Abstract
Background
A needle trap device (NTD) was packed with Carbotrap/silica composite sorbent and applied for field sampling of halogenated volatile organic compounds (HVOCs) followed by gas chromatography/mass spectrometry (GC/MS) separation and determination.
Methods
Carbotrap B, as a highly pure surface sorbent, was prepared using sol-gel method to improve its surface properties for adsorption/desorption of the target analytes. The effects of important experimental variables on the sampling and determination of trichloroethylene (thrCE) and tetrachloroethylene (tetCE) using the proposed NTD-GC/MS strategy were evaluated and optimized.
Results
The results showed that sampling temperature and relative humidity interfered with sampling efficiency of the developed method and peak area responses of the analytes decreased with increasing temperature and relative humidity. The peak areas of the analytes increased with raising desorption temperature from 180 to 250 °C, and increasing desorption time from 1 to 3 min. The carryover experiments showed that the carryover effect disappeared after 3 min of desorption time. The Limits of Detection (LODs) and Limits of Quantitation (LOQs) of the analytes were in the range 0.01–0.03 and 0.05–0.09, respectively.
Conclusions
The results indicated that the developed NTD-GC/MS procedure can be used as a technology with high sensitivity for the field sampling and determination of HVOCs.
Electronic supplementary material
The online version of this article (10.1007/s40201-019-00418-2) contains supplementary material, which is available to authorized users.
Keywords: Needle trap device, Carbotrap B, Silica, Dry cleaning, Air
Background
Volatile organic compounds (VOCs) have a high vapor pressure and can easily be released into the ambient air. The presence of these compounds in ambient air and workplaces has become a major public health concern worldwide [1]. Halogenated volatile organic compounds (HVOCs) are a major group of the VOCs, which have at least one halogen atom. These compounds are commonly used in various industries as solvents, cleaning, disinfecting, blowing, and polymerization agents. HVOCs are considered as contaminants in air and water around the industries [2]. Trichloroethylene (thrCE) and tetrachloroethylene (tetCE) as two major compounds of HVOCs are widely used in dry cleaning from the 1930s to 1950s. They are still applied in dry cleaning shops, stain removal and chlorinated chemical production [3]. Epidemiological studies reported a positive relationship between the exposure of thrCE and tetCE and various cancers such as kidney, cervix, bladder, and oesophagus [3–5]. Due to their adverse effects on human heath, it is important to detect them in workplaces at extremely low concentrations. National Institute for Occupational Safety and Health (NIOHS) has recommended method-1003 for the sampling and analysis of this type of HVOCs [6]. The NIOSH method is based on the sampling of the analytes from air using a low-flow sampling pump, collecting analytes by a sorbent tube, and desorption of the analytes by a solvent. A small volume of the eluent solvent is then injected into the analytical instrument [6]. Furthermore, it can be concluded that this method has a low sensitivity for detecting low concentrations of HVOCs. In addition, the use of solvents for the preparation step can increase the exposure of individual to toxic solvents [7].
Nowadays, the use of single and solvent-free techniques for sampling of VOCs has gained an increasing attention. Needle trap device (NTD) is a green, solvent-free and single-step microextraction technique that can be used for simultaneous sampling and analysis of a wide range of organic compounds [8]. It is a robust and low-cost needle-based configuration of solid-phase microextraction (SPME), which has received much attention for analysis of VOCs over the last few years [8–11]. The initial principal of NTD was introduced by Qin et al. [8] and then its microextraction concept was established and developed by Pawliszyn et al. [9]. NTD is based on the headspace microextraction of analytes from the matrix using a narrow syringe packed with a small amount of sorbent [12]. Direct desorption and analysis are then happened by inserting the packed needle into the injection port of a gas chromatography (GC) device [13]. Therefore, the type of packed sorbent plays a key role for efficient sampling and proper desorption of analytes. Due to the small amount of NTD sorbent, its physical and chemical characteristics can have a substantial impact on the sampling efficiency. Sorbents with a high surface area can offer a high efficiency for sampling of trace amounts of analytes. Previous reported studies have used different materials for the sampling and analysis of VOCs, such as carbon nanotubes [14], graphene [15], polydimethylsiloxane (PDMS) and divinylbenzene (DVB) [13], and polyaniline/multi-walled carbon nanotube (PANI/MWCNT) [16].
Carbotrap is a carbon-based adsorbent with unique adsorption/desorption properties [17, 18]. Due to its high specific surface area, it has potential to provide a huge surface for the sampling trace concentrations of analytes from air. In addition, sol-gel technique is a new method for synthesis of the sorbent with better homogeneity. This method can provide a higher pore size and thermal stability [19–22]. To the best of our knowledge, no studies have been performed in application of NTD packed with Carbotrap B/silica composite for the sampling and analysis of VOCs from air.
The present study aimed to develop an efficient NTD for sampling HVOCs in air, prior to sensitive GC/MS analysis. For this purpose, the Carbotrap B was modified and prepared using sol-gel method. Then, the prepared Carbotrap/silica composite was used as a sorbent for packing the NTD. The proposed NTD was used for the sampling and analysis of thrCE and tetCE as two widely used HVOCs. Moreover, the effects of sampling temperature, relative humidity, and desorption conditions were investigated on the efficiency of the proposed NTD. The prepared sorbent was successfully applied through NTD-GC/MS strategy for field sampling and analysis of the studied HVOCs.
Methods
Chemicals
ThrCE and tetCE with a high purity were purchased from Sigma Aldrich (Germany). Carbotrap® Adsorbent (matrix Carbotrap® B, 20–40 mesh) was purchased from Supelco (Bellefonte, PA, USA). Trifluoroacetic acid (TFA), tetramethylorthosilicate (TMOS) and polymethyl hydrogensiloxane (PMHS) were obtained from Merck (Darmstadt, Germany). Sodium dodecylbanzenesulfunate (SDBS) was purchased from Fluka (Buchs, Switzerland). Bleed Temperature Optimized (BTO) injector septa, resistance up to 400 °C, was purchased from Cross Lab Agilent (PerkinElmer American Fork, UT, USA). Charcoal tubes were also purchased from Sigma Aldrich Company. Disposable medical syringes (2 and 10 mL) were provided from Sinamax (Tehran, Iran). Glass syringe (10 mL) was also provided from Shanghai branch (Jiangsu, China).
Instruments
A Varian Saturn 3800 Gas chromatography/mass spectrometry (GC/MS, Varian 3800) devise with a capillary column (RTX-624, 30 m × 0.25 mm × 1.4 μm) was applied. The temperature program was initially set at 50 °C and kept constant for 5 min. Then, it was ramped to 100 °C at a rate of 4 °C/min and finally ramped to 200 °C at 10 °C/min. Consequently, the total run time was 27.80 min. To keep the pressure of carrier gas constant in the injection port, a hand-made steel bar (with the same size of the NTD needle) was fabricated and used to cover the septum hole (Online Resource 1). Ultra-high purity helium (99.999%) was used as the carrier gas at 0.5 mL/min. A hand-made narrow neck glass liner (I.D.: 1.5 mm and neck diameter of 0.5 mm) was used instead of the standard liner in the GC/MS device. The performance accuracy of this liner in the delivery of volatile compounds to column of GC/MS has been confirmed by our previous studies [14, 15]. The mass transfer line was set at 220 °C. The injection port was set at 250 °C and operated in the split mode with a split ratio of 1:10. A calibrated low-volume sampling pump (SKC 222-3 portable personal air sampler) was used for gas sampling. After sampling, the packed NTD was connected to a 2-mL medical syringe containing 1 mL of pure helium gas. Next, the NTD connected to the syringe was injected into GC/MS without drawing the carrier gas for a specific time for complete desorption of the analytes. Finally, the plunger of the medical syringe was manually pressed to deliver the carrier gas along with the desorbed analytes to the GC-MS column (Online Resource 1).
Preparation of Carbotrap B/silica composite
To prepare the Carbotrap/silica composite, 2 mg of the Carbotrap B was added into a flask containing 50 μL of SDBS (5%). The obtained mixture was placed in an ultrasonic device for 15 min. Then, 400 μL TMOS and 50 μL PMHS were added, and then the mixture was sonicated for 30 min. Afterward, 50 μL of TFA was added to the mixture and sonicated for 15 min. The resulting mixture was refluxed with ethanol/dichloromethane (2:1) using a Soxhlet device and then centrifuged to remove possible impurities. The obtained Carbotrap B/silica precipitate was dried in an oven at 120 °C for 2 h. The prepared Carbotrap B/silica composite was crushed and granulated using ASTM standard sieves to obtain particles in the mesh size 40–60 [22]. ESM_2 shows the appearance of raw Carbotrap and Carbotrap/silica composite synthesized by sol-gel method (Online Resource 1).
Packing NTD with Carbotrap/silica composite
To prepare the packed NTD, a stainless-steel needle (spinal medical needle, 20-gauge with 90 mm L and 0.90 mm O.D.) was packed with the prepared sorbent. First, 3 mm glass wool was placed inside the needle, and then the proposed composite was packed inside the needle in 15 mm of length. In order to hold the sorbent firmly inside the needle, 3 mm of glass wool was placed after the sorbent, and 5 mm of the needle tip was empty. The packing operation was performed using the internal metal plungers of the medical spinal syringes which were cut in desired sizes. The passing flow rate of the packed NTD was determined using a soap bubble flow meter consisted of a 2 mL pipette. The sampling flow rate of the NTD packed with Carbotrap B/silica composite was determined to be 3 ± 1 mL min−1. Several NTDs were prepared according to the mentioned method, and finally three packed NTDs were chosen with a same flow rate (about 3 mL) for further experiments. In this study, the sampling preparation steps were as follows: first, the NTD was packed with Carbotrap/silica composite. Second, the packed NTD was used for the sampling of the HVOCs in the standard chamber. Third, the effects of sampling and desorption parameters were optimized on the proposed NTD. Next, the analytical parameters of the NTD were evaluated. Finally, the performance of the proposed NTD was investigated as field sampler.
Sampling and desorption parameters
The effects of sampling parameters such as sampling temperature and relative humidity were tested on the efficiency of new proposed sampler. For this purpose, a light bulb was used to provide the desired sampling temperature inside the standard sampling chamber. The sampling temperature was monitored using a digital thermometer (model SUN15-TI). The humidity was also adjusted using a flask containing distilled water placed on a heater, and a digital hygrometer (model SUN25-H) was applied to monitor the level of humidity inside the standard sampling chamber. The sampling efficiency was evaluated under three sampling temperatures: 10, 20, 35 °C, and three sampling relative humidities: 20, 50 and 80%. In addition, the effects of desorption time in the range 1–5 min and desorption temperature in the range 180–280 °C were investigated on the new proposed sampler under constant sampling conditions (10 °C of temperature and 20% of relative humidity).
Carryover effect
The carryover effect of the NTD packed with Carbotrap B/silica composite was evaluated under desorption times of 1, 2, 3 and 4 min. In this step, the NTD containing analyte was again injected into the GC injection port in a second desorption cycle with a constant interval (2 min after the first injection). The second desorption cycle was performed under constant conditions (desorption time: 3 min and desorption temperature: 250 °C).
Analytical parameters
In order to determine the analytical parameters of the proposed method, the values of Limit of Detection (LOD) and Limit of Quantitation (LOQ) of the proposed NTD coupled with GC-MS were determined for the studied HVOCs. The LOD and LOQ were defined as the concentrations corresponding to the peak area responses of the analyte with the signal-to-noise ratios (S/N) of 3 and 10, respectively. To obtain the linear dynamic range (LDR), the concentrations of the studied compounds were consecutively reduced to reach the lowest measurable concentrations through the proposed NTD (ranged from 0.01 to 100 ng/mL). In the present study, the repeatability of the proposed NTD was reported by the calculation of relative standard deviation percentage (RSD%) from three repeated measurements with a prepared NTD at various concentrations of the analytes. Moreover, the reproducibility of the proposed NTD was determined as RSD% from three measurements with three prepared NTDs under a constant concentration and condition.
Field study
In order to investigate the efficiency of the proposed NTD as field sampler, the prepared NTD was used to monitor the concentrations of thrCE and tetCE in a dry cleaning shop where used HVOCs as cleaning agents. Three stations were selected for the sampling of the analytes of interest. The sampling was performed using the developed NTD in the determined stations at a distance of 1 m from the washing machines, and 1 m from the ground at the reception station. Side by side sampling was also conducted with charcoal-sorbent tubes (according to the NIOSH method). In final, the results of measurements by the NTDs were compared with those obtained by the NIOSH method.
Results and discussion
Characterization of the Carbotrap B/silica composite
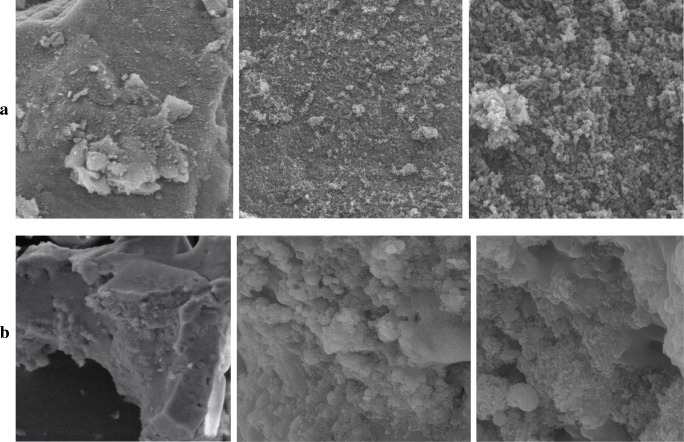
Scanning electron microscope (SEM) and Fourier-transform infrared spectroscopy (FTIR) were used to study the surface changes and functional groups of the sorbent after preparing by sol-gel technique. Figure 1a shows three magnifications of the surface of raw Carbotrap B, and Fig. 1b shows three magnifications of the synthetized composite. As can be seen here, the surface of raw Carbotrap B is heterogeneous and grainy textured. Whereas, the surface of Carbotrap B/silica composite composed of porous micro-structures and agglomerates of irregular shapes and sizes. According to the SEM images, the sorbent prepared by the sol-gel method has more pores and homogenous surface compared to the raw Carbotrap B. FT-IR spectra are shown in Fig. 2. The adsorption was recorded from 4000 to 450 cm−1. Figure 2a shows a peak in the region between 750 and 900 cm−1, which is assigned to the silica networks corresponding to Si-H groups, and peaks at 1264, 1368 and 1485.3 cm−1 might be attributed to the more adsorption bands of SDBS, which represented a good formation of the Carbotrap/silica composite. These results are in good agreement with the SEM findings, which indicated the important role of the SDBS in the formation of finer particles [21].
Fig. 1.
SEM analysis of a raw Carbotrap and b Carbotrap/silica composite synthesized by sol-gel method at different magnifications
Fig. 2.
FTIR spectra of a raw Carbotrap and b Carbotrap/silica composite synthesized by sol-gel method
Exploratory experiments
In this part, first the flow rate passing through the packed NTD was determined while it was connected to the low-flow sampling pump. Afterwards, three packed NTDs with a same flow rate (3 ± 1 mL/min) were selected for next experiments. The crystalline structure of the synthesized sorbent allowed the carrier gas to pass through sorbent packed without the need for using glass powder. Contrary to our previous study, because of the crystalline structure of the proposed sorbent, we did not use glass powder to prevent clogging inside the packed needle [17]. This certainly increases the absorbent capacity inside the NTD for absorbing more amount of the analytes compared to the NTD packed with a mixture of sorbent and glass powder. In order to test the breakthrough volume (BTV), two packed NTDs were connected in a series, and then the tip of the first needle was used for sampling from the standard chamber. The head of the second NTD was connected to the sampling pump. The results demonstrated that the breakthrough was observed in none of the packed NTDs in the concentration range.
Effect of sampling temperature and relative humidity
Sampling parameters such as environmental temperature and relative humidity affect the sampler efficiency. In this regard, it is necessary to investigate the effects of these parameters on the sampler efficiency as a field sampler. In this study, the sampling efficiency was tested under three sampling temperatures: 10, 20, 35 °C, and three sampling relative humidities: 20, 50 and 80%. The results showed that the peak area responses of the analytes decreased with sampling temperature raise (Fig. 3). This result may be due to the reduction of mass adsorption on the surface of sorbent used in the NTD. Because, adsorption is an exothermic process and has a bilateral effect; increasing temperature rises adsorption rate and decreases its affinity to trap analytes. Moreover, the results demonstrated that the relative humidity had a negative influence on the efficiency of the proposed NTD for the sampling of studied analytes. As can be seen from Fig. 4, a decrease trend was also observed in the peak area responses of compounds of interest with increasing relative humidity in the standard chamber. This finding is attributed to the occupying the available active sites of the sorbent inside NTD by water molecules [23, 24]. Comparison of the influence of sampling temperature and relative humidity showed that the negative effect of relative humidity on the reduction of peak area responses was greater than that of temperature.
Fig. 3.
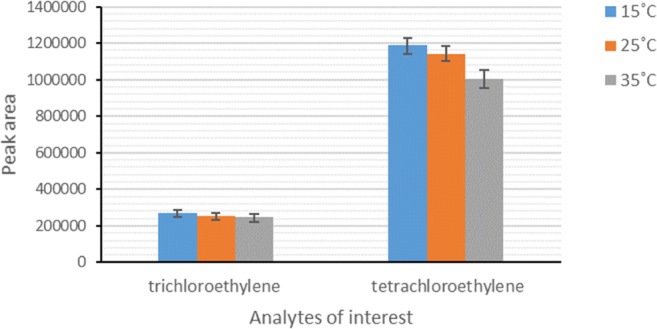
Effect of sampling temperature on the efficiency of NTD packed with Carbotrap B/silica composite for sampling of HVOCs
Fig. 4.
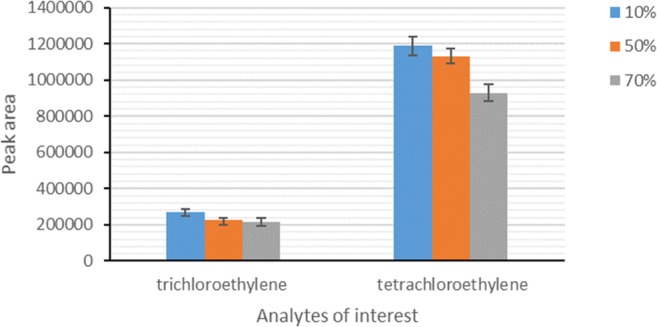
Effect of sampling relative humidity on the efficiency of NTD packed with Carbotrap B/silica composite for sampling of HVOCs
Effect of desorption temperature and time
Desorption time and temperature as two parameters that can be optimized during desorption process, have key roles in the thermal desorption of analytes from sorbent in solvent less extraction methods. High desorption times and temperatures can cause complete desorption of analytes from sorbent surface to GC-MS column, and subsequently reduce the possibility of carryover effect. By contrast, higher desorption times and temperatures may damage sorbent used and decrease its lifespan. Therefore, the effects of desorption time and temperature on the desorption of analytes of interest from the surface of Carbotrap B/silica composite were optimized under constant sampling conditions (15 °C of temperature and 10% of relative humidity) and a constant desorption time (3 min). Figure 5 shows the effect of desorption temperature on the analysis efficiency of the compounds with the proposed NTD. Results showed that the peak area responses of the compounds of interest increased with increasing desorption time from 180 to 250 °C, and further increase in the desorption temperature did not show an increase trend in the peak area responses. Increasing desorption temperature in a specified range can improve the rate of analytes desorption, and further increase in the temperature over the range can even have a reverse effect [24].
Fig. 5.
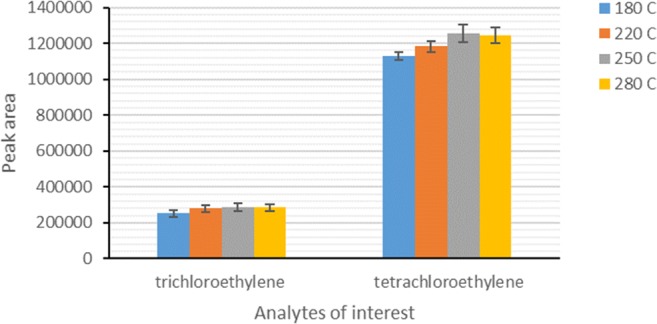
Effect of desorption temperature on the efficiency of NTD packed with Carbotrap B/silica composite for analysis of HVOCs
The effect of desorption time was also investigated on the peak area responses of compounds of interest under constant sampling conditions (15 °C of temperature and 10% of relative humidity). With respect to the previous studies, the influences of 1, 2, 3 and 5 min of desorption times were tested on the desorption of the studied compounds from the surface of Carbotrap B/silica composite packed in the NTD (at 250 °C of desorption temperature). Results indicated that the peak area responses of the studied compounds increased with increasing desorption time, and this increase was obvious in the range 1–3 min. As can be seen from Fig. 6, further increase in desorption time did not increase the peak area responses of the studied compounds. This finding indicated that 3 min was appropriate for desorption of the analytes trapped on the absorbent surface. With regard to the error bar levels of peak area responses corresponding to 3 and 5 min of desorption time, it can be concluded that there was no significant difference between these desorption times. Furthermore, to avoid damage to the sorbent packed inside the NTD, 3 min of desorption time was chosen as the optimal desorption time. This finding demonstrated that further desorption times higher than the optimal value does not increase the desorption efficiency of the analytes, and may increase the risk of sorbent damage.
Fig. 6.

Effect of desorption time on the efficiency of NTD packed with Carbotrap B/silica composite for analysis of HVOCs
Carryover effect
Carryover as a common problem in reusable samplers may interfere with subsequent measurements. In microextraction techniques, carryover effect has a dramatic effect on the NTD application. Carryover effect is associated with characteristics of the target analyte and its affinity to the sorbent surface. Since the carryover effect depends directly on desorption time and temperature, optimizing the desorption time and temperature could minimize its effect. For this reason, carryover effect was investigated under four desorption times (1, 2 and 3 min) and in a second desorption cycle with a constant interval (2 min after the first injection). The second desorption cycle was performed under constant conditions (desorption time: 3 min and desorption temperature: 250 °C). The results indicated that the carryover effect disappeared after 3 min of desorption time (Table 1). Therefore, it can be concluded that 3 min of desorption time and 250 °C of desorption temperature is sufficient to eliminate the memory effect of the HVOCs in the NTD packed with Carbotrap B/silica composite.
Table 1.
Carryover effect of NTD packed with Carbotrap B/silica composite for measurement of HOVCs in various desorption times
| Desorption time (min) | ThrCE (%) | TetCE (%) |
|---|---|---|
| 1 min | 0.33 | 0.39 |
| 2 min | 0.10 | 0.24 |
| 3 min | Not detected | Not detected |
Repeatability and reproducibility
Repeatability and reproducibility are two important parameters for measuring precision in NTD as a field sampler. In this study, the repeatability of the proposed NTD was measured as three repeated measurements from a proposed NTD for three concentrations. Also, the reproducibility was defined as three measurements from three NTDs for a constant concentration [25]. The results were reported as relative standard deviation (RSD). As can be seen from Table 2, the repeatability of the proposed NTD was investigated under different concentrations of 1, 10 and 100 ng/mL. The results demonstrated that the proposed NTD offered an acceptable repeatability (RSD <20) for the studied concentrations. The results also implied that the NTD had an acceptable reproducibility for the sampling and analysis of the HVOCs.
Table 2.
Repeatability and reproducibility of NTD packed with Carbotrap B/silica composite for sampling and analysis of HVOCs
| Analytes of interest | Percentage of RSD for four different concentrations | Percentage of RSD for three NTDs | |||||
|---|---|---|---|---|---|---|---|
| 0.01 ng/mL | 1 ng/mL | 10 ng/mL | 50 ng/mL | NTD1 | NTD2 | NTD3 | |
| (1 ng/mL) | |||||||
| ThrCE | 10.19 | 2.90 | 5.14 | 6.82 | 1.75 | 2.90 | 5.18 |
| TetCE | 3.80 | 3.44 | 3.39 | 12.67 | 1.23 | 3.44 | 7.45 |
Method validation
The sensitivity of the proposed method was defined as LOD and LOQ. In order to determine the value of LOD and LOQ of the proposed NTD, the concentrations of the analytes in the standard chamber were consecutively reduced to their smallest concentration corresponding o the signal to noise ratios of 3 and 10, respectively. Results showed that the LOD values for thrCE and tetCE were 0.01 and 0.03 ng/mL, respectively. Moreover, the LOQ values of the thrCE and tetCE were 0.05 and 0.09 ng/mL, respectively.
Comparative study
In order to compare the performance of the NTD packed with Carbotrap B/silica composite with other sorbents, the analytical parameters of the proposed NTD were compared with previously reported methods. As can be seen from Table 3, the proposed NTD coupled with GC-MS was able to detect 0.01 ng/mL of the HVOCs, which indicated high sensitivity of the proposed NTD in comparison with the previous applications of NTD packed with other sorbents. Moreover, the LDR of the proposed NTD was 0.01–50 ng/mL, which showed a suitable concentration range for the studied compounds.
Table 3.
Comparative study for analytical performance of the proposed NTD and previous applications of NTD for sampling and analysis of thrCE and tetCE (HVOCs)
| NTD packing | Target analytes | LOD (ng/mL) | LOQ (ng/mL) | LDR | Ref |
|---|---|---|---|---|---|
| Carbotrap B/silica composite | HVOCs | 0.01 | 0.05 | 0.01–50 | Current study |
| Graphene/silica composite | HVOCs | 0.02 | 0.08 | 0.01–80 | [15] |
| Graphene | HVOCs | 0.02 | 0.08 | 0.01–70 | [26] |
| PDMS* | HVOCs | 0.25 | 0.75 | 0.1–30 | [26] |
*Polydimethylsiloxane
Field study
The proposed NTD as a field sampler was used for the sampling of thrCE and tetCE in a dry-cleaning workplace which uses the target analytes (at sampling temperature: 28 ± 2 °C and relative humidity: 30%). Side by side sampling according to the NIOSH 1003 method was also performed to compare its results with the proposed NTD. Environmental sampling was carried out at three stations of the dry-cleaning shop (one station was selected near the reception and two other stations were near the washing machine). According to the results, thrCE as a target analyte was not found in the studied stations. While, a high concentration of tetCE was detected in the studied stations. Moreover, there was a consistency between the concentrations detected with the proposed NTD and the NIOSH method. This finding demonstrated that the NTD packed with Carbotrap B/silica composite can be successfully used as field sampler for the determination of HVOCs in workplaces (Table 4).
Table 4.
Field study of the performance of NTD packed with Carbotrap B/silica composite for sampling and analysis of tetCE in a dry cleaning shop
| Quantitation | Station 1 | Station 2 | Station 3 | |||
|---|---|---|---|---|---|---|
| NTD | NIOSH | NTD | NIOSH | NTD | NIOSH | |
| Average concentration (mg/m3) | 11.7 | 8.2 | 55 | 51 | 56 | 53 |
| RSD (%) | 2.54 | 5.87 | 1.88 | 8.23 | 5.20 | 10.12 |
Conclusion
In the present study, Carbotrap B as a sorbent with high specific surface area was prepared using sol-gel method to provide a higher pore size, surface area and thermal stability. In this study, NTD was packed with the Carbotrap B/silica composite and used for the determination of thrCE and tetCE in air. The effects of sampling temperature and relative humidity were evaluated on the NTD performance. Moreover, the effects of desorption time and temperature were also investigated on the performance of the proposed NTD. The results demonstrated that the performance of the proposed NTD decreased with increasing sampling temperature and relative humidity. The NTD performance increased with increasing desorption time from 180 to 250 °C, and increasing desorption temperature from 1 to 3 min. A suitable repeatability and reproducibility was achieved in the application of the NTD packed with Carbotrap B/silica composite. The results of carryover experiments indicated that the carryover effect was disappeared at desorption time of 3 min and temperature of 250 °C. Our findings proved that the NTD packed with Carbotrap B/silica composite can be used as field sampler with high sensitivity for the determination of HVOCs.
Electronic supplementary material
(DOC 837 kb)
Acknowledgements
The authors would like to thank the Hamadan University of Medical Sciences for supporting this research and the dry cleaning staff for their kind cooperation.
Authors’ contributions
AB supervised this research and he participated in all stages of study. AP performed the experiments and participated in all stages of this research work. ARG, FGS and MF as advisors of the study and participated in all stages of the present study. All authors read and approved the final manuscript.
Funding information
This work was supported by Center of Excellence for Occupational Health, and Research Center for Health Sciences, Hamadan University of Medical Sciences, Hamadan, Iran (Grant No. 9508185021).
Compliance with ethical standards
Competing interests
The authors declare that there is no conflict of interest regarding this paper.
Consent for publication
“Not applicable”.
Ethics approval and consent to participate
“Not applicable”.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Samarghandi MR, Babaee SA, Ahmadian M, Asgari G, Ghorbani Shahna F, Poormohammadi A. Performance catalytic ozonation over the carbosieve in the removal of toluene from waste air stream. J Res Health Sci. 2014;14:227–232. [PubMed] [Google Scholar]
- 2.Jakubowska N, Zygmunt B, Polkowska Ż, Zabiegała B, Namieśnik J. Sample preparation for gas chromatographic determination of halogenated volatile organic compounds in environmental and biological samples. J Chromatogr A. 2009;1216:422–441. doi: 10.1016/j.chroma.2008.08.092. [DOI] [PubMed] [Google Scholar]
- 3.Guha N, Loomis D, Grosse Y, Lauby-Secretan B, El Ghissassi F, Bouvard V, Benbrahim-Tallaa L, Baan R, Mattock H, Straif K. Carcinogenicity of trichloroethylene, tetrachloroethylene, some other chlorinated solvents, and their metabolites. Lancet Oncol. 2012;13:1192. doi: 10.1016/S1470-2045(12)70485-0. [DOI] [PubMed] [Google Scholar]
- 4.Scott CS, Jinot J. Trichloroethylene and cancer: systematic and quantitative review of epidemiologic evidence for identifying hazards. Int J Environ Res Public Health. 2011;8:4238–4272. doi: 10.3390/ijerph8114238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Raschdorf F. Short-term measurements of toxic substances at work site. Chimia. 1978;32:478–483. [Google Scholar]
- 6.NIOSH Manual of Analytical Methods (NMAM). Fourth Edition, Hydrocarbons, Halogenated: method 1003. 2003; Issue 3, dated 15 March: 2–7.
- 7.Stocka J, Tankiewicz M, Biziuk M, Namieśnik J. Green aspects of techniques for the determination of currently used pesticides in environmental samples. Int J Mol Sci. 2011;12:7785–7805. doi: 10.3390/ijms12117785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Qin T, Xu X, Polák T, Pacáková V, Štulík K, Jech L. A simple method for the trace determination of methanol, ethanol, acetone and pentane in human breath and in the ambient air by preconcentration on solid sorbents followed by gas chromatography. Talanta. 1997;44:1683–1690. doi: 10.1016/S0039-9140(97)00073-8. [DOI] [PubMed] [Google Scholar]
- 9.Koziel JA, Pawliszyn J. Air sampling and analysis of volatile organic compounds with solid phase microextraction. J Air Waste Manag Assoc. 2001;51:173–184. doi: 10.1080/10473289.2001.10464263. [DOI] [PubMed] [Google Scholar]
- 10.Ghiasvand AR, Heidari N, Abdolhosseini S, Hamdi A, Haddad PR. Evaluation of a cooling/heating-assisted microextraction instrument using a needle trap device packed with aminosilica/graphene oxide nanocomposite, covalently attached to cotton. Analyst. 143(11):2632–40. 10.1039/C8AN00063H. [DOI] [PubMed]
- 11.Niri VH, Eom IY, Kermani FR, Pawliszyn J. Sampling free and particle-bound chemicals using solid-phase microextraction and needle trap device simultaneously. J Sep Sci. 2009;32:1075–1080. doi: 10.1002/jssc.200800603. [DOI] [PubMed] [Google Scholar]
- 12.Ras MR, Marcé RM, Borrull F. Volatile organic compounds in air at urban and industrial areas in the Tarragona region by thermal desorption and gas chromatography–mass spectrometry. Environ Monit Assess. 2010;161:389–402. doi: 10.1007/s10661-009-0755-6. [DOI] [PubMed] [Google Scholar]
- 13.Trefz P, Kischkel S, Hein D, James ES, Schubert JK, Miekisch W. Needle trap micro-extraction for VOC analysis: effects of packing materials and desorption parameters. J Chromatogr A. 2012;1219:29–38. doi: 10.1016/j.chroma.2011.10.077. [DOI] [PubMed] [Google Scholar]
- 14.Heidari M, Bahrami A, Ghiasvand AR, Shahna FG, Soltanian AR. A novel needle trap device with single wall carbon nanotubes sol–gel sorbent packed for sampling and analysis of volatile organohalogen compounds in air. Talanta. 2012;101:314–321. doi: 10.1016/j.talanta.2012.09.032. [DOI] [PubMed] [Google Scholar]
- 15.Heidari M, Bahrami A, Ghiasvand AR, Shahna FG, Soltanian AR, Rafieiemam M. Application of graphene nanoplatelets silica composite, prepared by sol–gel technology, as a novel sorbent in two microextraction techniques. J Sep Sci. 2015;38:4225–4232. doi: 10.1002/jssc.201500975. [DOI] [PubMed] [Google Scholar]
- 16.Ghiasvand AR, Yazdankhah F. Single-step reinforced microextraction of polycyclic aromatic hydrocarbons from soil samples using an inside needle capillary adsorption trap with electropolymerized aniline/multi-walled carbon nanotube sorbent. J Chromatogr A. 2017;1487:47–53. doi: 10.1016/j.chroma.2017.01.056. [DOI] [PubMed] [Google Scholar]
- 17.Poormohammadi A, Bahrami A, Farhadian M, Shahna FG, Ghiasvand A. Development of Carbotrap B-packed needle trap device for determination of volatile organic compounds in air. J Chromatogr A. 2017;1527:33–42. doi: 10.1016/j.chroma.2017.10.062. [DOI] [PubMed] [Google Scholar]
- 18.Allego E, Roca FJ, Perales JF, Guardino X. Comparative study of the adsorption performance of a multi-sorbent bed (Carbotrap, Carbopack X, Carboxen 569) and a Tenax TA adsorbent tube for the analysis of volatile organic compounds (VOCs) Talanta. 2010;81:916–924. doi: 10.1016/j.talanta.2010.01.037. [DOI] [PubMed] [Google Scholar]
- 19.Kumar A, Malik AK, Tewary DK, Singh B. A review on development of solid phase microextraction fibers by sol–gel methods and their applications. Anal Chim Acta. 2008;610:1–4. doi: 10.1016/j.aca.2008.01.028. [DOI] [PubMed] [Google Scholar]
- 20.Liu M, Zeng Z, Xiong B. Preparation of novel solid-phase microextraction fibers by sol–gel technology for headspace solid-phase microextraction-gas chromatographic analysis of aroma compounds in beer. J Chromatogr A. 2005;1065:287–299. doi: 10.1016/j.chroma.2004.12.073. [DOI] [PubMed] [Google Scholar]
- 21.Mirjalili F, Mohamad H. Chuah l. Preparation of nano-scale α-Al2O3 powder by the sol-gel method. Ceramics–Silikáty. 2011;55:378–383. [Google Scholar]
- 22.Yarazavi M, Noroozian E. A novel sorbent based on carbon nanotube/amino-functionalized sol–gel for the headspace solid-phase microextraction of α-bisabolol from medicinal plant samples using experimental design. J Sep Sci. 2018;41(10):2229–2236. doi: 10.1002/jssc.201700993. [DOI] [PubMed] [Google Scholar]
- 23.Hartonen K, Helin A, Parshintsev J, Riekkola ML. Problems caused by moisture in gas chromatographic analysis of headspace SPME samples of short-chain amines. Chromatographia. 2019;82(1):307–316. doi: 10.1007/s10337-018-3641-y. [DOI] [Google Scholar]
- 24.Biagini D, Lomonaco T, Ghimenti S, Onor M, Bellagambi FG, Salvo P. Using labelled internal standards to improve needle trap micro-extraction technique prior to gas chromatography/mass spectrometry. Talanta. 2019;200:145–155. doi: 10.1016/j.talanta.2019.03.046. [DOI] [PubMed] [Google Scholar]
- 25.Magnusson B. The fitness for purpose of analytical methods: a laboratory guide to method validation and related topics. Eurachem. 2014:34–5.
- 26.Heidari M, Bahrami A, Ghiasvand AR, Emam MR, Shahna FG, Soltanian AR. Graphene packed needle trap device as a novel field sampler for determination of perchloroethylene in the air of dry cleaning establishments. Talanta. 2015;131:142–148. doi: 10.1016/j.talanta.2014.07.043. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOC 837 kb)