Abstract
Fenugreek (Trigonella foenum-graecum) is an annual herbaceous plant and a staple of traditional health remedies for metabolic conditions including high cholesterol and diabetes. While the mechanisms of the beneficial actions of fenugreek remain unknown, a role for intestinal microbiota in metabolic homeostasis is likely. To determine if fenugreek utilizes intestinal bacteria to offset the adverse effects of high fat diets, C57BL/6J mice were fed control/low fat (CD) or high fat (HFD) diets each supplemented with or without 2% (w/w) fenugreek for 16 weeks. The effects of fenugreek and HFD on gut microbiota were comprehensively mapped and then statistically assessed in relation to effects on metrics of body weight, hyperlipidemia, and glucose tolerance. 16S metagenomic analyses revealed robust and significant effects of fenugreek on gut microbiota, with alterations in both alpha and beta diversity as well as taxonomic redistribution under both CD and HFD conditions. As previously reported, fenugreek attenuated HFD-induced hyperlipidemia and stabilized glucose tolerance without affecting body weight. Finally, fenugreek specifically reversed the dysbiotic effects of HFD on numerous taxa in a manner tightly correlated with overall metabolic function. Collectively, these data reinforce the essential link between gut microbiota and metabolic syndrome and suggest that the preservation of healthy populations of gut microbiota participates in the beneficial properties of fenugreek in the context of modern Western-style diets.
Subject terms: Dyslipidaemias, Obesity
Introduction
Obesity linked to Western-style diets is the prototypical ailment of the modern era. Obesity currently affects more than 35% of Americans1; and in addition to ties with type 2 diabetes and cardiovascular disease, obesity increases the risk of all-cause mortality and exacerbates anxiety and depression2–5. While search for effective obesity treatments has become a priority in biomedical research, available pharmacological options for obesity are undermined by issues related to toxicity and off-target side6. Herbal medicine or phytotherapy has long been a source of traditional medicinal remedies, and indeed, interest in generally regarded as safe (GRAS) plant materials for the clinical treatment of obesity is growing (reviewed in7,8). Fenugreek (Trigonella foenum-graecum) is an annual herbaceous plant and a staple of traditional health remedies to treat hyperlipidemia and diabetes9–12, as well as mood disorders13. Laboratory studies demonstrate protective effects of fenugreek on diabetes14–18, and suggest that potential mechanisms might include inhibition of intestinal glucose absorption14–16, delayed gastric emptying15, and/or insulinotropic activity19,20,17,18. Protective effects of fenugreek on cholesterol and hyperlipidemia21 might be based on modulation of hepatic steatosis22–26, inflammation26–28, and/or oxidative stress secondary to diabetes29–32. While the exact mechanisms whereby fenugreek or its constituents confers metabolic resiliency are unknown, data show that fenugreek administration can also modulate intestinal microbiota, which can in turn impact metabolic physiology33,34.
A remarkably mutualistic relationship exists between gut microbiota and their mammalian hosts, with microbiota providing protection against ingested pathogens, neutralizing carcinogens, and metabolizing otherwise inaccessible lipids and polysaccharides into potent bioactive metabolites35. Sequencing data show that modern high fat/calorie diets can disrupt gut microbiota, reducing bacterial diversity and upsetting the balance of pathogenic and commensal bacteria36. Data from our lab and others show that such diet-induced gut dysbiosis is sufficient to impair both metabolic and neurologic function37,38, suggesting that preservation of healthy gut microbiota could offset the pathophysiologic effects of high fat diets39. As fenugreek has indeed been shown to modulate intestinal bacteria in several models33,34,40, studies were designed to determine if fenugreek could offset the effects of a high fat diet on gut dysbiosis, and to establish the relationship of fenugreek-shaped gut microbiota to clinically relevant metrics of metabolic function. To this end, data from our previously published study on the effects of fenugreek on mice given a high fat diet were extended to include sequencing and statistical assessment of gut microbiota. As reported in our previous study, high fat or nutritionally matched low fat diets supplemented with ground fenugreek seeds (2% w/w) were administered to male C57BL/6J mice for 16 weeks, and the metabolic effects of the various diets on adiposity, glycemic control, and hyperlipidemia were quantified41. Metagenomic sequencing of fecal microbiota collected from mice was conducted, and diet-related changes in gut microbiota were statistically analyzed in relation to established metrics of metabolic function.
Results
Fenugreek improves glucose tolerance and dyslipidemia in mice given high fat diet
Data in this manuscript is built on initial publication of the effects of whole fenugreek seed supplementation (2% w/w) on overall metabolic function in the context of a 16-week trial of high fat diet consumption41, and thus previously published data are only summarized in this report. Briefly, data show that fenugreek supplementation increased HDL and decreased LDL cholesterol levels in high fat fed-mice (Table 1). Furthermore, fenugreek significantly improved glucose tolerance (as measured 40 minutes after oral glucose loading), but did not affect HFD-induced changes in total cholesterol, body weight, amount of body fat, or fasting blood glucose (Table 1). Fenugreek administration did not cause changes in food intake41.
Table 1.
Summary of fenugreek-induced metabolic resiliency: decreased hyperlipidemia and improved glucose tolerance.
| CD | CD/FG | HFD | HFD/FG | |
|---|---|---|---|---|
| Total Cholesterol (mg/dl) | 148.9 ± 45.7 | 136.2 ± 31.7 | 255.4 ± 32.9*** | 245.7 ± 26.3 |
| LDL Cholesterol (mg/dl) | 9.62 ± 2.6 | 8.48 ± 2.1 | 17.98 ± 4.7*** | 13.83 ± 4.3# |
| HDL Cholesterol (%TC) | 45.15 ± 12.7 | 44.86 ± 5.8 | 28.28 ± 3.3*** | 33.3 ± 5.1# |
| Body Weight (gr) | 31.78 ± 3.2 | 31.57 ± 2.6 | 48.73 ± 2.7*** | 50.03 ± 2.2 |
| Body Fat (gr) | 5.15 ± 1.8 | 5.04 ± 1.2 | 16.61 ± 1.3*** | 16.86 ± 1.3 |
| Fasting Blood Glucose (mg/dl) | 153.4 ± 16.8 | 149.4 ± 23.4 | 211.1 ± 16.8*** | 219.1 ± 21.1 |
| Glucose Tolerance (40 min) | 234.0 ± 49.3 | 213.5 ± 23.3 | 386.1 ± 89.9*** | 311.6 ± 75.8# |
Adult male C57Bl/6 mice were given high fat (HFD) or nutritionally matched control diet (CD) with or without fenugreek (FG; 2% w/w), and subject to measures of metabolic function as described in Methods. Statistically significant differences in metabolic parameters in HFD-fed mice as compared to CD-fed mice are mice are noted by ***(p < 0.001), while significant changes in mice given HFD/FG as compared to HFD-fed mice are noted by #(p < 0.05). Adapted from previously published data41.
Fenugreek and high fat diet exert pronounced effects on gut microbial composition
The impact of fenugreek on intestinal microbiota was determined by 16S sequencing of fecal samples isolated from mice at euthanasia as described in Methods. Initial weighted and unweighted Unifrac phylogenetic analyses reveal that the microbiomes of fenugreek-fed mice were significantly different from non-fenugreek mice under both control diet and high fat-fed conditions (Table 2). Indeed, the magnitude of the effects of fenugreek were similar in that of the high fat dies as compared to control diet (Table 2). These robust shifts in beta-diversity were also apparent on principal component analysis plots generated from normalized read count data (Fig. 1). Finally, data show that fenugreek also significantly increased alpha diversity (Shannon metrics) in both control diet and high fat-fed mice (Fig. 2).
Table 2.
Differences in microbiota community composition in mice with CD- and HFD-shaped microbiota with and without fenugreek.
| Comparison | Score | P value | |
|---|---|---|---|
| Weighted Unifrac | CD vs CD/FG | 0.65012 | <0.001*** |
| HFD vs HFD/FG | 0.61915 | <0.001*** | |
| CD vs HFD | 0.796987 | <0.001*** | |
| Unweighted Unifrac | CD vs CD/FG | 0.90777 | <0.001*** |
| HFD vs HFD/FG | 0.885073 | 0.002009** | |
| CD vs HFD | 0.952579 | 0.001009** |
Operational taxonomical units (OTU) were identified based on sequence clustering as described in Methods, and generation of a read count table was performed with the software package ‘usearch’. Statistical tests for differential representation were performed with tools incorporated in ‘mothur’, and statistically significant differences in microbiota community composition between groups were detected using both weighted and unweighted Unifrac phylogenetic analysis tools.
Figure 1.
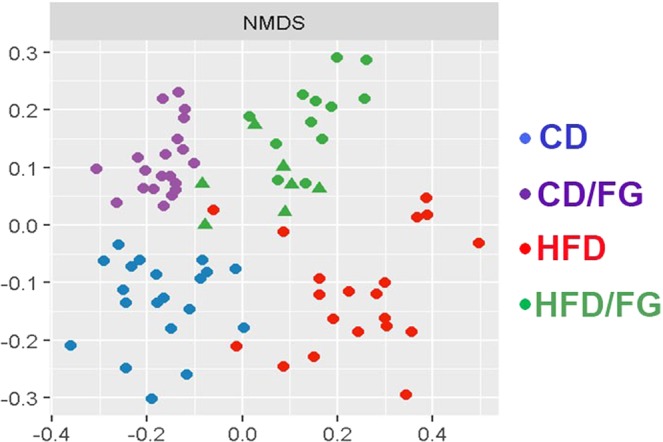
Fenugreek changes intestinal microbial populations in mice. Fecal microbiome populations from CD, CD/FG, HFD, and HFD/FG mice were analyzed using 16S rRNA sequencing, and multi-dimensional scaled principal coordinate analysis were used to visualize UniFrac distances of fecal samples from individual recipient mice. Samples from CD, CD/FG, HFD, and HFD/FG mice are depicted as blue, purple, red, and green symbols, respectively.
Figure 2.
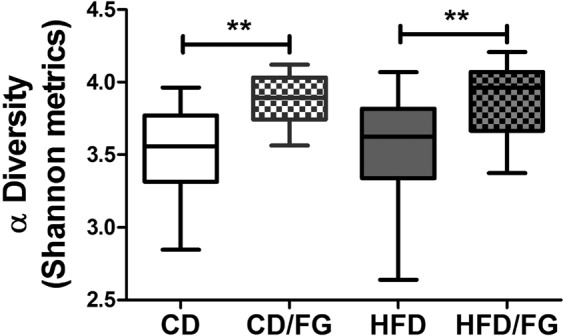
Fenugreek increases overall intestinal microbial diversity. Fecal microbiome populations from CD, CD/FG, HFD, and HFD/FG mice were analyzed using 16S rRNA sequencing, and box plots were generated to depict differences in Shannon α-diversity. Data show that mice supplemented with 2% fenugreek in their feed exhibited a statistically significant (**p < 0.01) increase in α-diversity compared to mice given CD or HFD alone.
Fenugreek can correct the dysbiotic effects of high fat diet on intestinal microbial populations
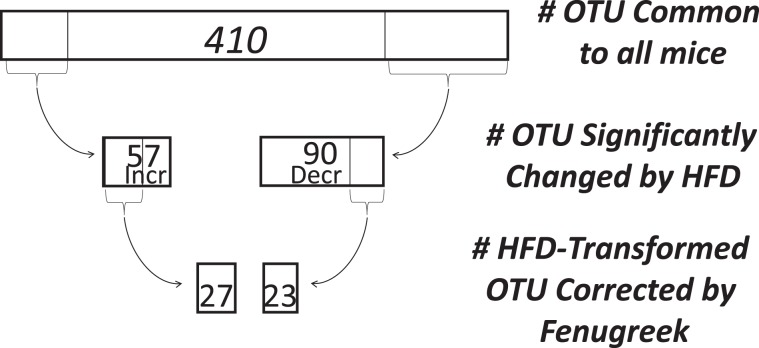
To assess the impact of fenugreek and HFD on gut microbial composition in greater statistical detail, a differential analysis of count data was conducted using DESeq. 2. Specifically, the specific individual operational taxonomic units (OTUs) whose relative representation was significantly (p < 0.05 adjusted with Benjamini-Hochberg correction) changed by high fat diet were identified using DESeq. 2. These analyses revealed that out of 410 Core OTUs (identified in all mice), the relative representation of 147 individual OTUs was significantly different in HFD-fed mice as compared to CD-fed mice (Fig. 3); with 57 increased and 90 decreased, respectively, by HFD. A similar DESeq. 2 analysis of these 147 “HFD-transformed” taxa was conducted to identify those that were significantly affected by fenugreek such that the direction of the change induced by high fat diet was reversed. This ivvestigation of “fenugreek-corrected” taxa revealed that fenugreek corrected the effects of HFD on 50 of these OTUs by reducing the representation of 27 HFD-increased OTUs and augmenting the representation of 23 OTUs reduced by HFD (Fig. 3). These analyses reflect the robust effect of both fenugreek and HFD on gut microbiota, and show that fenugreek is able to significantly correct much (greater than 34%) of the dysbiotic effects of HFD.
Figure 3.
Graphical representation of fenugreek’s ability to reverse HFD-induced changes in individual intestinal microbiota. The ability of high fat diet to significantly alter the representation of individual taxa was assessed as described in Methods showing that out of 410 Core OTUs, 57 were significantly increased and 90 significantly decreased by HFD. Fenugreek supplementation significantly reversed the effects of HFD on 50 of these OTUs by reducing the representation of 27 HFD-increased OTUs and bolstering the representation of 23 OTUs reduced by HFD.
Representation of Fenugreek-corrected taxa largely predicts overall metabolic function
In the final set of analyses, the 50 fenugreek-corrected taxa whose representation was skewed in one direction by HFD but in the opposite direction by fenugreek was examined in relation to metabolic function. Specifically, to determine if the relative representation of fenugreek-corrected taxa could be used to predict metabolic resiliency, the statistical relationship of fenugreek-corrected taxa representation to the metrics of metabolic function listed in Table 1 was determined. To this end, a matrix was built containing OTU count data for all 50 fenugreek-corrected taxa along with all metabolic data depicted in Table 1, including those indices not affected by fenugreek. Pairwise Pearson correlations indicate that the relative expression of many of these 50 OTUs were highly predictive of metabolic function. For example, of the 23 taxa decreased by HFD but increased with fenugreek supplementation, 8 taxa (all in the fermicutes phylum) significantly correlated with at least 1 metric of metabolic function (Table 3, see Supplementary Table 1 for additional details (log2FC and Pearson r values) on HFD-decreased, fenugreek corrected taxa). Likewise, of the 27 taxa increased by HFD but decreased with fenugreek supplementation, 20 taxa significantly correlated with selected metrics of metabolic function (Table 4, see Supplementary Table 2 for additional details (log2FC and Pearson r values) on HFD-increased, fenugreek corrected taxa). It is important to note that the representation of fenugreek-corrected taxa correlated frequently with aspects of metabolic function (e.g., body weight, body fat, total cholesterol, fasting blood glucose) that were not significantly improved in fenugreek-treated mice.
Table 3.
OTUs that predicts metabolic decline in high fat-fed mice.
| Correlation (Pearson) of HFD-Decreased, Fenugreek-Corrected OTU’s to Metrics of Metabolic Function | |||||||
|---|---|---|---|---|---|---|---|
| Individual OTUs Decreased by High Fat Diet (HF) Corrected by FG | Total Chol. | LDL Chol. | HDLChol. | Body Weight | Body Fat | Fasting Glucose | Glucose Tolerance |
| Firmicutes/Clostridia/Clostridiales/Lachnospiraceae/Clostridium_XlVa/Species1 | ns | ns | ns | ns | 0.0014 | ns | 0.0001 |
| Firmicutes/Clostridia/Clostridiales/Lachnospiraceae/Clostridium_XlVa/Species2 | ns | ns | ns | 0.0027 | 0.0025 | ns | ns |
| Firmicutes/Clostridia/Clostridiales/Lachnospiraceae/Clostridium_XlVa/Species3 | 0.0001 | 0.0034 | 0.0011 | ns | ns | 0.0006 | 0.0001 |
| Firmicutes/Clostridia/Clostridiales/Ruminococcaceae/Flavonifractor | 1.2E-05 | 0.0005 | 0.0001 | 1.6E-06 | 2.1E-06 | 2.2E-05 | 1.9E-05 |
| Firmicutes/Erysipelotrichia/Erysipelotrichales/Erysipelotrichaceae/Turicibacter | 0.0004 | ns | 0.0024 | ns | ns | ns | 0.0015 |
| Firmicutes/Clostridia/Clostridiales/Ruminococcaceae/Oscillibacter | 0.0002 | ns | 1.3E-05 | ns | 4.0E-05 | 0.0002 | 4.2E-05 |
| Firmicutes/Clostridia/Clostridiales/Ruminococcaceae/Intestinimonas | 0.0009 | 0.0021 | 0.0026 | ns | ns | 0.0004 | 0.0017 |
| Firmicutes/Clostridia/Clostridiales/Lachnospiraceae/Acetatifactor/Species 1 | ns | ns | ns | ns | ns | ns | 0.0012 |
Individual OTUs in which fenugreek administration reversed high fat diet-induced decreases in representation were correlated against measures of hyperlipidemia. Data are p values of Pearson correlation with total cholesterol (mg/dl), low-density lipoprotein (LDL Chol.; mg/dl), high-density lipoprotein (HDL Chol.; %TC), body weight (grams), body fat (grams), fasting blood glucose (mg/dl), and glucose tolerance (blood glucose levels 40 minutes after oral loading). See Supplementary Table 1 for additional details (log2FC and Pearson r values) on HFD-decreased, fenugreek corrected taxa.
Table 4.
OTUs that predicts metabolic decline in high fat-fed mice.
| Correlation (Pearson) of HFD-Increased, Fenugreek-Corrected OTU’s to Metrics of Metabolic Function | |||||||
|---|---|---|---|---|---|---|---|
| Individual OTUsDecreased by High Fat Diet (HF) Corrected by FG | Total Chol. | LDL Chol. | HDL Chol. | Body Weight | Body Fat | Fasting Glucose | Glucose Tolerance |
| Firmicutes/Clostridia/Clostridiales/Lachnospiraceae/Clostridium_XlVa/Species 4 | ns | 4.9E-05 | ns | ns | ns | ns | ns |
| Firmicutes/ClostridiaClostridiales/Ruminococcaceae/Anaerotruncus | 0.0004 | 1.3E-08 | ns | ns | 0.0016 | ns | 4.6E-06 |
| Firmicutes/Clostridia/Clostridiales/Lachnospiraceae/Clostridium_XlVa/Species 5 | ns | 5.8E-05 | 0.0024 | ns | ns | ns | ns |
| Firmicutes/Clostridia/Clostridiales/Lachnospiraceae/Clostridium_XlVa/Species 6 | 0.0002 | 4.9E-09 | 0.0051 | ns | ns | ns | 0.0002 |
| Bacteroidetes/Bacteroidia/Bacteroidales/Porphyromonadaceae/Barnesiella/Species 1 | 9.1E-05 | 4.1E-10 | ns | 0.0009 | 0.0009 | 0.0024 | 1.6E-06 |
| Bacteroidetes/Bacteroidia/Bacteroidales/Porphyromonadaceae/Barnesiella/Species 2 | 0.0005 | 4.2E-09 | ns | ns | ns | ns | 8.8E-06 |
| Firmicutes/Clostridia/Clostridiales/Lachnospiraceae/Clostridium_XlVa/Species 7 | 8.1E-07 | 5.7E-10 | 4.8E-05 | ns | ns | ns | 0.0001 |
| Firmicutes/Clostridia/Clostridiales/Lachnospiraceae/Clostridium_XlVa/Species 8 | 0.0001 | 0.0002 | 0.0030 | ns | 1.2E-05 | 0.0011 | 1.3E-05 |
| Firmicutes/Clostridia/Clostridiales/Lachnospiraceae/Clostridium_XlVa/Species 9 | 4.1E-06 | 5.9E-08 | 0.0007 | ns | 1.2E-06 | 4.1E05 | ns |
| Firmicutes/Bacilli/Lactobacillales/Streptococcaceae/Streptococcus | ns | ns | ns | ns | ns | ns | 0.0007 |
| Firmicutes/Clostridia/Clostridiales/Lachnospiraceae/Clostridium_XlVa/Species 10 | 0.0009 | 2.1E-05 | ns | ns | 0.0002 | ns | 5.0E-06 |
| Firmicutes/Bacilli/Lactobacillales/Lactobacillaceae/Lactobacillus/Species 1 | 4.2E-06 | ns | ns | 7.1E-08 | 4.3E-09 | 5.6E07 | 1.1E-06 |
| Firmicutes/Bacilli/Lactobacillales/Lactobacillaceae/Lactobacillus/Species 2 | 1.3E-06 | 0.0001 | ns | 2.9E-08 | 2.1E-09 | 2.9E-07 | 5.2E-07 |
| Actinobacteria/Actinobacteria/Coriobacteridae/Coriobacteriales/Coriobacterineae | ns | ns | ns | ns | ns | ns | 0.0033 |
| Firmicutes/Clostridia/Clostridiales/Lachnospiraceae/Roseburia | 6.0E-05 | 5.4E-07 | 0.0004 | ns | ns | ns | ns |
| Firmicutes/Clostridia/Clostridiales/Lachnospiraceae/Clostridium_XlVa/Species 11 | ns | ns | ns | 0.0030 | 0.0016 | ns | 0.0015 |
| Firmicutes/Clostridia/Clostridiales/Clostridiales_Incertae_Sedis_XI/Dethiosulfatibacter | 0.0039 | ns | ns | 0.0004 | ns | ns | |
| Bacteroidetes/Bacteroidia/Bacteroidales/Porphyromonadaceae/Barnesiella/Species 3 | ns | 1.4E-05 | ns | ns | ns | ns | 0.0040 |
| Firmicutes/Clostridia/Clostridiales/Lachnospiraceae/Acetatifactor/Species 2 | 0.0003 | 0.0002 | ns | ns | 1.6E-05 | ns | ns |
| Firmicutes/Clostridia/Clostridiales/Lachnospiraceae/Clostridium_XlVa/Species 12 | 5.2E-05 | 2.2E-07 | 0.0003 | ns | ns | ns | ns |
Individual OTUs in which fenugreek administration reversed high fat diet-induced increases in representation were correlated against measures of hyperlipidemia. Data are correlation coefficients (Pearson r) and p values of correlation with total cholesterol (mg/dl), low-density lipoprotein (LDL Chol.; mg/dl), high-density lipoprotein (HDL Chol.; %TC), body weight (grams), body fat (grams), fasting blood glucose (mg/dl), and glucose tolerance (blood glucose levels 40 minutes after oral loading). See Supplementary Table 2 for additional details (log2FC and Pearson r values) on HFD-increased, fenugreek corrected taxa.
Discussion
While beneficial effects of fenugreek on hyperlipidemia and hyperglycemia have been widely reported, the mechanisms of fenugreek-mediated actions on metabolic function are unknown. Here we demonstrate the robust effects of fenugreek on gut microbiota, and describe a novel combination of statistical analyses including Unifrac, iterative DESeq. 2, and pairwise correlation matrices to generate insight into the role of intestinal microbial changes in the protective effects of fenugreek. Specifically, sequencing analyses reveal that fenugreek significantly increased overall microbiome diversity in mice, and specifically reversed the actions of high dietary fat on key intestinal taxa. Furthermore, the representation of fenugreek-corrected taxa significantly correlated with metabolic function, including changes in body weight and composition, glucose regulation, and hyperlipidemia.These findings are in agreement with the extensive body of literature documenting the ability of high fat diet to reduce bacterial diversity and disrupt the balance of pathogenic/commensal bacteria within the intestine36,42,38,43. As data from our lab and others show that this pattern of gut dysbiosis is sufficient to impair both metabolic and neurologic function37,44–48, data in this paper suggest that the reported effects of fenugreek on gut microbiota33,34,40,49, may be fundamental to its beneficial properties. In light of the stubborn prevalence of obesity and the pervasive accessibility of unhealthy diets, it is both clinically significant and generally promising that these data suggest that partial reversal of gut dysbiosis and metabolic impairment can be achieved with botanical supplementation within the context of unhealthy, Western-style diets.
While the association of intestinal dysbiosis with metabolic disease is well established45–48 causal relationships have not been identified and it is not understood how microbiome constituents impact metabolic resilience/vulnerability. Three major phyla are the most abundant in the human distal intestine: Bacteroidetes (gram-negative), Firmicutes (gram-positive), and Actinobacteria (gram-positive). Early studies suggested that obesity causes reductions in Bacteroidetes and increases in Firmicutes38,43 and indeed further studies indicate that these changes are reversed with weight loss50,51. While these data suggest that the balance between these phyla might broadly impact host physiology, this binary distinction does not always occur52,53 and may be too simple to faithfully reflect the complexity of diet-induced changes to the gut microbiome54–56. In the present study as well as our previous studies54,57, differences between control and high fat groups did not manifest as phylum-level shifts but rather differential representation within taxa, particularly Firmicutes. Indeed, the majority of taxa included in Tables 3 and 4 arise from the Clostridium class (Clostridium cluster XIVa) or the Clostridium leptum group (Clostridium cluster IV). This is notable, as divergent shifts in the representation of Clostridia have been reported in other pathophysiological conditions. For example, while overall Clostridium representation generally increases with age, Clostridium XIVa clusters have been shown to be significantly reduced in the elderly58. The bacteria in Clostridium XIVa play major roles in the fermentation of carbohydrates within the gut59, and the major end products of hind-gut fermentation are short-chain fatty acids (SCFAs). Further, Fermicutes produce primarily butyrate as their metabolic end product60, and butyrate is the main source of nutrition for gut epithelium cells61. Depletion of butyrate is associated with impaired intestinal barrier integrity62, and loss of intestinal barrier function is in turn associated with a growing number of inflammatory disease states diseases, including obesity as well as autoimmune diseases and cancer (reviewed in63,64). While butyrate was not directly measured in the present study, decreased levels of butyrate and other SCFA are widely reported in the context of obesity while high-fiber plant products are known to increase colonic fermentation and the generation of SCFA65.
Our data indicate that fenugreek is particularly effective against hyperlipidemia, which is in keeping with results of both experimental and clinical studies14,22,30,66. While there are several mechanisms whereby changes in gut microbiota could mediate the effects of fenugreek on serum lipids, most ultimately impact the absorption of dietary fat. For example, intestinal microbiota alter the metabolism of diet-derived long-chain fatty acids such as conjugated linoleic acid, modulating absorption67. Gut microbiota can also moderate cholesterolemia by regulating cholesterol conversion into coprostanol68. Dietary cholesterol is largely incorporated into chylomicrons for absorption in the small intestine. However, significant quantities of cholesterol (as much as 1 gram per day) escape proximal absorption to enter the colon to be either be excreted or absorbed. Microbial-based metabolism of cholesterol into coprostanone/coprostanol reduces blood cholesterol by increasing fecal cholesterol excretion69,70. Interestingly, recent data suggest that taxa arising from Lachnospiraceae and Runinococcacea families of the phylum Fermicutes are uniquely associated with high coprostanol generation in healthy humans71, while other studies likewise link these gut microbiota to variation in blood lipid levels independently of age, sex, and host genetics72. It is important to note that seven of the eight of the taxa listed in Table 3 are members of Lachnospiraceae and Runinococcacea families, suggesting that reversal of HFD-induced decreases in these key taxa by fenugreek could remediate hyperlipidemia by promoting fecal fat excretion. In further support of this scenario, published data suggest that fenugreek supplementation can dose-dependently increase fecal excretion of cholesterol from rats given high fat/high calorie diets73. Collectively, these data raise the possibility that increased representation of coprostanoligenic taxa arising from Lachnospiraceae and Runinococcacea families could participate in the lipid-lowering effects of fenugreek, and further suggest that identification and analysis such strains could lead to improved understanding and management of hypocholesteremia.
Transformation and metabolism of bile acids is another key pathway whereby fenugreek-shaped intestinal bacteria could impact serum lipids (reviewed in74). Indeed, fenugreek has been reported to inhibit the intestinal absorption of primary and secondary bile acids75, and to increase bile acid excretion into feces30. While the bulk of bile acids released into the intestine are efficiently absorbed and recycled back to the liver, perhaps 5% of the total bile acid pool progresses into the colon. Bile acids reaching the colon are subject to several microbial-mediated reactions including transformation into secondary bile acids by dihydroxylation, and deconjugation by bile salt hydrolases. While data indicate that bile salt hydrolases are a pervasive microbial adaptation to the human gut environment with enrichment in major genera including Bacteroides, Clostridium, Lactobacillus, and Eubacterium76, probiotics with bile salt hydrolytic activity can lower serum cholesterol77,78. With regard to more broad metabolic benefits, secondary bile acids generated by microbial metabolism are potent ligands of the G-protein coupled receptor TGR5, the activation of which triggers release of GLP-1 and insulin, thereby modulating host glucose tolerance and energy expenditure79.
While this study is in keeping with an extensive body of literature on the beneficial effects of fenugreek, the use of whole seed supplementation precludes identification of the bioactive constituent(s) mediating changes to gut microbiota. Furthermore, only male mice were used, so sex-based differences in the effects of fenugreek or the relation of such to metabolic function cannot be resolved. This point is especially significant as female C57BL/6J mice are generally considered resistant to diet induced obesity80,81. Notwithstanding these limitations, these data indicate that fenugreek supplementation can stabilize metabolic function within the context of high fat consumption. Indeed, fenugreek did not affect food intake or alleviate diet-induced obesity, but rather was able to bolster resistance of the obese mice to hyperlipidemia and glucose intolerance. We use the term “metabolic resiliency” to describe this ability to preserve, at least in part, a healthy metabolic phenotype in the context of powerful external stressors – in this case sustained consumption of a high fat diet and the obese state. This is a significant finding, as while the components of a healthy lifestyle are generally well known, numerous societal factors including poverty, food deserts, irregular/sedentary work schedules combine to hinder a consistently healthy lifestyle for most Americans. Thus, use of fenugreek and/or other strategies to maintain a healthy population of intestinal microbes in the context of a high fat diet could foster metabolic resilience even when diets/lifestyles are not optimal. Indeed, while fenugreek conferred protection against hyperlipidemia and glucose intolerance, it is important to note that representation of specific fenugreek-corrected taxa correlated frequently with aspects of metabolic function (e.g., body weight, body fat, total cholesterol, fasting blood glucose) that were not significantly improved in fenugreek-treated mice (see Tables 3 and 4). While correlation does not equal causation, these data clearly illustrate the very close relationship of individual microbes and microbial balance with metabolic function, and raise the possibility that strategic manipulation of key intestinal taxa could result in a more complete reversal of the adverse action of high fat diet. Thus, the action of fenugreek could be possibly optimized by dose, preparation, or combination with additional factors (probiotics, etc) to have a greater effect on key microbiota, enhancing its beneficial profile. These data also suggest that perhaps intestinal microbial “fingerprints” could be generated to estimate vulnerability to metabolic dysfunction and/or the potential for efficacy of metabolic interventions. Overall, data in the manuscript strongly suggest that the development of microbially-targeted therapies – both primary and adjunctive – that are built upon safe, natural, plant-based products like fenugreek could be used to attain significant advancements in public health within the context of contemporary dietary environments.
Materials and Methods
Animals and diets
This study was carried out in strict accordance with PHS/NIH guidelines on the use of experimental animals, and all experimental protocols were approved by the Institutional Animal Care and Use Committee at Pennington Biomedical Research Center. Data in this manuscript follows an initial publication on the effects of whole fenugreek seed supplementation (2% w/w) on overall metabolic function in the context of a 16-week trial of high fat diet consumption41. As detailed in our initial report41, male, 9 week-old C57BL/6 J mice were purchased from Jackson Laboratories, and group-housed (4/cage) under standard conditions with ad libitum access to food/water. After 7 days acclimation, mice were randomly separated into the following 4 groups (20 mice each in each group): high fat diet ± fenugreek (HFD and HFD/FG) and control diet ± fenugreek (CD and CD/FG) for 16 weeks. HFD and HFD/FG mice were fed a diet with 60% kcal from fat without or with 2% fenugreek seed powder incorporated into the diets, respectively (Research Diets Inc. D12492, D16020410), while CD mice were fed a nutritionally matched control low fat diet (10% kcal from fat) with or without 2% fenugreek seed powder (Research Diet Inc.; D12450J, D16020408). All diets contained 10% kcal from protein with the balance in caloric intake provided by differences in carbohydrate content. T. foenum-graecum L. “Fenugreek” seeds were purchased from Johnny’s Selected Seeds, Winslow Maine, certified organic for sprouting. Fenugreek seeds were ground in the Department of Plant Biology at Rutgers University and hand-delivered to Research Diets Inc., (New Brunswick, NJ) for commercial incorporation into treatment diets at 2% of the diet by weight.
Metabolic phenotyping
Assessment of metabolic function is fully detailed in our previous report41. Briefly, body composition (fat mass, fat-free/lean mass, and water content) was measured by briefly placing in mice into Bruker minispec LF110 time domain NMR analyzer (Bruker Optics, Billerica MA) as described previously41. Glucose tolerance was measured using an oral glucose tolerance assay (OGTT) based on repeated sampling of tail blood using a glucometer (Ascensia Elite, Bayer, Mishawaka, IN) at 0, 20, 40, 60, and 120 minutes after oral glucose (2 gm/kg) administration. All mice remained in the study for the duration of the 16-week feeding trail, after which mice with euthanized following a 8-hr fast by decapitation under deep isoflurane anesthesia. Levels of total cholesterol, HDL cholesterol, LDL cholesterol, and triglycerides in serum collected at euthanasia were measured colorimetrically (Wako Chemicals, Richmond, VA).
16S Metagenomic sequencing
Fecal samples were collected at euthanasia, and DNA preparation, sequencing and bioinformatics were performed by the PBRC Genomics Core Facility. DNA was isolated using a commercial reagent system (MoBio Power Fecal Kit, MoBio Laboratories, Carlsbad, CA) augmented by enzymatic lysis using lysostaphin, mutanolysin, and lysozyme82. Sequencing libraries targeting V4 of the gene encoding the 16S ribosomal RNA were generated using a commercially available kit (NEXTflex™ 16S V4 Amplicon-Seq Library Prep Kit, BIOO Scientific, Austin, TX), relying on 16S gene-specific primer sequences V4F 5′-GTGCCAGCMGCCGCGGTAA-3′ and V4R 5′-GGACTACHVGGGTWTCTAAT-3′, and including Illumina adaptors and molecular barcodes as described by the manufacturer to produce 253 bp amplicons. Samples were sequenced with custom primers (BIOO Scientific, Austin, TX) on an Illumina MiSeq instrument using version 3 sequencing chemistry (300 bp paired end reads). Forward and reverse sequence reads were processed into double-stranded DNA contigs using quality control metrics implemented in the software package ‘mothur’83. Sequence clustering (at better than 97% identity) to identify operational taxonomical units (OTUs), removal of chimeric sequences, and generation of a read count table (i.e. tabulating the occurrence of each OTU in each sample) were performed with the software package ‘usearch’84. Taxonomical classification of each OTU sequence relied on the SILVA 16S rRNA sequence database version 123.185, and statistical tests for differential representation were performed with tools incorporated in ‘mothur’, as well as using the software package DESeq. 286. Relative abundance of each OTU was examined on the phylum, class, order, family and genus levels.
Statistical analyses
Biochemical data were analyzed using Prism software (GraphPad Software, Inc.), and displayed as mean ± standard error, and were analyzed by ANOVA. Statistical significance for all analyses was accepted at p < 0.05, and *, **, and *** represent p < 0.05, p < 0.01, and p < 0.001, respectively. Alpha diversity (chao1 metrics) and beta diversity (weighted UniFrac metrics87) were assessed using tools implemented in ‘mothur’ on the basis of 80,000 sequences per sample. Differential representation of OTUs was assessed using DESeq. 2 on the basis of sequence count data, relying on Wald statistics with Benjamini-Hochberg correction and a false discovery rate cutoff set at 0.1. Inter-sample relationships relying on Principal Component Analysis on the basis of DESeq. 2 output, and data visualizations were both performed using JMP Genomics software (SAS, Cary, NC). Pairwise Pearson correlations of individual metrics of metabolic function against individual HFD-transformed, fenugreek-corrected OTU expression were carried out using Prism software.
Supplementary information
Acknowledgements
The authors gratefully acknowledge Dr. Kem Singletary and Ms. Cynthia Kloster for expert veterinary assistance and insight into the described studies. These studies were supported by grants from the National Center For Complementary & Integrative Health and the Office of Dietary Supplements of the National Institutes of Health (RO1AT010279 and P50AT002776; which funds the Botanical Dietary Supplements Research Center of Pennington Biomedical Research Center and the Department of Plant Biology and Pathology in the School of Environmental and Biological Sciences (SEBS) of Rutgers University). These studies also utilized the facilities of the Animal Behavior/Phenotyping and Genomics Cores that are supported in part by COBRE (NIH P20-GM103528) and NORC (NIH P30-DK072476) center grants from the National Institutes of Health.
Author contributions
A.B.K. and J.M.S. conceived the experiments. D.M.R. procured and performed all quality control on the fenugreek seeds. S.F.K., A.J.R., and A.B.K. conducted the animal experiments. R.C. performed metagenomic sequencing, while S.N., J.M.S., and A.B.K. analyzed the data. A.B.K. and J.M.S. prepared the manuscript and figures. All authors reviewed the manuscript.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
is available for this paper at 10.1038/s41598-020-58005-7.
References
- 1.Flegal KM, Kruszon-Moran D, Carroll MD, Fryar CD, Ogden CL. Trends in Obesity Among Adults in the United States, 2005 to 2014. JAMA. 2016;315:2284–9. doi: 10.1001/jama.2016.6458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Atlantis E, Baker M. Obesity effects on depression: systematic review of epidemiological studies. Int. J. Obes. 2008;6:881–91. doi: 10.1038/ijo.2008.54. [DOI] [PubMed] [Google Scholar]
- 3.Ma J, Xiao L. Obesity and depression in US women: results from the 2005–2006 National Health and Nutritional Examination Survey. Obes. 2010;18:347–353. doi: 10.1038/oby.2009.213. [DOI] [PubMed] [Google Scholar]
- 4.Beydoun MA, Beydoun HA, Wang Y. Obesity and central obesity as risk factors for incident dementia and its subtypes: a systematic review and meta-analysis. Obes. Rev. 2008;9:204–18. doi: 10.1111/j.1467-789X.2008.00473.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Flegal KM, Kit BK, Orpana H, Graubard BI. Association of all-cause mortality with overweight and obesity using standard body mass index categories: a systematic review and meta-analysis. JAMA. 2013;309:71–82. doi: 10.1001/jama.2012.113905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nissen SE, Wolski K. Effect of rosiglitazone on the risk of myocardial infarction and death from cardiovascular causes. N. Engl. J. Med. 2007;356:2457–71. doi: 10.1056/NEJMoa072761. [DOI] [PubMed] [Google Scholar]
- 7.de Freitas Junior LM, de Almeida EBJ. Medicinal plants for the treatment of obesity: ethnopharmacological approach and chemical and biological studies. Am. J. Transl. Res. 2017;9:2050–2064. [PMC free article] [PubMed] [Google Scholar]
- 8.Kooti W, Farokhipour M, Asadzadeh Z, Ashtary-Larky D, Asadi-Samani M. The role of medicinal plants in the treatment of diabetes: A systematic review. Electron. Physician. 2016;8:1832–1842. doi: 10.19082/1832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Haber SL, Keonavong J. Fenugreek use in patients with diabetes mellitus. Am. J. Health-Sysyt Pharm. 2013;70:1198–2013. doi: 10.2146/ajhp120523. [DOI] [PubMed] [Google Scholar]
- 10.Nagulapalli Venkata, K. C., Swaroop, A., Bagchi, D. & Bishayee, A. A small plant with big benefits: Fenugreek (Trigonella foenum-graecum Linn.) for disease prevention and health promotion. Mol Nutr Food Res., 61 (2017). [DOI] [PubMed]
- 11.Basch E, Ulbricht C, Kuo G, Szapary P, Smith M. Therapeutic applications of fenugreek. Altern. Med. Rev. 2003;8:20–2. [PubMed] [Google Scholar]
- 12.Verma N, et al. A multicenter clinical study to determine the efficacy of a novel fenugreek seed (Trigonella foenum-graecum) extract (Fenfuro™) in patients with type 2 diabetes. Food Nutr. Res. 2016;60:32382. doi: 10.3402/fnr.v60.32382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Assad T, Khan RA. Effect of methanol extract of Trigonella foenum-graecum L. seeds on anxiety, sedation and motor coordination. Metab. Brain Dis. 2017;32:343–349. doi: 10.1007/s11011-016-9914-y. [DOI] [PubMed] [Google Scholar]
- 14.Al-Harbori and M., R., A. Antidiabetic and hypocholesterolaemic effects of fenugreek. Phytother Res., 12, 233–242 (1998).
- 15.Hannan JM, et al. Soluble dietary fibre fraction of Trigonella foenum-graecum (fenugreek) seed improves glucose homeostasis in animal models of type 1 and type 2 diabetes by delaying carbohydrate digestion and absorption, and enhancing insulin action. Br. J. Nutr. 2007;97:514–521. doi: 10.1017/S0007114507657869. [DOI] [PubMed] [Google Scholar]
- 16.Madar Z, Shomer I. Polysaccharide composition of a gel fraction derived from fenugreek and its effect on starch digestion and bile-acid absorption in rats. J. Agric. Food Chem. 1990;38:1535–1539. doi: 10.1021/jf00097a023. [DOI] [Google Scholar]
- 17.Jetté L, Harvey L, Eugeni K, Levens N. 4-Hydroxyisoleucine: a plant-derived treatment for metabolic syndrome. Curr. Opin. Investig. Drugs. 2009;10:353–358. [PubMed] [Google Scholar]
- 18.Sauvaire Y, et al. 4-Hydroxyisoleucine: a novel amino acid potentiator of insulin secretion. Diabetes. 1998;47:206–210. doi: 10.2337/diab.47.2.206. [DOI] [PubMed] [Google Scholar]
- 19.Broca C, et al. 4- Hydroxyisoleucine: experimental evidence of its insulinotropic and antidiabetic properties. Am. J. Physiol. 1999;277:E617–E623. doi: 10.1152/ajpendo.1999.277.4.E617. [DOI] [PubMed] [Google Scholar]
- 20.Broca C, et al. Insulinotropic agent ID-1101 (4-hydroxyisoleucine) activates insulin signaling in rat. Am. J. Physiol. Endocrinol. Metab. 2004;287:E463–E471. doi: 10.1152/ajpendo.00163.2003. [DOI] [PubMed] [Google Scholar]
- 21.Srinivasan K. Fenugreek (Trigonella foenum-graecum): A review of health beneficial physiological effects. Food Rev. Int. 2006;22:203–224. doi: 10.1080/87559120600586315. [DOI] [Google Scholar]
- 22.Reddy RL, Srinivasan K. Dietary fenugreek seed regresses preestablished cholesterol gallstones in mice. Can. J. Physiol. Pharmacol. 2009;87:684–93. doi: 10.1139/Y09-062. [DOI] [PubMed] [Google Scholar]
- 23.Kaviarasan S, Viswanathan P, Anuradha CV. Fenugreek seed (Trigonella foenum graecum) polyphenols inhibit ethanol-induced collagen and lipid accumulation in rat liver. Cell Biol. Toxicol. 2007;23:373–383. doi: 10.1007/s10565-007-9000-7. [DOI] [PubMed] [Google Scholar]
- 24.Raju J, Bird RP. Alleviation of hepatic steatosis accompanied by modulation of plasma and liver TNF-alpha levels by Trigonella foenum graecum (fenugreek) seeds in Zucker obese (fa/fa) rats. Int. J. Obes. 2006;30:1298–1307. doi: 10.1038/sj.ijo.0803254. [DOI] [PubMed] [Google Scholar]
- 25.Yadav UC, Moorthy K, Baquer NZ. Effects of sodium-orthovanadate and Trigonella foenum-graecum seeds on hepatic and renal lipogenic enzymes and lipid profile during alloxan diabetes. J. Biosci. 2004;29:81–91. doi: 10.1007/BF02702565. [DOI] [PubMed] [Google Scholar]
- 26.Liu YBRK, Nair MG. Compounds in functional food fenugreek spice exhibit anti-inflammatory and antioxidant activities. Food Chem. Toxicol. 2012;131:1187–1192. doi: 10.1016/j.foodchem.2011.09.102. [DOI] [Google Scholar]
- 27.Hirai S, et al. Diosgenin attenuates inflammatory changes in the interaction between adipocytes and macrophages. Mol. Nutr. Food Res. 2010;54:797–804. doi: 10.1002/mnfr.200900208. [DOI] [PubMed] [Google Scholar]
- 28.Choi KW, et al. Inhibition of TNF-alpha-induced adhesion molecule expression by diosgenin in mouse vascular smooth muscle cells via downregulation of the MAPK, Akt and NF-kappaB signaling pathways. Vasc. Pharmacol. 2010;53:273–280. doi: 10.1016/j.vph.2010.09.007. [DOI] [PubMed] [Google Scholar]
- 29.Marzouk M, Soliman AM, Omar TY. Hypoglycemic and antioxidative effects of fenugreek and termis seeds powder in streptozotocin-diabetic rats. Eur. Rev. Med. Pharmacol. Sci. 2013;17:559–565. [PubMed] [Google Scholar]
- 30.Chaturvedi U, Shrivastava A, Bhadauria S, Saxena JK, Bhatia G. A mechanism-based pharmacological evaluation of efficacy of Trigonella foenum graecum (fenugreek) seeds in regulation of dyslipidemia and oxidative stress in hyperlipidemic rats. J. Cardiovasc. Pharmacol. 2013;61:505–512. doi: 10.1097/FJC.0b013e31828b7822. [DOI] [PubMed] [Google Scholar]
- 31.Kumar P, Kale RK, Baquer NZ. Antihyperglycemic and protective effects of Trigonella foenum graecum seed powder on biochemical alterations in alloxan diabetic rats. Eur. Rev. Med. Pharmacol. Sci. 2012;16(Suppl 3):18–27. [PubMed] [Google Scholar]
- 32.Annida B, Stanely Mainzen PP. Supplementation of fenugreek leaves reduces oxidative stress in streptozotocin-induced diabetic rats. J. Med. Food. 2005;8:382–385. doi: 10.1089/jmf.2005.8.382. [DOI] [PubMed] [Google Scholar]
- 33.Zentek J, et al. Fenugreek seed affects intestinal microbiota and immunological variables in piglets after weaning. Br. J. Nutr. 2013;109:859–66. doi: 10.1017/S000711451200219X. [DOI] [PubMed] [Google Scholar]
- 34.Shtriker MG, et al. Fenugreek galactomannan and citrus pectin improve several parameters associated with glucose metabolism and modulate gut microbiota in mice. Nutr. 2018;46:134–142.e3. doi: 10.1016/j.nut.2017.07.012. [DOI] [PubMed] [Google Scholar]
- 35.Robles Alonso V, Guarner F. Linking the gut microbiota to human health. Br. J. Nutr. 2013;109(Suppl 2):S21–6. doi: 10.1017/S0007114512005235. [DOI] [PubMed] [Google Scholar]
- 36.Kim KA, Gu W, Lee IA, Joh EH, Kim DH. High fat diet-induced gut microbiota exacerbates inflammation and obesity in mice via the TLR4 signaling pathway. PLoS One. 2012;7(10):e47713. doi: 10.1371/journal.pone.0047713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Douglas-Escobar M, Elliott E, Neu J. Effect of intestinal microbial ecology on the developing brain. JAMA Pediatr. 2013;167:374–379. doi: 10.1001/jamapediatrics.2013.497. [DOI] [PubMed] [Google Scholar]
- 38.Turnbaugh PJ, et al. An obesity-associated gut microbiome with increased capacity for energy harvest. Nat. 2006;444:1027–31. doi: 10.1038/nature05414. [DOI] [PubMed] [Google Scholar]
- 39.Zhang YJ, et al. Impacts of gut bacteria on human health and diseases. Int. J. Mol. Sci. 2015;16:7493–519. doi: 10.3390/ijms16047493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Anwar S, et al. Trigonelline inhibits intestinal microbial metabolism of choline and its associated cardiovascular risk. J. Pharm. Biomed. Anal. 2018;159:100–112. doi: 10.1016/j.jpba.2018.06.027. [DOI] [PubMed] [Google Scholar]
- 41.Knott EJ, et al. Fenugreek supplementation during high-fat feeding improves specific markers of metabolic health. Sci. Rep. 2017;7:12770. doi: 10.1038/s41598-017-12846-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Everard A, et al. Responses of gut microbiota and glucose and lipid metabolism to prebiotics in genetic obese and diet-induced leptin-resistant mice. Diabetes. 2011;60:2775–86. doi: 10.2337/db11-0227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Turnbaugh PJ, Backhed F, Fulton L, Gordon JI. Diet-induced obesity is linked to marked but reversible alterations in the mouse distal gut microbiome. Cell Host Microbe. 2008;3:213–23. doi: 10.1016/j.chom.2008.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dinan TG, Quigley EM. Probiotics in the treatment of depression: science or science fiction? Psychiatry. 2011;45:1023–5. doi: 10.3109/00048674.2011.613766. [DOI] [PubMed] [Google Scholar]
- 45.Tillisch K. The effects of gut microbiota on CNS function in humans. Gut Microbes. 2014;5:404–10. doi: 10.4161/gmic.29232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Neufeld, K. M., Kang, N., Bienenstock, J. & Foster, J. A. Reduced anxiety-like behavior and central neurochemical change in germ-free mice. Neurogastroenterol. Motil., 255–64 (2011). [DOI] [PubMed]
- 47.Diaz Heijtz R, et al. Normal gut microbiota modulates brain development and behavior. Proc. Natl. Acad. Sci. USA. 2011;108:3047–52. doi: 10.1073/pnas.1010529108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bercik P, et al. The intestinal microbiota affect central levels of brain-derived neurotropic factor and behavior in mice. Gastroenterology. 2011;141:599–609. doi: 10.1053/j.gastro.2011.04.052. [DOI] [PubMed] [Google Scholar]
- 49.Mendis M, Leclerc E, Simsek S. Arabinoxylans, gut microbiota and immunity. Carbohydr. Polym. 2016;139:159–6. doi: 10.1016/j.carbpol.2015.11.068. [DOI] [PubMed] [Google Scholar]
- 50.Zhang H, et al. Human gut microbiota in obesity and after gastric bypass. Proc. Natl. Acad. Sci. USA. 2009;106:2365–70. doi: 10.1073/pnas.0812600106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sweeney TE, Morton JM. The human gut microbiome: a review of the effect of obesity and surgically induced weight loss. JAMA Surg. 2013;148:563–9. doi: 10.1001/jamasurg.2013.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Schwiertz A, et al. Microbiota and SCFA in lean and overweight healthy subjects. Obes. 2009;18:190–19. doi: 10.1038/oby.2009.167. [DOI] [PubMed] [Google Scholar]
- 53.Walters WA, Xu Z, Knight R. Meta‐analyses of human gut microbes associated with obesity and IBD. FEBS Lett. 2014;588:4223–4233. doi: 10.1016/j.febslet.2014.09.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bruce-Keller AJ, et al. Obese-type gut microbiota induce neurobehavioral changes in the absence of obesity. Biol. Psychiatry. 2015;77:607–15. doi: 10.1016/j.biopsych.2014.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Murphy EF, et al. Divergent metabolic outcomes arising from targeted manipulation of the gut microbiota in diet-induced obesity. Gut Pathog. 2013;62:220–6. doi: 10.1136/gutjnl-2011-300705. [DOI] [PubMed] [Google Scholar]
- 56.Johnson, E. L., Heaver, S. L., Walters, W. A. & Ley, R. E. Microbiome and metabolic disease: revisiting the bacterial phylum Bacteroidetes. J Mol Med (Berl) [Epub ahead of print], (2016). [DOI] [PMC free article] [PubMed]
- 57.Bruce-Keller AJ, et al. Maternal obese-type gut microbiota differentially impact cognition, anxiety and compulsive behavior in male and female offspring in mice. PLoS One. 2017;12:e0175577. doi: 10.1371/journal.pone.0175577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Salazar N, Valdés-Varela L, González S, Gueimonde M, de Los Reyes-Gavilán CG. Nutrition and the gut microbiome in the elderly. Gut Microbes. 2017;8:82–97. doi: 10.1080/19490976.2016.1256525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Pryde SE, Duncan SH, Hold GL, Stewart CS, Flint HJ. The microbiology of butyrate formation in the human colon. FEMS Microbiol. Lett. 2002;217:133–13. doi: 10.1111/j.1574-6968.2002.tb11467.x. [DOI] [PubMed] [Google Scholar]
- 60.Macfarlane S, Macfarlane GT. Regulation of short-chain fatty acid production. Proc. Nutr. Soc. 2003;62:67–7. doi: 10.1079/PNS2002207. [DOI] [PubMed] [Google Scholar]
- 61.Clausen, M. R. & Mortensen, P. B. Kinetic studies on colonocyte metabolism of short chain fatty acids and glucose in ulcerative colitis. Gut, 37, 684–689. [DOI] [PMC free article] [PubMed]
- 62.Mortensen PB, Clausen MR. Short-chain fatty acids in the human colon: relation to gastrointestinal health and disease. Scand. J. Gastroenterol. Suppl. 1996;216:132–148. doi: 10.3109/00365529609094568. [DOI] [PubMed] [Google Scholar]
- 63.Fasano A. Zonulin and its regulation of intestinal barrier function: the biological door to inflammation, autoimmunity, and cancer. Physiol. Rev. 2011;91:151–75. doi: 10.1152/physrev.00003.2008. [DOI] [PubMed] [Google Scholar]
- 64.Bischoff SC, et al. Intestinal permeability–a new target for disease prevention and therapy. BMC Gastroenterol. 2014;14:189. doi: 10.1186/s12876-014-0189-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.McNabney SM, Henagan TM. Short Chain Fatty Acids in the Colon and Peripheral Tissues: A Focus on Butyrate, Colon Cancer, Obesity and Insulin Resistance. Nutrients. 2017;9:E1348. doi: 10.3390/nu9121348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Roberts KT. The potential of fenugreek (Trigonella foenum-graecum) as a functional food and nutraceutical and its effects on glycemia and lipidemia. J. Med. Food. 2011;14:1485–9. doi: 10.1089/jmf.2011.0002. [DOI] [PubMed] [Google Scholar]
- 67.Kishino S, et al. Polyunsaturated fatty acid saturation by gut lactic acid bacteria affecting host lipid composition. Proc. Natl Acad. Sci. USA. 2013;110:17808–17813. doi: 10.1073/pnas.1312937110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kriaa A, et al. Microbial impact on cholesterol and bile acid metabolism: current status and future prospects. J. Lipid Res. 2019;60:323–332. doi: 10.1194/jlr.R088989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Lye HS, Rusul G, Liong MT. Removal of cholesterol by lactobacilli via incorporation and conversion to coprostanol. J. Dairy. Sci. 2010;93:1383–1392. doi: 10.3168/jds.2009-2574. [DOI] [PubMed] [Google Scholar]
- 70.Sekimoto H, Shimada O, Makanishi M, Nakano T, Katayama O. Interrelationship between serum and fecal sterols. Jpn. J. Med. 1983;22:14–20. doi: 10.2169/internalmedicine1962.22.14. [DOI] [PubMed] [Google Scholar]
- 71.Antharam VC, et al. An integrated metabolomic and microbiome analysis identified specific gut microbiota associated with fecal cholesterol and coprostanol in Clostridium difficile infection. PLoS One. 2016;11:e0148824. doi: 10.1371/journal.pone.0148824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Fu J, et al. The gut microbiome contributes to a substantial proportion of the variation in blood lipids. Circ. Res. 2015;117:817–824. doi: 10.1161/CIRCRESAHA.115.306807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Muraki E, Hayashi Y, Chiba H, Tsunoda N, Kasono K. Dose-dependent effects, safety and tolerability of fenugreek in diet-induced metabolic disorders in rats. Lipids Health Dis. 2011;10:240. doi: 10.1186/1476-511X-10-240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Fiorucci S, Distrutti E. Bile Acid-Activated Receptors, Intestinal Microbiota, and the Treatment of Metabolic Disorders. Trends Mol. Med. 2015;21:702–14. doi: 10.1016/j.molmed.2015.09.001. [DOI] [PubMed] [Google Scholar]
- 75.Stark A, Madar Z. The effect of an ethanol extract derived from fenugreek (Trigonella foenum-graecum) on bile acid absorption and cholesterol levels in rats. Br. J. Nutr. 1993;69:277–87. doi: 10.1079/BJN19930029. [DOI] [PubMed] [Google Scholar]
- 76.Jones BV, Begley M, Hill C, Gahan CG, Marchesi JR. Functional and comparative metagenomic analysis of bile salt hydrolase activity in the human gut microbiome. Proc. Natl Acad. Sci. USA. 2008;105:13580–5. doi: 10.1073/pnas.0804437105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Hepner G, Fried R, St Jeor S, Fusetti L, Morin R. Hypocholesterolemic effect of yogurt and milk. Am. J. Clin. Nutr. 1979;32:19–24. doi: 10.1093/ajcn/32.1.19. [DOI] [PubMed] [Google Scholar]
- 78.Hlivak P, et al. One-year application of probiotic strain Enterococcus faecium M-74 decreases serum cholesterol levels. Bratisl. Lek. Listy. 2005;106:67–72. [PubMed] [Google Scholar]
- 79.Thomas C, et al. TGR5-mediated bile acid sensing controls glucose homeostasis. Cell Metab. 2005;10:167–177. doi: 10.1016/j.cmet.2009.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Yang Y, Smith DLJ, Keating KD, Allison DB, Nagy TR. Variations in body weight, food intake and body composition after long-term high-fat diet feeding in C57BL/6J mice. Obes. 2014;22:2147–55. doi: 10.1002/oby.20811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Senthil Kumar SP, et al. Distinct metabolic effects following short-term exposure of different high-fat diets in male and female mice. Endocr. J. 2014;61:457–70. doi: 10.1507/endocrj.EJ13-0455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Yuan S, Cohen DB, Ravel J, Abdo Z, Forney LJ. Evaluation of methods for the extraction and purification of DNA from the human microbiome. PLoS One. 2012;7:e33865. doi: 10.1371/journal.pone.0033865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Kozich JJ, Westcott SL, Baxter NT, Highlander SK, Schloss PD. Development of a dual-index sequencing strategy and curation pipeline for analyzing amplicon sequence data on the MiSeq Illumina sequencing platform. Appl. Environ. Microbiol. 2013;79:5112–20. doi: 10.1128/AEM.01043-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Edgar RC. UPARSE: highly accurate OTU sequences from microbial amplicon reads. Nat. Methods. 2013;10:996–8. doi: 10.1038/nmeth.2604. [DOI] [PubMed] [Google Scholar]
- 85.Quast C, et al. The SILVA ribosomal RNA gene database project: improved data processing and web-based tools. Nucleic Acids Res. 2013;41:D590–6. doi: 10.1093/nar/gks1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Love MI, Huber W, Anders S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq. 2. Genome Biol. 2014;15:550. doi: 10.1186/s13059-014-0550-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Lozupone C, Hamady M, Knight R. UniFrac–an online tool for comparing microbial community diversity in a phylogenetic context. BMC Bioinforma. 2006;7:371. doi: 10.1186/1471-2105-7-371. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.