouts had significantly worse myopia than those who remained in the study at baseline (P = 0.01) |
| Selective reporting (reporting bias) | Low risk | Results were reported for outcomes described in the methods section of the paper |
| Other bias | Unclear risk | Unit of analysis (i.e. average value of both eyes or right eye only) was not reported |
Yen 1989.
| Study characteristics | ||
| Methods | Study design: parallel‐group RCT Study center: 1 (Refraction Clinic, Veterans General Hospital, Taipei, Taiwan) Number randomized: 247 children Study follow‐up: 1 year Exclusions and losses to follow‐up: 151 (61%) children were excluded or lost to follow‐up |
|
| Participants | Age: mean = 9 years (range 6 to 14 years) Gender: 118 boys, 129 girls Culture: children with simple myopia were randomly selected from clinic records Inclusion criteria: (1) age 6 to 14 years; (2) myopia with refractive error between ‐0.5 D and ‐4.0 D Exclusion criteria: (1) amblyopia or tropia; (2) cylinder refraction greater than 1.0 D |
|
| Interventions | Atropine: 1% atropine drops every other night; bifocal spectacles prescribed 2 weeks after treatment began Cyclopentolate: 1% cyclopentolate drops every night; single vision spectacles prescribed if necessary Saline control: normal saline eye drops every night; single vision spectacles prescribed if necessary |
|
| Outcomes |
Primary outcome:
Secondary outcomes:
Measurements taken at baseline and every 3 months for 1 year Note: baseline for atropine group was measured 2 weeks after treatment began Unit of analysis: right eyes only |
|
| Notes | Study dates: enrollment from July 1, 1985 to October 31, 1986 Trial registration: not reported Funding source: not reported Additional data: study author provided unpublished data via email correspondence |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Ophthalmic examinations were done by 3 doctors. Each doctor got a random table. Patients were assigned to 3 groups according to the sequence on the random table (via email communication with study author) |
| Allocation concealment (selection bias) | Unclear risk | Allocation was done via a random numbers table, but it is not clear whether the allocation sequence was concealed |
| Masking of participants (performance bias) | High risk | Masking of participants was not reported for the pharmaceutical agents; however, 1 group received bifocal spectacles rather than single vision spectacles; therefore this was not masked |
| Masking of outcome assessors (detection bias) Progression of myopia | Unclear risk | No details were given |
| Masking of outcome assessors (detection bias) Secondary outcomes | Unclear risk | "To avoid deviation during retinoscopy, all examinations were done by three doctors" |
| Masking of data analyzers | Unclear risk | No details were given |
| Incomplete outcome data (attrition bias) Incomplete outcome(s) data | High risk | "Patients who used the eye drops continuously for one year received another complete ophthalmologic examination...96 such patients were collected for evaluation, 32 in each group" Patients who discontinued using eye drops or who did not use them consistently were excluded from the study. Of the 247 children randomized in the study, 151 (61%) were not included in the analyses. The 96 patients analyzed included the first subset of children who followed the treatment protocol and were examined at 1 year (via email communication with study author) |
| Selective reporting (reporting bias) | High risk | Not all outcomes described in the methods section were reported (changes in vision, funduscopy, and IOP) |
| Other bias | High risk | "For statistical significance, each group should include at least 30 samples. Our aim was to evaluate the results after using the medication for 1 year. So, when we felt we had enough numbers of patients who had continuously used the eye drops for 1 year, we decided to analyze the data. It happened to be 32 in each group" (via email communication with study author) |
Yi 2015.
| Study characteristics | ||
| Methods | Study design: parallel‐group RCT Study center: 1 (The Third People’s Hospital of Chongqing City) Number randomized: 140 children Study follow‐up: 12 months Exclusions and losses to follow‐up: 6 (8%) in treatment group and 2 (3%) in control group withdrew from the study |
|
| Participants | Age: mean = 9.8 years Gender: 65 boys, 67 girls Culture: China Inclusion criteria: (1) children with low myopia: refractive error between ‐0.50 and ‐2.00 D in both eyes as measured by cycloplegic autorefraction; (2) normal binocular function and stereopsis; (3) normal intraocular pressure less than 21 mmHg; (4) willingness and ability to tolerate cycloplegia and mydriasis Exclusion criteria: (1) astigmatism more than ‐1.00 D; (2) other ocular disease, such as amblyopia, strabismus, congenital cataract, glaucoma, corneal scar, optic neuropathy, traumatic ocular injury, uveitis, or ocular tumor; (3) history of any ocular surgery; (4) any systemic disease or condition that could affect visual function and development, including diabetes mellitus and/or chromosome anomaly; (5) previous or current use of contact lenses, bifocals, progressive addition lenses, or other forms of treatment (including atropine) for myopia |
|
| Interventions | Atropine (n = 70): 1% atropine sulfate once nightly in both eyes Placebo (n = 70): vehicle eye drops (Tears Naturale Free; Alcon, Fort Worth, TX) once nightly in both eyes |
|
| Outcomes |
Primary outcomes:
Secondary outcomes:
Measurements taken at baseline and every 3 months up to 1 year Unit of analysis: individual (right eye) |
|
| Notes | Study dates: enrollment from January to October 2012 Trial registration: not reported Funding source: not reported Declarations of interest: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of randomization was not reported |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment before randomization was not reported |
| Masking of participants (performance bias) | High risk | "We could not make our study double‐masked. We had to inform subjects about dilation and cycloplegia from atropine at the beginning" |
| Masking of outcome assessors (detection bias) Progression of myopia | Low risk | "To minimize observational bias, both pupils of every child were dilated fully and checked by nurses before being examined by the study investigators, who were kept masked to the assigned trial medications" |
| Masking of outcome assessors (detection bias) Secondary outcomes | Low risk | "To minimize observational bias, both pupils of every child were dilated fully and checked by nurses before being examined by the study investigators, who were kept masked to the assigned trial medications" |
| Masking of data analyzers | Unclear risk | This was not reported |
| Incomplete outcome data (attrition bias) Incomplete outcome(s) data | Low risk | Eight (6%) of 140 children who dropped out were not included in the analysis |
| Selective reporting (reporting bias) | Low risk | Results were reported for outcomes described in the methods section of the paper |
| Other bias | Low risk | None was identified |
7‐mx: 7‐methylxanthine. AC/A: accommodative convergence (in prism diopters) to the stimulus/accommodation ratio. AL: axial length. BSCL: bifocal soft contact lens. D: diopters. DC: diopter cylinder. DF: dual focus. DISC: dual‐focus incorporated soft contact. IOP: intraocular pressure. logMAR: logarithm of the minimum angle of resolution. MZ: monozygotic. N/A: not applicable. OFG: ordinary frame glasses. PAL: progressive addition lens. PD: pupillary distance. PR: XXX. RCT: randomized controlled trial. RGPCL: rigid gas permeable contact lens. SA: XXX. SCL: soft contact lens. SER: XXX. SVL: single vision lens. SVSCL: single vision soft contact lens. VA: visual acuity. VT: vision training.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Abraham 1966 | Not randomized: case report |
| ACHIEVE Study 2008 | Not intended to control progression of myopia: glasses vs contacts for self‐esteem in schoolchildren |
| Aller 2008 | Interventional twin case series: included only 1 pair of twins: 1 randomized to wear bifocal SCLs and the other to wear SVSCLs for 1 year; both wore BSCLs for the second year |
| Andreo 1990 | Not randomized: not intended to control progression of myopia; participants older than 18 were included |
| ATOM 2 Study 2012 | Interventions not eligible: dosing study to compare doses of atropine, with no control group |
| Baldwin 1969 | Not randomized: patients selected treatment assignment |
| Baltimore Myopia Project 1946 | Interventions not eligible: vision training for myopia; interventions of vision training were not prespecified in the protocol |
| Baronet 1979 | Not randomized: retrospective review of patients treated with atropine at a medical practice with no comparison group |
| Bedrossian 1979 | Not randomized: method of allocation was not specified. Cross‐over study of atropine in 1 eye for 1 year, with the fellow eye serving as the control, then alternated treatment after each year for 4 years |
| Berkeley OK Study 1983 | Population not eligible: participants were 21 to 28 years old |
| Bier 1988 | Not randomized: sequential assignment to groups |
| Brodstein 1984 | Not randomized: "the lack of randomization permits a possibility for bias" |
| Chan 2014 | Interventional twin case series: included only 1 pair of twins: 1 randomized to wear orthokeratology lens and the other to wear SVLs for 2 years |
| Chen 2012 | Not randomized: allocation was done by parental decision |
| Chen 2014a | Not randomized: cohort study of children wearing SVLs with full correction or undercorrection |
| Chen 2016 | Not randomized: treatment group included participants who chose to wear orthokeratology lenses; controls included participants who had never worn orthokeratology lenses |
| Cho 2012 | Interventions not eligible: comparison of fenestrated orthokeratology lenses vs nonfenestrated orthokeratology lenses; interventions comparing types of orthokeratology lenses were not prespecified in the protocol |
| Cho 2017 | Interventions not eligible: comparison of continuing vs discontinuing orthokeratology wear after 2 years; interventions comparing length of orthokeratology wear were not prespecified in the protocol |
| Choi 2005 | Not randomized: study was reported only as a conference abstract and randomization was not specified ("We prescribed 1% atropine once a day with bifocal glasses to the treated group (41 patients) and prescribed only glasses to the control group (43 patients)") |
| Chou 1997 | Not randomized: allocation was by parental decision |
| Dumbleton 1999 | Interventions not eligible: lenses with different oxygen permeability; interventions comparing oxygen permeability not prespecified in the protocol |
| Dyer 1979 | Not randomized: case‐control study |
| Ebri 2007 | Not intended to control progression of myopia: cycloplegic effect and pupillary dilation outcomes, as well as cost‐effectiveness; follow‐up 3 days |
| Eissa 2018 | Interventions were not eligible |
| Filip 2000 | Population was not eligible: myopia progression in adults |
| Gimbel 1973 | Not randomized: comparison of patients vs an historical cohort |
| Goss 1984 | Not randomized: treatment group included patients with overcorrection; controls included random patients selected retrospectively |
| Grosvenor 1991 | Not randomized: historical control group |
| He 2016 | Not randomized: retrospective cohort study; comparison of orthokeratology lenses vs SVLs |
| He 2018 | Interventions were not eligible |
| Horner 1999 | Not intended to control progression of myopia: comparison of soft spherical contact lenses vs spectacles; SCLs not expected to slow myopia progression. In fact, the study was conducted because researchers believed that SCLs may increase myopia progression |
| Hosaka 1982 | Not randomized: interventional case series of children aged 6 to 14 years treated with labetalol ophthalmic solution |
| Hosaka 1988 | Not randomized: interventional case series |
| Hua 2017 | Interventions not eligible: cluster RCT of elevated light levels in classrooms to prevent myopia onset or progression; interventions of light levels were not prespecified in the protocol |
| Huffman 2002 | Not intended to control progression of myopia: aspheric vs spheric lenses; outcome to decrease spherical aberration; adults were included |
| Jiang 2018 | Not randomized |
| Kao 1988 | Not randomized: children were enrolled in 2 separate series of patients |
| Keller 1996 | Not randomized: all children wore RGPCLs |
| Kennedy 1995 | Not randomized: treatment was atropine; controls were patients matched by medical records |
| Khoo 1999 | Not randomized: study reported that "children were randomly selected from the various schools in Singapore. They were then randomly selected for contact lens wear" Children in the RGPCL cohort who completed 3 years of follow‐up were compared with a cohort of children who wore spectacles |
| Kubena 2002 | Not randomized: cohort study that compared spectacle lenses that filtered non‐visible light vs conventional spectacle lenses |
| Lakkis 2006 | Not intended to control progression of myopia: 2‐week randomized cross‐over trial to evaluate visual performance and satisfaction of clear and photochromic spectacle lenses in children aged 10 to 15 years wearing fully corrected spectacles |
| Lee 2016 | Interventions not eligible: dosing study conducted to compare 0.125% or 0.25% atropine; controls were patients who preferred SVLs |
| Leung 1999 | Not randomized: odd or even case numbers determined the 2 groups |
| Li 2005 | Not randomized: experimental group received progressive multifocal lenses; control group wore common glasses; participants were 6 to 23 years old |
| Liang 2008 | Interventions not eligible: RCT comparing atropine eye drops alone vs combined treatment with atropine and stimulation of the auricular acupoints in school‐aged children with myopia |
| Lu 2010 | Not randomized: case‐control study comparing myopic children treated with seasonal doses of atropine vs nonmyopic children |
| Ma 2014 | Interventions not eligible: cluster RCT with 3 groups: free spectacles provided in class; vouchers for free spectacles; and prescriptions for spectacles; interventions of accessibility to spectacles were not prespecified in the protocol |
| Mandell 1959 | Not randomized: historical cohort, including adults |
| Meythaler 1971 | Not randomized: interventional cases series (70 eyes in persons from 8 to 35 years of age were checked); 3 groups were based on age; youngest group was 8 to 19 years old |
| NCT00348166 | Not randomized |
| NCT03372551 | Wrong patient population |
| NCT03512626 | Wrong patient population |
| Neetens 1985 | Not randomized: control group consisted of participants who could not use bifocals |
| Nesterov 1990 | Not randomized: comparison of a group using cycloplegics and ocular hypotensives vs a reference group for progression of myopia |
| Oakley 1975 | Not randomized: control group consisted of children (or parents) who refused bifocals |
| Parker 1958 | Not randomized: comparison of author's practice vs other practices |
| Perrigin 1990 | Not randomized: treatment group was given silicone lenses; control consisted of an historical cohort |
| Pirenzepine 2003 | Not randomized: review of pirenzepine studies and mechanism of action |
| Plowright 2015 | Not intended to control progression of myopia: RCT to evaluate daily disposable contact lenses vs SVLs for 2 weeks |
| Pritchard 1999 | Not intended to control progression of myopia: extended wear for low Dk vs high Dk lenses in adults |
| Rah 2002 | Population not eligible: overnight orthokeratology in adults (LOOK study); not randomized |
| Rainey 2000 | Interventions not eligible: vision therapy vs control; interventions for vision training were not prespecified in the protocol |
| Ritchey 2005 | Population not eligible: included adults aged 18 and older (COLM study) |
| Sankaridurg 2003 | Not intended to control progression of myopia: RCT conducted to compare adverse events for SCLs vs SVLs (spectacles); participants were 16 to 35 years old |
| Santodomingo‐Rubido 2012 | Not randomized: allocation was done by parental decision |
| Savoliuk 1968 | Not randomized: comparison of groups using SVLs continuously or for distance‐use only vs no spectacles |
| Shen 2011 | Allocation method not clear, randomization not specified: compared groups using 0.25% atropine vs no atropine |
| Shimmyo 2003 | Allocation method not clear, randomization not specified: atropine vs control for 2 years |
| Shum 2003 | Not randomized: comparison of groups using orthokeratology vs no orthokeratology |
| SightGlass 2018 | Wrong patient population |
| SMART Study 2009 | Not randomized: comparison of groups using orthokeratology lenses vs daily wear silicone hydrogel SCLs |
| Soni 2006 | Not randomized: included adults |
| Stone 1976 | Not intended to control progression of myopia: study authors state that "the research team is not purposely attempting to flatten the cornea in order to arrest the myopia" |
| Sun 2007 | Not randomized: case‐control study of spectacle users vs controls |
| Syniuta 2001 | Not randomized: intervention group included patients whose parents requested treatment for myopic progression; control group comprised the next myopic child by alphabetical order after study child’s record number |
| Takano 1964 | Not randomized: cohort study comparing treatment with Mydrine (tropicamide + phenylephrine) eye drops with or without Neosynesin (phenylephrine) eye drops; included boys and girls with myopia ages 7 to 19 years; follow‐up was 20 days |
| Tan 2012 | Not randomized |
| Toki 1960 | Not randomized: cohort study of patients receiving 5% Neosynesin (phenylephrine) eye drops; included boys and girls with myopia ages 7 to 21 years; follow‐up was 14 to 28 days |
| Tokoro 1964 | Not randomized: nonrandomized study of treatment with Mydrine (tropicamide + phenylephrine) eye drops + 5% Neosynesin (phenylephrine) eye drops + low‐frequency electro stimulus in children ages 7 to 15 years; included children with hyperopia |
| Tokoro 1965 | Not randomized: retrospective cohort comparing full correction spectacles vs undercorrection (< ‐1 D) spectacles or full correction in case of need in children ages 7 to 14 years; included children with hyperopia |
| TO‐SEE Study 2013 | Not randomized: prospective cohort study of children wearing orthokeratology lenses vs SVLs |
| Xiao 2009 | Not randomized: observational study of 2 groups of children who wore RGPCLs vs spectacles |
| Yamada 2004 | Not randomized: review article with some cohort data on children with high myopia |
| Yamaji 1967 | Not randomized: observation of children treated with Mydrine‐M; no control group |
| Yang 2017 | Not intended to control progression of myopia: evaluated accommodative lag in groups using orthokeratology vs SVLs for 1 year |
| Yi 2011 | Interventions not eligible: RCT to evaluate near‐ and middle‐vision activities and outdoor activities in children with myopia; interventions of visual activities were not prespecified in the protocol |
| Young 1992 | Not intended to control progression of myopia: comparison of overnight lenses for 12 months in adults only |
| Zeng 2009 | Not intended to control progression of myopia: RCT to evaluate visual performance and satisfaction of ready‐made spectacles vs custom spectacles in Chinese school‐aged children with uncorrected refractive error |
| Zhou 2015 | Not intended to control progression of myopia: evaluated accommodative lag in groups using RGPCLs vs SVLs for 1 year |
| Zhou 2016 | Not randomized: 400 children wearing orthokeratology lenses or SVLs selected from patient records |
BSCL: bifocal soft contact lens. COLM: Comparison of Overnight Lens Modalities. Dk: oxygen permeability. LOOK: Lenses and Overnight Orthokeratology. RCT: randomized controlled trial. RGPCL: rigid gas permeable contact lens. SCL: soft contact lens. SVL: single vision lens. SVSCL: single vision soft contact lens.
Characteristics of studies awaiting classification [ordered by study ID]
Anderson 2016.
| Methods | Randomized parallel‐group design (participants were allowed to switch to intervention of their choice after 5 years) |
| Participants | Inclusion criteria: not reported Exclusion criteria: not reported |
| Interventions | Intervention: progressive addition lenses Comparison intervention: single vision lenses |
| Outcomes | Primary outcome: phoria magnitude Secondary outcomes: not reported Maximum follow‐up: 10 years |
| Notes | Study name: Evaluation of progressive addition lens wear and age‐related changes in phoria magnitude in myopic children |
Bakaraju 2015.
| Methods | Randomized parallel‐group design |
| Participants | Inclusion criteria: Chinese children, aged 8 to 13 years, with spherical equivalent refractive error between ‐0.75D and ‐3.50D Exclusion criteria: previous treatment for myopia and systemic or ocular disease |
| Interventions | Intervention: single vision control contact lenses Comparison intervention 1: extended depth of focus 1 prototype contact lenses Comparison intervention 2: extended depth of focus 2 prototype contact lenses |
| Outcomes | Primary outcome: cycloplegic autorefraction, axial length Secondary outcomes: not reported Maximum follow‐up: 3 years |
| Notes | Study name: Extended depth‐of‐focus contact lenses can slow the rate of progression of myopia |
BLINK Study 2017b.
| Methods | Not reported |
| Participants | Inclusion criteria: myopic children, aged 7 to 11, myopia (spherical component) of ‐0.75 D to ‐5.00 D (inclusive) and 1.00 D cylinder or less (corneal plane) Exclusion criteria: not reported |
| Interventions | Intervention: center‐distance soft multifocal contact lens with a +2.50 D add Comparison intervention: spectacle correction |
| Outcomes | Primary outcome: visual acuity Secondary outcomes: not reported Maximum follow‐up: not reported |
| Notes | Study name: Visual acuity and over‐refraction in myopic children fitted with soft multifocal contact lenses in the BLINK Study |
Cheung 2018.
| Methods | Observational |
| Participants | Inclusion criteria: not reported Exclusion criteria: not reported |
| Interventions | Intervention: orthokeratology lenses Comparison intervention: single‐vision spectacles |
| Outcomes | Primary outcome: endothelial cell density, coefficient of variation in cell size, hexagonality Secondary outcomes: not reported Maximum follow‐up: 2 years |
| Notes | Study name: Does a two‐year period of orthokeratology lead to changes in the endothelial morphology of children? |
ChiCTR1800018092.
| Methods | Randomized parallel‐group design |
| Participants | Inclusion criteria: children with myopia were included in the randomized control, with no gender limitation, aged 7‐12 years old, clear refractive media, equivalent spherical lens ≤‐5.00D, 40.00D≤ corneal base curvature <45.50D, and corneal astigmatism ≤1.50D Exclusion criteria: rule out basic eye diseases that may affect vision, corneal plasticizer and potion |
| Interventions | Intervention: orthokeratology glass Comparison intervention: 0.01% atropine eye drops once per night |
| Outcomes | Primary outcome: axial length Secondary outcomes: spherical equivalent, corneal curvature Maximum follow‐up: not reported |
| Notes | Study name: Comparison of myopia control effect between single use ortho‐k and combined with 0.01% atropine eye drops in children |
Diaz‐Llopis 2018.
| Methods | Randomized parallel‐group design |
| Participants | Inclusion criteria: aged 9 to 12 years, bilateral myopia of ‐0.5 to ‐2 D, less than 1.5 astigmatism Exclusion criteria: not reported |
| Interventions | Intervention: one drop of 0.01% atropine once a day in both eyes before going to bed Comparison intervention: no treatment |
| Outcomes | Primary outcome: progression of myopia, side effects Secondary outcomes: not reported Maximum follow‐up: 5 years |
| Notes | Study name: Superdiluted atropine at 0.01% reduces progression in children and adolescents. A 5 year study of safety and effectiveness |
EUCTR2016‐003340‐37‐IE.
| Methods | Randomized cross‐over design |
| Participants | Inclusion criteria: spherical equivalent refractive error of ‐1.0D or worse with myopia progression of at least ‐0.50DS over the last year, based on refractive or clinical evidence; astigmatism less than or equal to ‐2.50D and an intraocular difference in spherical equivalent <= 1D; corrected visual acuity must be better or equal to logMAR 0.2 in both eyes and difference between non‐cycloplegic and cycloplegic spherical refraction of less than 1.00 D; normal IOP (<= 21mmHg), normal ocular health and good general health with no history of cardiac/respiratory diseases; willingness to commit to the 2 year clinical trial as well as randomization to the placebo Exclusion criteria: ocular/systemic diseases/conditions affecting vision or refractive error; any ocular/systemic condition wherein atropine is contraindicated; known allergy to atropine, cyclopentolate hydrochloride and/or proxymetacaine hydrochloride; defective binocular vision, amblyopia or strabismus; any other conditions precluding adherence to the protocol including allergy to study eye drops (active agent or preservative); previous pharmaceutical or optical myopia control interventions; subjects (or parent/guardian) unable to provide written informed consent |
| Interventions | Intervention: atropine sulphate ophthalmic solution 0.01% Comparison intervention: placebo |
| Outcomes | Primary outcome: efficacy of treatment, the difference in QoL questionnaire scores between intervention and control groups, the occurrence of adverse events, proportion of participants needing bifocals, the drop‐out rate Secondary outcomes: not reported Maximum follow‐up: 24 months |
| Notes | Study name: Preventing the progression of shortsightedness in children using an eye drop called Atropine |
EUCTR2018‐001286‐16‐DK.
| Methods | Randomized parallel‐group design |
| Participants | Inclusion criteria: children aged =6 years <9 years: myopia =‐1 (spherical equivalent) in at least one eye; children aged =9 years <12 years: myopia =‐2 (spherical equivalent) in at least one eye; cylinder less than 1.5 diopters Exclusion criteria: myopia related to retinal dystrophies; collagen syndromes (Ehlers‐Danlos syndrome, Marfan syndrome and Stickler syndrome); other ocular pathology (e.g., amblyopia, strabismus); previous eye surgery; previous use of agents thought to affect myopia progression, e.g. atropine, pirenzepine or 7‐methylxanthine (metabolite of caffeine and theobromine) and orthokeratology contact lenses; known allergy to atropine or any of the contents of the trial medication (active and in‐active ingredients) used in the study; non‐compliance to eye examinations; serious systemic health troubles (e.g., cardiac or respiratory illness) and developmental disorders and delays |
| Interventions | Intervention 1: atropine 0.01% Comparison intervention 1: atropine 0.1% Comparison intervention 2: placebo eye drops |
| Outcomes | Primary outcome: axial length elongation; change in spherical equivalent Secondary outcomes: patient reported outcome; adverse effects and reactions; change in choroidal thickness; change in ocular biometry (i.e. keratometry, anterior chamber depth, lens thickness, vitreous axial distance); change in higher‐order aberrations Maximum follow‐up: 36 months |
| Notes | Study name: Low‐dose atropine for the prevention of nearsightedness in Danish children |
Jong 2015.
| Methods | Randomized parallel‐group design |
| Participants | Inclusion criteria: children aged between 8 to 13 years (spherical refractive error between ‐0.75 D to ‐5.00 D, cylinder ≤ ‐1.00 D) Exclusion criteria: previous myopia treatment and no systemic or ocular disease |
| Interventions | Intervention: novel, daily disposable extended depth of focus (EDOF) contact lenses: EDOF1 Comparison intervention 1: novel, daily disposable extended depth of focus (EDOF) contact lenses: EDOF2 Comparison intervention 2: single vision (SV) contact lenses |
| Outcomes | Primary outcome: rate of increase in axial length Secondary outcomes: not reported Maximum follow‐up: 6 months |
| Notes | Study name: A dose‐response relationship between duration of daily lens wear and reduction in rate of axial elongation |
Kinoshita 2017.
| Methods | Randomized parallel‐group design |
| Participants | Inclusion criteria: children aged 8 to 12 years with spherical equivalent refractions of ‐1.00 to ‐6.00 diopters (D) and astigmatism of ‐1.50 D or less Exclusion criteria: not reported |
| Interventions | Intervention: orthokeratology with 0.01% atropine Comparison intervention: orthokeratology alone |
| Outcomes | Primary outcome: axial length Secondary outcomes: not reported Maximum follow‐up: 1 year |
| Notes | Study name: Suppressive effect of combined treatment of orthokeratology and 0.01% atropine instillation on axial length elongation in childhood myopia |
Lam 2018.
| Methods | Randomized parallel‐group design |
| Participants | Inclusion criteria: healthy myopes, refractive errors between –4D to –5D sphere and astigmatism within –1.50D Exclusion criteria: long‐term contact lens wearers and individuals with a history of ocular diseases |
| Interventions | Intervention: orthokeratology lens in one eye Comparison intervention: conventional rigid gas permeable lens in the other eye |
| Outcomes | Primary outcome: corneal curvature, biomechanics, and thickness Secondary outcomes: not reported Maximum follow‐up: not reported |
| Notes | Study name: Influence of short‐term orthokeratology to corneal tangent modulus: A randomized study |
Maekawa 2016.
| Methods | Randomized parallel‐group design |
| Participants | Inclusion criteria: not reported Exclusion criteria: not reported |
| Interventions | Intervention: ortho‐k contact lenses made by Alpha Corp Comparison intervention: ortho‐k contact lenses made by Technopia Co., Ltd |
| Outcomes | Primary outcome: objective cycloplegic refraction, axial length Secondary outcomes: not reported Maximum follow‐up: 2 years and at 3‐weeks post discontinuance of the ortho‐k lens wear |
| Notes | Study name: Comparison of the correction effect to suppress the progression of myopia between two types of orthokeratology lenses |
NCT02055378.
| Methods | Randomized parallel‐group design |
| Participants | Inclusion criteria: children aged from 6 to 12 years with myopia, defined as spherical equivalent (SE) of ‐0.5 diopter (D) or less, were recruited from the outpatient clinics from January 2011 to June 2012 Exclusion criteria: abnormal IOP (>21 mmHg) at presentation, astigmatism or anisometropia of more than 1.5 D, amblyopia or strabismus, the presence of any related eyelid diseases, ocular diseases, or auricular diseases, the presence of hemostatic disorders or other related major systemic diseases, history of allergy to atropine, previous or current use of contact lenses, bifocals, progressive lenses, or other forms of treatment for myopia |
| Interventions | Intervention: topical 0.125% atropine with auricular acupoint stimulation Comparison intervention: topical 0.125% atropine |
| Outcomes | Primary outcome: the change in spherical equivalent Secondary outcomes: axial length (AL) elongation, anterior chamber depth (ACD) and intraocular pressure(IOP) Maximum follow‐up: 12 months |
| Notes | Study name: The effect of low‐concentration atropine combined with auricular acupoint stimulation in myopia control |
NCT02700139.
| Methods | Randomized parallel‐group design |
| Participants | Inclusion criteria: myopia between 0.75 ~ ‐ 4.50 D and with‐the‐rule astigmatism not more than 1.50 D; difference between eyes, no more than 1.25 spherical equivalent; best corrected visual acuity (VA) is equal to or better than 0.10 in logMAR scale (Snellen VA 6/7.5 or better); eyes straight at distance and near with best subjective correction; willing to be randomized and wear the study spectacles according to the instructions from practitioner; willing to come back for follow up; in the Optometry Clinic during the study period Exclusion criteria: abnormal ocular and general health; prior myopic treatment (e.g. refractive surgery and progressive lens wear for myopic control) before and during the study period; history of rigid contact lenses (including orthokeratology lenses) wearing; systemic condition which might affect refractive development (for example, Down syndrome, Marfan's syndrome); ocular conditions which might affect the refractive error (for example, cataract, ptosis) |
| Interventions | Intervention: aspheric lens Comparison intervention: single vision spheric/toric lens |
| Outcomes | Primary outcome: axial length Secondary outcomes: not reported Maximum follow‐up: 1 year |
| Notes | Study name: Shamir aspheric ophthalmic lenses (MyLens) for myopic control clinical trial |
NCT03519490.
| Methods | Randomized parallel‐group design |
| Participants | Inclusion criteria: myopia: ≥ 0.5 D in least myopic meridian, < 12.0 D in most myopic meridian);
Anisometropia (interocular difference in refractive error) ≤ 2D; astigmatism: ≤ 3D; myopia progression ≥ 0.5D in at least one eye based on available clinical records or based on habitual spectacle prescription; visual acuity: best corrected acuity of 20/20 or better in each eye; capable of proper handling, insertion and removal of hybrid contact lenses Exclusion criteria: ocular health: any pathology that may alter eye growth (e.g. history of retinal detachment & treatment for the same), and/or may adversely impact contact lens wear (e.g. chronic, poorly controlled allergic conjunctivitis) will be grounds for exclusion; strabismus, amblyopia; systemic disease that may affect vision, vision development or contact lens wear; chronic use of medications that may affect immunity, such as oral or topical corticosteroids rigid or hybrid contact lens wear within the preceding 3 months; prior ocular surgery, nursing or pregnant mothers; participants who cannot commit to the 24 month study period or who have a high likelihood of leaving the area within the 24 month study period |
| Interventions | Intervention: multifocal hybrid contact lens Comparison intervention: single vision hybrid contact lens |
| Outcomes | Primary outcome: myopia progress rate, axial length Secondary outcomes: subjective myopia progression rate, macular pigment optical density, tear film dynamics and meibomian gland health Maximum follow‐up: 24 months |
| Notes | Study name: Can distance center and near center multifocal contact lenses control myopia progression in children? |
Pärssinen 2017.
| Methods | Randomized parallel‐group design |
| Participants | Inclusion criteria: spherical refraction (SR) >−3 D, astigmatism ≥−2 D, spherical equivalent (SE) ≥−3 D Exclusion criteria: other eye diseases and previous glasses for myopia |
| Interventions | Intervention: continuous use spectacles Comparison intervention 1: only for distant use spectacles Comparison intervention 2: bifocal spectacles with a 1.75 D add |
| Outcomes | Primary outcome: anisometropia of spherical equivalent and astigmatism Secondary outcomes: spherical equivalent, corneal refractive power, anterior chamber depth, axial length Maximum follow‐up: 23 years |
| Notes | Study name: Anisometropia of spherical equivalent and astigmatism among myopes: a 23‐year follow‐up study of prevalence and changes from childhood to adulthood |
Ren 2017.
| Methods | Randomized parallel‐group design |
| Participants | Inclusion criteria: not reported Exclusion criteria: not reported |
| Interventions | Intervention: low concentration atropine Comparison intervention 1: orthokeratology Comparison intervention 2: spectacles |
| Outcomes | Primary outcome: refractive degree, ocular axial length Secondary outcomes: not reported Maximum follow‐up: not reported |
| Notes | Study name: Effects of low concentration atropine and orthokeratology on myopia prevention and control |
Sankaridurg 2017.
| Methods | Randomized parallel‐group design |
| Participants | Inclusion criteria: myopic children with cycloplegic spherical equivalents (SE) between ‐0.75 to ‐4.25D Exclusion criteria: not reported |
| Interventions | Intervention: control group wearing single‐vision, silicone hydrogel (SH) contact lens Comparison intervention 1: silicone hydrogel contact lens with a central treatment zone of relative +ve power of +1.00D combined with relative +ve powers of +2.50D Comparison intervention 2: silicone hydrogel contact lens with a central treatment zone of relative +ve power of +1.00D combined with relative +ve powers of +2.50D and +1.50D in the periphery Comparison intervention 3: hydrogel contact lens III Comparison intervention 4: hydrogel contact lens IV |
| Outcomes | Primary outcome: myopia progression Secondary outcomes: cycloplegic (1% tropicamide) autorefraction and axial length (AL) Maximum follow‐up: 12 months |
| Notes | Study name: Novel contact lenses designed to slow progress of myopia: 12 month results |
Tan 2019.
| Methods | Randomized parallel‐group design |
| Participants | Inclusion criteria: aged 6 to <11 years; Chinese ethnicity; myopia between 1.00–4.00 D; astigmatism (negative cylinder) not more than 2.50 D of axes 180 ± 30; astigmatism with other axes not more than 0.50 D; less than 1.00 D difference in spherical equivalent between the two eyes; best corrected logMAR visual acuity 0.10 or better in both eyes; symmetrical corneal topography with corneal toricity less than 2.00 D in either eye; normal ocular health other than myopia; agree to be randomized and to attend the scheduled and aftercare visits Exclusion criteria: contraindications to atropine: known allergies or cardiovascular disease, epilepsy; contraindications to contact lens wear and ortho‐k: history of ocular inflammation or infection, strabismus or amblyopia; history of myopia control treatment (e.g., soft or rigid contact lenses, bifocal or multifocal spectacles, atropine eye drops); systemic condition which might affect refractive development (e.g., Down syndrome, Marfan’s syndrome), or ocular conditions which might affect refractive error (e.g., cataract, ptosis) |
| Interventions | Intervention: combined atropine with orthokeratology Comparison intervention: orthokeratology alone |
| Outcomes | Primary outcome: lens performance, changes in refractive error, unaided vision, ocular adverse events, corneal staining, lens binding and centration, and axial length Secondary outcomes: not reported Maximum follow‐up: 2 years |
| Notes | Study name: Combined atropine with orthokeratology for myopia control: study design and preliminary results |
Tilia 2018.
| Methods | Randomized cross‐over design |
| Participants | Inclusion criteria: myopic; between 7 and 18 years of age; be correctable to at least 0.3 logMAR distance high‐contrast visual acuity (HCVA) at 6 m with spherical hydrogel contact lenses (power range ‐0.75 to ‐6.00 D); and have no ocular or systemic findings that would contraindicate contact lens wear Exclusion criteria: amblyopia or strabismus |
| Interventions | Intervention: single vision contact lens Comparison intervention 1: extended depth of focus contact lens low Comparison intervention 2: extended depth of focus contact lens high |
| Outcomes | Primary outcome: visual acuity, accommodative and binocular function, and objective static refraction Secondary outcomes: not reported Maximum follow‐up: one week |
| Notes | Study name: Vision performance and accommodative/binocular function in children wearing prototype extended depth‐of‐focus contact lenses |
Trier 2015.
| Methods | Randomized parallel‐group design |
| Participants | Inclusion criteria: not reported Exclusion criteria: not reported |
| Interventions | Intervention: 7‐methylxanthine Comparison intervention: placebo |
| Outcomes | Primary outcome: myopia progression, safety Secondary outcomes: not reported Maximum follow‐up: 8 years |
| Notes | Study name: 7‐methylxanthine treatment |
Wei 2017.
| Methods | Randomized parallel‐group design |
| Participants | Inclusion criteria: myopic children with the diopter of ‐0.50 to ‐6.00 D Exclusion criteria: not reported |
| Interventions | Intervention: orthokeratology Comparison intervention: spectacles |
| Outcomes | Primary outcome: ocular peripheral refraction, relative peripheral refraction Secondary outcomes: not reported Maximum follow‐up: not reported |
| Notes | Study name: A randomized controlled clinical trial on the effects of wearing orthokeratology and spectacles on ocular peripheral refraction in myopic children |
Wu 2018.
| Methods | Randomized parallel‐group design |
| Participants | Inclusion criteria: not reported Exclusion criteria: not reported |
| Interventions | Intervention: AC custom‐made orthokeratology lens Comparison intervention: standard orthokeratology lens |
| Outcomes | Primary outcome: uncorrected visual acuity (UCVA), corneal topography, and the complications of corneal epithelium Secondary outcomes: not reported Maximum follow‐up: 1 month |
| Notes | Study name: Effectiveness of AC custom‐made Ortho‐K lens |
Yam 2019.
| Methods | Randomized parallel‐group design |
| Participants | Inclusion criteria: aged 4 to 12 years with myopic refraction of at least 1.0 D in both eyes, astigmatism of less than 2.5 D, and documented myopic progression of at least 0.5 D in the past 1 year Exclusion criteria: ocular diseases (e.g., cataract, congenital retinal diseases, amblyopia, and strabismus), previous use of atropine or pirenzepine, or orthokeratology lens or other optical methods for myopia control, allergy to atropine, or systemic diseases (e.g., endocrine, cardiac, and respiratory diseases) |
| Interventions | Intervention: 0.05% atropine eye drops Comparison intervention 1: 0.025% atropine eye drops Comparison intervention 2: 0.01% atropine eye drops Comparison intervention 3: placebo eye drops |
| Outcomes | Primary outcome: spherical equivalent Secondary outcomes: axial length, accommodation amplitude, mesopic and photopic pupil sizes, distant best corrected visual acuity, near visual acuity Maximum follow‐up: 1 year |
| Notes | Study name: Low‐concentration atropine for myopia progression (LAMP) study: a randomized, double‐blinded, placebo‐controlled trial of 0.05%, 0.025%, and 0.01% atropine eye drops in myopia control |
Zhao 2017.
| Methods | Randomized parallel‐group design |
| Participants | Inclusion criteria: juveniles with ametropia and aged 11‐15 years Exclusion criteria: glaucoma, cataract, retinal detachment or denaturation, and other ocular diseases affecting vision |
| Interventions | Intervention: blue‐violet light filtering lenses Comparison intervention: ordinary aspherical lenses |
| Outcomes | Primary outcome: refractive power, axial length, contrast sensitivity (glare and non‐glare under bright and dark
conditions), accommodation, asthenopia, adverse reaction Secondary outcomes: not reported Maximum follow‐up: 1 year |
| Notes | Study name: Role of short‐wavelength filtering lenses in delaying myopia progression and amelioration of asthenopia in juveniles |
N/A: not applicable.
Characteristics of ongoing studies [ordered by study ID]
ACTRN12605000633684.
| Study name | Trial of an experimental soft contact lens designed to inhibit the progression of axial myopia in children |
| Methods | Randomized cross‐over design (within‐person study) |
| Participants |
Inclusion criteria: 40 children aged 11 to 14 years with progressing myopia, spherical equivalent refraction of ‐1.50 to ‐4.00, visual acuity of 6/6 or better Exclusion criteria: children with astigmatism > 0.75 D, anisometropia > 1.00 D, abnormal binocular vision, ocular pathology, systemic disease with ocular complications, active anterior surface disease that would preclude contact lens wear, inadequate fit of soft contact lenses |
| Interventions |
Intervention: frequent replacement soft contact lens that both corrects vision and simultaneously produces myopic retinal defocus Comparison intervention: standard single vision frequent replacement soft contact lens |
| Outcomes |
Primary outcome: myopia progression rate Secondary outcomes: refractive error, axial length Maximum follow‐up: 20 months |
| Starting date | November 2005 Estimated end date: not reported |
| Contact information | http://www.anzctr.org.au/ACTRN12605000633684.aspx |
| Notes |
ACTRN12608000566336.
| Study name | Myopia control lens efficacy trial |
| Methods | Randomized parallel‐group design |
| Participants |
Inclusion criteria: 300 children aged 6 to 12 years with spherical equivalent refractive error of –0.50 to ‐4.50 D, astigmatism of not more than ‐1.50 D, anisometropia of not more than ‐1.50 D in spherical or cylindrical error, best‐corrected visual acuity of at least 6/9 (20/30) in each eye, normal ocular health other than myopia, no prior use of bifocal or progressive lenses in the last 12 months, no rigid contact lenses or bifocal contact lens experience, willingness not to wear contact lenses, in satisfactory health, willingness and ability to tolerate cycloplegia, informed parental consent Exclusion criteria: no availability for follow‐up for at least 2 years, absence of parental consent to the random assignment of their child to 1 of 3 spectacle lens groups, any systemic condition that might affect refractive development or systemic disease that may affect vision or refractive error, previous use of contact lens/PALs or other treatment for myopia within the last 12 months, defective binocular function, amblyopia and or manifested squint, vestibular disorders or motor imbalance, any other conditions precluding adherence to the protocol |
| Interventions |
Intervention 1: binocular progressive 1.00 D addition lenses Intervention 2: binocular progressive 1.50 D addition lenses Comparison intervention: single vision binocular lens |
| Outcomes |
Primary outcomes: refractive error, axial length Secondary outcome: peripheral refractive error Maximum follow‐up: 24 months |
| Starting date | September 2008 Estimated end date: September 2009 |
| Contact information | https://www.anzctr.org.au/Trial/Registration/TrialReview.aspx?id=83124 |
| Notes |
ACTRN12611000499987.
| Study name | Duplex orthokeratology (DOK) and myopia progression in children |
| Methods | Randomized parallel‐group design |
| Participants |
Inclusion criteria: (1) 10 to 14 years of age; (2) spherical equivalent refractive error between ‐1.25 D and ‐4.00 D; (3) myopia progression of at least 0.50 D in previous 12 months; (4) astigmatism < 1.50 D; (5) anisometropia < 1.00 D; (6) best‐corrected visual acuity of 6/6 or better in both eyes; (7) good general and ocular health; (8) parents and child able to communicate in English Exclusion criteria: (1) recent rigid contact lens wear; (2) history of corneal surgery; (3) active eye disease including keratoconus; (4) severe dry eye symptoms; (5) systemic disease affecting visual acuity; (6) taking medication that could affect ocular health |
| Interventions |
Intervention: duplex (dual focus optic zone) orthokeratology lens in 1 eye (overnight wear) Intervention comparison: conventional orthokeratology lens in the other eye (overnight wear) Note: children were randomly assigned to wear the orthokeratology lens in the dominant eye or the nondominant eye |
| Outcomes |
Primary outcome: change in vitreous chamber depth, measured by non‐contact Optical Low‐Coherence Reflectometry (Lenstar LS 900, Haag Streit, Switzerland) Secondary outcomes: magnitude of central and peripheral refractive error, amplitude of accommodation, contrast sensitivity |
| Starting date | May 2011 Estimated end date: not reported |
| Contact information | John Phillips, PhD, or Martin Loertscher Department of Optometry and Vision Science The University of Auckland 85 Park Road Grafton, Auckland 1023 email: j.phillips@auckland.ac.nz; m.loertscher@auckland.ac.nz http://www.anzctr.org.au/ACTRN12611000499987.aspx |
| Notes |
ACTRN12611000582954.
| Study name | Myopia control with progressive spectacle lenses trial (MCPAL‐3) |
| Methods | Randomized parallel‐group design |
| Participants |
Inclusion criteria: 167 children aged 7 to 12 years with refractive error between ‐1.00 D and ‐4.50 D, best‐corrected visual acuity of at least 6/9 or 20/30 in each eye, and anisometropia not more than ‐1.50 D, astigmatism not greater than ‐1.50 D, no other ocular conditions, no history of using bifocal or progressive lenses in 12 months preceding study, and tolerant to cycloplegia, with parental consent Exclusion criteria: systemic condition affecting vision or refractive errors, history of contact lens or other treatment for myopia in the preceding 12 months, impaired binocular function, history of amblyopia, manifest squint, vestibular disorders or motor imbalance, other conditions that prevent adherence to protocol |
| Interventions |
Intervention: progressive addition lenses Comparison intervention: single vision lenses |
| Outcomes |
Primary outcome: progression in refractive error (spherical equivalent using cycloplegic autorefraction) Secondary outcome: axial length Maximum follow‐up: 24 months |
| Starting date | June 2011 Date of last participant enrolment: June 2012 |
| Contact information | https://www.anzctr.org.au/Trial/Registration/TrialReview.aspx?id=343027 |
| Notes |
ACTRN12611001148965.
| Study name | To determine the rate of refractive error change in children wearing multifocal soft contact lens as compared to those wearing single vision soft contact lenses |
| Methods | Randomized parallel‐group design |
| Participants |
Inclusion criteria: 40 children aged 8 to 14 years with cycloplegic autorefraction: sphere ‐0.50 D to ‐4.00 D; cylinder 0 to ‐0.75 D; best‐corrected visual acuity 6/9 or better; ability to safely wear contact lenses; distortion‐free keratometric readings; no active corneal infection, inflammation, or infection of the anterior chamber, eye disease, injury or abnormality of the cornea; conjunctiva or eyelids affecting wearing of contact lenses; no previous ocular surgery; no severe insufficiency of lacrimal secretion; no evidence of corneal hypoesthesia; no systemic disease or use of medications that may affect the eye or produce an adverse response by the wearing of contact lenses Exclusion criteria: binocular vision problems, strabismus, amblyopia, external ocular problems that may impact lens fit (i.e. lid ptosis, chalazia, swollen lids) |
| Interventions |
Intervention: multifocal soft contact lenses Comparison intervention: single vision soft contact lenses |
| Outcomes |
Primary outcome: rate of myopia progression Secondary outcomes: fitting characteristics of, and ocular response to, soft contact lenses Maximum follow‐up: 3 years |
| Starting date | November 2005 Estimated end date: not reported |
| Contact information | https://www.anzctr.org.au/Trial/Registration/TrialReview.aspx?id=347659 |
| Notes |
ACTRN12617000598381.
| Study name | A pilot study to evaluate the effectiveness of daily 0.01% atropine eye drop therapy in modifying the progression of myopia, in Australian children |
| Methods | Randomized parallel‐group design |
| Participants |
Inclusion criteria: aged 6 to 16 years, myopia with spherical equivalent refractive error greater or equal to ‐1.5 D in each eye, documented myopic progression of greater than or equal to ‐0.5 D over the previous 12 months in either eye, astigmatism less than ‐1.5 D, intraocular difference in spherical equivalent < 1 D, corrected visual acuity greater than logMar 0.2, normal IOP, normal ocular health, no history of cardiac/respiratory disease, willingness and ability to provide details of parents' country of origin, ability to provide appropriate parental/carer consent Exclusion criteria: astigmatism of 1.5 D or more; 1 D or more anisometropia; severe developmental delay (inability to participate in subjective refraction of testing); ocular comorbidities such as glaucoma, aphakia, pseudophakia, uveitis, keratoconus, or connective tissue disease (e.g. Marfan syndrome, vitreoretinal dystrophies); severe ocular surface disease; previous atropine treatment for amblyopia at any time in the past |
| Interventions |
Intervention: 0.01% atropine eye drops Comparison intervention: placebo eye drops |
| Outcomes |
Primary outcome: mean change in spherical equivalent refractive error Secondary outcomes: amplitude of accommodation, choroidal thickness, corneal curvature and axial length, Wilkins Rate of Reading test comparison, intraocular pressure, stereovision assessment, quality of life Maximum follow‐up: 24 months |
| Starting date | January 2017 Estimated end date: December 2020 |
| Contact information | https://www.anzctr.org.au/Trial/Registration/TrialReview.aspx?id=372668 |
| Notes |
ACTRN12618000242224.
| Study name | Prospective, contralateral, randomized, cross‐over dispensing clinical trial to compare the myopia progression rate between a myopia control contact lens and single vision contact lenses |
| Methods | Randomized cross‐over design (within‐person study) |
| Participants |
Inclusion criteria: 45 participants aged 6 to 17 years, spherical equivalent ‐0.75 D to ‐3.50 D, cylinder no more than ‐1.00 D, anisometropia ≤ 0.75 D, vision correctable to 6/9.5 or better Exclusion criteria: preexisting ocular irritation precluding contact lens fitting, systemic or ocular condition or injury, corneal refractive surgery, keratoconus, allergy to cyclopentolate, astigmatism > 1.00 D in either eye, strabismus, amblyopia, any ocular or systemic disease associated with myopia, retinopathy of prematurity, current orthoptic treatment or vision training, eye injury or surgery within 12 weeks before enrollment, atropine treatment for myopia control, previously worn bifocal or progressive spectacles or antimyopia contact or orthokeratology lenses, anisometropic by > 0.75 D |
| Interventions |
Intervention: experimental contact lens (lens type not reported) Comparison intervention: single vision contact lens |
| Outcomes |
Primary outcome: change in cycloplegic autorefraction spherical equivalent Secondary outcomes: change in axial length Maximum follow‐up: 12 months |
| Starting date | January 2018 Estimated end date: not reported |
| Contact information | https://www.anzctr.org.au/Trial/Registration/TrialReview.aspx?id=374450 |
| Notes |
BLINK Study 2017a.
| Study name | Bifocal lenses in nearsighted kids (BLINK) |
| Methods | Randomized parallel‐group design |
| Participants |
Inclusion criteria: 7 to 11 years of age, inclusive; at baseline examination, ‐0.75 to ‐5.00 D, inclusive; spherical component; cycloplegic autorefraction ≤ 1.00 DC; cycloplegic autorefraction; ≥ 2.00 D difference between sphere components of the 2 eyes (anisometropia); cycloplegic autorefraction; 0.1 logMAR or better best‐corrected visual acuity in each eye; 0.1 logMAR or better visual acuity OU distance and near with a +2.50 D add contact lens; +2.50 D add lens provides adequate fit with respect to movement and centration Exclusion criteria: eye disease or binocular vision problems (e.g. strabismus, amblyopia, oculomotor nerve palsies, corneal disease); systemic disease that may affect vision, vision development, or contact lens wear (e.g. diabetes, Down syndrome); previous gas permeable, soft bifocal, or orthokeratology contact lens wear or bifocal/PAL spectacle wear (longer than 1 month of wear); chronic use of medication that may affect immunity |
| Interventions |
Intervention 1: Biofinity Multifocal D +1.50 add Intervention 2: Biofinity Multifocal D +2.50 add Comparison intervention: Biofinity |
| Outcomes |
Primary outcomes: ocular shape change, eye growth, peripheral defocus, axial length Secondary outcomes: not reported Maximum follow‐up: 3 years |
| Starting date | September 2014 Estimated end date: July 2019 |
| Contact information | https://clinicaltrials.gov/ct2/show/NCT02255474 |
| Notes | Study author |
ChiCTR1800016504.
| Study name | Clinical effect of vitamin B12 eye drops on myopia in children |
| Methods | Randomized parallel‐group design |
| Participants |
Inclusion criteria: age 6 to 12 years; the refractive power of the eyes after dilation is between ‐1.0 and ‐3.0d; no refractive error (binocular diopter within ‐1.0d); binocular astigmatism < ‐1.5d; far vision of the eyes can be corrected to at least 0.8; the intraocular pressure is lower than 21mmHg; no allergy to dilated pupils; no corneal plasticizer has been used to treat myopia; no amblyopia, squint, etc. Exclusion criteria: failing to meet the inclusion criteria; unwilling to participate in this study |
| Interventions |
Intervention: vitamin B12 eye drop Comparison intervention: no intervention |
| Outcomes |
Primary outcome: diopter Secondary outcomes: not reported Maximum follow‐up: 12 months |
| Starting date | July 2018 Estimated end date: June 2019 |
| Contact information | http://www.chictr.org.cn/showprojen.aspx?proj=26962 |
| Notes |
ChiCTR1800017683.
| Study name | A double‐masked comparative study of peripheral defocus lenses |
| Methods | Randomized parallel‐group design |
| Participants |
Inclusion criteria: age 8 to 13 years; spherical refractive error of ‐0.75 to ‐4.75 D in each eye, as measured by cycloplegic autorefraction; astigmatism of not more than 1.50 D; anisometropia of not more than 1.00 D; best corrected visual acuity of equal or better than 0.05 LogMAR (≥ 0.9 as Snellen) Exclusion criteria: history of PALs or bifocal use and no prior use of contact lenses; strabismus by cover test at near and distance; ocular disease with full ophthalmic examination, such as retinal disease, cataract and ptosis; systemic or neurodevelopmental conditions; ocular or systemic medicine, which might affect myopia progression or visual acuity through known effects on retina, accommodation or significant elevation of intraocular pressure |
| Interventions |
Intervention 1: "defocus lenses" Intervention 2: "defocus lenses" Comparison intervention: single vision lenses |
| Outcomes |
Primary outcome: refractive power; axial Length; contrast visual acuity Secondary outcomes: not reported Maximum follow‐up: not reported |
| Starting date | July 2018 Estimated end date: November 2020 |
| Contact information | http://www.chictr.org.cn/hvshowproject.aspx?id=13585 |
| Notes |
ChiCTR1900021316.
| Study name | Clinical observation for auricular acupoint stimulation combined with low‐concentration atropine in myopia control and its effect on accommodative microfluctuations |
| Methods | Randomized parallel‐group design |
| Participants |
Inclusion criteria: age 6 to 11 years children; male or female; with simple myopia; 0.5% tocarbamide mydriatic optometry: +0.5DS to‐6.0DS; corneal topography Kmax: 42‐44D; astigmatism of less than 1.50D, anisometropia of less than 1.00D, intraocular pressure of 10 to 21mmHg; patient with good compliance who volunteers to join the subject and signs informed consent Exclusion criteria: patient with other ocular diseases (e.g., cataract, congenital retinal disease, strabismus, amblyopia) or systemic diseases; patient with active eye lesions or undergo eye surgery; allergy to atropine;patient whose skin of the auricular acupoint area is broken or patient who has allergy to auricular plaster; guardians do not hold reasonable expectations |
| Interventions |
Intervention: 0.01% atropine eyedrops combined with auricular acupoint stimulation Comparison intervention: 0.01% atropine eyedrops |
| Outcomes |
Primary outcome: uncorrected distance visual acuity; diopter; axial length Secondary outcomes: anterior chamber depth; accommodation amplitude; accommodative microfluctuations Maximum follow‐up: not reported |
| Starting date | February 2019 Estimated end date: May 2020 |
| Contact information | http://www.chictr.org.cn/hvshowproject.aspx?id=15141 |
| Notes |
ChiCTR‐INR‐17013794.
| Study name | The effectiveness safety of corneal contact lens used to correct myopia: a multi‐center, randomized, open and positive parallel control clinical trial |
| Methods | Randomized parallel‐group design |
| Participants |
Inclusion criteria: 41 patients aged 8 to 40 years with myopia ≤ 4.00 D, astigmatism with‐the‐rule of < 1.75 D, and astigmatism against‐the‐rule of < 1.00 D; best‐corrected visual acuity not less than 20/20; corneal curvature at 40.00 D to 46.00 D; diopter stay stability before trial; has not worn hard contact lenses in the past 2 months Exclusion criteria: systemic disease that causes low immunity or effects on corneal shape; corneal abnormality; corneal surgery; history of corneal or ocular trauma; hypocorneal sensory impairment; intraocular surgery; fundus lesions; ocular disease; pregnant or lactating; use of drugs that cause dry eyes or affect corneal curvature; allergy to contact lens or its solution; pupil diameter > 6.2 mm |
| Interventions |
Intervention: corneal contact lens 2 (not specified) Comparison intervention: corneal contact lens 2 (not specified) |
| Outcomes |
Primary outcome: visual acuity Secondary outcomes: not reported Maximum follow‐up: not reported |
| Starting date | May 2017 Estimated end date: December 2018 |
| Contact information | http://www.chictr.org.cn/showprojen.aspx?proj=23702 |
| Notes |
ChiCTR‐INR‐17013853.
| Study name | Effects of orthokeratology and combined with 0.01% atropine on myopia control: a multicenter comparative study |
| Methods | Randomized parallel‐group design |
| Participants |
Inclusion criteria: 216 children aged 8 to 15 years; spherical degree without dilation ≥ ‐1.00 D and ≤ ‐5.50 D; equivalent spherical degree ≥ ‐1.00 D and ≤ ‐5.50 D; astigmatism ≤ ‐1.50 D; best‐corrected visual acuity ≥ 1.0 D; no strabismus; no contact lens wearing history; no history of myopia control by optical or drug route; no active inflammation or ocular surface disease; no serious ocular appendage lesions and eye organic disease; cooperation with researchers Exclusion criteria: systemic connective tissue disease and autoimmune disease; history of ocular trauma or surgery; history of severe ocular infection |
| Interventions |
Intervention 1: orthokeratology at night Intervention 2: orthokeratology at night and 0.01% atropine eye drops before sleep Comparison intervention: single vision spectacles |
| Outcomes |
Primary outcomes: axial length, refraction, eyesight Secondary outcomes: IOP, corneal topography Maximum follow‐up: 12 months |
| Starting date | December 2017 Estimated end date: June 2019 |
| Contact information | http://www.chictr.org.cn/showprojen.aspx?proj=22940 |
| Notes |
ChiCTR‐IOR‐17010432.
| Study name | Myopia progression with invisible round segment bifocal spectacle lenses |
| Methods | Randomized parallel‐group design |
| Participants |
Inclusion criteria: best‐corrected vision of 6/9.5 or better with spectacles in each eye; normal ocular health; ability to comply with trial protocol; parental ability to understand English and Mandarin and parental consent Exclusion criteria: history of allergy to topical anesthetics; strabismus; eye surgery; ocular or systemic condition affecting vision; ocular injury; use of bifocals, spectacles, orthokeratology, vision training, orthoptic training, or conditions that affect ability to wear spectacles |
| Interventions |
Intervention: bifocal spectacles Comparison intervention: single vision spectacles |
| Outcomes |
Primary outcome: spherical equivalent Secondary outcome: axial length Maximum follow‐up: not reported |
| Starting date | February 2017 Estimated end date: September 2018 |
| Contact information | http://www.chictr.org.cn/showproj.aspx?proj=17727 |
| Notes |
ChiCTR‐IOR‐17011993.
| Study name | Prospective, masked, contralateral, randomized, cross‐over dispensing clinical trial to compare the myopia progression rate between myopia control contact lenses and single vision contact lenses |
| Methods | Randomized cross‐over design |
| Participants |
Inclusion criteria: aged between 7 years and 13 years (7 years and 13 years inclusive); spherical component ‐0.75 D to ‐3.50 D with cylinder no more than ‐0.75 D; anisometropia ≤ 0.75 D; informed consent; parent or guardian who is able to read and comprehend Mandarin and give informed consent as demonstrated by signing a record of informed consent by both parent/guardian and participant; ocular health findings considered to be normal and that would not prevent patient from safely wearing contact lenses; vision correctable to 6/9.5 or better in each eye with study contact lenses Exclusion criteria: preexisting ocular irritation that would preclude contact lens fitting; any systemic or ocular condition or ocular injury that may preclude safe wearing of contact lenses; having undergone corneal refractive surgery; at baseline, astigmatism more than 0.75 D in either eye; past strabismus and/or current ongoing amblyopia; any ocular, systemic, or other condition or disease with possible associations with myopia or affecting refractive development; current orthoptic treatment or vision training; eye injury or surgery within 12 weeks immediately before enrollment for this trial; having undergone atropine treatment for myopia control, worn bifocal or progressive addition spectacles or antimyopia contact lenses previously; having worn orthokeratology lenses previously; requiring anticholinergic medication for gastrointestinal or other conditions; at baseline, anisometropic by more than 0.75 D |
| Interventions |
Intervention 1: single vision contact lenses in both eyes Intervention 2: myopia control contact lens in 1 eye, and single vision contact lens in the other eye; contact lenses swapped between eyes after 6 months Comparison intervention: myopia control contact lens in 1 eye, and single vision contact lens in the other eye; contact lenses swapped between eyes after 6 months |
| Outcomes |
Primary outcome: spherical equivalent, axial length Secondary outcomes: not reported Maximum follow‐up: not reported |
| Starting date | Not reported Estimated end date: not reported |
| Contact information | http://www.chictr.org.cn/showprojen.aspx?proj=20301 |
| Notes |
ChiCTR‐IPD‐16008844.
| Study name | Clinical study of low‐concentration atropine in controlling child myopia |
| Methods | Randomized parallel‐group design |
| Participants |
Inclusion criteria: 400 children aged 6 to 12 years; myopia spherical equivalent degree: ‐1.25 to ‐6.0; astigmatism less than 2.0; distance corrected visual acuity greater than or equal to 0.8, without significant skew and other eye disease; no ocular inflammation; no history of ocular trauma; no history of ocular surgery; no systemic and ocular implement qualitative sex pathological change Exclusion criteria: congenital myopia and pathological myopia; premature and low birth weight myopia patients, with no other related myopia drugs and training method in the past 6 months |
| Interventions |
Intervention 1: 0.005% concentration atropine Intervention 2: 0.01% concentration atropine Intervention 3: 0.02% concentration atropine Intervention 4: 0.02% concentration atropine, once every 2 days Comparison intervention: spectacles |
| Outcomes |
Primary outcomes: "myopia degree" Secondary outcome: not reported Maximum follow‐up: not reported |
| Starting date | July 2016 Estimated end date: July 2020 |
| Contact information | http://www.chictr.org.cn/com/25/hvshowproject.aspx?id=11127 |
| Notes |
ChiCTR‐TRC‐07000029.
| Study name | Double‐blinded, randomized controlled trial about the influence of new lenses on the progress of children's myopia |
| Methods | Randomized parallel‐group design |
| Participants |
Inclusion criteria: 200 children aged 6 to 16 years; degree of myopia > ‐0.50 D and < ‐4.50 D; astigmatism degree < ‐1.50 D; binocular anisometropic degree < 1 D; healthy ocular region; visual acuity can be corrected to 6/9 (20/30) or higher Exclusion criteria: strabismus or amblyopia; history of allergy to tropicamide; any ophthalmopathy, previous ophthalmic surgery, systemic disease that may be related to myopia; using anticholinergic drugs; taking part in other myopia‐controlled study; previous wearing of orthokeratology lenses in the last 2 weeks; accepted or are participating in orthophoria treatment or vision training |
| Interventions |
Intervention 1: type A lenses Intervention 2: type B lenses Intervention 3: type C lenses Comparison intervention: routine lenses |
| Outcomes |
Primary outcomes: axial length Secondary outcome: "diopter" Maximum follow‐up: not reported |
| Starting date | October 2007 Estimated end date: November 2009 |
| Contact information | http://www.chictr.org.cn/showproj.aspx?proj=9496 |
| Notes |
ChiCTR‐TRC‐07000044.
| Study name | Clinical randomized controlled trial of progressive addition lenses on control of myopia in Chinese adolescents |
| Methods | Randomized parallel‐group design |
| Participants |
Inclusion criteria: 178 adolescents aged 7 to 18 years; computer optometry after cycloplegia; binocular myopia; spherical equivalent degree between ‐0.75 and ‐3.00 D; astigmatism degree less than ‐1.50 D; binocular anisometropic degree less than 1.00 D; bilateral corrected visual acuity more than 1.0; normal intraocular pressure: binocular intraocular pressure less than 21 mmHg, and difference less than 2 mmHg; no history of wearing contact lenses, bifocals, or multifocal lenses; term infants; birth weight more than 1250 g; agree to wear lenses and follow up for more than 2 years; understand the study objective and accept the randomized allocation Exclusion criteria: manifest strabismus or other ophthalmopathy; systematic disease; use of drugs that may influence the refractive status; myopia degree of either parent more than 3 D; use of contact lenses or other myopia treatment methods in the study |
| Interventions |
Intervention: gradual focal lens Comparison intervention: routine single lens |
| Outcomes |
Primary outcomes: myopic degree, eyeball biotest Secondary outcome: heterophoria Maximum follow‐up: not reported |
| Starting date | July 2004 Estimated end date: May 2007 |
| Contact information | http://www.chictr.org.cn/showproj.aspx?proj=9481 |
| Notes |
ChiCTR‐TRC‐09000476.
| Study name | Novel spectacle lenses vs single vision spectacle lenses on progression of myopia in children: a randomized clinical trial |
| Methods | Randomized parallel‐group design |
| Participants |
Inclusion criteria: children aged 6 to 12 years with spherical equivalent refraction between ‐0.75 D and ‐3.50 D; astigmatism less than or equal to ‐1.50 D; best‐corrected vision of at least 6/9.5 with spectacles; ability to comply with study protocol; normal ocular health Exclusion criteria: anisometropia less than or equal to 1.00 D; history of allergy to topical anesthetics; strabismus; eye surgery; ocular or systemic conditions affecting vision; ocular injury; use of bifocals, spectacles, orthokeratology, vision training, orthoptic training, or conditions that affect ability to wear spectacles; concurrent participation in another clinical trial |
| Interventions |
Intervention: not reported (“Iteration E”) Intervention: not reported (“Iteration G”) Intervention: not reported (“Iteration F”) Intervention: not reported (“Iteration H”) Comparison intervention: single vision spectacle lenses |
| Outcomes |
Primary outcomes: cycloplegic autorefraction Secondary outcome: not reported Maximum follow‐up: not reported |
| Starting date | August 2009 Estimated end date: December 2011 |
| Contact information | http://chictr.org.cn/showprojen.aspx?proj=9058 |
| Notes |
ChiCTR‐TRC‐10000914.
| Study name | Progression of refractive error in myopic Chinese children wearing commercially available single vision spectacles |
| Methods | Randomized parallel‐group design |
| Participants |
Inclusion criteria: children aged 7 to 14 years; spherical equivalent refraction between ‐0.50 D and ‐3.50 D; astigmatism less than or equal to 0.75 D; best‐corrected vision in each eye of at least 6/9.5; ability to comply with protocol; parental ability to comprehend Mandarin; parental ability to consent Exclusion criteria: anisometropia not greater than 1.50 D; prior use of atropine for myopia control; prior use of bifocal or progressive addition spectacles or concurrent use of orthokeratology contact lenses in the previous 12 months; prior eye surgery or ocular trauma; history of ocular or systematic condition that affects refractive development |
| Interventions |
Intervention: spherical profile spectacle lenses Comparison intervention: aspheric front surface spectacle lenses |
| Outcomes |
Primary outcomes: spherical equivalent refraction, axial length Secondary outcome: not reported Maximum follow‐up: not reported |
| Starting date | July 2010 Estimated end date: September 2013 |
| Contact information | http://www.chictr.org.cn/showprojen.aspx?proj=8624 |
| Notes |
ChiCTR‐TRC‐11001463.
| Study name | Efficacy of MyoVision spectacle lenses for slowing the progression of myopia |
| Methods | Randomized parallel‐group design |
| Participants |
Inclusion criteria: 200 children aged 6 to 12 years; myopic; spherical component ‐0.75 D to ‐3.50 D with astigmatism no more than ‐1.50 D; having at least 1 parent who is myopic; willingness to comply with wearing and visit schedule; having normal ocular health findings; having vision correctable to 6/9.5 or better in each eye with spectacles Exclusion criteria: allergy to tropicamide or topical anaesthetics; anisometropic by more than 1.00 D; strabismus or amblyopia; previous eye surgery; ocular or systemic disease with possible associations with myopia; any ocular injury or condition of the cornea or conjunctiva or eyelids; having worn bifocals or MyoVision spectacles in the last 12 months; having worn orthokeratology or bifocal contact lenses in the last 12 months; current orthoptic treatment or vision training |
| Interventions |
Intervention: MyoVision spectacles Comparison intervention: single vision spectacles |
| Outcomes |
Primary outcome: myopia progression Secondary outcome: axial length Maximum follow‐up: not reported |
| Starting date | August 2011 Estimated end date: January 2014 |
| Contact information | http://www.chictr.org.cn/hvshowproject.aspx?id=1096 |
| Notes |
ChiCTR‐TRC‐11001746.
| Study name | Assessment of myopia progression rates in children wearing either a multifocal center near or single vision soft contact lens |
| Methods | Randomized parallel‐group design |
| Participants |
Inclusion criteria: 100 children aged 10 to 17 years; Chinese ethnicity; myopic (short‐sighted) up to ‐8.00 D of spherical equivalent; willingness to comply with wearing and clinical trial visit schedule as directed by the investigator; having ocular health findings considered to be “normal” and that would prevent the patient from safely wearing contact lenses; having distance vision correctable to 6/9.5 or better in each eye with study contact lenses Exclusion criteria: preexisting ocular irritation, injury, or condition; any systemic disease that adversely affects ocular health; eye surgery within 12 weeks immediately before enrollment for this trial; previous corneal refractive surgery; keratoconus; known allergy to, or history of, intolerance to tropicamide or topical anaesthetics; past strabismus and/or amblyopia; any ocular, systemic, or other condition or disease with possible associations with myopia or affecting refractive development; current orthoptic treatment or vision training; having undergone atropine treatment for myopia control; having worn bifocal or progressive addition spectacles in the previous 12 months; having worn orthokeratology lenses in the previous 12 months; requiring anticholinergic medication for gastrointestinal or other conditions; pregnant or lactating females |
| Interventions |
Intervention 1: multifocal silicone hydrogel contact lens Intervention 2: spherical silicone hydrogel contact lens |
| Outcomes |
Primary outcomes: cycloplegic autorefraction, axial length Secondary outcomes: not reported Maximum follow‐up: not reported |
| Starting date | December 2011 Estimated end date: December 2015 |
| Contact information | http://www.chictr.org.cn/hvshowproject.aspx?id=1766 |
| Notes |
ChiCTR‐TRC‐13003396.
| Study name | Myopia progression with sedentary use, small segment, concentric bifocals |
| Methods | Randomized parallel‐group design |
| Participants |
Inclusion criteria: children aged 6 to 12 years, with spherical equivalent of ‐0.75 D to ‐3.50 D; astigmatism not greater than ‐1.50 D; normal ocular health; parental willingness to comply with the protocol; ability to consent Exclusion criteria: anisometropia less than or equal to 1.00 D; history of allergy to topical anesthetics; strabismus; eye surgery; ocular or systemic conditions affecting vision; ocular injury; use of bifocals, spectacles, orthokeratology, vision training, orthoptic training, or condition that affects ability to wear spectacles; concurrent participation in another clinical trial |
| Interventions |
Intervention: intermittent alternate use of spectacles with concentric bifocal lenses and single vision lenses Comparison intervention: single vision lens spectacles |
| Outcomes |
Primary outcome: change in spherical equivalent Secondary outcome: change in axial length Maximum follow‐up: not reported |
| Starting date | August 2013 Estimated end date: March 2015 |
| Contact information | http://www.chictr.org.cn/hvshowproject.aspx?id=6324 |
| Notes |
ChiCTR‐TRC‐13004032.
| Study name | Chinese University Low dose Atropine for Myopia Progression Study (CU‐LAMP) |
| Methods | Randomized parallel‐group design |
| Participants |
Inclusion criteria: age 4 to 12 years; myopia: SE ‐1 to ‐10 D; astigmatism: < 2.5 D; anisometropia: < 2.0 D; myopia progression > 1 D for BE in one year; informed parental consent Exclusion criteria: ophthalmic diseases other than refractive errors; previous use of treatment of atropine; allergy or intolerance to atropine; inability to attend regular follow up assessment |
| Interventions |
Intervention 1: 0.05% atropine eye drops Intervention 2: 0.025% atropine eye drops Intervention 3: 0.01% atropine eye drops Comparison intervention: 0.9% normal saline eye drops |
| Outcomes |
Primary outcome: spherical equivalent refraction (cycloplegic refraction); axial length Secondary outcomes: safety variable: best corrected visual acuity, pupil size, intraocular pressure Maximum follow‐up: not reported |
| Starting date | January 2014 Estimated end date: not reported |
| Contact information | http://www.chictr.org.cn/hvshowproject.aspx?id=14749 |
| Notes |
ChiCTR‐TRC‐14004227.
| Study name | Assessment rate of progression of myopia with contact lenses in Chinese children |
| Methods | Randomized parallel‐group design |
| Participants |
Inclusion criteria: 450 children aged 8 to 12 years; Chinese ethnicity; myopic (short‐sighted); ‐0.75 D to ‐3.50 D of cycloplegic spherical equivalent with astigmatism no more than 0.75 D; preferably progressive myopia; ocular health findings considered to be “normal”; vision correctable to 6/9.5 or better in each eye with study contact lenses Exclusion criteria: preexisting ocular irritation that would preclude contact lens fitting; any systemic or ocular condition or ocular injury that may preclude safe wearing of contact lenses; having undergone corneal refractive surgery; keratoconus; allergy to or history of intolerance to tropicamide or topical anesthetics; astigmatism more than 0.75 D in either eye; past strabismus and/or current ongoing amblyopia; any ocular, systemic, or other condition or disease with possible associations with myopia or affecting refractive development; eye injury or surgery within 12 weeks immediately before enrollment for this trial; having undergone atropine treatment for myopia control; having worn bifocal or progressive addition spectacles or antimyopia contact lenses previously; having worn orthokeratology lenses previously; requiring anticholinergic medication for gastrointestinal or other conditions; anisometropic by more than 1.50 D; current enrollment in another clinical trial/research project |
| Interventions |
Intervention 1: Clariti contact lenses Intervention 2: Aquamax contact lenses |
| Outcomes |
Primary outcome: myopia progression Secondary outcomes: not reported Maximum follow‐up: not reported |
| Starting date | February 5, 2014 Estimated end date: October 30, 2017 |
| Contact information | http://www.chictr.org.cn/hvshowproject.aspx?id=8971 |
| Notes |
ChiCTR‐TRC‐14004990.
| Study name | Low‐concentration atropine to slow myopic progression in children |
| Methods | Randomized parallel‐group design |
| Participants |
Inclusion criteria: 100 children aged 8 to 12 years with myopia of spherical equivalent ‐1 D to ‐6 D; astigmatism less than 1.5 D; anisometropia less than 2D; best‐corrected visual acuity larger than 0.8; intraocular pressure less than 21 mmHg; myopia progression more than 0.5 D in 1 year Exclusion criteria: ophthalmic disease other than refractive error or systematic disease; previous use of treatment of atropine, RGP, or ortho‐k; allergy or intolerance to atropine or tropicamide |
| Interventions |
Intervention: 0.01% atropine eye drops Comparison intervention: placebo eye drops |
| Outcomes |
Primary outcome: refraction Secondary outcomes: axial length, pupil size, residue accommodation Maximum follow‐up: not reported |
| Starting date | July 2014 Estimated end date: not reported |
| Contact information | http://www.chictr.org.cn/showproj.aspx?proj=4584 |
| Notes |
CTRI/2016/11/007450.
| Study name | Atropine eye drops to decrease myopia progression in children |
| Methods | Randomized parallel‐group design |
| Participants |
Inclusion criteria: 40 children aged 6 to 12 years; refractive error of spherical equivalent between ‐2 D and ‐6 D in each eye; distance vision correctable to logMAR 0.2 or better in both eyes; normal ocular health other than myopia; informed consent; willingness to follow up Exclusion criteria: astigmatism more than ‐1.5 D; amblyopia; strabismus; allergy to atropine or homatropine; previous or concurrent use of contact lenses, bifocals, progressive addition lenses, or other forms of treatment for myopia; history of cardiac, neurological, or significant respiratory disease; unwillingness to give consent/follow‐up |
| Interventions |
Intervention 1: 0.01% atropine eye drop Comparison intervention: 0.5% carboxymethylcellulose eye drop |
| Outcomes |
Primary outcome: myopia progression Secondary outcomes: side effects Maximum follow‐up: 1 year |
| Starting date | January 2016 Estimated end date: not reported |
| Contact information | http://ctri.nic.in/Clinicaltrials/pdf_generate.php?trialid=15817&EncHid=&modid=&compid=%27,%2715817det%27 |
| Notes |
IRCT20100414003714N3.
| Study name | Study of the effect of atropine eye drops with concentration of 0.1% & 0.01% and placebo in natural course of myopia progression in children 6 to 18 years old |
| Methods | Randomized parallel‐group design |
| Participants |
Inclusion criteria: aged 6 to 18 years; myopia or astigmatism (2 to 6 diopters); no amblyopia Exclusion criteria: strabismus |
| Interventions |
Intervention 1: 0.1% atropine eye drops for 12 months Intervention 2: 0.11% atropine eye drops for 12 months Comparison intervention: artificial eye drops for 12 months |
| Outcomes |
Primary outcomes: percentage of myopic power, axial length changes Secondary outcomes: not reported Maximum follow‐up: 6 months |
| Starting date | June 2018 Estimated end date: December 2019 |
| Contact information | http://en.irct.ir/trial/31944 |
| Notes |
IRCT20180216038747N1.
| Study name | Controlling myopia progression |
| Methods | Not reported |
| Participants |
Inclusion criteria: myopia ‐0.50 D to ‐6.00 D; astigmatism ≤ 0.75 D Exclusion criteria: myopic children with any ocular disease such as cataract, glaucoma, uveitis, strabismus; history of trauma; history of any ocular surgery systemic disease |
| Interventions |
Intervention 1: 0.01% atropine eye drops for 1 year Intervention 2: 0.02% atropine eye drops for 1 year Comparison intervention: artificial tear drops for 1 year |
| Outcomes |
Primary outcomes: axial length of the eye, accommodation amplitude, pupil size Secondary outcomes: not reported Maximum follow‐up: 12 months |
| Starting date | April 2018 Estimated end date: May 2019 |
| Contact information | https://www.irct.ir/trial/30096 |
| Notes |
ISRCTN36732601.
| Study name | Efficacy, safety, and mechanisms of atropine eye drops in slowing the progression of shortsightedness (myopia) in children |
| Methods | Randomized cross‐over design (within‐person study) |
| Participants |
Inclusion criteria: 250 children aged 6 to 16 years; myopia of ‐1.0 D or worse in each eye; astigmatism refractive error less than ‐1.50 D; progressive myopia of at least ‐0.50 D over the last year; intraocular difference in spherical difference equal to or less than 1.00 D; corrected visual acuity better than or equal to logMAR 0.2 in both eyes; normal IOP; normal ocular health Exclusion criteria: ocular or systemic disease affecting vision; allergy to study‐related drugs; defective binocular vision; previous pharmaceutical or optical myopia control interventions |
| Interventions |
Intervention: 0.01% atropine eye drops Comparison intervention: placebo eye drops |
| Outcomes |
Primary outcome: spherical equivalent refraction Secondary outcomes: axial length, off‐axis refraction, ocular growth, visual performance, ocular function, quality of life, adverse effects Maximum follow‐up: 24 months |
| Starting date | October 2017 Estimated end date: May 2023 |
| Contact information | http://www.isrctn.com/ISRCTN36732601 |
| Notes |
JPRN‐UMIN000005054.
| Study name | Clinical trial to evaluate effect of spectacle lens that reduces myopia progression |
| Methods | Randomized parallel‐group design |
| Participants |
Inclusion criteria: 6 to 12 years of age; myopic refractive error between ‐1.50 D and ‐4.50 D; astigmatism < 1.5 D; best‐corrected visual acuity 1.0 or better; father or mother with myopia Exclusion criteria: strabismus; having worn bifocals or progressive addition lenses in previous year; history of orthokeratology lens wear; prior participation in myopia trials; any eye disease other than myopia |
| Interventions |
Intervention: eyeglasses that reduce myopic progression Control: normal eyeglasses |
| Outcomes | Not reported |
| Starting date | February 2011 Estimated end date: not reported |
| Contact information | Takeshi Morimoto Department of Applied Visual Science Osaka University School of Medicine 2‐2 Yamadaoka, Suita, Osaka, Japan email: takeshi.morimoto@ophthal.med.osaka‐u.ac.jp http://www.umin.ac.jp/ctr/index.htm |
| Notes |
JPRN‐UMIN000007989.
| Study name | Clinical trial to prevent myopia progression by progressive additional soft contact lens compared with monofocal soft contact lens in children |
| Methods | Randomized cross‐over design |
| Participants |
Inclusion criteria: 20 children age 6 to 16 years; refractive error ‐0.75 D to ‐3.5 D; corrected visual acuity by spherical Spectacle lens: better than (0.7) Exclusion criteria: anisometropia greater than 1.0 D; amblyopia; strabismus |
| Interventions |
Intervention: wearing progressive additional soft contact lens Comparison intervention: wearing monofocal soft contact lens |
| Outcomes |
Primary outcome: ocular refraction Secondary outcome: axial length Maximum follow‐up: not reported |
| Starting date | January 2011 Estimated end date: not reported |
| Contact information | https://upload.umin.ac.jp/cgi-open-bin/ctr/ctr.cgi?function=brows&action=brows&recptno=R000009401&type=summary&language=E |
| Notes |
JPRN‐UMIN000013698.
| Study name | Examination of the nearsighted progress depression effect of the low‐concentrated atropine in the Japanese primary school child |
| Methods | Randomized parallel‐group design |
| Participants |
Inclusion criteria: 90 children aged 6 to 12 years with no eye disease except refractive error Exclusion criteria: children with contact lens; history of myopia progress suppression treatment |
| Interventions |
Intervention 1: 0.01% atropine eye drops Intervention 2: 0.025% cyclopentolate eye drops Comparison intervention: raw diet instillation |
| Outcomes |
Primary outcomes: refractive error, axial length Secondary outcomes: not reported Maximum follow‐up: not reported |
| Starting date | March 2014 Estimated end date: August 2017 |
| Contact information | https://upload.umin.ac.jp/cgi‐open‐bin/ctr/ctr.cgi?function=brows&action=brows&type=summary&recptno=R000015991&language=E |
| Notes |
JPRN‐UMIN000014362.
| Study name | Examination of suppressive effect by combined treatment of orthokeratology and atropine 0.01% ophthalmic solution on myopia progression |
| Methods | Randomized parallel‐group design |
| Participants |
Inclusion criteria: cycloplegic spherical equivalent refractive error of ‐1.00 to ‐6.00 D in both eyes; astigmatism of less than 1.50 D in both eyes; anisometropia of less than 1.50 D; best corrected visual acuity of more than 1.0 in both eyes Exclusion criteria: eye disorders such as strabismus and amblyopia; systemic disorders such as cardiac or respiratory illness; birth weight of less than 1500 g; history of hypersensitivity to atropine 5) using of orthokeratology and/or atropine ophthalmic solutions |
| Interventions |
Intervention: orthokeratology contact lens Comparison intervention: atropine 0.01% ophthalmic solution |
| Outcomes |
Primary outcomes: axial length Secondary outcomes: corneal endothelial cell density; corneal endothelial cell density Maximum follow‐up: 2 years |
| Starting date | June 2014 Estimated end date: March 2019 |
| Contact information | https://rctportal.niph.go.jp/en/detail?trial_id=UMIN000014362 |
| Notes |
JPRN‐UMIN000018041.
| Study name | The efficacy of 0.01% atropine ophthalmic solution for controlling the progression of childhood myopia (ATOM‐J Study) |
| Methods | Randomized parallel‐group design |
| Participants |
Inclusion criteria: 180 children aged 6 to 12 years; decrease in visual within the past year; cycloplegic objective spherical equivalent of ‐1.00 D to ‐6.00 D in each eye; anisometropia within 1.50 D; astigmatism within ± 1.50 D; corrected visual acuity of at least 1.0; no intraocular pressure abnormalities; capable of undergoing cycloplegia Exclusion criteria: abnormal visual function; amblyopia or manifest strabismus; difference in objective spherical equivalent with and without cycloplegia > 1.00 D in each eye; ocular disorders other than myopia; ocular or systemic disorders that potentially affect myopia or refractive power; previous treatment for myopia including atropine therapy, contact lenses, bifocal lenses, or progressive lenses with atropine therapy (does not apply to children who discontinued 0.4% tropicamide ophthalmic solution at least 3 months previously); history of cardiovascular or respiratory disease; children who have received pharmacotherapy for asthma in the past year; allergy to atropine, cyclopentolate, or benzalkonium; children who cannot instill medication into the eye, requiring contact lenses, bifocal lenses, or progressive lenses during the clinical study period |
| Interventions |
Intervention: 0.01% atropine ophthalmic solution Comparison intervention: placebo ophthalmic solution |
| Outcomes |
Primary outcome: change in objective spherical equivalent Secondary outcome: none reported Maximum follow‐up: 24 months |
| Starting date | July 2015 Estimated end date: not reported |
| Contact information | http://apps.who.int/trialsearch/Trial2.aspx?TrialID=JPRN‐UMIN000018041 |
| Notes |
JPRN‐UMIN000019237.
| Study name | Effect of dual‐focus soft contact lens wear on myopia progression |
| Methods | Randomized parallel‐group design |
| Participants |
Inclusion criteria: 28 children aged 10 to 14 years; no previous wearing of contact lenses; ‐1.0 D to ‐6.0 D refraction in each eye under nonaccommodative palsy; total astigmatism diopter within ‐1.5 D in each eye; corrected visual acuity > 1.0 D in each eye; no eye misalignment; not a premature infant; no ocular or systemic maldevelopment; no drug use; ability to wear contact lens for 1 week Exclusion criteria: as deemed appropriate by study investigators |
| Interventions |
Intervention: bifocal contact lenses Comparison intervention: spectacles |
| Outcomes |
Primary outcomes: refractivity, optic axis length Secondary outcomes: not reported Maximum follow‐up: not reported |
| Starting date | May 2015 Estimated end date: not reported |
| Contact information | https://rctportal.niph.go.jp/en/detail?trial_id=UMIN000019237 |
| Notes |
JPRN‐UMIN000023386.
| Study name | Clinical trial on the use of outdoor environment glasses for a suppressive effect on myopia progression |
| Methods | Randomized parallel‐group design |
| Participants |
Inclusion criteria: 140 children, aged 6 to 12 years; paralysis of accommodation in both eyes; spherical equivalent of each is between ‐1.50 D and 4.50 D; at least 1 parent who has myopia; no eye disease other than refractive error Exclusion criteria: history of wearing bifocal or progressive power lenses; history of wearing orthokeratology lenses; unequal parallax exceeding 1.50 D; astigmatism exceeding 1.50 D; overt strabismus; received refractive surgery in the past; keratoconus or herpes conjunctivitis; papillary proliferation; participating in other similar clinical research |
| Interventions |
Intervention: wearing outdoor environment glasses Comparison intervention: wearing normal glasses |
| Outcomes |
Primary outcome: change in axial length Secondary outcome: none reported Maximum follow‐up: 24 months |
| Starting date | July 2016 Estimated end date: not reported |
| Contact information | https://upload.umin.ac.jp/cgi‐open‐bin/ctr_e/ctr_view.cgi?recptno=R000026874 |
| Notes |
JPRN‐UMIN000027940.
| Study name | Clinical study on the effect of multifocal contact lens on myopia progression in myopia school children |
| Methods | Randomized parallel‐group design |
| Participants |
Inclusion criteria: 100 children aged 6 to 12 years; moderate myopia (objective equivalent spherical power ‐1.00 D to ‐6.00 D) Exclusion criteria: anisometropia; astigmatism beyond 1.5 D |
| Interventions |
Intervention: multifocal contact Comparison intervention: normal contact |
| Outcomes |
Primary outcome: refractive power change Secondary outcome: change in axial length Maximum follow‐up: not reported |
| Starting date | August 2017 Estimated end date: not reported |
| Contact information | https://upload.umin.ac.jp/cgi‐open‐bin/ctr/ctr_view.cgi?recptno=R000032004 |
| Notes |
Kinoshita 2018.
| Study name | Additive effects of orthokeratology and atropine 0.01% ophthalmic solution in slowing axial elongation in children with myopia: first year results |
| Methods | Randomized parallel‐group design |
| Participants |
Inclusion criteria: aged 8 to 12; spherical equivalent refractive error of ‐1.00 to ‐6.00 diopters Exclusion criteria: not reported |
| Interventions |
Intervention: orthokeratology (OK) and atropine 0.01% ophthalmic solution Comparison intervention: orthokeratology (OK) |
| Outcomes |
Primary outcome: axial length Secondary outcomes: not reported Maximum follow‐up: one year |
| Starting date | Not reported Estimated end date: not reported |
| Contact information | Nozomi Kinoshita, Department of Ophthalmology, Saitama Medical Center, Jichi Medical University, 1‐847 Amanuma‐cho, Omiya‐ku, Saitama‐shi, Saitama, 330‐8503, Japan. Email: nozomik@omiya.jichi.ac.jp |
| Notes |
Li 2013.
| Study name | The full correction and undercorrection of myopia evaluation trial (FUMET) |
| Methods | Randomized parallel‐group design |
| Participants | Inclusion criteria: 7 to 15 years of age; 6/6 or better in each eye; spherical error between ‐1.5 and ‐6.0 D; astigmatism below 1.5 D in each eye; anisometropia below 1.0 D between the 2 eyes; no history of contact lens use, strabismus, amblyopia, or other ocular and systematic disease that influences refractive growth Exclusion criteria: inability to live close to study center for 2 years; inability to cooperate with examinations or surveys; allergy to mydriatic drugs; use of other treatments to prevent myopia progression |
| Interventions | Intervention: full correction Intervention comparison: undercorrection (blurred by +0.5 D) |
| Outcomes |
Primary outcomes: change in cycloplegic autorefraction; change in axial length after 2 years Secondary outcomes: not specified Maximum follow‐up: 2 years |
| Starting date | November 2010 Estimated end date: January 2013 |
| Contact information | Professor Ning‐Li Wang, Beijing Tongren Eye Center, Beijing Tongren Hospital, Capital Medical University, Beijing 100730, China. Email: wningli@vip.163.com |
| Notes | Registration number ChiCTR‐TRC‐10001122 Funding source: grants from “Major State Basic Research Development Program of China (‘973’ Program, 2011CB504601) of the Ministry of Science and Technology”; “Major International (Regional) Joint Research Project (81120108807) of the National Natural Science Foundation of China”; “China Postdoctoral Science Foundation (20110490247)”; Research Foundation of Beijing Tongren Hospital Affiliated to Capital Medical University (2012‐YJJ‐019)” |
MASS 2018.
| Study name | MiSight Assessment Study Spain (MASS) |
| Methods | Randomized parallel‐group design |
| Participants |
Inclusion criteria: aged 8 to 12 with myopia (‐0.75 to ‐4.00 D sphere) and astigmatism (< ‐1.00 D cylinder) Exclusion criteria: current or prior contact lenses wear; current or prior use of bifocals, progressive addition lenses, atropine, pirenzepine, or any other myopia control treatment; regular use of ocular medications and artificial tears; current use of systemic medications, which may significantly affect contact lens wear, tear film production, pupil size, accommodation, or refractive state; known allergy to fluorescein, benoxinate, proparacaine, or tropicamide; history of corneal hypoesthesia, corneal ulcer, corneal infiltrates, ocular viral or fungal infection, or other recurrent ocular infection; strabismus by cover test at far (4 m) or near (40 cm); wearing distance correction; systemic or ocular disease affecting ocular health; keratoconus or an irregular cornea; CCLRU grade ≥ 2 for any given anterior segment ocular clinical signs; having pathological myopia; connective tissue disorder |
| Interventions |
Intervention: lens study group (MiSight) Comparison intervention: control group (single vision) |
| Outcomes |
Primary outcomes: visual acuity, subjective refraction Secondary outcomes: axial length, anterior chamber, corneal power, cycloplegic autorefraction Maximum follow‐up: 24 months |
| Starting date | September 2013 Estimated end date: June 2016 |
| Contact information | Alicia Ruiz‐Pomeda, Department of Pharmacy, Biotechnology, Optics and Optometry, European University of Madrid, C/Tajo s/n, Villaviciosa de Odón, 28670, Madrid, Spain. Email: alicia.ruiz@universidadeuropea.es. |
| Notes |
NCT00214487.
| Study name | Bifocal soft contact lenses and their effect on myopia progression in children and adolescents |
| Methods | Randomized parallel‐group design |
| Participants |
Inclusion criteria: myopia between ‐0.50 and ‐6.00; eso fixation disparity at 33 cm with distance correction; astigmatism 1.00 or less; ability to wear soft contact lenses Exclusion criteria: presence of ocular disease preventing wear of contacts; pregnancy or nursing; use of certain medications |
| Interventions |
Intervention: bifocal contact lenses Comparison intervention: single vision soft contact lenses |
| Outcomes |
Primary outcomes: cycloplegic autorefraction, cycloplegic subjective refraction, axial length Secondary outcomes: keratometric changes, manifest refraction Maximum follow‐up: 1 year |
| Starting date | October 2003 Estimated end date: March 2006 |
| Contact information | https://clinicaltrials.gov/ct2/show/record/NCT00214487 |
| Notes |
NCT00627874.
| Study name | Trial of myopia prevention using +3 D lenses (PLS) |
| Methods | Randomized parallel‐group design |
| Participants |
Inclusion criteria: 1200 children (age reported), with juvenile‐onset myopia Exclusion criteria: hyperopia > +2.0 D; high myopia > ‐6.0 D; astigmatism > 1.5 D; anisometropia > 1.5 D; strabismus and amblyopia; any ocular, systemic, or neurodevelopmental conditions that could influence refractive development; chronic medication use that might affect myopia progression or visual acuity; already receiving other treatment for progressing myopia |
| Interventions |
Intervention: +3 D lenses Comparison intervention: not reported |
| Outcomes |
Primary outcome: axial length of eyes Secondary outcome: autorefraction Maximum follow‐up: not reported |
| Starting date | April 2010 Estimated end date: April 2012 |
| Contact information | https://clinicaltrials.gov/ct2/show/NCT00627874 |
| Notes |
NCT00762970.
| Study name | Controlling my;opia progression with soft contact lenses |
| Methods | Randomized parallel‐group design |
| Participants |
Inclusion criteria: myopic subjects between 8 and 12 years of age; best sphere contact lens correction must lie between ‐0.75 D (best of the 2 eyes) and ‐5.00 D (worst of the 2 eyes); astigmatism must be less than or equal to 1.00 D; 1.00 D or less difference in spherical equivalent between the 2 eyes; best‐corrected visual acuity of 0.8 + 2 (20/25 + 2); spherical equivalent refraction visual acuity of 0.820/25 or better in both eyes; at least 8 D of accommodation Exclusion criteria: any ocular or systemic allergy or disease that may interfere with contact lens wear; systemic disease or autoimmune disease or use of medication (e.g. antihistamine) that may interfere with lens wear; clinically significant (grade 3 or 4) abnormality of the cornea that may contraindicate contact lens wear; clinically significant (grade 3 or 4) tarsal abnormalities or bulbar injection; any ocular infection; any corneal distortion; any infectious disease (e.g. hepatitis, tuberculosis) or immunosuppressive disease (e.g. HIV); diabetes; anisometropia greater than 1.00 D; astigmatism greater than 1.00 D in either eye; eye injury or surgery within 8 weeks immediately before enrollment for this study; previous refractive surgery; rigid contact lens wear; orthokeratology; keratoconus or other corneal irregularity in either eye; aphakia; strabismus; central corneal scar or pupil/lid abnormality in either eye; contraindications to contact lens; surgically altered eyes; ocular infection of any type; ocular inflammation; anterior chamber angle grade 2 or narrower |
| Interventions |
Intervention 1: soft contact lens, Test Lens 1 Intervention 2: soft contact lens, Test Lens 2 Comparison intervention: spectacle lenses |
| Outcomes |
Primary outcomes: spherical equivalent refraction, axial length Secondary outcomes: not reported Maximum follow‐up: 2 years |
| Starting date | April 2007 Estimated end date: February 2010 |
| Contact information | https://clinicaltrials.gov/ct2/show/NCT00762970 |
| Notes |
NCT01704729.
| Study name | The children's WEAR trial (phases 1 & 2) |
| Methods | Randomized parallel‐group design |
| Participants |
Inclusion criteria: aged 12 to 17 years; ≤ ‐1.00 D of myopic refractive error in each eye, with uncorrected vision ≤ 6/12 in at least 1 eye thought to be due to refractive error Exclusion criteria: significant strabismus or vision abnormality; vision deficiency |
| Interventions |
Intervention 1: noncycloplegic self‐refraction and conventional glasses Intervention 2: cycloplegic subjective refraction by experienced optometrist and conventional glasses Intervention 3: cycloplegic subjective refraction by rural refractionist program and conventional glasses Comparison intervention: cycloplegic subjective refraction by experienced optometrist and ready‐made glasses |
| Outcomes |
Primary outcome: visual acuity Secondary outcomes: visual functioning, frequency of glasses‐wear, accuracy of spectacles, value and satisfaction Maximum follow‐up: 2 months |
| Starting date | September 2012 Estimated end date: January 2013 |
| Contact information | https://clinicaltrials.gov/ct2/show/NCT01704729 |
| Notes |
NCT01729208.
| Study name | An evaluation of the effectiveness of dual focus soft contact lenses in slowing myopia progression |
| Methods | Randomized parallel‐group design |
| Participants |
Inclusion criteria: 300 children aged 8 to 12 years; best‐corrected visual acuity by manifest refraction of +0.10 logMAR; spherical equivalent refractive error between =0.75 and ‐4.00 D inclusive; astigmatism < ‐0.75 D; anisometropia < 1.00 D; possess wearable and visually functional eyeglasses; agree to wear assigned contact lenses for a minimum of 10 hours per day at least 6 days per week, for the duration of the 3‐year study Exclusion criteria: previously wore or currently wears contact lenses or rigid gas permeable contact lenses, including orthokeratology lenses; currently or within 30 days before this study has been an active participant in another clinical study; current or prior use of bifocals, progressive addition lenses, atropine, pirenzepine, or any other myopia control treatment; regular use of ocular medications, artificial tears, or wetting agents; current use of systemic medications that may significantly affect contact lens wear, tear film production, pupil size, accommodation, or refractive state; allergy to fluorescein, benoxinate, proparacaine, or tropicamide; strabismus; any ocular, systemic, or neurodevelopmental condition that could influence refractive development |
| Interventions |
Intervention: dual focus soft contact lens Comparison Intervention: single vision soft contact lens |
| Outcomes |
Primary outcomes: change in refractive error relative to baseline, change in axial length relative to baseline Secondary outcomes: incidence of adverse events Maximum follow‐up: 3 years |
| Starting date | November 2012 Estimated end date: May 2019 |
| Contact information | https://clinicaltrials.gov/ct2/show/NCT01729208 |
| Notes |
NCT01787760.
| Study name | Controlling myopia progression with soft contact lenses |
| Methods | Randomized parallel‐group design |
| Participants |
Inclusion criteria: myopic subjects between 8 and 12 years of age; best sphere contact lens correction must lie between ‐0.75 D (best of the 2 eyes) and ‐5.00 D (worst of the 2 eyes); astigmatism must be less than or equal to 1.00 D; 1.00 D or less difference in spherical equivalent between the 2 eyes; best‐corrected visual acuity of 0.8 + 2 (20/25 + 2); spherical equivalent refraction visual acuity of 0.820/25 or better in both eyes; at least 8 D of accommodation Exclusion criteria: any ocular or systemic allergy or disease that may interfere with contact lens wear; systemic disease or autoimmune disease or use of medication (e.g. antihistamine) that may interfere with lens wear; clinically significant (grade 3 or 4) abnormality of the cornea, which may contraindicate contact lens wear; clinically significant (grade 3 or 4) tarsal abnormalities or bulbar injection; any ocular infection; any corneal distortion; any infectious disease (e.g. hepatitis, tuberculosis) or immunosuppressive disease (e.g. HIV); diabetes; anisometropia greater than 1.00 D; astigmatism greater than 1.00 D in either eye; eye injury or surgery within 8 weeks immediately before enrollment for this study; previous refractive surgery; rigid contact lens wear; orthokeratology; keratoconus or other corneal irregularity in either eye; aphakia; strabismus; central corneal scar or pupil/lid abnormality in either eye; contraindications to contact lens; surgically altered eyes; ocular infection of any type; ocular inflammation; anterior chamber angle grade 2 or narrower |
| Interventions |
Intervention 1: soft contact lens, Test Lens B Intervention 2: soft contact lens, Test Lens C Comparison intervention: spectacle lenses |
| Outcomes |
Primary outcomes: spherical equivalent refraction, axial length Secondary outcomes: not reported Maximum follow‐up: 3 years |
| Starting date | April 2007 Estimated end date: April 2010 |
| Contact information | https://clinicaltrials.gov/ct2/show/NCT01787760 |
| Notes |
NCT01829191.
| Study name | Controlling myopia progression with soft contact lenses (contact lens control) |
| Methods | Randomized parallel‐group design |
| Participants |
Inclusion criteria: myopic subjects between 8 and 12 years of age; best sphere contact lens correction must lie between ‐0.75 D (best of the 2 eyes) and ‐5.00 D (worst of the 2 eyes); astigmatism less than or equal to 1.00 D; 1.00 D or less difference in spherical equivalent between the 2 eyes; best‐corrected visual acuity of 0.8 + 2 (20/25 + 2); spherical equivalent refraction visual acuity of 0.820/25 or better in both eyes; at least 8 D of accommodation Exclusion criteria: any ocular or systemic allergy or disease that may interfere with contact lens wear; systemic disease or autoimmune disease or use of medication (e.g. antihistamine) that may interfere with lens wear; clinically significant (grade 3 or 4) abnormality of the cornea, which may contraindicate contact lens wear; clinically significant (grade 3 or 4) tarsal abnormalities or bulbar injection; any ocular infection; any corneal distortion; any infectious disease (e.g. hepatitis, tuberculosis) or immunosuppressive disease (e.g. HIV); diabetes; anisometropia greater than 1.00 D; astigmatism greater than 1.00 D in either eye; eye injury or surgery within 8 weeks immediately before enrollment for this study; previous refractive surgery; rigid contact lens wear; orthokeratology; keratoconus or other corneal irregularity in either eye; aphakia; strabismus; central corneal scar or pupil/lid abnormality in either eye; contraindications to contact lens; surgically altered eyes; ocular infection of any type; ocular inflammation; anterior chamber angle grade 2 or narrower |
| Interventions |
Intervention 1: soft contact lens, Test Lens A Intervention 2: soft contact lens, Test Lens C Comparison intervention: spectacle lenses |
| Outcomes |
Primary outcome: spherical equivalent refractive error Secondary outcomes: axial length Maximum follow‐up: 2 years |
| Starting date | April 2008 Estimated end date: May 2010 |
| Contact information | https://clinicaltrials.gov/ct2/show/NCT01829191 |
| Notes |
NCT01923675.
| Study name | The role of cone opsin mutations & glasses that control axial elongation |
| Methods | Randomized parallel‐group design |
| Participants |
Inclusion criteria: nearsightedness with refractive error of at least ‐0.5 diopters; myopia progression at least ‐.50 D per year in previous year; astigmatism and anisometropia not more than 1.5 D; distance monocular acuity 6/6 or better; near monocular acuity of 0.4 M or better; stereoacuity not more than 40 seconds of arc at 40 cm; no contact lens use during the study; willingness to donate a blood sample or a buccal swab for genetic analysis; can be refracted to 20/20 or 20/15 Exclusion criteria: glaucoma; amblyopia; strabismus; ocular disease; developmental delay; history of wearing bifocal lenses; many types of eye surgery; color vision deficiency |
| Interventions |
Intervention 1: spectacles with red‐blocking tint Intervention 2: spectacles with holographic diffuser and color neutral tint Intervention 3: spectacles with holographic diffuser and red‐blocking tint Comparison intervention: spectacles with color neutral tint |
| Outcomes |
Primary outcome: axial elongation Secondary outcomes: cycloplegic autorefraction Maximum follow‐up: 18 months |
| Starting date | September 2013 Estimated end date: November 2016 |
| Contact information | https://clinicaltrials.gov/ct2/show/record/NCT01923675 |
| Notes |
NCT02001415.
| Study name | Efficacy study of different lens treatments on Chinese adolescent myopia (DLTCAM) |
| Methods | Randomized parallel‐group design |
| Participants |
Inclusion criteria: 120 adolescent myopia patients aged 10 to 15; myopic refraction between ‐1.00 D and ‐4.50 D; astigmatism equal to or less than ‐1.50 D; normal break‐up time of tear film Exclusion criteria: existence of any ocular disease except ametropia, hyperopia, severe dry eye |
| Interventions |
Intervention 1: MyoVision spectacles Intervention 2: orthokeratology lenses at night Comparison intervention: spectacles |
| Outcomes |
Primary outcome: ocular axial length Secondary outcomes: spherical equivalent refraction Maximum follow‐up: 12 months |
| Starting date | November 2013 Estimated end date: September 2016 |
| Contact information | https://clinicaltrials.gov/ct2/show/NCT02001415 |
| Notes |
NCT02130167.
| Study name | Low‐concentration atropine for myopia progression in schoolchildren |
| Methods | Randomized parallel‐group design |
| Participants |
Inclusion criteria: 60 children aged 6 to 12 years with myopia of at least 0.5 diopters (D) and astigmatism of ‐1.50 D or less Exclusion criteria: children with strabismus, amblyopia, cataract, glaucoma, or any ocular disease; any ocular surgery; history of systemic disease |
| Interventions |
Intervention: 0.01% atropine eye drops Comparison intervention: 0.05% atropine eye drops |
| Outcomes |
Primary outcome: cycloplegic spherical refraction Secondary outcomes: axial change, pupil size, accommodation, questionnaire Maximum follow‐up: 1 year |
| Starting date | August 2012 Estimated end date: August 2017 |
| Contact information | https://clinicaltrials.gov/ct2/show/NCT02130167 |
| Notes |
NCT02186184.
| Study name | Effect of orthokeratology vs spectacles on myopia progression in Chinese children: a crossover trial |
| Methods | Randomized cross‐over design |
| Participants |
Inclusion criteria: aged 7 to 14 years; visual acuity 20/20 or better in each eye; spherical error ranging from ‐0.5 D to ‐5.0 D and astigmatism less than 1.5 D in each eye; anisometropia less than 1.0 D between the 2 eyes; no strabismus, amblyopia, or any other ocular or systematic disease that may affect refractive development Exclusion criteria: currently using or history of using other interventions to control myopia progression (acupuncture, drugs, contact lenses, ear needles, and so on); inability to cooperate with the ocular examination; questionnaire survey; orthokeratology wearing |
| Interventions |
Intervention: orthokeratology Comparison intervention: spectacles |
| Outcomes |
Primary outcomes: refraction, axial length Secondary outcomes: tear film break‐up time, self‐evaluation of comfort, corneal endothelial cell density Maximum follow‐up: 2 years |
| Starting date | June 2014 Estimated end date: June 2017 |
| Contact information | https://clinicaltrials.gov/ct2/show/record/NCT02186184 |
| Notes |
NCT02206217.
| Study name | Myopia control with the multisegment lens |
| Methods | Randomized parallel‐group design |
| Participants |
Inclusion criteria: estimated 183 children aged 8 to 13 years with spherical equivalent refraction between ‐1.00 D and ‐5.00 D; anisometropia and astigmatism not greater than 1.50 D; best‐corrected logMAR visual acuity of 0 or better using spectacles; parental understanding of random allocation Exclusion criteria: ocular or systemic condition affecting vision or refractive development; prior treatment with any intervention for control of myopia |
| Interventions |
Intervention: multisegment spectacle lens Comparison intervention: single vision spectacle lens |
| Outcomes |
Primary outcome: cycloplegic refraction Secondary outcome: axial length Maximum follow‐up: 2 years |
| Starting date | August 2014 Estimated end date: July 2017 |
| Contact information | https://clinicaltrials.gov/ct2/show/NCT02206217 |
| Notes |
NCT02544529.
| Study name | Echothiophate iodide for the prevention of progression of myopia |
| Methods | Randomized parallel‐group design |
| Participants |
Inclusion criteria: between 8 and 15 years of age; documentation of progression of myopia within the 12 months before enrollment Exclusion criteria: any history of retinopathy of prematurity, glaucoma, cataracts, corneal disease, uveitis, manifest strabismus, nystagmus,or ocular trauma; any history of unstable asthma, diabetes, or juvenile idiopathic arthritis; systemic muscarinic agents, steroids, or anticholinesterase agents; benzalkonium chloride preservative allergy; astigmatism > 0.75 D; anisometropia > 1.50 D; pregnancy or positive pregnancy test at the screening visit |
| Interventions |
Intervention: echothiophate iodide 0.03% ophthalmic solution Comparison intervention: carboxymethylcellulose sodium (0.5%) |
| Outcomes |
Primary outcome: cycloplegic refraction Secondary outcomes: axial length, choroidal thickness Maximum follow‐up: 12 weeks |
| Starting date | June 2016 Estimated end date: June 2017 |
| Contact information | https://clinicaltrials.gov/ct2/show/NCT02544529 |
| Notes |
NCT02643342.
| Study name | A 2‐year longitudinal study on the structural and optical effects of orthokeratology treatment on eye |
| Methods | Randomized parallel‐group design |
| Participants |
Inclusion criteria: 90 children aged 6 to 10 years; myopia between 0.50 D and 4.00 D in both eyes; astigmatism < 1.50 D; ≤ 1.25 D for with‐the‐rule astigmatism (axes 180 ± 30); ≤ 0.50 D for astigmatism of other axes in both eyes; anisometropia ≤ 1.50 D; symmetrical corneal topography with corneal toricity < 2.00 D in both eyes; agree for randomization Exclusion criteria: contraindications for orthokeratology wear (e.g. limbus‐to‐limbus corneal cylinder, dislocated corneal apex); any type of strabismus or amblyopia; myopic treatment (e.g. refractive surgery, progressive lens wear for myopic control) before and during the study period; rigid contact lenses (including orthokeratology lenses); systemic condition that might affect refractive development (e.g. Down syndrome, Marfan’s syndrome); ocular condition that might affect the refractive error (e.g. cataract, ptosis); poor compliance with lens wear to follow‐up |
| Interventions |
Intervention 1: orthokeratology with normal compression factor Intervention 2: orthokeratology with increased compression factor Comparison intervention: single vision glasses |
| Outcomes |
Primary outcome: axial length Secondary outcomes: ocular aberration, corneal biomechanics, accommodation lag, choroidal thickness Maximum follow‐up: 2 years |
| Starting date | June 2016 Estimated end date: December 2019 |
| Contact information | https://clinicaltrials.gov/ct2/show/NCT02643342 |
| Notes |
NCT02643758.
| Study name | Myopia control using soft bifocal lenses |
| Methods | Randomized parallel‐group design |
| Participants |
Inclusion criteria: 97 children aged 6 to 12 years with refractive sphere ‐0.75 D to ‐4.50 D; refractive cylinder not to exceed 1.00 D; spherical equivalent: ‐0.75 D to ‐5.00 D; best‐corrected distance VA (logMAR) 0.14 or better in each eye and 0.10 or better in both eyes; difference in refractive error (spherical equivalent) in the 2 eyes not to exceed 1.00 D Exclusion criteria: children with prior history of myopia control treatment; contraindication to contact lens wear; binocular anomalies (e.g. strabismus) |
| Interventions |
Intervention: bifocal soft contact lenses Comparison intervention: single vision spectacles |
| Outcomes |
Primary outcomes: axial length, cycloplegic refractive error Secondary outcomes: wavefront aberrations, accommodation responses Maximum follow‐up: 2 years |
| Starting date | January 2016 Estimated end date: September 2018 |
| Contact information | https://clinicaltrials.gov/ct2/show/NCT02643758 |
| Notes |
NCT02955927.
| Study name | Combined atropine with orthokeratology in childhood myopia control (AOK): a randomized controlled trial |
| Methods | Randomized parallel‐group design |
| Participants |
Inclusion criteria: 60 children aged 6 to 11 years; myopia between 1.00 and 4.00 D in both eyes; astigmatism ≤ 2.50 D; with‐the‐rule astigmatism (axes 180 ± 30) ≤ 2.50 D; astigmatism with other axes ≤ 0.50 D in both eyes; < 1.00 D difference in manifest spherical equivalent (SE); cycloplegic objective refraction between 1.00 and 4.00 D in sphere; astigmatism ≤ 2.50 D; < 1.00 D difference in manifest SE between the 2 eyes; best‐corrected logMAR visual acuity 0.10 or better in both eyes; symmetrical corneal topography with corneal toricity < 2.00 D in either eye; normal ocular health other than myopia; agree to be randomized and to attend scheduled visits and aftercare Exclusion criteria: contraindications to atropine (known allergies or cardiovascular disease, epilepsy); contraindications to contact lens wear and ortho‐k; strabismus or amblyopia; history of myopia control treatment; rigid contact lens (including ortho‐k) wear experience; systemic condition that might affect refractive development; ocular condition that might affect refractive, poor response to lens wear including poor lens handling, poor vision and/ocular response after lens modifications, and poor compliance with scheduled visits |
| Interventions |
Interventions: ortho‐k and 0.01% atropine eye drops Comparison intervention: ortho‐k |
| Outcomes |
Primary outcomes: changes in axial length Secondary outcomes: none reported Maximum follow‐up: 24 months |
| Starting date | November 2016 Estimated end date: April 2020 |
| Contact information | https://clinicaltrials.gov/ct2/show/NCT02955927 |
| Notes |
NCT03242226.
| Study name | The effect of +3.00 ADD on myopia progression in Chinese children |
| Methods | Randomized parallel‐group design |
| Participants |
Inclusion criteria: 440 children aged 8 to 12 years; refractive error (cycloplegic autorefraction); spherical equivalent ‐1.00 to ‐6.00 D in both eyes; astigmatism ≤ 2.00 D in both eyes; spherical equivalent anisometropia ≤ 1.50 D; best‐corrected visual acuity ≥ 6/9.5 Exclusion criteria: allergy to tropicamide or topical anesthetic drugs; eye disease causing visual impairment including strabismus, amblyopia, ocular surface–related disease, cataract, trauma, ocular fundus disease, ocular surgery; previous wearing of rigid gas permeable contact lenses, progressive addition lenses, bifocal spectacles lens, peripheral defocus modifying contact lenses; receiving visual function training |
| Interventions |
Intervention: single vision spectacles (distant vision) and +3.00 ADD spectacles (near vision) Comparison intervention: single vision spectacles |
| Outcomes |
Primary outcome: spherical equivalent refraction Secondary outcomes: axial length, corneal curvature, binocular vision Maximum follow‐up: 3 years |
| Starting date | October 2016 Estimated end date: December 2018 |
| Contact information | https://clinicaltrials.gov/ct2/show/NCT03242226 |
| Notes |
NCT03246464.
| Study name | Clinical study of nearsightedness treatment with orthokeratology lenses (CONTROL) |
| Methods | Randomized parallel‐group design |
| Participants |
Inclusion criteria: 50 children aged 6 to 12 years; myopia ‐0.5 to ‐4.75 diopters spherical in 1 or both eyes; regular astigmatism ≤ ‐2.5 diopters in 1 or both eyes; anisometropia < 1.5 D spherical equivalent; best‐corrected visual acuity 0.1 logMAR or better in both eyes; acceptance of treatment randomization Exclusion criteria: manifest or latent squint; contraindications to use of OKL comprising keratoconus, allergic conjunctivitis, and keratoconjunctivitis sicca; previous eye surgery; chronic eye disease demanding daily use of eye drops; noncompliance with eye examinations (unstable fixation or intolerance to OKL); 1 or both parents being ethnical Asian, African, Hispanic, or Spanish |
| Interventions |
Intervention: orthokeratology lenses Comparison intervention: regular single vision spectacles |
| Outcomes |
Primary outcome: axial length Secondary outcomes: quality of life, safety Maximum follow‐up: 18 months |
| Starting date | March 2017 Estimated end date: October 2020 |
| Contact information | https://ichgcp.net/clinical‐trials‐registry/NCT03246464 |
| Notes |
NCT03329638.
| Study name | A study assessing the efficacy and safety of DE‐127 ophthalmic solution in subjects with mild or moderate myopia (APPLE) |
| Methods | Randomized parallel‐group design |
| Participants |
Inclusion criteria: 100 children aged 6 to 11 years; refractive error of spherical equivalent ‐1.0 diopter to ‐6.0 diopter in both eyes; anisometropia of spherical equivalent less than or equal to 1.50 diopters in both eyes; distance vision correctable to logMAR 0.2 or better in both eyes; normal intraocular pressure not greater than 21 mmHg in both eyes; no allergy to atropine, cyclopentolate, proparacaine, and benzalkonium chloride Exclusion criteria: amblyopia or manifest strabismus including intermittent tropia; ocular disorder that potentially affects myopia or refractive power; previous or current use of contact lenses, bifocal lenses, progressive addition lenses, or other forms of treatment (including atropine and pirenzepine) for myopia; systemic disorder that potentially affects myopia or refractive power |
| Interventions |
Intervention 1: DE‐127 ophthalmic solution low dose Intervention 2: DE‐127 ophthalmic solution medium dose Intervention 3: DE‐127 ophthalmic solution high dose Comparison intervention: placebo ophthalmic solution |
| Outcomes |
Primary outcome: spherical equivalent Secondary outcomes: not reported Maximum follow‐up: 12 months |
| Starting date | October 2017 Estimated end date: December 2019 |
| Contact information | https://clinicaltrials.gov/ct2/show/NCT03329638 |
| Notes |
NCT03334253.
| Study name | Low‐dose atropine for treatment of myopia |
| Methods | Randomized parallel‐group design |
| Participants |
Inclusion criteria: 186 children aged 5 to 12 years; myopia ‐1.00 D to ‐6.00 D spherical equivalent in both eyes; astigmatism ≤ 1.50 D in both eyes; anisometropia < 1.00 D spherical equivalent; gestational age ≥ 32 weeks; birth weight > 1500 g; understanding of the protocol and willingness to accept randomization to atropine or placebo by parents; willingness to participate in a 2‐ to 4‐week run‐in phase using daily artificial tear eye drops; ability to return in 2 to 4 weeks for possible randomization; accessible to phone; willingness to be contacted by Investigator's site staff Exclusion criteria: current or previous use of bifocals, progressive addition lenses, or multifocal contact lenses; current or previous use of orthoK, rigid gas permeable, or other contact lenses to reduce myopia progression; known atropine allergy; abnormality of the cornea, lens, central retina, iris, or ciliary body; current or prior history of manifest strabismus, amblyopia, or nystagmus; prior eyelid, strabismus, intraocular, or refractive surgery; Down syndrome or cerebral palsy; females who are pregnant, lactating, or intending to become pregnant within the next 30 months; negative urine pregnancy test (required for all females who have experienced menarche); current or previous myopia treatment with atropine, pirenzepine, or other antimuscarinic agent within 4 weeks of 13th birthday |
| Interventions |
Intervention: 0.01% atropine eye drops Comparison intervention: placebo eye drops |
| Outcomes |
Primary outcome: spherical equivalent refractive error Secondary outcome: spherical equivalent Maximum follow‐up: 30 months |
| Starting date | June 2018 Estimated end date: October 2022 |
| Contact information | https://clinicaltrials.gov/ct2/show/NCT03334253 |
| Notes |
NCT03350620.
| Study name | CHAMP: study of NVK‐002 in children with myopia |
| Methods | Randomized cross‐over design (within‐person study) |
| Participants |
Inclusion criteria: 483 children aged 3 to 17 years; myopia SER of at least ‐0.50 D and no greater than ‐6.00 D myopia in each eye Exclusion criteria: astigmatism more than ‐1.50 D in either eye; current or history of amblyopia or strabismus; history of any disease or syndrome that predisposes the patient to severe myopia; history in either eye of abnormal ocular refractive anatomy; serious systemic illness that, in the investigator’s opinion, would render the patient ineligible; chronic use (more than 3 days per week) of any topical ophthalmic medication (prescribed or over‐the‐counter) other than the assigned study medication |
| Interventions |
Intervention 1: NVK‐002 concentration 1 Intervention 2: NVK‐002 concentration 2 Comparison intervention: vehicle (placebo) |
| Outcomes |
Primary outcome: myopia progression Secondary outcomes: mean progression rates, proportion of patients who show < ‐ 0.75 D progression, median time to change in myopia < ‐ 0.75 D Maximum follow‐up: 36 months |
| Starting date | November 2017 Estimated end date: November 2022 |
| Contact information | https://clinicaltrials.gov/ct2/show/NCT03350620 |
| Notes |
NCT03374306.
| Study name | Topical application of low‐concentration (0.01%) atropine on the human eye with fast and slow myopia progression rate |
| Methods | Randomized parallel‐group design |
| Participants |
Inclusion criteria: 80 children aged 7 to 10 years; good general health; no family history of ocular disease; no current or history of epilepsy or asthma; myopia ‐0.50 to ‐1.00 D (inclusive, both eyes); astigmatism ≤ 0.50 D; no hyperopia, amblyopia, or strabismus; no reported ocular eye disease or disorder or drug allergy Exclusion criteria: not reported |
| Interventions |
Intervention: atropine 0.01% Comparison intervention: artificial tears |
| Outcomes |
Primary outcomes: refractive errors Secondary outcome: axial length Maximum follow‐up: 24 months |
| Starting date | January 2018 Estimated end date: June 2020 |
| Contact information | https://clinicaltrials.gov/ct2/show/NCT03374306 |
| Notes |
NCT03402100.
| Study name | Eye drops study for myopia control in schoolchildren |
| Methods | Randomized parallel‐group design |
| Participants |
Inclusion criteria: 150 children aged 6 to 12 years with myopia diagnosed with spherical equivalent refraction at least ‐0.5 diopter (D); able to use eye drops Exclusion criteria: children with astigmatism ‐1.50 D or greater; strabismus, amblyopia, cataract, glaucoma, any ocular disease, any ocular surgery; history of systemic disease; contact lens user; orthokeratology user |
| Interventions |
Intervention 1: 0.01% atropine eye drops Intervention 2: 0.005% atropine eye drops Intervention 3: 0.25% ketorolac eye drops Intervention 4: 0.01% atropine plus 0.25% ketorolac eye drops Intervention 5: 0.005% atropine plus 0.25% ketorolac eye drops |
| Outcomes |
Primary outcome: cycloplegic spherical refraction, axial length Secondary outcome: intraocular pressure, accommodation (diopter), pupil size, anterior chamber depth, posterior chamber depth Maximum follow‐up: 1 year |
| Starting date | October 2014 Estimated end date: December 2019 |
| Contact information | https://clinicaltrials.gov/ct2/show/NCT03402100 |
| Notes |
NCT03413085.
| Study name | To evaluate the efficacy and safety of multifocal soft contact lens in myopia control |
| Methods | Randomized parallel‐group design |
| Participants |
Inclusion criteria: 59 schoolchildren aged 6 to 15 years, with spherical equivalent refractive error between ‐1.00 D and ‐10.00 D; visual acuity with contact lens of 20/25 or better in each eye; astigmatism ≤ 1.50 D; anisometropia ≤ 1.00 D Exclusion criteria: eye disease interfering with contact lens wearing, use of bifocals, progressive addition lenses, rigid gas permeable contact lenses, orthokeratology lenses; myopia control treatment within 1 month before screening visit; systemic disease affecting vision or contact lens wearing; autoimmune disease, infectious disease, or immunosuppressive disease; surgically altered eyes; receiving medication for long‐term use that interferes with contact lens wearing, tear film production, pupil size, accommodation, or refractive state; nasal decongestants, antihistamines, prednisolone, or methylphenidate |
| Interventions |
Intervention: multifocal soft contact lens Comparison intervention: single vision soft contact lens |
| Outcomes |
Primary outcomes: objective cycloplegic refractive error, axial length Secondary outcomes: myopia progression, axial elongation, patient self‐assessment, average wearing hours across the study period, reasons and rates for discontinued wear during the study period Maximum follow‐up: 48 weeks |
| Starting date | May 2018 Estimated end date: March 2020 |
| Contact information | https://clinicaltrials.gov/ct2/show/NCT03413085 |
| Notes |
NCT03465748.
| Study name | Effectiveness of orthokeratology in myopia control |
| Methods | Randomized parallel‐group design |
| Participants |
Inclusion criteria: myopia progression more than ‐1.00 D in 1 year; prescription between ‐1.00 D and ‐6.00 D; best‐corrected visual acuity 20/25 or better; at least 1 eye with refractive astigmatism < 1.50 D Exclusion criteria: contraindications for orthoK; refractive surgery; current gas permeable contact lens wearers |
| Interventions |
Intervention: orthoK Comparison intervention: single vision spectacles |
| Outcomes |
Primary outcomes: visual acuity, axial length, myopia progression Secondary outcomes: not reported Maximum follow‐up: 2 years |
| Starting date | May 2017 Estimated end date: May 2019 |
| Contact information | https://clinicaltrials.gov/ct2/show/record/NCT03465748 |
| Notes |
NCT03508817.
| Study name | Atropine 0.01% eye drops in myopia study (AIMS) |
| Methods | Randomized parallel‐group design |
| Participants | Inclusion criteria: age 6 to 15 years; myopia ≥ 2.00 D (cycloplegic refraction; spherical equivalent); no prior or current treatment for preventing myopia progression (bifocals / progressive addition lenses / orthokeratology) Exclusion criteria: best corrected visual acuity < 0.5 (6/12); refractive myopia; astigmatism ≥ 1.5 D; amblyopia; ocular hypertension / glaucoma; prior intraocular surgery; allergy to atropine eye drops; systemic diseases associated with myopia such as Marfan syndrome, Stickler syndrome; history of cardiac or significant respiratory diseases; lack of consent for participating in the study |
| Interventions |
Intervention: atropine sulfate 0.01% eye drops Comparison intervention: control |
| Outcomes |
Primary outcomes: spherical equivalent refractive error Secondary outcomes: axial length; adverse events Maximum follow‐up: 2 years |
| Starting date | December 2018 Estimated end date: January 2022 |
| Contact information | https://clinicaltrials.gov/ct2/show/NCT03508817 |
| Notes |
NCT03538002.
| Study name | The effect of blue‐light filtering spectacle lenses on myopia progression in schoolchildren |
| Methods | Randomized parallel‐group design |
| Participants |
Inclusion criteria: refraction: myopia of ‐1.00 diopters (D) to ‐5.00D; astigmatism: equal or less than ‐1.50D; anisometropia: equal or less than 1.00D; best corrected monocular visual acuity: 0.0 LogMAR or better after full correction; parents' understanding and acceptance of random allocation of grouping Exclusion criteria: any ocular and systemic abnormalities might affect visual functions or refractive development; prior treatment of myopic control, e.g. drugs, orthokeratology, progressive addition lenses, bifocal lenses, drugs (e.g. atropine), etc. |
| Interventions |
Intervention: blue‐light filtering spectacle lenses Comparison intervention: conventional anti‐reflection coated spectacle lens |
| Outcomes |
Primary outcomes: cycloplegic refraction Secondary outcomes: axial length Maximum follow‐up: 2 years |
| Starting date | September 2018 Estimated end date: January 2021 |
| Contact information | https://clinicaltrials.gov/ct2/show/NCT03538002 |
| Notes |
NCT03552016.
| Study name | Evaluation of progression of myopia in children treated with vitamin B2 and outdoor sunlight exposure |
| Methods | Randomized parallel‐group design |
| Participants |
Inclusion criteria: age 6 to12 years old with myopia more than 0.50 D and astigmatism no more than 1.5 D; caretakers who choose to enroll their child in the study must agree to participate in the study on their own will after knowledge of potential alternatives (spectacle correction, orthokeratology, atropine eye drops, etc.) are explained to the patient's caretaker Exclusion criteria: known allergy to riboflavin; birth history of premature birth; developmental delay or other neurological or mental conditions; major systemic health problems; significant anisometropia more than 1.5 Diopters; any other eye condition which may complicate interpretation of data including: congenital glaucoma, congenital cataract, ectatic corneal condition, amblyopia or strabismus |
| Interventions |
Intervention 1: 200 mg Riboflavin (oral) Intervention 2: 400 mg Riboflavin (oral) Comparison intervention: 0 mg Riboflavin (oral) |
| Outcomes |
Primary outcomes: cycloplegic refraction Secondary outcomes: axial length, keratometry values, uncorrected best visual acuity Maximum follow‐up: 3 years |
| Starting date | October 2018 Estimated end date: October 2021 |
| Contact information | https://clinicaltrials.gov/ct2/show/NCT03552016 |
| Notes |
NCT03623074.
| Study name | Control of myopia using novel spectacle lens designs (CYPRESS) |
| Methods | Randomized parallel‐group design |
| Participants |
Inclusion criteria: age 6 to 10 years (day prior to 10th birthday) at time of informed consent/assent; spherical equivalent refraction error between ‐0.75 and ‐4.50 D; spherical equivalent refraction power between the two eyes must be less than or equal to 1.50 D;willingness to participate in the trial for 3 years without content lens wear Exclusion criteria: previous or current use of contact lenses; previous or current use of bifocals, progressive addition spectacles lenses; previous or current use of myopia control treatment; astigmatism worse then ‐1.25 DC in either eye |
| Interventions |
Intervention: novel spectacle lens design Comparison intervention: spectacle lenses |
| Outcomes |
Primary outcomes: axial length; spherical equivalent refraction Secondary outcomes: not reported Maximum follow‐up: 36 months |
| Starting date | July 2018 Estimated end date: January 2022 |
| Contact information | https://clinicaltrials.gov/ct2/show/NCT03623074 |
| Notes |
NCT03681366.
| Study name | Myopia control using optimized optical defocus RCTs |
| Methods | Randomized parallel‐group design |
| Participants |
Inclusion criteria: age at enrolment 8 to 13 years; Hong Kong Chinese; spherical equivalent refractions (SER): ‐1.00 to ‐5.00D; astigmatism: ‐1.00D or less; anisometropia: 1.25D or less; spectacle corrected monocular visual acuity (VA): 0.0 logMAR or better; contact lens corrected monocular VA: 0.1 logMAR or better; normal binocular function; willingness to wear contact lenses regularly; parents' understanding and acceptance of random allocation of grouping and masking Exclusion criteria: prior myopia control treatment, e.g. orthokeratology, defocus soft contact lenses, progressive addition lenses, bifocal lenses, drugs (e.g. atropine), etc.; strabismus or decompensated phoria (checked by cover test at far and near in screening); known contraindications for contact lens wear; have any ocular and systemic diseases and abnormalities that might affect visual function or refractive development |
| Interventions |
Intervention: single vision soft contact lens Comparison intervention: DISC3.5 Plus lens |
| Outcomes |
Primary outcomes: spherical equivalent Secondary outcomes: axial length Maximum follow‐up: 12 months |
| Starting date | October 2018 Estimated end date: April 2021 |
| Contact information | https://clinicaltrials.gov/ct2/show/NCT03681366 |
| Notes |
NCT03690089.
| Study name | Low‐dose atropine eye drops to reduce progression of myopia in children in the United Kingdom (CHAMP‐UK) |
| Methods | Randomized parallel‐group design |
| Participants |
Inclusion criteria: age 6 to 12 years (at the time of consenting); myopia of ‐0.5 D or greater (spherical equivalent refractive error) in both eyes; best‐corrected distance visual acuity (BCDVA) 0.20 logMAR or better in both eyes Exclusion criteria: other ocular morbidities; myopia of ‐10 D or greater in either eye; astigmatism of 2 D or higher in either eye; amblyopia; significant health problems that can compromise the ability to attend research visits or complete the trial |
| Interventions |
Intervention: atropine sulfate 0.01% eye drops Comparison intervention: placebo eye drops |
| Outcomes |
Primary outcome: spherical equivalent refractive error Secondary outcomes: axial length, best‐corrected distance visual acuity, near visual acuity, reading speed, pupil diameter, accommodation, spectacle correction, eye drop tolerability, adverse events, quality of life Maximum follow‐up: 24 months |
| Starting date | April 2019 Estimated end date: December 2024 |
| Contact information | https://clinicaltrials.gov/ct2/show/record/NCT03690089 |
| Notes |
NCT03690414.
| Study name | Evaluation of short‐term use of experimental eye drops BHVI2, 0.02% atropine, and BHVI2 plus 0.02% atropine eye drops |
| Methods | Randomized parallel‐group design |
| Participants |
Inclusion criteria: age 6 to 13 years; myopic; normal ocular findings; spherical equivalent between ‐0.50 diopter and ‐6.00 diopter; vision correctable to at least 20/25 or better in each eye with spectacles Exclusion criteria: preexisting ocular irritation, systematic disease, eye trauma, myopia control interventions |
| Interventions |
Intervention 1: experimental BHVI2 Intervention 2: atropine sulfate 0.02% eye drops Comparison intervention: combination eye drops |
| Outcomes |
Primary outcomes: pupillary diameter, accommodative amplitude Secondary outcomes: not reported Maximum follow‐up: 1 month |
| Starting date | October 2018 Estimated end date: February 2019 |
| Contact information | https://clinicaltrials.gov/ct2/show/NCT03690414 |
| Notes |
PACT Study 2017.
| Study name | Personalized addition lenses clinical trial |
| Methods | Randomized controlled trial |
| Participants |
Inclusion criteria: 7 to 12 years of age; myopic refractive error between ‐0.75 D and ‐4.00 D; cycloplegic spherical equivalent; astigmatism < 1.50 D; best‐corrected visual acuity logMAR +0.05 or better in each; anisometropia < 1.00 D; at least 0.50 D progression by cycloplegic autorefraction over the past year Exclusion criteria: strabismus with or without add; ocular or systemic condition that may affect refractive error development |
| Interventions |
Intervention 1: individualized add power Intervention 2: +2.00 D add power Comparison intervention: single vision |
| Outcomes |
Primary outcome: change in cycloplegic spherical equivalent refractive error Secondary outcome: change in axial elongation Maximum follow‐up: 2 years |
| Starting date | July 2014 Estimated end date: March 2017 |
| Contact information | Eye Hospital of Wenzhou Medical University |
| Notes | None |
D: diopters. logMAR: logarithm of the minimum angle of resolution.
Differences between protocol and review
We modified the interventions under review compared with the published protocol. Single vision soft contact lenses (SVSCLs) are considered a control intervention, and we did not study them as an active treatment intervention for the purposes of this review. Thus, we did not include in this review studies that compared SVSCLs with single vision lenses (SVLs) (spectacles).
We added methods for the Summary of findings table and the GRADE assessment, both of which we incorporated in this review update.
Contributions of authors
Contributions of authors to previous versions of this review have been published (Walline 2011).
For this version of the review: JJW contributed to screening search results, appraising studies, extracting data, analyzing and interpreting results, and writing the manuscript; KL contributed to screening search results, appraising studies, extracting data, analyzing and interpreting results, and writing the manuscript; SAC, DOM, and JDT contributed to interpreting results and providing substantial comments and edits to the manuscript. All authors provided final approval of the manuscript.
Sources of support
Internal sources
Ohio State University, College of Optometry, USA
External sources
National Eye Institute, National Institutes of Health, Grant UG1‐EY020522, USA
National Eye Institute, National Institutes of Health, Grant K23‐EY00383, USA
National Eye Institute, National Institutes of Health, Grant U10‐08893, USA
Research to Prevent Blindness, USA
-
National Institute for Health Research (NIHR), UK
o Richard Wormald, Co‐ordinating Editor for Cochrane Eyes and Vision (CEV) acknowledges financial support for his CEV research sessions from the Department of Health through the award made by the National Institute for Health Research to Moorfields Eye Hospital NHS Foundation Trust and UCL Institute of Ophthalmology for a Specialist Biomedical Research Centre for Ophthalmology. o This review updated was supported by the NIHR, via Cochrane Infrastructure funding to the CEV UK editorial base.
The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, NHS, or the Department of Health.
Declarations of interest
Jeffrey Walline was the Principal Investigator (PI) of the Contact Lens and Myopia Progression (CLAMP) Study, which was a randomized controlled trial conducted to examine the effects of rigid gas permeable contact lenses (RGPCLs) on myopia progression in children. Susan Cotter, OD, MS, was a clinical site PI and served on the writing committee for the PIR‐205 study—a trial evaluating pirenzepine ophthalmic gel for slowing myopia progression in children. Dr. Cotter was also a clinical site PI and served on the steering committee and the writing committee for the Correction of Myopia Evaluation Trial‐2 (COMET‐2) study evaluating progressive addition lenses versus single vision lenses for slowing progression of myopia in children with high accommodative lag and near esophoria. Both studies were included in this review.
Jeffrey Walline received research funding, consulted for companies, received honoraria from companies, and has pending grants with companies, all related to myopia and/or myopia progression. Susan Cotter's institution received grant funding for participation in the NIH/NEI‐funded multicenter study COMET‐2 and the industry‐sponsored PIR‐205 study. Susan Cotter and J. Daniel Twelker are clinical site PIs for an industry‐sponsored multicenter trial—the Childhood Atropine for Myopia Progression (CHAMP) Study, which evaluates low‐dose atropine for myopia progression in children. Donald O. Mutti received research funding, consulted for companies, and has received honoraria from companies with interests in myopia and/or myopia progression.
Kristina Lindsley and Swaroop Vedula were methodologists employed by CEV@US; CEV@US has been funded by various grants/contracts over years from the National Eye Institute, National Institutes of Health.
Edited (no change to conclusions)
References
References to studies included in this review
Adler 2006 {published data only}
- Adler D, Millodot M. The possible effect of undercorrection on myopic progression in children. Clinical and Experimental Optometry 2006;89(5):315-21. [DOI] [PubMed] [Google Scholar]
Anstice 2011 {published data only}
- Anstice NS, Phillips JR. Effect of dual-focus soft contact lens wear on axial myopia progression in children. Ophthalmology 2011;118(6):1152-61. [DOI] [PubMed] [Google Scholar]
ATOM Study 2006 {published data only}
- Chia A, Chua WH, Tan D. Effect of topical atropine on astigmatism. British Journal of Ophthalmology 2009;93(6):799-802. [DOI] [PubMed] [Google Scholar]
- Chua W, Balakrishnan V, Chan Y. Analysis of safety data for atropine in the treatment of myopia (ATOM) study. In: Investigative Ophthalmology and Visual Science. Vol. 43. 2002:ARVO E-abstract 329.
- Chua W, Balakrishnan V, Tan D, Chan Y. Efficacy results from the atropine in the treatment of myopia (ATOM) study. In: Investigative Ophthalmology and Visual Science. Vol. 44. 2003:ARVO E-abstract 3119.
- Chua WH, Balakrishnan V, Chan YH, Tong L, Ling Y, Quah BL, et al. Atropine for the treatment of childhood myopia. Ophthalmology 2006;113(12):2285-91. [DOI] [PubMed] [Google Scholar]
- Chua WH, Tan D, Balakrishnan V, Chan YH, ATOM Study Group. Progression of childhood myopia following cessation of atropine treatment. In: Investigative Ophthalmology and Visual Science. Vol. 46. 2005:ARVO E-abstract 4625.
- Kumaran A, Htoon HM, Tan D, Chia A. Analysis of changes in refraction and biometry of atropine- and placebo-treated eyes. Investigative Ophthalmology and Visual Science 2015;56(9):5650-5. [DOI] [PubMed] [Google Scholar]
- Loh KL, Lu Q, Tan D, Chia A. Risk factors for progressive myopia in the atropine therapy for myopia study. American Journal of Ophthalmology 2015;159(5):945-9. [DOI] [PubMed] [Google Scholar]
- Luu CD, Lau AM, Koh AH, Chua WH, Balakrishnan V, Tan D. Effects of long-term atropine usage on retinal function. In: Investigative Ophthalmology and Visual Science. Vol. 44. 2003:ARVO E-abstract 4790.
- Luu CD, Lau AM, Koh AH, Tan D. Multifocal electroretinogram in children on atropine treatment for myopia. British Journal of Ophthalmology 2005;89(2):151-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tong L, Huang XL, Koh AL, Zhang X, Tan DT, Chua WH. Atropine for the treatment of childhood myopia: effect on myopia progression after cessation of atropine. Ophthalmology 2009;116(3):572-9. [DOI] [PubMed] [Google Scholar]
Cambridge Anti‐Myopia Study 2013 {published data only}
- Allen PM, Radhakrishnan H, Price H, Rae S, Theagarayan B, Calver RI, et al. A randomised clinical trial to assess the effect of a dual treatment on myopia progression: the Cambridge Anti-Myopia Study. Ophthalmic & Physiological Optics 2013;33(3):267-76. [DOI] [PubMed] [Google Scholar]
- Price H, Allen PM, Radhakrishnan H, Calver R, Rae S, Theagarayan B, et al. The Cambridge Anti-myopia Study: variables associated with myopia progression. Optometry and Vision Science 2013;90(11):1274-83. [DOI] [PubMed] [Google Scholar]
Charm 2013 {published data only}
- Charm J, Cho P. High myopia-partial reduction ortho-k: a 2-year randomized study. Optometry and Vision Science 2013;90(6):530-9. [DOI] [PubMed] [Google Scholar]
- Charm J, Cho P. High myopia-partial reduction orthokeratology (HM-PRO): study design. Contact Lens & Anterior Eye 2013;36(4):164-70. [DOI] [PubMed] [Google Scholar]
- Charm J, Cho P. High myopia-partial reduction orthokeratology (HM-PRO) study: recruitment and one year result. Contact Lens & Anterior Eye 2011;34:S3. [DOI] [PubMed] [Google Scholar]
Cheng 2010 {published data only}
- Cheng D, Schmid KL, Woo GC, Drobe B. Randomized trial of effect of bifocal and prismatic bifocal spectacles on myopic progression: two-year results. Archives of Ophthalmology 2010;128(1):12-9. [DOI] [PubMed] [Google Scholar]
- Cheng D, Woo GC, Drobe B, Schmid KL. Effect of bifocal and prismatic bifocal spectacles on myopia progression in children: three-year results of a randomized clinical trial. JAMA Ophthalmology 2014;132(3):258-64. [DOI] [PubMed] [Google Scholar]
Cheng 2016 {published data only}
- Cheng X, Xu J, Chehab K, Exford J, Brennan N. Soft contact lenses with positive spherical aberration for myopia control. Optometry and Vision Science 2016;93(4):353-66. [DOI] [PubMed] [Google Scholar]
Chung 2002 {published data only}
- Chung K, Mohidin N, O'Leary DJ. Undercorrection of myopia enhances rather than inhibits myopia progression. Vision Research 2002;42(22):2555-9. [DOI] [PubMed] [Google Scholar]
CLAMP Study 2004 {published data only}
- Jones-Jordan LA, Walline JJ, Mutti DO, Rah MJ, Nichols KK, Nichols JJ, et al. Gas permeable and soft contact lens wear in children. Optometry and Vision Science 2010;87(6):414-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walline JJ, Jones LA, Mutti DO, Zadnik K. A randomized trial of the effects of rigid contact lenses on myopia progression. Archives of Ophthalmology 2004;122(12):1760-6. [DOI] [PubMed] [Google Scholar]
- Walline JJ, Jones LA, Mutti DO, Zadnik K. The Contact Lens And Myopia Progression (CLAMP) study. Ophthalmic and Physiological Optics 2006;26:29. [Google Scholar]
- Walline JJ, Jones LA, Mutti DO, Zadnik K. Use of a run-in period to decrease loss to follow-up in the Contact Lens And Myopia Progression (CLAMP) study. Controlled Clinical Trials 2003;24(6):711-8. [DOI] [PubMed] [Google Scholar]
- Walline JJ, Mutti DO, Jones LA, Rah MJ, Nichols KK, Watson R, et al. The Contact Lens And Myopia Progression (CLAMP) study: design and baseline data. Optometry and Vision Science 2001;78(4):223-33. [DOI] [PubMed] [Google Scholar]
- Walline JJ, Mutti DO, Jones LA, Zadnik K. Baseline characteristics of the Contact Lens And Myopia Progression (CLAMP) study. In: American Academy of Optometry. 2000:213. [DOI] [PubMed]
- Walline JJ, Zadnik K. Child and parent agreement on report of contact lens wearing time and comfort. In: American Academy of Optometry. 1999:158.
- Walline JJ, Zadnik K. Retention of subjects in clinical trials: the Contact Lens And Myopia Progression (CLAMP) example. In: American Academy of Optometry. 1997:89.
COMET2 Study 2011 {published data only}
- Correction of Myopia Evaluation Trial 2 Study Group for the Pediatric Eye Disease Investigator Group. Progressive-addition lenses versus single-vision lenses for slowing progression of myopia in children with high accommodative lag and near esophoria. Investigative Ophthalmology and Visual Science 2011;52(5):2749-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
COMET Study 2003 {published data only}
- Comet Study Group. The design of the correction of myopia evaluation trial. In: American Academy of Optometry. 1997:130.
- Deng L, Gwiazda J, Manny RE, Scheiman M, Weissberg E, Fern KD, et al. Limited change in anisometropia and aniso-axial length over 13 years in myopic children enrolled in the Correction of Myopia Evaluation Trial. Investigative Ophthalmology & Visual Science 2014;55(4):2097-105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng L, Manny R, Crossnoe C, Gwiazda J. Longitudinal measures of intraocular pressure and axial length in a subgroup of children in the Correction of Myopia Evaluation Trial (COMET). In: Investigative Ophthalmology & Visual Science. Vol. 48. 2007:ARVO E-abstract 4829.
- Dias L, Hyman L, Manny RE, Fern K, COMET Group. Evaluating the self-esteem of myopic children over a three-year period: the COMET experience. Optometry and Vision Science 2005;82(4):338-47. [DOI] [PubMed] [Google Scholar]
- Dias L, Manny R, Hyman L, Fern K, COMET Group. Ocular factors and self perception in myopic children. In: Investigative Ophthalmology & Visual Science. Vol. 42. 2001:ARVO E-abstract 2101.
- Dias L, Manny RE, Hyman L, Fern K. The relationship between self-esteem of myopic children and ocular and demographic characteristics. Optometry and Vision Science 2002;79(11):688-96. [DOI] [PubMed] [Google Scholar]
- Dias L, Manny RE, Weissberg E, Fern KD. Myopia, contact lens use and self-esteem. Ophthalmic & Physiological Optics 2013;33(5):573-80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dias L, Schoenfeld E, Thomas J, Baldwin C, Mcleod J, Smith J, et al. Reasons for high retention in pediatric clinical trials: Comparison of participant and staff responses in the Correction of Myopia Evaluation Trial. Clinical Trials 2005;2(5):443-52. [DOI] [PubMed] [Google Scholar]
- Dong L, Gwiazda J, Hyman L, Kurtz D, Manny R, Marsh-Tootle W, et al. Myopia stabilization in the Correction of Myopia Evaluation Trial (COMET) cohort. In: Investigative Ophthalmology & Visual Science. Vol. 48. 2007:ARVO E-abstract 2385.
- Dong L, Thorn F, Gwiazda J, Hyman L, Norton T, the COMET Group. Use of the Gompertz function to describe the course of myopia progression and stabilization in the correction of myopia evaluation trial (COMET). Optometry and Vision Science 2006;83:E-abstract 065421. [Google Scholar]
- Dong LM, Hyman L, Manny RE, Thomas J, Dias L, McLeod J, et al. Evaluating masking in a randomized, double-masked clinical trial in children with myopia. Optometry and Vision Science 2006;83(1):46-52. [DOI] [PubMed] [Google Scholar]
- Fern KD, Manny RE, Gwiazda J, Hyman L, Weise K, Marsh-Tootle W, et al. Intraocular pressure and central corneal thickness in the COMET cohort. Optometry and Vision Science 2012;89(8):1225-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gwiazda J, Deng L, Dias L, Marsh-Tootle W, COMET Study Group. Association of education and occupation with myopia in COMET parents. Optometry and Vision Science 2011;88(9):1045-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gwiazda J, Deng L, Manny R, Norton TT, COMET Study Group. Seasonal variations in the progression of myopia in children enrolled in the Correction of Myopia Evaluation Trial. Investigative Ophthalmology & Visual Science 2014;55(2):752-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gwiazda J, Hyman L, Dong LM, Everett D, Norton T, COMET Group. Factors associated with high myopia after 7 years of follow-up in the Correction of Myopia Evaluation Trial (COMET) Cohort. Ophthalmic Epidemiology 2007;14(4):230-7. [DOI] [PubMed] [Google Scholar]
- Gwiazda J, Hyman L, Hussein M, Everett D, Norton TT, Kurtz D, et al. A randomized clinical trial of progressive addition lenses versus single vision lenses on the progression of myopia in children. Investigative Ophthalmology and Visual Science 2003;44(4):1492-500. [DOI] [PubMed] [Google Scholar]
- Gwiazda J, Marsh-Tootle WL, Hyman L, Hussein M, Norton TT, COMET Group. Baseline refractive and ocular component measures of children enrolled in the Correction of Myopia Evaluation Trial (COMET). Investigative Ophthalmology and Visual Science 2002;43(2):314-21. [PubMed] [Google Scholar]
- Gwiazda J, Norton TT, Hou W, Hyman L, Manny R, COMET Group. Longitudinal changes in lens thickness in myopic children enrolled in the Correction of Myopia Evaluation Trial (COMET). Current Eye Research 2016;41(4):492-500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gwiazda JE, Hyman L, Everett D, Norton T, Kurtz D, Manny R, et al. Five-year results from the Correction of Myopia Evaluation Trial (COMET). In: Investigative Ophthalmology & Visual Science. Vol. 47. 2006:ARVO E-abstract 1166.
- Gwiazda JE, Hyman L, Norton T, Hussein M, Marsh-Tootle W, COMET Group. Baseline accommodation and related risk factors associated with myopia progression and their interaction with treatment in COMET children. In: Investigative Ophthalmology and Visual Science. Vol. 45. 2004:ARVO E-abstract 2740. [DOI] [PubMed]
- Gwiazda JE, Hyman L, Norton TT, Hussein ME, Marsh-Tootle W, COMET Group. Accommodation and related risk factors associated with myopia progression and their interaction with treatment in COMET children. Investigative Ophthalmology & Visual Science 2004;45(7):2143-51. [DOI] [PubMed] [Google Scholar]
- Harb E, Hyman L, Fazzari M, Gwiazda J, Marsh-Tootle W. Factors associated with macular thickness in the COMET myopic cohort. Optometry and Vision Science 2012;89(5):620-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harb E, Hyman L, Gwiazda J, Marsh-Tootle W, Zhang Q, Hou W, et al. Choroidal thickness profiles in myopic eyes of young adults in the Correction of Myopia Evaluation Trial cohort. American Journal of Ophthalmology 2015;160(1):62-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardy R, Hillis A, Mutti D, Stone R, Taylor C Sr, Hyman L, et al. Myopia stabilization and associated factors among participants in the Correction of Myopia Evaluation Trial (COMET). Investigative Ophthalmology & Visual Science 2013;54(13):7871-83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hussein M, Gwiazda J, Hyman L. Reliability of measurements of phoria and accommodation in the Correction of Myopia Evaluation Trial (COMET). In: Investigative Ophthalmology & Visual Science. Vol. 42. 2001:ARVO E-abstract 2110.
- Hyman L, Gwiazda J, Hussein M, Norton TT, Wang Y, Marsh-Tootle W, et al. Relationship of age, sex, and ethnicity with myopia progression and axial elongation in the correction of myopia evaluation trial. Archives of Ophthalmology 2005;123(7):977-87. [DOI] [PubMed] [Google Scholar]
- Hyman L, Gwiazda J, Hussein M, Norton TT, Wang Y, Marsh-Tootle W, et al. Relationship of baseline age, gender and ethnicity with 3-year myopia progression and axial elongation in the Correction of Myopia Evaluation Trial (COMET). In: Investigative Ophthalmology & Visual Science. Vol. 45. 2004:ARVO E-abstract 2734.
- Hyman L, Gwiazda J, Marsh-Tootle WL, Norton TT, Hussein M, COMET Group. The Correction of Myopia Evaluation Trial (COMET): design and general baseline characteristics. Controlled Clinical Trials 2001;22(5):573-92. [DOI] [PubMed] [Google Scholar]
- Hyman L, Gwiazda J, Marsh-Tootle WL, Norton TT, COMET Group. The Correction of Myopia Evaluation Trial (COMET): design and baseline characteristics. In: Investigative Ophthalmology & Visual Science. Vol. 40. 1999:S754.
- Hyman L, Gwiazda J. The Correction of Myopia Evaluation Trial: lessons from the study design. Annals of the Academy of Medicine Singapore 2004;33(1):44-8. [PubMed] [Google Scholar]
- Hyman L, Hussein M, Gwiazda J, COMET Group. Validity of cycloplegic autorefraction and axial length measurements in a clinical trial. In: Investigative Ophthalmology and Visual Science. Vol. 39. 1998:4348.
- Kowalski PM, Wang Y, Owens RE, Bolden J, Smith JB, Hyman L. Adaptability of myopic children to progressive addition lenses with a modified fitting protocol in the Correction of Myopia Evaluation Trial (COMET). Optometry and Vision Science 2005;82(4):328-37. [DOI] [PubMed] [Google Scholar]
- Kurtz D, Hyman L, Gwiazda JE, Manny R, Dong LM, COMET Group. Role of parental myopia in the progression of myopia and its interaction with treatment in COMET children. Investigative Ophthalmology and Visual Science 2007;48(2):562-70. [DOI] [PubMed] [Google Scholar]
- Kurtz D, Manny R, Hussein M, COMET Group. Reliability of ocular components measured by A-scan in children. In: Optometry and Vision Science. Vol. 77. 2000:S286.
- Kurtz D, Manny R, Hussein M. Variability of the ocular component measurements in children using A-scan ultrasonography. Optometry and Vision Science 2004;81(1):35-43. [DOI] [PubMed] [Google Scholar]
- Manny RE, Deng L, Crossnoe C, Gwiazda J. Intraocular pressure (IOP) and myopic progression in a subgroup of COMET (Correction of Myopia Evaluation Trial) children. In: American Academy of Optometry. 2006.
- Manny RE, Deng L, Crossnoe C, Gwiazda J. IOP, myopic progression and axial length in a COMET subgroup. Optometry and Vision Science 2008;85(2):97-105. [DOI] [PubMed] [Google Scholar]
- Manny RE, Hussein M, Gwiazda J, Marsh-Tootle W, COMET Group. Repeatability of ETDRS visual acuity in children. Investigative Ophthalmology and Visual Science 2003;44(8):3294-300. [DOI] [PubMed] [Google Scholar]
- Manny RE, Hussein M, Marsh-Tootle Wl, Gwiazda J. Reliability of ETDRS visual acuity (VA) in the Correction of Myopia Evaluation Trial (COMET). In: American Academy of Optometry. 2000:279.
- Manny RE, Hussein M, Scheiman M, Kurtz D, Niemann K, COMET Group. Tropicamide (1%): an effective cycloplegic agent for myopic children. Investigative Ophthalmology and Visual Science 2001;42(8):1728-35. [PubMed] [Google Scholar]
- Manny RE, Hussein ME, Grice K, Scheiman M, Kurtz D, Niemann K, et al. Tropicamide 1% as a cycloplegic agent in the Correction of Myopia Evaluation Trial (COMET). In: Investigative Ophthalmology & Visual Science. Vol. 40. 1999:ARVO E-abstract 3673.
- Marsh-Tootle WL, Gwiazda J, Hyman L, Norton TT. Refractive, phoria, and axial measures at baseline in children enrolled in the correction of myopia evaluation trial (COMET). In: Investigative Ophthalmology & Visual Science. Vol. 40. 1999:ARVO E-abstract 3992. [PubMed]
- Marsh-Tootle WL, Hyman L, Dong LM, Gwiazda J, Zhang Q. Myopia progression in adolescents in the correction of myopia evaluation trial (COMET) who switched to contact lenses versus remained in spectacles. In: American Academy of Optometry. 2007.
- Marsh-Tootle WL, Li MD, Weise KK, Fern KD, Gwiazda J, Norton T, et al. Myopia progression in children wearing spectacles vs. switching to contact lenses. Optometry and Vision Science 2009;86(6):741-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norton TT, Hyman L, Dong L, Gwiazda J. Longitudinal changes in lens thickness in myopic children enrolled in the Correction of Myopia Evaluation Trial (COMET). In: Investigative Ophthalmology and Visual Science. Vol. 49. 2008:ARVO E-abstract 2603.
- Scheiman M, Gwiazda J, Zhang Q, Deng L, Fern K, Manny RE, et al. Longitudinal changes in corneal curvature and its relationship to axial length in the Correction of Myopia Evaluation Trial (COMET) cohort. Journal of Optometry 2016;9(1):13-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheiman M, Zhang Q, Gwiazda J, Hyman L, Harb E, Weissberg E, et al. Visual activity and its association with myopia stabilisation. Ophthalmic & Physiological Optics 2014;34(3):353-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
CONTROL Study 2016 {published data only}
- Aller TA, Liu M, Wildsoet CF. Myopia control with bifocal contact lenses: a randomized clinical trial. Optometry and Vision Science 2016;93(4):344-52. [DOI] [PubMed] [Google Scholar]
- Aller TA, Wildsoet C. Results of a one-year prospective clinical trial (CONTROL) of the use of bifocal soft contact lenses to control myopia progression. Ophthalmic and Physiological Optics 2006;26(Suppl 1):8-9. [Google Scholar]
- Aller TA. Design of a prospective clinical trial of the use of bifocal soft contact lenses to control myopia progression (CONTROL). In: 10th International Myopia Conference. 2004.
DISC Study 2011 {published data only}
- Lam CS, Tang WC, Tse DY, Tang YY, To CH. Defocus Incorporated Soft Contact (DISC) lens slows myopia progression in Hong Kong Chinese schoolchildren: a 2-year randomised clinical trial. British Journal of Ophthalmology 2014;98(1):40-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lam CSY, Tang WC, Tang YY. Randomised clinical trial of myopia control in myopic schoolchildren using the defocus incorporated soft contact (DISC) lens. Optometry and Vision Science 2011;88(3):444-5. [Google Scholar]
Edwards 2002 {published data only}
- Edwards MH, Li RW, Lam CS, Lew JK, Yu BS. The Hong Kong progressive lens myopia control study: study design and main findings. Investigative Ophthalmology and Visual Science 2002;43(9):2852-8. [PubMed] [Google Scholar]
Fujikado 2014 {published data only}
- Fujikado T, Ninomiya S, Kobayashi T, Suzaki A, Nakada M, Nishida K. Effect of low-addition soft contact lenses with decentered optical design on myopia progression in children: a pilot study. Clinical Ophthalmology 2014;8:1947-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
Fulk 1996 {published data only}
- Fulk GW, Cyert LA. Can bifocals slow myopia progression? Journal of the American Optometric Association 1996;67(12):749-54. [PubMed] [Google Scholar]
Fulk 2002 {published data only}
- Fulk GW, Cyert LA, Parker DE, West RW. The effect of changing from glasses to soft contact lenses on myopia progression in adolescents. Ophthalmic and Physiological Optics 2003;23(1):71-7. [DOI] [PubMed] [Google Scholar]
- Fulk GW, Cyert LA, Parker DE. A 3-year clinical trial of bifocals in children with near point esophoria: myopia progression during the first year. In: Investigative Ophthalmology & Visual Science. Vol. 39. 1998:ARVO E-abstract 2982.
- Fulk GW, Cyert LA, Parker DE. A 3-year clinical trial of bifocals to slow myopia progression in children with near-point esophoria: baseline characteristics. In: Investigative Ophthalmology & Visual Science. Vol. 38. 1997:ARVO E-abstract 5404.
- Fulk GW, Cyert LA, Parker DE. A clinical trial of bifocals in children: myopia progression after two years. In: Investigative Ophthalmology & Visual Science. Vol. 40. 1999:ARVO E-abstract 3665.
- Fulk GW, Cyert LA, Parker DE. A randomized clinical trial of bifocal glasses for myopic children with esophoria: results after 54 months. Optometry 2002;73(8):470-6. [PubMed] [Google Scholar]
- Fulk GW, Cyert LA, Parker DE. A randomized trial of the effect of single-vision vs. bifocal lenses on myopia progression in children with esophoria. Optometry and Vision Science 2000;77(8):395-401. [DOI] [PubMed] [Google Scholar]
- Fulk GW, Cyert LA, Parker DE. Baseline characteristics in the Myopia Progression Study, a clinical trial of bifocals to slow myopia progression. Optometry and Vision Science 1998;75(7):485-92. [DOI] [PubMed] [Google Scholar]
- Fulk GW, Cyert LA, Parker DE. Progression of childhood myopia in soft contact lenses and glasses. In: Investigative Ophthalmology & Visual Science. Vol. 43. 2002:ARVO E-abstract 1507.
- Fulk GW, Cyert LA, Parker DE. Seasonal variation in myopia progression and ocular elongation. Optometry and Vision Science 2002;79(1):46-51. [DOI] [PubMed] [Google Scholar]
Han 2018 {published data only}
- Han X, Xu D, Ge W, Wang Z, Li X, Liu W. A comparison of the effects of orthokeratology lens, Medcall lens, and ordinary frame glasses on the accommodative response in myopic children. Eye & Contact Lens 2018;44(4):268-71. [DOI] [PubMed] [Google Scholar]
Hasebe 2008 {published data only}28611140
- Fujiwara M, Hasebe S, Nakanishi R, Tanigawa K, Ohtsuki H. Seasonal variation in myopia progression and axial elongation: an evaluation of Japanese children participating in a myopia control trial. Japanese Journal of Ophthalmology 2012;56(4):401-6. [DOI] [PubMed] [Google Scholar]
- Hasebe S, Nakatsuka C, Hamasaki I, Ohtsuki H. Downward deviation of progressive addition lenses in a myopia control trial. Ophthalmic and Physiological Optics 2005;25(4):310-4. [DOI] [PubMed] [Google Scholar]
- Hasebe S, Nonaka F, Nakatsuka C, Ohtsuki H. Myopia control trial with progressive addition lenses in Japanese schoolchildren: baseline measures of refraction, accommodation, and heterophoria. Japanese Journal of Ophthalmology 2005;49(1):23-30. [DOI] [PubMed] [Google Scholar]
- Hasebe S, Ohtsuki H, Nonaka T, Nakatsuka C, Miyata M, Hamasaki I, et al. Effect of progressive addition lenses on myopia progression in Japanese children: a prospective, randomized, double-masked, crossover trial. Investigative Ophthalmology and Visual Science 2008;49(7):2781-9. [DOI] [PubMed] [Google Scholar]
- Hasebe S. A clinical trial to evaluate progressive addition lenses on myopia control. Study design. Japanese Journal of Vision Science 2002;23:63-8. [Google Scholar]
- Nakatsuka C, Hasebe S, Nonaka F, Ohtsuki H. Assessment of downward deviation of progressive addition lenses in a myopia control study. In: Investigative Ophthalmology and Visual Science. Vol. 45. 2004:ARVO E-abstract 2732.
- Suemaru J, Hasebe S, Ohtsuki H. Visual symptoms and compliance with spectacle wear in myopic children: double-masked comparison between progressive addition lenses and single vision lenses. Acta Medica Okayama 2008;62(2):109-17. [DOI] [PubMed] [Google Scholar]
Hasebe 2014 {published data only}
- Hasebe S, Jun J, Varnas SR. Myopia control with positively aspherized progressive addition lenses: a 2-year, multicenter, randomized, controlled trial. Investigative Ophthalmology & Visual Science 2014;55(11):7177-88. [DOI] [PubMed] [Google Scholar]
Houston Study 1987 {published data only}
- Grosvenor T, Maslovitz B, Perrigin DM, Perrigin J. The Houston myopia control study: a preliminary report by the patient care team. Journal of the American Optometric Association 1985;56(8):636-43. [PubMed] [Google Scholar]
- Grosvenor T, Perrigin DM, Perrigin J, Maslovitz B. Houston Myopia Control Study: a randomized clinical trial. Part II. Final report by the patient care team. American Journal of Optometry and Physiological Optics 1987;64(7):482-98. [PubMed] [Google Scholar]
- Young FA, Leary GA, Grosvenor T, Maslovitz B, Perrigin DM, Perrigin J, et al. Houston myopia control study: a randomized clinical trial. Part I. Background and design of the study. American Journal of Optometry and Physiological Optics 1985;62(9):605-13. [PubMed] [Google Scholar]
Jensen 1991 {published data only}
- Jensen H. Myopia progression in young school children. A prospective study of myopia progression and the effect of a trial with bifocal lenses and beta blocker eye drops. Acta Ophthalmologica 1991;200(Suppl):1-79. [PubMed] [Google Scholar]
- Jensen H. Timolol maleate in the control of myopia. A preliminary report. Acta Ophthalmologica 1988;185(Suppl):128-9. [DOI] [PubMed] [Google Scholar]
Katz 2003 {published data only}
- Chew SJ, Saw SM, Rajan U, Lau C, O'Brien L, Chan TK, et al. RGP contact lenses in the control of myopia progression. In: Investigative Ophthalmology & Visual Science. Vol. 38. 1997:ARVO E-abstract 5402.
- Katz J, Schein OD, Levy B, Cruiscullo T, Saw SM, Rajan U, et al. A randomized trial of rigid gas permeable contact lenses to reduce progression of children's myopia. American Journal of Ophthalmology 2003;136(1):82-90. [DOI] [PubMed] [Google Scholar]
Koomson 2016 {published data only}
- Koomson NY, Amedo AO, Opoku-Baah C, Ampeh PB, Ankamah E, Bonsu K. Relationship between reduced accommodative lag and myopia progression. Optometry and Vision Science 2016;93(7):683-91. [DOI] [PubMed] [Google Scholar]
Lu 2015 {published data only}
- Lu YM, Ma SS, Luo M, Liang N. Clinical effect of the midperiphery additional designed lenses combined adjustment training on myopia in childhood. International Eye Science 2015;15(11):1960-3. [Google Scholar]
MIT Study 2001 {published data only}
- Hsiao CK, Chen CJ, Shih YF, Lin LL, Hung PT, Yao CL, et al. Design and statistical analysis for the Myopia Intervention Trial in Taiwan. In: Lin LLK, Shih YF, Hung PT , editors(s). Myopia Updates II, 7th International Conference on Myopia, Taipei, Taiwan, Nov-Dec, 1998. Springer-Verlag, 2000:161-4.
- Hsiao CK, Tsai MY, Chen HM. Inference of nested variance components in a longitudinal myopia intervention trial. Statistics in Medicine 2005;24(21):3251-67. [DOI] [PubMed] [Google Scholar]
- Shih YF, Hsiao CK, Chen CJ, Chang CW, Hung PT, Lin LL. An intervention trial on efficacy of atropine and multi-focal glasses in controlling myopic progression. Acta Ophthalmologica Scandinavica 2001;79(3):233-6. [DOI] [PubMed] [Google Scholar]
Pärssinen 1989 {published data only}
- Hemminki E, Pärssinen O. Prevention of myopic progress by glasses. Study design and the first-year results of a randomized trial among schoolchildren. American Journal of Optometry and Physiological Optics 1987;64(8):611-6. [DOI] [PubMed] [Google Scholar]
- Pärssinen O, Hemminki E, Klemetti A. Effect of spectacle use and accommodation on myopic progression: final results of a three-year randomised clinical trial among schoolchildren. British Journal of Ophthalmology 1989;73(7):547-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pärssinen O, Hemminki E. Spectacle-use, bifocals and prevention of myopic progression. The two-years results of a randomized trial among schoolchildren. Acta Ophthalmologica 1988;185(Suppl):156-61. [DOI] [PubMed] [Google Scholar]
- Pärssinen O, Kauppinen M, Viljanen A. Astigmatism among myopics and its changes from childhood to adult age: a 23-year follow-up study. Acta Ophthalmologica 2015;93(3):276-83. [DOI] [PubMed] [Google Scholar]
- Pärssinen O, Kauppinen M, Viljanen A. The progression of myopia from its onset at age 8-12 to adulthood and the influence of heredity and external factors on myopic progression. A 23-year follow-up study. Acta Ophthalmologica 2014;92(8):730-9. [DOI] [PubMed] [Google Scholar]
- Pärssinen O, Kauppinen M. What is the influence of parents' myopia on their children's myopic progression? A 22-year follow-up study. Acta Ophthalmologica 2017;94(6):579-85. [DOI] [PubMed] [Google Scholar]
- Pärssinen O, Lyyra AL. Myopia and myopic progression among schoolchildren: a three-year follow-up study. Investigative Ophthalmology and Visual Science 1993;34(9):2794-802. [PubMed] [Google Scholar]
- Pärssinen O. Astigmatism and school myopia. Acta Ophthalmologica 1991;69(6):786-90. [DOI] [PubMed] [Google Scholar]
- Pärssinen O. Corneal refraction and topography in school myopia. CLAO Journal 1993;19(1):69-72. [DOI] [PubMed] [Google Scholar]
- Pärssinen TO. Long-term connection of myopic progression with different background variables. In: Lin LL, Shih YF, Hung PT , editors(s). Myopia Updates II: Proceedings of the 7th International Conference on Myopia, Taipei, Taiwan, 1998. Springer-Verlag, 2000:21-3.
- Pärssinen TO. Progression of school myopia from its onset at the mean age of 10.9 years: 13-year follow-up study. In: Lin LLK, Shih YF, Hung PT , editors(s). Myopia Updates II: Proceedings of the 7th International Conference on Myopia, Taipei, Taiwan, 1998. Springer-Verlag, 2000:25-8.
PIR‐205 Study 2004 {published data only}
- Bartlett JD, Niemann K, Houde B, Allred T, Edmondson MJ, Crockett RS. A tolerability study of pirenzepine ophthalmic gel in myopic children. Journal of Ocular Pharmacology and Therapeutics 2003;19(3):271-9. [DOI] [PubMed] [Google Scholar]
- Bartlett JD, Niemann K, Houde B, Allred T, Edmondson MJ. Safety and tolerability of pirenzepine ophthalmic gel in pediatric, myopic patients. In: Investigative Ophthalmology & Visual Science. Vol. 41. 2000:ARVO E-abstract 1598.
- Bartlett JD, Voce M, Than TP, Edmondson M, Novack GD. Electronic monitoring system to assess patient adherence in pediatric drug studies. In: Investigative Ophthalmology and Visual Science. Vol. 44. 2003:ARVO E-abstract 1930.
- Chu R, Cotter S, Kwon S, PIR-205 Investigator Group. Pirenzepine 2% ophthalmic gel retards myopia progression in 8-12 year old children. In: American Academy of Optometry. 2003:170.
- Cotter SA, Chu RH, Kwon S. Pirenzepine 2% ophthalmic gel retards myopia progression in 8- to 12-year-old children. Optometry 2003;74:382-3. [Google Scholar]
- Kwon S, Cotter S, Chu R, Flores Y. Safety and efficacy of 2% pirenzepine ophthalmic gel in children with myopia: year two. In: American Academy of Optometry. 2001:144.
- Kwon S, Cotter S, Flores Y. Collaborative Assessment of Myopia Progression with pirenzepine (CAMP) study: recruitment underway for FDA (PIR-205) clinical trial. In: American Academy of Optometry. 2000:99.
- Siatkowski RM, Cotter SA, Crockett RS, Miller JM, Novack GD, Zadnik K, et al. Two-year multicenter, randomized, double-masked, placebo-controlled, parallel safety and efficacy study of 2% pirenzepine ophthalmic gel in children with myopia. Journal of AAPOS 2008;12(4):332-9. [DOI] [PubMed] [Google Scholar]
- Siatkowski RM, Cotter SA, Miller JM, Scher CA, Crockett RS, Novack GD. Pirenzepine 2% ophthalmic gel retards myopic progression in 8-12 year old children. In: Investigative Ophthalmology and Visual Science. Vol. 44. 2003:ARVO E-abstract 4778.
- Siatkowski RM, Cotter SA, Miller JM, Scher CA, Crockett RS, Novack GD. Safety and efficacy of 2% pirenzepine ophthalmic gel in children with myopia: a 1-year, multicenter, double-masked, placebo-controlled parallel study. Ophthalmology 2004;122(11):1667-74. [DOI] [PubMed] [Google Scholar]
- Siatkowski RM, Cotter SA, Miller JM, Scher CA, Crockett RS, US Pirenzepine Study Group. Pirenzepine 2% ophthalmic gel retards myopic progression in 8-12 year old children over two years. In: Investigative Ophthalmology and Visual Science. Vol. 45. 2004:ARVO E-abstract 2733.
- Tan DT, Lam D, Chua WH, Crockett RS. Pirenzepine ophthalmic gel (PIR): safety and efficacy for pediatric myopia in a one-year study in Asia. In: Investigative Ophthalmology & Visual Science. Vol. 44. 2003:ARVO E-abstract 801.
ROMIO Study 2012 {published data only}
- Chan KY, Cheung SW, Cho P. Clinical performance of an orthokeratology lens fitted with the aid of a computer software in Chinese children. Contact Lens & Anterior Eye 2012;35(4):180-4. [DOI] [PubMed] [Google Scholar]
- Cheung SW, Cho P. Long-term effect of orthokeratology on the anterior segment length. Contact Lens & Anterior Eye 2016;39(4):262-5. [DOI] [PubMed] [Google Scholar]
- Cheung SW, Cho P. Validity of axial length measurements for monitoring myopic progression in orthokeratology. Investigative Ophthalmology & Visual Science 2013;54(3):1613-5. [DOI] [PubMed] [Google Scholar]
- Cho P, Cheung SW. Orthokeratology for slowing myopic progression: a randomised controlled trial. Contact Lens & Anterior Eye 2011;34:S2-3. [DOI] [PubMed] [Google Scholar]
- Cho P, Cheung SW. Protective role of orthokeratology in reducing risk of rapid axial elongation: a reanalysis of data from the ROMIO and TO-SEE studies. Investigative Ophthalmology and Visual Science 2017;58(3):1411-6. [DOI] [PubMed] [Google Scholar]
- Cho P, Cheung SW. Retardation of Myopia in Orthokeratology (ROMIO) study: a 2-year randomized clinical trial. Investigative Ophthalmology & Visual Science 2012;53(11):7077-85. [DOI] [PubMed] [Google Scholar]
Sankaridurg 2010 {published data only}
- Donovan L, Sankaridurg P, Ho A, Chen X, Lin Z, Thomas V, et al. Myopia progression in Chinese children is slower in summer than in winter. Optometry and Vision Science 2012;89(8):1196-202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sankaridurg P, Chen X, Naduvilath T, la Jara PL, Lin Z, Li L, et al. Adverse events during 2 years of daily wear of silicone hydrogels in children. Optometry and Vision Science 2013;90(9):961-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sankaridurg P, Donovan L, Varnas S, Ho A, Chen X, Martinez A, et al. Spectacle lenses designed to reduce progression of myopia: 12-month results. Optometry and Vision Science 2010;87(9):631-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sankaridurg P, Holden B, Smith E, Naduvilath T, Chen X, Jara PL, et al. Decrease in rate of myopia progression with a contact lens designed to reduce relative peripheral hyperopia: one-year results. Investigative Ophthalmology and Visual Science 2011;52(13):9362-7. [DOI] [PubMed] [Google Scholar]
Schwartz 1981 {published data only}
- Schwartz JT. A monozygotic cotwin control study of a treatment for myopia. Acta Geneticae Medicae et Gemellologiae 1974;23(spec. nr.):26. [DOI] [PubMed] [Google Scholar]
- Schwartz JT. A monozygotic co-twin control study of a treatment for myopia. Acta Geneticae Medicae et Gemellologiae 1976;25:133-6. [DOI] [PubMed] [Google Scholar]
- Schwartz JT. Results of a monozygotic co-twin control study on a treatment for myopia. Acta Geneticae Medicae et Gemellologiae 1980;29(1):30. [DOI] [PubMed] [Google Scholar]
- Schwartz JT. Results of a monozygotic co-twin control study on a treatment for myopia. Progress in Clinical & Biological Research 1981;Pt C:249-58. [PubMed] [Google Scholar]
Shih 1999 {published data only}
- Chen CH, Shih YF, Chou AC, Ho TC, Lin LLK, Hung PT. The effect of different concentrations of atropine on myopia controlling in myopic children. In: Investigative Ophthalmology & Visual Science. Vol. 38. 1997:ARVO E-abstract 4537.
- Shih YF, Chen CH, Chou AC, Ho TC, Lin LL, Hung PT. Effects of different concentrations of atropine on controlling myopia in myopic children. Journal of Ocular Pharmacology and Therapeutics 1999;15(1):85-90. [DOI] [PubMed] [Google Scholar]
STAMP Study 2012 {published data only}
- Berntsen D, Mutti D, Zadnik K. The effect of bifocal add, correction type, and adaptation on accommodative lag in myopic children. In: American Academy of Optometry. 2009.
- Berntsen D, Mutti DO, Zadnik K. Baseline characteristics of the study of theories about myopia progression (STAMP). In: American Academy of Optometry. 2008. [DOI] [PMC free article] [PubMed]
- Berntsen DA, Barr CD, Mutti DO, Zadnik K. Peripheral defocus and myopia progression in myopic children randomly assigned to wear single vision and progressive addition lenses. Investigative Ophthalmology and Visual Science 2013;54(8):5761-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berntsen DA, Mutti DO, Zadnik K. Study of Theories About Myopia Progression (STAMP) design and baseline data. Optometry and Vision Science 2011;87(11):823-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berntsen DA, Mutti DO, Zadnik K. The effect of bifocal add on accommodative lag in myopic children with high accommodative lag. Investigative Ophthalmology and Visual Science 2010;51(12):6104-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berntsen DA, Sinnott LT, Mutti DO, Zadnik K. A randomized trial using progressive addition lenses to evaluate theories of myopia progression in children with a high lag of accommodation. Investigative Ophthalmology and Visual Science 2012;53(2):640-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Swarbrick 2015 {published data only}
- Swarbrick HA, Alharbi A, Lum E, Watt K. Overnight orthokeratology for myopia control: short-term effects on axial length and refractive error. Contact Lens & Anterior Eye 2011;34:S3. [Google Scholar]
- Swarbrick HA, Alharbi A, Watt K, Lum E, Kang P. Myopia control during orthokeratology lens wear in children using a novel study design. Ophthalmology 2015;122(3):620-30. [DOI] [PubMed] [Google Scholar]
Tan 2005 {published data only}
- Tan D, Chia A, Han CW, Bun CY. Author reply: one-year multicenter, double-masked, placebo-controlled, parallel safety and efficacy study of 2% pirenzepine ophthalmic gel in children with myopia. Ophthalmology 2012;119(12):2653-4. [DOI] [PubMed] [Google Scholar]
- Tan DT, Lam D, Chua WH, Crockett RS. Pirenzepine ophthalmic gel (PIR): safety and efficacy for pediatric myopia in a one-year study in Asia. In: Investigative Ophthalmology and Visual Science. Vol. 44. 2003:ARVO E-abstract 801.
- Tan DT, Lam DS, Chua WH, Shu-Ping DF, Crockett RS, Asian Pirenzepine Study Group. One-year multicenter, double-masked, placebo-controlled, parallel safety and efficacy study of 2% pirenzepine ophthalmic gel in children with myopia. Ophthalmology 2005;112(1):84-91. [DOI] [PubMed] [Google Scholar]
Trier 2008 {published data only}
- Trier K, Munk Ribel-Madsen S, Cui D, Brøgger Christensen S. Systemic 7-methylxanthine in retarding axial eye growth and myopia progression: a 36-month pilot study. Journal of Ocular Biology, Diseases, and Informatics 2008;1(2-4):85-93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trier K, Ribel-Madsen SM. Effect of 7-methylxanthine on axial eye growth in myopic children—24 months follow-up. In: Investigative Ophthalmology & Visual Science. Vol. 48. 2007:ARVO E-abstract 4421.
- Trier K, Ribel-Madsen SM. Effect of 7-methylxanthine on eye growth in myopic children—36 months follow-up. In: Investigative Ophthalmology & Visual Science. Vol. 49. 2008:ARVO E-abstract 2609.
Wang 2005 {published data only}
- Wang X, Chu R. Wearing progressive addition lenses (PALs) to slow down the progression of juvenile myopia. In: Investigative Ophthalmology & Visual Science. Vol. 46. 2005:ARVO E-abstract 5595.
Wang 2017 {published data only}
- Wang YR, Bian HL, Wang Q. Atropine 0.5% eyedrops for the treatment of children with low myopia: a randomized controlled trial. Medicine 2017;96(27):e7371. [DOI] [PMC free article] [PubMed] [Google Scholar]
Yang 2009 {published data only}
- Lan W, Yang Z, Ge J, Liu W, Chen X. The effectiveness of progressive additional lens on Chinese juvenile-onset acquired myopia: the first year report. Ophthalmic & Physiological Optics 2006;26:8. [Google Scholar]
- Yang Z, Lan W, Ge J, Liu W, Chen X, Chen L, et al. The effectiveness of progressive addition lenses on the progression of myopia in Chinese children. Ophthalmic & Physiological Optics 2009;29(1):41-8. [DOI] [PubMed] [Google Scholar]
Yen 1989 {published data only}
- Yen MY, Liu JH, Kao SC, Shiao CH. Comparison of the effect of atropine and cyclopentolate on myopia. Annals of Ophthalmology 1989;21(5):180-2, 187. [PubMed] [Google Scholar]
Yi 2015 {published data only}
- Yi S, Huang Y, Yu SZ, Chen XJ, Yi H, Zeng XL. Therapeutic effect of atropine 1% in children with low myopia. Journal of AAPOS 2015;19(5):426-9. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Abraham 1966 {published data only}
- Abraham SV. Control of myopia with tropicamide. Journal of Pediatric Ophthalmology 1966;3:10-22. [Google Scholar]
ACHIEVE Study 2008 {published data only}
- Jones-Jordan LA, Chitkara M, Coffey B, Jackson JM, Manny RE, Rah MJ, et al. A comparison of spectacle and contact lens wearing times in the ACHIEVE study. Clinical and Experimental Optometry 2010;93(3):157-63. [DOI] [PubMed] [Google Scholar]
- Rah MJ, Walline JJ, Jones-Jordan LA, Sinnott LT, Jackson JM, ACHIEVE Study Group. Vision specific quality of life of pediatric contact lens wearers. Optometry and Vision Science 2010;87(8):560-6. [DOI] [PubMed] [Google Scholar]
- Walline JJ, Jones LA, Chitkara M, Coffey B, Jackson JM, ACHIEVE Study Group. The Adolescent and Child Health Initiative to Encourage Vision Empowerment (ACHIEVE) study. In: American Academy of Optometry. 2004. [DOI] [PubMed]
- Walline JJ, Jones LA, Chitkara M, Coffey B, Jackson JM, ACHIEVE Study Group. The Adolescent and Child Health Initiative to Encourage Vision Empowerment (ACHIEVE) study design and baseline data. Optometry and Vision Science 2006;83(1):37-45. [DOI] [PubMed] [Google Scholar]
- Walline JJ, Jones LA, Sinnott L, Chitkara M, Coffey B, Jackson JM, et al. Soft contact lenses do not increase myopia progression in children. In: Investigative Ophthalmology & Visual Science. Vol. 49. 2008:ARVO E-abstract 2021. [DOI] [PubMed]
- Walline JJ, Jones LA, Sinnott L, Chitkara M, Coffey B, Jackson JM, et al. The effect of contact lens wear on children's self-perceptions. In: American Academy of Optometry. 2009.
- Walline JJ, Jones LA, Sinnott L, Chitkara M, Coffey B, ACHIEVE Study Group. Randomized trial of the effect of contact lens wear on self-perception in children. Optometry and Vision Science 2009;86(3):222-32. [DOI] [PubMed] [Google Scholar]
- Walline JJ, Jones LA, Sinnott L, Manny RE, Gaume A, ACHIEVE Study Group. A randomized trial of the effect of soft contact lenses on myopia progression in children. Investigative Ophthalmology and Visual Science 2008;49(11):4702-6. [DOI] [PubMed] [Google Scholar]
Aller 2008 {published data only}
- Aller TA, Wildsoet C. Bifocal soft contact lenses as a possible myopia control treatment: a case report involving identical twins. Clinical and Experimental Optometry 2008;91(4):394-9. [DOI] [PubMed] [Google Scholar]
Andreo 1990 {published data only}
- Andreo LK. Long-term effects of hydrophilic contact lenses on myopia. Annals of Ophthalmology 1990;22(6):224-7. [PubMed] [Google Scholar]
ATOM 2 Study 2012 {published data only}
- Chia A, Chua WH, Cheung YB, Wong WL, Lingham A, Fong A, et al. Atropine for the treatment of childhood myopia: safety and efficacy of 0.5%, 0.1%, and 0.01% doses (Atropine for the Treatment of Myopia 2). Ophthalmology 2012;119(2):347-54. [DOI] [PubMed] [Google Scholar]
- Chia A, Chua WH, Wen L, Fong A, Goon YY, Tan D. Atropine for the treatment of childhood myopia: changes after stopping atropine 0.01%, 0.1% and 0.5%. American Journal of Ophthalmology 2014;157(2):451-7. [DOI] [PubMed] [Google Scholar]
- Chia A, Li W, Tan D, Luu CD. Full-field electroretinogram findings in children in the Atropine Treatment for Myopia (ATOM2) study. Documenta Ophthalmologica. Advances in Ophthalmology 2013;126(3):177-86. [DOI] [PubMed] [Google Scholar]
- Chia A, Lu QS, Tan D. Five-year clinical trial on Atropine for the Treatment of Myopia 2: myopia control with atropine 0.01% eyedrops. Ophthalmology 2016;123(2):391-9. [DOI] [PubMed] [Google Scholar]
- Chia A, Luu CD, Li W, Cheung YB, Tan D. Full-field electroretinogram findings in myopic children on topical atropine. Documenta Ophthalmologica. Advances in Ophthalmology 2011;123(Suppl 1):42. [DOI] [PubMed] [Google Scholar]
- Chua WH. Establishing an optimal dose of atropine for slowing progression of childhood myopia. Ophthalmic and Physiological Optics 2006;26:29. [Google Scholar]
Baldwin 1969 {published data only}
- Baldwin WR, West D, Jolley J, Reid W. Effects of contact lenses on refractive corneal and axial length changes in young myopes. American Journal of Optometry and Archives of American Academy of Optometry 1969;46(12):903-11. [DOI] [PubMed] [Google Scholar]
Baltimore Myopia Project 1946 {published data only}
- Betts AE. An evaluation of the Baltimore myopia control project part A. Experimental procedures. Journal of the American Optometric Association 1947;18:481-5. [Google Scholar]
- Ewalt HW Jr. The Baltimore myopia control project. Journal of the American Optometric Association 1946;17:167-85. [Google Scholar]
Baronet 1979 {published data only}
- Baronet, Lecaillon-Thiobon. Longitudinal study of developing myopia and effects of treatment with atropine and Difrarel E [Étude longitudinale des myopies évolutives et effets du traitement par atropine et Difrarel E]. Bulletin des Societes d Ophtalmologie de France 1979;79(4-5):417-21. [PubMed] [Google Scholar]
Bedrossian 1979 {published data only}
- Bedrossian RH. The effect of atropine on myopia. Annals of Ophthalmology 1971;3(8):891-7. [PubMed] [Google Scholar]
- Bedrossian RH. The effect of atropine on myopia. Ophthalmology 1979;86(5):713-9. [DOI] [PubMed] [Google Scholar]
- Bedrossian RH. Treatment of progressive myopia with atropine. In: Weigelin E , editors(s). XX Concilium Ophthalmologicum Germania, International Congress Series No. 146. Amsterdam: Excerpta Medica Foundation, 1966:612-7.
Berkeley OK Study 1983 {published data only}
- Brand RJ, Polse KA, Schwalbe JS. The Berkeley Orthokeratology Study, Part I. General conduct of the study. American Journal of Optometry and Physiological Optics 1983;60(3):175-86. [DOI] [PubMed] [Google Scholar]
- Polse KA, Brand RJ, Keener RJ, Schwalbe JS, Vastine DW. The Berkeley Orthokeratology Study, Part III. Safety. American Journal of Optometry and Physiological Optics 1983;60(4):321-8. [DOI] [PubMed] [Google Scholar]
- Polse KA, Brand RJ, Schwalbe JS, Vastine DW, Keener RJ. The Berkeley Orthokeratology Study, Part II. Efficacy and duration. American Journal of Optometry and Physiological Optics 1983;60(3):187-98. [DOI] [PubMed] [Google Scholar]
- Polse KA, Brand RJ, Vastine DW, Schwalbe JS. Corneal change accompanying orthokeratology. Plastic or elastic? Results of a randomized controlled clinical trial. Archives of Ophthalmology 1983;101(12):1873-8. [DOI] [PubMed] [Google Scholar]
- Polse KA, Brand RJ. Contact lens effects on ametropia: a current example of the clinical trial. American Journal of Optometry and Physiological Optics 1981;58(4):281-8. [PubMed] [Google Scholar]
Bier 1988 {published data only}
- Bier N, Lowther G. Myopia Control Study: effect of different contact lens refractive corrections on the progression of myopia. Optometry Today 1988;28:38-40. [Google Scholar]
Brodstein 1984 {published data only}
- Brodstein RS, Brodstein DE, Olson RJ, Hunt SC, Williams RR. The treatment of myopia with atropine and bifocals: a long-term prospective study. Ophthalmology 1984;91(11):1373-9. [DOI] [PubMed] [Google Scholar]
Chan 2014 {published data only}
- Chan KY, Cheung SW, Cho P. Orthokeratology for slowing myopic progression in a pair of identical twins. Contact Lens & Anterior Eye 2014;37(2):116-9. [DOI] [PubMed] [Google Scholar]
Chen 2012 {published data only}
- Chen Z, Niu LL, Xue F, Qu XM, Zhou ZM, Zhou XT, et al. Impact of pupil diameter on axial growth in orthokeratology. Optometry and Vision Science 2012;89(11):1636-40. [DOI] [PubMed] [Google Scholar]
Chen 2014a {published data only}
- Chen YH. Clinical observation of the development of juvenile myopia wearing glasses with full correction and under-correction. International Eye Science 2014;14(8):1553-4. [Google Scholar]
Chen 2016 {published data only}
- Chen Z, Xue F, Zhou J, Qu X, Zhou X. Effects of orthokeratology on choroidal thickness and axial length. Optometry and Vision Science 2016;93(9):1064-71. [DOI] [PubMed] [Google Scholar]
Cho 2012 {published data only}
- Cho P, Chan B, Cheung SW, Mountford J. Do fenestrations affect the performance of orthokeratology lenses? Optometry and Vision Science 2012;89(4):401-10. [DOI] [PubMed] [Google Scholar]
Cho 2017 {published data only}
- Cho P, Cheung SW. Discontinuation of orthokeratology on eyeball elongation (DOEE). Contact Lens & Anterior Eye 2017;40(2):82-7. [DOI] [PubMed] [Google Scholar]
Choi 2005 {published data only}
- Choi YY, Jeong JW, Park DJ. Effect of 1% atropine with tinted bifocal glasses in slowing the progression of myopia in children. In: American Academy of Ophthalmology. 2005:234.
Chou 1997 {published data only}
- Chou AC, Shih YF, Ho TC, Lin LL. The effectiveness of 0.5% atropine in controlling high myopia in children. Journal of Ocular Pharmacology and Therapeutics 1997;13(1):61-7. [DOI] [PubMed] [Google Scholar]
Dumbleton 1999 {published data only}
- Dumbleton KA, Chalmers RL, Richter DB, Fonn D. Changes in myopic refractive error with nine months' extended wear of hydrogel lenses with high and low oxygen permeability. Optometry and Vision Science 1999;76(12):845-9. [DOI] [PubMed] [Google Scholar]
- Dumbleton KA, Richter D, Fonn D, Chalmers R. Refractive and keratometric changes following extended wear. In: American Academy of Optometry. 1998:170.
Dyer 1979 {published data only}
- Dyer JA. Role of cycloplegics in progressive myopia. Ophthalmology 1979;86(5):692-4. [DOI] [PubMed] [Google Scholar]
Ebri 2007 {published data only}
- Ebri A, Kuper H, Wedner S. Cost-effectiveness of cycloplegic agents: results of a randomized controlled trial in Nigerian children. Investigative Ophthalmology and Visual Science 2007;48(3):1025-31. [DOI] [PubMed] [Google Scholar]
Eissa 2018 {published data only}
- Eissa S, Badr Eldin N. ICL versus SMILE in management of anisometropic myopic amblyopia in children. Canadian Journal of Ophthalmology 2018;53:560-7. [DOI] [PubMed] [Google Scholar]
Filip 2000 {published data only}
- Filip M, Stefaniu I, Stefan C. Changes in myopic refractive errors after 9 months of extensive wear of hydrogel lenses with high oxygen permeability and compared with those with low permeability. Oftalmologia 2000;51(2):35-40. [PubMed] [Google Scholar]
Gimbel 1973 {published data only}
- Gimbel HV. The control of myopia with atropine. Canadian Journal of Ophthalmology 1973;8(4):527-32. [PubMed] [Google Scholar]
Goss 1984 {published data only}
- Goss DA. Overcorrection as a means of slowing myopic progression. American Journal of Optometry and Physiological Optics 1984;61(2):85-93. [DOI] [PubMed] [Google Scholar]
Grosvenor 1991 {published data only}
- Grosvenor T, Perrigin D, Perrigin J, Quintero S. Do rigid gas-permeable contact lenses control the progress of myopia? Contact Lens and Spectacles 1991;6(7):29-35. [Google Scholar]
He 2016 {published data only}
- He MM, Du YR, Liu QY, Ren CD, Liu JL, Wang QY, et al. Short-term effects of orthokeratology on the development of low-to-moderate myopia in Chinese children. International Eye Science 2016;16(2):237-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
He 2018 {published data only}
- He X, Sankaridurg P, Xiong S, Li W, Zhang B, Weng R, Zhu J, et al. Shanghai time outside to reduce myopia trial: design and baseline data. Clinical & Experimental Ophthalmology 2019;47:171-8. [DOI] [PubMed] [Google Scholar]
Horner 1999 {published data only}
- Horner DG, Gross DA, Soni PS, Schroeder TL. Principal axis analysis to determine the contribution of axial elongation to myopia progression. In: Investigative Ophthalmology & Visual Science. Vol. 38. 1997:ARVO E-abstract 4546.
- Horner DG, Salmon TO, Soni PS. Junior high age children's myopia progression in soft lenses vs. spectacles. In: American Academy of Optometry. 1994:78.
- Horner DG, Salmon TO, Soni PS. Junior high age children's myopia progression in soft lenses vs. spectacles. In: American Academy of Optometry. 1995:96.
- Horner DG, Soni PS, Ross J. Junior high age children's myopia progresses equally in soft lenses and spectacles. Investigative Ophthalmology & Visual Science 1994;35:ARVO E-abstract 735. [Google Scholar]
- Horner DG, Soni PS, Salmon TO, Schroeder T. Junior high age children's myopia progression in soft lenses vs. spectacles. In: Investigative Ophthalmology & Visual Science. Vol. 37. 1996:ARVO E-abstract 4610.
- Horner DG, Soni PS, Salmon TO, Swartz TS. Myopia progression in adolescent wearers of soft contact lenses and spectacles. Optometry and Vision Science 1999;76(7):474-9. [DOI] [PubMed] [Google Scholar]
- Terry RL, Soni PS, Horner DG. Spectacles, contact lenses, and children's self-concepts: a longitudinal study. Optometry and Vision Science 1997;74(12):1044-8. [PubMed] [Google Scholar]
Hosaka 1982 {published data only}
- Hosaka A, Abiko Y, Teranishi C. Topical use of labetalol in the treatment of pseudomyopia. In: Proceedings from the 2nd International Conference on Myopia, San Francisco, 1978. New York: Myopia International Research Foundation Proceedings, 1982:339-52.
Hosaka 1988 {published data only}
- Hosaka A. Myopia prevention and therapy. The role of pharmaceutical agents. Japanese studies. Acta Ophthalmologica 1988;185(Suppl):130-1. [DOI] [PubMed] [Google Scholar]
Hua 2017 {published data only}
- Hua WJ, Jin JX, Wu XY, Yang JW, Jiang X, Gao GP, et al. Elevated light levels in schools have a protective effect on myopia. Ophthalmic & Physiological Optics 2015;35(3):252-62. [DOI] [PubMed] [Google Scholar]
Huffman 2002 {published data only}
- Huffman KD, Ross S, Pack L, Salmon T, Hoenes R. Visual and optical performance of frequency 55 aspheric vs. spheric contact lenses. In: American Academy of Optometry. 2002.
Jiang 2018 {published data only}
- Jiang J. Effect of orthokeratology, low concentration atropine and frame glasses on juvenile myopia prevention and control. International Eye Science 2018;18:1349-52. [Google Scholar]
Kao 1988 {published data only}
- Kao SC, Lu HY, Liu JH. Atropine effect on school myopia. A preliminary report. Acta Ophthalmologica 1988;185(Suppl):132-3. [DOI] [PubMed] [Google Scholar]
Keller 1996 {published data only}
- Keller J. Myopia control with RGPs in children. Contact Lens and Spectacles 1996;11(12):45-8. [Google Scholar]
Kennedy 1995 {published data only}
- Kennedy RH. Progression of myopia. Transactions of the American Ophthalmological Society 1995;93:755-800. [PMC free article] [PubMed] [Google Scholar]
Khoo 1999 {published data only}
- Khoo CY, Chong J, Chew SJ, Cheng HM, Rajan U. The effect of RGP contact lens wear on school myopia: a three-year study. In: Investigative Ophthalmology & Visual Science. Vol. 39. 1998:ARVO E-abstract 1289.
- Khoo CY, Chong J, Rajan U. A 3-year study on the effect of RGP contact lenses on myopic children. Singapore Medical Journal 1999;40(4):230-7. [PubMed] [Google Scholar]
Kubena 2002 {published data only}
- Kubena T, Kubena K Jr, Kubena K. Effect of TLT absorptive eyeglasses on progression of myopia in children. Ceska a Slovenska Oftalmologie 2002;58(6):377-81. [PubMed] [Google Scholar]
Lakkis 2006 {published data only}
- Lakkis C, Weidemann K. Evaluation of the performance of photochromic spectacle lenses in children and adolescents aged 10 to 15 years. Clinical and Experimental Optometry 2006;89(4):246-52. [DOI] [PubMed] [Google Scholar]
Lee 2016 {published data only}
- Lee CY, Sun CC, Lin YF, Lin KK. Effects of topical atropine on intraocular pressure and myopia progression: a prospective comparative study. BMC Ophthalmology 2016;16:114. [DOI] [PMC free article] [PubMed] [Google Scholar]
Leung 1999 {published data only}
- Brown B, Edwards MH, Leung JT. Is esophoria a factor in slowing of myopia by progressive lenses? Optometry and Vision Science 2002;79(10):638-42. [DOI] [PubMed] [Google Scholar]
- Brown B, Edwards MH. Is esophoria a factor in slowing of myopia by progressive lenses? Author's response. Optometry and Vision Science 2003;80(3):199. [DOI] [PubMed] [Google Scholar]
- Leung JT, Brown B. Progression of myopia in Hong Kong Chinese schoolchildren is slowed by wearing progressive lenses. Optometry and Vision Science 1999;76(6):346-54. [DOI] [PubMed] [Google Scholar]
- Leung JT, Brown B. The effect of progressive lenses on the progression of myopia in Chinese school children. In: American Academy of Optometry. 1997:130.
Li 2005 {published data only}
- Li JP, Wang W. Effect of progressive multifocal lenses for juvenile myopia in 876 cases. International Journal of Ophthalmology 2005;5(3):599-601. [Google Scholar]
Liang 2008 {published data only}
- Liang CK, Ho TY, Li TC, Hsu WM, Li TM, Lee YC, et al. A combined therapy using stimulating auricular acupoints enhances lower-level atropine eyedrops when used for myopia control in school-aged children evaluated by a pilot randomized controlled clinical trial. Complementary Therapies in Medicine 2008;16(6):305-10. [DOI] [PubMed] [Google Scholar]
- Liang CK, Ho TY, Li TC, Hsu WM, Li TM, Lee YC. Auricular acupoints enhance atropine eyedrops in myopic children. Journal of the Australian Traditional-Medicine Society 2009;15:151. [Google Scholar]
Lu 2010 {published data only}
- Lu PC, Chen JC. Retarding progression of myopia with seasonal modification of topical atropine. Journal of Ophthalmic and Vision Research 2010;5(2):75-81. [PMC free article] [PubMed] [Google Scholar]
Ma 2014 {published data only}ISRCTN03252665
- Ma X, Congdon N, Yi H, Zhou Z, Pang X, Meltzer ME, et al. Safety of spectacles for children's vision: a cluster-randomized controlled trial. American Journal of Ophthalmology 2015;160(5):897-904. [DOI] [PubMed] [Google Scholar]
- Ma X, Zhou Z, Yi H, Pang X, Shi Y, Chen Q, et al. Effect of providing free glasses on children's educational outcomes in China: cluster randomized controlled trial. BMJ 2014;349:g5740. [DOI] [PMC free article] [PubMed] [Google Scholar]
Mandell 1959 {published data only}
- Mandell RB. Myopia control with bifocal correction. American Journal of Optometry and Archives of American Academy of Optometry 1959;36:652-8. [DOI] [PubMed] [Google Scholar]
Meythaler 1971 {published data only}
- Meythaler H, Ruppert W. The myopic and miotic effect of pilocarpin and glaucostat [Vergleichende Untersuchungen über den myopisierenden und miotischen Effekt von Pilocarpin und Aceclydine (Glaucostat)]. Albrecht Von Graefe's Archive for Clinical and Experimental Ophthalmology 1971;181(3):234-45. [DOI] [PubMed] [Google Scholar]
NCT00348166 {published data only}
- NCT00348166. Does undercorrection of myopia retard myopia progression among kindergarten children? clinicaltrials.gov/ct2/show/NCT00348166 (first received October 5, 2011).
NCT03372551 {published data only}
- NCT03372551. Comparison of Somofilcon a daily disposable test contact lens and Somofilcon a daily disposable control contact lens. clinicaltrials.gov/ct2/show/NCT03372551 (first received July 23, 2019).
NCT03512626 {published data only}
- NCT03512626. Clinical evaluation of multifocal intraocular lens: OPTIVIS.TM. clinicaltrials.gov/ct2/show/NCT03512626 (first received May 1, 2018).
Neetens 1985 {published data only}
- Neetens A, Evens P. The use of bifocals as alternative in the management of low grade myopia. Bulletin de la Societe Belge d Ophtalmologie 1985;214:79-85. [PubMed] [Google Scholar]
Nesterov 1990 {published data only}
- Nesterov AP, Svirin AV, Lapochkin VI. Drug therapy of progressive myopia. Vestnik Oftalmologii 1990;106(2):25-8. [PubMed] [Google Scholar]
Oakley 1975 {published data only}
- Oakley KH, Young FA. Bifocal control of myopia. American Journal of Optometry and Physiological Optics 1975;52(11):758-64. [DOI] [PubMed] [Google Scholar]
Parker 1958 {published data only}
- Parker MW. Protective-corrective program for young myopes. Optometry Weekly 1958;49:681-3. [Google Scholar]
Perrigin 1990 {published data only}
- Grosvenor T, Perrigin J, Perrigin D, Quintero S. Use of silicone-acrylate contact lenses for the control of myopia: results after two years of lens wear. Optometry and Vision Science 1989;66(1):41-7. [DOI] [PubMed] [Google Scholar]
- Perrigin J, Perrigin D, Quintero S, Grosvenor T. Silicone-acrylate contact lenses for myopia control: 3-year results. Optometry and Vision Science 1990;67(10):764-9. [DOI] [PubMed] [Google Scholar]
Pirenzepine 2003 {published data only}
- No author. Pirenzepine for the treatment of myopia [Schärfer sehen mit Pirenzepin]. Deutsche Apotheker Zeitung 2003;143(12):54. [Google Scholar]
Plowright 2015 {published data only}
- Plowright AJ, Maldonado-Codina C, Howarth GF, Kern J, Morgan PB. Daily disposable contact lenses versus spectacles in teenagers. Optometry and Vision Science 2015;92(1):44-52. [DOI] [PubMed] [Google Scholar]
Pritchard 1999 {published data only}
- Pritchard N, Fonn D. Myopia associated with extended wear of low-oxygen-transmissible hydrogel lenses. In: American Academy of Optometry. 1999:169.
Rah 2002 {published data only}
- Rah MJ, Jackson JM, Jones LA, Marsden HJ, Bailey MD, Barr JT. Overnight orthokeratology: preliminary results of the Lenses and Overnight Orthokeratology (LOOK) study. Optometry and Vision Science 2002;79(9):598-605. [DOI] [PubMed] [Google Scholar]
Rainey 2000 {published data only}
- Rainey BB, Goss DA. The effect of vision therapy on myopia progression. In: American Academy of Optometry. 2000:283.
Ritchey 2005 {published data only}
- Ritchey ER, Barr JT, Mitchell GL. The comparison of overnight lens modalities (COLM) study. Eye and Contact Lens 2005;31(2):70-5. [DOI] [PubMed] [Google Scholar]
Sankaridurg 2003 {published data only}
- Sankaridurg PR, Sweeney DF, Holden BA, Naduvilath T, Velala I, Gora R, et al. Comparison of adverse events with daily disposable hydrogels and spectacle wear: results from a 12-month prospective clinical trial. Ophthalmology 2003;110(12):2327-34. [DOI] [PubMed] [Google Scholar]
Santodomingo‐Rubido 2012 {published data only}
- Santodomingo-Rubido J, Villa-Collar C, Gilmartin B, Gutierrez-Ortega R, Sugimoto K. Long-term efficacy of orthokeratology contact lens wear in controlling the progression of childhood myopia. Current Eye Research 2017;42(5):713-20. [DOI] [PubMed] [Google Scholar]
- Santodomingo-Rubido J, Villa-Collar C, Gilmartin B, Gutierrez-Ortega R. Factors preventing myopia progression with orthokeratology correction. Optometry and Vision Science 2013;90(11):1225-36. [DOI] [PubMed] [Google Scholar]
- Santodomingo-Rubido J, Villa-Collar C, Gilmartin B, Gutierrez-Ortega R. Myopia control with orthokeratology contact lenses in Spain: a comparison of vision-related quality-of-life measures between orthokeratology contact lenses and single-vision spectacles. Eye & Contact Lens 2013;39(2):153-7. [DOI] [PubMed] [Google Scholar]
- Santodomingo-Rubido J, Villa-Collar C, Gilmartin B, Gutierrez-Ortega R. Myopia control with orthokeratology contact lenses in Spain: refractive and biometric changes. Investigative Ophthalmology and Visual Science 2012;53(8):5060-5. [DOI] [PubMed] [Google Scholar]
- Santodomingo-Rubido J, Villa-Collar C, Gilmartin B, Gutierrez-Ortega R. Myopia control with orthokeratology contact lenses in Spain (MCOS): design, baseline findings and 12 month data. In: American Academy of Optometry. 2009.
- Santodomingo-Rubido J, Villa-Collar C, Gilmartin B, Gutierrez-Ortega R. Myopia control with orthokeratology contact lenses in Spain (MCOS): predictive factors associated with myopia progression. Contact Lens & Anterior Eye 2012;35:e16. [Google Scholar]
- Santodomingo-Rubido J, Villa-Collar C, Gilmartin B, Gutierrez-Ortega R. Orthokeratology vs. spectacles: adverse events and discontinuations. Optometry and Vision Science 2012;89(8):1133-9. [DOI] [PubMed] [Google Scholar]
- Santodomingo Rubido J, Villa-Collar C. Myopia control with orthokeratology contact lenses in Spain (MCOS): design, baseline findings and 6 month data. In: American Academy of Optometry. 2008.
Savoliuk 1968 {published data only}
- Savoliuk MM. Optical correction and progressive myopia. Vestnik Oftalmologii 1968;81(1):82-3. [PubMed] [Google Scholar]
Shen 2011 {published data only}
- Shen EP, Hsieh YT, Hsu WC. Axial length and optical coherence tomography (OCT) characteristics of myopic children with atropine treatment [Presented at the 43rd Annual Scientific Congress of The Royal Australian and New Zealand College of Ophthalmologists Canberra, ACT Australia]. Clinical and Experimental Ophthalmology 2011;39(Suppl 1):68. [Google Scholar]
Shimmyo 2003 {published data only}
- Shimmyo M, Rho DS. Retardation of myopic progression and axial growth in human children by muscarinic inhibitor. In: American Academy of Ophthalmology. 2003:143-4.
Shum 2003 {published data only}
- Shum P. Control of myopia by using overnight orthokeratology. In: Investigative Ophthalmology and Visual Science. Vol. 44. 2003:ARVO E-abstract 3718.
SightGlass 2018 {published data only}
- Tolerability of a novel spectacle design with reduced peripheral contrast. NCT03761758.
SMART Study 2009 {published data only}
- Eiden S, Davis R. Stabilization of Myopia by Accelerated Reshaping Technique (SMART) study: one year results. In: American Academy of Optometry. 2009.
- Gerowitz RS. SMART study: three year outcomes. Contact Lens & Anterior Eye 2012;35(Suppl):e40. [Google Scholar]
Soni 2006 {published data only}
- Soni PS, Nguyen TT. Overnight orthokeratology experience with XO material. Eye & Contact Lens 2006;32(1):39-45. [DOI] [PubMed] [Google Scholar]
Stone 1976 {published data only}
- Stone J, Powell Cullingford G. Myopia control after contact lens wear. British Journal of Physiological Optics 1974;29(3):93-108. [PubMed] [Google Scholar]
- Stone J. Contact lens wear in the young myope. British Journal of Physiological Optics 1973;28:90-134. [Google Scholar]
- Stone J. The possible influence of contact lenses on myopia. British Journal of Physiological Optics 1976;31(3):89-114. [PubMed] [Google Scholar]
Sun 2007 {published data only}
- Sun J, Li Y. Study on near-distance reading addition controlling the aggravating of adolescent myopia. Chinese Ophthalmic Research 2007;25(6):462-4. [Google Scholar]
Syniuta 2001 {published data only}
- Syniuta LA, Isenberg SJ. Atropine and bifocals can slow the progression of myopia in children. Binocular Vision and Strabismus Quarterly 2001;16(3):203-8. [PubMed] [Google Scholar]
Takano 1964 {published data only}
- Takano J. Treatment of myopia by the instillation of tropicamide. Japanese Journal of Clinical Ophthalmology 1964;18:45-50. [PubMed] [Google Scholar]
Tan 2012 {published data only}
- Tan D, Chia A, Han CW, Bun CY. Author reply: one-year multicenter, double-masked, placebo-controlled, parallel safety and efficacy study of 2% pirenzepine ophthalmic gel in children with myopia. Ophthalmology 2012;119:2653-4. [DOI] [PubMed] [Google Scholar]
Toki 1960 {published data only}
- Toki T. Treatment of myopia with local use of Neosynephrine hydrochloride. Japanese Journal of Clinical Optometry 1960;14:248-52. [Google Scholar]
Tokoro 1964 {published data only}
- Tokoro T, Kabe S. Treatment of myopia and changes in optical components. I. Topical application of Neosynephrine and N-ethyln9gamma-picolyl)-tropamide. Nippon Ganka Gakkai Zasshi 1964;68(13):1958-61. [PubMed] [Google Scholar]
Tokoro 1965 {published data only}
- Tokoro T, Kabe S. Treatment of the myopia and the changes in optical components. Report II. Full- or under-correction of myopia by glasses. Nippon Ganka Gakkai Zasshi 1965;69(2):140-4. [PubMed] [Google Scholar]
TO‐SEE Study 2013 {published data only}
- Chen C, Cheung S, Cho P. Toric orthokeratology for slowing eye elongation (TO-SEE) study. Contact Lens & Anterior Eye 2013;36:e10. [Google Scholar]
Xiao 2009 {published data only}
- Xiao ZG, Tao LJ, Guo Y, Wang P. Effect of rigid gas permeable contact lenses in controlling the progress of high myopia in children. International Journal of Ophthalmology 2009;9(5):991-3. [Google Scholar]
Yamada 2004 {published data only}
- Yamada Y. Myopia in primary school children. Japanese Journal of Clinical Ophthalmology 2004;58(2):125-9. [Google Scholar]
Yamaji 1967 {published data only}
- Yamaji R, Sakiyama A, Yoshihara M, Furuta I, Nakamura R. Clinical study of the effect of tropic acid amide on the visual acuity and refraction in myopic children. Part VII. Nippon Ganka Kiyo - Folia Ophthalmologica Japonica - Bulletin of Japanese Ophthalmology 1967;18(3):333-45. [PubMed] [Google Scholar]
- Yamaji R, Yoshihara M, Sakiyama A, Ishikawa K, Furuta I. Group therapy with tropic acid amide (Mydrin M) against pseudo-myopia in primary school children. Bulletin of the Osaka Medical School 1967;13(1):42-51. [PubMed] [Google Scholar]
- Yamaji R, Yoshihara M, Yoshida I, Nakamura R, Arisawa M. Clinical study on the effect of tropic acid amide on the visual acuity and refraction in myopic children. Nippon Ganka Kiyo - Folia Ophthalmologica Japonica - Bulletin of Japanese Ophthalmology 1968;19(3):419-34. [PubMed] [Google Scholar]
Yang 2017 {published data only}
- Yang Y, Wang L, Liu WL, Yan J. Comparison of the accommodative response with two refractive corrections for myopic teenagers. International Eye Science 2017;17(2):302-5. [Google Scholar]
Yi 2011 {published data only}
- Yi JH, Li RR. Influence of near-work and outdoor activities on myopia progression in school children. Chinese Journal of Contemporary Pediatrics 2011;13(1):32-5. [PubMed] [Google Scholar]
Young 1992 {published data only}
- Young G, Port M. Rigid gas-permeable extended wear: a comparative clinical study. Optometry and Vision Science 1992;69(3):214-26. [PubMed] [Google Scholar]
Zeng 2009 {published data only}
- Zeng Y, Keay L, He M, Mai J, Munoz B, Brady C, et al. A randomized, clinical trial evaluating ready-made and custom spectacles delivered via a school-based screening program in China. Ophthalmology 2009;116(10):1839-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
Zhou 2015 {published data only}
- Zhou C, Yan BX. Comparison of accommodative lag between rigid gas permeable contact lens and spectacles in adolescents after 1 year. International Eye Science 2015;15(5):924-7. [Google Scholar]
Zhou 2016 {published data only}
- Zhou ZX, Xu SS, Yi SP. Clinical effect of orthokeratology for juvenile with myopia astigmatism and its effects on corneal endothelial cells. International Eye Science 2016;16(8):1525-7. [Google Scholar]
References to studies awaiting assessment
Anderson 2016 {published and unpublished data}
- Anderson HA, Benoit J, Manny RE. Evaluation of progressive addition lens wear and age-related changes in phoria magnitude in myopic children. Investigative Ophthalmology and Visual Science 2016;57:ARVO E-abstract 1523.
Bakaraju 2015 {published and unpublished data}
- Bakaraju RC, Xu P, Song S, Ma M, Chen X, Jong M, et al. Extended depth-of-focus contact lenses can slow the rate of progression of myopia. Investigative Ophthalmology and Visual Science 2015;56:ARVO E-abstract 1728.
BLINK Study 2017b {published and unpublished data}
- Berntsen DA, Schulle KL, Sinnott LT, Bickle KM, Group TBS . Visual acuity and over-refraction in myopic children fitted with soft multifocal contact lenses in the BLINK Study. Investigative Ophthalmology and Visual Science 2017;58:ARVO E-Abstract 3052. [DOI] [PMC free article] [PubMed] [Google Scholar]
Cheung 2018 {published data only}
- Cheung SW, Cho P. Does a two-year period of orthokeratology lead to changes in the endothelial morphology of children? Contact Lens & Anterior Eye 2018;41:214-8. [DOI] [PubMed] [Google Scholar]
ChiCTR1800018092 {published and unpublished data}
- ChiCTR1800018092. Comparison of myopia control effect between single use ortho-k and combined with 0.01% atropine eye drops in children. ChiCTR1800018092.
Diaz‐Llopis 2018 {published and unpublished data}
- Diaz-Llopis M, Pinazo-Duran MD. Superdiluted atropine at 0.01% reduces progression in children and adolescents. A 5 year study of safety and effectiveness. Archivos de la Sociedad Espanola de Oftalmologia 2018;93:182-5. [DOI] [PubMed] [Google Scholar]
EUCTR2016‐003340‐37‐IE {published and unpublished data}36732601
- EUCTR2016-003340-37-IE. Preventing the progression of shortsightedness in children using an eye drop called atropine. EUCTR2016-003340-37-IE.
EUCTR2018‐001286‐16‐DK {published and unpublished data}
- EUCTR2018-001286-16-DK. Low-dose atropine for the prevention of nearsightedness in Danish children. EUCTR2018-001286-16-DK.
Jong 2015 {published data only}
- Jong M, Xu P, Bakaraju RC, Chen X, Sankaridurg P, Ma M, et al. A dose-response relationship between duration of daily lens wear and reduction in rate of axial elongation. Investigative Ophthalmology & Visual Science 2015;56:2941. [Google Scholar]
Kinoshita 2017 {published data only}
- Kinoshita N, Konno Y, Hamada N, Kakehashi A. Suppressive effect of combined treatment of orthokeratology and 0.01% atropine instillation on axial length elongation in childhood myopia. Investigative Ophthalmology & Visual Science 2017;58:2386. [Google Scholar]
Lam 2018 {published data only}
- Lam AK, Leung SY, Hon Y, Shu-Ho L, Wong KY, Tiu PK, Lam DC. Influence of short-term orthokeratology on corneal tangent modulus: a randomized study. Current Eye Ressearch 2018;43:474-81. [DOI] [PubMed] [Google Scholar]
Maekawa 2016 {published and unpublished data}
- Maekawa H, Hieda O, Nakamura Y, Koizumi N, Sotozono C, Kinoshita S. Comparison of the correction effect to suppress the progression of myopia between two types of orthokeratology lenses. Investigative Ophthalmology and Visual Science 2016;57:2485. [Google Scholar]
NCT02055378 {published and unpublished data}
- NCT02055378. The effect of low-concentration atropine combined with auricular acupoint stimulation in myopia control. clinicaltrials.gov/ct2/show/NCT02055378 (first received February 5, 2014). [DOI] [PubMed]
NCT02700139 {published and unpublished data}
- NCT02700139. Shamir aspheric ophthalmic lenses (MyLens) for myopic control clinical trial. clinicaltrials.gov/ct2/show/NCT02700139 (first received March 7, 2016).
NCT03519490 {published and unpublished data}
- NCT03519490. Can distance center and near center multifocal contact lenses control myopia progression in children? clinicaltrials.gov/ct2/show/NCT03519490 (first received May 9, 2019).
Pärssinen 2017 {published and unpublished data}
- Pärssinen O, Kauppinen M. Anisometropia of spherical equivalent and astigmatism among myopes: a 23-year follow-up study of prevalence and changes from childhood to adulthood. Acta Ophthalmologica 2017;95:518-24. [DOI] [PubMed] [Google Scholar]
Ren 2017 {published data only}
- Ren QJ, Yue H, Wang P, Liu RJ, Lu P. Effects of low concentration atropine and orthokeratology on myopia prevention and control. International Eye Science 2017;17:794-6. [Google Scholar]
Sankaridurg 2017 {published and unpublished data}
- Sankaridurg P, Bakaraju RC, Morgan J, Chen X, Tilia D, Ho A, et al. Novel contact lenses designed to slow progress of myopia: 12 month results. Investigative Ophthalmology & Visual Science 2017;58:2391. [Google Scholar]
Tan 2019 {published data only}
- Tan Q, Ng AL, Cheng GP, Woo VC, Cho P. Combined atropine with orthokeratology for myopia control: study design and preliminary results. Current Eye Research 2019;44:671-8. [DOI] [PubMed] [Google Scholar]
Tilia 2018 {published data only}
- Tilia D, Sha J, Thomas V, Bakaraju RC. Vision performance and accommodative/binocular function in children wearing prototype extended depth-of-focus contact lenses. Eye Contact Lens 2019;27:260-70. [DOI] [PubMed] [Google Scholar]
Trier 2015 {published data only}
- Trier K. 7-methylxanthine treatment. Acta Ophthalmologica 2015;93:NR. [Google Scholar]
Wei 2017 {published data only}
- Wei S, Li S, Sun Y, Kang M, Meng B, Ran A, et al. A randomized controlled clinical trial on the effects of wearing orthokeratology and spectacles on ocular peripheral refraction in myopic children. Chinese Journal of Experimental Ophthalmology 2017;35:930-5. [Google Scholar]
Wu 2018 {published and unpublished data}
- Wu WH, Wang HS. Effectiveness of AC custom-made Ortho-K lens. International Eye Science 2018;18:1742-5. [Google Scholar]
Yam 2019 {published and unpublished data}
- Yam JC, Jiang Y, Tang SM, Law AKP, Chan JJ, Wong E, et al. Low-concentration atropine for myopia progression (LAMP) study: a randomized, double-blinded, placebo-controlled trial of 0.05%, 0.025%, and 0.01% atropine eye drops in myopia control. Ophthalmology 2019;126:113-24. [DOI] [PubMed] [Google Scholar]
Zhao 2017 {published and unpublished data}
- Zhao HL, Jiang J, Yu J, Xu HM. Role of short-wavelength filtering lenses in delaying myopia progression and amelioration of asthenopia in juveniles. International Journal of Ophthalmology 2017;10:1261-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to ongoing studies
ACTRN12605000633684 {published and unpublished data}
- ACTRN12605000633684. Trial of an experimental soft contact lens designed to inhibit the progression of axial myopia in children. apps.who.int/trialsearch/Trial2.aspx?TrialID=ACTRN12605000633684 (date registered October 13, 2005).
ACTRN12608000566336 {published and unpublished data}
- ACTRN12608000566336. Myopia control lens efficacy trial. www.anzctr.org.au/Trial/Registration/TrialReview.aspx?id=83124 (date registered November 12, 2008).
ACTRN12611000499987 {unpublished data only}
- ACTRN12611000499987. Overnight contact lens treatment for myopia (short-sight) progression in children. www.anzctr.org.au/Trial/Registration/TrialReview.aspx?id=336636 (date registered May 12, 2011).
ACTRN12611000582954 {published and unpublished data}
- ACTRN12611000582954. Myopia control with progressive spectacle lenses trial. https://www.anzctr.org.au/Trial/Registration/TrialReview.aspx?id=343027 (date registered June 6, 2011).
ACTRN12611001148965 {published and unpublished data}
- ACTRN12611001148965. To determine the rate of refractive error change in children wearing multifocal soft contact lens as compared to those wearing single vision soft contact lenses. www.anzctr.org.au/Trial/Registration/TrialReview.aspx?ACTRN=12611001148965 (date registered November 2, 2011).
ACTRN12617000598381 {published and unpublished data}
- ACTRN12617000598381. Western Australian ATOM pilot study: atropine for the treatment of myopia. www.anzctr.org.au/Trial/Registration/TrialReview.aspx?ACTRN=12617000598381 (date registered April 27, 2017).
ACTRN12618000242224 {published and unpublished data}
- ACTRN12618000242224. Contralateral myopia progression trial. anzctr.org.au/Trial/Registration/TrialReview.aspx?ACTRN=12618000242224 (date registered February 14, 2018).
BLINK Study 2017a {published data only}
- Walline JJ, Gaume Giannoni A, Sinnott LT, Chandler MA, Huang J, Mutti DO, et al. A randomized trial of soft multifocal contact lenses for myopia control: baseline data and methods. Optometry and Vision Science 2017;94(9):856-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
ChiCTR1800016504 {published and unpublished data}
- ChiCTR1800016504. Clinical effect of vitamin B12 eye drops on myopia in children. chictr.org.cn/showprojen.aspx?proj=26962 ChiCTR1800016504.
ChiCTR1800017683 {published and unpublished data}
- ChiCTR1800017683. A double-masked comparative study of peripheral defocus lenses. ChiCTR1800017683.
ChiCTR1900021316 {published and unpublished data}
- ChiCTR1900021316. Clinical observation for acupuncture in myopia control and its effect on accommodative microfluctuations. ChiCTR1900021316.
ChiCTR‐INR‐17013794 {published and unpublished data}
- ChiCTR-INR-17013794. The effectiveness safety of corneal contact lens used to correct myopia: a multi-center, randomized, open and positive parallel control clinical trials. www.chictr.org.cn/showprojen.aspx?proj=23702 (date registered December 9, 2017).
ChiCTR‐INR‐17013853 {published and unpublished data}
- ChiCTR-INR-17013853. Effects of orthokeratology and combined with 0.01% atropine on myopia control: a multicenter comparative study. www.chictr.org.cn/showprojen.aspx?proj=22940 (date registered December 11, 2017).
ChiCTR‐IOR‐17010432 {published and unpublished data}
- ChiCTR-IOR-17010432. Myopia progression with Invisible round segment bifocal spectacle lenses. www.chictr.org.cn/com/25/showprojen.aspx?proj=17727 (date registered January 14, 2017).
ChiCTR‐IOR‐17011993 {published and unpublished data}
- ChiCTR-IOR-17011993. Prospective, masked, contralateral, randomised, crossover dispensing clinical trial to compare the myopia progression rate between myopia control contact lens and single vision contact lenses. www.chictr.org.cn/showprojen.aspx?proj=20301 (date registered July 14, 2017).
ChiCTR‐IPD‐16008844 {published and unpublished data}
- ChiCTR-IPD-16008844. Clinical study of low concentration atropine in controlling children myopia. www.chictr.org.cn/showprojen.aspx?proj=14705 (date registered July 14, 2016).
ChiCTR‐TRC‐07000029 {published and unpublished data}
- ChiCTR-TRC-07000029. Double-blinded, randomized controlled trial about the influence of new lenses on the progress of children's myopia. www.chictr.org.cn/showprojen.aspx?proj=9496 (date registered November 30, 2007).
ChiCTR‐TRC‐07000044 {published and unpublished data}
- ChiCTR-TRC-07000044. Clinical randomized controlled trial of progressive addition lenses on the control of myopia in Chinese adolescents. http://www.chictr.org.cn/showproj.aspx?proj=9481 (date registered December 12, 2007).
ChiCTR‐TRC‐09000476 {published and unpublished data}
- ChiCTR-TRC-09000476. Novel spectacle lenses versus single-vision spectacle lenses on the progression of myopia in children: a randomised clinical trial. chictr.org.cn/showprojen.aspx?proj=9058 (date registered August 4, 2009).
ChiCTR‐TRC‐10000914 {published and unpublished data}
- ChiCTR-TRC-10000914. Progression of refractive error in myopic Chinese children wearing commercially-available single vision spectacles. www.chictr.org.cn/showproj.aspx?proj=8624 (date registered June 27, 2007).
ChiCTR‐TRC‐11001463 {published and unpublished data}
- ChiCTR-TRC-11001463. Efficacy of MyoVision spectacle lenses. www.chictr.org.cn/showproj.aspx?proj=8076 (date registered August 2, 2011).
ChiCTR‐TRC‐11001746 {published and unpublished data}
- ChiCTR-TRC-11001746. Assessment of myopia progression rates in children wearing either a multifocal centre near or single vision soft contact lens. www.chictr.org.cn/showprojen.aspx?proj=7799 (date registered December 1, 2011).
ChiCTR‐TRC‐13003396 {published and unpublished data}
- ChiCTR-TRC-13003396. Myopia progression with sedentary-use, small segment, concentric bifocals. www.chictr.org.cn/showprojen.aspx?proj=6163 (date registered August 1, 2013).
ChiCTR‐TRC‐13004032 {published and unpublished data}
- ChiCTR-TRC-13004032. Chinese University low dose atropine for myopia progression study (CU-LAMP). ChiCTR-TRC-13004032.
ChiCTR‐TRC‐14004227 {published and unpublished data}
- ChiCTR-TRC-14004227. Assessment of rate of progression of myopia with contact lenses in Chinese children. www.chictr.org.cn/hvshowproject.aspx?id=8971 (date registered August 9, 2016).
ChiCTR‐TRC‐14004990 {published and unpublished data}
- ChiCTR-TRC-14004990. Low concentration atropine to slow myopic progression in children. www.chictr.org.cn/showproj.aspx?proj=4584 (date registered July 3, 2014).
CTRI/2016/11/007450 {published and unpublished data}
- CTRI/2016/11/007450. Atropine eye drops to decrease myopia progression in children. ctri.nic.in/Clinicaltrials/pdf_generate.php?trialid=15817&EncHid=&modid=&compid=%27,%2715817det%27 (date registered November 8, 2016).
IRCT20100414003714N3 {published and unpublished data}
- IRCT20100414003714N3. Effect of atropine eye drop for inhibition of myopic progression. http://en.irct.ir/trial/31944 (date registered October 7, 2018).
IRCT20180216038747N1 {published and unpublished data}
- IRCT20180216038747N1. Controlling myopia progression. https://www.irct.ir/trial/30096 (date registered April 17, 2018).
ISRCTN36732601 {published and unpublished data}
- ISRCTN36732601. Efficacy, safety and mechanisms of atropine eyedrops in slowing the progression of shortsightedness (myopia) in children. www.isrctn.com/ISRCTN36732601 (date assigned October 4, 2017).
JPRN‐UMIN000005054 {unpublished data only}
- JPRN-UMIN000005054. Clinical trial to evaluate effect of spectacle lens that reduces myopia progression. apps.who.int/trialsearch/Trial2.aspx?TrialID=JPRN-UMIN000005054 (date registered February 8, 2011).
JPRN‐UMIN000007989 {published and unpublished data}
- JPRN-UMIN000007989. Clinical trial to prevent myopia progression by progressive additional soft contact lens compared with monofocal soft contact lens in children. apps.who.int/trialsearch/Trial2.aspx?TrialID=JPRN-UMIN000007989 (date registered June 1, 2012).
JPRN‐UMIN000013698 {published and unpublished data}
- JPRN-UMIN000013698. Examination of the nearsighted progress depression effect of the low-concentrated atropine in the Japanese primary schoolchild. apps.who.int/trialsearch/Trial2.aspx?TrialID=JPRN-UMIN000013698 (date registered April 11, 2014).
JPRN‐UMIN000014362 {published and unpublished data}
- JPRN-UMIN000014362. Examination of the myopia progress suppressive effect by combined treatment of orthokeratology and 0.01% atropine instillation. JPRN-UMIN000014362.
JPRN‐UMIN000018041 {published and unpublished data}
- JPRN-UMIN000018041. The efficacy of 0.01% atropine ophthalmic solution for controlling the progression of childhood myopia (ATOM-J Study). apps.who.int/trialsearch/Trial2.aspx?TrialID=JPRN-UMIN000018041 (date registered June 23, 2015).
JPRN‐UMIN000019237 {published and unpublished data}
- JPRN-UMIN000019237. Effect of dual-focus soft contact lens wear on myopia progression. apps.who.int/trialsearch/Trial2.aspx?TrialID=JPRN-UMIN000018041 (date registered June 23, 2013).
JPRN‐UMIN000023386 {published and unpublished data}
- JPRN-UMIN000023386. Clinical trial on the use of outdoor environment glasses for a suppressive effect on myopia progression. apps.who.int/trialsearch/Trial2.aspx?TrialID=JPRN-UMIN000023386 (date registered July 29, 2016).
JPRN‐UMIN000027940 {published and unpublished data}
- JPRN-UMIN000027940. Clinical study on the effect of multifocal contact lens on myopia progression in myopia school children. apps.who.int/trialsearch/Trial2.aspx?TrialID=JPRN-UMIN000027940 (date registered July 21, 2017).
Kinoshita 2018 {published data only}
- Kinoshita N, Konno Y, Hamada N, Kanda Y, Shimmura-Tomita M, Kakehashi A. Additive effects of orthokeratology and atropine 0.01% ophthalmic solution in slowing axial elongation in children with myopia: first year results. Japanese Journal of Ophthalmology 2018;62:544-53. [DOI] [PubMed] [Google Scholar]
Li 2013 {published data only}
- Li SM, Li SY, Liu LR, Guo JY, Chen W, Wang Nl, et al. Full correction and undercorrection of myopia evaluation trial: design and baseline data of a randomized, controlled, double-blind trial. Clinical & Experimental Ophthalmology 2013;41(4):329-38. [DOI] [PubMed] [Google Scholar]
MASS 2018 {published and unpublished data}
- Ruiz-Pomeda A, Perez-Sanchez B, Canadas P, Prieto-Garrido FL, Gutierrez-Ortega R, Villa-Collar C. Binocular and accommodative function in the controlled randomized clinical trial MiSight Assessment Study Spain (MASS). Graefe's Archive for Clinical and Experimental Ophthalmology 2018;257:207-15. [DOI] [PubMed] [Google Scholar]
- Ruiz-Pomeda A, Perez-Sanchez B, Prieto-Garrido FL, Gutierrez-Ortega R, Villa-Collar C. MiSight assessment study Spain: adverse events, tear film osmolarity, and discontinuations. Eye Contact Lens 2018;12:S180-6. [DOI] [PubMed] [Google Scholar]
- Ruiz-Pomeda A, Perez-Sanchez B, Valls I, Prieto-Garrido FL, Gutierrez-Ortega R, Villa-Collar C. MiSight Assessment Study Spain (MASS). A 2-year randomized clinical trial. Graefes Archive for Clinical & Experimental Ophthalmology 2018;3:1011-21. [DOI] [PubMed] [Google Scholar]
NCT00214487 {published and unpublished data}
- NCT00214487. Bifocal soft contact lenses and their effect on myopia progression in children and adolescents. clinicaltrials.gov/ct2/show/NCT00214487 (first received September 22, 2005).
NCT00627874 {published and unpublished data}
- NCT00627874. Trial of myopia prevention using +3D lenses. clinicaltrials.gov/ct2/show/NCT00627874 (first received March 4, 2008).
NCT00762970 {published and unpublished data}
- NCT00762970. Controlling myopia progression with soft contact lenses. clinicaltrials.gov/ct2/show/NCT00762970 (first received September 30, 2008).
NCT01704729 {published and unpublished data}
- NCT01704729. The children's WEAR trial (Phase 1 & 2). clinicaltrials.gov/ct2/show/NCT01704729 (first received October 11, 2012).
NCT01729208 {published and unpublished data}
- NCT01729208. An evaluation of the effectiveness of dual focus soft contact lenses in slowing myopia progression. clinicaltrials.gov/ct2/show/NCT01729208 (first received November 20, 2012).
NCT01787760 {published and unpublished data}
- NCT01787760. Controlling myopia progression with soft contact lenses. clinicaltrials.gov/ct2/show/NCT01787760 (first received July 29, 2015).
NCT01829191 {published and unpublished data}
- NCT01829191. Controlling myopia progression with soft contact lenses (contact lens control). clinicaltrials.gov/ct2/show/NCT01829191 (first received July 29, 2015).
NCT01923675 {published and unpublished data}
- NCT01923675. Myopia: the role of cone opsin mutations & glasses that control axial elongation. clinicaltrials.gov/ct2/show/NCT01923675 (first received August 15, 2013).
NCT02001415 {published and unpublished data}
- NCT02001415. Efficacy study of different lens treatments on Chinese adolescent myopia. clinicaltrials.gov/ct2/show/NCT02001415 (first received December 4, 2013).
NCT02130167 {published and unpublished data}
- NCT02130167. Low concentration atropine for myopia progression in school children. clinicaltrials.gov/ct2/show/NCT02130167 (first received May 5, 2014).
NCT02186184 {published and unpublished data}
- NCT02186184. Effect of orthokeratology versus spectacles on myopia progression in Chinese children: a crossover trial. clinicaltrials.gov/ct2/show/NCT02186184 (first received July 10, 2014).
NCT02206217 {published and unpublished data}
- NCT02206217. Myopia control with the multi-segment lens. clinicaltrials.gov/ct2/show/NCT02206217 (first received August 1, 2014).
NCT02544529 {published and unpublished data}
- NCT02544529. Echothiophate iodide for the prevention of progression of myopia. clinicaltrials.gov/ct2/show/NCT02544529 (first received September 9, 2015).
NCT02643342 {published and unpublished data}
- NCT02643342. A 2-year longitudinal study on the structural and optical effects of orthokeratology treatment on eye. clinicaltrials.gov/ct2/show/NCT02643342 (first received December 31, 2015).
NCT02643758 {published and unpublished data}
- NCT02643758. Myopia control using soft bifocal lenses. clinicaltrials.gov/ct2/show/NCT02643758 (first received December 31, 2015).
NCT02955927 {published and unpublished data}
- NCT02955927. Combined atropine with orthokeratology in childhood myopia control (AOK)—a randomized controlled trial. clinicaltrials.gov/ct2/show/NCT02955927 (first received November 4, 2016).
NCT03242226 {published and unpublished data}
- NCT03242226. The effect of +3.00ADD on myopia progression in Chinese children. clinicaltrials.gov/ct2/show/NCT03242226 (first received August 8, 2017).
NCT03246464 {published and unpublished data}
- NCT03246464. Clinical study of nearsightedness; treatment with orthokeratology lenses (CONTROL). clinicaltrials.gov/ct2/show/NCT03246464 (first received August 11, 2017).
NCT03329638 {published and unpublished data}
- NCT03329638. A study assessing the efficacy and safety of DE-127 ophthalmic solution in subjects with mild or moderate myopia (APPLE). clinicaltrials.gov/ct2/show/NCT03329638 (first received November 6, 2016).
NCT03334253 {published and unpublished data}
- NCT03334253. Low-dose atropine for treatment of myopia. clinicaltrials.gov/ct2/show/NCT03334253 (first received November 7, 2017).
NCT03350620 {published and unpublished data}
- NCT03350620. CHAMP: study of NVK-002 in children with myopia. clinicaltrials.gov/ct2/show/NCT03350620 (first received November 22, 2017).
NCT03374306 {published and unpublished data}
- NCT03374306. Topical application of low-concentration (0.01%) atropine on the human eye with fast and slow myopia progression rate. clinicaltrials.gov/ct2/show/NCT03374306 (first received December 15, 2017).
NCT03402100 {published and unpublished data}
- NCT03402100. Eye drops study for myopia control in schoolchildren. clinicaltrials.gov/ct2/show/NCT03402100 (first received January 18, 2019).
NCT03413085 {published and unpublished data}
- NCT03413085. To evaluate the efficacy and safety of multifocal soft contact lens in myopia control. clinicaltrials.gov/ct2/show/NCT03413085 (first received January 29, 2018).
NCT03465748 {published and unpublished data}
- NCT03465748. Effectiveness of orthokeratology in myopia control. clinicaltrials.gov/ct2/show/NCT03465748 (first received March 14, 2018).
NCT03508817 {published and unpublished data}
- NCT03508817. Atropine 0.01% eye drops in myopia study. clinicaltrials.gov/ct2/show/NCT03508817 (first received April 26, 2018).
NCT03538002 {published and unpublished data}
- NCT03538002. The effect of blue-light filtering spectacle lenses on myopia progression in schoolchildren. clinicaltrials.gov/ct2/show/NCT03538002 (first received May 25, 2018).
NCT03552016 {published and unpublished data}
- NCT03552016. Evaluation of progression of myopia in children treated with vitamin B2 and outdoor sunlight exposure. clinicaltrials.gov/ct2/show/NCT03552016 (first received June 11, 2018).
NCT03623074 {published and unpublished data}
- NCT03623074. Control of myopia using novel spectacle lens designs (CYPRESS). clinicaltrials.gov/ct2/show/NCT03623074 (first received August 9, 2018).
NCT03681366 {published and unpublished data}
- NCT03681366. Myopia control using optimized optical defocus RCTs. clinicaltrials.gov/ct2/show/NCT03681366 (first received September 24, 2018).
NCT03690089 {published and unpublished data}
- ISRCTN99883695. Low-dose atropine eye drops to reduce progression of short-sightedness (myopia) in children in the United Kingdom. ISRCTN99883695.
- NCT03690089. Low-dose atropine eye drops to reduce progression of myopia in children in the United Kingdom. clinicaltrials.gov/ct2/show/NCT03690089 (first received October 1, 2018).
NCT03690414 {published and unpublished data}
- NCT03690414. Evaluation of short term use of experimental eye drops BHVI2, 0.02% atropine and BHVI2 plus 0.02% atropine eye drops. clinicaltrials.gov/ct2/show/NCT03690414 (first received October 1, 2018).
PACT Study 2017 {published data only}
- Yu X, Zhang B, Bao J, Zhang J, Wu G, Xu J, et al. Design, methodology, and baseline data of the Personalized Addition Lenses Clinical Trial (PACT). Medicine 2017;96(11):e6069. [DOI] [PMC free article] [PubMed] [Google Scholar]
Additional references
Barsam 2010
- Barsam A, Allan BDS. Excimer laser refractive surgery versus phakic intraocular lenses for the correction of moderate to high myopia. Cochrane Database of Systematic Reviews 2010, Issue 5. Art. No: CD007679. [DOI: 10.1002/14651858.CD007679.pub2] [DOI] [PubMed] [Google Scholar]
Braun 1996
- Braun CI, Freidlin V, Sperduto RD, Milton RC, Strahlman ER. The progression of myopia in school age children: data from the Columbia Medical Plan. Ophthalmic Epidemiology 1996;3(1):13-21. [DOI] [PubMed] [Google Scholar]
Chalmers 2011
- Chalmers RL, Wagner H, Mitchell GL, Lam DY, Kinoshita BT, Jansen ME, et al. Age and other risk factors for corneal infiltrative and inflammatory events in young soft contact lens wearers from the Contact Lens Assessment in Youth (CLAY) study. Investigative Ophthalmology & Visual Science 2011;52(9):6690-6. [DOI] [PubMed] [Google Scholar]
Charman 2006
- Charman WN, Mountford J, Atchison DA, Markwell EL. Peripheral refraction in orthokeratology patients. Optometry and Vision Science 2006;83(9):641-8. [DOI] [PubMed] [Google Scholar]
Chen 1985
- Chen CJ, Cohen BH, Diamond EL. Genetic and environmental effects on the development of myopia in Chinese twin children. Ophthalmic Paediatrics and Genetics 1985;6(1-2):353-9. [PubMed] [Google Scholar]
Deeks 2017
- Deeks JJ, Higgins JPT, Altman DG (editors), on behalf of the Cochrane Statistical Methods Group. Chapter 9. Analysing data and undertaking meta-analyses. In: Higgins JPT, Churchill R, Chandler J, Cumpston MS (editors), Cochrane Handbook for Systematic Reviews of Interventions version 5.2.0 (updated June 2017), Cochrane, 2017. Available from www.training.cochrane.org/handbook.
Dirani 2009
- Dirani M, Tong L, Gazzard G, Zhang X, Chia A, Young TL, et al. Outdoor activity and myopia in Singapore teenage children. British Journal of Ophthalmology 2009;93(8):997-1000. [DOI] [PubMed] [Google Scholar]
Duffey 2003
- Duffey RJ, Leaming D. US trends in refractive surgery: 2002 ISRS survey. Journal of Refractive Surgery 2003;19(3):357-63. [DOI] [PubMed] [Google Scholar]
Fonn 1988
- Fonn D, Holden BA. Rigid gas-permeable vs. hydrogel contact lenses for extended wear. American Journal of Optometry and Physiological Optics 1988;65(7):536-44. [DOI] [PubMed] [Google Scholar]
Garner 1999
- Garner LF, Owens H, Kinnear RF, Frith MJ. Prevalence of myopia in Sherpa and Tibetan children in Nepal. Optometry and Vision Science 1999;76(5):282-5. [DOI] [PubMed] [Google Scholar]
Glanville 2006
- Glanville JM, Lefebvre C, Miles JN, Camosso-Stefinovic J. How to identify randomized controlled trials in MEDLINE: ten years on. Journal of the Medical Library Association 2006;94(2):130-6. [PMC free article] [PubMed] [Google Scholar]
Goss 1987
- Goss DA. Cessation age of childhood myopia progression. Ophthalmic and Physiological Optics 1987;7(2):195-7. [DOI] [PubMed] [Google Scholar]
Goss 1990
- Goss DA. Variables related to the rate of childhood myopia progression. Optometry and Vision Science 1990;67(8):631-6. [DOI] [PubMed] [Google Scholar]
Guggenheim 2012
- Guggenheim JA, Northstone K, McMahon G, Ness AR, Deere K, Mattocks C, et al. Time outdoors and physical activity as predictors of incident myopia in childhood: a prospective cohort study. Investigative Ophthalmology & Visual Science 2012;53(6):2856-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
Guo 2013
- Guo Y, Liu LJ, Xu L, Lv YY, Tang P, Feng Y, et al. Outdoor activity and myopia among primary students in rural and urban regions of Beijing. Ophthalmology 2013;120(2):277-83. [DOI] [PubMed] [Google Scholar]
Guyatt 2011
- Guyatt GH, Oxman AD, Schunemann HJ, Tugwell P, Knottnerus A. GRADE guidelines: a new series of articles in the Journal of Clinical Epidemiology. Journal of Clinical Epidemiology 2011;64:380-2. [DOI] [PubMed] [Google Scholar]
Gwiazda 2009
- Gwiazda J. Treatment options for myopia. Optometry and Vision Science 2009;86(6):624-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hammond 2001
- Hammond CJ, Snieder H, Gilbert CE, Spector TD. Genes and environment in refractive error: the twin eye study. Investigative Ophthalmology and Visual Science 2001;42(6):1232-6. [PubMed] [Google Scholar]
He 2015
- He M, Xiang F, Zeng Y, Mai J, Chen Q, Zhang J, et al. Effect of time spent outdoors at school on the development of myopia among children in China: a randomized clinical trial. JAMA 2015;314(11):1142-8. [DOI] [PubMed] [Google Scholar]
Higgins 2017
- Higgins JP, Altman DG, Sterne JAC, editor(s). Chapter 8. Assessing risk of bias in included studies. In: Higgins JPT, Churchill R, Chandler J, Cumpston MS (editors), Cochrane Handbook for Systematic Reviews of Interventions version 5.2.0 (updated June 2017), Cochrane, 2017. Available from www.training.cochrane.org/handbook.
Holden 2016
- Holden BA, Fricke TR, Wilson DA, Jong M, Naidoo KS, Sankaridurg P, et al. Global prevalence of myopia and high myopia and temporal trends from 2000 through 2050. Ophthalmology 2016;123(5):1036-42. [DOI] [PubMed] [Google Scholar]
Jones 2007
- Jones LA, Sinnott LT, Mutti DO, Mitchell GL, Moeschberger ML, Zadnik K. Parental history of myopia, sports and outdoor activities, and future myopia. Investigative Ophthalmology and Visual Science 2007;48:3524-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kang 2011
- Kang P, Swarbrick H. Peripheral refraction in myopic children wearing orthokeratology and gas-permeable lenses. Optometry and Vision Science 2011;88(4):476-82. [DOI] [PubMed] [Google Scholar]
Lin 1999
- Lin LL, Shih YF, Tsai CB, Chen CJ, Lee LA, Hung PT, et al. Epidemiologic study of ocular refraction among schoolchildren in Taiwan in 1995. Optometry and Vision Science 1999;76(5):275-81. [DOI] [PubMed] [Google Scholar]
MacRae 1991
- MacRae S, Herman C, Stulting RD, Lippman R, Whipple D, Cohen E, et al. Corneal ulcer and adverse reaction rates in premarket contact lens studies. American Journal of Ophthalmology 1991;111(4):457-65. [DOI] [PubMed] [Google Scholar]
Maul 2000
- Maul E, Barroso S, Munoz SR, Sperduto RD, Ellwein LB. Refractive Error Study in Children: results from La Florida, Chile. American Journal of Ophthalmology 2000;129(4):445-54. [DOI] [PubMed] [Google Scholar]
Moore 2017
- Moore KE, Benoit JS, Berntsen DA. Spherical soft contact lens designs and peripheral defocus in myopic eyes. Optometry and Vision Science 2017;94(3):370-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Mutti 2006
- Mutti DO, Mitchell GL, Hayes JR, Jones LA, Moeschberger ML, Cotter SA, et al. Accommodative lag before and after the onset of myopia. Investigative Ophthalmology and Visual Science 2006;47(3):837-46. [DOI] [PubMed] [Google Scholar]
Pararajasegaram 1998
- Pararajasegaram R. Vision 2020—the right to sight: from strategies to action. American Journal of Ophthalmology 1999;128(3):359-60. [DOI] [PubMed] [Google Scholar]
Park 2004
- Park DJ, Congdon NG. Evidence for an "epidemic" of myopia. Annals of the Academy of Medicine Singapore 2004;33(1):21-6. [PubMed] [Google Scholar]
Pineles 2017
- Pineles SL, Kraker RT, VanderVeen DK, Hutchinson AK, Galvin JA, Wilson LB, et al. Atropine for the prevention of myopia progression in children: a report by the American Academy of Ophthalmology. Ophthalmology 2017;124(12):1857-66. [DOI] [PubMed] [Google Scholar]
Review Manager 2014 [Computer program]
- Nordic Cochrane Centre, The Cochrane Collaboration Review Manager 5 (RevMan 5). Version 5.3. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Rose 2008
- Rose KA, Morgan IG, Ip J, Kifley A, Huynh S, Smith W, et al. Outdoor activity reduces the prevalence of myopia in children. Ophthalmology 2008;115(8):1279-85. [DOI] [PubMed] [Google Scholar]
Saw 2000
- Saw SM, Nieto FJ, Katz J, Schein OD, Levy B, Chew SJ. Factors related to the progression of myopia in Singaporean children. Optometry and Vision Science 2000;77(10):549-54. [DOI] [PubMed] [Google Scholar]
Saw 2001
- Saw SM, Wu HM, Seet B, Wong TY, Yap E, Chia KS, et al. Academic achievement, close up work parameters, and myopia in Singapore military conscripts. British Journal of Ophthalmology 2001;85(7):855-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Saw 2002a
- Saw SM, Gazzard G, Au Eong KG, Tan DT. Myopia: attempts to arrest progression. British Journal of Ophthalmology 2002;86(11):1306-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
Saw 2002b
- Saw SM, Shih-Yen EC, Koh A, Tan D. Interventions to retard myopia progression in children: an evidence-based update. Ophthalmology 2002;109(3):415-21. [DOI] [PubMed] [Google Scholar]
Schein 1989
- Schein OD, Glynn RJ, Poggio EC, Seddon JM, Kenyon KR. The relative risk of ulcerative keratitis among users of daily-wear and extended-wear soft contact lenses. A case-control study. Microbial Keratitis Study Group. New England Journal of Medicine 1989;321(12):773-8. [DOI] [PubMed] [Google Scholar]
Schulz 2010
- Schulz KF, Altman DG, Moher D, CONSORT Group. CONSORT 2010 statement: updated guidelines for reporting parallel group randomised trials. BMJ 2010;340:340:c332. [DOI] [PMC free article] [PubMed] [Google Scholar]
Shortt 2006
- Shortt AJ, Allan BDS. Photorefractive keratectomy (PRK) versus laser-assisted in-situ keratomileusis (LASIK) for myopia. Cochrane Database of Systematic Reviews 2006, Issue 2. Art. No: CD005135. [DOI: 10.1002/14651858.CD005135.pub2] [DOI] [PubMed] [Google Scholar]
Smith 2009
- Smith EL 3rd, Hung LF, Huang J. Relative peripheral hyperopic defocus alters central refractive development in infant monkeys. Vision Research 2009;49(19):2386-92. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ticak 2013
- Ticak A, Walline JJ. Peripheral optics with bifocal soft and corneal reshaping contact lenses. Optometry and Vision Science 2013;90(1):3-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Troilo 1987
- Troilo D, Gottlieb MD, Wallman J. Visual deprivation causes myopia in chicks with optic nerve section. Current Eye Research 1987;6(8):993-9. [DOI] [PubMed] [Google Scholar]
Vitale 2009
- Vitale S, Sperduto RD, Ferris FL 3rd. Increased prevalence of myopia in the United States between 1971-1972 and 1999-2004. Archives of Ophthalmology 2009;127(12):1632-9. [DOI] [PubMed] [Google Scholar]
Voo 1998
- Voo I, Lee DA, Oelrich FO. Prevalences of ocular conditions among Hispanic, white, Asian, and black immigrant students examined by the UCLA Mobile Eye Clinic. Journal of the American Optometric Association 1998;69(4):255-61. [PubMed] [Google Scholar]
Wagner 2011
- Wagner H, Chalmers RL, Mitchell GL, Jansen ME, Kinoshita BT, Lam DY, et al. Risk factors for interruption to soft contact lens wear in children and young adults. Optometry and Vision Science 2011;88(8):973-80. [DOI] [PubMed] [Google Scholar]
Wu 2010
- Wu PC, Tsai CL, Hu CH, Yang YH. Effects of outdoor activities on myopia among rural school children in Taiwan. Ophthalmic Epidemiology 2010;17(5):338-42. [DOI] [PubMed] [Google Scholar]
Young 1969
- Young FA, Leary GA, Baldwin WR, West DC, Box RA, Harris E, et al. The transmission of refractive errors within eskimo families. American Journal of Optometry and Archives of American Academy of Optometry 1969;46(9):676-85. [DOI] [PubMed] [Google Scholar]
Zadnik 1994
- Zadnik K, Satariano WA, Mutti DO, Sholtz RI, Adams AJ. The effect of parental history of myopia on children's eye size. JAMA 1994;271(17):1323-7. [PubMed] [Google Scholar]
Zadnik 2003
- Zadnik K, Manny RE, Yu JA, Mitchell GL, Cotter SA, Quiralte JC, et al. Ocular component data in schoolchildren as a function of age and gender. Optometry and Vision Science 2003;80(3):226-36. [DOI] [PubMed] [Google Scholar]
Zhan 2000
- Zhan MZ, Saw SM, Hong RZ, Fu ZF, Yang H, Shui YB, et al. Refractive errors in Singapore and Xiamen, China—a comparative study in school children aged 6 to 7 years. Optometry and Vision Science 2000;77(6):302-8. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Walline 2008
- Walline JJ, Vedula SS, Mutti DO, Twelker JD, Cotter SA. Interventions to slow progression of myopia in children. Cochrane Database of Systematic Reviews 2008, Issue 1. Art. No: CD004916. [DOI: 10.1002/14651858.CD004916.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Walline 2011
- Walline JJ, Lindsley K, Vedula SS, Cotter SA, Mutti DO, Twelker JD. Interventions to slow progression of myopia in children. Cochrane Database of Systematic Reviews 2011, Issue 12. Art. No: CD004916. [DOI: 10.1002/14651858.CD004916.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]


