Abstract
Background
Heel lance has been the conventional method of blood sampling in neonates for screening tests. Neonates undergoing heel lance experience pain which cannot be completely alleviated.
Objectives
To determine whether venepuncture or heel lance is less painful and more effective for blood sampling in term neonates.
Search methods
Randomized or quasi‐randomised controlled trials comparing pain response to venepuncture versus heel lance were identified by searching the Cochrane Central Regsiter of Controlled Trials (CENTRAL, The Cochrane Library), MEDLINE, EMBASE, CINAHL, and clinical trials registries in May 2011.
Selection criteria
Trials comparing pain response to venepuncture versus heel lance with or with out the use of a sweet tasting solution as a co‐intervention in term neonates.
Data collection and analysis
Outcomes included pain response to venepuncture versus heel lance with or without the use of a sweet tasting solution using validated pain measures, the need of repeat sampling and cry characteristics. Analyses included typical relative risk (RR), risk difference (RD), number needed to treat (NNT), weighted mean difference (WMD) and standardized mean difference (SMD) with their 95% confidence intervals (CI). Between study heterogeneity was reported including the I squared (I2) test.
Main results
Six studies (n = 478) of variable quality were included. A composite outcome of Infant Pain Scale (NIPS), Neonatal Facial Action Coding System (NFCS) and/or Premature Infant Pain Profile (PIPP) score was reported in 288 infants, who did not receive a sweet tasting solution. Meta‐analysis showed a significant reduction in the venepuncture versus the heel lance group (SMD ‐0.76, 95% CI ‐1.00 to ‐0.52; I2 = 0%). When a sweet tasting solution was provided the SMD remained significant favouring the venepuncture group (SMD ‐ 0.38, 95% CI ‐0.69 to ‐0.07). The typical RD for requiring more than one skin puncture for venepuncture versus heel lance (reported in 4 studies; n = 254) was ‐0.34 (95% CI ‐0.43 to ‐0.25; I2 = 97%). The NNT to avoid one repeat skin puncture was 3 (95% CI 2 to 4). Cry characteristics favoured the venepuncture group but the differences were reduced by the provision of sweet tasting solutions prior to either procedure.
Authors' conclusions
Venepuncture, when performed by a skilled phlebotomist, appears to be the method of choice for blood sampling in term neonates. The use of a sweet tasting solution further reduces the pain.
Further well designed randomised controlled trials should be conducted in settings where several individuals perform the procedures.
Plain language summary
Venepuncture versus heel lance for blood sampling in term neonates
In most countries, a blood sample from newborn babies is needed for screening tests. A heel lance is the standard way of taking blood, but it is a painful procedure with no optimal method of pain relief known. This review of trials found evidence that venepuncture, when done by a trained practitioner, caused less pain than heel lance. The use of a sweet tasting solution given to the baby prior to the event reduced pain further. The evidence included outcome measures using pain scales, how long the baby cried and how the mother rated their baby's pain.
Background
Description of the condition
Every year millions of neonates require diagnostic blood sampling. Neonates undergoing these procedures cry (Owens 1984; Brown 1987) and exhibit facial expression and body movements (Izard 1979; Grunau 1987; Johnston 1986) that are indicative of pain. Until recently, it was believed that infants and young children could not appreciate pain due to the immaturity of the central nervous system. However, it is now well established that the anatomical, physiological and neurochemical structures which convey pain are well developed in neonates (Fitzgerald 1989, CPS 2000). Recent research suggests that babies' early pain experience may alter their pain response in later infancy (Taddio 1995; Taddio 1997).
Heel lance (HL) has been the conventional method of blood sampling in neonates for screening tests (phenylketonuria and hypothyroidism) or measurements of serum bilirubin or glucose. Sick neonates (preterm and term infants) admitted to neonatal intensive care units (NICUs) undergo this procedure repeatedly as part of routine care. Barker 1995 reviewed the nature and frequency of invasive procedures in a NICU and showed that HL was the most common procedure being performed in a NICU. Apart from discomfort to the infant associated with HL, there are concerns regarding the possibility of puncturing the calcaneus and causing osteochondritis, ecchymosis or haemolyzed samples and the possibility of accidental injuries to personnel (Moxley 1989, Meehan 1998).
Description of the intervention
Various pharmacological and non‐pharmacological interventions have been investigated for management of pain associated with HL. Automated piercing devices (Harpin 1983, Paes 1993), behavioural interventions such as pacifiers (Field 1984) and rocking (Campos 1994), sucrose (Stevens 2010), glucose (Skogdal 1997), non‐sucrose sweet tasting solution (Ramenghi 1996), anaesthetic cream such as lignocaine (Rushforth 1995) and EMLA (Larsson 1995, McIntosh 1994), and paracetamol (Shah 1998) have been studied. The use of a mechanical lancet (Autolet) caused less physiological instability than manual HL (Harpin 1983, Paes 1993). The total volume of blood collected with automated device was significantly larger than with lancet device. The time required for blood sampling was significantly reduced and there was reduced haemolysis in the automated device group. Comforting measures were associated with less crying (Field 1984, Campos 1994) and use of sucrose (2 ml of 12% solution) two minutes prior to the procedure reduced composite pain measures (Stevens 2010). Anaesthetic cream (Rushforth 1995, Larsson 1995, McIntosh 1994) and paracetamol (Shah 1998) have been ineffective in decreasing pain scores with HL. Topical amethocaine gel does not have a clinically important effect on pain from HL blood sampling (Jain 2001). Despite various studies to date, there are no effective and practical methods to alleviate pain from HL (Ohlsson 2000).
How the intervention might work
Venepuncture (VP) is a common procedure performed in older infants and children (McKay 1966). The advantages of VP include a reduced risk of a haemolyzed or clotted sample, increased sample volume and possibly less pain (McKay 1966). The disadvantage of VP is the need for a skilled phlebotomist to perform the procedure (the phlebotomist will have to spend time training and the amount of time required depends on the skill of the individual). In contrast, the benefit of HL is the perceived ease to perform the task even by paramedical personnel.
Why it is important to do this review
The aim of this review is to compare pain response from VP versus HL, the success rate of obtaining an adequate blood sample and sample collection times and possible adverse effects.
Objectives
The primary objective was to compare pain response to VP versus HL in term neonates. The secondary objectives were to compare the need of repeated sampling, adverse effects, if any, to these interventions, sample collection times and the parent's perception of their infant's pain.
Methods
Criteria for considering studies for this review
Types of studies
Randomized and quasi‐randomised controlled trials in which pain response from VP was compared to HL.
Types of participants
Healthy neonates of > 37 weeks post menstrual age (PMA) subjected to blood sampling.
Types of interventions
Venepuncture or heel lance.
Types of outcome measures
For this update of the review in 2011 we changed the primary outcome to be a combination of different validated behavioural pain measures (neonatal Infant Pain Score (NIPS) (Lawrence 1993), Neonatal Facial Coding System (NFCS) (Grunau 1987; Craig 1994) and Premature Infant Pain Profile (PIPP) (Stevens 1996). For this update we included populations that received a sweet tasting solution (dextrose, glucose, sucrose) in both the VP and the HL group.
Comparison 1: Infants who did not receive sweet tasting solutions:
Primary outcome
Pain response using a combination of one or more of NIPS, NFCS and/or PIPP.
Secondary outcomes
Duration of first cry (seconds).
Total duration of cry (seconds).
Number of neonates who cried during the procedure.
First crying time (seconds)/total procedure time (seconds) (%).
Duration of cry (seconds) in the first three minutes after skin puncture.
Sampling time (seconds).
Need for more than one skin puncture.
Need for more than two skin punctures.
Bruising/hematoma at local site.
Maternal anxiety score prior to the procedure.
Infant's pain score as rated by the mother.
Comparison 2: Infants who received sweet tasting solutions:
Primary outcome
Pain response using a combination of one or more of NIPS, NFCS and or PIPP.
Secondary Outcomes
Duration of first cry (seconds).
Total duration of cry (seconds).
Number of neonates who cried during the procedure.
First crying time (seconds)/total procedure time (seconds) (%).
Duration of cry (seconds) in the first three minutes after skin puncture.
Sampling time (seconds).
Need for more than one skin puncture.
Need for more than two skin punctures.
Bruising/hematoma at local site.
Maternal anxiety score prior to the procedure.
Infant's pain score as rated by the mother.
Search methods for identification of studies
See: Cochrane Neonatal Collaborative Review Group search strategy. We searched MEDLINE (1966 to May 12, 2011) using the terms: venepuncture, heel lance (prick), pain, newborn ‐ infant, blood sampling. We searched other databases including: the Cochrane Central Register of Controlled Trials (CENTRAL, The Cochrane Library, Issue 5, 2011), EMBASE (1980 to May 12, 2011), CINAHL (1982 to May 12, 2011) and reference lists of identified trials. In addition electronic abstracts from the Pediatric Academic Societies Annual Meeting were searched from 2000 to 2011. Clinical trials registries were searched for ongoing or recently completed trials (clinicaltrials.gov and controlled‐trials.com).
Data collection and analysis
Standard methods of the Cochrane Neonatal Collaborative Review Group and for this update in addition for certain sections the standard methods of the Cochrane Pregnancy and Childbirth Review Group were used as guidance for the methods sections reported below including all the headings from "Selection of studies" to "Sensitivity analysis". Retrieved articles were assessed and data were abstracted independently by the two review authors.
Selection of studies
Two review authors independently assessed for inclusion all the potential studies we identified as a result of the search strategy. We resolved any disagreement through discussion or, if required, we would have consulted a third person. Dr. R. Soll was consulted for the inclusion of the study by Saththasivam 2009.
Data extraction and management
We designed a form to extract data. For eligible studies, the two review authors extracted the data using the agreed form. We resolved discrepancies through discussion or, if required, we would have consulted a third person. We entered data into Review Manager software 5.1 and checked for accuracy. When information regarding any of the above was unclear, we contacted authors of the original reports to provide further details.
Assessment of risk of bias in included studies
The two review authors independently assessed the risk of bias for each study using the criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We resolved any disagreement by discussion or would have involved a third assessor if required.
For the original review the quality of included trials was evaluated independently by the two reviewers, using the following criteria: Blinding of randomisation? Blinding of intervention? Blinding of outcome measure assessment? Completeness of follow up?
There were three potential answers to these questions ‐ yes, can't tell, no
For the update in 2011, the following issues were evaluated and entered into the Risk of Bias table:
Selection bias (random sequence generation and allocation concealment): For each included study, we categorized the risk of selection bias as:
‐ Random sequence generation:
Low risk ‐ adequate (any truly random process e.g. random number table; computer random number generator);
High risk ‐ inadequate (any non random process e.g. odd or even date of birth; hospital or clinic record number);
Unclear risk ‐ no or unclear information provided.
‐ Allocation concealment: For each included study, we categorized the risk of bias regarding allocation concealment as:
Low risk ‐ adequate (e.g. telephone or central randomisation; consecutively numbered, sealed, opaque envelopes);
High risk ‐ inadequate (open random allocation; unsealed or non‐opaque envelopes, alternation; date of birth);
Unclear risk ‐ no or unclear information provided.
Performance bias: For each included study, we categorized the methods used to blind study personnel from knowledge of which intervention a participant received. As our study population consisted of neonates they would all be blinded to the study intervention:
Low risk ‐ adequate for personnel (a placebo that could not be distinguished from the active drug was used in the control group);
High risk ‐ inadequate personnel aware of group assignment;
Unclear risk ‐ no or unclear information provided.
Detection bias: For each included study, we categorized the methods used to blind outcome assessors from knowledge of which intervention a participant received. (As our study population consisted of neonates they would all be blinded to the study intervention). Blinding was assessed separately for different outcomes or classes of outcomes. We categorized the methods used with regards to detection bias as:
Low risk ‐ adequate follow‐up was performed with assessors blinded to group assignment;
High risk ‐ inadequate assessors at follow‐up were aware of group assignment;
Unclear risk ‐ no or unclear information provided.
Attrition bias: For each included study and for each outcome, we described the completeness of data including attrition and exclusions from the analysis. We noted whether attrition and exclusions were reported, the numbers included in the analysis at each stage (compared with the total randomised participants), reasons for attrition or exclusion where reported, and whether missing data were balanced across groups or were related to outcomes. Where sufficient information was reported or supplied by the trial authors, we re‐included missing data in the analyses. We categorized the methods with respect to the risk attrition bias as:
Low risk ‐ adequate (< 10% missing data);
High risk ‐ inadequate (> 10% missing data);
Unclear risk ‐ no or unclear information provided.
Reporting bias: For each included study, we described how we investigated the risk of selective outcome reporting bias and what we found. We assessed the methods as:
Low risk ‐ adequate (where it is clear that all of the study's pre‐specified outcomes and all expected outcomes of interest to the review have been reported);
High risk ‐ inadequate (where not all the study's pre‐specified outcomes have been reported; one or more reported primary outcomes were not pre‐specified; outcomes of interest are reported incompletely and so cannot be used; study fails to include results of a key outcome that would have been expected to have been reported);
Unclear risk ‐ no or unclear information provided (the study protocol was not available).
Other bias: For each included study, we described any important concerns we had about other possible sources of bias (for example, whether there was a potential source of bias related to the specific study design or whether the trial was stopped early due to some data‐dependent process). We assessed whether each study was free of other problems that could put it at risk of bias as:
Low risk ‐ no concerns of other bias raised;
High risk ‐ concerns raised about multiple looks at the data with the results made known to the investigators, difference in number of patients enrolled in abstract and final publications of the paper;
Unclear ‐ concerns raised about potential sources of bias that could not be verified by contacting the authors.
If needed, we planned to explore the impact of the level of bias through undertaking sensitivity analyses.
Measures of treatment effect
Dichotomous data: For dichotomous data the statistical methods included relative risk (RR), risk difference (RD), number needed to treat to benefit (NNT) and to harm (NNH). Continuous data: For continuous outcomes we used weighted mean difference (WMD) along with 95% confidence intervals (CI) were used. We used the standardised mean difference (STD) to combine trials that measured the same outcome, but used different pain assessment tools.
If present, statistically significant between study heterogeneity including the I squared (I2) test was reported. We categorized the level of heterogeneity as suggested by Higgins (Higgins 2003 ) as adjectives of low, moderate, and high to I2 values of 25%, 50%, and 75%. All data were analysed using RevMan 5.1. We converted median and ranges to means and standard deviations using the method suggested by Hozo et al (Hozo 2005). For some studies we estimated the means and standard deviations, and medians and ranges from graphs presented by the authors.
Unit of analysis issues
Cluster‐randomised trials: As expected we did not encounter any cluster randomised trials. Cross‐over Trials: If identified we would have used information in the Handbook section 16.4, that describes methods for risk of bias assessment and analysis (Higgins 2011).
Dealing with missing data
For included studies, we noted levels of attrition. We would have explored the impact of including studies with high levels of missing data in the overall assessment of treatment effect by using sensitivity analysis. For all outcomes, we carried out analyses, as far as possible, on an intention‐to‐treat basis, i.e. we attempted to include all participants randomised to each group in the analyses, and all participants were analysed in the group to which they were allocated, regardless of whether or not they received the allocated intervention. The denominator for each outcome in each trial was the number randomised minus any participants whose outcomes were known to be missing.
Assessment of heterogeneity
We assessed statistical heterogeneity in each meta‐analysis using the I‐squared (I²) and Chi² statistics. Heterogeneity tests including the I‐ squared test (I2) were performed to assess the appropriateness of pooling the data. The degree of heterogeneity was roughly categorized according to Higgins and co‐workers (Higgins 2003) as 25% = low, 50% = moderate, and 75% = high.
Assessment of reporting biases
If there were 10 or more studies in the meta‐analysis we would have investigated reporting biases (such as publication bias) using funnel plots. We would assess funnel plot asymmetry visually, and use formal tests for funnel plot asymmetry. For continuous outcomes we would use the test proposed by Egger 1997 (Egger 1997) , and for dichotomous outcomes we would use the test proposed by Harboard 2006 (Harboard 2006 ). If asymmetry was detected in any of these tests or was suggested by a visual assessment, we would perform exploratory analyses to investigate it.
Data synthesis
We carried out statistical analyses using the Review Manager 5.1 software. We use fixed‐effect meta‐analysis for combining data where it was reasonable to assume that studies were estimating the same underlying treatment effect: i.e. where trials were examining the same intervention, and the trials’ populations and methods were judged sufficiently similar.
Subgroup analysis and investigation of heterogeneity
If we identified substantial heterogeneity, we would investigate it using subgroup analyses and sensitivity analyses. As the maximum number of included studies for any one outcome was five studies or less we did not perform any subgroup analyses.
Sensitivity analysis
As the maximum number of included studies for any one outcome was five studies or less we did not perform any subgroup analyses.
Results
Description of studies
For details see the table 'Characteristics of Included Studies'.
For each trial, information was sought from the published report regarding method of randomisation, blinding, and reporting of all outcomes for all infants enrolled in the trial. As noted in the Risk of Bias tables the quality varied with many studies not providing enough information to judge the level of bias and for some item there was clearly a high risk of bias. Unpublished data were included from the studies by Shah (Shah 1997), Eriksson (Eriksson 1999), Kvist (Kvist 2002) and by Saththasivam (Saththasivam 2009). Unpublished data on the six patients that were excluded (because more than two skin punctures were required) from the analyses by Kvist et al (Kvist 2002) could not be obtained. Data regarding infants enrolled in the VP and HL groups with or without the administration of glucose were available from Eriksson (Eriksson 1999). From the study by Saththasiwam (Saththasivam 2009) the authors provided us with data analysed as means and standard deviations as the published study reported the outcomes as medians and ranges.
One additional trial was identified for this review (Saththasivam 2009). With the addition of this study, six trials enrolling 523 neonates were included. These studies were performed in four countries [UK, Japan, Sweden (n = 3) and Malaysia]. Of the 523 neonates enrolled, information on 478 neonates relevant to this systematic review were included.
All studies except the one by Larsson 1998 strictly fulfilled our inclusion criteria of PMA > 37 weeks. In the Larsson 1998 study, the PMAs ranged from 36 weeks to 43 weeks; the median PMA was 40 weeks. The decision was made to include this study in the review as most infants were term. Two studies compared the effects of sweet tasting solution (Eriksson 1999; and Ogawa 2005) on VP and HL in sub‐samples of the study populations. Saththasivam 2009 gave all infants sucrose prior to HL or VP. We included the results for these three studies that used a sweet tasting solution prior to the intervention in separate analyses.
Pain assessments were made in these trials using various validated tools. Shah 1997 and Kvist 2002 used the NIPS. The NIPS includes five behavioral groupings (facial expression, crying, movement of arms and legs, and state of arousal) and one physiological indicator (breathing pattern) along with the descriptors for the scores within each grouping (Lawrence 1993). The total score ranges from 0 (relaxed and calm) to seven (crying dissatisfied infant). The scale has been tested for validity and reliability in preterm and term infants subjected to capillary, venous or arterial punctures (Lawrence 1993).
Larsson 1998 used the Neonatal Facial Coding System (NFCS) (Grunau 1987). The presence or absence of six facial actions [brow bulge, eyes squeezed shut, deepening of the naso‐labial furrow, open lips, a taut cupped tongue, and stretching of the mouth (vertically and horizontally)] were recorded and presented as percent positive scores with a total range of 0 to 600%. The scale has been validated (Craig 1994). The results of the NFCS scores for the study by Larsson 1998 are not included in the meta‐analysis of pain response as they were presented as median scores in a table and the centiles in graphic form. Ogawa 2005 used the NFCS to assess pain. These authors assessed the 10 facial actions (brow bulge, eye squeeze, nasolabial furrow, open lips, lip purse, vertical and horizontal mouth stretch, taut tongue, tongue protrusion and chin quiver). The data from Ogawa et al (Ogawa 2005) could not be incorporated in the meta‐analysis as results of the NFCS score were reported as median and interquartile ranges. The data from the study by Saththasivam 2009 used NFCS score and we obtained data converting medians and range to means and standard deviations.
Eriksson 1999 used the PIPP score to assess pain. The PIPP score assigns points for changes in three facial expressions (brow bulge, eye squeeze and naso‐labial fold), heart rate, oxygen saturation, PMA and behavioral state with a higher score indicating more pain. A score of 0 to 6 points indicates minimal or no pain, while a score of 12 or more indicates moderate to severe pain.
The blood sampling techniques (VP or HL) were performed by one investigator in the trials of Shah 1997, Larsson 1998 and Eriksson 1999. For the trial by Kvist 2002 two investigators performed the procedure while for the trial of Ogawa 2005 seven experienced nurses were trained to perform VP or HL. In the study by Saththasivam 2009 HL was performed by a single experienced staff nurse while VP was performed by two senior paediatric registrars.
Unpublished data on the six patients that were excluded (because more than two skin punctures were required) in the trial by Kvist 2002 could not be obtained. The analyses presented from this trial do not represent an "intention to treat analysis". The results were presented as median test and odds ratios with 95% CIs. The authors provided us with unpublished data for means and standard deviations for NIPS scores for the small calibre VP needle group and the HL group.
Included Studies:
Shah 1997 was a single centre study performed in Bristol, UK.
Objective: To compare the pain response to different methods of blood sampling (VP versus HL) in full term infants and the incidence of adverse effects.
Population: Healthy neonates (> 37 weeks GA) having blood taken for measurement of bilirubin or glucose.
Intervention: HL was performed using a commercially available lancet (Becton Dickinson VACUTAINER Systems Eur. 36129). VP was performed using a 21 gauge needle from a vein on the dorsum of the hand.
Outcomes assessed: Pain assessments were made by nurses using Neonatal Infant Pain Scale (NIPS). Infant's pain score as rated by the mother was assessed using a three point scale where 0 = no pain at all, 1 = a little pain and 2 = a lot of pain. Maternal anxiety score prior to the procedure was assessed using a three point scale where 0 = not worried at all, 1 = a little worried and 2 = very worried. The number, reasons for multiple attempts, and occurrence of adverse effects were noted.
Larsson 1998 was a single centre study performed in Stockholm, Sweden.
Objective: To compare the pain response to VP compared to HL either with a standardized lancet or a large lancet.
Population: Healthy neonates (36 ‐ 43 weeks GA) undergoing the phenylketonuria (PKU) screening test.
Intervention: VP was performed using a Microlance needle measuring 0.9 x 40 mm (Becton‐ Dickinson, Madrid, Spain). Two devices were used for heel lancing. In the small lancet group (SL) a CCS Minilancet (Clean Chemical, Borlänge, Sweden) was used. In the large lancet group (LL) a Microlance (Becton‐Dickinson, Meylan Cedex, France) was used.
Outcomes assessed: Pain assessments were made using NFCS (Grunau 1987) and cry [latency (cry within 60 seconds of the skin puncture) and duration of the first cry as well as the total cry duration for the procedure]. The need for more than one skin puncture and sampling times were recorded.
Eriksson 1999 was a single centre study performed in Örebro, Sweden.
Objective: To identify the least painful method to obtain blood sample for PKU test by VP or HL with or without 30% glucose.
Population: Healthy neonates (> 37 weeks GA) undergoing the metabolic screening blood test.
Intervention: HL was performed using a microtainer safety lancet (Microtainer Brand Safety Flow Lancet, Becton Dickinson, Meylan Cedex, France). VP was performed using a 21‐gauge needle (Terumo, Leuven, Belgium). Half the total population received glucose prior to the blood sampling.
Outcomes assessed: Pain assessments were made using the Premature Infant Pain Profile (PIPP) score, duration of crying within the first 3 minutes after the skin puncture and by changes in heart rate. The need for more than one skin puncture and sampling times were recorded.
Kvist 2002 was a single centre study performed in Helsingborg, Sweden.
Objective: To identify the least painful method of blood sampling for the PKU test.
Population: Healthy neonates (> 37 weeks GA) with normal weight (i.e. > 2,500 g) and breast fed.
Intervention: VP was performed either with a small calibre needle (0.6 x 25 mm) or a large calibre needle (0.9 x 40 mm) and HL was performed using microtainer lancet with a depth of 2 mm).
Outcomes assessed: Pain assessments were made using the NIPS score and the need for more than one skin puncture was recorded.
Ogawa 2005 was a single centre study performed in Osaka, Japan.
Objective: To identify the least painful and most effective method for blood sampling by VP or HL with or without sucrose.
Population: Healthy neonates (> 37 weeks GA) undergoing screening blood test for inborn errors of metabolism.
Intervention: HL was performed using a standard lancet with a sharp triangular edge that was 2.5 mm long and 1 mm wide (Feather Safety Razor Co, Ltd, Osaka, Japan) while VP was performed using a 23 gauge needle.
Outcomes assessed: Pain assessments were performed using NFCS and duration of first cry (the percentage of the first crying time relative to the total procedure time and the number of crying babies). Adverse events of the procedure itself and those occurring after completion including bruising and hematoma were recorded.
Saththasivam 2009 (new inclusion) was a single centre study performed in Kubang Kerian, Malaysia.
Objective: To determine whether there was a difference in the pain indicators and effectiveness between venipuncture and heel lance for blood glucose monitoring in term neonates.
Population: Healthy full‐term neonates undergoing blood glucose monitoring.
Intervention: HL was performed by a single experienced staff nurse using an automatic disposable lancing device, Unistik 2 Neonatal (Omega Health Care, London, UK) while VP was performed by two senior paediatric registrars using a 23 gauge needle.
Outcomes assessed: Pain assessments were performed using NFCS, duration of first cry, total duration of cry, total duration of procedure, number of punctures 1 or > 1. No adverse events occurred during or after the blood‐taking.
Risk of bias in included studies
For details see the Risk of Bias Table. Adequate random sequence generation was only reported in one trial (Shah 1997). Allocation concealment was unclear in five trials (Kvist 2002; Larsson 1998; Ogawa 2005; Shah 1997 ) and there was high risk for bias in one trial in which the neonates were allocated to either the VP or the HL group depending on the availability of th assigned staff (Saththasivam 2009). Intention to treat analysis was not reported in the trials by Larsson (Larsson 1998) and Kvist (Kvist 2002).
Shah (Shah 1997) ‐ Neither blinding of the intervention (not possible) nor of outcome assessments was ensured. Outcomes were given for all neonates enrolled in the study.
Larsson (Larsson 1998) ‐ Blinding of the intervention was not possible, while blinding of outcome assessment was ensured. Video and audio tapes were analysed by two observers unaware of the group to which the infant had been allocated. All infants in the study were accounted for. Three infants in the small lancet group were excluded as one infant was of 35 weeks gestation and two infants had screamed before the puncture was performed. Outcomes were reported for 117 out of 120 infants.
Eriksson (Eriksson 1999) ‐ Neither blinding of the intervention (not possible) nor of outcome assessments was ensured. Outcomes were given for all neonates enrolled in the study (Figure 1).
1.
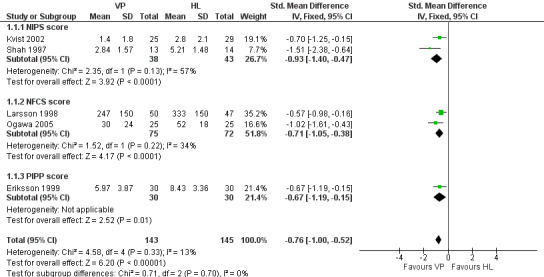
Forest plot of comparison: 1 Pain response to VP VS HL in infants who did not receive a sweet tasting solution, outcome: 1.1 Behavioural pain scores for VP VS HL.
Kvist (Kvist 2002) ‐ Neither blinding of the intervention (not possible) nor of outcome assessments was ensured. Ten of the 30 infants randomised to VP with large calibre VP needle required more than two attempts at VP and had their blood sampling carried out by HL. NIPS scores were not provided for this group. The authors compared the NIPS scores for 25 infants in the small calibre VP needle group (five infants were excluded because of unsuccessful sampling) and 29 infants in the HL group (one was excluded because of several punctures). Thus complete follow‐up was not available from all patients enrolled.
Ogawa (Ogawa 2005) ‐ Randomization was performed using sealed envelopes. Blinding of the intervention was not possible while blinding of outcome assessment was ensured. Audio‐video tapes were analysed by a single investigator who was unaware of the sampling method. All infants in the study were accounted for.
Saththasivam (Saththasivam 2009) Randomization was based on the availability of staff who could perform VP or HL. Blinding of the intervention was not possible while blinding of outcome assessment was ensured. Audio‐video tapes were analysed by two investigators who were unaware of the sampling method. All infants in the study were accounted for. Sixty six infants were randomised but three in each group were excluded as they cried just before the skin was punctured. NFCS scores were reported for all 60 infants randomised as was the total duration of the procedure and the number of skin punctures. Duration of the first cry and total duration of cry were reported for 15 infants in the VP group and 14 in the HL group.
Effects of interventions
VENEPUNCTURE VERSUS HEEL LANCE IN INFANTS WHO DID NOT RECEIVE SWEET TASTING SOLUTIONS PRIOR TO BLOOD SAMPLING (Comparison 01):
Primary outcomes:
Pain response using a combination of one or more of NIPS, NFCS and or PIPP (Outcome 1.1)
Two studies (Kvist 2002; Shah 19970 enrolling 81 infants reported on the NIPS score. Two studies (Larsson 1998; Ogawa 2005) enrolling 147 infants reported on the NFCS score and one study (Eriksson 1999) enrolling 60 infants reported on the PIPP score. Combining all studies (n = 288 infants) the SMD for pain scores was significantly reduced in the VP group versus the HL group (‐0.76, 95% CI ‐1.00 to ‐0.52). There was no statistically significant heterogeneity for this outcome, (p = 0.70, I2 = 0%). Figure 1
Secondary Outcomes:
Duration of first cry (seconds) (Outcome 1.2)
Duration of first cry was reported in one study (Ogawa 2005) enrolling 50 neonates. There was a significant reduction in the duration of the first cry in infants in the VP versus the HL group (mean difference ‐112 seconds, 95% CI ‐164 to ‐60). Test for heterogeneity not applicable.
Total duration of cry (Outcome 1.3)
One study (Larsson 1998) reported on this outcome in 100 infants. There was a significant reduction in the total duration of cry in the VP group compared to the HL group [mean difference ‐188 seconds, (95% CI ‐228 to ‐148)]. Test for heterogeneity not applicable.
Number of neonates who cried during the procedure (Outcome 1:4)
Three studies (Eriksson 1999; Larsson 1998; Ogawa 2005) enrolling 207 infants reported on this outcome. There was a significant reduction in VP group compared to the HL group (typical RR 0.59, 95% CI 0.49 to 0.73; typical RD ‐0.35, 95% CI ‐0.46 to 0.23; NNT 3, 95% CI 2 to 4). There was no significant heterogeneity for this outcome (p = 0.85, I2 = 0%) for RR and for RD (p = 0.54, I2 = 0%).
First crying time (seconds)/total procedure time (seconds) (%) (Outcome 1.5)
One study (Ogawa 2005) enrolling 50 infants reported on this outcome. There was a significant reduction in the VP versus the HL group (mean difference ‐65%, 95% CI ‐110 to ‐20).
Test for heterogeneity not applicable.
Duration of cry (seconds) in the first three minutes after skin puncture (Outcome 1.6)
One study (Eriksson 1999) enrolling 60 infants reported on this outcome. The outcome was significantly reduce in the VP versus the HL group (mean difference ‐106 seconds, 95% CI ‐129 to ‐84). Test for heterogeneity not applicable.
Sampling time (seconds) (Outcome 1.7)
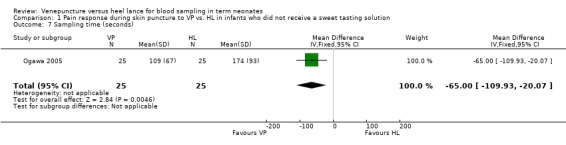
One study (Ogawa 2005) reported on this outcome. There was a significant reduction in the sampling time in the VP versus the HL group [mean difference ‐65 seconds, 95% CI ‐110 to ‐20). Test for heterogeneity not applicable.
Need for more than one skin puncture (Outcome 1.8)
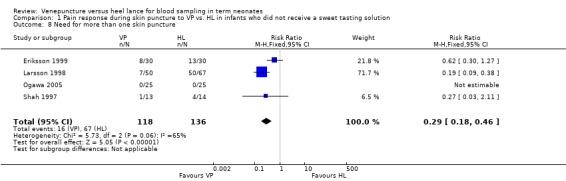
This outcome was reported in four studies (Eriksson 1999; Larsson 1998; Ogawa 2005; Shah 1997) enrolling 254 infants. There was a significant reduction in the typical RR and RD favouring the VP group (typical RR 0.29, 95% CI 0.18 to 0.46; typical RD ‐0.34, 95% CI ‐0.43 to ‐0.25; NNT 3, 95% CI 2 to 4). There was significant heterogeneity for this outcome (for RR p= 0.06, I2 = 65%; for RD p , 0.00001, I2 = 97%, high).
Need for more than two skin punctures (Outcome 1.9)
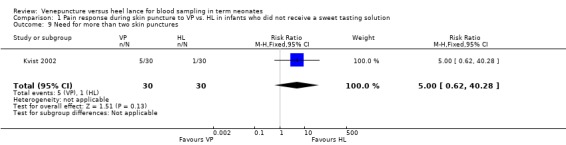
This outcome was reported in one study (Kvist 2002) enrolling 60 infants. There was no significant difference in the RR between the VP and the HL groups (RR 5.00, 95% CI 0.62 to 40.28; RD 0.13, 95% CI ‐0.01 to 0.28).
Bruising/hematoma at local site (Outcome 1.10)
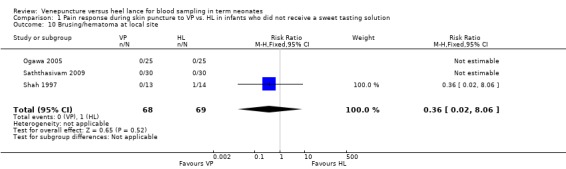
This outcome was reported in three studies (Shah 1997; Ogawa 2005; Saththasivam 2009) in 137 infants. There was no significant difference between the VP vs the HL groups (typical RR 0.36, 95% CI 0.02 to 8.06; typical RD ‐0.01, 95% CI ‐0.07 to 0.04). Test for heterogeneity not applicable for RR. Test for heterogeneity for RD showed p = 0.70, I2 = 0%.
Maternal anxiety score prior to the procedure (Outcome 1.11)
One study (Shah 1997) enrolling 27 infants reported on this outcome. The maternal anxiety score was significantly higher for VP versus HL prior to the procedure (mean difference 0.80, 95% CI 0.34 to 1.26). Test for heterogeneity not applicable.
Infant's pain score as rated by the mother (Outcome 1.12):
One study (Shah 1997) enrolling 27 infants reported on this outcome. The mothers rated the pain scores lower in the VP group versus the HL group (mean difference ‐0.80, 95% CI ‐1.18 to ‐0.42). Test for heterogeneity not applicable.
VENEPUNCTURE VERSUS HEEL LANCE IN INFANTS WHO RECEIVED SWEET TASTING SOLUTIONS PRIOR TO BLOOD SAMPLING (Comparison 2):
Pain response using a combination of one or more of NIPS, NFCS and or PIPP scores (Outcome 2.1) (Figure 2)
2.
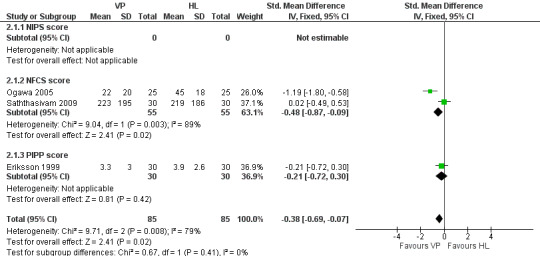
Forest plot of comparison: 2 Pain response during skin puncture to VP VS HL in infants who received a sweet tasting solution, outcome: 2.1 Behavioural pain scores for VP vs. HL.
None of the studies reported on the NIPS score. Two trials (Ogawa 2005; Saththasivam 2009) enrolling 110 infants reported on the NFCS score and one trial (Eriksson 1999) enrolling 60 infants reported on the PIPP score. Combining all studies (n= 170 infants) the SMD for pain scores was significantly reduced in the VP group compared to the HL group (‐0.38, 95% CI ‐0.69 to ‐ 0.07). There was no significant heterogeneity for this outcome, (p = 0.41, I2 = 0%).
Duration of first cry (seconds) (Outcome 2.2)
Two trials (Ogawa 2005; Saththasivam 2009) including 79 neonates reported on the duration of first cry. There was no significant difference in the duration of the first cry between the VP and the HL groups (WMD ‐4.46 seconds, 95% CI ‐18.00 to 9.08)]. There was statistically significant heterogeneity for this outcome (p = 0.0002; I2 = 93%).
Total duration of cry (seconds) (Outcome 2.3)
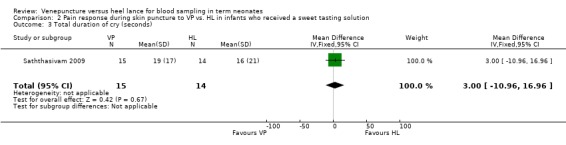
One trial (Saththasivam 2009) reported on this outcome in 29 infants. There was no statistically significant difference for total duration of cry between the VP and the HL groups [mean difference 3.0 seconds (95% CI ‐10.96 to 16.96). Test for heterogeneity not applicable.
Number of neonates who cried during the procedure (Outcome 2.4)
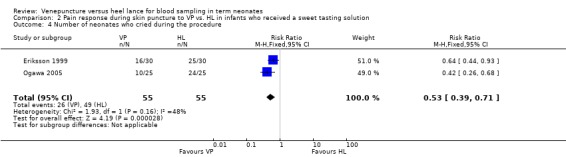
Two trials (Eriksson 1999; Ogawa 2005) enrolling 110 neonates reported on this outcome. There was a significantly lower number of neonates who cried in the VP group than the HL group (typical RR 0.53, 95% CI 0.39, to 0.71; typical RD ‐0.42, 95% CI ‐0.57 to ‐0.26; NNT 2, 95% CI 2 to 4). There was no significant heterogeneity between studies (RR p = 0.16, I2 = 48%; RD p =0.09, I2 = 65%).
First crying time/total procedure time (%) (Outcome 2.5)
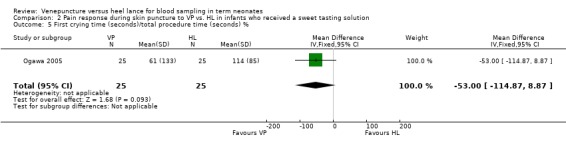
One trial (Ogawa 2005) enrolling 50 neonates reported on this outcome. The percentage of the first crying time relative to the total procedure time was not significantly different between the VP group and the HL group (mean difference ‐53%, 95% CI ‐115 to 9). Test for heterogeneity not applicable.
Duration of cry in the first three minutes after skin puncture (Outcome 2.6)
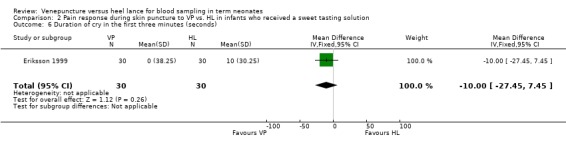
One trial (Eriksson 1999) enrolling 60 neonates reported on this outcome. There was no significant difference between the groups (mean difference ‐10 seconds, 95% CI ‐28 to 8). Test for heterogeneity not applicable.
Sampling time (seconds) (Outcome 2.7)
Two trials (Ogawa 2005; Saththasivam 2009) with 79 enrolled neonates reported sampling time. There was no statistically significant difference in the sampling time between the groups (typical WMD 1 second, 95% CI ‐27 to 30). There was statistically significant heterogeneity for this outcome (p = 0.001, I2 = 90%).
Need for more than one skin puncture (Outcome 2.8)
Two trials ( Ogawa 2005; Saththasivam 2009;) enrolling 110 neonates reported on this outcome. There was no significant difference in the number of neonates who required one additional skin puncture in the VP group compared to the HL group (typical RR 7.00, 95% CI 0.38 to 0.129.93; typical RD ‐0.05, 95% CI ‐0.02 to ‐0.13). There was statistically significant heterogeneity between the studies for RD (p = 0.11, I2 = 62%, moderate). For RR the test for heterogeneity was not applicable.
Need for more than two skin punctures
This outcome was not reported in any of the trials that used a sweet tasting solution as a co‐intervention.
Maternal anxiety score prior to the procedure
This outcome was not reported in any of the trials that used a sweet tasting solution as a co‐intervention.
Infant's pain score as rated by the mother
This outcome was not reported in any of the trials that used a sweet tasting solution as a co‐intervention.
Discussion
In this update of the review we included one additional study (Saththasivam 2009) and subgroups from two previously included studies (Ogawa 2005; Eriksson 1999) that used a sweet solution prior to the skin puncture. The results of the study by Saththasivam and co‐workers (Saththasivam 2009) differed with the results of previous studies. The only significant results reported in that study was an increase in the total duration of the procedure (sampling time) for the VP group. There were serious concerns about bias for that study (Saththasivam 2009). However, the review of all studies indicates that VP is preferable to HL to obtain a blood sample from term neonates. The combined meta‐analyses of studies that used NIPS, NFCS or PIPP scores showed a significant reduction in the SMD in favour of VP versus HL (SMD‐0.76, 95% CI ‐1.00 to ‐0.52). The increase in power by combining three different validated pain scores confirms that VP is less painful than HL. When sweet tasting solutions were given prior to skin puncture the SMD was significantly reduced (SMD ‐0.38, 95% CI ‐0.69, 0.07) in favour of VP but the effect size was smaller, indicating that sweet tasting solutions do reduce the pain for both VP and HL. This supports the findings of the Cochrane review of sucrose to reduce pain in neonates (Stevens 2010). Cry characteristics such as duration of first cry, total duration of cry, number of neonates who cried during the procedure, duration of cry in the first three minutes after skin puncture and sampling time were all significantly reduced in favour of VP in infants who did not receive a sweet tasting solution. For infants who received a sweet tasting solution (in both VP and HL groups) only the outcome of "Number of neonates who cried during the procedure" was statistically significant in favour of VP. The number of infants included in the various cry outcomes was small ranging from 29 to110 neonates.
The results of this review need to be interpreted with some caution. In three studies (Kvist 2002; Larsson 1998; Saththasivam 2009) the outcomes were not reported on all infants enrolled and therefore intention to treat analyses were not performed. In three (Larsson 1998; Shah 1997; Eriksson 1999) of the five studies included in this review, the procedures were performed by a single investigator, respectively a paediatrician or a nurse. In one study, the procedures were performed by two midwives who were relatively inexperienced in performing VPs (according to the authors) (Kvist 2002), while in the trial by Ogawa 2005, seven experienced nurses were trained to perform the procedures. In the study by Saththasivam 2009 two senior paediatric registrars were assigned to do the VPs and a single experienced staff nurse was assigned to do the HPs. There was statistically significant between study heterogeneity for several outcomes. The between study heterogeneity may in part be explained by the variable skills among the phlebotomists. The reproducibility of the results from these studies needs to be tested in a large study with multiple health care workers obtaining the blood samples. Before embarking on such a study, adequate training of the personnel undertaking heel lances and venipunctures would be required.
All studies used validated pain measures and showed that infants in the VP group had lower scores as compared to the HL group. Even though the changes in pain scores are statistically significant, the question remains as to whether these changes represent a clinically significant difference. The conventional method to determine this would be to ask patients how painful the procedure was as perceived by them. This is impossible in neonates and, therefore, clinicians have to rely on surrogate measures of pain. One study measured parents' judgement regarding their own anxiety and their infant's pain using a categorical rating scale. Parents favoured VP. This observation requires confirmation.
In the study by Larsson (Larsson 1998), the success rate with either the SL or the LL was poor as compared to the study by Shah (Shah 1997). This finding is hard to explain by poor technique, as neonatal nurses are well trained to perform HL. Larsson (Larsson 1998) evaluated the role of EMLA to reduce pain from VP in term neonates. In comparison to placebo, neonates in the EMLA group were noted to have significantly lower pain scores. However, when this study was compared to a previous study by the same investigators (Larsson 1998a), pain scores were higher in the VP‐EMLA group as compared to VP group without EMLA (Shah 1999a).
In conclusion, VP is less painful as assessed by validated pain measures and parental rating and is associated with less maternal anxiety. In view of the limitations of the studies performed to date, these promising results need confirmation in a study of appropriate sample size using multiple operators on the postnatal floor. Sweet tasting solutions appear to reduce pain responses both to VP and HL.
Authors' conclusions
Implications for practice.
When performed by a trained phlebotomist, venepuncture appears to be the method of choice for blood sampling in term neonates. Sweet tasting solutions should be provided to reduce pain responses both to VP and HL.
Implications for research.
Further well designed randomised controlled trials need to be conducted. The interventions should be compared in settings where several individuals perform the VP and the HL. Both groups should receive sweet tasting solutions prior to the procedures.
What's new
| Date | Event | Description |
|---|---|---|
| 27 January 2020 | Amended | Arne Ohlsson deceased. |
History
Protocol first published: Issue 2, 1999 Review first published: Issue 2, 1999
| Date | Event | Description |
|---|---|---|
| 7 December 2011 | Amended | Minor amendment to correct Outcome numbering in text, 'Effects of Interventions', Comparison 2. |
| 8 July 2011 | New citation required and conclusions have changed | For this update we included comparisons for studies that did or did not include a sweet tasting solution as a co‐intervention for venepuncture and heel lance. As before, venepuncture proved to be less painful than heel lance, but the addition of a sweet tasting solution prior to skin puncture reduced the difference between the two methods used for blood sampling. |
| 8 July 2011 | New search has been performed | This updates the review 'Venepuncture versus heel lance for blood sampling in term neonates' published in the Cochrane Database of Systematic Reviews (Shah 2007). Search updated May 12, 2011. One additional trial was identified. |
| 11 June 2008 | Amended | Converted to new review format. |
| 27 July 2007 | New citation required but conclusions have not changed | Substantive amendment |
| 27 July 2007 | New search has been performed | This updates the review "Venepuncture versus heel lance for blood sampling in term neonates", published in The Cochrane Library, Issue 2, 1999 (Shah 1999b) and updated in 2004 (Shah 2004). One additional randomized controlled trial was identified for inclusion. The data from this additional study strengthen the evidence that venepuncture is less painful compared to heel lance to obtain a blood sample from healthy term neonates. However, in the newly identified study, a team of seven trained nurses performed the two procedures and obtained blood samples on the first attempt. |
Acknowledgements
We are thankful to Dr. Mats Eriksson (Eriksson 1999) and Ms. Linda J Kvist (Kvist 2002) who provided clarifications and unpublished information regarding their studies. For the study by Saththasivam (Saththasivam 2009) we were provided results as means and standard deviations from the statistician Ms K.Voralu. Ms Yolanda Montagne conducted the literature searches in May, 2011.
Data and analyses
Comparison 1. Pain response during skin puncture to VP vs. HL in infants who did not receive a sweet tasting solution.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Behavioural pain scores for VP vs. HL | 5 | 288 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.76 [‐1.00, ‐0.52] |
| 1.1 NIPS score | 2 | 81 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.93 [‐1.40, ‐0.47] |
| 1.2 NFCS score | 2 | 147 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.71 [‐1.05, ‐0.38] |
| 1.3 PIPP score | 1 | 60 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.67 [‐1.19, ‐0.15] |
| 2 Duration of first cry (seconds) | 1 | 50 | Mean Difference (IV, Fixed, 95% CI) | ‐112.0 [‐163.99, ‐60.01] |
| 3 Total duration of cry (seconds) | 1 | 100 | Mean Difference (IV, Fixed, 95% CI) | ‐188.0 [‐228.42, ‐147.58] |
| 4 Number of neonates who cried during the procedure | 3 | 207 | Risk Difference (M‐H, Fixed, 95% CI) | ‐0.35 [‐0.46, ‐0.23] |
| 5 First crying time (seconds)/total procedure time (seconds) (%) | 1 | 50 | Mean Difference (IV, Fixed, 95% CI) | ‐65.0 [‐110.12, ‐19.88] |
| 6 Duration of cry in the first three minutes after skin puncture | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | ‐106.0 [‐128.52, ‐83.48] |
| 7 Sampling time (seconds) | 1 | 50 | Mean Difference (IV, Fixed, 95% CI) | ‐65.0 [‐109.93, ‐20.07] |
| 8 Need for more than one skin puncture | 4 | 254 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.29 [0.18, 0.46] |
| 9 Need for more than two skin punctures | 1 | 60 | Risk Ratio (M‐H, Fixed, 95% CI) | 5.0 [0.62, 40.28] |
| 10 Brusing/hematoma at local site | 3 | 137 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.36 [0.02, 8.06] |
| 11 Maternal anxiety score prior to the procedure | 1 | 27 | Mean Difference (IV, Fixed, 95% CI) | 0.80 [0.34, 1.26] |
| 12 Infant's pain score as rated by the mother | 1 | 27 | Mean Difference (IV, Fixed, 95% CI) | ‐0.80 [‐1.18, ‐0.42] |
1.1. Analysis.
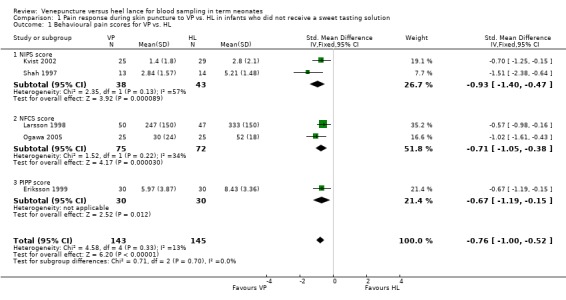
Comparison 1 Pain response during skin puncture to VP vs. HL in infants who did not receive a sweet tasting solution, Outcome 1 Behavioural pain scores for VP vs. HL.
1.2. Analysis.
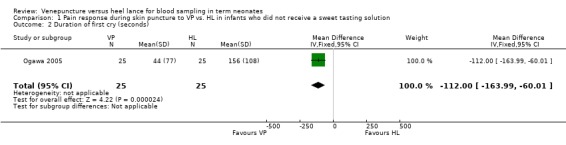
Comparison 1 Pain response during skin puncture to VP vs. HL in infants who did not receive a sweet tasting solution, Outcome 2 Duration of first cry (seconds).
1.3. Analysis.
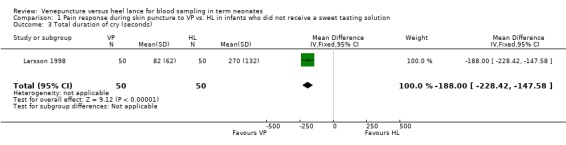
Comparison 1 Pain response during skin puncture to VP vs. HL in infants who did not receive a sweet tasting solution, Outcome 3 Total duration of cry (seconds).
1.4. Analysis.
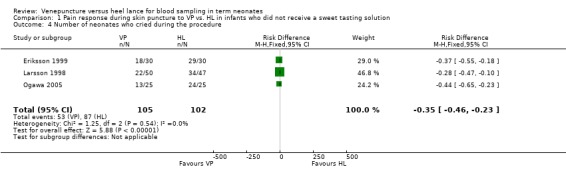
Comparison 1 Pain response during skin puncture to VP vs. HL in infants who did not receive a sweet tasting solution, Outcome 4 Number of neonates who cried during the procedure.
1.5. Analysis.
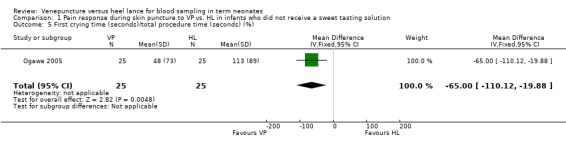
Comparison 1 Pain response during skin puncture to VP vs. HL in infants who did not receive a sweet tasting solution, Outcome 5 First crying time (seconds)/total procedure time (seconds) (%).
1.6. Analysis.
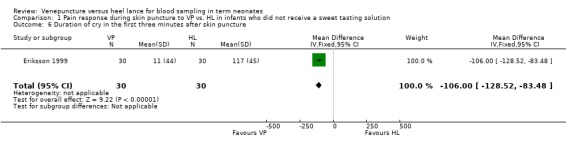
Comparison 1 Pain response during skin puncture to VP vs. HL in infants who did not receive a sweet tasting solution, Outcome 6 Duration of cry in the first three minutes after skin puncture.
1.7. Analysis.
Comparison 1 Pain response during skin puncture to VP vs. HL in infants who did not receive a sweet tasting solution, Outcome 7 Sampling time (seconds).
1.8. Analysis.
Comparison 1 Pain response during skin puncture to VP vs. HL in infants who did not receive a sweet tasting solution, Outcome 8 Need for more than one skin puncture.
1.9. Analysis.
Comparison 1 Pain response during skin puncture to VP vs. HL in infants who did not receive a sweet tasting solution, Outcome 9 Need for more than two skin punctures.
1.10. Analysis.
Comparison 1 Pain response during skin puncture to VP vs. HL in infants who did not receive a sweet tasting solution, Outcome 10 Brusing/hematoma at local site.
1.11. Analysis.
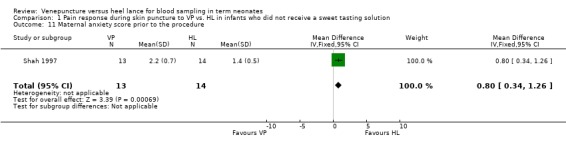
Comparison 1 Pain response during skin puncture to VP vs. HL in infants who did not receive a sweet tasting solution, Outcome 11 Maternal anxiety score prior to the procedure.
1.12. Analysis.
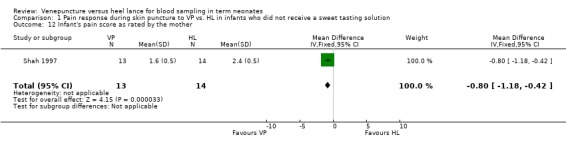
Comparison 1 Pain response during skin puncture to VP vs. HL in infants who did not receive a sweet tasting solution, Outcome 12 Infant's pain score as rated by the mother.
Comparison 2. Pain response during skin puncture to VP vs. HL in infants who received a sweet tasting solution.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Behavioural pain scores for VP vs. HL | 3 | 170 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.38 [‐0.69, ‐0.07] |
| 1.1 NIPS score | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 1.2 NFCS score | 2 | 110 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.48 [‐0.87, ‐0.09] |
| 1.3 PIPP score | 1 | 60 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.21 [‐0.72, 0.30] |
| 2 Durration of first cry (seconds) | 2 | 79 | Mean Difference (IV, Fixed, 95% CI) | ‐4.46 [‐16.00, 9.08] |
| 3 Total duration of cry (seconds) | 1 | 29 | Mean Difference (IV, Fixed, 95% CI) | 3.0 [‐10.96, 16.96] |
| 4 Number of neonates who cried during the procedure | 2 | 110 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.53 [0.39, 0.71] |
| 5 First crying time (seconds)/total procedure time (seconds) % | 1 | 50 | Mean Difference (IV, Fixed, 95% CI) | ‐53.0 [‐114.87, 8.87] |
| 6 Duration of cry in the first three minutes (seconds) | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | ‐10.0 [‐27.45, 7.45] |
| 7 Sampliing time (seconds) | 2 | 79 | Mean Difference (IV, Fixed, 95% CI) | 1.36 [‐25.00, 29.71] |
| 8 Need for more than one skin puncture | 2 | 110 | Risk Ratio (M‐H, Fixed, 95% CI) | 7.0 [0.38, 129.93] |
2.1. Analysis.
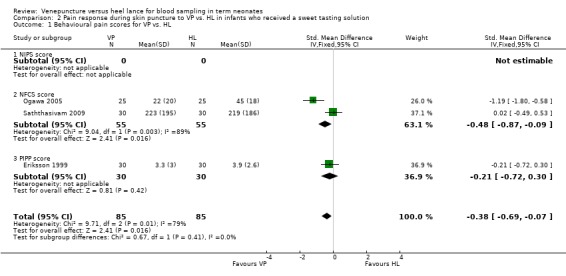
Comparison 2 Pain response during skin puncture to VP vs. HL in infants who received a sweet tasting solution, Outcome 1 Behavioural pain scores for VP vs. HL.
2.2. Analysis.
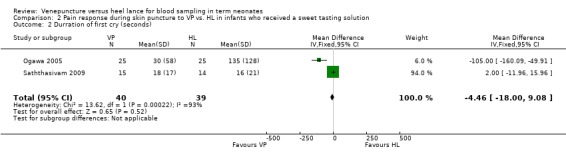
Comparison 2 Pain response during skin puncture to VP vs. HL in infants who received a sweet tasting solution, Outcome 2 Durration of first cry (seconds).
2.3. Analysis.
Comparison 2 Pain response during skin puncture to VP vs. HL in infants who received a sweet tasting solution, Outcome 3 Total duration of cry (seconds).
2.4. Analysis.
Comparison 2 Pain response during skin puncture to VP vs. HL in infants who received a sweet tasting solution, Outcome 4 Number of neonates who cried during the procedure.
2.5. Analysis.
Comparison 2 Pain response during skin puncture to VP vs. HL in infants who received a sweet tasting solution, Outcome 5 First crying time (seconds)/total procedure time (seconds) %.
2.6. Analysis.
Comparison 2 Pain response during skin puncture to VP vs. HL in infants who received a sweet tasting solution, Outcome 6 Duration of cry in the first three minutes (seconds).
2.7. Analysis.
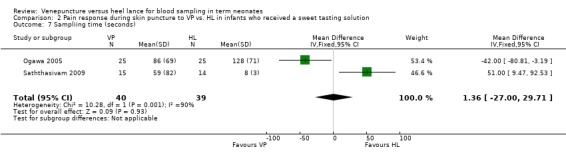
Comparison 2 Pain response during skin puncture to VP vs. HL in infants who received a sweet tasting solution, Outcome 7 Sampliing time (seconds).
2.8. Analysis.
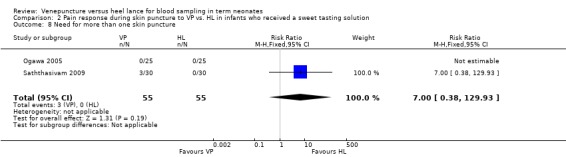
Comparison 2 Pain response during skin puncture to VP vs. HL in infants who received a sweet tasting solution, Outcome 8 Need for more than one skin puncture.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Eriksson 1999.
| Methods | Randomized controlled trial Blinding of randomisation: Can't tell Blinding of intervention: No Complete follow‐up: Yes Blinding of outcome: Can't tell | |
| Participants | Healthy full term infants undergoing metabolic screening blood test (n = 120)
Demographic data: Values are presented as mean (SD) or percentage (%) VP group: n = 30 Birth weight (g) 3667 (449) PMA (wks) 39.7 (1.4) Sex (%) male 40 HL group: n = 30 Birth weight (g) 3578 (465) PMA (wks) 40.2 (1.2) Sex (%) male 53 VP with 1 ml of 30% glucose: n = 30 Birth weight (g) 3598 (444) PMA (wks) 40.0 (1.2) Sex (%) male 43 HL group with 1 ml of 30% glucose: n = 30 Birth weight (g) 3533 (341) PMA (wks) 39.8 (1.1) Sex (%) male 53 |
|
| Interventions | VP (n=60). A 21‐gauge needle (Terumo, Leuven, Belgium) was used to access the vein. VP was performed by one investigator HL (n=60). The heel was lanced with a microtainer safety lancet (Microtainer Brand Safety Flow Lancet, Becton Dickinson, Meylan Cedex, France). HL was performed by one investigator. Sixty infants received 1 ml of 30% glucose (30 infants randomised to the VP group and 30 to the HL group) | |
| Outcomes | Pain assessments were made using the duration of cry within the first 3 minutes after the skin puncture, the Premature Infant Pain Profile (PIPP) score and by changes in the heart rate | |
| Notes | Infants were randomised using a block randomisation technique with sealed envelopes into one of the four groups ‐ HL and VP with and without oral administration of 1 ml of 30% glucose | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information provided about sequence generation, except for using a block randomisation technique |
| Allocation concealment (selection bias) | Unclear risk | Sealed envelops were used but no information if they were opaque and sequentially numbered |
| Blinding (performance bias and detection bias) All outcomes | High risk | High risk for VP versus HL; Low risk for glucose administration or not |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Outcomes reported for all randomised infants |
| Selective reporting (reporting bias) | Unclear risk | The study was not registered in a trials registry and the protocol was not available to us |
| Other bias | Low risk | Appears free of other bias |
Kvist 2002.
| Methods | Randomized controlled trial Blinding of randomisation: Can't tell Blinding of intervention: No Complete follow‐up: No Blinding of outcome: No | |
| Participants | Healthy full term infants undergoing PKU screening (n = 90) Demographic data for the small calibre VP needle group (n = 25) and the HL group (n = 29) are presented. Values are presented as mean (range) or percentage (%) Small calibre VP needle group; Birth weight 3613 g (range 2690 ‐ 4740) PMA (40, 37‐42) Age 4 days (3‐6) Sex % male 44 For the HL group (n = 29) Birth weight 3525 g (2710 ‐ 4650) PMA 39 weeks (37 ‐ 42) Age 5 days (3 ‐ 6) Sex % male 66 |
|
| Interventions | VP with large calibre needle (n = 30) (results not reported) VP with small calibre needle (n = 30; 5 infants excluded). A 0.6 x 25 mm needle was used HL (n = 30; 1 infant excluded). A microtainer lancet, depth 2 mm was used | |
| Outcomes | NIPS (Neonatal Infant Pain Scale) reported at the beginning of each minute, the first minute starting at the moment of puncture All infants were observed for a minimum of 5 minutes Number of punctures were noted |
|
| Notes | Infants were randomised using consecutively numbered envelopes
No envelope was taken out of sequence
Data for the large calibre venepuncture needle group were not presented as in 33% of the infants the venepuncture was unsuccessful
5 infants in the small calibre venepuncture needle group and 1 infant in the heel lance group were excluded from the analyses because more than two skin punctures were required Blood sampling was carried out by two of the authors who were registered nurses |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information provided about sequence generation |
| Allocation concealment (selection bias) | Unclear risk | Sealed opaque lottery tickets, identical on the out side were used but no information if the were sequentially numbered |
| Blinding (performance bias and detection bias) All outcomes | High risk | Personnel‐knew group assignment |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Outcome data not reported on all randomised infants |
| Selective reporting (reporting bias) | Unclear risk | The study was not registered in a trials registry |
| Other bias | Low risk | Appears free of other bias |
Larsson 1998.
| Methods | Randomized controlled trial Blinding of randomisation: Can't tell Blinding of intervention: No Complete follow up: No Blinding of outcome measurement: No | |
| Participants | Healthy term infants undergoing blood testing for phenylketonuria (n=120)
Three infants in the small lancet group were excluded as one infant was of 35 weeks gestation and two infants screamed prior to heel lance
Demographic data: Values are presented as median (range) or % VP group: n = 50 Birth weight (g) 3410 (2440‐5035) PMA (wks) 40 (36‐43) Age (days) 3 (3‐7) Sex (%) male 44 Small HL group: n = 47 Birth weight (g) 3570 (2650‐4540) PMA (wks) 40 (36‐43) Age (days) 4 (3‐6) Gender (%) male 55 Large HL group: n = 20 Birth weight (g) 3398 (2160‐4330) PMA (wks) 40 (37‐42) Age (days) 4 (3‐7) Gender (%) male 45 |
|
| Interventions | VP (n=50). A Microlance needle measuring 0.9 x 40 mm (Becton‐Dickinson, Madrid, Spain) was used for VP HL with a small lancet (n=47). A CCS Minilancet (Clean Chemical, Borlänge, Sweden) was used HL with a large lancet (n=20). A Microlance (Becton‐Dickinson, Meylan Cedex, France) was used The authors do not explain the reasons for the unequal group sizes | |
| Outcomes | Pain assessments were made using Neonatal Facial Action Coding System (NFCS) and cry [latency (cry within 60 seconds of the skin puncture) and duration of first cry and total duration of cry] Audiotapes were reviewed to determine the latency to cry from the skin puncture, duration of first cry and total time the infant cried during the procedure Cry was defined as high‐pitched vocalization | |
| Notes | Infants were randomised using envelopes to receive VP, HL using a small lancet (SL) [three later excluded (one was a preterm infant and two infants screamed prior to heel lance)] or a large lancet (LL) One investigator (neonatal nurse) performed all procedures | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information provided about sequence generation |
| Allocation concealment (selection bias) | Unclear risk | Envelopes were used but no information on whether they were sealed or not, opaque or not. |
| Blinding (performance bias and detection bias) All outcomes | High risk | High risk for venepuncture versus heel lance; Low risk for glucose administration or not. The assessors knew whether the infant had a venepuncture or a heel lance |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Outcomes reported for all randomised infants |
| Selective reporting (reporting bias) | Unclear risk | The study was not registered in a trials registry |
| Other bias | Low risk | Appears free of other bias |
Ogawa 2005.
| Methods | Randomized, controlled trial Blinding of randomisation: Can't tell Blinding of intervention: No Complete follow up: Yes Blinding of outcome assessment: Yes | |
| Participants | Healthy full term neonates of > = 37 weeks PMA undergoing the newborn screening test
Demograpic data: Values are presented as median (range) VP group: n = 25 Birth weight (g) 3274 (2295‐3715) PMA (weeks) 39 (37‐41) Male sex (n) 12/25 HL group: n = 25 Birth weight (g) 3030 (2530‐3550) PMA (weeks) 40 (38‐42) Male sex (n) 12/25 |
|
| Interventions | Venepuncture (n=25) was performed using a 23 gauge needle Heel lance (n=25). Heel lance was performed using a standard lancet with a sharp triangular edge that was 2.5 mm long and 1 mm wide (Feather Safety Razor Co, Ltd, Osaka, Japan | |
| Outcomes | Pain assessments were made using NFCS and duration of first cry | |
| Notes | Infants were randomised using sealed envelopes A team of seven nurses performed the procedure A single investigator blinded to the sampling techniques performed the outcome assessments | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information provided about sequence generation |
| Allocation concealment (selection bias) | Unclear risk | Unclear risk, sealed envelopes but no information if the were opaque and sequentially numbered |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Video recordings assessed blinded to groups |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Outcomes reported for all randomised infants |
| Selective reporting (reporting bias) | Unclear risk | The study was not registered in a trials registry |
| Other bias | Low risk | Appears free of other bias |
Saththasivam 2009.
| Methods | Quasi randomised controlled trial Blinding of randomisation: No Blinding of intervention: No Complete follow up: Yes, for some outcomes Blinding of outcome assessment: Yes | |
| Participants | Healthy full‐term neonates undergoing blood glucose monitoring. VP group: n = 30 Birth weight (g) 3158 (SD 645) PMA (weeks) 39 (SD 1) Male sex (n) 18/30 Apgar scores: 5 minutes 9 (SD 0.5) HL group: n = 30 Birth weight (g) 3066 (SD 636) PMA (weeks) 40 (SD 2) Male sex (n) 20/30 Apgar scores: 5 minutes 9 (SD 1.5) |
|
| Interventions | HL (n = 30) was performed by a single experienced staff nurse using an automatic disposable lancing device, Unistik 2 Neonatal (Omega Health Care, London, UK) while VP (n = 30) was performed by two senior paediatric registrars using a 23 gauge needle. Both groups received 2 ml of 25% dextrose orally via a sterile syringe. |
|
| Outcomes | Pain assessments were performed using NFCS, duration of first cry, total duration of cry, total duration of procedure, number of punctures 1 or > 1. No adverse events occurred during or after the blood‐taking. | |
| Notes | The authors provided us with outcomes data as means and standard deviations | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | There was no sequence generation |
| Allocation concealment (selection bias) | High risk | Neonates were allocated to either the VP group or the HP group depending on the availability of the assigned staff |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Performance bias ‐ high; Detection bias low ‐ Video recordings assessed blinded to groups |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | NFCS scores, total duration of cry and number of punctures reported for all infants; duration of first cry and total duration of cry reported for 15 infants in the VP group and 15 in the HL group |
| Selective reporting (reporting bias) | Unclear risk | The study was not registered in a trials registry |
| Other bias | Low risk | Appears free of other bias |
Shah 1997.
| Methods | Randomized controlled trial Blinding of randomisation: Can't tell Blinding of intervention: No Complete follow up: Yes Blinding of outcome measurement: No | |
| Participants | Healthy neonates of > =37 weeks PMA (n=27)
Demographic data: Values are mean (SD) or number (%) HL group: Age (days) 3.1 (1.1) Sex (% male) 8 (57%) State (% awake before procedure) 5 (36%) Reason for test (% bilirubin) 13 (93%) VP group: Age (days) 2.8 (1.2) Sex (% male) 8 (62%) State (% awake before procedure) 7 (58%) Reason for test (% bilirubin) 12 (97%) |
|
| Interventions | VP (n=13) HL (n=14) 0.25 ml of blood was obtained with either method | |
| Outcomes | Pain assessments were made using Neonatal Infant Pain Scale (NIPS) Parental rating of their own anxiety was assessed using a three point scale where 0= not worried at all, 1= a little worried and 2= very worried and infant's pain using a scale where 0= no pain at all, 1= a little pain and 2= a lot of pain | |
| Notes | One investigator (well‐trained paediatrician) performed all procedures One infant in each group had the procedure performed while being breat fed One of the reviewers (V. Shah) for this systematic review is the primary author of this paper | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer generated |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding (performance bias and detection bias) All outcomes | High risk | Personnel knew group assignment |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Outcomes reported for all 27 infants included |
| Selective reporting (reporting bias) | Unclear risk | The study was not registered in a trials registry |
| Other bias | High risk | The study was stopped after 27 infants had been enrolled from the preset sample of 50 infants |
Differences between protocol and review
For this update the outcome of "Total duration of cry (seconds)" was added.
Contributions of authors
Both review authors contributed to all sections of this review update in 2011 and to the updates conducted in 2004 and 2007.
Sources of support
Internal sources
Mount Sinai Hospital, Toronto, Ontario, Canada.
External sources
No sources of support supplied
Declarations of interest
Dr. Vibhuti Shah is the principal author of one of the included trials in this review (Shah 1997).
Deceased
Edited (no change to conclusions)
References
References to studies included in this review
Eriksson 1999 {published and unpublished data}
- Eriksson M, Gradin M, Schollin J. Oral glucose and venepuncture reduce blood sampling pain in newborns. Early Human Development 1999;55:211‐8. [DOI] [PubMed] [Google Scholar]
Kvist 2002 {published and unpublished data}
- Kvist LJ, Jonsson K, Tornestrand BM, Edwinson Mansson M. Can venepuncture reduce the pain of neonatal PKU‐sampling? A randomised study. Vard i Norden 2002;22:27‐30. [Google Scholar]
Larsson 1998 {published data only}
- Larsson BA, Tannfeldt G, Lagercrantz H, Olsson GL. Venipuncture is more effective and less painful than heel lancing for blood tests in neonates. Pediatrics 1998;101:882‐6. [DOI] [PubMed] [Google Scholar]
Ogawa 2005 {published data only}
- Ogawa S, Ogihara T, Fujiwara E, Ito K, Nakano M, Nakayama S, et al. Venepuncture is preferable to heel lance for blood sampling in term neonates. Archives of Disease in Childhood 2005;90:F432‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Saththasivam 2009 {published data only}
- Saththasivam P, Umadevan D, Ramli N, Voralu K, Naing NN, Ilias MI, et al. Venipuncture versus heel prick for blood glucose monitoring in neonates. Singapore Medical Journal 2009;50:1004‐7. [PubMed] [Google Scholar]
Shah 1997 {published and unpublished data}
- Shah VS, Taddio A, Bennett S, Speidel BD. Neonatal pain response to heel stick vs. venepuncture for routine blood sampling. Archives of Disease in Childhood 1997;77:F143‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Additional references
Barker 1995
- Barker DP, Rutter N. Exposure to invasive procedures in neonatal intensive care unit admissions. Archives of Disease in Childhood 1995;72:F47‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Brown 1987
- Brown L. Physiologic responses to cutaneous pain in neonates. Neonatal Network 1987;5:18‐21. [PubMed] [Google Scholar]
Campos 1994
- Campos GR. Rocking and pacifiers: Two comforting interventions for heel stick pain. Research in Nursing and Health 1994;17:321‐31. [DOI] [PubMed] [Google Scholar]
CPS 2000
- Fetus and Newborn Committee, Canadian Paediatric Society, and Committee on Fetus and Newborn, Committee on Drugs, Section on Anesthesiology, Section on Surgery. American Academy of Pediatrics. Prevention and management of pain and stress in the neonate. Journal of Paediatrics and Child Health 2000;5:31‐8. [Google Scholar]
Craig 1994
- Craig KD, Hadjistavropoulos HD, Grunau RVE. A comparison of two measures of facial activity during pain in the newborn child. Journal of Pediatric Psychology 1995;19:305‐18. [DOI] [PubMed] [Google Scholar]
Egger 1997
- Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta‐analysis detected by a simple, graphical test. British Medical Journal 1997;315:629‐34. [DOI] [PMC free article] [PubMed] [Google Scholar]
Field 1984
- Field T, Goldson E. Pacifying effects of non‐nutritive sucking on term and preterm neonates during heel stick procedures. Pediatrics 1984;74:1012‐5. [PubMed] [Google Scholar]
Fitzgerald 1989
- Fitzgerald M, McIntosh N. Pain and analgesia in the newborn. Archives of Disease in Childhood 1989;64:441‐3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Grunau 1987
- Grunau RVE, Craig KD. Pain expression in neonates: facial action and cry. Pain 1987;28:395‐410. [DOI] [PubMed] [Google Scholar]
Harboard 2006
- Harbord RM, Egger M, Sterne JA. A modified test for small‐study effects in meta‐analyses of controlled trials with binary endpoints. Statistics in Medicine 2006;25:3443‐57. [DOI] [PubMed] [Google Scholar]
Harpin 1983
- Harpin VA, Rutter N. Making heel pricks less painful. Archives of Disease in Childhood 1983;58:226‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2003
- Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analyses. British Medical Journal 2003;327:557‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration. Available from www.cochrane‐handbook.org.
Hozo 2005
- Hozo SP, Djulbegovic B, Hozo I. Estimating the mean and variance from the median,range , and the size of a sample. Bio Med Central Medical Research Methodology 2005;5:5‐13. [DOI] [PMC free article] [PubMed] [Google Scholar]
Izard 1979
- Izard CE. The maximally discriminative facial movement coding system (MAX). Newark DE: University of Delaware Instructional Resources Centre, 1979. [Google Scholar]
Jain 2001
- Jain A, Rutter N, Ratnayaka M. Topical amethocaine gel for pain relief of heel prick blood sampling: a randomized double blind controlled trial. Archives of Disease in Childhood Fetal Neonatal Edition 2001;84:F56‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Johnston 1986
- Johnston CC, Strada ME. Acute pain response in infants: a multidimensional description. Pain 1986;24:373‐82. [DOI] [PubMed] [Google Scholar]
Larsson 1995
- Larsson BA, Jylli L, Lagercrantz H, Olsson GL. Does a local anaesthetic cream (EMLA) alleviate pain from heel lancing in neonate?. Acta Anaesthesiologica Scandinavica 1995;39:1028‐31. [DOI] [PubMed] [Google Scholar]
Larsson 1998a
- Larsson BA, Tannfeldt G, Lagercrantz H, Olsson GL. Alleviation of the pain of venepuncture in neonates. Acta Paediatrica 1998;87:774‐9. [DOI] [PubMed] [Google Scholar]
Lawrence 1993
- Lawrence J, Alcock D, McGrath P, Kay J, MacMurray SB, Dulberg C. The development of a tool to assess neonatal pain. Neonatal Network 1993;12:59‐66. [PubMed] [Google Scholar]
McIntosh 1994
- McIntosh N, Vee L, Brameyer H. Alleviation of the pain of heel prick in preterm infants. Archives of Disease in Childhood 1994;79:177‐81. [DOI] [PMC free article] [PubMed] [Google Scholar]
McKay 1966
- McKay RJ. Diagnosis and treatment: risks of obtaining samples of venous blood in infants. Pediatrics 1966;38:906‐8. [PubMed] [Google Scholar]
Meehan 1998
- Meehan RM. Heelsticks in neonates for capillary blood sampling. Neonatal Network 1998;17:17‐24. [PubMed] [Google Scholar]
Moxley 1989
- Moxley S. Neonatal heel puncture. The Canadian Nurse 1989;1:25‐7. [PubMed] [Google Scholar]
Ohlsson 2000
- Ohlsson A, Taddio A, Jadad AR, Stevens BJ. Evidence‐based decision making, systematic reviews and the Cochrane Collaboration: implications for neonatal analgesia. In: Anand KJS, Stevens BJ, McGrath PJ editor(s). Pain in neonates. 2nd Edition. Vol. 10, Amsterdam: Elsevier, 2000:251‐268. [Google Scholar]
Owens 1984
- Owens ME, Todt EH. Pain in infancy: neonatal reaction to heel lance. Pain 1984;20:77‐86. [DOI] [PubMed] [Google Scholar]
Paes 1993
- Paes B, Janes M, Vegh P, LeDuca F, Andrew M. A comparative study of heel stick devices for infant blood collection. American Journal of Diseases of Children 1993;147:346‐8. [DOI] [PubMed] [Google Scholar]
Ramenghi 1996
- Ramenghi LA, Griffiths GC, Wood CM, Levene MI. Effect of non‐sucrose sweet tasting solution on neonatal heel prick responses. Archives of Disease in Childhood 1996;74:F129‐31. [DOI] [PMC free article] [PubMed] [Google Scholar]
Rushforth 1995
- Rushforth JA, Griffiths G, Thorpe H, Levene MI. Can topical lignocaine reduce behavioural response to heel prick?. Archives of Disease in Childhood 1995;72:F49‐51. [DOI] [PMC free article] [PubMed] [Google Scholar]
Shah 1998
- Shah VS, Taddio A, Ohlsson A. Randomized controlled trial of paracetamol for heel prick pain in neonates. Archives of Disease in Childhood 1998;79:F209‐11. [DOI] [PMC free article] [PubMed] [Google Scholar]
Shah 1999a
Skogdal 1997
- Skogdal Y, Eriksson M, Schollin J. Analgesia in newborns given oral glucose. Acta Paediatrica 1997;86:217‐20. [DOI] [PubMed] [Google Scholar]
Stevens 1996
- Stevens B, Johnston C, Petryshen P, Taddio A. Premature infant pain profile: development and initial validation. The Clinical Journal of Pain 1996;12:13‐22. [DOI] [PubMed] [Google Scholar]
Stevens 2010
- Stevens B, Yamada J, Ohlsson A. Sucrose for analgesia in newborn infants undergoing painful procedures. Cochrane Database of Systematic Reviews 2010, Issue 1. [DOI: 10.1002/14651858.CD001069.pub3] [DOI] [PubMed] [Google Scholar]
Taddio 1995
- Taddio A, Goldbach M, Ipp M, Stevens B, Koren G. Effect of neonatal circumcision on pain responses during vaccination in boys. Lancet 1995;345:291‐2. [DOI] [PubMed] [Google Scholar]
Taddio 1997
- Taddio A, Katz J, Ilersich AL, Koren G. Effect of neonatal circumcision on pain response during subsequent routine vaccination. Lancet 1997;349:599‐603. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Shah 1999b
- Shah V, Ohlsson A. Venepuncture versus heel lance for blood sampling in term neonates. Cochrane Database of Systematic Reviews 1999, Issue 2. [DOI: 10.1002/14651858.CD001452] [DOI] [PubMed] [Google Scholar]
Shah 2001
- Shah V, Ohlsson A. Venepuncture versus heel lance for blood sampling in term neonates. Cochrane Database of Systematic Reviews 2001, Issue 1. [DOI: 10.1002/14651858.CD001452] [DOI] [PubMed] [Google Scholar]
Shah 2004
- Shah V, Ohlsson A. Venepuncture versus heel lance for blood sampling in term neonates. Cochrane Database of Systematic Reviews 2004, Issue 4. [DOI: 10.1002/14651858.CD001452.pub2] [DOI] [PubMed] [Google Scholar]
Shah 2007
- Shah V, Ohlsson A. Venepuncture versus heel lance for blood sampling in term neonates. Cochrane Database of Systematic Reviews 2007, Issue 4. [DOI: 10.1002/14651858.CD001452.pub3] [DOI] [PubMed] [Google Scholar]


