Abstract
Objective
The aim of the study was to identify the survival of patients with locally advanced pancreatic cancer (LAPC) and assess the effect of surgical resection after neoadjuvant therapy on patient outcomes.
Background
An increasing number of LAPC patients who respond favorably to neoadjuvant therapy undergo surgical resection. The impact of surgery on patient survival is largely unknown.
Materials and Methods
All LAPC patients who presented to the institutional pancreatic multidisciplinary clinic (PMDC) from January 2013 to September 2017 were included in the study. Demographics and clinical data on neoadjuvant treatment and surgical resection were documented. Primary tumor resection rates after neoadjuvant therapy and overall survival (OS) were the primary study endpoints.
Results
A total of 415 LAPC patients were included in the study. Stratification of neoadjuvant therapy in FOLFIRINOX-based, gemcitabine-based, and combination of the two, and subsequent outcome comparison did not demonstrate significant differences in OS of 331 non-resected LAPC patients (P = 0.134). Eighty-four patients underwent resection of the primary tumor (20%), after a median duration of 5 months of neoadjuvant therapy. FOLFIRINOX-based therapy and stereotactic body radiation therapy correlated with increased probability of resection (P = 0.006). Resected patients had better performance status, smaller median tumor size (P =0.029), and lower median CA19–9 values (P < 0.001) at PMDC. Patients who underwent surgical resection had significant higher median OS compared with those who did not (35.3 vs 16.3 mo, P < 0.001). The difference remained significant when non-resected patients were matched for time of neoadjuvant therapy (19.9 mo, P < 0.001).
Conclusions
Surgical resection of LAPC after neoadjuvant therapy is feasible in a highly selected cohort of patients (20%) and is associated with significantly longer median overall survival.
Keywords: FOLFIRINOX, gemcitabine, locally advanced, neoadjuvant chemotherapy, pancreatic cancer, stereotactic body radiation therapy, survival
Pancreatic ductal adenocarcinoma (PDAC) is historically characterized by poor prognosis and high rates of disease-specific mortality. For 2017, the American Cancer Society estimated 53,670 new cases and 43,090 PDAC-related deaths in the United States.1 Mortality continues to increase, and it is projected that by 2030, PDAC will be the second most common cancer-related cause of death.2 The advanced stage of disease at the time of diagnosis is the main driver for decreased survival.3 Systemic chemotherapy is the treatment of choice for patients presenting with advanced disease with a potential role for consolidative radiation, yet overall survival remains low compared with those who are eligible for upfront surgical resection.
Locally advanced pancreatic cancer (LAPC) accounts for 30% of all newly diagnosed pancreatic cancer cases and is considered surgically unresectable, due to local involvement of adjacent vessels. Current guidelines for LAPC recommend nonoperative treatment for patients with good performance status, through a multidisciplinary approach.4 Randomized prospective trials have not yet provided definitive data to favor a specific chemotherapy regimen. Previous studies have shown that for patients with metastatic PDAC, the combination of leucovorin, fluorouracil, irinotecan, and oxaliplatin (FOLFIRINOX) is associated with improved survival.5 A recent meta-analysis also suggested a potential survival benefit of FOLFIRINOX in patients with LAPC.6 Similar results have also been reported with gemcitabine-based multiagent therapy.7
Recent retrospective series have suggested the possibility of potentially curative resection after induction chemotherapy in patients with LAPC.8–11 Data from these studies indicated a survival benefit after tumor resection in patients with imaging response or no progression of disease after neoadjuvant therapy. In addition, contemporary radiation modalities, such as stereotactic body radiation therapy (SBRT), can complement induction chemotherapy and improve tumor resectability in patients with LAPC.10 However, these reports were limited by cohort heterogeneity because they included patients with borderline resectable and/or metastatic PDAC, and by the lack of survival comparison with patients who were treated with nonoperative therapy. In this large retrospective series, we sought to overcome these limitations by studying exclusively LAPC patients, who were reviewed in a single high-volume pancreatic multidisciplinary clinic (PMDC) within the past 5 years. The primary goal of this study is to determine if surgical resection is associated with improved survival compared with aggressive nonoperative management in patients with LAPC. We secondarily sought to identify potential predictive factors for surgical resection.
PATIENTS AND METHODS
Patient Cohort
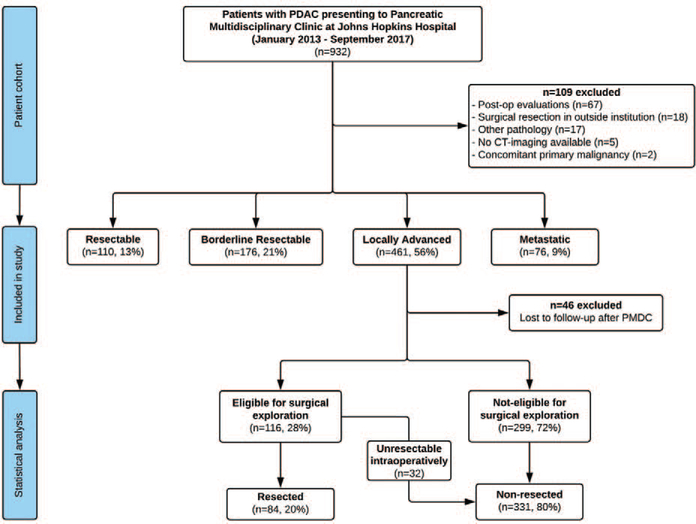
The initial patient pool included all patients seen at the Johns Hopkins PMDC from January 1, 2013 to September 30, 2017. The primary eligibility criterion was the diagnosis of LAPC at the time of PMDC evaluation, based on the National Comprehensive Cancer Network guidelines.12 More specifically, all patients who presented with encasement (>180° contact) of the celiac artery (CA) or superior mesenteric artery (SMA), unreconstructible portal or superior mesenteric vein, or tumor involvement of the first jejunal SMA branch were staged as LAPC and were included in the study. Most of these patients were seen more than once at PMDC along the course of their disease, to reassess tumor resectability and reevaluate treatment response. Primarily, all non-LAPC patients were excluded from the study. The rest of the exclusion criteria are shown in Figure 1; to ensure cohort homogeneity for resection eligibility, all LAPC patients who did not undergo standardized pancreatic protocol multidetector computed tomography (MDCT) in our institution were excluded from the study. Follow-up data were documented until patient death, or date of last follow-up. Major eligibility criteria for surgical exploration included the absence of local tumor extension and major vessel involvement, no deteriorating patient performance status, and/or response to neoadjuvant treatment per RECIST criteria (Response Evaluation Criteria in Solid Tumors),13 assessed at PMDC. Subsequently, 2 cohorts were generated: LAPC patients who underwent surgical resection of the primary tumor, and LAPC patients who did not reach eligibility for resection and were treated with non-operative therapy. The same team of surgeons, specialized in pancreatic and hepatobiliary surgery, performed all surgical resections of LAPC patients. The institutional review board has approved this retrospective study.
FIGURE 1.
Patient study selection flowchart.
Data Collection
All patient data were collected from the PMDC disposition notes and the prospectively maintained institutional database. Patient demographics included age, sex, race, and performance status based on the Eastern Cooperative Oncology Group (ECOG) scale. Data on tumor site and size, major vessel involvement, the presence of regional lymphadenopathy, tumor involvement of the gastrointestinal tract, and the presence or suspicion of distant metastases, as assessed by MDCT, were documented. Information about the type and timing of induction therapy were collected, including chemotherapy regimens, radiation therapy modalities, and treatment changes or complications, where applicable.
For patients who underwent surgical resection of the primary tumor, the type of surgery and postoperative outcomes were evaluated. Tumor pathology characteristics, such as grade of differentiation, nodal disease, and resection margin status, were extracted from final pathology reports. Resections were characterized as R1, when malignant cells were identified within 1 mm (≤1 mm) from the surgical margin. Patient appointments at the institutional outpatient clinic and medical notes from local oncologists for out-of-state patients were utilized for tracking of adjuvant therapy plans and disease recurrence patterns. The date of death was obtained from institutional medical records, the Social Security Death Index, or online obituaries.
Definitions and Statistical Analysis
Summary statistics were obtained using established methods. Categorical variables were compared by the χ2 test, whereas continuous variables were compared using the Mann–Whitney U test. Overall survival (OS) and post-resection survival were defined as the time from date of diagnosis (OS) or resection (post-resection survival) to either death or last follow-up. Recurrence-free survival (RFS) was defined as the time interval between date of operation and either date of recurrence or last follow-up, if recurrence was not observed. Kaplan–Meier curves were used to estimate median survival with corresponding 95% confidence intervals (CIs). The log-rank test was utilized for subgroup comparison. A 2-sided P value of <0.05 was considered statistically significant. Statistical analysis was performed with SPSS statistical software version 25.0 (SPSS Inc., Chicago, IL).
RESULTS
Patient Cohort
The flowchart for the study selection cohort is available in Figure 1. Within the studied period, 932 patients were evaluated at the institutional PMDC. In total, 109 patients (12%) met the exclusion criteria; most of them had undergone resection of the primary tumor before initial PMDC presentation (n = 85). The remaining 823 patients were stratified based on their clinical disease stage and resectability status. According to the NCCN guidelines, 461 patients were characterized as LAPC (56%). Induction chemotherapy and/or chemoradiation, and re-evaluation were recommended in all LAPC cases, on an individual basis. After initial presentation, 46 patients (10%) were lost to follow-up and were excluded.
Resected LAPC Patients
One hundred and sixteen LAPC patients (28%) were deemed eligible for surgical exploration within the studied period. The decision for exploration was made based on tumor response to neoadjuvant therapy (n = 13 with complete or partial response according to RECIST 1.1 criteria,13 Table 1), or offered to patients without signs of local disease progression or metastases after completion of ≥4 months of neoadjuvant therapy (n = 103). Resection of the primary tumor was achieved in 84 patients (20% of all LAPC patients, 72% of those eligible for exploration). For the remainder, resection was aborted intraoperatively, due to occult abdominal metastatic disease (n = 12, 10%), or local extension of the tumor (n = 20, 17%).
TABLE 1.
Tumor Response Postneoadjuvant Therapy in All Explored LAPC Patients, According to RECIST 1.1 Criteria13
| Variable | All Explored Patients (n = 116) | Resected (n = 84) | Non-resected (n = 32) | P-value |
|---|---|---|---|---|
| RECIST 1.1, grade | 0.463 | |||
| Complete response | 2 (2%) | 2 (2%) | 0 (0%) | |
| Partial response (<30%) | 11 (9%) | 8 (10%) | 3 (9%) | |
| Stable disease | 96 (83%) | 70 (83%) | 26 (81%) | |
| Progressive disease | 7 (6%) | 4 (5%) | 3 (9%) |
LAPC indicates locally advanced pancreatic cancer.
All resected LAPC patients had an ECOG performance status of 0 or 1 (Table 2). Most common tumor site was at the right side of the pancreas (head, uncinate process, and pancreatic neck, n = 57, 68%), and mean tumor size was 35 mm (±11 mm, SD). The median value of cancer antigen 19–9 (CA19–9) at presentation was 72U/mL (IQR 35–268). A pancreaticoduodenectomy was the most common type of performed operation (n = 44, 53%), followed by distal pancreatectomy with en bloc celiac axis resection (n = 23, 27%). Postoperative outcomes are detailed in Table 3. Thirty-day morbidity per Clavien-Dindo classification14 was 59% (n = 49), and 90-day mortality 4% (n = 3): one patient died from sepsis and subsequent multiorgan failure, one from a cardiovascular event, and another from failure to thrive postoperatively. Margin-negative resection (R0) was achieved in 89% of cases (n = 75).
TABLE 2.
Demographics, Tumor Characteristics, and Neoadjuvant Treatment Data of Resected and Non-resected Locally Advanced Pancreatic Cancer Patients
| Variable | All Patients (n = 415) | Resected (n = 84) | Non-resected (n = 331) | P-value |
|---|---|---|---|---|
| Male, n (%) | 195 (47%) | 36 (43%) | 159 (48%) | 0.396 |
| Age, years | ||||
| Mean (SD) | 65.2 (10.1) | 63.3 (9.1) | 65.6 (10.3) | 0.050 |
| ECOG performance status, n (%) | <0.001 | |||
| ECOG 0 | 131 (32%) | 42 (50%) | 89 (27%) | |
| ECOG 1 | 260 (63%) | 42 (50%) | 218 (66%) | |
| ECOG 2 | 21 (5%) | 0 (0%) | 21 (6%) | |
| ECOG 3 | 3 (1%) | 0 (0%) | 3 (1%) | |
| CT-scan characteristics, n (%) | ||||
| Tumor size, mm, mean (SD) | 38 (14) | 35 (11) | 39 (15) | 0.029 |
| Regional lymphadenopathy, n (%) | 111 (27%) | 24 (29%) | 87 (26%) | 0.672 |
| GI involvement, n (%) | 91 (22%) | 10 (12%) | 81 (25%) | 0.013 |
| Duodenum | 68 (75%) | 6 (60%) | 62 (77%) | |
| Stomach | 22 (24%) | 3 (30%) | 19 (23%) | 0.987 |
| Small bowel | 1 (1%) | 1 (10%) | 0 (0%) | |
| Tumor site, n (%) | 0.002 | |||
| Head | 174 (42%) | 33 (39%) | 141 (43%) | |
| Uncinate | 62 (15%) | 13 (16%) | 49 (15%) | |
| Neck | 51 (12%) | 11 (13%) | 40 (12%) | |
| Body | 115 (28%) | 24 (29%) | 91 (28%) | |
| Tail | 13 (3%) | 3 (4%) | 10 (3%) | |
| Atrophy of the pancreas, n (%) | 258 (62%) | 40 (48%) | 218 (66%) | |
| CA 19–9 at PMDC* (U/ml) | ||||
| Median (IQR) | 181 (56–628) | 72 (35–268) | 206 (72–693) | <0.001 |
| Final neoadjuvant CHT, n (%) | 378 (91%) | 84 (100%) | 294 (89%) | 0.001 |
| Stratified, n (%) | ||||
| FFX-based | 189 (50%) | 53 (63%) | 136 (46%) | 0.018† |
| FFX-gemcitabine combination | 72 (19%) | 14 (17%) | 58 (20%) | |
| Gemcitabine-based | 117 (31%) | 17 (20%) | 100 (34%) | |
| Time of therapy administered, mo | <0.001 | |||
| Median (IQR) | 4 (3–6) | 5 (4–6) | 4 (3–6) | |
| Final neoadjuvant RT, n (%) | 222 (54%) | 81 (96%) | 141 (43%) | <0.001 |
| Modality, n (%) | ||||
| SBRT | 160 (72%) | 67 (80%) | 93 (66%) | |
| IMRT/EBRT | 62 (28%) | 14 (17%) | 48 (34%) | |
Thirty patients had CA 19–9 = 1 (not expressing the Lewis antigen).
Post hoc analysis (resected vs non-resected): FFX-based and Gemcitabine-based: P = 0.006, FFX-gemcitabine combination and gemcitabine-based: P = 0.376, FFX-based and FFX-gemcitabine combination: P = 0.155.
CA 19–9 indicates cancer antigen 19–9; CHT, chemotherapy; CT, computed tomography; EBRT, external beam radiation therapy; ECOG, Eastern Cooperative Oncology Group; FFX, FOLFIRINOX; IMRT, intensity-modulated radiation therapy; IQR, interquartile range; PMDC, pancreatic multidisciplinary clinic; RT, radiation therapy; SBRT, stereotactic body radiation therapy; SD, standard deviation.
TABLE 3.
Perioperative Data and Outcomes of Resected Locally Advanced Pancreatic Cancer Patients
| Variable | Resected Patients (n = 84) |
|---|---|
| Type of operation, n (%) | |
| Whipple | 44 (53%) |
| DP-CAR | 23 (27%) |
| Distal pancreatectomy | 16 (19%) |
| Total pancreatectomy | 1 (1%) |
| Length of hospital stay, days | |
| Median (IQR) | 8 (6–11) |
| Morbidity, n (%) | |
| ≤ Clavien-Dindo grade IIIa | 45 (54%) |
| ≥ Clavien-Dindo grade IIIb | 4 (5%) |
| Thirty-day readmission, n (%) | 24 (29%) |
| Thirty-day mortality, n (%) | 1 (1%) |
| Ninety-day mortality, n (%) | 3 (4%) |
| Adjuvant chemotherapy, n (%) | 25 (30%) |
| Months of adjuvant chemotherapy administered | |
| Median (IQR) | 3 (2–4) |
| Disease recurrence, n (%) | 40 (48%) |
| Recurrence site, n (%) | |
| Local | 13 (33%) |
| Liver | 9 (23%) |
| Carcinomatosis | 7 (18%) |
| Multiple sites | 7 (18%) |
| Lung | 4 (10%) |
| Tumor size in pathology report, mm | |
| Mean (SD) | 20 (16) |
| Tumor differentiation, n (%) | |
| Well | 2 (2%) |
| Moderate | 45 (54%) |
| Poor | 16 (19%) |
| Unknown | 19 (23%) |
| Resection margin status | |
| Negative (R0, >1 mm), n (%) | 75 (89%) |
| Positive (R1, ≤1 mm), n (%) | 9 (11%) |
| Positive lymph nodes, n (%) | 21 (25%) |
| Perineural invasion, n (%) | 48 (57%) |
| Perivascular invasion, n (%) | 18 (21%) |
| Treatment response, n (%) | |
| Complete (grade 0) | 5 (6%) |
| Extensive (grade 1) | 27 (32%) |
| Moderate (grade 2) | 33 (39%) |
| Poor (grade 3) | 13 (16%) |
| Unknown | 6 (7%) |
DP-CAR indicates distal pancreatectomy with en bloc celiac artery resection (modified Appleby procedure); IQR, interquartile range; SBRT, stereotactic body radiation therapy; SD, standard deviation.
Significant variations were noted in the neoadjuvant chemotherapy regimens (Supplemental Table 1, http://links.lww.com/SLA/B399): most of the patients received FOLFiRiNOX5 (n = 44, 52%), or “modified” FOLFIRINOX (omitted bolus doses of 5-FU or leucovorin, n = 7, 8%), and regimens were adapted individually, depending on drug toxicity and tolerance. In 16 patients (19%), gemcitabine/nab-paclitaxel was preferred.15 Fourteen patients (17%) received both FOLFIRINOX and gemcitabine/nab-paclitaxel at different time points, due to either drug toxicity (n = 8), or increase in CA19–9 and disease progression on imaging under one regimen (n = 6). Neoadjuvant therapy scheme was decided by a medical oncologist on an individual basis, taking multiple factors under consideration, including patient performance status and personal medical history. The median time of chemotherapy received for resected LAPC patients was 5 months (IQR 4–6). Radiation therapy was added as an adjunct after induction chemotherapy in almost all patients who were offered surgical exploration (n = 111, 96%), to maximize the probability of margin-negative resection. SBRT was the modality of choice for the resected cohort (n = 67, 80%). The median total time from diagnosis to surgery was 10 months (IQR 9–11).
Twenty-five patients received additional chemotherapy postoperatively (30%) for a median time of 3 months (IQR 2–4). The decision for adjuvant therapy was also made by the medical oncologist based on final pathology and patient status. The administered adjuvant regimens are summarized in Supplementary Table 1 (http://links.lww.com/SLA/B399). Disease recurrence after resection was recorded in 40 patients (48%), with local recurrence (n = 13, 33%) being the most common, followed by distant liver metastases (n = 9, 23%).
Non-resected LAPC Patients
Overall, 331 LAPC patients were treated only with chemotherapy and radiation therapy: 299 deemed not eligible for exploration and 32 who were declared unresectable intraoperatively. These patients developed distant metastases or significant local disease progression during neoadjuvant treatment. At the time of initial evaluation at the PMDC, non-resected patients were marginally older (P = 0.050) and were more often characterized as ECOG 1 instead of ECOG 0 (P < 0.001). In addition, mean tumor size in baseline MDCT was larger compared to resected LAPC patients (39 mm vs 35 mm, P = 0.029), and gastrointestinal tract involvement was more frequent (P = 0.013). Median CA 19–9 values were also significantly higher in the non-resected group (206 U/mL, IQR 72–693, P < 0.001).
Variations in chemotherapy regimen combinations were also common in the non-resected cohort. Thirty-seven patients (11%) in total did not receive chemotherapy after diagnosis. ECOG status of 2 or 3 was associated with no (P = 0.004) or less chemotherapy (2.5 vs 4 mo for ECOG 0 or 1, P = 0.020). When compared with the resected group, non-resected patients most often underwent gemcitabine-based chemotherapy (P = 0.006). Moreover, the median duration of administered chemotherapy was shorter (4 vs 5 mo, P < 0.001). Less than half of the non-resected patients received complementary radiation therapy (n = 141, 43%). However, the distribution of SBRT versus traditional external beam and intensity-modulated radiation therapy was similar in both cohorts.
Survival Rates in LAPC Patients
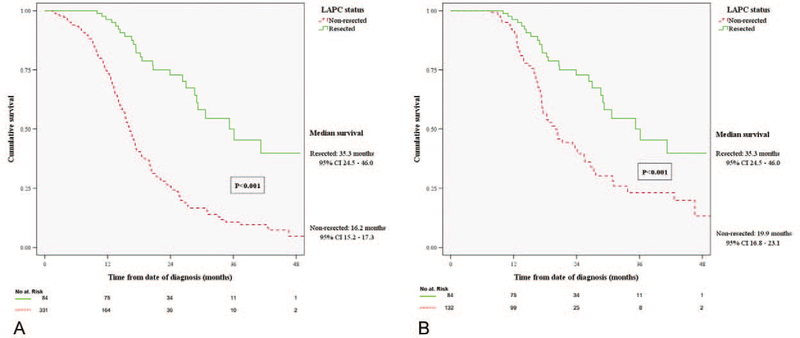
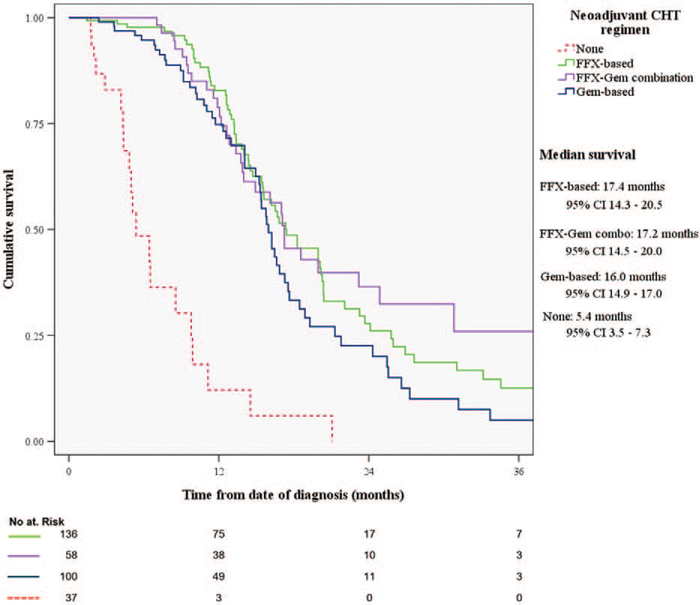
In this highly selected patient cohort, median survival from the time of diagnosis was 35.3 months (95% CI, 24.5–46.0) for the resected, and 16.2 months (95% CI, 15.2–17.3) for the non-resected group (P < 0.001; Fig. 2A). In the non-resected cohort, OS was not associated with the type of chemotherapy regimen (gemcitabine-based 16 mo vs FOLFIRINOX-based 17.4 mo, P = 0.134; Fig. 3). Patients who switched chemotherapy during the course of their treatment also had comparable OS (17.2 mo, 95% CI, 14.5–20; P = 0.099 vs gemcitabine-based, and P = 0.675 vs FOLFIRINOX-based only). Patients who received complementary neoadjuvant radiation therapy after chemotherapy had increased OS (20 vs 14 mo, P < 0.001). As expected, patients who did not receive any treatment after diagnosis, due to poor performance status or personal preference (n = 37), had worse survival than any treatment approach (5.4 mo, IQR 3.5–7.3, P < 0.001).
FIGURE 2.
(A) Comparison of overall survival between resected and non-resected LAPC patients. (B) Comparison of overall survival between resected LAPC patients, and non-resected who underwent ≥5 months of chemotherapy (: non-resected patients who died, or progressed within 5 mo, or received <5 mo of neoadjuvant chemotherapy). LAPC indicates locally advanced pancreatic cancer.
FIGURE 3.
Comparison of overall survival between different chemotherapy regimens in non-resected locally advanced pancreatic cancer patients. CHT indicates chemotherapy; FFX, FOLFIRINOX; Gem, gemcitabine.
The plateau identified in the resected group survival curve (Fig. 2A) depicts the median time interval between diagnosis and surgery (10 mo, IQR 9–11). In an attempt to limit selection bias, all non-resected patients who progressed or died within this same time interval were excluded, and thus a matched group of non-resected patients who received ≥5 months of chemotherapy (n = 132) was compared with the resected cohort: OS remained significantly different, favoring surgical resection (35.3 mo vs 19.9 mo, P < 0.001; Fig. 2B). Statistical significance was preserved when we further limited the non-resected cohort to patients who received 5 months of chemotherapy and SBRT (n = 74, 25.5 mo, P = 0.019). The impact of surgical resection on OS is also demonstrated in the percentage of alive patients at the 1-year and 3-year time points: in the resected group, 96% (n = 81) and 50% (n = 42) were alive at 1 year and 3 years, whereas in the non-resected group 74% (n = 246, P < 0.001) and 11% (n = 37, P < 0.001), respectively.
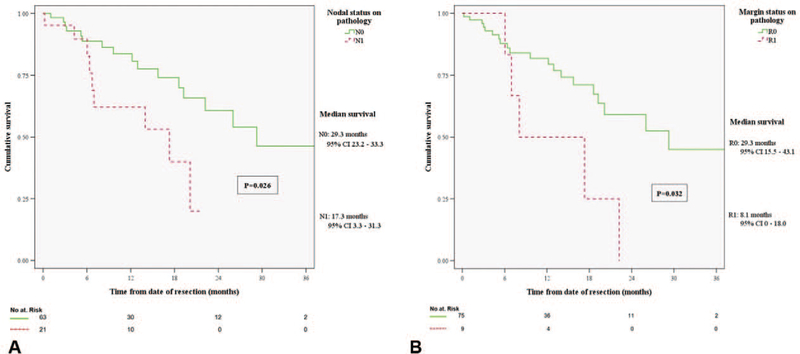
In the resected group, median postoperative RFS was 11.3 months (95% CI, 9.3–13.3). Patients who received adjuvant chemotherapy did not have significantly longer postoperative RFS (15.2 vs 9.9 mo, P = 0.135) or OS (36.1 vs 28.6 mo, P = 0.577). However, patients with positive nodal disease in final pathology had significantly lower post-resection survival compared with node-negative patients (17.3 vs 29.3 mo, P = 0.026; Fig. 4A). Similarly, post-resection survival was worse with margin-positive resection (8.1 vs 29.3 mo, P = 0.032; Fig. 4B).
FIGURE 4.
(A) Comparison of overall survival in patients with negative (N0) versus positive (N1) nodal disease in final pathology report. (B) Comparison of overall survival in patients with negative (R0) versus positive (R1) resection margin.
DISCUSSION
This study provides detailed insight on the management and outcomes of patients with LAPC and clearly identifies a survival advantage in a selected cohort who underwent resection of the primary tumor, compared with those who did not. Moreover, we have overcome the confounding factor of previous reports that combined borderline resectable and LAPC patients. In previous retrospective studies, surgical resection of the primary tumor was the main driver for improved OS. Ferrone et al showed increased resection rates after FOLFIRINOX therapy in 25 LAPC patients.8 Hackert et al also demonstrated that FOLFIRINOX resulted in primary tumor resectability of >60% in a large mixed cohort of borderline resectable, locally advanced, and metastatic PDAC patients.9 In our cohort, FOLFIRINOX-based therapy correlated with increased resection rates (P = 0.006). SBRT after FOLFIRINOX further contributed to that increase (P < 0.001). SBRT as adjunct modality in LAPC has been previously associated with improvement of local disease control and increased likelihood of margin-negative resection.10,16–18
The decision to offer surgical exploration was driven by 3 main factors: vessel involvement by the tumor, ≥4 months of chemotherapy, and/or absence of disease progression. After completion of scheduled treatment, patients with good performance status and no disease progression were re-reviewed and offered surgical exploration, with a median time to the operating room of 6 weeks. As Ferrone et al previously suggested, imaging response alone may be inadequate to declare resectability after neoadjuvant treatment,8 and this also proves to be the case in our series: only 12% of resected patients had partial or complete response according to RECIST criteria.13
Surgical resection of the primary tumor correlated with significant improvement in OS in LAPC patients, with a median time of approximately 3 years from diagnosis. Moreover, half of the resected patients were alive at the 3-year time point. The decision to give adjuvant chemotherapy (n = 25, 30%) was mainly based on patient preference, performance status, and final pathology findings. Interestingly, administration of adjuvant chemotherapy did not seem to further improve the post-resection survival of these patients (P = 0.577). In addition, we did not identify a correlation between administration of adjuvant chemotherapy and the time of disease recurrence. This conclusion coincides with previous reports9,19; however, data on adjuvant chemotherapy after resection in LAPC patients are scarce. Increased resection rates with current neoadjuvant therapies make this an interesting field for future studies.
Distal pancreatectomy with en bloc celiac axis resection (DP-CAR) was the second most common operation performed in our study. Until recently, involvement of the CA in LAPC resulted to unresectability. The development of DP-CAR allowed surgical resection of body or tail tumors that involved the CA. The rationale behind this surgical approach is that the liver arterial supply will be preserved via retrograde blood flow from the gastroduodenal to the proper hepatic artery, after resection of the CA and ligation of the common hepatic artery. Despite an increased risk of liver ischemia, advancements in assessment of hepatic supply and optimized surgical technique allow improved postoperative morbidity and mortality rates in high-volume centers. Currently, DP-CAR, also known as the “modified Appleby” procedure, is the most common operation that involves arterial resection in PDAC.20
Positive nodal status (N1) and margin-positive resection (R1) had a direct impact on post-resection survival. In the resected cohort, 25% of the patients had N1 disease, a lower percentage than historically reported in patients with resectable PDAC.21 Regional lymph node metastases correlated with lower median RFS and post-resection survival (P = 0.047 and 0.026, respectively), in concordance with studies on stage I/II disease.22,23 Furthermore, resected patients who underwent R0 resection (88%) had significantly higher median post-resection survival (P = 0.032). Increased R0 resection rates have been previously identified after neoadjuvant therapy,9 compared with upfront resection in resectable PDAC,24 due to extensive necrosis of the tumor. Margin-positive resection is associated with increased risk of local recurrence and consequently shorter post-resection survival.25,26
The survival benefit of FOLFIRINOX5 and gemcitabine/nab-paclitaxel15 against gemcitabine monotherapy in metastatic PDAC patients paved the road for anecdotal treatment of LAPC in recent years. Multiple studies have associated both regimens with increased OS in patients with unresectable pancreatic cancer (borderline resectable and locally advanced).9,16,17,27–30 In a recent meta-analysis, Suker et al reported a median overall survival of 24.2 months after FOLFIRINOX therapy in LAPC patients.6 Data on the role of radiation and chemoradiation therapy after induction chemotherapy, however, are more controversial. The LAP07 trial failed to show a survival benefit with the addition of chemoradiotherapy (54 Gy plus capecitabine) after 4 months of chemotherapy,31 but even in this setting the chemoradiation arm experienced a local control benefit. Moreover, recent retrospective studies have demonstrated a potentially beneficial role of SBRT in LAPC,10,18,32 both for local control, as well as to help achieve a margin-negative resection. In this study, we focused exclusively on LAPC patients and came across significant treatment variations, characterized by extensive micromanagement, due to drug toxicity or disease progression. Treatment stratification based on the main chemotherapy agent provided a valid comparison basis. Except for patients who did not receive any treatment, OS was similar across different chemotherapy regimens in the non-resected cohort. A further positive effect of radiation therapy on OS was noted, most likely due to selection bias: patients whose disease did not progress during induction chemotherapy had an option for additional radiation therapy, under the prism of future potential surgical resection.
In this large LAPC cohort, one can identify tumor biology as the common denominator for patient outcomes. In all stage IIIPDAC patients, a major goal at the time of diagnosis is potential surgical resection of the primary tumor. Our retrospective data indicate that regardless of neoadjuvant chemotherapy regimen, approximately 25% of patients had the chance to undergo surgical exploration with curative intent, among which 75% were resected, leading to a final patient ratio of 1:5 for surgical resection versus no resection in LAPC. This resection rate seems to be low compared with previous retrospective studies,27,28,33,34 and we can acknowledge 2 distinct factors for this difference: (1) borderline resectable PDAC patients were not part of the studied cohort (historically the resection rate for these patients in our institution is 65%), and (2) all LAPC patients who presented at PMDC were included in the initial pool, including those who did not receive neoadjuvant chemotherapy, or progressed early in the course of their disease. Most importantly however, surgical resection in LAPC patients was associated with an additional survival advantage that was comparable to their resected stage I/II counterparts.35 At the moment, we cannot identify with scientific certainty demographic and clinical parameters that will suggest which patients will become eligible for surgery. Genetic identification of PDAC subtypes may provide additional information about treatment response patterns,36 whereas optimal neoadjuvant treatment schemes will need to be determined through randomized prospective trials.37
This study has several limitations. First, it is a retrospective study and eligible LAPC patients were not prospectively randomized to surgical resection or observation after neoadjuvant therapy. Therefore, it is subject to selection bias on multiple levels and its conclusions are observational. At PMDC, decision-making for neoadjuvant therapy was based on patient characteristics and expert consensus. In addition, micromanagement of neoadjuvant therapy and lack of adherence to standardized treatment protocols introduced further selection bias on which patients may respond, and how many will proceed to radiation therapy and surgical exploration. Specific data on regimen toxicity were also limited because many out-of-state patients were not treated in our institution. Moreover, lead-time bias is possible because a minority of patients were followed up for a shorter period than the median time required from diagnosis to surgery, and they may be eligible for resection later in time. However, our data emanate from a realistic multidisciplinary approach in a high-volume institution for PDAC and reflect the current landscape of LAPC treatment. Randomized prospective trials are needed to identify the optimal neoadjuvant chemotherapy regimen for improved resection rates and further assess the impact on overall survival in LAPC patients.
CONCLUSIONS
This large retrospective study demonstrates the current trends in LAPC treatment. Improved overall survival does not seem to correlate with a specific neoadjuvant regimen. Patients who receive ≥4 months of neoadjuvant treatment without signs of disease progression may be eligible for surgical exploration and resection. In our study, this resected cohort is 20% of all LAPC patients. Patients who underwent surgical resection of the primary tumor had significantly better overall survival, reaching a median of 35 months. A multidisciplinary approach in high-volume centers is necessary to identify these patients. Future prospective trials are required to define the optimal neoadjuvant treatment for LAPC.
Supplementary Material
ACKNOWLEDGMENTS
The authors thank Lindsay Parish and Mary Hodgin for their help in data acquisition from the Johns Hopkins Pancreatic Multidisciplinary Clinic.
This work was supported by SPORE in GI Cancer CA 62924.
Footnotes
The authors report no conflicts of interest.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal’s Web site (www.annalsofsurgery.com).
REFERENCES
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2017. CA Cancer J Clin. 2017;67:7–30. [DOI] [PubMed] [Google Scholar]
- 2.Rahib L, Smith BD, Aizenberg R, et al. Projecting cancer incidence and deaths to 2030: the unexpected burden of thyroid, liver, and pancreas cancers in the United States. Cancer Res. 2014;74:2913–2921. [DOI] [PubMed] [Google Scholar]
- 3.Wolfgang CL, Herman JM, Laheru DA, et al. Recent progress in pancreatic cancer. CA Cancer J Clin. 2013;63:318–348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Balaban EP, Mangu PB, Khorana AA, et al. Locally advanced, unresectable pancreatic cancer: American Society of Clinical Oncology Clinical Practice Guideline. J Clin Oncol. 2016;34:2654–2668. [DOI] [PubMed] [Google Scholar]
- 5.Conroy T, Desseigne F, Ychou M, et al. FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N Engl J Med. 2011;364:1817–1825. [DOI] [PubMed] [Google Scholar]
- 6.Suker M, Beumer BR, Sadot E, et al. FOLFIRINOX for locally advanced pancreatic cancer: a systematic review and patient-level meta-analysis. Lancet Oncol. 2016;17:801–810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhu C-P, Shi J, Chen Y-X, et al. Gemcitabine in the chemoradiotherapy for locally advanced pancreatic cancer: a meta-analysis. Radiother Oncol. 2011;99:108–113. [DOI] [PubMed] [Google Scholar]
- 8.Ferrone CR, Marchegiani G, Hong TS, et al. Radiological and surgical implications of neoadjuvant treatment with FOLFIRINOX for locally advanced and borderline resectable pancreatic cancer. Ann Surg. 2015;261: 12–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hackert T, Sachsenmaier M, Hinz U, et al. Locally advanced pancreatic cancer: neoadjuvant therapy with folfirinox results in resectability in 60% of the patients. Ann Surg. 2016;264:457–463. [DOI] [PubMed] [Google Scholar]
- 10.Moningi S, Dholakia AS, Raman SP, et al. The role of stereotactic body radiation therapy for pancreatic cancer: a single-institution experience. Ann Surg Oncol. 2015;22:2352–2358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Michelakos T, Pergolini I, Castillo CF, et al. Predictors of resectability and survival in patients with borderline and locally advanced pancreatic cancer who underwent neoadjuvant treatment with FOLFIRINOX. Ann Surg. 2017. doi: 10.1097/SLA.0000000000002600 [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 12.Tempero MA, Malafa MP, Al-Hawary M, et al. Pancreatic adenocarcinoma, Version 2. 2017, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw. 2017;15:1028–1061. [DOI] [PubMed] [Google Scholar]
- 13.Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009;45:228–247. [DOI] [PubMed] [Google Scholar]
- 14.Clavien PA, Barkun J, de Oliveira ML, et al. The Clavien-Dindo classification of surgical complications: five-year experience. Ann Surg. 2009;250:187–196. [DOI] [PubMed] [Google Scholar]
- 15.Von Hoff DD, Ervin T, Arena FP, et al. Increased survival in pancreatic cancer with nab-paclitaxel plus gemcitabine. N Engl J Med. 2013;369:1691–1703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mellon EA, Hoffe SE, Springett GM, et al. Long-term outcomes of induction chemotherapy and neoadjuvant stereotactic body radiotherapy for borderline resectable and locally advanced pancreatic adenocarcinoma. Acta Oncol. 2015;54:979–985. [DOI] [PubMed] [Google Scholar]
- 17.Kluger MD, Rashid MF, Rosario VL, et al. Resection of locally advanced pancreatic cancer without regression of arterial encasement after modern-era neoadjuvant therapy. J Gastrointest Surg. 2018;22:235–241. [DOI] [PubMed] [Google Scholar]
- 18.Zhong J, Patel K, Switchenko J, et al. Outcomes for patients with locally advanced pancreatic adenocarcinoma treated with stereotactic body radiation therapy versus conventionally fractionated radiation. Cancer. 2017;123: 3486–3493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.de Geus SWL, Kasumova GG, Eskander MF, et al. Is neoadjuvant therapy sufficient in resected pancreatic cancer patients? A National Study. J Gastrointest Surg. 2018;22:214–225. [DOI] [PubMed] [Google Scholar]
- 20.Peters NA, Javed AA, Cameron JL, et al. Modified appleby procedure for pancreatic adenocarcinoma: does improved neoadjuvant therapy warrant such an aggressive approach? Ann Surg Oncol. 2016;23:3757–3764. [DOI] [PubMed] [Google Scholar]
- 21.Cameron JL, He J. Two thousand consecutive pancreaticoduodenectomies. J Am Coll Surg. 2015;220:530–536. [DOI] [PubMed] [Google Scholar]
- 22.Wentz SC, Zhao Z-G, Shyr Y, et al. Lymph node ratio and preoperative CA 19–9 levels predict overall survival and recurrence-free survival in patients with resected pancreatic adenocarcinoma. World J Gastrointest Oncol. 2012;4: 207–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tol JAMG, Brosens LAA, van Dieren S, et al. Impact of lymph node ratio on survival in patients with pancreatic and periampullary cancer. Br J Surg. 2015;102:237–245. [DOI] [PubMed] [Google Scholar]
- 24.Neoptolemos JP, Stocken DD, Dunn JA, et al. Influence of resection margins on survival for patients with pancreatic cancer treated by adjuvant chemoradiation and/or chemotherapy in the ESPAC-1 randomized controlled trial. Ann Surg. 2001;234:758–768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Groot VP, Rezaee N, Wu W, et al. Patterns, timing, and predictors of recurrence following pancreatectomy for pancreatic ductal adenocarcinoma. Ann Surg. 2017. doi: 10.1097/SLA.0000000000002234. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 26.Konstantinidis IT, Warshaw AL, Allen JN, et al. Pancreatic ductal adenocarcinoma: is there a survival difference for R1 resections versus locally advanced unresectable tumors? What is a “true” R0 resection? Ann Surg. 2013;257:731–736. [DOI] [PubMed] [Google Scholar]
- 27.Sadot E, Doussot A, O’Reilly EM, et al. FOLFIRINOX induction therapy for stage 3 pancreatic adenocarcinoma. Ann Surg Oncol. 2015;22:3512–3521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Blazer M, Wu C, Goldberg RM, et al. Neoadjuvant modified (m) FOLFIRINOX for locally advanced unresectable (LAPC) and borderline resectable (BRPC) adenocarcinoma of the pancreas. Ann Surg Oncol. 2015;22: 1153–1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Reni M, Zanon S, Balzano G, et al. Selecting patients for resection after primary chemotherapy for non-metastatic pancreatic adenocarcinoma. Ann Oncol. 2017;28:2786–2792. [DOI] [PubMed] [Google Scholar]
- 30.Marthey L, Sa-Cunha A, Blanc JF, et al. FOLFIRINOX for locally advanced pancreatic adenocarcinoma: results of an AGEO multicenter prospective observational cohort. Ann Surg Oncol. 2015;22:295–301. [DOI] [PubMed] [Google Scholar]
- 31.Hammel P, Huguet F, van Laethem J-L, et al. Effect of chemoradiotherapy vs chemotherapy on survival in patients with locally advanced pancreatic cancer controlled after 4 months of gemcitabine with or without erlotinib: the LAP07 randomized clinical trial. JAMA. 2016;315:1844–1853. [DOI] [PubMed] [Google Scholar]
- 32.Dohopolski MJ, Glaser SM, Vargo JA, et al. Stereotactic body radiotherapy for locally-advanced unresectable pancreatic cancer-patterns of care and overall survival. J Gastrointest Oncol. 2017;8:766–777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sui K, Okabayashi T, Shima Y, et al. Clinical effects of chemoradiotherapy in pursuit of optimal treatment of locally advanced unresectable pancreatic cancer. Br J Radiol 1075;90:20170165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rombouts SJ, Walma MS, Vogel JA, et al. Systematic review of resection rates and clinical outcomes after FOLFIRINOX-based treatment in patients with locally advanced pancreatic cancer. Ann Surg Oncol. 2016;23: 4352–4360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mokdad AA, Minter RM, Zhu H, et al. Neoadjuvant therapy followed by resection versus upfront resection for resectable pancreatic cancer: a propensity score matched analysis. J Clin Oncol. 2017;35:515–522. [DOI] [PubMed] [Google Scholar]
- 36.Waddell N, Pajic M, Patch A-M, et al. Whole genomes redefine the mutational landscape of pancreatic cancer. Nature. 2015;518:495–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.ClinicalTrials.gov. Bethesda (MD): National Library of Medicine (US) NCT02125136, Trial to Investigate Intensified Neoadjuvant Chemotherapy in Locally Advanced Pancreatic Cancer (NEOLAP); April 29, 2014. Available from https://clinicaltrials.gov/ct2/show/NCT02125136. Accessed March 4, 2018. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.