Abstract
Mesenchymal stem cells (MSCs) can be derived from various adult tissues with multipotent and self‐renewal abilities. The characteristics of presenting no major ethical concerns, having low immunogenicity and possessing immune modulation functions make MSCs promising candidates for stem cell therapies. MSCs could promote inflammation when the immune system is underactivated and restrain inflammation when the immune system is overactivated to avoid self‐overattack. These cells express many immune suppressors to switch them from a pro‐inflammatory phenotype to an anti‐inflammatory phenotype, resulting in immune effector cell suppression and immune suppressor cell activation. We would discuss the mechanisms governing the immune modulation function of these cells in this review, especially the immune‐suppressive effects of MSCs.
Keywords: immune modulation, immune modulators, mesenchymal stem cell, stem cell therapy
1. INTRODUCTION
Mesenchymal stem cells (MSCs), also known as mesenchymal stromal cells, are spindle‐shaped cells with multipotent (chondrocyte, osteoblast and adipocyte) and self‐renewal abilities.1, 2 These cells are derived from various adult tissues,3, 4 attach to tissue culture dishes and express certain cell surface markers (positive for CD73, CD90 and CD105; negative for CD45, CD34, CD14 or CD11b, CD79alpha or CD19, and HLA‐DR).2 MSCs can be safely harvested with no major ethical concerns and have low immunogenicity.3 Therefore, MSCs have been proposed as effective and safe cell sources for stem cell therapy.
Although MSCs have differentiation abilities, the main mechanism of their therapeutic effects in pre‐clinical and clinical studies is believed to be paracrine effects. These paracrine effects include promoting angiogenesis, preventing apoptosis, suppressing inflammation and modulating extracellular matrix dynamics. One of the ways that these cells improve the tissue microenvironments is by modulating immune system components, such as macrophages and neutrophils. After the tissues or cells are injured, the MSCs activate or suppress the immune system to control the whole‐tissue regeneration process.3, 4, 5, 6
Mesenchymal stem cells have been successfully applied in treating various diseases such as diabetes,7 cardiovascular diseases,8 graft‐versus‐host diseases 9 and autoimmune diseases.10 Although many questions remain unanswered the immune modulation effects of MSCs make them promising candidates for cell therapy–based tissue repair and disease treatment, especially for immune system abnormalities, such as cancer and autoimmune diseases. Thus, we will discuss the mechanisms of immune modulation by MSCs. Given the important roles of MSCs in immune suppression to help cancer to escape immune surveillance and their potential roles in immune tolerance re‐establishment, we mainly focus on the immune‐suppressive function of MSCs in the current review.
2. IMMUNE MODULATION BY MSCS
Mesenchymal stem cells could promote inflammation when the immune system is underactivated and restrain inflammation while the immune system is overactivated to avoid self‐overattack. This activity is also known as the function of “sensor and switcher of the immune system” (Figure 1).11 The MSCs could sense different danger signals through TLRs (Toll‐like receptors).12, 13, 14, 15, 16 MSCs express TLR2, TLR3, TLR4, TLR7 and TLR9. The expression levels of these TLRs vary significantly based on their tissue origin.17 TLRs recognize molecules from injured cells or pathogens acting as the first line of the immune defence system. TLR activation can further stimulate immune cells and MSCs.17 Activated MSCs respond to TLR ligands and release anti‐inflammatory factors. Thus, TLRs play an important role in sensing and switching immune responses by MSCs.17 The allogeneic MSCs would be eliminated by NK cells slowly. However, once the MSCs are activated via TLR3 ligand, they could escape from this clearance process by NK cells.18 The type of TLR (TLR3 or TLR4) activation could also induce a pro‐inflammatory or anti‐inflammatory phenotype of MSCs.12, 13, 14 For example, TLR3 activation induces an anti‐inflammatory phenotype of MSCs (also known as the MSC2 phenotype), while TLR4 activation induces a pro‐inflammatory phenotype (also known as the MSC1 phenotype).3, 14
Figure 1.
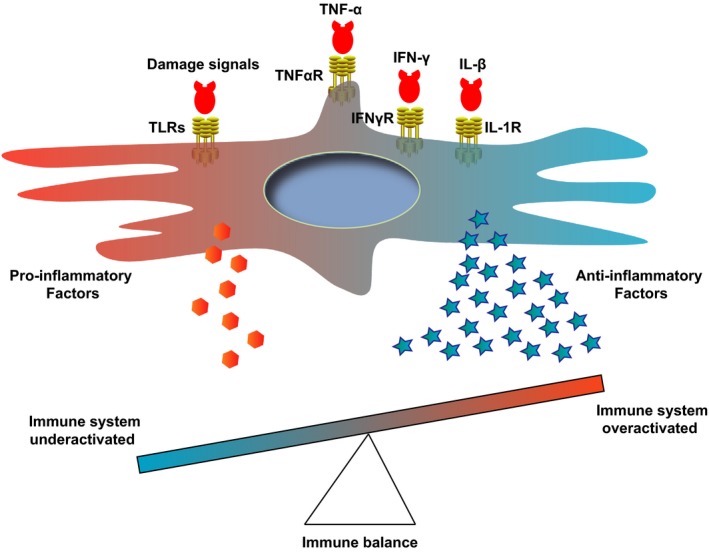
Sensor and switcher model of MSCs. MSCs could sense danger signals through different receptors (such as TLRs) and also respond to excessive pro‐inflammatory signals via receptors for TNF‐α, IFN‐γ and IL‐1β. Depending on the signal types or strength, MSCs secrete cytokines to promote or suppress the immune responses for maintaining the immune balance. IFN‐γ, interferon gamma; IFNγR, interferon gamma receptor; IL‐1R, interleukin‐1 receptor; IL‐1β, interleukin‐1 beta; TLR, Toll‐like receptor; TNF‐α, tumour necrosis factor alpha; TNFαR, tumour necrosis factor alpha receptor
Furthermore, the MSC microenvironment could switch the MSCs between pro‐inflammatory and anti‐inflammatory phenotypes. MSCs have pro‐inflammatory functions in the early stage of inflammation through recruiting neutrophils.19 Pro‐inflammatory MSCs activate T cells by secreting MIP‐1 (macrophage inflammatory protein‐1), CCL5 (C‐C motif ligand 5), CXCL9 (C‐X‐C motif ligand 9) and CXCL10 (C‐X‐C motif ligand 10) and recruiting more lymphocytes.3 At this stage, there are only low levels of inflammation signals, such as TNF‐α and IFN‐λ. MSCs derived from bone marrow and umbilical cord promote immune response when they are treated with low levels of IFN‐γ and TNF‐α, which could not produce sufficient iNOS or IDO to suppress the lymphocytes.20 However, when these two cytokines reach a high level, they stimulate MSCs to secrete iNOS (mice) or IDO (human), resulting in T‐cell proliferation inhibition and Treg induction. Therefore, the iNOS or IDO level has been proposed as the switcher between the pro‐ and anti‐inflammatory effects of MSCs.3 TNF‐α and IFN‐λ are often used for MSC activation.21
3. IMMUNE SUPPRESSION BY MSCS
Immune system components, such as immune molecules and immune cells, protect the host against exogenous pathogen invasion and endogenous cancer development. The understimulated immune system could not protect the host. However, overstimulation would attack the healthy cells and tissues of the host, resulting in tissue or organ destruction. Thus, the immune response must be tightly regulated through different pathways. Uncovering the detailed mechanisms of this regulatory network is critical for understanding the pathogenesis of immune dysfunction–related diseases and developing new therapy strategies. Several cell populations have been demonstrated to prevent immune system overstimulation, including natural and induced CD4+ Treg (T regulatory cells),22 CD8+ Treg,23 Breg (B regulatory cells),24 M2 macrophages25 and suppressive dendritic cells.26 These cells modulate the immune reaction through secreting suppressive cytokines, such as IL‐10, TGF‐β, IL‐35, inhibitory ligand and receptors (such as PD‐1 and PD‐L1), and by directly regulating immune cell differentiation, maturation and survival.
It has been demonstrated that MSCs also represent one type of cell to prevent overstimulation of the immune system. The immune‐suppressive activities of MSCs are primarily stimulated by pro‐inflammatory factors, such as IFN‐γ (interferon gamma), TNF‐α (tumour necrosis factor alpha) and IL‐1β (interleukin‐1 beta).4, 5, 6 Among these factors, IFN‐γ is even more crucial for the immune‐suppressive function of MSCs.27 IFN‐γ stimulates MSCs to express the immune inhibitors PD‐L1 and PD‐L2 (programmed cell death ligands 1 and 2) and downregulates ILTRs (immunoglobulin‐like transcript receptors).28
The immunosuppressive MSCs have downregulated antigen‐presenting molecules (MHC‐I, MHC‐II), co‐stimulators (CD80, CD86, CD40, CD40L) and FasL.3, 21 MSCs also express many chemokines and adhesion proteins to recruit immune cells, such as CXCR3 (C‐X‐C motif chemokine receptor 3) ligands, CCR5 (C‐C motif chemokine receptor 5) ligands, ICAM‐1 (intercellular adhesion molecule 1) and VCAM‐1 (vascular cell adhesion molecule 1).3, 21 MSCs could suppress the inflammation process, partly through downregulating pro‐inflammatory factors and upregulating anti‐inflammatory factors. Furthermore, these cells could suppress immune reactions through direct cell contact.
3.1. Immune modulators expressed by MSCs
Although direct cell contact is important for the immune‐suppressive effects of MSCs, studies have shown that the immune modulators expressed by MSCs are more critical, including indoleamine 2,3‐dioxygenase (IDO), prostaglandin E2 (PGE2), inducible nitric oxide synthase (iNOS), transforming growth factor beta (TGF‐β), interleukin‐10 (IL‐10), hepatocyte growth factor (HGF), histocompatibility locus antigen‐G (HLA‐G), CD39 and CD73, galectins, C‐C motif chemokine ligand 2 (CCL2), programmed cell death ligands 1 and 2 (PD‐L1 and PD‐L2), haem oxygenase 1 (HO‐1), tumour necrosis factor‐stimulated gene 6 (TSG6), interleukin‐1 receptor antagonist (IL1RA) and complement system–related factors (Figure 2).
Figure 2.
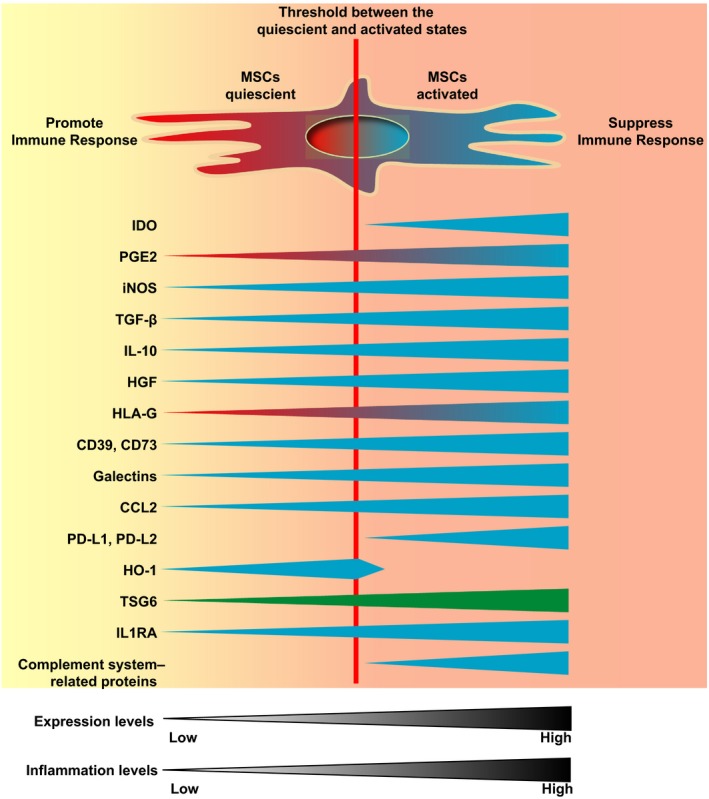
Immune modulators expressed by MSCs. MSCs express many immune modulators under different conditions. Some modulators are expressed in both quiescent and activated states, including PGE2, iNOS, TGF‐β, IL‐10, HGF, CD39 and CD73, galectins, CCL2, TSG6 and IL1RA. Some are expressed only in the activated state, including IDO, PD‐L1 and PD‐L2, and complement system–related proteins. And the HO‐1 is mainly expressed in the quiescent state and decreased sharply in the activated state of MSCs. However, they all are upregulated by pro‐inflammatory factors in the concentration‐dependent manner. Data have shown that low levels of PGE2 and HLA‐G have pro‐inflammation effects, while high levels have anti‐inflammation effects. TSG6 is mainly expressed in the MSC sphere state. Blue and green indicate the anti‐inflammation function; red indicates the pro‐inflammation function. CCL2, C‐C motif chemokine ligand 2; HGF, hepatocyte growth factor; HLA‐G, histocompatibility locus antigen‐G; HO‐1, haem oxygenase 1; IDO, indoleamine 2,3‐dioxygenase; IL‐10, interleukin‐10; IL1RA, interleukin‐1 receptor antagonist; iNOS, inducible nitric oxide synthase; MSCs, mesenchymal stem cells; PD‐L1 and PD‐L2, programmed cell death ligands 1 and 2; PGE2, prostaglandin E2; TGF‐β, transforming growth factor beta; TSG6, tumour necrosis factor‐stimulated gene 6
3.1.1. IDO
IDO has two isoforms, IDO1 and IDO2. These isoforms catalyse tryptophan, an important essential amino acid, into different metabolites, resulting in tryptophan depletion.29 Because tryptophan is essential for T‐cell proliferation,30 tryptophan depletion switches the metabolic pathway from glycolysis to oxidative phosphorylation, resulting in T‐cell arrest.31 Tryptophan reduction also induces the accumulation of uncharged tryptophan tRNA in immune cells, which could activate stress‐response kinase GCN2 (general control nonderepressible 2) and eIF2 (eukaryotic translation initiation factor 2)‐mediated pathways, leading to protein synthesis reduction, cell proliferation inhibition and Fas‐mediated lymphocyte apoptosis.32 GCN2 pathway activation also promotes Treg differentiation while suppressing Th17 conversion through downregulating IL‐6.33 Tryptophan deprivation could induce Treg generation through producing tolerogenic DCs, with downregulation of co‐stimulatory molecules and upregulation of the inhibitory receptors ILT3 (immunoglobulin‐like transcript 3) and ILT4 (immunoglobulin‐like transcript 4) on DCs.34 The tryptophan metabolites (kynurenine, quinolinic acid and picolinic acid) are more toxic to CD4+ Th1 and CD8+ T cells and less toxic to Th2 cells, thereby switching T helper cells from Th1 to Th2.35 Furthermore, the tryptophan metabolite kynurenine could directly bind to AhR (aryl hydrocarbon receptor) and promote CD4+Foxp3+ Treg differentiation while suppressing Th17 generation and decreasing DC immunogenicity.36
IDO is primarily expressed by antigen‐presenting cells.32 MSCs also express and utilize IDO to mediate immune suppression.37 IDO is not expressed in MSCs in the quiescent state but could be induced by IFN‐γ and enhanced by PGE2. Under IFN‐γ stimulation, activated STAT1 (signal transducer and activator of transcription 1), IRF‐1 (interferon regulatory factor‐1) and NF‐B (nuclear factor kappa‐light‐chain‐enhancer of activated B cells) bind to the upstream IFN‐γ–responsive elements of the IDO gene and promote IDO gene expression.38, 39
3.1.2. PGE2
PGE2 is produced by COX‐1 (cyclooxygenase‐1, the constitutive isoform) or COX‐2 (cyclooxygenase‐2, the inducible isoform) from the arachidonic acid released from the membrane phospholipids. PGE2 interacts with EP2 and EP4 receptors expressed on the surface of immune cells and exerts its anti‐inflammatory effects. The interaction between PGE2 and EP2 or EP4 receptors induces cyclic AMP (cAMP) upregulation, which then activates the PKA (protein kinase A) and PI3K (phosphatidylinositol‐3 kinase) pathways. cAMP induces the expression of anti‐inflammatory factors (IL‐4, IL‐5 and IL‐10) and inhibits the expression of pro‐inflammatory factors (IL‐12p70, TNF‐α, CCL3 and CCL4) through IL‐2 pathway suppression. In addition, cAMP promoted M2 macrophage and Th2 cell differentiation and inhibited Th1 production.40, 41, 42 However, some studies have shown that PGE2 has pro‐inflammatory effects with enhancing DC maturation and T‐cell proliferation.43 Later studies have demonstrated that a low concentration of PGE2 promotes an inflammatory response, while a high concentration inhibits.43 PGE2 promotes Foxp3+ Treg cell production.44 PGE2 also promotes TGF‐β secretion from monocytes and induces MDSC (myeloid‐derived suppressor cells) generation, which could suppress NK cell and CD8+ T‐cell activities.45, 46
PGE2 suppresses IL‐12 and promotes IL‐23 expression. IL‐12 (IL‐12p70) is composed of IL‐12p35 and IL‐12p40. The suppression of IL‐12 by PGE2 is mediated through inhibiting IL‐12p35 but not IL‐12p40. PGE2 could increase IL‐23p19 expression, which could form IL‐23 with IL‐12p40. Thus, PGE2 induces IL‐23 expression, which is important for Th17 production.47, 48
MSCs express COX‐2 and produce PGE2,11, 49 which could be further enhanced by inflammatory stimuli or the combination of IFN‐γ and TNF‐α treatment.50 Therefore, these cells produce high amounts of PGE2 to suppress the immune response.51
3.1.3. iNOS
Mesenchymal stem cells express iNOS, which metabolizes L‐arginine to generate NO (nitric oxide).37, 52 NO suppresses the IL‐2 pathways (Janus kinase 3, signal transducer and activator of transcription 5, extracellular signal–regulated kinases and protein kinase B), resulting in T‐cell proliferation and function inhibition.52, 53, 54, 55 NO also induces T‐cell apoptosis and inhibits the expression of MHC‐II.56 NO suppresses the secretion of Th1 and Th2 cytokines.57, 58 When MSCs are stimulated with inflammatory factors, the iNOS gene is upregulated. These cells produce high amounts of NO to suppress the immune response.21, 51 Interestingly, the pro‐inflammatory cytokine IL‐17 could stabilize the iNOS protein in MSCs derived from bone marrow, resulting in immune suppression.59
MSCs from mice, rabbits, rats and hamsters mainly exert suppressive functions through iNOS, while MSCs derived from humans, pigs and monkeys primarily exert suppressive functions through IDO.60 Thus, the mechanism of immune‐suppressive functions of MSCs from different species might differ in the detailed pathways.
3.1.4. TGF‐β
TGF‐β and IL‐10 are the main immune‐regulatory cytokines generated by quiescent MSCs.61, 62 TGF‐β is constitutively secreted by MSCs 63 and further upregulated by inflammatory factors, such as IFN‐γ and TNF‐α.50, 64, 65 TGF‐β inhibits IL‐2, MHC‐II (major histocompatibility complex II) and co‐stimulatory factor expression in DCs and T cells.61, 62 Both Th1 differentiation and Th2 differentiation could be inhibited by TGF‐β.66, 67 TGF‐β promotes Treg and Breg production.61 TGF‐β is one of the key regulators of Foxp3 expression.61, 62 However, it has also been shown that the immune suppression effects of bone marrow‐derived MSCs stimulated with IFN‐γ and TNF‐α are abolished by adding TGF‐β through inhibiting iNOS and IDO expression.68
3.1.5. IL‐10
In addition to TGF‐β, IL‐10 is another main immune‐suppressive cytokine generated by quiescent MSCs. IL‐10 expression could be further enhanced by TLR ligands and PEG2.69 IL‐10 could inhibit antigen‐presenting cell (APC) maturation and the expression of MHC and co‐stimulatory factors.70 IL‐10 inhibits pro‐inflammatory production, T‐cell proliferation and memory T‐cell formation.70 IL‐10 suppresses Th17 generation and promotes Treg formation.71 IL‐10 exerts its anti‐inflammatory effects through the JAK1‐TYK2‐STAT3‐SOCS3 pathway.72
3.1.6. HGF
MSCs express HGF, which exhibits immune suppression effects. HGF induces IL‐10 expression in monocytes, inhibits Th1 and DC activities, and promotes IL‐10–positive Treg cells.73, 74 HGF generated by MSCs also promotes immune‐suppressive MDSC expansion.75
3.1.7. HLA‐G
MSCs secrete HLA‐G5 (one secreted isoform of non‐classical class I MHC with immune‐suppressive functions) under the stimulation of IL‐10, IFN‐γ and TNF‐α.76 HLA‐G binds to the receptors of ILT2 and ILT4, which are widely expressed by monocytes/macrophages, DCs, CD4+ and CD8+ T cells, B cells and NK cells.77 HLA‐G inhibits the cytotoxic function of CD8+ T and NK cells, cytokine production of Th1 and Th17 cells, and induces Treg generation and MDSC expansion.76, 78, 79 However, the immune‐suppressive effects of HLA‐G might also be concentration‐dependent. It has been shown that a high concentration of HLA‐G induces Treg generation, while a low concentration promotes Th1 development.80 HLA‐G also confers the immune privilege characteristics of MSC differentiated derivatives 81, 82
3.1.8. CD39 and CD73
MSCs express CD39 and CD73. CD39 catabolizes ATP to AMP, and CD73 catabolizes AMP to adenosine. Extracellular ATP has pro‐inflammatory effects, while adenosine has anti‐inflammatory effects through the cAMP and PKA pathways. Thus, CD39 and CD73 could cleave extracellular ATP to adenosine and switch pro‐inflammation to anti‐inflammation.83, 84
3.1.9. Galectins
Galectins (Gal) are soluble proteins that bind to cell surface glycoproteins. MSCs express three isoforms of Gal, Gal‐1, Gal‐3 and Gal‐9.85, 86, 87 Gal‐1 binds to Th1 and Th17 but not Th2 cells and induces cell apoptosis.88 Furthermore, Gal‐1 promotes IL‐10 production in Th1 and Th17 cells.89 Gal‐1 suppresses the migration of immunogenic DCs.89 Gal‐1 and Sema‐3A bind to NRP1 (neuropilin 1, expressed on the T‐cell surface) and arrest the T cells in the G0/G1 phase.90 Gal‐9 suppresses B‐ and T‐cell proliferation and is upregulated by IFN‐γ.91
3.1.10. CCL2
Mesenchymal stem cells express CCL2 and the related metalloproteinases that are responsible for CCL2 cleavage. The truncated CCL2 functions as a CCR2 antagonist and inhibits immune cell migration. While the full‐length CCL2 binds to its receptor CCR2, which is expressed by activated Th1, Th17 and NK cells, and recruits them into the inflammation sites, the truncated CCL2 plays a critical role in the autoimmunity suppression by MSCs.
3.1.11. PD‐L1 and PD‐L2
MSCs express PD‐L1 (B7H1) and PD‐L2 (B7DC) under IFN‐γ and TNF‐α stimulation.92, 93, 94 Blocking the PD‐L1 and PD‐L2 pathways significantly impairs the immune‐suppressive effects of MSCs.92, 93 MSCs secreted PD‐L1/L2 bind to PD‐1 and inhibit lymphocyte proliferation.94, 95 PD‐L1 and PD‐L2 could suppress CD4+ T‐cell activation, reduce IL‐2 secretion, silence T cells and induce T‐cell death.94 These factors could also inhibit AKT phosphorylation and upregulate Foxp3 expression, resulting in Treg production.94
3.1.12. HO‐1
Both human and rat MSCs express a high level of HO‐1 in the quiescent state.96 Blocking HO‐1 reduced the immune‐suppressive effects of MSCs.96 HO‐1 could promote IL10+ Tr1 and TGFβ+ Tr3 generation, two types of Treg.97 However, once MSCs are activated by pro‐inflammatory factors, HO‐1 expression decreases rapidly, and the immune‐suppressive function of MSCs is taken over by other suppressive factors, such as iNOS.97
3.1.13. TSG6
The aggregated MSCs and MSC spheres express TSG6, an important immune‐suppressive factor.98, 99 TSG6 could reduce lymphocyte and neutrophil proliferation and decrease metalloproteinase activity and the expression of IL‐6 and IFN‐γ. On the other hand, TSG6 could promote Foxp3+ Treg and IL10+iNOS+ regulatory macrophage expansion.98
3.1.14. IL1RA
IL1RA expressed by MSCs could promote M2 macrophage polarization and Treg generation with elevated IL‐10 expression and suppress CD4+ T‐cell activities. Furthermore, IL1RA could suppress B‐cell differentiation and antibody production.100, 101
3.1.15. Complement system–related proteins
MSCs express C3aR (C3a receptor) and C5aR (C5a receptor), which could be activated by C3a and C5a produced in the inflammation sites. The activated C3aR/C5aR could enhance the resistance to oxidative stress and apoptosis of MSCs.102 On the other hand, CD46, CD55 and CD59 expressed on the surface of MSCs could inhibit complement system activation and prevent MSCs from cell lysis.102, 103 However, once cell lysis is activated by the complementary system, this protection is not sufficient to stop the cell death process.104 Combining IFN‐γ treatment with TNF‐α significantly increases the ability of MSCs to secrete factor H, which is a key molecule involved in the inhibition of complement activation.103
3.2. Immune cells modulated by MSCs
MSCs regulate many types of immune cells through the immune modulators expressed by them, including DC (dendritic cell), monocyte/macrophage, B, Breg (regulatory B cell), T, Treg (regulatory T cell), Th1 (T helper cell, type 1), Th2 (T helper cell, type 2), Th17 (T helper cell, type 17), NK (natural killer cell), NKT (natural killer T cell), ILC (innate lymphoid cell), MDSC (myeloid‐derived suppressor cells), neutrophils and mast cells (Figure 3).62
Figure 3.
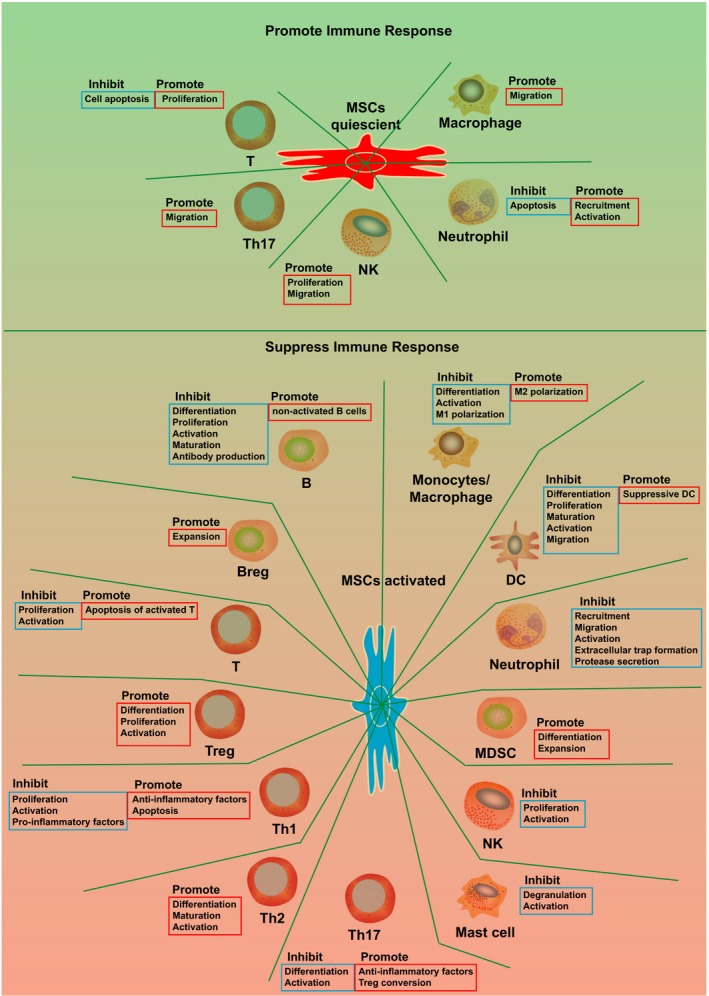
Immune cells modulated by MSCs. MSCs regulate many immune cells from different perspectives, either promoting or suppressing the immune cells. Blue frame indicates inhibiting the functions; red frame indicates promoting the functions. Breg: regulatory B cell; DC: dendritic cell; MDSC: myeloid‐derived suppressor cells; MSCs: mesenchymal stem cells; NK: natural killer cell; Th1: T helper cell, type 1; Th17: T helper cell, type 17; Th2: T helper cell, type 2; Treg: regulatory T cell
3.2.1. DC
Mesenchymal stem cells suppress DC differentiation, maturation and activation and compromise their antigen presentation abilities.105, 106, 107, 108, 109 MSCs inhibit DC differentiation from monocytes or CD34+ HSCs (hematopoietic stem cells), resulting in immature DC production and immune suppression.105, 110 MSCs could downregulate the expression of HLA II, CD80, CD86 and IL‐12 in DCs, resulting in the inhibition of DC maturation and activation.110, 111, 112
For mature DCs, MSCs could inhibit DC migration by downregulating CCR7 and CD49dβ1 and decreasing inflammatory factor expression and antigen presentation abilities.109, 113, 114, 115 MSCs could switch the mature DCs into a suppressive immature phenotype through the Jagged1 or IL‐10‐SOCS3 pathway.116, 117, 118 MSCs promote IL‐10–positive pDCs (plasmacytoid DCs) differentiation,11 which would promote Treg development.119
Mesenchymal stem cells could also induce DCs into an anti‐inflammatory phenotype through downregulating the pro‐inflammatory factors (TNF‐α and IL‐12) and upregulating the anti‐inflammatory factors, such as IL‐10,11, 120 PGE2121, 122 and M‐CSF (macrophage colony–stimulating factor).123, 124 MSCs induce DCs to secrete IL‐10 and then inhibit T‐cell activation.11, 120 MSCs also express high levels of PGE2, which binds to its receptor EP4 on DCs and exerts inhibitory effects.121, 122
The direct contact between MSCs and DCs activates the Notch pathway and suppresses DC generation106 and proliferation.125 Furthermore, MSCs block the cell interaction between DCs and lymphocytes.126 On the other hand, DCs support MSC survival through lymphotoxin‐β expression.127
3.2.2. Monocytes/Macrophages
Monocyte modulation is the critical step in the immune modulation process, as depleting monocytes would abolish the immune‐suppressive effects of MSCs.128, 129 These results support the hypothesis that the immune‐suppressive effects of MSCs are mainly induced through monocyte/macrophage modulation by MSCs.
Mesenchymal stem cells inhibit monocyte differentiation from CD34+ HSCs.105, 122, 123 These cells could also induce M2 macrophage polarization, which expresses high levels of immune‐suppressive factors (such as IL‐10) and low levels of immune activators (such as IL‐6, IL‐12, TNF‐α, IL‐1β, IL‐23, CD86 and MHC‐II).108, 128, 129, 130, 131, 132, 133 MSCs derived from bone marrow and placenta could induce tolerogenic monocytes and M2 macrophages through IL‐10 and B7‐H4 expression.108, 134, 135 The TGF‐β pathway is also involved in the M2 macrophage polarization process mediated by MSCs.136
Under an inflammatory environment, MSCs could recruit macrophages to inflamed sites and enhance tissue regeneration and immune regulation.137, 138 MSCs sensitize inflammatory factors and switch macrophages from the M1 (pro‐inflammatory) to the M2 (anti‐inflammatory) phenotype through IDO (indoleamine 2,3‐dioxygenase), CCL18 (C‐C motif ligand 18) and PGE2 (prostaglandin E2).63 The M2 macrophage polarization effect of MSCs is further enhanced by pro‐inflammatory factor stimulation.101, 128, 134, 139, 140, 141, 142, 143 Inflammatory factors (such as IFN‐γ, TNF‐α and LPS) could stimulate MSCs to express immune‐suppressive factors, such as PGE2, IDO and COX2.42, 143 The PGE2 released by MSCs binds to its receptors EP2 and EP4 on macrophages and activates downstream pathways to polarize macrophages to M2 phenotype.134 MSCs also express IL‐1RA to suppress the immune response and induce M2 macrophage polarization.101, 140
Furthermore, the activated MSCs secrete the immune‐suppressive factor TSG‐6 and inhibit the activation of newly differentiated macrophages.139 TSG‐6 interacts with CD44 expressed on macrophages and decreases the nuclear translocation of NF‐κB.139
3.2.3. B cells
Although the detailed mechanisms are still lacking, it has been demonstrated that MSCs inhibit B‐cell differentiation, proliferation, activation and antibody production indirectly or directly.101, 144, 145, 146, 147, 148, 149, 150, 151, 152, 153, 154, 155
MSCs secrete IL‐1RA to inhibit B‐cell maturation.101 MSCs secrete CCL2, which inhibits STAT3 activation and induces PAX5 expression, to suppress antibody production in B cells.150 MSCs also directly inhibit B‐cell activity through the PD‐1/PD‐L1 pathway.156
Furthermore, MSCs promote non‐activated B cells (naive, transitional and memory subsets) formation.148, 150, 157 Non‐activated B cells promote Treg differentiation.158 However, it was also shown that MSCs could induce purified B‐cell proliferation and differentiation.157 Later studies have demonstrated that B‐cell suppression by MSCs requires signals from T cells.147
3.2.4. Regulatory B cells
Mesenchymal stem cells could promote Breg production (CD19+CD24highCD38high in humans and CD19+CD1dhighCD5+ in mice) with IL‐10 expression.9, 101, 148, 154, 159, 160 The expansion of Breg cells promoted by MSCs might account for the total B‐cell population expansion in some studies.152, 161
3.2.5. T cells
Mesenchymal stem cells inhibit T‐cell proliferation and activation regardless of the species and tissue origins.162, 163, 164, 165, 166, 167, 168, 169, 170, 171 MSCs inhibit T cells directly or indirectly by inducing the suppressive Tr1 (CD4+IL‑10+ cells) 172 and Treg cells (CD4+CD25+Foxp3+ or CD4+IL‐10+IFN‐γ+),6, 173 which would further inhibit the T cells.174, 175 MSCs express erythropoietin‐producing hepatocellular (EPH) receptor B2 (EPHB2) and ephrinB2, while T cells express EPHB4 and ephrinB1.163 The direct interaction between MSCs and T cells through EPHB2/ephrinB1 and ephrinB2/EPHB4 is essential for the immune‐suppressive effects, as blocking these interactions reduces the suppressive effects.163 Furthermore, these interactions would enhance the expression of IDO and iNOS.163 EPHB2 and ephrinB2 treatment decreased the expression of TNF‐α, IL‐2 and IL‐17 in T cells.176
The T‐cell suppression effects of MSCs rely on the high cell ratio of MSCs to T cells.166 The low cell ratio stimulates T‐cell proliferation.162 MSCs even protect T cells from apoptosis in the quiescent state.177 Activated MSCs express PD‐L1 and FasL, which inhibit CD69 expression and T‐cell proliferation.178 MSCs also express HLA‐G1, TGF‐β and HGF to inhibit T‐cell proliferation through downregulating phosphoretinoblastoma (pRb), cyclin D and cyclin A while upregulating cyclin‐dependent kinase inhibitor 1B (p27Kip1), resulting in cycle arrest to G1 phase.168, 169, 170, 179 MSCs could induce activated T cells into apoptosis through converting tryptophan into kynurenine 180 and the Fas/FasL pathway.181 MSCs secrete galectin‐1, galectin‐3 and galectin‐9 to inhibit T‐cell activities.85, 86, 87 The interactions between galectin‐9 and TIM3 (T‐cell immunoglobulin domain and mucin domain 3) lead to cell apoptosis.91, 182, 183 Furthermore, MSCs inhibit the antigen‐specific proliferation of memory T cells 166 and induce memory Treg cells (CD3+CD45RO+).184 However, the T‐cell suppression effects of MSCs could be abolished by treatment with IL‐2.168
3.2.6. Regulatory T cells
Mesenchymal stem cells promote Treg differentiation through both direct cell contact and paracrine effects (such as PGE2, TGF‐β, HLA‐G5 and IL‐10).76, 185, 186 MSCs could directly induce Treg differentiation through the TLR‐Notch pathway118, 187, 188 and the secretion of TGF‐β1 (transforming growth factor beta 1), IDO and iNOS (inducible nitric oxide synthase).63 MSCs also promote IL‐10 production and inhibit IFN‐γ and IL‐17 secretion, resulting in promoting Treg differentiation of CD4+ T cells and suppressing Th1 and Th17 differentiation.76, 189 Furthermore, MSCs express GILZ (glucocorticoid‐induced leucine zipper) to induce regulatory Th17 cells with immune‐suppressive effects190 and Treg cells.191 MSCs promote CD8+CD28‐ Treg generation and activities192 through upregulating IL‐10 and FasL.192 MSCs also promote IL10+ Tr1 and TGFβ+ Th3 production through HO‐1.97
3.2.7. Th1
Mesenchymal stem cells exert immune‐suppressive effects through inhibiting Th1 type pro‐inflammatory factor expression (such as IFN‐γ, TNF‐α and IL‐1β) and enhancing Th2 type factor expression.11 MSCs promote Th1 cells to secrete the immune suppressor IL‐10 and thus repress the immune responses.193 MSCs also inhibit Th1 cell activation indirectly through suppressing DC and NK cells.113
3.2.8. Th2
Mesenchymal stem cells induce the differentiation and maturation of Th2 cells through IDO expression, which causes tryptophan depletion and tryptophan metabolite production.194 The tryptophan metabolites also induced Th1 cell apoptosis.195
3.2.9. Th17
Mesenchymal stem cells could inhibit Th17 cell differentiation and function directly or indirectly.196 MSCs enhance CD54 expression and recruit Th17 cells onto MSCs through CCR6‐CCL20.186 MSCs could inhibit Th17 differentiation through upregulating PD‐1, IL‐10, CCL2 or SOCS3186, 197, 198, 199 and inhibiting the STAT3 pathway.198, 199 STAT3 pathway inhibition reduces Th17 differentiation through downregulating RORt and IL‐17 expression.200 Th17 inhibition by MSCs also involves PGE2.186 The MSCs could even convert the Th17 cells into Treg cells.201 Although some studies have shown that MSCs could promote Th17 expansion,202, 203 these studies have limitations that might require further investigation. For example, Guo et al202 demonstrated that bone marrow‐derived MSCs promoted IL‐17 expression and Th17 cell differentiation in mixed lymphocyte reaction experiments. However, these researchers did not find the upregulation of Treg cell,202 which should be normally observed in this assay. Thus, the quality and quantity of the MSCs might affect the findings.
3.2.10. NK
MSCs could inhibit the proliferation, activation and activities of NK cells.11, 204, 205, 206, 207, 208, 209 However, the inhibitory effects of MSCs on NK cells also require a high cell ratio of MSCs to NK cells.205 IDO and PGE2 play important roles in these suppressive effects.205, 206 MSCs induce NK cells to upregulate CD73 expression.210 CD73 could convert AMP into adenosine, the anti‐inflammation inducer.211 The TLR4 expressed on MSCs also mediates direct contact with NK cells.208, 212 Furthermore, MSCs inhibit the activation and proliferation of γδT cells and invariant NKT cells.213
On the other hand, it has been demonstrated that MSCs support NK cell proliferation at the low cell ratio of MSCs to NK cells.214 IL‐12‐ or IL‐18–stimulated MSCs promote IFN‐γ secretion from NK cells.215 Furthermore, the NK cell–secreted IFN‐γ promotes MSCs expressing CCL2, which would further enhance IFN‐γ secretion from NK cells.216 NK cells also secrete CCL5 and CXCL7 to recruit MSCs.217 Activated NK cells could induce MSC death.205, 218
3.2.11. NKT
MSCs inhibit the expansion and activity of NKT cells through both direct cell contact and paracrine modulators,219, 220 such as PGE2,221 IDO222 and iNOS.223
3.2.12. ILC
Mesenchymal stem cells support the differentiation of ILC2 (group 2 innate lymphoid cells),224 and the expansion and activity of ILC3 (group 3 innate lymphoid cells).225 Furthermore, ILC3 also promotes the activity of MSCs.225 Both ILC2 and ILC3 have tissue‐protective functions, such as anti‐inflammation and promoting tissue regeneration.226, 227 Recently, it has been demonstrated that ILC3 could support and generate Treg cells by secreting IL‐2.228
3.2.13. MDSC
MSCs promote MDSC (CD14‐CD11b+CD33+ in humans and Gr‐1+CD11b+ in mice) generation and expansion.75 MSCs could induce MDSCs to express iNOS and arginase, which suppress T‐cell activity and promote Treg expansion. These supportive effects on MDSCs occur through HGF secretion from MSCs. HGF interacts with its receptor c‐Met expressed by MDSCs, induces phosphorylation of STAT3 and thus promotes MDSC proliferation.75
3.2.14. Neutrophils
MSCs suppress neutrophil recruitment, activation, extracellular trap formation and protease secretion by secreting superoxide dismutase‐3.229, 230, 231 However, some reports have shown that MSCs protect neutrophils from apoptosis, promote their function through the IL‐6 and STAT3 pathways,232, 233, 234 and promote neutrophil recruitment through IL‐8 and MIF (macrophage migration inhibitory factor) secreted by MSCs.235 This feature is correlated with the pro‐inflammation phenotype of MSCs.12, 107, 236, 237 Thus, neutrophil modulation by MSCs might also depend on the pro‐inflammatory or anti‐inflammatory phenotype of MSCs.
3.2.15. Mast cells
Mesenchymal stem cells could inhibit the immune activities of mast cells, including inflammatory cytokine expression, degranulation and chemotaxis abilities, via COX2‐PGE2 and TGF‐β1 pathways.238, 239, 240, 241, 242 MSCs secrete PGE2 via upregulating COX2. Then, PGE2 recognizes and activates the EP4 receptor expressed on mast cells, resulting in mast cell suppression.240
4. CONCLUSION
Although further efforts should be made to understand the biological roles of MSCs in immunological modulation, the basic concept about the function of MSCs is becoming clear. Perivascular MSCs sense danger signals through receptors, such as TLRs. Then, the MSCs recruit immune cells and promote inflammation. At the later stage of inflammation, MSCs are activated by excessive pro‐inflammatory factors and begin to suppress inflammation to avoid self‐attack. These cells express many immune suppressors to switch them from a pro‐inflammatory phenotype to an anti‐inflammatory phenotype, resulting in immune effector cell suppression and immune suppressor cell activation.
CONFLICT OF INTEREST
The authors declare no commercial or financial conflict of interest.
AUTHOR CONTRIBUTION
WJ collected the updated references; JX wrote the manuscript and draw the figures.
FUTURE PERSPECTIVES
Since the first demonstration of MSCs, many achievements have been made to understand their localization, function and underlying mechanisms. However, many unresolved issues still need to be addressed. In this section, we would like to raise three main questions that should be answered in the coming future. First, what is the specific cell marker for MSCs? Several MSC markers have been demonstrated, but none of them are specific.243 Second, what is the role of MSCs in situ? Most of the studies to date are based on in vitro experiments or transplanting the in vitro expanded MSCs into the host. Whether or how much the in vitro culture conditions could affect the phenotype or function of MSCs requires further investigation. Third, is it the time for us to reconsider the appropriateness of the MSC definition? It has been understood for a long time that MSCs are heterogeneous. The MSCs defined by current widely used criteria actually contain several cell populations. Heterogeneity may make the research conclusions controversial. Instead of studying the mixed populations of MSCs, in the future, it might be better to focus on specific subpopulations with specific cell markers and functions.
ACKNOWLEDGEMENTS
This work was supported by Medical Foundation of Guangdong (A2018308), Natural Science Foundation of SZU (2017083), Natural Science Foundation of Shenzhen (JCYJ20180305163407913, JCYJ20170302152735071, KQJSCX20180328093434771) and Natural Science Foundation of Guangdong Province (2017A030313904, 2018A030310643).
Jiang W, Xu J. Immune modulation by mesenchymal stem cells. Cell Prolif. 2020;53:e12712 10.1111/cpr.12712
REFERENCES
- 1. Pittenger MF, Mackay AM, Beck SC, et al. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284(5411):143‐147. [DOI] [PubMed] [Google Scholar]
- 2. Dominici M, Le Blanc K, Mueller I, et al. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8(4):315‐317. [DOI] [PubMed] [Google Scholar]
- 3. Wang Y, Chen X, Cao W, Shi Y. Plasticity of mesenchymal stem cells in immunomodulation: pathological and therapeutic implications. Nat Immunol. 2014;15(11):1009‐1016. [DOI] [PubMed] [Google Scholar]
- 4. Uccelli A, Moretta L, Pistoia V. Mesenchymal stem cells in health and disease. Nat Rev Immunol. 2008;8(9):726‐736. [DOI] [PubMed] [Google Scholar]
- 5. Keating A. Mesenchymal stromal cells: new directions. Cell Stem Cell. 2012;10(6):709‐716. [DOI] [PubMed] [Google Scholar]
- 6. Bernardo ME, Fibbe WE. Mesenchymal stromal cells: sensors and switchers of inflammation. Cell Stem Cell. 2013;13(4):392‐402. [DOI] [PubMed] [Google Scholar]
- 7. Jurewicz M, Yang S, Augello A, et al. Congenic mesenchymal stem cell therapy reverses hyperglycemia in experimental type 1 diabetes. Diabetes. 2010;59(12):3139‐3147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Xu J‐Y, Lee Y‐K, Ran X, et al. Generation of Induced Cardiospheres via Reprogramming of Skin Fibroblasts for Myocardial Regeneration. Stem Cells. 2016;34(11):2693‐2706. [DOI] [PubMed] [Google Scholar]
- 9. Peng Y, Chen X, Liu Q, et al. Mesenchymal stromal cells infusions improve refractory chronic graft versus host disease through an increase of CD5+ regulatory B cells producing interleukin 10. Leukemia. 2015;29(3):636‐646. [DOI] [PubMed] [Google Scholar]
- 10. Cipriani P, Carubbi F, Liakouli V, et al. Stem cells in autoimmune diseases: Implications for pathogenesis and future trends in therapy. Autoimmun Rev. 2013;12(7):709‐716. [DOI] [PubMed] [Google Scholar]
- 11. Aggarwal S, Pittenger MF. Human mesenchymal stem cells modulate allogeneic immune cell responses. Blood. 2005;105(4):1815‐1822. [DOI] [PubMed] [Google Scholar]
- 12. Romieu‐Mourez R, Francois M, Boivin MN, Bouchentouf M, Spaner DE, Galipeau J. Cytokine modulation of TLR expression and activation in mesenchymal stromal cells leads to a proinflammatory phenotype. J Immunol. 2009;182(12):7963‐7973. [DOI] [PubMed] [Google Scholar]
- 13. Zhao X, Liu D, Gong W, et al. The toll‐like receptor 3 ligand, poly(I:C), improves immunosuppressive function and therapeutic effect of mesenchymal stem cells on sepsis via inhibiting MiR‐143. Stem Cells. 2014;32(2):521‐533. [DOI] [PubMed] [Google Scholar]
- 14. Waterman RS, Tomchuck SL, Henkle SL, Betancourt AM. A new mesenchymal stem cell (MSC) paradigm: polarization into a pro‐inflammatory MSC1 or an Immunosuppressive MSC2 phenotype. PLoS ONE. 2010;5(4):e10088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Opitz CA, Litzenburger UM, Lutz C, et al. Toll‐like receptor engagement enhances the immunosuppressive properties of human bone marrow‐derived mesenchymal stem cells by inducing indoleamine‐2,3‐dioxygenase‐1 via interferon‐beta and protein kinase R. Stem Cells. 2009;27(4):909‐919. [DOI] [PubMed] [Google Scholar]
- 16. Tomchuck SL, Zwezdaryk KJ, Coffelt SB, Waterman RS, Danka ES, Scandurro AB. Toll‐like receptors on human mesenchymal stem cells drive their migration and immunomodulating responses. Stem Cells. 2008;26(1):99‐107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Delarosa O, Dalemans W, Lombardo E. Toll‐like receptors as modulators of mesenchymal stem cells. Front Immunol. 2012;3:182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Giuliani M, Bennaceur‐Griscelli A, Nanbakhsh A, et al. TLR ligands stimulation protects MSC from NK killing. Stem Cells. 2014;32(1):290‐300. [DOI] [PubMed] [Google Scholar]
- 19. Kolaczkowska E, Kubes P. Neutrophil recruitment and function in health and inflammation. Nat Rev Immunol. 2013;13(3):159‐175. [DOI] [PubMed] [Google Scholar]
- 20. Li W, Ren G, Huang Y, et al. Mesenchymal stem cells: a double‐edged sword in regulating immune responses. Cell Death Differ. 2012;19(9):1505‐1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ren G, Zhang L, Zhao X, et al. Mesenchymal stem cell‐mediated immunosuppression occurs via concerted action of chemokines and nitric oxide. Cell Stem Cell. 2008;2(2):141‐150. [DOI] [PubMed] [Google Scholar]
- 22. Miyara M, Ito Y, Sakaguchi S. TREG‐cell therapies for autoimmune rheumatic diseases. Nat Rev Rheumatol. 2014;10(9):543‐551. [DOI] [PubMed] [Google Scholar]
- 23. Li S, Xie Q, Zeng Y, et al. A naturally occurring CD8(+)CD122(+) T‐cell subset as a memory‐like Treg family. Cell Mol Immunol. 2014;11(4):326‐331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Mauri C, Bosma A. Immune regulatory function of B cells. Annu Rev Immunol. 2012;30:221‐241. [DOI] [PubMed] [Google Scholar]
- 25. Vieira‐Potter VJ. Inflammation and macrophage modulation in adipose tissues. Cell Microbiol. 2014;16(10):1484‐1492. [DOI] [PubMed] [Google Scholar]
- 26. Garcia‐Gonzalez P, Ubilla‐Olguin G, Catalan D, Schinnerling K, Aguillon JC. Tolerogenic dendritic cells for reprogramming of lymphocyte responses in autoimmune diseases. Autoimmun Rev. 2016;15(11):1071‐1080. [DOI] [PubMed] [Google Scholar]
- 27. Krampera M. Mesenchymal stromal cell 'licensing': a multistep process. Leukemia. 2011;25(9):1408‐1414. [DOI] [PubMed] [Google Scholar]
- 28. Parolini O, Alviano F, Bagnara GP, et al. Concise review: isolation and characterization of cells from human term placenta: outcome of the first international Workshop on Placenta Derived Stem Cells. Stem Cells. 2008;26(2):300‐311. [DOI] [PubMed] [Google Scholar]
- 29. Meisel R, Zibert A, Laryea M, Gobel U, Daubener W, Dilloo D. Human bone marrow stromal cells inhibit allogeneic T‐cell responses by indoleamine 2,3‐dioxygenase‐mediated tryptophan degradation. Blood. 2004;103(12):4619‐4621. [DOI] [PubMed] [Google Scholar]
- 30. Munn DH, Shafizadeh E, Attwood JT, Bondarev I, Pashine A, Mellor AL. Inhibition of T cell proliferation by macrophage tryptophan catabolism. J Exp Med. 1999;189(9):1363‐1372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Böttcher M, Hofmann AD, Bruns H, et al. Mesenchymal stromal cells disrupt mTOR‐signaling and aerobic glycolysis during T‐cell activation. Stem Cells. 2016;34(2):516‐521. [DOI] [PubMed] [Google Scholar]
- 32. Munn DH, Mellor AL. Indoleamine 2,3 dioxygenase and metabolic control of immune responses. Trends Immunol. 2013;34(3):137‐143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Sharma MD, Hou D‐Y, Liu Y, et al. Indoleamine 2,3‐dioxygenase controls conversion of Foxp3+ Tregs to TH17‐like cells in tumor‐draining lymph nodes. Blood. 2009;113(24):6102‐6111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Brenk M, Scheler M, Koch S, et al. Tryptophan deprivation induces inhibitory receptors ILT3 and ILT4 on dendritic cells favoring the induction of human CD4+CD25+ Foxp3+ T regulatory cells. J Immunol. 2009;183(1):145‐154. [DOI] [PubMed] [Google Scholar]
- 35. Xu H, Oriss TB, Fei M, et al. Indoleamine 2,3‐dioxygenase in lung dendritic cells promotes Th2 responses and allergic inflammation. Proc Natl Acad Sci USA. 2008;105(18):6690‐6695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Nguyen NT, Kimura A, Nakahama T, et al. Aryl hydrocarbon receptor negatively regulates dendritic cell immunogenicity via a kynurenine‐dependent mechanism. Proc Natl Acad Sci USA. 2010;107(46):19961‐19966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Ren G, Su J, Zhang L, et al. Species variation in the mechanisms of mesenchymal stem cell‐mediated immunosuppression. Stem Cells. 2009;27(8):1954‐1962. [DOI] [PubMed] [Google Scholar]
- 38. Chon SY, Hassanain HH, Gupta SL. Cooperative role of interferon regulatory factor 1 and p91 (STAT1) response elements in interferon‐gamma‐inducible expression of human indoleamine 2,3‐dioxygenase gene. J Biol Chem. 1996;271(29):17247‐17252. [DOI] [PubMed] [Google Scholar]
- 39. Dai W, Gupta SL. Regulation of indoleamine 2,3‐dioxygenase gene expression in human fibroblasts by interferon‐gamma. Upstream control region discriminates between interferon‐gamma and interferon‐alpha. J Biol Chem. 1990;265(32):19871‐19877. [PubMed] [Google Scholar]
- 40. Betz M, Fox BS. Prostaglandin E2 inhibits production of Th1 lymphokines but not of Th2 lymphokines. J Immunol. 1991;146(1):108‐113. [PubMed] [Google Scholar]
- 41. Meyer F, Ramanujam KS, Gobert AP, James SP, Wilson KT. Cutting edge: cyclooxygenase‐2 activation suppresses Th1 polarization in response to Helicobacter pylori. J Immunol. 2003;171(8):3913‐3917. [DOI] [PubMed] [Google Scholar]
- 42. Ylostalo JH, Bartosh TJ, Coble K, Prockop DJ. Human mesenchymal stem/stromal cells cultured as spheroids are self‐activated to produce prostaglandin E2 that directs stimulated macrophages into an anti‐inflammatory phenotype. Stem Cells. 2012;30(10):2283‐2296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Sreeramkumar V, Fresno M, Cuesta N. Prostaglandin E2 and T cells: friends or foes? Immunol Cell Biol. 2012;90(6):579‐586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Sharma S, Yang SC, Zhu L, et al. Tumor cyclooxygenase‐2/prostaglandin E2‐dependent promotion of FOXP3 expression and CD4+ CD25+ T regulatory cell activities in lung cancer. Cancer Res. 2005;65(12):5211‐5220. [DOI] [PubMed] [Google Scholar]
- 45. Mao Y, Sarhan D, Steven A, Seliger B, Kiessling R, Lundqvist A. Inhibition of tumor‐derived prostaglandin‐e2 blocks the induction of myeloid‐derived suppressor cells and recovers natural killer cell activity. Clin Cancer Res. 2014;20(15):4096‐4106. [DOI] [PubMed] [Google Scholar]
- 46. Fujita M, Kohanbash G, Fellows‐Mayle W, et al. COX‐2 blockade suppresses gliomagenesis by inhibiting myeloid‐derived suppressor cells. Cancer Res. 2011;71(7):2664‐2674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Rodriguez M, Domingo E, Municio C, et al. Polarization of the innate immune response by prostaglandin E2: a puzzle of receptors and signals. Mol Pharmacol. 2014;85(1):187‐197. [DOI] [PubMed] [Google Scholar]
- 48. Khayrullina T, Yen JH, Jing H, Ganea D. In vitro differentiation of dendritic cells in the presence of prostaglandin E2 alters the IL‐12/IL‐23 balance and promotes differentiation of Th17 cells. J Immunol. 2008;181(1):721‐735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Jarvinen L, Badri L, Wettlaufer S, et al. Lung resident mesenchymal stem cells isolated from human lung allografts inhibit T cell proliferation via a soluble mediator. J Immunol. 2008;181(6):4389‐4396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. English K, Barry FP, Field‐Corbett CP, Mahon BP. IFN‐gamma and TNF‐alpha differentially regulate immunomodulation by murine mesenchymal stem cells. Immunol Lett. 2007;110(2):91‐100. [DOI] [PubMed] [Google Scholar]
- 51. Crop MJ, Baan CC, Korevaar SS, et al. Inflammatory conditions affect gene expression and function of human adipose tissue‐derived mesenchymal stem cells. Clin Exp Immunol. 2010;162(3):474‐486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Sato K, Ozaki K, Oh I, et al. Nitric oxide plays a critical role in suppression of T‐cell proliferation by mesenchymal stem cells. Blood. 2007;109(1):228‐234. [DOI] [PubMed] [Google Scholar]
- 53. Bingisser RM, Tilbrook PA, Holt PG, Kees UR. Macrophage‐derived nitric oxide regulates T cell activation via reversible disruption of the Jak3/STAT5 signaling pathway. J Immunol. 1998;160(12):5729‐5734. [PubMed] [Google Scholar]
- 54. Mazzoni A, Bronte V, Visintin A, et al. Myeloid suppressor lines inhibit T cell responses by an NO‐dependent mechanism. J Immunol. 2002;168(2):689‐695. [DOI] [PubMed] [Google Scholar]
- 55. Moriggl R, Topham DJ, Teglund S, et al. Stat5 is required for IL‐2‐induced cell cycle progression of peripheral T cells. Immunity. 1999;10(2):249‐259. [DOI] [PubMed] [Google Scholar]
- 56. Harari O, Liao JK. Inhibition of MHC II gene transcription by nitric oxide and antioxidants. Curr Pharm Des. 2004;10(8):893‐898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Taylor‐Robinson AW, Liew FY, Severn A, et al. Regulation of the immune response by nitric oxide differentially produced by T helper type 1 and T helper type 2 cells. Eur J Immunol. 1994;24(4):980‐984. [DOI] [PubMed] [Google Scholar]
- 58. Bauer H, Jung T, Tsikas D, Stichtenoth DO, Frolich JC, Neumann C. Nitric oxide inhibits the secretion of T‐helper 1‐ and T‐helper 2‐associated cytokines in activated human T cells. Immunology. 1997;90(2):205‐211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Han X, Yang Q, Lin L, et al. Interleukin‐17 enhances immunosuppression by mesenchymal stem cells. Cell Death Differ. 2014;21(11):1758‐1768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Su J, Chen X, Huang Y, et al. Phylogenetic distinction of iNOS and IDO function in mesenchymal stem cell‐mediated immunosuppression in mammalian species. Cell Death Differ. 2014;21(3):388‐396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Mattar P, Bieback K. Comparing the immunomodulatory properties of bone marrow, adipose tissue, and birth‐associated tissue mesenchymal stromal cells. Front Immunol. 2015;6:560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Najar M, Raicevic G, Fayyad‐Kazan H, Bron D, Toungouz M, Lagneaux L. Mesenchymal stromal cells and immunomodulation: A gathering of regulatory immune cells. Cytotherapy. 2016;18(2):160‐171. [DOI] [PubMed] [Google Scholar]
- 63. Melief SM, Schrama E, Brugman MH, et al. Multipotent stromal cells induce human regulatory T cells through a novel pathway involving skewing of monocytes toward anti‐inflammatory macrophages. Stem Cells. 2013;31(9):1980‐1991. [DOI] [PubMed] [Google Scholar]
- 64. Ryan JM, Barry F, Murphy JM, Mahon BP. Interferon‐gamma does not break, but promotes the immunosuppressive capacity of adult human mesenchymal stem cells. Clin Exp Immunol. 2007;149(2):353‐363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Engela AU, Baan CC, Peeters AM, Weimar W, Hoogduijn MJ. Interaction between adipose tissue‐derived mesenchymal stem cells and regulatory T‐cells. Cell Transplant. 2013;22(1):41‐54. [DOI] [PubMed] [Google Scholar]
- 66. Heath VL, Murphy EE, Crain C, Tomlinson MG, O'Garra A. TGF‐beta1 down‐regulates Th2 development and results in decreased IL‐4‐induced STAT6 activation and GATA‐3 expression. Eur J Immunol. 2000;30(9):2639‐2649. [DOI] [PubMed] [Google Scholar]
- 67. Gorelik L, Constant S, Flavell RA. Mechanism of transforming growth factor beta‐induced inhibition of T helper type 1 differentiation. J Exp Med. 2002;195(11):1499‐1505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Xu C, Yu P, Han X, et al. TGF‐beta promotes immune responses in the presence of mesenchymal stem cells. J Immunol. 2014;192(1):103‐109. [DOI] [PubMed] [Google Scholar]
- 69. Saraiva M, O'Garra A. The regulation of IL‐10 production by immune cells. Nat Rev Immunol. 2010;10(3):170‐181. [DOI] [PubMed] [Google Scholar]
- 70. Moore KW, de Waal MR, Coffman RL, O'Garra A. Interleukin‐10 and the interleukin‐10 receptor. Annu Rev Immunol. 2001;19:683‐765. [DOI] [PubMed] [Google Scholar]
- 71. Chaudhry A, Samstein R, Treuting P, et al. Interleukin‐10 signaling in regulatory T cells is required for suppression of Th17 cell‐mediated inflammation. Immunity. 2011;34(4):566‐578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Walter MR. The molecular basis of IL‐10 function: from receptor structure to the onset of signaling. Curr Top Microbiol Immunol. 2014;380:191‐212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Benkhoucha M, Santiago‐Raber M‐L, Schneiter G, et al. Hepatocyte growth factor inhibits CNS autoimmunity by inducing tolerogenic dendritic cells and CD25+Foxp3+ regulatory T cells. Proc Natl Acad Sci USA. 2010;107(14):6424‐6429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Chen P‐M, Liu K‐J, Hsu P‐J, et al. Induction of immunomodulatory monocytes by human mesenchymal stem cell‐derived hepatocyte growth factor through ERK1/2. J Leukoc Biol. 2014;96(2):295‐303. [DOI] [PubMed] [Google Scholar]
- 75. Yen BL, Yen ML, Hsu PJ, et al. Multipotent human mesenchymal stromal cells mediate expansion of myeloid‐derived suppressor cells via hepatocyte growth factor/c‐met and STAT3. Stem Cell Reports. 2013;1(2):139‐151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Selmani Z, Naji A, Zidi I, et al. Human leukocyte antigen‐G5 secretion by human mesenchymal stem cells is required to suppress T lymphocyte and natural killer function and to induce CD4+CD25highFOXP3+ regulatory T cells. Stem Cells. 2008;26(1):212‐222. [DOI] [PubMed] [Google Scholar]
- 77. Carosella ED, Gregori S, LeMaoult J. The tolerogenic interplay(s) among HLA‐G, myeloid APCs, and regulatory cells. Blood. 2011;118(25):6499‐6505. [DOI] [PubMed] [Google Scholar]
- 78. Agaugue S, Carosella ED, Rouas‐Freiss N. Role of HLA‐G in tumor escape through expansion of myeloid‐derived suppressor cells and cytokinic balance in favor of Th2 versus Th1/Th17. Blood. 2011;117(26):7021‐7031. [DOI] [PubMed] [Google Scholar]
- 79. Rizzo R, Campioni D, Stignani M, et al. A functional role for soluble HLA‐G antigens in immune modulation mediated by mesenchymal stromal cells. Cytotherapy. 2008;10(4):364‐375. [DOI] [PubMed] [Google Scholar]
- 80. Kapasi K, Albert SE, Yie S, Zavazava N, Librach CL. HLA‐G has a concentration‐dependent effect on the generation of an allo‐CTL response. Immunology. 2000;101(2):191‐200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Du W‐J, Reppel L, Leger L, et al. Mesenchymal stem cells derived from human bone marrow and adipose tissue maintain their immunosuppressive properties after chondrogenic differentiation: Role of HLA‐G. Stem Cells Dev. 2016;25(19):1454‐1469. [DOI] [PubMed] [Google Scholar]
- 82. Morandi F, Raffaghello L, Bianchi G, et al. Immunogenicity of human mesenchymal stem cells in HLA‐class I‐restricted T‐cell responses against viral or tumor‐associated antigens. Stem Cells. 2008;26(5):1275‐1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Kerkelä E, Laitinen A, Räbinä J, et al. Adenosinergic immunosuppression by human mesenchymal stromal cells requires co‐operation with T cells. Stem Cells. 2016;34(3):781‐790. [DOI] [PubMed] [Google Scholar]
- 84. Chen M, Su W, Lin X, et al. Adoptive transfer of human gingiva‐derived mesenchymal stem cells ameliorates collagen‐induced arthritis via suppression of Th1 and Th17 cells and enhancement of regulatory T cell differentiation. Arthritis Rheum. 2013;65(5):1181‐1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Gieseke F, Bohringer J, Bussolari R, Dominici M, Handgretinger R, Muller I. Human multipotent mesenchymal stromal cells use galectin‐1 to inhibit immune effector cells. Blood. 2010;116(19):3770‐3779. [DOI] [PubMed] [Google Scholar]
- 86. Liu GY, Xu Y, Li Y, Wang LH, Liu YJ, Zhu D. Secreted galectin‐3 as a possible biomarker for the immunomodulatory potential of human umbilical cord mesenchymal stromal cells. Cytotherapy. 2013;15(10):1208‐1217. [DOI] [PubMed] [Google Scholar]
- 87. Gieseke F, Kruchen A, Tzaribachev N, Bentzien F, Dominici M, Muller I. Proinflammatory stimuli induce galectin‐9 in human mesenchymal stromal cells to suppress T‐cell proliferation. Eur J Immunol. 2013;43(10):2741‐2749. [DOI] [PubMed] [Google Scholar]
- 88. Toscano MA, Bianco GA, Ilarregui JM, et al. Differential glycosylation of TH1, TH2 and TH‐17 effector cells selectively regulates susceptibility to cell death. Nat Immunol. 2007;8(8):825‐834. [DOI] [PubMed] [Google Scholar]
- 89. Thiemann S, Man JH, Chang MH, Lee B, Baum LG. Galectin‐1 regulates tissue exit of specific dendritic cell populations. J Biol Chem. 2015;290(37):22662‐22677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Lepelletier Y, Lecourt S, Renand A, et al. Galectin‐1 and semaphorin‐3A are two soluble factors conferring T‐cell immunosuppression to bone marrow mesenchymal stem cell. Stem Cells Dev. 2010;19(7):1075‐1079. [DOI] [PubMed] [Google Scholar]
- 91. Ungerer C, Quade‐Lyssy P, Radeke HH, et al. Galectin‐9 is a suppressor of T and B cells and predicts the immune modulatory potential of mesenchymal stromal cell preparations. Stem Cells Dev. 2014;23(7):755‐766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Sheng H, Wang Y, Jin Y, et al. A critical role of IFNgamma in priming MSC‐mediated suppression of T cell proliferation through up‐regulation of B7–H1. Cell Res. 2008;18(8):846‐857. [DOI] [PubMed] [Google Scholar]
- 93. Chinnadurai R, Copland IB, Patel SR, Galipeau J. IDO‐independent suppression of T cell effector function by IFN‐gamma‐licensed human mesenchymal stromal cells. J Immunol. 2014;192(4):1491‐1501. [DOI] [PubMed] [Google Scholar]
- 94. Davies LC, Heldring N, Kadri N, Le Blanc K. Mesenchymal stromal cell secretion of programmed death‐1 ligands regulates T cell mediated immunosuppression. Stem Cells. 2017;35(3):766‐776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Augello A, Tasso R, Negrini S, et al. Bone marrow mesenchymal progenitor cells inhibit lymphocyte proliferation by activation of the programmed death 1 pathway. Eur J Immunol. 2005;35(5):1482‐1490. [DOI] [PubMed] [Google Scholar]
- 96. Chabannes D, Hill M, Merieau E, et al. A role for heme oxygenase‐1 in the immunosuppressive effect of adult rat and human mesenchymal stem cells. Blood. 2007;110(10):3691‐3694. [DOI] [PubMed] [Google Scholar]
- 97. Mougiakakos D, Jitschin R, Johansson CC, Okita R, Kiessling R, Le Blanc K. The impact of inflammatory licensing on heme oxygenase‐1‐mediated induction of regulatory T cells by human mesenchymal stem cells. Blood. 2011;117(18):4826‐4835. [DOI] [PubMed] [Google Scholar]
- 98. Sala E, Genua M, Petti L, et al. Mesenchymal stem cells reduce colitis in mice via release of TSG6, independently of their localization to the intestine. Gastroenterology. 2015;149(1):163‐176.e120. [DOI] [PubMed] [Google Scholar]
- 99. Bartosh TJ, Ylostalo JH, Bazhanov N, Kuhlman J, Prockop DJ. Dynamic compaction of human mesenchymal stem/precursor cells into spheres self‐activates caspase‐dependent IL1 signaling to enhance secretion of modulators of inflammation and immunity (PGE2, TSG6, and STC1). Stem Cells. 2013;31(11):2443‐2456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Martino MM, Maruyama K, Kuhn GA, et al. Inhibition of IL‐1R1/MyD88 signalling promotes mesenchymal stem cell‐driven tissue regeneration. Nat Commun. 2016;7:11051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Luz‐Crawford P, Djouad F, Toupet K, et al. Mesenchymal stem cell‐derived Interleukin 1 receptor antagonist promotes macrophage polarization and inhibits B cell differentiation. Stem Cells. 2016;34(2):483‐492. [DOI] [PubMed] [Google Scholar]
- 102. Le Blanc K, Mougiakakos D. Multipotent mesenchymal stromal cells and the innate immune system. Nat Rev Immunol. 2012;12(5):383‐396. [DOI] [PubMed] [Google Scholar]
- 103. Tu Z, Li Q, Bu H, Lin F. Mesenchymal stem cells inhibit complement activation by secreting factor H. Stem Cells Dev. 2010;19(11):1803‐1809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Li Y, Lin F. Mesenchymal stem cells are injured by complement after their contact with serum. Blood. 2012;120(17):3436‐3443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Nauta AJ, Kruisselbrink AB, Lurvink E, Willemze R, Fibbe WE. Mesenchymal stem cells inhibit generation and function of both CD34+‐derived and monocyte‐derived dendritic cells. J Immunol. 2006;177(4):2080‐2087. [DOI] [PubMed] [Google Scholar]
- 106. Li YP, Paczesny S, Lauret E, et al. Human mesenchymal stem cells license adult CD34+ hemopoietic progenitor cells to differentiate into regulatory dendritic cells through activation of the Notch pathway. J Immunol. 2008;180(3):1598‐1608. [DOI] [PubMed] [Google Scholar]
- 107. Chen H‐W, Chen H‐Y, Wang L‐T, et al. Mesenchymal stem cells tune the development of monocyte‐derived dendritic cells toward a myeloid‐derived suppressive phenotype through growth‐regulated oncogene chemokines. J Immunol. 2013;190(10):5065‐5077. [DOI] [PubMed] [Google Scholar]
- 108. Abumaree MH, Al Jumah MA, Kalionis B, et al. Human placental mesenchymal stem cells (pMSCs) play a role as immune suppressive cells by shifting macrophage differentiation from inflammatory M1 to anti‐inflammatory M2 macrophages. Stem Cell Rev. 2013;9(5):620‐641. [DOI] [PubMed] [Google Scholar]
- 109. English K, Barry FP, Mahon BP. Murine mesenchymal stem cells suppress dendritic cell migration, maturation and antigen presentation. Immunol Lett. 2008;115(1):50‐58. [DOI] [PubMed] [Google Scholar]
- 110. Jiang X‐X, Zhang YI, Liu B, et al. Human mesenchymal stem cells inhibit differentiation and function of monocyte‐derived dendritic cells. Blood. 2005;105(10):4120‐4126. [DOI] [PubMed] [Google Scholar]
- 111. Du Rocher B, Mencalha AL, Gomes BE, Abdelhay E. Mesenchymal stromal cells impair the differentiation of CD14(++) CD16(‐) CD64(+) classical monocytes into CD14(++) CD16(+) CD64(++) activate monocytes. Cytotherapy. 2012;14(1):12‐25. [DOI] [PubMed] [Google Scholar]
- 112. Zhang W, Ge W, Li C, et al. Effects of mesenchymal stem cells on differentiation, maturation, and function of human monocyte‐derived dendritic cells. Stem Cells Dev. 2004;13(3):263‐271. [DOI] [PubMed] [Google Scholar]
- 113. Consentius C, Akyüz L, Schmidt‐Lucke JA, et al. Mesenchymal stromal cells prevent allostimulation in vivo and control checkpoints of Th1 priming: Migration of human DC to lymph nodes and NK cell activation. Stem Cells. 2015;33(10):3087‐3099. [DOI] [PubMed] [Google Scholar]
- 114. Chiesa S, Morbelli S, Morando S, et al. Mesenchymal stem cells impair in vivo T‐cell priming by dendritic cells. Proc Natl Acad Sci USA. 2011;108(42):17384‐17389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Li H, Guo Z, Jiang X, Zhu H, Li X, Mao N. Mesenchymal stem cells alter migratory property of T and dendritic cells to delay the development of murine lethal acute graft‐versus‐host disease. Stem Cells. 2008;26(10):2531‐2541. [DOI] [PubMed] [Google Scholar]
- 116. Zhang B, Liu R, Shi D, et al. Mesenchymal stem cells induce mature dendritic cells into a novel Jagged‐2‐dependent regulatory dendritic cell population. Blood. 2009;113(1):46‐57. [DOI] [PubMed] [Google Scholar]
- 117. Liu X, Qu X, Chen Y, et al. Mesenchymal stem/stromal cells induce the generation of novel IL‐10‐dependent regulatory dendritic cells by SOCS3 activation. J Immunol. 2012;189(3):1182‐1192. [DOI] [PubMed] [Google Scholar]
- 118. Cahill EF, Tobin LM, Carty F, Mahon BP, English K. Jagged‐1 is required for the expansion of CD4+ CD25+ FoxP3+ regulatory T cells and tolerogenic dendritic cells by murine mesenchymal stromal cells. Stem Cell Res Ther. 2015;6:19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Maccario R, Podesta M, Moretta A, et al. Interaction of human mesenchymal stem cells with cells involved in alloantigen‐specific immune response favors the differentiation of CD4+ T‐cell subsets expressing a regulatory/suppressive phenotype. Haematologica. 2005;90(4):516‐525. [PubMed] [Google Scholar]
- 120. Favaro E, Carpanetto A, Caorsi C, et al. Human mesenchymal stem cells and derived extracellular vesicles induce regulatory dendritic cells in type 1 diabetic patients. Diabetologia. 2016;59(2):325‐333. [DOI] [PubMed] [Google Scholar]
- 121. Zhang YI, Cai W, Huang Q, et al. Mesenchymal stem cells alleviate bacteria‐induced liver injury in mice by inducing regulatory dendritic cells. Hepatology. 2014;59(2):671‐682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Spaggiari GM, Abdelrazik H, Becchetti F, Moretta L. MSCs inhibit monocyte‐derived DC maturation and function by selectively interfering with the generation of immature DCs: central role of MSC‐derived prostaglandin E2. Blood. 2009;113(26):6576‐6583. [DOI] [PubMed] [Google Scholar]
- 123. Djouad F, Charbonnier L‐M, Bouffi C, et al. Mesenchymal stem cells inhibit the differentiation of dendritic cells through an interleukin‐6‐dependent mechanism. Stem Cells. 2007;25(8):2025‐2032. [DOI] [PubMed] [Google Scholar]
- 124. Deng Y, Yi S, Wang G, et al. Umbilical cord‐derived mesenchymal stem cells instruct dendritic cells to acquire tolerogenic phenotypes through the IL‐6‐mediated upregulation of SOCS1. Stem Cells Dev. 2014;23(17):2080‐2092. [DOI] [PubMed] [Google Scholar]
- 125. Ramasamy R, Fazekasova H, Lam EW, Soeiro I, Lombardi G, Dazzi F. Mesenchymal stem cells inhibit dendritic cell differentiation and function by preventing entry into the cell cycle. Transplantation. 2007;83(1):71‐76. [DOI] [PubMed] [Google Scholar]
- 126. Aldinucci A, Rizzetto L, Pieri L, et al. Inhibition of immune synapse by altered dendritic cell actin distribution: a new pathway of mesenchymal stem cell immune regulation. J Immunol. 2010;185(9):5102‐5110. [DOI] [PubMed] [Google Scholar]
- 127. Chia JJ, Zhu T, Chyou S, et al. Dendritic cells maintain dermal adipose‐derived stromal cells in skin fibrosis. J Clin Invest. 2016;126(11):4331‐4345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Francois M, Romieu‐Mourez R, Li M, Galipeau J. Human MSC suppression correlates with cytokine induction of indoleamine 2,3‐dioxygenase and bystander M2 macrophage differentiation. Mol Ther. 2012;20(1):187‐195. [DOI] [PubMed] [Google Scholar]
- 129. Cutler AJ, Limbani V, Girdlestone J, Navarrete CV. Umbilical cord‐derived mesenchymal stromal cells modulate monocyte function to suppress T cell proliferation. J Immunol. 2010;185(11):6617‐6623. [DOI] [PubMed] [Google Scholar]
- 130. Zhang Q‐Z, Su W‐R, Shi S‐H, et al. Human gingiva‐derived mesenchymal stem cells elicit polarization of m2 macrophages and enhance cutaneous wound healing. Stem Cells. 2010;28(10):1856‐1868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Cho D‐I, Kim MR, Jeong H‐Y, et al. Mesenchymal stem cells reciprocally regulate the M1/M2 balance in mouse bone marrow‐derived macrophages. Exp Mol Med. 2014;46:e70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Selleri S, Bifsha P, Civini S, et al. Human mesenchymal stromal cell‐secreted lactate induces M2‐macrophage differentiation by metabolic reprogramming. Oncotarget. 2016;7(21):30193‐30210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Kim J, Hematti P. Mesenchymal stem cell‐educated macrophages: a novel type of alternatively activated macrophages. Exp Hematol. 2009;37(12):1445‐1453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Németh K, Leelahavanichkul A, Yuen PST, et al. Bone marrow stromal cells attenuate sepsis via prostaglandin E(2)‐dependent reprogramming of host macrophages to increase their interleukin‐10 production. Nat Med. 2009;15(1):42‐49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Hof‐Nahor I, Leshansky L, Shivtiel S, et al. Human mesenchymal stem cells shift CD8+ T cells towards a suppressive phenotype by inducing tolerogenic monocytes. J Cell Sci. 2012;125(Pt 19):4640‐4650. [DOI] [PubMed] [Google Scholar]
- 136. Song X, Xie S, Lu K, Wang C. Mesenchymal stem cells alleviate experimental asthma by inducing polarization of alveolar macrophages. Inflammation. 2015;38(2):485‐492. [DOI] [PubMed] [Google Scholar]
- 137. Chaturvedi P, Gilkes DM, Takano N, Semenza GL. Hypoxia‐inducible factor‐dependent signaling between triple‐negative breast cancer cells and mesenchymal stem cells promotes macrophage recruitment. Proc Natl Acad Sci USA. 2014;111(20):E2120‐E2129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Liu W, Zhang S, Gu S, Sang L, Dai C. Mesenchymal stem cells recruit macrophages to alleviate experimental colitis through TGFbeta1. Cell Physiol Biochem. 2015;35(3):858‐865. [DOI] [PubMed] [Google Scholar]
- 139. Choi H, Lee RH, Bazhanov N, Oh JY, Prockop DJ. Anti‐inflammatory protein TSG‐6 secreted by activated MSCs attenuates zymosan‐induced mouse peritonitis by decreasing TLR2/NF‐kappaB signaling in resident macrophages. Blood. 2011;118(2):330‐338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Chen Y, Song Y, Miao H, et al. Gene delivery with IFN‐gamma‐expression plasmids enhances the therapeutic effects of MSCs on DSS‐induced mouse colitis. Inflamm Res. 2015;64(9):671‐681. [DOI] [PubMed] [Google Scholar]
- 141. Zullo JA, Nadel EP, Rabadi MM, et al. The secretome of hydrogel‐coembedded endothelial progenitor cells and mesenchymal stem cells instructs macrophage polarization in endotoxemia. Stem Cells Transl Med. 2015;4(7):852‐861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Xie Z, Hao H, Tong C, et al. Human umbilical cord‐derived mesenchymal stem cells elicit macrophages into an anti‐inflammatory phenotype to alleviate insulin resistance in type 2 diabetic rats. Stem Cells. 2016;34(3):627‐639. [DOI] [PubMed] [Google Scholar]
- 143. Braza F, Dirou S, Forest V, et al. Mesenchymal stem cells induce suppressive macrophages through phagocytosis in a mouse model of asthma. Stem Cells. 2016;34(7):1836‐1845. [DOI] [PubMed] [Google Scholar]
- 144. Rasmusson I, Le Blanc K, Sundberg B, Ringden O. Mesenchymal stem cells stimulate antibody secretion in human B cells. Scand J Immunol. 2007;65(4):336‐343. [DOI] [PubMed] [Google Scholar]
- 145. Comoli P, Ginevri F, Maccario R, et al. Human mesenchymal stem cells inhibit antibody production induced in vitro by allostimulation. Nephrol Dial Transplant. 2008;23(4):1196‐1202. [DOI] [PubMed] [Google Scholar]
- 146. Liu O, Xu J, Ding G, et al. Periodontal ligament stem cells regulate B lymphocyte function via programmed cell death protein 1. Stem Cells. 2013;31(7):1371‐1382. [DOI] [PubMed] [Google Scholar]
- 147. Rosado MM, Bernardo ME, Scarsella M, et al. Inhibition of B‐cell proliferation and antibody production by mesenchymal stromal cells is mediated by T cells. Stem Cells Dev. 2015;24(1):93‐103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Franquesa M, Mensah FK, Huizinga R, et al. Human adipose tissue‐derived mesenchymal stem cells abrogate plasmablast formation and induce regulatory B cells independently of T helper cells. Stem Cells. 2015;33(3):880‐891. [DOI] [PubMed] [Google Scholar]
- 149. Asari S, Itakura S, Ferreri K, et al. Mesenchymal stem cells suppress B‐cell terminal differentiation. Exp Hematol. 2009;37(5):604‐615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150. Rafei M, Hsieh J, Fortier S, et al. Mesenchymal stromal cell‐derived CCL2 suppresses plasma cell immunoglobulin production via STAT3 inactivation and PAX5 induction. Blood. 2008;112(13):4991‐4998. [DOI] [PubMed] [Google Scholar]
- 151. Corcione A, Benvenuto F, Ferretti E, et al. Human mesenchymal stem cells modulate B‐cell functions. Blood. 2006;107(1):367‐372. [DOI] [PubMed] [Google Scholar]
- 152. Tabera S, Perez‐Simon JA, Diez‐Campelo M, et al. The effect of mesenchymal stem cells on the viability, proliferation and differentiation of B‐lymphocytes. Haematologica. 2008;93(9):1301‐1309. [DOI] [PubMed] [Google Scholar]
- 153. Che N, Li X, Zhou S, et al. Umbilical cord mesenchymal stem cells suppress B‐cell proliferation and differentiation. Cell Immunol. 2012;274(1–2):46‐53. [DOI] [PubMed] [Google Scholar]
- 154. Cho KA, Lee JK, Kim YH, Park M, Woo SY, Ryu KH. Mesenchymal stem cells ameliorate B‐cell‐mediated immune responses and increase IL‐10‐expressing regulatory B cells in an EBI3‐dependent manner. Cell Mol Immunol. 2017;14(11):895‐908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155. Franquesa M, Hoogduijn MJ, Bestard O, Grinyo JM. Immunomodulatory effect of mesenchymal stem cells on B cells. Front Immunol. 2012;3:212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156. Schena F, Gambini C, Gregorio A, et al. Interferon‐gamma‐dependent inhibition of B cell activation by bone marrow‐derived mesenchymal stem cells in a murine model of systemic lupus erythematosus. Arthritis Rheum. 2010;62(9):2776‐2786. [DOI] [PubMed] [Google Scholar]
- 157. Traggiai E, Volpi S, Schena F, et al. Bone marrow‐derived mesenchymal stem cells induce both polyclonal expansion and differentiation of B cells isolated from healthy donors and systemic lupus erythematosus patients. Stem Cells. 2008;26(2):562‐569. [DOI] [PubMed] [Google Scholar]
- 158. Reichardt P, Dornbach B, Rong S, et al. Naive B cells generate regulatory T cells in the presence of a mature immunologic synapse. Blood. 2007;110(5):1519‐1529. [DOI] [PubMed] [Google Scholar]
- 159. Guo Y, Chan KH, Lai WH, et al. Human mesenchymal stem cells upregulate CD1dCD5(+) regulatory B cells in experimental autoimmune encephalomyelitis. NeuroImmunoModulation. 2013;20(5):294‐303. [DOI] [PubMed] [Google Scholar]
- 160. Park MJ, Kwok SK, Lee SH, Kim EK, Park SH, Cho ML. Adipose tissue‐derived mesenchymal stem cells induce expansion of interleukin‐10‐producing regulatory B cells and ameliorate autoimmunity in a murine model of systemic lupus erythematosus. Cell Transplant. 2015;24(11):2367‐2377. [DOI] [PubMed] [Google Scholar]
- 161. Healy ME, Bergin R, Mahon BP, English K. Mesenchymal stromal cells protect against caspase 3‐mediated apoptosis of CD19(+) peripheral B cells through contact‐dependent upregulation of VEGF. Stem Cells Dev. 2015;24(20):2391‐2402. [DOI] [PubMed] [Google Scholar]
- 162. Le Blanc K, Tammik L, Sundberg B, Haynesworth SE, Ringden O. Mesenchymal stem cells inhibit and stimulate mixed lymphocyte cultures and mitogenic responses independently of the major histocompatibility complex. Scand J Immunol. 2003;57(1):11‐20. [DOI] [PubMed] [Google Scholar]
- 163. Ramasamy R, Tong CK, Seow HF, Vidyadaran S, Dazzi F. The immunosuppressive effects of human bone marrow‐derived mesenchymal stem cells target T cell proliferation but not its effector function. Cell Immunol. 2008;251(2):131‐136. [DOI] [PubMed] [Google Scholar]
- 164. Krampera M, Pasini A, Pizzolo G, Cosmi L, Romagnani S, Annunziato F. Regenerative and immunomodulatory potential of mesenchymal stem cells. Curr Opin Pharmacol. 2006;6(4):435‐441. [DOI] [PubMed] [Google Scholar]
- 165. Petrini I, Pacini S, Petrini M, Fazzi R, Trombi L, Galimberti S. Mesenchymal cells inhibit expansion but not cytotoxicity exerted by gamma‐delta T cells. Eur J Clin Invest. 2009;39(9):813‐818. [DOI] [PubMed] [Google Scholar]
- 166. Krampera M, Glennie S, Dyson J, et al. Bone marrow mesenchymal stem cells inhibit the response of naive and memory antigen‐specific T cells to their cognate peptide. Blood. 2003;101(9):3722‐3729. [DOI] [PubMed] [Google Scholar]
- 167. Djouad F, Plence P, Bony C, et al. Immunosuppressive effect of mesenchymal stem cells favors tumor growth in allogeneic animals. Blood. 2003;102(10):3837‐3844. [DOI] [PubMed] [Google Scholar]
- 168. Di Nicola M, Carlo‐Stella C, Magni M, et al. Human bone marrow stromal cells suppress T‐lymphocyte proliferation induced by cellular or nonspecific mitogenic stimuli. Blood. 2002;99(10):3838‐3843. [DOI] [PubMed] [Google Scholar]
- 169. Glennie S, Soeiro I, Dyson PJ, Lam EW, Dazzi F. Bone marrow mesenchymal stem cells induce division arrest anergy of activated T cells. Blood. 2005;105(7):2821‐2827. [DOI] [PubMed] [Google Scholar]
- 170. Bartholomew A, Sturgeon C, Siatskas M, et al. Mesenchymal stem cells suppress lymphocyte proliferation in vitro and prolong skin graft survival in vivo. Exp Hematol. 2002;30(1):42‐48. [DOI] [PubMed] [Google Scholar]
- 171. Beyth S, Borovsky Z, Mevorach D, et al. Human mesenchymal stem cells alter antigen‐presenting cell maturation and induce T‐cell unresponsiveness. Blood. 2005;105(5):2214‐2219. [DOI] [PubMed] [Google Scholar]
- 172. Lin R, Ma H, Ding Z, et al. Bone marrow‐derived mesenchymal stem cells favor the immunosuppressive T cells skewing in a Helicobacter pylori model of gastric cancer. Stem Cells Dev. 2013;22(21):2836‐2848. [DOI] [PubMed] [Google Scholar]
- 173. Hsu WT, Lin CH, Chiang BL, Jui HY, Wu KK, Lee CM. Prostaglandin E2 potentiates mesenchymal stem cell‐induced IL‐10+IFN‐gamma+CD4+ regulatory T cells to control transplant arteriosclerosis. J Immunol. 2013;190(5):2372‐2380. [DOI] [PubMed] [Google Scholar]
- 174. Roncarolo MG, Gregori S, Battaglia M, Bacchetta R, Fleischhauer K, Levings MK. Interleukin‐10‐secreting type 1 regulatory T cells in rodents and humans. Immunol Rev. 2006;212:28‐50. [DOI] [PubMed] [Google Scholar]
- 175. Vignali DA, Collison LW, Workman CJ. How regulatory T cells work. Nat Rev Immunol. 2008;8(7):523‐532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176. Nguyen TM, Arthur A, Hayball JD, Gronthos S. EphB and Ephrin‐B interactions mediate human mesenchymal stem cell suppression of activated T‐cells. Stem Cells Dev. 2013;22(20):2751‐2764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177. Benvenuto F, Ferrari S, Gerdoni E, et al. Human mesenchymal stem cells promote survival of T cells in a quiescent state. Stem Cells. 2007;25(7):1753‐1760. [DOI] [PubMed] [Google Scholar]
- 178. Gu Y‐Z, Xue Q, Chen Y‐J, et al. Different roles of PD‐L1 and FasL in immunomodulation mediated by human placenta‐derived mesenchymal stem cells. Hum Immunol. 2013;74(3):267‐276. [DOI] [PubMed] [Google Scholar]
- 179. Giuliani M, Fleury M, Vernochet A, et al. Long‐lasting inhibitory effects of fetal liver mesenchymal stem cells on T‐lymphocyte proliferation. PLoS ONE. 2011;6(5):e19988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180. Plumas J, Chaperot L, Richard MJ, Molens JP, Bensa JC, Favrot MC. Mesenchymal stem cells induce apoptosis of activated T cells. Leukemia. 2005;19(9):1597‐1604. [DOI] [PubMed] [Google Scholar]
- 181. Akiyama K, Chen C, Wang DD, et al. Mesenchymal‐stem‐cell‐induced immunoregulation involves FAS‐ligand‐/FAS‐mediated T cell apoptosis. Cell Stem Cell. 2012;10(5):544‐555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182. Kim SN, Lee HJ, Jeon MS, Yi T, Song SU. Galectin‐9 is involved in immunosuppression mediated by human bone marrow‐derived clonal mesenchymal stem cells. Immune Netw. 2015;15(5):241‐251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183. Zhu C, Anderson AC, Schubart A, et al. The Tim‐3 ligand galectin‐9 negatively regulates T helper type 1 immunity. Nat Immunol. 2005;6(12):1245‐1252. [DOI] [PubMed] [Google Scholar]
- 184. Di Ianni M, Del Papa B, De Ioanni M, et al. Mesenchymal cells recruit and regulate T regulatory cells. Exp Hematol. 2008;36(3):309‐318. [DOI] [PubMed] [Google Scholar]
- 185. English K, Ryan JM, Tobin L, Murphy MJ, Barry FP, Mahon BP. Cell contact, prostaglandin E(2) and transforming growth factor beta 1 play non‐redundant roles in human mesenchymal stem cell induction of CD4+CD25(High) forkhead box P3+ regulatory T cells. Clin Exp Immunol. 2009;156(1):149‐160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186. Ghannam S, Pene J, Moquet‐Torcy G, Jorgensen C, Yssel H. Mesenchymal stem cells inhibit human Th17 cell differentiation and function and induce a T regulatory cell phenotype. J Immunol. 2010;185(1):302‐312. [DOI] [PubMed] [Google Scholar]
- 187. Del Papa B, Sportoletti P, Cecchini D, et al. Notch1 modulates mesenchymal stem cells mediated regulatory T‐cell induction. Eur J Immunol. 2013;43(1):182‐187. [DOI] [PubMed] [Google Scholar]
- 188. Rashedi I, Gomez‐Aristizabal A, Wang XH, Viswanathan S, Keating A. TLR3 or TLR4 activation enhances mesenchymal stromal cell‐mediated treg induction via notch signaling. Stem Cells. 2017;35(1):265‐275. [DOI] [PubMed] [Google Scholar]
- 189. Luz‐Crawford P, Kurte M, Bravo‐Alegría J, et al. Mesenchymal stem cells generate a CD4+CD25+Foxp3+ regulatory T cell population during the differentiation process of Th1 and Th17 cells. Stem Cell Res Ther. 2013;4(3):65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190. Luz‐Crawford P, Tejedor G, Mausset‐Bonnefont AL, et al. Glucocorticoid‐induced leucine zipper governs the therapeutic potential of mesenchymal stem cells by inducing a switch from pathogenic to regulatory Th17 cells in a mouse model of collagen‐induced arthritis. Arthritis Rheumatol. 2015;67(6):1514‐1524. [DOI] [PubMed] [Google Scholar]
- 191. Yang N, Baban B, Isales CM, Shi XM. Crosstalk between bone marrow‐derived mesenchymal stem cells and regulatory T cells through a glucocorticoid‐induced leucine zipper/developmental endothelial locus‐1‐dependent mechanism. FASEB J. 2015;29(9):3954‐3963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192. Liu Q, Zheng H, Chen X, et al. Human mesenchymal stromal cells enhance the immunomodulatory function of CD8(+)CD28(‐) regulatory T cells. Cell Mol Immunol. 2015;12(6):708‐718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193. Selleri S, Dieng MM, Nicoletti S, et al. Cord‐blood‐derived mesenchymal stromal cells downmodulate CD4+ T‐cell activation by inducing IL‐10‐producing Th1 cells. Stem Cells Dev. 2013;22(7):1063‐1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194. Ge W, Jiang J, Arp J, Liu W, Garcia B, Wang H. Regulatory T‐cell generation and kidney allograft tolerance induced by mesenchymal stem cells associated with indoleamine 2,3‐dioxygenase expression. Transplantation. 2010;90(12):1312‐1320. [DOI] [PubMed] [Google Scholar]
- 195. Fallarino F, Grohmann U, Vacca C, et al. T cell apoptosis by tryptophan catabolism. Cell Death Differ. 2002;9(10):1069‐1077. [DOI] [PubMed] [Google Scholar]
- 196. Duffy MM, Pindjakova J, Hanley SA, et al. Mesenchymal stem cell inhibition of T‐helper 17 cell‐ differentiation is triggered by cell‐cell contact and mediated by prostaglandin E2 via the EP4 receptor. Eur J Immunol. 2011;41(10):2840‐2851. [DOI] [PubMed] [Google Scholar]
- 197. Luz‐Crawford P, Noël D, Fernandez X, et al. Mesenchymal stem cells repress Th17 molecular program through the PD‐1 pathway. PLoS ONE. 2012;7(9):e45272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198. Rafei M, Campeau PM, Aguilar‐Mahecha A, et al. Mesenchymal stromal cells ameliorate experimental autoimmune encephalomyelitis by inhibiting CD4 Th17 T cells in a CC chemokine ligand 2‐dependent manner. J Immunol. 2009;182(10):5994‐6002. [DOI] [PubMed] [Google Scholar]
- 199. Liu X, Ren S, Qu X, Ge C, Cheng K, Zhao RC. Mesenchymal stem cells inhibit Th17 cells differentiation via IFN‐gamma‐mediated SOCS3 activation. Immunol Res. 2015;61(3):219‐229. [DOI] [PubMed] [Google Scholar]
- 200. Laurence A, Tato CM, Davidson TS, et al. Interleukin‐2 signaling via STAT5 constrains T helper 17 cell generation. Immunity. 2007;26(3):371‐381. [DOI] [PubMed] [Google Scholar]
- 201. Obermajer N, Popp FC, Soeder Y, et al. Conversion of Th17 into IL‐17A(neg) regulatory T cells: a novel mechanism in prolonged allograft survival promoted by mesenchymal stem cell‐supported minimized immunosuppressive therapy. J Immunol. 2014;193(10):4988‐4999. [DOI] [PubMed] [Google Scholar]
- 202. Guo Z, Zheng C, Chen Z, et al. Fetal BM‐derived mesenchymal stem cells promote the expansion of human Th17 cells, but inhibit the production of Th1 cells. Eur J Immunol. 2009;39(10):2840‐2849. [DOI] [PubMed] [Google Scholar]
- 203. Eljaafari A, Tartelin M‐L, Aissaoui H, et al. Bone marrow‐derived and synovium‐derived mesenchymal cells promote Th17 cell expansion and activation through caspase 1 activation: contribution to the chronicity of rheumatoid arthritis. Arthritis Rheum. 2012;64(7):2147‐2157. [DOI] [PubMed] [Google Scholar]
- 204. Spaggiari GM, Capobianco A, Becchetti S, Mingari MC, Moretta L. Mesenchymal stem cell‐natural killer cell interactions: evidence that activated NK cells are capable of killing MSCs, whereas MSCs can inhibit IL‐2‐induced NK‐cell proliferation. Blood. 2006;107(4):1484‐1490. [DOI] [PubMed] [Google Scholar]
- 205. Sotiropoulou PA, Perez SA, Gritzapis AD, Baxevanis CN, Papamichail M. Interactions between human mesenchymal stem cells and natural killer cells. Stem Cells. 2006;24(1):74‐85. [DOI] [PubMed] [Google Scholar]
- 206. Spaggiari GM, Capobianco A, Abdelrazik H, Becchetti F, Mingari MC, Moretta L. Mesenchymal stem cells inhibit natural killer‐cell proliferation, cytotoxicity, and cytokine production: role of indoleamine 2,3‐dioxygenase and prostaglandin E2. Blood. 2008;111(3):1327‐1333. [DOI] [PubMed] [Google Scholar]
- 207. Qu M, Cui J, Zhu J, et al. Bone marrow‐derived mesenchymal stem cells suppress NK cell recruitment and activation in PolyI:C‐induced liver injury. Biochem Biophys Res Commun. 2015;466(2):173‐179. [DOI] [PubMed] [Google Scholar]
- 208. Michelo CM, Fasse E, van Cranenbroek B, et al. Added effects of dexamethasone and mesenchymal stem cells on early Natural Killer cell activation. Transpl Immunol. 2016;37:1‐9. [DOI] [PubMed] [Google Scholar]
- 209. DelaRosa O, Sánchez‐Correa B, Morgado S, et al. Human adipose‐derived stem cells impair natural killer cell function and exhibit low susceptibility to natural killer‐mediated lysis. Stem Cells Dev. 2012;21(8):1333‐1343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210. Chatterjee D, Tufa DM, Baehre H, Hass R, Schmidt RE, Jacobs R. Natural killer cells acquire CD73 expression upon exposure to mesenchymal stem cells. Blood. 2014;123(4):594‐595. [DOI] [PubMed] [Google Scholar]
- 211. Chen X, Shao H, Zhi Y, et al. CD73 Pathway contributes to the immunosuppressive ability of mesenchymal stem cells in intraocular autoimmune responses. Stem Cells Dev. 2016;25(4):337‐346. [DOI] [PubMed] [Google Scholar]
- 212. Lu Y, Liu J, Liu Y, et al. TLR4 plays a crucial role in MSC‐induced inhibition of NK cell function. Biochem Biophys Res Commun. 2015;464(2):541‐547. [DOI] [PubMed] [Google Scholar]
- 213. Prigione I, Benvenuto F, Bocca P, Battistini L, Uccelli A, Pistoia V. Reciprocal interactions between human mesenchymal stem cells and gammadelta T cells or invariant natural killer T cells. Stem Cells. 2009;27(3):693‐702. [DOI] [PubMed] [Google Scholar]
- 214. Boissel L, Tuncer HH, Betancur M, Wolfberg A, Klingemann H. Umbilical cord mesenchymal stem cells increase expansion of cord blood natural killer cells. Biol Blood Marrow Transplant. 2008;14(9):1031‐1038. [DOI] [PubMed] [Google Scholar]
- 215. Thomas H, Jager M, Mauel K, Brandau S, Lask S, Flohe SB. Interaction with mesenchymal stem cells provokes natural killer cells for enhanced IL‐12/IL‐18‐induced interferon‐gamma secretion. Mediators Inflamm. 2014;2014:143463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216. Cui R, Rekasi H, Hepner‐Schefczyk M, et al. Human mesenchymal stromal/stem cells acquire immunostimulatory capacity upon cross‐talk with natural killer cells and might improve the NK cell function of immunocompromised patients. Stem Cell Res Ther. 2016;7(1):88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217. Almeida CR, Caires HR, Vasconcelos DP, Barbosa MA. NAP‐2 secreted by human NK cells can stimulate mesenchymal stem/stromal cell recruitment. Stem Cell Reports. 2016;6(4):466‐473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218. Gotherstrom C, Lundqvist A, Duprez IR, Childs R, Berg L, le Blanc K. Fetal and adult multipotent mesenchymal stromal cells are killed by different pathways. Cytotherapy. 2011;13(3):269‐278. [DOI] [PubMed] [Google Scholar]
- 219. Zhu XS, He BX, Zhou XN, Ren J. Effects of transplanted bone‐marrow‐derived mesenchymal stem cells in animal models of acute hepatitis. Cell Tissue Res. 2013;351(3):477‐486. [DOI] [PubMed] [Google Scholar]
- 220. Wang BA, Wu SM, Wang T, Ma ZS, Liu K. Bone marrow‐derived mesenchymal stem cells‐mediated protection against organ dysfunction in disseminated intravascular coagulation is associated with peripheral immune responses. J Cell Biochem. 2017;118(10):3184‐3192. [DOI] [PubMed] [Google Scholar]
- 221. Prigione I, Benvenuto F, Bocca P, Battistini L, Uccelli A, Pistoia V. Reciprocal interactions between human mesenchymal stem cells and gamma delta T cells or invariant natural killer T cells. Stem Cells. 2009;27(3):693‐702. [DOI] [PubMed] [Google Scholar]
- 222. Milosavljevic N, Gazdic M, Simovic Markovic B, et al. Mesenchymal stem cells attenuate acute liver injury by altering ratio between interleukin 17 producing and regulatory natural killer T cells. Liver Transplant. 2017;23(8):1040‐1050. [DOI] [PubMed] [Google Scholar]
- 223. Gazdic M, Simovic Markovic B, Vucicevic L, et al. Mesenchymal stem cells protect from acute liver injury by attenuating hepatotoxicity of liver natural killer T cells in an inducible nitric oxide synthase‐ and indoleamine 2,3‐dioxygenase‐dependent manner. J Tissue Eng Regen Med. 2018;12(2):E1173‐E1185. [DOI] [PubMed] [Google Scholar]
- 224. Koga S, Hozumi K, Hirano K, et al. Peripheral PDGFR alpha(+)gp38(+) mesenchymal cells support the differentiation of fetal liver‐derived ILC2. J Exp Med. 2018;215(6):1609‐1626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225. van Hoeven V, Munneke JM, Cornelissen AS, et al. Mesenchymal stromal cells stimulate the proliferation and IL‐22 production of group 3 innate lymphoid cells. Journal of Immunology. 2018;201(4):1165‐1173. [DOI] [PubMed] [Google Scholar]
- 226. Eberl G, Colonna M, Di Santo JP, McKenzie AN. Innate lymphoid cells. Innate lymphoid cells: a new paradigm in immunology. Science. 2015;348(6237):aaa6566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227. Vivier E, Artis D, Colonna M, et al. Innate lymphoid cells: 10 years on. Cell. 2018;174(5):1054‐1066. [DOI] [PubMed] [Google Scholar]
- 228. Zhou L, Chu C, Teng F, et al. Innate lymphoid cells support regulatory T cells in the intestine through interleukin‐2. Nature. 2019;568(7752):405‐409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229. Munir H, Luu NT, Clarke LS, Nash GB, McGettrick HM. Comparative ability of mesenchymal stromal cells from different tissues to limit neutrophil recruitment to inflamed endothelium. PLoS ONE. 2016;11(5):e0155161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230. Zhu YG, Feng XM, Abbott J, et al. Human mesenchymal stem cell microvesicles for treatment of Escherichia coli endotoxin‐induced acute lung injury in mice. Stem Cells. 2014;32(1):116‐125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231. Jiang D, Muschhammer J, Qi YU, et al. Suppression of neutrophil‐mediated tissue damage‐A novel skill of mesenchymal stem cells. Stem Cells. 2016;34(9):2393‐2406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232. Raffaghello L, Bianchi G, Bertolotto M, et al. Human mesenchymal stem cells inhibit neutrophil apoptosis: a model for neutrophil preservation in the bone marrow niche. Stem Cells. 2008;26(1):151‐162. [DOI] [PubMed] [Google Scholar]
- 233. Hall SRR, Tsoyi K, Ith B, et al. Mesenchymal stromal cells improve survival during sepsis in the absence of heme oxygenase‐1: the importance of neutrophils. Stem Cells. 2013;31(2):397‐407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234. Cassatella MA, Mosna F, Micheletti A, et al. Toll‐like receptor‐3‐activated human mesenchymal stromal cells significantly prolong the survival and function of neutrophils. Stem Cells. 2011;29(6):1001‐1011. [DOI] [PubMed] [Google Scholar]
- 235. Brandau S, Jakob M, Hemeda H, et al. Tissue‐resident mesenchymal stem cells attract peripheral blood neutrophils and enhance their inflammatory activity in response to microbial challenge. J Leukoc Biol. 2010;88(5):1005‐1015. [DOI] [PubMed] [Google Scholar]
- 236. Yu PF, Huang Y, Han YY, et al. TNFalpha‐activated mesenchymal stromal cells promote breast cancer metastasis by recruiting CXCR2+ neutrophils. Oncogene. 2017;36(4):482‐490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237. Brandau S, Jakob M, Bruderek K, et al. Mesenchymal stem cells augment the anti‐bacterial activity of neutrophil granulocytes. PLoS ONE. 2014;9(9):e106903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238. Brown JM, Nemeth K, Kushnir‐Sukhov NM, Metcalfe DD, Mezey E. Bone marrow stromal cells inhibit mast cell function via a COX2‐dependent mechanism. Clin Exp Allergy. 2011;41(4):526‐534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239. Kim HS, Yun JW, Shin TH, et al. Human umbilical cord blood mesenchymal stem cell‐derived PGE2 and TGF‐beta1 alleviate atopic dermatitis by reducing mast cell degranulation. Stem Cells. 2015;33(4):1254‐1266. [DOI] [PubMed] [Google Scholar]
- 240. Liu J, Kuwabara A, Kamio Y, et al. Human mesenchymal stem cell‐derived microvesicles prevent the rupture of intracranial aneurysm in part by suppression of mast cell activation via a PGE2‐dependent mechanism. Stem Cells. 2016;34(12):2943‐2955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241. Kim A, Yu HY, Heo J, et al. Mesenchymal stem cells protect against the tissue fibrosis of ketamine‐induced cystitis in rat bladder. Sci Rep. 2016;6:30881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242. Su WR, Zhang QZ, Shi SH, Nguyen AL, Le AD. Human gingiva‐derived mesenchymal stromal cells attenuate contact hypersensitivity via prostaglandin E2‐dependent mechanisms. Stem Cells. 2011;29(11):1849‐1860. [DOI] [PubMed] [Google Scholar]
- 243. Lv FJ, Tuan RS, Cheung KM, Leung VY. Concise review: the surface markers and identity of human mesenchymal stem cells. Stem Cells. 2014;32(6):1408‐1419. [DOI] [PubMed] [Google Scholar]


