Graphical abstract
Keywords: Superior vena cava, Saccular aneurysm, Venolymphatic malformation, Sirolimus
Highlights
-
•
Superior vena cava saccular aneurysms are a rare clinical entity.
-
•
Diagnosis of superior vena cava aneurysm includes multiple advanced imaging modalities.
-
•
Management options for venolymphatic malformations.
-
•
Symptoms were reduced following surgical resection and treatment with sirolimus.
Introduction
Superior vena cava (SVC) aneurysms are a rare clinical entity. Diagnosis is completed with advanced imaging, and treatment remains controversial, often dictated by the size and morphologic classification of the aneurysm as either saccular or fusiform. Fusiform aneurysms tend to receive conservative medical management, whereas saccular aneurysms often require prophylactic surgical resection to prevent rupture, thrombosis, or venous obstruction. We present a case of a large saccular SVC aneurysm causing shortness of breath in an otherwise healthy 36-year-old woman.
Case Presentation
A 36-year-old white woman came to urgent care with new-onset shortness of breath and chest wall discomfort. She had a history of asthma and had delivered a healthy baby girl by cesarean section 5 days prior. A chest radiograph was obtained that revealed a large right perihilar mass measuring 8.4 × 7.5 × 6.9 cm (Figure 1).
Figure 1.
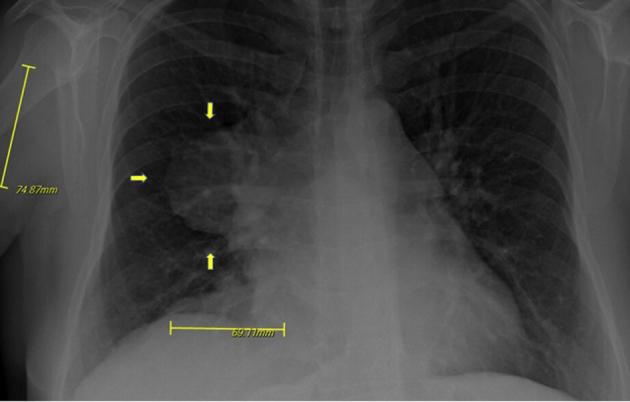
Chest radiograph with arrows indicating a large right perihilar mass measuring 8.4 cm anteroposterior × 7.5 cm cephalocaudal × 6.9 cm transverse.
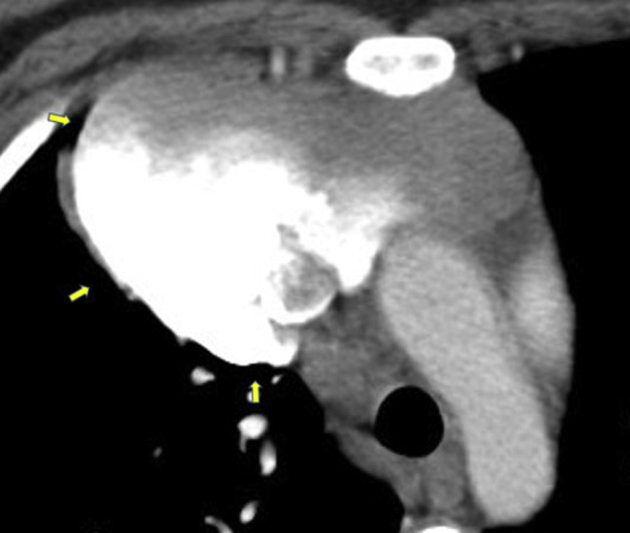
Subsequent chest computed tomographic (CT) imaging revealed right jugular vein and SVC thromboses (Figure 2) and a large transspatial hypervascular superior mediastinal mass with extensive lobular soft tissue densities measuring 12.2 × 8.0 × 10.8 cm that partially encircled the SVC and extended toward the perihepatic space (Figures 3 and 4). The patient was started on a heparin drip for treatment of thrombi and admitted to the hospital. The initial differential diagnosis included metastatic gestational trophoblastic disease and vascular malformation. Results of a β human chorionic gonadotropin measurement showed a level of 280 U/L, trending downward, effectively ruling out gestational trophoblastic disease. Chest CT venography revealed filling defects to the large mediastinal mass (Figure 5) and the presence of a right lower lobe pulmonary embolism. Cardiothoracic surgery was consulted and recommended magnetic resonance imaging of the chest and abdomen. Magnetic resonance imaging revealed a large lobular soft tissue lesion within the chest and abdomen, appearing to have direct communication with the SVC, presence of numerous calcified phleboliths, and infiltrative appearance likely representing a vascular anomaly (Figure 6). Transthoracic echocardiography revealed normal right and left ventricular size and function, a mildly dilated left atrium, and an irregular, inhomogeneous, lobulated mass visualized over the anterior mediastinal space and adjacent to the SVC (Videos 1 and 2). Gated cardiac CT scan revealed the aforementioned mediastinal anomaly with the upper cystic section of the enhancing mass arising from the junction of the brachiocephalic veins with appearance of an SVC saccular aneurysm (Figure 7). The patient clinically improved while on heparin and was subsequently started on apixaban for planned 3-month treatment for provoked pulmonary embolism. A right upper extremity and right hemidiaphragmatic mass venogram revealed a 5-cm, wide-neck saccular aneurysm arising from the midportion of the SVC, as well as a similar perihepatic venous malformation (Figure 8, Video 3). With these findings, treatment options were discussed with the patient, and through the shared decision-making process, surgical treatment was planned. Resection of the large saccular SVC aneurysm and partial excision of the low-flow perihepatic and right pleural cavity venous malformation were successfully completed on cardiopulmonary bypass. The patient's postoperative course was uneventful. On postoperative day 1, 12.5 mg metoprolol twice daily was started. Apixaban was restarted on postoperative day 4. The patient was discharged home in stable condition on postoperative day 5. The patient was seen for follow-up at the vascular anomalies clinic, at which time magnetic resonance angiography of the chest and upper abdomen revealed a slow-flow vascular malformation located to the mediastinum with extension into the upper abdomen that had significantly decreased in size from previous imaging (Figure 9). It was believed that this lesion likely represented a venolymphatic malformation exacerbated during pregnancy. She was continued on apixaban, a copper intrauterine device was chosen pursuant to a discussion of family planning, and sirolimus was initiated for stabilization and potential reduction of the venolymphatic malformation. The patient has remained stable and without significant symptoms since hospital discharge.
Figure 2.
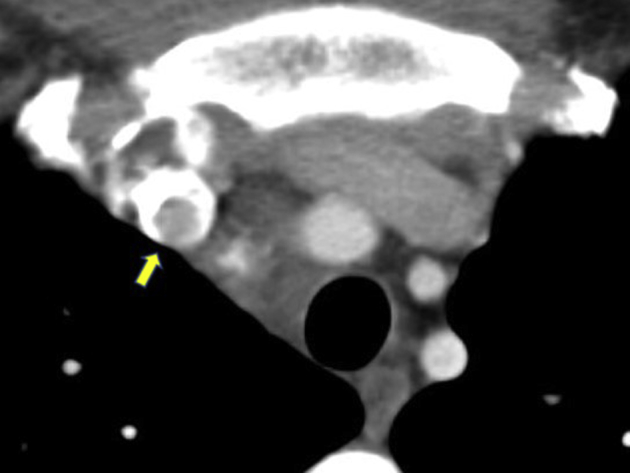
Chest CT scan, axial view, arrow indicating SVC thrombus.
Figure 3.
CT scan, axial view, arrows denoting a large, hypervascular superior mediastinal mass measuring 9.98 cm caudal to cranial and 12.2 cm laterally.
Figure 4.
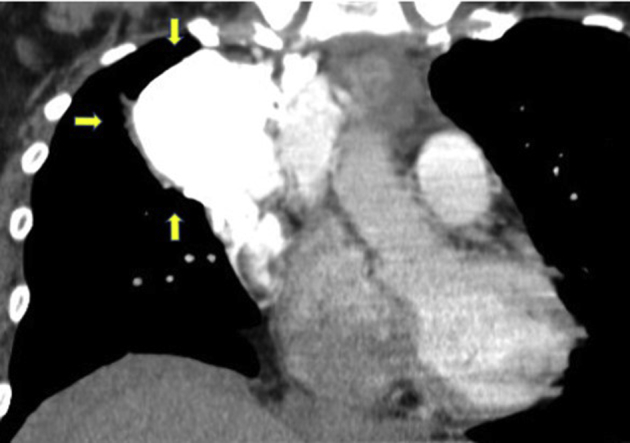
CT scan, coronal view, arrows indicating hypervascular mass.
Figure 5.
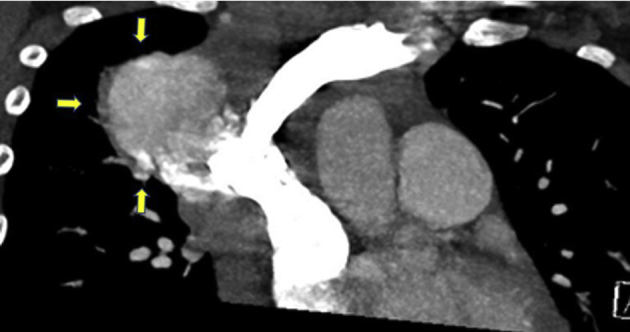
Chest CT venography, coronal view, arrows indicating a large mediastinal complex mass with filling defects noted to the SVC.
Figure 6.
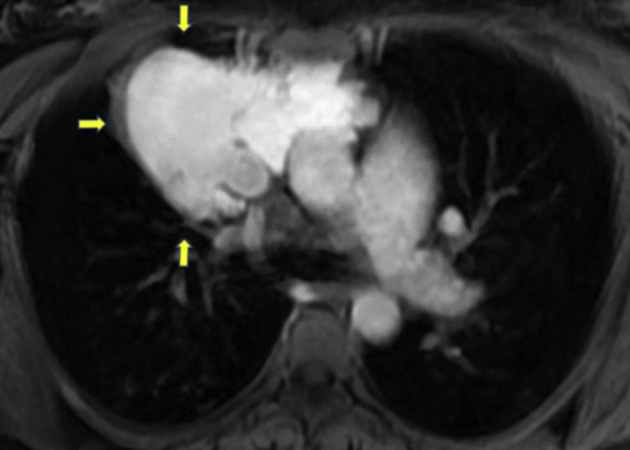
Magnetic resonance imaging scan, chest, axial view, arrows denoting a large lobular soft tissue lesion within the chest demonstrating direct communication with the SVC, representing a vascular anomaly.
Figure 7.
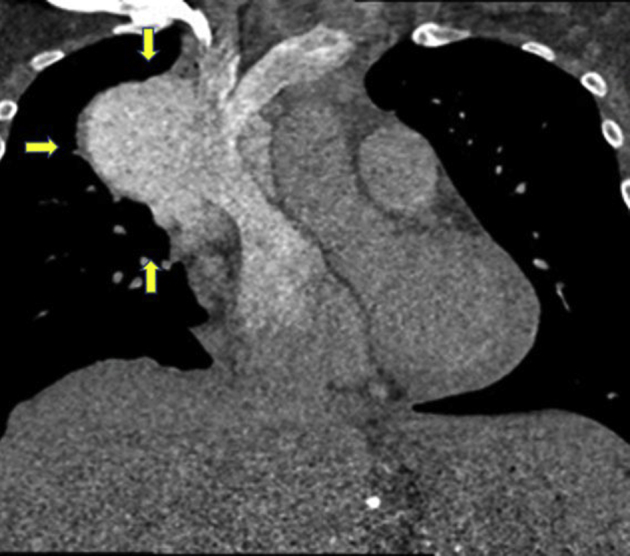
Cardiac CT scan, coronal view, arrows showing a large lobulated mass in the anterior and right mediastinum adjacent to the SVC, with the upper cystic section of the enhancing mass arising from the junction of the brachiocephalic veins and SVC with appearance of SVC saccular aneurysm.
Figure 8.
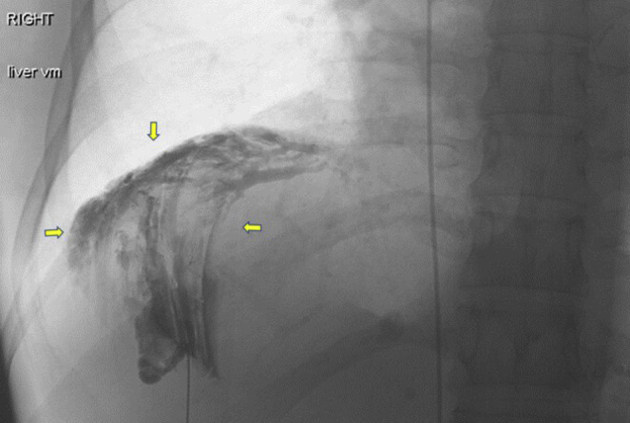
Right hemidiaphragmatic mass venogram. Arrows indicate perihepatic venous malformation.
Figure 9.
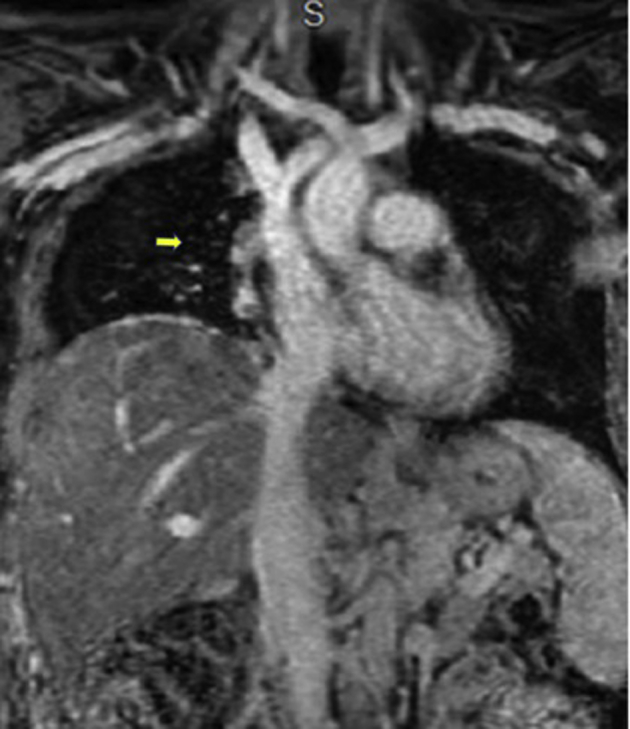
Magnetic resonance angiogram revealing slow-flow vascular malformation, arrow showing significant decrease in size following surgical reduction.
Discussion
SVC aneurysms are a rare cause of incidentally found mediastinal widening on chest radiography.1, 2 SVC aneurysms are classified morphologically as either fusiform or saccular, with the majority being fusiform. The exact mechanism by which these aneurysms form has not been elucidated, but suggested theories include congenital abnormalities, inflammation, infection, mechanical trauma, and pathologic deficiencies of the SVC longitudinal muscle wall adventia.3, 4, 5 An association with cystic hygromas has been suggested because of the similar embryologic origins of the venous and lymphatic systems.3 Often, the exact etiology of SVC aneurysms cannot be confirmed.
Diagnosis often involves advanced imaging, including chest radiography, ultrasonography, chest CT scan, magnetic resonance imaging, venography, or aortography.6 Potential complications of major venous aneurysms include thrombosis, pulmonary emboli, venous obstruction, and rupture.2 Complications rarely occur with fusiform aneurysms, whereas saccular aneurysms can be more problematic.7
Treatment for fusiform SVC aneurysms often follows a conservative management approach with anticoagulation and regular observation2, 8; however, treatment for saccular SVC aneurysms, as in our patient, requires more clinical acumen because of the increased risk for thromboembolism and aneurysmal rupture as the aneurysm increases in size. Treatment to decrease thrombus formation warrants anticoagulation, but anticoagulation with agents such as warfarin or the newer drug class of direct-acting oral anticoagulants carries an increased risk for bleeding and mortality, should the aneurysm rupture. Thus, aspirin therapy has been recommended as the anticoagulant modality of choice for reduction of thrombotic events in patients without evidence of thrombus formation.3, 6 When thrombus is present, the treatment plan should reflect standardized protocol, weighing risks and benefits concerning the initiation of anticoagulation. Our patient developed a symptomatic pulmonary embolism, so we chose apixaban for further clot prevention, with a good result. Prophylactic surgical resection to prevent aneurysmal rupture is recommended for saccular aneurysms that are >40 mm, growing, symptomatic, or contain thrombus.1, 4, 9
The classification of our patient's thoracic lesion was believed to likely represent a venolymphatic malformation, on the basis of the International Society for the Study of Vascular Anomalies classification system. Truncal venolymphatic malformations are thought to arise sporadically in most cases, but rare familial inheritance patterns do exist.6 Such lesions are often present from birth and grow dramatically during times of hormone excess,6 as in our recently pregnant patient. When these truncal lesions become large and aneurysm has formed, surgical resection is often the treatment modality of choice because these anomalies have a low chance of recurrence after resection. Other adjuvant treatment options include sclerotherapy and mammalian target of rapamycin (mTOR) inhibitors. In a systematic review of 28 studies, sirolimus, an mTOR inhibitor, led to partial remission and/or volume reduction of 85% of low-flow vascular malformations treated.10 Because mTOR inhibitors are systemic medications, they carry elevated risks for infection and bone marrow suppression. Thus, sclerotherapy is indicated unless the anomaly is large, resistant to sclerotherapy, or would require an unrealistic number of treatment sessions to achieve a significant result.
When mediastinal masses are found incidentally on routine imaging, vascular malformations, such as SVC aneurysms, should be included in the differential diagnosis. Advanced imaging can help delineate the morphology and hemodynamics of the malformation. Treatment with anticoagulation should be individualized to characteristics of the patient and of the vascular anomaly itself. If a large saccular SVC aneurysm is discovered, causing symptoms or containing thrombus, we recommend surgical resection of the aneurysm to decrease the risk for rupture and potential future thrombotic events. Subsequent treatment with sclerotherapy or mTOR inhibition may play a role in treatment for large SVC saccular aneurysms following resection.
Footnotes
Conflicts of interest: The authors reported no actual or potential conflicts of interest relative to this document.
Supplementary data related to this article can be found at https://doi.org/10.1016/j.case.2019.08.001.
Supplementary Data
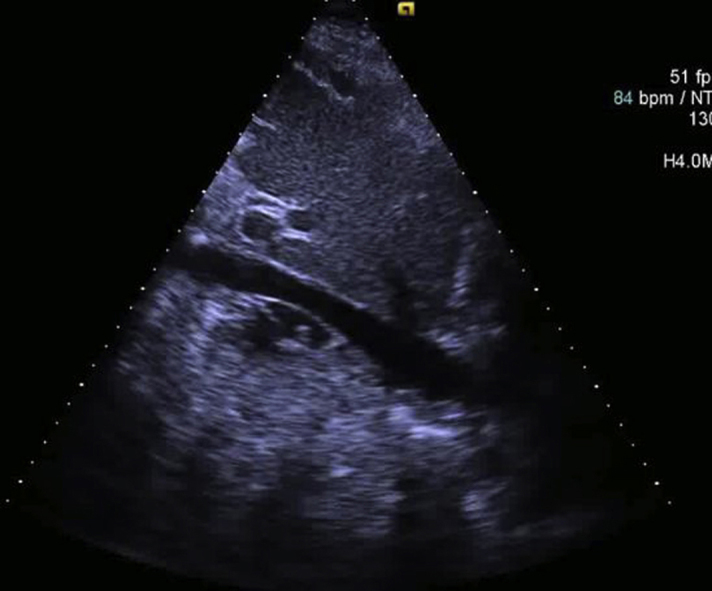
Transthoracic echocardiogram revealing normal left ventricular size and function (left ventricular ejection fraction 68%) with irregular inhomogeneous lobulated mass appreciated over the surface of the right ventricular pericardium, right ventricular outflow tract, and aortic root.
Transthoracic echocardiogram revealing mobile fibrinous intravascular anomaly appreciated within the inferior vena cava.
Right hemidiaphragmatic mass venogram with enhanced imaging revealing perihepatic venous malformation.
References
- 1.Haddad R., Joni H., Geske J. Images in vascular medicine. Twenty-eight years later: a case of superior vena cava aneurysm secondary to cystic hygroma. Vasc Med. 2014;19:417–418. doi: 10.1177/1358863X14531640. [DOI] [PubMed] [Google Scholar]
- 2.Oh S.G., Kim K.H., Seon H.J., Yoon H.J., Ahn Y., Jeong M.H. Unusual cause of acute right ventricular dysfunction: rapid progression of superior vena cava aneurysm complicated by thrombosis and pulmonary thromboembolism. J Korean Med Sci. 2011;26:690–693. doi: 10.3346/jkms.2011.26.5.690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Patel A., Cobb R., Rivera V., Simpson S. Untreated superior vena cava aneurysm: radiological significance and review of the literature. Case Rep Radiol. 2016;2016:6960757. doi: 10.1155/2016/6960757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Janczak D., Skiba J., Gemel M., Mak M., Ziomek A., Malinowski M. Giant saccular superior vena cava aneurysm-a rare and difficult clinical case. J Thorac Dis. 2016;8:E247–E249. doi: 10.21037/jtd.2016.02.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gabrielli R., Vitale S., Costanzo A., Carra A. Our experience of popliteal vein aneurysm. Interact Cardiovasc Thorac Surg. 2010;11:835–837. doi: 10.1510/icvts.2010.248823. [DOI] [PubMed] [Google Scholar]
- 6.Behravesh S., Yakes W., Gupta N., Naidu S., Chong B.W., Khademhosseini A. Venous malformations: clinical diagnosis and treatment. Cardiovasc Diagn Ther. 2016;6:557–569. doi: 10.21037/cdt.2016.11.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Koga S., Ikeda S., Sanuki Y., Ninomiya A., Izumikawa T., Miyahara Y. A case of asymptomatic fusiform aneurysm of the superior vena cava detected by magnetic resonance imaging. Int J Cardiol. 2006;113:E39–E41. doi: 10.1016/j.ijcard.2006.04.009. [DOI] [PubMed] [Google Scholar]
- 8.Panduranga P., Thomas E., Al-Maskari S., Al-Farqani A. Giant superior vena caval aneurysm in a post-Glenn patient. Interact Cardiovasc Thorac Surg. 2012;14:878–879. doi: 10.1093/icvts/ivs090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Varma P.K., Dharan B.S., Ramachandran P., Neelakandhan K.S. Superior vena caval aneurysm. Interact Cardiovasc Thorac Surg. 2003;2:331–333. doi: 10.1016/S1569-9293(03)00076-8. [DOI] [PubMed] [Google Scholar]
- 10.Wiegand S., Wichmann G., Dietz A. Treatment of lymphatic malformations with the mTOR inhibitor sirolimus: a systematic review. Lymphat Res Biol. 2018;16:330–339. doi: 10.1089/lrb.2017.0062. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Transthoracic echocardiogram revealing normal left ventricular size and function (left ventricular ejection fraction 68%) with irregular inhomogeneous lobulated mass appreciated over the surface of the right ventricular pericardium, right ventricular outflow tract, and aortic root.
Transthoracic echocardiogram revealing mobile fibrinous intravascular anomaly appreciated within the inferior vena cava.
Right hemidiaphragmatic mass venogram with enhanced imaging revealing perihepatic venous malformation.


