Abstract
Coronary artery bypass graft (CABG) is one of the primary methods of treating coronary heart disease (CHD); however, vein graft restenosis is a major limiting factor of the effectiveness of CABG. Emerging evidence has indicated that miR-423 is associated with vascular diseases. Additionally, upregulation of a disintegrin and metalloproteinase with thrombospondin motifs-7 (ADAMTS-7) contributes to neointima formation by promoting the proliferation and migration of vascular smooth muscle cells and inhibiting the proliferation and migration of endothelial cells. The aim of the present study was to examine the effects of miR-423 target, ADAMTS-7, on regulating vein graft disease and identify novel biomarkers for use in therapy of vein graft failure (VGF). Aberrant expression of miR-423 in plasma of patients with CHD prior to and following CABG confirms that miR-423 may be a suitable target for preventing VGF. Furthermore, a dual-luciferase reporter gene assay indicated that miR-423 directly interacted with ADAMTS-7 and suppressed its expression. Ectopic expression of miR-423 suppressed ADAMTS-7, resulting in decreased proliferation and migration rates of human umbilical vein smooth muscle cells by targeting ADAMTS-7, but resulted in increased proliferation and migration of human umbilical vein endothelial cells in vitro. Overexpression of miR-423 also enhanced re-endothelialization and decreased neointimal formation in a rat vein graft model. In conclusion, the results of the present study demonstrated that the miR-423/ADAMTS-7 axis may possess potential clinical value for the prevention and treatment of restenosis in patients with CHD following CABG.
Keywords: coronary heart disease, coronary artery bypass grafting, vein graft restenosis, a disintegrin and metalloproteinase with thrombospondin motifs-7, microRNA-423, endothelial cells, vascular smooth muscle cells
Introduction
In recent years, the incidence and mortality rates of patients with coronary heart disease (CHD) have increased annually, and it is one of the primary causes of mortality of hospitalized patients (1,2). Coronary artery bypass graft (CABG) is one of the primary means of treating patients with CHD (3,4). At present, the great saphenous veins are the most frequently used vessel for graft material for CABG. Although significant improvements have been made in surgical techniques and surgical instruments, vein graft failure (VGF) remains a frequent outcome in patients who have undergone CABG, and seriously limits the effectiveness of surgery and consequent prognosis (5,6). Studies analyzing CABG operations over a 10-year period have indicated that the incidence of VGF is >50%. Of these failures, >90% are acute VGF that occurred within 1 month following surgery and were the result of an acute thrombosis (7-9). Regular anticoagulation therapy may decrease the incidence of acute VGF. However, >60% of chronic VGF occurring in a 10 year period following CABG was due to the proliferation and migration of smooth muscle cells (SMCs) (10,11). Furthermore, the proliferation and migration of SMCs may mediate platelet adhesion and atheromatous plaque formation, which may aggravate VGF (12). However, the mechanism of SMCs proliferation and migration in VGF remains unclear. There are no effective therapeutic means of inhibiting the proliferation and migration of SMCs. Therefore, determining the mechanism of the proliferation and migration of SMCs in VGF has been the subject of increased attention in cardiac surgery research.
MicroRNA (miRNA) are a class of non-coding RNAs, ~22 nucleotides in length, with regulatory functions that serve a pivotal role in the regulation of developmental timing, cell proliferation and migration. Continuous studies investigating the functions of miRNAs have demonstrated the association between miRNA and cardiovascular diseases (13,14). A number of studies have indicated that miRNAs regulate the proliferation and migration of vascular endothelial cells (ECs) and SMCs (15-18). Inhibition of vascular SMC is an effective method of preventing VGF (19,20). The plasma expression level of miR-423 was upregulated in patients with CHD following CABG (21). Previous studies revealed abnormal expression of miR-423 in heart disease (22-24). In addition, miR-423 has been reported to be involved in regulating the development of various vascular diseases, including atherosclerosis (25), myocardial infarction (26), diabetic vascular complications (27) and lymphoma (28). However, the role of miR-423 in regulating the proliferation and migration of ECs and SMCs to mediate VGF remains unclear.
A disintegrin and metalloproteinase with thrombospondin motifs-7 (ADAMTS-7), the 7th member of the ADAMTS family to be identified, serves a vital role in multiple biological processes, including embryogenesis, vasculogenesis and blood coagulation. For example, increased expression of ADAMTS-7 enhanced the proliferation and migration capacity of SMCs (29). Upregulation of ADAMTS-7 promoted atherosclerosis by regulating degradation and remodeling of the vascular matrix (30). Wang et al (31) suggested that ADAMTS-7 overexpression accelerated the progression of carotid artery injury in rats by promoting the proliferation and migration of SMCs. Furthermore, ADAMTS-7 is involved in intima hyperplasia following vascular injury (32). However, the underlying mechanism is not yet understood. Therefore, the importance of ADAMTS-7 in vein graft restenosis requires additional investigation.
In the present study, reverse transcription-quantitative polymerase chain reaction (RT-qPCR) was used to measure the expression levels of miR-423 in plasma, and in human umbilical vein endothelial cells (HUVECs) and human umbilical vein smooth muscle cells (HUVSMCs), and the association between miR-423 and ADAMTS-7 was assessed. A potential interaction between miR-423 and ADAMTS-7 was hypothesized to regulate the proliferation and migration of HUVSMCs and HUVECs in vitro. Furthermore, the role of miR-423 overexpression in a rat vein graft model was examined in vivo.
Materials and methods
Ethics statement
The present study obtained approval from The Ethics Committee of The First People's Hospital of Yunnan Province (approval no. 2017YYLH018) and complied with the guidelines and principles of the Declaration of Helsinki. All participants provided written informed consent. Experiments involving animals were conducted according to the guidelines of the Animal Care and Use Committees at The First People's Hospital of Yunnan Province.
Plasma specimens
For the clinical part of the present study, 15 patients (age, 62.279.04 years; 9 males and 6 females) who were preparing to undergo CABG surgery and 10 healthy volunteers (age, 58.6±4.48 years; 5 males and 5 females) from urban and rural areas were recruited. The patients were recruited at the Department of Cardiovascular Surgery of The First People's Hospital of Yunnan Province between December 2017 to March 2018. The healthy volunteers were recruited at the medical examination center of The First People's Hospital of Yunnan Province between December 2017 to March 2018. Peripheral blood samples (2 ml) were collected from the patients into EDTA tubes 1 day prior to and 1, 5, 10 and 20 days following surgery in both patients and healthy volunteers. Samples were frozen in liquid nitrogen immediately and stored at −80°C for subsequent experiments.
Cell culture
HUVECs and HUVSMCs were obtained from the BeNa Culture Collection and 293T cells were purchased from the American Type Culture Collection. Cells were cultured in Dulbecco's modified Eagle's medium (DMEM; Thermo Fisher Scientific, Inc.) containing 1% penicillin-streptomycin and 10% FBS (Gibco; Thermo Fisher Scientific, Inc.) at 37°C and 5% CO2.
Cell transfection
A total of 24 h prior to transfection, HUVECs and HUVSMCs were seeded in 6-well plates at a density of 2×105 cells/well and incubated overnight. Subentry, 50 nM miR-423 mimic, 100 nM miR-423 inhibitor and 50 nM negative control were transfected into HUVECs and HUVSMCs using Lipofectamine™ 3000 reagent and Opti-MEM medium (Invitrogen; Thermo Fisher Scientific, Inc.) according to the manufacturer's protocols, and incubated for 4 h. The mixed solution was then replaced with complete growth medium and cultured for 24 h. The miR-423 mimics, inhibitor, pcDNA3.1 ADAMTS-7, and control (blank plasmid) were purchased from Tolo Biotech Co., Ltd. The Sequences corresponding to miR-423 mimic were: 5′-GCCTGAGGGGCAGAGAGC-3′, miR-423 inhibitor, 5′-ATCTTTGGTGGCCGTAGACCT-3′, and scrambled negative control, 5′-GCCTAACTGTGTCAGAAGGAA-3′.
RT-qPCR
Total RNA was isolated from cultured plasma and cells using TRIzol® reagent (Qiagen GmbH) and revere-transcribed into cDNA using the PrimeScript™ RT reagent kit using a gDNA Eraser (Takara Bio, Inc.). RT-qPCR Master mix was purchased from (Takara Bio, Inc.). The sequence of the primers for qPCR are presented in Table I. U6 was used as an internal control. The 2−∆∆Cq method (33) was used to calculate the relative expression of miR-423. qPCR was performed in triplicate.
Table I.
Name and sequences of the primers.
| Name | Primer sequences |
|---|---|
| miR-423 | F: 5′-GCCTGAGGGGCAGAGAGC-3′ |
| R: 5′-CCACGTGTCGTGGAGTC-3′ | |
| U6 | F: 5′-CTCGCTTCGGCAGCACA-3′ |
| R: 5′-AACGCTTCACGAATTTGCGT-3′ | |
| ADAMTS-7 | F: 5′-GTCATCGACTTCCCTTCCATAC-3′ |
| R: 5′-TGTCCATGTCATCGCAGAAG-3′ | |
| GAPDH | F: 5′-ATGCCTCCTGCACCACCA-3′ |
| R: 5′-AGTCCCTCCACGATGCCAA-3′ |
F, forward; R, reverse primer; miR, microRNA; ADAMTS-7, a disin-tegrin and metalloproteinase with thrombospondin motifs-7.
CCK-8 assay
Cell Counting Kit-8 (Sigma-Aldrich; Merck KGaA) was used to assess the proliferation rates of the HUVECs and HUVSMCs. Cells were seeded in 96-well plates at a density of 1×105 cells per well and cultured with 5% CO2 at 37°C for 2 h to allow cells to adhere. A total of 10 µl CCK-8 solution was added to each well and mixed, and cells were incubated for an additional 2 h at 37°C. A dual-wavelength microplate reader was used to measure proliferation at 450 nm (Beckman Coulter, Inc.). Assays were performed in triplicate.
Transwell migration assay
A total of 2×105 cells/ml HUVECs or HUVSMCs were plated in 200 µl serum-free medium in the upper layer of the Transwell chambers (Corning, Inc.) and 800 µl medium supplemented with 10% FBS was added to the bottom chamber. After incubation for 24 h at 37°C, the cells that had migrated were fixed with 4% paraformaldehyde at 37°C, rinsed three times with PBS, stained with 0.1% crystal violet for 10 min at 37°C and rinsed three times with PBS. The images of migrated cells were taken using a light microscope (Olympus Corporation). For quantification, 5 randomly selected fields were analyzed at magnification, ×40.
miR-423 target prediction
TargetScan 7.2 (http://www.Targetscan.org) was performed to predict the target genes of miR-423 according to the previous studies (34-37).
Dual-luciferase reporter gene assay
The 3′-UTR fragments of ADAMTS-7 containing the wild-type (WT) miR-423-binding site or a mutant (MUT) miR-423-binding site were amplified by Shanghai GenePharma Co., Ltd. and cloned into the pmirGLO luciferase reporter gene vector (Promega Corporation). The generated luciferase reporter plasmids were designated as WT-ADAMTS-7-3′UTR and MUT-ADAMTS-7-3′UTR. WT-ADAMTS-7-3′UTR or MUT-ADAMTS-7-3′UTR were co-transfected with miR-423 mimics (50 nM) or mimics control (50 nM) into 293T cells using Lipofectamine® 3000 reagent (Invitrogen; Thermo Fisher Scientific, Inc.). After 48 h of incubation at 37°C, the activity of luciferase was determined using a dual-luciferase reporter assay system (cat. no. PR-E1960; Promega Corporation). The firefly lucif-erase activity was normalized to Renilla luciferase activity.
Western blot analysis
Total protein was extracted for western blot analysis. Briefly, total proteins were extracted from tissues samples or cells using RIPA lysis buffer (Beijing Solarbio Science & Technology Co., Ltd) and protein concentration was determined using a BCA Protein assay kit (Thermo Fisher Scientific, Inc.). Protein (40 µg/lane) from each sample was separated by 12% SDS-PAGE and transferred onto PVDF membranes (Sigma-Aldrich; Merck KGaA). The membranes were blocked at 37°C for 2 h with 5% fat-free milk diluted with TBS containing 0.1% Tween-20. Subsequently, the PVDF membranes were incubated overnight at 4°C with the primary antibodies against ADAMTS-7 (cat. no. ab203027; 1:1,000; Abcam), α-smooth muscle actin (α-SMA; cat. no. ab32575; 1:1,000; Abcam), matrix metalloproteinase (MMP2; cat. no. ab215986; 1:1,000; Abcam), MMP9 (cat. no. ab219372; 1:1,000; Abcam) and proliferating cell nuclear antigen (PCNA; cat. no. ab92552; 1:1,000; Abcam). After washing, the membranes were incubated for 1 h at 37°C with horseradish peroxidase-conjugated secondary antibodies (cat. nos. ab6721 and ab6728; 1:500; Abcam) at 37°C for 2 h. The signal was visualized using enhanced chemiluminescence reagent (Bio-Rad Laboratories, Inc.) according to the manufacturer's protocol. Densitometric analysis was performed using ImageJ 1.8.0 software (National Institute of Health). The expression levels of protein in each sample were normalized to GAPDH.
Immunohistochemistry assay
Vessel graft wall tissues were collected and fixed in 4% paraformaldehyde for 48 h at 37°C. Subsequently, tissues were embedded in paraffin, and 4 µm-thick slices were dewaxed and rehydrated in a graded series of ethanol solutions (50, 75, 85, 95 and 100%; 5 min per solution). Xylene and a graded series of ethanol (100, 95, 85, and 75%) were used to dewax and hydrate the samples, followed by 30 min of antigen retrieval in Tris-EDTA (pH 9.0) in a 720 W microwave. Subsequently, a DAB horseradish peroxidase color development kit (Beyotime Institute of Biotechnology) with a Ki-67 antibody (cat. no. ab15580; 1:1,000; Abcam) was used to stain the samples at room temperature for 15 min. The, slides were then dyed with hematoxylin for 30 sec at 37°C, dehydrated and fixed, and sealed with neutral glue. Stained images were observed and photographed under a fluorescence microscope (Olympus Corporation) at magnification, ×400.
Hematoxylin and eosin (H&E) staining and Masson staining
Whole vessel graft lumen tissues obtained from the rat vein graft models were dissected and fixed with 4% paraformaldehyde for 24 h at room temperature. Subsequently, samples were dehydrated and embedded in paraffin blocks. Blocks of 6-µm thickness were cut and stained at room temperature using hematoxylin for 20 min and eosin for 5 min to measure the neointimal thickness. Meanwhile, sections of rat vein grafts were also stained with Masson's trichrome for the evaluation of collagen expression. Masson's trichrome kit (cat. no. Mst-8003/8004; Fuzhou Maixin Biotech Co., Ltd.) was used at room temperature according to the manufacturer's protocol, and observed under a light microscope (magnification, ×200; Olympus Corporation).
Rat vein graft models
A total of 30 male Sprague-Dawley rats (8-9 week-old; weight, 250-300 g) were purchased from the Experimental Animal Center of Kunming Medical University. All animals were housed in an animal facility with a 12:12 h light: Dark cycle at 18-22°C and 40-60% humidity, and had ad libitum access to rodent chow and water. Subsequently, all rats were anesthetized with 300 mg/kg chloral hydrate by intraperitoneal injection and systemically heparinized, and no rats exhibited signs of peritonitis following the administration of chloral hydrate. Next, all rats were randomly separated into three groups, with 10 rats per group. A right sternocleidomastoid incision was performed in all the groups. Subsequently, exposure of the right jugular vein of a sufficient size was performed and a section ~2 cm was trimmed for vessel graft. Then, the vessel graft to the right carotid artery was replaced. During surgery, 8 nmol miR-423 agomir dissolved in normal saline (0.9%) according to the manufacturer's instructions, was perfused into the vein graft under a distending pressure of 20 mmHg for 10 min at room temperature prior to the arteriovenous anastomosis. Rats in the sham group were injected with the same volume of saline at the same location. Rats in all groups were sacrificed immediately, and the jugular vein was harvested and further analyzed.
Statistical analysis
All statistical analyses were performed using SPSS v.22.0 software (IBM Corp.). Data are presented as the mean ± standard deviation. Differences between multiple groups were analyzed using a one-way analysis of variance followed by Tukey's post hoc test. A Student's t-test was used to analyze differences between two groups. P<0.05 was considered to indicate a statistically significant difference.
Results
Expression of miR-423 in patients with CHD following CABG surgery
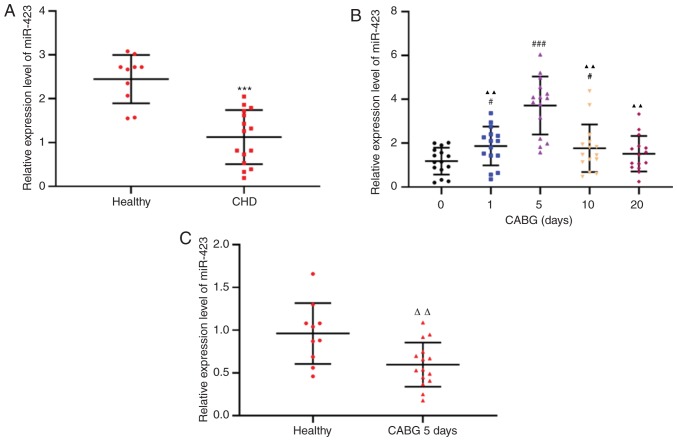
To investigate the effects of miR-423 on autologous vein graft restenosis, the clinicopathological characteristics were compared. There were no significant differences in sex, age, smoking habits, drinking habits, family history, diabetes mellitus and hypertension between the patients who underwent CABG and healthy controls (Table II). RT-qPCR was used to detect miR-423 expression levels in the plasma of patients with CHD (n=15) and healthy controls (n=10). The expression levels of miR-423 in the plasma of patients with CHD were decreased compared with the healthy subjects (P<0.001; Fig. 1A). The expression levels of plasma miR-423 in all samples from patients with CHD 1 day prior to CABG surgery and 1, 5, 10 and 20 days following CABG surgery were decreased by the 10th day following CABG and returned to the preoperative levels 20 days after CABG (Fig. 1B). In addition, even the maximum expression levels of miR-423 in patients with CHD following CABG were decreased compared with those in the healthy subjects (P<0.01; Fig. 1C). Therefore, an abnormal increase in miR-423 expression in patients with CHD prior to and following CABG may be involved in the protection of transplanted blood vessels in surgery, although the mechanism remains unclear.
Table II.
Clinicopathological characteristics.
| Characteristics | Healthy people (n=10) | CABG patients (n=15) | P-value |
|---|---|---|---|
| Sex | |||
| Male, n (%) | 5 (50) | 9 (60) | 0.5836 |
| Female, n (%) | 5 (50) | 6 (40) | 0.6124 |
| Age, years (range) | 58.6±4.48 (50-65) | 62.27±9.04 (45-72) | 0.1293 |
| Drinking, n (%) | 4 (40) | 7 (46.67) | 0.6502 |
| Smoking, n (%) | 4 (40) | 6 (40) | 0.7954 |
| Family history, n (%) | 3 (30) | 5 (30) | 0.8135 |
| Diabetes mellitus, n (%) | 2 (20) | 3 (20) | 0.7106 |
| Hypertension, n (%) | 2 (20) | 4 (26.67%) | 0.6341 |
CABG, coronary artery bypass graft.
Figure 1.
Relative expression levels of miR-423. (A) Expression levels of miR-423 in the plasma of healthy volunteers (n=10) and patients with CHD. ***P<0.001 vs. healthy group. (B) Expression of miR-423 in the plasma of patients with CHD at 1 day prior to and 1, 5, 10 and 20 days following CABG. #P<0.05 and ###P<0.001 vs. 1 day prior to CABG. ▲▲P<0.01 vs. 5 days following surgery. (C) Expression of miR-423 in the plasma from healthy control and patients with CHD 5 days after CABG. ΔΔP<0.01 vs. healthy control group. miR, miRNA; CHD, coronary heart disease; CABG, coronary artery bypass graft; miR, microRNA.
Effect of miR-423 on the proliferation and migration of HUVECs
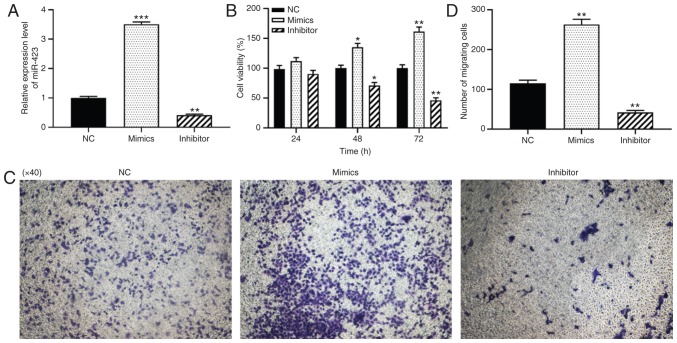
To further verify the function of miR-423 in patients with CHD following CABG surgery, the effects of miR-423 on the proliferation and migration levels of HUVECs were examined using CCK-8 and Transwell assays. HUVECs were transfected with miR-423 mimics or inhibitor to alter the expression of miR-423. RT-qPCR analysis indicated that, compared with the control group, the expression levels of miR-423 were significantly increased in cells transfected with miR-423 mimics (P<0.001; Fig. 2A) and significantly decreased in cells transfected with miR-423 inhibitor (P<0.01; Fig. 2A). The CCK-8 assay results indicated that the over-expression of miR-423 notably promoted cell viability in HUVECs at 48 (P<0.05) and 72 h (P<0.01) compared with the NC group (Fig. 2B). Data from the Transwell migration assays demonstrated that the upregulation of miR-423 markedly increased the migration of HUVECs compared with the NC group. (P<0.01; Fig. 2C and D), and knockdown of miR-423 resulted in the opposite effects on proliferation and migration (P<0.01). Taken together, the overexpression of miR-423 was demonstrated to increase the proliferation and migration levels of HUVECs.
Figure 2.
Effect of miR-423 on the proliferation and migration of HUVECs in vitro. (A) Expression levels of miR-423. (B) Proliferation of HUVECs was evaluated using a Cell Counting Kit-8 assay; (C and D) Migration capacity of HUVECs was determined using a Transwell migration assay. (C) Images are at magnification, ×40. (D) The number of migrating cells was quantified. *P<0.05, **P<0.01 and ***P<0.001 vs. NC group. miR, miRNA; CHD, coronary heart disease; NC, negative control; miR, microRNA; NC, negative control; HUVECs, human umbilical vein endothelial cells.
Effect of miR-423 on the proliferation and migration of HUVSMCs
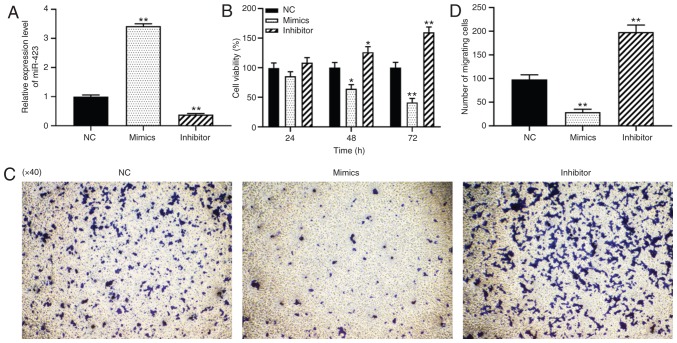
To investigate the loss- and gain-of-function of miR-423 in vascular SMCs of vein grafts, miR-423 mimics or inhibitors were transfected into HUVSMCs, and the results are presented in Fig. 3A. CCK-8 and Transwell migration assays were used to detect the effects of miR-423 on the proliferation and migration of HUVSMCs. As indicated in Fig. 3B-D, knockdown of miR-423 significantly promoted the proliferation at 48 (P<0.05) and 72 h (P<0.01; Fig. 3B) and migration capacity of HUVSMCs (P<0.01; Fig. 3C and D), whereas overexpression of miR-423 resulted in the opposite effect (P<0.01). These results suggest that miR-423 overexpression significantly inhibited the proliferation and migration of HUVSMCs in vitro.
Figure 3.
Effect of miR-423 on the proliferation and migration of HUVSMCs in vitro. (A) Expression of miR-423 was detected using reverse transcription-quantitative polymerase chain reaction. (B) Proliferation of HUVSMCs was evaluated using a Cell Counting Kit-8 assay; (C and D) Migration capacity of HUVSMCs was measured using a Transwell migration assay. (C) Images are at magnification, ×40. (D) The number of migrating cells was quantified. *P<0.05 and **P<0.01 vs. NC group. miR, miRNA; CHD, coronary heart disease; NC, negative control.
miR-423 directly targets ADAMTS-7
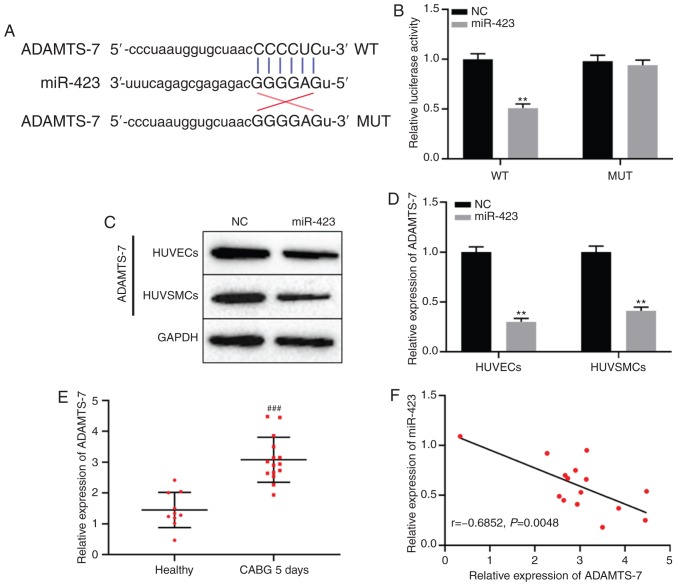
TargetScan database analysis indicated that miR-423 may target ADAMTS-7 directly (Fig. 4A). To determine whether miR-423 specifically bound to the 3′-untranslated region of ADAMTS-7 mRNA and regulated the expression of ADAMTS-7, a dual-luciferase reporter gene assay was used. The results demonstrated that luciferase activity in the ADAMTS-7-WT + miR-423 mimics group was decreased compared with the ADAMTS-7-WT + miR-NC group, but there was no significant difference between miR-423 mimics or NC combined with ADAMTS-7-MUT group (P<0.01; Fig. 4B). Protein expression levels of ADAMTS-7 in cells transfected with miR-423 mimic compared with the NC group (P<0.01; Fig. 4C and D). Additionally, the expression levels of ADAMTS-7 were significantly increased in patients with CHD following CABG after 5 days compared with the healthy subjects (P<0.001; Fig. 4E). Spearman's correlation analysis results revealed that there was a remarkable negative correlation between the expression of miR-423 and ADAMTS-7 in patients with CHD following CABG after 5 days (r=−0.685; P<0.01; Fig. 4F). These results suggest that ADAMTS-7 was directly targeted by miR-423, and negatively regulated ADAMTS-7 expression.
Figure 4.
miR-423 directly targets ADAMTS-7. (A) miR-423 predicted binding site with the 3′UTR of ADAMTS-7 using the TargetScan database. (B) Dual-luciferase reporter gene assay was used to verify the interaction between miR-423 and ADAMTS-7. (C and D) The effect of miR-423 on protein expression levels of ADAMTS-7 in HUVECs and HUVSMCs were detected using western blot analysis. (C) Representative blot gel. (D) Densitometric analysis of the ADAMTS-7 protein expression levels. (E) The levels of mRNA expression of ADAMTS-7 was measured using reverse transcription-quantitative polymerase chain reaction. (F) Associated between miR-423 and ADAMTS-7 in patients 5 days after CABG was evaluated using a Spearman's correlation analysis. **P<0.01 vs. NC group. ###P<0.001 vs. healthy control group. ADAMTS-7, a disintegrin and metalloproteinase with thrombospondin motifs-7; miR, miRNA; UTR, untranslated region; CABG, coronary artery bypass graft; NC, negative control; WT, wild type; MUT, mutant.
Effect miR-423/ADAMTS-7 axis on the proliferation and migration of HUVECs and HUVSMCs
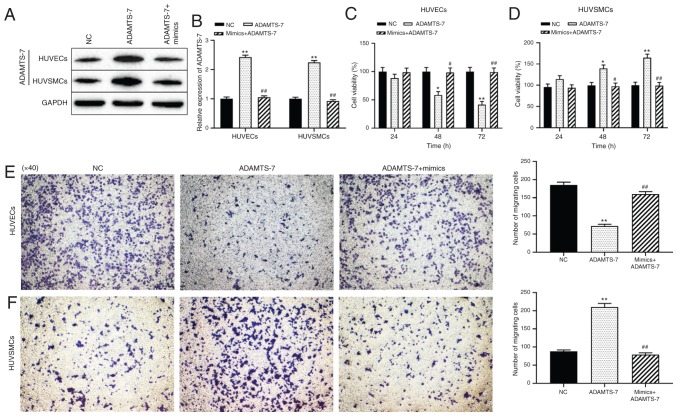
The mechanism by which miR-423 modulated cell proliferation and migration of HUVECs and HUVSMCs was then assessed. Western blot analysis demonstrated that transfection of ADAMTS-7 was successful and protein expression levels were increased, and that the increase in expression was reversed in cells co-transfected with miR-423 mimics + pcDNA-ADAMTS-7 (P<0.01; Fig. 5A and B). Compared with the NC group, overexpression of ADAMTS-7 significantly decreased the proliferation and migration ability of HUVECs at 48 (P<0.05) and 72 h (P<0.01; Fig. 5C and E), but promoted the proliferation and migration of HUVSMCs at 48 (P<0.05) and 72 h (P<0.01; Fig. 5D and F). The effect of increased ADAMTS-7 levels on proliferation and migration of HUVSMCs or HUVECs was reversed by transfection with miR-423 mimics. These results suggest that upregulation of miR-423 decreases ADAMTS-7 expression, thereby promoting the proliferation and migration of HUVECs and decreasing these behaviors in HUVSMCs in vitro.
Figure 5.
Effect of the miR-423/ADAMTS-7 axis on the proliferation and migration of HUVECs and HUVSMCs. (A and B) Expression levels of ADAMTS-7 were detected using western blot analysis. (A) Representative blot gel. (B) Densitometric analysis of the ADAMTS-7 protein expression levels. (C and D) The proliferation rates of (C) HUVECs and (D) HUVSMCs were evaluated using a Cell Counting Kit-8 assay. (E and F) Migration capacity of (E) HUVECs and (F) HUVSMCs were measured using a Transwell migration assay. Images are at magnification, ×40. *P<0.05 and **P<0.01 vs. NC group. #P<0.05 and ##P<0.01 vs. ADAMTS-7 overexpression group. ADAMTS-7 overexpression group. ADAMTS-7, a disintegrin and metalloproteinase with thrombospondin motifs-7; miR, miRNA; NC, negative control; HUVECs, human umbilical vein endothelial cells; HUVSMCs, human umbilical vein smooth muscle cells.
Effect of miR-423 upregulated on autologous vein graft restenosis in vivo
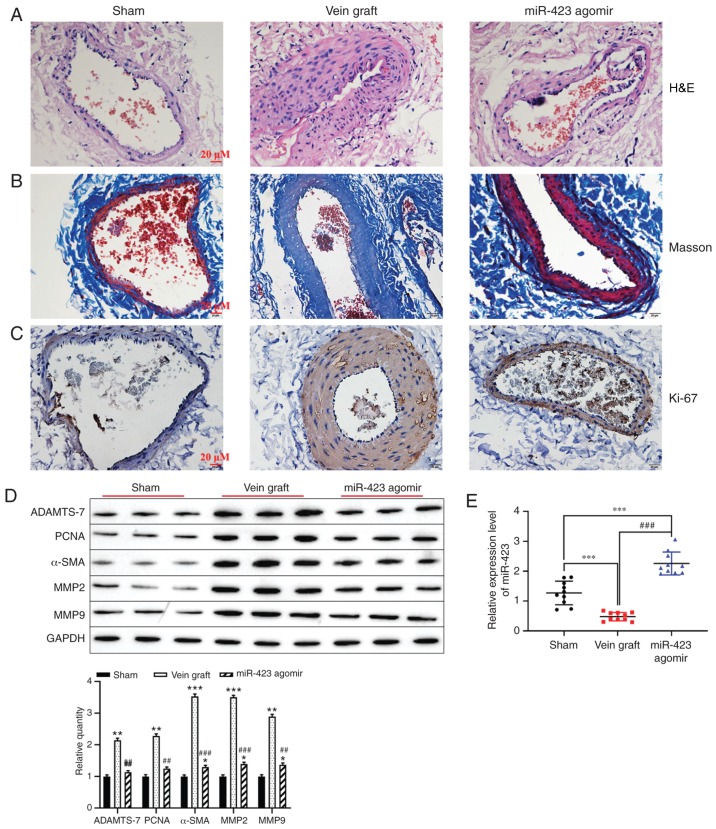
Based on the effect of miR-423 on the proliferation and migration in HUVSMCs or HUVECs, the function of miR-423 in the distension on vein graft restenosis in vivo was determined. Hematoxylin and eosin staining indicated that overexpression of miR-423 markedly decreased neointimal thickness compared with the vein graft group (Fig. 6A). However, Masson staining results revealed that the percentage of the neointima area occupied by blue-colored fibers (collagen) was significantly different between the miR-423 agomir-treated group and the Sham group or Vein graft group (Fig. 6B). Immunohistochemistry staining demonstrated that increased levels of miR-423 decreased the expression of Ki-67 in carotid arteries compared with the vein graft group (Fig. 6C). Furthermore, western blot analysis was applied to detect the effect of miR-423 overexpression on the proliferation of vascular SMCs. Upregulation of miR-423 decreased the expression vein graft-induced ADAMTS-7, PCNA, MMP2, MMP9 and α-SMA (P<0.01; Fig. 6D). Expression of miR-423 was upregulated in the miR-423 agomir group compared with the vein graft group or the Sham group (P<0.001; Fig. 6E). These results suggest that overexpression of miR-423 may attenuate autologous vein graft restenosis via targeting ADAMTS-7 in vivo.
Figure 6.
Effect of miR-423 on autologous vein graft restenosis in vivo. (A) Effect of miR-423 overexpression on the thickening of vascular intima in vivo was evaluated using H&E staining. Images are at magnification, ×20. (B) Masson-trichrome staining was applied to evaluate the percentage of collagen in the neointima area. Images are at magnification, ×20. (C) Proliferation of vascular cells, visualized using immunostaining. Images are at magnification, ×20. (D) Expression of ADAMTS-7, PCNA, α-SMA, MMP2, MMP9 were detected using western blot analysis. (E) Expression of miR-423 was detected using reverse transcription-quantitative polymerase chain reaction. *P<0.05, **P<0.01 and ***P<0.001 vs. Sham group. ##P<0.01 and ###P<0.001 vs. vein graft group. ADAMTS-7, a disintegrin and metalloproteinase with thrombospondin motifs-7; PCNA, proliferating cell nuclear antigen; α-SMA, α-smooth muscle actin; MMP, matrix metalloproteinase; miR, miRNA; H&E, hematoxylin and eosin.
Discussion
CHD is a common disease that affects the quality of life patients and causes great economic burden to the patients and the whole society (38). In the present study, the role of the miR-423/ADAMTS-7 axis in the proliferation and migration of HUVECs and HUVSMCs in vitro and in vivo was examined. miR-423 was downregulated in the plasma of patients with CHD compared with healthy volunteers. Upregulation of miR-423 decreased the expression level of ADAMTS-7, resulting in increases in the rates of proliferation and migration of HUVECs, and accelerated the endothelialization of the artificial vessel in vivo. In the past decade, numerous studies in the field of miRNAs have provided evidence to support the role of miRNAs in cardiovascular function and disease, and miRNAs have exhibited significant clinical potential (39,40).
The biological behavior of HUVSMCs and HUVECs allows them to serve an important role in restenosis and long-term occlusion of vein bridges following coronary angioplasty (41-44). Increasing evidence has indicated that the abnormal expression of miRNAs serves an important role in transplanted vascular diseases and they have received much interest in the research of various diseases (21,45-47). Proliferation, migration and phenotypic transformation of SMCs or endothelial cells are associated with various miRNAs (48) including miR-145 (49,50), miR-222 (51), miR-195 (52) and miR-21 (53). For example, Liu et al (54) confirmed that the upregulation of miR-378a targeted cyclin-dependent kinase 1 to promote the proliferation and migration of vascular SMCs and increase the incidence of in-stent restenosis following stenting. Huang et al (55) suggested that miR-22-3p overexpression inhibited proliferation and migration of human artery vascular SMCs and prevented neointimal hyperplasia by targeting high mobility group box-1. Endothelial cells-derived miR-195 significantly suppressed the proliferation and migration of vascular SMCs (52). Qu et al (56) identified that all patients with severe CHD, who were preparing for CABG had a significantly decreased level of miR-126-3p expression compared with the healthy subjects. Taken together, inhibition of the biological function of SMCs may be possible using specific drugs or biomolecules and this may prevent graft vein failure. The results of the present study revealed that the expression levels of miR-423 the plasma of patients with CHD were decreased compared with those in the healthy subjects, and that upregulation of miR-423 increased proliferation and migration of HUVECs and had anti-proliferative and anti-migratory effects on HUVSMCs. This result is in agreement with previous studies indicating that the expression levels of miRNAs related to the regulation of endothelial cell biological behavior and vasculoprotective are significantly decreased in patients with stable CHD (57).
Previous studies have demonstrated that ADAMTS-7 is an important member of the depolymerized protein-like metal-loproteinase family containing platelet binding protein motifs, and its gene polymorphism is significantly associated with the susceptibility to human coronary artery disease (30,58). Recently, ADAMTS-7 levels were demonstrated to be associated with the severity of disease in patients with CHD or saphenous vein grafts restenosis following CABG (59,60). Furthermore, abnormally upregulated expression levels of ADAMTS-7 promoted the proliferation and migration of vascular SMCs and induced neointima formation in vitro and in vivo (29,61). ADAMTS-7 promoted the proliferation and migration of vascular SMCs by decomposing the extracellular matrix, and thereby accelerating intimal hyperplasia, resulting in restenosis (62,63). In addition, upregulation of ADAMTS-7 decreased the proliferation and migration of endothelial cells and restenosis following vascular injury by interrupting the homeostasis between thrombospondin-1 and its natural inhibitor cartilage oligomeric matrix protein (COMP) (31,32). In the present study, overexpression of ADAMTS-7 significantly promoted the proliferation and migration of HUVSMCs and induced neointima formation following aorta injury. Knockdown of ADAMTS-7 significantly decreased the expression of metalloproteinases such as MMP-2 and MMP-9. Previous studies have indicated that MMPs contributed to the activation of vascular SMCs proliferation (29,64). Overexpression of ADAMTS-7 degrades matrix COMP in vessels (31), and COMP decreases vascular SMCs migration by maintaining them in a quiescent/contractile state (65). Based on the results of previous studies, upregulation of ADAMTS-7 induces neointima formation following injury to the aorta through promoting growth and migration of vascular SMCs, perhaps as a result of degradation of COMP. Furthermore, ADAMTS-7 was significantly highly expressed in the neointima in vein grafts, and the expression level was markedly decreased in vein grafts treated with miR-423 agomir.
In conclusion, the miR-423/ADAMTS-7 axis may be involved in regulating the proliferation and migration of HUVSMCs and HUVECs in vitro and in vivo, and may serve a pivotal role in neointima formation in response to injury. The present study may highlight a novel molecular target for treating patients with CHD following CABG and new biomarkers for diagnosis and prognosis.
Acknowledgments
Not applicable.
Funding
The present study was supported by the Applied Basic Research Project of Yunnan Provincial Science and Technology Department, Kunming Medical University [Kunming, China; grant no. 2018FE001(−290)].
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Authors' contributions
YL conceived and designed the study. WR, LL, FW, and NM performed the experiments and collected the data. WR, LZ, WH, DX, and YC contributed to the analysis of the data and data acquisition. HL contributed to the analysis of data and manuscript preparation. WR and LL drafted the paper and revised manuscript. All authors read and approved the final manuscript.
Ethics approval and consent to participate
The present study obtained approval from The Ethics Committee of The First People's Hospital of Yunnan Province (approval no. 2017YYLH018) and complied with the guidelines and principles of the Declaration of Helsinki. All participants provided written informed consent. Experiments involving animals were conducted according to the guidelines of the Animal Care and Use Committees at The First People's Hospital of Yunnan Province.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Yang X, Li Y, Ren X, Xiong X, Wu L, Li J, Wang J, Gao Y, Shang H, Xing Y. Effects of exercise-based cardiac rehabilitation in patients after percutaneous coronary intervention: A meta-analysis of randomized controlled trials. Sci Rep. 2017;7:44789. doi: 10.1038/srep44789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Anderson L, Oldridge N, Thompson DR, Zwisler AD, Rees K, Martin N, Taylor RS. Exercise-based cardiac rehabilitation for coronary heart disease: Cochrane systematic review and meta-analysis. J Am Coll Cardiol. 2016;67:1–12. doi: 10.1016/j.jacc.2015.10.044. [DOI] [PubMed] [Google Scholar]
- 3.Kaur K, Bedi G, Kaur M, Vij A, Kaur I. Lipid peroxidation and the levels of antioxidant enzymes in coronary artery disease. Indian J Clin Biochem. 2008;23:33–37. doi: 10.1007/s12291-008-0008-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yang X, He T, Han S, Zhang X, Sun Y, Xing Y, Shang H. The role of traditional chinese medicine in the regulation of oxidative stress in treating coronary heart disease. Oxid Med Cell Longev. 2019;2019:3231424. doi: 10.1155/2019/3231424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hausenloy DJ, Boston-Griffiths E, Yellon DM. Cardioprotection during cardiac surgery. Cardiovasc Res. 2012;94:253–265. doi: 10.1093/cvr/cvs131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hegewald J, Wegewitz UE, Euler U, van Dijk JL, Adams J, Fishta A, Heinrich P, Seidler A. Interventions to support return to work for people with coronary heart disease. Cochrane Database Syst Rev. 2019;3:CD010748. doi: 10.1002/14651858.CD010748.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McKavanagh P, Yanagawa B, Zawadowski G, Cheema A. Management and prevention of saphenous vein graft failure: A review. Cardiol Ther. 2017;6:203–223. doi: 10.1007/s40119-017-0094-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dianati Maleki N, Ehteshami Afshar A, Parikh PB. Management of saphenous vein graft disease in patients with prior coronary artery bypass surgery. Curr Treat Options Cardiovasc Med. 2019;21:12. doi: 10.1007/s11936-019-0714-7. [DOI] [PubMed] [Google Scholar]
- 9.Virk HUH, Lakhter V, Ahmed M, O'Mucrchu B, Chatterjee S. Radial artery versus saphenous vein grafts in coronary artery bypass surgery: A literature review. Curr Cardiol Rep. 2019;21:36. doi: 10.1007/s11886-019-1112-1. [DOI] [PubMed] [Google Scholar]
- 10.Kenagy RD, Kikuchi S, Chen L, Wijelath ES, Stergachis AB, Stamatoyannopoulos J, Tang GL, Clowes AW, Sobel M. A single nucleotide polymorphism of cyclin-dependent kinase inhibitor 1B (p27(Kip1)) associated with human vein graft failure affects growth of human venous adventitial cells but not smooth muscle cells. J Vasc Surg. 2018;67:309–317. e7. doi: 10.1016/j.jvs.2016.12.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wadey K, Lopes J, Bendeck M, George S. Role of smooth muscle cells in coronary artery bypass grafting failure. Cardiovasc Res. 2018;114:601–610. doi: 10.1093/cvr/cvy021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Campos LCG, Ribeiro-Silva JC, Menegon AS, Barauna VG, Miyakawa AA, Krieger JE. Clin Sci (Lond) Feb 2, 2018. Cyclic stretch-induced Crp3 sensitizes vascular smooth muscle cells to apoptosis during vein arterialization remodeling. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 13.Gu H, Liu Z, Zhou L. Roles of miR-17-92 cluster in cardiovascular development and common diseases. Biomed Res Int. 2017;2017:9102909. doi: 10.1155/2017/9102909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Moghaddam AS, Afshari JT, Esmaeili SA, Saburi E, Joneidi Z, Momtazi-Borojeni AA. Cardioprotective microRNAs: Lessons from stem cell-derived exosomal microRNAs to treat cardiovascular disease. Atherosclerosis. 2019;285:1–9. doi: 10.1016/j.atherosclerosis.2019.03.016. [DOI] [PubMed] [Google Scholar]
- 15.Zhang W, Liu D, Han X, Ren J, Zhou P, Ding P. MicroRNA-451 inhibits vascular smooth muscle cell migration and intimal hyperplasia after vascular injury via Ywhaz/p38 MAPK pathway. Exp Cell Res. 2019;379:214–224. doi: 10.1016/j.yexcr.2019.03.033. [DOI] [PubMed] [Google Scholar]
- 16.Zheng B, Yin WN, Suzuki T, Zhang XH, Zhang Y, Song LL, Jin LS, Zhan H, Zhang H, Li JS, Wen JK. Exosome-mediated miR-155 transfer from smooth muscle cells to endothelial cells induces endothelial injury and promotes atherosclerosis. Mol Ther. 2017;25:1279–1294. doi: 10.1016/j.ymthe.2017.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 17.Gabani M, Liu J, Ait-Aissa K, Koval O, Kim YR, Castaneda D, Vikram A, Jacobs JS, Grumbach I, Trebak M, et al. MiR-204 regulates type 1 IP3R to control vascular smooth muscle cell contractility and blood pressure. Cell Calcium. 2019;80:18–24. doi: 10.1016/j.ceca.2019.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang Y, Dong CQ, Peng GY, Huang HY, Yu YS, Ji ZC, Shen ZY. MicroRNA-134-5p regulates media degeneration through inhibiting VSMC phenotypic switch and migration in thoracic aortic dissection. Mol Ther Nucleic Acids. 2019;16:284–294. doi: 10.1016/j.omtn.2019.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cao BJ, Zhu L, Wang XW, Zou RJ, Lu ZQ. MicroRNA-365 Promotes the contractile phenotype of venous smooth muscle cells and inhibits neointimal formation in rat vein grafts. IUBMB Life. 2019;71:908–916. doi: 10.1002/iub.2022. [DOI] [PubMed] [Google Scholar]
- 20.Elsayed Y, Lekakou C, Tomlins P. Modeling, simulations, and optimization of smooth muscle cell tissue engineering for the production of vascular grafts. Biotechnol Bioeng. 2019;116:1509–1522. doi: 10.1002/bit.26955. [DOI] [PubMed] [Google Scholar]
- 21.Engler A, Dreja F, Koberle S, Thielmann M, Peters J, Frey UH. Establishment of an easy and straight forward heparinase protocol to analyse circulating and myocardial tissue micro-RNA during coronary artery-bypass-graft surgery. Sci Rep. 2018;8:1361. doi: 10.1038/s41598-018-19748-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.van Boven N, Kardys I, van Vark LC, Akkerhuis KM, de Ronde MWJ, Khan MAF, Merkus D, Liu Z, Voors AA, Asselbergs FW, et al. Serially measured circulating microRNAs and adverse clinical outcomes in patients with acute heart failure. Eur J Heart Fail. 2018;20:89–96. doi: 10.1002/ejhf.950. [DOI] [PubMed] [Google Scholar]
- 23.Miyamoto S, Usami S, Kuwabara Y, Horie T, Baba O, Hakuno D, Nakashima Y, Nishiga M, Izuhara M, Nakao T, et al. Expression patterns of miRNA-423-5p in the serum and pericardial fluid in patients undergoing cardiac surgery. PLoS One. 2015;10:e0142904. doi: 10.1371/journal.pone.0142904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tutarel O, Dangwal S, Bretthauer J, Westhoff-Bleck M, Roentgen P, Anker SD, Bauersachs J, Thum T. Circulating miR-423-5p fails as a biomarker for systemic ventricular function in adults after atrial repair for transposition of the great arteries. Int J Cardiol. 2013;167:63–66. doi: 10.1016/j.ijcard.2011.11.082. [DOI] [PubMed] [Google Scholar]
- 25.Zeng JF, Zeng ZL, Zhang K, Zhao Y, Liu YM, Chen JJ, Tong H, Wei DH, Jiang ZS, Wang Z. miR-23b-3p and miR-125b-5p downregulate apo(a) expression by targeting Ets1 in HepG2 cells. Cell Biol Int. 2018;42:313–323. doi: 10.1002/cbin.10896. [DOI] [PubMed] [Google Scholar]
- 26.Bauters C, Kumarswamy R, Holzmann A, Bretthauer J, Anker SD, Pinet F, Thum T. Circulating miR-133a and miR-423-5p fail as biomarkers for left ventricular remodeling after myocardial infarction. Int J Cardiol. 2013;168:1837–1840. doi: 10.1016/j.ijcard.2012.12.074. [DOI] [PubMed] [Google Scholar]
- 27.Hirota K, Keino H, Inoue M, Ishida H, Hirakata A. Comparisons of microRNA expression profiles in vitreous humor between eyes with macular hole and eyes with proliferative diabetic retinopathy. Graefes Arch Clin Exp Ophthalmol. 2015;253:335–342. doi: 10.1007/s00417-014-2692-5. [DOI] [PubMed] [Google Scholar]
- 28.Ayoubian H, Ludwig N, Fehlmann T, Menegatti J, Groger L, Anastasiadou E, Trivedi P, Keller A, Meese E, Grasser FA. Epsteinbarr virus infection of cell lines derived from diffuse large B-cell lymphomas alters microrna loading of the Ago2 complex. J Virol. 2019;93:e1297–e1318. doi: 10.1128/JVI.01297-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhang L, Yu F, Wang L, Zheng J, Du Y, Huang Y, Liu B, Wang X, Kong W. ADAMTS-7 promotes vascular smooth muscle cells proliferation in vitro and in vivo. Sci China Life Sci. 2015;58:674–681. doi: 10.1007/s11427-015-4843-2. [DOI] [PubMed] [Google Scholar]
- 30.Bengtsson E, Hultman K, Duner P, Asciutto G, Almgren P, Orho-Melander M, Melander O, Nilsson J, Hultgardh-Nilsson A, Goncalves I. ADAMTS-7 is associated with a high-risk plaque phenotype in human atherosclerosis. Sci Rep. 2017;7:3753. doi: 10.1038/s41598-017-03573-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang L, Zheng J, Bai X, Liu B, Liu CJ, Xu Q, Zhu Y, Wang N, Kong W, Wang X. ADAMTS-7 mediates vascular smooth muscle cell migration and neointima formation in balloon-injured rat arteries. Circ Res. 2009;104:688–698. doi: 10.1161/CIRCRESAHA.108.188425. [DOI] [PubMed] [Google Scholar]
- 32.Kessler T, Zhang L, Liu Z, Yin X, Huang Y, Wang Y, Fu Y, Mayr M, Ge Q, Xu Q, et al. ADAMTS-7 inhibits re-endothelialization of injured arteries and promotes vascular remodeling through cleavage of thrombospondin-1. Circulation. 2015;131:1191–1201. doi: 10.1161/CIRCULATIONAHA.114.014072. [DOI] [PubMed] [Google Scholar]
- 33.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 34.Agarwal V, Bell GW, Nam J, Bartel DP. Predicting effective microRNA target sites in mammalian mRNAs. Elife. 2015;4:e05005. doi: 10.7554/eLife.05005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.García DM, Baek D, Shin C, Bell GW, Grimson A, Bartel DP. Weak seed-pairing stability and high target-site abundance decrease the proficiency of lsy-6 and other miRNAs. Nat Struct Mol Biol. 2011;18:1139–1146. doi: 10.1038/nsmb.2115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Friedman RC, Farh KK, Burge CB, Bartel DP. Most mammalian mRNAs are conserved targets of MicroRNAs. Genome Res. 2009;19:92–105. doi: 10.1101/gr.082701.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Grimson A, Farh KK, Johnston WK, Garrett-Engele P, Lim LP, Bartel DP. MicroRNA targeting specificity in mammals: Determinants beyond seed pairing. Mol Cell. 2007;27:91–105. doi: 10.1016/j.molcel.2007.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shah T, Palaskas N, Ahmed A. An update on gender disparities in coronary heart disease care. Curr Atheroscler Rep. 2016;18:28. doi: 10.1007/s11883-016-0574-5. [DOI] [PubMed] [Google Scholar]
- 39.Nouraee N, Mowla SJ. miRNA therapeutics in cardiovascular diseases: Promises and problems. Front Genet. 2015;6:232. doi: 10.3389/fgene.2015.00232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.van Rooij E, Olson EN. MicroRNA therapeutics for cardiovascular disease: Opportunities and obstacles. Nat Rev Drug Discov. 2012;11:860–872. doi: 10.1038/nrd3864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kostina D, Zverev D, Grebennik V, Gordeev M, Ignatieva E, Voronkina I, Kostareva A, Malashicheva A. aortic graft at coronary artery bypass surgery as a source of human aortic smooth muscle cells. Cell Transplant. 2017;26:1663–1668. doi: 10.1177/0963689717721226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhu Y, Feng B, He S, Su Z, Zheng G. Resveratrol combined with total flavones of hawthorn alleviate the endothelial cells injury after coronary bypass graft surgery. Phytomedicine. 2018;40:20–26. doi: 10.1016/j.phymed.2017.12.037. [DOI] [PubMed] [Google Scholar]
- 43.Hu X, Wang Z, Wu H, Jiang W, Hu R. Ras ssDNA aptamer inhibits vascular smooth muscle cell proliferation and migration through MAPK and PI3K pathways. Int J Mol Med. 2015;35:1355–1361. doi: 10.3892/ijmm.2015.2139. [DOI] [PubMed] [Google Scholar]
- 44.Wang D, Wang Y, Ma J, Wang W, Sun B, Zheng T, Wei M, Sun Y. MicroRNA-20a participates in the aerobic exercise-based prevention of coronary artery disease by targeting PTEN. Biomed Pharmacother. 2017;95:756–763. doi: 10.1016/j.biopha.2017.08.086. [DOI] [PubMed] [Google Scholar]
- 45.Frey UH, Klaassen M, Ochsenfarth C, Murke F, Thielmann M, Kottenberg E, Kleinbongard P, Klenke S, Engler A, Heusch G, et al. Remote ischaemic preconditioning increases serum extracellular vesicle concentrations with altered micro-RNA signature in CABG patients. Acta Anaesthesiol Scand. 2019;63:483–492. doi: 10.1111/aas.13296. [DOI] [PubMed] [Google Scholar]
- 46.Wang Z, Li X, Shen J, Tian D, Ji Q, Xia L, Lv Q. Plasma microRNAs reflecting cardiac and inflammatory injury in coronary artery bypass grafting surgery. J Surg Res. 2018;224:58–63. doi: 10.1016/j.jss.2017.11.036. [DOI] [PubMed] [Google Scholar]
- 47.Ram TP, Fomison-Nurse I, Gandhi S, Coffey S, Saxena P, Galvin I, Bunton R, Williams MJA, Lamberts RR, Katare R. The diagnostic sensitivity of circulating cardio-enriched microRNAs is increased after normalization of high-density lipoprotein levels. Int J Cardiol. 2017;236:498–500. doi: 10.1016/j.ijcard.2017.01.119. [DOI] [PubMed] [Google Scholar]
- 48.Santulli G. microRNAs Distinctively regulate vascular smooth muscle and endothelial cells: Functional implications in angiogenesis, atherosclerosis, and in-stent restenosis. Adv Exp Med Biol. 2015;887:53–77. doi: 10.1007/978-3-319-22380-3_4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yeh YT, Wei J, Thorossian S, Nguyen K, Hoffman C, Del Alamo JC, Serrano R, Li YJ, Wang KC, Chien S. MiR-145 mediates cell morphology-regulated mesenchymal stem cell differentiation to smooth muscle cells. Biomaterials. 2019;204:59–69. doi: 10.1016/j.biomaterials.2019.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hall IF, Climent M, Quintavalle M, Farina FM, Schorn T, Zani S, Carullo P, Kunderfranco P, Civilini E, Condorelli G, Elia L. Circ_Lrp6, a circular RNA enriched in vascular smooth muscle cells, acts as a sponge regulating miRNA-145 function. Circ Res. 2019;124:498–510. doi: 10.1161/CIRCRESAHA.118.314240. [DOI] [PubMed] [Google Scholar]
- 51.Yasmeen S, Kaur S, Mirza AH, Brodin B, Pociot F, Kruuse C. miRNA-27a-3p and miRNA-222-3p as novel modulators of phosphodiesterase 3a (PDE3A) in cerebral microvascular endothelial cells. Mol Neurobiol. 2019;56:5304–5314. doi: 10.1007/s12035-018-1446-5. [DOI] [PubMed] [Google Scholar]
- 52.Gu J, Zhang H, Ji B, Jiang H, Zhao T, Jiang R, Zhang Z, Tan S, Ahmed A, Gu Y. Vesicle miR-195 derived from endothelial cells inhibits expression of serotonin transporter in vessel smooth muscle cells. Sci Rep. 2017;7:43546. doi: 10.1038/srep43546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Fang Q, Tian M, Wang F, Zhang Z, Du T, Wang W, Yang Y, Li X, Chen G, Xiao L, et al. Amlodipine induces vasodilation via Akt2/Sp1-activated miR-21 in smooth muscle cells. Br J Pharmacol. 2019;176:2306–2320. doi: 10.1111/bph.14679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Liu S, Yang Y, Jiang S, Xu H, Tang N, Lobo A, Zhang R, Liu S, Yu T, Xin H. MiR-378a-5p regulates proliferation and migration in vascular smooth muscle cell by targeting CDK1. Front Genet. 2019;10:22. doi: 10.3389/fgene.2019.00022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Huang SC, Wang M, Wu WB, Wang R, Cui J, Li W, Li ZL, Li W, Wang SM. Mir-22-3p inhibits arterial smooth muscle cell proliferation and migration and neointimal hyperplasia by targeting HMGB1 in arteriosclerosis obliterans. Cell Physiol Biochem. 2017;42:2492–2506. doi: 10.1159/000480212. [DOI] [PubMed] [Google Scholar]
- 56.Qu Q, Bing W, Meng X, Xi J, Bai X, Liu Q, Guo Y, Zhao X, Bi Y. Upregulation of miR-126-3p promotes human saphenous vein endothelial cell proliferation in vitro and prevents vein graft neointimal formation ex vivo and in vivo. Oncotarget. 2017;8:106790–106806. doi: 10.18632/oncotarget.22365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Fichtlscherer S, De Rosa S, Fox H, Schwietz T, Fischer A, Liebetrau C, Weber M, Hamm CW, Röxe T, Müller-Ardogan M, et al. Circulating microRNAs in patients with coronary artery disease. Circ Res. 2010;107:677–684. doi: 10.1161/CIRCRESAHA.109.215566. [DOI] [PubMed] [Google Scholar]
- 58.Pu X, Xiao Q, Kiechl S, Chan K, Ng FL, Gor S, Poston RN, Fang C, Patel A, Senver EC, et al. ADAMTS7 cleavage and vascular smooth muscle cell migration is affected by a coronary-artery-disease-associated variant. Am J Hum Genet. 2013;92:366–374. doi: 10.1016/j.ajhg.2013.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wu W, Wang H, Yu C, Li J, Gao Y, Ke Y, Wang Y, Zhou Y, Zheng J. Association of ADAMTS-7 levels with cardiac function in a rat model of acute myocardial infarction. Cell Physiol Biochem. 2016;38:950–958. doi: 10.1159/000443047. [DOI] [PubMed] [Google Scholar]
- 60.Yu J, Zhou B, Yu H, Han J, Cui M, Zhang F, Wang G, Guo L, Gao W. Association between plasma ADAMTS-7 levels and severity of disease in patients with stable obstructive coronary artery disease. Medicine (Baltimore) 2016;95:e5523. doi: 10.1097/MD.0000000000005523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Jansen F, Stumpf T, Proebsting S, Franklin BS, Wenzel D, Pfeifer P, Flender A, Schmitz T, Yang X, Fleischmann BK, et al. Intercellular transfer of miR-126-3p by endothelial microparticles reduces vascular smooth muscle cell proliferation and limits neointima formation by inhibiting LRP6. J Mol Cell Cardiol. 2017;104:43–52. doi: 10.1016/j.yjmcc.2016.12.005. [DOI] [PubMed] [Google Scholar]
- 62.Wu W, Zhou Y, Li Y, Li J, Ke Y, Wang Y, Zheng J. Association between plasma ADAMTS-7 levels and ventricular remodeling in patients with acute myocardial infarction. Eur J Med Res. 2015;20:27. doi: 10.1186/s40001-015-0118-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Du Y, Gao C, Liu Z, Wang L, Liu B, He F, Zhang T, Wang Y, Wang X, Xu M, et al. Upregulation of a disintegrin and metallo-proteinase with thrombospondin motifs-7 by miR-29 repression mediates vascular smooth muscle calcification. Arterioscler Thromb Vasc Biol. 2012;32:2580–2588. doi: 10.1161/ATVBAHA.112.300206. [DOI] [PubMed] [Google Scholar]
- 64.Bendeck MP, Conte M, Zhang M, Nili N, Strauss BH, Farwell SM. Doxycycline modulates smooth muscle cell growth, migration, and matrix remodeling after arterial injury. Am J Pathol. 2002;160:1089–1095. doi: 10.1016/S0002-9440(10)64929-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wang L, Zheng J, Du Y, Huang Y, Li J, Liu B, Liu CJ, Zhu Y, Gao Y, Xu Q, et al. Cartilage oligomeric matrix protein maintains the contractile phenotype of vascular smooth muscle cells by interacting with alpha(7)beta(1) integrin. Circ Res. 2010;106:514–525. doi: 10.1161/CIRCRESAHA.109.202762. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.