Abstract
As a common therapy for prostate cancer, androgen deprivation therapy (ADT) is effective for the majority of patients. However, prolonged ADT promotes drug resistance and progression to an aggressive variant with reduced androgen receptor signaling, so called neuroendocrine prostate cancer (NEPC). Until present, NEPC is still poorly understood, and lethal with no effective treatments. Elevated expression of neuroendocrine related markers and increased angiogenesis are two prominent phenotypes of NEPC, and both of them are positively associated with cancers progression. However, direct molecular links between the two phenotypes in NEPC and their mechanisms remain largely unclear. Their elucidation should substantially expand our knowledge in NEPC. This knowledge, in turn, would facilitate the development of effective NEPC treatments. We recently showed that a single critical pathway regulates both ADT-enhanced angiogenesis and elevated expression of neuroendocrine markers. This pathway consists of CREB1, EZH2, and TSP1. Here, we seek new insights to identify molecules common to pathways promoting angiogenesis and neuroendocrine phenotypes in prostate cancer. To this end, our focus is to summarize the literature on proteins reported to regulate both neuroendocrine marker expression and angiogenesis as potential molecular links. These proteins, often described in separate biological contexts or diseases, include AURKA and AURKB, CHGA, CREB1, EZH2, FOXA2, GRK3, HIF1, IL-6, MYCN, ONECUT2, p53, RET, and RB1. We also present the current efforts in prostate cancer or other diseases to target some of these proteins, which warrants testing for NEPC, given the urgent unmet need in treating this aggressive variant of prostate cancer.
Keywords: new therapeutics, molecular mechanisms, angiogenesis, neuroendocrine prostate cancer, neuroendocrine phenotype
Introduction
In the United States, prostate cancer is responsible for the second most cancer death in men, behind lung cancer. It is estimated that about 31,620 deaths in 2019 in USA are caused by prostate cancer (www.cancer.org). Androgen deprivation therapies (ADT) that target the androgen receptor (AR) is the main treatment for prostate cancer (1–4). ADT is effective initially. However, the majority of tumors invariably relapse and progress, becoming castration resistant prostate cancer (CRPC) (1–4). Frequently associated with ADT resistance is the emergence of neuroendocrine prostate cancers (NEPC) that have a poor prognosis with no effective treatment (5–8). With the common use of new generation potent ADT into clinic, the incidence of NEPC is rising (6, 9–12).
NEPC are highly vascularized (13, 14). Increased angiogenesis and expression of NE markers are two prominent phenotypes of NEPC (13–16) and are expected to be molecularly linked. However, direct molecular connections between these two phenotypes in NEPC remain largely unclear. The main purpose of this review is to summarize the reported and potential connections between the regulation of increased angiogenesis and expression of NE markers. Further, we analyze the implications of these connections for prostate cancer. Our goal is to identify key regulators of both characteristics as potential targets for NEPC, with the hope of hitting two birds with one stone to achieve better therapeutic efficacy and fewer side effects.
Neuroendocrine Phenotype in Prostate Cancer
Approximately 20% of lethal CRPCs have a neuroendocrine (NE) phenotype, and thus are called NEPC or CRPC-NE (5, 17–19). NEPCs often lose AR signaling, become resistant to ADT, and express NE markers, such as neuron-specific enolase 2 (ENO2), synaptophysin (SYP), chromogranin A and B (CHGA and CHGB) (5–8). Features of NEPC include elevated angiogenesis, high proliferative rates, and metastatic propensity (20). Unfortunately, there is no effective therapy against NEPCs. They respond only transiently to chemotherapy (17, 20–24).
Clinical data, including genomic analyses of metastatic tumors, and preclinical studies suggest an evolution of CRPC-NE from a prostate adenocarcinoma precursor (25–27). Researchers are beginning to unfold the signaling events involved in NEPC development (6, 17, 24). General knowledge of NEPC has been elegantly reviewed (19, 20, 24, 28–32). A number of proteins have been reported to contribute to NEPC progression. These proteins include Aurora kinase A and B, BRN2, CREB1, DEK, EZH2, FOXA2, GRK3, HIF1, IL-6, MYCN, ONECUT2, PEG10, p53, REST, RB1, SRRM4, SOX2 et al. (33–44).
Angiogenesis in Prostate Cancer
As a basic physiological process, angiogenesis usually occurs in embryonic development, tissue repair and fertility to form new blood vessels resulted from the extension of pre-existing vasculature. In addition, angiogenesis is also accompanied by chronic inflammation, tumor growth and metastasis (19). Actually, angiogenesis is a dynamic process involves interaction between endothelial cells and their extracellular environment. There are two main types of angiogenesis in vivo, including sprouting angiogenesis (sprouting of vascular endothelia from pre-existing capillary endothelia into the surrounding tissues) and non-sprouting angiogenesis (division of pre-existing capillaries by tissue pillars into new daughter vessels) (19, 45–47). The formation of new blood vessels depends on a balanced process that are regulated by many factors (48). Angiogenic activators include angiopoietins, CCL2, EGFL6, endothelins, FGF, HIF1, IGF1, MMPs, PDGF, TGF, VEGF, and et al. (48–56). On the other hand, angiostatin, endostatin, TSP1, and PAI2 are among the endogenous inhibitors of angiogenesis (57–60).
Angiogenesis is involved in prostate cancer survival, progression, and metastasis (61). Its importance in prostate cancer has been established (62, 63). Higher microvessel density is associated with worse prognosis in prostate cancer (64, 65). VEGF as well as some neurosecretory peptides, e.g., serotonin, bombesin, and gastrin, have been shown to boost angiogenesis in NEPC (15). We recently reported that ADT repression of thrombospondin 1 (TSP1 or THBS1), a potent endogenous angiogenesis inhibitor, contributes to angiogenic phenotype in NEPC (66). Several reviews have already described the current knowledge and therapeutic development targeting angiogenesis in prostate cancer (61, 67, 68).
Clinical Correlation of Neuroendocrine Phenotype, Angiogenesis and Prognosis in Prostate Cancer
Several research groups have shown positive correlations between NE marker expression and angiogenesis in prostate tumors. Higher neovascularization and VEGF staining are observed in prostate tumors with more NE tumor cells (16, 69, 70). Grobholz et al. detected NE marker CHGA and angiogenic marker CD34 in 102 prostatectomy prostate tumor specimens. They found that poorer pathological staging correlates with increased neovascularization and stronger NE marker expression (16). Harper et al. found a positive correlation between VEGF and CHGA levels in 45 prostatic carcinoma specimens (67, 70–72). Borre et al. analyzed VEGF and CHGA expression in 221 prostate tumors (62). They found only tumors with strong expression of both VEGF and NE showed significantly poor clinical characteristics such as higher microvessel density, T stage, dedifferentiation, and shorter disease-specific survival.
Proteins and Pathways Regulating Both Ne Phenotype and Angiogenesis
It remains largely unclear whether neuroendocrine differentiation and angiogenesis regulate each other in NEPC. It is also unclear what proteins directly link these two prominent characteristics of NEPC. Our literature search did not yield reports showing direct involvement of pro-angiogenic factors VEGF and neurosecretory peptides (serotonin, bombesin, and gastrin) in promoting NE marker expression. On the other hand, among the NE marker proteins, only CHGA (73, 74) has been shown to directly contribute to angiogenesis. As summarized below and depicted in Figure 1, several signaling proteins have been reported to regulate both angiogenesis and NE marker expression, often in separate diseases or biological contexts. These proteins are potential molecular links between the two important characteristics of NEPC.
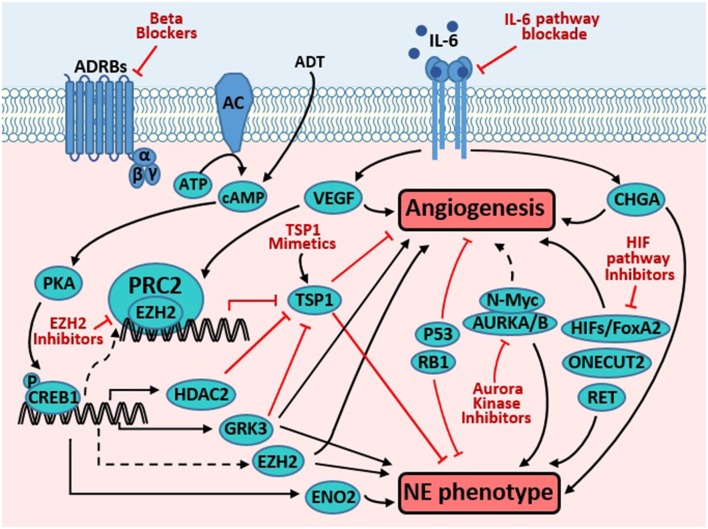
Figure 1.
Targeting molecules common to pathways promoting angiogenesis and neuroendocrine phenotype in prostate cancer. Androgen derivation therapy (ADT) elevates cAMP level, which activates PKA, resulting in phosphorylation and activation of CREB1. Activated CREB1 directly induces transcription of several genes involved in neuroendocrine differentiation (NED) and angiogenesis, such as VEGF, ENO2, GRK3, and HDAC2. VEGF is a potent pro-angiogenic factor, while ENO2 is a neuroendocrine marker. GRK3 promotes angiogenesis, NE marker expression, and prostate cancer progression. HDAC2 is critical for prostate cancer progression that is induced by chronical bio-behavioral stress and signals from beta adrenergic receptors (ADRBs). GRK3 and HDAC2 promotes angiogenesis, at least in part through downregulating TSP1. TSP1 is well-established as an anti-angiogenesis factor. Through unclear mechanisms, CREB1 activation enhances the PRC2 function of EZH2, which is critical for NED and angiogenesis induced by ADT. In endothelial cells, VEGF induces EZH2 expression and activity, which contributes to VEGF's action in promoting angiogenesis. Loss of p53 and RB1, alone or in cooperation, promote angiogenesis and NE phenotype through multiple mechanisms (detailed in text). IL-6 pathway activation enhances angiogenesis (through inducing VEGF) and NE phenotype (through inducing CHGA). AURKA interacts with N-Myc and regulates the stability of the latter, which promotes NED. AURKA and AURKB regulate angiogenesis in endothelial and neuroblastoma cells. HIF1A promotes angiogenesis through inducing VEGF. Moreover, it also cooperates with FoxA2 to promote NED and tumorigenesis. ONECUT2 has recently emerged as a master regulator of NED. Recent studies have also implicated receptor tyrosine kinase RET in regulating NED and angiogenesis. Novel strategies targeting the proteins and pathways that regulate both prominent phenotypes may be effective to treat NEPC (detailed in text).
CHGA
CHGA is one of the classic markers for NEPC. It is a secreted glycoprotein that shows paradoxical properties in angiogenesis (71, 73–75). Recent studies showed CHGA can be proteolytically cleaved into active peptides by thrombin. This cleavage shifts its function from anti- to pro-angiogenesis under pathophysiologic conditions, which could be observed in prothrombin activation or multiple myeloma (73, 74). Its function in angiogenesis in NEPC is still unclear.
p53 and RB1
p53 and RB1, two most prominent tumor suppressors, have been implicated in both angiogenesis and NE marker expression in separate studies. Mutations and loss of p53 or RB1 are common alterations in prostate cancer patients (76). Tumors containing p53 mutations are usually more vascularized than tumors harboring wild type p53. This pattern has been observed in several independent clinical studies on prostate, colon, and breast cancers (77–80). Some basic mechanisms underlying p53's inhibition of angiogenesis have been detailed. Ravi et al. found that, under hypoxic conditions, p53 inhibits the HIF1A activity that is required for VEGF transcription (81). Besides VEGF, p53 also inhibits other pro-angiogenetic factors, such as bFGF-BP (bFGF-binding protein) and COX-2 (cyclooxygenase-2). In addition, p53 also induces anti-angiogenetic factors, such as TSP-1 and EPHA2 (ephrin receptor A2) (82). However, it is not clear whether or how p53 itself plays a role in regulating NE phenotype.
RB1 has also been reported to regulate tumor angiogenesis (83–85). For example, Lasorella et al. reported Id2 (inhibitor of differentiation 2), a target of RB1, mediates angiogenesis of pituitary tumors from Rb1+/− mice (86). RB1 loss is one of the most critical events in neuroendocrine carcinoma (12, 87, 88), but the mechanism by which RB1 contributes to NE phenotype is largely unclear. A recent study reported RB1 takes part in regulating both angiogenesis and NE phenotypes. Labrecque et al. found, under hypoxic conditions, RB1-loss deregulates the expression of genes that govern angiogenesis, metastasis and NE differentiation. These effects led to a more invasive phenotype as well as NE protein markers expression in human prostate cancer cells (40).
Growing evidence implies a cooperative function of p53 and RB1 in tumor angiogenesis. Martinez-Cruz et al. found that combinatorial deletion of p53 and RB1 augmented tumor angiogenesis in a spontaneous squamous cell carcinoma mouse model, comparing with loss of p53 alone (89). Similarly, inactivation of p53 and RB1 leads to a pro-angiogenic transcriptional response in keratinocytes (90). In a xenograft model of retinoblastoma, p53 was shown to increase VEGF expression and promote angiogenesis in cells deficient for p21/RB1 pathway (91). All these observations underline the possibility of p53 and RB1 cooperation in promoting prostate cancer angiogenesis.
Interestingly, p53 and RB1 are also both connected to NE marker expression in prostate cancer. In a NEPC xenograft model LTL-331R that relapsed upon castration resistance of prostate adenocarcinoma patient-derived xenograft LTL-331, genomic alterations of both p53 and RB1 were observed (39). Of note, Beltran et al. showed (25) that “concurrent loss of RB1 and p53 was present in 53.3% of NEPC patient tumors vs. 13.7% of CRPC-adenocarcinoma samples (P < 0.0004, proportion test).” In a classic NEPC genetically engineered mouse (GEM) model called TRAMP, p53 and RB1 are both inactivated in the prostate by SV40 large T antigen oncoprotein, which induces the development of prostate cancers that subsequently progress to NEPC (92). Using GEM model and human cell models, loss of p53 and RB1 has been shown to promote linear plasticity and a phenotypic shift from AR-dependent luminal epithelial cells to AR-independent NEPC with resistance to enzalutamide (an antiandrogen drug) (26, 36).
PKA-CREB1 Axis
Both angiogenesis and NE marker expression can be induced by increased cellular cAMP level (93–95). Androgen deprivation therapy (ADT) increases cAMP level in prostate cancer cells, which activates the PKA-CREB1 pathway that in turn regulates both phenotypes. VEGF and ENO2 have been identified as targets of CREB1 and regulate angiogenesis and NE marker expression, respectively (96–98). However, targets of CREB1 that regulate both phenotypes were largely unknown. We recently reported two direct targets of CREB1, GRK3 (G protein coupled receptor kinase 3) and HDAC2 (histone deacetylase 2). GRK3 was shown to promote both angiogenesis and NE marker expression in separate studies (detailed below). Induction of HDAC2 by CREB1 is critical for prostate cancer progression promoted by chronical bio-behavioral stress that activates PKA-CREB1 pathway though beta adrenergic signaling (99). It is still unknown whether HDAC2 is involved in NE phenotype regulation in prostate cancer. In another study, we found that PKA-CREB1 signaling enhances the epigenetic repressive activity of EZH2 (enhanced zeste homolog 2) that in turn induces NE phenotype and angiogenesis (detailed below). In short, the PKA-CREB1 axis seems to be a master upstream regulator for both NE phenotype and angiogenesis in prostate cancer.
GRK3
We initially uncovered GRK3 as a key regulator of the progression of prostate cancer through unbiased shRNA and focused cDNA screening of human kinases (100). GRK3 is essential for metastatic prostate cancer cells in culture and in mouse xenografts. Further, its overexpression promotes orthotropic prostate tumor growth in mouse xenografts. Mechanistically, GRK3 promotes prostate cancer progression in part through repressing two anti-angiogenic factors TSP1 and PAI2, thus inducing angiogenesis in prostate cancer cells (100). Genomic profiling and immunohistochemical staining of human prostate cancers showed that GRK3 is upregulated in advanced prostate cancers (100, 101). Of note, we found a strong trend between GRK3 protein level and glomeruloid microvascular proliferation, a marker of VEGFA–driven angiogenesis, in prostate cancer patient samples. This result further supports a role of GRK3 in stimulating angiogenesis.
We recently reported that GRK3 promotes ADT resistance and NE marker expression of prostate adenocarcinoma cells (101). The kinase dead form of GRK3 abolished these phenotypes, indicating a requirement of GRK3's kinase activity (100, 101). Moreover, GRK3 is positively associated with NE marker expression in human cancer cell lines and patient tumors. Upon GRK3 silencing, expression of NE markers induced by ADT was reduced. These results suggest that GRK3 is a key regulator of both NE phenotype and angiogenesis in prostate cancer. It is worth further investigating the molecular mechanisms of GRK3 and the potential of inhibiting GRK3 as a novel strategy to block NEPC.
EZH2
Polycomb repressive complex 2 (PRC2) is another important regulator for both angiogenesis and NEPC. PRC2 usually renders transcriptional repression by tri-methylating lysine 27 of histone H3 (H3K27me3) on target genes (102, 103). As the key catalytic subunit of PRC2, EZH2 is widely overexpressed in many tumors, including prostate cancer (102). Overexpression of EZH2 and elevated PRC2 activity promote prostate cancer cell proliferation and migration (103). Clermont et al. found that EZH2 is one of the most upregulated epigenetic regulators in NEPC across multiple datasets from clinical to xenograft tissues (104). Dardenne et al. reported that high catalytic activity of EZH2 promotes N-Myc-AR-PRC2 complex formation and promotes NE phenotype (37). Ku et al. emphasized that overexpressed EZH2 in prostate-specific Pten-Rb1-p53 triple knockout mice plays a pivotal role in promoting prostate cancer lineage plasticity, antiandrogen resistance, and neuroendocrine phenotype (26). We recently demonstrated that EZH2 presents a critical molecular link for NE phenotype and angiogenesis, downstream of ADT-activated PKA-CREB1 signaling (66). EZH2 is activated by ADT and PKA-CREB1 signaling, which in turn induces NE markers and reduces TSP1 in prostate cancer cells. Our study also fills in a gap of knowledge how EZH2 overexpression in cancer cells directly contributes to tumor angiogenesis. Lu et al. have showed that EZH2 is induced by VEGF in endothelial cells, which contributes to angiogenesis (105).
TSP1
TSP1 is found to have various specific biological activities in different specific tumor environments. The role, regulation and expression patterns of TSP1 in human malignancies are highly context dependent and complicated. On general knowledge of TSP1 in urological cancers, please refer to this outstanding review (106). TSP1 is the first identified endogenous inhibitor of angiogenesis. It suppresses endothelial cell proliferation, migration, and tube formation, as well as induces endothelial apoptosis (107–109). While TSP1's role in angiogenesis is well-known, we recently established its role and regulation in NEPC (66). As expected, TSP1 inhibits angiogenesis induced by NEPC cells. Furthermore, the expression of TSP1 in NEPC is significantly lower than that in CRPC-adenocarcinoma, and NE markers negatively correlate with TSP1 in several prostate cancer datasets (66). Interestingly, TSP1 silence increase NE marker expression in PC3 prostate cancer cells, which suggests that TSP1 may directly regulate NE phenotype. This intriguing observation supports an intimate relation between NE phenotype and angiogenesis in prostate cancer cells (66). The molecular mechanisms underlying TSP1's role of NE phenotype warrants further investigation.
IL-6
As a pro-inflammatory cytokine, interleukin-6 (IL-6) is expressed in both of prostate tumors and the stromal tumor microenvironment. IL-6 is well-known to participate in cellular angiogenesis. Recently, Culig and Puhr have elegantly reviewed the role and regulation of IL-6 in prostate cancer (110). Several signaling pathways downstream of IL-6 orchestrate angiogenesis and NE phenotype in prostate cancer. For example, Ishii et al. showed that IL-6 promotes angiogenesis by up-regulating VEGF through PI3K/AKT pathway (111). On the other hand, IL-6 boosts NE phenotype by inducing CHGA and ENO2 expression through JAK/STAT3 and MAPK pathways (112, 113), as well as AMPK activation and autophagy induction (114). Detailed molecular mechanisms that connect IL-6 induced angiogenesis and NE phenotype need to be further elucidated.
MYCN
As a key oncogene driver in neuroblastoma, MYCN (N-Myc) is also a critical regulator of NEPC and SCLC (small cell lung cancer, a poorly differentiated neuroendocrine lung cancer) (21, 37, 71, 115). While convincing evidence supporting a direct role of N-Myc in regulating angiogenesis is scarce, NDRG1 (N-Myc downstream-regulated gene 1) has demonstrated pleiotropic roles in angiogenesis and cancer progression, depending on cancer types (71, 116).
Aurora Kinases A and B
Aurora kinase A and B (AURKA/B) phosphorylate and stabilize N-Myc protein, which sustains N-Myc function in promoting NE phenotypes in neuroblastoma (117). AURKA and AURKB have been shown to regulate VEGF production and angiogenesis in endothelial cells directly and in neuroblastoma cells (118, 119). It is postulated that AURKA and/or AURKB may regulate angiogenesis of NEPC, although direct evidences are needed.
HIF1A-FOXA2 Axis
HIF1 and HIF2 are well-known key regulators of angiogenesis (48, 50, 120). Recent studies have also implicated them, especially HIF1A, in regulating neuroendocrine phenotype in prostate cancer. HIF1A cooperates with FOXA2, a transcription factor expressed in NE tissue, to induce several HIF1A target genes that are required for hypoxia-mediated NE phenotype and metastasis in prostate cancer (41, 43).
ONECUT2 (OC2)
According to recent reports by Rotinen et al. and Guo et al., ONECUT2 plays a critical role in poorly differentiated neuroendocrine prostate tumors as a master transcriptional regulator (41, 121). As a survival factor in mCRPC models, ONECUT2 depresses AR transcription-related program and activates NE differentiation genes and progression to lethal disease (121). Besides, overexpression of ONECUT2 in prostate adenocarcinoma under hypoxia condition is able to inhibit AR signaling and induce NE phenotype (41). Given the crucial role of hypoxia in angiogenesis, we postulate that ONECUT2 may also contribute to the angiogenic phenotype of NEPC, which warrants further study. One study in ovarian cancer demonstrated that silencing ONECUT2 reduces VEGF expression and vascularization in xenograft tumors (122).
RET
RET mutations are found to enrich in lung adenocarcinoma with NE differentiation (123, 124). Knockdown of RET inhibits prostate tumor growth in vivo (125). A recent study from Justin Drake's lab has showed that RET phosphopeptides and mRNA levels are higher in NEPC than in prostate adenocarcinoma, while RET inhibitor AD80 blocks NEPC cell growth in culture and in mouse xenografts (126). Further experiments on gain and loss of function of RET protein will need to be carried out in NEPC cell models. While a role of RET in angiogenesis is well-established in medullary thyroid cancers (127), it is still unclear whether it is critical for the angiogenic phenotype in poorly differentiated neuroendocrine tumors, such as NEPC.
Targeting the Molecular Links Between Angiogenesis and Ne Phenotype For Developing New Therapies
As summarized above, elevated angiogenesis and NE marker expression are two important interconnected phenotypes. Targeting key molecules linking these two phenotypes may be effective therapeutic strategies for neuroendocrine prostate cancers. Potential therapeutic agents targeting some of these molecules include beta blockers inhibiting PKA-CREB1 signaling, TSP1 mimetic peptides, inhibitors of EZH2 and HIF1 pathway, and IL-6 pathway blockade. It is paramount to evaluate these and other related agents, alone and in combinations, for NEPC, given the reported contributions of their targets in this lethal variant of prostate cancer that has no effective treatment.
Beta Blockers
Beta blockers which inhibit beta adrenergic signaling and PKA-CREB1 activation, have been used to treat patients with cardiovascular diseases for decades. According to epidemiology studies, cancer patients who have used beta blockers for cardiovascular diseases have better clinical outcomes than the matched patients who do not use, in multiple cancer types, including melanoma, prostate, lung, and breast cancers (128–130). Results from these retrospective investigations are consistent with emerging evidences supporting anti-tumor effects of beta blockers in cancer cells in vitro and in mouse xenografts (99, 131–133). Because that beta blockers have been already applied in hypertension and heart diseases for years, they may also become efficient and safe therapies for NEPC. Beta blockers propranolol and carvedilol are tested in several cancer clinical trials (clinicaltrials.gov). However, major obstacles of beta blockers in clinical studies include incomplete understandings of their mechanisms of action in cancers, as well as a shortage of biomarkers for patient selection and efficacy monitoring (129, 134). We recently reported that propranolol downregulates NE marker expression and inhibits angiogenesis and growth of NEPC cell-derived xenografts by blocking a critical pathway CREB1-EZH2-TSP1 (66). This finding suggests that this pathway's activity level may serve as a biomarker for future cancer clinical trials of beta blockers. The therapeutic value of propranolol and other PKA-CREB1 signaling inhibitors in prostate cancer treatment should be further tested.
EZH2 Inhibitors
Based on the driving role and significant overexpression of EZH2 in many tumors, several inhibitors targeting EZH2 have been developed, such as GSK126, GSK343, GSK503, EPZ6438, CPI-1205, PF-06821497, and DZNeP. Some of these EZH2 inhibitors have demonstrated anti-tumor activity against NEPC in vitro and in vivo. Beltran et al. found that GSK343 preferentially inhibited cell viability of NEPC cells, while minimally affecting non-NEPC cells (25). Ku et al. reported GSK503 restored enzalutamide sensitivity of prostate tumors from castrated Pten-Rb1 double knockout mouse (26). DZNeP has also shown some anti-tumor activity in preclinical studies of several cancer types, including prostate cancer (135, 136). We recently demonstrated that conditioned media from prostate cancer cells expressing EZH2 shRNA or treated with GSK126 or EPZ6438 inhibit in vitro angiogenesis of endothelial cells (66). In addition, GSK126 and DZNeP were shown to decrease NE marker expression (66). Several EZH2 inhibitors are currently in clinical trials for multiple types of lymphoma, synovial sarcoma, and solid epithelial tumors: NCT03010982 and NCT01897571 (EPZ6438), NCT03480646 (CPI-1205), and NCT03460977 (PF-06821497). It is conceivable that the existing EZH2 inhibitors or other new drugs under development may have positive efficacy targeting NEPC.
HIF Pathway Inhibitors
Pathways of hypoxia-inducible factors (HIF) play key roles in development of resistance to different treatment modalities. Thereby, HIF pathway inhibitors targeting advanced cancers warrant further clinical studies either as a single agent or in combination with other therapeutic agents (137). Specifically for prostate cancer, two mCRPC clinical trials of HIF pathway inhibitors, including 2ME2 nanocrystal dispersion (panzem) and 17-AAG (tanespimycin), have been reported, which unfortunately showed little efficacy (138, 139). However, given the critical roles of HIF in control both angiogenesis and neuroendocrine phenotypes in NEPC, future testing of other inhibitors of HIF pathway, alone or in combinations, is still justifiable for NEPC. Interestingly, Guo et al. recently showed that TH-302 (evofosfamide), a prodrug activated by hypoxia, significantly inhibits NEPC tumor growth (41). An ongoing immunotherapy study combines ipilimumab (targeting CTLA-4) and evofosfamide for the treatment several solid tumor types, including confirmed metastatic or locally advanced prostate cancers (NCT03098160).
Aurora Kinase Inhibitor
Phase II trial of Alisertib (MLN8237), an Aurora Kinase A inhibitor, for castration resistant and neuroendocrine prostate cancers was recently completed (140). Although the report did not meet its primary endpoint of significantly extending 6-month radiographic progression-free survival (rPFS), a subset of advanced prostate cancer patients with AURKA and N-Myc activation achieved significant clinical benefits.
TSP-1 Mimetic Peptides
ABT-510, a TSP-1 mimetic peptide, has been tested in phase I and II clinical trials for many cancer types, including soft tissue sarcoma, metastatic melanoma, renal cell carcinoma, and advanced solid tumors (141–144). ABT-510 failed to show significant clinical benefits as a single agent, suggesting a combinatory strategy is needed. Combination of ABT-510 and cytoxan leads to a delay in progression of PC-3 tumor xenografts (145). Notably, in a phase I study of glioblastoma, combination of ABT-510 with temozolomide and radiotherapy moderately extended overall survival time (146). These findings suggest that combination of ABT-510 with other standard anti-tumor therapies may be an effective strategy to yield better clinical efficacy. Recently, a new TSP-1 mimetic peptide, ABT-898, with greatly increased potency over ABT-510, has been generated. Its efficacy has been tested in rodents and dogs (147–149), and have showed more notable antiangiogenic efficacy than ABT-510 (147). Investigation of the therapeutic potential of ABT-510 and ABT-898 in prostate cancers, especially in NEPC, warrants additional studies.
IL-6 Pathway Blockade
Given its critical contributions to cancer progression, IL-6 signaling pathway (IL6-/IL6R/JAK/STAT3) is being actively pursued for novel cancer therapies. Recent progress and obstacles in targeting IL-6 to treat cancers have been well-summarized (150–152). Agents blocking IL-6/IL-6R or inhibiting JAK/STAT3 to block tumor progression have been or are being tested in clinical trials, such as siltuximab (an anti-IL-6 mAb), tocilizumab (an anti-IL-6R mAb), Ruxolitinib (a JAK signaling inhibitor). Although many evidences confirmed a key role of IL-6 cascades in regulating the growth of malignant cells in preclinical studies, anti-IL6 or anti-IL6R mAbs have not demonstrated clinical efficacy in several cancer types. The lack of efficacy of IL-6 pathway blockade in cancer is likely due to tumor cells plasticity, displaying different tumor clones in tumor samples in vivo (153). Testing IL-6 pathway inhibitors, in combination with standard or other targeted therapies, is still favored for NEPC.
Future Directions
Besides the knowledge gaps and future directions abovementioned for individual regulators or therapeutic developments, we believe that the following three directions warrant further investigation to fully understand and target the molecules common to pathways promoting angiogenesis and neuroendocrine differentiation of prostate cancer.
Do Neuroendocrine Differentiation and Angiogenesis Promote Each Other?
We have described several genes reported to regulate both neuroendocrine and angiogenic phenotypes. Much of the knowledge for both phenotypes was in different biological contexts or cancer types. It is largely unclear whether induction of one phenotype leads to increase in another phenotype in the same biological system, such as in NEPC. It is conceivable that induction of neuroendocrine phenotype may promote angiogenesis, in part due to secretion of pro-angiogenic factors by neuroendocrine cells, such as VEGF and neuropeptides bombesin and gastrin, although the roles of these factors in neuroendocrine phenotype are still unclear (15).
Do Critical Regulators Established in One Phenotype Contribute to the Other Phenotype?
This review mainly focuses on genes that have been implicated in regulating both angiogenesis and neuroendocrine differentiation, although in separate contexts for many genes. To better understand these two phenotypes and to facilitate the development of effective treatments for NEPC, a systematic investigation is necessary to define the roles of these regulators in a shared context. Moreover, studies have characterized the function of several other proteins in regulating either neuroendocrine differentiation (such as BRN2, PEG10, SRRM4, REST, and DEK) or angiogenesis (such as FGF, TGF, EGFL6, PDGF, MMPs, and CCL2). Given the intimate links between the two characteristics as we summarize, it is worthwhile to investigate the roles of critical regulators of neuroendocrine differentiation in regulating angiogenesis, and vice versa.
Anti-angiogenesis Therapy and Combination Treatments for NEPC?
Positive results in anti-angiogenic therapy were observed in pancreatic neuroendocrine tumors (PNET), another type of neuroendocrine tumors that are well-differentiated with better prognosis than SCLC and NEPC. Sunitinib is a multi-targeted tyrosine kinase receptor inhibitor of VEGFR1-3, PDGFR, c-kit, RET, CSF-1R, and FLT3. It has demonstrated direct antitumor and antiangiogenic effects, and has received FDA approval for the treatment of locally advanced or metastatic PNETs (154, 155).
In SCLC, it was demonstrated that higher VEGF is associated with poor prognosis, which makes it a reasonable strategy to block VEGF pathway for inhibiting angiogenesis and tumor progression. However, only limited clinical benefits in this attempt was observed (156). As far as we know, no result has been reported on clinical trials of anti-angiogenic therapy for NEPC. Due to the striking pathological similarity between SCLC and NEPC, it is likely that, for NEPC, finding the right combinations of anti-angiogenesis and other therapies will be key to achieve significant efficacy for NEPC. Several strategies of combining anti-angiogenic regimens with targeted/chemo/immune therapies have been or are being tested clinically in several cancer types (59). These strategies include combining different anti-angiogenic regimens, simultaneously inhibiting angiogenesis and driving oncogenes, or combining anti-angiogenic regimens with immunotherapy. It is conceivable that similar combinatorial strategies are applicable to NEPC.
Another strategy for NEPC is to target key regulators for both NEPC phenotypes that we have discussed, i.e., neuroendocrine differentiation and angiogenesis, hitting two birds with one stone. In section Targeting the Molecular Links Between Angiogenesis and NE Phenotype for Developing New Therapies, we have summarized some opportunities for developing therapeutics to target pathways involved in both angiogenesis and neuroendocrine phenotypes. It may be necessary to co-target multiple key regulators of both phenotypes to simultaneously block alternative pathways that NEPC cells may use to escape.
Conclusion
NEPC is lethal without effective treatment. It is still poorly understood. They often have both elevated neuroendocrine marker expression and increased angiogenesis, the mechanisms of which remain largely elusive. Here, we summarize the literature on several proteins and pathways that regulate both angiogenesis and neuroendocrine phenotype in prostate cancer and other contexts. Bridging the mechanistic gaps between regulation of angiogenesis and neuroendocrine phenotype will facilitate better understanding of NEPC progression. We also discuss the opportunities of targeting some of these key regulators to inhibit both angiogenesis and neuroendocrine phenotype for treatments of patients with NEPC. Furthermore, many of the molecular mechanisms that we discuss here for NEPC are also dysregulated in small cell lung cancer (SCLC), a poorly differentiated aggressive neuroendocrine lung carcinoma. Therefore, we expect that much of the current knowledge and new therapeutic potentials summarized here for NEPC are relevant to SCLC.
Author Contributions
ZW, YZ, ZA, and WL wrote the paper.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We thank Dr. Georgina T. Salazar for editing the manuscript.
Footnotes
Funding. This work was supported by awards from American Cancer Society (RSG-17-062-01) and Cancer Prevention and Research Institute of Texas (CPRIT, RP170330) to WL. It was also supported by CPRIT (RP150551) and the Welch Foundation (AU-0042-20030616) to ZA.
References
- 1.Agoulnik IU, Vaid A, Nakka M, Alvarado M, Bingman WE, III, Erdem H, et al. Androgens modulate expression of transcription intermediary factor 2, an androgen receptor coactivator whose expression level correlates with early biochemical recurrence in prostate cancer. Cancer Res. (2006) 66:10594–602. 10.1158/0008-5472.CAN-06-1023 [DOI] [PubMed] [Google Scholar]
- 2.Komiya A, Yasuda K, Watanabe A, Fujiuchi Y, Tsuzuki T, Fuse H. The prognostic significance of loss of the androgen receptor and neuroendocrine differentiation in prostate biopsy specimens among castration-resistant prostate cancer patients. Mol Clin Oncol. (2013) 1:257–62. 10.3892/mco.2013.69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wang Q, Li W, Zhang Y, Yuan X, Xu K, Yu J, et al. Androgen receptor regulates a distinct transcription program in androgen-independent prostate cancer. Cell. (2009) 138:245–56. 10.1016/j.cell.2009.04.056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhu ML, Kyprianou N. Role of androgens and the androgen receptor in epithelial-mesenchymal transition and invasion of prostate cancer cells. Faseb J. (2010) 24:769–77. 10.1096/fj.09-136994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Aparicio A, Logothetis CJ, Maity SN. Understanding the lethal variant of prostate cancer: power of examining extremes. Cancer Disc. (2011) 1:466–8. 10.1158/2159-8290.CD-11-0259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Beltran H, Tagawa ST, Park K, MacDonald T, Milowsky MI, Mosquera JM, et al. Challenges in recognizing treatment-related neuroendocrine prostate cancer. J Clin Oncol. (2012) 30:e386–9. 10.1200/JCO.2011.41.5166 [DOI] [PubMed] [Google Scholar]
- 7.Hirano D, Okada Y, Minei S, Takimoto Y, Nemoto N. Neuroendocrine differentiation in hormone refractory prostate cancer following androgen deprivation therapy. Eur Urol. (2004) 45:586–92. 10.1016/j.eururo.2003.11.032 [DOI] [PubMed] [Google Scholar]
- 8.Papandreou CN, Daliani DD, Thall PF, Tu SM, Wang X, Reyes A, et al. Results of a phase II study with doxorubicin, etoposide, and cisplatin in patients with fully characterized small-cell carcinoma of the prostate. J Clin Oncol. (2002) 20:3072–80. 10.1200/JCO.2002.12.065 [DOI] [PubMed] [Google Scholar]
- 9.Wang HT, Yao YH, Li BG, Tang Y, Chang JW, Zhang J. Neuroendocrine prostate cancer (NEPC) progressing from conventional prostatic adenocarcinoma: factors associated with time to development of NEPC and survival from NEPC diagnosis-a systematic review and pooled analysis. J Clin Oncol. (2014) 32:3383–90. 10.1200/JCO.2013.54.3553 [DOI] [PubMed] [Google Scholar]
- 10.Goodman OB, Jr, Flaig TW, Molina A, Mulders PF, Fizazi K, Suttmann H, et al. Exploratory analysis of the visceral disease subgroup in a phase III study of abiraterone acetate in metastatic castration-resistant prostate cancer. Prostate Cancer Prostat Dis. (2014) 17:34–9. 10.1038/pcan.2013.41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Scher HI, Fizazi K, Saad F, Taplin ME, Sternberg CN, Miller K, et al. Increased survival with enzalutamide in prostate cancer after chemotherapy. N Engl J Med. (2012) 367:1187–97. 10.1056/NEJMoa1207506 [DOI] [PubMed] [Google Scholar]
- 12.Tan HL, Sood A, Rahimi HA, Wang W, Gupta N, Hicks J, et al. Rb loss is characteristic of prostatic small cell neuroendocrine carcinoma. Clin Cancer Res. (2014) 20:890–903. 10.1158/1078-0432.CCR-13-1982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Villaume K, Blanc M, Gouysse G, Walter T, Couderc C, Nejjari M, et al. VEGF secretion by neuroendocrine tumor cells is inhibited by octreotide and by inhibitors of the PI3K/AKT/mTOR pathway. Neuroendocrinology. (2010) 91:268–78. 10.1159/000289569 [DOI] [PubMed] [Google Scholar]
- 14.Yazdani S, Kasajima A, Tamaki K, Nakamura Y, Fujishima F, Ohtsuka H, et al. Angiogenesis and vascular maturation in neuroendocrine tumors. Hum Pathol. (2014) 45:866–74. 10.1016/j.humpath.2013.09.024 [DOI] [PubMed] [Google Scholar]
- 15.Heinrich E, Trojan L, Friedrich D, Voss M, Weiss C, Michel MS, et al. Neuroendocrine tumor cells in prostate cancer: evaluation of the neurosecretory products serotonin, bombesin, and gastrin - impact on angiogenesis and clinical follow-up. Prostate. (2011) 71:1752–8. 10.1002/pros.21392 [DOI] [PubMed] [Google Scholar]
- 16.Grobholz R, Bohrer MH, Siegsmund M, Junemann KP, Bleyl U, Woenckhaus M. Correlation between neovascularisation and neuroendocrine differentiation in prostatic carcinoma. Pathol Res Pract. (2000) 196:277–84. 10.1016/S0344-0338(00)80056-4 [DOI] [PubMed] [Google Scholar]
- 17.Beltran H, Tomlins S, Aparicio A, Arora V, Rickman D, Ayala G, et al. Aggressive variants of castration-resistant prostate cancer. Clin Cancer Res. (2014) 20:2846–50. 10.1158/1078-0432.CCR-13-3309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Aggarwal R, Huang J, Alumkal JJ, Zhang L, Feng FY, Thomas GV, et al. Clinical and genomic characterization of treatment-emergent small-cell neuroendocrine prostate cancer: a multi-institutional prospective study. J Clin Oncol. (2018) 36:2492–503. 10.1200/JCO.2017.77.6880 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Puca L, Vlachostergios PJ, Beltran H. Neuroendocrine differentiation in prostate cancer: emerging biology, models, and therapies. Cold Spring Harbor Persp Med. (2019) 9:a030593. 10.1101/cshperspect.a030593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Conteduca V, Aieta M, Amadori D, De Giorgi U. Neuroendocrine differentiation in prostate cancer: current and emerging therapy strategies. Crit Rev Oncol Hematol. (2014) 92:11–24. 10.1016/j.critrevonc.2014.05.008 [DOI] [PubMed] [Google Scholar]
- 21.Beltran H, Rickman DS, Park K, Chae SS, Sboner A, MacDonald TY, et al. Molecular characterization of neuroendocrine prostate cancer and identification of new drug targets. Cancer Disc. (2011) 1:487–95. 10.1158/2159-8290.CD-11-0130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jongsma J, Oomen MH, Noordzij MA, Van Weerden WM, Martens GJ, van der Kwast TH, et al. Kinetics of neuroendocrine differentiation in an androgen-dependent human prostate xenograft model. Am J Pathol. (1999) 154:543–51. 10.1016/S0002-9440(10)65300-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Terry S, Beltran H. The many faces of neuroendocrine differentiation in prostate cancer progression. Front Oncol. (2014) 4:60. 10.3389/fonc.2014.00060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vlachostergios PJ, Papandreou CN. Targeting neuroendocrine prostate cancer: molecular and clinical perspectives. Front Oncol. (2015) 5:6. 10.3389/fonc.2015.00006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Beltran H, Prandi D, Mosquera JM, Benelli M, Puca L, Cyrta J, et al. Divergent clonal evolution of castration-resistant neuroendocrine prostate cancer. Nat Med. (2016) 22:298–305. 10.1038/nm.4045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ku SY, Rosario S, Wang Y, Mu P, Seshadri M, Goodrich ZW, et al. Rb1 and Trp53 cooperate to suppress prostate cancer lineage plasticity, metastasis, and antiandrogen resistance. Science. (2017) 355:78–83. 10.1126/science.aah4199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zou M, Toivanen R, Mitrofanova A, Floch N, Hayati S, Sun Y, et al. Transdifferentiation as a mechanism of treatment resistance in a mouse model of castration-resistant prostate cancer. Cancer Dis. (2017) 7:736–49. 10.1158/2159-8290.CD-16-1174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bishop JL, Davies A, Ketola K, Zoubeidi A. Regulation of tumor cell plasticity by the androgen receptor in prostate cancer. Endocr Relat Cancer. (2015) 22:R165–82. 10.1530/ERC-15-0137 [DOI] [PubMed] [Google Scholar]
- 29.Chen R, Dong X, Gleave M. Molecular model for neuroendocrine prostate cancer progression. BJU Int. (2018) 122:560–70. 10.1111/bju.14207 [DOI] [PubMed] [Google Scholar]
- 30.Davies AH, Beltran H, Zoubeidi A. Cellular plasticity and the neuroendocrine phenotype in prostate cancer. Nat Rev Urol. (2018) 15:271–86. 10.1038/nrurol.2018.22 [DOI] [PubMed] [Google Scholar]
- 31.Soundararajan R, Paranjape AN, Maity S, Aparicio A, Mani SA. EMT, stemness and tumor plasticity in aggressive variant neuroendocrine prostate cancers. Biochim Biophys Acta Rev Cancer. (2018) 1870:229–38. 10.1016/j.bbcan.2018.06.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hu CD, Choo R, Huang J. Neuroendocrine differentiation in prostate cancer: a mechanism of radioresistance and treatment failure. Front Oncol. (2015) 5:90. 10.3389/fonc.2015.00090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Li Y, Donmez N, Sahinalp C, Xie N, Wang Y, Xue H, et al. SRRM4 drives neuroendocrine transdifferentiation of prostate adenocarcinoma under androgen receptor pathway inhibition. Eur Urol. (2016) 71:68–78. 10.1016/j.eururo.2016.04.028 [DOI] [PubMed] [Google Scholar]
- 34.Zhang X, Coleman IM, Brown LG, True LD, Kollath L, Lucas JM, et al. SRRM4 expression and the loss of REST activity may promote the emergence of the neuroendocrine phenotype in castration-resistant prostate cancer. Clin Cancer Res. (2015) 21:4698–708. 10.1158/1078-0432.CCR-15-0157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lin D, Dong X, Wang K, Wyatt AW, Crea F, Xue H, et al. Identification of DEK as a potential therapeutic target for neuroendocrine prostate cancer. Oncotarget. (2015) 6:1806–20. 10.18632/oncotarget.2809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mu P, Zhang Z, Benelli M, Karthaus WR, Hoover E, Chen CC, et al. SOX2 promotes lineage plasticity and antiandrogen resistance in TP53- and RB1-deficient prostate cancer. Science. (2017) 355:84–8. 10.1126/science.aah4307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dardenne E, Beltran H, Benelli M, Gayvert K, Berger A, Puca L, et al. N-Myc induces an EZH2-mediated transcriptional program driving neuroendocrine prostate cancer. Cancer Cell. (2016) 30:563–77. 10.1016/j.ccell.2016.09.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bishop JL, Thaper D, Vahid S, Davies A, Ketola K, Kuruma H, et al. The master neural transcription factor BRN2 is an androgen receptor-suppressed driver of neuroendocrine differentiation in prostate cancer. Cancer Disc. (2017) 7:54–71. 10.1158/2159-8290.CD-15-1263 [DOI] [PubMed] [Google Scholar]
- 39.Akamatsu S, Wyatt AW, Lin D, Lysakowski S, Zhang F, Kim S, et al. The placental gene PEG10 promotes progression of neuroendocrine prostate cancer. Cell Rep. (2015) 12:922–36. 10.1016/j.celrep.2015.07.012 [DOI] [PubMed] [Google Scholar]
- 40.Labrecque MP, Takhar MK, Nason R, Santacruz S, Tam KJ, Massah S, et al. The retinoblastoma protein regulates hypoxia-inducible genetic programs, tumor cell invasiveness and neuroendocrine differentiation in prostate cancer cells. Oncotarget. (2016) 7:24284–302. 10.18632/oncotarget.8301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Guo H, Ci X, Ahmed M, Hua JT, Soares F, Lin D, et al. ONECUT2 is a driver of neuroendocrine prostate cancer. Nat Commun. (2019) 10:278. 10.1038/s41467-018-08133-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Park JW, Lee JK, Witte ON, Huang J. FOXA2 is a sensitive and specific marker for small cell neuroendocrine carcinoma of the prostate. Modern Pathol. (2017) 30:1262–72. 10.1038/modpathol.2017.44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Qi J, Nakayama K, Cardiff RD, Borowsky AD, Kaul K, Williams R, et al. Siah2-dependent concerted activity of HIF and FoxA2 regulates formation of neuroendocrine phenotype and neuroendocrine prostate tumors. Cancer Cell. (2010) 18:23–38. 10.1016/j.ccr.2010.05.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mirosevich J, Gao N, Gupta A, Shappell SB, Jove R, Matusik RJ. Expression and role of Foxa proteins in prostate cancer. Prostate. (2006) 66:1013–28. 10.1002/pros.20299 [DOI] [PubMed] [Google Scholar]
- 45.Risau W. Mechanisms of angiogenesis. Nature. (1997) 386:671–4. 10.1038/386671a0 [DOI] [PubMed] [Google Scholar]
- 46.Burri PH, Djonov V. Intussusceptive angiogenesis–the alternative to capillary sprouting. Mol Aspects Med. (2002) 23:S1–27. 10.1016/S0098-2997(02)00096-1 [DOI] [PubMed] [Google Scholar]
- 47.Carmeliet P. Angiogenesis in life, disease and medicine. Nature. (2005) 438:932–6. 10.1038/nature04478 [DOI] [PubMed] [Google Scholar]
- 48.Carmeliet P, Jain RK. Angiogenesis in cancer and other diseases. Nature. (2000) 407:249–57. 10.1038/35025220 [DOI] [PubMed] [Google Scholar]
- 49.Marech I, Leporini C, Ammendola M, Porcelli M, Gadaleta CD, Russo E, et al. Classical and non-classical proangiogenic factors as a target of antiangiogenic therapy in tumor microenvironment. Cancer Lett. (2016) 380:216–26. 10.1016/j.canlet.2015.07.028 [DOI] [PubMed] [Google Scholar]
- 50.Krock BL, Skuli N, Simon MC. Hypoxia-induced angiogenesis: good and evil. Genes Cancer. (2011) 2:1117–33. 10.1177/1947601911423654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rundhaug JE. Matrix metalloproteinases and angiogenesis. J Cell Mol Med. (2005) 9:267–85. 10.1111/j.1582-4934.2005.tb00355.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.An J, Du Y, Fan X, Wang Y, Ivan C, Zhang XG, et al. EGFL6 promotes breast cancer by simultaneously enhancing cancer cell metastasis and stimulating tumor angiogenesis. Oncogene. (2019) 38:2123–34. 10.1038/s41388-018-0565-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ridiandries A, Tan JT, Bursill CA. The role of CC-chemokines in the regulation of angiogenesis. Int J Mol Sci. (2016) 17:1856. 10.3390/ijms17111856 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Fagiani E, Christofori G. Angiopoietins in angiogenesis. Cancer Lett. (2013) 328:18–26. 10.1016/j.canlet.2012.08.018 [DOI] [PubMed] [Google Scholar]
- 55.Bagnato A, Spinella F. Emerging role of endothelin-1 in tumor angiogenesis. Trends Endocrinol Metab. (2003) 14:44–50. 10.1016/S1043-2760(02)00010-3 [DOI] [PubMed] [Google Scholar]
- 56.Guerrero PA, McCarty JH. TGF-β Activation and Signaling in Angiogenesis. London, UK: IntechOpen; (2017). 10.5772/66405 [DOI] [Google Scholar]
- 57.Al-Abd AM, Alamoudi AJ, Abdel-Naim AB, Neamatallah TA, Ashour OM. Anti-angiogenic agents for the treatment of solid tumors: potential pathways, therapy and current strategies - a review. J Adv Res. (2017) 8:591–605. 10.1016/j.jare.2017.06.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Yadav L, Puri N, Rastogi V, Satpute P, Sharma V. Tumour angiogenesis and angiogenic inhibitors: a review. J Clin Diagn Res. (2015) 9:XE01–5. 10.7860/JCDR/2015/12016.6135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Comunanza V, Bussolino F. Therapy for cancer: strategy of combining anti-angiogenic and target therapies. Front Cell Dev Biol. (2017) 5:101. 10.3389/fcell.2017.00101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Noh K, Mangala LS, Han HD, Zhang N, Pradeep S, Wu SY, et al. Differential effects of EGFL6 on tumor versus wound angiogenesis. Cell Rep. (2017) 21:2785–95. 10.1016/j.celrep.2017.11.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Li Y, Cozzi PJ. Angiogenesis as a strategic target for prostate cancer therapy. Med Res Rev. (2010) 30:23–66. 10.1002/med.20161 [DOI] [PubMed] [Google Scholar]
- 62.Borre M, Offersen BV, Nerstrom B, Overgaard J. Microvessel density predicts survival in prostate cancer patients subjected to watchful waiting. Br J Cancer. (1998) 78:940–4. 10.1038/bjc.1998.605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Bono AV, Celato N, Cova V, Salvadore M, Chinetti S, Novario R. Microvessel density in prostate carcinoma. Prostate Cancer Prostat Dis. (2002) 5:123–7. 10.1038/sj.pcan.4500572 [DOI] [PubMed] [Google Scholar]
- 64.Strohmeyer D, Rossing C, Strauss F, Bauerfeind A, Kaufmann O, Loening S. Tumor angiogenesis is associated with progression after radical prostatectomy in pT2/pT3 prostate cancer. Prostate. (2000) 42:26–33. [DOI] [PubMed] [Google Scholar]
- 65.Vartanian RK, Weidner N. Endothelial cell proliferation in prostatic carcinoma and prostatic hyperplasia: correlation with Gleason's score, microvessel density, and epithelial cell proliferation. Lab Invest. (1995) 73:844–50. [PubMed] [Google Scholar]
- 66.Zhang Y, Zheng D, Zhou T, Song H, Hulsurkar M, Su N, et al. Androgen deprivation promotes neuroendocrine differentiation and angiogenesis through CREB-EZH2-TSP1 pathway in prostate cancers. Nat Commun. (2018) 9:4080. 10.1038/s41467-018-06177-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.van Moorselaar RJ, Voest EE. Angiogenesis in prostate cancer: its role in disease progression and possible therapeutic approaches. Mol Cell Endocrinol. (2002) 197:239–50. 10.1016/S0303-7207(02)00262-9 [DOI] [PubMed] [Google Scholar]
- 68.Russo G, Mischi M, Scheepens W, De la Rosette JJ, Wijkstra H. Angiogenesis in prostate cancer: onset, progression and imaging. BJU Int. (2012) 110:E794–808. 10.1111/j.1464-410X.2012.11444.x [DOI] [PubMed] [Google Scholar]
- 69.Revelos K, Petraki C, Scorilas A, Stefanakis S, Malovrouvas D, Alevizopoulos N, et al. Correlation of androgen receptor status, neuroendocrine differentiation and angiogenesis with time-to-biochemical failure after radical prostatectomy in clinically localized prostate cancer. Anticancer Res. (2007) 27:3651–60. [PubMed] [Google Scholar]
- 70.Harper ME, Glynne-Jones E, Goddard L, Thurston VJ, Griffiths K. Vascular endothelial growth factor (VEGF) expression in prostatic tumours and its relationship to neuroendocrine cells. Br J Cancer. (1996) 74:910–6. 10.1038/bjc.1996.456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Bae DH, Jansson PJ, Huang ML, Kovacevic Z, Kalinowski D, Lee CS, et al. The role of NDRG1 in the pathology and potential treatment of human cancers. J Clin Pathol. (2013) 66:911–7. 10.1136/jclinpath-2013-201692 [DOI] [PubMed] [Google Scholar]
- 72.Roberts E, Cossigny DA, Quan GM. The role of vascular endothelial growth factor in metastatic prostate cancer to the skeleton. Prostate Cancer. (2013) 2013:418340. 10.1155/2013/418340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Crippa L, Bianco M, Colombo B, Gasparri AM, Ferrero E, Loh YP, et al. A new chromogranin A-dependent angiogenic switch activated by thrombin. Blood. (2013) 121:392–402. 10.1182/blood-2012-05-430314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Bianco M, Gasparri AM, Colombo B, Curnis F, Girlanda S, Ponzoni M, et al. Chromogranin A is preferentially cleaved into proangiogenic peptides in the bone marrow of multiple myeloma patients. Cancer Res. (2016) 76:1781–91. 10.1158/0008-5472.CAN-15-1637 [DOI] [PubMed] [Google Scholar]
- 75.Helle KB, Corti A. Chromogranin A: a paradoxical player in angiogenesis and vascular biology. Cell Mol Life Sci. (2015) 72:339–48. 10.1007/s00018-014-1750-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Khemlina G, Ikeda S, Kurzrock R. Molecular landscape of prostate cancer: implications for current clinical trials. Cancer Treat Rev. (2015) 41:761–6. 10.1016/j.ctrv.2015.07.001 [DOI] [PubMed] [Google Scholar]
- 77.Yu EY, Yu E, Meyer GE, Brawer MK. The relation of p53 protein nuclear accumulation and angiogenesis in human prostatic carcinoma. Prostate Cancer Prostat Dis. (1997) 1:39–44. 10.1038/sj.pcan.4500205 [DOI] [PubMed] [Google Scholar]
- 78.Takahashi Y, Bucana CD, Cleary KR, Ellis LM. p53, vessel count, and vascular endothelial growth factor expression in human colon cancer. Int J Cancer. (1998) 79:34–38. [DOI] [PubMed] [Google Scholar]
- 79.Gasparini G, Weidner N, Bevilacqua P, Maluta S, Dalla Palma P, Caffo O, et al. Tumor microvessel density, p53 expression, tumor size, and peritumoral lymphatic vessel invasion are relevant prognostic markers in node-negative breast carcinoma. J Clin Oncol. (1994) 12:454–66. 10.1200/JCO.1994.12.3.454 [DOI] [PubMed] [Google Scholar]
- 80.Faviana P, Boldrini L, Spisni R, Berti P, Galleri D, Biondi R, et al. Neoangiogenesis in colon cancer: correlation between vascular density, vascular endothelial growth factor (VEGF) and p53 protein expression. Oncol Rep. (2002) 9:617–20. 10.3892/or.9.3.617 [DOI] [PubMed] [Google Scholar]
- 81.Ravi R, Mookerjee B, Bhujwalla ZM, Sutter CH, Artemov D, Zeng Q, et al. Regulation of tumor angiogenesis by p53-induced degradation of hypoxia-inducible factor 1alpha. Genes Dev. (2000) 14:34–44. 10.1101/gad.14.1.34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Teodoro JG, Evans SK, Green MR. Inhibition of tumor angiogenesis by p53: a new role for the guardian of the genome. J Mol Med. (2007) 85:1175–86. 10.1007/s00109-007-0221-2 [DOI] [PubMed] [Google Scholar]
- 83.Gabellini C, Del Bufalo D, Zupi G. Involvement of RB gene family in tumor angiogenesis. Oncogene. (2006) 25:5326–32. 10.1038/sj.onc.1209631 [DOI] [PubMed] [Google Scholar]
- 84.Schaal C, Pillai S, Chellappan SP. The Rb-E2F transcriptional regulatory pathway in tumor angiogenesis and metastasis. Adv Cancer Res. (2014) 121:147–82. 10.1016/B978-0-12-800249-0.00004-4 [DOI] [PubMed] [Google Scholar]
- 85.Bakker WJ, Weijts BG, Westendorp B, de Bruin A. HIF proteins connect the RB-E2F factors to angiogenesis. Transcription. (2013) 4:62–6. 10.4161/trns.23680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Lasorella A, Rothschild G, Yokota Y, Russell RG, Iavarone A. Id2 mediates tumor initiation, proliferation, and angiogenesis in Rb mutant mice. Mol Cell Biol. (2005) 25:3563–74. 10.1128/MCB.25.9.3563-3574.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Yachida S, Vakiani E, White CM, Zhong Y, Saunders T, Morgan R, et al. Small cell and large cell neuroendocrine carcinomas of the pancreas are genetically similar and distinct from well-differentiated pancreatic neuroendocrine tumors. Am J Surg Pathol. (2012) 36:173–84. 10.1097/PAS.0b013e3182417d36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Rickman DS, Beltran H, Demichelis F, Rubin MA. Biology and evolution of poorly differentiated neuroendocrine tumors. Nat Med. (2017) 23:1–10. 10.1038/nm.4341 [DOI] [PubMed] [Google Scholar]
- 89.Martinez-Cruz AB, Santos M, Lara MF, Segrelles C, Ruiz S, Moral M, et al. Spontaneous squamous cell carcinoma induced by the somatic inactivation of retinoblastoma and Trp53 tumor suppressors. Cancer Res. (2008) 68:683–92. 10.1158/0008-5472.CAN-07-3049 [DOI] [PubMed] [Google Scholar]
- 90.Toussaint-Smith E, Donner DB, Roman A. Expression of human papillomavirus type 16 E6 and E7 oncoproteins in primary foreskin keratinocytes is sufficient to alter the expression of angiogenic factors. Oncogene. (2004) 23:2988–95. 10.1038/sj.onc.1207442 [DOI] [PubMed] [Google Scholar]
- 91.Farhang Ghahremani M, Goossens S, Nittner D, Bisteau X, Bartunkova S, Zwolinska A, et al. p53 promotes VEGF expression and angiogenesis in the absence of an intact p21-Rb pathway. Cell Death Differ. (2013) 20:888–97. 10.1038/cdd.2013.12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Gingrich JR, Barrios RJ, Kattan MW, Nahm HS, Finegold MJ, Greenberg NM. Androgen-independent prostate cancer progression in the TRAMP model. Cancer Res. (1997) 57:4687–91. [PubMed] [Google Scholar]
- 93.Zhang Y, Daaka Y. PGE2 promotes angiogenesis through EP4 and PKA Cgamma pathway. Blood. (2011) 118:5355–64. 10.1182/blood-2011-04-350587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Garg J, Feng YX, Jansen SR, Friedrich J, Lezoualc'h F, Schmidt M, et al. Catecholamines facilitate VEGF-dependent angiogenesis via beta2-adrenoceptor-induced Epac1 and PKA activation. Oncotarget. (2017) 8:44732–48. 10.18632/oncotarget.17267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Bang YJ, Pirnia F, Fang WG, Kang WK, Sartor O, Whitesell L, et al. Terminal neuroendocrine differentiation of human prostate carcinoma cells in response to increased intracellular cyclic AMP. Proc Natl Acad Sci USA. (1994) 91:5330–4. 10.1073/pnas.91.12.5330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Jeon SH, Chae BC, Kim HA, Seo GY, Seo DW, Chun GT, et al. The PKA/CREB pathway is closely involved in VEGF expression in mouse macrophages. Mol Cells. (2007) 23:23–9. [PubMed] [Google Scholar]
- 97.Rhee SH, Ma EL, Lee Y, Tache Y, Pothoulakis C, Im E. Corticotropin releasing hormone and urocortin 3 stimulate vascular endothelial growth factor expression through the cAMP/CREB pathway. J Biol Chem. (2015) 290:26194–203. 10.1074/jbc.M115.678979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Park MH, Lee HS, Lee CS, You ST, Kim DJ, Park BH, et al. p21-Activated kinase 4 promotes prostate cancer progression through CREB. Oncogene. (2013) 32:2475–82. 10.1038/onc.2012.255 [DOI] [PubMed] [Google Scholar]
- 99.Hulsurkar M, Li Z, Zhang Y, Li X, Zheng D, Li W. Beta-adrenergic signaling promotes tumor angiogenesis and prostate cancer progression through HDAC2-mediated suppression of thrombospondin-1. Oncogene. (2017) 36:1525–36. 10.1038/onc.2016.319 [DOI] [PubMed] [Google Scholar]
- 100.Li W, Ai N, Wang S, Bhattacharya N, Vrbanac V, Collins M, et al. GRK3 is essential for metastatic cells and promotes prostate tumor progression. Proc Natl Acad Sci USA. (2014) 111:1521–6. 10.1073/pnas.1320638111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Sang M, Hulsurkar M, Zhang X, Song H, Zheng D, Zhang Y, et al. GRK3 is a direct target of CREB activation and regulates neuroendocrine differentiation of prostate cancer cells. Oncotarget. (2016) 7:45171–85. 10.18632/oncotarget.9359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Varambally S, Dhanasekaran SM, Zhou M, Barrette TR, Kumar-Sinha C, Sanda MG, et al. The polycomb group protein EZH2 is involved in progression of prostate cancer. Nature. (2002) 419:624–9. 10.1038/nature01075 [DOI] [PubMed] [Google Scholar]
- 103.Yang YA, Yu J. EZH2, an epigenetic driver of prostate cancer. Protein Cell. (2013) 4:331–41. 10.1007/s13238-013-2093-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Clermont PL, Lin D, Crea F, Wu R, Xue H, Wang Y, et al. Polycomb-mediated silencing in neuroendocrine prostate cancer. Clin Epigenetics. (2015) 7:40. 10.1186/s13148-015-0074-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Lu C, Han HD, Mangala LS, Ali-Fehmi R, Newton CS, Ozbun L, et al. Regulation of tumor angiogenesis by EZH2. Cancer Cell. (2010) 18:185–97. 10.1016/j.ccr.2010.06.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Miyata Y, Sakai H. Thrombospondin-1 in urological cancer: pathological role, clinical significance, and therapeutic prospects. Int J Mol Sci. (2013) 14:12249–72. 10.3390/ijms140612249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Taraboletti G, Roberts D, Liotta LA, Giavazzi R. Platelet thrombospondin modulates endothelial cell adhesion, motility, and growth: a potential angiogenesis regulatory factor. J Cell Biol. (1990) 111:765–72. 10.1083/jcb.111.2.765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Tolsma SS, Stack MS, Bouck N. Lumen formation and other angiogenic activities of cultured capillary endothelial cells are inhibited by thrombospondin-1. Microvasc Res. (1997) 54:13–26. 10.1006/mvre.1997.2015 [DOI] [PubMed] [Google Scholar]
- 109.Jimenez B, Volpert OV, Crawford SE, Febbraio M, Silverstein RL, Bouck N. Signals leading to apoptosis-dependent inhibition of neovascularization by thrombospondin-1. Nat Med. (2000) 6:41–8. 10.1038/71517 [DOI] [PubMed] [Google Scholar]
- 110.Culig Z, Puhr M. Interleukin-6 and prostate cancer: current developments and unsolved questions. Mol Cell Endocrinol. (2018) 462:25–30. 10.1007/978-1-4939-7845-8 [DOI] [PubMed] [Google Scholar]
- 111.Ishii K, Sasaki T, Iguchi K, Kajiwara S, Kato M, Kanda H, et al. Interleukin-6 induces VEGF secretion from prostate cancer cells in a manner independent of androgen receptor activation. Prostate. (2018) 78:849–56. 10.1002/pros.23643 [DOI] [PubMed] [Google Scholar]
- 112.Spiotto MT, Chung TD. STAT3 mediates IL-6-induced neuroendocrine differentiation in prostate cancer cells. Prostate. (2000) 42:186–95. [DOI] [PubMed] [Google Scholar]
- 113.Deeble PD, Murphy DJ, Parsons SJ, Cox ME. Interleukin-6- and cyclic AMP-mediated signaling potentiates neuroendocrine differentiation of LNCaP prostate tumor cells. Mol Cell Biol. (2001) 21:8471–82. 10.1128/MCB.21.24.8471-8482.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Chang PC, Wang TY, Chang YT, Chu CY, Lee CL, Hsu HW, et al. Autophagy pathway is required for IL-6 induced neuroendocrine differentiation and chemoresistance of prostate cancer LNCaP cells. PLoS ONE. (2014) 9:e88556. 10.1371/journal.pone.0088556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Nau MM, Brooks BJ, Jr, Carney DN, Gazdar AF, Battey JF, Sausville EA, et al. Human small-cell lung cancers show amplification and expression of the N-myc gene. Proc Natl Acad Sci USA. (1986) 83:1092–6. 10.1073/pnas.83.4.1092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Park KC, Paluncic J, Kovacevic Z, Richardson DR. Pharmacological targeting and the diverse functions of the metastasis suppressor, NDRG1, in cancer. Free Rad Biol Med. (2019). 10.1016/j.freeradbiomed.2019.05.020 [DOI] [PubMed] [Google Scholar]
- 117.Otto T, Horn S, Brockmann M, Eilers U, Schuttrumpf L, Popov N, et al. Stabilization of N-Myc is a critical function of Aurora A in human neuroblastoma. Cancer Cell. (2009) 15:67–78. 10.1016/j.ccr.2008.12.005 [DOI] [PubMed] [Google Scholar]
- 118.Sun X, Niu S, Zhang Z, Wang A, Yang C, Guo Z, et al. Aurora kinase inhibitor VX680 suppresses the proliferation and migration of HUVECs and angiogenesis. Mol Med Rep. (2019) 19:3841–7. 10.3892/mmr.2019.9996 [DOI] [PubMed] [Google Scholar]
- 119.Romain C, Paul P, Kim KW, Lee S, Qiao J, Chung DH. Targeting Aurora kinase-A downregulates cell proliferation and angiogenesis in neuroblastoma. J Pediatr Surg. (2014) 49:159–65. 10.1016/j.jpedsurg.2013.09.051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Keith B, Simon MC. Hypoxia-inducible factors, stem cells, and cancer. Cell. (2007) 129:465–72. 10.1016/j.cell.2007.04.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Rotinen M, You S, Yang J, Coetzee SG, Reis-Sobreiro M, Huang WC, et al. ONECUT2 is a targetable master regulator of lethal prostate cancer that suppresses the androgen axis. Nat Med. (2018) 24:1887–98. 10.1038/s41591-018-0241-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Lu T, Wu B, Yu Y, Zhu W, Zhang S, Zhang Y, et al. Blockade of ONECUT2 expression in ovarian cancer inhibited tumor cell proliferation, migration, invasion and angiogenesis. Cancer Sci. (2018) 109:2221–34. 10.1111/cas.13633 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 123.Kosari F, Ida CM, Aubry MC, Yang L, Kovtun IV, Klein JL, et al. ASCL1 and RET expression defines a clinically relevant subgroup of lung adenocarcinoma characterized by neuroendocrine differentiation. Oncogene. (2014) 33:3776–83. 10.1038/onc.2013.359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Rudin CM, Drilon A, Poirier JT. RET mutations in neuroendocrine tumors: including small-cell lung cancer. J Thor Oncol. (2014) 9:1240–2. 10.1097/JTO.0000000000000301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Ban K, Feng S, Shao L, Ittmann M. RET signaling in prostate cancer. Clin Cancer Res. (2017) 23:4885–96. 10.1158/1078-0432.CCR-17-0528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.VanDeusen H, Ramroop JR, Morel KL, Sheahan AV, Sychev Z, Lau NA, et al. Targeting RET kinase in neuroendocrine prostate cancer. biorxiv [Preprint]. (2019). 10.1101/622415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Verrienti A, Tallini G, Colato C, Boichard A, Checquolo S, Pecce V, et al. RET mutation and increased angiogenesis in medullary thyroid carcinomas. Endocr Relat Cancer. (2016) 23:665–76. 10.1530/ERC-16-0132 [DOI] [PubMed] [Google Scholar]
- 128.Choi CH, Song T, Kim TH, Choi JK, Park JY, Yoon A, et al. Meta-analysis of the effects of beta blocker on survival time in cancer patients. J Cancer Res Clin Oncol. (2014) 140:1179–88. 10.1007/s00432-014-1658-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Cole SW, Sood AK. Molecular pathways: beta-adrenergic signaling in cancer. Clin Cancer Res. (2012) 18:1201–6. 10.1158/1078-0432.CCR-11-0641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Holmes S, Griffith EJ, Musto G, Minuk GY. Antihypertensive medications and survival in patients with cancer: a population-based retrospective cohort study. Cancer Epidemiol. (2013) 37:881–5. 10.1016/j.canep.2013.09.001 [DOI] [PubMed] [Google Scholar]
- 131.Thaker PH, Han LY, Kamat AA, Arevalo JM, Takahashi R, Lu C, et al. Chronic stress promotes tumor growth and angiogenesis in a mouse model of ovarian carcinoma. Nat Med. (2006) 12:939–44. 10.1038/nm1447 [DOI] [PubMed] [Google Scholar]
- 132.Palm D, Lang K, Niggemann B, Drell TLt, Masur K, Zaenker KS, et al. The norepinephrine-driven metastasis development of PC-3 human prostate cancer cells in BALB/c nude mice is inhibited by beta-blockers. Int J Cancer. (2006) 118:2744–9. 10.1002/ijc.21723 [DOI] [PubMed] [Google Scholar]
- 133.Hassan S, Karpova Y, Baiz D, Yancey D, Pullikuth A, Flores A, et al. Behavioral stress accelerates prostate cancer development in mice. J Clin Invest. (2013) 123:874–86. 10.1172/JCI63324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Powe DG, Entschladen F. Targeted therapies: using beta-blockers to inhibit breast cancer progression. Nat Rev Clin Oncol. (2011) 8:511–2. 10.1038/nrclinonc.2011.123 [DOI] [PubMed] [Google Scholar]
- 135.Crea F, Fornaro L, Bocci G, Sun L, Farrar WL, Falcone A, et al. EZH2 inhibition: targeting the crossroad of tumor invasion and angiogenesis. Cancer Metastasis Rev. (2012) 31:753–61. 10.1007/s10555-012-9387-3 [DOI] [PubMed] [Google Scholar]
- 136.Smits M, Mir SE, Nilsson RJ, van der Stoop PM, Niers JM, Marquez VE, et al. Down-regulation of miR-101 in endothelial cells promotes blood vessel formation through reduced repression of EZH2. PLoS ONE. (2011) 6:e16282. 10.1371/journal.pone.0016282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Fallah J, Rini BI. HIF inhibitors: status of current clinical development. Curr Oncol Rep. (2019) 21:6. 10.1007/s11912-019-0752-z [DOI] [PubMed] [Google Scholar]
- 138.Harrison MR, Hahn NM, Pili R, Oh WK, Hammers H, Sweeney C, et al. A phase II study of 2-methoxyestradiol (2ME2) NanoCrystal(R) dispersion (NCD) in patients with taxane-refractory, metastatic castrate-resistant prostate cancer (CRPC). Invest New Drugs. (2011) 29:1465–74. 10.1007/s10637-010-9455-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Heath EI, Hillman DW, Vaishampayan U, Sheng S, Sarkar F, Harper F, et al. A phase II trial of 17-allylamino-17-demethoxygeldanamycin in patients with hormone-refractory metastatic prostate cancer. Clin Cancer Res. (2008) 14:7940–6. 10.1158/1078-0432.CCR-08-0221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Beltran H, Oromendia C, Danila DC, Montgomery B, Hoimes C, Szmulewitz RZ, et al. A Phase II trial of the aurora kinase A inhibitor alisertib for patients with castration-resistant and neuroendocrine prostate cancer: efficacy and biomarkers. Clin Cancer Res. (2019) 25:43–51. 10.1158/1078-0432.CCR-18-1912 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Baker LH, Rowinsky EK, Mendelson D, Humerickhouse RA, Knight RA, Qian J, et al. Randomized, phase II study of the thrombospondin-1-mimetic angiogenesis inhibitor ABT-510 in patients with advanced soft tissue sarcoma. J Clin Oncol. (2008) 26:5583–88. 10.1200/JCO.2008.17.4706 [DOI] [PubMed] [Google Scholar]
- 142.Gordon MS, Mendelson D, Carr R, Knight RA, Humerickhouse RA, Iannone M, et al. A phase 1 trial of 2 dose schedules of ABT-510, an antiangiogenic, thrombospondin-1-mimetic peptide, in patients with advanced cancer. Cancer. (2008) 113:3420–9. 10.1002/cncr.23953 [DOI] [PubMed] [Google Scholar]
- 143.Markovic SN, Suman VJ, Rao RA, Ingle JN, Kaur JS, Erickson LA, et al. A phase II study of ABT-510 (thrombospondin-1 analog) for the treatment of metastatic melanoma. Am J Clin Oncol. (2007) 30:303–9. 10.1097/01.coc.0000256104.80089.35 [DOI] [PubMed] [Google Scholar]
- 144.Ebbinghaus S, Hussain M, Tannir N, Gordon M, Desai AA, Knight RA, et al. Phase 2 study of ABT-510 in patients with previously untreated advanced renal cell carcinoma. Clin Cancer Res. (2007) 13:6689–95. 10.1158/1078-0432.CCR-07-1477 [DOI] [PubMed] [Google Scholar]
- 145.Yap R, Veliceasa D, Emmenegger U, Kerbel RS, McKay LM, Henkin J, et al. Metronomic low-dose chemotherapy boosts CD95-dependent antiangiogenic effect of the thrombospondin peptide ABT-510: a complementation antiangiogenic strategy. Clin Cancer Res. (2005) 11:6678–85. 10.1158/1078-0432.CCR-05-0621 [DOI] [PubMed] [Google Scholar]
- 146.Nabors LB, Fiveash JB, Markert JM, Kekan MS, Gillespie GY, Huang Z, et al. A phase 1 trial of ABT-510 concurrent with standard chemoradiation for patients with newly diagnosed glioblastoma. Arch Neurol. (2010) 67:313–9. 10.1001/archneurol.2010.16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Sahora AI, Rusk AW, Henkin J, McKeegan EM, Shi Y, Khanna C. Prospective study of thrombospondin-1 mimetic peptides, ABT-510 and ABT-898, in dogs with soft tissue sarcoma. J Vet Intern Med. (2012) 26:1169–76. 10.1111/j.1939-1676.2012.00966.x [DOI] [PubMed] [Google Scholar]
- 148.Nakamura DS, Edwards AK, Virani S, Thomas R, Tayade C. Thrombospondin-1 mimetic peptide ABT-898 affects neovascularization and survival of human endometriotic lesions in a mouse model. Am J Pathol. (2012) 181:570–82. 10.1016/j.ajpath.2012.05.010 [DOI] [PubMed] [Google Scholar]
- 149.Campbell N, Greenaway J, Henkin J, Petrik J. ABT-898 induces tumor regression and prolongs survival in a mouse model of epithelial ovarian cancer. Mol Cancer Ther. (2011) 10:1876–85. 10.1158/1535-7163.MCT-11-0402 [DOI] [PubMed] [Google Scholar]
- 150.Song Z, Ren D, Xu X, Wang Y. Molecular cross-talk of IL-6 in tumors and new progress in combined therapy. Thorac Cancer. (2018) 9:669–75. 10.1111/1759-7714.12633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Masjedi A, Hashemi V, Hojjat-Farsangi M, Ghalamfarsa G, Azizi G, Yousefi M, et al. The significant role of interleukin-6 and its signaling pathway in the immunopathogenesis and treatment of breast cancer. Biomed Pharmacother. (2018) 108:1415–24. 10.1016/j.biopha.2018.09.177 [DOI] [PubMed] [Google Scholar]
- 152.Rossi JF, Lu ZY, Jourdan M, Klein B. Interleukin-6 as a therapeutic target. Clin Cancer Res. (2015) 21:1248–57. 10.1158/1078-0432.CCR-14-2291 [DOI] [PubMed] [Google Scholar]
- 153.Melchor L, Brioli A, Wardell CP, Murison A, Potter NE, Kaiser MF, et al. Single-cell genetic analysis reveals the composition of initiating clones and phylogenetic patterns of branching and parallel evolution in myeloma. Leukemia. (2014) 28:1705–15. 10.1038/leu.2014.13 [DOI] [PubMed] [Google Scholar]
- 154.Capozzi M, VON Arx C, DE Divitiis C, Ottaiano A, Tatangelo F, Romano GM, et al. Antiangiogenic therapy in pancreatic neuroendocrine tumors. Anticancer Res. (2016) 36:5025–30. 10.21873/anticanres.11071 [DOI] [PubMed] [Google Scholar]
- 155.Raymond E, Dahan L, Raoul JL, Bang YJ, Borbath I, Lombard-Bohas C, et al. Sunitinib malate for the treatment of pancreatic neuroendocrine tumors. N Engl J Med. (2011) 364:501–13. 10.1056/NEJMoa1003825 [DOI] [PubMed] [Google Scholar]
- 156.Gadgeel SM. Targeted therapy and immune therapy for small cell lung cancer. Curr Treatm Opt Oncol. (2018) 19:53. 10.1007/s11864-018-0568-3 [DOI] [PubMed] [Google Scholar]