Pulmonary infection with the multidrug-resistant Mycobacterium abscessus complex (MABSC) is difficult to treat in individuals with cystic fibrosis (CF). MABSC grows as biofilm aggregates in CF patient lungs, which are known to have anaerobic niches. How aggregation and anoxic conditions affect antibiotic tolerance is not well understood. We sought to determine whether disaggregation and oxygen availability sensitize MABSC isolates to recommended antibiotics.
KEYWORDS: Mycobacterium abscessus complex, oxygenation, cystic fibrosis, antimicrobial resistance, biofilm
ABSTRACT
Pulmonary infection with the multidrug-resistant Mycobacterium abscessus complex (MABSC) is difficult to treat in individuals with cystic fibrosis (CF). MABSC grows as biofilm aggregates in CF patient lungs, which are known to have anaerobic niches. How aggregation and anoxic conditions affect antibiotic tolerance is not well understood. We sought to determine whether disaggregation and oxygen availability sensitize MABSC isolates to recommended antibiotics. We tested the susceptibilities of 33 isolates from 22 CF patients with MABSC infection and a reference strain to the following antibiotics: amikacin, azithromycin, cefoxitin, ciprofloxacin, clarithromycin, imipenem, kanamycin, linezolid, moxifloxacin, rifampin, tigecycline, and sulfamethoxazole-trimethoprim. Isolates were grown in Mueller-Hinton broth with and without the disaggregating detergent Tween 80 (5%). Time-kill curves at days 1 and 3 were generated for oxic and anoxic amikacin treatment in 4-fold dilutions ranging from 2 to 512 mg liter−1. Scanning electron microscopy was used to visualize the aggregation patterns, while confocal laser scanning microscopy and microrespirometry were used to visualize biofilm growth patterns. Disruption of MABSC aggregates increased susceptibility to amikacin, tigecycline, kanamycin, azithromycin, imipenem, cefoxitin, and clarithromycin (P < 0.05, n = 29 to 31). Oxygenation enhanced the killing of disaggregated MABSC isolates by amikacin (P < 0.05) by 1 to 6 log units when 2 to 512 mg liter−1 of amikacin was used. This study explains why current drug susceptibility testing results correlate poorly with treatment outcomes. The conditions achieved by oxic culturing of planktonic isolates in vitro do not resemble the hypoxic conditions in CF patient lungs. Biofilm disruption and increased O2 availability during antibiotic therapy may be new therapeutic strategies for chronic MABSC infection.
INTRODUCTION
The Mycobacterium abscessus complex (MABSC) is part of the rapidly growing pathogenic nontuberculous mycobacteria (NTMs) and is increasingly isolated from sputum samples from patients with cystic fibrosis (CF) (1, 2). The MABSC severely impacts lung function and life expectancy in patients with CF (3). It is clinically challenging to treat MABSC infections (4), and antibiotic therapy often fails (5, 6). Intensive, multidrug treatment regimens are required for months to years, but cure rates are still disappointing (7–9). It is recommended that several antibiotics be combined in an initial, intravenous phase, followed by a long maintenance phase with two or more antibiotics, administered orally. Susceptibility testing is common, but there are only weak correlations between the results of in vitro susceptibility studies and clinical outcomes (10).
The genus Mycobacterium is comprised of more than 170 species (11), the majority of which are not implicated in human infection, while Mycobacterium tuberculosis is the most well-known. NTMs include species that are ubiquitous in soil and aquatic environments, as well as human pathogens (12, 13). NTMs may exist as free-living, planktonic cells or embedded in large, biofilm aggregates. Aggregation was clearly demonstrated in the early days of mycobacteriology (14, 15), with the subsequent focus being on growth patterns, cord formation, and other manifestations of complex bacterial communities (16–19). Bacteria in a biofilm differ from planktonic cells, are more tolerant to antimicrobial treatments, and can resist challenges from inflammatory cells (20). Recently, it was demonstrated that cells of the MABSC cluster together in aggregates in mucus and in the endothelial wall of patients with CF and that the resulting infection induces a humoral immune response, which is correlated with disease severity (21, 22). MABSC exhibits two distinct morphotypes when plated on agar media: the smooth morphotype (SM) and the rough morphotype (RG). The main difference between these two morphotypes is the complete lack of surface-associated glycopeptidolipids (GPL; molecules that localize to the outermost layer of the mycobacterial cell wall) in the rough morphotype (23). These morphotypes appear to differ in their virulence and pathogenicity traits, as well as in their ability to form biofilms (24).
The most severe complication in CF patients is chronic bacterial lung infection, characterized by antibiotic-tolerant biofilms in the endobronchial mucus. These biofilms are dominated by the pathogen Pseudomonas aeruginosa and are present in zones of oxygen (O2) depletion (25), a consequence of O2 consumption by polymorphonuclear leukocytes during their respiratory burst (26, 27). Slow metabolism is favored in these environments, and under the right conditions, the mycobacteria can enter a dormant state (28) with almost no metabolic activity. Aggregation in metabolically dormant biofilms offers protection from the host immune response and antibiotic challenges (29, 30). The mechanisms of the antibiotic tolerance of biofilms remain unclear, but it has been shown that the efficiency of several bactericidal antibiotics is enhanced by stimulating aerobic respiration. A lack of O2, on the other hand, increases bacterial antibiotic tolerance (31–35). The activity of antibiotics depends on bacterial metabolic activity. Bacterial metabolic activity may lead to the increased uptake of antibiotics and expression to antibiotic targets. In addition, active aerobic metabolism enables the formation of reactive O2 radicals (reactive oxygen species [ROS]), which may in part contribute to the bactericidal effect of antibiotics (30, 34–36). In bacteria surviving treatment with antibiotics, the formation of mutagenic ROS may lead to the development of antibiotic resistance (36). Mycobacterial growth has been shown to be O2 dependent, and therefore, ROS formation may provide an explanation for why MABSC quickly develops resistance to bactericidal drugs, such as aminoglycosides, during chronic lung infection in patients with CF (37).
In Pseudomonas aeruginosa, zone formation during agar disk susceptibility testing is due to the transformation of the bacteria from single cells to a biofilm, in which the resistance to antibiotics increases when the growth of the inoculum reaches aggregates of approximately 64 cells (38, 39). Since M. abscessus grows as aggregates, we hypothesized that this is the reason for the high level of antimicrobial resistance of this species and why the results of susceptibility testing have a low predictive value for determination of the clinical effect of antibiotic treatment. An additional reason could be the very slow growth of the bacteria (40) because of the dormancy of the bacteria due to a lack of O2.
The objective of this investigation was therefore to determine whether disaggregation and O2 availability sensitize MABSC to the recommended antibiotics.
RESULTS
Aggregation of MABSC.
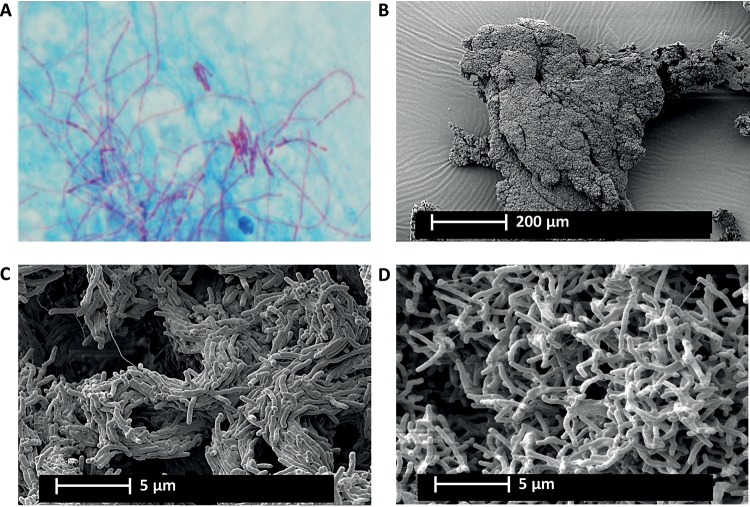
Ziehl-Neelsen staining demonstrated the presence of MABSC aggregation and the more abundant cording in a sputum sample from a CF patient (Fig. 1A). However, scanning electron microscopy (SEM) shows that MABSC readily aggregates in shaken cultures if no detergent is added. This indicates biofilm formation by MABSC (Fig. 1B). From the SEM images, it was possible to see the cording formation of the rough morphotype (Fig. 1C) and a chaotically scattered distribution of the cells with a smooth morphotype (Fig. 1D).
FIG 1.
Aggregation and cording formation by MABCS with rough morphotypes. (A) Cord formation of MABSC in Ziehl-Neelsen-stained CF sputum sample. (B) MABSC aggregate with cells of the rough morphotype isolated from a CF patient sputum sample visualized by scanning electron microscopy (SEM). (C and D) Enlarged images of organized cord formation in the MABSC aggregate with cells of the rough morphotype (C) and smooth morphotype (D) isolated from a CF patient sputum sample visualized by SEM.
The aggregation of MABSC isolates is influenced by Tween 80.
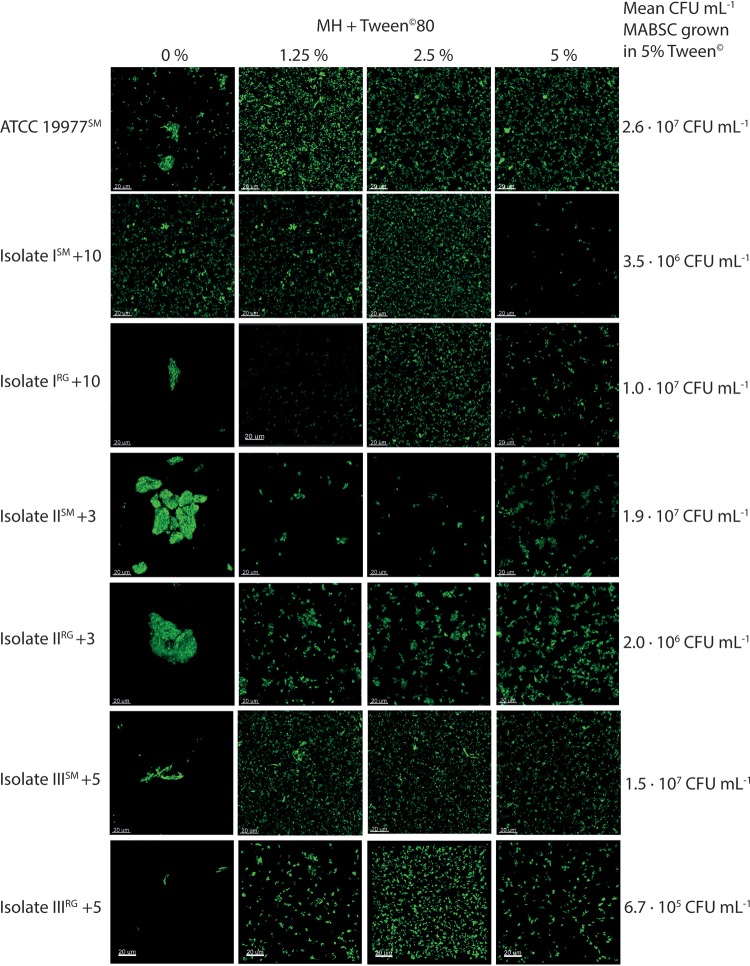
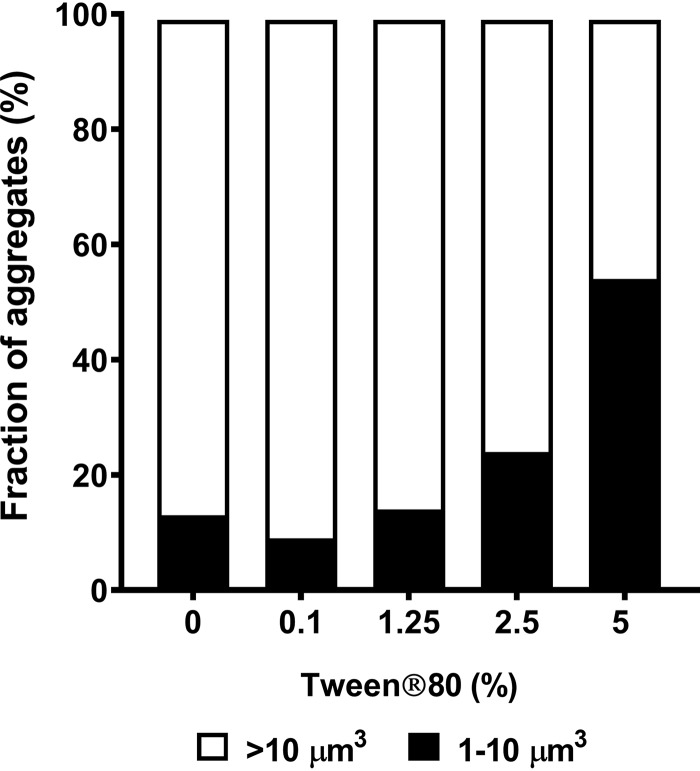
The aggregate size and shape of MABSC isolates as well as reference strain ATCC 19977 in the presence of increasing concentrations of Tween 80 were compared by confocal microscopy (Fig. 2). Variations in aggregate size were seen when cells were incubated in medium without Tween 80. Visually, the cells formed pellicles in the liquid medium (Fig. 2). Increasing the concentration of Tween 80 in the liquid medium caused cells to disperse as Tween 80 altered the structural integrity of the membrane, lipids, and proteins (41). The fraction of aggregates of <10 μm was the highest for cells incubated in 5% Tween 80, indicating more disaggregated cells (Fig. 3). Furthermore, 5% Tween 80 had no detectable effect on the viability of MABSC (see Fig. S1A in the supplemental material), nor did Tween 80 in combination with amikacin affect bacterial killing (Fig. S1B).
FIG 2.
The growth of MABSC is influenced by disaggregation. The images show the effect of Tween 80, which breaks up MABSC isolates from liquid cultures. Bacterial aggregation at four different Tween 80 concentrations is shown. Samples were stained with Syto 9, and the images were obtained using a 63× (numerical aperture [NA], 1.4) Zeiss objective on a Zeiss 880 CLSM. Bars, 20 μm at a magnification of ×630. Isolates I to III were isolated from three CF patients +10, +3, and +5 years after collection of the first sample positive for M. abscessus.
FIG 3.
The size of MABSC aggregates from liquid cultures decreases as the concentration of Tween 80 increases. The fractions of large (>10 μm3; white) versus small (1 to 10 μm3; black) aggregates of the MABSC ATCC 19977 smooth reference strain are shown.
Aggregation increases antibiotic tolerance.
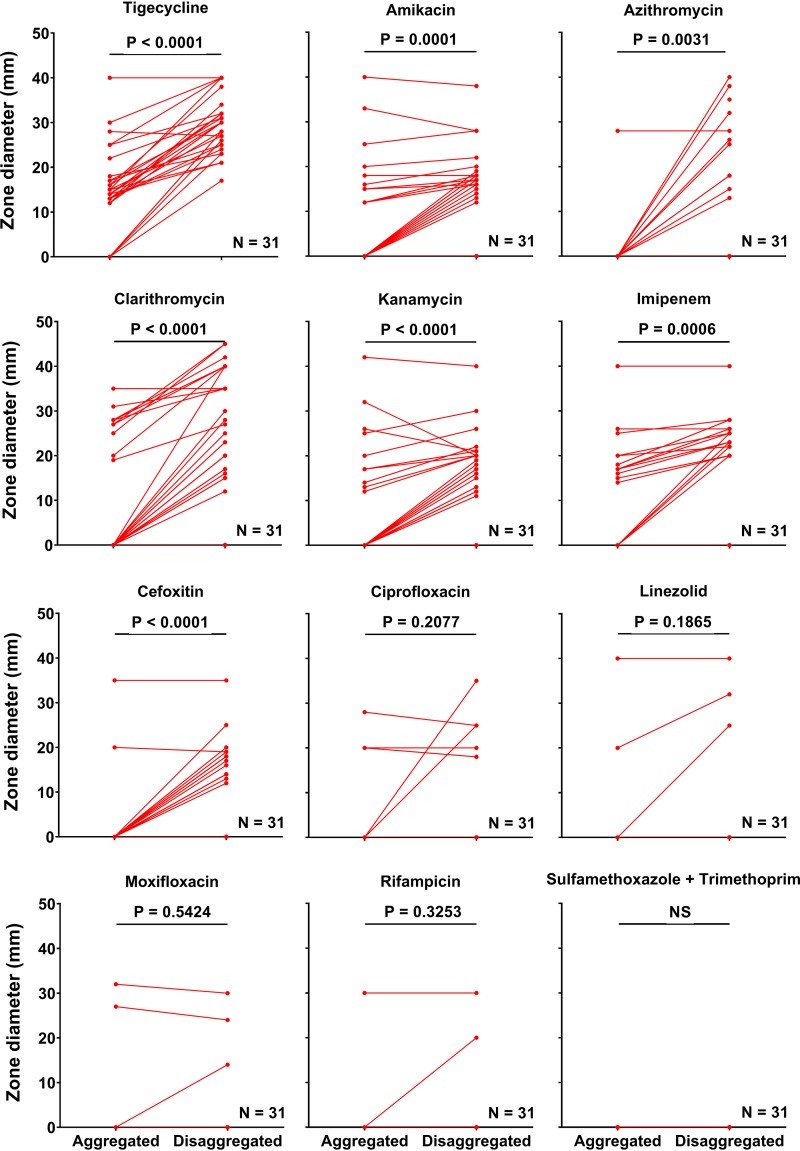
In total, 31 CF patient isolates of MABSC were tested for antimicrobial susceptibility using the disk diffusion assay. Isolates were crudely classified into two groups, based on zone diameters: the susceptible (zone diameter > 0 mm) and resistant (zone diameter = 0 mm) groups. It was decided to use these divisions as breakpoints due to a lack of breakpoints approved by the European Committee on Antimicrobial Susceptibility Testing (EUCAST) and the Clinical and Laboratory Standards Institute (CLSI) for the antibiotics used for the treatment of infections caused by MABSC. The susceptibility results for the MABSC isolates were based on data obtained from samples being aggregated and disaggregated in 5% Tween 80 using plate spreading. The following 12 antibiotics were tested: amikacin, azithromycin, cefoxitin, ciprofloxacin, clarithromycin, imipenem, kanamycin, linezolid, moxifloxacin, rifampin, tigecycline, and the combination of sulfamethoxazole and trimethoprim. Disaggregation of MABSC CF patient isolates with 5% Tween 80 resulted in significantly increased zone diameters for tigecycline, amikacin, azithromycin, clarithromycin, kanamycin, imipenem, and cefoxitin (Fig. 4). No significant effect of disaggregation on the zone diameter was observed for ciprofloxacin, linezolid, moxifloxacin, rifampin, and sulfamethoxazole-trimethoprim (Fig. 4).
FIG 4.
Disaggregation with Tween 80 sensitizes MABSC to antimycobacterial drugs. The effect of 5% Tween 80 on MABSC isolates (n = 31) was tested by disk diffusion susceptibility testing using amikacin, azithromycin, cefoxitin, ciprofloxacin, clarithromycin, imipenem, kanamycin, linezolid, moxifloxacin, rifampin, tigecycline, and sulfamethoxazole-trimethoprim. Aggregated MABSC isolates were grown and incubated in MH and plated by normal plate spreading on blood agar plates. Disaggregated MABSC isolates were suspended in MH plus 5% Tween 80, incubated for 2 to 5 days, and subsequently added onto blood agar plates and uniformly distributed by gently tipping the plates. Excess liquid was discharged. All isolates that were completely resistant regardless of Tween 80 are represented by the flat red line on the x axis. Statistical significance was determined using a paired t test (P ≤ 0.05). NS, not significant.
All aggregated isolates were resistant to 4 or more antibiotics.
In fact, 29/31 aggregated isolates were multidrug resistant to between 8 and 12 antibiotics. Nevertheless, when the isolates were disaggregated, the isolates become susceptible to 2.97 more drugs, on average (95% confidence interval, 2.37, 3.56; P < 0.0001; n = 30) (Fig. 5). These results indicate that the effect of Tween 80 on susceptibility depends on the type of antibiotic used. This decrease in the susceptibility of aggregating MABSC is in line with that seen in other bacteria that have formed biofilms (42). However, some bias may have been introduced by the lack of a consistent methodology for applying the bacteria onto the plates.
FIG 5.
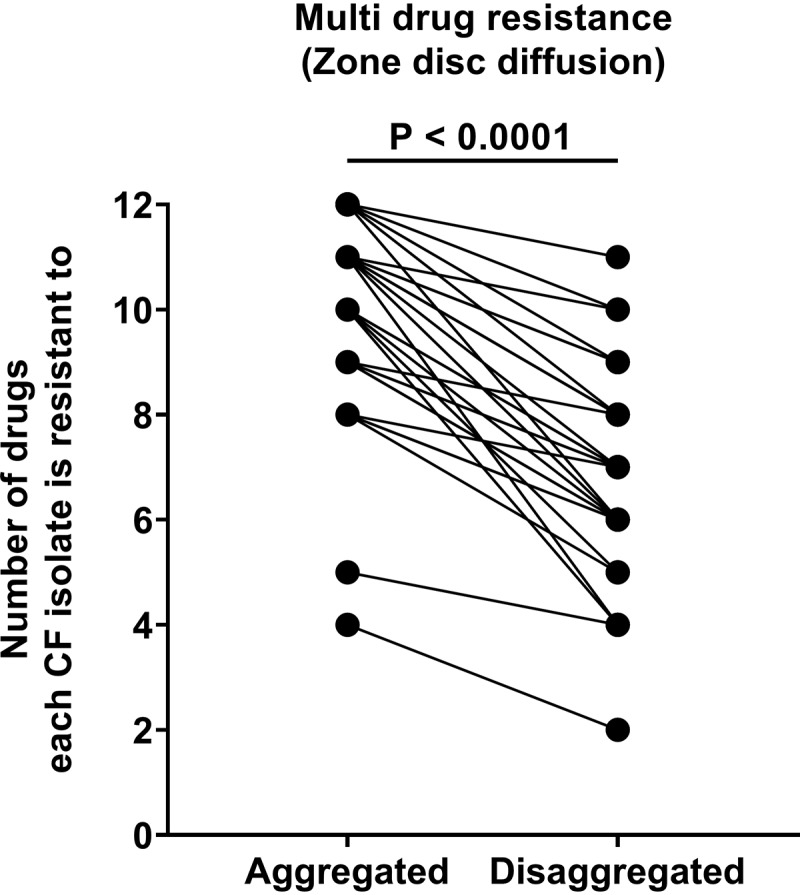
Multidrug resistance to antimycobacterial drugs in MABSC is lowered by disaggregation of the isolates with Tween 80. MABSC isolates (n = 31) were tested by disk diffusion susceptibility testing, using (per disk) 30 μg amikacin, 15 μg azithromycin, 30 μg cefoxitin, 5 μg ciprofloxacin, 15 μg clarithromycin, 10 μg imipenem, 30 μg kanamycin, 10/30 μg linezolid, 5 μg moxifloxacin, 5 μg rifampin, 15 μg tigecycline, and 1.25 μg/23.75 μg sulfamethoxazole-trimethoprim. Isolates with zone diameters of 0 mm were considered resistant. Aggregated MABSC isolates were grown and incubated in MH and plated by normal plate spreading on blood agar plates. Disaggregated MABSC isolates were suspended in MH plus 5% Tween 80, incubated for 2 to 5 days, and subsequently added onto blood agar plates and uniformly distributed by gently tipping the plates. Excess liquid was discharged. Statistical significance was determined using a paired t test (P ≤ 0.05).
To examine if the greater drug sensitivity in response to the high concentration of Tween 80 could result from an increased permeability of the cell envelope, the effect of Tween 80 on the penetration of the membrane-impermeable dye, propidium iodide (PI), was examined by flow cytometry (Fig. S1C). No difference in the PI fluorescence was observed between MABSC bacteria cultured without or with 5% Tween 80, indicating the absence of an effect of 5% Tween 80 on the permeability of the cell envelope.
Low O2 consumption reflects dormant subpopulations.
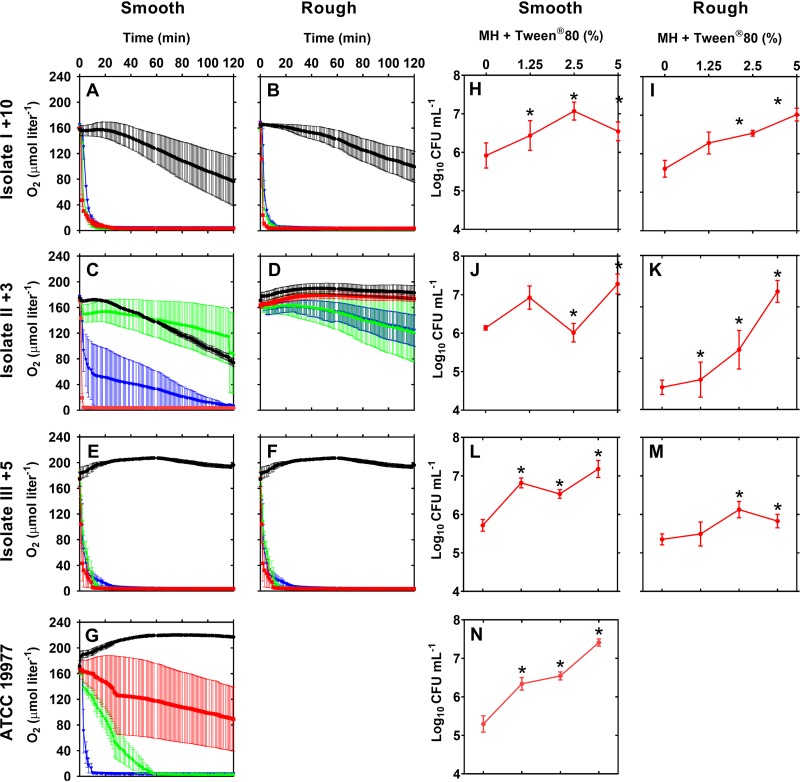
Smooth and rough MABSC isolates from 3 CF patients were dissolved in oxygenated, liquid media with various concentrations of Tween 80 and were incubated for 120 min, while O2 consumption was recorded. It was found that all smooth isolates consumed all the O2 present within 120 min when dissolved with 5% Tween 80 (Fig. 6A, C, E, and G) (P < 0.0001), while O2 consumption by the rough isolates varied between the isolates (Fig. 6B, D, and F). On the contrary, when cells were allowed to aggregate without Tween 80, O2 consumption was inhibited, which indicates that subpopulations of bacteria were slow growing or dormant. Furthermore, MABSC isolates grown in increasing concentrations of Tween 80 demonstrated increased bacterial growth after 120 min of incubation, as measured by plate counting (P < 0.05, n = 3) (Fig. 6H to N). Compared to the findings for the aggregated isolates, disruption resulted in increased aerobic respiration and increased bacterial growth (Fig. 6). Control experiments demonstrated that Tween 80 alone had no effect on O2 consumption in Mueller-Hinton II bouillon medium (MH) with increasing concentrations of Tween 80 or in the supernatant of an MABSC culture grown in increasing concentrations of Tween 80 (Fig. S1).
FIG 6.
The oxygen consumption of MABSC is influenced by the degree of bacterial aggregation. The graphs show the microrespirometry of O2 in aerobic cultures with smooth and rough CF MABSC isolates and the ATCC 19977 strain during different stages of bacterial disaggregation, as determined by the Tween 80 concentrations: 0% (black), 1.25% (red), 2.5% (green), 5% (blue). Isolates I to III were isolated from three CF patients +10, +3, and +5 years after the first sample was positive for MABSC. (A to G) The oxygen consumption with 5% Tween 80 was significantly higher than the O2 consumption with 0% Tween 80 for all isolates (P < 0.0001). Error bars indicate the mean ± standard error of the mean (n = 3). Statistical significance was assessed using two-way ANOVA, followed by Bonferroni multiple-comparison tests. (H to N) Effect of the Tween 80 dilution on the numbers of CFU per milliliter. Error bars indicate the mean ± standard error of the mean (n = 3). Statistical significance was determined using one-way ANOVA, followed by Dunnett's multiple-comparison test (*, P ≤ 0.05).
Aerobic conditions increase bacterial killing by amikacin.
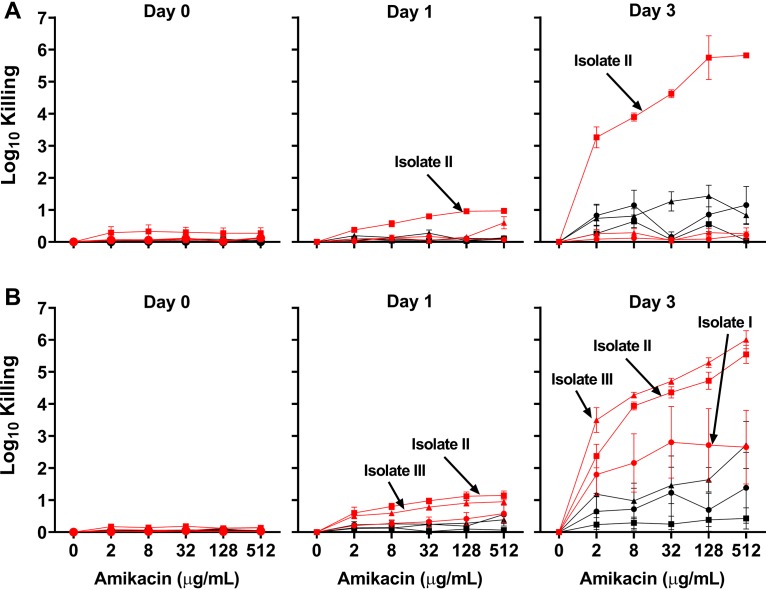
We investigated the impact of normoxic and anoxic conditions on the efficacy of amikacin on clinical MABSC isolates. To obtain a uniform suspension of cells, all MABSC isolates were cultured in medium with 5% Tween 80. We tested both smooth and rough morphotypes isolated from 3 CF patients 10, 5, 3, and 2 years after their first sample was positive for MABCS. After 3 days of incubation with 5% Tween 80, increased killing was observed for cells treated with amikacin under oxic conditions compared to that observed for cells treated with amikacin under anoxic conditions (P < 0.05) (Fig. 7). Two exceptions were seen, for smooth morphotype isolates ISM+10 and IIISM+5, indicating variations between morphotypes (Fig. S2A and C). Oxic conditions increased the killing of both smooth and rough isolates, with maximum bacterial killing exceeding a 2- to 4-log10 reduction when the bacteria were treated with 8 μg ml−1 amikacin compared to the level of killing seen under anoxic conditions.
FIG 7.
Aerobic conditions increase the bacterial killing of M. abscessus complex isolates during amikacin treatment. Isolates from three CF patients were cultured for 0, 1, or 3 days with amikacin, as indicated. On each day, the number of CFU per milliliter was recorded for each amikacin treatment for triplicate individual experiments. Killing was calculated as the log reduction relative to the number of CFU per milliliter of untreated samples on each day. Isolates I to III were from three CF patients +10, +3, and +5 years after first sample was positive for M. abscessus. (A) Effect of increasing amikacin concentrations ranging from 0 to 512 μg ml−1 on smooth CF isolates ISM+10, IISM+3, and IIISM+5 incubated for 0, 1, and 3 days under oxic (red) and anoxic (black) conditions. (B) Effect of increasing amikacin concentrations ranging from 0 to 512 μg ml−1 on rough CF isolates IRG+10, IIRG+3, and IIIRG+5 incubated for 0, 1, and 3 days under oxic (red) and anoxic (black) conditions. Error bars indicate the mean ± standard error of the mean (n = 3). The statistical significance of the difference between oxic and anoxic treatments was assessed using a two-way ANOVA, followed by Bonferroni multiple-comparison tests (P ≤ 0.05).
DISCUSSION
MABSC is recognized as a severe pulmonary pathogen in people with CF, in whom it leads to chronic infection and an accelerated loss of lung function. Clearing of the infection is difficult, but not impossible (3, 43). Conventional antibiotic resistance mechanisms are insufficient to explain the ability of susceptible MABSC strains to survive in the presence of antibiotic concentrations above the MIC, and this study proposes that other mechanisms are in play, namely, antibiotic tolerance through aggregation and metabolic dormancy (44, 45).
When reproducing the O2 depletion known to be created by the host response to invading pathogens within the endobronchial mucus, we were able to establish anaerobic MABSC cultures with slow growth and tolerance to amikacin, which is a common anti-NTM antibiotic inhibiting translation (Fig. 7). This is in line with the effect of anaerobiosis on the tolerance to antibiotics in other CF patient pathogens (30). Under similar circumstances, MABSC has found a successful niche in the endobronchial mucus in CF lungs to aggregate in megaclusters (21), offering essential biofilm protection by means of slow growth and low metabolic activity (46). Our results show that bacterial aggregation leads to diminished O2 consumption, while disaggregated cells deplete O2 within 30 min (Fig. 6). This low O2 consumption in MABSC aggregates indicates slow-growing or metabolically inactive subpopulations with low levels of aerobic respiration. The aminoglycoside amikacin belongs to a class of antibiotics targeting 16S rRNA and is an essential drug for the treatment of the multidrug-resistant NTM infections (47). We show that treatment with amikacin induces higher levels of killing under oxic conditions than under anoxic conditions, which can be explained by the increased bacterial growth typically associated with increased ribosomal activity (48, 49). Bacterial aggregation and the availability of O2 are thus essential to consider when searching for novel treatment targets.
In this study, we confirmed that both smooth and rough CF isolates aggregate into large clusters (Fig. 2), a phenomenon also recently demonstrated in the MABSC ATCC 19977 laboratory strain (45). Most noticeably, rough isolates have pronounced cord formation, whereas smooth aggregates appear as chaotically scattered clusters. Cord formation is required for mycobacterial persistence in the host (50), whereas GPL expression in the smooth morphotype has been suggested to play a role in environmental colonization, as seen by the sliding ability and biofilm formation (51). However, one study reported that the infecting MABSC strain was of the smooth morphotype (21), indicating the controversies in the detection and characterization of MABSC morphotypes. More studies are needed, though, to disentangle the virulence of the smooth and rough morphotypes of MABCS.
However, MABSC strains are intrinsically resistant to most antibiotics that are currently available, and it is proposed that MABSC develops resistance to common antibiotics (52, 53). In addition to these well-accepted mechanisms of antimicrobial resistance, our results indicate that in vivo-like conditions, such as aggregation and O2 depletion, contribute significantly to the resistance of MABSC against amikacin. Our finding of reduced susceptibility to amikacin in MABSC when O2 is missing is predictable due to a lack of respiration (54). We have, however, no knowledge of previous reports on the effect of the absence of O2 on the susceptibility of MABSC to amikacin. However, other studies have demonstrated that the minimal bactericidal concentrations of amikacin are higher for mature biofilms of MABSC than for planktonic cells (55). In this respect, the effect of O2 on susceptibility to other antibiotics may add even more information for the implications of low metabolic activity due to a lack of O2 in antimicrobial resistance in MABSC.
Delays of treatment of several weeks or even months may enable aggregate formation and O2 restriction and thereby represent the difference between eradication and chronic progressive infection. Hence, new treatment strategies that reduce both aggregation and the metabolic inactivity of MABSC are crucial for a successful eradication strategy. We believe that early diagnosis is necessary for successful eradication, as was originally shown with P. aeruginosa in CF patients (56). The present study has provided in vitro evidence for the reasons why susceptibility testing of MABSC does not give reliable results and that the improved efficacy of antimycobacterial drugs can be obtained on disaggregated MABSC isolates from CF patients. The mechanism may be ascribed to the biofilm aggregation associated with lower metabolism, which can be reverted by disaggregation by Tween 80, leading to increased antibiotic uptake. In this respect, it may be highly relevant to determine the minimum number of bacteria providing an aggregate with tolerance. On the basis of the relation between the lower tolerance and the reduced frequency of aggregates exceeding 10 μm3 induced by treatment with 5% Tween 80 (Fig. 3 to 5) and by assuming that the average volume of MABSC is 0.6 μm3, our results indicate that the aggregation of only 15 cells is sufficient to provide increased tolerance.
New therapeutic strategies in CF patients with chronic MABSC infection could therefore be early antibiotic eradication before biofilm formation combined with the disaggregation of bacterial clusters and increasing O2 availability in the endobronchial mucus.
MATERIALS AND METHODS
Patients.
As defined by the Danish Act on Research Ethics Review of Health Research Projects, Section 2, the project did not constitute a health research project and was thus initiated without approval from the Committees on Health Research Ethics in the Capital Region of Denmark. The study was carried out with 36 bacterial isolates from 22 chronically infected CF patients (Table 1). Chronic infection with MABSC was defined as fulfilling the ATS/IDSA criteria (7).
TABLE 1.
Characteristics of 22 patients with cystic fibrosis and chronic MABSC infectiona
| Characteristic | Value |
|---|---|
| Median (IQR) age (yr) | 19 (12–24) |
| No. (%) of female patients | 9 (42.9) |
| Median (IQR) body mass index (kg/m2) | 20 (16–23)b |
| No. (%) of patients homozygous for F508del mutation | 20 (95.2) |
| Median (IQR) % FEV1 | 70 (62.5–84.1) |
| Median (IQR) duration of chronic MABSC infection (yr) | 3 (2–6) |
| No. (%) of patients with chronic Gram-negative bacterial infection | 10 (58.8) |
| No. (%) of patients receiving: | |
| Intravenous treatment for a minimum of 14 days | 10 (62.5) |
| Oral treatment for a minimum of 90 days | 10 (62.5) |
Abbreviations: IQR, interquartile range; FEV1, forced expiratory volume in 1 s; MABSC, Mycobacterium abscessus complex.
Data are missing for one patient.
Bacterial strains and media.
Wild-type M. abscessus subsp. abscessus reference strain ATCC 19977, obtained from the American Type Culture Collection by Statens Serum Institute (SSI), Denmark, and 36 MABSC isolates with smooth or rough morphotypes from CF patients were used (Table 1). All experiments were initiated by streaking bacteria on chocolate blood agar plates (CBA; 36.0 g liter−1 GC agar base [15 g liter−1 peptone, 1.0 g liter−1 corn starch, 4 g liter−1 dipotassium phosphate, 1 g liter−1 monopotassium phosphate, 5 g liter−1 sodium chloride, 10 g liter−1 agar], 10.0 g liter−1 IsoVitaleX enrichment, 10.0 g liter−1 hemoglobin; SSI), a nonselective, enriched growth medium used for the isolation of pathogenic bacteria. Bacteria were incubated until visible growth (5 to 8 days) at 37°C with 5% CO2. MABSC was grown in Mueller-Hinton II bouillon medium (MH; 17.5 g liter−1 of acid hydrolysate of casein, 3 g liter−1 of beef extract, 1.5 g liter−1 starch; SSI) with various concentrations of Tween 80 (Croda International PLC, United Kingdom) to reach final concentrations of 1.25%, 2.5%, and 5%. A single colony was transferred from CBA plates into 20 ml MH plus 5% Tween 80 in 50-ml culture flasks (TPP, Switzerland) and incubated for 5 days (37°C, 150 rpm) before each experiment.
Bacterial susceptibility and resistance test.
Aggregated MABSC isolates were grown and incubated in MH and plated by normal plate spreading, in which one MABSC colony was suspended in 2 ml 0.85% NaCl medium (bioMérieux, Ballerup, Denmark) and thereafter streaked with a sterile cotton swap onto blood agar plates (SSI) with a plate rotator (Retro C-80; bioMérieux). Disaggregated MABSC isolates were tested in MH plus 5% Tween 80, in which one MABSC colony was suspended in 12 ml MH plus 5% Tween 80 and incubated (for 2 to 5 days, depending on the isolate, at 150 rpm and 37°C in 5% CO2). The MABSC cultures were then diluted 10-fold in MH plus 5% Tween 80 and subsequently added onto cation-adjusted horse blood agar susceptibility medium (SSI Diagnostica, Hillerød, Denmark) and uniformly distributed by gently tipping the plates. Excess liquid was discharged. First, tests for susceptibility to the following antibiotics (containing the indicated amounts per disk) were performed by the agar disk diffusion method: 30 μg amikacin, 15 μg azithromycin, 30 μg cefoxitin, 5 μg ciprofloxacin, 15 μg clarithromycin, 10 μg imipenem, 30 μg kanamycin, 10/30 μg linezolid, 5 μg moxifloxacin, 5 μg rifampin, 15 μg tigecycline, and 1.25 μg sulfamethoxazole plus 23.75 μg trimethoprim (Neo-Sensitabs; Rosco Diagnostica, Denmark). Second, Etests were performed with the following antibiotics: amikacin, azithromycin, clarithromycin, kanamycin, and tigecycline (bioMérieux). The plates were incubated for 4 to 5 days, depending on the isolate (37°C, 5% CO2), before determining the inhibition zones and MICs by visual inspection.
Anaerobic growth.
The MABSC isolates were grown and treated under anoxic conditions in an anaerobic growth chamber (Concept 400 anaerobic workstation; Ruskinn Technology Ltd., UK). The gas atmosphere consisted of N2-H2-CO2 (ratio, 80:10:10). Anoxia was confirmed with an optical O2 sensor (HQ40d multi; Hach Company, CO, US). To remove traces of O2, all media and chemical solutions used for anaerobic work were equilibrated in the anaerobic chamber for 5 days prior to the experiment.
Killing of MABSC.
Killing curves were generated to investigate the effect of O2 on MABSC treated with amikacin for 3 days. Five-day-old cultures of reference strain ATCC 19977 and 9 MABSC isolates from CF patients were adjusted to an optical density at 600 nm (OD600) of 0.005, corresponding to approximately 105 CFU ml−1. Treatment was initiated by diluting amikacin in 20 ml oxic or anoxic MH plus 5% Tween 80 in 4-fold dilutions ranging from 2 to 512 mg liter−1. Cultures were aliquoted in 20-ml glass vials (Schott, Germany), after which the anoxic cultures were sealed with airtight lids, while oxic cultures were sealed with Parafilm (Sigma-Aldrich, Denmark). All O2-depleted cultures were prepared in an anaerobic bench using equilibrated MH plus 5% Tween 80. The cultures were then incubated at 37°C and 150 rpm for up to 3 days. Samples were taken from the same cultures at days 0, 1, and 3, viable plate counts were performed by standard microbiological methods, and the plates were incubated for up to 7 days at 37°C.
Microscopy and image analysis.
One milliliter of untreated, 5-day-old cultures of ATCC 19977 and each of six MABSC isolates from patients with CF grown in MH supplemented with 0%, 1.25%, 2.5%, or 5% Tween 80 was mixed with 1 μl of Syto 9 (Molecular Probes, USA). The stained samples were incubated in the dark for 15 min at room temperature. After incubation, 20 μl of each sample was placed in the wells of a μ-slide VI channel slide (Ibidi, Germany). Samples were visualized by confocal laser scanning microscopy (CLSM) on an LSM 880 Zeiss inverted microscope running the Zen (version 2.1) program (Zeiss, Germany). Imaging was performed at a ×63 magnification by use of a 488-nm laser exciting Syto 9 and an emission filter of 495 to 580 nm. Samples were imaged at 7 different randomly selected regions in images measuring 135 μm by 135 μm by 15 μm per scan. The z-stacks obtained were rendered into three-dimensional projections with Imaris (version 9.2) software (Bitplane, Switzerland). Aggregate sizes were measured with the use of the Measure Pro Expansion tool for Imaris (version 9.2) software. An iso-surface was applied over the Syto 9-stained biomass. Iso-surface particles of between 1 and 10 μm3 were distinguished from particles larger than 10 μm3, and the fraction of the two parts was used as a measurement of aggregation versus single cells/small aggregates.
To visualize MABSC in CF patient lungs, a CF patient sputum sample was transferred to 4% formaldehyde before further preparation for microscopic investigation. The biopsy material was embedded in paraffin, cut into 4-μm sections, and mounted on glass slides. Prior to microscopy, the sections were deparaffinized, and the tissue sections were analyzed by staining with Ziehl-Neelsen strain (an acid-fast stain). Microscopy was performed using an LSM 880 Zeiss inverted microscope. Representative MABSC bacteria isolated from a CF patient sputum sample were prepared for scanning electron microscopy (SEM) as previously described (39). Briefly, bacteria were cultured in MH without Tween 80 and harvested and fixed in 2% glutaraldehyde, postfixed in 1% OsO4, dried to the critical point using CO2, and sputter coated with gold according to standard procedures. Specimens for SEM were investigated with a Philips XL Feg30 SEM operated at a 2- to 5-kV accelerating tension.
Flow cytometry.
The effect of 5% Tween 80 on the permeability of the cell envelope of MABSC was investigated using flow cytometry by comparison of the propidium iodide (PI) fluorescence of ATCC 19977 grown for 3 days in MH without 5% Tween 80 to the fluorescence of ATCC 19977 grown for 3 days in MH with 5% Tween 80. Before flow cytometry, PI (catalog number P4170; Sigma-Aldrich) was added to the samples to a final concentration of 20 μM and the mixture was incubated for 15 min. Stained samples were analyzed on an Attune Next flow cytometer (Thermo Fisher Scientific) by exciting PI-stained samples at 561 nm and collecting the fluorescence through a 585/16-nm band-pass filter.
Oxygen respirometry.
Respiration vials (Oxvial4) with integrated strips of O2-sensitive, Redflash indicators glued to the inner wall (PyroScience, GmbH) were positioned on a magnetic stirring head connected to a magnetic stirrer (model 300; Rank Brothers, Cambridge, UK). Adapter rings (Advial4; Pyro-Science) were fitted around the vials to allow for the easy placement of bare optical fibers (Spfib-Bare; PyroScience) connected to an optical O2 meter (catalog number FSO2-4; FireStingO2; Pyro Science). The oxygen concentration was measured with Pure Oxygen Logger software (version 3.206) with the optical O2 meter (FireStingO2; Firmware, version 3.07; PyroScience) in the continuous mode with sampling every 1 s. The temperature was fixed at 37°C, and the pressure was fixed at 1.013 × 105 Pa. The factory calibration was used to calibrate the sensors (Oxvial4) per the manufacturer’s recommendation. The oxygen concentrations were measured in cultures of reference strain ATCC 19977 and 6 MABSC isolates from CF patients that had been grown in MH with final concentrations of 0%, 1.25%, 2.5%, and 5% Tween 80. All samples were homogenized with O2 before measurements were taken. Quantitation of bacterial growth was performed.
Statistical methods.
Statistical significance was evaluated by Fisher’s exact test, Student’s t test, and ordinary one-way analysis of variance (ANOVA) followed by Dunnett’s or Bonferroni’s multiple-comparison test. A P value of ≤0.05 was considered statistically significant. Data from at least 3 independent experiments were compared. Tests were performed with GraphPad Prism (version 6.1) software (GraphPad Software Inc., La Jolla, CA) and Microsoft Excel software (Microsoft Corp., Redmond, WA).
Supplementary Material
ACKNOWLEDGMENTS
The skillful assistance of Klavs Qvortrup, head director of the Core Facility for Integrated Microscopy (CFIM), University of Copenhagen, Copenhagen, Denmark, is gratefully appreciated and recognized as being crucial for this study.
This work was supported by grants from the American Cystic Fibrosis Foundation (grant HOIBY05A0) through grant 50061804231-F16 to Mette Kolpen, Human Frontiers Science Program grant RGY0081/2012, and the Lundbeck Foundation through grant R105-A9791 to Thomas Bjarnsholt, as well as Technology and Production Sciences (FTP) through grant DFF-4184-00515 to Peter Østrup Jensen.
The funders had no role in experimental design, data analysis and interpretation, or the decision to submit the work for publication.
Conceived and designed the experiments: M.K. and P.Ø.J. Generated the data: M.K., C.R., K.N.K., B.G.F., and U.R.J. Analyzed the data: M.K., P.Ø.J., C.R., K.N.K., B.G.F., and U.R.J. Wrote the first draft: M.K., P.Ø.J., C.R., T.Q., K.N.K., B.G.F., T.B., and N.H.
Footnotes
Supplemental material is available online only.
REFERENCES
- 1.Adjemian J, Olivier KN, Prevots DR. 2018. Epidemiology of pulmonary nontuberculous mycobacterial sputum positivity in patients with cystic fibrosis in the United States, 2010-2014. Ann Am Thorac Soc 15:817–826. doi: 10.1513/AnnalsATS.201709-727OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Qvist T, Gilljam M, Jonsson B, Taylor-Robinson D, Jensen-Fangel S, Wang M, Svahn A, Kotz K, Hansson L, Hollsing A, Hansen CR, Finstad PL, Pressler T, Høiby N, Katzenstein TL, Scandinavian Cystic Fibrosis Study Consortium (SCFSC). 2015. Epidemiology of nontuberculous mycobacteria among patients with cystic fibrosis in Scandinavia. J Cyst Fibros 14:46–52. doi: 10.1016/j.jcf.2014.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Qvist T, Taylor-Robinson D, Waldmann E, Olesen HV, Hansen CR, Mathiesen IH, Høiby N, Katzenstein TL, Smyth RL, Diggle PJ, Pressler T. 2016. Comparing the harmful effects of nontuberculous mycobacteria, and Gram negative bacteria on lung function in patients with cystic fibrosis. J Cyst Fibros 15:380–385. doi: 10.1016/j.jcf.2015.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nessar R, Cambau E, Reyrat JM, Murray A, Gicquel B. 2012. Mycobacterium abscessus: a new antibiotic nightmare. J Antimicrob Chemother 67:810–818. doi: 10.1093/jac/dkr578. [DOI] [PubMed] [Google Scholar]
- 5.Maurer FP, Bruderer VL, Ritter C, Castelberg C, Bloemberg GV, Bottger EC. 2014. Lack of antimicrobial bactericidal activity in Mycobacterium abscessus. Antimicrob Agents Chemother 58:3828–3836. doi: 10.1128/AAC.02448-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ferro BE, Srivastava S, Deshpande D, Pasipanodya JG, van Soolingen D, Mouton JW, van Ingen J, Gumbo T. 2016. Failure of the amikacin, cefoxitin, and clarithromycin combination regimen for treating pulmonary Mycobacterium abscessus infection. Antimicrob Agents Chemother 60:6374–6376. doi: 10.1128/AAC.00990-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Floto RA, Olivier KN, Saiman L, Daley CL, Herrmann JL, Nick JA, Noone PG, Bilton D, Corris P, Gibson RL, Hempstead SE, Koetz K, Sabadosa KA, Sermet-Gaudelus I, Smyth AR, van Ingen J, Wallace RJ, Winthrop KL, Marshall BC, Haworth CS, US Cystic Fibrosis Foundation, European Cystic Fibrosis Society. 2016. US Cystic Fibrosis Foundation and European Cystic Fibrosis Society consensus recommendations for the management of non-tuberculous mycobacteria in individuals with cystic fibrosis. Thorax 71(Suppl 1):i1–i22. doi: 10.1136/thoraxjnl-2015-207360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Griffith DE, Aksamit T, Brown-Elliott BA, Catanzaro A, Daley C, Gordin F, Holland SM, Horsburgh R, Huitt G, Iademarco MF, Iseman M, Olivier K, Ruoss S, von Reyn CF, Wallace RJ Jr, Winthrop K, ATS Mycobacterial Diseases Subcommittee, American Thoracic Society, Infectious Disease Society of America. 2007. An official ATS/IDSA statement: diagnosis, treatment, and prevention of nontuberculous mycobacterial diseases. Am J Respir Crit Care Med 175:367–416. doi: 10.1164/rccm.200604-571ST. [DOI] [PubMed] [Google Scholar]
- 9.Koh WJ, Jeong BH, Kim SY, Jeon K, Park KU, Jhun BW, Lee H, Park HY, Kim DH, Huh HJ, Ki CS, Lee NY, Kim HK, Choi YS, Kim J, Lee SH, Kim CK, Shin SJ, Daley CL, Kim H, Kwon OJ. 2017. Mycobacterial characteristics and treatment outcomes in Mycobacterium abscessus lung disease. Clin Infect Dis 64:309–316. doi: 10.1093/cid/ciw724. [DOI] [PubMed] [Google Scholar]
- 10.van Ingen J, Boeree MJ, van Soolingen D, Mouton JW. 2012. Resistance mechanisms and drug susceptibility testing of nontuberculous mycobacteria. Drug Resist Updat 15:149–161. doi: 10.1016/j.drup.2012.04.001. [DOI] [PubMed] [Google Scholar]
- 11.Tortoli E. 2014. Microbiological features and clinical relevance of new species of the genus Mycobacterium. Clin Microbiol Rev 27:727–752. doi: 10.1128/CMR.00035-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pontiroli A, Khera TT, Oakley BB, Mason S, Dowd SE, Travis ER, Erenso G, Aseffa A, Courtenay O, Wellington EM. 2013. Prospecting environmental mycobacteria: combined molecular approaches reveal unprecedented diversity. PLoS One 8:e68648. doi: 10.1371/journal.pone.0068648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang W, Zhai Y, Cao L, Tan H, Zhang R. 2016. Illumina-based analysis of core actinobacteriome in roots, stems, and grains of rice. Microbiol Res 190:12–18. doi: 10.1016/j.micres.2016.05.003. [DOI] [PubMed] [Google Scholar]
- 14.Calmette A. 1936. L’infection bacillaire et la tuberculose. Masson et Cie, Paris, France. [Google Scholar]
- 15.Löwenstein E. 1920. Vorlesungen über Bakteriologie, Immunität, spezifische Diagnostik und Therapie der Tuberkulose. Fischer, Jena, Germany. [Google Scholar]
- 16.Koch R. 1982. Classics in infectious diseases. The etiology of tuberculosis: Robert Koch. Berlin, Germany. Rev Infect Dis 4:1270–1274. doi: 10.1093/clinids/4.6.1270. [DOI] [PubMed] [Google Scholar]
- 17.Ojha AK, Baughn AD, Sambandan D, Hsu T, Trivelli X, Guerardel Y, Alahari A, Kremer L, Jacobs WR Jr, Hatfull GF. 2008. Growth of Mycobacterium tuberculosis biofilms containing free mycolic acids and harbouring drug-tolerant bacteria. Mol Microbiol 69:164–174. doi: 10.1111/j.1365-2958.2008.06274.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zambrano MM, Kolter R. 2005. Mycobacterial biofilms: a greasy way to hold it together. Cell 123:762–764. doi: 10.1016/j.cell.2005.11.011. [DOI] [PubMed] [Google Scholar]
- 19.Esteban J, Martín-de-Hijas NZ, Kinnari TJ, Ayala G, Fernández-Roblas R, Gadea I. 2008. Biofilm development by potentially pathogenic non-pigmented rapidly growing mycobacteria. BMC Microbiol 8:184. doi: 10.1186/1471-2180-8-184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Høiby N, Bjarnsholt T, Moser C, Bassi GL, Coenye T, Donelli G, Hall-Stoodley L, Hola V, Imbert C, Kirketerp-Møller K, Lebeaux D, Oliver A, Ullmann AJ, Williams C, ESCMID Study Group for Biofilms and Consulting External Expert Werner Zimmerli. 2015. ESCMID guideline for the diagnosis and treatment of biofilm infections 2014. Clin Microbiol Infect 21(Suppl 1):S1–S25. doi: 10.1016/j.cmi.2014.10.024. [DOI] [PubMed] [Google Scholar]
- 21.Qvist T, Eickhardt S, Kragh KN, Andersen CB, Iversen M, Høiby N, Bjarnsholt T. 2015. Chronic pulmonary disease with Mycobacterium abscessus complex is a biofilm infection. Eur Respir J 46:1823–1826. doi: 10.1183/13993003.01102-2015. [DOI] [PubMed] [Google Scholar]
- 22.Qvist T, Pressler T, Taylor-Robinson D, Katzenstein TL, Høiby N. 2015. Serodiagnosis of Mycobacterium abscessus complex infection in cystic fibrosis. Eur Respir J 46:707–716. doi: 10.1183/09031936.00011815. [DOI] [PubMed] [Google Scholar]
- 23.Kreutzfeldt KM, McAdam PR, Claxton P, Holmes A, Seagar AL, Laurenson IF, Fitzgerald JR. 2013. Molecular longitudinal tracking of Mycobacterium abscessus spp. during chronic infection of the human lung. PLoS One 8:e63237. doi: 10.1371/journal.pone.0063237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Claeys TA, Robinson RT. 2018. The many lives of nontuberculous mycobacteria. J Bacteriol 200:e00739-17. doi: 10.1128/JB.00739-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Worlitzsch D, Tarran R, Ulrich M, Schwab U, Cekici A, Meyer KC, Birrer P, Bellon G, Berger J, Weiss T, Botzenhart K, Yankaskas JR, Randell S, Boucher RC, Döring G. 2002. Effects of reduced mucus oxygen concentration in airway Pseudomonas infections of cystic fibrosis patients. J Clin Invest 109:317–325. doi: 10.1172/JCI13870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kolpen M, Hansen CR, Bjarnsholt T, Moser C, Christensen LD, van Gennip M, Ciofu O, Mandsberg L, Kharazmi A, Döring G, Givskov M, Høiby N, Jensen PØ. 2010. Polymorphonuclear leucocytes consume oxygen in sputum from chronic Pseudomonas aeruginosa pneumonia in cystic fibrosis. Thorax 65:57–62. doi: 10.1136/thx.2009.114512. [DOI] [PubMed] [Google Scholar]
- 27.Kragh KN, Alhede M, Jensen PØ, Moser C, Scheike T, Jacobsen CS, Seier Poulsen S, Eickhardt-Sørensen SR, Trøstrup H, Christoffersen L, Hougen HP, Rickelt LF, Kühl M, Høiby N, Bjarnsholt T. 2014. Polymorphonuclear leukocytes restrict growth of Pseudomonas aeruginosa in the lungs of cystic fibrosis patients. Infect Immun 82:4477–4486. doi: 10.1128/IAI.01969-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lim A, Eleuterio M, Hutter B, Murugasu-Oei B, Dick T. 1999. Oxygen depletion-induced dormancy in Mycobacterium bovis BCG. J Bacteriol 181:2252–2256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Moser C, Pedersen HT, Lerche CJ, Kolpen M, Line L, Thomsen K, Høiby N, Jensen PØ. 2017. Biofilms and host response—helpful or harmful. APMIS 125:320–338. doi: 10.1111/apm.12674. [DOI] [PubMed] [Google Scholar]
- 30.Jensen PØ, Kolpen M, Kragh KN, Kühl M. 2017. Microenvironmental characteristics and physiology of biofilms in chronic infections of CF patients are strongly affected by the host immune response. APMIS 125:276–288. doi: 10.1111/apm.12668. [DOI] [PubMed] [Google Scholar]
- 31.Kohanski MA, Dwyer DJ, Hayete B, Lawrence CA, Collins JJ. 2007. A common mechanism of cellular death induced by bactericidal antibiotics. Cell 130:797–810. doi: 10.1016/j.cell.2007.06.049. [DOI] [PubMed] [Google Scholar]
- 32.Brochmann RP, Toft A, Ciofu O, Briales A, Kolpen M, Hempel C, Bjarnsholt T, Høiby N, Jensen PØ. 2014. Bactericidal effect of colistin on planktonic Pseudomonas aeruginosa is independent of hydroxyl radical formation. Int J Antimicrob Agents 43:140–147. doi: 10.1016/j.ijantimicag.2013.10.015. [DOI] [PubMed] [Google Scholar]
- 33.Jensen PØ, Briales A, Brochmann RP, Wang H, Kragh KN, Kolpen M, Hempel C, Bjarnsholt T, Høiby N, Ciofu O. 2014. Formation of hydroxyl radicals contributes to the bactericidal activity of ciprofloxacin against Pseudomonas aeruginosa biofilms. Pathog Dis 70:440–443. doi: 10.1111/2049-632X.12120. [DOI] [PubMed] [Google Scholar]
- 34.Kolpen M, Lerche CJ, Kragh KN, Sams T, Koren K, Jensen AS, Line L, Bjarnsholt T, Ciofu O, Moser C, Kühl M, Høiby N, Jensen PØ. 2017. Hyperbaric oxygen sensitizes anoxic Pseudomonas aeruginosa biofilm to ciprofloxacin. Antimicrob Agents Chemother 61:e01024-17. doi: 10.1128/AAC.01024-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kolpen M, Mousavi N, Sams T, Bjarnsholt T, Ciofu O, Moser C, Kühl M, Høiby N, Jensen PØ. 2016. Reinforcement of the bactericidal effect of ciprofloxacin on Pseudomonas aeruginosa biofilm by hyperbaric oxygen treatment. Int J Antimicrob Agents 47:163–167. doi: 10.1016/j.ijantimicag.2015.12.005. [DOI] [PubMed] [Google Scholar]
- 36.Dwyer DJ, Belenky PA, Yang JH, MacDonald IC, Martell JD, Takahashi N, Chan CT, Lobritz MA, Braff D, Schwarz EG, Ye JD, Pati M, Vercruysse M, Ralifo PS, Allison KR, Khalil AS, Ting AY, Walker GC, Collins JJ. 2014. Antibiotics induce redox-related physiological alterations as part of their lethality. Proc Natl Acad Sci U S A 111:E2100–E2109. doi: 10.1073/pnas.1401876111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Boshoff HI, Barry CE III. 2005. Tuberculosis—metabolism and respiration in the absence of growth. Nat Rev Microbiol 3:70–80. doi: 10.1038/nrmicro1065. [DOI] [PubMed] [Google Scholar]
- 38.Høiby N, Henneberg KA, Wang H, Stavnsbjerg C, Bjarnsholt T, Ciofu O, Johansen UR, Sams T. 2019. Formation of Pseudomonas aeruginosa inhibition zone during tobramycin disk diffusion is due to a transition from planktonic to biofilm mode of growth. Int J Antimicrob Agents 53:564–573. doi: 10.1016/j.ijantimicag.2018.12.015. [DOI] [PubMed] [Google Scholar]
- 39.Qvortrup K, Rostgaard J, Bretlau P. 1995. Surface morphology of the endolymphatic duct in the rat. A scanning electron microscopy study. Ann Otol Rhinol Laryngol 104:120–126. doi: 10.1177/000348949510400207. [DOI] [PubMed] [Google Scholar]
- 40.Tuomanen E, Cozens R, Tosch W, Zak O, Tomasz A. 1986. The rate of killing of Escherichia coli by beta-lactam antibiotics is strictly proportional to the rate of bacterial growth. J Gen Microbiol 132:1297–1304. doi: 10.1099/00221287-132-5-1297. [DOI] [PubMed] [Google Scholar]
- 41.Teixeira PC, Leite GM, Domingues RJ, Silva J, Gibbs PA, Ferreira JP. 2007. Antimicrobial effects of a microemulsion and a nanoemulsion on enteric and other pathogens and biofilms. Int J Food Microbiol 118:15–19. doi: 10.1016/j.ijfoodmicro.2007.05.008. [DOI] [PubMed] [Google Scholar]
- 42.Høiby N, Bjarnsholt T, Givskov M, Molin S, Ciofu O. 2010. Antibiotic resistance of bacterial biofilms. Int J Antimicrob Agents 35:322–332. doi: 10.1016/j.ijantimicag.2009.12.011. [DOI] [PubMed] [Google Scholar]
- 43.Chacko A, Wen SC, Hartel G, Kapur N, Wainwright CE, Clark JE. 2019. Improved clinical outcome following treatment of Mycobacterium abscessus complex pulmonary disease in children with cystic fibrosis. Pediatr Infect Dis J 33:660–666. doi: 10.1097/INF.0000000000002274. [DOI] [PubMed] [Google Scholar]
- 44.Hunt-Serracin AC, Parks BJ, Boll J, Boutte CC. 2019. Mycobacterium abscessus cells have altered antibiotic tolerance and surface glycolipids in artificial cystic fibrosis sputum medium. Antimicrob Agents Chemother 63:e02488-18. doi: 10.1128/AAC.02488-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Clary G, Sasindran SJ, Nesbitt N, Mason L, Cole S, Azad A, McCoy K, Schlesinger LS, Hall-Stoodley L. 2018. Mycobacterium abscessus smooth and rough morphotypes form antimicrobial-tolerant biofilm phenotypes but are killed by acetic acid. Antimicrob Agents Chemother 62:e01782-17. doi: 10.1128/AAC.01782-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sønderholm M, Bjarnsholt T, Alhede M, Kolpen M, Jensen PØ, Kühl M, Kragh KN. 2017. The consequences of being in an infectious biofilm: microenvironmental conditions governing antibiotic tolerance. Int J Mol Sci 18:E2688. doi: 10.3390/ijms18122688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sander P, Bottger EC. 1999. Mycobacteria: genetics of resistance and implications for treatment. Chemotherapy 45:95–108. doi: 10.1159/000007171. [DOI] [PubMed] [Google Scholar]
- 48.Bosdriesz E, Molenaar D, Teusink B, Bruggeman FJ. 2015. How fast-growing bacteria robustly tune their ribosome concentration to approximate growth-rate maximization. FEBS J 282:2029–2044. doi: 10.1111/febs.13258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Scott M, Gunderson CW, Mateescu EM, Zhang Z, Hwa T. 2010. Interdependence of cell growth and gene expression: origins and consequences. Science 330:1099–1102. doi: 10.1126/science.1192588. [DOI] [PubMed] [Google Scholar]
- 50.Glickman MS, Cox JS, Jacobs WR Jr. 2000. A novel mycolic acid cyclopropane synthetase is required for cording, persistence, and virulence of Mycobacterium tuberculosis. Mol Cell 5:717–727. doi: 10.1016/S1097-2765(00)80250-6. [DOI] [PubMed] [Google Scholar]
- 51.Recht J, Kolter R. 2001. Glycopeptidolipid acetylation affects sliding motility and biofilm formation in Mycobacterium smegmatis. J Bacteriol 183:5718–5724. doi: 10.1128/JB.183.19.5718-5724.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ballarino GJ, Olivier KN, Claypool RJ, Holland SM, Prevots DR. 2009. Pulmonary nontuberculous mycobacterial infections: antibiotic treatment and associated costs. Respir Med 103:1448–1455. doi: 10.1016/j.rmed.2009.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Jeon K, Kwon OJ, Lee NY, Kim B-J, Kook Y-H, Lee S-H, Park YK, Kim CK, Koh W-J. 2009. Antibiotic treatment of Mycobacterium abscessus lung disease. A retrospective analysis of 65 patients. Am J Respir Crit Care Med 180:896–902. doi: 10.1164/rccm.200905-0704OC. [DOI] [PubMed] [Google Scholar]
- 54.Lobritz MA, Belenky P, Porter CB, Gutierrez A, Yang JH, Schwarz EG, Dwyer DJ, Khalil AS, Collins JJ. 2015. Antibiotic efficacy is linked to bacterial cellular respiration. Proc Natl Acad Sci U S A 112:8173–8180. doi: 10.1073/pnas.1509743112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Greendyke R, Byrd TF. 2008. Differential antibiotic susceptibility of Mycobacterium abscessus variants in biofilms and macrophages compared to that of planktonic bacteria. Antimicrob Agents Chemother 52:2019–2026. doi: 10.1128/AAC.00986-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Valerius NH, Koch C, Høiby N. 1991. Prevention of chronic Pseudomonas aeruginosa colonization in cystic fibrosis by early treatment. Lancet 338:725–726. doi: 10.1016/0140-6736(91)91446-2. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.