Abstract
Background
Non‐oliguric hyperkalaemia of the newborn is defined as a plasma potassium level > 6.5 mmol/L in the absence of acute renal failure. Hyperkalaemia is a common complication in the first 48 hours of life in very low birth weight (VLBW) (birth weight < 1500 g) and/or very preterm newborns (< 32 weeks gestational age).
Objectives
To determine the effectiveness and safety of interventions for non‐oliguric hyperkalaemia [for the purpose of this review defined as serum potassium > 6.0 mmol/L (the clinical setting in which interventions would likely be introduced prior to reaching a grossly abnormal level) and urine output > 0.5 ml/kg/hour] in preterm or VLBW infants during their first 72 hours of life.
Search methods
The Cochrane Central Register of Controlled Trials (CENTRAL, The Cochrane Library, Issue 2, 2006) was searched to identify relevant randomised and quasi‐randomised controlled trials. The following data bases were searched in June 2006; MEDLINE from 1966, EMBASE from 1980, CINAHL from 1982. Search updated in June 2011.
Selection criteria
Randomised or quasi‐randomised controlled trials conducted in preterm and/or VLBW neonates with a diagnosis of non‐oliguric hyperkalaemia. Interventions included were those aimed at redistributing serum potassium (sodium bicarbonate or insulin and glucose) or increasing the elimination of potassium from the body [diuretics (any type) or ion exchange resins (any type), or exchange transfusion, or peritoneal dialysis, or salbutamol, or albuterol] or counteracting potential arrhythmias from hyperkalaemia (calcium) versus placebo or no intervention; or comparing any two of these interventions. Primary outcome measure was 'All cause mortality during initial hospital stay'. Secondary outcomes included common adverse outcomes seen in preterm infants.
Data collection and analysis
We used the standard review methods of the Cochrane Neonatal Review Group. Two authors assessed all studies identified as potentially relevant by the literature search for inclusion in the review. Statistical methods included relative risk (RR), risk difference (RD), number needed to treat to benefit (NNTB) or number needed to treat to harm (NNTH) for dichotomous and weighted mean difference (WMD) for continuous outcomes reported with 95% confidence intervals (CI). We used a fixed effect model for meta‐analysis. Heterogeneity was assessed using the I squared (I2 ) statistic.
Main results
Three randomised trials, enrolling 74 preterm infants (outcome data available on 71 infants) evaluated interventions for hyperkalaemia. Urine output was ascertained in only one study (Hu 1999). In none of the trials could we ascertain that allocation to the comparison groups was concealed. The sample sizes of the three trials were very small with 12 (Malone 1991), 19 (Singh 2002) and 40 infants enrolled (Hu 1999). The intervention and the outcome assessments could not be blinded to the clinical staff in two trials (Malone 1991; Hu 1999).
One study (Malone 1991), glucose and insulin, compared to cation‐exchange resin, caused a reduction in all cause mortality that was of borderline statistical significance: RR 0.18 (95% CI 0.03 to 1.15); RD ‐0.66 (95% CI ‐1.09 to ‐0.22); NNTB 2 (95% CI 1 to 5)]. In the study of Hu (Hu 1999), the incidence of intraventricular haemorrhage ≥ grade 2 was significantly reduced [RR 0.30 (95% CI 0.10 to 0.93); RD ‐0.35 (95% CI ‐0.62 to ‐0.08); NNTB 3 (95% CI 2 to 13).
Albuterol inhalation versus saline inhalation changed serum K+ from baseline at four hours [WMD ‐0.69 mmol/L (95% CI ‐0.87 to ‐0.51)] and at eight hours [WMD ‐0.59 mmol/L (95% CI ‐0.78 to ‐0.40)] after initiation of treatment. No differences noted in mortality or other clinical outcomes (Singh 2002).
No serious side effects were noted with either the combination of insulin and glucose or albuterol inhalation. Other interventions listed in our objectives have not been studied to date.
Authors' conclusions
In view of the limited information from small studies of uncertain quality, no firm recommendations for clinical practice can be made. It appears that the combination of insulin and glucose is preferred over treatment with rectal cation‐resin for hyperkalaemia in preterm infants. Both the combination of insulin and glucose and albuterol inhalation deserve further study. The two interventions could possibly be tested against each other. The effectiveness of other potentially effective interventions for non‐oliguric hyperkalaemia (diuretics, exchange transfusion, peritoneal dialysis and calcium) have not been tested in randomised controlled trials.
Plain language summary
Interventions for non‐oliguric hyperkalaemia in preterm neonates
Elevated levels of potassium (an important salt for normal body functions) are common in infants born very preterm or with birth weight less than 1500 g. High potassium levels in the blood may lead to irregular or rapid heart rate that may result in bleedings in the brain and/or sudden death. The objective of this review was to determine the effectiveness and safety of interventions for this serious condition. Two studies enrolling 52 infants that assessed the use of a combination of insulin and sugar to reduce the blood levels of potassium were identified. This combination reduced the duration of high blood levels of potassium and the risk for bleeds in the brains of the infants. One study that enrolled 19 patients reported on the use of albuterol (a medication that helps to move potassium from the blood to the body cells). Albuterol lowered the blood levels of potassium both at four and at eight hours after the treatment had started. Because of the few infants enrolled in the studies to date, no firm recommendations for the treatment of too high blood levels of potassium in neonates can be made. Further research is needed.
Background
Description of the condition
Definition: The normal range for plasma (serum) potassium in the neonate is 4.5 to 6.5 mmol/L (Lackmann 1992; Shaffer 1992; Chevalier 1998). Reversible hyperkalaemia in preterm infants, first reported by Usher in 1959 (Usher 1959), is now referred to as non‐oliguric hyperkalaemia of the preterm infant (Gruskay 1988). Plasma potassium levels depend on the balance between potassium intake, intracellular/extra cellular distribution and renal and fecal excretion (Chevalier 1998). Non‐oliguric hyperkalaemia of the newborn is defined as a plasma potassium level > 6.5 mmol/L in the absence of acute renal failure (Gruskay 1988; Kilbride 1988). As interventions to decrease plasma potassium levels would most likely be introduced in the clinical setting prior to reaching > 6.5 mmol/ L, a plasma (serum) level of > 6.0 mmol/L was chosen as the cut‐off for the definition of hyperkalaemia for this review. Burden of illness: Hyperkalaemia is a common complication in the first 48 hours of life in very low birth weight (BW < 1500 g) and/or very preterm newborns (< 32 weeks gestational age). Omar (Omar 2000), quoting studies published between 1988 and 1997, reported that non‐oliguric hyperkalaemia affected 30% to 50% of very low birth weight (VLBW) infants. In a cohort of 32 infants with birth weight < 800 g who survived at least 24 hours, 16 (50 %) developed hyperkalaemia (Kilbride 1988). All infants of less than 25 weeks' gestation developed hyperkalaemia (Kilbride 1988). The incidence of hyperkalaemia (defined in this study as two successive serum potassium measurements of > 7.5 mmol/L) in an unselected cohort of 200 VLBW infants was 3.5% (Shortland 1987). Hyperkalaemic infants have a high incidence of cardiac arrhythmias (60%), impaired renal function (50%) (based on abnormal serum urea or creatinine concentrations) and changes on cerebral ultrasonography (88%) (Shortland 1987). A temporal association between hyperkalaemia, cardiac arrhythmias and periventricular leukomalacia suggests a causal association (Shortland 1987). Among seven infants with cardiac arrhythmias secondary to hyperkalaemia, only one survived (Sychlowy 1990). In a descriptive review of perinatal mortality in a regional perinatal centre in Canada between 1980 to 1984, 11 of 89 (12%) early neonatal deaths in infants with a BW < 1000 grams were associated with hyperkalaemia and cardiac arrhythmia (Ohlsson 1987a). In 2002, 17 neonatal intensive care units reported that one or more deaths related to non‐oliguric hyperkalaemia of the preterm infant had occurred in their units (Mildenberger 2002b). Antenatal maternal corticosteroid treatment may prevent non‐oliguric hyperkalaemia in extremely low birth weight infants (Omar 2000; Uga 2003). With an increased use of antenatal steroids especially in the very early gestations the incidence of non‐oliguric hyperkalaemia may now be lower than previously reported. With the high rates of mortality, cardiac arrhythmias and need for emergency measures associated with non‐oliguric hyperkalaemia, it is likely that many of the common morbidities of VLBW infants (intraventricular haemorrhage, periventricular leukomalacia, bronchopulmonary dysplasia, retinopathy of prematurity, necrotizing enterocolitis, adverse long‐term outcomes) would be influenced by the condition and possibly reduced by interventions for its treatment.
Pathophysiology: Non‐oliguric hyperkalaemia in VLBW infants is not a result of increased potassium intake or decreased potassium excretion. It is due mainly to a shift of potassium from the intracellular to the extracellular space associated with a decrease in the erythrocyte Na+, K+ ‐ ATPase activity (Stefano 1993). Non‐oliguric hyperkalaemia of the preterm infant is unrelated to leakage of potassium from cell disruption associated with bruising, intracranial haemorrhage, or haemolysis, perinatal asphyxia or acidosis, glucose tolerance and catabolism (Gruskay 1988; Kilbride 1988; Brion 1989; Fukuda 1989; Shaffer 1992; Stefano 1993; Stefano 1993a; Sato 1995; Mildenberger 1996; Lorenz 1997).
Description of the intervention
Treatment: Postnatal therapeutic measures aim to redistribute potassium from the extracellular to the intracellular space, to remove potassium from the body and/or to decrease the arrhythmogenicity of hyperkalaemia. Bicarbonate therapy has been recommended for the acute treatment of neonatal hyperkalaemia (Brion 1989), as acidosis decreases the renal excretion of potassium and increases the arrhythmogenicity of hyperkalaemia (Perkin 1980). Both insulin and glucose decrease serum potassium by facilitating potassium transport into the intracellular space. The use of insulin for hyperkalaemia has been documented in one case report (Heyman 1989) and two retrospective studies (Sychlowy 1990; Lui 1992). Of the diuretics, furosemide exerts the most pronounced kaliuretic effect and its use to treat hyperkalaemia has been reported in one preterm infant (Gruskay 1988).
Potassium elimination can be enhanced by ion resins, but hyperkalaemic neonates have developed gastro‐intestinal obstruction and/or perforation following oral or rectal administration of exchange resins (Ohlsson 1987; Sychlowy 1990; Bennett 1996; Grammatikopoulos 03). Exchange transfusion (Setzer 1984) and peritoneal dialysis (Shortland 1987; Kilbride 1988) provide treatment options after failure of other interventions. The effect of salbutamol on transmembrane potassium flux has been studied in neonatal red blood cells under hyperkalaemic conditions and resulted in a 50% increase in net transmembrane potassium flux (Angelopoulous 1996). Salbutamol infusion (but not inhalation) for hyperkalaemia has been reported in neonates (Greenough 1992; Dilmen 1992; Avenarius 1996).
Preterm infants commonly experience hypocalcaemia during their first 24 to 48 hours of life, at a time when non‐oliguric hyperkalaemia occurs, and case reports support the administration of intravenous calcium for cardiac arrhythmias secondary to hyperkalaemia (Kilbride 1988; Bennett 1996).
Why it is important to do this review
The pathogenesis and therapy of non‐oliguric hyperkalaemia of the preterm infant has recently been the topic of a narrative review (Mildenberger 2002a). However, the topic of non‐oliguric hyperkalaemia in preterm infants has not been the subject of a systematic review.
Objectives
Primary objective: To determine the effectiveness and safety of interventions for non‐oliguric hyperkalaemia in preterm or very low birth weight infants during their first 72 hours of life.
Separate comparisons were planned to assess the effectiveness and safety of the following interventions:
Interventions aimed at redistributing serum potassium: Sodium bicarbonate; Insulin and glucose; Salbutamol or albuterol.
Interventions aimed at increasing the elimination of potassium from the body: Diuretics (any type); Ion exchange resins (any type); Exchange transfusion; Peritoneal dialysis
Interventions aimed at counteracting potential arrhythmias from hyperkalaemia; Calcium.
Comparisons included all interventions to placebo or no intervention; or comparisons of any two interventions to each other. Each drug or intervention was assessed separately. For specific drugs any dosage, duration of treatment and route of administration was included and when possible was categorized and assessed separately and in combination using the subcategory feature of RevMan 5.1. Secondary objectives: To determine in subgroup analyses the effectiveness and safety of interventions listed above for non‐oliguric hyperkalaemia in relation to the following criteria: Gestational age (< 25 weeks and > 25 weeks) or birth weight (< 1000 g and > 1000 g).
Methods
Criteria for considering studies for this review
Types of studies
Randomised or quasi‐randomised controlled trials.
Types of participants
Preterm (< 32 weeks) and/or very low birth weight (< 1500 g) neonates with a diagnosis of non‐oliguric (urine output > 0.5 ml/kg/hour) hyperkalaemia (serum potassium > 6.0 mmol/L) and less than 72 hours old.
Types of interventions
Treatment of hyperkalaemia in neonates by use of any intervention aimed at redistributing serum potassium (sodium bicarbonate or insulin and glucose) or increasing the elimination of potassium from the body [diuretics (any type) or ion exchange resins (any type), or exchange transfusion, or peritoneal dialysis, or salbutamol, or albuterol] or counteracting potential arrhythmias from hyperkalaemia (calcium) versus placebo or no intervention; or comparing any two of these interventions. Each drug or intervention was assessed separately. For specific drugs any dosage, duration of treatment and route of administration was included and when possible will be categorized and assessed separately and in combination using the subcategory feature of RevMan 5.1.
Types of outcome measures
Primary outcomes
All cause mortality during initial hospital stay.
All cause mortality at 28 days of age.
Secondary outcomes
Mortality due to hyperkalaemia during initial hospitalisation.
Mortality due to hyperkalaemia at 28 days of age.
Cardiac arrhythmias.
Normalization of serum/plasma potassium levels (K+ < 6.0 mmol/L).
Intraventricular haemorrhage (IVH); all grades and grades III and IV (according to Papile) (Papile 1978).
Periventricular leukomalacia (PVL); cystic changes in the periventricular areas of the brain.
Bronchopulmonary dysplasia (BPD) (supplementary oxygen at 28 days of age) and (supplementary oxygen at 36 weeks postmenstrual gestational age).
Retinopathy of prematurity (ROP) (any stage and stage > 3 or more).
Necrotizing enterocolitis (NEC) (Bell's stage II or more) (Bell 1978).
Length of hospital stay (days).
Duration of assisted ventilation (days).
Duration of oxygen requirement > 0.21 (days).
Long term outcomes assessed at any age beyond one year of age by a validated cognitive, motor, language, or behavioural/school/social interaction/adaptation test.
Known side effects from the interventions (hypoglycaemia ‐ from insulin, intestinal obstruction from resins).
Any side effects reported in the trials (post‐hoc analysis).
Search methods for identification of studies
The Cochrane Central Register of Controlled Trials (CENTRAL, The Cochrane Library, Issue 2, 2006) was searched to identify relevant randomised and quasi‐randomised controlled trials. The following data bases were searched in June 2006; MEDLINE from 1966, EMBASE from 1980, CINAHL from 1982.
For MEDLINE(R) (1966 to June Week 1 2006) the search strategy was:
hyperkalemia/co, dh, dt, ep, th, et [complications, diet therapy, drug therapy, epidemiology, therapy, etiology]or hyperkalaemia. mp.
insulin/ or Albuterol/ or gluconates/ or calcium gluconate/ or Calcium/ or resonium.mp. or bicarbonates/ or sodium bicarbonate/ or sodium bicarbonate (nm) or exchange transfusion/ or peritoneal dialysis/
1 and 2
limit 3 to [humans and "newborn infant (birth to 1 month)"]
the final set was combined with all three phases of the Robinson and Dickersin strategy (Robinson 2002). This is a highly sensitive search strategy to retrieve reports of controlled trials using the MEDLINE database
For EMBASE the search strategy was:
hyperkalemia/ep, et, dt, th [epidemiology, etiology, drug therapy, therapy]or hyperkalaemia:.mp.
insulin/ or bicarbonate/ or Bicarbonate Sodium Cotransporter/ or salbutamol/ or salbutamol sulfate/ or Gluconate Calcium/ or calcium/ or polystyrenesulfonate calcium/ or polystyrenesulfonate sodium/ or exchange blood transfusion/ or peritoneal dialysis/
1 and 2
limit 3 to human
(newborn: or preterm: or prematur: or neonate: or preemie:).mp.
4 and 5
For CINAHL the search strategy was:
hyperkalemia/dt, ep, et, th [drug therapy, epidemiology, etiology, therapy] or hyperkalaemia:.mp. (124)
insulin/ or Albuterol/ or gluconate:.mp. or calcium gluconate:.mp. or calcium/ or resonium.mp. or bicarbonates/ or sodium bicarbonate/ or Exchange Transfusion, Whole Blood/ or (exchange adj2 transfusion:).mp. or Peritoneal Dialysis/
1 and 2
limit 3 to newborn infant <birth to 1 month>
In addition, manual searches of bibliographies of identified trials were performed. Personal files were searched. No language restrictions were applied. Abstracts published from the Pediatric Academic Societies' Meetings and the European Society of Pediatric Research Meetings (published in Pediatric Research) were hand searched from 1980 to June 2006.
This search was updated in May, 2011. See: Appendix 1
Data collection and analysis
We used the methods of the Cochrane Neonatal Review Group for data collection and analysis.
Selection of studies
We included all randomised and quasi‐randomised controlled trials that fulfilled the selection criteria described in the previous section. The review authors independently reviewed the results of the search and selected studies for inclusion. We resolved any disagreement by discussion.
Data extraction and management
Each author extracted data separately on pre‐designed data abstraction forms. We sought information regarding the method of randomisation, blinding and reporting of all outcomes of all the infants enrolled in the trial for each trial. We obtained data from the primary investigator for unpublished trials or when published data were incomplete. The information was compared and differences were resolved by consensus. One review author (AO) entered data into RevMan 5.1 and the other (PV) cross checked the printout against his own data abstraction forms and any detected errors were corrected.
Assessment of risk of bias in included studies
The methodological quality of each trial was assessed by each review author using the criteria of the Cochrane Collaboration, focusing on concealment of allocation, blinding of the intervention, completeness of follow‐up and blinding of the outcome assessors.
The review authors independently assessed the risk of bias for each study using the criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We resolved any disagreement by discussion.
The methodological quality of the studies was assessed using the following criteria:
-
Sequence generation (checking for possible selection bias): For each included study, we categorized the method used to generate the allocation sequence as:
low risk (any truly random process e.g. random number table; computer random number generator);
high risk (any non random process e.g. odd or even date of birth; hospital or clinic record number);
unclear risk.
-
Allocation concealment (checking for possible selection bias): For each included study, we categorized the method used to conceal the allocation sequence as:
low risk (e.g. telephone or central randomisation; consecutively numbered sealed opaque envelopes);
high risk (open random allocation; unsealed or non‐opaque envelopes, alternation; date of birth);
unclear risk.
-
Blinding (checking for possible performance bias): For each included study, we categorized the methods used to blind study participants and personnel from knowledge of which intervention a participant received. Blinding was assessed separately for different outcomes or classes of outcomes. We characterised the methods used for blinding as:
low risk, high risk or unclear risk for participants;
low risk, high risk or unclear risk for personnel;
low risk, high risk or unclear risk for outcome assessors.
-
Incomplete outcome data (checking for possible attrition bias through withdrawals, dropouts, protocol deviations): For each included study and for each outcome, we described the completeness of data including attrition and exclusions from the analysis. We noted whether attrition and exclusions were reported, the numbers included in the analysis at each stage (compared with the total randomised participants), reasons for attrition or exclusion where reported, and whether missing data were balanced across groups or were related to outcomes. Where sufficient information was reported or supplied by the trial authors, we re‐included missing data in the analyses. We categorized the methods as:
low risk (< 20% missing data);
high risk (≥ 20% missing data);
unclear risk.
-
Selective reporting bias: For each included study, we described how we investigated the possibility of selective outcome reporting bias and what we found. We assessed the methods as:
low risk (where it is clear that all of the study’s pre‐specified outcomes and all expected outcomes of interest to the review have been reported);
high risk (where not all the study’s pre‐specified outcomes have been reported; one or more reported primary outcomes were not pre‐specified; outcomes of interest are reported incompletely and so cannot be used; study fails to include results of a key outcome that would have been expected to have been reported);
unclear risk.
-
Other sources of bias: For each included study, we described any important concerns we had about other possible sources of bias (for example, whether there was a potential source of bias related to the specific study design or whether the trial was stopped early due to some data‐dependent process). We assessed whether each study was free of other problems that could put it at risk of bias as:
low risk;
high risk;
unclear risk.
Overall risk of bias [described in Table 8.5c in the Handbook]
We made explicit judgements regarding whether studies were at high risk of bias, according to the criteria given in the Cochrane Handbook (Higgins 2011). With reference to (1) to (6) above, we assessed the likely magnitude and direction of the bias and whether we considered it is likely to impact on the findings. If needed, we planned to explore the impact of the level of bias through undertaking sensitivity analyses (see 'Sensitivity analysis' below).
Measures of treatment effect
We analysed the data using the standard methods of the Neonatal Review Group.
We performed statistical analyses using Review Manager software. We analysed dicotomous data using relative risk (RR), risk difference (RD) and the number needed to benefit (NNTB) or number needed to harm (NNTH). We reported the 95% Confidence Interval (CI) on all estimates.
We analysed continuous data using weighted mean difference (WMD) or the standardized mean difference to combine trials that measure the same outcome but use different methods.
Unit of analysis issues
We analysed the data as proportion of neonates having one or more episodes for clinical outcomes such as episodes of sepsis.
Dealing with missing data
For included studies, levels of attrition were noted. The impact of including studies with high levels of missing data in the overall assessment of treatment effect was explored by using sensitivity analysis.
All outcome analyses were on an intention to treat basis, i.e. we included all participants randomised to each group in the analyses. The denominator for each outcome in each trial was the number randomised minus any participants whose outcomes were known to be missing.
Assessment of heterogeneity
We examined heterogeneity between trials by inspecting the forest plots and quantifying the impact of heterogeneity using the I2 statistic. If noted, we planned to explore the possible causes of statistical heterogeneity using pre‐specified subgroup analysis (for example, differences in study quality, participants, intervention regimens, or outcome assessments).
Assessment of reporting biases
We assessed possible publication bias and other biases using symmetry/asymmetry of funnel plots.
For included trials that were recently performed (and therefore prospectively registered), we explored possible selective reporting of study outcomes by comparing the primary and secondary outcomes in the reports with the primary and secondary outcomes proposed at trial registration, using the web sites www.clinicaltrials.gov and www.controlled‐trials.com. If such discrepancies were found, we planned to contact the primary investigators to obtain missing outcome data on outcomes pre‐specified at trial registration.
Data synthesis
Meta‐analysis was done using Review Manager software, supplied by the Cochrane Collaboration. We used the Mantel‐Haenszel method for estimates of typical relative risk and risk difference. We analysed continuous measures using the inverse variance method.
We used the fixed effect model for all meta‐analyses.
Subgroup analysis and investigation of heterogeneity
We planned separate comparisons to assess the effectiveness and safety of the following interventions:
Interventions aimed at redistributing serum potassium: Sodium bicarbonate; Insulin and glucose; Salbutamol or albuterol.
Interventions aimed at increasing the elimination of potassium from the body: Diuretics (any type); Ion exchange resins (any type); Exchange transfusion; Peritoneal dialysis
Interventions aimed at counteracting potential arrhythmias from hyperkalaemia; Calcium.
Comparisons included all interventions to placebo or no intervention; or comparisons of any two interventions to each other. Each drug or intervention was assessed separately. For specific drugs any dosage, duration of treatment and route of administration was included and when possible was categorized and assessed separately and in combination using the subcategory feature of RevMan 5.1. Subgroup analyses were to be performed for infants < 1000 g versus > 1000 < 1500 g birthweight and for infants < 25 weeks versus > 25 < 32 weeks gestational age at birth.
Sensitivity analysis
We planned sensitivity analyses for in situations where this might affect the interpretation of significant results (for example, where there is risk of bias associated with the quality of some of the included trials or missing outcome data). None were thought necessary in this review.
Results
Description of studies
Results of the search
Three studies enrolling 74 infants and reporting on 71 infants with hyperkalaemia were included (Malone 1991; Hu 1999; Singh 2002). These studies were all identified through the prespecified search strategy. One study was excluded (Leslie 1993). No additional trial was found in personal files. Details are noted below and in the table 'Characteristics of included studies'.
Included studies
Three studies enrolling 74 infants and reporting on 71 infants with hyperkalaemia were included (Malone 1991; Hu 1999; Singh 2002).
Two studies evaluated the effectiveness of glucose and insulin versus cation‐exchange resin (Kayexalate). All preset inclusion criteria were met in the study by Hu et al (Hu 1999). In the study by Malone et al (Malone 1991) urine output prior to study entry was not reported. We chose to include this study in spite of this shortcoming. We excluded one study (Leslie 1993), reported in abstract form only. For details see table 'Characteristics of excluded studies'.
Hu 1999 was a single centre study conducted in Taichung, Taiwan.
Objective: To compare insulin and glucose infusion with rectal administration of a cation‐exchange resin for the treatment of hyperkalaemia in very low birth weight infants.
Population: Very low birth weight infants with non‐oliguric hyperkalaemia (serum K+ > 6 mmol/L) in the first few days after birth. The infants were not oliguric.
Intervention: One group (n = 20) received insulin infusion and the other group (n = 20) received sodium polystyrene sulphonate (Kayexalate) prepared in a 25% sorbitol solution and given rectally in a dose of 1 g/kg every six hours.
Outcomes assessed: Duration of hyperkalaemia (hours), peak serum K+ levels during treatment, grade >II or more IVH, cardiac dysrhythmia, hyperglycaemia and hypoglycaemia.
Malone 1991 was a single centre study performed in one hospital in the US.
Objective: To compare insulin and glucose infusion with rectal administration of a cation‐exchange resin for the treatment of hyperkalaemia in very low birth weight infants.
Population: Preterm infants < 28 weeks GA with hyperkalaemia defined as a central non‐haemolyzed serum K+ level of > 7.0 mmol/L. Urine output was not reported as an inclusion criterion.
Intervention: One group (n = 7) received human insulin with 5% albumin. The insulin was "piggybacked" into the existing i.v. fluids by means of a syringe infusion pump, starting at a rate of 0.1 ml/hr to deliver a dose of 0.05 to 0.1 IU/kg/hr. The cation‐exchange group (n = 5) received sodium polystyrene sulphonate (Kayexalate) prepared in a 25% sorbitol solution and given rectally in a dose of 1 g/kg every six hours.
Outcomes assessed: Treatment failure (defined as a rise in serum K+ concentration of more than 0.5 mmol/L after treatment initiation or failure to decrease serum K+ to < 7 mmol/L within 24 hours, mortality and hypoglycaemia.
One study evaluated the use of inhaled albuterol in hyperkalaemic infants (Singh 2002). In the study by Singh et al (Singh 2002) the age of the infants enrolled varied from two to 22 days (the mean age was not reported). Some infants were therefore > 72 hours old at the time of enrolment. Urine out put prior to study entry was not reported. As this was the only study evaluating the effects of albuterol inhalation versus saline inhalation we decided to include the study.
Singh 2002 was a single centre study performed in one hospital in the US.
Objective: To evaluate the efficacy of inhaled albuterol for the treatment of hyperkalaemia in neonates born preterm.
Population: Preterm neonates < 2000 g (gestational age ranged from 23 to 27 weeks and weight ranged from 486 g to 1330 g; thus fulfilling our inclusion criteria for birth weight and gestational age) receiving mechanical ventilation with central serum K+ > 6.0 mmol/L. Urine output was not reported as an inclusion criterion.
Intervention: The albuterol group (n = 8) received 400 microgram by nebulization and the control group (n = 11) received saline by nebulization. Treatment was given every two hours until serum K+ levels fell to < 5 mmol/L or if the maximum of 12 doses of the study drug was reached.
Outcomes assessed: Mortality, IVH grade 3 or 4, pulmonary haemorrhage and pneumothorax. Change in serum K+ from baseline. Adverse effects related to albuterol (tremors, twitching, abnormal motor activity).
Excluded studies
One trial was identified that was excluded from the analysis. Leslie and colleagues reported on a comparison of resonium versus dextrose‐insulin for treatment of hyperkalaemia in extremely premature infants; however, this was not a randomised controlled trial (Leslie 1993).
Risk of bias in included studies
Only one study fulfilled all the inclusion criteria (Hu 1999). None of the studies provided information on whether the allocation to the study groups was concealed or not. In both studies of insulin and glucose versus rectal Kayexalate (Malone 1991; Hu 1999) the intervention could not be blinded. In the study by Singh 2002, a placebo was used and the intervention and the outcome assessments were performed blinded to the two assigned groups. Only one study (Singh 2002) reported on a predetermined sample size. However, the study was terminated after 22 patients out of the estimated 40 patients had been enrolled. In that study, three randomised patients were withdrawn and the data for these patients were not reported. There was complete follow‐up in the other two studies (Malone 1991; Hu 1999). The sample sizes of the studies were small ranging from 12 to 40 infants enrolled.
Effects of interventions
Few of our predetermined outcomes were reported. We included additional relevant outcomes as defined and reported by the authors.
The results are reported separately for the two identified comparisons; "Insulin and glucose versus cation‐exchange resin (Kayexalate)" and for "Albuterol versus normal saline (placebo)".
COMPARISON 1: INSULIN AND GLUCOSE VERSUS CATION‐EXCHANGE RESIN (KAYEXALATE)
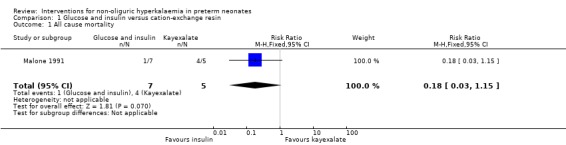
Primary outcome: Outcome 1.1: All cause mortality during initial hospital stay
Only one study, enrolling 12 infants, reported this outcome (Malone 1991). Glucose and insulin, compared to cation‐exchange resin, caused a reduction in all cause mortality that was of borderline statistical significance: RR 0.18 (95% CI 0.03 to 1.15); RD ‐0.66 (95% CI ‐1.09 to ‐0.22); NNTB 2 (95% CI 1 to 5)].
Secondary outcomes:
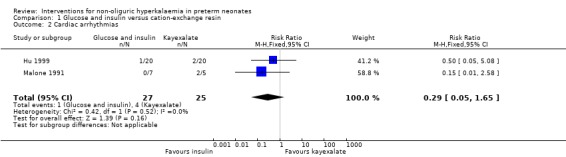
Outcome 1.2: Cardiac arrhythmias
Two studies enrolling 52 infants reported on this outcome (Malone 1991; Hu 1999). Neither study showed a significant difference and the typical RR was 0.29 (95% CI 0.05 to 1.65) and the typical RD was ‐0.13 (95% CI ‐0.30 to 0.04) favouring the combination of insulin and glucose versus cation‐exchange resin (Kayexalate). There was no statistically significant heterogeneity for this outcome.
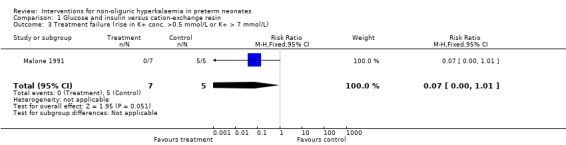
Outcome 1.3: Treatment failure (rise in K+ concentration > 0.5 mmol/L or serum K+ > 7 mmol/L)
Only one study enrolling 12 infants reported on this outcome (Malone 1991). Glucose and insulin, compared to cation‐exchange resin, caused a reduction in treatment failure that was of borderline statistical significance: RR 0.07 (95% CI 0.00 to 1.01); RD ‐1.00 (95% CI ‐1.28 to ‐0.72); NNTB 1 (95% CI 1 to 1).
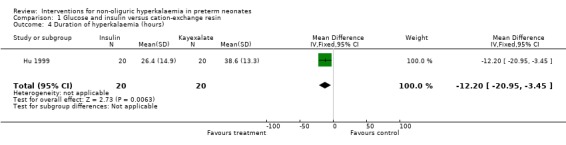
Outcome 1.4: Duration of hyperkalaemia (hours) Only one study enrolling 40 infants reported on this outcome (Hu 1999). Glucose and insulin, compared to cation‐exchange resin, caused reduced duration of hyperkalaemia that was statistically significant: MD ‐12 hours (95% CI ‐21 to ‐3).
Outcome 1.5: Peak serum K+ level (mmol/L)
Only one study enrolling 40 infants reported on this outcome (Hu 1999). Glucose and insulin, compared to cation‐exchange resin, caused reduced in the peak serum K+ level that was not statistically significant: MD ‐0.10 (95% CI ‐0.57 to 0.37).
Outcome 1.6: Intraventricular haemorrhage (grade >2)
Only one study enrolling 40 infants reported on this outcome (Hu 1999). Insulin and glucose versus cation‐exchange resin resulted in a statistically significant reduction: RR 0.30 (95% CI 0.10 to 0.93); RD ‐0.35 (95% CI ‐0.62 to ‐0.08); NNTB 3 (95% CI 2 to 13).
Outcome 1.7: Hyperglycaemia
Only one study enrolling 40 infants reported on this outcome (Hu 1999). The RR was not estimable as there were no outcomes in either group. The RD was 0.00 (95% CI ‐0.09 to 0.09).
Outcome 1.8. Hypoglycemia Two studies enrolling 52 infants reported on this outcome (Malone 1991; Hu 1999). Neither showed a statistically significant difference. There were no outcomes in one of the studies. The typical RR was 2.25 (95% CI 0.11 to 46.13). The typical RD was 0.03 (95% CI ‐0.09 to 0.15) . Heterogeneity tests not applicable for RR. For RD the p‐value was 0.36 and the I2 was 0% (both non‐significant). COMPARISON 2: ALBUTEROL INHALATION VERSUS PLACEBO (SALINE INHALATION)
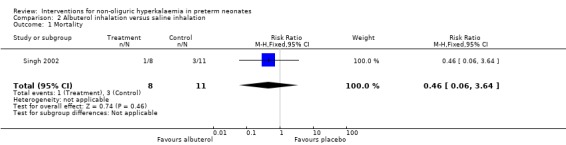
One study enrolling 19 infants was included for this comparison, and all results reported below apply to this study that compared albuterol inhalation versus placebo (saline inhalation) (Singh 2002). Primary outcome:
Outcome 2.1: All cause mortality during initial hospital stay
There was no statistically significant difference for all cause mortality during initial hospital stay; RR 0.46 (95% CI 0.06 to 3.64); RD ‐0.15 (95% CI ‐0.50 to 0.20).
SECONDARY OUTCOMES
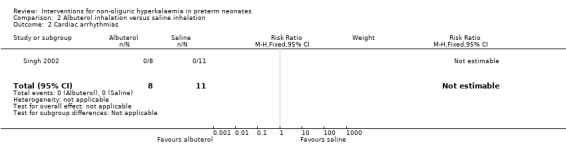
Outcome 2.2: Cardiac arrhythmias
Cardiac arrhythmias did not occur in either of the two groups [RD 0.00 (95% CI ‐0.19 to 0.19)]
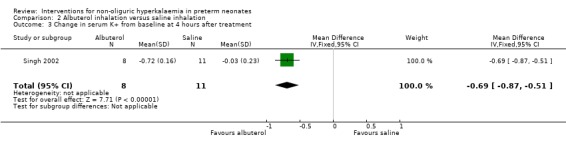
Outcome 2.3: Change in serum K+ (mmol/L) from baseline at 4 hours of treatment
Albuterol inhalation versus saline inhalation caused a statistically significant decrease in serum K+ from baseline at 4 hours of treatment favouring albuterol inhalation; MD ‐0.69 mmol/L (95% CI ‐0.87 to ‐0.51).
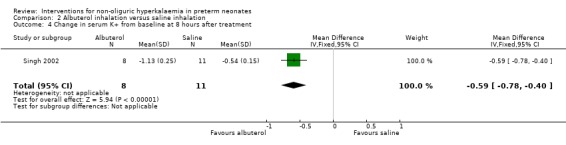
Outcome 2.4: Change in serum K+ (mmol/L) from baseline at 8 hours of treatment
Albuterol inhalation versus saline inhalation caused a statistically significant decrease in serum K+ from baseline at 8 hours of treatment favouring albuterol inhalation; MD ‐0.59 mmol/L (95% CI ‐0.78 to ‐0.40) favouring albuterol inhalation.
Outcome 2.5: IVH (grade > 2)
The statistically non‐significant RR was 0.69 (95% CI 0.07 to 6.34) and the RD was ‐0.06 (95% CI ‐0.38 to 0.27).
Outcome 2.6: Tremors/twitching
The statistically non‐significant RR was 0.69 (95% CI 0.07 to 6.34) and the RD was ‐0.06 (95% CI ‐0.38 to 0.27).
Outcome 2.7: Hyperglycaemia (>11.1 mmol/L)
The statistically non‐significant RR was 1.38 (95% CI 0.37 to 5.13) and the RD was 0.10 (95% CI ‐0.32 to 0.53).
Outcome 2.8: Pulmonary haemorrhage
The statistically non‐significant RR was 0.44 (95% CI 0.02 to 9.69) and the RD was ‐0.09 (95% CI ‐0.33 to 0.15).
SUBGROUP ANALYSES
Subgroup analyses based on gestational age (< 25 weeks and > 25 weeks) or birth weight (< 1000 g and > 1000 g) could not be performed as the authors of the primary studies did not report the data in this way.
Discussion
To date, only 74 preterm infants have been enrolled in trials of interventions for non‐oliguric hyperkalaemia and outcome data have been reported for 71 of these infants. Only one study (Hu 1999) fulfilled all our inclusion criteria. We chose to include the other two studies as the populations were likely to be representative of infants with non‐oliguric hyperkalaemia during the first few days of life. In none of the trials could we ascertain that allocation to the comparison groups was concealed. In only one trial was a sample size calculation performed, but the study was terminated after only 22 of the 40 infants had been enrolled (Singh 2002). The sample sizes of the three trials were very small with 12 (Malone 1991), 19 (Singh 2002) and 40 infants enrolled (Hu 1999). The intervention and the outcome assessments could not be blinded to the clinical staff in two trials (Hu 1999; Malone 1991). For some of the outcomes, there were discrepancies in statistical significance between the results for RR and RD. Thus, the results of these meta‐analyses have to be interpreted with caution.
It appears that the combination of insulin and glucose may provide benefits compared to rectal cation‐resin (Kayexalate) with statistically significantly reduced RD or WMD for all cause mortality, treatment failure, duration of hyperkalaemia and IVH (grade > 2). However, the RR was statistically reduced only for duration of hyperkalaemia and IVH. The results should be interpreted with caution as the statistical significance changed with the use of two different statistics (RR and RD). The results could be classified as of borderline statistical significance.
Albuterol inhalation when compared to placebo (saline inhalation), changed (decreased) serum K+ from baseline at four and eight hours after initiation of treatment.
No serious side effects were noted with either the combination of insulin and glucose or albuterol inhalation.
Other interventions listed in our objectives have not been studied to date. These include interventions aimed at increasing the elimination of potassium from the body (diuretics, exchange transfusion and peritoneal dialysis) and interventions aimed at counteracting potential arrhythmias from hyperkalaemia (calcium).
Both the combination of insulin and glucose and albuterol inhalation appear to be promising interventions and deserve further study.
Authors' conclusions
Implications for practice.
In view of the limited information from small studies of uncertain quality no firm recommendations for clinical practice can be made. Until further evidence is ascertained from larger well designed and executed randomised controlled trials, it appears that the combination of insulin and glucose is preferred over treatment with rectal cation‐resin intervention for hyperkalaemia in preterm infants.
Implications for research.
Both the combination of insulin and glucose and albuterol inhalation deserve further study in well designed and executed randomised controlled trials. The two interventions could be tested against each other. Other interventions that have not been studied to date include interventions aimed at increasing the elimination of potassium from the body (diuretics, exchange transfusion and peritoneal dialysis) and interventions aimed at counteracting potential arrhythmias from hyperkalaemia (calcium).
What's new
| Date | Event | Description |
|---|---|---|
| 27 January 2020 | Amended | Arne Ohlsson deceased. |
History
Protocol first published: Issue 2, 2005 Review first published: Issue 1, 2007
| Date | Event | Description |
|---|---|---|
| 9 June 2011 | New search has been performed | This review updates the existing review "Interventions for non‐oliguric hyperkalaemia in preterm neonates" published in the Cochrane Database of Systematic Reviews (Vemgal 2007). Updated search in June 2011 found no new trials. No changes to conclusions. |
| 9 June 2011 | New citation required but conclusions have not changed | Updated search found no new trials. No changes to conclusions. |
| 18 September 2008 | Amended | Converted to new review format. |
| 30 August 2006 | New citation required and conclusions have changed | Substantive amendment |
Acknowledgements
We are thankful to Ms. Elizabeth Uleryk, Chief Librarian at the Hospital for Sick Children, Toronto, Ontario, Canada, for developing the search strategy for this review.
Appendices
Appendix 1. Search Strategy ‐ May 2011
PubMed
(Hyperkalemia OR complications OR diet therapy OR drug therapy OR epidemiology OR therapy OR etiology OR hyperkalaemia) AND (Insulin OR Albuterol OR gluconates OR calcium gluconate OR Calcium OR resonium OR bicarbonates OR sodium bicarbonate OR sodium bicarbonate OR exchange transfusion OR peritoneal dialysis) AND (sepsis OR septicemia OR septicaemia OR septic shock OR infection OR sepsis syndrome) AND ((infant, newborn[MeSH] OR newborn OR neon* OR neonate OR neonatal OR premature OR low birth weight OR VLBW OR LBW) AND (randomized controlled trial [pt] OR controlled clinical trial [pt] OR randomized [tiab] OR placebo [tiab] OR clinical trials as topic [mesh: noexp] OR randomly [tiab] OR trial [ti]) NOT (animals [mh] NOT humans [mh])) AND (("2006"[PDat] : "3000"[PDat]))
Embase
1 (infant, newborn or newborn or neonate or neonatal or premature or very low birth weight or low birth weight or VLBW or LBW).mp. [mp=title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer, device trade name, keyword] (607975)
2 (human not animal).mp. [mp=title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer, device trade name, keyword] (11910500)
3 (randomized controlled trial or controlled clinical trial or randomized or placebo or clinical trials as topic or randomly or trial or clinical trial).mp. [mp=title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer, device trade name, keyword] (1266847)
4 (Hyperkalemia or complications or diet therapy or drug therapy or epidemiology or therapy or etiology or hyperkalaemia).mp. [mp=title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer, device trade name, keyword] (3108682)
5 (Insulin or Albuterol or gluconates or calcium gluconate or Calcium or resonium or bicarbonates or sodium bicarbonate or exchange transfusion or peritoneal dialysis).mp. [mp=title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer, device trade name, keyword] (940142)
6 1 and 2 and 3 and 4 and 5 (890)
7 limit 6 to yr="2006 ‐Current" (385)
Cinahl
( (Hyperkalemia OR complications OR diet therapy OR drug therapy OR epidemiology OR therapy OR etiology OR hyperkalaemia) AND (Insulin OR Albuterol OR gluconates OR calcium gluconate OR Calcium OR resonium OR bicarbonates OR sodium bicarbonate OR sodium bicarbonate OR exchange transfusion OR peritoneal dialysis) ) and ( ( infant, newborn OR newborn OR neonate OR neonatal OR premature OR low birth weight OR VLBW OR LBW) AND ( randomized controlled trial OR controlled clinical trial OR randomized OR placebo OR clinical trials as topic OR randomly OR trial OR PT clinical trial) 2006 ‐ Current
Cochrane Central Register of Controlled Trials
"(infant or newborn or neonate or neonatal or premature or very low birth weight or low birth weight or VLBW or LBW)and (Hyperkalemia OR complications OR diet therapy OR drug therapy OR epidemiology OR therapy OR etiology OR hyperkalaemia) and (Insulin OR Albuterol OR gluconates OR calcium gluconate OR Calcium OR resonium OR bicarbonates OR "sodium bicarbonate" OR "exchange transfusion" OR "peritoneal dialysis") 2006 ‐ Current
clinicaltrials.gov
(infant OR newborn) AND (Hyperkalemia OR complications OR epidemiology OR therapy OR etiology OR hyperkalaemia) AND (Insulin OR Albuterol OR gluconates OR Calcium OR resonium OR bicarbonate OR exchange transfusion OR dialysis)
controlled‐trials.com
(infant OR newborn) AND (Hyperkalemia OR complications OR epidemiology OR therapy OR etiology OR hyperkalaemia) AND (Insulin OR Albuterol OR gluconates OR Calcium OR resonium OR bicarbonate OR exchange transfusion OR dialysis)
Data and analyses
Comparison 1. Glucose and insulin versus cation‐exchange resin.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 All cause mortality | 1 | 12 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.18 [0.03, 1.15] |
| 2 Cardiac arrhythmias | 2 | 52 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.29 [0.05, 1.65] |
| 3 Treatment failure (rise in K+ conc. >0.5 mmol/L or K+ > 7 mmol/L) | 1 | 12 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.07 [0.00, 1.01] |
| 4 Duration of hyperkalaemia (hours) | 1 | 40 | Mean Difference (IV, Fixed, 95% CI) | ‐12.20 [‐20.95, ‐3.45] |
| 5 Peak serum K + level (mmol/L) | 1 | 40 | Mean Difference (IV, Fixed, 95% CI) | ‐0.10 [‐0.57, 0.37] |
| 6 IVH | 1 | 40 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.3 [0.10, 0.93] |
| 6.1 IVH (grades >/= 2) | 1 | 40 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.3 [0.10, 0.93] |
| 7 Hyperglycaemia | 1 | 40 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 8 Hypoglycaemia | 2 | 52 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.25 [0.11, 46.13] |
1.1. Analysis.
Comparison 1 Glucose and insulin versus cation‐exchange resin, Outcome 1 All cause mortality.
1.2. Analysis.
Comparison 1 Glucose and insulin versus cation‐exchange resin, Outcome 2 Cardiac arrhythmias.
1.3. Analysis.
Comparison 1 Glucose and insulin versus cation‐exchange resin, Outcome 3 Treatment failure (rise in K+ conc. >0.5 mmol/L or K+ > 7 mmol/L).
1.4. Analysis.
Comparison 1 Glucose and insulin versus cation‐exchange resin, Outcome 4 Duration of hyperkalaemia (hours).
1.5. Analysis.
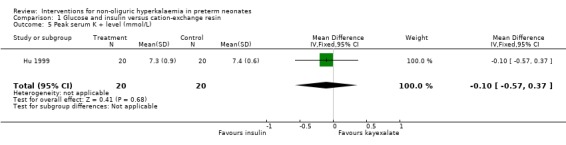
Comparison 1 Glucose and insulin versus cation‐exchange resin, Outcome 5 Peak serum K + level (mmol/L).
1.6. Analysis.
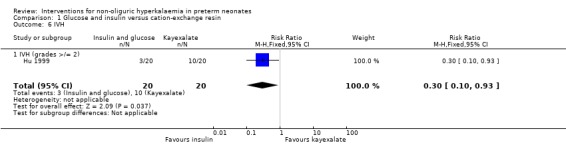
Comparison 1 Glucose and insulin versus cation‐exchange resin, Outcome 6 IVH.
1.7. Analysis.
Comparison 1 Glucose and insulin versus cation‐exchange resin, Outcome 7 Hyperglycaemia.
1.8. Analysis.
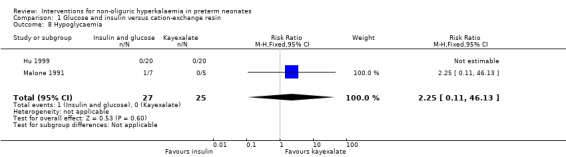
Comparison 1 Glucose and insulin versus cation‐exchange resin, Outcome 8 Hypoglycaemia.
Comparison 2. Albuterol inhalation versus saline inhalation.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Mortality | 1 | 19 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.46 [0.06, 3.64] |
| 2 Cardiac arrhythmias | 1 | 19 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 3 Change in serum K+ from baseline at 4 hours after treatment | 1 | 19 | Mean Difference (IV, Fixed, 95% CI) | ‐0.69 [‐0.87, ‐0.51] |
| 4 Change in serum K+ from baseline at 8 hours after treatment | 1 | 19 | Mean Difference (IV, Fixed, 95% CI) | ‐0.59 [‐0.78, ‐0.40] |
| 5 IVH (grade > 2) | 1 | 19 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.69 [0.07, 6.34] |
| 6 Tremors/twitching | 1 | 19 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.69 [0.07, 6.34] |
| 7 Hyperglycaemia (> 11.1 mmol/L) | 1 | 19 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.38 [0.37, 5.13] |
| 8 Pulmonary haemorrhage | 1 | 19 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.44 [0.02, 9.69] |
2.1. Analysis.
Comparison 2 Albuterol inhalation versus saline inhalation, Outcome 1 Mortality.
2.2. Analysis.
Comparison 2 Albuterol inhalation versus saline inhalation, Outcome 2 Cardiac arrhythmias.
2.3. Analysis.
Comparison 2 Albuterol inhalation versus saline inhalation, Outcome 3 Change in serum K+ from baseline at 4 hours after treatment.
2.4. Analysis.
Comparison 2 Albuterol inhalation versus saline inhalation, Outcome 4 Change in serum K+ from baseline at 8 hours after treatment.
2.5. Analysis.
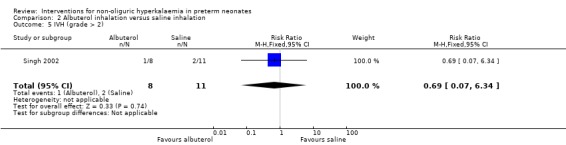
Comparison 2 Albuterol inhalation versus saline inhalation, Outcome 5 IVH (grade > 2).
2.6. Analysis.
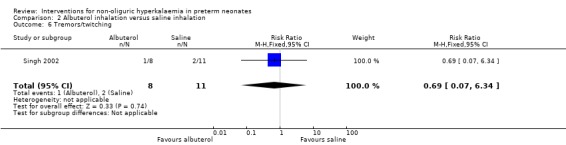
Comparison 2 Albuterol inhalation versus saline inhalation, Outcome 6 Tremors/twitching.
2.7. Analysis.
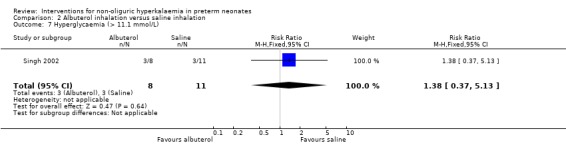
Comparison 2 Albuterol inhalation versus saline inhalation, Outcome 7 Hyperglycaemia (> 11.1 mmol/L).
2.8. Analysis.
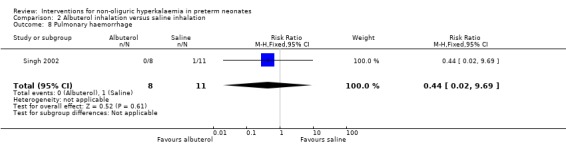
Comparison 2 Albuterol inhalation versus saline inhalation, Outcome 8 Pulmonary haemorrhage.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Hu 1999.
| Methods | Randomised, controlled study I. Blinding of randomisations ‐ can't tell (See notes) II. Blinding of intervention ‐ no III. Blinding of outcome measure assessment ‐ no IV. Complete follow‐up‐ yes | |
| Participants | 40 VLBW infants with non‐oliguric hyperkalaemia (K+ > 6 mmol/L) in the first few days of life after birth Single centre, Taichung, Taiwan Study period not stated | |
| Interventions | 20 infants mean (SD) GA 27.4 (1.8) weeks; BW mean (SD) 935 (259) g; mean (SD) urine output 5.4 (1.3) (ml/kg/hr) received regular insulin (Actrapid Human, Novo Nordisk) The ratio of infused glucose to regular insulin was 10‐15 g of glucose to 1 unit of regular insulin, and the total glucose infusion rate of each infant was maintained at greater than 6 mg/kg/min. Insulin administration was adjusted according to blood sugar levels 20 infants mean (SD) GA 28.4 (2.4) weeks; BW mean (SD) 1065 (214); mean (SD) urine output 5.5 (0.9) (ml/kg/hr) received sodium polystyrene sulphonate (Kayexalate) Kayexalate was prepared in normal saline solution with the ratio of g 1 g Kayexalate to 2 ml of normal saline and the administered dose was 1 g/kg body weight given rectally every 4 hours Both groups received electrolyte free solution of glucose in the first 3‐4 days or until hyperkalaemia subsided Therapy was discontinued when serum K+ was < 6 mmol/L for 6 hours No infant had IVH prior to enrolment | |
| Outcomes | Cardiac dysrhythmias IVH grade >/= II by cerebral ultrasound scan Peak serum K+, duration of hyperkalaemia Hypo (< 40 mg/dl) and hyperglycaemia (> 150 mg/dl) | |
| Notes | Infants were randomly assigned to one of two groups, no details were provided None of the infants had IVH prior to study entry | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised, controlled study |
| Allocation concealment (selection bias) | Unclear risk | Blinding of randomisations ‐ can't tell (see notes) |
| Blinding (performance bias and detection bias) All outcomes | High risk | Blinding of intervention ‐ no |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Blinding of intervention ‐ no |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Blinding of outcome measure assessment ‐ no |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Complete follow‐up ‐ yes |
Malone 1991.
| Methods | Randomised, open controlled study I. Blinding of randomisations ‐ can't tell II. Blinding of intervention ‐ no III. Blinding of outcome measure assessment ‐ no IV. Complete follow‐up‐ yes | |
| Participants | 12 infants born at </= 28 weeks GA with hyperkalaemia defined as central non haemolyzed serum K+ of > 7 mmol/L Urine output (ml/kg/hr) was not reported Study period May 1, 1989, and December 31, 1989 Single centre, the US | |
| Interventions | 7 infants [mean (SD) GA 24.8 +/‐ 1.5 weeks, BW 780 +/‐ 160 g ] received glucose‐insulin (human insulin with 5% albumin). The insulin was "piggybacked" into the existing i.v. fluids by means of a syringe infusion pump, starting at a rate of 0.1 ml/hr to deliver a dose of 0.05 to 0.1 IU/kg/hr 5 infants [mean (SD) GA 23.8 +/‐ 0.8 weeks, BW 650 +/‐ 60 g] received sodium polystyrene sulphonate (Kayexalate) prepared in a 25% sorbitol solution and given rectally in a dose of 1 gm/kg every 6 hours | |
| Outcomes | Mortality, treatment failure (increase in K+ concentration > 0.5 mmol/L or failure to decrease K+ to < 7mmol/L, cardiac arrhythmia, hypoglycaemia (< 40 mg/dl; 2.2 mmol/L) | |
| Notes | In 4 of the 5 Kayexalate‐treated infants in whom treatment was considered to have failed, a glucose‐insulin therapy was added. All 4 responded with decreased central K+ concentration within 6 hours after glucose‐insulin was added | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised, open controlled study |
| Allocation concealment (selection bias) | Unclear risk | Blinding of randomisations ‐ can't tell |
| Blinding (performance bias and detection bias) All outcomes | High risk | Blinding of intervention ‐ no |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Blinding of intervention ‐ no |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Blinding of outcome measure assessment ‐ no |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Complete follow‐up ‐ yes |
Singh 2002.
| Methods | Randomised, controlled study I. Blinding of randomisations ‐ yes II. Blinding of intervention ‐ yes III. Blinding of outcome measure assessment ‐ yes IV. Complete follow‐up‐ yes (see notes) | |
| Participants | 22 preterm infants receiving assisted ventilation and with a serum potassium >/= 6.0 mmol/L. GA at birth ranged from 23 to 27 weeks and their birth weights ranged from 486 to 1330 g. Age at enrolment varied from 2 to 22 days. Three infants were withdrawn from the study, two at the discretion of the attending physicians and another at the request of the parents Urine output (ml/kg/hr) was not reported | |
| Interventions | 8 infants received albuterol inhalation (400 microgram of albuterol in 2 ml of saline solution) administered by the respiratory therapists through a T‐connection inserted at the inspiratory limb of the ventilator tubing at the endotracheal tube ventilator connection by using a 10‐cm tube with an oxygen flow of 6 LPM. Treatment was given every 2 hours until serum K+ levels fell below 5 mmol/L or if the maximum of 12 doses was reached 11 infants received 2 ml of saline solution inhalation in the same fashion | |
| Outcomes | Change in serum K+ from baseline at 4 and 8 hours after treatment Grade 3 or 4 IVH Pulmonary haemorrhage Pneumothorax Dysrhythmia Mortality Tremors/twitching | |
| Notes | Two patients were withdrawn from the saline group and one infant from the albuterol group Treatment with polystyrene sulphonate and/or glucose‐insulin and/or furosemide occurred in 2 patients in the albuterol group and 5 patients in the control group | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised, controlled study |
| Allocation concealment (selection bias) | Low risk | Blinding of randomisations ‐ yes |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Blinding of intervention ‐ yes Blinding of outcome measure assessment ‐ yes Complete follow‐up ‐ yes (see notes) |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Blinding of intervention ‐ yes |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Blinding of outcome measure assessment ‐ yes |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Complete follow‐up ‐ yes (see notes) |
BW = birth weight g = grams GA = gestational age K+ = serum/plasma potassium IVH = intraventricular haemorrhage VLBW = very low birth weight
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Leslie 1993 | This study was identified through the search of the Cochrane Central Register of Controlled Trials Database in the Cochrane Library. This was a controlled but not a randomised trial |
Contributions of authors
Prakash Vemgal (PV) and Arne Ohlsson (AO) contributed equally to all sections of the protocol for this review. The literature search of databases was developed with the help of an experienced librarian. Both authors identified potentially eligible studies from the printouts and agreed on which trials to include. Quality assessments were conducted and data were abstracted by both authors independently and compared. One author (AO) entered the data into RevMan and the other author (PV) checked for accuracy. One author (AO) wrote the sections of the full review and the other author (PV) read and made changes. Changes were made by both authors following feedback from the editors of the review group.
The May 2011 update was conducted centrally by the Cochrane Neonatal Review Group staff (Yolanda Montagne, Diane Haughton, and Roger Soll). This update was reviewed and approved by PV and AO.
Sources of support
Internal sources
Mount Sinai Hospital, Toronto, Canada.
External sources
-
Eunice Kennedy Shriver National Institute of Child Health and Human Development National Institutes of Health, Department of Health and Human Services, USA.
Editorial support of the Cochrane Neonatal Review Group has been funded with Federal funds from the Eunice Kennedy Shriver National Institute of Child Health and Human Development National Institutes of Health, Department of Health and Human Services, USA, under Contract No. HHSN275201100016C.
Declarations of interest
None
Deceased
Edited (no change to conclusions)
References
References to studies included in this review
Hu 1999 {published data only}
- Hu P‐S, Su B‐H, Peng C‐T, Tsai C‐H. Glucose and insulin infusion versus kayexalate for the early treatment of non‐oliguric hyperkalemia in very‐low‐birth‐weight infants. Acta Paediatrica Taiwanica 1999;40:314‐8. [PubMed] [Google Scholar]
Malone 1991 {published data only}
- Malone TA. Glucose and insulin versus cation‐exchange resin for the treatment of hyperkalemia in very low birth weight infants. Journal of Pediatrics 1991;118:121‐3. [DOI] [PubMed] [Google Scholar]
Singh 2002 {published data only}
- Singh BS, Sadiq HF, Noguchi A, Keenan WJ. Efficacy of albuterol inhalation in treatment of hyperkalemia in premature neonates. Journal of Pediatrics 2002;141:16‐20. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Leslie 1993 {published data only}
- Leslie GI, Koumantakis G, Arnold J, Bowen J. Resonium vs dextrose‐insulin for treatment of hyperkalaemia in extremely premature infants. Journal of Paediatrics and Child Health 1993;29:A16. [Google Scholar]
Additional references
Angelopoulous 1996
- Angelopoulous M, Leitz H, Lambert G, MacGilvary S. In vitro analysis of the Na(+) ‐K+ ATPase activity in neonatal and adult red blood cells. Biology of the Neonate 1996;69:140‐5. [DOI] [PubMed] [Google Scholar]
Avenarius 1996
- Avenarius S, Gosch G. Postnatale Hyperkaliamie sehrkleiner Fruhgeborener. Monatsschrift fur Kinderheilkunde 1996;144:1374. [Google Scholar]
Bell 1978
- Bell MJ, Ternberg JL, Feigin RD, Keating JP, Marshall R, Barton L, Brotherton T. Neonatal necrotizing enterocolitis. Therapeutic decisions based upon clinical staging. Annals of Surgery 1978;187:1‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bennett 1996
- Bennett LN, Myres TF, Lambert GH. Cecal perforation associated with sodium polystyrene sulfonate‐sorbital enemas in a 650 gram infant with hyperkalemia. American Journal of Perinatology 1996;13:167‐70. [DOI] [PubMed] [Google Scholar]
Brion 1989
- Brion LP, Schwartz GJ, Campell D, Fleischman AR. Early hyperkalaemia in very low birthweight infants in absence of oliguria. Archives of Disease in Childhood 1989;64:270‐82. [DOI] [PMC free article] [PubMed] [Google Scholar]
Chevalier 1998
- Chevalier RL. What are normal potassium concentrations in the neonate? What is a reasonable approach to hyperkalemia in the newborn with normal renal function?. Seminars in Nephrology 1998;18:360‐1. [PubMed] [Google Scholar]
Dilmen 1992
- Dilmen U, Toppare M, Senses DA, Kaya IS. Salbutamol in the treatment of neonatal hyperkalemia. Biology of the Neonate 1992;62:424‐6. [DOI] [PubMed] [Google Scholar]
Fukuda 1989
- Fukuda Y, Kojima T, Ono A, Matsuzaki S, Iwase S, Kobayashi Y. Factors causing hyperkalemia in premature infants. American Journal of Perinatology 1989;6:76‐9. [DOI] [PubMed] [Google Scholar]
Grammatikopoulos 03
- Grammatikopoulos T, Greenough A, Pallidis C, Davenport M. Benefits and risks of calcium resonium therapy in hyperkalaemic preterm infants. Acta Paediatrica 2003;92:118‐20. [DOI] [PubMed] [Google Scholar]
Greenough 1992
- Greenough A, Emery EF, Brooker R, Gamsu HR. Salbutamol infusion to treat neonatal hyperkalaemia. Journal of Perinatal Medicine 1992;20:437‐41. [DOI] [PubMed] [Google Scholar]
Gruskay 1988
- Gruskay J, Costarino AT, Polin RA, Baumgart S. Nonoliguric hyperkalemia in premature infants weighing less than 1000 grams. Journal of Pediatrics 1988;113:381‐6. [DOI] [PubMed] [Google Scholar]
Heyman 1989
- Heyman E, Shennan A, Ohlsson A. Aggressive, early insulin and glucose treatment for hyperkalemia in extremely low gestation newborns (Abstract). Pediatric Research 1989;25:218A. [Google Scholar]
Higgins 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Kilbride 1988
- Kilbride HW, Cater G, Warady BA. Early onset hyperkalemia in extremely low birth infants. Journal of Perinatology 1988;8:211‐4. [PubMed] [Google Scholar]
Lackmann 1992
- Lackmann GM, Tollner U. Strategies to reduce hyperkalemia‐induced cardiac arrythmias in premature infants and newborns. Pediatrics 1992;89:1130‐1. [PubMed] [Google Scholar]
Lorenz 1997
- Lorenz JM, Kleinman LI, Markarian K. Potassuim metabolism in extremely low birth weight infants in the first week of life. Journal of Pediatrics 1997;131:81‐6. [DOI] [PubMed] [Google Scholar]
Lui 1992
- Lui K, Thungappa U, Nair A, John E. Treatment with hypertonic dextrose and insulin in severe hyperkalaemia of immature infants. Acta Paediatrica 1992;81:213‐6. [DOI] [PubMed] [Google Scholar]
Mildenberger 1996
- Mildenberger E, Mansmann U, Versmold HT. Postnatal hyperkalemia of the very low birthweight infant: trait of immaturity. Monatsschrift Kinderheilkunde 1996;144:267‐70. [Google Scholar]
Mildenberger 2002a
- Mildenberger E, Versmold HT. Pathogenesis and therapy of non‐oliguric hyperkalaemia of the premature infant. European Journal of Pediatrics 2002;161:415‐22. [DOI] [PubMed] [Google Scholar]
Mildenberger 2002b
- Mildenberger E, Versmold H. Results of national survey in Germany on incidence and therapy of the nonoliguric hyperkalemia of the premature infant. Zeitschrift Geburtshielfe Neonatolgie 2002;206:9‐14. [DOI] [PubMed] [Google Scholar]
Ohlsson 1987
- Ohlsson A, Hosking M. Complications following oral administration of exchange resins in extremely low‐birth weight infants. European Journal of Pediatrics 1987;146:571‐4. [DOI] [PubMed] [Google Scholar]
Ohlsson 1987a
- Ohlsson A, Shennan T, Rose TH. Review of causes of perinatal mortality in a regional perinatal center, 1980 to 1984. American Journal of Obstetrics and Gynecology 1987;157:443‐5. [DOI] [PubMed] [Google Scholar]
Omar 2000
- Omar SA, DeCristofaro JD, Agarwal BI, LaGamma EF. Effects of prenatal steroids on potassium balance in extremely low birth weight neonates. Pediatrics 2000;106:561‐7. [DOI] [PubMed] [Google Scholar]
Papile 1978
- Papile LA, Burstein J, Burstein R, Koffler H. Incidence and evolution of subependymal and intraventricular hemorrhage: a study of infants with birth weights less than 1,500 gm. Journal of Pediatrics 1978;92:529‐34. [DOI] [PubMed] [Google Scholar]
Perkin 1980
- Perkin RM, Levin DL. Common fluid and electrolyte problems in the pediatric intensive care unit. Pediatric Clinics of North America 1980;27:567‐86. [DOI] [PubMed] [Google Scholar]
Robinson 2002
- Robinson KA, Dickersin K. Development of a highly sensitive search strategy for the retrieval of reports of controlled trials using PubMed. International Journal of Epidemiology 2002;31:150‐3. [DOI] [PubMed] [Google Scholar]
Sato 1995
- Sato K, Kondo T, Iwao H, Honda S, Ueda K. Internal potassium shift in premature infants: cause of nonoliguric hyperkalemia. Journal of Pediatrics 1995;126:109‐13. [DOI] [PubMed] [Google Scholar]
Setzer 1984
- Setzer ES, Ahmed F, Goldberg RN, Hellman RL, Moscoso P, Ferrer PL, et al. Exchange transfusion using washed red blood cells reconstituted with fresh‐frozen plasma for treatment of severe hyperkalemia in the neonate. Journal of Pediatrics 1984;104:443‐6. [DOI] [PubMed] [Google Scholar]
Shaffer 1992
- Shaffer SG, Kilbride HW, Hayen LK, Meade VM, Warady BA. Hyperkalemia in very low birth weight infants. Journal of Pediatrics 1992;121:275‐9. [DOI] [PubMed] [Google Scholar]
Shortland 1987
- Shorland D, Trounce JQ, Levene MI. Hyperkalaemia, cardiac arrhythmias, and cerebral lesions in high risk neonates. Archives of Disease in Childhood 1987;62:1139‐43. [DOI] [PMC free article] [PubMed] [Google Scholar]
Stefano 1993
- Stefano JL, Norman ME, Morales MC, Goplerud JM, Mishra OP, Delivoria‐Papadopoulos M. Decreased erythrocyte Na+, K(+) ‐ATPase activity associated with cellular potassium loss in extremely low birth weight infants with nonoliguric hyperkalemia. Journal of Pediatrics 1993;122:276‐84. [DOI] [PubMed] [Google Scholar]
Stefano 1993a
- Stefano JL, Norman ME. Nitrogen balance in extremely low birth weight infants with nonoliguric hyperkalaemia. Journal of Pediatrics 1993;123:632‐5. [DOI] [PubMed] [Google Scholar]
Sychlowy 1990
- Sychlowy A, Gaag H, Hannen‐Hofheinz I. Hyperkalemia ‐ a life threatening early complication of asphyxia in premature infants [Hyperkaliamie ‐ lebensbedrohliche Frukomplikation asphyktischer Frugeborener]. Monatsschrift Kinderheilkunde 1990;138:62‐5. [PubMed] [Google Scholar]
Uga 2003
- Uga N, Nemoto Y, Ishii T, Kawase Y, Arai H, Tada H. Antenatal steroid treatment prevents severe hyperkalemia in very low‐birthweight infants. Pediatrics International 2003;45:656‐60. [DOI] [PubMed] [Google Scholar]
Usher 1959
- Usher R. The respiratory distress syndrome of prematurity. I. Changes in potassium in the serum and the electrocardiogram and effects of therapy. Pediatrics 1959;24:562‐76. [PubMed] [Google Scholar]
References to other published versions of this review
Vemgal 2007
- Vemgal P, Ohlsson A. Interventions for non‐oliguric hyperkalaemia in preterm neonates. Cochrane Database of Systematic Reviews 2007, Issue 1. [DOI: 10.1002/14651858.CD005257.pub2] [DOI] [PubMed] [Google Scholar]


