In the past decades, the incidence of cryptococcosis has increased dramatically, which poses a new threat to human health. However, only a few drugs are available for the treatment of cryptococcosis. Here, we described a leading compound, NT-a9, an analogue of isavuconazole, that showed strong antifungal activities in vitro and in vivo.
KEYWORDS: NT-a9, triazole compound, Cryptococcus neoformans, disseminated cryptococcosis, fungal burden
ABSTRACT
In the past decades, the incidence of cryptococcosis has increased dramatically, which poses a new threat to human health. However, only a few drugs are available for the treatment of cryptococcosis. Here, we described a leading compound, NT-a9, an analogue of isavuconazole, that showed strong antifungal activities in vitro and in vivo. NT-a9 showed a wide range of activities against several pathogenic fungi in vitro, including Cryptococcus neoformans, Cryptococcus gattii, Candida albicans, Candida krusei, Candida tropicalis, Candida glabrata, and Candida parapsilosis, with MICs ranging from 0.002 to 1 μg/ml. In particular, NT-a9 exhibited excellent efficacy against C. neoformans, with a MIC as low as 0.002 μg/ml. NT-a9 treatment resulted in changes in the sterol contents in C. neoformans, similarly to fluconazole. In addition, NT-a9 possessed relatively low cytotoxicity and a high selectivity index. The in vivo efficacy of NT-a9 was assessed using a murine disseminated-cryptococcosis model. Mice were infected intravenously with 1.8 × 106 CFU of C. neoformans strain H99. In the survival study, NT-a9 significantly prolonged the survival times of mice compared with the survival times of the control group or the isavuconazole-, fluconazole-, or amphotericin B-treated groups. Of note, 4 and 8 mg/kg of body weight of NT-a9 rescued all the mice, with a survival rate of 100%. In the fungal-burden study, NT-a9 also significantly reduced the fungal burdens in brains and lungs, while fluconazole and amphotericin B only reduced the fungal burden in lungs. Taken together, these data suggested that NT-a9 is a promising antifungal candidate for the treatment of cryptococcosis infection.
INTRODUCTION
In recent years, with the increases in high-risk populations like solid organ transplant and chemotherapy patients and patients with liver failure and immunosuppression, the incidence of invasive fungal infections (IFIs) has increased dramatically (1–3), which has also brought enormous medical and economic burdens to our communities. It is estimated that in the United States alone, the cost of fungal diseases exceeded $7.2 billion in 2017 (4). Among all IFIs, more than 50% are caused by Candida spp. (5). Although patients with cryptococcosis are fewer than those with candidiasis, cryptococcal infections are more serious and difficult to treat, because cryptococcal infections often invade the central nervous system, leading to cryptococcal meningitis. In addition, cryptococcosis often leaves survivors with central nervous system damage, which does not occur with candidiasis. Cryptococcal meningitis has become one of the leading causes of AIDS-related deaths (6–8). A study of HIV-related cryptococcal meningitis found that more than 220,000 cases of cryptococcal meningitis and approximately 180,000 deaths caused by cryptococcal meningitis occurred worldwide in 2014, with sub-Saharan Africa accounting for more than 70% of the estimated cases and deaths (9).
The development of antifungal drugs in the past decades has been extremely slow (10). For years, there was no new drug available for the treatment of cryptococcosis. Currently, the gold standard treatment for cryptococcal meningitis is based on amphotericin B. The treatment consists of induction therapy with amphotericin B and 5-flucytosine, followed by consolidation and maintenance therapy with fluconazole (11). However, the current treatment regime has disadvantages, including the adverse effects of amphotericin B and the lack of availability of therapy in resource-limited settings (12, 13). Therefore, there is an urgent need for new drugs to be available for the treatment of cryptococcosis.
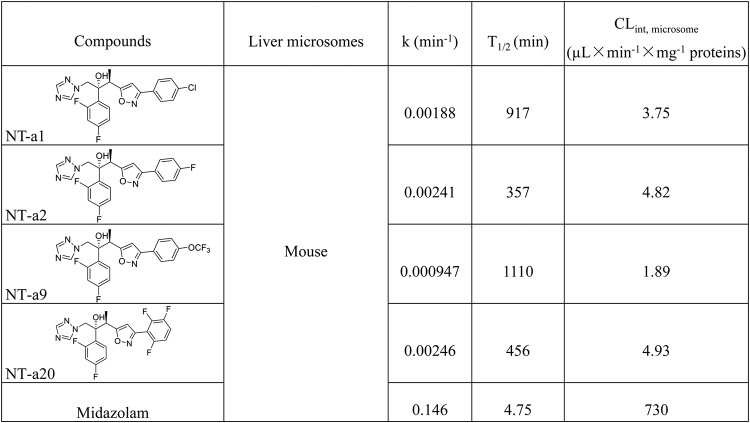
In our previous study, a series of isavuconazole analogues bearing an isoxazole motif were synthesized. A preliminary antifungal study found that these compounds had a wide range of antifungal ability against several pathogenic fungi (14). In this study, before translation of the in vitro antifungal activities to an in vivo disseminated-cryptococcosis mouse model, the metabolic stability of four selected compounds, NT-a1, NT-a2, NT-a9, and NT-a20, was assessed. According to the results shown in Table 1, compound NT-a9 showed low clearance when exposed to mouse liver microsomes, making it more worthy of further evaluation. Therefore, in this study, we investigated the in vitro efficacy of NT-a9 against several Cryptococcus and Candida strains. The in vivo efficacy of NT-a9 against disseminated cryptococcosis was also evaluated.
TABLE 1.
Stability of four compounds in mouse liver microsomes
RESULTS
NT-a9 showed significant antifungal activity against Candida species, while its strongest efficacy was against Cryptococcus neoformans.
In our previous study, NT-a9 had antifungal activity against Candida albicans strains with MICs of 0.0313 and 0.125 μg/ml, against Candida parapsilosis with a MIC of 0.5 μg/ml, against Candida glabrata with a MIC of 0.0625 μg/ml, and against Aspergillus fumigatus with a MIC of 8 μg/ml (14). In this study, we further investigated the in vitro susceptibility to NT-a9 of more strains of Cryptococcus neoformans, Cryptococcus gattii, Candida albicans, Candida krusei, Candida tropicalis, Candida glabrata, and Candida parapsilosis. The results showed that NT-a9 was highly active against all 32 strains tested, with MICs ranging from 0.002 to 1 μg/ml (Table 2). The efficacy of NT-a9 against Candida strains was comparable to that of isavuconazole and superior to those of fluconazole and amphotericin B. The MIC50s of NT-a9 ranged from 0.002 to 0.25 μg/ml. For most strains tested, the MICs of NT-a9 were only twice the MIC50 or even equal to it. In particular, NT-a9 exhibited its strongest effect against C. neoformans (2 standard reference strains and 16 clinical isolates), with MICs even lower than those of isavuconazole, 8- to 2,000-fold lower than those of fluconazole, and 8- to 250-fold lower than those of amphotericin B. Therefore, we further evaluated the in vivo effects of NT-a9 against C. neoformans.
TABLE 2.
MICs of NT-a9 against common pathogenic fungi
| Speciesa | Strain | Value (μg/ml) forb
: |
||||||
|---|---|---|---|---|---|---|---|---|
| NT-a9 |
ISA |
FLC |
AMB | |||||
| MIC | MIC50 | MIC | MIC50 | MIC | MIC50 | MIC | ||
| C. neoformans | H99 | 0.031 | 0.004 | 0.13 | 0.031 | 4 | 2 | 1 |
| C. neoformans | ATCC 32609 | 0.016 | 0.008 | 0.016 | 0.004 | 0.5 | 0.13 | 0.13 |
| C. neoformans | SCZ50100 | 0.008 | 0.002 | 0.063 | 0.016 | 4 | 2 | 1 |
| C. neoformans | SCZ50101 | 0.002 | 0.002 | 0.031 | 0.004 | 4 | 1 | 0.5 |
| C. neoformans | SCZ50102 | 0.008 | 0.004 | 0.063 | 0.031 | 8 | 4 | 1 |
| C. neoformans | SCZ50104 | 0.008 | 0.002 | 0.063 | 0.016 | 4 | 2 | 1 |
| C. neoformans | SCZ50106 | 0.008 | 0.002 | 0.063 | 0.031 | 8 | 4 | 1 |
| C. neoformans | SCZ50107 | 0.008 | 0.002 | 0.063 | 0.031 | 8 | 4 | 1 |
| C. neoformans | HN2-40 | 0.063 | 0.031 | 0.13 | 0.063 | 4 | 2 | 2 |
| C. neoformans | HN15 | 0.25 | 0.25 | 0.063 | 0.063 | 2 | 1 | 4 |
| C. neoformans | HN17 | 0.063 | 0.063 | 0.063 | 0.063 | 4 | 2 | 1 |
| C. neoformans | HN19 | 0.063 | 0.063 | 0.063 | 0.031 | 4 | 2 | 1 |
| C. neoformans | HN20 | 0.063 | 0.063 | 0.13 | 0.063 | 4 | 2 | 2 |
| C. neoformans | BJ3 | 0.063 | 0.063 | 0.13 | 0.008 | 4 | 0.13 | 2 |
| C. neoformans | BJ72 | 0.063 | 0.063 | 0.13 | 0.031 | 4 | 1 | 2 |
| C. neoformans | BJ95 | 0.063 | 0.063 | 0.13 | 0.008 | 4 | 0.5 | 2 |
| C. neoformans | SH68 | 0.063 | 0.063 | 0.13 | 0.008 | 4 | 0.13 | 1 |
| C. gattii | WM178 | 0.13 | 0.063 | 0.13 | 0.063 | 8 | 4 | 1 |
| C. gattii | WM179 | 0.063 | 0.016 | 0.13 | 0.016 | 4 | 2 | 2 |
| C. gattii | E566 | 0.063 | 0.063 | 0.13 | 0.031 | 4 | 2 | 2 |
| C. gattii | SCZ20024 | 0.13 | 0.063 | 0.13 | 0.031 | 4 | 1 | 4 |
| C. gattii | SCZ20031 | 0.063 | 0.063 | 0.13 | 0.031 | 4 | 1 | 2 |
| C. albicans | SC5314 | 0.13 | 0.063 | 0.13 | 0.031 | 0.5 | 0.13 | 0.13 |
| C. albicans | 333 | 0.25 | 0.13 | 0.13 | 0.031 | 1 | 0.25 | 0.25 |
| C. krusei | 463 | 1 | 0.13 | 0.13 | 0.063 | 4 | 1 | 0.5 |
| C. krusei | 397 | 0.13 | 0.063 | 0.13 | 0.063 | 2 | 1 | 1 |
| C. krusei | 467 | 0.13 | 0.13 | 0.13 | 0.13 | 0.5 | 0.25 | 0.25 |
| C. tropicalis | ATCC 750 | 0.25 | 0.13 | 0.5 | 0.13 | 2 | 1 | 0.5 |
| C. tropicalis | 112936 | 0.25 | 0.13 | 1 | 0.13 | 2 | 0.5 | 0.5 |
| C. glabrata | ATCC 2001 | 0.13 | 0.063 | 0.13 | 0.063 | 2 | 1 | 1 |
| C. glabrata | 537 | 0.13 | 0.063 | 0.13 | 0.063 | 0.5 | 0.25 | 0.5 |
| C. parapsilosis | 9018 | 0.13 | 0.063 | 0.13 | 0.063 | 0.25 | 0.13 | 0.13 |
Cryptococcus species: C. neoformans and C. gattii. Candida species: C. albicans, C. krusei, C. tropicalis, C. glabrata, and C. parapsilosis.
ISA, isavuconazole; FLC, fluconazole; AMB, amphotericin B.
NT-a9 is fungistatic against C. neoformans and C. albicans.
A time-kill assay was carried out against C. neoformans and C. albicans. As shown by the results in Fig. 1, compared with the results for the control, NT-a9 significantly inhibited the growth of C. neoformans H99 (Fig. 1A) and C. albicans strain SC5314 (Fig. 1B). NT-a9 showed an inhibitory effect similar to but slightly better than that of fluconazole, exhibiting a fungistatic effect against both strains.
FIG 1.
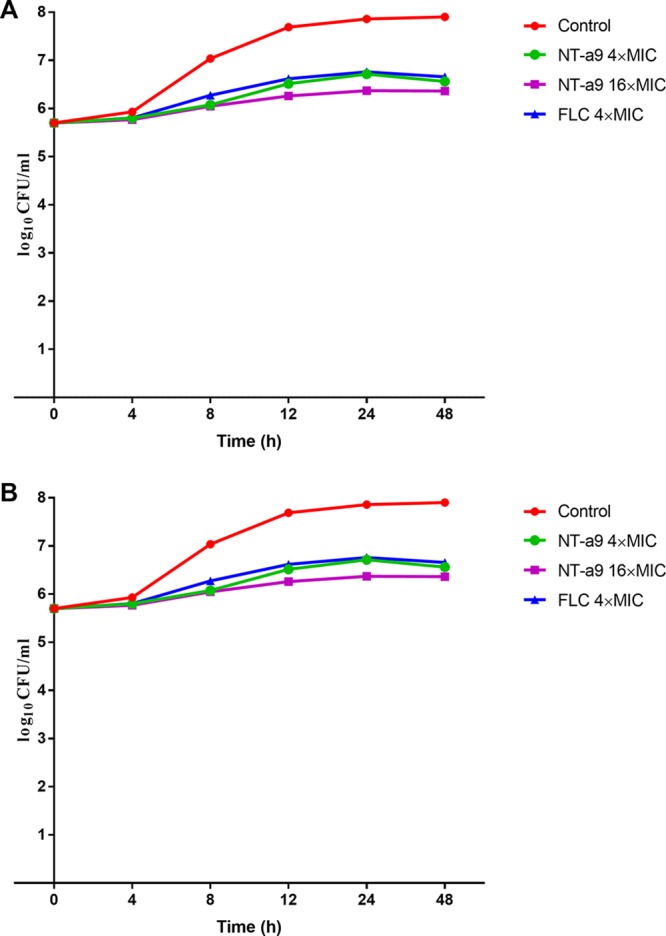
Time-kill curves of C. neoformans strain H99 (A) and C. albicans strain SC5314 (B) treated with NT-a9 or fluconazole (FLC). Strains were grown in RPMI 1640 medium with a starting inoculum of 5 × 105 CFU/ml. Aliquots were obtained at the predetermined time points, and serial dilutions were spread on SDA plates. Colony counts were determined after incubation. The results are calculated from two independent experiments.
NT-a9 treatment affected the sterol biosynthesis of C. neoformans.
To reveal the antifungal mechanism of NT-a9 against C. neoformans, total sterols of C. neoformans H99 were extracted and analyzed after treatment with NT-a9 or fluconazole. As shown by the results in Fig. 2, in the control group, most of the sterol contents were ergosterol, accounting for more than 90%. Treatment with NT-a9 resulted in a significant decrease in ergosterol content, to less than 10%. Meanwhile, the content of obtusifoliol increased and eburicol became the main component of the total sterols. Importantly, these changes were consistent with those in fluconazole-treated groups. These results showed that NT-a9 treatment affected the sterol biosynthesis of C. neoformans and suggested the compound might inhibit fungal CYP51 similarly to fluconazole.
FIG 2.
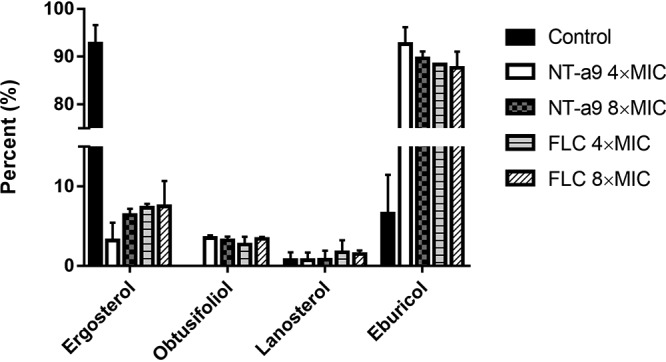
Sterol analysis of C. neoformans cells by GC-MS. C. neoformans H99 was treated with NT-a9 or fluconazole (FLC) for 8 h. Cells were collected, and total sterols were extracted. Sterol compositions were analyzed by GC-MS. Data are shown as the mean values ± standard deviations from two independent experiments.
NT-a9 had relatively low toxicity against mammalian cells and a high selectivity index.
The cytotoxicity of NT-a9 was assessed using human umbilical vein endothelial cells (HUVECs). A concentration of 4 μg/ml of NT-a9 had no cytotoxicity against HUVECs, and 8 μg/ml of NT-a9 began to show an observable inhibitory effect on cell viability (Fig. 3). The selectivity index, which here is the ratio of the IC50 against HUVECs and the MIC against C. neoformans, can indicate the safety of a compound to some extent (15). The 50% inhibitory concentration (IC50) of NT-a9 against HUVECs was 13.66 μg/ml, and the MIC against C. neoformans was 0.031, which gave NT-a9 a high selectivity index of 440. In conclusion, NT-a9 showed good safety properties.
FIG 3.
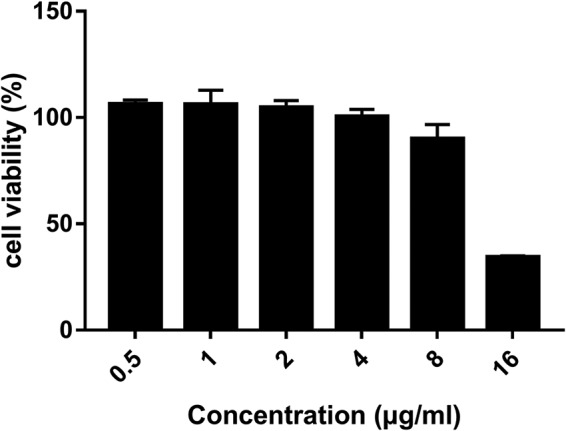
Cytotoxicity results for NT-a9 against HUVECs. A total of 1 × 105 cells/ml of HUVECs in DMEM containing 10% fetal bovine serum (FBS) were seeded in 96-well tissue culture plates and incubated for 3 h for adhesion. After incubation, the supernatant was removed and fresh DMEM with different concentrations of NT-a9 was added. The plates were incubated for an additional 24 h at 37°C with 5% CO2. After incubation, 10 μl of CCK-8 solution was added to each well and the plates were incubated at 37°C for 2 h. Cell viability was assessed by measuring absorbance at 450 nm. Data are shown as the mean values ± standard deviations for three independent experiments.
NT-a9 increased the survival rate and prolonged the survival time in the disseminated-cryptococcosis mouse model.
In the 20-day survival experiment, NT-a9 exhibited potent efficacy in the disseminated-cryptococcosis mouse model. First, we assessed the in vivo effects of NT-a9 at doses of 4 and 8 mg/kg of body weight, with isavuconazole, fluconazole, and amphotericin B as positive controls (Fig. 4A). The results showed that 4 mg/kg and 8 mg/kg of NT-a9 protected all the mice through the observation period and significantly improved the survival rates. NT-a9 extended the median survival time of mice by 12.1 days compared with the median survival time of the control group. In contrast, isavuconazole showed no therapeutic effect against disseminated cryptococcosis. In order to determine the effective dose of NT-a9, we reduced the dosage to 0.5, 1, 2, and 4 mg/kg (Fig. 4B). All the mice in the vehicle control group were dead between 6 and 9 days postinoculation, with a median survival time of 7.4 days. Compared with the survival time for the control group, NT-a9 significantly prolonged the survival time in mice at all four doses tested (P < 0.001 for all comparisons). The median survival times of groups that were treated with 0.5, 1, and 2 mg/kg of NT-a9 were 11.8, 15.9, and 16 days, respectively. Similarly, 4 mg/kg of NT-a9 rescued all the mice without death. In addition, there were statistically significant differences in survival times between the NT-a9-treated groups and the fluconazole- and amphotericin B-treated groups (P < 0.001 for all comparisons). Both the fluconazole-treated mice and the amphotericin B-treated ones died within 14 days postinfection. Therefore, it can be concluded that NT-a9 has a much stronger protective effect than fluconazole or amphotericin B in mice with C. neoformans infection.
FIG 4.
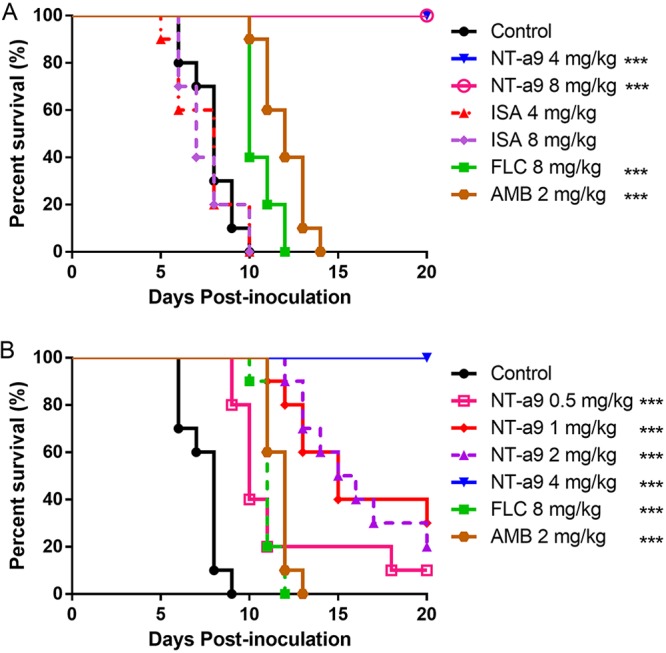
Survival curves for mice with disseminated cryptococcosis treated with antifungal agents. Mice were inoculated intravenously with C. neoformans H99 at 1.8 × 106 CFU per animal and then treated with NT-a9, isavuconazole (ISA), fluconazole (FLC), or amphotericin B (AMB) (n = 10 mice per group). The differences in in vivo effects between high doses (A) and low doses (B) of NT-a9 were investigated. Antifungal monotherapy was initiated 2 h after inoculation and continued for 7 days. Mice were monitored without therapy until day 20. Amphotericin B was administered intraperitoneally, while other antifungal treatments were performed through oral gavage. ***, P < 0.001 versus the results for the control. One representative result out of two independent experiments is shown.
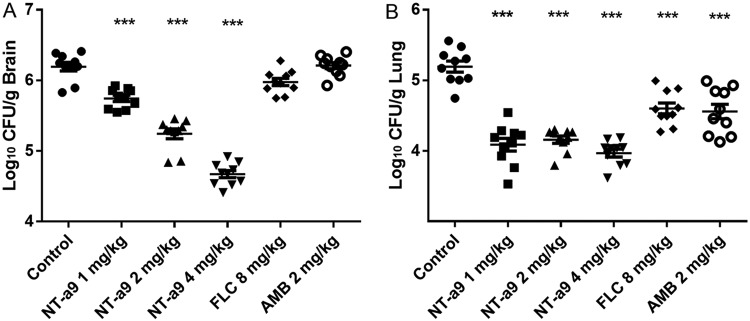
NT-a9 reduced the fungal burden in both brain and lungs in the disseminated-cryptococcosis mouse model.
To further investigate the in vivo effects of NT-a9, a fungal burden study was also carried out in the disseminated-cryptococcosis mouse model. Following 3 days of antifungal treatment, we examined the changes in the fungal burdens in the brains and lungs, which are the organs most vulnerable to C. neoformans infection. As shown by the results in Fig. 5, significant reductions in fungal burdens were observed with the NT-a9 treatment. In brains, the CFU counts for all doses in the NT-a9-treated groups were significantly lower than that of the control group: the mean values for the treatment groups were 5.75, 5.24, and 4.67 Log10 CFU/g, while the mean value for the control group was 6.19 Log10 CFU/g (P < 0.001 for all comparisons) (Fig. 5A). However, fluconazole and amphotericin B did not demonstrate significant reductions in fungal burdens in brains compared with the fungal burden in the control group. In contrast, all the treatments demonstrated significant reductions in fungal burdens in lungs (P < 0.001 for all comparison) (Fig. 5B). Each dose of NT-a9 showed a similar reduction in the fungal burden in lungs. Fluconazole and amphotericin B also led to significant decreases in fungal burdens in lungs. Still, there were significant differences between the NT-a9 treatment groups and the fluconazole or amphotericin B group.
FIG 5.
Fungal burdens in mice with disseminated cryptococcosis treated with antifungal agents. Mice were inoculated intravenously with C. neoformans H99 at 1.8 × 106 CFU per animal and treated with NT-a9, fluconazole, or amphotericin B (n = 10 mice per group). Antifungal monotherapy was initiated 1 day after inoculation and continued for 3 days. Mice were humanely euthanized 1 day after the last treatment, and brain (A) and lung (B) samples were collected for fungal burden analysis. Amphotericin B was administered intraperitoneally, while other treatments were performed through oral gavage. Horizontal lines represent mean values, and whiskers represent standard deviations. ***, P < 0.001 versus the result for the control. One representative result out of two independent experiments is shown.
DISCUSSION
Currently, fluconazole is the first-line drug for the treatment of cryptococcosis in the clinic. However, Cryptococcus strains have developed resistance to fluconazole in recent years (16, 17). In this study, the triazole compound NT-a9 exhibited potent antifungal activity in vitro and in vivo. The in vitro activities against C. neoformans and C. gattii were comparable to those of VT-1129, a new tetrazole compound that is undergoing clinical trials (18). Not surprisingly, the time-kill curve experiment demonstrated that NT-a9 is fungistatic rather than fungicidal, like most of the azole compounds (19, 20). The classical antifungal mechanism of azole drugs is to inhibit CYP51 enzyme activity and ergosterol biosynthesis, resulting in changes in cell membrane components. Sterol analysis by gas chromatography-mass spectrometry (GC-MS) revealed that NT-a9 treatment decreased the ergosterol content of C. neoformans. Some azole drugs have cytotoxicity, such as hepatocellular toxicity, which is responsible for the clinical liver damage caused by antifungal therapy (21, 22). A cytotoxicity assay showed that NT-a9 had relatively low toxicity for mammalian cells, indicating that it is relatively safe.
The results of survival and fungal burden experiments demonstrated that NT-a9 also has potent efficacy in the murine model of disseminated cryptococcosis caused by C. neoformans. NT-a9 significantly improved the survival times, and the survival benefit reached 100% at the doses of 4 and 8 mg/kg. In the assessment of brain fungal burdens, NT-a9 decreased the fungal burden in a dose-dependent manner. In contrast, treatment with fluconazole and amphotericin B did not reduce the brain fungal burdens. We hypothesized that the poor efficacy of amphotericin B treatment could be attributed to the high CFU count in the brain and the resistance of the blood-brain barrier to passage of amphotericin B. In lungs, fungal burdens were reduced in all the antifungal treatment groups.
NT-a9 is a triazole compound, and thus, it may exert antifungal effects by inhibiting the CYP enzyme. Isavuconazole, the latest triazole drug, is effective against C. neoformans in vitro, but it did not demonstrate a protective effect in disseminated cryptococcosis in mice. The reason for this difference in in vivo efficacies between isavuconazole and NT-a9 and the mechanism of the antifungal action of NT-a9 will need further study to reveal. In future studies, we will examine the effects of NT-a9 on the fungal CYP51 enzyme and its pharmacokinetic characteristics, such as plasma and brain tissue drug concentrations.
The synthesis of NT-a9 is not difficult. Therefore, if large-scale production is carried out, it could be more cost effective than the production of amphotericin B, considering that the production cost of the amphotericin B lipid formulations is high and out of the reach of patients (23). Further study of oral bioavailability will be required to determine whether NT-a9 is suitable as an oral formulation. According to the current research results, NT-a9 inhibits the biosynthesis of sterols and is a fungistatic agent. Therefore, the long-term use of NT-a9 may also result in resistance, similarly to other azole drugs (24).
In conclusion, this study has demonstrated the potent in vitro efficacy of the triazole derivative compound NT-a9 against C. neoformans, C. gattii, C. albicans, C. krusei, C. tropicalis, C. glabrata, and C. parapsilosis. More importantly, NT-a9 is especially effective in a murine disseminated-cryptococcosis model and is superior to the current standard-of-care regimen. Therefore, NT-a9 will be studied further as a promising antifungal candidate.
MATERIALS AND METHODS
Strains, media, and reagents.
C. neoformans strains H99 and ATCC 32609 and C. albicans, C. krusei, C. tropicalis, C. glabrata, and C. parapsilosis strains were from the fungus collection of our laboratory. C. neoformans clinical isolates (strains SCZ50100, SCZ50101, SCZ50102, SCZ50104, SCZ50106, SCZ50107, HN2-40, HN15, HN17, HN19, HN20, BJ3, BJ72, BJ95, and SH68), C. gattii standard reference strains (strains WM178, WM179, and E566), and C. gattii clinical isolates (strains SCZ20024 and SCZ20031) were obtained from Shanghai Changzheng Hospital. Prior to each in vitro and in vivo experiment, colonies of each strain were cultured in yeast extract-peptone-dextrose (YEPD) medium overnight at 30°C in a shaking incubator. The cells were then collected by centrifugation and washed three times in phosphate-buffered saline (PBS). The cell concentrations were determined using a hemocytometer. Fluconazole was purchased from Pfizer. Dulbecco’s modified Eagle’s medium (DMEM), cell counting kit-8 (CCK-8), and amphotericin B were purchased from Meilun Biotechnology. Isavuconazole powder (≥99% pure) was obtained from Shanghai Pharmaceuticals Holding Co., Ltd.
Compound acquisition and synthesis.
The new triazole derivative NT-a9 was prepared by using an efficient and straightforward 1,3-dipolarcyclo addition reaction, which has been previously reported by our group (14).
In vitro susceptibility test.
To determine the in vitro antimicrobial activity, a broth microdilution susceptibility assay was carried out in RPMI 1640 medium in accordance with the Clinical and Laboratory Standards Institute (CLSI) guidelines in CLSI document M27-A3 (25). In brief, strains in RPMI 1640 medium (final concentration, ∼1 × 103 cells/ml) were prepared in 96-well plates, and antifungal agents at 2× concentrations were added. The final concentrations ranged from 0.002 to 1 μg/ml for NT-a9, from 0.008 to 4 μg/ml for isavuconazole, and from 0.03 to 16 μg/ml for fluconazole and amphotericin B. The plates were incubated at 35°C for 48 h (C. albicans, C. krusei, C. tropicalis, C. glabrata, and C. parapsilosis) or 72 h (C. neoformans and C. gattii). The results were determined visually after incubation. The MIC and MIC50 were determined according to inhibition of visible growth compared to that in the control and reduction in turbidity compared to that of the control, respectively. All experiments were done in duplicate.
Time-kill curves.
Strains were grown in RPMI 1640 medium with a starting inoculum of 5 × 105 CFU/ml. NT-a9 was tested over a range of concentrations of 0 (control), 4, and 16 times the MIC for each tested strain. Fluconazole was tested at 4 times the MIC for each tested strain. At predetermined time points after incubation with agitation at 30°C, 100-μl aliquots were obtained from each solution and serially diluted in PBS. A 100-μl aliquot from each dilution was spread on a Sabouraud dextrose agar (SDA) plate. Colony counts were determined after incubation at 35°C for 72 h (C. neoformans) or 48 h (C. albicans). Fungicidal activity was defined as a ≥3-log reduction in CFU (26). All experiments were performed in duplicate.
Sterol extraction and analysis.
The effect of NT-a9 treatment on sterol biosynthesis in C. neoformans was determined by a method reported previously, with slight modifications (27). Briefly, overnight-cultured C. neoformans H99 cells were inoculated into 100 ml of YEPD at a final concentration of 5 × 106 cells/ml with NT-a9 or fluconazole. After incubation at 30°C for 8 h, cells were collected and washed twice with distilled water. Then, 6 ml of 15% NaOH resolved in 90% ethanol was added to 0.5 g (wet weight) cells. The mixture was incubated at 80°C for 1 h. Sterols were extracted three times with 5 ml of hexane. The extract was washed once with water and evaporated in a water bath to obtain the total sterols. Sterols were analyzed by gas chromatography-mass spectrometry (GC-MS) and identified by relative retention times and mass spectra compared with the sterol profiles of NIST. All experiments were performed in duplicate.
Cytotoxicity test.
HUVECs were used to evaluate the toxicity of NT-a9 (28). A total of 1 × 105 cells/ml of HUVECs in DMEM containing 10% fetal bovine serum (FBS) was seeded in 96-well tissue culture plates and incubated for 3 h for adhesion. After incubation, the supernatant was removed and fresh DMEM with different concentrations of NT-a9 was added. The plates were incubated for an additional 24 h at 37°C with 5% CO2. Cytotoxicity was assessed by using the CCK-8 assay (29). After incubation, 10 μl of CCK-8 solution was added to each well and the plates were incubated at 37°C for 2 h. Cell viability was assessed by measuring absorbance at 450 nm. Cells incubated in DMEM without NT-a9 treatment were used as the standard for 100% viability. Three independent experiments were conducted.
Animal model and ethics.
All animal experiments were done according to institutional guidelines and were approved by the Institutional Animal Care and Use Committee (IACUC) of Second Military Medical University. An established murine model of disseminated cryptococcosis with some modifications was used to evaluate the in vivo efficacy of NT-a9 (30). ICR female mice weighing 18 to 22 g were used in the study. To establish disseminated cryptococcosis, mice were injected with 1.8 × 106 CFU of C. neoformans H99 intravenously via the lateral tail vein.
Survival.
In the survival study, antifungal treatments began 2 h after inoculation of C. neoformans and lasted for 7 days. The different treatment groups received the vehicle control, 0.5, 1, 2, 4, or 8 mg/kg of NT-a9, 4 or 8 mg/kg of isavuconazole, 8 mg/kg of fluconazole, or 2 mg/kg of amphotericin B. Amphotericin B was dissolved in normal saline, and the other agents were dissolved in 0.5% sodium carboxymethyl cellulose (CMC-Na). The fluids were administered to mice in 0.2-ml amounts. Amphotericin B was administered intraperitoneally, while other antifungal treatments were performed through oral gavage. Mice were monitored and recorded for survival conditions twice daily for a total period of 20 days postinfection. At the end of the observation period, the surviving mice were humanely sacrificed. All experiments were performed in duplicate.
Fungal burdens.
In the fungal burden study, antifungal treatments began 1 day after fungal inoculation and continued for 3 days. The day after therapy had stopped, mice were humanely euthanized and brains and lungs were collected for quantitative determination of the tissue fungal burdens. After brain and lung weights were determined, tissues were homogenized in 1 ml PBS. Homogenates were serially diluted in 10-fold steps, and aliquots (100 μl) of the homogenates were plated on SDA plates. The plates were incubated at 30°C for 72 h, and the numbers of CFU were counted. The fungal burdens were indicated as Log10 CFU/g. All experiments were performed in duplicate.
Statistics.
Survival was plotted by Kaplan-Meier analysis, and the log rank test was used to assess whether differences in median survival times were significant. Differences in fungal burdens between groups were assessed for significance by analysis of variance (ANOVA) with Tukey’s posttest for multiple comparisons. A P value of ≤0.05 was considered statistically significant.
ACKNOWLEDGMENTS
We thank Wan-Qing Liao (Shanghai Institute of Medical Mycology, Changzheng Hospital, Second Military Medical University, Shanghai, China) for providing clinical isolates of C. neoformans and C. gattii strains.
This work was supported by the National Natural Science Foundation of China (grants no. 81673280, 81573473, and 81830106) and Natural Science Foundation of Shanghai (grant no. 17ZR1437700).
REFERENCES
- 1.Baker AW, Maziarz EK, Arnold CJ, Johnson MD, Workman AD, Reynolds JM, Perfect JR, Alexander BD. 25 February 2019. Invasive fungal infection after lung transplantation: epidemiology in the setting of antifungal prophylaxis. Clin Infect Dis doi: 10.1093/cid/ciz156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Netelenbos T, Massey E, de Wreede LC, Harding K, Hamblin A, Sekhar M, Li A, Ypma PF, Ball L, Zwaginga JJ, Stanworth SJ. 2019. The burden of invasive infections in neutropenic patients: incidence, outcomes, and use of granulocyte transfusions. Transfusion 59:160–168. doi: 10.1111/trf.14994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Verma N, Singh S, Taneja S, Duseja A, Singh V, Dhiman RK, Chakrabarti A, Chawla YK. 2019. Invasive fungal infections amongst patients with acute-on-chronic liver failure at high risk for fungal infections. Liver Int 39:503–513. doi: 10.1111/liv.13981. [DOI] [PubMed] [Google Scholar]
- 4.Benedict K, Jackson BR, Chiller T, Beer KD. 2018. Estimation of direct healthcare costs of fungal diseases in the United States. Clin Infect Dis 68:1791–1797. doi: 10.1093/cid/ciy776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Webb BJ, Ferraro JP, Rea S, Kaufusi S, Goodman BE, Spalding J. 2018. Epidemiology and clinical features of invasive fungal infection in a US health care network. Open Forum Infect Dis 5:ofy187. doi: 10.1093/ofid/ofy187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Park BJ, Wannemuehler KA, Marston BJ, Govender N, Pappas PG, Chiller TM. 2009. Estimation of the current global burden of cryptococcal meningitis among persons living with HIV/AIDS. AIDS 23:525–530. doi: 10.1097/QAD.0b013e328322ffac. [DOI] [PubMed] [Google Scholar]
- 7.Lortholary O, Poizat G, Zeller V, Neuville S, Boibieux A, Alvarez M, Dellamonica P, Botterel F, Dromer F, Chene G. 2006. Long-term outcome of AIDS-associated cryptococcosis in the era of combination antiretroviral therapy. AIDS 20:2183–2191. doi: 10.1097/01.aids.0000252060.80704.68. [DOI] [PubMed] [Google Scholar]
- 8.Armstrong-James D, Meintjes G, Brown GD. 2014. A neglected epidemic: fungal infections in HIV/AIDS. Trends Microbiol 22:120–127. doi: 10.1016/j.tim.2014.01.001. [DOI] [PubMed] [Google Scholar]
- 9.Rajasingham R, Smith RM, Park BJ, Jarvis JN, Govender NP, Chiller TM, Denning DW, Loyse A, Boulware DR. 2017. Global burden of disease of HIV-associated cryptococcal meningitis: an updated analysis. Lancet Infect Dis 17:873–881. doi: 10.1016/S1473-3099(17)30243-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Roemer T, Krysan DJ. 2014. Antifungal drug development: challenges, unmet clinical needs, and new approaches. Cold Spring Harb Perspect Med 4:a019703. doi: 10.1101/cshperspect.a019703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Perfect JR, Dismukes WE, Dromer F, Goldman DL, Graybill JR, Hamill RJ, Harrison TS, Larsen RA, Lortholary O, Nguyen MH, Pappas PG, Powderly WG, Singh N, Sobel JD, Sorrell TC. 2010. Clinical practice guidelines for the management of cryptococcal disease: 2010 update by the Infectious Diseases Society of America. Clin Infect Dis 50:291–322. doi: 10.1086/649858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Molloy SF, Kanyama C, Heyderman RS, Loyse A, Kouanfack C, Chanda D, Mfinanga S, Temfack E, Lakhi S, Lesikari S, Chan AK, Stone N, Kalata N, Karunaharan N, Gaskell K, Peirse M, Ellis J, Chawinga C, Lontsi S, Ndong JG, Bright P, Lupiya D, Chen T, Bradley J, Adams J, van der Horst C, van Oosterhout JJ, Sini V, Mapoure YN, Mwaba P, Bicanic T, Lalloo DG, Wang D, Hosseinipour MC, Lortholary O, Jaffar S, Harrison TS. 2018. Antifungal combinations for treatment of cryptococcal meningitis in Africa. N Engl J Med 378:1004–1017. doi: 10.1056/NEJMoa1710922. [DOI] [PubMed] [Google Scholar]
- 13.Lofgren S, Abassi M, Rhein J, Boulware DR. 2017. Recent advances in AIDS-related cryptococcal meningitis treatment with an emphasis on resource limited settings. Expert Rev Anti Infect Ther 15:331–340. doi: 10.1080/14787210.2017.1285697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Xie F, Ni T, Zhao J, Pang L, Li R, Cai Z, Ding Z, Wang T, Yu S, Jin Y, Zhang D, Jiang Y. 2017. Design, synthesis, and in vitro evaluation of novel antifungal triazoles. Bioorg Med Chem Lett 27:2171–2173. doi: 10.1016/j.bmcl.2017.03.062. [DOI] [PubMed] [Google Scholar]
- 15.Lazzarini C, Haranahalli K, Rieger R, Ananthula HK, Desai PB, Ashbaugh A, Linke MJ, Cushion MT, Ruzsicska B, Haley J, Ojima I, Del Poeta M. 2018. Acylhydrazones as antifungal agents targeting the synthesis of fungal sphingolipids. Antimicrob Agents Chemother 62:e00156-18. doi: 10.1128/AAC.00156-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bongomin F, Oladele RO, Gago S, Moore CB, Richardson MD. 2018. A systematic review of fluconazole resistance in clinical isolates of Cryptococcus species. Mycoses 61:290–297. doi: 10.1111/myc.12747. [DOI] [PubMed] [Google Scholar]
- 17.Nasri H, Kabbani S, Bou Alwan M, Wang YF, Rebolledo PA, Kraft CS, Nguyen ML, Anderson AM, Rouphael N. 2016. Retrospective study of cryptococcal meningitis with elevated minimum inhibitory concentration to fluconazole in immunocompromised patients. Open Forum Infect Dis 3:ofw076. doi: 10.1093/ofid/ofw076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lockhart SR, Fothergill AW, Iqbal N, Bolden CB, Grossman NT, Garvey EP, Brand SR, Hoekstra WJ, Schotzinger RJ, Ottinger E, Patterson TF, Wiederhold NP. 2016. The investigational fungal Cyp51 inhibitor VT-1129 demonstrates potent in vitro activity against Cryptococcus neoformans and Cryptococcus gattii. Antimicrob Agents Chemother 60:2528–2531. doi: 10.1128/AAC.02770-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Manavathu EK, Cutright JL, Chandrasekar PH. 1998. Organism-dependent fungicidal activities of azoles. Antimicrob Agents Chemother 42:3018–3021. doi: 10.1128/AAC.42.11.3018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Calabrese EC, Castellano S, Santoriello M, Sgherri C, Quartacci MF, Calucci L, Warrilow AG, Lamb DC, Kelly SL, Milite C, Granata I, Sbardella G, Stefancich G, Maresca B, Porta A. 2013. Antifungal activity of azole compounds CPA18 and CPA109 against azole-susceptible and -resistant strains of Candida albicans. J Antimicrob Chemother 68:1111–1119. doi: 10.1093/jac/dks506. [DOI] [PubMed] [Google Scholar]
- 21.Kyriakidis I, Tragiannidis A, Munchen S, Groll AH. 2017. Clinical hepatotoxicity associated with antifungal agents. Expert Opin Drug Saf 16:149–165. doi: 10.1080/14740338.2017.1270264. [DOI] [PubMed] [Google Scholar]
- 22.Haegler P, Joerin L, Krahenbuhl S, Bouitbir J. 2017. Hepatocellular toxicity of imidazole and triazole antimycotic agents. Toxicol Sci 157:183–195. doi: 10.1093/toxsci/kfx029. [DOI] [PubMed] [Google Scholar]
- 23.Kleinberg M. 2006. What is the current and future status of conventional amphotericin B? Int J Antimicrob Agents 27(Suppl 1):12–16. doi: 10.1016/j.ijantimicag.2006.03.013. [DOI] [PubMed] [Google Scholar]
- 24.Cuenca-Estrella M. 2014. Antifungal drug resistance mechanisms in pathogenic fungi: from bench to bedside. Clin Microbiol Infect 20(Suppl 6):54–59. doi: 10.1111/1469-0691.12495. [DOI] [PubMed] [Google Scholar]
- 25.CLSI. 2008. Reference method for broth dilution antifungal susceptibility testing of yeasts; approved standard, 3rd ed CLSI document M27-A3 Clinical and Laboratory Standards Institute, Wayne, PA. [Google Scholar]
- 26.Scorneaux B, Angulo D, Borroto-Esoda K, Ghannoum M, Peel M, Wring S. 2017. SCY-078 is fungicidal against Candida species in time-kill studies. Antimicrob Agents Chemother 61:e01961-16. doi: 10.1128/AAC.01961-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Venkateswarlu K, Denning DW, Manning NJ, Kelly SL. 1996. Comparison of D0870, a new triazole antifungal agent, to fluconazole for inhibition of Candida albicans cytochrome P-450 by using in vitro assays. Antimicrob Agents Chemother 40:1382–1386. doi: 10.1128/AAC.40.6.1382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li MY, Lv YC, Tong LJ, Peng T, Qu R, Zhang T, Sun YM, Chen Y, Wei LX, Geng MY, Duan WH, Xie H, Ding J. 2016. DW10075, a novel selective and small-molecule inhibitor of VEGFR, exhibits antitumor activities both in vitro and in vivo. Acta Pharmacol Sin 37:398–407. doi: 10.1038/aps.2015.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Xie X, Zhang Y, Ma W, Shao X, Zhan Y, Mao C, Zhu B, Zhou Y, Zhao H, Cai X. 2019. Potent anti-angiogenesis and anti-tumour activity of pegaptanib-loaded tetrahedral DNA nanostructure. Cell Prolif 52:e12662. doi: 10.1111/cpr.12662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wiederhold NP, Najvar LK, Bocanegra R, Kirkpatrick WR, Sorrell TC, Patterson TF. 2013. Limited activity of miltefosine in murine models of cryptococcal meningoencephalitis and disseminated cryptococcosis. Antimicrob Agents Chemother 57:745–750. doi: 10.1128/AAC.01624-12. [DOI] [PMC free article] [PubMed] [Google Scholar]