Abstract
Background
Glutamate is the principal excitatory neurotransmitter in the brain. Injury to the brain can cause an ionic imbalance in cerebral tissue, creating an excitotoxic cascade involving glutamate and other excitatory amino acids, that leads to neuronal death in the tissue surrounding the original injury site. Research has centred around inhibiting this increase in excitatory amino acid during injury either pre‐ or post‐synaptically. Animal studies appeared promising, but as yet, those results have not been repeated in human clinical trials.
Objectives
To assess systematically the efficacy of excitatory amino acid inhibitors on improving patient outcome following traumatic brain injury.
Search methods
Searches of the databases; CENTRAL, MEDLINE, EMBASE, IDdb3, and Science Citation Index were carried out. Searches were also carried out on online clinical trial registers; ClinicalTrials (http://clinicaltrials.gov) and Current Controlled Trials (http://controlled‐trials.com/mrct). General Internet searches were carried out using selected terms from the original search strategy and individual drug names. Authors of published works and associated pharmaceutical companies were contacted. Searches were last carried out in January 2003.
Selection criteria
Trials were included if they were randomised, double‐blind, controlled trials where excitatory amino acid inhibitors were administered to patients with traumatic brain injury, within 24 hours of sustaining that injury, and compared to a control group.
Data collection and analysis
Twelve trials, involving eight compounds, were identified that appeared to fit the inclusion criteria. Further investigation excluded three of these trials. Two of the remaining trials are ongoing. Of the seven included studies, one trial did not report GOS data and we were unable to acquire them. Three trials have not been published and the data were not made available to us. One trial is currently being prepared for publication, leaving two trials where data were available. Data were extracted by two independent reviewers.
Main results
Data were available for two of the seven relevant trials identified, with 760 recruited participants. Mortality is similar between patients who receive excitatory amino acid inhibitors and those that receive placebo: odds ratio (OR) 1.11; 95% confidence interval (CI) 0.78, 1.60. Patients who have a favourable outcome six months after injury are also similar between treatment and placebo groups: OR 0.86; 95% CI 0.64, 1.16.
Authors' conclusions
The case for efficacy of excitatory amino acid inhibitor therapy remains unproven. To date, no product has proven to be efficacious (as determined by the criteria applied) for improving the outcomes of brain‐injured patients. Early termination, unpublished, and underpowered studies limit a clear appreciation of the merits of this form of intervention. Additional studies, some of which remain in progress, may more clearly define the efficacy and effectiveness issues.
Keywords: Humans, Brain Injuries, Brain Injuries/drug therapy, Excitatory Amino Acid Antagonists, Excitatory Amino Acid Antagonists/therapeutic use, Randomized Controlled Trials as Topic
Plain language summary
Excitatory amino acid inhibiting drugs for traumatic brain injury
Brain injury can start a cascade of damage to brain tissue. Release into the brain of excess excitatory amino acids is thought to begin this process. Drugs which stop the release of excitatory amino acids or which block them may reduce brain damage. Studies have been done in patients with stroke as well as traumatic brain injury. The review found that the results from most drug trials with brain injured patients have not been published. There is therefore not enough evidence about the effects of excitatory amino acid inhibiting drugs for traumatic brain injury, and more published trials are needed.
Background
Brain injury is a leading cause of mortality and disability (Murray 1996). It has been observed that the neuro‐anatomical damage may not be immediately evident at the moment of impact; for example, evidence suggests that up to 40% of patients are initially able to speak before subsequently deteriorating (Blumbergs 1989).
The mechanics by which damage is initiated is complex. It is proposed that reduced blood flow depletes energy stores and causes membrane depolarisation. Excitatory amino acids (EAA) (primarily glutamate) are released into the synapse in supra‐physiological concentrations and overstimulate (primarily) the N‐methyl‐D‐aspartate (NMDA) receptor (Hickenbottom 1998). Ionic imbalance occurs with Na+ and Ca2+ influx and K+ efflux, leading to further depolarisation which can overcome the Mg2+ blockade of the NMDA receptor (Gentile 1993). Glutamate re‐uptake is diminished due to the ionic imbalance, and the concentration is further increased. The increase in Ca2+ leads to neuronal death, while the efflux of K+ leads to swelling in the brain. Therefore, cells surrounding the initial damage are less compromised, but may succumb to deleterious neurochemical events (Duhaime 1994). Neuroprotective therapy is aimed at interrupting the excitotoxic cascade in tissue before neuronal toxicity is irreversible (Hickenbottom 1998), leading to a reduction in severity of damage, with long term benefits to the individual and the community.
Excitatory amino acid inhibitors fall into several categories: pre‐ and post‐synaptic blockers, which inhibit the activation of different receptors such as NMDA; AMPA/kinate and metabotropic receptors; competitive antagonists, which bind directly to the receptor site; and non‐competitive antagonists (open‐channel blockers), which do not compete with the massive concentrations of excitatory neurotransmitters in the synapse (Gentile 1993; Muir 1995).
Recent results have confirmed elevated excitatory amino acids in the cerebrospinal fluid of head‐injured patients (Zhang 2001). In this study, both glutamate and aspartate were measured by high performance liquid chromatography (HPLC). Findings showed statistically significant, higher concentrations of the amino acids in traumatic brain‐injured patients than controls, and that those with severe head injuries had statistically significant, higher concentrations than those mildly injured. These higher concentrations persisted in the cerebrospinal fluid for at least seven days in severely brain‐injured patients. Patients with poor outcomes at three months had higher concentrations of excitatory amino acids (only significant for glutamate). Another study used HPLC to measure glutamate and aspartate (from microdialysis probe sample) from 83 severely brain‐injured patients (Koura 1998). This study also found a correlation between excitatory amino acid concentration and six‐month patient outcome.
It has been postulated that, for any phase III traumatic brain injury trial to succeed, six theoretical requirements must be fulfilled (Bullock 1999). In brief, they are that: 1) the mechanism of cellular damage should have been demonstrated in human head injuries, as well as in animal models; 2) it should be demonstrated in animal models that the mechanism of cellular damage is blocked effectively by the drug and that the improvement is clinically relevant; 3) it should be demonstrated in brain injured humans that the drug is safe and tolerable; 4) the dose is effective in brain injured humans; 5) the drug is administered within the right time frame; 6) outcome measures should be sensitive to detect meaningful effects of the drug.Although results from animal studies and phase II trials have appeared promising, most phase III trials of excitatory amino acid inhibitors to date have not fulfilled one or more of these requirements. Further to those requirements, another possible explanation is that, due to small sample sizes, the trials lacked sufficient statistical power. These studies have also proposed an absolute improvement of 10% in favourable outcome, which may be too high. A smaller absolute improvement in this population might still be clinically significant, due to the number of people who suffer traumatic brain injury (Dickinson 2000). An alternative explanation for the apparent lack of efficacy may involve one or more of the following:
The extent of injury is rated on the Glasgow coma scale (GCS) from 3 (severe injury) to 15 (mild injury), therefore some patients will recover to pre‐injury levels and some will never recover, regardless of treatment (Teasdale 1999). Treatment should be directed to the injured population who will most benefit from the intervention.
That the brain‐injured patients are not homogeneous (Doppenberg 1997; Faden 1996; Maas 2000).
The targeted mechanism may not occur in all patients. It has been shown in severely brain‐injured patients that glutamate concentrations correspond with type of injury (Bullock 1998). Those patients with contusions, or who had hypoxaemic or ischaemic events, had higher levels of post‐injury glutamate in their dialysate than those patients who suffered subdural or epidural hematomas. This suggests the possibility that it may be difficult to show efficacy of a single neuroprotectant in the overall brain‐injured population.
There is insufficient information to investigate these last two possibilities. Due to the negative results of the completed phase III trials, the efficacy of excitatory amino acid inhibitors as a class has been questioned (Maas 2000). However, many trials demonstrate a non‐significant increase in favourable outcome and efficacy is not disproved (Maas 2000; Teasdale 1999). These explanations provide a rational basis for combining data across trials and performing a meta analysis.
Objectives
To assess the efficacy of excitatory amino acid inhibitors on improving patient outcome following traumatic brain injury. The primary outcome is the Glasgow outcome score (GOS) or disability rating scale (DRS) within the first year.
Methods
Criteria for considering studies for this review
Types of studies
All truly randomised, double‐blind, human trials where excitatory amino acid inhibitors were administered and compared to a control group were appraised. Any trial which fulfilled the above requirements and had a GOS or DRS within the first year post injury, was considered. It has not been shown whether EAA inhibitors are effective when given more than 24 hours after the time of injury. Studies where the drug was administered greater than 24 hours after injury were, therefore, excluded. This review was not restricted by language or status of publication (i.e. published/non‐published).
We compared EAA inhibitors versus placebo.
Types of participants
All patients clinically diagnosed as having mild, moderate or severe brain injury as rated by the GCS.
In future reviews (as data become available), a subgroup analysis will be undertaken:
between the different types of brain injury
with studies that involve patients who suffer multiple injuries
with studies that investigate children with traumatic brain injury.
Types of interventions
All types of excitatory amino acid inhibitors given within 24 hours of injury by i.v. administration, including agents that modify the release of EAAs, or block EAA receptors.
Drug classes included:
NMDA receptor blockers
AMPA/Kinate receptor blockers
metabotropic receptor blockers.
Types of outcome measures
GOS or DRS ‐ within the first year. (So far, most published studies have been of six months duration.) At present there does not seem to be an established methodology for comparing DRS with GOS, so we propose the conversion shown in Table 1.
1. Conversion for Glasgow outcome score (GOS) and disability rating scale (DRS).
| GOS | DRS |
| 5: good recovery | 0 none + 1 mild |
| 4: moderate disability | 2‐3 partial + 4‐6 moderate |
| 3: severe disability | 7‐11 moderately severe + 12‐16 severe + 17‐21 extremely severe |
| 2: persistent vegetative state | 22‐24 vegetative state + Extreme vegetative state |
| 1: death | death |
5 good recovery = 0 none + 1 mild 4 moderate disability = 2‐3 partial + 4‐6 moderate 3 severe disability = 7‐11 moderately severe + 12‐16 severe + 17‐21 extremely severe 2 persistent vegetative state= 22‐24 vegetative state + Extreme vegetative state 1 death = death.
These conversions are based on correlations between DRS and GOS reported by Choi 1998.
Drug associated adverse effects were noted.
Search methods for identification of studies
Electronic searches
We searched the following databases:
MEDLINE (1966 to 10th January 2003),
EMBASE (1966 to 10th January 2003),
CENTRAL (The Cochrane Library 2003, issue 1),
ClinicalTrials (http://clinicaltrials.gov) searched 10th January 2003,
Current Controlled Trials (http://controlled‐trials.com/mrct) searched 10th January 2003,
Investigational Drugs (until 8th May 2002).
The search strategy can be found in Appendix 1.
Searching other resources
Authors names, derived from published work, were used to search the Science Citation Index to uncover any previous trials in this field. References from within published works were also investigated.
The proceedings from the 'International conference of neuroprotective agents: Clinical and experimental aspects', Volumes 2‐5 were handsearched.
General searches of the Internet were conducted throughout the course of the data collection period (up until the 10th January 2003).
Authors of published works and pharmaceutical companies were contacted to request data from completed but unpublished studies. As yet, no additional information has been released to us regarding completed but unpublished trials.
Data collection and analysis
One reviewer (CW) located trials that were possibly relevant to the review. Two reviewers (CW and SL) independently applied the selection criteria. There was no disagreement regarding trials that were eligible for inclusion. The same two reviewers then independently assessed the included trials for methodological quality using the Jadad scale (Jadad 1996), with no disagreements, and extracted relevant data. The following information was recorded: number of patients in each treatment (drug vs placebo) group; outcome data for each group including mortality and favourable outcome. Favourable outcome is defined as good recovery or moderate disability on the Glasgow outcome scale. Where percentages were reported, original figures were calculated from those percentages. So that the studies are comparable, we used a dichotomized GOS at six months as the outcome. In the dichotomised scale, 'favourable outcome' includes 'good' and 'moderate disability' combined.
Results
Description of studies
Nine trials were identified in the literature search, that met the inclusion criteria. Two trials are ongoing (Dexanabinol phase III and magnesium salts). The seven completed trials describe six excitatory amino acid inhibitor compounds and involved over 2700 brain‐injured patients. Of the phase III trials, three (CP‐101,606; EAA 494; Eliprodil) were completed, while two trials (Selfotel, and Cerestat) were terminated early due to extrapolation of side effect profiles, or futility analysis.
NMDA antagonists
Selfotel (CGS 19755) Selfotel is a competitive glutamate antagonist. Trials with this compound have also been conducted in stroke patients. Two separate randomised, double‐blind, controlled phase III trials were conducted simultaneously with nearly identical protocols. The domestic study (protocol 008) had sites in the United States and Israel, while the international study (protocol 011) had sites in Europe, Canada, Australia and Argentina. A target sample size of 920 patients was selected (both trials) to demonstrate a 10% improvement (treatment compared with placebo) in the dichotomised GOS score, with 80% power. The number of patients recruited was 693. Patients were given 5mg/kg of Selfotel: four doses, 24 hours apart. (Selfotel Phase III).
Dexanabinol (HU‐211) Dexanabinol is a synthetic, non‐psychotropic cannabinoid. It acts as a non‐competitive NMDA receptor antagonist, as well as having antioxidant and anti‐inflammatory properties. A phase II placebo controlled, escalating dose study was completed, but was not powered for efficacy. Sixty‐seven patients were randomised to receive a single administration of dexanabinol (n = 30) or placebo (n = 37). Of those receiving dexanabinol, 10 people received 48mg of the drug and 20 people received 150mg. The results from both cohorts were then combined (HU 211 Phase II). A phase III multinational trial commenced in Europe and Israel in December 2000. This trial will involve approximately 30 centres. It is anticipated that several hundred patients with TBI will be enrolled.
EAA 494 (D‐CPPene) EAA 494 is a competitive NMDA antagonist. A double‐blind, placebo controlled phase III trial (the "SAPHIR" trial) was initiated, with 924 patients enrolled by November 1997 (EBIC News). Data were not available for inclusion.
Cerestat (CNS‐1102) Cerestat is a high affinity, non‐competitive NMDA receptor antagonist. A phase III trial was initiated in 1995. 512 patients were enrolled in this study. Those randomised to the treatment arm were initially given 15mg of drug, followed by 3 mg/h infusion over three days. The investigators were looking for a 12% improvement in favourable outcome when compared with placebo (EBIC News). Data were not available for inclusion.
CP‐101,606 CP‐101,606 is a postsynaptic NMDA antagonist which only interacts with receptors bearing the NR2B regulatory site. A phase II trial comparing drug versus placebo was completed and published, but did not include GOS scores. The primary objective was to assess the safety, pharmacokinetics and tolerability of CP‐101,606. 53 patients were recruited, 45 of whom had mild or moderate traumatic brain injury. Administration was by i.v. infusion for 2 hours, then either stopped, continued for 22 hours, or continued for 70 hours. GOS data is unable to be acquired at this time for inclusion (Merchant 1999). A double‐blind, placebo controlled phase III trial was concluded in early 2001. A paper describing the results of the trial is currently being prepared (personal communication).
Polyamine site antagonists
Eliprodil (SL82.0715) Eliprodil is a non‐competitive NMDA receptor antagonist. An international multi‐centre, randomised, double‐blind, phase III trial was completed in 452 severely brain‐injured patients comparing Eliprodil to placebo, but is unpublished. The primary objective was to evaluate the efficacy of Eliprodil on the improvement of functional status at six months post‐injury. Treatment lasted for 20 days. The first seven days involved the drug being administered intravenously while, for the following 13 days, the drug was administered orally or by nasogastric tube. The investigators were looking for a 15% improvement in favourable outcome when compared with placebo (Bolland 1998). Data were not available for inclusion in this review.
Mechanism of action yet to be described
Magnesium salts (ongoing) The NMDA receptor is blocked by the magnesium ion in a voltage‐dependent fashion. It is also thought that extracellular magnesium acts as a non‐competitive NMDA antagonist. Currently a phase III, double blind, randomised, placebo‐controlled, parallel design trial investigating magnesium sulfate is recruiting patients. For inclusion in the trial, patients must be 14 years and over, with an injury less than 8 hours old. The first dose of magnesium sulfate is 1meq/kg i.v., then a five‐day continuous i.v. infusion at 0.24meq/kg/h. Patient blood levels are to be approximately 4meq/L (ClinicalTrials db).
Risk of bias in included studies
All included trials of excitatory amino acid inhibitors are randomised, double‐blind trials that have compared product to placebo. Specifically, the Selfotel and HU 211 trials used a standard double‐blind format. Identical ampules were used for the treatment drug and placebo and blinding remained intact throughout the duration of the trial. For the remaining five trials, we were unable to confirm whether the allocation concealment was adequate and it has therefore been recorded as unclear.
Effects of interventions
Six excitatory amino acid inhibitors have been involved in seven completed phase II and III trials. Of these trials, three have been published. However one trial did not report the GOS data, although the authors have confirmed that the data had been collected.
Data were available for two of the seven relevant trials identified, involving 760 subjects in total. Mortality is similar between patients who receive excitatory amino acid inhibitors and those that receive placebo (OR 1.11, 95% CI 0.78 ‐ 1.60). Patients who have a favourable outcome six months after injury are also similar between those patients who receive treatment as compared to those who receive placebo (OR 0.86, 95% CI 0.64 ‐ 1.16).
In the Selfotel trial, nearly all patients experienced adverse events (335/339 of the Selfotel group and 353/354 of the placebo), which were reported as follows: intracranial hypertension, anaemia, pneumonia, hypotension, hypokalaemia, hypertension, hypothermia and agitation. Over 60% of mortality, in both groups, was related to central nervous system events, most frequent was intracranial hypertension.
The most common adverse events in the dexanabinol trial were, for both treatment and placebo groups: fever, anaemia, hypokalaemia, pneumonia and tachycardia. Most patients experienced adverse events. There were no statistically significant differences in frequency of adverse events, for any event, between the treatment and placebo groups. These results must be interpreted with caution, as the Selfotel trial was terminated before full recruitment was completed and the dexanabinol trial was not powered for efficacy. Both studies were therefore under powered.
No subgroup analyses were undertaken.
Discussion
Although, seven phase III trials were undertaken using excitatory amino acid inhibitors (two of which are still ongoing), only one has been published. This is disappointing, since the general results of three of the unpublished trials are public knowledge; that is, it is well known that the trials were ceased due to lack of efficacy. As discussed earlier, the reasons for this lack of efficacy may be due to small sample sizes, or that absolute improvement of 10% in favourable outcome may be too high. A smaller absolute improvement in this population might still be clinically significant due to the number of people who suffer traumatic brain injury and the severity of the clinical condition (Dickinson 2000). Also, futility analyses assess whether, if continued, a trial would reach statistical significance between placebo and treatment. Due to lowered recruitment, there is the potential to miss a clinically useful effect of a drug (Teasdale 1999).
Selfotel and Cerestat were also undergoing Phase III trials in stroke patients and, therefore, decisions to terminate the brain‐injury trial were somewhat influenced by the results from those trials. This is regrettable, as stroke patients and traumatic brain‐injured patients differ from one another in a number of aspects, including the biology of damage and patient age. TBI patients are often managed with ventilation and sedation and as such, some side effects which are relevant in stroke trials, such as behavioural events, are not problematic in TBI trials(Bullock 1999), and it is easier to reach therapeutic drug levels in TBI patients (Teasdale 1999).
As the investigators for five of the seven trials have not released their trial data, this review is not well placed to comment for or against the place of excitatory amino acid inhibitors in traumatic brain injury. The additional data is needed to determine effect sizes of treatments on brain injured patients. At this stage, there is not enough evidence to confirm or refute claims for the efficacy of excitatory amino acid inhibitors in moderating the damage caused by traumatic brain injury.
Authors' conclusions
Implications for practice.
Excitatory amino acid inhibitors are not registered for use in traumatic brain injury.
Implications for research.
Previous research needs to be published. As the public access knowledge of therapeutic failure is often known, sponsors need to provide data for analysis, regardless of their ability or desire to publish results. Transparency of previous research may lead to future improvements in drug formulation or trial design.
Further research in this area is warranted. The efficacy of trial products to date is not disproven. Therapeutic benefit does appear possible with this class of drug, given the non‐significant increase in favourable outcome of some trial products when compared with placebo.
Some modification of phase III entry and/or outcome criteria may be appropriate, for example:
better guidelines for phase III trials may need to be devised
better patient targeting (type of injury) and determining a 'clinically significant' favourable improvement outcome may need to be addressed
an absolute improvement of 10% in favourable outcome may be too high in this heterogeneous population; a smaller absolute improvement in this population might still be clinically significant, due to the number of people who suffer traumatic brain injury (Dickinson 2000).
TBI trials need to be conducted independently of stroke trial research, despite the initial similarities between the patient groups. Based on publications to date, there is evidence to suggest benefit in the separation of these protocols.
What's new
| Date | Event | Description |
|---|---|---|
| 19 April 2012 | Amended | An update of this review is in progress, and a new protocol for the update has been published: Gauden AJ, Pitt V, Gruen RL. Excitatory amino acid inhibitors for traumatic brain injury (Protocol). Cochrane Database of Systematic Reviews 2012, Issue 2. Art. No.: CD009661. DOI: 10.1002/14651858.CD009661. This version of the review will be withdrawn upon publication of the updated review. |
History
Protocol first published: Issue 1, 2003 Review first published: Issue 1, 2004
| Date | Event | Description |
|---|---|---|
| 8 September 2008 | Amended | Converted to new review format. |
Notes
An update of this review is in progress, and a new protocol for the update has been published: Gauden AJ, Pitt V, Gruen RL. Excitatory amino acid inhibitors for traumatic brain injury (Protocol). Cochrane Database of Systematic Reviews 2012, Issue 2. Art. No.: CD009661. DOI: 10.1002/14651858.CD009661.
This version of the review will be withdrawn upon publication of the updated review.
Appendices
Appendix 1. Search strategy
The electronic bibliographic databases MEDLINE and EMBASE were searched until 10th January 2003. Using (in combination and singularly) the following search terms:
brain damage head injur* brain injur* TBI traumatic brain injury
random* control* trial random* control* study control* clinical trial multicenter study systematic review* meta analys* placebo double‐blind RCT
EAA excitatory amino acid neuroprotect* neuroprotective agent NMDA n‐methyl‐D‐aspartate Antagon*
We also searched for individual drug names.
CENTRAL (The Cochrane Library 2003, issue 1) was searched using the terms 'excitatory amino acid', 'traumatic brain injury', 'head injury'.
The trial registers ClinicalTrials (http://clinicaltrials.gov) and Current Controlled Trials (http://controlled‐trials.com/mrct) were searched using the terms 'brain injury', 'head injury', 'NMDA' and 'excitatory amino acid'.
The Investigational Drugs database (IDdb3) was searched until the 8th May 2002, to find drugs in clinical testing. We looked under 'drug actions' and exploded all subheadings under "neurotransmitter modulator drugs". We also searched the 'drug' section using the terms 'head injury' and 'brain injury'. For both terms we exploded the sections 'drugs', 'meetings' and 'references'.
Data and analyses
Comparison 1. EAA inhibitors.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Mortality | 2 | 722 | Odds Ratio (M‐H, Random, 95% CI) | 1.11 [0.78, 1.60] |
| 2 Favourable outcome as determined by GOS or DRS | 2 | 722 | Odds Ratio (M‐H, Random, 95% CI) | 0.86 [0.64, 1.16] |
1.1. Analysis.
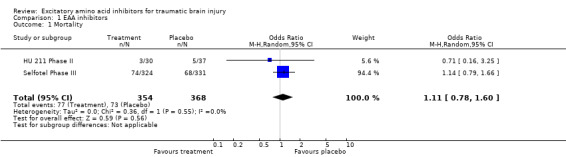
Comparison 1 EAA inhibitors, Outcome 1 Mortality.
1.2. Analysis.
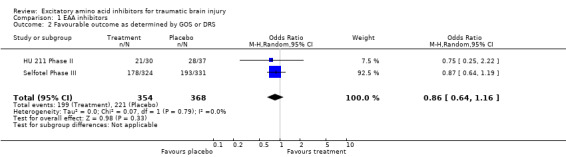
Comparison 1 EAA inhibitors, Outcome 2 Favourable outcome as determined by GOS or DRS.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Cerestat Phase III.
| Methods | Prospective, randomised, double blind, placebo controlled. | |
| Participants | 512 patients. | |
| Interventions | 15mg of Cerestat follwed by 3mg/h infusion over three days. | |
| Outcomes | 12% improvement in favourable outcome when compared with placebo. | |
| Notes | Trial stopped due to lack of efficacy and no data were released. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | Unclear |
CP 101,606 Phase II.
| Methods | Prospective, randomised, double blind, placebo controlled. | |
| Participants | 45 patients who had mild or moderate brain injury. | |
| Interventions | i.v. infusion for 2 hours which was then either stopped, or continued for 22 hours, or continued for 70 hours. | |
| Outcomes | Safety, pharmacokinetics and tolerability. | |
| Notes | GOS could not be accquired from investigators. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | Unclear |
CP 101,606 Phase III.
| Methods | Prospective, randomised, double blind, placebo controlled. | |
| Participants | ||
| Interventions | ||
| Outcomes | ||
| Notes | Results of the trial are currently being prepared for publication. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | Unclear |
EAA 494 Phase III.
| Methods | Prospective, randomised, double blind, placebo controlled. | |
| Participants | 924 patients enrolled. | |
| Interventions | ||
| Outcomes | ||
| Notes | No significant effect of treatment. Data not released. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | Unclear |
Eliprodil Phase III.
| Methods | Prospective, randomised, double blind, multi‐centre, placebo controlled. | |
| Participants | 452 patients with a severe brain injury. | |
| Interventions | Seven days of i.v. infusion followed by 13 days of oral administration or by naso‐gastric tube. | |
| Outcomes | 15% improvement in favourable outcome when compared with placebo. | |
| Notes | Data not released. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | Adequate |
HU 211 Phase II.
| Methods | Prospective, randomised, double blind, placebo controlled, multicenter, intention to treat. | |
| Participants | 67 patients with severe blunt head trauma who could be enrolled and treated within 6 hours of injury. | |
| Interventions | IV fast infusion (15 minutes) of 48mg or 150mg of drug in 1ml or 3ml vehicle. | |
| Outcomes | Primary: ICP, cardiovascular function, clinical laboratory tests and adverse medical events. Secondary: GOAT, GOS and DRS. | |
| Notes | Escalating dose study (sequential safe passage design). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | Adequate |
Selfotel Phase III.
| Methods | Prospective, multicentre, randomised, double blind, placebo controlled, between patient matched trial, intention to treat. Protocols 008 (Unitied States and Israel) and 011 (Europe, Canada, Australia, and Argentina) were combined in the main publication. | |
| Participants | 693 brain injuried patients with a GCS or 4‐8, at least one reactive pupil, abnormal CT scan. | |
| Interventions | i.v. infusion of 5mg/kg Selfotel once a day for four days, starting no later than 8 hours from time of injury. | |
| Outcomes | Primary: 6 month GOS scores supported by the DRS. Secondary: ICP and CPP during 1st week of hospitalisation and the 3‐month GOS AND DRS. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | Adequate |
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Cerestat Phase II | There were conflicting reports as to whether study was randomised and no mention that the study was double blind. |
| Gacyclidine | No evidence that the study was randomised. |
| Selfotel Phase II | No evidence that the study was randomised. |
Characteristics of ongoing studies [ordered by study ID]
Dexanabinol.
| Trial name or title | Multinational Phase III |
| Methods | |
| Participants | Approximately 800 (400 persons per group). |
| Interventions | Single dose of 150mg of drug or placebo within six hours of brain injury. |
| Outcomes | A 10% shift or more in dichotomised GOS. |
| Starting date | December 2000 |
| Contact information | |
| Notes |
Magnesium salts.
| Trial name or title | Phase III |
| Methods | |
| Participants | Participants must be 14 years and over with a brain injury less than 8 hours old. |
| Interventions | 1meq/kg i.v. of magnesium sulfate then a five day continuous i.v. infusion at 0.24meg/kg/h. |
| Outcomes | |
| Starting date | August 1999 |
| Contact information | |
| Notes |
Contributions of authors
NB and SL selected the topic. CW designed and wrote the protocol. NB and SL copyedited the protocol. CW screened records, obtained reports, chose included trials, extracted the data, quality assessed the trials, performed the analyses and wrote the review. SL chose included trials, extracted the data, quality assessed the trials and copyedited the review. NB was to be the adjudicator for any disagreements, but none occurred. NB copyedited the review.
Declarations of interest
None known
Edited (no change to conclusions)
References
References to studies included in this review
Cerestat Phase III {unpublished data only}
- EBIC News. European Brain Injury Consortium. Newsletter 5: Winter. http://www.brainit.gla.ac.uk/ebic/downloads/Newsletter%205.pdf 1997.
CP 101,606 Phase II {published data only}
- Merchant RE, Bullock MR, Carmack CA, Shah AK, Wilner KD, Ko G, Williams SA. A double‐blind, placebo‐controlled study of the safety, tolerability and pharmacokinetics of CP‐101,606 in patients with a mild or moderate traumatic brain injury. Annals of the New York Academy of Sciences 1999;890:42‐50. [DOI] [PubMed] [Google Scholar]
CP 101,606 Phase III {unpublished data only}
- Merchant RE. Personal communication.
EAA 494 Phase III {published data only (unpublished sought but not used)}
- EBIC News. European Brain Injury Consortium. Newsletter 5: Winter. http://www.brainit.gla.ac.uk/ebic/downloads/Newsletter%205.pdf 1997. [Google Scholar]
- Maas AIR. Assessment of agents for the treatment of head injury: Problems and pitfalls in trial design. CNS Drugs 2000;13(2):139‐54. [Google Scholar]
Eliprodil Phase III {published data only (unpublished sought but not used)}
- Bolland K, Sooriyarachchi MR, Whitehead J. Sample size review in a head injury trial with ordered categorical responses. Statistics in Medicine 1998;17:2835‐47. [DOI] [PubMed] [Google Scholar]
HU 211 Phase II {published data only}
- Knoller N, Levi L, Shoshan I, Reichenthal E, Razon N, Rappaport ZH, Biegon A. Dexanabinol (HU‐211) in the treatment of severe closed head injury: A randomized, placebo‐controlled, phase II clinical trial. Critical Care Medicine 2002;30(3):548‐54. [DOI] [PubMed] [Google Scholar]
- Pop E. Dexanabinol: Pharmos. Current Opinion in Investigational Drugs 2000;1(4):494‐503. [PubMed] [Google Scholar]
Selfotel Phase III {published data only}
- Ananda A, Morris GF, Juul N, Marshall SB, The Executive Committee of the International Selfotel Trial, Marshall LF. The frequency, antecedent events, and causal relationships of neurologic worsening following severe head injury. Acta Neurochirurgica 1999;73([Suppl]):99‐102. [DOI] [PubMed] [Google Scholar]
- Juul N, Morris GF, Marshall SB, The Executive Committee of the International Selfotel Trial, Marshall LF. Intracranial hypertension and cerebral perfusion pressure: influence on neurological deterioration and outcome in severe head injury. Journal of Neurosurgery 2000;92(1):1‐6. [DOI] [PubMed] [Google Scholar]
- Morris GF, Bullock R, Marshall SB, Marmarou A, Maas A, The Selfotel Investigators and Marshall LF. Failure of the competitive N‐methyl‐D‐aspartate antagonist Selfotel (CGS 19755) in the treatment of severe head injury: results of two Phase III clinical trials. Journal of Neurosurgery 1999;91:737‐43. [DOI] [PubMed] [Google Scholar]
- Morris GF, Juul N, Marshall SB, Benedict B, The Executive Committee of the International Selfotel Trial, Marshall LF. Neurological deterioration as a potential alternative endpoint in human clinical trials of experimental pharmacological agents for treatment of severe traumatic brain injuries. Neurosurgery 1998;43(6):1369‐74. [PubMed] [Google Scholar]
References to studies excluded from this review
Cerestat Phase II {unpublished data only}
- McBurney RN. Development of the NMDA ion‐channel blocker, aptiganel hydrochloride, as a neuroprotective agent for acute CNS injury. Neuroprotective agents and Cerebral Ischaemia. Internation Review of Neurobiolgy edition. Academic Press Limited, 1997; Vol. 40. [DOI] [PubMed]
- Teasdale G, Wagstaff A. Safety of extended administration to head injured patients of aptiganel HCL (Cerestat), A non‐competitive NMDA antagonist (Abstract). Journal of Neurology, Neurosurgery and Psychiatry. 1996:547‐8.
Gacyclidine {published data only}
- Mitha APA, Maynard KI. Gacyclidine. Current Opinion in Investigational Drugs 2001;2(6):814‐19. [PubMed] [Google Scholar]
Selfotel Phase II {unpublished data only}
- Bullock R, Marmarow A, Kotake A, Falleck H. Experience with the competitive NMDA antagonist CGS19755 (Selfotel) in severe human head injury: A Phase II study (Abstract). 3rd International Neurotrauma Symposium. 1995.
References to ongoing studies
Dexanabinol {published data only (unpublished sought but not used)}
- Multinational Phase III. Ongoing study December 2000.
Magnesium salts {published data only (unpublished sought but not used)}
- Phase III. Ongoing study August 1999.
Additional references
Blumbergs 1989
- Blumbergs P, Jones NR, North JB. Diffuse axonal injury in head trauma. Journal of Neurology Neurosurgery and Psychiatry 1989;52(7):838‐41. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bolland 1998
- Bolland K, Sooriyarachchi MR, Whitehead J. Sample size review in a head injury trial with ordered categorical responses. Statistics in Medicine 1998;17:2835‐47. [DOI] [PubMed] [Google Scholar]
Bullock 1995
- Bullock R, Marmarou A, Kotake A, Falleck H. Experience with the competitive NMDA antagonist CGS19755 (Selfotel) in severe human head injury: A Phase II study (Abstract). 3rd International Neurotrauma Symposium. 1995.
Bullock 1997
- Bullock R, Marshall L, Marmarou A, Faleck H, Kotake A. Failure of the competitive NMDA antagonist Selfotel (CGS 19755) to improve outcome after severe head injury: results of phase III trials (Abstract). Intercranial Pressure. 1997.
Bullock 1998
- Bullock R, Zauner A, Woodward JJ, Myseros J, Choi SC, Ward JD, Marmarou A, Young HF. Factors affecting excitatory amino acid release following severe human head injury. Journal of Neurosurgery 1998;89(4):507‐18. [DOI] [PubMed] [Google Scholar]
Bullock 1999
- Bullock MR, Lyeth BG, Muizelaar JP. Current status of neuroprotection trials for traumatic brain injury: Lessons from animal models and clinical studies. Neurosurgery 1999;45(2):207‐20. [DOI] [PubMed] [Google Scholar]
Choi 1998
- Choi SC, Marmarou A, Bullock R, Nichols JS, Wei X, Pitts LH and the ABIC study group. Primary end points in phase III clinical trials of severe head trauma: DRS versus GOS. Journal of Neurotrauma 1998;15(10):771‐6. [DOI] [PubMed] [Google Scholar]
ClinicalTrials db
- National Institutes of Health. ClinicalTrials. http://clinicaltrials.gov 2001. [Google Scholar]
Dickinson 2000
- Dickinson K, Bunn F, Wentz R, Edwards P, Roberts I. Size and quality of randomised controlled trials in head injury: review of published studies. BMJ 2000;320(7245):1308‐11. [DOI] [PMC free article] [PubMed] [Google Scholar]
Doppenberg 1997
- Doppenberg EMR, Choi SC, Bullock R. Clinical trials in traumatic brain injury: what can we learn from previous studies?. Annals of the New York Academy of Sciences 1997;825:305‐22. [DOI] [PubMed] [Google Scholar]
Duhaime 1994
- Duhaime AC. Exciting your neurons to death: Can we prevent cell loss after brain injury?. Pediatric Neurosurgery 1994;21(2):117‐23. [DOI] [PubMed] [Google Scholar]
EBIC News
- European Brain Injury Consortium. Newsletter 5: Winter. http://www.brainit.gla.ac.uk/ebic/downloads/Newsletter%205.pdf 1997. [Google Scholar]
Faden 1996
- Faden AI. Pharmacologic treatment of acute traumatic brain injury. Journal of the American Medical Association 1996;276(7):569‐70. [PubMed] [Google Scholar]
Gentile 1993
- Gentile NT, McIntosh TK. Antagonists of excitatory amino acids and endogenous opioid peptides in the treatment of experimental central nervous system injury. Annals of Emergency Medicine 1993;22(6):1028‐34. [DOI] [PubMed] [Google Scholar]
Hickenbottom 1998
- Hikenbottom MD, Grotta J. Neuroprotective therapy. Seminars in Neurology 1998;18(4):485‐92. [DOI] [PubMed] [Google Scholar]
IDdb3 database
- IDdb3. The investigational drugs database. http://www.iddb3.com 2002.
Jadad 1996
- Jadad AR, Moore RA, Carroll D, Jenkinson C, Reynolds DJM, Gavaghan DJ, McQuay HJ. Assessing the quality of reports of randomized clinical trials: Is blinding nesessary?. Controlled Clinical Trials 1996;17:1‐12. [DOI] [PubMed] [Google Scholar]
Koura 1998
- Koura SS, Doppenberg EMR, Marmarou A, Choi S, Young HF, Bullock R. Relationship between excitatory amino acid release and outcome after severe human head injury. Acta Neurochir 1998;71(Suppl):244‐6. [DOI] [PubMed] [Google Scholar]
Maas 2000
- Maas AIR. Assessment of agents for the treatment of head injury: Problems and pitfalls in trial design. CNS Drugs 2000;13(2):139‐54. [Google Scholar]
McBurney 1997
- McBurney RN. Development of the NMDA ion‐channel blocker, aptiganel hydrochloride, as a neuroprotective agent for acute CNS injury. In: Harris RA, Bradley RJ, Jenner P editor(s). Neuroprotective agents and Cerebral Ischaemia. International Review of Neurobiology. Vol. 40, Academic Press Limited, 1997:173‐95. [DOI] [PubMed] [Google Scholar]
Merchant 1999
- Merchant RE, Bullock MR, Carmack CA, Shah AK, Wilner KD, Ko G, Williams SA. A double‐blind, placebo‐controlled study of the safety, tolerability and pharmacokinetics of CP‐101,606 in patients with a mild or moderate traumatic brain injury. Annals of the New York Academy of Sciences 1999;890:42‐50. [DOI] [PubMed] [Google Scholar]
Mitha 2001
- Mitha APA, Maynard KI. Gacyclidine Beaufour‐Ipsen. Current Opinion in Investigational Drugs 2001;2(6):814‐9. [PubMed] [Google Scholar]
Muir 1995
- Muir KW, Lees KR. Clinical experience with excitatory amino acid antagonist drugs. Stroke 1995;26(3):503‐13. [DOI] [PubMed] [Google Scholar]
Murray 1996
- Murray CJL, Lopez AD. Global health statistics: a compendium of incidence, prevalence and mortality estimates for over 200 conditions. Boston: Harvard University Press, 1996. [Google Scholar]
Pop 2000
- Pop E. Dexanabinol Pharmos. Current Opinion in Investigational Drugs 2000;1(4):494‐503. [PubMed] [Google Scholar]
Teasdale 1996
- Teasdale G, Wagstaff A. Safety of extended administration to head injured patients of aptiganel HCl (Cerestat), A non‐competitive NMDA antagonist (Abstract). Journal of Neurology, Neurosurgery and Psychiatry. 1996:547‐8.
Teasdale 1999
- Teasdale GM, Maas A, Iannotti F, Ohman J, Unterberg A. Challenges in translating the efficacy of neuroprotective agents in experimental models into knowledge of clinical benefits in head injured patients. Acta Neurochir 1999;73(Suppl):111‐6. [DOI] [PubMed] [Google Scholar]
Zhang 2001
- Zhang H, Zhang X, Zhang T, Chen L. Excitatory amino acids in cerebrospinal fluid of patients with acute head injuries. Clinical Chemistry 2001;47(8):1458‐62. [PubMed] [Google Scholar]


