Abstract
To establish the contribution of glucose-dependent insulinotropic polypeptide (GIP) and glucagon-like peptide-1 (GLP-1) for the incretin effect after oral glucose, studies were undertaken in female mice with genetic deletion of receptors for GIP and GLP-1 (double incretin receptor knockout [DIRKO] mice) and their wild-type (WT) counterparts. Insulin secretion was explored after oral glucose (doses ranging from 0 to 100 mg), after intravenous glucose (doses ranging from 0 to 0.75 g/kg), and after oral and intravenous glucose at matching circulating glucose. DIRKO mice had glucose intolerance after oral glucose challenges in association with impaired beta-cell function. Suprabasal area under the curve for C-peptide (AUCC-peptide) correlated linearly with suprabasal AUCglucose both in WT (r = 0.942, P = .017) and DIRKO mice (r = 0.972, P = .006). The slope of this regression was lower in DIRKO than in WT mice (0.012 ± 0.006 vs 0.031 ± 0.006 nmol C-peptide/mmol glucose, P = .042). In contrast, there was no difference in the insulin response to intravenous glucose between WT and DIRKO mice. Furthermore, oral and intravenous glucose administration at matching glucose levels showed that the augmentation of insulin secretion after oral glucose (the incretin effect) in WT mice (11.8 ± 2.3 nmol/L min) was entirely absent in DIRKO mice (3.3 ± 1.2 nmol/L min). We conclude that GIP and GLP-1 are required for normal glucose tolerance and beta-cell function after oral glucose in mice, that they are the sole incretin hormones after oral glucose at higher dose levels, and that they contribute by 65% to insulin secretion after oral glucose.
Keywords: incretin hormones, animal models, insulin secretion, incretin effect, glucose tolerance
In the early 1900s, the term incretin was introduced by Starling for the stimulation of the pancreatic islets by gut hormones (1). Experimental evidence supporting the existence of an incretin concept was presented in the 1930s by La Barre and Still (2) and Heller (3) when they reported that administration of gut extracts lowered circulating glucose in experimental animals, which they suggested was mediated by an increase in insulin secretion. The final evidence of the incretin concept had to await the development of the radioimmunoassay technique to measure insulin that was used in the landmark studies in 1964 showing that oral glucose elicits a stronger increase in circulating insulin than intravenous glucose (4, 5). This was confirmed in the key 1967 study by Perley and Kipnis (6). Further studies have suggested that the main incretin hormones are glucose-dependent insulinotropic polypeptide (GIP) and glucagon-like peptide-1 (GLP-1) (7, 8). Moreover, it has been estimated that approximately 70% of insulin secretion after oral glucose is dependent on the incretin effect in humans (9, 10). However, whether GIP and GLP-1 are the sole incretin hormones after oral glucose is not established.
To study the contribution of GIP and GLP-1 as incretin hormones, mice with genetic deletion of receptors both for GIP and GLP-1 have been developed (double incretin receptor knockout [DIRKO] mice). Studies have demonstrated that these mice have glucose intolerance with impaired insulin response after oral administration of glucose (11-13). Although these studies demonstrated that GIP and GLP-1 are incretin hormones also in mice, they did not establish the quantitative contribution of the 2 hormones for glucose tolerance and the incretin effect after oral glucose. Studies with this aim would require oral administration of glucose at several different doses considering that the release both of GIP and GLP-1 are dependent on the amount of glucose entering the gut (14). Furthermore, to establish and quantify the incretin effect, it is also important to examine effects of oral and intravenous glucose at matching glucose levels (10). We have previously performed such isoglycemic studies in normal mice and showed that the beta-cell response is approximately 50% lower after intravenous compared to oral glucose (15). Such studies have not, however, been performed in DIRKO mice and therefore the contribution by GIP and GLP-1 is still not established.
To establish the contribution of GIP and GLP-1 for the insulin response to oral glucose in mice, we have therefore performed oral glucose tests over a wide range of glucose loads and estimated insulin secretion by using C-peptide measurements. Using C-peptide for estimation of beta-cell function is preferred in the DIRKO mouse because of its established increased insulin clearance (16). Therefore, using insulin as a surrogate for beta-cell function in DIRKO mice would falsely underestimate insulin secretion. We also determined the beta-cell response to intravenous glucose in wild-type (WT) and DIRKO mice to compare the contribution of GIP and GLP-1 and, finally, we estimated the incretin effect in WT and DIRKO mice by assessing the beta-cell response to oral vs intravenous glucose at matching glucose levels.
1. Materials and Methods
A. Animals
The generation of DIRKO mice on a C57BL6J background has been described previously (11). Briefly, mice heterozygous for the deletion both of the Glp1r and Gipr genes were generated from double homozygous deletion mutant mice by rederivation at Taconic Europe. Heterozygotes were mated to yield DIRKO and WT mice. The resulting offspring was used to establish breeding pairs, whose offspring was used in the experiments. All experiments were undertaken in mice age 4 to 6 months. The animals were maintained in a temperature-controlled room (22°C) on a 12:12-hour light-dark cycle (light on at 7:00 am). Mice were fed a standard pellet diet (total energy 15.1 MJ/kg with 11.5% from fat, 61.2% from carbohydrate, and 27.3% from protein; Special Diets Services, Scanbur) and tap water ad libitum. During experimental days, food was removed from the cages at 7:30 am and the actual experiments started at 12:30 pm, that is, during the light cycle. We used female mice to avoid the stress of single housing, which is used in male mice, and to be in line with the previous study in DIRKO mice (16). We used the mice randomly during the estrous cycle. The study was approved by the Lund/Malmö Animal Ethics Committee (Approval No. M166-15) and performed according to Good Laboratory Practice.
B. Animal Disposition
A total of 278 animals were allocated for the experiments (137 WT mice and 141 DIRKO mice). In the oral glucose tests and the intravenous glucose tests, studies were undertaken in batches of 6 to 8 mice each experimental day, whereas for the matching oral/intravenous studies, 1 to 3 animals were examined each experimental day. All experiments were undertaken by one experienced technician. In 7 mice (4 WT and 3 DIRKO mice) the experiment was insufficient (intravenous injection was missed or insufficient volume for analysis was obtained) and 4 DIRKO mice died during the matching oral/intravenous studies before the final blood sample was taken. These mice were not included in the analysis. All individual results from the completer population were included in the final analysis and statistics (133 WT mice and 134 DIRKO mice).
C. Experiments
C-1. Oral glucose test
After a 5-hour fast during the late morning hours, mice were anesthetized with an intraperitoneal injection of a fixed-dose combination of fentanyl (0.02 mg/mouse)–fluanisone (0.5 mg/mouse; Hypnorm, Vetpharma) and midazolam (0.125 mg/mouse; Roche). After 30 minutes, a blood sample (40 µL) was taken from the retrobulbar, intraorbital sinus capillary plexus in pipettes that had been prerinsed in heparin solution (100 U/mL in 0.9% NaCl; Orifarm). Thereafter, a gastric tube (outer diameter 1.2 mm) was placed in the stomach and saline (0.3 mL) or glucose (25, 50, 75, or 100 mg in volume of 0.3 mL) was administered. New blood samples (40 µL each) were then taken at 15, 30, and 60 minutes.
C-2. Intravenous glucose test
After a 5-hour fast, mice were anesthetized as previously described, and after a first blood sample mice were given an intravenous bolus dose of saline or D-glucose over 3 seconds (dissolved in saline; Sigma-Aldrich) in a tail vein at the doses of 0 (only saline), 0.35, and 0.75 g/kg. Whole blood was sampled as previously described 1, 5, 10, 20, and 50 minutes after glucose injection.
C-3. Combined oral/intravenous glucose test
The animals were anesthetized as previously described. Ten minutes later, the right jugular vein and the left carotid artery were catheterized. The venous catheter was used for infusion of glucose and the arterial catheter for sample collection. After another 20 minutes, a variable-rate glucose infusion was started (solution 20 g/dL) or a gavage tube was inserted in the stomach as previously described and glucose (35-75 mg) was administered. The oral dose varied between 35 and 75 mg to achieve good glycemic matching of the 2 tests. Plasma glucose was determined every 5 minutes. In the intravenous test, the rate of glucose infusion was adjusted to achieve a similar glucose level as obtained after oral glucose. Blood samples for analysis of C-peptide were taken after another 15, 30, and 60 minutes.
D. Assays
Plasma was immediately separated after collection and stored at –20°C until analysis. Insulin concentration was determined by enzyme-linked immunosorbent assay (Mercodia). The intra-assay coefficient of variation (CV) of the method is 4% both at low and high levels, and the interassay CV is 5% both at low and high levels. The lower limit of the assay is 6 pmol/L. Mouse C-peptide was determined by enzyme-linked immunosorbent assay (Crystal Chem). The intra-assay and interassay CVs of the method are less than 10% both at low and high levels. The lower limit of the assay is 25 pmol/L. Glucose was measured using Accu-Chek Aviva (Hoffman-La Roche).
E. Data Analysis and Statistics
Data are presented as means ± SE. Areas under the curves (AUCs) were calculated with the trapezoid rule, either for measured values (total AUC) or for incremental values greater than baseline (suprabasal AUC). Early insulin response was estimated by subtracting the baseline insulin levels from the 15-minute insulin values after oral glucose. The acute insulin secretion after intravenous glucose was estimated as the increase in mean of 1- and 5-minute C-peptide levels greater than baseline. Differences between experimental groups were determined using the Student unpaired t test. Statistics on insulin and C-peptide were performed after logarithmic transformation because they do not display normal distribution in mice (17). Linear regression analysis was performed for test of correlation. For all analyses, statistical significance was defined as P < .05. Analyses were carried out using SPSS, version 25.
2. Results
A. Baseline Body Weight, Glucose, Insulin, and C-Peptide
A total of 133 WT mice and 134 DIRKO mice comprised the completer population (54 WT mice and 54 DIRKO mice in the oral glucose test [OGTT], 58 WT mice, and 60 DIRKO mice in the intravenous glucose test [IVGTT] and 21 WT mice and 20 DIRKO mice in the clamp studies). Body weight was slightly lower in DIRKO mice than in WT mice (21.3 ± 0.2 vs 22.6 ± 0.2 g, P < .001). In the OGTT and IVGTT series (112 WT mice and 114 DIRKO mice), baseline glucose (8.4 ± 0.1 vs 7.5 ± 0.1 mmol/L, P < .001), insulin (256 ± 10 pmol/L vs 204 ± 8 pmol/L, P = .001), and C-peptide (194 ± 6 vs 136 ± 4 pmol/L, P < .001) were higher in DIRKO mice.
B. Glucose, Insulin, and C-Peptide Responses to Oral Glucose
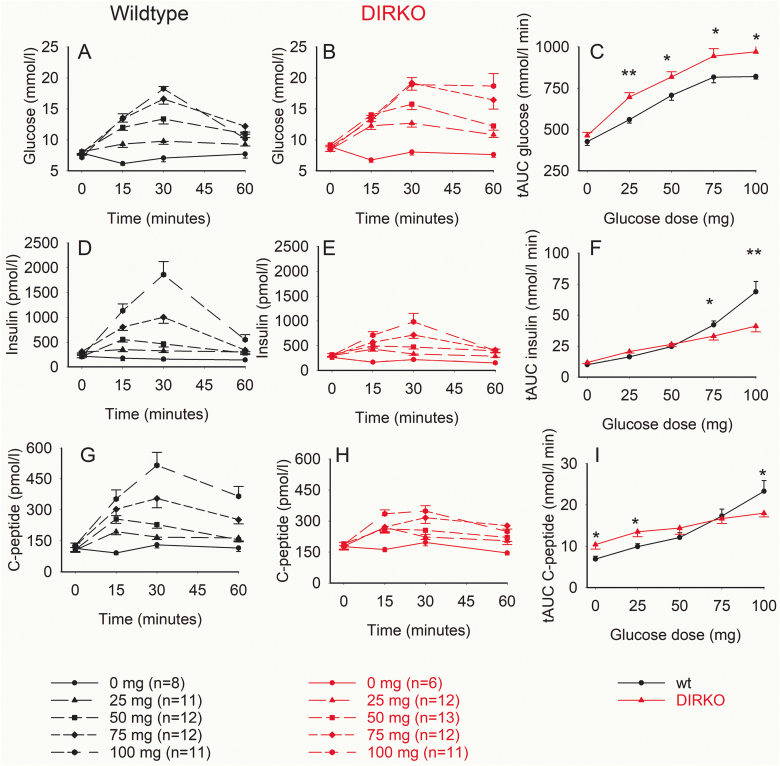
Plasma glucose, insulin, and C-peptide levels increased after oral glucose both in WT and DIRKO mice, whereas there was virtually no change in glucose, insulin, and C-peptide after oral saline administration (Fig. 1A, 1B, 1D, 1E, 1G, and 1H). There was a clear dose-dependent increase in total AUCglucose, total AUCinsulin, and total AUCC-peptide after increasing the glucose dose in both groups (Fig. 1C, 1F, and 1I). AUCglucose was significantly higher in DIRKO mice than in WT mice after administration of 25, 50, 75, and 100 mg glucose (Fig. 1C), whereas AUCinsulin was lower in DIRKO mice than in WT mice after 75 mg and 100 mg glucose and AUCC-peptide was higher in DIRKO mice than in WT mice after 0 and 25 mg but significantly lower after 100 mg glucose (Fig. 1F and 1I).
Figure 1.
Plasma glucose, insulin, and C-peptide levels, and total area under the curve (AUC)glucose, AUCinsulin, and AUCC-peptide after oral glucose administration of 0, 25, 50, 75, and 100 mg glucose in wild-type (wt) mice and double incretin receptor knockout (DIRKO mice). Means ± SEM are shown. Asterisks indicate the probability level of random difference between the groups. *P < .05, **P < .01.
C. Glucose and C-Peptide Responses to Intravenous Glucose
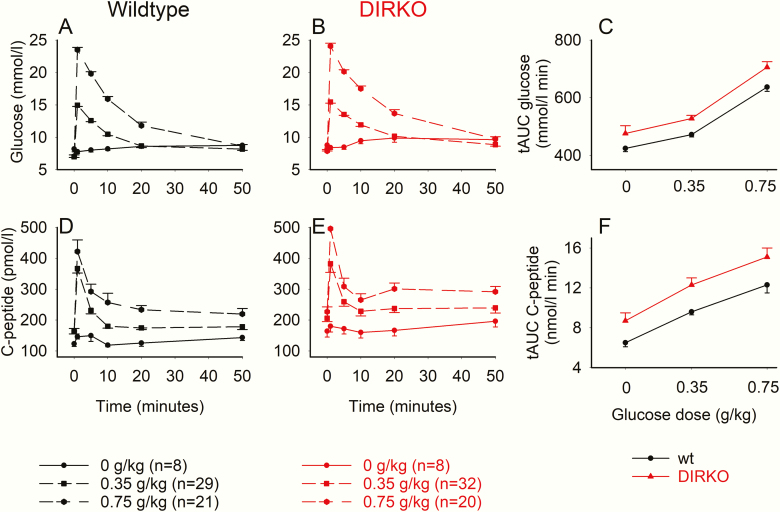
Plasma glucose and C-peptide levels increased after intravenous glucose both in WT and DIRKO mice, whereas there was virtually no change in glucose and C-peptide after intravenous saline administration (Fig. 2A, 2B, 2D, and 2E). There was a clear dose-dependent increase in total AUCglucose and total AUCC-peptide after increasing the glucose dose in both groups but with no differences between the groups (Fig. 2C and 2F).
Figure 2.
Plasma glucose and C-peptide levels and suprabasal area under the curve (AUC)glucose and AUCC-peptide after intravenous glucose administration of 0, 0.35, and 0.75 g/kg in wild-type (wt) mice and double incretin receptor knockout (DIRKO) mice. Means ± SEM are shown.
D. Beta-Cell Response to Oral and Intravenous Glucose
D-1. Beta-cell response to oral glucose
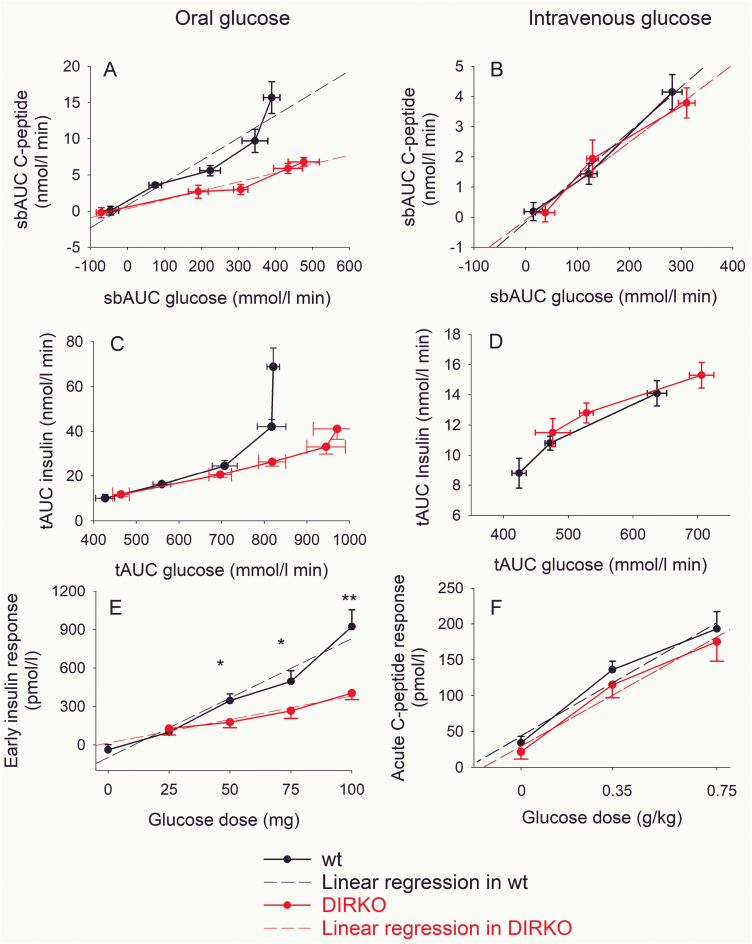
Suprabasal AUCC-peptide increased with higher suprabasal AUCglucose after oral glucose both in WT and DIRKO mice (Fig. 3A). There was a significant linear correlation between suprabasal AUCC-peptide and suprabasal AUCglucose both in WT mice (r = 0.942, P = .017) and DIRKO mice (r = 0.972, P = .006). However, the slope of the regression was higher in WT mice (0.031 ± 0.006 nmol C-peptide/mmol glucose) than in DIRKO mice (0.012 ± 0.006 nmol C-peptide/mmol glucose; P = .042). There was also a lower AUCinsulin in relation to AUCglucose at higher glucose levels after oral glucose (Fig. 3C) but not after intravenous glucose (Fig. 3D). Furthermore, the early insulin response to oral glucose (ie, the increase in insulin at min 15 compared to baseline) was dose-dependently increased after oral glucose, with significantly higher responses in WT mice than in DIRKO mice after 50, 75, and 100 mg glucose (Fig. 3E). There was a significant correlation between early insulin response and administered dose of glucose both in WT mice (r = 0.978, P = .004) and DIRKO mice (r = 0.974, P = .005) (Fig. 3C). The slope of the regression was higher in WT mice (9.3 ± 1.1 pmol insulin/mg glucose) than in DIRKO mice (4.6 ± 0.6 pmol insulin/mg glucose, P = .020).
Figure 3.
Suprabasal area under the curve (AUC)C-peptide in relation to suprabasal AUCglucose after oral glucose administration at A, 0, 25, 50, 75, and 100 mg or after intravenous glucose administration at B, 0, 0.35, and 0.75 g/kg, total AUCinsulin in relation to total AUCglucose after C, oral, and D, intravenous glucose, early (15-min) insulin response to oral glucose at E, 0, 25, 50, 75, and F, 100 mg and acute C-peptide response (5-min) to intravenous glucose at 0, 0.35, and 0.75 g/kg in wild-type (wt) mice and double incretin receptor knockout (DIRKO) mice. Means ± SEM and linear regressions are shown. Asterisks indicate the probability level of random difference between the groups; *P < .05, **P < .01.
D-2. Beta-cell response to intravenous glucose
Suprabasal AUC both of glucose and C-peptide increased by increasing the intravenous glucose load. with a significant correlation between them both in WT mice (r = 0.332; P = .011) and DIRKO mice (r = 0.260; P = .049) (Fig. 3B). The slope of the regression did not differ between the groups (0.015 ± 0.001 and 0.013 ± 0.002 nmol C-peptide/mmol glucose in WT and DIRKO mice). The acute C-peptide response to intravenous glucose (ie, mean increase in C-peptide at 1 and 5 min) was dose-dependently increased after intravenous glucose but with no significant difference between the 2 groups (Fig. 3D); the slope of regression between acute C-peptide response and dose of glucose was 210 ± 42 and 203 ± 34 nmol C-peptide/g/kg glucose in WT and DIRKO mice.
E. Oral vs Intravenous Glucose Tests at Matching Glucose
E-1. Baseline glucose, insulin, and C-peptide
After the anesthesia and insertion of catheters in the jugular vein and carotid artery, glucose (9.4 ± 0.4 vs 8.1 ± 0.3 mmol/l, P = .008) and C-peptide levels (286 ± 17 vs 233 ± 16 pmol/l, P = .028) were higher in DIRKO mice (n = 20) than in WT mice (n = 21).
E-2. Glucose levels during oral and intravenous administration
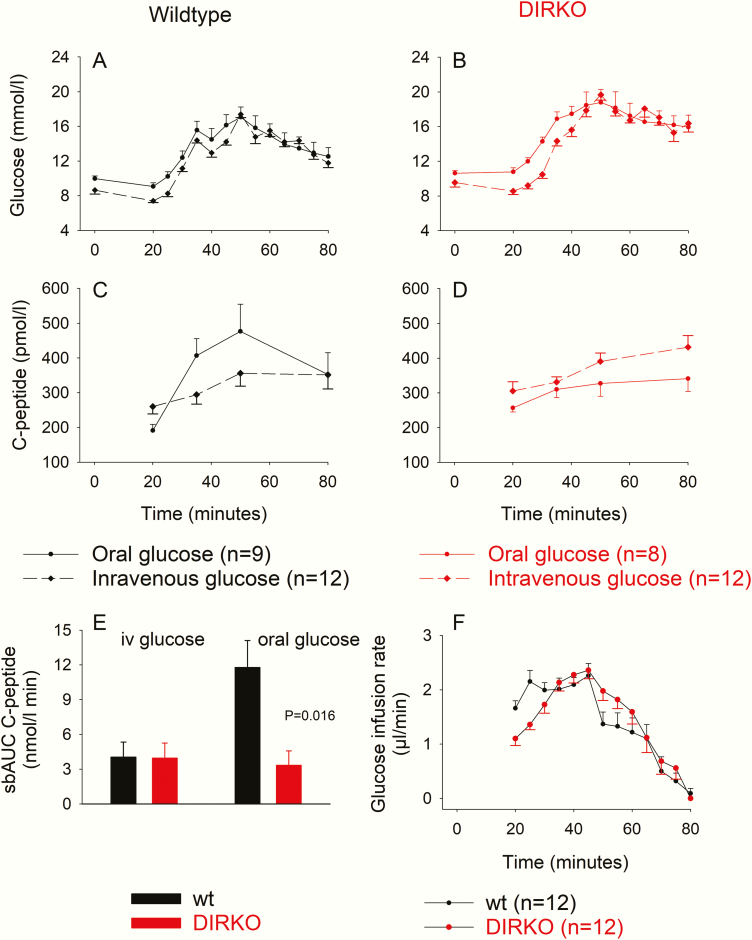
Fig. 4A and 4B show that both in WT and DIRKO mice, glucose levels were well matched during oral vs intravenous glucose administration. In WT mice, there was no significant difference between glucose levels at any time point. In DIRKO mice, glucose levels were slightly lower in the intravenous test than in the oral test at baseline and during the first 15 minutes of glucose administration. From minute 15, the glucose levels were well matched between the 2 groups with no significant difference.
Figure 4.
Plasma glucose and C-peptide levels and suprabasal area under the curve (AUC)C-peptide after oral and intravenous glucose administration in A to E, wild-type (wt) and double incretin receptor knockout (DIRKO) mice and F, glucose infusion rate during intravenous glucose administration. Means ± SEM are shown.
E-3. C-peptide responses to oral and intravenous glucose
C-peptide levels increased after both oral and intravenous glucose both in WT (Fig. 4C) and DIRKO mice (Fig. 4D). The beta-cell response to oral and intravenous glucose was determined by estimating the suprabasal AUCC-peptide (Fig. 4E). In WT mice, the C-peptide response to oral glucose was 11.8 ± 2.3 nmol/L minute and the response to intravenous glucose was 4.0 ± 1.3 nmol/L minute, that is, only 34% of that after oral glucose. In DIRKO mice, the C-peptide response to intravenous glucose was 4.0 ± 1.2 nmol/L minute, which was not significantly different from WT mice. After oral glucose, however, the C-peptide response was only 3.3 ± 1.2 nmol/L minute, which was not significantly different from the response after intravenous glucose and significantly lower than in WT mice (P = .016).
E-4. Glucose infusion rates
The glucose infusion rate was initially higher in WT mice (to counteract their lower baseline glucose), but after 10 minutes there was no difference in glucose infusion rate between the 2 groups (Fig. 4F). The total amount of infused glucose was not significantly different in the 2 groups (90.9 ± 5.5 µL in WT mice and 85.9 ± 6.0 µL in DIRKO mice).
3. Discussion
We show in the present study that DIRKO mice have glucose intolerance after administration of oral glucose over a wide dose range from 0 to 100 mg per mouse in association with reduced beta-cell response. Earlier studies have shown glucose intolerance and suppressed insulin response to oral glucose at single doses (11-13). A novel finding in this study is therefore that GIP and GLP-1 are major incretin hormones over a full range of oral glucose loads ranging from slight to marked increases in glucose levels. Another novelty of this study is that we analyzed the beta-cell response to oral glucose by using C-peptide and not insulin levels. This is important when estimating beta-cell function in vivo in DIRKO mice because, as we have previously demonstrated, DIRKO mice have higher insulin clearance than WT mice (16). Therefore, using insulin levels as a surrogate for insulin secretion in DIRKO mice will erroneously underestimate insulin secretion. We found that there was a linear relation between glucose and C-peptide levels both in WT and DIRKO mice, illustrating the beta-cell glucose sensitivity. Interestingly, this slope was reduced by approximately 70% in DIRKO mice, showing that the 2 incretin hormones are important for glucose sensitivity after oral glucose. In contrast, there was no difference in beta-cell glucose sensitivity to intravenous glucose between the groups, which shows that the insulin response to glucose per se is not different between WT and DIRKO mice. This confirms previous data after intravenous (16) or intraperitoneal (12) glucose in DIRKO mice. The impairment of insulin secretion after oral glucose in DIRKO mice is thus explained by GIP and GLP-1.
It has been demonstrated that it is the early insulin response to oral glucose that is of particular importance for glucose tolerance after oral glucose or meal ingestion. Thus, an early insulin response rapidly suppresses glucose release from the liver, which is important for the postload glucose tolerance (18). We therefore estimated also the 15-minute increase in insulin levels after oral glucose administration to measure early insulin response. We found that this early increase was lower in DIRKO mice than in WT mice. Therefore, the augmentation of early insulin response by increasing the oral glucose load seems dependent on incretin hormones, the release of which are glucose dependent (10, 14). An effect through glucagon may also be possible because recent studies have indicated that islet GLP-1 receptors are important for the insulin secretory effect of glucagon (19), that is, the lower insulin levels in relation to glucose in DIRKO mice may also be mediated by an impaired action of glucagon to stimulate insulin secretion. At 25 mg, however, the early insulin response did not differ significantly between WT and DIRKO mice. A higher glucose level in DIRKO mice may explain this, although mechanisms of GIP and GLP-1 other than the increase in insulin may also contribute to glucose tolerance, such as changes in hepatic glucose delivery caused by changes in glucagon levels, which are effects of GIP and GLP-1 (10, 20). It is also possible that gut hormones other than GIP and GLP-1 are important for glucose tolerance at low glucose loads. Because low glucose loads that raise glucose levels by only a few millemole per liter may represent common glucose challenges during daily living, these potential mechanisms need to be studied in more detail.
To verify the existence of an incretin effect, and also for its quantification, studies with oral and intravenous glucose administration at matching glucose levels have to be undertaken (10). A novelty of this study is that we performed such experiments in WT and DIRKO mice. The experimental procedure of placing carotid and jugular arteries raised glucose and insulin levels, and therefore the oral glucose load had to be re-performed in operated-on animals. The main finding of this experimental series is that, again, there was no difference in beta-cell response to intravenous glucose between WT and DIRKO mice. In contrast, there was a huge difference in beta-cell response to oral glucose administration between the 2 groups. In fact, whereas there was a marked augmentation of the beta-cell response to oral glucose compared to the response to intravenous glucose in WT mice, there was no difference at all in beta-cell response to oral vs intravenous glucose in DIRKO mice. The conclusion from this is that DIRKO mice completely lack an incretin effect. In WT mice, the difference in C-peptide response to oral vs intravenous glucose shows that approximately 65% of insulin secretion after oral glucose is dependent on incretin hormones, and the remainder depends on the increase in circulating glucose. This is in a similar range as we previously reported in mice (15) and that also has been estimated in humans (9, 10, 21). The result that this incretin effect was completely absent in DIRKO mice suggests that GIP and GLP-1 are the only incretin hormones after oral glucose at these dose levels in mice. Other existing gut hormones and potential gut incretin factors (22) seem therefore to lack physiological roles as incretin factors after oral glucose administration in mice. This does not, however, exclude that they might have incretin effects after other gut-derived challenges, such as oral protein, fat, or low-dose glucose challenges.
There are a number of strengths of this study: the use of a high number of mice, the test of several different doses of glucose, both orally and intravenously, and not just one dose, the performance of each individual mouse test by one experienced technician, the use of C-peptide for estimation of insulin secretion, and the matching of glucose levels after intravenous and glucose challenges. A limitation, however, is that only female mice were used, which restricts the conclusion to female mice. On the other hand, the use of female mice avoided the stress of single housing, which is used in male mice, and, also, the results are comparable to our previous study in DIRKO mice, which also used female mice (16). Another limitation is the use of anesthesia during the test, which on one hand reduces the stress of the animals but on the other hand opens the possibility of effects related to anesthesia. Importantly, however, the same anesthesia was used in all tests.
In summary, this study in WT mice and mice with double genetic deletion of receptors for GIP and GLP-1 have shown that GIP and GLP-1 are important incretin hormones after oral glucose loads over a wide dose range, contribute by approximately 70% to beta-cell sensitivity after oral glucose, do not contribute to glucose sensitivity after intravenous glucose, contribute by approximately 65% to insulin secretion after oral glucose in normal mice, and contribute to 100% of the augmenting effect of insulin secretion by oral vs intravenous glucose. Based on these data we conclude that GIP and GLP-1 are important incretin hormones for beta-cell response to oral glucose and that they seem to be the sole incretin hormones after oral glucose challenge in mice.
Acknowledgments
We thank Kristina Andersson of Lund University for expert technical assistance and Dr Daniel J. Drucker of the Lunenfeld-Tanenbaum Research Institute of Mount Sinai Hospital, Toronto, Canada, for provision of the GLP-1 receptor knockout mice used in the creation of the DIRKO strain.
Financial Support:This work was supported by the Lund University Medical Faculty, Region Skåne, and the Swedish Research Council (to B.A.).
Author Contributions: B.A. conceived and designed the research and analyzed the data. B.A., Y.Y., and Y.S. interpreted the results of the experiments, edited the manuscript, and approved the final version of the manuscript. B.A. performed the experiments, prepared the figures, and drafted the manuscript.
Glossary
Abbreviations
- DIRKO
double incretin receptor knockout
- GIP
glucose-dependent insulinotropic polypeptide
- GLP-1
glucagon-like peptide-1
- WT
wild-type
Additional Information
Disclosure Summary: The authors have nothing to disclose.
Data Availability: The datasets generated during and/or analyzed during the current study are not publicly available but are available from the corresponding author on reasonable request.
References
- 1. Moore B. On the treatment of diabetus mellitus by acid extract of duodenal mucous membrane. Biochem J. 1906;1(1):28–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. La Barre J, Still EU. Studies on the physiology of secretin. III. Further studies on the effects of secretin on the blood sugar. Am J Physiol. 1930;91:649–653. [Google Scholar]
- 3. Heller H. Über das insulinotropic Hormon der Darmschleimhaut (Duodenin). Naunyn-Schm Arch Pharmacol. 1935;177:127–133. [Google Scholar]
- 4. Mcintyre N, Holdsworth CD, Turner DS. New interpretation of oral glucose tolerance. Lancet. 1964;2(7349):20–21. [DOI] [PubMed] [Google Scholar]
- 5. Elrick H, Stimmler L, Hlad CJ Jr, Arai Y. Plasma insulin response to oral and intravenous glucose administration. J Clin Endocrinol Metab. 1964;24:1076–1082. [DOI] [PubMed] [Google Scholar]
- 6. Perley MJ, Kipnis DM. Plasma insulin responses to oral and intravenous glucose: studies in normal and diabetic subjects. J Clin Invest. 1967;46(12):1954–1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Dupre J, Ross SA, Watson D, Brown JC. Stimulation of insulin secretion by gastric inhibitory polypeptide in man. J Clin Endocrinol Metab. 1973;37(5):826–828. [DOI] [PubMed] [Google Scholar]
- 8. Kreymann B, Williams G, Ghatei MA, Bloom SR. Glucagon-like peptide-1 7-36: a physiological incretin in man. Lancet. 1987;2(8571):1300–1304. [DOI] [PubMed] [Google Scholar]
- 9. Nauck M, Stöckmann F, Ebert R, Creutzfeldt W. Reduced incretin effect in type 2 (non-insulin-dependent) diabetes. Diabetologia. 1986;29(1):46–52. [DOI] [PubMed] [Google Scholar]
- 10. Holst JJ. The incretin system in healthy humans: the role of GIP and GLP-1. Metabolism. 2019;96:46–55. [DOI] [PubMed] [Google Scholar]
- 11. Hansotia T, Baggio LL, Delmeire D, et al. Double incretin receptor knockout (DIRKO) mice reveal an essential role for the enteroinsular axis in transducing the glucoregulatory actions of DPP-IV inhibitors. Diabetes. 2004;53(5):1326–1335. [DOI] [PubMed] [Google Scholar]
- 12. Preitner F, Ibberson M, Franklin I, et al. Gluco-incretins control insulin secretion at multiple levels as revealed in mice lacking GLP-1 and GIP receptors. J Clin Invest. 2004;113(4):635–645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Omar B, Ahlkvist L, Yamada Y, Seino Y, Ahrén B. Incretin hormone receptors are required for normal beta cell development and function in female mice. Peptides. 2016;79:58–65. [DOI] [PubMed] [Google Scholar]
- 14. Bagger JI, Knop FK, Lund A, Vestergaard H, Holst JJ, Vilsbøll T. Impaired regulation of the incretin effect in patients with type 2 diabetes. J Clin Endocrinol Metab. 2011;96(3):737–745. [DOI] [PubMed] [Google Scholar]
- 15. Ahrén B, Winzell MS, Pacini G. The augmenting effect on insulin secretion by oral versus intravenous glucose is exaggerated by high-fat diet in mice. J Endocrinol. 2008;197(1):181–187. [DOI] [PubMed] [Google Scholar]
- 16. Tura A, Bizzotto R, Yamada Y, Seino Y, Pacini G, Ahrén B. Increased insulin clearance in mice with double deletion of glucagon-like peptide-1 and glucose-dependent insulinotropic polypeptide receptors. Am J Physiol Regul Integr Comp Physiol. 2018;314(5):R639–R646. [DOI] [PubMed] [Google Scholar]
- 17. Tura A, Pacini G, Yamada Y, Seino Y, Ahrén B. Glucagon and insulin secretion, insulin clearance, and fasting glucose in GIP receptor and GLP-1 receptor knockout mice. Am J Physiol Regul Integr Comp Physiol. 2019;316(1):R27–R37. [DOI] [PubMed] [Google Scholar]
- 18. Mitrakou A, Kelley D, Mokan M, et al. Role of reduced suppression of glucose production and diminished early insulin release in impaired glucose tolerance. N Engl J Med. 1992;326(1):22–29. [DOI] [PubMed] [Google Scholar]
- 19. Zhu L, Dattaroy D, Pham J, et al. Intra-islet glucagon signaling is critical for maintaining glucose homeostasis. JCI Insight. 2019;4:e127994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ahrén B, Foley JE. Improved glucose regulation in type 2 diabetic patients with DPP-4 inhibitors: focus on alpha and beta cell function and lipid metabolism. Diabetologia. 2016;59(5):907–917. [DOI] [PubMed] [Google Scholar]
- 21. Nauck MA, Homberger E, Siegel EG, et al. Incretin effects of increasing glucose loads in man calculated from venous insulin and C-peptide responses. J Clin Endocrinol Metab. 1986;63(2):492–498. [DOI] [PubMed] [Google Scholar]
- 22. Rehfeld JF. The origin and understanding of the incretin concept. Front Endocrinol (Lausanne). 2018;9:387. [DOI] [PMC free article] [PubMed] [Google Scholar]