Abstract
Background
Patients undergoing Total Knee Arthroplasty (TKA) typically have early postoperative pain and decreased mobility, especially so in the first 24 h. Achieving a pain free knee in the immediate postoperative period and reducing complications using multimodal pain and blood management protocols forms a keystone in early mobilization and functional recovery. Enhanced Recovery after Surgery pathways (ERASp) since their inception, have significantly improved perioperative care and functional outcomes, thereby reducing the average length of stay (ALOS), complications and overall healthcare costs. ERASp modified suitably for TKA have had encouraging results. We have retrospectively analyzed the outcomes of the ERASp for TKA at our tertiary care centre with equal emphasis on pre-hospital preparations, in-hospital care, and post-hospital discharge.
Methods
All TKA patients operated by the senior author between July 2016 and January 2018 with a minimum one year follow up were included. The outcomes measured were: Visual Analogue Score (VAS) for pain at rest and on movement, milestones, transfusion requirements, postoperative complications, ALOS and functional scores at one year follow-up.
Results
775 patients (392 unilateral TKA {UTKA} and 383 bilateral {BTKA}) met our inclusion criteria. Both groups were comparable demographically. Mean VAS pain scores at rest were 3.15 ± 2.15 on the day of surgery, 2.5 ± 1.86 on the first postoperative day and 2.08 ± 1.81 on the second day, and 6.2 ± 2.38, 5.77 ± 2.34 and 4.71 ± 2.48 on movement respectively in the UTKA group. In the BTKA group, the mean VAS pain scores at rest were 4.39 ± 2.25 on the day of surgery, 3.98 ± 2.36 on the first postoperative day and 3.05 ± 2.12 on the second day and 6.21 ± 2.38, 5.77 ± 2.34 and 4.71 ± 2.48 on movement respectively. 85.49% of UTKA and 77.22% of BTKA patients walked on the day of surgery. Decrease in haemoglobin and transfusion rates were 1.25 ± 0.41 g% and 0.5%, 1.85 ± 0.62 and 3.9% in the UTKA and BTKA groups respectively.
The average length of hospital stay (LOS) was 3.98 days. LOS was 3.17 and 4.78 days with 1.55% and 6.05% major complications in the UTKA and BTKA groups respectively.
There was a significant improvement in Oxford Knee and WOMAC scores at 3, 6 and 12 months in both groups.
Conclusions
Pain following TKA is a major deterrent in early mobilization thereby delaying functional recovery and increasing ALOS. We recommend our multimodal interdisciplinary protocol to achieve early mobilization, better pain scores and minimize complications, resulting in overall reduced LOS.
Keywords: Enhanced recovery, Fast tracking, Early mobilization, Clinical pathways, Multimodal pain management, Length of stay
1. Introduction
The joints registry of the Indian Society of Hip and Knee surgeons noted that approximately 1, 70, 000 total knee arthroplasties (TKA) were done between 2006 and 2017.1 This number is growing as osteoarthritis becomes widespread and more surgeons report to the registry. Unlike other surgeries, patients undergoing TKA typically have pain and decreased mobility postoperatively, especially so in the first 24 h which increases the morbidity and hospital average length of stay.2,3 Achieving a pain free knee in the immediate postoperative period following TKA forms a keystone in early mobilization and functional recovery. In the past, pain after TKA was managed by epidurals, femoral nerve blocks and opioids. These techniques had side effects which hindered faster recovery.4 Enhanced Recovery after Surgery pathways (ERASp) first described by Kehlet H5 are being gradually modified and used for perioperative management of hip and knee replacements.6 The advent of adductor canal block (ACB), local infiltration analgesia (LIA), other multimodal pain and blood management protocols have significantly enhanced the immediate recovery following TKA.2,7,8
We present an interdisciplinary ERASp for patients undergoing TKA at our tertiary care centre which address pre-hospital preparations, in-hospital care, and post-hospital discharge and has shown outstanding results.
2. Methods
We retrospectively analyzed data of all patients who underwent primary TKA (unilateral TKA {UTKA} and bilateral TKA {BTKA}) between July 2016 and January 2018 at tertiary care centre with a minimum follow up of 12 months. All patients were operated by the senior author, following the same perioperative protocol implemented by an interdisciplinary team. We excluded cases of revision arthroplasty.
-
•
In the preoperative period, all the patients were educated about the surgery using visual aids and information brochures. Prehabilitation was done through quadriceps strengthening exercises and hamstring stretching. Comorbidities such as diabetes, hypertension, asthma and anaemia were optimized. The Preoperative protocol is described in Table 1.
-
•
Most patients were given spinal anaesthesia, while general anaesthesia was given to those patients who had a contraindication to spinal anaesthesia or refused spinal anaesthesia.
-
•
After the spinal or general anaesthesia was in place, an ultrasound guided Adductor canal block (ACB) was given and urinary catheterization was done.
-
•
Posterior stabilized/posterior cruciate substituting prosthesis was implanted using a midvastus approach. Patellaplasty was done in all.9 After checking the trial implant, periarticular local infiltration analgesia (LIA) was injected in the posterior capsule, collateral attachments, synovium, Hoffa's fat pad, tissue adjoining the arthrotomy incision and subcutaneous tissue. The drug mixture is detailed in Table 1. Final implantation was done with cement and the wound was closed in layers over a negative suction drain. Entire surgical procedure was done with a pneumatic tourniquet (>100 mm Hg from baseline systolic blood pressure). The drain was clamped for 4 h after release of the tourniquet as we did not release tourniquet prior to closure. Subsequently the drains were removed 4 h after opening the clamps (8 h postoperative).10 Urinary catheter was removed 6 h after surgery. Postoperative protocol is as described in Table 1.
-
•
All patients were shifted to the recovery room in the theatre complex which functioned as a post-anaesthesia care unit (PACU). They were observed for complete recovery from anaesthesia, adequate blood and fluid management.11 This functioned as a careful transition between the operation theatre and the patient ward.
-
•
Visual analogue pain scores (VAS) were monitored for the first 48 h. DVT socks and intermittent compression devices were applied to the lower limbs along with low molecular weight heparin till discharge.
-
•
All patients were encouraged to sit, stand, walk and climb stairs on the day of surgery as well as on subsequent postoperative days.
-
•
Patients were assessed for functional status using the Oxford knee (OKS) score and Western Ontario and McMaster's Universities Osteoarthritis index (WOMAC) score. OKS and WOMAC scores and any complications were noted at follow up of patients up to 1 year and analyzed using Paired T test.
Table 1.
Preoperative and postoperative protocol for TKA.
| Intervention by time point | Dose | Route | Frequency | Notes | |
|---|---|---|---|---|---|
| Preoperative | |||||
| Gabapentin | 300 mg | Oral | 3 doses | Given at night, starting on night before surgery | |
| Intraoperative | |||||
| Subarachnoid block 0.5%Bupivacaine Heavy + Buprenorphine General Anaesthesia |
15–20 mg 1 μg/kg |
Intrathecal Intrathecal |
Contraindication/failure of subarachnoid block, standard general anaesthesia with endotracheal intubation | ||
| Antibiotic Cefuroxime |
1.5 g | Iv | Prior to surgery, prior to 2nd knee, 2 doses thereafter, 12 hourly | ||
| Adductor canal block Ropivacaine |
12 ml of 0.2% | Adductor canal | 1 dose | Ultrasound guided, prior to surgery, on each side for bilateral TKA | |
| Midazolam | 0.02 mg/kg | Iv | 1-2 doses | ||
| Pantaprazole | 40 mg | iv/oral postop | 1 dose | Continued I dose daily before breakfast | |
| Paracetamol | 1 g | Iv | 1 dose | ||
| Tranexamic acid | 500–1000 mg (10 mg/kg) | Iv | 1 dose | ||
| Methylprednisolone | 500 mg | Iv | 1 dose | ||
| Ondansetron | 4–8 mg | Iv | 1 dose | ||
| Local infiltration | Unilateral | Bilateral | Intra articular | The mixture is diluted to 100 ml and infiltration is done with 20 ml syringes and 18 g needles | |
| Each Knee | |||||
| Levobupivacaine 0.5% | 30 ml | 15 ml | |||
| Clonidine | 75 μg | 75 μg | |||
| Fentanyl | 100 μg | 100 μg | |||
| Adrenaline 1:1000 | 2 drops | 2 drops | |||
| Ketorolac | 30 mg | 30 mg | |||
| Tranexamic acid | 1 g | 500 mg | |||
| Postoperative on day of surgery | Drain kept clamped for 4 h, then released | ||||
| Paracetamol | 1 g | Iv | 2-3doses | ||
| Ice application | Locally | 4 times a day | For 2 days | ||
| Postoperative day one | |||||
| Paracetamol | 1 g | Oral | 2-3 times | ||
| Tramadol | 50 mg | IV | Rescue analgesic | ||
| Dalteparin sodium | 5000U | Sc | Once daily | Till discharge | |
| Buprenorphine patch | 10 μg/h | Transdermal | once | ||
| Decadurobolin | 100 mg | Im | 1 dose | ||
| Vitamin C | 2 g | Oral | 5 doses | One dose a day | |
| Multivitamin with Zinc | Oral | 5 doses | One dose a day | ||
3. Results
775 patients, 392 UTKA and 383 BTKA met our inclusion criteria. Both groups were comparable demographically, including ASA physical status and comorbidities as shown in Table 2. Spinal anaesthesia was administered in 708 patients and 67 patients received general anaesthesia.
Table 2.
Patient characteristics.
| Characteristic | Unilateral | Bilateral |
|---|---|---|
| Age (years) | 62.74 ± 8.965 | 64 ± 8.68 |
| Male | 145 | 81 |
| Female | 247 | 299 |
| Osteoarthritis | 365 | 353 |
| Rheumatoid arthritis | 27 | 27 |
| ASA class 3 | 17 | 17 |
| Diabetes | 86 | 85 |
| Hypertension | 91 | 88 |
| Cardiac morbidity | 41 | 41 |
| COPD | 10 | 9 |
| Other comorbidities | 4 | 3 |
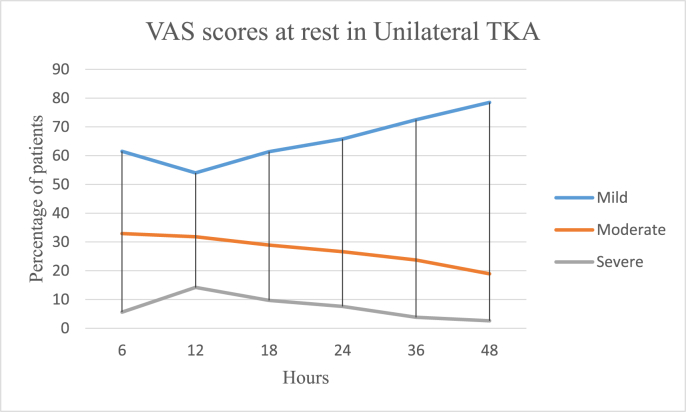
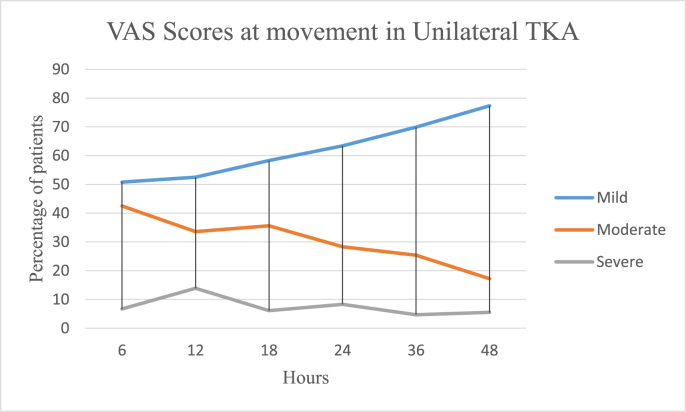
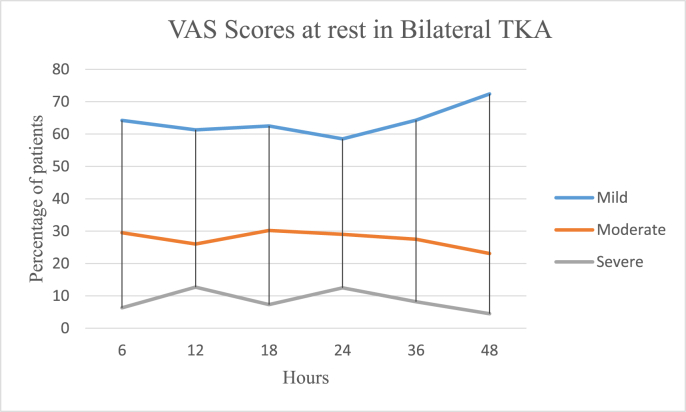
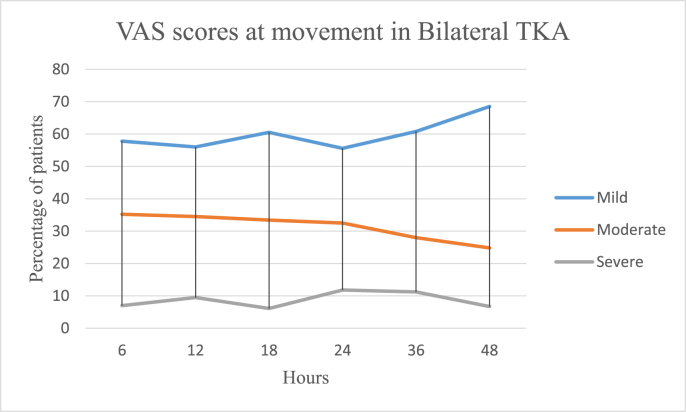
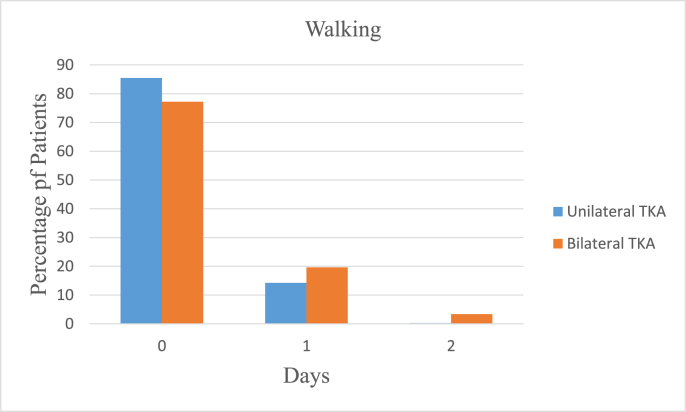
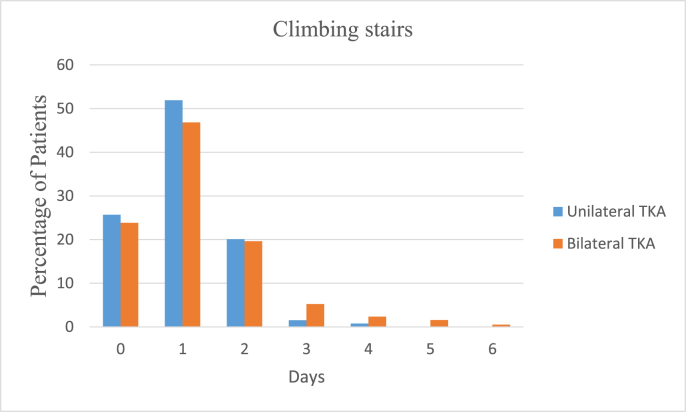
In the UTKA group the mean VAS pain scores at rest were 3.15 ± 2.15 on the day of surgery, 2.5 ± 1.86 on the first postoperative day and 2.08 ± 1.81 on the second day, and 6.2 ± 2.38, 5.77 ± 2.34 and 4.71 ± 2.48 on movement respectively. In the BTKA group the mean VAS pain scores at rest were 4.39 ± 2.25 on the day of surgery, 3.98 ± 2.36 on the first postoperative day and 3.05 ± 2.12 on the second day and 6.21 ± 2.38, 5.77 ± 2.34 and 4.71 ± 2.48 on movement respectively. Further details are as described in Table 3 and Fig. 1a, b, 1c and 1d. Mobilization on the day of surgery was achieved in 85.49 and 77.22% cases of UTKA and BTKA groups respectively; details are displayed in Fig. 2, Fig. 3.
Table 3.
Visual analogue scale pain scores.
|
Unilateral |
6 |
12 |
18 |
24 |
36 |
48 |
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mild | mod | sev | mild | mod | sev | mild | mod | sev | mild | mod | sev | Mild | mod | sev | mild | Mod | sev | ||
| 393 |
VAS r | 3.0 ± 1.76 | 5.81 ± 2.62 | 7.85 ± 1.72 | 2.94 ± 1.83 | 5.22 ± 1.9 | 8.34 ± 1.32 | 3.67 ± 1.19 | 5.64 ± 0.67 | 7.23 ± 2.61 | 2.78 ± 1.74 | 4.88 ± 2.01 | 7.9 ± 0.55 | 2.01 ± 1.56 | 3.68 ± 1.89 | 6.78 ± 2.04 | 1.85 ± 1.24 | 3.98 ± 2.33 | 6.8 ± 0.83 |
| % | 61.5 | 32.9 | 5.6 | 54.0 | 31.8 | 14.2 | 61.4 | 28.9 | 9.7 | 65.8 | 26.6 | 7.6 | 72.5 | 23.7 | 3.8 | 78.5 | 18.9 | 2.6 | |
| VAS m | 2.66 ± 2.23 | 5.78 ± 2.50 | 7.64 ± 2.06 | 3.22 ± 1.56 | 6.02 ± 1.48 | 7.88 ± 1.80 | 3.22 ± 1.65 | 5.8 ± 1.55 | 7.68 ± 2.07 | 3.05 ± 1.66 | 5.12 ± 2.31 | 7.99 ± 1.59 | 2.55 ± 1.17 | 3.84 ± 1.44 | 6.51 ± 2.64 | 2.11 ± 1.66 | 3.77 ± 2.41 | 6.88 ± 1.41 | |
| % |
50.8 |
42.5 |
6.7 |
52.5 |
33.6 |
13.9 |
58.3 |
35.6 |
6.1 |
63.4 |
28.3 |
8.3 |
69.9 |
25.4 |
4.7 |
77.3 |
17.2 |
5.5 |
|
| Bilateral |
Mild |
mod |
sev |
mild |
mod |
sev |
mild |
mod |
sev |
mild |
mod |
sev |
Mild |
mod |
sev |
mild |
Mod |
sev |
|
| 382 | VAS r | 2.38 ± 1.58 | 4.54 ± 0.61 | 7.54 ± 1.74 | 2.94 ± 1.83 | 4.64 ± 2.08 | 7.5 ± 1.6 | 3.32 ± 0.9 | 5.88 ± 1.42 | 7.41 ± 2.48 | 2.5 ± 1.08 | 4.18 ± 2.04 | 8.01 ± 1.1 | 3.4 ± 1.78 | 2.45 ± 1.47 | 7.03 ± 1.22 | 2.23 ± 1.29 | 4.55 ± 2.13 | 7.53 ± 1.22 |
| % | 64.2 | 29.5 | 6.3 | 61.3 | 26.0 | 12.7 | 62.5 | 30.2 | 7.3 | 58.5 | 29 | 12.5 | 64.3 | 27.5 | 8.2 | 72.4 | 23.1 | 4.5 | |
| VAS m | 2.33 ± 2.1 | 4.44 ± 2.31 | 7.12 ± 1.6 | 3.66 ± 1.9 | 5.77 ± 1.76 | 7.25 ± 1.77 | 2.9 ± 1.81 | 5.34 ± 1.62 | 7.28 ± 2.12 | 3.22 ± 1.81 | 5.12 ± 1.9 | 7.4 ± 1.22 | 2.8 ± 1.65 | 3.66 ± 1.8 | 7.02 ± 2.75 | 2.38 ± 1.62 | 3.42 ± 1.82 | 7.1 ± 1.8 | |
| % | 57.8 | 35.2 | 7.0 | 56 | 34.5 | 9.5 | 60.5 | 33.4 | 6.1 | 55.6 | 32.54 | 11.86 | 60.8 | 28.0 | 11.2 | 68.5 | 24.8 | 6.7 | |
Fig. 1.
a: Postoperative Visual analogue pain scores in Unilateral TKA at rest.
Mild is VAS score 0–3, Moderate is VAS score 4–6 and Severe is VAS score 7–10.
1b: Postoperative Visual analogue pain scores in Unilateral TKA on movement.
1c: Postoperative Visual analogue pain scores in Bilateral TKA at rest.
1d: Postoperative Visual analogue pain scores in Bilateral TKA on movement.
Fig. 2.
Post-operative mobilization.
Fig. 3.
Post-operative mobilization.
Postoperative Hemoglobin (Hb) levels and drop in Hb were 11.56 ± 1.22 and 1.25 ± 0.4, 10.96 ± 1.43 and 1.85 ± 0.62 in the UTKA and BTKA groups respectively. Two patients (0.51%) in the unilateral group needed blood transfusions and 15 (3.94%) patients in the bilateral group required transfusions.
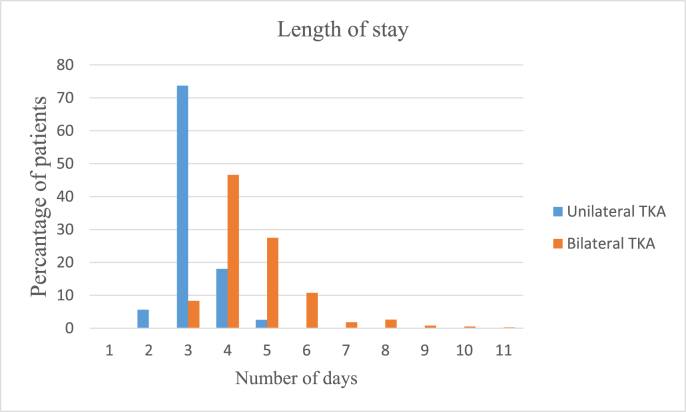
Average hospital length of stay (ALOS) was 3.98 days. LOS was 3.17 days with 79.38% patients getting discharged by postoperative day 3 in the UTKA group. LOS was 4.78 days with 82.39% patients getting discharged by postoperative day 5 in the BTKA group. The distribution of LOS is shown in Fig. 4.
Fig. 4.
Hospital length of stay.
Table 4 details the major and minor postoperative complications. Major complications were seen in 1.55% and 6.05% of unilateral and bilateral cases respectively.
Table 4.
Complication rates.
| Complication | Unilateral TKA No. of patients |
Bilateral TKA No. of patients |
|---|---|---|
| Major | ||
| Cardiac complications | 1 (0.26%) | 6 (1.58%) |
| Pulmonary complications | 0 | 1 (0.26%) |
| Infection | 2 (0.51%) | 6 (1.58%) |
| Periprosthetic fracture | 1 (0.26%) | 1 (0.26%) |
| Revision | 0 | 2 (0.53%) |
| Mortality at 1 year | 0 | 3 (0.79%) |
| Deep vein thrombosis | 1 (0.26%) | 4 (1.05%) |
| Minor | ||
| Electrolyte imbalance | 5 (1.28%) | 7 (1.84%) |
| Urine retention | 6 (1.53%) | 14 (3.68%) |
| Urinary tract infection | 0 | 2 (0.53%) |
| Blood transfusion | 2 (0.51%) | 15 (3.95%) |
| Postoperative ICU | 8 (2.04%) | 33 (8.68%) |
Table 5 shows a significant improvement in the follow-up OKS and WOMAC scores.
Table 5.
OKS and WOMAC scores preoperatively and at follow up.
| Time |
OKS |
WOMAC Score |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Unilateral TKA |
Bilateral TKA |
Unilateral TKA |
Bilateral TKA |
|||||||||
| Right knee | Left knee | Right Knee |
Left knee | |||||||||
| Preoperative | 15.33 ± 2.94 | 14.24 ± 2.95 | 13.97 ± 3.00 | 43.54 ± 4.41 | 43.48 ± 4.40 | 44.93 ± 5.15 | ||||||
| 3 months postoperative | 36.78 ± 4.80 | P < 0.001 | 36.18 ± 4.131 | P < 0.001 | 36.49 ± 2.57 | P < 0.001 | 80.55 ± 7.69 | P < 0.001 | 78.40 ± 5.49 | P < 0.001 | 78.66 ± 5.36 | P < 0.001 |
| 6 months postoperative | 43.01 ± 1.71 | P < 0.001 | 42.87 ± 2.38 | P < 0.001 | 42.62 ± 2.22 | P < 0.001 | 92.06 ± 2.05 | P < 0.001 | 91.53 ± 3.48 | P < 0.001 | 91.35 ± 3.27 | P < 0.001 |
| 12 months postoperative | 46.79 ± 0.94 | P < 0.001 | 46.51 ± 1.80 | P < 0.001 | 46.32 ± 1.71 | P < 0.001 | 97.57 ± 1.02 | P < 0.001 | 96.96 ± 3.02 | P < 0.001 | 96.80 ± 2.89 | P < 0.001 |
Statistically significant improvement in OKS and WOMAC scores at 3, 6 and 12 months when compared with scores at previous follow up (P < 0.001).
4. Discussion
ERASp have proven their efficacy in various surgical fields.12 They effectively reduced perioperative morbidity, pain, complications and thereby improved the functional outcomes. This led to reduced ALOS and healthcare costs.13
The patient and surgeon expectations following TKA have undergone a major change with prime focus on early mobilization, discharge from hospital and return to function.14 There are a few studies which describe utilizing the ERASp and modifying them to enable fast tracking of individuals undergoing TKA.6,15, 16, 17 At our institute, we have implemented an interdisciplinary pathway focusing on preoperative patient education and optimization, anaesthesia and surgical technique, multimodal pain and blood management, early mobilization and reducing ALOS and complications.
-
1.
Preoperative patient education and prehabilitation form a key component to allay anxiety.15 Wang et al. showed that though prehabilitation can slightly improve early postoperative pain and function in patients undergoing joint replacement, their effects remain too small and short-term to be considered clinically-important, and did not affect key outcomes such as length of stay and costs.18 This may be due to the fact that patients with osteoarthritis which is severe enough to need surgery may be unable to achieve the exercise levels needed to show large benefits. However we considered even the small benefit valuable enough to prehabilitate our patients and do so with visual aids and information brochures.
-
2.
Preoperative optimization of anemia, hypertension, diabetes mellitus, asthma and nutritional status when optimized give a good outcome. Cessation of smoking 4 weeks before surgery lowers the risk of a poor outcome to the level of nonsmokers.15 All our patients underwent a medical, cardiac, endocrinology and anaesthesia fitness prior to surgery and were optimized as indicated.
-
3.
Spinal and regional anaesthesia have proven benefits in fast tracking over general anaesthesia.4 We encouraged all our patients to undergo spinal anaesthesia and used general anaesthesia only where spinal was contraindicated or on patient refusal for the same. We added 1 μg/kg of Buprenorphine to 15–20 mg of 0.5% Bupivacaine to prolong the duration of anaesthesia as it acts at the spinal and supra spinal level.19
-
4.
Modifications in the surgical technique such as a midvastus approach and circumferential electrocautery with Patellaplasty have also helped reduce pain and an earlier return to normal functional status.20, 21, 22 Our drain clamping technique has shown a significant reduction in drop in Hb and transfusion rates.10
-
5.
Multimodal blood management protocols included drain clamping for 4 h and the use of tranexamic acid both in the LIA and intravenously.10,23 We were able to keep the number of blood transfusions down to 0.51% patients in the UTKA group and 3.94% patients in the BTKA group. Morais et al. reported no transfusions using a multimodal blood loss prevention approach in their study.24
-
6.
Multimodal analgesia combines analgesics with different mechanisms or sites of action in order to improve analgesia, reduce opioid requirements and/or reduce adverse effects. It is the synergistic effects of combinations of systemically and locally administered analgesic drugs that improves pain relief.25 All our patients received perioperative doses of Gabapentinoids, Nonsteroidal anti-inflammatory drugs, intravenous Methylprednisolone and intravenous Paracetamol, the benefits of which have been well established in literature.26, 27, 28 ACB and LIA were used in all our cases. Numerous studies have cited the benefit of these modalities.29, 30, 31 Acute pain has various deleterious effects such as hypercoagulability, immunosuppression, poor wound healing, myocardial ischaemia, paralytic ileus and potential to develop chronic pain.11 Reduction in pain scores in the first 24 h significantly improves the short to medium term functional outcomes as well as reduces the incidence of deep vein thrombosis.31 Ice application around the wound helps in the control of pain and may increase range of movement.32 A 10 μg/h Buprenorphine transdermal patch was applied on the first postoperative day as it is an ideal drug for use in a transdermal patch and provides adequate analgesia without the systemic adverse effects of opioids.33
-
7.
Mobilizing patients on the day of surgery results in higher functional outcomes, improved pain control and shorter hospital stay. A robust multimodal pain management protocol ensures better mental and physical readiness to participate in early mobilization and physiotherapy.11 The majority of our patients in both the unilateral and bilateral groups were able to mobilize postoperatively on the day of surgery.34
-
8.
Visual Analogue Score: The use of a multimodal technique for postoperative pain management helped in keeping the Visual analogue scores down and a high percentage were mobilized on the day of surgery itself. Our VAS score findings were similar to those found by Danoff et al., where their overall mean and average highest visual analogue scale for pain during the postoperative stay were 42.6 mm and 66.1 mm on a scale of 0–100.35
-
9.
Our ALOS of 3.98 days- 3.17 and 4.78 days in UTKA and BTKA groups respectively was significantly low and comparable with international literature. 79.38% patients in the UTKA group were discharged by day 3 and 82.39% in the BTKA group were discharged by day 5. Medicare in the USA recognizes a 3 day hospital length of stay as a standard of care after TKA.36 Gwynne Jones et al., recently reported an average LOS of 4.8 days following TKA using ERASp.8
-
10.
Major complications were minimized by implementing multimodal blood a nd pain management protocols. The observed types of complications were similar to other studies done on total joint arthroplasties. Berger et al. reported 7 medical complications {gastrointestinal bleed, deep vein thrombosis (DVT), anemia} within 3 months of outpatient TKA in 86 patients at a rate of 8.1%.37 Lovecchio et al. had an overall complication rate of 7.1% in inpatient and 10.1% outpatient TKA and THA patients.7 A meta-analysis done by Zhang et al. found that an indwelling urinary catheter, removed 24–48 h postoperatively in patients undergoing total joint arthroplasty was better than intermittent catheterization in preventing postoperative urinary retention. Moreover the indwelling urinary catheter removed 24–48 h postoperatively did not increase the risk of urinary tract infection.38 We catheterised all our patients which were removed 6 h after surgery. DVT socks and intermittent pneumatic compression (IPC) devices are known to reduce the incidence of DVT and were combined with Dalteparin in all our cases and thus could keep the rates of DVT at 0.26% in the unilateral TKA group and 1.05% in the bilateral TKA group.39 Wound healing and patient outcomes were enhanced with supplemental oral Vitamin C and multivitamins with Zinc.40, 41, 42
-
11.
The WOMAC score is an outcome measure that has validated responder definitions and cut-off points which are specific for TKA and the OKS is a simple and brief one.43 We had a significant improvement in both the OKS and WOMAC scores at follow up which showed that our protocol had long lasting and positive effects on our patients.
Our study is not without limitations. It is a retrospective analysis with no comparative group. The strength of the study would be much better with a randomized controlled trial between ERASp and non- ERASp groups. This however at times is not clinically feasible. Moreover, a longer follow up would be needed to know the morbidity and mortality rates over a medium to long term period.
5. Conclusion
Pain following TKA is a major deterrent in early mobilization thereby delaying functional recovery and increasing ALOS. We recommend our multimodal interdisciplinary protocol to achieve early mobilization, better pain scores and minimize complications, resulting in overall reduced average length of hospital stay.
Conflicts of interest
None.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jcot.2019.05.007.
Contributor Information
Sanjay Agarwala, Email: drsa2011@gmail.com.
Manju Butani, Email: butanimanju@yahoo.co.in.
Jacqueline D'Mello, Email: jackie.dmello@gmail.com.
Shalini Saksena, Email: shalinisaksena64@gmail.com.
Aditya Menon, Email: docmenon83@gmail.com.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Total Hip Arthroplasty. 2018. http://www.ishks.com/pdf/ISHKS-Regitsry-Update-2018.pdf
- 2.Grosu I., Lavand’homme P., Thienpont E. Pain after knee arthroplasty: an unresolved issue. Knee Surgery, Sport Traumatol Arthrosc. 2014;22(8):1744–1758. doi: 10.1007/s00167-013-2750-2. [DOI] [PubMed] [Google Scholar]
- 3.Gerbershagen H.J., Aduckathil S., van Wijck A.J.M., Peelen L.M., Kalkman C.J., Meissner W. Pain intensity on the first day after surgery. Anesthesiology. 2013;118(4):934–944. doi: 10.1097/ALN.0b013e31828866b3. [DOI] [PubMed] [Google Scholar]
- 4.O'Donnell R., Dolan J. Anaesthesia and analgesia for knee joint arthroplasty. BJA Educ. 2018;18(1):8–15. doi: 10.1016/j.bjae.2017.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kehlet H. Multimodal approach to control postoperative pathophysiology and rehabilitation. Br J Anaesth. 1997;78(5):606–617. doi: 10.1093/bja/78.5.606. http://www.ncbi.nlm.nih.gov/pubmed/9175983 [DOI] [PubMed] [Google Scholar]
- 6.Soffin E.M., Yadeau J.T. Enhanced recovery after surgery for primary hip and knee arthroplasty: a review of the evidence. Br J Anaesth. 2016;117(S3):62–72. doi: 10.1093/bja/aew362. [DOI] [PubMed] [Google Scholar]
- 7.Lovecchio F., Alvi H., Sahota S., Beal M., Manning D. Is outpatient Arthroplasty as safe as fast-track inpatient Arthroplasty? A propensity score matched analysis. J Arthroplast. 2016;31(9):197–201. doi: 10.1016/j.arth.2016.05.037. [DOI] [PubMed] [Google Scholar]
- 8.Gwynne-Jones D.P., Martin G., Crane C. Enhanced recovery after surgery for hip and knee replacements. Orthop Nurs. 2017;36(3):203–210. doi: 10.1097/NOR.0000000000000351. [DOI] [PubMed] [Google Scholar]
- 9.Agarwala S., Sobti A., Naik S. Patellaplasty, as an alternative to replacing patella in total knee arthroplasty. Open J Orthoped. 2015;05(09):277–282. [Google Scholar]
- 10.Agarwala S., Jhaveri M., Menon A. Advantages of clamping and drainage over continuous drainage in a total knee arthroplasty. J Clin Orthop Trauma. 2020;11(1):133–135. doi: 10.1016/j.jcot.2019.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Urban M.K. The role of the post-anesthesia care unit in the perioperative care of the orthopedic patient. In: Mackenzie, editor. Textbook of Perioperative Care of the Orthopedic Patient. Springer New York; 2014. pp. 91–100. [Google Scholar]
- 12.Ljungqvist O., Scott M., Fearon K.C. Enhanced recovery after surgery. JAMA Surg. 2017;152(3):292. doi: 10.1001/jamasurg.2016.4952. [DOI] [PubMed] [Google Scholar]
- 13.Brown J.K., Singh K., Dumitru R., Chan E., Kim M.P. The benefits of enhanced recovery after surgery programs and their application in cardiothoracic surgery. Methodist Debakey Cardiovasc J. 2018;14(2):77–88. doi: 10.14797/mdcj-14-2-77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Feng J.E., Novikov D., Anoushiravani A.A., Schwarzkopf R. Total knee arthroplasty: improving outcomes with a multidisciplinary approach. J Multidiscip Healthc. 2018;11:63–73. doi: 10.2147/JMDH.S140550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kumar L., Kumar A.H., Grant S.A., Gadsden J. Updates in enhanced recovery pathways for total knee arthroplasty. Anesthesiol Clin. 2018;36(3):375–386. doi: 10.1016/j.anclin.2018.04.007. [DOI] [PubMed] [Google Scholar]
- 16.Ghosh A., Chatterji U. An evidence-based review of enhanced recovery after surgery in total knee replacement surgery. J Perioper Pract. 2018;0(0) doi: 10.1177/1750458918791121. 175045891879112. [DOI] [PubMed] [Google Scholar]
- 17.Ye C., Zhu S., Chen X., Jiang C., Qian W. Enhanced recovery after surgery for hip and knee arthroplasty: a systematic review and meta-analysis. Postgrad Med. 2017 doi: 10.1136/postgradmedj-2017-134991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang L., Lee M., Zhang Z., Moodie J., Cheng D., Martin J. Does preoperative rehabilitation for patients planning to undergo joint replacement surgery improve outcomes? A systematic review and meta-analysis of randomised controlled trials. BMJ Open. 2016;6(2):e009857. doi: 10.1136/bmjopen-2015-009857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rabiee S.M., Alijanpour E., Jabbari A., Rostami S. Benefits of using intrathecal buprenorphine. Casp J Intern Med. 2014;5(3):143–147. http://www.ncbi.nlm.nih.gov/pubmed/25202441 [PMC free article] [PubMed] [Google Scholar]
- 20.Aslam M., Sabir A., Tiwari V., Abbas S., Tiwari A., Singh P. Approach to total knee replacement: a randomized double blind study between medial parapatellar and midvastus approach in the early postoperative period in asian population. J Knee Surg. 2017;30(08):793–797. doi: 10.1055/s-0036-1597978. [DOI] [PubMed] [Google Scholar]
- 21.Agarwala S., Sobti A., Naik S. Patellaplasty, as an alternative to replacing patella in total knee arthroplasty. Open J Orthoped. 2015;05(09):277–282. [Google Scholar]
- 22.Fan L., Ge Z., Zhang C. Circumferential electrocautery of the patella in primary total knee replacement without patellar replacement: a meta-analysis and systematic review. Sci Rep. 2015;5(1):9393. doi: 10.1038/srep09393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shinde A., Sobti A., Maniar S., Mishra A., Gite R., Shetty V. Tranexamic acid reduces blood loss and need of blood transfusion in total knee arthroplasty: a prospective, randomized, double-blind study in Indian population. Asian J Transfus Sci. 2015;9(2):168–172. doi: 10.4103/0973-6247.154251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Moráis S., Ortega-Andreu M., Rodríguez-Merchán E.C. Blood transfusion after primary total knee arthroplasty can be significantly minimised through a multimodal blood-loss prevention approach. Int Orthop. 2014;38(2):347–354. doi: 10.1007/s00264-013-2188-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Joshi G.P., Schug S.A., Kehlet H. Procedure-specific pain management and outcome strategies. Best Pract Res Clin Anaesthesiol. 2014;28(2):191–201. doi: 10.1016/j.bpa.2014.03.005. [DOI] [PubMed] [Google Scholar]
- 26.Buvanendran A., Kroin J.S., Della Valle C.J., Kari M., Moric M., Tuman K.J. Perioperative oral pregabalin reduces chronic pain after total knee arthroplasty: a prospective, randomized, controlled trial. Anesth Analg. 2010;110(1):199–207. doi: 10.1213/ANE.0b013e3181c4273a. [DOI] [PubMed] [Google Scholar]
- 27.Ong C.K.S., Seymour R.A., Lirk P., Merry A.F. Combining Paracetamol (acetaminophen) with nonsteroidal antiinflammatory drugs: a qualitative systematic review of analgesic efficacy for acute postoperative pain. Anesth Analg. 2010;110(4):1. doi: 10.1213/ANE.0b013e3181cf9281. [DOI] [PubMed] [Google Scholar]
- 28.Lunn T.H., Kristensen B.B., Andersen L.Ø. Effect of high-dose preoperative methylprednisolone on pain and recovery after total knee arthroplasty: a randomized, placebo-controlled trial. Br J Anaesth. 2011;106(2):230–238. doi: 10.1093/bja/aeq333. [DOI] [PubMed] [Google Scholar]
- 29.Sardana V., Burzynski J.M., Scuderi G.R. Adductor canal block or local infiltrate analgesia for pain control after total knee arthroplasty? A systematic review and meta-analysis of randomized controlled trials. J Arthroplast. 2019;34(1):183–189. doi: 10.1016/j.arth.2018.09.083. [DOI] [PubMed] [Google Scholar]
- 30.Ma J., Gao F., Sun W., Guo W., Li Z., Wang W. Combined adductor canal block with periarticular infiltration versus periarticular infiltration for analgesia after total knee arthroplasty. Medicine (Baltim) 2016 Dec;95(52):e5701. doi: 10.1097/MD.0000000000005701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Perlas A., Kirkham K.R., Billing R. The impact of analgesic modality on early ambulation following total knee arthroplasty. Reg Anesth Pain Med. 2013;38(4):334–339. doi: 10.1097/AAP.0b013e318296b6a0. [DOI] [PubMed] [Google Scholar]
- 32.Thacoor A., Back D.L., Na S. Cryotherapy following total knee arthroplasty: what is the evidence? Journal of Rheumatology and Arthritic Diseases. 2018;3(1):1–4. [Google Scholar]
- 33.Kress H.G. Clinical update on the pharmacology, efficacy and safety of transdermal buprenorphine. Eur J Pain. 2009;13(3):219–230. doi: 10.1016/j.ejpain.2008.04.011. [DOI] [PubMed] [Google Scholar]
- 34.Tayrose G., Newman D., Slover J., Jaffe F., Hunter T., Bosco J. Rapid mobilization decreases length-of-stay in joint replacement patients. Bull Hosp Jt Dis. 2013;71(3):222–226. http://www.ncbi.nlm.nih.gov/pubmed/24151950 [PubMed] [Google Scholar]
- 35.Danoff J.R., Goel R., Sutton R., Maltenfort M.G., Austin M.S. How much pain is significant? Defining the minimal clinically important difference for the visual analog scale for pain after total joint arthroplasty. J Arthroplast. 2018;33(7):S71–S75. doi: 10.1016/j.arth.2018.02.029. e2. [DOI] [PubMed] [Google Scholar]
- 36.Parcells B.W., Giacobbe D., Macknet D. Total joint arthroplasty in a stand-alone ambulatory surgical center: short-term outcomes. Orthopedics. 2016;39(4):223–228. doi: 10.3928/01477447-20160419-06. [DOI] [PubMed] [Google Scholar]
- 37.Berger R.A., Kusuma S.K., Sanders S.A., Thill E.S., Sporer S.M. The feasibility and perioperative complications of outpatient knee arthroplasty. Clin Orthop Relat Res. 2009;467(6):1443–1449. doi: 10.1007/s11999-009-0736-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhang W., Liu A., Hu D. Indwelling versus intermittent urinary catheterization following total joint arthroplasty: a systematic review and meta-analysis. In: Hsieh Y.-H., editor. Vol. 10. 2015. p. e0130636. (PLoS One). 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ho K.M., Tan J.A. Stratified meta-analysis of intermittent pneumatic compression of the lower limbs to prevent venous thromboembolism in hospitalized patients. Circulation. 2013;128(9):1003–1020. doi: 10.1161/CIRCULATIONAHA.113.002690. [DOI] [PubMed] [Google Scholar]
- 40.Cuevas L.E., Koyanagi A.I. Zinc and infection: a review. Ann Trop Paediatr. 2005 Sep;25(3):149–160. doi: 10.1179/146532805X58076. [DOI] [PubMed] [Google Scholar]
- 41.Sadeghpour A., Alizadehasl A., Kyavar M. Impact of vitamin C supplementation on post-cardiac surgery ICU and hospital length of stay. Anesthesiol Pain Med. 2015;5(1):e25337. doi: 10.5812/aapm.25337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.MacKay D., Miller A.L. Nutritional support for wound healing. Altern Med Rev. 2003;8(4):359–377. http://www.ncbi.nlm.nih.gov/pubmed/14653765 [PubMed] [Google Scholar]
- 43.Dowsey M.M., Choong P.F.M. The utility of outcome measures in total knee replacement surgery. Internet J Rheumatol. 2013:8. doi: 10.1155/2013/506518. Article ID 506518. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.