Abstract
Background
Inhaled fluticasone propionate (FP) is a high‐potency inhaled corticosteroid used in the treatment of asthma.
Objectives
1. To assess the efficacy and safety outcomes of inhaled fluticasone at different nominal daily doses in the treatment of chronic asthma. 2. To test for the presence of a dose‐response effect.
Search methods
We searched the Cochrane Airways Group Trials Register (January 2008).
Selection criteria
Randomised trials in children and adults comparing fluticasone at different nominal daily doses in the treatment of chronic asthma. Two reviewers independently assessed articles for inclusion and methodological quality.
Data collection and analysis
One review author extracted data. These were checked and verified by a second reviewer. Quantitative analyses where undertaken using Review Manager.
Main results
Fifty‐one published and unpublished trials (representing 55 group comparisons, 10,797 participants) met the inclusion criteria. In asthmatics with mild to moderate disease who were not on oral steroids, FP did not exhibit a dose‐response effect in the lower dose comparisons in FEV1 (50mcg, 100mcg, 200mcg and 4‐500mcg daily). There were no statistically significant differences between 4‐500mcg and 800‐1000mcg, and between 50‐100 and 800‐1000mcg of FP. When 200mcg was compared with 800‐1000mcg daily FEV1 favoured the four/five fold increase. For PEF, a dose response was present with FP when low and moderate, and low and high doses of FP were compared. There was no evidence of a dose‐response effect on symptoms or rescue beta‐2 agonist use. The likelihood of hoarseness and oral candidiasis was significantly greater for the higher doses (800 to 1000 µg/day). People with oral steroid‐dependent asthma treated with FP (2000 µg/day) were significantly more likely to reduce oral prednisolone than those on 1000 to 1500 µg/day (Peto odds Ratio 2.8, 95% CI 1.3 to 6.3). The highest dose also allowed a significant reduction in daily oral prednisolone dose compared to 1000 to 1500 µg/day (WMD 2.0 mg/day, 95% CI 0.1 to 4.0 mg/day).
Authors' conclusions
We have not found evidence of a pronounced dose response in FEV1 with increasing doses of fluticasone. The number of studies contributing to our primary outcomes was low. At dose ratios of 1:2, there are statistically significant differences in favour of the higher dose in morning peak flow across the low dose range. The clinical impact of these differences is open to interpretation. Patients with moderate disease achieve similar levels of asthma control on medium doses of fluticasone (400 to 500 µg/day) as they do on high doses (800 to 1000 µg/day). More work in severe asthma would help to confirm that doses of FP above 500 µg/day confer greater benefit in this subgroup than doses of around 200 µg/day. In oral corticosteroid‐dependent asthmatics, reductions in prednisolone requirement may be gained with FP 2000 µg/day.
Plain language summary
Fluticasone at different doses for chronic asthma in adults and children
Fluticasone (FP) is an inhaled corticosteroid commonly used to treat inflammation of the airways (passages to the lungs) and improve breathing in people with asthma. This review examined the effectiveness of FP when given at different doses for treating asthma in children and adults. High doses (800 to 1000 microgram per day) led to small improvements in measures of airway opening compared to low doses (50 to 100 microgram per day) in people with mild to moderate asthma. High dose FP did not lead to clear improvements in symptoms over the lower dose and increased the risk of a hoarse voice and fungal mouth infections. In people with severe asthma, very high doses FP (2000 microgram per day) appeared to allow more people on oral steroids to stop or reduce their dose of oral steroid tablets compared to lower doses of FP (1000 to 1500 microgram per day).
Background
Fluticasone propionate (FP) is an anti‐inflammatory inhaled corticosteroid (ICS) used for the treatment of childhood and adult asthma. It is licensed for use over a range of nominal daily doses and is widely used in the UK, Europe, Northern America and other areas of the world. Current asthma management guidelines produced by leading respiratory societies and organisations recommend a dose titration approach to the use of all ICSs, including FP (BTS 1997; GINA 1995; NHLBI 1997). For patients with persistent evidence of sub‐optimal control as judged by frequency of symptoms, rescue bronchodilator requirement and measures of airway calibre (forced expiratory volume in one minute (FEV1), peak expiratory flow (PEF)) consideration should be given to increasing the daily dose of FP in the hope of achieving improved control. This recommendation is borne out of assuming a dose‐response effect, in other words, that larger doses lead to improved measures of control. The best way of determining whether such an approach has a sound foundation is to undertake a trial in which patients are randomised to different doses of FP. The purpose of this review was, therefore, to evaluate all the available evidence from randomised trials that have compared FP at different nominal daily doses in order to assess whether a clinically relevant dose‐response effect is present.
Objectives
1. To assess the efficacy and safety outcomes in studies that compared inhaled FP at different nominal daily doses for the treatment of chronic asthma in adults and children.
2. To test for the presence of a dose‐response effect.
Methods
Criteria for considering studies for this review
Types of studies
Only prospective, randomised studies were considered. Double, single and unblinded studies were eligible for inclusion. Both parallel‐group design and crossover studies were considered.
Types of participants
Studies including children and/or adults with a clinical diagnosis of chronic asthma were reviewed. Participants needed to be at least two years of age or older and have a diagnosis of chronic asthma. Diagnosis based on the physician opinion alone was acceptable, as well as asthma diagnosed using objective criteria related to asthma symptoms, airway reversibility, and all bronchial hyper‐responsiveness. Treatment in the setting of primary care, hospital outpatients clinics, or institutional care was considered.
Types of interventions
Inhaled fluticasone at one nominal daily dose versus fluticasone (FP) at a second nominal daily dose. Treatment periods had to be for at least one week. Delivery of FP by a metered dose inhaler (MDI) or an MDI with a chamber or dry powder inhaler (DPI) was acceptable. Studies using nebulisers were specifically excluded. Patients receiving any two interventions were acceptable, including the use of oral corticosteroids (OCS).
Types of outcome measures
Primary outcomes
The primary outcome for this review is FEV1. We have subgrouped only on this outcome, and done so for the measurement of FEV1 with the highest number of effect estimates available (mostly change from baseline in Litres).
Secondary outcomes
Measurements of lung function other than FEV1 (i.e. PEFR, FVC)
Symptoms
Rescue medication use
Health status/health related quality of life (HRQOL);
Exacerbations (primary care physician visits, emergency room visits, hospital admission and days loss from work or school)
Adverse events
Growth and measurements of bone turnover are considered in other Cochrane reviews and we do not summarise evidence for these effects of therapy here.
Search methods for identification of studies
Electronic searches
Trials were identified using the Cochrane Airways Group Specialised Register of trials, which is derived from systematic searches of bibliographic databases including the Cochrane Central Register of Controlled Trials (CENTRAL), MEDLINE, EMBASE, CINAHL, AMED and PsycINFO, and handsearching of respiratory journals and meeting abstracts. All records in the Specialised Register coded as 'asthma' were searched using the following terms:
steroid* OR glucocorticoid* OR corticosteroid* OR fluticasone OR Flixotide OR Flovent
Searches are current to January 2008
Searching other resources
Reference lists of all included studies and relevant narrative reviews were searched for additional RCTs.
The UK headquarters of GSK (manufacturers of Becotide, Becloforte and Flixotide) and the Swedish headquarters of Astra Zeneca (manufacturers of Pulmicort) were asked if they were aware of further missed trials.
We handsearched the GSK clinical trials register (http://ctr.gsk.co.uk) for unpublished data
Authors of studies were asked if they were aware of further missed trials.
The British Journal of Clinical Research and the European Journal of Clinical Research (journals not electronically indexed on MEDLINE or EMBASE) were hand‐searched.
Proceedings of the British Thoracic Society (1997 to 2003), European Respiratory Society (1997 to 2003) and the American Thoracic Society (1997 to 2003) were searched for relevant trials.
Data collection and analysis
Selection of studies
The decision to exclude studies prior to full paper retrieval was made independently by two reviewers (NPA and JB). In cases of disagreement, the full text article was retrieved. Papers retrieved in full text were assessed by two reviewers independently (NPA and JB), any disagreement regarding eligibility was resolved by consensus. Two reviewers (NPA and JB) who were blinded to the author's names, institution and funding sources, independently assessed included studies for methodological quality. Data that were not available for inclusion in the meta‐analysis has been listed in Table 18.
2. Data not included in the meta‐analysis.
| Study ID | Data |
| Allen 2000 | Steroid consumption Side effects Unclear reporting (no response from trialists) |
| Ayres 1995 | Symptom free days and nights Rescue beta2 agonist free days and nights Daytime and night‐time symptom scores Above outcomes analysed by investigators using non‐parametric statistics Change in FEV1 compared to baseline Change in FVC compared to baseline Change in morning PEFR compared to baseline Change in evening PEFR compared to baseline Change in diurnal variability in PEFR compared to baseline Change in clinic PEFR compared to baseline No SD values available for above outcomes Morning plasma cortisol Data log transformed and reported using geometric means by investigators: log transformed values not available |
| Boner 1999 | Methacholine BHR (PC20 FEV1) Log transformed data not available FEV1 No SD values available Overnight urinary cortisol No numerical data available |
| Bukovskis 2002 | FEV1 change from baseline Unclear reporting (no response from trialists) |
| Chervinsky 1994 | Change in urinary free cortisol compared to baseline Change in urinary 17‐hydroxy steroids compared to baseline Change in morning plasma cortisol compared to baseline Change in plasma cortisol 60 min post co‐syntropin No SD values available for above outcomes |
| Chetta 2002 | FEV1 Unclear reporting (no response from trialists) |
| Dahl 1993 | Morning plasma cortisol Plasma cortisol 30 min post 250 mcg ACTH Diurnal variation in PEFR FVC Daily beta2 agonist use (puffs/day) No SD values available for above outcomes |
| Derom 1999 | Cortisol suppression Unclear reporting (no response from trialists) |
| Derom 2001 | Cortisol suppression PC20 Unclear reporting (no response from trialists) |
| Gershman 2000 | PC20 ECP Data reported as medians |
| Hofstra 2000 | PD20 No SDs presented. |
| Ind 2003 | Medication usage Symptoms Data presented as medians |
| Katz 1998 | Change in FEV1 compared to baseline Change in FVC compared to baseline Change in FEF25‐75 compared to baseline Change in evening PEFR compared to baseline Change in night‐time awakening score compared to baseline No SD values available for above outcomes |
| Meijer 1999 | FEV1 PEF Symptoms Medication usage Cortisol Data presented as medians |
| Nieto 2001 | PC20 Unclear reporting (no response from trialists) |
| Noonan 1998 | Change in log e methacholine bronchial responsiveness PD20 FEV1 Error bars plotted on graphical display of results, but unclear whether these represent SD or SEM values |
| Pauwels 2002 | Cortisol suppression PC20 Unclear reporting (no response from trialists) |
| Pearlman 1997 | Change in evening PEFR compared to baseline Medical Outcomes Study Short Form (SF‐36A) Living with asthma questionnaire No SD values available for above outcomes Morning serum cortisol No numerical data available for above outcome Physician rated global assessment of efficacy Data not presented in a form suitable for meta‐analysis |
| SAM40012 | % symptom free days rescue medication usage Data reported as medians. |
| Verona 2003 | Medication usage Symptoms Data reported as medians |
| Wallin 2003 | am PEF/pm PEF Data reported as medians |
| Wasserman 1996 | Physician‐rated global assessment of effectiveness Data not presented in a form suitable for meta‐analysis |
| Wolfe 1996 | Change FEV1 compared to baseline No SD values available for above outcome Change in morning PEFR compared to baseline Change in evening PEFR compared to baseline Daily wheeze, cough, shortness of breath scores Daily beta2 agonist use Morning plasma cortisol No numerical data available for above outcomes |
Data extraction and management
Two authors (NPA and TL) extracted data for each outcome from the published results of included trials. In the case of continuous outcomes (such as FEV1), only data from the last evaluable time point was used. Where data had to be extracted from graphical plots, an attempt was made to verify the data by contacting authors.
Assessment of risk of bias in included studies
We assessed the risk of bias for each included study according to recommendations described in the Cochrane Handbook. We have assessed the risk of bias for the generation and concealment of allocation schedules for the eligible studies, and blinding of treatment preparations. We have judged the degree of bias for each domain to be of high risk (No), low risk (Yes) or unclear risk (Unclear). Our previous approach is described in Appendix 1.
Dealing with missing data
Authors were written to (by mail, fax and/or electronic mail) in an attempt to clarify details of methods, and to request missing or incomplete outcome data. Attempts were made to send requests to correct current addresses by searching MEDLINE, EMBASE and hospital web sites for up to date contact details. Glaxo Wellcome (UK) was also approached for data concerning trials in which contact authors did not initially reply, or when authors suggested doing so. Data that were not available for inclusion in the meta‐analysis have been listed in Table 18
We have imputed a number of missing standard deviations for a number of studies. Our methods for doing so are described in Table 19. We have maintained an approach consistent with the recommendations regarding imputation in the Cochrane Handbook, whereby these studies represent a small proportion of the studies included in a given outcome. We report the findings of outcomes that contain imputed estimates, but outcomes with unimputed data are also available.
3. Methods of imputations and estimates.
| Outcome | WMD/GIV | Study | Method |
| 07:03 | WMD | Pinnas 2005 | Published means. SDs based on other studies. |
| 07:07 | GIV | Pinnas 2005 | Published means. SDs based on other studies. |
| 07:07 | GIV | Wolfe 1996 | Published P values (versus placebo), assumed same SEM between two FP groups. |
| 20:01 | WMD | Pinnas 2005 | Published means. SDs based on other studies. |
| 20:03 | WMD | Ind 2003; Pinnas 2005 | Published means. SDs based on other studies. |
Assessment of heterogeneity
We assessed heterogeneity with I square measurement. Sensitivity analysis with random effects modelling was used where this value exceeded 20%.
Data synthesis
A weighted treatment effect across trials was calculated using the Cochrane statistical package RevMan 5. For continuous outcomes, a weighted mean difference (WMD) or standardised mean difference (SMD) was calculated, as appropriate. For binary or dichotomous outcomes odds ratios (OR) were calculated. Pooled treatment effects were expressed with their 95% confidence intervals (95% CI).
A number of conditions were established a priori regarding the comparisons to be made, as follows:
Adult and paediatric lung function data reported as litres (i.e. FEV1 and PEF) were not combined due to the different lung volumes in these populations. Where data were presented on a % predicted scale which takes account of age, we combined paediatric and adult data.
Parallel and crossover trials were not pooled together.
Studies were categorised based on the presence or absence of regular oral corticosteroid (OCS) use at participant enrolment. It was expected that most trials with patients on regular oral steroids would use a steroid‐sparing design in which daily dose of OCS was progressively reduced. In such studies the principal outcome variable is the dose of oral steroid needed to maintain asthma control. Conversely, studies in which patients were not treated with regular OCS would be more likely to have designs aimed at detecting improvements in asthma control. It would be inappropriate to combine trials with these different designs and aims
Subgroup analysis and investigation of heterogeneity
For each reported outcome, subgroup analyses have been undertaken. These are based on patient age (children or adults); treatment duration (one to four weeks, one to five months, six months or longer); delivery device (MDI or DPI); asthma severity (mild, mild‐to‐moderate, moderate). These analyses have been used to explore variations in treatment response according to these factors. In particular, for outcomes where heterogeneity exists between studies, subgroup analyses have been used to try to identify factors that may account for heterogeneity. These are discussed as appropriate in the following section.
Results
Description of studies
Results of the search
For details of the search history see Table 20. From searches conducted in January 2008, six new studies met the inclusion criteria FLIP01; FLIP01a; FLIP39; FLTA3014; FLTA3022/FLTA3022a; Pinnas 2005. Additional unpublished data were identified from the GSK online repository of trial data for the following studies: Agertoft 1997; Allen 1998; Boner 1999; Chervinsky 1994; Dahl 1993; Kemp 2004; Katz 1998; Nathan 2000; Nelson 1999; Pearlman 1999; Lumry 2006; Peden 1998; Verona 2003.
4. Search History Detail.
| Date | N included/excluded |
| All Years searching to March 1999 | Initial version of the review (All Years searching to March 1999): 6494 citations retrieved, 2162 unique citations imported to Inhaled Steroid Register. From this a fluticasone register was created consisting of 258 citations. 180 excluded on basis of abstract: 150 not RCT; 30 not chronic asthma in humans; 78 papers retrieved in full text form; 57 excluded on basis of full paper: 6 not RCT; 1 infants; 3 delivery device comparison; 1 treatment period < 1 week; 46 not a comparison of 2 or more doses of FP; 21 publications meeting inclusion criteria; 16 unique studies meeting inclusion criteria One study (Raphael 1999) was identified by Glaxo Wellcome. This study was published after the date of the final electronic search (March 1999). Three studies (Boner 1999, Hofstra 2000, Ind 2003) were identified as a result of searching respiratory society meeting abstracts. |
| Update (March 1999‐January 2005) | From hand searching the updated inhaled steroids search results (additional 1301 references), a 'fluticasone' register was created consisting of 196 citations (121 references excluded from abstracts as irrelevant comparisons). Forty‐six references pertaining to 34 studies were retrieved in full for this review. One study reported findings from two data‐sets and these studies have been given two identifiers (Sorkness 1999; Sorkness 1999a). We excluded 11 studies for the following reasons: Wrong comparator (9), outcomes not relevant (2) and varying dose of FP (1). 24 new studies met the inclusion criteria for the review (Allen 2000; Bukovskis 2002; Casale 2001; Chetta 2002; Derom 1999; Derom 2001; Falcoz 2000; Gershman 2000; Giannini 2003; Kemp 2004; Li 1999; Meijer 1999; Nathan 2000; Nielsen 2002; Nieto 2001; O'Sullivan 2002; Pauwels 2002; Pearlman 1999; Pearlman 2002; SAM40012; Sorkness 1999; Sorkness 1999a; Verona 2003; Wallin 2003). Data for two studies previously included as abstracts were published in full text form (Hofstra 2000; Ind 2003). One study was identified from an online repository of unpublished clinical trials (SAM40012). |
| January 2005‐January 2006 | References identified: 411 Number assessed for further scrutiny: 55 |
Included studies
A total of 55 randomised group comparisons (derived from 51 studies, represented by 89 published and unpublished references) are now included in the review.
Populations
The majority of studies were multicentre trials that recruited patients from the USA, Europe and Canada. One study (Katz 1998) also included patients from Asia. Two single centre studies were conducted in Denmark (Agertoft 1997) and The Netherlands (Hofstra 2000). Only one study (Raphael 1999) recruited patients from primary as well as a secondary care/hospital outpatient clinic setting. All other studies were conducted in secondary care. The majority of studies assessed adults, with only seven studies recruiting children.
Study Design
Two studies (Agertoft 1997; Derom 1999) were of crossover design, all others were parallel group studies. The parallel studies were of varying length. The majority were of six to 12 weeks duration. One study (Dahl 1993) lasted four weeks, two studies (Nelson 1999, Noonan 1995) were of 16 weeks, and three studies (Ind 2003; SAM40012; Verona 2003) were of six months duration. Two studies (Allen 1998; Verona 2003) lasted a year.
Interventions
A range of daily doses of FP were compared. These included less than 2‐fold dose comparisons (e.g. 1000 versus 1500 mcg/d), two‐fold comparisons, (e.g. 50 versus 100, 200 versus 400, 1000 versus 2000 mcg/d), four‐fold dose comparisons (e.g. 200 versus 800 mcg/d) and greater than five‐fold dose comparisons (e.g. 50 versus 1000 mcg/d). Ten studies (Casale 2001; Chervinsky 1994, Dahl 1993, Nathan 2000; O'Sullivan 2002; Pearlman 1997, Lumry 2006; Sheffer 1996, Wasserman 1996, Wolfe 1996) assessed three or more doses as randomised treatment arms within the same trial. One study (Peden 1998) compared two doses of FP (100 versus 200 mcg/d) administered using two different delivery devices (Diskhaler DPI and Diskus/Accuhaler DPI). Patients were randomised to receive either FP 100 or 200 mcg/d delivered via either delivery device. A number of studies also included treatment arms with either a placebo or other inhaled corticosteroid. These interventions have not been assessed in this review. Details of these interventions are provided in the notes section of the included studies table.
Delivery device
Eighteen studies used a dry powder inhaler (either Diskhaler or Diskus/Accuhaler) and 19 used a metered dose inhaler. In one study (Ayres 1995), patients were given the option of using an MDI, with or without an additional spacer/chamber device provided that this was consistently throughout the trial. In six studies (Boner 1999; Bukovskis 2002; Derom 2005; Giannini 2003; Nieto 2001; Pauwels 2002) the delivery device used was not stated.
Prior treatment with oral corticosteroids (OCS)
Three studies (FLTA3022/FLTA3022a; Noonan 1995; Nelson 1999) recruited oral steroid dependent asthmatics. Use of oral prednisolone was an inclusion criterion for all studies.
Prior treatment with inhaled corticosteroids (ICS)
In 48 studies, patients were not receiving oral steroids at enrolment. In 16 of these (Allen 1998; Ayres 1995; Chervinsky 1994; Dahl 1993; Ind 2003; Lawrence 1997; Meijer 1999; Nathan 2000; Pearlman 1997; Lumry 2006; Peden 1998, Raphael 1999; SAM40012; Verona 2003; Wallin 2003; Wolfe 1996) patients were receiving a regular ICS, however in all cases this was discontinued at the point of randomisation. In six studies, recent use of ICS was a specific exclusion criterion (Galant 1996; Hofstra 2000; Katz 1998; Kemp 2004; Noonan 1998; Sheffer 1996; Wasserman 1996). In two studies (Agertoft 1997; Boner 1999) it was unclear if any patients were receiving an ICS at the time of enrolment.
Asthma severity
Patients with a range of asthma severity were studied. Table 21 provides a breakdown of included studies according to baseline FEV1 (% predicted), symptom frequency reported at baseline and the stated opinion of investigators regarding asthma severity. An overall approximation of severity based on these features is given, related to the current GINA 1995/NHLBI 1997 classification. In summary, six studies recruited patients with mild asthma, 11 studies recruited patients with mild to moderate asthma and 17 studies assessed moderately severe asthmatics. Three studies (Ayres 1995; Ind 2003; Verona 2003) assessed patients with moderate to severe asthma, one study (Raphael 1999) included patients with a spectrum of disease from mild to severe whilst three studies (Allen 2000; Nelson 1999, Noonan 1995) assessed severe, oral steroid dependent asthmatics. In the case of 10 studies not enough details were available to make an estimation.
5. Asthma severity: characteristics of included patients at baseline.
| Study ID | FEV1: incl. criteria | Basline FEV1 | Symptom frequency | OCS treatment | ICS treatment | Author opinion | Overall estimation |
| Agertoft 1997 | Not stated | Not stated | No | No | Not stated | Mild | Mild |
| Allen 1998 | >60 | 88‐89% | Not stated | No | Approx. 50% patients ICS naive at baseline, 50% previous regular ICS use | Mild to moderate | Mild to moderate |
| Allen 2000 | Not stated | 61% | Not stated | Yes (non‐OCS dependents excluded) | Not stated | Severe | Severe |
| Ayres 1995 | Not stated | Mean baseline morning PEFR 73‐77 (% predicted) | Need for 2 or more doses beta2 agonist on 2 out of 7 days of run in period | Proportion of patients using OCS (<10 mg/d) | Yes: BDP 1‐2 mg/d or BUD 0.8‐1.6 mg/d | moderate to severe | Moderate to severe |
| Boner 1999 | Not stated | Not stated | Not stated | No | Not stated | Not stated | Unclear |
| Bukovkis 2002 | Not stated | Not stated | Not stated | Not stated | Not stated | Not stated | Unclear |
| Casale 2001 | >/=65% | 3‐3.2 L | Not stated | No | Not stated | Mild to moderate | Mild to moderate |
| Chervinsky 1994 | 60‐90 | 71‐73% | Not stated | No | Yes: at least 1 month regular treatment with BDP prior to study | Mild to moderate | Mild to moderate |
| Chetta 2002 | >70% | 100‐110% | Well documented history of asthma | No | Not stated | Mild to moderate | Mild |
| Dahl 1993 | Not stated | 73‐75% | daytime wheezing or night‐time symptoms on at least 4 days of 7 day run‐in period or PEFR variability 20% or greater | No | Yes: BDP 1000 mcg/d or less | Moderate | Moderate |
| Derom 1999 | >/=40% | 80% | Not stated | No | Not within 6 months | Not stated | Mild |
| Derom 2001 | Not stated | Not stated | Not stated | Not stated | Not stated | Unclear | Unclear |
| Falcoz 2000 | 50‐80% | Not stated | Not stated | Not stated | Not stated | Mild‐to‐moderate | Unclear |
| FAP30001 | >/=45% | 74‐5% | Not stated | No | Yes | Not stated | Moderate |
| FLIC15 | >/=60% predicted | Not stated | Not stated | No | No | Mild to moderate | Mild |
| FLIP01/a | Not reported | Not reported | Not reported | No | Yes | Not reported | Moderate |
| FLIP39 | Not reported | Not reported | Perennial symptoms requiring ICS | No | Yes ‐ up to 400mcg/d | Not stated | Moderate |
| FLTA3014 | 50‐85% | Not reported | Not reported | No | Yes | Not stated | Unclear |
| FLTA3020/a | 60‐90% | Not reported | Not reported | No | No (low dose ceased 30 days prior to study entry) | Not stated | Mild to moderate |
| FLTA3022/a | 40‐85% predicted | Not reported | Not stated | Yes | Yes | Not stated | Severe |
| FLTA4030 | 50‐80% | Not stated | Stable during 7 day run‐in, controlled with SABA alone | No | No | Not stated | Mild to moderate |
| Galant 1996 | 45‐75 | 60‐62% | > 8 puffs/d beta2 agonist or 2‐4 night‐time awakenings in week run‐in | No | No | Mild to moderate | Moderate |
| Gershman 2000 | Not stated | 66‐69% | Not stated | Not stated | Not stated | Not stated | Unclear |
| Giannini 2003 | Not stated | 3.23 L | Requirement for beta‐agonist treatment during run‐in | Not stated | Not stated | Moderate | Mild to moderate |
| Hofstra 2000 | Not stated | 96.6‐93.2% | Not stated | No | No | Not stated | Unclear |
| Ind 2003 | FEV1 not stipulated at inclusion | No details | Symptomatic despite ICS treatment. History of exacerbations | No | Yes: 1000‐1600 mcg/d of BDP or BUD | Moderate‐severe | Moderate‐severe |
| Katz 1998 | Not stated | PEFR 75 (% predicted) or less | Asthma symptoms on at least 4 out of 10 days of run in period or at least one night‐time awakening in 10 days or 4 or puffs beta2 agonist on at least 4 days | No | No | Not stated | Moderate |
| Kemp 2004 | 50‐100% predicted | 82‐85% predicted | Mild stable asthma | No | No | Mild | Mild |
| Lawrence 1997 | 50‐80 | 65‐68% | "Mean beta2 agonist use 3.2 ‐ 4.2 puffs/d " | No | Yes: 3 months treatment or longer prior to study | Not stated | Moderate |
| Li 1999 | FEV1 </=50% predicted | 82.5‐88.2% | Not stated | No | No | Not stated | Mild |
| Lumry 2006 | 45‐80% | 65.3‐65.5 | Not stated | No | Yes | Not stated | Moderate |
| Meijer 1999 | Not stipulated | 79‐81% | Participants who exacerbated needing OCS during run‐in were excluded | No | Yes ‐ treatment tapered prior to randomisation | Mild‐moderate | Mild to moderate |
| Nathan 2000 | 45‐75% predicted | 63.3‐64.3 | Not stated | No | Yes | Moderate | Moderate |
| Nelson 1999 | 40‐80 | 60‐62% | Not stated | Yes | Almost 100% of patients receiving ICS | Severe | Severe |
| Nieto 2001 | Not stated | Not stated | Not stated | Not stated | Not stated | Not stated | Unclear |
| Noonan 1998 | 60‐85 | 73‐76% | "No more than 12 puffs/d beta2 agonist and no more than 3 nights with awakening due to asthma" | No | No | Mild to moderate | Moderate |
| Noonan 1995 | 40‐80 | 56‐57.4% | Requirement for rescue beta2 agonist for 2 weeks prior to study due to symptoms | Yes | 87% of patients receiving ICS | Severe | Severe |
| O'Sullivan 2002 | >/=60% | 79‐86% | Not stated | No | No | Mild‐moderate | Mild to moderate |
| Pauwels 2002 | Not stated | Not stated | Not stated | Not stated | Not stated | Not stated | Unclear |
| Pearlman 1997 | 50‐80 | 66‐67% | "Mean beta2 agonist use 3.4‐4.1 puffs/dNo more than 12 puffs/d beta2 agonist and no more than 2 nights with awakening due to asthma symptoms in last 7 days" | No | Yes: at least 3 months | Moderate | Moderate |
| Pearlman 1999 | 50‐80% | 65‐69% | Not stated | No | No | Mild‐moderate | Mild to moderate |
| Peden 1998 | 50‐85 | 72‐73% | "No more than 12 puffs/d beta2 agonist and no more than 3 nights with awakening due to asthmaMean awakenings per night due to asthma 0.05 to 0.09Mean beta2 agonist use 1.4 to 2.0 puffs/d" | No | Some patients: amount and type of ICS not stated | Not stated | Moderate |
| Pinnas 2005 | 45‐80 | 67% | 'during the week before randomization, patients could not have had more than 3 days in which more than 12 inhalations of albuterol were used, more than 3 nights with awakenings due to asthma requiring albuterol, or asthma exacerbations requiring systemic corticosteroids and/or hospitalization.' | No | No | Moderate to severe | Moderate |
| Raphael 1999 | 45‐65 | 64.7‐65.7% | > 8 puffs/week beta 2 agonist or diurnal variability in PEFR > 20% during run‐in if FEV > 65‐80 (% predicted) | No | Yes: BDP or TA 8‐12 puffs/d | mild/moderate and severe | mild/moderate and severe |
| SAM40012 | Not stated | Not stated | Symptom score greater than 2 on at least 3 of previous 7 days | Not stated | Yes | Not stated | Moderate |
| Sheffer 1996 | 45‐75 | 62‐64% | "During 7 day run‐in:> 2 night‐time awakenings due to asthma in last 7 days20% or greater PEFR diurnal variability at least one day in which 8 puffs beta2 agonist used " | No | No | Mild to moderate | Moderate |
| Sorkness 1999a | >/=50% | 86‐88% | Not stated | No | No | Mild to moderate | Mild to moderate |
| Sorkness 1999 | >/=50% | 83‐88% | Not stated | No | No | Mild to moderate | Mild to moderate |
| Verona 2003 | Not stated | Not stated | Exacerbation in last year requiring hospitalisation | No | Yes | Moderate to severe | Moderate to severe |
| Wallin 2003 | Not stated | 91‐2% | Symptomatoic during run‐in period despite medication | Not stated | Yes | Mild to moderate | Mild to moderate |
| Wasserman 1996 | 50‐80% | Not stated | "Mean beta2 agonist use 3.1 to 3.3 puffs/dDuring last 7 days run‐in no more than 12 puffs/d beta2 agonist and no more than 2 nights with awakening due to asthma" | No | No | Not stated | Moderate |
| Wolfe 1996 | 50‐80% | 64‐66% | During 2 week run‐in period no more than 12 puffs/d beta2 agonist and no more than 2 nights with awakening due to asthma | No | Yes: dose not stated | Moderate | Moderate |
Outcomes
A range of efficacy and safety outcomes was assessed. Those that have not been considered include growth assessment (Agertoft 1997, Allen 1998) and biochemical markers of bone turnover (Ayres 1995). All other outcomes were considered. A significant amount of data could not be included in the meta‐analysis. This is listed in Table 18. This was requested from the authors who either did not respond or were unwilling/unable to provide it.
Excluded studies
Risk of bias in included studies
An overview of our judgements (high, low or unclear risk of bias) for each of three domains relating to allocation (generation and concealment), and blinding is given in Figure 1.
1.
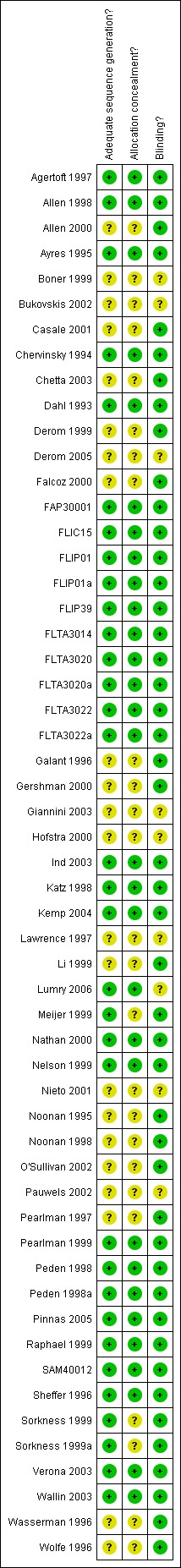
Methodological quality summary: review authors' judgements about each methodological quality item for each included study.
The procedures for allocating participants randomly were satisfactory, although we could not ascertain the risk of bias for this aspect of study design in 23 of 55 studies. Similarly in 25 studies we could not determine how well concealed the allocation procedures were.
Adequate masking of treatment group assignment with blinding was a common feature of the studies, as might be anticipated for trials of different doses: 46 out of 55 studies assessed different doses of FP through identical inhaler devices.
Effects of interventions
The results were grouped by the dose comparisons used. We report data for outcomes with the lower dose of FP taken to be the active treatment group, and the higher dose of FP the control group.
Efficacy measures: lower dose range comparisons
FP 50 versus 100 µg/day
A single study (Sheffer 1996) reported the results of a comparison of FP 50 versus 100 µg/day. This study assessed the effects of treatment over a 12‐week period in adults with moderately severe asthma. A number of outcomes were reported including FEV1, morning PEF, asthma symptoms, night‐time awakenings, rescue beta‐2 agonist use and the number of patients withdrawn due to lack of efficacy. No significant differences between the two doses were apparent for any outcome.
FP 50 versus 200 µg/day
Two studies included a comparison of FP 50 versus 200 µg/day. One study (Chervinsky 1994) included adults with mild to moderate asthma who were treated for eight weeks. Sheffer 1996 recruited adults with moderate asthma who were treated over a 12‐week period. A number of common outcomes were reported, all were expressed as a change compared to baseline. No significant differences between doses were apparent for FEV1, morning PEF, evening PEF, daily use of rescue beta‐2 agonist or trial withdrawal due to lack of efficacy. However, there were significantly greater reductions in asthma symptom score (SMD 0.32, 95% CI 0.10 to 0.54) and number of night‐time awakenings per week (WMD 0.09, 95% CI 0.02 to 0.16) with the higher dose. No heterogeneity was apparent when studies were pooled.
FP 100 versus 200 µg/day
Primary outcome
There was no statistically significant change between the lower and higher doses of FP in FEV1 in paediatric (‐0.04 Litres, 95% CI ‐0.09 to 0.01, three studies: Analysis 3.1), or adult (0.03 Litres, 95% CI ‐0.02 to 0.07, six studies: Analysis 3.1) studies. The level of I square measurements were 0 for both of these outcomes.
3.1. Analysis.
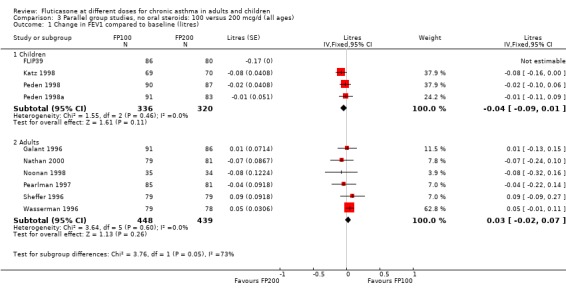
Comparison 3 Parallel group studies, no oral steroids: 100 versus 200 mcg/d (all ages), Outcome 1 Change in FEV1 compared to baseline (litres).
The remaining outcomes are reported below:
Secondary outcomes
Children
The higher doses were more effective than lower doses for peak flow measurements (change in am PEF from baseline: ‐3.67 Litres/min, 95% CI ‐9.81 to 2.46, four studies: Analysis 3.8; change in pm PEF from baseline: ‐4.50 Litres/min, 95% CI ‐11.77 to 2.77, two studies: Analysis 3.9)
3.8. Analysis.
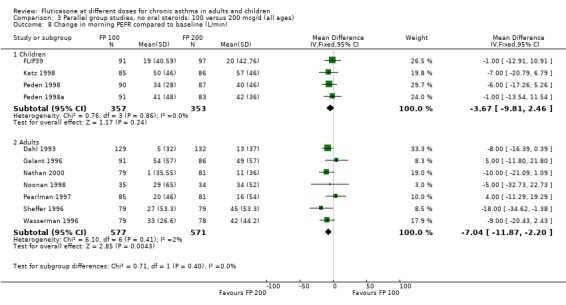
Comparison 3 Parallel group studies, no oral steroids: 100 versus 200 mcg/d (all ages), Outcome 8 Change in morning PEFR compared to baseline (L/min).
3.9. Analysis.
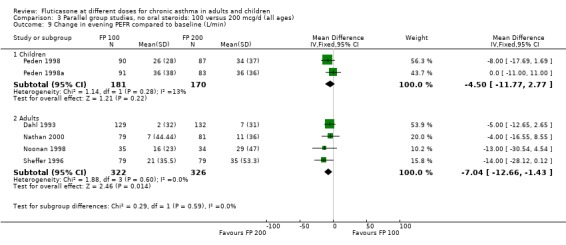
Comparison 3 Parallel group studies, no oral steroids: 100 versus 200 mcg/d (all ages), Outcome 9 Change in evening PEFR compared to baseline (L/min).
Adults
For adult studies, the higher doses were more effective than lower doses in improving peak flow (change in am PEF from baseline: ‐7.04 Litres/min, 95% CI ‐11.87 to ‐2.20, six studies: Analysis 3.8; change in pm PEF from baseline: ‐7.04 Litres/min, 95% CI ‐12.66 to ‐1.43, four studies: Analysis 3.9)
Outcomes pooled for adults and children
Daily symptom score: SMD 0.04 (95% CI ‐0.07 to 0.15), eight studies: Analysis 3.13.
3.13. Analysis.
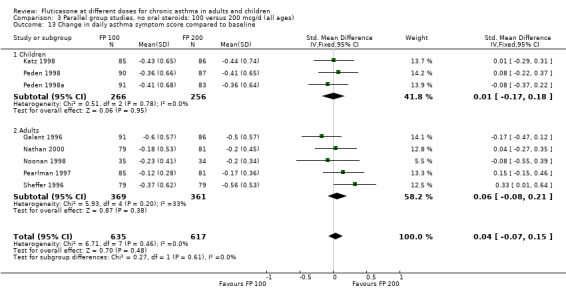
Comparison 3 Parallel group studies, no oral steroids: 100 versus 200 mcg/d (all ages), Outcome 13 Change in daily asthma symptom score compared to baseline.
Daily use of beta‐2 agonist: WMD 0.14 puffs per day (95% CI ‐0.09 to 0.37), nine studies: Analysis 3.11
3.11. Analysis.
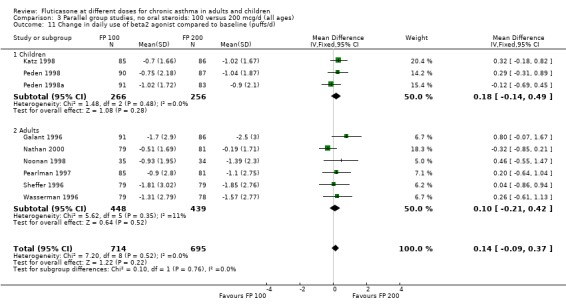
Comparison 3 Parallel group studies, no oral steroids: 100 versus 200 mcg/d (all ages), Outcome 11 Change in daily use of beta2 agonist compared to baseline (puffs/d).
Number of night‐time awakenings per week): WMD 0.05 (95% CI 0.01 to 0.1), four studies: Analysis 3.14
3.14. Analysis.
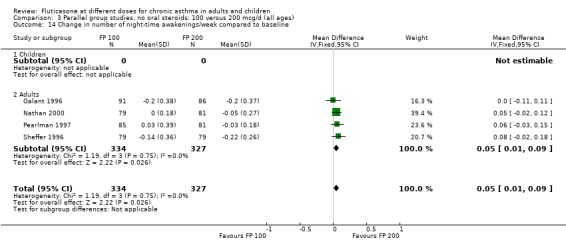
Comparison 3 Parallel group studies, no oral steroids: 100 versus 200 mcg/d (all ages), Outcome 14 Change in number of night‐time awakenings/week compared to baseline.
Night‐time awakening score: SMD 0.17 (95% CI 0.04 to 0.30), 5 studies, n=921: Analysis 3.16
3.16. Analysis.
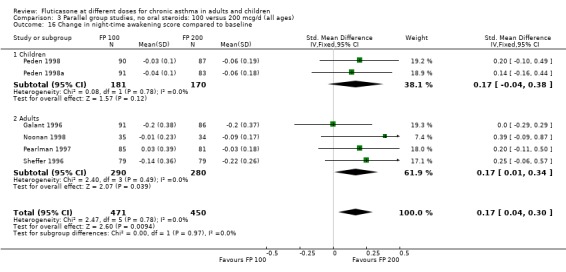
Comparison 3 Parallel group studies, no oral steroids: 100 versus 200 mcg/d (all ages), Outcome 16 Change in night‐time awakening score compared to baseline.
The number of people withdrawn due to lack of efficacy criteria (see Table 22 for studies reporting a priori defined criteria) was reported in eight studies. No significant difference between treatments was apparent (Peto OR 1.01, 95% CI 0.76 to 1.36).
6. Criteria for withdrawal due to lack of efficacy.
| Study ID | FEV1 | PEFR | Beta2 agonist use | Night‐time awakening | Exacerbations |
| Chervinsky 1994 | 20% or greater decrease compared to baseline | 20% decrease in morning or evening PEFR on 4 or more days out of 7 in week prior to clinic visit | 12 or more puffs on 3 or more days out of 7 in week prior to clinic visit | 2 or more nights with 2 awakening out of 7 in week prior to clinic visit | Any clinical exacerbation requiring emergency treatment, hospital admission or additional asthma medication other than rescue beta 2 agonist |
| Galant 1996 | 15% or greater decrease compared to baseline | 20% or greater decrease in morning PEFR on 3 or more days out of 7 in week prior to clinic visit | 12 or more puffs on 3 or more days out of 7 in week prior to clinic visit | 3 or more awakening in week prior to clinic visit | |
| Katz 1997 | 15% or greater decrease compared to baseline | 15% or greater decrease in morning PEFR on 3 or more days out of 7 in week prior to clinic visit | 8 or more puffs on 2 or more days out of 7 in week prior to clinic visit | 2 or more nights with awakening out of 7 in week prior to clinic visit | |
| Lawrence 1997 | 20% or greater decrease compared to baseline | 20% or greater decrease in morning PEFR on 3 or more days out of 7 in week prior to clinic visit | 12 or more puffs on 2 or more days out of 7 in week prior to clinic visit | 2 or more nights with awakening out of 7 in week prior to clinic visit | any clinical exacerbation requiring emergency treatment, hospital admission or additional asthma medication other than rescue beta 2 agonist |
| Nathan 2000 | 20% or greater decrease compared to baseline | 20% or greater decrease compared to baseline | 12 or more puffs on 2 or more days out of 7 in week prior to clinic visit | 2 or more awakening in week prior to clinic visit | Any clinical exacerbation requiring emergency treatment, hospital admission or additional asthma medication other than rescue beta 2 agonist |
| Pearlman 1997 | 20% or greater decrease compared to baseline | 20% or greater decrease in morning PEFR on 3 or more days out of 7 in week prior to clinic visit | 12 or more puffs on 2 or more days out of 7 in week prior to clinic visit | 2 or more awakening in week prior to clinic visit | Any clinical exacerbation requiring emergency treatment, hospital admission or additional asthma medication other than rescue beta 2 agonist |
| Peden 1997 | 15% or greater decrease compared to baseline | 20% or greater decrease in morning PEFR on 3 or more days out of 7 in week prior to clinic visit | 12 or more puffs on 3 or more days out of 7 in week prior to clinic visit | 3 or more awakening in week prior to clinic visit | Any clinical exacerbation requiring emergency treatment, hospital admission or additional asthma medication other than rescue beta 2 agonist |
| Raphael 1999 | 20% or greater decrease compared to baseline | 20% or greater decrease in morning PEFR on 3 or more days out of 7 in week prior to clinic visit | 12 or more puffs on 3 or more days out of 7 in week prior to clinic visit | 3 or more nights with awakening out of 7 in week prior to clinic visit | |
| Sheffer 1996 | 15% or greater decrease compared to baseline | 20% or greater decrease in morning PEFR on 3 or more days out of 7 in week prior to clinic visit | 12 or more puffs on 3 or more days out of 7 in week prior to clinic visit | 3 or more nights with awakening out of 7 in week prior to clinic visit | Any clinical exacerbation requiring emergency treatment, hospital admission or additional asthma medication other than rescue beta 2 agonist |
| Wasserman 1996 | 20% or greater decrease compared to baseline | 20% or greater decrease in morning PEFR on 3 or more days out of 7 in week prior to clinic visit | 12 or more puffs on 3 or more days out of 7 in week prior to clinic visit | 2 or more nights with awakening out of 7 in week prior to clinic visit | any clinical exacerbation requiring emergency treatment, hospital admission or additional asthma medication other than rescue beta 2 agonist |
| Wolfe 1996 | 20% or greater decrease compared to baseline | 20% or greater decrease in morning PEFR on 3 or more days out of 7 in week prior to clinic visit | 12 or more puffs on 3 or more days out of 7 in week prior to clinic visit | 2 or more nights with awakening out of 7 in week prior to clinic visit | Any clinical exacerbation requiring emergency treatment, hospital admission or additional asthma medication other than rescue beta 2 agonist |
Allen 1998 reported health status in 205 children with mild to moderate asthma who were randomised to receive FP 100 or 200 µg/day over a 12‐month treatment period. The health status of the children was assessed using: the Functional Status IIR (FSIIR) and the Sleep‐scale Children Questionnaire (SLP‐C). The effect of the children having asthma on their parents' lives was evaluated using the Quality of Life of Parents of Asthmatic Children Questionnaire (QOL‐PAC). No significant differences between FP doses were apparent at the 12‐month time point for the FSIIR, SLP‐C or any domain of the QOL‐PAC.
Pearlman 1997 assessed these doses of FP over a 12‐week period in adults. This study reported health status using a general instrument, the Medical Outcomes Short Form 36 (SF‐36), and an asthma‐specific instrument, the Living with Asthma Questionnaire (LWA‐20). No significant differences between treatment groups were apparent.
FP 200 versus 400 to 500 µg/day
Primary outcome
There was no statistically significant difference between lower and higher doses of FP from 11 studies in adults (0.01 litres, 95% CI ‐0.04 to 0.05; Analysis 5.3). Two paediatric and two adult studies assessed FEV1 as percent predicted, with no significant difference observed between the doses (FEV1 predicted: MD ‐0.96% (95% CI ‐3.45 to 1.53), Analysis 5.1).
5.3. Analysis.
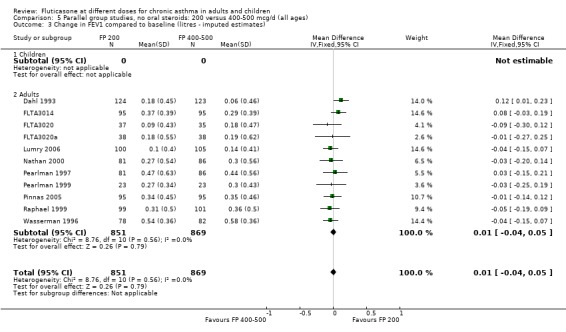
Comparison 5 Parallel group studies, no oral steroids: 200 versus 400‐500 mcg/d (all ages), Outcome 3 Change in FEV1 compared to baseline (litres ‐ imputed estimates).
5.1. Analysis.
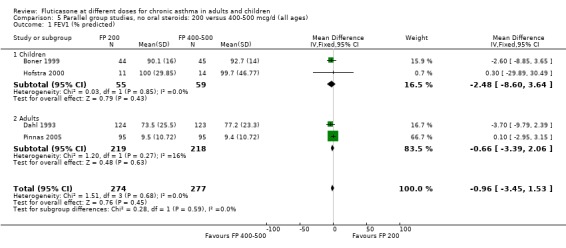
Comparison 5 Parallel group studies, no oral steroids: 200 versus 400‐500 mcg/d (all ages), Outcome 1 FEV1 (% predicted).
Secondary outcomes
Children
The lower doses were significantly less effective than higher doses for improving peak flow measurements (change in am PEF: ‐7.92 L/min, 95% CI ‐12.93 to ‐2.91, two studies: Analysis 5.6; change in pm PEF: ‐9.36 L/min, 95% CI ‐14.37 to ‐4.35, two studies: Analysis 5.8).
5.6. Analysis.
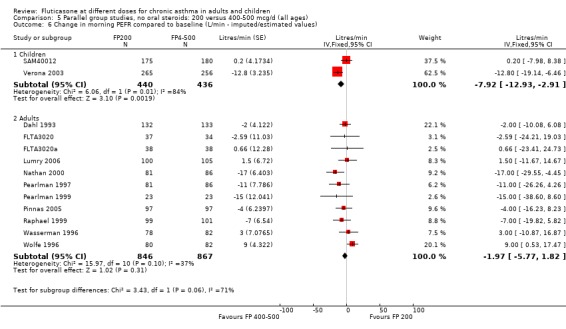
Comparison 5 Parallel group studies, no oral steroids: 200 versus 400‐500 mcg/d (all ages), Outcome 6 Change in morning PEFR compared to baseline (L/min ‐ imputed/estimated values).
5.8. Analysis.
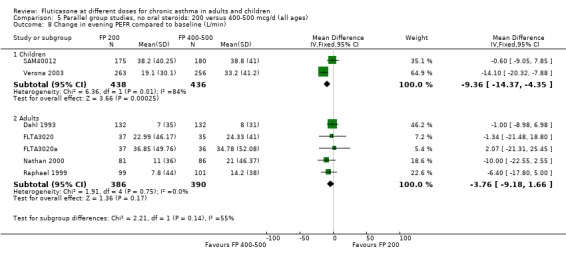
Comparison 5 Parallel group studies, no oral steroids: 200 versus 400‐500 mcg/d (all ages), Outcome 8 Change in evening PEFR compared to baseline (L/min).
Adults
For adult participants, there was no statistically significant differences between the doses compared (change in am PEF: ‐1.97 L/min, 95% CI ‐5.77 to 1.82, 11 studies: Analysis 5.6; change in pm PEF: ‐3.76 L/min, 95% CI ‐9.18 to 1.66, five studies: Analysis 5.8)
Outcomes pooled for adults and children
The 95% confidence intervals for the pooled estimates included no difference for the following outcomes:
Daily symptom score: 0.11 SMD, 95% CI ‐0.02 to 0.24, seven studies: Analysis 5.14
5.14. Analysis.
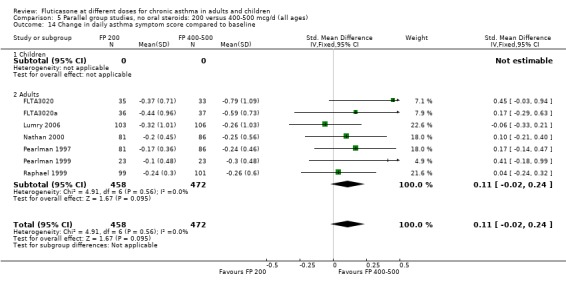
Comparison 5 Parallel group studies, no oral steroids: 200 versus 400‐500 mcg/d (all ages), Outcome 14 Change in daily asthma symptom score compared to baseline.
Daily use of rescue beta‐2 agonist: WMD 0.18 puffs/day, 95% CI ‐0.08 to 0.43, seven studies: Analysis 5.11
5.11. Analysis.
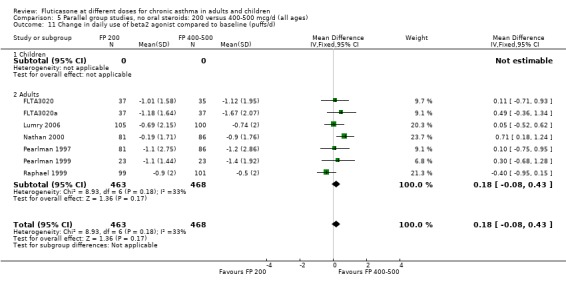
Comparison 5 Parallel group studies, no oral steroids: 200 versus 400‐500 mcg/d (all ages), Outcome 11 Change in daily use of beta2 agonist compared to baseline (puffs/d).
Number of night‐time awakenings per week: WMD ‐0.01 awakenings (95% CI ‐0.05 to 0.04), two studies: Analysis 5.12
5.12. Analysis.
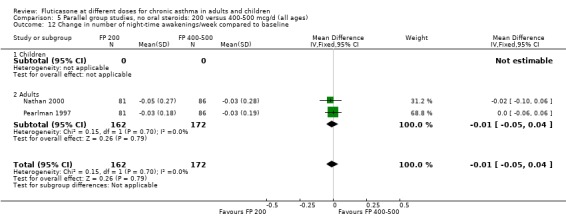
Comparison 5 Parallel group studies, no oral steroids: 200 versus 400‐500 mcg/d (all ages), Outcome 12 Change in number of night‐time awakenings/week compared to baseline.
Exacerbations requiring OCS: Peto OR: 1.21 (95% CI 0.72 to 2.05), two studies: Analysis 5.18
5.18. Analysis.
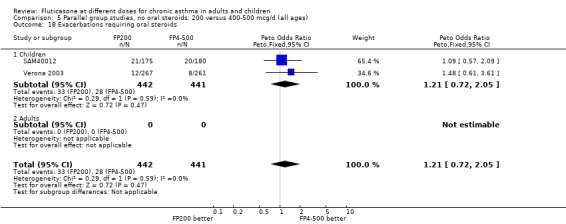
Comparison 5 Parallel group studies, no oral steroids: 200 versus 400‐500 mcg/d (all ages), Outcome 18 Exacerbations requiring oral steroids.
No significant differences were present when comparing doses for FVC and FEF 25 to 75 (Raphael 1999).
For the following outcomes the confidence intervals excluded no statistically significant difference in favour of the higher dose of FP:
Number of patients withdrawn due to lack of efficacy: Peto OR 1.40 (95% CI 1.01 to 2.13), seven studies: Analysis 5.17
5.17. Analysis.
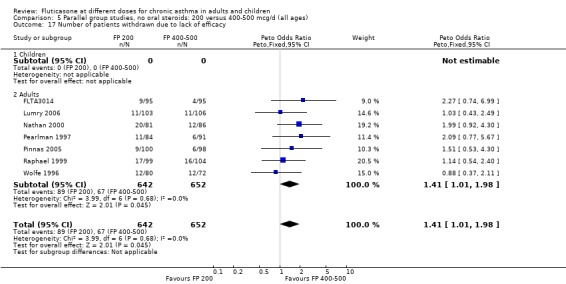
Comparison 5 Parallel group studies, no oral steroids: 200 versus 400‐500 mcg/d (all ages), Outcome 17 Number of patients withdrawn due to lack of efficacy.
FP 100 versus 400 to 500 µg/day
Primary outcome
No significant difference between doses was apparent for the change in FEV1 from baseline (0.01 litres (95% CI ‐0.09 to 0.1), three studies: Analysis 7.1. These studies were conducted in adults with moderate and mild asthma.
7.1. Analysis.
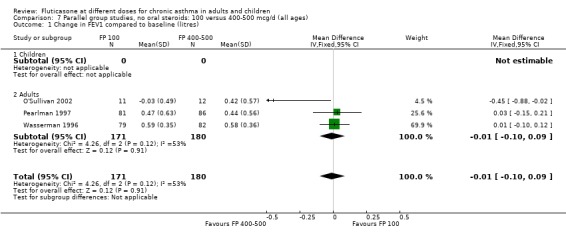
Comparison 7 Parallel group studies, no oral steroids: 100 versus 400‐500 mcg/d (all ages), Outcome 1 Change in FEV1 compared to baseline (litres).
Secondary outcomes
The lower dose of FP was significantly less effective than the higher dose in improving morning PEF (‐8 litres/min (95% CI ‐15 to ‐1), three studies: Analysis 7.3. Symptom scores also favoured higher doses (SMD 0.31 (95% CI 0.03 to 0.6), two studies: Analysis 7.6.
7.3. Analysis.
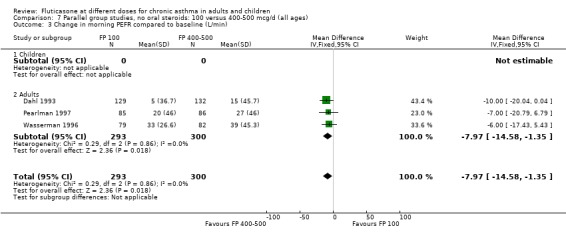
Comparison 7 Parallel group studies, no oral steroids: 100 versus 400‐500 mcg/d (all ages), Outcome 3 Change in morning PEFR compared to baseline (L/min).
7.6. Analysis.
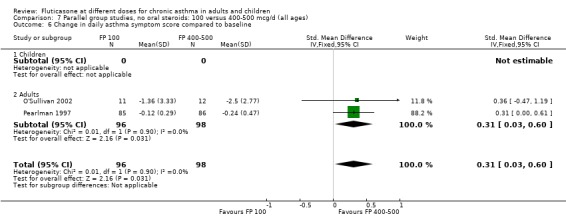
Comparison 7 Parallel group studies, no oral steroids: 100 versus 400‐500 mcg/d (all ages), Outcome 6 Change in daily asthma symptom score compared to baseline.
Middle versus higher dose range comparisons
FP 400 to 500 versus 800 to 1000 µg/day
Primary outcome
There was no significant difference between moderate and high doses of FP in the change in FEV1 (‐0.01 litres, 95% CI ‐0.06 to 0.04), five studies: Analysis 9.1). These five studies were conducted in adults with predominantly moderate and severe asthma.
9.1. Analysis.
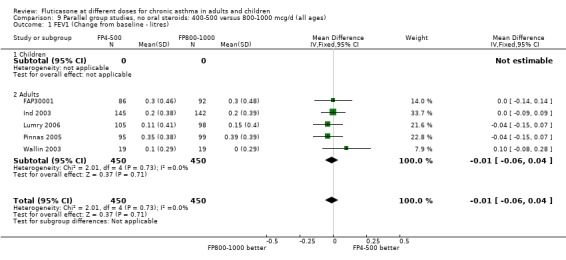
Comparison 9 Parallel group studies, no oral steroids: 400‐500 versus 800‐1000 mcg/d (all ages), Outcome 1 FEV1 (Change from baseline ‐ litres).
Secondary outcomes
Change in morning PEF: ‐2.3 litres/min (95% CI ‐7.94 to 3.35), four studies: Analysis 9.2
9.2. Analysis.
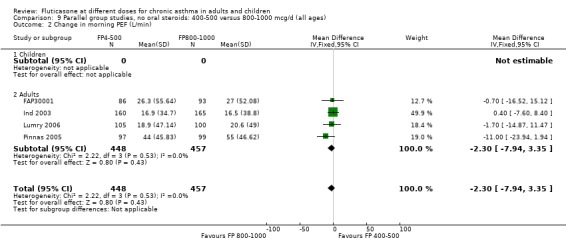
Comparison 9 Parallel group studies, no oral steroids: 400‐500 versus 800‐1000 mcg/d (all ages), Outcome 2 Change in morning PEF (L/min).
Change in evening PEF: 5.83 litres/min (95% CI ‐2.94 to 14.60), two studies: Analysis 9.3
9.3. Analysis.
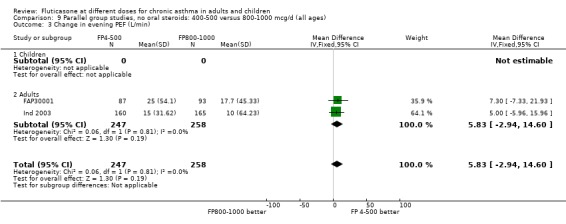
Comparison 9 Parallel group studies, no oral steroids: 400‐500 versus 800‐1000 mcg/d (all ages), Outcome 3 Change in evening PEF (L/min).
Exacerbations requiring treatment with oral steroids: Peto OR 1.24 (95% CI 0.77 to 1.98), two studies: Analysis 9.9
9.9. Analysis.
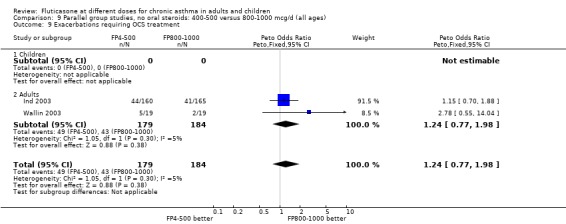
Comparison 9 Parallel group studies, no oral steroids: 400‐500 versus 800‐1000 mcg/d (all ages), Outcome 9 Exacerbations requiring OCS treatment.
Withdrawals due to any reason: Peto OR 1.27 (95% CI 0.88 to 1.83), five studies: Analysis 9.10
9.10. Analysis.
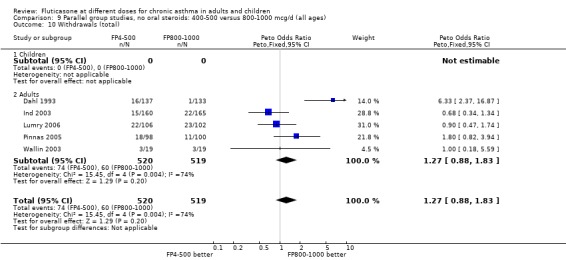
Comparison 9 Parallel group studies, no oral steroids: 400‐500 versus 800‐1000 mcg/d (all ages), Outcome 10 Withdrawals (total).
Withdrawals due to adverse events: Peto OR 0.44 (95% CI 0.17 to 1.25), four studies: Analysis 9.11
9.11. Analysis.
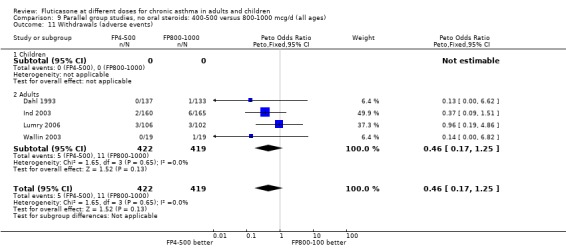
Comparison 9 Parallel group studies, no oral steroids: 400‐500 versus 800‐1000 mcg/d (all ages), Outcome 11 Withdrawals (adverse events).
Lower versus higher dose‐range comparisons
FP 50 to 100 versus 800 to 1000 µg/day
Primary outcome
There was no significant difference in the change in FEV1 predicted (WMD 0.43% predicted, 95% CI ‐4.87 to 5.72; 2 studies: Analysis 11.2).
11.2. Analysis.
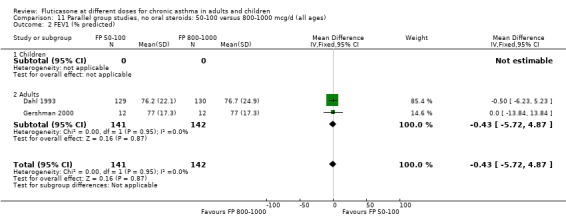
Comparison 11 Parallel group studies, no oral steroids: 50‐100 versus 800‐1000 mcg/d (all ages), Outcome 2 FEV1 (% predicted).
Secondary outcomes
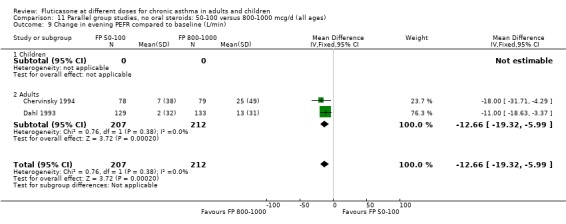
The lower doses of FP were significantly less effective than the higher doses in improving morning PEF: WMD ‐22 litres/min (95% CI ‐29 to ‐15, two studies: Analysis 11.8), and evening PEF: WMD ‐13 litres/min (95% CI ‐19 to ‐6, two studies: Analysis 11.9).
11.8. Analysis.
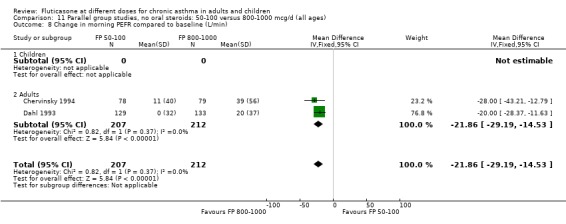
Comparison 11 Parallel group studies, no oral steroids: 50‐100 versus 800‐1000 mcg/d (all ages), Outcome 8 Change in morning PEFR compared to baseline (L/min).
11.9. Analysis.
Comparison 11 Parallel group studies, no oral steroids: 50‐100 versus 800‐1000 mcg/d (all ages), Outcome 9 Change in evening PEFR compared to baseline (L/min).
Withdrawals due to lack of efficacy: Peto OR 5.31, 95% CI 2.18 to 12.96: Analysis 11.20.
11.20. Analysis.
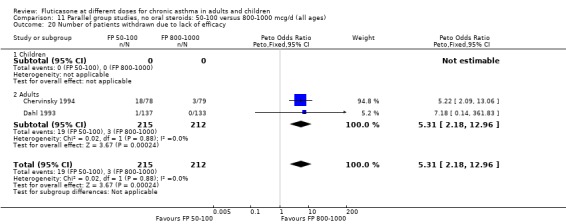
Comparison 11 Parallel group studies, no oral steroids: 50‐100 versus 800‐1000 mcg/d (all ages), Outcome 20 Number of patients withdrawn due to lack of efficacy.
Withdrawals (any reason): Peto OR 5.29, 95% CI 1.59, 17.60: Analysis 11.19.
11.19. Analysis.
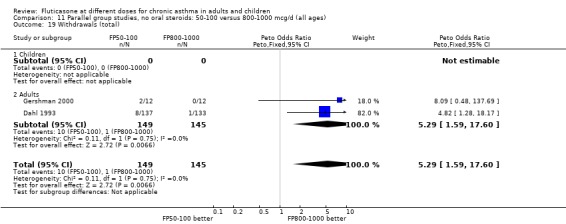
Comparison 11 Parallel group studies, no oral steroids: 50‐100 versus 800‐1000 mcg/d (all ages), Outcome 19 Withdrawals (total).
FP 200 versus 800 to 1000 µg/day
Primary outcome
Change from baseline in FEV1 was significantly lower with 200mcg of FP than at higher dose (‐0.11 litres, 95% CI ‐0.19 to ‐0.04, three studies: Analysis 12.1).
12.1. Analysis.
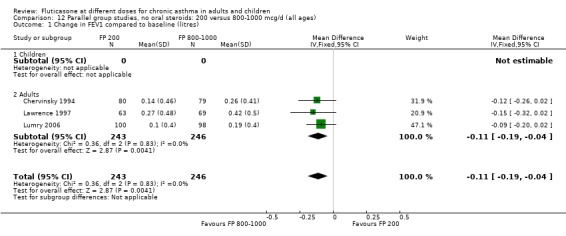
Comparison 12 Parallel group studies, no oral steroids: 200 versus 800‐1000 mcg/d (all ages), Outcome 1 Change in FEV1 compared to baseline (litres).
Secondary outcomes The higher doses of FP led to greater improvements in morning and evening PEF (8.07 litres/min (95% CI 1.61 to 14.53, four studies: Analysis 12.5*; and 8 litres/min (95% CI 1 to 15), two studies: Analysis 12.6).
12.5. Analysis.
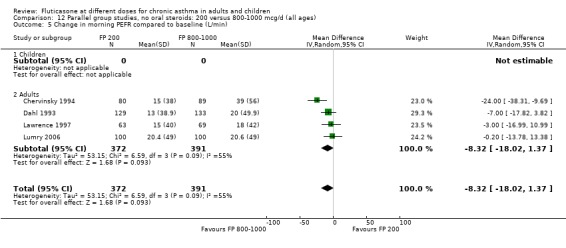
Comparison 12 Parallel group studies, no oral steroids: 200 versus 800‐1000 mcg/d (all ages), Outcome 5 Change in morning PEFR compared to baseline (L/min).
12.6. Analysis.
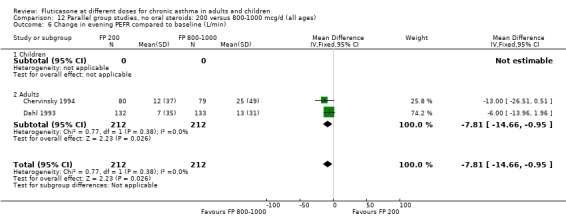
Comparison 12 Parallel group studies, no oral steroids: 200 versus 800‐1000 mcg/d (all ages), Outcome 6 Change in evening PEFR compared to baseline (L/min).
No significant difference between treatment groups was apparent for the following measures; when expressed as a change compared to baseline and reported in favour of the higher dose:
Daily asthma symptom score: SMD 0.22 (95% CI ‐0.09 to 0.54), two studies: Analysis 12.10
12.10. Analysis.
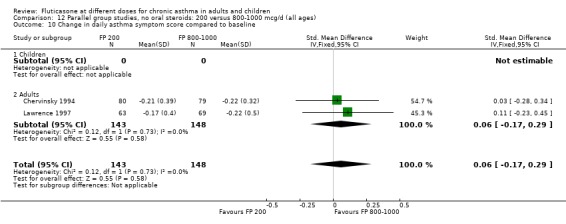
Comparison 12 Parallel group studies, no oral steroids: 200 versus 800‐1000 mcg/d (all ages), Outcome 10 Change in daily asthma symptom score compared to baseline.
Rescue beta‐2 agonist use: WMD 0.11 puffs/day (95% CI ‐0.29 to 0.50), two studies: Analysis 12.13
12.13. Analysis.
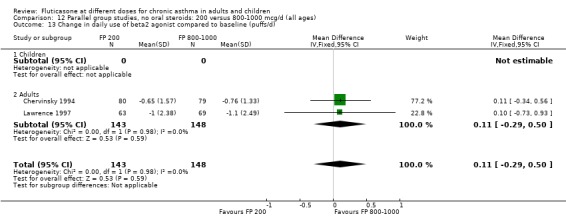
Comparison 12 Parallel group studies, no oral steroids: 200 versus 800‐1000 mcg/d (all ages), Outcome 13 Change in daily use of beta2 agonist compared to baseline (puffs/d).
Physician‐rated efficacy and responses judged to be ineffective: Peto OR 1.3 (95% CI 0.9 to 2.0), two studies: Analysis 12.12.
12.12. Analysis.
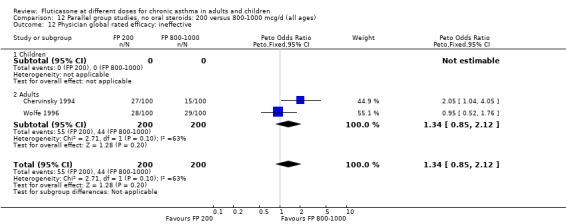
Comparison 12 Parallel group studies, no oral steroids: 200 versus 800‐1000 mcg/d (all ages), Outcome 12 Physician global rated efficacy: ineffective.
Number of patients withdrawn due to lack of efficacy: Peto OR 1.32 (95% CI 0.73 to 2.4), four studies: Analysis 12.14
12.14. Analysis.
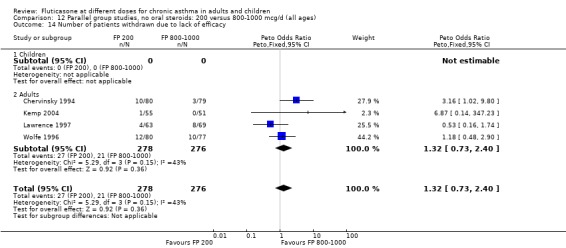
Comparison 12 Parallel group studies, no oral steroids: 200 versus 800‐1000 mcg/d (all ages), Outcome 14 Number of patients withdrawn due to lack of efficacy.
*Denotes significant heterogeneity (I2 54.5%). Random‐effects modelling for this outcome resulted in a non‐significant difference (WMD 8.32 litres/min, 95% CI ‐1.37 to 18.02). When the study conducted in more mild participants was removed from the analysis (Chervinsky 1994), the heterogeneity resolved completely and the pooled‐effect estimate was non‐significant (WMD ‐4 litres/min, 95% CI ‐11.04 to 3.24). This may not be the sole reason for variation between the studies. Indeed, one area of uncertainty is the relative potency of FP using older propellants compared with the more recently developed CFC‐free propellants. Lumry 2006 compared CFC‐free (HFA) preparations of FP. While questions pertaining to the relative efficacy with CFC and HFA are not fully answered, there is a theoretical possibility that better deposition of the steroid particles in the small airways of the lungs enhances the potency of the drug. This would make FP more effective when propelled with HFA than older preparations of FP, especially in lower dose ranges.
Safety measures
Results were reported as the number of patients experiencing at least one of the following events during the treatment period. The following table summarises the findings for the dose comparisons that were assessed. A Peto odds ratio below one indicated fewer people with side effects at the lower FP dose.
Oropharyngeal side effects
Sore throat or pharyngitis FP 100 versus 200 µg/day: Peto OR 1.87 (95% CI 0.71 to 4.93) FP 200 versus 400 to 500 µg/day: Peto OR 0.98 (95% CI 0.6 to 1.63) FP 100 versus 400 to 500 µg/day: Peto OR 1.02 (95% CI 0.29 to 3.56) FP 200 versus 800 to 1000 µg/day: Peto OR 0.51 (95% CI 0.18 to 1.39)
Hoarseness and dysphonia FP 100 versus 200 µg/day: Peto OR 0.65 (95% CI 0.27 to 1.57) FP 200 versus 400 to 500 µg/day: Peto OR 0.76 (95% CI 0.4 to 1.46) FP 100 versus 400 to 500 µg/day: Peto OR 0.28 (95% CI 0.08 to 0.92) FP 200 versus 800 to 1000 µg/day: Peto OR 0.51 (95% CI 0.24 to 1.08) FP 50 to 100 versus 800 to 1000 µg/day: Peto OR 0.18 (95% CI 0.05 to 0.59)
Oral candidiasis FP 100 versus 200 µg/day: Peto OR 0.98 (95% CI 0.46 to 2.08) FP 200 versus 400 to 500 µg/day: Peto OR 0.87 (95% CI 0.47 to 1.61) FP 100 versus 400 to 500 µg/day: Peto OR 0.89 (95% CI 0.32 to 2.49) FP 200 versus 800 to 1000 µg/day: Peto OR 0.33 (95% CI 0.16 to 0.70) FP 50 to 100 versus 800 to 1000 µg/day: Peto OR 0.32 (95% CI 0.11 to 0.97)
No heterogeneity was present for any comparison. With regard to the likelihood of sore throat, there were no significant differences between FP daily doses over a wide dose range. However, higher doses were associated with a significantly greater risk of hoarseness and dysphonia. The studies comparing FP 50 to 100 versus 800 to 1000 µg/day (Chervinsky 1994; Dahl 1993) were of four and eight‐weeks duration respectively. A significantly greater likelihood of hoarseness and oral candidiasis was apparent for patients treated with FP at the higher dose.
Hypothalamo‐pituitary adrenal (HPA) function Basal adrenocortical activity, as assessed by morning plasma cortisol, was reported in a number of studies (Chervinsky 1994; Dahl 1993; Lawrence 1997; Li 1999; Pearlman 1997; Peden 1998/Peden 1998a; Sorkness 1999; Sorkness 1999a; Wolfe 1996). Data were rarely presented in a form suitable for inclusion in a meta‐analysis. However, no significant differences were seen in any study.
Two studies reported plasma cortisol levels following the standard short ACTH stimulation test (cortisol levels measured 30 to 60 minutes after intravenous injection of cosyntropin, 250 µg). No significant differences in levels were apparent when comparing four daily doses of FP (100, 200, 400, 800 µg/day) (Dahl 1993), or three daily doses of FP (100, 200, 500 µg/day) (Pearlman 1997).
Non‐oral steroid treated asthmatics: crossover studies
Two studies (Agertoft 1997; Derom 1999) were conducted using a crossover design. No significant differences between FP doses were apparent for FEV1 or morning PEF in either study. Agertoft 1997 also reported no significant differences in evening PEF, asthma symptoms and 24‐hour urinary free cortisol excretion.
Oral steroid‐treated asthmatics
Oral steroid‐sparing design
Two parallel group studies (Nelson 1999; Noonan 1995) were conducted in oral steroid‐treated patients using an oral steroid‐sparing design. Both were large, high quality (Jadad score 3 or 4) multicentre trials in adults with severe asthma and conducted in the USA. An inclusion criterion for both studies was that participants were treated with, and dependent on, oral prednisolone for asthma control at the time of enrolment. Two nominal daily doses of FP were compared in each trial: FP 1000 µg/day or 2000 µg/day (Nelson 1999) delivered via the Accuhaler DPI; and FP 1500 µg/day or 2000 µg/day delivered via MDI (Noonan 1995). The mean daily baseline dose of oral prednisolone for the two studies was 13.0 to 13.6 mg/day and 9.5 to 10.2 mg/day respectively. In both studies a high proportion of patients (> 80%) were also receiving treatment with regular inhaled corticosteroids (beclomethasone, triamcinolone or flunisolide) at enrolment, although this was not an inclusion criterion in either case. Reduction in daily dose of oral prednisolone was the primary outcome measure and both trials were of 16‐weeks duration. Criteria for prednisolone dose reduction were established a priori and were based on maintenance of stable asthma control determined in relation to baseline control. This was assessed using changes in FEV1, PEF, rescue beta‐2 agonist use and frequency of symptoms at clinic visits. Pooled with unpublished data (FLTA3022; FLTA3022a) there was no significant difference between the higher and lower dose of FP (FP 2000 µg/day compared with FP 1000 to 1500 µg/day) in terms of the number of participants who were able to stop OCS therapy (Peto OR 1.53, 95% CI 0.88 to 2.68). There was also no significant difference in the change in daily oral prednisolone requirement (MD 1 mg/day, 95% CI ‐0.45 to 2.45).
The studies were designed to maintain stable asthma control and this may explain the non‐significant effects observed with random effects with moderate levels of heterogeneity for the outcomes (four studies, N = 274): Change in FEV1 (‐0.14 L (95% CI ‐0.32 to 0.04), I square 54.5%) Change in am PEF (‐21.17 L/min (95% CI ‐51 to 8.87), I square 74%) Change in pm PEF (‐21.73 L/min (95% CI ‐47.87 to 4.41), I square 72.1%) Change in symptom scores (SMD 0.15 (95% CI ‐0.09 to 0.39), I square 34%) Change in daily rescue medication use (‐0.14 puffs/d (95% CI ‐1.05 to 0.78), I square 36%)
There was a low level of heterogeneity present in the analysis change in asthma quality of life questionnaire (‐0.12 AQLQ units (95% CI ‐0.48 to 0.23), I square 5.7%).
Health status was assessed in both studies. Nelson 1999 reported the results of the Asthma Quality of Life Questionnaire (AQLQ). No significant difference between FP doses was apparent for overall score. A significant improvement in the symptoms domain that favoured the higher dose was apparent (mean difference 0.74, 95% CI 0.15 to 1.33); no significant differences between doses were seen for other domains. Significantly greater improvements in a number of domains of the Medical Outcomes Short Study Form (SF‐36), a general health status questionnaire, were found for FP 2000 µg/day compared to FP 1500 µg/day by Noonan 1995. These included physical functioning and role limitations because of physical health problems. Other domains did not show any significant dose‐dependent differences.
Trials not using an oral steroid‐sparing design
A single study (Ayres 1995) compared FP 1000 versus 2000 µg/day over a six‐week treatment period. This was a large multicentre trial in 671 adults with moderate to severe asthma and was of fair methodological quality (Jadad score 3). One in eight patients randomised to receive FP treatment were receiving oral prednisolone (< 10 mg/day) at the time or enrolment. A number of outcome measures were reported. Only the evening PEF showed a significantly greater improvement favouring higher dose FP (mean difference 7 litre/min, 95% CI 0 to 15). No significant differences between the two doses were apparent for FEV1, morning PEF, improvement in symptom‐free days, or daytime/night‐time rescue beta‐2 agonist use. There were no significant differences between treatment groups with regard to the incidence of oropharyngeal side effects, including oral candidiasis.
Discussion
We analysed data from 51 studies (yielding 55 randomised comparisons) with participants. The overall risk of bias from these trials was low. The purpose of the review was to test whether a dose‐response effect is evident for fluticasone (FP), i.e. whether significantly greater improvements were present with higher as opposed to lower doses of FP. When asking such a question an important distinction needs to be made between statistically significant dose‐dependent changes and those that are also of clinical significance. In a previous systematic review (Adams 2008) we assessed the efficacy and safety of FP when compared with placebo. That review was a quantitative synthesis of all the available randomised clinical trial evidence. Comparisons between active drug and placebo have the greatest likelihood of detecting clinically worthwhile improvements in asthma control and treatment‐associated side effects. A significant proportion of the studies included in the current review included a placebo treatment arm and were also analysed in the previous review. As a result, the findings from that review can be considered as a benchmark whereby present findings can be better put into context. They are referred to in the following discussion, which considers dose response by outcome.
FEV1
For dose comparisons over the lower‐to‐middle part of the dose range (FP 50 and 400 to 500 µg/day) no dose‐response effect was apparent in people who had asthma and were not receiving oral steroids. In such people, these doses of FP have been shown to produce clinically significant improvements in FEV1 compared to placebo (0.31 to 0.41 litres) (Adams 2008). A statistically significant dose‐response effect was apparent when comparing high doses (800 to 1000 µg/day) to the lower dose range (200 µg/day), although additional benefit with the high dose FP was relatively small (110 mls).
Diary card peak expiratory flow (PEF)
For diary card PEF, a statistically significant dose‐response effect was seen over most parts of the dose range. This was apparent when comparing the lower and middle part of the dose range. In comparison with FP 100 µg/day, FP 200 µg/day led to small improvements in morning and evening PEF. Curiously, FP 400 to 500 µg/day was not superior to 100mcg/day but was when compared with 200 µg/day in adults. Given that the difference in both comparisons was not likely to be noticeable (2 and 8 litres for 100 and 200mcg respectively), this apparent discordant finding may be attributable to differential statistical power, or may be artefactual.
When moderate doses of FP were compared with higher doses the dose‐response effect was less apparent overall. There was no significant difference between FP (400 to 500 µg/day) and FP (800 to 1000 µg/day) in morning PEF. These data suggest that there is a consistent dose response when doses of FP are increased in the lower dose ranges but at higher doses of FP (400 to 500 µg/day) the dose‐dependent response became less apparent.
With a four to five‐fold dose increase (200 and 800 to 1000 µg/day) a greater difference favouring the higher dose was seen for both morning and evening PEF. Similarly with a ten‐fold plus increase in dose of FP (50 to 100 versus 800 to 1000 µg/day).
Symptoms and rescue beta‐2 agonist use
Although all daily doses of FP led to significant improvements in symptoms scores when compared with placebo (Adams 2008), no dose response effect was apparent when making comparisons of individual doses across any part of the dose range. The same pattern of response was present for rescue beta‐2 agonist requirement. All daily doses of FP led to significantly greater reductions in use compared to placebo (Adams 2008) but the dose relationship for FP was flat and there was no significant difference in benefit between high (1000 µg/day) and low dose (200 µg/day).
Health status
Measures of health status were reported infrequently and did not cover the whole of the FP dose range. Where reported, however, no dose response was apparent. This included measures of child and parent well‐being at doses over the lower part of the dose range, using disease specific instruments (Allen 1998) and comparisons over the lower and mid‐dose range using general and disease specific instruments (Lumry 2006; Pearlman 1997).
Oropharyngeal side effects
A significant dose‐response effect was apparent for the likelihood of participants experiencing oral candidiasis. This was apparent when comparing high dose FP (1000 µg/day) with low dose FP (50 to 100 µg/day): the higher the dose the greater the risk. No significant difference in likelihood was seen when comparing doses in the lower part of the dose range. A similar picture was seen for risk of experiencing hoarseness. No dose‐response effect was seen for likelihood of sore throat. This is consistent with the finding that an increased likelihood of sore throat was only apparent when the highest dose of FP was compared to placebo. Doses of 500 µg/day or less did not lead to an increased incidence of this side effect (Adams 2008).
Hypothalamo‐pituitary‐adrenal axis function
Measures of basal adrenal function (using morning plasma cortisol levels and 24‐hour urinary cortisol excretion) and dynamic tests of adrenal reserve (post ACTH plasma cortisol levels) were reported in a number of studies. These studies covered most of the FP dose range and no differences were seen. However, non‐randomised evidence does suggest that adrenal suppression has been documented as a serious side‐effect of fluticasone (Tattersfield 2004).
Clinical significance and generalisability of findings
Children and adults treated with FP using a variety of dry powder and aerosol delivery devices were included in this review. Participants were treated for varying lengths of time, from four weeks up to one year. A notable feature of this analysis is that the addition of new studies increased heterogeneity in outcomes that had previously been homogeneous. This enabled us to revisit our a priori‐defined subgroup analyses. Where sufficient data have been assembled, overall subgroup analyses have failed to identify groups who appeared to respond to treatment in a consistently different way. The findings appear to apply to children (over two years of age) and adults treated with all delivery devices (keeping in mind the use of nebulisers was excluded) for periods of one month up to a year. An important feature of the participants included in this analysis is that most appeared to have mild‐to‐moderate or moderate asthma, based on the current GINA 1995/NHLBI 1997 criteria (using the baseline characteristics of the patients, listed in Table 21). It appears that, as a population, people with mild to moderate asthma who are not receiving oral corticosteroids do not experience any further gains in terms of symptom relief or needs for rescue beta‐2 agonists when doses of FP are increased above 100 µg/day. However, people can be expected to gain some incremental improvements in measures of airway function on higher rather than lower daily doses. However, these additional improvements are small and it is difficult to judge whether they are clinically worthwhile.
Current UK guidelines recommend that adults and children with asthma should be prescribed low‐dose steroids if their symptoms are severe enough to warrant initiation of preventative therapy. Subsequently, if they are inadequately controlled on low‐dose steroids, a trial of additive therapy, such as long‐acting beta agonists, is recommended (BTS 2003), although use of LABA as a monotherapy does carry risks (Cates 2008). This is based on the assumption that dose‐dependent improvements in asthma control (symptoms, exacerbations, rescue beta‐2 agonist use, PEF variability) can be achieved. The findings of this review provided evidence to support this approach. However, it also suggests that most people with mild to moderate asthma achieve similar levels of control, certainly in terms of symptom relief and bronchodilator requirements, in the lower part of the dose range for fluticasone as they do when up to five‐fold increases in daily doses are prescribed. The use of high‐dose FP was accompanied by an increased risk of oral side effects. This needs to be borne in mind when advising patients about possible side effects and when choosing a prescribed dose.
Oral steroid‐treated asthma
High‐dose FP was effective in allowing people who were dependent on oral steroids and receiving inhaled steroids to reduce or stop the use of prednisolone (Adams 2008). In this group of patients, a dose‐response effect was apparent for likelihood of both stopping prednisolone completely and reducing the daily dose. Very high dose FP (2000 µg/day) led to significantly greater improvements in these outcomes compared to FP (1000 to 1500 µg/day). A NNT of 6 (95% CI 3 to 25) implied that only six patients needed to be treated at the higher daily dose over 16 weeks to allow an extra patient to stop prednisolone completely compared to those treated with FP 1000 to 1500 µg/day. The highest dose of FP also allowed an additional 2 mg/day (95% CI 0.1 to 4.0 mg/day) reduction in prednisolone dose compared to FP 1000 to 1500 µg/day, in people with a baseline prednisolone use of between 9 and 14 mg/day. Such improvements may be beneficial. An interesting finding of this analysis was that a clear dose‐response effect over this range was also apparent in terms of improvement in FEV1, morning PEF, evening PEF and daily asthma symptom score. In fact, the additional improvements seen for the highest dose of FP, compared to 1000 to 1500 µg/day, for these patients were substantially greater than those seen in mild to moderately severe asthmatics over a 10‐fold plus increase in dose (FP 100 and FP 800 to 1000 µg/day). The explanation for this difference may lie in the fact that people with severe asthma had a greater scope for improvement in these measures, as judged by their significantly impaired baseline lung function (Table 21).
Methodological limitations
The search for trials covered a large volume of literature but it is possible that relevant, published trials were missed. However, Glaxo Smith Kline (GSK) who have the sole licence for the manufacture of fluticasone (Flixotide) and who have sponsored many of the trials included in this review only alerted us to one further study, published after the date of the last electronic search. This provided a degree of reassurance that the search strategy was effective and that no further studies were missed.
Many trials included in this review were conducted with the objective of assessing whether FP exhibits a dose‐response effect. In this context, failure to detect dose differences could be interpreted as a negative finding. It is possible that relevant, negative trials exist but have not been published. Failure to include such studies could lead to a biased over‐estimate of dose differences. However, this possibility seems unlikely as authors who are experts in the area and GSK did not alert us to such studies.
This review does not include data on bone turnover. Given the concerns raised about the short and long‐term side‐effect profile of steroids (BTS 2003), maintaining patients on the lowest possible dose is recommended in order to minimise the risk of potential adverse events without compromising asthma control.
Studies including infants under the age of two years were specifically excluded. The diagnosis of asthma and administration of inhaled medication are especially difficult in this group of patients, so the findings of this review should not be extrapolated to this group.
FP has been licensed for use in the UK in the form of a nebulised solution. Trials using nebulisers were specifically excluded from this review and the findings from this review should not be extrapolated to the use of fluticasone using a nebuliser.
Authors' conclusions
Implications for practice.
Inhaled fluticasone (FP) is an effective treatment for chronic asthma when administered over a wide range of daily doses. Although a shallow dose‐response effect is evident for a number of measures of asthma control where oral steroids are not given, the findings of this review would suggest that most children and adults with mild to moderate asthma do not achieve clinically worthwhile improvements in FEV1 with higher doses (more than 500 µg/day) as compared to lower doses (up to 200 µg/day). Small increases in peak flow have been shown with higher doses of FP, but although these are statistically significant they may not be large enough to be clinically important. Severe, oral steroid‐dependent asthmatics gain clinically worthwhile benefit in terms of oral steroid cessation and reduction in daily dose of prednisolone on very high doses of FP (2000 µg/day). People who cannot be weaned off oral steroids using lower doses should have a trial of FP 2000 µg/day.
Implications for research.
A large number of people have been studied in the assessment of the relative efficacy of fluticasone (FP) at different doses. Clinically‐relevant outcome measures related to airway calibre and symptoms have been assessed in both children and adults. However, a number of possible avenues of further research can be identified. Heath status (patient‐centred evaluation of the impact of asthma on functional state) has been reported infrequently and has not been assessed over the whole of the available dose range. There is a place for further studies over the available dose range using disease‐specific instruments and where clinically meaningful change scores can be established. Other important outcomes have not been assessed. These include those that have health economic significance, including hospital and primary care physician attendance rates due to clinical asthma exacerbations that necessitate additional treatment or hospital admission. Longer‐term studies (six months or greater) would be needed to reliably assess these outcomes. Such trials are likely to be difficult to set up and very expensive.
The identified upper limits of the dose‐response effect require some confirmatory work. In particular, the assessment of FP at doses between 400 to 500 µg/day compared with a two‐fold increase in children and in more severe asthma would help to extend the validity of the evidence. In the present review the trial populations were predominantly adult populations with moderately severe asthma.
The long‐term effects of different doses of FP on HPA axis and any associated risk of adrenal insufficiency are unknown. Studies that assess these risks will need to include patients treated for a number of years and will need to include many patients in order to have adequate power of detecting differences because such events are likely to be rare. Prospective RCTs will almost certainly be unable to answer these questions due to limitations of cost. Well‐conducted retrospective cohort studies will probably be the only way to evaluate the differential risk, if any, of different doses of FP in terms of clinically meaningful disturbances to HPA function.
What's new
| Date | Event | Description |
|---|---|---|
| 27 April 2009 | Amended | Software problem identified and bug fixed. |
History
Protocol first published: Issue 1, 1999 Review first published: Issue 3, 2001
| Date | Event | Description |
|---|---|---|
| 24 July 2008 | New citation required and conclusions have changed | Unpublished data: Agertoft 1997; Dahl 1993; Ind 2003; Kemp 2004; Katz 1998; Boner 1999; Allen 1998; Chervinsky 1994; Nathan 2000; Nelson 1999; Pearlman 1999; Peden 1998; Verona 2003
New trials: N = 10 (representing 11 randomised comparisons: FLIP39; Pinnas 2005; FAP30001; FLIP01; FLIP01a; FLTA3014; FLTA3020; FLTA3020a; FLTA3022; Lumry 2006; SLGF75) Previous descriptions of What's New are given in Table 17. |
| 24 July 2008 | Amended | Converted to new review format. |
| 1 January 2008 | New search has been performed | Literature search re‐run |
| 1 April 2005 | New citation required and conclusions have changed | Substantive amendment |
1. What's New.
| Issue | Detail |
| 3, 2005 | Impact of new evidence on findings of the review The addition of new data on the review has largely increased the precision of the effect estimates. The existence of a small dose response in the lower dose ranges of fluticasone (FP) has not been challenged by the new trials included in the review. The inclusion of data from three studies comparing FP at doses of 400 to 500 µg/day with 800 to 1000 µg/day raises the possibility that the upper limits of the dose response occur at medium doses for adults with moderate asthma. Evidence from one trial suggested that the response to higher doses of FP is greater in more severe asthma. Further work would help to confirm this. Detail of update ‐ latest search date January 2005 A total of 23 new trials, contributing data for an additional 2370 participants met the inclusion criteria of the review (Allen 2000; Bukovskis 2002; Casale 2001; Chetta 2002; Derom 1999; Derom 2001; Falcoz 2000; Gershman 2000; Giannini 2003; Kemp 2004; Li 1999; Meijer 1999; Nathan 2000; Nielsen 2002; Nieto 2001; O'Sullivan 2002; Pauwels 2002; Pearlman 1999; Pearlman 2002; SAM40012; Sorkness 1999; Sorkness 1999a; Verona 2003; Wallin 2003). The latest search date was January 2004. Data from the full publications of studies previously available as abstracts were included (Ind 1998 (now Ind 2003); Hofstra 1998 (now Hofstra 2000)). Several trials contributed to more than one comparison as they were multi‐arm studies. One unpublished trial was identified (SAM40012). Unless otherwise indicated, the addition of new data tightened confidence intervals around existing significant or non‐significant pooled estimates. In the following summary of outcomes for different dose comparisons, outcomes marked * denote those that became significant with the addition of new data. Fluticasone 100 versus 200 µg/day (4 new studies) Change in forced expiratory volume in one minute (FEV1); change in morning peak expiratory flow (PEF); change in evening PEF; change in daily use of rescue medication; change in daily asthma symptoms; change in number of nocturnal awakenings*; withdrawal due to lack of efficacy; sore throat or pharyngitis Fluticasone 100 versus 400 to 500 µg/day (2 new studies) Change in FEV1; change in morning PEF; change in daily symptoms Fluticasone 200 versus 400 to 500 µg/day (9 new studies) Change in morning PEF; change in evening PEF; change in FEV1; change in daily symptoms; change in rescue medication usage; number of nocturnal awakenings; number of patients withdrawn due to lack of efficacy; sore throat or pharyngitis; hoarseness or dysphonia; oral candidiasis Fluticasone 400 to 500 versus 800 to 1000 µg/day (3 new studies, no studies previously included) Change in morning PEF; change in FEV1; exacerbations requiring oral corticosteroids (OCS); withdrawals Fluticasone 50 to 100 versus 800 to 1000 µg/day (2 new studies) FEV1 Fluticasone 200 versus 800 to 1000 µg/day (4 new studies) Change in FEV1; change in morning PEF; sore throat or pharyngitis; oral candidiasis* (fewer instances of candidiasis in lower dose group); withdrawals due to lack of efficacy, hoarseness or dysphonia |
Acknowledgements
We would like to thank the support staff of the Cochrane Airways Group, Liz Arnold, Anna Bara and Jane Dennis for assistance in the electronic search and retrieval of papers and Steve Milan for statistical support. We would like to thank the authors who were kind enough to provide extra details concerning their studies, including Professor DB Allen, Professor JG Ayres, Professor R Dahl, Professor E Derom, Dr Y Katz and Dr AL Sheffer. We would like to thank Julia Earnshaw and Karen Richardson who co‐coordinated efforts on behalf of Glaxo Smith Kline to search for additional studies and who provided further information regarding a number of included studies that were sponsored by the company. We are grateful to the Nederlands Astma Fonds who provided a grant which enabled us to maintain this review. We are grateful to Janet Wale who copy‐edited this review.
Appendices
Appendix 1. Previous assessment of study quality
Two authors (NPA and JB) were blinded to the authors' names, institutions and funding sources and independently assessed the methodological quality of included studies. Trials were scored using the following approach.
Grade A: adequate allocation concealment. Grade B: unclear allocation concealment. Grade C: clearly inadequate concealment.
Studies were also assessed using a five‐point scoring instrument (Jadad 1996). 1. Was the study described as randomised? (yes = 1, no = 0). 2. Was the study described as double blind? (yes = 1, no = 0). 3. Was there a description of withdrawals and dropouts? (yes = 1, no = 0). 4. Was the method of randomisation well described and appropriate? (yes = 1, no = 0). 5. Was the method of double blinding well described and appropriate? (yes = 1, no = 0). 6. Deduct one point if method of randomisation or blinding inappropriate. Inter‐rater agreement was measured using the kappa statistic. Disagreement was resolved by consensus.
Appendix 2. GSK randomisation procedures
The procedures for randomising GSK sponsored studies has been detailed in correspondence between Richard Follows and TL, the details of which are given below:
The randomisation software is a computer‐generated, centralised programme (RandAll). After verification that the randomisation sequence is suitable for the study design (crossover, block or stratification), Clinical Supplies then package the treatments according to the randomisation list generated. Concealment of allocation is maintained by a third party, since the sites phone in and are allocated treatments on that basis. Alternatively a third party may dispense the drug at the sites. Unblinding of data for interim analyses can only be done through RandAll, and are restricted so that only those reviewing the data are unblinded to treatment group allocation.
Data and analyses
Comparison 1. Parallel group studies, no oral steroids: 50 versus 100 mcg/d (all ages).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Change in FEV1 compared to baseline (litres) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 1.1 Children | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 Adults | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Change in morning PEFR compared to baseline (L/min) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2.1 Children | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.2 Adults | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Change in morning PEFR compared to baseline (L/min) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 3.1 Children | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.2 Adults | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4 Change in daily asthma symptom score compared to baseline | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 4.1 Children | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.2 Adults | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5 Change in number of night‐time awakenings/week compared to baseline | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 5.1 Children | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.2 Adults | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 6 Change in daily use of beta2 agonist compared to baseline (puffs/d) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 6.1 Children | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 6.2 Adults | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 7 Number of patients withdrawn due to lack of efficacy | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Totals not selected | |
| 7.1 Children | 0 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 7.2 Adults | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
1.1. Analysis.
Comparison 1 Parallel group studies, no oral steroids: 50 versus 100 mcg/d (all ages), Outcome 1 Change in FEV1 compared to baseline (litres).
1.2. Analysis.
Comparison 1 Parallel group studies, no oral steroids: 50 versus 100 mcg/d (all ages), Outcome 2 Change in morning PEFR compared to baseline (L/min).
1.3. Analysis.
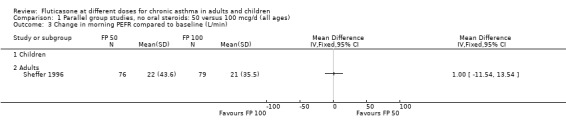
Comparison 1 Parallel group studies, no oral steroids: 50 versus 100 mcg/d (all ages), Outcome 3 Change in morning PEFR compared to baseline (L/min).
1.4. Analysis.
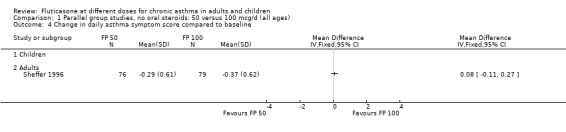
Comparison 1 Parallel group studies, no oral steroids: 50 versus 100 mcg/d (all ages), Outcome 4 Change in daily asthma symptom score compared to baseline.
1.5. Analysis.
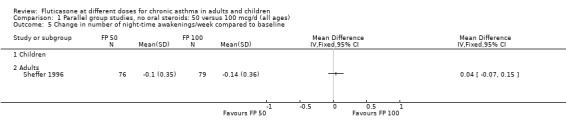
Comparison 1 Parallel group studies, no oral steroids: 50 versus 100 mcg/d (all ages), Outcome 5 Change in number of night‐time awakenings/week compared to baseline.
1.6. Analysis.
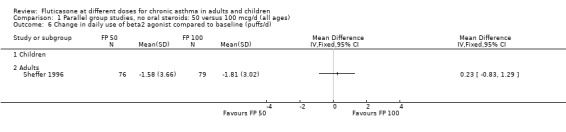
Comparison 1 Parallel group studies, no oral steroids: 50 versus 100 mcg/d (all ages), Outcome 6 Change in daily use of beta2 agonist compared to baseline (puffs/d).
1.7. Analysis.
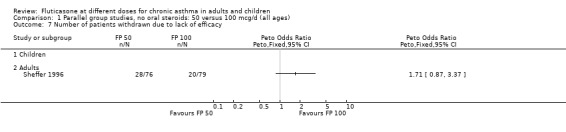
Comparison 1 Parallel group studies, no oral steroids: 50 versus 100 mcg/d (all ages), Outcome 7 Number of patients withdrawn due to lack of efficacy.
Comparison 2. Parallel group studies, no oral steroids: 50 versus 200 mcg/d (all ages).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Change in FEV1 compared to baseline (litres) | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 1.1 Children | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 1.2 Adults | 2 | 313 | Mean Difference (IV, Fixed, 95% CI) | ‐0.04 [‐0.16, 0.08] |
| 2 Change in FVC compared to baseline (litres) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2.1 Children | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.2 Adults | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Change in FEF25‐75 compared to baseline (L/second) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 3.1 Children | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.2 Adults | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4 Change in morning PEFR compared to baseline (L/min) | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 4.1 Children | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 4.2 Adults | 2 | 313 | Mean Difference (IV, Fixed, 95% CI) | ‐7.49 [‐17.31, 2.33] |
| 5 Change in evening PEFR compared to baseline (L/min) | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 5.1 Children | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 5.2 Adults | 2 | 313 | Mean Difference (IV, Fixed, 95% CI) | ‐7.95 [‐17.24, 1.34] |
| 6 Change in daily asthma symptom score compared to baseline | 2 | 313 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.32 [0.10, 0.54] |
| 6.1 Children | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 6.2 Adults | 2 | 313 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.32 [0.10, 0.54] |
| 7 Change in number of night‐time awakenings/week compared to baseline | 2 | 313 | Mean Difference (IV, Fixed, 95% CI) | 0.09 [0.02, 0.16] |
| 7.1 Children | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 7.2 Adults | 2 | 313 | Mean Difference (IV, Fixed, 95% CI) | 0.09 [0.02, 0.16] |
| 8 Change in daily use of beta2 agonist compared to baseline (puffs/d) | 2 | 313 | Mean Difference (IV, Fixed, 95% CI) | 0.07 [‐0.37, 0.51] |
| 8.1 Children | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 8.2 Adults | 2 | 313 | Mean Difference (IV, Fixed, 95% CI) | 0.07 [‐0.37, 0.51] |
| 9 Number of patients withdrawn due to lack of efficacy | 2 | 313 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.48 [0.88, 2.46] |
| 9.1 Children | 0 | 0 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 9.2 Adults | 2 | 313 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.48 [0.88, 2.46] |
| 10 Hoarseness or dysphonia (No. of patients) | 2 | 322 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.52 [0.14, 1.97] |
| 10.1 Children | 0 | 0 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 10.2 Adults | 2 | 322 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.52 [0.14, 1.97] |
| 11 Oral Candidiasis (No. of patients) | 2 | 322 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.27 [0.05, 1.35] |
| 11.1 Children | 0 | 0 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 11.2 Adults | 2 | 322 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.27 [0.05, 1.35] |
| 12 Physician global rated efficacy: ineffective | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Totals not selected | |
| 12.1 Children | 0 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 12.2 Adults | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
2.1. Analysis.
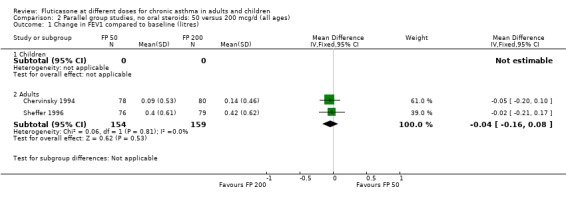
Comparison 2 Parallel group studies, no oral steroids: 50 versus 200 mcg/d (all ages), Outcome 1 Change in FEV1 compared to baseline (litres).
2.2. Analysis.
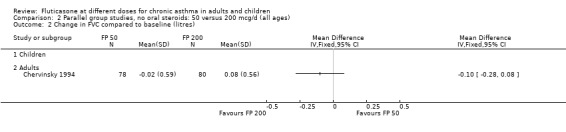
Comparison 2 Parallel group studies, no oral steroids: 50 versus 200 mcg/d (all ages), Outcome 2 Change in FVC compared to baseline (litres).
2.3. Analysis.
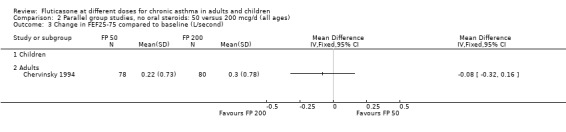
Comparison 2 Parallel group studies, no oral steroids: 50 versus 200 mcg/d (all ages), Outcome 3 Change in FEF25‐75 compared to baseline (L/second).
2.4. Analysis.
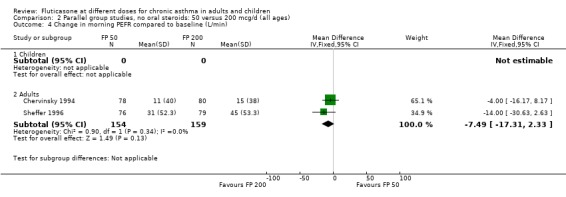
Comparison 2 Parallel group studies, no oral steroids: 50 versus 200 mcg/d (all ages), Outcome 4 Change in morning PEFR compared to baseline (L/min).
2.5. Analysis.
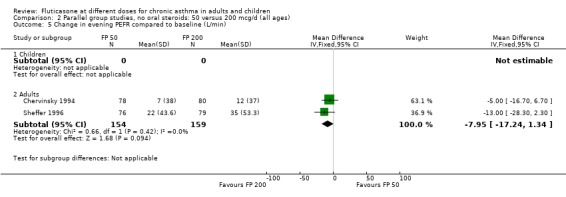
Comparison 2 Parallel group studies, no oral steroids: 50 versus 200 mcg/d (all ages), Outcome 5 Change in evening PEFR compared to baseline (L/min).
2.6. Analysis.
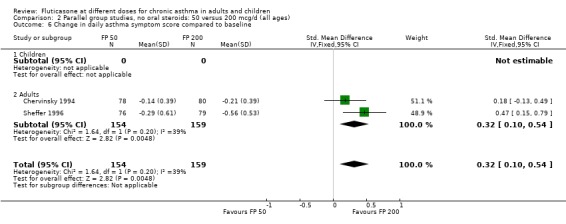
Comparison 2 Parallel group studies, no oral steroids: 50 versus 200 mcg/d (all ages), Outcome 6 Change in daily asthma symptom score compared to baseline.
2.7. Analysis.
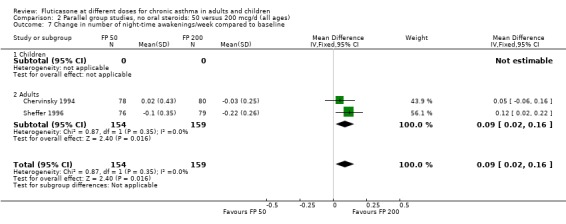
Comparison 2 Parallel group studies, no oral steroids: 50 versus 200 mcg/d (all ages), Outcome 7 Change in number of night‐time awakenings/week compared to baseline.
2.8. Analysis.
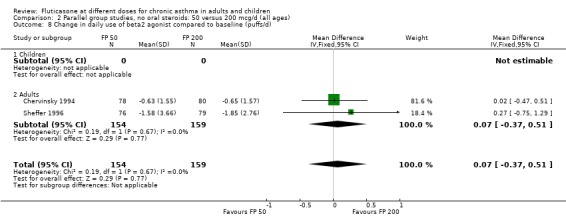
Comparison 2 Parallel group studies, no oral steroids: 50 versus 200 mcg/d (all ages), Outcome 8 Change in daily use of beta2 agonist compared to baseline (puffs/d).
2.9. Analysis.
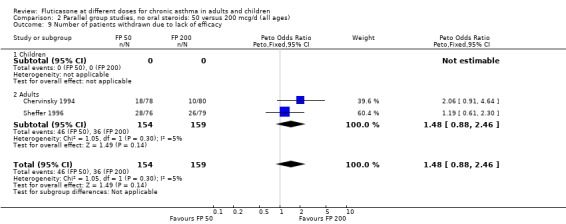
Comparison 2 Parallel group studies, no oral steroids: 50 versus 200 mcg/d (all ages), Outcome 9 Number of patients withdrawn due to lack of efficacy.
2.10. Analysis.
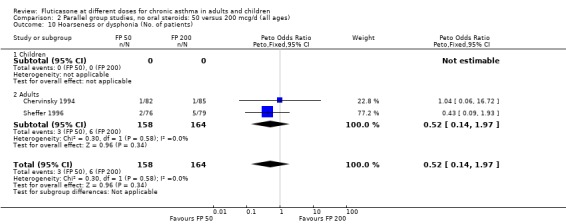
Comparison 2 Parallel group studies, no oral steroids: 50 versus 200 mcg/d (all ages), Outcome 10 Hoarseness or dysphonia (No. of patients).
2.11. Analysis.
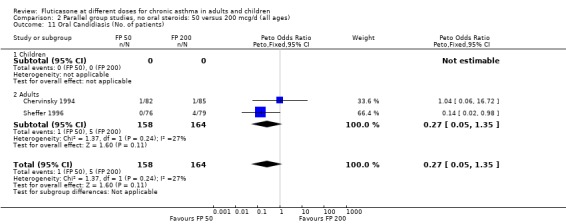
Comparison 2 Parallel group studies, no oral steroids: 50 versus 200 mcg/d (all ages), Outcome 11 Oral Candidiasis (No. of patients).
2.12. Analysis.
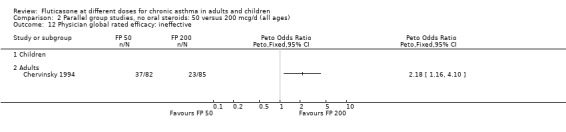
Comparison 2 Parallel group studies, no oral steroids: 50 versus 200 mcg/d (all ages), Outcome 12 Physician global rated efficacy: ineffective.
Comparison 3. Parallel group studies, no oral steroids: 100 versus 200 mcg/d (all ages).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Change in FEV1 compared to baseline (litres) | 10 | Litres (Fixed, 95% CI) | Subtotals only | |
| 1.1 Children | 4 | 656 | Litres (Fixed, 95% CI) | ‐0.04 [‐0.09, 0.01] |
| 1.2 Adults | 6 | 887 | Litres (Fixed, 95% CI) | 0.03 [‐0.02, 0.07] |
| 2 Change in FEV1 compared to baseline (litres) | 10 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 2.1 Children | 4 | 656 | Mean Difference (IV, Fixed, 95% CI) | ‐0.03 [‐0.08, 0.02] |
| 2.2 Adults | 6 | 887 | Mean Difference (IV, Fixed, 95% CI) | 0.01 [‐0.05, 0.07] |
| 3 FEV1 (% predicted) | 2 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 3.1 Children | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.2 Adults | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4 FEV1 (% predicted) | 2 | 432 | % (Fixed, 95% CI) | ‐0.52 [‐3.83, 2.79] |
| 4.1 Children | 1 | 179 | % (Fixed, 95% CI) | ‐2.0 [‐6.00, 2.00] |
| 4.2 Adults | 1 | 253 | % (Fixed, 95% CI) | 2.70 [‐3.19, 8.59] |
| 5 FEV1 Litres | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 5.1 Children | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.2 Adults | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 6 PEF (L/min) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 6.1 Children | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 6.2 Adults | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 7 Change in clinic PEFR compared to baseline (L/min) | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 7.1 Children | 2 | 351 | Mean Difference (IV, Fixed, 95% CI) | 3.00 [‐6.84, 12.84] |
| 7.2 Adults | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 8 Change in morning PEFR compared to baseline (L/min) | 11 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 8.1 Children | 4 | 710 | Mean Difference (IV, Fixed, 95% CI) | ‐3.67 [‐9.81, 2.46] |
| 8.2 Adults | 7 | 1148 | Mean Difference (IV, Fixed, 95% CI) | ‐7.04 [‐11.87, ‐2.20] |
| 9 Change in evening PEFR compared to baseline (L/min) | 6 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 9.1 Children | 2 | 351 | Mean Difference (IV, Fixed, 95% CI) | ‐4.50 [‐11.77, 2.77] |
| 9.2 Adults | 4 | 648 | Mean Difference (IV, Fixed, 95% CI) | ‐7.04 [‐12.66, ‐1.43] |
| 10 Rescue medication usage | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 10.1 Children | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 10.2 Adults | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 11 Change in daily use of beta2 agonist compared to baseline (puffs/d) | 9 | 1409 | Mean Difference (IV, Fixed, 95% CI) | 0.14 [‐0.09, 0.37] |
| 11.1 Children | 3 | 522 | Mean Difference (IV, Fixed, 95% CI) | 0.18 [‐0.14, 0.49] |
| 11.2 Adults | 6 | 887 | Mean Difference (IV, Fixed, 95% CI) | 0.10 [‐0.21, 0.42] |
| 12 Symptom scores | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 12.1 Children | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 12.2 Adults | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 13 Change in daily asthma symptom score compared to baseline | 8 | 1252 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.04 [‐0.07, 0.15] |
| 13.1 Children | 3 | 522 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.01 [‐0.17, 0.18] |
| 13.2 Adults | 5 | 730 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.06 [‐0.08, 0.21] |
| 14 Change in number of night‐time awakenings/week compared to baseline | 4 | 661 | Mean Difference (IV, Fixed, 95% CI) | 0.05 [0.01, 0.09] |
| 14.1 Children | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 14.2 Adults | 4 | 661 | Mean Difference (IV, Fixed, 95% CI) | 0.05 [0.01, 0.09] |
| 15 Percentage of symptom‐free days | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 15.1 Children | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 15.2 Adults | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 16 Change in night‐time awakening score compared to baseline | 6 | 921 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.17 [0.04, 0.30] |
| 16.1 Children | 2 | 351 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.17 [‐0.04, 0.38] |
| 16.2 Adults | 4 | 570 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.17 [0.01, 0.34] |
| 17 HRQOL: Functional Status IIR questionaire (short version) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 17.1 Children | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 17.2 Adults | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 18 HRQOL: Sleep Scale Children questionnaire | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 18.1 Children | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 18.2 Adults | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 19 HRQOL: Quality of Life of Parents of Asthmatic Children questionnaire, burden dimension | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 19.1 Children | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 19.2 Adults | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 20 HRQOL: Quality of Life of Parents of Asthmatic Children questionnaire, subjective norms dimension | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 20.1 Children | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 20.2 Adults | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 21 HRQOL: Quality of Life of Parents of Asthmatic Children questionnaire, subjective norms dimension | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 21.1 Children | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 21.2 Adults | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 22 Physician global rated efficacy: ineffective | 2 | 357 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.37 [0.77, 2.44] |
| 22.1 Children | 0 | 0 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 22.2 Adults | 2 | 357 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.37 [0.77, 2.44] |
| 23 Withdrawal due to asthma exacerbation (No. of patients) | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Totals not selected | |
| 23.1 Children | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 23.2 Adults | 0 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 24 Withdrawals due to adverse events | 3 | 627 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.16 [0.54, 2.47] |
| 24.1 Children | 0 | 0 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 24.2 Adults | 3 | 627 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.16 [0.54, 2.47] |
| 25 Number of patients withdrawn due to lack of efficacy | 9 | 1657 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.01 [0.76, 1.35] |
| 25.1 Children | 3 | 522 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.25 [0.79, 1.97] |
| 25.2 Adults | 6 | 1135 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.88 [0.61, 1.28] |
| 26 Oral Candidiasis (No. of patients) | 6 | 1150 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.98 [0.46, 2.08] |
| 26.1 Children | 2 | 391 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.33 [0.30, 5.90] |
| 26.2 Adults | 4 | 759 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.88 [0.37, 2.11] |
| 27 Headaches | 3 | 511 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.62 [0.15, 2.60] |
| 27.1 Children | 0 | 0 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 27.2 Adults | 3 | 511 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.62 [0.15, 2.60] |
| 28 Sore throat or pharyngitis (No. of patients) | 5 | 841 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.87 [0.71, 4.93] |
| 28.1 Children | 0 | 0 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 28.2 Adults | 5 | 841 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.87 [0.71, 4.93] |
| 29 Hoarseness or dysphonia (No. of patients) | 7 | 1365 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.65 [0.27, 1.57] |
| 29.1 Children | 2 | 391 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.00 [0.20, 4.99] |
| 29.2 Adults | 5 | 974 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.54 [0.19, 1.55] |
| 30 Urinary free cortisol excretion (mcg/24 hours) | 2 | 228 | Mean Difference (IV, Fixed, 95% CI) | ‐0.70 [‐3.73, 2.32] |
| 30.1 Children | 2 | 228 | Mean Difference (IV, Fixed, 95% CI) | ‐0.70 [‐3.73, 2.32] |
| 30.2 Adults | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 31 Morning plasma cortisol (mcg/dL) | 3 | 377 | Mean Difference (IV, Fixed, 95% CI) | ‐0.06 [‐1.01, 0.89] |
| 31.1 Children | 2 | 333 | Mean Difference (IV, Fixed, 95% CI) | ‐0.07 [‐1.14, 0.99] |
| 31.2 Adults | 1 | 44 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [‐2.09, 2.09] |
3.2. Analysis.
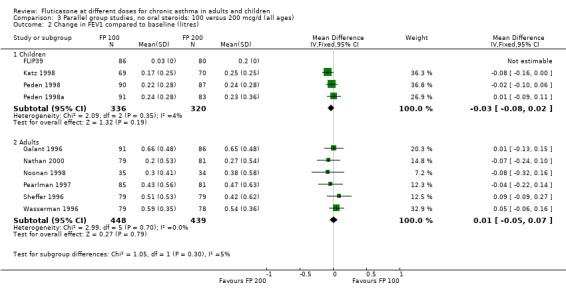
Comparison 3 Parallel group studies, no oral steroids: 100 versus 200 mcg/d (all ages), Outcome 2 Change in FEV1 compared to baseline (litres).
3.3. Analysis.
Comparison 3 Parallel group studies, no oral steroids: 100 versus 200 mcg/d (all ages), Outcome 3 FEV1 (% predicted).
3.4. Analysis.
Comparison 3 Parallel group studies, no oral steroids: 100 versus 200 mcg/d (all ages), Outcome 4 FEV1 (% predicted).
3.5. Analysis.
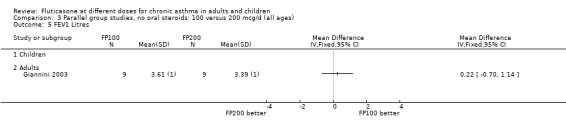
Comparison 3 Parallel group studies, no oral steroids: 100 versus 200 mcg/d (all ages), Outcome 5 FEV1 Litres.
3.6. Analysis.
Comparison 3 Parallel group studies, no oral steroids: 100 versus 200 mcg/d (all ages), Outcome 6 PEF (L/min).
3.7. Analysis.
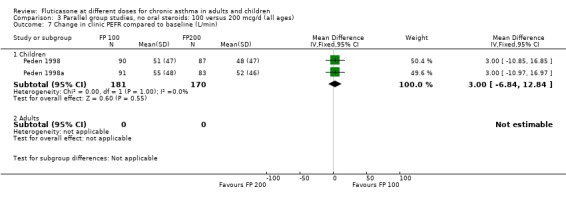
Comparison 3 Parallel group studies, no oral steroids: 100 versus 200 mcg/d (all ages), Outcome 7 Change in clinic PEFR compared to baseline (L/min).
3.10. Analysis.
Comparison 3 Parallel group studies, no oral steroids: 100 versus 200 mcg/d (all ages), Outcome 10 Rescue medication usage.
3.12. Analysis.
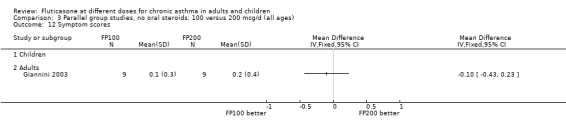
Comparison 3 Parallel group studies, no oral steroids: 100 versus 200 mcg/d (all ages), Outcome 12 Symptom scores.
3.15. Analysis.
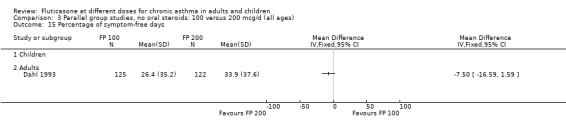
Comparison 3 Parallel group studies, no oral steroids: 100 versus 200 mcg/d (all ages), Outcome 15 Percentage of symptom‐free days.
3.17. Analysis.
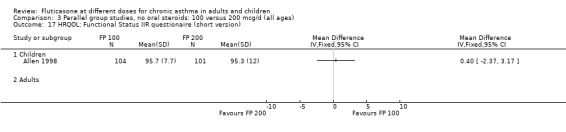
Comparison 3 Parallel group studies, no oral steroids: 100 versus 200 mcg/d (all ages), Outcome 17 HRQOL: Functional Status IIR questionaire (short version).
3.18. Analysis.
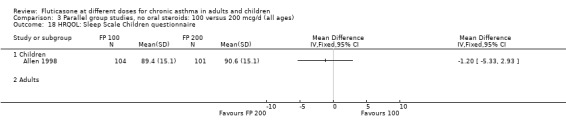
Comparison 3 Parallel group studies, no oral steroids: 100 versus 200 mcg/d (all ages), Outcome 18 HRQOL: Sleep Scale Children questionnaire.
3.19. Analysis.
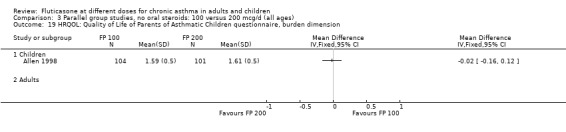
Comparison 3 Parallel group studies, no oral steroids: 100 versus 200 mcg/d (all ages), Outcome 19 HRQOL: Quality of Life of Parents of Asthmatic Children questionnaire, burden dimension.
3.20. Analysis.
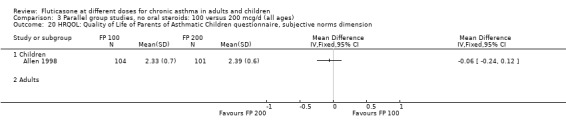
Comparison 3 Parallel group studies, no oral steroids: 100 versus 200 mcg/d (all ages), Outcome 20 HRQOL: Quality of Life of Parents of Asthmatic Children questionnaire, subjective norms dimension.
3.21. Analysis.
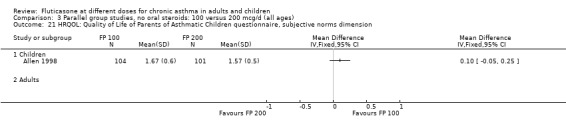
Comparison 3 Parallel group studies, no oral steroids: 100 versus 200 mcg/d (all ages), Outcome 21 HRQOL: Quality of Life of Parents of Asthmatic Children questionnaire, subjective norms dimension.
3.22. Analysis.
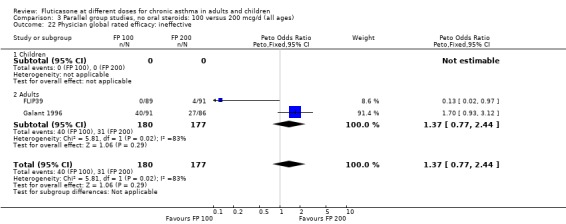
Comparison 3 Parallel group studies, no oral steroids: 100 versus 200 mcg/d (all ages), Outcome 22 Physician global rated efficacy: ineffective.
3.23. Analysis.
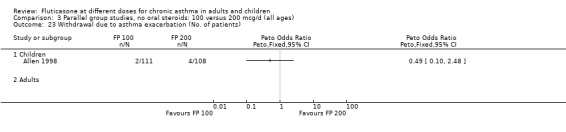
Comparison 3 Parallel group studies, no oral steroids: 100 versus 200 mcg/d (all ages), Outcome 23 Withdrawal due to asthma exacerbation (No. of patients).
3.24. Analysis.
Comparison 3 Parallel group studies, no oral steroids: 100 versus 200 mcg/d (all ages), Outcome 24 Withdrawals due to adverse events.
3.25. Analysis.
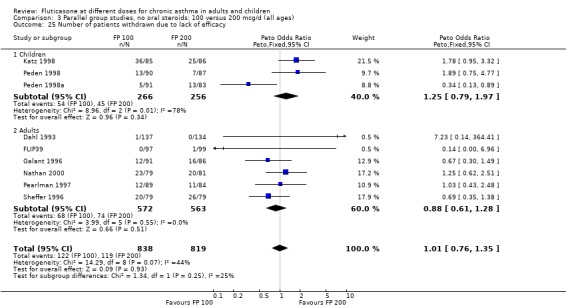
Comparison 3 Parallel group studies, no oral steroids: 100 versus 200 mcg/d (all ages), Outcome 25 Number of patients withdrawn due to lack of efficacy.
3.26. Analysis.
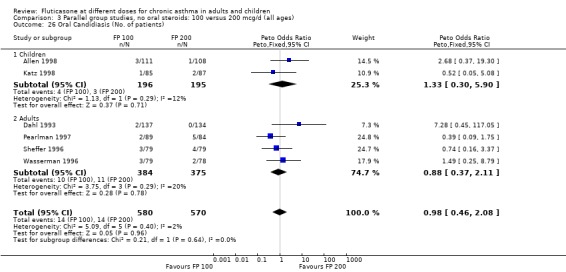
Comparison 3 Parallel group studies, no oral steroids: 100 versus 200 mcg/d (all ages), Outcome 26 Oral Candidiasis (No. of patients).
3.27. Analysis.
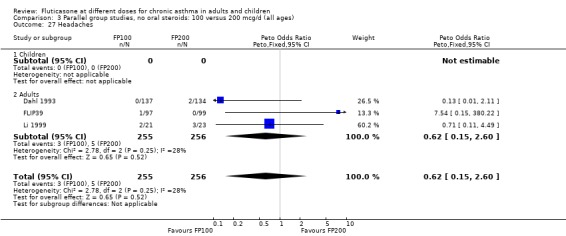
Comparison 3 Parallel group studies, no oral steroids: 100 versus 200 mcg/d (all ages), Outcome 27 Headaches.
3.28. Analysis.
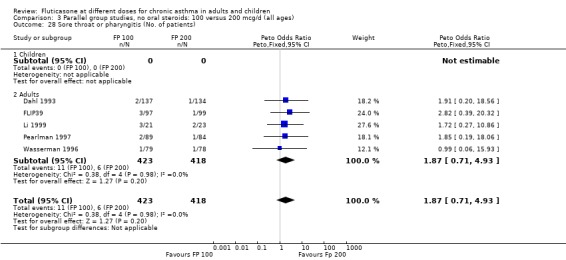
Comparison 3 Parallel group studies, no oral steroids: 100 versus 200 mcg/d (all ages), Outcome 28 Sore throat or pharyngitis (No. of patients).
3.29. Analysis.
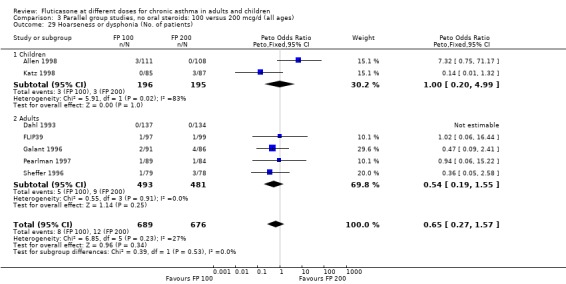
Comparison 3 Parallel group studies, no oral steroids: 100 versus 200 mcg/d (all ages), Outcome 29 Hoarseness or dysphonia (No. of patients).
3.30. Analysis.
Comparison 3 Parallel group studies, no oral steroids: 100 versus 200 mcg/d (all ages), Outcome 30 Urinary free cortisol excretion (mcg/24 hours).
3.31. Analysis.
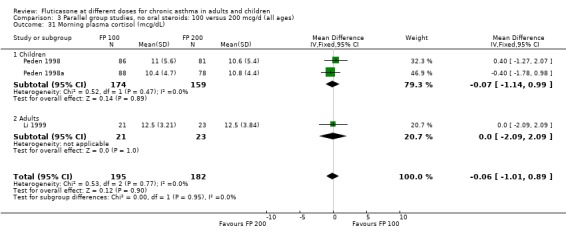
Comparison 3 Parallel group studies, no oral steroids: 100 versus 200 mcg/d (all ages), Outcome 31 Morning plasma cortisol (mcg/dL).
Comparison 4. Parallel group studies, no oral steroids: 100 versus 200 mcg/d (subgroups).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Change in FEV1 compared to baseline based on study duration(litres) ‐ children | 3 | 490 | Litres (Fixed, 95% CI) | ‐0.01 [‐0.07, 0.05] |
| 1.1 1‐4 weeks | 0 | 0 | Litres (Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 1.2 1‐5 months | 3 | 490 | Litres (Fixed, 95% CI) | ‐0.01 [‐0.07, 0.05] |
| 1.3 6 months or more | 0 | 0 | Litres (Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2 Change in FEV1 compared to baseline based on study duration (litres) ‐ adults | 6 | Litres (Fixed, 95% CI) | 0.03 [‐0.02, 0.07] | |
| 2.1 1‐4 weeks | 0 | Litres (Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.2 1‐5 months | 6 | Litres (Fixed, 95% CI) | 0.03 [‐0.02, 0.07] | |
| 2.3 6 months or more | 0 | Litres (Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Change in FEV1 compared to baseline (litres) based on delivery devices ‐ children | 3 | Litres (Fixed, 95% CI) | ‐0.01 [‐0.07, 0.05] | |
| 3.1 MDI | 0 | Litres (Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.2 DPI | 3 | Litres (Fixed, 95% CI) | ‐0.01 [‐0.07, 0.05] | |
| 4 Change in FEV1 compared to baseline based on delivery devices (litres) ‐ adults | 6 | Litres (Fixed, 95% CI) | 0.03 [‐0.02, 0.07] | |
| 4.1 MDI | 3 | Litres (Fixed, 95% CI) | 0.02 [‐0.08, 0.12] | |
| 4.2 DPI | 3 | Litres (Fixed, 95% CI) | 0.03 [‐0.02, 0.08] | |
| 5 Change in FEV1 compared to baseline based on degrees of severity (litres) ‐ children | 3 | Litres (Fixed, 95% CI) | ‐0.01 [‐0.07, 0.05] | |
| 5.1 Mild to moderate | 0 | Litres (Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.2 Moderate | 3 | Litres (Fixed, 95% CI) | ‐0.01 [‐0.07, 0.05] | |
| 6 Change in FEV1 compared to baseline based on degrees of severity (litres) ‐ adults | 6 | Litres (Fixed, 95% CI) | 0.03 [‐0.02, 0.07] | |
| 6.1 Mild to moderate | 0 | Litres (Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 6.2 Moderate | 6 | Litres (Fixed, 95% CI) | 0.03 [‐0.02, 0.07] |
4.1. Analysis.
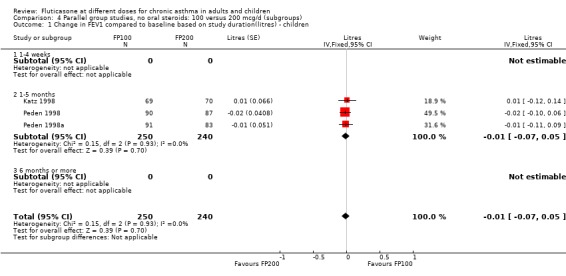
Comparison 4 Parallel group studies, no oral steroids: 100 versus 200 mcg/d (subgroups), Outcome 1 Change in FEV1 compared to baseline based on study duration(litres) ‐ children.
4.2. Analysis.
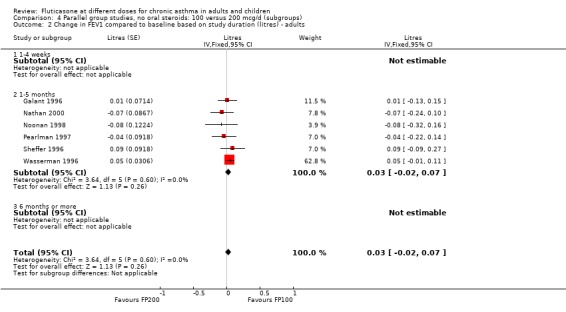
Comparison 4 Parallel group studies, no oral steroids: 100 versus 200 mcg/d (subgroups), Outcome 2 Change in FEV1 compared to baseline based on study duration (litres) ‐ adults.
4.3. Analysis.
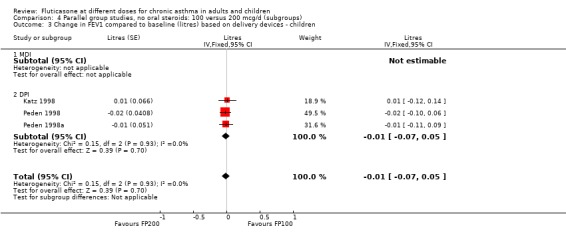
Comparison 4 Parallel group studies, no oral steroids: 100 versus 200 mcg/d (subgroups), Outcome 3 Change in FEV1 compared to baseline (litres) based on delivery devices ‐ children.
4.4. Analysis.
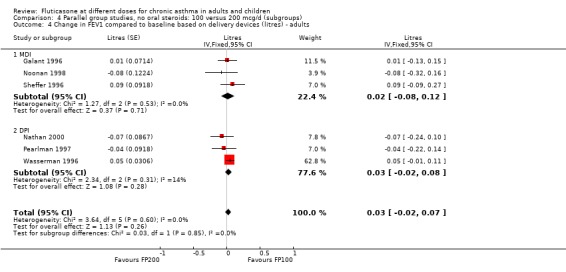
Comparison 4 Parallel group studies, no oral steroids: 100 versus 200 mcg/d (subgroups), Outcome 4 Change in FEV1 compared to baseline based on delivery devices (litres) ‐ adults.
4.5. Analysis.
Comparison 4 Parallel group studies, no oral steroids: 100 versus 200 mcg/d (subgroups), Outcome 5 Change in FEV1 compared to baseline based on degrees of severity (litres) ‐ children.
4.6. Analysis.
Comparison 4 Parallel group studies, no oral steroids: 100 versus 200 mcg/d (subgroups), Outcome 6 Change in FEV1 compared to baseline based on degrees of severity (litres) ‐ adults.
Comparison 5. Parallel group studies, no oral steroids: 200 versus 400‐500 mcg/d (all ages).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 FEV1 (% predicted) | 4 | 551 | Mean Difference (IV, Fixed, 95% CI) | ‐0.96 [‐3.45, 1.53] |
| 1.1 Children | 2 | 114 | Mean Difference (IV, Fixed, 95% CI) | ‐2.48 [‐8.60, 3.64] |
| 1.2 Adults | 2 | 437 | Mean Difference (IV, Fixed, 95% CI) | ‐0.66 [‐3.39, 2.06] |
| 2 Change in FEV1 compared to baseline (litres) | 9 | 1283 | Mean Difference (IV, Fixed, 95% CI) | ‐0.01 [‐0.06, 0.04] |
| 2.1 Children | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2.2 Adults | 9 | 1283 | Mean Difference (IV, Fixed, 95% CI) | ‐0.01 [‐0.06, 0.04] |
| 3 Change in FEV1 compared to baseline (litres ‐ imputed estimates) | 11 | 1720 | Mean Difference (IV, Fixed, 95% CI) | 0.01 [‐0.04, 0.05] |
| 3.1 Children | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 3.2 Adults | 11 | 1720 | Mean Difference (IV, Fixed, 95% CI) | 0.01 [‐0.04, 0.05] |
| 4 Change in FVC compared to baseline (litres) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 4.1 Children | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.2 Adults | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5 Change in FEF25‐75 compared to baseline (L/second) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 5.1 Children | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.2 Adults | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 6 Change in morning PEFR compared to baseline (L/min ‐ imputed/estimated values) | 13 | Litres/min (Fixed, 95% CI) | Subtotals only | |
| 6.1 Children | 2 | 876 | Litres/min (Fixed, 95% CI) | ‐7.92 [‐12.93, ‐2.91] |
| 6.2 Adults | 11 | 1713 | Litres/min (Fixed, 95% CI) | ‐1.97 [‐5.77, 1.82] |
| 7 Change in morning PEFR compared to baseline (L/min) | 13 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 7.1 Children | 2 | 876 | Mean Difference (IV, Fixed, 95% CI) | ‐7.92 [‐12.93, ‐2.91] |
| 7.2 Adults | 11 | 1703 | Mean Difference (IV, Fixed, 95% CI) | ‐4.84 [‐9.37, ‐0.31] |
| 8 Change in evening PEFR compared to baseline (L/min) | 7 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 8.1 Children | 2 | 874 | Mean Difference (IV, Fixed, 95% CI) | ‐9.36 [‐14.37, ‐4.35] |
| 8.2 Adults | 5 | 776 | Mean Difference (IV, Fixed, 95% CI) | ‐3.76 [‐9.18, 1.66] |
| 9 PC20 | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 9.1 Children | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 9.2 Adults | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 10 PD20 | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 10.1 Children | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 10.2 Adults | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 11 Change in daily use of beta2 agonist compared to baseline (puffs/d) | 7 | 931 | Mean Difference (IV, Fixed, 95% CI) | 0.18 [‐0.08, 0.43] |
| 11.1 Children | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 11.2 Adults | 7 | 931 | Mean Difference (IV, Fixed, 95% CI) | 0.18 [‐0.08, 0.43] |
| 12 Change in number of night‐time awakenings/week compared to baseline | 2 | 334 | Mean Difference (IV, Fixed, 95% CI) | ‐0.01 [‐0.05, 0.04] |
| 12.1 Children | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 12.2 Adults | 2 | 334 | Mean Difference (IV, Fixed, 95% CI) | ‐0.01 [‐0.05, 0.04] |
| 13 Change in % nights without waking | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 13.1 Children | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 13.2 Adults | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 14 Change in daily asthma symptom score compared to baseline | 7 | 930 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.11 [‐0.02, 0.24] |
| 14.1 Children | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 14.2 Adults | 7 | 930 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.11 [‐0.02, 0.24] |
| 15 Percentage of symptom‐free days | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 15.1 Children | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 15.2 Adults | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 16 Health related quality of life ‐ AQLQ (absolute scores) | 0 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 16.1 Children | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 16.2 Adults | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 17 Number of patients withdrawn due to lack of efficacy | 7 | 1294 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.41 [1.01, 1.98] |
| 17.1 Children | 0 | 0 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 17.2 Adults | 7 | 1294 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.41 [1.01, 1.98] |
| 18 Exacerbations requiring oral steroids | 2 | 883 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.21 [0.72, 2.05] |
| 18.1 Children | 2 | 883 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.21 [0.72, 2.05] |
| 18.2 Adults | 0 | 0 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 19 Exacerbations requiring hospitalisation | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 19.1 Children | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 19.2 Adults | 0 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 20 Oral Candidiasis (No. of patients) | 8 | 1752 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.87 [0.47, 1.61] |
| 20.1 Children | 1 | 528 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.65 [0.19, 2.27] |
| 20.2 Adults | 7 | 1224 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.95 [0.47, 1.95] |
| 21 Sore throat or pharyngitis (No. of patients) | 8 | 1825 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.98 [0.60, 1.63] |
| 21.1 Children | 2 | 883 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.34 [0.58, 3.08] |
| 21.2 Adults | 6 | 942 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.83 [0.44, 1.55] |
| 22 Hoarseness or dysphonia (No. of patients) | 7 | 1693 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.76 [0.40, 1.46] |
| 22.1 Children | 1 | 528 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.30 [0.29, 5.79] |
| 22.2 Adults | 6 | 1165 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.67 [0.32, 1.38] |
| 23 Withdrawals due to adverse events | 5 | 1161 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.65 [0.22, 1.92] |
| 23.1 Children | 1 | 528 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.14, 6.99] |
| 23.2 Adults | 4 | 633 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.55 [0.15, 2.03] |
| 24 No. patients with </=18 mcg/dL poststimulation cortisol | 2 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 24.1 Children | 0 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 24.2 Adults | 2 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 25 AUC serum cortisol | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 25.1 Children | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 25.2 Adults | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 26 Change in peak plasma cortisol expression | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 26.1 Children | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 26.2 Adults | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
5.2. Analysis.
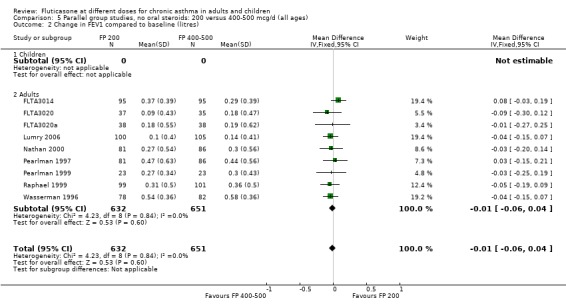
Comparison 5 Parallel group studies, no oral steroids: 200 versus 400‐500 mcg/d (all ages), Outcome 2 Change in FEV1 compared to baseline (litres).
5.4. Analysis.
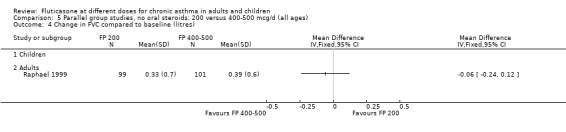
Comparison 5 Parallel group studies, no oral steroids: 200 versus 400‐500 mcg/d (all ages), Outcome 4 Change in FVC compared to baseline (litres).
5.5. Analysis.
Comparison 5 Parallel group studies, no oral steroids: 200 versus 400‐500 mcg/d (all ages), Outcome 5 Change in FEF25‐75 compared to baseline (L/second).
5.7. Analysis.
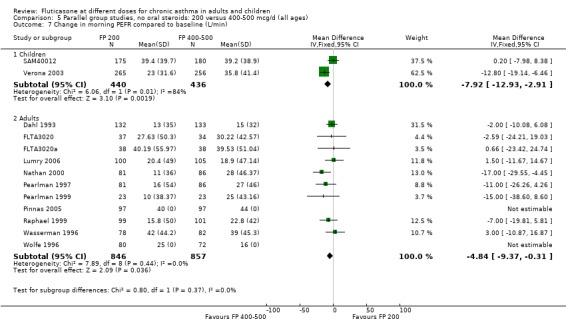
Comparison 5 Parallel group studies, no oral steroids: 200 versus 400‐500 mcg/d (all ages), Outcome 7 Change in morning PEFR compared to baseline (L/min).
5.9. Analysis.
Comparison 5 Parallel group studies, no oral steroids: 200 versus 400‐500 mcg/d (all ages), Outcome 9 PC20.
5.10. Analysis.
Comparison 5 Parallel group studies, no oral steroids: 200 versus 400‐500 mcg/d (all ages), Outcome 10 PD20.
5.13. Analysis.
Comparison 5 Parallel group studies, no oral steroids: 200 versus 400‐500 mcg/d (all ages), Outcome 13 Change in % nights without waking.
5.15. Analysis.
Comparison 5 Parallel group studies, no oral steroids: 200 versus 400‐500 mcg/d (all ages), Outcome 15 Percentage of symptom‐free days.
5.19. Analysis.
Comparison 5 Parallel group studies, no oral steroids: 200 versus 400‐500 mcg/d (all ages), Outcome 19 Exacerbations requiring hospitalisation.
5.20. Analysis.
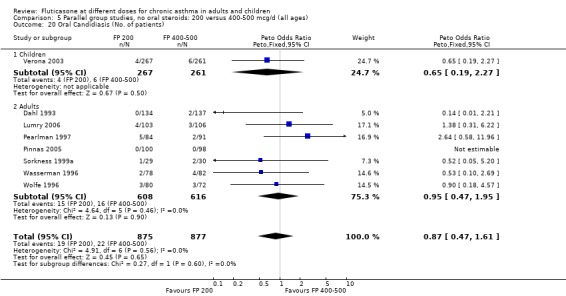
Comparison 5 Parallel group studies, no oral steroids: 200 versus 400‐500 mcg/d (all ages), Outcome 20 Oral Candidiasis (No. of patients).
5.21. Analysis.
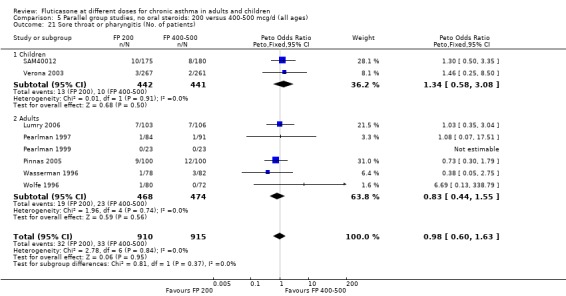
Comparison 5 Parallel group studies, no oral steroids: 200 versus 400‐500 mcg/d (all ages), Outcome 21 Sore throat or pharyngitis (No. of patients).
5.22. Analysis.
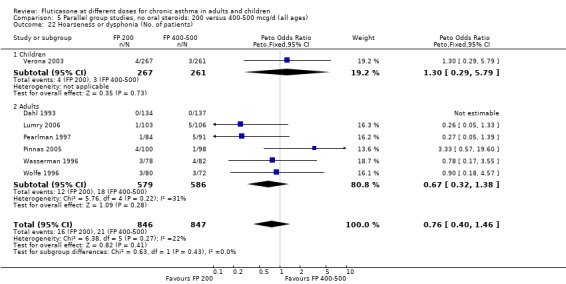
Comparison 5 Parallel group studies, no oral steroids: 200 versus 400‐500 mcg/d (all ages), Outcome 22 Hoarseness or dysphonia (No. of patients).
5.23. Analysis.
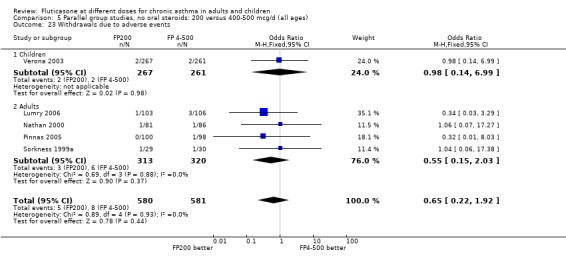
Comparison 5 Parallel group studies, no oral steroids: 200 versus 400‐500 mcg/d (all ages), Outcome 23 Withdrawals due to adverse events.
5.24. Analysis.
Comparison 5 Parallel group studies, no oral steroids: 200 versus 400‐500 mcg/d (all ages), Outcome 24 No. patients with </=18 mcg/dL poststimulation cortisol.
5.25. Analysis.
Comparison 5 Parallel group studies, no oral steroids: 200 versus 400‐500 mcg/d (all ages), Outcome 25 AUC serum cortisol.
5.26. Analysis.
Comparison 5 Parallel group studies, no oral steroids: 200 versus 400‐500 mcg/d (all ages), Outcome 26 Change in peak plasma cortisol expression.
Comparison 6. Parallel group studies, no oral steroids: 200 versus 400‐500 mcg/d (subgroups).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Change in FEV1 compared to baseline based on study duration (litres) ‐ adults | 11 | 1720 | Mean Difference (IV, Fixed, 95% CI) | 0.01 [‐0.04, 0.05] |
| 1.1 1‐4 weeks | 2 | 293 | Mean Difference (IV, Fixed, 95% CI) | 0.09 [‐0.01, 0.19] |
| 1.2 1‐5 months | 8 | 1237 | Mean Difference (IV, Fixed, 95% CI) | ‐0.03 [‐0.08, 0.02] |
| 1.3 6 months or longer | 1 | 190 | Mean Difference (IV, Fixed, 95% CI) | 0.08 [‐0.03, 0.19] |
| 2 Change in FEV1 compared to baseline based on delivery devices (litres) ‐ adults | 11 | 1720 | Mean Difference (IV, Fixed, 95% CI) | 0.01 [‐0.04, 0.05] |
| 2.1 MDI | 8 | 1226 | Mean Difference (IV, Fixed, 95% CI) | 0.02 [‐0.03, 0.07] |
| 2.2 DPI | 3 | 494 | Mean Difference (IV, Fixed, 95% CI) | ‐0.02 [‐0.11, 0.06] |
| 3 Change in FEV1 compared to baseline based on degrees of severity (litres) ‐ adults | 11 | 1720 | Mean Difference (IV, Fixed, 95% CI) | 0.01 [‐0.04, 0.05] |
| 3.1 Mild to moderate | 3 | 194 | Mean Difference (IV, Fixed, 95% CI) | ‐0.05 [‐0.18, 0.08] |
| 3.2 Moderate | 6 | 1136 | Mean Difference (IV, Fixed, 95% CI) | 0.01 [‐0.05, 0.06] |
| 3.3 Mixed | 1 | 200 | Mean Difference (IV, Fixed, 95% CI) | ‐0.05 [‐0.19, 0.09] |
| 3.4 Unclear | 1 | 190 | Mean Difference (IV, Fixed, 95% CI) | 0.08 [‐0.03, 0.19] |
6.1. Analysis.
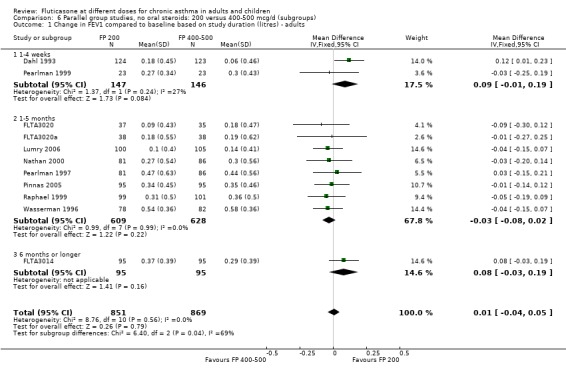
Comparison 6 Parallel group studies, no oral steroids: 200 versus 400‐500 mcg/d (subgroups), Outcome 1 Change in FEV1 compared to baseline based on study duration (litres) ‐ adults.
6.2. Analysis.
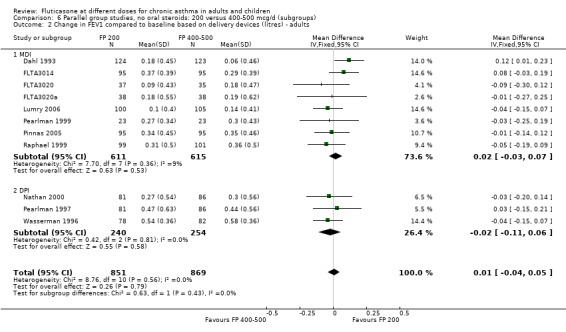
Comparison 6 Parallel group studies, no oral steroids: 200 versus 400‐500 mcg/d (subgroups), Outcome 2 Change in FEV1 compared to baseline based on delivery devices (litres) ‐ adults.
6.3. Analysis.
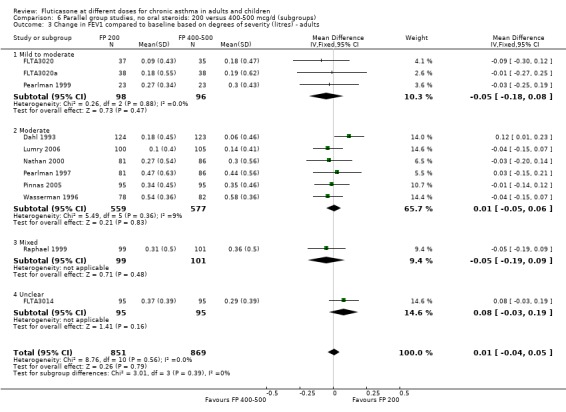
Comparison 6 Parallel group studies, no oral steroids: 200 versus 400‐500 mcg/d (subgroups), Outcome 3 Change in FEV1 compared to baseline based on degrees of severity (litres) ‐ adults.
Comparison 7. Parallel group studies, no oral steroids: 100 versus 400‐500 mcg/d (all ages).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Change in FEV1 compared to baseline (litres) | 3 | 351 | Mean Difference (IV, Fixed, 95% CI) | ‐0.01 [‐0.10, 0.09] |
| 1.1 Children | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 1.2 Adults | 3 | 351 | Mean Difference (IV, Fixed, 95% CI) | ‐0.01 [‐0.10, 0.09] |
| 2 FEV1 (% predicted) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2.1 Children | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.2 Adults | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Change in morning PEFR compared to baseline (L/min) | 3 | 593 | Mean Difference (IV, Fixed, 95% CI) | ‐7.97 [‐14.58, ‐1.35] |
| 3.1 Children | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 3.2 Adults | 3 | 593 | Mean Difference (IV, Fixed, 95% CI) | ‐7.97 [‐14.58, ‐1.35] |
| 4 Change in evening PEFR compared to baseline (L/min) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 4.1 Children | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.2 Adults | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5 Percentage of symptom‐free days | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 5.1 Children | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.2 Adults | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 6 Change in daily asthma symptom score compared to baseline | 2 | 194 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.31 [0.03, 0.60] |
| 6.1 Children | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 6.2 Adults | 2 | 194 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.31 [0.03, 0.60] |
| 7 Change in number of night‐time awakenings/week compared to baseline | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 7.1 Children | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 7.2 Adults | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 8 Change in daily use of beta2 agonist compared to baseline (puffs/d) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 8.1 Children | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 8.2 Adults | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 9 Physician global rated efficacy: ineffective | 2 | 400 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.25 [0.81, 1.93] |
| 9.1 Children | 0 | 0 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 9.2 Adults | 2 | 400 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.25 [0.81, 1.93] |
| 10 Number of patients withdrawn due to lack of efficacy | 2 | 454 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 2.30 [0.90, 5.90] |
| 10.1 Children | 0 | 0 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 10.2 Adults | 2 | 454 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 2.30 [0.90, 5.90] |
| 11 Sore throat or pharyngitis (No. of patients) | 3 | 615 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.02 [0.29, 3.56] |
| 11.1 Children | 0 | 0 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 11.2 Adults | 3 | 615 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.02 [0.29, 3.56] |
| 12 Hoarseness or dysphonia (No. of patients) | 3 | 615 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.28 [0.08, 0.92] |
| 12.1 Children | 0 | 0 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 12.2 Adults | 3 | 615 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.28 [0.08, 0.92] |
| 13 Oral Candidiasis (No. of patients) | 3 | 615 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.89 [0.32, 2.49] |
| 13.1 Children | 0 | 0 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 13.2 Adults | 3 | 615 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.89 [0.32, 2.49] |
| 14 Plasma Cortisol (AUC) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 14.1 Children | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 14.2 Adults | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 15 Clinic PEF (L/min ‐ change from baseline) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 15.1 Children | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 15.2 Adults | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
7.2. Analysis.
Comparison 7 Parallel group studies, no oral steroids: 100 versus 400‐500 mcg/d (all ages), Outcome 2 FEV1 (% predicted).
7.4. Analysis.
Comparison 7 Parallel group studies, no oral steroids: 100 versus 400‐500 mcg/d (all ages), Outcome 4 Change in evening PEFR compared to baseline (L/min).
7.5. Analysis.
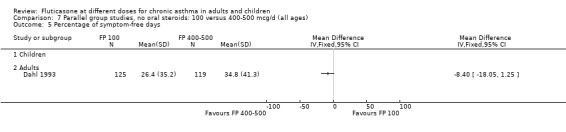
Comparison 7 Parallel group studies, no oral steroids: 100 versus 400‐500 mcg/d (all ages), Outcome 5 Percentage of symptom‐free days.
7.7. Analysis.
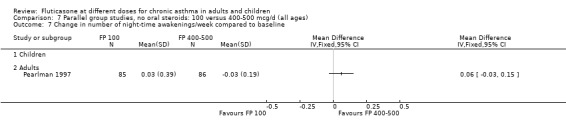
Comparison 7 Parallel group studies, no oral steroids: 100 versus 400‐500 mcg/d (all ages), Outcome 7 Change in number of night‐time awakenings/week compared to baseline.
7.8. Analysis.
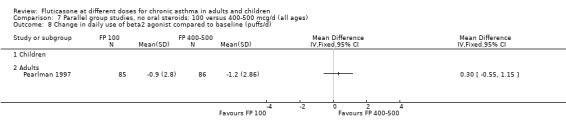
Comparison 7 Parallel group studies, no oral steroids: 100 versus 400‐500 mcg/d (all ages), Outcome 8 Change in daily use of beta2 agonist compared to baseline (puffs/d).
7.9. Analysis.
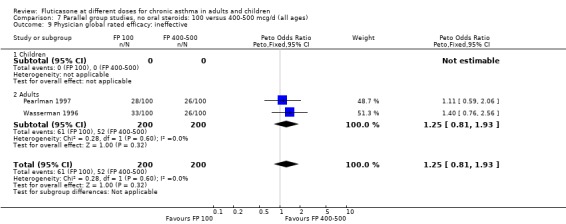
Comparison 7 Parallel group studies, no oral steroids: 100 versus 400‐500 mcg/d (all ages), Outcome 9 Physician global rated efficacy: ineffective.
7.10. Analysis.
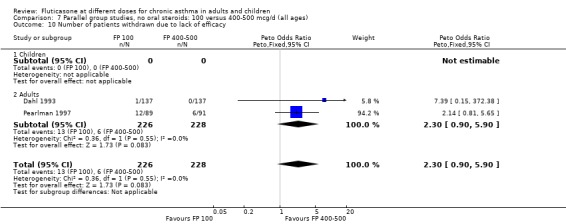
Comparison 7 Parallel group studies, no oral steroids: 100 versus 400‐500 mcg/d (all ages), Outcome 10 Number of patients withdrawn due to lack of efficacy.
7.11. Analysis.
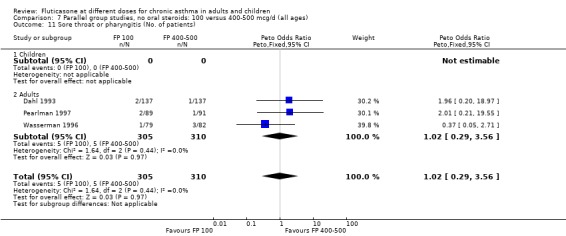
Comparison 7 Parallel group studies, no oral steroids: 100 versus 400‐500 mcg/d (all ages), Outcome 11 Sore throat or pharyngitis (No. of patients).
7.12. Analysis.
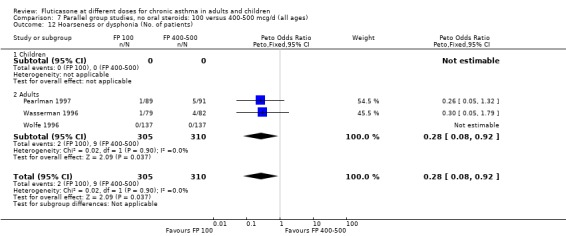
Comparison 7 Parallel group studies, no oral steroids: 100 versus 400‐500 mcg/d (all ages), Outcome 12 Hoarseness or dysphonia (No. of patients).
7.13. Analysis.
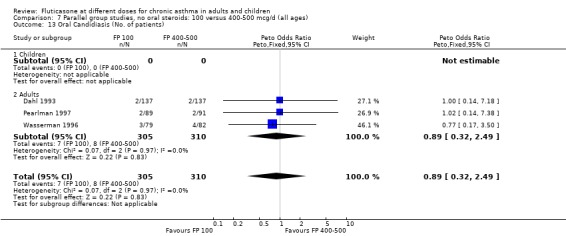
Comparison 7 Parallel group studies, no oral steroids: 100 versus 400‐500 mcg/d (all ages), Outcome 13 Oral Candidiasis (No. of patients).
7.14. Analysis.
Comparison 7 Parallel group studies, no oral steroids: 100 versus 400‐500 mcg/d (all ages), Outcome 14 Plasma Cortisol (AUC).
7.15. Analysis.
Comparison 7 Parallel group studies, no oral steroids: 100 versus 400‐500 mcg/d (all ages), Outcome 15 Clinic PEF (L/min ‐ change from baseline).
Comparison 8. Parallel group studies, no oral steroids: 100 versus 400‐500 mcg/d (subgroups).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Change in FEV1 compared to baseline based on study duration (litres) ‐ adults | 3 | 351 | Mean Difference (IV, Fixed, 95% CI) | ‐0.01 [‐0.10, 0.09] |
| 1.1 1‐4 weeks | 1 | 23 | Mean Difference (IV, Fixed, 95% CI) | ‐0.45 [‐0.88, ‐0.02] |
| 1.2 1‐5 months | 2 | 328 | Mean Difference (IV, Fixed, 95% CI) | 0.02 [‐0.08, 0.11] |
| 1.3 6 months or longer | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2 Change in FEV1 compared to baseline based on delivery devices (litres) ‐ adults | 3 | 351 | Mean Difference (IV, Fixed, 95% CI) | ‐0.01 [‐0.10, 0.09] |
| 2.1 MDI | 1 | 23 | Mean Difference (IV, Fixed, 95% CI) | ‐0.45 [‐0.88, ‐0.02] |
| 2.2 DPI | 2 | 328 | Mean Difference (IV, Fixed, 95% CI) | 0.02 [‐0.08, 0.11] |
| 3 Change in FEV1 compared to baseline based on degrees of severity (litres) ‐ adults | 3 | 351 | Mean Difference (IV, Fixed, 95% CI) | ‐0.01 [‐0.10, 0.09] |
| 3.1 Mild to moderate | 1 | 23 | Mean Difference (IV, Fixed, 95% CI) | ‐0.45 [‐0.88, ‐0.02] |
| 3.2 Moderate | 2 | 328 | Mean Difference (IV, Fixed, 95% CI) | 0.02 [‐0.08, 0.11] |
8.1. Analysis.
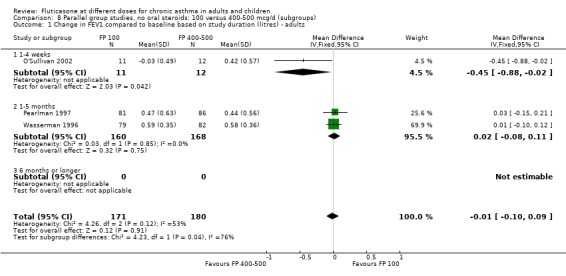
Comparison 8 Parallel group studies, no oral steroids: 100 versus 400‐500 mcg/d (subgroups), Outcome 1 Change in FEV1 compared to baseline based on study duration (litres) ‐ adults.
8.2. Analysis.
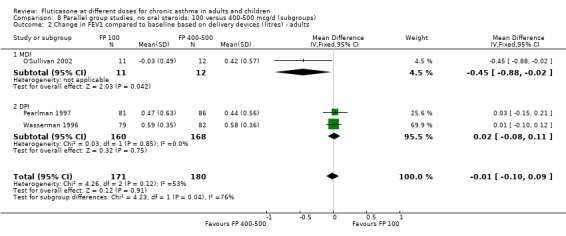
Comparison 8 Parallel group studies, no oral steroids: 100 versus 400‐500 mcg/d (subgroups), Outcome 2 Change in FEV1 compared to baseline based on delivery devices (litres) ‐ adults.
8.3. Analysis.
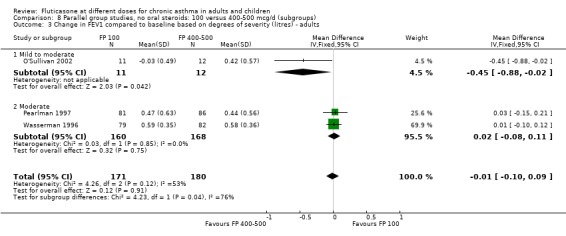
Comparison 8 Parallel group studies, no oral steroids: 100 versus 400‐500 mcg/d (subgroups), Outcome 3 Change in FEV1 compared to baseline based on degrees of severity (litres) ‐ adults.
Comparison 9. Parallel group studies, no oral steroids: 400‐500 versus 800‐1000 mcg/d (all ages).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 FEV1 (Change from baseline ‐ litres) | 5 | 900 | Mean Difference (IV, Fixed, 95% CI) | ‐0.01 [‐0.06, 0.04] |
| 1.1 Children | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 1.2 Adults | 5 | 900 | Mean Difference (IV, Fixed, 95% CI) | ‐0.01 [‐0.06, 0.04] |
| 2 Change in morning PEF (L/min) | 4 | 905 | Mean Difference (IV, Fixed, 95% CI) | ‐2.30 [‐7.94, 3.35] |
| 2.1 Children | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2.2 Adults | 4 | 905 | Mean Difference (IV, Fixed, 95% CI) | ‐2.30 [‐7.94, 3.35] |
| 3 Change in evening PEF (L/min) | 2 | 505 | Mean Difference (IV, Fixed, 95% CI) | 5.83 [‐2.94, 14.60] |
| 3.1 Children | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 3.2 Adults | 2 | 505 | Mean Difference (IV, Fixed, 95% CI) | 5.83 [‐2.94, 14.60] |
| 4 Change in rescue medication (puffs/d) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 4.1 Children | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.2 Adults | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5 Health‐related quality of life ‐ AQLQ (absolute scores) | 0 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 5.1 Children | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.2 Adults | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 6 Change in daily symptoms compared with baseline | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 6.1 Children | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 6.2 Adults | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 7 Nocturnal awakenings (awakenings per night) | 0 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 7.1 Children | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 7.2 Adults | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 8 Exacerbations requiring hospitalisation | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Totals not selected | |
| 8.1 Children | 0 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 8.2 Adults | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 9 Exacerbations requiring OCS treatment | 2 | 363 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.24 [0.77, 1.98] |
| 9.1 Children | 0 | 0 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 9.2 Adults | 2 | 363 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.24 [0.77, 1.98] |
| 10 Withdrawals (total) | 5 | 1039 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.27 [0.88, 1.83] |
| 10.1 Children | 0 | 0 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 10.2 Adults | 5 | 1039 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.27 [0.88, 1.83] |
| 11 Withdrawals (adverse events) | 4 | 841 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.46 [0.17, 1.25] |
| 11.1 Children | 0 | 0 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 11.2 Adults | 4 | 841 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.46 [0.17, 1.25] |
| 12 Withdrawals (lack of efficacy) | 2 | 537 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.67 [0.73, 3.81] |
| 12.1 Children | 0 | 0 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 12.2 Adults | 2 | 537 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.67 [0.73, 3.81] |
| 13 Drug‐related adverse events | 0 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Totals not selected | |
| 13.1 Children | 0 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 13.2 Adults | 0 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 14 HPA function data (am cortisol <5mcg/dL) | 0 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Totals not selected | |
| 14.1 Children | 0 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 14.2 Adults | 0 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
9.4. Analysis.
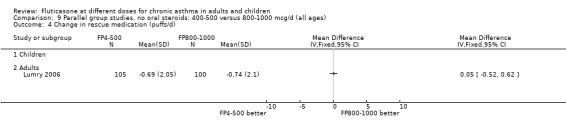
Comparison 9 Parallel group studies, no oral steroids: 400‐500 versus 800‐1000 mcg/d (all ages), Outcome 4 Change in rescue medication (puffs/d).
9.6. Analysis.
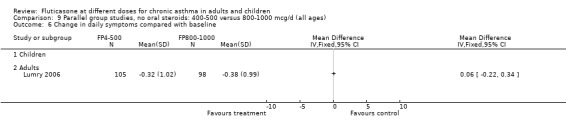
Comparison 9 Parallel group studies, no oral steroids: 400‐500 versus 800‐1000 mcg/d (all ages), Outcome 6 Change in daily symptoms compared with baseline.
9.8. Analysis.
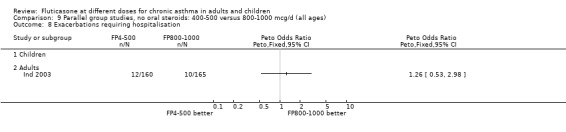
Comparison 9 Parallel group studies, no oral steroids: 400‐500 versus 800‐1000 mcg/d (all ages), Outcome 8 Exacerbations requiring hospitalisation.
9.12. Analysis.
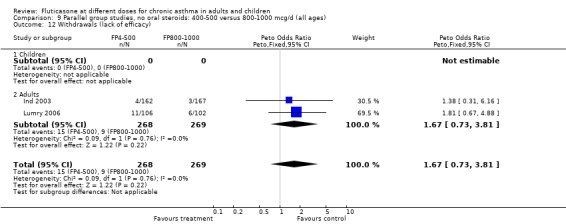
Comparison 9 Parallel group studies, no oral steroids: 400‐500 versus 800‐1000 mcg/d (all ages), Outcome 12 Withdrawals (lack of efficacy).
Comparison 10. Parallel group studies, no oral steroids: 400‐500 versus 800‐1000 mcg/d (subgroups).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Change from baseline in FEV1 based on study duration (litres) ‐ adults | 5 | 900 | Mean Difference (IV, Fixed, 95% CI) | ‐0.01 [‐0.06, 0.04] |
| 1.1 1‐4 weeks | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 1.2 1‐5 months | 3 | 435 | Mean Difference (IV, Fixed, 95% CI) | ‐0.02 [‐0.09, 0.05] |
| 1.3 6 months or longer | 2 | 465 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [‐0.07, 0.07] |
| 2 Change from baseline in FEV1 based on delivery devices (litres) ‐ adults | 5 | 900 | Mean Difference (IV, Fixed, 95% CI) | ‐0.01 [‐0.06, 0.04] |
| 2.1 MDI | 3 | 435 | Mean Difference (IV, Fixed, 95% CI) | ‐0.02 [‐0.09, 0.05] |
| 2.2 DPI | 2 | 465 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [‐0.07, 0.07] |
| 3 Change from baseline in FEV1 based on degree of severity (litres) ‐ adults | 5 | 900 | Mean Difference (IV, Fixed, 95% CI) | ‐0.01 [‐0.06, 0.04] |
| 3.1 Mild to moderate | 1 | 38 | Mean Difference (IV, Fixed, 95% CI) | 0.1 [‐0.08, 0.28] |
| 3.2 Moderate | 3 | 575 | Mean Difference (IV, Fixed, 95% CI) | ‐0.03 [‐0.10, 0.04] |
| 3.3 Moderate to severe | 1 | 287 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [‐0.09, 0.09] |
10.1. Analysis.
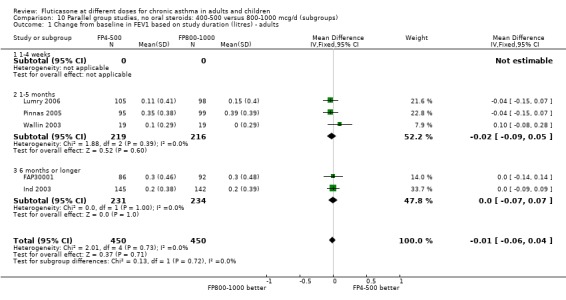
Comparison 10 Parallel group studies, no oral steroids: 400‐500 versus 800‐1000 mcg/d (subgroups), Outcome 1 Change from baseline in FEV1 based on study duration (litres) ‐ adults.
10.2. Analysis.
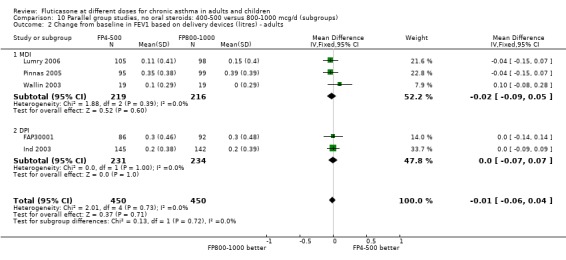
Comparison 10 Parallel group studies, no oral steroids: 400‐500 versus 800‐1000 mcg/d (subgroups), Outcome 2 Change from baseline in FEV1 based on delivery devices (litres) ‐ adults.
10.3. Analysis.
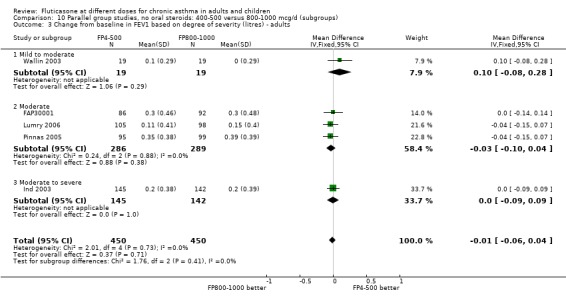
Comparison 10 Parallel group studies, no oral steroids: 400‐500 versus 800‐1000 mcg/d (subgroups), Outcome 3 Change from baseline in FEV1 based on degree of severity (litres) ‐ adults.
Comparison 11. Parallel group studies, no oral steroids: 50‐100 versus 800‐1000 mcg/d (all ages).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Change in FEV1 compared to baseline (litres) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 1.1 Children | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 Adults | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 FEV1 (% predicted) | 2 | 283 | Mean Difference (IV, Fixed, 95% CI) | ‐0.43 [‐5.72, 4.87] |
| 2.1 Children | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2.2 Adults | 2 | 283 | Mean Difference (IV, Fixed, 95% CI) | ‐0.43 [‐5.72, 4.87] |
| 3 FEV1 (Litres) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 3.1 Children | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.2 Adults | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4 Change in FVC compared to baseline (litres) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 4.1 Children | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.2 Adults | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5 FEF25‐75 | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 5.1 Children | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.2 Adults | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 6 Change in FEF25‐75 compared to baseline (L/second) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 6.1 Children | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 6.2 Adults | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 7 am PEF (Litres/min) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 7.1 Children | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 7.2 Adults | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 8 Change in morning PEFR compared to baseline (L/min) | 2 | 419 | Mean Difference (IV, Fixed, 95% CI) | ‐21.86 [‐29.19, ‐14.53] |
| 8.1 Children | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 8.2 Adults | 2 | 419 | Mean Difference (IV, Fixed, 95% CI) | ‐21.86 [‐29.19, ‐14.53] |
| 9 Change in evening PEFR compared to baseline (L/min) | 2 | 419 | Mean Difference (IV, Fixed, 95% CI) | ‐12.66 [‐19.32, ‐5.99] |
| 9.1 Children | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 9.2 Adults | 2 | 419 | Mean Difference (IV, Fixed, 95% CI) | ‐12.66 [‐19.32, ‐5.99] |
| 10 Physician global rated efficacy: ineffective | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Totals not selected | |
| 10.1 Children | 0 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 10.2 Adults | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 11 Symptoms | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 11.1 Children | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 11.2 Adults | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 12 Percentage of symptom‐free days | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 12.1 Children | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 12.2 Adults | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 13 Change in daily asthma symptom score compared to baseline | 1 | Std. Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 13.1 Children | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 13.2 Adults | 1 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 14 Change in number of night‐time awakenings/week compared to baseline | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 14.1 Children | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 14.2 Adults | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 15 Rescue medication usage | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 15.1 Children | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 15.2 Adults | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 16 Change in daily use of beta2 agonist compared to baseline (puffs/d) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 16.1 Children | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 16.2 Adults | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 17 Oral Candidiasis (No. of patients) | 2 | 433 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.32 [0.11, 0.97] |
| 17.1 Children | 0 | 0 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 17.2 Adults | 2 | 433 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.32 [0.11, 0.97] |
| 18 Hoarseness or dysphonia (No. of patients) | 2 | 433 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.18 [0.05, 0.59] |
| 18.1 Children | 0 | 0 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 18.2 Adults | 2 | 433 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.18 [0.05, 0.59] |
| 19 Withdrawals (total) | 2 | 294 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 5.29 [1.59, 17.60] |
| 19.1 Children | 0 | 0 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 19.2 Adults | 2 | 294 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 5.29 [1.59, 17.60] |
| 20 Number of patients withdrawn due to lack of efficacy | 2 | 427 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 5.31 [2.18, 12.96] |
| 20.1 Children | 0 | 0 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 20.2 Adults | 2 | 427 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 5.31 [2.18, 12.96] |
11.1. Analysis.
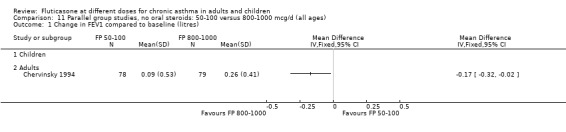
Comparison 11 Parallel group studies, no oral steroids: 50‐100 versus 800‐1000 mcg/d (all ages), Outcome 1 Change in FEV1 compared to baseline (litres).
11.3. Analysis.
Comparison 11 Parallel group studies, no oral steroids: 50‐100 versus 800‐1000 mcg/d (all ages), Outcome 3 FEV1 (Litres).
11.4. Analysis.
Comparison 11 Parallel group studies, no oral steroids: 50‐100 versus 800‐1000 mcg/d (all ages), Outcome 4 Change in FVC compared to baseline (litres).
11.5. Analysis.
Comparison 11 Parallel group studies, no oral steroids: 50‐100 versus 800‐1000 mcg/d (all ages), Outcome 5 FEF25‐75.
11.6. Analysis.
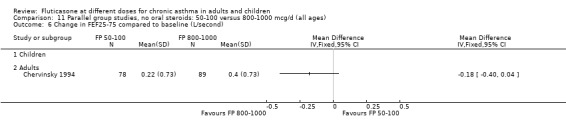
Comparison 11 Parallel group studies, no oral steroids: 50‐100 versus 800‐1000 mcg/d (all ages), Outcome 6 Change in FEF25‐75 compared to baseline (L/second).
11.7. Analysis.
Comparison 11 Parallel group studies, no oral steroids: 50‐100 versus 800‐1000 mcg/d (all ages), Outcome 7 am PEF (Litres/min).
11.10. Analysis.
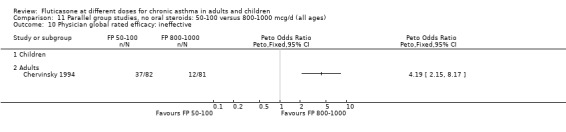
Comparison 11 Parallel group studies, no oral steroids: 50‐100 versus 800‐1000 mcg/d (all ages), Outcome 10 Physician global rated efficacy: ineffective.
11.11. Analysis.
Comparison 11 Parallel group studies, no oral steroids: 50‐100 versus 800‐1000 mcg/d (all ages), Outcome 11 Symptoms.
11.12. Analysis.
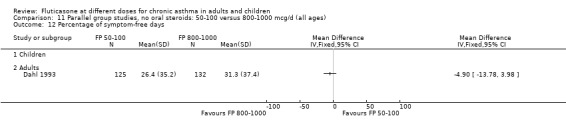
Comparison 11 Parallel group studies, no oral steroids: 50‐100 versus 800‐1000 mcg/d (all ages), Outcome 12 Percentage of symptom‐free days.
11.13. Analysis.
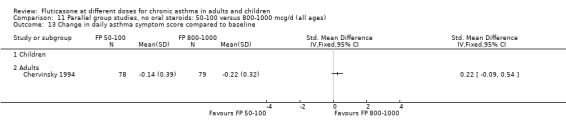
Comparison 11 Parallel group studies, no oral steroids: 50‐100 versus 800‐1000 mcg/d (all ages), Outcome 13 Change in daily asthma symptom score compared to baseline.
11.14. Analysis.
Comparison 11 Parallel group studies, no oral steroids: 50‐100 versus 800‐1000 mcg/d (all ages), Outcome 14 Change in number of night‐time awakenings/week compared to baseline.
11.15. Analysis.
Comparison 11 Parallel group studies, no oral steroids: 50‐100 versus 800‐1000 mcg/d (all ages), Outcome 15 Rescue medication usage.
11.16. Analysis.
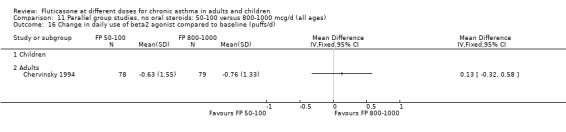
Comparison 11 Parallel group studies, no oral steroids: 50‐100 versus 800‐1000 mcg/d (all ages), Outcome 16 Change in daily use of beta2 agonist compared to baseline (puffs/d).
11.17. Analysis.
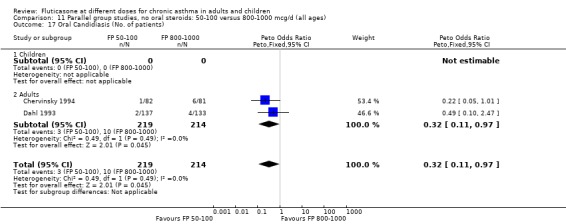
Comparison 11 Parallel group studies, no oral steroids: 50‐100 versus 800‐1000 mcg/d (all ages), Outcome 17 Oral Candidiasis (No. of patients).
11.18. Analysis.
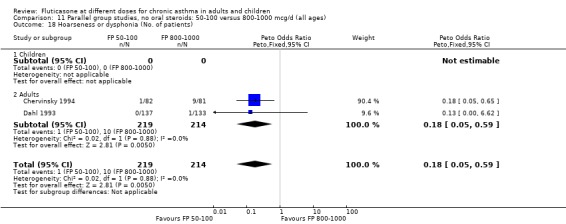
Comparison 11 Parallel group studies, no oral steroids: 50‐100 versus 800‐1000 mcg/d (all ages), Outcome 18 Hoarseness or dysphonia (No. of patients).
Comparison 12. Parallel group studies, no oral steroids: 200 versus 800‐1000 mcg/d (all ages).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Change in FEV1 compared to baseline (litres) | 3 | 489 | Mean Difference (IV, Fixed, 95% CI) | ‐0.11 [‐0.19, ‐0.04] |
| 1.1 Children | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 1.2 Adults | 3 | 489 | Mean Difference (IV, Fixed, 95% CI) | ‐0.11 [‐0.19, ‐0.04] |
| 2 Change in FEV1 compared to baseline (%) | 1 | Std. Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2.1 Children | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.2 Adults | 1 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Change in FEV1 % predicted | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 3.1 Children | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.2 Adults | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4 FEV1 (% predicted) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 4.1 Children | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.2 Adults | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5 Change in morning PEFR compared to baseline (L/min) | 4 | 763 | Mean Difference (IV, Random, 95% CI) | ‐8.32 [‐18.02, 1.37] |
| 5.1 Children | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 5.2 Adults | 4 | 763 | Mean Difference (IV, Random, 95% CI) | ‐8.32 [‐18.02, 1.37] |
| 6 Change in evening PEFR compared to baseline (L/min) | 2 | 424 | Mean Difference (IV, Fixed, 95% CI) | ‐7.81 [‐14.66, ‐0.95] |
| 6.1 Children | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 6.2 Adults | 2 | 424 | Mean Difference (IV, Fixed, 95% CI) | ‐7.81 [‐14.66, ‐0.95] |
| 7 Change in FVC compared to baseline (litres) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 7.1 Children | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 7.2 Adults | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 8 Change in FEF25‐75 compared to baseline (L/second) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 8.1 Children | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 8.2 Adults | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 9 PD20 | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 9.1 Children | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 9.2 Adults | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 10 Change in daily asthma symptom score compared to baseline | 2 | 291 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.06 [‐0.17, 0.29] |
| 10.1 Children | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 10.2 Adults | 2 | 291 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.06 [‐0.17, 0.29] |
| 11 Change in number of night‐time awakenings/week compared to baseline | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 11.1 Children | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 11.2 Adults | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 12 Physician global rated efficacy: ineffective | 2 | 400 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.34 [0.85, 2.12] |
| 12.1 Children | 0 | 0 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 12.2 Adults | 2 | 400 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.34 [0.85, 2.12] |
| 13 Change in daily use of beta2 agonist compared to baseline (puffs/d) | 2 | 291 | Mean Difference (IV, Fixed, 95% CI) | 0.11 [‐0.29, 0.50] |
| 13.1 Children | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 13.2 Adults | 2 | 291 | Mean Difference (IV, Fixed, 95% CI) | 0.11 [‐0.29, 0.50] |
| 14 Number of patients withdrawn due to lack of efficacy | 4 | 554 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.32 [0.73, 2.40] |
| 14.1 Children | 0 | 0 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 14.2 Adults | 4 | 554 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.32 [0.73, 2.40] |
| 15 Oral Candidiasis (No. of patients) | 5 | 618 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.33 [0.16, 0.70] |
| 15.1 Children | 0 | 0 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 15.2 Adults | 5 | 618 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.33 [0.16, 0.70] |
| 16 Sore throat or pharyngitis (No. of patients) | 4 | 452 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.49 [0.18, 1.39] |
| 16.1 Children | 0 | 0 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 16.2 Adults | 4 | 452 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.49 [0.18, 1.39] |
| 17 Hoarseness or dysphonia (No. of patients) | 4 | 561 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.51 [0.24, 1.08] |
| 17.1 Children | 0 | 0 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 17.2 Adults | 4 | 561 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.51 [0.24, 1.08] |
| 18 Change in peak plasma cortisol compared to baseline (mcg/dL) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 18.1 Children | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 18.2 Adults | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 19 Change in morning plasma cortisol compared to baseline (mcg/dL) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 19.1 Children | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 19.2 Adults | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
12.2. Analysis.
Comparison 12 Parallel group studies, no oral steroids: 200 versus 800‐1000 mcg/d (all ages), Outcome 2 Change in FEV1 compared to baseline (%).
12.3. Analysis.
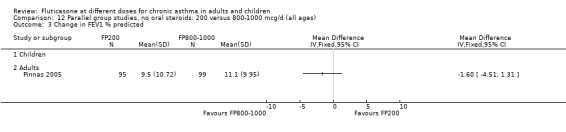
Comparison 12 Parallel group studies, no oral steroids: 200 versus 800‐1000 mcg/d (all ages), Outcome 3 Change in FEV1 % predicted.
12.4. Analysis.
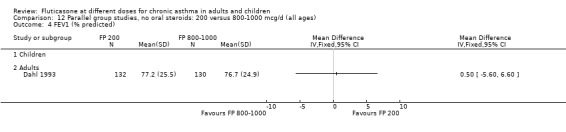
Comparison 12 Parallel group studies, no oral steroids: 200 versus 800‐1000 mcg/d (all ages), Outcome 4 FEV1 (% predicted).
12.7. Analysis.
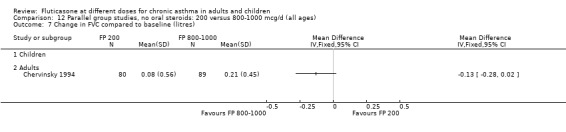
Comparison 12 Parallel group studies, no oral steroids: 200 versus 800‐1000 mcg/d (all ages), Outcome 7 Change in FVC compared to baseline (litres).
12.8. Analysis.
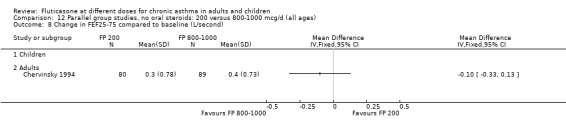
Comparison 12 Parallel group studies, no oral steroids: 200 versus 800‐1000 mcg/d (all ages), Outcome 8 Change in FEF25‐75 compared to baseline (L/second).
12.9. Analysis.
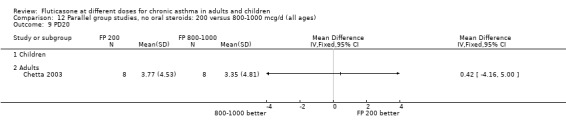
Comparison 12 Parallel group studies, no oral steroids: 200 versus 800‐1000 mcg/d (all ages), Outcome 9 PD20.
12.11. Analysis.
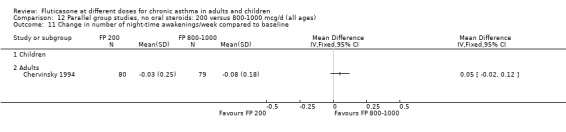
Comparison 12 Parallel group studies, no oral steroids: 200 versus 800‐1000 mcg/d (all ages), Outcome 11 Change in number of night‐time awakenings/week compared to baseline.
12.15. Analysis.
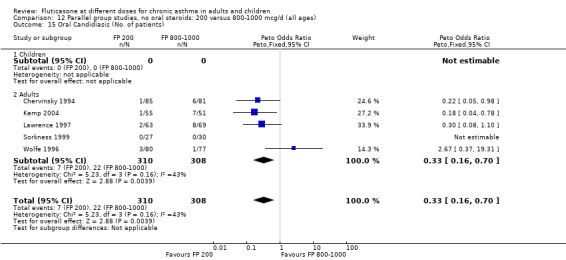
Comparison 12 Parallel group studies, no oral steroids: 200 versus 800‐1000 mcg/d (all ages), Outcome 15 Oral Candidiasis (No. of patients).
12.16. Analysis.
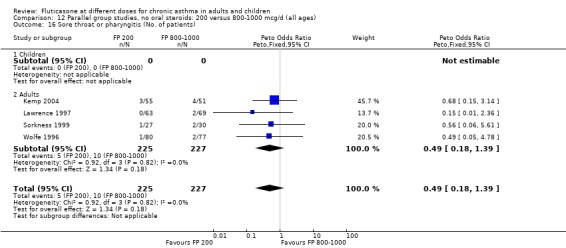
Comparison 12 Parallel group studies, no oral steroids: 200 versus 800‐1000 mcg/d (all ages), Outcome 16 Sore throat or pharyngitis (No. of patients).
12.17. Analysis.
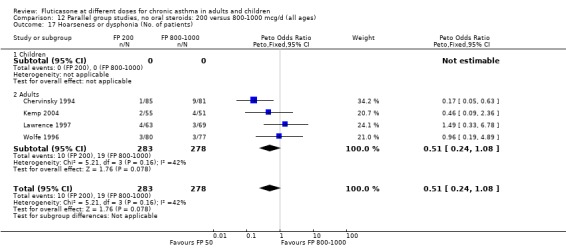
Comparison 12 Parallel group studies, no oral steroids: 200 versus 800‐1000 mcg/d (all ages), Outcome 17 Hoarseness or dysphonia (No. of patients).
12.18. Analysis.
Comparison 12 Parallel group studies, no oral steroids: 200 versus 800‐1000 mcg/d (all ages), Outcome 18 Change in peak plasma cortisol compared to baseline (mcg/dL).
12.19. Analysis.
Comparison 12 Parallel group studies, no oral steroids: 200 versus 800‐1000 mcg/d (all ages), Outcome 19 Change in morning plasma cortisol compared to baseline (mcg/dL).
Comparison 13. Parallel group studies, no oral steroids: 200 versus 800‐1000 mcg/d (subgroups).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Change in FEV1 compared to baseline based on study duration (litres) ‐ adults | 3 | 489 | Mean Difference (IV, Fixed, 95% CI) | ‐0.11 [‐0.19, ‐0.04] |
| 1.1 1‐4 weeks | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 1.2 1‐5 months | 3 | 489 | Mean Difference (IV, Fixed, 95% CI) | ‐0.11 [‐0.19, ‐0.04] |
| 1.3 6 months or longer | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2 Change in FEV1 compared to baseline based on delivery devices (litres) ‐ adults | 3 | 489 | Mean Difference (IV, Fixed, 95% CI) | ‐0.11 [‐0.19, ‐0.04] |
| 2.1 MDI | 2 | 357 | Mean Difference (IV, Fixed, 95% CI) | ‐0.10 [‐0.19, ‐0.02] |
| 2.2 DPI | 1 | 132 | Mean Difference (IV, Fixed, 95% CI) | ‐0.15 [‐0.32, 0.02] |
| 3 Change in FEV1 compared to baseline based on degrees of severity (litres) ‐ adults | 3 | 489 | Mean Difference (IV, Fixed, 95% CI) | ‐0.11 [‐0.19, ‐0.04] |
| 3.1 Mild to moderate | 1 | 159 | Mean Difference (IV, Fixed, 95% CI) | ‐0.12 [‐0.26, 0.02] |
| 3.2 Moderate | 2 | 330 | Mean Difference (IV, Fixed, 95% CI) | ‐0.11 [‐0.20, ‐0.02] |
13.1. Analysis.
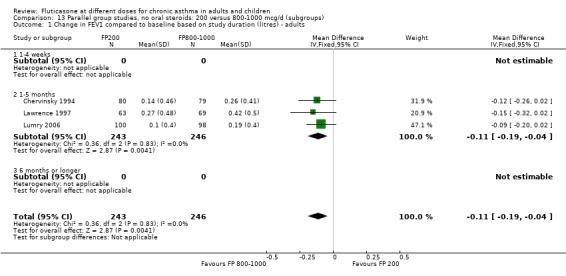
Comparison 13 Parallel group studies, no oral steroids: 200 versus 800‐1000 mcg/d (subgroups), Outcome 1 Change in FEV1 compared to baseline based on study duration (litres) ‐ adults.
13.2. Analysis.
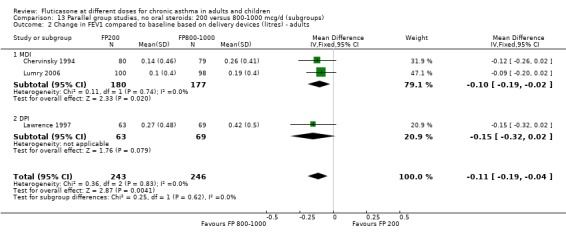
Comparison 13 Parallel group studies, no oral steroids: 200 versus 800‐1000 mcg/d (subgroups), Outcome 2 Change in FEV1 compared to baseline based on delivery devices (litres) ‐ adults.
13.3. Analysis.
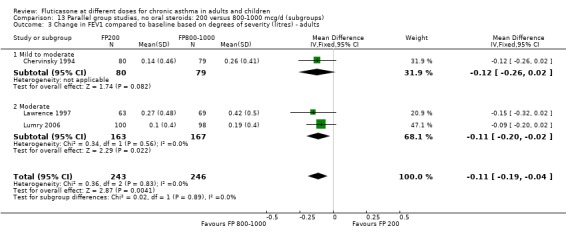
Comparison 13 Parallel group studies, no oral steroids: 200 versus 800‐1000 mcg/d (subgroups), Outcome 3 Change in FEV1 compared to baseline based on degrees of severity (litres) ‐ adults.
Comparison 14. Parallel group studies, no oral steroids: 4‐500 versus 1500‐2000mcg/d (all ages).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 FEV1 (Change from baseline ‐ litres) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 1.1 Children | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 Adults | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Clinic PEF (L/min ‐ change from baseline) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2.1 Children | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.2 Adults | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 PC20 (methacholine) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 3.1 Children | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.2 Adults | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4 PC20 AMP | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 4.1 Children | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.2 Adults | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5 Symptoms (change from baseline) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 5.1 Children | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.2 Adults | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
14.1. Analysis.
Comparison 14 Parallel group studies, no oral steroids: 4‐500 versus 1500‐2000mcg/d (all ages), Outcome 1 FEV1 (Change from baseline ‐ litres).
14.2. Analysis.
Comparison 14 Parallel group studies, no oral steroids: 4‐500 versus 1500‐2000mcg/d (all ages), Outcome 2 Clinic PEF (L/min ‐ change from baseline).
14.3. Analysis.
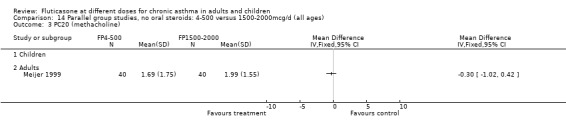
Comparison 14 Parallel group studies, no oral steroids: 4‐500 versus 1500‐2000mcg/d (all ages), Outcome 3 PC20 (methacholine).
14.4. Analysis.
Comparison 14 Parallel group studies, no oral steroids: 4‐500 versus 1500‐2000mcg/d (all ages), Outcome 4 PC20 AMP.
14.5. Analysis.
Comparison 14 Parallel group studies, no oral steroids: 4‐500 versus 1500‐2000mcg/d (all ages), Outcome 5 Symptoms (change from baseline).
Comparison 15. Parallel studies, oral steroid treated: 1000‐1500 versus 2000 mcg/d.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Change in daily dose of oral prednisolone compared to baseline (mg) | 4 | 274 | Mean Difference (IV, Fixed, 95% CI) | 1.00 [‐0.45, 2.45] |
| 2 Number of patients unable to discontinue OCS completely | 4 | 274 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.53 [0.88, 2.68] |
| 3 Change in FEV1 compared to baseline (litres) | 4 | 274 | Mean Difference (IV, Fixed, 95% CI) | ‐0.13 [‐0.24, ‐0.01] |
| 4 Change in morning PEFR compared to baseline (L/min) | 4 | 274 | Mean Difference (IV, Fixed, 95% CI) | ‐19.63 [‐34.98, ‐4.27] |
| 5 Change in evening PEFR compared to baseline (L/min) | 4 | 274 | Mean Difference (IV, Fixed, 95% CI) | ‐21.58 [‐35.20, ‐7.97] |
| 6 Change in daily asthma symptom score compared to baseline | 4 | 274 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.15 [‐0.09, 0.39] |
| 7 Change in rescue beta2 agonist use compared to baseline (puffs/d) | 4 | 274 | Mean Difference (IV, Fixed, 95% CI) | ‐0.14 [‐1.05, 0.78] |
| 8 Asthma Quality of Life Questionnaire: change in overall score compared to baseline | 3 | 187 | Mean Difference (IV, Fixed, 95% CI) | ‐0.12 [‐0.48, 0.23] |
| 9 Asthma Quality of Life Questionnaire: change in activity limitation domain compared to baseline | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 10 Asthma Quality of Life Questionnaire: change in symptom domain compared to baseline | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 11 Asthma Quality of Life Questionnaire: change in emotional function domain compared to baseline | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 12 Asthma Quality of Life Questionnaire: change in environmental exposure domain compared to baseline | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 13 Sore throat (No. of patients) | 2 | 141 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.70 [0.15, 3.18] |
| 14 Hoarseness/dysphonia (No. of patients) | 2 | 141 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.92 [0.28, 2.99] |
| 15 Oral Candidiasis (No. of patients) | 2 | 141 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.96 [0.41, 2.24] |
15.1. Analysis.
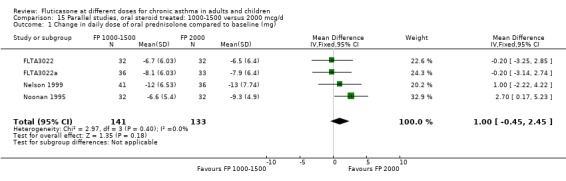
Comparison 15 Parallel studies, oral steroid treated: 1000‐1500 versus 2000 mcg/d, Outcome 1 Change in daily dose of oral prednisolone compared to baseline (mg).
15.2. Analysis.
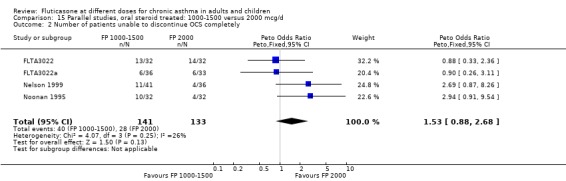
Comparison 15 Parallel studies, oral steroid treated: 1000‐1500 versus 2000 mcg/d, Outcome 2 Number of patients unable to discontinue OCS completely.
15.3. Analysis.
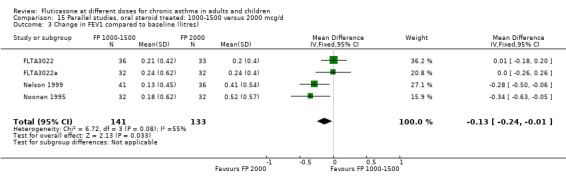
Comparison 15 Parallel studies, oral steroid treated: 1000‐1500 versus 2000 mcg/d, Outcome 3 Change in FEV1 compared to baseline (litres).
15.4. Analysis.
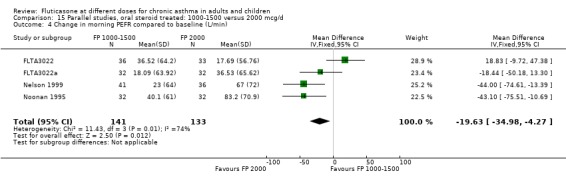
Comparison 15 Parallel studies, oral steroid treated: 1000‐1500 versus 2000 mcg/d, Outcome 4 Change in morning PEFR compared to baseline (L/min).
15.5. Analysis.
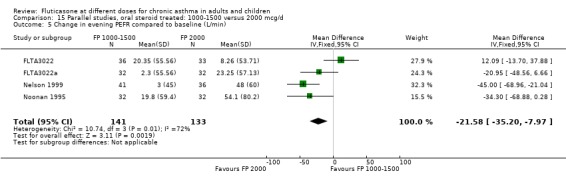
Comparison 15 Parallel studies, oral steroid treated: 1000‐1500 versus 2000 mcg/d, Outcome 5 Change in evening PEFR compared to baseline (L/min).
15.6. Analysis.
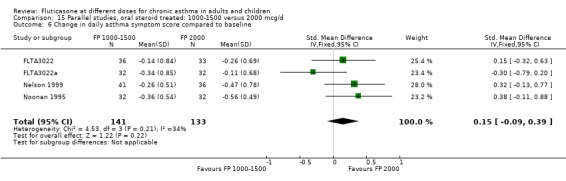
Comparison 15 Parallel studies, oral steroid treated: 1000‐1500 versus 2000 mcg/d, Outcome 6 Change in daily asthma symptom score compared to baseline.
15.7. Analysis.
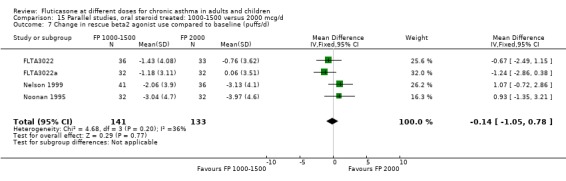
Comparison 15 Parallel studies, oral steroid treated: 1000‐1500 versus 2000 mcg/d, Outcome 7 Change in rescue beta2 agonist use compared to baseline (puffs/d).
15.8. Analysis.
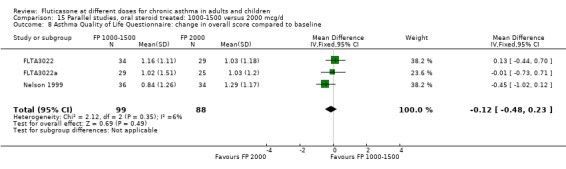
Comparison 15 Parallel studies, oral steroid treated: 1000‐1500 versus 2000 mcg/d, Outcome 8 Asthma Quality of Life Questionnaire: change in overall score compared to baseline.
15.9. Analysis.
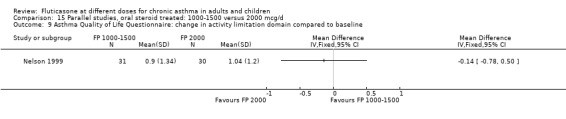
Comparison 15 Parallel studies, oral steroid treated: 1000‐1500 versus 2000 mcg/d, Outcome 9 Asthma Quality of Life Questionnaire: change in activity limitation domain compared to baseline.
15.10. Analysis.
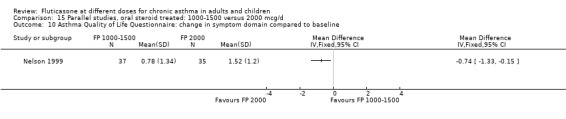
Comparison 15 Parallel studies, oral steroid treated: 1000‐1500 versus 2000 mcg/d, Outcome 10 Asthma Quality of Life Questionnaire: change in symptom domain compared to baseline.
15.11. Analysis.
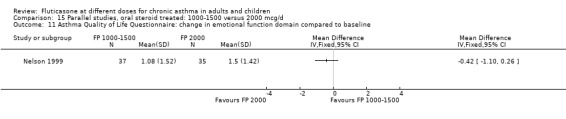
Comparison 15 Parallel studies, oral steroid treated: 1000‐1500 versus 2000 mcg/d, Outcome 11 Asthma Quality of Life Questionnaire: change in emotional function domain compared to baseline.
15.12. Analysis.
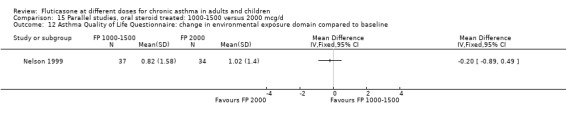
Comparison 15 Parallel studies, oral steroid treated: 1000‐1500 versus 2000 mcg/d, Outcome 12 Asthma Quality of Life Questionnaire: change in environmental exposure domain compared to baseline.
15.13. Analysis.
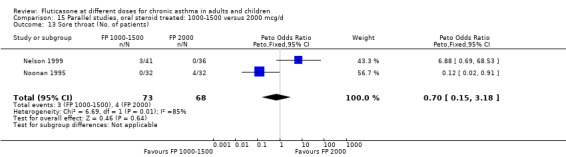
Comparison 15 Parallel studies, oral steroid treated: 1000‐1500 versus 2000 mcg/d, Outcome 13 Sore throat (No. of patients).
15.14. Analysis.
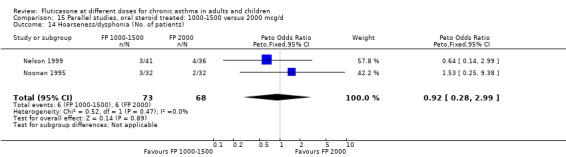
Comparison 15 Parallel studies, oral steroid treated: 1000‐1500 versus 2000 mcg/d, Outcome 14 Hoarseness/dysphonia (No. of patients).
15.15. Analysis.
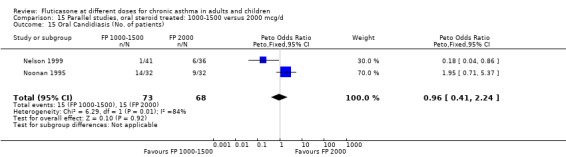
Comparison 15 Parallel studies, oral steroid treated: 1000‐1500 versus 2000 mcg/d, Outcome 15 Oral Candidiasis (No. of patients).
Comparison 16. Crossover studies, no oral steroids: 200 versus 1000 mcg (all ages).
16.1. Analysis.

Comparison 16 Crossover studies, no oral steroids: 200 versus 1000 mcg (all ages), Outcome 1 FEV1.
16.2. Analysis.
Comparison 16 Crossover studies, no oral steroids: 200 versus 1000 mcg (all ages), Outcome 2 PEF.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Agertoft 1997.
| Methods | Setting: Denmark, paediatric outpatient clinic Design: crossover, 2 week washout Length of intervention period: 2 weeks Randomisation: yes, computer generated random sequence with balanced blocks Masking: double‐blind Excluded: stated (none) Withdrawals: stated (one child from low dose group due to sore throat) Baseline characteristics: comparable between groups Jadad score: 5 | |
| Participants | 48 children: 27M 21F Age range: 6‐12 years Inclusion criteria: Pre‐pubertal children 'Mild' asthma requiring treatment with as needed beta2 agonists only Exclusion criteria: Inhaled or oral steroid use in last 2 months | |
| Interventions | 1. FP 200mcg/d 2. FP 400mcg/d Delivery device: Accuhaler DPI |
|
| Outcomes | FEV1 Morning PEFR Evening PEFR Daily asthma symptom score Daily use of beta2agonist 24 hour urinary cortisol excretion Growth by lower leg knemometry | |
| Notes | Patients also received BUD and placebo in a randomised fashion: results not considered in this review | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | Computer generated random sequence with balanced blocks |
| Allocation concealment? | Low risk | See Appendix 2 |
| Blinding? All outcomes | Low risk | Identical inhaler devices |
Allen 1998.
| Methods | Setting: multicentre study USA, paediatric outpatient clinic Length of intervention period: 12 months Randomisation: yes, method not stated Allocation concealment: yes (randomisation code generated off site and concealed using sealed envelopes) Design: parallel group Masking: double blind Excluded: stated Withdrawals: stated Baseline characteristics: comparable Jadad score: 3 | |
| Participants | 344 children enrolled, 325 randomised: 81M 244F Age range: M 4‐11 years, F 4‐9 years ) Inclusion criteria: Pre‐pubescent children with mild to moderate asthma (ATS criteria 1987) for at least 3 months FEV1 60 (% predicted) or greater Exclusion criteria: Systemic, intra‐nasal or opthalmic corticosteroids in last month More than 60 days of systemic corticosteroid use in last 2 years | |
| Interventions | 1. FP 50 mcg 2xdaily (100 mcg/d) 2. FP 100 mcg 2xdaily (200 mcg/d) Delivery device: Diskhaler DPI |
|
| Outcomes | Height assessment HRQOL: Functional Status IIR (FSII) questionnaire, Sleep Scale Children (SLP‐C) questionnaire, Quality of Life of Parents of Asthmatic Children Questionniare (QOL‐PAC) Oral corticosteroids for asthma exacerbation (No. of courses or prednisolone) Withdrawal due to asthma exacerbation Oro‐pharyngeal side effects | |
| Notes | Authors confirmed use of allocation concealment Criteria for withdrawal due to lack of efficacy: requirement for more than two seven day courses of oral corticosteroid Placebo treatment arm also included: results not considered in this review Data available from www.clinicalstudyresults.org |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | See Appendix 2 |
| Allocation concealment? | Low risk | Randomisation code generated off site and concealed using sealed envelopes |
| Blinding? All outcomes | Low risk | Double blind; identical inhaler devices |
Allen 2000.
| Methods | Randomised, double‐blind parallel group trial. Withdrawals: not stated. Jadad score: 2 |
|
| Participants | N = 111. Distribution between groups not clear. Mean FEV1: 61% Inclusion criteria: adolescent/adult asthmatics; OCS dependent Exclusion criteria: not clear |
|
| Interventions | i) FP 1000mcg BiD (2000); ii) FP500mcg BiD (1000); iii) Placebo. Inhaler device: Diskus. Study duration: 52 weeks. | |
| Outcomes | Steroid consumption; lung function; adverse events | |
| Notes | Conference abstract | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Described as randomised; no other information available |
| Allocation concealment? | Unclear risk | Information not available |
| Blinding? All outcomes | Low risk | Double blind; identical inhaler devices |
Ayres 1995.
| Methods | Setting: multicentre study worldwide, hospital outpatient clinics Design: parallel group Length of intervention period: 6 weeks Randomisation: computer generated random sequence Allocation concealment: yes (central coding by pharmaceutical company sponsor) Masking: double blind Withdrawals: adverse event rates reported, unclear if any led to patient withdrawal Baseline characteristics: comparable between groups Jadad score: 3 | |
| Participants | 862 adults enrolled, 671 randomised Age range: 18‐70 years Inclusion criteria: Adults with a clinical history of severe asthma Requiring BDP 1‐2 mg/d or BUD 0.8‐1.6 mg/d BUD for asthma control During run‐in period: Asthma symptom scores of 1 or more on 4 out of last 7 days and either: 1. At least 15% reversibility FEV1 post beta2 agonist or: 2. Diurnal variation in PEFR 15% or greater on 4 out of last 7 days or: 3. Need for 2 or more doses beta2 agonist each of last 7 days with either a). % predicted FEV1 80% or greater b) Mean morning PEFR 80% or greater in last 7 days Exclusion criteria: Alteration of normal asthma medication during run‐in period Hospital admission due to asthma exacerbation in last month Systemic corticosteroids > 10mg daily Suspected of being steroid hypersensitive Concomitant disease likely to complicate evaluation of drug Current smokers | |
| Interventions | 1. FP 125 mcg 4 puffs 2xdaily (1000 mcg/d) 2. FP 250 mcg 4 puffs 2xdaily (2000 mcg/d) Delivery device: MDI +/‐ spacer |
|
| Outcomes | Outcomes reported as change compared to baseline: FEV1 FVC Clinic PEFR Morning PEFR Evening PEFR Diurnal variation in PEFR Symptom free days Symptom free nights Rescue beta2 agonist free days Asthma exacerbations Morning plasma cortisol Biochemical markers of bone turnover |
|
| Notes | Details concerning randomisation method provided by Glaxo Wellcome 12.5% of patients randomised to FP treatment arms receiving oral prednisolone (< 10mg/d) at the time of enrolment. No attempt was made to reduce dose in these patients Patients were given the option of using spacer device BUD treatment arm also included: results not considered in this review |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | Computer generated random sequence |
| Allocation concealment? | Low risk | Central coding by pharmaceutical company sponsor) |
| Blinding? All outcomes | Low risk | Double blind; identical inhaler devices |
Boner 1999.
| Methods | Setting: multicentre study Europe and New Zealand Length of intervention period: 6 weeks Randomisation: yes, method not stated Allocation concealment: not stated Design: parallel group Masking: no details Excluded: no details Withdrawals: no details Baseline characteristics: no details Jadad score: 1 | |
| Participants | 89 children Age range: 5‐16 years Inclusion criteria: Children with asthma, no further details Exclusion criteria: No details | |
| Interventions | 1. FP 200 mcg/d 2. FP 400 mcg/d Delivery device: no details |
|
| Outcomes | Methacholine BHR (PC20 FEV1) FEV1 | |
| Notes | Study presented in abstract form only | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Described as randomised; no other information available |
| Allocation concealment? | Unclear risk | Information not available |
| Blinding? All outcomes | Unclear risk | Information not available |
Bukovskis 2002.
| Methods | Randomised, double‐blind parallel group trial. Method of randomisation: not reported Withdrawals: not stated Jadad score: 2 |
|
| Participants | N=19 (FP500: N: 10; FP100: N: 9) | |
| Interventions | FP100 v FP500. Inhaler device not specified. Study duration: 24 weeks. Inhaler device: unclear. | |
| Outcomes | FEV1; PD20; am/pm PEF; ß‐2 agonist use; symptoms; blood eosinophils; sputum cell count; | |
| Notes | Conference abstract | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Described as randomised; no other information available |
| Allocation concealment? | Unclear risk | Information not available |
| Blinding? All outcomes | Unclear risk | Information not available |
Casale 2001.
| Methods | Multicentre, single‐blind, randomised open label controlled trial. Method of randomisation not reported. Open label study with exception of flunisolide and placebo treatment groups. Withdrawals not described. Jadad score: 1 |
|
| Participants | N=78 (PLA: 15; FP110: 14; FP220: 12; FP330: 20; FP440: 17); Mean age (SD): PLA: 31.5 (10.09); FP110: 36.1 (8.70); FP220: 32.2 (8.66); FP330: 29.1 (8.66); FP440: 29.4 (10.20); M/F (%): PLA: 47/53; FP110: 57/43; FP220: 50/50; FP330: 40/60; FP440: 35/65; Mean FEV1 (SD): PLA: 3.0 (0.66); FP110: 3.3 (0.86); FP220: 3.2 (0.67); FP330: 3.0 (0.69); FP440: 3.2 (0.77) Inclusion criteria: non‐smokers; 18‐50 years; diagnosis of persistent mild to moderate asthma confirmed within previous 12 months by response to SABA (increase in FEV1 >/= 12%)/methacholine challenge <8mg/mL); FEV1 >/=65% predicted; no OCS/nasal/ICS use in previous 6 months Exclusion criteria: significant pulmonary disease (e.g. COPD); exacerbation within 6 weeks; URTI within 30 days screening; oestrogen usage; current condition that might confound data interpretation |
|
| Interventions | PLA versus FP220 versus FP440 versus FP660 versus FP880 . Study duration: 3 weeks. Inhaler device: pMDI | |
| Outcomes | HPA function | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Described as randomised; no other information available |
| Allocation concealment? | Unclear risk | Information not available |
| Blinding? All outcomes | Low risk | Identical inhaler devices |
Chervinsky 1994.
| Methods | Setting: multicentre study USA, hospital outpatient clinic Length of intervention period: 8 weeks Randomisation: yes, method not stated Allocation concealment: unclear Design: parallel group Masking: double blind Excluded: stated Withdrawals: stated Baseline characteristics: comparable Jadad score: 3 | |
| Participants | 331 adults Mean age: 48‐59 years Inclusion criteria: Mild to moderate asthma (as defined by the Committee on Diagnositic standards for Non‐Tuberculous Respiratory Diseases 1962) Treatment with BDP for at least 1 month prior to study and daily beta2 agonist, or daily theophylline for at least 2 weeks prior to study FEV1 60‐90 (% predicted) Exclusion criteria: Not stated | |
| Interventions | 1. FP 50 mcg 2xdaily (100 mcg/d) 2. FP 100 mcg 2xdaily (200mcg/d) 3. FP 500 mcg 2xdaily (1000 mcg/d) Delivery device: MDI |
|
| Outcomes | Probability of remaining in study All outcomes expressed as change compared to baseline: FEV1 FVC FEF25‐75 Morning PEFR Evening PEFR Daily symptom score Daily beta2 agonist use Night awakenings Morning plasma cortisol Urinary free cortisol Plasma cortisol 60 min post ACTH Physician‐rated global assessment of efficacy Oropharyngeal side‐effects |
|
| Notes | No reply from author to clarify details of randomisation method For continuous outcomes change scores from baseline to endpoint (i.e. point of withdrawal) were reported A priori criteria for withdrawal due to lack of efficacy were established based on FEV1, morning PEFR, night‐time awakenings or clinical exacerbation requiring emergency hospital treatment Placebo treatment arm also included: results not considered in this review |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | See Appendix 2 |
| Allocation concealment? | Low risk | See Appendix 2 |
| Blinding? All outcomes | Low risk | Identical inhaler devices |
Chetta 2003.
| Methods | Randomised, double‐blind, parallel group trial. Method of randomisation: not reported. Withdrawals: 12. Non‐ITT Jadad score: 3 |
|
| Participants | N=30 randomised. Data only presented on those completing the study. FP100: N=8; FP1000: N=8. Mean age (SD): FP100: 28 (8); FP1000: 26 (8); Atopic (%): 100/100; Mean duration of asthma (years): FP100: 11 (7); FP1000: 13 (9); Mean FEV1 (% predicted): FP100: 100 (SD 18); FP1000: 110 (22); Asthma severity score: FP100: 7 (2); FP1000: 6 (2). Inclusion criteria: mild to moderate asthma; well‐documented history of asthma; baseline FEV1>70% predicted Exclusion criteria: exacerbations within 2 months; CS within 6 months of study; free from respiratory infections in 4 weeks prior to study |
|
| Interventions | FP100 (BID) versus FP500 (BID). Inhaler device: pMDI + spacer. Study duration: 6 weeks | |
| Outcomes | PD20; FEV1; Mast cells; eosinophils; vessels; membrane thickness; vascular area | |
| Notes | High attrition rate | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Described as randomised; no other information available |
| Allocation concealment? | Unclear risk | Information not available |
| Blinding? All outcomes | Low risk | Identical inhaler devices |
Dahl 1993.
| Methods | Setting: world‐wide multicentre study, hospital outpatient clinic Design: parallel group Length of intervention period: 4 weeks Randomisation: yes, computer generated sequence Allocation concealment: yes (central coding by pharmaceutical company sponsors) Masking: double blind Excluded: not stated Withdrawals: stated Baseline characteristics: comparable Jadad score: 4 | |
| Participants | 825 adults: 297M 528F Age range: 17‐74 years Inclusion criteria: Adults with moderately severe chronic asthma requiring BDP 1000 mcg/d or less. During run‐in period: Daytime or night‐time symptoms during at least 4 days or: Diurnal variation in PEFR of 20% or more Exclusion criteria: Systemic steroids within the last month Serious concurrent disease Baseline asthma control: See inclusion criteria | |
| Interventions | 1. FP 100 mcg/d 2. FP 200 mcg/d 3. FP 400 mcg/d 4. FP 800 mcg/d Delivery device: MDI |
|
| Outcomes | Change in morning PEFR compared to baseline Change in evening PEFR compared to baseline Diurnal variation in PEFR FEV1 (% predicted) FVC % symptom free days Rescue beta2 agonist use (puffs/day) Plasma cortisol Plasma cortisol 30 mins after 250 mcg ACTH Incidence of oral candidiasis Incidence of oropharyngeal side effects | |
| Notes | Details of randomisation method and SD values for FEV1 (% predicted) provided by Glaxo Wellcome A BDP treatment arm also included: results not considered in this review |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | See Appendix 2 |
| Allocation concealment? | Low risk | See Appendix 2 |
| Blinding? All outcomes | Low risk | Identical inhaler devices |
Derom 1999.
| Methods | Randomised, crossover double‐blind, placebo controlled trial. Withdrawals stated. Non‐ITT. Jadad score: 3 | |
| Participants | N = 23. 8F; Mean age: 33 (19‐57); FEV1: 2.95 (SD 0.83) (FEV1 % predicted: 80.0 (SD 21.4)); Mean FVC: 4.42 L (SD 0.94). Inclusion criteria: either sex; 18‐60 years of age; ATS defined asthma; >/=40% predicted value; Either post‐BD increase in FEV1 of at least 200ml or >/=12% of baseline, OR diurnal variation of PEF >/=15% on at least 2 days/week during run‐in. Exclusion criteria: Exacerbation 4 weeks before inclusion; use of oral steroids within 4 weeks; use of ICS within 6 months; other systemic steroids within 4 weeks. |
|
| Interventions | FP 200mcg; FP 1000mcg; BUD: 200mcg; BUD 800mcg; Placebo administered over 1 week. Inhaler device: DPI Concomitant therapy: IP, xanthines, sodium cromoglycate permitted provided doses kept at constant level 4 weeks prior to inclusion |
|
| Outcomes | FEV1; PEFR; Serum cortisol; White blood cell count; Neutrophils; Basophils | |
| Notes | Data reported for effects after 24hours @ 1 week | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Described as randomised; other information not available |
| Allocation concealment? | Unclear risk | Information not available |
| Blinding? All outcomes | Low risk | Identical inhaler devices |
Derom 2005.
| Methods | Randomised double‐blind placebo‐controlled double dummy crossover trial. Jadad score: 2 | |
| Participants | N=25 | |
| Interventions | FP500 versus FP1000 versus PLA; washout period: 3 weeks. Study duration: 6 treatment periods unclear duration . Inhaler device: unclear | |
| Outcomes | Cortisol suppression; PC20 | |
| Notes | Conference abstract | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Described as randomised; other information not available |
| Allocation concealment? | Unclear risk | Information not available |
| Blinding? All outcomes | Unclear risk | Information not available |
Falcoz 2000.
| Methods | Randomised, double‐blind, parallel group study, Non‐ITT. Jadad score 3. | |
| Participants | N randomised = 232 (230 evaluable: FP 100mcg: 78; FP 500mcg: 79; Placebo: 73); Mean age 38 years; Participants suffered from mild‐to‐moderate asthma Inclusion criteria: Mild‐to‐moderate asthma (defined as FEV1 50‐80%) Exclusion criteria: Not stated |
|
| Interventions | 1) FP 100mcg 2) FP 500mcg 3) Placebo. Duration 6 weeks. Inhaler device: DPI. | |
| Outcomes | Plasma concentrations | |
| Notes | Data taken only for study 1. Study 2 assessed equal dose of FP given via different inhalers | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Described as randomised; other information not available |
| Allocation concealment? | Unclear risk | Information not available |
| Blinding? All outcomes | Low risk | Identical inhaler devices |
FAP30001.
| Methods | Setting: multicentre study in USA Design: parallel group Length of intervention period: 26 weeks Randomisation: yes, method unclear Allocation concealment: unclear Masking: double‐blind Excluded: not stated Withdrawals: stated Baseline characteristics: comparable Jadad score: 3 | |
| Participants | N = 182. Mean age: 37‐39. Inclusion criteria: >12 years; ATS‐defined asthma; FEV1 >/=45% predicted; able to use MDI Exclusion criteria: History of life threatening asthma; systemic steroids within 6 months of study entry; immunosuppressive agents prior to study entry |
|
| Interventions | Run‐in period: 1‐2 weeks 1) FP HFA 220mcg bid (440mcg/d) 2) FPHFA 440mcg bid (880mcg/d) Inhaler device: MDI |
|
| Outcomes | Withdrawals Change in FEV1 % predicted Change in FEV1 Litres Change in am PEF L/min Change in pm PEF L/min Change in symptom scores Change in symptom free days Change in number of awakenings/nt Adverse events | |
| Notes | Unpublished study downloaded from www.clinicalstudyresults.org | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | See Appendix 2 |
| Allocation concealment? | Low risk | See Appendix 2 |
| Blinding? All outcomes | Low risk | Identical inhaler devices |
FLIC15.
| Methods | Setting: multicentre study in Italy Design: parallel group Length of intervention period: 4 weeks Randomisation: yes, method unclear Allocation concealment: unclear Masking: double‐blind Excluded: not stated Withdrawals: stated Baseline characteristics: comparable Jadad score: 3 | |
| Participants | 33 participants. Mean age 37‐44 years. Inclusion criteria: M/F participants; age 18‐60 years; persistent mild‐moderate asthma (ATS). Exclusion criteria: Preventer medication 4 weeks prior to study entry. |
|
| Interventions | Run‐in period: 2 weeks i) FP100mcg bid (200mcg/d) ii) FP500mcg bid (1000mcg/d) iii) Placebo Inhaler device: DPI |
|
| Outcomes | Withdrawals; FEV1; PEFR; FVC | |
| Notes | Unpublished study downloaded from www.clinicalstudyresults.org | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | See Appendix 2 |
| Allocation concealment? | Low risk | See Appendix 2 |
| Blinding? All outcomes | Low risk | Identical inhaler devices |
FLIP01.
| Methods | Setting: multicentre study in Belgium, Netherlands, Germany, Switzerland Design: parallel group Length of intervention period: 5 weeks Randomisation: yes, method unclear Allocation concealment: unclear Masking: double‐blind Excluded: not stated Withdrawals: stated Baseline characteristics: comparable Jadad score: 3 | |
| Participants | 110 participants ‐ age not reported Inclusion criteria: Moderate asthma (no steroids) | |
| Interventions | i) FP 50mcg/d ii) FP 100mcg/d iii) FP 200mcg/d iv) BDP 100mcg/d v) BDP 200mcg/d Inhaler device: MDI |
|
| Outcomes | Withdrawals; am & pm PEF; symptoms; adverse events | |
| Notes | Unpublished study | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | See Appendix 2 |
| Allocation concealment? | Low risk | See Appendix 2 |
| Blinding? All outcomes | Low risk | Identical inhaler devices |
FLIP01a.
| Methods | As Above | |
| Participants | As above | |
| Interventions | As above | |
| Outcomes | As above | |
| Notes | Unpublished study | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | As for FLIP01 |
| Allocation concealment? | Low risk | As for FLIP01 |
| Blinding? All outcomes | Low risk | As for FLIP01 |
FLIP39.
| Methods | Setting: multicentre study in Europe Length of intervention period: 12 weeks Randomisation: yes, method not stated Allocation concealment: unclear Design: parallel group Masking: double blind Excluded: not stated Withdrawals: stated Baseline characteristics: comparable Jadad score: 3 | |
| Participants | 196 children. Median age: 10 (6‐17) Inclusion criteria: 6‐16 years of age; established history of childhood asthma; perennial symptoms requiring treatment with up to 400mcg/d ICS; history of recurrent episode of bronchoconstriction or cough; and >/=10% reversibility in FEV1 post‐SABA; Prior to randomisation, participants were required to show two fo the following in last 12 days of run‐in period: mean of 4 lowest PEFR </=85% predicted or mean PEFR </=95% predicted; ii) diurnal variation in PEFR at least 20% on >/=4 days; iii) asthma symptoms on >/=4 days; iv) bronchodilator use on at least 2 of 4 days. Exclusion criteria: systemic CS in previous 4 weeks/run‐in or on >3 times in last 6 months; acute lower RTI in last 14 days that would affect baseline lung function/symptoms |
|
| Interventions | 2 week run‐in period (200mcg/d BDP) followed by randomisation to: 1. FP 50mcg BID (100mcg/d) 2. FP 100mcg BID (200mcg/d) Inhaler device: DPI |
|
| Outcomes | am PEF pm PEF clinic PEF FEV1 Assessment of efficacy Symptoms Rescue medication usage Adverse events Withdrawal | |
| Notes | Sourced from http://ctr.gsk.co.uk | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | See Appendix 2 |
| Allocation concealment? | Low risk | See Appendix 2 |
| Blinding? All outcomes | Low risk | Identical inhaler devices |
FLTA3014.
| Methods | Setting: 21 centres in USA Length of intervention period: 26 weeks Randomisation: yes, method not described Allocation concealment: unclear Design: parallel group Masking: double blind Excluded: not stated Withdrawals: stated Baseline characteristics: comparable Jadad score: 3 | |
| Participants | 379 adults and adolescents. Mean age: 37‐39 Inclusion criteria: >/=12 years; 6 month history of asthma requiring pharmacotherapy; FEV1 50‐85%; use of SABA and/or ICS | |
| Interventions | 1. FP 100mcg BID (without spacer) 2. FP250mcg BID (with spacer) 3. FP250mcg BID (without spacer) 4. Placebo (with or without spacer) Inhaler device: MDI |
|
| Outcomes | FEV1; am PEF; pm PEF; withdrawals; SABA usage; symptoms; adverse events; withdrawals | |
| Notes | Sourced from http://ctr.gsk.co.uk | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | See Appendix 2 |
| Allocation concealment? | Low risk | See Appendix 2 |
| Blinding? All outcomes | Low risk | Identical inhaler devices |
FLTA3020.
| Methods | Setting: 25 centres in USA Length of intervention period: 12 weeks Randomisation: yes, method not described Allocation concealment: unclear Design: parallel group Masking: double blind Excluded: not stated Withdrawals: stated Baseline characteristics: comparable Jadad score: 3 | |
| Participants | 191 adults and adolescents
Mean age: 31‐36 years. Inclusion criteria: >/=12 years; 6 month history of asthma requiring pharmacotherapy; FEV1 50‐85%; use of SABA and/or ICS; pre‐BD FEV1% predicted 60‐90%; SABA prn or regular use only; effective use of MDI. Exclusion criteria: ICS within 30 days of screening; hospitalisation due to asthma on 2+ occasions in 12 months prior to screening; significant other medication within 30 days of screening |
|
| Interventions | 1. HFA‐FP 110mcg BID 2. HFA‐FP 220mcg BID 3. CFC‐FP 110mcg BID 4. CFC‐FP 220mcg BID 5. Placebo Inhaler device: MDI |
|
| Outcomes | FEV1; am PEF; pm PEF; withdrawals; symptoms; rescue medication usage; adverse events | |
| Notes | Sourced from www.clinicalstudyresults.org | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | See Appendix 2 |
| Allocation concealment? | Low risk | See Appendix 2 |
| Blinding? All outcomes | Low risk | Identical inhaler devices |
FLTA3020a.
| Methods | As above | |
| Participants | As above | |
| Interventions | As above | |
| Outcomes | As above | |
| Notes | As above | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | As for FLTA3020 |
| Allocation concealment? | Low risk | As for FLTA3020 |
| Blinding? All outcomes | Low risk | As for FLTA3020 |
FLTA3022.
| Methods | Setting: 39 centres in USA Design: parallel group Length of intervention period: 16 weeks (plus 2 week run‐in) Randomisation: yes, method not described Masking: double‐blind (identical devices) Excluded: not stated Withdrawals: stated (placebo: 22; FP440hfa bid: 6; FP880hfa bid: 13; FP440cfc bid: 6; FP880cfc bid: 8) Baseline characteristics: comparable between groups Jadad score: 4 | |
| Participants | 168 adolescents/adults: 78M/90F Age range: >12 (mean age: 50 years) Inclusion criteria: 12 years of age or older FEV1 40‐85 (% predicted); oral steroid dependent asthma Exclusion criteria: History of life‐threatening asthma; 3 or more hospitalisations in past year; therapy with antileukotrienes, nedocromil and/or ipratropium bromide within 4 weeks of randomisation | |
| Interventions | 1. FP440mcg CFC bid 2. FP880mcg CFC bid 3. FP440mcg HFA bid 4. FP880mcg HFA bid 5. Placebo Inhaler device: MDI |
|
| Outcomes | Oral steroid reduction FEV1 am PEF pm PEF Symptoms Rescue medication usage AQLQ Withdrawals Adverse events | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | See Appendix 2 |
| Allocation concealment? | Low risk | See Appendix 2 |
| Blinding? All outcomes | Low risk | Identical inhaler devices |
FLTA3022a.
Galant 1996.
| Methods | Setting: multicentre study USA, hospital outpatient clinic Length of intervention period: 12 weeks Randomisation: yes, method not stated Allocation concealment: unclear Design: parallel group Masking: double blind Excluded: not stated Withdrawals: stated Baseline characteristics: comparable Jadad score: 4 | |
| Participants | 353 adolescents/adults: 236M 117F Age range: 12‐75 years Inclusion criteria: 12 years of age or older FEV1 45‐75 (% predicted) 15% or greater reversibility in FEV1 after inhaled beta2 agonist Significant asthma symptoms during run‐in period: e.g. daily asthma symptoms with > 8 puffs beta2 agonist/day or 2‐ 4 weekly nighttime awakenings due to asthma Exclusion criteria: History of life‐threatening asthma Smokers of 10 pack years or greater Previous use of inhaled, oral, injectable or intra‐nasal corticosteroids Pregnancy | |
| Interventions | 1. FP 25 mcg 2 puffs 2xdaily (100 mcg/d) 2. FP 50 mcg 2 puffs 2xdaily (200 mcg/d) Delivery device: MDI |
|
| Outcomes | Probability of remaining in study All outcomes expressed as change compared to baseline: FEV1 Morning PEFR Daily use of beta2 agonist Daily asthma symptom score Night‐time awakenings per week 'Effective or very effective' Physician rated global assessment of efficacy (No. of patients) Oropharyngeal side effects |
|
| Notes | No reply from author to clarify details of randomisation method For continuous outcomes change scores from baseline to endpoint (i.e. point of withdrawal) were reported A priori criteria for withdrawal due to lack of efficacy were established based on diurnal variability in PEFR, night‐time awakenings, rescue beta2 agonist use and FEV1 Placebo treatment arm also included: results not considered in this review Study also included an oral theophylline treatment arm: results not considered in this review |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Described as randomised; other information not available |
| Allocation concealment? | Unclear risk | Information not available |
| Blinding? All outcomes | Low risk | Identical inhaler devices |
Gershman 2000.
| Methods | Randomised, double‐blind, single dummy, parallel group study. Participants randomised according to their entry in to the treatment phase. 2 participants withdrew from the low dose FP. ITT population Jadad score: 3 |
|
| Participants | N = 24 (FP1000mcg group: 12; FP100mcg group: N = 12. FP1000: 3F/9M; FP100: 12M). 23/24 atopic asthma. Mean FEV1 (% pred): FP1000 group: 69; FP100 group: 66; FEF25‐75%: FP1000 group: 1.87 (SEM 0.17); FP100 group: 2.18 (SEM 0.22); PC20: FP1000 group: 0.95 (0.1 to 11.2); FP 100 group: 0.63 (0.3 to 2.5); ECP ng/mL: FP 1000 group: 84 (24 to 165); FP 100 group: 154 (24 to 282) | |
| Interventions | FP100mcg daily versus FP1000mcg daily. Duration: 6 weeks. Inhaler device: MDI+spacer | |
| Outcomes | Lung function (FEV1; PEF; FEF); PC20; ECP; Symptoms | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Described as randomised; other information not available |
| Allocation concealment? | Unclear risk | Information not available |
| Blinding? All outcomes | Low risk | Identical inhaler devices |
Giannini 2003.
| Methods | Randomised, double‐blind parallel group study. Method of randomisation: Not reported. Withdrawals: No withdrawals occurred. Jadad score: 3 |
|
| Participants | N=27. Mean age: 38.67 (SD 16.97). M/F: 15/12; history of atopy: 18/6; FEV1: 3.23 (SD 0.91); FEV1 % predicted (median (range)): 96 (76‐122); PD20: 0.220 Inclusion criteria: diurnal/nocturnal symptoms=0, low PEF variability [maximal amplitude(‐MA) <10%). Exclusion criteria: no use of ß‐agonists throughout run‐in |
|
| Interventions | FP100 versus FP250 versus PLA. Inhaler device: unclear | |
| Outcomes | FEV1; PD20; Sputum eosinophils; max amplitude; PEF; Symptoms; Rescue medication use | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Described as randomised; other information not available |
| Allocation concealment? | Unclear risk | Information not available |
| Blinding? All outcomes | Unclear risk | Information not available |
Hofstra 2000.
| Methods | Randomised, double‐blind, placebo‐controlled, 3‐arm parallel group trial. Methods of randomisation: not reported. ITT population Jadad score: 2 | |
| Participants | N = 37 (PLA: 12; FP100: 11; FP250: 14); Age: PLA: 9.8 (SD 2.4); FP100: 9.9 (SD 1.6); FP250: 11.1 (SD 2.4); FEV1 (% predicted): PLA: 92.1 (SD 12.5); FP100: 96.6 (SD 6.9); FP250: 93.2 (SD 13.3) Exclusion criteria: ICS use in last 4 months | |
| Interventions | Inhaled FP100 BID (200mcg/d)versus inhaled FP250 BID (500mcg/d) versus placebo, via MDI with a volumatic spacer Duration: 6 weeks (FP100 versus FP250 versus PLA); subsequent 12 weeks, PLA group re‐allocated at random to FP100 or FP250 group. Data extracted up until 6 weeks (subsequent time points have data from participants who had been treated with PLA for preceding 6 weeks) |
|
| Outcomes | FEV1 (% predicted); EIB; PD20 | |
| Notes | Patients were randomised to receive FP or placebo and treated for 6 weeks. After 6 weeks patients receiving placebo were re‐randomised to either dose of FP for a further 18 weeks. Placebo treatment arm also included: results not considered in this review. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Described as randomised; other information not available |
| Allocation concealment? | Unclear risk | Information not available |
| Blinding? All outcomes | Unclear risk | Information not available |
Ind 2003.
| Methods | Setting: multicentre study Canada, Denmark, Iceland, Italy, UK Length of intervention period: 24 weeks Randomisation: yes, not reported Allocation concealment: unclear Design: parallel group Masking: double blind Excluded: not stated Withdrawals: stated Baseline characteristics: comparable Jadad score: 3 | |
| Participants | 496 adults 266 F; 230M Age range: 16‐75 years Inclusion criteria: 16‐75 years Requirement for high dose BDP Poor control Two exacerbations in past year requiring a change of therapy Symptomatic (assessed during run‐in) | |
| Interventions | i) FSC (not covered in this review) ii) FP 500mcg/d iii) FP 1000mcg/d Inhaler device: MDI | |
| Outcomes | Withdrawals (n) am PEF pm PEF Exacerbations Symptoms Relief medication usage Clinic FEV1 Clinic FVC Physician assessment of effectiveness (n) Subjects assessment of effectiveness (n) Adverse events (n) | |
| Notes | Unpublished trial | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | See Appendix 2 |
| Allocation concealment? | Low risk | See Appendix 2 |
| Blinding? All outcomes | Low risk | Identical inhaler devices used |
Katz 1998.
| Methods | Setting: multicentre study, Europe, Middle East and Asia, hospital outpatient clinics Length of intervention period: 12 weeks Randomisation: yes, method not stated Allocation concealment: unclear Design: parallel group Masking: double blind Excluded: not stated Withdrawals: stated Baseline characteristics: comparable Jadad score: 3 | |
| Participants | 263 children: 166M 97F Age range: 4‐11 years Inclusion criteria: Diagnosis of asthma (not otherwise defined) Recurrent episodes of bronchoconstriction and cough Able to use delivery device and peak flow meter satisfactorily PEFR 75 (% predicted)or less, or PEFR 75‐90 (% predicted) with asthma symptoms during run‐in period Exclusion criteria: Treatment with inhaled corticosteroids within last 3 months Oral steroids in last month Continuous treatment with oral steroids over 2 months or more in past Hospital admission due to asthma in last 3 months | |
| Interventions | 1. FP 50 mcg 2 x daily (100 mcg/d) 2. FP 100 mcg 2 x daily (200 mcg/d) Delivery device: Diskhaler DPI |
|
| Outcomes | Outcomes expressed as change compared to baseline: FEV1 FVC FEF 25‐75 Morning PEFR Evening PEFR Daily asthma symptom score Night‐time wakening score Daily use of beta2 agonists Probability of remaining in study | |
| Notes | Reply from author but unable to clarify details of randomisation method For continuous outcomes change scores from baseline to endpoint (i.e. point of withdrawal) were reported A priori criteria for withdrawal due to lack of efficacy were established based on FEV1, PEFR, sleep disturbance or rescue beta2 agonist use Placebo treatment arm also included: results not considered in this review |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | See Appendix 2 |
| Allocation concealment? | Low risk | See Appendix 2 |
| Blinding? All outcomes | Low risk | Identical inhaler devices used |
Kemp 2004.
| Methods | Setting: multicentre study, USA
Length of intervention period: 2 years
Randomisation: yes (randomisation code generated off‐site)
Allocation concealment: adequate
Design: parallel group
Masking: double blind (identical)
Excluded: yes
Withdrawals: stated Jadad score: 5 |
|
| Participants | 190 adults and adolescents screened, 160 randomised (three arm study; PLA: N = 54; FP400: N = 55; FP1000: 51), Age range: 18‐50; Mean baseline FEV1 (% predicted): PLA: 83; FP100: 82; FP500: 85 Inclusion criteria: 18‐50 years (F: 18‐40); mild asthma (6 months); FEV1: 50‐100% predicted Exclusion criteria: Significant co‐morbidity of bone; alterations in body weight; reversal of nocturnal sleeping hours; substance abuse |
|
| Interventions | FP200 BID (400) versus FP1000 BID (1000) versus PLA. Inhaler device: MDI | |
| Outcomes | Bome mineral density; withdrawals; adverse events | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | See Appendix 2 |
| Allocation concealment? | Low risk | Randomisation code generated off‐site |
| Blinding? All outcomes | Low risk | Idenitcal inhaler devices |
Lawrence 1997.
| Methods | Setting: multicentre study USA, hospital outpatient clinics Length of intervention period: 6 weeks Randomisation: yes, method not stated Allocation concealment: unclear Design: parallel group Masking: double blind, double dummy Excluded: not stated Withdrawals: stated Baseline characteristics: comparable Jadad score: 4 | |
| Participants | 261 adults Age range: 18‐71 years Inclusion criteria: Diagnosis of asthma (ATS criteria 1987) Treatment with ICS for 3 months or longer Treatment with BDP 336 mcg/d or TA 800 mcg/d at stable dose for 2 weeks FEV1 50‐80 (% predicted) 15% or greater reversibility in FEV1 after inhaled beta2 agonist Exclusion criteria: Systemic, intra‐nasal or opthalmic corticosteroids in last 2 months Oral corticosteroids for > 2 months in last 6 months Pregnancy | |
| Interventions | 1. FP100 mcg 2xdaily (200 mcg/d) 2. FP 500 mcg 2xdaily (1000 mcg/d) Delivery device: Diskhaler DPI |
|
| Outcomes | Probability of remaining in study Outcomes expressed as change compared to baseline: FEV1 Morning PEFR Daily asthma symptom score Daily use of beta2 agonist Morning plasma cortisol Oro‐pharyngeal side effects | |
| Notes | No reply from author to clarify details of randomisation method Results for continuous outcomes expressed as change to endpoint (point of withdrawal) A priori criteria for withdrawal due to lack of efficacy were established based on FEV1, morning PEFR, night‐time awakenings or clinical exacerbation requiring emergency hospital treatment Placebo treatment arm also included: results not considered in this review |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Described as randomised; no other information available |
| Allocation concealment? | Unclear risk | Information not available |
| Blinding? All outcomes | Unclear risk | Information not available |
Li 1999.
| Methods | Randomised, double‐blind, triple dummy, placebo‐controlled trial. Methods of randomisation not reported. Withdrawals: Placebo: 0; FP88: 1; FP220: 1. Non‐ITT. Jadad score: 4 |
|
| Participants | N = 128 (N for treatments considered by this review: 63; Placebo: 17; FP88: 22; FP220: 24). M/F ratio (%): PLA: 82/18; FP88: 68/32; FP220: 58/42; Mean age (range): PLA: 31 (19‐41); FP88: 30 (19‐42); FP220: 33 (18‐53); Ethnic origin (%) (White/Other): PLA: 94/6; FP88: 95/5; FP220: 88/13; FEV1 % Predicted: PLA: 89.1; FP88: 82.5; FP220: 88.2; Concurrent medication: Salmeterol: PLA: 0; FP88: 0; FP220: 1; Theophylline: PLA: 2; FP88: 0; FP220: 3; Cromolyn: PLA: 0; FP88: 1; FP220: 1; Nedocromil: PLA: 0; FP88: 0; FP220: 1 Inclusion criteria: Non‐smokers; asthma according to ATS criteria; duration of disease >6 months; FEV1 >/=50% predicted Exclusion criteria: Pregnancy/lactation; use of methotrexate/gold salts; use of inhaled cromolyn/nedocromil; use of oral, intranasal, inhaled or injectable steroids <4 weeks of study commencement; use of >/= 140mg prednisone or equivalent dosage in past year; significant concomitant illness; immunotherapy requiring change in dosage regimen within 12 weeks; reversal of nocturnal sleeping hours; concurrent use of over‐the‐counter medication that might affect course of asthma or interact with sympathomimetic amines or confound cortisol assay |
|
| Interventions | FP88 versus FP220 versus Placebo. Inhaler device: pMDI + spacer. Duration: 28 days. | |
| Outcomes | HPA axis function; plasma concentration; area under the curve; adverse events | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Described as randomised; other information not available |
| Allocation concealment? | Unclear risk | Information not available |
| Blinding? All outcomes | Low risk | Identical inhaler devices |
Lumry 2006.
| Methods | Randomised, double‐blind, parallel group study. Method of randomisation: not reported; blinding: not reported. Withdrawals ‐ Last observation carried forward. Missing: PLA: 2; FP88 BID: 3; FP220 BID: 1; FP440 BID: 2 Jadad score: 3 |
|
| Participants | N=415 (PLA: 104; FP172: 103; FP440: 106; FP880: 102); Mean FEV1 % predicted: PLA: 65.6; FP172: 65.3; FP440: 65.5; FP880: 66.2; mean am PEF (l/min): PLA: 346; FP172: 334; FP440: 329; FP880: 333.1 Inclusion criteria: >/=12 years; asthma for >6 months requiring tx with ICS for >/=3 months; FEV1: 45‐80% predicted; >/=12% reversibility Exclusion criteria: not reported |
|
| Interventions | HFA FP88 BID (172 mcg/d) versus HFA FP220 BID (440) versus HFA FP440 BID (880). Study duration: 12 weeks. Inhaler device: MDI | |
| Outcomes | am PEF; FEV1 (% predicted) | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | See Appendix 2 |
| Allocation concealment? | Low risk | See Appendix 2 |
| Blinding? All outcomes | Unclear risk | Identical inhaler devices used |
Meijer 1999.
| Methods | Randomised, double‐blind, double‐dummy parallel group trial. Method of randomisation: computerised minimisation method. Participants stratified according to age, previous dose of ICS, FEV % pred, reversibility to 200mcg sal, smoking status, serum IgE and PC20 methacholine. ITT population (all participants who contributed one reading). ICS tapered down at least 3 weeks prior to randomisation. If symptoms deteriorated during tapering phase thy were asked to present earlier for randomisation. Jadad score: 4 |
|
| Participants | N = 120 (Prednisolone: 40; FP2000: 40; FP500: 40; ); Median age: Prednisolone: 28 (18‐53); FP2000: 27 (18‐48); FP500: 27 (18‐56); M/F: Prednisolone: 14/26; FP2000: 14/26; FP500: 13/27; Smokers (%) (Current:Ex‐smoker:Non‐smoker): Prednisolone: 28:23:49; FP2000: 31:15:54; FP500: 28:23:49; FEV1 (% predicted): Prednisolone: 80 (65 to 91); FP2000: 79 (67 to 91); FP500: 81 (70 to 96); Reversibility (% pred): Prednisolone: 12 (9.2 to 17.8); FP2000: 11.4 (9 to 17.2); FP500: 12.3 (9.2 to 14.4); Log2 PC20 methacholine (mg/ml): Prednisolone: ‐0.86 (0.36); FP2000: ‐0.83 (0.37); FP500: ‐0.83 (0.36); Log2 PC20 AMP (mg/ml): Prednisolone: 1.89 (0.56); FP2000: 3.02 (0.54); FP500: 2.59 (0.67); IgE (IU/ml): Prednisolone: 251 (157 to 615); FP2000: 251 (85 to 550); FP500: 181 (97 to 631); Blood eosinophils (%): Prednisolone: 5.8 (3.6 to 8.0); FP2000: 5.0 (4.0 to 6.8); FP500: 5.1 (2.8 to 7.9); Sputum eosinophils (%): Prednisolone: 5.5 (2.0 to 14.7); FP2000: 5.0 (1.0 to 8.0); FP500: 5.0 (1.67 to 12); Serum ECP (mcg/l): Prednisolone: 19.5 (10.4 to 26.8); FP2000: 13.3 (9.9 to 22); FP500: 17.1 (9.3 to 24.9); Serum ECP Prednisolone (mcg/l): 78.4 (28 to 292); FP2000: 73.6 (33 to 250); FP500: 95.8 (46 to 233); Serum cortisol (nmol/l): Prednisolone: 420 (302 to 563); FP2000: 425 (320 to 725); FP500: 445 (265 to 740). Inclusion criteria: 18‐56 years; diagnosis of asthma; concentration of methacholine causing 20% fall in FEV1 (PC20) of 8mg/ml; 1 +ve skin prick test to 17 most common aeroallergens; reversibility to ß2 agonist (>/= 9% of predicted FEV1); ability to expectorate after hypertonic saline. Exclusion criteria: Participants who experienced exacerbation during run‐in phase which required course of oral steroids. |
|
| Interventions | Inhaled FP 500mcg versus Inhaled FP 2000mcg versus oral prednisolone. Duration: 2 weeks. Inhaler device: DPI. | |
| Outcomes | FEV (% predicted); PC20 methacholine (DC); PC20 AMP (DC); PEF (L/min); Daytime symptoms; rescue medication (puffs/day); Sputum eosinophils (%); Serum ECP (mcg/l); Sputum ECP (mcg/l); Serum cortisol | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | Computerised minimisation method. Participants stratified according to age, previous dose of ICS, FEV % predicted, reversibility to 200mcg SABA, smoking status, serum IgE and PC20 methacholine |
| Allocation concealment? | Unclear risk | Information not available |
| Blinding? All outcomes | Low risk | Double dummy design |
Nathan 2000.
| Methods | Randomised, double‐blind, placebo controlled multi‐centre trial. Method of randomisation not reported. Participants randomised according to baseline therapy: ICS or ß‐2. Withdrawals: PLA: 43; FP100: 34; FP200: 28; FP500: 20. Jadad score: 3 |
|
| Participants | N = 330. (PLA: 84; FP100mcg: 79; FP200mcg: 81; FP500mcg: 86); Gender (% M:F): PLA: 56:44; FP100: 65:35; FP200: 56:44; FP500: 55:45; Age range: 12‐75; Mean age: PLA: 38; FP100: 34; FP200: 38; FP500: 37; FEV1 L: PLA: 2.22 (SEM 0.06); FP100: 2.40 (0.07); FP200: 2.21 (SEM 0.07); FP500: 2.26 (SEM 0.05); FEV1 % predicted: PLA: 62.6 (SEM 1.07); FP100: 64.3 (SEM 0.89); FP200: 63.3 (SEM 1.03); FP500: 63.7 (SEM 0.96); am PEF (L/min): PLA: 394 (SEM 10); FP100: 397 (SEM 10); FP200: 395 (SEM 10); FP500: 379 (SEM 10); pm PEF (L/min): PLA: 412 (SEM 10); FP100: 420 (SEM 10); FP200: 414 (SEM 10); FP500: 404 (SEM 10); Asthma symptom scores: PLA: 1.10 (SEM 0.07); FP100: 1.18 (SEM 0.06); FP200: 1.03 (SEM 0.07); FP500: 1.08 (SEM 0.07); Albuterol use (puffs/d): PLA: 3.05 (SEM 0.26); FP100: 3.43 (SEM 0.26); FP200: 2.62 (SEM 0.24); FP500: 3.18 (SEM 0.26); Nighttime awakenings, No. (%): PLA: 0.09 (SEM 0.02); FP100: 0.08 (SEM 0.02); FP200: 0.12 (SEM 0.02); FP500: 0.10 (SEM 0.02) . | |
| Interventions | Inhaled FP100mcg QD versus FP200mcg QD versus FP500mcg QD versus placebo. Diskus inhaler. Duration: 12 weeks (plus open label extension) | |
| Outcomes | Lung function (FEV1; am PEF; pm PEF); asthma symptoms; albuterol use; nighttime awakenings; withdrawals; safety; HPA axis function | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | See Appendix 2 |
| Allocation concealment? | Low risk | See Appendix 2 |
| Blinding? All outcomes | Low risk | Identical inhaler devices |
Nelson 1999.
| Methods | Setting: multicentre study USA, hospital outpatient clinics Length of intervention period: 16 weeks Randomisation: yes, method not stated Allocation concealment: unclear Design: parallel group Masking: double blind Excluded: not stated Withdrawals: stated Baseline characteristics: comparable Jadad score: 3 | |
| Participants | 111 adults: 44M 67F Age range: 12‐77 years Inclusion criteria: 12 years of age or older Diagnosis of asthma (ATS criteria 1987) Dependent on oral corticosteroids for asthma control for 6 months or longer Requiring 5‐40 mg/day oral prednisolone FEV1 40‐80 (% predicted) 15% or greater reversibility in FEV1 after inhaled beta2 agonist Exclusion criteria: Life‐threatening asthma or other severe concurrent disease Use of intra‐nasal, injectable, topical corticosteroids Methotrexate, cyclosporin, azathioprine, troleandomycin within last 3 months | |
| Interventions | 1. FP 500 mcg 2xdaily (1000 mcg/d) 2. FP 1000 mcg 2xdaily (2000 mcg/d) Delivery device: Accuhaler DPI |
|
| Outcomes | 100% reduction in daily dose oral prednisolone (No. of patients) 1‐49% reduction in daily dose oral prednisolone (No. of patients) 0% reduction or increase in daily dose oral prednisolone (No. of patients) Outcomes reported as a change compared to baseline: Daily dose oral prednisolone FEV1 Morning PEFR Evening PEFR Daily asthma symptom score Daily beta2 agonist use Night‐time awakenings Health status: asthma Quality of Life Questionnaire (AQLQ) Plasma cortisol < 5mcg/L (No. of patients) Peak plasma cortisol < 18 mcg/dL during 6 hour iv infusion with 250 mcg co‐syntropin (No. of patients) Change in plasma cortisol < 7 mcg/dL following co‐syntropin infusion (No. of patients) Change in morning plasma cortisol compared to baseline | |
| Notes | No reply from author to clarify details of randomisation method Usual ICS discontinued at randomisation A priori criteria for prednisolone dose reduction based on FEV1 (% predicted), PEFR (% predicted), number of night‐time awakenings, beta‐2 agonist use compared to run in period values Patients were withdrawn from the study if they experienced asthma exacerbation requiring hospital admission, or 3 bursts of oral prednisolone due to exacerbation Placebo treatment arm also included: results not considered in this review |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | See Appendix 2 |
| Allocation concealment? | Low risk | See Appendix 2 |
| Blinding? All outcomes | Low risk | Identical inhaler devices |
Nieto 2001.
| Methods | Randomised controlled trial. Method of randomisation not reported; blinding not reported. Withdrawals not reported. Jadad score: 1 |
|
| Participants | N=18 (distribution between groups unclear). M/F: 9/9; mean age: 30 (SD 8); PC20: 1.14 (1.38). Inclusion criteria: not reported. Exclusion criteria: not reported. |
|
| Interventions | FP100 BID (200) versus FP250 BID (500). Inhaler device: unclear | |
| Outcomes | PC20; exhaled nitric oxide | |
| Notes | Unpublised conference abstract | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Described as randomised; other information not available |
| Allocation concealment? | Unclear risk | Information not available |
| Blinding? All outcomes | Unclear risk | Information not available |
Noonan 1995.
| Methods | Setting: multicentre study USA, hospital outpatient clinics Length of intervention period: 16 weeks Randomisation: yes, method not stated Allocation concealment: unclear Design: parallel group Masking: double blind Excluded: stated Withdrawals: Baseline characteristics: comparable Jadad score: 4 | |
| Participants | 96 adults: 46M 50F Mean age: 50‐52 years Inclusion criteria: 12 years of age or older Diagnosis of asthma (ATS criteria 1987) Dependent on oral corticosteroids for asthma control for 6 months or longer FEV1 40‐80 (% predicted) Documented evidence of previous attempts to reduce oral steroid dose Exclusion criteria: Use of methotrexate, gold salts or troleandomycin in last 3 months Nasal corticosteroid use 10 pack year history of smoking or greater Pregnancy or lactation | |
| Interventions | 1. FP 750 mcg 2xdaily (1500 mcg/d) 2. FP 1000 mcg 2xdaily (2000 mcg/d) Delivery device: MDI |
|
| Outcomes | 100% reduction in daily oral steroid use (% patients) 1‐49% reduction in daily oral steroid use (% patients) 0% or increase in daily oral steroid use (% patients) Outcomes expressed as change compared to baseline: Daily oral FEV1 Morning PEFR Evening PEFR Daily use of beta2 agonists Daily asthma symptom score Quailty of life: Medical Outcomes Study Short Form (SF‐36) | |
| Notes | No reply from author to clarify details of randomisation method Usual ICS discontinued at randomisation Daily dose oral prednisolone reduced according to pre‐defined criteria An uncontrolled one year open label study was undertaken following the randomised 16 week trial, when all patients received FP 2000 mcg/d. Results not considered in this review Placebo treatment arm also included: results not considered in this review |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Described as randomised; other information not available |
| Allocation concealment? | Unclear risk | Information not available |
| Blinding? All outcomes | Low risk | Identical inhaler devices |
Noonan 1998.
| Methods | Setting: multicentre study USA, hospital outpatient clinic Length of intervention period: 8 weeks Randomisation: yes, method not stated Allocation concealment: unclear Design: parallel group Masking: double blind Excluded: not stated Withdrawals: stated Baseline characteristics: comparable Jadad score: 3 | |
| Participants | 138 adults: 84M 54F Age range: 12‐59 years Inclusion criteria: 12 years of age or older Diagnosis of asthma (ATS criteria 1987) 6 months or longer FEV1 60 (% predicted) or greater 15% or greater reversibility in FEV1 after inhaled beta2 agonist Methacholine BHR (PD20 FEV1) < 18 mg Asthma stability during run in period based on a priori defined criteria related to PEFR, medication requirement and symptoms Exclusion criteria: Recent hospitalisation due to asthma exacerbation Treatment with corticosteroids, theophylline, sodium cromoglycate, nedocromil Pregnancy | |
| Interventions | 1. FP 50 mcg 2xdaily (100 mcg/d) 2. FP 100 mcg 2xdaily (200 mcg/d) Delivery device: MDI |
|
| Outcomes | Outcomes expressed as change compared to baseline: FEV1 Morning PEFR Evening PEFR Methacholine BHR (log e PD20 FEV1) Daily asthma symptom score Daily use of beta2 agonist Night‐time awakenings Probability of remaining in study Oro‐pharyngeal side effects | |
| Notes | No reply from author to clarify details of randomisation method For continuous outcomes change scores from baseline to endpoint (i.e. point of withdrawal) were reported A priori criteria for withdrawal due to lack of efficacy were established based on FEV1, morning PEFR, night‐time awakenings or clinical exacerbation requiring emergency hospital treatment MDI's used for all interventions. Formulations of FP with 1% lecithin and 10% lecithin used. Only data for 1% formulation included in meta‐analysis Placebo treatment arm also included: results not considered in this review |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Described as randomised; other information not available |
| Allocation concealment? | Unclear risk | Information not available |
| Blinding? All outcomes | Low risk | Identical inhaler devices |
O'Sullivan 2002.
| Methods | Randomised, double‐blind, parallel group trial. Method of randomisation not reported; blinding not reported. Withdrawals: N = 1. nonITT population Jadad score: 3 |
|
| Participants | N=36. Mean age: FP100: 32 (SEM 2.8); FP500: 32 (SEM 2.1); FP2000: 34 (SEM 2.8); FEV1% predicted: FP100: 81 (SEM 4.2); FP500: 86 (SEM 2.7); FP2000: 79 (SEM 3.3); Mean PC20: FP100: 1.11 (0.41); FP500: 0.55 (SEM 0.18); FP2000: 0.86 (SEM 0.51). Inclusion criteria: Atopic asthma as determined by skin prick test; fev1 >/=60% predicted; change in FEV1 >/=12% post SABA; PC20 fall of 4mg/mL; all participants were steroid naive; SABA prn Exclusion criteria: RTI in previous 6 months; steroid (I/O) use in 6 weeks prior to enrolment |
|
| Interventions | FP100 versus FP500 versus FP2000. Study duration: 2 weeks. 2 week run‐in period with placebo inhalers. Inhaler device: MDI | |
| Outcomes | FEV1; FEF; FEV1/FVC; PEF L/min; Symptoms; bronchial biopsy; PC20; adverse events | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Described as randomised; other information not available |
| Allocation concealment? | Unclear risk | Information not available |
| Blinding? All outcomes | Low risk | Identical inhaler devices |
Pauwels 2002.
| Methods | Randomised, double‐blind, double‐dummy crossover study. Method of randomisation: not reported; blinding: not reported. Jadad score: 2 |
|
| Participants | N=26, Other details not reported | |
| Interventions | Ciclesonide (400mcg QID; 800mcg QID; 800mcg QID), FP500 BID & FP1000 BID or PLA. Study duration: 6 x 1 week treatment period. Inhaler device: unclear | |
| Outcomes | % Cortisol suppression; PC20 | |
| Notes | Unpublished conference abstract | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Described as randomised; other information not available |
| Allocation concealment? | Unclear risk | Information not available |
| Blinding? All outcomes | Unclear risk | Information not available |
Pearlman 1997.
| Methods | Setting: multicentre study USA, hospital outpatient clinic Length of intervention period: 12 weeks Randomisation: yes, method not stated Allocation concealment: unclear Design: parallel group Masking: double blind Excluded: not stated Withdrawals: stated Baseline characteristics: comparable Jadad score: 3 | |
| Participants | 342 subjects randomised Age range: 12‐72 years Inclusion criteria: Diagnosis of asthma (ATS criteria 1987) Required maintenance inhaled corticosteroids for at least 3 months FEV! 50‐80 (%predicted) 15% or greater reversibility in FEV1 after inhaled beta2 agonist During last 7 days of run‐in period: No more than 12 puffs per day of albuterol No more than 4 morning PEFR 20% less than previous evenings No more than 2 nights wakening due to asthma requiring inhaled albuterol adequate compliance with study medication Exclusion criteria: Previous use of gold or methotrexate for control of asthma Inhaled cromoglycate or oral steroids in the last 4 weeks Significant co‐existent illness Pregnancy or lactation | |
| Interventions | 1. FP 50 mcg 1 actuation 2xdaily (100 mcg/d) 2. FP 100mcg 1 actuation 2xdaily (200 mcg/d) 3. FP 250 mcg 1 actuation 2xdaily (500 mcg/d) Delivery device: Diskhaler DPI |
|
| Outcomes | Outcomes expressed as change compared to baseline: FEV1 Morning PEFR Evening PEFR Daily asthma symptom score Night‐time awakenings Daily use of beta2 agonist Medical Outcomes Study Short Form (SF‐36A) Living with Asthma Questionnaire (LWA) Validated sleep scale Probability of remaining in study Physician global assessment of efficacy Serum cortisol Oro‐pharyngeal side effects | |
| Notes | No reply from author to clarify details of randomisation method For continuous outcomes change scores from baseline to endpoint (i.e. point of withdrawal) were reported A priori criteria for withdrawal due to lack of efficacy were established based on FEV1, morning PEFR, night‐time awakenings or clinical exacerbation requiring emergency hospital treatment Placebo treatment arm also included: results not considered in this review |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Described as randomised; other information not available |
| Allocation concealment? | Unclear risk | Information not available |
| Blinding? All outcomes | Low risk | Identical inhaler devices used |
Pearlman 1999.
| Methods | Randomised, double‐blind, double‐dummy, parallel group multi‐centre trial. Method of randomisation not reported. ITT population. Withdrawals: PLA 1; SAL42mcg: 2; FP88mcg: 1; FP220mcg: 1; SL42mcg/FP88mcg: 2; SAL42mcg/FP220mcg: 21 Jadad score: 3 |
|
| Participants | N = 136 (PLA: 23; FP88: 23; FP220: 23; SAL: 21; SAL/FP88: 25; SAL/FP220: 21).Mean age (range): PLA: 35 (12‐62); SAL: 29 (15‐57); FP88: 27 (13‐50); FP220: 32 (14‐61); SAL/FP88: 33 (14‐60); SAL/FP220: 26 (13‐52); Gender (M:F %): PLA: 43:57; SAL42: 67:33; FP88: 74:26; FP220: 57:43; SAL/FP88: 40:60; SAL/FP220: 67:33; Mean FEV1 (% predicted): PLA: 68; SAL: 70; FP88: 69; FP220: 65; SAL/FP88: 67; SAL/FP220: 69; Reversibility: PLA: 32; SAL: 27; FP88: Inclusion criteria: >/=12 years of age; ATS defined asthma (at least 6 months), requiring medical treatment; FEV1 between 50‐80% predicted; >/=15% increase in FEV1 post‐SABA; treatment with prn SABA; female participants had ‐ve pregnancy tests and either surgically sterile, postmenopausal at 1 year or using acceptable birth control for 1 month prior to participation Exclusion criteria: History of life‐threatening asthma; hypersensitivity reaction to beta‐agonists/corticosteroids; smoking within previous year/history >10 pack years; use of OCS/ICS or parenteral steroids (except for Flonase); use of steroid therapy in previous month; OCS treatment in previous 6 months; use of OTC medication that may affect the course of asthma; abnormal CXR; clinically significant abnormal 12‐lead ECG; history of concurrent disease (glaucoma, diabetes + hypertension) |
|
| Interventions | PLA versus FP88mcg BID versus FP220mcg BID versus SAL42mcg/FP88mcg BID versus SAL42mcg/FP220mcg BID daily. Inhaler device: MDI. Duration: 4 weeks | |
| Outcomes | FEV1; Am PEF; Symptoms; % days without asthma; % nights awakening due to asthma; rescue medication use; adverse effects | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | See Appendix 2 |
| Allocation concealment? | Low risk | See Appendix 2 |
| Blinding? All outcomes | Low risk | Identical inhaler devices |
Peden 1998.
| Methods | Setting: multicentre study USA, paediatric outpatient clinic Length of intervention period: 12 weeks Randomisation: yes, method not stated Allocation concealment: unclear Design: parallel group Masking: double blind, double dummy Excluded: not stated Withdrawals: stated Baseline characteristics: comparable Jadad score: 3 | |
| Participants | 177 children: 112M 65F Age range: 4‐11 years Inclusion criteria: History of chronic asthma (ATS criteria 1987) Symptoms requiring maintenance therapy for 3 months or more PEFR 85 (% predicted) or greater FEV1 50‐85 (% predicted) 15% or greater improvement in FEV1 after inhaled beta2 agonist Asthma stability during run‐in period, based on a priori beta2 agonist use and morning PEFR Exclusion criteria: Life‐threatening asthma Severe concurrent disease Systemic steroids in last month Previous treatment with methotrexate or gold | |
| Interventions | 1. FP 50mcg 2xdaily (100 mcg/d) via Accuhaler DPI 2. FP 100mcg 2xdaily (200mcg/d) via Accuhaler DPI |
|
| Outcomes | Outcomes expressed as change compared to baseline: FEV1 FEV1 (% predicted) Morning PEFR Morning PEFR (% predicted) Evening PEFR Daily asthma symptom score Daily use of beta2 agonist Night‐time awakening score Morning plasma cortisol Total urinary free cortisol excretion (mcg/24 hours) Probability of remaining in study | |
| Notes | No reply from author to clarify details of randomisation method For continuous outcomes change scores from baseline to endpoint (i.e. point of withdrawal) were reported A placebo treatment arm was also included: results not considered in this review |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | See Appendix 2 |
| Allocation concealment? | Low risk | See Appendix 2 |
| Blinding? All outcomes | Low risk | Double dummy design |
Peden 1998a.
| Methods | See Peden 1998 | |
| Participants | 174 children: 100M 74F See Peden 1998a for inclusion and exclusion criteria | |
| Interventions | 1. FP 50 mcg 2xdaily (100 mcg/d) via Diskhaler DPI 2. 100 mcg 2xdaily (200 mcg/d) via Diskhaler DPI |
|
| Outcomes | See Peden 1998 | |
| Notes | See Peden 1998 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | See Peden 1998 |
| Allocation concealment? | Low risk | See Peden 1998 |
| Blinding? All outcomes | Low risk | See Peden 1998 |
Pinnas 2005.
| Methods | Setting: multicentre study USA. Length of intervention period: 12 weeks. Randomisation: yes, method not stated. Allocation concealment: unclear. Design: parallel group Masking: double blind Excluded: not stated Withdrawals: stated Baseline characteristics: comparable Jadad score: 3 | |
| Participants | 397 adults randomised, 312 completed: 212F
Age range: >/=12 years
Inclusion criteria: treatment with SABA only for 6 months previously; am pre‐SABA FEV1 of 45‐80% predicted; >/=12% reversibility. No run‐in period described. |
|
| Interventions | FP:
1. 88mcg 2 x daily 2. 110mcg 2 x daily 2. 220mcg 2 x daily 3. Placebo Inhaler device: MDI |
|
| Outcomes | Change in FEV1; am PEF; rescue medication usage; symptoms; quality of life; adverse events | |
| Notes | Conference abstract Sourced from www.clinicalstudyresults.org |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | See Appendix 2 |
| Allocation concealment? | Low risk | See Appendix 2 |
| Blinding? All outcomes | Low risk | Identical inhaler devices |
Raphael 1999.
| Methods | Setting: multicentre study USA, primary care and hospital outpatient clinics Design: parallel group Length of intervention period: 12 weeks Randomisation: yes, computer generated sequence Allocation concealment: yes (central coding by pharmaceutical company sponsors) Masking: double blind Excluded: not stated Withdrawals: stated Baseline characteristics: comparable Jadad score: 5 | |
| Participants | 399 adolescents and adults: 167M 232F Age range: 12‐83 years Inclusion criteria: 12 years of age or older with established diagnosis of asthma (no further details) At end of run‐in period: FEV1 of 45‐65 (% predicted), or if FEV1 65‐80 (% predicted) additional evidence of sub‐optimal control (> 8 puffs rescue beta2 agonist/week, diurnal PEFR variability > 20%, any night‐time wakening due to asthma symptoms requiring beta2 agonist) 12% or greater increase in FEV1 after inhaled beta2 agonist Regular treatment with BDP or TA 8‐12 puffs/day for one month or longer Exclusion criteria: Use of systemic steroids, leukotriene modifiers, sodium cromoglycate or nedocromil within last month Smokers Asthma exacerbation during run‐in period Baseline asthma control: Reduced FEV1 of 45‐65 (% predicted) or significant symptoms (see above) | |
| Interventions | 1. FP 44 mcg 2 puffs 2xdaily (176 mcg/d) 2. FP 110 mcg 2 puffs 2xdaily (440 mcg/d) Delivery device: MDI |
|
| Outcomes | Change in FEV1 compared to baseline Change in FEF25‐75 compared to baseline Change in FVC compared to baseline Change in morning PEFR compared to baseline Change in evening PEFR compared to baseline Change in rescue beta2 agonist use compared to baseline (puffs/day) Change in daily asthma symptom score compared to baseline Change % days with no rescue beta2 agonist use compared to baseline Change in % days with no symptoms compared to baseline Withdrawal due to asthma exacerbation (No. of patients) Oropharyngeal side effects Oropharyngeal Candidiasis | |
| Notes | Study also included two further treatment arms with BDP: results not considered in this review | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | Computer generated sequence |
| Allocation concealment? | Low risk | Central coding by pharmaceutical company sponsors |
| Blinding? All outcomes | Low risk | Identical inhaler devices |
SAM40012.
| Methods | Setting: multicentre study Europe and Israel Design: parallel group Length of intervention period: 24 weeks Randomisation: yes Allocation concealment: not reported Masking: double blind, double dummy Excluded: not stated Withdrawals: stated Baseline characteristics: comparable Jadad score: 3 | |
| Participants | 367 children (ITT population: 355); 245M 110F Age range: 4‐11 years Inclusion criteria: 4‐11 years of age; participants symptomatic despite moderate dose of ICS for at least 4 weeks; symptom score during run‐in >/=2 on at least 3 of last 7 days; mean am PEF during run‐in of >50 to <85% of post SABA at randomisation | |
| Interventions | 1. FP100 BD (200mcg/d) 2. FP200 BD (400mcg/d) Inhaler device: DPI |
|
| Outcomes | % symptom free days and nights; use of reliever medication; am PEF (L/min); pm PEF (L/min); Clinic PEF; exacerbations; adverse events | |
| Notes | Unpublished study ‐ data retrieved and extracted from study detailed online | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | See Appendix 2 |
| Allocation concealment? | Low risk | See Appendix 2 |
| Blinding? All outcomes | Low risk | Identical inhaler devices |
Sheffer 1996.
| Methods | Setting: multicentre study USA, hospital outpatient clinics Length of intervention period: 12 weeks Randomisation: yes, computer generated sequence Allocation concealment: yes Design: parallel group Masking: double blind Excluded: not stated Withdrawals: stated Baseline characteristics: comparable Jadad score: 5 | |
| Participants | 307 patients: 185M 122F Age range: 12‐72 years Inclusion criteria: Diagnosis of asthma requiring at least 3 months of regular therapy FEV1 45‐75 (% predicted) 15% or greater reversibility in FEV1 after inhaled beta2 agonist Exclusion criteria: More than 1 month's use of oral steroids in the past Any oral, topical or inhaled steroid or cromoglycate in last month Previous history of life threatening asthma Pregnancy or lactation | |
| Interventions | 1. FP 25 mcg 1 puff 2xdaily (50 mcg/d) 2. FP 50 mcg 1 puff 2xdaily (100 mcg/d) 3. FP 50 mcg 2 puffs 2xdaily (200 mcg/d) Delivery device: MDI |
|
| Outcomes | Outcomes expressed as change compared to baseline: FEV1; Morning PEFR; Evening PEFR; Night‐time awakenings; Daily wheeze score; Daily cough score; Daily breathlessness score; Daily use of beta2 agonists; Probability of remaining in study | |
| Notes | Randomisation details confirmed by author For continuous outcomes change scores from baseline to endpoint (i.e. point of withdrawal) were reported A priori criteria for withdrawal due to lack of efficacy were established based on FEV1, morning and evening PEFR, diurnal variability in PEFR, night‐time awakenings or clinical exacerbation requiring emergency hospital treatment | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | Computer generated sequence |
| Allocation concealment? | Low risk | Off site by third party |
| Blinding? All outcomes | Low risk | Identical inhaler devices |
Sorkness 1999.
| Methods | Randomised, double‐blind, triple dummy, placebo controlled, parallel group study. Method of randomisation: computer‐generated randomisation. Blinding: matching inhalers. Withdrawals: Placebo: 0; FP100: 1; FP500: 1. ITT population. Jadad score: 5 |
|
| Participants | N = 168 (N for treatment groups considered by the review: Placebo: 30; FP100: 27; FP500: 30); Mean age (SE): PLA: 27.9 (1.6); FP100: 27.7 (1.7); FP500: 28.2 (1.6); Gender (M/F): PLA: 26/4; FP100: 26/1; FP500: 24/6; Race (White/other %): PLA: 67/33; FP100: 81/19; FP500: 77/23; FEV1 % predicted (SE): PLA: 87 (2.5); FP100: 88 (3.1); FP500: 83 (3.9) Inclusion criteria: 18‐51 years of age; documented diagnosis of asthma (>/=6 months according to ATS criteria; FEV1 at least 50% predicted Exclusion criteria: Pregnancy or lactation; corticosteroid/immunosuppressive therapy for 3 months prior to study entry; use of 140mg prednisone or equivalent in any dosage or form in previous year; current/prior use of antiasthma medication other than beta‐agonists, theophylline or cromolyn sodium; historical or current evidence of significant concomitant disease; use of oral contraceptives or other hormonal therapy; current use of prescription or over the counter medication known to interact with corticosteroids or to cause an abnormal response to exogenous glucocorticoids or reversal of normal nocturnal sleeping hours |
|
| Interventions | FP200 versus FP1000 versus Placebo. Delivery device: Rotadisk. Duration of study: 4 weeks | |
| Outcomes | AUC; Plasma cortisol; withdrawals; adverse events | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | Computer‐generated randomisation. |
| Allocation concealment? | Unclear risk | Information not available |
| Blinding? All outcomes | Low risk | Triple dummy design |
Sorkness 1999a.
| Methods | Randomised, double‐blind, triple dummy, placebo controlled, parallel group study. Method of randomisation: computer‐generated randomisation. Blinding: matching inhalers. Withdrawals: PLA: 1; FP100: 3; FP500: 2. ITT population. Jadad score: 5 |
|
| Participants | N = 119 (N for treatment groups considered by the review: PLA: 31; FP200: 29; FP500: 30); Mean age (SE): PLA: 32.1 (1.7); FP200: 31.4 (1.8); FP500: 33 (1.6); Gender (M/F): PLA: 25/6; FP200: 26/3; FP500: 26/4; Race (White/other %): PLA: 94/6; FP200: 93/7; FP500: 90/10; FEV1 % predicted (SE): PLA: 87 (2.7); FP200: 86 (2.7); FP500: 88 (3) Inclusion criteria: 18‐51 years of age; documented diagnosis of asthma (>/=6 months according to ATS criteria; FEV1 at least 50% predicted Exclusion criteria: Pregnancy or lactation; corticosteroid/immunosuppressive therapy for 3 months prior to study entry; use of 140mg prednisone or equivalent in any dosage or form in previous year; current/prior use of antiasthma medication other than beta‐agonists, theophylline or cromolyn sodium; historical or current evidence of significant concomitant disease; use of oral contraceptives or other hormonal therapy; current use of prescription or over the counter medication known to interact with corticosteroids or to cause an abnormal response to exogenous glucocorticoids or reversal of normal nocturnal sleeping hours |
|
| Interventions | FP200 versus FP500 versus Placebo. Delivery device: Rotadisk. Duration of study: 4 weeks | |
| Outcomes | AUC; Plasma cortisol; withdrawals; adverse events | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | Computer‐generated randomisation. |
| Allocation concealment? | Unclear risk | Information not available |
| Blinding? All outcomes | Low risk | Identical inhaler devices |
Verona 2003.
| Methods | Randomised, double‐blind parallel group, multi‐centre (Eastern Europe) study. Method of randomisation: computer‐generated randomisation schedule; Blinding ‐ both FP doses administered via Diskus. Withdrawals: FP200: 97/267; FP400: 83/261 ‐ High withdrawal rate as some trial centres did not participate in extension beyond 16 weeks. Ns used based on correspondence with GSK. Jadad score: 5 |
|
| Participants | N = 528 (FP200: 267; FP400: 261); Mean age: FP200: 7.8 (SD 2.1); FP400: 7.9 (SD 2); M/F (%): FP200: 72/28; FP400: 72/28; Ethnicity: White/non‐white (%): FP200: >99/<1; FP400: 100/0; mean duration of asthma symptoms (years): FP200: 3.82 (SD 2.2); FP400: 4.05 (SD 2.37); Treatment with BUD/FP/BDP/FLUN (%): FP200: 38/26/25/1; FP400: 41/33/24/<1; mean clinic PEF (% predicted): FP200: 105.1 (21.7); FP400: 101.6 (22.4); mean am PEF (L/min): FP200: 256.9 (SD 75); FP400: 255.4 (SD 72.2); pm PEF (L/min): 265.9 (SD 73.1); FP400: 261.3 (SD 72.2) | |
| Interventions | FP200 versus FP400 via Diskus inhaler. Study duration 52 weeks (2 week run‐in). Participants were allowed to take: oral theophylline, SABAs, DSCG or nedocromil sodium | |
| Outcomes | Asthma exacerbations; clinic PEF; diary PEF (am& pm); symptoms; adverse events | |
| Notes | GSK responded with data on Ns and means/SEMs 060904 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | See Appendix 2 |
| Allocation concealment? | Low risk | See Appendix 2 |
| Blinding? All outcomes | Low risk | Identical inhaler devices |
Wallin 2003.
| Methods | Randomised, double‐blind, parallel group trial. Method of randomisation: not reported. Blinding: identical inhalers. Withdrawals: FP400: N = 3; FP1000: N = 3. Unclear population for analysis ‐ assumed non‐ITT Jadad score: 4 |
|
| Participants | N = 56 (FP400: 19; FP1000: 19; FP+SAL: 18 ‐ baseline characteristics reported only for FP groups); M/F: FP400: 8/11; FP1000: 9/10; Mean age: FP400: 42 (SEM 12); FP1000: 40 (SEM 15); Asthma duration (months): FP400: 206 (SEM 130); FP1000: 176 (SEM 169); FEV1 L: FP400: 3.0 (SEM 0.9); FP1000: 3.3 (SEM 0.9); FEV1 % predicted: FP400: 91 (SEM 20); FP1000: 92 (SEM 12); PC20 mg/mL: FP400: 1.86 (SEM 2.33); FP1000: 6.22 (SEM 7.54); Reversibility (%): FP400: FEV1: 12 (SEM 11); PEF: 24 (SEM 19); FP1000: FEV1: 12 (SEM 11); PEF: 20 (SEM 17). Run‐in treatment: FP400: BUD: 2; BDP: 2; FP: 5; FP1000: BUD: 14; BDP: 2; FP: 3 Inclusion criteria: Symptomatic asthma during run‐in period in spite of normal medication (frequent asthma symptoms, need for SABAs, >/=20% variation between am and pm PEF); Lung function: 15% increase in FEV1 post‐SABA; PC20 methacholine <4mg/mL Exclusion criteria: RTI in previous four weeks |
|
| Interventions | FP200 BID (400mcg/d) versus FP500 BID (1000 mcg/d) via Diskus inhaler. SABA prn concomitant therapy. Study duration: 12 weeks (2 week run‐in period) | |
| Outcomes | PEF; FEV1; Bronchial lavage; immunohistochemistry | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | See Appendix 2 |
| Allocation concealment? | Low risk | See Appendix 2 |
| Blinding? All outcomes | Low risk | Identical inhaler devices |
Wasserman 1996.
| Methods | Setting: multicentre study USA, primary care and hospital outpatient clinics Length of intervention period: 12 weeks Randomisation: yes, method not stated Allocation concealment: unclear Design: parallel group Masking: double blind Excluded: not stated Withdrawals: stated Baseline characteristics: comparable Jadad score: 3 | |
| Participants | 331 adults randomised, 265 completed: 265M 66F Age range 12‐74 years Inclusion criteria: Diagnosis of asthma (ATS criteria 1987) for at least 6 months FEV1 50‐80 (% predicted) 15% or greater reversibility in FEV1 after inhaled beta2 agonist During run‐in: 12 or less puffs/day albuterol 4 or less mornings when PEFR decreased 20% or less than previous night PEFR 2 or less nights wakening requiring albuterol Good compliance Exclusion criteria: Smoking Use of any oral, inhaled or topical steroid within last month of study Oral steroids for 2 months or longer within last 6 month | |
| Interventions | 1. FP 50 mcg 1 actuation 2xdaily (100 mcg/d) 2. FP 100 mcg 1 actuation 2xdaily (200 mcg/d) 3. FP 250 mcg 1 actuation 2xdaily (500 mcg/d) Delivery device: Diskhaler DPI |
|
| Outcomes | Outcomes expressed as change compared to baseline: FEV1 FVC FEF 25‐75% Morning PEFR Evening PEFR Daily asthma score Change in night time awakenings Daily use of beta2 agonist Probability of remaining in study Physician global assessment of efficacy Oro‐pharyngeal side effects | |
| Notes | No reply from author to clarify details of randomisation method For continuous outcomes change scores from baseline to endpoint (i.e. point of withdrawal) were reported A priori criteria for withdrawal due to lack of efficacy were established based on FEV1, morning PEFR, night‐time awakenings or clinical exacerbation requiring emergency hospital treatment Study also included a placebo treatment arm: results not considered in this review |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Described as randomised; other information not available |
| Allocation concealment? | Unclear risk | Information not available |
| Blinding? All outcomes | Low risk | Identical inhaler devices |
Wolfe 1996.
| Methods | Setting: multicentre study USA, hospital outpatient clinics Length of intervention period: 12 weeks Randomisation: yes, method not stated Allocation concealment: unclear Design: parallel group Masking: double blind Excluded: not stated Withdrawals: stated Baseline characteristics: comparable Jadad score: 3 | |
| Participants | 304 adults: 169M 135F Age range: 12‐87 years Inclusion criteria: 12 years of age or older Diagnosis of moderate asthma, for at least 6 months Current treatment with inhaled corticosteroids and regular/as needed beta2 agonists Exclusion criteria: During run‐in period: More than 12 puffs albuterol daily for 3 or more days Diurnal variation in PEFR > 20% for 4 or more days Awakening more than 2 nights due to asthma symptoms And: Systemic steroids in last month Significant concurrent disease Pregnancy or lactation | |
| Interventions | 1. FP 100 mcg 2xdaily (200 mcg/d) 2. FP 250 mcg 2xdaily (500 mcg/d) 3. FP 500 mcg 2xdaily (1000 mcg/d) Delivery device: MDI |
|
| Outcomes | Outcomes expressed as change compared to baseline: FEV1 Morning PEFR Evening PEFR Daily use of beta2 agonist Daily cough score Daily wheezing score Daily breathlessness score Daily asthma symptom score Probability of remaining in the study Physician related global assessment of efficacy Oro‐pharyngeal side effects Morning plasma cortisol | |
| Notes | No reply from author to clarify details of randomisation method For continuous outcomes change scores from baseline to endpoint (i.e. point of withdrawal) were reported A priori criteria for withdrawal due to lack of efficacy were established based on FEV1, morning PEFR, night‐time awakenings or clinical exacerbation requiring emergency hospital treatment Study also included a placebo treatment arm: results not considered in this review |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Described as randomised; no other information available |
| Allocation concealment? | Unclear risk | Information not available |
| Blinding? All outcomes | Low risk | Identical inhaler devices |
ACTH: adrenocorticotropic hormone; ATS: American Thoracic Society; BDP: beclomethasone dipropionate; BHR: bronchial hyperresponsiveness; BUD: budesonide; DPI: dry powder inhaler; FEF25‐75: forced expiratory flow at 25 to 75% of FVC; FEV1: forced expired volume in one second; FP: fluticasone propionate; FSC: fluticasone/salmeterol combination; FVC: forced vital capacity; ICS: inhaled corticosteroid; ITT: intension‐to‐treat; mcg/d: micrograms per day; MDI: metered dose inhaler; PC20 FEV1: provocative concentration of inhalant required to produce a 20% fall in FEV1; PD20 FEV1: provocative dose of inhalant required to produce a 20% fall in FEV1; PEFR: peak expiratory flow rate; TA: triamcinolone acetonide
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Ayres 2000 | Delivery device comparison |
| Bisgaard 1999 | This study assessed a group of young children including infants under the age of two years |
| Dorini 2001 | Outcomes not relevant |
| FLTA2007 | Placebo controlled study |
| Fowler 2002 | Wrong comparison |
| Harrison 2001 | Wrong comparison |
| Kelly 2001 | Varying dose of FP |
| Laforce 2000 | Wrong comparison |
| Lipworth 1997 | Crossover study with intervention periods of only 4 days |
| Lundback 1993 | Delivery device comparison |
| Lundback 1994 | Delivery device comparison |
| Medici 2000 | Outcomes not relevant |
| Murray 1998 | Wrong comparison |
| Pieters 1998 | Delivery device comparison |
| Visser 2001 | Wrong comparison |
| Wittmann 1999 | Wrong comparison |
| Wolfe 2000 | Comparison of QID versus BID administration of 200mcg FP |
| ZuWallack 2000 | Once versus twice daily administration of FP (same dosage with different dosing strategy compared) |
Differences between protocol and review
We have opted against pooling data from studies in adults and children where outcomes are reported as litres (such as FEV1 and PEF). Heterogeneity in lung function outcomes such as FEV1 and PEF, are likely to be explained by differences in lung volume between these age groups since lower lung volumes in children may naturally yield results to those given by adults. However where metrics convert litres to percent predicted, and take into account age (as well as height and gender) we have retained combined estimates for adult and paediatric studies.
Contributions of authors
Nick Adams retrieved papers identified by the electronic search, handsearched additional sources for relevant studies, assessed trials for methodological quality, contacted authors to clarify details of trial design and/or request missing data, extracted data from included trials and wrote the text of the review.
Janine Bestall retrieved papers identified by the search, assessed trials for methodological quality, contacted authors for clarification of trial details and/or to request missing data.
Paul Jones provided editorial support.
Toby Lasserson and Benedict Griffiths assisted with study selection, study assessment, data extraction, data entry and analysis, and interpretation and writing of the update of the review, in November 2004, and August 2008.
Chris Cates provided methodological support and guidance with the content for the November 2004 and August 2008 updates.
Sources of support
Internal sources
No sources of support supplied
External sources
NHS Research and Development, UK.
Garfield Weston Foundation, UK.
Declarations of interest
None known.
Edited (no change to conclusions)
References
References to studies included in this review
Agertoft 1997 {published data only}
- Agertoft L, Pedersen S. Bone, growth and collagen markers in children treated with fluticasone propionate and budesonide.. European Respiratory Journal 1996;9(23 Suppl):295s. [DOI] [PubMed] [Google Scholar]
- Agertoft L, Pedersen S. Short‐term knemometry and urine cortisol excretion in children treated with fluticasone propionate and budesonide: a dose response study. European Respiratory Journal 1997;10(7):1507‐12. [DOI] [PubMed] [Google Scholar]
- FLIS‐02. http://ctr.gsk.co.uk 2005.
Allen 1998 {published data only}
- Allen DB, Bronsky EA, LaForce CF, Nathan RA, Tinkelman DG, Vandewalker ML, Konig P. Growth in asthmatic children treated with fluticasone propionate. Fluticasone Propionate Asthma Study Group. Journal of Pediatrics 1998;132(3 Pt 1):472‐7. [DOI] [PubMed] [Google Scholar]
- FLD‐220. http://ctr.gsk.co.uk 2005.
- Konig P, Ford L, Galant S, Lawrence M, Lemanske R, Mendelson L, et al. A 1‐year comparison of the effects of inhaled fluticasone propionate (FP) and placebo on growth in pre‐pubescent children with asthma. European Respiratory Journal 1996;9:S294. [Google Scholar]
- Mahajan P, Pearlman D, Okamoto L. The effect of fluticasone propionate on functional status and sleep in children with asthma and on the quality of life of their parents. Journal of Allergy & Clinical Immunology 1998;102(1):19‐23. [DOI] [PubMed] [Google Scholar]
Allen 2000 {published data only}
- Allen T, Wire P, Wolford J, Harding S. Fluticasone propionate (FP) via the diskus device maintained prednisone reduction beyond one year. American Journal of Respiratory and Critical Care Medicine 2000;161(3 Suppl):A185. [Google Scholar]
Ayres 1995 {published data only}
- Ayres JG, Bateman ED, Lundback B, Harris TAJ. High dose fluticasone propionate, 1 mg daily, versus fluticasone propionate, 2 mg daily, or budesonide, 1.6 mg daily, in patients with chronic severe asthma. European Respiratory Journal 1995;8:579‐86. [PubMed] [Google Scholar]
Boner 1999 {published data only}
- Boner A, Benedictis F, Rosa M, Decimo F, Piacentini G, Berg A, et al. Clincial Trial to compare the efficacy of two doses of fluticasone propionate (FP) 200 mcg/day and 400 mcg/day, administered for 6 weeks to asthmatic children, as assessed by bronchial responsiveness to methacholine. American Journal of Respiratory & Critical Care Medicine 1999;159(3 pt 2):A139. [Google Scholar]
- FLTB3051. Report on a multicentre, randomised, double‐blind, parallel‐group clinical trial to compare the efficacy and safety of the dry powder formulation of fluticasone propionate (FP) 200µg/day with fluticasone propionate 400µg/day administered for 6 weeks from a multidose powder device (Diskus™) to paediatric patients with chronic asthma. http://ctr.gsk.co.uk 2005.
Bukovskis 2002 {unpublished data only}
- Bukovskis M, Jurka N, Taivans I. Influence of large and small dose of fluticasone propionate on clinical symptoms of bronchial asthma, lung function, bronchial hyperreactivity and eosinophilia in induced sputum. European Respiratory Journal 2002;20(Suppl 38):P755. [Google Scholar]
Casale 2001 {published data only}
- Casale T B, Nelson HS, Stricker WE, Raff H, Newman KB. Suppression of hypothalamic‐pituitary‐adrenal axis activity with inhaled flunisolide and fluticasone propionate in adult asthma patients. Annals of Allergy, Asthma and Immunology 2001;87(5):379‐85. [DOI] [PubMed] [Google Scholar]
- Casale TB, Nelson HS, Stricker W, Fourre JA, Newman KB. Dose response of the effect of inhale fluticasone and flunisolide on endogenous cortisol secretion. American Journal of Respiratory and Critical Care Medicine 2000;161(3 Suppl):A776. [Google Scholar]
Chervinsky 1994 {published data only}
- Chervinsky P, As A, Bronsky EA, Dockhorn R, Noonan M, LaForce C, Pleskow W. Fluticasone propionate aerosol for the treatment of adults with mild to moderate asthma. The Fluticasone Propionate Asthma Study Group. Journal of Allergy & Clinical Immunology 1994;94(4):676‐83. [DOI] [PubMed] [Google Scholar]
- FLI201. A randomized, double‐blind comparative trial of three dosage regimens of inhaled fluticasone propionate versus placebo in adult patients with asthma. http://ctr.gsk.co.uk 2005. [Google Scholar]
Chetta 2003 {published data only}
- Chetta A, Zanini A, Foresi A, Donno M, Castagnaro A, D'Ippolito R, et al. Vascular component of airway remodelling in asthma is reduced by high dose of fluticasone. American Journal of Respiratory and Critical Care Medicine 2003;167(5):751‐7. [DOI] [PubMed] [Google Scholar]
- Foresi A, Pelucchi A, Dorini S, Mastropasqua B, Chetta A, D'Ippolito R, et al. Low‐dose (100µg, b.i.d.) inhaled fluticasone propionate (FP) is as affective as high‐dose (500µg, b.i.d.) in mild symptomatic asthma. European Respiratory Journal 2001;18(Supp 33):96s. [Google Scholar]
- Foresi A, Pelucchi A, Dorini S, Mastropasqua B, Chetta A, Ippolito RD, et al. Effect of low and high dose inhaled fluticasone propionate fp in symptomatic asthma. Annual Thoracic Society 97th International Conference; San Francisco CA ,May 18‐23, www.abstracts2view.com 2001. [Google Scholar]
Dahl 1993 {published data only}
- Dahl R, Lundback B, Malo JL, Mazza JA, Nieminen MM, Saarelainen P, Barnacle H. A dose‐ranging study of fluticasone propionate in adult patients with moderate asthma. International Study Group. Chest 1993;104:1352‐8. [DOI] [PubMed] [Google Scholar]
- FLIT05. www.clinicalstudyresults.org.
Derom 1999 {published data only}
- Derom E, Schoor J, Verhaeghe W, Vincken W, Pauwels R. Systemic effects of inhaled fluticasone propionate and budesonide in adult patients with asthma. American Journal of Respiratory & Critical Care Medicine 1999;160(1):157‐61. [DOI] [PubMed] [Google Scholar]
Derom 2005 {published data only}
- Derom E, Velde V, Marissens S, Engelstatter R, Vincken W, Pauwels R. Effects of inhaled ciclesonide and fluticasone propionate on cortisol secretion and airway responsiveness to adenosine 5'monophosphate in asthmatic patients. Pulmonary Pharmacology & Therapeutics 2005;18(5):328‐36. [DOI] [PubMed] [Google Scholar]
- Derom E, Velde V, Marissens S, Vincken W, Pauwels RA. Efficacy and systemic effects of ciclesonide and fluticasone in asthma patients. European Respiratory Journal 2001;18(Suppl 33):147s. [Google Scholar]
- Pauwels RA, Derom E, Velde V, Marissens S, Vincken W. Effects of inhaled ciclesonide and fluticasone propionate on cortisol secretion and PC²° for adenosine in asthma patients. American Journal of Respiratory and Critical Care Medicine 2002;165(Suppl 8):A768.. [Google Scholar]
Falcoz 2000 {published data only}
- Falcoz C, Horton J, Mackie A, Harding S, Daley‐Yates PT. Pharmacokinetics of fluticasone propionate inhaled via the diskhaler and diskus powder devices in patients with mild‐to‐moderate asthma. Cinical Pharmacokinetics 2000;39(Suppl 1):31‐7. [DOI] [PubMed] [Google Scholar]
FAP30001 {unpublished data only}
- FAP30001. A randomized, double‐blind, parallel‐group trial of inhaled fluticasone propionate/GR106642X 220mcg BID and 440mcg BID in adolescent and adult subjects with asthma. www.clinicalstudyresults.org 2005 [Accessed 14/11/2007].
FLIC15 {unpublished data only}
- FLIC15. www.clinicalstudyresults.org 2005.
FLIP01 {published data only}
- FLIP01. http://ctr.gsk.co.uk 2005. [Google Scholar]
FLIP01a {published data only}
- FLIP01. http://ctr.gsk.co.uk 2005. [Google Scholar]
FLIP39 {unpublished data only}
- FLIP39. http://ctr.gsk.co.uk 2005.
FLTA3014 {published data only}
- FLTA3014. www.clinicalstudyresults.org 2005.
FLTA3020 {published data only}
- FLTA3020. http:ctr.gsk.co.uk 2005. [Google Scholar]
FLTA3020a {published data only}
- FLTA3020. http:ctr.gsk.co.uk 2005.
FLTA3022 {unpublished data only}
- FLTA3022. A Randomized, Double‐Blind, Placebo‐Controlled Comparative Trial of Fluticasone Propionate 440mcg BID or 880mcg BID versus Placebo Administered via Metered‐Dose Inhaler in Propellant 11/12 or GR106642X in Adolescent and Adult Oral Corticosteroid Dependent Asthmatics. http://ctr.gsk.co.uk 2005. [Google Scholar]
FLTA3022a {unpublished data only}
- FLTA3022. A Randomized, Double‐Blind, Placebo‐Controlled Comparative Trial of Fluticasone Propionate 440mcg BID or 880mcg BID versus Placebo Administered via Metered‐Dose Inhaler in Propellant 11/12 or GR106642X in Adolescent and Adult Oral Corticosteroid Dependent Asthmatics. http://ctr.gsk.co.uk 2005. [Google Scholar]
Galant 1996 {published data only}
- Galant SP, Lawrence M, Meltzer EO, Tomasko M, Baker KA, Kellerman DJ. Fluticasone propionate compared with theophylline for mild‐to‐moderate asthma. Annals of Allergy Asthma & Immunology 1996;77(2):112‐8. [DOI] [PubMed] [Google Scholar]
Gershman 2000 {published data only}
- Gershman NH, Wong HH, Liu JT, Fahy JV. Low‐ and high‐dose fluticasone propionate in asthma; effects during and after treatment. European Respiratory Journal 2000;15(1):11‐8. [DOI] [PubMed] [Google Scholar]
Giannini 2003 {published data only}
- Giannini D, Franco A, Bacci E, Bartoli ML, Carnevali S, Cianchetti S, et al. Different doses of inhaled fluticasone propionate FP in the management of moderate asthmatic subjects. Annual Thoracic Society 97th International Conference; San Francisco CA ,May 18‐23; www.abstracts2view.com 2001. [Google Scholar]
- Giannini D, Franco A, Tonelli M, Bartoli ML, Carnevali S, Cianchetti S. Fifty mug b.i.d. of inhaled fluticasone propionate (FP) are effective in stable asthmatics previously treated with a higher dose of FP. Respiratory Medicine 2003;97(5):463‐7. [DOI] [PubMed] [Google Scholar]
Hofstra 2000 {published data only}
- Hofstra WB, Neijens HJ, Duiverman EJ, Kouwenberg JM, Mulder PG, Kuethe MC, Sterk PJ. Dose‐responses over time to inhaled fluticasone propionate treatment of exercise‐ and methacholine‐induced bronchoconstriction in children with asthma. Pediatric Pulmonology 2000;29(6):415‐23. [DOI] [PubMed] [Google Scholar]
- Hofstra WB, Sterk PJ, Neijens HJ, Kuethe MC, Mulder PGH, Duiverman EJ. The effect of 24 weeks treatment with 100 mcg or 250 mcg b.d. fluticasone propionate in reducing exercise‐induced bronchoconstriction in childhood asthma. American Journal of Respiratory & Critical Care Medicine 1997;155(4 (pt 2)):A267. [Google Scholar]
Ind 2003 {published data only}
- Ind PW, Dal Negro R, Colman N, Fletcher CP, Browning DC, James MH. Inhaled fluticasone propionate and salmeterol in moderate adult asthma I: lung function and symptoms. American Journal of Respiratory & Critical Care Medicine 1998;157(3):A416. [Google Scholar]
- Ind PW, Dal Negro R, Colman NC, Fletcher CP, Browning D, James MH. Addition of salmeterol to fluticasone propionate treatment in moderate‐ to‐severe asthma. Respiratory Medicine 2003;97(5):555‐62. [DOI] [PubMed] [Google Scholar]
- Ind PW, Dal Negro R, Fletcher CP, Browning DC, James MH. Inhaled salmeterol and fluticasone propionate therapy in moderate adult asthma. European Respiratory Journal 1997;10(Suppl 25):1S. [Google Scholar]
- SLGQ97. http://ctr.gsk.co.uk 2005.
Katz 1998 {published data only}
- FLIT85. http://ctr.gsk.co.uk 2005.
- Katz Y, Lebas FX, Medley HV, Robson R. Fluticasone propionate 50 micrograms BID versus 100 micrograms BID in the treatment of children with persistent asthma. Fluticasone Propionate Study Group. Clinical Therapeutics 1998;20(3):424‐37. [DOI] [PubMed] [Google Scholar]
Kemp 2004 {published data only}
- FLTA3001. http://ctr.gsk.co.uk 2005.
- Kemp JP, Osur S, Shrewsbury SB, Herje NE, Duke SP, Harding SM, et al. Potential effects of fluticasone propionate on bone mineral density in patients with asthma: a 2‐year randomized, double‐blind, placebo‐controlled trial. Mayo Clinic Proceedings 2004;79(4):458‐66. [DOI] [PubMed] [Google Scholar]
- Osur S, Chervinsky P, Herie N, Harding S, Kellerman D. Long term effects of fluticasone propionate (FP) inhalation aerosol in subjects with asthma. American Journal of Respiratory and Critical Care Medicine 1998;157(Suppl 3):A405. [Google Scholar]
Lawrence 1997 {published data only}
- Lawrence M, Wolfe J, Webb DR, Chervinsky P, Kellerman D, Schaumberg JP, Shah T. Efficacy of inhaled fluticasone propionate in asthma results from topical and not from systemic activity. American Journal of Respiratory & Critical Care Medicine 1997;156(3 Pt 1):744‐51. [DOI] [PubMed] [Google Scholar]
- Nielsen K, Okamoto L, Shah T. Importance of selected inhaler characteristics and acceptance of a new breath‐actuated powder inhalation device. Journal of Asthma 1997;34(3):249‐53. [DOI] [PubMed] [Google Scholar]
Li 1999 {published data only}
- Li JTC, Goldstein MF, Gross GN, Noonan MJ, Weisberg S, Edwards L, et al. Effects of fluticasone propionate, triamcinalone acetonide, prednisolone, and placebo on the hypothalamic‐pituatary axis. Journal of Allergy and Clinical Immunology 1999;103(4):622‐9. [DOI] [PubMed] [Google Scholar]
Lumry 2006 {published data only}
- FAP3007. A randomized, double‐blind, parallel‐group, placebo‐controlled 12‐week trial of inhaled fluticasone propionate 88mcg BID, 220mcg BID, and 440mcg BID versus placebo in propellant GR106642X in adolescent and adult subjects with asthma who are maintained on inhaled corticosteroid therapy. http://ctr.gsk.co.uk 2005.
- Lumry WR, Conway MM, LaForce CF, Pearlman DS, Scott CA, Herje NE, et al. Fluticasone propionate hydrofluoroalkane inhalation aerosol in patients receiving inhaled corticosteroids. Annals of allergy, asthma & immunology 2006;96(1):51‐9. [DOI] [PubMed] [Google Scholar]
- Nielsen K, Herje N, Wu W. Inhaled fluticasone propionate via metered‐dose inhaler with alternate propellant improves asthma‐related quality of life. American Journal of Respiratory and Critical Care Medicine 2002;165(Suppl 8):A107. [Google Scholar]
- Pearlman D, Kerwin E, Kim K, Murray A, Fischer T, Wu W, et al. Fluticasone propionate HFA‐134 A significantly improves asthma control in inhaled corticosteroid dependent asthmatics. American Journal of Respiratory and Critical Care Medicine 2002;165(Suppl 8):A770. [Google Scholar]
Meijer 1999 {published data only}
- Meijer RJ, Kerstjens HA, Arends LR, Kauffman HF, Koeter GH, Postma DS. Effects of inhaled fluticasone and oral prednisolone on clinical and inflammatory parameters in patients with asthma. Thorax 1999;54(10):894‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Nathan 2000 {published data only}
- FLTA2016. http://ctr.gsk.co.uk 2005.
- Nathan RA, Li JTC, Finn A, Jones R, Payne E, Wolford JP, et al. A dose ranging study of fluticasone propionate administered once daily via multidose powder inhaler to patients with moderate asthma. Chest 2000;118(2):296‐302. [DOI] [PubMed] [Google Scholar]
Nelson 1999 {published data only}
- FLTA2002. http://ctr.gsk.co.uk 2005.
- Nelson HS, Busse WW, deBoisblanc BP, Berger WE, Noonan MJ, Webb DR, Wolford JP, Mahajan PS, Hamedani AG, Shah T, Harding SM. Fluticasone propionate powder: oral corticosteroid‐sparing effect and improved lung function and quality of life in patients with severe chronic asthma. Journal of Allergy & Clinical Immunology 1999;103(2 Pt 1):267‐75. [DOI] [PubMed] [Google Scholar]
- Nimmagadda SR, Spahn JD, Nelson HS, Jenkins J, Szefler SJ, Leung DY. Fluticasone propionate results in improved glucocorticoid receptor binding affinity and reduced oral glucocorticoid requirements in severe asthma. Annals of Allergy Asthma & Immunology 1998;81(1):35‐40. [DOI] [PubMed] [Google Scholar]
Nieto 2001 {published data only}
- Nieto ML, Diego A, Perpina M, Compte L, Macian V. Are bronchial hyperresponsiveness and exhaled nitric oxide related in asthma? Effects of fluticasone. European Respiratory Journal 2001;18(Supp 33):335s. [Google Scholar]
Noonan 1995 {published data only}
- Noonan M, Chervinsky P, Busse WW, Weisberg SC, Pinnas J, Boisblanc BP, Boltansky H, Pearlman D, Repsher L, Kellerman D. Fluticasone propionate reduces oral prednisone use while it improves asthma control and quality of life. American Journal of Respiratory & Critcal Care Medicine 1995;152(5 Pt 1):1467‐73. [DOI] [PubMed] [Google Scholar]
- Okamoto LJ, Noonan M, DeBoisblanc BP, Kellerman DJ. Fluticasone propionate improves quality of life in patients with asthma requiring oral corticosteroids. Annals of Allergy Asthma & Immunology 1996;76(5):455‐61. [DOI] [PubMed] [Google Scholar]
Noonan 1998 {published data only}
- Noonan MJ, Chervinsky P, Wolfe J, Liddle R, Kellerman DJ, Crescenzi KL. Dose‐related response to inhaled fluticasone propionate in patients with methacholine‐induced bronchial hyperresponsiveness: a double‐blind, placebo‐controlled study. Journal of Asthma 1998;35(2):153‐64. [DOI] [PubMed] [Google Scholar]
O'Sullivan 2002 {published data only}
- O'Sullivan S, Cormican L, Murphy M, Poulter LW, Burke CM. Effects of Varying Doses of Fluticasone Propionate on the Physiology and Bronchial Wall Immunopathology in Mild‐to‐Moderate Asthma. Chest 2002;122(6):1966‐72. [DOI] [PubMed] [Google Scholar]
Pauwels 2002 {unpublished data only}
- Pauwels RA, Derom E, Velde V, Marissens S, Vincken W. Effects of inhaled ciclesonide and fluticasone propionate on cortisol secretion and PC²° for adenosine in asthma patients. American Journal of Respiratory and Critical Care Medicine 2002;165(Suppl 8):A768. [Google Scholar]
Pearlman 1997 {published data only}
- Mahajan P, Okamoto LJ, Schaberg A, Kellerman D, Schoenwetter WF. Impact of fluticasone propionate powder on health‐related quality of life in patients with moderate asthma. Journal of Asthma 1997;34(3):227‐34. [DOI] [PubMed] [Google Scholar]
- Pearlman DS, Noonan MJ, Tashkin DP, Goldstein MF, Hamedani AG, Kellerman DJ, Schaberg A. Comparative efficacy and safety of twice daily fluticasone propionate powder versus placebo in the treatment of moderate asthma. Annals of Allergy Asthma & Immunology 1997;78(4):356‐62. [DOI] [PubMed] [Google Scholar]
Pearlman 1999 {published data only}
- FLTA3015. http://ctr.gsk.co.uk. 2005.
- Pearlman DS, Stricker W, Weinstein S, Gross G, Chervinsky P, Woodring A, et al. Inhaled salmeterol and fluticasone: a study comparing monotherapy and combination therapy in asthma. Annals of Allergy, Asthma, & Immunology 1999;82(3):257‐65. [DOI] [PubMed] [Google Scholar]
Peden 1998 {published data only}
- FLTA2006. http://ctr.gsk.co.uk 2005.
- Noonan M, Berger W, Thomas R, Pinnas A, Nayak A, Hendricks V, et al. Inhaled fluticasone propionate dry powder administered via Diskus or Diskhaler is safe and effective in pediatric patients with chronic asthma. European Respiratory Journal 1997;10(Suppl 25):221S. [Google Scholar]
- Peden DB, Berger WE, Noonan MJ, Thomas MR, Hendricks VL, Hamedani AG, et al. Inhaled fluticasone propionate delivered by means of two different multidose powder inhalers is effective and safe in a large pediatric population with persistent asthma. Journal of Allergy & Clinical Immunology 1998;102(1):32‐8. [DOI] [PubMed] [Google Scholar]
Peden 1998a {published data only}
- FLTA2006. http://ctr.gsk.co.uk 2005.
- Noonan M, Berger W, Thomas R, Pinnas A, Nayak A, Hendricks V, et al. Inhaled fluticasone propionate dry powder administered via Diskus or Diskhaler is safe and effective in pediatric patients with chronic asthma. European Respiratory Journal 1997;10(Suppl 25):221S. [Google Scholar]
- Peden DB, Berger WE, Noonan MJ, Thomas MR, Hendricks VL, Hamedani AG, et al. Inhaled fluticasone propionate delivered by means of two different multidose powder inhalers is effective and safe in a large pediatric population with persistent asthma. Journal of Allergy & Clinical Immunology 1998;102(1):32‐8. [DOI] [PubMed] [Google Scholar]
Pinnas 2005 {published and unpublished data}
- FAP3008. A randomized, double‐blind, parallel group, placebo‐controlled 12‐week trial of inhaled fluticasone propionate 88mcg BID, 220mcg BID, and 440mcg BID versus placebo in propellant GR106642X in adolescent and adult subjects with asthma who are maintained on bronchodilator therapy. www.clinicalstudyresults.org.
- Pinnas JL, Noonan MJ, Weinstein SF, Chervinsky P, Scott CA, Herje NE, et al. Fluticasone propionate HFA‐134a pressurized metered‐dose inhaler in adolescents and adults with moderate to severe asthma. Journal of Asthma 2005;42(10):865‐71. [DOI] [PubMed] [Google Scholar]
- Weinstein S, Korenblat P, Raphael G. Fluticasone propionate HFA 134‐a significantly improves asthma control in inhaled corticosteroid naïve asthmatics. American Journal of Respiratory Critical Care Medicine 2002;165(8):A770. [Google Scholar]
Raphael 1999 {published data only}
- Raphael GD, Lanier RQ, Baker J, Edwards L, Rickard K, Lincourt WR. A comparison of multiple doses of fluticasone propionate and beclomethasone dipropionate in subjects with persistent asthma. Journal of Allergy & Clinical Immunology 1999;103(5 Pt 1):796‐803. [DOI] [PubMed] [Google Scholar]
SAM40012 {unpublished data only}
- SAM40012. www.clinicalstudyresults.org 2001.
Sheffer 1996 {published data only}
- Sheffer AL, LaForce C, Chervinsky P, Pearlman D, Schaberg A. Fluticasone propionate aerosol: efficacy in patients with mild to moderate asthma. Fluticasone Propionate Asthma Study Group. Journal of Family Practice 1996;42(4):369‐75. [PubMed] [Google Scholar]
Sorkness 1999 {published data only}
- Sorkness CA, LaForce C, Storms W, Lincourt WR, Edwards L, Rogenes PR. Effects of the inhaled corticosteroids fluticasone propionate, triamcinolone acetonide, and flunisolide and oral prednisone on the hypothalamic‐pituitary‐adrenal axis in adult patients with asthma. Clinical Therapeutics 1999;21(2):353‐67. [DOI] [PubMed] [Google Scholar]
Sorkness 1999a {published data only}
- Sorkness CA, LaForce C, Storms W, Lincourt WR, Edwards L, Rogenes PR. Effects of the inhaled corticosteroids fluticasone propionate, triamcinolone acetonide, and flunisolide and oral prednisolone on the hypothalamic‐pituitary‐adrenal axis in adult patients with asthma. Clinical Therapeutics 1999;21(2):353‐67. [DOI] [PubMed] [Google Scholar]
Verona 2003 {published data only}
- FAS30006. http://ctr.gsk.co.uk 2005.
- Verona E, Petrov D, Cserhati E, Hofman J, Geppe N, Davies P. A long term study to compare the safety and efficacy of Fluticasone Propionate (FP) 200mcg/day with 400mcg/day in asthmatic children aged 4‐11 years. Journal of Allergy and Clinical Immunology 2001;107(2):s105. [Google Scholar]
- Verona E, Petrov D, Cserhati E, Hofman J, Geppe N, Davies P. Fluticasone propionate in asthmatic children:a long term dose comparison of safety and efficacy. European Respiratory Journal 2001;18(Suppl 33):288s. [Google Scholar]
- Verona E, Petrov D, Cserhati E, Hofman J, Geppe N, Medley H, et al. Fluticasone propionate in asthma: a long term dose comparison study. Archives of Disease in Childhood 2003;88(6):503‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Wallin 2003 {published data only}
- Wallin A, Sue‐Chu M, Bjermer L, Ward J, Sandstrom T, Lindberg A, et al. Effect of inhaled fluticasone with and without salmeterol on airway inflammation in asthma. Journal of Allergy & Clinical Immunology 2003;112(1):72‐8. [DOI] [PubMed] [Google Scholar]
- Wilson SJ, Ward JA, Djukanovic R, Wallin A, Sue‐Chu M, Sandstrom T, et al. Effects of high and low dose inhaled fluticasone propionate (FP) compared to low dose FP combined with salmeterol (SAL) on airway inflammation in asthma. American Journal of Respiratory and Critical Care Medicine 2000;161(Suppl 3):A196. [Google Scholar]
Wasserman 1996 {published data only}
- Wasserman SI, Gross GN, Schoenwetter WF, Munk ZM, Kral KM, Schaberg A, Kellerman DJ. A 12‐week dose‐ranging study of fluticasone propionate powder in the treatment of asthma. Journal of Asthma 1996;33(4):265‐74. [DOI] [PubMed] [Google Scholar]
Wolfe 1996 {published data only}
- Wolfe JD, Selner JC, Mendelson LM, Hampel FJr, Schaberg A. Effectiveness of fluticasone propionate in patients with moderate asthma: a dose‐ranging study. Clinical Therapeutics 1996;18(4):635‐46. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Ayres 2000 {published data only}
- Ayres JG, Millar AB, Sykes AP. Clinical efficacy and safety of fluticasone propionate 1 mg twice daily administered via a HFA 134a pressurized metered dose inhaler to patients with severe asthma. Respiratory Medicine. 2000;94(Suppl B):S42‐50. [PubMed] [Google Scholar]
Bisgaard 1999 {published data only}
- Bisgaard H. Efficacy of inhaled fluticasone propionate in the treatment of young children with asthmatic symptoms: a dose comparison study. American Journal of Respiratory & Critical Care Medicine 1998;157(3):A711. [DOI] [PubMed] [Google Scholar]
- Bisgaard H, Gillies J, Groenewald M, Maden C. The effect of inhaled fluticasone propionate in the treatment of young asthmatic children: a dose comparison study. American Journal of Respiratory & Critical Care Medicine 1999;160(1):126‐31. [DOI] [PubMed] [Google Scholar]
Dorini 2001 {published data only}
- Dorini S, Pelucchi A, Leone C, Mastropasqua B, Guarnieri R, Chetta A, et al. Eosinophil apoptosis in induced sputum (IS) from patients with mild symptomatic asthma during treatment with Fluticasone propionate (FP). European Respiratory Journal 2001;18(Suppl 33):337s. [Google Scholar]
FLTA2007 {unpublished data only}
- FLTA2007. http://ctr.gsk.co.uk.
Fowler 2002 {published data only}
- Fowler SJ, Orr LC, Sims EJ, Wilson AM, Currie GP, McFarlane L, et al. Therapeutic ratio of hydrofluoroalkane and chlorofluorocarbon formulations of fluticasone propionate. Chest 2002;122(2):618‐23. [DOI] [PubMed] [Google Scholar]
Harrison 2001 {published data only}
- Harrison TW, Wisnieswki A, Honour J, Tattersfield AE. Comparison of the systemic effects of fluticasone propionate and budesonide given by dry powder inhaler in healthy and asthmatic subjects. Thorax 2001;56:186‐91. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kelly 2001 {unpublished data only}
- Kelly MM, Leigh R, Parameswaran K, Jayaram L, Belda J, Goodwin S, et al. Establishing dose response and relative potencies of inhaled corticosteroids. Annual Thoracic Society 97th International Conference; San Francisco CA, May 18‐23. 2001.
Laforce 2000 {published data only}
- Laforce CF, Pearlman DS, Ruff ME, Silvers WS, Weinstein SW, Clements DS, et al. Efficacy and safety of dry powder fluticasone propionate in children with persistent asthma. Annals of Allergy, Asthma and Immunology 2000;85(5):407‐15. [DOI] [PubMed] [Google Scholar]
Lipworth 1997 {published data only}
- Lipworth BJ, Clark DJ, McFarlane LC. Adrenocortical activity with repeated twice daily dosing of fluticasone propionate and budesonide given via a large volume spacer to asthmatic school children. Thorax 1997;52(8):686‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lundback 1993 {published data only}
- Lundback B, Alexander M, Day J, Hebert J, Holzer R, Uffelen R, et al. Evaluation of fluticasone propionate (500 micrograms day‐1) administered either as dry powder via a Diskhaler inhaler or pressurized inhaler and compared with beclomethasone dipropionate (1000 micrograms day‐1) administered by pressurized inhaler. Respiratory Medicine 1993;87:609‐20. [DOI] [PubMed] [Google Scholar]
Lundback 1994 {published data only}
- Lundback B, Dahl R, Jonghe M, Hyldebrandt N, Valta R, Payne SL. A comparison of fluticasone propionate when delivered by either the metered‐dose inhaler or the Diskhaler in the treatment of mild‐to‐moderate asthma. European Journal of Clinical Research 1994;5:11‐19. [Google Scholar]
Medici 2000 {published data only}
- Medici TC, Grebski E, Häcki M, Rüegsegger P, Maden C, Efthimiou J. Effect of one year treatment with inhaled fluticasone propionate or beclomethasone dipropionate on bone density and bone metabolism: a randomised parallel group study in adult asthmatic subjects. Thorax 2000;55:375‐82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medici TG, Grebski E, Hacki M, Ruegsegger P, Maden C, Efthimiou J. One‐year treatment with inhaled fluticasone propionate or beclomethasone dipropionate does not affect bone density and bone metabolism. A randomised, parallel group study in adult asthmatics. American Journal of Respiratory & Critical Care Medicine 1998;157:A407. [DOI] [PMC free article] [PubMed] [Google Scholar]
Murray 1998 {unpublished data only}
- Murray JJ, Friedman B, Chervinsky P, Fogarty C, Baker JW, Rogenes PR, et al. Fluticasone propionate (FP) is more effective than higher doses of beclomethasone dipropionate (BDP) in patients with moderate asthma. American Journal of Respiratory and Critical Care Medicine 1998;157(Suppl 3):A407. [Google Scholar]
Pieters 1998 {published data only}
- Pieters WR, Stallaert RA, Prins J, Greefhorst AP, Bosman HG, Uffelen R, et al. A study on the clinical equivalence and patient preference of fluticasone propionate 250 mcg twice daily via the Diskus/Accuhaler inhaler or the Diskhaler in adult asthmatic patients. Journal of Asthma 1998;35(4):337‐45. [DOI] [PubMed] [Google Scholar]
Visser 2001 {published and unpublished data}
- Visser MJ, Brand PLP, Kamps AWA, Duiverman EJ, Kauffman HF, Postma DS. One year treatment with different schedules of inhaled fluticasone propionate in childhood asthma: effects on lung function and immunological parameters. Annual Thoracic Society 97th International Conference; San Francisco CA ,May 18‐23; www.abstracts2view.com 2001. [Google Scholar]
- Visser MJ, Duiverman EJ, Postma DS, Arends LR, Brand PLP. High doses of inhaled fluticasone propionate dose‐dependently suppress adrenal cortical function in asthmatic children. European Respiratory Journal 2001;18(Supp 33):290s. [Google Scholar]
- Visser MJ, Postma DS, Arends LR, Vries TW, Duiverman EJ, Brand PL. One‐year treatment with different dosing schedules of fluticasone propionate in childhood asthma. American Journal of Respiratory and Critical Care Medicine 2001;164:2073‐7. [DOI] [PubMed] [Google Scholar]
- Visser MJ, Postma DS, Duiverman EJ, Arends LR, Kauffman HF, Brand PLP. Influence of fluticasone propionate on levels of exhaled nitric oxide in asthmatic children: a dose response study. European Respiratory Journal 2001;18(Supp 33):40s. [Google Scholar]
Wittmann 1999 {unpublished data only}
- Wittmann M, Thomas A, Petro W. Does doubling the inhaled steroid dosage at the beginning of an asthma therapy lead to a faster improvement of pathology or pulmonary function? [Führt die Verdopplung der inhalativen Steroiddosis zu Beginn einer Asthmatherapie zu enier schnelleren Verbesserung von Symptomatik oder Lungenfunktion?]. Pneumologie 1999;53(SH1). [Google Scholar]
Wolfe 2000 {published data only}
- Stanford R, Wightman D, Lincourt W, Edwards L, Crim C. Patient satisfaction with fluticasone propionate 250µg once daily. American Journal of Respiratory and Critical Care Medicine 2002;165(Suppl 8):A769. [Google Scholar]
- Wolfe J, Rooklin A, Grady J, Munk ZM, Stevens A, Prillaman B, et al. Comparison of once‐ and twice‐daily dosing of fluticasone propionate 200 micrograms per day administered by diskus device in patients with asthma treated with or without inhaled corticosteroids. Journal of Allergy and Clinical Immunology 2000;105(6 pt 1):1153‐61. [DOI] [PubMed] [Google Scholar]
ZuWallack 2000 {published data only}
- ZuWallack R, Adelglass J, Clifford DP, Duke SP, Wire PD, Faris M, Harding SM. Long‐term efficacy and safety of fluticasone propionate powder administered once or twice daily via inhaler to patients with moderate asthma. Chest 2000;118(2):303‐12. [DOI] [PubMed] [Google Scholar]
References to studies awaiting assessment
Bukovskis 2005 {published data only}
- Bukovskis M, Jurka N. Increase of neutrophil leukocyte count in induced sputum in patients on the treatment with high dose inhaled steroids [Abstract 303]. XIX World Allergy Organization Congress. 2005.
Osur 1998 {unpublished data only}
- Osur S, Chervinsky P, Herie N, Harding S, Kellerman D. Long term effects of fluticasone propionate (FP) inhalation aerosol in subjects with asthma. American Journal of Respiratory and Critical Care Medicine 1998;157(Suppl 3):A405. [Google Scholar]
Additional references
Adams 2008
- Adams NP, Bestall JC, Lasserson TJ, Jones PW. Inhaled fluticasone propionate versus placebo for chronic asthma in adults and children. Cochrane Database of Systematic Reviews 2008, Issue 4. [DOI] [PubMed] [Google Scholar]
Ayres 2000
- Ayres JG, Millar AB, Sykes AP. [Clinical efficacy and safety of fluticasone propionate 1 mg twice daily administered via a HFA 134a pressurized metered dose inhaler to patients with severe asthma. U.K. study group]. Respiratory Medicine 2000;94(Suppl B):S42‐50. [PubMed] [Google Scholar]
BTS 1997
- British Thoracic Society. The British guidelines on asthma management 1995 review and position statement. Thorax 1997;52(Suppl 1):S1‐20. [Google Scholar]
BTS 2003
- The British Thoracic Society/Scottish Intercollegiate Guidelines Network. British Guideline on the Management of Asthma. Thorax 2003;58(Suppl 1). [DOI] [PMC free article] [PubMed] [Google Scholar]
Cates 2008
- Cates CJ, Cates MJ. Regular treatment with salmeterol for chronic asthma: serious adverse events. Cochrane Database of Systematic Reviews 2008, Issue 3. [DOI: 10.1002/14651858.CD006363.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Egger 2001
- Egger M, Davey Smith G, Altman DG. Systematic Reviews in Healthcare: Meta‐analysis in Context. BMJ Books, 2001. [Google Scholar]
GINA 1995
- National Asthma Education and Prevention Program. Global initiative for asthma management and prevention NHBLI/WHO workshop report. National Institute of Health, Bethseda, MD 1995, issue NIH Publication No. 95‐3659.
Gross 1999
- Gross G, Thompson PJ, Chervinsky P, Vanden Burgt J. [Hydrofluoroalkane‐134a beclomethasone dipropionate, 400 mug, is as effective as chlorofluorocarbon beclomethasone dipropionate, 800 mug, for the treatment of moderate asthma]. Chest 1999;115(2):343‐51. [DOI] [PubMed] [Google Scholar]
NHLBI 1997
- National Asthma Education and Prevention Program. Guidelines for the Diagnosis and Managment of Asthma, Expert Panel Report No. 2. Bethesda MD: NIH/National Heart, Lung and Blood Institute 1997, issue NIH Publication No. 97‐4051.
Tattersfield 2004
- Tattersfield AE, Harrison TW, Hubbard RB, Mortimer K. Safety of inhaled corticosteroids. Proceedings of the American Thoracic Society 2004;1:171‐5. [DOI] [PubMed] [Google Scholar]


