Abstract
Atrial fibrillation (AF) is a complex cardiac arrhythmia with diverse etiology that negatively affects morbidity and mortality of millions of patients. Technological and experimental advances have provided a wealth of information on the pathogenesis of AF, highlighting a multitude of mechanisms involved in arrhythmia initiation and maintenance, and disease progression. However, it remains challenging to identify the predominant mechanisms for specific subgroups of AF patients, which, together with an incomplete understanding of the pleiotropic effects of antiarrhythmic therapies, likely contributes to the suboptimal efficacy of current antiarrhythmic approaches. Computer modeling of cardiac electrophysiology has advanced in parallel to experimental research and provides an integrative framework to attempt to overcome some of these challenges. Multi-scale cardiac modeling and simulation integrate structural and functional data from experimental and clinical work with knowledge of atrial electrophysiological mechanisms and dynamics, thereby improving our understanding of AF mechanisms and therapy.
In this review, we describe recent advances in our quantitative understanding of AF through mathematical models. We discuss computational modeling of AF mechanisms and therapy using detailed, mechanistic cell/tissue-level models, including approaches to incorporate variability in patient populations. We also highlight efforts using whole-atria models to improve catheter ablation therapies. Finally, we describe recent efforts and suggest future extensions to model clinical concepts of AF using patient-level models.
Keywords: antiarrhythmic drugs, atrial fibrillation, catheter ablation, computational modeling, arrhythmia mechanisms
1. Introduction
The pathogenesis of atrial fibrillation (AF) involves a multitude of mechanisms that control its initiation, maintenance and progression, including alterations in electrical, structural, mechanical, neurohumoral, and metabolic properties [1]. The current management and treatment of AF remain unsatisfactory, likely due to incomplete understanding of the mechanisms underlying arrhythmogenesis and disease progression in an individual patient, and pleiotropic effects of current therapies.
Several factors have made it challenging to translate basic research findings into improved clinical management [2, 3]. Simulation and modeling have proven tremendously valuable in physical sciences and engineering, and in the past five decades, computational approaches have also contributed to our understanding of cardiac physiology [4, 5]. Multi-scale models of cardiac electrophysiology integrate structural and functional data from experimental and clinical work with knowledge of atrial electrophysiological mechanisms and dynamics to improve our mechanistic understanding of this complex arrhythmia. Imaging-based whole-atria simulations have emerged as promising tools for AF management and treatment strategies tailored to each patient [6, 7], contributing to the ongoing efforts towards precision medicine in cardiology.
Here, we review recent advances in our quantitative understanding of AF therapy with antiarrhythmic drugs (AADs) and catheter ablation through mechanism-based integrative mathematical models. We also discuss how approaches to incorporate variability might help to dissect mechanisms of disease and impact of treatment for specific patient populations. Finally, we describe recent efforts and suggest future extensions to model clinical AF concepts using patient-level models.
2. AF modeling approaches
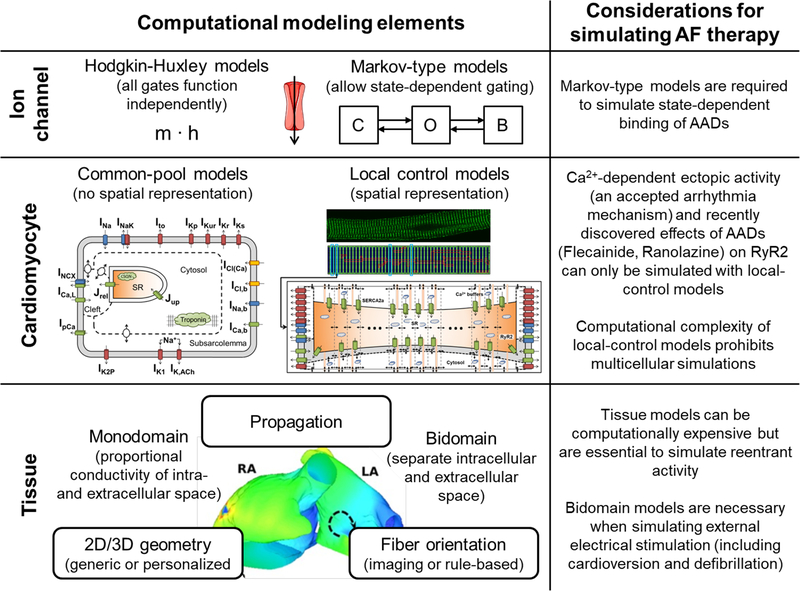
Multi-scale mechanistic models are systems of differential equations in which dynamic quantities, so-called ‘state variables’ (e.g., ion channel states, membrane potential, intracellular ion concentrations), are iteratively updated depending on their current values. For example, the current membrane potential can determine the change in open probability of an ion channel. The most important modeling approaches at the level of the ion channel (Hodgkin-Huxley and Markov-type models), single cell (common pool and local control models) and tissue (mono- and bidomain approaches) are summarized in Figure 1. Interested readers are referred to other reviews for methodological details [8–10]. In contrast to experimental work, simulation of mechanistic models provides perfect control over parameters, allowing observability of all model components, making them highly suitable to test for cause-effect relationships and to explore AF mechanisms.
Figure 1.
Schematic overview of the most important computational modeling approaches and elements at the ion channel, cardiomyocyte and tissue levels. The right column highlights a number of methodological considerations for simulating AF therapy. The whole-atria model figure was reproduced from [58].
2.1. Cell models
The main characteristics and evolution of several commonly used human atrial cardiomyocyte models [11–15] have recently been reviewed [9, 16]. The first human atrial cardiomyocyte models (Courtemanche et al. [11]; Nygren et al. [15]) focused on dynamic atrial electrophysiological properties and are still widely used for multi-scale simulations, along with their subsequent refinements and variants replicating regional atrial heterogeneity [14, 17]. Subsequently, the Grandi and Koivumaki models have focused on the simulation of atrial Ca2+-handling, emphasizing the importance of Ca2+-and Na+-homeostasis in atrial electrophysiology [12, 13]. The Grandi model also allows simulating the consequences of sympathetic and vagal stimulation (as in the Maleckar model [14, 18]). The Koivumäki model enables simulating centripetal Ca2+ diffusion, providing a first spatial representation of Ca2+-cycling [13]. The Grandi model has also been merged with a spatial Ca2+-handling model incorporating both transverse and longitudinal Ca2+ compartmentation [19], which has recently been employed to study the proarrhythmic effects of a heterogeneous distribution of Ca2+-handling proteins [20]. Other extensions of the Grandi model include regulation of the basal and acetylcholine-activated inward-rectifier K+-currents IK1 and IK,ACh, as well as atrial-specific two-pore-domain K+-channel (K2P) models and their regulation in AF [21, 22], and Markov-type Na+-channel and ultra-rapid delayed-rectifier K+-current (IKur) models [23–25]. Thus, although contemporary cardiomyocyte models reproduce a wide range of experimental findings, there is no single model that incorporates all key components. Each model has its own limitations, which should be taken into account when applying them to experimental findings.
2.2. Populations of models
Accounting for phenotypic diversity has become an important consideration when building models of cardiac electrophysiology and interpreting simulation results under (patho)physiological conditions. New tools, such as population-based approaches, have recently been developed to address some of the limitations of traditional modeling approaches [26, 27]. Populations of models are generally built from a baseline “average” model by varying sets of parameters within their experimental range or between theoretical upper/lower bounds. Statistical analyses of these populations have contributed to our understanding of the relative roles of the underlying parameters in modulating physiological properties of interest (i.e., sensitivity analysis), or revealing associations of certain parameter ranges or properties with specific physiological behaviors (e.g., repolarization abnormalities, ectopic activity, drug response) [28], as reviewed in [26]. Notably, although baseline variants of different atrial electrophysiological models exhibit important differences in action potential (AP) morphology and rate dependence [16, 29], recent population-based simulations showed remarkable similarities in the ionic determinants of inter-subject variability in three different human atrial AP models [30]. Furthermore, populations of models with wide variations in ionic densities can yield APs consistent with experiments, even if baseline models are initially far away from the specific experimental cohort [31]. Although, there are requirements for the baseline model employed for population-based simulations, for example the ability to recapitulate the AF mechanisms being studied (e.g., not all models can simulate abnormal automaticity or ectopy), the population-based approach may 1) reflect natural cell-to-cell variability, even within a single right-atrial sample; 2) address uncertainty in measurements, whereby several expression patterns may produce the same AP under basal conditions but respond differently to challenges; and 3) reduce the impact of the selected parameters of the baseline model.
2.3. Tissue and organ (image-based) models
Atrial-tissue models can simulate the major arrhythmogenic mechanisms and such models have been used to investigate the ionic mechanisms of clinically observed AF mechanisms involving steep APD gradients [32]. Given the small atrial-wall thickness, 2D geometries have been used extensively as approximations of atrial tissue for simplified AF simulations. Patient-specific anatomical models of the human atria have been constructed from diffusion tensor magnetic resonance imaging (MRI) reconstructions of tissue geometry. Recently, organ-level atrial models have also begun to represent fibrotic remodeling associated with AF. These geometries can be combined with heterogeneous AP models and recapitulate diverse forms of AF dynamics, including stable rotors, wavelets broken by repolarization heterogeneities, and multiple unstable meandering wavelets [17, 33–37]. The 3D atria can also be incorporated into a torso model to simulate body-surface electrocardiogram (ECG) patterns [33] and have the potential to provide in-depth insights into AF mechanisms beyond current experimental or clinical technical capabilities. For example, integrating ex-vivo imaging and functional data in a 3D high-resolution whole-atria model has enabled the identification of specific structural properties (“fingerprints”) underlying localized AF drivers [38]. Image-based organ models and their applications have been reviewed recently [6, 7] and are the focus of a separate review in this issue.
3. Anti-AF drug therapy: what can we learn from modeling?
Atrial efficacy and safety.
The development of effective and safe AADs against AF remains an important unmet clinical need. Recently, both atrial-predominant and multi-channel block have emerged as promising strategies for AF therapy. Atrial-specific ion-channel block may enable antiarrhythmic effects while avoiding ventricular proarrhythmia. Combination therapy may allow synergistic antiarrhythmic drug responses and thus reduction of therapeutic doses, thereby minimizing deleterious side effects. Several modeling and simulation examples have highlighted their usefulness to predict safety and efficacy of anti-AF therapeutic strategies [39]. Many of these studies suggest that accurately accounting for drug-channel interactions, instead of relying on steady-state concentration response curves or EC50 values, might be necessary to understand how AADs interact with a dynamically changing atrial (and ventricular) substrate [23, 40–42]. These considerations might play an important role in the prospective design of novel AADs, whereby an optimal AF-selective pharmacological approach would aim at maximizing drug effects at fast atrial rates without impacting normal sinus rhythm.
One strategy to achieve atrial selectivity is to exploit differences in atrial vs. ventricular electrophysiology. Atrioventricular differences in AP- and INa-gating properties render certain Na+-channel blockers atrial-selective [40, 43]. For example, ranolazine prolongs atrial AP duration (APD), slows conduction and suppresses AF, without affecting ventricular parameters [43]. Computational modeling has shown that ranolazine may also prevent proarrhythmic Ca2+ overload- and INa-mediated phase-3 afterdepolarizations in an atrial-selective manner [23]. Kinetics and state dependence of drug-channel interactions also modulate Na+-channel rate selectivity. In a combined experimental and computational study, anti-AF properties of Na+-channel blockers were potentiated by concomitant K+-channel blockade [40], prolonging the AP plateau and increasing APD. These effects led to a synergistic reduction of INa-dependent parameters (upstroke and conduction velocities), a more rapid termination of AF, and reduced AF inducibility [40]. Modeling has also demonstrated that AF-selectivity of INa inhibition is greatly augmented by blocking multiple atrial K+-currents (e.g., by acacetin) and translates into an enhanced termination of reentrant excitation waves. Importantly, virtual human ventricular cardiomyocytes and tissue were only modestly affected by acacetin and INa blockers [44].
Nevertheless, blocking K+ channels that are also abundantly expressed in ventricular tissue might pose safety concerns due to the increased risk of torsade-des-pointes arrhythmias, making atrial-predominant ion-channels attractive anti-AF targets. The atrial-predominant small-conductance Ca2+-activated K+-current (IK,Ca) blocker ICAGEN indeed enhances the anti-arrhythmic properties of the INa inhibitors flecainide and ranolazine in guinea pig atria [45]. However, the role of IK,Ca in either promoting or opposing AF is controversial, highlighting a role for modeling approaches to establish conditions in which IK,Ca inhibition or stimulation might be anti-arrhythmic [46]. Atrial-predominant K2P channels (e.g., K2P3.1) are upregulated in AF and their inhibition reduces the stability of reentry in 2D-tissue simulations incorporating electrical remodeling observed in AF patients with preserved left-ventricular function, but not AF patients with left-ventricular dysfunction, where K2P3.1 channels are downregulated [22]. These results demonstrate how combined experimental and computational studies can be used to predict therapeutic efficacy of new targets in specific patient populations. Numerous compounds targeting the atrial-predominant IKur have been screened in vitro and in AF animal models. However, evidence of antiarrhythmic efficacy in clinical trials is still lacking. A novel Markov-type model of IKur gating and drug-channel interaction revealed the ideal binding properties of IKur inhibitors that maximize AF-selectivity. The identified drug characteristics favor APD prolongation at fast atrial rates, while causing limited or no effect during normal heart rhythm. Despite being strongly downregulated in chronic AF (cAF) simulations, IKur contributes more prominently to APD in cAF than in sinus rhyhtm, and IKur inhibition in cAF displayed less cardiotoxic effects with increased efficacy [24, 25]. Thus, these computational modeling results suggest that the lack of clinically effective IKur inhibitors might be partly explained by the fact that preclinical assessment of candidate drugs overly relies on steady-state drug concentration-response curves rather than accounting for channel-state specificity and kinetics of drug binding.
IK1 is upregulated in cAF, promoting reentry stabilization in experiments [47] and simulations [48, 49]. Pentamidine analogue-6 (PA-6) inhibits IK1, restores sinus rhythm in goats with persistent AF, and decreases AF complexity before cardioversion [50], without affecting QT interval or heart rate in dogs, thus representing a potential safe therapeutic candidate for AF [50].
Role of variability.
Applying population-based approaches incorporating variability to study the effects of AADs substantially advances traditional approaches: it allows interpreting drug effects on electrophysiological properties at a population level and identifying the factors underlying the variability in drug-responses [26]. Indeed, a set of whole-atria models with identical structure (geometry and fiber orientation), but distinct electrophysiological properties selected from an experimentally calibrated population of AP models shows remarkable differences in AF properties [51]. Liberos et al. compared 3D-atrial tissue models with sustained versus self-terminating reentry circuits [52], showing that AF maintenance correlates with large L-type Ca2+-current (ICaL) and INa, and that ICaL block could be an effective treatment depending on the basal availability of Na+ and Ca2+ channels, with INa depression increasing overall efficacy. Thus, understanding the causes of variability in organ-level electrophysiological behavior and arrhythmia proclivity [28] may allow developing specific antiarrhythmic approaches for different arrhythmia phenotypes, potentially limiting the contribution of parameter- or model-dependent findings.
4. Computational modeling of AF ablation
Catheter ablation appears more effective than AAD therapy at maintaining sinus rhythm [53], and recent data suggest that AF ablation may even improve mortality in selected populations [54]. Overall, ablation results remain suboptimal, particularly in cAF patients [55, 56]. Recently several ablation strategies have been proposed with no additional benefit to pulmonary vein isolation alone. Thius, the most effective ablation strategy remains unclear and is likely patient specific. Personalized computational models represent a promising tool to determine optimal ablation strategies in a given patient [7, 10]. Simulation studies have provided information on the effectiveness of different surgical- or catheter-based ablation strategies to terminate AF in representative virtual atria [57, 58]. Other studies have employed personalized computed-tomography [59] or MRI [37, 60]-derived anatomies to retrospectively identify optimal patient-specific ablation strategies. The use of MRI also enables personalization of fibrosis patterns, which are a critical component of AF initiation and maintenance [37]. Recently, the practical feasibility of model-guided selection of predetermined AF ablation-lesion sets has been shown in a prospective randomized study, albeit only using personalization of computed-tomography-derived endocardial-wall anatomy [61].
These examples provide an important proof-of-concept and highlight the potential of image-based models for improving AF ablation, although it remains to be demonstrated whether they can improve patients’ outcomes. Several other challenges remain. First, only a handful of labs worldwide have the available expertise, computing power and required collaboration between clinicians and engineers to perform these interdisciplinary studies. However, with technological advances, simulations will become faster and easier to use, with commercial applications facilitating a more wide-spread use. Second, the extent of personalization of whole-atria models remains limited. Although late-gadolinium-enhancement MRI can provide information on patient-specific structural abnormalities, its current spatial resolution is insufficient to detect microscopic structural abnormalities, which may alter atrial conduction producing microreentry circuits maintaining AF [38]. Similarly, fiber orientation in whole-atria models is currently rule-based or adapted from an atlas of ex-vivo data. Finally, most whole-atria models employ a generic AP model (typically a version of the Courtemanche model [11]) to simulate electrophysiological properties [37, 58, 59]. Variability in electrophysiological parameters has a significant impact on reentrant driver localization, suggesting that simulation-predicted ablation strategies from patient-specific atrial models only incorporating individual fibrosis patterns alongside an average representation of AF-remodeled electrophysiology may not fully reproduce the individual phenotype [62]. Patient-specific electrophysiological information can be obtained invasively using mapping systems, but integrating this information in whole-atria models within the time constraints of a single ablation procedure will remain challenging. Moreover, perhaps one of the most important future contributions of whole-atria models may be the identification of patients in whom ablation is unlikely to be successful, and therefore should not undergo invasive procedures in the first place. Besides population-based modeling approaches (discussed above), non-invasive electrocardiographic imaging [63] or information from patient-specific induced pluripotent stem-cell-derived cardiomyocytes [6] may be used in the future to constrain some electrophysiological properties, although both have their own limitations.
5. Modeling long-term outcomes of AF patients
Although cellular, tissue and whole-atria models have provided important information about AF mechanisms and the acute electrophysiological effects of antiarrhythmic therapies, they do not provide information on the long-term clinical outcomes of AF patients. Patient- or population-level models representing dynamic transitions between different clinical states over extended time-periods have been employed to study cost-effectiveness of treatment strategies. These patient/population-level models are often based on Markov-type decision models, methodologically similar to the ion-channel formulations employed in modern cardiomyocyte models [9, 64], and can be employed to simulate the dynamic distribution of a large population over different states, or the stochastic transitions of a single virtual individual or ion channel [9, 64].
Patient-level Markov models, typically covering a period from several years up to the lifetime of a virtual population with 1-month intervals, have been used to determine the cost-effectiveness of non-vitamin-K oral anticoagulants and warfarin (reviewed in [65]), more extensive ablation strategies [66] and screening for asymptomatic AF [67]. Patient-level models have also been used to design optimal screening strategies [67] and to identify the impact of differences in patient populations and the associated heterogeneity in treatment effects between different studies [68]. For example, in a patient-level model for anticoagulation of AF patients with dabigatran vs. warfarin, differences in patient characteristics between the RE-LY trial and subsequent observational studies in real-world populations could largely explain discrepancies in bleeding rates, but not the rates of ischemic stroke [68]. Most AF-related patient-level models simulate the clinical outcomes of patients with AF, rather than the arrhythmia itself. One notable exception studied AF progression at a much higher temporal resolution (minutes), simulating the stochastic transitions between sinus rhythm and AF during the lifetime of a virtual patient [69]. However, this model does not consider the proper epidemiology of AF, with every individual progressing to permanent AF by 80 years of age.
Thus, patient-level models enable simulations of clinically relevant time scales and outcomes. Current patient-level models predominantly focus on health-economics applications and are mainly based on statistical properties derived from clinical trials rather than fundamental mechanistic understanding. However, future ‘hybrid’ mechanistic cell/tissue/whole-atria and patient-level models may help to bridge the widely different time-scales relevant for linking AF pathophysiology, epidemiology and clinical management, thereby contributing to improved tailored AF therapy.
6. Computational modeling in the era of big data
Statistical models already play a central role in the clinical management of AF, for example to assess stroke/bleeding risks. Although there are certain commonalities between mechanistic and statistical models (e.g., their dependence on clinical/experimental data), a number of important differences should be considered. Most statistical models reflect a static association between inputs and outputs, which are entirely derived from clinical or experimental data. The significant increase in data availability and advances in machine-learning techniques to derive models has promoted a strong interest in “big data”, which has been suggested to radically improve the diagnosis and treatment of cardiovascular diseases in the upcoming years [70]. Conversely, mechanistic models integrate information from clinical or experimental data (e.g., through parameter estimation procedures) with known biophysical laws to produce dynamic cause-effect simulations, and thus are essential to improve our understanding of the complex dynamics of AF, its underlying mechanisms and the effectiveness of specific therapeutic approaches. Ultimately, both strategies likely provide complimentary information and might even be integrated, whereby simulation output from a mechanistic model is combined with other clinical data in a statistical model to guide AF management in an individual patient.
7. Clinical relevance and conclusions
Computational modeling of AF has undergone substantial advances in the last 20 years. Although individual models are developed to address specific questions and usually operate on a limited subset of scales (e.g., addressing molecular and cellular AF mechanisms, whole-atria electrophysiology, or different clinical states), together these models have covered AF pathophysiology from molecule to patient (Figure 2). Statistical models (e.g., for stroke risk) already play a major role in clinical AF management and will be likely further refined with the data growth. At present, the relevance of mechanistic computational models is only indirect, serving as a plausibility check for mechanisms proposed based on experimental observations and helping to generate new hypothesis that can subsequently be tested experimentally. Their recent use in safety pharmacology may further affect clinical practice by guiding the preclinical development of novel AADs. Similarly, patient-level cost-effectiveness models may also affect clinical practice by influencing reimbursement policies. The direct clinical application of mechanistic models to guide AF therapy (notably ablation) is emerging, but is currently restricted to a few expert centers. Taken together, currently available models have provided insight into all major components of AF therapy, including AADs, ablation and anticoagulation, but their role in the disease management of AF patients is still in its infancy and there are numerous challenges that need to be overcome (Table 1). Nonetheless, technological advances and interconnection of different types of models are expected to further increase the relevance of computational modeling for clinical AF management in the years to come.
Figure 2. Overview of types of models and their most important experimental /clinical inputs and applications.
The whole-atria model figure was reproduced from [58].
Table 1.
Summary of notable results and challenges for computational modeling of AF.
| Notable results |
|
| Notable challenges |
|
Grant support:
This work is supported by the American Heart Association grant 15SDG24910015 (to E.G.), the National Institutes of Health Stimulating Peripheral Activity to Relieve Conditions (SPARC) grants 1OT2OD023848-01 and OT2 OD026580-01 (to E.G.), the National Heart, Lung, And Blood Institute (NHLBI) grants R01HL131517 (to E.G. and D.D), R01HL41214 (to E.G.), R01HL136389 (to D.D.), the UC Davis School of Medicine Dean’s Fellow award (to E.G.), the German Research Foundation (DFG, Do 769/4-1 to D.D.), the Netherlands Organization for Scientific Research (ZonMW Veni 91616057 to J.H.), and the CardioVascular Onderzoek Nederland and Netherlands Heart Foundation PREDICT project (Young Talent Program to J.H.)
Footnotes
Disclosures: none (all authors)
References
- [1].Andrade J, Khairy P, Dobrev D, Nattel S, The clinical profile and pathophysiology of atrial fibrillation: relationships among clinical features, epidemiology, and mechanisms, Circ Res 114 (2014) 1453–68. [DOI] [PubMed] [Google Scholar]
- [2].Heijman J, Algalarrondo V, Voigt N, Melka J, Wehrens XH, Dobrev D, et al. , The value of basic research insights into atrial fibrillation mechanisms as a guide to therapeutic innovation: a critical analysis, Cardiovasc Res 109 (2016) 467–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Heijman J, Guichard JB, Dobrev D, Nattel S, Translational Challenges in Atrial Fibrillation, Circ Res 122 (2018) 752–73. [DOI] [PubMed] [Google Scholar]
- [4].Bers DM, Grandi E, Human atrial fibrillation: insights from computational electrophysiological models, Trends Cardiovasc Med 21 (2011) 145–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Noble D, Cardiac action and pacemaker potentials based on the Hodgkin-Huxley equations, Nature 188 (1960) 495–7. [DOI] [PubMed] [Google Scholar]
- [6].Barichello S, Roberts JD, Backx P, Boyle PM, Laksman Z, Personalizing therapy for atrial fibrillation: the role of stem cell and in silico disease models, Cardiovasc Res 114 (2018) 931–43. [DOI] [PubMed] [Google Scholar]
- [7].Boyle PM, Zahid S, Trayanova NA, Using personalized computer models to custom-tailor ablation procedures for atrial fibrillation patients: are we there yet?, Expert Rev Cardiovasc Ther 15 (2017) 339–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Clayton RH, Bernus O, Cherry EM, Dierckx H, Fenton FH, Mirabella L, et al. , Models of cardiac tissue electrophysiology: progress, challenges and open questions, Prog Biophys Mol Biol 104 (2011) 22–48. [DOI] [PubMed] [Google Scholar]
- [9].Heijman J, Erfanian Abdoust P, Voigt N, Nattel S, Dobrev D, Computational models of atrial cellular electrophysiology and calcium handling, and their role in atrial fibrillation, J Physiol 594 (2016) 537–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Jacquemet V, Lessons from computer simulations of ablation of atrial fibrillation, J Physiol 594 (2016) 2417–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Courtemanche M, Ramirez RJ, Nattel S, Ionic mechanisms underlying human atrial action potential properties: insights from a mathematical model, Am J Physiol 275 (1998) H301–21. [DOI] [PubMed] [Google Scholar]
- [12].Grandi E, Pandit SV, Voigt N, Workman AJ, Dobrev D, Jalife J, et al. , Human atrial action potential and Ca2+ model: sinus rhythm and chronic atrial fibrillation, Circ Res 109 (2011) 1055–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Koivumaki JT, Korhonen T, Tavi P, Impact of sarcoplasmic reticulum calcium release on calcium dynamics and action potential morphology in human atrial myocytes: a computational study, PLoS Comput Biol 7 (2011) e1001067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Maleckar MM, Greenstein JL, Giles WR, Trayanova NA, K+ current changes account for the rate dependence of the action potential in the human atrial myocyte, Am J Physiol Heart Circ Physiol 297 (2009) H1398–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Nygren A, Fiset C, Firek L, Clark JW, Lindblad DS, Clark RB, et al. , Mathematical model of an adult human atrial cell: the role of K+ currents in repolarization, Circ Res 82 (1998) 63–81. [DOI] [PubMed] [Google Scholar]
- [16].Wilhelms M, Hettmann H, Maleckar MM, Koivumaki JT, Dossel O, Seemann G, Benchmarking electrophysiological models of human atrial myocytes, Front Physiol 3 (2012) 487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Colman MA, Aslanidi OV, Kharche S, Boyett MR, Garratt C, Hancox JC, et al. , Pro-arrhythmogenic effects of atrial fibrillation-induced electrical remodelling: insights from the three-dimensional virtual human atria, J Physiol 591 (2013) 4249–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Maleckar MM, Greenstein JL, Trayanova NA, Giles WR, Mathematical simulations of ligand-gated and cell-type specific effects on the action potential of human atrium, Prog Biophys Mol Biol 98 (2008) 161–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Voigt N, Heijman J, Wang Q, Chiang DY, Li N, Karck M, et al. , Cellular and molecular mechanisms of atrial arrhythmogenesis in patients with paroxysmal atrial fibrillation, Circulation 129 (2014) 145–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Sutanto H, van Sloun B, Schonleitner P, van Zandvoort MAMJ, Antoons G, Heijman J, The Subcellular Distribution of Ryanodine Receptors and L-Type Ca2+ Channels Modulates Ca2+-Transient Properties and Spontaneous Ca2+-Release Events in Atrial Cardiomyocytes, Front Physiol 9 (2018) 1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Heijman J, Kirchner D, Kunze F, Chretien EM, Michel-Reher MB, Voigt N, et al. , Muscarinic type-1 receptors contribute to IK,ACh in human atrial cardiomyocytes and are upregulated in patients with chronic atrial fibrillation, Int J Cardiol 255 (2018) 61–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Schmidt C, Wiedmann F, Zhou XB, Heijman J, Voigt N, Ratte A, et al. , Inverse remodelling of K2P3.1 K+ channel expression and action potential duration in left ventricular dysfunction and atrial fibrillation: implications for patient-specific antiarrhythmic drug therapy, Eur Heart J 38 (2017) 1764–74. [DOI] [PubMed] [Google Scholar]
- [23].Morotti S, McCulloch AD, Bers DM, Edwards AG, Grandi E, Atrial-selective targeting of arrhythmogenic phase-3 early afterdepolarizations in human myocytes, J Mol Cell Cardiol 96 (2016) 63–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Ellinwood N, Dobrev D, Morotti S, Grandi E, In Silico Assessment of Efficacy and Safety of IKur Inhibitors in Chronic Atrial Fibrillation: Role of Kinetics and State-Dependence of Drug Binding, Front Pharmacol 8 (2017) 799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Ellinwood N, Dobrev D, Morotti S, Grandi E, Revealing kinetics and state-dependent binding properties of IKur-targeting drugs that maximize atrial fibrillation selectivity, Chaos 27 (2017) 093918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Ni HB, Morotti S, Grandi E, A Heart for Diversity: Simulating Variability in Cardiac Arrhythmia Research, Front Physiol 9 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Muszkiewicz A, Britton OJ, Gemmell P, Passini E, Sanchez C, Zhou X, et al. , Variability in cardiac electrophysiology: Using experimentally-calibrated populations of models to move beyond the single virtual physiological human paradigm, Prog Biophys Mol Biol 120 (2016) 115–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Morotti S, Grandi E, Logistic regression analysis of populations of electrophysiological models to assess proarrythmic risk, MethodsX 4 (2017) 25–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Cherry EM, Hastings HM, Evans SJ, Dynamics of human atrial cell models: restitution, memory, and intracellular calcium dynamics in single cells, Prog Biophys Mol Biol 98 (2008) 24–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Sanchez C, Bueno-Orovio A, Wettwer E, Loose S, Simon J, Ravens U, et al. , Inter-subject variability in human atrial action potential in sinus rhythm versus chronic atrial fibrillation, PLoS One 9 (2014) e105897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Muszkiewicz A, Liu X, Bueno-Orovio A, Lawson BAJ, Burrage K, Casadei B, et al. , From ionic to cellular variability in human atrial myocytes: an integrative computational and experimental study, Am J Physiol Heart Circ Physiol (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Krummen DE, Bayer JD, Ho J, Ho G, Smetak MR, Clopton P, et al. , Mechanisms of human atrial fibrillation initiation: clinical and computational studies of repolarization restitution and activation latency, Circ Arrhythm Electrophysiol 5 (2012) 1149–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Aslanidi OV, Colman MA, Stott J, Dobrzynski H, Boyett MR, Holden AV, et al. , 3D virtual human atria: A computational platform for studying clinical atrial fibrillation, Prog Biophys Mol Biol 107 (2011) 156–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Krueger MW, Rhode KS, O’Neill MD, Rinaldi CA, Gill J, Razavi R, et al. , Patient-specific modeling of atrial fibrosis increases the accuracy of sinus rhythm simulations and may explain maintenance of atrial fibrillation, J Electrocardiol 47 (2014) 324–8. [DOI] [PubMed] [Google Scholar]
- [35].Matene E, Jacquemet V, Fully automated initiation of simulated episodes of atrial arrhythmias, Europace 14 Suppl 5 (2012) v17–v24. [DOI] [PubMed] [Google Scholar]
- [36].Zhao J, Butters TD, Zhang H, LeGrice IJ, Sands GB, Smaill BH, Image-based model of atrial anatomy and electrical activation: a computational platform for investigating atrial arrhythmia, IEEE Trans Med Imaging 32 (2013) 18–27. [DOI] [PubMed] [Google Scholar]
- [37].McDowell KS, Zahid S, Vadakkumpadan F, Blauer J, MacLeod RS, Trayanova NA, Virtual electrophysiological study of atrial fibrillation in fibrotic remodeling, PLoS One 10 (2015) e0117110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Zhao J, Hansen BJ, Wang Y, Csepe TA, Sul LV, Tang A, et al. , Three-dimensional Integrated Functional, Structural, and Computational Mapping to Define the Structural “Fingerprints” of Heart-Specific Atrial Fibrillation Drivers in Human Heart Ex Vivo, J Am Heart Assoc 6 (2017) e005922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Grandi E, Maleckar MM, Anti-arrhythmic strategies for atrial fibrillation: The role of computational modeling in discovery, development, and optimization, Pharmacol Ther 168 (2016) 126–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Aguilar M, Xiong F, Qi XY, Comtois P, Nattel S, Potassium Channel Blockade Enhances Atrial Fibrillation-Selective Antiarrhythmic Effects of Optimized State-Dependent Sodium Channel Blockade, Circulation 132 (2015) 2203–11. [DOI] [PubMed] [Google Scholar]
- [41].Lee W, Mann SA, Windley MJ, Imtiaz MS, Vandenberg JI, Hill AP, In silico assessment of kinetics and state dependent binding properties of drugs causing acquired LQTS, Prog Biophys Mol Biol 120 (2016) 89–99. [DOI] [PubMed] [Google Scholar]
- [42].Moreno JD, Zhu ZI, Yang PC, Bankston JR, Jeng MT, Kang C, et al. , A computational model to predict the effects of class I anti-arrhythmic drugs on ventricular rhythms, Sci Transl Med 3 (2011) 98ra83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Burashnikov A, Di Diego JM, Barajas-Martinez H, Hu D, Cordeiro JM, Moise NS, et al. , Ranolazine effectively suppresses atrial fibrillation in the setting of heart failure, Circ Heart Fail 7 (2014) 627–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Ni H, Whittaker DG, Wang W, Giles WR, Narayan SM, Zhang H, Synergistic Anti-arrhythmic Effects in Human Atria with Combined Use of Sodium Blockers and Acacetin, Front Physiol 8 (2017) 946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Kirchhoff JE, Diness JG, Sheykhzade M, Grunnet M, Jespersen T, Synergistic antiarrhythmic effect of combining inhibition of Ca2+-activated K+ (SK) channels and voltage-gated Na+ channels in an isolated heart model of atrial fibrillation, Heart Rhythm 12 (2015) 409–18. [DOI] [PubMed] [Google Scholar]
- [46].Morotti S, Ellinwood N, Ni H, Koivumaki JT, Maleckar MM, Heijman J, et al. , Effects of Modulation of Small-Conductance Calcium-Activated Potassium Current on Atrial Electrophysiology and Arrhythmogenesis: A Population-Based Computational Study, Biophys J 114 (2018) Abstract 473A. [Google Scholar]
- [47].Filgueiras-Rama D, Martins RP, Mironov S, Yamazaki M, Calvo CJ, Ennis SR, et al. , Chloroquine terminates stretch-induced atrial fibrillation more effectively than flecainide in the sheep heart, Circ Arrhythm Electrophysiol 5 (2012) 561–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Koivumaki JT, Seemann G, Maleckar MM, Tavi P, In silico screening of the key cellular remodeling targets in chronic atrial fibrillation, PLoS Comput Biol 10 (2014) e1003620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Pandit SV, Berenfeld O, Anumonwo JM, Zaritski RM, Kneller J, Nattel S, et al. , Ionic determinants of functional reentry in a 2-D model of human atrial cells during simulated chronic atrial fibrillation, Biophys J 88 (2005) 3806–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Ji Y, Varkevisser R, Opacic D, Bossu A, Kuiper M, Beekman JDM, et al. , The inward rectifier current inhibitor PA-6 terminates atrial fibrillation and does not cause ventricular arrhythmias in goat and dog models, Br J Pharmacol 174 (2017) 2576–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Sanchez C, Bueno-Orovio A, Pueyo E, Rodriguez B, Atrial Fibrillation Dynamics and Ionic Block Effects in Six Heterogeneous Human 3D Virtual Atria with Distinct Repolarization Dynamics, Front Bioeng Biotechnol 5 (2017) 29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Liberos A, Bueno-Orovio A, Rodrigo M, Ravens U, Hernandez-Romero I, Fernandez-Aviles F, et al. , Balance between sodium and calcium currents underlying chronic atrial fibrillation termination: An in silico intersubject variability study, Heart Rhythm 13 (2016) 2358–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Hakalahti A, Biancari F, Nielsen JC, Raatikainen MJ, Radiofrequency ablation vs. antiarrhythmic drug therapy as first line treatment of symptomatic atrial fibrillation: systematic review and meta-analysis, Europace 17 (2015) 370–8. [DOI] [PubMed] [Google Scholar]
- [54].Marrouche NF, Brachmann J, Andresen D, Siebels J, Boersma L, Jordaens L, et al. , Catheter Ablation for Atrial Fibrillation with Heart Failure, N Engl J Med 378 (2018) 417–27. [DOI] [PubMed] [Google Scholar]
- [55].Scherr D, Khairy P, Miyazaki S, Aurillac-Lavignolle V, Pascale P, Wilton SB, et al. , Five-year outcome of catheter ablation of persistent atrial fibrillation using termination of atrial fibrillation as a procedural endpoint, Circ Arrhythm Electrophysiol 8 (2015) 18–24. [DOI] [PubMed] [Google Scholar]
- [56].Verma A, Jiang CY, Betts TR, Chen J, Deisenhofer I, Mantovan R, et al. , Approaches to catheter ablation for persistent atrial fibrillation, N Engl J Med 372 (2015) 1812–22. [DOI] [PubMed] [Google Scholar]
- [57].Ruchat P, Dang L, Virag N, Schlaepfer J, von Segesser LK, Kappenberger L, A biophysical model of atrial fibrillation to define the appropriate ablation pattern in modified maze, Eur J Cardiothorac Surg 31 (2007) 65–9. [DOI] [PubMed] [Google Scholar]
- [58].Bayer JD, Roney CH, Pashaei A, Jais P, Vigmond EJ, Novel Radiofrequency Ablation Strategies for Terminating Atrial Fibrillation in the Left Atrium: A Simulation Study, Front Physiol 7 (2016) 108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Hwang M, Kwon SS, Wi J, Park M, Lee HS, Park JS, et al. , Virtual ablation for atrial fibrillation in personalized in-silico three-dimensional left atrial modeling: comparison with clinical catheter ablation, Prog Biophys Mol Biol 116 (2014) 40–7. [DOI] [PubMed] [Google Scholar]
- [60].Zahid S, Whyte KN, Schwarz EL, Blake RC 3rd, Boyle PM, Chrispin J, et al. , Feasibility of using patient-specific models and the “minimum cut” algorithm to predict optimal ablation targets for left atrial flutter, Heart Rhythm 13 (2016) 1687–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Shim J, Hwang M, Song JS, Lim B, Kim TH, Joung B, et al. , Virtual In-Silico Modeling Guided Catheter Ablation Predicts Effective Linear Ablation Lesion Set for Longstanding Persistent Atrial Fibrillation: Multicenter Prospective Randomized Study, Front Physiol 8 (2017) 792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Deng D, Murphy MJ, Hakim JB, Franceschi WH, Zahid S, Pashakhanloo F, et al. , Sensitivity of reentrant driver localization to electrophysiological parameter variability in image-based computational models of persistent atrial fibrillation sustained by a fibrotic substrate, Chaos 27 (2017) 093932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Boyle PM, Hakim JB, Zahid S, Franceschi WH, Murphy MJ, Vigmond EJ, et al. , Comparing Reentrant Drivers Predicted by Image-Based Computational Modeling and Mapped by Electrocardiographic Imaging in Persistent Atrial Fibrillation, Front Physiol 9 (2018) 414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Sonnenberg FA, Beck JR, Markov models in medical decision making: a practical guide, Med Decis Making 13 (1993) 322–38. [DOI] [PubMed] [Google Scholar]
- [65].Limone BL, Baker WL, Kluger J, Coleman CI, Novel anticoagulants for stroke prevention in atrial fibrillation: a systematic review of cost-effectiveness models, PLoS One 8 (2013) e62183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Baykaner T, Duff S, Hasegawa JT, Mafilios MS, Turakhia MP, Cost effectiveness of focal impulse and rotor modulation guided ablation added to pulmonary vein isolation for atrial fibrillation, J Cardiovasc Electrophysiol 29 (2018) 526–36. [DOI] [PubMed] [Google Scholar]
- [67].Aronsson M, Svennberg E, Rosenqvist M, Engdahl J, Al-Khalili F, Friberg L, et al. , Designing an optimal screening program for unknown atrial fibrillation: a cost-effectiveness analysis, Europace 19 (2017) 1650–6. [DOI] [PubMed] [Google Scholar]
- [68].Najafzadeh M, Schneeweiss S, Choudhry NK, Wang SV, Gagne JJ, Simulation for Predicting Effectiveness and Safety of New Cardiovascular Drugs in Routine Care Populations, Clin Pharmacol Ther (2018). [DOI] [PubMed] [Google Scholar]
- [69].Chang ET, Lin YT, Galla T, Clayton RH, Eatock J, A Stochastic Individual-Based Model of the Progression of Atrial Fibrillation in Individuals and Populations, PLoS One 11 (2016) e0152349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Beam AL, Kohane IS, Big Data and Machine Learning in Health Care, JAMA 319 (2018) 1317–8. [DOI] [PubMed] [Google Scholar]