Abstract
Background
Polycyclic aromatic hydrocarbons (PAH) are considered to be one of the major contaminants of drinking water and natural water bodies. Some of the well documented polycyclic aromatic hydrocarbons that are water pollutants and were considered for analysis in this study included benzo[b]fluoranthene (BbF), benzo[k]fluoranthene (BkF), benzo[a]pyrene (BaP), benzo[g,h,i]perylene (BgP), and indeno[1,2,3-c,d]pyrene (InD). This study aimed at determining the levels of concentrations of basically five polycyclic aromatic hydrocarbons in 57 drinking water bodies located around Samsun, Ordu, Giresun, Çorum, Amasya, Kastamonu and Sinop provinces.
Materials and method
In this study, the Environmental Protection Agency (EPA) method 550.1 for the determination of polycyclic aromatic hydrocarbons in drinking by Liquid-Solid Extraction (LSE) and High Performance Liquid Chromatography (HPLC) with Coupled Ultraviolet (CD) and Fluorescence Detection (FD) was used. Sampling procedures were done according to the validated method specified by the Turkish Ministry of Enivironment and Forestry. Prior to the determination of concentrations by HPLC, PAHs contained in the samples were separated from the solid phase by Solid-Phase Extraction (SPE). All data analyses were conducted using SPSS and Excel.
Results
Obtained results from the investigation revealed that the average total PAH and benzo[a]pyrene (BaP) concentration levels in drinking water samples taken from the central districts of Samsun were 2.73 ± 1.51 and 0.35 ± 0.24 ng/L respectively. In drinking water samples taken from Ordu, Giresun, Çorum, Amasya, Kastamonu and Sinop, the average total PAH concentrations were found to be 5.85 ± 3.82 ng/L, 3.79 ± 1.27 ng/L, 1.08 ± 0.62 ng/L, 2.42 ± 1.04 ng/L; 1.92 ± 0.35 ng/L and 4.07 ± 2.33 ng/L respectively. The average (BaP) concentrations for the same named locations were determined as 0.97 ± 0.75 ng/L; 0.55 ± 0.29 ng/L; 0.11 ± 0.08 ng/L; 0.35 ± 0.10 ng/L; 0.14 ± 0.04 ng/L; 0.39 ± 0.23 ng/L, respectively. It is therefore evident that the values of PAH and BaP in drinking water were below the limits of 100 and 10 ng/L specified in the Regulation on Water Intended for Human Consumption. These values are below the set limits proposed by Turkish legislation and WHO.
Conclusion
All the results for drinking water, usable water and natural spring water were below the values specified in the Regulation on Water Intended for Human Consumption and WHO. The PAH content of the studied river waters as well were below the limits proposed by Turkish legislation and WHO.
Keywords: PAH, BaP, Drinking water, Samsun, Natural spring water
Introduction
Water required for sustenance of all forms of life must be highly hygienic. This implies that for it to be considered suitable for consumption, it must be odourless, colourless, clear and slightly alkaline. The levels of heavy metals such as lead, arsenic, cadmium, mercury, nickel and the presence of other substances such as organic matter, ammonia, nitrate, nitrite, detergents, cynide, pesticides and polycyclic hydrocarbons (PAHs) should be within acceptable limits. Also, there should be no presence of bacteria such as E.coli or coliform in the water. Today, due to human activities and various natural processes, there is a great deal of organic pollution taking place in various water sources. For example, PAH compounds which tend to be miscible in various ways end up as natural and anthropogenic sources of pollutants to the aquatic environment [1]. It is therefore imperative that chemical, physical and bacteriological analyses be undertaken continuously for drinking water and usable water. Organic compounds that are a cause of water pollution, many of which are toxic and carcinogenic have been of concern worldwide[2]. Among these organic compounds, PAHs have the lowest solubility in water hence they tend to absorb strongly to particles and sediments. [3]. Polycyclic aromatic hydrocarbons (PAHs) often appear to be highly hazardous to both the environment and animals as seen from their carcinogenic effects [4]. Benzo[a]fluoranthene (BaF), benzo[b]fluoranthene (BbF) and indeno[1, 2, 3-c, d]pyrene (InD) have been listed in the class of possible carcinogenic substances that are harmful to human beings while benzo[g,h, i]perylene (B[ghi]P) has not been classified as carcinogenic [5].
When a comparison is made, it is worth noting that the priority PAHs listed in Europe are different from those considered in Turkey and the USA. For example, dibenzo[a,h]anthracene is given priority by EPA while it is not included in Europe [6]. A European Community directive 80/778/ECC (1980) set a maximum level for PAHs in drinking water at 200 ng/L with fluoranthene, benzo[k]pyrene, benzo[b] fluoranthene, benzo[k]fluoranthene, benzo[ghi]perylene and indeno[1,2,3-cd]pyrene as reference compounds. Another European Communities Regulation 835/2011 set new limits for a range of foodstuffs also taking into account benzo[b]fluoranthene and the other four PAHs (benzo[a]pyrene, benz[a]anthracene, benzo[b]fluoranthene, and chrysene) as reference compounds. However, other food categories; especially dried food products, infusions and drinking water, were not covered by this legislation. Due to this, a different European Union Directive (98/83/CE) was taken into account.
According to the Turkish Legislation (Regulation on Water Intended for Human Consumption) implemented in line with the directive of the European Union, five PAHs (benzo[b]fluoranthene, benzo[k]fluoranthene, benzo[a]pyrene, benzo[g,h,i]perylene, and indeno[1,2,3-c,d]pyrene) should be determined in drinking water. This regulation also stipulates that BaP concentration should not exceed 10 ng/L while the concentration for the other four PAHs (BbF, BkF,BgP and InD) should remain below 100 ng/L.
Many studies for the determination of PAHs have been carried out and several analytical methods have been applied [3, 8–18] such as high-performance liquid chromatography (HPLC) with ultraviolet (UV) or fluorescence detection (FLD), and gas chromatography (GC) with a mass spectrophotometer (MS) or flame ionization detector (FID). Girell et al. reported that although both methods are adequate for the analysis of PAHs in water samples, a higher sensitivity with a lower relative standard deviation percentage value is only achievable using HPLC-FLD [7]. However, In Turkey, there have been very few studies conducted on monitoring of PAHs in drinking water [13, 14]. Therefore, it was for this reason that this research aimed at determining the concentration of five PAHs in drinking water sources in Samsun, Ordu, Giresun, Corum, Amasya, Kastamonu and Sinop provinces. The Environmental Protection Agency (EPA) method 550.1 for the determination of polycyclic hydrocarbons (PAHs) in drinking water using liquid-solid extraction and HPLC coupled with ultraviolet and fluorescence detection method was employed for analysis. Considering the fact that the Turkish legislation had previously intended that five PAHs be analyzed in drinking water sources, we as well aimed at analyzing the amount of PAHs in eight different natural spring water pet bottles. Lastly, this study also aimed at evaluating the results by comparing the obtained data with the standards determined by the Turkish legislation and the World Health Organization (WHO).
Materials and methods
Chemicals and instruments
A mixture of five PAHs (benzo[b]fluoranthene (BbF), benzo[k]fluoranthene (BkF), benzo[a]pyrene (BaP), benzo[g,h,i]perylene (BgP), and indeno[1,2,3-c,d]pyrene (InD)) obtained from Dr. Ehrenstorfer GmbH (Germany). All solvents that were used were of HPLC grade or equivalent. Ultra pure water was used for HPLC-FLD.
PAHs were analysized using HPLC method coupled with a fluorescence detector and a reverse-phase C18 column (4.6 mm × 250 mm, 5 μm particle size, Agilent Corp, USA). The flow rate of the mobile phase was kept at 1.0 mL. The temperature of the column oven was kept at 25 °C.
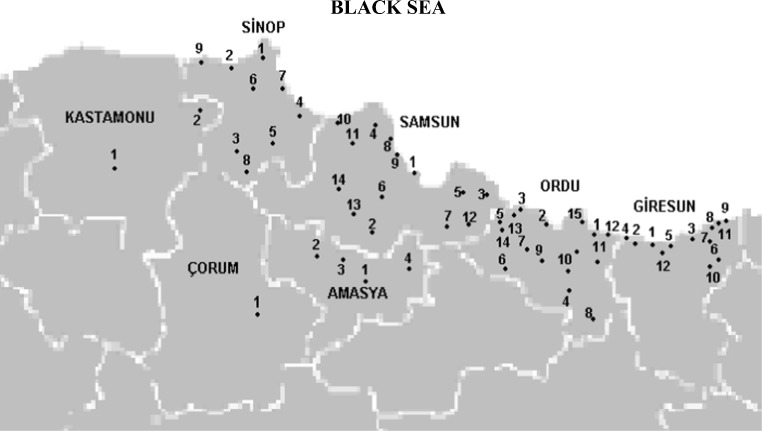
All the drinking water samples were collected by the Institute of Samsun Refik Saydam National Public Laboratory from several locations in Samsun and the nearby regions totaling up to 57 sampling sites. Samples taken from Sumsun were 48, 124 samples were taken from Ordu, 30 samples were taken from Giresun, 12 samples were taken from Central Çorum, 5 samples were taken from a Amasya, 3 samples from central Kastamonu and Hanönü district and 9 samples were taken from Sinop respectively (2010 February-2011 Aprail). The map of 57 sampling sites is illustrated in Fig. 1. A total number of 8 brands of natural spring water in pet bottles were purchased from markets in Samsun. All sampling procedures were done according to the method described in the official newspaper for the Turkish Ministry of Enivironment and Forestry [19]. The collected samples were stored in a dark room at 4 °C and processed within a period of 7 days of collection without any prior treatment or filtration. Quantification of analytes was done by external calibration where a series of standard solutions is prepared by dilution of the stock solution and analysis was done using HPLC-FLD to obtain linear calibration plots for each analyte based on the chromatographic peak areas.
Fig. 1.
The study area and sampling locations in Samsun and nearby regions. Samsun: 1. Centrum 2. Ladik 3. Terme 4. Bafra 5. Carsamba 6. Kavak 7. Ayvacik 8. Ondokuz Mayis 9. Atakum 10. Yakakent 11. Alacam 12. Salipazari 13. Havza 14. Vezirkopru; Ordu: 1. Centrum 2. Fatsa 3. Unye 4. Golkoy 5. Ikizce 6. Akkus 7. Kumru 8. Mesudiye 9. Korgan 10. Gurgentepe 11. Kabaduz 12. Gulyalı 13. Catalpinar 14. Caybasıi 15. Persembe; Giresun: 1. Centrum 2. Bulancak 3. Espiye 4. Piraziz 5. Kesap 6. Canakcı 7. Guce 8. Tirebolu 9. Eynesil 10. Dogankent 11. Görele 12. Yaglidere; Corum: 1. Centrum; Amasya: 1. Centrum 2. Merzifon 3. Suluova 4. Tasova; Kastamonu: 1. Centrum 2. Hanonu; Sinop: 1. Centrum 2. Ayancik 3. Boyabat 4. Dikmen 5. Duragan 6. Erfelek 7. Gerze 8. Sarayduzu 9. Turkeli
Extraction and clean-up of PAHs from water samples
All analyses of PAH from the water samples was done using High Performance Liquid Chromatography (HPLC) and all procedures were in accordance with the Environmental Protection Agency (EPA) method 550.1 [20]. A Dionex Auto Trace 280 SPE system was used for extratction of PAHs from water samples. Prior to the extraction, the C18-bonded phase containing 1000 mg of reversed phase octadecyl (Finisterre by Teknokroma, Teknokroma S. Coop. Ltda., Barcelona Spain) was first washed four times with 10 mL of dichloromethane, then four times with 10 mL methanol four times and lastly, four times with 10 mL ultra pure water respectively. After each washing, the cartridges were dried with nitrogen gas. A water sample (1 L) was then percolated through the cartridges with a flow rate of 10 mL/min. After extraction, the trapped PAHs were eluted into a glass vial by using 5 mL dichloromethane two times. The eluate was carefully evaporated with a gentle stream of nitrogen. Afterwards 4 mL acetonitrile was placed into the glass vial and then transferred to a 4-mL amber vial. A blank analysis of 1000 mL deionized was first performed followed by the procedure that was applied in sample analysis. No detectable concentrations of PAHs were present in any of the procedural blanks known amounts of 5 individual PAH compounds were added to the sample and the same experimental procedure was applied.
Results and discussion
Five priority PAHs were determined in drinking water samples from Samsun, Ordu, Giresun, Çorum, Amasya, Kastamonu and Sinop provinces. At the same time, PAHs were determined in water samples taken from the Abdal River which is a source of drinking water and usable water for Central Samsun. Also, various brands of natural spring water in pet bottles sold in stores were analyzed for the prescence of PAHs.
The limit of detection values (LOD) for the analyzed PAHs in the samples are given in Table 1. The accuracy of the method was determined by recovery studies. As illustrated in Table 2, the recoveries of the applied method are in the range of 82 to 103%, implying that the method can be accepted as being accurate. The repeatability of the method was determined by relative standard deviation (RSD). The RSD values, which are a measure of the precission of the method, were found to be below 1.5%. The results presented in Table 2 indicate that the performance of the method can be regarded as being reliable.
Table 1.
Method limit of detection (MDLs) for all PAHs studied in water samples
| PAHs | MDLs (ng/L) |
|---|---|
| BbF | 0.29 |
| BkF | 0.033 |
| BaP | 0.048 |
| InD + BgP | 0.66 |
Table 2.
Recovery values of PAHs in spiked water samples (n = 5)
| PAHs | Added PAH (μg/L) | Found PAH (μg/L) | Recovery % | Standard deviation (s) | Relative standard deviation (RSD) |
|---|---|---|---|---|---|
| BbF | 1.000 | 0.820 | 82.0 | 107 | 1.30 |
| BkF | 1.000 | 0.902 | 90.2 | 115 | 1.27 |
| BaP | 1.000 | 0.870 | 87.0 | 49 | 0.56 |
| InD + BgP | 2.000 | 2.056 | 102.8 | 153 | 1.49 |
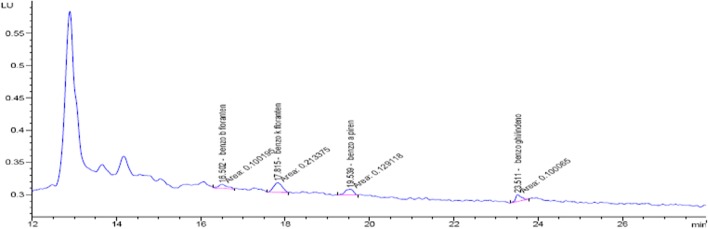
The concentration ranges and mean values of individual PAHs in drinking water are shown in Table 3. The chromatogram of a sample of drinking water taken from Central Samsun is presented in Fig. 2. For drinking water samples taken from Central Samsun, the average total PAH and BaP concentrations were found to be 2.73 ± 1.51 and 0.35 ± 0.24 ng/L, respectively. As for drinking water samples taken from Ordu, Giresun, Corum, Amasya, Kastamonu and Sinop, the average total PAH concentrations were found to be 5.85 ± 3.82 ng/L; 3.79 ± 1.27 ng/L; 1.08 ± 0.62 ng/L; 2.42 ± 1.04 ng/L; 1.92 ± 0.35 ng/L; 4.07 ± 2.33 ng/L respectively. The average BaP concentrations on the other hand were found to be 0.97 ± 0.75 ng/L; 0.55 ± 0.29 ng/L; 0.11 ± 0.08 ng/L; 0.35 ± 0.10 ng/L; 0.14 ± 0.04 ng/L; 0.39 ± 0.23 ng/L, respectively. Consequently, the values of PAH in drinking water were found below the values specified in the Regulation on Water Intended for Human Consumption. According to this regulation, BaP concentration should not exceed 10 ng/L, while total PAH concentration of the other 4 compounds (BbF, BkF, BgP and InD) should not be higher than 100 ng/L [20]. It is also important to note that the obtained results did not exceed the specified standards by the European Union (EU) (1998) (BaP: 10 ng/L, total PAH: 100 ng/L) and World Health Organization (WHO) (1999,1998) (BaP: 700 ng/L) for drinking water. Also the total concentration of the six specific PAHs (fluoranthene, BbF, BkF, BaP, BgP and InD) did not exceed the limit of 200 ng/L [16, 21]. However, there are no WHO standards specified in the year 2017 for PAH concentration [22] hence, the results of the study for total PAH concentration were evaluated according to the old standards.
Table 3.
Concentration ranges and mean values of PAHs in drinking water from Samsun, Ordu, Giresun, Corum, Amasya, Kastamonu and Sinop provinces
| PAH compounds | Range (ng/L) | Mean (ng/L) |
|---|---|---|
| Samsun | ||
| BbF | nd – 2.86 | 1.04 ± 0.61 (n = 48) |
| BkF | nd – 1.42 | 0.41 ± 0.18 (n = 48) |
| BaP | nd – 1.06 | 0.35 ± 0.24 (n = 48) |
| BgP + InD | nd – 2.56 | 0.94 ± 0.68 (n = 48) |
| ∑ PAHs | nd – 6.74 | 2.73 ± 1.51 (n = 48) |
| Ordu | ||
| BbF | 0.34–4.87 | 2.10 ± 1.43 (n = 124) |
| BkF | 0.18–3.83 | 1.25 ± 0.91 (n = 124) |
| BaP | 0.05–3.04 | 0.97 ± 0.75 (n = 124) |
| BgP + InD | nd – 5.84 | 1.53 ± 1.08 (n = 124) |
| ∑ PAHs | 0.63–17.5 | 5.85 ± 3.82 (n = 124) |
| Giresun | ||
| BbF | 0.73–5.10 | 1.64 ± 0.63 (n = 30) |
| BkF | 0.34–2.72 | 0.98 ± 0.34 (n = 30) |
| BaP | 0.11–1.70 | 0.55 ± 0.29 (n = 30) |
| BgP + InD | nd – 1.18 | 0.62 ± 0.45 (n = 30) |
| ∑ PAHs | 1.52–9.52 | 3.79 ± 1.27 (n = 30) |
| Çorum Central | ||
| BbF | nd – 2.38 | 0.41 ± 0.34 (n = 12) |
| BkF | nd – 0.39 | 0.15 ± 0.11 (n = 12) |
| BaP | nd – 0.37 | 0.11 ± 0.08 (n = 12) |
| BgP + InD | nd – 0.95 | 0.41 ± 0.28 (n = 12) |
| ∑ PAHs | nd – 2.93 | 1.08 ± 0.62 (n = 12) |
| Amasya | ||
| BbF | 0.88–2.31 | 1.40 ± 0.31 (n = 5) |
| BkF | nd – 0.95 | 0.41 ± 0.20 (n = 5) |
| BaP | 0.12–0.72 | 0.35 ± 0.10 (n = 5) |
| BgP + InD | nd – 1.08 | 0.27 ± 0.24 (n = 5) |
| ∑ PAHs | 1.00–3.98 | 2.42 ± 1.04 (n = 5) |
| Kastamonu Central and Hanonu Subprovince | ||
| BbF | 0.76–1.10 | 0.93 ± 0.22 (n = 3) |
| BkF | 0.25–0.66 | 0.46 ± 0.18 (n = 3) |
| BaP | 0.11–0.17 | 0.14 ± 0.04 (n = 3) |
| BgP + InD | nd – 0.78 | 0.39 ± 0.15 (n = 3) |
| ∑ PAHs | 1.90–1.93 | 1.92 ± 0.35 (n = 3) |
| Sinop | ||
| BbF | nd – 5.66 | 2.31 ± 2.01 (n = 9) |
| BkF | nd – 0.69 | 0.37 ± 0.26 (n = 9) |
| BaP | 0.13–0.75 | 0.39 ± 0.23 (n = 9) |
| BgP + InD | nd – 1.35 | 1.00 ± 0.41 (n = 9) |
| ∑ PAHs | 1.39–7.93 | 4.07 ± 2.33 (n = 9) |
BbF:Benzo[b]fluoranthene; BkF:Benzo[k]fluoranthene; BaP:Benzo[a]pyrene; BgP: Benzo[g,h,i]perylene; InD:Indeno[1,2,3 c,d]pyrene
nd: Not detected
n: The number of samples used in the calculation of standard deviation
Fig. 2.
Chromatogram of drinking water samples taken from the central of Samsun
The obtained results in descending order from lowest to highest value for the studied districts in each province are presented in Table 4. Amongst the studied provinces, the highest average total PAH and highest average BaP concentration were for Ordu province while the lowest was for Çorum, Amasya and Kastamonu provinces. The reason for the high concentration determined in water samples from Ordu may be due to a constant rainy climate and constant floods or due to usage of coal as a source heating instead of natural gas. The other reason could be that Ordu is a city located along the main road on the Black Sea.
Table 4.
Maximum and mininum values of PAH in drinking according studies provinces
| ∑ PAH ng/L | BaP ng/L | ||||
|---|---|---|---|---|---|
| Merkez | 0.64 | Minimum | Bafra | 0.1 | SAMSUN |
| Havza | 6.47 | Maximum | Kavak | 1.06 | |
| Akkus | 0.63 | Minimum | Akkus | 0.12 | ORDU |
| Ikizce | 17.5 | Maximum | Korgan | 0.42 | |
| Tirebolu | 1.52 | Minimum | Espiye | 0.11 | GİRESUN |
| Kesap | 9.52 | Maximum | Kesap | 1.7 | |
| Tasova | 1 | Minimum | Tasova | 0.12 | AMASYA |
| Merkez | 3.98 | Maximum | Merkez | 0.72 | |
| Hanonu | 1.9 | Minimum | Hanonu | 0.11 | KASTAMONU |
| Merkez | 1.93 | Maximum | Merkez | 0.17 | |
| Gerze | 1.39 | Minimum | Ayancik | 0.13 | SİNOP |
| Boyabat | 7.93 | Maximum | Dikmen | 0.75 | |
| Merkez ortalama | 0.67 | Merkez ortalama | 0.11 | ÇORUM |
Obtained results were compared with the available literature on the determination of PAH concentrations in drinking water and surface water in other regions in Turkey. The results for this study were in line with the previous studies done in Turkey. As reported by Caylak and Tokar in 2012, the total PAH concentration in drinking water samples in Cankiri region was found to be below the detection limits of the applied method in our study [13]. Also in another study done by Demir and Ergin in 2011 in the city of Tunceli in eastern Turkey, the total PAH concentrations were as well found to be below the detection limits set in the method applied in this study for drinking water [14]. It is worth noting that the PAHs concentrations for drinking water in Samsun were found to be much less than the concentrations reported for other Turkish cities such as Tunceli and Cankiri. BaP is an important indicator of carcinogenic PAHs. The average BaP concentration that was found for drinking water in Samsun and other cities was lower while the concentration found for Ordu was higher than the concentration reported for Cankiri.
The results obtained in this study were observed to be lower than those found in literature for studies conducted in other countries. For example, the mean PAH level reported in a study that was carried out by Karyab et al. (2013) [23] in Tehran (Iran) was 36.30 ng/L in drinking water which was higher than the results for this study. In another study carried out in Lodz by Kabzinsk et al. (2002) in Poland, the total PAHs concentrations specifically for benzo[a]pyrene, benzol[g.h,i] perylene, benzo[b]fluoranthene, benzo[k]fluoranthene, indeno [1,2,3-c,d)pyrene,benzo[a]anthracene, chrysene and dibenzo[a,h]anthracene in drinking water samples were detected to be in the range of 126–9.815 ng/L [24] which is higher than the values that were obtained in our study.
The results for concentration values of PAHs in water samples taken from Abdal, Kizilirmak and Yesilirmak rivers in Samsun are shown in Table 5. Data from the table clearly indicates that water samples taken from Abdal River between the month of January and April 2011 were high with total PAH concentrations of 119 and 16.7 ng/L respectively. BaP concentrations obtained with the same period also were determined as 13.2 and 0.09 ng/L. As for water samples taken from Kizilirmak and Yesilirmak in the month of April 2011, the total PAH concentrations were found to be 45.8 and 15 7 ng/L while the BaP concentrations were determined as 0.23and 0.09 ng/L respectively.It is thought that the high PAHs concentration values obtained in the month of January are as a result of high rainfall and snowfall which brings about an increase in the usage of coal as a fuel.
Table 5.
Concentration values of PAHs in water samples taken from Abdal river, Kizilirmak and Yesilirmak
| BbF (ng/L) * |
BkF (ng/L) * |
BaP (ng/L) * |
BgP + InD (ng/L) * |
Total PAH (ΣPAH) (ng/L) * |
|
|---|---|---|---|---|---|
| Abdal river-January 2011 | 14.0 ± 4.9 | 72.9 ± 25.5 | 13.2 ± 4.62 | 18.7 ± 6.5 | 119 ± 43 |
| Abdal river-April 2011 | 1.02 ± 0.41 | 14.4 ± 5.8 | 0.09 ± 0.03 | 1.10 ± 0.44 | 16.7 ± 7.2 |
| Kizilirmak- April 2011 | 2.51 ± 0.80 | 41.8 ± 13.4 | 0.23 ± 0.07 | 1.31 ± 0.41 | 45.8 ± 14.8 |
| Yesilirmak- April 2011 | 0.66 ± 0.20 | 13.6 ± 4.2 | 0.09 ± 0.03 | 1.36 ± 0.42 | 15.7 ± 4.9 |
*The mean and standard deviation values (n = 3)
The results obtained from the studied rivers were compared with studies carried out in other countries. For example Zhu et al. (2008) reported the average total PAH (for BbF, BkF, BaP, Ind, BgP) concentrations in water samples taken from Qiantang River, which is a source of drinking water for China’s Zhejiang province, to be 13.3 ng/L [17]. Sun et al. (2009) also reported the mean total PAH (BbF, BkF. BaP, Ind, BgP) concentration of water samples from the Yellow River of China to be 2.669 ng/L [18]. In another study done by Li et al. (2010), the mean total PAH (BbF, BkF. BaP, Ind, BgP) concentration of water samples taken from Aojiang River in China was reported as 16.1 ng/L [25]. Wang et al. (2018) also reported the mean total PAH (BbF, BkF, BaP, Ind, BgP) concentration of water samples from Liaohe River in China as 48.48 ng/L during the flooding period [26]. The total PAH concentration for water samples obtained in January 2011 from Abdal river in Samsun was found to be significantly higher than the values above. However, the total PAH concentration in the water samples taken from the Kizilirmak River in April 2011 was found to be similar to the values in the literature mentioned.
Additionally, the results of this study revealed that the concentrations of total PAH in water sampled from Abdal river, Kizilirmak and Yesilirmak were significantly lower than the mean value which was reported as 92.908 ng/L for water samples taken from Jinsha River in Southwest China by Huang et al. (2003) [27]. Also, our values were lower than the values found by Cao et al. (2005) [28] for surface waters (main rivers, tributaries, trenches, etc.) in Tianjin in North China (367 ng/L).
Various brands of natural spring water sold in pet bottles in stores were analyzed for PAHs and the obtained concentration values are shown in Table 6. All the results for natural spring water samples presented here were seen to be below the specified values in the Regulation on Water Intended for Human Consumption and EU Directive 98/93 [8]. The results in our study were compared with other studies done on various brands of natural spring water in pet bottles since there was no data on PAHs concentrations for five PAH compounds that is acceptable according to the Turkish legislation. The BaP concentrations found for bottled water in our study were lower than the concentrations range of 3.41–7.78 ng/L reported by Karyab et al. (2016) [29].
Table 6.
Concentration values of PAHs in various brands of natural spring water in pet bottles sold in stores
| NATURAL SPRING WATER | |||||
|---|---|---|---|---|---|
| Samples | BbF (ng/L)* |
BkF (ng/L)* |
BaP (ng/L)* |
BgP + InD (ng/L)* |
Total PAH (ΣPAH) (ng/L)* |
| (I) | 2.13 ± 0.24 | 1.39 ± 0.16 | 0.44 ± 0.09 | 2.19 ± 0.25 | 6.14 ± 0.80 |
| (II) | 0.80 ± 0.09 | 0.35 ± 0.05 | 0.22 ± 0.04 | 0.78 ± 0.10 | 2.15 ± 0.38 |
| (III) | 4.99 ± 0.93 | 2.86 ± 0.68 | 1.50 ± 0.47 | 5.05 ± 1.53 | 14.4 ± 5.66 |
| (IV) | 0.74 ± 0.11 | 0.15 ± 0.06 | 0.34 ± 0.09 | nd | 1.23 ± 0.17 |
| (V) | 0.75 ± 0.12 | 0.83 ± 0.11 | 0.45 ± 0.08 | 2.58 ± 0.37 | 4.61 ± 0.76 |
| (VI) | 0.89 ± 0.24 | 0.48 ± 0.12 | 0.22 ± 0.06 | 0.98 ± 0.17 | 2.57 ± 0.94 |
| (VII) | 1.01 ± 0.24 | 0.60 ± 0.10 | 0.16 ± 0.03 | 0.79 ± 0.15 | 2.56 ± 0.42 |
| (VIII) | 1.51 ± 0.22 | 0.19 ± 0.03 | 0.32 ± 0.05 | 0.91 ± 0.13 | 2.93 ± 0.47 |
*The mean and standard deviation values (n = 3)
nd: not detected
The total PAH concentrations in drinking and surface water from Samsun, Ordu, Giresun, Amasya Kastamonu and Sinop provinces were compared with one-way ANOVA calculations primarily on a provincial basis (i.e comparison of provincial centers and some of their districts). Sample averages for all cities were seen to be significantly different from each other (p < 0.05). When the average total PAH concentrations found in the city centers of Samsun, Ordu, Giresun, Çorum, Amasya, Kastamonu, and Sinop were compared, it was seen that the results of the samples were also significantly different from each other.
When the PAH concentrations for the different natural spring water samples were compared using one-way ANOVA, a significant difference (p < 0.05) was found. There was also a significant difference in PAH concentrations among the three rivers when a one-way ANOVA was done. All statistical calculations were done using SPSS and Excel program.
Conclusion
Five priority PAHs were determined in drinking and usable water samples from Samsun, Ordu, Giresun, Corum, Amasya, Kastamonu and Sinop provinces and water samples which were taken from Abdal, the river is a source of drinking water to the center of Samsun, Kizilirmak and Yesilirmak and various brands of natural spring water in pet bottles were purchased from a market in Samsun. The water PAH contamination levels were assessed by comparing the obtained values with the national and WHO drinking water quality guidelines. All the results except Abdal river january 2011 (for drinking, natural spring water and surface water) were detected as below the values specified in the Turkish legislation, EU legislation and WHO.
Although the concentration of PAH compounds in the analyzed drinking and usable water samples was found to be below the values specified in the Regulation on Water for Human Consumption and WHO, these values also appear to be high compared to drinking water in other places in the world. However, it is advised that water treatment and wastewater treatment facilities in the municipalities (in terms of PAHs) should be reviewed and improved. Furthermore, the use of natural gas as a fuel in Samsun and the surrounding provinces could immensly decrease the PAH concentration in the water sources. It is therefore recommended that necessary steps be taken to switch to the use of natural gas within the shortest period of time. However, it is thought that the amount of PAHs might increase in drinking water sources located around industries in time. Therefore, changes in the total amount of PAHs in drinking water and water sources should be monitored periodically every 10 years.
Acknowledgements
This study is summarized from the master thesis which was accepted by the Ondokuz Mayis University Natural Sciences Institutes. A vote of thanks goes to the Institute of Samsun Refik Saydam National Public Health Agency for allowing the of their laboratory and the help rendered during the sampling stage.
Compliance with ethical standards
Conflict of interest
The authors declare that there are no conflicts of interest.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Guo H, Lee SC, Ho KF, Wang XM, Zou SC. Particle-Associated Polycyclic Aromatic Hydrocarbons in Urban Air of Hong Kong. Atmos Environ. 2003;37:5307–5317. doi: 10.1016/j.atmosenv.2003.09.011. [DOI] [Google Scholar]
- 2.Celino JJ, Corseuil HX, Fernandes M, Garcia KS. Distribution and Sources of Polycyclic Aromatic Hydrocarbons in the Aquatic Environment: A Multivariate Analysis. Rem Revista Escola de Minas. 2010;63(2):211–218. doi: 10.1590/S0370-44672010000200003. [DOI] [Google Scholar]
- 3.Guo W, He M, Yang Z, Lin C, Quan X, Wang H. Distribution of Polycyclic Aromatic Hydrocarbons in Water, Suspended Particulate Matter and Sediment from Daliao River Watershed, China. Chemospher. 2007;68:93–104. doi: 10.1016/j.chemosphere.2006.12.072. [DOI] [PubMed] [Google Scholar]
- 4.Amoako J, Osmund D, Ansa-Asare OD, Karikari AY, Dartey G. Levels of Polycyclic Aromatic Hydrocarbons (PAHs) in the Densu River Basin of Ghana. Environ Monitor Assess. 2011;174:471–480. doi: 10.1007/s10661-010-1471-y. [DOI] [PubMed] [Google Scholar]
- 5.Yurchenko S, Molder U. The Determination of Polycyclic Hydrocarbons in Smoked Fish by Gas chromatography Mass Spektrometry with Positive-ion Chemical Ionization. J Food Compos and Anal. 2005;18:857–869. doi: 10.1016/j.jfca.2004.11.004. [DOI] [Google Scholar]
- 6.Goodarzi PK. Mukhopadhyay (Muki): Metals and Polyaromatic Hydrocarbons in the Drinking Water of the Sydney Basin, Nova Scotia,Canada: A Preliminary Assessment of their Source. Int J Coal Geology. 2000;43:357–372. doi: 10.1016/S0166-5162(99)00067-1. [DOI] [Google Scholar]
- 7.Girelli AM, Apriceno A, Tarola AM, Tortora F. Determination of Polycyclic Aromatic Hydrocarbons in Tea Infusions Samples by High Performance Liquid Chromatography with Fluorimetric Detection. J Food Quality. 2017;2017:1–7. doi: 10.1155/2017/1076876. [DOI] [Google Scholar]
- 8.Urbe I, Ruana J. Application of Solid-Phase Extraction Discs with a Glass Fiber Matrix to Fast Determination of Polycyclic Aromatic Hydrocarbons in Water. J Chromatogr A. 1997;778:337–345. doi: 10.1016/S0021-9673(97)00539-6. [DOI] [PubMed] [Google Scholar]
- 9.Hii TM, Basheer C, Lee HK. Commercial Polymeric Fiber as Sorbent for Solid-Phase Microextraction Combined with High-Performance Liquid Chromatography for the Determination of Polycyclic Aromatic Hydrocarbons in Water. J Chromatogr A. 2009;1216:7520–7526. doi: 10.1016/j.chroma.2009.09.019. [DOI] [PubMed] [Google Scholar]
- 10.Olivella MA, Ribalta TG, de Febrer AR. Mollet JM, de las Heras FXC: Distribution of Polycyclic Aromatic Hydrocarbons in Riverine Waters after Mediterranean Forest Fires. Sci Total Environ. 2006;355:156–166. doi: 10.1016/j.scitotenv.2005.02.033. [DOI] [PubMed] [Google Scholar]
- 11.Tillner J, Hollard C, Bach C, Rosin C, Munoz JF, Dauchy X. Simultaneous Determination of Polycyclic Aromatic Hydrocarbons and their Chlorination by-Products in Drinking Water and the Coatings of Water Pipes by Automated Solid Phase Microextraction Followed by Gas Chromatography- Mass Spectrometry. J Chromatogr A. 2013;1315:36–46. doi: 10.1016/j.chroma.2013.09.047. [DOI] [PubMed] [Google Scholar]
- 12.Caylak E, Tokar M. Investigating Chemical and Microbiological Contaminants in Drinking Water of Cankiri Province, Turkey. Environ Earth Sci. 2012;67(7):2015–2025. doi: 10.1007/s12665-012-1641-z. [DOI] [Google Scholar]
- 13.Demir V, Ergin S. Occurrence and Assessment of Chemical Contaminants in Drinking Water in Tunceli, Turkey. J Chem. 2013;2013:1–6. doi: 10.1155/2013/238374. [DOI] [Google Scholar]
- 14.Wilson WB, Campiglia AD. Determination of Polycyclic Aromatic Hydrocarbons with Molecular Weight 302 in Water Samples by Solid-Phase Nano-Extraction and Laser Excited Time-Resolved Shpol’skii Spectroscopy. Analyst. 2011;136:3366–3374. doi: 10.1039/c1an15309a. [DOI] [PubMed] [Google Scholar]
- 15.Badawy MI, Emababy MA. Distribution of Polycyclic Aromatic Hydrocarbons in Drinking Water in Egypt. Desalination. 2010;251(1–3):34–40. doi: 10.1016/j.desal.2009.09.148. [DOI] [Google Scholar]
- 16.Zhu L, Chen Y, Zhou R. Distribution of polycyclic aromatic hydrocarbons in water, sediment and soil in drinking water resource of Zhejiang Province, China. J Hazar Mater. 2008;150:308–316. doi: 10.1016/j.jhazmat.2007.04.102. [DOI] [PubMed] [Google Scholar]
- 17.Sun JH, Wang GL, Chai Y, Zhang G, Li J, Feng J. Distribution of Polycyclic Aromatic Hydrocarbons (PAHs) in Henan Reach of the Yellow River, Middle China. Ecotoxicol and Environ Safety. 2009;72:1614–1624. doi: 10.1016/j.ecoenv.2008.05.010. [DOI] [PubMed] [Google Scholar]
- 18.Srogi K. Monitoring of Environmental Exposure to Polycyclic Aromatic Hydrocarbons: a Review. Environ Chem Lett. 2007;5:169–195. doi: 10.1007/s10311-007-0095-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Anonym: Su kirliliğiyönetmeliği numune alma ve analiz metodları tebliği, 2004.https://mevzuattakip.com.tr/mevzuat/su-kirliligi-kontrolu-yonetmeligi-numune-alma-ve- analiz-metodlari-tebligi
- 20.Hodgeson JW, Bashe WJ, Baker TV: Method 550.1 Determination of Polycyclic Aromatic Hydrocarbons in Drinking Water by Liquid-Solid Extraction and HPLC with Coupled Ultraviolet and Fluorescence Detection, Environmental Monitoring Systems Laboratory Office Of Research And Development, U.S.EPA, Cincinnati, Ohio 45,268, 1990, p.
- 21.Anonym: Regulation on Water Intended for Human Consumption, 2005. http://www.mevzuat.net/legislation/item/5458.
- 22.World Health Organizations (WHO): Guidelines for Drinking-water Quality, 4th edition. World Health Organization; 2011.https://apps.who.int/iris/bitstream/handle/10665/44584/9789241548151_eng .pdf;jsessionid = B8AF3CA0F8903EF918F0D217861D8A23?sequence = 1
- 23.Karyab H, Yunesian M, Nasseri S, Mahvi AH, Ahmadkhaniha R, Rastkari N, Nabizadeh R. Polycyclic Aromatic Hydrocarbons in Drinking Water of Tehran, Iran. Iran J Environ Health Sci Eng. 2013;25(11):1–7. doi: 10.1186/2052-336X-11-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kabzinski AKM, Cyran J, Juszczak R: Determination of Polycyclic Aromatic Hydrocarbons in Water (Including Drinking Water) of Lodz. Pol J Environ Stud 2002, 11(6): 695–706. http://www.pjoes.com/Determination-of-Polycyclicaromatic- hydrocarbons-in-water-including-drinking-water,87,510,0,2.html
- 25.Li J, Shang X, Zhao Z, Tanguay RL, Dong Q, Huang C. Polycyclic Aromatic Hydrocarbons in Water, Sediment, Soil, and Plants of the Aojiang River Waterway in Wenzhou, China. J Hazar Mater. 2010;173(1–3):75–81. doi: 10.1016/j.jhazmat.2009.08.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang H, Liu Z, Sun L, Wang Y, Luo Q, Wu H, Wang X. Characterization and Risk Assessment of Polycyclic Aromatic Hydrocarbons in Surface Water from Liaohe River, Northeast China. Polycyclic Aromatic Compounds. 2018;5:389–401. doi: 10.1080/10406638.2016.1220960. [DOI] [Google Scholar]
- 27.Huang J, Zhang Z, Yu G. Occurrence of Dissolved PAHs in the Jinsha River (Panzhihua)-Upper Reaches of the Yangtze River, Southwest China. J Environ Monit. 2003;5:604–609. doi: 10.1039/B210670A. [DOI] [PubMed] [Google Scholar]
- 28.Cao Z, Wang Y, Ma Y, Xu Z, Shi G, Zhuang Y, Zhu T. Occurrence and Distribution of Polycyclic Aromatic Hydrocarbons in Reclaimed Water and Surface Water of Tianjin, China. J Hazar Mater. 2005;122(1–2):51–59. doi: 10.1016/j.jhazmat.2005.04.003. [DOI] [PubMed] [Google Scholar]
- 29.Karyab H, Yunesian M, Nasseri S, Rastkari N, Mahvi AH, Nabizadeh R. Carcinogen Risk Assessment of Polycyclic Aromatic Hydrocarbons in Drinking Water, Using Probabilistic Approaches, Iran. Iran J Public Health. 2016;45(11):1455–1464. [PMC free article] [PubMed] [Google Scholar]