The activities of meropenem-vaborbactam and comparators against 152 (1.1%) carbapenem-resistant Enterobacterales (CRE) isolates identified among 13,929 Enterobacterales isolates collected from U.S. hospitals during 2016 to 2018 were evaluated. CRE rates were higher in the Middle Atlantic census division (3.5%) than in the other divisions (range, 0.0% for the West North Central division to 1.4% for the West South Central division).
KEYWORDS: CRE, Enterobacterales, outer membrane protein
ABSTRACT
The activities of meropenem-vaborbactam and comparators against 152 (1.1%) carbapenem-resistant Enterobacterales (CRE) isolates identified among 13,929 Enterobacterales isolates collected from U.S. hospitals during 2016 to 2018 were evaluated. CRE rates were higher in the Middle Atlantic census division (3.5%) than in the other divisions (range, 0.0% for the West North Central division to 1.4% for the West South Central division). Among the CRE isolates, 134 carried carbapenemase genes, and these included 72 isolates carrying blaKPC-3, 51 isolates carrying blaKPC-2, 4 isolates carrying blaNDM-1, 3 isolates carrying blaSME-4, 2 isolates carrying blaVIM-1, 1 isolate carrying blaOXA-232, and 1 isolate carrying blaKPC-4. Meropenem-vaborbactam was active against 95.4% of the CRE isolates and 94.8% of the carbapenem-producing Enterobacterales (CPE) isolates when applying the CLSI breakpoints. All isolates producing serine carbapenemases were inhibited by meropenem-vaborbactam at ≤8 mg/liter. One Citrobacter freundii isolate carrying blaKPC-3 had a meropenem-vaborbactam MIC of 8 mg/liter and was resistant according to CLSI breakpoints (the isolate was susceptible when the EUCAST criterion of an MIC of ≤8 mg/liter for susceptible was applied), had disrupted OmpC and OmpF sequences, and overexpressed AcrAB-TolC. All carbapenemase-negative CRE isolates (n = 18) were inhibited by meropenem-vaborbactam at ≤4 mg/liter, and the MIC values of this combination ranged from 0.25 to 4 mg/liter. Among 7 isolates carrying metallo-β-lactamases and/or oxacillinases with carbapenemase activity, meropenem-vaborbactam susceptibility was 14.3% and 57.1% when applying CLSI and EUCAST breakpoints, respectively. CRE isolates were resistant to many comparator agents, and the most active agents were tigecycline, colistin, and amikacin (to which 63.2% to 96.7% of the isolates were susceptible). Understanding the epidemiology of CRE isolates in U.S. hospitals and the resistance mechanisms among these isolates is important to form guidelines for the treatment of infections caused by these organisms, which have high mortality rates.
INTRODUCTION
Carbapenem-resistant Enterobacterales (CRE) isolates have emerged worldwide, have been observed in all states within the United States, and are considered endemic in a few U.S. regions (1). Although determining the exact burden of antimicrobial resistance is challenging, the CDC attributes 600 deaths and over 9,000 infection episodes every year in the United States to CRE organisms (2). Serious infections caused by CRE organisms have a higher attributable mortality rate than those caused by isolates susceptible to carbapenems (3, 4). Patients infected with a CRE organism are less likely to receive early appropriate therapy, and this delay can be associated with the elevated mortality rates (4–6).
Until recently, the treatment of CRE infections consisted in many cases of combinations that included colistin, tigecycline, and aminoglycosides (4–6). In 2015, ceftazidime-avibactam was approved by the FDA, and this combination displayed good activity against some CRE isolates, including KPC-producing organisms (7). Despite the good in vitro activity of ceftazidime-avibactam (8), no randomized trials specific for CRE were performed for this combination agent, and shortly after its approval, KPC-producing Enterobacterales isolates resistant to ceftazidime-avibactam emerged during therapy (9–11).
Meropenem-vaborbactam was approved by the FDA in 2017 for the treatment of complicated urinary tract infections (UTIs) and pyelonephritis caused by Enterobacterales isolates and more recently was approved by the European Medicines Agency for the treatment of complicated UTI (including pyelonephritis), complicated intra-abdominal infections, and hospital-acquired pneumonia (including ventilator-associated pneumonia) caused by Enterobacterales and Pseudomonas aeruginosa isolates (12). Meropenem-vaborbactam was developed to be active against KPC-producing Enterobacterales isolates, and its dosing regimen was designed to cover isolates with MIC values up to 8 mg/liter (13, 14). Beyond the clinical trial that led to the approval of meropenem-vaborbactam (15), the activity of this combination agent against CRE infections was also evaluated in the TANGO II trial (16). Despite the small number of isolates in both arms, 32 patients were randomized in the meropenem-vaborbactam arm and 15 were treated with the best available therapy, which included combination regimens. The analysis of that study concluded that meropenem-vaborbactam monotherapy showed significant improvement in clinical cure rates, lower nephrotoxicity, and lower mortality rates compared to the best available therapy, which consisted of multiple agents combined and included tetracyclines, aminoglycosides, colistin, and high carbapenem doses (16). Moreover, the authors highlighted that meropenem-vaborbactam considerably improved mortality rates among immunocompromised patients, who can be at a higher risk of these infections and who are rarely addressed in clinical trial studies (16).
The in vitro activity of meropenem-vaborbactam has been evaluated against worldwide Enterobacterales isolates, including CRE, multidrug-resistant, and extensively drug-resistant isolates (17, 18), and in studies that targeted isolates from more limited collections (19, 20). In this study, we expanded that knowledge by evaluating the activity of meropenem-vaborbactam and comparator agents against 152 CRE isolates collected among 13,929 isolates from 31 U.S. hospitals distributed in all 9 census divisions from 2016 to 2018. Isolates were screened for carbapenemases and other β-lactam resistance mechanisms using whole-genome sequencing analysis and evaluation of the transcription levels of genes involved in β-lactam resistance.
RESULTS
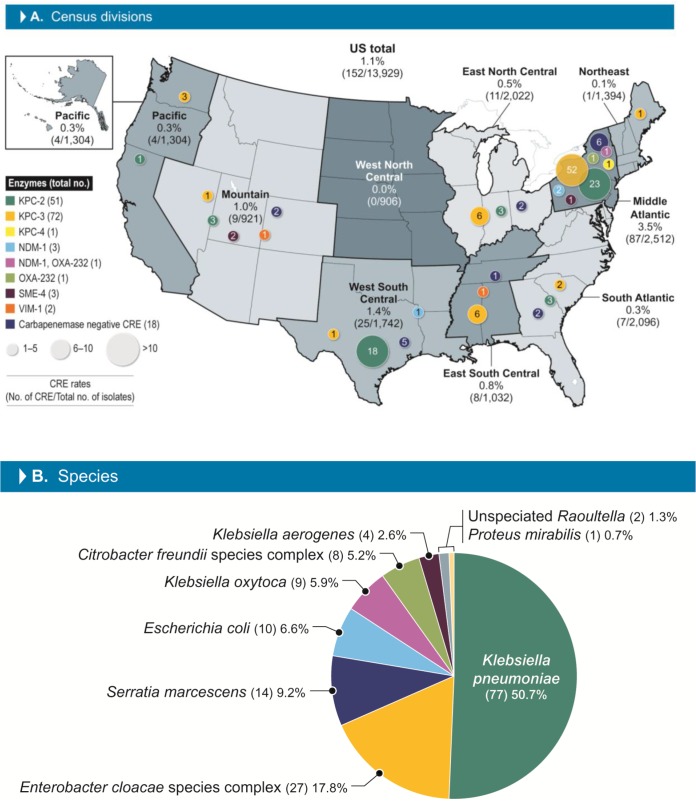
A total of 152 CRE isolates (1.1% of the overall isolates) were observed among 13,929 isolates collected in 31 U.S. hospitals distributed in all 9 census divisions of the United States from 2016 to 2018. More than half of these isolates were detected in the Middle Atlantic division (n = 87; 57.2% of the CRE isolates; Fig. 1). This was reflected in the overall meropenem susceptibility rates for Enterobacterales, which were lower in the Middle Atlantic division (96.5% by use of the CLSI breakpoint; data not shown) than in the other U.S. census divisions. CRE rates were 1.4% in the West South Central division (25/1,742 isolates) and 1.0% in the Mountain division (9/921) and ranged from 0.0% in the West North Central division (0/906 isolates collected) to 0.8% in the East South Central division (8/1,032). The CRE isolates consisted of 77 Klebsiella pneumoniae isolates, 27 Enterobacter cloacae species complex isolates, 14 Serratia marcescens isolates, 10 Escherichia coli isolates, 9 Klebsiella oxytoca isolates, 8 Citrobacter freundii species complex isolates, 4 Klebsiella aerogenes isolates, 2 Raoultella isolates, and 1 Proteus mirabilis isolate.
FIG 1.
CRE distribution in U.S. census divisions (A) and species (B). CRE, carbapenem-resistant Enterobacterales.
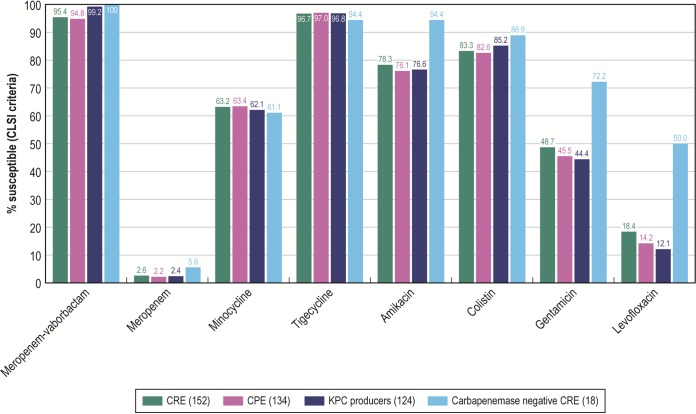
Meropenem-vaborbactam activity (MIC50/90, 0.06/2 mg/liter) was greater than meropenem activity (Fig. 2) and the activity of all β-lactam agents against CRE isolates. Meropenem-vaborbactam inhibited 95.4% of the CRE isolates when the CLSI breakpoint was applied and 98.0% of the isolates when the EUCAST interpretative criteria were used, whereas the other β-lactams inhibited only up to 6.6% of the CRE isolates (determined using CLSI breakpoints; data not shown). Among the other antimicrobial classes, tigecycline, colistin, amikacin, and minocycline inhibited 96.7%, 83.3%, 78.3%, and 63.2% of the CRE isolates, respectively (CLSI breakpoints were used for all antimicrobials except colistin, for which EUCAST breakpoints were applied) (Fig. 2). For all other agents, <50% of these isolates were susceptible (data not shown).
FIG 2.
Activity of meropenem-vaborbactam and comparator agents tested against Enterobacterales and CRE isolates. CRE, carbapenem-resistant Enterobacterales; CPE, carbapenemase-producing Enterobacterales.
Most CRE isolates carried carbapenemase genes (134/152; 88.2%) and included 72 isolates carrying blaKPC-3, 51 isolates carrying blaKPC-2, 4 isolates carrying blaNDM-1 (1 isolate coharbored blaOXA-232), 3 isolates carrying blaSME-4 (all were S. marcescens isolates), 2 isolates carrying blaVIM-1, and 1 isolate each carrying of blaOXA-232 and blaKPC-4 (Fig. 1). Eighteen isolates were negative for carbapenemase genes. Isolates carrying blaKPC-3 were observed in all U.S. census divisions except the West North Central division, and blaKPC-2-carrying organisms were noted in all divisions except the Northeast, West North Central, and East South Central divisions. The isolates carrying blaKPC-4, the 3 isolates carrying blaNDM-1, the 1 isolate carrying blaOXA-232, and the 1 isolate carrying blaSME-4 were all detected in the Middle Atlantic division. The remaining isolate carrying blaNDM-1 was observed in the West South Central division.
Among 134 carbapenemase-producing Enterobacterales (CPE) isolates, meropenem-vaborbactam (MIC50/90, 0.03/1 mg/liter) inhibited 94.8% and 97.8% of the isolates when FDA and EUCAST breakpoints were applied, respectively (Table 1). Among the 7 isolates (<0.1% of the overall isolates and 4.6% of the CRE isolates) displaying nonsusceptible meropenem-vaborbactam MIC results when applying the CLSI breakpoint, 4 carried blaNDM-1 (and 1 of these 4 isolates also harbored an oxacillinase with carbapenemase activity [blaOXA-232]), 1 carried blaOXA-232 alone, 1 carried blaVIM-1, and 1 Citrobacter freundii isolate carried blaKPC-3. The last isolate was further investigated for additional resistance mechanisms (Table 2).
TABLE 1.
Activity of meropenem-vaborbactam against Enterobacterales isolates collected in 31 U.S. hospitals during 2016 to 2018c
| Organism (no. of isolates) | MIC (mg/liter) |
% of isolates susceptible to meropenem-vaborbactam applying interpretative criteria of: |
|||
|---|---|---|---|---|---|
| 50% | 90% | Range | CLSIa | EUCASTb | |
| CRE (152) | 0.06 | 2 | ≤0.015 to >32 | 95.4 | 98.0 |
| KPC producers (124) | 0.03 | 0.5 | ≤0.015 to 8 | 99.2 | 100.0 |
| KPC-2 producers (51) | 0.03 | 1 | ≤0.015 to 2 | 100.0 | 100.0 |
| KPC-3 producers (72) | 0.03 | 0.5 | ≤0.015 to 8 | 98.6 | 100.0 |
| MBL and OXA-48-like producers (7) | 8 | 4 to >32 | 14.3 | 57.1 | |
| CPE (134) | 0.03 | 1 | ≤0.015 to >32 | 94.8 | 97.8 |
| Carbapenemase negative (18) | 1 | 4 | 0.25 to 4 | 100.0 | 100.0 |
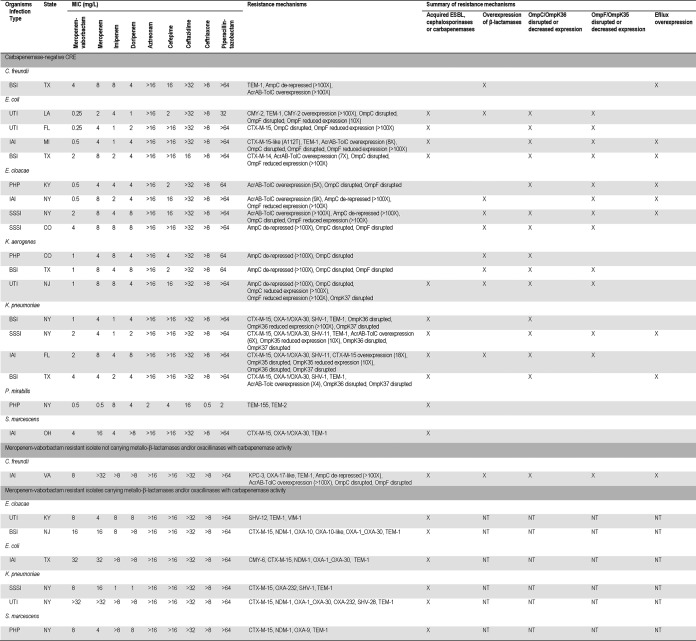
TABLE 2.
Mechanisms of resistance to β-lactams detected among carbapenemase-negative CRE isolates and isolates resistant to meropenem-vaborbactama
CRE, carbapenem-resistant Enterobacterales; BSI, bloodstream infection; UTI, urinary tract infection; IAI, intra-abdominal infection; PHP, patients hospitalized with pneumonia; SSSI, skin and skin structure infection; NT, not tested.
Meropenem-vaborbactam activity was similar for isolates carrying blaKPC-2 (51 isolates; MIC50/90, 0.03/1 mg/liter) and blaKPC-3 (72 isolates; MIC50/90, 0.03/0.5 mg/liter), and all blaKPC-2-carrying isolates and all but 1 of the blaKPC-3-carrying isolates were inhibited by meropenem-vaborbactam at ≤2 mg/liter. Isolates carrying the genes encoding KPC-4 and SME-4 displayed meropenem-vaborbactam MIC values ranging from 0.03 to 0.06 mg/liter.
One C. freundii isolate carrying blaKPC-3, in addition to blaTEM-1 and blaOXA-17-like, displayed a meropenem-vaborbactam MIC value of 8 mg/liter (intermediate by the use of CLSI breakpoints and susceptible by the use of EUCAST breakpoints). This isolate had missense mutations in OmpC and OmpF that caused the disruption of these genes and the overexpression of AcrAB-TolC and AmpC (Table 2).
Isolates negative for carbapenemase genes were also noted in all but 2 census divisions and included 4 E. cloacae species complex isolates, 4 E. coli isolates, 4 K. pneumoniae isolates, 3 K. aerogenes isolates, and 1 isolate each of the C. freundii species complex, P. mirabilis, and S. marcescens. The carbapenem resistance mechanisms among these isolates were diverse and for E. coli, E. cloacae, K. aerogenes, and K. pneumoniae included a combination of acquired β-lactamases or AmpC overexpression and disrupted sequences or reduced expression of the genes encoding OmpC/OmpK36 and/or OmpF/OmpK35. Additionally, AcrAB-TolC overexpression was observed among 2 E. coli isolates, 3 E. cloacae isolates, and 2 K. pneumoniae isolates. A noteworthy finding was that all K. pneumoniae isolates and 2 of the 4 E. coli isolates carried blaCTX-M-15-like. Among the remaining species, the C. freundii isolate had a combination of elevated expression of AmpC and AcrAB-TolC and the P. mirabilis and the S. marcescens isolates only harbored acquired β-lactamases among the mechanisms of β-lactam resistance analyzed. Meropenem-vaborbactam (MIC50/90, 1/4 mg/liter) inhibited all CRE isolates that did not carry carbapenemases at ≤4 mg/liter, and the MIC values for this combination ranged from 0.25 to 4 mg/liter (the MIC was 4 mg/liter for only 4 isolates). The activity of meropenem-vaborbactam was lower against these isolates than against the CRE isolates overall (MIC50/90, 0.06/2 mg/liter).
Meropenem-vaborbactam inhibited 1 (a 14.3% rate of susceptibility) of the 7 isolates carrying metallo-β-lactamases (MBL) and/or oxacillinases with carbapenemase activity at the current CLSI breakpoint, but 57.1% of the isolates were susceptible to this combination when using the EUCAST breakpoint criteria. One S. marcescens isolate carrying blaVIM-1 had a meropenem-vaborbactam MIC of 4 mg/liter, and 1 E. cloacae isolate harboring blaVIM-1, 1 S. marcescens isolate carrying blaNDM-1, and 1 K. pneumoniae isolate carrying blaOXA-232 had MIC values of 8 mg/liter for this combination. These isolates were resistant to meropenem alone and were susceptible to tigecycline. Minocycline and colistin inhibited 71.4% of these isolates when the CLSI and EUCAST breakpoints, respectively, were applied (data not shown).
DISCUSSION
In a recent study by Satlin et al. (21) investigating the CRE epicenter in the United States, patients with CRE infections had a 47-h delay in appropriate therapy and 49% died within 30 days. In another U.S. investigation, mortality rates among patients with CRE infections ranged from 13.3%, when using combination therapy that included colistin, tigecycline, and aminoglycosides, to 57.8%, when patients were treated with monotherapy (22). Similar data were replicated in Greece and Italy (4, 6). These studies highlight the threat of CRE infections for patients and the importance of understanding the rates of CRE in individual institutions to establish protocols and methods to identify CRE isolates and evaluate their susceptibility patterns to implement effective therapies promptly.
While other carbapenemases have been reported in U.S. hospitals, KPC-producing organisms are still the most frequent. These isolates have been reported in high numbers in the New York City area and Texas but have been encountered in every U.S. state (https://www.cdc.gov/hai/organisms/cre/trackingcre.html). In this survey, most carbapenemase-producing isolates from 31 U.S. hospitals carried KPC-encoding genes. These isolates were observed in all U.S. census divisions, except the West North Central division. Our results confirm that meropenem-vaborbactam was very active against these isolates, and since its activity against KPC producers is remarkably greater than that of most comparator agents, the combination agent should be considered an effective alternative for the treatment of CRE infections in U.S. hospitals.
Furthermore, meropenem-vaborbactam displays activity against KPC-producing organisms regardless of the KPC variant produced; in contrast to these findings, Satlin et al. (21) observed that 42% of the K. pneumoniae isolates carrying KPC-3 had ceftazidime-avibactam MIC values of ≥4 mg/liter, while the MIC results for this combination were lower for KPC-2 producers.
Despite the elevated prevalence of isolates carrying blaKPC, isolates carrying the gene encoding NDM have been observed in 34 states, according to the CDC website (https://www.cdc.gov/hai/organisms/cre/trackingcre.html), and other carbapenemases have been sporadically detected in the United States. Meropenem-vaborbactam, like ceftazidime-avibactam and other β-lactam agents, with the exception of monobactams, is not active against isolates carrying MBLs; meropenem-vaborbactam has limited activity against isolates carrying genes encoding oxacillinases with carbapenemase activity, such as OXA-48. Noteworthy in this study was the finding that >50% of the isolates carrying MBLs and/or oxacillinases with carbapenemase activity had a meropenem-vaborbactam MIC value of ≤8 mg/liter. It would be of utmost importance to understand the efficacy of meropenem-vaborbactam for the treatment of infections caused by isolates harboring genes encoding MBLs or oxacillinases with carbapenemase activity and displaying lower MIC values against this combination. Regardless, safe and efficacious therapeutic options are still needed for the treatment of infections caused by isolates producing MBLs and some isolates producing OXA-48-like enzymes, for which treatment options are limited.
Few studies have analyzed the mechanisms of resistance among carbapenemase-negative CRE isolates, and it is assumed that overexpression of β-lactamase and impaired permeability are the main mechanisms among these isolates. We noticed that these mechanisms can be diverse, vary in different species, and might be difficult to predict. Despite the presence of a combination of these mechanisms, meropenem-vaborbactam was active against these isolates, which are more susceptible to comparator agents than carbapenemase-producing isolates but still present a challenge for the selection of appropriate antimicrobial therapy.
In clinical and in vitro studies (17–19, 23, 24), meropenem-vaborbactam has been shown to be more effective than combination therapy and to have greater activity than comparator agents. This new agent should be considered as a treatment option for CRE infections in the United States, where KPC-producing isolates constitute the majority of the CRE; however, diagnostic methods for determining the type of carbapenemase and/or the susceptibility to meropenem-vaborbactam or other agents active against CRE isolates must be available to confirm that the isolate is susceptible to this agent.
MATERIALS AND METHODS
Bacterial isolates.
A total of 13,929 Enterobacterales clinical isolates were consecutively collected from 2016 to 2018 in 31 U.S. hospitals as part of the SENTRY Antimicrobial Surveillance Program according to standardized protocols. Only clinically significant isolates were included in the study (1 per patient episode). Among these isolates, CRE were defined as any isolate exhibiting a doripenem, imipenem (Proteus mirabilis and members of the indole-positive tribe Proteeae were not included due to their intrinsically elevated MIC values), and/or meropenem MIC value of ≥4 mg/liter and were selected for further analysis. Species identification was confirmed by matrix-assisted laser desorption ionization–time of flight mass spectrometry using a Bruker Daltonics MALDI Biotyper apparatus (Billerica, MA, USA) according to the manufacturer’s instructions.
Antimicrobial susceptibility testing.
All isolates were susceptibility tested using the CLSI reference broth microdilution method (25). CLSI (26), EUCAST (27), or FDA (28) categorical interpretations were applied. Quality control (QC) was performed using E. coli ATCC 25922 and ATCC 35218 and K. pneumoniae ATCC 700603, BAA-1705, and BAA-2814. All QC MIC results were within acceptable ranges, as published in CLSI documents (26).
Carbapenemase screening.
CRE isolates were submitted to whole-genome sequencing using the Nextera XT library construction protocol and index kit (Illumina, San Diego, CA, USA) according to the manufacturer’s instructions and were sequenced on a MiSeq sequencer (Illumina) with a target coverage of 30 times. FASTQ format files for each sample set were assembled independently using the de novo assembler SPAdes (version 3.9.0) (29) with K values of 21, 33, 55, 77, and 99 and the careful mode on to reduce the number of mismatches, producing a FASTA format file of contiguous sequences with the best N50 value. Software designed in-house using the target assembled sequences (30) as queries for alignment against numerous resistance determinants from the NCBI Bacterial Antimicrobial Resistance Reference Gene Database (https://www.ncbi.nlm.nih.gov/bioproject/PRJNA313047) was used to search for β-lactamase genes, and potential matches were generated with the criteria of >94% identity and 40% minimum coverage length.
Analysis of intrinsic β-lactam resistance mechanisms.
Carbapenemase-negative CRE isolates and 1 isolate that did not carry MBLs or oxacillinases with carbapenemase activity but that displayed a meropenem-vaborbactam MIC value of 8 mg/liter were further evaluated. OmpK35/OmpF and OmpK36/OmpC sequences were analyzed and compared to the reference sequences for β-lactam-susceptible isolates using whole-genome sequencing data. The transcription levels of genes encoding the intrinsic cephalosporinases, porin, efflux, and the main acquired β-lactamases (extended-spectrum β-lactamases, transferrable cephalosporinases, and carbapenemases) were measured as previously described (31, 32).
ACKNOWLEDGMENTS
This study was performed by JMI Laboratories and supported by Melinta Therapeutics, Inc., which included funding for services related to preparing the manuscript.
JMI Laboratories contracted to perform services in 2018 for Achaogen, Inc.; the Albany College of Pharmacy and Health Sciences; Allecra Therapeutics; Allergan; American Proficiency Institute; AmpliPhi Biosciences Corp.; Amplyx; Antabio; Arietis Corp.; Arixa Pharmaceuticals, Inc.; Astellas Pharma Inc.; Athelas; Basilea Pharmaceutica Ltd.; Bayer AG; Becton, Dickinson and Company; bioMérieux SA; Boston Pharmaceuticals; Bugworks Research Inc.; CEM-102 Pharmaceuticals; Cepheid; Cidara Therapeutics, Inc.; CorMedix Inc.; DePuy Synthes; Destiny Pharma; Discuva Ltd.; Dr. Falk Pharma GmbH; Emery Pharma; Entasis Therapeutics; Eurofarma Laboratorios SA; Fox Chase Chemical Diversity Center, Inc.; Gateway Pharmaceutical LLC; GenePOC Inc.; Geom Therapeutics, Inc.; GlaxoSmithKline plc; Harvard University; Helperby; HiMedia Laboratories; F. Hoffmann-La Roche Ltd.; ICON plc; Idorsia Pharmaceuticals Ltd.; Iterum Therapeutics plc; Laboratory Specialists, Inc.; Melinta Therapeutics, Inc.; Merck & Co., Inc.; Microchem Laboratory; Micromyx; MicuRx Pharmaceuticals, Inc.; Mutabilis Co.; Nabriva Therapeutics plc; NAEJA-RGM; Novartis AG; Oxoid Ltd.; Paratek Pharmaceuticals, Inc.; Pfizer, Inc.; Pharmaceutical Product Development, LLC; Polyphor Ltd.; Prokaryotics Inc.; Qpex Biopharma, Inc.; Ra Pharmaceuticals, Inc.; Roivant Sciences, Ltd.; Safeguard Biosystems; Scynexis, Inc.; SeLux Diagnostics, Inc.; Shionogi and Co., Ltd.; SinSa Labs; Spero Therapeutics; Summit Pharmaceuticals International Corp.; Synlogic; T2 Biosystems, Inc.; Taisho Pharmaceutical Co., Ltd.; TenNor Therapeutics Ltd.; Tetraphase Pharmaceuticals; The Medicines Company; Theravance Biopharma; University of Colorado; University of North Texas Health Science Center; University of Southern California—San Diego; the U.S. Food and Drug Administration; VenatoRx Pharmaceuticals, Inc.; Vyome Therapeutics Inc.; Wockhardt; Yukon Pharmaceuticals, Inc.; Zai Lab; and Zavante Therapeutics, Inc. There are no speakers’ bureaus or stock options to declare.
Footnotes
[This article was published on 27 January 2020 with a standard copyright line (“© 2020 American Society for Microbiology. All Rights Reserved.”). The authors elected to pay for open access for the article after publication, necessitating replacement of the original copyright line with the one above, and this change was made on 18 February 2020.]
REFERENCES
- 1.Logan LK, Weinstein RA. 2017. The epidemiology of carbapenem-resistant Enterobacteriaceae: the impact and evolution of a global menace. J Infect Dis 215:S28–S36. doi: 10.1093/infdis/jiw282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Centers for Disease Control and Prevention. 2013. Antibiotic resistance threats in the United States, 2013. https://www.cdc.gov/drugresistance/threat-report-2013/pdf/ar-threats-2013-508.pdf.
- 3.Martin A, Fahrbach K, Zhao Q, Lodise T. 2018. Association between carbapenem resistance and mortality among adult, hospitalized patients with serious infections due to Enterobacteriaceae: results of a systematic literature review and meta-analysis. Open Forum Infect Dis 5:ofy150. doi: 10.1093/ofid/ofy150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Daikos GL, Tsaousi S, Tzouvelekis LS, Anyfantis I, Psichogiou M, Argyropoulou A, Stefanou I, Sypsa V, Miriagou V, Nepka M, Georgiadou S, Markogiannakis A, Goukos D, Skoutelis A. 2014. Carbapenemase-producing Klebsiella pneumoniae bloodstream infections: lowering mortality by antibiotic combination schemes and the role of carbapenems. Antimicrob Agents Chemother 58:2322–2328. doi: 10.1128/AAC.02166-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lodise TP, Berger A, Altincatal A, Wang R, Bhagnani T, Gillard P, Bonine NG. 2019. Antimicrobial resistance or delayed appropriate therapy—does one influence outcomes more than the other among patients with serious infections due to carbapenem-resistant versus carbapenem-susceptible Enterobacteriaceae? Open Forum Infect Dis 6:ofz194. doi: 10.1093/ofid/ofz194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tumbarello M, Viale P, Viscoli C, Trecarichi EM, Tumietto F, Marchese A, Spanu T, Ambretti S, Ginocchio F, Cristini F, Losito AR, Tedeschi S, Cauda R, Bassetti M. 2012. Predictors of mortality in bloodstream infections caused by Klebsiella pneumoniae carbapenemase-producing K. pneumoniae: importance of combination therapy. Clin Infect Dis 55:943–950. doi: 10.1093/cid/cis588. [DOI] [PubMed] [Google Scholar]
- 7.Zhanel GG, Lawson CD, Adam H, Schweizer F, Zelenitsky S, Lagace-Wiens PR, Denisuik A, Rubinstein E, Gin AS, Hoban DJ, Lynch JP III, Karlowsky JA. 2013. Ceftazidime-avibactam: a novel cephalosporin/β-lactamase inhibitor combination. Drugs 73:159–177. doi: 10.1007/s40265-013-0013-7. [DOI] [PubMed] [Google Scholar]
- 8.Shields RK, Nguyen MH, Chen L, Press EG, Potoski BA, Marini RV, Doi Y, Kreiswirth BN, Clancy CJ. 2017. Ceftazidime-avibactam is superior to other treatment regimens against carbapenem-resistant Klebsiella pneumoniae bacteremia. Antimicrob Agents Chemother 61:e00883-17. doi: 10.1128/AAC.00883-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shields RK, Chen L, Cheng S, Chavda KD, Press EG, Snyder A, Pandey R, Doi Y, Kreiswirth BN, Nguyen MH, Clancy CJ. 2017. Emergence of ceftazidime-avibactam resistance due to plasmid-borne blaKPC-3 mutations during treatment of carbapenem-resistant Klebsiella pneumoniae infections. Antimicrob Agents Chemother 61:e02097-16. doi: 10.1128/AAC.02097-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nelson K, Hemarajata P, Sun D, Rubio-Aparicio D, Tsivkovski R, Yang S, Sebra R, Kasarskis A, Nguyen H, Hanson BM, Leopold S, Weinstock G, Lomovskaya O, Humphries RM. 2017. Resistance to ceftazidime-avibactam is due to transposition of KPC in a porin-deficient strain of Klebsiella pneumoniae with increased efflux activity. Antimicrob Agents Chemother 61:e00989-17. doi: 10.1128/AAC.00989-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Castanheira M, Arends SJR, Davis AP, Woosley LN, Bhalodi AA, MacVane SH. 2018. Analyses of a ceftazidime-avibactam-resistant Citrobacter freundii isolate carrying blaKPC-2 reveals a heterogenous population and reversible genotype. mSphere 3:e00408-18. doi: 10.1128/mSphere.00408-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhanel GG, Lawrence CK, Adam H, Schweizer F, Zelenitsky S, Zhanel M, Lagace-Wiens PRS, Walkty A, Denisuik A, Golden A, Gin AS, Hoban DJ, Lynch JP III, Karlowsky JA. 2018. Imipenem-relebactam and meropenem-vaborbactam: two novel carbapenem-beta-lactamase inhibitor combinations. Drugs 78:65–98. doi: 10.1007/s40265-017-0851-9. [DOI] [PubMed] [Google Scholar]
- 13.Griffith DC, Sabet M, Tarazi Z, Lomovskaya O, Dudley MN. 2019. Pharmacokinetics/pharmacodynamics of vaborbactam, a novel beta-lactamase inhibitor, in combination with meropenem. Antimicrob Agents Chemother 63:e01659-18. doi: 10.1128/AAC.01659-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lomovskaya O, Sun D, Rubio-Aparicio D, Nelson K, Tsivkovski R, Griffith DC, Dudley MN. 2017. Vaborbactam: spectrum of beta-lactamase inhibition and impact of resistance mechanisms on activity in Enterobacteriaceae. Antimicrob Agents Chemother 61:e01443-17. doi: 10.1128/AAC.01443-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kaye KS, Bhowmick T, Metallidis S, Bleasdale SC, Sagan OS, Stus V, Vazquez J, Zaitsev V, Bidair M, Chorvat E, Dragoescu PO, Fedosiuk E, Horcajada JP, Murta C, Sarychev Y, Stoev V, Morgan E, Fusaro K, Griffith D, Lomovskaya O, Alexander EL, Loutit J, Dudley MN, Giamarellos-Bourboulis EJ. 2018. Effect of meropenem-vaborbactam vs piperacillin-tazobactam on clinical cure or improvement and microbial eradication in complicated urinary tract infection: the TANGO I randomized clinical trial. JAMA 319:788–799. doi: 10.1001/jama.2018.0438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wunderink RG, Giamarellos-Bourboulis EJ, Rahav G, Mathers AJ, Bassetti M, Vazquez J, Cornely OA, Solomkin J, Bhowmick T, Bishara J, Daikos GL, Felton T, Furst MJL, Kwak EJ, Menichetti F, Oren I, Alexander EL, Griffith D, Lomovskaya O, Loutit J, Zhang S, Dudley MN, Kaye KS. 2018. Effect and safety of meropenem-vaborbactam versus best-available therapy in patients with carbapenem-resistant Enterobacteriaceae infections: the TANGO II randomized clinical trial. Infect Dis Ther 7:439–455. doi: 10.1007/s40121-018-0214-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Castanheira M, Huband MD, Mendes RE, Flamm RK. 2017. Meropenem-vaborbactam tested against contemporary Gram-negative isolates collected worldwide during 2014, including carbapenem-resistant, KPC-producing, multidrug-resistant, and extensively drug-resistant Enterobacteriaceae. Antimicrob Agents Chemother 61:e00567-17. doi: 10.1128/AAC.00567-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pfaller MA, Huband MD, Mendes RE, Flamm RK, Castanheira M. 2018. In vitro activity of meropenem/vaborbactam and characterization of carbapenem resistance mechanisms among carbapenem-resistant Enterobacteriaceae from the 2015 meropenem/vaborbactam surveillance program. Int J Antimicrob Agents 52:144–150. doi: 10.1016/j.ijantimicag.2018.02.021. [DOI] [PubMed] [Google Scholar]
- 19.Wilson WR, Kline EG, Jones CE, Morder KT, Mettus RT, Doi Y, Nguyen MH, Clancy CJ, Shields RK. 2019. Effects of KPC variant and porin genotype on the in vitro activity of meropenem-vaborbactam against carbapenem-resistant Enterobacteriaceae. Antimicrob Agents Chemother 63:e02048-18. doi: 10.1128/AAC.02048-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hackel MA, Lomovskaya O, Dudley MN, Karlowsky JA, Sahm DF. 2018. In vitro activity of meropenem-vaborbactam against clinical isolates of KPC-positive Enterobacteriaceae. Antimicrob Agents Chemother 62:e01904-17. doi: 10.1128/AAC.01904-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Satlin MJ, Chen L, Patel G, Gomez-Simmonds A, Weston G, Kim AC, Seo SK, Rosenthal ME, Sperber SJ, Jenkins SG, Hamula CL, Uhlemann AC, Levi MH, Fries BC, Tang YW, Juretschko S, Rojtman AD, Hong T, Mathema B, Jacobs MR, Walsh TJ, Bonomo RA, Kreiswirth BN. 2017. Multicenter clinical and molecular epidemiological analysis of bacteremia due to carbapenem-resistant Enterobacteriaceae (CRE) in the CRE epicenter of the United States. Antimicrob Agents Chemother 61:e02349-16. doi: 10.1128/AAC.02349-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Qureshi ZA, Paterson DL, Potoski BA, Kilayko MC, Sandovsky G, Sordillo E, Polsky B, Adams-Haduch JM, Doi Y. 2012. Treatment outcome of bacteremia due to KPC-producing Klebsiella pneumoniae: superiority of combination antimicrobial regimens. Antimicrob Agents Chemother 56:2108–2113. doi: 10.1128/AAC.06268-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bassetti M, Giacobbe DR, Patel N, Tillotson G, Massey J. 2019. Efficacy and safety of meropenem-vaborbactam versus best available therapy for the treatment of carbapenem-resistant Enterobacteriaceae infections in patients without prior antimicrobial failure: A post hoc analysis. Adv Ther 36:1771–1777. doi: 10.1007/s12325-019-00981-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Athans V, Neuner EA, Hassouna H, Richter SS, Keller G, Castanheira M, Brizendine KD, Mathers AJ. 2018. Meropenem-vaborbactam as salvage therapy for ceftazidime-avibactam-resistant Klebsiella pneumoniae bacteremia and abscess in a liver transplant recipient. Antimicrob Agents Chemother 63:e01551. doi: 10.1128/AAC.01551-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Clinical and Laboratory Standards Institute. 2018. M07Ed11. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically; approved standard, 11th ed Clinical and Laboratory Standards Institute, Wayne, PA. [Google Scholar]
- 26.Clinical and Laboratory Standards Institute. 2019. M100Ed29. Performance standards for antimicrobial susceptibility testing: 29th informational supplement. Clinical and Laboratory Standards Institute, Wayne, PA. [Google Scholar]
- 27.EUCAST. 2019. Breakpoint tables for interpretation of MICs and zone diameters, version 9.0, January 2019. http://www.eucast.org/fileadmin/src/media/PDFs/EUCAST_files/Breakpoint_tables/v_9.0_Breakpoint_Tables.pdf. Accessed January 2019.
- 28.FDA. 2019. Antibacterial susceptibility test interpretive criteria. https://www.fda.gov/drugs/development-resources/antibacterial-susceptibility-test-interpretive-criteria. Accessed 5 May 2019.
- 29.Bankevich A, Nurk S, Antipov D, Gurevich AA, Dvorkin M, Kulikov AS, Lesin VM, Nikolenko SI, Pham S, Prjibelski AD, Pyshkin AV, Sirotkin AV, Vyahhi N, Tesler G, Alekseyev MA, Pevzner PA. 2012. SPAdes: a new genome assembly algorithm and its applications to single-cell sequencing. J Comput Biol 19:455–477. doi: 10.1089/cmb.2012.0021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Camacho C, Coulouris G, Avagyan V, Ma N, Papadopoulos J, Bealer K, Madden TL. 2009. BLAST+: architecture and applications. BMC Bioinformatics 10:421. doi: 10.1186/1471-2105-10-421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Castanheira M, Mendes RE, Sader HS. 2017. Low frequency of ceftazidime-avibactam resistance among Enterobacteriaceae Isolates Carrying blaKPC collected in U.S. hospitals from 2012 to 2015. Antimicrob Agents Chemother 61:e02369-16. doi: 10.1128/AAC.02369-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Castanheira M, Costello SE, Woosley LN, Deshpande LM, Davies TA, Jones RN. 2014. Evaluation of clonality and carbapenem resistance mechanisms among Acinetobacter baumannii-Acinetobacter calcoaceticus complex and Enterobacteriaceae isolates collected in European and Mediterranean countries and detection of two novel β-lactamases, GES-22 and VIM-35. Antimicrob Agents Chemother 58:7358–7366. doi: 10.1128/AAC.03930-14. [DOI] [PMC free article] [PubMed] [Google Scholar]