Omadacycline is an effective therapy for community-acquired bacterial pneumonia (CABP). Given its potent activity against methicillin-susceptible Staphylococcus aureus (MSSA) and methicillin-resistant S. aureus (MRSA), we sought to determine the pharmacodynamic activity and target pharmacokinetic/pharmacodynamic (PK/PD) exposures associated with a therapeutic effect in the neutropenic mouse pneumonia model against 10 MSSA/MRSA strains.
KEYWORDS: Staphylococcus aureus, omadacycline, pharmacodynamics, pneumonia
ABSTRACT
Omadacycline is an effective therapy for community-acquired bacterial pneumonia (CABP). Given its potent activity against methicillin-susceptible Staphylococcus aureus (MSSA) and methicillin-resistant S. aureus (MRSA), we sought to determine the pharmacodynamic activity and target pharmacokinetic/pharmacodynamic (PK/PD) exposures associated with a therapeutic effect in the neutropenic mouse pneumonia model against 10 MSSA/MRSA strains. The area under the concentration-time curve (AUC)/MIC associated with 1-log kill was noted at 24-h epithelial lining fluid (ELF) and plasma AUC/MIC exposures of ∼2 (ELF range, <0.93 to 19; plasma range, <1.06 to 17) and 2-log kill was noted at 24-h ELF and plasma AUC/MIC exposures of ∼12 (ELF range, 2.5 to 130; plasma range, 3.5 to 151).
TEXT
Omadacycline (NUZYRA; Paratek Pharmaceuticals), an aminomethylcycline antibiotic, has potent in vitro and in vivo activity against Gram-positive pathogens, in particular, Staphylococcus aureus, including methicillin-resistant S. aureus (MRSA) (1–4). It has been approved in the United States for the treatment of adults with acute bacterial skin and skin structure infection (ABSSSI) and community-acquired bacterial pneumonia (CABP) given its demonstrated efficacy in large randomized control trials (5–7). Based on these preclinical and clinical findings, a logical consideration is to what extent omadacycline could be useful for S. aureus pneumonia. Therefore, we attempted to answer this question by examining the pharmacodynamics of omadacycline against a diverse group of S. aureus strains in the neutropenic mouse pneumonia model to delineate the pharmacodynamic activity and target exposures associated with efficacy.
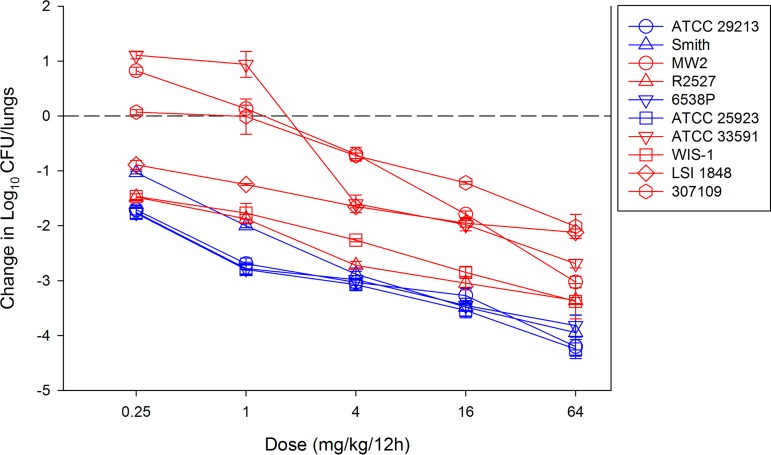
Ten S. aureus strains were utilized (Table 1) that included 6 MRSA and 4 MSSA strains. Susceptibility testing was performed in triplicates according to CLSI guidelines (8). The MIC range was relatively narrow at 0.25 to 0.5 mg/liter, consistent with previous in vitro surveillance studies (1–4). The neutropenic mouse lung infection model was used for all animal studies (9). Animals were maintained in accordance with American Association for Accreditation of Laboratory Animal Care (AAALAC) criteria, and all studies were approved by the University of Wisconsin and the William S. Middleton Memorial VA Hospital animal research committees. Neutropenic mice were infected with an inoculum of 8.01 ± 0.06 CFU/ml by the intranasal route resulting in a zero hour burden of 6.41 ± 0.11 CFU/lungs. The burden in untreated control mice increased to 8.91 ± 0.14 CFU/lungs over 24 h; thus, on average, there was an approximately 2.5 log10 CFU increase in burden, demonstrating fitness in the model. Two hours after inoculation into lungs, groups of 3 mice were treated by the subcutaneous route with 4-fold increasing doses of omadacycline (0.25 to 64 mg/kg/12 h). The dose range was selected based upon prior study with this drug and these strains in a similar murine thigh infection model. After 24 h, the burden of organisms was enumerated from lung homogenates. The dose-response curves are shown in Fig. 1. Low doses of omadacycline resulted in net cidal activity, even at the lowest dose tested, against 7 of 10 strains. As doses were increased, there was a further increase in killing activity, and there was a ≥2-log kill achieved against all organisms over the dose range.
TABLE 1.
Staphylococcus aureus strains, fitness in the model, in vitro susceptibility results, and pharmacodynamic target exposures for each strain in the neutropenic mouse pneumonia model
| Phenotype | Organism | 24-h growth in untreated controls (log10 CFU/lungs) | MIC (mg/liter) | Static dose (mg/kg q12h)a | Stasis 24-h ELF AUC/MIC | Stasis 24-h plasma tAUC/MICb | 1-Log kill |
2-Log kill |
||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Dose (mg/kg q12h) | 24-h ELF AUC/MIC | 24-h plasma tAUC/MIC | Dose (mg/kg q12h) | ELF AUC/MIC | 24-h plasma tAUC/MIC | |||||||
| Able | 307109 | 2.59 | 0.5 | 0.56 | 2.01 | 2.27 | 6.75 | 19.49 | 16.97 | 63.90 | 130.45 | 151.02 |
| LSI 1848 | 2.51 | 0.5 | NAc | 0.93 | <1.06 | 0.38 | 1.43 | 1.63 | 33.12 | 67.61 | 78.26 | |
| Wis-1 | 2.23 | 0.5 | NA | 0.93 | <1.06 | NA | 0.93 | <1.06 | 1.23 | 3.73 | 3.93 | |
| ATCC 33591 | 2.31 | 0.5 | 1.45 | 4.28 | 4.47 | 3.89 | 11.17 | 10.35 | 12.25 | 30.59 | 29.77 | |
| MW2 | 2.35 | 0.5 | 1.21 | 3.68 | 3.89 | 4.48 | 12.90 | 11.74 | 17.87 | 40.48 | 42.82 | |
| R2527 | 2.59 | 0.5 | NA | 0.93 | <1.06 | NA | 0.93 | <1.06 | 0.96 | 3.04 | 3.27 | |
| MSSA | ATCC 25923 | 2.64 | 0.25 | NA | 1.86 | <2.12 | NA | 1.86 | <2.12 | 0.34 | 2.52 | 3.47 |
| ATCC 29213 | 2.62 | 0.25 | NA | 1.86 | <2.12 | NA | 1.86 | <2.12 | 0.38 | 2.86 | 3.69 | |
| SMITH | 2.62 | 0.25 | NA | 1.86 | <2.12 | 0.25 | 1.87 | 2.14 | 0.99 | 6.21 | 6.66 | |
| 6538P | 2.48 | 0.25 | NA | 1.86 | <2.12 | NA | 1.86 | <2.12 | 0.33 | 2.50 | 3.45 | |
q12h, every 12 h.
tAUC/MIC, total drug AUC/MIC.
NA, not available
FIG 1.
Dose-response curves for omadacycline against 10 S. aureus (blue symbols, MSSA; red symbols, MRSA) strains in the neutropenic mouse pneumonia model. Each symbol represents the mean and standard deviation from three mice. Five different dose levels were administered by the subcutaneous route every 12 h. The burden of organisms was enumerated at the start and end of antimicrobial therapy over a 24-h experiment duration. The horizontal dashed line at 0 represents the burden of organisms at the start of therapy. Data points above the line represent net growth (i.e., increase) in burden, and those below the line represent net cidal activity (i.e., decrease) in bacterial burden.
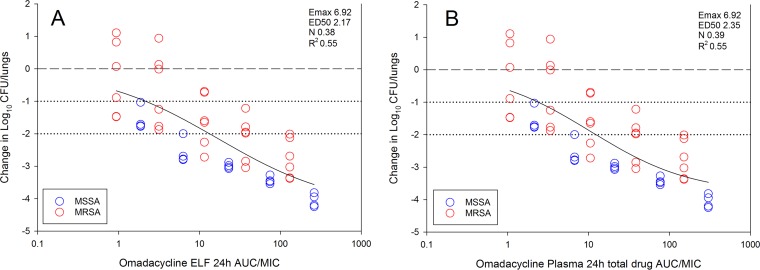
We assessed the pharmacodynamic relationship for omadacycline epithelial lining fluid (ELF) and plasma area under the concentration-time curve over 24 h (AUC)/MIC, as this has been shown to be the predictive index of therapeutic effect for tetracyclines, including omadacycline (9–13). Previously published ELF and plasma pharmacokinetic data for omadacycline from mice in this model from our laboratory was utilized to estimate drug exposures (9). Treatment effect relative to the pharmacokinetic/pharmacodynamic (PK/PD) index AUC/MIC was modeled utilizing the Hill equation sigmoid maximum effect (Emax) model and is shown in Fig. 2A based on ELF AUC/MIC and in Fig. 2B based on plasma AUC/MIC. One will note the exposure response curves are quite similar between ELF and plasma exposures. This is due to the uniform and nearly complete penetration of plasma into the ELF, with ELF/plasma AUC ratios ranging from 0.86 to 1.17 over the dose range. Additionally, protein binding in plasma is very low for omadacycline (14). Due to the low protein binding, we report total drug concentrations in plasma. Based on the best-fit line (see Fig. 2A and B), a 1-log kill was noted at 24-h AUC/MIC exposures of approximately 2 based on ELF and plasma drug exposures, and a 2-log kill was noted at 24-h AUC/MIC exposures of approximately 12. We did observe more variability in treatment effects for MRSA than for MSSA organisms. Individual AUC/MIC exposures, using both ELF and plasma exposures, for each organism for the various microbiological endpoints are shown in Table 1. Due to the unexpected efficacy over the dose range, static targets were not able to be accurately identified; however, 1-log kill was noted at 24-h ELF AUC/MIC exposures of <0.93 to 19 and 24-h plasma AUC/MIC exposures of <1.06 to 17. A 2-log kill was observed at 24-h ELF AUC/MIC exposures of 2.5 to 130 and 24-h plasma AUC/MIC exposures of 3.5 to 151.
FIG 2.
In vivo exposure-response relationships between the pharmacodynamic parameter 24-h ELF AUC/MIC and 24-h plasma AUC/MIC and treatment effect for 10 S. aureus strains in the neutropenic mouse pneumonia model. Each symbol is the mean from three mice per treatment group. Five total drug dosing regimens were fractionated into an every-12-h regimen. The omadacycline exposure is represented on the x axes as ELF AUC/MIC (A) and plasma total drug AUC/MIC (B). Given the protein binding is very low, only total drug concentrations were considered in the analysis as they closely approximate free drug values. The burdens of organisms were measured at the start and end of therapy over a 24-h experiment duration. The changes (i.e., differences) between the start and end of therapy are represented on the y axes. The horizontal dashed lines at 0 represent the burden of organisms at the start of therapy, and dotted lines represent 1- and 2-log kill, respectively. Data points above the dashed line represent a net increase, and those below the line represent a net decrease in bacterial burden. The sigmoid lines drawn through the data are the best-fit lines based on the Emax model (Hill equation). Also shown are the pharmacodynamic parameters Emax (maximum effect), ED50 (50% maximal effect point), N (slope of the line), and coefficient of determination (R2).
Omadacycline demonstrated a potent in vivo effect in the mouse pneumonia model. The target exposures were relatively similar to those in our previous study in the pneumonia model against Streptococcus pneumoniae (9). In both studies, even the lowest doses of omadacycline tested in the pneumonia model demonstrated net killing activity against numerous test organisms. Additionally, in the previous study, we also experienced moderate variability in exposure response relationships, as the 1-log-kill ELF AUC/MIC target for S. pneumoniae ranged from 6 to 180, and the 2-log kill ranged from 19 to >486. In the present study, the S. aureus 1-log-kill ELF AUC/MIC ranged from <0.93 to 19, and for 2-log kill, from 2.5 to 130. This variability in exposure-response relationships within a species in mouse pneumonia models has been consistently demonstrated in a number of labs and across the tetracycline class (9, 15–18). The reasons for this variability are not well understood. There are a few model factors that we speculate are unlikely to be the etiology of the observed variability, including organism fitness and inoculum effect. In this and previous studies, fitness was similar based upon equal growth measurements in untreated control animals (e.g., >2-log increase in burden for all organisms was noted in this study). Similarly, start-of-therapy control groups (at zero hour) were extremely closely matched.
Previous in vivo mouse pneumonia model studies have been undertaken for a number of tetracycline derivatives. For example, in a similar neutropenic mouse model, Koomanachai and colleagues demonstrated that the 1-log-kill plasma AUC/MIC for free, unbound drug (fAUC/MIC) (including lower and upper bounds) for tigecycline against 6 S. aureus strains was 1 to >50 (15). This range of plasma AUC/MIC exposures associated with 1-log kill in this study are very similar to those observed in the present study of omadacycline, in which 1-log kill was observed at plasma AUC/MIC exposures of <1.06 to 17 and at ELF exposures of <0.93 to 19. An additional interesting finding is the demonstration of lower ELF AUC/MIC targets than for thigh targets. In our previous thigh infection study, using the same set of S. aureus strains, we observed a 1-log kill at 24-h plasma AUC/MIC targets, on average, 3- to 4-fold higher than what was observed in the present pneumonia model studies (13). This difference in targets between thigh and lung infection models has been noted for other drugs and, in particular, for ribosomal inhibitors, including tetracyclines, oxazolidinones, and streptogramins (11, 15, 19–23). For example, tigecycline targets for net stasis against S. aureus in the thigh were a 24-h fAUC/MIC of 38 in a study by Van Ogtrop and colleagues (11); whereas, in a separate murine lung infection model against S. aureus, the fAUC/MIC target for net stasis was approximately 2 (15). It has been well established that many compounds in the tetracycline class penetrate well into the lung and, in particular, into ELF (20). They also concentrate in immune system cells, including resident alveolar cells and macrophages (24, 25). Whether the enhanced pharmacodynamic efficacy noted in lung infection models is due to site-specific factors, pharmacokinetic properties, including enhanced killing, postantibiotic effect, or intracellular accumulation, or to class effects, including synergizing with innate immune defenses, is unclear but should be further explored.
Clinical studies have demonstrated that outcomes in patients are significantly improved when PK/PD target AUC/MIC ratios are optimized based on preclinical PK/PD target studies. For example, patients with hospital-acquired pneumonia (HAP) treated with tigecycline with plasma fAUC/MIC of ≥0.9 had an 8.42-fold higher odds of clinical success than those with plasma fAUC/MIC of <0.9 (26). This clinical PK/PD threshold fits extremely well with the lower bound fAUC/MIC threshold observed for 1-log kill (fAUC/MIC of 1) in the mouse model study by Koomanachai and colleagues (15). Therefore, we also explored the PK/PD targets identified in this study in the context of human PK and surveillance MIC distribution data. Human plasma and ELF pharmacokinetic exposures have been published for intravenous and oral omadacycline (27, 28). ELF penetration in humans is slightly higher (ELF/plasma of >1) than we observed in mice and result in mean steady-state plasma and ELF AUC0–24 of approximately 10 and 17 mg·h/liter, respectively, using the currently approved human dosing regimens, which consist of 100 mg intravenously (i.v.) daily or 300 mg orally (p.o.) daily (27, 28). Numerous large surveillance studies examining omadacycline in vitro activity against S. aureus have demonstrated an MIC90 of 0.25 mg/liter (1–4, 29). Thus, the clinical doses of omadacycline would produce, on average, an ELF 24-h AUC/MIC exposure of approximately 69 and plasma 24-h AUC/MIC exposures of approximately 40 at the MIC90 value of 0.25 mg/liter. These values exceed all 1-log kill targets for both ELF and plasma observed in the present study. Given the previous correlation between tigecycline 1-log-kill animal model PK/PD targets and PK/PD targets associated with clinical success in patients with pneumonia, we project omadacycline may be a very useful option for S. aureus pneumonia. Future clinical data sets incorporating drug PK, organism MIC, and clinical outcome will be necessary to verify the clinical translation of the identified PK/PD targets from this study.
ACKNOWLEDGMENT
This study was funded by Paratek Pharmaceuticals.
REFERENCES
- 1.Huband MD, Pfaller MA, Shortridge D, Flamm RK. 2019. Surveillance of omadacycline activity tested against clinical isolates from the United States and Europe: results from the SENTRY Antimicrobial Surveillance Programme, 2017. J Glob Antimicrob Resist 19:56–63. doi: 10.1016/j.jgar.2019.02.017. [DOI] [PubMed] [Google Scholar]
- 2.Pfaller MA, Huband MD, Rhomberg PR, Flamm RK. 2017. Surveillance of omadacycline activity against clinical isolates from a global collection (North America, Europe, Latin America, Asia-Western Pacific), 2010–2011. Antimicrob Agents Chemother 61:e00018-17. doi: 10.1128/AAC.00018-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pfaller MA, Huband MD, Shortridge D, Flamm RK. 2018. Surveillance of omadacycline activity tested against clinical isolates from the United States and Europe as part of the 2016 SENTRY Antimicrobial Surveillance Program. Antimicrob Agents Chemother 62:e02327-17. doi: 10.1128/AAC.02327-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pfaller MA, Rhomberg PR, Huband MD, Flamm RK. 2017. Activities of omadacycline and comparator agents against Staphylococcus aureus isolates from a surveillance program conducted in North America and Europe. Antimicrob Agents Chemother 61:e02411-16. doi: 10.1128/AAC.02411-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.O'Riordan W, Green S, Overcash JS, Puljiz I, Metallidis S, Gardovskis J, Garrity-Ryan L, Das AF, Tzanis E, Eckburg PB, Manley A, Villano SA, Steenbergen JN, Loh E. 2019. Omadacycline for acute bacterial skin and skin-structure infections. N Engl J Med 380:528–538. doi: 10.1056/NEJMoa1800170. [DOI] [PubMed] [Google Scholar]
- 6.Stets R, Popescu M, Gonong JR, Mitha I, Nseir W, Madej A, Kirsch C, Das AF, Garrity-Ryan L, Steenbergen JN, Manley A, Eckburg PB, Tzanis E, McGovern PC, Loh E. 2019. Omadacycline for community-acquired bacterial pneumonia. N Engl J Med 380:517–527. doi: 10.1056/NEJMoa1800201. [DOI] [PubMed] [Google Scholar]
- 7.O’Riordan W, Cardenas C, Shin E, Sirbu A, Garrity-Ryan L, Das AF, Eckburg PB, Manley A, Steenbergen JN, Tzanis E, McGovern PC, Loh E, Investigators O. 2019. Once-daily oral omadacycline versus twice-daily oral linezolid for acute bacterial skin and skin structure infections (OASIS-2): a phase 3, double-blind, multicentre, randomised, controlled, non-inferiority trial. Lancet Infect Dis 19:1080–1090. doi: 10.1016/S1473-3099(19)30275-0. [DOI] [PubMed] [Google Scholar]
- 8.CLSI. 2018. Methods for dilutional antimicrobial susceptibility tests for bacteria that grow aerobically, 11th ed. CLSI standard M07 Clinical and Laboratory Standards Institute, Wayne, PA. [Google Scholar]
- 9.Lepak AJ, Zhao M, Marchillo K, VanHecker J, Andes DR. 2017. In vivo pharmacodynamic evaluation of omadacycline (PTK 0796) against Streptococcus pneumoniae in the murine pneumonia model. Antimicrob Agents Chemother 61:e02368-16. doi: 10.1128/AAC.02368-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Agwuh KN, MacGowan A. 2006. Pharmacokinetics and pharmacodynamics of the tetracyclines including glycylcyclines. J Antimicrob Chemother 58:256–265. doi: 10.1093/jac/dkl224. [DOI] [PubMed] [Google Scholar]
- 11.van Ogtrop ML, Andes D, Stamstad TJ, Conklin B, Weiss WJ, Craig WA, Vesga O. 2000. In vivo pharmacodynamic activities of two glycylcyclines (GAR-936 and WAY 152,288) against various gram-positive and gram-negative bacteria. Antimicrob Agents Chemother 44:943–949. doi: 10.1128/aac.44.4.943-949.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhao M, Lepak AJ, Marchillo K, VanHecker J, Andes DR. 2017. In vivo pharmacodynamic target assessment of eravacycline against Escherichia coli in a murine thigh infection model. Antimicrob Agents Chemother 61:e00250-17. doi: 10.1128/AAC.00250-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lepak AJ, Zhao M, Marchillo K, VanHecker J, Andes DR. 2019. In vivo pharmacodynamics of omadacycline against Staphylococcus aureus in the neutropenic murine thigh infection model. Antimicrob Agents Chemother 63: e00624-19. doi: 10.1128/AAC.00624-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chaturvedi P, Esposito C, Koroma J, Cannon EP, Tanaka SK. 2003. In vitro assessment of plasma protein binding and metabolic stability of PTK 0796. 43rd Interscience Conference on Antimicrobial Agents and Chemotherapy. American Society for Microbiology, Washington, D.C. [Google Scholar]
- 15.Koomanachai P, Crandon JL, Banevicius MA, Peng L, Nicolau DP. 2009. Pharmacodynamic profile of tigecycline against methicillin-resistant Staphylococcus aureus in an experimental pneumonia model. Antimicrob Agents Chemother 53:5060–5063. doi: 10.1128/AAC.00985-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Koomanachai P, Kim A, Nicolau DP. 2009. Pharmacodynamic evaluation of tigecycline against Acinetobacter baumannii in a murine pneumonia model. J Antimicrob Chemother 63:982–987. doi: 10.1093/jac/dkp056. [DOI] [PubMed] [Google Scholar]
- 17.Lepak AJ, Zhao M, Liu Q, Wang P, Wang Y, Bader JC, Ambrose PG, Andes DR. 2019. Pharmacokinetic/pharmacodynamic evaluation of a novel aminomethylcycline antibiotic, KBP-7072, in the neutropenic murine pneumonia model against Staphylococcus aureus and Streptococcus pneumoniae. Antimicrob Agents Chemother 63:e02404-18. doi: 10.1128/AAC.02404-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhou J, Ledesma KR, Chang KT, Abodakpi H, Gao S, Tam VH. 2017. Pharmacokinetics and pharmacodynamics of minocycline against Acinetobacter baumannii in a neutropenic murine pneumonia model. Antimicrob Agents Chemother 61:e02371-16. doi: 10.1128/AAC.02371-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Andes D, Craig WA. 2006. Pharmacodynamics of a new streptogramin, XRP 2868, in murine thigh and lung infection models. Antimicrob Agents Chemother 50:243–249. doi: 10.1128/AAC.50.1.243-249.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Andes D, Craig W. 2007. Pharmacokinetics and pharmacodynamics of tetracyclines, p 267–278. In Nightingale CH, Ambrose PG, Drusano GL, Murakawa T (ed), Antimicrobial pharmacodynamics in theory and clinical practice, 2nd ed Informa Healthcare USA, New York, NY. [Google Scholar]
- 21.Andes D, van Ogtrop ML, Peng J, Craig WA. 2002. In vivo pharmacodynamics of a new oxazolidinone (linezolid). Antimicrob Agents Chemother 46:3484–3489. doi: 10.1128/aac.46.11.3484-3489.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lepak AJ, Marchillo K, Pichereau S, Craig WA, Andes DR. 2012. Comparative pharmacodynamics of the new oxazolidinone tedizolid phosphate and linezolid in a neutropenic murine Staphylococcus aureus pneumonia model. Antimicrob Agents Chemother 56:5916–5922. doi: 10.1128/AAC.01303-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Louie A, Liu W, Kulawy R, Drusano GL. 2011. In vivo pharmacodynamics of torezolid phosphate (TR-701), a new oxazolidinone antibiotic, against methicillin-susceptible and methicillin-resistant Staphylococcus aureus strains in a mouse thigh infection model. Antimicrob Agents Chemother 55:3453–3460. doi: 10.1128/AAC.01565-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ong CT, Babalola CP, Nightingale CH, Nicolau DP. 2005. Penetration, efflux and intracellular activity of tigecycline in human polymorphonuclear neutrophils (PMNs). J Antimicrob Chemother 56:498–501. doi: 10.1093/jac/dki260. [DOI] [PubMed] [Google Scholar]
- 25.Connors KP, Housman ST, Pope JS, Russomanno J, Salerno E, Shore E, Redican S, Nicolau DP. 2014. Phase I, open-label, safety and pharmacokinetic study to assess bronchopulmonary disposition of intravenous eravacycline in healthy men and women. Antimicrob Agents Chemother 58:2113–2118. doi: 10.1128/AAC.02036-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bhavnani SM, Rubino CM, Hammel JP, Forrest A, Dartois N, Cooper CA, Korth-Bradley J, Ambrose PG. 2012. Pharmacological and patient-specific response determinants in patients with hospital-acquired pneumonia treated with tigecycline. Antimicrob Agents Chemother 56:1065–1072. doi: 10.1128/AAC.01615-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gotfried MH, Horn K, Garrity-Ryan L, Villano S, Tzanis E, Chitra S, Manley A, Tanaka SK, Rodvold KA. 2017. Comparison of omadacycline and tigecycline pharmacokinetics in the plasma, epithelial lining fluid, and alveolar cells of healthy adult subjects. Antimicrob Agents Chemother 61:e01135-17. doi: 10.1128/AAC.01135-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rodvold KA, Pai MP. 2019. Pharmacokinetics and pharmacodynamics of oral and intravenous omadacycline. Clin Infect Dis 69:S16–S22. doi: 10.1093/cid/ciz309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Karlowsky JA, Steenbergen J, Zhanel GG. 2019. Microbiology and preclinical review of omadacycline. Clin Infect Dis 69:S6–S15. doi: 10.1093/cid/ciz395. [DOI] [PMC free article] [PubMed] [Google Scholar]