Abstract
Human heat-shock protein 60 (HSP60) is an autoantigen involved in the pathogenesis of rheumatoid arthritis (RA). Epitopes derived from HSP60 can trigger activation of regulatory T cells (Treg). CIGB-814 is an altered peptide ligand (APL) derived from HSP60. In preclinical models, this peptide had anti-inflammatory effects and increased Treg. The results from phase I clinical trial indicated that CIGB-814 was safe and activated mechanisms associated with induction of tolerance. Biodistribution profile for inducers of tolerance is crucial for triggering its effects. The primary goal of this study in Lewis rats was to identify (1) the target organs of CIGB-814 and (2) the pharmacokinetics (PK) profile. 125I-CIGB-814 administered subcutaneously at three dose levels was distributed in the thyroid gland, but also at considerable levels to the stomach and small and large intestines. In addition, concentration of CIGB-814 was increased in lymph nodes (LNs) at 24 h, compared with 4-h post-administration. Small intestine and LNs are excellent sites for induction of tolerance, due to the characteristics of dendritic cells in these tissues. Maximum concentration of CIGB-814 in blood of Lewis rats at 0.5 to 1 h agrees with PK profile determined for patients. Altogether, these results support therapeutic possibilities of CIGB-814 for RA.
Keywords: Heat-shock protein 60, Altered peptide ligands, Biodistribution and pharmacokinetic profiles, Rheumatoid arthritis, Treg
Introduction
Heat-shock proteins (Hsps) are among the most conserved and immunogenic proteins shared by microbes and mammals. T and B cells induced by a microbial HSP may cross-react with corresponding human Hsp and trigger an autoimmune response, which if unrestricted can lead to immune pathology (Rajaiah and Moudgil 2009). Cohen and Young postulated that HSP could be part of the immunological homunculus, which includes a few dominant self-antigens encoded in a cell regulatory network comprising the immune system’s picture of self (Cohen and Young 1991).
Specifically, HSP60s can act as stimulators of the adaptive immune response through their ability to bind antigenic peptides during intracellular antigen processing (Srivastava 2005; Tukaj and Kaminski 2019). Epitopes from HSP60 involved in inhibition of T and B cells in adjuvant induced arthritis (AA) models have been identified (van Eden et al. 1988; Alberta et al. 2000; Kim et al. 2016; Domínguez et al. 2011). Some authors have postulated that peptides derived from HSP60 can trigger activation of Treg (Prakken et al. 2003; Cohen et al. 2004; van Eden et al. 2005).
In addition, epitopes from human HSP60 were selected with the aim of inducing tolerance in patients with RA (Prakken et al. 2004; Koffeman et al. 2009; Prada et al. 2018).
RA is a systemic autoimmune disease mediated by activation of TH1 and TH17 (Kotake et al. 2017). In the last two decades, significant progresses have been achieved in the knowledge of the immunological and molecular mechanisms of RA. These were translated into a generation of biological therapeutic agents that target pro-inflammatory cytokines (Breedveld et al. 2006), inhibition of T cell co-stimulation (Blair and Deeks 2017), and the depletion of B cells (Chatzidionysiou et al. 2011). However, these approaches remain vulnerable by limitations associated with serious infections (including tuberculosis), lymphoma and other malignancies, demyelinating disorders, hepatotoxicity, hyperlipidemia, and cardiotoxicity (Rubbert-Roth 2012).
Induction of peripheral tolerance using autoantigens involved in the pathogenesis of RA remains considered an interesting therapeutic approach for this disease. Unlike current biological therapies, induction of tolerance is not aimed at suppressing the immune system, but at regulating the magnitude of inflammation.
We previously designed novel APL (called previously APL-1 and here called CIGB-814) derived from human HSP60 using bioinformatics tools. This peptide increased the frequency of Treg and their suppressive capacity against antigen responding effector CD4+ T cells from RA patients. In addition, this peptide inhibits significantly IL-17 levels produced by effector CD4+ T cells from peripheral blood mononuclear cells (PBMCs) of RA patients (Kim et al. 2016; Barberá et al. 2016).
Additionally, CIGB-814 reduced the course of AA in Lewis rats model (Breedveld et al. 2006). Likewise, this APL efficiently inhibits collagen-induced arthritis in DBA/1 mice, similar to Methotrexate, which is the current standard treatment for RA. In both animal models, the therapeutic effect induced by CIGB-814 was associated with a decrease of tumor necrosis factor α (TNFα) down to physiological levels and the induction of Treg (Lorenzo et al. 2017).
Recently, in a phase I clinical trial, safety and PK were evaluated as primary end points for the subcutaneous (SC) administration of CIGB-814. Preliminary evidences of efficacy were observed and documented (Koffeman et al. 2009; Corrales et al. 2019). In particular, PK study showed that CIGB-814 reached the maximum concentration in plasma in 30 min and was cleared mostly after 4 h (Cabrales-Rico et al. 2017).
A biodistribution profile of this therapeutic candidate is crucial for triggering the mechanisms that mediate peripheral tolerance. For this reason, the primary intention of this study was to identify into which organs CIGB-814 was distributed, when inoculated by SC route in Lewis rats, using three different doses, as well as identify the PK profile of this peptide.
Material and methods
CIGB-814 labeling procedure
Peptide was labeled with 125I following a standard procedure previously reported (Schumacher and Tsomides 2001). At the end of the reaction, [125I]-CIGB-814 was purified by solid phase extraction using SepPak C18 cartridge (Waters, USA). Samples were weighted in analytic scales (Precise, Sweden) and measured in automatic gamma counter (Wallac, Sweden) calibrated for the emission energy of 125I. [125I]-CIGB-814 showed a specific activity of 27.3 MBq/μg and a total activity of 37.0 MBq/mL in 2 mL. In these conditions, three solutions were prepared (A, B, and C) to be administered to animals. Table 1 shows the characteristics of these solutions. Three reference samples of 100 μL from each of the dose solution A, B, and C were tested for total radioactive content.
Table 1.
Formulation of dose solutions
| Solution | Concentration | Peptide (cold) | Solution A | Peptide [125I] | PBS | Used for group # |
|---|---|---|---|---|---|---|
| A | 1.00 mg/mL | 19.8 mg | NA | 2 mL | 18 mL | 1, 4, and 5 |
| B | 0.20 mg/mL | NA | 4 mL | NA | 16 mL | 2, 6, and 7 |
| C | 0.05 mg/mL | NA | 1 mL | NA | 19 mL | 3, 8, and 9 |
NA not applicable
Animals
All animal procedures were performed in accordance with the guidelines approved by the Ethical Committee and National Regulations for experiments with animals and by the European Union guidelines for animal experimentation (Directive 2010/63/EU, 2010). Twenty-seven male Lewis rats of about 150 g were supplied from Charles River Europe. Rats were caged in European standard cages type III. The air was exchanged approximately 12 times per hour in the animal house. Temperature, controlled via the ambient ventilation system, was 20 to 24 °C. Light cycle was 12-h dark and 12-h light (lights on 06.00). Diet and water were administered ad libitum.
Dosing for biodistribution and pharmacokinetic studies
For each animal [125I]-CIGB-814 was administered SC 5 mL/kg of either formulation A, B, or C (Table 2) according to the group. The same syringe was used for all animals given the same dose solution in order to avoid losses by dead volume. The dosing volume was checked by weighing the syringe before each injection.
Table 2.
Animal’s allocation according to the dosing groups, and different experiments carried out
| Gr. no. | Animal no. | Dose solution (xmg/mL) | Dose (mg/kg) | Experiments |
|---|---|---|---|---|
| 1 | 1–3 | A (1) | 5 | Pharmacokinetic |
| 2 | 4–6 | B (0.2) | 1 | Pharmacokinetic |
| 3 | 7–9 | C (0.05) | 0.25 | Pharmacokinetic |
| 4 | 10–12 | A (1) | 5 | Biodistribution (4 h) |
| 5 | 13–15 | A (1) | 5 | Biodistribution (24 h) |
| 6 | 16–18 | B (0.2) | 1 | Biodistribution (4 h) |
| 7 | 19–21 | B (0.2) | 1 | Biodistribution (24 h) |
| 8 | 22–24 | C (0.05) | 0.25 | Biodistribution (4 h) |
| 9 | 25–27 | C (0.05) | 0.25 | Biodistribution (24 h) |
At the end of experimentation, the animals were euthanized by exposure to CO2 (medical quality) from a cylinder with the compressed gas and via in-house pipelines. More than 100 μL of blood were drawn at each time point and considerable more was drawn at the last time point (24 h). Each blood sample was weighed. For the biodistribution study, animals were euthanized at either 4 h or 24 h after the administration and the tissues were dissected free immediately, weighed and frozen at − 20 °C. The amount of radioactivity was determined in each blood and tissue sample. The weights were used to normalize the content of radioactivity of each sample.
Biodistribution study
Eighteen rats (6 animals per group) were inoculated each with dose solution according to Table 2. Fourteen tissues from 9 animals (3 animals per group) were isolated at 4 and 24 h, after administration. The body weights were recorded (186 to 212 g) at the day of dosing and clinical observations were logged during the test period.
Pharmacokinetic study
Nine rats (3 animals per group) were inoculated each with dose solution according to Table 2. Blood was collected by retro orbital bleeding under CO2/O2 anesthesia. Samples were drawn at 8 time points: pre-dose, 1 min, 15 min, 30 min and 1, 4, 8 and 24 h post-administration.
Data analysis
The descriptive statistic package provided by Microsoft Excel was used for biodistribution and pharmacokinetic data analysis. The PKsolver add-ins of the Microsoft Excel was used for the pharmacokinetic parameters estimation. A significance of α < 0.05 was taken into account for dose dependency analysis.
Results
Biodistribution study
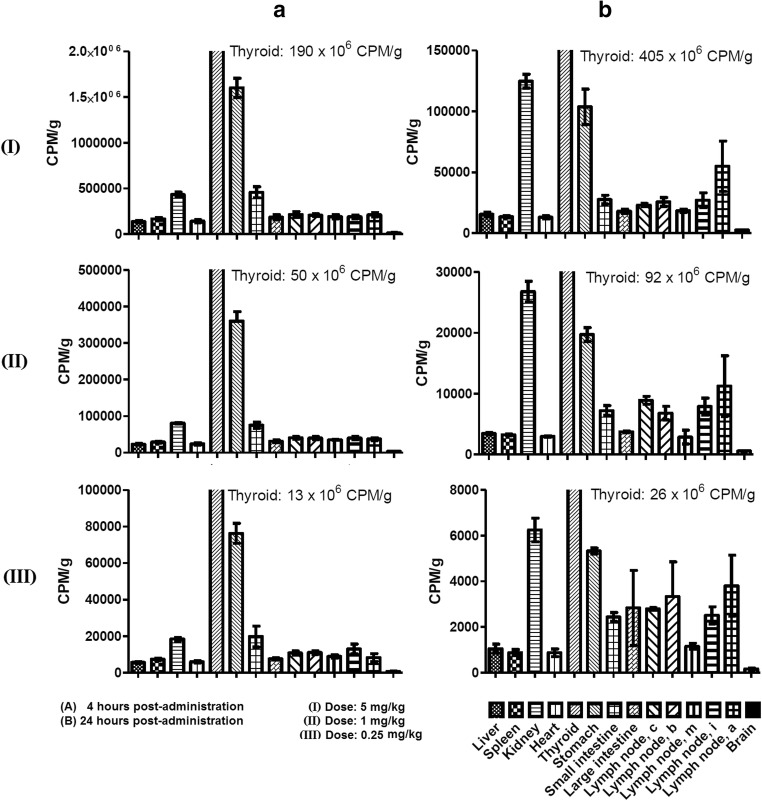
Biodistribution profile of [125I]-CIGB-814 at 4 and 24 h post-administration is shown in Fig. 1. The peptide concentration was high in thyroid at these two times while in the kidney, the peptide level was appreciable at 24 h. [125I]-CIGB-814 was widely distributed in the stomach and small intestine, even 4 h post-administration. Peptide concentration was low in the liver, brain, heart, and spleen. In addition, [125I]-CIGB-814 was detected in a wide distribution of lymph nodes at 24 h that was increased from that at 4 h.
Fig. 1.
Biodistribution profiles of [125I]-labeled CIGB-814 at 4 h (a) and 24 h (b) post-administration. Each bar represents the average normalized [125I]-labeled peptide content in the specific tissue (indicated by different format in the bars). Three doses of CIGB-814 studied are indicated in romans numerals: 5 (I), 1 (II) and 0.25 (III) mg/kg of body weight. The legend explaining the meaning of bars is shown at the bottom of the figure
Pharmacokinetic study
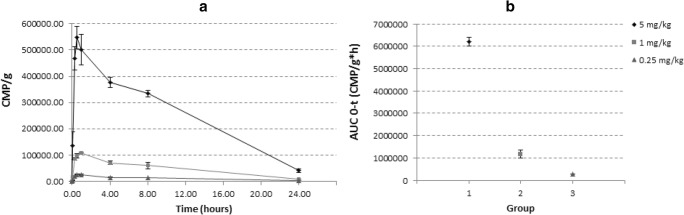
Plasmatic concentration of [125I]-CIGB-814 over time was in agreement with the extravascular drug administration (SC). Figure 2 (panel a) shows the graphic plots for three dose levels (5 mg/kg, 1 mg/kg, and 0.25 mg/kg). According to data analysis, the inter-individual variation, expressed as standard deviation of the average concentration value at each time point, was suitable, displaying higher values in the higher dose level (5 mg/kg).
Fig. 2.
Pharmacokinetic study. a Distribution of [125I]-labeled peptide in blood over time. The peptide was administered at 5 (rhombuses), 1 (squares), and 0.25 (triangles) mg/kg. b Inter-individual variation, expressed as standard deviation of the average concentration value at each time point
The fitting to compartmental pharmacokinetic models was not homogeneous; thus, PK analysis was performed by non-compartmental analysis, considering single subcutaneous administration of assessed drug candidate. Main pharmacokinetic parameters are listed in the Table 3, for the three dose levels. Dose dependency for AUC, based on the narrow 95% confidence interval, was obtained for each dose level (Fig. 2, panel b).
Table 3.
Pharmacokinetic parameters calculated by using “PK solver 2.0”
| Pharmacokinetic parameters calculated by using “PK solver 2.0” dose | T½ (h) | Tmax (h) | Cmax (CPM/g) | AUC (h*CPM/g) |
|---|---|---|---|---|
| 5 mg/kg | 6.3 | 0.5 | 546,975.5 | 6,481,488.8 |
| 1 mg/kg | 6.4 | 1.0 | 109,019.8 | 1,233,026.6 |
| 0.25 mg/kg | 6.9 | 1.0 | 26,623.8 | 297,516.5 |
Discussion
A significant progress in understanding of the mechanisms of RA has taken place in last years and such knowledge has been translated into novel anti-cytokines approaches. However, these treatments have some limitations such as induction of adverse events, approximately 40% of patients do not respond and the high cost of the treatment (Rubbert-Roth 2012).
Antigen-specific immunomodulation is a different approach; it provides a tool for inducing peripheral tolerance to pathogenic T cell clones. APLs are an option for this approach. Conceptually, said peptides mediate their therapeutic intervention by inhibiting specific antigenic T cells, whereby lower toxicity is expected, compared with agents targeting broadly active inflammatory cytokines (Garrood and Pitzalis 2006).This therapy aims to regulate the magnitude of the immune response, without causing immunosuppression.
Previously, we showed that CIGB-814 induced regulatory properties associated with inhibition of inflammation in several experimental models (Domínguez et al. 2011; Barberá et al. 2016; Lorenzo et al. 2017).
A phase I clinical trial with CIGB-814 was carried out in 20 moderately active RA patients. Sequential dose-escalation of 1, 2.5, and 5 mg of CIGB-814 was studied. This treatment was well tolerated at all doses. These patients showed decreases of clinical activity of RA. CIGB-814 significantly decreased IL-17 in patients treated with 2.5 mg. Therapy with 1 mg and 2.5 mg of CIGB-814 led to a significant reduction of interferon gamma (IFN-γ) (Prada et al. 2018).
On other hand, CIGB-814 induced a significant reduction of autoantibodies against cyclic citrullinated peptides (anti-CCP antibodies), this decrease correlated with clinical activity of RA (Corrales et al. 2019). Results from preclinical studies and phase I clinical trial indicate that CIGB-814 induces mechanism associated with induction of tolerance.
We think that this therapeutic approach is closely associated with biodistribution profile of antigenic molecule, since depending on the tissue where the molecule is located, this can cross talk with antigen presenting cells. In addition, it can contact with cytokines, as well as other cells and molecules essential to induce tolerance.
Biodistribution of a peptide can be influenced by antigen, doses, and the route of inoculation. The end points of this study were to determine the biodistribution and PK profile of three different CIGB-814 doses in Lewis rats, inoculated by SC route.
Here, concentration of peptide was high in the thyroid and kidneys at 4 h after the administration by SC route. This result is due to the peptide being labeled with iodine and this element is sequestered by the thyroid and then is eliminated through the kidneys. Concentration in thyroid was higher at 24 h after administration in comparison with 4 h, probably indicating the peptide degradation and the accumulation of 125I in this tissue due to the physiologic/metabolic processing reported for this radioisotope. (Chien-Chih et al. 2017).
Interestingly, [125I]-labeled peptide was distributed mainly to the stomach and small intestine, as early as 4 h post-administration. At 24 h, concentration of CIGB-814 was high in the stomach and small and large intestines.
Radioactivity was at relatively low levels in the liver, spleen, heart, and brain regardless time post-administration of [125I]-labeled peptide. These experimental facts could indicate a low-toxicity of CIGB-814 seen in preclinical models (mice and dogs, manuscript in preparation) as well as the very favorable safety profile observed in phase I clinical trial (Prada et al. 2018).
Biodistribution profile of CIGB-814 was practically identical for two other administration routes studied: intravenous and intradermal (data not shown). These results are different from those previously reports for other synthetic peptides obtained in our Institute and evaluated in clinical trials for other therapeutic interventions.
For instance, CIGB-300 an antitumor cell-penetrating peptide that binds to several Casein Kinase II substrates impairing their phosphorylation, when labeled with 99mTc regardless the administration route either intravenously or intraperitoneally, showed the same biodistribution pattern mainly in lungs, blood, and tumor tissues (Perera et al. 2008).
Likewise CIGB-552, another antitumor cell-penetrating peptide targeting the COMMD1 molecule, labeled with [131I] and administered subcutaneously, was mainly distributed to the liver and kidneys (excretions pathways), but it was detected immediately (as early as 20 min post-administration) in the tumor tissue, having a prevalence there up until the 24 h post-administration (Vallespí et al. 2014).
Moreover, another peptide known as growth hormone-releasing hexapeptide having wide cytoprotective effects, when it was labeled with [125I] and administered intravenously, displayed a biodistribution profile to large intestine, according to the metabolic and excretion pathways proposed for this secretagogue peptide family (Davis et al. 1994; Roumi et al. 2000).
Although these peptides were obtained by chemical synthesis like CIGB-814, it is important to remark that no matter the administration route or even labeling strategy used in the PK-BD studies, the biodistribution profile was strongly related to biological activity and action mechanism of the specific peptide.
This particular biodistribution of CIGB-814 to the gut could be associated with the fact that this peptide is derived from a conserved region of HSP60 (Kim et al. 2016). This protein is expressed by commensal bacteria in gastro-intestinal tract. HSPs derived from commensal microbes can stimulate immune-regulatory pathways for the maintenance of intestinal homeostasis (Ohue et al. 2011). Repeated contacts to microbiota-associated HSP in the gut mucosa may induce HSP-specific Tregs to conserved epitopes from this molecule (Lathrop and Bloom 2011; van Eden et al. 2017).
In addition, the small intestine is an excellent site for induction of tolerance, due to characteristics of dendritic cells in this tissue (Steimle and Frick 2016).
On the other hand, concentration of CIGB-814 was increased in LNs at 24 h, regarding to 4-h post-administration.
LNs are unique organs distributed all over the body. They have to filter the lymph for incoming antigens from the draining area to defend the body. Interestingly, removing the lymph nodes (LNs) leads to various different responses in the body; for example, the immune response in the gut system is enhanced after LNs dissection (Buettner and Bode 2012).
Therapeutic effect induced by CIGB-814 in two animal models for RA was associated with an increase of Treg cells and a decrease of TNFα level down to physiological levels. Also, this peptide inoculated subcutaneously induced an increase of Treg in the draining lymph nodes of healthy mice (Domínguez et al. 2011). These results are in agreement with biodistribution profile described in this work.
On the other hand, maximum concentration of CIGB-814 in blood from rats was at 0.5 to 1 h; and the t½ was calculated to be 6.3, 6.4, and 6.9 h for the three different doses. The amount of peptide inoculated was correlated with the amount detected in the blood.
These results agreed with PK profile described in the phase I clinical trial. This PK study showed that CIGB-814 inoculated by SC route was cleared from plasma in 4 h for patients inoculated with 1 mg and 2.5 mg; and approximately in 6 h for patients treated with 5 mg. Cmax was reached at 0.5 h for the three doses, showing a wide dispersion, probably associated with the biological variability among patients and the non-normalized doses respect to the body weight used in this clinical trial (Cabrales-Rico et al. 2017).
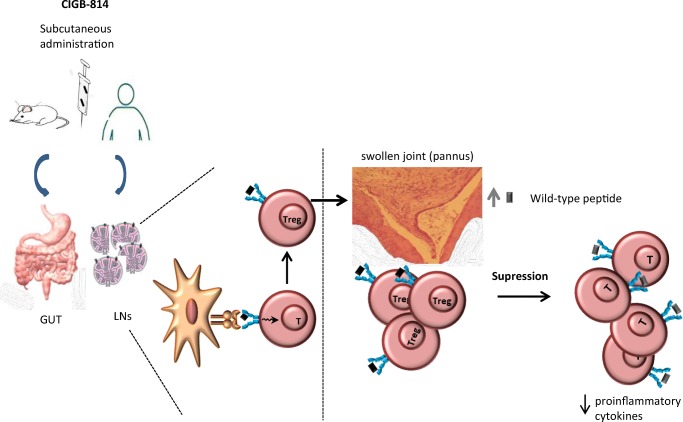
Probably, therapeutic effect of CIGB-814 in animal models and patients is due to the processing and presentation of this altered peptide ligand by dendritic cells to T-lymphocytes in the intestine or LNs could induce the expansion of Treg. These activated cells migrate to inflammation site and they could cross-recognize wild-type epitope from HSP60, where it is highly expressed due to the inflammation process. This new contact with HSP60 autoantigen may induce potent immune-regulatory effect, attenuating autoreactive T cells responsible of RA pathogenesis and inhibiting inflammatory process (Fig. 3).
Fig. 3.
Processing and presentation of CIGB-814 by dendritic cells to T-lymphocytes in the gut or LNs could induce the expansion of Treg. These activated cells migrate to inflammation site (joints) and could cross-recognize wild-type epitope from HSP60, where it is highly expressed due to the inflammation process. This new contact with this autoantigen may induce potent immuno-regulatory effect, attenuating autoreactive T cells responsible of RA pathogenesis and inhibiting inflammatory cytokines
CIGB-814 is being studied for other autoimmune diseases in which HSP60 is considered an autoantigen. This therapeutic intervention with low doses of an immunomodulatory peptide should not cause immunosuppression and should restore the tolerance that is lost in the course of an autoimmune disease.
Funding information
This work was supported by Biomedical Research Department at Center for Genetic Engineering and Biotechnology.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflicts of interest.
Disclaimer
The authors alone are responsible for the content and writing of the paper.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Alberta GA, van der Zee R, Taams LS, et al. A self-Hsp60 peptide acts as a partial agonist inducing expression of B7-2 on mycobacterial hsp60-specific T cells: a possible mechanism for inhibitory T cell regulation of adjuvant arthritis. Int Immunol. 2000;12:1041–1050. doi: 10.1093/intimm/12.7.1041. [DOI] [PubMed] [Google Scholar]
- Barberá A, Lorenzo N, van Kooten P, van Roon J, de Jager W, Prada D, Gómez J, Padrón G, van Eden W, Broere F, del Carmen Domínguez M. APL1, an altered peptide ligand derived from human heat-shock protein 60, increases the frequency of Tregs and its suppressive capacity against antigen responding effector CD4+T cells from rheumatoid arthritis patients. Cell Stress Chaperones. 2016;21:735–744. doi: 10.1007/s12192-016-0698-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blair HA, Deeks ED. Abatacept: a review in rheumatoid arthritis. Drugs. 2017;11:1221–1233. doi: 10.1007/s40265-017-0775-4. [DOI] [PubMed] [Google Scholar]
- Breedveld FC, Weisman MH, Kavanaugh AF, Cohen SB, Pavelka K, van Vollenhoven R, Sharp J, Perez JL, Spencer-Green GT. The PREMIER study: a multicenter, randomized, double-blind clinical trial of combination therapy with adalimumab plus methotrexate versus methotrexate alone or adalimumab alone in patients with early, aggressive rheumatoid arthritis who had not had previous methotrexate treatment. Arthritis Rheum. 2006;54:26–37. doi: 10.1002/art.21519. [DOI] [PubMed] [Google Scholar]
- Buettner M, Bode U. Lymph node dissection – understanding the immunological function of lymph nodes. Clin Exp Immunol. 2012;169:205–212. doi: 10.1111/j.1365-2249.2012.04602.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabrales-Rico A, Ramos Y, Besada V, et al. Development and validation of a bioanalytical method based onLC–MS/MS analysis for the quantitation of CIGB-814 peptide in plasma from rheumatoid arthritis patients. J Pharm Biomed Anal. 2017;143:130–140. doi: 10.1016/j.jpba.2017.05.030. [DOI] [PubMed] [Google Scholar]
- Chatzidionysiou K, Lie E, Nasonov E, et al. Highest clinical effectiveness of rituximab in autoantibody-positive patients with rheumatoid arthritis and in those for whom no more than one previous TNF antagonist has failed: pooled data from 10 European registries. Ann Rheum Dis. 2011;9:1575–1580. doi: 10.1136/ard.2010.148759. [DOI] [PubMed] [Google Scholar]
- Chien-Chih K, Zi-Ming H, Ya-Ju H, et al. Quantitative measurement of the thyroid uptake function of mouse by Cerenkov luminescence imaging. Sci Rep. 2017;7:5717. doi: 10.1038/s41598-017-05516-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen IR, Young DB. Autoimmunity, microbial immunity and the immunological homunculus. Immunol Today. 1991;12:105–110. doi: 10.1016/0167-5699(91)90093-9. [DOI] [PubMed] [Google Scholar]
- Cohen IR, Quintana FJ, Mimran A. T regs in T cell vaccination: exploring the regulation of regulation. J Clin Invest. 2004;114:1227–1232. doi: 10.1172/JCI200423396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corrales O, Hernández L, Prada D, Gómez J, Reyes Y, López AM, González LJ, del Carmen Domínguez Horta M. CIGB-814, an altered peptide ligand derived from human heat-shock protein 60, decreases anti-cyclic citrullinated peptides antibodies in patients with rheumatoid arthritis. Clin Rheumatol. 2019;38:955–960. doi: 10.1007/s10067-018-4360-3. [DOI] [PubMed] [Google Scholar]
- Davis CB, Crysler CS, Boppana VK, et al. Disposition of grow hormonereleasing peptide (SK&F 110679) in rat and dog following intravenous or subcutaneous administration. Drug Metab Disp. 1994;22:90–98. [PubMed] [Google Scholar]
- Directive 2010/63/EU of the European Parliament and of the Council of 22 September 2010 on the protection of animals used for scientific purposes Text with EEA relevance. OJ L 276, 20.10.2010, p. 33–79. http://data.europa.eu/eli/dir/2010/63/oj
- Domínguez MC, Lorenzo N, Barberá A, et al. An altered peptide ligand corresponding to a novel epitope from heat-shock protein 60 induces regulatory T cells and suppresses pathogenic response in an animal model of adjuvant induced arthritis. Autoimmunity. 2011;44:471–482. doi: 10.3109/08916934.2010.550590. [DOI] [PubMed] [Google Scholar]
- Garrood T, Pitzalis C. Targeting the inflamed synovium: the quest for specificity. Arthritis Rheum. 2006;54:1055–1060. doi: 10.1002/art.21720. [DOI] [PubMed] [Google Scholar]
- Kim EY, Durai M, Mia Y, et al. Modulation of adjuvant arthritis by cellular and humoral immunity to Hsp65. Front Immunol. 2016;7:203. doi: 10.3389/fimmu.2016.00203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koffeman EC, Genovese M, Amox D, et al. Epitope-specific immunotherapy of rheumatoid arthritis. Clinical responsiveness occurs with immune deviation and relies on the expression of a cluster of molecules associated with T cell tolerance in a double-blind, placebo-controlled, pilot phase II trial. Arthritis Rheum. 2009;60:3207–3321. doi: 10.1002/art.24916. [DOI] [PubMed] [Google Scholar]
- Kotake S, Yago T, Kobashigawa T, et al. The plasticity of Th17 cells in the pathogenesis of rheumatoid arthritis. J Clin Med. 2017;7:67. doi: 10.3390/jcm6070067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lathrop SK, Bloom SM, Rao SM, et al. Peripheral education of the immune system by colonic commensal microbiota. Nature. 2011;7368:250–254. doi: 10.1038/nature10434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorenzo N, Altruda F, Silengo L, del Carmen Dominguez M. APL-1, an altered peptide ligand derived from heat-shock protein, alone or combined with methotrexate attenuates murine collagen induced arthritis. Clin Exp Med. 2017;17:209–216. doi: 10.1007/s10238-016-0412-7. [DOI] [PubMed] [Google Scholar]
- Ohue R, Hashimoto K, Nakamoto M, Furukawa Y, Masuda T, Kitabatake N, Tani F. Bacterial heat shock protein 60, GroEL, can induce the conversion of naïve T cells into a CD4 + CD25 + Foxp3-expressing phenotype. J Innate Immun. 2011;3:605–613. doi: 10.1159/000330786. [DOI] [PubMed] [Google Scholar]
- Perera Y, Farina G, Hernández I, et al. Systemic administration of a peptide that impairs the protein kinase (CK2) phosphorylation reduces solid tumor growth in mice. Int J Cancer. 2008;122:57–62. doi: 10.1002/ijc.23013. [DOI] [PubMed] [Google Scholar]
- Prada D, Gómez J, Lorenzo N, et al. Phase I clinical trial with a novel altered peptide ligand derived from human heat-shock protein 60 for treatment of rheumatoid arthritis: safety, pharmacokinetics and preliminary therapeutic effects. J Clin Trials. 2018;8:339. doi: 10.4172/2167-0870.1000339. [DOI] [Google Scholar]
- Prakken BJ, Roord S, Ronaghy A, Wauben M, Albani S, van Eden W. Heat shock protein 60 and adjuvant arthritis: a model for T cell regulation in human arthritis. Springer Semin Immunopathol. 2003;25:47–63. doi: 10.1007/s00281-003-0128-7. [DOI] [PubMed] [Google Scholar]
- Prakken BJ, Samodal R, Le TD, et al. Epitope specific immunotherapy induces immune deviation of proinflammatory T cells in rheumatoid arthritis. Proc Natl Acad Sci U S A. 2004;101:4228–4233. doi: 10.1073/pnas.0400061101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajaiah R, Moudgil KD. Heat-shock proteins can promote as well as regulate autoimmunity. Autoimmun Rev. 2009;8:388–393. doi: 10.1016/j.autrev.2008.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roumi M, Marleau S, Souichi PD, et al. Kinetics and disposition of hexarelin, a peptidic growth hormone secretagogue, in rats. Drug Metab Disp. 2000;28:44–50. [PubMed] [Google Scholar]
- Rubbert-Roth A. Assessing the safety of biologic agents in patients with rheumatoid arthritis. Rheumatology. 2012;51:38–47. doi: 10.1093/rheumatology/kes114. [DOI] [PubMed] [Google Scholar]
- Schumacher TN, Tsomides TJ. In vitro radiolabeling of peptides and proteins. Curr Protoc Protein Sci. 2001;Chapter 3:unit 3.3. doi: 10.1002/0471140864.ps0303s00. [DOI] [PubMed] [Google Scholar]
- Srivastava PK. Immunotherapy for human cancer using heat shock protein-peptide complexes. Curr Oncol. 2005;7:104–108. doi: 10.1007/s11912-005-0035-8. [DOI] [PubMed] [Google Scholar]
- Steimle Alex, Frick Julia-Stefanie. Molecular Mechanisms of Induction of Tolerant and Tolerogenic Intestinal Dendritic Cells in Mice. Journal of Immunology Research. 2016;2016:1–12. doi: 10.1155/2016/1958650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tukaj S, Kaminski M. Heat shock proteins in the therapy of autoimmune diseases: too simple to be true? Cell Stress Chaperones. 2019;3:475–479. doi: 10.1007/s12192-019-01000-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vallespí Maribel G., Pimentel Gilmara, Cabrales-Rico Ania, Garza Julio, Oliva Brizaida, Mendoza Osmani, Gomez Yolanda, Basaco Tais, Sánchez Iraida, Calderón Carlos, Rodriguez Juan C., Markelova Maria Rivera, Fichtner Iduna, Astrada Soledad, Bollati-Fogolín Mariela, Garay Hilda E., Reyes Osvaldo. Antitumor efficacy, pharmacokinetic and biodistribution studies of the anticancer peptide CIGB-552 in mouse models. Journal of Peptide Science. 2014;20(11):850–859. doi: 10.1002/psc.2676. [DOI] [PubMed] [Google Scholar]
- van Eden W, Thole JER, van der Zee R, Noordzij A, van Embden J, Hensen EJ, Cohen IR. Cloning of the mycobacterial epitope recognized by T lymphocytes in adjuvant arthritis. Nature. 1988;331:171–173. doi: 10.1038/331171a0. [DOI] [PubMed] [Google Scholar]
- van Eden W, van der Zee R, Prakken B. Heat-shock proteins induce T-cell regulation of chronic inflammation. Nat Rev Immunol. 2005;5:318–330. doi: 10.1038/nri1593. [DOI] [PubMed] [Google Scholar]
- van Eden W, Jansen MAA, Ludwig I, et al. The enigma of heat shock proteins in immune tolerance. Front Immunol. 2017;8:1599. doi: 10.3389/fimmu.2017.01599. [DOI] [PMC free article] [PubMed] [Google Scholar]