Abstract
Background
Successful transition from parenteral nutrition to full enteral feedings during the immediate neonatal period is associated with improved growth in preterm infants. Lactase is the last of the major intestinal disaccharidases to develop in preterm infants. Because of inadequate lactase activity, preterm infants are unable to digest lactose. Lactase preparations could potentially be used to hydrolyse lactose in formulas and breast milk to minimize lactose malabsorption in preterm infants.
Objectives
To assess the effectiveness and safety of the addition of lactase to milk compared to placebo or no intervention for the promotion of growth and feeding tolerance in preterm infants.
Primary outcomes: weight gain expressed as grams/kg/day, growth expressed as weight, length and head circumference percentile for postmenstrual age (PMA), assessed at birth and at 40 weeks PMA, days to achieve full enteral feeds. Secondary outcomes: several common outcomes associated with preterm birth, and adverse effects.
Search methods
Electronic and manual searches were conducted in January 2005 of the Cochrane Central Register of Controlled Trials (CENTRAL, The Cochrane Library, 2004, Issue 4), MEDLINE (1966 to Jan 2005), EMBASE (1980 to Jan 2005) and CINAHL (1982 to Jan 2005), personal files, bibliographies of identified trials and abstracts by the Pediatric Academic Societies' Meetings and the European Society of Pediatric Research Meetings published in Pediatric Research. The searches were repeated in May 2012 of The Cochrane Library, MEDLINE, EMBASE and CINAHL and abstracts from the Pediatric Academic Societies' Annual Meetings from 2000 to 2012 (Abstracts2View). The Web of Science was searched using the only previously identified trial by Erasmus 2002 as the starting point to search for additional trials that cited this trial.
Selection criteria
Types of studies: randomized or quasi‐randomized controlled trials. Participants: preterm infants < 37 weeks PMA. Intervention: addition of lactase to milk versus placebo or no intervention.
Data collection and analysis
The standard methods of the Cochrane Neonatal Review Group were followed independently by the review authors to assess study quality and report outcomes. Treatment effects, calculated using Review Manager 5, included risk ratio (RR), risk difference (RD) and mean difference (MD), all with 95% confidence intervals (CI). A fixed‐effect model was used for meta‐analyses. We did not perform heterogeneity tests as only one study was identified.
Main results
The repeat searches conducted in May 2012 did not identify any additional studies for inclusion. One study enrolling 130 infants of 26 to 34 weeks PMA (mean postnatal age at entry 11 days) was identified and no identified study was excluded. The study was a double blind randomized controlled trial of high quality. Lactase treated feeds were initiated when enteral feedings provided > 75% of daily intake. None of the primary outcomes outlined in the protocol for this review and only one of the secondary outcomes, necrotizing enterocolitis (NEC) were reported on. The RR for NEC was 0.32 (95% CI 0.01 to 7.79); the RD was ‐0.02 (95% CI ‐0.06 to 0.03) (a reduction which was not statistically significant). There was a statistically significant increase in weight gain at study day 10 in the lactase treated feeds group but not at any other time points. Overall, there was not a statistically significant effect on weight gain. No adverse effects were noted.
Authors' conclusions
The only randomized trial to date provides no evidence of significant benefit to preterm infants from adding lactase to their feeds. Further research regarding effectiveness and safety are required before practice recommendations can be made. Randomized controlled trials comparing lactase versus placebo treated feeds and enrolling infants when enteral feeds are introduced are required. The primary and secondary outcomes for effectiveness and safety should include those identified in this review.
Plain language summary
Lactase treated feeds to promote growth and feeding tolerance in preterm infants
Very low birth weight preterm infants are often fed through a tube into a vein (parenterally) as adequate growth and nutrition is important for lung and brain development. Early feeding via the gut (enterally) stimulates motility and digestive activity and is associated with improved growth, but this is not always possible. Lactase is an intestinal enzyme that helps digest milk and is slow to develop in preterm infants after birth. Breast milk contains components that help with lactose digestion. Lactose intolerance is often managed in infants born at term with low‐lactose or lactose‐free formulas, but these do not fulfil the nutrition requirements for preterm infants. Feeding intolerance leaves residual feeds in the stomach prior to the next scheduled feeding and causes abdominal distension, bile stained fluid in the lungs (aspirates), and vomiting. Preparations of lactase could potentially be added to formula or breast milk for preterm infants. There was not a significant effect on weight gain in the one randomized controlled trial identified that investigated addition of lactase. The review authors searched the medical literature thoroughly but found only this one high quality trial enrolling 130 preterm infants. No adverse effects were noted and lactase treated feeds appeared to be well tolerated.
Background
Description of the condition
Successful transition from parenteral nutrition to full enteral feedings during the immediate neonatal period is associated with improved growth outcomes in preterm infants (Wright 1993; Lee 1996; Ehrenkranz 1999). Adequate growth and nutrition in this population has been shown to be closely linked to outcomes such as chronic lung disease and neurodevelopment, and has implications for future adult health (Hack 2003). Early postnatal nutrition in the very low birth weight (VLBW) preterm infant is primarily supported parenterally, but this does little to support the function of the gastrointestinal tract. Early enteral feedings are beneficial to the gastrointestinal tract because of their trophic effects, positive effect on motility, and stimulation of gastrointestinal hormone secretion (Berseth 1995). However, studies have shown that full enteral feeds are not established in critically ill VLBW preterm infants until an average of 30 days of age (Shulman 1998; Ehrenkranz 1999; Griffin 1999; Steward 2002).
One of the primary setbacks to establishing full enteral feeds in preterm infants is feeding intolerance. This presents clinically as residual feeds in the stomach prior to the next scheduled feeding, sometimes associated with abdominal distension, bile stained aspirates, or emesis. Consequences of feeding difficulties include withholding of feedings, reductions in the amount of feeding, and the need for repeated abdominal radiographs to rule out the possibility that the feeding intolerance is related to necrotizing enterocolitis. In addition, the slow advancement of enteral feeding often leads to prolonged use of parenteral nutrition which predisposes these infants to nosocomial infections, hepatic dysfunction, and prolonged hospitalizations (Schanler 1996).
Description of the intervention
Lactose intolerance is often managed with low‐lactose or lactose‐free formulas (Sinden 1991). However, these formulas ‐ developed for term infants ‐ do not meet the requirements for growth and development of the preterm infant (NCCPS 1995; AAP 1998). In the adult and paediatric population, lactose malabsorption is often treated with commercial lactase preparations. The recommended technique of lactase supplementation suggested by the manufacturer of commercially available lactase is to store milk with added enzyme (crushed tablet or drops of liquid) for 24 hours in the refrigerator before its use (PDR 2003).
Commercial lactase preparations can be used to hydrolyse lactose in formulas and breast milk to minimize lactose malabsorption in preterm infants. The concern, however, is that the addition of lactase enzyme following the manufacturer‐recommended 24‐hour incubation period can increase osmolality to levels that exceed current guidelines for preterm infant feedings. There is evidence that hyperosmolar solutions may place the preterm population at higher risk for development of necrotizing enterocolitis (Book 1975; Willis 1977). The 24‐hour incubation period has also raised concerns regarding bacterial contamination of milk (Malone 1999) which has been shown to occur within eight hours in an unsterile environment (White 1979). This lengthy incubation, which results in near elimination of lactose, may not be necessary for the purpose of improving feeding tolerance in the preterm infant. Studies have demonstrated that incubating milk with lactase for 15 minutes at 37 degrees Celsius was sufficient to accomplish over half of the 24‐hour digestion (Fenton 2002) and minimize the rise in osmolality to levels that are still within current guidelines (Carlson 1991).
How the intervention might work
Enhanced endogenous lactase activity is associated with improved feeding tolerance in preterm infants, with a decrease in the time to transition to full enteral feeds (Shulman 1998). Breast milk, shown to be better tolerated by preterm infants, induces higher lactase activity than formula (Shulman 1998). Direct and indirect trophic effects of minimal enteral nutrition in the early postnatal period demonstrated by human and animal studies can be related to its effects of increasing intestinal lactase activity (Shulman 1998; McClure 2002).
Why it is important to do this review
To our knowledge, the topic of lactase‐treated feeds for preterm infants and its effects on growth and nutrition has not been systematically reviewed.
Objectives
To assess the effectiveness and safety of the addition of lactase to milk compared to placebo or no intervention for the promotion of growth and feeding tolerance in preterm infants.
Methods
Criteria for considering studies for this review
Types of studies
Randomized or quasi‐randomized controlled trials.
Types of participants
Preterm infants < 37 weeks PMA.
Types of interventions
Addition of lactase to milk versus placebo or no intervention.
Types of outcome measures
Primary outcomes
Weight gain expressed as grams/kg/day.
Growth expressed as weight, length and head circumference percentile for PMA, assessed at birth and at 40 weeks postmenstrual age.
Days to achieve full enteral feeds.
Secondary outcomes
Duration of parenteral nutrition (PN) expressed in number of days.
Days enteral feeds held.
Number of times enteral feeding is interrupted for gastric residuals.
Days to regain birthweight.
Duration of hospitalisation expressed in total days since birth.
Incidence of necrotizing enterocolitis (NEC) defined as suspected or confirmed positive Bell's Stage II or greater.
Incidence of bacteraemia defined as blood cultures positive for bacteria.
Incidence of sepsis defined as signs and symptoms of infection and positive culture from blood.
Incidence of chronic lung disease defined as requiring supplemental oxygen at 28 days of age or 36 weeks corrected PMA.
Adverse effects reported by the investigators.
Search methods for identification of studies
Electronic searches
See: Collaborative Review Group search strategy (http://neonatal.cochrane.org/).
The review started by review of personal files. We searched MEDLINE (1966 to January 2005) using MESH terms: beta‐galactosidase/therapeutic use, lactase, weight gain, feeding tolerance, infant nutrition/physiology, newborn, infant, premature (or preterm). We searched other databases including: the Cochrane Central Register of Controlled Trials (CENTRAL, The Cochrane Library 2004, Issue 4), EMBASE (1980 to January 2005), CINAHL (1982 to January 2005) and the reference list of identified trials and abstracts published in Pediatric Research (1991 to 2004) from conference proceedings of the Academic Pediatric Societies (American Pediatric Society, Society of Pediatric Research) and the European Society of Pediatric Research. For any abstracts identified in Pediatric Research, authors performed searches in MEDLINE and EMBASE to identify any corresponding full manuscripts published. We entered identified trials into Science Citation Index to find articles that quoted the original studies and to ascertain any additional potential studies for inclusion in the review. We sought unpublished data. We planned to contact authors of published trials to clarify or provide additional information. We did not apply any language restriction.
The searches were repeated in May 2012 of The Cochrane Library, Issue 4, MEDLINE, EMBASE and CINAHL (see Appendix 1). In June 2012, we searched abstracts from the Pediatric Academic Societies' Annual Meetings from 2000 to 2012 (Abstracts2View). We searched the Web of Science in June 2012 using the only previously identified trial by Erasmus 2002 as the starting point to search for additional trials that cited this trial.
Searching other resources
We scanned the reference list of the only identified trial (Erasmus 2002) for any additional trial. Reference lists of published narrative and systematic reviews were to be reviewed.
Data collection and analysis
Selection of studies
We assessed all abstracts and published full reports identified as potentially relevant by the literature search for inclusion in the review. Each review author extracted data separately using pre‐designed data abstraction forms. Review authors then compared and resolved differences.
We assessed retrieved articles and abstracted data independently. We conducted independent quality assessment and were not blinded to authors, institution or journal of publication.
Data extraction and management
One review author (AO) entered data into Review Manager (RevMan) (RevMan 2011) and the other author (TD) cross checked the printout against her own data abstraction forms. Errors were corrected by consensus.
For studies identified as abstracts, primary authors were to be contacted to ascertain whether a full publication was available, if the full paper had not been identified in an electronic database.
Assessment of risk of bias in included studies
We used the standardized review methods of the Cochrane Neonatal Review Group to assess the methodological quality of studies.
Quality of included trials were evaluated independently by the review authors, using the following criteria:
Blinding of randomization?
Blinding of intervention?
Blinding of outcome measure assessment?
Completeness of follow‐up?
There were three potential answers to these questions ‐ yes, can't tell, no.
For this update we used the Risk of Bias (RoB) table to report detailed information about the only included trial.
Selection bias (random sequence generation and allocation concealment)
Random sequence generation
For each included study, we categorized the risk of bias regarding random sequence generation as:
low risk ‐ adequate (any truly random process e.g. random number table; computer random number generator);
high risk ‐ inadequate (any non random process e.g. odd or even date of birth; hospital or clinic record number);
unclear risk ‐ no or unclear information provided.
Allocation concealment
For each included study, we categorized the risk of bias regarding allocation concealment as:
low risk ‐ adequate (e.g. telephone or central randomization; consecutively numbered sealed opaque envelopes);
high risk ‐ inadequate (open random allocation; unsealed or non‐opaque envelopes, alternation; date of birth);
unclear risk ‐ no or unclear information provided.
Performance bias
For each included study, we categorized the methods used to blind study personnel from knowledge of which intervention a participant received. (As our study population consisted of neonates they would all be blinded to the study intervention.) We categorized performance bias as:
low risk ‐ adequate for personnel (a placebo that could not be distinguished from the active drug was used in the control group).
high risk ‐ inadequate ‐ personnel aware of group assignment.
unclear risk ‐ no or unclear information provided.
Detection bias
For each included study, we categorized the methods used to blind outcome assessors from knowledge of which intervention a participant received. (As our study population consisted of neonates they would all be blinded to the study intervention.) Blinding was assessed separately for different outcomes or classes of outcomes. We categorized the methods used with regards to detection bias as:
low risk ‐ adequate; follow‐up was performed with assessors blinded to group;
high risk ‐ inadequate; assessors at follow‐up were aware of group assignment;assignment;
unclear risk ‐ no or unclear information provided.
Attrition bias
For each included study and for each outcome, we described the completeness of data including attrition and exclusions from the analysis. We noted whether attrition and exclusions were reported, the numbers included in the analysis at each stage (compared with the total randomized participants), reasons for attrition or exclusion where reported, and whether missing data were balanced across groups or were related to outcomes. Where sufficient information was reported or supplied by the trial authors, we re‐included missing data in the analyses. We categorized the methods with respect to the risk attrition bias as:
low risk ‐ adequate (< 10% missing data);
high risk ‐ inadequate (> 10% missing data);
unclear risk ‐ no or unclear information provided.
Reporting bias
For each included study, we described how we investigated the risk of selective outcome reporting bias and what we found. We assessed the methods as:
low risk ‐ adequate (where it is clear that all of the study's pre‐specified outcomes and all expected outcomes of interest to the review have been reported);
high risk ‐ inadequate (where not all the study's pre‐specified outcomes have been reported; one or more reported primary outcomes were not pre‐specified; outcomes of interest were reported incompletely and so cannot be used; study failed to include results of a key outcome that would have been expected to have been reported);
unclear risk ‐ no or unclear information provided (the study protocol was not available).
Other bias
For each included study, we described any important concerns we had about other possible sources of bias (e.g. whether there was a potential source of bias related to the specific study design or whether the trial was stopped early due to some data‐dependent process). We assessed whether each study was free of other problems that could put it at risk of bias as:
low risk ‐ no concerns of other bias raised;
high risk ‐ concerns raised about multiple looks at the data with the results made known to the investigators, difference in number of patients enrolled in abstract and final publications of the paper;
unclear ‐ concerns raised about potential sources of bias that could not be verified by contacting the authors.
If needed, we planned to explore the impact of the level of bias through undertaking sensitivity analyses.
Measures of treatment effect
The statistical analyses followed the recommendations of the Cochrane Neonatal Review Group. A mean treatment effect was calculated using RevMan (Higgins 2011; RevMan 2011). The statistical methods included risk ratio (RR), risk difference (RD), number needed to treat for an additional beneficial outcome (NNTB) or number needed to treat for an additional harmful outcome (NNTH) for dichotomous outcomes, and mean difference (WMD) for continuous outcomes. All estimates of treatment effects are reported with 95% confidence intervals (CI). We used a fixed‐effect model for meta‐analyses. We did not plan additional sensitivity analyses a priori but exploratory (post‐hoc) analyses were to be performed depending on the results of the review. We did not plan any subgroup analyses. We included outcomes reported by the authors but these outcomes had not been included in our protocol.
Unit of analysis issues
The unit of analysis was the individual randomized infant. It is unlikely that we will identify any cluster randomized trials for this intervention.
Dealing with missing data
We planned to obtain further information from the primary author if a published article provided inadequate information for the review. Dr. Koravangattu Sankaran, Royal University Hospital, Saskatoon, Saskatchewan, Canada provided additional information on the included study.
Assessment of heterogeneity
Heterogeneity tests were not performed as only one study was identified. If more than one trial had been identified we had planned to report on heterogeneity including the I2 statistic (Higgins 2003). We would have reported on the percentages of heterogeneity as: < 25% ‐ no heterogeneity; > 25% to 49% ‐ low heterogeneity; 50% to 74% ‐ moderate heterogeneity, 75% or more ‐ high heterogeneity.
Assessment of reporting biases
If we had included at least 10 trials we would have performed a funnel plot and we would have looked for asymmetry in the funnel plot. Asymmetry could indicate that trials showing no effect of the intervention might not have been published.
Subgroup analysis and investigation of heterogeneity
We did not plan any subgroup analyses a priori.
Sensitivity analysis
As we have commonly‐noted discrepancies between numbers enrolled in trials as reported in abstracts and full text reports (Walia 2000), sensitivity analyses were to be performed excluding abstracts.
Results
Description of studies
The repeat search of the literature in May 2012 did not identify any additional trials for inclusion. The literature search did not identify any study that was later excluded. One study enrolling 130 preterm infants in a prospective, double‐blind, randomized controlled trial in one neonatal intensive care unit in Canada between April 1997 and July 2000 was identified. For further details see the table 'Characteristics of included studies'. The inclusion criteria were 26 weeks to 34 weeks PMA at birth, ≥ 75% estimated energy requirement from enteral feeds, absence of major congenital malformations or gastrointestinal diseases, including necrotizing enterocolitis (NEC), and no postnatal steroids or diuretics. Small, appropriate and large for PMA infants were eligible for the study. The study intervention started when enteral feedings provided > 75% of the daily intake and was terminated when the infant reached 36 weeks or was discharged from the unit, whichever came first. Infants randomly assigned to the 'lactase treated feeds group' received feeds treated with Lactacid drops (McNeil Consumer Products Company). According to a previous study by Carlson et al (Carlson 1991) this would result in a 70% decrease in lactose concentration (from 35.3 to 10.3 grams/kg) after a two‐hour incubation period. A study placebo solution composed of the identical carrier agent as in Lactacid was used in the control group. The enzyme and matched placebo solutions were packaged in identical bottles labelled "lactase study drops" and were identifiable only by the research nurse according to assigned code numbers. Researchers and care givers remained blinded for the duration of the study. The primary outcome measure was weight gain (grams/day) measured at study day seven, 10, 14 and at study exit. Additional outcomes included gains in length and head circumference; serum concentrations of protein, albumin, sodium and potassium; and measurements of feeding tolerance. In addition withdrawal from the study because of feeding intolerance or NEC was recorded.
Risk of bias in included studies
Although not clearly stated by the authors, we assumed that there was concealed allocation of the infants to one of the two groups. The authors write "...only the research nurse and central food production staff had access to randomization information and did not participate in patient care". Of the 66 infants randomly assigned to the lactase group 52 reached study day 14. Of the 64 infants randomly assigned to the control group 50 reached study day 14. The average (mean ± standard error of the mean) length of the study in the lactase group was 24.1 ± 1.7 days and in the placebo group 25.7 ± 1.9 days. None of the important primary outcomes that we had identified in the protocol and only one secondary outcome (the incidence of NEC) were reported on. The protocol for the study was not available to us. A sample size calculation was performed to allow for a 33% increase in mean weight gain per day in the treatment group, with a power of 0.80. However, it is unclear if the authors had decided a priori at what points in time after study entry to measure growth.
Effects of interventions
Primary outcomes
1. Weight gain expressed as grams/kilogram/day (Outcomes 1.1 to 1.4)
Data for this outcome were not reported. Weight gain (grams/day) was reported at seven days, 10 days, 14 days after entry to study (or at study exit if this occurred earlier) and at study exit (Analysis 1.1; Analysis 1.2; Analysis 1.3; Analysis 1.4). Weight gain (grams/day) on day 7 after study entry was higher in the lactase treated feeds group (mean difference (MD) 4.5 grams; 95% CI ‐0.76 to 9.76), but this was not statistically significant. Weight gain (grams/day) on day 10 after study entry was significantly higher in the lactase treated feeds group (MD 4.9 grams/day; 95% CI 0.18 to 9.62). Weight gain (grams/day) on day 14 after study entry was higher in the lactase treated feeds group (MD 2.7 grams/day; 95% CI ‐1.47 to 6.87) and on study exit it was higher in the lactase treated feeds group (MD 2.2 grams/day; 95% CI ‐0.98 to 5.38). Neither of these were statistically significant.
1.1. Analysis.
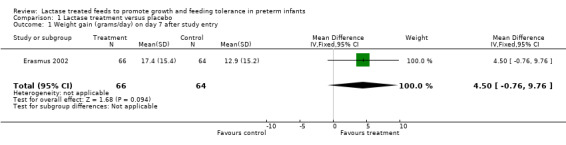
Comparison 1 Lactase treatment versus placebo, Outcome 1 Weight gain (grams/day) on day 7 after study entry.
1.2. Analysis.
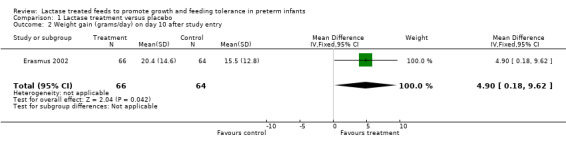
Comparison 1 Lactase treatment versus placebo, Outcome 2 Weight gain (grams/day) on day 10 after study entry.
1.3. Analysis.
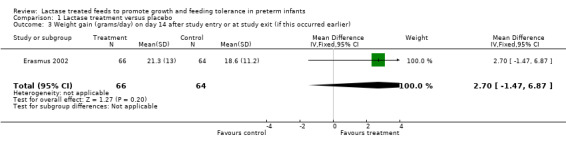
Comparison 1 Lactase treatment versus placebo, Outcome 3 Weight gain (grams/day) on day 14 after study entry or at study exit (if this occurred earlier).
1.4. Analysis.
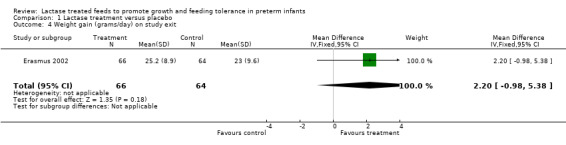
Comparison 1 Lactase treatment versus placebo, Outcome 4 Weight gain (grams/day) on study exit.
2. Growth expressed as weight, length and head circumference percentile for PMA, assessed at birth and at 40 weeks PMA (Outcomes 1.5 and 1.6)
Data for these outcomes were not reported. Length gain (cm/week) (Analysis 1.5) and head circumference gain (cm/week) (Analysis 1.6) were reported on study day 14 or study exit if this occurred earlier. Both were higher in the lactase treated feeds group but not significantly so. Mean difference for length gain was 0.30 cm/week (95% CI ‐0.13 to 0.73). Mean difference for head circumference gain (cm/week) was 0.10 (95% CI ‐0.18 to 0.38).
1.5. Analysis.
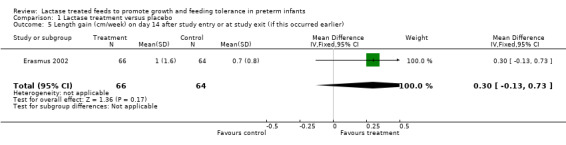
Comparison 1 Lactase treatment versus placebo, Outcome 5 Length gain (cm/week) on day 14 after study entry or at study exit (if this occurred earlier).
1.6. Analysis.
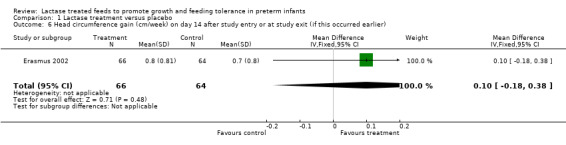
Comparison 1 Lactase treatment versus placebo, Outcome 6 Head circumference gain (cm/week) on day 14 after study entry or at study exit (if this occurred earlier).
3. Days to achieve full enteral feeds
Data for this outcome were not reported. Secondary outcomes
1. Duration of parenteral nutrition (PN) expressed in number of days
Data for this outcome were not reported.
2. Days enteral feeds held
Data for this outcome were not reported.
3. Number of times enteral feeding was interrupted for gastric residuals (Outcome 1.7)
Data for this outcome were not reported. The authors reported that feeding intolerance (not defined) (Analysis 1.7) was lower in the lactase group than in the control group. The RR was 0.65 (95% CI 0.19 to 2.18) and RD was ‐0.03 (95% CI ‐0.12 to 0.06); neither of these were statistically significant.
1.7. Analysis.
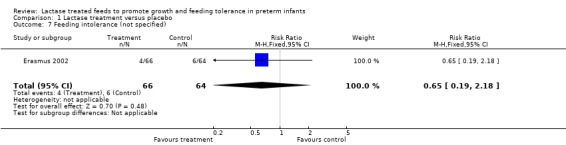
Comparison 1 Lactase treatment versus placebo, Outcome 7 Feeding intolerance (not specified).
4. Days to regain birthweight
Data for this outcome were not reported.
5. Duration of hospitalisation expressed in total days since birth
Data for this outcome were not reported.
6. Incidence of necrotizing enterocolitis (NEC) defined as suspected or confirmed positive Bell's Stage II or greater (Outcome 1.8)
NEC (stage not mentioned) was reported as lower in the lactase treated feeds group. The RR was 0.32 (95% CI 0.01 to 7.79); the RD was ‐ 0.02 (95% CI ‐0.06 to 0.03); neither of these were statistically significant.
7. Incidence of bacteraemia defined as blood cultures positive for bacteria
Data for this outcome were not reported.
8. Incidence of sepsis defined as signs and symptoms of infection and positive culture from blood
Data for this outcome were not reported.
9. Incidence of chronic lung disease defined as requiring supplemental oxygen at 28 days of age or 36 weeks corrected PMA
Data for this outcome were not reported.
10. Adverse effects reported by the investigators (Outcome 1.9)
The authors reported on the number of infants with at least one episode of emesis during the study period (Analysis 1.9) and the number was smaller in the lactase treated feeds group. The RR was 0.95 (95% CI 0.80 to 1.13) and the RD was ‐0.04 (95% CI ‐0.18 to 0.10); neither of these were statistically significant.
1.9. Analysis.
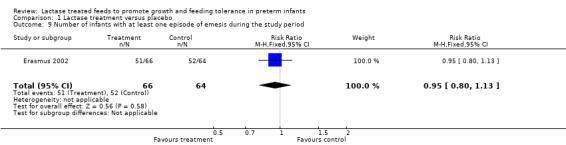
Comparison 1 Lactase treatment versus placebo, Outcome 9 Number of infants with at least one episode of emesis during the study period.
11. Serum albumin on study day 14 or at study exit if this occurred earlier (Outcome 1.10)
This outcome was not predetermined in our protocol. There was a statistically significant increase in serum albumin (g/L) on study day 14 in the lactase treated feeds group with a mean difference of 2.20 g/L (95% CI 0.78 to 3.62) (Analysis 1.10).
1.10. Analysis.
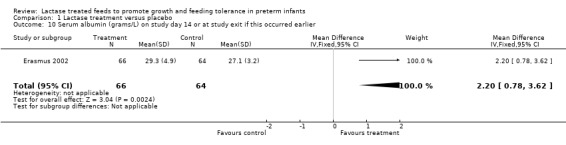
Comparison 1 Lactase treatment versus placebo, Outcome 10 Serum albumin (grams/L) on study day 14 or at study exit if this occurred earlier.
Discussion
The repeat search of the literature in May 2012 did not identify any additional trials for inclusion. To date, only one study has been included in this review and no identified studies have been excluded. The included study enrolled 130 infants of approximately 31 weeks PMA at around 11 days of age, when they were tolerating ≥ 75% of enteral feeds. The study was of high quality but none of the predetermined primary outcomes in our protocol for this review were reported on. Only one of the secondary outcomes (NEC) was reported on. The authors reported on growth at seven, 10 and 14 days after study entry and at exit from the study. It is uncertain whether the time points for measuring growth were predetermined. None of these individual analyses can be interpreted as showing a significant effect on weight gain. There was a statistically significant increase in serum albumin on study day 14 or at study exit if this occurred earlier. There were no serious side effects reported. Lactase treated feeds appear to be well tolerated by preterm infants of approximately 31 weeks PMA at initiation of treatment. Further studies should include the most immature preterm infants (24 to 25 weeks PMA) as the lactase levels in the intestinal tract are even lower in that group of infants. Likewise in future studies lactase should be introduced at the initiation of enteral feeds to confer a potentially greater benefit. The studies need to be of adequate sample size to confirm the potential effectiveness of lactase treated feeds.
Authors' conclusions
Implications for practice.
The only randomized trial to date provides no evidence of significant benefit to preterm infants from adding lactase to their feeds. Further research is required before benefits and risks can be reliably determined.
Implications for research.
Randomized controlled trials of lactase treated feeds enrolling very preterm infants when enteral feeds are started are recommended. The primary and secondary outcomes should include those identified in this review.
What's new
| Date | Event | Description |
|---|---|---|
| 27 January 2020 | Amended | Arne Ohlsson deceased. |
History
Protocol first published: Issue 1, 2004 Review first published: Issue 2, 2005
| Date | Event | Description |
|---|---|---|
| 5 September 2012 | New citation required but conclusions have not changed | Updated search in May 2012 found no new trials. No changes to conclusions. |
| 16 May 2012 | New search has been performed | This review updates the existing review "Lactase treated feeds to promote growth and feeding tolerance in preterm infants" published in the Cochrane Database of Systematic Reviews (Tan‐Dy 2005). |
| 15 October 2008 | Amended | Converted to new review format. |
Acknowledgements
We are thankful to Dr. Koravangattu Sankaran, Royal University Hospital, Saskatoon, Saskatchewan, Canada for providing additional information on the study.
Appendices
Appendix 1. Search Strategy ‐ May 2012
MEDLINE (via PubMed)
(beta‐galactosidase OR lactase OR weight gain OR feeding tolerance OR infant nutrition/physiology) AND ((infant, newborn[MeSH] OR newborn OR neon* OR neonate OR neonatal OR premature OR low birth weight OR VLBW OR LBW) AND (randomised controlled trial [pt] OR controlled clinical trial [pt] OR randomised [tiab] OR placebo [tiab] OR clinical trials as topic [mesh: noexp] OR randomly [tiab] OR trial [ti]) NOT (animals [mh] NOT humans [mh])) AND (("2005"[PDat] : "3000"[PDat]))
CINAHL
((beta‐galactosidase OR lactase OR weight gain OR feeding tolerance OR infant nutrition OR infant physiology) ) and ( ( infant, newborn OR newborn OR neonate OR neonatal OR premature OR low birth weight OR VLBW OR LBW) AND ( randomized controlled trial OR controlled clinical trial OR randomized OR placebo OR clinical trials as topic OR randomly OR trial OR PT clinical trial)) Limiters ‐ Published Date from: 20050101‐20111231
EMBASE
(infant, newborn or newborn or neonate or neonatal or premature or very low birth weight or low birth weight or VLBW or LBW).mp. [mp=title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer, device trade name, keyword] (607975)
(human not animal).mp. [mp=title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer, device trade name, keyword] (11910500)
(randomized controlled trial or controlled clinical trial or randomized or placebo or clinical trials as topic or randomly or trial or clinical trial).mp. [mp=title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer, device trade name, keyword] (1266847)
(beta‐galactosidase or lactase or weight gain or feeding tolerance or infant nutrition or infant physiology).mp. [mp=title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer, device trade name, keyword] (101811)
1 and 2 and 3 and 4 (1050)
limit 5 to yr="2005 ‐Current" (482)
The Cochrane Library
(infant or newborn or neonate or neonatal or premature or very low birth weight or low birth weight or VLBW or LBW)and (beta‐galactosidase OR lactase OR "weight gain" OR "feeding tolerance" OR "infant nutrition" OR "infant physiology") 2005 ‐ 2011
clinicaltrials.gov
(infant OR newborn) AND (beta‐galactosidase OR lactase) AND (weight gain OR feeding tolerance OR infant nutrition OR infant physiology)
controlled‐trials.com
(infant OR newborn) AND (beta‐galactosidase OR lactase) AND (weight gain OR feeding tolerance OR infant nutrition OR infant physiology)
Data and analyses
Comparison 1. Lactase treatment versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Weight gain (grams/day) on day 7 after study entry | 1 | 130 | Mean Difference (IV, Fixed, 95% CI) | 4.50 [‐0.76, 9.76] |
| 2 Weight gain (grams/day) on day 10 after study entry | 1 | 130 | Mean Difference (IV, Fixed, 95% CI) | 4.90 [0.18, 9.62] |
| 3 Weight gain (grams/day) on day 14 after study entry or at study exit (if this occurred earlier) | 1 | 130 | Mean Difference (IV, Fixed, 95% CI) | 2.70 [‐1.47, 6.87] |
| 4 Weight gain (grams/day) on study exit | 1 | 130 | Mean Difference (IV, Fixed, 95% CI) | 2.20 [‐0.98, 5.38] |
| 5 Length gain (cm/week) on day 14 after study entry or at study exit (if this occurred earlier) | 1 | 130 | Mean Difference (IV, Fixed, 95% CI) | 0.30 [‐0.13, 0.73] |
| 6 Head circumference gain (cm/week) on day 14 after study entry or at study exit (if this occurred earlier) | 1 | 130 | Mean Difference (IV, Fixed, 95% CI) | 0.10 [‐0.18, 0.38] |
| 7 Feeding intolerance (not specified) | 1 | 130 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.65 [0.19, 2.18] |
| 8 Necrotizing enterocolitis | 1 | 130 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.32 [0.01, 7.79] |
| 9 Number of infants with at least one episode of emesis during the study period | 1 | 130 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.95 [0.80, 1.13] |
| 10 Serum albumin (grams/L) on study day 14 or at study exit if this occurred earlier | 1 | 130 | Mean Difference (IV, Fixed, 95% CI) | 2.20 [0.78, 3.62] |
1.8. Analysis.
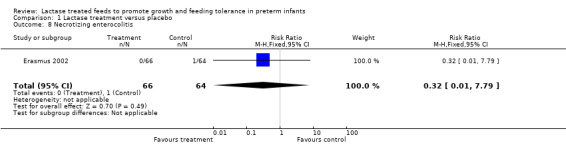
Comparison 1 Lactase treatment versus placebo, Outcome 8 Necrotizing enterocolitis.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Erasmus 2002.
| Methods | Randomized, double‐blind, controlled trial Blinding of randomization? ‐ Yes Blinding of intervention? ‐ Yes Blinding of outcome measure assessment? ‐ Yes Completeness of follow‐up? Yes ‐ see Notes section | |
| Participants | 130 preterm infants (PMA 26 to 34 weeks) admitted to one NICU in Saskatoon, Saskatchewan, Canada were enrolled between April 1997 and July 2000 Entry characteristics expressed as mean and SEM 66 infants were assigned to the lactase group (numbers indicate mean ± SEM); PMA (weeks) 31.4 ± 0.3; BW (grams) 1394.0 ± 49.1; Age at study entry (d) 11.2 ± 0.9; Weight at study entry (grams) 1408.8 ± 41.6; Body length at study entry (cm) 40.7 ± 0.5; Head circumference at study entry (cm) 27.9 ± 0.3 64 infants were assigned to the placebo group; PMA (weeks) 31.4 ± 0.2; BW (grams) 1420.9 ± 56.3; Age at study entry (d) 10.8 ± 0.9; Weight at study entry (grams) 1434.2 ± 48.7; Body length at study entry (cm) 41.0 ± 0.4; Head circumference at study entry (cm) 27.9 ± 0.3 | |
| Interventions | Infants randomly assigned to the lactase group received feeds treated with Lactacid drops (McNeil Consumer Products Company) which according to Carlson et al (Carlson 1991) would result in a 70% decrease in lactose concentration. A study placebo solution composed of the identical carrier agent as in Lactacid was used in the control group. The enzyme and matched placebo solutions were packaged in identical bottles labelled "lactase study drops" and were identifiable only by the research nurse according to assigned code numbers. Researchers and care givers remained blinded for the duration of the study. |
|
| Outcomes | Primary outcome was weight gain (grams per day). Secondary outcomes included gains in length and head circumference, biochemical indexes of nutritional status, feeding intolerance, NEC | |
| Notes | 52 of 66 infants assigned to the lactase group reached study day 14; 50 of 64 infants assigned to the control group reached study day 14.
One of the authors of the study was contacted and confirmed that outcomes were reported as per the numbers of infants randomized.
The study intervention started when enteral feedings provided > 75% of the daily intake. The study was terminated when the infant reached 36 weeks or was discharged from the unit, whichever came first. Study infants were fed according to parental choice. Infants fed human milk received human milk alone on study day 1 and 2. On study day 3, each infant received a 1:1 ratio of human milk and a liquid human milk fortifier (Natural Care, Ross Laboratories) (providing 81 kcal/100 mL) and continued with this feeding regimen for the duration of the study. Infants fed formula received the preterm formula Similac Special Care (SSC) (Ross Laboratories). On study days 1 and 2 these infants received SSC 20 (68 kcal/100 mL) and on study day 3 they were advanced to SSC 24 (81 kcal/100 mL). |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information provided |
| Allocation concealment (selection bias) | Unclear risk | Infants were randomly assigned |
| Blinding (performance bias and detection bias) All outcomes | Low risk | "Researchers and care givers remained blinded for the duration of the study; only the research nurse and central food production staff had access to randomization information and did not participate in patients care" |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "Researchers and care givers remained blinded for the duration of the study; only the research nurse and central food production staff had access to randomization information and did not participate in patients care" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "Researchers and care givers remained blinded for the duration of the study; only the research nurse and central food production staff had access to randomization information and did not participate in patients care" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Sixty‐six infants were randomly assigned to the lactase group, 52 reached study day 14. Sixty‐four infants were randomly assigned to the control group, of which 50 reached study day 14 |
| Selective reporting (reporting bias) | Unclear risk | The study protocol was not available to us |
BW = birth weight; d = days; NEC = necrotizing enterocolitis; NICU = neonatal intensive care unit; PMA = postmenstrual age; SEM = standard error of the mean.
Contributions of authors
Dr. Cherrie Rose Y Tan‐Dy (CRYT‐D) and Dr. Arne Ohlsson (AO) contributed to all the stages of the protocol development. Both authors reviewed the search printout and identified the one eligible study. One author (AO) contacted one of the authors of the identified study and clarified some details. One author (AO) entered the data into Review Manager and the other author (CRYT‐D) checked the entered data for accuracy. One author (AO) wrote the sections of the full review not included in the protocol and both authors (AO & CRYT‐D) made revisions following editorial comments. Both authors read and approved the final submission.
CRYT‐D and AO wrote the original review. The June 2012 update was conducted by CRYT‐D and AO. Ms. Yolanda Montagne conducted the main literature searches in May 2012.
Sources of support
Internal sources
Department of Paediatrics, Mount Sinai Hospital, Toronto, Ontario, Canada.
External sources
-
Eunice Kennedy Shriver National Institute of Child Health and Human Development National Institutes of Health, Department of Health and Human Services, USA, under Contract No. HHSN275201100016C , USA.
Editorial support of the Cochrane Neonatal Review Group has been funded with Federal funds from the Eunice Kennedy Shriver National Institute of Child Health and Human Development National Institutes of Health, Department of Health and Human Services, USA, under Contract No. HHSN275201100016C
Declarations of interest
None known.
Deceased
Edited (no change to conclusions)
References
References to studies included in this review
Erasmus 2002 {published data only}
- Erasmus HD, Ludwig‐Auser HM, Paterson PG, Sun D, Sankaran K. Enhanced weight gain in preterm infants receiving lactase‐treated feeds: a randomized, double blind, controlled trial. Journal of Pediatrics 2002;141:532‐7. [DOI] [PubMed] [Google Scholar]
Additional references
AAP 1998
- American Academy of Pediatrics, Committee on Nutrition. Nutritional needs of preterm infants. Pediatric Nutrition Handbook. Elk Grove Village, IL: American Academy of Pediatrics, 1998. [Google Scholar]
Antonowicz 1974
- Antonowicz I, Chang SK, Grand RJ. Development and distribution of lysosomal enzymes and disaccharidases in human fetal intestine. Gastroenterology 1974;67:51‐8. [PubMed] [Google Scholar]
Auricchio 1965
- Auricchio S, Rubino A, Murset G. Intestinal glycosidase activities in the human embryo, fetus, and newborn. Pediatrics 1965;35:944‐54. [PubMed] [Google Scholar]
Berseth 1995
- Berseth CL. Minimal enteral feeds. Clinics in Perinatology 1995;22:195‐205. [PubMed] [Google Scholar]
Book 1975
- Book LS, Herbst JJ, Atherton SO, Jung AL. Necrotizing enterocolitis in low‐birth‐weight infants fed an elemental formula. Journal of Pediatrics 1975;87:602‐5. [DOI] [PubMed] [Google Scholar]
Carlson 1991
- Carlson SJ, Rogers RR, Lombard KA. Effect of a lactase preparation on lactose content and osmolality of preterm and term infant formulas. Journal of Parenteral and Enteral Nutrition 1991;15:564‐6. [DOI] [PubMed] [Google Scholar]
Ehrenkranz 1999
- Ehrenkranz RA, Younes N, Lemons JA, Fanaroff AA, Donovan EF, Wright LL, et al. Longitudinal growth of hospitalized very low birth weight infants. Pediatrics 1999;104:280‐9. [DOI] [PubMed] [Google Scholar]
Fenton 2002
- Fenton T, Belik J. Routine handling of milk fed to preterm infants can significantly increase osmolality. Journal of Pediatric Gastroenterology and Nutrition 2002;35:298‐302. [DOI] [PubMed] [Google Scholar]
Griffin 1999
- Griffin MP, Hansen JW. Can the elimination of lactose from formula improve feeding tolerance in premature infants?. Journal of Pediatrics 1999;135:587‐92. [DOI] [PubMed] [Google Scholar]
Hack 2003
- Hack M, Schluchter M, Cartar L, Rahman M, Cuttler L, Borawski E. Growth of very low birth weight infants to age 20 years. Pediatrics 2003;112:e30‐8. [DOI] [PubMed] [Google Scholar]
Hamosh 1996
- Hamosh M. Digestion in the newborn. In: Neu J editor(s). Neonatal Gastroenterology. Philadelphia, PA: WB Saunders, 1996:191‐210. [Google Scholar]
Higgins 2003
- Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analyses. BMJ 2003;327:557‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Hughes 1980
- Hughes CA, Dowling RH. Speed of onset of adaptive mucosal hypoplasia and hypofunction in the intestine of parenterally fed rats. Clinical Science 1980;59:317‐27. [DOI] [PubMed] [Google Scholar]
Kien 1996
- Kien CL. Digestion, absorption, and fermentation of carbohydrates in the newborn. Clinics in Perinatology 1996;23:211‐28. [PubMed] [Google Scholar]
Lee 1996
- Lee JK, Yu VY. Calorie intake in sick versus respiratory stable very low birthweight babies. Acta Paediatrica Japonica 1996;38:449‐54. [DOI] [PubMed] [Google Scholar]
Levine 1974
- Levine GN, Deren JJ, Steiger E, Zinno R. Role of oral intake in maintenance of gut mass and disaccharidase activity. Gastroenterology 1974;67:975‐82. [PubMed] [Google Scholar]
Malone 1999
- Malone A, Kearney PJ, Duggan PF. The effect of lactase and formula reconstitution on milk osmolality. International Journal of Food Sciences and Nutrition 1999;50:311‐7. [DOI] [PubMed] [Google Scholar]
McClure 2002
- McClure RJ, Newell SJ. Randomized controlled study of digestive enzyme activity following trophic feeding. Acta Paediatrica 2002;91:292‐6. [DOI] [PubMed] [Google Scholar]
NCCPS 1995
- Nutrition Committee, Canadian Paediatric Society. Nutrient needs and feeding of premature infants. Canadian Medical Association Journal 1995;152:1765‐85. [PMC free article] [PubMed] [Google Scholar]
Neu 1996
- Neu J, Koldovsky O. Nutrient absorption in the preterm neonate. Clinics in Perinatology 1996;23:229‐43. [PubMed] [Google Scholar]
PDR 2003
- Medical Economics Company (editor). Physician Desk Reference. Montvale, NJ: PDR network, 2003. [ISBN‐13: 978 1563634457] [Google Scholar]
Raul 1986
- Raul F, Lacroix B, Aprahamian M. Longitudinal distribution of brush border hydrolases and morphological maturation in the intestine of the preterm infant. Early Human Development 1986;13:225‐34. [DOI] [PubMed] [Google Scholar]
RevMan 2011 [Computer program]
- Nordic Cochrane Centre. The Cochrane Collaboration. Review Manager (RevMan). Version 5.1. Copenhagen: Nordic Cochrane Centre. The Cochrane Collaboration, 2011.
Schanler 1996
- Schanler RJ. The low‐birth‐weight infant. In: Walker WA, Watkins JB editor(s). Nutrition in pediatrics: Basic science and clinical application. 2nd Edition. Hamilton, Ontario, Canada: BC Decker Inc, 1996:392‐412. [Google Scholar]
Shulman 1998
- Shulman RJ, Schanler RJ, Lau C, Heitkemper M, Ou C‐N, O'Brian Smith E. Early feeding, feeding tolerance, and lactase activity in preterm infants. Journal of Pediatrics 1998;133:645‐9. [DOI] [PubMed] [Google Scholar]
Sinden 1991
- Sinden AA, Sutphen JL. Dietary treatment of lactose intolerance in infants and children. Journal of the American Dietetic Association 1991;91:1567‐71. [PubMed] [Google Scholar]
Steward 2002
- Steward DK, Pridham KF. Growth patterns of extremely low‐birth‐weight hospitalized preterm infants. Journal of Obstetric, Gynecological and Neonatal Nursing 2002;31:57‐65. [DOI] [PubMed] [Google Scholar]
Walia 2000
- Walia R, Ohlsson A. All is gold, is it? Differences between abstracts of randomised controlled trials in neonates submitted to a conference and their final publication ‐ implications for meta‐analysis. Archives of Disease in Childhood 2000;82(Suppl 1):A3. [Google Scholar]
White 1979
- White WT 3rd, Acuff T, Sykes TR, Dobbie RP. Bacterial contamination of enteral nutrient solution: a preliminary report. Journal of Parenteral and Enteral Nutrition 1979;3:459‐61. [DOI] [PubMed] [Google Scholar]
Willis 1977
- Willis DM, Chabot J, Radde IC, Chance GW. Unsuspected hyperosmolality of oral solutions contributing to necrotizing enterocolitis in very‐low‐birth‐weight infants. Pediatrics 1977;60:535‐8. [PubMed] [Google Scholar]
Wright 1993
- Wright K, Dawson JP, Fallis D, Vogt E, Lorch V. New postnatal growth grids for very low birth weight infants. Pediatrics 1993;91:922‐6. [PubMed] [Google Scholar]
References to other published versions of this review
Tan‐Dy 2005
- Tan‐Dy CRY, Ohlsson A. Lactase treated feeds to promote growth and feeding tolerance in preterm infants. Cochrane Database of Systematic Reviews 2005, Issue 2. [DOI: 10.1002/14651858.CD004591.pub2] [DOI] [PubMed] [Google Scholar]


