Abstract
Tuberculosis (TB), an infectious disease caused by Mycobacterium tuberculosis, is one of the top ten infectious diseases worldwide, and is the leading cause of morbidity from a single infectious agent. M. tuberculosis can cause infection in several species of animals in addition to humans as the natural hosts. Although animal models of TB disease cannot completely simulate the occurrence and development of human TB, they play an important role in studying the pathogenesis, immune responses, and pathological changes as well as for vaccine research. This review summarizes the commonly employed animal models, including mouse, guinea pig, rabbit, rat, goat, cattle, and nonhuman primates, and their characteristics as used in TB vaccine research, and provides a basis for selecting appropriate animal models according to specific research needs. Furthermore, some of the newest animal models used for TB vaccine research (such as humanized animal models, zebrafish, Drosophila, and amoeba) are introduced, and their characteristics and research progress are discussed.
1. Introduction
Tuberculosis (TB) is a major human infectious disease caused by a single organism, and was responsible for 1.6 million deaths, including human immunodeficiency virus (HIV)-associated TB deaths, with 10 million new TB cases diagnosed in 2017 worldwide [1]. The development of novel vaccines is considered a high priority in protecting human beings against TB disease worldwide. Currently, 22 new TB vaccines are being evaluated in clinical trials, four of which [Vaccae (Mycobacterium vaccae for injection) in patients with latent TB infection (LTBI), Mycobacterium indicus pranii (MIP)/Mw, Utilins (Mycobacterium phlei), and VPM1002 (rBCG ΔureC::hly)] have reached Phase III clinical trials [2–4]. Furthermore, three therapeutic vaccines [Vaccae, Utilins, and BCG Polysaccharide and Nucleic Acid Injection (BCG-PSN)] have obtained registration certificates from the China Food and Drug Administration (http://eng.sfda.gov.cn/WS03/CL0755/) and have been widely used to clinically treat TB in China [4]. In comparison with TB vaccines at the stage of clinical trials, there are many more vaccine candidates emerging in preclinical stages of development.
Promotion of the development of TB vaccines using humans as experimental subjects is fraught with challenges. Accumulation of clinical research is not only limited by time and space but also the several ethical and methodological restrictions of experiments with human subjects. The main advantage of an animal model is that it overcomes these deficiencies, and this essential role in the preclinical research of TB vaccines is receiving increasing attention. The superiority of using an animal model is mainly manifested in the following aspects: (1) the risks of experimentation on humans are avoided; (2) experimental conditions can be strictly controlled, and comparability of experimental materials is enhanced; (3) experimental operation and sample collection are simplified; and (4) a more comprehensive understanding of the nature of TB can be achieved.
Because of these advantages, various animal models have been generated for testing TB vaccines. However, the strategy of using animal models has begun to shift from an empirical-based approach to focus on the 3Rs principle (replacement, reduction, and refinement) [5]. Therefore, establishing methods to evaluate the immune protective efficiency and safety of TB vaccines using the smallest number of animals possible has become a scientific priority. Herein, we review the advantages and disadvantages of animal models, as well as clinical trials for TB vaccine research, and suggest that the goal of realizing a successful TB vaccine to the market stage is inseparable from the selection of appropriate animal models in preclinical testing.
2. Current Animal Models Used in TB Vaccine Research
Animal models are not only valuable for understanding the humoral and cellular immune responses against M. tuberculosis but are also essential to evaluate the safety, immunogenicity, and protective efficacy of TB vaccine candidates. The main animal models used in TB vaccine research according to a search of the PubMed database are schematically presented in Figure 1 and listed in Table 1. Each of these animal models has its own characteristics that make it suitable for studying candidate TB vaccines; therefore, the choice and utilization of animal models should depend on the purpose of the experiment, availability of space, stage of the vaccine, financial resources, trained staff, laboratory conditions, and other available resources (Table 1). In addition, pathological characteristics are the consequence of host-pathogen interactions mediated by immunologic responses; thus, these features are directly relevant to the strengths and limitations of the different models used in evaluating vaccine candidates. Previous studies have suggested that classical granulomas with similarity to those in humans could be observed in guinea pig, rabbit, rat, nonhuman primate (NHP), cattle, and goat animal models, but not in common mouse, fruit fly, and amoeba animal models (Table 1). In general, small animal models are used for large-scale screening of TB vaccines, such as mice, guinea pigs, rabbits, and zebrafish, which are not only economical but also readily available. Once a vaccine with good protective efficacy has been identified, it can be further evaluated in large animal models such as NHPs, which, although expensive, can more closely mimic the immune responses of humans to reliably test the protective efficacy of the potential TB vaccine. Furthermore, these animal models play key roles in evaluating the safety of vaccines, including mice for acute toxicity and drug distribution, monkeys for chronic toxicity, guinea pigs for skin allergic reactions, and rabbits for skin irritation.
Figure 1.
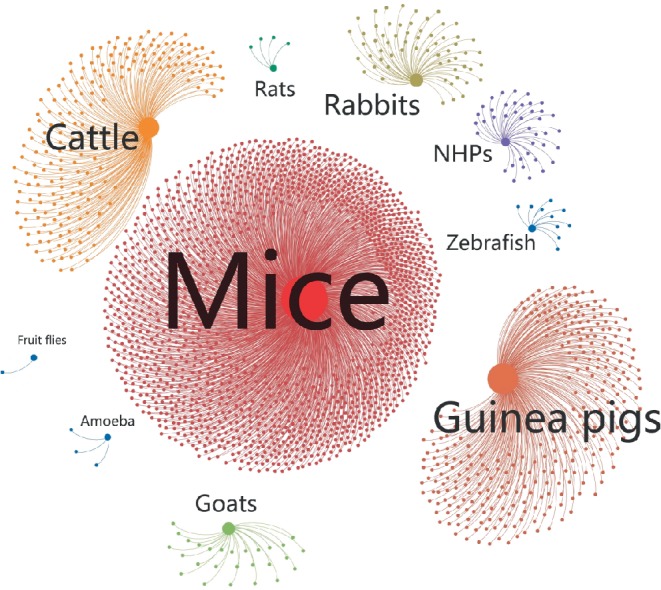
Statistical map of the utilization of different animal models in preclinical studies of TB vaccines. The source of the publications was an NCBI (National Center for Biotechnology Information) PubMed search using the keywords (vaccine AND tuberculosis AND ten categories shown in figure). The statistics were plotted using an open source graph visualization and manipulation software termed Gehpi. Each study is represented by a blue dot, and each animal model is represented by a circle of different color. The circle size represents the frequency of use of the animal model.
Table 1.
Main animal models used in TB vaccine research.
| Model kinds | Animal kinds | Mycobacterium | Granulomas (necrosis) | Advantages | Disadvantages | Main references |
|---|---|---|---|---|---|---|
| Small mammalian models | Mice | M. tuberculosis | No | Small and low cost | M. tuberculosis not a natural pathogen | [6–9] |
| Genetically tractable | No granulomas formation | |||||
| Mature immunological evaluation indexes | ||||||
| More abundant commercial reagents | ||||||
| Suitable for large-scale screening of vaccines and drugs | ||||||
| Guinea pigs | M. tuberculosis | Yes (+) | Highly susceptible to M. tuberculosis | Large and high cost | [10–13] | |
| Classical granulomas are similar to those in humans | Lack of reagents | |||||
| Suitable for vaccine and drug studies | Genetic manipulation difficult | |||||
| Rabbits | M. tuberculosis | Yes (+) | Highly susceptible to M. tuberculosis | Large and high cost | [14–20] | |
| M. bovis | Granulomas, liquefaction, and cavities are similar to those in humans | Lack of reagents | ||||
| Suitable for vaccine and drug studies | Genetic manipulation difficult | |||||
| Rats | M. tuberculosis | Yes (−) | Easy manipulation | Cannot mimic human lung pathological changes | [21–26] | |
| Low cost | Without caseous necrosis, fibrosis, calcification and cavitation | |||||
| Easy blood collection | ||||||
| Suitable for vaccine and drug studies | ||||||
|
| ||||||
| Large mammalian models | Cattle | M. bovis | Yes (±) | M. bovis is a natural pathogen | Large and high cost | [8, 27–30] |
| Granulomatous reactions and immune responses are similar to those in humans | Genetic manipulation difficult | |||||
| Secondary screening of TB vaccines | ||||||
| Availability of reagents | ||||||
| Goats | M. caprae | Yes (+) | M. caprae is a natural pathogen | Large and high cost | [31–33] | |
| Typical caseous necrotizing granulomas with liquefactive necrosis and cavities | Genetic manipulation difficult | |||||
| Vaccination studies | ||||||
| Nonhuman primates | M. tuberculosis | Yes (+) | Susceptible to M. tuberculosis | Large and high cost | [8, 30, 34, 35] | |
| Availability of reagents | Space requirements | |||||
| Disease pathology like human TB | Ethical concerns | |||||
| Small sample size | ||||||
|
| ||||||
| Invertebrate models | Zebrafish | M. marinum | Yes (−) | Disease progression and pathology like humans | Lack of cell lines | [7, 36–39] |
| Genetically tractable | Anatomy and physiology are unlike humans | |||||
| Suitable for large-scale screening of vaccines and drugs | ||||||
| Fruit flies | M. marinum | No | Innate immunity well conserved | Mycobacteria are not natural pathogens | [40–42] | |
| Wasting phenotype like human TB | No granulomas | |||||
| Genetically tractable | No adaptive immunity | |||||
M. marinum: Mycobacterium marinum, M. tuberculosis: Mycobacterium tuberculosis, M. bovis: Mycobacterium tuberculosis, M. caprae: Mycobacterium caprae, BCG: Bacille-Calmette–Guerin, TB: tuberculosis. +: Necrosis within granulomas, −: no necrosis within granulomas, ±: granulomas in cattle animal model were staged (I–IV) based on cellular composition and the presence or absence of necrosis and peripheral fibrosis.
2.1. Small Mammalian Models
Small mammals are the most widely used type of animal models in preclinical studies of TB vaccines for several reasons, including easy operation, easy access, clear genetic background, low cost, easy feeding, and more abundant commercial reagents. The most profound advantage of these models is their cost-effectiveness, allowing for numerous applications and detailed characterization. However, small mammalian animal models differ from humans with respect to genetics and immunology. Therefore, such models, especially murine models, are more suitable for screening candidate vaccines for TB on a large scale.
2.1.1. Mice
Mice have been the most widely used small animal model in the initial screening of TB vaccine candidates and for evaluating the efficacy of new vaccine candidates because of their low cost, rapid propagation, feasibility of use in the laboratory, long-term survival, mature immunological evaluation indices, and more abundant commercial reagents. The most popular mouse strains used for these purposes are BALB/c and C57BL/6, which both show variations in the susceptibility to infection of the M. tuberculosis H37Rv strain according to different challenge routes, with doses of tail vein injection, intraperitoneal injection, and aerosol attack of 1–5 × 105 colony-forming units (CFUs), 1 × 106 CFUs, and 0.5–1 × 102 CFUs [43, 44], respectively. Both of these mouse strains also show equivalent protective efficacy for evaluating the Bacillus Calmette–Guérin (BCG) vaccine (the current clinically used TB vaccine) [45]. Moreover, the differences in animal models and immunization routes will affect the protective response induced by vaccines. Stylianou et al. [46] reported that when BALB/c and C57BL/6 mice were primed with BCG and boosted 10 weeks later with ChAdOx1.PPE15 vaccine, followed by challenge with aerosolized M. tuberculosis, the booster ChAdOx1.PPE15 only improved the protection provided by BCG in C57BL/6 mice and not in BALB/c mice. A recent study compared the effects of different immunization routes [intranasal (i.n.), subcutaneous (s.c.), and intramuscular (i.m.)] on immune responses against the recombinant protein ESAT-6/CFP-10 of M. tuberculosis in a mouse model, and found that the titers of specific antibodies were quickly elevated in s.c. and i.m. immunized mice compared to those in i.m. immunized mice, whereas the i.n. immunized mice showed lower levels of interleukin (IL)-5 production [47]. Some previous studies also suggested that the BCG vaccine could induce similar immune responses and protection by rectal and parenteral immunization routes in BALB/c mice [48]; s.c. and i.n./oral immunization with Ag85A-Mtb32 exhibited the strongest boosting effects for BCG-primed systemic and pulmonary cell-mediated immunity responses in C57BL/6 mice [49], respectively. These results highlight the importance of considering differences between mouse models as well as immunization routes when evaluating TB vaccine in mice.
Interestingly, a growing number of studies have suggested that immunization with most BCG or recombinant BCG (rBCG) vaccines could induce a significantly strong Th1-type immune response, characterized by enhanced IgG2a/IgG1, IgG2b/IgG1, or IgG2c/IgG1 ratios, as well as a high expression level of Th1 cytokines [interferon (IFN)-γ, tumor necrosis factor (TNF)-α, and IL-2) in C57BL/6 or BALB/c mouse models [50–57]. Additionally, a previous study reported that immunization of a new recombinant BCG vaccine, rBCG-CMX (composed of immune-dominant epitopes from Ag85C, MPT51, and HspX), could present higher amounts of Th1, Th17, and polyfunctional specific T cells in a murine model [58]. In contrast, a small number of BCG or rBCG vaccines led to a relatively high Th2 response, as evidenced by the high IgG1/IgG2a ratio and the low IFN-γ levels in these murine models [59–62]. We suggest that the type of immune responses induced by BCG or rBCG vaccines might be dependent on the adjuvants, vaccine types, immunization routes, and immunization doses used in these mouse models.
A further advantage of mouse models is their ease for genetic manipulation. Recently, several immunodeficient and gene knockout mouse models, including severe combined immune deficiency (SCID) mice [63], C3HeB/FeJ mice (model of liquefactive necrosis and necrotic granulomas) [64, 65], CBA/J IL-10(−/−) mice (mature, fibrotic M. tuberculosis-containing pulmonary granulomas) [66], C57BL/6 RAG(−/−) mice (small and diffuse lesions, with the majority of the lung retaining the typical lacy alveolar appearance of normal lung tissue) [67], C57BL/6 IL-17(−/−) mice (less densely packed granulomas with mononuclear cells) [68], and iNOS knockout mice (granulomas similar to those that form in humans) [69], have been used to study particular immune responses to mycobacterial infections. However, accumulating evidence shows that M. tuberculosis infection could induce neither caseous granuloma nor central necrosis in the most widely used mouse models (except for C3HeB/FeJ mice) [70, 71], which was entirely different to the pattern observed in humans and guinea pigs [30]. Moreover, some mouse models have disadvantages for studying various stages of TB progression in human pathologies, including granuloma formation, liquefaction, cavity formation, and hematogenous spread of the disease [30, 72].
2.1.2. Guinea Pigs
Guinea pigs were first used for mycobacterial infection studies as a very useful animal model for lymphocyte proliferation assays, and for evaluating dermal reactivity, new TB vaccine candidates, and the capacity of naturally transmitted multidrug-resistant M. tuberculosis because of their high susceptibility to M. tuberculosis infection via the airways [73, 74]. Pathological lesions that form on the inside and outside of the lungs of guinea pigs infected with M. tuberculosis have been widely studied, offering fundamental insight into pulmonary TB in guinea pigs [75, 76]. We and others reported that distinct gross pathological tubercles could be observed in the spleen of guinea pigs infected by M. tuberculosis, which were not observed in mice (Figure 2), whereas slight gross pathological tubercles could be observed in the lungs of both guinea pigs and mice (Figure 2) [35]. In particular, guinea pigs can develop classical granulomas that are structurally similar to those in humans, and Langerhans giant cells that are formed from macrophages and epithelioid cells after mycobacterium infection have been observed [10].
Figure 2.
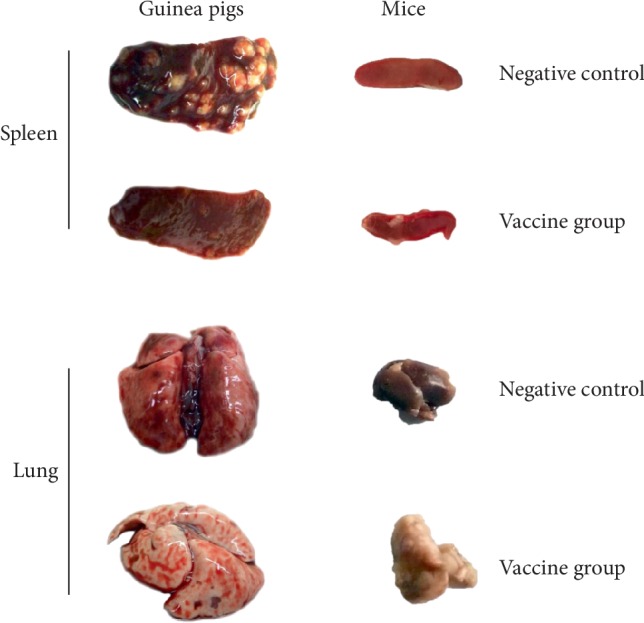
Tubercles of spleen or lung collected from guinea pigs or mice infected with M. tuberculosis H37Rv strain. BALB/c mice or guinea pigs were challenged with M. tuberculosis H37Rv strain (2 × 105 CFUs or 5 × 103 CFUs) to construct M. tuberculosis infected mouse or guinea pig TB model, respectively. After 3 days or 1 week, mice or guinea pigs were immunized intramuscularly three times at 2-weeks intervals with M. tuberculosis Ag85A/B chimeric DNA vaccine (vaccine group) or normal saline (negative control), respectively. Three weeks after last immunization, the mice or guinea pigs were sacrificed and their spleen and lung were collected to observe pathological lesions.
Furthermore, guinea pigs can be subsequently used to screen skin-test antigens, and to evaluate promising vaccines previously tested in a mouse model. A previous study also found that guinea pigs could be used as a long-term challenge model (with survival after 12 months) in assessment of TB vaccine efficacy [11]. Moreover, some vaccine candidates may be deemed to not be promising in the mouse model, but show satisfactory protection in guinea pigs as well as in humans. The immune responses of TB vaccines in guinea pigs have been studied by several methods such as antibody blocking, flow cytometry, bioassays, and microarray [12, 77], demonstrating that M. tuberculosis infection could initially activate responding T cells (mostly CD4 cells), which dramatically decreased in number 30 days after the infection and were gradually replaced by steadily increasing B cells and granulocytes [12]. Hiromatsu et al. [78] also found that immunization with the lipid antigens of mycobacteria induced a CD1-restricted immune response in guinea pigs. However, in comparison with the reagents available for other animal models, there are limited immunological reagents specific for this animal model available, which affects the utility of guinea pigs in the evaluation of TB vaccines. Therefore, there is an urgent need to develop specific immunological reagents for guinea pigs. Recently, a range of immunological reagents for guinea pigs have been developed, such as cloned guinea pig IL-17A cDNA and its recombinant protein [79], IL-10 cDNA and its recombinant protein [80], IL-4 cDNA [81], and IFN-γ cDNA [82].
2.1.3. Rabbits
Rabbit models were first widely used in molecular immunology, and have since been gradually replaced by rodents such as mice. However, rabbits are still an excellent animal model for human TB vaccine research because of the similar manifestations of lesions (granulomas, liquefaction, and cavities) to those observed in humans [14, 15]. In particular, rabbit models have been extensively used to screen and evaluate potential vaccine candidates (such as BCG, M. vaccae, M. microti and subunit vaccines), and to determine the pathogenic factors and pathogenesis of cavities induced by M. tuberculosis H37Rv infection [15–20, 83]. In addition, Tsenova et al. [84] reported large confluent granulomas with expansive areas of central necrosis in the lungs of rabbits infected with M. tuberculosis HN878 strain. Furthermore, a recent review article reported that infection of M. tuberculosis Erdman, M. tuberculosis H37Rv, and M. tuberculosis CDC1551 in New Zealand white rabbits resulted in different pulmonary pathologies, which indicated that the virulence of M. tuberculosis strains will determine the lesion severity in rabbit models [6]. Some recent studies suggested that a BCG-challenge rabbit skin model could be a valuable method for selecting therapeutic agents [20] and evaluating TB vaccines [19]. Collectively, these data suggest that rabbit animal models can be used not only for H37Rv strain infection but also for infection of other strains such as M. tuberculosis HN878, M. tuberculosis Erdman, M. tuberculosis CDC1551, and M. bovis, which provides new insights into the selection of animal models for evaluation of TB vaccines. Although guinea pigs and rabbits have many desirable features as models for TB, the high cost, lack of reagents, difficult gene manipulation, and ethical considerations involving these models often preclude their suitability for long-term survival studies [85].
2.1.4. Rats
Initially, it was widely believed that rats were insensitive to M. tuberculosis and that high doses of M. tuberculosis could neither kill rats nor induce typical TB pathological lesions and tuberculin susceptibility [86–88]. However, this view has changed. A large number of studies have found that rats are not only sensitive to M. tuberculosis but also show delayed hypersensitivity [89]. Compared with mice and guinea pigs, rats have several advantages as models, such as easy manipulation, relatively low cost, strong resistance, and easy blood collection [21]. Therefore, this animal model has been widely used in evaluating vaccine- or drug-induced resistance [22, 23], for determining anaerobic drug activity [90], estimating the efficacy of BCG vaccination [91], and discovering new TB drugs [21]. Previous studies have indicated that granulomatous lesions (which lack central necrosis) could be observed in the lungs, spleens, lymph nodes, and livers of M. tuberculosis-infected American cotton rats, Lewis rats, Wistar rats, and Sprague-Dawley rats [21, 24–26]. Interestingly, microelement deficiency (such as zinc) in the diet of rats could affect their humoral and cellular immune responses to BCG and ESAT-6/CFP-10 vaccination [92]. In addition to this limitation, similar to the situation with mice, the rat animal model has certain drawbacks, including not being able to mimic human pathological lung changes such as caseous necrosis, fibrosis, calcification, and cavitation.
2.2. Large Mammalian Models
Small mammalian animal models play important roles in the preliminary screening of new vaccine candidates. However, large mammalian animal models can effectively confirm the protective efficacy of the initially screened vaccines in systems that are more similar to humans. Additionally, compared to small mammalian models, large mammalian models are more like humans with respect to the genetic background and characteristics of immune responses; however, their disadvantages include few available commercial reagents, ethical limitations, high cost, and difficult genetic manipulation. As a rule, NHPs are always used in evaluating human TB vaccines, while other large mammalian animal models are usually used in testing animal TB vaccines.
2.2.1. NHPs
NHPs are naturally susceptible to M. tuberculosis and their use in vaccine and drug development has a long history. The biggest differences between NHPs and other animal models are the close evolutionary relationship with humans [8] and the quite similar pathology as well as disease condition between NHPs and human beings [34], which indicates that the immune responses of NHP models are very similar to those of humans. In infected monkeys, widespread caseous necrosis and liquefaction of the caseous material with cavity formation have been observed [30], along with granulomas containing giant cells with a similar structure to that of human lung granulomas [34]. It is widely accepted that improved TB vaccines should be able to avoid interfering with TB diagnoses such as the tuberculin skin test (TST), interferon-gamma release assay (IGRA), and GeneXpert. As early as 1998, an additional test called the PRIMAGAM-IFN-γ test was developed to distinguish TB disease among NHPs by detecting cellular immune responses to a purified protein derivative antigen via the IFN-γ concentration in whole-blood samples [93]. However, the reliability of the IFN-γ response to tuberculin antigen in cynomolgus macaques remains controversial [94]. Based on the immunological characteristics mentioned above, NHPs have become one of the best animal models for screening and evaluating improved TB vaccines with no interference with the diagnosis of TB.
To date, a large number of novel TB vaccines have been evaluated in NHP animal models by gastrointestinal or respiratory mucosal delivery, and the delivery method of vaccination appears to have an influence on the protective efficacy of TB vaccines in these models. Jeyanathan et al. [95] reported that respiratory mucosal boost immunization with AdHu5Ag85A vaccine could improve the protective efficacy and enhance the antigen-specific IFN-γ+ T cell responses in BCG-primed NHPs. IFN-γ is a cytokine that is critical for innate and adaptive immunity against mycobacterial infection. Another study demonstrated that the BCG vaccine induced multifunctional CD4+ T-cells producing IFN-γ and TNF-α, which are associated with reduced disease pathology following subsequent M. tuberculosis infection [96]. However, a previous study suggested that IFN-γ production was not a reliable correlate of immune protection for vaccination protocols and might be more relevant for active disease [97].
Although primates are more similar to humans with respect to genetic background, pathogenesis, clinical symptoms, and the immune mechanisms of TB, they are generally only used to test vaccine candidates that have been identified as promising during pre-screening in small animal models, because the use of NHPs is limited by ethical concerns, high cost, time consumption, enormous variance among individuals, lack of necessity for new drug approval, and space requirements [8, 35]. An additional challenge in using NHPs to test new vaccine candidates for improved performance compared to BCG is the potential for variable responses after BCG vaccination, depending on which NHP species is used [98]. Moreover, it is difficult to obtain statistically significant results from NHP animal models because of the typical small sample sizes, and large individual and genetic differences involved.
2.2.2. Cattle
Cattle are the natural host of M. bovis, and these infections are a major cause of economic losses and problems with animal welfare, along with a zoonotic risk, especially in developing countries [99]. BCG-vaccinated cattle always show a higher IFN-γ response, fewer lesions, and fewer bacilli per lesion [100, 101]. Compared with nonvaccinated cattle, the microscopically visible bacterial load, CD68+ macrophages, CD3+ T lymphocytes, WC1+γδ T cells, and CD4+ IFN-γ+ T cells were significantly reduced in lymph node granulomas [102, 103], and the expression of indoleamine 2,3-dioxygenase (considered to play an immunoregulatory role in the immune response to M. tuberculosis) was decreased in the granulomas of BCG-vaccinated cattle [27]. A more recent study showed that the protective efficacy of BCG in cattle gradually weakened, and the level of antigen-specific IFN-γ remained above baseline levels at two years post-vaccination [104]. Fortunately, this issue could be solved by BCG revaccination [105, 106], which supported the hypothesis that revaccination of BCG in humans might be effective in populations showing a negative response in the TST.
This model is also well-suited for the secondary screening of TB vaccines [28] and measuring elements of immune responses against mycobacteria [101]. Indeed, the cattle model has several advantages in TB vaccine research, including the fact that the clinical disease develops slowly, the granulomatous reactions and immune responses are similar to those observed in humans, and the possibility of vaccination involving neonatal calves [8, 29]. However, this model also has certain drawbacks, including high costs and absence of cavitations, which are seen in infected humans [30].
2.2.3. Goats
Goats can be naturally infected by Mycobacterium caprae or M. bovis [107] and are used to evaluate vaccine efficacy by differences in body weight, gross pathology, and bacterial loads. Indeed, the typical caseous necrotizing granulomas with liquefactive necrosis and cavities can be observed in the goat model infected with M. caprae [31], which is similar to that of active TB in humans. Recently, some studies have demonstrated that BCG vaccination of goats afforded a certain degree of protection against experimental challenge with M. bovis or M. caprae by reducing the volume of gross lung lesions and the bacterial loads in pulmonary lymph nodes, and increasing weight gain [32, 108, 109]. Interestingly, we found that the differences in BCG vaccination route might have an impact on the resulting immunoresponse characteristics. Accumulating data show that positivity to the single intradermal test and IGRA was observed in subcutaneously, intramuscularly [108, 110], or intranasally [111] vaccinated kid goats, but not in orally vaccinated goats [112]. These studies also indicated that the goat could be a more feasible model than cattle and NHPs because of its smaller size, lower cost, and caseous granulomatous and cavitary lesions that resemble those found in human TB patients [32, 33].
2.3. Invertebrate Models
Although mammals have been widely used as experimental animal models in TB vaccine development, recent studies on Mycobacterium marinum infection in invertebrates have offered valuable insight into strategies for developing novel animal models. Furthermore, invertebrate models show several benefits in terms of resources, costs, technical convenience, and ethical acceptance.
2.3.1. Zebrafish
Zebrafish (Danio rerio) can be naturally infected by M. marinum (a close relative of M. tuberculosis and the etiological agent of TB in humans), and is widely used as an animal model in vaccine research owing to its advantages of small size, easy reproduction, and low cost [36]. After infection by M. marinum, both adult zebrafish and larvae can form granulomas that are very similar to those observed in humans, and the innate and adaptive immune responses elicited against mycobacteria are composed of the same primary components found in humans [37–39]. In addition, the transparent characteristic of zebrafish larvae is also suitable for fluorescence imaging. Although zebrafishes are very different to humans in genetic terms, the above characteristics of this model have helped to bridge the gap between fish and humans. Data obtained from zebrafish studies have already shown that BCG vaccination, as well as DNA vaccination, can protect adult zebrafish from M. marinum infection by reducing both the mortality and bacterial counts in a manner dependent on the adaptive immune response and enhanced production of IFN-γ [38, 113]. In addition to its use for the preclinical screening of vaccines, the zebrafish model has been used in clarifying the mechanisms underlying granuloma formation [114]. Recently, several studies have indicated that this animal model provides a feasible tool for examining the mechanisms underlying reactivation in mycobacterial infections, and confirmed its suitability for the preclinical screening of TB vaccine candidates [38, 115–117]. However, a recent review indicated that the zebrafish model has significant differences in anatomy and physiology from those of humans [7], which warrant attention when using this animal model to evaluate TB vaccines.
2.3.2. Fruit Fly
The fruit fly Drosophila melanogaster is also a good model for studying the innate immune responses to M. marinum infection, understanding the physiological consequences of such infection and the associated immune responses, along with anti-mycobacterial drug discovery [41]. As an animal model for studying host-pathogen interactions, D. melanogaster has significant advantages such as being easy to breed and handle, strong fecundity, short generation time, low cost, technical convenience, ethical acceptability, and genetic amenability [40, 41]. D. melanogaster can be infected by M. marinum through anesthetizing with CO2 and injection in the abdomen using an individually calibrated pulled glass needle, as characterized by widespread tissue damage and low bacterial loads [118]. Additionally, a previous study suggested that M. marinum-infected D. melanogaster showed a diabetes-like state with reduced levels of circulating insulin or increased turnover of activated Akt [119]. These pathological characteristics are similar to those found in the early stages of M. marinum infection in fish [42]. Thus, this model may be valuable in testing interactions between the pathogen and the host. However, the drawback of this model is that the fruit fly can only be used to study innate immunity because of the absence of adaptive immunity; therefore, experimental results still need to be confirmed in mammals.
2.3.3. Amoeba
The amoeba species Dictyostelium discoideum is widely distributed in forest soil and can be infected by M. marinum, M. tuberculosis, and M. bovis [35, 120, 121]. D. discoideum has a haploid genome and a simple life cycle, which provides a genetically tractable single-cell model for studying conserved host–pathogen interactions [35]. As early as 2009, Soldati et al. [122] used D. discoideum as a genetically tractable host of M. tuberculosis and M. marinum, and discovered a conserved nonlytic spreading mechanism, in which pathogenic mycobacteria are ejected from the amoeba cell through the ejectosome, providing the opportunity for research into the spreading of tubercular mycobacteria infections in mammalian cells. Recently, the D. discoideum host model was developed to quantitatively monitor M. marinum growth, and to quantify the recruitment of host proteins to the bacterium-containing compartment [123, 124], assess the virulence of M. marinum, identify compounds inhibiting mycobacterial virulence [125], and recover new species of Mycobacteria from environmental and clinical specimens [126]. However, its application is limited, since it is single-cell model.
3. Lessons from Preclinical Experiments in Animal Models and Clinical Trials in Humans
The potential for a candidate vaccine to progress to the stage of efficacy evaluation in humans depends on the following main criteria: protection and safety in animal models, and safety as well as immunogenicity in Phase I/IIa clinical trials [127]. To date, BCG has been used as a “gold-standard” control vaccine to evaluate and compare the protection efficacy of new TB vaccine candidates in both preclinical animal models and clinical trials. Compared with BCG immunization in isolation, a good candidate TB vaccine should offer improvements in safety, immunogenicity, and protective efficacy (Table 2) [127]. A recent study showed that more than 85% of candidate drugs or vaccines that have passed preclinical testing failed in Phase I clinical trials [128]. Five well-known TB vaccine candidates that were successful in animal models but failed in clinical trials are recombinant BCG30 (rBCG30), AERAS-422, H1:LTK63, MVA85A, and SRL-172 (heat-killed M. vaccae) [3, 4, 129]. All five vaccines showed significant immunological protection and safety in animal models, but were terminated in clinical trials due to their poor protective efficacy and safety issues such as an antibiotic resistance gene in the case of rBCG30 [130], painful skin herpes for AERAS-422 [131], transient peripheral facial nerve palsies for H1:LTK63 [132], the absence of efficacy against TB for MVA85A [129], and technical issues for SRL-172 [133]. These data indicated that some negative results in terms of safety, immunogenicity, and protection efficacy were not observed in animal models. The following reasons were used to explain inconsistencies between animal preclinical data and clinical trials: (1) species differences between animal models and humans [134]; (2) differences in methodology between animal challenge experiments and natural infection in humans [134]; (3) fundamental differences in study schemes, protection efficacy definitions, and immunization strategies [135]; and (4) environmental differences such as environmental mycobacteria infection, BCG vaccination, and exposure level [135].
Table 2.
Comparison of safety, immunogenicity, and protective efficacy of current TB vaccines and BCG in preclinical and clinical trials.
| Status | Vaccine | Animal models or populations | Immunization route and dosea | Safety | Immunogenicity | Efficacy | Failure reasons | References or NCT Nob |
|---|---|---|---|---|---|---|---|---|
| Preclinical | VV-tPA-85B | Mice | Unknown | Unknown | IFN-γ two times higher | Less | NA | [136] |
| PcDNA3.1-Rv1769 or PcDNA3.1-Rv1772 | BALB/c mice | 50 μg DNA, s.c. | Unknown | Induce long-lasting Th1-type responses and CD8+ T-cell response | Unknown | NA | [137] | |
| rBCGΔais1/zmp1 | Guinea pig | 5 × 104 CFUs, s.c. | Safe | Stimulate high immunogenicity and strong memory immune responses | Improved BCG protection by 0.91 log10 | NA | [138] | |
| BCGΔBCG1419c | BALB/c mice | 2.5 × 103 CFUs, s.c. | Safe | A better activation of specific T-lymphocytes population | Similar | NA | [139] | |
| rBCG: CysVac2 | C57BL/6 mice | 5 × 105 CFUs, s.c. | Safe | Enhanced antigen-specific CD4+ T cell priming | Similar | NA | [140] | |
| rBCG-CMX | BALB/c mice | 1 × 106 CFUs, s.c. | Safe | Higher levels of CD4+IL−17+ and CD4+IFN-γ+T cells | Improved | NA | [58] | |
| ChAdOx1.PPE15 | C57BL/6 and BALB/c mice | 1 × 108 infectious units, i.n. or i.d. | Safe | More lung parenchymal CD4+ and CD8+ CXCR3+ KLRG1− T cells | Improved BCG protection by 0.52 log10 in C57bl/6 mice | NA | [46] | |
| BER opt | BALB/c mice | 100 μg DNA, i.m./EP | Safe | Induce surprisingly high frequencies of Ag85B tetramer+ CD8+ T cells and IFN-γ-secreting CD8+ T cells | Similar | NA | [141] | |
| MtbΔlpqS | Guinea pigs | 50–100 CFUs, respiratory | Safe | Expression of IFN-γ and IL-10 was lower in the lungs | Superior protection than BCG by 1 log10 | NA | [142] | |
|
| ||||||||
| Phase I | Ad5Ag85A | Both BCG-naïve and previously BCG-immunized healthy adults (24 participants) | 1 × 108 PFUs (low dose), 1 × 109 PFUs (high dose), i.m. | Well tolerated, no serious adverse effects | Markedly increased antigen-specific responses of both polyfunctional CD4+ and CD8+ T cells | NA | NA | NCT00800670, [143] |
| ChAdOx1.85A | Healthy BCG-vaccinated adults (42 participants) | Starter group (n = 6), 5 × 109 vp; Group A (n = 12), 2.5 × 1010 vp, Group B (n = 12), 2.5 × 1010 vp + 1 × 108 PFU of MVA85A, Group C (n = 12), 2.5 × 1010 vp + 1 × 108 PFU of MVA85A, i.m. | No data | No data | NA | NA | NCT01829490 | |
| H1:LTK63 | BCG vaccine-naïve healthy subjects (9 participants) | 100 µg H1 mixed with 30 µg LTK63, i.n. | Two volunteers experienced transient peripheral facial nerve palsies | No data | NA | Transient facial paralysis | NCT00440544, [132] | |
| rBCG30 | PPD−/HIV− healthy adults (35 participants) | 5 × 105 CFUs (n = 35), i.d. | Well tolerated, no serious adverse effects | Significantly improved antigen-specific T cell expansion capacity, IFN-γ secretion, and phenotypes of CD4+ and CD8+ T cells | NA | Potential danger from antibiotic resistance gene | [130, 144] | |
| AERAS-422 | HIV negative BCG naïve healthy adults (24 participants) | >105–<106 CFUs (low dose, n = 8),106–107 CFUs (high dose, n = 8), 1–8 × 105 CFUs (BCG, n = 8) i.d. | Unexpectedly, VZV reactivation (zoster) occurred in 2/8 volunteers | Stronger immune response in CD8+ T cells | NA | Painful skin herpes | [131, 145] | |
|
| ||||||||
| Phase IIa | H56:IC31 | HIV-negative, BCG-vaccinated adults (98 participants) | 3 dose levels: 5, 15, and 50 µg of H56 antigen with 500 nmol IC31, i.m. | Well tolerated, no serious adverse effects | Induced functional profiles of antigen-specific CD4 T cells | NA | NA | NCT01865487 [146] |
| ID93 + GLA-SE | HIV negative TB patients (60 participants) | 2 or 10 mcg ID93 + 2 mcg GLA-SE Vaccine (low dose) and 2 mcg ID93 + 5 mcg GLA-SE Vaccine (low dose), i.m. | No data | No data | NA | NA | NCT02465216 | |
| TB/FLU-04L | Unknown | Unknown | Ongoing | Ongoing | NA | NA | [1] | |
| MTBVAC | HIV unexposed, BCG naïve newborns (99 participants) | 2.5 × 104 CFUs (intermediate dose, n = 25), 2.5 × 105 CFUs (high dose, n = 25), 2.5 × 106 CFUs (highest dose, n = 25), BCG 2.5 × 105 CFUs (n = 24), i.d. | Well tolerated, no serious adverse effects | A greater frequency of polyfunctional CD4+ central memory T cells | NA | NA | [147], NCT03536117 | |
|
| ||||||||
| Phase IIb | DAR-901 booster | BCG-vaccinated, IGRA-negative healthy adolescents (650 participants) | 0.1 ml intradermal injection of 1 mg DAR-901 (n = 325), saline control (n = 325) | Ongoing | Ongoing | NA | NA | NCT02712424 |
| M72/AS01E | TB-naïve adults (n = 80), adults previously treated for TB (n = 49), and adults who have completed the intensive phase of TB treatment (n = 13), total 142 participants | Two doses of M72/AS01E (n = 71) or placebo (n = 71) and followed-up until six months post-dose 2 | Unexplained local reactions were observed, and further recruitment and vaccination in this study was discontinued | Stronger CD4+ T cell immune responses, rather than CD8+ T cell responses | NA | NA | NCT01424501 [148] | |
| MVA85A | BCG-vaccinated, HIV-negative healthy infants (2797 participants) | 1 × 108 PFUs MVA85A (n = 1399), placebo control (n = 1398), i.d. | Well tolerated, no serious adverse effects | Induced highly durable Th1 responses | Failed to improve BCG efficacy in infants | Unreasonable design, inappropriate study subjects, and too short observation time | NCT00953927 [129] | |
|
| ||||||||
| Phase III | VPM1002 | Pulmonary TB patients completed ATT and declared cured (2000 participants) | Single dose of VPM1002 (n = 1000), placebo control (n = 1000), i.d. | Safe, no serious adverse events | Stimulated multifunctional T cells producing IFN-γ or B cells producing antibodies | Ongoing | NA | [149], NCT03152903 |
| Vaccae™ | Cases whose skin tests of PPD are strongly positive (10000 participants) | One vial of Vaccae diluted with 1.0 ml sterile water, i.m., once every 2 weeks, 6 times totally | Safe and well-tolerated, no serious adverse events | Improved immunity and phagocytosis, and reduced pathological damage | TB incidence and degree of pathological changes of experimental group are lower than those of control group | NA | [150], NCT01979900 | |
| MIP/Mw | Cat II PTB patients (1020 participants) | 1 × 109 heat killed organisms followed 6 months later with a 2nd dose of 5 × 108 organisms | Safe, no serious adverse events | Higher IL-2 and IFN-gamma secretion | Significantly higher number of patients in the MIP group showing sputum culture conversion as early as 4 weeks after initiation of therapy | NA | NCT00265226, [2] | |
| SRL-172 (M. vaccae) | BCG-vaccinated, HIV-infected patients with CD4 cell counts of at least 200 cells/ml (1962 participants) | 0.1 ml M. vaccae (SRL-172, 1 mg, 109 CFUs, n = 983), placebo control (n = 979), i.d. | Safe, no adverse effect, and no increase in the rate of serious adverse events | SRL-172 immunization boostsIFN-γ and LPA responses to MV sonicate, and antibody responses to LAM | Protection was significant for the secondary endpoint of definite TB but not for probable TB | Technical reasons related to the method of production | NCT00052195 [3, 133] | |
as.c., i.n., i.d., i.m./EP: animal models were immunized subcutaneously, intranasally, intradermally, or by intramuscular electroporation, respectively.
bClinicalTrials.gov Identifier NCT number.
NA, not available; No data, data cannot be obtained from the public database.
Although there are some barriers in translating the results of animal models to clinical trials, animal models are still the most effective tool for testing the safety and efficacy of TB vaccines, and they are still widely used by researchers worldwide. Herein, we take VPM1002 and MVA85A as examples to review the preclinical studies in the context of human clinical trials. VPM1002 is a recombinant BCG vaccine in which the urease C gene has been replaced by the listeriolysin O (LLO) gene [4]. VPM1002 can secrete LLO to accelerate the transport of BCG-derived antigens into the cytosol and promote the apoptosis and xerophagy of host cells in vitro. A growing number of studies have shown that the protective efficiency and/or safety of VMP1002 were improved compared with those of BCG tested in mice, guinea pigs, rabbits, and NHPs [4, 151–153]. After extensive preclinical development, the safety and immunogenicity of VPM1002, in comparison with BCG, have been successfully evaluated in two Phase I clinical trials conducted in adults and infants in South Africa (NCT01113281) and Germany (NCT00749034) [154]. The results showed that VPM1002 was safe and immunogenic, which is consistent with two subsequent Phase II clinical trials carried out in HIV-exposed/unexposed newborn infants in South Africa (NCT02391415) [149], and in adults in Germany (NCT02371447). At present, a Phase II/III clinical trial is being conducted in India to assess the efficacy and safety of VPM1002 (NCT03152903). In contrast, previous studies reported that MVA85A, a booster vaccine, showed protection efficacy in animal models, but failed to show better protective efficacy than BCG in Phase II clinical trials, which might be attributed to the fact that the clinical trial design did not include the same route of administration as that used in mice where efficacy was observed [155]. In summary, the above comparison of animal and human data of VPM1002 and MVA85A vaccines suggests that preclinical results in animal models will be more predictive and consistent if the study design is optimized to more closely reflect the targeted effects of vaccines in clinical trials.
4. Current Challenges and Future Opportunities
Over 22 new TB vaccines have passed through animal experiments to evaluation in clinical trials. However, development and further evaluations of four TB vaccine candidates were terminated owing to their disappointing results after Phase I or II clinical trials. Why are these hidden dangers not found early in animal models, but only later on in human volunteers? The answer to this question is rather complicated, but the main reasons may be the lack of suitable animal models for TB vaccines, experimental design defects, vaccine adverse events, and lack of a complete understanding of host immunity to TB [127]. Although animal models are indispensable tools for human TB vaccine research, no animal model can fully mimic the real situation of human TB disease. Therefore, the experimental results of animal models are only an indirect indication, and the protective effects of the vaccine need to be verified by clinical trials. The following sections will discuss the challenges and opportunities related to the use of animal models in TB vaccine development.
4.1. Interactions Between the Host and M. tuberculosis are Still Unclear
Previous studies have indicated that innate immunity and adaptive immunity play critical roles in controlling M. tuberculosis infection in humans [4, 156, 157]. Thus, to develop a suitable animal model for TB vaccine development, it is important to first understand the interplay between M. tuberculosis and the host. At the early stage of M. tuberculosis infection, M. tuberculosis can be first recognized and controlled by the innate immune cells such as macrophages, dendritic cells, neutrophils, and natural killer cells via pattern recognition receptors, phagocytosis, inflammasome activation, reactive oxygen species, autophagy, apoptosis, and production of nonspecific cytokines and chemokines [158–162]. However, M. tuberculosis has a special ability to escape from the immune surveillance of these innate immune cells [163]. Fortunately, this innate immunity “negligence” is overcome by adaptive immunity, especially cellular immunity. Class I or Class II major histocompatibility complex molecules bridge the gap between innate immunity and adaptive immunity by presenting M. tuberculosis antigens to CD4+ T cells such as Th1 and Th17 cells, or CD8+ T cells [164, 165]. A growing number of studies have suggested that Th1 and Th17 cells play a central role in host protection by secreting IFN-γ, TNF-α, and IL-17 [4, 166–172]. However, disappointingly, some vaccines have good immune protection and safety in animal models, but unexpected safety issues still arise in clinical trials. The reasons behind this variability in protective efficacy and safety are largely unknown, but we hypothesize that the differences could be due to differences in immune system biology between mice, NHPs, and humans.
4.2. Immunization Strategies Should Be Optimized Based on Different Animal Models
No clinical studies have established immunologic requirements for protection against TB. Despite endless immunologic observations, in the absence of controlled trials comparing immunologic responses among successful and unsuccessful vaccines (or controls), these observations do not meet established vaccine requirements. For this reason, there is increasing acknowledgement that it is problematic to extrapolate the findings from “successful” animal studies to clinical efficacy. To overcome this problem, immunization strategies should be optimized and improved. Currently, three immunization strategies are used in the development of new TB vaccine candidates, including an immunotherapy strategy, prime strategy, and BCG prime-boost strategy [173], and the TB vaccine candidates in clinical development can be divided into two groups: BCG replacements and BCG boosters [174]. This issue has also been explicitly addressed in recent World Health Organization position papers (https://apps.who.int/iris/handle/10665/273089) and the general conclusion is that the most efficient and cost-effective approach will be a BCG booster vaccine. BCG was used for TB prevention as early as 1921, and since then many clinical trials conducted worldwide have evaluated the efficacy of BCG in preventing TB. These tests have shown that BCG can continue to protect children from TB meningitis and disseminated TB [4]. However, a large number of studies have also shown that the protective effect of a BCG vaccine varies in different regions [175]. In addition, a large randomized controlled trial in Brazil showed that revaccinating BCG at adolescence did not improve the protective efficacy of BCG vaccination at birth [176]. Our recent study found that the main TB vaccine immunization strategy is BCG for primary immunization, followed by selection of appropriate subunit vaccines for boosting immunization [4], which is consistent with previous studies [177–179]. Therefore, we strongly recommend that further TB vaccine research should focus on a BCG booster vaccine, and animal models will provide an opportunity for conducting preclinical studies to demonstrate the protective efficacy of booster vaccines.
4.3. M. tuberculosis/HIV Co-Infection Has Become a Major Barrier for Fighting TB with Limited Appropriate Animal Models
LTBI is a condition characterized by a persistent immune response to stimulation by M. tuberculosis antigens without evidence of clinical manifestations of active TB [1]. However, when the immunity of patients with LTBI is weakened, the possibility of LTBI transforming into TB is greatly increased. Unfortunately, the decreased CD4+ T cell level of HIV patients provides an opportunity for LTBI reactivation to active TB [180]. According to published data, people infected with HIV are 16–27-times more likely to develop TB than healthy people, and HIV co-infection in individuals with LTBI enhances the risk of developing active TB from 10% over a lifetime to 10% per year [181]. Compounding this situation is the unique increased susceptibility of this population to any mycobacterial infection, which poses extraordinary challenges to the use of any live TB vaccines. Therefore, there is an urgent need to establish appropriate animal models to evaluate M. tuberculosis/HIV co-infection vaccines. Although HIV does not cause disease in rodents and NHPs, complementary mouse models and simian immunodeficiency virus (SIV; a retrovirus causing immunodeficiency similar to AIDS in Asian macaques) macaque models have been used for studies on M. tuberculosis/HIV co-infection [180]. Previous studies have reported two kinds of complementary mouse models, including a humanized mouse model and HIV transgenic mouse model. The first humanized mice were generated by reconstituting the immune system of immunodeficient mice using human hematopoietic progenitor cells (CD34+) from human cord blood [182]. The second one is a bone marrow, liver, thymic (BLT) mouse model in which NOD/scid-IL-2Rgammacnull mice are engrafted with human lymphoid tissue after CD34+ hematopoietic stem cell reconstitution [183]. These humanized mice gained human immunity by producing more proper humanized T cells, and have been used to evaluate new approaches for the prevention or treatment of HIV and/or M. tuberculosis infection [184–186]. An HIV transgenic mouse model was generated by incorporating the entire viral genome of HIV, which has been used to study the effect of M. tuberculosis infection on the induction of HIV gene expression [187]. In addition, a recent study found that NHPs could be infected by SIV, and SIV-infected macaques have been used as a model for AIDS and TB [188].
4.4. New Technologies and Tools Open New Avenues for the Use of Animal Models in TB Vaccine Research
The traditional use of animal models for vaccine research follows the conventional testing route through mouse models, then into guinea pigs or rabbits, which may be followed by testing in NHPs before moving to humans. However, traditional research methods are somewhat insufficient for the dynamic study of living experimental animals. Therefore, new technologies and tools are needed to observe the physiological, biochemical, and pathological changes in these living animal models, which will accelerate the development of TB vaccine research. Fortunately, new technologies and equipment have been employed in studies of animal models of TB and other diseases, including fluorescence microscopy to detect infection by M. marinum; robotic injection technology used in zebrafish embryos for high-throughput screening in disease models, which can greatly improve the injection efficiency and accuracy, and reduce errors caused by manual operation [189]; fluorescence-based methods for serial quantitative assessments of drug efficacy and toxicity [190]; photodynamic therapy technology in the treatment of localized mycobacterial infections such as pulmonary granulomas and cavities [191]; a three-dimensional granuloma model for studying bacterial-host interactions, drug-susceptibility, and resuscitation of dormant mycobacteria [192]; a small animal SPECT/PET/CT system for real-time dynamic observation of living animals, and for recording pathological changes, which can not only be more convenient and allow for objective evaluation of vaccine efficiency but also reduces the number of animals required as well as the impact of individual differences on vaccine evaluation [193]; and differentiating infected from vaccinated animals (DIVA) reagents in the skin test and IFN-γ assay in cattle and goats to differentiate TB-infected from vaccinated animals [194, 195]. Moreover, targeted genome editing technology has become a hot research area in animal models. Specifically, CRISPR/Cas9 technology, which greatly improves the efficiency of constructing gene-targeted animal models, has been widely used to construct genetically modified mouse models such as knockout/knockin models, and somatic cell genome-editing models [196].
4.5. Humanized Transgenic Animal Models Bring New Hope for TB Vaccine Research
TB vaccine candidates cannot be tested directly in human beings for ethical and safety reasons. Thus, humanized animal models could be useful to bridge the gap between preclinical and clinical studies, and to gain relevant insight into the determinants of TB vaccine development. Humanized mice (defined as mice engrafted with functional human genes, cells, or tissues) have become an essential tool in validating the results of infectious disease research in recent years because of their small size, easy access, low cost, clear genetic background, and easy manipulation. To date, large numbers of human cells or tissues have been engrafted in mouse models, including immune system components, hepatocytes, skin tissue, pancreatic islets, uterine endometrium, and neural cells [197]. Recently, some new humanized mouse models have been developed to identify potential TB or other vaccine candidates, including humanized NOD/shi-scid/γcnull (NOG) mice [198], NOD/SCID/γcnull (NSG) mice engrafted with human fetal liver and thymus tissues, and CD34+cells [199], DRAG mice (NSG mice transgenic for human DR4, RRID:IMSR_JAX:017914) [200], HSC-engrafted NSG mice [201], HLA-A2 transgenic NSG-BLT mice [201], and NOD.Cg-Prkdcscid Il2rgtm1Wjl/SzJ mice [202]. As early as 2013, a BLT-humanized mouse model was developed to evaluate its feasibility as a model for experimental TB, demonstrating human T cells in the lung, liver, and spleen, and the formation of granuloma lesions with central necrosis and cholesterol crystals in the lung lesions [199]. Recently, a study compared the ability of the BCG vaccine to affect the immune response to infection with M. tuberculosis in C57BL/6 mice, Hartley guinea pigs, and humanized NOG mouse models, and the results indicated that the BCG vaccine could induce a human T cell response in the humanized NOG mouse model but not in the C57BL/6 mouse and Hartley guinea pig models [198]. Although the use of humanized animal models is limited by some shortcomings such as high cost, slow reproduction, and strict feeding conditions, the development of more novel humanized animal models will be important to create a crucial pre-clinical platform for evaluating the protective efficacy of TB vaccines, and for screening antigens, epitopes, and targets of TB vaccines.
4.6. Transmission of M. tuberculosis Infection among Different Animal Models Needs to be Considered
For thousands of years, animals have become important hosts of M. tuberculosis because of the close relationship between animals and the primary host human beings via coughing, sneezing, expectoration, or contaminated food [203–205]. We previously reported an infectious disease among 84 rhesus macaques at a Chinese zoo, and determined that the potential pathogen of this outbreak was likely M. tuberculosis [157]; the source of the infection may have been inhalation of airborne droplet nuclei from visitors infected with M. tuberculosis. In another study, Cadmus et al. [206] confirmed M. tuberculosis as the cause of pulmonary TB among livestock workers in southwestern Nigeria, and the mycobacteria pathogens likely originated from infected animals. M. tuberculosis can be transmitted not only between humans and animals but also between animals through similar transmission routes found in humans. Ramos et al. [207] reported that when W-Beijing M. tuberculosis HN878 infected or unchallenged minipigs were housed together, active M. tuberculosis could be identified in the pulmonary and thoracic lymph nodes from both infected and unchallenged minipigs. Furthermore, it is gradually becoming recognized that vaccination of domestic animals may be an alternative long-term strategy for controlling the transmission of TB [208].
5. Conclusion
Development of novel TB vaccines is urgently needed to fight TB disease. The animal models commonly used in human TB vaccine research include mice, guinea pigs, rabbits, rats, and NHPs, along with cattle, goats, and zebrafish in animal TB vaccine research. Along with recent developments in genetics, immunology, and molecular biology, some novel animal models have been introduced into TB vaccine research, such as fruit flies, amoeba, and humanized mouse models. The usage of humanized mouse models could overcome the disadvantages of NHPs and other animal models. In particular, the BLT humanized mouse has facilitated pioneering studies of TB pathogenesis, pathology, and vaccines [197, 199, 201, 209]. Although the cost of BLT mice is higher than that of small mammalian animal models such as mice, guinea pigs, and rabbits, the expense of BLT mice is considerably lower than that of NHPs. Therefore, the immune protective efficacy of TB vaccine candidates could be evaluated in humanized mouse models, which could shorten the research process and reduce costs [210]. Furthermore, the lack of protective biomarkers and understanding of the detailed host–pathogen relationship are the main obstacles hindering the evaluation of TB vaccines in animal models [211]. A recent review recommended some protective biomarkers such as survival, cytokines, pulmonary pathology, and bacterial load [6]. Therefore, these should be firmly integrated into future TB research, which will make the evaluation of a TB vaccine in animal models more diversified and objective, and will provide novel opportunities for the discovery of new TB vaccines.
Acknowledgments
This study was supported by National Natural Science Foundation of China under Grant 81801643, National Key Program for Infectious Disease of China under Grant 2018ZX10731301-005, Beijing Municipal Science & Technology Commission under Grant Z181100001718005, and Medical Science and Technology Youth Cultivation Program of PLA under Grant 16QNP075.
Conflicts of Interest
The authors do not have commercial or other associations that might pose a conflict of interest.
Authors' Contributions
Wenping Gong wrote the main manuscript text, Xueqiong Wu reviewed and revised the paper, and Yan Liang prepared Figures 1 and 2.
References
- 1.WHO. Global Tuberculosis Report 2018. Geneva: World Health Organization; 2018. pp. 1–243. [Google Scholar]
- 2.Sharma S. K., Katoch K., Sarin R., et al. Efficacy and safety of Mycobacterium indicus pranii as an adjunct therapy in category II pulmonary tuberculosis in a randomized trial. Scientific Reports. 2017;7(1):p. 3354. doi: 10.1038/s41598-017-03514-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.von Reyn C. F., Mtei L., Arbeit R. D., et al. Prevention of tuberculosis in Bacille Calmette–Guerin-primed, HIV-infected adults boosted with an inactivated whole-cell mycobacterial vaccine. AIDS. 2010;24(5):675–685. doi: 10.1097/QAD.0b013e3283350f1b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gong W., Liang Y., Wu X. The current status, challenges, and future developments of new tuberculosis vaccines. Human Vaccines & Immunotherapeutics. 2018;14(7):1697–1716. doi: 10.1080/21645515.2018.1458806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tanner R., McShane H. Replacing, reducing and refining the use of animals in tuberculosis vaccine research. ALTEX. 2017;34(1):157–166. doi: 10.14573/altex.1607281. [DOI] [PubMed] [Google Scholar]
- 6.Mantilla Galindo A., Ocampo M., Patarroyo M. A. Experimental models used in evaluating anti-tuberculosis vaccines: the latest advances in the field. Expert Review of Vaccines. 2019;18(4):365–377. doi: 10.1080/14760584.2019.1583558. [DOI] [PubMed] [Google Scholar]
- 7.Singh A. K., Gupta U. D. Animal models of tuberculosis: lesson learnt. Indian Journal of Medical Research. 2018;147(5):456–463. doi: 10.4103/ijmr.IJMR_554_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cardona P. J., Williams A. Experimental animal modelling for TB vaccine development. International Journal of Infectious Diseases. 2017;56:268–273. doi: 10.1016/j.ijid.2017.01.030. [DOI] [PubMed] [Google Scholar]
- 9.Fonseca K. L., Rodrigues P. N. S., Olsson I. A. S., Saraiva M. Experimental study of tuberculosis: from animal models to complex cell systems and organoids. PLoS Pathogens. 2017;13(8):p. e1006421. doi: 10.1371/journal.ppat.1006421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liu J. Tuberculosis and the tubercle bacillus. Emerging Infectious Diseases. 2005;11(8):1331–1331. doi: 10.3201/eid1108.050611. [DOI] [Google Scholar]
- 11.Horwitz M. A., Harth G. A new vaccine against tuberculosis affords greater survival after challenge than the current vaccine in the guinea pig model of pulmonary tuberculosis. Infection and Immunity. 2003;71(4):1672–1679. doi: 10.1128/iai.71.4.1672-1679.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ordway D., Palanisamy G., Henao-Tamayo M., et al. The cellular immune response to Mycobacterium tuberculosis infection in the guinea pig. Journal of Immunology. 2007;179(4):2532–2541. doi: 10.4049/jimmunol.179.4.2532. [DOI] [PubMed] [Google Scholar]
- 13.Clark S., Hall Y., Williams A. Animal models of tuberculosis: guinea pigs. Cold Spring Harbor Perspectives in Medicine. 2015;5(5):p. a018572. doi: 10.1101/cshperspect.a018572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dannenberg Jr. A. M. Pathogenesis of pulmonary Mycobacterium bovis infection: basic principles established by the rabbit model. Tuberculosis. 2001;81(1–2):87–96. doi: 10.1054/tube.2000.0260. [DOI] [PubMed] [Google Scholar]
- 15.Dannenberg Jr. A. M. Liquefaction and cavity formation in pulmonary TB: a simple method in rabbit skin to test inhibitors. Tuberculosis. 2009;89(4):243–247. doi: 10.1016/j.tube.2009.05.006. [DOI] [PubMed] [Google Scholar]
- 16.Bishai W. R., Dannenberg Jr. A. M., Parrish N., et al. Virulence of Mycobacterium tuberculosis CDC1551 and H37Rv in rabbits evaluated by Lurie’s pulmonary tubercle count method. Infection and Immunity. 1999;67(9):4931–4934. doi: 10.1128/iai.67.9.4931-4934.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tsenova L., Sokol K., Freedman V. H., Kaplan G. A combination of thalidomide plus antibiotics protects rabbits from mycobacterial meningitis-associated death. The Journal of Infectious Diseases. 1998;177(6):1563–1572. doi: 10.1086/515327. [DOI] [PubMed] [Google Scholar]
- 18.Dorman S. E., Hatem C. L., Tyagi S., et al. Susceptibility to tuberculosis: clues from studies with inbred and outbred New Zealand White rabbits. Infection and Immunity. 2004;72(3):1700–1705. doi: 10.1128/iai.72.3.1700-1705.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen H., Liu X., Ma X., et al. A new rabbit-skin model to evaluate protective efficacy of tuberculosis vaccines. Frontiers in Microbiology. 2017;8:p. 842. doi: 10.3389/fmicb.2017.00842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sun H., Ma X., Zhang G., et al. Effects of immunomodulators on liquefaction and ulceration in the rabbit skin model of tuberculosis. Tuberculosis. 2012;92(4):345–350. doi: 10.1016/j.tube.2012.03.005. [DOI] [PubMed] [Google Scholar]
- 21.Singhal A., Aliouat E. M., Herve M., et al. Experimental tuberculosis in the wistar rat: a model for protective immunity and control of infection. PLoS One. 2011;6(4):p. e18632. doi: 10.1371/journal.pone.0018632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McFarland C. T., Ly L., Jeevan A., et al. BCG vaccination in the cotton rat (Sigmodon hispidus) infected by the pulmonary route with virulent Mycobacterium tuberculosis. Tuberculosis. 2010;90(4):262–267. doi: 10.1016/j.tube.2010.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kumar N., Vishwas K. G., Kumar M., et al. Pharmacokinetics and dose response of anti-TB drugs in rat infection model of tuberculosis. Tuberculosis. 2014;94(3):282–286. doi: 10.1016/j.tube.2014.02.004. [DOI] [PubMed] [Google Scholar]
- 24.Elwood R. L., Wilson S., Blanco J. C., et al. The American cotton rat: a novel model for pulmonary tuberculosis. Tuberculosis. 2007;87(2):145–154. doi: 10.1016/j.tube.2006.07.001. [DOI] [PubMed] [Google Scholar]
- 25.Sugawara I., Yamada H., Mizuno S. Pathological and immunological profiles of rat tuberculosis. International Journal of Experimental Pathology. 2004;85(3):125–134. doi: 10.1111/j.0959-9673.2004.00379.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Huang C. W., Feng Q. X., Wang F. H., Li Z. B., Lin S. M., Zhang C. S. Effect of different pathways on establishment of experimental tuberculosis in SD rats. International Journal of Respiration. 2011;8:588–591. [Google Scholar]
- 27.Garcia-Jimenez W. L., Villarreal-Ramos B., Grainger D., Hewisnon R. G., Vordermeier H. M., Salguero F. J. The expression of Indoleamine 2, 3-dioxygenase (IDO) is reduced in granulomas from BCG vaccinated cattle compared to granulomas from unvaccinated controls after experimental challenge with Mycobacterium bovis. Veterinary Immunology and Immunopathology. 2018;203:52–56. doi: 10.1016/j.vetimm.2018.08.002. [DOI] [PubMed] [Google Scholar]
- 28.Buddle B. M., Skinner M. A., Wedlock D. N., de Lisle G. W., Vordermeier H. M., Glyn Hewinson R. Cattle as a model for development of vaccines against human tuberculosis. Tuberculosis. 2005;85(1–2):19–24. doi: 10.1016/j.tube.2004.09.003. [DOI] [PubMed] [Google Scholar]
- 29.Siddiqui N., Price S., Hope J. BCG vaccination of neonatal calves: potential roles for innate immune cells in the induction of protective immunity. Comparative Immunology, Microbiology and Infectious Diseases. 2012;35(3):219–226. doi: 10.1016/j.cimid.2011.11.003. [DOI] [PubMed] [Google Scholar]
- 30.Gupta U. D., Katoch V. M. Animal models of tuberculosis for vaccine development. Indian Journal of Medical Research. 2009;129(1):11–18. [PubMed] [Google Scholar]
- 31.de Val Perez B., Lopez-Soria S., Nofrarias M., et al. Experimental model of tuberculosis in the domestic goat after endobronchial infection with Mycobacterium caprae. Clinical and Vaccine Immunology. 2011;18(11):1872–1881. doi: 10.1128/CVI.05323-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vidal E., Arrieta-Villegas C., Grasa M., Mercader I., Domingo M., Perez de Val B. Field evaluation of the efficacy of Mycobacterium bovis BCG vaccine against tuberculosis in goats. BMC Veterinary Research. 2017;13(1):p. 252. doi: 10.1186/s12917-017-1182-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sanchez J., Tomas L., Ortega N., et al. Microscopical and immunological features of tuberculoid granulomata and cavitary pulmonary tuberculosis in naturally infected goats. Journal of Comparative Pathology. 2011;145(2–3):107–117. doi: 10.1016/j.jcpa.2010.12.006. [DOI] [PubMed] [Google Scholar]
- 34.Flynn J. L., Capuano S. V., Croix D., et al. Non-human primates: a model for tuberculosis research. Tuberculosis. 2003;83(1–3):116–118. doi: 10.1016/S1472-9792(02)00059-8. [DOI] [PubMed] [Google Scholar]
- 35.Myllymaki H., Niskanen M., Oksanen K. E., Ramet M. Animal models in tuberculosis research — where is the beef? Expert Opinion on Drug Discovery. 2015;10(8):871–883. doi: 10.1517/17460441.2015.1049529. [DOI] [PubMed] [Google Scholar]
- 36.Swaim L. E., Connolly L. E., Volkman H. E., Humbert O., Born D. E., Ramakrishnan L. Mycobacterium marinum infection of adult zebrafish causes caseating granulomatous tuberculosis and is moderated by adaptive immunity. Infection and Immunity. 2006;74(11):6108–6117. doi: 10.1128/IAI.00887-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Traver D., Herbomel P., Patton E. E., et al. The zebrafish as a model organism to study development of the immune system. Advances in Immunology. 2003;81:253–330. [PubMed] [Google Scholar]
- 38.Myllymaki H., Niskanen M., Oksanen K. E., et al. Identification of novel antigen candidates for a tuberculosis vaccine in the adult zebrafish (Danio rerio) PLoS One. 2017;12(7):p. e0181942. doi: 10.1371/journal.pone.0181942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Martinot A. J. Microbial offense vs host defense: who controls the TB granuloma? Veterinary Pathology. 2018;55(1):14–26. doi: 10.1177/0300985817705177. [DOI] [PubMed] [Google Scholar]
- 40.Igboin C. O., Griffen A. L., Leys E. J. The Drosophila melanogaster host model. Journal of Oral Microbiology. 2012;4(1):p. 10368. doi: 10.3402/jom.v4i0.10368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Oh C. T., Moon C., Choi T. H., Kim B. S., Jang J. Mycobacterium marinum infection in Drosophila melanogaster for antimycobacterial activity assessment. Journal of Antimicrobial Chemotherapy. 2013;68(3):601–609. doi: 10.1093/jac/dks425. [DOI] [PubMed] [Google Scholar]
- 42.Lopez Hernandez Y., Yero D., Pinos-Rodriguez J. M., Gibert I. Animals devoid of pulmonary system as infection models in the study of lung bacterial pathogens. Frontiers in Microbiology. 2015;6:p. 38. doi: 10.3389/fmicb.2015.00038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lowrie D. B. DNA vaccines for therapy of tuberculosis: where are we now? Vaccine. 2006;24(12):1983–1989. doi: 10.1016/j.vaccine.2005.11.010. [DOI] [PubMed] [Google Scholar]
- 44.Liang Y., Zhang J., Yang Y., et al. Immunogenicity and therapeutic effects of recombinant Ag85AB fusion protein vaccines in mice infected with Mycobacterium tuberculosis. Vaccine. 2017;35(32):3995–4001. doi: 10.1016/j.vaccine.2017.05.083. [DOI] [PubMed] [Google Scholar]
- 45.Garcia-Pelayo M. C., Bachy V. S., Kaveh D. A., Hogarth P. J. BALB/c mice display more enhanced BCG vaccine induced Th1 and Th17 response than C57BL/6 mice but have equivalent protection. Tuberculosis. 2015;95(1):48–53. doi: 10.1016/j.tube.2014.10.012. [DOI] [PubMed] [Google Scholar]
- 46.Stylianou E., Harrington-Kandt R., Beglov J., et al. Identification and evaluation of novel protective antigens for the development of a candidate tuberculosis subunit vaccine. Infection and Immunity. 2018;86(7):p. e00014-18. doi: 10.1128/IAI.00014-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Namvarpour M., Tebianian M., Mansouri R., Ebrahimi S. M., Kashkooli S. Comparison of different immunization routes on the immune responses induced by Mycobacterium tuberculosis ESAT-6/CFP-10 recombinant protein. Biologicals. 2019;59:6–11. doi: 10.1016/j.biologicals.2019.04.002. [DOI] [PubMed] [Google Scholar]
- 48.Abolhassani M., Lagranderie M., Chavarot P., Balazuc A. M., Marchal G. Mycobacterium bovis BCG induces similar immune responses and protection by rectal and parenteral immunization routes. Infection and Immunity. 2000;68(10):5657–5662. doi: 10.1128/iai.68.10.5657-5662.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhang Y., Feng L., Li L., et al. Effects of the fusion design and immunization route on the immunogenicity of Ag85A-Mtb32 in adenoviral vectored tuberculosis vaccine. Human Vaccines & Immunotherapeutics. 2015;11(7):1803–1813. doi: 10.1080/21645515.2015.1042193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yang E., Lu Y., Xu Y., et al. Recombinant BCG coexpressing Ag85B, ESAT-6 and Rv3620c elicits specific Th1 immune responses in C57BL/6 mice. Microbial Pathogenesis. 2014;69–70:53–59. doi: 10.1016/j.micpath.2014.03.011. [DOI] [PubMed] [Google Scholar]
- 51.Lu Y., Xu Y., Yang E., Wang C., Wang H., Shen H. Novel recombinant BCG coexpressing Ag85B, ESAT-6 and Rv2608 elicits significantly enhanced cellular immune and antibody responses in C57BL/6 mice. Scandinavian Journal of Immunology. 2012;76(3):271–277. doi: 10.1111/j.1365-3083.2012.02726.x. [DOI] [PubMed] [Google Scholar]
- 52.Christy A. J., Dharman K., Dhandapaani G., et al. Epitope based recombinant BCG vaccine elicits specific Th1 polarized immune responses in BALB/c mice. Vaccine. 2012;30(7):1364–1370. doi: 10.1016/j.vaccine.2011.12.059. [DOI] [PubMed] [Google Scholar]
- 53.Roupie V., Romano M., Zhang L., et al. Immunogenicity of eight dormancy regulon-encoded proteins of Mycobacterium tuberculosis in DNA-vaccinated and tuberculosis-infected mice. Infection and Immunity. 2007;75(2):941–949. doi: 10.1128/IAI.01137-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yuan X., Teng X., Jing Y., et al. A live attenuated BCG vaccine overexpressing multistage antigens Ag85B and HspX provides superior protection against Mycobacterium tuberculosis infection. Applied Microbiology and Biotechnology. 2015;99(24):10587–10595. doi: 10.1007/s00253-015-6962-x. [DOI] [PubMed] [Google Scholar]
- 55.Shen H., Wang C., Yang E., et al. Novel recombinant BCG coexpressing Ag85B, ESAT-6 and mouse TNF-alpha induces significantly enhanced cellular immune and antibody responses in C57BL/6 mice. Microbiology and Immunology. 2010;54(8):435–441. doi: 10.1111/j.1348-0421.2010.00232.x. [DOI] [PubMed] [Google Scholar]
- 56.Gouveia A. C., Brugiolo A. S., Alves C. C., et al. Th2 responses in OVA-sensitized BALB/c mice are down-modulated by Mycobacterium bovis BCG treatment. Journal of Clinical Immunology. 2013;33(1):235–245. doi: 10.1007/s10875-012-9746-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Dobakhti F., Naghibi T., Taghikhani M., et al. Adjuvanticity effect of sodium alginate on subcutaneously injected BCG in BALB/c mice. Microbes and Infection. 2009;11(2):296–301. doi: 10.1016/j.micinf.2008.12.003. [DOI] [PubMed] [Google Scholar]
- 58.da Costa A. C., Costa-Junior Ade O., de Oliveira F. M., et al. A new recombinant BCG vaccine induces specific Th17 and Th1 effector cells with higher protective efficacy against tuberculosis. PLoS One. 2014;9(11):p. e112848. doi: 10.1371/journal.pone.0112848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Shibata Y., Henriksen R. A., Honda I., Nakamura R. M., Myrvik Q. N. Splenic PGE2-releasing macrophages regulate Th1 and Th2 immune responses in mice treated with heat-killed BCG. Journal of Leukocyte Biology. 2005;78(6):1281–1290. doi: 10.1189/jlb.0605321. [DOI] [PubMed] [Google Scholar]
- 60.Bonanni D., Rindi L., Lari N., Garzelli C. Immunogenicity of mycobacterial PPE44 (Rv2770c) in Mycobacterium bovis BCG-infected mice. Journal of Medical Microbiology. 2005;54(Pt 5):443–448. doi: 10.1099/jmm.0.45960-0. [DOI] [PubMed] [Google Scholar]
- 61.Haile M., Schroder U., Hamasur B., et al. Immunization with heat-killed Mycobacterium bovis bacille Calmette-Guerin (BCG) in Eurocine L3 adjuvant protects against tuberculosis. Vaccine. 2004;22(11–12):1498–1508. doi: 10.1016/j.vaccine.2003.10.016. [DOI] [PubMed] [Google Scholar]
- 62.Dhar N., Rao V., Tyagi A. K. Skewing of the Th1/Th2 responses in mice due to variation in the level of expression of an antigen in a recombinant BCG system. Immunology Letters. 2003;88(3):175–184. doi: 10.1016/S0165-2478(03)00043-9. [DOI] [PubMed] [Google Scholar]
- 63.Tran V., Ahn S. K., Ng M., Li M., Liu J. Loss of lipid virulence factors reduces the efficacy of the BCG vaccine. Scientific Reports. 2016;6:p. 29076. doi: 10.1038/srep29076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Driver E. R., Ryan G. J., Hoff D. R., et al. Evaluation of a mouse model of necrotic granuloma formation using C3HeB/FeJ mice for testing of drugs against Mycobacterium tuberculosis. Antimicrobial Agents and Chemotherapy. 2012;56(6):3181–3195. doi: 10.1128/aac.00217-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lanoix J. P., Lenaerts A. J., Nuermberger E. L. Heterogeneous disease progression and treatment response in a C3HeB/FeJ mouse model of tuberculosis. Disease Models & Mechanisms. 2015;8(6):603–610. doi: 10.1242/dmm.019513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Cyktor J. C., Carruthers B., Kominsky R. A., Beamer G. L., Stromberg P., Turner J. IL-10 inhibits mature fibrotic granuloma formation during Mycobacterium tuberculosis infection. The Journal of Immunology. 2013;190(6):2778–2790. doi: 10.3410/f.717977278.793471932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wozniak T. M., Saunders B. M., Ryan A. A., Britton W. J. Mycobacterium bovis BCG-specific Th17 cells confer partial protection against Mycobacterium tuberculosis infection in the absence of gamma interferon. Infection and Immunity. 2010;78(10):4187–4194. doi: 10.1128/IAI.01392-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Umemura M., Yahagi A., Hamada S., et al. IL-17-mediated regulation of innate and acquired immune response against pulmonary Mycobacterium bovis Bacille Calmette-Guerin infection. The Journal of Immunology. 2007;178(6):3786–3796. doi: 10.4049/jimmunol.178.6.3786. [DOI] [PubMed] [Google Scholar]
- 69.Krahenbuhl J., Adams L. B. Exploitation of gene knockout mice models to study the pathogenesis of leprosy. Leprosy Review. 2000;71:S170–S175. doi: 10.5935/0305-7518.20000090. [DOI] [PubMed] [Google Scholar]
- 70.Irwin S. M., Gruppo V., Brooks E., et al. Limited activity of clofazimine as a single drug in a mouse model of tuberculosis exhibiting caseous necrotic granulomas. Antimicrobial Agents and Chemotherapy. 2014;58(7):4026–4034. doi: 10.1128/AAC.02565-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Irwin S. M., Prideaux B., Lyon E. R., et al. Bedaquiline and pyrazinamide treatment responses are affected by pulmonary lesion heterogeneity in Mycobacterium tuberculosis infected C3HeB/FeJ mice. ACS Infectious Diseases. 2016;2(4):251–267. doi: 10.1021/acsinfecdis.5b00127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Liang Y., Wu X. Q., Li N., et al. Wang. Creation and evaluation of mouse models for Mycobacterium tuberculosis infection. Journal of Pathogen Biology. 2014;9(9):775–778. doi: 10.3389/fmicb.2017.00717. [DOI] [Google Scholar]
- 73.Haslov K., Closs O., Moller S., Bentzon M. W. Studies on the development of tuberculin sensitivity in immunized guinea pigs with demonstration of a close relationship between results of skin tests and the lymphocyte transformation technique. International Archives of Allergy and Immunology. 2004;73(2):114–122. doi: 10.1159/000233450. [DOI] [PubMed] [Google Scholar]
- 74.Dharmadhikari A. S., Basaraba R. J., Van Der Walt M. L., et al. Natural infection of guinea pigs exposed to patients with highly drug-resistant tuberculosis. Tuberculosis. 2011;91(4):329–338. doi: 10.1016/j.tube.2011.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Palanisamy G. S., Smith E. E., Shanley C. A., Ordway D. J., Orme I. M., Basaraba R. J. Disseminated disease severity as a measure of virulence of Mycobacterium tuberculosis in the guinea pig model. Tuberculosis. 2008;88(4):295–306. doi: 10.1016/j.tube.2007.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Basaraba R. J. Experimental tuberculosis: the role of comparative pathology in the discovery of improved tuberculosis treatment strategies. Tuberculosis (Edinburgh,Scotland) 2008;88(Suppl 1):S35–S47. doi: 10.1016/S1472-9792(08)70035-0. [DOI] [PubMed] [Google Scholar]
- 77.Tree J. A., Elmore M. J., Javed S., Williams A., Marsh P. D. Development of a guinea pig immune response-related microarray and its use to define the host response following Mycobacterium bovis BCG vaccination. Infection and Immunity. 2006;74(2):1436–1441. doi: 10.1128/IAI.74.2.1436-1441.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Hiromatsu K., Dascher C. C., LeClair K. P., et al. Induction of CD1-restricted immune responses in guinea pigs by immunization with mycobacterial lipid antigens. The Journal of Immunology. 2002;169(1):330–339. doi: 10.4049/jimmunol.169.1.330. [DOI] [PubMed] [Google Scholar]
- 79.Dirisala V. R., Jeevan A., Ramasamy S. K., McMurray D. N. Molecular cloning, expression, and in silico structural analysis of guinea pig IL-17. Molecular Biotechnology. 2013;55(3):277–287. doi: 10.1007/s12033-013-9679-z. [DOI] [PubMed] [Google Scholar]
- 80.Dirisala V. R., Jeevan A., Bix G., Yoshimura T., McMurray D. N. Molecular cloning and expression of the IL-10 gene from guinea pigs. Gene. 2012;498(1):120–127. doi: 10.1016/j.gene.2012.01.076. [DOI] [PubMed] [Google Scholar]
- 81.Jeevan A., Yoshimura T., Ly L. H., Dirisala V. R., McMurray D. N. Cloning of guinea pig IL-4: reduced IL-4 mRNA after vaccination or Mycobacterium tuberculosis infection. Tuberculosis. 2011;91(1):47–56. doi: 10.1016/j.tube.2010.11.006. [DOI] [PubMed] [Google Scholar]
- 82.Jeevan A., Yoshimura T., Lee K. E., McMurray D. N. Differential expression of gamma interferon mRNA induced by attenuated and virulent Mycobacterium tuberculosis in guinea pig cells after Mycobacterium bovis BCG vaccination. Infection and Immunity. 2003;71(1):354–364. doi: 10.1128/IAI.71.1.354-364.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Dannenberg Jr. A. M., Collins F. M. Progressive pulmonary tuberculosis is not due to increasing numbers of viable bacilli in rabbits, mice and guinea pigs, but is due to a continuous host response to mycobacterial products. Tuberculosis. 2001;81(3):229–242. doi: 10.1054/tube.2001.0287. [DOI] [PubMed] [Google Scholar]
- 84.Tsenova L., Harbacheuski R., Sung N., Ellison E., Fallows D., Kaplan G. BCG vaccination confers poor protection against M. tuberculosis HN878-induced central nervous system disease. Vaccine. 2007;25(28):5126–5132. doi: 10.1016/j.vaccine.2006.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Sampson S. L., Dascher C. C., Sambandamurthy V. K., et al. Protection elicited by a double leucine and pantothenate auxotroph of Mycobacterium tuberculosis in guinea pigs. Infection and Immunity. 2004;72(5):3031–3037. doi: 10.1128/iai.72.5.3031-3037.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Gb O., Mm S. The resistance of the albino rat to infection with tubercle bacilli. American Review of Tuberculosis. 1925;12(1):77–86. [Google Scholar]
- 87.Bodkin G. E. A case of tuberculosis in a rat. Journal of Hygiene. 1918;17(1):10–12. doi: 10.1017/S0022172400007038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Gabler E., Pendl O. Inborn resistance to tubercle bacilli (H 37 Rv) in albino rats. Annals of Thoracic Medicine. 1966;23(1):36–51. [PubMed] [Google Scholar]
- 89.Gray D. F. The relative natural resistance of rats and mice to experimental pulmonary tuberculosis. Epidemiology and Infection. 1961;59(4):471–478. doi: 10.1017/S0022172400039164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Heng Y., Seah P. G., Siew J. Y., et al. Mycobacterium tuberculosis infection induces hypoxic lung lesions in the rat. Tuberculosis. 2011;91(4):339–341. doi: 10.1016/j.tube.2011.05.003. [DOI] [PubMed] [Google Scholar]
- 91.Singhal A., Mathys V., Kiass M., et al. BCG induces protection against Mycobacterium tuberculosis infection in the Wistar rat model. PLoS One. 2011;6(12):p. e28082. doi: 10.1371/journal.pone.0028082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Shi L., Zhang L., Li C., et al. Dietary zinc deficiency impairs humoral and cellular immune responses to BCG and ESAT-6/CFP-10 vaccination in offspring and adult rats. Tuberculosis. 2016;97:86–96. doi: 10.1016/j.tube.2016.01.002. [DOI] [PubMed] [Google Scholar]
- 93.Desem N., Jones S. L. Development of a human gamma interferon enzyme immunoassay and comparison with tuberculin skin testing for detection of Mycobacterium tuberculosis infection. Clinical and Diagnostic Laboratory Immunology. 1998;5(4):531–536. doi: 10.1128/cdli.5.4.531-536.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Garcia M. A., Bouley D. M., Larson M. J., et al. Outbreak of Mycobacterium bovis in a conditioned colony of rhesus (Macaca mulatta) and cynomolgus (Macaca fascicularis) macaques. Comparative Medicine. 2004;54(5):578–584. [PubMed] [Google Scholar]
- 95.Jeyanathan M., Shao Z., Yu X., et al. AdHu5Ag85A respiratory mucosal boost immunization enhances protection against pulmonary tuberculosis in BCG-primed non-human primates. PLoS One. 2015;10(8):p. e0135009. doi: 10.1371/journal.pone.0135009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Sharpe S., White A., Sarfas C., et al. Alternative BCG delivery strategies improve protection against Mycobacterium tuberculosis in non-human primates: protection associated with mycobacterial antigen-specific CD4 effector memory T-cell populations. Tuberculosis. 2016;101:174–190. doi: 10.1016/j.tube.2016.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Larsen M. H., Biermann K., Chen B., et al. Efficacy and safety of live attenuated persistent and rapidly cleared Mycobacterium tuberculosis vaccine candidates in non-human primates. Vaccine. 2009;27(34):4709–4717. doi: 10.1016/j.vaccine.2009.05.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Harris S. A., White A., Stockdale L., et al. Development of a non-human primate BCG infection model for the evaluation of candidate tuberculosis vaccines. Tuberculosis. 2018;108:99–105. doi: 10.1016/j.tube.2017.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Palmer M. V. Mycobacterium bovis: characteristics of wildlife reservoir hosts. Transboundary and Emerging Diseases. 2013;60(Suppl. 1):1–13. doi: 10.1111/tbed.12115. [DOI] [PubMed] [Google Scholar]
- 100.Canto Alarcon G. J., Rubio Venegas Y., Bojorquez Narvaez L., et al. Efficacy of a vaccine formula against tuberculosis in cattle. PLoS One. 2013;8(10):p. e76418. doi: 10.1371/journal.pone.0076418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Villarreal-Ramos B., Berg S., Chamberlain L., et al. Development of a BCG challenge model for the testing of vaccine candidates against tuberculosis in cattle. Vaccine. 2014;32(43):5645–5649. doi: 10.1016/j.vaccine.2014.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Salguero F. J., Gibson S., Garcia-Jimenez W., et al. Differential cell composition and cytokine expression within lymph node granulomas from BCG-vaccinated and non-vaccinated cattle experimentally infected with Mycobacterium bovis. Transboundary and Emerging Diseases. 2017;64(6):1734–1749. doi: 10.1111/tbed.12561. [DOI] [PubMed] [Google Scholar]
- 103.Maggioli M. F., Palmer M. V., Thacker T. C., Vordermeier H. M., Waters W. R. Characterization of effector and memory T cell subsets in the immune response to bovine tuberculosis in cattle. PLoS One. 2015;10(4):p. e0122571. doi: 10.1371/journal.pone.0122571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Thom M. L., McAulay M., Vordermeier H. M., et al. Duration of immunity against Mycobacterium bovis following neonatal vaccination with bacillus Calmette-Guerin Danish: significant protection against infection at 12, but not 24, months. Clinical and Vaccine Immunology. 2012;19(8):1254–1260. doi: 10.1128/CVI.00301-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Parlane N. A., Shu D., Subharat S., et al. Revaccination of cattle with bacille calmette-guerin two years after first vaccination when immunity has waned, boosted protection against challenge with Mycobacterium bovis. PLoS One. 2014;9(9):p. e106519. doi: 10.1371/journal.pone.0106519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Dye C. Making wider use of the world’s most widely used vaccine: bacille calmette-guerin revaccination reconsidered. Journal of The Royal Society Interface. 2013;10(87):p. 20130365. doi: 10.1098/rsif.2013.0365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Aranaz A., Cousins D., Mateos A., Dominguez L. Elevation of Mycobacterium tuberculosis subsp. caprae Aranaz, et al. 1999 to species rank as Mycobacterium caprae comb. nov., sp. nov. International Journal of Systematic and Evolutionary Microbiology. 2003;53(Pt 6):1785–1789. doi: 10.1099/ijs.0.02532-0. [DOI] [PubMed] [Google Scholar]
- 108.Perez de Val B., Villarreal-Ramos B., Nofrarias M., et al. Goats primed with Mycobacterium bovis BCG and boosted with a recombinant adenovirus expressing Ag85A show enhanced protection against tuberculosis. Clinical and Vaccine Immunology. 2012;19(9):1339–1347. doi: 10.1128/CVI.00275-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.de Val B. P., Vidal E., Nofrarías M., López-Soria S., Cardona P.-J., Domingo M. Assessment of goat tuberculosis model for use in vaccine trials. Procedia in Vaccinology. 2014;8:43–49. doi: 10.1016/j.provac.2014.07.008. [DOI] [Google Scholar]
- 110.Arrieta-Villegas C., Peralvarez T., Vidal E., et al. Efficacy of parenteral vaccination against tuberculosis with heat-inactivated Mycobacterium bovis in experimentally challenged goats. PLoS One. 2018;13(5):p. e0196948. doi: 10.1371/journal.pone.0196948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Bezos J., Casal C., Puentes E., et al. Evaluation of the immunogenicity and diagnostic interference caused by M. tuberculosis SO2 vaccination against tuberculosis in goats. Research in Veterinary Science. 2015;103:73–79. doi: 10.1016/j.rvsc.2015.09.017. [DOI] [PubMed] [Google Scholar]
- 112.Roy A., Risalde M. A., Casal C., et al. Oral vaccination with heat-inactivated Mycobacterium bovis does not interfere with the antemortem diagnostic techniques for tuberculosis in goats. Frontiers in Veterinary Science. 2017;4:p. 124. doi: 10.3389/fvets.2017.00124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Oksanen K. E., Halfpenny N. J., Sherwood E., et al. An adult zebrafish model for preclinical tuberculosis vaccine development. Vaccine. 2013;31(45):5202–5209. doi: 10.1016/j.vaccine.2013.08.093. [DOI] [PubMed] [Google Scholar]
- 114.Lugo-Villarino G., Balla K. M., Stachura D. L., Banuelos K., Werneck M. B., Traver D. Identification of dendritic antigen-presenting cells in the zebrafish. Proceedings of the National Academy of Sciences of the United States of America. 2010;107(36):15850–15855. doi: 10.1073/pnas.1000494107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Myllymaki H., Niskanen M., Luukinen H., Parikka M., Ramet M. Identification of protective postexposure mycobacterial vaccine antigens using an immunosuppression-based reactivation model in the zebrafish. Disease Models & Mechanisms. 2018;11(3) doi: 10.1242/dmm.033175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Chen Z., Shao X. Y., Wang C., et al. Mycobacterium marinum infection in zebrafish and microglia imitates the early stage of Tuberculous Meningitis. Journal of Molecular Neuroscience. 2018;64(2):321–330. doi: 10.1007/s12031-018-1026-1. [DOI] [PubMed] [Google Scholar]
- 117.Takaki K., Ramakrishnan L., Basu S. A zebrafish model for ocular tuberculosis. PLoS One. 2018;13(3):p. e0194982. doi: 10.1371/journal.pone.0194982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Dionne M. S., Ghori N., Schneider D. S. Drosophila melanogaster is a genetically tractable model host for Mycobacterium marinum. Infection and Immunity. 2003;71(6):3540–3550. doi: 10.1128/IAI.71.6.3540-3550.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Dionne M. S., Pham L. N., Shirasu-Hiza M., Schneider D. S. Akt and FOXO dysregulation contribute to infection-induced wasting in Drosophila. Current Biology. 2006;16(20):1977–1985. doi: 10.1016/j.vaccine.2013.08.093. [DOI] [PubMed] [Google Scholar]
- 120.Solomon J. M., Leung G. S., Isberg R. R. Intracellular replication of Mycobacterium marinum within Dictyostelium discoideum: efficient replication in the absence of host coronin. Infection & Immunity. 2003;71(6):3578–3586. doi: 10.1128/IAI.71.6.3578-3586.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Sanchez-Hidalgo A., Obregon-Henao A., Wheat W. H., Jackson M., Gonzalez-Juarrero M. Mycobacterium bovis hosted by free-living-amoebae permits their long-term persistence survival outside of host mammalian cells and remain capable of transmitting disease to mice. Environmental Microbiology. 2017;19(10):4010–4021. doi: 10.1111/1462-2920.13810. [DOI] [PubMed] [Google Scholar]
- 122.Hagedorn M., Rohde K. H., Russell D. G., Soldati T. Infection by tubercular mycobacteria is spread by nonlytic ejection from their amoeba hosts. Science. 2009;323(5922):1729–1733. doi: 10.1126/science.1169381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Barisch C., Lopez-Jimenez A. T., Soldati T. Live imaging of Mycobacterium marinum infection in Dictyostelium discoideum. Methods in Molecular Biology. 2015;1285:369–385. doi: 10.1007/978-1-4939-2450-9_23. [DOI] [PubMed] [Google Scholar]
- 124.Arafah S., Kicka S., Trofimov V., et al. Setting up and monitoring an infection of Dictyostelium discoideum with mycobacteria. Methods in Molecular Biology. 2013;983:403–417. doi: 10.1007/978-1-62703-302-2_22. [DOI] [PubMed] [Google Scholar]
- 125.Ouertatani-Sakouhi H., Kicka S., Chiriano G., et al. Inhibitors of Mycobacterium marinum virulence identified in a Dictyostelium discoideum host model. PLoS One. 2017;12(7):p. e0181121. doi: 10.1371/journal.pone.0181121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Drancourt M. Looking in amoebae as a source of mycobacteria. Microbial Pathogenesis. 2014;77:119–124. doi: 10.1016/j.micpath.2014.07.001. [DOI] [PubMed] [Google Scholar]
- 127.O’Shea M. K., McShane H. A review of clinical models for the evaluation of human TB vaccines. Human Vaccines & Immunotherapeutics. 2016;12(5):1177–1187. doi: 10.1080/21645515.2015.1134407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Mak I. W., Evaniew N., Ghert M. Lost in translation: animal models and clinical trials in cancer treatment. American Journal of Translational Research. 2014;6(2):114–118. [PMC free article] [PubMed] [Google Scholar]
- 129.Tameris M. D., Hatherill M., Landry B. S., et al. Safety and efficacy of MVA85A, a new tuberculosis vaccine, in infants previously vaccinated with BCG: a randomised, placebo-controlled phase 2b trial. Lancet. 2013;381(9871):1021–1028. doi: 10.1016/S0140-6736(13)60177-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Da C. A., Nogueira S. V., Kipnis A., Junqueirakipnis A. P. Recombinant BCG: innovations on an old vaccine. Scope of BCG strains and strategies to improve long-lasting memory. Frontiers in Immunology. 2014;5:p. 152. doi: 10.3389/fimmu.2014.00152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Hoft D. F., Blazevic A., Selimovic A., et al. Safety and immunogenicity of the recombinant BCG vaccine AERAS-422 in healthy BCG-naive adults: a randomized, active-controlled, first-in-human phase 1 trial. EBioMedicine. 2016;7(C):278–286. doi: 10.1016/j.ebiom.2016.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Lewis D. J., Huo Z., Barnett S., et al. Transient facial nerve paralysis (Bell’s palsy) following intranasal delivery of a genetically detoxified mutant of Escherichia coli heat labile toxin. PLoS One. 2009;4(9):p. e6999. doi: 10.1371/journal.pone.0006999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Lahey T., Arbeit R. D., Bakari M., et al. Immunogenicity of a protective whole cell mycobacterial vaccine in HIV-infected adults: a phase III study in Tanzania. Vaccine. 2010;28(48):7652–7658. doi: 10.1016/j.vaccine.2010.09.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Regenberg A., Mathews D. J., Blass D. M., et al. The role of animal models in evaluating reasonable safety and efficacy for human trials of cell-based interventions for neurologic conditions. Journal of Cerebral Blood Flow & Metabolism. 2009;29(1):1–9. doi: 10.1038/jcbfm.2008.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.McShane H., Williams A. A review of preclinical animal models utilised for TB vaccine evaluation in the context of recent human efficacy data. Tuberculosis. 2014;94(2):105–110. doi: 10.1016/j.tube.2013.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.CDC K. Recombinant vaccinia virus strain and vaccine composition comprising the same. 2016. p. p. 17. K. P. Office, Ed., Korea.
- 137.Lu M., Xia Z. Y., Bao L. A Mycobacterium bovis BCG-naked DNA prime-boost vaccination strategy induced CD4(+) and CD8(+) T-cell response against Mycobacterium tuberculosis immunogens. Journal of Immunology Research. 2014;2014:p. 395626. doi: 10.1155/2014/395626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Sander P., Clark S., Petrera A., et al. Deletion of zmp1 improves Mycobacterium bovis BCG-mediated protection in a guinea pig model of tuberculosis. Vaccine. 2015;33(11):1353–1359. doi: 10.1016/j.vaccine.2015.01.058. [DOI] [PubMed] [Google Scholar]
- 139.Pedroza-Roldan C., Guapillo C., Barrios-Payan J., et al. The BCGDeltaBCG1419c strain, which produces more pellicle in vitro, improves control of chronic tuberculosis in vivo. Vaccine. 2016;34(40):4763–4770. doi: 10.1016/j.vaccine.2016.08.035. [DOI] [PubMed] [Google Scholar]
- 140.Counoupas C., Pinto R., Nagalingam G., Britton W. J., Triccas J. A. Protective efficacy of recombinant BCG over-expressing protective, stage-specific antigens of Mycobacterium tuberculosis. Vaccine. 2018;36(19):2619–2629. doi: 10.1016/j.vaccine.2018.03.066. [DOI] [PubMed] [Google Scholar]
- 141.Tang J., Cai Y., Liang J., et al. In vivo electroporation of a codon-optimized BER(opt) DNA vaccine protects mice from pathogenic Mycobacterium tuberculosis aerosol challenge. Tuberculosis. 2018;113:65–75. doi: 10.1016/j.tube.2018.07.003. [DOI] [PubMed] [Google Scholar]
- 142.Sakthi S., Palaniyandi K., Gupta U. D., Gupta P., Narayanan S. Lipoprotein LpqS deficient M. tuberculosis mutant is attenuated for virulence in vivo and shows protective efficacy better than BCG in guinea pigs. Vaccine. 2016;34(6):735–743. doi: 10.1016/j.vaccine.2015.12.059. [DOI] [PubMed] [Google Scholar]
- 143.Smaill F., Jeyanathan M., Smieja M., et al. A human type 5 adenovirus-based tuberculosis vaccine induces robust T cell responses in humans despite preexisting anti-adenovirus immunity. Science Translational Medicine. 2013;5(205):p. 205ra134. doi: 10.1126/scitranslmed.3006843. [DOI] [PubMed] [Google Scholar]
- 144.Hoft D. F., Blazevic A., Abate G., et al. A new recombinant bacille Calmette-Guerin vaccine safely induces significantly enhanced tuberculosis-specific immunity in human volunteers. The Journal of Infectious Diseases. 2008;198(10):1491–1501. doi: 10.1086/592450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Henao-Tamayo M., Shanley C. A., Verma D., et al. The Efficacy of the BCG vaccine against newly emerging clinical strains of Mycobacterium tuberculosis. PLoS One. 2015;10(9):p. e0136500. doi: 10.1371/journal.pone.0136500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Suliman S., Luabeya A. K. K., Geldenhuys H., et al. Dose optimization of H56:IC31 vaccine for tuberculosis-endemic populations. A double-blind, placebo-controlled, dose-selection trial. American Journal of Respiratory and Critical Care Medicine. 2019;199(2):220–231. doi: 10.1164/rccm.201802-0366OC. [DOI] [PubMed] [Google Scholar]
- 147.Spertini F., Audran R., Chakour R., et al. Safety of human immunisation with a live-attenuated Mycobacterium tuberculosis vaccine: a randomised, double-blind, controlled phase I trial. The Lancet Respiratory Medicine. 2015;3(12):953–962. doi: 10.1016/S2213-2600(15)00435-X. [DOI] [PubMed] [Google Scholar]
- 148.Gillard P., Yang P. C., Danilovits M., et al. Safety and immunogenicity of the M72/AS01E candidate tuberculosis vaccine in adults with tuberculosis: a phase II randomised study. Tuberculosis. 2016;100:118–127. doi: 10.1016/j.tube.2016.07.005. [DOI] [PubMed] [Google Scholar]
- 149.Loxton A. G., Knaul J. K., Grode L., et al. Safety and immunogenicity of the recombinant Mycobacterium bovis BCG vaccine VPM1002 in HIV-unexposed newborn infants in South Africa. Clinical and Vaccine Immunology. 2017;24(2) doi: 10.1128/CVI.00439-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Sun Z. P., Liu H., Liu R. Evaluation of the efficacy of Mycobacterium bacillus in the treatment of latent TB infection. Modern Preventive Medicine. 2013;40(15):2894–2896. [Google Scholar]
- 151.Nieuwenhuizen N. E., Kulkarni P. S., Shaligram U., et al. The recombinant Bacille Calmette-Guerin vaccine VPM1002: ready for clinical efficacy testing. Frontiers in Immunology. 2017;8:p. 1147. doi: 10.3389/fimmu.2017.01147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Scriba T. J., Kaufmann S. H., Henri Lambert P., Sanicas M., Martin C., Neyrolles O. Vaccination against tuberculosis with whole-cell mycobacterial vaccines. Journal of Infectious Diseases. 2016;214(5):659–664. doi: 10.1093/infdis/jiw228. [DOI] [PubMed] [Google Scholar]
- 153.Vogelzang A., Perdomo C., Zedler U., et al. Central memory CD4+ T cells are responsible for the recombinant Bacillus Calmette–Guerin DeltaureC:hly vaccine’s superior protection against tuberculosis. Journal of Infectious Diseases. 2014;210(12):1928–1937. doi: 10.1093/infdis/jiu347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Grode L., Ganoza C. A., Brohm C., Weiner 3rd. J., Eisele B., Kaufmann S. H. Safety and immunogenicity of the recombinant BCG vaccine VPM1002 in a phase 1 open-label randomized clinical trial. Vaccine. 2013;31(9):1340–1348. doi: 10.1016/j.vaccine.2012.12.053. [DOI] [PubMed] [Google Scholar]
- 155.Macleod M. Learning lessons from MVA85A, a failed booster vaccine for BCG. BMJ. 2018;360:p. k66. doi: 10.1136/bmj.k66. [DOI] [PubMed] [Google Scholar]
- 156.Russell D. G. Who puts the tubercle in tuberculosis? Nature Reviews Microbiology. 2007;5(1):39–47. doi: 10.1038/nrmicro1538. [DOI] [PubMed] [Google Scholar]
- 157.Gong W., Yang Y., Luo Y., et al. An alert of Mycobacterium tuberculosis infection of rhesus macaques in a wild zoo in China. Experimental Animals. 2017;66(4):357–365. doi: 10.1538/expanim.16-0095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Khan A., Jagannath C. Analysis of host-pathogen modulators of autophagy during Mycobacterium tuberculosis infection and therapeutic repercussions. International Reviews of Immunology. 2017;36(5):271–286. doi: 10.1080/08830185.2017.1356924. [DOI] [PubMed] [Google Scholar]
- 159.Tang J., Yam W. C., Chen Z. Mycobacterium tuberculosis infection and vaccine development. Tuberculosis. 2016;98:30–41. doi: 10.1016/j.tube.2016.02.005. [DOI] [PubMed] [Google Scholar]
- 160.Hu S., He W., Du X., et al. IL-17 production of neutrophils enhances antibacteria ability but promotes arthritis development during Mycobacterium tuberculosis infection. EBioMedicine. 2017;23:88–99. doi: 10.1016/j.ebiom.2017.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Junqueirakipnis A. P., Kipnis A., Jamieson A., et al. NK cells respond to pulmonary infection with Mycobacterium tuberculosis, but play a minimal role in protection. Journal of Immunology. 2003;171(11):p. 6039. doi: 10.4049/jimmunol.171.11.6039. [DOI] [PubMed] [Google Scholar]
- 162.Bhatt K., Salgame P. Host innate immune response to Mycobacterium tuberculosis. Journal of Clinical Immunology. 2007;27(4):347–362. doi: 10.1007/s10875-007-9084-0. [DOI] [PubMed] [Google Scholar]
- 163.Srivastava S., Ernst J. D., Desvignes L. Beyond macrophages: the diversity of mononuclear cells in tuberculosis. Immunological Reviews. 2014;262(1):179–192. doi: 10.1111/imr.12217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Mourik B. C., Lubberts E., de Steenwinkel J. E. M., Ottenhoff T. H. M., Leenen P. J. M. Interactions between type 1 interferons and the Th17 response in tuberculosis: lessons learned from autoimmune diseases. Frontiers in Immunology. 2017;8(Suppl 4) doi: 10.3389/fimmu.2017.00294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Lazarevic V., Flynn J. CD8+ T cells in tuberculosis. American Journal of Respiratory and Critical Care Medicine. 2002;166(8):1116–1121. doi: 10.1164/rccm.2204027. [DOI] [PubMed] [Google Scholar]
- 166.Gong W., Wang P., Xiong X., Jiao J., Yang X., Wen B. Chloroform-methanol residue of coxiella burnetii markedly potentiated the specific immunoprotection elicited by a recombinant protein fragment rOmpB-4 derived from outer membrane protein B of Rickettsia rickettsii in C3H/HeN Mice. PLoS One. 2015;10(4):p. e0124664. doi: 10.1371/journal.pone.0124664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Gong W., Wang P., Xiong X., Jiao J., Yang X., Wen B. Enhanced protection against Rickettsia rickettsii infection in C3H/HeN mice by immunization with a combination of a recombinant adhesin rAdr2 and a protein fragment rOmpB-4 derived from outer membrane protein B. Vaccine. 2015;33(8):985–992. doi: 10.1016/j.vaccine.2015.01.017. [DOI] [PubMed] [Google Scholar]
- 168.Gong W., Qi Y., Xiong X., Jiao J., Duan C., Wen B. Rickettsia rickettsii outer membrane protein YbgF induces protective immunity in C3H/HeN mice. Human Vaccines & Immunotherapeutics. 2015;11(3):642–649. doi: 10.1080/21645515.2015.1011572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Gong W., Xiong X., Qi Y., Jiao J., Duan C., Wen B. Identification of novel surface-exposed proteins of Rickettsia rickettsii by affinity purification and proteomics. PLoS One. 2014;9(6):p. e100253. doi: 10.1371/journal.pone.0100253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Gong W., Xiong X., Qi Y., Jiao J., Duan C., Wen B. Surface protein Adr2 of Rickettsia rickettsii induced protective immunity against Rocky Mountain spotted fever in C3H/HeN mice. Vaccine. 2014;32(18):2027–2033. doi: 10.1016/j.vaccine.2014.02.057. [DOI] [PubMed] [Google Scholar]
- 171.Liang Y., Zhang X., Xiao L., et al. Immunogenicity and therapeutic effects of pVAX1-rv1419 DNA from Mycobacterium tuberculosis. Current Gene Therapy. 2016;16(4):249–255. doi: 10.2174/1566523216666161102170123. [DOI] [PubMed] [Google Scholar]
- 172.Wu X., Yang Y., Han Y., et al. Effect of recombinant Rv1009 protein on promoting the growth of Mycobacterium tuberculosis. Journal of Applied Microbiology. 2008;105(4):1121–1127. doi: 10.1111/j.1365-2672.2008.03850.x. [DOI] [PubMed] [Google Scholar]
- 173.Ohara N. Current status of tuberculosis and recombinant bacillus Calmette-Guérin vaccines. Journal of Oral Biosciences. 2012;54(2):92–95. doi: 10.1016/j.job.2012.04.002. [DOI] [Google Scholar]
- 174.McShane H. Tuberculosis vaccines: beyond bacille Calmette-Guerin. Philosophical Transactions of the Royal Society B. 2011;366(1579):2782–2789. doi: 10.1098/rstb.2011.0097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Hussey G., Hawkridge T., Hanekom W. Childhood tuberculosis: old and new vaccines. Paediatric Respiratory Reviews. 2007;8(2):148–154. doi: 10.1016/j.prrv.2007.04.009. [DOI] [PubMed] [Google Scholar]
- 176.Rodrigues L. C., Pereira S. M., Cunha S. S., et al. Effect of BCG revaccination on incidence of tuberculosis in school-aged children in Brazil: the BCG-REVAC cluster-randomised trial. Lancet. 2005;366(9493):1290–1295. doi: 10.1016/S0140-6736(05)67145-0. [DOI] [PubMed] [Google Scholar]
- 177.Buddle B. M., Parlane N. A., Wedlock D. N., Heiser A. Overview of vaccination trials for control of tuberculosis in cattle, wildlife and humans. Transboundary and Emerging Diseases. 2013;60(Suppl 1):136–146. doi: 10.1111/tbed.12092. [DOI] [PubMed] [Google Scholar]
- 178.Ferraz J. C., Stavropoulos E., Yang M., et al. A heterologous DNA priming-Mycobacterium bovis BCG boosting immunization strategy using mycobacterial Hsp70, Hsp65, and Apa antigens improves protection against tuberculosis in mice. Infection and Immunity. 2004;72(12):6945–6950. doi: 10.1128/IAI.72.12.6945-6950.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Skinner M. A., Buddle B. M., Wedlock D. N., et al. A DNA prime-Mycobacterium bovis BCG boost vaccination strategy for cattle induces protection against bovine tuberculosis. Infection and Immunity. 2003;71(9):4901–4907. doi: 10.1128/iai.71.9.4901-4907.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Guo M., Ho W. Z. Animal models to study Mycobacterium tuberculosis and HIV co-infection. Dongwuxue Yanjiu. 2014;35(3):163–169. doi: 10.11813/j.issn.0254-5853.2014.3.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Aaron L., Saadoun D., Calatroni I., et al. Tuberculosis in HIV-infected patients: a comprehensive review. Clinical Microbiology and Infection. 2004;10(5):388–398. doi: 10.1111/j.1469-0691.2004.00758.x. [DOI] [PubMed] [Google Scholar]
- 182.Traggiai E., Chicha L., Mazzucchelli L., et al. Development of a human adaptive immune system in cord blood cell-transplanted mice. Science. 2004;304(5667):104–107. doi: 10.1126/science.1093933. [DOI] [PubMed] [Google Scholar]
- 183.Gorantla S., Makarov E., Finke-Dwyer J., et al. CD8+ cell depletion accelerates HIV-1 immunopathology in humanized mice. The Journal of Immunology. 2010;184(12):7082–7091. doi: 10.4049/jimmunol.1000438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Joseph A., Zheng J. H., Chen K., et al. Inhibition of in vivo HIV infection in humanized mice by gene therapy of human hematopoietic stem cells with a lentiviral vector encoding a broadly neutralizing anti-HIV antibody. Journal of Virology. 2010;84(13):6645–6653. doi: 10.1128/JVI.02339-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Kumar P., Ban H. S., Kim S. S., et al. T cell-specific siRNA delivery suppresses HIV-1 infection in humanized mice. Cell. 2008;134(4):577–586. doi: 10.1016/j.cell.2008.06.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Heuts F., Gavier-Widen D., Carow B., Juarez J., Wigzell H., Rottenberg M. E. CD4+ cell-dependent granuloma formation in humanized mice infected with mycobacteria. Proceedings of the National Academy of Sciences of the United States of America. 2013;110(16):6482–6487. doi: 10.1073/pnas.1219985110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Scanga C. A., Bafica A., Sher A. Viral gene expression in HIV transgenic mice is activated by Mycobacterium tuberculosis and suppressed after antimycobacterial chemotherapy. The Journal of Infectious Diseases. 2007;195(2):246–254. doi: 10.1086/510244. [DOI] [PubMed] [Google Scholar]
- 188.Pawlowski A., Jansson M., Skold M., Rottenberg M. E., Kallenius G. Tuberculosis and HIV co-infection. PLoS Pathogens. 2012;8(2):p. e1002464. doi: 10.1371/journal.ppat.1002464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Spaink H. P., Cui C., Wiweger M. I., et al. Robotic injection of zebrafish embryos for high-throughput screening in disease models. Methods. 2013;62(3):246–254. doi: 10.1016/j.ymeth.2013.06.002. [DOI] [PubMed] [Google Scholar]
- 190.Takaki K., Cosma C. L., Troll M. A., Ramakrishnan L. An in vivo platform for rapid high-throughput antitubercular drug discovery. Cell Reports. 2012;2(1):175–184. doi: 10.1016/j.celrep.2012.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.O'Riordan K., Sharlin D. S., Gross J., et al. Photoinactivation of Mycobacteria in vitro and in a new murine model of localized Mycobacterium bovis BCG-induced granulomatous infection. Antimicrobial Agents and Chemotherapy. 2006;50(5):1828–1834. doi: 10.1128/AAC.50.5.1828-1834.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Fitzgerald L. E., Abendano N., Juste R. A., Alonso-Hearn M. Three-dimensional in vitro models of granuloma to study bacteria-host interactions, drug-susceptibility, and resuscitation of dormant mycobacteria. Biomed Research International. 2014;2014:p. 623856. doi: 10.1155/2014/623856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Esquinas P. L., Rodriguez-Rodriguez C., Carlos De La Vega J., et al. (188)Re image performance assessment using small animal multi-pinhole SPECT/PET/CT system. Physica Medica. 2017;33:26–37. doi: 10.1016/j.ejmp.2016.11.105. [DOI] [PubMed] [Google Scholar]
- 194.Jones G. J., Coad M., Khatri B., et al. Tuberculin skin testing boosts interferon gamma responses to DIVA reagents in Mycobacterium bovis-infected cattle. Clinical and Vaccine Immunology. 2017;24(5) doi: 10.1128/CVI.00551-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Buddle B. M., Vordermeier H. M., Chambers M. A., de Klerk-Lorist L. M. Efficacy and safety of BCG vaccine for control of tuberculosis in domestic livestock and wildlife. Frontiers in Veterinary Science. 2018;5:259. doi: 10.3389/fvets.2018.00259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Shao M., Xu T. R., Chen C. S. The big bang of genome editing technology: development and application of the CRISPR/Cas9 system in disease animal models. Dongwuxue Yanjiu. 2016;37(4):191–204. doi: 10.13918/j.issn.2095-8137.2016.4.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Fujiwara S. Humanized mice: a brief overview on their diverse applications in biomedical research. Journal of Cellular Physiology. 2018;233(4):2889–2901. doi: 10.1002/jcp.26022. [DOI] [PubMed] [Google Scholar]
- 198.Grover A., Troy A., Rowe J., et al. Humanized NOG mice as a model for tuberculosis vaccine-induced immunity: a comparative analysis with the mouse and guinea pig models of tuberculosis. Immunology. 2017;152(1):150–162. doi: 10.1111/imm.12756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Calderon V. E., Valbuena G., Goez Y., et al. A humanized mouse model of tuberculosis. PLoS One. 2013;8(5):p. e63331. doi: 10.1371/journal.pone.0063331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Yi G., Xu X., Abraham S., et al. A DNA vaccine protects human immune cells against Zika virus infection in humanized mice. EBioMedicine. 2017;25:87–94. doi: 10.1016/j.ebiom.2017.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Walsh N. C., Kenney L. L., Jangalwe S., et al. Humanized mouse models of clinical disease. Annual Review of Pathology: Mechanisms of Disease. 2017;12(1):187–215. doi: 10.1146/annurev-pathol-052016-100332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Oh H., Kim C. Y., Kim C. H., et al. Humanized mice for the evaluation of Francisella tularensis vaccine candidates. Journal of Microbiology and Biotechnology. 2018;28(1):157–164. doi: 10.4014/jmb.1707.07075. [DOI] [PubMed] [Google Scholar]
- 203.Xia A., Li X., Xu Z., et al. Prevalence and transmission of Mycobacterium tuberculosis in animals. Journal of Microbes and Infections. 2017;12(4):243–247. [Google Scholar]
- 204.Toth A., Fackelmann J., Pigott W., Tolomeo O. Tuberculosis prevention and treatment. Can Nurse. 2004;100(9):27–30. [PubMed] [Google Scholar]
- 205.Donald P. R., Diacon A. H., Lange C., Demers A. M., von Groote-Bidlingmaier F., Nardell E. Droplets, dust and guinea pigs: an historical review of tuberculosis transmission research, 1878–1940. The International Journal of Tuberculosis and Lung Disease. 2018;22(9):972–982. doi: 10.5588/ijtld.18.0173. [DOI] [PubMed] [Google Scholar]
- 206.Cadmus S., Akinseye V., Adegbulu A., et al. Isolation of Mycobacterium tuberculosis from livestock workers and implications for zooanthroponotic transmission in Ibadan, South-western Nigeria. Journal of Preventive Medicine and Hygiene. 2018;59(3):E212–E218. doi: 10.15167/2421-4248/jpmh2018.59.3.882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Ramos L., Obregon-Henao A., Henao-Tamayo M., Bowen R., Lunney J. K., Gonzalez-Juarrero M. The minipig as an animal model to study Mycobacterium tuberculosis infection and natural transmission. Tuberculosis. 2017;106:91–98. doi: 10.1016/j.tube.2017.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Perez de Val B., Vidal E., Lopez-Soria S., et al. Assessment of safety and interferon gamma responses of Mycobacterium bovis BCG vaccine in goat kids and milking goats. Vaccine. 2016;34(7):881–886. doi: 10.1016/j.vaccine.2016.01.004. [DOI] [PubMed] [Google Scholar]
- 209.Akkina R. Human immune responses and potential for vaccine assessment in humanized mice. Current Opinion in Immunology. 2013;25(3):403–409. doi: 10.1016/j.coi.2013.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Aguilar L. D., Infante E., Bianco M. V., Cataldi A., Bigi F., Pando R. H. Immunogenicity and protection induced by Mycobacterium tuberculosis mce-2 and mce-3 mutants in a Balb/c mouse model of progressive pulmonary tuberculosis. Vaccine. 2006;24(13):2333–2342. doi: 10.1016/j.vaccine.2005.11.051. [DOI] [PubMed] [Google Scholar]
- 211.Wang C. C., Zhu B., Fan X., Gicquel B., Zhang Y. Systems approach to tuberculosis vaccine development. Respirology. 2013;18(3):412–420. doi: 10.1111/resp.12052. [DOI] [PubMed] [Google Scholar]


