Abstract
Recently, increasing evidences have shown that the exposure to phthalates can adversely affect health status of pregnant women and their newborns. However, only a limited number of studies have investigated the concentrations of these compounds in the body fluids of pregnant women. In the present study, we aimed to evaluate the concentrations of phthalate metabolites in urinary samples of pregnant women in correlation with the population characteristics and different lifestyle factors. The study was conducted in 2018–2019 and urinary samples were taken from 121 pregnant women during their first pregnancy trimester who lived in Isfahan, Iran. The concentrations of monobutyl phthalate (MBP), mono-benzyl phthalate (MBzP), mono-2-ethylhexyl phthalate (MEHP), and mono (2-ethyl-5hydroxyhexyl) phthalate (MEHHP) metabolites in urinary samples were determined by gas chromatography mass spectrometry (GC/MS). The socio-demographic profile of the participants (maternal education, age, family income, pre-pregnancy BMI), their lifestyle information (smoking habit, food pattern, and physical activity), cleaning products use data (cosmetic and household cleaning products) were collected by the use of PERSIAN birth cohort questionnaire. MBzP, MBP, MEHP, and MEHHP were detected in 100% of participated pregnant women with the mean concentration of 342.5 ± 193.8, 308.5 ± 229.4, 126.5 ± 118.3, and 866.5 ± 307.6 μg/g creatinine, respectively. Significant correlations were observed between the mean concentration of urinary phthalate metabolites with the following variables: using plastic packaging (for bread, lemon juice, pickle, leftover, and water), lower physical activity, passive smoking exposure during pregnancy (p value<0.05). Furthermore, the pre-pregnancy BMI (r = 0.27, r = 0.3, r = 0.26, and r = 0.26), use of the household cleaning products (r = 0.2, r = 0.22, r = 0.3, and r = 0.26), utilize of the cosmetic products (r = 0.46, r = 0.48, r = 0.49, and r = 0.54), and passive smoking status (r = 0.5, r = 0.44, r = 0.44, and r = 0.26) directly correlated with the urinary concentrations of MBP, MBzP, MEHP, and MEHHP, respectively. No significant association was seen between the concentration of urinary phthalate metabolites with the maternal education level and family income. According to our findings, higher amounts of phthalate metabolites were detected in urinary samples of pregnant women who were passive smokers, or had higher pre-pregnancy BMI and lower physical activity, as well as those women who used higher amounts of cosmetic and household cleaning products, or used plastic packaging for food and non-food products.
Keywords: Phthalate, Pregnancy, Urinary metabolites, Birth cohort
Introduction
Phthalates or Phthalic acid esters (PAEs) are considered as synthetic chemical groups utilized in several consumables products during processing measures. Low molecular-weight of phthalates such as diethyl phthalate (DEP), and dibutyl phthalate (DBP) are commonly applied in personal care products such as cosmetics, lotions, perfumes, pesticides as stabilizing agents while high- molecular- weight of phthalates with ≥8 carbons in the alkyl chain di-2-ethyl hexyl phthalate (DEHP), and butyl benzyl phthalate (BBzP) are widely used in food packaging, polyvinylchloride (PVC), and even in medical applications like blood transfusion devices and catheters [1]. Dietary intake has been considered as the main route of exposure to phthalates in human beings. Besides, these hazardous compounds can conveniently release from materials containing phthalates, thus can be inhaled, ingested, or adsorbed via the skin into the human body [2, 3]. After absorption, the di-ester phthalates are immediately hydrolyzed into their corresponding monoesters (monoester (mono-(2-ethylhexyl) phthalate, MEHP)) then oxidized into the secondary metabolites such as MEHHP (mono(2-ethyl-5-hydroxyhexyl), which subsequently are excreted in urine and feces [4].
Phthalates have been detected in cord blood and amniotic fluid, indicating that these compounds can easily cross through the placenta [5]. Therefore, the exposure of fetus to these hazardous substances may affect fetal growth through changing the metabolic and homeostatic mechanisms [6]. Some epidemiological studies indicated relationship between maternal phthalate exposure and the consequent risk such as shortened gestational length or preterm delivery and some poor birth outcomes for infants. Because of the toxic effects, the use of phthalates such as DEHP, DBP, and BBzP have been prohibited in most of the developed countries (Europe Commission) [7], while these substances are widely used in developing countries, including Iran. Due to the behavioral and physiological changes during the pregnancy, the female body may negatively be affected by the exposure to environmental chemicals [8]. To the best of our knowledge, there is no evidence or previous publication on the biomonitoring of phthalate metabolites among pregnant women in Iran. Therefore, the present study was done with the aim to measure the concentrations of phthalate metabolites in pregnant women and to investigate the correlation of urinary phthalate metabolites with the socio-demographic profile and lifestyle information of the participants, as well as cleaning products use during the pregnancy.
Materials and methods
Study population
This cross-sectional study was conducted between January 2018 and February 2019 to assess the concentrations of phthalate metabolites in urinary samples from pregnant women lived in Isfahan city, Iran. The data was obtained from the PERSIAN Birth Cohort study, targeting 121 pregnant women in their first trimester. In brief, multiple urine samples were obtained from the participants during their first trimester of pregnancy. The urine samples of pregnant women were randomly collected from public and private clinics in Isfahan city. The urine samples were poured in borosilicate containers and stored at −20 °C until the use. All the ethical issues related to the study were observed by Ethics Committees of Isfahan university of Medical sciences. Moreover, a signed informed consent letter was taken from each participant. The distribution of participants’ location are given in Fig. 1.
Fig. 1.
The distribution of participants’ location in Isfahan city
Data collection
The PERSIAN Birth Cohort questionnaire was utilized to scroll information on socio-demographic (maternal education, age, family income), lifestyle factors (pre-pregnancy BMI, smoking habit, and physical activity (PA)), and consumer products use (cosmetic such as lipstick, eye linear, nail polish, mascara, rouge, sunscreen, lotion, perfume, and etc., and household cleaning products such as bleach, waxes, glass cleaners, stain liquid, air fresher, oven cleaning sprays and degreasing products, bugs spray) [9]. The data was collected by two of our expert researchers.
Information about the food intake of participants was obtained using a reliable and valid food frequency questionnaire (FFQ). The consumption of fried foods (as daily, 1–2 per week, 1–3 per month, seldom, and never) and plastic packaging used for certain foods including bread, lemon juice, pickle, leftover and water were asked form the participants.
Maternal body mass index (BMI) was determined as a ratio of pregnancy weight (kg) to the square of height (m). An overall total physical activity (MET-minutes/week) score were computed by IPAQ (International Physical Activity Questionnaire) [10]. Three levels of PA proposed to categorize populations – “high as an overall physical activity of 1500-3000 MET-minutes/week “, “moderate as 3 or more days of high activity at least 20 minutes per day, 5 or more days of moderate activity for at least 30 minutes per day, or an overall physical activity of at least 600 MET-minutes/week “, and “low as those persons who not meet criteria of classes 2 or 3.
Measuring urinary metabolites of phthalate
Standard solutions from MBP, MBzP, MEHP, and MEHHP in methanol were separately provided and stored in Teflon capped amber glass containers at temperatures below zero. Afterwards, 10 ml from each of the urine samples were defreezed and digested by b-glucuronidase enzyme (incubated for 18 h at 37 C). Then, the samples were diluted and 10% sulfuric acid was used to adjust the pH. At the next step, dispersive liquid-liquid microextraction (DLLME) was performed to extract the investigated metabolites. Finally, gas chromatography mass spectrometry (GC/MS) was used to measure the concentration of phthalate metabolites in urine samples [11].
Quality assurance and quality control (QA/QC)
The quality assurance and quality control (QA/QC) was applied to ensure the reliability of the analytical data generated and increase confidence in the relevance of possible responses. The linear regression gave a good fit (R2 ≥ 0.98) with high precision (≤ 13.2% RSD). The limit of detection (LOD) and limit of quantification (LOQ) were estimated from the signal-to-noise ratio of 3 and 10, respectively. During statistical analyzes, when metabolite concentrations were under the LOD, one-half of LOD was used [12]. The concentration of phthalate metabolites were adjusted using urine creatinine. The R2, precision (% RSD), LOD, LOQ, and mean recovery are summarized in Table 1.
Table 1.
QA/QC parameters of Phthalate metabolites
| Phthalate metabolites | R2 | Precision (% RSD) | LOD (μg/L) | LOQ (μg/L) | Recovery (%) |
|---|---|---|---|---|---|
| MBP | 0.99 | 7 | 0.017 | 0.06 | 83 |
| MBzP | 0.99 | 6.9 | 0.013 | 0.04 | 94 |
| MEHP | 0.99 | 13.2 | 0.018 | 0.06 | 90 |
| MEHHP | 0.98 | 6.2 | 0.019 | 0.07 | 69 |
Data analysis
Continuous variables have been presented as mean ± SD and median (minimum-maximum) while categorical variables as frequencies (percentages). Normality of continuous data was evaluated by using Kolmogorov-Smirnov test and Q-Q plot. One-way Analysis of variance (ANOVA) or independent samples t-test was used to compare the mean values of phthalate metabolite concentrations across categories of possible demographic and lifestyle determinants while Pearson or Spearman rank correlation coefficient were used for evaluating the association of phthalate metabolite concentrations with quantitative determinants All statistical analyses were done using SPSS software version 23 (IBM SPSS Inc., Chicago, IL). P < 0.05 was considered as statistically significant.
Results and discussion
The characteristics of participants are depicted in Table 2. Among the participants, 59% (n = 71) were ≥ 30-years old, 41% (n = 50) were ≤ 30 years old, 69% of subjects (n = 83) were categorized into overweight, while 29% of subjects (n = 35) had normal weight and only 2% (n = 3) were classified as underweight. The majority of the participants had an academic education (n = 105, 86.8%). Most of our participants were categorized into the middle income group with 100–300 $ family income per month (n = 69, 57%). Based on the initial findings, cosmetic products and household cleaning products were respectively utilized by 92.6% and %100 (n = 112 and 121) of pregnant women. Furthermore, more than half of the participants (51.26%) used plastic packaging for bread (n = 76, 62.8%), lemon juice (n = 67, 55.4%), pickle (n = 55, 45.5%), leftover (n = 52, 43%) and water (n = 60, 49.6%). Only 31.4% (n = 38) of the pregnant women used fried foods 1–2 times per week, which was the highest frequency for fried foods consumption, while 4.1% (n = 5) never used this food item. About more than half of the individuals (n = 65, 53.7%) were categorized into the low physical activity group, however only 6.6% of subjects (n = 8) were classified into the high physical activity group.
Table 2.
Maternal characteristics of 121 pregnant women enrolled in the study
| Variables | Categories | n (%) | |
|---|---|---|---|
| Maternal age (years) | <25 | 8(6) | |
| 25–29 | 42(35) | ||
| 30–34 | 47(39) | ||
| >34 | 24(20) | ||
| Pre-pregnancy BMI (kg/m2) | Underweight (<18.5) | 3(2) | |
| Normal weight (18.5–23.9) | 35(29) | ||
| Overweight (≥24) | 83(69) | ||
| Education | College | 105(86.8) | |
| High school | 9(7.4) | ||
| Less than high school | 7(5.8) | ||
| Family income ($/month) | Low (<100) | 40(33.1) | |
| Middle (100–300) | 69(57) | ||
| High (>300) | 12(9.9) | ||
| Cosmetic usage | Yes | 112(92.6) | |
| No | 9(7.4) | ||
| Using household cleaning products | Yes | 121(100) | |
| No | 0(0) | ||
| Plastic packaging usage | Bread | Yes | 76(62.8) |
| No | 45(37.2) | ||
| Lemon juice | Yes | 67(55.4) | |
| No | 54(44.6) | ||
| Pickle | Yes | 55(45.5) | |
| No | 66(54.5) | ||
| Leftover | Yes | 52(43) | |
| No | 69(57) | ||
| Water | Yes | 60(49.6) | |
| No | 61(50.4) | ||
| Total | Yes | (51.26) | |
| No | (48.74) | ||
| Eating fried foods | Never | 5(4.1) | |
| Seldom (<once a month) | 13(10.7) | ||
| 1–3 per month | 35(28.9) | ||
| 1–2 per week | 38(31.4) | ||
| daily | 30(24.8) | ||
| Smoking during pregnancy | Yes | 0(0) | |
| No | 121(100) | ||
| Passive smoking during pregnancy | Yes | 65(53.7) | |
| No | 56(46.3) | ||
| Physical activity | Low | 65(53.7) | |
| Moderate | 48(39.7) | ||
| High | 8(6.6) | ||
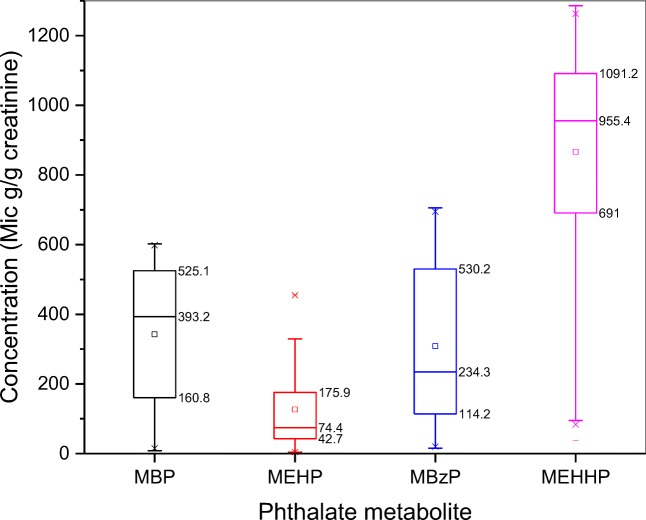
The mean, quartile, minimum, and maximum of creatinine-adjusted concentration of phthalate metabolites are presented in Table 3. Accordingly, phthalate metabolites were detected in 100% of urine samples (n = 121). The highest and lowest mean concentration values were observed for MEHHP and MEHP with 866.5 ± 307.6 and 126.5 ± 118.3 μg/g creatinine, respectively. The distribution of urinary phthalate metabolites concentration was analyzed in terms of quartiles (25th, 50th, and 75th) and mean value and the results are shown in Fig. 2. As indicated, the highest concentration and variation values were seen for MEHHP, while the lowest values were obtained for MEHP. In the present study, the mean concentration of MBP, MBzP, MEHP, and MEHHP were found to be 13, 30, 20, and 44 times higher than those concentrations reported by other studies in the US and European countries [8, 4, 13]. Such higher levels may be in line with the higher exposure to phthalate metabolites in Iranian population was reported by Amin et al. [11].
Table 3.
Mean concentration of phthalate metabolites (μg/g creatinine) in pregnant women urine
| Creatinine-adjusted phthalate metabolites(μg/g) | LOD (μg/L) |
% > LOD* | Min | Percentile | Max | Mean (SD) | ||
|---|---|---|---|---|---|---|---|---|
| 25th | 50th | 75th | ||||||
| MBP | 0.017 | 100 | 8.5 | 160.8 | 393.2 | 525.1 | 602.2 | 342.5 (193.8) |
| MBzP | 0.013 | 100 | 15.6 | 114.2 | 234.3 | 530.2 | 705.4 | 308.5 (229.4) |
| MEHP | 0.018 | 100 | 4.2 | 42.7 | 74.4 | 175.9 | 455.9 | 126.5 (118.3) |
| MEHHP | 0.019 | 100 | 37.8 | 691 | 955.4 | 1091.2 | 1285.9 | 866.5 (307.6) |
*Counts of detectable sample] / [Total sample counts] × 100(%)
Fig. 2.
The distribution of urinary phthalate metabolites concentration (μg/g creatinine) and variation among the study population depicted in box plots
The distribution of urinary phthalate metabolite concentrations in correlation with some influencing variables including maternal education level, family income per month, plastic packaging (bread, lemon juice, pickle, leftover, water) are presented in Table 4. According to the results, the maternal education level has not significant influence on the distribution of different urinary phthalate metabolite concentrations (p value>0.05). Likewise, another research performed among pregnant women in Peru showed no significant difference between phthalate metabolites and maternal education [14]. On the contrast some studies in developed countries have indicated a direct correlation between urinary phthalate metabolites concentrations with the maternal education [15, 16]. Opposite effects of education on this issue in developing versus developed countries may stem from this fact that, although the maternal education is an important factor, but the influence of education is strongly associated with sociocultural factors [17]. As mentioned, about 90% of the participated pregnant women were categorized into the low and middle income category (<300$ per month), however no significant difference was observed between family income with phthalate metabolite concentrations (p value>0.05). Similar findings were also reported by Zhu et al. [18]. There are also some reports that indicate family income is positively associated with urinary phthalate metabolites concentration among pregnant women [19]. As stated for education, the effect of family income on urinary phthalate metabolites among pregnant women can be mediated by socioeconomic and cultural factors. Since the education status, family income and different sociocultural factors are somehow interrelated, therefore they may result in different effects in developed countries versus developing countries [8].
Table 4.
The mean value of urinary phthalate metabolite concentrations across categories of some possible determinants
| Variables | MBP | MBzP | MEHP | MEHHP | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Mean(SD) | p value | Mean(SD) | p value | Mean (SD) | p value | Mean (SD) | p value | |||
| Education | College | 316 (187) | 0.3 | 404 (252) | 0.48 | 120 (55) | 0.85 | 1043(127) | 0.26 | |
| High school | 250 (176) | 269 (232) | 148(144) | 904(286) | ||||||
| <high school | 352 (195) | 305 (228) | 125(120) | 851(317) | ||||||
| Family income per month | <100 $ | 343 (189) | 0.79 | 316 (216) | 0.97 | 127(115) | 0.3 | 895(288) | 0.22 | |
| 100–300 $ | 349 (195) | 304(238) | 135(125) | 875(307) | ||||||
| >300 $ | 305 (217) | 309(243) | 78(72) | 721(360) | ||||||
| Plastic packaging | Bread | Yes | 371 (183) | 0.03 | 340(221) | 0.04 | 141(118) | 0.09 | 915(283) | 0.02 |
| No | 293 (204) | 254(235) | 103(117) | 785(332) | ||||||
| Lemon juice | Yes | 351 (197) | 0.6 | 351(228) | 0.02 | 154(124) | 0.004 | 923(279) | 0.02 | |
| No | 332 (192) | 256(222) | 92(102) | 796(329) | ||||||
| Pickle | Yes | 357 (191) | 0.45 | 373(238) | 0.005 | 163(134) | 0.002 | 925(293) | 0.06 | |
| No | 330 (196) | 255(209) | 96(94) | 817(313) | ||||||
| Leftover | Yes | 387 (193) | 0.02 | 372(249) | 0.007 | 173(138) | <0.001 | 915(297) | 0.13 | |
| No | 308 (189) | 260(202) | 91(87) | 830(312) | ||||||
| Water | Yes | 375 (196) | 0.07 | 363(233) | 0.008 | 160(127) | 0.002 | 919(307) | 0.06 | |
| No | 310 (188) | 254(215) | 93(99) | 815(301) | ||||||
| Passive smoking during pregnancy | Yes | 432 (152) | <0.001 | 395(240) | <0.001 | 171(132) | <0.001 | 974(266) | <0.001 | |
| No | 238 (156) | 280(170) | 75(72) | 742(308) | ||||||
| Physical activity | Low | 373 (172) | 0.12 | 360 (230) | 0.03 | 146(118) | 0.13 | 937(270) | 0.02 | |
| Moderate | 311 (209) | 248 (211) | 104(112) | 773(333) | ||||||
| High | 284 (246) | 254 (256) | 99(141) | 850(323) | ||||||
Bold results show significant association (p-value < 0.05)
Phthalates has been used widely as plasticizers in food and water packaging for many years, but due to adverse health effects, their use in food processing is prohibited in some developed countries [20]. However, these materials are extensively used in several developing countries such as Iran. The distribution of urinary phthalate metabolites was assessed in relation with the use of plastic packaging for different purposes. In this regard, the results of current study showed that the mean concentration of urinary phthalate metabolites was significantly different among those participants who used plastic packaging for bread as: MBP (p value = 0.03), MBzP (p value = 0.04), and MEHHP (p value = 0.02), those used plastic packaging for lemon juice was as: MBzP (p value = 0.02), MEHP (p value = 0.004), and MEHHP (p value = 0.02),those who used for leftover was as: MBP (p value = 0.02), MBzP (p value = 0.007), and MEHP (p value<0.001), and those that used for water and pickle was MBzP (p value = 0.008, p value = 0.005) and MEHP (p value = 0.002, p value = 0.002), respectively. Consistent with these findings, Ebrahimi et al. detected the presence of some phthalates (DEHP) in bottled waters in Isfahan [21]. In another study, Pourzamani et al. examined several brands of packed mineral water in PET containers and have found that all the investigated brands contained DEHP metabolites [22]. DEHP is considered as the parent compound for MEHP, MEHHP, and MEOHP metabolites and BBP can produce MBzP metabolite. Furthermore, the presence of DEP in a variety of plastic bottles have reported been reported in several previous studies that, and it assumed that the concentration of these substances may be affected by storage conditions [23]. The phthalates compounds can released from the plastic due to some chemical reaction and plastic corrosion which finally enter to foods and water and may consequently lead to disrupt endocrine in human body [24].
Our results indicated that the mean values of urinary MBzP and MEHHP were significantly (p value = 0.03 and p value = 0.02, respectively) lower in individuals who had high physical activity. We assumed that the harsh physical activity may decrease the exposure to phthalates. There are only limited studies which have addressed the association between having physical activity and the exposure to phthalates. In this regard, Amin et al. have shown that higher levels of physical activity is associated with lower phthalates exposure levels [11]. Additionally, low concentration of urinary phthalates (or metabolites) in urine samples of those participates who had high physical activity can be interpreted by the fact that, the physical activity remarkably can influence the toxicokinetics of hazardous materials and result in higher excretion rates [25].
Table 5 presents the results of correlation between quantitative determinants and urinary phthalate metabolites.
Table 5.
Correlation between phthalate metabolite concentrations with the quantitative studied determinants
| Variables | MBP | MBzP | MEHP | MEHHP | ||||
|---|---|---|---|---|---|---|---|---|
| r | p value | r | p value | r | p value | r | p value | |
| Maternal age | −0.05 | 0.6 | 0.27 | 0.002 | 0.13 | 0.17 | 0.18 | 0.05 |
| Pre-pregnancy BMI | 0.27 | 0.003 | 0.3 | <0.001 | 0.26 | 0.003 | 0.26 | 0.003 |
| Cosmetic usage | 0.46 | <0.001 | 0.48 | <0.001 | 0.49 | <0.001 | 0.54 | <0.001 |
| household cleaning products | 0.2 | 0.03 | 0.22 | 0.01 | 0.3 | <0.001 | 0.26 | 0.003 |
| Fried food usage | −0.06 | 0.53 | −0.06 | 0.52 | −0.05 | 0.57 | −0.12 | 0. 9 |
| Passive smoking | 0.5 | <0.001 | 0.44 | <0.001 | 0.44 | <0.001 | 0.26 | 0.003 |
r: Correlation coefficient
Bold results show significant correlation (p-value < 0.05)
In the present study, we also observed a significantly positive correlation between maternal age with MBzP (r = 0.27) and MEHHP (r = 0.18). However, controversial results have been reported by the previous studies regarding the influence of maternal age on the concentration of urinary phthalate metabolites. There are some evidences indicating no correlation between age and phthalate concentration [26], while several others demonstrating a correlation between phthalate metabolites such as MBzP, MEOHP and MEHHP with maternal age [19, 27].
Furthermore, positive correlations were also found between pre-pregnancy BMI and the urinary phthalate metabolites including MBP, MBzP, MEHP, and MEHHP (r = 0.27, r = 0.3, r = 0.26, and r = 0.26, respectively). Similar findings were also observed in other study by Buser et al. [28]. According to their results, the higher levels of MBzP, MEHHP, and MBP were found in urinary samples of obese individuals compared to normal bodyweight controls. In the other study conducted by Hatch et al. positive relationships have been demonstrated between BMI and urinary phthalate metabolites (MBzP, MEHHP, and MBP) [29]. Since the phthalates have some extent of lipophilic properties and can be stored in adipose tissue, thus obese people may exhibit higher concentrations of urinary phthalate metabolites [30].
Moreover, dermal absorption of phthalates can also occur as these materials are widely utilized as stabling agents in cosmetic products [31]. The highest consumption of cosmetics in the Middle East has been reported in Iran [32]. The above mentioned points are in line with the positive correlations were found in the present study between the use of personal care products (such as cosmetics) with urinary concentrations of MBP (r = 0.46), MBzP (r = 0.48), MEHP (r = 0.49) and MEHHP (r = 0.54). Several previous studies have also reported consistent results [33, 34]. However, on the contrast, Valvi et al. have shown no relationship between the use of cosmetics and the levels of phthalate metabolite in urine samples [4]. A possible explanation for these discrepancies could be due to different legislative actions applied in different countries for the production of cosmetics and care products. For instance, the use of some phthalates including DEHP, di-n-butyl phthalate, di-iso-butyl phthalate and BBP are prohibited by the EU (European Union) legislation in the production of cosmetic ingredients [35]. As mentioned, a large proportion of the participants in this study were the high consumer of cosmetic products. Additionally, the majority of the study population was the individuals with low or middle income. Therefore, it can be concluded that the majority of this population may use inexpensive with low-quality cosmetic products containing higher grades of phthalates.
In spite of cosmetic products, household cleaning products is considered as another important source of phthalate exposure in pregnant women [36]. In our study, it was found that the use of household cleaning products with the concentrations of urinary phthalates were positively associated with the elevated urinary concentrations of MBP (r = 0.2), MBzP (r = 0.22), MEHP (r = 0.3), and MEHHP (r = 0.26). Valvi et al. have also obtained consistent results [4]. They showed that the use of household chemical products is associated with the urinary concentrations of MEHHP, MEHP and MBzP. Collectively, because the females are mainly involved in household works, thus they may expose to higher levels of phthalates through the use of chemical products. Furthermore, due to the physiological changes during the pregnancy high concentration of these pollutants can endanger the health of pregnant women and their newborns as well [30].
No correlation was found between the uses of fried foods with the concentrations urinary phthalate metabolites. In contrast, Wenzel et al. have declared that processed fast foods are major sources of phthalate exposure which may result from the inappropriate processing and storage of fast foods [8].
Moreover, a strong significant correlation was observed between exposure to smoke with the urinary concentrations of MBP, MBzP, MEHP, and MEHHP (p value<0.05). Although all the subjects in this study were non-smokers, but the majority of them (n = 65, 53.7%) were passively exposed to smoke. In addition, positive correlation was noted between passive smoking exposure with urinary concentrations of MBP (r = 0.5), MBzP (r = 0.44), MEHP (r = 0.44), and MEHHP (r = 0.26). In accordance, previous studies have also linked smoking habits to higher phthalates exposure [4, 19]. Casas et al. have found that smoking is directly associated with elevated urinary phthalate concentrations [27]. Some evidences indicated that the ambient air pollutants may affect the pregnancy progress and consequently fetus growth, and may lead to poor birth outcome [37]. Besides, exposure to harmful chemical and physical factors have shown strong association with embryonic development cessation. During the pregnancy, detrimental substances extremely present in the tobacco and cigarette smoke which influence hormone secretion, and may cause serious effect on fetus. Phthalates are considered as an environmental endocrine disruptor with estrogen activity which can induce the reproductive developmental and fetus growth [38].
Jackson and Darnell et al. have demonstrated that cigarette smoke and filters contain phthalates in the form of di-2-methoxyethyl phthalate [39]. Additionally, low-quality cigarettes may also further increase the urinary concentration of phthalates metabolites in both first hand smokers and passive smokers.
One of the main limitations of the present study was its cross-sectional design. However, to extent of our knowledge, there is limited number of studies conducted in Iran to address this issue, thus the findings of this can be helpful in the improvement of health policies at the country level and in planning for the future investigations. Collectively, a high concentration of these metabolites can be considered as a health threatening alarm for exposed population especially for those vulnerable groups such as pregnant women or newborn infants. Here, only a population of pregnant women during their first trimester of pregnancy was studied. It is recommended that the monitoring of phthalate metabolites in pregnant women should be done at second and third trimester as well. Moreover, the relationships between higher concentrations of urinary phthalate metabolites among pregnant women with the adverse birth outcomes have to be considered.
Conclusion
This study was conducted to determine the possible associations between the lifestyle factors and characteristics of pregnant women in Iran with the concentrations of urinary phthalate metabolites. According to the findings, the following conclusions can be made:
MBzP, MBP, MEHP, and MEHHP were detected in 100% of pregnant women.
The concentration of studied metabolites in the Iranian pregnant women urine was higher than other countries.
The urinary phthalate metabolites were higher in pregnant women who were passive smokers, had higher pre-pregnancy BMI, lower physical activity, and among the users of cosmetic and household cleaning products, and the users of plastic packaging for food and water.
The lifestyle characteristics and consumer product use are better predictors of urinary phthalate metabolites in pregnant women than socio-demographic determinants.
Acknowledgements
The authors are grateful to the staff at the laboratory of the Department of Environmental Health Engineering, Isfahan University of Medical Sciences (IUMS).
Funding information
This research was conducted as a part of the Ph.D. dissertation of the first author, supported by Isfahan University of Medical Sciences.
Compliance with ethical standards
This study was approved by the Ethics Committee, Isfahan University of Medical Sciences (Code: IR.MUI.RESEARCH.REC.1397.441) and project number #397573.
Conflict of interest
The authors declare that they have no conflict of interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Bijan Bina, Email: bbina123@yahoo.com.
Roya Kelishadi, Email: Roya.kelishadi@gmail.com.
References
- 1.Li X, Sun H, Yao Y, Zhao Z, Qin X, Duan Y, Wang L. Distribution of phthalate metabolites between paired maternal–fetal samples. Environ Sci Technol. 2018;52(11):6626–6635. doi: 10.1021/acs.est.8b00838. [DOI] [PubMed] [Google Scholar]
- 2.Hauser R, Duty S, Godfrey-Bailey L, Calafat AM. Medications as a source of human exposure to phthalates. Environ Health Perspect. 2004;112(6):751–753. doi: 10.1289/ehp.6804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Darvishmotevalli M, Moradnia M, Noorisepehr M, Fatehizadeh A, Fadaei S, Mohammadi H, Salari M, Jamali HA, Daniali SS. Evaluation of carcinogenic risks related to nitrate exposure in drinking water in Iran. MethodsX. 2019;6:1716–1727. doi: 10.1016/j.mex.2019.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Valvi D, Monfort N, Ventura R, Casas M, Casas L, Sunyer J, Vrijheid M. Variability and predictors of urinary phthalate metabolites in Spanish pregnant women. Int J Hyg Environ Health. 2015;218(2):220–231. doi: 10.1016/j.ijheh.2014.11.003. [DOI] [PubMed] [Google Scholar]
- 5.Huang Y, Li J, Garcia JM, Lin H, Wang Y, Yan P, Wang L, Tan Y, Luo J, Qiu Z. Phthalate levels in cord blood are associated with preterm delivery and fetal growth parameters in Chinese women. PLoS One. 2014;9(2):e87430. doi: 10.1371/journal.pone.0087430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Magnusson C, Jugdaohsingh R, Hulthen L, Westerlund A, Powell JJ, Ransjö M. Urinary excretion of silicon in men, non-pregnant women, and pregnant women: a cross-sectional study. Biol Trace Elem Res. 2019:1–7. [DOI] [PMC free article] [PubMed]
- 7.Becker K, Güen T, Seiwert M, Conrad A, Pick-Fuß H, Müller J, Wittassek M, Schulz C, Kolossa-Gehring M. GerES IV: phthalate metabolites and bisphenol a in urine of German children. Int J Hyg Environ Health. 2009;212(6):685–692. doi: 10.1016/j.ijheh.2009.08.002. [DOI] [PubMed] [Google Scholar]
- 8.Wenzel AG, Brock JW, Cruze L, Newman RB, Unal ER, Wolf BJ, Somerville SE, Kucklick JR. Prevalence and predictors of phthalate exposure in pregnant women in Charleston, SC. Chemosphere. 2018;193:394–402. doi: 10.1016/j.chemosphere.2017.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kelishadi R, Majdzadeh R, Motlagh ME, Heshmat R, Aminaee T, Ardalan G, Esmaillzadeh A, Azadbakht L, Poursafa P, Movahedian M, Baraz S. Development and evaluation of a questionnaire for assessment of determinants of weight disorders among children and adolescents: the CASPIAN-IV study. Int J Prev Med. 2012;3(10):699–705. [PMC free article] [PubMed] [Google Scholar]
- 10.Cleland C, Ferguson S, Ellis G, Hunter RF. Validity of the international physical activity questionnaire (IPAQ) for assessing moderate-to-vigorous physical activity and sedentary behaviour of older adults in the United Kingdom. BMC Med Res Methodol. 2018;18(1):176. doi: 10.1186/s12874-018-0642-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Amin MM, Ebrahimpour K, Parastar S, Shoshtari-Yeganeh B, Hashemi M, Mansourian M, Poursafa P, Fallah Z, Rafiei N, Kelishadi R. Association of urinary concentrations of phthalate metabolites with cardiometabolic risk factors and obesity in children and adolescents. Chemosphere. 2018;211:547–556. doi: 10.1016/j.chemosphere.2018.07.172. [DOI] [PubMed] [Google Scholar]
- 12.Schuhmacher M, Nadal M, Domingo JL. Environmental monitoring of PCDD/Fs and metals in the vicinity of a cement plant after using sewage sludge as a secondary fuel. Chemosphere. 2009;74(11):1502–1508. doi: 10.1016/j.chemosphere.2008.11.055. [DOI] [PubMed] [Google Scholar]
- 13.Berman T, Hochner-Celnikier D, Calafat AM, Needham LL, Amitai Y, Wormser U, Richter E. Phthalate exposure among pregnant women in Jerusalem, Israel: results of a pilot study. Environ Int. 2009;35(2):353–357. doi: 10.1016/j.envint.2008.08.010. [DOI] [PubMed] [Google Scholar]
- 14.Irvin EA, Calafat AM, Silva MJ, Aguilar-Villalobos M, Needham LL, Hall DB, Cassidy B, Naeher LP. An estimate of phthalate exposure among pregnant women living in Trujillo, Peru. Chemosphere. 2010;80(11):1301–1307. doi: 10.1016/j.chemosphere.2010.06.048. [DOI] [PubMed] [Google Scholar]
- 15.Adibi JJ, Hauser R, Williams PL, Whyatt RM, Calafat AM, Nelson H, Herrick R, Swan SH. Maternal urinary metabolites of di-(2-ethylhexyl) phthalate in relation to the timing of labor in a US multicenter pregnancy cohort study. Am J Epidemiol. 2009;169(8):1015–1024. doi: 10.1093/aje/kwp001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wolff MS, Engel SM, Berkowitz GS, Ye X, Silva MJ, Zhu C, Wetmur J, Calafat AM. Prenatal phenol and phthalate exposures and birth outcomes. Environ Health Perspect. 2008;116(8):1092–1097. doi: 10.1289/ehp.11007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Auger N, Luo Z, Platt R, Daniel M. Do mother’s education and foreign born status interact to influence birth outcomes? Clarifying the epidemiological paradox and the healthy migrant effect. J Epidemiol Community Health. 2008;62(5):402–409. doi: 10.1136/jech.2007.064535. [DOI] [PubMed] [Google Scholar]
- 18.Zhu Y, Wan Y, Li Y, Zhang B, Zhou A, Cai Z, Qian Z, Zhang C, Huo W, Huang K. Free and total urinary phthalate metabolite concentrations among pregnant women from the healthy baby cohort (HBC), China. Environ Int. 2016;88:67–73. doi: 10.1016/j.envint.2015.12.004. [DOI] [PubMed] [Google Scholar]
- 19.Arbuckle TE, Davis K, Marro L, Fisher M, Legrand M, LeBlanc A, Gaudreau E, Foster WG, Choeurng V, Fraser WD. Phthalate and bisphenol a exposure among pregnant women in Canada—results from the MIREC study. Environ Int. 2014;68:55–65. doi: 10.1016/j.envint.2014.02.010. [DOI] [PubMed] [Google Scholar]
- 20.European Food Safety Authority Opinion of the scientific panel on food additives, flavourings, processing aids and materials in contact with food (AFC) on a request related to a 18th list of substances for food contact materials.
- 21.Ebrahimi A, Esfandiari Z, Estaki F, Majd AMS, Mirlohi M, Moghadam ZA, Falahati M, Pourzamani H. Qualitative evaluation of bottled water stored in polyethylene terephtalate based on organic chemical compounds. Anu Inst Geocienc. 2016;39(2):29–35. [Google Scholar]
- 22.Pourzamani H, Falahati M, Rastegari F, Ebrahim K. Freeze–melting process significantly decreases phthalate ester plasticizer levels in drinking water stored in polyethylene terephthalate (PET) bottles. Water Sci Technol Water Supply. 2017;17(3):745–751. doi: 10.2166/ws.2016.172. [DOI] [Google Scholar]
- 23.Al-Saleh I, Shinwari N, Alsabbaheen A. Phthalates residues in plastic bottled waters. J Toxicol Sci. 2011;36(4):469–478. doi: 10.2131/jts.36.469. [DOI] [PubMed] [Google Scholar]
- 24.Gallo F, Fossi C, Weber R, Santillo D, Sousa J, Ingram I, Nadal A, Romano D. Marine litter plastics and microplastics and their toxic chemicals components: the need for urgent preventive measures. Environ Sci Eur. 2018;13(1):30. doi: 10.1186/s12302-018-0139-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Council NR (2009) Systemic exposures to volatile organic compounds and factors influencing susceptibility to their effects. In: contaminated water supplies at camp Lejeune: assessing potential health effects. National Academies Press (US). [PubMed]
- 26.Duty SM, Ackerman RM, Calafat AM, Hauser R. Personal care product use predicts urinary concentrations of some phthalate monoesters. Environ Health Perspect. 2005;113(11):1530–1535. doi: 10.1289/ehp.8083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Casas L, Fernández MF, Llop S, Guxens M, Ballester F, Olea N, Irurzun MB, Rodríguez LSM, Riaño I, Tardón A. Urinary concentrations of phthalates and phenols in a population of Spanish pregnant women and children. Environ Int. 2011;37(5):858–866. doi: 10.1016/j.envint.2011.02.012. [DOI] [PubMed] [Google Scholar]
- 28.Buser MC, Murray HE, Scinicariello F. Age and sex differences in childhood and adulthood obesity association with phthalates: analyses of NHANES 2007–2010. Int J Hyg Environ Health. 2014;217(6):687–694. doi: 10.1016/j.ijheh.2014.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hatch EE, Nelson JW, Qureshi MM, Weinberg J, Moore LL, Singer M, Webster TF. Association of urinary phthalate metabolite concentrations with body mass index and waist circumference: a cross-sectional study of NHANES data, 1999–2002. Environ Health. 2008;7(1):27. doi: 10.1186/1476-069X-7-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Philips EM, Jaddoe VW, Trasande L. Effects of early exposure to phthalates and bisphenols on cardiometabolic outcomes in pregnancy and childhood. Reprod Toxicol. 2017;68:105–118. doi: 10.1016/j.reprotox.2016.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Aydemir D, Karabulut G, Şimşek G, Gok M, Barlas N, Ulusu NN. Impact of the Di (2-Ethylhexyl) phthalate administration on trace element and mineral levels in relation of kidney and liver damage in rats. Biol Trace Elem Res. 2018;186(2):474–488. doi: 10.1007/s12011-018-1331-0. [DOI] [PubMed] [Google Scholar]
- 32.Volpe M, Nazzaro M, Coppola R, Rapuano F, Aquino R. Determination and assessments of selected heavy metals in eye shadow cosmetics from China, Italy, and USA. Microchem Journal. 2012;101:65–69. doi: 10.1016/j.microc.2011.10.008. [DOI] [Google Scholar]
- 33.Parlett LE, Calafat AM, Swan SH. Women’s exposure to phthalates in relation to use of personal care products. J Expo Sci Environ Epidemiol. 2013;23(2):197. doi: 10.1038/jes.2012.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Romero-Franco M, Hernández-Ramírez RU, Calafat AM, Cebrián ME, Needham LL, Teitelbaum S, Wolff MS, López-Carrillo L. Personal care product use and urinary levels of phthalate metabolites in Mexican women. Environ Int. 2011;37(5):867–871. doi: 10.1016/j.envint.2011.02.014. [DOI] [PubMed] [Google Scholar]
- 35.Wittassek M, Koch HM, Angerer J, Brüning T. Assessing exposure to phthalates–the human biomonitoring approach. Mol Nutr Food Res. 2011;55(1):7–31. doi: 10.1002/mnfr.201000121. [DOI] [PubMed] [Google Scholar]
- 36.Harley KG, Kogut K, Madrigal DS, Cardenas M, Vera IA, Meza-Alfaro G, She J, Gavin Q, Zahedi R, Bradman A. Reducing phthalate, paraben, and phenol exposure from personal care products in adolescent girls: findings from the HERMOSA intervention study. Environ Health Perspect. 2016;124(10):1600–1607. doi: 10.1289/ehp.1510514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhao R, Wu Y, Zhao F, Lv Y, Huang D, Wei J, et al. The risk of missed abortion associated with the levels of tobacco, heavy metals and phthalate in hair of pregnant woman: a case control study in Chinese women. Medicine. 2017;96(51). [DOI] [PMC free article] [PubMed]
- 38.Chen M, Fan Z, Zhao F, Gao F, Mu D, Zhou Y, Shen H, Hu J. Occurrence and maternal transfer of chlorinated bisphenol a and nonylphenol in pregnant women and their matching embryos. Environ Sci technol. 2015;50(2):970–977. doi: 10.1021/acs.est.5b04130. [DOI] [PubMed] [Google Scholar]
- 39.Jackson Jr WJ, Darnell WR (1985) Process for foaming cellulose acetate rod. US patent 4,507,256. Eastman Kodak.