Abstract
Objective:
Characterize failure and resistance above and below guidelines-recommended 1,000 copies/mL virologic threshold, upon 2nd-line failure.
Design:
Cross-sectional study.
Methods:
Kenyan adults on lopinavir/ritonavir-based 2nd-line were enrolled at AMPATH (Academic Model Providing Access to Healthcare). Charts were reviewed for demographic/clinical characteristics and CD4/viral load (VL) were obtained. Participants with detectable VL had a second visit and pol genotyping was attempted in both visits. Accumulated resistance was defined as mutations in the second, not the first visit. Low level viremia (LLV) was detectable VL<1,000 copies/mL. Failure and resistance associations were evaluated using logistic and Poisson regression, Fisher Exact and t-tests.
Results:
Of 394 participants (median age 42, 60% female, median 1.9 years on 2nd-line) 48% had detectable VL; 21% had VL>1,000 copies/mL, associated with younger age, tuberculosis treatment, shorter time on 2nd-line, lower CD4 count/percent, longer 1st-line treatment interruption and pregnancy. In 105 sequences from the first visit (35 with LLV), 79% had resistance (57% dual-, 7% triple-class; 46% with intermediate-high-level resistance to ≥1 future drug option). LLV was associated with more overall and NRTI-associated mutations and with predicted resistance to more next-regimen drugs. In 48 second-visit sequences (after median 55 days; IQR 28–33), 40% accumulated resistance and LLV was associated with more mutation accumulation.
Conclusions:
High resistance upon 2nd-line failure exists at levels above and below guidelines-recommended virologic-failure threshold, impacting future treatment options. Optimization of care should include increased VL monitoring, resistance testing and 3rd-line ART access, and consideration of lowering the virologic failure threshold, though this demands further investigation.
Keywords: HIV, AIDS, Treatment Failure, Drug Resistance, Second Line, Kenya
Introduction
More than 35 million people are infected with HIV worldwide, most in resource-limited settings (RLS), predominantly sub-Saharan Africa[1]. Access and eligibility to antiretroviral therapy (ART) are increasing globally, however only 46% of ART-eligible individuals receive it, a vast treatment gap[2],[3]. Treatment failure in RLS upon non-nucleoside reverse transcriptase inhibitor (NNRTI)-based 1st-line ART is estimated at 24% within 12 months and 33% within 24 months[4]; and upon protease inhibitor (PI)-based 2nd-line ART at 23% within 12 months and 26% within 24 months[5]. As the treatment gap narrows, more individuals in settings where routine sustainable virological monitoring capacity and 3rd-line salvage regimens are limited will fail therapy with potential rises in transmitted and acquired drug resistance[3, 6]. This concerning scenario, which may result in poor clinical outcomes, mandates strategic planning to minimize resistance evolution and transmission[7, 8].
Adult HIV prevalence in Kenya (5.6%−6.1%; 2012) is the 12th highest worldwide, representing a high health burden[9, 10] of an epidemic with multiple circulating subtypes[11]. ART access has significantly increased in Kenya since 2001, with positive clinical outcomes[1, 12]. As of June 2017 70% of eligible adults were receiving ART, 93% on 1st-line (www.nascop.or.ke). Standard 1st-line regimens included zidovudine (AZT) or stavudine (d4T); lamivudine (3TC); and nevirapine (NVP) or efavirenz (EFV), with tenofovir (TDF) substituting d4T in 2013, per WHO guidelines[3]. Data on transmitted[11, 13–16] and 1st-line[17–21] resistance are limited and non-existent for 2nd-line, which mostly includes AZT or TDF, 3TC, and lopinavir/ritonavir (LPV/r)[22], with atazanavir available since 2014.
At the Academic Model Providing Access to Healthcare (AMPATH), a large HIV program in sub-Saharan Africa[23], patients are suspected to fail ART with two consecutive viral load (VL)>1,000 copies/mL, despite adherence counseling. Patients failing 1st-line are switched to 2nd-line, the use of which has consistently increased, with 3,943 patients receiving it in 2010, and 6,968 in 2016. Third-line options (e.g. darunavir, etravirine, raltegravir) have been available since 2015, with a recent addition of dolutegravir, however this process is still restricted, and data on the extent and impact of resistance upon continued 2nd-line, particularly at VL below the WHO-defined failure threshold of 1,000 copies/mL[3], are limited[24].
To address the lack of data on failure and resistance in experienced subjects failing 2nd-line in Kenya and other RLS, we determined the prevalence and correlates of 2nd-line virologic failure in a large AMPATH adult cohort, examined resistance patterns among those with detectable VL above and below 1,000 copies/mL, and ascertained their accumulation and longitudinal effect on future drug options. We hypothesized substantial resistance accumulation over time with impact on 3rd-line salvage regimen options at both high and low-level viremia (LLV).
Methods
Study Setting
At the time of this study, 165,461 HIV-infected persons had received care at AMPATH. Of those, 91,548 were actively in care, 70,224 (77%) of whom received ART: 65,577 NNRTI-based 1st-line and 4,647 LPV/r based 2nd-line. Study enrollment was at the Moi Teaching and Referral Hospital (MTRH) clinic, AMPATH’s largest, where 18,282 adults (≥18 years) were followed, 76% (n=13,983) of whom started ART; 12,741 1st-line and 1,242 2nd-line.
AMPATH patients are managed with an electronic medical record[25] according to locally-developed protocols per WHO guidelines. At the time of the study CD4s were done at HIV diagnosis and every 6 months, and VL access was limited. Subjects suspected as failing 1st-line by consecutive VL>1,000 copies/mL or immunological failure were switched to a standard 2nd-line regimen. Subjects failing 2nd-line remained on this regimen for lack of 3rd-line options.
Participant Enrollment
Inclusion criteria were: (i) HIV-positive (ii) ≥18 years; (iii) on ≥24 weeks LPV/r-based 2nd-line; (iv) ≥6 months prior 1st-line (AZT/d4T+3TC+NVP/EFV); and (v) >50% self-reported adherence in the prior month and seven days. Between June-2011 and May-2012, participants meeting inclusion criteria were offered enrollment, consenting participants were enrolled sequentially and interviewed, charts were reviewed for demographic, clinical and laboratory characteristics, and blood samples were obtained for CD4, VL and pol genotyping. Participants with detectable VL (>40 copies/mL) at enrollment (first visit) were invited for a second visit one month later for repeat pol genotyping, after verification of unchanged ART and adherence. Lifespan and Moi University ethics committees approved the study.
Laboratory Methods
CD4 (FACSCaliber system; Becton Dickenson, San Jose, CA, USA) and HIV VL (Amplicor, Version 1.5); Roche Molecular, Pleasanton, CA, USA) testing was done at the AMPATH laboratory, where they are routinely performed for clinical and research purposes. Plasma samples from both visits were frozen (−80°C), batched and shipped to the Kantor lab for pol genotyping, as described[21]. Briefly, viral RNA was extracted from 400–1000uL plasma via Biomerieux’s MiniMAG, (Durham, NC), followed by reverse transcription and polymerase chain reaction using SuperScript III First-Strand Synthesis System and Platinum Taq DNA Polymerase High Fidelity (ThermoFisher Scientific). A 1.3 kilobase fragment spanning the pol gene (2147–3503, HXB2) was Sanger-sequenced and assembled with Sequencher v4.10.1.
Statistical Analysis
Demographic and clinical data included age, gender, ART history including prevention of mother to child transmission (pMTCT) and treatment interruptions (TIs), prior pregnancy, tuberculosis (TB) treatment and previous CD4s and VLs. Sequence quality control was performed with SQUAT[26]. Resistance interpretation and predicted susceptibilities were evaluated using Stanford Database tools[27]. Measures of resistance at visits 1 and 2 included number of mutations and number and type of drug classes with associated resistance. Patients were classified as having resistance to current regimens if ≥1 medication had low-level or higher predicted resistance; and to future regimens if they had low-level or higher predicted resistance to ≥1 future medication (etravirine, rilpivirine, tenofovir, didanosine, abacavir, atazanavir, darunavir). Counts of resistance to future medications omitted currently-prescribed drug; counts of susceptibility to future medications included current drugs if susceptible. Accumulated resistance was defined as new mutations, seen in the second but not first visit. Visit 2 susceptibility was taken as the worse of the two visits. Pol subtyping was derived with REGA[28].
VL groups, based on visit 1 VL, were defined as ‘Undetectable’ - VL below lower limit of detection (≤40 copies/mL); ‘LLV’ - detectable VL≤1,000 copies/mL; and ‘Failure’ - VL>1,000 copies/mL. We assessed relationships between VL group and age, gender, WHO stage, TB treatment, time on 1st-line, time on 2nd-line, adherence, CD4, VL, TIs, unique regimens (non-traditional 1st/2nd-line), number of 1st/2nd-line regimens, previous pregnancies, PMTCT treatment, and subtype using unadjusted logistic regression. One multivariable logistic regression was fit for each comparison using the covariates found to be most highly correlated with VL group. Odds Ratios (ORs) compare Failure vs non-failure, Detectable vs Undetectable, and Failure vs LLV. To examine representativeness of genotype data, demographic and clinical measures for those with and without genotypes were compared using Fisher Exact and Wilcoxon Rank Sum tests, stratified by VL group.
Multiple statistical methods were used to examine associations between failure group (LLV vs. Failure) at visit 1 as the predictor and various resistance measures as outcomes. At visit 1, dichotomous resistance mutation outcomes (e.g. any resistance) were examined using unadjusted logistic regression; categorical measures (e.g. resistance category) using Fisher Exact Tests; and mutation counts using unadjusted Poisson regression. Tests for mutation outcomes at visit 2 were adjusted for the value of the measure at visit 1. At both visits, current and future medications (finite quantitative measures) were compared using t-tests. 95% confidence intervals that exclude the value of the null hypothesis (i.e. 0 for t-tests and 1 for logistic and Poisson regression) were considered statistically significantly different.
Results
Study Population
A total of 394 eligible participants were enrolled (Table 1), lower than the planned 438 due to slow enrollment. The median age at enrollment was 42 years (IQR 35–48), 60% were female, and 70% had WHO stage 3–4. Before enrollment participants were on 2nd-line for median 1.9 years (IQR 0.8–4.1). Most common regimens included LPV/r + abacavir + didanosine +/− lamivudine (52%), LPV/r + AZT or abacavir + didanosine +/− lamivudine (23%) and LPV/r + tenofovir + lamivudine +/− abacavir (19%) (other regimens ≤1%). Median time on 1st-line was 2.9 years (IQR 1.9–4.1), most commonly lamivudine, zidovudine/stavudine and nevirapine (78%). Twenty four percent were exposed to several 2nd-line regimens and 39% to several 1st-line regimens. TI on 2nd-line was experienced by 3% (median 47 days, IQR 40–63), 4% between 1st/2nd-line (median 69 days, IQR 32–125), and 11% on 1st-line (median 49 days, IQR 28–70). Most participants reported complete adherence in the prior month (97%) and seven days (94%). Eight percent received anti-TB medications on 2nd-line. Among females, 38% had previous pregnancies, 70% once, and 16% received pMTCT ART. Median CD4 values were 282 cells/μL (IQR 182–419) and 17% (IQR 12–23), 28% with AIDS-defining CD4 count <200.
Table 1:
Demographic, clinical, and laboratory characteristics of the study cohort according to viral load threshold a
| VL≤40 (Copies/mL) (n=203) | VL > 40 & ≤1,000 (Copies/mL) (n=109) | VL>1,000 (Copies/mL) (n=82) | Total Cohort (n=394) | |
|---|---|---|---|---|
| Continuous Measures: Median(range) | ||||
| Age | 42.2 (26.5, 68.9) | 41.5 (22.2, 72.6) | 38.8 (18.6, 69.8) | 41.7 (18.6, 72.6) |
| Years on 1st-line | 2.7 (0.5, 10.6) | 2.7 (0.6, 10.4) | 3.3 (0.7, 8.7) | 2.9 (0.5, 10.6) |
| Years on 2nd line | 2.4 (0.5, 8.8) | 1.8 (0.4, 7.5) | 1.3 (0.5, 7.2) | 1.9 (0.4, 8.8) |
| Days of TI on 1st-line b | 40 (16, 110) | 54 (14, 448) | 72 (14, 382) | 49 (14, 448) |
| Days of TI on 2nd-line b | 45.5 (14, 370) | 50 (44, 56) | 48 (35, 126) | 46.5 (14, 370) |
| Days of TI between 1st and 2nd-line b | 65 (14, 399) | 58 (24, 165) | 106 (14, 130) | 69 (14, 399) |
| CD4 Count (cells/μL) | 313 (35, 1424) | 278 (31, 1377) | 171.5 (8, 855) | 282 (8, 1424) |
| CD4 Percent | 19 (2, 40) | 16 (2, 36) | 14 (1, 35) | 17 (1, 40) |
| Categorical Measures: number, % | ||||
| Female | 130, 64% | 62, 57% | 46, 56% | 239, 60% |
| WHO Stage 1 | 30, 15% | 12, 11% | 12, 15% | 54, 14% |
| WHO Stage 2 | 35, 17% | 21, 20% | 9, 11% | 65, 17% |
| WHO Stage 3 | 86, 43% | 50, 47% | 50, 61% | 186, 48% |
| WHO Stage 4 | 50, 25% | 24, 22% | 11, 13% | 85, 22% |
| >One 2nd-Line Regimen | 50, 25% | 25, 23% | 19, 23% | 94, 24% |
| >One 1st-Line Regimen | 89, 44% | 38, 35% | 28, 34% | 155, 39% |
| Unique ARV regimen | 7, 3% | 3, 3% | 5, 6% | 15, 4% |
| TI on 1st-line b | 19, 9% | 11, 10% | 13, 16% | 43, 11% |
| TI on 2nd-line b | 6, 3% | 2, 2% | 4, 5% | 12, 3% |
| TI between 1st-and 2nd-line b | 6, 3% | 3, 3% | 6, 7% | 15, 4% |
| Incomplete 1-Month Adherence | 6, 3% | 3, 3% | 4, 5% | 13, 3% |
| Incomplete 7-day Adherence | 16, 8% | 1, 1% | 6, 7% | 23, 6% |
| TB treatment on 2nd-line | 12, 6% | 7, 6% | 12, 15% | 31, 8% |
| Previous Pregnancies (n=238 females) | 46, 35% | 18, 29% | 26, 57% | 90, 38% |
| pMTCT c | 7, 15% | 6, 33% | 1, 4% | 14, 16% |
Measures presented as median (range) for continuous and n, % for categorical measures;
TI times are summarized only among those who had a TI;
pMTCT data unknown for 2/90.
Abbreviations: ARV, antiretroviral; pMTCT, prevention of mother to child transmission; TB, tuberculosis; TI, treatment interruption; VL, viral load. Odds ratios, confidence intervals and p-values for comparisons of the VL groups are provided in Supplemental Table 1.
Virologic Treatment Failure
Detectable VL was seen in 48% (n=191; median 521 copies/mL, IQR 116–3,876), and 21% had viral failure (VL>1,000 copies/mL; n=82; median 5,424 copies/mL, IQR 1,826–93,660; Table 1). Higher likelihood of VL>1,000 copies/mL vs. ≤1,000 copies/mL was associated with younger age, concurrent TB treatment, shorter time on 2nd-line, lower CD4 count and percent, and longer TI while on 1st-line, with similar results in the univariable and multivariable analyses for age, concurrent TB treatment, time on 2nd-line and CD4 count. Among females, prior pregnancy was significantly associated with VL>1,000 copies/mL (Supplementary Table 1).
Participants with detectable VL were similar to those with undetectable VL on most variables, except less time on 2nd-line in univariable analysis and lower CD4 values in both analyses. In univariable analysis, compared to participants with LLV, those with viral failure were younger, less likely with complete 1-week adherence, and had lower CD4 values. CD4 count remained significant in the multivariable model. Among females, those with failure were more likely to have ever been pregnant, but if pregnant, less likely to have taken ART for pMTCT (Supplementary Table 1).
Drug Resistance and Diversity
Pol sequences were available for 105/191 (55%) participants with detectable VL, 35/109 (32%) with VL≤1,000 copies/mL and 70/82 (85%) with VL>1,000 copies/mL. Clinical and demographic characteristics of participants with/without genotypes did not differ, other than association with increased VL in participants with available genotypes (Supplementary Tables 2 and 3).
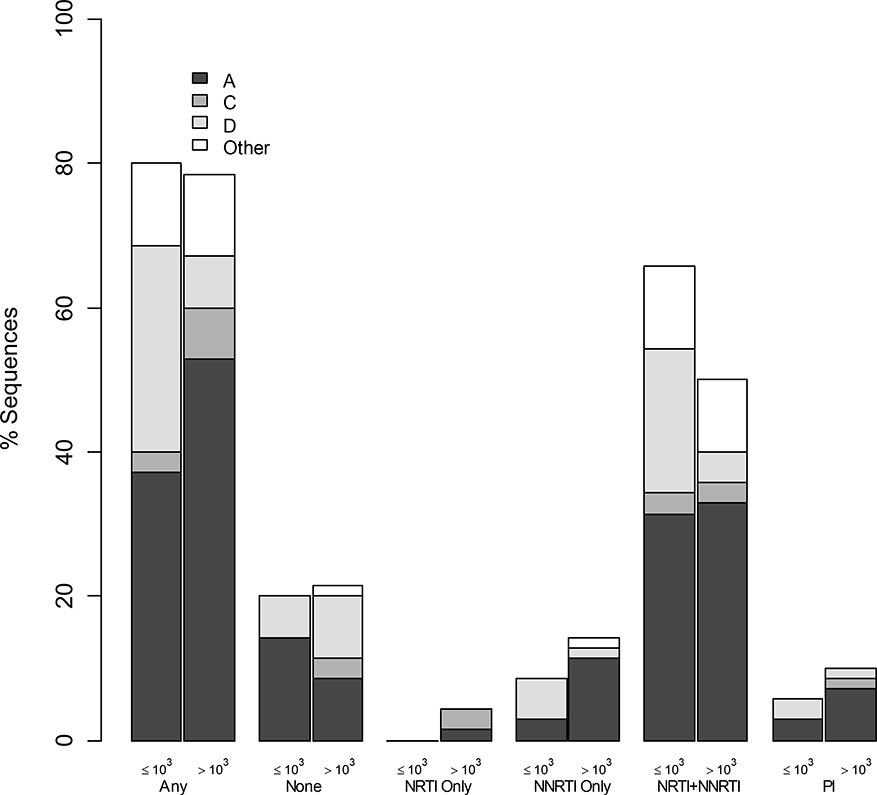
Seventy-nine percent of participants with genotypes had ≥1 resistance mutation; 67% NRTIs, 74% NNRTIs, 9% PIs, 57% dual-class and 7% triple-class resistance. Figure 1 demonstrates resistance prevalence by VL threshold, drug class category and subtype. Of genotyped viruses 58% were subtype A, 22% D, 8% C and 12% others (4% G, 2% AC, 6% AD, 1% DC). The most prevalent (>20%) RT mutations included NRTI-associated M184V, T215Y/F/N/V, D67N/G, M41L and K219Q/R/N/E; and NNRTI-associated K103N/S, Y181C/V and G190A/S. The two most common PI-associated mutations were M46L/I and V82A/L (Supplementary Figure 1). Higher VL was associated with having fewer mutations (Rate Ratio (RR) 0.84 per 1-log10 higher VL, CI=0.74– 0.97, p<0.05). Table 2 provides further information on resistance measures according to VL threshold. Other than the (very low) number of PI mutations, the LLV group had worse resistance in every examined measure. Statistical significance was observed for more overall as well as NRTI-associated mutations and more predicted resistance to next-regimen drugs. Demographic, clinical and laboratory characteristics of genotyped participants with/without resistance to current/future medications were not different in either VL category. Participants with VL>1,000 copies/mL and subtype D were significantly less likely to have resistance mutations compared to participants with VL>1,000 copies/mL and subtype A (OR 0.14, CI=0.03 to 0.61, p<0.05).
Figure 1: Prevalence of drug resistance by drug-class, viral load category, and HIV-1 subtype.
The figure demonstrates the proportion of patients with sequences (Y axis) that contained resistance mutations associated with different combinations of drug resistance categories (X axis). For each category left bars represent VL≤1,000 copies/mL (represented by ‘≤103’) and right bars represent VL>1,000 copies/mL (represented by ‘>103’). Major subtypes are also shown (A-black, C-dark gray, D-light gray, Other-white; per the legend). Abbreviations: NRTI, nucleoside reverse transcriptase inhibitor; NNRTI, non-nucleoside reverse transcriptase inhibitor; PI, protease inhibitor.
Table 2:
Various measures of resistance overall and stratified by VL groupa.
| Resistance Measure | Total (n=105; 48/105 visit 2) | 40 < VL ≤ 1000 (n=35; 17/35 visit 2) | VL > 1000 (n=70; 31/70 visit 2) | VL ≤ 1000 vs VL > 1000 Effect (95%, CI) |
|---|---|---|---|---|
| Resistance category (visit 1) | Fisher Exact Test p=0.31 | |||
| None | 22, 21% | 7, 20% | 15, 21% | |
| NRTI Only | 3, 3% | 0, 0% | 3, 4% | |
| NNRTI Only | 13, 12% | 3, 9% | 10, 14% | |
| NRTI/NNRTI Only | 58, 55% | 23, 66% | 35, 50% | |
| PI | 9, 9% | 2, 6% | 7, 10% | |
| # drug classes with resistance (visit 1) | Fisher Exact Test p=0.56 | |||
| 0 | 22, 21% | 7, 20% | 15, 21% | |
| 1 | 16, 15% | 3, 9% | 13, 19% | |
| 2 | 60, 57% | 24, 69% | 36, 51% | |
| 3 | 7, 7% | 1, 3% | 6, 9% | |
| NRTI/NNRTI/PI Resistanceb | ||||
| Any, visit 1 | 83, 79% | 28, 80% | 55, 79% | OR = 1.09 (0.40, 2.98) |
| Any accumulation | 19, 40% | 8, 47% | 11, 35% | AOR = 1.59 (0.45, 5.59) |
| # of mutations, visit 1 | 3.53 (0, 13) | 4.57 (0, 13) | 3.01 (0, 11) | RR = 1.52 (1.23, 1.86) |
| # of accumulated mutations | 0.92 (0, 5) | 1.41 (0, 5) | 0.65 (0, 4) | ARR = 2.48 (1.34, 4.58) |
| NRTI Resistanceb | ||||
| Any, visit 1 | 70, 67% | 25, 71% | 45, 64% | OR = 1.39 (0.58, 3.35) |
| Any accumulation | 10, 21% | 5, 29% | 5, 16% | AOR = 3.81 (0.69, 21.02) |
| # of mutations, visit 1 | 2.04 (0, 9) | 3.03 (0, 9) | 1.54 (0, 6) | RR = 1.96 (1.50, 2.57) |
| # of accumulated mutations | 0.44 (0, 4) | 0.76 (0, 4) | 0.26 (0, 2) | ARR = 4.18 (1.72, 10.16) |
| NNRTI Resistanceb | ||||
| Any, visit 1 | 78, 74% | 27, 77% | 51, 73% | OR = 1.26 (0.49, 3.25) |
| Any accumulation | 16, 33% | 7, 41% | 9, 29% | AOR = 1.57 (0.43, 5.72) |
| # of mutations, visit 1 | 1.28 (0, 4) | 1.37 (0, 4) | 1.23 (0, 4) | RR = 1.12 (0.78, 1.59) |
| # of accumulated mutations | 0.4 (0, 2) | 0.47 (0, 2) | 0.35 (0, 2) | ARR = 1.39 (0.54, 3.57) |
| Major PI Resistanceb | ||||
| Any, visit 1 | 9, 9% | 2, 6% | 7, 10% | OR = 0.55 (0.11, 2.78) |
| Any accumulation | 2, 4% | 1, 6% | 1, 3% | AOR = 1.56 (0.09, 26.8) |
| # of mutations, visit 1 | 0.22 (0, 4) | 0.17 (0, 4) | 0.24 (0, 4) | RR = 0.71 (0.28, 1.79) |
| # of accumulated mutations | 0.08 (0, 3) | 0.18 (0, 3) | 0.03 (0, 1) | ARR = 4.59 (0.48, 44.11) |
| Resistance to Current Medicationsc | ||||
| Any, visit 1 | 65, 62% | 25, 71% | 40, 57% | OR = 1.39 (0.58, 3.35) |
| Any, visit 2 | 29, 60% | 14, 82% | 15, 48% | AOR = 6.55 (0.83, 52.06) |
| # with resistance, visit 1 | 1.19 (0, 4) | 1.43 (0, 3) | 1.07 (0, 4) | Δ = 0.36 (−0.08, 0.80) |
| # resistance, visit 2 | 1.23 (0, 4) | 1.65 (0, 3) | 1.00 (0, 4) | Δ = 0.65 (0.00, 1.29) |
| Resistance to Future Medicationsc | ||||
| Any, visit 1 | 65, 62% | 24, 69% | 41, 59% | OR = 1.54 (0.65, 3.64) |
| Any, visit 2 | 29, 60% | 12, 71% | 17, 55% | AOR = 2.23 (0.45, 11.05) |
| # with resistance, visit 1 | 1.44 (0, 5) | 1.83 (0, 5) | 1.24 (0, 5) | Δ = 0.59 (−0.03, 1.21) |
| # resistance, visit 2 | 1.48 (0, 4) | 1.65 (0, 4) | 1.39 (0, 4) | Δ = 0.26 (−0.68, 1.2) |
Measures presented as mean (range) for continuous and n, % for categorical measures.
Resistance: OR = Odds Ratio from unadjusted logistic regression; RR = Rate Ratio from unadjusted Poisson Regression; ARR = Rate Ratio from Poisson Regression, adjusted for the number of mutations at visit 1; AOR = Odds Ratio from logistic regression adjusted for presence of resistance at visit 1.;
Medications: Δ = difference from t-test; OR = Odds Ratio from unadjusted logistic regression; AOR = Odds Ratio from logistic regression adjusted for presence of resistance to medications at visit 1. Abbreviations: #, number; NRTI, nucleoside reverse transcriptase inhibitor; NNRTI, non-nucleoside reverse transcriptase inhibitor; PI, protease inhibitor.
Drug Resistance Accumulation
Of 105 genotyped participants, 48 also had second visit sequences, median 55 days after the first visit (range 14–243 days). All sequence-pairs clustered well phylogenetically, with high (>85%) bootstrap values. Of these, 19 (40%) had 51 resistance mutations detected in the second visit, not in the first; eight of 48 (17%) with LLV (Table 3). Ten patients (53% of 19) accumulated NRTI mutations, 16 (84%) NNRTI mutations, and two (11%) PI mutations. The most common position where mutations accumulated was RT 184 (six patients). Of 51 accumulated mutations, 26 (51%) were mixtures. In 11/48 (23%) participants, 28 resistance mutations detected in the first visit were not detected in the second.
Table 3:
Drug resistance mutations in participants with resistance accumulationa
| ID | VL (c/mL) | Months Between Visits | ART | NRTI-Associated | NNRTI-Associated | PI-Associated |
|---|---|---|---|---|---|---|
| 1 | 66 | 2.8 | 3,T,L/r | D67N, M184V, T215F, K219Q | K103N, V106I, M230L | None |
| 2 | 69 | 3 | A,D,L/r | None | G190AG | None |
| 3 | 77 | 1.9 | A,D,L/r | D67N, K70R, M184V, K219Q | K103N | None |
| 4 | 87 | 2.8 | Z,A,L/r | M41LM, D67DN, M184MV, T215FISTY | V108I, Y181C, H221Y | None |
| 5 | 125 | 1.1 | 3,T,A,L/r | D67N, T69N, K70R, V75LV, M184V, K219Q | K101E, V106I, G190S | M46I, I54V, V82A |
| 6 | 161 | 8.1 | 3,A,D,L/r | A62AV, V75IV, Q151KLMQ, M184MV | K103KN | None |
| 7 | 360 | 3.5 | A,D, L/r | D67N, M184MVI, T215ST | K103KN, E138EQ | None |
| 8 | 384 | 0.9 | A,D,L/r | M41L, D67N, V75M, M184V, L210W, T215FY | V90I, G190A | None |
| 9 | 1697 | 0.9 | Z,3,L/r | K70R, M184V | V106A, F227L | None |
| 10 | 1858 | 2.8 | A,D,L/r | D67DN, K70KR, M184V, K219KQ | Y181C | None |
| 11 | 2215 | 2.1 | A,D,L/r | M184MV, K219N | K103KN, Y181C, K238KT | None |
| 12 | 9478 | 1.4 | Z,D,L/r | D67DGN, K70R, M184MV, K219Q | Y181C, H221Y | None |
| 13 | 31999 | 2.8 | 3,T,A,L/r | M41L, D67N, L74V, M184V, L210W, T215Y | K103S, G190A | M46I, I54V, V82A, L90M |
| 14 | 42415 | 1.9 | A,D,L/r | M184V, T215Y | V108IV, Y181C, H221Y | M46I, I54IV, V82A |
| 15 | 140298 | 0.9 | Z,D,L/r | None | V90IV, K103KN | None |
| 16 | 167586 | 1.9 | Z,3,L/r | M184V | A98G, K103N, Y181CY, P225H | None |
| 17 | 193742 | 2.8 | Z,3,L/r | M184V | K103N, E138Q | None |
| 18 | 341778 | 1.9 | Z,A,L/r | T215NT, M41LM, M184MV, T215F | V108IV, Y181CY | None |
| 19 | 3379111 | 2.8 | 3,T,L/r | None | None | I54IL |
Bold indicates mutations detected at the second visit but not in the first. Abbreviations: ‘3’ in ART column, 3TC=lamivudine; ART, antiretroviral therapy; c/mL, copies/mL; ‘D’ in ART column, didanosine; ‘L’ in ART column, lopinavir; NRTI, nucleoside reverse transcriptase inhibitor; NNRTI, non-nucleoside reverse transcriptase inhibitor; PI, protease inhibitor; ‘r’ in ART column, ritonavir; ‘T’ in ART column, tenofovir; VL, viral load; ‘Z’ in ART column, zidovudine.
At the second visit, those with LLV were on average resistant to 1.65 medications in their current regimen compared to 1.00 medication among those with VL>1000 copies/mL (p=0.049, Table 2). Additionally, those with LLV were susceptible to fewer of the medications in their current regimen at visit 1 and 2 (p=0.053). Those with LLV were susceptible to fewer future medications at visit 1 (CI=−1.81to −0.11, p<0.028) and visit 2 (CI=−2.23 to 0.2, p=0.100) than those with VL>1,000 copies/mL. Those with LLV accumulated significantly more mutations (RR=2.48, CI=1.34–4.59, p<0.05) than those with VL>1,000, specifically NRTI mutations (RR=4.18, CI=1.72–10.16, p>0.05). While not statistically significant, those with LLV also accumulated more NNRTI (RR=1.39, CI=0.54–3.57, p=0.50) and PI (RR=4.59, CI=0.48–44.11, p=0.187) mutations.
Older participants were at increased risk of accumulating mutations (OR 2.35 per 10 years older, CI=1.06–5.20, p<0.05), whereas females were at decreased risk (OR 0.2, CI=0.05–0.84, p<0.05, data not shown). There was a non-significant trend towards increased risk of accumulation in participants with higher CD4 counts (OR 1.65 per 100 cells higher, CI=0.99–2.77, p=0.064). Resistance accumulation occurred in 36% (10/28) of participants with subtype A, 25% (1/4) subtype C, 54% (7/13) subtype D, and 33% (1/3) other subtypes (p>0.05).
Predicted Susceptibilities to Future Options
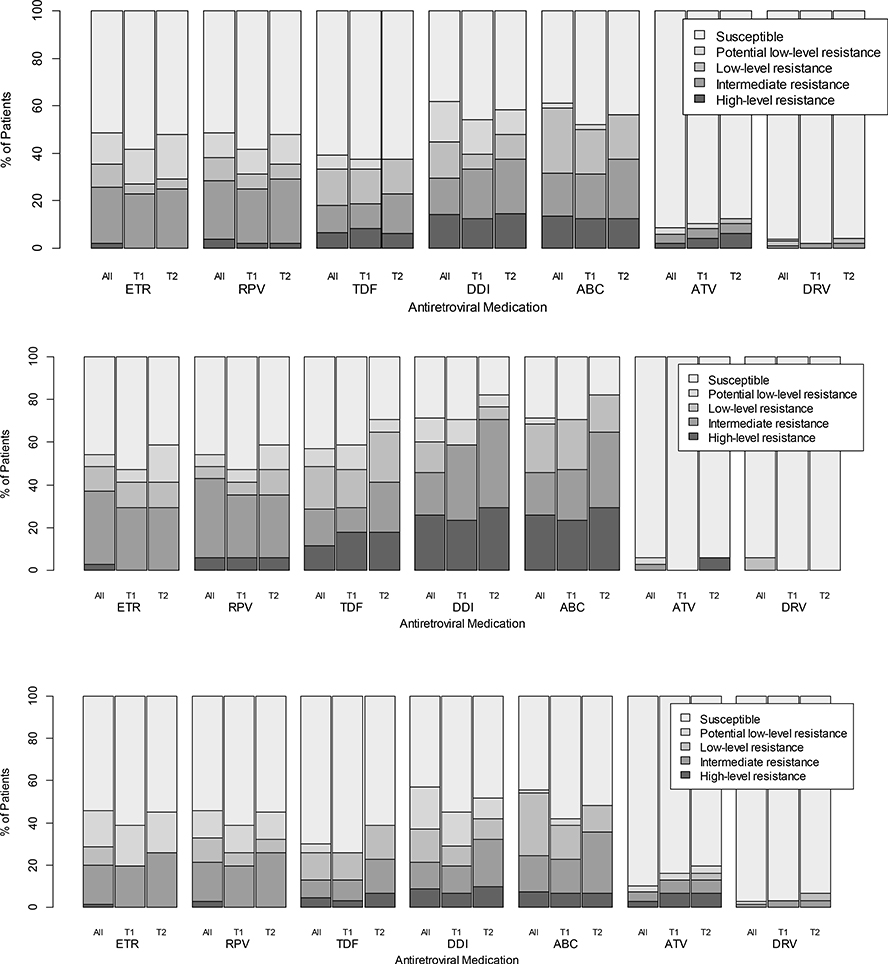
At enrollment, 46% (48/105) of genotyped participants, in both VL categories, had predicted intermediate-high level resistance to at least one future drug option: 26% etravirine, 29% rilpivirine, 18% tenofovir, 30% didanosine, 31% abacavir, 6% atazanavir, and 1% darunavir. Of the 48 patients with two available genotypes, 21% (10/48) had increased levels of predicted resistance at the second visit to intermediate-high levels, to at least one future drug option: 4% etravirine, 4% rilpivirine, 10% tenofovir, 13% didanosine, 15% abacavir and 2% atazanavir, more pronounced at LLV (Figure 2).
Figure 2: Predicted susceptibility to future drug options upon 2nd-line failure.
The figure demonstrates the proportion of patients (Y axis) that had one of five levels of predicted susceptibility (legend) to antiretroviral medications that might be considered for future treatment options (X axis). Results are provided for (i) 105 patients with genotypes at the first visit (All; left bars for each drug); (ii) Timepoint 1 (T1=first visit; center bars for each drug; for 48/105 participants with genotypes at both visits); and (iii) Timepoint 2 (T2=second visit; for 48/105 participants with genotypes at both visit right bars). Data are provided for all participants (top graph); those with low level viremia (middle graph); and those with viral load >1,000 copies/mL (lower graph).
Discussion
The goal of this study, the first of its kind in Kenya, was to guide management of HIV-infected persons failing 2nd-line ART. In an analysis of 394 adults on 2nd-line, 48% had detectable VL and 21% had VL>1,000 copies/mL. Participants had low-level (9%) PI resistance, but extensive NRTI (67%), NNRTI (74%) and dual/triple-class (64%) resistance, at VL levels above and below the WHO threshold of 1,000 copies/mL. Moreover, 40% of participants with follow up sequences after an average 59 days accumulated resistance mutations, even at low-level VLs, compromising potential future treatment options.
To our knowledge, this is the first report of resistance development at LLV in RLS. Almost a third of participants in this study had LLV, consistent with current literature[29]. Although we and others identified correlates of persistent LLV, these vary widely and no specific risk factors have been identified and broadly accepted[29]. In RLS, resistance mutations at LLV upon 2nd-line failure have not yet been investigated, mostly since individuals at this VL range are not considered as failing ART per current WHO guidelines and their genotyping is more challenging. LLV studies in developed settings report resistance in 17–72%, suggesting detrimental outcomes of allowing patients to remain on therapy with low-level viral replication[24, 30, 31]. Participants with LLV in our study had 79% resistance and 72% dual-class resistance, higher than reported in non-RLS, with an even significantly higher prevalence of NRTI mutations compared to participants with VL>1,000 copies/mL. This is concerning, since these individuals are not considered as failing ART in most RLS, even with the now recommended annual VL monitoring[10, 32]. The 42% of participants with LLV who demonstrated resistance mutation accumulation further emphasize the potential impact of replication at this level. Additional investigation is needed to examine the impact of LLV resistance and the need to consider lowering the VL threshold definition for ART failure and the sensitivity requirements for drug resistance testing assays.
In this study, the first to longitudinally examine intra-patient resistance accumulation upon 2nd-line failure in RLS, 40% of participants accumulated mutations. A prior study in Nigeria demonstrated a similar concept, but using cross-sectional data[33]. Despite low overall PI resistance, two participants accumulated new PI mutations in the short time period between study visits. Accumulation of PI mutations can lead to darunavir resistance[34], and indeed four patients (4%) in our cohort were found to have some resistance to darunavir, and one (1%) had intermediate resistance to this 3rd-line drug. Interestingly, most participants who accumulated resistance had new NNRTI mutations in the second visit, despite not being on this ART class as part of 2nd-line. This may be explained, though not examined here, by selection of more fit viral quasispecies with linked NNRTI mutations on the same viral genome[35], and the low impact NNRTI mutations may have on viral fitness[36]. The distinction between mutation accumulation and re-emergence must therefore be further examined.
Virological failure rates of 2nd-line ART in RLS vary widely (5–72%) across studies, settings and methodologies[5, 37–39]. Our findings, from a large HIV program in western Kenya, fall within this wide spectrum. Prior reports associate 2nd-line failure with poor adherence[5, 38, 39], longer time on 1st-line[5] and shorter time on 2nd-line ART[39], lower CD4 and higher VL at 2nd-line initiation[39, 40], being female[41] and delayed switch to 2nd-line[41]. We confirm some of these observations and extend them with other correlations of 2nd-line failure: First, longer TIs while on 1st-line, consistent with reports of its association with 1st-line failure, poor adherence and/or resistance mutation accumulation[21, 42]. Second, concurrent TB treatment, possibly resulting from medication interactions, particularly rifampicin and its induction of the CYP 450 enzymes, and the ensuing lower PI concentrations[43]. This is an important finding in light of the high TB prevalence in RLS, which was previously shown in children[44], and recently in South-African adults[45]. Third, prior pregnancies among females, and if pregnant, less pMTCT exposure, possibly related to low-engagement in care. The finding that younger age is associated with 2nd-line failure, contrasts prior reports [39, 40] and may be related to specific settings. Altogether, these highly variable findings point to potential interventions to improve treatment monitoring and sustain 2nd-line ART.
The low (9%) PI resistance upon 2nd-line failure is consistent with reports from RLS, ranging from 6–25%[34, 38, 46–48], as well as earlier reports, mostly from developed countries, with a slightly wider (0–33%), though still low, range[49–54]. As expected, considering future ART options, almost all participants with LPV/r resistance were also resistant to atazanavir, and darunavir seems an appropriate 3rd-line option, as suggested by current Kenya ART guidelines[10]. The implications of low PI resistance upon 2nd-line failure on subsequent ART susceptibilities and ability to recycle PIs, and the impact of postulated etiologies to such low resistance, including adherence[48, 55], minority resistance variants[56], and newer resistance mechanisms[57–59], are under investigation. Higher rates (63–87%) of PI resistance have been reported from India, Vietnam and Nigeria[33, 37, 60]; and reasons for this discordance, possibly associated to past ART exposure, differential treatment monitoring, viral subtype and host factors, should be further examined. The high RT resistance (65–95%) found here was associated with almost 30% predicted resistance to rilpivirine and etravirine, the newer-generation NNRTIs being considered in 3rd-line regimens[10]. This augments similar reports from India (29%)[37], Mali (38%)[46], and Nigeria (at least 26%, based on mutational analysis of published genotypes)[33]. Though high, these data indicate that some patients may still be susceptible to these medications, if genotyping was available in this setting. Importantly, following the report of the first case of a multi-drug resistant isolate in sub-Saharan Africa[61], two patients in this study had intermediate-high-level resistance to all 1st/2nd-line drugs available in the public sector (3TC, FTC, TDF, ABC, DDI, AZT, d4T, EFV, NVP, ATV and LPV), substantiating and supporting the strategic need for 3rd-line ART in RLS[10, 62, 63].
HIV-1 subtype may be relevant to clinical care and to resistance development[64–67]. Few studies have assessed 2nd-line resistance in non-B subtypes[33, 37, 46, 47]. Grossman et al. found no significant differences in 2nd-line resistance development between subtypes B and C[52], and other studies have not included enough subtype diversity to allow inter-subtype comparisons. In this study, allowing resistance evaluation upon 2nd-line failure in a RLS with diverse circulating subtypes, we found that at VL>1,000 copies/mL, subtype D viruses had significantly less resistance compared to subtype A. These findings contrast data from Uganda demonstrating more resistance in subtype D vs. A, though not solely upon 2nd-line failure[68]. A larger study assessing differences in 2nd-line failure and resistance between subtypes could elucidate the cause of such findings, and help guide clinical care in areas with diverse subtypes.
The major limitations of the present study are primarily its cross-sectional nature and the lack of continuous virologic monitoring and confirmed testing for VL failure, limiting the ability to rule out viral blips, estimate time to virologic failure and determine duration of time on a failing regimen. This study design however allowed for accrual of a large number of patients, and unfortunately the limited monitoring is representative of routine circumstances in many RLS, even today[69]. Additionally, adherence was based on self-report; pre-ART and pre-2nd-line resistance testing were not available; and resistance testing sensitivity was limited to conventional, population-based pol genotyping, with no consideration of gag/env mutations or effects of minority variants. Lastly, treatment and monitoring guidelines evolve rapidly and new and improved ART as well as more routine VL testing are available in some RLS, different than the study’s settings. However, concepts presented here hold across guidelines and treatments, and will still be relevant in many settings for years to come.
In conclusion, in this large study in Kenyans on 2nd-line ART, we found high rates of failure, extensive overall but low PI resistance and resistance accumulation at VL levels above and below the WHO treatment-failure threshold, that impact treatment options. Considering increased global ART access, the aging HIV-infected population and existing cost constraints in RLS, optimization of adherence and treatment monitoring (e.g. VL/resistance testing) are required to support current local and global HIV cascade goals[70–72]. Continued investigation into resistance mechanisms, in particular when PI resistance is absent and good adherence is documented, is needed to improve resistance detection and ART strategies[73]. As in resource-rich settings, treatment goals in RLS should be geared towards achieving virologic suppression in ART-experienced patients with resistance, making ART available to all and strategically planning for 3rd-line regimens.
Supplementary Material
The figure presents frequencies of mutations (Y axis) at positions associated with resistance to NRTIs (left mutation block, X axis); NNRTIs (middle block) and PIs (right block), in patients with VL > 1,000 copies/ML (gray) and VL<1,000 copies/mL (black).
Correlates of viral load failure according to viral load thresholda
Characteristics of patients with and without sequences at VL≤1,000 copies/mLa.
Characteristics of patients with and without sequences at VL>1,000 copies/mLa.
Acknowledgements
RK conceptualized the study; RK and AD curated and managed the data; RK, AK, LS, MRe, KB and MC analyzed the data, with specific development of methods and statistical analysis by AD and JH; EK, SO and RB consented, enrolled and interviewed participants, supervised by LD; LS, MO and MRo conducted laboratory experiments, supervised by RK, WE, MC and NB; RK, MRe and KB wrote the initial draft; all authors critically reviewed the paper.
Conflicts of Interest and Source of Funding: no conflict of interest is declared by all authors. This work was supported by National Institutes of Health grants R01AI108441 and P30AI042853.
References
- 1.UNAIDS. Available at http://www.unaids.org; accessed August 15, 2017. In.
- 2.UNAIDS. Global AIDS Update 2016. Available at http://www.unaids.org/en/resources/documents/2016/Global-AIDS-update-2016; accessed August 15, 2017.
- 3.WHO. Consolidated guidelines on the use of antiretroviral drugs for treating and preventing HIV infection. Recommendations for a public health approach. Available at http://www.who.int/hiv/pub/guidelines/arv2013/download/en/; accessed August 15, 2017.
- 4.Barth RE, van der Loeff MF, Schuurman R, Hoepelman AI, Wensing AM. Virological follow-up of adult patients in antiretroviral treatment programmes in sub-Saharan Africa: a systematic review. Lancet Infect Dis 2010,10:155–166. [DOI] [PubMed] [Google Scholar]
- 5.Ajose O, Mookerjee S, Mills EJ, Boulle A, Ford N. Treatment outcomes of patients on second-line antiretroviral therapy in resource-limited settings. Aids 2012,26:929–938. [DOI] [PubMed] [Google Scholar]
- 6.WHO. HIV drug resistance report 2017. Available at http://www.who.int/hiv/pub/drugresistance/hivdr-report-2017/en/; accessed October 16, 2017.
- 7.WHO. Guidelines on the public health response to pretreatment HIV drug resistance, July 2017. Available at http://apps.who.int/iris/handle/10665/255880; accessed October 16, 2017.
- 8.WHO. Global action plan on HIV drug resistance 2017–2021. Available at http://who.int/hiv/pub/drugresistance/hivdr-action-plan-2017-2021/en/; accessed October 16, 2017.
- 9.Maina WK, Kim AA, Rutherford GW, Harper M, K’Oyugi BO, Sharif S, et al. Kenya AIDS Indicator Surveys 2007 and 2012: implications for public health policies for HIV prevention and treatment. J Acquir Immune Defic Syndr 2014,66 Suppl 1:S130–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.NASCOP. Guidelines on Use of Antiretroviral Drugs for Treating and Preventing HIV Infection in Kenya. 2016. Edition. Available at https://aidsfree.usaid.gov/sites/default/files/kenya_art_2016.pdf. Accessed October 3, 2017.
- 11.Kantor R, DeLong A, Balamane M, Schreier L, Lloyd RM Jr., Injera W, et al. HIV diversity and drug resistance from plasma and non-plasma analytes in a large treatment programme in western Kenya. J Int AIDS Soc 2014,17:19262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wools-Kaloustian K, Kimaiyo S, Diero L, Siika A, Sidle J, Yiannoutsos CT, et al. Viability and effectiveness of large-scale HIV treatment initiatives in sub-Saharan Africa: experience from western Kenya. AIDS 2006,20:41–48. [DOI] [PubMed] [Google Scholar]
- 13.Hassan AS, Mwaringa SM, Obonyo CA, Nabwera HM, Sanders EJ, Rinke de Wit TF, et al. Low prevalence of transmitted HIV type 1 drug resistance among antiretroviral-naive adults in a rural HIV clinic in Kenya. AIDS Res Hum Retroviruses 2013,29:129–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kiptoo M, Brooks J, Lihana RW, Sandstrom P, Ng’ang’a Z, Kinyua J, et al. HIV-1 drug resistance-associated mutations among HIV-1 infected drug-naive antenatal clinic attendees in rural Kenya. BMC Infect Dis 2013,13:517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lihana RW, Khamadi SA, Lubano K, Lwembe R, Kiptoo MK, Lagat N, et al. HIV type 1 subtype diversity and drug resistance among HIV type 1-infected Kenyan patients initiating antiretroviral therapy. AIDS Res Hum Retroviruses 2009,25:1211–1217. [DOI] [PubMed] [Google Scholar]
- 16.Nyamache AK, Waihenya R, Ng’ang’a ZW, Muigai AW, Khamadi SA. Reverse transcriptase inhibitors drug resistance mutations in drug-naive HIV type 1 positive Kenyans. East Afr Med J 2011,88:4–8. [PubMed] [Google Scholar]
- 17.Crawford KW, Njeru D, Maswai J, Omondi M, Apollo D, Kimetto J, et al. Occurrence of etravirine/rilpivirine-specific resistance mutations selected by efavirenz and nevirapine in Kenyan patients with non-B HIV-1 subtypes failing antiretroviral therapy. AIDS 2014,28:442–445. [DOI] [PubMed] [Google Scholar]
- 18.Hassan AS, Nabwera HM, Mwaringa SM, Obonyo CA, Sanders EJ, Rinke de Wit TF, et al. HIV-1 virologic failure and acquired drug resistance among first-line antiretroviral experienced adults at a rural HIV clinic in coastal Kenya: a cross-sectional study. AIDS Res Ther 2014,11:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Osman S, Lihana RW, Kibaya RM, Ishizaki A, Bi X, Okoth FA, et al. Diversity of HIV type 1 and drug resistance mutations among injecting drug users in Kenya. AIDS Res Hum Retroviruses 2013,29:187–190. [DOI] [PubMed] [Google Scholar]
- 20.Steegen K, Luchters S, Dauwe K, Reynaerts J, Mandaliya K, Jaoko W, et al. Effectiveness of antiretroviral therapy and development of drug resistance in HIV-1 infected patients in Mombasa, Kenya. AIDS Res Ther 2009,6:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mann M, Diero L, Kemboi E, Mambo F, Rono M, Injera W, et al. Antiretroviral Treatment Interruptions Induced by the Kenyan Postelection Crisis Are Associated With Virological Failure. J Acquir Immune Defic Syndr 2013,64:220–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.UNAIDS. Office of the President National AIDS Control Council. The Kenya AIDS Epidemic. Available at http://www.unaids.org/en/dataanalysis/knowyourresponse/countryprogressreports/2012countries/ce_KE_Narrative_Report.pdf; accessed October 16, 2017.
- 23.AMPATH. Academic Model Providing Access to Healthcare. Available at http://www.who.int/hiv/pub/prev_care/ampath/en/. Accessed October 16, 2017.
- 24.Swenson LC, Min JE, Woods CK, Cai E, Li JZ, Montaner JS, et al. HIV drug resistance detected during low-level viraemia is associated with subsequent virologic failure. AIDS 2014,28:1125–1134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tierney WM, Rotich JK, Hannan TJ, Siika AM, Biondich PG, Mamlin BW, et al. The AMPATH medical record system: creating, implementing, and sustaining an electronic medical record system to support HIV/AIDS care in western Kenya. Stud Health Technol Inform 2007,129:372–376. [PubMed] [Google Scholar]
- 26.Delong AK, Wu M, Bennett D, Parkin N, Wu Z, Hogan JW, et al. Sequence quality analysis tool for HIV type 1 protease and reverse rranscriptase. AIDS Res Hum Retroviruses 2012,28:894–901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shafer R Stanford HIV Sequence Database. Available at http://hivdb.stanford.edu; acccessed October 16, 2017.
- 28.Pineda-Pena AC, Faria NR, Imbrechts S, Libin P, Abecasis AB, Deforche K, et al. Automated subtyping of HIV-1 genetic sequences for clinical and surveillance purposes: performance evaluation of the new REGA version 3 and seven other tools. Infect Genet Evol 2013,19:337–348. [DOI] [PubMed] [Google Scholar]
- 29.Ryscavage P, Kelly S, Li JZ, Harrigan PR, Taiwo B. Significance and clinical management of persistent low-level viremia and very-low-level viremia in HIV-1-infected patients. Antimicrob Agents Chemother 2014,58:3585–3598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Taiwo B, Gallien S, Aga E, Ribaudo H, Haubrich R, Kuritzkes DR, et al. Antiretroviral drug resistance in HIV-1-infected patients experiencing persistent low-level viremia during first-line therapy. J Infect Dis 2011,204:515–520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gonzalez-Serna A, Min JE, Woods C, Chan D, Lima VD, Montaner JS, et al. Performance of HIV-1 drug resistance testing at low-level viremia and its ability to predict future virologic outcomes and viral evolution in treatment-naive individuals. Clin Infect Dis 2014,58:1165–1173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.WHO. Consolidated guidelines on the use of antiretroviral drugs for treating and preventing HIV infection. Availabe at http://www.who.int/hiv/pub/arv/arv-2016/en/. Accessed October 1, 2017.
- 33.Rawizza HE, Chaplin B, Meloni ST, Darin KM, Olaitan O, Scarsi KK, et al. Accumulation of protease mutations among patients failing second-line antiretroviral therapy and response to salvage therapy in Nigeria. PLoS One 2013,8:e73582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Steegen K, Bronze M, Papathanasopoulos MA, van Zyl G, Goedhals D, Van Vuuren C, et al. Prevalence of Antiretroviral Drug Resistance in Patients Who Are Not Responding to Protease Inhibitor-Based Treatment: Results From the First National Survey in South Africa. J Infect Dis 2016,214:1826–1830. [DOI] [PubMed] [Google Scholar]
- 35.Palmer S, Kearney M, Maldarelli F, Halvas EK, Bixby CJ, Bazmi H, et al. Multiple, linked human immunodeficiency virus type 1 drug resistance mutations in treatment-experienced patients are missed by standard genotype analysis. J Clin Microbiol 2005,43:406–413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Joly V, Descamps D, Peytavin G, Touati F, Mentre F, Duval X, et al. Evolution of human immunodeficiency virus type 1 (HIV-1) resistance mutations in nonnucleoside reverse transcriptase inhibitors (NNRTIs) in HIV-1-infected patients switched to antiretroviral therapy without NNRTIs. Antimicrob Agents Chemother 2004,48:172–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Saravanan S, Vidya M, Balakrishnan P, Kantor R, Solomon SS, Katzenstein D, et al. Viremia and HIV-1 drug resistance mutations among patients receiving second-line highly active antiretroviral therapy in Chennai, Southern India. Clinical Infectious Diseases 2012,54:995–1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Boender TS, Hamers RL, Ondoa P, Wellington M, Chimbetete C, Siwale M, et al. Protease Inhibitor Resistance in the First 3 Years of Second-Line Antiretroviral Therapy for HIV-1 in Sub-Saharan Africa. J Infect Dis 2016,214:873–883. [DOI] [PubMed] [Google Scholar]
- 39.Pujades-Rodriguez M, Balkan S, Arnould L, Brinkhof MA, Calmy A. Treatment failure and mortality factors in patients receiving second-line HIV therapy in resource-limited countries. JAMA 2010,304:303–312. [DOI] [PubMed] [Google Scholar]
- 40.Johnston V, Fielding K, Charalambous S, Mampho M, Churchyard G, Phillips A, et al. Second-line antiretroviral therapy in a workplace and community-based treatment programme in South Africa: determinants of virological outcome. PLoS One 2012,7:e36997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Levison JH, Orrell C, Losina E, Lu Z, Freedberg KA, Wood R. Early outcomes and the virological effect of delayed treatment switching to second-line therapy in an antiretroviral roll-out programme in South Africa. Antivir Ther 2011,16:853–861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kranzer K, Ford N. Unstructured treatment interruption of antiretroviral therapy in clinical practice: a systematic review. Trop Med Int Health 2011,16:1297–1313. [DOI] [PubMed] [Google Scholar]
- 43.McIlleron H, Meintjes G, Burman WJ, Maartens G. Complications of antiretroviral therapy in patients with tuberculosis: drug interactions, toxicity, and immune reconstitution inflammatory syndrome. J Infect Dis 2007,196 Suppl 1:S63–75. [DOI] [PubMed] [Google Scholar]
- 44.Walters E, Reichmuth K, Dramowski A, Marais BJ, Cotton MF, Rabie H. Antiretroviral regimens containing a single protease inhibitor increase risk of virologic failure in young HIV-infected children. Pediatr Infect Dis J 2013,32:361–363. [DOI] [PubMed] [Google Scholar]
- 45.Collier D, Iwuji C, Derache A, de Oliveira T, Okesola N, Calmy A, et al. Virological Outcomes of Second-line Protease Inhibitor-Based Treatment for Human Immunodeficiency Virus Type 1 in a High-Prevalence Rural South African Setting: A Competing-Risks Prospective Cohort Analysis. Clin Infect Dis 2017,64:1006–1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Maiga AI, Fofana DB, Cisse M, Diallo F, Maiga MY, Traore HA, et al. Characterization of HIV-1 antiretroviral drug resistance after second-line treatment failure in Mali, a limited-resources setting. J Antimicrob Chemother 2012,67:2943–2948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Van Zyl GU, Liu TF, Claassen M, Engelbrecht S, de Oliveira T, Preiser W, et al. Trends in Genotypic HIV-1 Antiretroviral Resistance between 2006 and 2012 in South African Patients Receiving First- and Second-Line Antiretroviral Treatment Regimens. PLoS One 2013,8:e67188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.van Zyl GU, van Mens TE, McIlleron H, Zeier M, Nachega JB, Decloedt E, et al. Low lopinavir plasma or hair concentrations explain second-line protease inhibitor failures in a resource-limited setting. J Acquir Immune Defic Syndr 2011,56:333–339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Madruga JV, Berger D, McMurchie M, Suter F, Banhegyi D, Ruxrungtham K, et al. Efficacy and safety of darunavir-ritonavir compared with that of lopinavir-ritonavir at 48 weeks in treatment-experienced, HIV-infected patients in TITAN: a randomised controlled phase III trial. Lancet 2007,370:49–58. [DOI] [PubMed] [Google Scholar]
- 50.Boyd MA, Kumarasamy N, Moore CL, Nwizu C, Losso MH, Mohapi L, et al. Ritonavir-boosted lopinavir plus nucleoside or nucleotide reverse transcriptase inhibitors versus ritonavir-boosted lopinavir plus raltegravir for treatment of HIV-1 infection in adults with virological failure of a standard first-line ART regimen (SECOND-LINE): a randomised, open-label, non-inferiority study. Lancet 2013,381:2091–2099. [DOI] [PubMed] [Google Scholar]
- 51.Barber TJ, Harrison L, Asboe D, Williams I, Kirk S, Gilson R, et al. Frequency and patterns of protease gene resistance mutations in HIV-infected patients treated with lopinavir/ritonavir as their first protease inhibitor. J Antimicrob Chemother 2012,67:995–1000. [DOI] [PubMed] [Google Scholar]
- 52.Grossman Z, Schapiro JM, Levy I, Elbirt D, Chowers M, Riesenberg K, et al. Comparable long-term efficacy of Lopinavir/Ritonavir and similar drug-resistance profiles in different HIV-1 subtypes. PLoS One 2014,9:e86239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mo H, King MS, King K, Molla A, Brun S, Kempf DJ. Selection of resistance in protease inhibitor-experienced, human immunodeficiency virus type 1-infected subjects failing lopinavir- and ritonavir-based therapy: mutation patterns and baseline correlates. J Virol 2005,79:3329–3338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kempf DJ, King MS, Bernstein B, Cernohous P, Bauer E, Moseley J, et al. Incidence of resistance in a double-blind study comparing lopinavir/ritonavir plus stavudine and lamivudine to nelfinavir plus stavudine and lamivudine. J Infect Dis 2004,189:51–60. [DOI] [PubMed] [Google Scholar]
- 55.El-Khatib Z, Ekstrom AM, Ledwaba J, Mohapi L, Laher F, Karstaedt A, et al. Viremia and drug resistance among HIV-1 patients on antiretroviral treatment: a cross-sectional study in Soweto, South Africa. AIDS 2010,24:1679–1687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Fisher R, van Zyl GU, Travers SA, Kosakovsky Pond SL, Engelbrech S, Murrell B, et al. Deep sequencing reveals minor protease resistance mutations in patients failing a protease inhibitor regimen. J Virol 2012,86:6231–6237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Nijhuis M, van Maarseveen NM, Lastere S, Schipper P, Coakley E, Glass B, et al. A novel substrate-based HIV-1 protease inhibitor drug resistance mechanism. PLoS Med 2007,4:e36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Rabi SA, Laird GM, Durand CM, Laskey S, Shan L, Bailey JR, et al. Multi-step inhibition explains HIV-1 protease inhibitor pharmacodynamics and resistance. J Clin Invest 2013,123:3848–3860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Gatanaga H, Suzuki Y, Tsang H, Yoshimura K, Kavlick MF, Nagashima K, et al. Amino acid substitutions in Gag protein at non-cleavage sites are indispensable for the development of a high multitude of HIV-1 resistance against protease inhibitors. J Biol Chem 2002,277:5952–5961. [DOI] [PubMed] [Google Scholar]
- 60.Thao VP, Quang VM, Day JN, Chinh NT, Shikuma CM, Farrar J, et al. High prevalence of PI resistance in patients failing second-line ART in Vietnam. J Antimicrob Chemother 2016,71:762–774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Magambo B, Nazziwa J, Bbosa N, Gupta RK, Kaleebu P, Parry CM. The arrival of untreatable multidrug-resistant HIV-1 in sub-Saharan Africa. Aids 2014,28:1373–1374. [DOI] [PubMed] [Google Scholar]
- 62.Ouattara EN, Ross EL, Yazdanpanah Y, Wong AY, Robine M, Losina E, et al. Clinical impact and cost-effectiveness of making third-line antiretroviral therapy available in sub-Saharan Africa: a model-based analysis in Cote d’Ivoire. J Acquir Immune Defic Syndr 2014,66:294–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lorenzana SB, Hughes MD, Grinsztejn B, Collier AC, Luz PM, Freedberg KA, et al. Genotype assays and third-line ART in resource-limited settings: a simulation and cost-effectiveness analysis of a planned clinical trial. Aids 2012,26:1083–1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kantor R, Katzenstein D, Efron B, Carvalho AP, Wynhoven B, Soares M, et al. Impact of HIV-1 subtype and antiretroviral therapy on protease and reverse transcriptase genotypic evolution: results of a global collaboration. PLOS Medicine 2005,2:e112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wainberg MA, Zaharatos GJ, Brenner BG. Development of antiretroviral drug resistance. N Engl J Med 2011,365:637–646. [DOI] [PubMed] [Google Scholar]
- 66.Taylor BS, Sobieszczyk ME, McCutchan FE, Hammer SM. The challenge of HIV-1 subtype diversity. N Engl J Med 2008,358:1590–1602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Huang A, Hogan JW, Luo X, DeLong A, Saravanan S, Wu Y, et al. Global comparison of drug resistance mutations after first-line antiretroviral therapy across Human Immunodeficiency Virus-1 subtypes. Open Forum Infectious Diseases 2016,3: ofv158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kyeyune F, Nankya I, Metha S, Akao J, Ndashimye E, Tebit DM, et al. Treatment failure and drug resistance is more frequent in HIV-1 subtype D versus subtype A-infected Ugandans over a 10-year study period. AIDS 2013,27:1899–1909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Roberts T, Cohn J, Bonner K, Hargreaves S. Scale-up of Routine Viral Load Testing in Resource-Poor Settings: Current and Future Implementation Challenges. Clin Infect Dis 2016,62:1043–1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Genberg BL, Naanyu V, Wachira J, Hogan JW, Sang E, Nyambura M, et al. Linkage to and engagement in HIV care in western Kenya: an observational study using population-based estimates from home-based counselling and testing. Lancet HIV 2015,2:e20–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Hill A, Pozniak A. HIV treatment cascades: how can all countries reach the UNAIDS 90–90-90 target? Aids 2015,29:2523–2525. [DOI] [PubMed] [Google Scholar]
- 72.Estill J, Ford N, Salazar-Vizcaya L, Haas AD, Blaser N, Habiyambere V, et al. The need for second-line antiretroviral therapy in adults in sub-Saharan Africa up to 2030: a mathematical modelling study. Lancet HIV 2016,3:e132–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Coetzer M, Ledingham L, Diero L, Kemboi E, Orido M, Kantor R. Gp41 and Gag amino acids linked to HIV-1 protease inhibitor-based second-line failure in HIV-1 subtype A from Western Kenya. J Int AIDS Soc 2017,20. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The figure presents frequencies of mutations (Y axis) at positions associated with resistance to NRTIs (left mutation block, X axis); NNRTIs (middle block) and PIs (right block), in patients with VL > 1,000 copies/ML (gray) and VL<1,000 copies/mL (black).
Correlates of viral load failure according to viral load thresholda
Characteristics of patients with and without sequences at VL≤1,000 copies/mLa.
Characteristics of patients with and without sequences at VL>1,000 copies/mLa.