Abstract
Our previous study had shown that chronic corticosterone (CORT) exposure causes excessive fat deposition in chicken liver, yet it remains unknown whether it is associated with inflammation and fibrosis. In general, heat shock proteins (HSPs) are activated in response to acute stress to play a cytoprotective role, and this activation is associated with m6A-mediated post-transcriptional regulation. However, changes of HSPs and the m6A methylation on their mRNAs in response to chronic CORT treatment in chicken liver have not been reported. In this study, chronic CORT exposure induced inflammation and fibrosis in chicken liver, associated with significantly modulated expression of HSPs that was significantly upregulated at mRNA level yet downregulated at protein level. Concurrently, m6A methyltransferases METTL3 content was upregulated together with the level of m6A methylation on HSPs transcripts. The m6A-seq analysis revealed 2-6 significantly (P < 0.05) hypermethylated m6A peaks in the mRNA of 4 different species of HSPs in CORT-treated chicken liver. HSP90B1 transcript had 6 differentially methylated m6A peaks among which peaks on exon 16 and exon 17 showed 3.14- and 4.72-fold of increase, respectively. Mutation of the 8 predicted m6A sites on exon 16 and exon 17 resulted in a significant (P < 0.05) increase in eGFP-fused content of HSP90B1 exon 16 and exon 17 fragment in 293 T cells, indicating a possible role of m6A in post-transcriptional regulation of HSPs. In conclusion, chronic CORT exposure induces inflammation and fibrosis in chicken liver along with an increase in the levels and m6A methylation of several HSPs mRNAs; HSPs levels were however reduced under the indicated conditions. Results presented suggest that the reduction in HSPs levels may be associated with m6A methylation in CORT-exposed chickens.
Electronic supplementary material
The online version of this article (10.1007/s12192-019-01034-7) contains supplementary material, which is available to authorized users.
Keywords: Chicken liver, Inflammation, Fibrosis, Heat shock proteins, N6-methyladenosine
Introduction
Modern intensive poultry production attracts accusations of poor welfare concerning exposure of chickens to high temperature, insistent humidity, and over density, which causes chicken long-term exposure to these stressors (Cohen et al. 2011; Dawkins et al. 2004; Liu and Zhao 2014). Long-term stress is a high-risk factor for nonalcoholic fatty liver disease (NAFLD) in rodents (Cheng et al. 2017; Czech et al. 2013; Fu et al. 2010) and zebrafish (Cinaroglu et al. 2011). NAFLD ranges from excessive fat deposition in hepatocytes (steatosis) to steatohepatitis, which can progress to fibrosis, cirrhosis, and ultimately, hepatocellular carcinoma (Cohen et al. 2011). Inflammation is a characteristic feature of NAFLD, which is associated with the development of fibrosis, cirrhosis, and hepatocellular carcinoma (Seki and Schwabe 2015). Previous studies indicated that chronic corticosterone (CORT) administration could cause excessive fat deposition in chicken livers (Hu et al. 2018; Lin et al. 2006a; Lin et al. 2006b). However, it remains unknown whether CORT-induced fatty liver is accompanied with inflammation and fibrosis in the chicken.
In mammals, glucocorticoid response and heat shock proteins (HSPs) play a critical role in regulation of the biphasic, pro-inflammatory and anti-inflammatory, immune response (Stolte et al. 2009). Activated hypothalamic–pituitary–adrenal axis induces the release of cortisol from the adrenal cortex which is bound to glucocorticoid receptor (GR) to inhibit nuclear factor-κB (NF-κB) nuclear translocation and inflammatory cytokine production through direct and indirect genomic effects and non-genomic mechanism (Rhen and Cidlowski 2005). Additionally, GR function is regulated by coordinated action of the HSP90 and HSP70 chaperone cycles (Kirschke et al. 2014). HSP90 is required for GR to enhance cortisol affinity and become active (Picard et al. 1990), while HSP70 inactivates GR through protein aggregation. Furthermore, HSPs could regulate cellular immune response by itself. Extracellular HSP70 acts as damage-associated molecular pattern and induces pro-inflammatory cytokine release, such as interleukin (IL)-1, IL-6, IL-8, and tumor necrosis factor (TNF-α), in THP-1 cells (Hulina et al. 2018) and human peripheral blood monocytes (Ferat-Osorio et al. 2014). Whereas intracellular HSP70 interacting with IKK-γ impairs NF-κB signaling (Ran et al. 2004). Also, Chen et al. (2002) reported that HSP90 is an essential stability factor of IKK signalosome to inhibit NF-κB signaling. On the other hand, other reports indicate that HSPs protect against pulmonary, renal, dermal and intestinal fibrosis in mice through inhibition of aberrant transforming growth factor (TGF)-β signaling (Kitamura et al. 2011; Noh et al. 2012; Tanaka et al. 2010; Tomcik et al. 2014), which is a central mediator of fibrogenesis. However, less is known whether HSPs are involved in hepatic inflammation and fibrosis by CORT treatment in chicken.
N6-methyladenosine (m6A) is the most prevalent and internal modification occurring in RNAs, which plays an important role in RNA splicing, degradation, and translation (Maity and Das 2016). The METTL3-METTL14 protein complex is the enzymatic core of the m6A writer, while Wilms’ tumor–associated protein (WTAP) and other factors regulate the methylation process (Liu et al. 2014). Demethylases fat mass and obesity-associated protein (FTO) and AlkB Homolog 5 (ALKB5) are erasers that remove m6A marks from RNAs (Zheng et al. 2013). Additionally, the m6A reader proteins (e.g., YTHDF family proteins) recognize the m6A code to regulate both mRNA stability and translation (Wang et al. 2015; Zhou et al. 2015). Previous studies demonstrated that HSPs are modulated by m6A RNA methylation at the post-transcriptional level when cells (Yu et al. 2018; Zhou et al. 2015) and sheep (Lu et al. 2019) are subjected to acute heat shock. However, it is still unknown whether m6A RNA methylation regulates HSPs gene expression in the liver of chickens chronically treated with CORT.
In this study, we aimed to know whether chronic CORT administration could induce hepatic inflammation and fibrosis in juvenile chickens, and to determine whether such effects are related to the translational regulation of HSPs through m6A modifications.
Materials and methods
Ethics statement
All the experiments were approved by the Animal Ethics Committee of Nanjing Agricultural University (31672512). The sampling procedures were as per the “Guidelines on Ethical Treatment of Experimental Animals” (2006) No. 398 set by the Ministry of Science and Technology, China.
Animals and treatment
Twenty-four male chickens (at 8 weeks of age) were selected from Poultry Institute of Yangzhou, Jiangsu, China, and were randomly divided into control (CON) and corticosterone (CORT) groups (12 in each group), being subjected to either vehicle or corticosterone treatment with daily subcutaneous injection of solvent (15% ethanol) or CORT (C2505, Sigma-Aldrich, USA) in a dose of 4.0 mg/kg body mass for 7 days (twice per day, 9:00–10:30 and 21:00–22:30), according to Lin et al. 2004. At 9 weeks of age, all the chickens were weighed and killed by rapid decapitation which is considered to be acceptable for euthanasia of birds according to the American Veterinary Medical Association (AVMA) Guidelines for the Euthanasia of Animals: 2013 Edition. Blood samples were taken and plasma samples were separated and stored at − 20 °C. The liver (without the gall bladder) samples were rapidly frozen in liquid nitrogen and keep at − 80 °C for further analysis.
Aspartate aminotransferase, alanine aminotransferase, and interleukin 1β concentration in serum
Serum aspartate aminotransferase (AST) and alanine aminotransferase (ALT) concentrations were measured using an automatic biochemical analyzer (7020, Hitachi, Tokyo, Japan), following the manufacturer’s instructions. Serum contents of IL-1β were measured by using the IL-1β ELISA Kit according to the manufacturer’s instructions (RGB-60013C, Rigor Bioscience, Beijing, China). A microplate reader (Synergy BioTek, USA) was used to measure the absorbance of each well at 450 nm. All assays were done in duplicates. The detection limit of the assay was 7.5–120 ng/L. The intra-assay and inter-assay CVs were 4.5% and 8.4%, respectively.
Histological analysis of liver tissue
Fresh liver tissue was fixed with 4% paraformaldehyde and paraffin-embedded, and then the sections of liver were stained with hematoxylin eosin (HE) and Sirius red.
Total RNA isolation and real-time PCR
Total RNA was isolated from 30-mg liver samples by using TRIzol Reagent (3101, Shanghai Pufei Biotechnology Co., Ltd., China), and 1 μg of RNA was reverse-transcribed into cDNA by using a HiScript® II Reverse Transcriptase kit (R233-01, Vazyme, China) according to the manufacturer’s protocol. Two microliters of diluted cDNA (1:20, v:v) was used for real-time quantitative PCR by using a Mx3000P Real-Time PCR System (Stratagene, USA). All primers (Table 1) were synthesized by Genewiz Biotech (Suzhou, China). 18S rRNA was chosen as a reference gene and was not affected by CORT treatment. Data were analyzed by using the method of 2−ΔΔCT.
Table 1.
Nucleotide sequences of primers
| Target genes | Primer sequences (5′to 3′) | |
|---|---|---|
| TLR1 | F: ACCCGTTCAAGTGTTCGTG | R: TTCCGCTCAAGTCTTCTGG |
| TLR2 | F: GGTGTTCCTGTTCATCCTCATC | R: GTTGGAGTCGTTCTCACTGTAGG |
| TLR3 | F: TCAGTACATTTGTAACACCCCGCC | R: TCAGTACATTTGTAACACCCCGCC |
| TLR4 | F: ATCTTTCAAGGTGCCACATC | R: GGATATGCTTGTTTCCACCA |
| TLR5 | F: ACTCCCTTCCTTCCCACATCT | R: GTTTGCGAGCCAGTTTCTCTCT |
| TLR7 | F: TTCTGGCCACAGATGTGACC | R: CCTTCAACTTGGCAGTGCAG |
| MyD88 | F: AGCGTGGAGGAGGACTGCAAGAAG | R: CCGATCAAACACACACAGCTTCAG |
| NLRP3 | F: GGTTTACCAGGGGAAATGAGG | R: TTGTGCTTCCAGATGCCGT |
| IL-1β | F: CAAACTGCTGCGGAGGC | R: AGGACTGTGAGCGGGTGTAG |
| IL-4 | F: CTGCCTGGATGCTGTTCT | R: TGCCCTGTCCTCAGTGCTTT |
| IL-8 | F: CAAGCCAAACACTCCT | R: TTTAGGGTGGATGAACT |
| IL-10 | F: GCTGAGGGTGAAGTTTGAG | R: CAGGTGAAGAAGCGGTGA |
| IL-12 | F: GGGAACAGAACTGAAAGG | R: GCTGATAATCTCGTGGG |
| IL-17α | F: ACATGGGAAGGTGATACGGC | R: TGGGTTAGGCATCCAGCATC |
| IFN-α | F: ATCCTGCTGCTCACGCTCCTTCT | R: GGTGTTGCTGGTGTCCAGGATG |
| IFN-β | F: GCCTCCAGCTCCTTCAGAATACG | R: CTGGATCTGGTTGAGGAGGCTGT |
| P65 | F: AGGACTTAAAATGGCAGGAGA | R: GCTGTTCGTAGTGGTAAGTCT |
| COL1A1 | F: ACCTCAGCAAGAACCCCAAG | R: CTCACCGCCGTACTCAAACT |
| COL1A2 | F: GCGGTTTCTACTGGATTGA | R: AGCGAGACGGCTTATTTG |
| TGFβ1 | F: GCAAACTGCGTCTGACCG | R: ACGAAGAAGATGCTGTGGC |
| α-SMA | F: AAGCACCACTGAATCCCAAAG | R: CCAGAGTCAAGCACAATCCCT |
| MMP2 | F: CGATGCTGTCTACGAGTCCC | R: TAGCCCCTATCCAGGTTGCT |
| TIMP2 | F: CGACGTAGTGATCCGAGCAA | R: ACTGGATTCGCTTGATGGGG |
| HSP60 | F: GGACGGAAAGGTGTAATC | R: TTCAAGAGCTGGAACTATG |
| HSP70 | F: CGTCAGTGCTGTGGACAAGAGTA | R: CCTATCTCTGTTGGCTTCATCCT |
| HSP90AA1 | F: GAGTTTGACTGACCCGAGCA | R: CAGCTGATGACTCCCAAGCA |
| HSP90B1 | F: GCCAGTTTGGTGTTGGCTTT | R: TTAGCCAGTCGTGTGCGATT |
| GAPDH | F: ATCGTCAAGGCTGAGAACGGG | R: ATCTGCCCATTTGATGTTGCT |
Protein extraction and western blot assay
Total protein was extracted from 60-mg frozen liver samples as previously described (Hu et al. 2015). The protein concentrations were measured by using BCA Protein Assay kit (No.23225, Thermo Scientific, USA) according to the manufacturer’s instructions. 40 μg of proteins were used for electrophoresis on a 10% SDS-PAGE gel and transferred onto a nitrocellulose membrane. The membrane was blocked in 4% milk for 2 h, then incubated with primary antibodies overnight and secondary antibodies 2 h. Western blot analysis for GR (Antibody was customized by Genecreate, China, diluted 1:1000), p65 (sc-372, Santa Cruz, UK, diluted 1:200), NLRP3 (13158, Cell Signaling Technology, USA, diluted 1:1000), TIMP1 (BS1697, Bioworld, USA, diluted 1:1000), TIMP2 (BS1366, Bioworld, USA, diluted 1:1000), MMP2 (BS1236, Bioworld, USA, diluted 1:1000), HSP60 (As a gift from Northeast Agricultural University, diluted 1:1000), HSP70 (As a gift from Northeast Agricultural University, diluted 1:1000), HSP90α (As a gift from Northeast Agricultural University, diluted 1:1000), HSP90β (BS1851, Bioworld, USA, diluted 1:1000), HSP90B1 (BS1155, Bioworld, USA, diluted 1:1000), METTL3 (AB98009, Abcam, USA, diluted 1:1000), METTL14 (AB98116, Abcam, USA, diluted 1:1000), WTAP (BS2062, Bioworld, USA, diluted 1:1000) was carried out according to the manufacturer’s instructions. Tubulin-β (AP0064, Bioworld, USA, diluted 1:5000) was selected as an internal control. Images were captured by VersaDoc 4000MP system (Bio-Rad, USA) and the band density was analyzed with Quantity One software (Bio-Rad, USA).
m6A sequencing
m6A-IP and library preparation were performed according to the reported protocol (Dominissini et al. 2012). Briefly, poly-A-purified RNA was fragmented and incubated with m6A primary antibody for 2 h at 4 °C. The mixture was then immunoprecipitated by incubation with Protein A beads (21348, Thermo Fisher) for 2 h at 4 °C. Captured RNA was washed 3 times, then eluted with m6A nucleotide solution and purified by a RNA clean and concentrator kit (R1013, Zymo Research, USA). The m6A-sequencing and data analysis were performed by Novogene (Beijing, China).
m6A mini-gene construction
The m6A mini-gene was constructed based on a pEGFP-C1 vector by Applied Biological Materials Inc. (Nanjing, China). Exon16 and exon17 of HSP90B1 containing 8 m6A motifs (DRACH) was cloned in-frame into the mini-gene following the coding region of EGFP by ClonExpress®II One Step Cloning Kit (C112-01, Vazyme, China). Synonymous point mutations were introduced to disrupt m6A RAC core motif without changing any codons (Supplemental 1). HEK293Tl cells maintained at 37 °C in a humidified incubator containing 5% CO2. Twenty-four hours prior to transfection, cells were grown in medium supplemented with 10% FBS at a suitable density in 6-well plates. Before transfection, the culture medium was replaced with fresh DMEM. The transfection was conducted with a mixture of Lipofectamine® 2000 Transfection Reagent (11668-019, Thermo Scientific, US) and Opti-MEM (31985, Thermo Scientific, USA) according to the manufacturer’s protocol. Whole cell lysates were extracted and the protein concentration was determined by using Pierce BCA Protein Assay kit (No.23225, Thermo Scientific, USA) according to the manufacturer’s instruction. Thirty-microgram whole cell proteins were loaded on a 10% SDS-PAGE gel for electrophoresis. Mouse monoclonal anti-GFP tag antibody (G096, Applied Biological Materials Inc., China, diluted 1:1000) was used for western blot.
Statistical analysis
All data are presented as means ± SEM and the differences between groups were analyzed using independent-samples t test with SPSS 20.0 software (SPSS Inc., Chicago, IL, USA). The differences were considered statistically significant when P < 0.05.
Results
Body weight, liver weight, liver index, and serum AST, ALT, and IL-1β levels in CORT-treated chickens
It was shown that chronic CORT administration decreased the body weight of chickens as compared with untreated group (P < 0.05, Table 2). Liver weight and liver index were however significantly increased in the treated group (P < 0.05, Table 2). In addition, serum AST, ALT, and IL-1β were also elevated the CORT group (P < 0.05, Table 2).
Table 2.
The body weight, liver weight, liver index, and serum AST, ALT, and IL-1β levels
| Parameters | Control | CORT | P value |
|---|---|---|---|
| Initial body weight (g) | 576.15 ± 5.49 | 581.54 ± 4.92 | 0.47 |
| Final body weight (g) | 656.23 ± 8.67 | 617.77 ± 7.04 | < 0.01 |
| Liver weight (g) | 14.49 ± 0.39 | 18.61 ± 0.79 | < 0.01 |
| Liver index (%) | 2.21 ± 0.05 | 3.00 ± 0.10 | < 0.01 |
| Plasma ALT (U/L) | 3.54 ± 0.45 | 5.17 ± 0.51 | 0.02 |
| Plasma AST (U/T) | 248.58 ± 11.10 | 295.92 ± 13.20 | 0.01 |
| Plasma IL-1β (ng/L) | 14.56 ± 2.53 | 24.61 ± 3.59 | 0.04 |
Encoding proinflammatory agents in livers of CORT treated chickens
Microscopic evaluation of HE-stained (Fig. 1a) liver section revealed that the chronic CORT-treated exhibited higher infiltration cells compared with untreated control. We also observed significant increase (P < 0.05) in the mRNA levels of proinflammatory cytokines IL-1β, IL-8, and IL-12 in the livers of CORT-treated chickens over control group (Fig. 1b). Transcript toll like receptors (TLR) 1, 2, 3, 4, MyD88 (adaptor protein required for TLR signaling), and NLRP3 (inflammasome constituent) were all significantly upregulated (P < 0.05) in the CORT-administered group (Fig. 1c). In addition, protein content of p65 and NLRP3 were also significantly up-regulated (P < 0.05, Fig. 1d) compared with control.
Fig. 1.
mRNA and protein levels of inflammatory related genes. a Histological images of inflammatory cell infiltration (H&E staining technique, black arrows); b, c mRNA levels of inflammatory related genes; d p65 and NLRP3 protein content. CON = control; CORT = corticosterone. Values are expressed as the mean ± SEM; *p < 0.05 (n = 6)
mRNA and protein levels of fibrosis-related genes in livers of CORT-treated chickens
Collagen fiber content as detected by Sirius red staining (Fig. 2a) was increased in the CORT group. In addition, mRNA levels of fibrosis-related genes, COL1A1, COL1A2, TGFβ1, and α-SMA (P < 0.05), were significantly increased by CORT administration (Fig. 2b). Whereas the protein levels of metallopeptidase inhibitor TIMP1 and TIMP 2 were significantly elevated (P < 0.05) in the CORT-treated group, those of the matrix metalloproteinase MMP2 was lower in the experimental group (P < 0.05, Fig. 2c).
Fig. 2.
mRNA and protein levels of fibrosis related genes. a Sirius red staining of collagen (black arrows). The red area indicated by the arrow is collagen; b Liver fibrosis–related mRNA expression; c Liver fibrosis–related protein content. CON = Control; CORT = Corticosterone. Values are expressed as the mean ± SEM; *p < 0.05 (n = 5)
mRNA and protein levels of HSPs genes in livers of CORT-treated chickens
The expression of hepatic HSP60, HSP70, HSP90α, and HSP90B1 were significantly increased (P < 0.05) at mRNA levels in the CORT group (Fig. 3a); however, the protein levels of HSP60, HSP70, HSP90α, and HSP90B1 were significantly decreased (P < 0.05) in the CORT group (Fig. 3b).
Fig. 3.
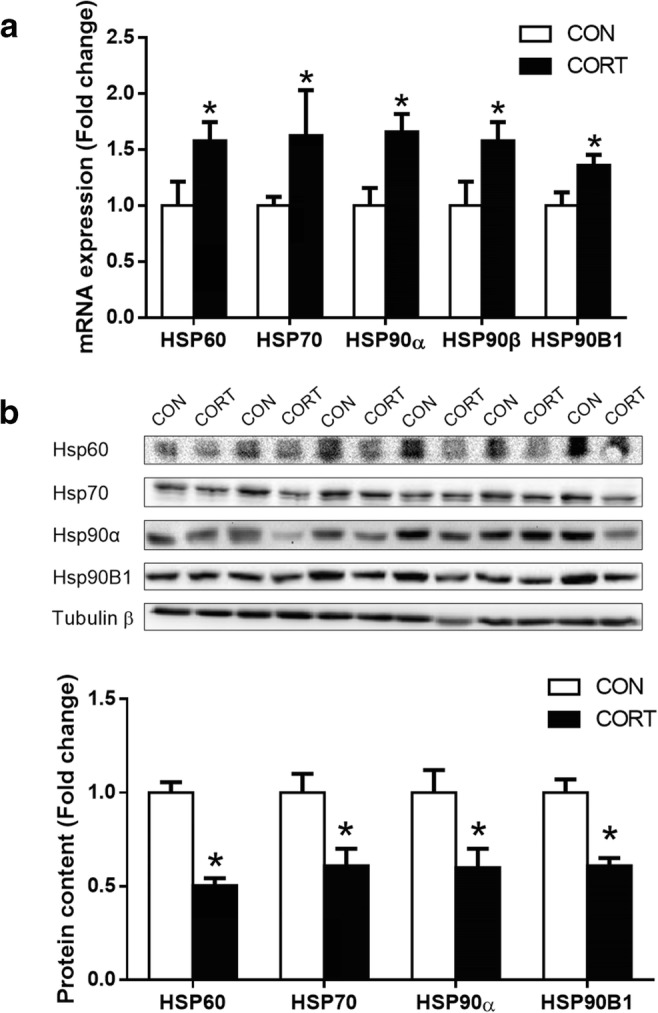
mRNA and protein levels of HSPs genes in liver. a HSPs mRNA expression; b HSPs protein content. CON = Control; CORT = Corticosterone. Values are expressed as the mean ± SEM; *p < 0.05 (n = 6)
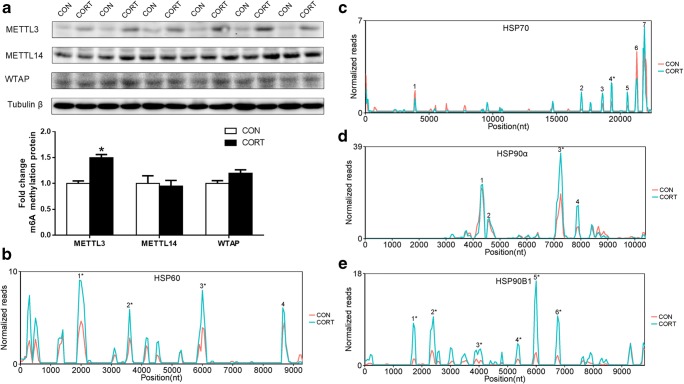
Protein content of m6A methyltransferase and profiling of m6A in HSPs transcripts
METTL3, METTL14, and WTAP are characterized as the methyltransferase core complex. Our results showed that the protein content of METTL3 (P < 0.05) was significantly increased in the CORT group, while no significant difference was observed in METTL14 or WTAP (Fig. 4a). Furthermore, we detected the m6A modification in HSPs genes through m6A-seq. It was found that the m6A sites of HSP60, HSP70, HSP90α, and HSP90B1 transcripts mainly distribute on exons. In addition, it was observed that the m6A sites were most enriched around stop codons in HSP70 and HSP90B1 transcripts. We have identified 3 of 4 m6A peaks were significantly increased in HSP60 transcript (P < 0.05), 1 of 7 m6A peaks in HSP70 transcript (P < 0.05), 1 of 4 m6A peaks in HSP90α transcript (P < 0.05), and 6 of 6 m6A peaks in HSP90B1 transcript (P < 0.05, Fig. 4b–e).
Fig. 4.
Protein content of m6A methyltransferase and profiling of m6A in HSPs transcripts. a Protein content of methyltransferase core complex, Values are expressed as the mean ± SEM; *p < 0.05 (n = 6); b–e m6A modification in HSPs genes through m6A-seq, *p < 0.05. CON = Control; CORT = Corticosterone
Functional verification of m6A modification in vitro
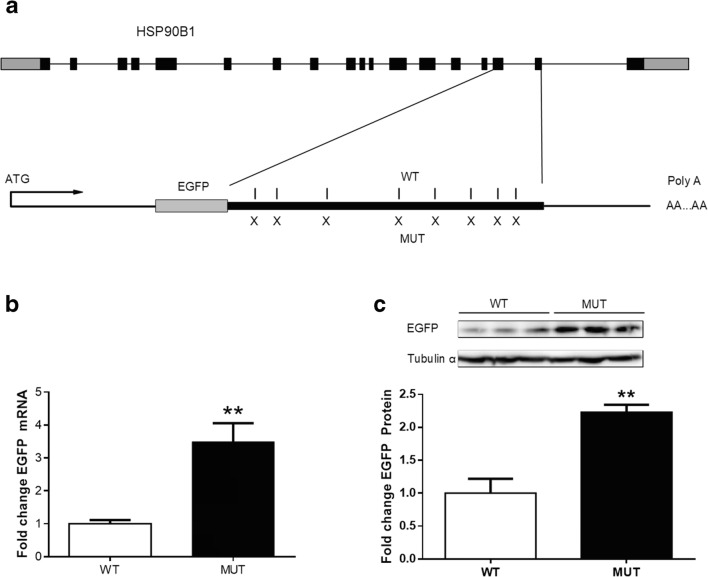
Since the major difference of m6A sites were identified in exon 16 to exon 17 of HSP90B1 transcript, we used the mini-gene experiment to verify the function of m6A sites. All predicted m6A sites (8 in total) of HSP90B1 transcripts in exon 16 and exon 17 were synonymous point mutated (Fig. 5a) and cloned to a mini-gene vector in frame with EGFP. It was found that both of mRNA and protein levels of EGFP was significantly increased by synonymous point mutation in m6A sites (P < 0.05, Fig. 5b, c).
Fig. 5.
Functional verification of m6A modification in vitro. a The mini-gene experiment to verify the function of m6A sites for HSP90B1; b EGFP mRNA expression after synonymous point mutation in m6A sites; c EGFP protein content after synonymous point mutation in m6A sites. CON = Control; CORT = Corticosterone. Values are expressed as the mean ± SEM; *p < 0.05 (n = 3)
Discussion
Previous reports demonstrated that chronic CORT treatment causes hepatic lipid accumulation and progresses to NAFLD in mice (Sun et al. 2013) and chicken (Hu et al. 2017). If fatty liver is accompanied with stress and inflammation, up to 30% of people with NAFLD will develop non-alcoholic steatohepatitis (NASH) (Rinella 2015; Younossi et al. 2016); If the liver function is further damaged by increased hepatic fibrosis, up to 20% of people with NASH die of liver cirrhosis (Rinella 2015). In this study, we found that chronic CORT treatment could cause liver injury and increase inflammatory cytokines and fibrotic biomarkers in the liver of chicken. Here, we may cautiously suppose that chronic stress, especially increased glucocorticoid, is a risk factor in the progression of fatty liver to liver cirrhosis.
In this study, we found that the protein contents of hepatic HSP60, HSP70, HSP90α, and HSP90B1 were all significantly decreased in CORT-treated chicken, which were associated with hepatic inflammation and fibrosis. HSP60 is a mitochondrial chaperonin that is essentially required for protein folding in mitochondria (Ostermann et al. 1989). However, recent research has indicated that HSP60 can activate macrophages and dendritic cells and can alter adaptive immune response (Rajaiah and Moudgil 2009). In addition, HSP60 can promote cytokine production through toll-like receptors and other ligands of innate immune receptors (Rajaiah and Moudgil 2009). In contrast, HSP70 collaborates with HSP90α to cope with excessive stress response through inhibiting NF-κB signaling (Ran et al. 2004; Chen et al. 2002). Furthermore, HSP90B1, an HSP90 paralogue that is found in the endoplasmic reticulum, has been considered as an essential immune chaperone to regulate both innate and adaptive immunity (Schild 2000). It seems that there are paradoxical results for HSPs protein levels in our study, and we supposed that acute stress could induce HSPs to repair the misfolding proteins to relieve cell injury, while chronic stress may cause sustained cell damage, and then the decreased HSPs may loss the protective function which induces hepatic inflammation and fibrosis. Sun et al. was reported that HSP70 inhibit NF-κB activation and cytokine production in murine Kupffer cells (Sun et al. 2005). Since liver is an organ with multitudinous cell components, further study should be performed to research the interaction between Kupffer and stellate cells.
Intriguingly, we found that mRNA levels of hepatic HSPs were significantly increased in CORT-treated chicken, which was inconsistent with their protein levels. It seems that there may be a post-transcriptional mechanism involved in regulation of HSPs’ translation by chronic CORT treatment. m6A is a prevalent post-transcriptional modification in mammalian mRNA impacting a variety of physiological events (Yue et al. 2015). m6A mRNA methylation are dynamically and reversibly regulated by m6A “writers” (METTL3, METTL14, WTAP) and the “eraser” FTO. In this study, we found that CORT treatment enhances the protein concentrations of hepatic METTL3 and WTAP in chicken; in addition, m6A-seq analysis revealed that m6A peaks of HSPs are mainly enriched on exons and near stop codons. Until now, there are limited information is available about the function of m6A modification in exons. Xiao et al. (2016) revealed that m6A reader YTHDC1 promotes exon inclusion to regulate targeted mRNA splicing. Whereas knockdown of METTL3 in mouse embryonic stem cells discovered that less than 100 exons containing an m6A site are alternatively spliced (Dominissini et al. 2012; Geula et al. 2015; Ke et al. 2017). Furthermore, previous research found that internal m6A residues increase the in vitro translation efficiency of dihydrofolate reductase mRNA (Heilman et al. 1996). For m6A located near stop codons, it was discovered that m6A residues in the last exons play an important role in regulating proximal alternative polyA choice (Ke et al. 2015) and mRNA stability (Ke et al. 2017). This m6A-containing region (Last exon) was cloned to a mini-gene vector in-frame with GFP, and they found that mutate the A in DRACH motif can increase mRNA half-life compared with wild type. Our results of synonymous mutation in exon DRACH motif were consistent with Ke’s report. It was suggested that m6A modifications near the last exon of HSP90B1 may have a similar mechanism to reduce the stability of mRNA.
In conclusion, chronic corticosterone administration induces post-transcriptional suppression of heat shock proteins with m6A modification in chicken liver. This finding would provide insights into the new regulatory mechanisms of HSPs-mediated liver lipid accumulation to inflammation and fibrosis transformation situations in chicken.
Electronic supplementary material
(DOCX 29 kb)
Funding information
This work was supported by the National Key Research and Development Program of China (2016YFD0500502), the National Natural Science Foundation of China (31672512) and the Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD).
Compliance with ethical standards
All the experiments were approved by the Animal Ethics Committee of Nanjing Agricultural University (31672512). The sampling procedures were as per the “Guidelines on Ethical Treatment of Experimental Animals” (2006) No. 398 set by the Ministry of Science and Technology, China.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Abu-Elsaad NM, Serrya MS, El-Karef AM, Ibrahim TM. The heat shock protein 90 inhibitor, 17-AAG, attenuates thioacetamide induced liver fibrosis in mice. Pharmacol Rep. 2016;68:275–282. doi: 10.1016/j.pharep.2015.08.015. [DOI] [PubMed] [Google Scholar]
- Ambade A, Catalano D, Lim A, Mandrekar P. Inhibition of heat shock protein (molecular weight 90 kDa) attenuates proinflammatory cytokines and prevents lipopolysaccharide-induced liver injury in mice. Hepatology. 2012;55:1585–1595. doi: 10.1002/hep.24802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Archer AE, Rogers RS, Von Schulze AT, Wheatley JL, Morris EM, McCoin CS, Thyfault JP, Geiger PC. Heat shock protein 72 regulates hepatic lipid accumulation. Am J Physiol Regul Integr Comp Physiol. 2018;315:R696–R707. doi: 10.1152/ajpregu.00073.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asea A, Kabingu E, Stevenson MA, Calderwood SK. HSP70 peptidembearing and peptide-negative preparations act as chaperokines. Cell Stress Chaperones. 2000;5:425–431. doi: 10.1379/1466-1268(2000)005<0425:hpbapn>2.0.co;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen G, Cao P, Goeddel DV. TNF-induced recruitment and activation of the IKK complex require Cdc37 and Hsp90. Mol Cell. 2002;9:401–410. doi: 10.1016/s1097-2765(02)00450-1. [DOI] [PubMed] [Google Scholar]
- Cheng F, Ma C, Wang X, Zhai C, Wang G, Xu X, Mu J, Li C, Wang Z, Zhang X. Effect of traditional Chinese medicine formula Sinisan on chronic restraint stress-induced nonalcoholic fatty liver disease: a rat study. BMC Complement Altern Med. 2017;17:203. doi: 10.1186/s12906-017-1707-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cinaroglu A, Gao C, Imrie D, Sadler KC. Activating transcription factor 6 plays protective and pathological roles in steatosis due to endoplasmic reticulum stress in zebrafish. Hepatology. 2011;54:495–508. doi: 10.1002/hep.24396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen JC, Horton JD, Hobbs HH. Human fatty liver disease: old questions and new insights. Science. 2011;332:1519–1523. doi: 10.1126/science.1204265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czech B, Neumann ID, Müller M, Reber SO, Hellerbrand C. Effect of chronic psychosocial stress on nonalcoholic steatohepatitis in mice. Int J Clin Exp Pathol. 2013;6:1585. [PMC free article] [PubMed] [Google Scholar]
- Dawkins MS, Donnelly CA, Jones TA. Chicken welfare is influenced more by housing conditions than by stocking density. Nature. 2004;427:342. doi: 10.1038/nature02226. [DOI] [PubMed] [Google Scholar]
- Di Naso FC, Porto RR, Fillmann HS, Maggioni L, Padoin AV, Ramos RJ, Mottin CC, Bittencourt A, Marroni NA, de Bittencourt PI., Jr Obesity depresses the anti-inflammatory HSP70 pathway, contributing to NAFLD progression. Obesity. 2015;23:120–129. doi: 10.1002/oby.20919. [DOI] [PubMed] [Google Scholar]
- Dominissini D, Moshitch-Moshkovitz S, Schwartz S, Salmon-Divon M, Ungar L, Osenberg S, Cesarkas K, Jacob-Hirsch J, Amariglio N, Kupiec M, et al. Topology of the human and mouse m6A RNA methylomes revealed by m6A-seq. Nature. 2012;485:201–206. doi: 10.1038/nature11112. [DOI] [PubMed] [Google Scholar]
- Ferat-Osorio E, Sanchez-Anaya A, Gutierrez-Mendoza M, Bosco-Garate I, Wong-Baeza I, Pastelin-Palacios R, Pedraza-Alva G, Bonifaz LC, Cortes-Reynosa P, Perez-Salazar E, et al. Heat shock protein 70 down-regulates the production of toll-like receptor-induced pro-inflammatory cytokines by a heat shock factor-1/constitutive heat shock element-binding factor-dependent mechanism. J Inflamm. 2014;11:19. doi: 10.1186/1476-9255-11-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu J-H, Sun H-S, Wang Y, Zheng W-Q, Shi Z-Y, Wang Q-J. The effects of a fat-and sugar-enriched diet and chronic stress on nonalcoholic fatty liver disease in male Wistar rats. Dig Dis Sci. 2010;55:2227–2236. doi: 10.1007/s10620-009-1019-6. [DOI] [PubMed] [Google Scholar]
- Geula S, Moshitch-Moshkovitz S, Dominissini D, Mansour AA, Kol N, Salmon-Divon M, Hershkovitz V, Peer E, Mor N, Manor YS, et al. Stem cells. m6A mRNA methylation facilitates resolution of naive pluripotency toward differentiation. Science. 2015;347:1002–1006. doi: 10.1126/science.1261417. [DOI] [PubMed] [Google Scholar]
- Heilman KL, Leach RA, Tuck MT. Internal 6-methyladenine residues increase the in vitro translation efficiency of dihydrofolate reductase messenger RNA. Int J Biochem Cell Biol. 1996;28:823–829. doi: 10.1016/1357-2725(96)00014-3. [DOI] [PubMed] [Google Scholar]
- Hu Y, Sun Q, Li X, Wang M, Cai D, Li X, Zhao R. In Ovo injection of betaine affects hepatic cholesterol metabolism through epigenetic gene regulation in newly hatched chicks. PloS one. 2015;10:e0122643. doi: 10.1371/journal.pone.0122643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu Y, Sun Q, Liu J, Jia Y, Cai D, Idriss AA, Omer NA, Zhao R. In ovo injection of betaine alleviates corticosterone-induced fatty liver in chickens through epigenetic modifications. Sci Rep. 2017;7:40251. doi: 10.1038/srep40251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu Y, Sun Q, Hu Y, Hou Z, Zong Y, Omer NA, Abobaker H, Zhao R. Corticosterone-induced lipogenesis activation and lipophagy inhibition in chicken liver are alleviated by maternal betaine supplementation. J Nutr. 2018;148:316–325. doi: 10.1093/jn/nxx073. [DOI] [PubMed] [Google Scholar]
- Hulina A, Grdic Rajkovic M, Jaksic Despot D, Jelic D, Dojder A, Cepelak I, Rumora L. Extracellular Hsp70 induces inflammation and modulates LPS/LTA-stimulated inflammatory response in THP-1 cells. Cell Stress Chaperones. 2018;23:373–384. doi: 10.1007/s12192-017-0847-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kampinga HH, Hageman J, Vos MJ, Kubota H, Tanguay RM, Bruford EA, Cheetham ME, Chen B, Hightower LE. Guidelines for the nomenclature of the human heat shock proteins. Cell Stress Chaperones. 2009;14:105–111. doi: 10.1007/s12192-008-0068-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ke S, Alemu EA, Mertens C, Gantman EC, Fak JJ, Mele A, Haripal B, Zucker-Scharff I, Moore MJ, Park CY, et al. A majority of m6A residues are in the last exons, allowing the potential for 3' UTR regulation. Genes Dev. 2015;29:2037–2053. doi: 10.1101/gad.269415.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ke S, Pandya-Jones A, Saito Y, Fak JJ, Vagbo CB, Geula S, Hanna JH, Black DL, Darnell JE, Jr, Darnell RB. m(6) A mRNA modifications are deposited in nascent pre-mRNA and are not required for splicing but do specify cytoplasmic turnover. Genes Dev. 2017;31:990–1006. doi: 10.1101/gad.301036.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirschke E, Goswami D, Southworth D, Griffin PR, Agard DA. Glucocorticoid receptor function regulated by coordinated action of the Hsp90 and Hsp70 chaperone cycles. Cell. 2014;157:685–1697. doi: 10.1016/j.cell.2014.04.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitamura H, Yamamoto S, Nakase H, Matsuura M, Honzawa Y, Matsumura K, Takeda Y, Uza N, Nagata K, Chiba T. Role of heat shock protein 47 in intestinal fibrosis of experimental colitis. Biochem Biophys Res Commun. 2011;404:599–604. doi: 10.1016/j.bbrc.2010.12.006. [DOI] [PubMed] [Google Scholar]
- Lin H, Decuypere E, Buyse J. Oxidative stress induced by corticosterone administration in broiler chickens (Gallus gallus domesticus) 1. Chronic exposure. Comparative Biochem Physiol Part B, Biochem Mol Biol. 2004;139:737–744. doi: 10.1016/j.cbpc.2004.09.013. [DOI] [PubMed] [Google Scholar]
- Lin H, Sui S, Jiao H, Buyse J, Decuypere E. Impaired development of broiler chickens by stress mimicked by corticosterone exposure. Comp Biochem Physiol A Mol Integr Physiol. 2006;143:400–405. doi: 10.1016/j.cbpa.2005.12.030. [DOI] [PubMed] [Google Scholar]
- Lin H, Sui SJ, Jiao HC, Buyse J, Decuypere E. Impaired development of broiler chickens by stress mimicked by corticosterone exposure. Comp Biochem Physiol A Mol Integr Physiol. 2006;143:400–405. doi: 10.1016/j.cbpa.2005.12.030. [DOI] [PubMed] [Google Scholar]
- Lin S, Choe J, Du P, Triboulet R, Gregory RI. The m(6)A methyltransferase METTL3 promotes translation in human cancer cells. Mol Cell. 2016;62:335–345. doi: 10.1016/j.molcel.2016.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu W, Zhao J. Insights into the molecular mechanism of glucose metabolism regulation under stress in chicken skeletal muscle tissues. Saudi J Biol Sci. 2014;21:197–203. doi: 10.1016/j.sjbs.2014.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Yue Y, Han D, Wang X, Fu Y, Zhang L, Jia G, Yu M, Lu Z, Deng X, et al. A METTL3-METTL14 complex mediates mammalian nuclear RNA N6-adenosine methylation. Nat Chem Biol. 2014;10:93–95. doi: 10.1038/nchembio.1432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu Z, Ma Y, Li Q, Liu E, Jin M, Zhang L, Wei C (2019) The role of N(6)-methyladenosine RNA methylation in the heat stress response of sheep (Ovis aries). Cell Stress Chaperones [DOI] [PMC free article] [PubMed]
- Mahmoud KZ, Edens FW, Eisen EJ, Havenstein GB. Ascorbic acid decreases heat shock protein 70 and plasma corticosterone response in broilers (Gallus gallus domesticus) subjected to cyclic heat stress. Comparative Biochem Physiol Part B, Biochem Mol Biol. 2004;137:35–42. doi: 10.1016/j.cbpc.2003.09.013. [DOI] [PubMed] [Google Scholar]
- Maity A, Das B. N6-methyladenosine modification in mRNA: machinery, function and implications for health and diseases. FEBS J. 2016;283:1607–1630. doi: 10.1111/febs.13614. [DOI] [PubMed] [Google Scholar]
- Najafi P, Zulkifli I, Jajuli NA, Farjam AS, Ramiah SK, Amir AA, O'Reily E, Eckersall D. Environmental temperature and stocking density effects on acute phase proteins, heat shock protein 70, circulating corticosterone and performance in broiler chickens. Int J Biometeorol. 2015;59:1577–1583. doi: 10.1007/s00484-015-0964-3. [DOI] [PubMed] [Google Scholar]
- Noh H, Kim HJ, Yu MR, Kim WY, Kim J, Ryu JH, Kwon SH, Jeon JS, Han DC, Ziyadeh F. Heat shock protein 90 inhibitor attenuates renal fibrosis through degradation of transforming growth factor-beta type II receptor. Laboratory investigation; a journal of technical methods and pathology. 2012;92:1583–1596. doi: 10.1038/labinvest.2012.127. [DOI] [PubMed] [Google Scholar]
- Ostermann J, Horwich AL, Neupert W, Hartl FU. Protein folding in mitochondria requires complex formation with hsp60 and ATP hydrolysis. Nature. 1989;341:125–130. doi: 10.1038/341125a0. [DOI] [PubMed] [Google Scholar]
- Picard D, Khursheed B, Garabedian MJ, Fortin MG, Lindquist S, Yamamoto KR. Reduced levels of hsp90 compromise steroid receptor action in vivo. Nature. 1990;348:166–168. doi: 10.1038/348166a0. [DOI] [PubMed] [Google Scholar]
- Rajaiah R, Moudgil KD. Heat-shock proteins can promote as well as regulate autoimmunity. Autoimmun Rev. 2009;8:388–393. doi: 10.1016/j.autrev.2008.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ran R, Lu A, Zhang L, Tang Y, Zhu H, Xu H, Feng Y, Han C, Zhou G, Rigby AC. Hsp70 promotes TNF-mediated apoptosis by binding IKK gamma and impairing NF-kappa B survival signaling. Genes Dev. 2004;18:1466–1481. doi: 10.1101/gad.1188204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhen T, Cidlowski JA. Antiinflammatory action of glucocorticoids--new mechanisms for old drugs. N Engl J Med. 2005;353:1711–1723. doi: 10.1056/NEJMra050541. [DOI] [PubMed] [Google Scholar]
- Rinella ME. Nonalcoholic fatty liver disease: a systematic review. Jama. 2015;313:2263–2273. doi: 10.1001/jama.2015.5370. [DOI] [PubMed] [Google Scholar]
- Schild H. Rammensee HG: gp96--the immune system’s Swiss army knife. Nat Immunol. 2000;1:100–101. doi: 10.1038/77770. [DOI] [PubMed] [Google Scholar]
- Seki E, Schwabe RF. Hepatic inflammation and fibrosis: functional links and key pathways. Hepatology. 2015;61:1066–1079. doi: 10.1002/hep.27332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stolte EH, Chadzinska M, Przybylska D, Flik G, Savelkoul HF, Verburg-van K, BM. The immune response differentially regulates Hsp70 and glucocorticoid receptor expression in vitro and in vivo in common carp (Cyprinus carpio L.) Fish Shellfish Immunol. 2009;27:9–16. doi: 10.1016/j.fsi.2008.11.003. [DOI] [PubMed] [Google Scholar]
- Sun D, Chen D, Du B, Pan J. Heat shock response inhibits NF-kappaB activation and cytokine production in murine Kupffer cells. J Surg Res. 2005;129:114–121. doi: 10.1016/j.jss.2005.05.028. [DOI] [PubMed] [Google Scholar]
- Sun X, Luo W, Tan X, Li Q, Zhao Y, Zhong W, Sun X, Brouwer C, Zhou Z. Increased plasma corticosterone contributes to the development of alcoholic fatty liver in mice. Am J Physiol Gastrointest Liver Physiol. 2013;305:G849–G861. doi: 10.1152/ajpgi.00139.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svensson PA, Asea A, Englund MC, Bausero MA, Jernas M, Wiklund O, Ohlsson BG, Carlsson LM, Carlsson B. Major role of HSP70 as a paracrine inducer of cytokine production in human oxidized LDL treated macrophages. Atherosclerosis. 2006;185:32–38. doi: 10.1016/j.atherosclerosis.2005.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka K, Tanaka Y, Namba T, Azuma A, Mizushima T. Heat shock protein 70 protects against bleomycin-induced pulmonary fibrosis in mice. Biochem Pharmacol. 2010;80:920–931. doi: 10.1016/j.bcp.2010.05.025. [DOI] [PubMed] [Google Scholar]
- Tomcik M, Zerr P, Pitkowski J, Palumbo-Zerr K, Avouac J, Distler O, Becvar R, Senolt L, Schett G, Distler JH. Heat shock protein 90 (Hsp90) inhibition targets canonical TGF-beta signalling to prevent fibrosis. Ann Rheum Dis. 2014;73:1215–1222. doi: 10.1136/annrheumdis-2012-203095. [DOI] [PubMed] [Google Scholar]
- Wang X, Zhao BS, Roundtree IA, Lu Z, Han D, Ma H, Weng X, Chen K, Shi H, He C. N(6)-methyladenosine Modulates Messenger RNA Translation Efficiency. Cell. 2015;161:1388–1399. doi: 10.1016/j.cell.2015.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitley D, Goldberg SP, Jordan WD. Heat shock proteins: a review of the molecular chaperones. J Vasc Surg. 1999;29:748–751. doi: 10.1016/s0741-5214(99)70329-0. [DOI] [PubMed] [Google Scholar]
- Xiao W, Adhikari S, Dahal U, Chen YS, Hao YJ, Sun BF, Sun HY, Li A, Ping XL, Lai WY, et al. Nuclear m(6)A reader YTHDC1 regulates mRNA splicing. Mol Cell. 2016;61:507–519. doi: 10.1016/j.molcel.2016.01.012. [DOI] [PubMed] [Google Scholar]
- Younossi ZM, Koenig AB, Abdelatif D, Fazel Y, Henry L, Wymer M. Global epidemiology of nonalcoholic fatty liver disease-meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology. 2016;64:73–84. doi: 10.1002/hep.28431. [DOI] [PubMed] [Google Scholar]
- Yu J, Li Y, Wang T, Zhong X. Modification of N6-methyladenosine RNA methylation on heat shock protein expression. PloS one. 2018;13:e0198604. doi: 10.1371/journal.pone.0198604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yue Y, Liu J, He C. RNA N6-methyladenosine methylation in post-transcriptional gene expression regulation. Genes Dev. 2015;29:1343–1355. doi: 10.1101/gad.262766.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng G, Dahl JA, Niu Y, Fedorcsak P, Huang CM, Li CJ, Vagbo CB, Shi Y, Wang WL, Song SH, et al. ALKBH5 is a mammalian RNA demethylase that impacts RNA metabolism and mouse fertility. Mol Cell. 2013;49:18–29. doi: 10.1016/j.molcel.2012.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou J, Wan J, Gao X, Zhang X, Jaffrey SR, Qian SB. Dynamic m(6)A mRNA methylation directs translational control of heat shock response. Nature. 2015;526:591–594. doi: 10.1038/nature15377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zulkifli I, Najafi P, Nurfarahin AJ, Soleimani AF, Kumari S, Aryani AA, O'Reilly EL, Eckersall PD. Acute phase proteins, interleukin 6, and heat shock protein 70 in broiler chickens administered with corticosterone. Poult Sci. 2014;93:3112–3118. doi: 10.3382/ps.2014-04099. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX 29 kb)