Burkholderia cepacia complex is an opportunistic pathogen capable of causing chronic pulmonary infections. These studies were conducted to demonstrate the activity of aerosolized levofloxacin in a chronic mouse lung infection model caused by B. cepacia isolates from patients with cystic fibrosis.
KEYWORDS: aerosolized levofloxacin, mouse lung infection, B. cepacia
ABSTRACT
Burkholderia cepacia complex is an opportunistic pathogen capable of causing chronic pulmonary infections. These studies were conducted to demonstrate the activity of aerosolized levofloxacin in a chronic mouse lung infection model caused by B. cepacia isolates from patients with cystic fibrosis. Treatment with aerosolized levofloxacin for 4 days produced at least 1 log CFU of bacterial killing against all strains tested, suggesting possible utility in the treatment of lung infections caused by B. cepacia isolates.
TEXT
Burkholderia cepacia complex is an opportunistic pathogen that is capable of causing various degrees of respiratory infection in patients with and without cystic fibrosis (1–3). These pulmonary infections often result in asymptomatic carrier, chronic infection, or “cepacia symptoms” (4–6). Although infections with B. cepacia complex are uncommon, infections in patients with cystic fibrosis can lead to a rapid decline in pulmonary function and, in some cases, morbidity (7, 8). In addition, therapeutic options for these infections are very limited due to the high level of resistance to multiple antibiotics, including tobramycin, aztreonam, imipenem, amikacin, trimethoprim-sulfamethoxazole, piperacillin-tazobactam, ceftazidime, and ciprofloxacin (4, 9–12).
Levofloxacin has potent activity against key cystic fibrosis pathogens, including Pseudomonas aeruginosa and B. cepacia complex, with no loss of in vitro activity in sputum (13). Furthermore, aerosol delivery of levofloxacin achieves high local concentrations at the site of infection, enhances bacterial killing, and reduces the potential for development of resistance (14).
Previous studies have shown that aerosolized levofloxacin administered once or twice daily produced more than 1 log of bacterial killing compared to that seen in untreated controls at the start of treatment and prevented mortality in acute lethal and chronic lung infection models caused by P. aeruginosa isolates (15). In the present study, we assessed the in vivo activity of aerosolized levofloxacin against five B. cepacia complex strains in a mouse chronic lung infection model.
(This work was presented in part at the 24th North American Cystic Fibrosis Conference, October 2010.)
Antimicrobial susceptibility was determined by a broth microdilution assay according to CLSI reference methods (16). As shown in Table 1, levofloxacin MICs for these strains ranged between 0.25 and 8 mg/liter, and levofloxacin was more active against these isolates than aztreonam, tobramycin, or amikacin.
TABLE 1.
MICs for the strains used in these studies
| Strain | MIC (mg/liter) of: |
|||
|---|---|---|---|---|
| Levofloxacin | Tobramycin | Aztreonam | Amikacin | |
| B. cepacia ATCC 25416 | 1 | 16 | 64 | 16 |
| B. cenocepacia (genomovar III) BC1020 | 8 | >128 | 128 | >128 |
| B. cenocepacia (genomovar III) BC1021 | 8 | >128 | 128 | >128 |
| B. multivorans (genomovar II) BC1013 | 0.25 | 16 | 4 | 16 |
| B. multivorans (genomovar II) BC1014 | 1 | 32 | 64 | 64 |
| B. multivorans (genomovar II) BC1012 | 4 | 32 | 64 | 64 |
For in vivo studies, female BALB/c mice (6 to 8 weeks of age) were obtained from Envigo Laboratories (Livermore, CA) and were provided food and water ad libitum in accordance with National Institutes of Health guidelines for the care and use of laboratory animals (17). All studies using animals were performed under protocols approved by an Institutional Animal Care and Use Committee. Mice were rendered transiently neutropenic by administration of 150 mg/kg of cyclophosphamide (Baxter, Deerfield, IL) intraperitoneally on days 4 and 1 before infection. Bacteria were grown overnight at 37°C for 20 h under constant aeration (300 rpm) in Mueller-Hinton broth (MHB). The infecting inoculum was prepared by removal of an aliquot from the overnight culture and subculturing into fresh MHB. The subculture was incubated at 37°C under constant aeration for 3 h to reach an absorbance of 0.30 to 0.35 at 600 nm (∼108 CFU/ml). The bacterial suspensions were diluted into Hanks balanced salt solution with 0.1% (vol/vol) gelatin (Sigma) suspension to yield ∼106 CFU/ml.
One day after the last dose of cyclophosphamide, mice were anesthetized by isoflurane administration (5% isoflurane in oxygen running at 4 liters/min) and then infected by intratracheal instillation of 0.05 ml of inoculum using a curved oral gavage tip attached to a 1-ml syringe. Treatments started 72 h postinfection and were administered once or twice daily for 4 days. Levofloxacin and saline were aerosolized using a microspray aerosol device (MicroSprayer model IA-C; Penn-Century, Philadelphia, PA) attached to a high-pressure syringe (FMJ-250; Penn-Century). For aerosol administration, each mouse was anesthetized with isoflurane (5% isoflurane in oxygen running at 4 liters/min) and positioned securely at a 45° to 50° angle by the upper teeth, the microspray aerosol tip was inserted into the bifurcation, and 50 μl of bacterial suspension was administered. An untreated group of mice (n = 4) was sacrificed before the initiation of treatment to determine baseline bacterial counts (72 h postinfection). The control and treated animals (n = 4 to 8) were sacrificed 12 to 16 h after the last dose by carbon dioxide asphyxiation. The lungs were removed aseptically and homogenized (Pro200 homogenizer; Pro Scientific, Monroe, CT) in 1 ml of sterile saline. Serial 10-fold dilutions of the homogenized lungs were plated on Mueller-Hinton agar plates. The plates were incubated overnight at 37°C, and then colonies were counted. Bacterial counts in lungs were analyzed using an unpaired t test (GraphPad Prism, version 6.03). A P value of <0.05 was considered statistically significant.
Model development experiments with all five strains used in this study established that the untreated bacterial burden in the lungs did not decline for up to 8 days under these experimental conditions (data not shown). Additionally, the Burkholderia multivorans isolates used in these studies were found to produce a persistent infection for up to 16 days in a neutropenic mouse chronic lung infection model (18).
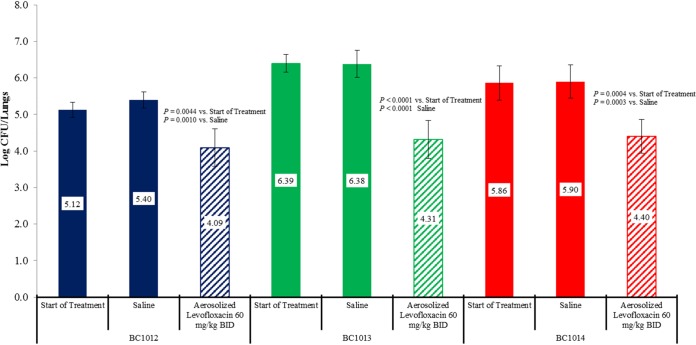
Figure 1 shows that treatment with 60 mg/kg of aerosolized levofloxacin twice daily for 4 days produced 1.03-, 2.08-, and 1.46-log CFU reductions in lung bacterial counts in mice infected with B. multivorans strains BC1012, BC1013, and BC1014, respectively. The bacterial killing produced by aerosol levofloxacin was statistically significant for all three strains compared with that in saline-treated controls at the end of treatment and untreated controls at the start of treatment.
FIG 1.
Activity of aerosolized levofloxacin administered twice daily for 4 consecutive days against three B. multivorans strains in a mouse chronic lung infection model.
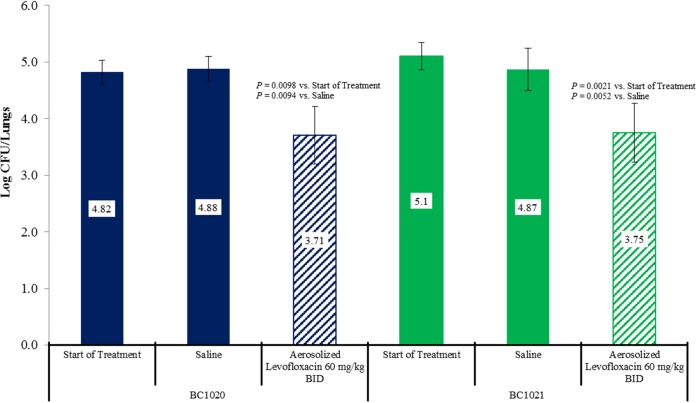
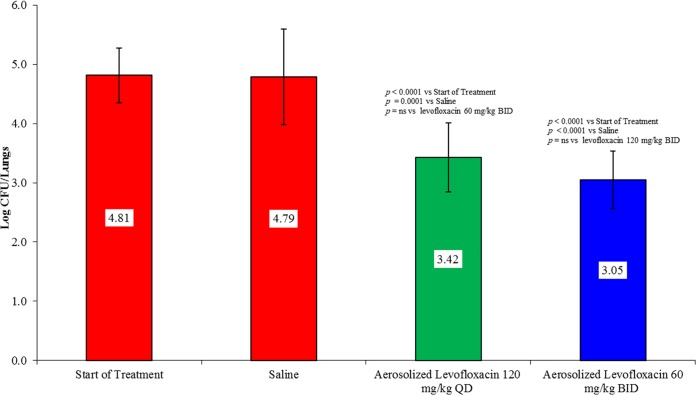
The lung bacterial counts for B. cenocepacia strains BC1020 and BC1021 are presented in Fig. 2. For strains BC1020 and BC1021, treatment with 60 mg/kg aerosolized levofloxacin twice daily produced 1.11- and 1.35-log CFU reductions in lung bacterial counts, respectively. The bacterial killing produced by aerosol levofloxacin was statistically significant for both strains compared with that in saline-treated controls at the end of treatment and untreated controls at the start of treatment and was similar to the level produced against B. multivorans strains despite having up to 32-fold higher MICs. Because levofloxacin was administered by inhalation, the lung levels were substantially higher than those after a systemic dose, allowing for coverage of organisms with MICs that are resistant to systemic treatment (15). Previous studies showed that aerosol administration of the same total daily dose of levofloxacin given once or twice daily against P. aeruginosa strains produced similar bacterial killing in mice (15). To determine whether B. cepacia isolates would respond the same way, we examined the activity 120 mg/kg of aerosolized levofloxacin administered as a single 120-mg/kg dose or divided into two 60-mg/kg doses administered 12 h apart in the mouse chronic lung infection model from B. cenocepacia strain BC1021. Figure 3 shows the activity of aerosolized levofloxacin administered at 120 mg/kg once daily or 60 mg/kg twice daily for 4 days. As observed previously with P. aeruginosa isolates, both regimens produced similar bacterial killing against B. cenocepacia strain BC1021.
FIG 2.
Activity of aerosolized levofloxacin administered twice daily for 4 consecutive days against two B. cenocepacia strains in a mouse chronic lung infection model.
FIG 3.
Comparative activity of aerosolized levofloxacin administered once (120 mg/kg) or twice (60 mg/kg) daily for 4 consecutive days in a mouse chronic lung infection model against B. cenocepacia strain BC1021.
In summary, aerosolized levofloxacin produced significant bacterial killing in this mouse chronic lung infection model against all five B. cepacia complex strains tested. Aerosol administration of the same total daily dose once or twice daily produced similar bacterial killing, as observed previously, suggesting some potential flexibility in the dosage regimen. Overall, these data suggest that aerosolized levofloxacin may be useful in the management of chronic pulmonary infections caused by B. cepacia complex.
ACKNOWLEDGMENTS
We are grateful to David P. Speert for providing the B. cepacia complex strains. We thank Courtney Miller for technical assistance with the in vitro studies and Dana Johnson for the animal care.
We have no conflicts of interest to declare.
REFERENCES
- 1.Abdallah M, Abdallah HA, Memish ZA. 2018. Burkholderia cepacia complex outbreaks among non-cystic fibrosis patients in the intensive care units: a review of adult and pediatric literature. Infez Med 26:299–307. [PubMed] [Google Scholar]
- 2.Kenna DTD, Lilley D, Coward A, Martin K, Perry C, Pike R, Hill R, Turton JF. 2017. Prevalence of Burkholderia species, including members of Burkholderia cepacia complex, among UK cystic and non-cystic fibrosis patients. J Med Microbiol 66:490–501. doi: 10.1099/jmm.0.000458. [DOI] [PubMed] [Google Scholar]
- 3.Scoffone VC, Chiarelli LR, Trespidi G, Mentasti M, Riccardi G, Buroni S. 2017. Burkholderia cenocepacia infections in cystic fibrosis patients: drug resistance and therapeutic approaches. Front Microbiol 8:1592. doi: 10.3389/fmicb.2017.01592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Goldmann DA, Klinger JD. 1986. Pseudomonas cepacia: biology, mechanisms of virulence, epidemiology. J Pediatr 108:806–812. doi: 10.1016/s0022-3476(86)80749-1. [DOI] [PubMed] [Google Scholar]
- 5.Isles A, Maclusky I, Corey M, Gold R, Prober C, Fleming P, Levison H. 1984. Pseudomonas cepacia infection in cystic fibrosis: an emerging problem. J Pediatr 104:206–210. doi: 10.1016/s0022-3476(84)80993-2. [DOI] [PubMed] [Google Scholar]
- 6.Reik R, Spilker T, Lipuma JJ. 2005. Distribution of Burkholderia cepacia complex species among isolates recovered from persons with or without cystic fibrosis. J Clin Microbiol 43:2926. doi: 10.1128/JCM.43.6.2926-2928.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Loutet SA, Valvano MA. 2010. A decade of Burkholderia cenocepacia virulence determinant research. Infect Immun 78:4088–4100. doi: 10.1128/IAI.00212-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mahenthiralingam E, Urban TA, Goldberg JB. 2005. The multifarious, multireplicon Burkholderia cepacia complex. Nat Rev Microbiol 3:144–156. doi: 10.1038/nrmicro1085. [DOI] [PubMed] [Google Scholar]
- 9.Chen JS, Witzmann KA, Spilker T, Fink RJ, LiPuma JJ. 2001. Endemicity and inter-city spread of Burkholderia cepacia genomovar III in cystic fibrosis. J Pediatr 139:643–649. doi: 10.1067/mpd.2001.118430. [DOI] [PubMed] [Google Scholar]
- 10.Golini G, Cazzola G, Fontana R. 2006. Molecular epidemiology and antibiotic susceptibility of Burkholderia cepacia-complex isolates from an Italian cystic fibrosis centre. Eur J Clin Microbiol Infect Dis 25:175–180. doi: 10.1007/s10096-006-0099-x. [DOI] [PubMed] [Google Scholar]
- 11.Horsley A, Jones AM. 2012. Antibiotic treatment for Burkholderia cepacia complex in people with cystic fibrosis experiencing a pulmonary exacerbation. Cochrane Database Syst Rev 10:CD009529. doi: 10.1002/14651858.CD009529.pub2. [DOI] [PubMed] [Google Scholar]
- 12.Horsley A, Jones AM, Lord R. 2016. Antibiotic treatment for Burkholderia cepacia complex in people with cystic fibrosis experiencing a pulmonary exacerbation. Cochrane Database Syst Rev 20 January:CD009529. doi: 10.1002/14651858.CD009529.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.King P, Lomovskaya O, Griffith DC, Burns JL, Dudley MN. 2010. In vitro pharmacodynamics of levofloxacin and other aerosolized antibiotics under multiple conditions relevant to chronic pulmonary infection in cystic fibrosis. Antimicrob Agents Chemother 54:143–148. doi: 10.1128/AAC.00248-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dudley MN, Loutit J, Griffith DC. 2008. Aerosol antibiotics: considerations in pharmacological and clinical evaluation. Curr Opin Biotechnol 19:637–643. doi: 10.1016/j.copbio.2008.11.002. [DOI] [PubMed] [Google Scholar]
- 15.Sabet M, Miller CE, Nolan TG, Senekeo-Effenberger K, Dudley MN, Griffith DC. 2009. Efficacy of aerosol MP-376, a levofloxacin inhalation solution, in models of mouse lung infection due to Pseudomonas aeruginosa. Antimicrob Agents Chemother 53:3923–3928. doi: 10.1128/AAC.00268-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Clinical and Laboratory Standards Institute. 2015. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically; approved standard—10th ed. CLSI document M7-A9. Clinical and Laboratory Standards Institute, Wayne, PA. [Google Scholar]
- 17.Institute of Laboratory Animal Resources, National Research Council. 1996. Guide for the care and use of laboratory animals. National Academies Press, Washington, DC. [Google Scholar]
- 18.Chu KK, Davidson DJ, Halsey TK, Chung JW, Speert DP. 2002. Differential persistence among genomovars of the Burkholderia cepacia complex in a murine model of pulmonary infection. Infect Immun 70:2715–2720. doi: 10.1128/iai.70.5.2715-2720.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]