Abstract
Extracellular vesicles (EVs) can be classified into several types based on their different biosyntheses or release pathways, including exosomes, microvesicles, apoptotic bodies, and large oncosomes. As they contain DNAs, RNAs, proteins, and other bioactive signals, EVs have been utilized in the diagnosis field for a long time. Considering the fact that stem cells have been widely used for tissue regeneration and EVs possess similar biological properties to their source cells, tissue regeneration abilities of EVs have recently attracted much attention in the regenerative medicine field. In this paper, recent advances and challenges of EVs applied in the repair and regeneration of damaged tissues, such as skin, heart, liver, kidney, bone, and central nervous system, have been summarized. Specifically, critical bioactive molecules, which are encapsulated within EVs and play significant roles in the tissue regeneration, have been highlighted. Finally, the prospects and future development directions of the application of EVs in the field of tissue regeneration have been discussed.
I. INTRODUCTION
Tissue regeneration refers to the physiological processes that necrotic cells and tissue are replaced by healthy cells and a newly formed healthy tissue. The strategies for promoting tissue regeneration mainly include cell therapy, gene therapy, and tissue engineering. A commonly applied definition of tissue engineering is “an interdisciplinary field that applies the principles of engineering and life sciences toward the development of biological substitutes that restore, maintain, or improve tissue function or a whole organ.”1 It has been widely accepted that there are three main factors in tissue engineering technology: seed cells, scaffolds, and growth factors. Since the concept of tissue engineering was proposed in the 1990s, tissue engineering technology has been applied to regenerate various tissues and/or organs, including bone tissue, skin tissue, cartilage tissue, etc.2,3 At the beginning, differentiated primary cells were used as seed cells for regenerating different tissue/organs. With the development of stem cell technologies, the advantages of stem cells over differentiated primary cells were widely recognized. Then, various kinds of stem cells, such as embryonic stem cells (ESCs), adult stem cells, have been widely used in tissue engineering.4 Among those stem cells, mesenchymal stem cells (MSCs) have shown certain advantages over other stem cells in terms of sources, proliferation ability, differentiation ability, and easy to be induced.5,6 For example, bone marrow (BM) mesenchymal stem cells have been the most common seed cells in tissue engineering as they are easy to be obtained and possess the abilities to differentiate into various kinds of cells to form the desired tissue/organs.7 In addition, according to the natural properties of regenerating tissue/organs, different biomaterials have been applied to fabricate porous scaffolds. For example, as the bone tissue is mainly composed of hydroxyapatite and collagen, bioactive ceramics, including tricalcium phosphate, hydroxyapatite, bioglasses, and silicate bioceramics, have been used to fabricate pure bioceramic scaffolds or composite scaffolds with polymers for bone tissue engineering.8–10 Differently, for cartilage tissue engineering, polymers, including synthesized polymer and some nature-derived polymers, have been widely used to form scaffolds with proper mechanical properties for cartilage tissue engineering.11,12 Furthermore, to help the seed cells grow within the scaffolds and induce the cells to differentiate into the desired cells to form regenerated tissue/organs, various kinds of growth factors have been studied and used in tissue engineering, such as vascular endothelial growth factor (VEGF) for inducing blood vessel formation and bone morphogenetic protein 2 (BMP-2) for bone tissue engineering.8–10
The basic principle of tissue engineering is as follows: First, cells isolated from patients are expanded to obtain enough seed cells. Then, the obtained cells are seeded in a biocompatible, biodegradable, porous, and three dimensional scaffold with certain growth factors. After the cell-growth factor-scaffold composites are in vitro cultured for 1 or 2 weeks, they are implanted into the defect site of the patients for inducing tissue regeneration. After implantation, the scaffold gradually degrades and newly formed healthy tissue grows. Finally, the defects are filled with healthy and functional tissues, and the tissue regeneration process is completed.13 Although this tissue engineering technology looks very promising for tissue regeneration, after about 30 years of development, this technology still faces some critical obstacles and “bottlenecks,” which limits its clinical translation. One of the major problems is that it is hard to obtain enough seed cells as the currently used in vitro cell expansion technology is not only time consumable, but also has the risks to change the inherent properties of the cells due to the differences between the in vitro culture condition and in vivo cell microenvironment. In addition, in vivo tumorigenic risk of stem cells has been a major concern for using stem cells as seed cells for tissue engineering or cell therapy.14 To avoid these problems, using a cell replacement might be an attractive and potential strategy for tissue regeneration.
In 1987, Johnstone et al. discovered extracellular vesicles (EVs) in the culture of reticulocytes at first.15 Since then, EVs have been widely utilized in the disease diagnosis field because of the DNAs, RNAs, proteins, and other bioactive signals carried in EVs.16 EVs can be classified into several types according to their different biosynthesis or release pathways, including exosomes released by multivesicular bodies, microvesicles directly released from plasma membrane, apoptotic body generated during cell apoptosis, and large oncosomes secreted from tumor cells. The property details of different extracellular vesicles are shown in Table I.17–23 In EVs, exosomes are the significant part. Exosomes are secreted by almost all types of cells, ranging from 30 nm to 100 nm in size and 1.10 and 1.18 g/ml in density.24 In addition, it has recently been reported that EVs play an important role in interactions between cells as they carry substances secreted by cells.25
TABLE I.
Details of different extracellular vesicles.
| Extracellular vesicles | Shape | Size | Origin |
|---|---|---|---|
| Exosome | Round shape | 40–100 nm | Endosomes from many cell types |
| Microvesicle | Irregular shape | 20–1000 nm | Plasma membrane of many cell types |
| Apoptotic body | Heterogeneous | 1–5 μm | Plasma membrane from the endoplasmic reticulum |
| A large oncosome | Amoeboid phenotype | 1–10 μm | Plasma membrane from cancer cells |
Considering the fact that EVs derive from the inward budding of the endosome membrane of cells, researchers in the field of regenerative medicine have shown great interest in studying the relationship between the EVs and their source cells, and more and more studies have recently demonstrated that the biological activities of EVs are similar to those of their source cells.26 As stem cells have been widely applied in the regenerative medicine field by cell therapy technology or tissue engineering technology and EVs from stem cells have similar biological properties to their source stem cells, researchers hypothesize that the EVs, including exosomes, may also possess the application potential for enhancing tissue regeneration in the field of regenerative medicine.
Recently, more and more studies have demonstrated that EVs can be used as cell replacements in tissue engineering technology or cell therapy technology for enhancing tissue regeneration. Based on these studies, the following advantages of EVs have been proposed in terms of the clinical translation purposes: (1) EVs can be constantly and steadily produced and supplied by immortalized stem cells, which can overcome the shortage of seeding cells or therapy cells for tissue engineering or cell therapy.27 (2) EVs can deliver numerous biological effects of stem cells but avoid potential side effects of stem cells, such as immunological rejection, tumor formation, or growth in stem cell therapy.28 (3) As a vesicle structure, the storage conditions of EVs are much more practical than those of stem cells since the processes of freezing and thawing have adverse effects on cells.29,30 (4) EVs containing specific miRNA, proteins, ncRNA fragments, or drugs can be easily obtained by simply editing the source cells for accelerating the process of therapy.20
In addition, some studies even reported that EVs might have higher therapy efficiency than their source cells according to the different therapy mechanisms. It has been well recognized that the stem cells improve the tissue regeneration by regulating the microenvironment of the tissue cells, rather than transforming into a part of the regenerated tissue.31 However, the survival rate of stem cells after implantation is usually limited by the crucial microenvironment in the wound areas as there are normally limited oxygen and nutrient due to the damages of blood vessels.32 A low survival rate of the stem cells is accompanied by a limited survival time of implanted stem cells, which further limits the communications between the implanted stem cells and surrounding cells. In addition, compared to the nanostructure of EVs, it is more difficult for microsized stem cells to overcome some barriers or move into wound areas and capillaries, which limits their applications in targeting therapies.33 Therefore, the therapy efficiency of the stem cells is reduced, and a large number of stem cells are needed in order to enhance the therapy efficiency. On the contrary, EVs have been reported to be able to maintain a high concentration in the wound area after injection as they have long-term persistence, low degradation, and high preservation of cargo.34
In addition, after being implanted into the wound areas, stem cells are sensitive to the microenvironment and their phenotype, including their stemness and differentiation, may be altered. Therefore, they may secrete undesirable growth factors and perform uncontrollable communications with the host cells in the damaged area and the surroundings, which may result in unexpected therapy effects on tissue regeneration. However, for EVs, they are relatively stable as compared to stem cells as they are not easily be influenced by the microenvironment.35 Besides, they are easily taken up by recipient cells in a very short time and directly exert their effects on target cells, while stem cells need to be activated to secrete growth factors or cytokines to interact with the host cells.33 Therefore, as compared to stem cells, their EVs are more stable and efficient for tissue regeneration.
Based on the mentioned hypothesis and advantages of EVs above, more and more studies have been performed to evaluate the therapeutic effects of EVs on the damaged tissue. The results have shown that EVs can enhance the repair and regeneration of damaged tissues, such as heart, liver, kidney, bone, and central nervous system,36–39 which prove the hypothesis that EVs can be used to improve tissue regeneration. To further understand the clinical application potential of EVs in tissue regeneration, in this work, the recent progress of EVs in various types of tissue regeneration has been reviewed. In addition, the challenges of EVs applied in the tissue regeneration have been analyzed. At the end, the prospects and future development directions of EVs in the field of regenerative medicine have been discussed.
II. ISOLATION AND PURIFICATION OF EVs
Normally, EVs are isolated from a culture medium of cells.40 Although EVs have been widely studied, no standard methods of isolation and purification of EVs from the protein, lipoparticles, and cell debris have been reported yet.41 As reported, the widely used isolation and purification techniques of EVs include ultracentrifugation, purification by differential centrifugation based on particle size, poly(ethylene glycol) (PEG) precipitation, and immunoprecipitation and by using some commercial kits.42–44 Among these methods, isolating the EVs by ultracentrifugation and purifying them through differential centrifugation based on particle size are most widely used as these methods require no specific agents.45 However, the main disadvantage of these isolation and purification methods is time consumable as it takes a long time to achieve enough dose of EVs for tissue regeneration with these ultracentrifugation and differential centrifugations, considering the doses of EVs used range from 106 to 1010 vesicles per target animal.31 PEG precipitation has also been frequently used as it allows rapid separation and sufficient purification of EVs to easily produce a large amount of EVs for clinical studies.46,47 As there are specific proteins and lipids in the membrane of EVs, magnetic beads conjugated with the antibodies that can react with the antigens in the membrane of the EVs have been used to isolate and purify EVs from the collected solution with a magnetic field, which is the principle of the immunoprecipitation method and some commercial kits.37 Based on this principle, types of EVs could be selected by using specific antibodies, and high purity of EVs could be obtained as the reactions between the reagents and the EVs are specific.48 Apart from these methods, recently, it has been reported that, to improve the isolation and purification efficiency of EVs, combinations of different techniques to take the advantages and avoid the disadvantages of each method may be an optimal choice.49
III. TISSUES REGENERATED BY EVs
A. Skin
Skin is the largest organ in the human body and is also the first line of the defense system against the invasion of pathogenic microorganisms in the human body. Under normal circumstances, skin wounds can heal themselves. However, other underlying diseases, such as diabetes, may cause delayed skin wound healing. Cell therapy and tissue engineering technologies have been applied to enhance the healing process of acute and chronic wounds.50 Wound healing is a complex process that involves four major phases: coagulation, inflammatory, proliferative, and remodeling.51 It has been reported that stem cell-derived EVs can participate in different stages of skin tissue repair and regeneration and stimulate wound healing and skin tissue regeneration by regulating the inflammatory response in the wound area and promoting skin cell migration, proliferation, and angiogenesis.52–54
The inflammatory response is a normal physiological process of wound healing happening within 24 h to 6 days after wound formation. In the case of burns or other chronic diseases, the process is usually prolonged. During the early stage of inflammation, macrophages mainly show M1 type of proinflammatory and transform into M2 type of anti-inflammatory in the late stage of inflammation, effectively controlling the excessive development of inflammation.51 Ti et al. reported that, after MSCs were treated by lipopolysaccharide, their EVs could stimulate macrophages to transform into M2 type. The authors then suggested that these EVs possessed a great immunotherapeutic potential for wound healing.55 In addition, it has been reported by several studies that EVs from MSCs could promote the activation, proliferation, and differentiation of B lymphocytes and the transformation of T lymphocytes into regulatory T cells, which synergistically exerted immunosuppressive effects.56–58 Furthermore, it has been reported that EVs from the umbilical cord mesenchymal stem cell (UC-MSC) could suppress immune responses by blocking the TLR4 signaling pathway in macrophages to adjust polarization of macrophages, downregulating the expression of inflammatory factors, and up-regulating the expression of MCP-1, IL-10, and other anti-inflammatory cytokines.59,60
The proliferation phase is a critical stage in wound healing, which occurs 3 days after the trauma and lasts for more than two weeks. Studies on the mechanisms through which stem cell-derived EVs enhance wound healing mainly focus on the proliferation phase.61–65 During this stage, physiological activities mainly include migration and proliferation of fibroblasts, epidermal cells and vascular endothelial cells, deposition of extracellular matrices, and formation of blood vessels.61 For example, Zhang et al.62 and Hu et al.63 reported that EVs from MSCs derived from induced pluripotent stem cells (iPSC) could not only improve the migration and proliferation of fibroblasts, but also stimulate collagen maturation and angiogenesis, which finally enhanced granulation tissue formation. Besides, EVs derived from UC-MSC stimulated proliferation of fibroblasts by activating WNT/β-catenin and Akt signaling pathways, thus inhibiting the apoptosis of fibroblasts and accelerating skin healing in deeply burned rats.64 In addition, the content of WNT11 in EVs from the UC-MSC treated with small molecule DIM was higher than that in EVs from normal UC-MSC, and subsequently, the EVs from those treated cells possess stronger efficient on skin tissue repair as compared to those from untreated cells.65
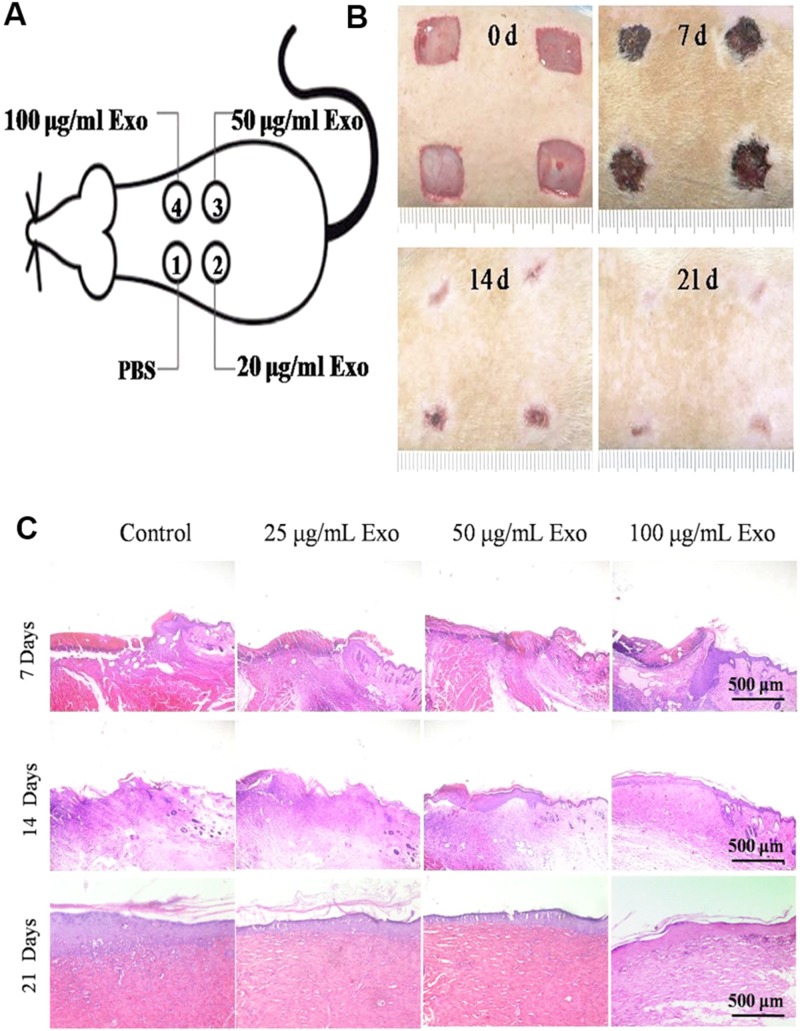
The remodeling phase is the final stage of wound healing, mainly including the contraction of the wound and the rearrangement of collagen fibers. This stage normally lasts for a year or even longer. In the case of severe trauma or deep burn wounds, excessive proliferation of myofibroblasts and deposition of extracellular matrices usually cause the formation of scars,66 which not only affects the appearance, but also influences the function of the organs. EVs have also been applied to avoid the scar formation.67,68 For example, it has been reported that 14-3-3 protein carried in EVs derived from UC-MSC could inhibit scar formation as they could inhibit the Wnt/catenin signaling pathway through phosphorylation of YAP and avoid excessive proliferation of fibroblasts and deposition of collagen.67 Besides, the EVs derived from human amniotic epithelial cells (hAECs) could upregulate the expression of matrix metalloproteinase-1, which further maintained the balance between synthesis and degradation of extracellular matrix and contributed to the scarless wound repair.68 It can be seen from Fig. 1 that authors used exosomes derived from hAECs (hAECs-Exo) to treat skin wounds on the mouse and further found that the concentration of hAECs-Exo at 100 μg/ml showed strongest stimulation effects on the wound closure among all concentrations [Figs. 1(a)–1(c)].68
FIG. 1.
(a) Wound model. (b) Representative photographs of full-thickness excisional wounds treated with PBS, hAECs-Exo (25 μg/ml), hAECs-Exo (50 μg/ml), and hAECs-Exo (100 μg/ml). (c) H&E staining of wounded skin sections in different groups at day 7, 14, and 21 postwounding. All images are reproduced with permission from Zhao et al., J. Mol. Histol. 48(2), 121–132 (2017). Copyright 2017 Springer Science+Business Media Dordrecht.
Taken together, stem cell-derived EVs can promote the regeneration of skin wounds by regulating the immune activity, stimulating the migration, proliferation, and differentiation of tissue regeneration cells, promoting angiogenesis, and avoiding the formation of scar tissues, which brings a new technology for the treatment of skin injury, especially the treatment of refractory skin wounds.
B. Heart
Cardiovascular disease is a worldwide problem with high mortality and a serious threat to human health. Although the survival rate of patients has been greatly enhanced by the diagnosis and intervention or bypass therapy, many patients still gradually suffer from heart failure or other infarct-related complications due to the massive death of myocardial cells and cardiac remodeling caused by ischemia.69 After the injury, cardiomyocytes could be updated at a rate of 0.3%–1% annually.70 Nevertheless, this rather low rate of cell renewal is obviously insufficient to repair a damaged heart. Therefore, stimulating the proliferation of cardiomyocytes and inhibiting their apoptosis have become the potential strategies of cardiac regeneration therapy.
Various stem cells and conditioned media of stem cells have been reported to achieve good effects on preclinical experiments for the therapy of ischemic cardiomyopathy and myocardial tissue repair.28 Recently, a large number of preclinical studies have found that EVs derived from stem cells have identical myocardial regeneration functions as transplanted stem cells.71–78 These EVs can synergistically promote myocardial regeneration and repair cardiac function by activating cardiac precursor cells, promoting survival and proliferation of cardiomyocytes, inhibiting apoptosis of cardiomyocytes, promoting cardiac angiogenesis, reducing the infarct size and tissue fibrosis, and regulating the inflammatory response in areas of heart injury.71–76 Khan et al. found that, in a mice model with acute myocardial infarction, mouse embryonic stem cell-derived EVs could promote angiogenesis, cardiomyocyte survival and proliferation, reduce cardiac fibrosis, enhance left ventricular contraction, and improve heart function. During these progresses, miR-294-3p in the EVs played an important role.71 Besides, some EVs, such as iPSC-derived EVs containing miR-21 and miR-210, hypoxia-treated bone mesenchymal stem cells (BMSC)-derived EVs containing miR-125b as well as sca-1 positive cells-derived EVs containing miR-21, have been reported to be able to protect cardiomyocytes by inhibiting cardiomyocyte apoptosis.77,78
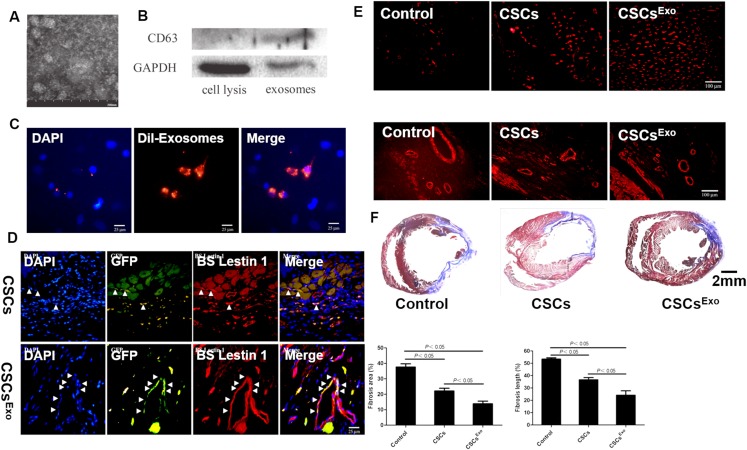
After myocardial infarction, macrophages are activated to release inflammatory factors, and neutrophils are recruited to the wound site because of the necrosis and apoptosis of numerous myocardial cells, which triggers a series of inflammatory reactions. EVs have also been proved to be able to modulate the inflammatory responses in heart tissue regeneration.74–76,79–82 For example, it has been reported that EVs derived from MSCs had an ability of immunomodulatory, which could regulate local and systemic immune responses, reduce tissue inflammation, and promote tissue regeneration after myocardial injury.74,79 EVs derived from MSCs have also been reported to be able to reduce the infiltration of cardiac neutrophils and macrophages in mice myocardial infarction areas as well as the number of white blood cells in peripheral blood after ischemia-reperfusion injury, which improved cardiac function.80 EVs derived from adipose-derived mesenchymal stem cells (ADSCs) have similar effects to those derived from BMSCs in regulating the inflammatory response of the myocardial infarction and protecting the function of the heart.75 Some specific miRNAs in the EVs play critical roles in their immunomodulatory effects in cardiac regeneration. For example, overexpression of miR-93-5p in EVs could reduce the autophagy response and the expression of inflammatory factors, such as IL-6, IL-1β, and TNF-α, by targeting Atg7 and TLR4 after myocardial infarction, which had a positive effect on myocardial protection.75 Besides, overexpression of miR-30d in EVs inhibited autophagy and promoted polarization of macrophages to anti-inflammatory M2 type, and overexpression of miR-126 could downregulate the expression of inflammatory factors, promote angiogenesis, and reduce the area of myocardial infarction.76,81 Zhang et al. successfully collected exosomes from mesenchymal stem cells (MSC-Exo). Results showed that the obtained MSC-Exo had a mean diameter of 46.55 ± 11.64 nm and were positive for CD63 [Figs. 2(a) and 2(b)].82 In addition, they found that the MSC-Exo could be internalized into cardiac stem cells (CSCs) to form CSCs encapsulating MSC-Exo (CSCsExo) [Fig. 2(c)].82 They then transplanted the CSCsExo into a peri-infarct myocardium and found that the neovascularization in the CSCExo group was significantly improved as compared with those in the CSCs and control groups, which contributed to the reduced fibrotic tissue in ischemic hearts in the CSCExo group [Figs. 2(d)–2(f)].82
FIG. 2.
(a) The morphology of MSC-Exo was observed under an electron microscope. (b) Western blot analysis of CD63 protein was detected in MSC-Exo. (c) Cellular internalization of mesenchymal stem cell (MSC)-Exo into c-kit + cardiac stem cells (CSCs). (d) Differentiation of transplanted cardiac stem cells (CSCs) into the neovasculature in the peri-infarct myocardium. (e) Histological analysis for capillary density in the peri-infarct myocardium. (f) Histological analysis for myocardial infarction (MI) sizes in each group. All images are reproduced from Zhang et al., J. Am. Heart Assoc. 5(1), e002856 (2016). Copyright 2016 Author(s), licensed under a Creative Commons Attribution (CC BY) License.
Angiogenesis plays a critical role in the repair or regeneration of the damaged tissue, especially in the myocardial ischemic area, which can reduce the necrosis or/and apoptosis of cardiomyocytes, and maintain the survival of myocardial cells. Therefore, angiogenesis is currently a research hot spot in the ischemic cardiomyopathy. Recently, preclinical studies on a large number of ischemic cardiomyopathy have found that EVs derived from stem cells, such as embryonic stem cells (ESCs),71 MSCs,79,82–86 and myocardial precursor cells,87–89 could effectively increase vascular density, reduce tissue fibrosis, and regain the heart function. In promoting angiogenesis in the damaged tissue area, mRNAs, miRNAs, and protein factors in EVs play important roles. For example, EVs derived from ESCs contained VEGF that could bind to the receptors on endothelial cells to activate the signaling pathway of PI3 K/AKT and subsequently promoted proliferation and migration of endothelial cells and angiogenesis.90 EVs derived from mouse iPSCs were abundant in miR-21 that could promote angiogenesis by targeting phosphatase and tensin homolog to activate Akt and ERK1/2 signaling pathways for increasing the expression of hypoxia inducible factor-1α and VEGF.91 Besides, Gray et al. demonstrated that hypoxic preconditioning of cardiac progenitor cells could increase angiogenesis-related miRNAs in EVs, which was an effective method to promote angiogenesis in ischemic myocardium.73
Therefore, EVs derived from stem cells can protect myocardium and improve the regeneration of the heart tissue by improving the microenvironment of the myocardial infarction through anti-inflammatory and pro-angiogenic effects.
C. Nervous system
Many injuries and diseases in the nervous system have a serious impact on the life quality of patients, which includes stroke, brain trauma, spinal cord injury, neurodegenerative diseases, glioma, and so on. EVs possess obvious therapeutic effect advantages in the repair of the damaged nerve. For example, due to the specific membrane-bound structure, EVs can be effectively ingested by cells, thus reducing the barrier of the blood–brain barrier.33,92,93 However, since the results about this aspect have been rarely reported, more studies are necessary to be carried out to verify these claims. In addition, EVs have extremely low immunogenicity, allowing them to exert immunoregulatory effects of stem cells while avoiding elimination.
Stroke is a common acute cerebrovascular damage, which often causes different degrees of nerve dysfunction. In stroke lesions, there are not only apoptotic brain cells but also repairing cells, so that enhancing functions of these repairing cells becomes a way of treating stroke. Doeppner et al. compared the therapeutic effects of MSCs with the EVs derived from MSCs (MSC-EVs) in stroke mice and demonstrated that MSC-EVs could achieve the same functional tissue regeneration as MSCs, indicating the feasibility of replacing stem cells with their EVs in repairing stroke.92 In another study, results show that, as compared with MSCs, MSC-EVs had better rehabilitation effects in stroke repair, which might result from the higher blood–brain barrier permeability of MSC-EVs than MSCs.93 As EVs have good permeability in the blood–brain barrier, various strategies have been used to edit stem cells in order to endow the EVs from those edited stem cells therapeutically miRNAs.94 For example, Xin et al. found that, in stem cell therapy of stroke mice, the level of miR-133b significantly increased in the ipsilateral hemisphere, which suggested that miR-133b carried in MSC-EVs was critical in neuron regeneration. Then, they edited MSCs to obtain EVs carrying a high amount of miR-133b in order to regulate glial cell function and promote neuronal regeneration.95,96
Brain trauma is a serious neurological injury that often leads to death or long-term functional defects. Previous studies have attempted to repair the brain tissue by using stem cell transplantation. However, the blood–brain barrier and the unintended consequences of cerebral embolism make the stem cell therapy unpromising. Recently, EVs have been used to treat the brain trauma.97–100 For example, Zhang et al. tried to treat brain trauma in mice by using MSC-EVs first, and they discovered that MSC-EVs treatment could promote the regeneration of nerve cells and vascular endothelial cells in the lesions and reduce the local inflammatory response.97 EVs derived from human MSCs were used to improve cognitive function and memory function in mice.98 In addition, to control the neuroinflammatory response in the treatment of traumatic brain injury, the inflammation modulatory abilities of EVs have also been applied. For instance, Kim et al. found that the MSC-EVs could significantly reduce the inflammatory response of the brain within 12 h after injury, which made the recovery of memory and cognition in mice better as compared with the MSCs.99 Patel et al. loaded the ncRNA MALAT1 into the EVs of human adipose-derived stem cells (hASC-EXOs) and found that motor function could be improved, and cerebral cortical injury could be alleviated after the brain trauma was treated by these EVs due to the inflammatory response regulatory effects of ncRNA MALAT1.100
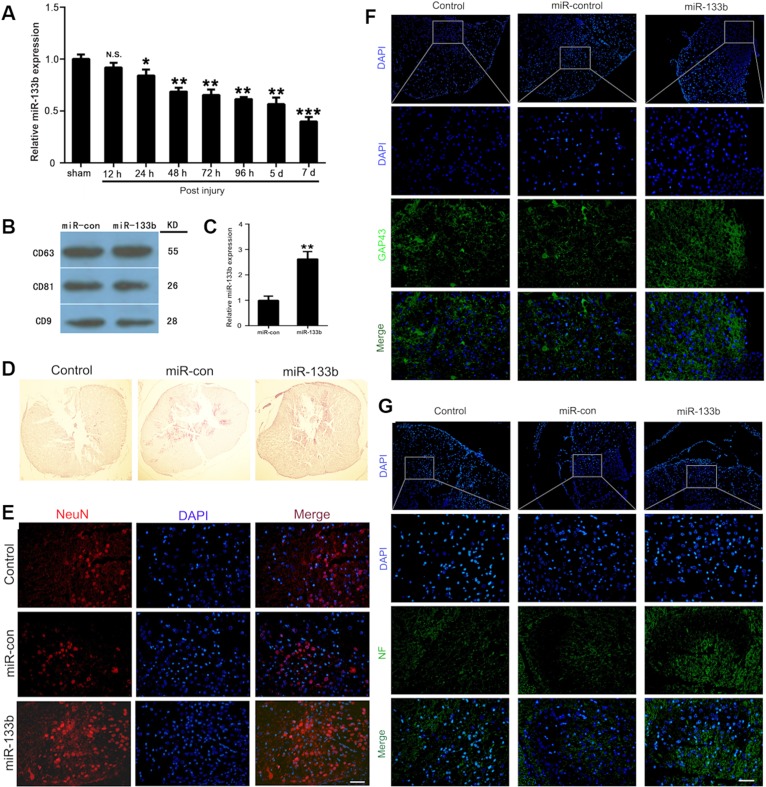
Spinal cord injury (SCI) is a common neurological injury after spinal trauma, which causes conscious cognitive dysfunction. It becomes one of major clinical problems because of the residual effects, such as paralysis and incontinence. Several studies have reported that EVs could be used to treat SCI.101–106 For example, Huang et al.101 and Lankford et al.102 confirmed first that systemic injection of MSC-EVs could inhibit apoptosis and neuroinflammatory reaction, promote local angiogenesis, and target spinal cord lesions in mice after injury, which was conducive to functional recovery after SCI. As the expression of miR-133b in injured spinal cords was significantly downregulated after SCI, Lankford et al. transfected the MSCs with miR-133b mimic by Lipofectamine 3000 to achieve exosomes containing abundant of miR-133b. They found that the exosomes from transfected MSCs still contained the exosomal markers, such as CD9, CD63, and CD81, but the expression levels of miR-133b in the exosomes from transfected MSCs (miR-133b exosomes) were much higher than that in the normal MSCs (miR-con exosomes) [Figs. 3(a)–3(c)].103 They then used these miR-133b exosomes to treat the injured spinal cord, and the results indicated that miR-133b exosomes significantly decreased the area of the lesion cavity but increased the mature neuron numbers, the expression of growth-associated protein 43, and neurofilament in the injured spinal cord as compared to miR-con exosomes [Figs. 3(d)–3(g)].103 In addition, Wang et al.104 and Liu et al.105 found that MSC-EVs could relieve nerve damages by inhibiting the activation of A1 astrocytes after spinal cord injury. Furthermore, considering the high expression and repair ability of miR-133b in many neurological lesions, Li et al.103 and Ren et al.106 both injected MSC-EVs carrying miR-133b intravenously into a mouse model and found that the treatment could reduce the volume of lesions, protect neurons and promote axonal growth.
FIG. 3.
(a) Expression levels of miR-133b in injured spinal cords were measured by quantitative real-time polymerase chain reaction (qRT-PCR) after acute SCI. (b) Expressions of CD63, CD81, and CD9 in exosomes derived from miR-133b- or miR-con-transfected MSCs were evaluated by western blot. (c) Expression of miR-133b in exosomes derived from miR-133b- or miR-con-transfected MSCs was assessed by qRT-PCR. D) H&E staining results for sections of the injured spinal cord. (e) The effect of miR-133b exosomes on NeuN+ neurons was evaluated by immunofluorescence staining. (f) Effect of miR-133b exosomes on GAP43 expression was measured by immunofluorescence staining. (g) Effect of miR-133b exosomes on NF expression was measured by immunofluorescence staining. All images are reproduced from Li et al., Front. Neurosci. 12, 845 (2018). Copyright 2018 Author(s), licensed under a Creative Commons Attribution (CC BY) license.
The accumulation of Aβ protein in nerve cells is currently recognized as the pathogenesis of Alzheimer's disease. Thus, reducing Aβ protein becomes a possible treatment for Alzheimer's disease. Katsuda et al. first discovered that EVs secreted by MSCs carried enzymes that could degrade Aβ protein.107 Furthermore, Godoy et al.108 and Wang et al.109 confirmed that MSC-EVs could effectively reduce Aβ protein accumulation in nerve cells and improve cognitive function in mice. In addition, Cui et al. found that the MSC-EVs obtained in hypoxia had better anti-inflammatory effects and cognitive function improvement on Alzheimer's disease as compared to the MSC-EVs collected in normal.110
Glioma is the most common type of malignant tumor in the nervous system. Nevertheless, the treatment of glioma has been a major problem due to the drug resistance of the tumor itself and the obstruction of the blood–brain barrier. Kim et al. found that the expression of metalloproteinase −2 protein associated with the pathogenesis of glioma decreased by 2.5 times with the treatment of MSC-EVs carrying miR-584 in vitro and the intervention of EVs in vivo could significantly inhibit tumor growth.111 Zhu et al. utilized ESC-EVs to targeted treat glioblastoma (GBM) and confirmed that ESC-EVs modified with targeted logo cRGyDK of GBM significantly induced cell necrosis in vitro and reduced tumor volume in vivo.112
In conclusion, current research results suggest that EVs from the stem cell can efficiently target the nervous system lesions by miRNAs as the EVs can easily permeate the blood–brain barrier and activate the biological functions of the target cells while avoiding the risk of cell therapy.
D. Bone
The failure in the bone repair process usually prolongs the regeneration cycle and ultimately leads to pathologically destructive bone diseases, such as osteoporosis, osteoarthritis (OA), and rheumatoid arthritis (RA). These disorders in repair are associated with functions of related cells in the bone tissue, including abnormal proliferation or apoptosis of osteoblasts and osteoclasts, and deviant activity of vascular endothelial cells and bone cells. Due to the lack of effective therapies, bone trauma seriously lessens the quality of life of patients. However, studies have shown that EVs derived from bone cells can effectively promote the process of bone repair by transmitting specific proteins, RNA, and other molecules.113–120
Angiogenesis, inflammation response, and osteogenesis are three critical stages in the bone regeneration. Angiogenesis is essential for almost all tissue regeneration, as the vascular provides nutrients, supplies oxygen, and metabolizes the waste produced by cells. Recently, EVs derived from MSCs have been shown to promote endothelial cell proliferation, migration, and tube formation in vitro.114,115 Although there are no direct studies of the angiogenic ability of EVs in bone, the angiogenic abilities of EVs reported in other tissues and organs indicate that EVs have potential in stimulating bone growth and regeneration by increasing vessel formation.85 For example, Zhang et al. found that exosomes derived from umbilical cord mesenchymal stem cells in a hypoxic environment not only promoted the formation of endothelial cell network structure on Matrigel, but also improved blood flow reperfusion in rat hind limb ischemia model in vivo.115 Therefore, EVs might be used to improve angiogenesis in bone regeneration.
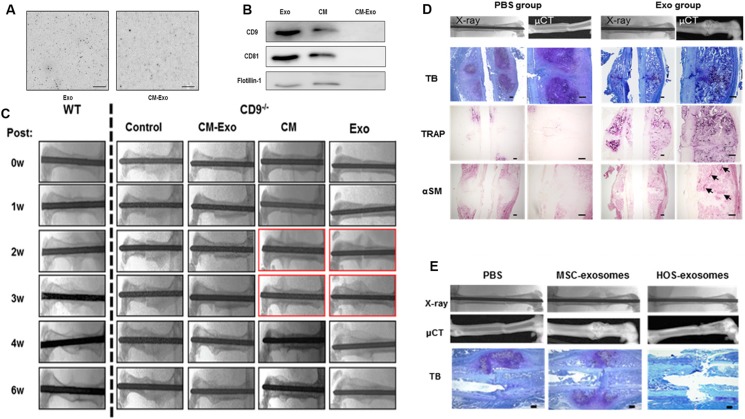
RA is mainly caused by autoimmune problems where the infiltration of inflammatory cells at the joints triggers chronic inflammation, leading to bone and cartilage necrosis and loss of function at the joints.116 Furuta et al. collected exosomes from MSC-conditioned medium by ultracentrifugation and determined that the exosomes (Exo) and conditioned medium (CM) contained exosomal markers, including CD9, CD81, and flotillin-1, which were missing in the supernatant after ultracentrifugation (CM-Exo) [Figs. 4(a) and 4(b)].117 Next, they injected Exo, CM, and CM-Exo into the fracture site of the CD9−/− mice delayed fracture healing and found that, 3 weeks after injection, Exo and CM rescued the CD9−/− mice as there were more callus and bone union in the CD9−/− mice treated with Exo and CM as compared to those in the CD9−/− mice treated with CM-Exo [Fig. 4(c)].117 In contrast to phosphate buffer saline (PBS)-injected mice (PBS group), more trap and α-SM positive cells, hypertrophic chondrocytes as well as woven bone tissue were observed in the exosome-injected mice, which indicated that exosomes assisted the fracture healing [Fig. 4(d)].117 The exosomes derived from the human osteosarcoma cell line HOS (HOS-exosomes) were also utilized to treat the CD9−/− mice. However, they did not facilitate the formation of the callus, which further demonstrated that the exosomes from MSCs were specific for stimulating the bone fracture healing [Fig. 4(e)].117 Kim et al. demonstrated that EVs from dendritic cells differentiated from MSCs after IL-10 stimulation were capable of inhibiting collagen-induced arthritis in mice and significantly mitigated the severity of arthritis.118
FIG. 4.
(a) Images of the untreated Exo and CM-Exo in solution by the frequency transmission electric-field system. (b) Immunoblotting for exosome markers, CD9, CD81, and flotillin-1, in Exo, CM, and CM-Exo. (c) Representative radiologic images of control, CM, CM-Exo, and Exo treated CD9−/− mice after the fracture. (d) Representative histological evaluation of callus formation after Exo injection in CD9−/− mice. (e) Representative radiologic images and μCT images of femurs. All images are reproduced with permission from Furuta et al., Stem Cells Transl. Med. 5(12), 1620–1630 (2016). Copyright 2016 AlphaMed Press.
Most bone defects can self-repair by the methods of intramembranous osteogenesis and endochondral ossification. However, overdamage of periosteum would prolong the repair process. The imbalance of osteogenesis can lead to deterioration of microstructure after the formation of new bone, which increases bone fragility and eventually leads to diseases such as osteoporosis.119 Furuta et al. demonstrated that MSC-EVs could repair bone defects by promoting endochondral ossification and had better effects on bone defects in CD9−/− mice as compared with conditioned media collected from the same MSC without EVs.117 Qi et al. found that exosomes secreted by MSCs derived from human induced pluripotent stem cells could effectively promote the proliferation and differentiation of bone marrow MSCs derived from ovariectomized, which improved bone regeneration in the critical dimension skull defects in a rat model by enhancing osteogenesis and angiogenesis in vivo.120
In short, EVs, especially MSC-EVs, have great application potential for treating various bone diseases and enhancing bone regeneration due to their immunomodulatory effects, proangiogenesis ability, and stimulatory effects on osteogenic cells.
E. Liver
Liver is the most active metabolic and excretion organ in the body, and various imbalances of steady state in the body may cause different degrees of damage to the liver. Since stem cell therapy has been shown to be effective in the recovery of liver function in acute liver injury, as a functional product of stem cells, EVs also have application potential for the treatment of damaged liver.
Studies have shown that EVs can promote regeneration of drug-induced liver injury by enhancing the expression of proliferation genes in hepatocytes.121 EVs also inhibited the inflammatory response and cell necrosis in the lesion by increasing the mRNA expression of anti-inflammatory factors and the number of regulatory T cells.122 In addition, EVs had antioxidant damage effects,123 which had been proved in the treatment of ischemic liver injury in many studies.124–126 When EVs were used to repair the liver injury, lncRNA H19 and miR-17 in EVs of stem cells had been proved to play important roles in anti-inflammation, antioxidation, and regeneration.127–130 In addition, MSC-EVs were confirmed to be beneficial to relieve fibrosis caused by various types of liver damages, which indicated that stem cell EVs could be used to alleviate or reverse chronic liver injury.131–133
F. Kidney
Although the kidney has strong compensatory properties, it still has serious problems, such as progressive deterioration of renal function, difficulty in regenerating nephrons, and systemic complications, which are often caused by unstable metabolism of the body. EVs, as the important products secreted by stem cells, have been proved to be an advanced alternative to stem cells for acute kidney injury. Previous studies have confirmed that MSC-EVs and MSCs have the same therapeutic effects on mild acute kidney injury.134–138 Especially, in the treatment of ischemic and severe nephrotoxic drug kidney injuries, it has been proved that MSC-EVs had the effects on inhibiting epithelial cell apoptosis and promoting wound regeneration, which could protect chronic renal damage caused in the later stage.134,135 In addition, it has been reported that MSC-EVs could regulate the apoptosis and proliferation of epithelial cells by the cell cycle-associated mRNA, and the miRNAs involved in the regulation of antiapoptotic and proliferation pathways and VEGF transmitted by EVs play a significant role in angiogenesis in the lesions.136–138
In addition to their alleviation effects on symptoms of the acute kidney injury, MSC-EVs have been reported to have therapeutic effects on chronic kidney disease. MSC-EVs improved the function of the residual nephron and delayed the chronic injury of the residual nephron.139 For example, MSC-EVs delayed the renal function deterioration and reduced the renal interstitial fibrosis caused by TGF-β1 in a chronic kidney disease model.140 In addition, MSC-EVs promoted renal tubular cell regeneration and played a critical role in reducing apoptosis and inflammation response.141 For an instance, Eirin et al. demonstrated that MSC-EVs could relieve renal inflammatory damage by transmitting IL-10 in renal vasculopathy animals, thereby improving renal perfusion, maintaining filtration rate, and reducing fibrotic lesions in renal vascular disease caused by hypertension disease.142
Therefore, the studies show that the naturally secreted EVs have a good renal repair effects, which may provide a promising strategy for renal repair and regeneration.
G. Diabetes and related tissues
The pathogenesis and duration of diabetes are related to immune damage and insulin resistance. MSC-EVs can be used for treating diabetes and diabetes related complications by the immunomodulatory effects. Type 1 Diabetes Mellitus (T1DM) is considered as an autoimmune disorder that causes the lack of islet function. In the treatment of type 1 diabetes, MSC-EVs have been reported to be able to upregulate the expression of IL-4 and IL-10 and decrease the splenic mononuclear cells in the production of inflammatory factors to inhibit immune damages. Finally, the islet function was improved, suggesting that MSC-EVs have a good therapeutic effect on T1DM.143 Type 2 Diabetes Mellitus (T2DM) is caused by the resistance of peripheral tissues on insulin due to various reasons and the main cause is the changes in the signal pathway of target cells in insulin. MSCs have been shown to be able to improve the condition of T2DM animals and patients, and recent studies have found that MSC-EVs play pleotropic roles in improving insulin resistance.144–146 Specifically, MSC-EVs improved the activity of cell signal transduction pathway by restoring the phosphorylated region of insulin receptor and protein kinase B, thereby alleviating insulin resistance, promoting the expression of glucose transporter, increasing hepatic glycogen reserve, and finally stabilizing blood glucose.147
Multiple complications of diabetes also become another focus in the clinical treatment of diabetes. Human urinary stem cell EVs (USC-EVs) could inhibit podocyte necrosis and promote angiogenesis under the environment of high glucose. Besides, USC-EVs could reduce urine volume and urinary albumin in animal experiments, which indicated that USC-EVs had great potential therapeutic effects on diabetic nephropathy.148 Recent studies have reported that MSC-EVs had antiapoptosis and vascular endothelial protection effects in diabetic nephropathy, which could alleviate renal damage from hyperglycemia by up-regulating the expression of autophagy markers.149,150
IV. CONCLUSION AND PERSPECTIVE
EVs have been showing their great application potential in tissue regeneration as they can regulate host immune responses, promote the migration and proliferation of repair cells, and facilitate the neovascularization and other specific functions in different tissues. So far, EVs have been demonstrated to have preliminary stimulatory effects in the regeneration of skin, heart, nerves, liver, kidney, bone, diabetes, and related tissue disease. The summary of specific regenerative contents in the EVs that aids in tissue regeneration has been concluded in Table II. Besides the similar or even possible higher therapy effects, EVs show advantages over stem cells with their low carcinogenicity, high permeability to tissue barriers, and convenience in preservation due to its nanovesicle structure. Therefore, EVs have great potential to be used as an alternative to stem cells in cell therapy and seeding cells in tissue engineering. Nevertheless, there are some shortcomings in the treatment of damaged tissues or organs by EVs, such as complex extraction steps and low harvest rates. In addition, as there are mixtures in the cargo of EVs, it is hard to tell the roles of each component in the EVs during enhancing the tissue regeneration as it is hard to separate the bioactive components. Subsequently, the treatment mechanism of EVs on various types of tissue or organs is hard to be fully elucidated.
TABLE II.
Summary of specific regenerative contents in the EVs that aid in tissue regeneration.
| Type/source of EV | Regeneration tissue | Specific contents | Target effects | Reference |
|---|---|---|---|---|
| UC-MSCs treated by lipopolysaccharide | Skin tissue | Let-7b | Alleviate inflammation through let-7b/TLR4 pathway | 55 |
| hUCMSCs pretreated with siPORT NeoFX containing miR-181c mimics | Skin tissue | miR-181c | Reduce burn-induced inflammation by downregulating the TLR4 signaling pathway | 59 |
| UC-MSC | Skin tissue | WNT4 | Stimulate proliferation of fibroblasts by activating WNT/β-catenin and Akt signaling pathways | 64 |
| UC-MSC treated with small molecule DIM | Skin tissue | WNT11 | Promote stemness by activating WNT/β-catenin signaling pathways | 65 |
| UC-MSC | Skin tissue | 14-3-3 protein | Inhibit scar formation by inhibiting the WNT/catenin signaling pathway | 67 |
| Mouse embryonic stem cell | Heart | miR-294-3p | Promote increased survival, cell cycle progression, and proliferation of cardiac progenitor cell | 71 |
| Murine BM-MSCs subjected to hypoxia | Heart | miR-125b-5p | Facilitate ischemic heart repair | 77 |
| Cardiac progenitor cell | Heart | miR-21 | Protect myocardial cells against oxidative stress-related apoptosis by the miR-21/PDCD4 pathway | 78 |
| Adipose-derived stromal cells | Heart | miR-93-5p | Suppress inflammatory cytokine expression by targeting Atg7 and TLR4 | 75 |
| miR-30d-5p-overexpressing adipose-derived stem cells | Heart | miR-30d-5p | Inhibit autophagy-mediated microglial polarization to M1 | 76 |
| miR-126-overexpressing adipose-derived stem cells | Heart | miR-126 | Protect myocardial cells from apoptosis, inflammation, fibrosis, and increased angiogenesis | 81 |
| Embryonic stem cells | Heart | VEGF | Activate signaling pathway of PI3K/AKT and promote angiogenesis | 90 |
| MSCs | Nervous system | miR-133b | Regulate glial cell function and promote neuronal regeneration | 95 |
| Human adipose-derived stem cells | Nervous system | ncRNA MALAT1 | Regulate inflammation pathways, cell cycle, cell death | 100 |
| MSCs transfected with miR-133b mimic | Nervous system | miR-133b | Decrease the area of the lesion cavity but increase the mature neuron numbers | 103 |
| MSCs | Nervous system | miR-584 | Inhibit tumor growth | 111 |
| hMSC | Liver | Y-RNA-1 | Modulate the inflammatory response and activate protective mechanisms to limit cell death | 128 |
| Human adipose-derived stem cells | Liver | lncRNA H19 | Promote hepatocytes’ proliferation through the hepatocyte growth factor/c-Met pathway | 129 |
| Adipose-derived stem cells | Liver | miR-17 | Suppress TXNIP expression and consequent inflammasome activation | 130 |
As the applications of EVs extend from disease diagnosis to tissue regeneration, in the future, several approaches can be taken to improve the application of EVs in clinical: (1) As the low yield of EVs restricts their wide use clinically, enhancing the EV production ability of cells is the top priority. 2. To improve the therapeutic effects of EVs, different ways can be used to edit cells to achieve modified EVs with specific miRNAs, IncRNAs, or drugs for precise treatments. 3. To tackle the defects of low retention while directly utilizing solutions containing EVs, encapsulating EVs within tissue engineering scaffolds, such as hydrogels, is a promising method.
ACKNOWLEDGMENTS
This work was supported by the National Natural Science Foundation of China (NNSFC) (Grant Nos. 31771024 and 31971274) and the Interdisciplinary Program of Shanghai Jiao Tong University (No. ZH2018ZDA20).
REFERENCES
- 1.Shieh S. J. and Vacanti J. P., Surgery 137(1), 1–7 (2005). 10.1016/j.surg.2004.04.002 [DOI] [PubMed] [Google Scholar]
- 2.Koller M. R. and Palsson B. O., Biotechnol. Bioeng. 42(8), 909–930 (1993). 10.1002/bit.260420802 [DOI] [PubMed] [Google Scholar]
- 3.Tabata Y., J. R. Soc. Interface 6, S311–S324 (2009). 10.1098/rsif.2008.0448.focus [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Guillot P. V., Cui W., Fisk N. M., and Polak D. J., J. Cell. Mol. Med. 11(5), 935–944 (2007). 10.1111/j.1582-4934.2007.00106.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhao S. M., Wehner R., Bornhauser M., Wassmuth R., Bachmann M., and Schmitz M., Stem Cells Dev. 19(5), 607–614 (2010). 10.1089/scd.2009.0345 [DOI] [PubMed] [Google Scholar]
- 6.DiMarino A. M., Caplan A. I., and Bonfield T. L., Front. Immunol. 4, 9 (2013). 10.3389/fimmu.2013.00201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bara J. J., Richards R. G., Alini M., and Stoddart M. J., Stem Cells 32(7), 1713–1723 (2014). 10.1002/stem.1649 [DOI] [PubMed] [Google Scholar]
- 8.Peric Kacarevic Z., Rider P., Alkildani S., Retnasingh S., Pejakic M., Schnettler R., Gosau M., Smeets R., Jung O., and Barbeck M., “An introduction to bone tissue engineering,” Int. J. Artif. Organs (published online). 10.1177/0391398819876286 [DOI] [PubMed] [Google Scholar]
- 9.Qu H., Fu H., Han Z., and Sun Y., RSC Adv. 9(45), 26252–26262 (2019). 10.1039/C9RA05214C [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yin S., Zhang W., Zhang Z., and Jiang X., Adv. Healthc. Mater. 8(10), e1801433 (2019). 10.1002/adhm.201801433 [DOI] [PubMed] [Google Scholar]
- 11.Rai V., Dilisio M. F., Dietz N. E., and Agrawal D. K., J. Biomed. Mater. Res. A 105(8), 2343–2354 (2017). 10.1002/jbm.a.36087 [DOI] [PubMed] [Google Scholar]
- 12.Santos A. R. C., Almeida H. A., and Bartolo P. J., Virtual Phys. Prototyp. 8(3), 175–186 (2013). 10.1080/17452759.2013.838825 [DOI] [Google Scholar]
- 13.Nerem R. M., Biomaterials 28(34), 5074–5077 (2007). 10.1016/j.biomaterials.2007.07.032 [DOI] [PubMed] [Google Scholar]
- 14.Lee H. Y. and Hong I. S., Cancer Sci. 108(10), 1939–1946 (2017). 10.1111/cas.13334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Johnstone R. M., Adam M., Hammond J. R., Orr L., and Turbide C., J. Biol. Chem. 262(19), 9412–9420 (1987). [PubMed] [Google Scholar]
- 16.Raimondo F., Morosi L., Chinello C., Magni F., and Pitto M., Proteomics 11(4), 709–720 (2011). 10.1002/pmic.201000422 [DOI] [PubMed] [Google Scholar]
- 17.Di Vizio D., Kim J., Hager M. H., Morello M., Yang W., Lafargue C. J., True L. D., Rubin M. A., Adam R. M., Beroukhim R., Demichelis F., and Freeman M. R., Cancer Res. 69(13), 5601–5609 (2009). 10.1158/0008-5472.CAN-08-3860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Meehan B., Rak J., and Di Vizio D., J. Extracell. Vesicles 5 (2016). 10.3402/jev.v5.33109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wu Z., Zhang Z., Xia W., Cai J., Li Y., and Wu S., Cell Prolif. 52, e12659 (2019). 10.1111/cpr.12659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Raposo G. and Stoorvogel W., J. Cell Biol. 200(4), 373–383 (2013). 10.1083/jcb.201211138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lai R. C., Chen T. S., and Lim S. K., Regen. Med. 6(4), 481–492 (2011). 10.2217/rme.11.35 [DOI] [PubMed] [Google Scholar]
- 22.van der Pol E., Boing A. N., Harrison P., Sturk A., and Nieuwland R., Pharmacol. Rev. 64(3), 676–705 (2012). 10.1124/pr.112.005983 [DOI] [PubMed] [Google Scholar]
- 23.Lee T. H., D'Asti E., Magnus N., Al-Nedawi K., Meehan B., and Rak J., Semin. Immunopathol. 33(5), 455–467 (2011). 10.1007/s00281-011-0250-3 [DOI] [PubMed] [Google Scholar]
- 24.Lai R. C., Yeo R. W. Y., and Lim S. K., Semin. Cell Dev. Biol. 40, 82–88 (2015). 10.1016/j.semcdb.2015.03.001 [DOI] [PubMed] [Google Scholar]
- 25.Zhang X., Yuan X., Shi H., Wu L. J., Qian H., and Xu W. R., J. Hematol. Oncol. 8, 83 (2015). 10.1186/s13045-015-0181-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liang X. T., Ding Y., Zhang Y. L., Tse H. F., and Lian Q. Z., Cell Transplant 23(9), 1045–1059 (2014). 10.3727/096368913X667709 [DOI] [PubMed] [Google Scholar]
- 27.Ferreira J. R., Teixeira G. Q., Santos S. G., Barbosa M. A., Almeida-Porada G., and Goncalves R. M., Front. Immunol. 9, 2837 (2018). 10.3389/fimmu.2018.02837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Thery C., Ostrowski M., and Segura E., Nat. Rev. Immunol. 9(8), 581–593 (2009). 10.1038/nri2567 [DOI] [PubMed] [Google Scholar]
- 29.Matthay M. A., Calfee C. S., Zhuo H. J., Thompson B. T., Wilson J. G., Levitt J. E., Rogers A. J., Gotts J. E., Wiener-Kronish J. P., Bajwa E. K., Donahoe M. P., McVerry B. J., Ortiz L. A., Exline M., Christman J. W., Abbott J., Delucchi K. L., Caballero L., McMillan M., McKenna D. H., and Liu K. D., Lancet Respir. Med. 7(2), 154–162 (2019). 10.1016/S2213-2600(18)30418-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ock S. A. and Rho G. J., Cell Transplant 20(8), 1231–1239 (2011). 10.3727/096368910X552835 [DOI] [PubMed] [Google Scholar]
- 31.Borrelli D. A., Yankson K., Shukla N., Vilanilam G., Ticer T., and Wolfram J., J. Control. Release 273, 86–98 (2018). 10.1016/j.jconrel.2018.01.022 [DOI] [PubMed] [Google Scholar]
- 32.Terrovitis J. V., Smith R. R., and Marban E., Circ. Res. 106(3), 479–494 (2010). 10.1161/CIRCRESAHA.109.208991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Phinney D. G. and Pittenger M. F., Stem Cells 35(4), 851–858 (2017). 10.1002/stem.2575 [DOI] [PubMed] [Google Scholar]
- 34.Smith J. A., Leonardi T., Huang B., Iraci N., Vega B., and Pluchino S., Biogerontology 16(2), 147–185 (2015). 10.1007/s10522-014-9510-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lener T., Gimona M., Aigner L., Borger V., Buzas E., Camussi G., Chaput N., Chatterjee D., Court F. A., del Portillo H. A., O'Driscoll L., Fais S., Falcon-Perez J. M., Felderhoff-Mueser U., Fraile L., Gho Y. S., Gorgens A., Gupta R. C., Hendrix A., Hermann D. M., Hill A. F., Hochberg F., Horn P. A., de Kleijn D., Kordelas L., Kramer B. W., Kramer-Albers E. M., Laner-Plamberger S., Laitinen S., Leonardi T., Lorenowicz M. J., Lim S. K., Lotvall J., Maguire C. A., Marcilla A., Nazarenko I., Ochiya T., Patel T., Pedersen S., Pocsfalvi G., Pluchino S., Quesenberry P., Reischl I. G., Rivera F. J., Sanzenbacher R., Schallmoser K., Slaper-Cortenbach I., Strunk D., Tonn T., Vader P., van Balkom B. W. M., Wauben M., El Andaloussi S., Thery C., Rohde E., and Giebel B., J. Extracell. Vesicles 4, 30087 (2015). 10.3402/jev.v4.30087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Burke J., Kolhe R., Hunter M., Isales C., Hamrick M., and Fulzele S., Stem Cells Int. 6, 5802529 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Suzuki E., Fujita D., Takahashi M., Oba S., and Nishimatsu H., World J. Stem Cells 8(9), 297–305 (2016). 10.4252/wjsc.v8.i9.297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lou G. H., Chen Z., Zheng M., and Liu Y. N., Exp. Mol. Med. 49, 1–9 (2017). 10.1038/emm.2017.63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lopez-Verrilli M. A., Caviedes A., Cabrera A., Sandoval S., Wyneken U., and Khoury M., Neuroscience 320, 129–139 (2016). 10.1016/j.neuroscience.2016.01.061 [DOI] [PubMed] [Google Scholar]
- 40.Gardiner C., Di Vizio D., Sahoo S., Thery C., Witwer K. W., Wauben M., and Hill A. F., J. Extracell. Vesicles 5, 32945 (2016). 10.3402/jev.v5.32945 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.van Niel G., D'Angelo G., and Raposo G., Nat. Rev. Mol. Cell Biol. 19(4), 213–228 (2018). 10.1038/nrm.2017.125 [DOI] [PubMed] [Google Scholar]
- 42.van der Pol E., Boing A. N., Gool E. L., and Nieuwland R., J. Thromb. Haemost. 14(1), 48–56 (2016). 10.1111/jth.13190 [DOI] [PubMed] [Google Scholar]
- 43.Gheinani A. H., Vogeli M., Baumgartner U., Vassella E., Draeger A., Burkhard F. C., and Monastyrskaya K., Sci. Rep. 8, 3945 (2018). 10.1038/s41598-018-22142-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Szatanek R., Baran J., Siedlar M., and Baj-Krzyworzeka M., Int. J. Mol. Med. 36(1), 11–17 (2015). 10.3892/ijmm.2015.2194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Baek G., Choi H., Kim Y., Lee H. C., and Choi C., Stem Cells Transl. Med. 8(9), 880–886 (2019). 10.1002/sctm.18-0226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kordelas L., Rebmann V., Ludwig A. K., Radtke S., Ruesing J., Doeppner T. R., Epple M., Horn P. A., Beelen D. W., and Giebel B., Leukemia 28(4), 970–973 (2014). 10.1038/leu.2014.41 [DOI] [PubMed] [Google Scholar]
- 47.Ludwig A. K., De Miroschedji K., Doeppner T. R., Borger V., Ruesing J., Rebmann V., Durst S., Jansen S., Bremer M., Behrmann E., Singer B. B., Jastrow H., Kuhlmann J. D., El Magraoui F., Meyer H. E., Hermann D. M., Opalka B., Raunser S., Epple M., Horn P. A., and Giebel B., J. Extracell. Vesicles 7(1), 1528109 (2018). 10.1080/20013078.2018.1528109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Shao H. L., Chung J., Balaj L., Charest A., Bigner D. D., Carter B. S., Hochberg F. H., Breakefield X. O., Weissleder R., and Lee H., Nat. Med. 18(12), 1835 (2012). 10.1038/nm.2994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mateescu B., Kowal E. J. K., van Balkom B. W. M., Bartel S., Bhattacharyya S. N., Buzas E. I., Buck A. H., de Candia P., Chow F. W. N., Das S., Driedonks T. A. P., Fernandez-Messina L., Haderk F., Hill A. F., Jones J. C., Van Keuren-Jensen K. R., Lai C. P., Lasser C., di Liegro I., Lunavat T. R., Lorenowicz M. J., Maas S. L. N., Mager I., Mittelbrunn M., Momma S., Mukherjee K., Nawaz M., Pegtel D. M., Pfaffl M. W., Schiffelers R. M., Tahara H., Thery C., Tosar J. P., Wauben M. H. M., Witwer K. W., and Nolte-'T Hoen E. N. M., J. Extracell. Vesicles 6, 33 (2017). 10.1080/20013078.2017.1286095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhao C., Tan A., Pastorin G., and Ho H. K., Biotechnol. Adv. 31(5), 654–668 (2013). 10.1016/j.biotechadv.2012.08.001 [DOI] [PubMed] [Google Scholar]
- 51.Velnar T., Bailey T., and Smrkoli V., J. Int. Med. Res. 37(5), 1528–1542 (2009). 10.1177/147323000903700531 [DOI] [PubMed] [Google Scholar]
- 52.d A., Ferreira F., and Gomes D. A., Bioengineering 6(1), 4 (2018). 10.3390/bioengineering6010004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wu P. P., Zhang B., Shi H., Qian H., and Xu W. R., Cytotherapy 20(3), 291–301 (2018). 10.1016/j.jcyt.2017.11.002 [DOI] [PubMed] [Google Scholar]
- 54.Than U. T. T., Guanzon D., Leavesley D., and Parker T., Int. J. Mol. Sci. 18(5), 956 (2017). 10.3390/ijms18050956 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ti D. D., Hao H. J., Tong C. A., Liu J. J., Dong L., Zheng J. X., Zhao Y. L., Liu H. L., Fu X. B., and Han W. D., J. Transl. Med. 13, 308 (2015). 10.1186/s12967-015-0642-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lo Sicco C., Reverberi D., Balbi C., Ulivi V., Principi E., Pascucci L., Becherini P., Bosco M. C., Varesio L., Franzin C., Pozzobon M., Cancedda R., and Tasso R., Stem Cells Transl. Med. 6(3), 1018–1028 (2017). 10.1002/sctm.16-0363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Nosbaum A., Prevel N., Truong H. A., Mehta P., Ettinger M., Scharschmidt T. C., Ali N. H., Pauli M. L., Abbas A. K., and Rosenblum M. D., J. Immunol. 196(5), 2010–2014 (2016). 10.4049/jimmunol.1502139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Monguio-Tortajada M., Roura S., Galvez-Monton C., Pujal J. M., Aran G., Sanjurjo L., Franquesa M., Sarrias M. R., Bayes-Genis A., and Borras F. E., Theranostics 7(2), 270–284 (2017). 10.7150/thno.16154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Li X., Liu L., Yang J., Yu Y., Chai J., Wang L., Ma L., and Yin H., EBioMedicine 8, 72–82 (2016). 10.1016/j.ebiom.2016.04.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Yu B., Shao H., Su C., Jiang Y., Chen X., Bai L., Zhang Y., Li Q., Zhang X., and Li X., Sci. Rep. 6, 34562 (2016). 10.1038/srep34562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Gurtner G. C., Werner S., Barrandon Y., and Longaker M. T., Nature 453(7193), 314–321 (2008). 10.1038/nature07039 [DOI] [PubMed] [Google Scholar]
- 62.Zhang J., Guan J., Niu X., Hu G., Guo S., Li Q., Xie Z., Zhang C., and Wang Y., J. Transl. Med. 13, 49 (2015). 10.1186/s12967-015-0417-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hu L., Wang J., Zhou X., Xiong Z., Zhao J., Yu R., Huang F., Zhang H., and Chen L., Sci. Rep. 6, 32993 (2016). 10.1038/srep32993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zhang B., Wang M., Gong A., Zhang X., Wu X., Zhu Y., Shi H., Wu L., Zhu W., Qian H., and Xu W., Stem Cells 33(7), 2158–2168 (2015). 10.1002/stem.1771 [DOI] [PubMed] [Google Scholar]
- 65.Shi H., Xu X., Zhang B., Xu J., Pan Z., Gong A., Zhang X., Li R., Sun Y., Yan Y., Mao F., Qian H., and Xu W., Theranostics 7(6), 1674–1688 (2017). 10.7150/thno.18082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Bonnans C., Chou J., and Werb Z., Nat. Rev. Mol. Cell Biol. 15(12), 786–801 (2014). 10.1038/nrm3904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zhang B., Shi Y., Gong A., Pan Z., Shi H., Yang H., Fu H., Yan Y., Zhang X., Wang M., Zhu W., Qian H., and Xu W., Stem Cells 34(10), 2485–2500 (2016). 10.1002/stem.2432 [DOI] [PubMed] [Google Scholar]
- 68.Zhao B., Zhang Y., Han S., Zhang W., Zhou Q., Guan H., Liu J., Shi J., Su L., and Hu D., J. Mol. Histol. 48(2), 121–132 (2017). 10.1007/s10735-017-9711-x [DOI] [PubMed] [Google Scholar]
- 69.Timmis A., Townsend N., Gale C., Grobbee R., Maniadakis N., Flather M., Wilkins E., Wright L., Vos R., Bax J., Blum M., Pinto F., Vardas P., and ESC Scientific Document Group, Eur. Heart J. 39(7), 508 (2018). 10.1093/eurheartj/ehx628 [DOI] [PubMed] [Google Scholar]
- 70.Bergmann O., Bhardwaj R. D., Bernard S., Zdunek S., Barnabe-Heider F., Walsh S., Zupicich J., Alkass K., Buchholz B. A., Druid H., Jovinge S., and Frisen J., Science 324(5923), 98–102 (2009). 10.1126/science.1164680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Khan M., Nickoloff E., Abramova T., Johnson J., Verma S. K., Krishnamurthy P., Mackie A. R., Vaughan E., Garikipati V. N., Benedict C., Ramirez V., Lambers E., Ito A., Gao E., Misener S., Luongo T., Elrod J., Qin G., Houser S. R., Koch W. J., and Kishore R., Circ. Res. 117(1), 52–64 (2015). 10.1161/CIRCRESAHA.117.305990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wang Y., Zhang L., Li Y., Chen L., Wang X., Guo W., Zhang X., Qin G., He S. H., Zimmerman A., Liu Y., Kim I. M., Weintraub N. L., and Tang Y., Int. J. Cardiol. 192, 61–69 (2015). 10.1016/j.ijcard.2015.05.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Gray W. D., French K. M., Ghosh-Choudhary S., Maxwell J. T., Brown M. E., Platt M. O., Searles C. D., and Davis M. E., Circ. Res. 116(2), 255–263 (2015). 10.1161/CIRCRESAHA.116.304360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Pan W., Zhu Y., Meng X., Zhang C., Yang Y., and Bei Y., J. Cardiovasc. Transl. Res. 12(1), 28–36 (2019). 10.1007/s12265-018-9836-7 [DOI] [PubMed] [Google Scholar]
- 75.Liu J., Jiang M., Deng S., Lu J., Huang H., Zhang Y., Gong P., Shen X., Ruan H., Jin M., and Wang H., Mol. Ther. Nucleic Acids 11, 103–115 (2018). 10.1016/j.omtn.2018.01.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Luo Q., Guo D., Liu G., Chen G., Hang M., and Jin M., Cell. Physiol. Biochem. 44(6), 2105–2116 (2017). 10.1159/000485949 [DOI] [PubMed] [Google Scholar]
- 77.Zhu L. P., Tian T., Wang J. Y., He J. N., Chen T., Pan M., Xu L., Zhang H. X., Qiu X. T., Li C. C., Wang K. K., Shen H., Zhang G. G., and Bai Y. P., Theranostics 8(22), 6163–6177 (2018). 10.7150/thno.28021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Xiao J., Pan Y., Li X. H., Yang X. Y., Feng Y. L., Tan H. H., Jiang L., Feng J., and Yu X. Y., Cell Death Dis. 7(6), e2277 (2016). 10.1038/cddis.2016.181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Teng X., Chen L., Chen W., Yang J., Yang Z., and Shen Z., Cell. Physiol. Biochem. 37(6), 2415–2424 (2015). 10.1159/000438594 [DOI] [PubMed] [Google Scholar]
- 80.Arslan F., Lai R. C., Smeets M. B., Akeroyd L., Choo A., Aguor E. N., Timmers L., van Rijen H. V., Doevendans P. A., Pasterkamp G., Lim S. K., and de Kleijn D. P., Stem Cell Res. 10(3), 301–312 (2013). 10.1016/j.scr.2013.01.002 [DOI] [PubMed] [Google Scholar]
- 81.Jiang M., Wang H., Jin M., Yang X., Ji H., Jiang Y., Zhang H., Wu F., Wu G., Lai X., Cai L., Hu R., Xu L., and Li L., Cell. Physiol. Biochem. 47(2), 864–878 (2018). 10.1159/000490078 [DOI] [PubMed] [Google Scholar]
- 82.Zhang Z., Yang J., Yan W., Li Y., Shen Z., and Asahara T., J. Am. Heart Assoc. 5(1), e002856 (2016). 10.1161/JAHA.115.002856 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Kang K., Ma R., Cai W., Huang W., Paul C., Liang J., Wang Y., Zhao T., Kim H. W., Xu M., Millard R. W., Wen Z., and Wang Y., Stem Cells Int. 2015, 659890 10.1155/2015/659890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Ma J., Zhao Y., Sun L., Sun X., Zhao X., Sun X., Qian H., Xu W., and Zhu W., Stem Cells Transl. Med. 6(1), 51–59 (2017). 10.5966/sctm.2016-0038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Bian S., Zhang L., Duan L., Wang X., Min Y., and Yu H., J. Mol. Med. 92(4), 387–397 (2014). 10.1007/s00109-013-1110-5 [DOI] [PubMed] [Google Scholar]
- 86.Wang N., Chen C., Yang D., Liao Q., Luo H., Wang X., Zhou F., Yang X., Yang J., Zeng C., and Wang W. E., Biochim. Biophys. Acta Mol. Basis Dis. 1863(8), 2085–2092 (2017). 10.1016/j.bbadis.2017.02.023 [DOI] [PubMed] [Google Scholar]
- 87.Barile L., Lionetti V., Cervio E., Matteucci M., Gherghiceanu M., Popescu L. M., Torre T., Siclari F., Moccetti T., and Vassalli G., Cardiovasc. Res. 103(4), 530–541 (2014). 10.1093/cvr/cvu167 [DOI] [PubMed] [Google Scholar]
- 88.Izarra A., Moscoso I., Levent E., Canon S., Cerrada I., Diez-Juan A., Blanca V., Nunez-Gil I. J., Valiente I., Ruiz-Sauri A., Sepulveda P., Tiburcy M., Zimmermann W. H., and Bernad A., Stem Cell Rep. 3(6), 1029–1042 (2014). 10.1016/j.stemcr.2014.10.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Agarwal U., George A., Bhutani S., Ghosh-Choudhary S., Maxwell J. T., Brown M. E., Mehta Y., Platt M. O., Liang Y., Sahoo S., and Davis M. E., Circ. Res. 120(4), 701–712 (2017). 10.1161/CIRCRESAHA.116.309935 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Ratajczak J., Miekus K., Kucia M., Zhang J., Reca R., Dvorak P., and Ratajczak M. Z., Leukemia 20(5), 847–856 (2006). 10.1038/sj.leu.2404132 [DOI] [PubMed] [Google Scholar]
- 91.Liu L. Z., Li C., Chen Q., Jing Y., Carpenter R., Jiang Y., Kung H. F., Lai L., and Jiang B. H., PLoS One 6(4), e19139 (2011). 10.1371/journal.pone.0019139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Doeppner T. R., Herz J., Gorgens A., Schlechter J., Ludwig A. K., Radtke S., de Miroschedji K., Horn P. A., Giebel B., and Hermann D. M., Stem Cells Transl. Med. 4(10), 1131–1143 (2015). 10.5966/sctm.2015-0078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Moon G. J., Sung J. H., Kim D. H., Kim E. H., Cho Y. H., Son J. P., Cha J. M., and Bang O. Y., Transl. Stroke Res. 1, 509–521 (2018). [DOI] [PubMed] [Google Scholar]
- 94.Xiao Y., Geng F., Wang G., Li X., Zhu J., and Zhu W., J. Cell. Biochem. 1, 2109–2118 (2018). [DOI] [PubMed] [Google Scholar]
- 95.Xin H., Li Y., Buller B., Katakowski M., Zhang Y., Wang X., Shang X., Zhang Z. G., and Chopp M., Stem Cells 30(7), 1556–1564 (2012). 10.1002/stem.1129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Xin H., Li Y., Liu Z., Wang X., Shang X., Cui Y., Zhang Z. G., and Chopp M., Stem Cells 31(12), 2737–2746 (2013). 10.1002/stem.1409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Zhang Y. L., Chopp M., Meng Y. L., Katakowski M., Xin H. Q., Mahmood A., and Xiong Y., J. Neurosurg. 122(4), 856–867 (2015). 10.3171/2014.11.JNS14770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Zhang Y. L., Chopp M., Zhang Z. G., Katakowski M., Xin H. Q., Qu C. S., Ali M., Mahmood A., and Xiong Y., Neurochem. Int. 111, 69–81 (2017). 10.1016/j.neuint.2016.08.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Kim D. K., Nishida H., An S. Y., Shetty A. K., Bartosh T. J., and Prockop D. J., Proc. Natl. Acad. Sci. U.S.A. 113(1), 170–175 (2016). 10.1073/pnas.1522297113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Patel N. A., Moss L. D., Lee J. Y., Tajiri N., Acosta S., Hudson C., Parag S., Cooper D. R., Borlongan C. V., and Bickford P. C., J. Neuroinflammation 15(1), 204 (2018). 10.1186/s12974-018-1240-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Huang J. H., Yin X. M., Xu Y., Xu C. C., Lin X., Ye F. B., Cao Y., and Lin F. Y., J. Neurotrauma 34(24), 3388–3396 (2017). 10.1089/neu.2017.5063 [DOI] [PubMed] [Google Scholar]
- 102.Lankford K. L., Arroyo E. J., Nazimek K., Bryniarski K., Askenase P. W., and Kocsis J. D., PLoS One 13(1), e0190358 (2018). 10.1371/journal.pone.0190358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Li D., Zhang P., Yao X., Li H., Shen H., Li X., Wu J., and Lu X., Front. Neurosci. 12, 845 (2018). 10.3389/fnins.2018.00845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Wang L., Pei S., Han L., Guo B., Li Y., Duan R., Yao Y., Xue B., Chen X., and Jia Y., Cell. Physiol. Biochem. 50(4), 1535–1559 (2018). 10.1159/000494652 [DOI] [PubMed] [Google Scholar]
- 105.Liu W., Wang Y., Gong F., Rong Y., Luo Y., Tang P., Zhou Z., Zhou Z., Xu T., Jiang T., Yang S., Yin G., Chen J., Fan J., and Cai W., J. Neurotrauma 36(3), 469–484 (2019). 10.1089/neu.2018.5835 [DOI] [PubMed] [Google Scholar]
- 106.Ren Z. W., Zhou J. G., Xiong Z. K., Zhu F. Z., and Guo X. D., Eur. Rev. Med. Pharmacol. 23(1), 52–60 (2019). [DOI] [PubMed] [Google Scholar]
- 107.Katsuda T., Oki K., and Ochiya T., Methods Mol. Biol. 1212, 171–181 (2015). 10.1007/7651_2014_98 [DOI] [PubMed] [Google Scholar]
- 108.de Godoy M. A., Saraiva L. M., de Carvalho L. R. P., Vasconcelos-Dos-Santos A., Beiral H. J. V., Ramos A. B., Silva L. R. P., Leal R. B., Monteiro V. H. S., Braga C. V., de Araujo-Silva C. A., Sinis L. C., Bodart-Santos V., Kasai-Brunswick T. H., Alcantara C. L., Lima A., da Cunha E. S. N. L., Galina A., Vieyra A., De Felice F. G., Mendez-Otero R., and Ferreira S. T., J. Biol. Chem. 293(6), 1957–1975 (2018). 10.1074/jbc.M117.807180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Wang S. S., Jia J., and Wang Z., J. Alzheimers Dis. 61(3), 1005–1013 (2018). 10.3233/JAD-170848 [DOI] [PubMed] [Google Scholar]
- 110.Cui G. H., Wu J., Mou F. F., Xie W. H., Wang F. B., Wang Q. L., Fang J., Xu Y. W., Dong Y. R., Liu J. R., and Guo H. D., FASEB J. 32(2), 654–668 (2018). 10.1096/fj.201700600R [DOI] [PubMed] [Google Scholar]
- 111.Kim R., Lee S., Lee J., Kim M., Kim W. J., Lee H. W., Lee M. Y., Kim J., and Chang W., BMB Rep. 51(8), 406–411 (2018). 10.5483/BMBRep.2018.51.8.105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Zhu Q. W., Ling X. Z., Yang Y. L., Zhang J. T., Li Q., Niu X., Hu G. W., Chen B., Li H. Y., Wang Y., and Deng Z. F., Adv. Sci. 6(6), 1801899 (2019). 10.1002/advs.201801899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Xie Y., Chen Y., Zhang L., Ge W., and Tang P., J. Cell. Mol. Med. 21(5), 1033–1041 (2017). 10.1111/jcmm.13039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Salomon C., Ryan J., Sobrevia L., Kobayashi M., Ashman K., Mitchell M., and Rice G. E., PLoS One 8(7), e68451 (2013). 10.1371/journal.pone.0068451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Zhang H. C., Liu X. B., Huang S., Bi X. Y., Wang H. X., Xie L. X., Wang Y. Q., Cao X. F., Lv J., Xiao F. J., Yang Y., and Guo Z. K., Stem Cells Dev. 21(18), 3289–3297 (2012). 10.1089/scd.2012.0095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Tomita T., Hashimoto H., and Yoshikawa H., Curr. Drug Targets 4(8), 609–612 (2003). 10.2174/1389450033490777 [DOI] [PubMed] [Google Scholar]
- 117.Furuta T., Miyaki S., Ishitobi H., Ogura T., Kato Y., Kamei N., Miyado K., Higashi Y., and Ochi M., Stem Cells Transl. Med. 5(12), 1620–1630 (2016). 10.5966/sctm.2015-0285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Kim S. H., Lechman E. R., Bianco N., Menon R., Keravala A., Nash J., Mi Z., Watkins S. C., Gambotto A., and Robbins P. D., J. Immunol. 174(10), 6440–6448 (2005). 10.4049/jimmunol.174.10.6440 [DOI] [PubMed] [Google Scholar]
- 119.Verron E., Gauthier O., Janvier P., Pilet P., Lesoeur J., Bujoli B., Guicheux J., and Bouler J. M., Biomaterials 31(30), 7776–7784 (2010). 10.1016/j.biomaterials.2010.06.047 [DOI] [PubMed] [Google Scholar]
- 120.Qi X., Zhang J., Yuan H., Xu Z., Li Q., Niu X., Hu B., Wang Y., and Li X., Int. J. Biol. Sci. 12(7), 836–849 (2016). 10.7150/ijbs.14809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Tan C. Y., Lai R. C., Wong W., Dan Y. Y., Lim S. K., and Ho H. K., Curr. Stem Cell Res. Ther. 5, 76 (2014). 10.1186/scrt465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Tamura R., Uemoto S., and Tabata Y., Inflammation Regener. 36, 26 (2016). 10.1186/s41232-016-0030-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Jiang W., Tan Y., Cai M., Zhao T., Mao F., Zhang X., Xu W., Yan Z., Qian H., and Yan Y., Stem Cells Int. 2018, 6079642 10.1155/2018/6079642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Nong K., Wang W., Niu X., Hu B., Ma C., Bai Y., Wu B., Wang Y., and Ai K., Cytotherapy 18(12), 1548–1559 (2016). 10.1016/j.jcyt.2016.08.002 [DOI] [PubMed] [Google Scholar]
- 125.Haga H., Yan I. K., Borrelli D. A., Matsuda A., Parasramka M., Shukla N., Lee D. D., and Patel T., Liver Transpl. 23(6), 791–803 (2017). 10.1002/lt.24770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Yao J., Zheng J., Cai J., Zeng K., Zhou C., Zhang J., Li S., Li H., Chen L., He L., Chen H., Fu H., Zhang Q., Chen G., Yang Y., and Zhang Y., FASEB J. 33(2), 1695–1710 (2019). 10.1096/fj.201800131RR [DOI] [PubMed] [Google Scholar]
- 127.Chen L., Xiang B., Wang X., and Xiang C., Curr. Stem Cell Res. Ther. 8(1), 9 (2017). 10.1186/s13287-016-0453-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Haga H., Yan I. K., Takahashi K., Matsuda A., and Patel T., Stem Cells Transl. Med. 6(4), 1262–1272 (2017). 10.1002/sctm.16-0226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Jin Y., Wang J., Li H., Gao S., Shi R., Yang D., Wang X., Wang X., Zhu L., Wang X., Chen C., Ning K., Gao Z., Xu J., and Fu Q., EBioMedicine 34, 231–242 (2018). 10.1016/j.ebiom.2018.07.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Liu Y., Lou G., Li A., Zhang T., Qi J., Ye D., Zheng M., and Chen Z., EBioMedicine 36, 140–150 (2018). 10.1016/j.ebiom.2018.08.054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Mardpour S., Hassani S. N., Mardpour S., Sayahpour F., Vosough M., Ai J., Aghdami N., Hamidieh A. A., and Baharvand H., J. Cell. Physiol. 233(12), 9330–9344 (2018). 10.1002/jcp.26413 [DOI] [PubMed] [Google Scholar]
- 132.Chen L., Lu F. B., Chen D. Z., Wu J. L., Hu E. D., Xu L. M., Zheng M. H., Li H., Huang Y., Jin X. Y., Gong Y. W., Lin Z., Wang X. D., and Chen Y. P., Mol. Immunol. 93, 38–46 (2018). 10.1016/j.molimm.2017.11.008 [DOI] [PubMed] [Google Scholar]
- 133.Ohara M., Ohnishi S., Hosono H., Yamamoto K., Yuyama K., Nakamura H., Fu Q., Maehara O., Suda G., and Sakamoto N., Stem Cells Int. 2018, 3212643 10.1155/2018/3212643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Gatti S., Bruno S., Deregibus M. C., Sordi A., Cantaluppi V., Tetta C., and Camussi G., Nephrol. Dial. Transplant. 26(5), 1474–1483 (2011). 10.1093/ndt/gfr015 [DOI] [PubMed] [Google Scholar]
- 135.Bruno S., Grange C., Collino F., Deregibus M. C., Cantaluppi V., Biancone L., Tetta C., and Camussi G., PLoS One 7(3), e33115 (2012). 10.1371/journal.pone.0033115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Bruno S., Tapparo M., Collino F., Chiabotto G., Deregibus M. C., Soares Lindoso R., Neri F., Kholia S., Giunti S., Wen S., Quesenberry P., and Camussi G., Tissue Eng. Part A 23(21-22), 1262–1273 (2017). 10.1089/ten.tea.2017.0069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Zou X. Y., Zhang G. Y., Cheng Z. L., Yin D. M., Du T., Ju G. Q., Miao S., Liu G. H., Lu M. J., and Zhu Y. J., Curr. Stem Cell Res. Ther. 5, 40 (2014). 10.1186/scrt428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Zou X. Y., Gu D., Xing X. Y., Cheng Z. L., Gong D. L., Zhang G. Y., and Zhu Y. J., Am. J. Transl. Res. 8(10), 4289–4299 (2016). [PMC free article] [PubMed] [Google Scholar]
- 139.He J., Wang Y., Sun S., Yu M., Wang C., Pei X., Zhu B., Wu J., and Zhao W., Nephrology 17(5), 493–500 (2012). 10.1111/j.1440-1797.2012.01589.x [DOI] [PubMed] [Google Scholar]
- 140.He J., Wang Y., Lu X., Zhu B., Pei X., Wu J., and Zhao W., Nephrology 20(9), 591–600 (2015). 10.1111/nep.12490 [DOI] [PubMed] [Google Scholar]
- 141.Choi H. Y., Lee H. G., Kim B. S., Ahn S. H., Jung A., Lee M., Lee J. E., Kim H. J., Ha S. K., and Park H. C., Curr. Stem Cell Res. Ther. 6, 18 (2015). 10.1186/s13287-015-0012-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Eirin A., Zhu X. Y., Puranik A. S., Tang H., McGurren K. A., van Wijnen A. J., Lerman A., and Lerman L. O., Kidney Int. 92(1), 114–124 (2017). 10.1016/j.kint.2016.12.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Nojehdehi S., Soudi S., Hesampour A., Rasouli S., Soleimani M., and Hashemi S. M., J. Cell Biochem. 119(11), 9433–9443 (2018). 10.1002/jcb.27260 [DOI] [PubMed] [Google Scholar]
- 144.Xie M., Hao H. J., Cheng Y., Xie Z. Y., Yin Y. Q., Zhang Q., Gao J. Q., Liu H. Y., Mu Y. M., and Han W. D., Biochem. Biophys. Res. Commun. 483(1), 435–441 (2017). 10.1016/j.bbrc.2016.12.125 [DOI] [PubMed] [Google Scholar]
- 145.Guan L. X., Guan H., Li H. B., Ren C. A., Liu L., Chu J. J., and Dai L. J., Exp. Ther. Med. 9(5), 1623–1630 (2015). 10.3892/etm.2015.2339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Thomou T., Mori M. A., Dreyfuss J. M., Konishi M., Sakaguchi M., Wolfrum C., Rao T. N., Winnay J. N., Garcia-Martin R., Grinspoon S. K., Gorden P., and Kahn C. R., Nature 542(7642), 450 (2017). 10.1038/nature21365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Sun Y., Shi H., Yin S., Ji C., Zhang X., Zhang B., Wu P., Shi Y., Mao F., Yan Y., Xu W., and Qian H., ACS Nano 12(8), 7613–7628 (2018). 10.1021/acsnano.7b07643 [DOI] [PubMed] [Google Scholar]
- 148.Jiang Z. Z., Liu Y. M., Niu X., Yin J. Y., Hu B., Guo S. C., Fan Y., Wang Y., and Wang N. S., Curr. Stem Cell Res. Ther. 7, 24 (2016). 10.1186/s13287-016-0287-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Nagaishi K., Mizue Y., Chikenji T., Otani M., Nakano M., Konari N., and Fujimiya M., Sci. Rep. 6, 34842 (2016). 10.1038/srep34842 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Ebrahim N., Ahmed I. A., Hussien N. I., Dessouky A. A., Farid A. S., Elshazly A. M., Mostafa O., Gazzar W. B. E., Sorour S. M., Seleem Y., Hussein A. M., and Sabry D., Cells 7(12), 226 (2018). 10.3390/cells7120226 [DOI] [PMC free article] [PubMed] [Google Scholar]