Abstract
Heat acclimation (HA) in humans promotes thermoregulatory adaptations that support management of core temperature in hot environments and reduces the likelihood of heat related illness. Another adaptation to HA is thermotolerance through induction of the heat shock protein (HSP) stress system, which provides protection against thermal insult. However, whether or not HA leads to upregulation of the intracellular HSP system, namely intracellular HSP70 (HSP70), is unclear in humans. Therefore, the purposes of this meta-analysis were to determine if HA leads to HSP70 induction among humans and to evaluate how methodological differences among HA studies influence findings regarding HA-induced HSP70 accumulation. Several databases were searched to identify studies that measured HSP70 (protein and mRNA) changes in response to HA among humans. The effect of HA on HSP70 was analyzed. Differences in the effect of HA were assessed between protein and mRNA. The moderating effect of several independent variables (HA frequency, HA duration, core temperature, exercise intensity) on HSP70 was also evaluated. Data were extracted from 12 studies including 118 participants (mean age 24 years, 98% male). There was a significant effect of HA on HSP70 expression, g = 0.97 (95% CI, 0.08–1.89). The effect of HA was different between subgroups (protein vs. mRNA), g = 1.51 (95% CI, 0.71–2.31), and g = − 0.39 (95% CI, − 1.36), respectively. The frequency of HA (in days) moderated HSP70 protein expression. There was a significant effect of heat acclimation on HSP70 induction in humans. The only factor among identified studies that may moderate this response was the frequency (number of days) of heat exposure.
Keywords: Heat Acclimation, Thermotolerance, Heat Illness, heat shock protein 70/72, HSP70
Introduction
Repeated elevations in core temperature resulting from either passive heat exposure (Beaudin et al. 2009) or facilitated by exercise in hot conditions (Nadel et al. 2017) induce various physiological adaptations collectively referred to as heat acclimation (HA) (Sawka et al. 2011) or heat acclimatization (HAC) (Robinson et al. 1943). HAC is the result of repeated exposure to natural environmental heat stress (Robinson et al. 1943) while HA is induced through manipulation of ambient conditions in a laboratory setting (Sawka et al. 2011). HA improves physical performance in hot conditions (Lorenzo et al. 2010) and reduces susceptibility to heat-related illnesses (see Sawka et al. 2011 for review) through improvements in thermoregulation (Lorenzo and Minson 2010) and a decrease in cardiovascular strain (Garrett et al. 2009, 2011; Gibson et al. 2015b; Fox et al. 2017). The thermoregulatory adaptations of HA include an earlier onset of cutaneous vasodilation, higher sweat rates, and increases in plasma volume (Taylor 2014). The latter is thought to decrease cardiovascular strain (evidenced by reductions in heart rate at a given workload) by maintaining stroke volume and consequently cardiac output during prolonged periods of sweat loss (Nielsen et al. 1993; Rowell et al. 2017).
Considering these improvements in thermoregulation, HA is recommended for those in various occupations (e.g., soldiers, field workers) and competitive athletes that perform strenuous exercise in hot environments in order to decrease the incidence of heat-related illness such as heat syncope and exertional heat stroke (Carter et al. 2005; Garrett et al. 2011). Exertional heat stroke is a life-threatening condition resulting from severe hyperthermia (core temperature ≥ 40 °C) and is characterized by impaired central nervous system function and organ damage (Leon and Bouchama 2015). The incidence of exertional heat stroke among firefighters, military personnel, and endurance athletes has been well documented, and safety guidelines have been established to prevent its occurrence (Carter et al. 2005; Casa et al. 2005; Murakoshi and Sekine 2012; Armed Forces Health Surveillance Branch 2019). The decline in mortality related to heat stroke among US military members has been attributed to the implementation of heat acclimation protocols by the US military (Carter et al. 2005). Despite the decline in mortality, the number of heat stroke-related hospitalizations has increased, suggesting that current countermeasures reduce fatalities but not the incidence of heat stroke. This report highlights the importance of heat acclimation for preventing heat illness in workers who are frequently exposed to heat stress. The thermoregulatory adaptations to HA allow for the preservation of core temperature during environmental heat exposure; however, another key assimilation is thermotolerance, a phenomenon in which animals or cultured cells acutely exposed to nonlethal heat stress survive a subsequent, otherwise lethal heat stress dose (Amorim et al. 2015).
The accumulation of the heat stress-inducible chaperone, heat shock protein 72 (HSP72/70) within cells (intracellular HSP70; hereafter referred to as HSP70), is a hallmark of thermotolerance in animal and cell models and protects against cell death via proteasomal maintenance (Beckham et al. 2008). Interestingly, HA has been found to increase basal HSP70 levels in humans (Yamada et al. 2007; Magalhães et al. 2010; Amorim et al. 2011; Gibson et al. 2015b). There is also evidence to suggest that heat acclimation influences levels of extracellular HSP70 (Sandström et al. 2008), though this response is not well established. Both intracellular and extracellular HSP70 play important, yet different roles in the body’s response to stress. An increase in extracellular HSP70 acts as a “warning signal,” stimulating an immune response by triggering the release of proinflammatory cytokines (Campisi et al. 2003). In contrast, HSP70 suppresses inflammatory signaling (Chen et al. 2005) and maintains cell integrity by refolding damaged proteins and preventing protein aggregation (Bittencourt and Porto 2017). Thus, HSP70 plays an important role in mediating cell damage following heat stress by maintaining protein integrity during hyperthermia and refolding denatured proteins to their native states (Parsell and Lindquist 1993; Craig et al. 1994). The protective role of HSP70 in response to heat stress is exemplified by the finding that supplementation with glutamine, an HSP70 activator, prevents endotoxin leakage from the small intestine following exercise in hot conditions (Zuhl et al. 2015). Protection of intestinal cells from heat damage results from HSP-70-mediated maintenance of occludin, a protein integral to tight junctions (Dokladny et al. 2006a) and suppression of proinflammatory cytokines that damage the small intestine (Malago et al. 2002; Papamichael and Tiligada 2008). Increases in HSP70 within peripheral blood mononuclear cells (PBMCs) following glutamine supplementation are similar to those shown after HA, suggesting that HA may confer thermotolerance in humans and thus prevent the release of inflammatory agents and suppresses pro-inflammatory signaling. Therefore, HA may prevent heat illness in occupational and competitive athletes not only through improvements in thermoregulation but also increasing the responsiveness of the protective intracellular heat shock protein system.
For obvious reasons, direct investigation of thermotolerance in humans is unfeasible, and it is important to mention that cellular HSP70 induction is a cellular adaption and may not reflect systemic benefits of HA. However, Kuennen et al. (2011) showed that suppression of the heat shock protein response through supplementation with quercetin inhibited the cytoprotective effect of HA in healthy men. Furthermore, Xiao et al. (2003) showed differential levels of basal HSP70 between individuals susceptible to heat illness and those who were resistant to it. This indicates that human thermotolerance may be mediated through the upregulation of the HSP system and identifies a key attribute of heat acclimation for those exposed to harsh environments (e.g., soldiers, field workers, athletes). However, despite the apparent role of HSP70 in conferring thermotolerance in humans, its accumulation is not consistently found in HA studies. We speculate that these inconsistencies may be due to differences in HA protocols, which vary in number of days, time spent in the heat, and intensity of heat stress. Further, detection of changes in HSP70 may be influenced by the cell type being studied and between measurements of protein or mRNA expression. Given the invasive nature of HA studies, investigations into the effect of HA on HSP70 are often conducted on few individuals and without nonexercise and exercise-only controls, making it difficult to determine whether findings are truly representative. Therefore, the purposes of this meta-analysis were to determine if HA leads to HSP70 induction among humans and to evaluate how methodological differences among HA studies influence findings regarding HA-induced HSP70 accumulation. The rationale to only study HSP70 induction rather than extracellular HSP70 in humans was based on the logic that upregulation of the HSP70 family is a proposed mechanism that explains the benefits of heat acclimation-mediated thermotolerance among workers and athletes (Périard et al. 2015). If so, efforts should be made to evaluate HA studies to determine methodological factors (e.g., heat exposure, core temperature) that facilitate the HSP response. In doing so, researchers, coaches, and trainers may implement more effective HA protocols to achieve the desired benefits.
Methods
Search strategy and selection criteria
A systemic literature review was performed following the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement. PubMed, ScienceDirect, Cochrane Library, and Google Scholar were searched using the key words “heat acclimation,” ‘heat shock protein,” “HSP,” and “HSP70/72” in various combinations. Studies published between 1984 and 2019 were included in the original search. In addition, reference lists of original and review articles were analyzed manually for studies not identified in the original search. After initial screening of titles and abstracts, studies were assessed for inclusion and quality for meta-analysis (Fig. 1).
Fig. 1.
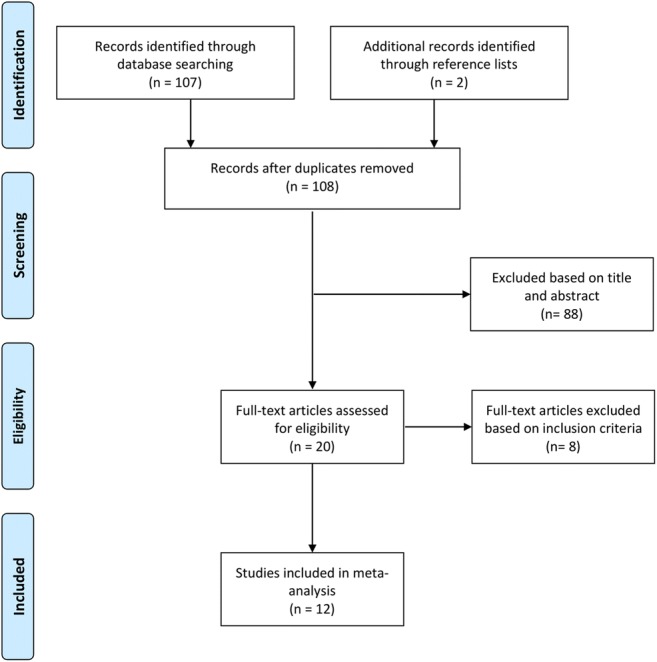
Flow diagram of literature search according to Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA)
Studies were included if they met the following criteria: (1) measured intracellular (leukocytes or skeletal muscle) HSP70/72 protein or mRNA expression and (2) human participants completed a heat acclimation protocol (either consecutive or nonconsecutive days). Exclusion criteria included (1) no post-PBMC HSP72 mRNA and post-heat acclimation measurement for HSP70 was performed and (2) no specific description of the heat acclimation protocol.
Data extraction and outcome assessment
Data were extracted from 14 studies. Two reviewers independently reviewed and extracted data. The primary outcome was HSP70 induction in skeletal muscle or immune cells from pre to post-heat acclimation among healthy men and women. For each study, participant characteristics (sample size, age), study characteristics (publication year), mean core temperature, HSP70 change (both protein and mRNA measures), and heat acclimation protocol (length, duration, environment, exercise intensity) were recorded.
The expression of HSP70 protein or mRNA was reported for each study by inputting the pre-heat acclimation and post heat acclimation values. In 10 studies, HSP70 was expressed as fold change from pre-heat acclimation, and 2 studies reported absolute values. Values for HSP70 not reported in the main text were extracted from representative figures using Plot Digitizer (http://plotdigitizer.sourceforge.net/).
The frequency of each heat acclimation protocol was reported in number of days. Total duration for each study was calculated by multiplying the number of heat acclimation days by the minutes of each exercise session and recorded in minutes. Average exercise intensity was primarily reported as a percentage of peak oxygen consumption (%VO2peak). There were differences between articles in the way maximal aerobic capacity was identified and reported. Some authors reported VO2peak (Yamada et al. 2007; Marshall et al. 2007; Watkins et al. 2008; Magalhães et al. 2010; Amorim et al. 2011; Gibson et al. 2015a, b; Lee et al. 2015, 2016) while others reported VO2max (Yamada et al. 2007; Kuennen et al. 2011; Hom et al. 2012). However, those that reported exercise intensity as %VO2max either did not report VO2max attainment criteria (Yamada et al. 2007) or used the attainment of VO2 plateau (change of < 150 ml/min in VO2 despite an increase in workload) and/or secondary criteria (achievement of > 90–95% of age-predicted maximal heart rate and respiratory exchange ratio > 1.1). Though there is some debate whether these secondary criteria can be used interchangeably with VO2 plateau to identify the attainment of VO2max, it was decided that in the absence of strict VO2max criteria using only VO2 plateau, the term VO2peak would be used to describe exercise intensities for all studies. However, whether the authors reported exercise intensity as a percentage of VO2peak or VO2max is specified in Table 1.
Table 1.
Article information and study characteristics of 12 heat acclimation studies included in the meta-analysis
| Author and year | Sample size | Population | HA protocol | Control | Outcome measure |
|---|---|---|---|---|---|
| Amorim (2011) | 9 total (7 male, 2 female) | Physically active (age 25 ± 3) | 10 sessions within 14 day period. 100 min/session (42 °C; 30% RH; WB:25.9 ± 0.4°), 56% VO2peak | NA | Pre-/post-PBMC HSP72 protein |
| Gibson (2015a) (Scan Med Sci) | 24 males | Physically active (age 26 ± 5) |
Group 1: 10 sessions, 90 min (40 °C; 39 ± 7.8%RH), 50% VO2peak (n = 8). Group 2: 10 sessions, 90 min (40 °C; 39 ± 7.8% RH), 65% VO2peak, controlled core temp at 38.5–39 °C (n = 8) |
NA | Pre-/post-leukocyte HSP72 mRNA. |
| Gibson (2015b) (JAP) | 16 males | Physically active (age 22 ± 4) | 10 sessions, 90 min (40 °C; 40% RH), 65% VO2peak (n = 8), controlled core temp ≥ 38.5 | Exercise matched control, 20 °C (n = 8) | Pre-/post-leukocyte HSP72 mRNA. |
| Hom (2012) | 11 males | Healthy (20 ± 1) | 11 sessions, 90 min (33 °C; 30–50% RH), 50% VO2max | NA | Pre-/post-monocyte HSP72 protein |
| Kuennen (2011) | 8 men | Healthy (28 ± 1) | 7 sessions, 100 min (46.5 °C; 20% RH) | NA | Pre-/post-PBMC HSP70 protein |
| Lee (2015) | 16 males | Healthy (22 ± 4) | 3 sessions, 60 min (40 °C; 20% RH), 50% VO2peak (n = 8) | Exercise matched control, 20 °C (n = 8) | Pre-/post-monocyte HSP72 protein |
| Lee (2016) | 7 males | Physically fit (25 ± 5) | 10 session, 60 min (40 °C; 25% RH), 50% VO2peak | NA | Pre-/post-monocyte HSP72 protein |
| Magalhães (2010) | 9 males | Healthy (25 ± 1) | 11 sessions, 60 min (40 °C; 45% RH). | NA | Pre-/post-leukocytes HSP72 protein |
| Marshall (2007) | 7 males | Healthy (30 ± 4) | 3 sessions, 120 min (38 °C; 60% RH) 38% VO2peak | NA |
Pre-/post-PBMC HSP72 mRNA Pre-/post-PBMC HSP72 protein |
| McClung (2007) | 6 total (male and female numbers not reported) | Healthy soldiers (23) | 10 sessions, 100 min (49 °C; 20% RH) | NA | Pre-/post-PBMC HSP72 |
| Watkins (2008) | 10 males | Physically active (23 ± 3) | 7 sessions, 30 min (39.5 °C; 27% RH), 75% VO2peak | NA | Pre-/post-skeletal muscle HSP72 |
| Yamada (2007) | 12 total (10 males, 2 females) | Healthy (24 ± 4) | 10 sessions, 100 min (42.5°; 27.9% RH; WB: 25.9 ± 0.4°), 56% VO2max | NA | Pre-/post-PBMC HSP72 |
RH: relative humidity, PBMC: peripheral blood mononuclear cells, VO2peak: peak oxygen consumption, VO2max: maximal oxygen consumption
Bias and limitations
A bias assessment was performed using the Cochrane Collaboration’s tool for assessing risk of bias (Higgins et al. 2011). We assessed the included studies for selection bias (sequence generation and allocation sequence concealment), performance bias (blinding of participants), detection bias (blinding of outcome assessors), attrition bias (incomplete outcome data), reporting bias (selective outcome reporting), and other biases.
Statistical analysis
Using a random-effects model, a meta-analysis was conducted using Meta-Essentials for Microsoft Excel (Van Rhee et al. 2018). Effect size for change in HSP70 was determined as mean difference of pre- and post-heat acclimation divided by pooled standard deviation. Each mean effect size was calculated as a weighted mean difference with 95% CIs. As the difference in pre- and post-heat acclimation means reflect within-subject effects, Hedges’ g was adjusted to account for the dependence between scores. For lack of correlation coefficients for included studies, a conservative estimate of r = 0.7 was used, as recommended by Metcalfe and Rosenthal (2006). A combined effect size was then calculated (Hedges’ g), weighting studies using a random effects model consistent with the pre–post-analysis. One subgroup analysis was performed to determine the difference in effect of heat acclimation on HSP70 protein versus HSP mRNA expression. Comparison was attempted within the cell type subgroup (PBMC vs. skeletal muscle), but only one study reported HSP70 expression in skeletal muscle (Watkins et al. 2008); therefore, the analysis could not be completed. Meta-regression analysis was performed using total duration of heat acclimation (minutes), protocol frequency (days), and exercise intensity (%VO2peak) as moderators (i.e., independent variables) with the change in HSP70 protein expression. The moderating effect of average core temperature (°C) was attempted but could not be completed because only one study measuring HSP70 reported average core temperature during heat acclimation (ref). Heterogeneity was assessed using the I2 test and was used for significant heterogeneity (I2 > 50%). Meta-regression analysis was performed within each subgroup independently using study duration (in weeks) as a moderator (i.e., independent variable). Statistical significance was set at p < 0.05.
Results
Literature search and publication Bias
A total of 12 studies involving 118 participants were included in the review (Fig. 1). The study characteristics are summarized in Table 1. The mean age was 24 ± 2 years, and 98% of subjects were males. Average heat acclimation protocol frequency was 8 ± 2 days, and duration per session was 83 ± 24 min. Ten studies reported HSP70 protein change while two reported HSP70 mRNA (one study reported both). Nine studies reported an average exercise intensity of 52 ± 11% of VO2peak, and among the HSP70 protein expression studies, seven reported exercise intensity.
Overall, the inferences from the included studies may be undermined by methodological flaws, and resulted in an over- or underestimation (e.g., bias) of the effect. Only two studies (Gibson et al. 2015b; Lee et al. 2015) included an exercise match control and randomization techniques were not detailed. Allocation concealment could not be assessed based on lack of control groups across the majority of studies (n = 10). Due to the nature of the exercise intervention, the participants and researchers were unblinded to the intervention in all the included studies; however, it is unlikely that the lack of blinding influenced the outcomes. There was no blinding of the outcome assessment (HSP70 expression), but again, it is unlikely that this caused bias. All included studies reported primary outcome, but two did not report attrition (McClung et al. 2007; Watkins et al. 2008). In addition, risk for selective reporting was found as several studies did not include expected key outcomes (e.g., mean core temperature; exercise intensity). We also found significant other bias due to small sample size, and only two studies included female participants (Yamada et al. 2007; Amorim et al. 2011). The likelihood of publication bias was also assessed using Egger regression test (p = 0.024).
The effect of heat acclimation on HSP70
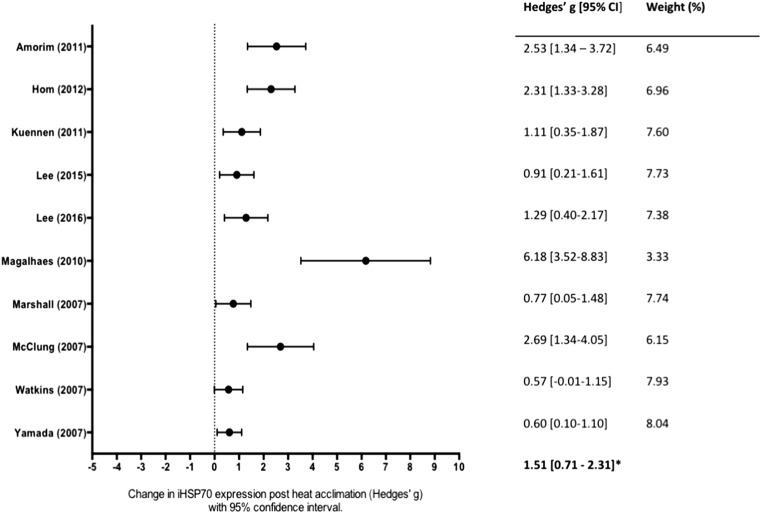
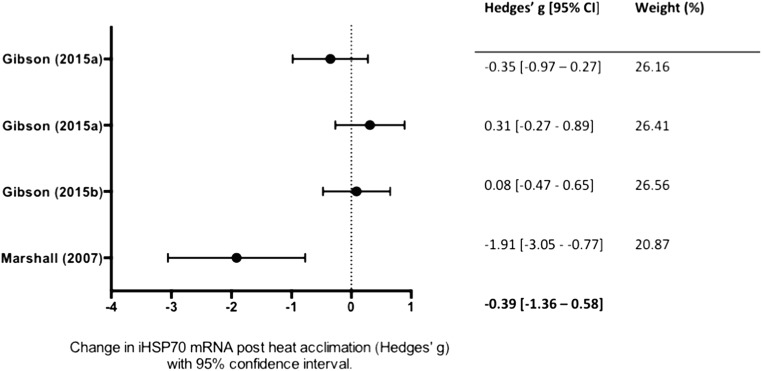
All studies reported both pre and post-heat acclimation HSP70 values. A total of fourteen mean, weighted ESs were derived from twelve studies. Ten studies measured HSP70 protein expression (Fig. 2), and four studies measured HSP70 mRNA (Fig. 3). Overall, heat acclimation induced a significant increase HSP70 (g = 0.97; 95% CI, 0.08–1.86; p = 0.018, combined data not shown). Significant heterogeneity (I2 = 89%) was identified, and reflects differences identified in samples.
Fig. 2.
Forest plot of studies investigating the effect of heat acclimation on intracellular heat shock protein 70/72 (HSP70) protein expression in humans. Effect sizes calculated from weighted means are listed on the right. The combined effect size is listed on the bottom right. Overall, heat acclimation induced a significant increase in HSP70 protein expression (p < 0.05). CI confidence interval
Fig. 3.
Forest plot of studies investigating the effect of heat acclimation on intracellular heat shock protein 70/72 (HSP70) mRNA. Effect sizes calculated from weighted means are listed on the right. The combined effect size is listed on the bottom right. There was no overall significant effect of heat acclimation on HSP70 mRNA
Subgroup analysis of expression
A subgroup analysis was performed to determine if the effect heat acclimation was different between HSP70 protein and mRNA. There was a significant difference in effect size between studies that measured mRNA, Hedges’ g = − 0.39 (95% CI, − 1.36–0.58, k = 3; Fig. 3) and studies that measured protein, Hedges’ g = 1.51 (95% CI, 0.71–2.31, k = 10; Fig. 2). The protein group demonstrated high heterogeneity (I2 = 80), while the mRNA group was homogenous (I2 = 0%).
Moderator analyses
Meta-regression analyses suggested that a greater number of heat acclimation days (frequency) was associated with a larger improvement in HSP70 protein (N = 10, n = 88, Z = 2.23, p = 0.02). Total duration (in minutes) of heat exposure trended with HSP70 protein expression but was nonsignificant (N = 10, n = 88, Z = 1.76, p = 0.07). Average exercise intensity during heat acclimation was not associated with HSP70 protein expression (Fig. 4).
Fig. 4.
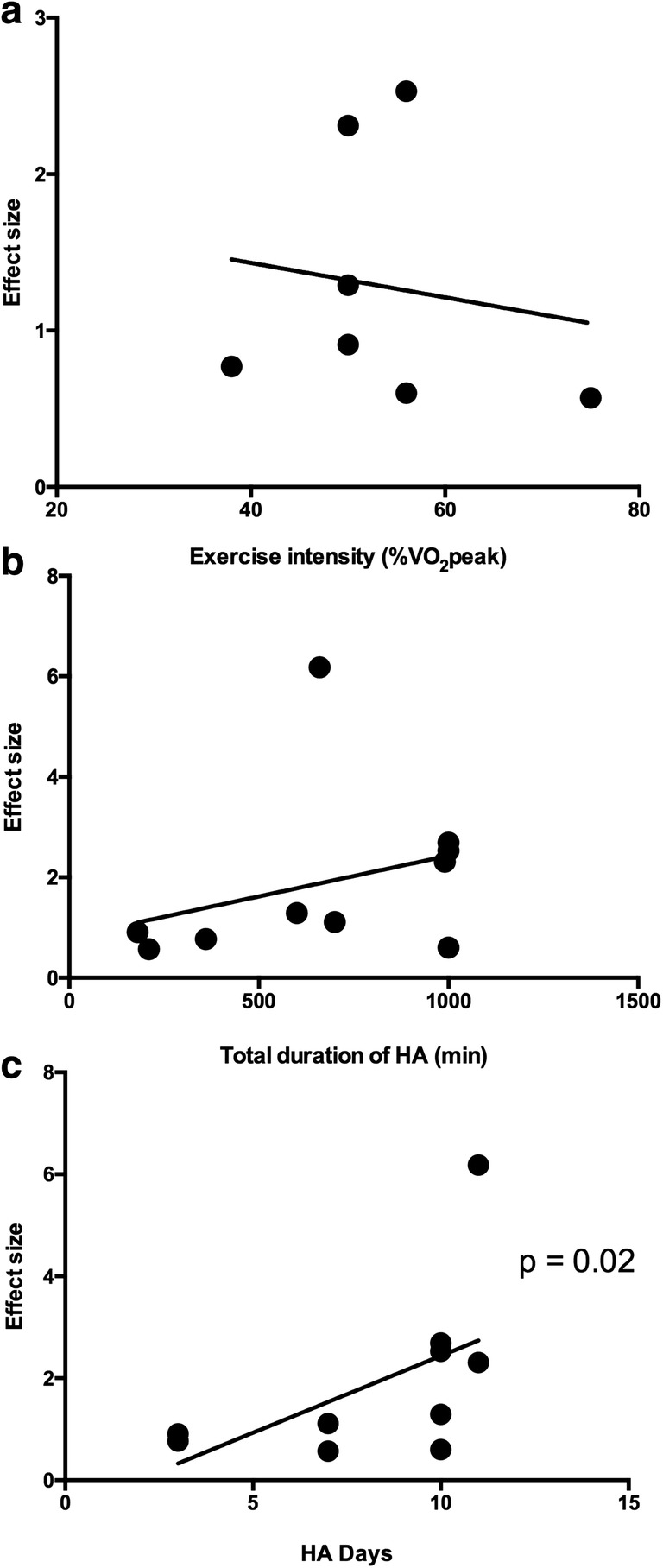
Moderating effects of exercise intensity (a), total duration of heat acclimation (HA) protocol in minutes (b), and number of heat acclimation days (c) on intracellular heat shock protein 70/72 (HSP70) protein expression. Meta-regression analysis suggested a significant association between the number of HA days and HSP70 expression (p < 0.05). Total duration and exercise intensity were not significantly associated with HSP70 expression (p > 0.05)
Discussion
The purpose of this meta-analysis was to determine the effect of heat acclimation on HSP70 protein and mRNA expression as well as the methodological variables that contribute to differences in the heat shock response among heat acclimation studies. According to these data, heat acclimation has a large and significantly positive effect on HSP70 (protein and mRNA combined) expression (Hedges’ g = 0.97). This increase is not reflected by HSP70 mRNA levels as these appear to decrease following heat acclimation (Hedges’ g = − 0.39). Increases in HSP70 protein expression following heat acclimation are moderated by the number of heat acclimation days and possibly the total duration of exposure, although the moderating effect of duration did not reach statistical significance (p = 0.07). Exercise intensity, however, did not appear contribute to heat acclimation-induced changes in HSP70 protein expression.
The large, positive effect of heat acclimation on HSP70 protein expression was consistent across most included studies, as effect sizes calculated from the results of all but two articles (Yamada et al. 2007; Watkins et al. 2008) were greater than 0.8. These heat acclimation-induced increases in human HSP70 protein substantiate those shown in cell (Li 1985; Laszlo 1988; Koishi et al. 1992; Dokladny et al. 2006b) and animal models (Maloyan and Horowitz 2002; Lee et al. 2006) in which the heat shock protein response may be necessary for acquired thermotolerance. The role of HSP70 in conferring resistance to heat illness in humans is perhaps best illustrated by the findings of Kuennen et al. (2011), who showed that inhibition of the heat shock response with quercetin during 7 days of heat acclimation resulted in increased gastrointestinal permeability and endotoxin leakage following acute heat stress (Kuennen et al. 2011). These markers of heat injury remained unchanged in the placebo group, suggesting that the heat shock response may be necessary for heat acclimation-mediated thermotolerance against gastrointestinal damage and cytokine production (Kuennen et al. 2011). This finding supports those displayed in transgenic animal models in which HSP70 null mice have lower survival rates than controls while overexpression of HSP70 increases survival rates above control mice (Lee et al. 2006). While the role of HSP70 is dependent on cell type, its role in protecting against exertional heat stroke via thermotolerance is associated with its regulation of inflammation (Dokladny et al. 2010) and protection against intestinal epithelial injury, both of which are characteristic of exertional heat stroke (Dokladny et al. 2006b; Armstrong et al. 2007).
The HSP70 system serves an immunoregulatory function by modulating the release of pro-inflammatory agents. HSP70 suppresses leukocyte release of inflammatory cytokines such as interleukin-6 (IL-6) and tumor necrosis factor alpha (TNF-α) through activation of IκB-α and inhibition of NF-κB in rats (Dokladny et al. 2010). Inducing HSP70 expression in humans via glutamine supplementation also increases IκB-α expression and suppressed inflammatory signaling in humans while also blunting the increase in gastrointestinal permeability otherwise shown following running (Zuhl et al. 2015) and simulated firefighting (Nava et al. 2019) in hot conditions. These findings highlight the protective role of HSP70 for workers and athletes exposed to heat stress. However, it is important to mention that the decrease in gastrointestinal permeability by HSP70 is attributed to maintenance of tight junction integrity by HSP70 in the small intestine (Zuhl et al. 2014), whereas the increased HSP70 reported in eleven of the twelve studies included in this analysis were measured in immune cells. While it is impossible to determine whether heat acclimation improves tight junction permeability by increasing HSP70 levels in human epithelium without invasive sampling of intestinal tissues, we previously showed that upregulation of HSP70 in human epithelial cells (Caco-2) increased occludin levels (a protein involved in tight junction integrity) (Zuhl et al. 2013) and decreased permeability in vivo (Dokladny et al. 2006a). These findings suggest that heat acclimation-induced increases in HSP70 may not be limited to immune cells but rather protect against heat illness in several cell types and at various stages of inflammatory response to heat stress. Again, this interpretation must be made with caution as the bulk of human research resides within circulation leukocytes (i.e., peripheral blood mononuclear cells).
Because expression of HSP70 has been reported to increase following heat stress in various cell types, we were interested in whether heat acclimation-induced HSP70 differed between immune cells and skeletal muscle. Unfortunately, only one included study (Watkins et al. 2008) measured the HSP70 protein in skeletal muscle while all others studied monocytes and thus this subgroup analysis was not possible.
The results from the subgroup analysis of HSP70 between mRNA and protein measurements suggest that the accumulation of HSP70 protein following heat acclimation may not be reflected by HSP70 mRNA levels. This discrepancy between protein and mRNA results is not surprising, as accumulation of HSP70 protein likely activates a negative feedback mechanism that inhibits further transcriptional activation of HSP70 mRNA. The induction of the heat shock response is regulated by activation of heat shock factor-1 (HSF1), a cytosolic-housed transcriptional factor (Morimoto 1998). Under normal conditions (nonstress), HSF1 is bound to several HSP chaperones within the cell cytoplasm (Zou et al. 1998). Upon exposure to heat stress, the chaperone complex dissociates from HSF1, allowing its trimerization, nuclear accumulation, and interaction with heat shock elements of various inducible HSPs such as HSP70 (Anckar and Sistonen 2011). Once the refolding requirements of HSPs are fulfilled, the HSP70 protein (and other HSP chaperones) interacts with HSF-1 in a manner that suppresses its trans-activating capacity and thus further HSP transcription is inhibited (Shi et al. 1998; Gómez et al. 2008). Because the increases in HSP70 protein expression reported here were measured at rest and therefore in the absence of immediate heat stress, the accumulation of HSP70 from the prior days of heat exposure likely inhibited further transcriptional activation of HSP70. Although HSF-1 was not measured in the included studies, the lack of change in HSP70 mRNA may be evidence of this negative feedback mechanism. Considering these data, researchers interested in the role of heat acclimation of HSP70 should consider measuring HSP70 protein expression as these measurements represent functional changes to cell function. Because only 3 studies (four effect sizes) reported mRNA data, researchers should also consider measuring HSP70 mRNA before and after HA protocols to further elucidate how HA may influence transcriptional regulation of the heat shock response.
The moderating effect of frequency on the degree of HSP70 expression is an interesting finding as short-term heat acclimation protocols have recently gained popularity among researchers. While HSP70 has been reported to be elevated up to 7 days following an acute bout of exercise in skeletal muscle (Morton et al. 2006), the time course of HSP70 induction and decay in immune cells is unknown. Here, it appears that short-term heat acclimation (3 days) has a large effect on HSP70 although not to the same degree as longer protocols (7–10 days).
It has been suggested that the physiological adaptations induced by HA are partially dependent on the exercise intensity employed (Périard et al. 2015). For example, Houmard et al. (1990) showed that a moderate intensity, short-duration HA protocol (35 min/day at 75% VO2peak) elicited similar reductions in heart rate and core temperature as a low intensity, long duration HA protocol (60 min/day at 50% VO2peak) where the number of days and environmental conditions remained equal. Despite the potential influence of exercise intensity on these physiological parameters, the results from the present analysis did not show a moderating effect of exercise intensity on HA-induced HSP70 protein. Although there were differences in exercise intensity between the studies included, the variability among the 9 studies that reported exercise intensity may be considered low (52 ± 11% of VO2peak). This finding, along with the moderating effect of number of days and potential moderating effect of exercise duration, may suggest that the induction and accumulation of HSP70 protein as a result of HA may depend on the length of time and number of days that core temperature is elevated rather than exercise intensity per se. It is worth mentioning that some authors reported VO2peak while others reported VO2max. The attainment of VO2max versus VO2peak could theoretically influence the absolute exercise intensity prescribed to individual participants within each study. However, we believe this difference would be negligible considering VO2peak values are statistically similar to VO2max values (Day et al. 2003).
Although the effect of core temperature on HA-induced HSP70 could not be included in this analysis due to the lack of available data, the greatest increase in HSP70, reported by Magalhães et al. (2010), was shown following a controlled hyperthermia protocol where the core temperature was guaranteed to remain ≥ 1 °C above baseline for a minimum of 30 min during each HA session. Two other groups (Kuennen et al. 2011; Gibson et al. 2015a) also employed similar controlled hyperthermia protocols but required fewer sessions than Magalhães’ group, which is consistent with the significant effect of the number of HA days on HSP70 reported here. From this perspective, the exercise intensity of a HA session may be arbitrary as long as it elicits a sustained increase in core temperature. If the induction of the heat shock response during HA is indeed dependent on these sustained and repeated increases in core temperature, perhaps exercise is not necessary, but rather facilitates a rise in core temperature. Furthermore, acute exercise alone has been shown to increase HSP70 in human skeletal muscle (Tupling et al. 2007) and luekocytes (Shin et al. 2004) and thus may independently contribute to the induction of the heat shock response during HA. Interestingly, Beaudin et al. (2009) and Brazaitis and Skurvydas (2010) showed that HA (confirmed by a decrease in resting and exercising core temperature) could be attained using passive heating protocols. Though there is no available evidence regarding passive HA-induced HSP70 in humans, passive HA increases HSP70 ubiquitously in rat tissues (Maloyan et al. 1999; Maloyan and Horowitz 2002; Sareh et al. 2011). Future researchers could isolate the effect of HA in humans on HSP70 by inducing increases in core temperature by only manipulating ambient temperature and humidity. This would elucidate whether the passive heat acclimation protocols employed in animal models have similar effects on the heat shock response in humans. Ideally, such experiments would be coupled with existing HA protocols and exercise-only controls to further determine that the relative contribution of each factor to HA and acquired thermotolerance.
There are number of potential limitations. First, there were limited studies that measured HSP70 expression in response to exercise-induced heat acclimation (n = 12). Due to the small number of studies identified, all results should be interpreted with caution. Second, only two (Gibson et al. 2015b; Lee et al. 2015) studies compared heat acclimation to a thermoneutral control group, and therefore, the results reported herein are from a single-arm analysis. Third, there was evidence of high levels of statistical heterogeneity (I2 = 80%) within several analyses among the included trials. This may be explained by heat acclimation protocols chosen in the included studies (e.g., duration, frequency, exercise intensity). Only the mRNA subgroup reported low heterogeneity (I2 = 0%), but only three studies were included. Fourth, the studies were moderately biased based on lack of randomization, allocation concealment, and selected reporting. Lastly, it is important to mention that the heat acclimation-induced increases in HSP70 protein levels described in these articles were found in immune cells, whereas the process of heat acclimation involves a host of systemic adaptations to heat exposure (e.g., decreased resting and exertion core temperature, earlier onset of sweating, and improved electrolyte balance) that may not depend on the upregulation of HSP70. Instead, increases in HSP70 within immune cells may indicate an increase in cellular tolerance to cellular stress and damage. Specifically, increases in HSP70 in immune cells correspond with decreases in hyperthermia-related gastrointestinal cell damage (Dokladny et al. 2010; Kuennen et al. 2011; Zuhl et al. 2015), which may prevent endotoxemia and suppresses inflammation following heat stress. However, it is important to interpret heat-acclimation mediated increases in HSP70 protein levels with caution as these only reflect changes within immune cells and not necessarily at the organismal level.
This study also highlights several emphasis areas for future research efforts. Only two studies included female participants, and when combining both studies, the total number of female participants was 2. Therefore, little is known about the differing HSP70 signaling in response to HA between men and women. The average age of research participants was ~ 25 and reveals a lack of effort placed on understanding the effect of HA among older humans. Also, the average core temperature for heat acclimation sessions was only reported in three studies. This information would provide insight into whether or not a daily core temperature threshold during acclimation is required to promote an HSP70 response. Further and surprisingly, HSP70 regulation in human skeletal muscle in response to heat acclimation is limited (only one study identified). Taken together, these factors highlight gaps in the literature related to HSP70 induction in response to HA. It is the opinion of the authors that initial steps should be made to develop or test already establish acclimation protocols using thorough measurement techniques (e.g., measuring and reporting core temperature, exercise intensity, and environmental conditions). Achieving consistent results from a standard protocol will then allow researchers to study differing HSP70 regulation between genders and age of participants.
In summary, there was a significant effect of heat acclimation on HSP70 induction in humans, although this appears specific to HSP70 protein expression and not mRNA expression. This finding is consistent with the transcriptional regulation of HSP70. The only factor among identified studies that may moderate this response was the frequency (number of days) of heat exposure. Though average core temperature data for individual heat acclimation sessions was not reported in all studies, it is possible that the accumulation of HSP70 during the HA is dependent on sustained and repeated elevations in core temperature. Identifying such threshold will require more detailed reporting of core temperature changes during individual HA session and will be useful to researchers interested in human thermotolerance.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Amorim F, Yamada P, Robergs R, Schneider S, Moseley P. Effects of whole-body heat acclimation on cell injury and cytokine responses in peripheral blood mononuclear cells. Eur J Appl Physiol. 2011;111:1609–1618. doi: 10.1007/s00421-010-1780-4. [DOI] [PubMed] [Google Scholar]
- Amorim Fabiano Trigueiro, Fonseca Ivana T, Machado-Moreira Christiano A, Magalhães Flávio de Castro. Insights into the role of heat shock protein 72 to whole-body heat acclimation in humans. Temperature. 2015;2(4):499–505. doi: 10.1080/23328940.2015.1110655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anckar Julius, Sistonen Lea. Regulation of HSF1 Function in the Heat Stress Response: Implications in Aging and Disease. Annual Review of Biochemistry. 2011;80(1):1089–1115. doi: 10.1146/annurev-biochem-060809-095203. [DOI] [PubMed] [Google Scholar]
- Armed Forces Health Surveillance Branch (2019) Update: heat illness, active component, U.S. Armed Forces, 2018. MSMR [PubMed]
- Armstrong Lawrence E., Casa Douglas J., Millard-Stafford Mindy, Moran Daniel S., Pyne Scott W., Roberts William O. Exertional Heat Illness during Training and Competition. Medicine & Science in Sports & Exercise. 2007;39(3):556–572. doi: 10.1249/MSS.0b013e31802fa199. [DOI] [PubMed] [Google Scholar]
- Beaudin Andrew E., Clegg Miriam E., Walsh Michael L., White Matthew D. Adaptation of exercise ventilation during an actively-induced hyperthermia following passive heat acclimation. American Journal of Physiology-Regulatory, Integrative and Comparative Physiology. 2009;297(3):R605–R614. doi: 10.1152/ajpregu.90672.2008. [DOI] [PubMed] [Google Scholar]
- Beckham Josh T., Wilmink Gerald J., Mackanos Mark A., Takahashi Keiko, Contag Chris H., Takahashi Takamune, Jansen E. Duco. Role of HSP70 in cellular thermotolerance. Lasers in Surgery and Medicine. 2008;40(10):704–715. doi: 10.1002/lsm.20713. [DOI] [PubMed] [Google Scholar]
- Bittencourt A, Porto RR (2017) eHSP70/iHSP70 and divergent functions on the challenge: effect of exercise and tissue specificity in response to stress. Clin Physiol Funct Imaging [DOI] [PubMed]
- Brazaitis M, Skurvydas A. Heat acclimation does not reduce the impact of hyperthermia on central fatigue. Eur J Appl Physiol. 2010;109:771–778. doi: 10.1007/s00421-010-1429-3. [DOI] [PubMed] [Google Scholar]
- Campisi J, Leem TH, Fleshner M (2003) Stress-induced extracellular Hsp72 is a functionally significant danger signal to the immune system. Cell Stress Chaperones. 10.1379/1466-1268(2003)008<0272:SEHIAF>2.0.CO;2 [DOI] [PMC free article] [PubMed]
- CARTER ROBERT, CHEUVRONT SAMUEL N., WILLIAMS JEFFREY O., KOLKA MARGARET A., STEPHENSON LOU A., SAWKA MICHAEL N., AMOROSO PAUL J. Epidemiology of Hospitalizations and Deaths from Heat Illness in Soldiers. Medicine & Science in Sports & Exercise. 2005;37(8):1338–1334. doi: 10.1249/01.mss.0000174895.19639.ed. [DOI] [PubMed] [Google Scholar]
- Casa Douglas J., Armstrong Lawrence E., Ganio Matthew S., Yeargin Susan W. Exertional Heat Stroke in Competitive Athletes. Current Sports Medicine Reports. 2005;4(6):309–317. doi: 10.1097/01.CSMR.0000306292.64954.da. [DOI] [PubMed] [Google Scholar]
- Chen Hsiang-Wen, Kuo Hung-Tien, Wang Shu-Jung, Lu Tzong-Shi, Yang Rei-Cheng. IN VIVO HEAT SHOCK PROTEIN ASSEMBLES WITH SEPTIC LIVER NF-??B/I-??B COMPLEX REGULATING NF-??B ACTIVITY. Shock. 2005;24(3):232–238. doi: 10.1097/01.shk.0000174020.87439.f2. [DOI] [PubMed] [Google Scholar]
- Craig EA, Weissman JS, Horwich AL (1994) Heat shock proteins and molecular chaperones: mediators of protein conformation and turnover in the cell. Cell [DOI] [PubMed]
- Day J. R., Rossiter H. B., Coats E. M., Skasick A., Whipp B. J. The maximally attainable V̇o2 during exercise in humans: the peak vs. maximum issue. Journal of Applied Physiology. 2003;95(5):1901–1907. doi: 10.1152/japplphysiol.00024.2003. [DOI] [PubMed] [Google Scholar]
- Dokladny Karol, Lobb Rebecca, Wharton Walker, Ma Thomas Y., Moseley Pope L. LPS-induced cytokine levels are repressed by elevated expression of HSP70 in rats: possible role of NF-κB. Cell Stress and Chaperones. 2009;15(2):153–163. doi: 10.1007/s12192-009-0129-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dokladny Karol, Moseley Pope L., Ma Thomas Y. Physiologically relevant increase in temperature causes an increase in intestinal epithelial tight junction permeability. American Journal of Physiology-Gastrointestinal and Liver Physiology. 2006;290(2):G204–G212. doi: 10.1152/ajpgi.00401.2005. [DOI] [PubMed] [Google Scholar]
- Dokladny Karol, Wharton Walker, Lobb Rebecca, Ma Thomas Y., Moseley Pope L. Induction of physiological thermotolerance in MDCK monolayers: contribution of heat shock protein 70. Cell Stress & Chaperones. 2006;11(3):268. doi: 10.1379/CSC-194R.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox R H, Goldsmith R, Hampton I F, Hunt T J. Heat acclimatization by controlled hyperthermia in hot-dry and hot-wet climates. Journal of Applied Physiology. 1967;22(1):39–46. doi: 10.1152/jappl.1967.22.1.39. [DOI] [PubMed] [Google Scholar]
- Garrett Andrew T., Goosens Niels G., Rehrer Nancy G., Patterson Mark J., Cotter James D. Induction and decay of short-term heat acclimation. European Journal of Applied Physiology. 2009;107(6):659–670. doi: 10.1007/s00421-009-1182-7. [DOI] [PubMed] [Google Scholar]
- Garrett AT, Rehrer NJ, Patterson MJ. Induction and decay of short-term heat acclimation in moderately and highly trained athletes. Med: Sport; 2011. [DOI] [PubMed] [Google Scholar]
- Gibson OR, Mee JA, Taylor L, et al. Isothermic and fixed-intensity heat acclimation methods elicit equal increases in Hsp72 mRNA. Scand J Med Sci Sports. 2015;25:259–268. doi: 10.1111/sms.12430. [DOI] [PubMed] [Google Scholar]
- Gibson OR, Turner G, Tuttle JA, Taylor L, Watt PW, Maxwell NS. Heat acclimation attenuates physiological strain and the HSP72, but not HSP90α, mRNA response to acute normobaric hypoxia. J Appl Physiol. 2015;119:889–899. doi: 10.1152/japplphysiol.00332.2015. [DOI] [PubMed] [Google Scholar]
- Gómez Andrea V., Galleguillos Danny, Maass Juan Cristóbal, Battaglioli Elena, Kukuljan Manuel, Andrés María Estela. CoREST Represses the Heat Shock Response Mediated by HSF1. Molecular Cell. 2008;31(2):222–231. doi: 10.1016/j.molcel.2008.06.015. [DOI] [PubMed] [Google Scholar]
- Higgins J. P. T., Altman D. G., Gotzsche P. C., Juni P., Moher D., Oxman A. D., Savovic J., Schulz K. F., Weeks L., Sterne J. A. C. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ. 2011;343(oct18 2):d5928–d5928. doi: 10.1136/bmj.d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hom LL, Lee ECH, Apicella JM, Wallace SD, Emmanuel H, Klau JF, Poh PY, Marzano S, Armstrong LE, Casa DJ, Maresh CM. Eleven days of moderate exercise and heat exposure induces acclimation without significant HSP70 and apoptosis responses of lymphocytes in college-aged males. Cell Stress Chaperones. 2012;17:29–39. doi: 10.1007/s12192-011-0283-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HOUMARD JOSEPH A., COSTILL DAVID L., DAVIS JACKIE A., MITCHELL JOEL B., PASCOE DAVID D., ROBERGS ROBERT A. The influence of exercise intensity on heat acclimation in trained subjects. Medicine & Science in Sports & Exercise. 1990;22(5):615–620. doi: 10.1249/00005768-199010000-00012. [DOI] [PubMed] [Google Scholar]
- Koishi Mototsugu, Hosokawa Nobuko, Sato Mamoru, Nakai Akira, Hirayoshi Kazunori, Hiraoka Masahiro, Abe Mitsuyuki, Nagata Kazuhiro. Quercetin, an Inhibitor of Heat Shock Protein Synthesis, Inhibits the Acquisition of Thermotolerance in a Human Colon Carcinoma Cell Line. Japanese Journal of Cancer Research. 1992;83(11):1216–1222. doi: 10.1111/j.1349-7006.1992.tb02748.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuennen Matthew, Gillum Trevor, Dokladny Karol, Bedrick Edward, Schneider Suzanne, Moseley Pope. Thermotolerance and heat acclimation may share a common mechanism in humans. American Journal of Physiology-Regulatory, Integrative and Comparative Physiology. 2011;301(2):R524–R533. doi: 10.1152/ajpregu.00039.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laszlo Andrei. The relationship of heat-shock proteins, thermotolerance, and protein synthesis. Experimental Cell Research. 1988;178(2):401–414. doi: 10.1016/0014-4827(88)90409-0. [DOI] [PubMed] [Google Scholar]
- Lee BJ, Mackenzie RWA, Cox V, et al. Human monocyte heat shock protein 72 responses to acute hypoxic exercise after 3 days of exercise heat acclimation. Biomed Res Int. 2015;2015:1–15. doi: 10.1155/2015/849809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee BJ, Miller A, James RS, Thake CD. Cross acclimation between heat and hypoxia: heat acclimation improves cellular tolerance and exercise performance in acute normobaric hypoxia. Front Physiol. 2016;7:1–15. doi: 10.3389/fphys.2016.00078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee W. C., Wen H. C., Chang C. P., Chen M. Y., Lin M. T. Heat shock protein 72 overexpression protects against hyperthermia, circulatory shock, and cerebral ischemia during heatstroke. Journal of Applied Physiology. 2006;100(6):2073–2082. doi: 10.1152/japplphysiol.01433.2005. [DOI] [PubMed] [Google Scholar]
- Leon LR, Bouchama A (2015) Heat stroke. Compr Physiol. 10.1002/cphy.c140017 [DOI] [PubMed]
- Li Gloria C. Elevated levels of 70,000 dalton heat shock protein in transiently thermotolerant chinese hamster fibroblasts and in their stable heat resistant variants. International Journal of Radiation Oncology*Biology*Physics. 1985;11(1):165–177. doi: 10.1016/0360-3016(85)90376-1. [DOI] [PubMed] [Google Scholar]
- Lorenzo Santiago, Halliwill John R., Sawka Michael N., Minson Christopher T. Heat acclimation improves exercise performance. Journal of Applied Physiology. 2010;109(4):1140–1147. doi: 10.1152/japplphysiol.00495.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorenzo Santiago, Minson Christopher T. Heat acclimation improves cutaneous vascular function and sweating in trained cyclists. Journal of Applied Physiology. 2010;109(6):1736–1743. doi: 10.1152/japplphysiol.00725.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magalhães FDC, Amorim FT, Passos RLF, et al. Heat and exercise acclimation increases intracellular levels of Hsp72 and inhibits exercise-induced increase in intracellular and plasma Hsp72 in humans. Cell Stress Chaperones. 2010;15:885–895. doi: 10.1007/s12192-010-0197-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malago JJ, Koninkx JFJG, Van Dijk JE (2002) The heat shock response and cytoprotection of the intestinal epithelium. Cell Stress Chaperones [DOI] [PMC free article] [PubMed]
- Maloyan Alina, Horowitz Michal. β-Adrenergic signaling and thyroid hormones affect HSP72 expression during heat acclimation. Journal of Applied Physiology. 2002;93(1):107–115. doi: 10.1152/japplphysiol.01122.2001. [DOI] [PubMed] [Google Scholar]
- Maloyan Alina, Palmon Aaron, Horowitz Michal. Heat acclimation increases the basal HSP72 level and alters its production dynamics during heat stress. American Journal of Physiology-Regulatory, Integrative and Comparative Physiology. 1999;276(5):R1506–R1515. doi: 10.1152/ajpregu.1999.276.5.R1506. [DOI] [PubMed] [Google Scholar]
- Marshall HC, Campbell SA, Roberts CW, Nimmo MA. Human physiological and heat shock protein 72 adaptations during the initial phase of humid-heat acclimation. J Therm Biol. 2007;32:341–348. doi: 10.1016/j.jtherbio.2007.04.003. [DOI] [Google Scholar]
- McClung JP, Hasday JD, He J, et al. Exercise-heat acclimation in humans alters baseline levels and ex vivo heat inducibility of HSP72 and HSP90 in peripheral blood mononuclear cells. Am J Physiol Integr Comp Physiol. 2007;294:R185–R191. doi: 10.1152/ajpregu.00532.2007. [DOI] [PubMed] [Google Scholar]
- Metcalfe Andrew, Rosenthal R. Meta-Analytic Procedures for Social Research. The Statistician. 1994;43(3):466. doi: 10.2307/2348600. [DOI] [Google Scholar]
- Morimoto RI (1998) Regulation of the heat shock transcriptional response: cross talk between a family of heat shock factors, molecular chaperones, and negative regulators. Genes Dev [DOI] [PubMed]
- Morton James P., MacLaren Don P. M., Cable Nigel T., Bongers Thomas, Griffiths Richard D., Campbell Iain T., Evans Louise, Kayani Anna, McArdle Anne, Drust Barry. Time course and differential responses of the major heat shock protein families in human skeletal muscle following acute nondamaging treadmill exercise. Journal of Applied Physiology. 2006;101(1):176–182. doi: 10.1152/japplphysiol.00046.2006. [DOI] [PubMed] [Google Scholar]
- Murakoshi M, Sekine M (2012) [Measures by local government--actions to take in dealing with heat stroke for firefighters]. Nihon Rinsho [PubMed]
- Nadel E R, Pandolf K B, Roberts M F, Stolwijk J A. Mechanisms of thermal acclimation to exercise and heat. Journal of Applied Physiology. 1974;37(4):515–520. doi: 10.1152/jappl.1974.37.4.515. [DOI] [PubMed] [Google Scholar]
- Nava Roberto C., Zuhl Micah N., Moriarty Terence A., Amorim Fabiano T., Bourbeau Kelsey C., Welch Anna M., McCormick James J., King Kelli E., Mermier Christine M. The Effect of Acute Glutamine Supplementation on Markers of Inflammation and Fatigue During Consecutive Days of Simulated Wildland Firefighting. Journal of Occupational and Environmental Medicine. 2019;61(2):e33–e42. doi: 10.1097/JOM.0000000000001507. [DOI] [PubMed] [Google Scholar]
- Nielsen B, Hales J R, Strange S, Christensen N J, Warberg J, Saltin B. Human circulatory and thermoregulatory adaptations with heat acclimation and exercise in a hot, dry environment. The Journal of Physiology. 1993;460(1):467–485. doi: 10.1113/jphysiol.1993.sp019482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papamichael K, Tiligada E (2008) Heat shock proteins in adaptive and protective physiology and pathophysiology of the gastrointestinal mucosa. In: Heat shock proteins: new research
- Parsell DA, Lindquist S. The function of heat-shock proteins in stress tolerance: degradation and reactivation of damaged proteins. Annu Rev Genet. 1993;27:437–496. doi: 10.1146/annurev.ge.27.120193.002253. [DOI] [PubMed] [Google Scholar]
- Périard JD, Racinais S, Sawka MN. Adaptations and mechanisms of human heat acclimation: applications for competitive athletes and sports. Sport: Scand. J. Med. Sci; 2015. [DOI] [PubMed] [Google Scholar]
- Robinson S, Turrell ES, Belding HS, Horvath SM. Rapid acclimatization to work in hot climates. Am J Phys. 1943;140:168–176. doi: 10.1152/ajplegacy.1943.140.2.168. [DOI] [Google Scholar]
- Rowell L. B., Kraning K. K., Kennedy J. W., Evans T. O. Central circulatory responses to work in dry heat before and after acclimatization. Journal of Applied Physiology. 1967;22(3):509–518. doi: 10.1152/jappl.1967.22.3.509. [DOI] [PubMed] [Google Scholar]
- Sandström Marie E., Siegler Jason C., Lovell Ric J., Madden Leigh A., McNaughton Lars. The effect of 15 consecutive days of heat–exercise acclimation on heat shock protein 70. Cell Stress and Chaperones. 2008;13(2):169–175. doi: 10.1007/s12192-008-0022-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sareh H, Tulapurkar ME, Shah NG, Singh IS, Hasday JD. Response of mice to continuous 5-day passive hyperthermia resembles human heat acclimation. Cell Stress Chaperones. 2011;16:297–307. doi: 10.1007/s12192-010-0240-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawka MN, Leon LR, Montain SJ, Sonna LA (2011) Integrated physiological mechanisms of exercise performance, adaptation, and maladaptation to heat stress. Compr Physiol. 10.1002/cphy.c100082 [DOI] [PubMed]
- Shi Y., Mosser D. D., Morimoto R. I. Molecularchaperones as HSF1-specific transcriptional repressors. Genes & Development. 1998;12(5):654–666. doi: 10.1101/gad.12.5.654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin YO, Oh JK, Sohn HS, et al. Expression of exercise-induced HSP70 in long-distance runner’s leukocytes. In: Journal of Thermal Biology; 2004. [Google Scholar]
- Taylor NAS (2014) Human heat adaptation. Compr Physiol. 10.1002/cphy.c130022 [DOI] [PubMed]
- Tupling A. R., Bombardier E., Stewart R. D., Vigna C., Aqui A. E. Muscle fiber type-specific response of Hsp70 expression in human quadriceps following acute isometric exercise. Journal of Applied Physiology. 2007;103(6):2105–2111. doi: 10.1152/japplphysiol.00771.2007. [DOI] [PubMed] [Google Scholar]
- van Rhee H, Suurmond R, Hak T (2018) User manual for meta-essentials: workbooks for meta-analysis. SSRN Electron J. 10.2139/ssrn.3241355
- Watkins AM, Cheek DJ, Harvey AE, Blair KE, Mitchell JB. Heat acclimation and HSP-72 expression in exercising humans. Int J Sports Med. 2008;29:269–276. doi: 10.1055/s-2007-965331. [DOI] [PubMed] [Google Scholar]
- Xiao C, Wu T, Ren A et al (2003) Basal and inducible levels of Hsp70 in patients with acute heat illness induced during training. Cell Stress Chaperones. 10.1379/1466-1268(2003)8<86:BAILOH>2.0.CO;2 [DOI] [PMC free article] [PubMed]
- Yamada PM, Amorim FT, Moseley P, Robergs R, Schneider SM. Effect of heat acclimation on heat shock protein 72 and interleukin-10 in humans. J Appl Physiol. 2007;103:1196–1204. doi: 10.1152/japplphysiol.00242.2007. [DOI] [PubMed] [Google Scholar]
- Zou Jiangying, Guo Yongle, Guettouche Toumy, Smith David F, Voellmy Richard. Repression of Heat Shock Transcription Factor HSF1 Activation by HSP90 (HSP90 Complex) that Forms a Stress-Sensitive Complex with HSF1. Cell. 1998;94(4):471–480. doi: 10.1016/S0092-8674(00)81588-3. [DOI] [PubMed] [Google Scholar]
- Zuhl M, Dokladny K, Mermier C, Schneider S, Salgado R, Moseley P. The effects of acute oral glutamine supplementation on exercise-induced gastrointestinal permeability and heat shock protein expression in peripheral blood mononuclear cells. Cell Stress Chaperones. 2015;20:85–93. doi: 10.1007/s12192-014-0528-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuhl M, Schneider S, Lanphere K et al (2014) Exercise regulation of intestinal tight junction proteins. Br J Sports Med [DOI] [PubMed]
- Zuhl Micah N., Lanphere Kathryn R., Kravitz Len, Mermier Christine M., Schneider Suzanne, Dokladny Karol, Moseley Pope L. Effects of oral glutamine supplementation on exercise-induced gastrointestinal permeability and tight junction protein expression. Journal of Applied Physiology. 2014;116(2):183–191. doi: 10.1152/japplphysiol.00646.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]