Abstract
Purpose: Age-related macular degeneration (AMD) is a common disease trending towards epidemic proportions and is a leading cause of irreversible vision loss in people over the age of 65. A pathomechanism of AMD is death and/or dysfunction of retinal pigment epithelial (RPE) cells; RPE loss invariably results in photoreceptor atrophy. Treatment options for AMD are very limited, and include vitamin supplements and lifestyle changes. An exciting potential therapy currently being tested in clinical trials is transplantation of stem cell-derived RPE.
Methods: We developed a NIH-registered embryonic stem line (CR-4), and in this study set out to determine if CR4-RPE are tolerated in normal mice and in murine models of retinal degeneration by injecting a bolus of CR4-RPE cells in the subretinal space of immunosuppressed wild-type, Mer mutant (Merkd), and Elovl4 deficient mice.
Results: Mice with CR-RPE grafts were monitored daily, were examined routinely using OCT, and histology was prepared and examined at terminal end-points. Based on the parameters of the study, none of the animals with CR-RPE grafts (n=36) experienced any obvious adverse reactions.
Conclusions: We conclude that transplanted CR-4 hES-derived RPE cells are well tolerated in immunosuppressed healthy and dystrophic murine retinas.
Keywords: macular degeneration, stem cell, retinal pigment epithelium, cell therapy
Introduction
Age-related macular degeneration (AMD) is a major global public health problem. In the United States alone, AMD is the leading cause of blindness in people over the age of 65. Treatment options are very limited, and consist of Age-related Eye Disease Study (AREDS) vitamin supplements and lifestyle changes.1–3 The number of patients with AMD is predicted to dramatically increase in the United States and globally.4–6
Transplantation of retinal pigment epithelial (RPE) cells derived from human embryonic stem cells (hESCs) or induced pluripotent stem cells may be a promising new treatment option for this devastating disease.6 RPE cells are a supporting cell type for photoreceptors and indispensable for vision. In fact, RPE dysfunction and/or death is characteristic of AMD. Previous studies have shown that hES-RPE cells implanted in human subjects are well tolerated, and may even reverse some vision loss in AMD.7–9
We developed a robust, new NIH-registered embryonic stem cell line (CR4), and previously demonstrated that CR4-RPE can be readily generated from these cells.10 The CR4 stem cell line, available at low passages, has been independently tested to ensure a stable karyotype up to at least passage 63, is pluripotent and Mycoplasma free. The goal of this study is to further characterize CR4-RPE cells and to determine their preclinical safety profiles by implanting them into wild-type and dystrophic murine retinas.
Methods
CR4-RPE derivation
Divided differentiation of CR4 hESC cells to RPE was carried out using sterile technique according to protocols previously described.10,11 Briefly, CR4 hESCs were grown to confluence on 10-cm dishes in mTeSR1 media (StemCell Technologies Catalog No. 85850) and the media were then changed to differentiation media composed of CTS KnockOut DMEM (ThermoFisher No. A12861-01) supplemented with 1% penicillin-streptomycin (Catalog No. 15070-063), 1% glutamax-1 supplement (Catalog No. A12860-01), 1% nonessential amino acids (Catalog No. 11140-050), 16% knockout serum replacement (Catalog No. 10828-028), and 0.1 mM β-mercaptoethanol (Millipore Sigma; Catalog No. M6250). Cells were maintained in culture for ∼22 weeks, at which point embryoid bodies began to form. These were manually dissociated using a Pasteur pipette and transferred to 24-well plates coated with Corning Matrigel (Catalog No. 354277).
Gene expression of CR4-RPE cells
RPE cell-specific gene expression of the CR4-RPE cells was confirmed by qPCR. When differentiation to RPE was complete, RNA was extracted from the cells using the Qiagen RNeasy Plus Mini kit, and cDNA was synthesized from RNA using the High Capacity cDNA Reverse Transcription Kit from Applied Biosystems. qPCR was performed using primers from Invitrogen, iTaq Universal SYBR Green Supermix (Bio-Rad), and an ABI-7900 thermal cycler. Expression of the following RPE-specific genes was measured using qRT-PCR: OTX-2, VMD, LRAT, Connexin43, MerTK, RPE65, MiTF, Pmel, and CRALBP.12 The following primers used for this experiment were supplied by ThermoFisher Scientific: OTX-2 (Catalog No. Hs00222238_m1), VMD (Best1) (Catalog No. Hs04397293_m1), LRAT (Catalog No. Hs00428109_m1), Connexin43 (Catalog No. Hs00748445_s1), MerTK (Catalog No. Hs01031979_m1), RPE65 (Catalog No. Hs01071462), MiTF (Catalog No. Hs01117294), Pmel (Catalog No. Hs00173854_m1), and CRALBP (Catalog No. Hs00165632_m1).
β-actin primers were purchased from Integrated DNA Technologies. The primer sequences used are as follows: forward: 5′-GGA TGC AGA AGG AGA TCA CTG-3′ reverse: 5′-CGA TCC ACA CGG AGT ACT TG-3′.
Fold difference in expression is compared with the level of expression in fetal RPE (fRPE) cDNA obtained from ScienCell Research Laboratories (Catalog No. 6544). The level of expression for fRPE is set to 1.0, and the CR4-RPE cells are compared with this level. Fold changes in expression are detailed in Fig. 1.
FIG. 1.
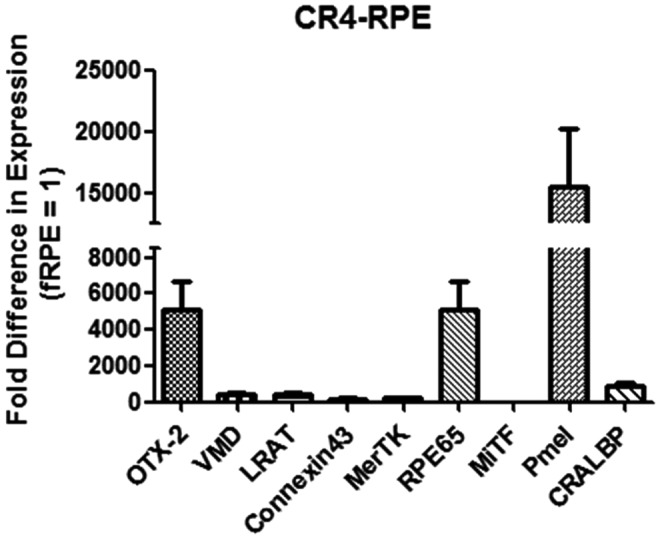
Results of qPCR showing fold-change expression of genes in CR4-RPE cells compared with fetal RPE and normalized to β-actin. RPE, retinal pigment epithelium.
Animals and experimental design
The animal models chosen for this study include the Merkd (Jackson Laboratory Catalog No. 011122) (n = 12) and Elovl4−/− (Jackson Laboratory Catalog No. 027977) (n = 12) mouse models for retinal degeneration.13–16 Mutations in the MerTK gene are responsible for causing retinal degeneration in the RCS rat because the mutated MerTK gene renders RPE cells incapable of carrying out phagocytosis, thus inducing progressive retinal degeneration.17 The Elovl4 mouse model has been used in previous preclinical studies to demonstrate the safety of stem cell-derived RPE cells that have been used in Phase I clinical trials.18
Six males and 6 females of each strain were injected subretinally in the right eye with 5 × 104 (50,000) CR4-RPE cells suspended in 0.5-μL sterile phosphate-buffered saline (PBS). Left eyes remained uninjected to serve as an internal control. Immunosuppression was achieved in the main experimental animals using cyclosporine A dissolved in the drinking water at a concentration of 210 mg/L.17,19
After injection, these animals were housed under the supervision of the Institutional Animal Care and Use Committee (IACUC) of Baylor College of Medicine. All experiments were carried out with institutional IACUC approval and in compliance with the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research.
Subretinal injections
Mature RPE cells were freshly harvested immediately before injection. The cells were dissociated using 0.5% trypsin and manually dissociated from the culture plates with a 2-mL glass pipet. The cell suspension was then diluted to a concentration of 100,000 cells/μL in sterile PBS.
To carry out the surgical procedure, animals were anesthetized using a ketamine-xylazine mixture of 87.5 mg/kg ketamine-12.5mg/kg xylazine delivered intraperitoneally. When complete anesthesia was achieved, the right eye was proptosed and a scleral hole was made using a sharp 31-gauge needle directly posterior the limbus inferior temporally. Immediately after withdrawal of the sharp needle, a blunt 34-gauge needle attached to a preloaded Hamilton syringe with 0.5 μL (50,000 cells) was inserted through the scleral hole and passed transvitreally to the contralateral (superior-nasal) side of the eye, where cells were injected into the subretinal space.20
The injections of CR4-RPE cells took place at postnatal day 21 in the Elovl4−/− mice. This day was chosen because retinal degeneration is documented to begin in these mice after P21.15,16 Merkd mice were injected at postnatal day 14.
Optical coherence tomography
Assessment of the induced retinal detachment was performed using optical coherence tomography (OCT) imaging on the Bioptigen Envisu 4110. Both eyes were imaged using OCT 1 day after injection and again at 5, 8, and 14 days. The injection site and subretinal bleb (induced detachment) were visualized at 1 day postinjection. Progression of healing was observed, and the detachment was observed to be resolved by 14 days postinjection (Fig. 2).
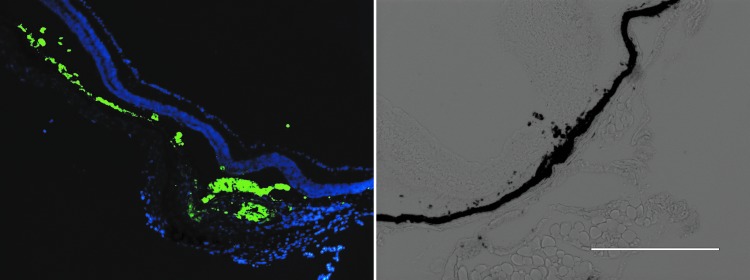
FIG. 2.
Left: The CR4-RPE cells have unique spectral qualities that can be distinguished from host cells in the subretinal space of a male mouse 60 days after injection. DAPI-stained section, 10 × . Right: Pigmented CR4-RPE cells at injection site. Bright field, 20 ×.
Histology
Two males and 2 females of each strain were then sacrificed at each of 3 time points for histological analysis of retinal tissue. The time points selected for sacrificing animals were as follows: 5 days postinjection, 14 days postinjection, and 60 days postinjection. After applying fixative, eyes were cryopreserved, sectioned, and subjected to immunohistochemistry. The uninjected left eyes were used as controls, while the right eyes were stained with DAPI, hematoxylin–eosin, or Giemsa. Since the CR4-RPE cells have unique spectral qualities, they were readily identifiable using a EVOS microscope with a filter for these specifications: 470/22 nm excitation; 510/42 nm emission.
Results
Our principal objective in this study was to examine if our CR4-RPE cells can be safely injected into the subretinal space of mouse models for retinal degeneration without invoking obvious adverse events. The major findings of this study are summarized in Table 1. We used 2 models of spontaneous retinal degeneration: Merkd and Elovl4 mutant mice. We used MerTK mutants in previous studies,17,19,21 and Elovl4 were used by other groups.22 Our major concerns were that the implanted cells might elicit detrimental immune reactions, form teratomas, or migrate into other regions of the retina. We also examined the retinas for complications of subretinal injections.
Table 1.
Findings of No Common Surgical Complications in the Subject Animals During This Study
| C57/BL6 wild-type | MerTK−/− | Elovl4−/− | |
|---|---|---|---|
| 5 days | n = 4 (2 males, 2 females) | n = 4 (2 males, 2 females) | n = 4 (2 males, 2 females) |
| 14 days | n = 4 (2 males, 2 females) | n = 4 (2 males, 2 females) | n = 4 (2 males, 2 females) |
| 60 days | n = 4 (2 males, 2 females) | n = 4 (2 males, 2 females) | n = 4 (2 males, 2 females) |
| Uveitis | None | None | None |
| Proliferative vitreoretinopathy | None | None | None |
| Cataract formation | None | None | None |
| Tumor formation (teratoma) | None | None | None |
| Induced retinal degeneration | None | None | None |
In this study, we examined the animals on a daily basis for any overt manifestations of uveitis, cataracts, or illness/discomfort. All animals survived the procedures, showed no overt adverse effects, and were sacrificed on schedule.
The injection site and subretinal bleb (induced detachment) were visualized at 1 day postinjection using OCT, and reattachment/healing was observed in all animals by 14 days postinjection.
Histological examination of harvested eyes showed persistence of the CR4-RPE cells in the subretinal space without infiltration of inflammatory cells. Eyes harvested from mice at early time points permitted visualization of well-demarcated injection sites. Eyes from mice harvested at later time points showed resolved injection sites with appropriate formation of retinal scarring. Although refluxed cells were observed inside the vitreous space and directly above the neural retina in several animals, no evidence of proliferative vitreoretinopathy was observed in any animals. In addition, cells appeared to migrate only minimally from the initial subretinal bleb to form a new RPE monolayer above the native RPE layer in the mice. Some pigmented cells also remain in the path of the initial retinal injection site as a result of tracking through the path of the needle.
Discussion
The results of this short-term safety study demonstrate that CR4 hES-derived RPE cells can be safely implanted in the subretinal space of healthy and diseased murine eyes. After performing this study, we are more confident that CR4-RPE cells can be implanted safely and well tolerated when delivered to the appropriate site.
The animals included in this study were observed daily for signs of cataract formation, ocular inflammation, conjunctival injection, photophobia, or behavioral changes. The absence of any of these symptoms throughout the study, along with our ability to obtain clear images on OCT examinations, is convincing evidence that our injections healed well and did not lead to surgical complications such as uveitis or traumatic cataract formation.
As previously mentioned, the injection site was easily visualized on histological examination of the injected eyes. This is compelling evidence that our technique is able to deliver the cells precisely to the subretinal space without extensive damage to the surrounding retinal architecture.
One of the most serious potential complications when working with stem cell-derived tissues is the threat of teratoma formation. Although it is theoretically possible that a teratoma could grow from a single undifferentiated cell, in practice a threshold exists, and spiking studies have shown that a certain percentage of undifferentiated cells is required for tumor growth.18,23 We were able to conclude that our differentiation protocols result in a sufficiently pure cell population of fully differentiated RPE cells so as to prevent teratoma formation. Furthermore, in this study and others we have implanted cells into animals without immunosuppressive therapy, and these animals are currently healthy at 8 months of age (7-month postinjection) without any evidence of tumor formation.21
Conclusion
The results of this study provide the foundation for future investigations, examining the effects of subretinal implantation of CR4-RPE cells at longer time points, and in a larger animal model to prove a higher degree of safety. Further, useful studies may include demonstrating the preservation or restoration of vision after injection of the CR4-RPE cells. This study serves as an important line of evidence, showing that CR4-RPE cells can be implanted safely to the subretinal space of mouse models for the treatment of retinal degeneration.
Acknowledgments
We wish to acknowledge Dr. David Marshak (UT Health), and Guofu Shen (BCM) for their valuable contributions to this project. We also wish to pay a special tribute to Clive Runnells (1926–2019). Not only did his generous donations fund this study, but also funded several other important medical research studies as well. We will miss his positive energy, passion for innovation, and long-standing desire to help others.
Author Disclosure Statement
No competing financial interests exist.
Funding Information
This work was funded by the Clive and Nancy Runnells Embryonic Stem Cell Research Fund (in memory of Pierce Runnells) at The University of Texas Health Science Center at Houston, by Core Grant 2P30EY002520 (Baylor College of Medicine), and by institutional grants to the Department of Ophthalmology at Baylor College of Medicine.
References
- 1. Group A-REDSR. A randomized, placebo-controlled, clinical trial of high-dose supplementation with vitamins C and E, beta carotene, and zinc for age-related macular degeneration and vision loss: AREDS report no. 8. Arch. Ophthalmol. 119:1417, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Evans J.R., and Lawrenson J.G.. Antioxidant vitamin and mineral supplements for slowing the progression of age-related macular degeneration. Cochrane Database Syst. Rev. 7:Cd000254, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Mares J.A., Voland R.P., Sondel S.A., et al. Healthy lifestyles related to subsequent prevalence of age-related macular degeneration. Arch. Ophthalmol. 129:470–480, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Brechner R.J., Rosenfeld P.J., Babish J.D., and Caplan S.. Pharmacotherapy for neovascular age-related macular degeneration: an analysis of the 100% 2008 medicare fee-for-service part B claims file. Am. J. Ophthalmol. 151:887–895. e1, 2011 [DOI] [PubMed] [Google Scholar]
- 5. Wong W.L., Su X., Li X., et al. Global prevalence of age-related macular degeneration and disease burden projection for 2020 and 2040: a systematic review and meta-analysis. Lancet Global Health. 2:e106–e116, 2014 [DOI] [PubMed] [Google Scholar]
- 6. Pan C.K., Heilweil G., Lanza R., and Schwartz S.D.. Embryonic stem cells as a treatment for macular degeneration. Expert Opin. Biol. Ther. 13:1125–1133, 2013 [DOI] [PubMed] [Google Scholar]
- 7. da Cruz L., Fynes K., Georgiadis O., et al. Phase 1 clinical study of an embryonic stem cell-derived retinal pigment epithelium patch in age-related macular degeneration. Nat. Biotechnol. 36:328–337, 2018 [DOI] [PubMed] [Google Scholar]
- 8. Schwartz S.D., Hubschman J.P., Heilwell G., et al. Embryonic stem cell trials for macular degeneration: a preliminary report. Lancet. 379:713–720, 2012 [DOI] [PubMed] [Google Scholar]
- 9. Schwartz S.D., Regillo C.D., Lam B.L., et al. Human embryonic stem cell-derived retinal pigment epithelium in patients with age-related macular degeneration and Stargardt's macular dystrophy: follow-up of two open-label phase 1/2 studies. Lancet. 385:509–516, 2015 [DOI] [PubMed] [Google Scholar]
- 10. Mazzilli J.L., Domozhirov A.Y., Mueller-Ortiz S.L., Garcia C.A., Wetsel R.A., and Zsigmond E.M.. Derivation and characterization of the human embryonic stem cell line CR-4: differentiation to human retinal pigment epithelial cells. Stem Cell Res. 18:37–40, 2017 [DOI] [PubMed] [Google Scholar]
- 11. Klimanskaya I., Hipp J., Rezai K.A., West M., Atala A., and Lanza R.. Derivation and comparative assessment of retinal pigment epithelium from human embryonic stem cells using transcriptomics. Cloning Stem Cells. 6:217–245, 2004 [DOI] [PubMed] [Google Scholar]
- 12. Song W.K., Park K.M., Kim H.J., et al. Treatment of macular degeneration using embryonic stem cell-derived retinal pigment epithelium: preliminary results in Asian patients. Stem Cell Rep. 4:860–872, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Duncan J.L., LaVail M.M., Yasumura D., et al. An RCS-like retinal dystrophy phenotype in mer knockout mice. Invest. Ophthalmol. Vis. Sci. 44:826–838, 2003 [DOI] [PubMed] [Google Scholar]
- 14. Lu Q., Gore M., Zhang Q., et al. Tyro-3 family receptors are essential regulators of mammalian spermatogenesis. Nature. 398:723–728, 1999 [DOI] [PubMed] [Google Scholar]
- 15. Karan G., Lillo C., Yang Z., et al. Lipofuscin accumulation, abnormal electrophysiology, and photoreceptor degeneration in mutant ELOVL4 transgenic mice: a model for macular degeneration. Proc. Natl. Acad. Sci. U. S. A. 102:4164–4169, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Vasireddy V., Jablonski M.M., Mandal M.N., et al. Elovl4 5-bp-deletion knock-in mice develop progressive photoreceptor degeneration. Invest. Ophthalmol. Vis. Sci. 47:4558–4568, 2006 [DOI] [PubMed] [Google Scholar]
- 17. Westenskow P.D., Bucher F., Bravo S., et al. iPSC-derived retinal pigment epithelium allografts do not elicit detrimental effects in rats: a follow-up study. Stem Cells Int. 2016:8470263, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Lu B., Malcuit C., Wang S., et al. Long-term safety and function of RPE from human embryonic stem cells in preclinical models of macular degeneration. Stem Cells. 27:2126–2135, 2009 [DOI] [PubMed] [Google Scholar]
- 19. Krohne T.U., Westenskow P.D., Kurihara T., et al. Generation of retinal pigment epithelial cells from small molecules and OCT4 reprogrammed human induced pluripotent stem cells. Stem Cells Transl. Med. 1:96–109, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Westenskow P.D., Kurihara T., Bravo S., et al. Performing subretinal injections in rodents to deliver retinal pigment epithelium cells in suspension. J. Vis. Exp. 95:52247, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Zhao T., Zhang Z.N., Westenskow P.D., et al. Humanized mice reveal differential immunogenicity of cells derived from autologous induced pluripotent stem cells. Cell Stem Cell. 17:353–359, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Lund R.D., Wang S., Klimanskaya I., et al. Human embryonic stem cell-derived cells rescue visual function in dystrophic RCS rats. Cloning Stem Cells. 8:189–199, 2006 [DOI] [PubMed] [Google Scholar]
- 23. Gropp M., Shilo V., Vainer G., et al. Standardization of the teratoma assay for analysis of pluripotency of human ES cells and biosafety of their differentiated progeny. PLoS One. 7:e45532, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]