Multidrug-resistant Enterobacteriaceae (MRE) colonize the intestine asymptomatically from where they can breach into the bloodstream and cause life-threatening infections, especially in heavily colonized patients. Despite the clinical relevance of MRE colonization levels, we know little about how they vary in hospitalized patients and the clinical factors that determine those levels.
KEYWORDS: Enterobacteriaceae, antibiotic resistance, beta-lactams, intestinal colonization
ABSTRACT
Multidrug-resistant Enterobacteriaceae (MRE) colonize the intestine asymptomatically from where they can breach into the bloodstream and cause life-threatening infections, especially in heavily colonized patients. Despite the clinical relevance of MRE colonization levels, we know little about how they vary in hospitalized patients and the clinical factors that determine those levels. Here, we conducted one of the largest studies of MRE fecal levels by tracking longitudinally 133 acute leukemia patients and monitoring their MRE levels over time through extensive culturing. MRE were defined as Enterobacteriaceae species that acquired nonsusceptibility to ≥1 agent in ≥3 antimicrobial categories. In addition, due to the selective media used, the MRE had to be resistant to third-generation cephalosporins. MRE were detected in 60% of the patients, but their fecal levels varied considerably among patients and within the same patient (>6 and 4 orders of magnitude, respectively). Multivariate analysis of clinical metadata revealed an impact of intravenous beta-lactams (i.e., meropenem and piperacillin-tazobactam), which significantly diminished the fecal MRE levels in hospitalized patients. Consistent with a direct action of beta-lactams, we found an effect only when the patient was colonized with strains sensitive to the administered beta-lactam (P < 0.001) but not with nonsusceptible strains. We report previously unobserved inter- and intraindividual heterogeneity in MRE fecal levels, suggesting that quantitative surveillance is more informative than qualitative surveillance of hospitalized patients. In addition, our study highlights the relevance of incorporating antibiotic treatment and susceptibility data of gut-colonizing pathogens for future clinical studies and in clinical decision-making.
INTRODUCTION
Resistant Enterobacteriaceae, most notably those that are multidrug resistant (MRE; acquired nonsusceptibility to ≥1 agent in ≥3 antimicrobial categories) (1), are a major threat for hospitalized patients. Infections by Enterobacteriaceae such as Klebsiella pneumoniae frequently begin by colonization of the intestinal tract (2), from where they can disseminate to the bloodstream and endanger patients’ lives (3). Intestinal colonization by multidrug-resistant pathogens can promote their dissemination to other patients through fecal contamination of the environment (4). Understanding what influences MRE intestinal colonization is key to prevent MRE infections.
The clinical variables associated with intestinal carriage of resistant Enterobacteriaceae in hospitalized patients have been analyzed before (5–10), but previous studies based on culture analysis defined carriage as the detectable presence of resistant bacteria in fecal samples irrespective of their levels: the question of how MRE levels varied within and between patients remained open. Intestinal levels, and not just detectable presence, are crucial for pathogen dissemination from the gut to the bloodstream (intradissemination) and dissemination between patients (interdissemination) (11–13). Leukemia patients carrying higher levels (>106 CFU/g of fecal sample) of resistant Enterobacteriaceae have 5-fold higher risk of developing bacteremia provoked by these Enterobacteriaceae (11). In addition, patients carrying higher relative abundance of Enterococcus or Klebsiella pneumoniae in feces, as determined through microbiota sequencing analysis, have increased risk of developing bloodstream infections by these organisms (12, 13). On the other hand, studies with vancomycin-resistant Enterococcus (VRE) showed that high intestinal pathogen loads (>104 CFU/g) enhance contamination of a patient’s environment (4), which facilitates dissemination to other patients (14).
While it is important to identify and understand the factors that impact intestinal levels of resistant pathogens, few clinical studies have sought to quantify pathogen loads.
Two studies—performed in a single medical center—concluded that the administration of antibiotics with activity against anaerobic bacteria can increase the fecal density of VRE and Gram-negative-resistant bacilli (4, 15). This effect may have been caused by depletion of anaerobic commensal microbes, thought to be important in providing colonization resistance (16, 17). However, it is not clear if all antibiotics with antianaerobic activity will increase MRE intestinal colonization levels to the same extent. For example, antibiotics administered intravenously (i.v.) to treat bloodstream infections must be excreted through the bile in order to reach the intestinal tract. Thus, certain i.v. antianaerobic therapies may not reach concentrations that are sufficiently high in the gut to promote MRE intestinal colonization. Besides, while certain antianaerobic therapies mainly affect anaerobes (i.e., metronidazole and clindamycin), others, such as beta-lactams, could impact the growth of MRE directly if they are excreted into the gut in sufficient amounts and that particular MRE is sensitive to the antibiotic.
Here, we conducted an extensive prospective study to examine the effect of specific antibiotics, as well as other factors such as antifungal treatments or neutropenia, on the gut expansion of MRE. We focused on acute leukemia patients, who are frequently colonized by resistant Enterobacteriaceae (11), to evaluate the range of colonization levels within and between patients and to further elucidate the impact of i.v.-administered antibiotics and other clinical factors on the intestinal levels of MRE.
RESULTS
Study population.
We initiated a study of 133 acute leukemia patients (see Tables S1 and S2 in the supplemental material), which lasted 18 months. Patients were tracked over multiple hospitalizations (median hospitalizations per patient, 3; median hospitalization length, 26 days; median number of days a patient was followed, 70). The most frequent causes of hospital admission were chemotherapy, infection, and transplantation (see Tables S3 and S5).
Acute leukemia patients received multiple antibiotic treatments during their hospitalization periods (Tables S3, S4, and S6). All antibiotics were i.v. administered except ciprofloxacin, which was administered orally. Ciprofloxacin, administered as prophylaxis immediately upon hospital admission for chemotherapy or transplantation, was the most frequently received antibiotic. Other frequently used antibiotics administered for empirical treatment of suspected bacteremia included beta-lactams, mainly, piperacillin-tazobactam (PTZ) and meropenem, the aminoglycoside amikacin, and the glycopeptide vancomycin. Similar results were obtained when days of therapy (DOT) per 1,000 patient days were calculated (Table S4). Here, ciprofloxacin was the therapy with the most DOT, followed by meropenem, PTZ, vancomycin, and amikacin.
MRE prevalence in fecal samples from acute leukemia patients.
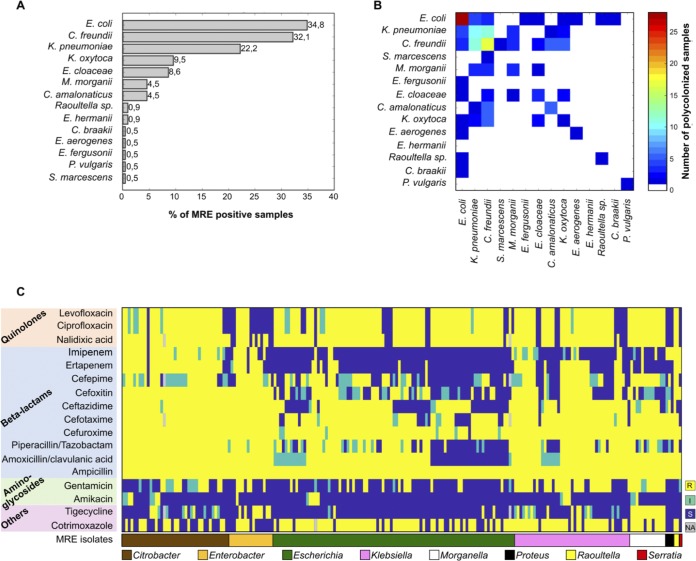
A total of 802 fecal samples were collected from 133 patients during the study period (6 samples on average per patient). MRE were detected in 221 samples (27.6%) collected from 80 (60.1%) of the analyzed patients. In 67.8% of the colonized patients, MRE were isolated in one or more consecutive samples obtained during the same hospital admission period. The most frequently isolated MRE were Escherichia coli, Citrobacter freundii, and K. pneumoniae (Fig. 1A). Polymicrobial MRE colonization was relatively frequent: 20.4% of the MRE-positive fecal samples were colonized by more than one MRE species (Fig. 1B). In addition, MRE strains belonging to the same species but with different antibiotic resistance patterns were identified in 31.7% of the MRE-positive samples. A summary of the detected resistances is shown in Fig. 1C and in Tables S7 and S8. As expected, considering the media used for MRE isolation, the majority of MRE isolates were resistant to penicillins and third-generation cephalosporins (i.e., 100% were resistant to ampicillin and 93.9% were resistant to cefotaxime). Consistent with the use of ciprofloxacin as a prophylactic agent, most MRE isolates were resistant to ciprofloxacin (86.6%). In addition, the majority of isolates were resistant to PTZ (82.3%), and approximately 50% of the isolates were resistant to carbapenems (i.e., imipenem and/or ertapenem). The proportion of resistance varied considerably among different species (Table S7).
FIG 1.
MRE-positive samples show diverse compositions and antibiotic resistance patterns of detected MRE isolates. MRE were detected in 221 samples collected from 80 of the analyzed patients. (A) The most frequently isolated MRE belonged to the species Escherichia coli. Other detected MRE in order of prevalence were Citrobacter freundii, Klebsiella pneumoniae, Klebsiella oxytoca, Enterobacter cloacae, Morganella morganii, Citrobacter amalonaticus, Raoultella spp., and Escherichia hermannii. Other MRE species, including Citrobacter braakii, Proteus vulgaris, Serratia marcescens, Enterobacter aerogenes, and Escherichia fergusonii, were detected in only one sample. (B) In 45 of the 221 MRE-positive samples (20.4%), MRE belonging to more than one bacterial species were identified, and in 70 of the MRE-positive samples (31.7%), MRE strains belonging to the same species but with different antibiotic resistance pattern were identified. (C) Antibiotic resistance patterns of MRE isolates detected in 221 positive samples. When two isolates from the same sample had exactly the same resistance patterns and taxonomies, only one of the two isolates was shown. Columns and rows were grouped based on MRE taxonomy and antibiotics’ class. S, susceptible; I, intermediate resistance phenotype; R, resistant. NA, not analyzed.
MRE fecal levels differ significantly within and between patients.
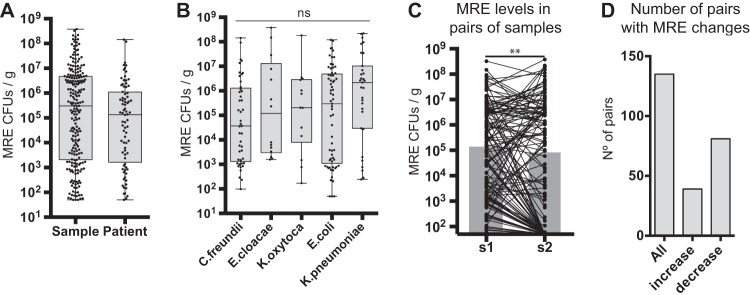
The levels of MRE (CFU per gram of feces) varied significantly among different samples from the same patient (acquired at different days) and also between patients (Fig. 2; see also Table S9). Detected MRE reached high levels in some patients (1.43 × 108 CFU/g), while in others, levels stayed scarcely within the limit of detection (50 CFU/g of feces). We saw no statistical significant differences in colonization levels among different MRE species (P = 0.2) (Fig. 2B). To analyze the dynamics of MRE colonization levels, we studied the changes in MRE levels in pairs of fecal samples. The total number of analyzed pairs was 135, obtained from 59 patients. The analysis showed that the levels of MRE changed over time (Fig. 2C and D; see also Fig. S1), with a pattern suggesting that once MRE had colonized the intestinal tract, their levels tended to decrease (Fig. 2C and D) (P = 0.004).
FIG 2.
MRE fecal levels differ significantly between patients but also within a patient. (A) MRE levels identified in all colonized fecal samples included in the study (N = 221 samples) or the mean (obtained from log10 CFU data) of the MRE levels identified in colonized samples from each patient (N = 80 patients). Whiskers represent minimum and maximum values. Horizontal lines represent the medians and 25th to 75th percentiles. (B) MRE levels in fecal samples colonized exclusively with the indicated species (low-abundant species [N ≤ 5] are not included). No significant (ns) differences in MRE levels were detected between different species (Kruskal-Wallis test). N = 45 (C. freundii), 14 (E. cloacae), 13 (K. oxytoca), 65 (E. coli), and 31 (K. pneumoniae) samples. (C) Changes in MRE levels among 135 pairs of consecutive fecal samples (see Materials and Methods for definition) collected from 59 patients. The gray bars represent the medians of MRE levels. **, P < 0.01 by two-tailed paired Wilcoxon test. (D) Number of the total pairs of consecutive samples included in the study (all) and numbers of pairs of samples in which an increase in MRE levels (>1 log2 fold change [FC]; N = 39) or a decrease in MRE levels (<−1 log2 FC; N = 81) was detected.
Antibiotic intravenous therapies that include the beta-lactam PTZ and/or meropenem decrease MRE fecal levels.
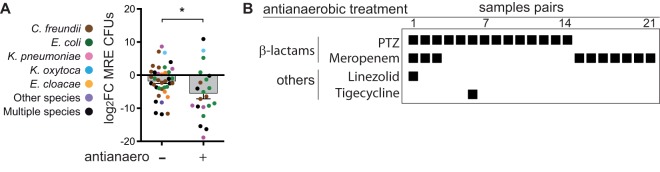
We next studied clinical factors that could explain the detected variability in MRE levels, focusing first on antibiotics. Based on previous studies that have detected an effect of antianaerobic therapies on the intestinal levels of VRE and resistant Gram-negative bacilli, including MRE (4, 15), we first asked whether the introduction of an antibiotic with activity against anaerobes between two consecutive samples acquired from the same patient changed the MRE levels (see Table S10 for the antibiotic administered in each pair of samples). As previously described (15), we did not include in this analysis those pairs of samples that had received a therapy with antianaerobic antibiotics in the previous week. Interestingly, introduction of antibiotics with activity against anaerobes between two consecutive samples (21 pairs of samples from 18 patients) diminished the intestinal levels of MRE (Fig. 3A) (P = 0.026) significantly more on average than in pairs of samples in which an antianaerobic treatment was not administered (38 pairs of samples from 21 patients).
FIG 3.
Antibiotic therapies including the beta-lactams piperacillin-tazobactam and meropenem decrease MRE fecal levels. (A) MRE log2 FC values among pairs of consecutive samples between which a therapy with an antibiotic with activity against anaerobic bacteria was initiated (+) compared to pairs of samples in which no antianaerobic antibiotics were administered (−). N = 38 pairs of samples from 21 patients and 21 pairs of samples from 18 patients for the antianaerobic (−) and (+) groups, respectively. *, P < 0.05 by two-tailed t test. Bars represents the means, whiskers represent the standard errors of the means (SEMs). (B) Antibiotics with antianaerobic activity that were administered between each pair of samples shown in panel A. Colors in panel A indicate the taxonomy of the MRE identified in each pair of samples. Detailed taxonomies and antibiotic resistance patterns of all the MRE identified within each pair of samples are shown in Table S10 in the supplemental material. PTZ, piperacillin-tazobactam.
In our hospital unit, the most frequently used antibiotics with activity against anaerobes were i.v. beta-lactams (mainly PTZ and meropenem), which can also directly inhibit the growth of some MRE strains. Indeed, in the 21 pairs of samples included in the group that received antianaerobic therapies, the patients had received at least one of these two beta-lactams (Fig. 3B), suggesting a major role for these antibiotics in the detected reduction of MRE levels.
Impact of i.v. beta-lactams (PTZ and meropenem) on MRE fecal levels depends on the MRE resistance profile.
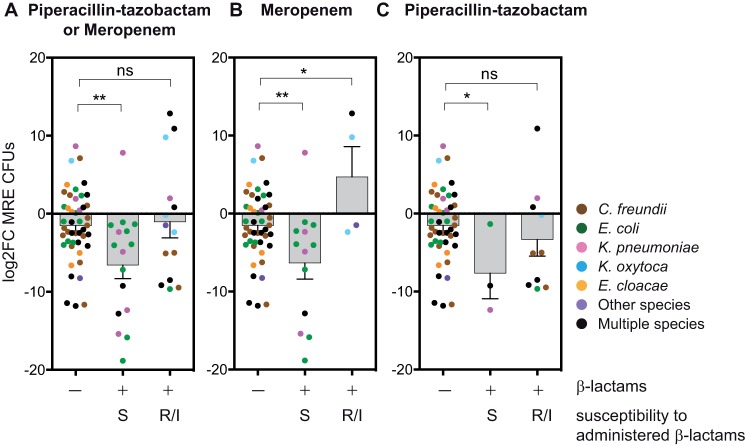
The role of i.v. beta-lactams (i.e., PTZ and meropenem) in decreasing MRE levels could be indirect (e.g., through changes in the microbiome that promote MRE depletion) or it could be direct (by inhibiting MRE growth). To test if i.v. beta-lactam administration was directly reducing MRE levels, we analyzed the effect of these antibiotics, taking into account the resistance pattern of the MRE isolated (see Fig. 4 and Table S11 for the antibiotic administered in each pair of samples and the resistant patterns of the MRE isolated). Notably, beta-lactam administration did not affect MRE levels when the MRE isolated in a pair of samples were nonsusceptible to the administered antibiotic (Fig. 4A) (P = 0.77; 14 pairs of samples from 9 patients). In contrast, beta-lactam administration significantly diminished MRE fecal levels when all the strains isolated within a pair of samples were susceptible to the beta-lactam administered (Fig. 4A) (P = 0.002; 16 pairs of samples from 15 patients). We carried out the same analysis but excluded the patients who had received in the previous week another beta-lactam therapy and saw a similar effect: the levels of MRE strains susceptible to the antibiotic administered decreased (P = 0.001), but we detected no change in nonsusceptible strains (P = 0.33) (not shown). Notably, this inhibitory effect of beta-lactams on susceptible MRE strains was not associated with treatment length (see Fig. S2A).
FIG 4.
Impact of i.v. beta-lactams (piperacillin-tazobactam and meropenem) on MRE fecal levels depends on the MRE resistance profile. (A) MRE log2 FC among pairs of consecutive samples between which a beta-lactam (i.e., piperacillin-tazobactam or meropenem) was administered (+) or not (−). MRE strains detected in the consecutive pairs of samples analyzed were either susceptible (S) or nonsusceptible (R/I) toward the administered beta-lactam. (B, C) Same as in panel A but only including pairs of samples in which the beta-lactam therapy initiated between the samples of a pair was exclusively meropenem (B) or exclusively piperacillin-tazobactam (C). *, P < 0.05; **, P < 0.01 versus group not receiving beta-lactams by two-tailed t test. The results show that beta-lactams (i.e., meropenem and piperacillin-tazobactam) reduce the levels of MRE strains susceptible to the beta-lactam administered. Colors indicate the taxonomies of the MRE identified in each pair of samples. Detailed taxonomies and antibiotic resistance patterns of all the MRE identified within each pair of samples are shown in Table S11. The numbers of pairs of samples (S) and patients (P) included in each group are as follows: no beta-lactam, S = 46, P = 27; susceptible to beta-lactams, S = 16, P = 15; nonsusceptible to beta-lactams, S = 14, P = 9; susceptible to meropenem, S = 13, P = 13; nonsusceptible to meropenem, S = 4, P = 3; susceptible to PTZ, S = 3, P = 3; nonsusceptible to PTZ, S = 10, P = 8.
We noticed that the proportion of MRE species within each group of analyzed samples differed (Fig. 4A; Table S11). For example, C. freundii was common in samples not receiving beta-lactams but was undetected in samples receiving beta-lactams that contained beta-lactam-susceptible strains from other species. To corroborate that the inhibition of susceptible strains was due to the beta-lactams administered and not to confounding differences in their composing MRE species, we reanalyzed the samples exclusively colonized with E. coli MRE strains, the most abundant MRE species in our cohort. We saw a similar inhibitory effect of beta-lactams in samples exclusively colonized with E. coli (Fig. S3) (P = 0.035; 9 pairs of samples from 9 patients).
The cultivation medium used for MRE isolation selects for strains that are producers of extended-spectrum beta-lactamases (ESBLs). We confirmed that most of the isolated strains before and after beta-lactam administration were ESBL producers (80% of analyzed strains) (Table S11). Most importantly, a similar impact of beta-lactams on MRE levels was detected when we included in the analysis only those pairs of samples containing ESBL+ strains (see Fig. S4).
Consistent with the inhibitory effect of i.v. beta-lactams on MRE susceptible strains, MRE were not detected after beta-lactam administration in 75% of the cases in which the patient was colonized with MRE strains susceptible to the administered beta-lactam (Table 1) (N = 16 pairs of samples from 15 patients). In contrast, MRE colonization persisted in the majority of the cases in which the patient was not receiving a beta-lactam (only 26.1% clearance in 46 pairs of samples analyzed from 27 patients) or when the patient was colonized with strains nonsusceptible to the administered beta-lactam (28.5% clearance in 14 pairs of samples analyzed from 9 patients) (Table 1).
TABLE 1.
MRE clearance after beta-lactam (piperacillin-tazobactam or meropenem) administration
| Beta-lactam(s)a | Susceptibilityb | MRE clearance rate (no./total no. [%])c | Significanced |
|---|---|---|---|
| None | NA | 12/46 (26.1) | |
| PTZ and/or meropenem | S | 12/16 (75) | *** |
| PTZ and/or Meropenem | R/I | 4/14 (28.5) | NS |
| PTZ | S | 2/3 (66.6) | NS |
| PTZ | R/I | 4/10 (40) | NS |
| Meropenem | S | 10/13 (77) | * |
| Meropenem | R/I | 0/4 (0%) | NS |
Beta-lactam(s) that was administered between a pair of samples. PTZ, piperacillin-tazobactam.
Susceptibility of the MRE to the administered antibiotic. S, susceptible; R/I, nonsusceptible (resistant/intermediate phenotype); NA, not applicable.
Number of pairs of samples from the total analyzed in which the MRE was not detected in the second sample of the pair.
*, P < 0.05; ***, P < 0.001; NS, P > 0.05; Fischer test comparing with the group in which no beta-lactams were administered.
Interestingly, pairs of samples colonized with MRE susceptible strains that were not cleared after i.v. beta-lactam administration were associated with shorter antibiotic treatment than those pairs of samples where the clearance was detected (P = 0.11) (Fig. S2B).
A separate analysis of the two most frequently administered beta-lactams (i.e., meropenem and PTZ) provided similar results. Compared to pairs of samples in which a beta-lactam was not administered (46 pairs of samples from 27 patients), introduction of meropenem significantly reduced the MRE levels of strains sensitive to this antibiotic (P = 0.005; 13 pairs of samples from 13 patients) (Fig. 4B; Table 1). PTZ also reduced the levels of MRE strains susceptible to this antibiotic (Fig. 4C) (P = 0.03), although the number of pairs of samples available for this analysis was low (3 pairs of samples from 3 patients). PTZ did not significantly modify the levels of MRE strains nonsusceptible to this antibiotic (Fig. 4C) (P = 0.3; 10 pairs of samples from 8 patients). Interestingly, the levels of nonsusceptible strains increased, on average, with meropenem treatment (Fig. 4B) (P = 0.017; 4 pairs of samples from 3 patients). Nevertheless, the number of pairs of samples available for this last analysis was low, and the MRE expansion only occurred in 2 of the 4 analyzed cases (Fig. 4B). A similar impact of individual beta-lactams on MRE dynamics was detected when analyzing exclusively those pairs of samples containing ESBL-producing strains (Fig. S4).
Other drugs showed no impact on MRE colonization.
Consistent with its poor biliary excretion (18, 19), other frequently administered antibiotics such as aminoglycosides (i.e., amikacin) or glycopeptides (i.e., vancomycin and teicoplanin) did not induce observable changes in MRE levels (see Fig. S5) (P > 0.05). We also saw no impact for amikacin on strains sensitive to this antibiotic (P = 0.485) (not shown). The effect of amikacin on nonsusceptible strains was not evaluated due to the low number of pairs of samples available (N = 1). The effect of quinolones on MRE dynamics could not be evaluated, since they were administered immediately after hospital admission, before the first fecal sample was collected.
Administration of antifungals such as caspofungin or amphotericin did not impact MRE levels either (P > 0.25). In addition, initiation of neutropenia (P = 0.9), mucositis (P = 0.83), or starting parenteral feeding (P = 0.79) did not affect MRE dynamics in pairs of consecutive samples.
We next applied multivariate statistical analysis, taking into account all clinical variables previously analyzed and other possible confounding factors (i.e., sex, age, type of admission, leukemia type). This analysis confirmed that i.v.-administered beta-lactams are an independent variable associated with MRE reduction when the MRE is sensitive to the administered beta-lactam (P < 0.001).
Beta-lactams (i.e., meropenem or PTZ) select for MRE clones resistant to the administered antibiotic.
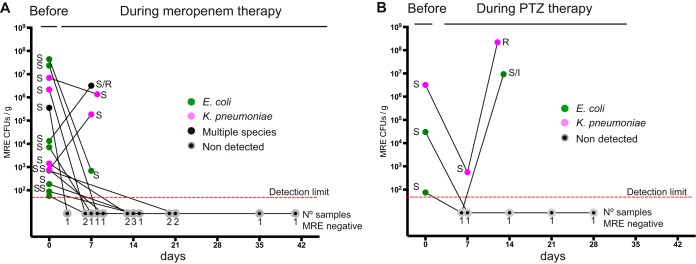
The results obtained suggest that i.v. beta-lactam therapy (i.e., meropenem or PTZ) could diminish the intestinal loads of MRE sensitive to the administered beta-lactam. However, beta-lactam therapy has the implied risk of selecting for resistant strains that could occupy the niche left by the sensitive ones. To investigate this possibility, we focused on patients that were initially colonized with MRE that were sensitive to the administered beta-lactam and asked whether their levels of MRE and their resistance patterns changed over time (Fig. 5, S6, and S7). As previously described, meropenem diminished the intestinal loads of meropenem-sensitive strains. During meropenem administration, MRE levels decreased in 12 of 14 analyzed cases, and MRE levels were below the detection limit in 10 cases. Moreover, MRE were no longer detected in any sample collected after clearance and during the treatment (8 samples from 4 patients that were collected in weeks following MRE elimination). Notably, in 1 of 4 events in which we detected MRE during the meropenem treatment, both sensitive and resistant strains to this antibiotic were detected, although the resistant strain was from a different species than the original susceptible strain. Introduction of PTZ decreased MRE levels in the 3 cases in which patients were colonized with strains susceptible to this antibiotic. However, strains nonsusceptible to PTZ arose and expanded during PTZ treatment in 2 of 3 analyzed cases. In this particular case, the nonsusceptible strains belonged to the same species as the original susceptible ones. Thus, although administration of meropenem and PTZ can overall reduce the levels of MRE susceptible strains, occasionally, nonsusceptible MRE strains may arise.
FIG 5.
Meropenem and piperacillin-tazobactam decrease fecal levels of susceptible MRE but, occasionally, resistant strains emerge. MRE levels and sensitivity patterns to the beta-lactam received before and during therapy with meropenem (A) or piperacillin-tazobactam (PTZ) (B). Consecutive samples collected from the same patient and admission period are connected with a line. Sensitivity to the antibiotic is indicated when the MRE was detected. Numbers of samples in which an MRE was not detected on each specific day are indicated. Note that the first sample always contained MRE strains susceptible to the beta-lactam received. S, susceptible; R, resistant; I, intermediate phenotype. Colors indicate the taxonomies of the MRE identified in each sample. Days are relative to the date of the first sample included in the figure for each patient. Graphs for each individual patient and samples collected after beta-lactam cessation are shown in Fig. S6 and S7. N = 14 patients for meropenem group and 3 patients for the PTZ group. Note that compared to Fig. 4, there is one more patient receiving meropenem in this figure. This is because in Fig. 4, a pair of samples was included in the group of pairs sensitive to meropenem only if all MRE strains characterized in the pair were sensitive to the antibiotic.
Finally, we asked whether cessation of beta-lactam treatment would enable the expansion of MRE strains in those cases in which we observed MRE clearance during treatment. We only had samples from two patients treated with meropenem for this type of analysis (Fig. S6). In one of these patients (i.e., 105), an MRE from a different species than the one eliminated during meropenem treatment was detected in fecal samples collected 21 days after meropenem cessation. Interestingly, in patient 75, an MRE isolate (from the same species and with the same antibiotic resistance pattern as the one eliminated during meropenem treatment) was detected 9 days after meropenem cessation, indicating that MRE reexpansion occurred after meropenem withdrawal.
DISCUSSION
We conducted an extensive survey on MRE intestinal colonization levels in patients hospitalized to receive cancer treatments, including the effect of a range of clinical factors on MRE dynamics. This is, to our knowledge, the first study to investigate quantitatively, through extensive culturing, MRE colonization in such a large cohort of patients, allowing us to investigate the factors associated with temporal changes of MRE levels in the intestinal tract of the same patient. The extensive data generated in this study is publicly available (see supplemental material) so that the scientific and clinical community can potentially investigate other factors influencing MRE dynamics within hospitalized patients besides the effect of beta-lactams that we revealed here.
Our analysis showed that MRE levels varied significantly between patients and between samples collected from the same patient during the same hospital admission period. Therefore, based on these results and previous studies indicating that higher levels of resistant bacteria increase the risk of pathogen dissemination to the bloodstream or to the environment (4, 11), it may be useful for clinical practice to accurately measure fecal MRE levels, beyond determining the presence or absence of fecal carriage.
When we started our investigation, we expected to find confirmation that antibiotics with antianaerobic activity promote higher intestinal colonization levels of multidrug-resistant pathogens such as VRE or resistant Gram-negative bacilli (4, 15). However, we found instead that the antibiotics with antianaerobic activity used in our hospital unit diminished, and not increased, the fecal levels of MRE. A separate analysis of specific antibiotics revealed that the beta-lactams (PTZ and meropenem) had the greatest effect. Beta-lactams, although administered intravenously, are partially excreted through the bile to the intestinal tract (18) and could directly affect the growth of MRE. Indeed, we were able to demonstrate that i.v. administration of PTZ and meropenem diminished the levels of MRE when the strain was sensitive and not when it was resistant to the administered antibiotic, pointing to the direct effect of these antimicrobials. In some cases, the impact of these beta-lactams was substantial, clearing MRE from the intestinal tract in patients previously colonized with more than 106 CFU/g of feces. This result agrees with previous observations in two patients that were highly colonized with Enterobacteriaceae strains sensitive to meropenem and that were undetectable after i.v. meropenem treatment (15). Notably, for a couple of patients in whom MRE had been cleared during meropenem treatment, fecal samples could be analyzed after meropenem cessation. In these two patients, MRE were detected again after meropenem withdrawal, suggesting that the inhibitory effect of meropenem was only effective during its administration. Additional studies should be performed in order to evaluate this in a larger number of patients. Since meropenem withdrawal usually occurred at the end of the hospitalization period, this type of analysis would require the collection and analysis of samples beyond the hospitalization period, which was not performed in this study.
The effectiveness of PTZ therapy against certain MRE strains (i.e., those producing ESBL) differs significantly depending on the study. Thus, its utility to treat infections by these pathogens remains controversial (20). Indeed, in the only randomized clinical trial performed, PTZ was shown to be less effective than meropenem in the treatment of bloodstream infections produced by Enterobacteriaceae ESBL-producer strains (21). Reasons that explain different PTZ treatment outcomes include the site of infection and susceptibility of the ESBL-producing strain to PTZ. Our results are consistent with these differential effects of PTZ on ESBL-producing strains. We found that PTZ decreased the fecal levels of ESBL-producing Enterobacteriaceae that are susceptible to the treatment, while it did not affect the levels of those nonsusceptible to this antibiotic. Nevertheless, the obtained results should be confirmed in an extended study, since only a few pairs of samples were included in this analysis. Our data also show occasional selection for strains resistant to PTZ (2 of 3 cases) and meropenem (1 of 14 cases). Thus, although both i.v. PTZ and meropenem decrease the intestinal levels of susceptible strains, their usage should be restricted to the treatment of suspected cases of infection rather than decontamination of MRE susceptible strains from the intestinal tract.
The administration of beta-lactams, and more specifically, PTZ, apparently did not promote large expansion of MRE strains nonsusceptible to the administered beta-lactam in acute leukemia patients. However, our analysis did suggest that the levels of MRE strains nonsusceptible to meropenem might increase after meropenem administration. This result, despite passing the common threshold of statistical significance, was based on only four pairs of samples, and a closer inspection shows that MRE expanded in only one-half of those cases. Thus, additional studies should be performed to validate this result. Nevertheless, this is consistent with the result obtained in a study in which carbapenem administration was associated with a higher relative abundance of carbapenem-resistant K. pneumoniae, measured by 16S rRNA sequencing, which was associated with increased risk of bloodstream infections (13). Thus, i.v. meropenem can reduce the fecal levels of susceptible strains, while it may promote the growth of resistant ones.
It is worth mentioning that our hospital unit rarely uses clindamycin and metronidazole, two antibiotics with major effects against anaerobic bacteria but with no direct effect against MRE. These antibiotics, in addition to PTZ, were some of the most frequently administered in a previous study showing higher levels of resistant Gram-negative bacilli during antianaerobic treatment (15). The different therapies administered in both studies may explain the different results obtained regarding the effect of antianaerobic antibiotics on the growth of resistant pathogens. Indeed, studies performed in mice have shown that parenteral administration of clindamycin consistently promotes high levels of MRE intestinal colonization. In contrast, intestinal density of MRE upon PTZ administration varies depending on the dose of the antibiotic administered, the pathogen inoculum concentration, and its level of antibiotic resistance (22). Future studies should elucidate how these variables influence the capacity of beta-lactams to promote intestinal colonization by MRE in patients.
We acknowledge several limitations of our study. First, our study was performed in a very specific human population (acute leukemia patients) that may already display an altered microbiota due to prior treatments. Nevertheless, we would expect that the beta-lactam effect on susceptible MRE strains would be the same in other types of hospitalized patients, since this effect is direct and probably not dependent on the microbiota. Moreover, acute leukemia patients are one of the cohorts with higher risk of infection due to intestinal colonization by MRE and could therefore benefit the most from this type of study. Second, our study was restricted to the subset of MRE that were able to grow on plates containing a third-generation cephalosporin, designed for the detection of ESBL-producing strains. Indeed, most of the analyzed strains were ESBL producers. In addition, most of the isolated strains were resistant to quinolones, probably because ciprofloxacin is administered in our hospital unit as prophylaxis immediately upon patient’s hospital admission. Additional work should be carried out in order to validate our findings on strains with a different resistance background. In addition, further work should be performed in order to study if the effect of beta-lactams on MRE levels depends on the different mechanisms of cephalosporin or carbapenem resistance, which were not studied here. Third, our results did not allow us to evaluate the impact of clinical factors on low-abundance MRE populations, since the MRE antibiotic resistant pattern and taxonomy were only characterized in a few representative isolates from each sample. Fourth, we were able to validate the inhibitory effect of specific beta-lactams on a particular individual MRE species (i.e., E. coli). However, the effect on other individual MRE species of interest (e.g., K. pneumoniae) could not be evaluated due the limited number of samples containing exclusively these other MRE. Additional studies should be performed to confirm the effect of beta-lactams on other MRE species of interest. Finally, we were not able to evaluate the effect of MRE levels on the risk of bacteremia due to the low number of samples available for this analysis (i.e., 6 fecal samples collected before bacteremia detection containing the same MRE species as the one isolated in the bloodstream). Nonetheless, previous studies have already pointed to a link between high intestinal levels of drug-resistant bacteria and increased risk of bloodstream infections provoked by these bacteria (11, 13). Additional work should be performed to corroborate those results using quantitative measurements to evaluate if a particular threshold leads to higher risk of bloodstream infections.
In summary, our results revealed a wide diversity of intestinal MRE levels and highlighted the relevance of quantitatively analyzing these levels in surveillance and/or clinical studies. Certain i.v.-administered antibiotics had a direct effect on the intestinal growth of MRE, and this effect depended on the sensitivity of the pathogen to the given antibiotic. Overall, our study highlights the relevance of incorporating antibiotic treatment and susceptibility data of gut-colonizing pathogens for future clinical studies and in clinical decision-making.
MATERIALS AND METHODS
Ethics.
This study was approved by the Ethics Committee of CEIC Dirección General de Salud Pública and Centro Superior de Investigación en Salud Pública (20130515/08). All included patients gave their consent to participate in the study.
Sample and metadata collection.
Fecal samples were collected weekly from November 2013 until April 2015 from all acute leukemia patients hospitalized at the Hospital La Fe (Valencia, Spain) who agreed to participate in this study. Procedures of sample collection and metadata acquisition are described in detail in the supplemental material.
Assessing MRE colonization levels and characterization of MRE isolates.
MRE levels, defined as the number of MRE CFU detected per gram of feces, were determined by culturing 10-fold dilutions of fecal samples in Brilliance ESBL agar plates (Oxoid), which contain cefpodoxime, a third-generation cephalosporin, as the selective agent. These plates allow for isolation of extended spectrum beta-lactamase (ESBL)-producing organisms while inhibiting the growth of non-ESBL Enterobacteriaceae and most AmpC organisms, allowing mainly for identification of ESBL-producing E. coli and the Klebsiella, Enterobacter, Serratia, and Citrobacter group (known as KESC). The number of colonies in each plate was normalized by dilution and fecal weight in order to calculate the levels of colonization of MRE per gram of feces. To confirm that the detected colonies were MRE, the taxonomy and resistance patterns of 5 isolated colonies per sample were determined through the matrix-assisted laser desorption ionization–time of flight (MALDI-TOF) and Vitek system (see methods in the supplemental material). In addition, production of extended-spectrum beta-lactamases was analyzed in a subset (11%) of the characterized MRE isolates (Table S11; Fig. S4) as described in the supplemental methods.
Defining pairs of samples and calculating the fold change among pairs of samples to study MRE dynamics.
For analyzing the dynamics of MRE colonization levels, we calculated the log2 fold change (FC) in MRE levels in pairs of fecal samples. The pairs were defined as two samples consecutively collected from the same patient and same hospital admission period. The first sample of the pair was always positive for MRE. Taking into account the weekly sampling strategy performed in this study, in most cases, the two samples were collected 1 week apart from each other.
Sometimes, MRE were not detected in the second sample of a pair of samples; we defined those cases as MRE clearance. To avoid infinite values that would arise by dividing by 0, the limit of the detection (i.e., 50 CFU/g) was added to the values obtained in each sample as pseudocounts before calculating the FC.
The MRE clearance rate was defined as the number of pairs of samples from the total number analyzed in which MRE were not detected in the second sample of the pair.
Description of the comparisons performed to evaluate the effect of clinical factors on MRE dynamics.
(i) Effect of antianaerobic antibiotics on MRE dynamics. The log2 FC obtained from pairs of samples in which a therapy with an antianaerobic antibiotic had been initiated was compared with the log2 FC of the group of pairs of samples that had not received such therapy. The group of pairs of samples that had received an antianaerobic therapy was defined, based on previous studies (4, 15), as those in which the MRE levels from the first sample of the pair were available within 2 weeks before the initiation of the regimen, no antianaerobic antibiotic had been administered within 7 days before the regimen was initiated, and the level of the second sample within the pair was determined at least 2 days after the initiation of the treatment but no more than 7 days after the completion of the antibiotic course. Although we decided to follow these previously established criteria dictating that the second sample in a pair should not have been collected more than 7 days after the cessation of the treatment, it is worth mentioning that in our study, all the second samples of analyzed pairs were collected while the treatment was ongoing. The length of the treatment was not accounted for as a variable for this type of analysis. The MRE log2 FC detected in this group of samples was compared with the MRE log2 FC obtained in pairs of samples in which no antibiotic with antianaerobic activity was received either between the two samples of a pair (including the days in which the samples were collected) or in the week before the date of collection of the first sample of the pair.
Antibiotics included in the analysis with activity against intestinal anaerobes were PTZ, amoxicillin-clavulanic acid, ampicillin, meropenem, ertapenem, imipenem, linezolid, metronidazole, and tigecycline. Antibiotics included in the analysis that were considered not active against intestinal anaerobes were gentamicin, amikacin, cloxacillin, aztreonam, co-trimoxazole, colistin, ciprofloxacin, levofloxacin, daptomycin, vancomycin, and teicoplanin (23–30).
Daptomycin, despite its in vitro effect against Gram-positive anaerobic bacteria (27), was included within the group of antibiotics with no activity against intestinal anaerobes. We made this choice because in mice, the parenteral administration of this antibiotic does not disrupt the anaerobic flora and does not promote the intestinal colonization of extended-spectrum-β-lactamase-producing K. pneumoniae (31). In addition, glycopeptides such as vancomycin and teicoplanin were included in the group with no effect against intestinal anaerobes because they were administered intravenously and their biliary excretion is low (18, 19). Nevertheless, similar results as those shown in Fig. 3 were obtained if glycopeptides were included within the group of antibiotics with activity against anaerobic bacteria (not shown).
Patients did not receive antibiotic prophylaxis while in an outpatient setting. Moreover, in cases where the patient required an antibiotic for other indications (fever or other symptoms), the patient was first hospitalized. For this reason, we did not account for the impact of outpatient antibiotic exposure on MRE levels, and we focused our analysis on those antibiotics received during the hospitalization period.
(ii) Effects of beta-lactam therapy and other clinical variables on MRE dynamics. A comparison was performed between the log2 FC obtained from pairs of samples in which a therapy with a beta-lactam antibiotic had been initiated and the value for the group of pairs of samples that had not received such therapy. The group of pairs of samples that had received the beta-lactam therapy was defined as those in which the MRE levels from the first sample of the pair were available within 2 weeks before the initiation of the regimen and the levels of the second sample within the pair were determined at least 2 days after the initiation of the treatment but no more than 7 days after the completion of the antibiotic course. As described above, although we decided to follow the criteria established by previous studies (4, 15), in our study, all the second samples of analyzed pairs were collected while the beta-lactam treatment was ongoing. The length of antibiotic treatment was not accounted for as a variable for this type of analysis. To study if the effect of beta-lactams on MRE change was direct (i.e., direct inhibition of MRE on susceptible strains), the pairs of samples selected were divided into two subgroups: (i) pairs of samples containing exclusively strains susceptible to the beta-lactam administered, and (ii) pairs of samples containing exclusively strains nonsusceptible to the beta-lactam administered. The MRE log2 FC detected in these two groups of pairs of samples was compared with the MRE log2 FC obtained in pairs of samples in which no beta-lactam was received between the two samples of a pair (including the days in which the samples were collected).
In addition to this type of analysis (Fig. 4), we performed the same analysis but excluding from the beta-lactam group those pairs of samples that had received another beta-lactam in the week before the initiation of the beta-lactam therapy, and we compared it with the groups of samples that had not received beta-lactams either between the samples of a pair (including the days in which the samples were collected) or in the week before the date of collection of the first sample of the pair. This type of analysis led to similar results as indicated in Results, although the number of samples included in this analysis was lower. For this reason and to avoid redundancy, only the first type of analysis is shown in Fig. 4.
The same comparisons as with beta-lactams were performed to assess the effect on MRE dynamics of other antibiotics (i.e., glycopeptides and aminoglycosides), antifungal treatments, and initiation of parenteral feeding, mucositis, or neutropenia. In the case of neutropenia, mucositis, or parenteral feeding, the log2 FC of pairs of samples in which these events were initiated was compared with that of pairs of samples collected during periods in which the patient did not have neutropenia, was not fed through the parenteral route, or did not develop mucositis.
Besides the analysis described above on the effect of antibiotics on MRE changes, previous studies have also compared the levels of resistant pathogens identified during the administration of different groups of antibiotics. Nonetheless, that approach had limitations: (i) it did not take into account those samples in which the pathogen was unable to colonize because of the presence of the antibiotic, and (ii) it did not consider those samples in which the pathogen was eliminated upon the introduction of the antibiotic. As seen in this study, some of the most frequently administered antibiotics in our hospital unit (i.e., meropenem and PTZ) often do eliminate MRE when the strain is sensitive to the antibiotic administered. For this reason, and to make our study more comprehensive, we focused our analysis on the changes in MRE after the introduction of specific antibiotics, which allowed us to account for cases in which the effect of the antibiotic was the complete elimination of the pathogen.
Statistical analysis.
In the case of this descriptive study without hypothesis to be tested, the comparisons and P values calculated were descriptive and exploratory.
Two-tailed Student's t test was applied in order to analyze if the log2 fold change of MRE levels between groups of samples were significantly different. Fischer’s exact test was applied to define if MRE clearance rate (Table 1) was significantly different among groups of samples.
Multivariate Lasso regression analysis was performed as described in the supplemental methods.
P values of <0.05 were considered statistically significant.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by the InfectERA-ERANET-Acciones complementarias grant (PCIN-2015-094), by a grant from the Spanish Ministerio de Economía y Competitividad (SAF2017-90083-R), by a Comunidad Valenciana grant from Consellería de d’Educació, Investigació, Cultura i Esport (AICO/2019/266), to C.U., by a Boehringer Ingelheim Fonds travel grant to A.D., by an FPU grant from the Spanish Ministerio de Educación, Cultura y Deporte to B.H., by an InfectERA-ERANET-Acciones complementarias de programación conjunta internacional grant (AC15/00070) to M.A.S. and J.S., by a BMBF FloraStopMRE grant (031L0089) to B.K., by a Fundação para a Ciência e a Tecnologia grant (Infect-ERA/0004/2015) to K.B.X., and by National Institutes of Health (NIH) grant U01 AI124275 and grant R01 AI137269 to J.B.X.
We thank Salvador Giner for help in determining the antibiotic resistance profiles of MRE strains.
We declare no conflict of interest.
Footnotes
Supplemental material is available online only.
REFERENCES
- 1.Magiorakos AP, Srinivasan A, Carey RB, Carmeli Y, Falagas ME, Giske CG, Harbarth S, Hindler JF, Kahlmeter G, Olsson-Liljequist B, Paterson DL, Rice LB, Stelling J, Struelens MJ, Vatopoulos A, Weber JT, Monnet DL. 2012. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: an international expert proposal for interim standard definitions for acquired resistance. Clin Microbiol Infect 18:268–281. doi: 10.1111/j.1469-0691.2011.03570.x. [DOI] [PubMed] [Google Scholar]
- 2.Gorrie CL, Mir Eta M, Wick RR, Edwards DJ, Thomson NR, Strugnell RA, Pratt NF, Garlick JS, Watson KM, Pilcher DV, McGloughlin SA, Spelman DW, Jenney AWJ, Holt KE. 2017. Gastrointestinal carriage is a major reservoir of Klebsiella pneumoniae infection in intensive care patients. Clin Infect Dis 65:208–215. doi: 10.1093/cid/cix270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Satlin MJ, Chavda KD, Baker TM, Chen L, Shashkina E, Soave R, Small CB, Jacobs SE, Shore TB, van Besien K, Westblade LF, Schuetz AN, Fowler VG, Jenkins SG, Walsh TJ, Kreiswirth BN. 2018. Colonization with levofloxacin-resistant extended-spectrum β-lactamase-producing Enterobacteriaceae and risk of bacteremia in hematopoietic stem cell transplant recipients. Clin Infect Dis 67:1720–1728. doi: 10.1093/cid/ciy363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Donskey CJ, Chowdhry TK, Hecker MT, Hoyen CK, Hanrahan JA, Hujer AM, Hutton-Thomas RA, Whalen CC, Bonomo RA, Rice LB. 2000. Effect of antibiotic therapy on the density of vancomycin-resistant enterococci in the stool of colonized patients. N Engl J Med 343:1925–1932. doi: 10.1056/NEJM200012283432604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Han JH, Bilker WB, Nachamkin I, Tolomeo P, Mao X, Fishman NO, Lautenbach E. 2013. Impact of antibiotic use during hospitalization on the development of gastrointestinal colonization with Escherichia coli with reduced fluoroquinolone susceptibility. Infect Control Hosp Epidemiol 34:1070–1076. doi: 10.1086/673155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bert F, Larroque B, Dondero F, Durand F, Paugam-Burtz C, Belghiti J, Moreau R, Nicolas-Chanoine MH. 2014. Risk factors associated with preoperative fecal carriage of extended-spectrum β-lactamase-producing Enterobacteriaceae in liver transplant recipients. Transpl Infect Dis 16:84–89. doi: 10.1111/tid.12169. [DOI] [PubMed] [Google Scholar]
- 7.Torres-Gonzalez P, Cervera-Hernandez ME, Niembro-Ortega MD, Leal-Vega F, Cruz-Hervert LP, García-García L, Galindo-Fraga A, Martinez-Gamboa A, Bobadilla-del Valle M, Sifuentes-Osornio J, Ponce-de-Leon A. 2015. Factors associated to prevalence and incidence of carbapenem-resistant Enterobacteriaceae fecal carriage: a cohort study in a Mexican tertiary care hospital. PLoS One 10:e0139883. doi: 10.1371/journal.pone.0139883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Arnan M, Gudiol C, Calatayud L, Liñares J, Domínguez MA, Batlle M, Ribera JM, Carratalà J, Gudiol F. 2011. Risk factors for, and clinical relevance of, faecal extended-spectrum β-lactamase producing Escherichia coli (ESBL-EC) carriage in neutropenic patients with haematological malignancies. Eur J Clin Microbiol Infect Dis 30:355–360. doi: 10.1007/s10096-010-1093-x. [DOI] [PubMed] [Google Scholar]
- 9.Rodríguez-Baño J, Lopez-Cerero L, Navarro MD, de Alba PD, Pascual A. 2008. Faecal carriage of extended-spectrum beta-lactamase-producing Escherichia coli: prevalence, risk factors and molecular epidemiology. J Antimicrob Chemother 62:1142–1149. doi: 10.1093/jac/dkn293. [DOI] [PubMed] [Google Scholar]
- 10.Titelman E, Hasan CM, Iversen A, Nauclér P, Kais M, Kalin M, Giske CG. 2014. Faecal carriage of extended-spectrum β-lactamase-producing Enterobacteriaceae is common 12 months after infection and is related to strain factors. Clin Microbiol Infect 20:O508–O515. doi: 10.1111/1469-0691.12559. [DOI] [PubMed] [Google Scholar]
- 11.Woerther P-L, Micol J-B, Angebault C, Pasquier F, Pilorge S, Bourhis J-H, de Botton S, Gachot B, Chachaty E. 2015. Monitoring antibiotic-resistant enterobacteria faecal levels is helpful in predicting antibiotic susceptibility of bacteraemia isolates in patients with haematological malignancies. J Med Microbiol 64:676–681. doi: 10.1099/jmm.0.000078. [DOI] [PubMed] [Google Scholar]
- 12.Taur Y, Xavier JB, Lipuma L, Ubeda C, Goldberg J, Gobourne A, Lee YJ, Dubin KA, Socci ND, Viale A, Perales M-A, Jenq RR, van den Brink MRM, Pamer EG. 2012. Intestinal Domination and the Risk of Bacteremia in Patients Undergoing Allogeneic Hematopoietic Stem Cell Transplantation. Clin Infect Dis 55:905–914. doi: 10.1093/cid/cis580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shimasaki T, Seekatz A, Bassis C, Rhee Y, Yelin RD, Fogg L, Dangana T, Cisneros EC, Weinstein RA, Okamoto K, Lolans K, Schoeny M, Lin MY, Moore NM, Young VB, Hayden MK, Centers for Disease Control and Prevention Epicenters Program. 2019. Increased relative abundance of Klebsiella pneumoniae carbapenemase-producing Klebsiella pneumoniae within the gut microbiota is associated with risk of bloodstream infection in long-term acute care hospital patients. Clin Infect Dis 68:2053–2059. doi: 10.1093/cid/ciy796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Datta R, Platt R, Yokoe DS, Huang SS. 2011. Environmental cleaning intervention and risk of acquiring multidrug-resistant organisms from prior room occupants. Arch Intern Med 171:491–494. doi: 10.1001/archinternmed.2011.64. [DOI] [PubMed] [Google Scholar]
- 15.Bhalla A, Pultz NJ, Ray AJ, Hoyen CK, Eckstein EC, Donskey CJ. 2003. Antianaerobic antibiotic therapy promotes overgrowth of antibiotic-resistant, Gram-negative bacilli and vancomycin-resistant enterococci in the stool of colonized patients. Infect Control Hosp Epidemiol 24:644–649. doi: 10.1086/502267. [DOI] [PubMed] [Google Scholar]
- 16.Caballero S, Kim S, Carter RA, Leiner IM, Sušac B, Miller L, Kim GJ, Ling L, Pamer EG. 2017. Cooperating commensals restore colonization resistance to vancomycin-resistant Enterococcus faecium. Cell Host Microbe 21:592.e4–602.e4. doi: 10.1016/j.chom.2017.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ubeda C, Bucci V, Caballero S, Djukovic A, Toussaint NC, Equinda M, Lipuma L, Ling L, Gobourne A, No D, Taur Y, Jenq RR, van den Brink MRM, Xavier JB, Pamer EG. 2013. Intestinal microbiota containing Barnesiella Species cures vancomycin-resistant Enterococcus faecium colonization. Infect Immun 81:965–973. doi: 10.1128/IAI.01197-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Karachalios G, Charalabopoulos K. 2002. Biliary excretion of antimicrobial drugs. Chemotherapy 48:280–297. doi: 10.1159/000069712. [DOI] [PubMed] [Google Scholar]
- 19.Wilson AP. 2000. Clinical pharmacokinetics of teicoplanin. Clin Pharmacokinet 39:167–183. doi: 10.2165/00003088-200039030-00001. [DOI] [PubMed] [Google Scholar]
- 20.Schuetz AN, Reyes S, Tamma PD. 2018. Point-Counterpoint: Piperacillin-tazobactam should be used to treat infections with extended-spectrum-beta-lactamase-positive organisms. J Clin Microbiol 56:2173. doi: 10.1128/JCM.01917-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Harris PNA, Tambyah PA, Lye DC, Mo Y, Lee TH, Yilmaz M, Alenazi TH, Arabi Y, Falcone M, Bassetti M, Righi E, Rogers BA, Kanj S, Bhally H, Iredell J, Mendelson M, Boyles TH, Looke D, Miyakis S, Walls G, Al Khamis M, Zikri A, Crowe A, Ingram P, Daneman N, Griffin P, Athan E, Lorenc P, Baker P, Roberts L, Beatson SA, Peleg AY, Harris-Brown T, Paterson DL, MERINO Trial Investigators and the Australasian Society for Infectious Disease Clinical Research Network (ASID-CRN). 2018. Effect of piperacillin-tazobactam vs meropenem on 30-day mortality for patients with E. coli or Klebsiella pneumoniae bloodstream infection and ceftriaxone resistance. JAMA 320:984. doi: 10.1001/jama.2018.12163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hoyen CK, Pultz NJ, Paterson DL, Aron DC, Donskey CJ. 2003. Effect of parenteral antibiotic administration on establishment of intestinal colonization in mice by Klebsiella pneumoniae strains producing extended-spectrum beta-lactamases. Antimicrob Agents Chemother 47:3610–3612. doi: 10.1128/aac.47.11.3610-3612.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nord CE, Brismar B, Kasholm-Tengve B, Tunevall G. 1992. Effect of piperacillin/tazobactam therapy on intestinal microflora. Scand J Infect Dis 24:209–213. doi: 10.3109/00365549209052614. [DOI] [PubMed] [Google Scholar]
- 24.Isaac S, Scher JU, Djukovic A, Jiménez N, Littman DR, Abramson SB, Pamer EG, Ubeda C. 2017. Short- and long-term effects of oral vancomycin on the human intestinal microbiota. J Antimicrob Chemother 72:128–136. doi: 10.1093/jac/dkw383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nord CE, Kager L, Philipson A, Stiernstedt G. 1984. Impact of imipenem/cilastatin therapy on faecal flora. Eur J Clin Microbiol 3:475–477. doi: 10.1007/bf02017379. [DOI] [PubMed] [Google Scholar]
- 26.Sakata H, Fujita K, Yoshioka H. 1986. The effect of antimicrobial agents on fecal flora of children. Antimicrob Agents Chemother 29:225–229. doi: 10.1128/aac.29.2.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Brook I, Wexler HM, Goldstein E. 2013. Antianaerobic antimicrobials: spectrum and susceptibility testing. Clin Microbiol Rev 26:526–546. doi: 10.1128/CMR.00086-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Greenwood D. 1988. Microbiological properties of teicoplanin. J Antimicrob Chemother 21 Suppl A:1–13. doi: 10.1093/jac/21.suppl_a.1. [DOI] [PubMed] [Google Scholar]
- 29.Behra-Miellet J, Calvet L, Dubreuil L. 2003. Activity of linezolid against anaerobic bacteria. Int J Antimicrob Agents 22:28–34. doi: 10.1016/s0924-8579(03)00087-6. [DOI] [PubMed] [Google Scholar]
- 30.Falagas ME, Kasiakou SK. 2005. Colistin: the revival of polymyxins for the management of multidrug-resistant Gram-negative bacterial infections. Clin Infect Dis 40:1333–1341. doi: 10.1086/429323. [DOI] [PubMed] [Google Scholar]
- 31.Pultz NJ, Stiefel U, Donskey CJ. 2005. Effects of daptomycin, linezolid, and vancomycin on establishment of intestinal colonization with vancomycin-resistant enterococci and extended-spectrum-beta-lactamase-producing Klebsiella pneumoniae in mice. Antimicrob Agents Chemother 49:3513–3516. doi: 10.1128/AAC.49.8.3513-3516.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.