We have recently shown that the detection of monogenic diabetes in a population can be improved by excluding individuals with type 1 diabetes identified by having low C-peptide levels and/or the presence of GAD autoantibodies (GADA) or IA-2 autoantibodies (IA-2A) in the UNITED study(1). Since completion of the study, there are now two new tests that also robustly identify T1D; Zinc Transporter 8 autoantibodies (ZnT8A)(2) and the Type 1 Diabetes Genetic Risk Score (T1D-GRS)(3,4). It is not known the extent to which the addition of these new tests will improve the diagnostic s(1). Autoantibodies assessment was performed at recruitment. The median duration of diabetes at recruitment was 7.4 years (IQR 2.5-17.3). GADA and IA-2A were measured as previously described(1). ZnT8A was measured by ELISA (RSR ltd, Cardiff, UK) on a Dynex DS2 ELISA robot (Dynex, Preston, UK). The RSR ZnT8A ELISA is capable of detecting, and quantifying, autoantibodies specific to R325 or to W325, or to residue 325 non-specific variants. We used >99th centile of a control population (n=1559, age range 1-69 years) to define the positivity of ZnT8A (≥126 units for age<30, ≥26 units for age≥30). The T1D-GRS was derived from genotyping 30 common polymorphisms as described previously. We used >50th centile of T1D-GRS, derived from large reference population of T1D from Wellcome Trust Case Control Consortium (n=1963), to define those at high genetic risk of T1D (3,4). All subjects who were GADA and IA-2A negative, had a next-generation targeted panel test for 35 gene causing monogenic diabetes(1). 15/212 individuals were identified with monogenic diabetes. Their mutations and clinical characteristics are described in table 1 of our previous paper (1) under the following ID numbers 175, 377, 540, 80089, 80173, 80541, 82003, 82006, 82010, 82013, 82014, 82038, 82316, 82352, 82399.
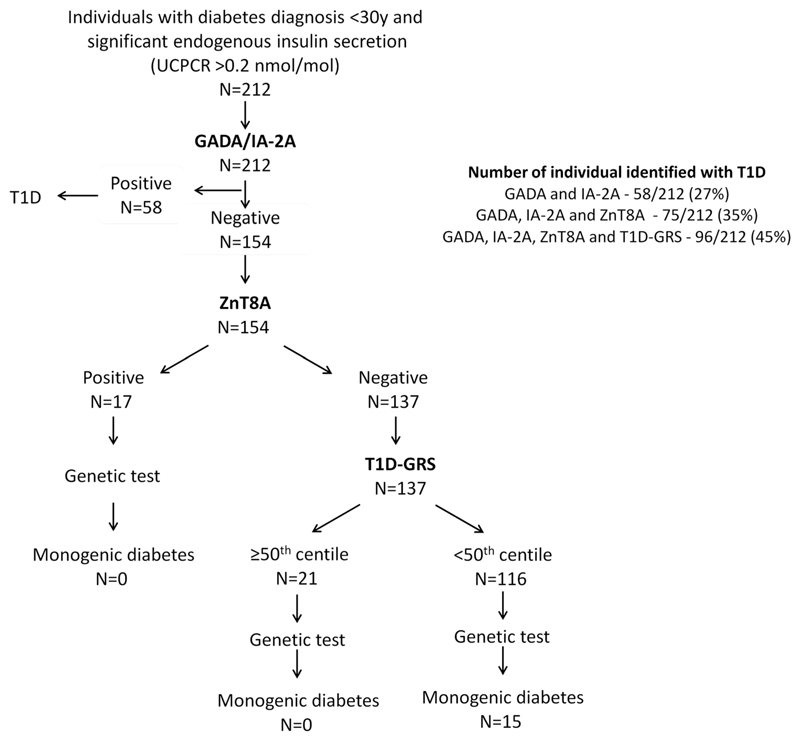
ZnT8A were present in 39/212 (18%) individuals. This increased the number of autoantibody positive individuals from 58 (27%, GAD and/or IA2 positive) to 75 (35%)(p=0.008)(Fig.1). A single autoantibody was found in 44, two in 26 and 5 had all three autoantibodies. .None of the individuals who were positive for the ZnT8A only, had monogenic diabetes.
Figure 1.
Benefit of ZnT8A and T1D-GRS in addition to GADA/IA-2A for excluding individuals with T1D from genetic test for monogenic diabetes who have significant endogenous secretion in the UNITED study.
The T1D-GRS 50th centile cut-off identified 48/212 individuals with probable T1D (those at high genetic risk of T1D). 21 of these were negative for all three autoantibodies (Fig.1). None of these 21 individuals had monogenic diabetes. Thus, addition of the T1D-GRS increased the number of people that can be excluded for genetic testing from 35% with all three autoantibodies to 45% (p=0.003)(Fig.1).
Overall, in individuals with significant endogenous insulin secretion, ZnT8A and T1D-GRS excluded an additional 18% individuals (p<0.001) without missing any monogenic diabetes. These individuals had the similar age of diagnosis, BMI, and UCPCR, from the 27% identified by GADA and IA-2A alone. However, the duration of diabetes was longer in autoantibodies negative T1D individuals who were identified by T1D-GRS compared to autoantibodies positive T1D [3.6 year (IQR 1.2-9.9) vs 14 years (5.8-21.1), p=0.002)]. These two additional tests reduced the number of patients with persistent insulin secretion who needed to be tested for monogenic diabetes from 73% to 55% (p<0.001). The main drawback of these new tests, like GADA and IA-2A antibodies, is that they do not discriminate between type 2 diabetes and monogenic diabetes.
In conclusion two new tests, ZnT8A and the T1D-GRS, helped identify an additional 18% of probable Type 1 diabetes in individuals with significant endogenous insulin secretion excluding the need for monogenic testing. The additional testing is likely to be cost effective as they cost approximately $15 each, which is approximately 1/100th of the cost of a full genetic test.
Acknowledgments
This study was supported by the Department of Health and Wellcome Trust Health Innovation Challenge Award (HICF-1009-041 and WT-091985). K.A.P. has a postdoctoral fellowship funded by the Wellcome Trust (110082/Z/15/Z). A.T.H. are Wellcome Trust Senior Investigators (WT098395/Z/12/Z), and A.T.H. is also supported by a NIHR Senior Investigator award. M.N.W. is supported by the Wellcome Trust Institutional Strategic Support Fund (WT097835MF) and the Medical Research Council (MR/M005070/1). B.M.S and A.T.H. are core members of the National Institute for Health Research (NIHR) Exeter Clinical Research Facility. T.J.M. is supported by an NIHR HEE Senior Clinical lectureship. The views expressed are those of the authors and not necessarily those of the Wellcome Trust, the National Health Service, the NIHR, or the Department of Health.
Footnotes
Author contributions. K.A.P and T.J.M designed the study. K.A.P., B.M.S and M.N.W. researched data and performed statistical analyses. K.A.P. and T.J.M. wrote the first draft of the manuscript, which was modified by all authors. All authors contributed to the discussion and reviewed or edited the manuscript. T.J.M. are the guarantors of this work and, as such, had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Duality of Interest. No potential conflicts of interest relevant to this article were reported.
References
- 1.Shields BM, Shepherd M, Hudson M, McDonald TJ, Colclough K, Peters J, et al. Population-Based Assessment of a Biomarker-Based Screening Pathway to Aid Diagnosis of Monogenic Diabetes in Young-Onset Patients. Diabetes Care. 2017 Aug;40(8):1017–25. doi: 10.2337/dc17-0224. American Diabetes Association. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vermeulen I, Weets I, Asanghanwa M, Ruige J, Van Gaal L, Mathieu C, et al. Contribution of antibodies against IA-2beta and zinc transporter 8 to classification of diabetes diagnosed under 40 years of age. Diabetes Care. 2011 Aug;34(8):1760–5. doi: 10.2337/dc10-2268. American Diabetes Association. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Patel KA, Oram RA, Flanagan SE, De Franco E, Colclough K, Shepherd M, et al. Type 1 Diabetes Genetic Risk Score: A Novel Tool to Discriminate Monogenic and Type 1 Diabetes. Diabetes. 2016 Jul;65(7):2094–9. doi: 10.2337/db15-1690. American Diabetes Association. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Oram RA, Patel K, Hill A, Shields B, McDonald TJ, Jones A, et al. A Type 1 Diabetes Genetic Risk Score Can Aid Discrimination Between Type 1 and Type 2 Diabetes in Young Adults. Diabetes Care. 2016 Mar;39(3):337–44. doi: 10.2337/dc15-1111. [DOI] [PMC free article] [PubMed] [Google Scholar]