Abstract
Background
Newborn infants have the ability to experience pain. Hospitalised infants are exposed to numerous painful procedures. Healthy newborns are exposed to pain if the birth process consists of assisted vaginal birth by vacuum extraction or by forceps and during blood sampling for newborn screening tests.
Objectives
To determine the efficacy and safety of paracetamol for the prevention or treatment of procedural/postoperative pain or pain associated with clinical conditions in neonates. To review the effects of various doses and routes of administration (enteral, intravenous or rectal) of paracetamol for the prevention or treatment of pain in neonates.
Search methods
We used the standard search strategy of the Cochrane Neonatal Review group to search the Cochrane Central Register of Controlled Trials (CENTRAL 2016, Issue 4), MEDLINE via PubMed (1966 to 9 May 2016), Embase (1980 to 9 May 2016), and CINAHL (1982 to 9 May 2016). We searched clinical trials' databases, Google Scholar, conference proceedings, and the reference lists of retrieved articles.
Selection criteria
We included randomised and quasi‐randomised controlled trials of paracetamol for the prevention/treatment of pain in neonates (≤ 28 days of age).
Data collection and analysis
Two review authors independently extracted data from the articles using pre‐designed forms. We used this form to decide trial inclusion/exclusion, to extract data from eligible trials and to request additional published information from authors of the original reports. We entered and cross‐checked data using RevMan 5 software. When noted, we resolved differences by mutual discussion and consensus. We used the GRADE approach to assess the quality of evidence.
Main results
We included nine trials with low risk of bias, which assessed paracetamol for the treatment of pain in 728 infants. Painful procedures studied included heel lance, assisted vaginal birth, eye examination for retinopathy of prematurity assessment and postoperative care. Results of individual studies could not be combined in meta‐analyses as the painful conditions, the use of paracetamol and comparison interventions and the outcome measures differed. Paracetamol compared with water, cherry elixir or EMLA cream (eutectic mixture of lidocaine and prilocaine) did not significantly reduce pain following heel lance. The Premature Infant Pain Profile score (PIPP) within three minutes following lancing was higher in the paracetamol group than in the oral glucose group (mean difference (MD) 2.21, 95% confidence interval (CI) 0.72 to 3.70; one study, 38 infants). Paracetamol did not reduce "modified facies scores" after assisted vaginal birth (one study, 119 infants). In another study (n = 123), the Échelle de Douleur et d'Inconfort du Nouveau‐Né score at two hours of age was significantly higher in the group that received paracetamol suppositories than in the placebo suppositories group (MD 1.00, 95% CI 0.60 to 1.40). In that study, when infants were subjected to a heel lance at two to three days of age, Bernese Pain Scale for Neonates scores were higher in the paracetamol group than in the placebo group, and infants spent a longer time crying (MD 19 seconds, 95% CI 14 to 24). For eye examinations, no significant reduction in PIPP scores in the first or last 45 seconds of eye examination was reported, nor at five minutes after the eye examination. In one study (n = 81), the PIPP score was significantly higher in the paracetamol group than in the 24% sucrose group (MD 3.90, 95% CI 2.92 to 4.88). In one study (n = 114) the PIPP score during eye examination was significantly lower in the paracetamol group than in the water group (MD −2.70, 95% CI −3.55 to 1.85). For postoperative care following major surgery, the total amount of morphine (µg/kg) administered over 48 hours was significantly less among infants assigned to the paracetamol group than to the morphine group (MD −157 µg/kg, 95% CI −27 to −288). No adverse events were noted in any study. The quality of evidence according to GRADE was low.
Authors' conclusions
The paucity and low quality of existing data do not provide sufficient evidence to establish the role of paracetamol in reducing the effects of painful procedures in neonates. Paracetamol given after assisted vaginal birth may increase the response to later painful exposures. Paracetamol may reduce the total need for morphine following major surgery, and for this aspect of paracetamol use, further research is needed.
Plain language summary
Paracetamol (acetaminophen) for prevention or treatment of pain in newborns
Review question: Is paracetamol effective and safe for the prevention or treatment of procedural or postoperative pain or pain associated with clinical conditions in newborn infants?
Background: Newborn infants have the ability to experience pain. Newborns treated in neonatal intensive care units are exposed to numerous painful procedures. Healthy newborns are exposed to pain if the birth process consists of assisted vaginal birth by vacuum extraction or by forceps and during blood sampling for newborn screening tests.
Study characteristics: We identified nine studies that reported comparisons in 728 infants of paracetamol versus placebo or other pain‐reducing interventions. The literature search was updated in May 2016.
Key results: Paracetamol for heel lance did not reduce pain compared with placebo (water or cherry elixir) or compared with EMLA cream (eutectic mixture of lidocaine and prilocaine). Paracetamol use was associated with a stronger response to pain than was seen with glucose. Paracetamol did not reduce pain in infants exposed to vacuum extraction or forceps at birth, and their response to a subsequent heel lance at two to three days of life was increased compared with placebo. For eye examination, paracetamol was effective in reducing pain compared with water in one study, but the pain response was stronger among paracetamol‐treated infants than in infants given 24% sucrose. In infants treated with paracetamol and morphine compared with morphine alone, the total amount of morphine required during the first 48 hours following major surgery to the chest or the abdomen was less in the paracetamol group. Paracetamol did not significantly reduce pain during heel lance. Paracetamol following assisted birth may increase the response to later exposure to painful interventions. Paraetamol may reduce the total need for morphine following major surgery, and further research is needed into this aspect of paracetamol use.
Quality of evidence: In general the studies were of good quality but the numbers of infants enrolled in the different studies were small. The overall quality of evidence was low.
Summary of findings
for the main comparison.
| Paracetamol compared with control for pain | ||||||
|
Patient or population: Neonates Settings: Any Intervention: Paracetamol Comparison: Control | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| [control] | Paracetamol | |||||
| Heel lance ‐ paracetamol (oral) compared to sterile water (oral) for prevention or treatment of pain in newborns | ||||||
| PIPP score (difference between baseline and heel lance period) | The mean PIPP score (difference between baseline and heel lance period) in the intervention groups was 1.4 higher (0.45 lower to 3.25 higher) | 72 (1) | ⊕⊕⊝⊝ low | Bias: there was low risk of bias in this study (see RoB tables) Consistency: N/A as there was only one study included Precision: there was low precision in the point estimate with a wide 95% CI Directness of the evidence: the trial was conducted in the target population Presence of publication bias: N/A as there was only one study included | ||
| Duration of crying (seconds) during the first 3 minutes | The mean duration of crying (seconds) during the first 3 minutes in the intervention groups was 8.1 higher (19.09 lower to 35.29 higher) | 72 (1) | ⊕⊕⊝⊝ low | Bias: there was low risk of bias in this study (see RoB tables) Consistency: N/A as there was only one study included Precision: there was low precision in the point estimate with a wide 95% CI Directness of the evidence: the trial was conducted in the target population Presence of publication bias: N/A as there was only one study included | ||
| Heel lance ‐ paracetamol (oral) compared to glucose (oral) for prevention or treatment of pain in newborns | ||||||
| PIPP (maximum score within 3 minutes following lancing) | The mean PIPP (maximum score within 3 minutes following lancing) in the intervention groups was 2.21 higher (0.72 to 3.7 higher) | 38 (1) | ⊕⊕⊝⊝ low | Bias: there was some concern about selection bias in this study (see RoB tables) Consistency: N/A as there was only one study included Precision: there was low precision in the point estimate with a wide 95% CI Directness of the evidence: the trial was conducted in the target population Presence of publication bias: N/A as there was only one study included | ||
| Heel lance ‐ paracetamol (oral) compared to EMLA (cream) for prevention or treatment of pain in newborns | ||||||
| PIPP (maximum score within 3 minutes following lancing) | The mean PIPP (maximum score within 3 minutes following lancing) in the intervention groups was 1.21 higher (0.38 lower to 2.8 higher) | 38 (1) | ⊕⊕⊝⊝ low | Bias: there was some concern about selection bias in this study (see RoB tables) Consistency: N/A as there was only one study included Precision: there was low precision in the point estimate with a wide 95% CI Directness of the evidence: the trial was conducted in the target population Presence of publication bias: N/A as there was only one study included | ||
| Eye examination ‐ paracetamol (oral) compared to placebo (sterile water) for eye examination for prevention or treatment of pain in newborns | ||||||
| PIPP score in first 45 seconds of eye examination | The mean PIPP score in first 45 seconds of eye examination in the intervention groups was 0.8 lower (1.69 lower to 0.09 higher) | 80 (1) | ⊕⊕⊝⊝ low | Bias: there was some concern about performance bias in this study (see RoB tables) Consistency: N/A as there was only one study included Precision: there was low precision in the point estimate with a wide 95% CI Directness of the evidence: the trial was conducted in the target population Presence of publication bias: N/A as there was only one study included | ||
| Eye examination ‐ paracetamol (oral) compared to 24% sucrose (oral) for prevention or treatment of pain in newborns | ||||||
| PIPP score in first 45 seconds of eye examination | The mean PIPP score in first 45 seconds of eye examination in the intervention groups was 3.9 higher (2.92 to 4.88 higher) | 81 (1) | ⊕⊕⊝⊝ low | Bias: there was some concern about performance bias in this study (see RoB tables) Consistency: N/A as there was only one study included Precision: there was low precision in the point estimate with a wide 95% CI Directness of the evidence: the trial was conducted in the target population Presence of publication bias: N/A as there was only one study included | ||
| Eye examination ‐ paracetamol compared to morphine for prevention or treatment of pain in newborns | ||||||
| PIPP score 5 minutes after eye examination | The mean PIPP score 5 minutes after eye examination in the intervention groups was 1.1 higher (0.7 lower to 2.9 higher) | 11 (1) | ⊕⊝⊝⊝ Very low |
Bias: there was low risk of bias in this study (see RoB tables) Consistency: N/A as there was only one study included Precision: there was low precision in the point estimate with a wide 95% CI. Only 11 infants were included in the analysis Directness of the evidence: the trial was conducted in the target population Presence of publication bias: N/A as there was only one study included | ||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; N/A: Not applicable; RoB: Risk of bias | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
Background
Evolving evidence suggests that neonates/infants experience pain (Ohlsson 2000; Ohlsson 2007). This was documented as early as 1518, when Jörgen Ratgeb painted the circumcision of Jesus. The picture of crying Jesus shows the same facial expressions that were later depicted in an etching of the same event by Rembrandt in 1630 (Schwartz 1977; Ohlsson 2007). In 1872, Darwin commissioned photographs of infants experiencing pain and described the facial, vocal and bodily expressions of infants in pain (Darwin 1872; Ohlsson 2007). Similar observations form the basis for several validated neonatal pain scales in use today (Ohlsson 2007).
Over the centuries, little progress was made in the prevention and management of infant pain. The first controlled trial of an intervention for pain in infants was probably that conducted in the 1960s by Palmer, who found in a double‐blind, controlled study involving 86 infants with teething pain that an active gel (choline salicylate) was more effective than placebo in reducing pain (Palmer 1962). Dorsal penile nerve block (DPNB) was introduced in 1978 for circumcision (Kirya 1978), and in 1983 in a double‐blind investigation, Holve and co‐workers demonstrated that circumcision following DPNB with an injection of lidocaine reduced the time spent crying and reduced the mean increase in heart rate compared with DPNB with saline or no DPNB (Holve 1983). In a trial that used random allocation for assignment of infants to study groups, Harpin and Rutter demonstrated that a mechanical heel lance was considerably less painful than a manual heel lance (Harpin 1983).
In 1987, Anand and co‐workers reported the results of a small randomised controlled trial (Anand 1987a). Preterm infants undergoing ligation of a patent ductus arteriosus were given nitrous oxide and d‐tubocurarine. Eight infants received additional intravenous fentanyl (10 µg/kg) to the anaesthetic regimen, and eight infants did not. Hormonal responses to the surgery were significantly greater in the non‐fentanyl group. In contrast to the fentanyl group, the non‐fentanyl group had circulatory and metabolic complications postoperatively (Anand 1987a).
Later the same year, Anand and Hickey published the very influential paper, 'Pain and its effects in the human neonate and fetus', in The New England Journal of Medicine (Anand 1987b). As of 16 May 2015, the paper had been quoted more than 700 times according to the Web of Science. Anand and Hickey provided evidence that fetuses that are mature enough to survive outside the womb with or without extensive life support have the anatomical, biochemical and physiological requisites in place to respond to painful stimuli (Anand 1987b). That same year, the American Academy of Pediatrics published a one‐page opinion paper on "neonatal anesthesia" and stated "The Committee on Fetus and Newborn, the Committee on Drugs, the Section on Anesthesiology, and the Section on Surgery believe that local or systemic pharmacological agents now available permit relatively safe administration of anesthesia or analgesia to neonates undergoing surgical procedures and that such administration is indicated according to the usual guidelines for the administration of anesthesia to high‐risk, potentially unstable patients" (AAP 1987).
Infants treated in neonatal intensive care units (NICUs) are exposed to numerous painful procedures. Did increased awareness in 1987 about neonatal pain and its treatment change how healthcare workers approach pain management? Many surveys on pain management have been conducted, but changes in clinical practice have not occurred quickly. A survey of 30 Canadian level III NICUs in 1992 with a 87% response rate concluded that procedural and disease‐related pain is frequently untreated (Fernandez 1994).
Between September 2005 and January 2006, data on all painful and stressful procedures and corresponding analgesic therapy from the first 14 days of admission were prospectively collected from 430 neonates admitted to 13 tertiary care centres in the Paris region of France (Carbajal 2008). The mean (standard deviation (SD)) postmenstrual age (PMA) of the infants and the length of the intensive care unit stay were 33.0 (4.6) weeks and 8.4 (4.6) days, respectively. Neonates experienced 60,969 first‐attempt procedures, of which 42,413 (69.6%) were painful and 18,556 (30.4%) were stressful procedures. Neonates experienced a median of 115 (range 4 to 613) procedures during the study period and 16 (range 0 to 62) procedures per day of hospitalisation (Carbajal 2008). In order of frequency, the five most common painful procedures to which the neonates were exposed consisted of nasal aspiration, tracheal aspiration, heel lance, adhesive removal and gastric tube insertion. The five most frequently performed stressful procedures to which infants were exposed included nursing care, oral aspiration, washing of the neonate, blood pressure measurement and x‐rays (Carbajal 2008).
In an observational, prospective study conducted between February 2009 and August 2009 in the level III NICU of Sophia Children's Hospital in Rotterdam, The Netherlands, bedside data were collected on all procedures that infants underwent during the first 14 days of admission (Roofthooft 2014). A procedure was defined as any medical, nursing, surgical, diagnostic or therapeutic intervention provided to a patient. Study authors did not distinguish between painful and stressful procedures. Invasive or painful procedures were defined as interventions that cause mucosal or skin injury from removal or introduction of foreign material (Roofthooft 2014). A total of 21,076 procedures were performed during 1730 patient‐days (mean 12.2 days) in the 175 neonates studied. The mean number of painful procedures per neonate per day was 11.4 (SD 5.7) — significantly fewer than the 14.3 (SD 4.0) painful procedures reported in a similar study in the unit in 2001. Use of analgesics was 36.6% compared with 60.3% in 2001. Sixty‐three per cent of all peripheral arterial line insertions failed versus 37.5% in 2001, and 38% of intravenous cannula insertions failed versus 30.9% in 2001. Study authors concluded that the mean number of painful procedures per NICU patient per day had declined over time (Roofthooft 2014).
To our knowledge, no surveys have been performed to determine how commonly newborns are exposed to clinically painful conditions such as, for example, birth trauma, congenital anomalies (myelomeningoceles, hydrocephalus, open cutaneous lesions), necrotizing enterocolitis and burns.
Description of the condition
The International Association for the Study of Pain has defined pain as "an unpleasant sensory and emotional experience associated with actual or potential tissue damage or described in terms of such damage" (Merskey 1979). Interpretation of pain is subjective. Each person forms an internal construct of pain through encountered injury. Several experts have suggested that the neonate's expression of pain does not fit within the strict definition of the International Association for the Study of Pain because of the requirement for self‐report. This lack of ability to report pain contributes to the failure of healthcare professionals to recognise and treat pain aggressively during infancy and early childhood. Neonates cannot verbalise their pain; therefore they depend on others to recognise, assess and manage their pain. Healthcare professionals therefore can diagnose neonatal pain only by recognising the neonate's associated behavioural and physiological responses (AAP 2000; CPS 2000).
Optimal pain management requires competent pain assessment, which can be especially difficult to perform in neonates. Pain assessment tools can be biochemical (salivary and serum cortisol); physiological (heart rate); and behavioural (facial observation). A pain assessment tool should be multi‐dimensional, including measurements of both physiological and behavioural indicators of pain, because neonates cannot self‐report (AAP 2006). Pain associated with chronic illness states, such as necrotizing enterocolitis, asphyxia, intracranial haemorrhage, hydrocephalus, sepsis and chronic hypoxaemia secondary to lung disease, has been largely ignored (Stevens 2000). Some infants will require pain management as part of palliative care (Stevens 2000). Neonatal postoperative pain scales have been developed (Ambuel 1992; Krechel 1995; Büttner 2000); of these, the COMFORT scale appears to perform the best (van Dijk 2009; Franck 2011). Examples of pain scores include the Neonatal Infant Pain Score (NIPS) (Lawrence 1993); the Neonatal Facial Coding System (NFCS) (Grunau 1998); the Facial Action Coding System (FACS) (Craig 1994); the Premature Infant Pain Profile (PIPP) (Stevens 1996); Échelle de Douleur et d'Inconfort du Nouveau‐Né (EDIN; neonatal pain and discomfort scale) (Debillon 2001); the Behavioral Indicators of Infant Pain Scale (BIIP) (Holsti 2007; Holsti 2008); the Bernese Pain Scale for Neonates (BPSN) (Cignacco 2004); and numerous others (AAP 2006). Specific postoperative pain scores include COMFORT (Ambuel 1992), CRIES (Krechel 1995) and the Children's and Infants' Postoperative Pain Scale (CHIPPS) (Büttner 2000).
Extremely low gestational age infants (less than 27 weeks' PMA) have pain responses similar to those of more mature infants, but their responses are dampened (Gibbins 2008). Facial activities are increased following painful procedures (heel lance) and the magnitude of responses is proportional to PMA, with the most immature infants (less than 27 weeks' PMA) showing the least amount of change (Gibbins 2008).
Description of the intervention
Paracetamol is the most commonly prescribed analgesic for the treatment of acute pain in adults and children (Tzortzopoulou 2011); it is also used to treat pain in infants. Paracetamol can be administered intravenously, orally (or via a gastric tube) or rectally (Wang 2014). In a survey by UK anaesthetists of intravenous paracetamol use in neonates and infants younger than one year of age, maintenance doses were 7.5 mg/kg or 10 mg/kg, with a dosing interval of six or eight hours in preterm infants (Wilson‐Smith 2009). In a study of intravenous acetaminophen pharmacokinetics conducted in Australia, the postoperative dose given every six hours was 10 mg/kg for infants with a PMA of 28 to 32 weeks, 12.5 mg/kg for infants with a PMA of 32 to 36 weeks and 15 mg/kg for infants with a PMA of 36 or more weeks (Palmer 2008). Following this study, the unit continued to use the reported doses based on PMA (Palmer 2008). Overall, paracetamol has a reasonable safety profile; however, it has been reported that unconjugated hyperbilirubinaemia impacts upon clearance of paracetamol (Palmer 2008). In addition, acetaminophen‐induced hepatic failure with encephalopathy has been described in a term newborn who received oral acetaminophen from his parents every four hours following circumcision (Walls 2007).
In a meta‐pharmacokinetic analysis of population pharmacokinetics of paracetamol from birth to adulthood, Wang and co‐workers included eight previously published studies that enrolled neonates (one to 76 days old), infants (0.11 to 1.33 years old), children (two to seven years old) and adults (19 to 34 years old) (Wang 2014). Their results showed that developmental changes in clearance were best described on the basis of a power function with an exponent that varied with body weight. This exponent was found to vary from a value of 1.2 for neonates to 0.75 for older children and adults (Wang 2014). Based on their model, the study authors presented dosing regimens of intravenous paracetamol, aiming for a target paracetamol concentration of 9 mg/L in individuals weighing between 0.5 kg and 50 kg. The loading dose for neonates varies from 5.6 mg/kg at a body weight of 0.5 kg to 38.3 mg at a body weight of 3.0 kg. The maintenance dose (administered four times daily) varies from 5.1 mg/kg at 0.5 kg body weight to 8.5 mg at 3.0 kg body weight (Wang 2014). These findings of developmental changes based on body weight justify subgroup analyses based on the weight of the infant.
Potentially more serious complications following perinatal/neonatal exposure to paracetamol have been reported. In an animal model, Viberg and co‐workers examined whether neonatal paracetamol exposure could affect the development of the brain, manifested as adult behaviour and cognitive deficits, as well as changes in the response to paracetamol (Viberg 2014). Ten‐day‐old mice were administered a single dose of paracetamol (30 mg/kg/body weight) or repeated doses of paracetamol (30 + 30 mg/kg body weight, four hours apart). Concentrations of paracetamol and brain‐derived neurotrophic factor (BDNF) were measured in the neonatal brain. Behavioural testing was done when the animals reached adulthood. Neonatal exposure to paracetamol (2 × 30 mg) resulted in altered locomotor activity on exposure to a novel home cage arena and failure to acquire spatial learning in adulthood, without affecting thermal nociceptive responding while exhibiting paracetamol‐induced antinociceptive and anxiogenic‐like behaviour in adulthood. Study authors suggested that behavioural alterations in adulthood may be due, in part, to paracetamol‐induced changes in BDNF levels in key brain regions at a critical time during development. They concluded that exposure to, and presence of, paracetamol during a critical period of brain development can induce long‐lasting effects on cognitive function and can alter the adult response to paracetamol in mice (Viberg 2014). As this study was conducted in mice, findings may not be relevant to human neonates; however, the study raises concerns about the safety of paracetamol which need to be evaluated.
In an ecological study conducted in humans and using country‐level data for the period 1984 to 2005, prenatal use of paracetamol was correlated with autism/autism spectrum disorder (ASD) (Bauer 2013). To explore the relationship of early neonatal paracetamol exposure to autism/ASD, population‐weighted average male autism‐prevalence rates for all available countries and US states were compared with male circumcision rates — a procedure for which paracetamol has been widely prescribed since the mid‐1990s. For studies including boys born after 1995, a strong correlation was noted between country‐level autism/ASD prevalence in males and a country's circumcision rate (r = 0.98) (Bauer 2013). "Taken together, these ecological findings and mechanistic evidence suggest the need for formal study of the role of paracetamol in autism" (Bauer 2013).
In a recent Spanish birth cohort study Avella‐Garcia and co‐workers reported on 2644 mother‐child pairs recruited during pregnancy of whom 43% of children evaluated at age one year (N = 2195) and 41% of those assessed at age five years (N = 2001) were exposed to acetaminophen up to 32 weeks PMA (Avella‐Garcia 2016). They concluded that prenatal exposure to acetaminophen may affect attention function at five years of age, affecting males and females differently. The results suggest an association with hyperactivity/impulsivity behaviours for all children and that the associations appear to be dependent on the frequency of exposure, but further dosage assessments are warranted (Avella‐Garcia 2016).
How the intervention might work
Paracetamol is a derivative of acetanilide with analgesic, antipyretic and weak anti‐inflammatory properties. It is used as a common analgesic in all age groups but may cause liver, blood cell and kidney damage (National Library of Medicine 2013). Paracetamol in low concentrations stimulates, and in high concentrations inhibits, the synthesis of prostaglandins. In vivo (in adults), 500 mg of paracetamol causes a pronounced reduction in prostacyclin synthesis but has no effect on thromboxane synthesis (Grèen 1989). In vitro paracetamol is a weak inhibitor of cyclo‐oxygenase (COX)‐1 and COX‐2; therefore the possibility exists that it inhibits a so‐far unidentified form of COX, perhaps a COX‐3 (Botting 2000). In adults and children, a single dose of paracetamol provides effective analgesia for about half of patients with acute postoperative pain, for a period of about four hours, and is associated with few, mainly mild, adverse events (Toms 2008). A single dose of both intravenous propacetamol and intravenous paracetamol provides around four hours of effective analgesia for about 37% of patients with acute postoperative pain.
Why it is important to do this review
Infants may be exposed to prolonged and repeated pain during lengthy hospitalisation in neonatal intensive care units (Grunau 1998). The low tactile threshold in preterm infants when they are in the neonatal intensive care unit, while their physiological systems are unstable and immature, potentially renders them more vulnerable to the effects of repeated invasive procedures (Grunau 2006). Animal and human studies have documented how neonatal pain is associated with short‐term and long‐term adverse consequences (Fitzgerald 2009; Hall 2012). Growing evidence suggests that not only do these early events induce acute changes, but permanent structural and functional changes may result (Porter 1999). Early procedural pain in very preterm infants may contribute to impaired growth and brain development (Brummelte 2012; Vinall 2012). Enhanced survival of extremely low‐birth‐weight infants makes them more susceptible to the effects of pain and stress because of increased exposure (Hall 2012). "Effective pain management in infants requires a specialist approach ‐ analgesic protocols that have been designed for older children cannot simply be scaled down for central nervous system pain pathways and analgesic targets that are in a state of developmental transition" (Fitzgerald 2009).
The most common non‐pharmacological techniques used to treat pain include non‐nutritive sucking with or without sucrose, kangaroo care, swaddling and massage therapy (Hall 2012). Drugs used to treat neonatal pain include opiates, benzodiazepines, barbiturates, ketamine, propofol, acetaminophen and local and topical anaesthetics (Hall 2012).
In the prospective study conducted in 13 intensive care units in Paris, France, of 42,413 painful procedures, 2.1% were performed with pharmacological therapy alone; 18.2% with non‐pharmacological interventions alone; 20.8% with pharmacological, non‐pharmacological or both types of therapy; 79.2% without specific analgesia; and 34.2% while the neonate was receiving concurrent analgesic or anaesthetic infusions for other reasons. Study authors concluded, "During neonatal intensive care in the Paris region, large numbers of painful and stressful procedures were performed, the majority of which were not accompanied by analgesia" (Carbajal 2008).
A similar prospective study was conducted in 14 Canadian neonatal intensive care units between February and October 2007 (Johnston 2011b). Infants (n = 582) were followed for one week for all invasive procedures. A total of 3508 tissue‐damaging (mean = 5.8, SD = 15) and 14,085 non‐tissue‐damaging (mean = 25.6, SD = 15) procedures were recorded. Half of the procedures (46% tissue‐damaging and 57% non‐tissue‐damaging) had no analgesic interventions (Johnston 2011b). Study authors noted that parental presence had a positive influence on comfort strategies, and they offered encouragement and support to parents to remain with their infant during procedures (Johnston 2011b). Non‐pharmacological interventions for procedural pain in neonates include sensory stimulation approaches, oral sweet solutions and maternal interventions (Johnston 2011a).
Surveys of procedural pain in neonates and associated analgesic interventions have been conducted in many countries, including Australia (Foster 2013), Canada (Johnston 2011b), France (Carbajal 2008), The Netherlands (Roofthooft 2014), Japan (Ozawa 2013), Korea (Jeong 2013), Italy (Lago 2013) and Sweden (Gradin 2011). Although adherence to national or international pain guidelines has increased, infant pain remains under‐treated.
Paracetamol offers an advantage over other pain‐reducing interventions in that it can be administered via nasogastric tube, intravenously or rectally. In a review of health policy and health economics related to neonatal pain, Lee was not able to identify any studies that examined quality of life adjustment strictly as a function of pain (Lee 2007).
Controversy continues regarding the safety and long‐term impact of many interventions aimed at reducing stress or pain (or both) in neonates (McPherson 2014). These interventions include sucrose, anaesthetics and pharmacological agents (benzodiazepines and opioids) (McPherson 2014).
The possible link between perinatal exposure to paracetamol and autism has recently been raised and needs to be explored further (Bauer 2013).
Researchers and healthcare providers working with neonates have an obligation to reduce painful stimuli and interventions and to identify effective pain‐reducing pharmacological and non‐pharmacological agents. Paracetamol may be one such agent. By performing this review, we hope to ascertain which types of pain are amenable to treatment with paracetamol.
Objectives
Primary objective
To determine the efficacy and safety of paracetamol for the prevention or treatment of procedural/postoperative pain or pain associated with clinical conditions in neonates.
Secondary objective
To review the effects of various doses and routes of administration (enteral, intravenous or rectal) of paracetamol for the prevention or treatment of pain in neonates. We designed the main comparisons according to intention of use, that is, paracetamol for prevention or treatment of pain. We included separate comparisons based on the painful intervention/procedure/condition (heel lance, insertion of nasogastric tube, insertion of intravenous catheter, lumbar puncture, assisted vaginal birth, postoperative pain, birth trauma, congenital anomalies such as myelomeningocoele and open cutaneous lesions) and the mode of administration of paracetamol. Within these comparisons, we planned to assess in subgroups (when possible) effects based on postmenstrual age (PMA) at the birth of randomly assigned infants (< 28 weeks, 28 weeks to 31 weeks, 32 weeks to 36 weeks and ≥ 37 weeks) or based on birth weight (or current weight) categories (≤ 1000 grams, 1001 to 1500 grams, 1501 to 2500 grams and ≥ 2501 grams).
Methods
Criteria for considering studies for this review
Types of studies
We included randomised and quasi‐randomised controlled trials of paracetamol for the prevention or treatment of pain in neonates. We planned to include cluster‐randomised trials. We did not include cross‐over trials. We did not include letters to editors, narrative reviews and editorials, but we read these to identify eligible articles.
Types of participants
Term or preterm neonates who underwent one or more of the following painful procedures during their hospital stay or as outpatients: heel lance, venipuncture, lumbar puncture, bladder tap, insertion of nasogastric tubes, insertion of endotracheal tubes, insertion of venous or arterial catheters/lines or chest drains, etc. or surgery (including any surgery performed in the operating room); or who have a clinical condition that is painful (such as a fractured long bone, myelomeningocoele, necrotizing enterocolitis, open skin lesions from an inherited skin disorder or pain from assisted vaginal birth, etc.). We did not include infant pain relief for neonatal circumcision, as a Cochrane review has been published, and it does include the use of acetaminophen (paracetamol) (Brady‐Fryer 2004). We included newborn infants born at term up to postnatal age of 30 days. We included studies in preterm infants if they were enrolled up to 30 days beyond the expected date of birth (i.e. after reaching 40 weeks' postmenstrual age (PMA)).
Types of interventions
Paracetamol at any dose, administered intravenously, orally (or via nasogastric tube) or rectally, compared with placebo, no intervention or another pain‐reducing intervention (non‐pharmacological (sucrose, glucose, other sweet‐tasting solution, breast milk, skin‐to‐skin care or other) or a pharmacological agent (morphine, local or regional anaesthesia, or other)) for the prevention or treatment of pain. We included studies that reported on single administration of paracetamol or multiple (repeated) doses of paracetamol over a prolonged period during the initial hospital stay. Analyses of repeat administration of paracetamol would focus on potential adverse effects.
Types of outcome measures
Primary outcomes
Pain scores/indicators as measured by a validated tool
Behavioural (cry duration, proportion of time crying, facial actions).
Physiological (heart rate, respiratory rate, saturation of peripheral oxygen in the blood (SpO₂), transcutaneous oxygen and carbon dioxide (gas exchange measured across the skin — TcpO₂, TcpCO₂).
Validated composite pain scores.
Combination of these.
We measured the change from baseline values or the difference between absolute scores in intervention and control groups following treatment with the first dose of paracetamol. In keeping with a post hoc decision, we included maximum scores within 3 minutes of the painful intervention.
Secondary outcomes
Short‐term outcomes
Plasma, salivary or urinary cortisol levels (n mol/L or µg/dL) as a change from baseline values or as the difference between absolute values in intervention and control groups in keeping with treatment with paracetamol.
Duration of ventilator support (days).
Duration of need for supplementary oxygen (days).
Intraventricular haemorrhage (IVH) (Grade I to IV).
Severe IVH (Grade III and IV).
Spontaneous intestinal perforation.
Gastrointestinal bleed.
Retinopathy of prematurity (ROP) (according to the international classification of ROP); any stage and stage ≥ 3.
Decreased urine output (defined as < 1 cc/kg/h) during treatment.
Peak serum/plasma levels of creatinine (mmol/L) after treatment.
Peak serum/plasma levels of aspartate transaminase (AST) (IU/L) following treatment.
AST/alanine transaminase (ALT) levels > 100 IU/mL.
Peak serum/plasma levels of ALT (IU/L) following treatment.
Peak serum bilirubin (mmol/L) following treatment.
Liver failure; evidence of acute liver injury combined with severe coagulopathy (international normalised ratio (INR) > 2.0 or prothrombin time (PT) > 20 seconds) or encephalopathy with moderate coagulopathy (INR ≥ 1.5 or PT ≥ 15 seconds) (Sundaram 2011).
Duration of hospitalisation (total length of hospitalisation from birth to discharge home or death) (days).
Parent satisfaction with care provided in the NICU (as measured by a validated instrument/tool) (Butt 2013).
Long‐term outcomes
Infant mortality (death during first year of life).
Neurodevelopmental outcome (assessed by a standardised and validated assessment tool, a child developmental specialist or both) reported at any age (we will group outcome data at 18 months and 24 months, if available).
Altered reactions to painful stimuli following NICU discharge.
Autism/autism spectrum disorder (ASD) in childhood (American Psychiatric Association 2013; Bauer 2013).
Other side effects reported by study authors (not prespecified).
Search methods for identification of studies
See the Collaborative Review Group search strategy (Cochrane Neonatal Group 2013).
Electronic searches
For the May 2016 update we used the criteria and standard methods of Cochrane and the Cochrane Neonatal Review Group (see the Cochrane Neonatal Group search strategy for specialized register).
We conducted a comprehensive search including: Cochrane Central Register of Controlled Trials (CENTRAL 2016, Issue 4) in the Cochrane Library; MEDLINE via PubMed (1966 to 9 May 2016); Embase (1980 to 9 May 2016); and CINAHL (1982 to 9 May 2016) using the following search terms: (paracetamol OR acetaminophen), plus database‐specific limiters for RCTs and neonates (see Appendix 1 for the full search strategies for each database). We did not apply language restrictions. We searched clinical trials' registries for ongoing or recently completed trials (clinicaltrials.gov; the World Health Organization’s International Trials Registry and Platform (www.whoint/ictrp/search/en/); and the ISRCTN Registry).
For the previous searches we used the standard search strategy of the Cochrane Neonatal Review Group as outlined in the Cochrane Library. This includes electronic searches of the Cochrane Central Register of Controlled Trials (CENTRAL), MEDLINE (1966 to 13 October 2014), Embase (1980 to 13 October 2014) and the Cumulative Index to Nursing and Allied Health Literature (CINAHL) (1982 to 13 October 2014). Key words and MeSH terms included infant/newborn/neonate, pain, paracetamol (acetaminophen) and (random allocation or randomised controlled trial or controlled trial). We identified relevant reviews related to the topic. We applied no language restriction.
We conducted electronic searches of abstracts from the meetings of the Pediatric Academic Societies (2000 to October 2014) and the Perinatal Society of Australia and New Zealand (2010 to October 2014).
We searched the following clinical trial registries for ongoing or recently completed trials: clinicaltrials.gov; controlled‐trials.com; anzctr.org.au; who.int/ictrp. We searched the Web of Science for articles quoting identified RCTs and published before 2012.
We searched the first 200 hits on Google ScholarTM to identify grey literature in November 2014 and May 2016. We limited the Google ScholarTM search to the first 200 hits as in our experience the yield after 200 hits is poor.
Searching other resources
We performed manual searches of the reference lists of full‐text versions of eligible articles (RCTs and reviews) identified in the primary search of the literature.
Data collection and analysis
We used the standard methods of Cochrane and its Neonatal Review Group.
Selection of studies
Two review authors (AO and PS) independently assessed study eligibility for inclusion in this review according to prespecified selection criteria.
Data extraction and management
Two review authors (AO and PS) independently extracted data from the full‐text articles using a specifically designed spreadsheet/customised form to manage information. We used these forms to decide trial inclusion/exclusion, to extract data from eligible trials and to request additional published information from authors of the original reports. We entered and cross‐checked data using RevMan 5 software (RevMan 2014). We compared the extracted data for differences. When noted, we resolved differences by mutual discussion and consensus.
Assessment of risk of bias in included studies
The two review authors evaluated the following headings and associated questions (based on questions in the 'Risk of bias' tables) and entered details into the 'Risk of bias' tables. We attempted to obtain the study protocol for each included study to ascertain deviations between the protocol and the full publication of the study.
Selection bias (random sequence generation and allocation concealment)
Sequence generation
For each included study, we categorised the risk of selection bias as follows.
Low risk – adequate (any truly random process, e.g. random number table, computer random number generator).
High risk – inadequate (any non‐random process, e.g. odd or even date of birth, hospital or clinic record number).
Unclear risk – no or unclear information provided.
Allocation concealment
For each included study, we categorised the risk of bias regarding allocation concealment as follows.
Low risk – adequate (e.g. telephone or central randomisation; consecutively numbered, sealed, opaque envelopes).
High risk – inadequate (e.g. open random allocation, unsealed or non‐opaque envelopes, alternation, date of birth).
Unclear risk – no or unclear information provided.
Blinding of participants, personnel and outcome assessors (performance and detection bias)
For each included study, we categorised methods used to blind study personnel from knowledge of which intervention a participant received (as our study population will consist of neonates, all will be blinded to the study intervention).
Low risk – adequate for personnel (a placebo that could not be distinguished from the active drug was used in the control group).
High risk – inadequate (personnel aware of group assignment).
Unclear risk – no or unclear information provided.
For each included study, we categorised the methods used to blind outcome assessors from knowledge of which intervention a participant received (as our study population consisted of neonates, all were blinded to the study intervention). We assessed blinding separately for different outcomes or classes of outcomes. We categorised the methods used with regards to detection bias as follows.
Low risk – adequate (follow‐up was performed with assessors blinded to group).
High risk – inadequate (assessors at follow‐up were aware of group assignment).
Unclear risk – no or unclear information provided.
Incomplete outcome data (attrition bias)
For each included study and for each outcome, we described the completeness of data including attrition and exclusions from analysis. We noted whether attrition and exclusions were reported, numbers included in the analysis at each stage (compared with total randomly assigned participants), whether reasons for attrition or exclusion were reported and whether missing data were balanced across groups or were related to outcomes. When sufficient information was reported or was supplied by trial authors, we planned to re‐include missing data in the analyses. We categorised the methods with respect to risk of attrition bias as follows.
Low risk – adequate (less than 10% missing data).
High risk – inadequate (more than 10% missing data).
Unclear risk – no or unclear information provided.
Selective outcome reporting (reporting bias)
For each included study, we described how we investigated the risk of selective outcome reporting bias and what we found. We assessed methods as follows.
Low risk – adequate (when it is clear that all of the study's prespecified outcomes and all expected outcomes of interest to the review have been reported).
High risk – inadequate (when not all of the study's prespecified outcomes have been reported; one or more reported primary outcomes were not prespecified; outcomes of interest are reported incompletely and so cannot be used; study fails to include results of a key outcome that would have been expected to have been reported).
Unclear risk – no or unclear information provided (the study protocol was not available).
Other bias
For each included study, we described important concerns that we have about other possible sources of bias (e.g. whether a potential source of bias was related to the specific study design, whether the trial was stopped early because of some data‐dependent process). We assessed whether each study was free of other problems that could put it at risk of bias as follows.
Low risk (no concerns of other bias raised).
High risk (concerns raised about multiple looks at the data with results made known to investigators, difference in numbers of participants enrolled, as stated in the abstract and in final publications of the paper).
Unclear (concerns raised about potential sources of bias that could not be verified by contacting study authors).
Overall risk of bias
We made explicit judgements about whether studies were at high risk of bias, according to criteria given in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We assessed the likely magnitude and direction of the bias, and whether we considered it was likely to impact the findings. We planned to explore the impact of the level of bias by undertaking sensitivity analyses.
Measures of treatment effect
We analysed treatment effects in the individual trials using RevMan 5 (RevMan 2014).
Dichotomous data
We reported dichotomous data using risk ratio (RR) and risk difference (RD) with respective 95% confidence intervals (CIs). For those outcomes with a statistically significant RD for the pooled estimate from the meta‐analysis, we planned to calculate the number needed to treat for an additional beneficial outcome (NNTB) or the number needed to treat for an additional harmful outcome (NNTH) with respective 95% CIs.
Continuous data
We reported continuous data using mean difference (MD) with 95% CI.
Unit of analysis issues
The unit of randomisation was the intended unit of analysis (the individual infant). We did not identify any cluster‐randomised trials. If we had identified cluster‐randomised trials, the unit of analysis would have been the cluster (an individual NICU or a section of an NICU that was randomly assigned to treatment with paracetamol or to a control group). We planned to include cluster‐randomised trials in the analyses, along with trials that randomly assigned individual participants. We planned to analyse them using methods described in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011), using an estimate of the intracluster correlation coefficient (ICC) derived from the trial (if possible) or from another source. We planned to report this and to conduct sensitivity analyses to investigate the effects of variation in the ICC if ICCs from other sources were used. We planned to synthesise the relevant information if we identified both cluster‐randomised trials and participant‐level randomised trials. We considered it reasonable to combine the results from both if little heterogeneity between study designs was noted, and if interaction between the effect of the intervention and the choice of randomisation unit was unlikely.
At the protocol stage, we raised concerns regarding 'multiple' measures within one randomisation or different randomisation, and the impact on pain outcomes. Even if the infant remained in one randomly assigned group with multiple data points for an intervention, how to handle such data in RevMan 5 can be an issue. Do we take mean or median and generate variance around estimates from means of multiple measures? These are problematic issues for trials as well as for meta‐analyses. To simplify the review and to avoid dependency of measures (if an individual is 'hyper or hypo algesic', it may affect the results several times if randomly assigned to the same group), the next best approach would be to take only the first procedure after randomisation. Thus, even if a study had randomly assigned a participant multiple times, we intended to seek results for the first procedure after randomisation and to only include these in the primary analyses. We intended to carry out further exploration in the form of sensitivity analyses to check what the change in effect size would be, when the means or medians of multiple procedures were combined. We intended to contact study authors to request data resulting from the first randomisation. If we could not separate data from the first randomisation, we would exclude the study. For the full review, we included only the first procedure after randomisation.
Dealing with missing data
We requested additional data from the authors of each trial if data on important outcomes were missing or needed clarification (see the Characteristics of included studies table for details). Analyses were performed by intention to treat. When data were still missing, we planned to report the number of infants and to examine the effects of losses in a sensitivity analysis using a best/worst‐case scenario.
Assessment of heterogeneity
We used RevMan 5 software to assess heterogeneity of treatment effects between trials. We used the following two formal statistics.
Chi² test to assess whether observed variability in effect sizes between studies was greater than would be expected by chance. As this test has low power when the number of studies included in the meta‐analysis is small, we set the alpha probability at the 10% level of significance.
I² statistic to ensure that pooling of data was valid. We graded the degree of heterogeneity as none, low, moderate or high for values of less than 25%, 25% to 49%, 50% to 74% and 75% and above, respectively (Higgins 2003). When we found evidence of apparent statistical heterogeneity, we planned to assess the source of the heterogeneity using sensitivity and subgroup analyses, looking for evidence of bias or methodological differences between trials.
As no single meta‐analysis included more than one trial, assessment of heterogeneity was not applicable for any of the comparisons/analyses.
Assessment of reporting biases
We identified the study protocols for many of the trials that we selected for inclusion (see the 'Risk of bias' tables). We planned to assess reporting and publication bias by examining the degree of asymmetry of a funnel plot in RevMan 5 provided that a sufficient number of studies were available (n = 10) (Higgins 2011). This did not apply to any of the meta‐analyses.
Data synthesis
We performed statistical analyses according to the recommendations of the Cochrane Neonatal Review Group (Cochrane Neonatal Group 2013). We analysed all infants randomly assigned on an intention‐to‐treat basis. We used a fixed‐effect model to combine the data in a meta‐analysis in the first instance. If substantial heterogeneity was identified, we planned to examine the potential cause in subgroup and sensitivity analyses. If we had judged meta‐analysis to be inappropriate, we planned to analyse and interpret individual trials separately. For estimates of typical RR and RD, we used the Mantel‐Haenszel method. For measured quantities, we used the inverse variance method. We used the mean difference (MD) with 95% CI for synthesis of the same continuous measures. We planned to calculate NNTB and NNTH with 95% CIs if the RD was statistically significant. We planned to use the standardised mean difference (SMD) to combine trials that measured the same outcome but used different scales. If results from different pain assessment scales were to be combined, the scales would need to include the same components. If results were presented as medians and interquartile ranges (IQRs) we transformed the data to means and SDs using the formulas published by Wan 2014.
Quality of evidence
We used the GRADE approach, as outlined in the GRADE Handbook (Schünemann 2013), to assess the quality of evidence for the following (clinically relevant) outcomes: validated pain score (PIPP score) after procedure (heel lance or eye examination) and duration of crying.
Two authors independently assessed the quality of the evidence for each of the outcomes above. We considered evidence from randomised controlled trials as high quality but downgraded the evidence one level for serious (or two levels for very serious) limitations based upon the following: design (risk of bias), consistency across studies, directness of the evidence, precision of estimates and presence of publication bias. We used the GRADEpro Guideline Development Tool to create a ‘Summary of findings’ table to report the quality of the evidence.
The GRADE approach results in an assessment of the quality of a body of evidence in one of four grades:
High: We are very confident that the true effect lies close to that of the estimate of the effect.
Moderate: We are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different.
Low: Our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect.
Very low: We have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect.
Subgroup analysis and investigation of heterogeneity
We designed the main comparisons according to intention of use: paracetamol for prevention or treatment of pain. We planned to include separate comparisons based on the painful intervention/procedure/condition (heel lance, insertion of nasogastric tubes, insertion of intravenous catheters, lumbar puncture, postoperative pain, birth trauma, congenital anomalies (myelomeningocoele, open cutaneous lesions)) and the mode of administration of paracetamol. Within these comparisons, we planned to assess in subgroups (when possible) effects based on PMA at the birth of randomly assigned infants (< 28 weeks, 28 weeks to 31 weeks, 32 weeks to 36 weeks and ≥ 37 weeks) and by birth weight or body weight categories (≤ 1000 grams, 1001 to 1500 grams, 1501 to 2500 grams and ≥ 2501 grams). We report our findings based on the painful intervention/procedure/condition, but we were not able to perform subgroup analyses based on PMA or weight criteria.
We planned to meta‐analyse the data from various studies only if sufficient homogeneity was identified in populations studied, types of painful procedures, route and timing of administration of paracetamol and types of outcomes reported. If significant heterogeneity was identified, we planned to conduct only a systematic review and not a meta‐analysis.
Sensitivity analysis
We planned to perform a sensitivity analysis to determine whether findings were affected by including only studies of adequate methodology, defined as adequate randomisation and allocation concealment, blinding of intervention and measurement and less than 10% losses to follow‐up. Because no two identified studies included identical treatment for a certain painful intervention, we were not able to include at least two studies in a meta‐analysis; therefore sensitivity analyses were not indicated.
Results
Description of studies
Results of the search
Our searches identified nine studies for inclusion (Shah 1998; van Lingen 2001; Bonetto 2008; Badiee 2009; Manjunatha 2009; Ceelie 2013; Seifi 2013; Tinner 2013; Kabataş 2016); and one ongoing study (NCT01938261). These studies were conducted in Iran (Badiee 2009; Seifi 2013), Argentina (Bonetto 2008), The Netherlands (van Lingen 2001; Ceelie 2013), the UK (Manjunatha 2009), Canada (Shah 1998), Switzerland (Tinner 2013) and Turkey (Kabataş 2016). An ongoing study is being conducted in Finland (NCT01938261). The results of previous and the current literature searches are shown in the Study flow diagram (Figure 1).
1.
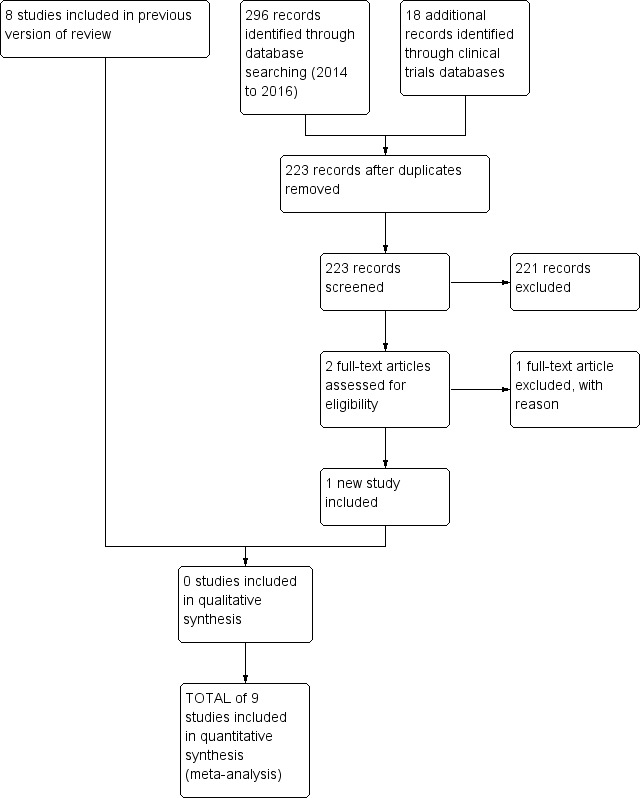
Study flow diagram: review update
Included studies
All studies applied paracetamol for the treatment of pain. No studies for the prevention of pain were identified. For details see the Table Characteristics of included studies.
Badiee 2009 was a single‐centre study conducted at Alzahra University Hospital, Isfahan, Iran, during the period of April 2007 to August 2007.
Objective: to evaluate whether high‐dose paracetamol (40 mg/kg orally) relieves pain in preterm infants.
Population: 72 preterm neonates (≤ 34 weeks' PMA, age ≥ 24 hours, no feeding for at least 30 minutes, Apgar scores > 3 at 5 minutes). 36 infants in each group.
Intervention: treatment group received oral paracetamol 40 mg/kg, and placebo group received sterile water 90 minutes before heel lance.
Outcomes: PIPP and crying time during the first 3 minutes of the procedure. Differences in SpO₂ and heart rate between baseline and heel lancing period.
Notes: Dr Badiee informed us on 5 January 2015 that the paracetamol solution looked similar to sterile water.
Bonetto 2008 was a single‐centre study conducted at Sanatorium Allende, Córdoba, Argentina, during November and December 2007.
Objective: to assess whether administration of oral glucose, paracetamol or EMLA, given individually, can reduce the pain caused in newborns by heel lance, in an outpatient setting.
Population: newborns of 36 weeks' PMA or more, more than 24 hours old and less than 30 days old, who needed blood tests for neonatal screening.
Intervention: oral glucose (n = 19) or oral paracetamol (20 mg/kg; 2 drops/kg) (n = 19) or EMLA to the heel (n = 19) and oral placebo (2 drops/kg distilled water) and placebo to the heel (n = 19).
Outcomes: maximum NIPS and PIPP scores from the start of the heel lance to 3 minutes after the heel lance procedure.
Ceelie 2013 was a single‐centre study conducted at a level III paediatric intensive care unit in Rotterdam, The Netherlands, between March 2008 and July 2010.
Objective: to determine whether intravenous paracetamol (acetaminophen) would significantly (30%) reduce morphine requirements in neonates and infants after major surgery.
Population: 71 neonates or infants younger than one year undergoing major thoracic (noncardiac) or abdominal surgery.
Intervention: all participants received a loading dose of morphine 30 minutes before the end of surgery, followed by continuous morphine or intermittent intravenous paracetamol up to 48 hours post surgery. Infants in both study groups received morphine (boluses and/or continuous infusion) as rescue medication on the guidance of the validated pain assessment instruments.
Outcomes: primary outcome was cumulative morphine dose (study and rescue dose). Secondary outcomes were pain scores and morphine‐related adverse effects.
Notes: we obtained unpublished information from Dr Saskia N de Wildt, one of the study authors, for the 41 infants who were 30 days of age or less at enrolment.
In a deviation from the protocol, we included this study that reported on infants randomly assigned to receive continuous morphine or intermittent intravenous paracetamol up to 48 hours post major thoracic or abdominal surgery (Ceelie 2013). The study reported on the following outcomes, which were not prespecified in our protocol.
Total amount of morphine administered (µg/kg) over 48 hours.
Any adverse event during postoperative care.
Re‐intubation during postoperative care.
Apnoea during postoperative care.
Apnoea with naloxone during postoperative care.
Bradycardia during postoperative care.
Urinary retention during postoperative care.
Kabataş 2016 was a single‐centre study conducted at the NICU of Dr. Sami Ulus Maternity and Children Research and Training Hospital, Ankara, Turkey. Study period: January 2013 through June 2014.
Objective: to investigate the efficacy of paracetamol in reducing pain during examination for retinopathy of prematurity (ROP) in preterm infants.
Population: very low birth weight (VLBW) infants (< 1500 g) who were candidates for ROP screening (n = 114).
Intervention: oral paracetamol 15 mg/kg (n = 58) or oral placebo (sterile water) (n = 56).
Outcomes: PIPP score during examination (median IQR); crying time (s) (mean and SD).
Notes: PIPP score during examination were presented as medians and IQRs. We transformed the data to means and SDs using the formulas presented by Wan 2014.
Manjunatha 2009 was a single‐centre study conducted at Wishaw General Hospital, Lanarkshire, UK, between 2003 and 2005.
Objective: to ascertain if and to what extent neonates experience pain and discomfort during ROP screening and to compare the effect of paracetamol, oral morphine or placebo on markers of pain in preterm infants.
Population: infants who satisfied the criteria for ROP screening (≤ 31 weeks of gestation, or ≤ 1.5 kg birth weight).
Intervention: infants were randomly assigned to one of three groups and were given placebo (n = 6), paracetamol (20 mg/kg) (n = 6) or oral morphine sulphate (200 μg/kg) (n = 6), one hour before the eye examination.
Outcomes: PIPP.
Notes: Dr Manjunatha and Ms Hazel Fisher (Senior Pharmacist) volunteered, "The 3 solutions looked identical (clear, colourless solutions). The diluent for all 3 solutions was hydroxybenzoate, with the placebo being made up with preserved water".
Seifi 2013 was a single‐centre study conducted in a tertiary level neonatal intensive care unit at Al Zahra Hospital, Tabriz, Iran, from October 2011 to October 2012.
Objective: to compare the efficacy of sucrose and acetaminophen in pain control during eye examination in preterm infants.
Population: preterm infants less than 32 weeks' PMA.
Intervention: Group A (n = 41) received oral acetaminophen 15 mg/kg 30 minutes before eye examination and 0.2 mL sterile water during initiation of eye examination. Group B (n = 40) received 0.2 mL sucrose (25%) during initiation of eye examination. Group C (n = 39) received 0.2 mL sterile water as placebo during initiation of eye examination.
Outcomes: PIPP during first 45 seconds and last 45 seconds of eye examination.
Shah 1998 was a single‐centre study conducted at the level III NICU at Women's College Hospital in Toronto, Ontario, Canada.
Objective: to evaluate the effectiveness of paracetamol in decreasing pain caused by heel lance.
Population: 75 term neonates undergoing heel lance.
Intervention: 60 to 90 minutes before the procedure, neonates received paracetamol orally at a dose of 20 mg/kg (n = 38) or an equal volume of placebo (n = 37).
Outcomes: per cent facial action (brow bulge, eye squeeze and nasolabial fold) (range 0% to 300%); and per cent of time spent crying (range 0% to 100%). Pain assessments were made from videotapes by a research assistant blinded to treatment allocation.
Tinner 2013 was a multi‐centre study conducted at the University Hospitals of Basel, Bern, and Zürich, Switzerland.
Objective: to assess the efficacy of paracetamol (acetaminophen) for neonatal pain relief.
Population: term and near‐term infants (n = 123) delivered by forceps or vacuum.
Intervention: Infants were randomly assigned to receive two suppositories with paracetamol (60/80/100 mg in infants < 3000 grams/3000 to 4000 grams/> 4000 grams birth weight) (n = 62) or placebo at two hours and eight hours of life (n = 61).
Outcomes: Pain and discomfort during the first 24 hours were assessed by the Échelle de Douleur et d’Inconfort du Nouveau‐né (neonatal pain and discomfort scale) score. The response to the subsequent heel prick for metabolic screening at days 2 to 3 of life was assessed by the Bernese Pain Scale for Neonates (BPSN).
The study by van Lingen 2001 was conducted at two level II hospitals in The Netherlands during a 15‐month period.
Objective: to evaluate whether paracetamol (20 mg/kg rectally) relieves pain in infants delivered by vacuum extraction, and if it improves the clinical condition.
Population: infants born by vacuum extraction with birth weight more than 2500 grams, gestational age more than 36 weeks, Apgar score at 5 minutes of 7 or more and absence of congenital anomalies of the newborn.
Intervention: 61 infants were given paracetamol suppositories rectally. The dose of paracetamol used was as close to 20 mg/kg as available strengths of suppository (50 mg for birth weight 2500 to 2749 grams, 60 mg for birth weight 2750 to 3249 grams, 70 mg for birth weight 3250 to 3749 grams and 80 mg for birth weight 3750 grams and above) would allow. At 6, 12 and 18 hours thereafter, they received another suppository from the same batch. Paracetamol was suspended in Witepof sol H‐15 as a fatty suppository base. In the placebo group, 61 infants received a rectal suppository with only a Witepsol H‐15 base.
Outcomes: a modified five‐point facies scale at 1, 7, 13 and 19 hours after the first suppository had been given.
Excluded studies
Hong 2010 enrolled infants/children who were six to 24 months old and therefore did not meet our inclusion criteria.
The median age of children participating in van der Marel 2007 was 0 months; 25th to 75th percentile 0 to 2 months. Some infants would therefore qualify for our review. We wrote to the first study author (van der Marel) in November 2014, but were unable to obtain data for infants who were less than 30 days of age at the time of randomisation. We wrote in May 2015 to two of the senior authors of the study (Drs van den Anker and Tibboel) to try to obtain the information but as of 24 June 2015 we have received no information on the infants who were less than 30 days of age (van der Marel 2007).
Härmä 2016 was a before‐and‐after study that showed that intravenous paracetamol decreases requirements of morphine in very preterm infants.
Two studies are awaiting classification as they were presented in abstract form only and no data could be used in our analyses (Foronda 2014; Garbi 2016).
Risk of bias in included studies
We report in the Characteristics of included studies table details of risk of bias in the included studies, and we provide summaries of risk of bias for the included studies in Figure 2 and Figure 3. Overall we consider the risk of bias for these studies to be low.
2.
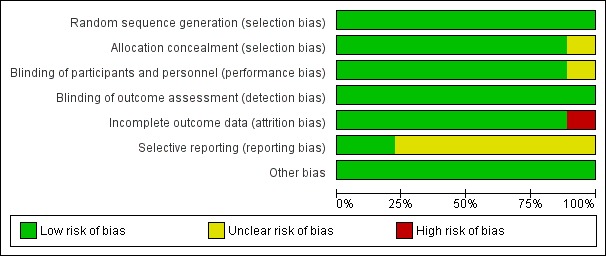
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
3.
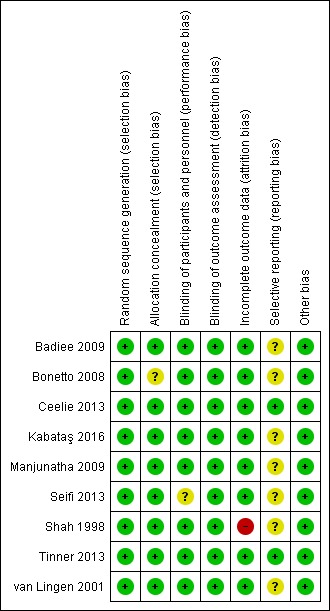
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
The random sequence was computer generated in eight trials (Shah 1998; van Lingen 2001; Bonetto 2008; Badiee 2009; Ceelie 2013; Seifi 2013; Tinner 2013; Kabataş 2016); and in one trial, the random sequence was generated by the pharmacist pulling folded tickets out of a bag with morphine, paracetamol or placebo on the ticket (Manjunatha 2009). Equal numbers of tickets were included in each arm in the bag, and all tickets were allocated: we judged the risk as low (Manjunatha 2009). Allocation to treatment groups was concealed in eight trials (Shah 1998; van Lingen 2001; Badiee 2009; Manjunatha 2009; Ceelie 2013; Seifi 2013; Tinner 2013; Kabataş 2016); and was unclear in one trial (Bonetto 2008).
Blinding
For blinding of participants and personnel (performance bias), risk of bias was low in eight trials (Shah 1998; van Lingen 2001; Bonetto 2008; Badiee 2009; Manjunatha 2009; Ceelie 2013; Tinner 2013; Kabataş 2016); and was unclear in one trial (Seifi 2013). For blinding of outcome assessment (detection bias), risk of bias was low for all included studies (Shah 1998; van Lingen 2001; Bonetto 2008; Badiee 2009; Manjunatha 2009; Ceelie 2013; Seifi 2013; Tinner 2013; Kabataş 2016).
Incomplete outcome data
We assessed risk of attrition bias as low for all bar one of included studies (van Lingen 2001; Bonetto 2008; Badiee 2009; Manjunatha 2009; Ceelie 2013; Seifi 2013; Tinner 2013; Kabataş 2016), the exception being Shah 1998, for which our judgement was 'high risk'. In that study, outcome data were missing for nine infants who had multiple heel lances; and in one infant, the video recording was of poor quality (missing data in 10 of 75 infants ‒ 13%).
Selective reporting
For seven studies, the study protocol was not available to us; we therefore judged risk of reporting bias as unclear for those studies (Shah 1998; van Lingen 2001; Bonetto 2008; Badiee 2009; Manjunatha 2009; Seifi 2013; Kabataş 2016). Two studies were entered into trials registries, and we did not detect deviations between the protocol and the full report. Therefore we judged risk of reporting bias as low in those trials (Ceelie 2013; Tinner 2013).
Other potential sources of bias
We judged the risk of other bias as low for all included trials.
Effects of interventions
See: Table 1
All studies applied paracetamol for the treatment of pain. We identified no studies on paracetamol for the prevention of pain.
Paracetamol for the treatment of pain
Only short‐term outcomes were reported.
As only one trial is included in any of the analyses below, tests for heterogeneity are not applicable.
Heel lance ‒ paracetamol (oral) vs sterile water (oral) (Comparison 1)
Primary outcomes
Pain scores
PIPP score (difference between baseline and heel lance period) ‒ Outcome 1.1 (Analysis 1.1)
1.1. Analysis.
Comparison 1 Heel lance ‐ paracetamol (oral) vs sterile water (oral), Outcome 1 PIPP score (difference between baseline and heel lance period).
One study including 72 preterm infants reported no significant differences in PIPP score between baseline and the heel lance period for the oral paracetamol group versus the oral sterile water group (MD 1.40, 95% CI −0.45 to 3.25) (Badiee 2009).
PIPP score (maximum score within three minutes following lancing) ‒ Outcome 1.5 (Analysis 1.2)
1.2. Analysis.
Comparison 1 Heel lance ‐ paracetamol (oral) vs sterile water (oral), Outcome 2 PIPP score (maximum score within 3 minutes following lancing).
One study including 38 infants reported no significant differences in PIPP score within three minutes following lancing (MD 1.48, 95% CI −0.11 to 3.07) for the oral paracetamol group versus the oral sterile water group (Bonetto 2008).
NIPS score (maximum score within three minutes following lancing) ‒ Outcome 1.6 (Analysis 1.3)
1.3. Analysis.
Comparison 1 Heel lance ‐ paracetamol (oral) vs sterile water (oral), Outcome 3 NIPS score (maximum score within 3 minutes following lancing).
One study including 38 infants reported no significant differences in NIPS score within three minutes following lancing (MD 0.85, 95% CI −0.14 to 1.84) for the oral paracetamol group versus the oral sterile water group (Bonetto 2008).
Physiological measures
Duration of crying (seconds) during first three minutes of the heel lance procedure ‒ Outcome 1.2 (Analysis 1.4)
1.4. Analysis.
Comparison 1 Heel lance ‐ paracetamol (oral) vs sterile water (oral), Outcome 4 Duration of crying (seconds) during the first 3 minutes.
One study including 72 preterm infants reported no significant differences in duration of crying for the oral paracetamol group versus the oral sterile water group (MD 8 seconds, 95% CI −19 to 35 seconds) (Badiee 2009).
Difference (%) in SpO₂between baseline and heel lance period – Outcome 1.3 (Analysis 1.5)
1.5. Analysis.
Comparison 1 Heel lance ‐ paracetamol (oral) vs sterile water (oral), Outcome 5 Difference in SpO2 between baseline and heel lance period (%).
One study including 72 preterm infants reported no significant differences (%) in SpO₂ between baseline and the heel lance period for the oral paracetamol group versus the oral sterile water group (MD 2.6%, 95% CI −0.58% to 5.78%) (Badiee 2009).
Difference in heart rate (beats/min) between baseline and heel lance period ‒ Outcome 1.4 (Analysis 1.6)
1.6. Analysis.
Comparison 1 Heel lance ‐ paracetamol (oral) vs sterile water (oral), Outcome 6 Difference in heart rate (beats/min) between baseline and heel lance period.
One study including 72 preterm infants reported no significant differences in heart rate (beats/min) between baseline and the heel lance period (MD 2 beats/min, 95% CI −5 to 9) for the oral paracetamol group versus the oral sterile water group (Badiee 2009).
Heel lance ‒ paracetamol (oral) vs glucose (oral) (Comparison 2)
Primary outcomes
Pain scores
PIPP score (maximum score within three minutes following lancing) ‒ Outcome 2.1 (Analysis 2.1) (Figure 4)
2.1. Analysis.
Comparison 2 Heel lance ‐ paracetamol (oral) vs glucose (oral), Outcome 1 PIPP (maximum score within 3 minutes following lancing).
4.
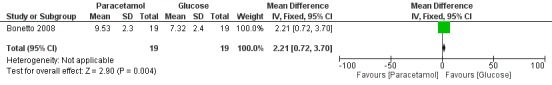
Forest plot of comparison: 2 Heel lance ‐ paracetamol (oral) vs glucose (oral), outcome: 2.1 PIPP (maximum score within 3 minutes following lancing).
One study including 38 infants reported significant differences in maximum PIPP score within three minutes following lancing (MD 2.21, 95% CI 0.72 to 3.70) for the oral paracetamol group versus the oral glucose group. PIPP score was higher in the oral paracetamol group than in the glucose group (Bonetto 2008).
NIPS score(maximum score within three minutes following lancing) ‒ Outcome 2.2 (Analysis 2.2)
2.2. Analysis.
Comparison 2 Heel lance ‐ paracetamol (oral) vs glucose (oral), Outcome 2 NIPS (maximum score within 3 minutes following lancing).
One study including 38 infants reported a significant difference in maximum NIPS score within three minutes following lancing (MD 1.32, 95% CI 0.40 to 2.24) for the oral paracetamol group versus the oral glucose group. NIPS score was higher in the oral paracetamol group than in the glucose group (Bonetto 2008).
Heel lance ‒ paracetamol (oral) vs EMLA (cutaneous) (Comparison 3)
Primary outcomes
Pain scores
PIPP score (maximum score within three minutes following lancing) ‒ Outcome 3.1 (Analysis 3.1)
3.1. Analysis.
Comparison 3 Heel lance ‐ paracetamol (oral) vs EMLA (cream), Outcome 1 PIPP (maximum score within 3 minutes following lancing).
One study including 38 infants reported no significant differences in maximum PIPP score within three minutes following lancing (MD 1.21, 95% CI −0.38 to 2.80) for the oral paracetamol group versus the EMLA (cutaneous) group (Bonetto 2008).
NIPS score (maximum score within three minutes following lancing) ‒ Outcome 3.1 (Analysis 3.1)
One study including 38 infants reported no significant differences in maximum NIPS score within three minutes following lancing (MD 0.58, 95% CI −0.34 to 1.50) for the oral paracetamol group versus the EMLA (cutaneous) group (Bonetto 2008).
Heel lance ‒ paracetamol (oral paracetamol cherry elixir) vs placebo (oral cherry elixir) (Comparison 4)
Primary outcomes
Pain scores
Facial action score (%) during heel lance (range 0% to 300%) ‒ Outcome 4.1 (Analysis 4.1)
4.1. Analysis.
Comparison 4 Heel lance ‐ paracetamol (oral paracetamol cherry elixir) vs placebo (cherry elixir), Outcome 1 Facial action score (%) during heel lance (range 0 to 300%).
One study including 65 infants reported no significant differences in facial action score (%) for the oral paracetamol cherry elixir group versus the oral cherry elixir (placebo) group (MD 12.4%, 95% CI −15.3 to 40.1) (Shah 1998).
Time spent crying (%) (range 0 to 100%) ‒ Outcome 4.2 (Analysis 4.2)
4.2. Analysis.
Comparison 4 Heel lance ‐ paracetamol (oral paracetamol cherry elixir) vs placebo (cherry elixir), Outcome 2 Time spent crying (%) (range 0 to 100%).
One study including 65 infants reported no significant differences in time spent crying (%) for the oral paracetamol cherry elixir group versus the cherry elixir (placebo) group (MD 2.6%, 95% CI −7.2 to 12.4) (Shah 1998).
Assisted vaginal birth (vacuum extraction or forceps) ‒ paracetamol suppositories vs placebo suppositories (Comparison 5)
Primary outcomes
Pain scores
Modified facies scores (possible score 0 to 4) 0, 1 or 2 at 1, 7, 13 and 19 hours) ‒ Outcome 5.1 (Analysis 5.1)
5.1. Analysis.
Comparison 5 Assisted vaginal birth (vacuum extraction or forceps) ‐ paracetamol suppositories vs placebo suppositories, Outcome 1 Modified facies scores (possible score 0 to 4) 0, 1 or 2 at 1, 7, 13 and 19 hours.
One study including 119 infants reported no significant differences in modified facies score of 0, 1 or 2 at 1, 7, 13 and 19 hours (RR 0.95, 95% CI 0.75 to 1.20; RD −0.04, 95% CI −0.20 to 0.13) for the paracetamol suppositories group versus the placebo suppositories group (van Lingen 2001).
Modified facies scores (possible score 0 to 4) 3 or 4 at 1, 7, 13 and 19 hours) ‒ Outcome 5.2 (Analysis 5.2)
5.2. Analysis.
Comparison 5 Assisted vaginal birth (vacuum extraction or forceps) ‐ paracetamol suppositories vs placebo suppositories, Outcome 2 Modified facies scores (possible score 0 to 4) 3 or 4 at 1, 7, 13 and 19 hours.
One study including 119 infants reported no significant differences in modified facies score of 3 or 4 at 1, 7, 13 and 19 hours (RR 1.14, 95% CI 0.66 to 1.96; RD 0.04, 95% CI −0.13 to 0.20) for the paracetamol suppositories group versus the placebo suppositories group (van Lingen 2001).
EDIN score at two hours of age ‒ Outcome 5.3 (Analysis 5.3)
5.3. Analysis.
Comparison 5 Assisted vaginal birth (vacuum extraction or forceps) ‐ paracetamol suppositories vs placebo suppositories, Outcome 3 EDIN score at 2 hours of age.
One study including 123 infants reported significantly higher EDIN scores at two hours of age in the paracetamol suppositories group compared with the placebo suppositories group (MD 1.00, 95% CI 0.60 to 1.40) (Tinner 2013).
EDIN score at four hours of age ‒ Outcome 5.4 (Analysis 5.4)
5.4. Analysis.
Comparison 5 Assisted vaginal birth (vacuum extraction or forceps) ‐ paracetamol suppositories vs placebo suppositories, Outcome 4 EDIN score at 4 hours of age.
One study including 123 infants reported no significant differences in EDIN score at four hours of age (MD 0.00, 95% CI −0.22 to 0.22) for the paracetamol suppositories group versus the placebo suppositories group (Tinner 2013).
Differences in acute pain score (BPSN) before and after heel lance at two to three days of life (by nurses) ‒ Outcome 5.5 (Analysis 5.5)
5.5. Analysis.
Comparison 5 Assisted vaginal birth (vacuum extraction or forceps) ‐ paracetamol suppositories vs placebo suppositories, Outcome 5 Difference in acute BPSN pain scores before and after heel lance at 2 to 3 days of life (by nurses).
One study including 123 infants reported significant differences in acute pain scores (BPSN) before and after heel lance at two to three days of life (as assessed by nurses) (MD 2.00, 95% CI 1.56 to 2.44) for the paracetamol suppositories group versus the placebo suppositories group (Tinner 2013).
Differences in acute pain score (BPSN) before and after heel lance at two to three days of life (by video) ‒ Outcome 5.6 (Analysis 5.6)
5.6. Analysis.
Comparison 5 Assisted vaginal birth (vacuum extraction or forceps) ‐ paracetamol suppositories vs placebo suppositories, Outcome 6 Difference in acute BPSN pain scores before and after heel lance at 2 to 3 days of life (by video).
One study including 123 infants reported significant differences in acute pain scores (BPSN) before and after heel lance at two to three days of life (as assessed by video) (MD 2.00, 95% CI 1.47 to 2.53) for the paracetamol suppositories group versus the placebo suppositories group (Tinner 2013).
Infants who cried following heel lance at two to three days of life ‒ Outcome 5.7 (Analysis 5.7)
5.7. Analysis.
Comparison 5 Assisted vaginal birth (vacuum extraction or forceps) ‐ paracetamol suppositories vs placebo suppositories, Outcome 7 Infants who cried following heel lance.
One study including 123 infants reported no significant differences in the number of infants who cried following heel lance (56% of infants in the paracetamol group and 41% of infants in the placebo group cried) (RR 1.38, 95% CI 0.95 to 2.00; RD 0.15, 95% CI −0.02 to 0.33) for the paracetamol suppositories group versus the placebo suppositories group (Tinner 2013).
Time spent crying (seconds) after heel lanceat two to three days of life ‒ Outcome 5.8 (Analysis 5.8)
5.8. Analysis.
Comparison 5 Assisted vaginal birth (vacuum extraction or forceps) ‐ paracetamol suppositories vs placebo suppositories, Outcome 8 Time spent crying (seconds) after heel lance.
One study including 123 infants reported significant differences in time spent crying (seconds) following heel lance at two to three days of life (MD 19 seconds, 95% CI 14 to 24) for the paracetamol suppositories group versus the placebo suppositories group (Tinner 2013).
Eye examination ‒ paracetamol (oral) vs placebo (sterile water) (Comparison 6)
Primary outcomes
Pain scores
PIPP score in first 45 seconds of eye examination ‒ Outcome 6.1 (Analysis 6.1)
6.1. Analysis.
Comparison 6 Eye examination ‐ paracetamol (oral) vs placebo (sterile water) for eye examination, Outcome 1 PIPP score in first 45 seconds of eye exam.
One study including 80 infants found no significant differences in PIPP score in the first 45 seconds of eye examination (MD −0.80, 95% CI −1.69 to 0.09) for the oral paracetamol group versus the oral placebo (sterile water) group (Seifi 2013).
PIPP score in last 45 seconds of eye examination ‒ Outcome 6.2 (Analysis 6.2)
6.2. Analysis.
Comparison 6 Eye examination ‐ paracetamol (oral) vs placebo (sterile water) for eye examination, Outcome 2 PIPP score in last 45 seconds of eye exam.
One study including 80 infants found no significant differences in PIPP score in the last 45 seconds of eye examination (MD 0.20, 95% CI −0.90 to 1.30) for the oral paracetamol group versus the oral placebo (sterile water) group (Seifi 2013).
PIPP score five minutes after eye examination ‒ Outcome 6.3 (Analysis 6.3)
6.3. Analysis.
Comparison 6 Eye examination ‐ paracetamol (oral) vs placebo (sterile water) for eye examination, Outcome 3 PIPP score 5 minutes after eye exam.
One study including 11 infants reported no significant differences in PIPP score five minutes after eye examination (MD −1.57, 95% CI −3.79 to 0.66) for the oral paracetamol group versus the oral placebo (sterile water) group (Manjunatha 2009).
PIPP score during eye examination ‒ Outcome 6.4 (Analysis 6.4) Figure 5
6.4. Analysis.
Comparison 6 Eye examination ‐ paracetamol (oral) vs placebo (sterile water) for eye examination, Outcome 4 PIPP score during eye examination.
5.
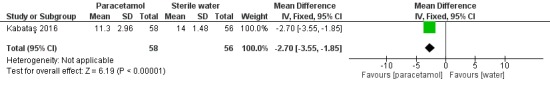
Forest plot of comparison: 6 Eye examination ‐ paracetamol (oral) vs placebo (sterile water) for eye examination, outcome: 6.4 PIPP score during eye examination.
One study including 114 infants reported a significantly lower PIPP score during eye examination (MD −2.70, 95% CI −3.55 to −1.85) for the oral paracetamol group versus the oral placebo (water) group (Kabataş 2016).
Crying time during eye examination ‒ Outcome 6.5 (Analysis 6.5)
6.5. Analysis.
Comparison 6 Eye examination ‐ paracetamol (oral) vs placebo (sterile water) for eye examination, Outcome 5 Crying time (s) during eye examination.
One study including 114 infants reported no significant difference in crying time (s) during eye examination (MD 5, 95% CI −2 to 11) for the oral paracetamol group versus the oral placebo (water) group (Kabataş 2016).
Infants with tachycardia during eye examination ‒ Outcome 6.6 (Analysis 6.6)
6.6. Analysis.
Comparison 6 Eye examination ‐ paracetamol (oral) vs placebo (sterile water) for eye examination, Outcome 6 Infants with tachycardia (>180 bpm).
One study including 114 infants reported no significant difference in the number of infants with tachycardia during eye examination (RR 1.64, 95% CI 0.82 to 3.27; RD 0.11, 95% CI −0.04 to 0.27) for the oral paracetamol group versus the oral placebo (water) group (Kabataş 2016).
Infants with bradycardia and desaturation during eye examination ‒ Outcome 6.7 (Analysis 6.7)
6.7. Analysis.
Comparison 6 Eye examination ‐ paracetamol (oral) vs placebo (sterile water) for eye examination, Outcome 7 Infants with bradycardia and desaturation.
One study including 114 infants reported no significant difference in the number of infants with bradycardia and desaturation during eye examination (RR 1.21, 95% CI 0.34 to 4.27; RD 0.01, 95% CI −0.08 to 0.11) for the oral paracetamol group versus the oral placebo (water) group (Kabataş 2016).
Eye examination ‒ paracetamol (oral) vs 24% sucrose (oral) (Comparison 7)
Primary outcomes
Pain scores
PIPP score in the first 45 seconds of eye examination ‒ Outcome 7.1 (Analysis 7.1) (Figure 6)
7.1. Analysis.
Comparison 7 Eye examination ‐ paracetamol (oral) vs 24% sucrose (oral), Outcome 1 PIPP score in first 45 seconds of eye exam.
6.
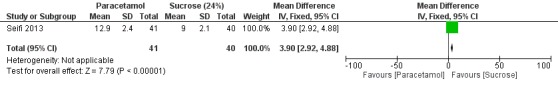
Forest plot of comparison: 7 Eye examination ‐ paracetamol (oral) vs 24% sucrose (oral), outcome: 7.1 PIPP score in first 45 seconds of eye examination.
One study including 81 infants reported significantly higher PIPP scores in the first 45 seconds of eye examination (MD 3.90, 95% CI 2.92 to 4.88) for the oral paracetamol group versus the oral 24% sucrose group (Seifi 2013).
PIPP score in the last 45 seconds of eye examination ‒ Outcome 7.2 (Analysis 7.2)
7.2. Analysis.
Comparison 7 Eye examination ‐ paracetamol (oral) vs 24% sucrose (oral), Outcome 2 PIPP score in last 45 seconds of eye exam.
One study including 81 infants reported no significant differences in PIPP score in the last 45 seconds of eye examination (MD 1.10, 95% CI −0.08 to 2.28) for the oral paracetamol group versus the oral 24% sucrose group (Seifi 2013).
Eye examination ‒ paracetamol (oral) vs morphine (oral) (Comparison 8)
Primary outcomes
Pain scores
PIPP score five minutes after eye examination ‒ Outcome 8.1 (Analysis 8.1)
8.1. Analysis.
Comparison 8 Eye examination ‐ paracetamol vs morphine, Outcome 1 PIPP score 5 minutes after eye exam.
One study including 11 infants reported no significant differences in PIPP score five minutes after eye examination (MD 1.10, 95% CI −0.70 to 2.90) for the oral paracetamol group versus the oral morphine group (Manjunatha 2009).
Postoperative care ‒ paracetamol vs morphine (Comparison 9)
Secondary outcomes
Total amount of morphine (µg/kg) administered over 48 hours ‒ Outcome 9.1 (Analysis 9.1)
9.1. Analysis.
Comparison 9 Postoperative care ‐ paracetamol vs morphine, Outcome 1 Total amount of morphine (µg/kg) administered over 48 hours.
One study including 41 infants reported a significantly lower total amount of morphine (µg/kg) administered over 48 hours following surgery (MD −157 µg/kg, 95% CI −27 to −288) for the paracetamol group versus the morphine group (Ceelie 2013).
Any adverse event ‒ Outcome 9.2 (Analysis 9.2)
9.2. Analysis.
Comparison 9 Postoperative care ‐ paracetamol vs morphine, Outcome 2 Any adverse event.
One study including 41 infants reported no significant differences in any adverse events during postoperative care (RR 0.95, 95% CI 0.50 to 1.78; RD −0.03, 95% CI −0.33 to 0.28) for the paracetamol group versus the morphine group (Ceelie 2013).
Re‐intubation ‒ Outcome 9.3 (Analysis 9.3)
9.3. Analysis.
Comparison 9 Postoperative care ‐ paracetamol vs morphine, Outcome 3 Re‐intubation.
One study including 41 infants reported no significant differences in reintubation during postoperative care (RR 3.45, 95% CI 0.15 to 80.03; RD 0.05, 95% CI −0.08 to 0.18) for the paracetamol group versus the morphine group (Ceelie 2013).
Apnoea ‒ Outcome 9.4 (Analysis 9.4)
9.4. Analysis.
Comparison 9 Postoperative care ‐ paracetamol vs morphine, Outcome 4 Apnoea.
One study including 41 infants reported no significant differences in apnoea during postoperative care (RR 0.77, 95% CI 0.26 to 2.33; RD −0.06, 95% CI −0.32 to 0.20) for the paracetamol group versus the morphine group (Ceelie 2013).
Apnoea with naloxone ‒ Outcome 9.5 (Analysis 9.5)
9.5. Analysis.
Comparison 9 Postoperative care ‐ paracetamol vs morphine, Outcome 5 Apnoea with naloxone.
One study including 41 infants reported no significant differences in apnoea with naloxone during postoperative care (RR 0.23, 95% CI 0.01 to 4.51; RD −0.09, 95% CI −0.24 to 0.05) for the paracetamol group versus the morphine group (Ceelie 2013).
Bradycardia ‒ Outcome 9.6 (Analysis 9.6)
9.6. Analysis.
Comparison 9 Postoperative care ‐ paracetamol vs morphine, Outcome 6 Bradycardia.
One study including 41 infants reported no significant differences in bradycardia during postoperative care (RR 1.16, 95% CI 0.33 to 4.01; RD 0.03, 95% CI −0.22 to 0.27) for the paracetamol group versus the morphine group (Ceelie 2013).
Urinary retention ‒ Outcome 9.7 (Analysis 9.7)
9.7. Analysis.
Comparison 9 Postoperative care ‐ paracetamol vs morphine, Outcome 7 Urinary retention.
One study including 41 infants reported no significant differences in urinary retention during postoperative care (RR 3.45, 95% CI 0.15 to 80.03; RD 0.05, 95% CI −0.08 to 0.18) for the paracetamol group versus the morphine group (Ceelie 2013).
We identified no studies for the prevention of pain.
Discussion
Summary of main results
We identified nine studies on treatment for pain with paracetamol. We found no studies that administered paracetamol for the prevention of pain. No evidence showed a reduction in pain associated with heel lance with the use of paracetamol compared with placebo (water or cherry elixir). Glucose appears to be more effective in reducing heel lance‐associated pain than paracetamol. The results for paracetamol compared with water to reduce pain associated with eye examinations performed to ascertain the presence of retinopathy of prematurity (ROP) were conflicting: one study found a reduction in the PIPP score during the examination for paracetamol versus water whereas another study did not find a significant reduction in PIPP scores in the first or last 45 seconds of eye examination. The PIPP scores were higher during eye examination for the paracetamol group than for the sucrose group. Paracetamol does not reduce pain associated with assisted vaginal birth (vacuum extraction or forceps) and may increase the pain response to a subsequent heel lance two to three days later.
Regular use of paracetamol may reduce the total amount of morphine required during the first 48 hours following major thoracic or abdominal surgery. However, one study that included infants up to a postnatal age of 10 months did not find that rectal paracetamol reduced morphine consumption after major surgery (van der Marel 2007). As we were not able to obtain data on infants younger than one month of age, the study is currently entered under Characteristics of studies awaiting classification.
We identified no studies that assessed the long‐term effects of paracetamol, including the possibility of an association with autism.
Overall completeness and applicability of evidence
We identified nine studies that reported comparisons in 728 infants of paracetamol versus placebo or other pain‐reducing interventions. Because no two trials assessing the effectiveness of paracetamol for a certain painful procedure used the same outcome assessment tool, we could not conduct a meta‐analysis. No evidence is available to support or refute the safety or effectiveness of paracetamol for the prevention of neonatal pain. Available evidence suggests that paracetamol is not effective for the treatment of neonatal pain. Evidence is inadequate to support or refute its safety for this purpose.
Quality of the evidence
The quality of the included trials, although they included small sample sizes, was good in general with low risk of bias; (Figure 2 and Figure 3). Using the GRADE criteria the quality of evidence was mostly low; Table 1.
Potential biases in the review process
We are not aware of any bias in our review process. As stated under 'Declarations of interest', Dr Arne Ohlsson is a co‐author of one of the included trials (Shah 1998).
Agreements and disagreements with other studies or reviews
As we could not perform meta‐analyses, our results are consistent with results of the individual studies included in this review. We are not aware of a similar systematic review on the topic.
Authors' conclusions
Implications for practice.
The paucity and low quality of existing data do not provide evidence for the effectiveness of paracetamol for neonates exposed to painful procedures such as heel lance and eye‐examination. Paracetamol given after assisted vaginal birth may increase the response to later painful exposures. The effectiveness of paracetamol for other painful procedures/conditions in newborn infants has not been studied in randomised controlled trials. The findings of our review provide insufficient evidence to establish the role of paracetamol in reducing the effects of painful procedures in neonates.
Implications for research.
Paracetamol may reduce the total amount of morphine required during the first 48 hours following major thoracic or abdominal surgery. Further trials are required to ascertain this possible beneficial effect of paracetamol for pain management in neonates.
What's new
| Date | Event | Description |
|---|---|---|
| 27 January 2020 | Amended | Arne Ohlsson deceased. |
| 27 January 2020 | New citation required but conclusions have not changed | Contact author changed, and contact details updated. |
History
Protocol first published: Issue 7, 2014 Review first published: Issue 6, 2015
| Date | Event | Description |
|---|---|---|
| 6 February 2017 | Amended | Added external source of support. |
| 16 August 2016 | New citation required but conclusions have not changed | Conclusions unchanged. |
| 24 May 2016 | New search has been performed | One additional study that assessed the effectiveness of paracetamol versus water for pain/stress associated with eye‐examination for retinopathy of prematurity (ROP) was identified. The study showed lower PIPP scores in the paracetamol group than the placebo (water group). The results for paracetamol for ROP exam are contradictory. Summary of findings tables have been added. |
Acknowledgements
We are thankful to Dr Badiee (Badiee 2009), Dr de Wildt (Ceelie 2013), Dr Manjunatha and Ms Fisher (Manjunatha 2009), and Dr van Lingen (van Lingen 2001), who provided additional information about their studies.
Appendices
Appendix 1. Standard search methodology
PubMed: ((infant, newborn[MeSH] OR newborn OR neonate OR neonatal OR premature OR low birth weight OR VLBW OR LBW or infan* or neonat*) AND (randomised controlled trial [pt] OR controlled clinical trial [pt] OR Clinical Trial[ptyp] OR randomised [tiab] OR placebo [tiab] OR clinical trials as topic [mesh: noexp] OR randomly [tiab] OR trial [ti]) NOT (animals [mh] NOT humans [mh]))
Embase: (infant, newborn or newborn or neonate or neonatal or premature or very low birth weight or low birth weight or VLBW or LBW or Newborn or infan* or neonat*) AND (human not animal) AND (randomized controlled trial or controlled clinical trial or randomized or placebo or clinical trials as topic or randomly or trial or clinical trial)
CINAHL: (infant, newborn OR newborn OR neonate OR neonatal OR premature OR low birth weight OR VLBW OR LBW or Newborn or infan* or neonat*) AND (randomized controlled trial OR controlled clinical trial OR randomized OR placebo OR clinical trials as topic OR randomly OR trial OR PT clinical trial)
Cochrane Library: (infant or newborn or neonate or neonatal or premature or very low birth weight or low birth weight or VLBW or LBW)
Data and analyses
Comparison 1. Heel lance ‐ paracetamol (oral) vs sterile water (oral).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 PIPP score (difference between baseline and heel lance period) | 1 | 72 | Mean Difference (IV, Fixed, 95% CI) | 1.40 [‐0.45, 3.25] |
| 2 PIPP score (maximum score within 3 minutes following lancing) | 1 | 38 | Mean Difference (IV, Fixed, 95% CI) | 1.48 [‐0.11, 3.07] |
| 3 NIPS score (maximum score within 3 minutes following lancing) | 1 | 38 | Mean Difference (IV, Fixed, 95% CI) | 0.85 [‐0.14, 1.84] |
| 4 Duration of crying (seconds) during the first 3 minutes | 1 | 72 | Mean Difference (IV, Fixed, 95% CI) | 8.10 [‐19.09, 35.29] |
| 5 Difference in SpO2 between baseline and heel lance period (%) | 1 | 72 | Mean Difference (IV, Fixed, 95% CI) | 2.60 [‐0.58, 5.78] |
| 6 Difference in heart rate (beats/min) between baseline and heel lance period | 1 | 72 | Mean Difference (IV, Fixed, 95% CI) | 1.90 [‐4.74, 8.54] |
Comparison 2. Heel lance ‐ paracetamol (oral) vs glucose (oral).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 PIPP (maximum score within 3 minutes following lancing) | 1 | 38 | Mean Difference (IV, Fixed, 95% CI) | 2.21 [0.72, 3.70] |
| 2 NIPS (maximum score within 3 minutes following lancing) | 1 | 38 | Mean Difference (IV, Fixed, 95% CI) | 1.32 [0.40, 2.24] |
Comparison 3. Heel lance ‐ paracetamol (oral) vs EMLA (cream).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 PIPP (maximum score within 3 minutes following lancing) | 1 | 38 | Mean Difference (IV, Fixed, 95% CI) | 1.21 [‐0.38, 2.80] |
| 2 NIPS score (maximum score within 3 minutes following lancing) | 1 | 38 | Mean Difference (IV, Fixed, 95% CI) | 0.58 [‐0.34, 1.50] |
3.2. Analysis.
Comparison 3 Heel lance ‐ paracetamol (oral) vs EMLA (cream), Outcome 2 NIPS score (maximum score within 3 minutes following lancing).
Comparison 4. Heel lance ‐ paracetamol (oral paracetamol cherry elixir) vs placebo (cherry elixir).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Facial action score (%) during heel lance (range 0 to 300%) | 1 | 65 | Mean Difference (IV, Fixed, 95% CI) | 12.40 [‐15.28, 40.08] |
| 2 Time spent crying (%) (range 0 to 100%) | 1 | 65 | Mean Difference (IV, Fixed, 95% CI) | 2.60 [‐7.15, 12.35] |
Comparison 5. Assisted vaginal birth (vacuum extraction or forceps) ‐ paracetamol suppositories vs placebo suppositories.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Modified facies scores (possible score 0 to 4) 0, 1 or 2 at 1, 7, 13 and 19 hours | 1 | 119 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.95 [0.75, 1.20] |
| 2 Modified facies scores (possible score 0 to 4) 3 or 4 at 1, 7, 13 and 19 hours | 1 | 119 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.14 [0.66, 1.96] |
| 3 EDIN score at 2 hours of age | 1 | 123 | Mean Difference (IV, Fixed, 95% CI) | 1.0 [0.60, 1.40] |
| 4 EDIN score at 4 hours of age | 1 | 123 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [‐0.22, 0.22] |
| 5 Difference in acute BPSN pain scores before and after heel lance at 2 to 3 days of life (by nurses) | 1 | 123 | Mean Difference (IV, Fixed, 95% CI) | 2.0 [1.56, 2.44] |
| 6 Difference in acute BPSN pain scores before and after heel lance at 2 to 3 days of life (by video) | 1 | 123 | Mean Difference (IV, Fixed, 95% CI) | 2.0 [1.47, 2.53] |
| 7 Infants who cried following heel lance | 1 | 123 | Risk Difference (M‐H, Fixed, 95% CI) | 0.15 [‐0.02, 0.33] |
| 8 Time spent crying (seconds) after heel lance | 1 | 123 | Mean Difference (IV, Fixed, 95% CI) | 19.0 [14.12, 23.88] |
Comparison 6. Eye examination ‐ paracetamol (oral) vs placebo (sterile water) for eye examination.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 PIPP score in first 45 seconds of eye exam | 1 | 80 | Mean Difference (IV, Fixed, 95% CI) | ‐0.80 [‐1.69, 0.09] |
| 2 PIPP score in last 45 seconds of eye exam | 1 | 80 | Mean Difference (IV, Fixed, 95% CI) | 0.20 [‐0.90, 1.30] |
| 3 PIPP score 5 minutes after eye exam | 1 | 11 | Mean Difference (IV, Fixed, 95% CI) | ‐1.57 [‐3.79, 0.66] |
| 4 PIPP score during eye examination | 1 | 114 | Mean Difference (IV, Fixed, 95% CI) | ‐2.70 [‐3.55, ‐1.85] |
| 5 Crying time (s) during eye examination | 1 | 114 | Mean Difference (IV, Fixed, 95% CI) | 4.80 [‐1.69, 11.29] |
| 6 Infants with tachycardia (>180 bpm) | 1 | 114 | Risk Difference (M‐H, Fixed, 95% CI) | 0.11 [‐0.04, 0.27] |
| 7 Infants with bradycardia and desaturation | 1 | 114 | Risk Difference (M‐H, Fixed, 95% CI) | 0.01 [‐0.08, 0.11] |
Comparison 7. Eye examination ‐ paracetamol (oral) vs 24% sucrose (oral).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 PIPP score in first 45 seconds of eye exam | 1 | 81 | Mean Difference (IV, Fixed, 95% CI) | 3.90 [2.92, 4.88] |
| 2 PIPP score in last 45 seconds of eye exam | 1 | 81 | Mean Difference (IV, Fixed, 95% CI) | 1.10 [‐0.08, 2.28] |
Comparison 8. Eye examination ‐ paracetamol vs morphine.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 PIPP score 5 minutes after eye exam | 1 | 11 | Mean Difference (IV, Fixed, 95% CI) | 1.10 [‐0.70, 2.90] |
Comparison 9. Postoperative care ‐ paracetamol vs morphine.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Total amount of morphine (µg/kg) administered over 48 hours | 1 | 41 | Mean Difference (IV, Fixed, 95% CI) | ‐157.0 [‐287.48, ‐26.52] |
| 2 Any adverse event | 1 | 41 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.95 [0.50, 1.78] |
| 3 Re‐intubation | 1 | 41 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.45 [0.15, 80.03] |
| 4 Apnoea | 1 | 41 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.77 [0.26, 2.33] |
| 5 Apnoea with naloxone | 1 | 41 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.23 [0.01, 4.51] |
| 6 Bradycardia | 1 | 41 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.16 [0.33, 4.01] |
| 7 Urinary retention | 1 | 41 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.45 [0.15, 80.03] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Badiee 2009.
| Methods | Prospective randomised double‐blind placebo‐controlled study | |
| Participants | 72 preterm neonates (≤ 34 weeks PMA, age ≥ 24 hours, no feeding for at least 30 minutes, Apgar scores > 3 at 5 minutes). 36 infants in each group. Infants were treated at the NICU of Alzahra University Hospital, Isfahan, Iran, during the period of April 2007 to August 2007 | |
| Interventions | The treatment group (n = 36) received oral paracetamol 40 mg/kg, and the placebo group (n = 36) received an equal volume of sterile water 90 minutes before heel lance | |
| Outcomes | PIPP and crying time during the first 3 minutes of the procedure. Differences in SpO₂ and heart rate between baseline and the heel lancing period | |
| Notes | Dr Badiee confirmed in an email on 5 January 2015 that the paracetamol solution looked the same as sterile water | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated block randomisation |
| Allocation concealment (selection bias) | Low risk | The research pharmacist randomly assigned newborns to treatment and placebo groups by computer‐generated block randomisation |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | The paracetamol oral solution looked similar to sterile water |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Neither the observers nor the nurse who carried out the heel lancing were aware of which treatment had been given to the neonate |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Outcomes were reported for all randomly assigned infants |
| Selective reporting (reporting bias) | Unclear risk | The protocol for the study was not available to us, so we cannot judge whether there were any deviations from the protocol |
| Other bias | Low risk | Study appears free of other bias |
Bonetto 2008.
| Methods | Randomised controlled trial | |
| Participants | Newborns of 36 weeks' PMA or more, more than 24 hours old and less than 30 days old, who needed blood tests for neonatal screening. Infants were born at Sanatorium Allende, Córdoba, Argentina, during November and December 2007 | |
| Interventions | Oral glucose or oral paracetamol (n = 19) or oral placebo (n = 19) or EMLA to the heel (n = 19), or placebo to the heel (n = 19) | |
| Outcomes | Maximum NIPS and PIPP scores from the start of the heel lance to 3 minutes after the heel lance procedure | |
| Notes | We used Google translation to translate the article from Spanish to English | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer generated |
| Allocation concealment (selection bias) | Unclear risk | No information was provided |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | A doctor was in charge of maintaining masking of the sample and knowing who administered treatment or placebo. Acetaminophen was administered at 20 mg/kg (2 drops/kg) orally 60 minutes before the extraction. Matching placebo consisted of 2 drops/kg of distilled water given with a masked dropper. Corresponding placebo for EMLA was a moisturiser of similar appearance to EMLA |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Pain assessment was performed independently by 2 observers (neonatal nurses) who used PIPP as the primary measure and NIPS as a secondary measure. Pain assessment began with the start of the puncture and ended after 3 minutes. Maximum scores achieved during these 3 minutes after the puncture were the outcome measures |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Outcomes were reported on all randomly assigned participants: 19 paracetamol + 19 placebo |
| Selective reporting (reporting bias) | Unclear risk | The protocol was not available to us, so we cannot judge whether selective reporting occurred |
| Other bias | Low risk | Study appears free of other bias |
Ceelie 2013.
| Methods | Randomised controlled trial | |
| Participants | 71 neonates or infants; PMA of 36 1/7 weeks or older to 1 year of age with body weight > 1500 grams undergoing major thoracic (non‐cardiac) or abdominal surgery. We obtained unpublished data on 41 infants who were ≤ 30 days of age and fulfilled our inclusion criteria. These infants are included in our review. Infants were admitted to a level III paediatric intensive care unit in Rotterdam, The Netherlands, between March 2008 and July 2010 | |
| Interventions | All participants received a loading dose of morphine 30 minutes before the end of surgery, followed by continuous morphine or intermittent intravenous paracetamol up to 48 hours post surgery. Infants in both study groups received morphine (boluses and/or continuous infusion) as rescue medication on the guidance of the validated pain assessment instruments. Participants were randomly assigned to receive morphine or paracetamol postoperatively. When participants were randomly assigned to receive paracetamol (30 mg/kg per day in 4 doses), a placebo infusion of normal saline was administered continuously at the same rate as an equivalent morphine infusion. When randomly assigned to receive morphine (participants ≤ 10 days of age, 2.5 µg/kg1.5 per hour; participants 11 days to 1 year of age, 5 µg/kg1.5 per hour), normal saline was administered 4 times daily as placebo in a volume similar to the intravenous paracetamol dose |
|
| Outcomes | Primary outcome was cumulative morphine dose (study and rescue dose). Secondary outcomes were pain scores and morphine‐related adverse effects | |
| Notes | We wrote to Dr Saskia N de Wildt, and she provided us with unpublished information on 41 infants who were ≤ 30 days of age and fulfilled our inclusion criteria | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | A hospital pharmacist carried out computer randomisation in advance |
| Allocation concealment (selection bias) | Low risk | Only the pharmacist had access to group allocation during the study period, for preparation of study medication |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Only the pharmacist had access to group allocation during the study period, for preparation of study medication. No other information was provided |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | All staff were blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Outcomes were reported for all randomly assigned infants |
| Selective reporting (reporting bias) | Low risk | This study was entered into a trials registry ‐ trialregister.nl Identifier: NTR1438 in The Netherlands. No deviations from the protocol are apparent |
| Other bias | Low risk | Study appears free of other bias |
Kabataş 2016.
| Methods | Randomised controlled trial. Study location: tertiary level neonatal intensive care unit (NICU) of Dr. Sami Ulus Maternity and Children Research and Training Hospital, Ankara, Turkey. Study period: January 2013 to June 2014 inclusive | |
| Participants | Very low birth weight (VLBW) infants (< 1500 g) who were candidates for ROP screening (n = 114) | |
| Interventions | Topical anaesthetic (Proparacaine; Alcaine® drop 0.5 %) was applied 30 seconds before the eye examination in all the infants Oral paracetamol 15 mg/kg (n = 58) or oral placebo (sterile water) (n = 56) |
|
| Outcomes | PIPP score during examination (median IQR) Crying time (seconds) (mean and SD) |
|
| Notes | PIPP score during examination were presented as medians and IQRs. We transformed the data to means and SDs using the formulas presented by Wan 2014 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐based randomisation process |
| Allocation concealment (selection bias) | Low risk | Sealed opaque envelopes |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Either paracetamol or sterile water was provided by the pharmacy in opaque syringes. The nurse administered the solutions 60 minutes before the onset of the examination. The parents, the ophthalmologist and investigators were blinded to the randomisation |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | The parents, the ophthalmologist and investigators were blinded to the randomisation |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All randomised infants were accounted for |
| Selective reporting (reporting bias) | Unclear risk | The protocol for the study was not available to us so we cannot judge if there were any deviations between the protocol and the report |
| Other bias | Low risk | Appears free of other bias |
Manjunatha 2009.
| Methods | Double‐blind randomised controlled trial. Study was conducted from 2003 to 2005 at Wishaw General Hospital, Lanarkshire, UK | |
| Participants | Infants who satisfied the criteria for ROP screening (≤ 31 weeks PMA, or ≤ 1.5 kg birth weight) were recruited into the study. Infants who were on morphine or paracetamol for other reasons, breast‐fed infants whose mothers were on methadone or other analgesics and infants with gastrointestinal problems such as ileostomy/colostomy were excluded | |
| Interventions | Neonates (n = 18) were randomly assigned to receive a single oral dose of morphine sulphate 200 μg/kg (n = 6) or paracetamol 20 mg/kg (n = 6) or placebo (n = 6) | |
| Outcomes | PIPP – The infant’s face, saturation monitor and time frame cards were recorded on videotape over a 1 to 2 minute period, at 5 minutes before, then at 5 minutes, 30 minutes, 1 hour, 2 hours and 3 hours after the procedure. 2 separate individuals subsequently scored the information independently. Main outcome was PIPP scores 5 minutes after ROP screening | |
| Notes | Dr Manjunatha and Ms Hazel Fisher (Senior Pharmacist) volunteered, "The 3 solutions looked identical (clear, colourless solutions). The diluent for all 3 solutions was hydroxybenzoate, with the placebo being made up with preserved water" | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The random sequence was generated by the pharmacist by pulling folded tickets out of a bag with morphine, paracetamol or placebo on the tickets. Equal numbers of tickets were provided in the bag for each arm and all tickets were allocated |
| Allocation concealment (selection bias) | Low risk | Blinded random assignment was done by picking up consecutive envelopes |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | The pharmacy department at Wishaw General Hospital supplied trial substances. Infants received the trial substance (placebo or medication) 1 hour before the procedure was performed. The dose of each trial substance was dispensed as 10 mL and was administered as 2 mL/kg/dose, 1 hour before the procedure. The 3 solutions looked identical (clear, colourless solutions). The diluent for all 3 solutions was hydroxybenzoate, and the placebo was made up with preserved water |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | The infant’s face, saturation monitor and time frame cards were recorded on videotape over a 1 to 2 minute period, at 5 minutes before, then at 5 minutes, 30 minutes, 1 hour, 2 hours and 3 hours after the procedure. 2 separate individuals subsequently scored the information independently |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 18 infants were enrolled. At 5 minutes post eye examination, the PIPP score was not reported for 1 infant in the paracetamol group |
| Selective reporting (reporting bias) | Unclear risk | The protocol for the study was available to us. No selective outcome reporting is apparent. However only 18 infants from a preset sample of 63 were enrolled |
| Other bias | Low risk | Study appears free of other bias |
Seifi 2013.
| Methods | Randomised controlled trial | |
| Participants | Preterm infants < 32 weeks PMA. Tertiary level neonatal intensive care unit (Al Zahra Hospital, Tabriz, Iran) from October 2011 to October 2012 | |
| Interventions | Group 1 – oral acetaminophen 15 mg/kg 30 minutes before eye examination and 0.2 mL sterile water during initiation of eye examination (n = 41) Group 2 – 0.2 mL sucrose (25%) during initiation of eye examination (n = 40) Group 3 – 0.2 mL sterile water as placebo (39) during initiation of eye examination |
|
| Outcomes | 2 neonatologists experienced in the use of PIPP scoring and blind to the intervention groups determined pain score by 30‐second observation of neonates and responses on videotape at the start of the eye examination and during the last 45 seconds of the eye examination | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐based randomisation process |
| Allocation concealment (selection bias) | Low risk | Sealed opaque envelopes |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | It is unclear who administered acetaminophen 30 minutes before the examination |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | 2 neonatologists experienced in the use of PIPP scoring and blind to the intervention groups determined pain score after 30 seconds of observation of neonates and responses on videotape at the start of the eye examination and during the last 45 seconds of the eye examination |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 1 neonate in group B and 1 infant in group C were excluded because of impaired videotape recording and covering of neonate's face with examiner's hand, making pain assessment impossible Outcomes were reported for all other infants |
| Selective reporting (reporting bias) | Unclear risk | The protocol for this study was not available to us, so we cannot judge whether selective reporting occurred |
| Other bias | Low risk | Study appears free of other bias |
Shah 1998.
| Methods | Randomised, double‐blind, controlled trial. Single‐centre study conducted at Women's College Hospital in Toronto, Ontario, Canada | |
| Participants | 75 term neonates undergoing heel lance for screening for newborn disease | |
| Interventions | Oral paracetamol cherry elixir 20 mg/kg, 60 to 90 minutes before heel lance (n = 38), or placebo (cherry elixir) (n = 37) | |
| Outcomes | Per cent facial action – brow bulge, eye squeeze and nasolabial fold (range 0% to 300%); per cent of time spent crying (range 0% to 100%) | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated random numbers list |
| Allocation concealment (selection bias) | Low risk | Neonates were randomly assigned by the pharmacy department to receive before‐heel lance oral paracetamol cherry elixir (32 mg/mL) at a dose of 20 mg/kg or an equal volume of placebo (cherry elixir) of identical appearance |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | See allocation concealment |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | See allocation concealment |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 10 infants were excluded from the efficacy analysis. In 9 cases, infants had multiple heel lances (n = 5 in group 1; n = 4 in group 2); in 1 case (group 1), the video recording was of poor quality. Data for 13% of enrolled infants were missing |
| Selective reporting (reporting bias) | Unclear risk | The protocol for the study was not available to us, so we cannot judge whether selective reporting occurred |
| Other bias | Low risk | Study appears free of other bias |
Tinner 2013.
| Methods | Randomised double‐blind placebo‐controlled trial | |
| Participants | Singleton newborns, born by assisted vaginal delivery (forceps or vacuum), with PMA > 35 weeks and BW > 2000 grams. Infants were admitted to one of 3 Swiss university hospitals | |
| Interventions | Paracetamol suppositories (60/80/100 mg in infants < 3000 grams/3000 to 4000 grams/> 4000 grams birth weight) at 2 hours and 8 hours of life, or placebo suppositories at 2 hours and 8 hours of life | |
| Outcomes | Prolonged pain and discomfort during the first 24 hours of life were assessed by the Échelle de Douleur et d’Inconfort du Nouveau‐né (neonatal pain and discomfort) (EDIN) scale Assessments were carried out by nurses or midwives in charge at baseline (2 hours), and at 4, 8, 12 and 24 hours of life Acute pain response to a short painful stimulus 2 to 3 days after birth was assessed by the 7 behavioural items of the Bernese Pain Scale for Newborns (BPSN), validated to measure acute pain in newborn infants on a scale between 0 (no pain) and 21 (maximum pain). Infants received 0.2 mL sucrose solution 1 minute before heel lance performed to obtain blood for metabolic screening at 2 to 3 days of life. BPSN was scored by nurse immediately before and after the heel lance. In addition, infants were videotaped and tapes were scored off‐site by a single reviewer. Whether infants cried after heel lance and, if so, the time infants spent crying after heel lance, were recorded |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The Pharmacy Institute held the randomisation key with a block random allocation |
| Allocation concealment (selection bias) | Low risk | At 2 hours and 8 hours of life, infants received 2 coded suppositories that contained paracetamol or placebo. The sequence remained concealed until the study was completed |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind placebo‐controlled study |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Double‐blind placebo‐controlled study |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All infants recruited were randomly assigned to receive placebo (n = 69) or paracetamol (n = 71), and all infants randomly assigned received the allocated intervention and continued treatment as planned (i.e. they received 2 suppositories at 2 hours and 8 hours of life). 17 subsequent drop‐outs (8 (3/5/0) infants transferred to the neonatal unit and 9 (0/2/7) infants who left the maternity hospital before metabolic screening). Thus, a total of 123 newborn infants completed the study; 62 had received paracetamol and 61 placebo |
| Selective reporting (reporting bias) | Low risk | The protocol is available at www.clinicaltrials.gov NCT 00488540; no deviations from the protocol are apparent |
| Other bias | Low risk | Study appears free of other bias |
van Lingen 2001.
| Methods | Randomised controlled study undertaken during a 15‐month period at 2 hospitals in The Netherlands | |
| Participants | Infants were eligible for the study if they fulfilled the following entry criteria: birth weight > 2500 grams, gestational age > 36 weeks, Apgar score at 5 minutes ≥ 7 and absence of congenital anomalies of the newborn. All infants were delivered by vacuum extraction. Infants admitted to the NICU for artificial ventilation because of respiratory insufficiency were excluded from the study. Furthermore, infants were excluded during the evaluation phase of the study if data were incomplete (e.g. in cases of early hospital discharge) | |
| Interventions | Paracetamol suppositories 20 mg/kg (n = 61) or placebo suppositories (n = 61) | |
| Outcomes | Modified 5 point facies scale at 1, 7, 13 and 19 hours after the first suppository had been given. Score of clinical condition | |
| Notes | Dr van Lingen provided us with his PhD thesis — 'Pain Assessment and Analgesia in the Newborn: An Integrated Approach' — which includes a chapter on this trial | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation procedure was carried out by one of the hospital pharmacists, who had no knowledge of the clinical status of the neonates |
| Allocation concealment (selection bias) | Low risk | In the delivery room, separate numbered boxes were available for birth‐weight categories; each contained 4 suppositories with paracetamol suspended in Witepsol H‐15 as a fatty suppository base or a placebo with only a Witepsol H‐15 base |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | See allocation concealment |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Scoring was performed by nurses from the obstetric and paediatric wards, who were blinded to the group to which the infant was assigned |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 18 infants were excluded as they failed to receive 3 or more doses of the study drug or placebo. 16 infants were discharged before all suppositories were given, and in 2 other infants, the study drug was stopped on parental request. 61 infants remained in each group; 59 in the paracetamol group and 60 in the placebo group underwent pain assessments |
| Selective reporting (reporting bias) | Unclear risk | The protocol for the study was not available to us; therefore we cannot judge whether selective reporting occurred |
| Other bias | Low risk | Study appears free of other bias |
BW = birth weight (grams).
NIPS = Neonatal Infant Pain Score.
PIPPS = Premature Infant Pain Profile.
PMA = postmenstrual age (weeks).
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Hong 2010 | Infants/children enrolled in this study were 6 to 24 months old |
| Härmä 2016 | Before after study. Not a randomised controlled trial. |
Characteristics of studies awaiting assessment [ordered by study ID]
Foronda 2014.
| Methods | A randomised, controlled and single‐blind study |
| Participants | Neonates (n = 129) undergoing heel lance |
| Interventions | Paracetamol (n = 42), dextrose (n = 47) and placebo (n = 40) |
| Outcomes | NIPS and PIPP |
| Notes | Study presented in abstract form. No numbers for NIPS and PIPP scores were presented. Pain scores were lowest in the dextrose group. |
Garbi 2016.
| Methods | Ongoing randomised trial |
| Participants | Infants 24 to 36 weeks' corrected PMA requiring continuous opioid infusion for pain control |
| Interventions | 96‐hour regimen of adjunct IV acetaminophen compared with controls receiving solely opioid infusion |
| Outcomes | Cumulative opioid exposure, measures of hepatic function, pain and sedation scores using the Neonatal Pain, Agitation Sedation Scale (NPASS), duration of mechanical ventilation, reintubation, time to full enteral feedings and number of apneic/bradycardic episodes |
| Notes | When the abstract was published, 24 (of 30) patients had been enrolled (11 Study, 13 Control) |
van der Marel 2007.
| Methods | Randomised controlled study |
| Participants | Infants 0 to 10 months old undergoing major surgery (thoracic ‒ non‐cardiac and abdominal surgery) |
| Interventions | Paracetamol or placebo given rectally, in addition to a morphine loading dose and continuous infusion |
| Outcomes | COMFORT score |
| Notes | We have written to study authors to request data for infants < 1 month of age. As of 23 May 2016 we have not received an answer |
Characteristics of ongoing studies [ordered by study ID]
NCT01938261.
| Trial name or title | Preterm infants' paracetamol study (PreParaS) |
| Methods | Randomised placebo‐controlled study |
| Participants | Preterm infants born at < 32 weeks PMA |
| Interventions | Placebo comparator: paracetamol effect on ductus. First drug dose is given before 24 hours of age. Masked study drug is paracetamol infusion solution 10 mg/mL (PERFALGAN®) or placebo, 0.45% saline solution. Loading dose is 20 mg/kg, and maintenance dose is 7.5 mg/kg every 6 hours for 4 days. Placebo comparator: paracetamol effect on pain. First drug dose is given before 24 hours of age. Masked study drug is paracetamol infusion solution 10 mg/mL (PERFALGAN®) or placebo, 0.45% saline solution. Loading dose is 20 mg/kg, and maintenance dose is 7.5 mg/kg every 6 hours for 4 days. |
| Outcomes | For PDA: ductus diameter mm/kg at postnatal age of 5 days For pain: cumulative dosage of morphine at postnatal age of 5 days |
| Starting date | August 2013 |
| Contact information | Outi Aikio, MD, PhD + 358 8 3155810 outi.aikio@ppshp.fi |
| Notes |
PMA = postmenstrual age.
Differences between protocol and review
In a deviation from the protocol, we included one study that reported the total amount of morphine administered to infants randomly assigned to receive continuous morphine or intermittent intravenous paracetamol up to 48 hours post major thoracic or abdominal surgery (Ceelie 2013). The study included infants who were 30 days of age or less at enrolment. In our opinion, this study provides important information relevant to the topic of the protocol. The study by Bonetto 2008 included infants less than 30 days of age. Our upper age limit was less than 28 days, but we decided to include these studies.
Contributions of authors
Both review authors contributed equally to this protocol and review.
Sources of support
Internal sources
Mount Sinai Hospital, Toronto, Ontario, Canada.
External sources
-
Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institutes of Health, Department of Health and Human Services, USA.
Editorial support of the Cochrane Neonatal Review Group has been funded with Federal funds from the Eunice Kennedy Shriver National Institute of Child Health and Human Development National Institutes of Health, Department of Health and Human Services, USA, under Contract No. HHSN275201100016C
-
National Institute for Health Research, UK.
Editorial support for Cochrane Neonatal has been funded with funds from a UK National Institute of Health Research Grant (NIHR) Cochrane Programme Grant (13/89/12). The views expressed in this publication are those of the authors and not necessarily those of the NHS, the NIHR, or the UK Department of Health.
Declarations of interest
Dr Arne Ohlsson is a co‐author of one of the included trials (Shah 1998).
Dr Prakeshkumar Shah reports no conflicts of interest.
Deceased
Edited (no change to conclusions)
References
References to studies included in this review
Badiee 2009 {published data only}
- Badiee Z, Torcan N. Effects of high dose orally administered paracetamol for heel prick pain in premature infants. Saudi Medical Journal 2009;30(11):1450‐3. [PUBMED: 19882059] [PubMed] [Google Scholar]
Bonetto 2008 {published data only}
- Bonetto G, Salvatico E, Varela N, Cometto C, Gómez PF, Calvo B. Pain prevention in term neonates: randomized trial for three methods [Prevención del dolor en recién nacidos de término: estudio aleatorizado sobre tres métodos]. Archivos Argentinos de Pediatría 2008;106(5):392‐6. [PUBMED: 19030637] [DOI] [PubMed] [Google Scholar]
Ceelie 2013 {published data only}
- Ceelie I, Wildt SN, Dijk M, Berg MM, Bosch GE, Duivenvoorden HJ, et al. Effect of intravenous paracetamol on postoperative morphine requirements in neonates and infants undergoing major noncardiac surgery: a randomized controlled trial. JAMA 2013;309(2):149‐54. [PUBMED: 23299606] [DOI] [PubMed] [Google Scholar]
Kabataş 2016 {published data only}
- Kabataş EU, Dursun A, Beken S, Dilli D, Zenciroğlu A, Okumuş N. Efficacy of single dose oral paracetamol in reducing pain during examination for retinopathy of prematurity: A blinded randomized controlled trial. Indian Journal of Pediatrics 2016;83(1):22‐6. [DOI] [PubMed] [Google Scholar]
Manjunatha 2009 {published data only}
- Manjunatha CM, Ibhanesebhor SE, Rennix C, Fisher H, Abara R. Pain control during retinopathy of prematurity screening: double‐blind, randomised, placebo‐controlled study. Infant 2009;5(5):155‐8. [Google Scholar]
Seifi 2013 {published data only}
- Seifi F, Peirovifar A, Gharehbaghi MM. Comparing the efficacy of oral sucrose and acetaminophen in pain relief for ophthalmologic screening of retinopathy of prematurity. American Journal of Medical Sciences and Medicine, 2013;1(2):24‐7. [Google Scholar]
Shah 1998 {published data only}
- Shah V, Taddio A, Ohlsson A. Randomised controlled trial of paracetamol for heel prick pain in neonates. Archives of Disease in Childhood. Fetal and Neonatal Edition 1998;79(3):F209‐11. [PUBMED: 10194994] [DOI] [PMC free article] [PubMed] [Google Scholar]
Tinner 2013 {published data only}
- Buehrer C, Glanzmann R, Hoesli I, Jost K, Schoebi N, Tinner EM. Mode of delivery and neonatal pain assessed by EDIN score. Journal of Maternal‐Fetal and Neonatal Medicine. 22nd European Congress of Perinatal Medicine; 2010 May 26‐29; Granada, Spain. 2010; Vol. 23:311.
- Tinner EM, Hoesli I, Jost K, Schöbi N, Ulrich Megged Y, Burkhardt T, et al. Rectal paracetamol in newborn infants after assisted vaginal delivery may increase pain response. The Journal of Pediatrics 2013;162(1):62‐6. [PUBMED: 22809664] [DOI] [PubMed] [Google Scholar]
- Visca E, Voekt C, Tinner E‐M, Burkhardt T, Krafft A, Surbek D, et al. Metal cups for vacuum‐assisted vaginal delivery increase neonatal pain response. International Journal of Gynaecology and Obstetrics. 20th FIGO World Congress of Gynecology and Obstetrics. 2012 Oct 7‐12; Rome, Italy. 2012; Vol. 119:S514.
van Lingen 2001 {published data only}
- Quak JME, Lingen RA, Deinum JT, an de Logt F, Eyck J, Okken A. Effects of administration of paracetamol versus placebo on the symptoms in neonates born after vacuum extraction; a prospective, double‐blind, randomised study [Effecten van paracetamol‐versus placebotoediening op de symptomen bij neonaten geboren na een vacuumextractie; een prospective, dubbelblinde, gerandomiseerde studie]. Tijdschrift voor Kindergeneeskunde 1965;63:82. [Google Scholar]
- Lingen R. Effect of rectally administered paracetamol in infants delivered by vacuum extraction. II.5 Pain assessment and analgesia in the newborn: an integrated approach. Den Haag: CIP‐gegevens Koninklijke Bibliotheek, 2000:89‐99. [Google Scholar]
- Lingen RA, Quak CM, Deinum HT, Logt F, Eyck J, Okken A, et al. Effects of rectally administered paracetamol on infants delivered by vacuum extraction. European Journal of Obstetrics, Gynecology, and Reproductive Biology 2001;94(1):73‐8. [PUBMED: 11134829] [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Härmä 2016 {published data only}
- Härmä A, Aikio O, Hallman M, Saarela T. Intravenous paracetamol decreases requirements of morphine in very preterm Infants. Journal of Pediatrics 2016;168:36‐40. [DOI] [PubMed] [Google Scholar]
Hong 2010 {published data only}
- Hong JY, Kim WO, Koo BN, Cho JS, Suk EH, Kil HK. Fentanyl‐sparing effect of acetaminophen as a mixture of fentanyl in intravenous parent‐/nurse‐controlled analgesia after pediatric ureteroneocystostomy. Anesthesiology 2010;113(3):672–7. [PUBMED: 20693884] [DOI] [PubMed] [Google Scholar]
References to studies awaiting assessment
Foronda 2014 {published data only}
- Foronda O, Rocha S, Grandy G. Efficacies of paracetamol and glucose in pain reduction in newborns: A randomized, controlled and single blind study. Pediatric Research. 2014; Vol. 75, issue 3:473.
Garbi 2016 {published data only}
- Garbi LR, Shah S. The effect of intravenous acetaminophen on opioid use in preterm infants. Pediatric Academic societies (PAS) Annual Meeting; 2016 Apr 30 ‐ May 3; Baltimore, USA. 2016:362.
van der Marel 2007 {published data only}
- Marel CD, Peters JW, Bouwmeester NJ, Jacqz‐Aigrain E, Anker JN, Tibboel D. Rectal acetaminophen does not reduce morphine consumption after major surgery in young infants. British Journal of Anaesthesia 2007;98(3):372‐9. [PUBMED: 17284514] [DOI] [PubMed] [Google Scholar]
References to ongoing studies
NCT01938261 {published data only}
- NCT01938261. The preterm infants' paracetamol study (PreParaS). clinicaltrials.gov/show/NCT01938261. ClinicalTrials.gov, (accessed 13 October 2013).
Additional references
AAP 1987
- American Academy of Pediatrics, Committee on Fetus and Newborn, Committee on Drugs, Section on Anesthesiology, Section on Surgery. Neonatal anesthesia. Pediatrics 1987;80:446. [Google Scholar]
AAP 2000
- American Academy of Pediatrics, Committee on Fetus and Newborn, Committee on Drugs, Section on Anesthesiology, Section on Surgery, Canadian Paediatric Society Fetus and Newborn Committee. Prevention and management of pain and stress in the neonate. Pediatrics 2000;105(2):454‐61. [PUBMED: 10654977] [PubMed] [Google Scholar]
AAP 2006
- American Academy of Pediatrics Committee on Fetus and Newborn, Section on Surgery, Canadian Paediatric Society Fetus and Newborn Committee, Batton DG, Barrington KJ, Wallman C. Prevention and management of pain in the neonate: an update. Pediatrics 2006;118(5):2231‐41. [PUBMED: 17079598] [DOI] [PubMed] [Google Scholar]
Ambuel 1992
- Ambuel B, Hamlett KW, Marx CM, Blumer JL. Assessing distress in pediatric intensive care environments: the COMFORT scale. Journal of Pediatric Psychology 1992;17(1):95‐109. [PUBMED: 1545324] [DOI] [PubMed] [Google Scholar]
American Psychiatric Association 2013
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th Edition. Arlington, VA: American Psychiatric Association, 2013. [Google Scholar]
Anand 1987a
- Anand KJS, Sippell WG, Aynsley‐Green A. Randomised trial of fentanyl anaesthesia in preterm babies undergoing surgery: effects on the stress response. The Lancet 1987;8527:243‐8. [DOI] [PubMed] [Google Scholar]
Anand 1987b
- Anand KJ, Hickey PR. Pain and its effects in the human neonate and fetus. The New England Journal of Medicine 1987;317(21):1321‐9. [DOI] [PubMed] [Google Scholar]
Avella‐Garcia 2016
Bauer 2013
- Bauer AZ, Kriebel D. Prenatal and perinatal analgesic exposure and autism: an ecological link. Environmental Health 2013;12:41. [PUBMED: 23656698] [DOI] [PMC free article] [PubMed] [Google Scholar]
Botting 2000
- Botting RM. Mechanism of action of acetaminophen: is there a cyclooxygenase 3?. Clinical Infectious Diseases 2000;31(Suppl 5):S202‐10. [PUBMED: 11113024] [DOI] [PubMed] [Google Scholar]
Brady‐Fryer 2004
- Brady‐Fryer B, Wiebe N, Lander JA. Pain relief for neonatal circumcision. Cochrane Database of Systematic Reviews 2004, Issue 4. [DOI: 10.1002/14651858.CD004217.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Brummelte 2012
- Brummelte S, Grunau RE, Chau V, Poskitt KJ, Brant R, Vinall J, et al. Procedural pain and brain development in premature newborns. Annals of Neurology 2012;71(3):385‐96. [PUBMED: 22374882] [DOI] [PMC free article] [PubMed] [Google Scholar]
Butt 2013
- Butt ML, McGrath JM, Samra HA, Gupta R. An integrative review of parent satisfaction with care provided in the neonatal intensive care unit. Journal of Obstetric, Gynecologic, and Neonatal Nursing 2013;42(1):105‐20. [PUBMED: 23316895] [DOI] [PubMed] [Google Scholar]
Büttner 2000
- Büttner W, Finke W. Analysis of behavioural and physiological parameters for the assessment of postoperative analgesic demand in newborns, infants and young children: a comprehensive report on seven consecutive studies. Paediatric Anaesthesia 2000;10(3):303‐18. [PUBMED: 10792748] [DOI] [PubMed] [Google Scholar]
Carbajal 2008
- Carbajal R, Rousset A, Danan C, Coquery S, Nolent P, Ducrocq S, et al. Epidemiology and treatment of painful procedures in neonates in intensive care units. JAMA 2008;300(1):60‐70. [PUBMED: 18594041] [DOI] [PubMed] [Google Scholar]
Cignacco 2004
- Cignacco E, Mueller R, Hamers JP, Gessler P. Pain assessment in the neonate using the Bernese Pain Scale for Neonates. Early Human Development 2004;78(2):125‐31. [PUBMED: 15223117] [DOI] [PubMed] [Google Scholar]
Cochrane Neonatal Group 2013
- Soll RF, Bracken MB, Horbar JD, Ohlsson A, Suresh G, Brosseau Y, et al. Cochrane Neonatal Group. About The Cochrane Collaboration (Cochrane Review Groups (CRGs)) 2013, Issue 5. Art. No.: NEONATAL.
CPS 2000
- American Academy of Pediatrics Fetus and Newborn Committee, Committee on Drugs, Section on Anesthesiology, Section on Surgery, Canadian Paediatric Society Committee on Fetus and Newborn. Prevention and management of pain and stress in the neonate. Paediatrics and Child Health 2000;5(1):31‐47. [PUBMED: 20107594] [DOI] [PMC free article] [PubMed] [Google Scholar]
Craig 1994
- Craig KD, Hadjistavropoulos HD, Grunau RV, Whitfield MF. A comparison of two measures of facial activity during pain in the newborn child. Journal of Pediatric Psychology 1994;19(3):305‐18. [PUBMED: 8071797] [DOI] [PubMed] [Google Scholar]
Darwin 1872
- Darwin C. The Expressions of the Emotions in Man and Animals. London: John Murry, Albemarle Street, 1872. [Google Scholar]
Debillon 2001
- Debillon T, Zupan V, Ravault N, Magny JF, Dehan M. Development and initial validation of the EDIN scale, a new tool for assessing prolonged pain in preterm infants. Archives of Disease in Childhood. Fetal and Neonatal Edition 2001;85(1):F36‐41. [PUBMED: 11420320] [DOI] [PMC free article] [PubMed] [Google Scholar]
Fernandez 1994
- Fernandez CV, Rees EP. Pain management in Canadian level 3 neonatal intensive care units. Canadian Medical Association Journal 1994;150(4):499‐504. [PMC free article] [PubMed] [Google Scholar]
Fitzgerald 2009
- Fitzgerald M, Walker SM. Infant pain management: a developmental neurobiological approach. Nature Clinical Practice. Neurology 2009;5(1):35‐50. [PUBMED: 19129789] [DOI] [PubMed] [Google Scholar]
Foster 2013
- Foster J, Spence K, Henderson‐Smart D, Harrison D, Gray PH, Bidewell J. Procedural pain in neonates in Australian hospitals: a survey update of practices. Journal of Paediatrics and Child Health 2013;49(1):E35‐9. [PUBMED: 23279125] [DOI] [PubMed] [Google Scholar]
Franck 2011
- Franck LS, Ridout D, Howard R, Peters J, Honour JW. A comparison of pain measures in newborn infants after cardiac surgery. Pain 2011;152(8):1758‐65. [PUBMED: 21514052] [DOI] [PubMed] [Google Scholar]
Gibbins 2008
- Gibbins S, Stevens B, McGrath PJ, Yamada J, Beyene J, Breau L, et al. Comparison of pain responses in infants of different gestational ages. Neonatology 2008;93(1):10‐8. [PUBMED: 17630493] [DOI] [PubMed] [Google Scholar]
GRADEpro [Computer program]
- McMaster University. GRADEpro. McMaster University, 2014.
Gradin 2011
- Gradin M, Eriksson M, NeoOpioid Investigations Group. Neonatal pain assessment in Sweden ‐ a fifteen‐year follow up. Acta Paediatrica 2011;100(2):204‐8. [PUBMED: 20804461] [DOI] [PubMed] [Google Scholar]
Grunau 1998
- Grunau RE, Oberlander T, Holsti L, Whitfield MF. Bedside application of the Neonatal Facial Coding System in pain assessment of premature neonates. Pain 1998;76(3):277‐86. [PUBMED: 9718246] [DOI] [PubMed] [Google Scholar]
Grunau 2006
- Grunau RE, Holsti L, Peters JW. Long‐term consequences of pain in human neonates. Seminars in Fetal & Neonatal Medicine 2006;11(4):268‐75. [PUBMED: 16632415] [DOI] [PubMed] [Google Scholar]
Grèen 1989
- Grèen K, Drvota V, Vesterqvist O. Pronounced reduction of in vivo prostacyclin synthesis in humans by acetaminophen (paracetamol). Prostaglandins 1989;37(3):311‐5. [PUBMED: 2664901] [DOI] [PubMed] [Google Scholar]
Hall 2012
- Hall RW. Anesthesia and analgesia in the NICU. Clinics in Perinatology 2012;39(1):239‐54. [PUBMED: 22341549] [DOI] [PMC free article] [PubMed] [Google Scholar]
Harpin 1983
- Harpin VA, Rutter N. Making heel pricks less painful. Archives of Disease in Childhood 1983;58(3):226‐8. [PUBMED: 6838257] [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2003
- Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analyses. BMJ 2003;327(7414):557‐60. [PUBMED: 12958120] [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Holsti 2007
- Holsti L, Grunau RE. Initial validation of the Behavioral Indicators of Infant Pain (BIIP). Pain 2007;132(3):264‐72. [PUBMED: 17382473] [DOI] [PMC free article] [PubMed] [Google Scholar]
Holsti 2008
- Holsti L, Grunau RE, Oberlander TF, Osiovich H. Is it painful or not? Discriminant validity of the Behavioral Indicators of Infant Pain (BIIP) scale. Clinical Journal of Pain 2008;24(1):83‐8. [PUBMED: 18180641] [DOI] [PMC free article] [PubMed] [Google Scholar]
Holve 1983
- Holve RL, Bromberger PJ, Groveman HD, Klauber MR, Dixon SD, Snyder JM. Regional anesthesia during newborn circumcision. Effect on infant pain response. Clinical Pediatrics 1983;22(12):813‐8. [PUBMED: 6354552] [DOI] [PubMed] [Google Scholar]
Jeong 2013
Johnston 2011a
- Johnston CC, Fernandes AM, Campbell‐Yeo M. Pain in neonates is different. Pain 2011;152(3 Suppl):S65‐73. [PUBMED: 20971562] [DOI] [PubMed] [Google Scholar]
Johnston 2011b
- Johnston C, Barrington KJ, Taddio A, Carbajal R, Filion F. Pain in Canadian NICUs: have we improved over the past 12 years?. Clinical Journal of Pain 2011;27(3):225‐32. [PUBMED: 21178602] [DOI] [PubMed] [Google Scholar]
Kirya 1978
- Kirya C, Werthman MW Jr. Neonatal circumcision and penile dorsal nerve block ‐ a painless procedure. Journal of Pediatrics 1978;92(6):998‐1000. [PUBMED: 660375] [DOI] [PubMed] [Google Scholar]
Krechel 1995
- Krechel SW, Bildner J. CRIES: a new neonatal postoperative pain measurement score. Initial testing of validity and reliability. Paediatric Anaesthesia 1995;5(1):53‐61. [PUBMED: 8521311] [DOI] [PubMed] [Google Scholar]
Lago 2013
- Lago P, Garreti E, Boccuzzo G, Merazzi D, Pirelli A, Pieragostini L, et al. Procedural pain in neonates: the state of the art in the implementation of national guidelines in Italy. Paediatric Anaesthesia 2013;23(5):407‐14. [PUBMED: 23301982] [DOI] [PubMed] [Google Scholar]
Lawrence 1993
- Lawrence J, Alcock D, McGrath P, Kay J, MacMurray SB, Dulberg C. The development of a tool to assess neonatal pain. Neonatal Network 1993;12(6):59‐66. [PUBMED: 8413140] [PubMed] [Google Scholar]
Lee 2007
- Lee SK. Health policy and health economics related to neonatal pain. In: Anand KJS, Stevens BJ, McGrath PJ editor(s). Pain in Neonates and Infants. 3rd Edition. Toronto: Elsevier, 2007:273‐8. [Google Scholar]
McPherson 2014
- McPherson C, Grunau RE. Neonatal pain control and neurologic effects of anesthetics and sedatives in preterm infants. Clinics in Perinatology 2014;41(1):209‐27. [PUBMED: 24524456] [DOI] [PMC free article] [PubMed] [Google Scholar]
Merskey 1979
- Merskey H, Albe‐Fessard DG, Bonica JJ, Carmon A, Dubner R, Kerr FWL, et al. Pain terms: a list with definitions and notes on usage. Recommended by the IASP Subcommittee on Taxonomy. Pain 1979;6(3):249‐52. [PUBMED: 460932] [PubMed] [Google Scholar]
National Library of Medicine 2013
- U.S. National Library of Medicine. National Institutes of Health. Acetaminophen/Paracetamol. Drug Information Portal. druginfo.nlm.nih.gov/drugportal (accessed 13 October 2013).
Ohlsson 2000
- Ohlsson A, Taddio A, Jadad AR, Stevens BJ. Evidence‐based decision making, systematic reviews and the Cochrane Collaboration: implications for neonatal analgesia. In: Anand KJS, Stevens BJ, McGrath PJ editor(s). Pain in Neonates. 2nd Edition. New York: Elsevier, 2000:251‐68. [Google Scholar]
Ohlsson 2007
- Ohlsson A, Shah V. Evidence‐based practice as a means for clinical decision making. In: Anand KJS, Stevens BJ, McGrath PJ editor(s). Pain in Neonates and Infants. 3rd Edition. Toronto: Elsevier, 2007:235‐50. [Google Scholar]
Ozawa 2013
- Ozawa M, Yokoo K. Pain management of neonatal intensive care units in Japan. Acta Paediatrica 2013;102(4):366‐72. [PUBMED: 23311590] [DOI] [PubMed] [Google Scholar]
Palmer 1962
- Palmer LE. Relief of pain in infant teething; double blind study of a new choline salicylate agent. Ohio Medicine 1962;58:434‐5. [PUBMED: 14483417] [PubMed] [Google Scholar]
Palmer 2008
- Palmer GM, Atkins M, Anderson BJ, Smith KR, Culnane TJ, McNally CM, et al. I.V. acetaminophen pharmacokinetics in neonates after multiple doses. British Journal of Anaesthesia 2008;101(4):523‐30. [PUBMED: 18628265] [DOI] [PubMed] [Google Scholar]
Porter 1999
- Porter FL, Grunau RE, Anand KJ. Long‐term effects of pain in infants. Journal of Developmental and Behavioral Pediatrics 1999;20(4):253‐61. [PUBMED: 10475600] [DOI] [PubMed] [Google Scholar]
RevMan 2014 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Roofthooft 2014
- Roofthooft DW, Simmons SH, Anand KJ, Tibboel D, Dijk M. Eight years later, are we still hurting newborn infants?. Neonatology 2014;105(3):218‐26. [PUBMED: 24503902] [DOI] [PubMed] [Google Scholar]
Schwartz 1977
- Schwartz G. Rembrandt. All the Etchings Reproduced in True Size. Maarsen, The Netherlands: Gary Schwartz, 1977. [Google Scholar]
Schünemann 2013
- Schünemann H, Brożek J, Guyatt G, Oxman A, editors. GRADE Working Group. GRADE handbook for grading quality of evidence and strength of recommendations. Available from www.guidelinedevelopment.org/handbook 2013.
Stevens 1996
- Stevens B, Johnston C, Petryshen P, Taddio A. Premature Infant Pain Profile: development and initial validation. Clinical Journal of Pain 1996;12(1):13‐22. [PUBMED: 8722730] [DOI] [PubMed] [Google Scholar]
Stevens 2000
- Stevens B, Johnston C, Gibbins S. Pain assessment in neonates. In: Anand KJS, Stevens BJ, McGrath PJ editor(s). Pain in Neonates. 2nd Edition. New York: Elsevier, 2000:101‐29. [Google Scholar]
Sundaram 2011
- Sundaram SS, Alonso EM, Narkewicz MR, Zhang S, Squires RH, Pediatric Acute Liver Failure Study Group. Characterization and outcomes of young infants with acute liver failure. Journal of Pediatrics 2011;159(5):813‐8. [PUBMED: 21621221] [DOI] [PMC free article] [PubMed] [Google Scholar]
Toms 2008
- Toms L, McQuay HJ, Derry S, Moore RA. Single dose oral paracetamol (acetaminophen) for postoperative pain in adults. Cochrane Database of Systematic Reviews 2008, Issue 4. [DOI: 10.1002/14651858.CD004602.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Tzortzopoulou 2011
- Tzortzopoulou A, McNicol ED, Cepeda MS, Francia MB, Farhat T, Schumann R. Single dose intravenous propacetamol or intravenous paracetamol for postoperative pain. Cochrane Database of Systematic Reviews 2011, Issue 10. [DOI: 10.1002/14651858.CD007126.pub2] [DOI] [PubMed] [Google Scholar]
van Dijk 2009
- Dijk M, Roofthooft DWE, Anand KJ, Guldemond F, Graaf J, Simons S, et al. Taking up the challenge of measuring prolonged pain in (premature) neonates: the COMFORTneo scale seems promising. Clinical Journal of Pain 2009;25(7):607‐16. [PUBMED: 19692803] [DOI] [PubMed] [Google Scholar]
Viberg 2014
- Viberg H, Eriksson P, Gordh T, Fredriksson A. Paracetamol (acetaminophen) administration during neonatal brain development affects cognitive function and alters its analgesic and anxiolytic response in adult male mice. Toxicological Sciences 2014;138(1):139‐47. [PUBMED: 24361869] [DOI] [PubMed] [Google Scholar]
Vinall 2012
- Vinall J, Miller SP, Chau V, Brummelte S, Synnes AR, Grunau RE. Neonatal pain in relation to postnatal growth in infants born very preterm. Pain 2012;153(7):1374‐81. [PUBMED: 22704600] [DOI] [PubMed] [Google Scholar]
Walls 2007
- Walls L, Baker CF, Sarkar S. Acetaminophen‐induced hepatic failure with encephalopathy in a newborn. Journal of Perinatology 2007;27(2):133‐5. [PUBMED: 17262050] [DOI] [PubMed] [Google Scholar]
Wan 2014
- Wan X, Wang W, Liu J, Tong T. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Medical Research Methodology 2014;14:135. [DOI] [PMC free article] [PubMed] [Google Scholar]
Wang 2014
- Wang C, Allegaert K, Tibboel D, Danhof M, Marel CD, Mathot RA, et al. Population pharmacokinetics of paracetamol across the human age‐range from (pre)term neonates, infants, children and adults. Journal of Clinical Pharmacology 2014;54(6):619‐29. [PUBMED: 24375166] [DOI] [PubMed] [Google Scholar]
Wilson‐Smith 2009
- Wilson‐Smith EM, Morton NS. Survey of i.v. paracetamol (acetaminophen) use in neonates and infants under 1 year of age by UK anaesthetists. Pediatric Anesthesia 2009;19(4):329‐37. [PUBMED: 19335345] [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Ohlsson 2015
- Ohlsson A, Shah PS. Paracetamol (acetaminophen) for prevention or treatment of pain in newborns. Cochrane Database of Systematic Reviews 2015, Issue 6. [DOI: 10.1002/14651858.CD011219.pub2] [DOI] [PubMed] [Google Scholar]


