Maraviroc-based regimens have been explored as preexposure prophylaxis (PrEP) against human immunodeficiency virus (HIV). In this study, we utilized mucosal tissue drug exposure data, combined with target concentrations generated in vitro, in a pharmacokinetic-pharmacodynamic analysis to predict the effects of drug combinations and adherence on PrEP efficacy. Mucosal tissue concentrations of maraviroc were measured in 24 healthy women.
KEYWORDS: HIV, antiretroviral, translational medicine, dose response, population pharmacokinetics-pharmacodynamics, quantitative pharmacology, preexposure prophylaxis, maraviroc, tenofovir, emtricitabine
ABSTRACT
Maraviroc-based regimens have been explored as preexposure prophylaxis (PrEP) against human immunodeficiency virus (HIV). In this study, we utilized mucosal tissue drug exposure data, combined with target concentrations generated in vitro, in a pharmacokinetic-pharmacodynamic analysis to predict the effects of drug combinations and adherence on PrEP efficacy. Mucosal tissue concentrations of maraviroc were measured in 24 healthy women. The 90% effective concentrations (EC90) of maraviroc (alone and combined with tenofovir and emtricitabine) for protection against HIV were identified in CD4+ T cells. Monte Carlo simulations were performed to identify dosing strategies to protect colorectal and female genital tract (FGT) tissues from HIV infection. Colorectal maraviroc concentrations were 350-fold higher than in the FGT. Under steady-state conditions, our model predicted that one 300-mg dose/week was sufficient to protect colorectal tissue from HIV in 99% of the population, while 300 mg daily would protect the FGT in only 63% of the population. FGT protection increased to >90% when maraviroc was used in combination with tenofovir (5 doses/week) or emtricitabine (3 doses/week). Poor adherence resulted in a drastic decrease in efficacy in the FGT but not colorectal tissue. However, greater forgiveness was seen when maraviroc was combined with tenofovir or emtricitabine, suggesting that maraviroc should not be used alone as PrEP.
INTRODUCTION
Truvada, a fixed-dose combination of 300 mg of tenofovir disoproxil fumarate (TDF) and 200 mg of emtricitabine (FTC), was approved in 2012 by the U.S. Food and Drug Administration (FDA) for use as a preexposure prophylaxis (PrEP) regimen against human immunodeficiency virus (HIV) (1). However, due to toxicity concerns surrounding the long-term use of TDF (2, 3), other antiretrovirals, such as maraviroc (MVC), raltegravir (RAL), and dapivirine (DPV), are also being explored for use as PrEP (4, 5). MVC was approved in 2007 by the FDA for the treatment of HIV (6). It is a C-chemokine receptor type 5 (CCR5) receptor antagonist that prevents coreceptor binding of HIV peptides and subsequently blocks the entry of HIV into target cells. MVC shows rapid, but variable, absorption (maximal plasma concentration from 0.5 to 4 h postdose) and is 76% bound to plasma proteins. It is a substrate for cytochrome P450 3A4 (CYP3A4) and P-glycoprotein (P-gp) and is primarily eliminated in the feces. MVC achieves higher concentrations in vulnerable tissues where HIV exposure occurs than in blood plasma, with 2-fold-higher concentrations in vaginal tissue (7) and 27-fold-higher concentrations in the rectal tissue (8) after multiple dosing orally. Furthermore, MVC exhibits good potency in peripheral blood mononuclear cells (PBMCs; PBMC concentration which leads to 90% of maximal inhibition response [IC90] from multiple [pooled] donors ranges from 1.2 to 2.6 nM against HIV type 1 [HIV-1] Ba-L) (9). Finally, MVC is generally safe and well tolerated for a long duration of use (>5 years) in treatment-experienced patients (10). However, despite favorable pharmacokinetic (PK) attributes and demonstrated in vitro potency, MVC has shown mixed results as PrEP. In a humanized mouse model for PrEP, oral MVC was 100% effective in preventing infection by intravaginal challenge (11). Similarly, in a rhesus macaque model, MVC vaginal microbicide gel showed dose- and time-dependent protection against vaginal simian-human immunodeficiency virus (SHIV) challenge, with six of seven macaques protected at the highest (6 mM) dose (12). However, rectal SHIV challenge in six rhesus macaques (13) dosed with MVC orally 24 h prior to challenge and 2 h postchallenge led to infections in five animals. Two phase IV trials reported limited ex vivo potency of a single dose of oral maraviroc in vaginal and colorectal tissue (14) and in colorectal tissue only (15). Furthermore, in HPTN069, a phase II clinical trial designed to evaluate the safety and tolerability of MVC alone or in combination with TDF or FTC as PrEP in men who have sex with men (MSM) and in high-risk women, five seroconversions were reported in the MSM cohort: four in the MVC-only arm and one in the MVC-TDF arm (16). This may have been related to issues with low medication adherence due to the finding of low or variable MVC concentrations in their plasma. Based on these data, a combination of MVC with other antiretrovirals (ARVs) may be a more suitable option for use as PrEP.
In this work, we developed a population PK model to characterize the disposition of MVC in plasma, colorectal tissue, and lower female genital tract (FGT) tissue. The model was combined with 90% effective concentration (EC90) targets against HIV derived from human CD4+ T cell culture systems to explore the effect of adherence on overall PrEP efficacy for MVC monotherapy. MVC exposure targets were combined with efficacy targets previously published for TFV and FTC alone (17) to generate synergy model-derived efficacy targets to explore the potential prophylactic efficacy of MVC combined with either TDF or FTC.
(Versions of this work were presented at the American Society of Clinical Pharmacology and Therapeutics Meeting, Orlando, FL, March 2018.)
RESULTS
Demographics of the clinical study.
Between April 2012 and August 2013, 25 healthy women were enrolled in the clinical trial. In the 300-mg cohort, one participant was not able to provide samples and was withdrawn from the analysis, leaving a total of 24 evaluable participants. Each dosing cohort included eight participants.
PK analysis and population pharmacokinetic model.
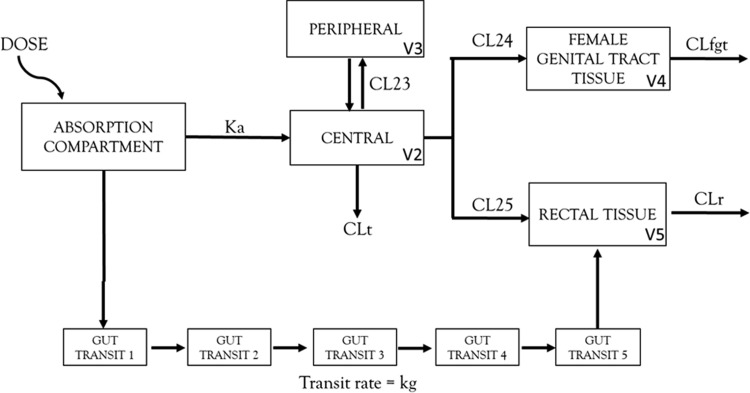
The population PK model was developed with a total of 264 plasma concentrations, 24 tissue concentrations in the female genital tract, and 24 tissue concentrations in the rectal tissue. The vaginal tissue and cervical tissue concentrations were not significantly different from each other (P = 0.07) and were thus averaged into one FGT tissue concentration per subject in order to simplify the model (see Fig. S1 in the supplemental material). At all doses, MCV concentrations were 100 times higher in the rectal tissue than in the FGT tissue. Concentrations also continued to increase at 48 h in the rectal tissue. A linear 10-compartment model best fit the clinical trial data (Fig. 1). Despite a 2-peak phenomenon shown in plasma with the 150-mg and 300-mg concentrations, models implementing complex absorption profiles were unstable, and a simple linear absorption profile was ultimately selected. Five transit compartments were used to describe the increasing rectal tissue concentrations past 24 h. The volume of distribution in both the tissue compartments was fixed to physiologically relevant volumes (100 ml for the FGT tissue and 170 ml for the rectal tissue) (Y. Fedoriw, personal communication, May 2014). Steady-state concentrations were predicted to occur after a single dose in the FGT tissue and after 10 doses in the rectal tissue based on the estimated half-life in the tissue. The PK model parameters are listed in Table S1, and goodness-of-fit plots are shown in Fig. S2. Exploratory analysis was performed to evaluate covariates, but because of the lack of significant relationships and the limited data, covariate modeling was not performed.
FIG 1.
Structure of the population pharmacokinetic model for maraviroc to describe the disposition in the plasma, female genital tract tissue, and rectal tissue. A 10-compartment population PK model was fitted to the data. Maraviroc distribution in plasma was described by a simple 2-compartment model with linear clearance. Distribution into rectal tissue was described by the use of 5 transit compartments with similar transit rates in order to account for delayed second peak in rectal tissue concentration. Volume of distribution in the female genital tract and rectal tissue was fixed to physiologically relevant values, and the clearance out of the two compartments was estimated. Ka, absorption constant; V2, central volume; V3, peripheral volume; V4, volume of female genital tract tissue; V5, volume of rectal tissue; CLt, total clearance; CL23, distributional clearance in plasma; CL24, distributional clearance from plasma to female genital tract; CL25, distributional clearance from plasma to rectal tissue; CLfgt, clearance out of female genital tract tissue; CLr, clearance out of rectal tissue.
Concentration-response relationships in CD4+ T cells.
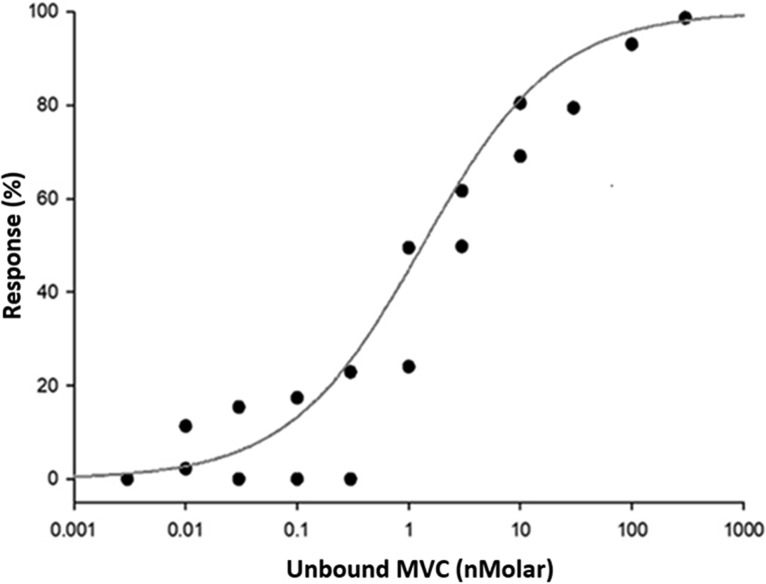
Unbound MVC targets for protective efficacy against HIV-1 pseudovirus were investigated, and the best fit for the exposure-response maximum-effect (Emax) model is shown in Fig. 2. The EC50 of protein-unbound MVC in CD4+ T cells was 0.68 ng/ml, and the Hill coefficient was 0.73. The EC90 protein-unbound target for inhibition of viral infection was 14.02 ng/ml.
FIG 2.
Concentration-response analysis and Emax model for maraviroc in CD4+ T cells. The EC50 of protein-unbound MVC in CD4+ T cells was 0.68 ng/ml, and the Hill coefficient was 0.73. The EC90 protein-unbound target for inhibition of viral infection was 14.02 ng/ml.
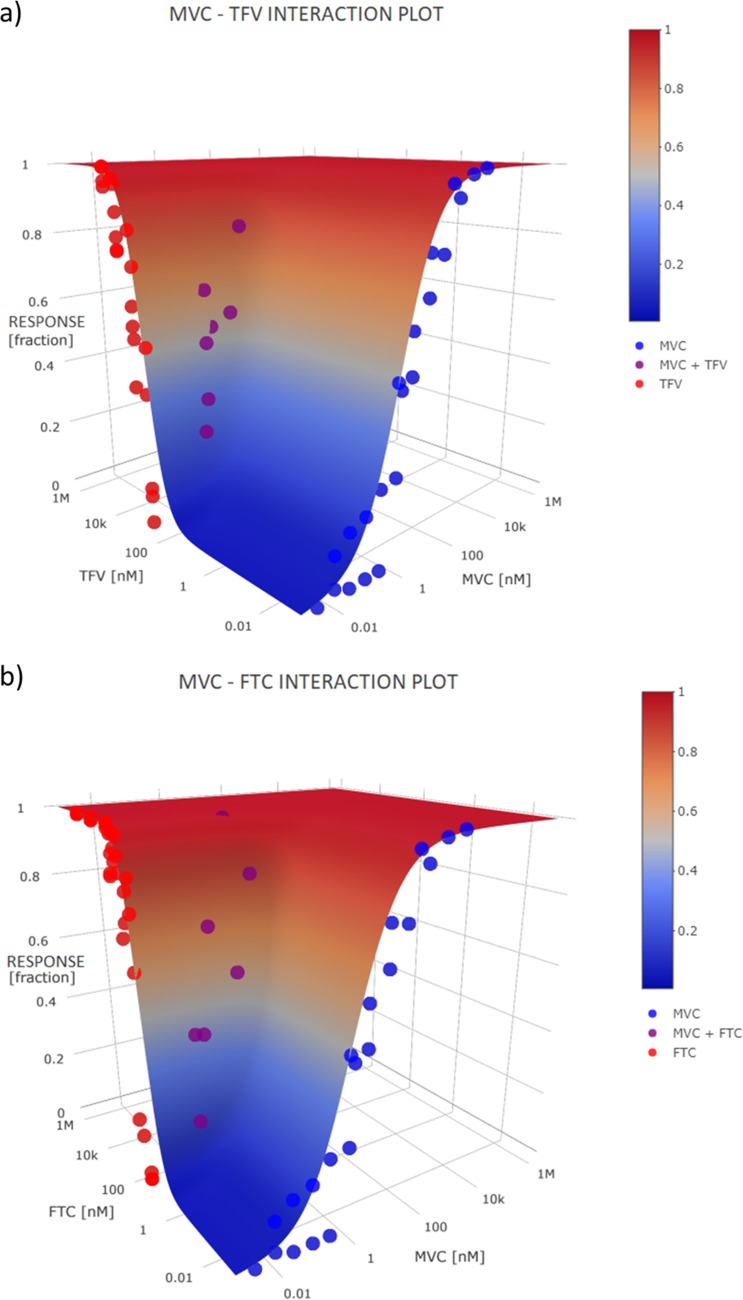
The noncompetitive-interaction model demonstrated a synergistic effect (Ψ < 1) for both combinations, MVC and TFV (Ψ = 0.51) (Fig. 3a) and MVC and FTC (Ψ = 0.48) (Fig. 3b). A 3-dimensional surface plot showed that the combinations fit the surface of the model predictions without bias.
FIG 3.
Three-dimensional surface plots showing the interaction between maraviroc (MVC) and tenofovir (TFV) (a) and MVC and emtricitabine (FTC) (b). The combinations fit the surface of the model predictions well, without any bias.
Efficacy simulations.
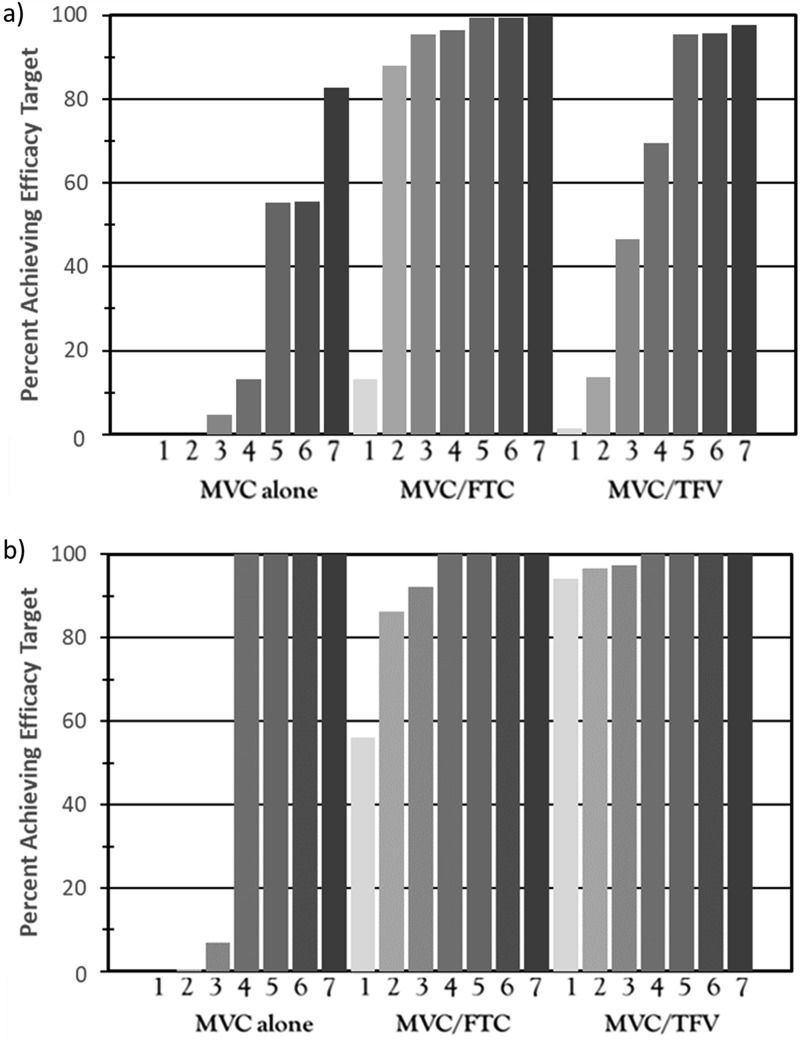
The results of the pharmacokinetic-pharmacodynamic (PK-PD) simulations are shown for the FGT tissue and rectal tissue across one to seven doses per week for MVC alone and for the two-drug combinations in Fig. 4. In the FGT, with MVC alone, 63% of the virtual subjects with 100% adherence achieved the target concentration needed for HIV-1 protection. With declining adherence (from six doses/week to one dose/week), the percentage of the population attaining the target concentration declined rapidly (Fig. 4a). The combination of MVC with FTC demonstrated >90% protection with at least 30% adherence. The combination of MVC with TFV demonstrated >90% protection with at least 85% adherence. In rectal tissue, however, administration of four doses/week of MVC alone was associated with >99% protection (Fig. 4b).
FIG 4.
Simulations for protection in female genital tract (FGT) tissue (a) and rectal tissue (b) based on patient adherence (given by number of doses per week). Maraviroc was predicted to show increased efficacy combined with emtricitabine in the FGT (>90% protection at two doses/week) and combined with tenofovir in the rectal tissue (>90% protection with one dose/week).
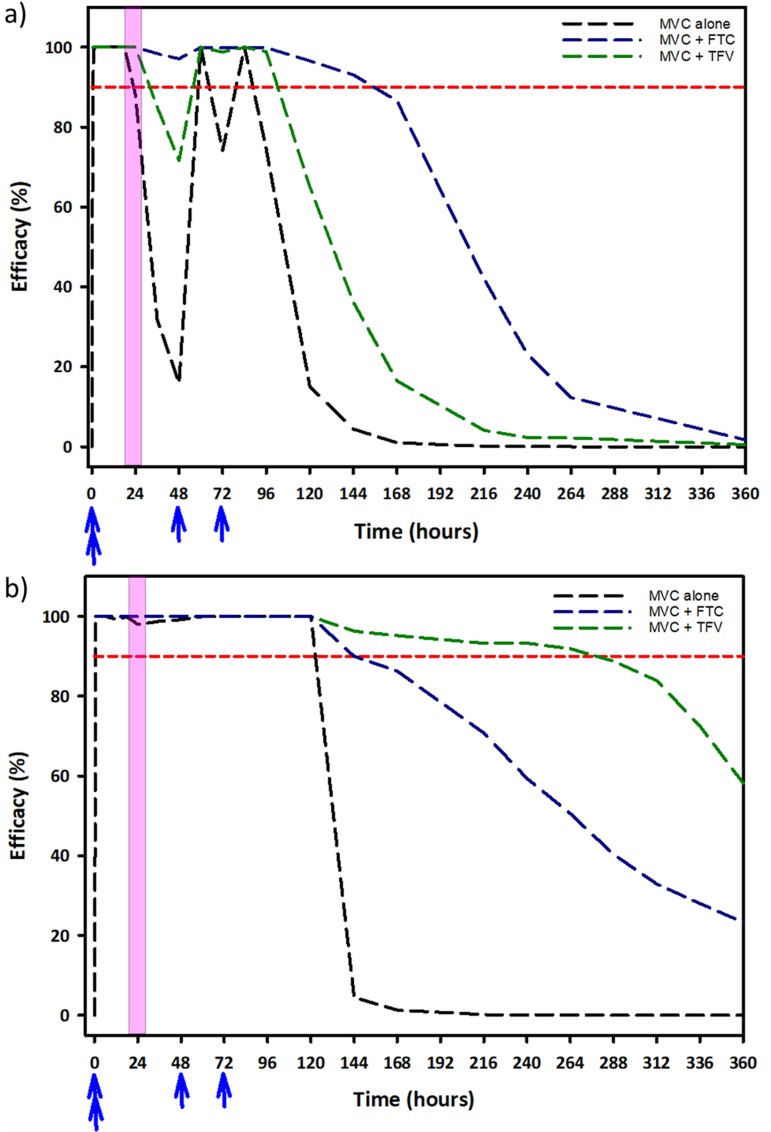
In order to predict the pericoital efficacy of MVC-based regimens, we simulated the concentration-time profile of MVC in vulnerable tissues (Fig. 5) after an on-demand Ipergay dosing regimen (two doses 2 to 24 h before coitus, one dose 24 h after coitus, and one dose 48 h after coitus) and up to 14 days postcoitus. When MVC was used alone, protection was achieved in 88% and 98% of the virtual population in FGT tissue (Fig. 5a) and rectal tissue (Fig. 5b), respectively. Protection dropped off rapidly in the FGT tissue, and only 15% of the virtual subjects were predicted to be protected by the 4th day postcoitus without additional doses of MVC. The combination of TFV and MVC in the FGT tissue led to an increase in efficacy predictions, with 65% of virtual subjects protected by the 4th day postcoitus. In comparison, with the combination of MVC and FTC, it was predicted that >96% of the virtual population would be protected by the 4th day postcoitus. In the rectal tissue, the use of MVC alone was predicted to protect 100% of the virtual population up to the 4th day postcoitus, followed by a sharp decline in efficacy. For the combination of TFV and MVC, it was predicted that >90% of the virtual population would be protected in rectal tissue up to 10 days postcoitus, while the combination of FTC and MVC was predicted to have only a marginal increase in efficacy compared to that of MVC alone (>90% of the virtual population protected up to the 5th day postcoitus).
FIG 5.
Simulations for protection by the IPERGAY strategy in female genital tract tissue (a) and rectal tract tissue (b). The blue arrows indicate time of dose of preexposure prophylaxis regimen (either maraviroc monotherapy or maraviroc in combination with tenofovir or emtricitabine), and the pink-shaded region represents exposure incident to HIV. The dashed red line represents the 90% efficacy threshold for protection against HIV infection.
DISCUSSION
While PrEP has shown efficacy in preventing HIV in high-risk populations (18), long-term use of TDF-based prevention strategies has been associated with renal nephropathy (2) and a reduction in bone mineral density (3) in 15% of PrEP users, highlighting the need for other approaches to prevent HIV. Furthermore, large HIV PrEP trials (19–22) have shown various efficacies in MSM and women. Using a similar model-based approach (17), we previously estimated that women would need at least 6 doses of Truvada per week to achieve protective concentrations in the FGT, while MSM would need more than two doses per week to reach adequate concentrations for protection in the rectal tissue. Therefore, while efficacy in HIV prevention trials has been linked with high adherence in women (23), adherence to oral TDF-based PrEP has generally been approximately 30% (20).
Results from the phase II HPTN069 study in women highlight the ongoing challenge of low adherence in HIV prevention trials. Despite a favorable reduction in side effects with MVC-based PrEP regimens, only 60% of women were adherent to daily doses of the study drug (24) (in the MSM group of HPTN069, adherence was 77%) (16). However, our analysis shows that less than 20% of the population would be protected by MVC monotherapy in the FGT with a similar adherence rate. Our model suggests that the combination of MVC and TFV may be slightly more forgiving of low adherence (∼40% protection at three doses/week), but MVC combined with FTC is the most forgiving of poor adherence (∼90% protection at two doses/week) in the FGT. This is consistent with results from our previous pharmacometric modeling analysis showing that FTC preferentially accumulates in the FGT compared to TFV (17). In the rectal tissue, our model predicted >90% protection with at least four doses/week of MVC, with protection quickly dropping off with poorer adherence. MVC in combination with either TFV or FTC was more forgiving of poorer adherence (90% protection achieved with 1 dose/week or 3 doses/week, respectively). These data suggest that MVC in combination with either TFV or FTC would show enhanced effectiveness as PrEP compared to that of the monotherapy regimen. Our conclusions agree with those of McGowan et al. (25), who showed that MVC alone showed limited viral suppression in an ex vivo rectal biopsy system compared to MVC in combination with either TFV or FTC.
While modeling and simulation analyses do not predict an individual’s risk of becoming infected, they may be useful to inform overall forgiveness of PrEP regimens in clinical trials or populations. The modeling framework that was developed in this study could be used to test other ARV drug combinations used in PrEP as well as different dosing regimens or strategies. Such models could also be used to explain biological differences in drug concentration or efficacy at the target site. For example, our group has previously identified pharmacological limitations that may explain the diverging results of TDF-based HIV prevention trials in MSM versus women, despite similar adherence patterns, and outlined estimated minimum adherence requirements for efficacy (17). Strict adherence in women (six or seven doses a week) but not men (two or three doses a week) resulted in at least 90% of the population achieving the efficacy target for protection.
In this work, we used a similar approach to look at MVC-based strategies for HIV prevention. The Emax parameters we report here for MVC in protecting CD4+ T cells from HIV infection, accounting for protein binding, have not been previously reported. Using an interaction model, we demonstrated that MVC showed synergistic antiviral activity in combination with both TDF and FTC. Work previously published by Dorr et al. showed that the antiviral activity of MVC in vitro was additive with TFV and mildly synergistic with certain protease inhibitors (9). In our model, we saw similar trends, with the combination of TFV and MVC being less synergistic (higher psi value) than the combination of FTC and MVC. In our simulation of 1,000 subjects, at least three doses of MVC-FTC per week were required for 90% of virtual subjects to attain the target concentration in FGT and rectal mucosal tissues. Given that FTC achieves high concentration in the FGT, the combination may be especially effective as a PrEP strategy in women.
Finally, we evaluated an Ipergay dosing strategy with MVC alone and in combination with FTC and TFV. MVC alone was predicted to protect greater than 90% of the population up to 4 to 5 days postcoitus in the FGT tissue and rectal tissue. While MVC in combination with TFV and FTC prolonged the duration of protection, the magnitude of this effect was dependent on the tissue type, as shown previously (17). Our model predicted that in the FGT, the combination of MVC and FTC would protect >90% of the virtual population up to a week postcoitus. The duration of protection of this combination in the FGT was predicted to be longer than for the combination of TDF and FTC (17), likely due to increased accumulation of MVC in the FGT compared to that of TDF. However, in the rectal tissue, the combination of MVC and FTC showed little additional benefit compared to MVC alone (>90% of virtual population would be protected up to 6 days postcoitus). Similarly, the combination of MVC-TFV was predicted to be highly effective in the rectal tissue, protecting greater than 90% of the virtual population for up to 11 days postcoitus, but was predicted to be only marginally effective in the FGT (>90% of virtual population protected for about 4 days postcoitus). Therefore, while MVC in combination could be an attractive option as an on-demand dosing strategy, different combinations may have to be utilized for men and women to be effective for longer durations, particularly since it is still not known how long the drug would need to be in the tissue in order to be completely protective.
This work has some important limitations. The model for MVC was not able to accurately describe the plasma absorption phase of the PK curve, due to high variability in the data at the lowest dose. However, using data from 24 subjects in this analysis did not support modeling complex absorption profiles to explain multiple peaks in plasma or to capture some of the highest concentrations, and this is shown in the final model diagnostics (Fig. S2b and c). Previously published models (26) have reported similar difficulties in modeling the absorption phase of MVC in plasma. Additionally, the 48-h sampling period did not allow enough time to capture the eventual decline in MVC concentrations in rectal tissue. Having this information would have better informed time to steady state. The tissue concentrations were highly variable, and as a result, the model was not able to adequately capture tissue concentrations at the 12-h postdose time point (particularly in the FGT). Finally, while all tissue concentrations were quantifiable, we used the M5 estimation method for our plasma concentrations below the lower limit of quantification (LLOQ) rather than likelihood estimation methods, and this could have introduced some bias into the model estimates that impact our plasma simulations (27).
In conclusion, PK-PD modeling was used to predict the tissue concentration profile of MVC alone and in combination with standard ARVs to predict efficacy in vulnerable populations with various levels of adherence. From our simulations, adherence significantly affected MVC’s efficacy in the FGT. Therefore, if MCV is to be used for PrEP, combining it with FTC might be an effective option in both men and women.
MATERIALS AND METHODS
Clinical trial.
A phase I dose-ranging PK study was conducted in healthy women (ClinicalTrials.gov identifier NCT01330199). Each woman was given a single dose of 150 mg, 300 mg, or 600 mg of MVC, which corresponded to 50%, 100%, or 200% of the clinical dose for HIV treatment. Intensive sampling was used for collection of plasma specimens over a 48-h period, including predose (0 h) and the following time points postdose: 0.5, 1, 2, 3, 4, 6, 9, 12, 18, 24, 36, and 48 h. In the case of tissue sampling, each woman contributed one rectal tissue biopsy specimen at a certain time point and one vaginal tissue and cervical tissue biopsy specimen at a distinct time point. Tissue biopsy specimens were collected at either 6, 12, 24, or 48 h. The clinical trial was conducted in accordance with good clinical practice and written consent was obtained from all participants before enrollment into the study. Detailed methodology about the clinical trial and sample collection methods have been published previously (17).
Pharmacokinetic sample analysis.
MVC was measured in the plasma, lower FGT tissue, and colorectal tissue by a validated liquid chromatography-tandem mass spectrometry (LC-MS/MS) assay which has been described previously (8). In the plasma, the dynamic range of the assay was 5 to 5,000 ng/ml, and accuracy and precision were within 15%. In the tissue, the dynamic range of the assay was 0.02 to 20 ng/ml of tissue homogenate. The precision and accuracy of this assay were within 15% (20% at the lower limit of quantification).
Development of the population pharmacokinetic model.
Nonlinear mixed-effects modeling (NONMEM version 7.4; ICON plc) was used to fit a population PK model to the MVC clinical trial concentration data. MVC concentrations in the plasma, female genital tract tissue, and rectal tissue were log transformed and a log additive error model was used to model residual variability. Samples that were below the limit of quantification (BLQ) were handled by the Beal M5 method (28). The model’s predictive performance was evaluated with standard goodness-of-fit plots and by evaluating the plausibility of parameter estimates. One thousand Monte Carlo simulations were performed at each dose using model estimates from the final model, and the model fit was assessed by using visual predictive checks (VPC), stratified by tissue type.
Concentration-response relationships in CD4+ T cells.
PBMCs were isolated from buffy coats obtained from 2 donors from the New York Blood Center (Long Island City, NY) and sorted for CD4+ T lymphocytes, using an EasySep negative-selection kit (Stemcell Technologies, Vancouver, Canada). CD4+ T cells were stimulated for 48 h in RPMI medium supplemented with 10% fetal bovine serum (FBS), antibiotics, phytohemagglutinin (PHA; 5 μg/ml), and interleukin 2 (10 U/ml). A total of 1 × 106 resuspended CD4+ T cells/ml were incubated in PHA-free RPMI medium after stimulation along with MVC (0.003 to 1,000 nM) either alone or in combination with TFV (0.3 to 10 μM) or FTC (0.03 to 30 μM) for 24 h. All drugs were obtained from the NIH AIDS Reagent Program. Pseudotyped virus was generated using NL4-3.Luc.R-.E backbone (NIH AIDS Reagent Program) (29, 30) and transfected with env expression plasmid from HIV-1JR-CSF, using X-tremeGENE HP (Roche Life Science, Indianapolis, IN). Virions were concentrated 10-fold using a Lenti-X concentrator (Clontech Laboratories, Mountain View, CA). Cells were incubated for 48 h in 25 μl of pseudotyped virus (multiplicity of infection [MOI] of 1) and then lysed with 100 μl of GloLysis buffer. A luciferase assay system kit (Promega) was used to measure relative light units on a Veritas microplate luminometer (Turner Biosystems, Sunnyvale, CA). Infectivity was normalized to infection in the absence of drug and reported as percent inhibition.
Protein binding of maraviroc in macaque mucosal tissue.
In order to determine the free fraction of MVC in the mucosal tissues, the protein binding of MVC was determined by rapid equilibrium dialysis in the lower FGT and colorectal tissue of six male and three female adult rhesus macaques. Details of this animal study have been described previously (31). In brief, six male and three female adult rhesus macaques were dosed to steady-state with TFV, FTC, MVC, and RAL. At day 10, the macaques were euthanized by a barbiturate overdose, and tissue reservoirs were collected at necropsy. The vaginal and colorectal tissue sections were snap-frozen in dry ice and placed in aluminum foil. A section of the tissue was reserved to determine the protein binding; the full details are described in the supplemental material.
Statistical analysis.
Drug exposure was plotted against percent inhibition of HIV-1 pseudovirus in the in vitro CD4+ T cell system and fit to a sigmoidal Emax model (SAS version 9.3) to calculate an EC90 target using equation 1. E0 is the response at zero concentration (fixed to 0), Emax is the maximal response (fixed to 100), [Cu] is the unbound concentration of maraviroc, β is the Hill slope, and E is the response. Previously published reports have shown that the protein binding of MVC in a cellular system containing 10% FBS is 46%. This protein correction factor was applied to our in vitro system to give a measure of unbound MVC concentration in the CD4+ T cell culture systems. The EC50 target for MVC was combined with previously determined EC50 targets for TDF and FTC (17) by using a noncompetitive interaction model (32) to determine if there was a synergistic interaction between MVC and TFV (equation 2) or MVC and FTC (equation 3). For the interaction models, the EC50 values and Hill slopes of MVC, TDF, and FTC were fixed and Ψ was the interaction term. A 3-dimensional goodness-of-fit plot was generated and visually inspected in order to check for bias in the model predictions.
| (1) |
| (2) |
| (3) |
Efficacy simulations.
Monte Carlo simulations were performed using the final population PK model at various levels of medication adherence to simulate 1,000 virtual subjects administered a 300-mg dose of MVC for PrEP under steady-state conditions. Adherence ranged from daily administration of MVC (seven doses per week) to once-weekly administration of MVC (one dose per week) and was modeled by using different values of interdose interval (II) in the NONMEM data set. For example, perfect adherence was modeled with an II of 24, while an adherence of six doses a week was modeled with an II of 28, and so on. The exposure in rectal tissue were further adjusted for unabsorbed MVC passing through the gut (50.5%) (33), and the exposures in the FGT and rectal tissue were adjusted for the measured tissue protein binding fraction. Under steady-state conditions, the concentrations in tissue at the end of the dosing interval were compared to efficacy targets either for MVC alone (EC90 target from Emax model) or for MVC in combination with TFV or FTV (synergy model). Protection was defined as a simulated tissue concentration at the end of the dosing interval that was above the efficacy target defined in the CD4+ T cells. The extent of protection in the virtual subjects was expressed as a percentage of the total simulated population.
Supplementary Material
ACKNOWLEDGMENTS
We sincerely thank John Schmitz of the UNC Center for AIDS Research Virology, Immunology and Microbiology Core, for his work isolating CD4+ lymphocytes from peripheral blood mononuclear cells. The following reagents were obtained through the NIH AIDS Reagent Program, Division of AIDS, NIAID, NIH: TZM-bl from John C. Kappes, Xiaoyun Wu, and Tranzyme Inc., HIV-1JR-CSF from Irvin Chen, and pNL4-3.Luc.R–.E– from Nathaniel Landau.
Angela D. M. Kashuba and her laboratory are part of the study teams for CAPRISA 004 and 008, FACTS 001, MTN 006, HPTN 066, FEM-PrEP, and CONRAD 113, 114, and 117. Grant funding from Gilead Sciences Inc. has been received by UNC on behalf of Angela D. M. Kashuba. No other authors have commercial interests that might pose a conflict of interest.
This work was supported by the National Institute of Allergy and Infectious Diseases (grant number U01 AU095031), the Centers for AIDS Research (grant number CFAR P30 AI50410), and the National Institute of General Medical Sciences (grant number T32 GM086330). N.S. was supported by the Royster Society of Fellows, University of North Carolina at Chapel Hill.
The content is solely the responsibility of the authors and does not necessarily represent the official views of the supporting agencies listed above.
Footnotes
Supplemental material is available online only.
REFERENCES
- 1.Gilead Sciences Inc. 2012. Truvada full prescribing information. Gilead Sciences Inc, Foster City, CA. [Google Scholar]
- 2.Tang EC, Vittinghoff E, Anderson PL, Cohen SE, Doblecki-Lewis S, Bacon O, Coleman ME, Chege W, Buchbinder S, Kolber MA, Elion R, Shlipak M, Liu AY. 2018. Changes in kidney function associated with daily tenofovir disoproxil fumarate/emtricitabine for HIV preexposure prophylaxis use in the United States demonstration project. J Acquir Immune Defic Syndr 77:193–198. doi: 10.1097/QAI.0000000000001566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Havens PL, Stephensen CB, Van Loan MD, Schuster GU, Woodhouse LR, Flynn PM, Gordon CM, Pan CG, Rutledge B, Liu N, Wilson CM, Hazra R, Hosek SG, Anderson PL, Seifert SM, Kapogiannis BG, Mulligan K. 2017. Decline in bone mass with tenofovir disoproxil fumarate/emtricitabine is associated with hormonal changes in the absence of renal impairment when used by HIV-uninfected adolescent boys and young men for HIV preexposure prophylaxis. Clin Infect Dis 64:317–325. doi: 10.1093/cid/ciw765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kovarova M, Swanson MD, Sanchez RI, Baker CE, Steve J, Spagnuolo RA, Howell BJ, Hazuda DJ, Victor-Garcia J. 2016. A long-acting formulation of the integrase inhibitor raltegravir protects humanized BLT mice from repeated high-dose vaginal HIV challenges. J Antimicrob Chemother 71:1586–1596. doi: 10.1093/jac/dkw042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Herrera C, Armanasco N, García-Pérez J, Ziprin P, Olejniczak N, Alcamí J, Nuttall J, Shattock RJ. 2016. Maraviroc and reverse transcriptase inhibitors combinations as potential preexposure prophylaxis candidates. AIDS 30:1015–1025. doi: 10.1097/QAD.0000000000001043. [DOI] [PubMed] [Google Scholar]
- 6.Pfizer Inc. 2007. Selzentry full prescribing information. Pfizer Inc, New York, NY. [Google Scholar]
- 7.Dumond JB, Patterson KB, Pecha AL, Werner RE, Andrews E, Damle B, Tressler R, Worsley J, Kashuba A. 2009. Maraviroc concentrates in the cervicovaginal fluid and vaginal tissue of HIV-negative women. J Acquir Immune Defic Syndr 51:546–553. doi: 10.1097/QAI.0b013e3181ae69c5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brown KC, Patterson KB, Malone SA, Shaheen NJ, Prince HMA, Dumond JB, Spacek MB, Heidt PE, Cohen MS, Kashuba A. 2011. Single and multiple dose pharmacokinetics of maraviroc in saliva, semen, and rectal tissue of healthy HIV-negative men. J Infect Dis 203:1484–1490. doi: 10.1093/infdis/jir059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dorr P, Westby M, Dobbs S, Griffin P, Irvine B, Macartney M, Mori J, Rickett G, Smith-Burchnell C, Napier C, Webster R, Armour D, Price D, Stammen B, Wood A, Perros M. 2005. Maraviroc (UK-427,857), a potent, orally bioavailable, and selective small-molecule inhibitor of chemokine receptor CCR5 with broad-spectrum anti-human immunodeficiency virus type 1 activity. Antimicrob Agents Chemother 49:4721–4732. doi: 10.1128/AAC.49.11.4721-4732.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gulick RM, Fatkenheuer G, Burnside R, Hardy WD, Nelson MR, Goodrich J, Mukwaya G, Portsmouth S, Heera JR. 2014. Five-year safety evaluation of maraviroc in HIV-1–infected treatment-experienced patients. J Acquir Immune Defic Syndr 65:78–81. doi: 10.1097/QAI.0b013e3182a7a97a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Neff PC, Ndolo T, Tandon A, Habu Y, Akkina R. 2010. Oral pre-exposure prophylaxis by anti-retrovirals raltegravir and maraviroc protects against HIV-1 vaginal transmission in a humanized mouse model. PLoS One 5:e15257. doi: 10.1371/journal.pone.0015257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Veazey R, Ketas T, Dufour J, Moroney-Rasmussen T, Green LC, Klasse PJ, Moore JP. 2010. Protection of rhesus macaques from vaginal infection by vaginally delivered Maraviroc, an inhibitor of HIV-1 entry via the CCR5 co-receptor. J Infect Dis 202:739–744. doi: 10.1086/655661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Massud I, Aung W, Martin A, Bachman S, Mitchell J, Aubert R, Solomon Tsegaye T, Kersh E, Pau C-P, Heneine W, García-Lerma JG. 2013. Lack of prophylactic efficacy of oral maraviroc in macaques despite high drug concentrations in rectal tissues. J Virol 87:8952–8961. doi: 10.1128/JVI.01204-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fox J, Tiraboschi JM, Herrera C, Else L, Egan D, Dickinson L, Jackson A, Olejniczak N, Back D, Khoo S, Shattock R, Boffito M. 2016. Brief report: pharmacokinetic/pharmacodynamic investigation of single-dose oral maraviroc in the context of HIV-1 pre-exposure prophylaxis. J Acquir Immune Defic Syndr 73:252–257. doi: 10.1097/QAI.0000000000001108. [DOI] [PubMed] [Google Scholar]
- 15.Coll J, Moltó J, Boix J, Gómez-Mora E, Else L, García E, Paredes R, Ouchi D, Carrillo A, Escrig R, Back D, Clotet B, Cabrera C. 2015. Single oral dose of maraviroc does not prevent ex-vivo HIV infection of rectal mucosa in HIV-1 negative human volunteers. AIDS 29:2149–2154. doi: 10.1097/QAD.0000000000000769. [DOI] [PubMed] [Google Scholar]
- 16.Gulick RM, Wilkin TJ, Chen YQ, Landovitz RJ, Amico KR, Young AM, Richardson P, Marzinke MA, Hendrix CW, Eshleman SH, McGowan I, Cottle LM, Andrade A, Marcus C, Klingman KL, Chege W, Rinehart AR, Rooney JF, Andrew P, Salata RA, Magnus M, Farley JE, Liu A, Frank I, Ho K, Santana J, Stekler JD, McCauley M, Mayer KH. 2017. Phase 2 study of the safety and tolerability of maraviroc-containing regimens to prevent HIV infection in men who have sex with men (HPTN 069/ACTG A5305). J Infect Dis 215:238–246. doi: 10.1093/infdis/jiw525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cottrell ML, Yang KH, Prince HMA, Sykes C, White N, Malone S, Dellon ES, Madanick RD, Shaheen NJ, Hudgens MG, Wulff J, Patterson KB, Nelson JAE, Kashuba A. 2016. A translational pharmacology approach to predicting outcomes of preexposure prophylaxis against HIV in men and women using tenofovir disoproxil fumarate with or without emtricitabine. J Infect Dis 214:55–64. doi: 10.1093/infdis/jiw077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jiang J, Yang X, Ye L, Zhou B, Ning C, Huang J, Liang B, Zhong X, Huang A, Tao R, Cao C, Chen H, Liang H. 2014. Pre-exposure prophylaxis for the prevention of HIV infection in high risk populations: a meta-analysis of randomized controlled trials. PLoS One 9:e87674. doi: 10.1371/journal.pone.0087674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Baeten JM, Donnell D, Ndase P, Mugo NR, Campbell JD, Wangisi J, Tappero JW, Bukusi EA, Cohen CR, Katabira E, Ronald A, Tumwesigye E, Were E, Fife KH, Kiarie J, Farquhar C, John-Stewart G, Kakia A, Odoyo J, Mucunguzi A, Nakku-Joloba E, Twesigye R, Ngure K, Apaka C, Tamooh H, Gabona F, Mujugira A, Panteleeff D, Thomas KK, Kidoguchi L, Krows M, Revall J, Morrison S, Haugen H, Emmanuel-Ogier M, Ondrejcek L, Coombs RW, Frenkel L, Hendrix C, Bumpus NN, Bangsberg D, Haberer JE, Stevens WS, Lingappa JR, Celum C, Partners PrEP Study Team. 2012. Antiretroviral prophylaxis for HIV-1 prevention among heterosexual men and women. N Engl J Med 367:399–410. doi: 10.1056/NEJMoa1108524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Marrazzo JM, Ramjee G, Richardson BA, Gomez K, Mgodi N, Nair G, Palanee T, Nakabiito C, van der Straten A, Noguchi L, Hendrix CW, Dai JY, Ganesh S, Mkhize B, Taljaard M, Parikh UM, Piper J, Mâsse B, Grossman C, Rooney J, Schwartz JL, Watts H, Marzinke MA, Hillier SL, McGowan IM, Chirenje ZM, VOICE Study Team. 2015. Tenofovir-based preexposure prophylaxis for HIV infection among African women. N Engl J Med 372:509–518. doi: 10.1056/NEJMoa1402269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Grant RM, Lama JR, Anderson PL, McMahan V, Liu AY, Vargas L, Goicochea P, Casapía M, Guanira-Carranza JV, Ramirez-Cardich ME, Montoya-Herrera O, Fernández T, Veloso VG, Buchbinder SP, Chariyalertsak S, Schechter M, Bekker LG, Mayer KH, Kallás EG, Amico KR, Mulligan K, Bushman LR, Hance RJ, Ganoza C, Defechereux P, Postle B, Wang F, McConnell JJ, Zheng JH, Lee J, Rooney JF, Jaffe HS, Martinez AI, Burns DN, Glidden DV, iPrEx Study Team. 2010. Preexposure chemoprophylaxis for HIV prevention in men who have sex with men. N Engl J Med 363:2587–2599. doi: 10.1056/NEJMoa1011205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Van Damme L, Corneli A, Ahmed K, Agot K, Lombaard J, Kapiga S, Malahleha M, Owino F, Manongi R, Onyango J, Temu L, Monedi MC, Mak'Oketch P, Makanda M, Reblin I, Makatu SE, Saylor L, Kiernan H, Kirkendale S, Wong C, Grant R, Kashuba A, Nanda K, Mandala J, Fransen K, Deese J, Crucitti T, Mastro TD, Taylor D, FEM-PrEP Study Group. 2012. Preexposure prophylaxis for HIV infection among African women. N Engl J Med 367:411–422. doi: 10.1056/NEJMoa1202614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hanscom B, Janes HE, Guarino PD, Huang Y, Brown ER, Chen YQ, Hammer SM, Gilbert PB, Donnell DJ. 2016. Preventing HIV-1 infection in women using oral pre-exposure prophylaxis: a meta-analysis of current evidence. J Acquir Immune Defic Syndr 73:606–608. doi: 10.1097/QAI.0000000000001160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gulick RM, Wilkin TJ, Chen YQ, Landovitz RJ, Amico KR, Young AM, Richardson P, Marzinke MA, Hendrix CW, Eshleman SH, McGowan I, Cottle LM, Andrade A, Marcus C, Klingman KL, Chege W, Rinehart AR, Rooney JF, Andrew P, Salata RA, Siegel M, Manabe YC, Frank I, Ho K, Santana J, Stekler JD, Swaminathan S, McCauley M, Hodder S, Mayer KH. 2017. Safety and tolerability of maraviroc-containing regimens to prevent HIV infection in women. Ann Intern Med 167:384–393. doi: 10.7326/M17-0520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McGowan I, Wilkin T, Landovitz RJ, Wu C, Chen Y, Marzinke MA, Hendrix CW, Richardson P, Eshleman SH, Andrade A, Chege W, Anderson PL, McCauley M, Farley J, Mayer KH, Anton P, Brand RM, Cranston RD, Gulick R. 2019. The pharmacokinetics, pharmacodynamics, and mucosal responses to maraviroc-containing pre-exposure prophylaxis regimens in MSM. AIDS 33:237–246. doi: 10.1097/QAD.0000000000002038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chan PLS, Weatherley B, McFadyen L. 2008. A population pharmacokinetic meta-analysis of maraviroc in healthy volunteers and asymptomatic HIV-infected subjects. Br J Clin Pharmacol 65(Suppl 1):6576–6585. doi: 10.1111/j.1365-2125.2008.03139.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bergstrand M, Karlsson MO. 2009. Handling data below the limit of quantification in mixed effect models. AAPS J 11:371–380. doi: 10.1208/s12248-009-9112-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Beal S. 2001. Ways to fit a PK model with some data below the quantification limit. J Pharmacokinet Pharmacodyn 28:481–504. doi: 10.1023/a:1012299115260. [DOI] [PubMed] [Google Scholar]
- 29.Jowett JBM, Planelles V, Poon B, Shah NP, Chen M-L, Chen I. 1995. The human immunodeficiency virus type 1 vpr gene arrests infected T cells in the G2 + M phase of the cell cycle. J Virol 69:6304–6313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Connor RI, Chen BK, Choe S, Landau NR. 1995. Vpr is required for efficient replication of human immunodeficiency virus type-1 in mononuclear phagocytes. Virology 206:935–944. doi: 10.1006/viro.1995.1016. [DOI] [PubMed] [Google Scholar]
- 31.Thompson CG, Fallon JK, Mathews M, Charlins P, Remling-Mulder L, Kovarova M, Adamson L, Srinivas N, Schauer A, Sykes C, Luciw P, Garcia JV, Akkina R, Smith PC, Kashuba A. 2017. Multimodal analysis of drug transporter expression in gastrointestinal tissue. AIDS 31:1669–1678. doi: 10.1097/QAD.0000000000001554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chakraborty A, Jusko WJ. 2002. Pharmacodynamic interaction of recombinant human interleukin-10 and prednisolone using in vitro whole blood lymphocyte proliferation. J Pharm Sci 91:1334–1342. doi: 10.1002/jps.3000. [DOI] [PubMed] [Google Scholar]
- 33.Weatherley B, McFadyen L. 2009. Maraviroc modelling strategy: use of early phase 1 data to support a semi-mechanistic population pharmacokinetic model. Br J Clin Pharmacol 68:355–369. doi: 10.1111/j.1365-2125.2009.03455.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.