Abstract
α-Mangostin (MAN) is a bioactive compound isolated from the inedible pericarp of a tropical fruit mangosteen (Garcinia mangostana Linn). It exhibits notable therapeutic potentials on lung cancers, but the underlying mechanisms are still largely unknown. This study was designed to further explore the mechanisms involved in cytotoxicity of MAN on A549 cells. Apoptosis and cell cycle distribution were analyzed by flow cytometry methods. The fluorescent probes DCFH-DA and JC-1 were used to assess the intracellular reactive oxidative species (ROS) and mitochondrial membrane potential statuses, respectively. The regulation of MAN on relevant pathways was investigated by immunoblotting assays. The results obtained indicated that MAN caused significant apoptosis and cell cycle arrest in A549 cells, which eventually resulted in inhibition on cell proliferation in vitro. All these phenomena were synchronized with escalated oxidative stress and downregulation of nicotinamide phosphoribosyltransferase/nicotinamide adenine dinucleotide (NAMPT/NAD). Supplementation with nicotinamide mononucleotide (NMN) and N-acetylcysteine (NAC) efficiently eased MAN-induced ROS accumulation, and potently antagonized MAN-elicited apoptosis and cell cycle arrest. The pro-apoptotic effect of MAN was further confirmed by increased expressions of cleaved caspase 3, 6, 7, and 9, and its effect on cell cycle progression was validated by the altered expressions of p-p38, p-p53, CDK4, and cyclin D1. The immunoblotting assays also demonstrated that NAC/NMN effectively restored these molecular changes elicited by MAN treatment. Collectively, this study revealed a unique anti-tumor mechanism of MAN by provoking ROS production through downregulation of NAMPT/NAD signaling and further validated MAN as a potential therapeutic reagent for lung cancer treatment.
Keywords: α-Mangostin, Lung cancer, Oxidative stress, Apoptosis, Reactive oxygen species, NAMPT/NAD
Introduction
Lung cancer (LC) is the most common cause of cancer-related mortality globally. The global survey in 2012 showed that 1.8 million people are diagnosed with LC each year and approximately 1.6 million patients die as a result of the disease (Ferlay et al. 2015; Wang et al. 2019). With the high incidence and high mortality rate, LC imposes a great threat to the global public health. Due to the large population size and heavy consumption of cigarettes, China contributes approximately 35.78% of all newly diagnosed cases of LC and 37.56% of LC-related mortality annually (Chen et al. 2015).
Two major subtypes of lung cancer have been identified, small-cell lung carcinoma (SCLC) and non-small-cell lung carcinoma (NSCLC). NSCLC is the most common form of LC as it accounts for 85% of all reported cases of LC (Wang et al. 2010; Xue et al. 2012; DeSantis et al. 2016; Miller et al. 2016; Siegel et al. 2016). Clinical efficacy of surgical resection is one of the promising approaches for the management of early stage NSCLC. However, due to lack of sensitive diagnostic biomarkers and obvious symptoms, majority of patients present in advanced stages and are not amenable to surgical procedures (Wang et al. 2010). As a result, chemotherapy is an indispensable option in NSCLC treatments. Platinum-based doublet therapy, which is a combination of one of the platinum agents such as cisplatin, carboplatin, or nedaplatin with one of the third-generation chemotherapy agents, has become the first line of treatment worldwide (Horita et al. 2017; Reck et al. 2014). Despite of the notable advancements of biotherapies achieved in recent years, chemotherapy has never been challenged and is still extensively used because of its effective and economic merits. However, platinum and other conventional drugs also have instinct shortages, such as potential toxicity, multidrug resistance, high rate of recurrence, and metastasis (Miller et al. 2016; Hirsch et al. 2017; Tan et al. 2019). Therefore, the priority of current NSCLC research attaches great importance to the exploration of new effective reagents with good safety profiles.
Nowadays, the application of natural products in tumor treatment is a common alternative for conventional therapies. This approach encourages scientists to screen out bioactive ingredients with anti-tumor potentials from natural resources. Mangosteen (Garcinia mangostana Linn.) is a tropical tree native to Southeast Asian countries. Its pulp as a fruit is famous for its highly palatable fruit and sweet flavor. In recent years, mangosteen has drawn intense attention from the pharmaceutical field as its inedible pericarp is a rich source of oxygenated and prenylated xanthones, which possess impressive bioactivities and have promising applications in treatments of many diseases including cancers (Pedrazachaverri et al. 2008; Ovalle-Magallanes et al. 2017; Abdallah et al. 2016; Mohamed et al. 2017). These compounds have displayed significant cytotoxicities on a panel of cancer cell lines in vitro (Akao et al. 2008; Watanapokasin et al. 2010; Chitra et al. 2010). Among the mangosteen-derived xanthone derivatives, α-mangostin (Fig. 1A) is the most important because of the high abundance and promising pharmaceutical potentials. It exhibited significant cytotoxic effect on NSCLC and efficiently inhibited the proliferation of A549 cells, as well as migration and invasion (Phan et al. 2018; Shih et al. 2010; Cheng et al. 2014). However, the mechanisms underlying its anti-NSCLC actions are still elusive. Available evidences suggested that α-mangostin universally induced cell cycle arrest and apoptosis in cancer cells (Kurose et al. 2012; Johnson et al. 2012; Kwak et al. 2016). We speculated that similar mechanisms could be involved in the inhibitory actions of α-mangostin on A549 cells. Thus, we tested this assumption in this study and further identified the possible target of α-mangostin on A549 cells.
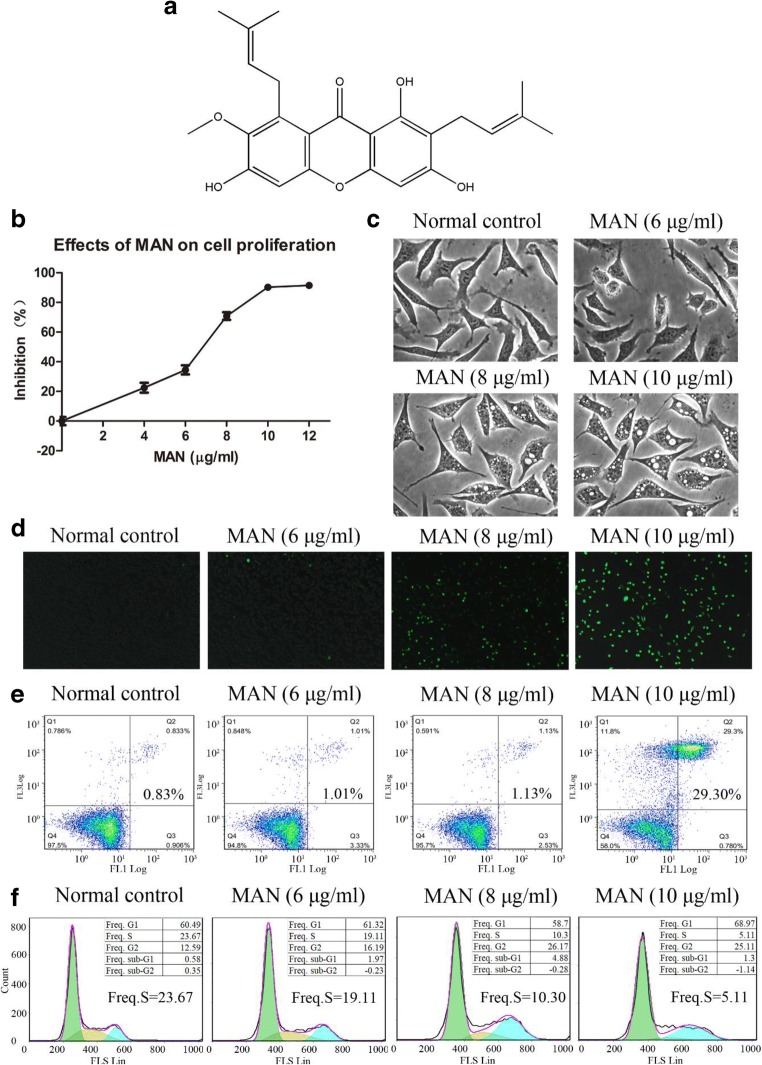
Fig. 1.
(A) Chemical structure of α-mangostin. (B) Inhibitory effect of MAN on A549 cell proliferation revealed by MTT assay. (C) Morphological observation of A549 cells under MAN treatments. (D) Effect of MAN on intracellular ROS accumulation in A549 cells. (E) The pro-apoptotic effect of MAN. (F) Effect of MAN treatments on cell cycle arrest
Materials and methods
Chemicals and reagents
MAN (98% purity) was purchased from SanHerb Bioscience Inc. (Chengdu, Sichuan, China). Fetal bovine serum (FBS) and bovine serum albumin (BSA) were procured from Thermo Scientific (Rockford, IL, USA). All the other reagents used for cell culture, including Dulbecco’s Modified Eagle Medium (DMEM), phosphate-buffered saline (PBS), and penicillin-streptomycin, were supplied by Keygen Biotech (Nanjing, Jiangsu, China). BCA protein quantitative kit, nicotinamide mononucleotide (NMN), 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl-tetrazolium-bromide (MTT), N-acetylcysteine (NAC), and HRP/biotin-conjugated secondary antibodies were purchased from Beyotime Biotech (Nantong, Jiangsu, China). Primary antibodies used in immunoblotting assay were purchased from Cell Signaling Technology (Beverly, MA, USA). Ultra-pure water was prepared using a Milli-Q purification system (Millipore, Bedford, MA, USA).
MTT assay and NAD determination
A549 cells were grown in DMEM supplemented with 10% (v/v) FBS, 80 U/ml penicillin, and 0.08 mg/ml streptomycin under humidified atmosphere with 5% CO2 at 37 °C. The cells were passaged every 2 days at the ratio of 1:3. Cells at exponential growth stage were seeded into a 96-well plate at a density of 10,000 cells/well and maintained overnight for attachment. The cells were treated with MAN at various concentrations or in the combination of NAC and NMN for 24 h. Afterwards, the supernatant was replaced by100 μl of fresh medium and 20 μl of MTT solution (5 mg/ml in PBS) was added. After an incubation period of 4 h, the medium in wells was carefully removed and the remaining crystals were dissolved with 150 μl of DMSO. The optical density (OD) of samples was determined by a micro-plate spectrophotometer (Bio-Rad Science Co. Ltd., USA) at 490 nm.
For NAD determination, cells seeded in 75-cm2 culture flasks were treated with MAN at various concentrations. After treatment, the cells were harvested and counted. Quantified cells were lysed in the extraction solution provided in the NAD determination kit (Solarbio Science & Technology Co., Ltd., Beijing, China). The obtained lysate was further denatured by boiling. After a centrifugation at 12,000 rpm for 10 min, the supernatant was collected for NAD quantitative analysis according to the protocol provided by the manufacturer.
Intracellular ROS and mitochondrial membrane potential evaluation
Intracellular ROS and mitochondrial membrane potential were assessed by using DCFH-DA and JC-1 fluorescent probe, respectively (Keygen Biotech, Nanjing, Jiangsu, China). All procedures were performed in accordance with the manufactures’ instructions. Briefly, cells seeded in 6-well plates (50,000 cells/well) were maintained overnight and treated with varying concentrations of MAN or in combination with NMN and NAC for 24 h. The attached cells were washed thrice with ice-cold PBS and then treated with DCFH-DA/JC-1 in serum-free medium. After further incubation at 37 °C for 20 min, the intracellular oxidative stress and mitochondrial membrane potential were evaluated based on the optical observations by using a BX53 fluorescence microscope (Olympus, Tokyo, Japan). DCFH-DA-stained cells were obtained and subjected to the quantitative analysis with the aid of a flow cytometry (FACSCalibur system, Becton & Dickinson, San Jose, CA, USA).
Apoptosis and cell cycle analyses
The pro-apoptotic effect and cell cycle distribution were analyzed based on Annexin V-FITC/PI double or PI single-labeling methods by using the corresponding staining kits provided by Keygen Biotech (Nanjing, Jiangsu, China). Cells were seeded into 6-well plates at suitable density and allowed to attach overnight. The attached cells were then subjected to the same treatments as described above and the cells used for cell cycle analysis were incubated in serum-free medium for 12 h before the treatment. Subsequently, the treated cells were collected with EDTA-free trypsin and washed twice with ice-cold PBS.
For apoptosis detection, the cells were re-suspended in 400 μl of binding buffer before incubation with Annexin V-FITC for 15 min at 2–8 °C in the dark. After further staining with PI in the dark for 5 min, the cells were subjected to qualitative assessment or quantitative analysis by using the fluorescence microscope and flow cytometry, respectively. The cells stained with both Annexin V-FITC and PI were deemed as apoptotic cells. To determine the cell cycle distribution, the obtained cells were fixed in ice-cold 75% ethanol and kept at − 20 °C for 24 h. Before analysis, the processed cells were washed twice with PBS, treated with RNase A, and then dyed with PI at 4 °C for 8 min. The stained cells were subjected to the flow cytometry analysis.
Immunoblotting assay
Cells seeded in 6-well plates were treated with MAN and lysed in RIPA buffer supplemented with 1% phosphatase inhibitor cocktail on ice. The supernatant from the lysates was collected after centrifuging at 12,000 rpm for 10 min at 4 °C, spiked into loading buffer at a ratio of 4:1, and denatured by boiling. Quantified proteins in samples were separated by SDS-PAGE and transferred to PVDF blotting membranes which were then blocked with 5% BSA and incubated with appropriate primary antibodies at 4 °C overnight. After the further incubation with secondary antibodies at room temperature for 1 h, the signals were developed using an ECL detection kit on a Tanon 5200 system (Bio-Tanon, Shanghai, China).
Statistical analysis
Results are expressed as means ± SD (n = 3). Statistical analyses were performed using the SPSS statistical analysis software version 14.0 (SPSS Inc. Chicago, IL, USA). The differences among different groups were evaluated based on one-way analysis of variance followed with Tukey post hoc test. Statistically significant was set at *P < 0.05 and **P < 0.01.
Results
MAN-induced cytotoxicity and ROS accumulation in A549 cells
As shown in Fig. 1 B, MAN inhibited the proliferation of A549 cells in vitro in a concentration-dependent manner. Initially, the effect was reinforced gradually with increase in treatment concentration; however, the proliferation inhibitory effect was significantly pronounced at a concentration above 6 μg/ml. The morphological observation found that intense MAN treatment caused significant cytotoxicity. It severely damaged the integrity of intracellular structures and elicited obvious vacuolization, swelling, and karyorrhexis in treated cells (Fig. 1C). Meanwhile, we noticed that all these negative effects on cells were synchronized with escalating oxidative stresses. Treatment with MAN (below 6 μg/ml for 24 h) had little influence on intracellular ROS generation, whereas at higher concentrations (8 and 10 μg/ml), the intracellular ROS level was significantly increased (Fig. 1D). As apoptosis and cell cycle arrest are the two most common factors involved in cytotoxicity, we carried out a flow cytometry analysis. As anticipated, MAN substantially promoted apoptosis and altered cell cycle distribution in A549 cells in a concentration-dependent manner. Furthermore, it was observed that cell cycle arrest occurred prior to apoptosis, as MAN at 8 μg/ml greatly reduced the frequency of S phase (Fig. 1F), while it induced significant apoptosis at 10 μg/ml (Fig. 1E).
MAN elicited ROS accumulation in A549 cells by downregulation of NAMPT/NAD
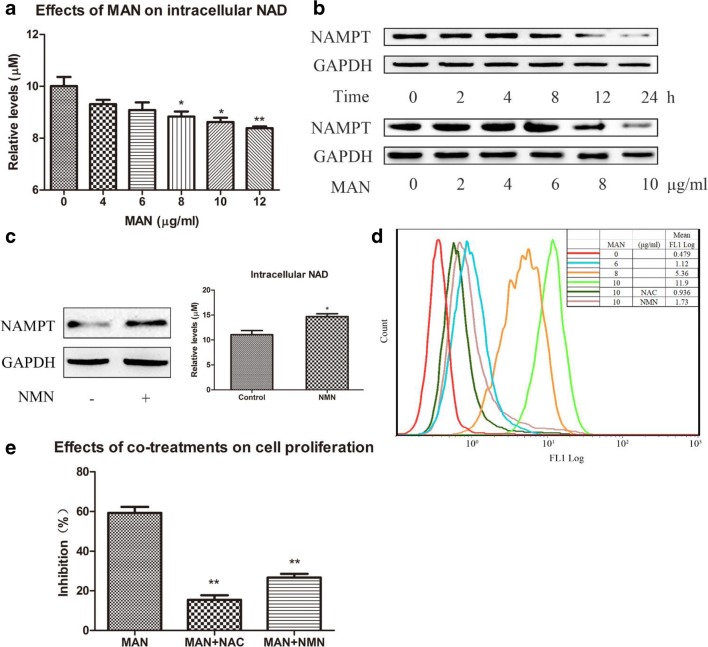
As NAMPT/NAD serves as an indispensable component in the ROS scavenging system, we speculated that intracellular ROS accumulation could be a critical factor responsible for the inhibitory effect of MAN on this signaling pathway taken its potent effect on NAMPT into consideration (Tao et al. 2018). To validate this hypothesis, we assessed the intracellular levels of NAD and found that MAN significantly reduced its level (Fig. 2A). Furthermore, MAN suppressed NAMPT expression in A549 cells in a time- and concentration-dependent manner (Fig. 2B). Based on these evidences, we optimized MAN treatment by adopting 10 μg/ml and 24 h as the treatment concentration and time in subsequent experiments. NMN is the dominant precursor for NAD biosynthesis in mammals. Theoretically, NMN will promote NAD production, which was validated by the in vitro experiment (Fig. 2C). Furthermore, NMN increased NAMPT expression in cells. The flow cytometry analysis also observed that MAN (8 μg/ml) promoted ROS production. Additionally, it was revealed that MAN-elicited ROS accumulation was reversed by NAC and NMN (Fig. 2D). These results indicated that MAN could increase oxidative stress by depleting NAD pool through the inhibition on NAMPT. The consequent MTT assay found that both NAC and NMN co-treatments weakened the effect of MAN on A549 cell proliferation in vitro, which further validated the plausible mechanism involved in the cytotoxicity of MAN by promoting ROS production through the downregulation of NAMPT/NAD signaling.
Fig. 2.
MAN elicited ROS accumulation in A549 cells by downregulation of NAMPT/NAD. (A) Effect of MAN on intracellular NAD levels. (B) The time-dependent inhibition of MAN on NAMPT expression (top) and the concentration-dependent inhibition of MAN on NAMPT expression (below). (C) NMN supplementation (200 μM for 24 h) upregulated NAMPT/NAD in A549 cells. (D) NAC and NMN co-treatment eased MAN-induced ROS accumulation. (E) The antagonistic effect of NAC and NMN against MAN-induced inhibition on proliferation of A549 cells. Data are expressed as means ± SD (n = 3). Statistical analyses were performed using the SPSS and differences among different groups were evaluated using ANOVA followed by Tukey post hoc test. *P < 0.05 compared with normal control (Fig. 2A and C), **P < 0.01 compared with normal control (Fig. 2A), **P < 0.01 compared with MAN-treated cells (Fig. 2E)
Pro-apoptotic effect of MAN was mediated by its inhibition on NAMPT/NAD
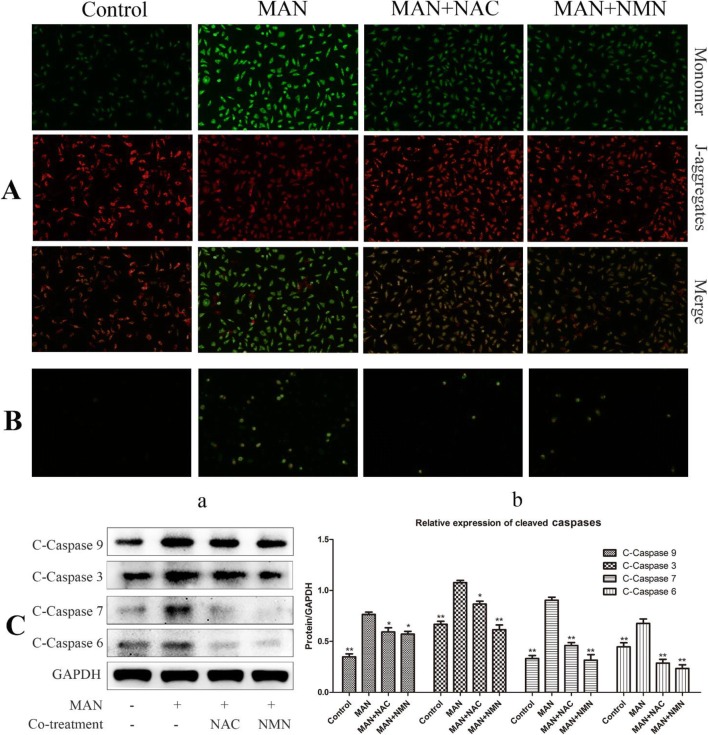
Mitochondrial membrane potential loss is a crucial cellular event implicated in ROS-relevant apoptosis. It was observed that treatment with MAN resulted in severe damages on the integrity of mitochondrial membrane potentials as suggested by the strong green fluorescence emitted by the monomer JC-1 probe. NAC and NMN exhibited similar protective effects on MAN-treated cells (Fig. 3A). It demonstrated that both NAC and NMN can protect cells from apoptosis by scavenging intracellular ROS and reducing the oxidative attack on mitochondrial membrane. The antagonistic effects of NAC and NMN against MAN-induced apoptosis were also confirmed by the Annexin V-FITC/PI staining assay (Fig. 3B). Results from immunoblotting assay provided direct evidences to support the conclusion above. Treatment with MAN increased the expression of a panel of cleaved caspases, including cleaved caspases 3, 6, 7, and 9, while both NAC and NMN significantly restored these changes (Fig. 3C).
Fig. 3.
MAN promoted apoptosis in A549 cells by regulation of NAMPT/NAD-controlled ROS accumulation. (A) Effect of MAN and co-treatments with NAC/NMN on mitochondrial membrane potentials. (B) NAC and NMN protected cells from apoptosis under MAN treatment indicated by Annexin V-FITC/PI double staining. (C) NAC and NMN restored MAN-induced increases in cleaved caspase expression: (a) results of the immunoblotting assay and (b) quantification of immunoblotting assay. Data are expressed as means ± SD (n = 3). Statistical analyses were performed using the SPSS and differences among different groups were evaluated using ANOVA followed by Tukey post hoc test. *P < 0.05 compared with MAN-treated cells, **P < 0.01 compared with MAN-treated cells
MAN-induced cell cycle arrest in A549 cells through downregulation of NAMPT/NAD
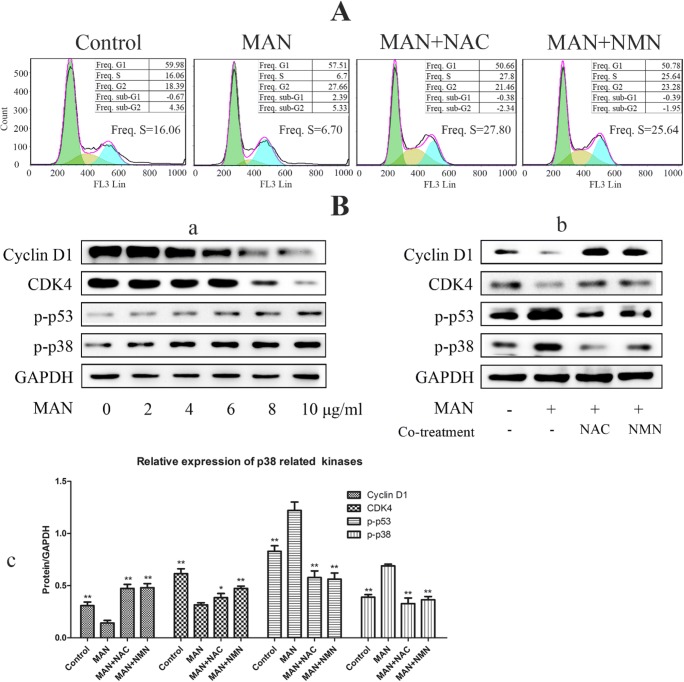
As ROS is an activator of p38 pathway and also a potent inducer of cell cycle arrest (Pan et al. 2016), we evaluated the effect of co-treatment of NAC and NMN with MAN on cell cycle distribution. Similar to their effect on apoptosis, NAC and NMN substantially reversed MAN-elicited cell cycle arrest in A549 cells (Fig. 4A). The mechanism underlying the effect of MAN on cell cycle distribution was partially elucidated by western blot analysis. As shown in Fig. 4 B, MAN promoted the phosphorylation of p38 and p53 and consequently led to the decline expression of cycling D1 and CDK4, which play central roles in cell cycle progression. Consistent with the results from flow cytometry analysis, it was observed that the regulatory effect of MAN on the proteins mentioned above mainly occurred at higher concentrations (not less than 8 μg/ml). To further validate the role of NAMPT/NAD-mediated ROS accumulation in the regulation of p38/p53 and consequent cell cycle arrest, we co-treated A549 cells with NAC/NMN and MAN. The results obtained solidly supported the assumption that p38 activation elicited cell cycle arrest under MAN treatment was achieved by the regulation on NAMPT/NAD-controlled ROS accumulation, as both NAC and NMN efficiently reversed the molecular changes related to cell cycle progression (Fig. 4B).
Fig. 4.
Downregulation of NAMPT/NAD involved in cell cycle arrest in A549 cells receiving MAN treatments. (A) NAC and NMN co-treatments restored phase S decline in cells induced by MAN stimulus. (B) regulatory effect of MAN on critical signals involved in cell cycle progression control: (a) MAN regulated the expressions of p-p38, p-p53, CDK4, and cyclin D1 in a concentration-dependent manner. (b) NAC and NMN reversed the regulation of MAN on cell cycle–related proteins. (c) Quantification of immunoblotting assay. Data are expressed as means ± SD (n = 3). Statistical analyses were performed using the SPSS and differences among different groups were evaluated using ANOVA followed by Tukey post hoc test. *P < 0.05 compared with MAN-treated cells; **P < 0.01 compared with MAN-treated cells
Discussion
The demand for food and fruit derivatives has greatly increased in recent years due to their invaluable usefulness as food supplements and multiple health benefit claims, especially in the treatment of metabolic disorders, cancer, and neurodegenerative diseases. Mangosteen is popularly referred to as the queen of fruits due to its unique flavor, pleasant aroma, and high nutritional value. The safety profile and multiple pharmacological properties of mangosteen have created so much interest in the fruit (Ovalle-Magallanes et al. 2017; Mohamed et al. 2014; Genovese et al. 2016). The pericarp possesses significant medicinal properties and has been used to treat various diseases for hundreds of years. Accumulating evidences suggested this plant has a promising application in the treatment of cancers (Kwak et al. 2016; Cheng et al. 2014; Shih et al. 2010). Because of its medicinal usage and safety merits, many mangosteen products have been developed and marketed to cancer patients as dietary supplements, although many fundamental issues especially concerning the therapeutic mechanisms are still not thoroughly addressed (Yeung 2006). Given the high volume as an agricultural byproduct, fully exploiting the medicinal value of mangosteen pericarp is not only technically feasible but also economically efficient and environmentally friendly. Alpha-mangostin (MAN) is the major compound from mangosteen and it exhibits versatile bioactivities and contributes mostly to the anti-tumor potentials (Phan et al. 2018; Kurose et al. 2012; Johnson et al. 2012; Cheng et al. 2014). Therefore, a better understanding of the relevant mechanisms involved in the therapeutic actions of MAN on cancers is of great clinical interest and also crucial for the exploitation of mangosteen as an anti-tumor remedy.
Cell cycle arrest and apoptosis are the two main cellular events occurring in cancer cells under MAN stimulus (Kurose et al. 2016; Johnson et al. 2012; Kwak et al. 2016). These changes lead to the proliferation inhibition and eventual death of cells and would have pronounced influence on the treated subjects with cancers. However, the upstream targets associated to these changes are still elusive. Many studies found that MAN can provoke ROS production in cancer cells, which substantially activates certain oxidative stress-sensitive and apoptosis-related pathways (Lee et al. 2017; Asif et al. 2018). In addition, ROS is also a potent inducer of cell cycle arrest in various cells including A549 cells (Wu et al. 2005). A recent study clearly illustrated that ROS-mediated cytotoxicity was greatly responsible for the inhibitory effects of MAN on NSCLC cells (Zhang et al. 2018). The bright red-orange fluorescence emitted by J-aggregates (the aggregate from accumulated JC-1 probe inside organelles) was observed in normal control cells, suggesting these cells were totally intact. These clues convince us that ROS is the upstream converge of some critical pathways manipulating the fate of cells, and ROS accumulation could be the common reason for MAN-induced cell cycle arrest and apoptosis in NSCLC cell lines. The inhibition of MAN on A549 cells seems to progress in two phases. Moderate MAN stimulus (below 8 μg/ml) caused accumulating ROS production in cells and further activated p38/p53 pathway leading to cell cycle arrest and proliferation inhibition. Upon increase in MAN concentration, the intracellular oxidative stress was further intensified. The hazardous radicals attack the mitochondrial membrane, which then causes loss of potentials. These changes inevitably resulted in caspase leakage and cleavage and subsequently initiated apoptosis processes. Thus, apoptosis other than cell cycle arrest may account for the cytotoxicity under intense MAN stimulus.
These findings revealed the importance of ROS in NSCLC treatment. However, it also raised another question. As a typical polyphenol, MAN is usually conceptualized as an antioxidant (Jung et al. 2006). Accordingly, MAN possesses notable antioxidative effects and it exhibits protective potentials against ROS-induced injuries (Pedraza-Chaverrí et al. 2009; Sánchez-Pérez et al. 2010). It seems to be contrary to our observation and similar reports, and the subtle effect of MAN on redox status should be further characterized. Previously, we found the exact outcomes from xanthones treatments were concentration- and time-dependent (Zuo et al. 2017). In other words, MAN usually acts as an antioxidant in most cases, while intense stimulus will result in ROS accumulation and cytotoxicity. Hafeez and colleagues found that MAN treatments under the same conditions selectively elicited cytotoxicity in cancer cells but not normal cells (Hafeez et al. 2014). Considering the profound influence of MAN on redox status, this phenomenon inspired us to hypothesize that metabolic differences should account for the selective inhibition on cancer cells by MAN. Overexpression of NAMPT has been recognized as a common characteristic of cancers (Garten et al. 2009; Bi & Che 2010). As the rate-limiting enzyme in the biosynthesis of NAD in mammals, NAMPT controls the metabolic rate in vivo. Upregulation of NAMPT/NAD reflects the high energy demand under cancer circumstances and accelerates the proliferation of the pathological cells (Garten et al. 2009; Bi & Che 2010). It also enhances the tolerance of cancer cells to ROS and allows them to survive under some extreme conditions such as chemotherapy and other therapies, since NAMPT/NAD is an important component of radical scavenging system (Bułdak et al. 2013; Cerna et al. 2012). Cancer cells are much more vulnerable to NAMPT inhibition than normal cells, and MAN could selectively elicit ROS accumulation in cancer cells rather than normal cells due to its effective effects on NAMPT expression.
Conclusion
In this study, the potentials of MAN in the treatment of NSCLC based on its significant cytotoxicity in A549 cells in vitro was investigated. MAN downregulated NAMPT/NAD signaling in treated cells, and therefore provoked ROS production, which should largely account for the notable apoptosis and cell cycle arrest. These facts revealed the crucial role of the subtle oxidative balance in determining the fate of cells, and demonstrated that NAMPT/NAD could be an effective target for cancer treatments.
Funding information
This work was supported by the National Natural Science Foundation of China (81603388), Talent Cultivation and International Academic Visiting Project for College Scholar of Anhui Province (gxfxZD2016163), Funding of “Peak” Training Program for Scientific Research of Yijishan Hospital, Wannan Medical College (GF2019J01), and Research Project on Traditional Chinese medicine of Anhui province (2016zy37).
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Yan-Yun Ding and Jia-Jie Luan contributed equally to this work.
References
- Abdallah HM, El-Bassossy H, Mohamed GA, El-Halawany AM, Alshali KZ, Banjar ZM. Phenolics from Garcinia mangostana alleviate exaggerated vasoconstriction in metabolic syndrome through direct vasodilatation and nitric oxide generation. BMC Complement Altern Med. 2016;16:359. doi: 10.1186/s12906-016-1340-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akao Y, Nakagawa Y, Nozawa Y. Anti-cancer effects of xanthones from pericarps of mangosteen. Int J Mol Sci. 2008;9:355–370. doi: 10.3390/ijms9030355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asif J, Sahabjada S, Amar D, Md A. Induction of apoptosis by alpha-mangostin in human hepatocellular carcinoma cells via nuclear fragmentation and ROS dependent mitochondrial pathway. J Clin Exp Hepatol. 2018;8:S116. [Google Scholar]
- Bi TQ, Che XM. Nampt/PBEF/visfatin and cancer. Cancer Biol Ther. 2010;10:119–125. doi: 10.4161/cbt.10.2.12581. [DOI] [PubMed] [Google Scholar]
- BUŁDAK RAFAŁ JAKUB, BUŁDAK ŁUKASZ, POLANIAK RENATA, KUKLA MICHAŁ, BIRKNER EWA, KUBINA ROBERT, KABAŁA-DZIK AGATA, DUŁAWA-BUŁDAK ANNA, ŻWIRSKA-KORCZALA KRYSTYNA. Visfatin affects redox adaptative responses and proliferation in Me45 human malignant melanoma cells: An in vitro study. Oncology Reports. 2012;29(2):771–778. doi: 10.3892/or.2012.2175. [DOI] [PubMed] [Google Scholar]
- Cerna David, Li Hongyun, Flaherty Siobhan, Takebe Naoko, Coleman C. Norman, Yoo Stephen S. Inhibition of Nicotinamide Phosphoribosyltransferase (NAMPT) Activity by Small Molecule GMX1778 Regulates Reactive Oxygen Species (ROS)-mediated Cytotoxicity in a p53- and Nicotinic Acid Phosphoribosyltransferase1 (NAPRT1)-dependent Manner. Journal of Biological Chemistry. 2012;287(26):22408–22417. doi: 10.1074/jbc.M112.357301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen W, Zheng R, Zeng H, Zhang S. Epidemiology of lung cancer in China. Thoracic Cancer. 2015;6:209–215. doi: 10.1111/1759-7714.12169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng K, Zhang G, Qiu S, Liu Y, Wang Y, Liu S, Shan Y, Yu B, Lu Y (2014) Synthesis and antitumor activities of α-,γ-mangostin derivatives. Lett Drug Des Discov 11:586–593
- Chitra S, Krithika MV, Pavithra S. Induction of apoptosis by xanthones from Garcinia mangostana in human breast and laryngeal carcinoma cell lines. IJPBS. 2010;1:1–8. [Google Scholar]
- DeSantis CE, Siegel RL, Sauer AG, Miller KD, Fedewa SA, Alcaraz KI, Jemal A. Cancer statistics for African Americans, 2016: progress and opportunities in reducing racial disparities. Cancer J Clin. 2016;66:290–308. doi: 10.3322/caac.21340. [DOI] [PubMed] [Google Scholar]
- Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, Parkin DM, Forman D, Bray F. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136:E359–E386. doi: 10.1002/ijc.29210. [DOI] [PubMed] [Google Scholar]
- Garten A, Petzold S, Körner A, Imai S, Kiess W. Nampt: linking NAD biology, metabolism and cancer. Trends Endocrinol Metab. 2009;20:130–138. doi: 10.1016/j.tem.2008.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genovese S, Fiorito S, Taddeo VA, Epifano F. Recent developments in the pharmacology of prenylated xanthones. Drug Discov Today. 2016;21:1814–1819. doi: 10.1016/j.drudis.2016.06.033. [DOI] [PubMed] [Google Scholar]
- Hafeez BB, Mustafa A, Fischer JW, Singh A, Zhong W, Shekhani MO, Meske L, Havighurst T, Kim K, Verma AK. α-Mangostin: a dietary antioxidant derived from the pericarp of Garcinia mangostana L. inhibits pancreatic tumor growth in xenograft mouse model. Antioxid Redox Signal. 2014;21:682–699. doi: 10.1089/ars.2013.5212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirsch FR, Scagliotti GV, Mulshine JL, Kwon R, Curran WJ, Jr, Wu YL, Paz-Ares L. Lung cancer: current therapies and new targeted treatments. Lancet. 2017;389:299–311. doi: 10.1016/S0140-6736(16)30958-8. [DOI] [PubMed] [Google Scholar]
- Horita N, Nagashima A, Nakashima K, Shibata Y, Ito K, Goto A, Yamanaka T, Kaneko T. The best platinum regimens for chemo-naive incurable non-small cell lung cancer: network meta-analysis. Sci Rep. 2017;7:13185. doi: 10.1038/s41598-017-13724-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson JJ, Petiwala SM, Syed DN, Rasmussen JT, Adhami VM, Siddiqui IA, Kohl AM, Mukhtar H. α-Mangostin, a xanthone from mangosteen fruit, promotes cell cycle arrest in prostate cancer and decreases xenograft tumor growth. Carcinogenesis. 2012;33:413–419. doi: 10.1093/carcin/bgr291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung HA, Su BN, Keller WJ, Mehta RG, Kinghorn AD. Antioxidant xanthones from the pericarp of Garcinia mangostana (mangosteen) J Agric Food Chem. 2006;54:2077–2082. doi: 10.1021/jf052649z. [DOI] [PubMed] [Google Scholar]
- Kwak HH, Kim IR, Kim HJ, Park BS, Yu SB (2016, 2016) α-Mangostin induces apoptosis and cell cycle arrest in oral squamous cell carcinoma cell. Evid Based Complement Alternat Med 5352412 [DOI] [PMC free article] [PubMed]
- Kurose H, Shibata MA, Iinuma M, Otsuki Y. Alterations in cell cycle and induction of apoptotic cell death in breast cancer cells treated with α-mangostin extracted from mangosteen pericarp. J Biomed Biotechnol. 2012;2012:672428. doi: 10.1155/2012/672428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee CH, Ying TH, Chiou HL, Hsieh SC, Wen SH, Chou RH, Hsieh YH. Alpha-mangostin induces apoptosis through activation of reactive oxygen species and ASK1/p38 signaling pathway in cervical cancer cells. Oncotarget. 2017;8:47425–47439. doi: 10.18632/oncotarget.17659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller KD, Siegel RL, Lin CC, Mariotto AB, Kramer JL, Rowland JH, Yabroff KR, Alfano CM, Jemal A, Kramer JL, Siegel RL. Cancer treatment and survivorship statistics, 2016. CA. Cancer J Clin. 2016;66:271–289. doi: 10.3322/caac.21349. [DOI] [PubMed] [Google Scholar]
- Mohamed GA, Al-Abd AM, El-Halawany AM, Abdallah HM, Ibrahim SRM. New xanthones and cytotoxic constituents from Garcinia mangostana fruit hulls against human hepatocellular, breast, and colorectal cancer cell lines. J Ethnopharmacol. 2017;198:302–312. doi: 10.1016/j.jep.2017.01.030. [DOI] [PubMed] [Google Scholar]
- Mohamed GA, Ibrahim SRM, Shaaban MIA, Ross SA. Mangostanaxanthones I and II, new xanthones from the pericarp of Garcinia mangostana. Fitoterapia. 2014;98:215–221. doi: 10.1016/j.fitote.2014.08.014. [DOI] [PubMed] [Google Scholar]
- Ovalle-Magallanes B, Eugenio-Pérez D, Pedraza-Chaverri J. Medicinal properties of mangosteen (Garcinia mangostana L.): a comprehensive update. Food Chem Toxicol. 2017;109:102–122. doi: 10.1016/j.fct.2017.08.021. [DOI] [PubMed] [Google Scholar]
- Pan B, Zhong W, Deng Z, Lai C, Chu J, Jiao G, Liu J, Zhou Q. Inhibition of prostate cancer growth by solanine requires the suppression of cell cycle proteins and the activation of ROS/P38 signaling pathway. Cancer Med. 2016;5:3214–3222. doi: 10.1002/cam4.916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedrazachaverri J, Cárdenasrodríguez N, Orozcoibarra M, Pérez-Rojas JM. Medicinal properties of mangosteen (Garcinia mangostana) Food Chem Toxicol. 2008;46:3227–3239. doi: 10.1016/j.fct.2008.07.024. [DOI] [PubMed] [Google Scholar]
- Pedraza-Chaverrí J, Reyes-Fermín LM, Nolasco-Amaya EG, Orozco-Ibarra M, Medina-Campos ON, González-Cuahutencos O, Rivero-Cruz I, Mata R. ROS scavenging capacity and neuroprotective effect of α-mangostin against 3-nitropropionic acid in cerebellar granule neurons. Exp Toxicol Pathol. 2009;61:491–501. doi: 10.1016/j.etp.2008.11.002. [DOI] [PubMed] [Google Scholar]
- Phan TKT, Shahbazzadeh F, Pham TTH, Kihara T. Alpha-mangostin inhibits the migration and invasion of A549 lung cancer cells. PeerJ. 2018;6:e5027. doi: 10.7717/peerj.5027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reck M., Popat S., Reinmuth N., De Ruysscher D., Kerr K.M., Peters S. Metastatic non-small-cell lung cancer (NSCLC): ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Annals of Oncology. 2014;25:iii27–iii39. doi: 10.1093/annonc/mdu199. [DOI] [PubMed] [Google Scholar]
- Sánchez-Pérez Yesennia, Morales-Bárcenas Rocío, García-Cuellar Claudia M., López-Marure Rebeca, Calderon-Oliver Mariel, Pedraza-Chaverri José, Chirino Yolanda I. The α-mangostin prevention on cisplatin-induced apoptotic death in LLC-PK1 cells is associated to an inhibition of ROS production and p53 induction. Chemico-Biological Interactions. 2010;188(1):144–150. doi: 10.1016/j.cbi.2010.06.014. [DOI] [PubMed] [Google Scholar]
- Shih YW, Chien ST, Chen PS, Lee JH, Wu SH, Yin LT. α-Mangostin suppresses phorbol 12-myristate 13-acetate-induced MMP-2/MMP-9 expressions via αvβ3 integrin/FAK/ERK and NF-κB signaling pathway in human lung adenocarcinoma A549 cells. Cell Biochem Biophys. 2010;58:31–44. doi: 10.1007/s12013-010-9091-2. [DOI] [PubMed] [Google Scholar]
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA. Cancer J Clin. 2016;66:7–30. doi: 10.3322/caac.21332. [DOI] [PubMed] [Google Scholar]
- Tan B, Huang Y, Lan L, Zhang B, Ye L, Yan W, Wang F, Lin N. Bruceine D induces apoptosis in human non-small cell lung cancer cells through regulating JNK pathway. Biomed Pharmacother. 2019;117:109089. doi: 10.1016/j.biopha.2019.109089. [DOI] [PubMed] [Google Scholar]
- Tao M, Jiang J, Wang L, Li Y, Mao Q, Dong J, Zuo J (2018) α-Mangostin alleviated lipopolysaccharide induced acute lung injury in rats by suppressing NAMPT/NAD controlled inflammatory reactions. Evid Based Complement Alternat Med 2018:5470187 [DOI] [PMC free article] [PubMed]
- Wang T, Nelson RA, Bogardus GFW., Jr Five-year lung cancer survival. Which advanced stage non-small cell lung cancer patients attain long-term survival. Cancer. 2010;116:1518–1525. doi: 10.1002/cncr.24871. [DOI] [PubMed] [Google Scholar]
- Wang L, Zhao D, Qin K, Rehman FUL, Zhang X. Effect and biomarker of nivolumab for non–small-cell lung cancer. Biomed Pharmacother. 2019;117:109199. doi: 10.1016/j.biopha.2019.109199. [DOI] [PubMed] [Google Scholar]
- Watanapokasin R, Jarinthanan F, Jerusalmi A, Suksamrarn S, Nakamura Y, Sukseree S, Uthaisang-Tanethpongtamb W, Ratananukul P, Sano T. Potential of xanthones from tropical fruit mangosteen as anti-cancer agents: caspase-dependent apoptosis induction in vitro and in mice. Appl Biochem Biotechnol. 2010;162:1080–1094. doi: 10.1007/s12010-009-8903-6. [DOI] [PubMed] [Google Scholar]
- Wu XJ, Kassie F, Mersch-Sundermann V. The role of reactive oxygen species (ROS) production on diallyl disulfide (DADS) induced apoptosis and cell cycle arrest in human A549 lung carcinoma cells. Mutat Res. 2005;579:115–124. doi: 10.1016/j.mrfmmm.2005.02.026. [DOI] [PubMed] [Google Scholar]
- Xue C, Hu Z, Jiang W, Zhao Y, Xu F, Huang Y, Zhao H, Wu J, Zhang Y, Zhao L, Zhang J, Chen L, Zhang L. National survey of the medical treatment status for non-small cell lung cancer (NSCLC) in China. Lung Cancer. 2012;77:371–375. doi: 10.1016/j.lungcan.2012.04.014. [DOI] [PubMed] [Google Scholar]
- Yeung Simon. Mangosteen for the Cancer Patient: Facts and Myths. Journal of the Society for Integrative Oncology. 2006;04(03):130. doi: 10.2310/7200.2006.022. [DOI] [PubMed] [Google Scholar]
- Zhang C, Yu G, Shen Y. The naturally occurring xanthone α-mangostin induces ROS-mediated cytotoxicity in non-small-scale lung cancer cells. Saudi J Biol Sci. 2018;25:1090–1095. doi: 10.1016/j.sjbs.2017.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuo J, Dou DY, Wang HF, Zhu YH, Li Y, Luan JJ. Reactive oxygen species mediated NF-κB/p38 feedback loop implicated in proliferation inhibition of HFLS-RA cells induced by 1,7-dihydroxy-3,4-dimethoxyxanthone. Biomed Pharmacother. 2017;94:1002–1009. doi: 10.1016/j.biopha.2017.07.164. [DOI] [PubMed] [Google Scholar]