The polymyxins are important agents for carbapenem-resistant Gram-negative bacilli. The United States Committee on Antimicrobial Susceptibility Testing breakpoint recommendations for colistin and polymyxin B are that isolates of Pseudomonas aeruginosa, Acinetobacter baumannii, and Enterobacteriaceae are considered susceptible at MIC values of ≤2 mg/liter. These recommendations are contingent upon dosing and testing strategies that are described in this commentary.
KEYWORDS: polymyxins, colistin, polymyxin B, breakpoints, USCAST
ABSTRACT
The polymyxins are important agents for carbapenem-resistant Gram-negative bacilli. The United States Committee on Antimicrobial Susceptibility Testing breakpoint recommendations for colistin and polymyxin B are that isolates of Pseudomonas aeruginosa, Acinetobacter baumannii, and Enterobacteriaceae are considered susceptible at MIC values of ≤2 mg/liter. These recommendations are contingent upon dosing and testing strategies that are described in this commentary. Importantly, these recommendations are not applicable to lower respiratory tract infections, for which we recommend no breakpoints. Furthermore, there is no breakpoint recommendation for polymyxin B for lower urinary tract infections.
TEXT
Polymyxin antimicrobial agents (i.e., polymyxin E [colistin] and polymyxin B) have been critical agents for the treatment of serious infections caused by multidrug-resistant (MDR) organisms, including carbapenem-resistant Gram-negative bacilli. Determining susceptibility test interpretive criteria (i.e., susceptibility breakpoints) is particularly challenging for agents in the polymyxin class. These challenges arise from (i) technical limitations in susceptibility testing methodologies, (ii) limited activity in in vivo infection models, (iii) an inability to reliably and safely achieve target serum exposures, and (iv) an absence of clinical efficacy data equating dose, MICs, and clinical outcome.
Recently, a joint EUCAST/CLSI task force recommended polymyxin susceptibility test interpretive criteria for Acinetobacter baumannii, Pseudomonas aeruginosa, and Enterobacteriaceae; however, the published breakpoints for these organizations have not been completely harmonized (1, 2). The interpretive criteria recommended were based largely on MIC population distributions and the results of pharmacokinetic-pharmacodynamic (PK-PD) target attainment analyses. While these recommendations are welcome improvements and modernizations of previous attempts, there are significant limitations to these breakpoints that warrant further consideration. The purpose of this United States Committee on Antimicrobial Susceptibility Testing (USCAST) review is to assess the evidence supporting susceptibility breakpoints for the polymyxins, to highlight the limitations surrounding variables that normally support breakpoint recommendations, and ultimately to provide breakpoint recommendations for the polymyxins.
POLYMYXIN BREAKPOINTS: A HISTORICAL PERSPECTIVE
Breakpoint criteria and technical details for susceptibility testing methods for the polymyxin class have been challenging from the beginning of standardization processes. The National Committee for Clinical Laboratory Standards (NCCLS; later to become CLSI) placed interpretive breakpoint criteria in their early documents between 1980 and 1982 (3–5). These publications provided susceptibility breakpoint criteria, driven primarily by MIC distribution data, for both agar disk diffusion and broth dilution methods (≤2 mg/liter) (3), and the M2-S2 disk diffusion document only suggested a resistance breakpoint at ≥4 mg/liter4 or ≥50 U/ml (≤8 mm) (4). The latter criteria were proposed due to the poor predictive value of disk diffusion methods with unacceptable intermethod correlations with the reference broth microdilution MIC (see Fig. S1 in the supplemental material, example scattergram) (6, 7). This led to the following comment cautioning the use of polymyxin disk test results (4): “Colistin and polymyxin B diffuse poorly in agar, and the diffusion method is thus, less accurate. Resistance is always significant, but when treatment of infections caused by a susceptible strain is being considered, results of a diffusion test should be confirmed with a dilution method. MIC correlates cannot be calculated reliably from regression analysis.”
Polymyxin interpretive criteria were subsequently removed from standards documents in the late 1980s, only to have these agents return as needed therapies for MDR Gram-negative pathogens. In vitro AST methods were reevaluated at that time and confirmed (i) unacceptable disk diffusion method results (7, 8), (ii) that agar dilution reference tests were also unacceptable and cumbersome to perform (7, 9), and (iii) that medium supplements such as polysorbate 80 did not improve reference MIC test performance (10). As modern-day PK-PD and clinical evidence was not available at this time, susceptibility breakpoints continued to be predominately driven by epidemiological cutoff values (ECOFF). This remained the situation until the recent joint task force from the CLSI/EUCAST reassessed breakpoints, although quality control guidances were added in 2005 (10). As previously described, these recent recommendations were based largely on ECOFF, microbiological, and PK-PD considerations. The remainder of this commentary highlights concerns with this strategy.
IS THE ECOFF A GOOD CHOICE FOR A CLINICAL BREAKPOINT?
A large amount of weight has been placed on ECOFF in the determination of EUCAST/CLSI/NCCLS susceptibility test interpretive criteria. An ECOFF is the antimicrobial MIC that discriminates between bacterial subpopulations by the presence or absence (so-called wild type [WT]) of resistance determinants. This approach led to a colistin EUCAST-based susceptibility test interpretive criterion of ≤2 mg/liter (susceptible) for Enterobacteriaceae, A. baumannii, and P. aeruginosa (despite an ECOFF of ≤4 mg/liter for this pathogen) (http://www.eucast.org/mic_distributions_and_ecoffs/). Unfortunately, the ECOFF alone without robust PK-PD information at clinically relevant doses may not be able to discriminate patients more likely to respond to therapy from those less likely. This is problematic since this is the very reason that clinicians rely on susceptibility breakpoints.
Furthermore, it is worrisome that isolates with values below the ECOFF may contain mcr-1, a recently recognized plasmid-mediated polymyxin resistance determinant (11, 12). Thus, the “drug susceptible WT population” contains isolates harboring resistance determinants. While most mcr-1-positive isolates are categorized as polymyxin resistant (MIC > 2 mg/liter) (13–23), there have been multiple reports of mcr-1-positive Enterobacteriaceae with MIC values of ≤2 mg/liter (24–27), including those with MIC values of ≤0.25 mg/liter (25). Moreover, there is recent in vitro evidence suggesting that mcr-1 can be induced in colistin-susceptible isolates at clinically relevant colistin exposures, with subsequent selection of drug resistance (26). These findings further call into question the overreliance on ECOFF in the setting of polymyxin breakpoints. Additionally, Table 1 shows the MIC distributions for colistin when tested by reference broth microdilution panels for 127,328 isolates in the United States (2011 to 2017), supplementing earlier reference result surveillance (28–30). Colistin MIC results for Enterobacteriaceae at 2 mg/liter are very uncommon (0.6% overall; 0.2% among Escherichia coli organisms), suggesting that even if an ECOFF were applied to Enterobacteriaceae, this value would be 1 mg/liter.
TABLE 1.
Colistin reference broth microdilution MIC distributions for U.S. isolates (128,573; SENTRY Program, 2011 to 2017)a
|
Organism(s) (no. tested) |
% at indicated MIC (mg/liter) |
MIC50/MIC90 | |||||
|---|---|---|---|---|---|---|---|
| ≤0.5 | 1 | 2 | 4 | ≥8 | |||
| Enterobacteriaceae (96,847) | 74.9 | 3.9b | 0.6 | 0.4 | 20.3 | ≤0.5/>4 | |
| WT group (79,474)c | 91.5 | 4.5b | 0.6 | 0.3 | 3.1 | ≤0.5/≤0.5 | |
| IR group (17,373)d | 0.8 | 0.8b | 0.3 | 0.1 | 98.0 | >4/>4 | |
| P. aeruginosa (23,685) | 20.0 | 50.8 | 28.6b | 0.5 | 0.1 | 1/2 | |
| Acinetobacter spp. (6,796) | 44.5 | 37.9 | 12.1b | 2.2 | 3.4 | 1/2 | |
aThe vertical line indicates the proposed breakpoint (≤2 mg/liter) for susceptibility.
bPerceived limit of WT population (ECOFF).
cWT, wild-type strain MICs, generally without resistance mechanisms (E. coli, Klebsiella spp., Enterobacter spp., Citrobacter spp.).
dIR, intrinsic resistance in genera/species (Proteus mirabilis, indole-positive Proteae, and Serratia marcescens).
SUSCEPTIBILITY TESTING ISSUES WITH POLYMYXINS
Given that mcr-1 is currently rare in the United States (31), perhaps the more important susceptibility testing issue relates to accuracy of commonly utilized polymyxin commercial test systems or products. A recent joint statement by the EUCAST/CLSI stated that “Reference testing of Enterobacteriaceae, P. aeruginosa and Acinetobacter spp. is by the ISO-standard broth microdilution method” and that “susceptibility testing by other methods, including agar dilution, disk diffusion, and gradient diffusion, cannot be recommended until historical data have been reviewed or new study data have been generated” (http://www.eucast.org/fileadmin/src/media/PDFs/EUCAST_files/General_documents/Recommendations_for_MIC_determination_of_colistin_March_2016.pdf). As previously discussed, this recommendation was based on two observations. First, disk diffusion test results remain unreliable due to poor and slow polymyxin diffusion out of the disk and through agar media, leading to unreliable results and a significant issue with false susceptibility. Second, nearly one-third of polymyxin-resistant isolates test susceptible when the Etest gradient diffusion test is utilized (24).
Unfortunately, limited laboratory resources to test isolates by the reference method and the lack of availability of colistin on most automated susceptibility systems (source of >90% of U.S. test results) severely compromise the ability of clinical laboratories to obtain a “reliable” MIC. Furthermore, very major error (false-susceptible) rates of 36% with Vitek 2 colistin panels have been described (24), leading the manufacturer (bioMérieux) to advise clinical microbiology laboratories to utilize an alternative method of testing in accordance with the current recommendations. Similarly, Vourli and colleagues documented false-susceptible colistin results among A. baumannii when this organism was tested by the BD Phoenix 100 and Vitek 2 products (32). A rapid polymyxin NP (Nordman-Poirel) test has been developed (33). This colorimetric test has achieved some favorable results, including 99.3% and 95.4% sensitivity and specificity, respectively (33, 34). Another commercial broth microdilution test (TREK Diagnostic systems, Cleveland, OH) is also available (35). While these data are encouraging for more reliable tests to differentiate susceptibility in the future, the ability of most clinical laboratories to utilize these technologies is currently limited.
The failure of clinical laboratories to be able to provide clinicians with reliable colistin susceptibility information was demonstrated by the results of a recent survey (36). Of the 59 U.S. institutions that responded to a question asking what methodology their laboratory utilized for determining colistin susceptibility, 66% responded that they used the Etest, 22% reported that they utilized automated broth microdilution, and 10% utilized the agar disk diffusion (Kirby-Bauer) test. This is obviously problematic since none of these methodologies reliably detects resistant isolates (assuming a breakpoint of ≤2 mg/liter). Therefore, any susceptibility breakpoint needs to be partnered with a test that can reliably detect that MIC value.
ARE PHARMACOKINETICS AND PHARMACODYNAMICS BEING APPROPRIATELY APPLIED TO POLYMYXIN BREAKPOINTS?
As mentioned above, the EUCAST/CLSI task force also utilized PK-PD target attainment analyses (described below) as decision support for polymyxin susceptibility test interpretive criteria. The PK-PD target attainment analyses utilized colistin exposure thresholds of the ratio of free-drug area under the plasma concentration-time curve from 0 to 24 h (AUC0–24) to MIC (AUC/MIC) derived from a neutropenic murine thigh infection model involving A. baumannii and P. aeruginosa (37). For A. baumannii, free-drug AUC/MIC ratios of 3.5 to 13.9 and 7.4 to 17.6 were associated with 1- and 2-log10 CFU reductions from baseline, respectively. Similar results were identified for P. aeruginosa, for which free-drug AUC/MIC ratios of 6.6 to 10.9 and 7.4 to 13.7 were associated with 1- and 2-log10 CFU reductions from baseline. Once the protein binding of colistin (∼50%) is taken into consideration, a total AUC/MIC ratio of ∼24 is needed to ensure 1- to 2-log10 CFU reductions in the thighs of neutropenic mice. Given that most current human colistin dosing strategies were recently derived to achieve a steady-state average total-drug AUC0–24 of 48 mg·h/liter, this would support a susceptibility breakpoint of 2 mg/liter, which is employed by both organizations (AUC0–24 of 48/MIC breakpoint of 2 = AUC/MIC ratio ≥ 24).
Unfortunately, in contrast to the thigh infection model, data from a neutropenic murine pneumonia infection model do not support a susceptibility breakpoint of 2 mg/liter (37). In the murine pneumonia model, the free-drug AUC/MIC ratio targets are much higher than in the murine thigh model. For P. aeruginosa, free-drug AUC/MIC ratios of 43.3 to 57.9 and 51.8 to 105 were associated with 1- and 2-log10 CFU reductions, respectively. Even more troubling, net bacterial stasis was not attained for 2 of the 3 A. baumannii challenge isolates despite the administration of maximal tolerated doses in mice. Poor efficacy in murine pneumonia models has also been demonstrated with polymyxin B, for which net bacterial stasis was not demonstrated against any of three challenge Enterobacteriaceae isolates at the highest dose tolerable (38).
Taken together, these data suggest that for pneumonia a more appropriate P. aeruginosa susceptibility breakpoint would be at least 4-fold lower, at 0.5 mg/liter, which would represent only 20% of P. aeruginosa isolates (Table 1). Furthermore, these data also suggest that no A. baumannii or Enterobacteriaceae susceptibility breakpoint should be put forward for this disease state. Finally, as invasive infections due to extensively drug-resistant (XDR) P. aeruginosa and A. baumannii are most commonly observed in the lung (39), there are significant concerns with the current breakpoint selections for these pathogens being routinely applied given that the data do not appear to support these recommendations.
CAN WE RELIABLY ATTAIN THESE TARGETS WITH CURRENT DOSING STRATEGIES IN THE UNITED STATES?
As previously stated, most experts suggest a target average AUC0–24 of 48 mg·h/liter (an average steady-state concentration of 2 mg/liter over 24 h) (40). This target is based on the PK-PD data described above and the extremely narrow colistin therapeutic index. With regard to toxicity, Sorli and colleagues identified a day 3 trough concentration of greater than 2.4 mg/liter to be an independent predictor of acute kidney injury (AKI) (41). These data are supported by those of Forrest and colleagues, who identified average steady-state concentrations greater than 1.9 mg/liter and 2.3 mg/liter (equivalent to AUC0–24 of 46 to 55 mg·h/liter), depending on baseline renal function, to be predictive of an increase in both incidence and severity of AKI (42). Therefore, it is important to note that although the target average steady-state concentration of 2 mg/liter is far from perfect for efficacy, there is a concern with our ability to safely target higher concentrations.
Attaining a target concentration with colistin is further complicated by significant interpatient variability. Pharmacokinetic data have demonstrated that at a given dose and creatinine clearance value, colistin serum concentrations attained in a patient population can vary up to 10-fold. This is due to complex interactions between the prodrug of colistimethate sodium and the active moiety colistin (43). Therefore, even if a target AUC0–24 of 48 mg·h/liter is used, there is little guarantee that these exposures will be attained in a given patient. While such a clinical scenario would present an opportunity for therapeutic drug monitoring, assays for measuring serum colistin levels are not readily available to clinicians. This wide interpatient variability and the unpredictable drug concentrations for colistin are among the reasons for the emerging preference of polymyxin B over colistin.
Colistin dosing (and subsequent appropriateness of susceptibility breakpoints) is further complicated by the existence of three different recommended dosing regimens, which vary in the ability to reliably achieve the target exposure (AUC0–24 of 48 mg·h/liter). The EUCAST breakpoints of 2 mg/liter were based on the current dosing in the EMA package insert for colistin (44). The EMA package insert describes a base maintenance dose of 9 million international units (MIU) (300 mg of colistin base activity [CBA])/day, with subsequent renal-function-based dosing recommendations. These dosing recommendations are largely in line with the final dosing recommendations from Nation and colleagues published in 2017, which were based on a pharmacokinetic study in critically ill patients (40). The U.S. product package insert is unique in that it offers a weight-based dosing recommendation, although current pharmacokinetic evidence does not support an association between patient weight, clearance, and subsequent dose requirements (45). The doses provided by the U.S. package insert (assuming a patient weight of 70 kg) are in the realm of those from the EMA and the dosing algorithm by Nation and colleagues for creatinine clearance values greater than 50 ml/min. However, due to dose reduction recommendations beyond what are necessary, patients with creatinine clearance values less than 50 ml/min will not reliably achieve target concentrations (46). This is most evident for patients with creatinine clearances less than 30 ml/min, of whom only 5 to 35% would be expected to achieve an AUC0–24 target of 48 mg·h/liter (46). Furthermore, due to rapid clearance of the colistimethate prior to conversion to the active moiety, none of the available dosing algorithms predict therapeutic exposures in over 40% of patients with creatinine clearances greater than 80 ml/min (46).
ARE WE SURE THAT THESE ARE THE BEST TARGET EXPOSURES?
Importantly, it warrants mentioning that it is not clear if any of the above-described dosing strategies are optimized. On one hand, there is a desire to achieve an average steady-state exposure target of ≥2 mg/liter (total drug AUC0–24 ≥ 48 mg·h/liter) in order to attain PK-PD targets at a proposed breakpoint of 2 mg/liter; on the other hand, these dose recommendations lead to median average steady-state concentrations of approximately 3 mg/liter (total drug AUC0–24 of 72 mg·h/liter) (40), which is an exposure above the aforementioned toxicity thresholds (41, 42). Furthermore, it is critical to remember that the free-drug AUC that is targeted by these dosing strategies is not associated with efficacy in perhaps the most relevant animal model: the murine pneumonia infection model.
Furthermore, although SENTRY Antimicrobial Surveillance Program data (derived from the reference broth microdilution MIC method, 2011 to 2017 [Table 1]) show that for P. aeruginosa (∼30%) and A. baumannii (∼10%) a substantial proportion of WT isolates have an MIC of 2 mg/liter, it is worth noting that for Enterobacteriaceae, <1% of isolates have MIC values of 2 mg/liter. Given that clinical laboratories cannot obtain reliable MIC values, the limitations of the PK-PD targets, the safety concerns with the targeted exposures, and the fact that other than for P. aeruginosa, and to a lesser extent A. baumannii, a very small fraction of WT MIC values fall at or above an MIC of 2 mg/liter, it remains to be determined if a breakpoint of 2 mg/liter is the appropriate balance between safety and efficacy. For all of these reasons, a rational argument could be made for a lower target colistin AUC0–24, coupled with a lower susceptibility breakpoint allowing the target AUC/MIC ratio to be more safely met and still covering a similar proportion of infecting isolates. This is particularly true if the polymyxins are accepted to be used as part of a combination regimen. This might allow safer usage of colistin without compromising clinical efficacy.
DO CLINICAL DATA SUPPORT THESE DOSING RECOMMENDATIONS?
The other important consideration that hinders the ability to confidently select polymyxin clinical breakpoints is the absence of any patient outcome data to demonstrate exposure-response relationships for efficacy. Additionally, data on the impact of differing dosing regimens are conflicting. This is perhaps not unexpected given (i) the large interpatient variability observed with colistin exposures even with the same dose; (ii) the complexity of the patient population and lack of clarity on the optimal endpoint to assess efficacy; (iii) the significant delays in time to appropriate therapy in patients with carbapenem-resistant organisms; (iv) the frequent use of poorly defined combination therapeutic regimens; (v) the unclear renal dosing adjustments performed in the studies; (vi) the differing sites of infection treated in these analyses, which confound the ability to assess efficacy; and (vii) the failure of most studies to report MIC values of the infecting pathogens.
In fact, the most robust study that assessed dose and outcome compared a dose similar to the current EMA package insert dosing regimen to “all other regimens.” The other regimens largely consisted of historic package insert dosing that entailed a median daily dose of 4 MIU (interquartile range [IQR], 3 to 6 MIU) or 133 mg of CBA (IQR, 100 to 200 mg of CBA). While the results of the study demonstrated no benefit for “high-dose” colistin with respect to mortality, the authors did demonstrate an increase in AKI with the more aggressive dose (47). Recently, data from a randomized controlled trial comparing colistin monotherapy to combination therapy for infections due to carbapenem-resistant Gram-negative bacilli demonstrated unacceptably high failure rates (∼75%) despite the use of “high-dose” colistin that included a loading dose of 9 MIU (300 mg of CBA) × 1 followed by 4.5 MIU (150 mg of CBA) every 12 h (48). Interestingly, data presented from this study suggested that this high failure rate occurred in spite of achieving higher-than-expected colistin average steady-state serum concentrations in the majority of patients (49).
Unfortunately, as it relates to the polymyxins, there is an absence of data suggesting that increased doses and/or exposures improve outcomes, nor are there data equating dose, MIC, and outcome. In fact, a subanalysis of the aforementioned randomized controlled trial found no difference in outcomes in patients who received colistin for “susceptible” isolates of A. baumannii (MIC ≤ 2 mg/liter) and those who had resistant ones (MICs of >2 mg/liter, where the majority were >8 mg/liter) (74). Conversely, there is direct evidence that this aggressive dosing strategy leads to increased toxicity.
CAN COMBINATION THERAPY RESCUE THE POLYMYXINS?
Breakpoint setting for the polymyxins is complicated with the currently available evidence. When one considers both the lack of predictability of response based on reported MIC values for polymyxin therapy and the synergistic mechanism of action that these agents display when combined with other classes of antimicrobials, combination therapy is a pragmatic therapeutic strategy.
Not surprisingly, clinical data to support combination therapy with polymyxins are often derived from studies that suffer from poor methodology and have conflicting results. While multiple studies have consistently demonstrated a survival advantage of combination therapy with multiple antimicrobials that demonstrate in vitro activity against bloodstream infections due to carbapenem-resistant Enterobacteriaceae (CRE) (50–54), the exact role of the polymyxins remains unclear in this setting, as combination regimens that excluded a polymyxin were also associated with enhanced survival. Despite these conflicting pieces of information, it remains prudent that if a polymyxin is being considered for an invasive CRE infection, combination therapy with another active agent should be utilized.
The data for polymyxin combination therapy outside of CRE are less convincing. Three open-label randomized trials comparing colistin monotherapy with colistin plus a synergistic second drug (rifampin, fosfomycin, or meropenem) (48, 55, 56) for invasive carbapenem-resistant A. baumannii infections failed to show a decrease in mortality or an improvement in clinical cure with the combination regimen. Importantly, no analysis has evaluated a polymyxin in combination with a second active agent for carbapenem-resistant A. baumannii, which, given the findings for CRE, might prove to be a more effective strategy. Therefore, it may be most practical to combine a polymyxin with an active second agent in these patients. Unfortunately, there are no data assessing polymyxin monotherapy versus a combination for P. aeruginosa infections.
USCAST COLISTIN CLINICAL BREAKPOINT RECOMMENDATIONS
There is no simple answer to the problem of setting colistin clinical breakpoint values. Given the aforementioned issues with each individual factor involved in breakpoint determination, and especially since there is significant concern with the accuracy of the MIC value reported from the microbiology laboratory, a very compelling case could be made for not setting breakpoint values for this class. However, this is not a stance that would be helpful for clinicians prescribing a U.S. FDA-approved antimicrobial drug, and thus, a recommendation should be made with certain defined limitations.
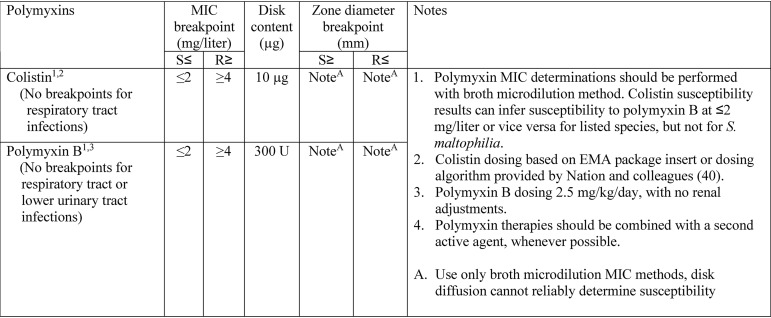
It is important to note that there are two major assumptions that underlie the breakpoint recommendations for colistin. These include MIC determination and drug exposure expected from dosing regimens used in clinical practice. The first assumption is that institutions with a high volume of colistin use must utilize a susceptibility testing methodology to improve MIC testing functionality and have a more accurate way of measuring MIC values than Etest, disk diffusion, or an automated system. In the absence of a reliable method of testing, no breakpoint recommendation is valid, as the MIC result cannot be guaranteed to be accurate, and false-susceptible isolates would be expected. The second assumption is that the institution is using either the dosing recommendations present in the EMA colistin package insert or those recommended via the dosing algorithm published by Nation and colleagues (40). Please note that USCAST is not recommending these doses as the appropriate balance between safety and efficacy; however, we are assuming that they will be utilized based off of international guideline recommendations (64). Both of these dosing methodologies should result in average colistin steady-state concentrations of 2 mg/liter, which results in an AUC0–24 of ≥48 mg·h/liter in ≥80% of patients. Although this is lower than the usual 90% target attainment goal, it is a reasonable compromise given increased safety concerns at higher doses. As was previously addressed, U.S. FDA-labeled doses do not meet these criteria for patients with creatinine clearances of ≤50 ml/min and therefore are invalid for these breakpoint recommendations.
We recommend that there be no formal breakpoints recommended for polymyxins when used for the treatment of respiratory tract infections. This stems from (i) the preclinical data suggesting the absence of reliable activity in murine pneumonia models, (ii) the high clinical failure rate when patients with pulmonary infections are treated with polymyxin monotherapy, (iii) the failure of synergistic combination regimens to improve clinical outcomes, and (iv) the impact of pulmonary surfactant on colistin activity (57). To provide breakpoints for polymyxins in respiratory infections would give the impression to clinicians that one could reasonably achieve a clinical/microbiological response with the current dosing recommendation, and we do not feel that this is supported by current evidence. Although the limitations of systemic polymyxin therapy for pneumonia have led experts and guidelines (58) to recommend inhaled polymyxins in this setting, insufficient evidence currently exists to support breakpoints with this route of administration. With regard to respiratory tract infections, polymyxins should not be considered a treatment option if alternative agents are available. If they are utilized, the polymyxin should not be relied upon as monotherapy.
For other systemic infections (e.g., primary bloodstream infections and complicated urinary tract infections), a breakpoint of ≤2 mg/liter appears to be reasonable. Current dosing recommendations should achieve free-drug AUC/MIC ratio target values associated with a 1- to 2-log10 CFU decrease from baseline for most patients. Although there is concern that due to rapid elimination of the prodrug, target concentrations might not be achieved if creatinine clearance values are >80 ml/min, recent evidence from the AIDA trial somewhat refutes this (49). In 55 patients with creatinine clearance values of 80 to 120 ml/min, the average (5th and 95th percentiles) colistin steady-state concentration was 3.7 mg/liter (1.0 to 6.9). However, in patients with augmented renal clearance (creatinine clearance values > 120 ml/min), the average (5th and 95th percentiles) steady-state concentration was 1.7 mg/liter (0.3 to 4.4). Furthermore, although mcr-1 might be present but not expressed at MIC values of <2 mg/liter, there is currently no evidence that suggests that patient outcomes would be worse in those treated with colistin than in those whose pathogens lack mcr-1. Therefore, breakpoints of ≤2 mg/liter appear to be appropriate for nonrespiratory tract infections at the current dosing regimens employed.
WHAT ABOUT POLYMYXIN B CLINICAL BREAKPOINTS AND SUSCEPTIBILITY TESTING?
Increased interest in polymyxin B use has emerged over the past few years. This has been driven by two main features. First, as polymyxin B is given as its active moiety, many of the prodrug-related pharmacokinetic limitations for colistin can be mitigated (59). In particular, this leads to more rapid achievement of therapeutic concentrations, a decrease in interpatient variability, and achievable therapeutic concentrations in patients with creatinine clearances of >80 ml/min. Second, emerging evidence has suggested that polymyxin B might be less nephrotoxic than colistin (60).
Unfortunately, very limited evidence exists regarding pharmacokinetic exposures with common dosing regimens. However, pharmacokinetic data from 23 critically ill patients that were used in a Monte Carlo simulation suggest that dosing at the upper end of the package insert-recommended range produces polymyxin B exposures similar to those obtained with EMA-based dosing of colistin (61). When simulating exposures with dosing of 1.25 mg/kg of body weight every 12 h, the average polymyxin B AUC0–24 was 72.0 mg·h/liter, with the 10th and 90th percentile of predicted exposures being 44.3 to 114 mg·h/liter. Given that these are similar to the target concentrations for colistin, and that the PK-PD targets, derived in Enterobacteriaceae, for polymyxin B appear to be similar to those of colistin (38), it is reasonable that the same breakpoints as described for colistin could be applied to polymyxin B. Of note, due to polymyxin B not being excreted in the urine (61), it is not appropriate therapy for lower urinary tract infections, and thus, no breakpoint should be recommended for that disease state. This is in contrast to the case with colistin, for which renally eliminated colistimethate may hydrolyze to colistin in the urine, allowing sufficient concentrations for the management of urinary tract infections (59). Importantly, however, these recommendations should be regarded as interim. As more pharmacokinetic, safety, and clinical data become available for polymyxin B, a reassessment will be warranted.
An important consideration for the setting of polymyxin B breakpoints is an understanding and appreciation that many clinical microbiology laboratories test only for colistin susceptibility. Polymyxin B appears to suffer from the same limitations as colistin when tested by Etest, with a recent publication demonstrating a very major error rate of 88% (62). Therefore, any consideration for colistin serving as a surrogate for polymyxin B susceptibility requires testing to be performed via the recommended reference broth microdilution method (63).
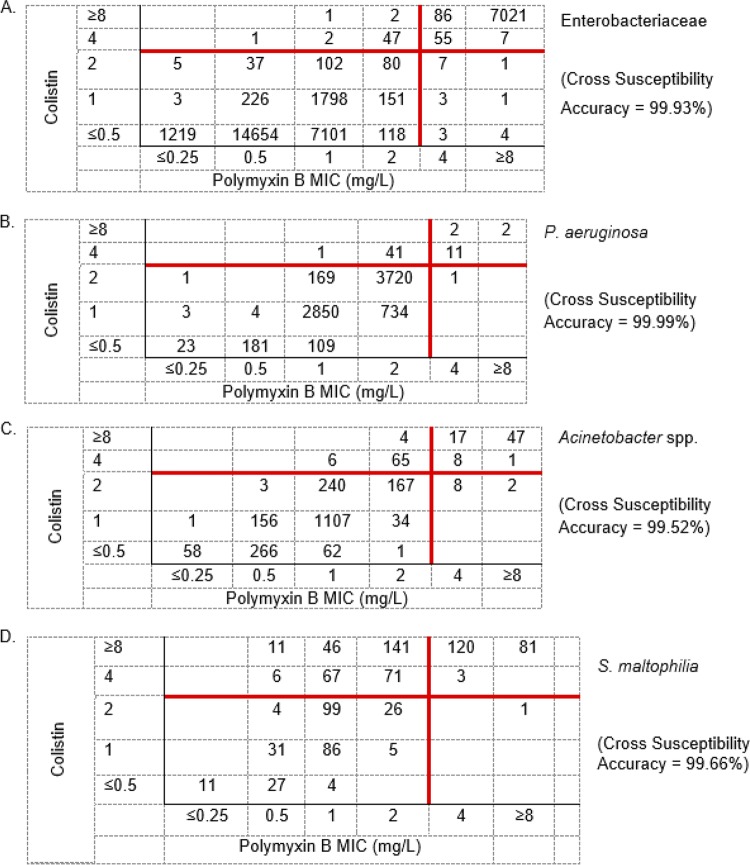
In order to confirm prior high-level essential agreement between colistin and polymyxin B MIC values (Fig. S2) (30) and to assess the ability of colistin to serve as a surrogate for polymyxin B susceptibility, USCAST members reviewed data on 43,893 P. aeruginosa, A. baumannii, Enterobacteriaceae, and Stenotrophomonas maltophilia isolates and both colistin and polymyxin B via the reference broth microdilution method. Cross-susceptibility analyses (Fig. 1) confirmed that colistin susceptibility results can serve as a surrogate for polymyxin B susceptibility when testing P. aeruginosa, A. baumannii, and Enterobacteriaceae. The accuracy rates of using colistin (≤2 mg/liter as susceptible) to infer polymyxin B activity (≤2 mg/liter as susceptible) across the various species are listed in Fig. 1. Cross-susceptibility rates range from 99.52% to 99.99% for all species, whereas false-nonsusceptibility (colistin resistance in the setting of polymyxin B susceptibility) rates account for <1% of polymyxin B-susceptible isolates outside of A. baumannii, where this occurred approximately 3.5% of the time. It is noteworthy that of these 75 A. baumannii isolates, 65 (87%) (Fig. 1C) displayed essential agreement (colistin MIC of 4 mg/liter [resistant] and polymyxin B MIC of 2 mg/liter [susceptible]). Therefore, we have validated and recommend that colistin susceptibility is an appropriate surrogate for polymyxin B susceptibility when testing these Gram-negative pathogens. Although we are not recommending clinical breakpoints for S. maltophilia due to an absence of data, it is important to note that the superior potency of polymyxin B over colistin against this pathogen (Fig. 1D) raises a unique pathogen-specific issue. If we apply a hypothetical breakpoint of 2 mg/liter, while colistin cross-susceptible surrogate accuracy is high (99.7% accuracy), the converse is not true, as the false-nonsusceptible (resistant) rate for polymyxin B was very high (54.6%) if this was assumed based on colistin nonsusceptibility.
FIG 1.
Cross-susceptibility analyses of utilizing colistin MIC results (y axis) at ≤2 mg/liter to predict polymyxin B at the same MIC (x axis). Data are from reference broth microdilution testing of 43,893 isolates focusing on (A) Enterobacteriaceae (32,928), (B) P. aeruginosa (7,852), (C) Acinetobacter spp. (2,253), and (D) S. maltophilia (832) from the SENTRY Antimicrobial Surveillance Program.
CONCLUSIONS
The polymyxins are suboptimal and somewhat flawed antimicrobial agents that have been used in clinical practice for over 6 decades. Antimicrobial stewardship programs and infectious diseases clinicians should consider them last-line therapeutic options. We recommend the use of newer, safer, and more effective antimicrobials with activity against resistant Gram-negative pathogens when available (65–67). Ceftazidime-avibactam (68–70), meropenem-vaborbactam (71), plazomicin (72), and ceftolozane-tazobactam (73) have all demonstrated increased efficacy and decreased toxicity compared to the polymyxins and therefore should be preferred in these clinical settings.
Based on the currently available data and commonly employed dosing strategies, we recommend a susceptibility breakpoint of ≤2 mg/liter for both colistin and polymyxin B (Table 2). Whether or not these are the optimal dosing strategies (and subsequently the optimal breakpoints) remains to be determined. Additionally, as more data become available for polymyxin B, a reassessment of these breakpoints would be warranted.
TABLE 2.
USCAST susceptibility breakpoint recommendations when testing the polymyxins against P. aeruginosa, A. baumannii, and Enterobacteriaceae
It is critical for clinicians to recognize that these breakpoint recommendations do not apply to respiratory tract infections, as systemic polymyxins do not have appreciable activity in the lung and should not be relied upon alone for the treatment of pulmonary infections. Also, since polymyxin B demonstrates extensive renal tubular reabsorption following glomerular filtration and does not undergo significant renal elimination, this drug should not be utilized for lower urinary tract infections.
Lastly, while the use of combination therapy with polymyxins was not thoroughly reviewed, it appears to represent a necessary therapeutic option against MDR Gram-negative pathogens. Given the current evidence, we would recommend the addition of a second agent with documented in vitro activity whenever possible. Further evidence is needed to optimize these current strategies for use in the absence of a second active agent.
Supplementary Material
The views expressed in this article do not necessarily reflect the views of the journal or of ASM.
Footnotes
Supplemental material is available online only.
REFERENCES
- 1.CLSI. 2019. M100Ed29. Performance standards for antimicrobial susceptibility testing: 29th informational supplement. Clinical and Laboratory Standards Institute, Wayne, PA. [Google Scholar]
- 2.EUCAST. 2019. Breakpoint tables for interpretation of MICs and zone diameters. Version 9.0, January 2019. http://www.eucast.org/fileadmin/src/media/PDFs/EUCAST_files/Breakpoint_tables/v_9.0_Breakpoint_Tables.pdf. Accessed January 2019.
- 3.NCCLS. 1981. M2-A02-S1. Performance standards for antimicrobial disc susceptibility tests; approved standard. First supplement. National Committee for Clinical Laboratory Standards, Villanova, PA. [Google Scholar]
- 4.NCCLS. 1982. M2-A02-S2. Performance standards for antimicrobial disc susceptibility tests; approved standard. Second supplement. National Committee for Clinical and Laboratory Standards, Villanova, PA. [Google Scholar]
- 5.NCCLS. 1980. PSM-7. Standard methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically. Proposed standard. National Committee for Clinical Laboratory Standards, Villanova, PA. [Google Scholar]
- 6.NCCLS. 1983. M7-T. Standard methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically. Tentative standard. National Committee for Clinical Laboratory Standards, Villanova, PA. [Google Scholar]
- 7.Gales AC, Reis AO, Jones RN. 2001. Contemporary assessment of antimicrobial susceptibility testing methods for polymyxin B and colistin: review of available interpretative criteria and quality control guidelines. J Clin Microbiol 39:183–190. doi: 10.1128/JCM.39.1.183-190.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Swenson JM, Killgore GE, Tenover FC. 2004. Antimicrobial susceptibility testing of Acinetobacter spp. by NCCLS broth microdilution and disk diffusion methods. J Clin Microbiol 42:5102–5108. doi: 10.1128/JCM.42.11.5102-5108.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Matuschek E, Davies L, Ahman J, Kahlmeter G, Wootton M. 2018. Can agar dilution be used for colistin MIC determination? Abstr Eur Congr ClinMicrobiol Infect Dis (ECCMID), 21 to 24 April, Madrid, Spain.
- 10.Sader HS, Rhomberg PR, Flamm RK, Jones RN. 2012. Use of a surfactant (polysorbate 80) to improve MIC susceptibility testing results for polymyxin B and colistin. Diagn Microbiol Infect Dis 74:412–414. doi: 10.1016/j.diagmicrobio.2012.08.025. [DOI] [PubMed] [Google Scholar]
- 11.Baron S, Hadjadj L, Rolain JM, Olaitan AO. 2016. Molecular mechanisms of polymyxin resistance: knowns and unknowns. Int J Antimicrob Agents 48:583–591. doi: 10.1016/j.ijantimicag.2016.06.023. [DOI] [PubMed] [Google Scholar]
- 12.Poirel L, Jayol A, Nordmann P. 2017. Polymyxins: antibacterial activity, susceptibility testing, and resistance mechanisms encoded by plasmids or chromosomes. Clin Microbiol Rev 30:557–596. doi: 10.1128/CMR.00064-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hasman H, Hammerum AM, Hansen F, Hendriksen RS, Olesen B, Agerso Y, Zankari E, Leekitcharoenphon P, Stegger M, Kaas RS, Cavaco LM, Hansen DS, Aarestrup FM, Skov RL. 2015. Detection of mcr-1 encoding plasmid-mediated colistin-resistant Escherichia coli isolates from human bloodstream infection and imported chicken meat, Denmark 2015. Euro Surveill 20:30085. doi: 10.2807/1560-7917.ES.2015.20.49.30085. [DOI] [PubMed] [Google Scholar]
- 14.Zhang X-F, Doi Y, Huang X, Li H-Y, Zhong L-L, Zeng K-J, Zhang Y-F, Patil S, Tian G-B. 2016. Possible transmission of mcr-1-harboring Escherichia coli between companion animals and human. Emerg Infect Dis 22:1679–1681. doi: 10.3201/eid2209.160464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Arcilla MS, van Hattem JM, Matamoros S, Melles DC, Penders J, de Jong MD, Schultsz C, COMBAT Consortium. 2016. Dissemination of the mcr-1 colistin resistance gene. Lancet Infect Dis 16:147–149. doi: 10.1016/S1473-3099(15)00541-1. [DOI] [PubMed] [Google Scholar]
- 16.Du H, Chen L, Tang YW, Kreiswirth BN. 2016. Emergence of the mcr-1 colistin resistance gene in carbapenem-resistant Enterobacteriaceae. Lancet Infect Dis 16:287–288. doi: 10.1016/S1473-3099(16)00056-6. [DOI] [PubMed] [Google Scholar]
- 17.Falgenhauer L, Waezsada SE, Yao Y, Imirzalioglu C, Kasbohrer A, Roesler U, Michael GB, Schwarz S, Werner G, Kreienbrock L, Chakraborty T, RESET Consortium. 2016. Colistin resistance gene mcr-1 in extended-spectrum beta-lactamase-producing and carbapenemase-producing Gram-negative bacteria in Germany. Lancet Infect Dis 16:282–283. doi: 10.1016/S1473-3099(16)00009-8. [DOI] [PubMed] [Google Scholar]
- 18.Liu Y-Y, Wang Y, Walsh TR, Yi L-X, Zhang R, Spencer J, Doi Y, Tian G, Dong B, Huang X, Yu L-F, Gu D, Ren H, Chen X, Lv L, He D, Zhou H, Liang Z, Liu J-H, Shen J. 2016. Emergence of plasmid-mediated colistin resistance mechanism MCR-1 in animals and human beings in China: a microbiological and molecular biological study. Lancet Infect Dis 16:161–168. doi: 10.1016/S1473-3099(15)00424-7. [DOI] [PubMed] [Google Scholar]
- 19.Malhotra-Kumar S, Xavier BB, Das AJ, Lammens C, Butaye P, Goossens H. 2016. Colistin resistance gene mcr-1 harboured on a multidrug resistant plasmid. Lancet Infect Dis 16:283–284. doi: 10.1016/S1473-3099(16)00012-8. [DOI] [PubMed] [Google Scholar]
- 20.Olaitan AO, Chabou S, Okdah L, Morand S, Rolain JM. 2016. Dissemination of the mcr-1 colistin resistance gene. Lancet Infect Dis 16:147. doi: 10.1016/S1473-3099(15)00540-X. [DOI] [PubMed] [Google Scholar]
- 21.Poirel L, Kieffer N, Liassine N, Thanh D, Nordmann P. 2016. Plasmid-mediated carbapenem and colistin resistance in a clinical isolate of Escherichia coli. Lancet Infect Dis 16:281. doi: 10.1016/S1473-3099(16)00006-2. [DOI] [PubMed] [Google Scholar]
- 22.Suzuki S, Ohnishi M, Kawanishi M, Akiba M, Kuroda M. 2016. Investigation of a plasmid genome database for colistin-resistance gene mcr-1. Lancet Infect Dis 16:284–285. doi: 10.1016/S1473-3099(16)00008-6. [DOI] [PubMed] [Google Scholar]
- 23.Yao X, Doi Y, Zeng L, Lv L, Liu J-H. 2016. Carbapenem-resistant and colistin-resistant Escherichia coli co-producing NDM-9 and MCR-1. Lancet Infect Dis 16:288–289. doi: 10.1016/S1473-3099(16)00057-8. [DOI] [PubMed] [Google Scholar]
- 24.Chew KL, La MV, Lin RTP, Teo J. 2017. Colistin and polymyxin b susceptibility testing for carbapenem-resistant and mcr-positive Enterobacteriaceae: comparison of Sensititre, MicroScan, Vitek 2, and Etest with broth microdilution. J Clin Microbiol 55:2609–2616. doi: 10.1128/JCM.00268-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Terveer EM, Nijhuis RHT, Crobach MJT, Knetsch CW, Veldkamp KE, Gooskens J, Kuijper EJ, Claas E. 2017. Prevalence of colistin resistance gene (mcr-1) containing Enterobacteriaceae in feces of patients attending a tertiary care hospital and detection of a mcr-1 containing, colistin susceptible E. coli. PLoS One 12:e0178598. doi: 10.1371/journal.pone.0178598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhou K, Luo Q, Wang Q, Huang C, Lu H, Rossen JWA, Xiao Y, Li L. 2018. Silent transmission of an IS1294b-deactivated mcr-1 gene with inducible colistin resistance. Int J Antimicrob Agents 51:822–828. doi: 10.1016/j.ijantimicag.2018.01.004. [DOI] [PubMed] [Google Scholar]
- 27.Pillonetto M, Mazzetti A, Becker GN, Siebra CA, Arend L, Barth AL. 2019. Low level of polymyxin resistance among nonclonal mcr-1-positive Escherichia coli from human sources in Brazil. Diagn Microbiol Infect Dis 93:140–142. doi: 10.1016/j.diagmicrobio.2018.08.009. [DOI] [PubMed] [Google Scholar]
- 28.Gales AC, Jones RN, Sader HS. 2006. Global assessment of the antimicrobial activity of polymyxin B against 54,731 clinical isolates of Gram-negative bacilli: report from the SENTRY antimicrobial surveillance programme (2001–2004). Clin Microbiol Infect 12:315–321. doi: 10.1111/j.1469-0691.2005.01351.x. [DOI] [PubMed] [Google Scholar]
- 29.Gales AC, Jones RN, Sader HS. 2011. Contemporary activity of colistin and polymyxin B against a worldwide collection of Gram-negative pathogens: results from the SENTRY Antimicrobial Surveillance Program (2006–09). J Antimicrob Chemother 66:2070–2074. doi: 10.1093/jac/dkr239. [DOI] [PubMed] [Google Scholar]
- 30.Sader HS, Rhomberg PR, Farrell DJ, Jones RN. 2015. Differences in potency and categorical agreement between colistin and polymyxin B when testing 15,377 clinical strains collected worldwide. Diagn Microbiol Infect Dis 83:379–381. doi: 10.1016/j.diagmicrobio.2015.08.013. [DOI] [PubMed] [Google Scholar]
- 31.Castanheira M, Griffin MA, Deshpande LM, Mendes RE, Jones RN, Flamm RK. 2016. Detection of mcr-1 among Escherichia coli and Klebsiella pneumoniae clinical isolates collected worldwide as part of the SENTRY Antimicrobial Surveillance Program during 2014–2015. Antimicrob Agents Chemother 60:5623–5624. doi: 10.1128/AAC.01267-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vourli S, Dafopoulou K, Vrioni G, Tsakris A, Pournaras S. 2017. Evaluation of two automated systems for colistin susceptibility testing of carbapenem-resistant Acinetobacter baumannii clinical isolates. J Antimicrob Chemother 72:2528–2530. doi: 10.1093/jac/dkx186. [DOI] [PubMed] [Google Scholar]
- 33.Nordmann P, Jayol A, Poirel L. 2016. Rapid detection of polymyxin resistance in Enterobacteriaceae. Emerg Infect Dis 22:1038–1043. doi: 10.3201/eid2206.151840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Giske CG, Kahlmeter G. 2018. Colistin antimicrobial susceptibility testing—can the slow and challenging be replaced by the rapid and convenient? Clin Microbiol Infect 24:93–94. doi: 10.1016/j.cmi.2017.10.007. [DOI] [PubMed] [Google Scholar]
- 35.Humphries RM. 2015. Susceptibility testing of the polymyxins: where are we now? Pharmacotherapy 35:22–27. doi: 10.1002/phar.1505. [DOI] [PubMed] [Google Scholar]
- 36.Wenzler E, Bunnell KL, Danziger LH. 2018. Clinical use of the polymyxins: the tale of the fox and the cat. Int J Antimicrob Agents 51:700–706. doi: 10.1016/j.ijantimicag.2017.12.023. [DOI] [PubMed] [Google Scholar]
- 37.Cheah SE, Wang J, Nguyen VT, Turnidge JD, Li J, Nation RL. 2015. New pharmacokinetic/pharmacodynamic studies of systemically administered colistin against Pseudomonas aeruginosa and Acinetobacter baumannii in mouse thigh and lung infection models: smaller response in lung infection. J Antimicrob Chemother 70:3291–3297. doi: 10.1093/jac/dkv267. [DOI] [PubMed] [Google Scholar]
- 38.Landersdorfer CB, Wang J, Wirth V, Chen K, Kaye KS, Tsuji BT, Li J, Nation RL. 2018. Pharmacokinetics/pharmacodynamics of systemically administered polymyxin B against Klebsiella pneumoniae in mouse thigh and lung infection models. J Antimicrob Chemother 73:462–468. doi: 10.1093/jac/dkx409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cai B, Echols R, Magee G, Arjona Ferreira JC, Morgan G, Ariyasu M, Sawada T, Nagata TD. 2017. Prevalence of carbapenem-resistant Gram-negative infections in the United States predominated by Acinetobacter baumannii and Pseudomonas aeruginosa. Open Forum Infect Dis 4:ofx176. doi: 10.1093/ofid/ofx176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nation RL, Garonzik SM, Thamlikitkul V, Giamarellos-Bourboulis EJ, Forrest A, Paterson DL, Li J, Silveira FP. 2017. Dosing guidance for intravenous colistin in critically-ill patients. Clin Infect Dis 64:565–571. doi: 10.1093/cid/ciw839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sorli L, Luque S, Grau S, Berenguer N, Segura C, Montero MM, Alvarez-Lerma F, Knobel H, Benito N, Horcajada JP. 2013. Trough colistin plasma level is an independent risk factor for nephrotoxicity: a prospective observational cohort study. BMC Infect Dis 13:380. doi: 10.1186/1471-2334-13-380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Forrest A, Garonzik SM, Thamlikitkul V, Giamarellos-Bourboulis EJ, Paterson DL, Li J, Silveira FP, Nation RL. 2017. Pharmacokinetic/toxicodynamic analysis of colistin-associated acute kidney injury in critically ill patients. Antimicrob Agents Chemother 61:e01367-17. doi: 10.1128/AAC.01367-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Garonzik SM, Li J, Thamlikitkul V, Paterson DL, Shoham S, Jacob J, Silveira FP, Forrest A, Nation RL. 2011. Population pharmacokinetics of colistin methanesulfonate and formed colistin in critically ill patients from a multicenter study provide dosing suggestions for various categories of patients. Antimicrob Agents Chemother 55:3284–3294. doi: 10.1128/AAC.01733-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.EMA. 2014. Annex III: amendments to relevant sections of the summary of product characteristics and package leaflets. https://www.ema.europa.eu/documents/referral/intravenous-iron-containing-medicinal-products-article-31-referral-annex-iii_en.pdf.
- 45.Monarch Pharmaceuticals. 2006. Coly-Mycin®M, package insert. Monarch Pharmaceuticals, Rochester, MI. [Google Scholar]
- 46.Nation RL, Garonzik SM, Li J, Thamlikitkul V, Giamarellos-Bourboulis EJ, Paterson DL, Turnidge JD, Forrest A, Silveira FP. 2016. Updated US and European dose recommendations for intravenous colistin: how do they perform? Clin Infect Dis 62:552–558. doi: 10.1093/cid/civ964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Benattar YD, Omar M, Zusman O, Yahav D, Zak-Doron Y, Altunin S, Elbaz M, Daitch V, Granot M, Leibovici L, Paul M. 2016. The effectiveness and safety of high-dose colistin: prospective cohort study. Clin Infect Dis 63:1605–1612. doi: 10.1093/cid/ciw684. [DOI] [PubMed] [Google Scholar]
- 48.Paul M, Daikos GL, Durante-Mangoni E, Yahav D, Carmeli Y, Benattar YD, Skiada A, Andini R, Eliakim-Raz N, Nutman A, Zusman O, Antoniadou A, Pafundi PC, Adler A, Dickstein Y, Pavleas I, Zampino R, Daitch V, Bitterman R, Zayyad H, Koppel F, Levi I, Babich T, Friberg LE, Mouton JW, Theuretzbacher U, Leibovici L. 2018. Colistin alone versus colistin plus meropenem for treatment of severe infections caused by carbapenem-resistant Gram-negative bacteria: an open-label, randomised controlled trial. Lancet Infect Dis 18:391–400. doi: 10.1016/S1473-3099(18)30099-9. [DOI] [PubMed] [Google Scholar]
- 49.Kristoffersson A, Brill M, Dishon Y, Daitch V, Andini R, Skiada A, Nutman A, Kotsaki A, Eliakim-Raz N, Zayyad H, Durante Mangoni E, Antoniadou A, Daikos G, Mouton J, Carmeli Y, Paul M, Friberg L. 2018. Colistin population pharmacokinetics (PK) in 237 critically ill patients in the AIDA colistin study, O-0803. European Congress of Clinical Microbiology and Infectious Diseases, (ECCMID), 21 to 28 April, Madrid, Spain.
- 50.Zarkotou O, Pournaras S, Tselioti P, Dragoumanos V, Pitiriga V, Ranellou K, Prekates A, Themeli-Digalaki K, Tsakris A. 2011. Predictors of mortality in patients with bloodstream infections caused by KPC-producing Klebsiella pneumoniae and impact of appropriate antimicrobial treatment. Clin Microbiol Infect 17:1798–1803. doi: 10.1111/j.1469-0691.2011.03514.x. [DOI] [PubMed] [Google Scholar]
- 51.Qureshi ZA, Paterson DL, Potoski BA, Kilayko MC, Sandovsky G, Sordillo E, Polsky B, Adams-Haduch JM, Doi Y. 2012. Treatment outcome of bacteremia due to KPC-producing Klebsiella pneumoniae: superiority of combination antimicrobial regimens. Antimicrob Agents Chemother 56:2108–2113. doi: 10.1128/AAC.06268-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tumbarello M, Viale P, Viscoli C, Trecarichi EM, Tumietto F, Marchese A, Spanu T, Ambretti S, Ginocchio F, Cristini F, Losito AR, Tedeschi S, Cauda R, Bassetti M. 2012. Predictors of mortality in bloodstream infections caused by Klebsiella pneumoniae carbapenemase-producing K. pneumoniae: importance of combination therapy. Clin Infect Dis 55:943–950. doi: 10.1093/cid/cis588. [DOI] [PubMed] [Google Scholar]
- 53.Daikos GL, Tsaousi S, Tzouvelekis LS, Anyfantis I, Psichogiou M, Argyropoulou A, Stefanou I, Sypsa V, Miriagou V, Nepka M, Georgiadou S, Markogiannakis A, Goukos D, Skoutelis A. 2014. Carbapenemase-producing Klebsiella pneumoniae bloodstream infections: lowering mortality by antibiotic combination schemes and the role of carbapenems. Antimicrob Agents Chemother 58:2322–2328. doi: 10.1128/AAC.02166-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gutiérrez-Gutiérrez B, Salamanca E, de Cueto M, Hsueh P-R, Viale P, Paño-Pardo JR, Venditti M, Tumbarello M, Daikos G, Cantón R, Doi Y, Tuon FF, Karaiskos I, Pérez-Nadales E, Schwaber MJ, Azap ÖK, Souli M, Roilides E, Pournaras S, Akova M, Pérez F, Bermejo J, Oliver A, Almela M, Lowman W, Almirante B, Bonomo RA, Carmeli Y, Paterson DL, Pascual A, Rodríguez-Baño J. 2017. Effect of appropriate combination therapy on mortality of patients with bloodstream infections due to carbapenemase-producing Enterobacteriaceae (INCREMENT): a retrospective cohort study. Lancet Infect Dis 17:726–734. doi: 10.1016/S1473-3099(17)30228-1. [DOI] [PubMed] [Google Scholar]
- 55.Durante-Mangoni E, Signoriello G, Andini R, Mattei A, De Cristoforo M, Murino P, Bassetti M, Malacarne P, Petrosillo N, Galdieri N, Mocavero P, Corcione A, Viscoli C, Zarrilli R, Gallo C, Utili R. 2013. Colistin and rifampicin compared with colistin alone for the treatment of serious infections due to extensively drug-resistant Acinetobacter baumannii: a multicenter, randomized clinical trial. Clin Infect Dis 57:349–358. doi: 10.1093/cid/cit253. [DOI] [PubMed] [Google Scholar]
- 56.Sirijatuphat R, Thamlikitkul V. 2014. Preliminary study of colistin versus colistin plus fosfomycin for treatment of carbapenem-resistant Acinetobacter baumannii infections. Antimicrob Agents Chemother 58:5598–5601. doi: 10.1128/AAC.02435-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Schwameis R, Erdogan-Yildirim Z, Manafi M, Zeitlinger MA, Strommer S, Sauermann R. 2013. Effect of pulmonary surfactant on antimicrobial activity in vitro. Antimicrob Agents Chemother 57:5151–5154. doi: 10.1128/AAC.00778-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kalil AC, Metersky ML, Klompas M, Muscedere J, Sweeney DA, Palmer LB, Napolitano LM, O’Grady NP, Bartlett JG, Carratalà J, El Solh AA, Ewig S, Fey PD, File TM, Restrepo MI, Roberts JA, Waterer GW, Cruse P, Knight SL, Brozek JL. 2016. Management of adults with hospital-acquired and ventilator-associated pneumonia: 2016 clinical practice guidelines by the Infectious Diseases Society of America and the American Thoracic Society. Clin Infect Dis 63:e61–e111. doi: 10.1093/cid/ciw353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Nation RL, Velkov T, Li J. 2014. Colistin and polymyxin B: peas in a pod, or chalk and cheese? Clin Infect Dis 59:88–94. doi: 10.1093/cid/ciu213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Vardakas KZ, Falagas ME. 2017. Colistin versus polymyxin B for the treatment of patients with multidrug-resistant Gram-negative infections: a systematic review and meta-analysis. Int J Antimicrob Agents 49:233–238. doi: 10.1016/j.ijantimicag.2016.07.023. [DOI] [PubMed] [Google Scholar]
- 61.Sandri AM, Landersdorfer CB, Jacob J, Boniatti MM, Dalarosa MG, Falci DR, Behle TF, Bordinhao RC, Wang J, Forrest A, Nation RL, Li J, Zavascki AP. 2013. Population pharmacokinetics of intravenous polymyxin B in critically ill patients: implications for selection of dosage regimens. Clin Infect Dis 57:524–531. doi: 10.1093/cid/cit334. [DOI] [PubMed] [Google Scholar]
- 62.Kulengowski B, Ribes JA, Burgess DS. 2019. Polymyxin B Etest compared with gold-standard broth microdilution in carbapenem-resistant Enterobacteriaceae exhibiting a wide range of polymyxin B MICs. Clin Microbiol Infect 25:92–95. doi: 10.1016/j.cmi.2018.04.008. [DOI] [PubMed] [Google Scholar]
- 63.CLSI. 2018. M07Ed11. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically; approved standard, 11th ed Clinical and Laboratory Standards Institute, Wayne, PA. [Google Scholar]
- 64.Tsuji BT, Pogue JM, Zavascki AP, Paul M, Daikos GL, Forrest A, Giacobbe DR, Viscoli C, Giamarellou H, Karaiskos I, Kaye D, Mouton JW, Tam VH, Thamlikitkul V, Wunderink RG, Li J, Nation RL, Kaye KS. 2019. International consensus guidelines for the optimal use of the polymyxins: endorsed by the American College of Clinical Pharmacy (ACCP), European Society of Clinical Microbiology and Infectious Diseases (ESCMID), Infectious Diseases Society of America (IDSA), International Society for Anti-infective Pharmacology (ISAP), Society of Critical Care Medicine (SCCM), and Society of Infectious Diseases Pharmacists (SIDP). Pharmacotherapy 39:10–39. doi: 10.1002/phar.2209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Mo Y, Lorenzo M, Farghaly S, Kaur K, Housman ST. 2019. What’s new in the treatment of multidrug-resistant gram-negative infections? Diagn Microbiol Infect Dis 93:171–181. doi: 10.1016/j.diagmicrobio.2018.08.007. [DOI] [PubMed] [Google Scholar]
- 66.Senchyna F, Gaur RL, Sandlund J, Truong C, Tremintin G, Kultz D, Gomez CA, Tamburini FB, Andermann T, Bhatt A, Tickler I, Watz N, Budvytiene I, Shi G, Tenover FC, Banaei N. 2019. Diversity of resistance mechanisms in carbapenem-resistant Enterobacteriaceae at a health care system in Northern California, from 2013 to 2016. Diagn Microbiol Infect Dis 93:250–257. doi: 10.1016/j.diagmicrobio.2018.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Pogue JM, Bonomo RA, Kaye KS. 2019. Ceftazidime/avibactam, meropenem/vaborbactam, or both? Clinical and formulary considerations. Clin Infect Dis 68:519–524. doi: 10.1093/cid/ciy576. [DOI] [PubMed] [Google Scholar]
- 68.Shields RK, Nguyen MH, Chen L, Press EG, Potoski BA, Marini RV, Doi Y, Kreiswirth BN, Clancy CJ. 2017. Ceftazidime-avibactam is superior to other treatment regimens against carbapenem-resistant Klebsiella pneumoniae bacteremia. Antimicrob Agents Chemother 61:e00883-17. doi: 10.1128/AAC.00883-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.van Duin D, Lok JJ, Earley M, Cober E, Richter SS, Perez F, Salata RA, Kalayjian RC, Watkins RR, Doi Y, Kaye KS, Fowler VG, Paterson DL, Bonomo RA, Evans S, Antibacterial Resistance Leadership Group. 2018. Colistin versus ceftazidime-avibactam in the treatment of infections due to carbapenem-resistant Enterobacteriaceae. Clin Infect Dis 66:163–171. doi: 10.1093/cid/cix783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Tumbarello M, Trecarichi EM, Corona A, De Rosa FG, Bassetti M, Mussini C, Menichetti F, Viscoli C, Campoli C, Venditti M, De Gasperi A, Mularoni A, Tascini C, Parruti G, Pallotto C, Sica S, Concia E, Cultrera R, De Pascale G, Capone A, Antinori S, Corcione S, Righi E, Losito AR, Digaetano M, Amadori F, Giacobbe DR, Ceccarelli G, Mazza E, Raffaelli F, Spanu T, Cauda R, Viale P. 2019. Efficacy of ceftazidime-avibactam salvage therapy in patients with infections caused by Klebsiella pneumoniae carbapenemase-producing K. pneumoniae. Clin Infect Dis 68:355–364. doi: 10.1093/cid/ciy492. [DOI] [PubMed] [Google Scholar]
- 71.Wunderink RG, Giamarellos-Bourboulis EJ, Rahav G, Mathers AJ, Bassetti M, Vazquez J, Cornely OA, Solomkin J, Bhowmick T, Bishara J, Daikos GL, Felton T, Furst MJL, Kwak EJ, Menichetti F, Oren I, Alexander EL, Griffith D, Lomovskaya O, Loutit J, Zhang S, Dudley MN, Kaye KS. 2018. Effect and safety of meropenem-vaborbactam versus best-available therapy in patients with carbapenem-resistant Enterobacteriaceae infections: the TANGO II randomized clinical trial. Infect Dis Ther 7:439–455. doi: 10.1007/s40121-018-0214-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Connolly L, Jubb A, O’Keeffe B, Serio A, Smith A, Gall J, Krause K, McKinnell JA, Zakynthinos E, Riddle V, Daikos G. 2017. Plazomicin is associated with improved survival and safety compared with colistin in the treatment of serious infections due to carbapenem-resistant Enterobacteriaceae (CRE) infections: results of the CARE study. Microbe, 1 to 5 June 2017, New Orleans, LA.
- 73.Pogue JM, Kaye KS, Veve MP, Gerlach A, Patel TS, Davis SL, Chaung E, Ray A, Puzniak L, Bonomo RA, Perez F. 2018. “Real world” treatment of multi-drug resistant (MDR) or extensively-drug resistant (XDR) P. aeruginosa infections with ceftolozane/tazobactam (C/T) versus a polymyxin or aminoglycoside (poly/AG) based regimen: a mutlicenter comparative effectiveness study, no. 2406. ID Week, 3 to 7 October 2018, San Francisco, CA.
- 74.Dickstein Y, Lellouche J, Dalak Amar MB, Schwartz D, Nutman A, Daitch V, Yahav D, Leibovici L, Skiada A, Antoniadou A, Daikos GL, Andini R, Zampino R, Durante-Mangoni E, Mouton JW, Friberg LE, Benattar YD, Bitterman R, Neuberger A, Carmeli Y, Paul M, AIDA Study Group. 2019. Treatment outcomes of colistin- and carbapenem-resistant Acinetobacter baumannii infections: an exploratory subgroup analysis of a randomized clinical trial. Clin Infect Dis 69:769–776. doi: 10.1093/cid/ciy988. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.