Abstract
The noble scallop Chlamys nobilis is an economically important marine bivalve cultivated in the southern sea of China since the 1980s. Unfortunately, mass mortality of this scallop species often occurs in summer. The present study was conducted to investigate whether the expression of heat shock protein 90 (HSP90) and level of carotenoids could enhance high-temperature stress resistance in scallop. First, the HSP90 homolog of C. nobilis (designated CnHSP90) was identified and cloned. The complete cDNA sequence of CnHSP90 was 2631 bp, including a 2181-bp open reading frame (ORF) encoding a 726 amino acid polypeptide with five HSP90 family signatures, and sharing high homology with members of the HSP90 family. CnHSP90 was ubiquitously expressed in all examined tissues including the intestine, kidney, adductor, mantle, gill, and gonad, with the highest in the gonad. Golden and brown scallops, which contain significantly different total carotenoid content (TCC), were subjected to acute thermal challenge, and the LTE50 (semi-lethal temperature at 36 h heat shock) and LTI50 (semi-lethal time after heat shock) as well as the correlation between CnHSP90 gene expression and TCC were determined. The LTE50 of golden scallop (32.14 °C) was higher than that of brown scallops (31.19 °C), with longer LTI50 at all tested temperatures, indicating that golden scallops were more resistant to thermal stress than brown scallops. Similarly, the mRNA expression levels of CnHSP90 in gill of golden scallops were significantly higher (P < 0.05) than that of brown scallops at 6, 12, 24, and 36 h, with a strong positive correlation between CnHSP90 expression level and TCC. This suggests that both carotenoids and HSP90 levels could improve thermal resistance in the noble scallops.
Keywords: Scallop, Chlamys nobilis, CnHSP90, Thermal stress, Carotenoids, Semi-lethal temperature, Semi-lethal time
Introduction
All aquatic animals are exposed to a wide range of abiotic environmental stressors including high-temperature fluctuations, salinity shifts, oxygen deprivations, and pollutions (Park et al. 2015), as well as disease-causing biotic stressors such as bacteria, virus, fungi, and parasites (Zhou et al. 2010). Temperature is considered one of the important environmental factors that affect biological processes in animals (Mubiana and Blust 2007). When animals are exposed to continuous thermal stress, heat shock proteins (HSPs) exert protective effects against the environmental perturbations (Cheng et al. 2019a). HSPs are divided into several families (based on molecular weight), including HSP110, HSP90, HSP70, HSP60, HSP40, and small HSPs (Joly et al. 2010). Among the HSPs, HSP90 is a ubiquitous, highly conserved and multi-functional molecular protein, which functions as part of a large complex with other chaperones or essential cofactors that corrects protein conformation and modifies misfolding of denatured proteins (Johnson and Brown 2009). Moreover, HSP90 is also involved in hormonal signal transduction, cell differentiation, cell proliferation, apoptosis, morphogenesis, immune response, and stress defense in organisms (Helmbrecht et al. 2000; Queitsch et al. 2002; Rutherford et al. 2007). Currently, the induced synthesis of HSP90 by external stress has been reported in various mollusks including Chlamys farreri (Gao et al. 2007), Argopecten irradians (Gao et al. 2008), Crassostrea gigas (Choi et al. 2008), Haliotis discus hannai (Zhang et al. 2011), Ruditapes philippinarum (Liu et al. 2015a, 2015b), and many others.
Carotenoids, which are essential nutrients for normal physiological functions of animals, not only are sources of vitamin A but also act as antioxidants to protect against injuries caused by free radicals and reactive oxygen species (Joanna and Burda 2014). Other proposed functions of carotenoids include stimulation of the immune system (Saks et al. 2003), improving stress tolerance (Babin et al. 2010; Lu et al. 2016), as well as enhancing embryonic development, growth, and gonadal maturation (Liñán-Cabello et al. 2002). Carotenoids also can directly regulate the expression of genes to protect against carcinogenesis and inflammation (Hix et al. 2004).
The noble scallop Chlamys nobilis is an economically importantly marine bivalve, which has been cultivated in the southern coastal areas of China since 1980s. In recent years, annual records of 60–80% mortalities of the noble scallop have been reported during summer, especially from July to September, which seems to be due to high water temperatures. The “Nan’ao Golden Scallop” is a new variety of scallop produced by selective breeding from cultured stock of the noble scallop in 2015. Individuals of this new variety do not only possess golden shells and golden muscles but also are enriched in carotenoids in their muscles. Meanwhile, brown scallops were also bred by selection from the same cultured stock of noble scallops, but these possess brown shells and white muscles with much lower total carotenoid content. Previous studies have shown that carotenoids could enhance resistance to low-temperature stress (Han et al. 2016) and immunity to Vibrio parahaemolyticus challenge (Lu et al. 2016) in scallops.
In the present study, golden scallops and brown scallops were exposed to an acute thermal challenge so as to investigate whether HSP90 and carotenoids could enhance the resistance of the noble scallop Chlamys nobilis to thermal stress. The LTE50 (semi-lethal temperature at 36 h heat shock) and LTI50 (semi-lethal time after heat shock) of both golden and brown scallops were determined. The full-length cDNA sequence of CnHSP90 was cloned and expressed, and the transcript levels and total carotenoid content (TCC) in gills of golden and brown scallops were examined. Finally, the correlation between the mRNA expression level of CnHSP90 and TCC was analyzed.
Materials and methods
Experimental animals and tissue collection
Adult (14 months old) golden and brown noble scallops Chlamys nobilis (Fig. 1) from the same stock and cultured in the same environment in the Nan’ao Marine Biology Experimental Station of Shantou University (Shantou, China) were used in the present study. Before the experiments, all scallops were randomly collected and acclimatized under constant aeration at ambient temperature of 25 ± 0.1 °C for 7 days. During the acclimation period, scallops were fed with diatom (Nitzschia closterium f. minutissima) and tetraselmis (Platymonas subcordiformis) cultured by our laboratory, and the water was changed daily. Tissues including those of the intestine, kidney, adductor, mantle, gill, and gonad were sampled and stored at − 80 °C for further tissue expression analyses.
Fig. 1.

Golden scallop (left) and brown scallop (right)
Experimental design
Determination of semi-lethal temperature (LTE50) and semi-lethal time (LTI50)
In the present study, the 36h-LTE50 (temperature of 50% scallops’ mortality after 36 h heat shock) and the LTI50 (time of 50% scallops’ mortality after heat shock) were first determined as follows: 100 golden scallops and 100 brown scallops were equally divided into five groups and cultured at five different temperatures of 25 (control), 30, 31, 32, and 33 °C, respectively. The mortality of each group was determined after 3, 6, 12, 24, and 36 h of heat exposure. Experiments were conducted in triplicates.
Acute thermal stress experiments
According to the predicted LTE50 from the above experiments, 32 °C was selected as the challenge temperature. Next, 60 golden and 60 brown scallops were equally divided into two separate tanks (30 golden and 30 brown scallops in each tank) and maintained at 32 °C and 25 °C (control), respectively. The gills of three golden and brown scallops were collected randomly at 3, 6, 12, 24, and 36 h. The gills of same color scallops were pooled together at equal amount and frozen in liquid nitrogen. The pooled samples were grounded in a mortar. Then the sub-samples were stored at − 80 °C and − 20 °C for subsequent RNA extraction and total carotenoid content determination, respectively. Along the experiment, seawater was aerated but feed was not provided. Experiments were conducted in four replicates.
Cloning full-length cDNA of CnHSP90
RNA isolation and cDNA synthesis
Total RNA was extracted from tissues using Trizol Reagent (Invitrogen, Carlsbad, CA) according to the manufacturer’s recommendations. The concentration of the total RNA was estimated by measuring the absorbance at 260 nm. Next, 1 μg of total RNA was used as template for cDNA synthesis by using SuperScript III reverse transcriptase (Invitrogen, USA) and Oligo(dT) as the primers to obtain the first strand following the manufacturer instructions.
Clone cDNA of CnHSP90
A cDNA fragment of CnHSP90 was obtained from our previous high-throughput transcriptome data (Liu et al. 2015a, 2015b), and using this sequence, gene-specific primers (Table 1) were designed for 3′ RACE and 5′ RACE of CnHSP90 using the SMART™ RACE cDNA amplification kit (Clontech, USA) and LA Taq polymerase (TaKaRa). All samples were prepared following the instructions of the manufacturers. A touchdown PCR program was performed to obtain the 3′ end and 5′ end of CnHSP90, with the following conditions: 94 °C for 2 min followed by 35 cycles of 94 °C for 30 s, 70–60 °C for 30 s in the initial 10 cycles decrementing 1 °C/cycle, 60 °C for 30 s for the remaining 25 cycles, 72 °C for 60 s, and a final extension step at 72 °C for 10 min. After the PCR, the purified DNA fragment was sub-cloned into the vector pMD18-T (TaKaRa, Guangzhou, China), transformed into Escherichia coli and positive clones sequenced (Sangon Biotech, Shanghai, China). The 3′- and 5′-RACE PCR fragment sequences were then aligned to assemble the full nucleotide sequence of the putative scallop CnHSP90 cDNA.
Table 1.
Primers used in the study of CnHSP90
| Primer name | Sequence (5′–3′) |
|---|---|
| cDNA synthesis | |
| Oligo(dT) | GGCCACGCGTCGACTAGTACT 17 |
| Gene cloning | |
| UPM (long) | CTAATACGACTCACTATAGGGCAAGCAGTGGTATCAACGCAGAGT |
| UPM (short) | CTAATACGACTCACTATAGGGC |
| NUP | AAGCAGTGGTATCAACGCAGAGT |
| HSP-GSP-F1 | TAGAGGAACACGAATCACCTTACAC |
| HSP-GSP-F2 | GGTGAGAGCCGAGAGGTTGTCCAAA |
| HSP-GSP-R1 | TTATCATTACTGTCAACTCGCACTT |
| HSP-GSP-R2 | TGCTGAACTTTGGACAACCTCTCGG |
| RT-PCR primers | |
| HSP-qRT-F | TCTGCTGGCGGCTCTTTCACT |
| HSP-qRT-R | CTTCTACCTTTGGCTTCTCAT |
| β-actin-F | CAAACAGCAGCCTCCTCGTCAT |
| β-actin-R | CTGGGCACCTGAACCTTTCGTT |
| Vector primers | |
| M13–47 | CGCCAGGGTTTTCCCAGTCACGAC |
| RV-M | GAGCGGATAACAATTTCACACAGG |
Bioinformatic analysis
The obtained CnHSP90 nucleotide sequence and the deduced amino acid sequence were analyzed using the BLAST program (http://blast.ncbi.nlm.nih.gov/Blast.cgi). The theoretical pI/Mw were calculated using the Compute PI/Mw program (http://web.expasy.org/compute_pi/). The deduced amino acid sequence of CnHSP90 was aligned with other species using the ClustalX multiple alignment program (version1.83), while the phylogenetic tree was generated by the neighbor-joining (NJ) method using MEGA 5.0 (Tamura et al. 2011).
Relative quantitative real-time PCR analysis
Transcripts levels of CnHSP90 were determined on the LightCycler 480 (Roche) using the SYBR®Premix Ex Taq™ II Kit (Perfect Real Time) (Takara, Japan). Primers for the real-time PCR analysis are listed in Table 1. The qRT-PCR amplifications were carried out in a total volume of 20 μL containing 10 μL SYBR®Premix Ex Taq™ II, 2 μL of the 1:5 diluted cDNA, 0.8 μL each of HSP-qRT-F and HSP-qRT-R primer to amplify CnHSP90 gene, 0.8 μL β-actin-F and β-actin-R to amplify the β-actin gene, and 6.4 μL PCR-grade water. The qRT-PCR program was 95 °C for 30 s, followed by 40 cycles of 95 °C for 5 s, 60 °C for 30 s. Melting curve analysis of amplification products was performed at the end of each PCR reaction to confirm that only one PCR product was amplified and detected, and the program was set as 95 °C for 15 s, 60 °C for 30 s, and 95 °C for 15 s. A no template control of RT-PCR reaction was also performed. Each sample was run in triplicate. Relative mRNA expression of CnHSP90 was determined using the 2−ΔΔCT algorithm with β-actin from Chlamys nobilis as the internal control (Livak and Schmittgen 2001).
Determination of total carotenoid content
The TCC was determined following the method of Zheng et al. (2010)). All samples were dried in a vacuum freeze-drying machine and then ground to fine powder in mortars. Homogenized samples of approximately 0.02 g were mixed with 1 mL acetone and shaken mildly in the dark at room temperature. After 1 h extraction, the mixture was centrifuged at 5000 rpm for 5 min and the supernatant scanned in a UV-vis recording spectrophotometer (UV2501PC, Japan) from 350 to 540 nm. Finally, TCC (μg/g dry weight) was calculated by using the extinction coefficient E (1%, 1 cm) of 1900 times the absorption value at 480 nm (Cheng et al. 2019b).
Statistical analysis
The differences in HSP90 transcript level and TCC between golden scallops and brown scallops at the same time points were analyzed by independent t test. The differences in HSP90 transcript levels and the TCC among different stress times were analyzed by a two-way ANOVA and Tukey’s multiple comparisons.
The correlation between mortality and thermal stress temperature or time was determined using logistic equation (1) or general linear equation (2) by MATLAB 8.3 (MathWork Inc., MA). The equations used are as follows:
| 1 |
| 2 |
where Y represents mortality, x represents time, K represents the saturation capacity of the mortality, and a and b are the equation parameters to be estimated via non-linear least squares estimation.
The 36h-LTE50 or LTI50 were then estimated by the above two equations.
A general linear model (3) was used to evaluate the effects of scallop color (S), stress time (T), and their interaction on the HSP90 gene expression levels:
| 3 |
where Yijm = HSP90 gene expression level or TCC of the m replicate in the i strain from the j treatment time; μ = overall constant; Si = the fixed effect of scallop (i = 1, 2); Tj = treatment time (j = 1, 2, 3, 4, 5, 6); (S × T)ij = interaction effect between scallop and treatment time; and eijm = random observation error (m = 1, 2, 3, 4).
In addition, the correlations between CnHSP90 gene expression level and TCC of gill were analyzed using liner regression and Pearson regression.
All statistical analyses were done by SPSS vision 20 (SPSS Inc., Chicago, USA) and significance for all analyses was set to P < 0.05 unless otherwise stated.
Results
Comparison of mortality, 36h-LTE50, and LTI50 between golden scallops and brown scallops
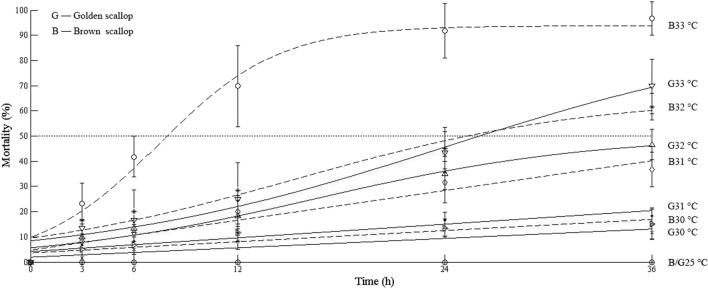
Mortalities of golden scallops and brown scallops at different temperature and times are illustrated in Fig. 2. All scallops survived at 25 °C; however, at higher temperatures, the mortalities of golden and brown scallops did not only increase with increasing temperature but also with time. Interestingly, the golden scallops had lower mortality compared to the brown scallops under the same temperature and time. Moreover, the 50% mortality of brown scallops was observed at temperatures of 32 °C and 33 °C, whereas for golden scallops it was only at 33 °C.
Fig. 2.
Mortalities of golden scallops and brown scallops at different temperatures and time points
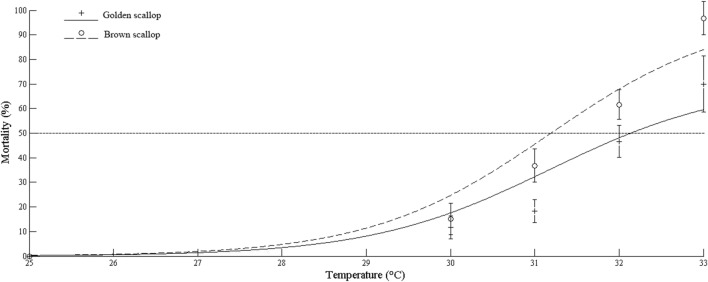
In Fig. 3, the mortality of golden and brown scallops at 36 h of thermal stress shows that golden scallops had lower mortality compared to brown scallops. Moreover, the LTE50 of golden scallops was higher (32.14 °C) than that of brown scallops (31.19 °C) (Table 2).
Fig. 3.
Mortality of golden scallops and brown scallops at 36 h thermal stress
Table 2.
Semi-lethal temperature estimates of golden scallops and browns scallops after 36 h acute thermal stress
| Groups | Equation | Coefficient (r) | Lethal temperature (LTE50) |
|---|---|---|---|
| Golden scallop | y = 70.32/(1 + 3e−0.9355(x−30)) | 0.8948 | 32.14 |
| Brown scallop | y = 98.83/(1 + 3e−0.9427(x−30)) | 0.9365 | 31.19 |
The LTI50 of golden and brown scallops are summarized in Table 3. It can be seen that at the same temperature, the LTI50 of golden scallops was much longer than that of brown scallops, with the ratio of golden scallops LTI50: brown scallops LTI50 increasing gradually with increase in temperature.
Table 3.
Semi-lethal time estimates of golden scallops and brown scallops under different temperature points
| Groups | Equation | Coefficient (r) | Lethal time (LTI50) | Ratio of LTI50 (G/B) |
|---|---|---|---|---|
| G 33 °C | y = 91.93/(1 + 9.910e−0.0949x) | 0.9684 | 26.02 | 3.29 |
| B 33 °C | y = 93.77/(1 + 8.547e−0.2878x) | 0.9800 | 7.92 | |
| G 32 °C | y = 50.22/(1 + 7.827e−0.1247x) | 0.9694 | 60.01 | 2.37 |
| B 32 °C | y = 65.64/(1 + 5.916e−0.1162x) | 0.9448 | 25.30 | |
| G 31 °C | y = 4.174 + 0.4521x | 0.8596 | 101.36 | 2.21 |
| B 31 °C | y = 4.755 + 0.9849x | 0.9409 | 45.94 | |
| G 30 °C | y = 1.925 + 0.3101x | 0.8517 | 155.03 | 1.23 |
| B 30 °C | y = 3.624 + 0.3694x | 0.8081 | 125.54 |
G golden scallop, B brown scallops
Sequence analysis of CnHSP90 cDNA
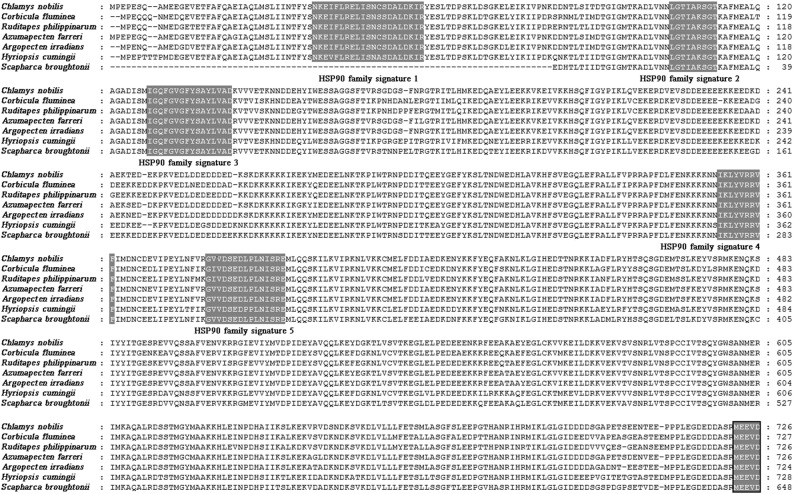
The full-length cDNA sequence of CnHSP90 determined by 5′ RACE and 3′ RACE was 2631 bp containing a 2181-bp open reading frame (ORF), a 120-bp 5′-terminal untranslated region (UTR) and a 330-bp 3′-UTR (Fig. 4). The ORF encoded a polypeptide of 726 amino acid residues (Fig. 4) with a theoretical molecular mass of 83.39 kDa and an isolectric point (pI) of 4.81. Several conserved motifs or domains were found in the deduced amino acids of CnHSP90, including five HSP90 family signatures, an ATP-binding GxxGxG motif, and a protein-binding leucine-zipper Lx6Lx5Lx6Lx6L motif (Fig. 4). The consensus sequence MEEVD at the C-terminus, which is characteristic of HSP90 proteins was also found in the CnHSP90 sequence. The cDNA sequence has submitted to GenBank under the accession number MH985699.
Fig. 4.
Nucleotide and deduced amino acid sequences of CnHSP90 cDNA. The start (ATG) and the termination (TAA) nucleotides are in bold. Five HSP90 family signature motifs are shadowed. The ATP-binding GxxGxG motif is boxed. The protein-binding leucine-zipper Lx6Lx5Lx6Lx6L motif is underlined, while the cytosolic HSP90 signature “MEEVD” is indicated with wave lines
Multiple sequence alignments and phylogenetic tree analysis
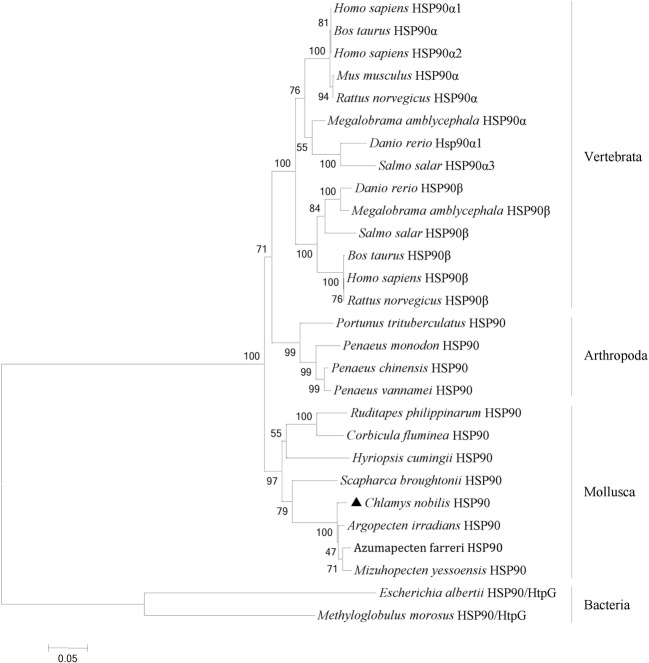
Multiple sequence alignments of the deduced amino acid sequence of CnHSP90 with the HSP90s of other species (Fig. 5) revealed that CnHSP90 shared high identity with the HSP90s of Hyriopsis cumingii (80%), Corbicula fluminea (83%), Ruditapes philippinarum (83%), Scapharca broughtonii (83%), Arogopecten irradians (95%), and Azumapecten farreri (97%). The multiple sequence alignment data also revealed that the HSP90s were highly conserved, especially the consensus sequence MEEVD at the C-terminus (shadow in Fig. 5). Phylogenetic tree analysis using the neighbor-joining method with 1000 bootstrap replications showed that the HSP90s analyzed grouped into four clusters: vertebrata, arthropoda, mollusca, and bacteria (Fig. 6). In the vertebrata cluster, HSP90α and HSP90β were clustered independently, while CnHSP90 clustered with other mollusca HSP90s and in close proximity with Argopecten irradians (Fig. 6).
Fig. 5.
Multiple sequence alignment of CnHSP90 with HSP90s of other species in GenBank. HSP90 family signature sequences are shaded with gray and the consensus sequence “MEEVD” at the C-terminal is boxed
Fig. 6.
A phylogenetic tree constructed using the neighbor-joining method based on amino acids sequence deduced from HSP90 gene of Chlamys nobilis and other species. Chlamys nobilis is labeled with black triangle. The scale bar under the tree represents the amino acid substitution rate, and the bootstrap values (%) of 1000 replicates are listed at the nodes. The GenBank accession numbers of all the HSP90 are as follows: Homo sapiens HSP90α1 (NP_001017963.2); Homo sapiens HSP90α2 (NP_005339.3); Homo sapiens HSP90β (NP_031381); Bos taurus HSP90α (NP_001012688.1); Bos taurus HSP90β (BAC82488); Rattus norvegicus HSP90α (NP_786937.1); Rattus norvegicus HSP90β (P34058); Mus musculus HSP90α (NP-034610.1); Danio rerio HSP90α (AAH75757.1); Danio rerio HSP90β (AAB96969); Megalobrama amblycephala HSP90α (AGV06257.1); Megalobrama amblycephala HSP90β (AGI97008); Salmo salar HSP90α (AGT57167.1); Salmo salar HSP90β (NP-001117004.1); Portunus trituberculatu HSP90 (ACQ90225); Penaeus monodon HSP90 (ACO83357); Fenner openaeus chinensis HSP90 (ABM92446.1); Penaeus vannamei HSP90 (ADU03767); Argopecten iradians HSP90 (ABS50431.1); Chlamys farreri HSP90 (AAR1781); Ruditapes philippinarum HSP90 (AHY27548); Corbicua fluminea HSP90(AMM04544); Mizuhopecten yessensis HSP90 (ASU91375.1); Scapharca broughtonii HSP90 (ALZ42089.1); Hyriopsis cumingii HSP90 (ALM87690.1); Methyloglobulus morosus HSP90/HtpG (WP-023494860); Escherichia albertii HSP90/HtpG (BAT34147.1)
Tissue distribution of CnHSP90 mRNA
The relative expression of CnHSP90 in different tissues (intestine, kidney, adductor, mantle, gill, and gonad) was determined by real-time PCR (Fig. 7). The results showed that CnHSP90 transcript could be detected in all examined tissues, albeit differently. The lowest expression of CnHSP90 was in the intestine, whereas the highest expression was detected in the gonad.
Fig. 7.
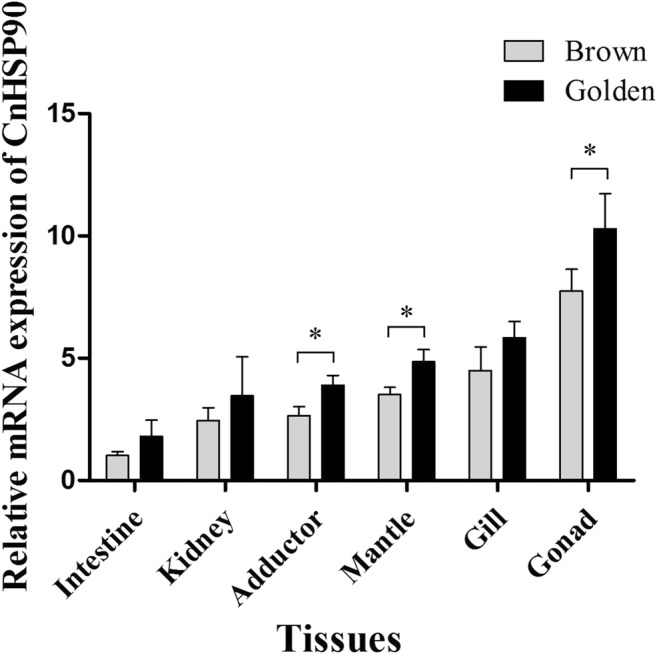
Tissue distribution of CnHSP90 mRNA expression. The mRNA expression of CnHSP90 was determined by real-time PCR with β-actin as internal control gene. The relative expression of CnHSP90 in each tissue (adductor, mantle, intestine, mantle, gonad, and kidney) was determined compared to the expression in the intestine. Vertical bars represent means ± SD (N = 4)
Comparison of mRNA expression levels of CnHSP90 in gill between golden and brown scallops
The temporal expression pattern of CnHSP90 in gill was examined after acute thermal stress treatment at 32 °C for 3, 6, 12, 24, and 36 h by RT-PCR. The result showed a time-dependent pattern (P < 0.05) (Fig. 8). The mRNA level of CnHSP90 increased with treatment time, with the highest level observed at 6 h for both golden and brown scallops. However, the transcript levels of CnHSP90 for golden scallops were significantly higher than that of brown scallops at 6, 12, 24, and 36 h (P < 0.05).
Fig. 8.
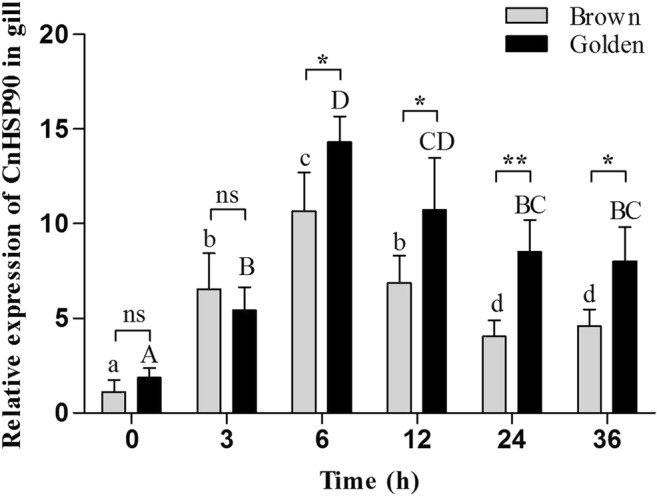
Comparison of the mRNA expression of CnHSP90 in the gill between golden and brown scallops in C. nobilis under acute thermal stress for 36 h. Vertical bars represent means ± SD (N = 4). Significant differences between golden and brown scallops at same time point are indicated by *P < 0.05, **P < 0.01, ns = no significance. Different upper case letters on bars indicate significant difference (P < 0.05) among different stress times in the same brown scallops, while different lower case letters on bars indicate significant difference (P < 0.05) among different stress times in the same golden scallops
Analysis of variance showed (Table 4) that color of scallops, stress time, and temperature all have significant effect on the mRNA expression level of CnHSP90 (P < 0.05).
Table 4.
Analyses of variance for CnHSP90 expression level in the gill among different treatment time between golden scallops and brown scallops
| Resource | df | MS | F | P |
|---|---|---|---|---|
| S | 1 | 75.752 | 31.220 | < 0.001 |
| T | 5 | 105.046 | 43.293 | < 0.001 |
| S × T | 5 | 9.641 | 3.973 | < 0.05 |
| Error | 36 | 2.426 |
Comparison of gill TCC between golden and brown scallops
In Fig. 9, it can be seen that the gill TCC of golden scallops was significantly higher than that of brown scallops (P < 0.05) at all time points. For golden scallops, there was a slight decrease in TCC at 3 h (P > 0.05), followed by a significant increase, reaching a peak at 6 h (P < 0.05). On the other hand, there was fluctuation in the TCC of brown scallops, with no significant difference compared to control (0 h) (P > 0.05). Analysis of variance also revealed (Table 5) that the two different color scallops and stress time had significant effect on TCC of gill (P < 0.05).
Fig. 9.
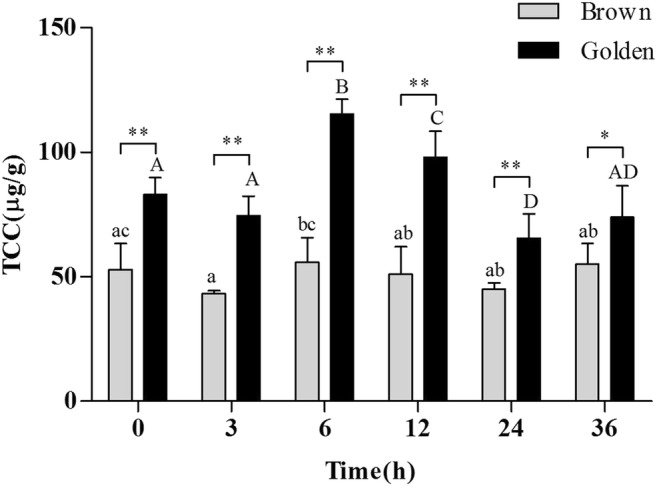
Comparison of TCC in gills of golden and brown scallops C. nobilis under acute thermal stress for 36 h. Vertical bars represent means ± SD (N = 4). Significant differences between golden and brown scallops at same time points are indicated by *P < 0.05, **P < 0.01, ns = no significance. Different upper case letters on bars indicate significant difference (P < 0.05) among different stress times in the same brown scallops, and different lower case letters on bars indicate significant difference (P < 0.05) among different stress times in the same golden scallops
Table 5.
Analyses of variance for TCC in the gill among different treatment time points between golden and brown scallops
| Resource | df | MS | F | P |
|---|---|---|---|---|
| S | 1 | 14,313.376 | 188.748 | < 0.001 |
| T | 5 | 985.273 | 12.993 | < 0.001 |
| S × T | 5 | 501.305 | 6.611 | < 0.001 |
| Error | 36 | 75.833 |
Correlations between HSP90 gene expression and TCC
In golden scallops, the level of CnHSP90 expression increased with increased TCC (Fig. 10a and Table 6), therefore exhibiting a strong positive correlation (P < 0.05) between them. On the other hand, no correlation was found between CnHSP90 expression level and TCC in brown scallops (Fig. 10b and Table 6).
Fig. 10.
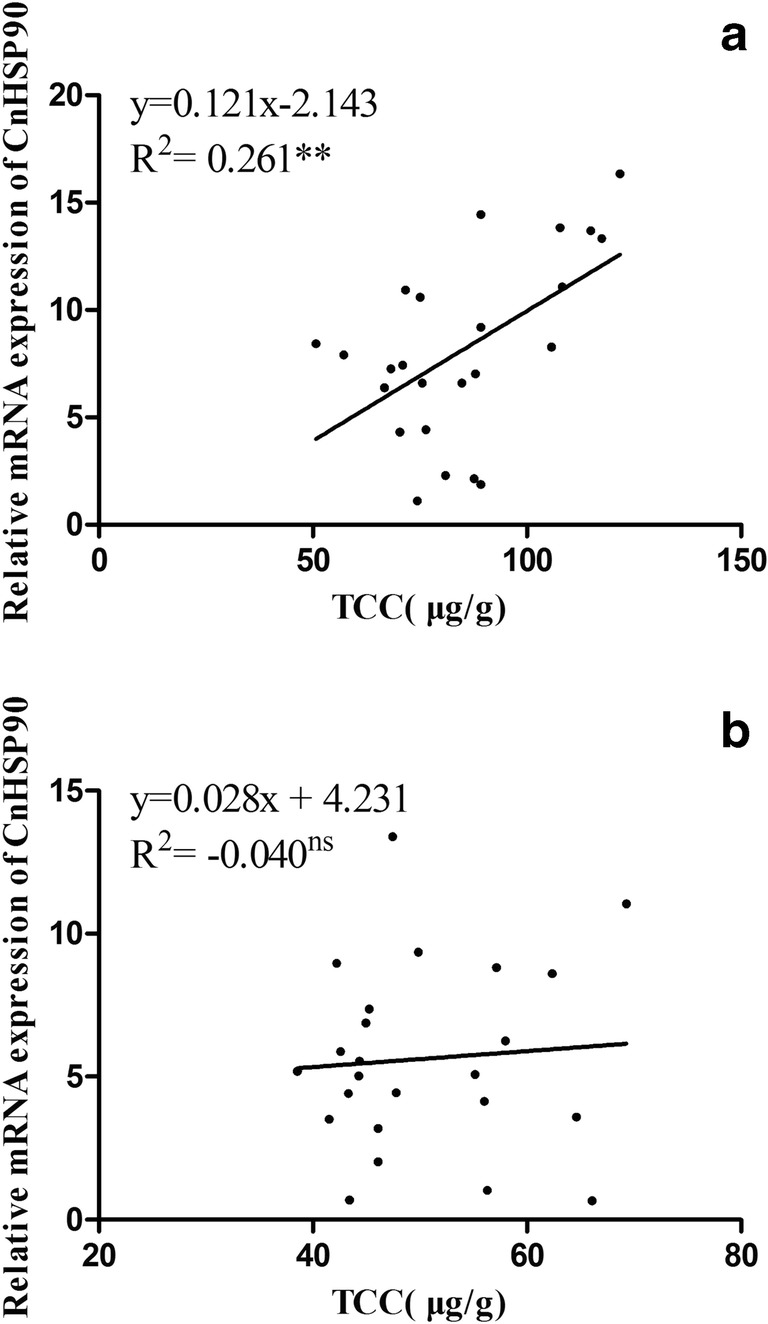
Linear correlation between CnHSP90 mRNA expression level and TCC in gill of golden scallops (a) and brown scallops (b). **P < 0.01), ns = no significance
Table 6.
Linear correlation (R2) and Pearson correlation (r) between the mRNA expression of CnHSP90 gene and TCC in the gill after acute thermal stress at different treatment time points
| y | x | a | b | R2 | r | |
|---|---|---|---|---|---|---|
| Golden scallops | CnHSP90 | TCC | − 2.143 | 0.121 | 0.261** | 0.542** |
| Brown scallops | CnHSP90 | TCC | 4.231 | 0.028 | − 0.040NS | 0.075NS |
NS not significant
**P < 0.01
Discussion
Temperature is an important environmental factor that influences every level of physiological and chemical changes in an organism (Wang et al. 2006). In the present study, different acute thermal stress treatments were applied on noble scallop Chlamys nobilis, a marine bivalve mollusk, so as to better understand the temperature adaptation mechanisms of marine invertebrates. The results reveal that both HSP90 and carotenoids (antioxidants) play important roles in adaptation to thermal stress in marine animals.
As subtropical marine invertebrates, noble scallops grow and survive in at temperatures within the ranges 15 to 28 °C (suitable) and 20 to 25 °C (optimum). The results from the present study provide direct evidence that shows that, beyond the optimum temperature, the mortality of both golden and brown scallops increases with increasing water temperature. Most importantly, the present data revealed that the 36h-LTE50 (temperature of 50% scallops’ mortality after 36 h heat shock) of golden scallops was about 1 °C higher compared with that of brown scallops, coupled with the fact that the LTI50 was prolonged in golden scallops. This phenomenon could partially be due to the significantly higher total carotenoid content (TCC) in golden scallops compared to brown scallops, as both scallops originated from the same population and were cultivated under the same conditions (Han et al. 2016). Carotenoids have potent antioxidant and immunomodulatory properties, including scavenging reactive oxygen species (ROS) and free radicals, protecting cells and tissues from oxidative damage, and enhancing humoral immune response (Stahl and Sies 2003; Astley et al. 2004). Besides, carotenoids have been linked with tolerance and adaptation to several stressors, such as low dissolved oxygen, elevated water temperature, ammonia levels, and harmful effects of light (Liñán-Cabello et al. 2002).
HSP90 is an important stress-response gene that has potential use as a new environmental stress biomarker because it is induced by a variety of environmental stressors such as temperature (Rosic et al. 2011; Cheng et al. 2016; Yan et al. 2017), hyperosmotic stress (Pan et al. 2000), bacterial infection (Xie et al. 2015), and heavy metals (Ma et al. 2012; Bai et al. 2014). In this study, the full-length cDNA of a HSP90 homolog (denoted CnHSP90) in C. nobilis was isolated and sequenced. CnHSP90 possessed conserved sequences and characteristic motifs of the HSP90 family including the ATP and geldanamycin binding domain, the major structural and functional domains typical of HSP90 (Gupta 1995; Prodromou et al. 1997). The presence of the MEEVD sequence in the C-terminus (Scheufler et al. 2000) of CnHSP90 further confirms that it is a functional gene and belongs to the cytosolic HSP90 family. Homology analysis revealed that CnHSP90 shares high similarity with the HSP90s of Hyriopsis cumingii, Corbicula flumines, Ruditapes philippinarum, Scapharca broughtonii, Arogopecten irradians, and Azumpapecten farreri, with identities ranging from 80 to 97%, therefore suggesting that CnHSP90 might function as other known HSP90. Further, sequence and phylogenetic analysis reveals that CnHSP90 was a member of the HSP90 family, which are ubiquitous and highly conserved molecular chaperones. In vertebrates two different isoforms of HSP90 are found and are designated as HSP90α and HSP90β. The α-isoforms of vertebrate HSP90 have characteristic glutamine-rich QTQDQ sequence at the N-terminus, which is absent in all the vertebrate β-isoforms (Lees-Miller and Anderson 1989; Theodoraki and Mintzas 2006). CnHSP90 had no glutamine-rich QTQDQ sequence at its N-terminus, suggesting that CnHSP90 was more closely related to the vertebrate HSP90 β-isoforms.
CnHSP90 was found to be ubiquitously expressed in all tested tissues, which indicates that the CnHSP90 gene was synthesized under unstimulated conditions. However, the transcript level of CnHSP90 was significantly different between the various tissues, with the highest found in the gonads of both golden and brown scallops, suggesting that CnHSP90 expression is tissue specific. Similar results have been reported in other invertebrates, such as Fenneropenaeus chinensis (Li et al. 2009), Portunus trituberculatus (Zhang et al. 2009), Crassostrea hongkonggensis (Fu et al. 2011), and Haliotis discus hannai (Zhang et al. 2011).
Exposure of organisms to thermal stress leads to the generation of ROS, which severely impairs normal cells function and could indirectly act as signaling molecules to cause DNA damage (Zhou et al. 2010). Gills are important respiratory organs, responsible for maintaining systemic homeostasis in the face of changing internal and environmental conditions (Evans et al. 2005). Thus, gill was chosen as the tissue for the thermal stress experiments in the present study. HSP90 is a general molecular chaperone involved in the folding of newly synthesized proteins and the re-folding of damaged proteins (Pratt and Toft 1997). A significant increase (P < 0.05) in the expression levels of CnHSP90 was observed in both golden and brown scallops throughout the entire thermal stress period compared to control (unstressed scallops), suggesting that CnHSP90 might play an important role in protecting organisms from further damage. This observation is consistent with earlier reports in Laternula elliptica exposed to thermal stress (Kim et al. 2009) and in Argopecten irradians after heat shock (Yang et al. 2014).
HSP90 is also believed to be important in the synthesizing of vitellogenin. For example, a study in shrimp Metapenaeus ensis indicated that HSP90 was involved in regulating the synthesis of vitellogenin (Wu and Chu 2008). Similarly, Osborne et al. (2007) blocked the chaperone activity of HSP90 with a specific HSP90 inhibitor in rainbow trout hepatocytes, which resulted in a significant decline in vitellogenin production. Vitellogenin is known to be a precursor of egg yolk protein, which acts as a carrier to transfer carotenoids into ovaries (Ando and Hatano 1991; Montorzi et al. 1995). It has therefore been speculated that probably the mRNA expression levels of HSP90 might be closely related to the total carotenoid content. In this study, there was a time-dependent increase in the TCC in gills of golden scallops. Interestingly, similar trends in TCC and transcript levels of CnHSP90 were observed in golden scallops when exposed to a temperature of 32 °C. Besides, correlation analysis showed a significant positive correlation between the mRNA level of CnHSP90 and TCC (P < 0.05). These results are consistent with our previous assumption. Another important finding in the present study was that golden scallops had significantly higher transcript levels of CnHSP90 compared to brown scallops at 6, 12, 24, and 36 h (P < 0.05). The question is, why do golden and brown scallops, which have the same genetic background and growth conditions, respond differently to the same thermal stress? The different levels of constitutively expressed carotenoids might be the reason, as numerous studies have reported that carotenoids could facilitate intercellular communication against inflammatory damage by regulating the expression of genes. For instance, carotenoids are reported to upregulate the expression level of connexin43, thereby inhibiting neoplastic transformation in the post-initiation phase (Bertram 1999; Livny et al. 2002). The expression of heme oxygenase-1 has also been shown to be enhanced by β-carotene, hence preventing UVA-irradiated cells from photodamage (Obermuller-Jevic et al. 1999). Similarly, the expression of CnTLR-1, CnTRX, Cnlec-1, and Ferritin gene in scallops could be upregulated under the influence of carotenoids to resist stress by pathogens (Lu et al. 2016; Zhang et al. 2018; Lu et al. 2018; Zhang et al. 2019). From the foregoing, it is therefore plausible to speculate that carotenoids upregulate the transcript level of CnHSP90 so as to increase the thermal tolerance of scallops.
In conclusion, the LTE50 and LTI50 in noble scallop Chlamys nobilis were determined using a logistic equation. Golden scallops had higher thermal tolerance than brown scallops, which could be due to the vast difference in their total carotenoid content. Moreover, CnHSP90 seems to play essential role in improving thermal tolerance in scallops, as its expression level had a significant positive correlation with TCC. This is the first report in aquatic animals linking improvement in thermal tolerance to total carotenoid content. The findings in the present study would provide important clues on how to breed new noble scallop varieties with high-temperature stress resistance.
Acknowledgments
We are very grateful to Dr. Aweya Jude Juventus (Institute of Marine Biology, Shantou University) for his careful revision and many constructive comments.
Funding information
This work was financially supported by the National Key R&D Program of China (2018YFD0901400), National Natural Science Foundation of China (31872563), China Modern Agro-industry Technology Research System (CARS-49), Guangdong Provincial Yangfan Plan (14600706), and Department of Education (2017KCXTD014), China.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Ando S, Hatano M. Distribution of carotenoids in the eggs from four species of salmonids. Comp Biochem Physiol B. 1991;99(2):341–344. doi: 10.1016/0305-0491(91)90052-f. [DOI] [PubMed] [Google Scholar]
- Astley SB, Hughes DA, Wright AJ, Elliott RM, Southon S. DNA damage and susceptibility to oxidative damage in lymphocytes: effects of carotenoids in vitro and in vivo. Brit J Nutr. 2004;91(1):53–61. doi: 10.1079/bjn20031028. [DOI] [PubMed] [Google Scholar]
- Babin A, Biard C, Moret Y. Dietary supplementation with carotenoids improves immunity without increasing its cost in a crustacean. Am Nat. 2010;176(2):234–241. doi: 10.1086/653670. [DOI] [PubMed] [Google Scholar]
- Bai C, Duan Y, Chen L, Liu Y, Zheng Y, Wang Y, Zhu X. Gene cloning and gene expression of Hsp90 from Meloidogyne incognita under the temperature and heavy metal stress. Int J Agric Biol. 2014;16(3):451–460. [Google Scholar]
- Bertram JS. Carotenoids and gene regulation. Nutr Rev. 1999;57(6):182–191. doi: 10.1111/j.1753-4887.1999.tb06941.x. [DOI] [PubMed] [Google Scholar]
- Cheng W, Li D, Wang Y, Liu Y, Salzman KZ. Cloning of heat shock protein genes (hsp70, hsc70 and hsp90) and their expression in response to larval diapause and thermal stress in the wheat blossom midge, Sitodiplosis mosellana. J Insect Physiol. 2016;95:66–77. doi: 10.1016/j.jinsphys.2016.09.005. [DOI] [PubMed] [Google Scholar]
- Cheng D, Liu H, Zhang H, Soon TK, Ye T, Li S, Ma H, Zheng H. Differential expressions of HSP70 gene between golden and brown noble scallops Chlamys nobilis under heat stress and bacterial challenge. Fish Shellfish Immunol. 2019;94:924–933. doi: 10.1016/j.fsi.2019.10.018. [DOI] [PubMed] [Google Scholar]
- Cheng D, Zhang Y, Liu H, Zhang H, Tan K, Ma H, Li S, Zheng H. An improving method for extracting total carotenoids in an aquatic animal Chlamys nobilis. Food Chem. 2019;280:45–50. doi: 10.1016/j.foodchem.2018.12.043. [DOI] [PubMed] [Google Scholar]
- Choi YK, Jo PG, Choi CY. Cadmium affects the expression of heat shock protein 90 and metallothionein mRNA in the Pacific oyster, Crassostrea gigas. Comp Biochem Physiol C. 2008;147(3):286–292. doi: 10.1016/j.cbpc.2007.11.002. [DOI] [PubMed] [Google Scholar]
- Evans DH, Piermarini PM, Choe KP. The multifunctional fish gill: dominant site of gas exchange, osmoregulation, acid-base regulation, and excretion of nitrogenous waste. Physiol Rev. 2005;85(1):97–177. doi: 10.1152/physrev.00050.2003. [DOI] [PubMed] [Google Scholar]
- Fu D, Chen J, Zhang Y, Yu Z. Cloning and expression of a heat shock protein (HSP) 90 gene in the haemocytes of Crassostrea hongkongensis under osmotic stress and bacterial challenge. Fish Shellfish Immunol. 2011;31:118–125. doi: 10.1016/j.fsi.2011.04.011. [DOI] [PubMed] [Google Scholar]
- Gao Q, Song L, Ni D, Wu L, Zhang H, Chang Y. cDNA cloning and mRNA expression of heat shock protein 90 gene in the haemocytes of Zhikong scallop Chlamys farreri. Comp Biochem Physiol B. 2007;147(4):704–715. doi: 10.1016/j.cbpb.2007.04.010. [DOI] [PubMed] [Google Scholar]
- Gao Q, Zhao J, Song L, Qiu L, Yu Y, Zhang H, Ni D. Molecular cloning, characterization and expression of heat shock protein 90 gene in the haemocytes of bay scallop Argopecten irradians. Fish Shellfish Immunol. 2008;24(4):379–385. doi: 10.1016/j.fsi.2007.08.008. [DOI] [PubMed] [Google Scholar]
- Gupta RS. Phylogenetic analysis of the 90 kD heat shock family of protein sequences and an examination of the relationship among animals, plants, and fungi species. Mol Biol Evol. 1995;12(6):1063–1073. doi: 10.1093/oxfordjournals.molbev.a040281. [DOI] [PubMed] [Google Scholar]
- Han J, Lu Y, Zheng H, Liu H, Deng H, Zhang B. Differential expression of CuZnSOD gene under low temperature stress in noble scallop Chlamys nobilis with different carotenoid content. Fish shellfish Immunol. 2016;54:30–39. doi: 10.1016/j.fsi.2016.03.160. [DOI] [PubMed] [Google Scholar]
- Helmbrecht K, Zeise E, Rensing L. Chaperones in cell cycle regulation and mitogenic signal transduction: a review. Cell Prolif. 2000;33(6):341–365. doi: 10.1046/j.1365-2184.2000.00189.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hix LM, Lockwood SF, Bertram JS. Bioactive carotenoids: potent antioxidants and regulators of gene expression. Redox Rep. 2004;9(4):181–191. doi: 10.1179/135100004225005967. [DOI] [PubMed] [Google Scholar]
- Joanna F, Burda K. Potential role of carotenoids as antioxidants in human health and disease. Nutrients. 2014;6(2):466–488. doi: 10.3390/nu6020466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson JL, Brown C. Plasticity of the Hsp90 chaperone machine in divergent eukaryotic organisms. Cell Stress Chaperones. 2009;14(1):83–94. doi: 10.1007/s12192-008-0058-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joly AL, Wettstein G, Mignot G, Ghiringhelli F, Garrido C. Dual role of heat shock proteins as regulators of apoptosis and innate immunity. J Innate Immun. 2010;2:238–247. doi: 10.1159/000296508. [DOI] [PubMed] [Google Scholar]
- Kim M, Ahn IY, Kim H, Cheon J, Park H. Molecular characterization and induction of heat shock protein 90 in the Antarctic bivalve Laternula elliptica. Cell Stress Chaperones. 2009;14(4):363–370. doi: 10.1007/s12192-008-0090-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lees-Miller SP, Anderson CW. The human double-stranded DNA-activated protein kinase phosphorylates the 90-kDa heat-shock protein, hsp90 alpha at two NH2-terminal threonine residues. J Biol Chem. 1989;264:17275–17280. [PubMed] [Google Scholar]
- Li F, Luan W, Zhang C, Zhang J, Wang B, Xie Y, Li S, Xiang J. Cloning of cytoplasmic heat shock protein 90 (FcHSP90) from Fenneropenaeus chinensis, and its expression response to heat shock and hypoxia. Cell Stress Chaperones. 2009;14(2):161–172. doi: 10.1007/s12192-008-0069-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liñán-Cabello MA, Paniagua-Michel J, Hopkins PM. Bioactive roles of carotenoids and retinoids in crustaceans. Aquac Nutr. 2002;8:299–309. [Google Scholar]
- Liu T, Pan L, Cai Y, Miao J. Molecular cloning and sequence analysis of heat shock proteins 70 (HSP70) and 90 (HSP90) and their expression analysis when exposed to benzo(a)pyrene in the clam Ruditapes philippinarum. Gene. 2015;555(2):108–118. doi: 10.1016/j.gene.2014.10.051. [DOI] [PubMed] [Google Scholar]
- Liu H, Zheng H, Zhang H, Deng L, Liu W, Wang S, Meng F, Wang Y, Guo Z, Li S, Zhang G. A de novo transcriptome of the noble scallop, Chlamys nobilis, focusing on mining transcripts for carotenoid-based coloration. BMC Genomics. 2015;16(1):44. doi: 10.1186/s12864-015-1241-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2-ΔΔCT method. Methods. 2001;25(4):402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Livny O, Kaplan I, Reifen R, Polak-Charcon S, Madar Z, Schwartz B. Lycopene inhibits proliferation and enhances gap-junction communication of KB-1 human oral tumor cells. J Nutr. 2002;132(12):3754–3759. doi: 10.1093/jn/132.12.3754. [DOI] [PubMed] [Google Scholar]
- Lu Y, Zheng H, Zhang H, Yang J, Wang Q. Cloning and differential expression of a novel toll-like receptor gene in noble scallop Chlamys nobilis with different total carotenoid content. Fish Shellfish Immunol. 2016;56:229–238. doi: 10.1016/j.fsi.2016.07.007. [DOI] [PubMed] [Google Scholar]
- Lu YQ, Zhang HK, Cheng DW, Liu HX, Li SK, Ma HY, Zheng HP. A multi-CRD C-type lectin gene Cnlec-1 enhance the immunity response in noble scallop Chlamys nobilis with higher carotenoids contents through up-regulating under different immunostimulants. Fish Shellfish Immunol. 2018;83:37–44. doi: 10.1016/j.fsi.2018.09.014. [DOI] [PubMed] [Google Scholar]
- Ma KX, Chen GW, Liu DZ. cDNA cloning of heat shock protein 90 gene and protein expression pattern in response to heavy metal exposure and thermal stress in planarian Dugesia japonica. Mol Biol Rep. 2012;39(6):7203–7210. doi: 10.1007/s11033-012-1552-9. [DOI] [PubMed] [Google Scholar]
- Montorzi M, Falchuk KH, Vallee BL. Vitellogenin and lipovitellin: zinc proteins of Xenopus laevis oocytes. Bioch. 1995;34(34):10851–10858. doi: 10.1021/bi00034a018. [DOI] [PubMed] [Google Scholar]
- Mubiana VK, Blust R. Effects of temperature on scope for growth andaccumulation of Cd, Co, Cu and Pb by the marine bivalve Mytilus edulis. Mar Environ Res. 2007;63:219–235. doi: 10.1016/j.marenvres.2006.08.005. [DOI] [PubMed] [Google Scholar]
- Obermuller-Jevic JU, Francz PI, Frank J, Flaccus A, Biesalski HK. Enhancement of the UVA induction of haem oxygenase-1 expression by β-carotene in human skin fibroblasts. FEBS Lett. 1999;460(2):212–216. doi: 10.1016/s0014-5793(99)01342-3. [DOI] [PubMed] [Google Scholar]
- Osborne N, Sherry J, Rendell JL, Currie S. The role of hsp90 in 17α-ethynylestradiol-induced endocrine disruption in rainbow trout hepatocytes. Ecotox Environ Safe. 2007;68(1):13–19. doi: 10.1016/j.ecoenv.2006.12.002. [DOI] [PubMed] [Google Scholar]
- Pan F, Zarate JM, Tremblay GC, Bradley TM. Cloning and characterization of salmon hsp90 cDNA: upregulation by thermal and hyperosmotic stress. J Exp Zool Part A. 2000;287(3):199–212. doi: 10.1002/1097-010x(20000801)287:3<199::aid-jez2>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- Park K, Lee JS, Kang JC, Kim JW, Kwak IS. Cascading effects from survival to physiological activities, and gene expression of heat shock protein 90 on the abalone Haliotis discus hannai responding to continuous thermal stress. Fish Shellfish Immunol. 2015;42(2):233–240. doi: 10.1016/j.fsi.2014.10.036. [DOI] [PubMed] [Google Scholar]
- Pratt WB, Toft DO. Steroid receptor interactions with heat shock protein and immunophilin chaperones. Endocr Rev. 1997;18(3):306–360. doi: 10.1210/edrv.18.3.0303. [DOI] [PubMed] [Google Scholar]
- Prodromou C, Roe SM, O’Brien R, Ladbury JE, Piper PW, Pearl LH. Identification and structural characterization of the ATP/ADP-binding site in the Hsp90 molecular chaperone. Cell. 1997;90:65–75. doi: 10.1016/s0092-8674(00)80314-1. [DOI] [PubMed] [Google Scholar]
- Queitsch C, Sangster TA, Lindquist S. Hsp90 as a capacitor of phenotypic variation. Nature. 2002;417(6889):618–624. doi: 10.1038/nature749. [DOI] [PubMed] [Google Scholar]
- Rosic NN, Pernice M, Dove S, Dunn S, Hoegh-Guldberg O. Gene expression profiles of cytosolic heat shock proteins Hsp70 and Hsp90 from symbiotic dinoflagellates in response to thermal stress: possible implications for coral bleaching. Cell Stress Chaperones. 2011;16(1):69–80. doi: 10.1007/s12192-010-0222-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutherford S, Knapp JR, Csermely P. Hsp90 and developmental networks. Adv Exp Med Biol. 2007;594(1):190–197. doi: 10.1007/978-0-387-39975-1_16. [DOI] [PubMed] [Google Scholar]
- Saks L, Ots I, Hõrak P. Carotenoid-based plumage coloration of male greenfinches reflects health and immunocompetence. Oecologia. 2003;134(3):301–307. doi: 10.1007/s00442-002-1125-z. [DOI] [PubMed] [Google Scholar]
- Scheufler C, Brinker A, Bourenkov G, Pegoraro S, Moroder L, Bartunik H, Hartl F, Moarefi L. Structure of TPR domain-peptide complexes: critical elements in the assembly of the Hsp70-Hsp90 multichaperone machine. Cell. 2000;101:199–210. doi: 10.1016/S0092-8674(00)80830-2. [DOI] [PubMed] [Google Scholar]
- Stahl W, Sies H. Antioxidant activity of carotenoids. Mol Asp Med. 2003;24(6):345–351. doi: 10.1016/s0098-2997(03)00030-x. [DOI] [PubMed] [Google Scholar]
- Tamura K, Peterson D, Peterson N, Stecher G, Nei M, Kumar S. MEGA5: molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol Biol Evol. 2011;28(10):2731–2739. doi: 10.1093/molbev/msr121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theodoraki MA, Mintzas AC. cDNA cloning, heat shock regulation and developmental expression of the hsp83 gene in the Mediterranean fruit fly Ceratitis capitata. Insect Mol Biol. 2006;15:839–852. doi: 10.1111/j.1365-2583.2006.00691.x. [DOI] [PubMed] [Google Scholar]
- Wang WN, Wang AL, Liu Y, Xiu J, Liu Z, Sun RY. Effects of temperature on growth, adenosine phosphates, ATPase and cellular defense response of juvenile shrimp Macrobrachium nipponense. Aquaculture. 2006;256:624–630. [Google Scholar]
- Wu LT, Chu KH. Characterization of heat shock protein 90 in the shrimp Metapenaeus ensis: evidence for its role in the regulation of vitellogenin synthesis. Mol Reprod Dev. 2008;75(5):952–959. doi: 10.1002/mrd.20817. [DOI] [PubMed] [Google Scholar]
- Xie Y, Song L, Weng Z, Liu S, Liu Z. Hsp90, Hsp60 and sHsp families of heat shock protein genes in channel catfish and their expression after bacterial infections. Fish Shellfish Immunol. 2015;44(2):642–651. doi: 10.1016/j.fsi.2015.03.027. [DOI] [PubMed] [Google Scholar]
- Yan J, Liang X, Zhang Y, Li Y, Cao X, Gao J. Cloning of three heat shock protein genes (HSP70, HSP90α and HSP90β) and their expressions in response to thermal stress in loach (Misgurnus anguillicaudatus) fed with different levels of vitamin C. Fish Shellfish Immunol. 2017;66:103–111. doi: 10.1016/j.fsi.2017.05.023. [DOI] [PubMed] [Google Scholar]
- Yang C, Wang L, Liu C, Zhou Z, Zhao X, Song L. The polymorphisms in the promoter of HSP90 gene and their association with heat tolerance of bay scallop. Cell Stress Chaperones. 2014;20(2):297–308. doi: 10.1007/s12192-014-0546-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang XY, Zhang MZ, Zheng CJ, Liu J, Hu HJ. Identification of two hsp90 genes from the marine crab, Portunus trituberculatus and their specific expression profiles under different environmental conditions. Comp Biochem Physiol C. 2009;150(4):465–473. doi: 10.1016/j.cbpc.2009.07.002. [DOI] [PubMed] [Google Scholar]
- Zhang W, Wu C, Mai K, Chen Q, Xu W. Molecular cloning, characterization and expression analysis of heat shock protein 90 from Pacific abalone, Haliotis discus hannai Ino in response to dietary selenium. Fish Shellfish Immunol. 2011;30(1):280–286. doi: 10.1016/j.fsi.2010.10.019. [DOI] [PubMed] [Google Scholar]
- Zhang HK, Cheng DW, Liu HX, Zheng HP. Differential responses of a thioredoxin-like protein gene to, Vibrio parahaemolyticus, challenge in the noble scallop, Chlamys nobilis, with different total carotenoids content. Fish Shellfish Immun. 2018;72:377–382. doi: 10.1016/j.fsi.2017.11.020. [DOI] [PubMed] [Google Scholar]
- Zhang HK, Cheng DW, Tan KS, Liu HX, Ye T, Li SK, Ma HY, Zheng HP. Identification of two ferritin genes and their expression profiles in response to bacterial challenge in noble scallop Chlamys nobilis with different carotenoids content. Fish Shellfish Immunol. 2019;88:9–16. doi: 10.1016/j.fsi.2019.02.051. [DOI] [PubMed] [Google Scholar]
- Zheng H, Liu H, Zhang T, Wang S, Sun Z, Liu W. Total carotenoid differences in scallop tissues of Chlamys nobilis (Bivalve: Pectinidae) with regard to gender and shell colour. Food Chem. 2010;122(4):1164–1167. doi: 10.1016/j.foodchem.2010.03.109. [DOI] [Google Scholar]
- Zhou J, Wang L, Xin Y, Wang WN, He WY, Wang AL, Liu Y. Effect of temperature on antioxidant enzyme gene expression and stress protein response in white shrimp, Litopenaeus vannamei. J Therm Biol. 2010;35:284–289. [Google Scholar]