Abstract
The complex scenario of multiple sclerosis (MS) pathology involves several mechanisms, including oxidative stress response. The heat shock proteins (HSPs) are important for the protection of the cells; however, their role in MS is not clear. The present research is focused on the response of peripheral blood mononuclear cells (PBMCs) to oxidative stress and to the involvement of HSP70-2 (a protein coded by the HSPA1B gene, located in the MHC class III). To this aim, we challenged PBMCs from MS patients and healthy controls with hydrogen peroxide. Specifically, PBMCs mitochondrial activity, HSP70-2 protein expression and the production of intracellular reactive oxygen species were assessed. These parameters were also related to the HSP70-2 rs1061581 polymorphism, which is linked to the risk of developing MS. Moreover, mitochondrial activity and HSP70-2 protein levels were also related to disease severity. Overall, our results indicate that PBMCs, from both MS patients and healthy controls, may display a similar response towards an oxidative insult; within this context, HSP70-2 does not seem to be central in the protection of PBMCs. Nevertheless, the HSP70-2 rs1061581 polymorphism is related to ROS levels and appears to have a role in the different expression of HSP70-2 under oxidative stimulus.
Keywords: Multiple sclerosis, HSP70-2, rs1061581, Oxidative stress, PBMCs, Hydrogen peroxide
Introduction
Multiple sclerosis (MS), a chronic autoimmune disease of the central nervous system (CNS), characterized by demyelination and neurodegeneration, affects mainly young people between 20 and 40 years (Dendrou et al. 2015). So far, the etiology of the disease is not fully understood, although it is well established that genetic and environmental factors play a fundamental role, influencing the risk of developing MS and its progression (Koch et al. 2013; Dendrou et al. 2015).
The alteration of the immune system is the main pathogenic mechanism (Herz et al. 2010). Autoreactive T cells disrupting the blood-brain barrier (BBB) invade the CNS, thus leading to an inflammatory process. This inflammatory event takes advantage of the secretion of pro-inflammatory chemokines and the recruitment of immune system cells, such as macrophages. Further, the relevant involvement of B cells in MS has also gained ground in the last years, and their importance is underscored by the development of B cell-depleting therapies (Sabatino et al. 2019). Indeed, B cells act as antigen-presenting cells activating T cells and regulate the inflammatory processes thought the production of cytokines (Bigaut et al. 2019). The resident microglia result activated as well, contributing to the inflammatory process, which in turn leads to demyelination, oligodendrocyte loss, axonal, and neuronal injury (Yong and Marks 2010). Moreover, with particular reference to MS, oxidative stress further contributes to neuroinflammation and neurodegeneration (Haider et al. 2011); indeed, the resident activated microglia and the infiltrated macrophages release reactive oxygen species (ROS) and reactive nitrogen species (Lassmann et al. 2012). These reactive species greatly contribute to the development of inflammation, triggering the immune response (van der Goes et al. 1998; Van der Goes et al. 2001), and axonal injury (van Horssen et al. 2011).
To counterbalance neuroaxonal injury, the upregulation of antioxidant enzymes at the level of lesions (van Horssen et al. 2010) and the production of protective molecules, such as the heat shock proteins (HSPs), occur (Stahnke et al. 2007).
HSPs are highly conserved molecular chaperones expressed in normal physiological conditions and exerting housekeeping functions; they also act in response to different stress stimuli, including heat shock, hypoxia, oxidative stress, and viral/bacterial infections (Njemini et al. 2007; Dai et al. 2007; Kalmar and Greensmith 2009). In physiological conditions, HSPs act as molecular chaperones, contributing, for example, to the correct folding and preventing protein aggregation (Craig et al. 1993; Hartl 1996). Inducible HSPs can trigger anti-apoptotic mechanisms (Beere 2004), promote ubiquitination and degradation of misfolded proteins (Park et al. 2007), and exert an extracellular action, being released by secretory mechanisms or after necrotic cell death (Turturici et al. 2011).
Among HSPs, the HSP70 family is the most evolutionary conserved (Muchowski and Wacker 2005). At the level of MS lesions, it has been demonstrated an increase in HSP70s protein levels, likely in response to inflammation and oxidative stress (Aquino et al. 1997; Chabas et al. 2001). HSP70s exert a protective role in the CNS, thanks to their chaperone functions (Turturici et al. 2011). In particular, in MS, the inflammatory and oxidative conditions can lead to the overexpression of HSP70s inside the plaques (Chabas et al. 2001; Mansilla et al. 2012). However, within the context of MS, HSP70s display a dual role: on one side, they can protect neurons when released by glial cells during the relapse neurodegenerative phase; on the other, they can act as pro-inflammatory cytokines and adjuvants for myelin peptides, thus exacerbating the auto-immune attack (Asea et al. 2000; Mansilla et al. 2012).
Three genes encoding HSP70 are located within the HLA class III region (chromosome 6p21.3): HSPA1A, HSPA1B, and HSPA1L. The two stress-inducible proteins, namely, HSP70-1 and HSP70-2, are encoded by HSPA1A and HSPA1B genes respectively; HSPA1L codes for a constitutively expressed non-inducible protein: HSP70-HOM (Daugaard et al. 2007). These genes are highly polymorphic and the variants may influence their function and expression (Favatier et al. 1997). The HSP70-2 +1267 A/G (rs1061581) polymorphism localizes in the coding region of HSPA1B gene and causes a synonymous mutation that seems to influence RNA translation (Goate et al. 1987); this polymorphism has been associated with other autoimmune diseases, such as systemic lupus erythematosus (Pablos et al. 1995) and Crohn’s disease (Klausz et al. 2005). We have published that, in the Caucasian population, the HSP70-2 rs1061581 polymorphism is related to the risk of developing MS, where the G allele frequency is increased in MS patients compared to healthy controls (Boiocchi et al. 2014). Moreover, a strong association between HSP70-HOM rs2227956 polymorphism and MS risk and disease severity was reported, independently from the association with HSP70-2 rs1061581 (Boiocchi et al. 2016).
Taking advantage of our previous works, the aim of the present study was to investigate ex vivo the influence of oxidative stress (hydrogen peroxide) on peripheral blood mononuclear cells (PBMCs) from MS patients and healthy controls and to assess the possible involvement of HSP70-2. PBMCs mitochondrial activity (MTT levels), HSP70-2 protein expression, and the production of intracellular ROS have been analyzed. In addition, HSP70-2 (rs1061581) polymorphism has been related to the MTT levels, to the expression of HSP70-2 protein, and to the intracellular ROS levels, and the progression of the disease was also taken into account.
Materials and methods
Subjects and ethics statement
In this study, we included patients with a diagnosis of MS, according to the 2010 McDonald criteria (Polman et al. 2011), randomly selected from the pool of clinically stable patients of the MS Center of the IRCCS National Neurological Institute C. Mondino, Pavia, Italy. Clinical stability (i.e., remission phase) was defined as the absence of clinical relapse and/or absence of treatment with steroids within 8 weeks prior to the inclusion time and was requested to decrease the potential variability of HSP70-2 levels. Healthy controls, as judged by regular checks, matched for Caucasian ethnicity, were provided by the Immunogenetics Laboratory, Immunohematology and Transfusion Centre, Fondazione IRCCS, Policlinico San Matteo, Pavia, Italy. MS patients’ clinical characteristics were recorded at the time of blood sampling. In particular, we detailed the type of MS (Polman et al. 2011) and recorded the number of clinical relapses that occurred 1 year before blood collection in order to calculate the annualized relapse rate (ARR). Neurological disability was quantified by the Expanded Disability Status Scale (EDSS) and the clinical impact of MS was calculated applying the Multiple Sclerosis Severity Score (MSSS), that correlates EDSS score to disease duration (Roxburgh et al. 2005).
The study was approved by the ethics committee of the IRCCS National Neurological Institute C. Mondino and was conducted according to the principles expressed in the Declaration of Helsinki.
Gene polymorphism analysis
Whole blood was collected by venipuncture in Vacutainer tubes containing ethylenediaminetetraacetic acid (EDTA, BD). Human genomic DNA was obtained from 200 μl of whole blood using the QIAamp DNA Blood Mini Kit (QIAGEN) following the manufacturer's instructions. The concentration and purity of DNA were determined by spectrophotometric analysis. In order to establish alleles and genotypes for the investigated polymorphism (rs1061581), a polymerase chain reaction-restriction fragment length polymorphism (PCR-RFLP) was used, followed by digestion with the appropriate restriction enzyme, as previously described (Boiocchi et al. 2016). Genotype frequencies of HSP70-2 rs1061581 polymorphism in the control groups did not deviate from the Hardy-Weinberg equilibrium (p > 0.05).
PBMCs isolation from whole blood
PBMCs were isolated from the blood of MS patients and healthy controls. Ten milliliters of blood was diluted 1:1 with Ficoll (Histopaque-1077, Sigma-Aldrich) and centrifuged at 450×g for 30 min. PBMCs were collected from the ring above Ficoll and were washed with phosphate-buffered saline 1× (PBS). PBMCs were resuspended in RPMI 1640 medium (EuroClone), supplemented with 10% fetal bovine serum, 1% L-glutamine, penicillin (100 U/ml), and streptomycin (100 μg/ml), and cultured at 37 °C in 5% CO2 atmosphere.
Cell treatments and MTT assay
To study the influence of oxidative stress on PBMCs from MS patients, we treated both PBMCs from MS and healthy subjects with H2O2 and performed an MTT assay.
Briefly, PBMCs were seeded into 96-well plates at a density of 5.0 × 104 cells/well in 100 μl of RPMI 1640 medium and incubated at 37 °C in 5% CO2 atmosphere. Cells were treated with 10 μM H2O2 (Sigma-Aldrich) for 15 min or 3 h. After the oxidative treatment, the medium with H2O2 was replaced with fresh RPMI 1640 medium, and the mitochondrial function of the cells was estimated using MTT [3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide] assay. Ten microliters of MTT (final concentration of 1 mg/ml, Sigma-Aldrich) was added to each well. Cells without H2O2 treatment were considered as internal control. After 4 h at 37 °C, formazan crystals were solubilized with 100 μl of lysis buffer [20% sodium dodecyl sulfate (SDS) in 50% dimethylformamide] and incubated at 37 °C overnight. Absorbance values were measured at 595 nm in a microplate reader (SynergyHT, BioTek Instruments, Inc.), and the results were expressed as arbitrary units (AU).
Cell treatments and western blotting analysis
PBMCs were seeded into 6-well plates at a density of 3.0 × 106 cells/well in 1 ml of RPMI 1640 medium and incubated at 37 °C in 5% CO2 atmosphere.
Cells were treated with an oxidative stress stimulus, as described in the “Cell treatments and MTT assay” section, washed with PBS and collected using a cell scraper. The cellular pellet was stored at − 80 °C for subsequent western blotting analysis.
PBMCs were directly resuspended in SDS protein gel loading solution, sonicated, and boiled for 5 min. Proteins were separated by 12% SDS-polyacrylamide gel electrophoresis and processed as previously described (Osera et al. 2011). The anti-HSP70-2 rabbit monoclonal antibody (Enzo Lifescience; ADI-SPA-812-D) was diluted 1:750, and the mouse monoclonal antibody anti-α-tubulin (Sigma-Aldrich; T9026) was diluted at 1:1000 in TBST buffer [10 mM Tris-HCl, 100 mM NaCl, 0.1% (v/v) Tween 20, pH 7.5] containing 6% (v/v) milk. The nitrocellulose membrane signals were detected by chemiluminescence; α-tubulin was used to normalize the data. The analyses were performed on the densitometric values obtained using the NIH Image software (http://rsb.info.nih.gov/nih-image) after image acquisition.
Cell treatments and detection of reactive oxygen species
ROS formation was measured by using the fluorescent probe 2′,7′-dichlorofluorescein diacetate (DCFH-DA; VWR International PBI, Milan, Italy), which forms 2′,7′-dichlorofluorescein when oxidized by ROS.
Cells were seeded into a 96-well plate at a density of 5.0 × 104 cells/well in 100 μl of RPMI 1640 medium and incubated at 37 °C in 5% CO2 atmosphere. After H2O2 treatment, the medium was removed and cells were washed with PBS, loaded with dimethylsulfoxide (DMSO), resuspended in PBS containing 10 μM DCFH-DA, and placed in the incubator. After 30 min, PBMCs were washed with PBS and the fluorescent intensity was measured at 485 nm in the microplate reader (SynergyHT, BioTek Instruments, Inc.).
Statistical analysis
The basic features of MS and healthy controls samples, cell treatments, and MTT assays will be summarized by the appropriate descriptive statistics.
For MTT, HSP70-2, and ROS endpoint variables, a non-parametric approach was chosen.
The association between the endpoint variables with gender was studied using the Mann-Whitney test, while the association with age using Spearman’s rank correlation coefficient. Friedman test for the evaluation of the effect of the time of exposure (T0, T15 min, T3 h) was used, followed, when significant, by a post hoc sign test with correction for multiple comparisons. Mann-Whitney test was carried out for the comparison of two groups (MS cases vs healthy controls and severe vs mild form of MS). Kruskal-Wallis test was performed for the comparison of the genotype categories (AA, AG, GG), followed, when significant, by a post hoc Mann-Whitney test with correction for multiple comparisons. The statistical interaction between time of exposure and clinical status (MS cases—healthy controls or severe—mild MS cases) or genotype factors was evaluated by graphical techniques.
A p value below 0.05 was considered statistically significant. All the analyses were performed using the Stata 14 statistical software (Stata Corporation, College Station, TX, USA).
Results
MTT levels
In order to investigate the influence of oxidative stress on PBMCs, the mitochondrial activity of PBMCs was analyzed in vitro by using the MTT assay.
We studied concentration/response curves to H2O2 of PBMCs cultures from 53 MS patients and 45 healthy controls.
Among MS patients, the mean age at the time of sample collection was 42.73 ± 12.38 years, 43.1% were males while among the healthy donors the mean age at the time of samples collection was 38.44 ± 13.96 years, where males were the 72.1% of the sample. 90.6% were relapse-remitting (RR) patients, 5.7% were primary progressive (PP), and 3.8% were secondary progressive (SP). The ARR range was 0–4 relapses. Specifically, in the year before blood sampling, 69.8% of the subjects were relapse-free, 9.4% had one relapse, 9.4% two relapses, 3.8% three relapses, and 1.9% four relapses.
We stratified the analysis by case-control status and HSP70-2 rs1061581 polymorphism. Due to the heterogeneity of the two groups (MS patients and healthy donors) with respect to gender and age, the association between MTT endpoint variable and gender or age was verified. Considering the different duration of oxidative treatment (time points: T0 without treatment, T15’ after 15 min of H2O2, T3h after 3 h of H2O2) separately, no significant association was found. Therefore, the following analyses were performed regardless of gender and age.
MTT levels stratified by case-control status
MTT levels of MS cases were not significantly different compared to healthy controls for every time point (U = 1138, p = 0.70 at T0, U = 1181, p = 0.94 at T15’, U = 1185, p = 0.96 at T3h); however, the analysis showed a statistically significant MTT variability over time both in cases (chi-square = 8.12, p = 0.017) and in controls (chi-square = 8.84, p = 0.011) (Fig. 1). Specifically, median MTT levels decreased after 15 min of oxidative stress among cases (z = − 2.20; p = 0.03) and controls (z = − 2.68; p = 0.007). Interestingly, after 3 h of oxidative stimulus, median basal levels were almost restored with respect to T0 in both groups: no significant difference was found between basal levels and 3 h of oxidative stress both in MS cases (z = − 0.97; p = 0.33) and controls (z = − 0.89; p = 0.371). MTT levels trend suggest no interaction effect between time and case-control status.
Fig. 1.
MTT levels in response to H2O2 treatment at different exposure times of PBMC cultures from MS patients and healthy controls. Median values (interquartile range) of MTT levels are reported
MTT levels stratified by HSP70-2 rs1061581 polymorphism genotype
We considered whether the HSP70-2 rs1061581 polymorphism could influence the PBMCs response to oxidative stress. We studied 40 AA subjects (41.2%), 39 AG subjects (40.2%), and 18 GG subjects (18.6%) because of a control subject with a missing genotype (Fig. 2).
Fig. 2.
MTT levels in response to H2O2 treatments at different exposure times of PBMC cultures from subjects (MS patients and healthy controls together) with different HSP70-2 rs1061581 genotype (AA, AG, and GG). Median values (interquartile range) of MTT levels are reported
The analysis did not show a statistically significant MTT variability among genotypes for each time point (chi-square = 0.67; p = 0.72 at T0; chi-square = 1.34; p = 0.51 at T15’; chi-square = 2.39; p = 0.30 at T3h) as well as it did not show any significant MTT variability over time for each genotype. Specifically, the median MTT levels, subdivided by genotype, appeared to decrease of some units after 15 min from the oxidative stress and increased afterward, but, even for the GG genotype, without statistically significant differences. MTT levels trend suggest no interaction effect between time and rs1061581 polymorphism genotype.
MTT levels stratified by MS severity
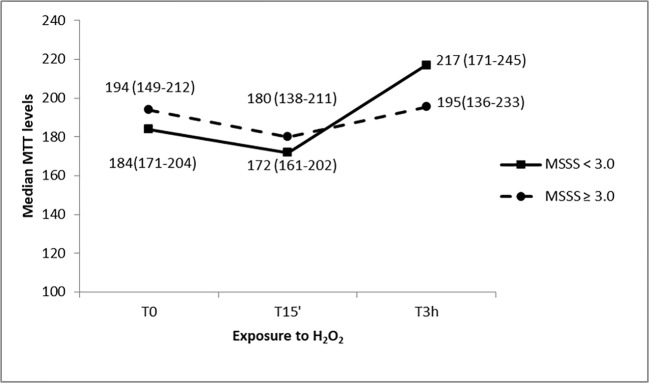
MS patients group was divided into two sub-groups: patients with a mild form of disease (MSSS < 3.0, N = 15) and subjects with a moderate to severe form of the disorder (MSSS ≥ 3.0, N = 16) (Fig. 3). The mean disease duration was 16.73 ± 8.43 and 12.88 ± 12.80 years, respectively. The analysis did not show a difference in MTT levels comparing mild vs severe MS patients at each time point (U = 113.5, p = 0.81 at T0; U = 119.0, p = 0.98 at T15’; U = 95, p = 0.34 at T3h); however, significance was borderline when evaluating MTT variability over time considering both mild (chi-square = 4.97; p = 0.089) and moderate/severe MS patients (chi-square = 4.98; p = 0.084), where PBMCs from patients with a mild form of MS showed higher MTT levels 3 h after oxidative stress with respect to the other time points.
Fig. 3.
MTT levels in response to H2O2 treatments at different exposure times of PBMC cultures from MS patients with a mild form of disease (MSSS < 3.0) and a moderate to severe form of the disorder (MSSS ≥ 3.0). Median values (interquartile range) of MTT levels are reported
HSP70-2 protein levels
HSP70-2 protein expression was evaluated following H2O2 treatments on PBMCs from 61 MS patients and 45 healthy donors. Protein expression was quantified by western blotting analysis. Among MS patients, the mean age at the time of sample collection was 47.77 ± 11.82 years, 29.8% were males while among the healthy donors the mean age at the time of sample collection was 32.69 ± 11.18 years, where males were the 61.4% of the sample. 91.8% were RR, 1.6% were PP and 6.6% were SP patients. ARR range was 0–3 relapses. 68.9% of the subjects were relapse-free, 13.1% had one relapse, 3.3% two relapses, and 3.3% three relapses in the year before blood sampling.
No significant association was found between HSP70-2 endpoint variable and gender or age; therefore, the following analyses were performed regardless of gender and age.
HSP70-2 protein levels stratified by case-control status
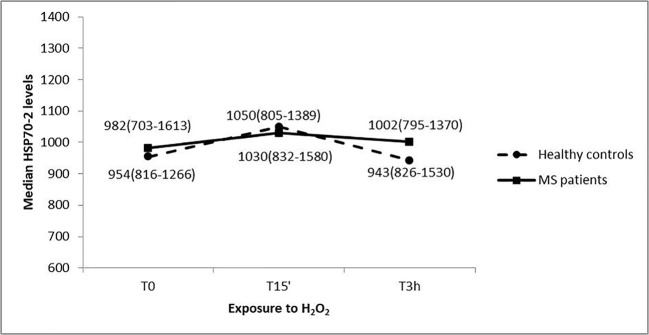
The HSP70-2 protein levels analysis showed no significant difference between cases and control status at each level of exposure time to oxidative treatment (U = 1331.5, p = 0.79 at T0; U = 1304.0, p = 0.66 at T15’; U = 1356.5 at T3h, p = 0.92) (Fig. 4).
Fig. 4.
HSP70-2 protein levels in response to H2O2 treatments at different exposure times of PBMC cultures from MS patients and healthy subjects. Median values (interquartile range) of HSP70-2 protein expression are reported
Furthermore, the exposure time to H2O2 did not change significantly the protein expression for both MS cases (Friedman’s chi-square = 1.70, p = 0.43) and healthy controls (Friedman’s chi-square = 1.11, p = 0.60). HSP70-2 protein levels trend suggest no interaction effect between time and case-control status.
HSP70-2 levels stratified by HSP70-2 rs1061581 polymorphism genotype
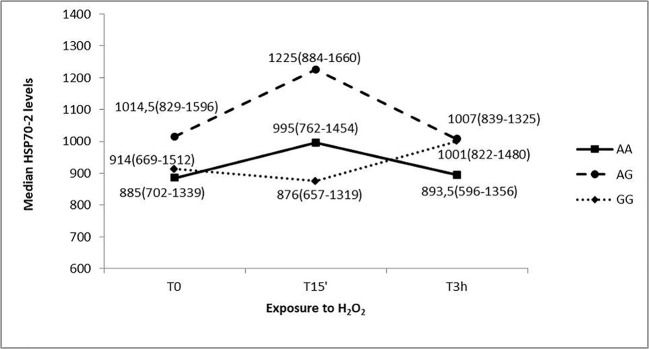
We also assessed the trend of the HSP70-2 protein expression pattern related to the HSP70-2 rs1061581 polymorphism genotype. We studied 36 AA subjects (34.6%), 48 AG subjects (46.2%), and 20 GG subjects (19.2%), with 2 missing genotypes (a control subject and a MS patient) (Fig. 5).
Fig. 5.
HSP70-2 protein levels in response to H2O2 treatments, at different exposure times, of PBMC cultures with different HSP70-2 rs1061581 genotype (AA, AG, and GG) (MS patients and healthy controls together). Median values (interquartile range) of HSP70-2 protein expression are reported
The analysis did not show a statistically significant variability among genotypes at T0 and T3h time points (chi-square = 0.92; p = 0.63; chi-square = 3.17; p = 0.20 respectively); while at T15’ the variability resulted borderline significant (chi-square = 6.01, p = 0.05). Moreover, the analysis did not show a statistically significant variability overtime points for every genotype. HSP70-2 protein levels trend suggest no interaction effect between time and rs1061581 polymorphism genotype.
HSP70-2 protein expression stratified by MS severity
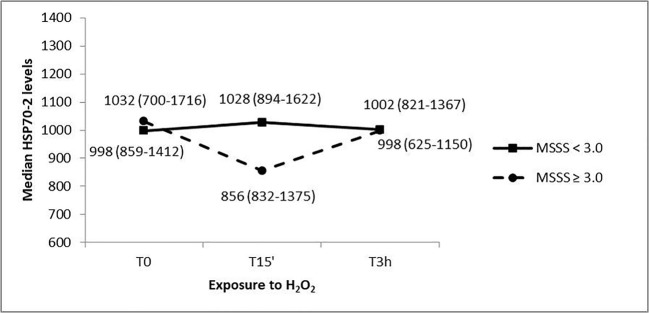
HSP70-2 protein expression was analyzed in the group of MS patients, dividing them into two sub-groups based on severity: patients with a mild form of disease (MSSS < 3.0, N = 23) and subjects with a moderate to severe form of the disorder (MSSS ≥ 3.0, N = 11) (Fig. 6). The mean disease duration was 12.59 ± 9.25 and 20.0 ± 13.73 years, respectively. A different trend in the variation of the HSP70-2 protein levels was detected: MS patients with higher severity showed a decrease in HSP70-2 after 15 min of oxidative treatment and then after 3 h of oxidative stress the protein levels came back to basal values. On the other hand, PBMCs from MS patients with a lower MSSS did not show any change in HSP70-2 protein expression at the different time points. Due to the reduced sample size and the high variability of the data, the HSP70-2 protein variation observed was not supported by a statistical significance.
Fig. 6.
HSP70-2 protein levels in response to H2O2 treatments at different exposure times of PBMC cultures from MS patients with a mild form of disease (MSSS < 3.0) and a moderate to severe form of disorder (MSSS ≥ 3.0). Median values (interquartile range) of HSP70-2 protein levels are reported
ROS formation
ROS formation was measured on PBMCs from a sub-group of subjects enrolled in the study (22 MS patients and 32 healthy controls) after treatment with H2O2. Among MS patients, the mean age at the time of samples collection was 34.25 ± 12.7 years, 22.7% were males, while among the healthy donors the mean age at the time of samples collection was 38.27 ± 12.0 years, where males were the 74.2% of the sample. 95.5% were RR and 4.5% were SP patients. The ARR was in the range 0–2. 72.7% of the subjects were relapse-free, 13.6% had one relapse, 4.5% two relapses.
The association between ROS endpoint variable and gender or age was not significant; therefore, the following analyses were performed regardless of gender and age.
ROS levels stratified by case-control status
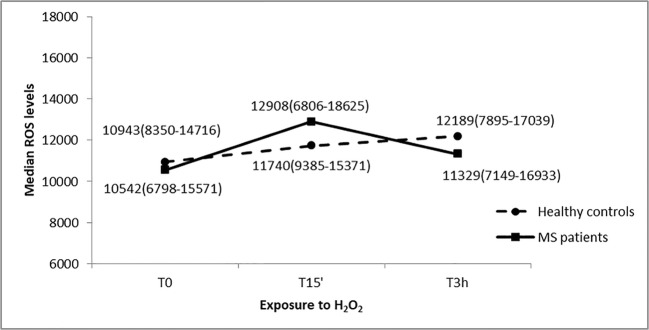
ROS levels of MS cases were not significantly different compared to healthy controls for every time point (U = 342, p = 0.87 at T0, U = 317, p = 0.55 at T15’, U = 327, p = 0.67 at T3h). Moreover, the analysis did not show any statistically significant ROS variability overtime points both in cases (chi-square = 2.46; p = 0.32) and in controls (chi-square = 2.69; p = 0.27) (Fig. 7). ROS levels trend suggest no interaction effect between time and case-control status.
Fig. 7.
ROS levels in response to H2O2 treatments at different exposure times of PBMC cultures from MS patients and healthy controls. Median values (interquartile range) of ROS levels are reported
ROS levels stratified by HSP70-2 rs1061581 polymorphism genotype
We considered whether the HSP70-2 rs1061581 polymorphism could influence the PBMCs response to oxidative stress in terms of ROS production. We studied 12 AA subjects (29.3%), 22 AG subjects (53.7%), and 7 GG subjects (17.0%), (Fig. 8).
Fig. 8.
ROS levels in response to H2O2 treatments at different exposure times of PBMC cultures with different HSP70-2 rs1061581 genotype (AA, AG, and GG) (MS patients and healthy controls together). Median values (interquartile range) of ROS levels are reported
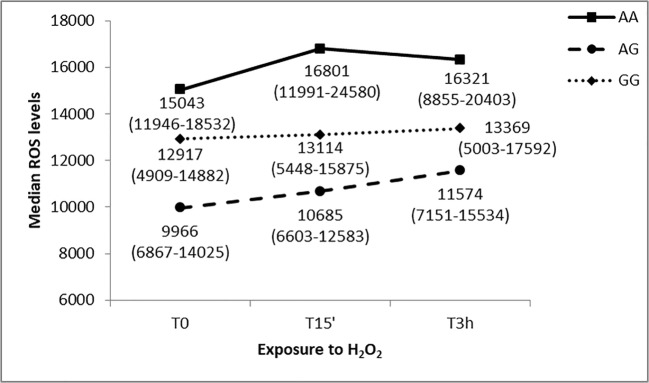
The analysis showed a statistically significant ROS variability among genotypes at T0 and T15 time point (chi-square = 6.58; p = 0.037 at T0; chi-square = 9.32; p = 0.009 at T15’). Specifically, the median AA level was statistically different from the median AG level both at T0 and T15’ time points (U = 60, p = 0.009; U = 52, p = 0.003 respectively). However, the analysis did not show a statistically significant ROS variability overtime points for every genotype. ROS levels trend suggest no interaction effect between time and rs1061581 polymorphism genotype.
Discussion
In the pathogenesis of MS, the immune attack plays a central role in the neurodegenerative process; however, several other factors, such as oxidative stress, contribute to it (Friese et al. 2014). The auto-inflammatory process involves ROS-mediated tissue injury: infiltrated macrophages, together with activated microglia and astrocytes, can generate pro-inflammatory mediators among which ROS, that are involved in MS autoimmune-mediated tissue damage (Ohl et al. 2016). In addition, mitochondrial dysfunction increases ROS levels which contribute to the damage of neurons and glia (Friese et al. 2014). In line with this concept, anti-oxidative drugs used in MS treatments, such as dimethyl fumarate, may induce the production of antioxidant enzymes, having a neuroprotective role combined to the anti-inflammatory effects (Pistono et al. 2017).
So far, one of the main interest of our research group has been focused on the MHC class III (Maggioli et al. 2014; Boiocchi et al. 2015). One of the sensors of the cell redox status is HSP70-2, whose gene is located in the MHC class III; indeed, this protein has been suggested to have a role in MS (Mansilla et al. 2014).
In this work, we investigated the effect of oxidative stress on PBMCs from MS patients and healthy controls, analyzing MTT levels, HSP70-2 protein levels, and intracellular ROS production. PBMCs from MS patients and healthy controls, randomly selected, were treated with H2O2 for 15 min and for a longer period of time (3 h) as described in the literature (Li et al. 2009; Nogueira-Pedro et al. 2013). The gender unbalance in the group of MS patients is consistent with the fact that MS affects more women than men with a female:male ratio usually ranging from 2:1 to 3:1 (Koch-Henriksen and Sørensen 2010).
PBMCs from both MS patients and healthy controls show a significant variation in their mitochondrial function, measured with the MTT assay, considering different exposure times to oxidative stress. This assay is extensively used for estimating the mitochondrial activity of cells, thus providing a first snapshot of the cell metabolic activity (Osera et al. 2011; Emamgholipour et al. 2016). In particular, after 15 min of oxidative stress, the mitochondrial activity decreases, and it is restored after 3 h of exposure to H2O2. We did not find any significant difference between the two groups, suggesting that both PBMCs from MS patients and healthy controls present an initial reduction in mitochondrial activity after an oxidative challenge, but they are able to restore the physiologic activity levels within a longer period of time (3 h). PBMCs are able to adapt to oxidative stress; our data are consistent with similar H2O2 treatments at increasing concentrations (50 μM, 100 μM, and 250 μM for 2 h) with no cytotoxic effect (Emamgholipour et al. 2016). In this study, performed on PBMCs from 14 healthy subjects, only 2 h of 500 μM H2O2 were able to significantly reduce the mitochondrial activity (Emamgholipour et al. 2016).
To assess whether the treatment with H2O2 could have an effect on the intracellular ROS levels, we investigated their levels in PBMCs from MS patients and healthy controls. No significant differences were found comparing PBMCs from MS patients and healthy controls. Moreover, ROS levels remained stable during the oxidative treatment. Considering that after 3 h of H2O2 exposure the mitochondrial activity is restored to the basal levels, we can hypothesize that, regardless of the MS pathology, all PBMCs are able to trigger a compensatory response to quench ROS production in the cell, thus facing the oxidative stress.
We then speculated that HSP70-2, a sensor of the cell redox status, could be involved in the response to the oxidative challenge and we analyzed the HSP70-2 protein levels in PBMCs from MS patients and healthy controls. Under stress conditions, it has been reported that HSP70-2 expression can vary quickly, HSP70-2 levels may increase within 1 h after heat shock (Maloyan et al. 1999; Doberentz et al. 2017). In our work, the exposure time to H2O2 does not change significantly the protein expression, both in PBMCs from MS patients and healthy controls, thus confirming that, in general, PBMCs respond in a similar way to the presence of an oxidative challenge, independently from the presence of the disease.
Although HSP70-2 is a key protein essential for the proper folding of proteins and with anti-apoptogenic proprieties (Beere 2004), the above-described data suggest that, in our model, HSP70-2 does not play a key role in PBMCs survival in the presence of an oxidative challenge. We may speculate that other compensatory cellular mechanisms may be involved in such a response, including a plethora of antioxidant enzymes. A putative mechanism of response to an oxidative stimulus is the activation of the nuclear factor erythroid 2-related factor 2 (Nrf2). Nrf2 modulates the expression of genes involved in detoxification pathways and in the defense against oxidative stress, such as superoxide dismutase, catalase, sulfaredoxin, thioredoxin, glutathione peroxidase, and glutathione reductase (Joshi and Johnson 2012). Also, we may speculate the involvement of several antioxidant enzymes that can act at different levels; for instance, in human PBMCs, it has been reported an increment in catalase activity after oxidative stress, but reduced activity of the manganese-dependent superoxide dismutase, MnSOD (Emamgholipour et al. 2016).
To better elucidate the role of the stress-inducible HSP70-2 in the response to an oxidative stimulus, we investigated the HSP70-2 rs1061581 polymorphism, in order to verify whether the presence of a variant may influence the response to oxidative stress, given that this specific polymorphism has been associated to autoimmune diseases (Zouari Bouassida et al. 2004; Wang et al. 2015). When the subjects of our study were divided for the HSP70-2 rs1061581 genotype, we noticed a lower percentage for GG genotype carriers in the healthy controls. This is consistent with the fact that the GG phenotype is less frequent in the Caucasian population (Giacconi et al. 2014). Nevertheless, the higher percentage of GG subjects in the group of MS patients compared to the healthy controls is in line with our previous findings indicating that the G allele is a risk allele for the development of MS (Boiocchi et al. 2014).
The correlation of MTT levels with HSP70-2 rs1061581 genotype does not show any significant difference. This polymorphism does not influence the response to oxidative stress, although it seems to be important in the susceptibility to develop MS (Boiocchi et al. 2014). In addition, the HSP70-2 levels were correlated to the HSP70-2 rs1061581 genotype. As shown in Fig. 5, PBMCs from GG subjects show a different profile with respect to the other genotypes, with a decrease in HSP70-2 levels at 15 min of oxidative treatment and a subsequent increase at 3 h. On the other hand, PBMCs from AA and AG carriers show on apposite trend, with an immediate increase in HSP70-2 levels and then a decrease at 3 h. This different profile is confirmed by the borderline significant variability (p = 0.05) noticed at T15’, when the HSP70-2 levels of the three groups were compared. Our data suggest that the HSP70-2 rs1061581 polymorphism may have a role in the expression of HSP70-2 in conditions of oxidative stress, indeed this nucleotide polymorphism leads to a silent mutation that has been suggested to influence the mRNA translation (Pociot et al. 1993).
The intracellular ROS levels were correlated as well to the HSP70-2 rs1061581 genotype. The three groups seem to have slightly different ROS levels; however, the difference is significant only when T0 and T15’ are considered. In particular, the median AA level is statistically different from the median AG level, both at T0 and T15’ time points. It is important to take into account that HSP70s can interact with other proteins involved in the protection against oxidative species; for example, inducible HSP70s were described to chaperone superoxide dismutases-2 to the mitochondria (Afolayan et al. 2014). Consistently, especially when considering AG carriers, we can hence hypothesize that in short-term oxidative stress conditions (i.e., H2O2 for 15 min), these PBMCs are able, on one side, to produce higher amounts of HSP70-2 and, on the other, to better counteract ROS generation. Moreover, we cannot exclude that this SNP may be in linkage with other variants that may explain the difference in ROS levels described by our analysis.
It is important also to notice that the majority of the MS subjects included in our study were RR patients in the remission phase. It will be interesting in the future to extend our analysis including also patients in the relapse phase, as it has already been shown that the disease phase can have an impact on HSP levels (Ce et al. 2011).
Finally, we considered MS progression, investigating MTT and HSP70-2 protein levels in a sub-group of patients, divided on the base of MSSS values: patients with a MSSS < 3.0 suffer from a mild form of disease, whereas patients with a MSSS ≥ 3.0 have a moderate to severe form. The two groups have a different trend in HSP70-2 protein expression: PBMCs from patients with a higher MSSS have a trend towards decreased HSP70-2 levels after 15 min of oxidative treatment, which then increase after 3 h of oxidative stress. On the other hand, PBMCs from MS patients with a lower MSSS do not vary the HSP70-2 protein expression, suggesting a different response of PBMCs to oxidative conditions in mild vs moderate to severe MS, which must be confirmed in a larger sample in future works.
Conclusions
The inflammatory process typical of MS is critically linked to oxidative stress. HSP70-2 is a sensor for the redox status of the cells, and its role in MS pathogenesis is still uncertain, since, on the one side, it can act as a neuroprotective element, and on the other, it may also promote a pro-inflammatory response.
Our work highlights that PBMCs, regardless of the presence of MS and the genotype of the HSP70-2 rs1061581 polymorphism, are able to adapt in oxidative stress conditions, even though the reduction of oxidative stress has a positive influence on the patients, as shown by the use of dimethyl fumarate.
Our results indicate that although HSP70-2 does not seem to play a key role in the protective reaction against oxidative stress, the presence of polymorphisms may dictate a different capability of PBMCs to face oxidative stress conditions. Nevertheless, taking into account that, as mentioned, MS patients benefit from anti-oxidative drugs, further studies are needed to identify which specific proteins significantly contribute to this defensive response in order to improve MS treatments. Moreover, considering that HSP70-2 rs1061581 polymorphism may have an impact on the HSP70-2 levels in response to oxidative stress, future analyses would be important to assess better its role in the pathogenesis of MS.
Funding information
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Afolayan AJ, Teng R-J, Eis A, Rana U, Broniowska KA, Corbett JA, Pritchard K, Konduri GG. Inducible HSP70 regulates superoxide dismutase-2 and mitochondrial oxidative stress in the endothelial cells from developing lungs. Am J Phys Lung Cell Mol Phys. 2014;306:L351–L360. doi: 10.1152/ajplung.00264.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aquino DA, Capello E, Weisstein J, Sanders V, Lopez C, Tourtellotte WW, Brosnan CF, Raine CS, Norton WT. Multiple sclerosis: altered expression of 70- and 27-kDa heat shock proteins in lesions and myelin. J Neuropathol Exp Neurol. 1997;56:664–672. doi: 10.1097/00005072-199706000-00004. [DOI] [PubMed] [Google Scholar]
- Asea A, Kraeft SK, Kurt-Jones EA, et al. HSP70 stimulates cytokine production through a CD14-dependant pathway, demonstrating its dual role as a chaperone and cytokine. Nat Med. 2000;6:435–442. doi: 10.1038/74697. [DOI] [PubMed] [Google Scholar]
- Beere HM. “The stress of dying”: the role of heat shock proteins in the regulation of apoptosis. J Cell Sci. 2004;117:2641–2651. doi: 10.1242/jcs.01284. [DOI] [PubMed] [Google Scholar]
- Bigaut K, De Seze J, Collongues N. Ocrelizumab for the treatment of multiple sclerosis. Expert Rev Neurother. 2019;19:97–108. doi: 10.1080/14737175.2019.1561284. [DOI] [PubMed] [Google Scholar]
- Boiocchi C, Osera C, Monti MC, Ferraro OE, Govoni S, Cuccia M, Montomoli C, Pascale A, Bergamaschi R. Are Hsp70 protein expression and genetic polymorphism implicated in multiple sclerosis inflammation? J Neuroimmunol. 2014;268:84–88. doi: 10.1016/j.jneuroim.2014.01.004. [DOI] [PubMed] [Google Scholar]
- Boiocchi C, Maggioli E, Monti MC, Zorzetto M, Sinforiani E, Cereda C, Ricevuti G, Cuccia M. The Possible Involvement of HLA Class III Haplotype (RAGE, HSP70 and TNF Genes) in Alzheimer’s Disease. Curr Alzheimer Res. 2015;12:997–1005. doi: 10.2174/1567205012666151027130635. [DOI] [PubMed] [Google Scholar]
- Boiocchi C, Monti MC, Osera C, Mallucci G, Pistono C, Ferraro OE, Nosari G, Romani A, Cuccia M, Govoni S, Pascale A, Montomoli C, Bergamaschi R. Heat shock protein 70-hom gene polymorphism and protein expression in multiple sclerosis. J Neuroimmunol. 2016;298:189–193. doi: 10.1016/j.jneuroim.2016.07.011. [DOI] [PubMed] [Google Scholar]
- Ce P, Erkizan O, Gedizlioglu M. Elevated HSP27 levels during attacks in patients with multiple sclerosis. Acta Neurol Scand. 2011;124:317–320. doi: 10.1111/j.1600-0404.2010.01475.x. [DOI] [PubMed] [Google Scholar]
- Chabas D, Baranzini SE, Mitchell D, Bernard CC, Rittling SR, Denhardt DT, Sobel RA, Lock C, Karpuj M, Pedotti R, Heller R, Oksenberg JR, Steinman L. The influence of the proinflammatory cytokine, osteopontin, on autoimmune demyelinating disease. Science. 2001;294:1731–1735. doi: 10.1126/science.1062960. [DOI] [PubMed] [Google Scholar]
- Craig EA, Gambill BD, Nelson RJ. Heat shock proteins: molecular chaperones of protein biogenesis. Microbiol Rev. 1993;57:402–414. doi: 10.1128/mr.57.2.402-414.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai C, Whitesell L, Rogers AB, Lindquist S. Heat shock factor 1 is a powerful multifaceted modifier of carcinogenesis. Cell. 2007;130:1005–1018. doi: 10.1016/j.cell.2007.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daugaard M, Rohde M, Jäättelä M. The heat shock protein 70 family: highly homologous proteins with overlapping and distinct functions. FEBS Lett. 2007;581:3702–3710. doi: 10.1016/j.febslet.2007.05.039. [DOI] [PubMed] [Google Scholar]
- Dendrou CA, Fugger L, Friese MA. Immunopathology of multiple sclerosis. Nat Rev Immunol. 2015;15:545–558. doi: 10.1038/nri3871. [DOI] [PubMed] [Google Scholar]
- Doberentz E, Genneper L, Wagner R, Madea B. Expression times for hsp27 and hsp70 as an indicator of thermal stress during death due to fire. Int J Legal Med. 2017;131:1707–1718. doi: 10.1007/s00414-017-1566-x. [DOI] [PubMed] [Google Scholar]
- Emamgholipour S, Hossein-Nezhad A, Ansari M. Can melatonin act as an antioxidant in hydrogen peroxide-induced oxidative stress model in human peripheral blood mononuclear cells? Biochem Res Int. 2016;2016:5857940. doi: 10.1155/2016/5857940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Favatier F, Bornman L, Hightower LE, et al. Variation in hsp gene expression and Hsp polymorphism: do they contribute to differential disease susceptibility and stress tolerance? Cell Stress Chaperones. 1997;2:141–155. doi: 10.1379/1466-1268(1997)002<0141:VIHGEA>2.3.CO;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friese MA, Schattling B, Fugger L. Mechanisms of neurodegeneration and axonal dysfunction in multiple sclerosis. Nat Rev Neurol. 2014;10:225–238. doi: 10.1038/nrneurol.2014.37. [DOI] [PubMed] [Google Scholar]
- Giacconi R, Costarelli L, Malavolta M, Piacenza F, Galeazzi R, Gasparini N, Basso A, Mariani E, Fulop T, Rink L, Dedoussis G, Kanoni S, Herbein G, Jajte J, Busco F, Mocchegiani E. Association among 1267 A/G HSP70-2, -308 G/A TNF-α polymorphisms and pro-inflammatory plasma mediators in old ZincAge population. Biogerontology. 2014;15:65–79. doi: 10.1007/s10522-013-9480-1. [DOI] [PubMed] [Google Scholar]
- Goate AM, Cooper DN, Hall C, Leung TK, Solomon E, Lim L. Localization of a human heat-shock HSP 70 gene sequence to chromosome 6 and detection of two other loci by somatic-cell hybrid and restriction fragment length polymorphism analysis. Hum Genet. 1987;75:123–128. doi: 10.1007/BF00591072. [DOI] [PubMed] [Google Scholar]
- Haider L, Fischer MT, Frischer JM, Bauer J, Höftberger R, Botond G, Esterbauer H, Binder CJ, Witztum JL, Lassmann H. Oxidative damage in multiple sclerosis lesions. Brain. 2011;134:1914–1924. doi: 10.1093/brain/awr128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartl FU. Molecular chaperones in cellular protein folding. Nature. 1996;381:571–579. doi: 10.1038/381571a0. [DOI] [PubMed] [Google Scholar]
- Herz J, Zipp F, Siffrin V. Neurodegeneration in autoimmune CNS inflammation. Exp Neurol. 2010;225:9–17. doi: 10.1016/j.expneurol.2009.11.019. [DOI] [PubMed] [Google Scholar]
- Joshi G, Johnson JA. The Nrf2-ARE pathway: a valuable therapeutic target for the treatment of neurodegenerative diseases. Recent Pat CNS Drug Discov. 2012;7:218–229. doi: 10.2174/157488912803252023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalmar B, Greensmith L. Induction of heat shock proteins for protection against oxidative stress. Adv Drug Deliv Rev. 2009;61:310–318. doi: 10.1016/j.addr.2009.02.003. [DOI] [PubMed] [Google Scholar]
- Klausz G, Molnár T, Nagy F, Gyulai Z, Boda K, Lonovics J, Mándi Y. Polymorphism of the heat-shock protein gene Hsp70-2, but not polymorphisms of the IL-10 and CD14 genes, is associated with the outcome of Crohn’s disease. Scand J Gastroenterol. 2005;40:1197–1204. doi: 10.1080/00365520510023350. [DOI] [PubMed] [Google Scholar]
- Koch MW, Metz LM, Agrawal SM, Yong VW. Environmental factors and their regulation of immunity in multiple sclerosis. J Neurol Sci. 2013;324:10–16. doi: 10.1016/j.jns.2012.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch-Henriksen N, Sørensen PS. The changing demographic pattern of multiple sclerosis epidemiology. Lancet Neurol. 2010;9:520–532. doi: 10.1016/S1474-4422(10)70064-8. [DOI] [PubMed] [Google Scholar]
- Lassmann H, van Horssen J, Mahad D. Progressive multiple sclerosis: pathology and pathogenesis. Nat Rev Neurol. 2012;8:647–656. doi: 10.1038/nrneurol.2012.168. [DOI] [PubMed] [Google Scholar]
- Li M, Zhao L, Liu J, Liu AL, Zeng WS, Luo SQ, Bai XC. Hydrogen peroxide induces G2 cell cycle arrest and inhibits cell proliferation in osteoblasts. Anat Rec (Hoboken) 2009;292:1107–1113. doi: 10.1002/ar.20925. [DOI] [PubMed] [Google Scholar]
- Maggioli E, Boiocchi C, Zorzetto M, et al. HLA class III genes involvement in Kawasaki disease: a case-control study in Caucasian population. Int J Immunogenet. 2014;41:44–53. doi: 10.1111/iji.12077. [DOI] [PubMed] [Google Scholar]
- Maloyan A, Palmon A, Horowitz M. Heat acclimation increases the basal HSP72 level and alters its production dynamics during heat stress. Am J Phys. 1999;276:R1506–R1515. doi: 10.1152/ajpregu.1999.276.5.R1506. [DOI] [PubMed] [Google Scholar]
- Mansilla MJ, Montalban X, Espejo C. Heat shock protein 70: roles in multiple sclerosis. Mol Med. 2012;18:1018–1028. doi: 10.2119/molmed.2012.00119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mansilla MJ, Comabella M, Río J, Castilló J, Castillo M, Martin R, Montalban X, Espejo C. Up-regulation of inducible heat shock protein-70 expression in multiple sclerosis patients. Autoimmunity. 2014;47:127–133. doi: 10.3109/08916934.2013.866104. [DOI] [PubMed] [Google Scholar]
- Muchowski PJ, Wacker JL. Modulation of neurodegeneration by molecular chaperones. Nat Rev Neurosci. 2005;6:11–22. doi: 10.1038/nrn1587. [DOI] [PubMed] [Google Scholar]
- Njemini R, Lambert M, Demanet C, Kooijman R, Mets T. Basal and infection-induced levels of heat shock proteins in human aging. Biogerontology. 2007;8:353–364. doi: 10.1007/s10522-006-9078-y. [DOI] [PubMed] [Google Scholar]
- Nogueira-Pedro A, Cesário TAM, Dias CC, Origassa CS, Eça LP, Paredes-Gamero EJ, Ferreira AT. Hydrogen peroxide (H2O2) induces leukemic but not normal hematopoietic cell death in a dose-dependent manner. Cancer Cell Int. 2013;13:123. doi: 10.1186/1475-2867-13-123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohl K, Tenbrock K, Kipp M. Oxidative stress in multiple sclerosis: Central and peripheral mode of action. Exp Neurol. 2016;277:58–67. doi: 10.1016/j.expneurol.2015.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osera C, Fassina L, Amadio M, Venturini L, Buoso E, Magenes G, Govoni S, Ricevuti G, Pascale A. Cytoprotective response induced by electromagnetic stimulation on SH-SY5Y human neuroblastoma cell line. Tissue Eng Part A. 2011;17:2573–2582. doi: 10.1089/ten.TEA.2011.0071. [DOI] [PubMed] [Google Scholar]
- Pablos JL, Carreira PE, Martín-Villa JM, et al. Polymorphism of the heat-shock protein gene HSP70-2 in systemic lupus erythematosus. Br J Rheumatol. 1995;34:721–723. doi: 10.1093/rheumatology/34.8.721. [DOI] [PubMed] [Google Scholar]
- Park S-H, Bolender N, Eisele F, Kostova Z, Takeuchi J, Coffino P, Wolf DH. The cytoplasmic Hsp70 chaperone machinery subjects misfolded and endoplasmic reticulum import-incompetent proteins to degradation via the ubiquitin-proteasome system. Mol Biol Cell. 2007;18:153–165. doi: 10.1091/mbc.E06-04-0338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pistono C, Osera C, Boiocchi C, Mallucci G, Cuccia M, Bergamaschi R, Pascale A. What’s new about oral treatments in Multiple Sclerosis? Immunogenetics still under question. Pharmacol Res. 2017;120:279–293. doi: 10.1016/j.phrs.2017.03.025. [DOI] [PubMed] [Google Scholar]
- Pociot F, Rønningen KS, Nerup J. Polymorphic analysis of the human MHC-linked heat shock protein 70 (HSP70-2) and HSP70-Hom genes in insulin-dependent diabetes mellitus (IDDM) Scand J Immunol. 1993;38:491–495. doi: 10.1111/j.1365-3083.1993.tb02593.x. [DOI] [PubMed] [Google Scholar]
- Polman CH, Reingold SC, Banwell B, Clanet M, Cohen JA, Filippi M, Fujihara K, Havrdova E, Hutchinson M, Kappos L, Lublin FD, Montalban X, O'Connor P, Sandberg-Wollheim M, Thompson AJ, Waubant E, Weinshenker B, Wolinsky JS. Diagnostic criteria for multiple sclerosis: 2010 revisions to the McDonald criteria. Ann Neurol. 2011;69:292–302. doi: 10.1002/ana.22366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roxburgh RHSR, Seaman SR, Masterman T, et al. Multiple sclerosis severity score: using disability and disease duration to rate disease severity. Neurology. 2005;64:1144–1151. doi: 10.1212/01.WNL.0000156155.19270.F8. [DOI] [PubMed] [Google Scholar]
- Sabatino Joseph J., Zamvil Scott S., Hauser Stephen L. B-Cell Therapies in Multiple Sclerosis. Cold Spring Harbor Perspectives in Medicine. 2018;9(2):a032037. doi: 10.1101/cshperspect.a032037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stahnke T, Stadelmann C, Netzler A, Brück W, Richter-Landsberg C. Differential upregulation of heme oxygenase-1 (HSP32) in glial cells after oxidative stress and in demyelinating disorders. J Mol Neurosci. 2007;32:25–37. doi: 10.1007/s12031-007-0005-8. [DOI] [PubMed] [Google Scholar]
- Turturici G, Sconzo G, Geraci F. Hsp70 and its molecular role in nervous system diseases. Biochem Res Int. 2011;2011:618127. doi: 10.1155/2011/618127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Goes A, Brouwer J, Hoekstra K, Roos D, van den Berg T, Dijkstra CD. Reactive oxygen species are required for the phagocytosis of myelin by macrophages. J Neuroimmunol. 1998;92:67–75. doi: 10.1016/S0165-5728(98)00175-1. [DOI] [PubMed] [Google Scholar]
- Van der Goes A, Wouters D, Van Der Pol SM, et al. Reactive oxygen species enhance the migration of monocytes across the blood-brain barrier in vitro. FASEB J. 2001;15:1852–1854. doi: 10.1096/fj.00-0881fje. [DOI] [PubMed] [Google Scholar]
- van Horssen J, Drexhage JAR, Flor T, Gerritsen W, van der Valk P, de Vries HE. Nrf2 and DJ1 are consistently upregulated in inflammatory multiple sclerosis lesions. Free Radic Biol Med. 2010;49:1283–1289. doi: 10.1016/j.freeradbiomed.2010.07.013. [DOI] [PubMed] [Google Scholar]
- van Horssen J, Witte ME, Schreibelt G, de Vries HE. Radical changes in multiple sclerosis pathogenesis. Biochim Biophys Acta. 2011;1812:141–150. doi: 10.1016/j.bbadis.2010.06.011. [DOI] [PubMed] [Google Scholar]
- Wang YP, Tang Z, Peng BK, Zhen Q, Zhou SL, Jin XF. Heat shock protein 70 polymorphisms in Chinese patients with Graves’ disease. Genet Mol Res. 2015;14:18376–18383. doi: 10.4238/2015.December.23.25. [DOI] [PubMed] [Google Scholar]
- Yong VW, Marks S. The interplay between the immune and central nervous systems in neuronal injury. Neurology. 2010;74(Suppl 1):S9–S16. doi: 10.1212/WNL.0b013e3181c97d04. [DOI] [PubMed] [Google Scholar]
- Zouari Bouassida K, Chouchane L, Jellouli K, Chérif S, Haddad S, Gabbouj S, Danguir J. Polymorphism of stress protein HSP70-2 gene in Tunisians: susceptibility implications in type 2 diabetes and obesity. Diabetes Metab. 2004;30:175–180. doi: 10.1016/S1262-3636(07)70104-0. [DOI] [PubMed] [Google Scholar]