Abstract
Context and Objective
Leptin treatment has dramatic clinical effects on glucose and lipid metabolism in leptin-deficient patients with lipodystrophy. Further elucidation of metabolic effects of exogenous leptin therapy will shed light on understanding leptin physiology in humans. Our objective was to utilize metabolomic profiling to examine the changes associated with administration of short-term metreleptin therapy in patients with lipodystrophy.
Study Design
We conducted a pre-post-treatment study in 19 patients (75% female) with varying forms of lipodystrophy (congenital generalized lipodystrophy, n = 10; acquired generalized lipodystrophy, n = 1; familial partial lipodystrophy, n = 8) who received daily subcutaneous metreleptin injections for a period of 16 to 23 weeks. A 3-hour oral glucose tolerance test and body composition measurements were conducted before and after the treatment period, and fasting blood samples were used for metabolomic profiling. The study outcome aimed at measuring changes in physiologically relevant metabolites before and after leptin therapy.
Results
Metabolomic analysis revealed changes in pathways involving branched-chain amino acid metabolism, fatty acid oxidation, protein degradation, urea cycle, tryptophan metabolism, nucleotide catabolism, vitamin E, and steroid metabolism. Fold changes in pre- to post-treatment metabolite levels indicated increased breakdown of fatty acids, branched chain amino acids proteins, and nucleic acids.
Conclusions
Leptin replacement therapy has significant effects on important metabolic pathways implicated in patients with lipodystrophy. Continued metabolomic studies may provide further insight into the mechanisms of action of leptin replacement therapy and provide novel biomarkers of lipodystrophy.
Abbreviations: 1,5-AG, 1,5-anhydroglucitol; 11βHSD1, 11-β hydroxysteroid dehydrogenase 1; BCAA, branched-chain amino acid; FFA, free fatty acid; GC-MS, gas chromatography mass spectrometry; IDO, indoleamine 2,3-dioxygenase; IFN-γ, interferon-γ; m/z, mass to charge ratio; OGTT, oral glucose tolerance test; TDO, tryptophan 2,3-dioxygenase; TNF-α, tumor necrosis factor-α; UPLC-MS/MS, ultra-performance liquid chromatography-tandem mass spectrometry.
Keywords: lipodystrophy, metabolomics, leptin, metreleptin
Introduction
Leptin is an adipokine that plays a key role in energy homeostasis, appetite, neuroendocrine function, and reproduction (1). Circulating leptin levels are primarily dependent on adipose tissue content and reflect body energy stores. Ob/ob mice, a rodent model of leptin deficiency, are insulin resistant, obese, hyperphagic, and diabetic (2). Leptin replacement in ob/ob mice improves these metabolic abnormalities independent of its effect on body weight (3,4). Lipodystrophy is an uncommon disorder characterized by deficiency of adipose tissue (complete or partial), and ectopic lipid deposition in liver and muscle resulting in severe metabolic complications including insulin resistance, hypertriglyceridemia, and hepatosteatosis (5). Diagnosis and treatment of lipodystrophy poses a substantial challenge, not only because it is a rare disease, but also because the underlying pathophysiologic mechanisms are not completely understood (5). The only United States Food and Drug Administration-approved treatment of lipodystrophy is the recombinant human leptin analogue, metreleptin (recombinant human methionyl leptin derived from Escherichia coli) (5–10).
Metreleptin has previously demonstrated favorable effects on the metabolic profile in patients with lipodystrophy (5). Clinically, metreleptin has been shown to reduce food intake, attenuate hypertriglyceridemia, and improve glycemic control and insulin sensitivity among lipodystrophy patients (5–10). However, a detailed analysis of the metabolic pathways and metabolites affected by exogenous leptin has not been performed till date.
Metabolomics is the study of a set of metabolites (small molecules) in the metabolome. The metabolome comprises the entirety of the metabolites in a cell, organ, or organism that are needed for normal function (8,11). Using analytical chemistry techniques such as nuclear magnetic resonance spectroscopy and mass spectrometry (MS), an untargeted approach provides comprehensive analysis of all measurable components in a sample (8,11). The effects of leptin replacement on the metabolome is largely unknown.
In this study, we used metabolomic analysis to delineate changes in the metabolome of patients with lipodystrophy at baseline and following leptin replacement therapy. The overall goal of this exploratory study was to decipher the changes induced by exogenous leptin in the metabolome, in order to further elucidate the mechanisms of action of leptin.
1. Subjects and Methods
A. Study Design and Study Subjects
This study was conducted at the Clinical Center of the National Institutes of Health in Bethesda, Maryland, and was approved by the Institutional Review Board of the National Institute of Diabetes and Digestive and Kidney Disease. All participants/guardians provided written informed consent/assent for the study procedures. The design of this study, the inclusion criteria, and the exclusion criteria have been previously described (12,13). A pre-poststudy design was implemented to evaluate the metabolomic effects of metreleptin therapy in patients with congenital generalized (n = 10), acquired generalized (n = 1), or familial partial lipodystrophy (n = 8). Metabolite profiling was an exploratory outcome of a larger study evaluating the effects of metreleptin therapy in lipodystrophy patients (ClinicalTrials.gov NCT00025883). Metreleptin was provided by Amgen (Thousand Oaks, CA) initially and Amylin Pharmaceuticals (San Diego, CA) subsequently. Metreleptin was provided as a self-administered once- or twice-daily subcutaneous injection in dosages as previously described (12,13).
B. Study Procedure
Blood samples were obtained at baseline before initiation of metreleptin and at a follow-up visit 16 to 23 weeks after initiation. Fasting blood samples were obtained for measurements of leptin, glucose, insulin, c-peptide, hemoglobin A1C (A1C), lipid panel, liver and renal function tests. Samples collected for future analysis were centrifuged to isolate serum, and then were stored at –80°C. After an overnight fast, participants underwent a 75-g oral glucose tolerance test (OGTT). Blood samples were taken at –10, 0, 30, 60, 90, 120, and 180 minutes for measurement of plasma glucose, insulin, and c-peptide. Leptin was measured using a commercial radioimmunoassay kit (Linco Research). Standard methodology was used for all other measurements and was conducted by the Department of Laboratory Medicine at the National Institutes of Health Clinical Center.
C. Sample Transfer and Preparation
Paired fasting samples, before and after metreleptin therapy, from the study participants were transferred to Metabolon, Inc. (Durham, NC) for examination of global metabolic profiles. All samples and aliquots were maintained at –80°C until analyzed. Sample preparation was done by the automated MicroLab STAR system (Hamilton Company). For quality control purposes, a recovery standard was added before the first step of the extraction process. Samples were precipitated with methanol under vigorous shaking, centrifuged, and separated into 5 fractions for analysis. A TurboVap (Zymark) was used to remove organic solvent from the fractions. Four of the 5 samples were analyzed by ultra-performance liquid chromatography-tandem mass spectrometry (UPLC-MS/MS) with positive ion mode electrospray ionization, UPLC-MS/MS with negative ion mode electrospray ionization, UPLC-MS/MS polar platform, and gas chromatography mass spectrometry (GC-MS) respectively. The fifth sample was stored for backup. Samples analyzed by LC were stored under nitrogen overnight before analysis, while samples for GC were dried overnight under vacuum before analysis.
D. Ultrahigh Performance Liquid Chromatography Tandem Mass Spectroscopy
Samples were run on a Waters ACQUITY UPLC-MS/MS and a THERMO Scientific Q-Exactive mass spectrometer with heated electrospray ionization (HESI-III) source and Orbitrap mass analyzer at 35 000 mass resolution. Samples were dried and then dissolved in acidic or basic conditions. The aliquots dissolved in acidic conditions were eluted through a C18 column (Waters UPLC BEH C18-2.1 × 100 mm, 1.7 µM) using gradient elution of water, methanol, and 0.1% formic acid. These samples were analyzed using acidic, positive ion optimized conditions. The aliquots dissolved in basic conditions were eluted in a separate dedicated C18 column and using a gradient of methanol, water, and 6.5 mM ammonium bicarbonate. A third aliquot in basic conditions was analyzed using a hydrophilic interaction chromatography column (Waters UPLC BEH Amide 2.1 × 150 mm, 1.7 µM) using a gradient of water, acetonitrile, and 10 mM ammonium formate. Samples in basic conditions were analyzed using basic, negative ion optimized conditions. Data were analyzed by MS and data-dependent MS2 scans using dynamic exclusion, with a scan range of 80 to 1000 mass to charge ratio (m/z).
E. Gas Chromatography Mass Spectroscopy
GC-MS samples were derivatized using bis(trimethylsilyl) trifluoroacetiamide under nitrogen gas. A 5% diphenyl/95% dimethylpolysiloxane fused silica column (20 m × 0.18 mm ID; 0.18 µm film thickness) was used to separate the samples. Helium was used as the carrier gas with a temperature ramp from 60° to 340°C over the course of 17.5 minutes. Analysis was done on a Thermo-Finnagan Trace DSQ single-quadrupole mass spectrometer. Scan range was 50 to 750 m/z.
F. Data Extraction, Compound Identification, and Quantification
Metabolon, Inc., extracted raw data, recognized peaks, ran quality control procedures, and identified compounds by comparison with the company’s own maintained library of authenticated and purified standards and recurrent unknown entries. The present dataset comprises a total of 620 compounds of known identity (named biochemicals). Three criteria were required for compound identification: the retention time must be within a narrow window of library standard, the mass must match library mass ± 0.005 atomic mass unit, and a comparison of MS/MS forward and reverse scores between experimental data and authenticated library data. Compounds which were matched to the library were verified for each sample, and if necessary were corrected. Data analysts at Metabolon, Inc., used proprietary visualization and interpretation software to verify the consistency of peak identification. Area under the curve was used to quantify biochemical peaks. Results for multiday studies were normalized to correct for interday instrument variation. For data visualization, each metabolite was rescaled to set the median to 1.0.
F. Statistical Analysis
Normality of baseline characteristics were determined using the D’Agostino and Person omnibus normality test. A paired t-test was performed if data were normally distributed. If not normally distributed, a Wilcoxon matched-pairs signed rank test determined significance. Metabolite measures were log transformed, and a matched pairs t-test was used to determine significance between before and after leptin replacement therapy. To account for false positives, a false discovery rate (q-value) was also calculated for each biochemical analyte. Metabolite measures that achieved P ≤ .05 were considered statistically significant, while measurements with P-values in the range .05 < P < .10 approached statistical significance. Statistical analyses were performed using GraphPad Prism (San Diego, CA), ArrayStudio (Cary, NC), R program (https://www.r-project.org/), and JMP (SAS; Cary, NC).
2. Results
A. Baseline Characteristics
Nineteen patients with lipodystrophy had samples analyzed before and after metreleptin treatment. Patients were diagnosed with either congenital generalized lipodystrophy (n = 10), acquired generalized lipodystrophy (n = 1), or familial partial lipodystrophy (n = 8). Before treatment, diabetes was managed with metformin (n = 9), insulin (n = 7), thiazolidinediones (n = 4), and sulfonylureas (n = 3). Elevated triglycerides were treated with fibrates (n = 8). After treatment with metreleptin, the comorbidities were treated with metformin (n = 12), insulin (n = 6), thiazolidinediones (n = 3), sulfonylureas (n = 1), and fibrates (n = 10). The clinical characteristics of the patient cohort before and after metreleptin treatment are presented in Table 1. Metreleptin increased the median plasma leptin concentrations from 5.2 ± 1.9 ng/mL to 84.8 ± 32.5 ng/mL (P = .03). Body mass index was significantly lower after leptin therapy. Following metreleptin therapy, fasting plasma glucose, 2-hour plasma glucose during OGTT, and A1C decreased significantly (Table 1). Plasma concentrations of total cholesterol and low-density lipoprotein cholesterol were significantly lower after leptin replacement therapy (Table 1). Plasma triglyceride levels tended to be lower (P = .06) after metreleptin therapy, while high-density lipoprotein cholesterol levels were not significantly different. There was no change in insulin sensitivity as measured by the surrogate index, QUICKI.
Table 1.
Clinical Parameters Before and After Metreleptin Treatment.
| Before leptin | After leptin | |
|---|---|---|
| Age, years | 25 ± 4 | 26 ± 4* |
| Sex, % female | 75% | 75% |
| Leptin, ng/mL | 1.5 (5.6) | 5.2 (4.5)* |
| Body composition | ||
| BMI, kg/m2 |
24.0 ± 1.0 |
23 ± 0.9* |
| Metabolic parameters | ||
| Fasting glucose, mg/dL | 159 (143) | 103 (71)* |
| 2-hr OGTT glucose, mg/dL |
273 ± 29 |
237 ± 22* |
| Hemoglobin A1C, % |
7.7 ± 0.5 |
7.0 ± 0.4* |
| Fasting insulin, µU/mL | 24.7 (58.1) | 18.5 (34.6) |
| Fasting C-peptide, ng/mL |
3.7 ± 0.5 |
3.5 ± 0.3 |
| QUICKI | 0.28 (0.05) | 0.28 (0.05) |
| Lipids | ||
| Total cholesterol, mg/dL | 168 (56) | 134 (75)* |
| LDL cholesterol, mg/dL |
84 ± 6 |
70 ± 10* |
| HDL cholesterol, mg/dL |
32 ± 3 |
32 ± 3 |
| Triglycerides, mg/dL | 257 (472) | 226 (163) |
Data expressed as mean ± standard error of the mean or median (interquartile range). *P < .05 compared with premetreleptin treatment.
B. Metabolomic Analysis
B-1. Branched-chain Amino Acids, Lipolysis, and β-Oxidation of Fatty Acids
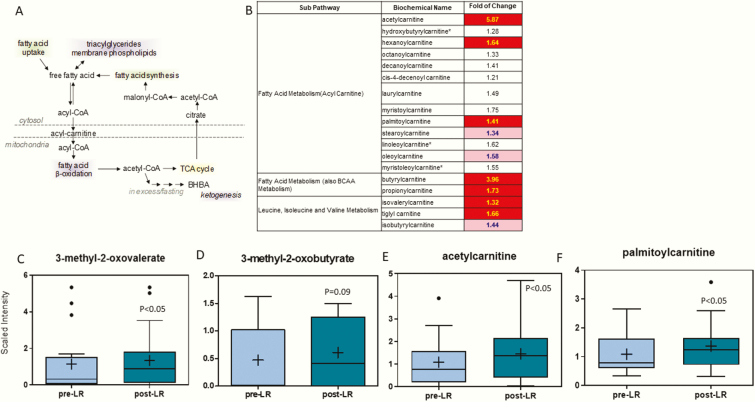
An illustration of fatty acid metabolism and the effects of leptin treatment on the various pathways involving fatty acid/branched-chain amino acid (BCAA) metabolism is in Figs. 1A and 1B, respectively. Various α-keto acids, which are the byproducts of BCAA catabolism, such as 3-methyl-2-oxovalerate, increased after leptin therapy (2.4-fold) and levels of 3-methyl-2-oxobutyrate (11-fold) tended to increase (Figs. 1C and 1D). Acylcarnitines, especially those with C3 and C5 acyl groups, are derived from α-keto acid products and contribute to fatty acid β-oxidation in the mitochondria as described in Fig. 1A (14). Several carnitine-conjugated derivatives increased following leptin treatment, including isovalerylacarnitine, tiglyl carnitine, isobutryrylcarnitine, butyrylcarnitine, propionylcarnitine, and steroylcarnitine (Fig. 1B). Other acyl carnitines that increased after leptin treatment were acetylcarnitine (5.9-fold) and palmitoylcarnitine (1.4-fold) (Figs. 1F and 1G). However, other metabolites associated with fatty acid metabolism such as free fatty acids (FFAs), glycerol, and beta-hydroxybutyrate (BHBA) were not significantly altered (all supplementary material and figures are located in a digital research materials repository (15)).
Figure 1.
(A) Pathway of free fatty acids. (B) Heat map of statistically significant biochemical markers profiled in this study. Red shaded cells indicate P ≤ .05 (red indicates that the mean values are significantly higher for that comparison). Light red shaded cells indicate .05 < P < .10 (light red indicates that the mean values trend higher for that comparison). α-Keto acids, (C) 3-methyl-2-oxovalerate and (D) 3-methyl-2-oxobutyrate and acylcarnitines, (E) acetylcarnitine, and (F) palmitoylcarnitine increased with metreleptin treatment. The X-axis shows the before and after leptin replacement (LR) and the Y-axis shows the relative scaled intensity for the metabolites measured. Within the boxplot, the mean value is represented by the plus sign, the median by the horizontal dividing line, and the top and bottom of the box represent the 75th and 25th percentile, with the whiskers indicating the maximum and minimum points and outlier points shown as filled circles.
B-2. Protein Degradation and the Urea Cycle
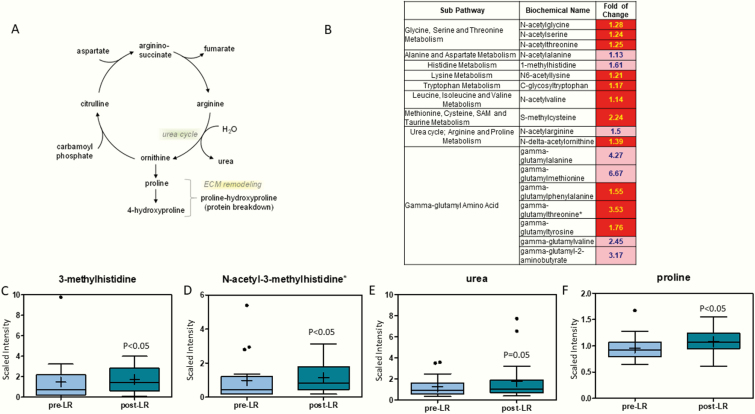
The urea cycle pathway and the effects of leptin replacement on various protein degradation and urea cycle byproducts is illustrated in Fig. 2 (16). Several modified free amino acids increased after leptin treatment (Fig. 2B). The increases in free amino acids modified with acetyl, methyl, and glycosyl functional groups indicate changes to the protein degradation process. To further examine this, we looked at markers of protein degradation, specifically gamma-glutamyl amino acids (17), 3-methylhistidine (10.4-fold), and N-acetyl-3-methylhistidine (2.1-fold), which were significantly higher after leptin treatment (Figs. 2B–2D). Metabolites involved in the urea cycle were also increased after leptin replacement (Figs. 2E and 2F).
Figure 2.
(A) Pathway of urea and protein degradation cycle. (B) Heat map of statistically significant biochemical markers profiled in this study. Red shaded cells indicate P ≤ .05 (red indicates that the mean values are significantly higher for that comparison). Light red shaded cells indicate .05 < P < .10 (light red indicates that the mean values trend higher for that comparison). (C) 3-Methylhistidine and (D) N-acetyl-3-methylhistidine and metabolites of the urea cycle, (E) urea and (F) proline are increased with leptin therapy. The X-axis shows before and after leptin replacement (LR) and the Y-axis shows the relative scaled intensity for the metabolites measured. Within the boxplot, the mean value is represented by the plus sign, the median by the horizontal dividing line, and the top and bottom of the box represent the 75th and 25th percentile, with the whiskers indicating the maximum and minimum points and outlier points shown as filled circles.
B-3. Tryptophan Catabolism
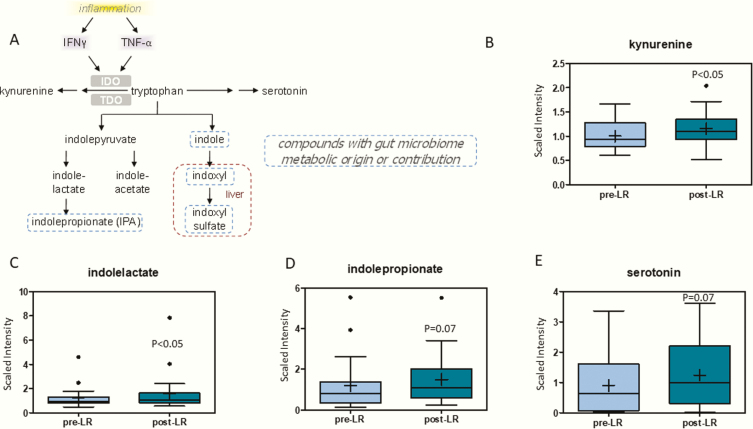
Metabolites in the tryptophan degradation pathway increased following leptin therapy (Fig. 3). Tryptophan can be degraded by tryptophan 2,3-dioxygenase (TDO) or indoleamine 2,3-dioxygenase (IDO), which is triggered by interferon-γ (IFN-γ) and tumor necrosis factor-α (TNF-α) (Fig. 3A). Kynurenine, the product of TDO and IDO tryptophan degradation increased (1.2-fold) following leptin replacement therapy (Fig. 3B). Other tryptophan degradation products, indolelactate (1.2-fold) and indolepropionate (2.2-fold), related to microbiome metabolic origin (18) tended to increase (Figs. 3C and 3D). Similarly, levels of serotonin, a derivative of tryptophan, increased (2.4 fold) after leptin replacement (Fig. 3E) (19).
Figure 3.
(A) Pathway of tryptophan degradation. Products of tryptophan degradation (B) kynurenine, (C) indolelactate, (D) indolepropionate, and (E) serotonin are increased following leptin therapy. The X-axis shows the before and after leptin replacement (LR) and the Y-axis shows the relative scaled intensity for the metzabolites measured. Within the boxplot, the mean value is represented by the plus sign, the median by the horizontal dividing line, and the top and bottom of the box represent the 75th and 25th percentile, with the whiskers indicating the maximum and minimum points and outlier points shown as filled circles.
B-4. Nucleotide Metabolism
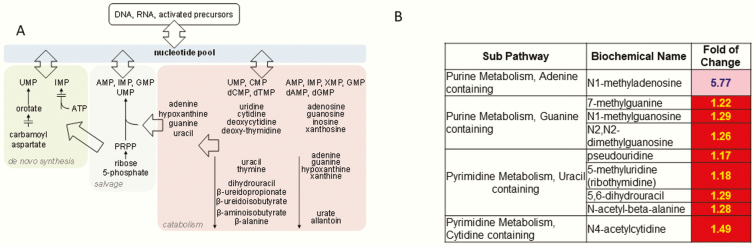
Figure 4A outlines the nucleotide metabolism pathway and demonstrates how purine and pyrimidine bases are incorporated into DNA and RNA (20). Various purine and pyrimidine nucleotide modified bases were elevated following therapy, including N1-methyladenosine, 7-methylguanine, 5-methyluridine, N4-acetylcytidine, N1-methylguanosine, and N-acetyl-beta-alanine, among others (Fig. 4B). Changes in modified nucleotide base metabolism could indicate that leptin may alter gene expression patterns.
Figure 4.
(A) pathway of nucleotide metabolism. (B) Heat map of statistically significant biochemicals profiled in this pathway. Red shaded cells indicate P ≤ .05 (red indicates that the mean values are significantly higher for that comparison). Light red shaded cells indicate .05 < P < .10 (light red indicates that the mean values trend higher for that comparison). Abbreviations: AMP, adenosine 5′-monophosphate; CMP, cytidine 5′-monophosphate; IMP, inosine 5′-monophosphate; PRPP, phosphoribosyl pyrophosphate; TMP, thymidine 5′-monophosphate; UMP, uridine 5′-monophosphate; XMP, xanthine 5′-monophosphate.
B-5. Vitamin E Metabolism
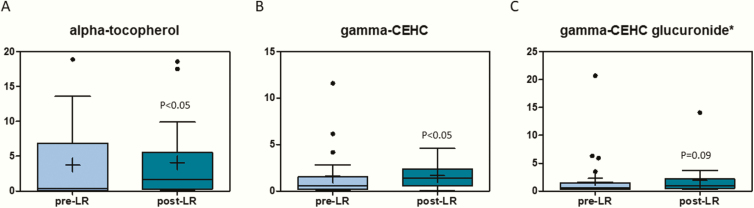
Various metabolites related to vitamin E metabolism displayed changes with leptin therapy (Fig. 5). α-Tocopherol, which is the vitamin E isoform that humans absorb favorably, had an almost 7-fold increase following leptin therapy (Fig. 5A). Similarly, gamma-carboxyethylhydrochroman, a product of vitamin E catabolism increased after leptin treatment (7-fold) (Figs. 5B and 5C).
Figure 5.
Vitamin E-related metabolites (A) alpha-tocopherol, (B) gamma-arboxyethylhydrochroman, and (C) gamma-CEHC glucuronide are elevated after leptin treatment. The X-axis shows the before and after leptin replacement (LR) and the Y-axis shows the relative scaled intensity for the metabolites measured. Within the boxplot, the mean value is represented by the plus sign, the median by the horizontal dividing line, and the top and bottom of the box represent the seventy-fifth and twenty-fifth percentile, with the whiskers indicating the maximum and minimum points and outlier points shown as filled circles.
B-6. Other Metabolites
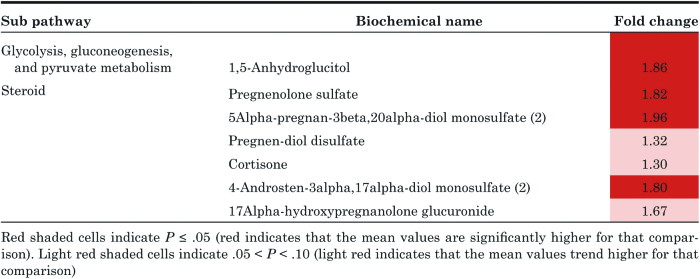
1,5-Anhydroglucitol (1,5-AG) increased after leptin replacement (Table 2). Several steroid hormone metabolites in the pregnenolone pathways were also increased with leptin replacement (Table 2).
Table 2.
Effect of metreleptin therapy on 1,5-anhydroglucitol levels and metabolites in the pregnenolone pathway.
3. Discussion
Our study demonstrated several changes in the metabolomic profile of lipodystrophy patients after leptin replacement. The biochemical alterations were especially identified in the protein and amino acid catabolism, fatty acid oxidation, nucleotide metabolism, vitamin E metabolism, glucose homeostasis, and steroid metabolism pathways.
Our data showed increases in levels of serum α-keto acids and related acylcarnitine derivatives after leptin therapy (Fig. 1). α-Keto acids are products of BCAA catabolism that occurs in the mitochondria (14). α-Keto acids are metabolized further and eventually yield acetyl-CoA and succinyl-CoA, which in turn act as substrates in various energy generation pathways such as the tricarboxylic acid cycle or anabolic pathways (ie, gluconeogenesis and fatty acid synthesis) (14). Therefore, the increase in plasma α-keto acids suggest improvement in mitochondrial function as a result of leptin treatment. Lipodystrophy is associated with impaired energy metabolism, β-oxidation, and mitochondrial dysfunction (21–23). Leptin treatment restores mitochondrial function through increased β-oxidation in vitro cell culture and in animal models, primarily mediated through activation of adenosine 5′-monophosphate (AMP)—activated protein kinase (AMPK) (24–27). Our data indicate leptin probably leads to an increased breakdown of BCAA in the mitochondria. BCAA catabolism has not been explored previously in relation to lipodystrophy in humans, and as per our knowledge, our study is the first one to report the effects of leptin replacement on BCAA in lipodystrophy.
We observed elevated serum levels of various acylcarnitine derivatives after leptin replacement. This observation might be reflective of leptin-mediated effects on AMPK, especially in adipose tissue and skeletal muscle. Leptin directly phosphorylates and activates AMPK, which then inhibits acetyl CoA carboxylase, which in turn leads to increased fatty acid oxidation through disinhibition of carnitine palmitoyltransferase I, which could concomitantly lead to increased generation of acylcarnitine (25,27). However, a study measuring fatty acid oxidation in men with HIV-associated dyslipidemic lipodystrophy showed no change in fatty acid oxidation with 4 months of leptin replacement therapy (28). Leptin treatment creates a state of negative energy which should technically increase triglyceride breakdown, FFA and ketone body (BHBA) production. But FFA and BHBA in our patients did not significantly change after leptin replacement. Interestingly, similar findings of unchanged FFA and BHBA levels were observed in rats treated with central nervous system leptin administration when compared with control rats (29). Further investigations are necessary to elucidate the effect of leptin replacement on fatty acid homeostasis.
Leptin replacement therapy was associated with increased plasma levels of modified free amino acids and gamma-glutamyl amino acids, and other markers of protein degradation. Leptin may potentially modulate protein metabolism through stimulation of insulin pathway proteins, PI3K and IRS-2(30,31). Studies on the effect of leptin on protein metabolism is limited with conflicting results. Leptin has been shown to reduce protein breakdown and stimulate protein synthesis in ob/ob mice and C2C12 myotubes and promote protein synthesis in chick embryonic myocytes and hepatocytes. In contrast, leptin showed no effect on protein synthesis or degradation in porcine myoblasts and in female Wistar rats (32–36). Protein metabolism data in patients with lipodystrophy is sparse. In a case report on a patient with generalized lipodystrophy, there was evidence of increased amino acid turnover (increase in both protein synthesis and breakdown) based on the rate of appearance and disposal measurements of peripherally infused [1-13C]leucine (37). In our study, the increase in protein degradation was further supported by an increase in urea cycle metabolites after leptin replacement therapy (16,38–40). These results suggest that lipodystrophy patients receiving leptin treatment may exhibit increased protein turnover.
Our metabolomic data further implicates that leptin replacement therapy has a significant impact on downstream pathways of tryptophan degradation. Leptin has previously been shown to be associated with increased tryptophan breakdown and increased levels of kynurenine (41). Similarly, we observed increased circulating levels of kynurenine following metreleptin treatment. Hypoleptinemia in lipodystrophy is associated with low TNF-α production in mononuclear cells, and leptin replacement therapy has been shown to normalize the levels of TNF-α, an inflammatory cytokine known to activate IDO (42). Therefore, the increased levels of kynurenine might be reflective of increased IDO activation due to leptin-mediated restoration of TNF-α levels (43). Furthermore, elevations in indolelactate and indoleproprionate, tryptophan metabolites generated by the gut microflora, may suggest changes in gut microbiome in response to metreleptin treatment. In addition, increased plasma levels of serotonin were observed after leptin replacement. This finding reflects the effects of leptin on regulation of serotonin metabolism which, in prior studies, has shown to play a role in bone mass accrual, appetite modulation, and energy expenditure. Leptin also has been shown to increase brain serotonin metabolism in mice through the nitric oxide synthase pathway (44,45). These findings demonstrate the potential influence of leptin on tryptophan, kynurenine and serotonin metabolism, gut microbiome, and immunomodulation.
Consistent elevations in several modified nucleosides and purine/pyrimidine bases were observed following leptin replacement therapy. Modified nucleosides and bases are produced from degradation of DNA and/or RNA, and some of these nucleosides also found in transfer and messenger RNAs. These findings may be suggestive of increased cellular turnover associated with leptin treatment (46–48).
As evidenced by elevated levels of α-tocopherol and metabolites of vitamin E catabolism, the subjects displayed increased vitamin E turnover. Vitamin E serves as a dietary-derived antioxidant, and alterations in vitamin E metabolites could be indicative of changes in oxidative stress brought about by leptin replacement. In addition, these changes could also be from altered dietary patterns after leptin replacement (49).
1,5-AG was significantly elevated after leptin replacement, indicating improved glycemic control as a result of metreleptin therapy. Glucose and 1,5-AG compete for reabsorption in the kidney and are their plasma levels are inversely proportional to each other, with serum 1,5-AG levels reducing in hyperglycemia. 1,5-AG serves as a useful alternative biomarker to A1C as it provides insight into short-term glycemic control, glycemic variability, and postprandial hyperglycemia (50,51). This finding is consistent with changes in A1C or 2-hour OGTT glucose in this study and previous studies (9,13,52).
Several steroid metabolites in the pregnenolone pathway were increased after leptin replacement. Previous studies have shown that leptin can regulate steroid metabolism through direct action on human adrenocortical cells (53). Also, in human preadipocytes, leptin has been shown to regulate 11-β hydroxysteroid dehydrogenase 1 (11βHSD1), an enzyme that converts cortisone to cortisol. The mRNA expression for 11βHSD1 is downregulated in female and is upregulated in male preadipocytes (54). This may explain the detection of elevated cortisone levels after leptin therapy given the female predominance in our study cohort. However, leptin’s effects on 11βHSD1 in nonadipose tissues is less clear.
Our study has several limitations. Firstly, the study lacked metabolomic data from a healthy control group for comparison. Secondly, a dietary history before and over the course of metreleptin treatment was not collected, and the diet of the patients were not controlled for. Thirdly, the small sample size of the study may not have the statistical power to identify all the physiologically significant changes due to leptin replacement therapy. Finally, it is also impossible to separate the direct effects of leptin versus indirect effects mediated by changes in not only diet, but also insulin sensitivity, etc. Further studies with larger cohorts could potentially delineate additional metabolic pathways affected by leptin replacement in lipodystrophy.
Management of lipodystrophy continues to pose a significant challenge to clinicians. The use of leptin replacement as a therapy for hypoleptinemia has been studied, yet many of its effects are still unknown and warrant further exploration. Our results from this global metabolic profiling study revealed perturbations in numerous metabolic pathways following leptin replacement therapy. In particular, changes in fatty acid oxidation, protein turnover, urea cycle, mitochondrial function, tryptophan metabolism related to the gut microbiome and inflammation, serotonin synthesis, nucleotide metabolism, vitamin E metabolism, glycemic control, and hepatic function were reflected in the plasma metabolic profile after leptin therapy. The results of this study can be utilized to further our understanding of the metabolic pathways potentially influenced by metreleptin therapy in patients with lipodystrophy.
Acknowledgments
Financial Support: This research was supported by the Intramural Research Program of the National Institute of Diabetes and Digestive and Kidney Disease, National Institutes of Health.
Author contributions: R.M. conceived and designed the study, acquired and analyzed data, and drafted and reviewed the manuscript. S.G., A.F., C.F., S.S., H.T., and R.M. analyzed data, and drafted and reviewed the manuscript. R.M. and R.J.B. designed the study, acquired data, and drafted and reviewed the manuscript.
Clinical Trial Information: ClinicalTrials.gov NCT00025883 (registered 29 October 2001)
Additional Information
Disclosure Summary: The authors have nothing to disclose.
References
- 1. Kelesidis T, Kelesidis I, Chou S, Mantzoros CS. Narrative review: the role of leptin in human physiology: emerging clinical applications. Ann Intern Med. 2010;152(2):93–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Pelleymounter MA, Cullen MJ, Baker MB, et al. Effects of the obese gene product on body weight regulation in ob/ob mice. Science. 1995;269(5223):540–543. [DOI] [PubMed] [Google Scholar]
- 3. Halaas JL, Gajiwala KS, Maffei M, et al. Weight-reducing effects of the plasma protein encoded by the obese gene. Science. 1995;269(5223):543–546. [DOI] [PubMed] [Google Scholar]
- 4. Campfield LA, Smith FJ, Guisez Y, Devos R, Burn P. Recombinant mouse OB protein: evidence for a peripheral signal linking adiposity and central neural networks. Science. 1995;269(5223):546–549. [DOI] [PubMed] [Google Scholar]
- 5. Brown RJ, Araujo-Vilar D, Cheung PT, et al. The diagnosis and management of lipodystrophy syndromes: a multi-society practice guideline. J Clin Endocrinol Metab. 2016;101(12):4500–4511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Brown RJ, Meehan CA, Cochran E, et al. Effects of metreleptin in pediatric patients with lipodystrophy. J Clin Endocrinol Metab. 2017;102(5):1511–1519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Diker-Cohen T, Cochran E, Gorden P, Brown RJ. Partial and generalized lipodystrophy: comparison of baseline characteristics and response to metreleptin. J Clin Endocrinol Metab. 2015;100(5):1802–1810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Gibney MJ, Walsh M, Brennan L, Roche HM, German B, van Ommen B. Metabolomics in human nutrition: opportunities and challenges. Am J Clin Nutr. 2005;82(3):497–503. [DOI] [PubMed] [Google Scholar]
- 9. Chong AY, Lupsa BC, Cochran EK, Gorden P. Efficacy of leptin therapy in the different forms of human lipodystrophy. Diabetologia. 2010;53(1):27–35. [DOI] [PubMed] [Google Scholar]
- 10. Meehan CA, Cochran E, Kassai A, Brown RJ, Gorden P. Metreleptin for injection to treat the complications of leptin deficiency in patients with congenital or acquired generalized lipodystrophy. Expert Rev Clin Pharmacol. 2016;9(1):59–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Wishart DS, Tzur D, Knox C, et al. HMDB: the Human Metabolome Database. Nucleic Acids Res. 2007;35(Database issue):D521–D526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Muniyappa R, Brown RJ, Mari A, et al. Effects of leptin replacement therapy on pancreatic β-cell function in patients with lipodystrophy. Diabetes Care. 2014;37(4):1101–1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Oral EA, Simha V, Ruiz E, et al. Leptin-replacement therapy for lipodystrophy. N Engl J Med. 2002;346(8):570–578. [DOI] [PubMed] [Google Scholar]
- 14. O’Connell TM. The complex role of branched chain amino acids in diabetes and cancer. Metabolites 2013; 3:931–945 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Grewal S, Gubbi S, Fosam A, Sedmak C, Sikder S, Talluru H, Brown RJ, Muniyappa R. Metabolomic analysis of the effects of leptin replacement therapy in patients with lipodystrophy. Dryad Digital Repository 2019, Deposited 12 November 2019, 10.5061/dryad.0p2ngf1w0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Morris SM Jr. Regulation of enzymes of the urea cycle and arginine metabolism. Annu Rev Nutr. 2002;22:87–105. [DOI] [PubMed] [Google Scholar]
- 17. Young VR, Munro HN. Ntau-methylhistidine (3-methylhistidine) and muscle protein turnover: an overview. Fed Proc. 1978;37(9):2291–2300. [PubMed] [Google Scholar]
- 18. Sinha R, Ahn J, Sampson JN, et al. Fecal microbiota, fecal metabolome, and colorectal cancer interrelations. Plos One. 2016;11(3):e0152126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Davis I, Liu A. What is the tryptophan kynurenine pathway and why is it important to neurotherapeutics? Expert Rev Neurother. 2015;15(7):719–721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Lane AN, Fan TW. Regulation of mammalian nucleotide metabolism and biosynthesis. Nucleic Acids Res. 2015;43(4):2466–2485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Vigouroux C, Caron-Debarle M, Le Dour C, Magré J, Capeau J. Molecular mechanisms of human lipodystrophies: from adipocyte lipid droplet to oxidative stress and lipotoxicity. Int J Biochem Cell Biol. 2011;43(6):862–876. [DOI] [PubMed] [Google Scholar]
- 22. Sleigh A, Stears A, Thackray K, et al. Mitochondrial oxidative phosphorylation is impaired in patients with congenital lipodystrophy. J Clin Endocrinol Metab. 2012;97(3):E438–E442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Vernochet C, Damilano F, Mourier A, et al. Adipose tissue mitochondrial dysfunction triggers a lipodystrophic syndrome with insulin resistance, hepatosteatosis, and cardiovascular complications. Faseb J. 2014;28(10):4408–4419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Blanquer-Rosselló MM, Santandreu FM, Oliver J, Roca P, Valle A. Leptin modulates mitochondrial function, dynamics and biogenesis in MCF-7 Cells. J Cell Biochem. 2015;116(9):2039–2048. [DOI] [PubMed] [Google Scholar]
- 25. Minokoshi Y, Kim YB, Peroni OD, et al. Leptin stimulates fatty-acid oxidation by activating AMP-activated protein kinase. Nature. 2002;415(6869):339–343. [DOI] [PubMed] [Google Scholar]
- 26. Suzuki A, Okamoto S, Lee S, Saito K, Shiuchi T, Minokoshi Y. Leptin stimulates fatty acid oxidation and peroxisome proliferator-activated receptor alpha gene expression in mouse C2C12 myoblasts by changing the subcellular localization of the alpha2 form of AMP-activated protein kinase. Mol Cell Biol. 2007;27(12):4317–4327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Steinberg GR, Bonen A, Dyck DJ. Fatty acid oxidation and triacylglycerol hydrolysis are enhanced after chronic leptin treatment in rats. Am J Physiol Endocrinol Metab. 2002;282(3):E593–E600. [DOI] [PubMed] [Google Scholar]
- 28. Sekhar RV, Jahoor F, Iyer D, et al. Leptin replacement therapy does not improve the abnormal lipid kinetics of hypoleptinemic patients with HIV-associated lipodystrophy syndrome. Metabolism. 2012;61(10):1395–1403. [DOI] [PubMed] [Google Scholar]
- 29. van Dijk G. The role of leptin in the regulation of energy balance and adiposity. J Neuroendocrinol. 2001;13(10):913–921. [DOI] [PubMed] [Google Scholar]
- 30. Kellerer M, Koch M, Metzinger E, Mushack J, Capp E, Häring HU. Leptin activates PI-3 kinase in C2C12 myotubes via Janus kinase-2 (JAK-2) and insulin receptor substrate-2 (IRS-2) dependent pathways. Diabetologia. 1997;40(11):1358–1362. [DOI] [PubMed] [Google Scholar]
- 31. Maroni P, Bendinelli P, Piccoletti R. Intracellular signal transduction pathways induced by leptin in C2C12 cells. Cell Biol Int. 2005;29(7):542–550. [DOI] [PubMed] [Google Scholar]
- 32. Will K, Kalbe C, Kuzinski J, et al. Effects of leptin and adiponectin on proliferation and protein metabolism of porcine myoblasts. Histochem Cell Biol. 2012;138(2):271–287. [DOI] [PubMed] [Google Scholar]
- 33. Lamosová D, Zeman M. Effect of leptin and insulin on chick embryonic muscle cells and hepatocytes. Physiol Res. 2001;50(2):183–189. [PubMed] [Google Scholar]
- 34. Ramsay TG. Porcine leptin inhibits protein breakdown and stimulates fatty acid oxidation in C2C12 myotubes. J Anim Sci. 2003;81(12):3046–3051. [DOI] [PubMed] [Google Scholar]
- 35. Carbó N, Ribas V, Busquets S, Alvarez B, López-Soriano FJ, Argilés JM. Short-term effects of leptin on skeletal muscle protein metabolism in the rat. J Nutr Biochem. 2000;11(9):431–435. [DOI] [PubMed] [Google Scholar]
- 36. Mao X, Zeng X, Huang Z, Wang J, Qiao S. Leptin and leucine synergistically regulate protein metabolism in C2C12 myotubes and mouse skeletal muscles. Br J Nutr. 2013;110(2):256–264. [DOI] [PubMed] [Google Scholar]
- 37. Klein S, Jahoor F, Wolfe RR, Stuart CA. Generalized lipodystrophy: in vivo evidence for hypermetabolism and insulin-resistant lipid, glucose, and amino acid kinetics. Metabolism. 1992;41(8):893–896. [DOI] [PubMed] [Google Scholar]
- 38. Yamashita H, Ichikawa T, Matsuyama D, et al. The role of the interaction of the vinculin proline-rich linker region with vinexin α in sensing the stiffness of the extracellular matrix. J Cell Sci. 2014;127(Pt 9):1875–1886. [DOI] [PubMed] [Google Scholar]
- 39. Javor ED, Moran SA, Young JR, et al. Proteinuric nephropathy in acquired and congenital generalized lipodystrophy: baseline characteristics and course during recombinant leptin therapy. J Clin Endocrinol Metab. 2004;89(7):3199–3207. [DOI] [PubMed] [Google Scholar]
- 40. Musso C, Javor E, Cochran E, Balow JE, Gorden P. Spectrum of renal diseases associated with extreme forms of insulin resistance. Clin J Am Soc Nephrol. 2006;1(4):616–622. [DOI] [PubMed] [Google Scholar]
- 41. Strasser B, Berger K, Fuchs D. Effects of a caloric restriction weight loss diet on tryptophan metabolism and inflammatory biomarkers in overweight adults. Eur J Nutr. 2015;54(1):101–107. [DOI] [PubMed] [Google Scholar]
- 42. Oral EA, Javor ED, Ding L, et al. Leptin replacement therapy modulates circulating lymphocyte subsets and cytokine responsiveness in severe lipodystrophy. J Clin Endocrinol Metab. 2006;91(2):621–628. [DOI] [PubMed] [Google Scholar]
- 43. Mangge H, Stelzer I, Reininghaus EZ, Weghuber D, Postolache TT, Fuchs D. Disturbed tryptophan metabolism in cardiovascular disease. Curr Med Chem. 2014;21(17):1931–1937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Calapai G, Corica F, Corsonello A, et al. Leptin increases serotonin turnover by inhibition of brain nitric oxide synthesis. J Clin Invest. 1999;104(7):975–982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Yadav VK, Oury F, Suda N, et al. A serotonin-dependent mechanism explains the leptin regulation of bone mass, appetite, and energy expenditure. Cell. 2009;138(5):976–989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Münzberg H, Huo L, Nillni EA, Hollenberg AN, Bjørbaek C. Role of signal transducer and activator of transcription 3 in regulation of hypothalamic proopiomelanocortin gene expression by leptin. Endocrinology. 2003;144(5):2121–2131. [DOI] [PubMed] [Google Scholar]
- 47. Kang X, Xie QY, Zhou JS, et al. C/EBP-α, involvement of a novel transcription factor in leptin-induced VCAM-1 production in mouse chondrocytes. FEBS Lett. 2014;588(7):1122–1127. [DOI] [PubMed] [Google Scholar]
- 48. Yaykasli KO, Hatipoglu OF, Yaykasli E, et al. Leptin induces ADAMTS-4, ADAMTS-5, and ADAMTS-9 genes expression by mitogen-activated protein kinases and NF-ĸB signaling pathways in human chondrocytes. Cell Biol Int. 2015;39(1):104–112. [DOI] [PubMed] [Google Scholar]
- 49. Stahl W, Sies H. Antioxidant defense: vitamins E and C and carotenoids. Diabetes. 1997;46 (Suppl. 2):S14–S18. [DOI] [PubMed] [Google Scholar]
- 50. Kim WJ, Park CY. 1,5-Anhydroglucitol in diabetes mellitus. Endocrine. 2013;43(1):33–40. [DOI] [PubMed] [Google Scholar]
- 51. Lee JE. Alternative biomarkers for assessing glycemic control in diabetes: fructosamine, glycated albumin, and 1,5-anhydroglucitol. Ann Pediatr Endocrinol Metab. 2015;20(2):74–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Chan JL, Lutz K, Cochran E, et al. Clinical effects of long-term metreleptin treatment in patients with lipodystrophy. Endocr Pract. 2011;17(6):922–932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Glasow A, Bornstein SR. Leptin and the adrenal gland. Eur J Clin Invest. 2000;30(Suppl. 3):39–45. [DOI] [PubMed] [Google Scholar]
- 54. Dieudonné MN, Sammari A, Dos Santos E, Leneveu MC, Giudicelli Y, Pecquery R. Sex steroids and leptin regulate 11beta-hydroxysteroid dehydrogenase I and P450 aromatase expressions in human preadipocytes: Sex specificities. J Steroid Biochem Mol Biol. 2006;99(4–5):189–196. [DOI] [PubMed] [Google Scholar]