Abstract
Background
Heavy metals and petroleum hydrocarbon pollution are important environmental problems. This research was conducted to evaluate the effect of nano Fe-oxide and endophytic fungus (P. indica) on petroleum hydrocarbons degradation in an arsenic and petroleum hydrocarbons contaminated soil using barley plant.
Methods
Treatments consisted of the presence (E+) and the absence (E−) of P.indica fungi, soil contaminated with As in the rates of 0 (AS0), 12 (AS12) and 24 (As24) mg As /kg of soil, and application of 0 (Fe0) and 1% (Fe1) (W/W) nano Fe-oxide. The plant used in this study was the barley plant. After 7 weeks, the root and shoot As concentration was measured using atomic absorption spectroscopy. The concentration of total soil petroleum hydrocarbon (TPHS) was measured using GC-mass.
Results
Application of nano Fe-oxide in soil treated with 12 and 24 mg As/kg soil decreased root As concentration by 30 and 20.6%, respectively. The presence of P.indica caused a significant reduction in the shoot As concentration. With increasing shoot Fe concentration the shoot As concentration was decreased. The highest TPHS degradation was observed in non As-polluted soil that containing 1% (W/W) nano Fe-oxide in the presence of P.indica, while the lowest that was in As polluted soil (24 mg As/kg soil) without applying nano Fe-oxide and in the absence of P.indica.
Conclusion
Increasing soil sorption properties due to nano Fe-oxide application had significant effect on TPHS degradation in the presence of P.indica. However the role of soil condition on the amount of TPHS degradation cannot be ignored.
Keywords: Heavy metal, Fungi, Petroleum, Environmental Pollution
Background
Soil contamination and pollutant removal methods which have been paid attention in recent years are important difficulties in environmental studies due to their effect on soil quality and human health. Human is beings at risk due to the consumption of heavy metals contaminated food and water. The use of contaminated food and water by humans, animals, and plants can lead to the accumulation of heavy metals in their body tissues, which makes serious problems.
High concentration of lead (Pb), copper (Cu), zinc (Zn), cadmium (Cd) and arsenic (As) in soil can increase the concentrations of these heavy metals in human food chain and thereby can harm the human health [1]. Organic compounds are also considered as the main pollutants of water and soil. Some of these materials have a medium to high solubility in water, and this property allows them to transfer from particles of the sub-soils and reach to groundwater, enter the human food chain and threaten their health [2].
Soil as a part of the environment is a unique habitat for different organisms. However, contaminated soils are not suitable to use as agricultural land, residential and recreational places. Therefore, removal of soil pollutant is necessary. At present, various methods have been used to reduce water and soil pollution, most of which are time-consuming and costly [3]. Up to now, many studies have been done to identify the mechanism of phyto-remediation and its effect on the removal or reduction of soil pollutants. In recent years, phyto-remediation has been introduced as a new strategy for soil remediation [4]. Awareness of the processes affecting the bioavailability of pollutants can help to increase phyto-remediation efficiency.
Application of organic amendments to soil can reduce the plant available forms of heavy metals through immobilization processes [5]. Baghaie et al., studied the effect of organic amendments application such as cow manure on heavy metals availability and stated that the use of such compounds can increase the soil sorption properties, such as cation exchange capacity (CEC), which plays an important role in reducing the availability of heavy metals [6]. Although the role of other soil physic-chemical properties such as pH in the changes of heavy metals availability could not be ignored [7]. Due to the fact that organic amendments mostly have pH-dependency, increasing soil pH due to the use of these compounds can reduce the soil heavy metals availability, while in many cases the use of some amendments, such as sewage sludge, reduces the local soil pH and leads to chelate heavy metals such as As, Cd, and Pb, and increase their bioavailability [8, 9]. Therefore, the choice of proper amendments that increase soil sorption properties is necessary. Additionally, organic pollutants such as polycyclic aromatic hydrocarbon may be degraded by the enzymes released from the plant’s roots or micro-organisms [7]. In addition, plant root exudate that releases into the rhizosphere can stimulate and increase the population of microorganisms and thereby increasing petroleum hydrocarbon degradation [10].
Today in most industrial areas there is simultaneous contamination of heavy metals and petroleum hydrocarbons, that their interactions can affect their availability. Arsenic (AS) is one of the most important heavy metals that cause different diseases such as skin damages and cancer, osteoporosis and disorders in the nervous system [11]. Therefore, it is necessary to find a suitable strategy to reduce the As availability in soil and plant.
In recent years, the influence of some organic compounds, such as nano oxides on decreasing the heavy metals availability in aquatic environments has been confirmed [12, 13], but the role of such compounds in soil environments has been less studied. On the other hand, some of the soil microorganisms such as endophyte fungi are capable to reduce the toxicity of heavy metals by secretion of different enzymes, storage the pollutants on their body, and using of some pollutant as their nutrients [14, 15].
Barley (Hordeum vulgare L. cv. Makoei) is one of the most important plants in the central regions of Iran, as this crop plant is compatible with arid and semi-arid regions. Additionally, barley has high ability to absorb heavy metals and its cultivation in contaminated soil to heavy metals and petroleum hydrocarbons can influence the plant growth process. So, it is necessary to find a suitable strategy to reduce the soil pollution with heavy metal and petroleum hydrocarbons. Thus this study was conducted to evaluate the effect of nano Fe -oxide and Piriformospora indica (P. indica) fungi on petroleum hydrocarbons degradation and reducing soil As concentration in an As and petrolium-contaminated soil under cultivation of barley plant.
Material and methods
Experimental design
A factorial experiment in the layout of randomized completely block design with the factors of P.indica fungi (the presence (E+) and the absence (E−)), soil contaminated with As in the rates of 0 (AS0), 12 (AS12) and 24 (As24) mg As /kg of soil and application of 0 (Fe0) and 1% (Fe1) (W/W) nano Fe-oxide was conducted with each treatment replicated three times (n = 3) in greenhouse condition.
Soil Preparation and Plant Cultivation
The petroleum polluted soil was collected from the tap 30 cm of land around the Tehran oil refinery. Soil physic-chemical properties are shown in Table 1. Selected soil samples were polluted with As at the mentioned levels and incubated for one month to equilibrium. After that, nano iron-oxide (prepared from the waste products of Mobarakeh Steel Complex which was converted into a nano-size particle) was added to the soil at the rate of 0 and 1% (W/W) and incubated for one month.
Table 1.
Selected soil physic-chemical properties in this research
| Characteristic | Unit | Amount |
|---|---|---|
| Soil texture | – | Sandy Loam |
| pH | – | 7.2 |
| EC | dS/m | 3.4 |
| DTPA- extractable AS | mg kg−1 | ND* |
| DTPA- extractable Cd | mg kg−1 | ND |
| DTPA- extractable Pb | mg kg−1 | ND |
| Organic carbon | % | 0.8 |
| CaCO3 | % | 3 |
*ND: Not detectable by atomic absorption spectroscopy
The studied soil was sterilized by autoclaving (at 121 °C for 45 min) [16] in order to eradicate the indigenous microbial communities. After that, the soil was re-inoculated with indigenous soil bacteria suspension (50 ml/kg soil), which was prepared from an aqueous suspension of the non-sterilized soil (2.5% w/v), filtered twice through Whatman No 1 filter paper [17]. This procedure of soil sterilization and microbial re-inoculation is using to prepare P. indica-free soil for analyses the effects of P.indica fungi. After that, about 5 kg of the soils in each combination of described treatments were filled in each pot and planted to 10 barley seedlings. Thereafter, half of all pots (combination of described treatments) were inoculated with P. indica spores. The spores of the inoculum were extracted by wet-sieving and decanting, and directly pipetted onto the roots of the transplanted barley seedlings. During the plant growth, soil moisture was maintained at the field capacity (FC). After 7 weeks of plant growth, plant roots and stems were harvested. .
The percentage of root colonization by P.indica was quantified using a microscope (100×) after the roots had been washed in a 10% KOH solution and stained in acid fuchsin according to the Liu et al. (2018) [18].
Chemical Analysis
The soil microbial respiration was measured according to the Besalatpour et al. [19]. Accordingly, soil samples of different treatment were incubated for one week at 25 °C in 250-ml glass containers and closed with rubber stoppers. The CO2 produced was trapped in NaOH solution and the excess in alkali was then titrated with HCl. To measure the Pb, Cd, Fe and As concentration in root and shoot of plants, the harvested plant were dried at 75 °C for 72 h and milled, 100 mg aliquots were incinerated at 550 °C for 6 h, and then dissolved in 1 ml of 13 M HNO3 and heated in 220 °C for 1 min. The soil (DTPA-extractable) and plant heavy metal concentrations were determined using atomic absorption spectroscopy [20]. The accuracies of heavy metal analyses were controlled by analyzing certified standard materials and including blanks in digestion batches.
The translocation factor (TF) of As from root to shoot was calculated using the following formula:
| 1 |
Where C shoot and C root are As concentrations in above and below ground plant parts, respectively.
The concentration of some aromatic polycyclic hydrocarbons (PAHs) and the total concentration of soil hydrocarbon (TPHS) were determined using the GC-mass with a Delsi DI 200 chromatograph equipped with a direct injection port and an FID detector at 340 °C 17) (Table 2). Catalase enzyme activity (CAT, EC 1.11.1.16) was determined as described by Khanna (2019) [21].
Table 2.
The concentration of some polycyclic aromatic hydrocarbons in the studied soil
| PAHs | mg/ kg soil |
|---|---|
| Phenanthrene | 43.2 |
| Anthracene | 1.5 |
| Benzo[k]fluoranthene | 0.8 |
| Pyrene | 22.5 |
| Benzo[g,h,i]perylene | 8,4 |
| Benz[a] pyrene | 0.65 |
| Naphthalene | 52.3 |
| Fluoranthene | 32.4 |
| TPHS | 55,348 |
Statistical Analysis
The statistical analyses of data were performed using the ANOVA procedure. Differences between means were evaluated using the least significant difference (LSD test). The 0.05 probability value was used to determine the significant difference.
Results and discussion
Based on the results of the Mobarakeh Steel Complex laboratory, about 73.5% of the nano-Fe oxide is Fe2O3 (Table 3). It should be noted that the concentration of heavy metals in this powder was not detectable by atomic absorption spectroscopy (AAS). On the other hand, the application of nano-Fe oxide has no significant effect on soil pH and soil electrical conductivity (EC).
Table 3.
Percentage of nano-Fe oxide components used in this study
| Element | Fe2O3 | FeO | CaO | SiO2 | MgO | Al2O3 | P2O5 | MnO | ZnO | V2O5 | S | Na2O | K2O |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Amount | 73.50 | 12.25 | 10.22 | 1.63 | 0.15 | 0.11 | 0.23 | 1.21 | 0.03 | 0.11 | 0.11 | 0.10 | 0.35 |
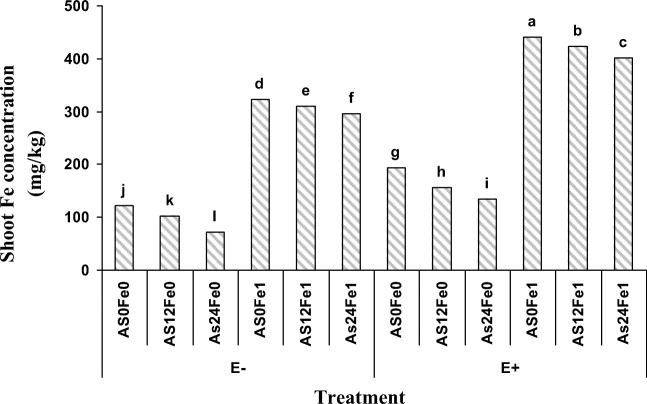
The highest shoot Fe concentration has belonged to the plants cultivated in the non As-polluted soil containing 1% (W/W) nano Fe-oxide in the presence of P. indica, while the least shoot Fe concentration was in As polluted soil (24 mg As/ kg soil) without applying nano Fe-oxide and in the absence of P.indica (Fig. 1).
Fig. 1.
Effect of nano Fe-oxide, As and P.indica on the shoot Fe concentration in a petroleum hydrocarbon polluted soil. As0, As12 and As24 are 0, 12 and 24 mg As/kg soil, Fe0 and Fe1 are 0 and 1% (W/W) nano-Fe oxide, E− and E+ indicate the presence and absence of P.indica, respectively
Presence of P.indica in the As polluted soil treated with nano Fe-oxide has played an important role on increasing the shoot Fe concentration, as the P.indica presence in the As polluted soil (12 mg As/kg soil) that containing 1% (W/W) nano Fe-oxide increased the shoot Fe concentration by 27% (Fig. 1). Increasing soil contamination with As decreased shoot Fe concentration. With increasing soil As level from 12 to 24 mg/kg in soil which nano Fe-oxide was added and that contained P.indica the shoot Fe concentration was decreased by 40.5%. However, the only 8% shoot Fe concentration was decreased in the presence of P.indica in that treatment.
Application of nano-Fe oxide has shown a significant increase in shoot Fe concentration. Application of 1% (W/W) nano Fe-oxide in the soil polluted with 12 mg As/kg (in the absence of P.indica) caused a 3 times increasing in the shoot Fe concentration by, while in soil which contains P.indica it was increased by 4.1 times. Ebrahimi et al., investigated the effect of humic organic material and mycorrhizal fungi on iron and zinc concentration in soybean and concluded that the presence of mycorrhizal fungi has an effective role on plant Fe and Zn uptake [22]. Although, element uptake efficiency can be dependent on plant physiology [23]. Jahandideh Mahjen Abadi et al. studied the effect of P.indica fungus inoculation on uptake and transportation of some nutrients in two wheat cultivars and revealed that inoculation of P.indica with plant roots has an important role in increasing the nutrients uptake [24].
The highest barley root As concentration was observed in the presence of P.indica in soil contaminated with 24 mg As/ kg soil which contains 1% (W/W) nano Fe-oxide (E+ Fe1As24), while the lowest root As concentration has belonged to the plant grown in soil contaminated with 12 mg of mg As/ kg soil and treated with 1% (W/W) nano Fe-oxide in the absence of P.indica (E−Fe0As12). As concentration in non-polluted soil was not detectable by atomic absorption spectroscopy (ASS) (Table 4).
Table 4.
Effect of nano Fe-oxide, As and P.indica on root and shoot As concentration (mg/kg soil) in barley plant in a petroleum hydrocarbon polluted soil
| Treatment | E−Fe0 | E−Fe1 | E+Fe0 | E+Fe1 | ||||
|---|---|---|---|---|---|---|---|---|
| Root | Shoot | Root | Shoot | Root | Shoot | Root | Shoot | |
| As0 | ND | ND | ND | ND | ND | ND | ND | ND** |
| As12 | 5.6g | 4.3c | 4.3h | 2.70e | 7.1e | 2.4f | 10.1c | 2.02g* |
| As24 | 8.7d | 7.1a | 6.9f | 4.8b | 15.5b | 4.03d | 20.3a | 2.4f |
As0, As12 and As24 are 0, 12 and 24 mg As /kg soil, Fe0 and Fe1 are 0 and 1% (W/W) nano-Fe oxide, E− and E+ indicate the presence and absence of P.indica, respectively. *Data with the similar letters in each parameter are not significantly different (P = 0.05, LSD test). **ND: Not detectable by atomic absorption spectroscopy
Application of 1% (W/W) nano Fe-oxide in soil treated with 12 and 24 mg As/kg soil decreased root As concentration by 30 and 20.6%, respectively, that maybe related to the increasing of soil sorption properties and thereby decreasing soil As availability. Mansouri et al. evaluated the effects of hematite nanoparticles on soil As immobilization and concluded that application of these products can decrease soil As concentration due to its high specific area [25]. The results of Bagherifam et al. confirm our results clearly [26].
On the other hand, the presence of P.indica caused a significant increase in the root As concentration, as the highest root As concentration was observed in the soil contaminated with 24 mg of As and in the presence of P.indica (E+Fe1As24). The lowest shoot As concentration was observed in the presence of P.indica in the soil contaminated with 12 mg As/kg soil that treated with 1% (W/W) nano Fe-oxide (E + Fe1As12), while the highest of that was measured in the absence of P.indica in polluted soil (24 mg As/kg soil) without receiving nano Fe-oxide (E−Fe0As24).
According to the results of this study, with increasing Fe concentration in plant the As concentration was decreased, which may be attributed to the competitive effect of As and Fe, which is a positive point in environmental studies. On the other hand, with increasing plant Fe concentration, the plant’s yield increased and thereby the Fe sorption by the plant increased. Principally, iron nanoparticles can release Fe in the soil solution due to their high specific surface, high reactivity and high mobility in the soil. On the other hand, due to their small size and high penetration through the cell membrane plant Fe uptake increases and simultaneously the As uptake decreases [27]. Although the role of soil physic-chemical properties on the changes of As availability could not be ignored. Ahmad et al. studied the model for heavy metal remediation from soil amended with petroleum wastewater by rye-grass L and concluded that the remediation efficiency of Pb, Zn, Ni, and Hg by this plant was 89.5, 72.1, 57,3 and 32.4, respectively [28].
Mansouri et al. by investigating the effect of AS on Fe concentration in soil and corn plant revealed that increasing soil As concentrations reduced plant Fe uptake [29]. Shaybur et al. studied the interaction of As and Fe in barley plants grown in hydroponic media and reported that by increasing the shoot As concentration up to 33.5 μmol /L, the shoot Fe concentration of barley plants was significantly decreased [30]. Mostafa et al. expressed that using of quartz-coated iron oxide is a suitable way for As removal from aqueous solution [31].
Lee et al. investigated the role of Fe-enriched industrial waste on As concentration in lettuce and concluded that the use of Fe-compounds is an effective way to reduce the plant As concentration. In addition, they mentioned that soil physic-chemical properties such as pH can effect on soil As availability considerably [32]. The results of Lee et al. (2001) on the role of heavy metal immobilization in soil by industrial iron wastes confirm our results clearly. Mayo et al. (2007) also showed that As can adsorb on hydroxides surface, metal oxides and clay minerals. They mentioned that reducing the metal oxides from 300 to 12 nm decreased the heavy metals availability by 200 times that are related to increase of surface area [33].
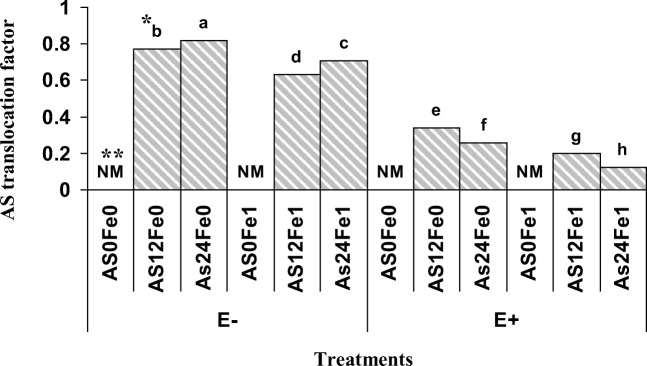
The highest TF value was recorded for the plant grown in soil polluted with24 mg As/kg soil, without receiving nano Fe-oxide and in the absence of P.indica (E−Fe0As24) (Fig. 2), while the lowest As TF value was observed for plants grown in soil to which the highest amount of As (24 mg As/kg soil) was applied and to which nano Fe-oxide was added and that contained P.indica (E+ Fe1As24). Based on the results, the presence of P.indica reduced the As TF value (Fig. 2), as the presence of P.indica in As polluted soil (24 mg As/kg soil) with 1% nano Fe-oxide reduced the TF value by 0.56 units.
Fig. 2.
Effect of nano Fe-oxide, As and P.indica on As translocation factor in a petroleum hydrocarbon polluted soil. As0, As12 and As24 are 0, 12 and 24 mg As /kg soil, Fe0 and Fe1 are 0 and 1% (W/W) nano-Fe oxide, E− and E+ indicate the presence and absence of P.indica, respectively. *Columns with the similar letters are not significantly different (P = 0.05, LSD test) **NM: not measured
Shahabivand et al., investigated the effect of P.indica on Cd accumulated in wheat plants and reported that the Cd TF was decreased in the presence of P.indica [34]. However, in their research, the Cd concentration in the aerial part of plants has been increased with increasing soil pollution that confirms our results. The remarkable point is that plant growth condition is an effective factor on heavy metals translocation to aerial part of plant. In this regard Ahmad et al. showed that the plant As concentrations and the bio-concentration factors were higher in the field experiment than those in the laboratory experiment. However, the element type has important role on TF value [35]. Furthermore, Ahmad el al. reported that the aquatic plants were most efficient in transferring metals into the biomass of plant compared with terrestrial plant samples [36].
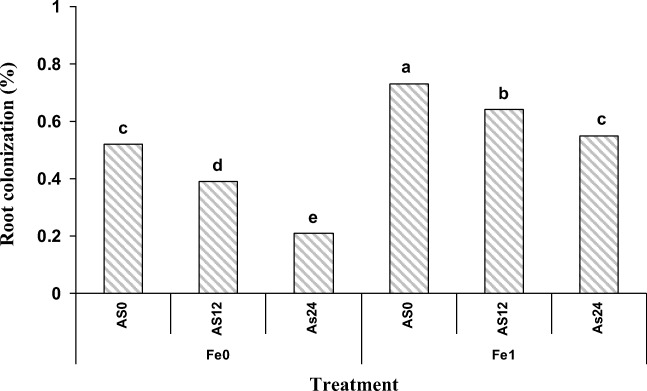
The highest root colonization was observed in As non –polluted soil containing 1% nano Fe-oxide, while the lowest of that was in As polluted soil (24 mg/kg soil) without applying nano Fe-oxide. With increasing the As level from 0 to 24 mg As/kg soil a significant decreasing in root colonization was observed in the soils treated with and without nano Fe-oxide by 0.18% and 0.31%, respectively (Fig. 3), that is attributed to the negative effect of As toxicity on the rate of spore germination or the growth of fungal mycelium. However, the cell wall of fungi is one of the main reasons of preventing heavy metals entry into the plant and simultaneously with the destruction of the cell wall, due to the toxicity of heavy metals, tolerance to the stress of heavy metals decreases [37]. The effect of heavy metal toxicity on plant growth has been reported by many researchers, due to the decrease in photosynthesis and nutrition uptake [38, 39].
Fig. 3.
Effect of nano Fe-oxide, As and P.indica on root colonization in a petroleum hydrocarbon polluted soil. As0, As12 and As24 are 0, 12 and 24 mg As /kg soil, Fe0 and Fe1 are 0 and 1% (W/W) nano-Fe oxide, respectively. Columns with the similar letters are not significantly different (P = 0.05, LSD test)
Based on the results of this study, the presence of P.indica had a significant effect on root dry weight. The highest root dry weight was observed in the As non-polluted soil treated with nano Fe-oxide in the presence of P.indica (E+Fe1As0) (Table 5). The lowest root dry weight has belonged to the plant cultivated in As polluted soil (24 mg As/kg soil) without addition of nano Fe-oxide in the absence of P.indica. The presence of P.indica plays an important role in increasing root dry weight, as, the presence of P.indica in As polluted soil (12 mg As/kg soil) treated with 1% (W/W) nano Fe-oxide increased the root dry weight by 31%. Similar results were observed for shoot dry weight (Table 5). Hosseini et al. investigated the effect of P. indica on physiological characteristics and root morphology of wheat under combined drought and mechanical stresses and stated that P.indica has an effective role on improving plant growth and yield under plant stress conditions [40]. The results of khanna et al. confirm this matter clearly [21]. Ahmad et al. investigated the remediation rates and translocation of heavy metals from contaminated soil amended with fly-ash through Parthenium hysterophorus and mentioned that it can efficiently reduce the heavy metal pollution in the soil. However, the plant biomass was affected by heavy metal concentration [41]. Biomass can express the tolerance of plants to toxic metals. The results of Ahmad et al. showed that the high length and biomass of rye-grass L is an important reason for their ability to phyto-remediate the metals from contaminated soil, and they introduced that plant as a tolerant plant to abiotic stress [28].
Table 5.
Effect of nano Fe-oxide, As and P.indica on shoot and root plant biomass (g) in a petroleum hydrocarbon polluted soil
| Treatment | E−Fe0 | E−Fe1 | E+Fe0 | E+Fe1 | ||||
|---|---|---|---|---|---|---|---|---|
| Root | Shoot | Root | Shoot | Root | Shoot | Root | Shoot | |
| As0 | 6.6e | 0.72j | 7.1c | 1.11g | 7.4b | 1.24d | 8.1a | 1.42a* |
| As12 | 5.3h | 0.54k | 6.3f | 0.95h | 6.9 d | 1.21e | 7.4b | 1.38b |
| As24 | 4.6i | 0.41l | 5.5g | 0.82i | 6.4f | 1.14f | 7.0c | 1.30c |
As0, As12 and As24 are 0, 12 and 24 mg As /kg soil, Fe0 and Fe1 are 0 and 1% (W/W) nano-Fe oxide, E− and E+ indicate the presence and absence of P.indica, respectively. *Data with the similar letters in each parameter are not significantly different (P = 0.05, LSD test)
The effect of P.indica presence on the TPHS degradation in soil was significant. The highest TPHS degradation was observed in As non-polluted soil that containing 1% (W/W) nano Fe-oxide in the presence of P.indica (E+ Fe1As0), However the lowest TPHS degradation was in As polluted soil (24 mg As/kg soil) without applying nano Fe-oxide and in the absence of P.indica (E−Fe0As24). The presence of P.indica in As polluted soil had a significant effect on TPHS degradation in soil. Increasing soil microbial respiration with reducing the soil TPHS confirms this matter clearly (Table 5). The P.indica mycelium penetration in the root of plants can improve the nutrient uptake by plant and hence has a positive effect on plant growth. In addition, plant root exudates significantly increase the soil microbial activity that helps THPS degradation [42]. It has been observed that symbiosis interaction between plant and fungi activated the plant antioxidant system and thereby the plant tolerance to biotic and abiotic stresses increases [43]. Meanwhile, the use of nano Fe-oxide also contributes to further soil THPS degradation by increasing the soil micro-organism activity.
Aghili et al. (2014) studied the influence of mycorrhizal fungi on plants nutrient uptake under stress conditions and concluded that micro-organisms require a minimum nutrient level for optimal growth under stress condition and the use of zinc compounds can be a useful factor on increasing microbial activity [44], which confirms our results. On the other hand, soil hydrocarbons can be also a good source of energy for P.indica activity [41]. Although, the high amount of petroleum hydrocarbon in soil has a toxic and inhibitory effect on micro-organisms activity [19].
According to the results of this study application of nano Fe-oxide had a significant effect on the soil TPHS degradation that is related to the role of nano Fe-oxide on decreasing soil As availability and consequently reducing the heavy metals toxicity for microorganisms. The reduction of heavy metals toxicity has allowed microorganisms to degradation the TPHS [45]. Samarghandi et al., studied the Cd adsorption on activated carbon granules coated with nano Fe-oxide in aqueous solutions and concluded that coating nano Fe-oxide on active carbon granules could change the chemical and physical properties of activated carbon granules and greatly increases the surface area for Cd adsorption. According to their results, with increasing time, the Cd removal efficiency had increased but the rate of Cd adsorption had been decreased and the high amount of Cd adsorption occurs during the initial time’s process [46].
According to the results of this study, the highest soil microbial respiration was observed in the presence of P.indica in the As non- polluted soil that containing 1% nano Fe-oxide (E+ Fe1As0) (Table 6), while the lowest that was in the soil contains 24 mg As/kg soil without receiving nano Fe-oxide and in the absence of P.indica (E−Fe0As24). The presence of P.indica in the As non-pollutes soil treated with 0 and 1% (w/w) nano Fe-oxide significantly increased soil microbial respiration by 47.6% and 34%, respectively. Regarding the presence or absence of P.indica increasing As contamination cause a significant decreasing in soil microbial respiration. With increasing soil As contamination from 0 to 24 mg/kg soil in soil without receiving nano Fe-oxide and in the absence of P.indica, the soil microbial respiration was decreased by 26.4%.
Table 6.
Effect of nano Fe-oxide, As and P.indica on soil microbial respiration (mg C-CO2/kg soil h) and TPHS degradation (%) in a petroleum hydrocarbon polluted soil
| Treatment | E−Fe0 | E−Fe1 | E+Fe0 | E+Fe1 | ||||
|---|---|---|---|---|---|---|---|---|
| Soil microbial respiration | TPHS degradation | Soil microbial respiration | TPHS degradation | Soil microbial respiration | TPHS degradation | Soil microbial respiration | TPHS degradation | |
| As0 | 4.6 h | 39.3 g | 6.4e | 50.3e | 8.6b | 68.1b | 9.4a | 75.3a* |
| As12 | 4.2i | 33.1 h | 5.9f | 44.9f | 8.0c | 62.4c | 8.7b | 69.2b |
| As24 | 3.6j | 28.9i | 5.3 g | 40.3 g | 7.2d | 54.3d | 8.1c | 61.3c |
As0, As12 and As24 are 0, 12 and 24 mg As /kg soil, Fe0 and Fe1 are 0 and 1% (W/W) nano-Fe oxide, E− and E+ indicate the presence and absence of P.indica, respectively. *Data with the similar letters in each parameter are not significantly different (P = 0.05, LSD test)
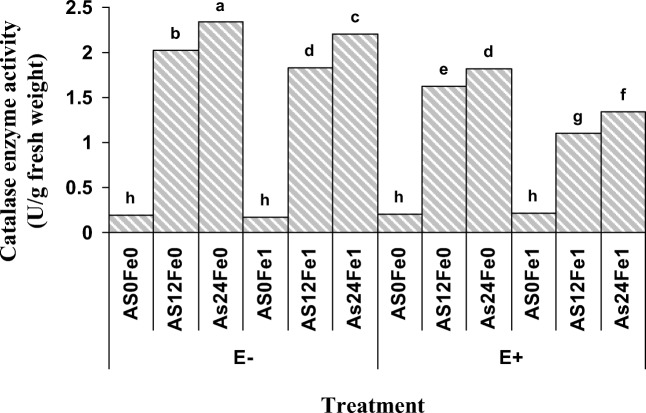
The highest catalase enzyme activity was belonged to the plants grown in the soil which was not contained P.indica and contaminated with As (24 mg As/kg soil) without receiving nano Fe- oxide (E−Fe0As24), while the lowest of that was observed in As non-polluted soil containing nano Fe-oxide in the presence of P.indica (E+Fe1As0) (Fig. 4). It should be noted that application of nanoFe- oxide or the presence of P.indica had no significant effect on catalase enzyme activity in As non-polluted soil which shows that catalase enzyme activity is highly dependent on environmental stresses such as heavy metals contamination [47].
Fig. 4.
Effect of nano Fe-oxide, As and P.indica on catalase enzyme activities in a petroleum hydrocarbon polluted soil. As0, As12 and As24 are 0, 12 and 24 mg As /kg soil, Fe0 and Fe1 are 0 and 1% (W/W) nano-Fe oxide, respectively. Columns with the similar letters are not significantly different (P = 0.05, LSD test)
With increasing As concentration from 12 to 24 mg AS/Kg of soil that was not received nano Fe-oxide and in the absence of P. indica the activity of catalase enzyme was increased by 2.1 units.
Hashemi et al., studied the antioxidant enzymes activity under Cd stress and concluded that the increase in antioxidant activity depends on the Cd concentration. In addition, their findings showed that the activity of antioxidant enzymes had increased when the Cd concentration was above 12.5 mM [48]. Increasing the antioxidant enzyme capacity is a general response to toxic heavy metals. Antioxidant enzymes are the most important compounds in preventing oxidative stress in plants [49].
Conclusion
The results of this study showed that using nano Fe-oxide has been able to reduce the plant As concentration, while the Plant Fe concentration has been increased,as applying 1% (W/W) nano Fe-oxide significantly increased and decreased the shoot Fe and As concentration by nearly 3 and 1.7 times, respectively. On the other hand, the presence of P.indica has been increased the As accumulation in the barley roots, but the TF has been shown a decreasing trend, which can be considered as an important point in environmental studies. In addition, the presence of P.indica played an important role in soil TPHS degradation. In this way, P.indica probably has been able to use petroleum hydrocarbon as a carbon source to decompose it. Accordingly, the presence of P.indica in soil containing 1% (W/W) nano Fe-oxide significantly increased the TPHS degradation by 33%. Despite of this, physic-chemical properties of soil such as pH can play an important role in the rate of petroleum degradation that needs to be investigated. Furthermore, it is also necessary to study the presence of P.indica on removal the other heavy metals from the contaminated soils in the future studies.
Acknowledgements
The authors would like to gratitude Islamic Azad University, Arak Branch for providing access to the laboratory facilities to perform this study.
Abbreviations
- TF
Translocation Factor
- TPHS
Total concentration of soil Petroleum Hydrocarbons
- As
(Arsenic)
- (Pb)
Lead
- (Cu)
copper
- (Zn)
zinc
- (Cd)
cadmium
- (As)
arsenic
Authors’ contributions
Amir Hossein Baghaie and Aminollah Aghilizefreei designed and performed the experiments, analyzed the data and wrote and approved the final manuscript.
Funding
No funding was received but this work was supported by Islamic Azad University, Arak Branch,.
Compliance with ethical standards
Ethics approval and consent to participate
Not applicable.
Conflict of interest
The authors declare that they have no competing interests.
Consent for publication
Not applicable.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Wu S, Zhou S, Bao H, Chen D, Wang C, Li B, et al. Improving risk management by using the spatial interaction relationship of heavy metals and PAHs in urban soil. J Hazard Mater. 2019;364:108–116. doi: 10.1016/j.jhazmat.2018.09.094. [DOI] [PubMed] [Google Scholar]
- 2.Soleimani M. Transport of polycyclic aromatic hydrocarbons in a calcareous wetland soil. Caspian J Environ Sci. 2013;11(2):131–140. [Google Scholar]
- 3.Li X, Li Y, Zhang X, Zhao X, Sun Y, Weng L, et al. Long-term effect of biochar amendment on the biodegradation of petroleum hydrocarbons in soil microbial fuel cells. Sci Total Environ. 2019;651:796–806. doi: 10.1016/j.scitotenv.2018.09.098. [DOI] [PubMed] [Google Scholar]
- 4.Cameselle C, Gouveia S. Phytoremediation of mixed contaminated soil enhanced with electric current. J Hazard Mater. 2019;361:95–102. doi: 10.1016/j.jhazmat.2018.08.062. [DOI] [PubMed] [Google Scholar]
- 5.Nzediegwu C, Prasher S, Elsayed E, Dhiman J, Mawof A, Patel R. Effect of biochar on heavy metal accumulation in potatoes from wastewater irrigation. J Environ Manag. 2019;232:153–164. doi: 10.1016/j.jenvman.2018.11.013. [DOI] [PubMed] [Google Scholar]
- 6.Baghaie A, Khoshgoftarmanesh AH, Afyuni M, Schulin R. The role of organic and inorganic fractions of cow manure and biosolids on lead sorption. Soil Sci Plant Nutr. 2011;57(1):11–18. doi: 10.1080/00380768.2010.548309. [DOI] [Google Scholar]
- 7.Soleimani M, Afyuni M, Hajabbasi MA, Nourbakhsh F, Sabzalian MR, Christensen JH. Phytoremediation of an aged petroleum contaminated soil using endophyte infected and non-infected grasses. Chemosphere. 2010;81(9):1084–1090. doi: 10.1016/j.chemosphere.2010.09.034. [DOI] [PubMed] [Google Scholar]
- 8.Rashid I, Murtaza G, Zahir ZA, Farooq M. Effect of humic and fulvic acid transformation on cadmium availability to wheat cultivars in sewage sludge amended soil. Environ Sci Pollut Res. 2018:1–9. [DOI] [PubMed]
- 9.Jiang M, Liu S, Li Y, Li X, Luo Z, Song H, et al. EDTA-facilitated toxic tolerance, absorption and translocation and phytoremediation of lead by dwarf bamboos. Ecotox Environ Safe. 2019;170:502–512. doi: 10.1016/j.ecoenv.2018.12.020. [DOI] [PubMed] [Google Scholar]
- 10.Jednak T, Avdalović J, Miletić S, Slavković-Beškoski L, Stanković D, Milić J, et al. Transformation and synthesis of humic substances during bioremediation of petroleum hydrocarbons. Int Biodeterior Biodegradation. 2017;122:47–52. doi: 10.1016/j.ibiod.2017.04.009. [DOI] [Google Scholar]
- 11.Bhowmick S, Pramanik S, Singh P, Mondal P, Chatterjee D, Nriagu J. Arsenic in groundwater of West Bengal, India: a review of human health risks and assessment of possible intervention options. Sci Total Environ. 2018;612:148–169. doi: 10.1016/j.scitotenv.2017.08.216. [DOI] [PubMed] [Google Scholar]
- 12.Vilardi G, Mpouras T, Dermatas D, Verdone N, Polydera A, Di Palma L. Nanomaterials application for heavy metals recovery from polluted water: The combination of nano zero-valent iron and carbon nanotubes. Competitive adsorption non-linear modeling. Chemosphere. 2018;201:716–729. doi: 10.1016/j.chemosphere.2018.03.032. [DOI] [PubMed] [Google Scholar]
- 13.Abebe B, Murthy HA. Fe-Oxide Nanomaterial: Synthesis, Characterization and Lead Removal. J Encapsulation Adsorp Sci. 2018;8:195–209. doi: 10.4236/jeas.2018.84010. [DOI] [Google Scholar]
- 14.Zamani J, Hajabbasi MA, Alaie E, Sepehri M, Leuchtmann A, Schulin R. The effect of Piriformospora indica on the root development of maize (Zea mays L.) and remediation of petroleum contaminated soil. Int J Phytoremediation. 2016;18(3):278–287. doi: 10.1080/15226514.2015.1085831. [DOI] [PubMed] [Google Scholar]
- 15.Zamani J, Hajabbasi MA, Mosaddeghi MR, Soleimani M, Shirvani M, Schulin R. Experimentation on Degradation of Petroleum in Contaminated Soils in the Root Zone of Maize (Zea Mays L.) Inoculated with Piriformospora Indica. Soil Sed Contam. 2018;27(1):13–30. doi: 10.1080/15320383.2018.1422693. [DOI] [Google Scholar]
- 16.Trevors J. Sterilization and inhibition of microbial activity in soil. J Microbiol Method. 1996;26(1–2):53–59. doi: 10.1016/0167-7012(96)00843-3. [DOI] [Google Scholar]
- 17.Aghili F, Gamper HA, Eikenberg J, Khoshgoftarmanesh AH, Afyuni M, Schulin R, et al. Green manure addition to soil increases grain zinc concentration in bread wheat. PLoS One. 2014;9(7):e101487. doi: 10.1371/journal.pone.0101487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liu L, Li J, Yue F, Yan X, Wang F, Bloszies S, et al. Effects of arbuscular mycorrhizal inoculation and biochar amendment on maize growth, cadmium uptake and soil cadmium speciation in Cd-contaminated soil. Chemosphere. 2018;194:495–503. doi: 10.1016/j.chemosphere.2017.12.025. [DOI] [PubMed] [Google Scholar]
- 19.Besalatpour A, Hajabbasi M, Khoshgoftarmanesh A, Dorostkar V. Landfarming process effects on biochemical properties of petroleum-contaminated soils. Soil Sediment Contam. 2011;20(2):234–248. doi: 10.1080/15320383.2011.546447. [DOI] [Google Scholar]
- 20.Mansouri T, Golchin A, Babaakbari SIM. The Effect of Arsenic on Phosphorus, Iron, Zinc and Manganese Concentrations in Soil and Corn Plant. J Water Soil. 2017;31(2):627–643. [Google Scholar]
- 21.Khanna K, Jamwal VL, Kohli SK, Gandhi SG, Ohri P, Bhardwaj R, et al. Plant growth promoting rhizobacteria induced Cd tolerance in Lycopersicon esculentum through altered antioxidative defense expression. Chemosphere. 2019;217:463–474. doi: 10.1016/j.chemosphere.2018.11.005. [DOI] [PubMed] [Google Scholar]
- 22.Ebrahimi Z, Sarcheshmehpour M, Hejazi MM. The effects of humic substances and mycorrhiza fungus on Fe and Zn uptake and some soybean growth characteristics under greenhouse conditions. J Sci Technol Greenhouse Culture. 2016;7(1):99–110. doi: 10.18869/acadpub.ejgcst.7.1.99. [DOI] [Google Scholar]
- 23.Singh AK, Hamel C, DePauw RM, Knox RE. Genetic variability in arbuscular mycorrhizal fungi compatibility supports the selection of durum wheat genotypes for enhancing soil ecological services and cropping systems in Canada. Can J Microbiol. 2012;58(3):293–302. doi: 10.1139/w11-140. [DOI] [PubMed] [Google Scholar]
- 24.Jahandideh mahjen abadi va, sepehri m. effect of Piriformospora indica fungus inoculation on uptake and transportation of some nutrients in two wheat cultivars. J Soil Manage Sustain Product. 2014; 4(3):155–73.
- 25.Mansouri T, Golchin A, Babaakbari Sari M, Ahmadi S. Reduction of arsenic mobilization in soil by applicationof hematite nanoparticles and acrylic polymers. J Water Soil Conserv. 2017;23:79–99. [Google Scholar]
- 26.Bagherifam S, Lakzian A, Fotovat A, Khorasani R, Komarneni S. In situ stabilization of As and Sb with naturally occurring Mn, Al and Fe oxides in a calcareous soil: bioaccessibility, bioavailability and speciation studies. J Hazard Mater. 2014;273:247–252. doi: 10.1016/j.jhazmat.2014.03.054. [DOI] [PubMed] [Google Scholar]
- 27.Casentini B, Michele G, Baldi F. Arsenate and arsenite removal from contaminated water by iron oxides nanoparticles formed inside a bacterial exopolysaccharide. J Environ Chem Eng. 2019;7:1–8. doi: 10.1016/j.jece.2019.102908. [DOI] [Google Scholar]
- 28.Ahmad A, Sreedhar Reddy S, Rumana G. Model for bioavailability and metal reduction from soil amended with petroleum wastewater by rye-grass L. Int J Phytoremediation. 2019;21(5):471–478. doi: 10.1080/15226514.2018.1537243. [DOI] [PubMed] [Google Scholar]
- 29.Mansouri T, Golchin A, Baba Akbari Sari M. The Effect of Arsenic on Phosphorus, Iron, Zinc and Manganese Concentrations in Soil and Corn Plant. J Water Soil. 2017;31:627–643. [Google Scholar]
- 30.Shaibur MR, Kitajima N, Huq SI, Kawai S. Arsenic–iron interaction: Effect of additional iron on arsenic-induced chlorosis in barley grown in water culture. Soil Sci Plant Nutr. 2009;55(6):739–746. doi: 10.1111/j.1747-0765.2009.00414.x. [DOI] [Google Scholar]
- 31.Mostafa M, Chen Y-H, Jean J-S, Liu C-C, Teng H. Adsorption and desorption properties of arsenate onto nano-sized iron-oxide-coated quartz. Water Sci Technol. 2010;62(2):378–386. doi: 10.2166/wst.2010.288. [DOI] [PubMed] [Google Scholar]
- 32.Lee S-H, Kim EY, Park H, Yun J, Kim J-G. In situ stabilization of arsenic and metal-contaminated agricultural soil using industrial by-products. Geoderma. 2011;161(1–2):1–7. doi: 10.1016/j.geoderma.2010.11.008. [DOI] [Google Scholar]
- 33.Mayo J, Yavuz C, Yean S, Cong L, Shipley H, Yu W, et al. The effect of nanocrystalline magnetite size on arsenic removal. Sci Technol Adv Mater. 2007;8(1–2):71. doi: 10.1016/j.stam.2006.10.005. [DOI] [Google Scholar]
- 34.Shahabivand S, Parvaneh A, Aliloo AA. Root endophytic fungus Piriformospora indica affected growth, cadmium partitioning and chlorophyll fluorescence of sunflower under cadmium toxicity. Ecotox Environ Safe. 2017;145:496–502. doi: 10.1016/j.ecoenv.2017.07.064. [DOI] [PubMed] [Google Scholar]
- 35.Ahmad A, Ghufran R, Wahid ZA. Metals Cd, As, Cu, and Zn transfer through dry to rehydrated biomass of Spirulina Platensis from wastewater. Pol J Environ Stud. 2010;19(5):674–682. [Google Scholar]
- 36.Ahmad A, Ghufran R, Zularisam A. Phytosequestration of metals in selected plants growing on a contaminated Okhla industrial areas, Okhla, New Delhi. India Water Air Soil Pollut. 2011;217(1–4):255–266. doi: 10.1007/s11270-010-0584-9. [DOI] [Google Scholar]
- 37.Zhao D, Li T, Wang J, Zhao Z. Diverse strategies conferring extreme cadmium (Cd) tolerance in the dark septate endophyte (DSE), Exophiala pisciphila: evidence from RNA-seq data. Microbiol Res. 2015;170:27–35. doi: 10.1016/j.micres.2014.09.005. [DOI] [PubMed] [Google Scholar]
- 38.Shri M, Singh PK, Kidwai M, Gautam N, Dubey S, Verma G, et al. Recent advances in arsenic metabolism in plants: current status, challenges and highlighted biotechnological intervention to reduce grain arsenic in rice. Metallomics. 2019. [DOI] [PubMed]
- 39.Pandey AK, Gautam A, Dubey RS. Transport and detoxification of metalloids in plants in relation to plant-metalloid tolerance. Plant Gene. 2019;100171.
- 40.Hosseini F, Mosaddeghi MR, Dexter AR. Effect of the fungus Piriformospora indica on physiological characteristics and root morphology of wheat under combined drought and mechanical stresses. Plant Physiol Biochem. 2017;118:107–120. doi: 10.1016/j.plaphy.2017.06.005. [DOI] [PubMed] [Google Scholar]
- 41.Ahmad A, Al-Othman AA. Remediation rates and translocation of heavy metals from contaminated soil through Parthenium hysterophorus. Chem Ecol. 2014;30(4):317–327. doi: 10.1080/02757540.2013.871269. [DOI] [Google Scholar]
- 42.Bagde US, Prasad R, Varma A. Influence of culture filtrate of Piriformospora indica on growth and yield of seed oil in Helianthus annus. Symbiosis. 2011;53(2):83. doi: 10.1007/s13199-011-0114-6. [DOI] [Google Scholar]
- 43.Varma A, Bakshi M, Lou B, Hartmann A, Oelmueller R. Piriformospora indica: a novel plant growth-promoting mycorrhizal fungus. Agr Res. 2012;1(2):117–131. doi: 10.1007/s40003-012-0019-5. [DOI] [Google Scholar]
- 44.Aghili F, Jansa J, Khoshgoftarmanesh AH, Afyuni M, Schulin R, Frossard E, et al. Wheat plants invest more in mycorrhizae and receive more benefits from them under adverse than favorable soil conditions. Appl Soil Ecol. 2014;84:93–111. doi: 10.1016/j.apsoil.2014.06.013. [DOI] [Google Scholar]
- 45.Moreira IT, Oliveira OM, Triguis JA, Queiroz AF, Barbosa RM, Anjos JA, et al. Evaluation of the effects of metals on biodegradation of total petroleum hydrocarbons. Microchem J. 2013;110:215–220. doi: 10.1016/j.microc.2013.03.020. [DOI] [Google Scholar]
- 46.Samarghandi MR, Azizi S. Cadmium Adsorption by Activated Carbon Granules Coated with Iron Nanoparticles from Aqueous Solution: Kinetics, Isotherms and Adsorption Mechanism Studies. J Mazandaran Univer Med Sci. 2014;24:109–121. [Google Scholar]
- 47.Guo J, Qin S, Rengel Z, Gao W, Nie Z, Liu H, et al. Cadmium stress increases antioxidant enzyme activities and decreases endogenous hormone concentrations more in Cd-tolerant than Cd-sensitive wheat varieties. Ecotox Environ Safe. 2019;172:380–387. doi: 10.1016/j.ecoenv.2019.01.069. [DOI] [PubMed] [Google Scholar]
- 48.Hashemi F, Kavousi H, Poorseyedi S. Effect of cadmium toxicity on gene expression and enzyme activity of superoxide dismutase and ascorbate peroxidas in chickpea (Cicer arietinum L.) seedlings. J Agr Biotechnol. 2017;8(4):99–111. [Google Scholar]
- 49.Javadzarin I, Motesharezadeh B, Ahmadi A. Evaluation Activity of some Antioxidant Enzymes under Cadmium Toxicity in Two wheat Cultivars. J Soil Manage Sustain Product. 2018;6(4):21–37. [Google Scholar]