The ex vivo bactericidal activity and pharmacodynamics of fosfomycin in urine were evaluated in 18 healthy subjects. Subjects received 3 g every other day (QOD) for 3 doses and then every day (QD) for 7 doses or vice versa. Serial urine samples were collected before and up to 24 h after dosing on days 1 and 5. Eight bacterial strains with various genotypic and phenotypic susceptibilities to fosfomycin were used for all experiments (5 Escherichia coli, 2 Klebsiella pneumoniae, and 1 Proteus mirabilis).
KEYWORDS: fosfomycin, urinary bactericidal titers, pharmacodynamics, urine, urinary tract infection
ABSTRACT
The ex vivo bactericidal activity and pharmacodynamics of fosfomycin in urine were evaluated in 18 healthy subjects. Subjects received 3 g every other day (QOD) for 3 doses and then every day (QD) for 7 doses or vice versa. Serial urine samples were collected before and up to 24 h after dosing on days 1 and 5. Eight bacterial strains with various genotypic and phenotypic susceptibilities to fosfomycin were used for all experiments (5 Escherichia coli, 2 Klebsiella pneumoniae, and 1 Proteus mirabilis). MICs were performed via agar dilution. Urinary bactericidal titers (UBTs) were performed via modified Schlichter test using participant’s drug-free urine as the diluent. Urinary time-kill analyses were performed on pooled 24-h urine aliquots from days 1 and 5. All experiments were performed in triplicate with and without the addition of 25 mg/liter of glucose-6-phosphate (G6P). Mean 24-h urine concentrations of fosfomycin ranged from 324.7 to 434.6 mg/liter regardless of study day or dosing regimen. The urinary antibacterial activity of fosfomycin was also similar across study days and dosing regimens. UBT values did not correlate with MICs determined in the presence of G6P. Fosfomycin was reliably bactericidal in urine only against the 5 E. coli strains, regardless of genotype or MIC value. Together, these data do not support the use of oral fosfomycin tromethamine for pathogens other than E. coli or at a dosing frequency higher than QOD. Fosfomycin MICs determined in the presence of G6P may not accurately reflect the in vivo activity given the lack of G6P in human urine. (This study has been registered at ClinicalTrials.gov under identifier NCT02570074.)
INTRODUCTION
Oral fosfomycin tromethamine is considered a first-line agent for the treatment of uncomplicated urinary tract infections (UTIs) (1) and is approved in the United States as a one-time 3-g oral dose for uncomplicated cystitis (2). Despite this narrow indication, fosfomycin maintains broad in vitro activity against many Gram-positive and -negative pathogens, including multidrug-resistant phenotypes (3). Consequently, the use of oral fosfomycin has increased dramatically in response to the rise in bacterial resistance and increase in more complicated UTIs (4–7). Its purported utility for these difficult-to-treat infections has also prompted the use of more aggressive dosing regimens, ranging from 3 g every other day (QOD) to 3 g daily (QD) for weeks to months (8–11). Despite being commercialized since 1971, there is a paucity of modern robust pharmacodynamic (PD), pharmacokinetic (PK), or safety data to support these clinical practices. As such, there is an urgent need to establish reliable PD, PK, and safety data to optimize the use of oral fosfomycin tromethamine.
Our recent work evaluated the PK and safety of oral fosfomycin tromethamine after repeated doses in healthy adults and failed to find a clear PK advantage to QD dosing compared to QOD dosing, while QD dosing resulted in significantly more diarrhea (12). As fosfomycin tromethamine is used clinically only for the treatment of UTIs, it is crucial to understand the antibacterial activity and PD of fosfomycin in human urine. Human urine is a complex and constantly dynamic medium that can alter bacterial growth and antimicrobial activity markedly compared to blood or plasma. Additionally, fosfomycin is unique in that it requires uptake into the bacterial cell via the l-alpha-glycerolphosphate (GlpT) or hexose-6-phosphate transport (UhpT) system, which are induced by their substrates glycerol-3-phosphate (G3P) and glucose-6-phosphate (G6P), respectively (3). Therefore, it is recommended to supplement in vitro fosfomycin susceptibility assays with G6P to mimic the physiologic environment in vivo and counteract excess phosphate present in laboratory media (13, 14). Human urine is naturally devoid of G6P, and it is unknown how this affects the antibacterial activity of fosfomycin in the target matrix. Finally, it is unclear if QD dosing provides a PD advantage over QOD dosing, and there is an overall dearth of published data regarding the activity of fosfomycin against Enterobacteriaceae spp. other than E. coli in human urine and after multiple repeated oral doses. Therefore, the purpose of this study was to determine the ex vivo urinary bactericidal activity and urinary pharmacodynamics of fosfomycin after two dosing regimens of oral fosfomycin tromethamine in a randomized, two-period crossover study in healthy subjects.
(This work was presented in part at the 2018 ECCMID meeting in Madrid, Spain.)
RESULTS
A total of 19 healthy adult subjects were enrolled in the study. Of the 19 subjects enrolled, 18 received both dosing regimens and were included in the PD analysis, while one subject completed only the QOD regimen due to scheduling conflicts. The mean (± standard deviation [SD]) baseline demographics of these subjects included age of 28 ± 7 years, body mass index (BMI) of 24.9 ± 2.5 kg/m2, and measured creatinine clearance (via 24-h urine collection) of 109 ± 30 ml/min. Urine fosfomycin concentrations peaked through the first 8 h of collection, and approximately 35 to 40% of the administered dose was excreted in the urine by 24 h postdose, regardless of study day or dosing regimen. Urine concentrations of fosfomycin were highly variable but similar between study days 1 and 5 and between the QOD and QD dosing regimen (see Table S1 in the supplemental material). The mean (±SD) pooled 24 urine concentration for all 18 subjects on days 1 and 5 of the QOD regimen were 361.7 ± 254.2 mg/liter and 434.6 ± 343.4 mg/liter, respectively, compared to 342.4 ± 324.7 mg/liter and 387.9 ± 224.8 mg/liter during the QD regimen. The mean (±SD) pooled 24 urine concentration for the 3 subjects used for G6P experiments on day 5 of the QOD and QD regimen were 313.5 ± 184.2 mg/liter and 381.9 ± 229.5 mg/liter, respectively. The mean (±SD) urine pH across all collection intervals, study days, and dosing regimens was 5.96 ± 0.8. No G6P was detected in any tested urine sample.
Susceptibility.
Genotypic and phenotypic susceptibilities and the interpretive category of each isolate against fosfomycin are displayed in Table 1. In the presence of 25 mg/liter of G6P, all 5 E. coli strains were susceptible to fosfomycin (15). Three of five E. coli strains were wild type (WT) (MIC, 2 to 32 mg/liter), while both strains (UIC45 and UIC46) with MIC values at the CLSI susceptibility breakpoint (64 mg/liter) harbored mutations in genes associated with the G3P/G6P uptake transport system. Despite possessing MICs of ≥256 mg/liter, P. mirabilis was wild type, as it intrinsically does not utilize the UhpT transport system, while both K. pneumoniae strains harbored multiple mutations in the uptake transport system, the target site murA, and/or possessed the fosfomycin-modifying enzyme fosA. When tested without G6P, a 16-fold increase in MIC was observed for both wild-type ATCC E. coli strains. MICs for the 3 clinical E. coli strains increased only 1 to 2 log2 dilutions without G6P, and no appreciable change in MIC was observed for the P. mirabilis and K. pneumoniae strains.
TABLE 1.
Genotypic and phenotypic susceptibilities obtained against isolates included in the study
| Organism | Resistance mechanism(s) | MIC in mg/liter (MIC interpretationa) in presence or absence of G6Pb
|
|
|---|---|---|---|
| Presence | Absence | ||
| E. coli ATCC 25922 | WT | 4 (S) | 64 (S) |
| E. coli BAA-2326 | WT | 2 (S) | 32 (S) |
| E. coli UIC44 | WT | 32 (S) | 128 (I) |
| E. coli UIC45 | uhpB, uhpC, cyaAc | 64 (S) | 128 (I) |
| E. coli UIC46 | ΔuhpABCd | 64 (S) | 128 (I) |
| P. mirabilis ATCC 35659 | WT | 256 (NA) | 256 (NA) |
| K. pneumoniae ATCC 33495 | fosA | 256 (NA) | ≥512 (NA) |
| K. pneumoniae ATCC 700603 | fosA, uhpA, uhpC, uhpT, glpt, ptsl, murAe | 512 (NA) | ≥512 (NA) |
According to CLSI M100-S29 (16). NA, CLSI breakpoints not available; S, susceptible; I, intermediate; R, resistant.
Reference broth microdilution method includes 25 mg/liter G6P. MIC results obtained in the absence of G6P were interpreted for comparison purposes only.
The following genes and respective alterations were observed in isolate UIC45 compared with wild-type ATCC 25922: uhpB (P84S, S374T, Q441H, G459D, Q463H, H482T), uhpC (T72P, A177S, S417A, A435T), and cyaA (S222G, A349E, S356K, G359E, E362D, D837E, T840A).
The transport system regulator UhpABC was not detected.
The following genes and respective alterations were observed in the ATCC 700603 compared with wild-type ATCC 43816: uhpA (T3I, A25T, T41A, E64A, I87V, S183N), uhpC (E55D, A185T, C192G, M236L, S237T, A240E, V415A, T438A), uhpT (V434I), glpT (G196A, K234E, E237Q, I260V, V337I, P344A, I429V), ptsI (S241N), and murA (S148N, T206S, S210T).
Urinary titers.
The urinary inhibitory titer (UIT) was the same as the urinary bactericidal titer (UBT) in all cases, so only UBT values are reported. The median reciprocal fosfomycin UBT against the initial 5 isolates tested from each collection interval on days 1 and 5 of both dosing regimens are displayed in Table 2, along with area under the 24-h UBT-time curve (AUBT24) values. Analogous to the urine concentrations, UBTs were similar between dosing regimens and study days and peaked during the 0- to 8-h collection interval postdose. UBTs and AUBTs were the highest for the two E. coli isolates with the lowest fosfomycin MICs. UBTs were consistently less than 2 and AUBTs less than 20 for both Klebsiella strains and less than 4 and 50, respectively, for P. mirabilis. Although the observed UBTs and corresponding AUBTs were correlative to the respective isolate’s fosfomycin MIC performed in the presence of 25 mg/liter of G6P, both were significantly lower than expected based on the known urine fosfomycin concentrations (>300 mg/liter over 24 h), likely due to the lack of G6P in human urine. To investigate this, UBTs were repeated with and without 25 mg/liter G6P and with the 3 additional clinical E. coli strains on 3 representative subjects’ urine from day 5 of each dosing regimen. Results of these experiments are displayed in Table 3. The addition of 25 mg/liter G6P increased the UBT value just 1- to 3-fold for the 5 isolates with known intrinsic or acquired mutations in the G3P/G6P uptake transport system. Conversely, for the E. coli strains without known mutations, the addition of G6P increases the AUBT24 values up to 30-fold. Notably, for the two wild-type E. coli strains exquisitely susceptible to fosfomycin (E. coli ATCC 25922 and BAA-2326), the difference between the UBT values with and without G6P (Table 3) was virtually identical to the difference in MIC with and without G6P (∼16-fold) (Table 1).
TABLE 2.
Median reciprocal fosfomycin UBT and AUBT24 for all 18 subjects against the first 5 isolates tested
| Collection interval (h) | Median (IQR) value by strain and dosing regimen |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
E. coli ATCC 25922 |
E. coli BAA-2326 |
P. mirabilis ATCC 35659 |
K. pneumoniae ATCC 33495 |
K. pneumoniae ATCC 700603 |
||||||
| QOD | QD | QOD | QD | QOD | QD | QOD | QD | QOD | QD | |
| Day 1 | ||||||||||
| 0–4 | 4 (15) | 8 (16) | 4 (15) | 8 (8) | 4 (7) | 2.5 (8) | 1 (2) | 0 (4) | 1 (2) | 0 (2) |
| 4–8 | 8 (30) | 4.5 (16) | 8 (14) | 2.5 (16) | 4 (7) | 0.5 (8) | 2 (2) | 0 (1) | 1 (2) | 0 (0) |
| 8–12 | 4 (8) | 3 (7) | 4 (8) | 3 (7) | 1 (4) | 1 (4) | 0 (0) | 0 (0) | 0 (1) | 0 (0) |
| 12–24 | 4 (7) | 6 (6) | 2 (3) | 4 (2) | 1 (3) | 2 (3) | 0 (1) | 1 (2) | 0 (1) | 0 (0) |
| AUBT24 | 92 (246) | 86 (224) | 72 (182) | 81 (138) | 40 (124) | 27 (64) | 10 (20) | 8 (12) | 10 (12) | 1 (8) |
| Day 5 | ||||||||||
| 0–4 | 8 (28) | 4 (15) | 16 (28) | 4 (6) | 4 (7) | 4 (8) | 2 (4) | 1.5 (2) | 1 (2) | 1 (2) |
| 4–8 | 16 (32) | 12 (31) | 8 (32) | 16 (28) | 2 (8) | 4 (15) | 1 (4) | 2 (4) | 1 (2) | 1 (2) |
| 8–12 | 4 (8) | 6 (15) | 4 (8) | 4 (7) | 2 (8) | 1 (4) | 0 (1) | 0 (0) | 0 (1) | 0 (0) |
| 12–24 | 4 (7) | 4 (7) | 4 (3) | 4 (6) | 1 (4) | 2 (2) | 0 (1) | 1 (1) | 1 (2) | 0 (0) |
| AUBT24 | 124 (350) | 104 (340) | 108 (288) | 104 (226) | 28 (100) | 46 (78) | 18 (34) | 12 (20) | 12 (14) | 8 (8) |
TABLE 3.
Median reciprocal fosfomycin UBT and AUBT24 for 5 representative subjects against all 8 isolates tested on study day 5 with and without 25 mg/liter G6P
| Collection interval (h) | Median (IQR) value by strain and dosing regimen |
|||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
E. coli ATCC 25922 |
E. coli BAA-2326 |
E. coli UIC44 |
E. coli UIC45 |
E. coli UIC46 |
P. mirabilis ATCC 35659 |
K. pneumoniae ATCC 33495 |
K. pneumoniae ATCC 700603 |
|||||||||
| QOD | QD | QOD | QD | QOD | QD | QOD | QD | QOD | QD | QOD | QD | QOD | QD | QOD | QD | |
| No G6P | ||||||||||||||||
| 0–4 | 6 (22) | 12 (7) | 6 (10) | 8 (3) | 2 (1.5) | 3 (1.5) | 3 (1) | 4 (1.3) | 3 (3.3) | 3 (1) | 8 (19.5) | 10 (9) | 0.5 (3) | 2 (0.8) | 0.5 (2.5) | 2 (1.5) |
| 4–8 | 16 (10) | 12 (6) | 12 (10) | 10 (6) | 2 (3.5) | 2 (2) | 3 (5) | 6 (1.5) | 4 (3.5) | 4 (4.5) | 16 (34) | 24 (29) | 3 (2) | 2.5 (1.8) | 2.5 (1) | 1 (0.5) |
| 8–12 | 8 (3.5) | 8 (6) | 6 (7.5) | 12 (14.5) | 1.5 (0.5) | 2 (1.3) | 1.5 (1.5) | 5 (6.5) | 1.5 (1.3) | 4 (3) | 6 (8) | 9 (4) | 0 (0.8) | 0 (0.5) | 0 (0.5) | 0.5 (0.5) |
| 12–24 | 6 (2) | 4 (3) | 3 (0.5) | 3 (7.8) | 2 (1.3) | 1.5 (0.5) | 2 (5.5) | 3 (3.5) | 4 (1) | 1.5 (0.8) | 8 (3.3) | 3 (2.3) | 0.5 (0.3) | 1 (0.5) | 0.5 (0.3) | 0.5 (0.5) |
| AUBT24 | 112 (95) | 156 (48) | 88 (93) | 128 (142) | 44 (14.5) | 46 (10.5) | 67 (33) | 66 (57.5) | 63 (24) | 74 (18) | 148 (236) | 190 (162) | 15 (18) | 18 (13.5) | 13 (10) | 14 (3.5) |
| G6P | ||||||||||||||||
| 0–4 | 96 (16) | 80 (64) | 64 (256) | 48 (8) | 8 (6.5) | 8 (6.5) | 2 (7) | 6 (2) | 4 (3.3) | 4 (4.5) | 32 (31) | 16 (43) | 1.5 (2.5) | 1.5 (0.8) | 1.5 (2.5) | 1.5 (1.8) |
| 4–8 | 640 (488) | 192 (128) | 96 (80) | 192 (224) | 16 (7) | 12 (10) | 6 (3.3) | 6 (4.5) | 4 (2) | 4 (7) | 32 (40) | 64 (54) | 2 (0.8) | 4 (3.3) | 2 (0.8) | 3 (3.3) |
| 8–12 | 48 (20) | 128 (80) | 72 (24) | 64 (52) | 4 (1) | 8 (4) | 2 (2.3) | 8 (3) | 2 (0.5) | 3 (1.5) | 16 (4) | 20 (8) | 1 (0.5) | 1 (0.5) | 1 (0.5) | 1 (3.8) |
| 12–24 | 48 (40) | 128 (80) | 48 (148) | 64 (8) | 6 (4) | 6 (2) | 2 (2) | 4 (3.5) | 2.5 (1) | 4 (3.5) | 16 (8) | 32 (8) | 1 (0.8) | 0.5 (1) | 1 (0.8) | 2 (1.8) |
| AUBT24 | 3280 (1968) | 3,200 (944) | 2,144 (968) | 1,920 (744) | 128 (63) | 184 (61) | 45 (47) | 128 (40) | 54 (17.5) | 70 (54) | 384 (230) | 608 (322) | 19 (9) | 30 (12) | 44 (42) | 27 (45) |
Urinary time-kill analyses.
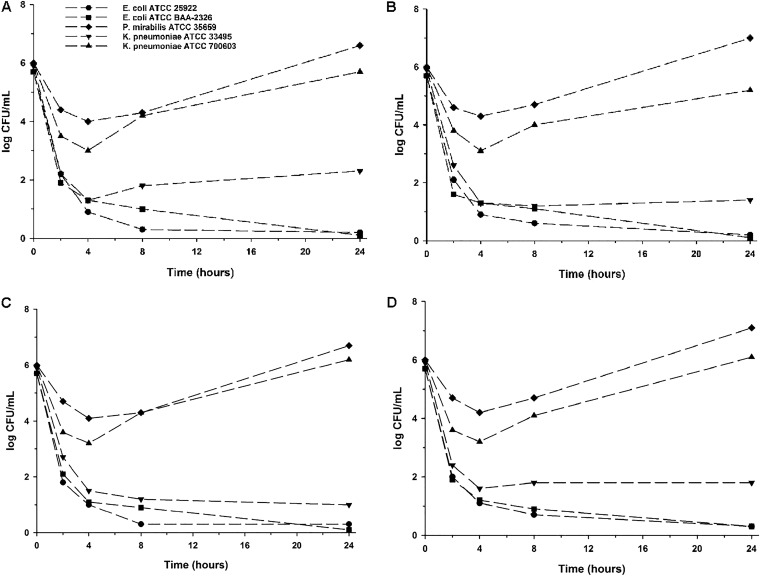
Results of time-kill experiments from pooled urine collected on days 1 and 5 of each dosing regimen against the initial 5 isolates tested are displayed in Fig. 1. Regardless of dosing regimen or study day, fosfomycin-laden urine was rapidly bactericidal in less than 2 h against both ATCC E. coli strains and K. pneumoniae ATCC 33495 and maintained bactericidality through 24 h. An initial ∼2-log10 CFU/ml inoculum reduction was observed against P. mirabilis ATCC 35659 and K. pneumoniae ATCC 700603 within the first 4 h, follow by rapid regrowth and 8- to 16-fold increases in MIC by 24 h (data not shown).
FIG 1.
Mean log10 CFU/ml versus time profile for each isolate against fosfomycin-laden urine collected from subjects during the QOD dosing regimen on day 1 (A) and day 5 (B) and during the QD dosing regimen on day 1 (C) and day 5 (D). Curves represent average concentrations for triplicate experiments. The y axis is in the log scale.
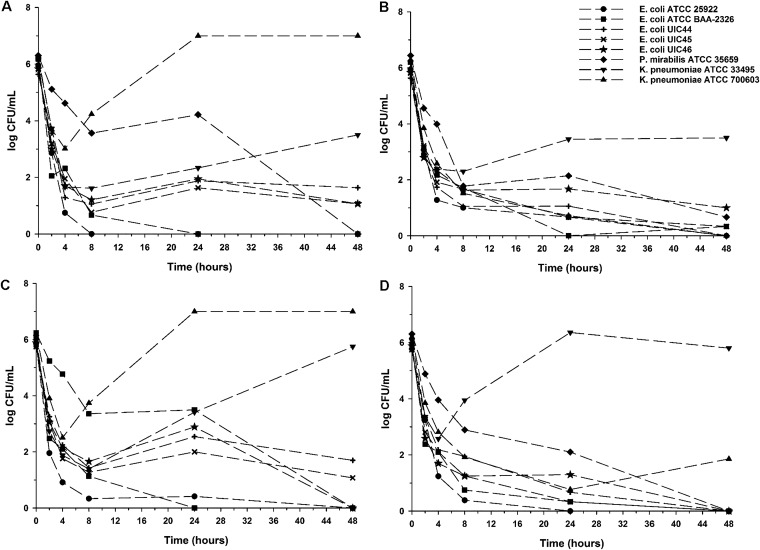
In order to assess the impact of G6P and any potential PD advantage of QD dosing, additional time-kill experiments were performed with and without 25 mg/liter G6P on a representative group of 3 subjects’ urine from day 5 of the QD dosing regimen and extended to 48 h (Fig. 2). No regrowth of any strain was observed between 24 and 48 h, while the addition of G6P improved the rate and extent of the bactericidal activity of the fosfomycin-laden urine against all 8 isolates tested except for the fosA-positive K. pneumoniae ATCC 33495 strain.
FIG 2.
Mean log10 CFU/ml versus time profile for each isolate against fosfomycin-laden urine collected from subjects during the QOD dosing regimen on day 5 without G6P (A) and with 25 mg/liter G6P (B) and during the QD dosing regimen on day 5 without G6P (C) and with 25 mg/liter G6P (D). Curves represent average concentrations for triplicate experiments. The y axis is in the log scale.
DISCUSSION
To our knowledge, this is the first study to evaluate the ex vivo urinary antibacterial activity and urinary PD of fosfomycin after repeated oral doses of fosfomycin tromethamine. There are several notable and clinically relevant findings from this study. First, fosfomycin MICs obtained in vitro in the presence of 25 mg/liter G6P as recommended (16) may not accurately reflect the antibacterial activity of fosfomycin in vivo. This study demonstrates that the antibacterial activity and PD of fosfomycin is significantly impaired in human urine due to the lack of G6P. As previously mentioned, the UIT and UBT values of fosfomycin were identical in all cases. This is expected given fosfomycin’s bactericidal activity and is consistent with previous studies (17). Therefore, barring any influence of urine on bacterial growth and/or antimicrobial activity, the UBT should be roughly equal to the corresponding urine drug concentration divided by the pathogen’s MIC for bactericidal drugs (18–21). In the present study, the average urine concentrations of fosfomycin during the 4- to 8-h collection interval were >400 mg/liter, and the MICs against the two wild-type E. coli strains were 4 and 2 mg/liter, respectively, which should translate into UBT values of ≥100 to 200. In fact, measured median UBT values without G6P peaked at 8 during the same 4- to 8-h interval. When G6P was added, UBT values increased into the range predicted. Importantly, the discordance in UBTs with and without G6P was almost identical to the discordance observed in MICs with and without G6P (∼16-fold) against E. coli isolates without mutations in the G6P transport system (Table 1). These data suggest that the properties of the urine itself, other than lacking G6P, do not attenuate the activity of fosfomycin. These results are supported by a recent study evaluating the ex vivo UBTs of fosfomycin in 40 healthy females given a single 3-g dose of oral fosfomycin (17). Wijma et al. also found that UBTs were not related to the strain’s MIC tested in the presence of G6P, although no further analyses were done to quantify the magnitude of this discrepancy.
Second, the antibacterial activity of fosfomycin was poor against Enterobacteriaceae isolates other than E. coli tested in this study, particularly K. pneumoniae. UBT values against K. pneumoniae were rarely above 2 at any time point, and the addition of G6P did not improve the activity of fosfomycin against these strains given the numerous resistance mechanisms present in both strains. This suboptimal activity against K. pneumoniae has been previously demonstrated in an in vitro dynamic bladder infection model where, despite MICs of ≤16 mg/liter and simulated peak urine fosfomycin concentrations of 1,053 to 4,415 mg/liter, 15/16 K. pneumoniae isolates tested demonstrated rapid regrowth and development of fosfomycin resistance (22). The aforementioned ex vivo fosfomycin UBT study was also unable to detect any antibacterial activity against the majority of K. pneumoniae isolates included (17). Together, these data support the FDA indication and the CLSI recommendation for susceptibility testing only against E. coli (16) and caution against the clinical use of oral fosfomycin for the treatment of UTIs not due to E. coli.
Third, given that multiple-dose regimens of oral fosfomycin are often employed clinically, it was important to evaluate the PK, PD, and safety of commonly used repeated-dose regimens. We observed similar urine concentrations of fosfomycin and correspondingly similar urinary antibacterial activity across dosing regimens (QOD and QD) and study days (1 and 5). Additionally, extending our time-kill analyses from 24 to 48 h did not demonstrate bacterial regrowth during that period. Collectively these PK/PD data, along with the higher rate of diarrhea observed in the QD dosing arm (12), do not support increasing the dosing interval of oral fosfomycin above QOD in an attempt to treat more complicated or resistant UTIs.
The data generated from this study are particularly opportune given the pending approval of intravenous (i.v.) fosfomycin disodium in the United States. We have previously demonstrated that a single 8-g i.v. dose of fosfomycin disodium produces a urinary maximum concentration (Cmax) of approximately 15,580.7 mg/liter in healthy volunteers, roughly 7-fold higher than that after a single 3-g dose of oral fosfomycin tromethamine (23). These results were used to inform the dose of 6 g every 8 h (q8h) i.v. utilized in the recently completed phase 2/3 trial in patients with complicated UTI or acute pyelonephritis, which demonstrated noninferiority compared to piperacillin-tazobactam (24). Importantly, clinical cure and microbiological eradication rates against E. coli were similar to those of other Gram-negative pathogens, including Klebsiella and Proteus spp., suggesting that i.v. fosfomycin disodium is preferred over oral fosfomycin tromethamine for the treatment of complicated UTIs due to less susceptible E. coli or other Enterobacteriaceae species.
Added strengths of the present study include use of an ex vivo model, which allowed us to evaluate the antibacterial activity and PD of fosfomycin in human urine based on real-world obtainable urine concentrations, and the use of whole-genome sequencing to select pathogens with a range of genotypic and phenotypic susceptibilities to fosfomycin. Limitations include the number of strains tested, the static nature of UBT and PD experiments, and the lack of a clear association between UBTs and clinical outcomes, although some thresholds for other bactericidal drugs have been suggested (25).
In summary, the significant variability in urine concentrations and corresponding urinary antibacterial activity may help to explain the suboptimal efficacy rates observed for oral fosfomycin in the treatment of uncomplicated UTIs (26, 27). Further, fosfomycin MICs determined in vitro in the presence of G6P may not accurately reflect the in vivo activity, given the lack of G6P in human urine observed in this study and others. Finally, our PK, PD, and safety results do not support the use of oral fosfomycin for pathogens other than E. coli or at a dosing frequency higher than QOD. Confirmation of these findings in controlled clinical trials is warranted.
MATERIALS AND METHODS
Study design and subjects.
This was an ex vivo annex to a phase I, randomized, open-label, two-period-crossover, multiple-dose study of oral fosfomycin tromethamine (Monurol; Forest Pharmaceuticals, Inc., St. Louis, MO) in healthy adult subjects (12). This study was approved by the University of Illinois at Chicago (UIC) Office for the Protection of Research Subjects Institutional Review Board and conducted in accordance with good clinical practices at the UIC Clinical Research Center. Written informed consent was obtained from each subject prior to the conduct of any study-related procedures.
Subjects were enrolled in study drug administration sequences in parallel so that each subject received both dosing regimens in a randomized, crossover fashion. The two regimens were 3 g QOD for 3 doses followed by 3 g QD for 7 doses or vice versa. Fosfomycin was delivered as a powder sachet mixed in 3 to 4 oz water under fasted conditions. Each administration sequence was separated by a 5- to 14-day washout period. Subjects’ fluid intake was allowed ad libitum.
Full details regarding eligibility criteria, plasma sampling, bioanalytical procedures, PK analysis, and safety assessments can be found in Wenzler et al. (12).
Urine sample collection.
Urine samples for PD analyses were collected before and at intervals of 0 to 4, 4 to 8, 8 to 12, and 12 to 24 h postdose on days 1 and 5. Single predose urine samples were also collected on days 3 and 7. Urine samples were stored at ≤4°C during collection intervals. After completion of the collection interval, aliquots of urine were extracted, frozen, and stored at –80°C until analyzed. Urine was thawed and filter sterilized prior to all PD experiments. Urine pH was measured and recorded prior to all experiments but was not adjusted. Urine G6P content was measured via G6P assay kit (Sigma-Aldrich, St. Louis, MO).
Bacteria and susceptibility testing.
Initially, five bacterial strains representing typical uropathogens were evaluated: E. coli ATCC 25922, E. coli ATCC BAA-2326 (CTX-M-15 producing), Proteus mirabilis ATCC 35659, Klebsiella pneumoniae ATCC 33495, and K. pneumoniae ATCC 700603 (sulphydryl variable-18 [SMV-18] producing). Subsequently, three clinical E. coli strains from patients with UTIs (UIC44, UIC45, and UIC46) were added in select experiments to elucidate the antibacterial activity of fosfomycin on clinical strains with higher fosfomycin MICs and underlying genotypic mutations. Isolates were maintained at –80°C in cation-adjusted Mueller-Hinton broth (CAMHB) with 20% glycerol and subcultured on tryptic soy agar with 5% sheep’s blood twice prior to use. Analytical-grade fosfomycin was purchased commercially (Sigma-Aldrich, St. Louis, MO). Fosfomycin MICs were performed in triplicate on the same day via agar dilution at standard inoculum, according to CLSI guidelines, with and without the addition of 25 mg/liter of G6P (28). Modal MIC values are reported. E. coli ATCC 25922 was included as a quality control, and susceptibilities to fosfomycin were interpreted according to CLSI interpretive criteria (16).
Determination of fosfomycin resistance mechanisms.
Genomic DNA was extracted and used for library preparation (Nextera XT). Paired-end genome sequencing was performed on an Illumina MiSeq (Illumina, San Diego, CA) in a 2× 150-bp configuration (JMI Laboratories, North Liberty, IA). Each raw sequencing data set was quality assured, error corrected, and assembled de novo using the SPAdes genome assembler (29). FASTQ format sequencing files for each sample set were applied to a JMI Laboratories-designed software workflow to align against a curated database containing known fosfomycin resistance genes, including fomA, fomB, fosA, fosA2, fosA4, fosA5, fosB, fosB1, fosB2, fosC, fosD, fosE, fosF, fosG, fosK, and fosX (30, 31). In addition, intrinsic genes associated with the fosfomycin binding site (MurA) and transport system regulators (glpT, uhpT, uhpABC, cyaA, crp, and ptsI) were extracted and amino acid sequences compared with the respective wild-type reference sequence.
Urinary titers.
Urinary inhibitory titers (UIT) and bactericidal titers (UBTs) were performed as previously described (18) via a modified Schlichter test (32), utilizing the participant’s drug-free urine as the diluent. Aliquots of urine from each subject obtained during each urine collection interval on study days 1 and 5 of both dosing regimens were inoculated with the test pathogen and then serially diluted with the same subject’s drug-free urine in a 96-well microtiter plate. Plates were then incubated at 35°C in ambient air for 24 h before being read. The UIT was defined as the highest dilution that inhibited visible bacterial growth. For UBTs, a 50-μl aliquot of subcultured urine from the first visibly clear well was then plated and incubated at 35°C for 24 h prior to enumeration. UBT was defined as the greatest urinary dilution that achieved a ≥3-log10 reduction in CFU/ml compared to the starting inoculum of 106 CFU/ml, ranging from 1:1,024. Reciprocal UIT and UBT values are reported. The area under the 24-h UBT-time curve (AUBT24) was calculated via the trapezoidal rule using reciprocal UBT values. In order to assess the impact of G6P, UBTs were repeated with the addition of 25 mg/liter of G6P on 3 representative subjects’ urine samples from each dosing regimen on study day 5.
Urinary time-kill analyses.
Urinary time-kill analyses were performed in triplicate according to CLSI guidelines (33), modified utilizing urine as the milieu in a total volume of 2 ml in deep-well plates. A direct suspension of 3 to 4 isolated colonies selected from a pure overnight culture was incubated at 35°C with shaking to ensure log-phase growth. Suspensions were then adjusted to a 0.5 McFarland standard in saline and further diluted to a starting inoculum of approximately 106 CFU/ml in CAMHB. Colony counts were performed to ensure starting inoculum densities.
An aliquot of each subject’s urine from each collection interval on days 1 and 5 of both dosing regimens was pooled in order to represent the activity of fosfomycin in the urine over 24 h and added to the bacterial suspension. A growth control without any fosfomycin-laden urine was included with each experiment. At the specified time points of 0, 2, 4, 8, and 24 h, aliquots of 20 μl were removed from the suspensions and serially diluted in log10 dilutions. A 100-μl aliquot was then plated on Mueller-Hinton agar and incubated at 35°C for at least 24 h prior to enumeration. Cystine-lactose-electrolyte-deficient (CLED) agar was used for experiments containing P. mirabilis to abate swarming. Time-kill curves were generated by plotting the average log10 CFU/ml from triplicate experiments versus time. Bactericidal activity was defined as a ≥3-log10 CFU/ml reduction in bacterial density compared to the starting inoculum. In order to assess the impact of G6P, time-kill analyses were repeated with the addition of 25 mg/liter of G6P on 3 representative subjects’ urine from each dosing regimen on study day 5.
Statistical analysis.
For each bacterial isolate, the time-specific reciprocal UBT and AUBT24 values are summarized separately for each dosing regimen and study day. Summary median and interquartile ranges (IQR) are reported. An artificial value of 0 indicates no bactericidal activity of undiluted urine.
Supplementary Material
ACKNOWLEDGMENTS
We thank the study participants and acknowledge the contributions of the staff at the UIC Clinical Research Center and Investigational Drug Service for their support of this work. We also thank Timothy B. Doyle from JMI Laboratories for assistance with sequencing and analysis of resistance mechanisms.
This study was supported in part by the National Institute of Allergy and Infectious Diseases of the National Institutes of Health (NIH) under award number 1UM1AI104681-01. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH. The Center for Clinical and Translational Science at the University of Illinois at Chicago is supported by the National Center for Advancing Translational Sciences NIH grant UL1TR002003.
E.W. serves on the scientific advisory board for GenMark Diagnostics and Shionogi and on the speaker’s bureau for Melinta Therapeutics and Astellas Pharma. We have no relevant conflicts of interest to report.
Footnotes
Supplemental material is available online only.
REFERENCES
- 1.Gupta K, Hooton TM, Naber KG, Wullt B, Colgan R, Miller LG, Moran GJ, Nicolle LE, Raz R, Schaeffer AJ, Soper DE. 2011. International clinical practice guidelines for the treatment of acute uncomplicated cystitis and pyelonephritis in women: a 2010 update by the Infectious Diseases Society of America and the European Society for Microbiology and Infectious Diseases. Clin Infect Dis 52:e103–e120. doi: 10.1093/cid/ciq257. [DOI] [PubMed] [Google Scholar]
- 2.Forest Pharmeceuticals, Inc. 2007. Monurol (fosfomycin tromethamine) [package insert]. Forest Pharmaceuticals, Inc, St. Louis, MO: http://www.accessdata.fda.gov/drugsatfda_docs/label/2008/050717s005lbl.pdf. Accessed 11 May 2015. [Google Scholar]
- 3.Falagas ME, Vouloumanou EK, Samonis G, Vardakas KZ. 2016. Fosfomycin. Clin Microbiol Rev 29:321–347. doi: 10.1128/CMR.00068-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.European Center for Disease Prevention and Control. 2018. Trend of antimicrobial consumption by country. European Center for Disease Prevention and Control, Solna, Sweden: https://ecdc.europa.eu/en/antimicrobial-consumption/database/trend-country. Accessed 5 August 2018. [Google Scholar]
- 5.Cai T, Cocci A, Verze P, Rizzo M, Palmieri A, Liguori G, Trombetta C, Adembri C, Carini M, Bartoletti R, Wagenlehner FM, Bonkat G, Mirone V, Bjerklund Johansen TE, Novelli A. 2018. The use of oral fosfomycin-trometamol in patients with catheter-associated urinary tract infections (CAUTI): new indications for an old antibiotic? J Chemother 30:290–295. doi: 10.1080/1120009X.2018.1500110. [DOI] [PubMed] [Google Scholar]
- 6.Bielen L, Likic R. 2019. Experience with fosfomycin in the treatment of complicated urinary tract infections caused by extended-spectrum beta-lactamase-producing Enterobacteriaceae. Ther Adv Infect Dis 6:2049936119858883. doi: 10.1177/2049936119858883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Babiker A, Clarke L, Doi Y, Shields RK. 2019. Fosfomycin for treatment of multidrug-resistant pathogens causing urinary tract infection: a real-world perspective and review of the literature. Diagn Microbiol Infect Dis 95:114856. doi: 10.1016/j.diagmicrobio.2019.06.008. [DOI] [PubMed] [Google Scholar]
- 8.Qiao LD, Zheng B, Chen S, Yang Y, Zhang K, Guo HF, Yang B, Niu YJ, Wang Y, Shi BK, Yang WM, Zhao XK, Gao XF, Chen M. 2013. Evaluation of three-dose fosfomycin tromethamine in the treatment of patients with urinary tract infections: an uncontrolled, open-label, multicentre study. BMJ Open 3:e004157. doi: 10.1136/bmjopen-2013-004157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Grayson ML, Macesic N, Trevillyan J, Ellis AG, Zeglinski PT, Hewitt NH, Gardiner BJ, Frauman AG. 2015. Fosfomycin for treatment of prostatitis: new tricks for old dogs. Clin Infect Dis 61:1141–1143. doi: 10.1093/cid/civ436. [DOI] [PubMed] [Google Scholar]
- 10.Moroni M. 1987. Monuril in lower uncomplicated urinary tract infections in adults. Eur Urol 13(Suppl 1):101–104. doi: 10.1159/000472872. [DOI] [PubMed] [Google Scholar]
- 11.Pullukcu H, Tasbakan M, Sipahi OR, Yamazhan T, Aydemir S, Ulusoy S. 2007. Fosfomycin in the treatment of extended spectrum beta-lactamase-producing Escherichia coli-related lower urinary tract infections. Int J Antimicrob Agents 29:62–65. doi: 10.1016/j.ijantimicag.2006.08.039. [DOI] [PubMed] [Google Scholar]
- 12.Wenzler E, Bleasdale SC, Sikka M, Bunnell KL, Finnemeyer M, Rosenkranz SL, Danziger LH, Rodvold KA. 2018. Phase I study to evaluate the pharmacokinetics, safety, and tolerability of two dosing regimens of oral fosfomycin tromethamine in healthy adult participants. Antimicrob Agents Chemother 62:e00464-18. doi: 10.1128/AAC.00464-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.CLSI. 2017. Performance standards for antimicrobial susceptibility testing: approved 27th ed. Document M100-S27 CLSI, Wayne, PA. [Google Scholar]
- 14.NCCLS. 1998. Subcommittee on antimicrobial susceptibility test meeting. NCCLS, Wayne, PA. [Google Scholar]
- 15.CLSI. 2018. Performance standards for antimicrobial susceptibility testing: approved 28th ed. Document M100-S28 CLSI, Wayne, PA. [Google Scholar]
- 16.CLSI. 2019. Performance standards for antimicrobial susceptibility testing: approved 28th ed. Document M100-S29 CLSI, Wayne, PA. [Google Scholar]
- 17.Wijma RA, Huttner A, van Dun S, Kloezen W, Abbott IJ, Muller AE, Koch BCP, Mouton JW. 2019. Urinary antibacterial activity of fosfomycin and nitrofurantoin at registered dosages in healthy volunteers. Int J Antimicrob Agents 54:435–441. doi: 10.1016/j.ijantimicag.2019.07.018. [DOI] [PubMed] [Google Scholar]
- 18.Naber KG, Theuretzbacher U, Kinzig M, Savov O, Sorgel F. 1998. Urinary excretion and bactericidal activities of a single oral dose of 400 milligrams of fleroxacin versus a single oral dose of 800 milligrams of pefloxacin in healthy volunteers. Antimicrob Agents Chemother 42:1659–1665. doi: 10.1128/AAC.42.7.1659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wagenlehner FME, Kinzig-Schippers M, Tischmeyer U, Wagenlehner C, Sörgel F, Naber KG. 2006. Urinary bactericidal activity of extended-release ciprofloxacin (1,000 milligrams) versus levofloxacin (500 milligrams) in healthy volunteers receiving a single oral dose. Antimicrob Agents Chemother 50:3947–3949. doi: 10.1128/AAC.00477-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Well M, Naber KG, Kinzig-Schippers M, Sörgel F. 1998. Urinary bactericidal activity and pharmacokinetics of enoxacin versus norfloxacin and ciprofloxacin in healthy volunteers after a single oral dose. Int J Antimicrob Agents 10:31–38. doi: 10.1016/s0924-8579(98)00014-4. [DOI] [PubMed] [Google Scholar]
- 21.Wagenlehner FME, Wydra S, Onda H, Kinzig-Schippers M, Sörgel F, Naber KG. 2003. Concentrations in plasma, urinary excretion, and bactericidal activity of linezolid (600 milligrams) versus those of ciprofloxacin (500 milligrams) in healthy volunteers receiving a single oral dose. Antimicrob Agents Chemother 47:3789–3794. doi: 10.1128/aac.47.12.3789-3794.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Abbott IJ, Meletiadis J, Belghanch I, Wijma RA, Kanioura L, Roberts JA, Peleg AY, Mouton JW. 2018. Fosfomycin efficacy and emergence of resistance among Enterobacteriaceae in an in vitro dynamic bladder infection model. J Antimicrob Chemother 73:709–719. doi: 10.1093/jac/dkx441. [DOI] [PubMed] [Google Scholar]
- 23.Wenzler E, Ellis-Grosse EJ, Rodvold KA. 2017. Pharmacokinetics, safety, and tolerability of single-dose intravenous (ZTI-01) and oral fosfomycin in healthy volunteers. Antimicrob Agents Chemother 61:e00775-17. doi: 10.1128/AAC.00775-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kaye KS, Rice LB, Dane AL, Stus V, Sagan O, Fedosiuk E, Das AF, Skarinsky D, Eckburg PB, Ellis-Grosse EJ. 2019. Fosfomycin for injection (ZTI-01) versus piperacillin-tazobactam for the treatment of complicated urinary tract infection including acute pyelonephritis: ZEUS, a phase 2/3 randomized trial. Clin Infect Dis 2019:ciz181. doi: 10.1093/cid/ciz181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wagenlehner FM, Wagenlehner C, Redman R, Weidner W, Naber KG. 2009. Urinary bactericidal activity of doripenem versus that of levofloxacin in patients with complicated urinary tract infections or pyelonephritis. Antimicrob Agents Chemother 53:1567–1573. doi: 10.1128/AAC.01133-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Anonymous. 1997. Fosfomycin for urinary tract infections. Med Lett Drugs Ther 39:66–68. [PubMed] [Google Scholar]
- 27.Huttner A, Kowalczyk A, Turjeman A, Babich T, Brossier C, Eliakim-Raz N, Kosiek K, Martinez de Tejada B, Roux X, Shiber S, Theuretzbacher U, von Dach E, Yahav D, Leibovici L, Godycki-Cwirko M, Mouton JW, Harbarth S. 2018. Effect of 5-day nitrofurantoin vs single-dose fosfomycin on clinical resolution of uncomplicated lower urinary tract infection in women: a randomized clinical trial. JAMA 319:1781–1789. doi: 10.1001/jama.2018.3627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.CLSI. 2015. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically; approved standard–10th ed . M07-A10 CLSI, Wayne, PA. [Google Scholar]
- 29.Bankevich A, Nurk S, Antipov D, Gurevich AA, Dvorkin M, Kulikov AS, Lesin VM, Nikolenko SI, Pham S, Prjibelski AD, Pyshkin AV, Sirotkin AV, Vyahhi N, Tesler G, Alekseyev MA, Pevzner PA. 2012. SPAdes: a new genome assembly algorithm and its applications to single-cell sequencing. J Comput Biol 19:455–477. doi: 10.1089/cmb.2012.0021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Takahata S, Ida T, Hiraishi T, Sakakibara S, Maebashi K, Terada S, Muratani T, Matsumoto T, Nakahama C, Tomono K. 2010. Molecular mechanisms of fosfomycin resistance in clinical isolates of Escherichia coli. Int J Antimicrob Agents 35:333–337. doi: 10.1016/j.ijantimicag.2009.11.011. [DOI] [PubMed] [Google Scholar]
- 31.Falagas ME, Athanasaki F, Voulgaris GL, Triarides NA, Vardakas KZ. 2019. Resistance to fosfomycin: mechanisms, frequency and clinical consequences. Int J Antimicrob Agents 53:22–28. doi: 10.1016/j.ijantimicag.2018.09.013. [DOI] [PubMed] [Google Scholar]
- 32.Edberg S. 1986. The measurement of antibiotics in human body fluids: techniques and significance, p 466–467. In Lorian V. (ed), Antibiotics and laboratory medicine. The Williams & Wilkins Co, Baltimore, MD. [Google Scholar]
- 33.CLSI. 1999. Methods for determining bactericidal activity of antimicrobial agents: approved guideline: document M26-A. CLSI, Wayne, PA. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.