Abstract
Background
The most common method of combating insects in low-income communities and developing countries, is the use of insecticides. The use of organophosphate insecticides is increasing due to their low prices and availability on the market. Chlorpyrifos is a medium-risk insecticide for human. The widespread use of organophosphorus insecticides, especially chlorpyrifos, in residential homes has undoubtedly created many health concerns. Babies have a high sensitivity to pesticides and environmental contaminants because of their evolutionary status. On the other hand, the main source of infants’ exposure who are breast-fed exclusively to environmental pollutants is through breast milk and oral contact with objects that are covered with dust and particles.
Methods
In this cross-sectional study, the concentration of chlorpyrifos in breast milk and its metabolite in urine samples of mothers and their under six months infants, feeding exclusively by breast milk in north of Iran have been investigated. The demographic data was collected through interviewing with selected mothers and completion of prepared data collecting forms. The data were statistically analyzed to investigate the relationships between exposure of mothers and their infants to chlorpyrifos.
Results
The mean concentration of chlorpyrifos and its metabolite in mothers’ urine and milk samples and infant’s urines were 1.3 ± 0.6, 2.1 ± 1.4 and 1.4 ± 0.7 μg/L, respectively. Also, the mean concentration of chlorpyrifos in the dust on the house floors was73.4 ± 49 ng/g. There are good correlations between the mean values of chlorpyrifos concentrations and its metabolite in mother’s milk and urine (r = 0.872, p = 0.001), and the mother’s milk and infant’s urine (r = 0.722, p = 0.001). Also, there was a significant correlation between the concentration of chlorpyrifos in floor dust and its metabolites in the infant’s urine (r = 0.554, p = 0.001).
Conclusion
Our study suggests that the infants are the recipient of concentrated forms of chlorpyrifos residues through breast milk and house dust and it is quite well known that OP pesticides are toxic and have different kinds of adverse health effects. However, further research needs to be done to determine what these chemicals are doing to our children.
Keywords: Breast milk, Urine, Household insecticide, Chlorpyrifos, Iran
Background
Nowadays, the use of organophosphate insecticides is increasing, due to their low prices and availability in the market [1] and they are applied widely to protect agricultural products in all over the world [2], so these days exposure to organophosphate (OP) pesticides is a serious concern [3]. Chlorpyrifos, with the trade name of Dursban, is a widely used Organophosphorus pesticide. Chlorpyrifos which has been introduced to the market since 1965, plays the key role in controlling a wide range of arthropods in agricultural products (rice, vegetables and fruits) [4]. However chlorpyrifos is a medium-risk insecticide for human, its consumption has been reported more than 25 million kg in the world during 2002–2006, due to its availability and low price. According to the latest reports, 11,000 tons of pesticides are consumed in Iran, including 4470 tons of insecticides and 1134 tons of chlorpyrifos [5]. Although chlorpyrifos has been illegal to use in home since 2001 in the United States [6] but because this insecticide controls Chilo Suppressalis effectively, it is widely applied for home use in Gilan province [7]. Chlorpyrifos is a non-polar molecule with low water solubility that its major metabolite in biological systems is 3, 5, 6-trichloro-2 pyridinol (TCPy) [8], and about 70% of it, is discharged by urine of mammals [9]. In human, cholinesterase enzymes can be inhibited in the central and peripheral nervous system by chlorpyrifos, causing side effects within hours of exposure. Many studies indicate that long-term occupational exposure to OP pesticides is associated with different adverse health effects. Exposure to OP is associated with symptoms such as disorders of the nervous system, cognitive impairment, changes in sexual and thyroid hormones [10–12], pulmonary distension [13, 14], breast cancer [15] and blood leukemia [14]. Since, after exposure, the pesticide residue of OP has been detected in blood, plasma and serum [16], the fetus can be exposed to these pesticides through the mother’s blood. Studies on animals have been indicated that some of OP pesticides can cross the placenta and affect the fetus [17]. Human studies on OP pesticides have identified the pesticide residues in umbilical cord blood and meconium after childbirth [18–22]. These pesticides can be stored in high fat content matrixes, such as mother’s milk [23]. Infants can be exposed to OP pesticides through breastfeeding after birth [24, 25]. In most developing countries, including Iran, the amount of OP pesticides in human biological samples has not been reported, while a high amount of these pesticides is used. The widespread use of organophosphate insecticides, especially chlorpyrifos, in residential buildings has undoubtedly led to health concerns for researchers. Besides, due to the possible side effects of chlorpyrifos in infants such as mental retardation, leukemia, respiratory distress, and the lack of studies on infants which are breastfeeding by exposed mothers, the following exposures were studied in this research:
The exposure of mothers to chlorpyrifos (through monitoring the concentration of its metabolite in the mother’s urine).
The Potential exposure of infants to chlorpyrifos (through detecting the chlorpyrifos residue in breast milk).
The exposure of infants to chlorpyrifos (through detecting of the chlorpyrifos metabolite in the urine of infants).
In addition, the exposure of infants to environment dust as the other conceivable source of contamination was investigated through monitoring of the concentration of chlorpyrifos in house dust, since at this age, the infants are usually in bed and at a distance of not more than one meter from the ground.
Materials and methods
Study population
This study was conducted on 61 urban households in Rasht, Iran, who had a breastfed baby under six months of age (They didn’t use any baby formula or even water). (Fig. 1). Mothers with under 6 month baby were listed by referring to 50 urban health centers in Rasht. Then, through interview, the infants feeding exclusively by breast milk were distinguished from infants who were fed by infant formula or both kind of feeding. The questions of prepared check list were filled out and collected for the 61 households who were interested to participate in this study.
Fig. 1.
The map of Iran, the location of Gilan province, and Rasht city
Subjects
During the sampling from households (August–November, 2017), the prepared check lists were filled out by interviewing with selected mothers and the information about demographic status (Table 1) and using insecticides in house (Table 2). Urine bags were used to collect infants’ urine samples. At the same time, urine and breast milk samples were collected from mothers. All the collected urine samples (mothers and infants) and breast milk samples were transported to laboratory under chilled conditions (4 °C) and stored at −20 °C until analysis. Dust samples were collected by vacuum cleaner, and then stored in plastic bags away from light at −20 °C.
Table 1.
Demographic characteristics of mothers and infants
| Groups | Personal character | Min | Max | Mean ± SD |
|---|---|---|---|---|
|
Infant (n = 61) Girls = 28 Boys = 33 |
Age(days) | 4 | 175 | 86.5 ± 51.9 |
| Weight(Kg) | 2.4 | 4.7 | 3.3 ± 0.6 | |
| Height(cm) | 43 | 55 | 49.6 ± 2.5 | |
|
Mothers (n = 61) |
Age(Years) | 20 | 40 | 29.7 ± 5.3 |
| Weight(Kg) | 46 | 108.5 | 73.3 ± 14.1 | |
| Height(m) | 1.5 | 1.8 | 1.6 ± 0.1 |
Table 2.
Frequency of using insecticides in house in this study (n = 61)
| Parameters | Description | Frequencies (%) |
|---|---|---|
| Usage of insecticide | Yes | 56(91.8) |
| No | 5(8.2) | |
| Insecticide composition | Chlorpyrifos | 35(61.4) |
| Pyrotroid | 17(29.8) | |
| Type of insecticide formulation | Spray | 29(50.9) |
| Powder | 11(19.3) | |
| Liquid | 12(21.0) | |
| Coil | 5(8.8) | |
| Insecticide Use Sequence | Monthly | 14(24.6) |
| Three months | 8(14.0) | |
| Six months and a years | 35(61.4) | |
| Return home after using insecticide | <24 h | 30(52.6) |
| 24 h | 8(14.0) | |
| 48 h | 19(33.4) | |
| Insecticide storage location | Kitchen Cabinet | 28(49.2) |
| Rest room and bath room | 2(3.5) | |
| Warehouse | 10(17.5) | |
| Others | 17(29.8) |
Standards and reagents
All solvents and reagents were HPLC grade and purchased from Sigma-Aldrich. Chlorpyrifos and its metabolite were purchased from Dr. Ehrenstorfer GmbH in Germany, with a purity of 99%.
Sample preparation
The urine samples were extracted by SPE, according to the method by Babina et al. [26]. In order to prepare the urine sample, 1 CC of urine was transferred to 10-mL glass screw-cap vials and spiked with 100 μL of 2, 6-di-bromophenol (1 mg/L) as internal standard. 5 mL Sodium acetate and 10 μL β glucuronidase/arylsulfatase were added. The samples were heated for 3 h at 80 °C in an oven in order to hydrolyzed conjugates of TCPy. Sample extraction was carried out using solid-phase extraction (SPE) procedure. SPE cartridges containing 50 mg of C18 media (United Chemical Technologies) were preconditioned using 1 mL methanol and then 1 mL of 1% acetic acid. Samples were passed through the SPE cartridge. To reduce interferences, cartridges were washed with 5% methanol in 1% acetic acid and the compound of interest was eluted with 2 mL 100% methanol. Separation and quantitative analysis are carried out by capillary gas chromatography and mass selective detection in selected ion monitoring mode.
The analytical conditions for GC/MS were as follows: injection amount, 1.0 μl; injection mode into the gas chromatograph, splitless; capillary column, DB-5 ms (30 m × 0.25 mm I.D., 0.25 μm film thickness); column oven temperature, 70 °C (2 min) – 10 °C/min – 280 °C; injection port temperature, 280 °C; carrier gas, helium; interface temperature, 300 °C; ionization energy, 70 eV; ion source temperature, 200 °C; analytical mode, selected ion monitoring (SIM).
The breast milk samples were extracted by HS-SPME, according to the method by Rodrigues et al. [27]. An aliquot of defrosted milk (15 mL), was put in a sealed 20 ml glass vial. PDMS-DVB fiber was exposed to the headspace after waiting for 30 min to reach gas–liquid equilibrium. Following extraction and pre-concentration, the fiber was then inserted directly into the GC injector for desorption at 250 °C, over 5 min. The initial column temperature was set to 70 °C during injection (held for 9 min) and then increased at 50 °C /min to 95 °C, then at 5 °C /min to 165 °C, then at 20 °C /min to 190 °C, and then finally raised to 300 °C at 100 °C/ min, which was held for 5 min.
The dust samples were extracted by liquid extraction followed SPE procedure, according to the method by Clot et al. [28]. The collected samples were sieved on a 100-μm sieve to remove the coarse fraction, and then a 0.5 g aliquot of fine dust was spiked with 50 μL of triphenyl phosphate (1 mg/L) as internal standard. The dust was extracted with 12 mL of 1:1 Hexane: Acetone in an ultrasonic bath for 15 min. The extract was solvent exchanged into hexane and applied to a silica SPE cartridge that had been conditioned with 20% acetone in ethyl acetate, dichloromethane and hexane. To reduce interferences, cartridges were washed with hexane and the analyte was eluted with 3 mL 100% dichloromethane. Extracted samples were analyzed using gas chromatography/ mass spectrometry. Sample extracts were analyzed using a DB-1 column (30 M, 0.25 mm id, 0.15 μm film) with the GC oven temperature programmed 130–220 °C (2 °C/min) and then 220–280 °C (10 °C/min).
Statistical analysis
SPSS20 software was used to analyze the data. Linear regression was used to determine the correlation between variables, the Pearson correlation coefficient was calculated for assessment of the correlation between normally distributed variables, and the Spearman correlation coefficient was calculated for variables whose distribution were not normal. In addition, the Shapiro-Wilk normality test was used to test the normality of the data.
Results
The demographic characteristics of participants were shown in Table 1. According to Table 1, 54.1 and 45.9% of infants were boys and girls, respectively, and the range of infants and their mothers’ age were 4–175 days and 20–40 years old, respectively. There were Fourteen (23%) infants younger than 1 month, 18 (29.5%) infants between 1 and 3 months old,and 29 (47.5%) infants between 3 and 6 months old. The mean of the infants and mothers’ BMI were 13.5 and 28 kg/m2, respectively. As can be seen in Table 2, based on the completed check lists more than 90% of participated mothers had exposure with different kind of insecticides which the major component of them was chlorpyrifos during their pregnancy. The frequency of spraying of insecticides by participated households has been reported from zero to five times per year. Only 4 mothers (6.6%) have reported that they did not use any insecticides during their pregnancy. Thirty eight mothers (66.6%) indicated that they have returned to their house 24 h after spraying insecticide. Moreover, none of mothers have performed the spraying themselves during their pregnancy. It is also important to mention that 28 households (49.2%) have kept the insecticide containers in the kitchen cabinet.
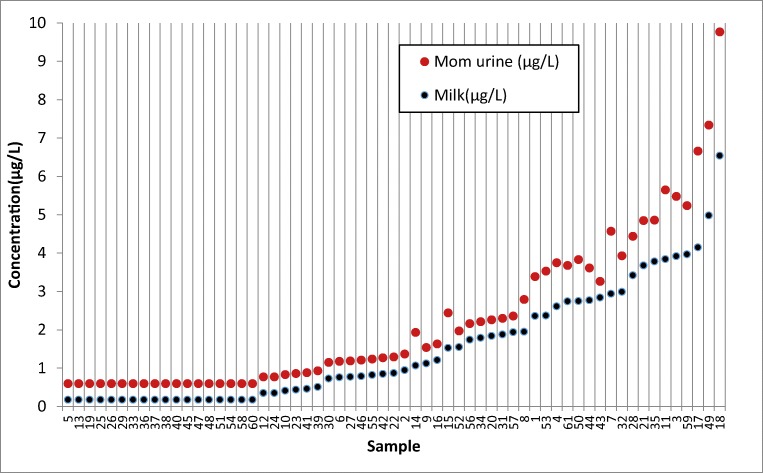
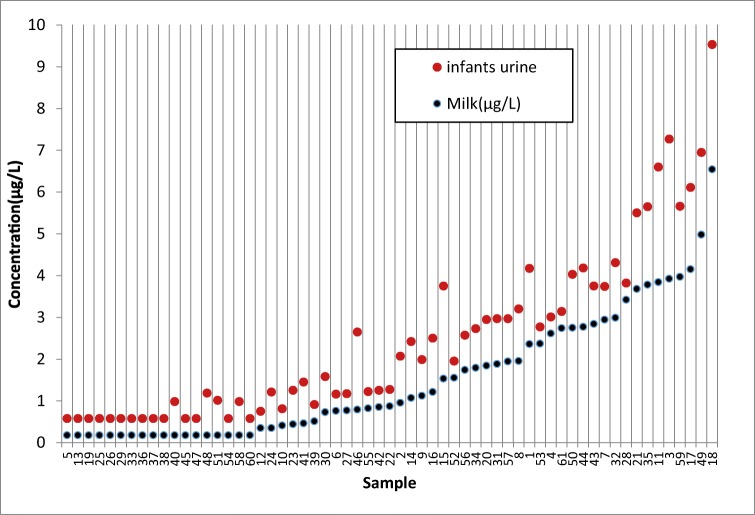
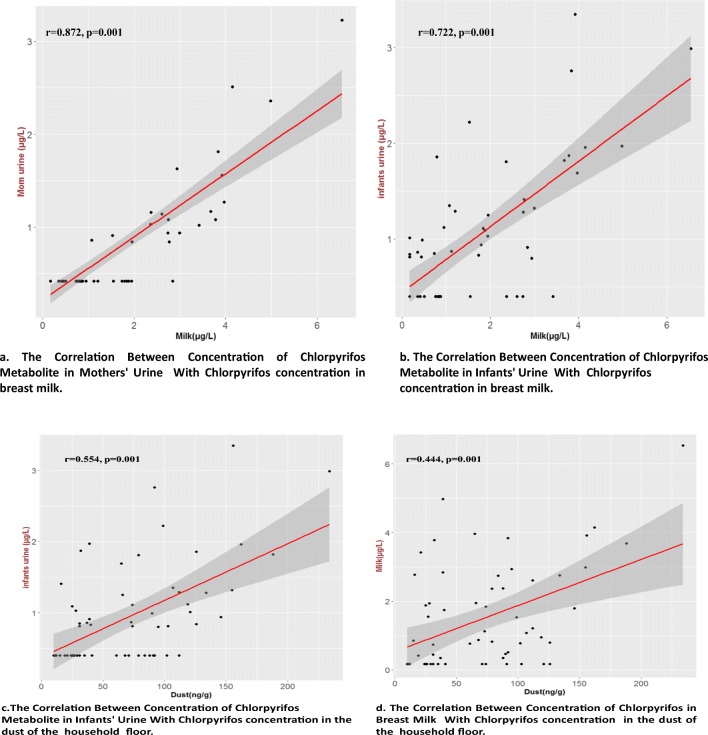
According to Table 3, TCPy, the major metabolite of chlorpyrifos was found in 32.8% and 55.7% of mothers and infants urine samples with mean concentration of 1.3 ± 0.6 and 1.4 ± 0.7 μg/L, respectively. The chlorpyrifos was detected in 43 samples (70.5%) of breast milk with mean concentration of 2.1 ± 1.4 μg/L. Moreover, the concentration of chlorpyrifos in 61 samples (100%) of dust was 10–234 ng/g. The data were analyzed for normal distribution by “Shapiro-Wilk normality test”. The interpretation of the results of this test and the normalization of the data is based on the lack of a significant difference in the value of p value. The result showed that the value of p value less than 0.001 and the non-compliance of urine chlorpyrifos of normal distribution. Therefore, the logarithm of the data was used. Consider the data logarithm and redo this test, the value of p value was greater than 0.05, so the logarithm of the data follows the normal distribution. Spearman correlation coefficient showed that there are significant correlations between the concentration of chlorpyrifos in breast milk with its metabolite concentration in mothers and infant’s urine samples (r = 0.872 and P = 0.001), (r = 0.722 and p = 0.001), respectively. (Figs. 2 and 3). There were also moderate correlations between the concentration of chlorpyrifos in dust and mother’s milk (P = 0.001, r = 0.444), and between the concentration of chlorpyrifos in dust and infant’s urine (P = 0.001, r = 0.552) (Fig. 4). The results of linear regression analysis according to Table 4 showed (R2 = 0.522, β = 0.722) that chlorpyrifos concentration in breast milk predicted the concentration of chlorpyrifos metabolism in the urine of infants. According to the results, there was a significant positive correlation between the age of mothers and the chlorpyrifos concentration in mother’s milk (P = 0.001, r = 0.308). There were none significant correlations between the level of chlorpyrifos in breast milk and other Variables such as type of insecticide formulation, frequency of use and storage location of chlorpyrifos.
Table 3.
Concentrations of chloropyrifos and its metabolite (TCPy) in breast milk and urine samples (n = 61)
| Compounds | Media | Detection frequency (%) | Min | Max | Mean ± SD | 50th | 75th | 90th |
|---|---|---|---|---|---|---|---|---|
| TCPy | Mother’s urine(μg/L) | 20(32.8) | 0.42 | 3.23 | 1.3 ± 0.6 | 0 | 0.9 | 1.5 |
| Infant’s urine(μg/L) | 34(55.7) | 0.4 | 3.35 | 1.4 ± 0.7 | 0.81 | 1.3 | 1.9 | |
| Chlorpyrifos | Breast milk (μg/L) | 43(70.5) | 0.2 | 6.54 | 2.1 ± 1.4 | 0.9 | 2.7 | 3.8 |
| Dust (ng/g) | 61(100) | 10 | 234 | 73.4 ± 49.0 | 71 | 102.5 | 143.6 |
Fig. 2.
The concentrations of chlorpyrifos in breast milks compared to mothers’ urines
Fig. 3.
The concentrations of chlorpyrifos in breast milks compared to infants’ urines
Fig. 4.
Correlation between the concentration of chlorpyrifos in breast milk, the urine of mothers and infants, and the dust of the household floor
Table 4.
Linear regression test result
| Model | Parameter | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| SS | R | R2 | F | P | B | β | t | ||
| 1 | regression | 15.294 | .872 | .761 | 187.754 | 0.0001 | 0.339 | 0.872 | 13.702 |
| Residual | 4.806 | ||||||||
| 2 | regression | 15.544 | .722 | .522 | 64.369 | 0.0001 | 0.342 | 0.722 | 8.023 |
| Residual | 14.247 | ||||||||
Discussion
Today organophosphate insecticides have played a critical role in expanding agricultural yield and control of vector borne sicknesses in Iran. The high levels of pesticides in human’s fluids are essentially through contaminated food products. In Iran and most developing countries, farmers harvest crops before the completion of the waiting periods of pesticides in the agricultural produce. As a result, vegetables and fruits that are consumed contain high levels of pesticides. Chlorpyrifos, was the most widely used pesticide in Iran and Information on associations between chlorpyrifos residues in breath milk, dust and urinary metabolite would be valuable for evaluating the relationship between personal exposure and possible health effects in infants. The results of this study have shown that chlorpyrifos is present in breast milk and dust and its metabolite is present in urine samples, in measurable concentrations. According to the results, chlorpyrifos was detected in 70.5% of breast milk samples, because this insecticide is a relatively non-polar molecule with low water solubility ((Log Kow ≥ 1).) and potentially is stored in the adipose tissue and discharged in milk during breast feeding period. A recent report from a population of mothers working in the farm in Thailand in 2016 shows, concentrations among our urban mothers population were lower than those measured in Thailand for chlorpyrifos (0.35–6.54 vs. 1.6–10 μg/L) [29]. In addition, in another research In Bhopal, India, in 2003 [30], the breast milk of 12 mothers with a baby less than 1 month of age contained 230 μg/L chlorpyrifos which is much more than our results. Srivastava et al. [31] analyzed the levels of OP pesticides in human breast milk among Indian populations. They reported that chlorpyrifos level was the highest among other OP pesticides, and also Weldon et al. reported similar results by analyzing the chlorpyrifos in another study, human milk samples from women residing in the agricultural region of Salinas and the urban San Francisco Bay Area in California [24]. It seems infants are exposed to the lipophilic OP pesticides like chlorpyrifos, by three routs: via inhalation, oral ingestion (breastfeeding), and dermal uptake. For infants who have very limited activity, the oral route and inhalation of their environment dust form low lying layers in the air, are the most common [32]. In our study since, the infants urine samples were taken from the infants with age less than six months with no other sources of food and water except breastmilk, and also because of a good and significant positive correlation between chlorpyrifos concentration in breast milk and urinary metabolite in infants urine samples, we can conclude that may be the main source of infants exposure to chloropyrifos is breast milk. Chlorpyrifos can be transmitted from mother’s milk to infant and after metabolism its metabolite can be discharged into the urine. In the present study, although the mean concentration of chlorpyrifos in breast milk was less than the threshold [33], due to its serious side effects on infant development,, it can be a potential risk to the infant’s health in long term. On the other hand, since chlorpyrifos was observed in all dust samples in detectable amount and also, there was a moderate and positive correlation (r = 0.552) between the concentration of chlorpyrifos in dust samples and its metabolite urinary concentration in infants, it can be concluded that the second source of exposure to chlorpyrifos in infants is environment dust, because, at this age, the infants are usually in bed and at a distance of not more than one meter from the ground. So they breathe dusts of air continuously. One report from North California which studied the relation between urinary concentration of metabolites of some OP insecticides and the concentration of parent compounds, as similar as our study chlorpyrifos had highest frequency of usage and showed a moderate correlation between chlorpyrifos concentration in floor napkins dust and its urinary metabolite concentration [34]. According to the results, the level of chlorpyrifos in the breast milk and its metabolite in the urine of mothers and infants is lower than the determined concentrations which were reported in National Health and Nutrition Examination Survey (NHANES) [35, 36].
Although the dietary exposure of infants is estimated between 0.45 to 0.87 microgram/kg bw/day (by considering mean concentration of chlorpyrifos in breast milk 2.1 μg/L and assuming daily intake of 1 L breast milk by the infants with body weight between 2.4 to 4.7 kg) and these estimated values are lower than the ADI of 1 microgram/kg bw/day and the ARfD of 5 microgram/kg bw day (EFSA 2014), but many substantial animal bioassay and mechanistic data and numerous human studies have been completed, and several have evaluated the possible association between chlorpyrifos exposure and neurodevelopmental effects in human. Infants and children are especially vulnerable for these effects since their brain is still developing. A number of studies show that children exposed to chlorpyrifos while in the womb or in early life can suffer neurodevelopmental effects at a later stage by causing structural changes in the developing brain and possibly resulting in a decrease of cognitive functions, such as IQ and working memory loss. So it is better to limit its exposure by eliminating of its usages [37].
This study was intended to be a pilot experiment, thus there are many limitations. The small sample size reduced the power of statistical analysis. Also, breast milk samples collected from mothers who are in different stages of lactation, so for persistent lipophilic organic compounds which may decrease over the period of breastfeeding, concentrations measured in the breast milk samples of women who have lactated longer, may underestimate infant exposures.
Conclusion
In summary, our study suggests that the infants are the recipient of concentrated forms of chlorpyrifos residues through breast milk and house dust, and it is quite well known that OP pesticides are toxic and have different kinds of adverse health effects, so exposure to pesticides should be minimized through appropriate regulatory policy and public education.
Acknowledgements
This research has been supported by Tehran University of Medical Sciences grant (project No. 96-04-46-36738). Hereby, the cooperation of the International Campus of University and also Institute for Environmental Research (IER) is highly appreciated.
Compliance with ethical standards
Conflict of interest
The authors declare that there is no conflict of interest.
Ethics approval and consent to participate
This research was approved by the Ethics Committee of the Tehran University of Medical Sciences (No.IR.TUMS.SPH.REC.1396. 3630). Participants are volunteers to participate in this research and their information is confidential.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Dehghani R, Moosavi SG, Esalmi H, Mohammadi M, Jalali Z, Zamini N. Surveying of pesticides commonly on the markets of Iran in 2009. J Environ Prot. 2011;2:1113–1117. doi: 10.4236/jep.2011.28129. [DOI] [Google Scholar]
- 2.Gonzalez-Alzaga B, et al. A systematic review of neurodevelopmental effects of prenatal and postnatal organophosphate pesticide exposure. Toxicol Lett. 2014;230:104–121. doi: 10.1016/j.toxlet.2013.11.019. [DOI] [PubMed] [Google Scholar]
- 3.Kamanyire R, Karalliedde L. Organophosphate toxicity and occupational exposure. Occup Med. 2004;54:69–75. doi: 10.1093/occmed/kqh018. [DOI] [PubMed] [Google Scholar]
- 4.Vanden Bilcke C. The Stockholm convention on persistent organic pollutants. RECIEL. 2002;11(3):328–342. [Google Scholar]
- 5.Morteza Z, Mousavi SB, Baghestani MA, Aitio A. An assessment of agricultural pesticide use in Iran, 2012-2014. J Environ Health Sci Eng. 2017. 10.1186/s40201-017-0272-4. [DOI] [PMC free article] [PubMed]
- 6.USEPA. Pesticides: Reregistration - Chlorpyrifos Facts. http://www.epa.gov/oppsrrd1/REDs/factsheets/chlorpyrifos_fs.htm (November1, 2013). Accessed 5 Aug 2017
- 7.Arjmandi A, Tavakol M, Shayeghi M. Determination of organoposporus insecticide residues in the rice paddies. Int Environ Sci Technol. 2010;7(2):175–182. doi: 10.1007/BF03326129. [DOI] [Google Scholar]
- 8.Bicker W, Lammerhofer M, Lindner W. Determination of chlorpyrifos metabolites in human urine by reversed-phase/weak anion exchange liquid chromatography–electrospray ionisation–tandem mass spectrometry. J Chromatogr B. 2005;822:160–169. doi: 10.1016/j.jchromb.2005.06.003. [DOI] [PubMed] [Google Scholar]
- 9.Nolan RJ, Rick DL, Freshour NL, Saunders JH. Chlorpyrifos: pharmacokinetics in human volunteers. Toxicol Appl Pharmacol. 1984;73:8–15. doi: 10.1016/0041-008X(84)90046-2. [DOI] [PubMed] [Google Scholar]
- 10.Aguilar-Garduno C, et al. Changes in male hormone profile after occupational organophosphate exposure. Toxicology. 2013;307:55–65. doi: 10.1016/j.tox.2012.11.001. [DOI] [PubMed] [Google Scholar]
- 11.R. Recio, et al. Pesticide exposure alters follicle-stimulating hormone levels in mexican agricultural workers. environmental health perspectives. VOLUME 113 NUMBERS 9 September 2005. [DOI] [PMC free article] [PubMed]
- 12.Lacasaña M, et al. Interaction between organophosphate pesticide exposure and PON1 activity on thyroid function. Toxicol Appl Pharmacol. 2010;249:16–24. doi: 10.1016/j.taap.2010.07.024. [DOI] [PubMed] [Google Scholar]
- 13.Lee WJ, et al. Cancer incidence among pesticide applicators exposed to chlorpyrifos in the agricultural health study. J Natl Cancer Inst. 2004;96(23):1781–1789. doi: 10.1093/jnci/djh324. [DOI] [PubMed] [Google Scholar]
- 14.Beane Freeman LE, et al. Cancer incidence among male pesticide applicators in the agricultural health study cohort exposed to diazinon. Am J Epidemiol. 2005;162(11):1070–1079. doi: 10.1093/aje/kwi321. [DOI] [PubMed] [Google Scholar]
- 15.Engel LS, Hill DA, Hoppin JA, Lubin JH, Lynch CF, Pierce J, Samanic C, Sandler DP, Blair A, Alavanja MC. Pesticide use and breast cancer risk among farmers’ wives in the agricultural health study. Am J Epidemiol. 2005;161:121–135. doi: 10.1093/aje/kwi022. [DOI] [PubMed] [Google Scholar]
- 16.Barr DB, Needham LL. Analytical methods for biological monitoring of exposure to pesticides. J Chromatogr B. 2002;778:5–29. doi: 10.1016/S1570-0232(02)00035-1. [DOI] [PubMed] [Google Scholar]
- 17.Villeneuve DC, et al. Placental transfer of C-parathion administered intravenously to sheep. Toxicol Appl Pharmacol. 1972;212:542–548. doi: 10.1016/0041-008X(72)90010-5. [DOI] [PubMed] [Google Scholar]
- 18.Whyatt RM, Camann D, Perera FP, Rauh VA, Tang D, Kinney PL, Garfinkel R, Andrews H, Hoepner L, Barr DB. Biomarkers in assessing residential insecticide exposures during pregnancy and effects on fetal growth. Toxicol Appl Pharmacol. 2005;206:246–254. doi: 10.1016/j.taap.2004.11.027. [DOI] [PubMed] [Google Scholar]
- 19.Perera FP, Rauh V, Tsai WY, Kinney P, Camann D, Barr D, Bernert T, Garfinkel R, Tu YH, Diaz D, Dietrich J, Whyatt RM. Effects of transplacental exposure to environmental pollutants on birth outcomes in a multiethnic population. Environ Health Perspect. 2003;111(2):201–205. doi: 10.1289/ehp.5742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Corrion ML, Ostrea EM, Jr, Bielawski DM, Posecion NC, Jr, Seagraves JJ. Detection of prenatal exposure to several classes of environmental toxicants and their metabolites by gas chromatography–mass spectrometry in maternal and umbilical cord blood. J Chromatogr B. 2005;822:221–229. doi: 10.1016/j.jchromb.2005.06.007. [DOI] [PubMed] [Google Scholar]
- 21.Ostrea EM, et al. Combined analysis of prenatal (maternal hair and blood) and neonatal (infant hair, cord blood and meconium) matrices to detect fetal exposure to environmental pesticides. Environ Res. 2009;109:116–122. doi: 10.1016/j.envres.2008.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Whyatt RM, Barr DB. Measurement of organophosphate metabolites in postpartum meconium as a potential biomarker of prenatal exposure. Environ Health Perspect. 2001;109(4):417–420. doi: 10.1289/ehp.01109417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rathod AL, Garg RK. Chlorpyrifos poisoning and its implications in human fatal cases. J Forensic Legal Med. 2017;47:29–34. doi: 10.1016/j.jflm.2017.02.003. [DOI] [PubMed] [Google Scholar]
- 24.Weldon RH, Barr DB, Trujillo C, Bradman A, Holland N, Eskenazi B. A pilot study of pesticides and PCBs in the breast milk of women residing in urban and agricultural communities of California. J Environ Monit. 2011;13:3136–3144. doi: 10.1039/c1em10469a. [DOI] [PubMed] [Google Scholar]
- 25.Paraíba LC, Castro VLSS, Maia AHN. Insecticide distribution model in human tissues viewing Worker’s health monitoring programs. Braz Arch Biol Technol. 2009;52:875–881. doi: 10.1590/S1516-89132009000400011. [DOI] [Google Scholar]
- 26.Babina K, Dollard M, Pilotto L, Edwards JW. Environmental exposure to organophosphorus and pyrethroid pesticides in south Australian preschool children: a cross sectional study. Environ Int. 2012;48:109–120. doi: 10.1016/j.envint.2012.07.007. [DOI] [PubMed] [Google Scholar]
- 27.González-Rodríguez MJ, Arrebola Liébanas FJ, Garrido Frenich A, Martínez Vidal JL, Sánchez López FJ. Determination of pesticides and some metabolites in different kinds of milk by solid-phase microextraction and low-pressure gas chromatography-tandem mass spectrometry. Anal Bioanal Chem. 2005;382:164–172. doi: 10.1007/s00216-005-3144-1. [DOI] [PubMed] [Google Scholar]
- 28.Colt JS, et al. Household vacuum cleaners vs. the high-volume surface sampler for collection of carpet dust samples in epidemiologic studies of children. Environ Health. 2008;7:6. doi: 10.1186/1476-069X-7-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Naksen W, Prapamontol T, Mangklabruks A, Chantara S, Thavornyutikarn P, Robson MG, Ryan PB, Barr DB, Panuwet P. A single method for detecting 11 organophosphate pesticides inhuman plasma and breast milk using GC-FPD. J Chromatogr B. 2016;1025:92–104. doi: 10.1016/j.jchromb.2016.04.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sanghi R, Pillai MK, Jayalekshmi TR, Nair A. Organochlorine and organophosphorus pesticide residues in breast milk from Bhopal, Madhya Pradesh, India. Hum Exp Toxicol. 2003;22:73–76. doi: 10.1191/0960327103ht321oa. [DOI] [PubMed] [Google Scholar]
- 31.Srivastava S, Narvi SS, Prasad SC. Levels of select organophosphates in human colostrum and mature milk samples in rural region of Faizabad district, Uttar Pradesh, India. Hum Exp Toxicol. 2011;30:1458–1463. doi: 10.1177/0960327110396525. [DOI] [PubMed] [Google Scholar]
- 32.Enrique M. Ostrea Jr, Esterlita Villanueva-Uy, Dawn Bielawski, Sarah Birn James J. Janisse. Analysis of house dust and children’s hair for pesticides: a comparison of markers of ongoing pesticide exposure in children. J Bioanal Biomed; 4. 10.4172/1948-593X.1000057. [DOI] [PMC free article] [PubMed]
- 33.Barr DB, et al. Concentrations of selective metabolites of organophosphorus pesticides in the United States population. Environ Res. 2005;99(3):314–326. doi: 10.1016/j.envres.2005.03.012. [DOI] [PubMed] [Google Scholar]
- 34.Trunnelle KJ, et al. Urinary pyrethroid and chlorpyrifos metabolite concentrations in northern California families and their relationship to indoor residential insecticide levels, part of the Study of Use of Products and Exposure Related Behavior (SUPERB) Environ Sci Technol. 2014;48:1931–1939. doi: 10.1021/es403661a. [DOI] [PubMed] [Google Scholar]
- 35.Barr DB, et al. Urinary concentrations of metabolites of pyrethroid insecticides in the general U.S. population: national health and nutrition examination survey 1999−2002. Environ. Health Perspect. 2010;118(6):742–748. doi: 10.1289/ehp.0901275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Berkowitz, G et al. Exposure to indoor pesticides during pregnancy in a multiethnic, urban cohort. Environ Health Perspect2003, 111 (1), 79–84. [DOI] [PMC free article] [PubMed]
- 37.Eaton DL, et al. Review of the toxicology of chlorpyrifos with an emphasis on human exposure and neurodevelopment. Crit Rev Toxicol. 2008;S2:1–125. doi: 10.1080/10408440802272158. [DOI] [PubMed] [Google Scholar]