Abstract
The management of heart failure has changed significantly over the last 30 years, leading to improvements in the quality of life and outcomes, at least for patients with a substantially reduced left ventricular ejection fraction (HFrEF). This has been made possible by the identification of various pathways leading to the development and progression of heart failure, which have been successfully targeted with effective therapies. Meanwhile, many other potential targets of treatment have been identified, and the list is constantly expanding. In this review, we summarise planned and ongoing trials exploring the potential benefit, or harm, of old and new pharmacological interventions that might offer further improvements in treatment for those with HFrEF and extend success to the treatment of patients with heart failure with preserved left ventricular ejection fraction (HFpEF) and other heart failure phenotypes.
Electronic supplementary material
The online version of this article (10.1007/s10741-019-09829-7) contains supplementary material, which is available to authorized users.
Keywords: Heart failure, Treatment, Trials, HFpEF, HFrEF
Introduction
Heart failure and its management have changed dramatically over the last 30 years. In the 1980s, patients were included in clinical trials of heart failure based purely on the clinical opinion of the investigator with no objective criteria to confirm the diagnosis. The patients were younger and had fewer comorbidities but a broad range of left ventricular ejection fraction (LVEF) compared with contemporary trials; quality of life was often poor and mortality rate high. Fluid retention, causing peripheral oedema and breathlessness, was the main therapeutic target. Digoxin and diuretics were the only available medical treatments, sometimes accompanied with bed rest and fluid restriction.
Subsequently, objective criteria such as LVEF and, more recently, natriuretic peptides were required to select patients for trials. Initially, trials targeted vasoconstriction, using nitrates and hydralazine [1], and pathologically activated neuro-hormonal systems, using angiotensin-converting enzyme (ACE) inhibitors, angiotensin II receptor blockers (ARBs), beta-blockers and mineralocorticoid antagonists (MRAs). These trials provided evidence that, for heart failure with reduced LVEF (HFrEF), treatment could improve ventricular function, symptoms and signs, as well as morbidity and mortality [2–5]. More recently, other targets and novel treatments have been identified for HFrEF. Ivabradine, an agent that slows the rate of sinus node discharge and therefore heart rate, improved ventricular function, symptoms and morbidity for patients who do not achieve a heart rate < 70 bpm on a beta-blocker; for those with a heart rate > 75 bpm or who were not treated with a beta-blocker, mortality was also reduced [6, 7]. Patients with HFrEF in sinus rhythm with a QRS duration > 130 msec benefitted from cardiac resynchronization therapy (CRT) [8, 9] with improvements in cardiac function, symptoms, morbidity and mortality. Patients who were at low risk of dying for any reason other than an arrhythmia benefitted from an implantable cardioverter-defibrillator (ICD) although its utility is currently being called into question [10, 11]. The development of dedicated specialist HF teams has also been of great importance to inform patients of their diagnosis, prognosis and need for therapy, to improve the implementation of and adherence to treatment and to facilitate titration of medications to target doses, all of which leads to greater patient-satisfaction and better long-term outcomes [12].
Despite these successes, the ‘war’ on heart failure is far from won. For patients hospitalised with worsening heart failure aged less than 75 years, mortality at 1 year may be as high as 20% and up to 40% in those aged > 85 years [13]. For patients with stable HFrEF who survive the initial 6 months after diagnosis and are enrolled in contemporary clinical trials, the annual risk of the composite of hospitalisation for heart failure or mortality is about 10% [14]. Outcome amongst patients who do not participate in clinical trials is much worse [15]. Older patients and those with a recent episode of decompensation despite guideline-recommended therapy who require intensification of therapy have a much worse prognosis. Disappointingly, many patients do not receive, and therefore cannot benefit from, guideline-recommended therapy [16, 17].
More appropriate use of investigations and less complex diagnostic algorithms are likely to reveal that there are many undiagnosed cases of heart failure in the community, particularly with preserved left ventricular (LV) ejection fraction (HFpEF) [18], a condition for which some insist no effective therapy exists as yet, although treatment with a thiazide diuretic and ACE inhibitor exerted remarkable benefits in the HYVET trial in a group of patients many of whom undoubtedly had undeclared HFpEF [19]. Of note, the European Society of Cardiology (ESC) heart failure registry suggested little difference in the therapies applied to patients with HFrEF and HFpEF in clinical practice; perhaps clinicians are sometimes wiser than the guidelines they are asked to follow [20].
The age-adjusted incidence of heart failure may be fairly stable but the total number of patients who will develop heart failure will rise substantially in the next few decades as the proportion of people aged > 60 years increases [21]. Nowadays, many people survive the onset of cardiovascular disease for long periods. Treatment of hypertension, diabetes, chronic kidney disease, atrial fibrillation and ischaemic heart disease might delay the onset of heart failure, but procrastination is not the same as prevention. It is likely that most people with cardiovascular disease will develop heart failure before they die [22, 23]. Strategies to diagnose and treat heart failure before it becomes clinically overt require much more research investment [24]. An increased awareness of what is important to older people may identify novel outcomes and treatments and define the future role of palliative care and euthanasia.
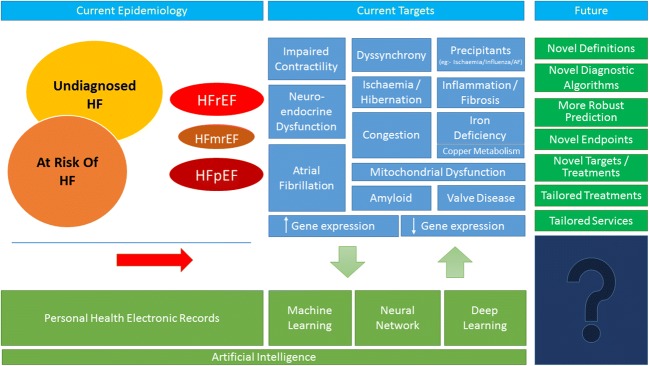
Enormous amounts of routinely collected personal health records, biochemical and imaging data are now available for novel analytical approaches such as machine-learning and artificial intelligence that will identify novel pathways leading to heart failure and redefine its epidemiology in the next decade (Fig. 1). The definition as well as management of heart failure might be transformed, with care and services personalised to the individual patient’s needs.
Fig. 1.
The present and future of heart failure. Conventionally, the prevalence of heart failure is thought to be about 1.5% in the adult population. However, it might be substantially greater than that, as many cases remain undiagnosed, particularly amongst older people, and are usually only identified when symptoms are severe enough to require hospital admission. Several ongoing trials target different pathways that might contribute to disease progression. Success provides tentative insights into the likely mechanisms of progression, although off-target effects may lead to serendipitous effects (this is probably true of most effective treatments for heart failure). There may be many reasons for failure other than the lack of importance of the targeted mechanism. This may include a smaller than anticipated benefit with consequent lack of power, lack of target engagement, a mechanism that is important but only works in a specific subgroup (e.g., heart rate reduction in sinus rhythm) or one that is overwhelmed by competing risks (e.g., rivaroxaban 2.5 mg bd for advanced heart failure in sinus rhythm). Processing large volumes of routinely collected electronic health records using novel analytical approaches, such as artificial intelligence and machine learning, will provide new insights into disease classification, mechanisms of progression and therapeutic targets. Epidemiology, definition and management of heart failure are likely to be transformed in the next decade, with care and services matched to the individual patient’s needs in a “precision-medicine” approach
Currently, there are many ongoing trials exploring the potential for benefit, or harm, of old and new treatments that might improve the management of HF: summarising novel pharmacological interventions is the purpose of this review; space precludes an in-depth review of devices (electrical, mechanical or valve) or biological interventions (other than influenza vaccination) although key trials are shown in the Table 1 (and in supplementary Table 1, if they aim to enrol fewer than 200 patients).
Table 1.
Ongoing trials in heart failure (HF). Only trials planned to recruit > 200 participants with HF are shown
| Name | ClinicalTrials.gov identifier | Expected completion | Phase | Participants | HF phenotype | Recruitment status |
|---|---|---|---|---|---|---|
| Willingness to participate | NCT03840499 | 2022 | NA | 400 | All | A |
| Neuro-endocrine interventions | ||||||
| Augmentation of natriuretic and other peptides: sacubitril/valsartan | ||||||
| PARAGON | NCT01920711 | 2019 | 3 | 4822 | HFpEF | T |
| PARALLEL-HF | NCT02468232 | 2020 | 3 | 225 | HFrEF | T |
| PERSPECTIVE | NCT02884206 | 2022 | 3 | 520 | HFpEF | A |
| PARALLAX | NCT03066804 | 2019 | 3 | 2500 | HFpEF | A |
| HFN-LIFE | NCT02816736 | 2020 | 4 | 400 | Severe HFrEF | A |
| Management of hyperkalaemia: patiromer and sodium zirconium cyclosilicate (SCZ) | ||||||
| DIAMOND (patiromer) | NCT03888066 | 2022 | 3 | 2388 | HFrEF | Not yet A |
| RELIEHF (patiromer) | ? | 2022/2024 | 4 | 400/2000 | All | Not yet A |
| PRIORITIZE HF (SZC) | NCT03532009 | Suspended | 2 | 280 | HFrEF | A |
| Vasodilators: vericiguat | ||||||
| VICTORIA | NCT02861534 | 2020 | 3 | 4872 | HFrEF | T |
| Vitality-HFpEF | NCT03547583 | 2020 | 2 | 735 | HFpEF | A |
| Vasodilators: nitroxyl | ||||||
| STANDUP-AHF | NCT03016325 | 2019 | 2 | 310 | HFrEF | A |
| Inotropic agents | ||||||
| Omecamtiv mecarbil | ||||||
| GALACTIC-HF | NCT02929329 | 2021 | 3 | 8000 | HFrEF | A |
| METEORIC-HF | NCT03759392 | 2021 | 3 | 270 | HFrEF | Not yet A |
| Levosimendan | ||||||
| LeoDOR | NCT03437226 | 2019 | 3 | 264 | HFrEF | A |
| Digoxin | ||||||
| DIG-START-AHF | NCT02544815 | 2019 | 3 | 1500 | AHF | A |
| DECISION | NCT03783429 | 2024 | 4 | 982 | LVEF < 50% | Not yet A |
| Recombinant human neuregulin-1β | ||||||
| NCT03388593 | 2023 | 3 | 1600 | HFrEF | A | |
| Congestion | ||||||
| Ultrasound guided treatment for congestion | ||||||
| JECICA | NCT02892227 | 2019 | NA | 250 | AHF | A |
| CAVA-ADHF | NCT03140566 | 2019 | NA | 388 | AHF | A |
| Device guided treatment for congestion | ||||||
| GUIDE-HF | NCT03387813 | 2023 | NA | 3600 | HFrEF and HFpEF | A |
| Torasemide | ||||||
| TRANSFORM-HF | NCT03296813 | 2022 | 3 | 6000 | HFrEF | A |
| Acetazolamide | ||||||
| ADVOR | NCT03505788 | 2021 | 4 | 519 | WHF | A |
| Other combinations of diuretic | ||||||
| CLOROTIC | NCT01647932 | 2019 | 4 | 304 | AHF | A |
| Spironolactone | ||||||
| SPIRRIT | NCT02901184 | 2022 | 3 | 3200 | HFpEF | A |
| SPIRIT-HF | 2017-000697-11* | ? | 3 | 1300 | HFmrEF/HFpEF | A |
| SGLT2i | ||||||
| Empagliflozin | ||||||
| EMPERIAL-R | NCT03448419 | 2019 | 3 | 300 | HFrEF | A |
| EMPERIAL-P | NCT03448406 | 2019 | 3 | 300 | HFpEF | A |
| EMMY | NCT03087773 | 2020 | 3 | 476 | HF (post AMI) | A |
| EMPEROR-P | NCT03057951 | 2021 | 3 | 6000 | HFpEF | A |
| EMPEROR-R | NCT03057977 | 2020 | 3 | 2850 | HFrEF | A |
| Sotagliflozin | ||||||
| SOLOIST-WHF | NCT03521934 | 2021 | 3 | 4000 | HFrEF and T2DM | A |
| Dapagliflozin | ||||||
| PRESERVED-HF | NCT03030235 | 2019 | 4 | 320 | HFpEF | A |
| DAPA-HF | NCT03036124 | 2019 | 3 | 4744 | HFrEF | T |
| DEFINE-HF | NCT02653482 | 2019 | 4 | 263 | HFrEF | T |
| DELIVER | NCT03619213 | 2021 | 3 | 4700 | HFpEF | A |
| Intravenous iron | ||||||
| IRONMAN | NCT02642562 | 2021 | 3 | 1300 | HFrEF | A |
| HEART-FID | NCT03037931 | 2022 | 3 | 3014 | HFrEF | A |
| FAIR-HF2 | NCT03036462 | 2020 | 4 | 1200 | HFrEF | A |
| FAIR-HFpEF | NCT03074591 | 2019 | 2 | 200 | HFpEF | A |
| Affirm-HF | NCT02937454 | 2019 | 4 | 1100 | AHF (LVEF < 50%) | A |
| Micronutrients: copper, selenium and co-enzyme Q10 | ||||||
| Q10 | NCT03133793 | 2020 | 2 | 250 | HFpEF | A |
| TRACER-HF | NCT03875183 | 2021 | 2 | 200 | HFrEF | Not yet A |
| Pulmonary hypertension and right ventricular dysfunction | ||||||
| Treprostinil | ||||||
| NCT03037580 | 2020 | 3 | 310 | HFpEF and PHT | A | |
| Macitentan | ||||||
| SERENADE | NCT03153111 | 2020 | 2 | 300 | HFpEF and RV Dysfunction and PHT | A |
| Cardiac amyloidosis | ||||||
| Tafamidis-long term | NCT02791230 | 2024 | 3 | 1400 | NA | A |
| Influenza vaccination | ||||||
| RCT-IVVE | NCT02762851 | 2020 | 4 | 5000 | NYHA II-IV | A |
| INVESTED | NCT02787044 | 2021 | 4 | 9300 | HFrEF | A |
| Hydralazine and metformin | ||||||
| DANHEART | NCT03514108 | 2023 | 4 | 1500 | HFrEF | A |
| Devices and others | ||||||
| AdaptResponse | NCT02205359 | 2023 | NA | 3700 | Adaptive CRT and HFrEF | A |
| APAF-CRT | NCT02137187 | 2021 | 2–3 | 1830 | Atrio-ventricular junction ablation for AF and HF | A |
| REVIVED-BCIS2 | NCT01920048 | 2022 | 3 | 700 | IHD and HFrEF (Revasc) | A |
| GUIDE-CMR | NCT01918215 | 2023 | NA | 428 | ICD v ILR for HF and LVEF 35–50% | A |
| RESET-ICD | NCT03494933 | 2021 | NA | 2030 | CRT-P vs CRT-D | A |
| RESHAPE-HF2 | NCT02444338 | 2021 | NA | 420 | MR and HFrEF | A |
| ADVENT-HF | NCT01128816 | 2020 | 4 | 860 | Sleep apnoea and LVEF < 45% | A |
| PURE-HF | NCT03161158 | 2021 | NA | 864 | HF and severe congestion (venous ultrafiltration) | A |
Smaller trials are summarised in Table 1 supplementary
*EUDRACT number
HFrEF, heart failure with reduced left ventricular ejection fraction (LVEF); HFpEF, heart failure with preserved left ventricular ejection fraction; HFmrEF, heart failure with mid-range left ventricular ejection fraction; AF, atrial fibrillation; MR, mitral regurgitation; IHD, ischaemic heart disease; T2DM, type 2 diabetes; ICD, implantable cardioverter-defibrillator; ILR, implantable loop recorder; CRT, cardiac resynchronization therapy; PHT, pulmonary hypertension; AHF, acute heart failure; AMI, acute myocardial infarction; A, active recruitment; T, recruitment terminated
Neuro-endocrine interventions
Augmentation of natriuretic and other peptides: sacubitril/valsartan
One of the key therapeutic successes for heart failure has been the inhibition of neuro-endocrine pathways with ACE-Is, ARBs, MRAs and beta-blockers. Recently, a new class of agents, angiotensin receptor neprilysin inhibitors (ARNI), has proved superior to ACE-Is for the treatment of HFrEF [14]. Neprilysin inhibitors retard the degradation of many peptides, including atrial (ANP) and B-type natriuretic peptides (BNP) and vasoactive intestinal polypeptide, which have diuretic, vasodilator and inotropic properties [25, 26]. In the Comparison of Sacubitril–Valsartan versus Enalapril on Effect on NT-proBNP in Patients Stabilized from an Acute Heart Failure Episode (PIONEER-HF) trial, initiation of sacubitril/valsartan for patients with either new-onset or chronic HFrEF (n = 881) during the in-hospital recovery phase after an acute decompensation was as safe as initiating enalapril, but led to a greater, and earlier (within 1 week), reduction in plasma concentrations of NT-proBNP, which was sustained until the end of 8 weeks follow-up [27]. A reduction in a composite of serious HF-related adverse clinical events was also observed [28]. However, about 20% of surviving patients discontinued treatment with either ACEi or ARNI and only 55% achieved guideline-recommended doses of the ARNI [27]. In the PRIME trial (n = 118), patients with HF, an LVEF < 50% and functional mitral regurgitation (MR) who were randomised to sacubitril/valsartan had a greater reduction in the effective regurgitant orifice area (EROA) compared with valsartan alone at 12 months follow-up [29]. Other trials are currently ongoing in specific populations with HFrEF, including those with symptoms at rest (NCT02816736), or an elevated pulmonary artery pressure (NCT02788656) or in Japan (NCT02468232).
The Prospective Comparison of ARNI with ARB Global Outcomes in HF With Preserved Ejection Fraction (PARAGON; NCT01920711) is a randomised, double-blind, event-driven trial comparing the efficacy and safety of valsartan vs sacubitril/valsartan in patients with HFpEF that has enrolled 4822 patients (mean age 73 ± 8 years, median NT-proBNP 911 (interquartile range 464–1610) pg/mL, > 2/3 in sinus rhythm) [30]. The results should be reported later in 2019. PARALLAX (NCT03066804) is another large (> 2,000 patients) randomised, double-blind trial of patients with HFpEF, comparing sacubitril/valsartan with a control group (the investigator can chose whether this is an ACE-I, an ARB or neither, in which case patients assigned to the control group receive placebo); the effect on plasma NT-proBNP and exercise capacity after 24 weeks of treatment and safety are the main outcomes of interest.
Concerns exist that the inhibition of neprilysin could interfere with breakdown of beta amyloid (βA) peptides, which might accumulate in the brain and contribute to the development of Alzheimer’s disease. The PERSPECTIVE trial (NCT02884206) is currently recruiting ~ 500 patients with HF and LVEF > 40%, to investigate whether chronic administration of sacubitril/valsartan for 3 years leads to a decline in cognitive function when compared with valsartan alone.
Management of hyperkalaemia: patiromer and sodium zirconium cyclosilicate
Currently, based on the evidence provided by clinical trials, guidelines recommend that ACEi, ARB and MRA should not be initiated if serum potassium is > 5.0 mmol/L (5.2 mmol/L for ARNI) and that doses should be reduced or treatment stopped if serum potassium is > 5.5 mmol/L. Accordingly, many patients with HFrEF do not receive guideline-recommended doses of these agents [16, 17, 31]. Older patients, those with type-2 diabetes mellitus and those with renal dysfunction are more likely to develop hyperkalaemia [32]. Patients who fail to achieve guideline-recommended doses of these medications due to hyperkalaemia have a worse prognosis, but this may be because of concomitant renal dysfunction or hypotension.
Patiromer and sodium zirconium cyclosilicate are novel oral treatments that bind potassium in the gastrointestinal (GI) tract and rapidly normalise serum potassium concentrations. Whether their use will allow doctors to prescribe and patients to achieve guideline-recommended doses of RAASi more often and whether this will improve outcomes are now being investigated. Results of substantial trials are not expected before 2021.
Vasodilators: vericiguat and nitroxyl
Nitric oxide (NO) activates soluble guanylate cyclase (sGC), causing an elevation of intracellular cyclic guanosine monophosphate (cGMP) in vascular and non-vascular tissues, such as the myocardium and kidney. In heart failure, production of NO is reduced and its degradation is increased, leading to an increase in systemic and pulmonary arteriolar and venous tone, thereby increasing the after-load and pre-load on the failing myocardium [33]. Vericiguat is an oral sGC stimulator which increases cGMP production. Phase 2 trials showed that vericiguat is well tolerated in patients with HFrEF [34]. A large (~ 4,500 patients) phase 3 trial (VICTORIA; NCT02861534) is currently evaluating whether vericiguat improves morbidity and mortality compared with placebo in patients with chronic HFrEF [35].
Nitroxyl is a second-generation donor of nitric oxide that causes vasodilatation and may have inotropic effects, which are only partially mediated by an increase in cGMP [36]. A phase 2 trial (STAND-UP; NCT03016325) is currently evaluating the safety and efficacy (changes in NT-proBNP and symptoms) of 48-h infusion of nitroxyl in 310 patients admitted with decompensated HFrEF. Smaller mechanistic trials are investigating its effects on cardiac and renal function.
Inotropic agents
Omecamtiv mecarbil, levosimendan, digoxin and recombinant human neuregulin-1
Omecamtiv mecarbil (OM) is a cardiac myosin activator that alters the kinetics of actin/myosin cross-bridges, prolonging the duration of the systole and, thus, stroke volume, without increasing ATP consumption [37]. Phase II trials showed that IV administration of OM in patients with acutely decompensated HFrEF had the expected haemodynamic effects but no clear clinical benefit [38]. In The Chronic Oral Study of Myosin Activation to Increase Contractility in Heart Failure (COSMIC-HF) trial, oral OM given for 20 weeks was safe and reduced LV size and plasma concentrations of NT-proBNP levels; the latter effect persisted for 4 weeks after treatment withdrawal suggesting that long-term favourable structural remodelling had occurred [39]. The Phase II trial programme has repeatedly shown small increases in serum troponin concentrations, raising concerns about safety that, so far, appears unfounded. Increases in troponin appear unrelated to any clinical evidence of myocardial ischaemia or adverse outcomes. A large (n ~ 8,000) phase III trial of patients with chronic HFrEF (with 25% planned to be enrolled during a hospitalisation for an episode of decompensation) is nearing completion of enrolment and should report in 2021 (GALACTIC-HF; NCT02929329).
Levosimendan, a vasodilator and calcium sensitiser, has been used to treat refractory HF in many countries despite two large neutral trials conducted in patients with acute HF and a large trial of an oral formulation in patients with chronic severe HF that showed reductions in NT-proBNP and an improvement in QoL but did not otherwise improve outcome [40, 41]. Recently, small trials have explored the effects of giving levosimendan intermittently to patients with chronic severe HFrEF and shown that this can reduce plasma concentrations of NT-proBNP [42]. Larger trials are now attempting to determine whether this strategy can improve symptoms, exercise capacity, morbidity and mortality in patients with HFrEF.
Neuregulin-1 proteins are important for the development and function of cardiac myocytes. Small phase II studies reported that recombinant human neuregulin-1 improved haemodynamics and promoted reverse LV remodelling in patients with HFrEF [43, 44]. A phase III study is currently testing whether, compared to placebo, use of daily (for 10 days) IV infusions, followed by weekly boluses, of recombinant human neuregulin-1 is feasible, safe and effective in reducing mortality in Chinese patients with mild to moderate chronic HFrEF.
Digoxin may be the oldest medicine still prescribed for heart failure, but controversies persist about its benefits. In the DIG trial, conducted before many current HF treatments were available, digoxin did not reduce mortality compared to placebo, although it did reduce HF hospitalisations by 28%. A retrospective analysis suggested that patients with serum concentrations of digoxin of 0.5–0.9 ng/mL were more likely to benefit [45, 46]. A prospective, randomised, placebo-controlled trial is testing whether lower doses of digoxin, guided by measurements of its plasma concentrations (0.5–0.9 ng/mL), will reduce HF hospitalisations and cardiovascular death in ~ 1,000 symptomatic patients with chronic HF and a reduced or mid-range LVEF (< 50%) (NCT03783429).
Congestion
Congestion is an important cause of the symptoms and signs of HF, leads to adverse atrial and ventricular remodelling, arrhythmias and worsening renal function and is associated with poor outcomes [47, 48]. Controlling congestion is a key therapeutic goal in the management of heart failure. However, clinical identification of congestion is challenging, unless severe. Up to 50% of outpatients with HF who were considered to be clinically dry had sub-clinical congestion on ultrasound, either in the pulmonary interstitium (lung B-lines) or in the intra-vascular space, as measured by a distended inferior vena cava (IVC). Sub-clinical congestion was associated with a poor outcome [49, 50]. Whether treatment guided by ultrasound assessments is feasible and effective for the management of congestion in patients with HF is currently being explored in several small- to medium-sized trials. Biomarker-guided management of congestion has met with mixed success, largely because treatment was similarly effective in each arm [51]. A large trial (GUIDE-HF; NCT03387813) is currently investigating whether pulmonary artery pressure monitoring using a small implanted device can help guide treatment of congestion.
Torasemide, acetazolamide and other diuretics
Loop diuretics are the most potent diuretic agents, and furosemide is the most widely used in patients with HF. However, other loop diuretics, such as bumetanide and torasemide, are either better absorbed or delivered more reliably to the renal tubule. Meta-analysis of small randomised trials and observational studies suggests that torasemide might be superior to furosemide, but no substantial randomised trial has yet compared these two agents [52–54]. TRANSFORM-HF (NCT03296813) is an ongoing, multi-centre, unblinded, trial that will randomise, prior to discharge, ~ 6000 patients admitted with decompensated heart failure to long-term treatment with oral torasemide or furosemide to investigate effects on morbidity and mortality.
Other options for treating resistant congestion in patients HF exist, such as combining different classes of diuretics, but their safety and efficacy have been rarely tested in clinical trials [55]. Most of the sodium filtered by kidneys is reabsorbed in the proximal tubule of the nephron. Acetazolamide, a carbonic anhydrase inhibitor, should decrease the amount of sodium reabsorbed in the proximal nephron and enhance the distal effects of loop diuretics. The Acetazolamide in Decompensated heart failure with Volume OveRload (ADVOR) is a randomised, double-blind, placebo-controlled trial which will test whether combining acetazolamide with a loop diuretic is more successful in achieving decongestion in ~ 500 patients admitted with HF and signs of fluid overload [56].
Sodium glucose co-transporter 2 inhibitors
Although not everyone would agree that it is the principal mechanism of action of sodium glucose co-transporter 2 inhibitors (SGLT2i), there is little doubt that diuresis contributes to their effects in HF. SGLT2i reduce glucose reabsorption in the proximal nephron, increasing delivery of glucose and sodium to the distal nephron and inducing an osmotic diuresis. Whether SGLT2i have additional metabolic effects on the heart and kidney by inhibiting carbonic anhydrase or increasing the availability of ketones as a metabolic substrate for the myocardium is uncertain [57]. Empagliflozin reduced all-cause mortality and hospitalisation for heart failure in patients with type 2 diabetes mellitus (T2DM) and ischaemic heart disease (IHD) [58]. Trials of canagliflozin and dapagliflozin also suggested a reduction in hospitalisations for HF [59–61]; although the relative risk reduction was substantial, the absolute benefits were very small, creating uncertainty about whether they are clinically meaningful. Interestingly, the programme of phase III trials for HF has not required patients to have T2DM and has enrolled a broad range of patients with HFrEF and HFpEF as well as in-patients and out-patients. The first of these trials is likely to report in 2019 (DAPA-HF) [62].
Intravenous iron
Up to 50% of patients with HF have iron deficiency (ID), with or without anaemia. ID is associated with adverse outcomes, even in the absence of anaemia, and is a potential target of treatment [63]. Oral iron is widely available and cheap but only a small amount of oral iron can be absorbed in a day (perhaps 2–10 mg/day compared with a total deficiency of > 1,000 mg) and many patients have GI intolerance to oral iron. Oral iron absorption may be impaired in heart failure, possibly due to increased secretion of hepatic hepcidin, but even if it is not, oral supplementation would take many months to correct iron deficiency [64]. Modern preparations of IV iron are safe and well tolerated and improve symptoms and exercise capacity in patients with HFrEF. An individual patient meta-analysis from four randomised controlled trials including 839 patients with HFrEF and ID, of whom 504 were randomised to IV ferric carboxymaltose, suggests that short-term (mean follow-up 31 weeks) treatment could also reduce HF hospitalisations when compared with placebo. However, the analysis included very few cardiovascular (n = 34) or other (n = 4) deaths and does not prove long-term safety [65]. Four substantial (> 1000 patients) randomised trials are currently investigating whether different formulations of IV iron (either iron isomaltoside or ferric carboxymaltose) improve morbidity and mortality in patients with chronic or acute HF. These trials have included far more patients and recorded far more events than the published evidence but have not yet been stopped for benefit. Phase II trials are also investigating the potential benefits of IV iron on symptoms, exercise tolerance and quality of life of patients with HFpEF and ID (NCT03074591).
Copper, selenium and co-enzyme Q10
Heart failure may be accompanied by high plasma copper concentrations but myocardial copper depletion. There is evidence from both animal models and a limited amount of human data that copper chelation may be beneficial [66]. However, an alternative view is that low doses of the chelating agent trientine might facilitate copper redistribution to tissues. This concept is currently being tested in a 200-patient, dose-ranging trial (NCT03875183).
Co-enzyme Q10 is an essential component of the mitochondrial electron transport chain and both co-enzyme Q10 and selenium have an important role in many metabolic processes. Lower plasma concentrations of Q10 and selenium have been associated with adverse outcomes in heart failure [67–69]. Two trials showed a reduction in mortality with co-enzyme Q10 supplements for patients with or at high-risk of heart failure and a broad range of LVEF [70, 71]. Randomised controlled trials are underway.
Other trials
Pulmonary hypertension and right ventricular dysfunction
Pulmonary hypertension (PHT) is common, especially in patients with advanced heart failure, due to a combination of left atrial hypertension, pulmonary arteriolar hypertrophy and pulmonary vasoconstriction. Small trials have shown that sildenafil, a selective inhibitor of type 5 phosphodiesterase, might improve haemodynamics and exercise performance in patients with HFrEF and PHT; other trials should report soon [72]. In HFpEF, sildenafil was not beneficial [73]. The effects of treprostinil, a synthetic analogue of prostacyclin with potent vasodilator properties, on exercise capacity and NT-proBNP are currently under investigation in a trial (n ~ 300) of HFpEF and PHT. However, trials in patients with HFrEF were stopped for harm. The safety, and effect on NT-proBNP levels of macitentan, an antagonist/blocker of endothelin receptors, will be also studied in 300 patients with HFpEF complicated by PHT or right ventricular dysfunction (SERENADE, NCT03153111).
Amyloidosis
Accumulation of wild-type or variant transthyretin amyloid occurs when fibrils become unstable and misfold. Recent reports suggest that 15–20% of patients with HFpEF may have TTR amyloidosis. These patients have a poor outcome and may not respond to conventional treatments [74]. A recent trial showed that treatment with tafamidis, which binds to transthyretin, preventing tetramer dissociation and amyloidogenesis, improves symptoms, quality of life and exercise capacity and reduces cardiovascular hospitalisations and mortality in patients with transthyretin amyloid cardiomyopathy [75]. The costs of tafamidis are currently prohibitive, preventing large-scale uptake. However, demonstration of the effectiveness of treatment will lead to changes in diagnostic pathways (at least to identify patients who may not benefit from some treatments or for selection into clinical trial even if treatment is unaffordable). In due course, the cost of tafamidis will fall.
Influenza vaccination
Influenza might be an important precipitant of HF hospitalisations [76]. A recent observational study from Denmark suggested that influenza vaccination might be associated with better outcomes in patients with heart failure, but it also reported that a large proportion (> 40%) of patients with heart failure do not receive influenza vaccination, which might reflect lack of evidence arising from trials and therefore weak recommendations from guidelines [77]. Two large trials investigating the ability of influenza vaccinations to reduce morbidity and mortality should report in the next few years. The Influenza Vaccine To Prevent Adverse Vascular Events (RCT-IVVE) will randomise ~ 5,000 patients with HF globally. The INfluenza Vaccine to Effectively Stop Cardio Thoracic Events and Decompensated Heart Failure (INVESTED) will compare high-dose trivalent influenza vaccine vs standard-dose quadrivalent influenza vaccine in almost 10,000 patients with a recent myocardial infarction or hospitalisation for HF.
Conclusions
Over the last 30 years, various pathways leading to the development and progression of heart failure have been identified and successfully targeted with effective therapies. This has improved the quality of life and survival for millions of individuals with HFrEF, globally. Hopefully, new treatments will offer further improvements and extend these successes to the treatment of HFpEF and other specific causes and phenotypes of HF. New concepts of how HF should be defined combined with new analytical approaches using large data-sets will re-shape its epidemiology and offer new therapeutic targets. However, old age rather than cardiac dysfunction may be the next great barrier to overcome.
Electronic supplementary material
(DOCX 23 kb)
Compliance with ethical standards
Conflict of interest
Dr. Cleland reports personal fees from Johnson & Johnson; grants and personal fees from Amgen; personal fees from AstraZeneca; grants and personal fees from Bayer; grants and personal fees from Bristol Myers Squibb; personal fees from GSK; grants, personal fees and non-financial support from Medtronic; personal fees from Myokardia; grants, personal fees and non-financial support from Novartis; grants and personal fees from Philips; grants and non-financial support from Pharmacosmos; grants and non-financial support from PharmaNord; personal fees from Sanofi; personal fees from Servier; grants and personal fees from Stealth Biopharmaceuticals; grants and personal fees from Torrent Pharmaceuticals; and grants, personal fees and non-financial support from Vifor.
Dr. Pellicori has received support for travel from Novartis and Vifor.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Cohn JN, Archibald DG, Ziesche S, Franciosa JA, Harston WE, Tristani FE, Dunkman WB, Jacobs W, Francis GS, Flohr KH, Goldman S, Cobb FR, Shah PM, Saunders R, Fletcher RD, Loeb HS, Hughes VC, Baker B. Effect of vasodilator therapy on mortality in chronic congestive heart failure. Results of a Veterans Administration Cooperative Study. N Engl J Med. 1986;314:1547–1552. doi: 10.1056/NEJM198606123142404. [DOI] [PubMed] [Google Scholar]
- 2.The CONSENSUS Trial Study Group (1987) Effects of enalapril on mortality in severe congestive heart failure. Results of the Cooperative North Scandinavian Enalapril Survival Study (CONSENSUS). N Engl J Med 316:1429–1435 [DOI] [PubMed]
- 3.The SOLVD Investigators (1991) Effect of enalapril on survival in patients with reduced left ventricular ejection fractions and congestive heart failure. N Engl J Med 325:293–302 [DOI] [PubMed]
- 4.Packer M, Bristow MR, Cohn JN, Colucci WS, Fowler MB, Gilbert EM, Shusterman NH. The effect of carvedilol on morbidity and mortality in patients with chronic heart failure. U.S. Carvedilol Heart Failure Study Group. N Engl J Med. 1996;334:1349–1355. doi: 10.1056/NEJM199605233342101. [DOI] [PubMed] [Google Scholar]
- 5.Pitt B, Zannad F, Remme WJ, Cody R, Castaigne A, Perez A, Palensky J, Wittes J. The effect of spironolactone on morbidity and mortality in patients with severe heart failure. Randomized Aldactone Evaluation Study Investigators. N Engl J Med. 1999;341:709–717. doi: 10.1056/NEJM199909023411001. [DOI] [PubMed] [Google Scholar]
- 6.Swedberg K, Komajda M, Böhm M, Borer JS, Ford I, Dubost-Brama A, Lerebours G, Tavazzi L. SHIFT Investigators. Ivabradine and outcomes in chronic heart failure (SHIFT): a randomised placebo-controlled study. Lancet. 2010;376:875–885. doi: 10.1016/S0140-6736(10)61259-7. [DOI] [PubMed] [Google Scholar]
- 7.Cleland et al. Effect of ivabradine on mortality in patients with heart failure and a reduced left ventricular ejection fraction not receiving a beta-blocker: an analysis from SHIFT. Eur Heart J, Volume 38, Issue suppl_1, August 2017, ehx501.246
- 8.Cleland JG, Daubert JC, Erdmann E, Freemantle N, Gras D, Kappenberger L, Tavazzi L, Cardiac Resynchronization-Heart Failure (CARE-HF) Study Investigators The effect of cardiac resynchronization on morbidity and mortality in heart failure. N Engl J Med. 2005;352:1539–1549. doi: 10.1056/NEJMoa050496. [DOI] [PubMed] [Google Scholar]
- 9.Cleland JG, Abraham WT, Linde C, Gold MR, Young JB, Claude Daubert J, Sherfesee L, Wells GA, Tang AS. An individual patient meta-analysis of five randomized trials assessing the effects of cardiac resynchronization therapy on morbidity and mortality in patients with symptomatic heart failure. Eur Heart J. 2013;34:3547–3556. doi: 10.1093/eurheartj/eht290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Moss AJ, Hall WJ, Cannom DS, Daubert JP, Higgins SL, Klein H, Levine JH, Saksena S, Waldo AL, Wilber D, Brown MW, Heo M. Improved survival with an implanted defibrillator in patients with coronary disease at high risk for ventricular arrhythmia. Multicenter Automatic Defibrillator Implantation Trial Investigators. N Engl J Med. 1996;335:1933–1940. doi: 10.1056/NEJM199612263352601. [DOI] [PubMed] [Google Scholar]
- 11.Køber L, Thune JJ, Nielsen JC, Haarbo J, Videbæk L, Korup E, Jensen G, Hildebrandt P, Steffensen FH, Bruun NE, Eiskjær H, Brandes A, Thøgersen AM, Gustafsson F, Egstrup K, Videbæk R, Hassager C, Svendsen JH, Høfsten DE, Torp-Pedersen C, Pehrson S, DANISH Investigators Defibrillator implantation in patients with nonischemic systolic heart failure. N Engl J Med. 2016;375:1221–1230. doi: 10.1056/NEJMoa1608029. [DOI] [PubMed] [Google Scholar]
- 12.Blue L, Lang E, McMurray JJ, Davie AP, McDonagh TA, Murdoch DR, Petrie MC, Connolly E, Norrie J, Round CE, Ford I, Morrison CE. Randomised controlled trial of specialist nurse intervention in heart failure. BMJ. 2001;323:715–718. doi: 10.1136/bmj.323.7315.715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cleland JG, McDonagh T, Rigby AS, Yassin A, Whittaker T, Dargie HJ, National Heart Failure Audit Team for England and Wales The national heart failure audit for England and Wales 2008-2009. Heart. 2011;97:876–886. doi: 10.1136/hrt.2010.209171. [DOI] [PubMed] [Google Scholar]
- 14.McMurray JJ, Packer M, Desai AS, Gong J, Lefkowitz MP, Rizkala AR, Rouleau JL, Shi VC, Solomon SD, Swedberg K, Zile MR. Angiotensin-neprilysin inhibition versus enalapril in heart failure. PARADIGM-HF Investigators and Committees. N Engl J Med. 2014;371:993–1004. doi: 10.1056/NEJMoa1409077. [DOI] [PubMed] [Google Scholar]
- 15.Clark AL, Lammiman MJ, Goode K, Cleland JG. Is taking part in clinical trials good for your health? A cohort study. Eur J Heart Fail. 2009;11:1078–1083. doi: 10.1093/eurjhf/hfp133. [DOI] [PubMed] [Google Scholar]
- 16.Pellicori P, Urbinati A, Shah P, MacNamara A, Kazmi S, Dierckx R, Zhang J, Cleland JGF, Clark AL. What proportion of patients with chronic heart failure are eligible for sacubitril-valsartan? Eur J Heart Fail. 2017;19:768–778. doi: 10.1002/ejhf.788. [DOI] [PubMed] [Google Scholar]
- 17.Greene Stephen J., Fonarow Gregg C., DeVore Adam D., Sharma Puza P., Vaduganathan Muthiah, Albert Nancy M., Duffy Carol I., Hill C. Larry, McCague Kevin, Patterson J. Herbert, Spertus John A., Thomas Laine, Williams Fredonia B., Hernandez Adrian F., Butler Javed. Titration of Medical Therapy for Heart Failure With Reduced Ejection Fraction. Journal of the American College of Cardiology. 2019;73(19):2365–2383. doi: 10.1016/j.jacc.2019.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.van Riet EE, Hoes AW, Limburg A, Landman MA, van der Hoeven H, Rutten FH. Prevalence of unrecognized heart failure in older persons with shortness of breath on exertion. Eur J Heart Fail. 2014;16:772–777. doi: 10.1002/ejhf.110. [DOI] [PubMed] [Google Scholar]
- 19.Beckett NS, Peters R, Fletcher AE, Staessen JA, Liu L, Dumitrascu D, Stoyanovsky V, Antikainen RL, Nikitin Y, Anderson C, Belhani A, Forette F, Rajkumar C, Thijs L, Banya W, Bulpitt CJ, HYVET Study Group Treatment of hypertension in patients 80 years of age or older. N Engl J Med. 2008;358:1887–1898. doi: 10.1056/NEJMoa0801369. [DOI] [PubMed] [Google Scholar]
- 20.Maggioni AP, Anker SD, Dahlström U, Filippatos G, Ponikowski P, Zannad F, Amir O, Chioncel O, Leiro MC, Drozdz J, Erglis A, Fazlibegovic E, Fonseca C, Fruhwald F, Gatzov P, Goncalvesova E, Hassanein M, Hradec J, Kavoliuniene A, Lainscak M, Logeart D, Merkely B, Metra M, Persson H, Seferovic P, Temizhan A, Tousoulis D, Tavazzi L. Heart Failure Association of the ESC. Are hospitalized or ambulatory patients with heart failure treated in accordance with European Society of Cardiology guidelines? Evidence from 12,440 patients of the ESC Heart Failure Long-Term Registry. Eur J Heart Fail. 2013;15:1173–1184. doi: 10.1093/eurjhf/hft134. [DOI] [PubMed] [Google Scholar]
- 21.Curtis LH, Whellan DJ, Hammill BG, Hernandez AF, Anstrom KJ, Shea AM, Schulman KA. Incidence and prevalence of heart failure in elderly persons, 1994-2003. Arch Intern Med. 2008;168:418–424. doi: 10.1001/archinternmed.2007.80. [DOI] [PubMed] [Google Scholar]
- 22.Torabi A, Cleland JG, Khan NK, Loh PH, Clark AL, Alamgir F, Caplin JL, Rigby AS, Goode K. The timing of development and subsequent clinical course of heart failure after a myocardial infarction. Eur Heart J. 2008;29:859–870. doi: 10.1093/eurheartj/ehn096. [DOI] [PubMed] [Google Scholar]
- 23.Torabi A, Rigby AS, Cleland JG. Declining in-hospital mortality and increasing heart failure incidence in elderly patients with first myocardial infarction. J Am Coll Cardiol. 2009;55:79–81. doi: 10.1016/j.jacc.2009.05.080. [DOI] [PubMed] [Google Scholar]
- 24.Conrad N, Judge A, Tran J, Mohseni H, Hedgecott D, Crespillo AP, Allison M, Hemingway H, Cleland JG, McMurray JJV, Rahimi K. Temporal trends and patterns in heart failure incidence: a population-based study of 4 million individuals. Lancet. 2018;391:572–580. doi: 10.1016/S0140-6736(17)32520-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Good JM, Peters M, Wilkins M, Jackson N, Oakley CM, Cleland JG. Renal response to candoxatrilat in patients with heart failure. J Am Coll Cardiol. 1995;25:1273–1281. doi: 10.1016/0735-1097(94)00561-4. [DOI] [PubMed] [Google Scholar]
- 26.Packer M, Califf RM, Konstam MA, Krum H, McMurray JJ, Rouleau JL, Swedberg K. Comparison of omapatrilat and enalapril in patients with chronic heart failure: the Omapatrilat Versus Enalapril Randomized Trial of Utility in Reducing Events (OVERTURE) Circulation. 2002;106:920–926. doi: 10.1161/01.cir.0000029801.86489.50. [DOI] [PubMed] [Google Scholar]
- 27.Velazquez EJ, Morrow DA, DeVore AD, Duffy CI, Ambrosy AP, McCague K, Rocha R, Braunwald E, PIONEER-HF Investigators Angiotensin-neprilysin inhibition in acute decompensated heart failure. N Engl J Med. 2019;380:539–548. doi: 10.1056/NEJMoa1812851. [DOI] [PubMed] [Google Scholar]
- 28.Morrow David A., Velazquez Eric J., DeVore Adam D., Desai Akshay S., Duffy Carol I., Ambrosy Andrew P., Gurmu Yared, McCague Kevin, Rocha Ricardo, Braunwald Eugene. Clinical Outcomes in Patients With Acute Decompensated Heart Failure Randomly Assigned to Sacubitril/Valsartan or Enalapril in the PIONEER-HF Trial. Circulation. 2019;139(19):2285–2288. doi: 10.1161/CIRCULATIONAHA.118.039331. [DOI] [PubMed] [Google Scholar]
- 29.Kang DH, Park SJ, Shin SH, Hong GR, Lee S, Kim MS, Yun SC, Song JM, Park SW, Kim JJ. Angiotensin receptor neprilysin inhibitor for functional mitral regurgitation. Circulation. 2019;139:1354–1365. doi: 10.1161/CIRCULATIONAHA.118.037077. [DOI] [PubMed] [Google Scholar]
- 30.Solomon SD, Rizkala AR, Lefkowitz MP, Shi VC, Gong J, Anavekar N, Anker SD, Arango JL, Arenas JL, Atar D, Ben-Gal T, Boytsov SA, Chen CH, Chopra VK, Cleland J, Comin-Colet J, Duengen HD, Echeverría Correa LE, Filippatos G, Flammer AJ, Galinier M, Godoy A, Goncalvesova E, Janssens S, Katova T, Køber L, Lelonek M, Linssen G, Lund LH, O’Meara E, Merkely B, Milicic D, Oh BH, Perrone SV, Ranjith N, Saito Y, Saraiva JF, Shah S, Seferovic PM, Senni M, Sibulo AS, Jr, Sim D, Sweitzer NK, Taurio J, Vinereanu D, Vrtovec B, Widimský J, Jr, Yilmaz MB, Zhou J, Zweiker R, Anand IS, Ge J, CSP L, Maggioni AP, Martinez F, Packer M, Pfeffer MA, Pieske B, Redfield MM, Rouleau JL, Van Veldhuisen DJ, Zannad F, Zile MR, McMurray JJV. Baseline characteristics of patients with heart failure and preserved ejection fraction in the PARAGON-HF trial. Circ Heart Fail. 2018;11(7):e004962. doi: 10.1161/CIRCHEARTFAILURE.118.004962. [DOI] [PubMed] [Google Scholar]
- 31.Beusekamp JC, Tromp J, van der Wal HH, Anker SD, Cleland JG, Dickstein K, Filippatos G, van der Harst P, Hillege HL, Lang CC, Metra M, Ng LL, Ponikowski P, Samani NJ, van Veldhuisen DJ, Zwinderman AH, Rossignol P, Zannad F, Voors AA, van der Meer P. Potassium and the use of renin-angiotensin-aldosterone system inhibitors in heart failure with reduced ejection fraction: data from BIOSTAT-CHF. Eur J Heart Fail. 2018;20:923–930. doi: 10.1002/ejhf.1079. [DOI] [PubMed] [Google Scholar]
- 32.Michel A, Martín-Pérez M, Ruigómez A, García Rodríguez LA. Risk factors for hyperkalaemia in a cohort of patients with newly diagnosed heart failure: a nested case-control study in UK general practice. Eur J Heart Fail. 2015;17:205–213. doi: 10.1002/ejhf.226. [DOI] [PubMed] [Google Scholar]
- 33.Stasch JP, Pacher P, Evgenov OV. Soluble guanylate cyclase as an emerging therapeutic target in cardiopulmonary disease. Circulation. 2011;123:2263–2273. doi: 10.1161/CIRCULATIONAHA.110.981738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gheorghiade M, Greene SJ, Butler J, Filippatos G, Lam CS, Maggioni AP, Ponikowski P, Shah SJ, Solomon SD, Kraigher-Krainer E, Samano ET, Müller K, Roessig L, Pieske B, SOCRATES-REDUCED Investigators and Coordinators Effect of vericiguat, a soluble guanylate cyclase stimulator, on natriuretic peptide levels in patients with worsening chronic heart failure and reduced ejection fraction: the SOCRATES-REDUCED randomized trial. JAMA. 2015;314:2251–2262. doi: 10.1001/jama.2015.15734. [DOI] [PubMed] [Google Scholar]
- 35.Armstrong PW, Roessig L, Patel MJ, Anstrom KJ, Butler J, Voors AA, Lam CSP, Ponikowski P, Temple T, Pieske B, Ezekowitz J, Hernandez AF, Koglin J, O’Connor CM. A Multicenter, randomized, double-blind, placebo-controlled trial of the efficacy and safety of the oral soluble guanylate cyclase stimulator. The VICTORIA Trial JACC Heart Fail. 2018;6:96–104. doi: 10.1016/j.jchf.2017.08.013. [DOI] [PubMed] [Google Scholar]
- 36.Tita Cristina, Gilbert Edward M., Van Bakel Adrian B., Grzybowski Jacek, Haas Garrie J., Jarrah Mohammad, Dunlap Stephanie H., Gottlieb Stephen S., Klapholz Marc, Patel Parag C., Pfister Roman, Seidler Tim, Shah Keyur B., Zieliński Tomasz, Venuti Robert P., Cowart Douglas, Foo Shi Yin, Vishnevsky Alexander, Mitrovic Veselin. A Phase 2a dose-escalation study of the safety, tolerability, pharmacokinetics and haemodynamic effects of BMS-986231 in hospitalized patients with heart failure with reduced ejection fraction. European Journal of Heart Failure. 2017;19(10):1321–1332. doi: 10.1002/ejhf.897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cleland JG, Teerlink JR, Senior R, Nifontov EM, Mc Murray JJ, Lang CC, Tsyrlin VA, Greenberg BH, Mayet J, Francis DP, Shaburishvili T, Monaghan M, Saltzberg M, Neyses L, Wasserman SM, Lee JH, Saikali KG, Clarke CP, Goldman JH, Wolff AA, Malik FI. The effects of the cardiac myosin activator, omecamtiv mecarbil, on cardiac function in systolic heart failure: a double-blind, placebo-controlled, crossover, dose-ranging phase 2 trial. Lancet. 2011;378(9792):676–683. doi: 10.1016/S0140-6736(11)61126-4. [DOI] [PubMed] [Google Scholar]
- 38.Teerlink JR, Felker GM, McMurray JJV, Ponikowski P, Metra M, Filippatos GS, Ezekowitz JA, Dickstein K, JGF C, Kim JB, Lei L, Knusel B, Wolff AA, Malik FI, Wasserman SM, ATOMIC-AHF Investigators Acute treatment with omecamtiv mecarbil to increase contractility in acute heart failure: the ATOMIC-AHF study. J Am Coll Cardiol. 2016;67:1444–1455. doi: 10.1016/j.jacc.2016.01.031. [DOI] [PubMed] [Google Scholar]
- 39.Teerlink JR, Felker GM, McMurray JJ, Solomon SD, Adams KF, Jr, Cleland JG, Ezekowitz JA, Goudev A, Macdonald P, Metra M, Mitrovic V, Ponikowski P, Serpytis P, Spinar J, Tomcsányi J, Vandekerckhove HJ, Voors AA, Monsalvo ML, Johnston J, Malik FI, Honarpour N, COSMIC-HF Investigators Chronic Oral Study of Myosin Activation to Increase Contractility in Heart Failure (COSMIC-HF): a phase 2, pharmacokinetic, randomised, placebo-controlled trial. Lancet. 2016;388:2895–2903. doi: 10.1016/S0140-6736(16)32049-9. [DOI] [PubMed] [Google Scholar]
- 40.Nieminen MS, Cleland JG, Eha J, Belenkov Y, Kivikko M, Põder P, Sarapohja T. Oral levosimendan in patients with severe chronic heart failure --the PERSIST study. Eur J Heart Fail. 2008;10:1246–1254. doi: 10.1016/j.ejheart.2008.09.006. [DOI] [PubMed] [Google Scholar]
- 41.Packer M, Colucci W, Fisher L, Massie BM, Teerlink JR, Young J, Padley RJ, Thakkar R, Delgado-Herrera L, Salon J, Garratt C, Huang B, Sarapohja T. REVIVE Heart Failure Study Group. Effect of levosimendan on the short-term clinical course of patients with acutely decompensated heart failure. JACC Heart Fail. 2013;1:103–111. doi: 10.1016/j.jchf.2012.12.004. [DOI] [PubMed] [Google Scholar]
- 42.Comín-Colet J, Manito N, Segovia-Cubero J, Delgado J, García Pinilla JM, Almenar L, Crespo-Leiro MG, Sionis A, Blasco T, Pascual-Figal D, Gonzalez-Vilchez F, Lambert-Rodríguez JL, Grau M, Bruguera J. LION-HEART Study Investigators. Efficacy and safety of intermittent intravenous outpatient administration of levosimendan in patients with advanced heart failure: the LION-HEART multicentre randomised trial. Eur J Heart Fail. 2018;20:1128–1136. doi: 10.1002/ejhf.1145. [DOI] [PubMed] [Google Scholar]
- 43.Jabbour A, Hayward CS, Keogh AM, Kotlyar E, McCrohon JA, England JF, Amor R, Liu X, Li XY, Zhou MD, Graham RM, Macdonald PS. Parenteral administration of recombinant human neuregulin-1 to patients with stable chronic heart failure produces favourable acute and chronic haemodynamic responses. Eur J Heart Fail. 2011;13:83–92. doi: 10.1093/eurjhf/hfq152. [DOI] [PubMed] [Google Scholar]
- 44.Gao R, Zhang J, Cheng L, Wu X, Dong W, Yang X, Li T, Liu X, Xu Y, Li X, Zhou M. A Phase II, randomized, double-blind, multicenter, based on standard therapy, placebo-controlled study of the efficacy and safety of recombinant human neuregulin-1 in patients with chronic heart failure. J Am Coll Cardiol. 2010;55:1907–1914. doi: 10.1016/j.jacc.2009.12.044. [DOI] [PubMed] [Google Scholar]
- 45.Ahmed A, Rich MW, Love TE, Lloyd-Jones DM, Aban IB, Colucci WS, Adams KF, Gheorghiade M. Digoxin and reduction in mortality and hospitalization in heart failure: a comprehensive post hoc analysis of the DIG trial. Eur Heart J. 2006;27(2):178–186. doi: 10.1093/eurheartj/ehi687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ahmed A, Pitt B, Rahimtoola SH, Waagstein F, White M, Love TE, Braunwald E. Effects of digoxin at low serum concentrations on mortality and hospitalization in heart failure: a propensity-matched study of the DIG trial. Int J Cardiol. 2008;123:138–146. doi: 10.1016/j.ijcard.2006.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pellicori P, Cleland JG, Zhang J, Kallvikbacka-Bennett A, Urbinati A, Shah P, Kazmi S, Clark AL. Cardiac dysfunction, congestion and loop diuretics: their relationship to prognosis in heart failure. Cardiovasc Drugs Ther. 2016;30:599–609. doi: 10.1007/s10557-016-6697-7. [DOI] [PubMed] [Google Scholar]
- 48.Pellicori P, Kallvikbacka-Bennett A, Dierckx R, Zhang J, Putzu P, Cuthbert J, Boyalla V, Shoaib A, Clark AL, Cleland JG. Prognostic significance of ultrasound-assessed jugular vein distensibility in heart failure. Heart. 2015;101:1149–1158. doi: 10.1136/heartjnl-2015-307558. [DOI] [PubMed] [Google Scholar]
- 49.Pellicori P, Clark AL, Kallvikbacka-Bennett A, Zhang J, Urbinati A, Monzo L, Dierckx R, Anker SD, Cleland JGF. Non-invasive measurement of right atrial pressure by near-infrared spectroscopy: preliminary experience. A report from the SICA-HF study. Eur J Heart Fail. 2017;19:883–892. doi: 10.1002/ejhf.825. [DOI] [PubMed] [Google Scholar]
- 50.Pellicori Pierpaolo, Shah Parin, Cuthbert Joe, Urbinati Alessia, Zhang Jufen, Kallvikbacka‐Bennett Anna, Clark Andrew L., Cleland John G.F. Prevalence, pattern and clinical relevance of ultrasound indices of congestion in outpatients with heart failure. European Journal of Heart Failure. 2019;21(7):904–916. doi: 10.1002/ejhf.1383. [DOI] [PubMed] [Google Scholar]
- 51.Felker GM, Anstrom KJ, Adams KF, Ezekowitz JA, Fiuzat M, Houston-Miller N, Januzzi JL, Jr, Mark DB, Piña IL, Passmore G, Whellan DJ, Yang H, Cooper LS, Leifer ES, Desvigne-Nickens P, O’Connor CM. Effect of natriuretic peptide-guided therapy on hospitalization or cardiovascular mortality in high-risk patients with heart failure and reduced ejection fraction: a randomized clinical trial. JAMA. 2017;318:713–720. doi: 10.1001/jama.2017.10565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mentz RJ, Hasselblad V, DeVore AD, Metra M, Voors AA, Armstrong PW, Ezekowitz JA, Tang WH, Schulte PJ, Anstrom KJ, Hernandez AF, Velazquez EJ, O’Connor CM. Torsemide versus furosemide in patients with acute heart failure (from the ASCEND-HF trial) Am J Cardiol. 2016;117:404–411. doi: 10.1016/j.amjcard.2015.10.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.DiNicolantonio JJ. Should torsemide be the loop diuretic of choice in systolic heart failure? Futur Cardiol. 2012;8:707–728. doi: 10.2217/fca.12.54. [DOI] [PubMed] [Google Scholar]
- 54.Miles Jeremy A., Hanumanthu Balaram K., Patel Kavisha, Chen Michelle, Siegel Robert M., Kokkinidis Damianos G. Torsemide versus furosemide and intermediate-term outcomes in patients with heart failure. Journal of Cardiovascular Medicine. 2019;20(6):379–388. doi: 10.2459/JCM.0000000000000794. [DOI] [PubMed] [Google Scholar]
- 55.Jentzer JC, DeWald TA, Hernandez AF. Combination of loop diuretics with thiazide-type diuretics in heart failure. J Am Coll Cardiol. 2010;56:1527–1534. doi: 10.1016/j.jacc.2010.06.034. [DOI] [PubMed] [Google Scholar]
- 56.Mullens W, Verbrugge FH, Nijst P, Martens P, Tartaglia K, Theunissen E, Bruckers L, Droogne W, Troisfontaines P, Damman K, Lassus J, Mebazaa A, Filippatos G, Ruschitzka F, Dupont M. Rationale and design of the ADVOR (Acetazolamide in Decompensated Heart Failure with Volume Overload) trial. Eur J Heart Fail. 2018;20:1591–1600. doi: 10.1002/ejhf.1307. [DOI] [PubMed] [Google Scholar]
- 57.Packer M, Anker SD, Butler J, Filippatos G, Zannad F. Effects of sodium-glucose cotransporter 2 inhibitors for the treatment of patients with heart failure: proposal of a novel mechanism of action. JAMA Cardiol. 2017;2:1025–1029. doi: 10.1001/jamacardio.2017.2275. [DOI] [PubMed] [Google Scholar]
- 58.Fitchett D, Zinman B, Wanner C, Lachin JM, Hantel S, Salsali A, Johansen OE, Woerle HJ, Broedl UC, Inzucchi SE, EMPA-REG OUTCOME® trial investigators Heart failure outcomes with empagliflozin in patients with type 2 diabetes at high cardiovascular risk: results of the EMPA-REG OUTCOME® trial. Eur Heart J. 2016;37:1526–1534. doi: 10.1093/eurheartj/ehv728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Perkovic Vlado, Jardine Meg J., Neal Bruce, Bompoint Severine, Heerspink Hiddo J.L., Charytan David M., Edwards Robert, Agarwal Rajiv, Bakris George, Bull Scott, Cannon Christopher P., Capuano George, Chu Pei-Ling, de Zeeuw Dick, Greene Tom, Levin Adeera, Pollock Carol, Wheeler David C., Yavin Yshai, Zhang Hong, Zinman Bernard, Meininger Gary, Brenner Barry M., Mahaffey Kenneth W. Canagliflozin and Renal Outcomes in Type 2 Diabetes and Nephropathy. New England Journal of Medicine. 2019;380(24):2295–2306. doi: 10.1056/NEJMoa1811744. [DOI] [PubMed] [Google Scholar]
- 60.Rådholm K, Figtree G, Perkovic V, Solomon SD, Mahaffey KW, de Zeeuw D, Fulcher G, Barrett TD, Shaw W, Desai M, Matthews DR, Neal B. Canagliflozin and heart failure in type 2 diabetes mellitus. Circulation. 2018;138:458–468. doi: 10.1161/CIRCULATIONAHA.118.034222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kato Eri T., Silverman Michael G., Mosenzon Ofri, Zelniker Thomas A., Cahn Avivit, Furtado Remo H.M., Kuder Julia, Murphy Sabina A., Bhatt Deepak L., Leiter Lawrence A., McGuire Darren K., Wilding John P.H., Bonaca Marc P., Ruff Christian T., Desai Akshay S., Goto Shinya, Johansson Peter A., Gause-Nilsson Ingrid, Johanson Per, Langkilde Anna Maria, Raz Itamar, Sabatine Marc S., Wiviott Stephen D. Effect of Dapagliflozin on Heart Failure and Mortality in Type 2 Diabetes Mellitus. Circulation. 2019;139(22):2528–2536. doi: 10.1161/CIRCULATIONAHA.119.040130. [DOI] [PubMed] [Google Scholar]
- 62.McMurray John J.V., DeMets David L., Inzucchi Silvio E., Køber Lars, Kosiborod Mikhail N., Langkilde Anna M., Martinez Felipe A., Bengtsson Olof, Ponikowski Piotr, Sabatine Marc S., Sjöstrand Mikaela, Solomon Scott D., McMurray John JV, DeMets David L, Inzucchi Silvio E, Køber Lars, Kosiborod Mikhail N, Langkilde Anna Maria, Martinez Felipe A, Ponikowski Piotr, Sabatine Marc S, Sjöstrand Mikaela, Solomon Scott D, Diez Mirta, Nicolau Jose, Katova Tzvetana, O'Meara Eileen, Howlett Jonathan, Verma Subodh, Ge Junbo, Belohlavek Jan, Schou Morten, Böhm Michael, Merkely Bela, Chopra Vijay, Kitakaze Masafumi, de Boer Rudolf A., Drozdz Jaroslaw, Tereshchenko Sergey, Dukat Andrej, Ljungman Charlotta, Chiang Chern‐En, Petrie Mark, Desai Akshay, Anand Inder, Pham Vinh Nguyen, Pfeffer Marc A., Pocock Stuart, Swedberg Karl, Rouleau Jean L., Chaturvedi Nishi, Ivanovich Peter, Levey Andrew S., Christ‐Schmidt Heidi, Held Claes, Varenhorst Christoph, Christersson Christina, Mann Johannes, Holmgren Pernilla, Hallberg Theresa, Langkilde AnnaMaria, Sjöstrand Mikaela, Denison Hans, Reicher Barry, Bengtsson Olof, Fox Ywonne, Forsby Mikael, Alenhag Eva‐Lena, Nilsson Ann, Kazanowska Kinga, Olofsson Eva Lavik, Karup Cathrine, Ekedahl‐Berggren Maria, Klockargård Anna‐Lena, Kempe Karin, Selvén Mathilda. A trial to evaluate the effect of the sodium–glucose co‐transporter 2 inhibitor dapagliflozin on morbidity and mortality in patients with heart failure and reduced left ventricular ejection fraction (DAPA‐HF) European Journal of Heart Failure. 2019;21(5):665–675. doi: 10.1002/ejhf.1432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Cleland JG, Zhang J, Pellicori P, Dicken B, Dierckx R, Shoaib A, Wong K, Rigby A, Goode K, Clark AL. Prevalence and outcomes of anemia and hematinic deficiencies in patients with chronic heart failure. JAMA Cardiol. 2016;1:539–547. doi: 10.1001/jamacardio.2016.1161. [DOI] [PubMed] [Google Scholar]
- 64.Lewis GD, Malhotra R, Hernandez AF, McNulty SE, Smith A, Felker GM, Tang WHW, LaRue SJ, Redfield MM, Semigran MJ, Givertz MM, Van Buren P, Whellan D, Anstrom KJ, Shah MR, Desvigne-Nickens P, Butler J, Braunwald E, NHLBI Heart Failure Clinical Research Network Effect of oral iron repletion on exercise capacity in patients with heart failure with reduced ejection fraction and iron deficiency: the IRONOUT HF randomized clinical trial. JAMA. 2017;317:1958–1966. doi: 10.1001/jama.2017.5427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Anker SD, Kirwan BA, van Veldhuisen DJ, Filippatos G, Comin-Colet J, Ruschitzka F, Lüscher TF, Arutyunov GP, Motro M, Mori C, Roubert B, Pocock SJ, Ponikowski P. Effects of ferric carboxymaltose on hospitalisations and mortality rates in iron-deficient heart failure patients: an individual patient data meta-analysis. Eur J Heart Fail. 2018;20:125–133. doi: 10.1002/ejhf.823. [DOI] [PubMed] [Google Scholar]
- 66.Zhang S, Liu H, Amarsingh GV, Cheung CC, Hogl S, Narayanan U, Zhang L, McHarg S, Xu J, Gong D, Kennedy J, Barry B, Choong YS, Phillips AR, Cooper GJ. Diabetic cardiomyopathy is associated with defective myocellular copper regulation and both defects are rectified by divalent copper chelation. Cardiovasc Diabetol. 2014;13:100. doi: 10.1186/1475-2840-13-100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.McMurray JJ, Dunselman P, Wedel H, Cleland JG, Lindberg M, Hjalmarson A, Kjekshus J, Waagstein F, Apetrei E, Barrios V, Böhm M, Kamenský G, Komajda M, Mareev V, Wikstrand J, CORONA Study Group Coenzyme Q10, rosuvastatin, and clinical outcomes in heart failure: a pre-specified substudy of CORONA (controlled rosuvastatin multinational study in heart failure) J Am Coll Cardiol. 2010;56:1196–1204. doi: 10.1016/j.jacc.2010.02.075. [DOI] [PubMed] [Google Scholar]
- 68.Alehagen U, Alexander J, Aaseth J. Supplementation with selenium and coenzyme Q10 reduces cardiovascular mortality in elderly with low selenium status. A secondary analysis of a randomised clinical trial. PLoS One. 2016;11:e0157541. doi: 10.1371/journal.pone.0157541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Alexanian I, Parissis J, Farmakis D, Pantziou C, Ikonomidis I, Paraskevaidis I, Ioannidou S, Sideris A, Kremastinos D, Lekakis J, Filippatos G. Selenium contributes to myocardial injury and cardiac remodeling in heart failure. Int J Cardiol. 2014;176:272–273. doi: 10.1016/j.ijcard.2014.06.095. [DOI] [PubMed] [Google Scholar]
- 70.Morisco C, Trimarco B, Condorelli M. Effect of coenzyme Q10 therapy in patients with congestive heart failure: a long-term multicenter randomized study. Clin Investig. 1993;71(8 Suppl):S134–S136. doi: 10.1007/BF00226854. [DOI] [PubMed] [Google Scholar]
- 71.Mortensen SA, Rosenfeldt F, Kumar A, Dolliner P, Filipiak KJ, Pella D, Alehagen U, Steurer G, Littarru GP, Q-SYMBIO Study Investigators The effect of coenzyme Q10 on morbidity and mortality in chronic heart failure: results from Q-SYMBIO: a randomized double-blind trial. JACC Heart Fail. 2014;2:641–649. doi: 10.1016/j.jchf.2014.06.008. [DOI] [PubMed] [Google Scholar]
- 72.Cooper TJ, Guazzi M, Al-Mohammad A, Amir O, Bengal T, Cleland JG, Dickstein K. Sildenafil in Heart failure (SilHF). An investigator-initiated multinational randomized controlled clinical trial: rationale and design. Eur J Heart Fail. 2013;15:119–122. doi: 10.1093/eurjhf/hfs152. [DOI] [PubMed] [Google Scholar]
- 73.Redfield MM, Chen HH, Borlaug BA, Semigran MJ, Lee KL, Lewis G, LeWinter MM, Rouleau JL, Bull DA, Mann DL, Deswal A, Stevenson LW, Givertz MM, Ofili EO, O’Connor CM, Felker GM, Goldsmith SR, Bart BA, McNulty SE, Ibarra JC, Lin G, Oh JK, Patel MR, Kim RJ, Tracy RP, Velazquez EJ, Anstrom KJ, Hernandez AF, Mascette AM, Braunwald E, RELAX Trial Effect of phosphodiesterase-5 inhibition on exercise capacity and clinical status in heart failure with preserved ejection fraction: a randomized clinical trial. JAMA. 2013;309:1268–1277. doi: 10.1001/jama.2013.2024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.González-López E, Gallego-Delgado M, Guzzo-Merello G, de Haro-Del Moral FJ, Cobo-Marcos M, Robles C, Bornstein B, Salas C, Lara-Pezzi E, Alonso-Pulpon L, Garcia-Pavia P. Wild-type transthyretin amyloidosis as a cause of heart failure with preserved ejection fraction. Eur Heart J. 2015;36:2585–2594. doi: 10.1093/eurheartj/ehv338. [DOI] [PubMed] [Google Scholar]
- 75.Maurer MS, Schwartz JH, Gundapaneni B, Elliott PM, Merlini G, Waddington-Cruz M, Kristen AV, Grogan M, Witteles R, Damy T, Drachman BM, Shah SJ, Hanna M, Judge DP, Barsdorf AI, Huber P, Patterson TA, Riley S, Schumacher J, Stewart M, Sultan MB, Rapezzi C, ATTR-ACT Study Investigators Tafamidis treatment for patients with transthyretin amyloid cardiomyopathy. N Engl J Med. 2018;379:1007–1016. doi: 10.1056/NEJMoa1805689. [DOI] [PubMed] [Google Scholar]
- 76.Kytömaa Sonja, Hegde Sheila, Claggett Brian, Udell Jacob A., Rosamond Wayne, Temte Jonathan, Nichol Kristin, Wright Jacqueline D., Solomon Scott D., Vardeny Orly. Association of Influenza-like Illness Activity With Hospitalizations for Heart Failure. JAMA Cardiology. 2019;4(4):363. doi: 10.1001/jamacardio.2019.0549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Modin D, Jørgensen ME, Gislason G, Jensen JS, Køber L, Claggett B, Hegde SM, Solomon SD, Torp-Pedersen C, Biering-Sørensen T. Influenza vaccine in heart failure. Circulation. 2019;139:575–586. doi: 10.1161/CIRCULATIONAHA.118.036788. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX 23 kb)