The discovery of antibiotics in the last century is considered one of the most important achievements in the history of medicine. Antibiotic usage has significantly reduced morbidity and mortality associated with bacterial infections. However, inappropriate use of antibiotics has led to emergence of antibiotic resistance at an alarming rate. Antibiotic resistance is regarded as a major health care challenge of this century.
KEYWORDS: adaptive resistance, antibiotic resistance, antibiotics, bacterial epigenetics, methylation
ABSTRACT
The discovery of antibiotics in the last century is considered one of the most important achievements in the history of medicine. Antibiotic usage has significantly reduced morbidity and mortality associated with bacterial infections. However, inappropriate use of antibiotics has led to emergence of antibiotic resistance at an alarming rate. Antibiotic resistance is regarded as a major health care challenge of this century. Despite extensive research, well-documented biochemical mechanisms and genetic changes fail to fully explain mechanisms underlying antibiotic resistance. Several recent reports suggest a key role for epigenetics in the development of antibiotic resistance in bacteria. The intrinsic heterogeneity as well as transient nature of epigenetic inheritance provides a plausible backdrop for high-paced emergence of drug resistance in bacteria. The methylation of adenines and cytosines can influence mutation rates in bacterial genomes, thus modulating antibiotic susceptibility. In this review, we discuss a plethora of recently discovered epigenetic mechanisms and their emerging roles in antibiotic resistance. We also highlight specific epigenetic mechanisms that merit further investigation for their role in antibiotic resistance.
INTRODUCTION
The discovery of antibiotics brought about a revolution in the field of medicine. Antibiotics have become the backbone of modern-day health care. Several classes of antibiotics are widely used today, and they target essential processes in bacteria, including cell wall synthesis, translation, transcription, etc. (1). However, bacteria are known to acquire drug resistance by various means. Mobilization of genetic elements from different strains and the environment allows horizontal transfer of resistance-conferring genes (2). Mutations which confer resistance can also negatively affect bacterial fitness as they have important roles in cellular processes. But off-site compensatory mutations which negate this cost of fitness can lead to the stable resistance status of bacterial strains. Resistance mutations that do not compromise fitness have also been reported (3). The genetic basis of antimicrobial resistance has been studied for several decades. In Fig. 1, we have summarized the genetic mechanisms underlying antimicrobial resistance; these mechanisms have been extensively reviewed elsewhere (4–7). In addition to genetic mechanisms, bacteria acquire resistance to antimicrobials by modulating the expression of chromosomally encoded proteins as well as plasmid-borne efflux pumps (8).
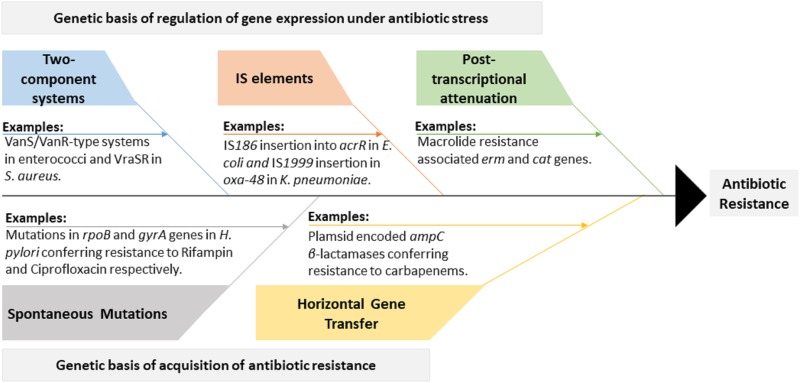
FIG 1.
An outline of the genetic basis of antibiotic resistance. Bacteria can acquire resistance to antibiotics by spontaneous mutations in the target genes or their regulators. Interchange of mobile genetic elements among bacteria (horizontal gene transfer [HGT]) also contributes to the dissemination of antibiotic resistance genes. In addition to acquisition of genetic modifications, bacteria can also survive antibiotic stress by modulating their gene expression. Well-known mediators of gene expression changes in response to antibiotic stress include two-component systems, insertion sequence (IS) elements, and posttranscriptional attenuation of gene expression.
In recent years, there has been a spike in interest to explore nonclassical mechanisms, including bacterial epigenetics which may contribute to antibiotic resistance in bacteria. Here, we introduce the readers to the latest findings in the field of bacterial epigenetics and provide a comprehensive account summarizing the biological roles of epigenetics and epigenetic modulators in the development of antibacterial resistance. In addition, we discuss multiple facets of bacterial epigenetics which have not been specifically explored in the context of antibiotic resistance. As genetic mechanisms cannot entirely explain the rapidity of resistance development or its transient nature (particularly in adaptive resistance), we believe that bacterial epigenetics may provide new answers.
BACTERIAL EPIGENETICS
In eukaryotic systems, an epigenetic trait has been defined as a “stably heritable phenotype resulting from changes in chromosomes without alterations in the DNA sequence” (9). Epigenetic mechanisms in eukaryotes, such as nucleotide modifications and histone modifications, have been studied extensively for decades. In contrast, bacterial DNA is not packaged in histones. Therefore, bacterial epigenetics is limited to modifications of bacterial DNA and RNA. In particular, methylation of bacterial DNA has been well studied. Methyl groups are transferred from S-adenosyl-l-methionine to adenine or cytosine by a group of enzymes called DNA methyltransferases (Mtases). The most commonly known DNA methyltransferases are those associated with the widely known defense mechanism in bacteria, the restriction-modification (R-M) system. As these methyltransferases add methyl groups only to specific sequences on the host bacterial DNA, unmethylated foreign DNA is recognized and degraded by endonucleases of these R-M systems. There are different groups of R-M systems depending on the proximity of the restriction site to the site of methylation and on the nature of polypeptides for restriction activity and modification activity (10). Bacterial DNA methyltransferases which do not belong to any R-M system are collectively called orphan methyltransferases. These orphan methyltransferases can regulate multiple cellular processes in bacteria, including cell cycle regulation, DNA mismatch repair, and regulation of gene expression (11).
Deoxyadenosine methylase (Dam) is an extensively studied orphan methyltransferase found in Escherichia coli; it is an adenine methyltransferase which adds a methyl group to the N6 position of the adenine residue in the palindromic sequence GATC. The Dam methyltransferase has been shown to play a crucial role in methyl-directed mismatch repair in E. coli and related gammaproteobacteria (12). The mismatch repair protein, MutH, recognizes unmethylated GATC and cleaves it. This ensures that only the newly synthesized DNA strand is cleaved; the parental strand is spared as the GATC sites in the parental strand are methylated (13). Dam-mediated methylation is also essential for regulation of replication initiation. The majority of the GATC sites in E. coli are completely methylated except for a transient period immediately after replication, during which they are present in a hemimethylated state. SeqA protein, which prevents reinitiation from hemimethylated oriC, allows only Dam to methylate the daughter strand after the removal of DnaA activity (responsible for initiation of replication) (14, 15). It has been postulated that Dam-mediated methylation is not a common mechanism of regulation of gene expression within the same generation. However, due to the ability of Dam to control methylation states transgenerationally, it can control phase variation (11, 16). Homologs of Dam with high sequence similarity have been found in several Gram-negative bacteria including Salmonella (17), Vibrio cholerae (18), etc. These homologs also methylate the adenine residue in the palindromic GATC sequence. YhdJ is a nonessential orphan methyltransferase, found in both E. coli and Salmonella enterica; this enzyme recognizes the sequence 5′-ATGCAT-3′ and adds a methyl group to the second adenine from the 5′ direction (19). Another well-studied orphan methyltransferase is CcrM; it recognizes and methylates the adenine residue in the sequence GANTC. CcrM utilizes hemimethylated DNA as a substrate, and its expression is cell cycle dependent. CcrM-mediated methylation is required for the functioning of some cell cycle regulators (such as GcrA and ctrA) (20, 21). CcrM is also reported in Agrobacterium tumefaciens (22) and other Alphaproteobacteria (23). In addition to adenines, orphan methyltransferases also methylate cytosines similar to their R-M counterparts. DNA cytosine methyltransferase (Dcm) found in E. coli can add a methyl group to the internal cytosine residue in the sequence 5′-CC(A/T)GG-3′ at the C5 position. Cytosine methylation mediated by Dcm is not essential for survival, but it can regulate gene expression of sigma factor RpoS (which is a major regulator of genes expressed in stationary phase) and some of its targets (24). VchM, an orphan methyltransferase found in Vibrio cholerae, methylates the sequence 5′-RCCGGY-3′ at the first cytosine, but it is not necessary for survival (25). There are several other orphan methyltransferases that have been discovered over the years apart from the ones mentioned in this review; due to limited space, discussing them is beyond the scope of this review.
R-M systems, which involve host DNA modifications, represent one of the mechanisms by which bacteria protect themselves (26). The phosphorothioate system is another lesser known defense mechanism that works in a way similar to that of the R-M system. It involves sequence-specific modification of the sugar-phosphate backbone of host DNA, wherein a sulfur replaces a nonbridging oxygen. This modification is mediated by the products of the dndABCDE gene cluster. Unmodified backbone of foreign DNA is recognized and cleaved by DndFGH proteins (27–29). In many bacterial strains, it is found that the phosphorothioate modification exists without the presence of the cognate restriction component (29).
In addition to DNA modification, nucleotide modifications in RNA are also widely found in bacteria. rRNA and tRNA harbor the majority of RNA modifications. Since bacterial rRNA and tRNA modifications have been extensively studied and reviewed in the past (30–32), we will not be discussing these modifications in this review. The presence of RNA modifications in bacterial mRNA has been discovered only recently. Methylation of adenine residue at the N6 position in the sequence 5′-GCCAG-3′ has been found to occur in the transcripts of E. coli and Pseudomonas aeruginosa. The m6A modification is found to be enriched in the open reading frames (ORFs) of the transcripts (33). The enzyme(s) which brings about m6A modification of mRNA in bacteria is yet to be identified. Another newly discovered modification of bacterial mRNA, a 5′ NAD cap, has been shown to prevent degradation of mRNA in vitro although the physiological significance is yet to be identified (30, 34). Modifications involved in bacterial epigenetics have been summarized in Fig. 2.
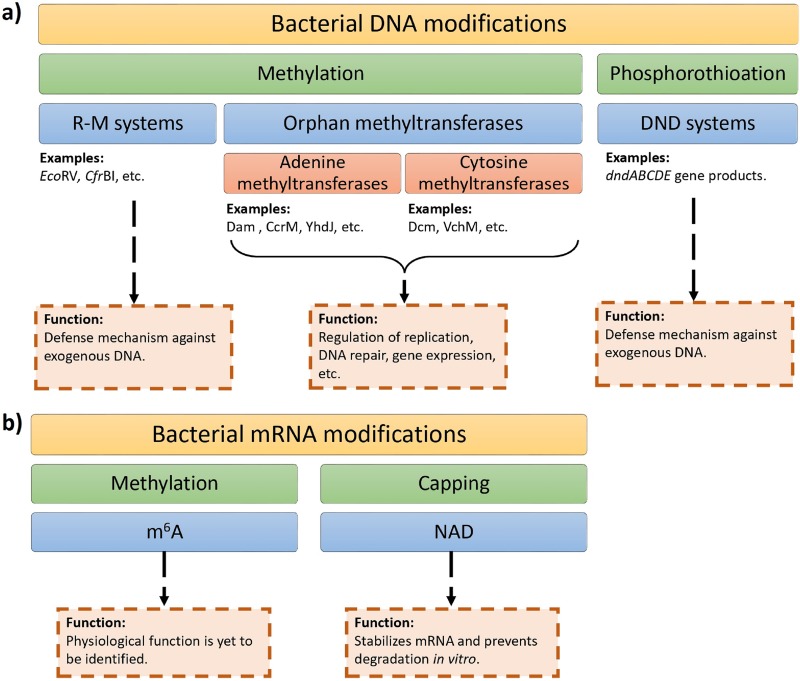
FIG 2.
Overview of bacterial epigenetics. (a) There are two broad classes of bacterial DNA modifications: methylation of adenines and cytosines and phosphorothioation of the DNA backbone, where a nonbridging oxygen gets replaced by sulfur. Bacterial DNA methylation is mediated by enzymes belonging to the restriction-modification (R-M) systems or orphan methyltransferases. Phosphorothioation is facilitated by the gene products of a DNA degradation (DND) system, dndABCDE. (b) Two classes of epigenetic modifications of bacterial mRNA have been discovered: methylation of adenine at N6 and NAD capping at the 5′ end.
EPIGENETIC TAGS AND BACTERIAL GENE EXPRESSION
Methylation brought about by DNA methyltransferases from R-M systems as well as orphan methyltransferases can alter gene expression by directly modulating the binding of RNA polymerase to promoters. For example, RNA polymerase cannot bind to the transposon Tn10 promoter with fully methylated GATC sites. RNA polymerase can bind only hemimethylated DNA, and, thus, Tn10 gets expressed only transiently following replication (35). Dam-mediated GATC methylation also represses the transcription of many genes including trpR and trpS (which control tryptophan operon in E. coli) due to decreased binding of RNA polymerase (10, 36). Dam-mediated regulation of gene expression during phase variation will be discussed later in this review with specific examples. Methyltransferase-mediated feedback repression is common in many bacteria (37–40). For example, in Shigella sonnei, a methyltransferase of the R-M system, SsoII, mediates its own repression through a negative-feedback loop (40). Negative regulation of the methyltransferases in R-M systems is crucial to ensure that the phage DNA does not get methylated along with that of the host.
Methylation can also promote gene expression. The essential cell cycle regulator in Caulobacter crescentus, GcrA, is a σ70 cofactor which binds to the majority of σ70-dependent promoters, but it is postulated that it can activate transcription only if a nearby methylated adenine stabilizes it (41). Methylation of the gene promoter of the phase-variable outer membrane protein antigen 43, which plays a role in biofilm formation (42), auto-aggregation, and colony morphology (43), prevents the binding of its repressor OxyR in E. coli. The repressor can bind to the promoter only after replication, when the DNA is present in a hemimethylated state (44). The tra (transfer) operon in the virulence plasmid pSLT in Salmonella is transcriptionally activated by the protein encoded by traJ. Dam methylation negatively regulates the expression of traJ. A bacterial small RNA (sRNA) encoded by finP, which represses TraJ expression, gets activated by Dam methylation (10, 45). As horizontal gene transfer (HGT) contributes significantly to acquisition of drug resistance, epigenetic control of transfer operons which can mediate conjugal gene transfer can perhaps indirectly aid in dissemination of antibiotic resistance-associated genes.
Methylation of cytosines is primarily repressive. In Vibrio cholerae, DNA-protein interactions, such as transcription factor binding, occur in regions which exhibit underrepresentation of cytosine methylation by VchM (an orphan Mtase) (46). Even in E. coli, expression of genes encoding ribosomal proteins is negatively regulated by Dcm-mediated methylation (24, 47).
EVIDENCE LINKING EPIGENETICS AND ANTIBIOTIC RESISTANCE
Over the years, literature has primarily focused on the genetic basis for the development of antimicrobial resistance in bacteria. But some findings suggest that changes in the DNA sequence alone cannot fully explain neither the rapidity of the development of resistance nor the reversibility to susceptible phenotype. There is evidence that suggests that epigenetic mechanisms may contribute to resistance development. They are discussed in the following sections.
Adaptive resistance.
When bacteria are subjected to increasing subinhibitory concentrations of an antibiotic, they develop adaptive resistance to the antibiotic (48–50). The characteristic feature of adaptive resistance is the rapidity with which the resistance phenotype emerges and reverts to a susceptible phenotype upon the withdrawal of the antibiotic. The survival rate of bacteria in subinhibitory concentrations of antibiotics cannot be explained by genetic mutations as expected mutation rates that lead to resistance are lower than the observed survival rates (48). Genetic changes also cannot explain the high reversion rates because for the bacteria to revert to susceptible phenotype, compensatory or back mutations are needed, and these are known to occur at a very low rate (51, 52). Adaptive resistance is the consequence of the presence of heritable phenotypic variations even in genetically identical bacteria from isogenic populations. Culture-based evolution studies, investigation of genetic knockout and transcriptomes, and modeling-based studies suggest that epigenetic inheritance and stochastic heterogeneity in gene expression patterns are responsible for this phenomenon (48, 51, 53–55).
As discussed in the previous section, epigenetic tags are known to influence the expression of genes in bacteria by modulating the binding of RNA polymerase or other factors such as transcription factors, repressors, etc. Figure 3 is a putative representation of the findings, which suggest that epigenetics can contribute to the development of adaptive resistance. Epigenetic inheritance is dynamic in nature and thus can explain the transience of adaptive resistance. The resistance which develops when bacteria grow in the presence of nonlethal concentrations of antibiotics could be the consequence of changes in the epigenetic landscape of the bacterial genome. As soon as the antibiotic stress is removed from the environment, the epigenetic changes are no longer sustained on the genome of the subsequent generations, and, thus, they retrogress to a susceptible phenotype.
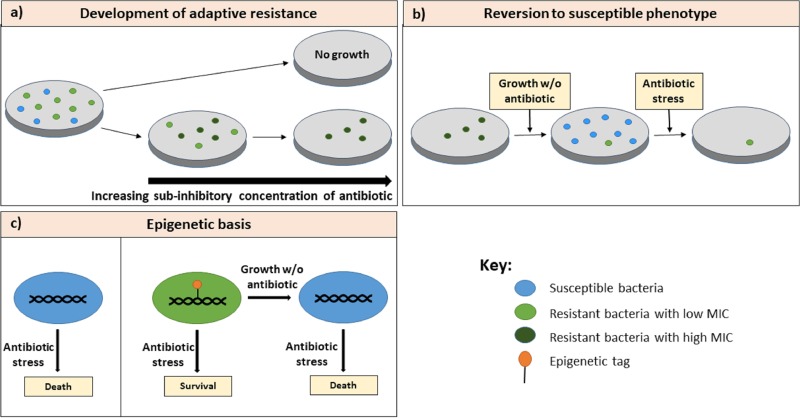
FIG 3.
Epigenetic basis of adaptive resistance. (a) When bacteria are exposed to subinhibitory concentrations of an antibiotic, they acquire adaptive resistance and are able to survive in increasing concentrations of the antibiotic. (b) When the antibiotic is withdrawn, the bacteria that have acquired adaptive resistance revert to the susceptible phenotype (48). (c) The instability of the resistance phenotype can be explained by the dynamic nature of epigenetic inheritance that governs gene expression. In the presence of the antibiotic, the epigenetic landscape of the resistant bacteria is passed on to subsequent generations, whereas in the absence of the antibiotic, the epigenetic tags are lost. Epigenetic changes thus modulate gene expression patterns, allowing the bacteria to switch between susceptible and resistant phenotypes.
Phase variation.
Bacteria can survive highly dynamic environments by rapidly modulating the expression of certain genes in a switch-on/switch-off manner, and this reversible switch is called phase variation. There are several ways by which phase variation is brought about, such as DNA inversion, methylation/demethylation of gene promoters or other regulatory sequences, slipped-strand mispairing, homologous recombination, and transposition (56, 57). Changes in methylation profiles of DNA regulate phase variation in bacteria, but the reverse is true as well, wherein phase variation leads to regulation of the expression of methyltransferases or changes in their target specificities (58–66).
Expression of the pyelonephritis-associated pilus (pap) operon in uropathogenic E. coli (67) and alteration of the length of lipopolysaccharide (LPS) O antigen in Salmonella enterica (which provides resistance to bacteriophages that use O antigen as receptor) (60) are well-studied examples of GATC methylation-mediated control of phase-variable gene expression. In Streptococcus pneumoniae, random switching that leads to genetic rearrangements in the hsdS gene of a type I restriction-modification system, called SpnD39III, generates six different variants of the gene, with each variant having a different target specificity. The six subpopulations that arise out of these rearrangements are phenotypically dissimilar due to global changes in methylation profiles (58).
Different DNA methyltransferases which exhibit phase-variable expression have been discovered in bacteria, including Helicobacter pylori (64), Neisseria (63, 68), and Haemophilus influenzae (66). Interestingly, phase-variable adenine DNA methyltransferases (Mod proteins) can influence susceptibility to certain antibiotics in Neisseria meningitidis. Common phase-variable methyltransferases found in N. meningitidis, ModA11 and ModA12, increase susceptibility to cloxacillin, ciprofloxacin, etc., which are widely used antibiotics for treatment of meningococcal disease. Absence of ModA11 has been associated with reduced susceptibility to ciprofloxacin and ceftazidime by 4-fold (62). It is unclear why bacteria retain methyltransferases that may increase drug susceptibility during their evolution. It is possible that these methyltransferases modulate the expression of other genes during phase variation to provide a hitherto unrecognized survival advantage to bacteria in a rapidly changing environment. Although the exact mechanisms of how these Mod proteins affect resistance or susceptible phenotype are not known, these results do indicate that epigenetic regulation of gene expression plays a role in antimicrobial resistance.
Persistence and heteroresistance.
A heterogenous response by members of an isogenic population to the same environmental cues resulting in varying fitness and survival under stress is called phenotypic heterogeneity (69, 70). This can sometimes lead to the generation of two distinct subpopulations, a phenomenon known as bistability (70, 71). Phenotypic heterogeneity and the resulting bistability can contribute to adaptive resistance (72) and formation of persister cells. Persister cells are typically described as slow-growing or growth-arrested cells in a bacterial population which can survive antibiotic treatment although recent evidence suggests that persister cells need not have altered growth rates (73). Upon the withdrawal of therapy, the persister cells can lead to a relapse of infection and thus are a major cause of recurrent infections. The presence of persister cells in a bacterial population is considered to be a bet-hedging strategy to ensure survival under changing milieus. But persister cells can also arise in response to environmental stresses. There are several mechanisms by which persisters arise, including the following: (i) spontaneous persistence via toxin/antitoxin systems and stochastic variability in expression of stress-related genes, (ii) environmental induction such as heat shock, nutrient deprivation, etc., (iii) inactivation of drug targets, (iv) reduction in drug uptake, (v) biofilm formation, (vi) quorum sensing from other persister cells, and (vii) a host immune response such as phagocytosis (74–78). Interestingly, epigenetic inheritance has been linked to the evolution of the persister phenotype. The plasticity of epigenetic inheritance along with within-generation heterogeneity (especially in stationary phase) can explain the rapid evolution of persister cells in changing environments (79). Genetic changes resulting in resistance to antibiotics has been suggested to follow persistence as a low rate of cell division coupled with stress-induced mutations provides a stable groundwork for adaptive evolution (74, 80, 81). The contribution of bacterial persistence to eventual development of resistance-conferring mutations has been illustrated in Fig. 4.
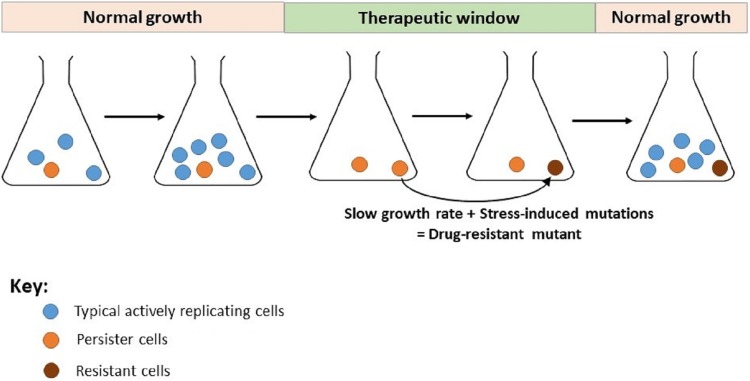
FIG 4.
Bacterial persistence can contribute to development of resistance-conferring mutations. Persister cells represent a small proportion of the bacterial population that are growth arrested or slow growing. Persister cells are neither defective nor have specific genetic changes; they are present in bacterial populations as a seed bank to survive rapidly changing environments. When the bacterial population is subjected to antibiotic therapy, typical bacterial cells rapidly decline in numbers, but the persister cells are able to survive in the presence of antibiotics. Persister cells can even be induced by environmental stresses or other factors. Epigenetic inheritance has been predicted to be a key player contributing to phenotypic drug tolerance in persister cells. Persister cells can lead to adaptive evolution of drug-resistant mutants due to the low rate of growth and mutations induced by stress conditions.
In an isogenic bacterial population, subpopulations can exhibit heterogeneous susceptibilities to an antibiotic, and this phenomenon is termed heteroresistance. Heteroresistance has been reported in both Gram-positive and Gram-negative bacteria (82–84), and it is a cause for concern as it can lead to antibiotic treatment failures (85). Epigenetic regulation of gene expression has been suggested to be one of the potential mechanisms that explain heteroresistance (86–88). Heteroresistance can increase the likelihood of the generation of genetic resistance to antibiotics (88).
Contrasting roles of Dam- and Dcm-mediated methylation in E. coli.
An inverse correlation exists between resistance to a fluoroquinolone (ciprofloxacin) and global m5C levels in genomic DNA of resistant E. coli. An analysis of 40 clinical isolates of E. coli, including 30 ciprofloxacin-resistant isolates, suggests that the global levels of m5C in genomic DNA inversely correlated to the MIC of ciprofloxacin. Ciprofloxacin-sensitive E. coli isolates had higher levels of m5C than ciprofloxacin-resistant isolates (89).
SugE is a well-studied multidrug efflux system protein belonging to the small multidrug resistance (SMR) protein family. Overexpression of SugE in E. coli has been linked to increased resistance to quaternary ammonium compounds (90). The promoter region and the gene body of the SugE gene in E. coli have one Dcm site [i.e., 5′-CC(A/T)GG-3′] and three Dcm sites, respectively. Interestingly, E. coli dcm mutants expressed SugE mRNA at 7-times-higher levels than dcm-expressing wild-type E. coli. Importantly, increased SugE expression led to increased resistance to ethidium bromide. Furthermore, dcm mutants of E. coli expressed much higher levels of a stationary-phase sigma factor, RpoS. Robust SugE expression in the early stationary phase of E. coli is dependent on RpoS expression. In other words, Dcm may (i) directly repress SugE expression by promoter/gene body methylation and (ii) indirectly repress SugE expression by promoter/gene body methylation of a sigma factor (RpoS) that modulates the transcription of SugE (91). This example highlights a multilayered control of efflux pump proteins by dcm-mediated methylation. Another study has linked the antimicrobial resistance phenotype in Enterobacter cloacae to SugE expression (92). It would be interesting to study the role of epigenetic players in modulation of SugE expression in E. cloacae.
Dam influences virulence of uropathogenic E. coli by regulating expression of the pap operon. The results of a recent study (93) indicate that Dam also influences resistance to several antibiotics. In dam mutants, a severalfold decrease in resistance was observed for ciprofloxacin, gentamicin, etc. This suggests that Dam-mediated N6-adenosine methylation facilitates resistance development in E. coli. An earlier study has also shown similar results wherein a 5-fold increase in survival rate in the presence of nalidixic acid was observed when dam mutants were complemented with expression plasmids carrying a functional dam gene (48). Investigation of the E. coli genome using single-molecule real-time (SMRT) sequencing has revealed that Dam-mediated GATC methylation is crucial for prevention of deleterious mutations and survival of bacteria under antibiotic stress and that dam strains exhibit hypersensitivity toward quinolone and β-lactam antibiotics (55).
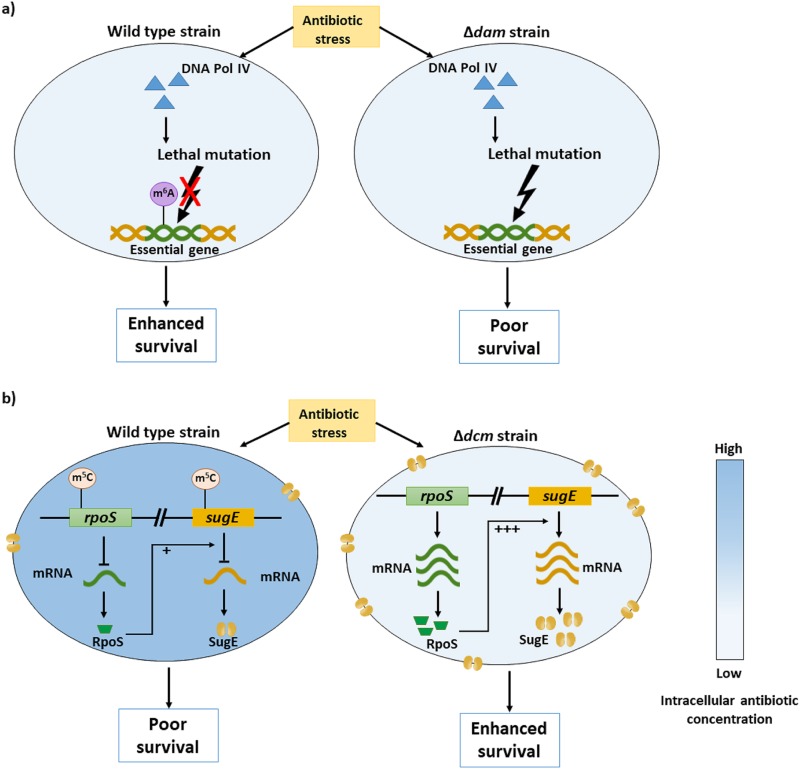
These studies clearly point toward the opposing roles of adenine and cytosine methylation in E. coli. While Dcm-mediated cytosine methylation increases susceptibility to antibiotics, GATC methylation by Dam gives a survival advantage to E. coli under antibiotic stress (Fig. 5).
FIG 5.
Contrasting roles of Dam- and Dcm-mediated methylation in antibiotic stress. Global methylation profiling identified increased adenine methylation and reduced cytosine methylation in antibiotic-resistant bacteria compared to levels in bacteria susceptible to antibiotics. (a) Dam-mediated adenine methylation facilitates DNA repair, reducing deleterious mutations in the bacterial genome and enhancing bacterial survival under antibiotic stress (55). (b) On the other hand, cytosine methylation has been linked to reduced expression of resistance-conferring genes such as sugE (a multidrug efflux pump) and rpoS (a sigma factor which modulates sugE expression), leading to poor survival under antibiotic stress. Robust rpoS expression from the Δdcm strain can enhance transcription of the sugE gene, representing a second layer of control or downstream effects linked to epigenetic control of gene expression in bacteria. In other words, sugE transcript levels are controlled by epigenetic mechanisms at two levels, as follows. (i) The presence of methylated cytosines in the sugE promoter or gene body is associated with reduced levels of sugE transcripts. (ii) The extent of inhibition of sugE transcription is further augmented by the lack of rpoS (a transcription factor) expression (91).
INTERPLAY BETWEEN EPIGENETICS AND GENETICS
Genetic changes or rearrangements in methyltransferases can change target specificities (59, 94), thus altering the epigenetic landscape of bacterial genomes. Conversely, methylation of adenine and cytosine residues can alter the genetic makeup by either facilitating or preventing mutations.
Methylated cytosines act as mutational hot spots.
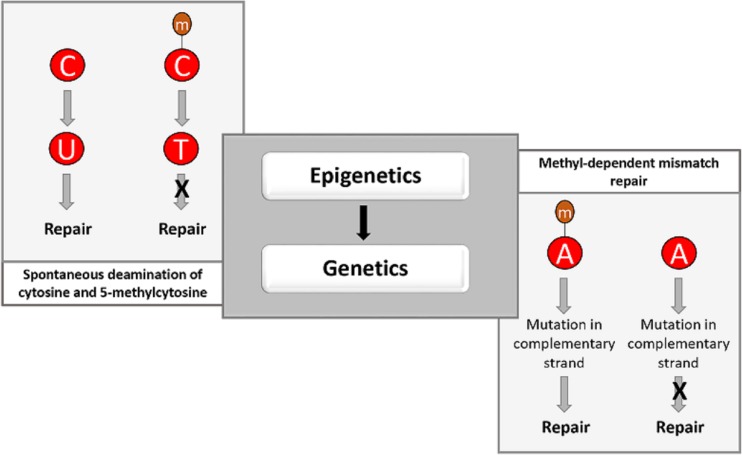
Cytosine undergoes spontaneous deamination to uracil. Since the presence of uracil is atypical in genomic DNA, it gets excised by uracil-DNA glycosylase, initiating the base excision repair pathway. But spontaneous deamination of 5-methylcytosine (m5C) yields thymine, which is not easily recognized by the DNA repair machinery and thus may not get excised or repaired (95). This results in fixing of the mutation in the genome (Fig. 6, left box). The frequency of deamination of m5C is 2 to 4 times higher than that of cytosine (96). Methylated cytosines are regarded as hot spots for cytosine to thymine mutations in bacteria (97) although very-short-patch (VSP) mismatch repair may reduce the frequency of such mutations (98).
FIG 6.
The epigenetic landscape influences bacterial genetics. Spontaneous deamination of cytosine yields uracil that is recognized by the DNA repair machinery, which corrects it back to cytosine. However, in case of methylated cytosines, deamination yields thymines which are not recognized as aberrant nucleotides in DNA, making an irreparable lesion; during subsequent DNA replication the thymine base pairs with adenine, and the lesion becomes permanent or fixed (left box). Methyl-dependent mismatch repair mechanisms correct mutations in the complementary strand during replication; this mode of repair is dependent on the presence of GATC sites with methylated adenines on the parental strand in the vicinity of the lesion on the complementary (nascent) strand. In the absence of methylated adenine in the parent strand, the mutation in the complementary (nascent) strand is not repaired (right box).
Overexpression of the methyltransferases, Dcm and EcoRII methylase, in E. coli resulted in almost a 50-fold increase in C-to-T transitions at the canonical site of methylation (i.e., 5′-CC(A/T)GG-3′; the substitution is underlined). Interestingly, the overexpression of these methyltransferases also led to a 10-fold increase in the frequency of C-to-T transitions at a noncanonical site (i.e., 5′-CCGGG-3′). This finding suggests that promiscuous methylation at noncanonical sites may significantly increase the extent of methylation-mediated genetic changes than previously predicted (99). In addition, methylated cytosine derivatives, including 5-hydroxymethylcytosine, have been shown to induce C-to-T transitions in E. coli (100). A recent report analyzing whole-genome sequences of thousands of bacteria suggests that cytosine methylation may increase the rate of C-to-T transitions by over 50-fold (101).
Environmental stresses, including exposure to antibiotics, induce the expression of error-prone polymerases to increase genetic variability of bacterial genomes (102). Occurrence of stable mutations that facilitate antibiotic resistance requires a high frequency of mutations. Methylation of cytosine provides a stable framework for the emergence of mutations which can potentially be either deleterious or beneficial for bacteria under antibiotic stress, depending on the site of mutation. Studies investigating the synergy between antibiotic stress-induced error-prone polymerases and C-to-T transitions of methylated cytosines may help us better understand the specific role of methylation-mediated genetic changes in regulating antimicrobial resistance.
Methyl-dependent mismatch repair requires GATC methylation.
In E. coli, a methyl-dependent mismatch repair system (also called MutHLS system) corrects insertion/deletion loops (which cause frameshifts) as well as base pair mismatches (103). These mismatches can arise due to multiple reasons, including spontaneous deamination of C/m5C, replication errors, etc. But their frequency increases under stress conditions due to the induction of error-prone DNA polymerases such as Pol IV (104) and Pol V (105). The MutHLS system requires Dam-mediated GATC methylation to differentiate between a newly synthesized strand and a parental strand; the GATC sites on the parental strands are methylated while the new strand lacks methylated GATC sites. MutH is the endonuclease which preferentially nicks the new strand at an unmethylated GATC site nearest to the mismatch. The repair machinery gets recruited to the site of the nick, and helicase displaces the new strand and is followed by exonuclease-mediated cleavage, resynthesis, and ligation (103). In the absence of methylated GATC sites, the MutHLS system generates lethal double-stranded breaks in the DNA (106) (Fig. 6, right box).
Poor survival rates of E. coli dam mutants in the presence of antibiotics has been reported (55). Antibiotic stress induces the expression of dinB which encodes the mutagenic DNA Pol IV; as a result, higher survival rates were documented for dam dinB mutants under antibiotic stress. However, as mismatch repair by the MutHLS system is dependent on Dam-mediated GATC methylation and as dam mutants lack methylated GATC sites, survival of dam mutants is compromised due to increased mutations generated by DNA Pol IV. Better survival of dam mutants in the presence of ampicillin after deletion of MutH- or MutS-encoding genes also clearly indicate that MutHLS-mediated double-stranded DNA breaks are fatal in the absence of GATC methylation.
AN EPIGENETIC LINK TO UNCHARTED MECHANISMS IN ANTIBIOTIC RESISTANCE
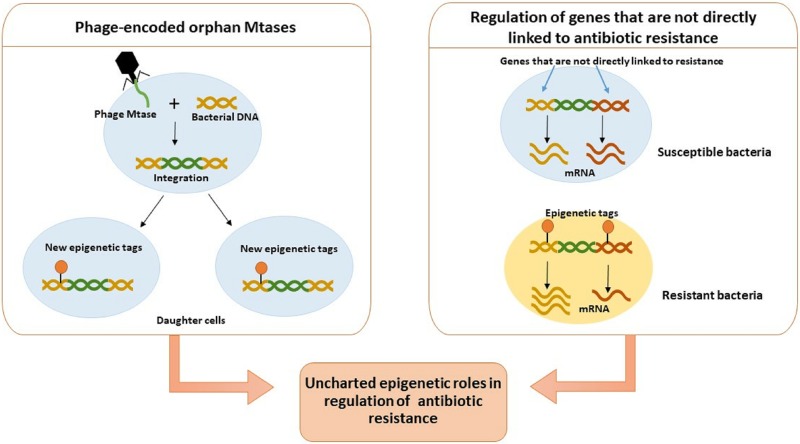
There are several gray areas in bacterial epigenetics which need to be explored further to understand if they have any influence on the rapidity and high frequency of antimicrobial resistance development. A potential role for epigenetic mechanisms will be discussed in two nonoverlapping themes (Fig. 7) in the following sections.
FIG 7.
Uncharted epigenetic mechanisms and their putative role in antibiotic resistance. Some epigenetic mechanisms are putatively associated with antibiotic resistance but remain poorly documented or partially understood. When methyltransferase-encoding phages infect bacteria, the phage DNA encoding methyltransferases (Mtases) can get integrated into the bacterial genome and influence the methylomes of the daughter cells. The link between altered methylomes and antibiotic resistance has been proposed by several groups, but direct evidence for this hypothesis is still lacking (left box). Epigenetic regulation of expression of genes that are not directly associated with antibiotic resistance has been predicted to contribute to the resistant phenotype, but the underlying mechanisms are poorly understood (right box).
Phage-encoded orphan methyltransferases.
Analysis of full-length bacterial genomes suggests that a sizable portion of their genome is made up of prophages or phage DNA that has integrated into bacterial genomes (107). Bacteriophages can incorporate bacterial genes into their own genome, including DNA methyltransferases. Not only does this facilitate HGT among bacteria, but it also helps these phages overcome the R-M systems of newer hosts, thus broadening their host range (108). Over 800 putative orphan methyltransferases are present in currently available annotated phage genomes, which account for almost 20% of these genomes, and they include m6A, m5C, and m4C classes of methyltransferases (108). Orphan adenine methyltransferases homologous to Dam are present in several bacteriophages such as phage T4, phage P1, phage Vibrio harveyi myovirus-like (VHML), 936-type phages, etc. (10, 108–112). The phage T4 adenine methyltransferase has the same target site (i.e., GATC) as Dam of E. coli, and it protects the viral DNA from cleavage by restriction endonucleases which recognize this site (109, 113). The methyltransferase of phage P1 also methylates the adenine residue in the sequence GATC (111), and it plays a role in the packaging of DNA in addition to protection from host endonuclease (108, 114). It has been proposed that in the bacteriophage VHML, GATC methylation in the ORF of the rha antirepressor gene mediates the switch from the lytic to lysogenic cycle (110). Evidently, methyltransferases encoded by phages have functions other than protection from host R-M defense systems. Furthermore, orphan methyltransferases in bacteriophages may methylate cytosines or adenines at multiple recognition sites (115). We speculate that since phages can integrate into host genomes and also mediate HGT among bacteria, genes which encode phage methyltransferases may integrate into host chromosomal DNA and potentially influence the bacterial methylome of subsequent generations as shown in Fig. 7 (left box). While the contribution of prophages to genomic diversification in bacteria (107), modulation of fitness (116), and virulence of bacteria (107) is known, their specific roles in contributing to antimicrobial resistance is not well studied.
Regulation of genes that are not directly associated with antibiotic resistance.
In several bacterial species, resistance-associated intrinsic genes such as efflux pumps are upregulated in the presence of antibiotic stress. Bacterial sRNAs, which can be regulated by DNA methylation (45), can influence the expression of efflux pumps under antibiotic stress. Recently, a genome-wide interrogation using single-molecule real-time (SMRT) sequencing was used to identify differentially methylated genes in laboratory-generated rifampin- and isoniazid-resistant Mycobacterium tuberculosis strains (117). The investigators found that in both rifampin- and isoniazid-resistant strains, 175 genes were hypermethylated while 160 genes exhibited hypomethylation compared to levels in sensitive strains. Interestingly, it was observed that the nitrogen metabolism pathway was enriched for differentially methylated genes in both rifampin- and isoniazid-resistant strains. Also, rifampin, which inhibits transcription by targeting RNA polymerase, induces differential methylation in genes of the ribosome pathway. Clearly, antibiotic stress influences the methylation profile of genes involved in essential pathways, but further investigation is required to understand the significance or the role of the differential methylation status of these genes in antibiotic-resistant bacteria. Additionally, we reflect that since conditionally essential genes (such as enzymes responsible for utilizing carbon sources, biosynthesis of membrane proteins, amino acids, etc.) aid in the survival of bacteria under nutrient deficit or other stress conditions (118), this group of genes should be scrutinized to discern if epigenetic regulation plays a role in their expression, leading to better survival under stress conditions, including antibiotic stress (Fig. 7, left box).
CHALLENGES IN UNDERSTANDING THE EPIGENETICS OF ANTIBIOTIC RESISTANCE
Our current understanding of bacterial epigenetics is still elementary. For example, the role of methylation in bacterial genomes is not well understood beyond R-M systems. Modifications in eukaryotic mRNAs regulate cellular processes such as translation, mRNA decay, etc. (119, 120). As nucleotide modifications in bacterial mRNAs are being increasingly reported (33), understanding the role of epigenetics in bacteria has become crucial. The need to understand the role of epigenetics in antimicrobial resistance in bacteria, genome- and transcriptome-wide interrogation and functional analyses of differentially methylated genes or regulatory elements are increasingly recognized. Genome-wide high-resolution mapping of epigenetic landscapes have started to emerge with the advent of single molecule real-time (SMRT) sequencing technology and nanopore-based sequencing technology. These technologies allow the detection of various types of nucleotide modifications in the same run, such as m6A, m5C, m4C, etc., without the need for prior chemical treatment to identify modified nucleotides (121–125). In addition, new sequencing technologies allow single-base resolution of bacterial transcriptomes, thus enabling direct identification of nucleotide modifications in mRNAs. But a major drawback of these sequencing technologies is the dependence on populations to achieve a consensus sequence which provides no information on the epigenetic heterogeneity which exists within different cells of isogenic populations. As discussed earlier in this review, epigenetic heterogeneity is a major driver of adaptive resistance. Researchers are now attempting to overcome this limitation by developing newer pipelines which allow better resolution and detection of epigenetic heterogeneity (122). Although curated databases for bacterial gene regulation are now available for a few bacteria (126, 127), epigenetic regulatory networks in bacteria remain poorly understood to be cataloged.
CONCLUSION AND FUTURE PERSPECTIVES
Since well-documented biochemical or genetic changes fail to fully explain mechanisms underlying antibiotic resistance, it is becoming increasingly evident that we need to shift our focus to newer, nonclassical mechanisms such as epigenetic regulation. The biological role of epigenetic modifications in modulating gene expression and other cellular processes is being increasingly recognized. New epigenetic modifications are being discovered in both prokaryotes and eukaryotes, including phosphorothioation in bacterial DNA backbone (27, 29) and acetylation of cytidine in eukaryal mRNA (120). Since it has been established that modifications in eukaryotic mRNAs can modulate a variety of cellular processes (119, 120), it may not be too speculative to suggest that such modifications in bacterial transcripts may be linked to critical functions in the bacterial life cycle. Even though m6A modification in bacterial mRNAs has been identified, the epigenetic writers (the enzymes that add the epigenetic tag to the nucleotide), readers, etc., for this modification are yet to be identified. The influence of epigenetics on the heterogeneity among members of isogenic bacterial populations merits detailed studies.
While the literature from the last decade reviewed here unambiguously indicates a role for epigenetics in antibiotic resistance among bacteria, we believe that precise mapping of epigenetic tags on bacterial genomes and their functional analysis will help create a paradigm shift in our understanding of this field. Furthermore, epigenetic modifications on bacterial genomes represent new diagnostic markers as well as novel drug targets.
ACKNOWLEDGMENTS
Dipannita Ghosh is a recipient of CSIR-Junior Research Fellowship from Council of Scientific and Industrial Research (CSIR), Government of India.
We received no specific funding for this work.
We have no conflicts of interest to report.
REFERENCES
- 1.Kohanski MA, Dwyer DJ, Collins JJ. 2010. How antibiotics kill bacteria: from targets to networks. Nat Rev Microbiol 8:423–435. doi: 10.1038/nrmicro2333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Skippington E, Ragan MA. 2011. Lateral genetic transfer and the construction of genetic exchange communities. FEMS Microbiol Rev 35:707–735. doi: 10.1111/j.1574-6976.2010.00261.x. [DOI] [PubMed] [Google Scholar]
- 3.Melnyk AH, Wong A, Kassen R. 2015. The fitness costs of antibiotic resistance mutations. Evol Appl 8:273–283. doi: 10.1111/eva.12196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Munita JM, Arias CA. 2016. Mechanisms of antibiotic resistance. Microbiol Spectr 4:VMBF-0016-2015. doi: 10.1128/microbiolspec.VMBF-0016-2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Depardieu F, Podglajen I, Leclercq R, Collatz E, Courvalin P. 2007. Modes and Modulations of Antibiotic Resistance Gene Expression. Clin Microbiol Rev 20:79–114. doi: 10.1128/CMR.00015-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Davies J, Davies D. 2010. Origins and evolution of antibiotic resistance. Microbiol Mol Biol Rev 74:417–433. doi: 10.1128/MMBR.00016-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Harbottle H, Thakur S, Zhao S, White DG. 2006. Genetics of antimicrobial resistance. Anim Biotechnol 17:111–124. doi: 10.1080/10495390600957092. [DOI] [PubMed] [Google Scholar]
- 8.Li X-Z, Plésiat P, Nikaido H. 2015. The challenge of efflux-mediated antibiotic resistance in Gram-negative bacteria. Clin Microbiol Rev 28:337–418. doi: 10.1128/CMR.00117-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Berger SL, Kouzarides T, Shiekhattar R, Shilatifard A. 2009. An operational definition of epigenetics. Genes Dev 23:781–783. doi: 10.1101/gad.1787609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Casadesús J, Low D. 2006. Epigenetic gene regulation in the bacterial world. Microbiol Mol Biol Rev 70:830–856. doi: 10.1128/MMBR.00016-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Adhikari S, Curtis PD. 2016. DNA methyltransferases and epigenetic regulation in bacteria. FEMS Microbiol Rev 40:575–591. doi: 10.1093/femsre/fuw023. [DOI] [PubMed] [Google Scholar]
- 12.Putnam CD. 2016. Evolution of the methyl directed mismatch repair system in Escherichia coli. DNA Repair (Amst) 38:32–41. doi: 10.1016/j.dnarep.2015.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li GM. 2008. Mechanisms and functions of DNA mismatch repair. Cell Res 18:85–98. doi: 10.1038/cr.2007.115. [DOI] [PubMed] [Google Scholar]
- 14.Bogan JA, Helmstetter CE. 1997. DNA sequestration and transcription in the oriC region of Escherichia coli. Mol Microbiol 26:889–896. doi: 10.1046/j.1365-2958.1997.6221989.x. [DOI] [PubMed] [Google Scholar]
- 15.Campbell JL, Kleckner N. 1990. E. coli oriC and the dnaA gene promoter are sequestered from dam methyltransferase following the passage of the chromosomal replication fork. Cell 62:967–979. doi: 10.1016/0092-8674(90)90271-F. [DOI] [PubMed] [Google Scholar]
- 16.Casadesús J, Low DA. 2013. Programmed heterogeneity: epigenetic mechanisms in bacteria. J Biol Chem 288:13929–13935. doi: 10.1074/jbc.R113.472274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McClelland M, Sanderson KE, Spieth J, Clifton SW, Latreille P, Courtney L, Porwollik S, Ali J, Dante M, Du F, Hou S, Layman D, Leonard S, Nguyen C, Scott K, Holmes A, Grewal N, Mulvaney E, Ryan E, Sun H, Florea L, Miller W, Stoneking T, Nhan M, Waterston R, Wilson RK. 2001. Complete genome sequence of Salmonella enterica serovar Typhimurium LT2. Nature 413:852–856. doi: 10.1038/35101614. [DOI] [PubMed] [Google Scholar]
- 18.Julio SM, Heithoff DM, Provenzano D, Klose KE, Sinsheimer RL, Low DA, Mahan MJ. 2001. DNA adenine methylase is essential for viability and plays a role in the pathogenesis of Yersinia pseudotuberculosis and Vibrio cholerae. Infect Immun 69:7610–7615. doi: 10.1128/IAI.69.12.7610-7615.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Broadbent SE, Balbontin R, Casadesus J, Marinus MG, van der Woude M. 2007. YhdJ, a nonessential CcrM-Like DNA methyltransferase of Escherichia coli and Salmonella enterica. J Bacteriol 189:4325–4327. doi: 10.1128/JB.01854-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zweiger G, Marczynski G, Shapiro L. 1994. A Caulobacter DNA methyltransferase that functions only in the predivisional cell. J Mol Biol 235:472–485. doi: 10.1006/jmbi.1994.1007. [DOI] [PubMed] [Google Scholar]
- 21.Fioravanti A, Fumeaux C, Mohapatra SS, Bompard C, Brilli M, Frandi A, Castric V, Villeret V, Viollier PH, Biondi EG. 2013. DNA binding of the cell cycle transcriptional regulator GcrA depends on N6-adenosine methylation in Caulobacter crescentus and other Alphaproteobacteria. PLoS Genet 9:e1003541. doi: 10.1371/journal.pgen.1003541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kahng LS, Shapiro L. 2001. The CcrM DNA methyltransferase of Agrobacterium tumefaciens is essential, and its activity is cell cycle regulated. J Bacteriol 183:3065–3075. doi: 10.1128/JB.183.10.3065-3075.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wright R, Stephens C, Shapiro L. 1997. The CcrM DNA methyltransferase is widespread in the alpha subdivision of proteobacteria, and its essential functions are conserved in Rhizobium meliloti and Caulobacter crescentus. J Bacteriol 179:5869–5877. doi: 10.1128/jb.179.18.5869-5877.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kahramanoglou C, Prieto AI, Khedkar S, Haase B, Gupta A, Benes V, Fraser GM, Luscombe NM, Seshasayee ASN. 2012. Genomics of DNA cytosine methylation in Escherichia coli reveals its role in stationary phase transcription. Nat Commun 3:886–889. doi: 10.1038/ncomms1878. [DOI] [PubMed] [Google Scholar]
- 25.Banerjee S, Chowdhury R. 2006. An orphan DNA (cytosine-5-)-methyltransferase in Vibrio cholerae. Microbiology 152:1055–1062. doi: 10.1099/mic.0.28624-0. [DOI] [PubMed] [Google Scholar]
- 26.Doron S, Melamed S, Ofir G, Leavitt A, Lopatina A, Keren M, Amitai G, Sorek R. 2018. Systematic discovery of antiphage defense systems in the microbial pangenome. Science 359:eaar4120. doi: 10.1126/science.aar4120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Xu T, Yao F, Zhou X, Deng Z, You D. 2010. A novel host-specific restriction system associated with DNA backbone S-modification in Salmonella. Nucleic Acids Res 38:7133–7141. doi: 10.1093/nar/gkq610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang L, Jiang S, Deng Z, Dedon PC, Chen S. 2019. DNA phosphorothioate modification–a new multi-functional epigenetic system in bacteria. FEMS Microbiol Rev 43:109–122. doi: 10.1093/femsre/fuy036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tong T, Chen SS, Wang L, Tang Y, Ryu JY, Jiang S, Wu X, Chen C, Luo J, Deng Z, Li Z, Lee SY, Chen S. 2018. Occurrence, evolution, and functions of DNA phosphorothioate epigenetics in bacteria. Proc Natl Acad Sci U S A 115:E2988–E2996. doi: 10.1073/pnas.1721916115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Marbaniang CN, Vogel J. 2016. Emerging roles of RNA modifications in bacteria. Curr Opin Microbiol 30:50–57. doi: 10.1016/j.mib.2016.01.001. [DOI] [PubMed] [Google Scholar]
- 31.Vendeix FAP, McCloskey JA, Rozenski J, Harris KA, Crain PF, Cantara WA, Zhang X, Fabris D, Agris PF. 2011. The RNA modification database, RNAMDB: 2011 update. Nucleic Acids Res 39:D195–D201. doi: 10.1093/nar/gkq1028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Machnicka MA, Milanowska K, Osman Oglou O, Purta E, Kurkowska M, Olchowik A, Januszewski W, Kalinowski S, Dunin-Horkawicz S, Rother KM, Helm M, Bujnicki JM, Grosjean H. 2013. MODOMICS: a database of RNA modification pathways–2013 update. Nucleic Acids Res 41:D262–D267. doi: 10.1093/nar/gks1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Deng X, Chen K, Luo GZ, Weng X, Ji Q, Zhou T, He C. 2015. Widespread occurrence of N6-methyladenosine in bacterial mRNA. Nucleic Acids Res 43:6557–6567. doi: 10.1093/nar/gkv596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cahová H, Winz ML, Höfer K, Nübel G, Jäschke A. 2015. NAD captureSeq indicates NAD as a bacterial cap for a subset of regulatory RNAs. Nature 519:374–377. doi: 10.1038/nature14020. [DOI] [PubMed] [Google Scholar]
- 35.Roberts D, Hoopes BC, McClure WR, Kleckner N. 1985. IS10 transposition is regulated by DNA adenine methylation. Cell 43:117–130. doi: 10.1016/0092-8674(85)90017-0. [DOI] [PubMed] [Google Scholar]
- 36.Marinus MG. 1985. DNA methylation influences trpR promoter activity in Escherichia coli K-12. Mol Gen Genet 200:185–186. doi: 10.1007/bf00383334. [DOI] [PubMed] [Google Scholar]
- 37.Beletskaya IV, Zakharova MV, Shlyapnikov MG, Semenova LM, Solonin AS. 2000. DNA methylation at the CfrBI site is involved in expression control in the CfrBI restriction-modification system. Nucleic Acids Res 28:3817–3822. doi: 10.1093/nar/28.19.3817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Christensen L, Josephsen J. 2004. The methyltransferase from the LlaDII restriction-modification system influences the level of expression of its own gene. J Bacteriol 186:287–295. doi: 10.1128/JB.186.2.287-295.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bogdanova E, Djordjevic M, Papapanagiotou I, Heyduk T, Kneale G, Severinov K. 2008. Transcription regulation of the type II restriction-modification system AhdI. Nucleic Acids Res 36:1429–1442. doi: 10.1093/nar/gkm1116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Karyagina A, Shilov I, Tashlitskii V, Khodoun M, Vasil'ev S, Lau PC, Nikolskaya I. 1997. Specific binding of SsoII DNA methyltransferase to its promoter region provides the regulation of SsoII restriction-modification gene expression. Nucleic Acids Res 25:2114–2120. doi: 10.1093/nar/25.11.2114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Haakonsen DL, Yuan AH, Laub MT. 2015. The bacterial cell cycle regulator GcrA is a σ70 cofactor that drives gene expression from a subset of methylated promoters. Genes Dev 29:2272–2286. doi: 10.1101/gad.270660.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Danese PN, Pratt LA, Dove SL, Kolter R. 2000. The outer membrane protein, sntigen 43, mediates cell-to-cell interactions within Escherichia coli biofilms. Mol Microbiol 37:424–432. doi: 10.1046/j.1365-2958.2000.02008.x. [DOI] [PubMed] [Google Scholar]
- 43.Henderson IR, Meehan M, Owen P. 2006. Antigen 43, a phase-variable bipartite outer membrane protein, determines colony morphology and autoaggregation in Escherichia coli K-12. FEMS Microbiol Lett 149:115–120. doi: 10.1111/j.1574-6968.1997.tb10317.x. [DOI] [PubMed] [Google Scholar]
- 44.Haagmans W, Van Der Woude M. 2000. Phase variation of Ag43 in Escherichia coli: Dam-dependent methylation abrogates OxyR binding and OxyR-mediated repression of transcription. Mol Microbiol 35:877–887. doi: 10.1046/j.1365-2958.2000.01762.x. [DOI] [PubMed] [Google Scholar]
- 45.Torreblanca J, Marqués S, Casadesús J. 1999. Synthesis of FinP RNA by plasmids F and pSLT is regulated by DNA adenine methylation. Genetics 152:31–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dalia AB, Lazinski DW, Camilli A. 2013. Characterization of undermethylated sites in Vibrio cholerae. J Bacteriol 195:2389–2399. doi: 10.1128/JB.02112-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Militello KT, Simon RD, Qureshi M, Maines R, van Horne ML, Hennick SM, Jayakar SK, Pounder S. 2012. Conservation of Dcm-mediated cytosine DNA methylation in Escherichia coli. FEMS Microbiol Lett 328:78–85. doi: 10.1111/j.1574-6968.2011.02482.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Adam M, Murali B, Glenn NO, Potter SS. 2008. Epigenetic inheritance based evolution of antibiotic resistance in bacteria. BMC Evol Biol 8:52. doi: 10.1186/1471-2148-8-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Fernández L, Breidenstein EBM, Hancock REW. 2011. Creeping baselines and adaptive resistance to antibiotics. Drug Resist Updat 14:1–21. doi: 10.1016/j.drup.2011.01.001. [DOI] [PubMed] [Google Scholar]
- 50.Levin-Reisman I, Ronin I, Gefen O, Braniss I, Shoresh N, Balaban NQ. 2017. Antibiotic tolerance facilitates the evolution of resistance. Science 355:826–830. doi: 10.1126/science.aaj2191. [DOI] [PubMed] [Google Scholar]
- 51.Motta SS, Cluzel P, Aldana M. 2015. Adaptive resistance in bacteria requires epigenetic inheritance, genetic noise, and cost of efflux pumps. PLoS One 10:e0118464-8. doi: 10.1371/journal.pone.0118464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Levin BR, Perrot V, Walker N. 2000. Compensatory mutations, antibiotic resistance and the population genetics of adaptive evolution in bacteria. Genetics 154:985–997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Vogt G. 2015. Stochastic developmental variation, an epigenetic source of phenotypic diversity with far-reaching biological consequences. J Biosci 40:159–204. doi: 10.1007/s12038-015-9506-8. [DOI] [PubMed] [Google Scholar]
- 54.Erickson KE, Otoupal PB, Chatterjee A. 2017. Transcriptome-level signatures in gene expression and gene expression variability during bacterial adaptive evolution. mSphere 2:e00009-17. doi: 10.1128/mSphere.00009-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Cohen NR, Ross CA, Jain S, Shapiro RS, Gutierrez A, Belenky P, Li H, Collins JJ. 2016. A role for the bacterial GATC methylome in antibiotic stress survival. Nat Genet 48:581–586. doi: 10.1038/ng.3530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Henderson IR, Owen P, Nataro JP. 1999. Molecular switches—the ON and OFF of bacterial phase variation. Mol Microbiol 33:919–932. doi: 10.1046/j.1365-2958.1999.01555.x. [DOI] [PubMed] [Google Scholar]
- 57.van der Woude MW, Bäumler AJ. 2004. Phase and antigenic variation in bacteria. Clin Microbiol Rev 17:581–611. doi: 10.1128/CMR.17.3.581-611.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Atack JM, Tan A, Bakaletz LO, Jennings MP, Seib KL. 2018. Phasevarions of bacterial pathogens: methylomics sheds new light on old enemies. Trends Microbiol 26:715–726. doi: 10.1016/j.tim.2018.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Manso AS, Chai MH, Atack JM, Furi L, De Ste Croix M, Haigh R, Trappetti C, Ogunniyi AD, Shewell LK, Boitano M, Clark TA, Korlach J, Blades M, Mirkes E, Gorban AN, Paton JC, Jennings MP, Oggioni MR. 2014. A random six-phase switch regulates pneumococcal virulence via global epigenetic changes. Nat Commun 5:5055. doi: 10.1038/ncomms6055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Cota I, Sánchez-Romero MA, Hernández SB, Pucciarelli MG, García-Del Portillo F, Casadesús J. 2015. Epigenetic control of Salmonella enterica O-antigen chain length: a tradeoff between Virulence and bacteriophage resistance. PLoS Genet 11:e1005667. doi: 10.1371/journal.pgen.1005667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Cascales E, Brunet YR, Bernard CS, Gavioli M, Lloube R. 2011. An epigenetic switch involving overlapping Fur and DNA methylation optimizes expression of a type VI secretion gene cluster. PLoS Genet 7:e1002205. doi: 10.1371/journal.pgen.1002205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Jen FEC, Seib KL, Jennings MP. 2014. Phasevarions mediate epigenetic regulation of antimicrobial susceptibility in Neisseria meningitidis. Antimicrob Agents Chemother 58:4219–4221. doi: 10.1128/AAC.00004-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Seib KL, Jen FEC, Tan A, Scott AL, Kumar R, Power PM, Chen LT, Wu HJ, Wang AHJ, Hill DMC, Luyten YA, Morgan RD, Roberts RJ, Maiden MC, Boitano M, Clark TA, Korlach J, Rao DN, Jennings MP. 2015. Specificity of the ModA11, ModA12 and ModD1 epigenetic regulator N6-adenine DNA methyltransferases of Neisseria meningitidis. Nucleic Acids Res 43:4150–4162. doi: 10.1093/nar/gkv219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Srikhanta YN, Gorrell RJ, Steen JA, Gawthorne JA, Kwok T, Grimmond SM, Robins-Browne RM, Jennings MP. 2011. Phasevarion mediated epigenetic gene regulation in Helicobacter pylori. PLoS One 6:e27569-9. doi: 10.1371/journal.pone.0027569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Van Der Woude M, Braaten B, Low D. 1996. Epigenetic phase variation of the pap operon in Escherichia coli. Trends Microbiol 4:5–9. doi: 10.1016/0966-842x(96)81498-3. [DOI] [PubMed] [Google Scholar]
- 66.Fox KL, Dowideit SJ, Erwin AL, Srikhanta YN, Smith AL, Jennings MP. 2007. Haemophilus influenzae phasevarions have evolved from type III DNA restriction systems into epigenetic regulators of gene expression. Nucleic Acids Res 35:5242–5252. doi: 10.1093/nar/gkm571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hernday A, Krabbe M, Braaten B, Low D. 2002. Self-perpetuating epigenetic pili switches in bacteria. Proc Natl Acad Sci U S A 99:16470–16476. doi: 10.1073/pnas.182427199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Srikhanta YN, Dowideit SJ, Edwards JL, Falsetta ML, Wu H-J, Harrison OB, Fox KL, Seib KL, Maguire TL, Wang AH-J, Maiden MC, Grimmond SM, Apicella MA, Jennings MP. 2009. Phasevarions mediate random switching of gene expression in pathogenic Neisseria. PLoS Pathog 5:e1000400. doi: 10.1371/journal.ppat.1000400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Davidson CJ, Surette MG. 2008. Individuality in bacteria. Annu Rev Genet 42:253–268. doi: 10.1146/annurev.genet.42.110807.091601. [DOI] [PubMed] [Google Scholar]
- 70.Grote J, Krysciak D, Streit WR. 2015. Phenotypic heterogeneity, a phenomenon that may explain why quorum sensing does not always result in truly homogenous cell behavior. Appl Environ Microbiol 81:5280–5289. doi: 10.1128/AEM.00900-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Dubnau D, Losick R. 2006. Bistability in bacteria. Mol Microbiol 61:564–572. doi: 10.1111/j.1365-2958.2006.05249.x. [DOI] [PubMed] [Google Scholar]
- 72.Sánchez-Romero MA, Casadesús J. 2014. Contribution of phenotypic heterogeneity to adaptive antibiotic resistance. Proc Natl Acad Sci U S A 111:355–360. doi: 10.1073/pnas.1316084111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Wakamoto Y, Dhar N, Chait R, Schneider K, Signorino-Gelo F, Leibler S, McKinney JD. 2013. Dynamic persistence of antibiotic-stressed mycobacteria. Science 339:91–95. doi: 10.1126/science.1229858. [DOI] [PubMed] [Google Scholar]
- 74.Cohen NR, Lobritz MA, Collins JJ. 2013. Microbial persistence and the road to drug resistance. Cell Host Microbe 13:632–642. doi: 10.1016/j.chom.2013.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Fisher RA, Gollan B, Helaine S. 2017. Persistent bacterial infections and persister cells. Nat Rev Microbiol 15:453–464. doi: 10.1038/nrmicro.2017.42. [DOI] [PubMed] [Google Scholar]
- 76.Harms A, Maisonneuve E, Gerdes K. 2016. Mechanisms of bacterial persistence during stress and antibiotic exposure. Science 354:aaf4268. doi: 10.1126/science.aaf4268. [DOI] [PubMed] [Google Scholar]
- 77.Moyed HS, Bertrand KP. 1983. hipA, a newly recognized gene of Escherichia coli K-12 that affects frequency of persistence after inhibition of murein synthesis. J Bacteriol 155:768–775. doi: 10.1128/jb.166.2.399-403.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Korch SB, Henderson TA, Hill TM. 2003. Characterization of the hipA7 allele of Escherichia coli and evidence that high persistence is governed by (p)ppGpp synthesis. Mol Microbiol 50:1199–1213. doi: 10.1046/j.1365-2958.2003.03779.x. [DOI] [PubMed] [Google Scholar]
- 79.Day T. 2016. Interpreting phenotypic antibiotic tolerance and persister cells as evolution via epigenetic inheritance. Mol Ecol 25:1869–1882. doi: 10.1111/mec.13603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Mwangi MM, Wu SW, Zhou Y, Sieradzki K, de Lencastre H, Richardson P, Bruce D, Rubin E, Myers E, Siggia ED, Tomasz A. 2007. Tracking the in vivo evolution of multidrug resistance in Staphylococcus aureus by whole-genome sequencing. Proc Natl Acad Sci U S A 104:9451–9456. doi: 10.1073/pnas.0609839104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Hayden HS, Lim R, Brittnacher MJ, Sims EH, Ramage ER, Fong C, Wu Z, Crist E, Chang J, Zhou Y, Radey M, Rohmer L, Haugen E, Gillett W, Wuthiekanun V, Peacock SJ, Kaul R, Miller SI, Manoil C, Jacobs MA. 2012. Evolution of Burkholderia pseudomallei in recurrent melioidosis. PLoS One 7:e36507. doi: 10.1371/journal.pone.0036507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.El-Halfawy OM, Valvano MA. 2015. Antimicrobial heteroresistance: an emerging field in need of clarity. Clin Microbiol Rev 28:191–207. doi: 10.1128/CMR.00058-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Alexander HE, Leidy G. 1947. Mode of action of streptomycin on type B Hemophilus influenzae. II. Nature of resistant variants. J Exp Med 85:607–621. doi: 10.1084/jem.85.6.607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Sutherland R, Rolinson GN. 1964. Characteristics of methicillin-resistant Staphylococci. J Bacteriol 87:887–899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Band VI, Crispell EK, Napier BA, Herrera CM, Tharp GK, Vavikolanu K, Pohl J, Read TD, Bosinger SE, Trent MS, Burd EM, Weiss DS. 2016. Antibiotic failure mediated by a resistant subpopulation in Enterobacter cloacae. Nat Microbiol 1:16053. doi: 10.1038/nmicrobiol.2016.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Hughes D, Andersson DI. 2017. Environmental and genetic modulation of the phenotypic expression of antibiotic resistance. FEMS Microbiol Rev 41:374–391. doi: 10.1093/femsre/fux004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Lyu F, Pan M, Patil S, Wang J-H, Matin AC, Andrews JR, Tang SKY. 2018. Phenotyping antibiotic resistance with single-cell resolution for the detection of heteroresistance. Sensors Actuators B Chem 270:396–404. doi: 10.1016/j.snb.2018.05.047. [DOI] [Google Scholar]
- 88.Sorg RA, Veening J-W. 2015. Microscale insights into pneumococcal antibiotic mutant selection windows. Nat Commun 6:8773. doi: 10.1038/ncomms9773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Yugendran T, Harish BN. 2016. Global DNA methylation level among ciprofloxacin-resistant clinical isolates of Escherichia coli. J Clin Diagn Res 10:DC27–DC30. doi: 10.7860/JCDR/2016/19034.7830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Chung YJ, Saier MHJ. 2002. Overexpression of the Escherichia coli sugE gene confers resistance to a narrow range of quaternary ammonium compounds. J Bacteriol 184:2543–2545. doi: 10.1128/JB.184.9.2543-2545.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Militello KT, Mandarano AH, Varechtchouk O, Simon RD. 2014. Cytosine DNA methylation influences drug resistance in Escherichia coli through increased sugE expression. FEMS Microbiol Lett 350:100–106. doi: 10.1111/1574-6968.12299. [DOI] [PubMed] [Google Scholar]
- 92.He G-X, Zhang C, Crow RR, Thorpe C, Chen H, Kumar S, Tsuchiya T, Varela MF. 2011. SugE, a new member of the SMR family of transporters, contributes to antimicrobial resistance in Enterobacter cloacae. Antimicrob Agents Chemother 55:3954–3957. doi: 10.1128/AAC.00094-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Stephenson SA-M, Brown PD. 2016. Epigenetic influence of Dam methylation on gene expression and attachment in uropathogenic Escherichia coli. Front Public Health 4:131. doi: 10.3389/fpubh.2016.00131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Tímár E, Groma G, Kiss A, Venetianer P. 2004. Changing the recognition specificity of a DNA-methyltransferase by in vitro evolution. Nucleic Acids Res 32:3898–3903. doi: 10.1093/nar/gkh724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Duncan BK, Miller JH, 1980. Mutagenic deamination of cytosine residues in DNA. Nature 287:560–561. doi: 10.1038/287560a0. [DOI] [PubMed] [Google Scholar]
- 96.Beletskii A, Bhagwat AS. 1996. Transcription-induced mutations: increase in C to T mutations in the nontranscribed strand during transcription in Escherichia coli. Proc Natl Acad Sci U S A 93:13919–13924. doi: 10.1073/pnas.93.24.13919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Lutsenko E, Bhagwat AS. 1999. Principal causes of hot spots for cytosine to thymine mutations at sites of cytosine methylation in growing cells. A model, its experimental support and implications. Mutat Res 437:11–20. doi: 10.1016/S1383-5742(99)00065-4. [DOI] [PubMed] [Google Scholar]
- 98.Lieb M. 1991. Spontaneous mutation at a 5-methylcytosine hotspot is prevented by very short patch (VSP) mismatch repair. Genetics 128:23–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Bandaru B, Gopal J, Bhagwat AS, Ecorii EM. 1996. Overproduction of DNA cytosine methyltransferases causes methylation and C to T mutations at non-canonical sites. J Biol Chem 271:7851–7859. doi: 10.1074/jbc.271.13.7851. [DOI] [PubMed] [Google Scholar]
- 100.Xing X-W, Liu Y-L, Vargas M, Wang Y, Feng Y-Q, Zhou X, Yuan B-F. 2013. Mutagenic and cytotoxic properties of oxidation products of 5-methylcytosine revealed by next-generation sequencing. PLoS One 8:e72993. doi: 10.1371/journal.pone.0072993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Cherry JL. 2018. Methylation-induced hypermutation in natural populations of bacteria. J Bacteriol 200:e00371-18. doi: 10.1128/JB.00371-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Foster PL. 2007. Stress-induced mutagenesis in bacteria. Crit Rev Biochem Mol Biol 42:373–397. doi: 10.1080/10409230701648494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Kunkel TA, Erie DA. 2005. DNA mismatch repair. Annu Rev Biochem 74:681–710. doi: 10.1146/annurev.biochem.74.082803.133243. [DOI] [PubMed] [Google Scholar]
- 104.Wagner J, Gruz P, Kim SR, Yamada M, Matsui K, Fuchs RPP, Nohmi T. 1999. The dinB gene encodes a novel E. coli DNA polymerase, DNA Pol IV, involved in mutagenesis. Mol Cell 4:281–286. doi: 10.1016/s1097-2765(00)80376-7. [DOI] [PubMed] [Google Scholar]
- 105.Tang M, Shen X, Frank EG, O'Donnell M, Woodgate R, Goodman MF. 1999. UmuD′2C is an error-prone DNA polymerase, Escherichia coli pol V. Proc Natl Acad Sci U S A 96:8919–8924. doi: 10.1073/pnas.96.16.8919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Doutriaux MP, Wagner R, Radman M. 1986. Mismatch-stimulated killing. Proc Natl Acad Sci U S A 83:2576–2578. doi: 10.1073/pnas.83.8.2576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Canchaya C, Proux C, Fournous G, Bruttin A, Brussow H. 2003. Prophage genomics. Microbiol Mol Biol Rev 67:238–276. doi: 10.1128/mmbr.67.2.238-276.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Murphy J, Mahony J, Ainsworth S, Nauta A, van Sinderen D. 2013. Bacteriophage orphan DNA methyltransferases: insights from their bacterial origin, function, and occurrence. Appl Environ Microbiol 79:7547–7555. doi: 10.1128/AEM.02229-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Schlagman SL, Miner Z, Fehér Z, Hattman S. 1988. The DNA [adenine-N6]methyltransferase (Dam) of bacteriophage T4. Gene 73:517–530. doi: 10.1016/0378-1119(88)90516-1. [DOI] [PubMed] [Google Scholar]
- 110.Bochow S, Elliman J, Owens L. 2012. Bacteriophage adenine methyltransferase: a life cycle regulator? Modelled using Vibrio harveyi myovirus like. J Appl Microbiol 113:1001–1013. doi: 10.1111/j.1365-2672.2012.05358.x. [DOI] [PubMed] [Google Scholar]
- 111.Coulby JN, Sternberg NL. 1988. Characterization of the phage P1 dam gene. Gene 74:191. doi: 10.1016/0378-1119(88)90284-3. [DOI] [PubMed] [Google Scholar]
- 112.Murphy J, Klumpp J, Mahony J, O'Connell-Motherway M, Nauta A, van Sinderen D. 2014. Methyltransferases acquired by lactococcal 936-type phage provide protection against restriction endonuclease activity. BMC Genomics 15:831. doi: 10.1186/1471-2164-15-831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Kossykh VG, Schlagman SL, Hattman S. 1995. Phage T4 DNA [N6-adenine]methyltransferase. Overexpression, purification, and characterization. J Biol Chem 270:14389–14393. doi: 10.1074/jbc.270.24.14389. [DOI] [PubMed] [Google Scholar]
- 114.Sternberg N, Coulby JN. 1990. Processing of the bacteriophage P1 packaging site (pac) is regulated by adenine methylation. Proc Natl Acad Sci U S A 87:8070–8074. doi: 10.1073/pnas.87.20.8070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Casey E, van Sinderen D, Mahony J. 2018. In vitro characteristics of phages to guide “real life” phage therapy suitability. Viruses 10:163. doi: 10.3390/v10040163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Shin J-EE, Lin C, Lim HN. 2016. Horizontal transfer of DNA methylation patterns into bacterial chromosomes. Nucleic Acids Res 44:4460–4471. doi: 10.1093/nar/gkw230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Chen L, Li HC, Chen T, Yu L, Guo HX, Chen YH, Chen M, Li ZY, Wu ZH, Wang XZ, Zhao J, Yan H, Wang X, Zhou L, Zhou J. 2018. Genome-wide DNA methylation and transcriptome changes in Mycobacterium tuberculosis with rifampicin and isoniazid resistance. Int J Clin Exp Pathol 11:3036–3045. [PMC free article] [PubMed] [Google Scholar]
- 118.Brown ED, Wright GD. 2016. Antibacterial drug discovery in the resistance era. Nature 529:336–343. doi: 10.1038/nature17042. [DOI] [PubMed] [Google Scholar]
- 119.Roignant JY, Soller M. 2017. m6A in mRNA: an ancient mechanism for fine-tuning gene expression. Trends Genet 33:380–390. doi: 10.1016/j.tig.2017.04.003. [DOI] [PubMed] [Google Scholar]
- 120.Arango D, Sturgill D, Alhusaini N, Dillman AA, Sweet TJ, Hanson G, Hosogane M, Sinclair WR, Nanan KK, Mandler MD, Fox SD, Zengeya TT, Andresson T, Meier JL, Coller J, Oberdoerffer S. 2018. Acetylation of cytidine in mRNA promotes translation efficiency. Cell 175:1872–1886.e24. doi: 10.1016/j.cell.2018.10.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Eid J, Fehr A, Gray J, Luong K, Lyle J, Otto G, Peluso P, Rank D, Baybayan P, Bettman B, Bibillo A, Bjornson K, Chaudhuri B, Christians F, Cicero R, Clark S, Dalal R, Dewinter A, Dixon J, Foquet M, Gaertner A, Hardenbol P, Heiner C, Hester K, Holden D, Kearns G, Kong X, Kuse R, Lacroix Y, Lin S, Lundquist P, Ma C, Marks P, Maxham M, Murphy D, Park I, Pham T, Phillips M, Roy J, Sebra R, Shen G, Sorenson J, Tomaney A, Travers K, Trulson M, Vieceli J, Wegener J, Wu D, Yang A, Zaccarin D, Zhao P, Zhong F, Korlach J, Turner S. 2009. Real-Time DNA sequencing from single polymerase molecules. Science 323:133–138. doi: 10.1126/science.1162986. [DOI] [PubMed] [Google Scholar]
- 122.Beaulaurier J, Zhang XS, Zhu S, Sebra R, Rosenbluh C, Deikus G, Shen N, Munera D, Waldor MK, Chess A, Blaser MJ, Schadt EE, Fang G. 2015. Single molecule-level detection and long read-based phasing of epigenetic variations in bacterial methylomes. Nat Commun 6:7438. doi: 10.1038/ncomms8438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Schatz MC. 2017. Nanopore sequencing meets epigenetics. Nat Methods 14:347–348. doi: 10.1038/nmeth.4240. [DOI] [PubMed] [Google Scholar]
- 124.McIntyre ABR, Alexander N, Grigorev K, Bezdan D, Sichtig H, Chiu CY, Mason CE. 2019. Single-molecule sequencing detection of N6-methyladenine in microbial reference materials. Nat Commun 10:579. doi: 10.1038/s41467-019-08289-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Beaulaurier J, Schadt EE, Fang G. 2019. Deciphering bacterial epigenomes using modern sequencing technologies. Nat Rev Genet 20:157–172. doi: 10.1038/s41576-018-0081-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Collado-Vides J, Salgado H, Morett E, Gama-Castro S, Jiménez-Jacinto V, Martínez-Flores I, Medina-Rivera A, Muñiz-Rascado L, Peralta-Gil M, Santos-Zavaleta A. 2009. Bioinformatics resources for the study of gene regulation in bacteria. J Bacteriol 191:23–31. doi: 10.1128/JB.01017-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Eckweiler D, Dudek CA, Hartlich J, Brötje D, Jahn D. 2018. PRODORIC2: the bacterial gene regulation database in 2018. Nucleic Acids Res 46:D320–D326. doi: 10.1093/nar/gkx1091. [DOI] [PMC free article] [PubMed] [Google Scholar]