This study investigated the in vivo efficacy of three bacteriophages combined compared with linezolid in two mouse models (nondiabetic and diabetic) of Staphylococcus aureus foot infection. In both models, a single injection of bacteriophages in the hindpaw showed significant antibacterial efficacy. Linezolid was as effective as bacteriophages in nondiabetic animals but ineffective in diabetic animals. These findings further support preclinical and clinical studies for the development of phage therapy.
KEYWORDS: Staphylococcus aureus, bacteriophages, diabetic foot infection, preclinical drug studies
ABSTRACT
This study investigated the in vivo efficacy of three bacteriophages combined compared with linezolid in two mouse models (nondiabetic and diabetic) of Staphylococcus aureus foot infection. In both models, a single injection of bacteriophages in the hindpaw showed significant antibacterial efficacy. Linezolid was as effective as bacteriophages in nondiabetic animals but ineffective in diabetic animals. These findings further support preclinical and clinical studies for the development of phage therapy.
TEXT
Diabetic foot infections are common and frequently polymicrobial, but Staphylococcus aureus remains the most frequently isolated pathogen (1–3). The emergence of antibiotic resistance and clinical failures requires the development and the evaluation of innovative alternatives against such infections. In this context, phage therapy is being reexamined as a complementary approach to antibiotics in clinical settings.
However, even though the potency of phage therapy was recently demonstrated in experimental models (4, 5), little is known about its in vivo success in this indication of diabetic foot infection. The assembly of the three bacteriophages described in the study has not been examined in a clinical trial, although it has already been successfully tested as an experimental treatment for compassionate use (6, 7).
The current study aimed to evaluate the pharmacokinetics of three lytic bacteriophages in treatment-naive mice and their therapeutic potential in the treatment of an acute experimental methicillin-susceptible S. aureus (MSSA) nondiabetic or diabetic foot infection model.
The clinical MSSA Hocil17 strain was used. Three anti-S. aureus purified bacteriophages were provided by Pherecydes Pharma (6).
Immunocompetent BALB/c female mice, weighing 20 to 25 g, were used (Charles River). The experimental protocol was approved by the ethics committee (APAFIS nos. 11757 and 11242) and performed in accordance with European Institute of Health EU directive 86/609.
For the pharmacokinetic study, animals received 1 × 108 or 1 × 109 PFU per phage locally (intra-articularly at the patella or in the hindpaw) or intravenously. Groups of five animals were sacrificed at 1, 2, 6, 24, 48, and 72 h postinjection. Blood, spleen, kidney, and liver were collected. The bacteriophage titers (PFU/ml) were determined by a validated spotting method.
Diabetes was induced in 80 mice by two intraperitoneal (i.p.) injections of 150 mg/kg streptozotocin (VWR) at 48 h apart. Two weeks after induction, blood glucose levels were recorded (glucometer Contour Next One) (8). Animals with insufficient levels (<300 mg/dl) before infection were excluded.
Nondiabetic (n = 80) and diabetic (n = 60) mice were randomized to different treatment groups. Mice were anesthetized and infected with a bacterial inoculum of 1 × 107 CFU/mouse at a depth of 2 to 4 mm into the planter proximal aspect of the hindpaw (4).
Thirty minutes postinfection, animals received a single dose of linezolid 25 mg/kg i.p., bacteriophages at 1 × 107 or 1 × 108 PFU/phage into the hindpaw, or the assembly of bacteriophages plus linezolid. The control animals were given vehicle. Bacterial counts were assessed on days 1, 2, 3, and 4.
Treatments were compared by one-way analysis of variance followed by a post hoc Bonferroni test (GraphPad Prism 7.0).
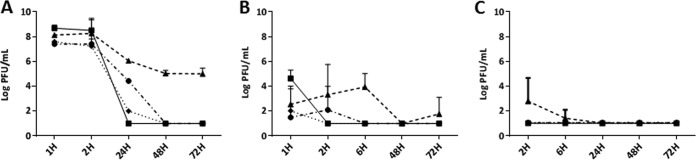
According to the pharmacokinetic study, a high concentration of bacteriophages was present in all tested organs 2 h after the intravenous injection (Fig. 1A). Bacteriophages were still detectable at 72 h in the spleen, whereas they rapidly decreased in blood, liver, and kidney to become undetectable at 48 h.
FIG 1.
Pharmacokinetics of an assembly of three bacteriophages after systemic injection (A) of 1 × 109 PFU/mouse or intraarticular injection at the kneecap (B) or in the hindpaw (C) of 108 PFU/mouse. Representation of mean ± SD (n = 5) PFU/ml in different organs or biological fluid at 1, 2, 6, 24, 48, and 72 h. ■, blood; ▲, spleen; ●, kidney; and ♦, liver. Detection threshold, 10 PFU/ml.
After local administration, phage titers in the organs and blood were low compared with those after systemic injection (Fig. 1B and C). With intra-articular administration, a positive bacteriophage titer was observed in the blood and organs 1 h postinjection; the bacteriophages then rapidly disappeared after 2 h in blood and liver. In comparison, positive bacteriophage titers were recorded in the kidney and spleen, respectively, at 2 and 6 h postinjection, before decreasing to undetectable levels at 6 and 48 h. With hindpaw administration, no phage was recovered in the organs, except in the spleen at 2 h postinjection, but this signal completely disappeared at 6 h.
Overall, no clinical signs of toxicity (mortality, loss of weight, reduced activity) were observed during the pharmacokinetic study.
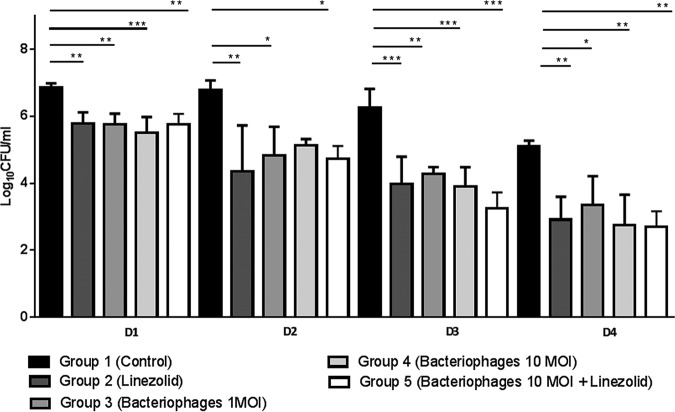
In nondiabetic controls, the bacterial load was stable over 3 days and started to decline on day 4 (Fig. 2). From day 1, regardless of dosage used, a single injection of the bacteriophage assembly was as effective as linezolid in nondiabetic infected animals. No antibacterial synergistic effect was observed in mice receiving the concomitant therapy (phages plus linezolid).
FIG 2.
Bacterial load (log10 CFU/ml), in hindpaws of nondiabetic BALB/c mice (n = 4/group and time point) after treatment with an assembly of three bacteriophages (1 or 10 MOI), linezolid (25 mg/kg i.p.), and the combination of phages plus linezolid. *, P < 0.05; **, P < 0.01; ***, P < 0.001.
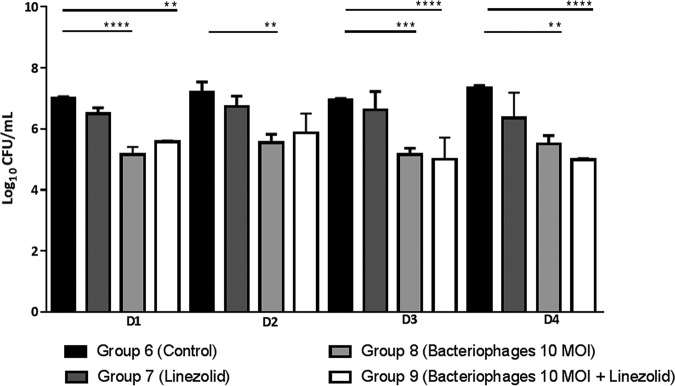
The bacterial load in untreated diabetic mice remained stable over 4 days (Fig. 3), thus longer than in nondiabetic mice. A single injection of bacteriophages at 10 MOI (multiplicity of infection) was significantly effective throughout the study. Surprisingly, linezolid was ineffective in treating diabetic mice. No antibacterial synergistic effect was recorded in diabetic mice receiving the concomitant therapy (phages plus linezolid).
FIG 3.
Bacterial load (log10 CFU/ml) in hindpaws of diabetic BALB/c mice (n = 3 or 4/group and time point) after treatment with an assembly of three bacteriophages, linezolid (25 mg/kg oral), and the combination of phages plus linezolid. **, P < 0.01; ***, P < 0.001; ****, P < 0.0001.
The data set detailed in this proof-of-concept preclinical study demonstrates that a single injection of three combined phages at the infection site was as effective as a single systemic injection of linezolid in reducing bacterial burden in the hindpaw of nondiabetic mice. Of note, in the diabetic model, the bacteriophage assembly was more active than linezolid, which failed to resolve the infection. Despite being the recommended treatment in the guidelines, linezolid has been associated to clinical failures (9, 10, 11).
In both models, no antibacterial synergistic effect was demonstrated in mice receiving the concomitant therapy (bacteriophages plus linezolid). Interestingly, the assembly of three lytic bacteriophages administered systemically or locally at high concentrations was very well tolerated and cleared rapidly from the bodies of noninfected mice.
These encouraging but preliminary findings suggest that bacteriophages may represent an interesting alternative for the treatment of such acute S. aureus infections. However, their potential requires further investigation in other relevant in vivo models, including biofilm-associated infections (e.g., chronic diabetic foot ulcer, orthopedic implant-associated infection models) to better define the most accurate administration parameters for future clinical trials. Even if the in vitro activity of this assembly of bacteriophages is found to be comparable in multidrug-resistant (MDR) or non-MDR S. aureus strains (data not shown), its potential needs to be assessed in MDR-induced preclinical models as well.
Finally, this assembly of bacteriophages has entered clinical development. It is currently being investigated in two trials, one involving diabetic foot ulcer (https://clinicaltrials.gov/ct2/show/NCT02664740) and the other involving prosthetic joint infection indications (http://www.infectiologie.com/UserFiles/File/formation/desc/2016/seminaire-octobre-2016/phagottt-16-09-pr-michel-dupon.pdf), to determine whether this activity translates into comparable broad clinical efficacy.
ACKNOWLEDGMENTS
Sewage water was provided by Les égouts de Paris.
This study was partially supported by PHOSA, a collaborative program financed by BPI France, the Conseil Régional de Bourgogne Franche Comté, and the Communauté Urbaine du Grand Dijon.
The funding bodies did not have any role in the design of the experiments; collection, analysis, and interpretation of data; writing of the manuscript; or decision to submit the manuscript for publication.
We do not have any financial conflicts of interest to declare.
S.A., D.L., D.H., D.B., N.A., and D.C. are employees of Vivexia.
REFERENCES
- 1.Singh N, Armstrong DG, Lipsky BA. 2005. Preventing foot ulcers in patients with diabetes. JAMA 293:217–228. doi: 10.1001/jama.293.2.217. [DOI] [PubMed] [Google Scholar]
- 2.Wu S, Yang T, Luo Y, Li X, Zhang X, Tang J, Ma X, Wang Z. 2014. Efficacy of the novel oxazolidinone compound FYL-67 for preventing biofilm formation by Staphylococcus aureus. J Antimicrob Chemother 69:3011–3019. doi: 10.1093/jac/dku240. [DOI] [PubMed] [Google Scholar]
- 3.Lipsky BA, Aragón-Sánchez J, Diggle M, Embil J, Kono S, Lavery L, Senneville É, Urbančič-Rovan V, Van Asten S, International Working Group on the Diabetic Foot, Peters E. 2016. IWGDF guidance on the diagnosis and management of foot infections in persons with diabetes. Diabetes Metab Res Rev 32 Suppl 1:45–74. doi: 10.1002/dmrr.2699. [DOI] [PubMed] [Google Scholar]
- 4.Chhibber S, Kaur T, Kaur S. 2013. Co-therapy using lytic bacteriophage and linezolid: effective treatment in eliminating methicillin resistant Staphylococcus aureus (MRSA) from diabetic foot infections. PLoS One 8:e56022. doi: 10.1371/journal.pone.0056022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pouillot F, Chomton M, Blois H, Courroux C, Noelig J, Bidet P, Bingen E, Bonacorsi S. 2012. Efficacy of bacteriophage therapy in experimental sepsis and meningitis caused by a clone O25b:H4-ST131 Escherichia coli strain producing CTX-M-15. Antimicrob Agents Chemother 56:3568–3575. doi: 10.1128/AAC.06330-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ferry T, Leboucher G, Fevre C, Herry Y, Conrad A, Josse J, Batailler C, Chidiac C, Medina M, Lustig S, Laurent F, Lyon BJI Study Group. 2018. Salvage debridement, antibiotics and implant retention (“DAIR”) with local injection of a selected cocktail of bacteriophages: is it an option for an elderly patient with relapsing Staphylococcus aureus prosthetic-joint infection? Open Forum Infect Dis 5:ofy269. doi: 10.1093/ofid/ofy269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ferry T. 2019. Alternative options to rescue patients: novel anti-persister antibiotics, local antibiotic delivery, phage therapy, presentation no. S0070. ECCMID, 13 to 16 April 2019, Amsterdam. [Google Scholar]
- 8.Oladeinde FO, Kinyua AM, Laditan AA, Michelin R, Bryant JL, Denaro F, Makinde JM, Williams AL, Kennedy AP, Bronner Y. 2007. Effect of Cnidoscolus aconitifolius leaf extract on the blood glucose and insulin levels of inbred type 2 diabetic mice. Cell Mol Biol (Noisy-le-grand) 53:34–41. [PubMed] [Google Scholar]
- 9.Stein GE, Schooley S, Peloquin CA, Missavage A, Havlichek DH. 2007. Linezolid tissue penetration and serum activity against strains of methicillin-resistant Staphylococcus aureus with reduced vancomycin susceptibility in diabetic patients with foot infections. J Antimicrob Chemother 60:819–823. doi: 10.1093/jac/dkm271. [DOI] [PubMed] [Google Scholar]
- 10.Dryden MS. 2011. Linezolid pharmacokinetics and pharmacodynamics in clinical treatment. J Antimicrob Chemother 66(Suppl 4):iv7–iv15. doi: 10.1093/jac/dkr072. [DOI] [PubMed] [Google Scholar]
- 11.Vardakas KZ, Horianopoulou M, Falagas ME. 2008. Factors associated with treatment failure in patients with diabetic foot infections: an analysis of data from randomized controlled trials. Diabetes Res Clin Pract 80:344–351. doi: 10.1016/j.diabres.2008.01.009. [DOI] [PubMed] [Google Scholar]