1. Introduction
A total hip replacement has been referred to as the “operation of the century” (The Lancet) and when performed in the right patient for the right reasons it is an incredibly successful operation.1, (1a) 1citation 1, 1aTotal hip replacement (THR) improves pain, ambulation and other quality of life related outcome measures for many years following implantation.1, (1a),2 Its success has led to a year on year increase in the number of procedures being performed, with over 105,000 being performed in 2017 alone.3
With greater numbers of primary procedures being performed there are increasing numbers of revision procedures, with 8589 being performed in England and Wales in a single year.3 3 There are many reasons why they can fail resulting in adverse clinical and functional outcomes. The diagnosis of the cause may be obvious and immediate or it may be obscure and only apparent after many years. To understand management options, one needs to understand the reason or the mechanism for failure and diagnose it early, in order to be able to correct it. This article provides a brief overview of the common modes of failure of total hip replacement and the clinical process of investigating and managing these problems.
Success of revision surgery relies on an early diagnosis and adequately treating the cause of failure in the first instance, and therefore clinicians should have an evidence based management algorithm for investigating and managing this problem. There are many causes for failure, including aseptic loosening, dislocation, bone or implant fracture. However, in almost all scenarios, concomitant infection should be ruled out as this plays a major role in influencing management.
2. History and examination
In the workup of a failed or painful THR, the clinical history forms a vital part. This includes the nature, location and severity of pain, whether it is similar to or different to the pain before surgery, and any aggravating or relieving factors. The location of pain may give a clue to the problem. Groin pain is typical of a hip pathology and may come from acetabular problems whereas thigh pain may indicate stem loosening. Pain in the trochanter may arise from tendinopathy, tendon rupture or bursitis and buttock pain may be referred from the back. A posterior pseudotumour or collection irritating the sciatic nerve may also cause buttock pain.
If pain was present right from the outset, it may indicate infection or a periprosthetic fracture. Impingement or early failure of osseointegration may also cause pain to have been present right from the first day of surgery. A pain free interval followed by pain may indicate loosening or late infection. Acetabular loosening can often be asymptomatic. Night pain or constant pain suggests infection or malignancy. Start-up pain that occurs when the patient gets up from the sitting position and starts walking or a typical history that the patient firmly impacts/bangs the foot into the ground a few times to get rid of the pain is typical of loosening. Progressive loss of length may indicate stem loosening and subsidence.
History should also include problems with wound healing, persistent ooze or need for antibiotics, fall or trauma, or foci of infection elsewhere such as the urinary tract or dental sepsis. Other causes and types of pain such as radiculopathy or vasculopathy should also be excluded.
Examination should include an assessment of the gait, abductor function, leg lengths, local skin and tissue condition, distal neurovascular function, spinal examination, and local tenderness around the hip and buttock. Careful assessment of movement is important and end of range pain may occur in impingement. Pain throughout movement may indicate infection or inflammatory problems.
3. Investigations
Our work up of a painful arthroplasty begins with obtaining pain radiographs in the clinic. An AP and lateral view of the hip, especially when compared to previous x-rays or serial x-rays can be quite helpful. They are good at demonstrating the presence of progressive of radiolucent lines at the bone cement interface or the bone-prosthesis interface in uncemented implants, osteolysis, component migration, osteopenia, fractures, and obvious component malalignment. The radiolucent lines should be systematically evaluated along the 3 zones of Charnley and DeLee or the 7 zones of Gruen.52, 53, 54, 55 Late stages of infection may be characterized by signs such as osteolysis, endosteal scalloping (especially neat the lesser trochanter) or periosteal new bone formation.
4. Blood tests
The next step consists of obtaining blood tests including a full blood count, ESR (erythrocyte sedimentation rate and CRP (C-reactive protein). A raised white cell count has been reported by some authors to have a low sensitivity in the diagnosis of infection. Elevated CRP beyond 3–4 weeks or a rising trend combined with a raised ESR may raise the suspicion of infection. Conversely, a normal ESR and normal CRP is highly specific in excluding infection. (see Summary Box 1, Summary box 2)
Summary Box 1.
Cemented femoral stem loosening - O’Neill and Harris.
Possible loosening - radiolucent line at the bone cement interface occupying between 50% to a 100% of the whole bone-cement interface.
Probable loosening - radiolucent line that is either continuous around the entire cement mantle or is 2 mm in width at some point.
Definite loosening is defined as component migration, cement or component fracture.
Alt-text: Summary Box 1
Summary box 2.
Cemented acetabular component loosening - Hodgkinson.
Type 0 no radiolucencies.
Type 1 involved the outer third.
Type 2 involved the outer and middle third.
Type 3 had complete demarcation.
Type 4 had a migrated socket.
Alt-text: Summary box 2
5. Isotope bone scans
Increased uptake after Tc-99MDP (technetium 99 -methylene diphosphonate) scans can be seen up to 2 years following normal uncomplicated THR. It has a low specificity in diagnosing aseptic loosening. We find a negative isotope bone scan of greater value in excluding loosening and eliminating some of the hip-related causes of pain. Indium-111 labelled white cell scans are done in our unit if Tc-99 scans are positive and they tend to have a higher sensitivity and specificity in the diagnosis of infection.
More recently we have started using and found SPECT/CT procedures (Single photon emission computed tomography/computed tomography (SPECT/CT)) more useful in the diagnosis of infection and loosening. It offers metabolic and morphologic information in one imaging step and it increases the diagnostic accuracy in the evaluation of aseptic and septic loosening in hip replacements compared with three-phase bone scintigraphy. Reports suggest that SPECT/CT with (111)In-WBC combined with (99 m)Tc-MDP or (99 m)Tc-sulfur colloid seems to be the best imaging technique for diagnosis of bone and joint infections.50, 51
6. HIP aspiration/Biopsy
Our next step in evaluation of the painful THR especially in cases of suspected infection consists of aspirating the hip under radiology image intensifier guidance in an aseptic environment, especially when the ESR and CRP is elevated. The probability of diagnosing infection is higher when the ESR and CRP are elevated. This procedure can be combined with injection a local anaesthetic which can also be a useful diagnostic test to confirm the source of pain. Occasionally in the case of a dry tap a repeat aspiration under ultrasound guidance can be quite useful to increase the yield of obtaining fluid for cultures.
7. Imaging
Cross-sectional imaging such as MRI scans can be quite useful in investigating some uncommon causes of post-operative pain such as abductor tendinopathy, soft tissue element of infection, or adverse reaction to metal debris.
We find CT scans with 3D reconstructions very helpful in assessing component position in cases of instability, and evaluation of bone loss in selective cases prior to revision surgery. Using specific protocols CT images can also be used to perform 3D planning and 3D printing of the hip or pelvic bone model to help with the planning of complex surgery. Ultrasound guided injections can be helpful in psoas impingement syndromes.
8. Common modes of failure and differential diagnoses
8.1. Infection/periprosthetic joint infection
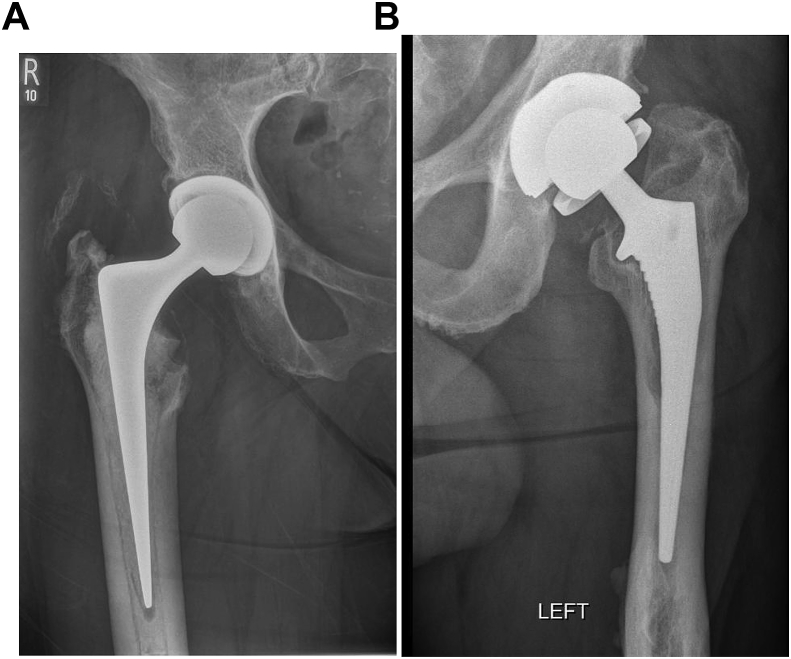
Infection (Fig. 1a and b) is a devastating complication and can be obvious as the cause for concern or indolent and hidden (see Summary box 3).5 Its incidence has been reported to be between 0 and 3%. It can be disastrous for the patient as it almost invariably necessitates further procedures and results in poorer patient satisfaction.4,5 The use of prophylactic antibiotics, clean air theatre systems and protective exhaust suits, much of which have been pioneered and used in this unit, 6,7,8,9 reduce the potential infection rate, but a small risk still remains.4,6, 7, 8, 9 The rise in peri-prosthetic joint infection (PJI) incidence can be partly attributed to the rise in primary procedures being performed but also to new resistant biofilm-forming pathogens which may be contributing to the burden.10,10
Fig. 1.
Infection in THR – Fig. 1a. Radiolucencies seen around the stem and scalloping near the lesser trochanter. Fig. 1b-septic loosening of uncemented stem.
Summary Box 3.
Fitzgerald classification of prosthetic joint infections:
Stage I Acute fulminating infections, usually presenting within six weeks.
Stage II Delayed sepsis or chronic indolent infection.
Stage III Late haematogenous infection in a previously well-functioning hip replacement.
Tsukayama added a fourth type where a positive culture is found at the time of revision without previous evidence of infection.
Alt-text: Summary Box 3
Diagnosis and management of PJI is a complex subject and should ideally be performed in specialised tertiary units with dedicated orthopaedic surgeons, radiologists, microbiologists, plastic surgeons, pharmacologists and other specialist staff as part of a good multi-disciplinary approach as recommended in the UK GIRFT guidelines.
When, the clinical picture is obvious, it can present with local symptoms of wound discharge or dehiscence, cellulitis or surgical site swelling or the presence of a sinus. Systemic symptoms of fevers, rigors or sweating may also be present. Radiological signs of implant loosening, particularly in the early post-operative period, should trigger suspicion of infection as should implant dislocation in the absence of obvious component mal-positioning.
The diagnosis of PJI remains problematic as there is no one test which is 100% specific and sensitive. The consensus statement from the International Consensus Meeting on Surgical Site and Periprosthetic Joint Infection stipulated that a PJI could be diagnosed with the presence of one major or 3 minor criteria. Major criteria include; two positive periprosthetic cultures with phenotypically identical organisms or the presence of a sinus communicating with the joint. Minor criteria include; elevated CRP and ESR, elevated synovial fluid white blood cell counts, elevated synovial fluid polymorphonuclear neutrophil percentage (PMN%), a positive leukocyte esterase test strip, positive histological analysis of peri-prosthetic tissue and a single positive culture.11
With this in mind, our initial workup begins with having a low threshold for suspicion followed by a clinical, biochemical, radiological, and microbiological assessments as may be appropriate in industrial cases.
A thorough history should elucidate patient related risk factors including, obesity, diabetes or other immune-compromising conditions amongst others. Additional history should delineate any early excessive or offensive wound discharge, a delay to wound healing or a need for additional antibiotics. One should also ask about the presence of pain felt in the groin, thigh or wound area and clarify for the presence of systemic symptoms.
Blood tests should screen for an elevated white cell count (WCC), (including a differential count) erythrocyte sedimentation rate (ESR) and C- reactive protein (CRP). When both ESR and CRP are negative a PJI can be ruled out with a sensitivity in excess of 90%.12 One should note that ESR can be normally elevated up to a year following surgery but starts to decline after about 4 weeks, whilst the CRP usually normalizes after 4–6 weeks. These markers can also be chronically raised in those with inflammatory arthritides such rheumatoid arthritis. Interleukin-6 (IL-6) has shown promise, is more sensitive13 than specific, not widely available, and is more expensive and therefore does not form part of our usual first line investigations.
Following the consensus guideline on PJI diagnosis, other newer synovial tests such as alpha-defensin immunoassay and leukocyte esterase colorimetric strip test, have proven useful in PJI diagnosis in certain studies but widespread reproducibility of these results is not available.14,15 A recent study looks at some scoring criteria which may aid clinicians to more quantitatively assess for the presence of a PJI in light of these new synovial biomarkers.11 We have some limited experience of using a commercially available alpha-defensin test more to exclude infection in an otherwise technically well-performed painful arthroplasty with uncertain indications for offering surgery.
Plain AP and lateral radiographs of the hip and implant should be taken and examined for obvious soft tissue swelling shadows, change in implant orientation, excessive subsidence, and lucent lines between fixation interfaces as described by DeLee and Charnley for the cup and Gruen for the femoral side.16,17 Periosteal reaction or new bone formation may also be present.18,19
If loosening is present, this could be due to mechanical and local biological causes as in aseptic loosening, or maybe due to an external pathogen. Should loosing occur in the first 2–3 years following implantation, infection should be excluded as the most likely cause.
A technetium-99 bone scan and labelled leukocyte scan may be useful to aid diagnosis. These tests have a reported sensitivity of between 65 and 80% on its own but can yield false positive results when performed within a year of the joint replacement.20, 21, 22 A negative scan may be more useful in excluding infection. The SPECT/CT scan, is gaining popularity and is most useful when other investigations have failed to identify a cause for symptoms.23
Identifying the organism using joint aspiration or biopsy under aseptic technique remains the mainstay of diagnosis. It is important to have agreed protocols of technique, transport of samples and their processing with advice from the microbiologists to ensure high yield using these approaches. Intra-operative samples should be obtained (5 samples) using agreed surgical techniques at the time of revision surgery. They should be sent for culture in order to identify the potential organism and guide antimicrobial management.11
9. Aseptic loosening and osteolysis
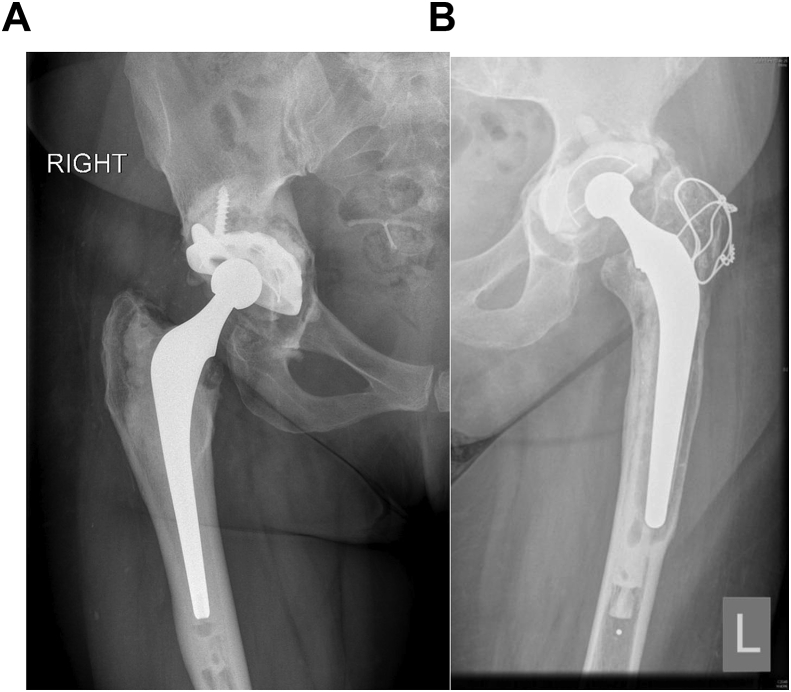
According to registry data, this represents the most common indication for revision hip surgery.3 Aseptic loosening (Fig. 2a and b) can be acute or chronic. Acute loosening is uncommon and results from either poor cementation or implantation techniques when using cemented components, or failure of osseous integration when using cementless implants. Some designs are associated with higher rates of aseptic loosening and attempt should be made to identify which prosthesis was used.
Fig. 2.
Aseptic loosening of the acetabular component in Fig. 2a and both components in Fig. 2b.
More commonly, aseptic loosening is chronic process resulting from a cell-mediated response to wear debris, which is generated over time.24,25 It may affect any prosthesis regardless of bearing couple or fixation method. The main mechanism appears to be the activation of receptor activator of nuclear factor-kB (RANK), and RANK ligand (RANKL) and expression of osteoprotegerin in periprosthetic membranes. Local osteoclastic activity is enhanced leading to osteolysis and eventual loosening.26,27 medical management of osteolysis has no practical role in our present treatment philosophy.28, 29, 30
Early osteolysis may be often asymptomatic especially on the acetabular side. Start-up pain has been reported to be a typical feature of stem loosening. Aseptic loosening can be associated with pelvic and femoral bone loss.
After excluding infection, it is important to evaluate the remaining bone stock for appropriate pre-operative planning before considering the reconstruction techniques in revision arthroplasty.
Early and progressive radiolucent lines at the bone cement interface or the prosthetic -bone interface on radiographic analysis could be a marker for early loosening. Where plain radiographs are insufficient Computed tomography is often useful to evaluate the degree of bone loss. Early asymptomatic radiolucencies may be amenable to regular clinical and radiological follow up and surveillance but if there is impending risk of peri-prosthetic fracture then a more pro-active approach is indicated.
The use of implant retention and bone grafting for osteolysis around stable implants has been reported upon but the long term results do not31 support this philosophy. And when present in the femoral diaphysis, it is not easily accessible.
In severely painful or life debilitating situations or when osteolysis is severe and threatening catastrophic fracture, it is very reasonable to consider revision. In the case of severe unexplained pain, but when plain radiographs do not give the diagnosis, a99mTc-labelled bone scan may be useful. In the case of obvious loosening on plain radiographs, we do not recommend the use of a bone scan. Instead a CT scan may be more useful to categorize the extent of bone loss and plan for the need for bone grafts, augments or revision prostheses.
The Wrightington philosophy revolves around using bone grafting techniques and “making our revisions look like primaries”. We use impaction allografting techniques to reconstruct bone loss especially in younger patients.32 This preserves bone stock and facilitates easier revision surgery in the future, should it be required.
10. Leg length discrepancies
Prevention is better than cure, and a careful and planned reconstruction of the femoral offset and leg length, will reduce the chance of patient dissatisfaction from leg length discrepancies (LLD). Patients with perceived longer legs following surgery have been found to have significantly worse patient reported outcomes.33 In addition, LLDs are associated with back pain, sciatica and gait disorders according to some, but not all authors.34, 35, 36 Failure to balance the soft tissues from LLD can also predispose to dislocations.37 These complications, have a significant financial impact as they are associated with large monitory payouts by National Health Service Litigation Authorities or health institutions every year.38
The assessment of suspected LLD following THA is pragmatic. In our practice, radiologically identified LLD which are asymptomatic are managed expectantly. Symptomatic LLD can be managed non-operatively or operatively depending on severity of symptoms extent of the LLD. The first step is to quantify the deformity. Traditionally, a pelvic view with the lower limbs in 20° of internal rotation, were used. However, one can be caught out using this method if there is shortening of the distal femur or tibia. Hence obtaining a CT scanogram is preferred. Care should be taken to account for/adjust for fixed flexion deformities, which may under-estimate deformities. Attention must be paid to assess the spine as well for spinal curvatures and spino-pelvic alignment as the discrepancy may actually not be arising from the hip at all. Extreme caution should be exercised before offering revision surgery for LLD in an otherwise well-fixed mechanically sound prosthesis.
11. Dislocation/instability
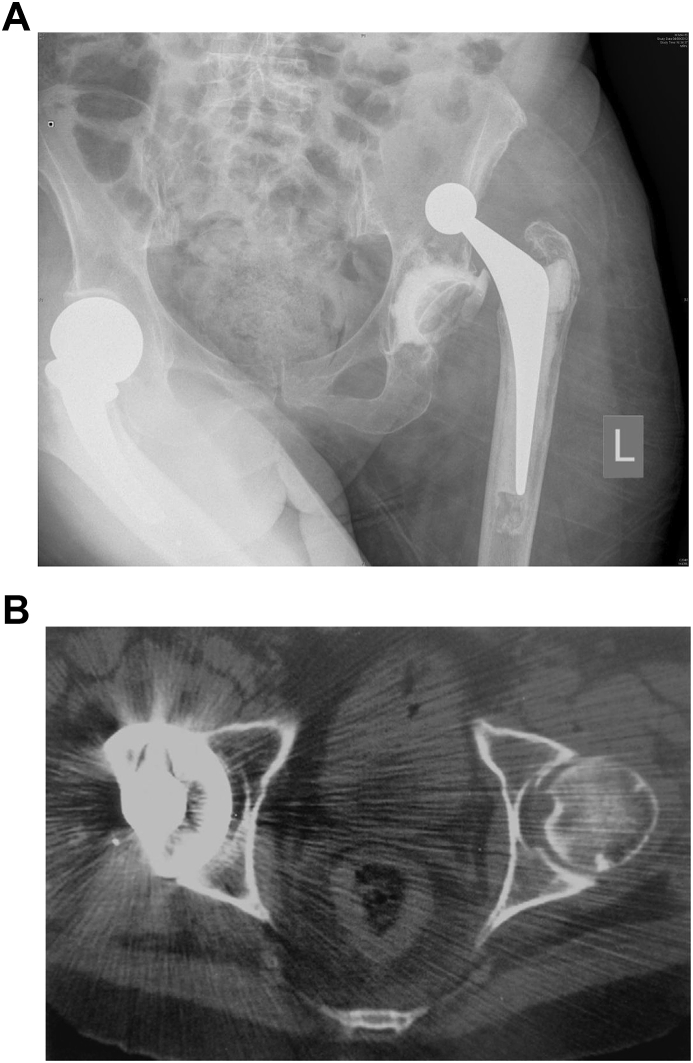
Dislocation (Fig. 3a and b) is the second most common reason for revision surgery in the England and Wales National Joint Registry.3 It can result from under or over tensioning the soft tissues or impingement. Failure of correct muscle tensioning occurs from a failure to reconstruct femoral offset, leg length or both.40, 41
Fig. 3.
a. Dislocation Fig. 3 a. b. Component malpositioning on CT scans Fig. 3b.
Impingement can occur between one implant and another, implant and host tissue (usually bone), or host tissue with host tissue. Impingement is usually the result of component malposition or failure to remove osteophytes or thickened capsule.
More uncommonly, it is due to patient related variables such as poor compliance with post-operative protective instructions, hypermobility conditions, poor coordination or muscle strength.39
Dislocation can be classified as to the direction of dislocation, or the timing of dislocation from the index procedure. Immediate dislocations can be considered to occur within the first 6 weeks, early dislocations between 3 and 6 months, and late dislocations after 5 years (which may occur due to wear in the acetabular component or patient related factors associated with aging such as cerebro-vascular accidents or dementia).
The early management of the dislocated hip replacement by relocation provides an opportunity to perform an examination under anaesthesia (EUA) to determine obvious instability or problems with laxity, impingement or offset. Post reduction x-rays should be examined for signs of loosening, obvious component malposition or wear of the polyethylene cup.42 Femoral offset and leg length relative to the opposite hip should be studied.
Should post reduction x-rays fail to delineate any obvious component malposition, then a CT scan can be useful. Axial images through the acetabulum, proximal femur and knee, will allow for assessment of component positioning. Obvious mal-positioning would necessitate a revision.43,44
Recurrent dislocation with the absence of component malposition, is due to abductor imbalance or impingement. Generally, one must be prepared to manage both of these problems, as the exact cause may only be evident at the time of revision.
12. Peri-prosthetic fractures
Acute periprosthetic fractures may occur around stable or loose implants. This problem is growing with the growth in the number of primary and revision hip replacements. History should elucidate whether the hip was pain free and well-functioning.
A carful history should establish whether there was any deterioration in pain in that limb, which might indicate a loose component. Plain x-rays should include the entire hip and femur to help clarify the extent of the fracture, extent of bone loss and whether the components were well fixed or loose.45
It is often quite difficult to get good quality radiographs in patients with periprosthetic fractures due to pain. CT scans are very helpful in assessing the fracture in a better fashion and may also help in assessing whether the components are well-fixed or loose. Infection should be excluded.
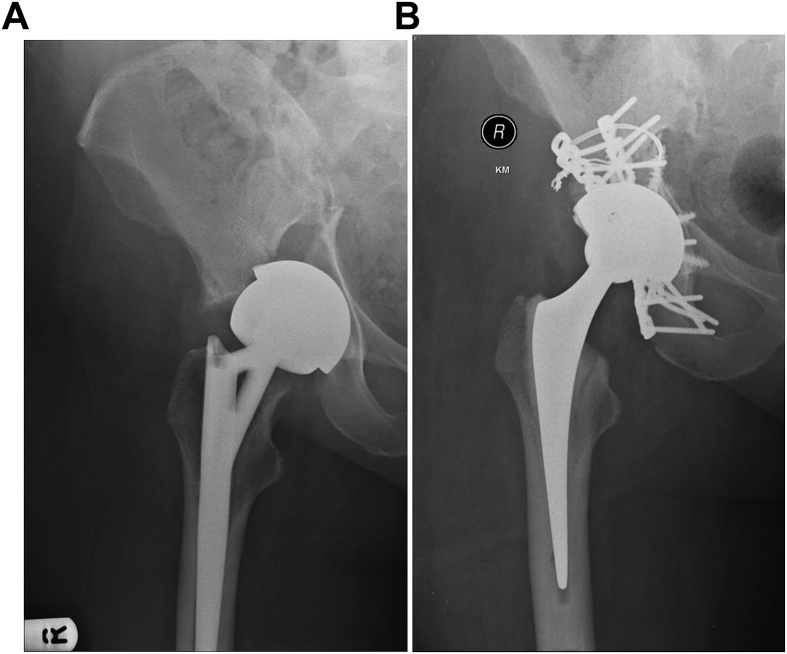
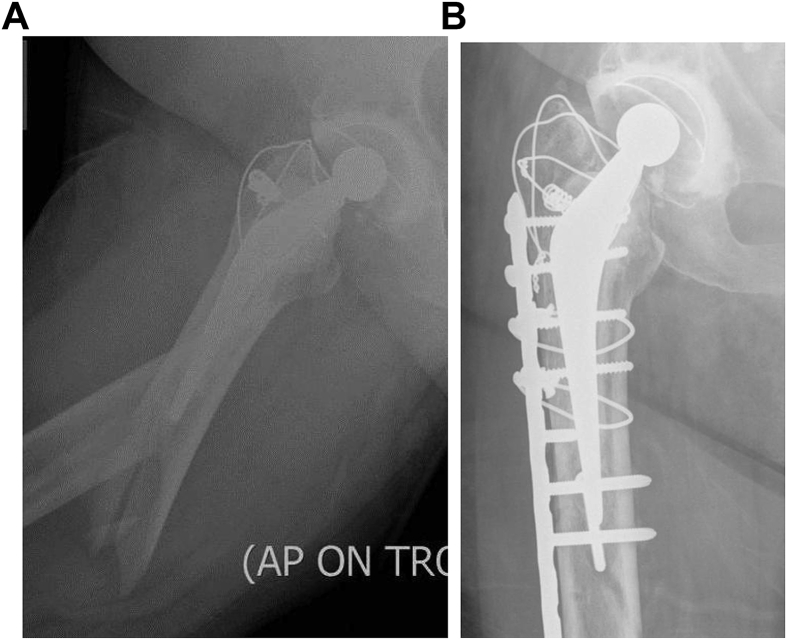
Periprosthetic fractures of the acetabulum (Fig. 4a and b) are increasingly being referred to our unit. Majority of the acute/early ones are around uncemented components. Non-displaced acetabula fractures with well-fixed components can also be managed expectantly. Displaced fractures of the acetabulum usually need a combined fixation bone grafting and revision procedure performed in a single or two stages.
Fig. 4.
a and b - Periprosthetic fracture of acetabulum treated with fixation and revision Fig.
Sometimes it is not possible to determine whether the implant is loose or not, and the final decision may need to be made at surgery. Therefore, the surgeon must be prepared to change tact to a full revision rather than performing an open reduction and internal fixation (Fig. 5a and b).
| The Vancouver classification is based on fracture location and implant stability |
| Type A the fracture involves the trochanteric region |
| Type B the fracture is around or just distal to the femoral stem |
| B1 the femoral implant is well fixed |
| B2 - the femoral implant is loose but the remaining bone stock is good |
| B3 severe bone stock loss in the presence of a loose implant. |
| Type C the fracture is so far below the stem that the treatment is independent of the hip replacement |
Fig. 5.
a and b – periprosthetic fracture of the femur.
13. Soft tissue problems
Iliopsoas tendinopathies is probably an under diagnosed cause of dissatisfaction following THA.46 The psoas tendon can impinge over the rim of an oversized or retroverted acetabular component or even a prominent neck of the prosthetic stem, causing it to become painful. This usually manifests as groin pain exacerbated by extension of the hip when the tendon is on maximum stretch. Occasionally, flexion abduction and extension hip examination may elicit a snapping hip sign. When suspected, an ultrasound scan or metal artifact reduction MRI scan may reveal an inflamed tendon or iliopsoas bursa. If the pain is relieved, with the aid of an image guided injection, the diagnosis is confirmed.
Trochanteric pain may occur due to bursitis or more commonly abductor tendinopathy or even partial or complete tears of the abductor tendons. History and clinical examination usually lead to the diagnosis. It is prudent in such cases also to exclude infection. MRI scans may be helpful in diagnosing tears of the tendons.
14. Adverse reaction to metal debris (ARMD)
ARMD (also referred to as Adverse local tissue reactions -ALTR) around metal-on-metal (MOM) hip arthroplasties have recently attracted a lot of attention as a cause of failures. Pain in the presence of a MOM bearing should raise suspicion of its presence. Risk factors include being female gender, or having an Articular Surface Replacement (ASR) cup.47 They can result in formation of large solid or liquid collections (pseudotumours), which themselves may be a cause of pain. Worryingly, ALTR can result in localized destruction of muscle or bone, and a progression of pain should point towards the possibility of this.
Total hips with large diameter metal on metal bearings are more at risk of this phenomenon compared to resurfacing implants due to the additional metal on metal interface at the head neck junction (trunnion).48
Infection should be excluded as there are reports of an increased incidence of infection in association with ARMD. X-rays may show osteolysis, around the femoral neck, trochanters or acetabulum. Raised cobalt and chromium levels in the blood (or a rising trend), is a cause of concern. Additional investigations include cross-sectional imaging with MAR MRI scans to look for cystic or solid pseudotumours, and assess for localized bony and soft tissue destruction.49 Rising metal ions levels, worsening of symptoms, and localized soft tissue destruction should prompt a discussion with the patient about early revision. Deficient abductor muscles on MRI are associated with increased risk of dislocation and poor functional outcomes.
15. Conclusion
This paper has described initial management algorithms required in evaluating the failing hip replacement. Whilst it does not exhaustively cover every potential cause of failure, it does depict an evidence based and methodological approach to identifying and planning further management of the most common causes of THA failure. The adage of “prepare for the worst, but hope for the best” is a safe strategy that ensures extensive planning and reduces the possibly of situations which are unexpected and unsalvageable due to a lack of preparation.
Declaration of competing interest
None.
References
- 1.Wroblewski B.M., Fleming P.A., Siney P.D. Charnley low-frictional torque arthroplasty of the hip. 20-to-30 year results. The Journal of bone and joint surgery British. 1999;81(3):427. doi: 10.1302/0301-620x.81b3.9521. [DOI] [PubMed] [Google Scholar]; (1a) Learmonth I.D., Young C., Rorabeck C. The operation of the century: total hip replacement. Lancet. 2007;370:1508–1519. doi: 10.1016/S0140-6736(07)60457-7. [DOI] [PubMed] [Google Scholar]
- 2.Aqil A., Wiik A., Zanotto M., Manning V., Masjedi M., Cobb J.P. The effect of hip arthroplasty on osteoarthritic gait: a blinded, prospective and controlled gait study at fast walking speeds. J Arthroplast. 2016;31(10):2337. doi: 10.1016/j.arth.2016.03.002. [DOI] [PubMed] [Google Scholar]
- 3.NJREnglandandWales . 2017. British Orthopaedic Association. National Joint Replacement Registry for England, Wales and Northern Ireland. 15th annual report. [Google Scholar]
- 4.Toms A.D., Davidson D., Masri B.A., Duncan C.P. The management of peri-prosthetic infection in total joint arthroplasty. The Journal of bone and joint surgery British. 2006;88(2):149. doi: 10.1302/0301-620X.88B2.17058. [DOI] [PubMed] [Google Scholar]
- 5.Jafari S.M., Coyle C., Mortazavi S.M., Sharkey P.F., Parvizi J. Revision hip arthroplasty: infection is the most common cause of failure. Clin Orthop Relat Res. 2010;468(8):2046. doi: 10.1007/s11999-010-1251-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Charnley J. Postoperative infection after total hip replacement with special reference to air contamination in the operating room. Clin Orthop Relat Res. 1972;87:167. doi: 10.1097/00003086-197209000-00020. [DOI] [PubMed] [Google Scholar]
- 7.Charnley J., Eftekhar N. Postoperative infection in total prosthetic replacement arthroplasty of the hip-joint. With special reference to the bacterial content of the air of the operating room. Br J Surg. 1969;56(9):641. doi: 10.1002/bjs.1800560902. [DOI] [PubMed] [Google Scholar]
- 8.Charnley J. [Surgical infection in orthopedic surgery. Wrightington’s "glass-house"] Revue de chirurgie orthopedique et reparatrice de l’appareil moteur. 1969;55(3):231. [PubMed] [Google Scholar]
- 9.Lynch M., Esser M.P., Shelley P., Wroblewski B.M. Deep infection in Charnley low-friction arthroplasty. Comparison of plain and gentamicin-loaded cement. The Journal of bone and joint surgery British. 1987;69(3):355. doi: 10.1302/0301-620X.69B3.3584184. [DOI] [PubMed] [Google Scholar]
- 10.Kurtz S.M., Lau E., Schmier J., Ong K.L., Zhao K., Parvizi J. Infection burden for hip and knee arthroplasty in the United States. J Arthroplast. 2008;23(7):984. doi: 10.1016/j.arth.2007.10.017. [DOI] [PubMed] [Google Scholar]
- 11.Parvizi J., Tan T.L., Goswami K. The 2018 definition of periprosthetic hip and knee infection: an evidence-based and validated criteria. J Arthroplast. 2018;33(5):1309. doi: 10.1016/j.arth.2018.02.078. [DOI] [PubMed] [Google Scholar]
- 12.Parvizi J., Della Valle C.J. AAOS Clinical Practice Guideline: diagnosis and treatment of periprosthetic joint infections of the hip and knee. J Am Acad Orthop Surg. 2010;18(12):771. doi: 10.5435/00124635-201012000-00007. [DOI] [PubMed] [Google Scholar]
- 13.Di Cesare P.E., Chang E., Preston C.F., Liu C.J. Serum interleukin-6 as a marker of periprosthetic infection following total hip and knee arthroplasty. The Journal of bone and joint surgery American. 2005;87(9):1921. doi: 10.2106/JBJS.D.01803. [DOI] [PubMed] [Google Scholar]
- 14.Wyatt M.C., Beswick A.D., Kunutsor S.K., Wilson M.J., Whitehouse M.R., Blom A.W. The alpha-defensin immunoassay and leukocyte esterase colorimetric strip test for the diagnosis of periprosthetic infection: a systematic review and meta-analysis. The Journal of bone and joint surgery American. 2016;98(12):992. doi: 10.2106/JBJS.15.01142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ahmad S.S., Hirschmann M.T., Becker R. A meta-analysis of synovial biomarkers in periprosthetic joint infection: synovasure is less effective than the ELISA-based alpha-defensin test. Knee Surg Sport Traumatol Arthrosc. 2018;26(10):3039. doi: 10.1007/s00167-018-4904-8. [DOI] [PubMed] [Google Scholar]
- 16.Gruen T.A., McNeice G.M., Amstutz H.C. Modes of failure" of cemented stem-type femoral components: a radiographic analysis of loosening. Clin Orthop Relat Res. 1979;141:17. [PubMed] [Google Scholar]
- 17.DeLee J.G., Charnley J. Radiological demarcation of cemented sockets in total hip replacement. Clin Orthop Relat Res. 1976;121(20) [PubMed] [Google Scholar]
- 18.Fitzgerald R.H., Jr. Total hip arthroplasty sepsis. Prevention and diagnosis. Orthop Clin N Am. 1992;23(2):259. [PubMed] [Google Scholar]
- 19.Lyons C.W., Berquist T.H., Lyons J.C., Rand J.A., Brown M.L. Evaluation of radiographic findings in painful hip arthroplasties. Clin Orthop Relat Res. 1985;195:239. [PubMed] [Google Scholar]
- 20.Levitsky K.A., Hozack W.J., Balderston R.A. Evaluation of the painful prosthetic joint. Relative value of bone scan, sedimentation rate, and joint aspiration. J Arthroplast. 1991;6(3):237. doi: 10.1016/s0883-5403(06)80170-1. [DOI] [PubMed] [Google Scholar]
- 21.Henderson J.J., Bamford D.J., Noble J., Brown J.D. The value of skeletal scintigraphy in predicting the need for revision surgery in total knee replacement. Orthopedics. 1996;19(4):295. doi: 10.3928/0147-7447-19960401-05. [DOI] [PubMed] [Google Scholar]
- 22.Love C., Marwin S.E., Palestro C.J. Nuclear medicine and the infected joint replacement. Semin Nucl Med. 2009;39(1):66. doi: 10.1053/j.semnuclmed.2008.08.007. [DOI] [PubMed] [Google Scholar]
- 23.Van den Wyngaert T., Paycha F., Strobel K. SPECT/CT in postoperative painful hip arthroplasty. Semin Nucl Med. 2018;48(5):425. doi: 10.1053/j.semnuclmed.2018.05.002. [DOI] [PubMed] [Google Scholar]
- 24.Jacobs J.J., Roebuck K.A., Archibeck M., Hallab N.J., Glant T.T. Osteolysis: basic science. Clin Orthop Relat Res. 2001;393:71. doi: 10.1097/00003086-200112000-00008. [DOI] [PubMed] [Google Scholar]
- 25.Archibeck M.J., Jacobs J.J., Roebuck K.A., Glant T.T. The basic science of periprosthetic osteolysis. Instr Course Lect. 2001;50:185. [PubMed] [Google Scholar]
- 26.Crotti T.N., Smith M.D., Findlay D.M. Factors regulating osteoclast formation in human tissues adjacent to peri-implant bone loss: expression of receptor activator NFkappaB, RANK ligand and osteoprotegerin. Biomaterials. 2004;25(4):565. doi: 10.1016/s0142-9612(03)00556-8. [DOI] [PubMed] [Google Scholar]
- 27.Ollivere B., Wimhurst J.A., Clark I.M., Donell S.T. Current concepts in osteolysis. The Journal of bone and joint surgery British. 2012;94(1):10. doi: 10.1302/0301-620X.94B1.28047. [DOI] [PubMed] [Google Scholar]
- 28.Zeng Y, Lai O, Shen B, et al. A systematic review assessing the effectiveness of alendronate in reducing periprosthetic bone loss after cementless primary THA. Orthopedics 34(4), 2011. [DOI] [PubMed]
- 29.Trevisan C., Nava V., Mattavelli M., Parra C.G. Bisphosphonate treatment for osteolysis in total hip arthroplasty. A report of four cases. Clin Cases Miner Bone Metab. 2013;10(1):61. doi: 10.11138/ccmbm/2013.10.1.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Childs L.M., Goater J.J., O’Keefe R.J., Schwarz E.M. Effect of anti-tumor necrosis factor-alpha gene therapy on wear debris-induced osteolysis. The Journal of bone and joint surgery American. 2001;83(12):1789. doi: 10.2106/00004623-200112000-00004. [DOI] [PubMed] [Google Scholar]
- 31.Maloney W.J. The surgical management of femoral osteolysis. J Arthroplast. 2005;20(4 Suppl 2):75. doi: 10.1016/j.arth.2005.03.019. [DOI] [PubMed] [Google Scholar]
- 32.Board T.N., Rooney P., Kearney J.N., Kay P.R. Impaction allografting in revision total hip replacement. The Journal of bone and joint surgery British. 2006;88(7):852. doi: 10.1302/0301-620X.88B7.17425. [DOI] [PubMed] [Google Scholar]
- 33.Konyves A., Bannister G.C. The importance of leg length discrepancy after total hip arthroplasty. The Journal of bone and joint surgery British. 2005;87(2):155. doi: 10.1302/0301-620x.87b2.14878. [DOI] [PubMed] [Google Scholar]
- 34.Friberg O. Clinical symptoms and biomechanics of lumbar spine and hip joint in leg length inequality. Spine. 1983;8(6):643. doi: 10.1097/00007632-198309000-00010. [DOI] [PubMed] [Google Scholar]
- 35.Friberg O. Biomechanical significance of the correct length of lower limb prostheses: a clinical and radiological study. Prosthet Orthot Int. 1984;8(3):124. doi: 10.3109/03093648409146072. [DOI] [PubMed] [Google Scholar]
- 36.Rosler J., Perka C. The effect of anatomical positional relationships on kinetic parameters after total hip replacement. Int Orthop. 2000;24(1):23. doi: 10.1007/s002640050006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Woo R.Y., Morrey B.F. Dislocations after total hip arthroplasty. The Journal of bone and joint surgery American. 1982;64(9):1295. [PubMed] [Google Scholar]
- 38.McWilliams A.B., Douglas S.L., Redmond A.C. Litigation after hip and knee replacement in the national health Service. The bone & joint journal. 2013;95-B(1):122. doi: 10.1302/0301-620X.95B1.30908. [DOI] [PubMed] [Google Scholar]
- 39.Padgett D.E., Warashina H. The unstable total hip replacement. Clin Orthop Relat Res. 2004;420:72. doi: 10.1097/00003086-200403000-00011. [DOI] [PubMed] [Google Scholar]
- 40.Sikes C.V., Lai L.P., Schreiber M., Mont M.A., Jinnah R.H., Seyler T.M. Instability after total hip arthroplasty: treatment with large femoral heads vs constrained liners. J Arthroplast. 2008;23(7 Suppl):59. doi: 10.1016/j.arth.2008.06.032. [DOI] [PubMed] [Google Scholar]
- 41.Williams J.F., Gottesman M.J., Mallory T.H. Dislocation after total hip arthroplasty. Treatment with an above-knee hip spica cast. Clin Orthop Relat Res. 1982;171:53. [PubMed] [Google Scholar]
- 42.Coventry M.B. Late dislocations in patients with Charnley total hip arthroplasty. The Journal of bone and joint surgery American. 1985;67(6):832. [PubMed] [Google Scholar]
- 43.Vaishya R., Sharma M., Chaudhary R.R. Bioball universal modular neck adapter as a salvage for failed revision total hip arthroplasty. Indian J Orthop. 2013;47(5):519. doi: 10.4103/0019-5413.118211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Novoa C.D., Citak M., Zahar A., Lopez R.E., Gehrke T., Rodrigo J.L. The Merete BioBall system in hip revision surgery: a systematic review. Orthopaedics & traumatology, surgery & research : OTSR. 2018;104(8):1171. doi: 10.1016/j.otsr.2018.06.016. [DOI] [PubMed] [Google Scholar]
- 45.Khan T., Grindlay D., Ollivere B.J., Scammell B.E., Manktelow A.R., Pearson R.G. A systematic review of Vancouver B2 and B3 periprosthetic femoral fractures. The bone & joint journal. 2017;17(4 suppl B) doi: 10.1302/0301-620X.99B4.BJJ-2016-1311.R1. 99-B. [DOI] [PubMed] [Google Scholar]
- 46.Lachiewicz P.F., Kauk J.R. Anterior iliopsoas impingement and tendinitis after total hip arthroplasty. J Am Acad Orthop Surg. 2009;17(6):337. doi: 10.5435/00124635-200906000-00002. [DOI] [PubMed] [Google Scholar]
- 47.Liow M.H.L., Kwon Y.M. Metal-on-metal total hip arthroplasty: risk factors for pseudotumours and clinical systematic evaluation. Int Orthop. 2017;41(5):885. doi: 10.1007/s00264-016-3305-1. [DOI] [PubMed] [Google Scholar]
- 48.Esposito C.I., Wright T.M., Goodman S.B., Berry D.J., Clinical B. Bioengineering study groups from carl TBW. What is the trouble with trunnions? Clin Orthop Relat Res. 2014;472(12):3652. doi: 10.1007/s11999-014-3746-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kwon Y.M., Tsai T.Y., Leone W.A., Liow M.H.L. Sensitivity and specificity of metal ion levels in predicting "pseudotumors" due to taper corrosion in patients with dual taper modular total hip arthroplasty. J Arthroplast. 2017;32(3):996. doi: 10.1016/j.arth.2016.08.040. [DOI] [PubMed] [Google Scholar]
- 50.Fitzgerald JrRH., NolanDR IlstrupDM. Deepwoundsepsis following total hip arthroplasty. J Bone Joint Surg Am. 1977;59-A:847e55. [PubMed] [Google Scholar]
- 51.Tsukayama D.T., Estrada R., Gustilo R.B. Infection after total hip arthroplasty: a study of the treatment of one hundred and six infections. J Bone Joint Surg Am. 1996;78-A:512e23. doi: 10.2106/00004623-199604000-00005. [DOI] [PubMed] [Google Scholar]
- 52.DeLee J.G., Charnley J. Radiological demarcation of cemented sockets in total hip replacement. Clin Orthop. 1976;121:20e32. [PubMed] [Google Scholar]
- 53.Gruen T.A., McNeice G.M., Amstutz H.C. ‘‘Modes of failure’’ of cemented stem-type femoral components: a radiographic analysis of loosening. Clin Orthop. 1979;141:17e27. [PubMed] [Google Scholar]
- 54.O’Neill D.A., Harris W.H. Failed total hip replacement: assessment by plain radiographs, arthrograms, and aspiration of the hip joint. J Bone Joint Surg Am. 1984;66:540e6. [PubMed] [Google Scholar]
- 55.Hodgkinson J.P., Shelley P., Wroblewski B.M. The correlation between the roentgenographic appearance and operative findings at the bone-cement junction of the socket in Charnley low friction arthroplasties. Clin Orthop. 1988;228:105e9. [PubMed] [Google Scholar]