Abstract
Implementing uncontrolled donation after circulatory determination of death (uDCDD) in the United States could markedly improve supply of donor lungs for patients in need of transplants. Evidence from U.S. pilot programs suggests families support uDCDD, but only if they are asked permission for using invasive organ preservation procedures prior to initiation. However, non-invasive strategies that confine oxygenation to lungs may be applicable to the overwhelming majority of potential uDCDD donors that have airway devices in place as part of standard resuscitation. We propose an ethical framework for lung uDCDD by: (1) initiating post mortem preservation without requiring prior permission to protect the opportunity for donation until an authorized party can be found; (2) using non-invasive strategies that confine oxygenation to lungs; and (3) maintaining strict separation between the healthcare team and the organ preservation team. Attempting uDCDD in this way has great potential to obtain more transplantable lungs while respecting donor autonomy and family wishes, securing public support, and enabling authorized persons to affirm or cease preservation decisions without requiring evidence of prior organ donation intent. It ensures prioritization of life-saving, the opportunity to allow willing donors to donate, and respect for bodily integrity while adhering to current ethical norms.
Despite the growth in the number of lung transplants performed in the United States, demand far exceeds organ supply. According to 2017 OPTN data, there were 2,439 lung transplants. Insufficient supply left over 1,462 patients waiting for this lifesaving procedure resulting in 194 deaths and 141 becoming too sick for transplantation while waiting. These numbers are inclusive of recipients who, as their conditions deteriorate, choose to receive lungs from less than ideal donors (i.e. older donors, donors with significant smoking history). To meet the overwhelming demand, one strategy is to expand the donor pool to include lung donation when death occurs unexpectedly in or out of hospital settings, what is often termed uncontrolled donation after circulatory determination of death (uDCDD). Widespread dissemination of uDCDD programs could markedly improve supply of donor lungs for patients in need of transplants. The objective of this paper is to propose an ethical framework for transplant programs to facilitate lung uDCDD in the United States.
Since 2000, European countries have utilized uDCDD to recover kidneys, livers, and lungs, leading to shorter wait times and thousands more saved lives (1). Foreign studies suggest uDCDD lung transplant outcomes are within acceptable range with some achieving results similar to BDD (2–4). Given the potential for saving lives, why has lung uDCDD not been widely utilized throughout the United States? Part of the reason stems from earlier unsuccessful uDCDD efforts.
uDCDD progress in the United States began with an in-hospital kidney program in Washington D.C. that achieved success through partnership with communities whereby the “city council for the District of Columbia amended the Uniform Anatomical Gift Act to allow initiation of in situ cold perfusion pending consent for organ donation, when families were not immediately available (5).” In three years of activity (1995–1997), kidneys from 19 donors who died from traumatic causes in the emergency department and surgical intensive care unit were recovered; 32 kidneys were successfully transplanted. The program ended due to insufficient funding and amid controversy after a deceased patient who had opted out of the program was mistakenly enrolled in the study.
In 2007, HRSA funded uDCDD pilot programs in NYC and Pittsburgh that used opt-in frameworks (6, 7). In NYC, government and community stakeholders established an out-of-hospital uDCDD program for cases when termination of resuscitation (TOR) decisions occurred in private residences (Figure 1: Maastricht type IIA). Legal considerations permitted home entry after TOR only if persons had previously consented to organ donation. From December 2010-May 2011, a dedicated organ preservation unit (OPU) approached 9 eligible donors after TOR decisions were made by treating Fire Department City of New York Emergency Medical Services providers who were unaware of OPU availability. No decedent had proof of prior authorization for donation. Operations notes revealed that all persons authorized to make organ donation decisions who were approached were not offended by being asked about organ donation soon after witnessing a loved one’s unexpected death. Four expressed disappointment at being excluded from donating a loved one’s organs (8). Pittsburgh investigators organized a dedicated rapid response team called Condition T to capture Maastricht II donors in hospitals. Condition T was activated for immediate donor preservation using acellular cold perfusion when registered donors were pronounced dead after failed resuscitation. In three years, only three kidneys and one liver were recovered from two donors; all organs failed biopsy tests after cold perfusion (6).
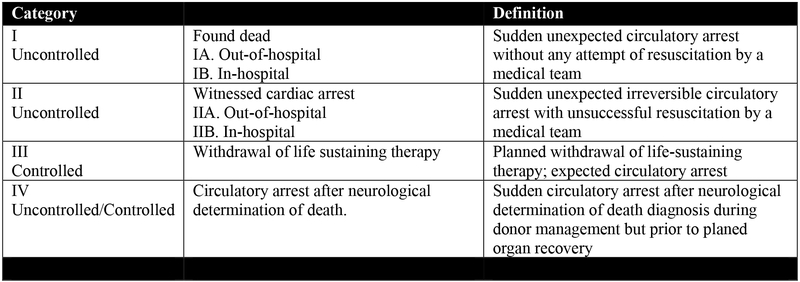
Figure 1. The Modified Maastricht Classification of DCD.
– Thuong et al, Transplant International 2016; 29: 749–759 (30).
uDCDD lung programs in the United States also had similar challenges. From September 2013-March 2016, a uDCDD lung program in North Carolina, funded by the U.S. National Institutes of Health, attempted to capture lungs from Maastricht IIA and IIB donors in coordination with EMS and hospital emergency departments (9). Mechanical ventilation post mortem was used for initial lung preservation. Lungs were recovered from 31 donors. Of these, 18 underwent ex-vivo lung perfusion (EVLP) with three passing performance testing. However, logistical and administrative barriers precluded these lungs from being transplanted. The University of Pennsylvania reported the first success with uDCDD lung transplantation in the United States from a donor with devastating brain injury who arrested prior to a second, confirmatory brain death evaluation (Maastricht IV) (10). Organs were preserved with continued cardiopulmonary support post mortem.
Why the mostly unsuccessful results? The main barriers to realizing transplants from the U.S. uDCDD experiences were requiring prior authorization for organ donation and restrictions on using in-situ normothermic extracorporeal membrane oxygenation (nECMO) for post-mortem kidney and liver preservation (11). To avoid organ degradation, uDCDD requires rapid initiation of organ preservation soon after unexpected deaths. In countries that operate under “opt-out” frameworks for donation – meaning that citizens are considered organ donors by default unless they explicitly opt out – there are lower barriers to implementing uDCDD programs. European protocols permit preservation efforts, such as femoral artery and vein cannulation to establish nECMO for preserving kidneys and livers, and bilateral chest tube insertion for administering cold preservation solutions for lung protection, without requiring explicit authorization from persons authorized to make organ donation decisions for the deceased; however, coroner and judicial authorizations from dedicated on-call personnel are required to proceed (12). Even countries that operate under opt-out frameworks for organ donation grant family members opportunities to object to donation after preservation is initiated, yet there are significantly fewer family objections to all forms of donation in opt-out countries than there are in the United States (13).
In the United States, organ “donation” rests on the norm that organs are a gift that one chooses whether or not to give. In opt-in countries like the United States, authorization is from donors who have specified their intent through registered wishes. When donation preferences are not registered, donation authorization must be obtained from family members or other identified authorized parties who, ideally, make their decisions based on what they believe the deceased would have wanted (14). At first glance, this framework appears inconsistent with uDCDD requirements, which include performing organ preservation rapidly on the newly deceased.
The consequences of preserving organs prior to permission must be weighed against choosing not to do so. If preservation is not initiated in time, then the opportunity to donate is lost, which might run counter to what the deceased and their family members would have wanted. Preserving prior to obtaining permission will either result in continued preservation and donation in accordance with donor/family wishes, or in the termination of preservation efforts if it is discovered that the deceased registered their wishes not to be a donor or if the person authorized to make donation decisions for the deceased objects.
There are international variations in acceptable uDCDD practice and related defintions. Although studies have shown that the U.S. public is largely supportive of uDCDD, the vast majority would require family permission specifically prior to invasive procedures performed strictly for organ preservation (7, 15). This requirement is grounded in concerns about treating the body respectfully after death and maintaining bodily integrity (7, 16). However, newer techniques for initial uDCDD lung preservation are notably non-invasive when compared to what are now standard procedures for preserving lungs and other organs, thus respecting the body and preserving bodily integrity. Use of non-invasive initial preservation measures would enable uDCDD lung preservation to work within ethical norms in the United States, provided an understanding that the family can still subsequently decide not to donate.
Instead of inserting chest tubes for cold in situ perfusion (3, 4, 17), recent uDCDD lung protocols advocate using post mortem mechanical ventilation for initial lung preservation (9, 18). Novel controlled DCDD protocols, translated from animal models, recently have preserved lungs for up to three hours after circulatory-respiratory arrest using positive end expiratory pressure [PEEP] to partially inflate the lungs without ventilation, with improvement in function by placing the body in prone positioning (19, 20). These non-invasive strategies are applicable to the overwhelming majority of potential donors that have airway devices already in place as part of standard cardiopulmonary resuscitation practice, and their use avoids the need for additional invasive procedures for which the public desires prior permission. Lung preservation is only initiated after failed resuscitation and declaration of death, and can be easily discontinued without any physical impact on the body. Therefore, because these new protocols respect the body, add no new invasive interventions and respect the autonomy of the family to act as organ donors, authorized persons and family might be far more accepting of lung preservation being initiated without requiring explicit permission. Continuing ventilation or partially inflating the lungs with the body in prone positioning merely preserves the future opportunity for donation, nothing more. Including cases without advanced airway devices having been used during the resuscitation could exacerbate medical mistrust, since their use would be solely for organ preservation.
Another concern with uDCDD is whether the application of organ preserving measures renders the preceding death determination invalid. The current evolution of death determination in the United States requires a “permanent” cessation of circulation, which leads to “irreversible” loss of total brain function. The justification of the standard is that circulatory function will not be restored because it will neither return spontaneously, nor return as a result of medical intervention because no resuscitation efforts will be attempted (11, 21). Because technologies such as nECMO applied for organ preservation have the potential to circulate oxygenated blood to the brain, some vocal detractors of using regional perfusion for organ preservation are concerned that using these interventions could undermine the permanent cessation of brain circulation rendering the prior determination of death invalid since “once death has been determined, no procedure that may resume brain circulation should be used, including cardiopulmonary resuscitation, artificial ventilation, and extra-corporeal membrane oxygenation” (11). Regional perfusion measures that use balloon catheters inserted after death determination and before nECMO initiation to prevent brain perfusion - an accepted procedure in countries with strong uDCDD programs that exist with opt out organ donation functioning within universal healthcare systems (22) - may not assuage the ethical concerns of those elsewhere who believe nECMO should not be used after death is declared. For these opponents, the problem is not whether brain perfusion is prevented, but whether doing so makes health providers complicit in the patient’s death, and is a technical work-around to avoid the fact that nECMO use would undermine the legal brain death determination (11, 21). Others do not agree with these analyses – as restoring some circulation to the brain after prolonged, exhaustive resuscitation attempts are unsuccessful, is exceedingly unlikely to restore meaningful neurological function (22) – but the concern is moot in considerations of lung uDCDD.
Emerging lung uDCDD protocols that solely partially inflate the lungs with PEEP in prone positioning confine oxygen delivery to the lungs thus completely avoiding any concern about recirculating oxygenated blood to the brain post mortem. Use of mechanical ventilation without circulating blood also confines oxygenation to the lungs. Each form of preservation only requires providing lung oxygenation through the oral airway without additional invasion of the body.
Some experts fear that allowing uDCDD might create incentives to either cease life saving measures early or not perform them fully in order to obtain lungs (23). An “integrated” approach to life saving measures and uDCDD preservation is operating in Portugal wherein the same clinicians perform both subject to an external office’s transition authorization. Portugal, as in other countries with successful uDCDD programs, offers universal healthcare and has an opt out organ donation system. Unfortunately, in the U.S., there remains significant (although unsupported by evidence) concern that registered organ donors receive less aggressive life-saving care (24). The pervasive mistrust with organ donation is reflective of mistrust with the U.S. healthcare system that does not provide equitable universal coverage. Until such misgivings are adequately addressed, medical practitioners should remain separate and shielded from organ preservation/procurement responders if uDCDD is to succeed in the United States. Separation ensures that treating providers are protected from any conflict of interest and will commit themselves fully to life-saving efforts. Further assurance of quality life-saving care and certainty of futility determination is aided in some countries by physicians on the EMS team, which might be considered for implementation in the United States; however, for integrated systems to exist, TOR decisions would need be made in hospitals where a separate team would be called in for transitioning to organ preservation. As additional assurance that separation is in no way breached, medical systems preparing to institute uDCDD should establish independent, educated, and transparent data safety monitoring boards (DSMBs) that are able to quickly convene to review resuscitation and organ preservation case data for compliance with standard resuscitation and TOR guidelines prior to lung preservation consideration (8).
Dissemination of nECMO for cardiopulmonary resuscitation in emergency departments and out-of-hospital settings has raised concerns that allowing uDCDD in cases where nECMO is not available does not incentivize future development of eCPR capacity and prioritizes organ preservation for donation over life sustaining efforts (21, 25). We disagree. nECMO for cardiac resuscitation is a resource intensive technology with equipoise in terms of selection of appropriate patients, duration, and outcomes (26). Forbidding uDCDD lung donation because an experimental intervention of uncertain benefit is unavailable (as in most clinical settings) is counter to the wishes of many willing donors and neglects those in dire need of life saving lung transplants. When eCPR is unavailable or contraindicated, lung uDCDD should be performed, so long as protocols are established in patients with demonstrated lack of spontaneous circulation/respiration, no invasive steps are taken, and with preservation methods that have no potential to circulate oxygen to the brain post mortem. When eCPR is available, as in the Portuguese protocol, strict guidelines should be implemented to direct practitioners when to use nECMO for resuscitation versus initiating organ preservation (27). If eCPR should fail, cases may be eligible for donation after neurological determination of death or cDCDD with thoughtful guidelines established based on clinical evidence dictating when it is clinically appropriate to disconue eCPR procedures. Establishing an on-call independent DSMB for immediate consultations would help ensure that patient care is always prioritized over organ preservation.
Another issue regarding lung uDCDD concerns the period of observation after resuscitation efforts are completed. Studies have examined the resuscitation duration in context of auto-resuscitation -- when a heart stops beating spontaneously and restarts -- and have shown cases of auto-resuscitation up to 10 minutes from TOR in adults and 2 minutes in children (28). Some authorities have questioned the requirement for “no-touch” periods, given the movement towards brain-based definitions of death with events preceding auto-resuscitation and its duration being inadequate to reverse the pending permanent and irreversible loss of brain function (29). Others follow a 5 minute no touch period. Given prudence, uDCDD lung protocols should follow a 5–10-minute “no-touch” period after TOR decisions are made to fulfill permanence/irreversibility criteria with timing considerations based on cultural norms and standard practice. Given the duration of resuscitation for uDCDD eligibility is at least 30 minutes, the likelihood of autoresuscitation is nearly negligible; in the unlikely event that autoresuscitation occurs, continuous monitoring of that event may be applied as is common practice in successful uDCDD programs (12, 22).
Previous uDCDD programs in the United States have relied on registered donors to avoid the challenge of obtaining authorization quickly enough for organ preservation, and have largely failed because of insufficient enrollment (6, 8, 9). The National Academy of Medicine originally advocated for presumed authorization for organ preservation in uDCDD citing the success of European protocols, but has since backed away from this position. But lungs are unique. Public disapproval of other organ preservation efforts without permission should not apply to new uDCDD lung preservation efforts because they are non-invasive, do not undermine the legal determination of death, and are easily stopped without any negative impact on the deceased.
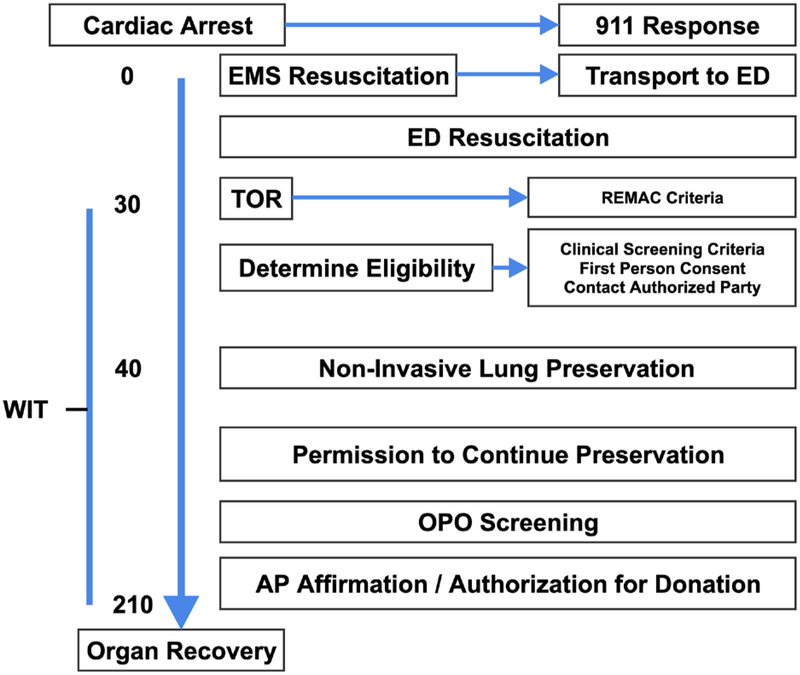
Lung uDCDD could have marked impact on the number of procured organs and number of lives saved while maintaining rigorous adherence to accepted life-saving and death determination protocols. These facts support the application of the lung uDCDD authorization process in Figure 2 and protocol timeline in Figure 3. The strategy for postmortem lung preservation has been successful in pre-clinical studies; therefore, translational research, including organ preservation optimization studies in clinical settings, is required prior to implementation. The protocol accepts cases only if there is potential to complete initial preservation and procedures within three hours of TOR. Planned viability assessments include macroscopic determination, radiological procedures (X-ray and Computed Tomography), fiber optic bronchoscopy, and Ex-Vivo lung perfusion. Post transplant outcomes are assessed using standard monitoring advocated by the International Society for Heart and Lung Transplantation including primary and delayed graft dysfunction.
Figure 2:
uDCDD LUNG AUTHORIZATION PROCESS
Figure 3. Sample Protocol Timeline.
- AP – Authorized Party; ED – Emergency Department; EMS – Emergency Medical Services; OPO – Organ Procurement Organization (NYODN); REMAC - Regional Emergency Medical Advisory Committee; TOR – Termination of Resuscitation; WIT – Warm Ischemic Time 180 minutes maximum allowable that is inclusive of a 5–10 minute hands off period after a TOR decision is made; Affirmation - act of stating positively, with confidence, or testifying, that the deceased would have desired this end of donation for those having previously registered for organ donation. An example of clinical screening criteria is described in Steen et al, 2003 (16).
In the United States, where authorization for organ donation is seen as an act of altruism, it is understandable why family members informed about organ preservation without their permission might be fearful that responders did not prioritize life-saving measures. This is why the establishment of uDCDD protocols and policy must be developed and evaluated with input from all stakeholders in program implementation, including secular and religious community organizations representing the population being served. The public must be informed of how uDCDD could lower organ waiting times and increase saved lives, how emergency responders adhere to strict life-saving protocols and how organ preservation and procurement teams would not be involved until after a prior independent determination that continued life-saving measures are futile and death has been pronounced. Success from transplanting uDCDD lungs with a more restrictive approach should hopefully lead the public to seek greater opportunities for donation of other organs. Since preservation methods for uDCDD lungs are noninvasive, easily reversed, do not involve circulatory measures that might challenge the determination of death, and preserve the right of authorized family members to participate in donation, the expansion of uDCDD as a possible source of donor lungs respects the current values surrounding organ donation and does not evoke concerns that some have raised about other forms of uDCDD organ procurement. Although previous uDCDD efforts, public concerns, and ethical considerations unique to the United States all inform the protocol described here, this effort must be continuously developed and refined in collaboration with community stakeholders to ensure transparency and public acceptance. Preservation of uDCDD lungs ought to be vigorously pursued in countries with opt-in systems including the United States, as a step that can hopefully lead toward ethical frameworks for uDCDD protocols for other organs.
Acknowledgments
Stephen P. Wall was supported in part by RO1DK098610 from the U.S. National Institutes of Health (NIH) National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) – PI Wall. Other authors received no financial support for the development of this article.
Abbreviations:
- BDD
brain death donors
- DSMBs
data safety monitoring boards
- EVLP
ex-vivo lung perfusion
- nECMO
normothermic extracorporeal membrane oxygenation
- OPU
organ preservation unit
- TOR
termination of resuscitation
- uDCDD
uncontrolled donation after circulatory determination of death
Footnotes
Disclosure
The authors of this manuscript have no conflicts of interest to disclose as described by the American Journal of Transplantation.
Sources:
- 1.Dominguez-Gil B, Duranteau J, Mateos A, Nunez JR, Cheisson G, Corral E, et al. Uncontrolled donation after circulatory death: European practices and recommendations for the development and optimization of an effective programme. Transpl Int. 2016;29(8):842–59. [DOI] [PubMed] [Google Scholar]
- 2.Cypel M, Levvey B, Van Raemdonck D, Erasmus M, Dark J, Love R, et al. International Society for Heart and Lung Transplantation Donation After Circulatory Death Registry Report. J Heart Lung Transplant. 2015;34(10):1278–82. [DOI] [PubMed] [Google Scholar]
- 3.Suberviola B, Mons R, Ballesteros MA, Mora V, Delgado M, Naranjo S, et al. Excellent long-term outcome with lungs obtained from uncontrolled donation after circulatory death. American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2019;19(4):1195–201. [DOI] [PubMed] [Google Scholar]
- 4.Valdivia D, Gomez de Antonio D, Hoyos L, Campo-Canaveral de la Cruz JL, Romero A, Varela de Ugarte A. Expanding the horizons: Uncontrolled donors after circulatory death for lung transplantation-First comparison with brain death donors. Clin Transplant. 2019:e13561. [DOI] [PubMed] [Google Scholar]
- 5.Light JA. The Washington, D.C. experience with uncontrolled donation after circulatory determination of death: promises and pitfalls. The Journal of law, medicine & ethics : a journal of the American Society of Law, Medicine & Ethics. 2008;36(4):735–40, 610. [DOI] [PubMed] [Google Scholar]
- 6.DeVita MA, Callaway CW, Pacella C, Brooks MM, Lutz J, Stuart S Experience With a New Process - Condition T - for Uncontrolled Donation After Circulatory Determination of Death in a University Emergency Department. Prog Transplant. 2016;26(1):21–7. [DOI] [PubMed] [Google Scholar]
- 7.Wall SP, Kaufman BJ, Gilbert AJ, Yushkov Y, Goldstein M, Rivera JE, et al. Derivation of the uncontrolled donation after circulatory determination of death protocol for New York City. American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2011;11(7):1417–26. [DOI] [PubMed] [Google Scholar]
- 8.Wall SP, Kaufman BJ, Williams N, Norman EM, Gilbert AJ, Munjal KG, et al. Lesson From the New York City Out-of-Hospital Uncontrolled Donation After Circulatory Determination of Death Program. Annals of emergency medicine. 2016;67(4):531–7 e39. [DOI] [PubMed] [Google Scholar]
- 9.Egan T, Blackwell J, Birchard K, Haithcock B, Long J, Gazda S, et al. Assessment of Lungs for Transplant Recovered from Uncontrolled Donation after Circulatory Determination of Death Donors. Ann Am Thorac Soc. 2017;14(Supplement_3):S251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Suzuki Y, Tiwari JL, Lee J, Diamond JM, Blumenthal NP, Carney K, et al. Should we reconsider lung transplantation through uncontrolled donation after circulatory death? American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2014;14(4):966–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bernat JL, Bleck TP, Blosser SA, Bratton SL, Capron AM, Cornell D, et al. Circulatory death determination in uncontrolled organ donors: a panel viewpoint. Annals of emergency medicine. 2014;63(4):384–90. [DOI] [PubMed] [Google Scholar]
- 12.Minambres E, Rubio JJ, Coll E, Dominguez-Gil B Donation after circulatory death and its expansion in Spain. Curr Opin Organ Transplant. 2018;23(1):120–9. [DOI] [PubMed] [Google Scholar]
- 13.Mahillo B, Carmona M, Alvarez M, White S, Noel L, Matesanz R 2009 global data in organ donation and transplantation: activities, laws, and organization. Transplantation. 2011;92(10):1069–74. [DOI] [PubMed] [Google Scholar]
- 14.Siminoff LA, Agyemang AA, Traino HM. Consent to organ donation: a review. Prog Transplant. 2013;23(1):99–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Volk ML, Warren GJ, Anspach RR, Couper MP, Merion RM, Ubel PA. Attitudes of the American public toward organ donation after uncontrolled (sudden) cardiac death. American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2010;10(3):675–80. [DOI] [PubMed] [Google Scholar]
- 16.Morgan SE, Harrison TR, Afifi WA, Long SD, Stephenson MT. In their own words: the reasons why people will (not) sign an organ donor card. Health Commun. 2008;23(1):23–33. [DOI] [PubMed] [Google Scholar]
- 17.Steen S, Liao Q, Wierup PN, Bolys R, Pierre L, Sjoberg T Transplantation of lungs from non-heart-beating donors after functional assessment ex vivo. Ann Thorac Surg. 2003;76(1):244–52; discussion 52. [DOI] [PubMed] [Google Scholar]
- 18.Valenza F, Citerio G, Palleschi A, Vargiolu A, Fakhr BS, Confalonieri A, et al. Successful Transplantation of Lungs From an Uncontrolled Donor After Circulatory Death Preserved In Situ by Alveolar Recruitment Maneuvers and Assessed by Ex Vivo Lung Perfusion. American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2016;16(4):1312–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kon Z Personal Communication with Marcelo Cypel, MD, Associate Professor Thoracic Surgery, University of Toronto. 2019. [Google Scholar]
- 20.Watanabe Y, Galasso M, Watanabe T, Ali A, Qaqish R, Nakajima D, et al. Donor prone positioning protects lungs from injury during warm ischemia. American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2019. [DOI] [PubMed] [Google Scholar]
- 21.Dalle Ave AL, Shaw DM, Bernat JL. Ethical Issues in the Use of Extracorporeal Membrane Oxygenation in Controlled Donation After Circulatory Determination of Death . American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2016;16(8):2293–9. [DOI] [PubMed] [Google Scholar]
- 22.Molina M, Dominguez-Gil B, Perez-Villares JM, Andres A Uncontrolled donation after circulatory death: ethics of implementation. Curr Opin Organ Transplant. 2019;24(3):358–63. [DOI] [PubMed] [Google Scholar]
- 23.Ortega-Deballon I, Hornby L, Shemie SD. Protocols for uncontrolled donation after circulatory death: a systematic review of international guidelines, practices and transplant outcomes. Crit Care. 2015;19:268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Patel MS, Raza SS, Bhakta A, Ewing T, Bukur M, Vagefi PA, et al. Patients on state organ donor registries receive similar levels of intensive care compared to those who are not: an opportunity to increase public intent to donate. Clin Transplant. 2016;30(6):682–7. [DOI] [PubMed] [Google Scholar]
- 25.Dalle Ave AL, Bernat JL. Uncontrolled Donation After Circulatory Determination of Death: A Systematic Ethical Analysis. J Intensive Care Med. 2018;33(11):624–34. [DOI] [PubMed] [Google Scholar]
- 26.Brooks SC, Shemie SD, Torrance S, Hornby L, Gillrie C, Grunau B, et al. Barriers and opportunities related to extracorporeal cardiopulmonary resuscitation for out-of-hospital cardiac arrest in Canada: A report from the first meeting of the Canadian ECPR Research Working Group. CJEM. 2018;20(4):507–17. [DOI] [PubMed] [Google Scholar]
- 27.Prabhu A, Parker LS, DeVita MA. Caring for Patients or Organs: New Therapies Raise New Dilemmas in the Emergency Department. Am J Bioeth. 2017;17(5):6–16. [DOI] [PubMed] [Google Scholar]
- 28.Hornby L, Dhanani S, Shemie SD. Update of a Systematic Review of Autoresuscitation After Cardiac Arrest. Critical care medicine. 2018;46(3):e268–e72. [DOI] [PubMed] [Google Scholar]
- 29.Veatch RM. Are Organ Donors Really Dead: The Near-Irrelevance of Autoresuscitation. Am J Bioeth. 2018;18(8):1–2. [DOI] [PubMed] [Google Scholar]
- 30.Thuong M, Ruiz A, Evrard P, Kuiper M, Boffa C, Akhtar MZ, et al. New classification of donation after circulatory death donors definitions and terminology. Transpl Int. 2016;29(7):749–59. [DOI] [PubMed] [Google Scholar]