Graphical abstract
Keywords: Right ventricular thrombus, Deep vein thrombus, Pulmonary embolus, Patent foramen ovale, Ovarian malignancy
Highlights
-
•
Right heart thrombi are rare and associated with significant mortality.
-
•
RV thrombus was complicated by recurrent pulmonary emboli and managed surgically.
-
•
Recurrent pulmonary emboli in anticoagulated patients may suggest malignancy.
-
•
The optimal treatment for right heart thrombi remains uncertain.
-
•
Surgical thrombectomy is an option, but treatment should be individualized.
Introduction
Right heart thrombi are uncommon, usually found with concurrent pulmonary emboli and associated with significant mortality. They are often “in transit,” originating from deep vein thrombi but may also be due to primary intracardiac processes such as heart failure, devices, and atrial fibrillation. Currently the optimal management of right ventricular (RV) thrombi is not well established, as there have been no large randomized clinical trials evaluating the various treatments, and the existing evidence has been controversial. We present a case of RV thrombus in transit originating from a deep vein thrombus, complicated by acute on chronic pulmonary emboli and embolic stroke in the setting of gynecologic malignancy. This case presented a complex therapeutic dilemma in which there were no clear or optimal management options.
Case Presentation
A 40-year-old obese woman with a history of hypertension, type 2 diabetes, unprovoked deep vein thrombus and pulmonary emboli being treated with rivaroxaban, and an untreated pelvic mass of unknown etiology presented with 3 weeks of progressive dyspnea on exertion. On admission she was hemodynamically stable but hypoxemic. She had normal results on cardiopulmonary examination and appeared euvolemic. Computed tomographic angiography of the chest showed nonocclusive thrombi in various branches of the right and left pulmonary arteries, likely representing chronic emboli with interval improvement. She was started on heparin and 4 L supplemental oxygen, with successful reversal of hypoxemia. Transthoracic echocardiography (TTE) showed mildly reduced RV function, RV fractional area change of 25%, tricuspid annular plane systolic excursion of 1.7 cm, and a new large RV mass suspicious for thrombus. Transesophageal echocardiography showed a large, mobile, multilobed RV thrombus extending from the tricuspid valve to the pulmonic valve, as well as a patent foramen ovale (PFO; Figure 1, Figure 2, Figure 3, Figure 4, Video 1, Video 2, Video 3). There was moderate to severe tricuspid regurgitation, a peak resting tricuspid regurgitation gradient of 94 mm Hg, and a mean tricuspid regurgitation gradient of 46 mm Hg (Figure 5). There was no evidence of pulmonic valve stenosis or regurgitation. Other echocardiographic findings included an estimated right atrial pressure of 8 mm Hg, RV systolic pressure (RVSP) of 102 mm Hg, and a mean pulmonary artery (PA) pressure of 54 mm Hg, which incongruently suggested normal or hyperdynamic RV function. Lower extremity duplex ultrasound showed chronic occlusive thrombi in various areas of the right lower extremity veins. Magnetic resonance imaging of the brain showed multiple tiny, acute infarcts of the bilateral cerebellar hemispheres, bilateral parietal lobes, and left frontal lobe (Figure 6). Results of a neurological examination were normal. A 12-cm ovarian mass was found incidentally 1 year before admission, but the patient was lost to follow-up. Computed tomography of the abdomen and pelvis now showed a 14.6 × 10.1 × 11.4 cm cystic and solid right ovarian mass. Cancer antigen 125 was elevated at 475 U/mL. The patient was transitioned to enoxaparin and started on sildenafil. An inferior vena cava filter was placed to prevent any further emboli. Repeat TTE 5 days after initial presentation showed a slight decrease in RV thrombus size and improvement in RVSP to 55 mm Hg and in mean PA pressure to 44 mm Hg. Emergent thrombolysis, surgery, and extracorporeal membrane oxygenation all were considered initially, but none of these options were pursued, because the patient was stable hemodynamically and improved with anticoagulation alone. She was discharged with enoxaparin and plans to pursue surgical resection of the pelvic mass as an outpatient.
Figure 1.
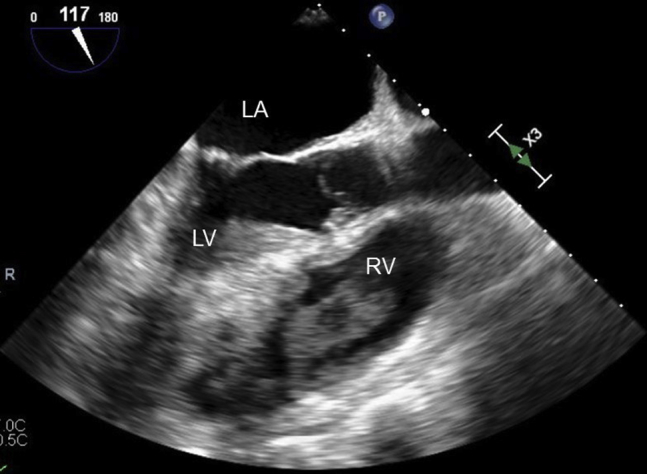
Transesophageal echocardiographic midesophageal 117° long-axis view of large right ventricular thrombus on initial presentation. LA, Left atrium; LV, left ventricle; RV, right ventricle.
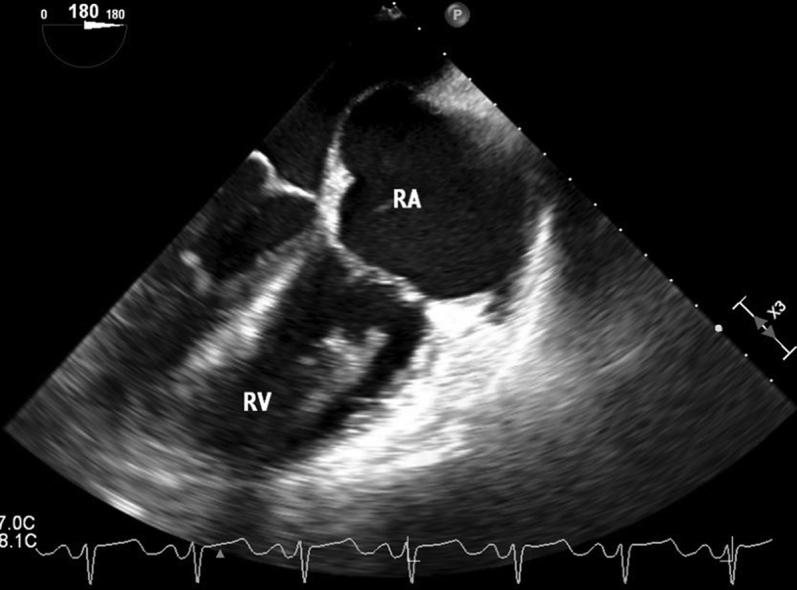
Figure 2.
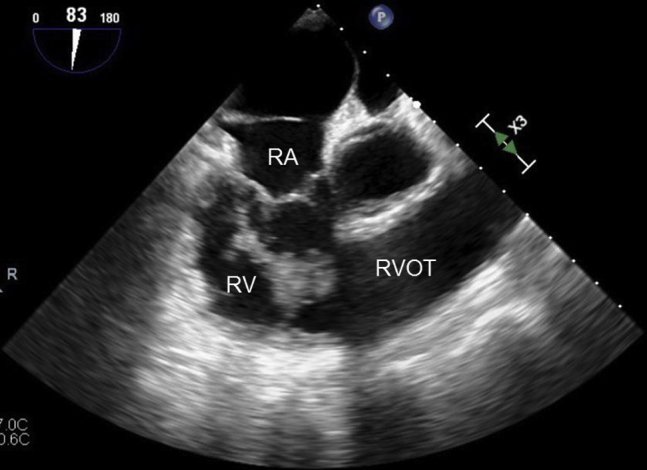
Transesophageal echocardiographic midesophageal 83° right ventricular inflow-outflow view of large right ventricular thrombus on initial presentation. RA, Right atrium; RV, right ventricle; RVOT, right ventricular outflow tract.
Figure 3.
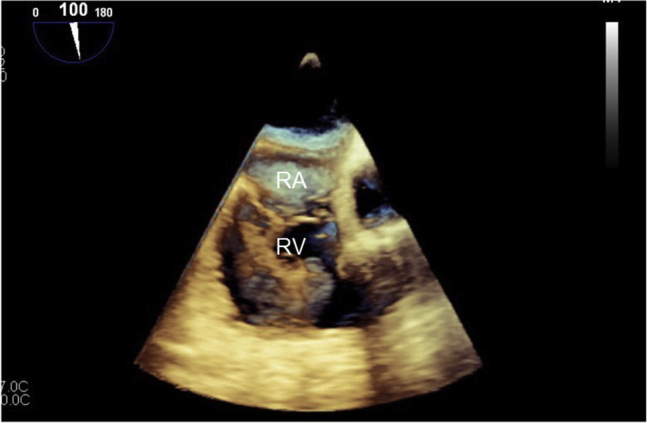
Transesophageal echocardiographic midesophageal 100° three-dimensional image of large right ventricular thrombus on initial presentation. RA, Right atrium; RV, right ventricle.
Figure 4.
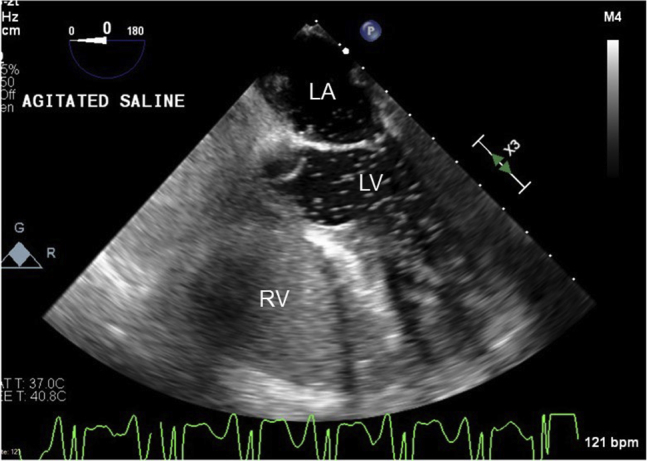
Transesophageal echocardiographic midesophageal 0° four-chamber view with agitated saline demonstrating presence of PFO. LA, Left atrium; LV, left ventricle; RV, right ventricle.
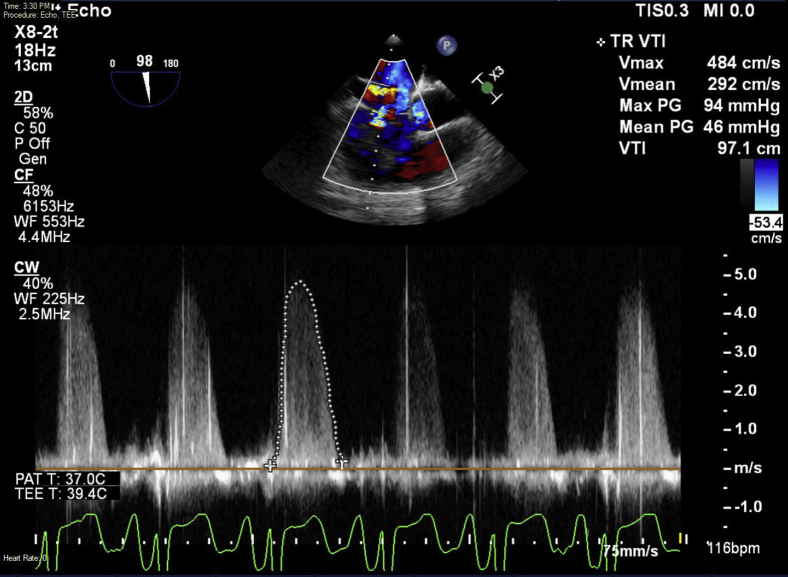
Figure 5.
Tricuspid regurgitation velocity-time integral showing peak pressure gradient of 94 mm Hg.
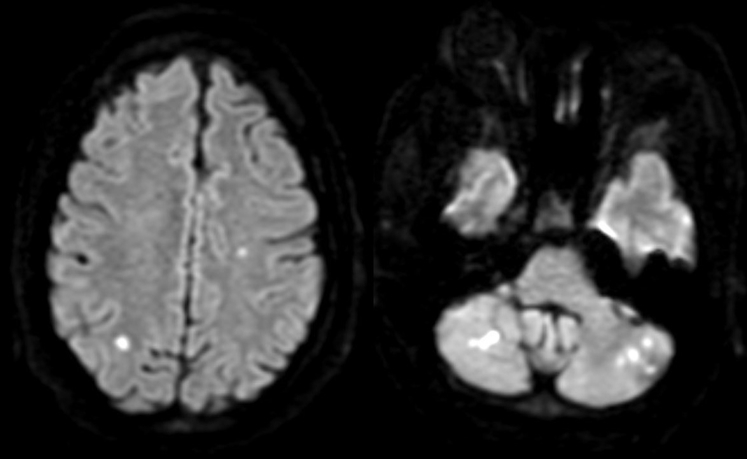
Figure 6.
Brain magnetic resonance imaging showing multiple tiny, acute emboli to the bilateral cerebellar hemispheres, parietal lobes, and left frontal lobe.
Forty-five days after initial presentation, the patient underwent laparoscopic tumor debulking, bilateral salpingo-oophorectomy, and total hysterectomy without any immediate complications. Intraoperative transesophageal echocardiography showed a stable RV thrombus, RVSP of 47 mm Hg, and mean PA pressure of 33 mm Hg (Figures 7 and 8). Pathology revealed synchronous endometrioid ovarian and endometrial adenocarcinoma. On the day after surgery, the patient was extubated successfully but subsequently developed progressive hypoxemia. Chest radiography showed clear lungs. Computed tomographic angiography of the chest showed new pulmonary emboli in the right upper lobe, right middle lobe, and left lower lobe arteries (Figure 9). Repeat TTE showed worsening right heart pressures, with RVSP of 91 mm Hg and mean PA pressure of 56 mm Hg. The patient was restarted on anticoagulation initially with a heparin drip, which was subsequently transitioned to enoxaparin. On the basis of these worsening pressures, continued risk for pulmonary and systemic emboli, and potential hemodynamic compromise, a decision was made to proceed with thrombectomy. The patient underwent sternotomy, resection of the RV thrombus, and PFO closure. On initial gross visualization, the RV mass was entwined in the chordae of the tricuspid valve, did not appear to be a thrombus, and was concerning for metastatic disease (Figure 10). Pathology showed that the mass was in fact an organizing thrombus without any malignant cells identified. Postoperative TTE showed improved RVSP of 53 mm Hg and mean PA pressure of 41 mm Hg, attributed to debulking of the RV mass, and no evidence of interatrial shunting. Postoperatively the patient remained hemodynamically stable and demonstrated good oxygen saturation on 3 L of supplemental oxygen. She was discharged with continued anticoagulation, sildenafil, and home oxygen. At the most recent outpatient follow-up 9 months after thrombectomy, the patient was asymptomatic, and TTE showed RVSP of 26 mm Hg, normal RV size, and mildly reduced RV function.
Figure 7.
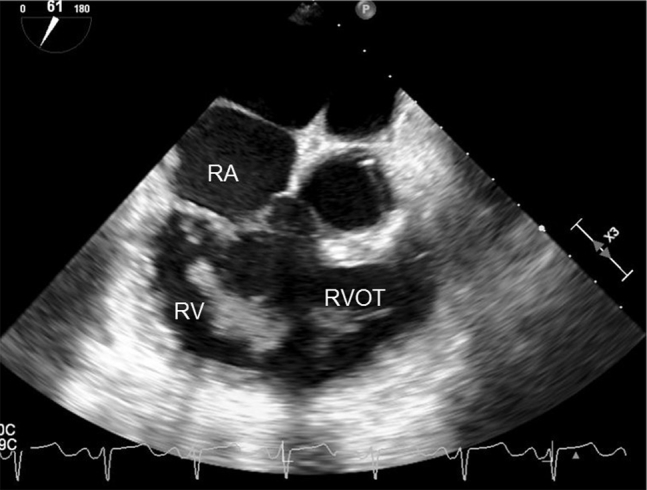
Intraoperative transesophageal echocardiographic midesophageal 61° right ventricular inflow-outflow view redemonstrating presence of right ventricular thrombus. RA, Right atrium; RV, right ventricle; RVOT, right ventricular outflow tract.
Figure 8.
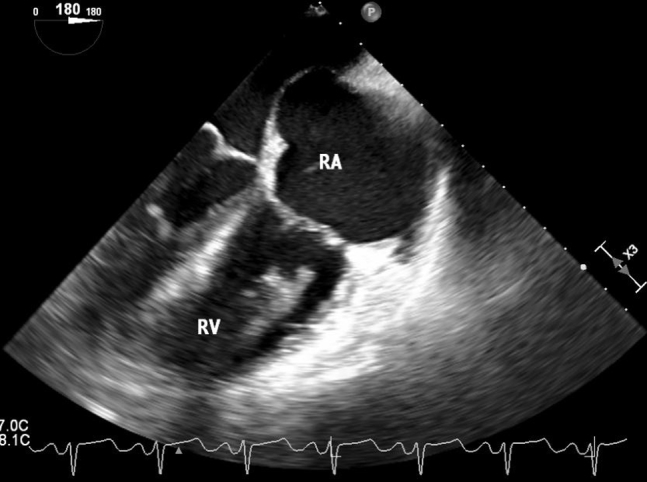
Intraoperative transesophageal echocardiographic midesophageal 180° four-chamber view redemonstrating presence of right ventricular thrombus. RA, Right atrium; RV, right ventricle.
Figure 9.
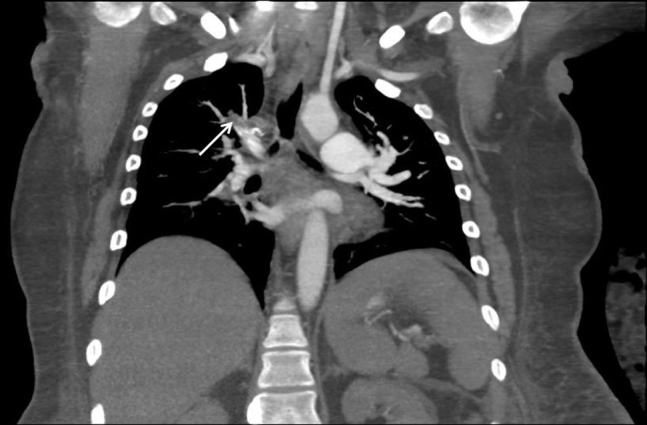
Computed tomographic angiography of the chest showing new pulmonary emboli in the right upper lobe arteries (arrow), right middle lobe artery, and left lower lobe arteries.
Figure 10.
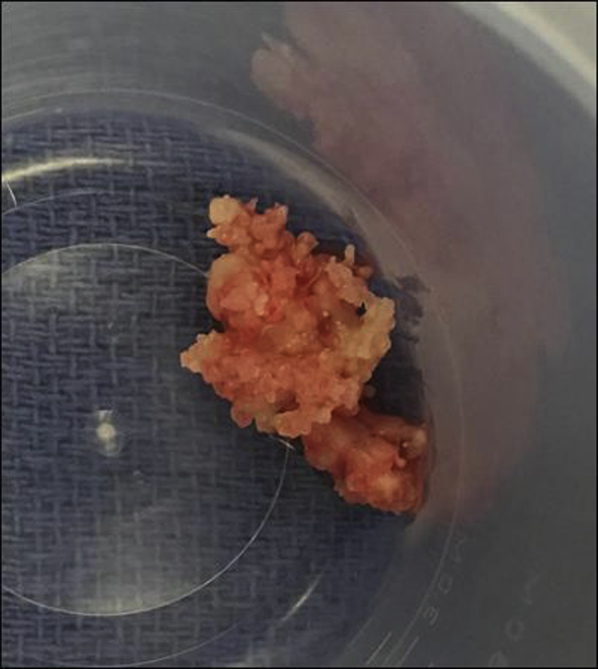
Gross image of sample taken from RV thrombus during surgical thrombectomy.
Discussion
Right heart thrombus is an uncommon condition that is found in about 4% of patients with pulmonary emboli.1, 2 It is often found in transit, originating from a systemic vein source, but it may also form within the cardiac chambers from primary processes such as atrial fibrillation. Right heart thrombi are associated with significantly increased mortality, with rates reported to be 27% to 45% despite treatment and rates near 100% in untreated patients.3, 4, 5 In comparison, the rate of mortality in patients with acute pulmonary emboli is approximately 2.5%.5 About 98% of cases of right heart thrombi are associated with concurrent pulmonary emboli.5
The treatment options for RV thrombus include anticoagulation, thrombolysis, and surgical thrombectomy. Optimal management remains unclear, as there are no randomized trials evaluating these modalities. Overall, the existing literature has shown conflicting evidence, with no clear consensus on management recommendations.2 Several studies have suggested a mortality benefit using a more aggressive approach with either thrombolysis or surgery.6 In 2015 Athappan et al.6 performed a pooled analysis of 328 patients showing that treatment with anticoagulation alone was associated with significantly greater mortality compared with surgery or thrombolysis.6 Other studies have shown better outcomes with use of thrombolysis, specifically over anticoagulation alone or surgery.3, 5 In 2002, Rose et al.5 performed a retrospective analysis of 177 reported cases of right heart thrombus, which showed that thrombolytic therapy was associated with significant improvement in mortality compared with either anticoagulation alone or surgery.5 There are also reports of similar mortality among all three treatments, as well as multiple cases managed successfully with anticoagulation alone.4, 7, 8, 9
This case presented a particularly difficult and complex therapeutic dilemma. The patient presented with severely elevated right heart pressures and RV dysfunction, suggesting a tenuous clinical status and high risk for hemodynamic instability. This was complicated further by a pelvic malignancy and PFO with resulting cerebral infarcts. There was concern that thrombolysis could lead to hemorrhagic conversion of her strokes. Studies have shown that in patients treated with thrombolysis for acute ischemic stroke, the rate of hemorrhagic transformation was 11% to 44%, and the rate of symptomatic hemorrhagic transformation was 6% to 20%.10, 11 Immediate surgery was also suboptimal because anticoagulation would have to be held in the setting of significant pulmonary embolic burden, a large RV thrombus, and significant right heart strain. In general, surgical embolectomy is known to carry a high risk for mortality. A 2017 systematic review of 56 studies and 1,579 patients who underwent surgical embolectomy revealed an in-hospital mortality rate of 26%.12 There was concern that either thrombolysis or surgery could cause the thrombus to dislodge, leading to hemodynamic compromise. Given the potentially poor outcomes and the patient's initial improvement in right heart pressures and thrombus size, a decision was made to start with anticoagulation alone. The patient's clinical improvement while on enoxaparin instead of rivaroxaban raised the question of whether low–molecular weight heparin is better for the management of venous thromboembolism (VTE) in the setting of malignancy. Unfortunately the patient developed recurrent acute pulmonary emboli after anticoagulation was interrupted for resection of the pelvic mass. In studies evaluating patients with cancer on anticoagulation, the rates of recurrent VTE over a 6- to 12-month period have been approximately 10% to 17% with vitamin K antagonists, 6% to 9% with low–molecular weight heparin, and 4% to 7% with novel oral anticoagulants.13, 14 One notable 2018 study randomized 406 patients to either rivaroxaban or dalteparin and found a significantly lower rate of recurrent VTE at 6-month follow-up in those on rivaroxaban, but at the cost of more bleeding.13 This patient with an ongoing, unresected RV thrombus was at particularly high risk because anticoagulation was interrupted and because malignancy and recent VTE in the past 3 months are known risk factors for recurrent thrombus.15 Because of the patient's worsening right heart pressures and continued high risk for pulmonary and systemic emboli, a multidisciplinary decision was made to proceed with surgery. The RV thrombus was surgically removed and the PFO was closed without any complications. Postoperatively and after discharge, the patient improved symptomatically and remained clinically stable, and serial echocardiography showed improving right heart pressures.
Despite this successful outcome, the optimal treatment for right heart thrombi remains uncertain and warrants additional studies. Until there is more definitive evidence, management decisions should be made on a case-by-case basis, with careful consideration of complicating factors such as hemodynamic instability, right heart function, PFO, and malignancy. This patient's stability allowed us to proceed with a trial of medical therapy first and defer aggressive intervention. Once medical therapy had failed, it became clear that she needed definitive reperfusion therapy. Given the presence of PFO and risk for recurrent systemic emboli, surgical thrombectomy with concomitant PFO closure was pursued. It is also important to recognize that recurrent unprovoked pulmonary emboli in anticoagulated patients should raise suspicion for malignancy. This patient's pelvic mass likely contributed significantly to the progression of her thrombus burden. The presence of malignancy should be carefully considered when assessing the risk for worsening thromboembolic disease and deciding on more aggressive treatment.
Conclusion
This was a case of RV thrombus in transit from deep vein thrombus in the setting of malignancy, complicated by recurrent pulmonary emboli and embolic stroke, that was successfully managed with surgical resection. However, the optimal treatment for right heart thrombi remains uncertain, and management decisions should be made on a case-by-case basis with multidisciplinary coordination. Providers should carefully consider complicating factors such as hemodynamic instability, RV dysfunction, PFO, and stroke. In particular, malignancy should be suspected in anticoagulated patients with recurrent pulmonary emboli and should be a significant factor in guiding more aggressive intervention.
Footnotes
Conflicts of interest: The authors reported no actual or potential conflicts of interest relative to this document.
Supplementary data related to this article can be found at https://doi.org/10.1016/j.case.2019.05.006.
Supplementary Data
Transesophageal echocardiographic midesophageal 54° RV inflow-outflow view showing large RV thrombus.
Transesophageal echocardiographic midesophageal 117° long-axis view showing large RV thrombus.
Transesophageal echocardiographic midesophageal 0° three-dimensional view showing large RV thrombus.
References
- 1.Agarwal V., Nalluri N., Shariff M.A., Akhtar M.S., Olkovsky Y., Kitsis P.E. Large embolus in transit—an unresolved therapeutic dilemma (case report and review of literature) Heart Lung. 2014;43:152–154. doi: 10.1016/j.hrtlng.2013.08.005. [DOI] [PubMed] [Google Scholar]
- 2.Otoupalova E., Dalal B., Renard B. Right heart thrombus in transit: a series of two cases. Crit Ultrasound J. 2017;9:14. doi: 10.1186/s13089-017-0069-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nkoke C., Faucher O., Camus L., Flork L. Free floating right heart thrombus associated with acute pulmonary embolism: an unsettled therapeutic difficulty. Case Rep Cardiol. 2015;2015:364780. doi: 10.1155/2015/364780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chartier L., Bera J., Delomez M., Asseman P., Beregi J.P., Bauchart J.J. Free-floating thrombi in the right heart: diagnosis, management, and prognostic indexes in 38 consecutive patients. Circulation. 1999;99:2779–2783. doi: 10.1161/01.cir.99.21.2779. [DOI] [PubMed] [Google Scholar]
- 5.Rose P.S., Punjabi N.M., Pearse D.B. Treatment of right heart thromboemboli. Chest. 2002;121:806–814. doi: 10.1378/chest.121.3.806. [DOI] [PubMed] [Google Scholar]
- 6.Athappan G., Sengodan P., Chacko P., Gandhi S. Comparative efficacy of different modalities for treatment of right heart thrombi in transit: a pooled analysis. Vasc Med. 2015;20:131–138. doi: 10.1177/1358863X15569009. [DOI] [PubMed] [Google Scholar]
- 7.Mollazadeh R., Ostovan M.A., Abdi Ardekani A.R. Right cardiac thrombus in transit among patients with pulmonary thromboemboli. Clin Cardiol. 2009;32:E27–E31. doi: 10.1002/clc.20386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Barrios D., Chavant J., Jiménez D., Bertoletti L., Rosa-Salazar V., Muriel A. Treatment of right heart thrombi associated with acute pulmonary embolism. Am J Med. 2017;130:588–595. doi: 10.1016/j.amjmed.2016.11.027. [DOI] [PubMed] [Google Scholar]
- 9.Charif F., Mansour M.J., Hamdan R., Najjar C., Nassar P., Issa M. Free-floating right heart thrombus with acute massive pulmonary embolism: a case report and review of the literature. J Cardiovasc Echogr. 2018;28:146–149. doi: 10.4103/jcecho.jcecho_64_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jaillard A., Cornu C., Durieux A., Moulin T., Boutitie F., Lees K.R., MAST-E Group Hemorrhagic transformation in acute ischemic stroke. The MAST-E study. Stroke. 1999;30:1326–1332. doi: 10.1161/01.str.30.7.1326. [DOI] [PubMed] [Google Scholar]
- 11.Zhang J., Yang Y., Sun H., Xing Y. Hemorrhagic transformation after cerebral infarction: current concepts and challenges. Ann Transl Med. 2014;2:81. doi: 10.3978/j.issn.2305-5839.2014.08.08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kalra R., Bajaj N.S., Arora P., Arora G., Crosland W.A., McGiffin D.C. Surgical embolectomy for acute pulmonary embolism: systematic review and comprehensive meta-analyses. Ann Thorac Surg. 2017;103:982–990. doi: 10.1016/j.athoracsur.2016.11.016. [DOI] [PubMed] [Google Scholar]
- 13.Al-Samkari H., Connors J.M. The role of direct oral anticoagulants in treatment of cancer-associated thrombosis. Cancers. 2018;10:271. doi: 10.3390/cancers10080271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang T.F., Li A., Garcia D. Managing thrombosis in cancer patients. Res Pract Thromb Haemost. 2018;2:429–438. doi: 10.1002/rth2.12102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kearon C., Hirsh J. Management of anticoagulation before and after elective surgery. N Engl J Med. 1997;336:1506–1511. doi: 10.1056/NEJM199705223362107. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Transesophageal echocardiographic midesophageal 54° RV inflow-outflow view showing large RV thrombus.
Transesophageal echocardiographic midesophageal 117° long-axis view showing large RV thrombus.
Transesophageal echocardiographic midesophageal 0° three-dimensional view showing large RV thrombus.